- 1Mycological Laboratory, Department of Dermatology, Peking University Third Hospital, Beijing, China
- 2Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands
- 3Center of Expertise in Mycology, Radboud University Medical Center/Canisius Wilhelmina Hospital, Nijmegen, Netherlands
Background: A 73-year-old female suffering from acute myeloid leukemia presented with progressive rhinofacial mycosis. Suspecting it to be mucormycosis, the antifungal amphotericin B (AMB) was administered empirically, but the patient did not respond as planned. The fungus was then isolated from the biopsied tissue and morphologically identified as a species of Aspergillus. Necrosis progressed and she died of cerebral hemorrhage. Since Aspergillus flavus is susceptible to AMB, and several other Aspergillus species can be misidentified as A. flavus, the observed resistance necessitated a re-examination of the fungal isolate.
Methods: The fungal strain was re-isolated and re-examined morphologically. Additionally, genomic DNA was extracted from the fungus and sequences were obtained from three genomic regions [the rDNA internal transcribed spacer (ITS) region, and portions of the β-tubulin and calmodulin genes] to more accurately identify this Aspergillus strain. Its antifungal susceptibility was assessed using multiple compounds and our findings were compared with literature data.
Results: The fungal culture again yielded an Aspergillus isolate morphologically identical to A. flavus. Molecular analyses, however, revealed the strain to be A. nomiae, a close relative of A. flavus in section Flavi, and it exhibited resistance to AMB. Reviewing the literature, only five other cases of A. nomiae infection in humans have been reported worldwide.
Conclusion and Clinical Importance: The rhinofacial mycosis of the patient was actually due to A. nomiae. The initial misidentification of the fungus, coupled with its resistance to AMB, could be the reason treatment did not help the patient. We postulate that clinical A. nomiae infections may be underreported and that accurate and speedy pathogen identification is important so that an effective antifungal regimen can be administered.
Introduction
Invasive aspergillosis, i.e., deep infections by an Aspergillus species, are among the most common opportunistic mold infections that can cause potentially life-threatening disease in those with chronic neutropenia, or inherited or acquired immunodeficiencies, as well as in those undergoing allogeneic hematopoietic stem cell transplant, solid organ transplant, or prolonged corticosteroid use (Li et al., 2008; Patterson et al., 2016). Among these, pulmonary infection and rhino-(facial)-orbital-cerebral mycosis (ROCM) are the most problematic types (Patterson et al., 2016; Leroy et al., 2020; Hu et al., 2021). Second only to Aspergillus fumigatus for lung infections and ROCM (Alsalman et al., 2017; Rudramurthy and Paul, 2019; Hu et al., 2021), Aspergillus flavus is the most frequently encountered species in invasive sinusitis (Bakhshaee et al., 2016; Alsalman et al., 2017); in 9% of cases of ROCM, A. flavus is listed as the causal organism (Hu et al., 2021), while other species have remained unidentified in the literature (Hu et al., 2021). Although clinical outcomes of treatment of aspergilloses have markedly improved with the availability of newer triazoles, the development of resistance to triazoles in A. fumigatus and the intrinsic resistance to polyenes in A. flavus is a growing concern (Rudramurthy and Paul, 2019; Wiederhold and Verweij, 2020). The understanding of antifungal drug resistance at the species level is of great significance for clinical selection of empirical drug therapy.
Aspergillus flavus has a number of similar molecular siblings. Phylogenetically, the Aspergillus section Flavi is split into eight series: Alliacei, Avenacei, Bertholletiarum, Coremiiformes, Flavi, Kitamyces, Leporum, and Nomiarum (Frisvad et al., 2019; Houbraken et al., 2020). A. flavus can have two morphotypes (L and S) based on sclerotium production; significant morphological, physiological, and pathological differences exist between the two morphotypes (Gilbert et al., 2018). Given that A. flavus shares a similar morphology with other closely related species, potential misidentification of an isolate as A. flavus is an issue. Molecular investigations of certain conserved loci (e.g., ITS, BenA, CaM) can help in determining the species. With high morphological similarities, conserved loci most often help to delineate species of fungi (Houbraken et al., 2020).
Aspergillus strains can produce B-series and G-series aflatoxins (Ehrlich et al., 2004), which are notorious for hepatocellular carcinoma development, lung adenocarcinoma, and chronic inflammatory changes. Infection by aflatoxin-producing species was reported in patients with leukemia (Mori et al., 1998; Cha and Dematteo, 2005; Liu et al., 2015). Being a new aflatoxin-producing species belonging to Aspergillus section Flavi, Aspergillus nomiae strains are able to produce both series B and G aflatoxins (Ehrlich et al., 2004). However, the relationship between aflatoxin-producing Aspergillus infection and leukocytosis or leukemia is unclear (Mori et al., 1998).
Rhinofacial fungal infections are almost entirely restricted to entomophthoromycoses and mucormycosis (Li and Lun, 2012; Li et al., 2014; Shaikh et al., 2016), very specific clinical entities that are usually susceptible to the antifungal compound amphotericin B (AMB) (Li et al., 2014). Here, we report an erosive case of invasive rhinofacial fungal infection that was resistant to antifungal treatment with AMB and contributed to the death of the patient after only 7 days in our hospital. A. flavus has been reported to be variably resistant to AMB (Hagiwara et al., 2016; Jalalian and Shokohi, 2020), the most commonly used antifungal in cases of severe infection in high-risk patients (Li and de Hoog, 2009; Li and Lun, 2012; Li et al., 2014). Antifungal resistance may be species-dependent, therefore rapid and accurate identification of resistant species is of great importance. The present case report of rhinofacial fungal infection concerns a fungus that was resistant to AMB. This resistant fungus warranted further study, which included both morphological and molecular investigations, supplemented with a literature search into previous clinical cases of fungal resistance to AMB. In this study, we revisited the original case file and determined the need to re-examine the fungal isolate using morphological and molecular techniques, to assess its antifungal susceptibility profile, and (once accurately identified) to search the literature for other cases involving antifungal resistance by this particular Aspergillus species in a clinical setting.
Materials and Methods
Case Report
In November of 2014, a 73-year-old female with acute myeloid leukemia presented to Peking University Third Hospital (Beijing, China) with progressive nose swelling and ulceration after a severe cold. The lesions had begun two months earlier as infiltrated erythema, then rapidly increased in size and ulcerated. The patient was initially diagnosed with skin and subcutaneous infection in the nose and face (rhinofacial) and treatment with antibiotics was ineffective. Next, the lesion was diagnosed as a malignant ulcer and chemotherapy was administered, but the necrotic ulcer became progressively enlarged. Subsequent clinical diagnosis of rhinofacial mucormycosis was made and the patient was treated with AMB (initial dose 5 mg/day, increased by 5 mg daily), but the ulcer still progressed. Two months after onset of the malignant ulcer, the patient was transferred to Peking University Third Hospital.
The patient had a 1-year history of acute myeloid leukemia and had received one cycle of DA chemotherapy (daunorubicin and cytarabine), two cycles of MA chemotherapy (mitoxantrone and cytarabine), one cycle of CAG chemotherapy (cytarabine, aclarubicin, and recombinant human granulocyte-colony stimulating factor), and one cycle of AA chemotherapy (aclarubicin and cytarabine). She denied any history of preceding injury or insect bite at the site of infection. Physical examination revealed erythema, edema, and ulceration over the right nasal alae and upper lip, extending to the soft tissue around the right side of the nose (Figure 1).
