- 1Laboratory of Mycology, Institute of Botany, Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan
- 2State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China
- 3Department of Ecology, University of Kassel, Kassel, Germany
- 4Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang, China
- 5Núcleo de Pesquisa em Micologia, Instituto de Botânica, São Paulo, Brazil
- 6Department of Ecology and Botany, Andijan State University, Andijan, Uzbekistan
- 7Tashkent State Agrarian University, Tashkent, Uzbekistan
- 8Faculty of Biological and Environmental Sciences, University of Helsinki, Helsinki, Finland
- 9Global Biodiversity Information Facility (GBIF), Secretariat, Universitetsparken, Copenhagen, Denmark
- 10School of Pharmaceutical Science and Technology, Health Sciences Platform, Tianjin University, Tianjin, China
- 11Graduate School of Geography, Clark University, Worcester, MA, United States
Uzbekistan, located in Central Asia, harbors high diversity of woody plants. Diversity of wood-inhabiting fungi in the country, however, remained poorly known. This study summarizes the wood-inhabiting basidiomycte fungi (poroid and corticoid fungi plus similar taxa such as Merismodes, Phellodon, and Sarcodon) (Agaricomycetes, Basidiomycota) that have been found in Uzbekistan from 1950 to 2020. This work is based on 790 fungal occurrence records: 185 from recently collected specimens, 101 from herbarium specimens made by earlier collectors, and 504 from literature-based records. All data were deposited as a species occurrence record dataset in the Global Biodiversity Information Facility and also summarized in the form of an annotated checklist in this paper. All 286 available specimens were morphologically examined. For 138 specimens, the 114 ITS and 85 LSU nrDNA sequences were newly sequenced and used for phylogenetic analysis. In total, we confirm the presence of 153 species of wood-inhabiting poroid and corticioid fungi in Uzbekistan, of which 31 species are reported for the first time in Uzbekistan, including 19 that are also new to Central Asia. These 153 fungal species inhabit 100 host species from 42 genera of 23 families. Polyporales and Hymenochaetales are the most recorded fungal orders and are most widely distributed around the study area. This study provides the first comprehensively updated and annotated the checklist of wood-inhabiting poroid and corticioid fungi in Uzbekistan. Such study should be expanded to other countries to further clarify species diversity of wood-inhabiting fungi around Central Asia.
Introduction
Central Asia is a biological crossroads at the most westerly part of the Himalayan range and supports both Palearctic species and others representative of more southerly subtropical latitudes. The peculiarity of fauna and flora is due to its mixed characters: Indo-Himalayan, Mongolian, Eurasian, and Mediterranean species are present (Anonymous, 2001). Uzbekistan in the heart of Central Asia has a diversity of habitats that are globally and regionally important in ecological functions. The varying landscapes of high mountain ranges, wide steppes, deserts, and riparian wetlands in Uzbekistan result in a high diversity of habitats. The mountain areas occupy 15% of the territory of Uzbekistan. Biodiversity of Uzbekistan includes more than 27,000 species, including over 15,000 animal species; plants, algae, and fungi total about 11,000 species (Anonymous, 1998). The flora of Uzbekistan includes 4500 species of vascular plants, of which about 400 species are endemic, rare, and relict. Many of the animals and higher plants are included in the Red List of the International Union for Conservation of Nature (IUCN) and the Red Book of the Republic of Uzbekistan.
In contrast to the great number of publications dealing with the flora, limited studies document the fungi in Uzbekistan and Central Asia in general (Gafforov, 2017; Gafforov et al., 2017). Current surveys in areas of high plant endemism, such as tropical and subtropical regions, are actually showing an even higher ratio of fungal to plant diversity and uncovering an extraordinary number of endemic fungi (e.g., Crous et al., 2006; Mueller and Schmit, 2007; Schmit and Mueller, 2007; Aime and Brearley, 2012; Hawksworth, 2012). Mountains of the Central Asia Biodiversity Hotspot consist of two major mountain systems, the Pamir and the Tien Shan. Both belong to the most diverse regions in the world with respect to fauna and flora and are regarded as areas of occurrence of many endemic, relict, and endangered species. Therefore, diverse and regionally limited fungi are expected to exist in the region. However, while knowledge of fungal diversity is developing rapidly in some areas of the world, data on the fungi in Central Asia are severely limited (Gafforov et al., 2017; Antonelli et al., 2020; Cheek et al., 2020): the current knowledge of Uzbekistan fungal biodiversity status and even a rough estimate of the number of fungal species in countries of Central Asia is unavailable. This knowledge gap has significantly impeded understanding the role of the region in biogeographic history of Asia and prevented conservation efforts in the region.
Fungi are essential components of ecosystems and are both directly and indirectly important for human cultures. Various fungal species are key symbionts of trees enabling the survival of the latter in the arid areas (Varma, 1995; Stutz et al., 2000). Fungal names used in other regional floras have often been applied to fungi in Uzbekistan. However, the Uzbek fungi often represent new, unrelated species as was shown for Uzbekistan ascomycetous microfungi (Solieva and Gafforov, 2001, 2002; Gafforov and Hoshino, 2015; Gafforov, 2002, 2010, 2015, 2016a,b; Gafforov and Rakhimov, 2017; Gafforov et al., 2019; Wanasinghe et al., 2017, 2018a,b; Samarakoon et al., 2018; Pem et al., 2018, 2019a,b; Hyde et al., 2019, 2020; Yuan et al., 2020). Basidiomycetous fungi have received even less attention than ascomycetous microfungi (Gafforov, 2014; Gafforov et al., 2014, 2017).
Among the basidiomycetous macrofungi, especially those with poroid fertile surface of fruiting bodies (poroid fungi) and corticioid fertile surface (corticioid fungi) play several essential roles in forest ecosystems (Swift, 1982). Most of these fungi are saprobes causing brown or white wood rot, whereas some of them form ectomycorrhizae with woody plants. Therefore, they play an important function in nutrient cycling and soil formation (Soudzilovskaia et al., 2019). Some of them are also known to be serious pathogenic disease agents of ecologically and economically important coniferous and deciduous woody plants. Regardless of the relationship, wood-inhabiting basidiomycetous fungi are often treated as a single research object by both taxonomists and ecologists.
The first mycological investigations on wood-inhabiting fungi in Uzbekistan were started by Sinadskiy and Bondartseva in 1950, who reported 21 polypore species (Sinadskiy and Bodartseva, 1956, 1960; Kleyner, 1958; Panfilova and Gaponenko, 1963; Gaponenko, 1965; Sinadskiy, 1968). The first study specifically in state reserves of Uzbekistan listed 71 polypore species (Baltaeva, 1992, 1993). In the study of macrofungi of Fergana valley (Andijan, Fergana and Namangan Provinces), Tashkent Province, 25 species of poroid and corticioid fungi were reported (Khalikova, 1989; Iminova, 2009). However, the fungal species recorded in these studies were identified solely by morphological characters and no specimen was preserved, which makes the reassessment of taxonomic affiliation of these records impossible.
Recent developments in DNA sequencing have revolutionized identification and systematics of fungi. This has rapidly advanced the mycological communities’ ability to document fungal biodiversity, distribution, ecological preferences, and biogeographic history (e.g., Hattori et al., 2012; Ovaskainen et al., 2013; Tsykun et al., 2013; Tedersoo et al., 2014). DNA barcodes can facilitate taxonomic research by increasing the ability to matching individuals regardless of the fruiting body, identifying specimens with morphological diagnostic characters either subtle, difficult to visualize, or absent, as well as reassessing intraspecific polymorphisms. With the aid of DNA sequences, research on the wood-inhabiting basidiomycetous fungi during the last decade has yielded some species previously unknown in Uzbekistan, as well as some species new to science (Gafforov, 2014; Gafforov et al., 2014, 2017; Yuan et al., 2017, 2020; Kan et al., 2017). Moreover, the first fungal checklist of the corticioid genus Hyphodontia from Central Asia was published (Gafforov et al., 2017). However, despite these steps forward, comprehensive information of the wood-inhabiting poroid and corticioid fungi is still unavailable in Uzbekistan.
On the basis of our own collections, literatures, and herbarium data reassessments, the present study aimed to recognize species diversity of wood-inhabiting poroid and corticioid fungi (plus similar taxa such as Merismodes, Phellodon and Sarcodon) in Uzbekistan from morphological and, where possible, phylogenetic perspectives, and also to provide comprehensive annotations for these species including host, substratum, distribution, and occurring frequency.
Materials and Methods
Vegetation and Climate of the Study Area
The Uzbekistan territory falls in the flora of Central Asian botanical region within the larger temperate Asia floral geographic region according to the World Geographical Scheme for Recording Plant Distributions’ system (Brummitt, 2001). The main ecological forest types in Uzbekistan are mountain, desert, and flood-plain forests (Figure 1). The majority of Uzbekistan forests are xerophytic open woodlands of deciduous trees and shrubs, constituting about 7.3% of the territory (Botman, 2009). These forests play an important role in the protection and prevention from environmental degradation, particularly land degradation and natural disasters, and also in the conservation of biodiversity and preservation of water quality.

Figure 1. Forest types in study area. (A) Mountain juniper forests. (B) Wild fruit tree forests in mountain. (C) Desert saxaul (Haloxylon spp.) forests. (D) Tugai Forests (Photo: Yusufjon Gafforov).
Uzbekistan has a continental climate with hot and dry summers and cold winters. Summer temperature often surpasses 40°C (104°F), and winter average temperature is about −2°C (28°F) but may fall as low as −40°C (−40°F). Most parts of the country are arid with average annual rainfall amounting to between 100 and 200 mm (3.9 and 7.9 in) and occurring mostly in winter and spring. Between July and September, little precipitation falls, essentially stopping the growth of vegetation during that period (Klein Tank et al., 2006; Lioubimtseva and Henebry, 2009).
Specimen Assembly
A total of 286 specimens of wood-inhabiting poroid and corticoid were examined. This includes 101 specimens from Mycological Herbarium of Estonian University of Life Sciences, Tartu, Estonia (TAAM); 3 specimens from Tashkent Mycological Herbarium, Institute of Botany of the Academy of Sciences of Uzbekistan, Tashkent (TASM); and 185 specimens from our own field surveys, which are deposited in TASM. Our own specimens were recently collected from Tashkent Botanical Garden (Tashkent city), Tashkent Province (Ugam-Chatkal State Nature National Park in Western Tien Shan Mountain), Jizzakh Province (Zaamin National Nature Park, Zaamin State Reserve in Turkestan range and Nurata State Reserve in Nurata range of Pamir-Alay), Surxondaryo Province (Baysun and Husar ranges in Pamir-Alay Mountains), and Fergana Valley (Namangan Province) (Figure 2). In addition, we reviewed 504 records of Uzbekistan fungi published between 1950 and 2020.
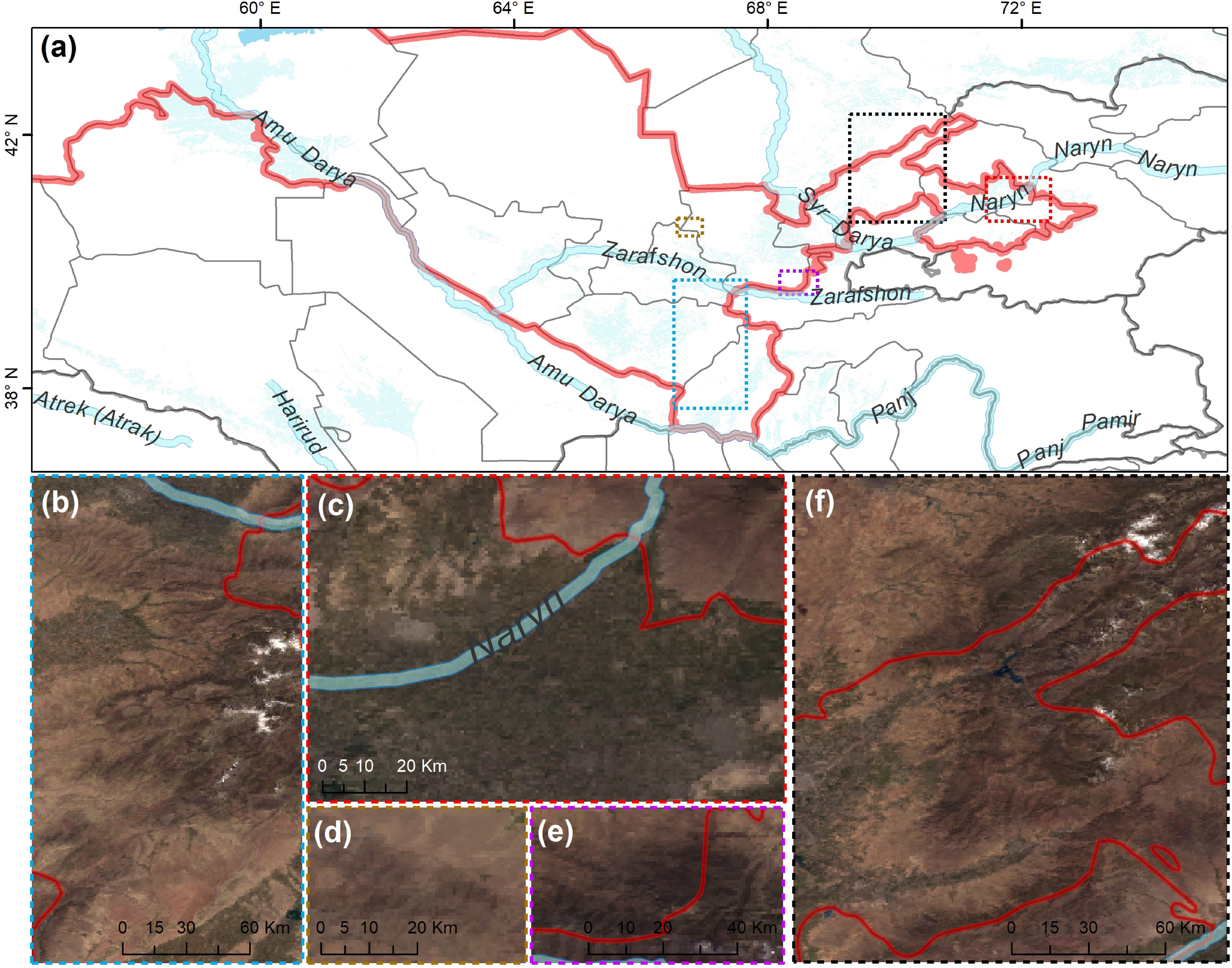
Figure 2. Map of (a) Uzbekistan labeled with five principal collecting areas shown in panels (b–f). The background map in panels (b–f) is the combination of bands 1, 4, 3 displays and R, G, B surface reflectance image using 500-m MODIS data (MODS09A1) acquired in July 2019 over Uzbekistan (by Yu Zhou).
Morphological Study
Morphological characters were described based on fresh and dried fruiting bodies. Microscopic characters of fruiting bodies were observed on dried specimens at a magnification up to 1000 × with a Leica DM 1000 (Tokyo, Japan) microscopes in 5% aqueous KOH plus 1% phloxine, Melzer’s reagent for amyloid or dextrinoid reactions, cotton blue in lactic acid for cyanophily, and 1% aqueous cresyl blue for metachromatism (Hawksworth et al., 1995). Macromorphological characters of fruiting bodies and hymenophores were observed under a Leica M165 FC stereomicroscope. Scientific names, both of the fungi and the host plants, were checked for potential synonyms in the databases Index Fungorum (2020) and ThePlantList (2013), respectively. Species whose taxonomic placement is not established are listed under incertae sedis.
DNA Extraction, Amplification, and Sequencing
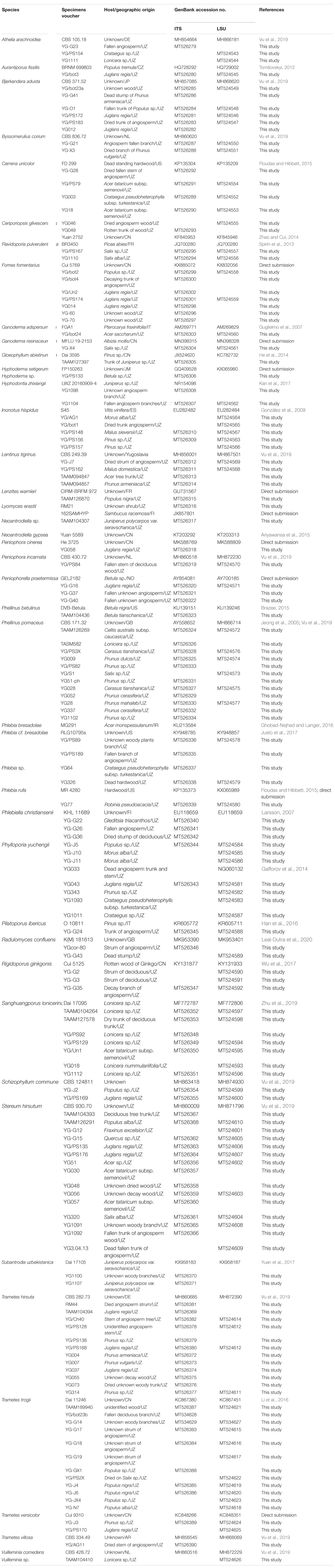
Genomic DNA was extracted from the dried basidiocarps of herbarium materials using DNeasy Plant Mini Kit (Qiagen, Valencia, CA, United States), QIAamp DNA Micro Kit (Qiagen), and the Extract-N-Amp Plant PCR Kit (Sigma-Aldrich, St. Louis, MO, United States), following protocols from the manufacturers, and was diluted as a template for subsequent amplification. PCR amplification targeted the internal transcribed spacer (ITS) region of the ribosomal RNA gene (rRNA), the universal DNA barcode for identification of fungi (Schoch et al., 2012), and the nuclear large ribosomal subunit (nLSU) region. Amplification was carried out using the fungal-specific primer sets ITS1F/ITS4b (Gardes and Bruns, 1996) and ITS1/ITS4 (White et al., 1990) for the ITS region and LR0R and LR5 for the nLSU region (Vilgalys and Hester, 1990; Rehner and Samuels, 1994). Purified PCR products were sequenced using DNA ABI 3730 XL automated sequencers (Applied Biosystems) by Macrogen Inc. (Seoul, Korea), by Eurofins Genomics (Ebersberg, Germany), and by the Beijing Genomics Institute (Beijing, China). All newly generated sequences of poroid and corticoid species from Uzbekistan were submitted to GenBank (Table 1).
Phylogenetic Analyses
After a preliminary BLAST search, 40 sequences related to those from Uzbekistan specimens were downloaded from GenBank to assist species identification (Table 1). The datasets of ITS and nLSU regions were separately aligned using MAFFT 7.110 (Katoh and Standley, 2013) under the G-INI-i option (Katoh et al., 2005) and then the two alignments were concatenated. The concatenated alignment, deposited in TreeBASE1 (accession number S26575), was subjected to an estimation of the best-fit evolutionary model using jModelTest (Guindon and Gascuel, 2003; Posada, 2008) with calculation under Akaike information criterion. Following this model, maximum likelihood (ML) and Bayesian inference (BI) methods were employed for phylogenetic analyses. The ML method was conducted using raxmlGUI 1.2 (Stamatakis, 2006; Silvestro and Michalak, 2012) with calculation of bootstrap (BS) replicates under the auto FC option (Pattengale et al., 2010). The BI method was conducted using MrBayes 3.2 (Ronquist et al., 2012). Two independent runs were employed. Each run had four chains and started from random trees. Trees were sampled every 1000th generation, of which the first 25% were removed and the other 75% were used for constructing a 50% majority consensus tree and calculating Bayesian posterior probabilities (BPPs). Tracer 1.52 was used to determine chain convergence. iTOL was used to visualize the tree to a circular form (Letunic and Bork, 2019).
GBIF Occurrence Dataset and Checklist Preparation
The occurrence data of wood-inhabiting poroid and corticioid fungi was extracted from 504 records in 19 publications as well as 185 records of our own recent collections in field surveys and 101 herbarium specimens from TAAM and TASM. All but collection data from TAAM (which are already displayed in GBIF) were formatted according to the Darwin Core Standard3 and published as an occurrence dataset (Gafforov and Ordynets, 2020, 4 alternative identifier5). When compiling the annotated species checklist for this paper, for the sake of conciseness, all occurrence records were linked to 50 localities that are listed in the study.
GIS Data Processing
A point distribution map of fungal orders was produced using the ArcGIS 10.7 desktop software (ArcGIS Desktop, 2020). A GPS navigation device and Google Earth software6 (2020) were used for geo-referencing all available occurrence data of wood-inhabiting poroid and corticioid fungi in the study sites. A WGS84 geographic coordinate system was used as a reference datum. The land cover data were adapted from the 500-m Moderate Resolution Imaging Spectroradiometer (MODIS) land cover product (MCD12Q1; Friedl et al., 2002) which has 17 IGBP classes, including water, evergreen needleleaf forests (ENF), evergreen broadleaf forests (EBF), deciduous needleleaf forests (DNF), deciduous broadleaf forests (DBF), mixed forests (MF), closed shrublands (CSH), open shrublands (OSH), woody savannas (WSA), savannas (SAV), grasslands (GRA), permanent wetlands, cropland (CRO), urban and built-up, cropland and natural vegetation mosaics (CNM), snow and ice, and barren. Considering the spatial distribution of irrigated and cultivated croplands, we further integrated these two classes from Klein et al. (2012). Data for roads, rivers, lake centerlines, and country boundaries were downloaded from the Natural Earth database (Natural Earth, 2020).
Results
Phylogenetic Placement of Collections of Poroid and Corticoid Fungi From Uzbekistan
In addition to morphological characters, DNA sequences were used to identify certain specimens. A total of 114 ITS and 85 LSU sequences from 138 specimens representing 40 species were newly generated for this study, and submitted to GenBank (Table 1). The alignment used for phylogenetic analysis included 178 collections (Table 1). The best-fit evolutionary model for this alignment was estimated as GTR + I + G. In the ML method, the BS search stopped after 250 replicates. In the BI method, all chains were converged after 6 million generations, where the average standard deviation of split frequencies is 0.006815, the estimated sample sizes of all parameters are above 700, and the potential scale reduction factor approaches 1.0. Both phylogenetic methods generated congruent topologies in main lineages, and thus only the topology from the ML method is visualized in a circle form with BS and BPP at the nodes (Figure 3). From a phylogenetic perspective, 36 species were recovered and four potential new lineages representing members of Hyphoderma, Neoantrodiella, Phlebia, and Vuilleminia were identified from the newly sequenced specimens.
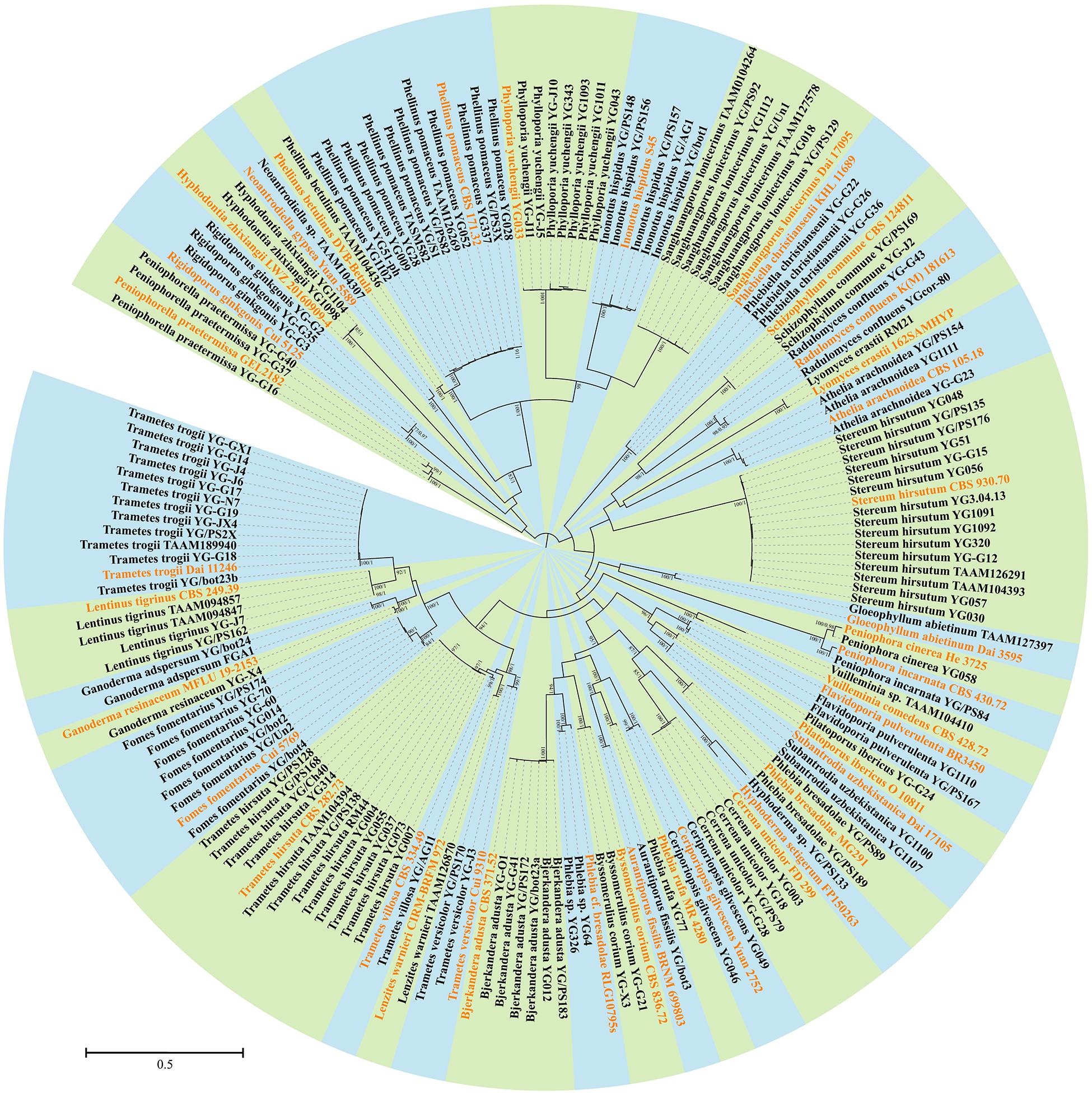
Figure 3. Phylogenetic position of newly sequenced Uzbekistan specimens of wood-inhabiting poroid and corticioid fungi based on a combination of ITS and nLSU sequences. Maximum likelihood tree with bootstrap support values and posterior probabilities inferred from Bayesian analysis is shown. The background for each identified species or undescribed lineage along with its reference sequence is set as one of the alternately appearing blue and green colors. The sequences of collections labeled in orange are downloaded from GenBank, while those in black are generated in this study.