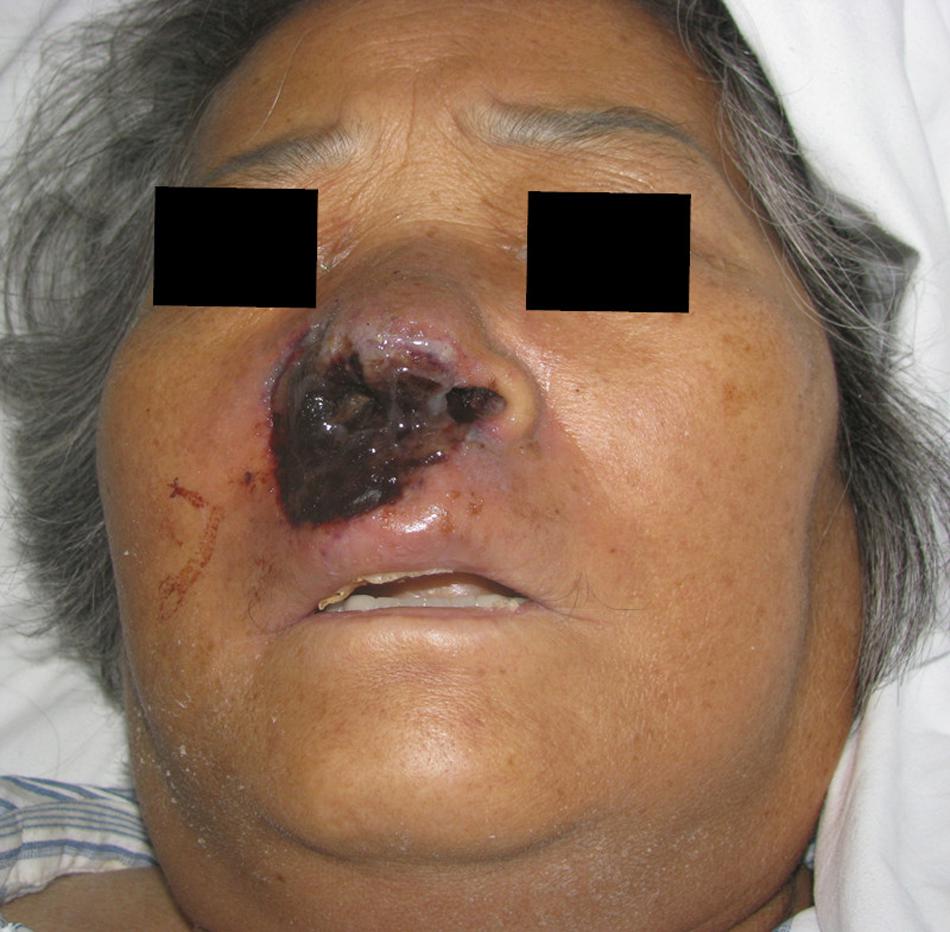
Figure 1. Image of patient exhibiting edematous erythema, ulcer, and necrosis over the right nasal alae and upper lip.
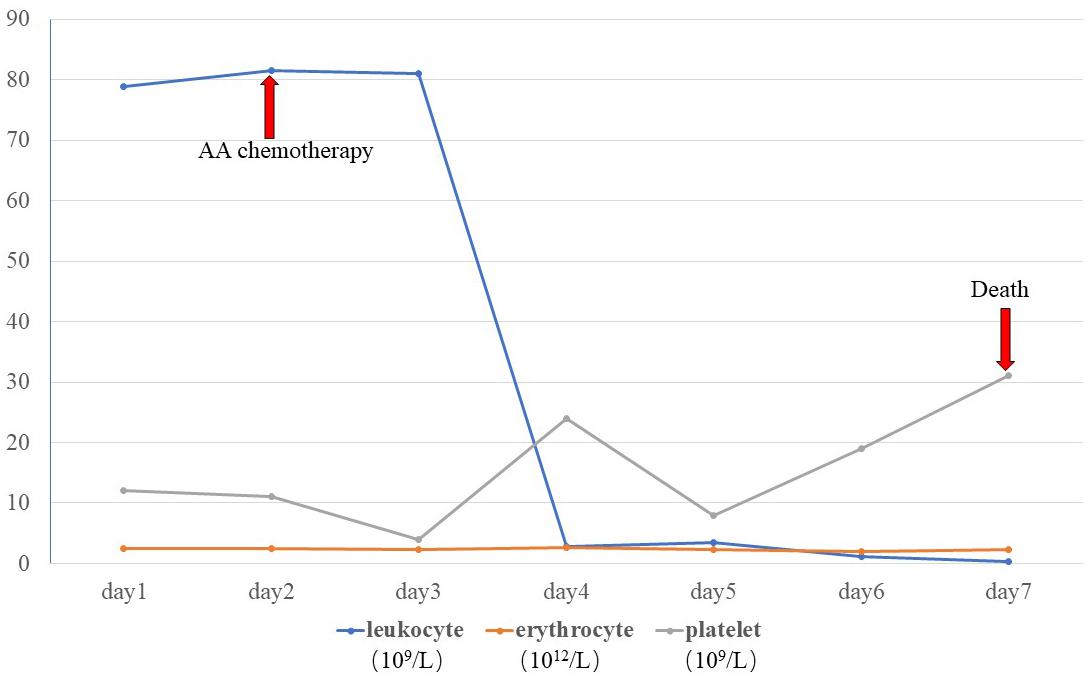
A skin biopsy was performed on the lesion of the nasal dorsum. Histopathological examination later revealed inflammation, necrosis, granuloma, and angiodestruction. In addition, numerous hyaline and septate hyphae with branches at acute angles were observed throughout the dermis with angioinvasion (Figure 2). The laboratory evaluation revealed hyperleukocytosis (78.9 × 109/L), erythrocytopenia (2.44 × 1012/L), thrombocytopenia (12 × 109/L), hypokalemia (2.38 mmol/L), an elevated level of C-reactive protein (31.2 mg/dL), and a positive serum galactomannan (GM) test (1.25 μg/L). The results of the antinuclear antibody test, T cell enzyme-linked immunospot assay, and serum (1-3)-β-d-glucan (G) test were all negative. Serum hepatitis B surface antigen and antibodies to human immunodeficiency virus, hepatitis C virus, and Treponema pallidum were all negative. Leukocyte counts decreased rapidly after receiving AA chemotherapy during hospitalization (Figure 3).
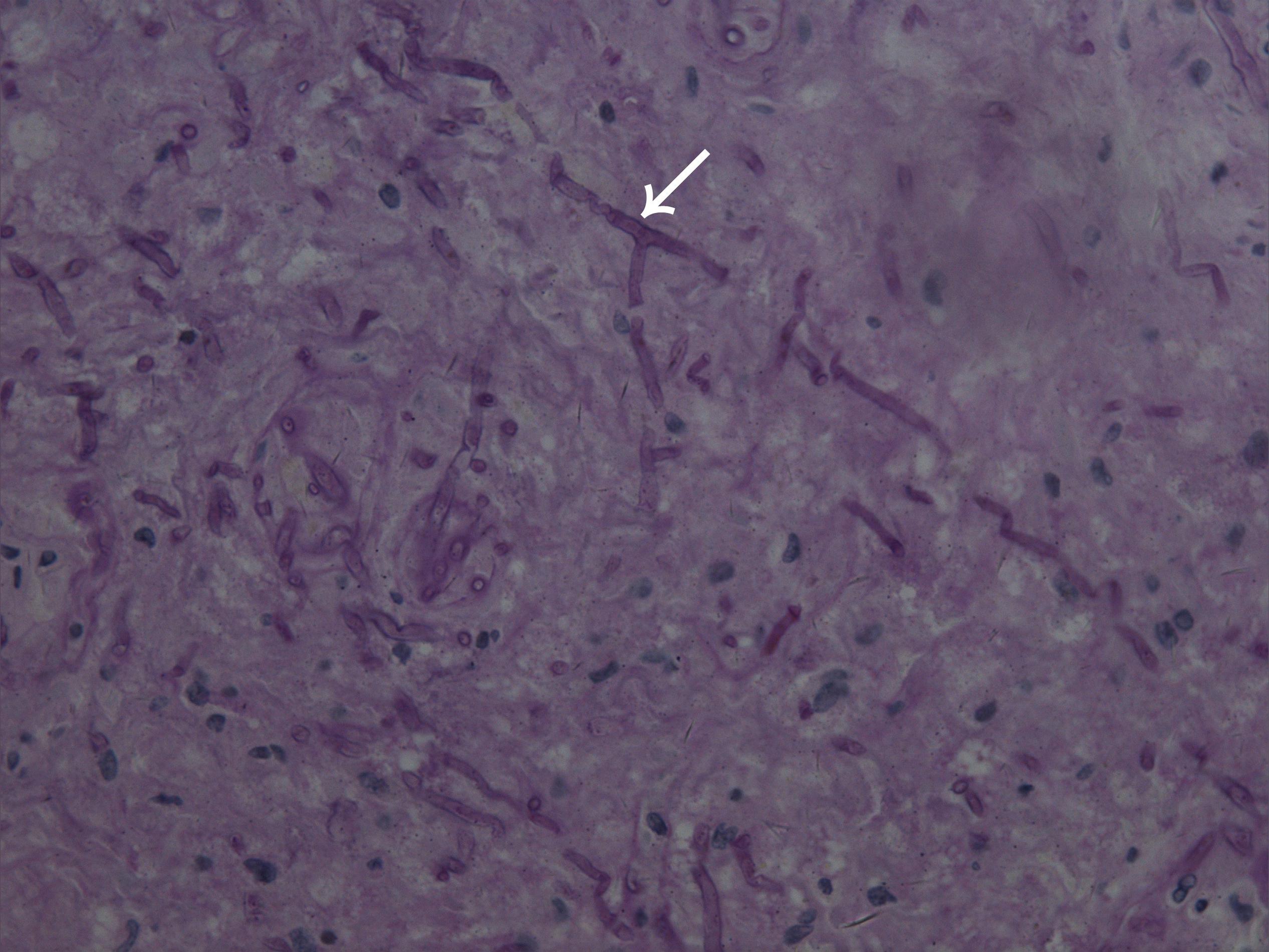
Figure 2. Microscope image of biopsied lesion tissue showing numerous hyaline and septate hyphae with branches at acute angles scattered in the dermis (arrowhead) (periodic acid–Schiff, original magnification × 400).
Despite 7 days of continuous treatment with AMB (before biopsy), the ulcer progressed and the patient died of cerebral hemorrhage. The biopsied tissue yielded an Aspergillus isolate that was initially identified by morphology as A. flavus. However, the antifungal susceptibility profile of the culture showed resistance to AMB.
Re-assessment of Fungal Morphology
The biopsied specimen was originally cultured and stored on Sabouraud’s glucose agar (SGA) medium in the Peking University Third Hospital collection under accession number PUTH 1342. For this study, we recovered the fungus from the original culture by inoculation onto potato dextrose agar (PDA) media and incubation at 37°C for 7 days. We then conducted macro- and micro-morphological examinations to identify the fungal pathogen. On the basis of these microscopic and macroscopic characteristics, the fungus was identified as a member of the Aspergillus section Flavi.
Molecular Investigations
Genomic DNA of the fungal isolate was extracted using the Biospin Fungus Genomic DNA Extraction kit (Bioer Technology, Hubei, China) in accordance with manufacturer’s instructions. The internal transcribed spacer (ITS) region was amplified using the ITS5 and ITS4 primers (Zhou et al., 2019), a segment of the β-tubulin gene (BenA) was amplified with βtub1 and βtub2 primers (Balajee et al., 2005), as well as a segment of the calmodulin gene (CaM) with cmd5 and cmd6 primers (Hong et al., 2005). Each PCR mixture contained 1 μL extracted fungal DNA, 0.08 μM each of the primers, and 12.5 μL 2 × Taq PCR MasterMix (Tiangen Biotech, Beijing, China) in a 25 μL reaction volume. PCR cycles comprised of an initial denaturation at 95°C for 5 min, followed by 35 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 1 min, a final extension at 72°C for 10 min, and cooling to 4°C. The amplicons were then sequenced by BGI Company (Beijing, China). The sequences obtained were compared with those deposited in GenBank by BLAST query.
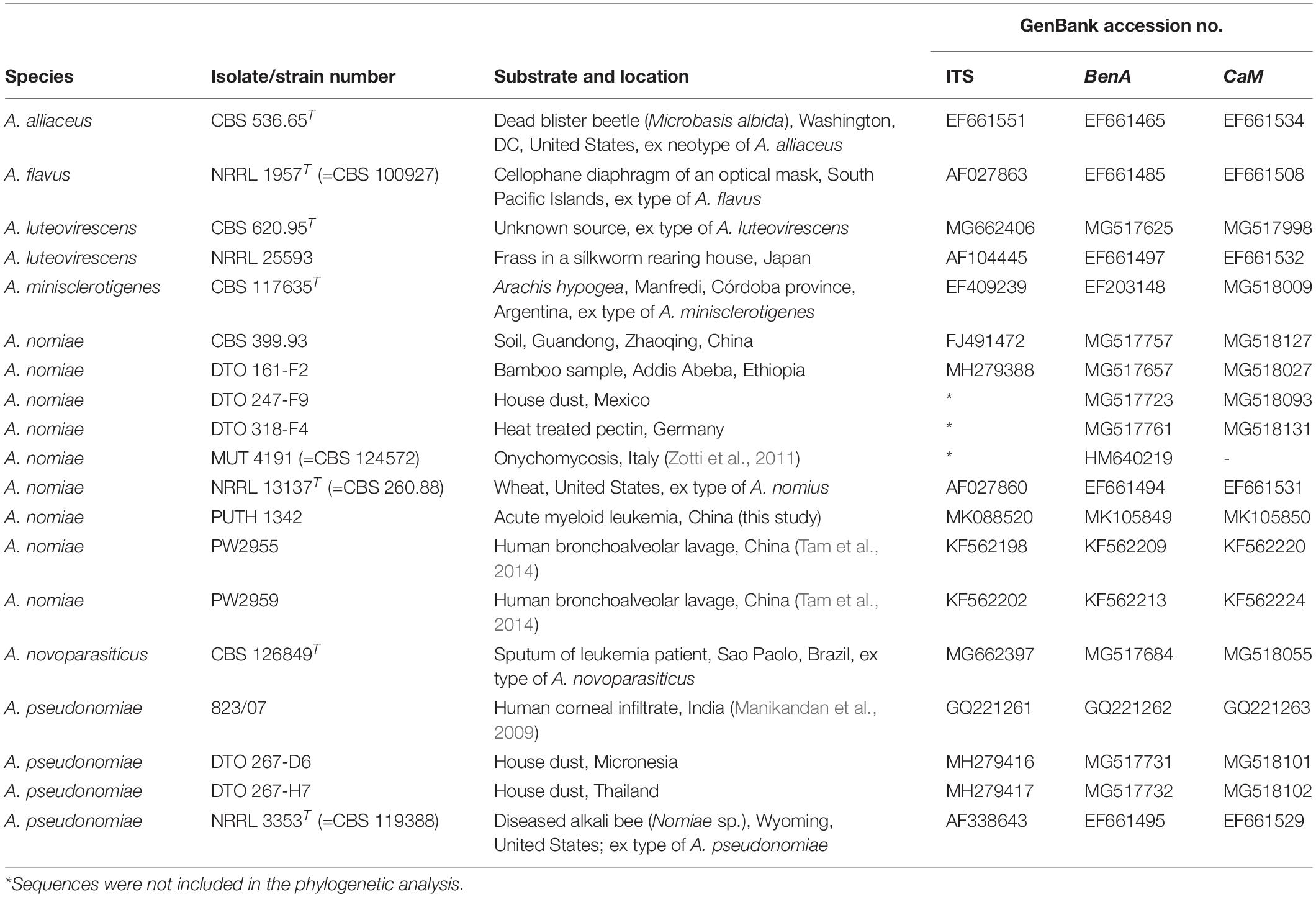
Based on BLAST findings, a phylogenetic analysis was performed based on concatenation of the three genomic regions (ITS, BenA, and CaM) to help determine the relationship between our isolate and seven reference type strains from section Flavi, as well as additional representatives of the identified species (Table 1). Maximum likelihood analysis of the individual datasets was performed using MEGA7 and the robustness of the trees was evaluated by 1000 bootstrap replicates.