Species Diversity of Wood-Inhabiting Poroid and Corticoid Fungi in Uzbekistan
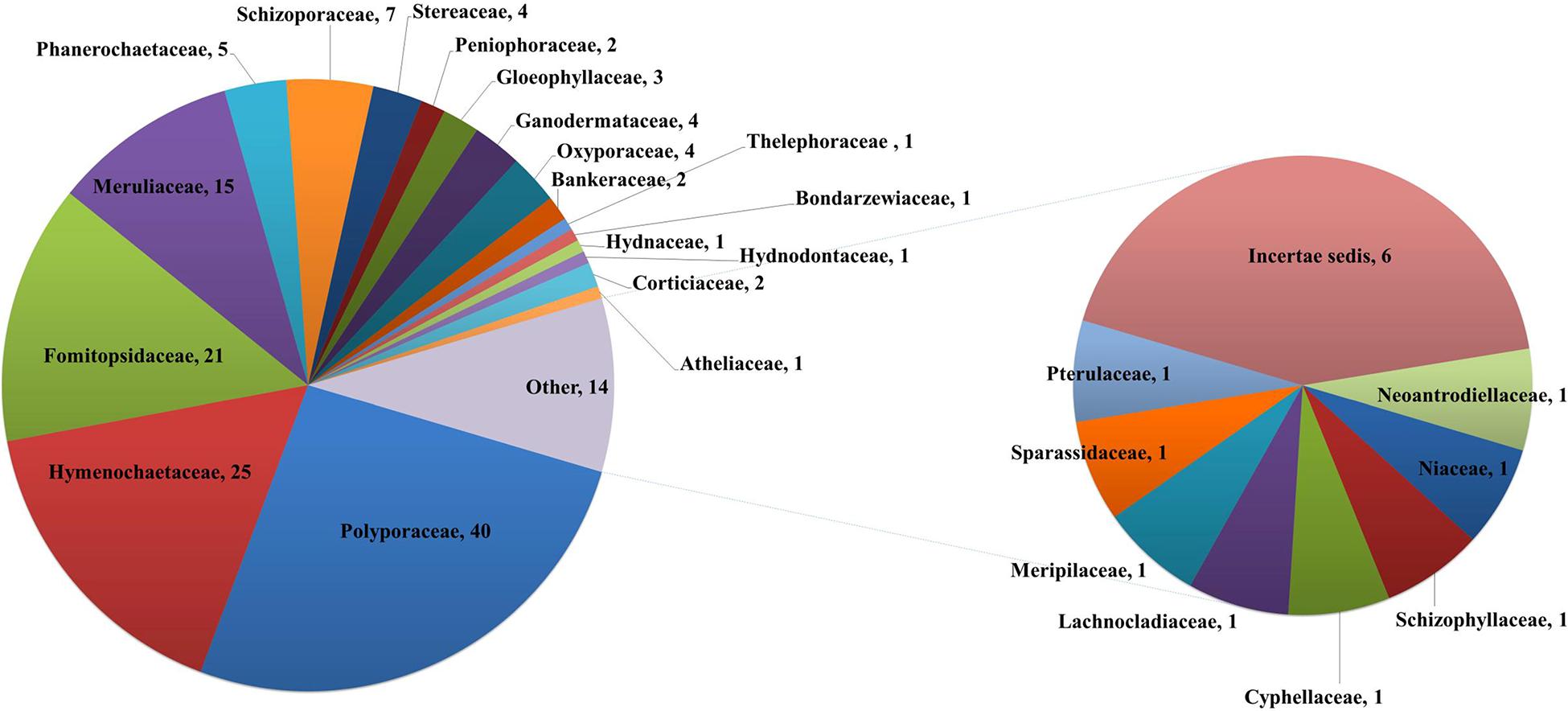
Taking literature information and morphological and phylogenetic evidence into consideration, we report 153 species of wood-inhabiting poroid and corticoid fungi including 149 described species and four single-specimen undescribed lineages belonging to 10 orders (Agaricales, Atheliales, Cantharellales, Corticiales, Gloeophyllales, Hymenochaetales, Russulales, Polyporales, Thelephorales and Trechisporales), 26 families, and 97 genera in Uzbekistan (Table 2 and Figure 4). Data on own specimens and extracted from literature records are accessible as an occurrence dataset (Gafforov and Ordynets, 2020,4 alternative identifier5). Among the 153 species, 31 are reported for the first time in Uzbekistan, including 19 also new to Central Asia. The orders represented by the most specimens are Polyporales (7 families, 59 genera, and 88 species) and Hymenochaetales (4; 20; 41). Together they contain 129 species or 84.3% of the total wood-inhabiting poroid and corticiod biota of Uzbekistan (Table 2). The most species-rich families are Polyporaceae (40 species in 25 genera), Hymenochaetaceae (25; 13), Fomitopsidaceae (21; 16), and Meruliaceae (15; 12) and contain 66 genera and 101 species that constitute 66% of the total poroid and corticoid species number. The genus with the highest number of recorded species are Trametes (9 species); Inonotus (5); Ganoderma, Lentinus, Phellinus, Rigidoporus, and Stereum (4 each); and Antrodia, Cerioporus, Gloeophyllum, Fomitiporia, Hyphodontia, Lyomyces, Phlebia, Phylloporia, Postia, and Trichaptum (3 each) that contain 64 species or 41.8%, and the other genera have one to two species (Table 2).
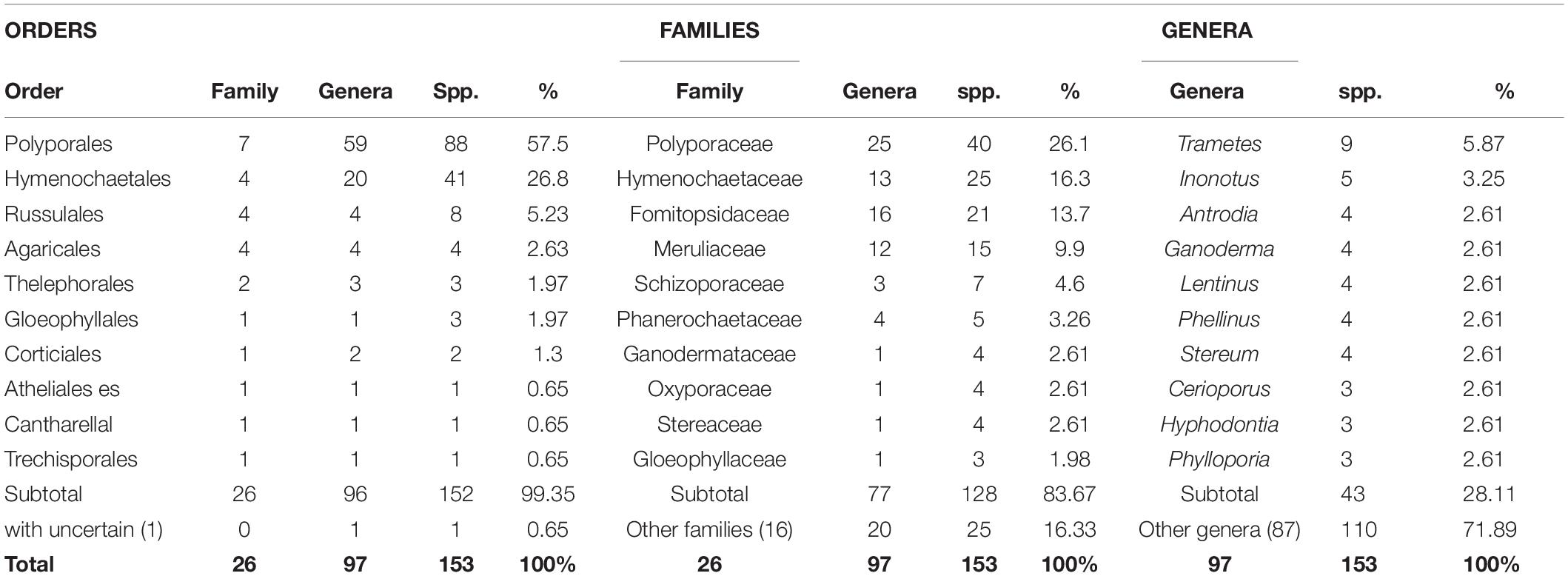
Table 2. Number of wood-inhabiting poroid and corticioid species in the most representative orders, families, and genera in the study area and proportion accounting for total species number.
Annotated Checklist of Wood-Inhabiting Poroid and Corticioid Species in Uzbekistan
The checklist of 153 species of wood-inhabiting poroid and corticioid fungi is arranged alphabetically by orders, family, and species. The currency sign (¤) indicates potentially new species to science and asterisk (•) denotes new fungal records to Central Asia and thus to Uzbekistan, while the new fungal records to Uzbekistan but not to Central Asia is indicated by a number sign (#). A filled circle (∙) means identification was DNA-assisted. Short notes are provided for some taxa. Photos of basidiocarps in situ are shown for some species (Figures 5–7).

Figure 5. Basidiocarps in situ. (A) Radulomyces confluens; (B) Schizophyllum commune; (C) Bjerkandera adusta; (D) Phlebia rufa; (E) Laetiporus sulphureus; (F) Cerioporus squamosus; (G) Cerrena unicolor; (H) Fomes fomentarius (Photo: Yusufjon Gafforov).

Figure 6. Basidiocarps in situ of (A) Lentinus tigrinus; (B) Pyrofomes demidoffii; (C) Trametes hirsuta; (D) Trametes trogii; (E) Ganoderma adspersum; (F) Ganoderma applanatum; (G) Inonotus hispidus; (H) Phellinus igniarius (Photo: Yusufjon Gafforov).

Figure 7. Basidiocarps in situ of (A) Phellinus pomaceus; (B) Phylloporia yuchengii; (C) Sanghuangporus lonicerinus; (D) Lyomyces erastii; (E) Lyomyces sambuci; (F) Stereum hirsutum (Photo: Yusufjon Gafforov).
All occurrence records considered in this study are attributed to 50 localities that are listed below and represented by numbers in brackets. Abbreviations used in these localities are as follows: Andijan Province—AP; Fergana Province—FP; Jizzakh Province; Karakalpakstan—K; Namangan Province—NMP; Navoi Province—NP; Qashqadaryo Province—QR; Samarkand Province—SP; Surkhandaryo Province—SRP; Syrdarya Province —SDR; Tashkent Province—TP; Tashkent botanical garden—TBG.
All territories of Uzbekistan except Kyzyl-kum desert (1); AP, Andijan District, Kutarma village (2); AP, Garden and Parks (3); AP, Shaxrixon district, Holdovonbek village (4); BP (5); FP (6); JP, Nurata State Reserve, Nurata Range, Pamir-Alay Mountain System (7); JP, Zaamin District, Zaamin National Park, Zaamin State Reserve in the South and South-east of the Turkestan Range (8); K, Lower-Amudarya Biosphere Reserve (9); Kyzyl-kum Desert (10); NMP, Chortoq District, Chortoq dam olishmaskani, Chortoq foothills (11); NMP, Haqiloobod District, Haquloobod village (12); NMP, Mingbuloq District, Qorasuv garden (13); NMP, National Parks and Gardens (14); NMP, Pop District, Chodaksay basin, Kurama Mountain Range of Western Tien Shan (15); NMP, Turaqurgon District, Kuymazor village, Pop and Chust foothills (16); NP, Sarmysh valley (17); NP. Tamdy District, Boymurot village, desert (18); QP, Hissar State Reserve in North-western of Hissar Range, Pamir-Alay Mountain System (19); QP, Yakkaobod village, Yakkabog forestry (20); SP, Zarafshan State Reserve, Zarafshan river valley, Pamir Mountains (21); SRP, Baysun District, Baysun village, Omonkhona, Baysun Mountain, South-western spurs of the Hissar Range in the Western part of the Pamir-Alay System (22); SRP, Baysun District, Darband village, Baysun Mountain, South-western spurs of the Hissar Range in the Western part of the Pamir-Alay System (23); SRP, Baysun District, Machay village, Baysun Mountain, South-western spurs of the Hissar Range in the Western part of the Pamir-Alay System (24); SRP, Hissar Range of Pamir-Alay Mountains (25); SRP, Surkhan State Reserve (26); SDR (27); TBG (28); Tashkent, olimlar shaxarchasi (29); TP, Angren, Yangibod village, South-eastern slope of Chatkal Mountain Range of Western Tien Shan (30); TP, Bekabad District, NW of Bekabad, Dalverzin village (31); TP, Bustonliq District, Beldersay, Greater Chimgan, Chatkal Mountain Range of Western Tien Shan (32); TP, Bustonliq District, Burchmulla village, Kulabsay, Western Tien Shan Mountains (33); TP, Bustonliq District, Gazalkent, spurs of the Western Tien Shan (34); TP, Bustonliq District, Kayinarsay and Sarvasay, Western Tien Shan (35); TP, Bustonliq District, Kuksu River, Pskem Mountain Range of Western Tien Shan (36); TP, Bustonliq District, Onaulgansoy, Pskem river, Pskem Mountain Range of Western Tien Shan (37); TP, Bustonliq District, Oqtosh village, Ugam Mountain Range of Western Tien Shan (38); TP, Bustonliq District, Xojikent village, Ugam Mountain Range of Western Tien Shan (39); TP, Bustonliq District, Xumson village, Xumsonsoy, Ugam Mountain Range of the Western Tien Shan (40); TP, Bustonliq District, Yubileyniy village, Chimyonsoy, Chimgan, Chatkal Mountain Range of Western Tien Shan (41); TP, Bustonliq District, Yusufhona village, Mazarsay, Charvak Reservoir, Western Tien Shan Mountains (42); TP, Karankulsay, Kungurbuka Mountain, Ugam Range of Western Tien Shan (43); TP, Oxangoron District, Oxangoron basin river (44); TP, Parkent District, Chatkal Biosphere Reserve, Chatkal Mountain Range of Western Tien Shan (45); TP, Parkent District, Kumyshkan village, Chatkal Mountain Range of Western Tien Shan Mountains (46); TP, Parkent District, Nivich and Qiziljar villages, Bashkyzylsay, Chatkal Biosphere Reserve, Chatkal Mountain Range of Western Tien Shan (47); TP, Tuyatashsoy, Western Tien Shan Mountains (48); TP, Ugam-Chatkal State Nature National Park, Western Tien Shan Mountains (49); TP, Yangikurgan village, Kurigansay river, Western Tien Shan Mountains (50).
AGARICALES Underw.
CYPHELLACEAE Lotsy
#Chondrostereum purpureum (Pers.) Pouzar, Česká Mykol. 13(1): 17 (1959)
Specimen examined: (24): on Acer pentapomicum Stewart ex Brandis, 17 May 2016, YG-B01.
PTERULACEAE CORNER
#•Radulomyces confluens (Fr.) M.P. Christ., Dansk bot. Ark. 19 (no. 2): 230 (1960)
Specimens examined: (39): on fallen rotten trunk, 2 Nov. 2011, YG006; (39): on trunks of angiosperm woody plant, 20 Nov. 2013, YGcor-80; (28): on dead stump, 2 Sept. 2013, YG-G43.
NIACEAE Jülich
∗Merismodes anomala (Pers.) Singer, Agaric. mod. Tax., Edn 3 (Vaduz): 665 (1975)
Specimen examined: (49): on dead branch of Prunus sp., 30 Apr. 1988, A. Kollom, TAAM127589.
Note: In TAAM, this specimen was originally labeled as Cyphellopsis anomala (Pers.) Donk.
SCHIZOPHYLLACEAE Quél.
•Schizophyllum commune Fr., Observ. mycol. (Havniae) 1: 103 (1815)
Specimens examined: (40): on Morus alba L., 26 May 2011, YG047; (37): on dead trunk of Juglans regia L., 20 Sept. 2014, YG/PS169; (45): on a dry branch of Celtis australis subsp. caucasica (Willd.) C.C. Towns., 30 Apr. 1980, A. Kollom, TAAM127588; (20): on Populus sp., Sept. 2012, YG-J2.
Literature: Khalikova (1989), (49): on Salix pentandra L. and Prunus armeniaca L.; Iminova (2009), (4): on Salix pentandra L., Ìàlus domestica, Prunus armeniaca, Jun. 2000, Jul. 2004.
ATHELIALES Jülich
ATHELIACEAE Jülich
#•Athelia arachnoidea (Berk.) Jülich, Willdenowia, Beih. 7: 53 (1972)
Specimens examined: (38): on fallen angiosperm branch, 3 Sept. 2013, YG-G23; (38): YG-G44; (37): on living branches of Crataegus sp., 18 Jun. 2014, YG/PS154; (8): on dried branches of Lonicera paradoxa Pojark., 26 May 2018, YG1111.
CANTHARELLALES Gäum.
HYDNACEAE Chevall.
#Sistotrema coroniferum (Höhn. and Litsch.) Donk, Fungus, Wageningen 26: 4 (1956)
Specimen examined: (37): on branches of Betula tree, 19 Jun. 2014, YG/PS95.
CORTICIALES K.H. Larss.
CORTICIACEAE Herter
#Corticium roseum Pers., Neues Mag. Bot. 1: 111 (1794)
Specimen examined: (35): on stumps of Juglans regia, 31 Aug. 1963, A. Raitviir, TAAM043491.
¤•Vuilleminia sp. Parmasto, Eesti NSV Tead. Akad. Toim., Biol. seer 16(4): 391 (1967)
Specimen examined: (36): on bark and at base of a trunk of Lonicera sp., E. Parmasto, 25 Apr. 1982, TAAM104410.
GLOEOPHYLLALES Thorn
GLOEOPHYLLACEAE Jülich
•Gloeophyllum abietinum (Bull.) P. Karst., Bidr. Känn. Finl. Nat. Folk 37: 80 (1882)
Specimens examined: (49): on trunk of Juniperus sp., 23 Apr. 1982, A. Kollom, TAAM127397.
Literature: Baltaeva (1992, 1993), [(45): on Pinus sp., 10 May 1987; (8): on Pinus sp., 17 Jun. 1988; (7): on dried stem of Pinus sp., 28 Jul. 1988; (21): on stem of Picea sp., 21 Oct. 1988; (19): on Juniperus sp., 29 Jul. 1989].
Note: Although the species is easily recognizable in the field, to our surprise, we failed to find it.
Gloeophyllum odoratum (Wulfen) Imazeki, Bull. Tokyo Sci. Mus. 6: 75 (1943)
Literature: Baltaeva (1992), [(19): on wet trunk of Biota sp., 5 Sept. 1990].
Gloeophyllum trabeum (Pers.) Murrill, N. Amer. Fl. (New York) 9(2): 129 (1908)
Literature: Baltaeva (1992, 1993), [(45): on stump and trunk of Quercus sp., 1 Jun. 1987; (8): on strum of unknown woody plants, 1 Jun. 1987; (7): on trunk of deciduous tree, 4 Oct. 1988; (21): on fallen stem of angiosperm, 22 Jun. 1988; (19): on dried stem of Quercus sp., 19 Mar. 1988; (9): on Quercus sp., 7 May 1988].
POLYPORALES Gäum.
MERULIACEAE Rea
Abortiporus biennis (Bull.) Singer, Mycologia 36(1): 68 (1944)
Literature: Baltaeva (1992), [(45): on dead attached branches of living Quercus sp., 12 Sept. 1990].
•Aurantiporus fissilis (Berk. and M.A. Curtis) H. Jahn ex Ryvarden, Polyp. N. Eur. (Oslo) 2: 222 (1978).
≡ Tyromyces fissilis (Berk. and M.A. Curtis) Donk.
Specimen examined: (28): on living stem of Juglans regia, 9 Jun. 2014, YG/bot3.
Literature: Kravtzev (1950), (as Tyromyces fissilis, (19): on stem of Malus sp.); Baltaeva (1992, 1993), (as Tyromyces fissilis, (1): on various deciduous wood).
•Bjerkandera adusta (Willd.) P. Karst., Meddn Soc. Fauna Flora fenn. 5: 38 (1879)
Specimens examined: (40): on dead stump of Juglans regia, 26 May 2011, YG012; (37): on dried Juglans regia log, 20 Jun. 2014, YG/PS172; (37): on dried trunk of angiosperm wood, 20 Jun. 2014, YG/PS183; (29): on dead stump of Prunus armeniaca, 2 Apr. 2013, YG-G41; (28): on unknown wood, 3 Sept. 2013, YG/bot23a; (47): on trunk of Populus alba L., 4 May 1988, I. Parmasto, TAAM126292; (19): on fallen trunk of Populus sp., 15 Jun. 2013, YG-O1.
Literature: Panfilova and Gaponenko (1963), [(44): on dried log of Salix sp.], Khalikova (1989), [(34): on decaying Prunus armeniaca log], Baltaeva (1992, 1993), [(49): on trunk of Salix sp., 13 Aug. 1986; (45): on dried stem and trunk of Populus sp., 25 Jul. 1986; (19): on decaying Quercus log, 6 May 1986; (6): on Tilia sp., 29 May 1989; (19): on stump of Ulmus sp., 28 Jun. 1988].
Note: This species is very common and occurs on dead and senescent deciduous wood. We mostly found it on Juglans, Populus, and Prunus species in Uzbekistan.
Bjerkandera fumosa (Pers.) P. Karst., Meddn Soc. Fauna Flora fenn. 5: 38 (1879)
Literature: Baymuratova (1963), [(27): on trunk of Quercus sp., 1962]; Khalikova (1989), [(28): on Quercus sp., Jun. 1983; (49): on dried trunk of Populus sp., Jul. 1984]; Baltaeva (1992, 1993), [(49): on Populus sp., 19 May 1985; (45): on stem of Populus sp., 6 Jun. 1986; (1): on trunk of hardwood].
Gelatoporia dichroa (Fr.) Ginns, Index Fungorum 156: 1 (2014)
≡ Gloeoporus dichrous (Fr.) Bres.
Literature: Panfilova and Gaponenko (1963), (as Gloeoporus dichrous, (49): dried stem of Morus alba, 1962); Baltaeva (1992, 1993), (as Gloeoporus dichrous, (45): on decaying Picea sp., 4 Jul. 1988; (8): on dried stem of Pinus sp., 6 Aug. 1987; (19): on Pinus sp., 18 Jul. 1989; (26): on Pinus sp., 10 Aug. 1989).
¤•Hyphoderma sp.
Specimen examined: (37): on decaying branch of Betula sp., 19 Jun. 2014, YG/PS133.
Irpex lacteus (Fr.) Fr., Elench. fung. (Greifswald) 1: 142 (1828)
Literature: Kleyner (1958), [(25): on branch of Pyrus sp., 1957]; Baltaeva (1992, 1993), [(19): on stump of Populus sp., 19 Aug. 1985, (19): on Ulmus sp., 12 Aug. 1989; (8): on branch of Alnus sp., 9 Sept. 1986; (7): on Quercus sp., 4 Aug. 1987; (45): on dried stem of Salix sp., 16 Oct. 1988].
Irpiciporus litschaueri (Lohwag) Zmitr., Folia Cryptogamica Petropolitana (Sankt-Peterburg) 6: 105 (2018).
≡ Spongipellis litschaueri Lohwag
Literature: Baltaeva (1992, 1993), (as Spongipellis litschaueri, 7: on stem of Malus sp., 7 Aug. 198; (7): on trunk of Quercus sp., 14 Aug. 1986; (8): on Fraxinus sp., 29 Jul. 1988; (9): on Quercus sp., 19 Aug. 1987; (45): on stems of Ulmus sp., 4 Sept. 1987; (21): on angiosperm fallen wood, 27 Sept. 1987; (26): on dead Fraxinus branch, 3 Sept. 1989).
∗Mycoacia aurea (Fr.) J. Erikss. and Ryvarden, Cortic. N. Eur. (Oslo) 4: 877 (1976).
≡ Phlebia aurea (Fr.) Nakasone
Specimen examined: (28): on a fallen rotten deciduous trunk, 20 Apr. 1982, E. Parmasto, TAAM104260.
Note: In TAAM, this specimen was originally labeled as Phlebia aurea.