Antifungal Susceptibility Testing
Antifungal susceptibility testing was performed using the CLSI M28-A2 microbroth dilution method. Minimal inhibitory concentrations (MIC) and minimum effective concentrations (MEC) were determined after growth at 35°C for 46–50 h for all antifungal agents tested. The MIC endpoints for itraconazole, voriconazole, posaconazole, and AMB were determined using a reading mirror as the lowest concentration of the drug that prevented any recognizable growth (100% inhibition). The MIC results were interpreted according to the revised clinical breakpoints v. 10.0 of the European Committee on Antimicrobial Susceptibility Testing (EUCAST), who defines new criteria for resistance to AMB (S ≤ / > R = 1/1) (Arendrup et al., 2020). The other revised breakpoints include itraconazole (ATU = 2) and isavuconazole against A. flavus (S usvuszolevucoATU = 2); isavuconazole (S ≤ / > R = 1/2, ATU = 2), itraconazole [S conazole (S oATU = 2)], posaconazole (ATU = 0.25), and voriconazole [S conazole (S iATU = 2)] against A. fumigatus, itraconazole (S conazoletracoATU = 2), and voriconazole [S conazole (S nATU = 2)] against Aspergillus nidulans, AMB against Aspergillus niger (S rgerst n B) against itraconazole [S conazole (S gATU = 2)], and posaconazole (ATU = 0.25) against Aspergillus terreus. MEC endpoints for caspofungin and micafungin were defined microscopically as the lowest concentration of drug that led to the growth of small, rounded, compact hyphal forms, compared to the long, unbranched hyphal clusters observed in the growth control. Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019, and A. flavus ATCC 204304 were used as quality controls.
Literature Search
After the species identity of our causal fungus was confirmed, an electronic search was performed using the correct species name and “human infection” in the PubMed database1.
Results
A velvety, yellow-green colony was observed on PDA (Figure 4A) after 1 week at 25°C. After prolonged incubation (>2 weeks), bullet-shaped sclerotia appeared. Microscopic examination of the fungus revealed hyaline and septate hyphae with branches at acute angles. Conidiophores had roughened stipes measuring 400–1000 μm; conidiophore heads had a spherical vesicle of 20–50 μm in diameter, fertile on more than three quarters of their surface, and carrying both metulae and phialides of 7–10 μm long. Conidia were rough-walled, subspherical, and measuring 2–5 μm diameter (Figures 4B–G). This morphology matched the description of the fungus given by de Hoog et al. (2019).
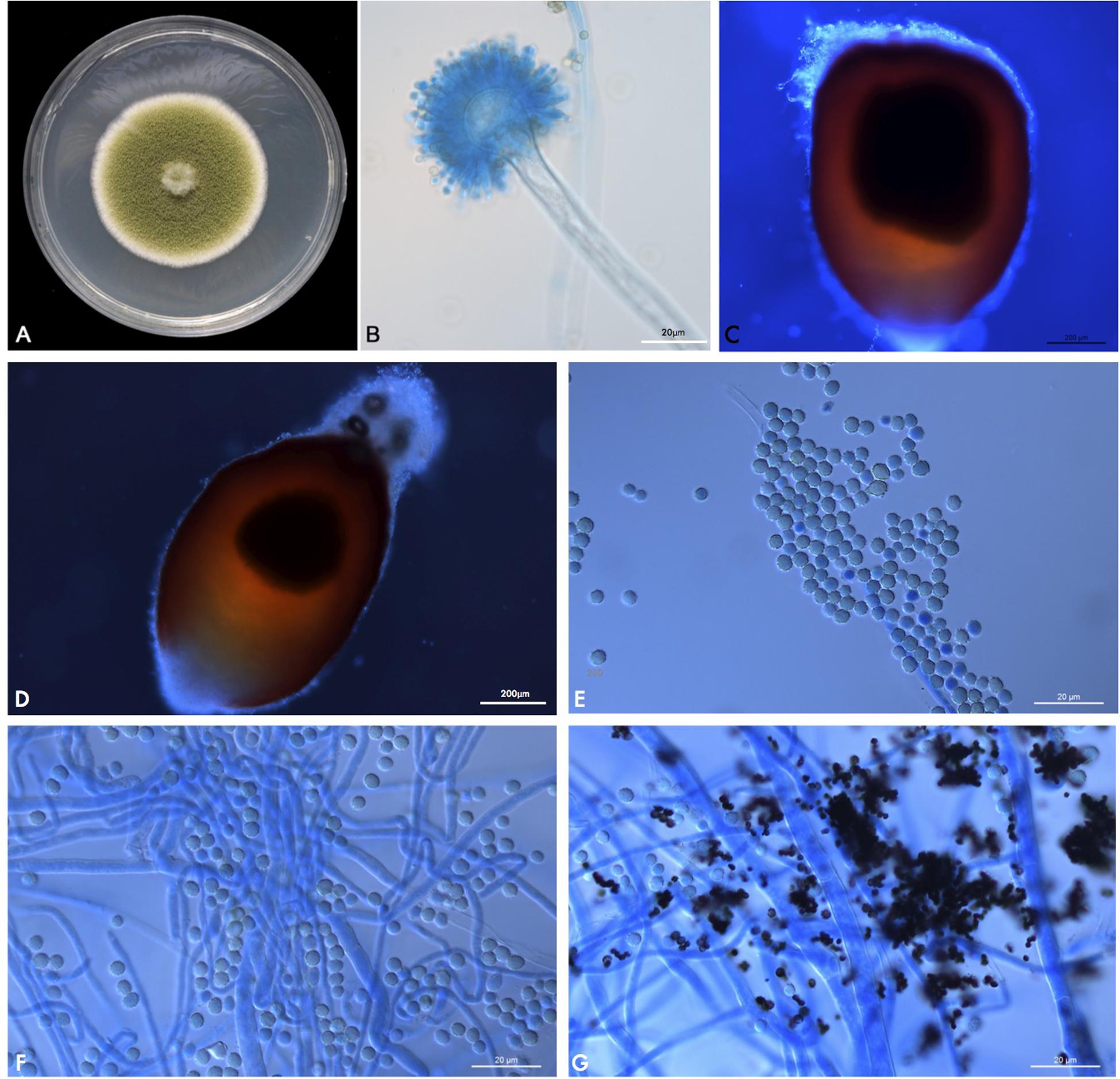
Figure 4. (A) PUTH 1342: Macromorphology on PDA at 37°C after 7 days of incubation. (B) Microscopic appearance of PUTH 1342 conidiophore (lactophenol cotton blue, original magnification × 400). (C,D) Bullet-shaped sclerotia. (E) Rough-walled subspherical conidia, measuring 2–5 μm diameter. (F,G) Hyaline and septate hyphae with branches at acute angles.