•Phlebia bresadolae Parmasto, Eesti NSV Tead. Akad. Toim., Biol. seer 16(4): 390 (1967)
Specimens examined: (37): on fallen branch of angiosperm, 18 Jun. 2014, YG/PS189; (37): on unknown woody plants branch, 19 Jun. 2014, YG/PS89.
¤•Phlebia sp. (P. Karst.) Ryvarden, Rept. Kevo subarct. Res. Stn 8: 151 (1971)
Specimens examined: (39): on dead hardwood, 2 Nov. 2011, YG326; (40): on living Crataegus pseudoheterophylla subsp. turkestanica, 26 May 2011, YG64.
∗•Phlebia rufa (Pers.) M.P. Christ., Dansk bot. Ark. 19(no. 2): 164 (1960)
Specimen examined: (40): on living stems of Robinia pseudoacacia L., 26 May 2011, YG77.
Resiniporus resinascens (Romell) Zmitr., Folia Cryptogamica Petropolitana (Sankt-Peterburg) 6: 98 (2018)
≡ Ceriporiopsis resinascens (Romell) Domański
Literature: Baltaeva (1992, 1993), (as Ceriporiopsis resinascens (Romell) Domański, (45): on fallen branch of Betula sp., 7 May 1987; (19): on fallen log of Populus sp., 30 May 1987).
Sarcodontia spumea (Sowerby) Spirin, Mycena 1(1): 64–71 (2001)
≡ Spongipellis spumeus (Sowerby) Pat.
Literature: Baltaeva (1992, 1993), (as Spongipellis spumeus (Sowerby) Pat., (45): on fallen stem of Ulmus sp., 4 Sept. 1987; (21): on trunk of Ulmus sp., 27 Sept. 1987; (7): on stem of Malus sp., 7 Aug. 1986, (7): on Quercus sp., 19 Aug. 1989; (8): on Fraxinus sp., 29 Jul. 1988; (19): on dried trunk of Fraxinus sp., 3 Sept. 1989; (9): on dried strum of Quercus sp., 14 Aug. 1986).
∗Steccherinum ciliolatum (Berk. and M.A. Curtis) Gilb. and Budington, J. Ariz. Acad. Sci. 6(2): 97 (1970).
Literature: Gafforov et al. (2017), [(45): on dead trunk of Prunus spinosissima (Bunge) Franch., 29 Apr. 1988, A. Kollom, TAAM127581].
FOMITOPSIDACEAE Jülich
Amyloporia sinuosa (Fr.) Rajchenb., Gorjón and Pildain, Aust. Syst. Bot. 24(2): 117 (2011)
≡ Antrodia sinuosa (Fr.) P. Karst.
Literature: Baltaeva (1992), (as Antrodia sinuosa (Fr.) P. Karst., (21): on wet trunk of Pinus sp., 15 Aug. 1990).
Antrodia albida (Fr.) Donk, Persoonia 4(3): 339 (1966)
Literature: Kravtzev (1950), [(27): on Quercus sp.]; Baltaeva (1992, 1993), [(49): on bark of Quercus fallen branches, 5 Apr. 1987; (45): on fallen Betula trunk, 18 May 1986; (26): on stumps of Populus sp., 25 Jun. 1988; (8): on wet woody plant, 5 Apr. 1987].
Antrodia heteromorpha (Fr.) Donk, Persoonia 4(3): 339 (1966)
Literature: Baltaeva (1992), [(21): on rotten Pinus fallen trunk, 17 Aug. 1990].
Antrodia xantha (Fr.) Ryvarden, Norw. Jl Bot. (20): 8 (1973)
Specimens examined: (33): on trunk of Juniperus polycarpos var. seravschanica, 22 Apr. 1982, E. Parmasto, TAAM104400; (50): on rotten trunk of Juniperus semiglobosa Regel, 24 Apr. 1982, E. Parmasto, TAAM104301; (33): on fallen rotten trunk of Juniperus semiglobosa, 24 Apr. 1982, E. Parmasto, TAAM104289.
Literature: Baltaeva (1992, 1993), [(45): on Pinus sp., 19 Aug. 1988; (19): on Juniperus polycarpos var. seravschanica, 15 Aug. 1988; (21): on Pinus sp., 3 Jul. 1989; (7): on stumps of Picea sp., 13 Jul. 1989; (7): on Juniperus sp., 15 Jul. 1989; (49): on Pinus sp., 20 Jul. 1989].
Note: This species appears to be common in the study area. However, we did not find fresh specimens during our field trips.
#Brunneoporus juniperinus (Murrill) Zmitr., Folia Cryptogamica Petropolitana (Sankt-Peterburg) 6: 86 (2018).
Specimen examined: (36): on base of tree of Juniperus semiglobosa, 25 Apr. 1982, M. Khalikova, TAAM104433.
Note: In TAAM, this specimen was originally labeled as Antrodia juniperina (Murrill) Niemelä and Ryvarden.
Climacocystis borealis (Fr.) Kotl. and Pouzar, Česká Mykol. 12(2): 103 (1958)
Literature: Baltaeva (1992, 1993), [(45): on old decaying trunks of Pinus sp., 24 Jul. 1988; (26): on fallen trunk of Picea sp., 26 Aug. 1989].
Daedalea quercina (L.) Pers., Syn. meth. fung. (Göttingen) 2: 500 (1801)
Literature: Baltaeva (1992), [(21): on stem of Juglans regia, 15 Aug. 1990].
∗•Flavidoporia pulverulenta (B. Rivoire) Audet, Mushrooms nomenclatural novelties 4: [1] (2017)
Specimens examined: (37): on trunk of Salix sp., 19 Jun. 2014, YG/PS167; (37): on rotten of branch of Salix alba L., 11 Sept. 2016, YG1110.
Fomitopsis betulina (Bull.) B.K. Cui, M.L. Han and Y.C. Dai, in Han, Chen, Shen, Song, Vlasák, Dai and Cui, Fungal Diversity 80: 359 (2016)
≡ Piptoporus betulinus (Bull.) P. Karst.
Literature: Panfilova and Gaponenko (1963); Khalikova (1989), (as Piptoporus betulinus (Bull.) P. Karst., (49): on stem of Betula sp.); Baltaeva (1992, 1993), (as Piptoporus betulinus, (45): on trunk of Betula sp., 14 Jul. 1987; (19): on wet Betula stems, 5 Aug. 1987; (26): on Betula sp., 30 Jun. 1988; (8): on dried stems of Betula sp., 24 Aug. 1989); Iminova (2009), (as Piptoporus betulinus, (3): on Betula tianschanica, Jul.–Nov. 2003–2005).
Fomitopsis pinicola (Sw.) P. Karst., Meddn Soc. Fauna Flora fenn. 6: 9 (1881)
Specimen examined: (48): on Juniperus polycarpos var. seravschanica, J.K. Rotkevich, Jul. 1956, TASM002.
Literature: Baltaeva (1992, 1993), [(45): on living trunk of Pinus sp., 25 Jul. 1986; (7): on living trunk of Pinus sp., 11 Jun. 1987; (8): on living stem of Picea sp.; (21): on living Pinus tree, 3 Aug. 1987; (19): on fallen stem of conifer tree, 19 Jul. 1988].
Laetiporus sulphureus (Bull.) Murrill, Annls mycol. 18(1/3): 51 (1920)
Specimens examined: (28): on stem of Salix sp., 2 Sept. 2011, YG031; (28): on dried strum angiosperm wood, 3 Oct. 2011, YG041; (24): on Acer tataricum subsp. semenovii (Regel and Herder) A.E. Murray, 20 Aug. 2016, YG-B10.
Literature: Panfilova and Gaponenko (1963); Khalikova (1989), [(49): on living stem of Prunus mahaleb L. and Juglans regia]; Baltaeva (1992, 1993), [(45): on trunk of Robinia pseudoacacia L., 16 Oct. 1986; (1): on trunks of Acacia, 30 Jul. 1989; (1): on trunk of Quercus, 12 Sept. 1989].
Neoantrodia serialis (Fr.) Audet, Mushrooms nomenclatural novelties 6: [2] (2017)
≡ Antrodia serialis (Fr.) Donk
Literature: Baltaeva (1992), (as Antrodia serialis, (19): on dried of Pinus trunk, 6 Apr. 1989).
Phaeodaedalea incerta (Curr.) Tura, Zmitr., Wasser and Spirin, Biodiversity of the Heterobasidiomycetes and non-gilled Hymenomycetes (former Aphyllophorales) of Israel: 401 (2011).
= Gloeophyllum sprucei (Berk.) Teixeira
Literature: Baltaeva (1992), (as Gloeophyllum sprucei, (19): on wet branches of Pinus sp., 25 Jul. 1990).
Phaeolus schweinitzii (Fr.) Pat., Essai Tax. Hyménomyc. (Lons-le-Saunier): 86 (1900)
Literature: Baltaeva (1992, 1993), [(49): on dried fallen stem of Pinus sp., 30 Jul. 1989; (45): on trunk of Picea sp., 29 Jul. 1989]; Iminova (2009), [(3): on Platanus orientalis, Jun.–Jul. 2005].
∗•Pilatoporus ibericus (Melo and Ryvarden) Kotl. and Pouzar, Cryptog. Mycol. 14(3): 217 (1993)
Specimens examined: (49): on trunk of angiosperm tree, 3 Sept. 2013, YG-G24.
Postia caesia (Schrad.) P. Karst., Revue mycol., Toulouse 3(no. 9): 19 (1881)
≡ Oligoporus caesius (Schrad.) Gilb. and Ryvarden
Literature: Baltaeva (1992, 1993), (as Oligoporus caesius, (45): on stump of Picea sp., 17 Oct. 1987, 6 Nov. 1987; (8): on trunk of Pinus sp., 28 Oct. 1987; (7): on Pinus fallen branch, 12 Nov. 1988).
Postia sericeomollis (Romell) Jülich, Persoonia 11(4): 423 (1982)
≡ Oligoporus sericeomollis (Romell) Bondartseva
= Chaetoporellus litschaueri (Pilát) Bondartsev
Literature: Baltaeva (1992, 1993), (as Oligoporus sericeomollis, Chaetoporellus litschaueri), [(45): on stump of Pinus sp., 18 Sept. 1986; (19): on Pinus sp., 21 Jun. 1989; (8): on trunk of Picea sp., 9 May 1987; (7): on fallen branch of Picea sp., 9 May 1987].
Postia stiptica (Pers.) Jülich, Persoonia 11(4): 424 (1982)
≡ Oligoporus stipticus (Pers.) Gilb. and Ryvarden
Literature: Baltaeva (1992, 1993), (as Oligoporus stipticus, (45): on stump of Pinus sp, 9 Oct. 1987; (19): on Pinus sp., 24 Oct. 1987; (21): on fallen trunk of Picea sp., 6 Nov. 1988).
Osteina obducta (Berk.) Donk, Schweiz. Z. Pilzk. 44: 86 (1966)
≡ Oligoporus obductus (Berk.) Gilb. and Ryvarden
Literature: Baltaeva (1992), (as Oligoporus obductus, (21): on root of Pinus sp., 15 Aug. 1990).
Rhodofomes roseus (Alb. and Schwein.) Vlasák, Ćeská Mykol. 44(4): 235 (1990)
≡ Fomitopsis rosea (Alb. and Schwein.) P. Karst
Specimens examined: (45): on conifer fallen trunk, 15 Jun. 1980, S.S. Ramazanova, N4 (TASM).
Literature: Baltaeva (1992, 1993), (as Fomitopsis rosea, (45): on dead standing Picea trunk, 7 Apr. 1987; (45): on Picea sp., 21 May 1988; (8): on trunk of Picea sp., 9 Jun. 1987; (7): on fallen branch of Pinus sp., 30 May 1988; (14): on Pinus sp., 5 May 1989).
•Subantrodia uzbekistanica (Yuan, Gafforov and F. Wu) Audet, Mushrooms nomenclatural novelties 9: [1] (2017).
≡ Antrodia uzbekistanica Yuan, Gafforov and F. Wu
Specimens examined: (8): on Juniper tree rotten wood, 8 Sept. 2016, YG1014; (8): on trunk of Juniperus sp., 4 Sept. 2017, YG1103; (8): on rotten stem of Juniperus polycarpos var. seravschanica (Kom.) Kitam., 10 Sept. 2017, YG1107; (8): on unknown woody branches, 9 Sept. 2016, YG1100.
Literature: Yuan et al. (2017), (same place: as Antrodia uzbekistanica).
PHANEROCHAETACEAE Jülich
∗•Byssomerulius corium (Pers.) Parmasto, Eesti NSV Tead. Akad. Toim., Biol. seer 16(4): 383 (1967)
Specimens examined: (28): on angiosperm fallen branch, 2 Sept. 2013, YG-G21; (7): on dried branch of Prunus vulgaris L., 28 Aug. 2013, YG-X3.
∗Ceriporia purpurea (Fr.) Donk, Proc. K. Ned. Akad. Wet., Ser. C, Biol. Med. Sci. 74(1): 28 (1971)
Specimen examined: (45): on a deciduous tree, 1 May 1988, A. Kollom, TAAM127605.
•Ceriporiopsis gilvescens (Bres.) Domański, Acta Soc. Bot. Pol. 32(4): 731 (1963)
≡ Tyromyces gilvescens (Bres.) Ryvarden
Specimens examined: (28): on dried angiosperm wood, 14 Oct. 2011, YG046; (36): on base of rotten trunk of wood, 8 Jun. 2011, YG049; (32): on Juniperus pseudosabina Fisch. et C.A. Mey., 26 May 2011, YG008.
Literature: Baltaeva (1992, 1993), (as Tyromyces gilvescens, (45): on fallen branch of Populus sp., 2 May 1987; (19): on Quercus trunk, 19 Aug., 1988; (26): on died fallen of Populus sp., 1 Sept. 1988; (9): on rotten trunk of Populus sp., 10 Jul. 1987; (21): on rotten trunk of Malus sp., 21 Jul. 1986; (6): on dead stump and trunk of deciduous wood, 13 Apr. 1986).
Ceriporiopsis mucida (Pers.) Gilb. and Ryvarden, Mycotaxon 22(2): 364 (1985)
Literature: Baltaeva (1992), [(5): on dried branch of Populus sp., 28 Aug. 1990].
#Efibula tuberculata (P. Karst.) Zmitr. and Spirin, in Zmitrovich, Malysheva and Spirin, Mycena 6: 33 (2006)
Specimen examined: (18): on fallen trunk of Haloxyllon sp., 6 Apr. 1979, K. Kalamees, TAAM120642.
Note: In TAAM, this specimen was originally labeled as Athelia sp.
POLYPORACEAE Fr. ex Corda
Cerioporus mollis (Sommerf.) Zmitr. and Kovalenko, Int. J. Med. Mushrooms 18(1): 33 (2016)
≡ Datronia mollis (Sommerf.) Donk
Literature: Baltaeva (1992, 1993), (as Datronia mollis, (45): on wet dead trunk of Populus sp., 10 May 1985; (19): on Populus sp., 21 Apr. 1986; (26): on Populus sp., 17 Jun. 1987).
Cerioporus squamosus (Huds.) Quél., Enchir. fung. (Paris): 167 (1886)
≡ Polyporus squamosus (Huds.) Fr.
Specimens examined: (50): on trunk of Juglans regia, 24 Apr. 1982, A. Kollom, TAAM127413; (36): on dried trunk and stem of angiosperm woody plant, 6 Jun. 2011, YG026; (37): on rotten trunk of Acer tataricum subsp. semenovii, 2 Sept. 2017, YG20170902; (22): on dried stump of Populus alba, 13 May 2015, YG-B02; (23): on Acer sp., 17 May 2015, YG-B05.
Literature: Panfilova and Gaponenko (1963), Akhmedova, 1966 (as Polyporus squamosus, (49): on trunk of Juglans regia); Khalikova (1989), (as Polyporus squamosus, (49): on Pistacia sp.); Baltaeva (1992, 1993), (as Polyporus squamosus, (1): on stump collar of Quercus, sp., 1988, on fallen big branch of Juglans regia, 1989); Iminova (2009), (as Polyporus squamosus, (3): on various woody plants, May–Jun. 2000–2004).
Note: This species is widespread on angiosperm woody plants across study area.
Cerioporus varius (Pers.) Zmitr. and Kovalenko, Int. J. Med. Mushrooms 18(1): 33 (2016)
≡ Polyporus varius (Pers.) Fr.
Literature: Baltaeva (1992), (as Polyporus varius, (45): on deadwood stem of Quercus sp., 23 Jun. 1990; (19): on fallen stem of Lonicera sp., 10 Aug. 1990).
•Cerrena unicolor (Bull.) Murrill, J. Mycol. 9(2): 91 (1903)
Specimens examined: (28): on dried fallen stem of angiosperm tree, 2 Sept. 2013, YG-G28; (32): on Acer tataricum subsp. semenovii, 15 May 2011, YG18; (32): on dried stem of Acer tataricum subsp. semenovii, 15 May 2011, YG027; (38): on Crataegus pseudoheterophylla subsp. turkestanica, 1 Jun. 2011, YG003; (41): on a trunk of Juglans regia, 22 Apr. 1982, E. Parmasto, TAAM104271; (41): on dry twig of Acer sp., 22 Apr. 1982, A. Kollom, TAAM127385; (50): on dead trunk of Salix sp., 24 Apr. 1982, A. Kollom, TAAM127405; (37): on Acer tataricum subsp. semenovii, 19 Sept. 2014, YG/PS79; (45): on dry branch of Celtis australis subsp. caucasica, 29 Apr. 1988, A. Kollom, TAAM127582; (45): on Celtis australis subsp. caucasica, 3 May 1988, A. Kollom, TAAM127632; (45): on dry trunk of Celtis australis subsp. caucasica, 1 May 1988, I. Parmasto, TAAM126263; (47): on trunk of Prunus mahaleb, 29 Apr. 1988, I. Parmasto, TAAM126248.
Literature: Akhmedova (1966), [(49): on Populus sp.]; Khalikova (1989), [(49): on trunk of Populus sp., Jul. 1988]; Baltaeva (1992, 1993), [(1): on stump of Quercus sp., Populus sp., Salix sp., Jul.–Aug. 1988–1989].
Note: This species is widespread and causes damage to Acer tataricum subsp. semenovii trees in Tien Shan Mountain.
Coriolopsis gallica (Fr.) Ryvarden, Norw. Jl Bot. 19: 230 (1973)
≡ Funalia gallica (Fr.) Bondartsev and Singer
Literature: Panfilova and Gaponenko (1963), (as Funalia gallica, (49): on Quercus sp.); Khalikova (1989), (as Funalia gallica, (28): on stumps and dried trunks of Fraxinus americana L., Jun. 1986, Sept. 1986, Dendropark, May, 1987); Baltaeva (1992, 1993), [(45): on dried stem and branches of Quercus sp., 6 Jun. 1985, (45): on Quercus sp., 3 Jun. 1988, (45): on Salix sp., 17 Jul. 1987, (47): on Populus sp., 27 Jul. 1987; (8): on Fraxinus sp., 4 Jul. 1989; (7): on Fraxinus sp., 1 Aug. 1989; (19): on stem of Fraxinus sp., 20 Jul. 1986; (21): on trunk of Populus sp., 27 Jul. 1987; (6): on Quercus sp., 3 Jun. 1988; (9): on Populus tremula L., 1 Aug. 1989]; Iminova (2009), (as Funalia gallica, (3): on fallen trunks of Platanus orientalis L., May 2005, Sept. 2005).
Daedaleopsis confragosa (Bolton) J. Schröt., in Cohn, Krypt.-Fl. Schlesien (Breslau) 3.1(25–32): 492 (1888) [1889]
Literature: Baltaeva (1992), [(45): on decaying stem of Betula sp., 11 Aug. 1990; (6): on Betula sp., 12 Sept. 1991; on fallen tree of Betula sp., 6 Aug. 1990, on Betula sp., 19 Jul. 1988].
Dichomitus squalens (P. Karst.) D.A. Reid, Revta Biol., Lisb. 5(1–2): 150 (1965) [1964–5]
Literature: Baltaeva (1992, 1993), [(45): on the bark of Pinus sp., 9 May 1987; (21): on Biota sp., 21 Jun. 1989; (21): on Pinus sp., 14 May 1988; (7): on Pinus sp., 9 Jun. 1989; (8): on Picea sp., 20 Jun. 1988; (19): on living stem of old Biota sp., 19 Jul. 1988].
Diplomitoporus flavescens (Bres.) Domański, Acta Soc. Bot. Pol. (39): 191 (1970)
≡ Antrodia flavescens (Bres.) Ryvarden
Literature: Khalikova (1989), (as Antrodia flavescens, (49): on fallen logs of Picea sp., 22 Apr. 1980); Baltaeva (1992), (as Diplomitoporus flavescens), Baltaeva, 1993 (as Antrodia flavescens, (7): on Picea stump, 20 Jul. 1987; (26): on Juniper fallen stems, 17 Jun. 1987; (21): on Pinus trunk, 21 Jun. 1989).
Fibroporia vaillantii (DC.) Parmasto, Consp. System. Corticiac. (Tartu): 177 (1968)
≡ Antrodia vaillantii (DC.) Ryvarden
Literature: Baltaeva (1992, 1993), (as Antrodia vaillantii, (45): on trunk of Picea sp., 18 Aug. 1989).
•Fomes fomentarius (L.) Fr., Summa veg. Scand., Sectio Post. (Stockholm): 321 (1849)
Specimens examined: (28): on living stem of Populus sp., 3 Sept. 2013, YG/bot2; (28): on decaying trunk of identified angiosperm, 4 Sept. 2013, YG/bot4; (40): on living trunk of Juglans regia, 26 May 2011, YG014; (28): on unknown wood, 7 Nov. 2014, YG-60, ibit., on unknown trunk decaying wood, YG-70; (38): on Juglans regia, 13 Sept. 2012, YG/Un2; (37): on dried Juglans regia trunk, 14 Sept. 2014, YG/PS174; (23): on living stem of Juglans regia, 13 Aug. 2015, YG-B03; (22): on dried stem of Salix alba, 17 Aug. 2016, YG-B04.
Literature: Panfilova and Gaponenko (1963); Akhmedova (1966); Khalikova (1989), [(49): on stem and trunk decaying and living Juglans regia]; Baltaeva (1992), [(1): on dead and living deciduous trees on Malus sp., Quercus sp., Populus sp.]; Iminova (2009), [(3): on living stem of Salix wilhelmsiana M. Bieb., Sept. 2000; (4): on died trunk of Salix alba, Oct. 2001].
Note: This species is widespread on living trees in the study area.
Hapalopilus rutilans (Pers.) Murrill, Bull. Torrey bot. Club 31(8): 416 (1904)
= Hapalopilus nidulans (Fr.) P. Karst.
Literature: Baltaeva (1992, 1993), (as Hapalopilus nidulans, (49): on dead branch of Betula sp., 1 Jul. 1989, (49): on fallen stem of Populus sp., 14 Jul. 1989; (19): on fallen strums of Populus sp., 29 Jul. 1989).
Lentinus arcularius (Batsch) Zmitr., Int. J. Med. Mushrooms 12(1): 88 (2010)
≡ Polyporus arcularius (Batsch) Fr.
Literature: Khalikova (1989), (as Polyporus arcularius, (45): on dead branch of Juglans regia, May, 1982, Nov. 1983; (41): on Salix interior Rowlee, Apr.–May 1983); Iminova (2009), (as Polyporus arcularius; (11): dried trunk of Juglans regia, Apr. 2000, Nov. 2004).
Lentinus brumalis (Pers.) Zmitr., Int. J. Med. Mushrooms 12(1): 88 (2010)
≡ Polyporus brumalis (Pers.) Fr.
Literature: Schwartzman (1964), (as Polyporus brumalis, (49): on Celtis australis subsp. Caucasica); Baltaeva (1993), (as Polyporus brumalis, (21): on stem of branch of Salix sp., 21 Jul. 1987; (3): on Salix sp., 15 Aug. 1987; (45): on Betula sp., 10 May 1987; (19): on Betula sp., 20 Jul. 1988; (8): on Populus sp., 6 Sept. 1988; (7): on small branches of Salix sp., 24 Aug. 1987; (27): 20 Jul. 1988; (10): on dried stem of Populus sp., 24 Aug. 1989).
Lentinus substrictus (Bolton) Zmitr. and Kovalenko, Int. J. Med. Mushrooms 18(1): 35 (2016)
= Polyporus ciliatus Fr.
Literature: Baltaeva (1992), (as Polyporus ciliatus, (45): on branches of Salix sp., 15 Aug. 1987; (8): on Salix sp., 21 Aug. 1987; (7): on strum and branch of Populus sp., 6 Nov. 1988; (19): on Betula fallen trumps, 10 May 1987; (21): on Betula sp., 30 Aug. 1988; (3): on Populus sp., 24 Aug. 1989).
•Lentinus tigrinus (Bull.) Fr., Syst. orb. veg. (Lundae) 1: 78 (1825)
≡ Panus tigrinus (Fr.) Sing.
Specimens examined: (28): on Salix sp., 24 Apr. 1989, K. Kalamees, TAAM144150; (28): on stump of Lonicera sp., 20 Apr. 1982, A. Kollom, TAAM104259; (40): on decaying Juglans regia, 26 May YG029; (33): on fallen trunk of angiosperm, 23 Apr. 1982, M. Khalikova, TAAM104290; (33): on trunk of Juglans regia, 23 Apr. 1982, E. Parmasto, TAAM104406; (33): on strum of Salix sp., 23 Apr. 1982, A. Kollom, TAAM127396; (41): on stump of unidentified wood, 23 Apr. 1982, A. Kollom, TAAM127381; (41): on trunk of angiosperm tree, 22 Apr. 1982, M. Khalikova, TAAM104275; (47): on Salix sp., 1 May 1988, A. Kollom, TAAM127603; (37): on trunk of Malus domestica, 18 Jun. 2014, YG/PS162; (17): on Salix sp., 7 May 1976, TAAM094856; (17): on stump of Prunus armeniaca, 7 May 1976, K. Kalamees and others, TAAM094857; (17): on Acer tree trunk, 7 May 1976, K. Kalamees and others, TAAM094847; (20): on dried trunks of angiosperm wood, 13 Jun. 2013, YG-J7.
Literature: Panfilova and Gaponenko (1963), [(44): on living trunk and stems of Lonicera sp., and on fallen dried trunk of Acer sp.]; Khalikova (1989), [(34): on dried stem of Malus domestica]; Iminova (2009), [(3): on Salix wilhelmsiana, on Populus euphratica Oliv., on living stem of Populus talassica Kom., Nov. 2005]; Iminova (2009), (as Panus tigrinus, (4): on Salix linearifalia Wolf.).
Note: This is a widespread species in the study area.
Lenzites betulinus (L.) Fr., Epicr. syst. mycol. (Upsaliae): 405 (1838) [1836–1838]
Literature: Baltaeva (1992, 1993), [(45): on fallen twigs of Betula sp., 17 Aug. 1987; (26): on Populus sp., 22 Jul. 1988; (10): on Populus sp., 10 Sept. 1988; (21): on Salix sp., 26 Sept. 1988, 18 Aug. 1989]
•Lenzites warnieri Durieu and Mont., Annls Sci. Nat., Bot., sér. 4 14: 182 (1860)
Specimen examined: (49): on branch of Populus nigra L., 15 Jul. 1985, E. Krall, Z. Narbal, TAAM126870.
Literature: Baltaeva (1992, 1993), [(45): on wet branch of Betula sp., 17 Aug. 1987; (19): on Populus sp., 22 Jul. 1988; (7): died trunk of Populus sp. 10 Sept. 1988; (8): on Salix sp., 26 Sept. 1988; (26): on Quercus sp., 10 Aug. 1990; (18): on Salix sp., 18 Aug. 1988].
Neolentinus lepideus (Fr.) Redhead and Ginns, Trans. Mycol. Soc. Japan 26(3): 357 (1985)
≡ Lentinus lepideus (Fr.) Fr.
Literature: Khalikova (1989), (as Lentinus lepideus, (28): on softwood conifer stumps, Sept. 1980, 1982); Baltaeva (1992), (as Lentinus lepideus, (45): on Populus sp. 13 Sept. 1990); Iminova (2009), (as Lentinus lepideus, (2): on stem of Populus talassica Kom., Nov. 2005).
Perenniporia fraxinea (Bull.) Ryvarden, Grundr. Krauterk. 2: 307 (1978)
Literature: Baltaeva (1992), [(45): on stem of Biota sp., 13 Sept. 1990].
Picipes badius (Pers.) Zmitr. and Kovalenko, International Journal of Medicinal Mushrooms (Redding) 18(1): 35 (2016)
≡ Polyporus badius (Pers.) Schwein
Literature: Baltaeva (1992), (as Polyporus badius, (19): on stump of woody plant, 13 Jul. 1989).
Picipes melanopus (Pers.) Zmitr. and Kovalenko, International Journal of Medicinal Mushrooms (Redding) 18(1): 36 (2016)
≡ Polyporus melanopus (Pers.) Fr.
Literature: Baltaeva (1992), (as Polyporus melanopus (Pers.) Fr., (21): on Prunus sp., 2 Sept. 1990).
Podofomes trogii (Fr.) Pouzar, Česká Mykol. 25(1): 19 (1971)
≡ Ischnoderma trogii (Fr.) Teixeira
Literature: Khalikova (1989), (as Ischnoderma trogii, (49): on Abies alba Mill., Oct. 1982).
Polyporus lipsiensis (Batsch) E.H.L. Krause, Basidiomycetum Rostochiensium: 54 (1928)
≡ Ganoderma lipsiense (Batsch) G.F. Atk.
Literature: Iminova (2009), (as Ganoderma lipsiense, (6): on dried stem of angiosperm wood, Sept. 2002).
Pycnoporus cinnabarinus (Jacq.) P. Karst., Revue mycol., Toulouse 3 (no. 9): 18 (1881)
Literature: Baltaeva (1992), [(45): on strum of Salix sp., 13 Jun. 1990].
Pyrofomes demidoffii (Lév.) Kotl. and Pouzar, Reprium nov. Spec. Regni veg. 69: 140 (1964)
Specimen examined: (45): on living trunk of Juniperus polycarpos var. seravschanica, 29 Apr. 1988, I. Parmasto, TAAM126251.
Literature: Panfilova and Gaponenko (1963); Akhmedova (1966); Khalikova (1989), [(49): on living trunk of Juniperus polycarpos var. seravschanica and Juniperus sp.]; Baltaeva (1992, 1993), [(49): on trunk of living Pinus sp., 21 Jul. 1987; (45): on living Juniper trunk, 21 Jul. 1987; (8): on Pinus sp., 4 Jul. 1987; (7): on Picea sp., 25 Aug. 1989; (9): on Pinus sp., 14 Oct. 1987; (21): on Pinus sp., 14 Jul. 1987; (19): on Picea sp., 27 Jul. 1988].
Note: This species causes severe infections of living Juniperus trees in the study area.
Szczepkamyces campestris (Quél.) Zmitr., Folia Cryptogamica Petropolitana (Sankt-Peterburg) 6: 52 (2018)
Literature: Baltaeva (1992, 1993), [(45): on broken trunk of Quercus sp., 11 Mar. 1985; (3): on Pyrus sp., 6 Jul. 1988; (19): on stem and branch of Aesculus sp., 15 May 1986; (7): on dried trunk of Populus sp., 21 Aug. 1989].
Skeletocutis amorpha (Fr.) Kotl. and Pouzar, Česká Mykol. 12(2): 103 (1958)
Literature: Baltaeva (1992, 1993), [(45): on trunk of Picea sp., 15 Aug. 1987; (19): on Picea sp., 19 Aug. 1987; (19): on dried stem of Abies alba; (8): on trunk of Pinus sp., 8 Jul. 1988; (7): on Abies alba, 6 Aug. 1989; (21): on Pinus sp., 21 Aug. 1988].
Skeletocutis nivea (Jungh.) Jean Keller, Persoonia 10(3): 353 (1979)
≡ Incrustoporia nivea (Jungh.) Ryvarden
Literature: Baltaeva (1992), (as Skeletocutis nivea), Baltaeva (1993), (as Incrustoporia nivea, (49): on wet branches of Salix sp., 10 Jun. 1988; (45): on stem of Fraxinus sp., 24 Jun. 1989).
#Tinctoporellus epimiltinus (Berk. and Broome) Ryvarden, Trans. Br. mycol. Soc. 73(1): 18 (1979)
Specimen examined: (47): on decayed branches of Populus sp., 19 May 1990, K. Kalamees, M. Vaasma, TAAM144614.
Note: In TAAM, this specimen was incorrectly labeled as Phellinus sp.
Trametes gibbosa (Pers.) Fr., Epicr. syst. mycol. (Upsaliae): 492 (1838) [1836–1838]
Literature: Baltaeva (1992, 1993), [(1): on wet trunk and stumps of deciduous woody plants: Populus sp., P. tremula, Salix sp., Betula sp., Ulmus sp.].
•Trametes hirsuta (Wulfen) Lloyd, Mycol. Writ. 7(Letter 73): 1319 (1924)
Specimens examined: (28): on dried stem of living Prunus vulgaris, 3 Oct. 2011, YG312; (38): on dried unknown woody trunk, 1 Jun. 2011, YG073; (38): on unknown dried wood, 1 Jun. 2011, YG032; (38): on fallen trunk of angiosperm wood, 1 Jun. 2011, YG042; (38): on unknown decaying wood, 1 Jun. 2011, YG055; (40): on decaying Juglans regia log, 26 May 2011, YG002; (40): on trunk and branch of Juglans regia, 26 May 2011, YG037; (40): on Prunus armeniaca, 2 Nov. 2011, YG004; (40): on Prunus vulgaris, 2 Nov. 2011, YG007; (39): on living Prunus tree, 2 Nov. 2011, YG314; (37): on decaying, unidentified angiosperm stem, 19 Jun. 2014, YG/PS128; (37): on dead Prunus sp., 19 Jun. 2014, YG/PS138; (37): on dried stem of Juglans regia, 20 Jun. 2014, YG/PS168; (15): on died angiosperm strum, 9 Jul. 2017, RM44; (41): on stem of angiosperm tree, 14 Jul. 2014, YG/Ch40; (33): on fallen trunk of Juglans regia, 23 Apr. 1982, E. Parmasto, TAAM104394 in TAAM reported as Antrodia sp.
Literature: Panfilova and Gaponenko (1963), [(44): on Prunus mahaleb]; Khalikova (1989), [(49): on decaying branch of the Quercus tree]; Sinadskiy (1968), [(18): on fallen log of Quercus]; Baltaeva (1992, 1993), [(45): 1988, on stem of Quercus sp.; (21): on dried trunk of Quercus tree; (1): on various deciduous woody plant]; Iminova (2009), (as Coriolus hirsutus (Wulfen) Pat., (14): on fallen decaying branch of Platanus orientalis L. Jun. 2002; (14): on Pinus brutia var. eldarica (Medw.) Silba, May-Jun. 2002; 3: on dried trunk of Prunus mahaleb, Oct. 2003, ibit., (3): on dried on Prunus mahaleb, Nov. 2003).
Note: This is one of the most common and widespread species in Uzbekistan. This species is mostly found on Prunus, Platanus, Pinus, Quercus, and Juglans species in the study area.
Trametes ochracea (Pers.) Gilb. and Ryvarden, N. Amer. Polyp., Vol. 2 Megasporoporia - Wrightoporia (Oslo): 752 (1987)
= Trametes zonatella Ryvarden
Specimen examined: (47): on fallen stems of Prunus sp., 17 May 1990, K.Kalamees, M. Vaasma, TAAM144571.
Literature: Panfilova and Gaponenko (1963); Kravtzev (1950); Khalikova (1989), (as Trametes zonatella, (49): on fallen branch of woody plants, Apr. 1985, May 1985); Iminova (2009), [(6): on unknown wood trunk, May 2000, Jul. 2005]; Baltaeva (1992), (as Trametes ochracea), Baltaeva (1993), (as Trametes zonatella, (1): on died trunks and stumps of deciduous woody plants); Iminova (2009), (as Coriolus zonatus (Nees) Quél., (2): on stump of woody plants, May, 2000, Jul. 2005).
Trametes pubescens (Schumach.) Pilát, in Kavina and Pilát, Atlas Champ. l’Europe, III, Polyporaceae (Praha) 1: 268 (1939)
Literature: Baltaeva (1992), [(1): on died trunks, 1992].
Trametes suaveolens (L.) Fr., Epicr. syst. mycol. (Upsaliae): 491 (1838) [1836–1838]
Literature: Baltaeva (1992, 1993), [(49): on trunk of Salix sp., 5 Sept. 1987; (45): on Salix sp., 16 Apr. 1987; (8): on Populus tremula, 11 May 1988; (19): on Populus tremula, 9 Jul. 1988; (21): on Populus tremula, 21 Sept. 1988].
Trametes tephroleuca Berk. Hooker’s J. Bot. Kew Gard. Misc. 6: 165 (1854)
Specimens examined: (33): on fallen trunk of Juglans regia, 23 Apr. 1982, E. Parmasto, TAAM104394a, ibit. TAAM104399; (33): on fallen trunk of Juglans regia, 23 Apr. 1982, K. Kalamees, TAAM104305; (41): on a trunk of Juglans regia, 22 Apr. 1982, E. Parmasto, TAAM104270a; (33): on a trunk of Lonicera sp., 22 Apr. 1982, E. Parmasto, TAAM104283; (45): on dry twig of Prunus sp., 29 Apr. 1988, I. Parmasto, TAAM126249; (45): on dead trunk of Prunus mahaleb, 2 May 1988, A. Kollom, TAAM127624; (45): on fallen twig of Prunus mahaleb, 3 May 1988, I. Parmasto, TAAM126285; (45): on a dry trunk of Crataegus sp., 29 Apr. 1988, A. Kollom, TAAM127579; (41): on dry branch of Crataegus pseudoheterophylla subsp. turkestanica, 22 Aprel 1988, A. Kollom, TAAM127382.
Literature: Khalikova (1989), [(33): on dried stem of Malus sieversii, 17 Apr. 1986]; Iminova (2009), (as Coriolus tephroleucus (Fr.) Bonk., (14): on Prunus vulgaris and Juglans regia, May 1999).
•Trametes trogii Berk., in Trog, Mittheil. d. schweiz. Naturf. Ges. in Bern 2: 52 (1850)
≡ Funalia trogii (Berk.) Bondartsev and Singer
Specimens examined: (28): on fallen deciduous branch, 2 Sept. 2013, YG/bot23b; 11 Sept. 2014; (38): on Acer tataricum subsp. semenovii, 9 Sept. 2016, YG1017; (49): on dried decaying trunk of Populus alba, 17 Sept. 2014, YG-N6, ibit. on trunk of Populus alba, 17 Sept. 2014, YG-N7; (31): on unidentified wood, 17 Sept. 2009, O. Kurina, TAAM189940 (as Funalia sp. in TAAM); (37): on dried on Salix sp. trunk, 14 Sept. 2014, YG/PS2X; (20): on Populus sp., 14 Sept. 2014, YG-JX4; (20): on unknown stump of angiosperm, 13 Jun. 2013, YG-G17, ibit. 13 Jun. 2013, YG-G18, ibit. 13 Jun. 2013, YG-G19; (20): on Populus nigra L., 16 Jun. 2013, YG-J4; (20): on dried stem of living Populus nigra, 15 Jun. 2013, YG-J6; (20): on Populus sp., 16 Jun. 2013, YG-GX1; (8): on unknown woody branches, 13 Jun. 2016, YG-G14, ibit., 13 Jun. 2016, YG1090.
Literature: Panfilova and Gaponenko (1963); Khalikova (1989), (as Funalia trogii, (30): on Populus sp.; (49): on unknown wood plant); Baltaeva (1992, 1993), [(45): on stem of Salix sp. 12 Sept. 1990; (1): mainly on dead stump and trunk of woody plants: on Salix sp., 27 Oct. 1992; (1): on stump and trunk of Fraxinus sp., 12 Sept. 1991; on fallen of Populus tremula, 15 Nov. 1992].
Note: First report of this species on stumps and trunks of Acer tataricum subsp. semenovii, and Populus nigra in Uzbekistan.
•Trametes versicolor (L.) Lloyd, Mycol. Notes (Cincinnati) 65: 1045 (1921) [1920]
≡ Coriolus versicolor (L.) Quél.
Specimens examined: (28): on trunk of Betula sp., 5 May 1988, I. Parmasto, TAAM126293; (28): on Betula trunk, 5 May 1988, A. Kollom, TAAM127635; (37): on dried stem of angiosperm wood, 19 Jun. 2014, YG/PS128-1; (37): on dried stem of angiosperm wood, 19 Jun. 2014, YG/PS170; (45): on trunk of Prunus sp., 22 Apr. 1982, A. Kollom, TAAM127389; (49): on a fallen deciduous trunk, 22 Apr. 1982, A. Kollom, TAAM127388; (35): on Crataegus pseudoheterophylla subsp. turkestanica (Pojark.) K.I.Chr., 31 Aug. 1963, A. Raitviir, TAAM043489; (50): on trunk of Lonicera sp., 24 Apr. 1982, A. Kollom, TAAM127403; (45): on dry trunk of Celtis australis subsp. caucasica, 3 May 1988, I. Parmasto, TAAM126287, (45): on dry twig of Prunus mahaleb, 2 May 1988, I. Parmasto, TAAM126284; (47): on fallen Prunus branch, 17 May 1990, K. Kalamees, M. Vaasma, TAAM144572; (47): 4 May 1988, I. Parmasto, TAAM126294; (20): on Prunus sp., 14 Jun. 2013, YG-J3.
Literature: Panfilova and Gaponenko (1963); Akhmedova (1966); Baltaeva (1992, 1993); Khalikova (1989), [(1): on Prunus mahaleb, Malus sp., Quercus sp., Juglans regia, Populus sp.]; Iminova (2009), (as Coriolus versicolor, (12): on dried and living stem of Prunus vulgaris, May 2003, Jun. 2004).
Note: This species was recorded for the first time in Uzbekistan, on Betula sp., Celtis australis subsp. caucasica, Crataegus pseudoheterophylla subsp. turkestanica, and Lonicera sp. in Uzbekistan.
∗•Trametes villosa (Sw.) Kreisel, Monografias, Ciencias, Univ. Habana, Ser. 4 16: 83 (1971)
Specimen examined: (29): on dried stem of angiosperm, 15 Jun. 2015, YG/AG11.
Trametopsis cervina (Schwein.) Tomšovský, Czech Mycol. 60(1): 7 (2008)
≡ Trametes cervina (Schwein.) Bres.
Literature: Baltaeva (1992, 1993), (as Trametes cervina, (19): on fallen branch of Juglans regia, 14 May 1987; (7): on Juglans regia, 5 Jul. 1987; (45): on dried stem of Morus alba, 15 May 1988; (21): on stem of Juglans regia, 5 Jul. 1987; (3): on Malus domestica, 27 Mar. 1988; (26): Morus alba trunk, 15 May 1988; (26): on dried Morus nigra, 4 Aug. 1988; (9): on rotten trunk of Morus alba, 6 Aug. 1989).
Note: We could not observe the species in the localities mentioned in Baltaeva (1992, 1993).
Tyromyces lacteus (Fr.) Murr, N. Amer. Fl. (New York) 9(1): 36 (1907)
Literature: Panfilova and Gaponenko (1963); Khalikova (1989), [(49): on died trunk of Betula pendula Roth].
SPARASSIDACEAE Herter
Sparassis crispa (Wulfen) Fr., Syst. mycol. (Lundae) 1: 465 (1821)
Literature: Iminova (2009), [(3): on trunk of angiosperm trees, Aug. 2000, Sept. 2003, Oct. 2003].
GANODERMATACEAE Donk
•Ganoderma adspersum (Schulzer) Donk, Proc. K. Ned. Akad. Wet., Ser. C, Biol. Med. Sci. 72(3): 273 (1969)
Specimens examined: (28): on died stump of Acer saccharum Marshall, 7 Jun. 2014, YG/bot24; (49): on strum of Acer sp., 12 Sept. 2011, YG/UG3; (49): on trunk of Acer sp., 12 Sept. 2014, YG/Gan1.
Literature: Gafforov (2014), [(28): on dead trunk of Acer saccharum, 14 Oct. 2011].
;Note: We found this species on decaying Acer saccharum, and this is the first report for Ganoderma adspersum on Acer from Central Asia. We collected Ganoderma adspersum only in Northeastern Uzbekistan. Outside of the study area, this species is mainly found in subatlantic or submediterranean regions and usually on trees such as Tilia, Quercus, Fagus, Platanus, and Aesculus.
Ganoderma applanatum (Pers.) Pat., Hyménomyc. Eur. (Paris): 143 (1887)
Specimen examined: (23): on Juglans regia, 18 Aug. 2016, YG-B06.
Literature: Panfilova and Gaponenko (1963); Khalikova (1989), [(49): on stump of Juglans regia]; Schwartzman (1964), [(49): on dried stem of angiosperm woody plants], Akhmedova (1966), [(49): on Populus sp.]; Baltaeva (1992), [(1): on deadwood stem and stumps of deciduous trees]; Baltaeva, 1993 [(45): on dead fallen trunk of Populus sp., 16 Jul. 1986]; Iminova (2009), [(14): on various angiosperm trunks, Sept. 2002].
Note: This species is widespread in the study area and causes root rot disease of walnut trees.
Ganoderma lucidum (Curtis) P. Karst., Revue mycol., Toulouse 3(no. 9): 17 (1881)
Literature: Panfilova and Gaponenko (1963); Schwartzman (1964); Khalikova (1989), [(49): on various deciduous wood]; Baltaeva (1992, 1993), [(49): on stump of deciduous wood, 13 Aug. 1989; (45): on living Quercus sp., 14 Aug. 1987; (19): on Quercus sp., 29 Jul. 1988; (19): on Ulmus sp., 2 Aug. 1988; (8): on stump of deciduous wood, 14 Aug. 1988; (6): on various deciduous woody plants, 9 Aug. 1989]; Iminova (2009), [(14): on trunks of angiosperm wood, 2000–2002].
#•Ganoderma resinaceum Boud., in Patouillard, Bull. Soc. mycol. Fr. 5(2,3): 72 (1889)
Specimen examined: (8): on living stem of Salix sp., 7 Sept. 2016, YG-X4.
MERIPILACEAE Jülich
Grifola frondosa (Dicks.) Gray, Nat. Arr. Brit. Pl. (London) 1: 643 (1821)
≡ Polyporus frondosus (Dicks.) Fr.
Literature: Iminova (2009), (as Polyporus frondosus, (3): on living Juglans regia, Jun. 2002, Jul. 2003).
FAMILY PLACEMENT UNCERTAIN (INCERTAE SEDIS)
∗•Phlebiella christiansenii (Parmasto) K.H. Larss. and Hjortstam, in Hjortstam and Larsson, Mycotaxon 29: 316 (1987)
Specimens examined: (28): on fallen woody plant branch, 2 Sept. 2013, YG-G4; (28): on Gleditsia triacanthos L., 2 Sept. 2013, YG-G22; (28): on stem of fallen angiosperm tree, 2 Sept. 2013, YG-G26; (28): on dried stump of deciduous tree, 3 Sept. 2013, YG-G36; (38): on stump of Juglans regia, 3 Sept. 2013, YG-G040.
HYMENOCHAETALES Oberw.
HYMENOCHAETACEAE Imazeki and Toki
Fomitiporia hippophaeicola (H. Jahn) Fiasson and Niemelä, Karstenia 24(1): 25 (1984)
≡ Phellinus hippophaeicola H. Jahn
Literature: Baltaeva (1992, 1993), (as Phellinus hippophaeicola, (49): on Elaeagnus rhamnoides (L.) A. Nelson, 26 Apr. 1989; (45): on Elaeagnus rhamnoides, 3 Sept. 1989; (21): on Elaeagnus rhamnoides, 3 Oct. 1989).
Fomitiporia punctata (P. Karst.) Murrill, Lloydia 10: 254 (1947)
≡ Phellinus punctatus (P. Karst.) Pilát
Literature: Baltaeva (1992, 1993), (as Phellinus punctatus, (49): on Ulmus sp., 12 May 1988; (45): on Crataegus sp., 7 Aug. 1989; (45): on Populus sp., 21 Mar. 1987; (9): on Populus sp., 16 Apr. 1987; (21): on Populus sp., 9 Jul. 1987; (8): on Ulmus sp., 14 Aug. 1987; (8): on Betula sp., 21 Jul. 1989; (7): on Ulmus sp., 14 Aug. 1987; (19): on Crataegus azarolus var. pontica (K.Koch) K.I.Chr., 17 Apr. 1988).
Fomitiporia robusta (P. Karst.) Fiasson and Niemelä, Karstenia 24(1): 25 (1984)
≡ Phellinus robustus (P. Karst.) Bourdot and Galzin
Literature: Panfilova and Gaponenko (1963); Khalikova (1989), (as Phellinus robustus, (49): on strums of Spiraea sp.); Baltaeva (1992, 1993), (as Phellinus robustus, (45): on stump of Pistacia sp., 7 Apr. 198; (20): on Castanea sp., 4 Mar. 1988; (19): on Quercus sp., 14 Mar. 1987; (21): on stem of Quercus sp., 24 Apr. 1987; (49): on Populus sp., 19 Aug. 1988; (8): on Castanea sp., 19 May 1987; (7): on Juglans regia, 30 Apr. 1988); Iminova (2009), (as Phellinus robustus, (16): on stem of Ìorus alba, Apr. 2001).
Fulvifomes rimosus (Berk.) Fiasson and Niemelä, Karstenia 24(1): 26 (1984)
≡ Phellinus rimosus (Berk.) Pilát
Literature: Panfilova and Gaponenko (1963), (as Phellinus rimosus, (49): on trunk of Pistacia vera, 1963); Baltaeva (1992, 1993), (as Phellinus rimosus, (9): on Quercus trunk, 6 Jul. 1985; (21): on Quercus, 12 Jul. 1985; (19): on Quercus sp., 30 Jul. 1985; (45): on dried trunk of Salix sp., 25 Apr. 1986; (7): on Salix sp., 6 Jul. 1987; (8): on Populus sp., 19 Aug. 1989; (19): on Populus sp., 10 Apr. 1989).
Fuscoporia contigua (Pers.) G. Cunn., Bull. N.Z. Dept. Sci. Industr. Res., Pl. Dis. Div. 73: 4 (1948)
≡ Phellinus contiguus (Pers.) Pat.
Literature: Baltaeva (1992, 1993), (as Phellinus contiguus, (21): on stem of Elaeagnus rhamnoides, 19 Sept. 1986; (45): on fallen Acacia trunk, 27 Aug. 1987, 6 Sept. 1987; (19): on stem of Alnus sp., 23 Sept. 1987; (7): on Alnus sp., 16 Aug. 1988; (8): on dried stem of Ulmus sp., 19 Sept. 1988; (9): on Ulmus sp., 30 Aug. 1989).