BLAST query of all three genomic regions indicated PUTH 1342 was actually Aspergillus nomius, which has recently been renamed as A. nomiae. These sequences have been deposited in GenBank under respective accession numbers MK088520, MK105849, and MK105850.
PUTH 1342 clustered most closely with the A. nomiae type strain (Figure 5) and formed a clade with other A. nomiae strains, including other clinical isolates that were identified as A. nomius in previous studies (PW 2955, PW 2959, and MUT 4191). PUTH 1342 was therefore confidently identified as A. nomiae (Table 2 and Figure 5) (Houbraken et al., 2020).
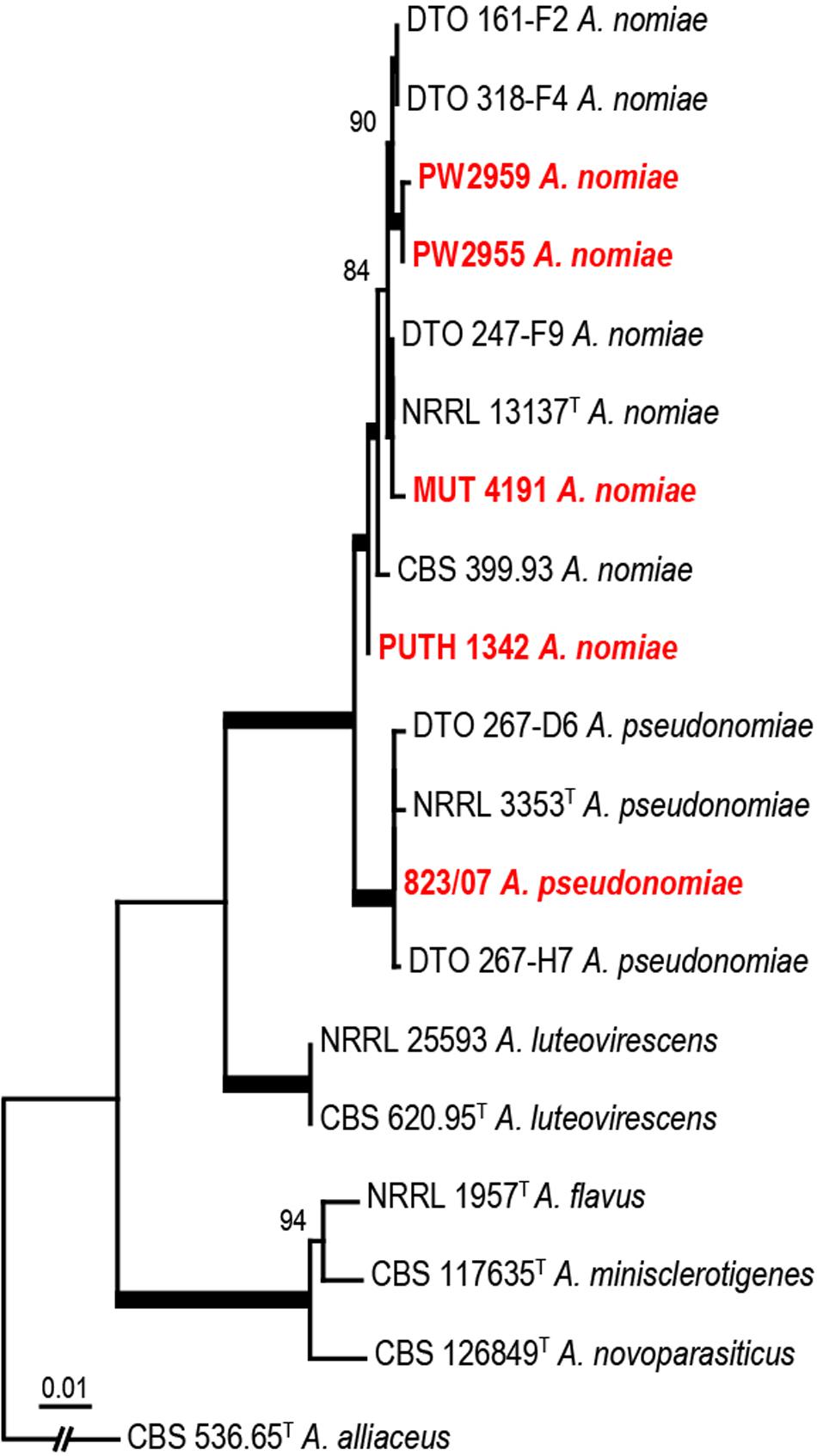
Figure 5. Phylogenetic analysis of three concatenated genomic regions (ITS, BenA, and CaM) involving PUTH 1342, seven reference strains, and closely related species from section Flavi. A. alliaceus were used as outgroup. The A. nomiae and A. pseudonomiae isolates in red were previously isolated from clinical specimens. The phylogram was constructed by the maximum likelihood method with 1000 bootstrap replicates.
With regard to PUTH 1342 resistance to antifungals, the MICs/MECs were as follows: AMB, 2 mg/L; voriconazole, 1 mg/L; itraconazole, 0.5 mg/L; posaconazole, 0.25 mg/L; caspofungin, ≤ 0.008 mg/L; and micafungin, ≤ 0.008 mg/L. According to antifungal criteria (EUCAST), A. flavus and A. nomiae are resistant to AMB and voriconazole, while other Aspergilli are susceptible. Therefore, PUTH 1342 was least susceptible to AMB, and most susceptible to the echinocandins (caspofungin and micafungin). The azoles appeared intermediate in their impact on PUTH 1342.