Fuscoporia torulosa (Pers.) T. Wagner and M. Fisch., Mycol. Res. 105(7): 780 (2001)
≡ Phellinus torulosus (Pers.) Bourdot and Galzin
Literature: Khalikova (1989), (as Phellinus torulosus (Pers.) Bourdot and Galzin, (28): on living and died stems of Quercus sp., Jun.1986, Sept. 1987); Iminova (2009), (as Phellinus torulosus, (3): on Betula tianschanica Rupr., on Salix babylonica L., on Pyrus communis L., on Morus nigra L., Sept.–Oct. 1999–2003).
Inocutis tamaricis (Pat.) Fiasson and Niemelä, Karstenia 24(1): 25 (1984)
≡ Inonotus tamaricis (Pat.) Maire
Literature: Gaponenko (1965); Sinadskiy and Bodartseva (1956), (as Inonotus tamaricis, (10): on living stem of Tamarix hispida Willd.); Baltaeva (1992, 1993), (as Inonotus tamaricis, (9): on Tamarix ramosissima Ledeb., 27 Aug. 1989; (9): on Tamarix sp., 24 Sept. 1986; (26): on Tamarix sp., 6 May 1987; (21): on stem of living Tamarix hispida, 31 Sept. 1987; (10): on dried stem of Tamarix hispida, 18 Mar. 1988).
Inonotus andersonii (Ellis and Everh.) Černý, Česká Mykol. 17(1): 1 (1963)
Literature: Baltaeva (1992, 1993), [(45): on trunk of Quercus sp., 14 Aug. 1988, 2 Sept. 1989; (19): on stump of Quercus sp., 16 Sept. 1988].
Inonotus cuticularis (Bull.) P. Karst., Meddn Soc. Fauna Flora fenn. 5: 39 (1879)
Literature: Baltaeva (1992), [(19): on Juglans regia, 10 Jul. 1990].
•Inonotus hispidus (Bull.) P. Karst., Meddn Soc. Fauna Flora fenn. 5: 39 (1879)
Specimens examined: (40): on stem of living Juglans regia, 26 May 2011, YG054; (38): on Juglans regia, 6 Jun. 2011, YG035; (38): on stem of Juglans regia, 11 Jun. 2014, YG/UG1; (37): on living Pinus sp., 19 Jun. 2014, YG/PS156; (37): on trunk of living Pinus sp., 19 Jun. 2014, YG/PS157; (37): on Malus sieversii, 14 Sept. 2014, YG/PS148; (39): on living Juglans regia, 9 Sept. 2016, YG1015; (22): on Juglans regia, 11 Aug. 2015, YG-B07; (28): on dried trunk angiosperm wood, 27 Sept. 2014, YG/bot1; (29): on living Morus alba stem, 17 Sept. 2015, YG/AG1; (23): on living stem of Juglans regia, 15 May 2016, YG-B08; (41): on a wood of Juglans regia, 22 Apr. 1982, E. Parmasto, TAAM207844; (17): on a wood of Morus alba, 8 May 1976, K. Kalamees, TAAM080947.
Literature: Panfilova and Gaponenko (1963); Akhmedova (1966); Khalikova (1989); Baltaeva (1992, 1993); Iminova (2009), [(1): on living trunks of deciduous woody plants: Malus domestica Borkh., M. sieversii (Ledeb.) M.Roem., Morus alba, Juglans regia, Prunus avium (L.) L.].
Inonotus obliquus (Fr.) Pilát, Atlas Champ. l’Europe, III, Polyporaceae (Praha) 1: 572 (1942)
Specimens examined: (28): unknown angiosperm fallen trunk, 14 Oct. 2011, YG001.
Literature: Baltaeva (1992, 1993), [(45): on living Betula sp., 28 Aug. 1987; (19): on stump of Fraxinus sp., 21 May 1986; (7): on Alnus sp., 9 Jul. 1986; (8): on dried fallen trunk of Salix sp., 13 Jun. 1987; (9): on Salix sp., 20 Apr. 1988; (3): on Salix sp., 29 Jun. 1989].
Inonotus pseudohispidus Kravtzev, Bull. Acad. Sci. Kazakh SSR 98: 128 (1950)
Literature: Sinadskiy and Bodartseva (1956, 1960), (on living trunk of and Populus pruinosa Schrenk, Populus euphratica Oliv.); Baltaeva (1992, 1993), [(26): on Populus sp., 18 Jul. 1988; (9): trunk of Populus sp., 9 Aug. 1988; (21): on Populus alba, 20 Jul. 1989; (26): on Populus sp., 26 Aug. 1989].
Mensularia radiata (Sowerby) Lázaro Ibiza, Revta R. Acad. Cienc. exact. fis. nat. Madr. 14(11): 736 (1916)
≡ Inonotus radiatus (Sowerby) P. Karst.
Literature: Baltaeva (1992, 1993), (as Inonotus radiatus, (6): on dried branch of Alnus tree, 3 Jul. 1986; (7): on stump of angiosperm woody plants, 19 May 1987; (8): on dried trunk and braches of Ulmus sp., 17 Jun. 1989; (45): on died trunk of Quercus sp., 7 Jul. 1987; (19): on Quercus sp., 17 Jun. 1987; (21): on Ulmus sp., 20 Aug. 1989).
Phellinidium ferrugineofuscum (P. Karst.) Fiasson and Niemelä, Karstenia 24(1): 26 (1984)
≡ Phellinus ferrugineofuscus (P. Karst.) Bourdot and Galzin
Literature: Baltaeva (1992, 1993), (as Phellinus ferrugineofuscus, (45): on wood of Pinus sp., 15 Sept. 1988; (8): on Pinus sp., 24 Oct. 1988); (20): on stump of Picea sp., 6 Nov. 1987; (19): on Picea sp., 28 Oct. 1987).
Phellinopsis conchata (Pers.) Y.C. Dai, Fungal Diversity 45: 309 (2010)
≡ Phellinus conchatus (Pers.) Quél.
Literature: Baltaeva (1992, 1993), (as Phellinus conchatus, (45): on dried stump Syringa sp., 10 Sept. 1988; (7): on Populus sp., 5 Oct. 1988; (6): on Alnus sp., 29 Aug. 1989; (21): on Ulmus sp., 13 Nov. 1988; (26): on dried stem of Populus sp., 21 Oct. 1988; (19): on Alnus sp., 8 Sept. 1989; (21): on Ulmus sp., 13 Oct. 1988).
∗•Phellinus betulinus (Murrill) Parmasto, Folia cryptog. Estonica 43: 41 (2007)
Specimens examined: (50): on a trunk of Betula tianschanica, 24 Apr. 1982, E.Parmasto, TAAM104436; (50): on a dead branch of Betula tianschanica, 24 Apr. 1982, E.Parmasto, TAAM104285.
Phellinus igniarius (L.) Quél., Enchir. fungi. (Paris): 177 (1886)
Specimen examined: (50): on a trunk of Salix sp., 24 Apr. 1982, A.Kollom, TAAM127406.
Literature: Panfilova and Gaponenko (1963); Khalikova (1989), [(41): on live trunk of Juglans regia, 1 Jun. 1980, Sept. 1984]; Baltaeva (1992, 1993), [(45): on trunk of Prunus vulgaris, 10 Sept. 1988; (21): on trunk of Prunus sp., 9 Aug. 1988; (9): on trunk of Acer sp., 12 Apr. 1987; (14): on Acer sp., 19 Aug. 1987; (8): on Salix sp., 6 Apr. 1988; (7): on Salix sp., 16 Jul. 1989; (19): on dried trunk of Salix sp., 24 Jul. 1988]; Iminova (2009), [(3): on Juglans regia, Apr.-May 2000].
Note: This species causes white rot of broad-leaved trees from many genera and is most common on Alnus, Betula, and Corylus spp. (all Betulaceae) and Salix spp. (Salicaceae), also fairly common on Acer (Sapindaceae) and Malus, Prunus, and Sorbus spp. (all Rosaceae), more rarely on Aesculus, Amelanchier, Carpinus, Carya, Castanea, Cratageus, Fraxinus, Hippophae, Hydrangea, Juglans, Laburnum, Morus, Populus, Pterocarya, Robinia, Pyrus, Syringa, Tilia, and Ulmus. Since this species is defined both in a wide and in a narrow sense, the lists of hosts should be interpreted with care.
•Phellinus pomaceus (Pers.) Maire, Mus. barcin. Scient. nat. Op., Ser. Bot. 15: 37 (1933)
= Phellinus tuberculosus Niemelä
Specimens examined: (33): on a living trunk of Prunus sp., 26 Apr. 1982, E. Parmasto, TAAM104413; (35): on Prunus cerasifera Ehrh., 31 Aug. 1963, A. Raitviir, TAAM043492; (33): on a fallen trunk of Prunus sp., 23 Apr. 1982, A. Kollom, TAAM127401; (41): on the base living fruit trees, 26 Apr. 1982, E. Parmasto, TAAM104434; (50): on a trunk of Prunus mahaleb, 24 Apr. 1982, A. Kollom, TAAM127411; (50): on a dry trunk of Prunus erythrocarpa (Nevski) Gilli, 24 Apr. 1982, A. Kollom, TAAM203618; (50): on a trunk of Salix sp., 24 Sept. 2014, YG/S1; (38): on Prunus cerasifera, 1 Jun. 2011, YG052; (38): on Prunus sp., 11 Sept. 2011, YG51-ph; (38): on dried Prunus tree, 12 Sept. 2014, YG/Ug01; (38): on living Prunus sp., 12 Sept. 2014, YG/Ug02; (39): on dried stem of Prunus dulcis (Mill.) D.A.Webb, 2 Nov. 2011, YG009; (39): on living stem of Cerasus tianshanica Pojark., 2 Nov. 2011, YG028, ibit on living stem of Cerasus tianshanica Pojark., 20 Sept. 2014, YG/PS3X; (39): on Prunus cerasifera, 2 Nov. 2011, YG337; (39): on living trunk of Prunus cerasifera, 2 Nov. 2011, YG338; (32): on Prunus mahaleb, 15 May 2011, YG28; (32): on dried trunk of Prunus sp., 13 Sept. 2014, YG/bil164; (37): on Prunus sp., 19 Sept. 2014, YG/PS82; (45): on a dry trunk of Crataegus altaica Ledeb., 2 May 1988, I. Parmasto, TAAM126247; (48): on Lonicera sp., Sept. 1982, N.I. Gaponenko, TASM582; (8): on dried stem of Prunus sp., 9 Sept. 2016, YG1102; (45): on dry branch of Juglans regia, 29 Apr. 1988, I. Parmasto, TAAM126253; (45): on dry trunk of Celtis australis subsp. caucasica, 1 May 1988, I. Parmasto, TAAM126269. In TAAM two specimens reported as Phellinus sp.
Literature: Panfilova and Gaponenko (1963); Khalikova (1989), (as Phellinus tuberculosus, (49): on trunks of Rosaceae family trees); Baltaeva (1992, 1993), (as Phellinus tuberculosis, (49): on Prunus spp., 10 May 1987; (45): on Malus domestica, 13 May 1987; (8): on Malus sp., 16 May 1989; (19): on Prunus sp., 26 May 1987); Iminova (2009), (as Phellinus pomaceus, (2): on Prunus persica (L.) Batsch, Apr. 2004); Iminova (2009), (as Phellinus tuberculosis, (15): on living stem of Cydonia oblonga Mill., May 2003; (2): on trunk of living Prunus domestica L. and on Malus domestica, May–Aug. 2002).
Note: First report of this species is on Celtis australis subsp. caucasica and Lonicera sp. from Uzbekistan. Usually it is largely confined to trees belonging to the Rosaceae, chiefly on Prunus and rarely on Malus, Pyrus, and Cydonia, and causes white rot of the heartwood of living fruit trees. This species is widespread in the northern hemisphere and probably occurs wherever native species of Prunus from the stone fruit group and where peaches, cherries, and plums are cultivated as fruit trees. This species is also reported on Acer, Alnus, Carpinus, Ceratonia, Cornus, Corylus, Crataegus, Fagus, Ficus, Juglans, Malus, Olea, Pyrus, Salix, and Ulmus.
Phellinus tremulae (Bondartsev) Bondartsev and P.N. Borisov, Trut. Grib Evrop. Chasti SSSR Kavkaza [Bracket Fungi Europ. U.S.S.R. Caucasus] (Moscow-Leningrad): 358 (1953)
Literature: Baltaeva (1992, 1993), [(45): on trunk of Populus tremula, 20 Jul. 1985; (19): on Populus tremula, 16 Aug. 1985; (21): on Populus tremula, 10 Aug. 1986; (7): on Populus sp., 25 Aug. 1986; (26): on dried fallen of Populus tremula, 19 Aug. 1987; (6): on living of Populus sp., 8 Aug. 1988]; Khalikova (1989), [(49): on live trunk of Populus sp., 20 Jul. 1985].
Phylloporia ampelina (Bondartsev and Singer) Bondartseva, Mikol. Fitopatol. 17(4): 279 (1983)
≡ Phellinus ampelinus Bondartsev and Singer
Literature: Bondarceva and Parmasto (1986), (as Phellinus ampelinus, (49): on dead and live trunk of Vitis vinifera L.).
∗Phylloporia ephedrae (Woron.) Parmasto, Proc. Indian Acad. Sci., Pl. Sci. 94(2–3): 377 (1985)
Specimens examined: (45): on stem of living Ephedra equisetina Bunge, 1 May 1988, I. Parmasto, TAAM126265; (45): on Ephedra equisetina, 2 May 1988, I. Parmasto, TAAM126279; (45): on stem of Ephedra equisetina, 1 May 1988, A. Kollom, TAAM127593.
Phylloporia yuchengii Gafforov, Tomšovský, Langer and L.W. Zhou, Cryptog. Mycol. 35(4): 318 (2015) [2014]
Specimens examined: (38): on trunk of Juglans regia, 1 Jun. 2011, YG043; (40): on Prunus sp., 11 Sept. 2011, YG343; (45): on a trunk of Juglans regia, 29 Apr. 1988, I. Parmasto, TAAM126260, in TAAM as Phellinus sp.; (7): on trunk of Crataegus pseudoheterophylla subsp. turkestanica, 11 Sept. 2015, YG1093; (39): on Crataegus sp., 9 Oct. 2016, YG1011; (8): on fallen unknown woody branches, 9 Sept. 2016, YG1101; (20): on trunk of Populus sp., 12 Jun. 2013, YG-J5; (20): on Morus alba, 13 Jun. 2013, YG-J10; (20): on Morus alba, 13 Jun. 2013, YG-J11.
Literature: Gafforov et al. (2014), [(38): on dead angiosperm trunk and stem, 1 Jun. 2011; (39): on dead angiosperm trunk, 2 Nov. 2011].
Note: This species was first described from northeastern Uzbekistan in 2014. Later, we collected this species in central and south Uzbekistan. It seems that this species is widespread in the study area. This species grows on Crataegus, Juglans, Morus, Populus, and Prunus, which represent four plant families.
Porodaedalea pini (Brot.) Murrill, Bull. Torrey bot. Club 32(7): 367 (1905)
≡ Phellinus pini (Brot.) Pilát
Literature: Baltaeva (1992, 1993), (as Phellinus pini, (1): on trunks and stumps of conifer tees); Khalikova (1989), (as Phellinus pini, (28): on live trunk of Pinus pallasiana D. Don, Oct. 1984); Iminova (2009), (as Phellinus pini, (13): on dried stem of angiosperm, May 2003).
•Sanghuangporus lonicerinus (Bondartsev) Sheng H. Wu, L.W. Zhou and Y.C. Dai, in Zhou, Vlasák, Decock, Assefa, Stenlid, Abate, Wu and Dai, Fungal Diversity 77: 340 (2015)
≡ Phellinus lonicerinus (Bondartsev) Bondartsev and Singer
Specimens examined: (49): on dried stem of Lonicera sp., 8 Nov. 2016, YG1095; (36): on Lonicera sp., 8 Nov. 2016, YG1096; (32): on stem of living Lonicera nummulariifolia Jaub. and Spach, 15 May 2011, YG018; (41): on the base of a living trunk of Lonicera sp., 22 Apr. 1982, E. Parmasto, TAAM203688; (36): on a trunk of Lonicera sp., 25 Apr. 1982, E. Parmasto, TAAM104407; (36): on the base of a living trunk of Lonicera sp., 25 Apr. 1982, E. Parmasto, TAAM104439; (50): on at the base of Lonicera sp., 24 Apr. 1982, E. Parmasto, TAAM0104264; (50): on a dry trunk of Lonicera sp., 24 Apr. 1982, A. Kollom, TAAM127410; (39): on Lonicera sp., 9 Oct. 2016, YG1012; (50): on Lonicera nummulariifolia, 9 Oct. 2016, YG1013; (38): on Lonicera sp., 9 Sept. 2016, YG1016; (37): 19 Jun. 2014, on living stem of Lonicera sp., YG/PS92; (37): 20 Jun. 2014, dried stem of Lonicera sp., YG/PS129; (37): on Acer sp., 20 Jun. 2014; (30): on living stem of Acer tataricum subsp. semenovii, 5 May 2014, YG/Un1; (46): on a dry trunk of deciduous trunk, 29 Apr. 1988, A. Kollom, TAAM127578; (7): on Lonicera sp., 11 Sept. 2016, YG1094; (8): on Lonicera sp., 26 May 2018, YG1097; (8): on living stem of Lonicera microphylla Willd. ex Schult., 26 May 2018, YG1112.
Literature: Panfilova and Gaponenko (1963); Khalikova (1989); Iminova (2009), (Phellinus lonicerinus, (1): on Lonicera spp.).
Note: This species was thought to grow exclusively on Lonicera, but a new host Acer tataricum subsp. semenovii is recorded here from Uzbekistan.
Tropicoporus linteus (Berk. and M.A. Curtis) L.W. Zhou and Y.C. Dai, in Zhou, Vlasák, Decock, Assefa, Stenlid, Abate, Wu and Dai, Fungal Diversity 77: 344 (2015)
≡ Phellinus linteus (Berk. and M.A. Curtis) Teng
Specimen examined: (42): on dry branch of Rosa fedtschenkoana Regel, 21 Apr. 1982, E. Parmasto, TAAM104272 (as Phellinus sp. in TAAM).
Literature: Panfilova and Gaponenko (1963); Khalikova (1989), (as Phellinus linteus, (49): on dead and living trunk and stem of angiosperm woody plants); Baltaeva (1992, 1993), (as Phellinus linteus, (49): on Salix sp., 16 Aug. 1985, (45): on living trunk of Lonicera sp., 9 Apr. 1985; (21): on Quercus sp., 12 Sept. 1986; (7): on Populus sp., 5 May 1987; (8): on Acer sp., 4 Sept. 1989; (19): on Ulmus sp., 27 Aug. 1987); Iminova (2009), (as Phellinus linteus, (3): on Salix wilhelmsiana, Apr. 2000, May 2003).
NEOANTRODIELLACEAE Y.C. Dai, B.K. Cui, Jia J. Chen and H.S. Yuan
¤•Neoantrodiella sp.
Specimen examined: (33): on trunk of Juniperus polycarpos var. seravschanica, 22 Apr. 1982, E. Parmasto, TAAM104307.
OXYPORACEAE Zmitr. and Malysheva
Rigidoporus corticola (Fr.) Pouzar, Folia geobot. phytotax. bohemoslov. 1(4): 368 (1966)
≡ Oxyporus corticola (Fr.) Ryvarden
Specimens examined: (49): on fallen trunk, 22 Sept. 2014, YG-P55, ibit., YG-P67; (49): on dried branch of angiosperm, 23 Sept. 2014, YG-P35.
Literature: Baltaeva (1992, 1993), (as Oxyporus corticola, (21): on stump of Quercus sp., 6 May, 1985; (21): on Fraxinus sp., 12 Aug. 1988; (45): on stem of Fraxinus sp., 6 May 1988; (20): on dried stem of living Salix alba, 29 Aug. 1989; (19): on dead trunk of Populus sp., 15 Apr. 1987; (9): on Populus sp., 30 Jul. 1987; (7): on Populus tremula, 19 Oct. 1987).
Rigidoporus latemarginatus (Durieu and Mont.) Pouzar, Folia geobot. phytotax. bohemoslov. 1(4): 368 (1966)
≡ Oxyporus latemarginatus (Durieu and Mont.) Donk
= Chaetoporus ambiguus (Bres.) Bondartsev and Singer
Literature: Baltaeva (1992), (as Oxyporus latemarginatus, (28): on stump of Pyrus sp., 16 Sept. 1990), Sinadskiy and Bodartseva, 1960 (as Chaetoporus ambiguus, (27): on Elaeagnus rhamnoides, Jun. 1960).
Rigidoporus populinus (Schumach.) Pouzar, Folia geobot. phytotax. bohemoslov. 1(4): 368 (1966)
≡ Oxyporus populinus (Schumach.) Donk
Literature: Panfilova and Gaponenko (1963), (as Oxyporus populinus, 49: on dead stem of Acer tataricum L.), Khalikova (1989), (as Oxyporus populinus, (49): on fallen branch and stem Populus sp., May 1987; on Quercus sp., Jun. 1988); Baltaeva (1992, 1993), (as Oxyporus populinus, (1): on dried stem of living Sorbus sp.).
•∗Rigidoporus ginkgonis (Y.C. Dai) F. Wu, Jia J. Chen and Y.C. Dai, in Wu, Chen, Ji, Vlasák and Dai, Mycologia 109(5): 761 (2017)
Specimens examined: (28): on fallen trunk and stump of deciduous woody plants, 2 Sept. 2013, YG-G2, ibid. on fallen trunk of deciduous woody plants, YG-G3; (28): on decaying branch of angiosperm, 3 Sept. 2013, YG-G35.
SCHIZOPORACEAE Jülich
Hyphodontia alutaria (Burt) J. Erikss., Symb. bot. upsal. 16(no. 1): 104 (1958)
Literature: Gafforov et al. (2017), [(28): on twigs and stem of Pterocarya pterocarpa Kunth ex I. Iljinsk, 2 Sept. 2013].
#Hyphodontia arguta (Fr.) J. Erikss., Symb. bot. upsal. 16(no. 1): 104 (1958)
Specimen examined: (49): on fallen tree, 21 Oct. 2015, YG-X66.
•Hyphodontia zhixiangii L.W. Zhou and Gafforov, Phytotaxa 299(2): 275 (2017)
Specimens examined: (8): on unknown angiosperm branch, 9 Sept. 2016, YG1098; (49): on fallen angiosperm branches, 7 Oct. 2016, YG1104.
Literature: Kan et al., 2017 [(8): on stem of Juniperus sp., 9 Sept. 2016].
Lyomyces crustosus (Pers.) P. Karst., Revue mycol., Toulouse 3(no. 9): 23 (1881)
Literature: Gafforov et al. (2017), [(38): on branch of living tree Fraxinus pennsylvanica Marshall, 3 Sept. 2013].
•Lyomyces erastii (Saaren. and Kotir.) Hjortstam and Ryvarden, Syn. Fung. (Oslo) 26: 43 (2009)
Specimen examined: (15): on unknown shrub, 12 Jul. 2017, RM21.
Literature: Gafforov et al. (2017), [(39): on deciduous wood, 2 Nov. 2011].
Lyomyces sambuci (Pers.) P. Karst., Bidr. Känn. Finl. Nat. Folk 37: 153 (1882)
Literature: Gafforov et al. (2017), [(28): on dead wood of Philadelphus sp., 20 Apr. 1982; (39): on a dead branch of angiosperm, 2 Sept. 2013].
Xylodon paradoxus (Schrad.) Chevall., Fl. gén. env. Paris (Paris) 1: 274 (1826)
≡ Schizopora paradoxa (Schrad.) Donk
Literature: Baltaeva (1992, 1993), (as Schizopora paradoxa, (49): on trunks of Quercus sp., 20 Aug. 1989; (45): on trunk of Quercus sp., 7 Oct. 1988; (8): on Quercus sp., 19 Nov. 1989).
TAXA WITH UNCERTAIN POSITION AT THE FAMILY LEVEL (INCERTAE SEDIS)
Sidera lenis (P. Karst.) Miettinen, in Miettinen and Larsson, Mycol. Progr. 10(2): 136 (2011)
≡ Diplomitoporus lenis (P. Karst.) Gilb. and Ryvarden
≡ Antrodia lenis (P. Karst.) Ryvarden
Literature: Baltaeva (1992), (as Diplomitoporus lenis), Baltaeva, 1993 (as Antrodia lenis, (45): on trunk of Pinus sp., 8 Jul. 1988; (8): on Pinus sp., 6 Jul. 1989; (19): on wet of trunk of Picea sp., 27 Aug. 1989; (19): on Picea sp., 14 Jul. 1987); Khalikova (1989), (as Antrodia lenis, (49): on living Juniperus tree, 17 Jul. 1988).
Trichaptum abietinum (Pers.) Ryvarden, Norw. Jl Bot. 19: 237 (1972)
Literature: Baltaeva (1992, 1993), [(45): on wet trunk of Pinus sp., 12 Mar. 1988; (7): on stump of Pinus sp., 15 Aug. 1988; (19): on Pinus sp., 9 Jul. 1989].
Trichaptum biforme (Fr.) Ryvarden, Norw. Jl Bot. 19(3–4): 237 (1972)
= Hirschioporus pergamenus (Fr.) Bondartsev and Singer
Literature: Panfilova and Gaponenko (1963), (as Hirschioporus pergamenus, (49): on dried woody plants); Khalikova (1989), [(49): on dead trunk of angiosperms tree]; Baltaeva (1992, 1993), [(45): on fallen stem of Populus sp., 18 Jul. 1988; (26): on Salix sp., 18 Jul. 1988; 9: on dead trunk of Populus sp., 7 Sept. 1987; (21): on Salix sp., 15 Sept. 1987].
Trichaptum fuscoviolaceum (Ehrenb.) Ryvarden, Norw. Jl Bot. 19: 237 (1972)
Specimen examined: (49): on a fallen trunk of Abies sibirica Ledeb., 29 Aug. 1958, E. Parmasto, TAAM009360.
Literature: Baltaeva (1992), [(45): on stump of Pinus sp., 17 Aug. 1990].
Note: This species is reported for the first time on Abies sibirica from Uzbekistan.
RUSSULALES Kreisel ex P.M.Kirk, P.F.Cannon and J.C.David
BONDARZEWIACEAE Kotl. and Pouzar
Heterobasidion annosum (Fr.) Bref., Unters. Gesammtgeb. Mykol. (Liepzig) (8): 154 (1888)
Literature: Baltaeva (1992, 1993), [(49): on Picea sp., 10 Sept. 1988, 30 Jul. 1989; (45): on root of Biota sp., 15 Nov. 1987; (19): on stump of Pinus sp., 6 Sept. 1988; (7): on Picea sp., 10 Sept. 1988].
LACHNOCLADIACEAE D.A. Reid
#Vararia parmastoi Boidin and Lanq., Persoonia 12(3): 257 (1984)
Specimens examined: (50): on decayed trunk of Juniperus semiglobosa, 24 Apr. 1982, E. Parmasto, TAAM104302 (Paratype); (50): on dead roots of Juniperus semiglobosa, 24 Apr. 1982, E. Parmasto TAAM104303 (Isotype); (50): on a fallen rotten trunk of Juniperus semiglobosa, 24 Apr. 1982, E. Parmasto, TAAM104294; (36): on trunk of Juniperus semiglobosa, 25 Apr. 1982, E. Parmasto, TAAM104440.
PENIOPHORACEAE Lotsy
∗Peniophora cinerea (Pers.) Cooke, Grevillea 8(no. 45): 20 (1879)
Specimens examined: (39): on fallen trunks of angiosperm woody plant, 2 Nov. 2011, YG039; (39): on stem and dried branch of Juglans regia, 1 Jun. 2011, YG058.
∗Peniophora incarnata (Pers.) P. Karst., Hedwigia 28: 27 (1889)
Specimen examined: (39): on fallen stem of deciduous wood, 16 Jun. 2014, YG/PS84.
STEREACEAE Pilát
∗Stereum gausapatum (Fr.) Fr., Hymenomyc. eur. (Upsaliae): 638 (1874)
Specimen examined: (49): on trunk of Picea schrenkiana Fisch. and C.A.Mey., 7 Sept. 2013, YG-Gxx.
Note: This species occurs on Quercus, Castanea, and Carpinus species in Europe, particularly in the Mediterranean area. Fruiting bodies develop on dead stems, rotten stumps, trunks, or more rarely on fallen branches and other debris of angiosperm woody plants. However, we found that species on the conifer tree, Picea schrenkiana from Western Tien Shan Mountains of Uzbekistan.
Stereum hirsutum (Willd.) Pers., Observ. mycol. (Lipsiae) 2: 90 (1800) [1799]
Specimens examined: (33): on deciduous tree trunk, 23 Apr. 1982, E. Parmasto, TAAM104393; (43): on unknown woody branch, 8 Sept. 2016, YG1091; (43): on fallen trunk of angiosperm wood, 8 Sept. 2016, YG1092; (40): on dried stem of Juglans regia, 26 May 2011, YG029; (46): on Crataegus sp., 13 May 1990, K. Kalamees and M. Vaasma, TAAM144492; (32): on Acer tataricum subsp. semenovii, 15 May 2011, YG030; (32): on Acer sp., 15 May 2011, YG51; (32): on unknown decaying wood, 15 May 2011 YG056; (32): on Acer tataricum subsp. semenovii, 15 May 2011, YG057; (32): on unknown dried wood, 7 Jun. 2011 YG048; (39): on stem of living Salix iliensis Regel, 2 Nov. 2011, YG034; (38): on Juglans regia, 1 Jun. 2011, YG109; (38): on dried stem of Fraxinus excelsior L., 3 Sept. 2013, YG-G12; (38): on Quercus sp., 3 Sept. 2013, YG-G15; (38): on dried stem of Salix alba, 3 Sept. 2013, YG320; (37): on living stem of Juglans regia, 19 Jun. 2014, YG/PS135; (37): on dried trunk of Juglans regia, 19 Jun. 2014, YG/PS176; (45): on a dry trunk of Celtis australis subsp. caucasica, 29 Apr. 1989, TAAM126246; (45): on Crataegus pseudoheterophylla subsp. turkestanica, 29 Apr. 1988, I. Parmasto, TAAM126255; (45): on trunk of Quercus sp., 29 Apr. 1988, A. Kollom, TAAM127585; (46): on trunk of Populus alba, 4 May 1988, I. Parmasto, TAAM126291 (as Coriolopsis sp.); (8): on fallen branch, 10 Sept. 2016, YG1099; (28): on dead fallen trunk of angiosperm, 3 Apr. 2013, YG3.04.13.
Literature: Panfilova and Gaponenko (1963), [(30): on Populus sp.]; Khalikova (1989), [(28): on dried branch of living Quercus robur L.]; Iminova (2009), [(14): on Salix interior, Apr. 2002; (3): on Juglans regia, May 2005].
∗Stereum rugosum Pers., Neues Mag. Bot. 1: 110 (1794)
Specimen examined: (50): on a dead trunk of Salix sp., 24 Apr. 1982, TAAM127402
Note: This species is very common and widespread on moist various deciduous and coniferous forests dead trees in the northern hemisphere especially in Europe (Breitenbach and Kränzlin, 1986). It is rarely reported from East and South Asia (Dai, 2011). This is the first report of the species from the mountainous area of Central Asia.
∗Stereum subtomentosum Pouzar, Ćeská Mykol. 18(3): 147 (1964)
Specimen examined: (28): on dead trunk of angiosperm, 24 Sept. 2014, YG-P37.
Note: This species is rare in the study area and generally reported in Betulaceae (Alnus, Betula, Carpinus) and Salicaceae (Populus, Salix) plant species from Europe. It is easily distinguished when fresh by the distinct yellowish exudate when cut. It is similar to Stereum ostrea, which, however, has a tropical or subtropical distribution and is distinguished by its oyster-like fruitbody and more brownish color.
THELEPHORALES Corner ex Oberw.
BANKERACEAE Donk
Phellodon fuligineoalbus (J.C. Schmidt) R.E. Baird, in Baird, Wallace, Baker and Scruggs, Fungal Diversity 62: 63 (2013)
≡ Bankera fuligineoalba (J.C. Schmidt) Coker and Beers.
Literature: Schwartzman (1964), (as Bankera fuligineoalba (49): on Salix caprea L., and on Betula pendula).
Sarcodon imbricatus (L.) P. Karst., Revue mycol., Toulouse 3(no. 9): 20 (1881)
= Sarcodon squamosus (Schaeff.) Quél.
Literature: Schwartzman (1964), (as Sarcodon squamosus (49): on old stump of Populus tremula).
THELEPHORACEAE Chevall.
#Pseudotomentella mucidula (P. Karst.) Svrćek, Ćeská Mykol. 12(2): 68 (1958)
Specimen examined: (47): on fallen trunk of Pinus sibirica Du Tour, 29 Aug. 1958, TAAM009363.
TRECHISPORALES K.H. Larss.
HYDNODONTACEAE Jülich
Fibuloporia desertorum (Kravtzev) Schwartzman, Flora Sporovykh Rastenii Kazakhstana [Cryptogamic Flora of Kazakhstan], 4, Auriculariales, Tremellales, Dacryomycetales, Exobasidiales, Aphyllophorales (Alma-Ata): 299 (1964)
≡ Dextrinosporium desertorum (Kravtzev) Bondartseva
Literature: Gaponenko (1965), (as Dextrinosporium desertorum, (10): on living Haloxylon persicum).
AGARICOMYCETES Doweld
ORDER AND FAMILY UNCERTAIN (INCERTAE SEDIS)
∗•Peniophorella praetermissa (P. Karst.) K.H. Larss., Mycol. Res. 111(2): 192 (2007)
Specimens examined: (38): on living stem of Juglans regia, 3 Sept. 2013, YG-G16; (38): on fallen unknown angiosperm branches, 3 Sept. 2013, YG-G40; (28): on dried stump of deciduous tree, 3 Sept. 2013, YG-G37.
Substrate Preferences of Wood-Inhabiting Poroid and Corticioid Species in Uzbekistan
In this study, poroid and corticoid fungal species were found on 100 woody plant species belonging to 23 families and 42 genera. One hundred wood-inhabiting species (accounting for 65.3% of the total wood-inhabiting poroid and corticoid species of Uzbekistan) were recorded exclusively on deciduous wood, 33 species (21.6%) were found exclusively on coniferous wood, and 5 species were recorded as inhabiting both groups of woody plants (Figure 8). The hosts were not determined for the remaining 15 species. These wood-inhabiting fungi were most frequently found on hosts belonging to Salicaceae (72 fungal species), Pinaceae (51), Rosaceae (48), Fagaceae (31), Juglandaceae (30), Betulaceae (25), Cupressaceae (16), Sapindaceae (16), and Ulmaceae and Oleaceae (each 11). Collectively, these families host about 70% of fungal species present in the study area; other plant families host one to nine species (Table 3).
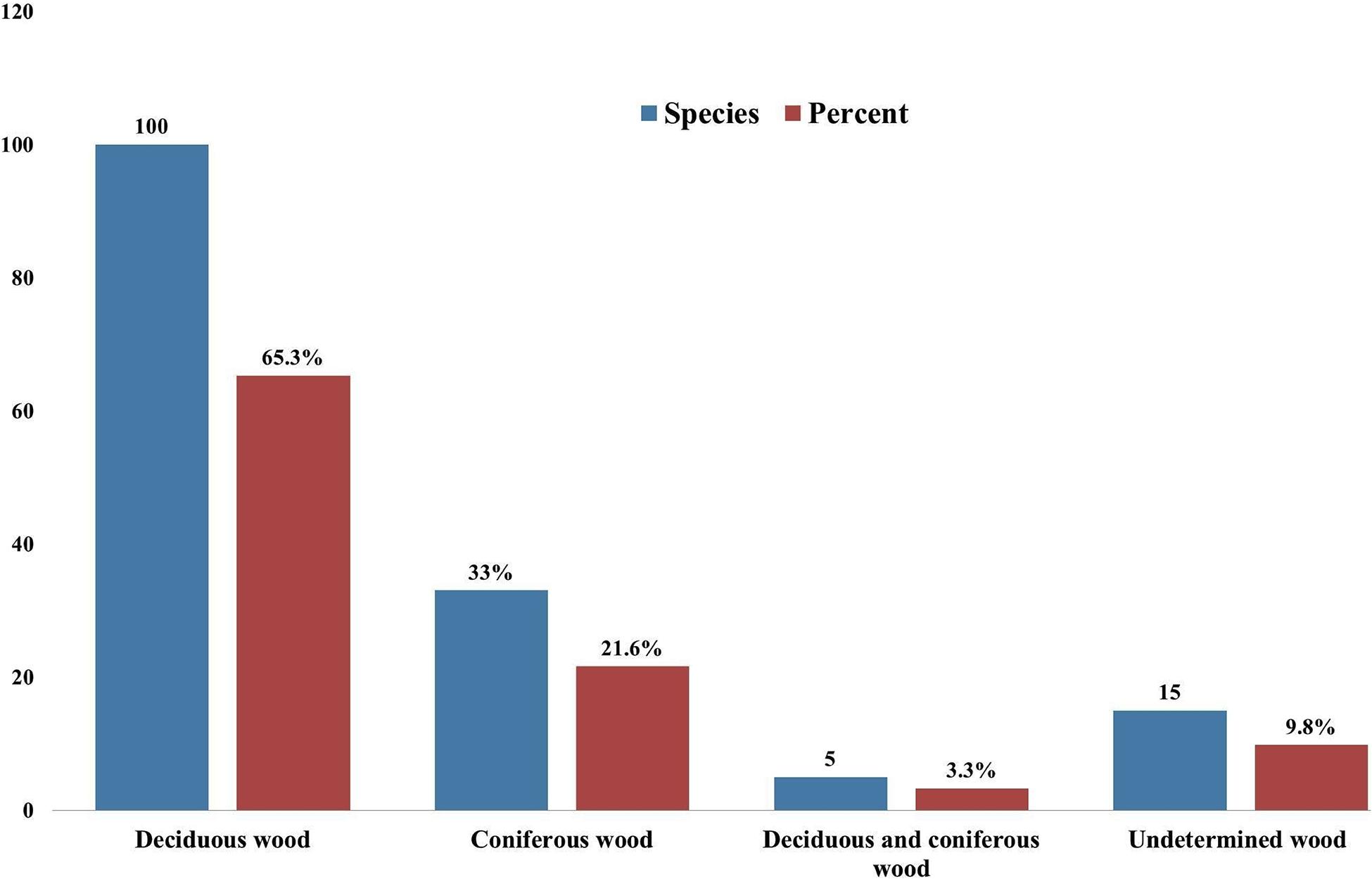
Figure 8. Species richness and proportions of wood-inhabiting poroid and corticioid fungi on different wood types.
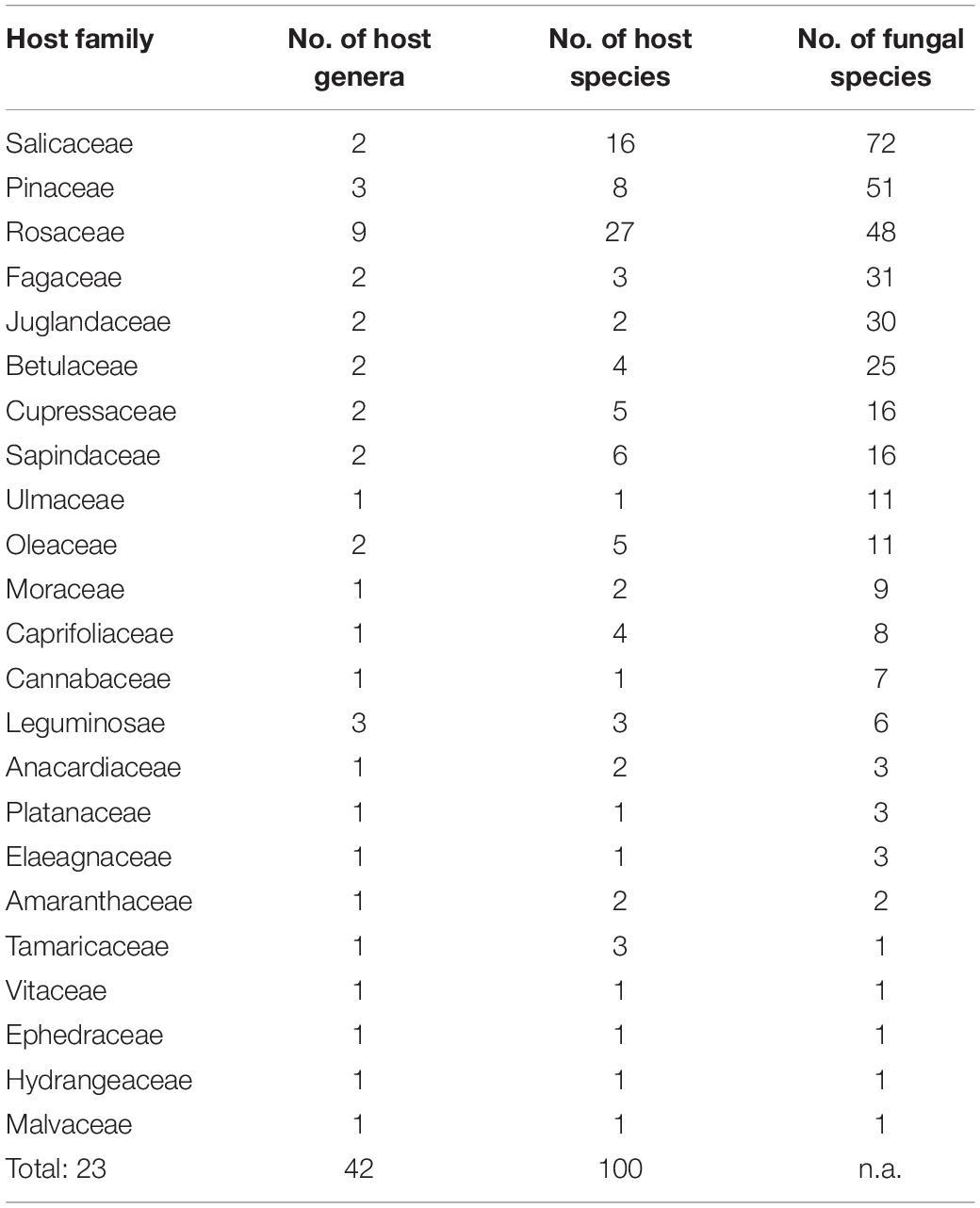
Table 3. Number of host family, genus, and species and number of wood-inhabiting poroid and corticioid species on host family in the study area.
The highest number of wood-inhabiting poroid and corticoid species is reported in the following host genera: Populus (40 species, 26.4% of the total species number), Quercus (30, 19.6%), Juglans, Pinus, and Salix (each 29, 18.9%), Betula (20, 13.0%), Picea (19, 12.4%), Prunus (17, 11.15%), Acer (14, 9.2%), Malus (12, 7.8%), Juniperus and Ulmus (each 11, 7.1%), Fraxinus (10, 6.5%); other plant genera host one to nine fungal species (Figure 9).
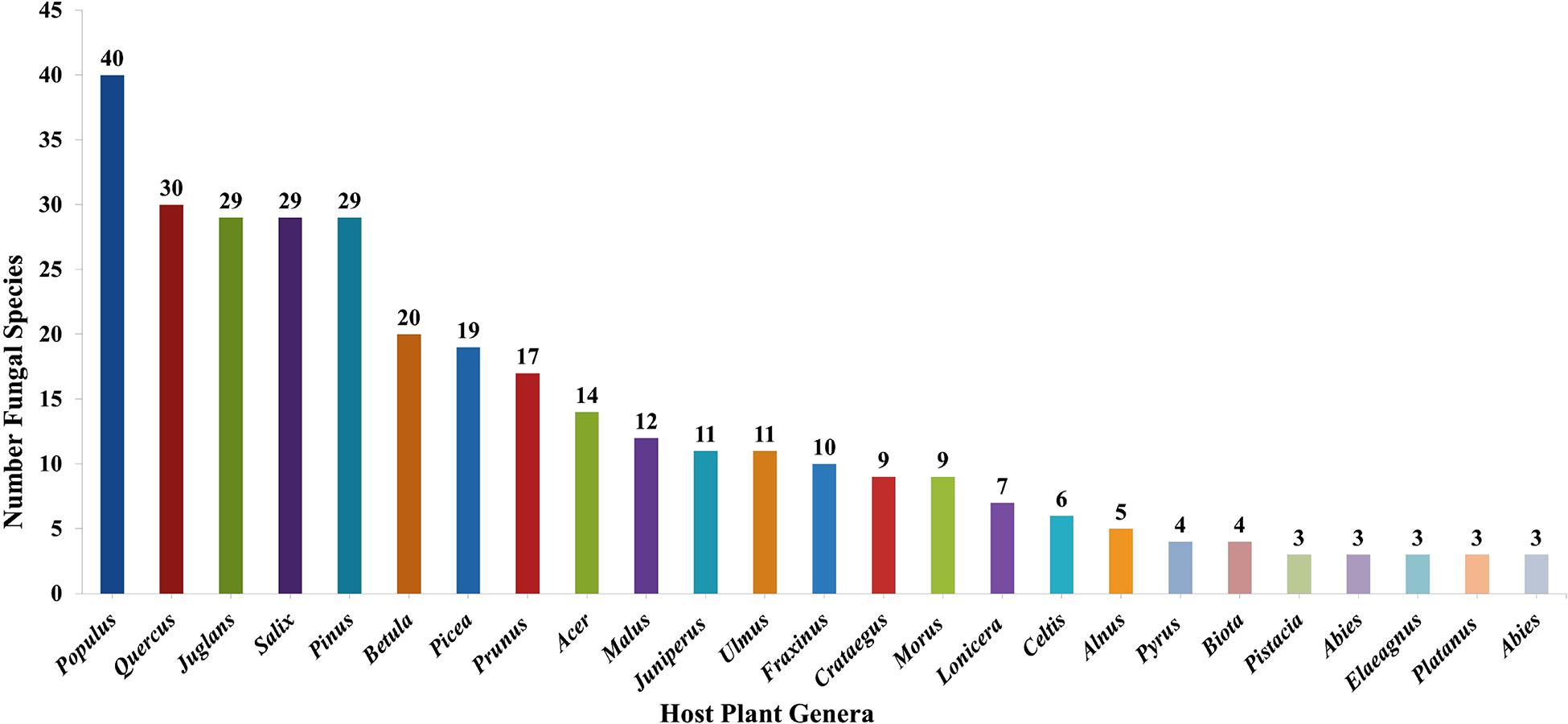
Figure 9. Occurence numbers of wood-inhabiting poroid and corticioid fungi on most representative host genera.
Among the wood-inhabiting poroid and corticioid fungi, 25 species were associated with a wide range of plant hosts, such as Phellinus pomaceus (16 host species), Stereum hirsutum (14), Trametes versicolor (11), Lentinus tigrinus (10), Cerrena unicolor (9), Bjerkandera adusta, Schizophyllum commune, Trametes hirsuta, T. tephroleuca, and T. trogii (8 hosts each); Coriolopsis gallica, Fomes fomentarius, Fomitiporia robusta, Tropicoporus linteus, and Laetiporus sulphureus (7 each); Irpex lacteus, Phylloporia yuchengii, Inonotus hispidus, and Cerioporus squamosus (6 each); and Fuscoporia torulosa, Trametes gibbosa, Antrodia xanthan, Phellinus igniarus, Lenzites warnieri, and Rigidoporus corticola (5 each) (Figure 10). The other 128 wood-inhabiting poroid and corticioid fungi studied each grew on one to four host species. Regarding the host preference, some wood-inhabiting poroid and corticioid species are tolerant, such as Phellinus pomaceus recorded on 16 plant species of nine genera (Prunus sp., P. mahaleb, P. cerasifera, P. erythrocarpa, P. dulcis, P. persica, P. domestica, Cerasus tianshanica, Cydonia oblonga, Celtis australis subsp. caucasica, Crataegus altaica, Juglans regia, Lonicera sp., Malus sp., M. domestica, and Salix sp.), followed by Stereum hirsutum on 14 species of seven genera (Juglans regia, Acer sp., A. tataricum subsp. semenovii, Salix iliensis, S. interior, S. alba, Fraxinus excelsior, Quercus sp., Q. robur, Celtis australis subsp. caucasica, Crataegus pseudoheterophylla subsp. turkestanica, Crataegus sp., Populus sp., and P. alba), and Trametes versicolor on 11 species of nine genera (Betula, Prunus, Crataegus, Lonicera, Quercus, Juglans, Populus, Malus, and Celtis). Other wood-inhabiting species each colonized 1 to 10 plant species (Figure 10).