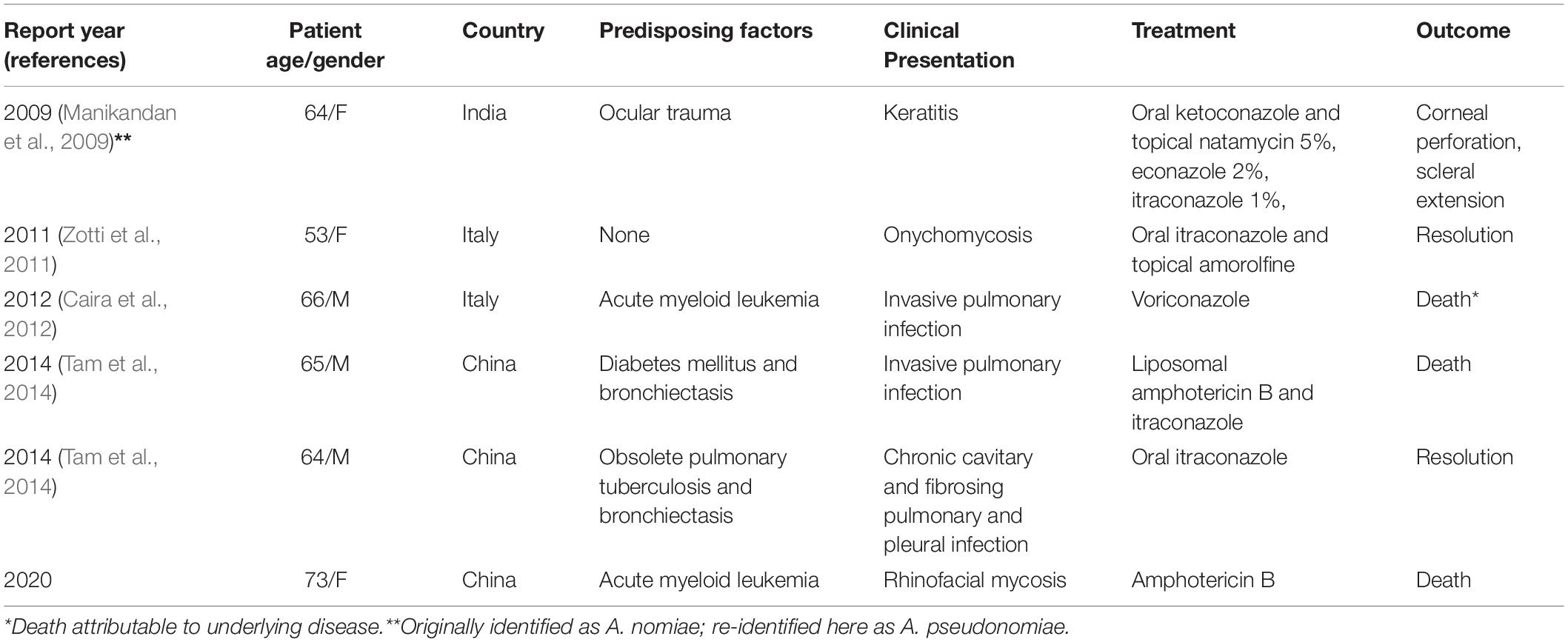
In a comprehensive literature search, we found five published cases of A. nomiae infection in humans (Manikandan et al., 2009; Zotti et al., 2011; Caira et al., 2012; Tam et al., 2014), two of which reported incidences of AMB (or any antifungal) resistance. The essential data from patients, including the present case, are summarized in Table 2.
Discussion
Aspergillus flavus is a relatively frequent cause of invasive sinusitis with a global distribution (Webb and Vikram, 2010), particularly in arid climate zones (Taj-Aldeen et al., 2003). Most patients are otherwise healthy, the sinusitis expressing nasal polyps and opacity of the sinus are evident upon CT scan (Taj-Aldeen et al., 2003). With underlying diseases, particularly those impairing the innate immune system, the disorder may become fulminant with potential extension into the brain (Ellis et al., 2002).
The present case was initiated with a rhinofacial ulcer by A. nomiae, a species closely related to A. flavus and also a member of section Flavi. Our findings showed that PUTH 1342 was misidentified, as it was morphologically similar to A. flavus. To our knowledge, this is the first report of a rhinofacial mycosis due to A. nomiae. Human infections due to A. nomiae seem to be rare. To date, infection in only five patients, including the present case, have been confirmed worldwide. Three of these cases were reported in China and two in Italy. The A. nomiae isolate from a corneal infiltrate in India (Manikandan et al., 2009) clustered more closely with Aspergillus pseudonomiae in our phylogenetic analysis (Figure 5), and this isolate is therefore here re-identified as such. A. pseudonomiae is phylogenetically and phenotypically closely related to A. nomiae, and was first described 2 years after the reported case (Table 2) (Manikandan et al., 2009). A. nomiae is another sibling of A. flavus and has repeatedly been misidentified with that species (Tam et al., 2014). Therefore, it may be significantly more common in aspergillosis infections than previously reported. A. flavus is susceptible to AMB. For example, Ellis et al. (2002) reported successful application of liposomal AMB in a case of a rhinocerebral infection by A. flavus. However, in the present case, AMB proved ineffective against PUTH 1342 and the strain appeared resistant in vitro. The limited information available (Manikandan et al., 2009; Caira et al., 2012) indicates that A. nomiae may be a species with enhanced resistance to AMB. Notably, angioinvasion was observed in our 73-year-old patient, which was characteristic of rhino-orbital cerebral mycosis and might have played an important role in her cerebral hemorrhage (Li and de Hoog, 2009; Li et al., 2014).
de Hoog et al. (2019), summarizing published antifungal studies against A. flavus, noted that for most compounds it showed a wide resistance profile, ranging from totally susceptible to totally resistant. Perhaps this variability is due to misidentification of resistant siblings of A. flavus, such as A. nomiae (Tam et al., 2014). Retrospective identification and susceptibility testing is required to resolve this uncertainty. The accurate diagnosis of A. nomiae infection requires close examination of micromorphology or identification with Maldi-tof (Tam et al., 2014), and unambiguous diagnostics through multilocus sequencing typing (Tam et al., 2014). Serum GM and G tests are widely used for the diagnosis of Aspergillus infection, but these tests are non-species-specific and sometimes insensitive (Boch et al., 2018). Although the serum G test was negative, the serum GM test in the case report was positive (1.25 μg/L > 0.5 μg/L) and might have led to certainty of Aspergillus infection.
In the limited number of reported human infection cases involving A. nomiae published to date, risk factors for fulminant disease included myeloid leukemia (Caira et al., 2012), trauma (Manikandan et al., 2009), diabetes mellitus (Tam et al., 2014), bronchiectasis (Tam et al., 2014), and Mycobacterium tuberculosis infection (Tam et al., 2014). Our patient had been diagnosed with acute myeloid leukemia, and during that first year received chemotherapy accordingly. Onset of the nasal necrosis occurred 10 months post-diagnosis during the course of chemotherapy. Reported infections known to involve A. nomiae were pulmonary (Caira et al., 2012), ocular (Manikandan et al., 2009), or involving the nails (Zotti et al., 2011). Half of the reported cases (3/6) were pulmonary, including two invasive infections and one chronic pulmonary and pleural infection (Caira et al., 2012). Our case showed rhinofacial involvement. Vascular invasion is an important feature of rhino-cerebral mycosis by Mucorales (Li and Lun, 2012; Li et al., 2014) and has been observed in rhino-orbital aspergillosis (Ben-Ami et al., 2009; Huang and Gui, 2019). Whether or not the lethal cerebral hemorrhage was induced by the angiodestruction of the fungus could not be determined; angioinvasive and angiodestructive characteristics have been observed biopsied in the rhinofacial area. Angioinvasion leading to cerebral destruction has played a role in the sudden death of immunocompromised patients before (Li and de Hoog, 2009; Li et al., 2014), as well as in A. fumigatus-triggered thrombosis, hypoxia, and proinflammatory cytokine release (Ben-Ami et al., 2009).