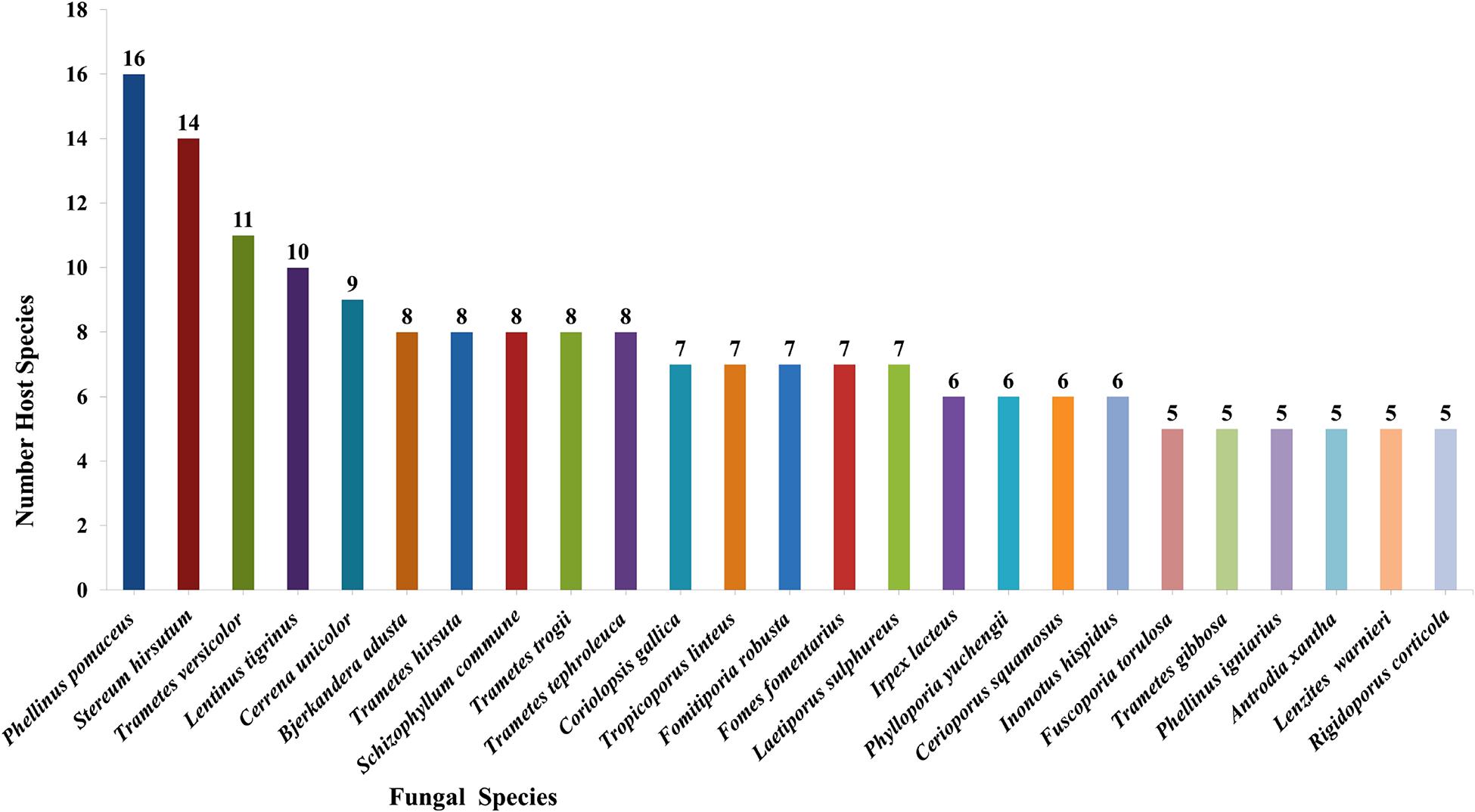
Figure 10. Host species numbers of 25 wood-inhabiting poroid and corticioid species with the widest host range.
The Distribution Range and Niches of Wood-Inhabiting Poroid and Corticioid Fungi in Uzbekistan
By importing all known records of wood-inhabiting poroid and corticioid fungi in Uzbekistan into ArcGIS, a distribution GIS map of wood-inhabiting poroid and corticoid species in Uzbekistan was produced (Figure 11). The distribution of all records in the whole study area was first mapped (Figure 11a), and then five subareas where fungal records are relatively dense were presented (Figures 11b–f). Fungal species were most commonly collected in subarea f with 445 records in Western Tien Shan Mountains in Tashkent province, followed by subarea b with 142 records in Pamir-Alay Mountain in Samarkand, Qashqadaryo, and Surkhandaryo Provinces of Central and Southern Uzbekistan. Subareas c, e, and d have 58, 51, and 46 records, respectively, in Turkestan, Nurata, Kurama, and Fergana ranges in Pamir Alay and Western Tien Shan Mountains in Navoi, Jizzakh, and Fergana valley of Uzbekistan (Figures 11c–e).
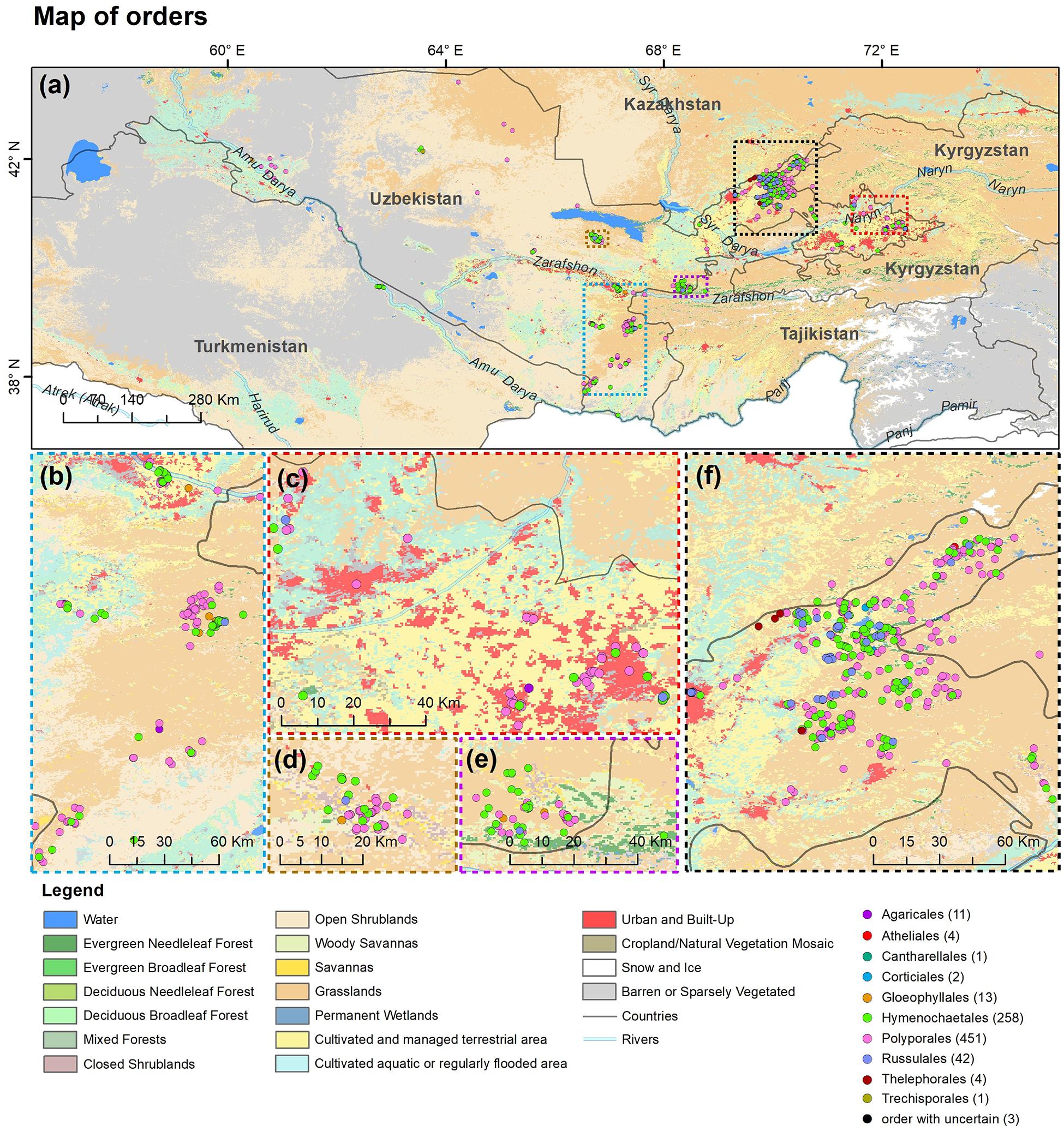
Figure 11. Spatial pattern of wood-inhabiting poroid and corticioid fungi at an order level based on 790 fungal records in (a) our study area, and five principal collecting areas shown zoom-in views in panels (b–f).
Polyporales is the most commonly collected fungal order with 451 records in urban and mountain areas of Uzbekistan. The next most abundant order is Hymenochaetales with 258 records mainly distributed in Tashkent Botanical Garden, Chatkal Biospheric, Nurata, Zarafshan, Surkhan, and Hissar State Reserves in North-eastren and southern Uzbekistan. The orders Russulales (42), Gleophyllales (13), and Agaricales (11) are distributed in coniferous and deciduous mixed forest trees in Ugam, Chatkal, Turkestan, Pskem, Nurata, and Hissar ranges in Pamiy-Alay and Westren Tien Shan Mountains. Other orders with fewer records include Atheliales (4), Thelephorales (4), Cantharellales (1), and Trechisporales (1). In addition, three records have uncertain taxonomic positions at the order level. The most frequently recorded species in Uzbekistan are Phellinus pomaceus (34 records); Stereum hirsutum (27); Trametes hirsuta (25); T. trogii (22); Lentinus tigrinus (21); Sanghuangporus lonicerinus and Trametes versicolor (20 each); Cerrena unicolor, Fomes fomentarius, and Inonotus hispidus (17 each); Bjerkandera adusta and Phellinus igniarius (14 each); Coriolopsis gallica, Fomitopsis betulina, and Trametes tephroleuca (13 each); Fuscoporia contigua (12); Phylloporia yuchengii and Tropicoporus linteus (each 11); Antrodia xantha, Cerioporus squamosus, Fomitiporia robusta, and Rigidoporus corticola (each 10); and Ceriporiopsis gilvescens (8).
Discussion
In this study, we compiled for the first time the species diversity of wood-inhabiting poroid and corticioid fungi in Uzbekistan. Comprehensive information of these species is provided, including taxonomic diversity, substrate preference, and distribution of geographic and landscape position, on the basis of 790 fungal records collected from 1950 to 2020.
A total of 153 wood-inhabiting poroid and corticioid species, belonging to 10 orders, 26 families, and 97 genera, were confirmed to be present mainly based on literature references, morphological examinations, and also on phylogenetic analysis wherever possible. Of these 153 species, 19 are new for mycobiota to Central Asia and 31 are reported for the first time in Uzbekistan. In addition, four taxa that may be new to science were discovered and must be examined further. The fungal diversity reported here for Uzbekistan is much lower than that in other regions where the diversity of wood-inhabiting poroid and corticoid fungi is well explored. For example, 1210 wood-inhabiting poroid and corticioid species, including ecologically similar hydnoid fungi, are recorded in China (Dai, 2011, 2012). Also, the number of wood-inhabiting poroid fungi recorded is 492 and 394 in North America and Europe, respectively (Ryvarden and Melo, 2014; Zhou et al., 2016). The low species number in Uzbekistan is partly due to the relatively small area, but also due to the lack of systematic field surveys and thorough identification with the aid of molecular sequencing. Few scientists study the ecologically important fungi of Uzbekistan. Although this report is the most comprehensive study of diversity of wood-inhabiting poroid and corticiod fungi in Uzbekistan today, it must be considered provisional. In contrast, tens of taxonomists have jointly contributed records of hundreds of (mainly new) wood-inhabiting poroid and corticioid species in China since the publications of Dai (2011, 2012). Therefore, even if we have reported the most comprehensive diversity of wood-inhabiting poroid and corticioid fungi in Uzbekistan to date, the current knowledge has to be considered as a provisional species checklist to be complemented. It is known that higher diversity of plant species results in a higher diversity of associated fungal species (Küffer and Senn-Irlet, 2005; Yamashita et al., 2010; Hawksworth and Lücking, 2017). Uzbekistan, with its mountainous landscape, is characterized by a high diversity of trees and shrubs, perhaps 500–600 species (Eastwood et al., 2009). Therefore, maybe more taxa of wood-inhabiting poroid and corticioid species, including new and even endemic species, still await to be revealed from Uzbekistan (Hyde et al., 2020; Yuan et al., 2020).
Most of the reported wood-inhabiting poroid and corticioid fungi in Uzbekistan are wood decomposers, which release matter and energy to the ecological system. These saprophytic species possess powerful enzymes, which can effectively degrade lignocellulose (Riley et al., 2014). In the current Uzbekistan mycota, several species in Trametes are considered to have potential biotechnological applications (Kneževič et al., 2013; Wang and Chen, 2019; Yang et al., 2020).
Although the resource recycling functions are generally considered beneficial to trees, forests, and humans, some of the wood-inhabiting poroid and corticioid fungi studied inhabit living trees as forest pathogens. According to previous studies (Kleyner, 1958; Sinadskiy and Bodartseva, 1960; Panfilova and Gaponenko, 1963; Sinadskiy, 1968; Khalikova, 1989; Baltaeva, 1992; Gafforov, 2014; Gafforov et al., 2014) and our field observations, Inonotus hispidus, Bjerkandera adusta, Cerrena unicolor, Lentinus tigrinus, Fomes fomentarius, Laetiporus sulphureus, Phylloporia yuchengii and Ganoderma applanatum, Aurantiporus fissilis, and some species from the genera Trametes and Phellinus can cause root rot disease. In addition, moreover, Cerrena unicolor also produces a stem canker of living Acer semenovii, Juglans regia, Crataegus pseudoheterophylla subsp. Turkestanica, and Celtis australis subsp. caucasica. Noteworthily, Inonotus hispidus is widespread in the walnut-fruit forests of Uzbekistan and damages up to 4% of the trees of Juglans regia and Malus sieversii trees in Baysun and Turkestan ranges of Pamir Alay Mountains (unpublished data Gafforov); Phellinus igniarius was observed both as a parasite and saprophyte of deciduous trees from the genera Juglans, Salix, and Acer in Ugam-Chatkal Natural State Park and Zaamin and Hissar State Reserve. The forest diseases caused by these wood-inhabiting poroid and corticioid fungi and the corresponding economic loss should be considered by relevant management departments.
In addition, some macrofungi including wood-inhabiting poroid and corticioid species are important edible and medicinal fungi (Wu et al., 2019; Zhou et al., 2020). Some poroid species known from Uzbekistan are recognized as valuable medicinal fungi, while Grifola frondosa, Laetiporus sulphureus, and Sarcodon imbricatus are important edible fungi. Cultivation of wood-inhabiting fungi is an important agricultural industry worldwide, especially in East Asia. Several medicinal and edible species, like Ganoderma spp. and Auricularia spp. in mainland China, Taiwanofungus in Taiwan, China, and Sanghuangporus spp. in South Korea, have huge economic value. In China, edible and medicinal fungi are the fifth largest crop industry (Dong et al., 2017). Industrial development of suitable endemic wood-inhabiting poroid and corticioid fungi will undoubtfully benefit the Uzbekistan economy. Uzbekistan materials must be directly studied for utilization of these fungal resources. For this purpose, strains of wood-inhabiting poroid and corticioid fungi firstly need to be isolated and preserved in public organizations.
The proportion of wood-inhabiting poroid and corticioid fungi found on different hosts differs in Uzbekistan from that in comparable temperate and warm temperate forest zones of China (Zhou et al., 2011). Compared with the proportions in temperate and warm temperate forest zone (Zhou et al., 2011), the proportion of wood-inhabiting poroid and corticioid species in Uzbekistan found on deciduous wood (72.46%) is similar, but the proportion on coniferous wood is much higher (23.91%) and that on both groups of wood is much lower (3.62%). The differences in the proportions on coniferous wood and both groups of wood compared to the Chinese case may be either the real status in Uzbekistan or a misleading failure to observe that some species recorded exclusively on coniferous wood do actually occur also on deciduous wood. Many more field surveys are needed to clarify this issue.
Among the 100 woody plant species belonging to 23 families and 42 genera recorded as hosts for wood-inhabiting poroid and corticioid species, Salicaceae, Pinaceae, and Rosaceae are the most favored, and Populus, Quercus, Juglans, and Salix are the most favored host genera.
To well understand the spatial patterns of wood-inhabiting poroid and corticioid fungi, their distributions associated with geography and landscapes were visualized using GIS maps (Figure 11). Wood-inhabiting poroid and corticioid species in Uzbekistan are distributed mostly in the regions of open shrublands and grasslands, and rarely in various kinds of forests. This distribution pattern is opposite to the natural habitats of such fungi as reported in previous studies. For example, Zhou and Dai (2012) reported that reserved forest with amounts of woody substrates for the growth of wood-inhabiting poroid fungi has significantly higher polypore diversity than unprotected forest. Moreover, normally, forests have higher species diversity of woody plants, which result in higher diversity of wood-inhabiting poroid fungi (Dai et al., 2015). This unusual phenomenon in Uzbekistan might be caused by a lower proportion of field surveys carried out in virginal forests due to difficult access. Therefore, more efforts should be made to reveal the diversity of wood-inhabiting poroid and corticioid fungi in the most primeval forests.
The two fungal orders with highest record diversity, viz., Polyporales and Hymenochaetales, are common in the whole studied area (Figure 11a). This reflects that geography and landscape factors do not have significant effects on the distribution of these two fungal orders. These two fungal orders have high species diversity (Table 2), resulting from a wide adaption to the environment. Species diversity of these two orders are also highest in other regions of the world (Dai, 2012; Ryvarden and Melo, 2014; Zhou et al., 2016). Russulales, the order with the third highest recorded diversity, also occurs in all five subareas, but mainly in the subarea f, viz., Western Tien Shan Mountains in Tashkent region (Figure 11f). Other orders and the species without a confirmed position at the order level are present only in small areas. Such species may be sensitive to environmental changes. So, to sustain species diversity of wood-inhabiting poroid and corticioid fungi, special attention should be paid to protecting their habitats. Using the GIS data, the potential future distribution of certain important (biotechnological, pathogenic, medicinal, and edible) wood-inhabiting poroid and corticioid species could be predicted. Similar studies have been performed on some wood-inhabiting poroid and corticioid species worldwide (Yuan et al., 2015; Elias et al., 2020). Generally, knowledge of species diversity and occurrence in an area is a baseline for benefiting from ecosystem services, monitoring environmental changes, and implementing conservation actions (Mueller and Schmit, 2007; Hibbett et al., 2007, 2011; Brock et al., 2009; Hyde et al., 2013; Osmundson et al., 2013; Truong et al., 2017). Therefore, the current GIS data are important for management and utilization of wood-inhabiting poroid and corticioid fungi in Uzbekistan.
In conclusion, this study provides the first comprehensive, thoroughly annotated checklist of species diversity of wood-inhabiting poroid and corticioid fungi in Uzbekistan. These species are ecologically and economically important as decomposers, pathogens, and sources of food and medicines. Beyond local scale, these data are also crucial as a supplement of the global knowledge of wood-inhabiting poroid and corticioid fungi and for elucidating the evolutionary history of wood-inhabiting poroid and corticioid fungi worldwide. More importantly, the current project exploring wood-inhabiting poroid and corticioid fungi in a less studied country may initiate similar explorations in other Central Asian countries and also other regions worldwide, which will largely fulfill the knowledge gap of wood-inhabiting poroid and corticioid fungi in certain rarely studied regions.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author Contributions
YG and MY collected fungal specimens and performed DNA lab work. YG and AO were responsible for morphological identification and management of collection data. L-WZ and YG performed the molecular phylogenetic analyses. YZ mapped the fungal taxa. YG, AO, and L-WZ drafted the manuscript. EL, AG, DS, LP, and LC improved and revised it. All authors have read the final manuscript version and approved it. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Ministry of Innovative Development of the Republic of Uzbekistan (Project Nos. P3-2014-0830174425 and P3-20170921183), CAS President’s International Fellowship Initiative (PIFI) for Visiting Scientist (Grant No. 2018VBB0021), German Academic Exchange Service (DAAD) for a Visiting Fellowship (Grant Nos. A/13/03723 and 57314018), Erasmus Mundus Action 2 CASIA Postdoctoral Fellowship (Grant No. UZGT2-P3), and a TWAS-CNPq postdoctoral fellowship (Grant No. 190136/2013-8). L-WZ thanks the financial support from the Youth Innovation Promotion Association of the Chinese Academy of Sciences (No. 2017240).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
YG thanks Prof. Jan Stenlid (Swedish University of Agricultural Sciences, Sweden) for the use of his laboratory facilities, Dr. Indrek Sell (Estonia) for organizing a trip to TAAM herbarium in Tartu, Dr. Ivan Zmitrovich (Komarov Botanical Institute of the Russian Academy of Sciences, Russia) for helping with literature search, and Dr. Viacheslav Spirin (Finnish Museum of Natural History, University of Helsinki, Finland) for helping identify Antrodia species. The authors warmly thank TAAM curator Dr. Ilmy Parmasto and other staff members for issuing permission and technical help while studying herbarium specimens at the Estonian University of Life Sciences, Tartu, Estonia. They also thank Dr. Barbara Wilson (Corvallis, Oregon) for reviewing and for valuable comments on an earlier version of the manuscript. Dr. Oleh Prylutskyi (V.N. Karazin Kharkiv National University, Ukraine) and the GBIF helpdesk are acknowledged for the help with data publishing.
Footnotes
- ^ http://www.treebase.org
- ^ http://tree.bio.ed.ac.uk/software/tracer/
- ^ https://dwc.tdwg.org
- ^ https://doi.org/10.15468/vsru5z
- ^ https://gbif.univer.kharkov.ua/resource?r=aphyllo_uz
- ^ google.com/earth/versions/
References
Aime, M. C., and Brearley, F. Q. (2012). Tropical fungal diversity: closing the gap between species estimates and species discovery. Biodivers. Conserv. 21, 2177–2180. doi: 10.1007/s10531-012-0338-7
Akhmedova, F. G. (1966). Mycoflora of South-Western Tien Shan Mountain. Ph.D. dissertation, Institute of Botany, Uzbekistan Academy of Sciences, Tashkent.
Anonymous (1998). UNDP/GEF: National Strategy and Action Plan. Tashkent: Biodiversity of Conservation of Republic of Uzbekistan.
Anonymous (2001). USAID Biodiversity Assessment for Uzbekistan. Almaty: USAID Central Asian Republics Mission, 57.
Antonelli, A., Fry, C., Smith, R. J., Simmonds, M. S. J., Kersey, P. J., Pritchard, H. W., et al. (2020). State of the World’s Plants and Fungi 2020. Kew: Royal Botanic Gardens. doi: 10.34885/172
ArcGIS Desktop (2020). Available online at: Desktop.arcgis.com/en
Ariyawansa, H. A., Hyde, K. D., Jayasiri, S. C., Buyck, B., Chethana, K. W. T., Dai, D. Q., et al. (2015). Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 75, 27–274. doi: 10.1007/s13225-015-0355-4
Baltaeva, G. M. (1992). Polypore Fungi (Polyporaceae s. lato) of Uzbekistan. Ph.D. thesis. Komarov Botanical Institute, Russian Academy of Sciences, Saint Petersburg.
Baltaeva, G. M. (1993). Polypore fungi (Polyporaceae, Ganodermataceae, Hymenochaetaceae) of UzSSR. Mikol. Fitopatol. 32–36.
Baymuratova, G. T. (1963). Mycoflora of Hungry Steppe. Ph.D. thesis, Institute of Botany, Uzbekistan Academy of Sciences, Tashkent.
Bondarceva, M. A., and Parmasto, E. H. (1986). Families Hymenochaetaceae, Lachnocladiaceae, Coniophoraceae, Schizophyllaceae (Order Aphyllophorales, Key to Fungi of the USSR), issue 1. Moscow: Nauka Press.
Botman, E. (2009). Forest Rehabilitation in the Republic of Uzbekistan. IUFRO World Ser. 20, 253–299.
Brazee, N. J. (2015). Phylogenetic relationships among species of Phellinus sensu stricto, cause of white trunk rot of hardwoods, from northern North America. Forests 6, 4191–4211. doi: 10.3390/f6114191
Breitenbach, J., and Kränzlin, F. (1986). Fungi of Switzerland, Vol. 2. Non Gilled Fungi - Heterobasidiomycetes, Aphyllophorales, Gasteromycetes. Mykologia. Luzern: Lucerne Press.
Brock, P. M., Döring, H., and Bidartondo, M. I. (2009). How to know unknown fungi: the role of a herbarium. New Phytol. 181, 719–724. doi: 10.1111/j.1469-8137.2008.02703.x
Brummitt, R. K. (2001). World Geographical Scheme for Recording Plant Distributions: Edition 2. International Working Group on Taxonomic Databases for Plant Sciences (TDWG). Pittsburgh: Carnegie Mellon University.
Cheek, M., Nic Lughadha, L., Kirk, P., Lindon, H., Carretero, J., Looney, B., et al. (2020). New discoveries: plants and Fungi. Plants People Planet. 2, 371–388. doi: 10.1002/ppp3.10148
Crous, P. W., Rong, I. H., Wood, A., Lee, S., Glen, H., Botha, W., et al. (2006). How many species of fungi are there at the tip of Africa? Stud. Mycol. 55, 13–33. doi: 10.3114/sim.55.1.13
Dai, Y. C. (2011). A revised checklist of corticioid and hydnoid fungi in China for 2010. Mycoscience 52, 69–79. doi: 10.1007/S10267-010-0068-1
Dai, Y. C. (2012). Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience 53, 49–80. doi: 10.1007/s10267-011-0134-3
Dai, Y. C., Wei, Y. L., and Zhou, L. W. (2015). Polypore richness along an elevational gradient: a case study in Changbaishan Nature Reserve, Northeastern China. Fungal Ecol. 13, 226–228. doi: 10.1016/j.funeco.2014.07.002
Dong, C. H., Liu, Q. Z., and Zhang, J. J. (2017). Research progress on important edible and medicinal fungi in China over the last decade. J. Microbiol. 37, 1–9.
Eastwood, A., Lazkov, G., and Newton, F. (2009). The Red List of Trees of Central Asia. Cambridge: Fauna & Flora International Press.