The A. nomiae isolate in our patient was resistant to AMB (S ≤ / > R = 1/1) according to the latest drug resistance criteria (Arendrup et al., 2020). This resistance permitted angioinvasion by the fungus, which led to treatment failure. This empirical treatment was chosen on the basis of clinical features of the rhinofacial mycosis, which is mostly caused by members of Mucorales that are generally susceptible to AMB. For invasive Aspergillus infection, voriconazole is typically recommended as the first-line drug (Vermeulen et al., 2013; Hagiwara et al., 2016). Limited published data on A. nomiae indicate insusceptibility to this compound (Manikandan et al., 2009; Caira et al., 2012). In this study, we found PUTH 1342 to be fairly resistant to voriconazole since it required a higher dose for fungal inhibition. Voriconazole therefore seems to be inappropriate as an initial therapy for A. nomiae infections. Correspondingly, due to the low values of in vitro drug sensitivity tests, caspofungin (≤0.008 mg/L) and micafungin (≤0.008 mg/L) would be good for first-line antifungals. At the same time, our case reflects the importance of developing a definitive antifungal criterion (EUCAST) for A. flavus and A. nomiae, especially in clinical practice.
Aspergillus nomiae strains are able to produce both series B and G aflatoxins (Ehrlich et al., 2004), which are notorious for hepatocellular carcinoma development, lung adenocarcinoma, and chronic inflammatory changes (Cha and Dematteo, 2005; Liu et al., 2015). Our patient’s white blood cell counts increased sharply after respiratory tract infection, and subsequently she was diagnosed with leukemia. Nasal necrosis by A. nomiae developed during chemotherapy. We are not sure whether the aflatoxins contributed to the malignancy. Given that the fungus had infiltrated her nasal cavity, perhaps she was swallowing mucus and fungal spores. Ingestion of aflatoxins is where the problems arise. They end up in the liver and cannot be removed so they accumulate and may lead to hepatocarcinoma. Depending on the concentration of aflatoxins, they may have contributed to her death as aflatoxicosis. The poor physical state of most patients with invasive fungal infection limits the efficiency of antifungal drugs. Of the patients with invasive infection due to A. nomiae, including the present one, all died. In contrast, patients with non-invasive infections, including chronic pulmonary infection, keratitis, and onychomycosis, have all been cured with antifungal treatment.
We report this case to increase the public awareness of A. nomiae infections. Given that the morphological characteristics of A. nomiae are similar to those of A. flavus and molecular identification of Aspergillus is not routinely performed in clinical mycology laboratories, A. nomiae infections are suspected to be underreported. Rhinofacial mycoses involving A. nomiae might be fatal if they cannot be identified in a clinical lab providing appropriate treatment and if they have reduced susceptibility to AMB.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
This research has been approved by Peking University Third Hospital IRB; approval #00006761-2015025. Written informed consent was obtained from the next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
DL diagnosed the patient, designed the experiments, and wrote partial manuscript. YZ did the experiment and wrote the manuscript. TS assisted in experiment. JH made the tree. GH reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC, C31770013, and H81571967).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Alsalman, J., Zaid, T., Makhlooq, M., Madan, M., Mohamed, Z., Alarayedh, A., et al. (2017). A retrospective study of the epidemiology and clinical manifestation of invasive aspergillosis in a major tertiary care hospital in Bahrain. J. Infect. Public Health 10, 49–58. doi: 10.1016/j.jiph.2016.02.015
Arendrup, M. C., Friberg, N., Mares, M., Kahlmeter, G., Meletiadis, J., Guinea, J., et al. (2020). How to: interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European Committee on Antimicrobial Susceptibility Testing (EUCAST). Clin. Microbiol. Infect. 26, 1464–1472. doi: 10.1016/j.cmi.2020.06.007
Bakhshaee, M., Bojdi, A., Allahyari, A., Majidi, M. R., Tavakol, S., Najafzadeh, M. J., et al. (2016). Acute invasive fungal rhinosinusitis: our experience with 18 cases. Eur. Arch. Otorhinolaryngol. 273, 4281–4287. doi: 10.1007/s00405-016-4109-z
Balajee, S. A., Gribskov, J. L., Hanley, E., Nickle, D., and Marr, K. A. (2005). Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot Cell 4, 625–632. doi: 10.1128/ec.4.3.625-632.2005
Ben-Ami, R., Lewis, R. E., Leventakos, K., and Kontoyiannis, D. P. (2009). Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood 114, 5393–5399. doi: 10.1182/blood-2009-07-231209
Boch, T., Reinwald, M., Spiess, B., Liebregts, T., Schellongowski, P., Meybohm, P., et al. (2018). Detection of invasive pulmonary aspergillosis in critically ill patients by combined use of conventional culture, galactomannan, 1-3-beta-D-glucan and Aspergillus specific nested polymerase chain reaction in a prospective pilot study. J. Crit. Care 47, 198–203. doi: 10.1016/j.jcrc.2018.07.001
Caira, M., Posteraro, B., Sanguinetti, M., De Carolis, E., Leone, G., and Pagano, L. (2012). First case of breakthrough pneumonia due to Aspergillus nomius in a patient with acute myeloid leukemia. Med. Mycol. 50, 746–750. doi: 10.3109/13693786.2012.660507
Cha, C., and Dematteo, R. P. (2005). Molecular mechanisms in hepatocellular carcinoma development. Best Pract. Res. Clin. Gastroenterol. 19, 25–37.
de Hoog, G. S., Guarro, J., Gené, J., Ahmed, S., Al-Hatmi, A. M. S., Figueras, M. J., et al. (2019). Atlas of Clinical Fungi, 3rd Edn. Utrecht: Westerdijk Fungal Biodiversity Institute.