Elias, S. G., Salvador-Montoya, C. A., Costa-Rezende, D. H., Guterres, D. C., Fernandes, M., and Drechsler-Santos, E. R. (2020). Studies on the biogeography of Phellinotus piptadeniae (Hymenochaetales, Basidiomycota): expanding the knowledge on its distribution and clarifying hosts relationships. Fungal Ecol. 45, 100912. doi: 10.1016/j.funeco.2020.100912
Floudas, D., and Hibbett, D. S. (2015). Revisiting the taxonomy of Phanerochaete (Polyporales, Basidiomycota) using a four gene dataset and extensive ITS sampling. Fungal Biol. 119, 679–719. doi: 10.1016/j.funbio.2015.04.003
Friedl, M. A., McIver, D. K., Hodges, J. C. F., Zhang, X. Y., Muchoney, D., Strahler, A. H., et al. (2002). Global land cover mapping from MODIS: algorithms and early results. Remote Sens. Environ. 83, 287–302. doi: 10.1016/S0034-4257(02)00078-0
Gafforov, Y. (2017). A preliminary checklist of Ascomycetous microfungi from Southern Uzbekistan. Mycosphere 8, 660–696. doi: 10.5943/mycosphere/8/4/12
Gafforov, Y., and Hoshino, T. (2015). Remarks on Typhula sp. in Uzbekistan. Mycoscience 56, 109–113. doi: 10.1016/j.myc.2014.04.003
Gafforov, Y., and Ordynets, A. (2020). Aphyllophoroid fu$ngi of Uzbekistan. Institute of Botany of the Academy of Sciences of the Republic of Uzbekistan. Occurrence Dataset. Available online at: GBIF.org (accessed August 13, 2020). doi: 10.15468/vsru5z
Gafforov, Y., Phookamsak, R., Jiang, H. B., Wanasinghed, D. N., and Juliev, M. (2019). Ophiobolus hydei sp. nov. (Phaeosphaeriaceae, Ascomycota) from Cirsium and Phlomoides in Uzbekistan. Botany 97, 671–680. doi: 10.1139/cjb-2019-0118
Gafforov, Y., and Rakhimov, D. (2017). Diplodia and Dothiorella species (Botryosphaeriaceae, Ascomycota) from Uzbekistan. J. Bot. Res. Inst. Texas 11, 455–467.
Gafforov, Y., Riebesehl, J., Ordynets, A., Langer, E., Yarasheva, M., Ghobad-Nejhad, M., et al. (2017). Hyphodontia (Hymenochaetales, Basidiomycota) and similar taxa from Central Asia. Botany 95, 1041–1056. doi: 10.1139/cjb-2017-0115
Gafforov, Y., Tomšovský, M., Langer, E., and Zhou, L. W. (2014). Phylloporia yuchengii sp. nov. (Hymenochaetales, Basidiomycota) from Western Tien Shan Mountains of Uzbekistan based on phylogeny and morphology. Cryptogamie Mycol. 35, 313–322. doi: 10.7872/crym.v35.iss4.2014.313
Gafforov, Y. S. (2010). Ecological peculiarities and mycodiversity of parasite hyphalic fungi (Hyphales) of Fergana Valley and their spread on host plants. Uzbekistan Boil. J. 2, 46–51.
Gafforov, Y. S. (2014). Taxonomy and diversity of the genus Ganoderma Karst. (Basidiomycota) species in Uzbekistan. Uzbekistan Biol. J. Spec. Issue 22–25.
Gafforov, Y. S. (2015). Species Diversity and Distribution of the Genus Ramularia Unger (Mycosphaerelaceae) in the Western Tien Shan. Plants and Animals of Ugam-Chatkal State National Natural Park. Tashkent: Chinor ERNk Press, 48–87.
Gafforov, Y. S. (2016a). Ascomycetous microfungi of Dendroflora of Boysun-botanical-geographic region. Uzbekistan J. Ecol. 12, 36–39.
Gafforov, Y. S. (2016b). Coniothyrium-like fungi (Ascomycota) from Western Tien Shan and South-Western Hissar mountains of Uzbekistan. Uzbekistan Boil. J. 4, 32–36.
Gardes, M., and Bruns, T. D. (1996). “ITS-RFLP matching for the indetification of fungi,” in Methods in Molecular in Biology: Species Diagnostics Protocols: PCR and Other Nucleic Acid Methods, ed. J. P. Clapp (Totowa, N.J: Humana Press. Inc), 177–186.
Ghobad-Nejhad, M., and Langer, E. (2016). First inventory of aphyllophoroid basidiomycetes of Zagros forests, W Iran. Plant Biosyst. 151, 844–854. doi: 10.1080/11263504.2016.1211199
González, V., Sánchez-Torres, P., Hinarejos, R., and Tuset, J. J. (2009). Inonotus hispidus fruiting bodies on grapevines with esca symptoms in Mediterranean areas of Spain. J. Plant Pathol. 91, 465–468. doi: 10.4454/jpp.v91i2.979
Guglielmo, F., Bergemann, S. E., Gonthier, P., Nicolotti, G., and Garbelotto, M. (2007). A multiplex PCR-based method for the detection and early identification of wood rotting fungi in standing trees. J. Appl. Microbiol. 103, 1490–1507. doi: 10.1111/j.1365-2672.2007.03378.x
Guindon, S., and Gascuel, O. (2003). A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704. doi: 10.1080/10635150390235520
Han, M. L., Chen, Y. Y., Shen, L. L., Song, J., Vlasák, J., Dai, Y. C., et al. (2016). Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Divers. 80, 343–373. doi: 10.1007/s13225-016-0364-y
Hattori, T., Yamashita, S., and Susee, L. (2012). Diversity and conservation of wood-inhabiting polypores and other aphyllophoraceous fungi in Malaysia. Biodivers. Conserv. 21, 2375–2396. doi: 10.1007/s10531-012-0238-x
Hawksworth, D., and Lücking, R. (2017). “Fungal diversity revisited: 2.2 to 3.8 million species,” in The Fungal Kingdom, Vol. 5, eds J. Heitman, B. Howlett, P. Crous, E. Stukenbrock, T. James, and N. Gow (Washington, DC: American Society of Microbiology), 79–95.
Hawksworth, D. L. (2012). Global species numbers of fungi: are tropical studies and molecular approaches contributing to a more robust estimate? Biodivers. Conserv. 21, 2425–2433. doi: 10.1007/s10531-012-0335-x
Hawksworth, D. L., Kirk, P. M., Sutton, B. C., and Pegler, D. N. (1995). Ainsworth& Bisby’s Dictionary of the Fungi, 8th Edn. Wallingford: CABI Publishing.
He, S. H., Vlasák, J., and Dai, Y. C. (2014). Hispidaedalea gen. nov. and Griseoporia taiwanense sp. nov. (Gloeophyllales, Basidiomycota) based on morphological and molecular characters. Mycol. Prog. 13, 833–839. doi: 10.1007/s11557-014-0966-2
Hibbett, D., Abarenkov, K., Kõljalg, U., Öpik, M., Chai, B., Cole, J., et al. (2011). Sequence-based classification and identification of Fungi. Mycologia 108, 1049–1068. doi: 10.3852/16-130
Hibbett, D. S., Binder, M., Bischoff, J. F., Blackwell, M., Cannon, P. F., Eriksson, O. E., et al. (2007). A higher-level phylogenetic classification of the Fungi. Mycol. Res. 111, 509–547. doi: 10.1016/j.mycres.2007.03.004
Hyde, K. D., Jeewon, R., Chen, Y., Bhunjun, C. S., Calabon, M. S., Jiang, H. B., et al. (2020). The numbers of fungi: is the descriptive curve flattening? Fungal Divers. 103, 219–271. doi: 10.1007/s13225-020-00458-2
Hyde, K. D., Tennakoon, D. S., Jeewon, R., Bhat, J., Maharachchikumbura, S. S. N., Rossi, W., et al. (2019). Fungal diversity notes 1036–1150: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 96, 1–242. doi: 10.1007/s13225-019-00429-2
Hyde, K. D., Udayanga, D., Manamgoda, D. S., Tedersoo, L., Larsson, E., Abarenkov, K., et al. (2013). Incorporating molecular data in fungal systematics: a guide for aspiring researchers. Curr. Res. Environ. Appl. Mycol. 3, 1–32. doi: 10.5943/cream/3/1/1
Iminova, M. M. (2009). Macrofungi of Fergana Valley (within Uzbekistan). Ph.D. dissertation, Institute of Botany, Uzbekistan Academy of Sciences, Tashkent.
Index Fungorum. (2020). Available online at: http://indexfungorum.org/Names/Names.asp (accessed May 27, 2020).
Jeong, W. J., Lim, Y. W., Lee, J. S., and Jung, H. S. (2005). Phylogeny of Phellinus and related genera inferred from combined data of ITS and mitochondrial SSU rDNA sequences. J. Microbiol. Biotechnol. 15, 1028–1038.
Justo, A., Miettinen, O., Floudas, D., Ortiz-Santana, B., Sjökvist, E., Lindner, D., et al. (2017). A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biol. 121, 798–824. doi: 10.1016/j.funbio.2017.05.010
Kan, Y. H., Gafforov, Y., Li, T., and Zhou, L. W. (2017). Hyphodontia zhixiangii sp. nov. (Schizoporaceae, Basidiomycota) from Uzbekistan. Phytotaxa 299, 273–279. doi: 10.11646/phytotaxa.299.2.12
Katoh, K., Kuma, K., Toh, H., and Miyata, T. (2005). MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic. Acids. Res. 33, 511–518. doi: 10.1093/nar/gki198
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Khalikova, M. M. (1989). Macrofungi of Tashkent Region. Ph.D. thesis, Institute of Botany, Uzbekistan Academy of Sciences, Tashkent.
Klein, I., Gessner, U., and Kuenzer, C. (2012). Regional land cover mapping and change detection in Central Asia using MODIS time-series. Appl. Geogr. 35, 219–234. doi: 10.1016/j.apgeog.2012.06.016
Klein Tank, A. M. G., Peterson, T. C., Quadir, D. A., Dorji, S., Zou, X., Tang, H., et al. (2006). Changes in daily temperature and precipitation extremes in central and south Asia. J. Geophys. Res. 111:D16105. doi: 10.1029/2005JD006316
Kneževič, A., Milovanovič, I., Stajič, M., and Vukojevič, J. (2013). Potential of Trametes species to degrade lignin. Int. Biodeter. Biodegr. 85, 52–56. doi: 10.1016/j.ibiod.2013.06.017
Kravtzev, B. I. (1950). Fungal diseases of popular in Kazakhstan. Bull. Acad. Sci. Kazakh SSR 98:128.
Küffer, N., and Senn-Irlet, B. (2005). Influence of forest management on the species richness and composition of wood-inhabiting basidiomycetes in Swiss forests. Biodivers. Conserv. 14, 2419–2435. doi: 10.1007/s10531-004-0151-z
Larsson, K. H. (2007). Re-thinking the classification of corticioid fungi. Mycol. Res. 111, 1040–1063. doi: 10.1016/j.mycres.2007.08.001
Leal-Dutra, C. A., Griffith, G. W., Neves, M. A., Mclaughlin, D. J., Mclaughlin, E. G., Clasen, L. A., et al. (2020). Reclassification of Pterulaceae Corner (Basidiomycota- Agaricales) introducing the ant-associated genus Myrmecopterula gen. nov., Phaeopterula Henn. and the corticioid Radulomycetaceae fam. nov. IMA Fungus 11:2.
Letunic, I., and Bork, P. (2019). Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 47, 256–259. doi: 10.1093/nar/gkz239
Li, H. J., Si, J., Zhang, Y. Z., Sun, J., and He, S. H. (2016). Taxonomic and phylogenetic studies reveal a new species from Funalia gallica complex (Polyporales, Basidiomycota). Mycol. Res. 15, 1–8. doi: 10.1007/s11557-016-1167-y
Lioubimtseva, A. E., and Henebry, G. M. (2009). Climate and environmental change in arid Central Asia: impacts, vulnerability, and adaptations. J. Arid Environ. 73, 963–977. doi: 10.1016/j.jaridenv.2009.04.022
Mueller, G. M., and Schmit, J. P. (2007). Fungal biodiversity: what do we know? What can we predict? Biodivers. Conserv. 16, 1–5. doi: 10.1007/s10531-006-9117-7
Natural Earth (2020). Available online at: http://www.naturalearthdata.com/ (accessed June 8, 2011).
Osmundson, T. W., Robert, V. A., Schoch, C. L., Baker, L. J., Smith, A., Robich, G., et al. (2013). Filling gaps in biodiversity knowledge for macrofungi: contributions and assessment of an herbarium collection DNA barcode sequencing project. PLoS One 8:e62419. doi: 10.1371/journal.pone.0062419
Ovaskainen, O., Schigel, D., Ali-Kovero, H., Auvinen, P., Paulin, L., Norden, B., et al. (2013). Combining high-throughput sequencing with fruit body surveys reveals contrasting life-history strategies in fungi. ISME J. 7, 1696–1709. doi: 10.1038/ismej.2013.611
Panfilova, T. S., and Gaponenko, N. I. (1963). Mycoflora of the Angren River Basin. Tashkent: AN UzSSR Press.
Pattengale, N. D., Alipour, M., Bininda-Emonds, O. R. P., Moret, B. M. E., and Stamatakis, A. (2010). How many bootstrap replicates are necessary? J. Comput. Biol. 17, 337–354.
Pem, D., Gafforov, Y., Jeewon, R., Hongsanan, S., Promputtha, I., Doilom, M., et al. (2018). Multigene phylogeny coupled with morphological characterization reveal two new species of Holmiella and taxonomic insights within Patellariaceae. Cryptogamie Mycol. 39, 193–209. doi: 10.7872/crym/v39.iss2.2018.193
Pem, D., Jeewon, R., Gafforov, Y., Hongsanan, S., Phukhamsakda, C., Promputtha, I., et al. (2019a). Melanocamarosporioides ugamica gen. et sp. nov., a novel member of the family Melanommataceae from Uzbekistan. Mycol. Prog. 18, 471–481. doi: 10.1007/s11557-018-1448-8
Pem, D., Jeewon, J., Bulgakov, T., Gafforov, Y., Hongsanan, S., Phookamsak, R., et al. (2019b). Taxonomy and molecular phylogeny of Thyrostroma ephedricola sp. nov. (Dothidotthiaceae) and proposal for Thyrostroma jaczewskii comb. nov. Phytotaxa 416, 243–256. doi: 10.11646/phytotaxa.416.4.3
Posada, D. (2008). jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 25, 1253–1256. doi: 10.1093/molbev/msn083
Rehner, S. A., and Samuels, G. J. (1994). Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 98, 625–634. doi: 10.1016/S0953-7562(09)80409-7
Riley, R., Salamov, A. A., Brown, D. W., Nagy, L. G., Floudas, D., Held, B. W., et al. (2014). Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc. Natl. Acad. Sci. U.S.A. 111, 9923–9928. doi: 10.1073/pnas.1400592111
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Samarakoon, M. C., Gafforov, Y., Liu, N. G., Maharachchikumbura, S. S. N., Bhat, J. D., Liu, J. K., et al. (2018). Combined multi-gene backbone tree for the genus Coniochaeta with two new species from Uzbekistan. Phytotaxa 336, 043–058. doi: 10.11646/phytotaxa.336.1.3
Schmit, J. P., and Mueller, G. M. (2007). An estimate of the lower limit of global fungal diversity. Biodivers. Conserv. 16, 99–111. doi: 10.1007/s10531-006-9129-3
Schoch, C. L., Seifert, K. A., Huhndorf, S., Robert, V., Spouge, J. L., Levesque, C. A., et al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. U.S.A. 109, 6241–6246. doi: 10.1073/pnas.1117018109
Schwartzman, S. R. (1964). Flora Sporowich Rastenii Kazachstana: Auriculariales, Tremellales, Dacryomycetales, Exobasidiales, Aphyllophorales. Alma Ata: Nauka Press.
Silvestro, D., and Michalak, I. (2012). raxmlGUI: a graphical front-end for RAxML. Org. Divers. Evol. 12, 335–337. doi: 10.1007/s13127-011-0056-0
Sinadskiy, Y. B. (1968). To the question of pathogenic mycoflora of tree-shrub species of desert zones of Central Asia and Kazakhstan. Mikol. Fitopotal. 2, 148–149.
Sinadskiy, Y. B., and Bodartseva, M. A. (1956). Little-known polypore on Populus and Tamarix and their significance in the Karakalpak S.S.R. Botanicheskii Zhur. 41, 1177–1183.
Sinadskiy, Y. B., and Bodartseva, M. A. (1960). Diseases of tree and shrub species in the tugai forests of the Syr Darya. Botanicheskii Zhur. 45, 423–429.
Solieva, Y. S., and Gafforov, Y. S. (2001). New records for mycoflora of Uzbekistan. J. Agrar Sci. Tashkent State Univ. 3, 36–39.
Solieva, Y. S., and Gafforov, Y. S. (2002). New species and genera of micromycetes for flora of Uzbekistan. Bull. Acad. Sci. Rep. Uzbekistan 4, 42–45.
Soudzilovskaia, N. A., Bodegom, P. M., Terrer, C., Zelfde, M. V., McCallum, I., McCormack, M. L., et al. (2019). Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nat. Commun. 10:5077. doi: 10.1038/s41467-019-13019-2
Spirin, V., Miettinen, O., Pennanen, J., Kotiranta, H., and Niemelä, T. (2013). Antrodia hyalina, a new polypore from Russia, and A. leucaena, new to Europe. Mycol. Prog. 12, 53–61. doi: 10.1007/s11557-012-0815-0
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. doi: 10.1093/bioinformatics/btl446
Stutz, J. C., Copeman, R., Martin, C., and Morton, J. B. (2000). Patterns of species composition and distribution of arbuscular mycorrhizal fungi in arid regions of southwestern North America and Namibia, Africa. Can. J. Bot. 78, 237–245. doi: 10.1139/b99-183
Swift, M. J. (1982). “Basidiomycetes as components of forest ecosystems,” in Decomposer Basidiomycetes, their Biology and Ecology, eds J. C. Frankland, J. N. Hedger, and M. J. Swift (Cambridge: Cambridge University Press).
Tedersoo, L., Bahram, M., Põlme, S., Kõljalg, U., Yorou, N. S., Wijesundera, R., et al. (2014). Global diversity and geography of soil fungi. Science 346:1256688. doi: 10.1126/science.1256688
ThePlantList (2013). Version 1.1. Available online at: http://www.theplantlist.org/ (accessed March 1, 2020).
Tomšovskyì, M. (2012). Delimitation of an almost forgotten species Spongipellis litschaueri (Polyporales, Basidiomycota) and its taxonomic position within the genus. Mycol. Prog. 11, 415–424. doi: 10.1007/s11557-011-0756-z
Truong, C., Mujic, A. B., Healy, R., Kuhar, F., Furci, G., Torres, D., et al. (2017). How to know the fungi: combining field inventories and DNA-barcoding to document fungal diversity. New Phytol. 3, 913–919. doi: 10.1111/nph.14509
Tsykun, T., Rigling, D., and Prospero, S. (2013). A new multilocus approach for reliable DNA-based identification of Armillaria species. Mycologia 105, 1059–1076. doi: 10.3852/12-209
Varma, A. (1995). “Ecophysiology and Application of Arbuscular Mycorrhizal Fungi in Arid Soils,” in Mycorrhiza, eds A. Varma and B. Hock (Berlin: Springer Press). doi: 10.1007/978-3-662-08897-5_24
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4239–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Vu, D., Groenewald, M., de Vries, M., Gehrmann, T., Stielow, B., Eberhardt, U., et al. (2019). Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 92, 135–154. doi: 10.1016/j.simyco.2018.05.001
Wanasinghe, D. N., Hyde, K. D., Crous, P. W., Wijayawardene, N. N., Jeewon, R., Jones, E. B. G., et al. (2017). Phylogenetic revision of Camarosporium (Pleosporinae, Dothideomycetes) and allied genera. Stud. Mycol. 87, 207–256. doi: 10.1016/j.simyco.2017.08.001
Wanasinghe, D. N., Jeewon, R., Peršoh, D., Jones, E. B. G., Camporesi, E., Bulgakov, T. S., et al. (2018a). Taxonomic circumscription and phylogenetics of novel didymellaceous taxa with brown muriform spores. Stud. Fungi 3, 152–175. doi: 10.5943/sif/3/1/17
Wanasinghe, D. N., Phukhamsakda, C., Hyde, K. D., Jeewon, R., Lee, H. B., Jones, E. B. G., et al. (2018b). Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers. 89, 1–236. doi: 10.1007/s13225-018-0395-7
Wang, Y., and Chen, Z. H. (2019). Bioinformatics and enzymatics investigation of Trametes laccase for optical biosensing application. J. Mater. Sci. 54, 4970–4983. doi: 10.1007/s10853-018-03187-9
White, T. J., Bruns, T. D., Lee, S., and Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR Protocols: A Guide to Methods and Applications, eds M. A. Innis and D. H. Gelfand (London: Academic Press), 315–322.
Wu, F., Chen, J. J., Ji, X. H., Vlasák, J., and Dai, Y. C. (2017). Phylogeny and diversity of the morphologically similar polypore genera Rigidoporus, Physisporinus, Oxyporus, and Leucophellinus. Mycologia 109, 749–765. doi: 10.1080/00275514.2017.1405215
Wu, F., Zhou, L. W., Yang, Z. L., Bau, T., Li, T. H., and Dai, Y. C. (2019). Resource diversity of Chinese macrofungi: edible, medicinal and poisonous species. Fungal Divers. 98, 1–76. doi: 10.1007/s13225-019-00432-7
Yamashita, S., Hattori, T., and Abe, H. (2010). Host preference and species richness of wood-Inhabiting aphyllophoraceous fungi in a cool temperate area of Japan. Mycologia 102, 11–19. doi: 10.3852/09-008
Yang, X., Wu, Y., Zhang, Y., Yang, E., Qu, Y., Xu, H., et al. (2020). A thermo-active laccase isoenzyme from Trametes trogii and its potential for dye decolorization at high temperature. Front. Microbiol. 11:241. doi: 10.3389/fmicb.2020.00241
Yuan, H. S., Wei, Y. L., and Wang, X. G. (2015). Maxent modeling for predicting the potential distribution of Sanghuang, an important group of medicinal fungi in China. Fungal Ecol. 17, 140–145. doi: 10.1016/j.funeco.2015.06.001
Yuan, Y., Gafforov, Y., Chen, Y. Y., and Wu, F. (2017). A new species of Antrodia (Basidiomycota, Polyporales) from juniper forest of Uzbekistan. Phytotaxa 303, 47–55. doi: 10.11646/phytotaxa.303.1.3
Yuan, Y. S., Lu, X., Dai, Y. C., Hyde, K. H., Kan, Y. H., et al. (2020). Fungal diversity notes 1276–1391: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. doi: 10.1007/s13225-020-00461-7
Zhao, C. L., and Cui, B. K. (2014). Phylogeny and taxonomy of Ceriporiopsis (Polyporales) with descriptions of two new species from southern China. Phytotaxa 164, 17–28. doi: 10.11646/phytotaxa.164.1.2
Zhou, L. W., and Dai, Y. C. (2012). Recognizing ecological patterns of wood-decaying polypores on gymnosperm and angiosperm trees in northeast China. Fungal Ecol. 5, 230–235. doi: 10.1016/j.funeco.2011.09.005
Zhou, L. W., Ghobad-Nejhad, M., Tian, X. M., Wang, Y. F., and Wu, F. (2020). Current status of ‘Sanghuang’ as a group of medicinal mushrooms and their perspective in industry development. Food Rev. Int. doi: 10.1080/87559129.2020.1740245
Zhou, L. W., Hao, Z. Q., Wang, Z., Wang, B., and Dai, Y. C. (2011). Comparison of ecological patterns of polypores in three forest zones in China. Mycology 2, 260–275. doi: 10.1080/21501203.2011.602726
Zhou, L. W., Nakasone, K. K., Burdsall, H. H., Ginns, J., Vlasák, J., and Miettinen, O. (2016). Polypore diversity in North America with an annotated checklist. Mycol. Prog. 15, 771–790. doi: 10.1007/s11557-016-1207-7
Keywords: Basidiomycota, Central Asia, distribution, substrate preferences, taxonomic diversity, GIS mapping
Citation: Gafforov Y, Ordynets A, Langer E, Yarasheva M, de Mello Gugliotta A, Schigel D, Pecoraro L, Zhou Y, Cai L and Zhou L-W (2020) Species Diversity With Comprehensive Annotations of Wood-Inhabiting Poroid and Corticioid Fungi in Uzbekistan. Front. Microbiol. 11:598321. doi: 10.3389/fmicb.2020.598321
Received: 24 August 2020; Accepted: 09 November 2020;
Published: 09 December 2020.
Edited by:
Peter Edward Mortimer, Kunming Institute of Botany, Chinese Academy of Sciences, ChinaReviewed by:
Masoomeh Ghobad-Nejhad, Iranian Research Organization for Science and Technology, IranBelle Damodara Shenoy, CSIR-National Institute of Oceanography, India
Copyright © 2020 Gafforov, Ordynets, Langer, Yarasheva, de Mello Gugliotta, Schigel, Pecoraro, Zhou, Cai and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yusufjon Gafforov, eXVnYWZmb3JvdkB5YWhvby5jb20=; Li-Wei Zhou, bGl3ZWlfemhvdTE5ODJAaW0uYWMuY24=
 Yusufjon Gafforov
Yusufjon Gafforov Alexander Ordynets
Alexander Ordynets Ewald Langer3
Ewald Langer3 Manzura Yarasheva
Manzura Yarasheva Adriana de Mello Gugliotta
Adriana de Mello Gugliotta Dmitry Schigel
Dmitry Schigel Lorenzo Pecoraro
Lorenzo Pecoraro Yu Zhou
Yu Zhou Lei Cai
Lei Cai Li-Wei Zhou
Li-Wei Zhou