Ehrlich, K. C., Chang, P. K., Yu, J., and Cotty, P. J. (2004). Aflatoxin biosynthesis cluster gene cypA is required for G aflatoxin formation. Appl. Environ. Microbiol. 70, 6518–6524. doi: 10.1128/aem.70.11.6518-6524.2004
Ellis, M., Watson, R., Mcnabb, A., Lukic, M. L., and Nork, M. (2002). Massive intracerebral aspergillosis responding to combination high dose liposomal amphotericin B and cytokine therapy without surgery. J. Med. Microbiol. 51, 70–75. doi: 10.1099/0022-1317-51-1-70
Frisvad, J. C., Hubka, V., Ezekiel, C. N., Hong, S. B., Nováková, A., Chen, A. J., et al. (2019). Taxonomy of Aspergillus section flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 93, 1–63. doi: 10.1016/j.simyco.2018.06.001
Gilbert, M. K., Mack, B. M., Moore, G. G., Downey, D. L., Lebar, M. D., Joardar, V., et al. (2018). Whole genome comparison of Aspergillus flavus L-morphotype strain NRRL 3357 (type) and S-morphotype strain AF70. PLoS One 13:e0199169. doi: 10.1371/journal.pone.0199169
Hagiwara, D., Watanabe, A., Kamei, K., and Goldman, G. H. (2016). Epidemiological and genomic landscape of azole resistance mechanisms in Aspergillus Fungi. Front. Microbiol. 7:1382. doi: 10.3389/fmicb.2016.01382
Hong, S. B., Go, S. J., Shin, H. D., Frisvad, J. C., and Samson, R. A. (2005). Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 97, 1316–1329. doi: 10.3852/mycologia.97.6.1316
Houbraken, J., Kocsubé, S., Visagie, C. M., Yilmaz, N., Wang, X. C., Meijer, M., et al. (2020). Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): an overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 95, 5–169. doi: 10.1016/j.simyco.2020.05.002
Hu, J. G., Li, D. M., and De Hoog, G. S. (2021). Rhinofacial and rhino-orbital-cerebral mycosis caused by Aspergillus spp. Mycosystema 39, 2218–2240. doi: 10.13346/j.mycosystema.200270
Huang, Y., and Gui, L. (2019). Cavernous sinus-orbital apex Aspergillus infection in a diabetic patient: a case report. Medicine (Baltimore) 98:e15041. doi: 10.1097/md.0000000000015041
Jalalian, R., and Shokohi, T. (2020). Fatal prosthetic valve endocarditis due to Aspergillus flavus in a diabetic patient. Infect. Drug Resistance 13, 2245–2250. doi: 10.2147/idr.s258637
Leroy, J., Vuotto, F., Le, V., Cornu, M., Francois, N., Marceau, L., et al. (2020). Invasive rhino-orbital-cerebral aspergillosis in an immunocompetent patient. J. Mycol. Med. 30:101002. doi: 10.1016/j.mycmed.2020.101002
Li, D. M., and de Hoog, G. S. (2009). Cerebral phaeohyphomycosis–a cure at what lengths? Lancet Infect. Dis. 9, 376–383. doi: 10.1016/s1473-3099(09)70131-8
Li, D. M., and Lun, L. D. (2012). Mucor irregularis infection and lethal midline granuloma: a case report and review of published literature. Mycopathologia 174, 429–439. doi: 10.1007/s11046-012-9559-2
Li, D. M., Shang, P. P., Zhu, L., and De Hoog, G. S. (2014). Rhino-orbital-cerebral mycosis and cavernous thromboses. Eur. J. Inflamm. 12, 1–10. doi: 10.1177/1721727x1401200101
Li, D. M., Xiu, D. R., Li, R. Y., Samson, R. A., De Hoog, G. S., and Wang, D. L. (2008). Aspergillus flavus myositis in a patient after liver transplantation. Clin. Transplant. 22, 508–511. doi: 10.1111/j.1399-0012.2008.00801.x
Liu, C., Shen, H., Yi, L., Shao, P., Soulika, A. M., Meng, X., et al. (2015). Oral administration of aflatoxin G(1) induces chronic alveolar inflammation associated with lung tumorigenesis. Toxicol. Lett. 232, 547–556. doi: 10.1016/j.toxlet.2014.11.002
Manikandan, P., Varga, J., Kocsubé, S., Samson, R. A., Anita, R., Revathi, R., et al. (2009). Mycotic keratitis due to Aspergillus nomius. J. Clin. Microbiol. 47, 3382–3385. doi: 10.1128/jcm.01051-09
Mori, T., Matsumura, M., Yamada, K., Irie, S., Oshimi, K., Suda, K., et al. (1998). Systemic aspergillosis caused by an aflatoxin-producing strain of Aspergillus flavus. Med. Mycol. 36, 107–112. doi: 10.1046/j.1365-280x.1998.00124.x
Patterson, T. F., Thompson, G. R. III, Denning, D. W., Fishman, J. A., Hadley, S., Herbrecht, R., et al. (2016). Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 63, e1–e60.
Rudramurthy, S. M., and Paul, R. A. (2019). Invasive Aspergillosis by Aspergillus flavus: epidemiology, diagnosis, antifungal resistance, and management. J. Fungi. (Basel). 5:55. doi: 10.3390/jof5030055
Shaikh, N., Hussain, K. A., Petraitiene, R., Schuetz, A. N., and Walsh, T. J. (2016). Entomophthoramycosis: a neglected tropical mycosis. Clin. Microbiol. Infect. 22, 688–694. doi: 10.1016/j.cmi.2016.04.005
Taj-Aldeen, S. J., Hilal, A. A., and Chong-Lopez, A. (2003). Allergic Aspergillus flavus rhinosinusitis: a case report from Qatar. Eur. Arch. Otorhinolaryngol. 260, 331–335. doi: 10.1007/s00405-002-0547-x
Tam, E. W., Chen, J. H., Lau, E. C., Ngan, A. H., Fung, K. S., Lee, K. C., et al. (2014). Misidentification of Aspergillus nomius and Aspergillus tamarii as Aspergillus flavus: characterization by internal transcribed spacer, β-Tubulin, and calmodulin gene sequencing, metabolic fingerprinting, and matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 52, 1153–1160. doi: 10.1128/jcm.03258-13
Vermeulen, E., Lagrou, K., and Verweij, P. E. (2013). Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr. Opin. Infect. Dis. 26, 493–500. doi: 10.1097/qco.0000000000000005
Webb, B. J., and Vikram, H. R. (2010). Chronic invasive sinus aspergillosis in immunocompetent hosts: a geographic comparison. Mycopathologia 170, 403–410. doi: 10.1007/s11046-010-9338-x
Wiederhold, N. P., and Verweij, P. E. (2020). Aspergillus fumigatus and pan-azole resistance: who should be concerned? Curr. Opin. Infect. Dis. 33, 290–297. doi: 10.1097/qco.0000000000000662
Zhou, Y. B., Zhang, G. J., Song, Y. G., Sun, L. N., Chen, Y. H., Sun, T. T., et al. (2019). Application of laser capture microdissection and polymerase chain reaction in the diagnosis of Trichoderma longibrachiatum infection: a promising diagnostic tool for ‘fungal contaminants’ infection. Med. Mycol. 58, 315–321. doi: 10.1093/mmy/myz055
Keywords: Aspergillus nomiae, rhinofacial mycosis, skin and soft tissue infections, leukemia, rhino-orbital-cerebral mucormycosis, antifungal resistance
Citation: Zhou YB, Li DM, Houbraken J, Sun TT and de Hoog GS (2020) Fatal Rhinofacial Mycosis Due to Aspergillus nomiae: Case Report and Review of Published Literature. Front. Microbiol. 11:595375. doi: 10.3389/fmicb.2020.595375
Received: 16 August 2020; Accepted: 27 November 2020;
Published: 22 December 2020.
Edited by:
Maurizio Sanguinetti, Catholic University of the Sacred Heart, ItalyReviewed by:
Geromy G. Moore, Southern Regional Research Center (USDA-ARS), United StatesBrunella Posteraro, Catholic University of the Sacred Heart, Italy
Copyright © 2020 Zhou, Li, Houbraken, Sun and de Hoog. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Ming Li, ZG9yaXNsaUAxMjYuY29t; RG9uZ21pbmdsaUBiam11LmVkdS5jbg==
 Ya Bin Zhou
Ya Bin Zhou Dong Ming Li
Dong Ming Li Jos Houbraken
Jos Houbraken Ting Ting Sun1
Ting Ting Sun1 G. Sybren de Hoog
G. Sybren de Hoog