- 1Robert Koch Institute, Unit 37: Nosocomial Infections, Surveillance of Antimicrobial Resistance and Consumption, Berlin, Germany
- 2Department of Oral and Maxillofacial Surgery, Plastic Surgery, University Medical Center of the Johannes Gutenberg-University Mainz, Mainz, Germany
- 3Institute of General Practice and Family Medicine, Jena University Hospital, Jena, Germany
Data on microbiological profiles in odontogenic infections are scarce. This study aimed to analyze the spectrum of pathogens and antimicrobial resistance in clinical isolates from dental and oral-maxillofacial clinical settings in Germany. We analyzed 20,645 clinical isolates (dental practices: n = 5,733; hospitals: n = 14,912) from patients with odontogenic infections using data (2012–2019) from the German Antimicrobial-Resistance-Surveillance (ARS) system. A total of 224 different species from 73 genera were found in clinical isolates from dental practices, and 329 different species from 97 genera were identified in isolates from hospital patients. In both hospitals and dental practices Streptococcus spp. (33 and 36%, respectively) and Staphylococcus spp. (21 and 12%, respectively) were the most frequently isolated microorganisms. In Streptococcus spp. isolates from hospitals, penicillin and aminopenicillin resistance proportions were 8.0% (95%CI 4.7–14.9%) and 6.9% (95%CI 4.7–9.9%), respectively. Substantially lower resistance proportions of penicillin and aminopenicillin were observed in dental practices [2.6% (95%CI 1.4–4.7%) and 2.1% (95%CI 1.1–4.0%), respectively]. Among Staphylococcus aureus isolates from hospital patients methicillin resistance proportions were 12.0% (95%CI 9.7–14.8%), which was higher than in isolates from dental practices (5.8% (95%CI 4.1–8.1%)]. High clindamycin and macrolide resistance proportions (>17%) were observed in Streptococcus spp. and Staphylococcus aureus isolates. In Klebsiella spp. isolates carbapenem resistance proportions were <1%. In sum, substantial antibiotic resistance was observed in isolates from odontogenic infections, which calls for strengthened efforts in antibiotic stewardship and infection prevention and control measures in both hospitals and dental practices.
Introduction
The oral flora, alongside the gastrointestinal microbiome, is one of the most diverse accumulations of microorganisms in the human body. The most common pathogens in the oral cavity in healthy people include Streptococcus spp., Granulicatella spp., and Veillonella spp. (Aas et al., 2005; Dewhirst et al., 2010), which can also cause dental and oral-maxillofacial infections under certain conditions, such as caries, periodontitis, endodontic infections and tonsillitis (Scannapieco, 2013; Wade, 2013; Døving et al., 2020). In addition to these commensals, other bacterial pathogens are associated with infections of the oral cavity, such as Staphylococcus spp. (3) and Candida spp. (Singh et al., 2014). Recent evidence indicates that oral microorganisms are also responsible for systemic diseases (Han and Wang, 2013), such as cardiovascular diseases (Desvarieux et al., 2010; Koren et al., 2011; El Kholy et al., 2015). According to the World Health Organization (WHO), many of the bacterial microorganisms associated with dental and oral-maxillofacial infections are also associated with resistance to antibiotics (Tacconelli et al., 2018). Antibiotic-resistant bacteria are associated with a significant mortality and morbidity (Cassini et al., 2019) and therefore pose a severe health threat worldwide (WHO, 2014).
Data on the spectrum of pathogens and their antibiotic resistance profiles are available in detail for various infections types, such as urinary tract (Tandogdu and Wagenlehner, 2016; Klingeberg et al., 2018) and bloodstream infections (Weiner et al., 2016; CDC, 2019; Diekema et al., 2019; ECDC, 2019; Markwart et al., 2019). Despite the clinical relevance and frequency of dental and oral-maxillofacial infections, there is a lack of recent data on the spectrum of clinical pathogens and associated antimicrobial resistance for those infections, especially from multicenter studies. Such data are necessary for the development of clinical recommendations and guidelines on the treatment dental and oral-maxillofacial infections.
This study therefore aimed to analyze the microbiological profile (i.e., pathogen spectrum and antimicrobial resistance) of clinical isolates from dental and oral-maxillofacial medicine and to compare microbiological profiles between dental practices and hospitals in Germany.
Materials and Methods
Study Design, Data Source and Outcomes
We conducted a retrospective observational study on clinical isolates from dental and oral-maxillofacial medicine from 2012 to 2019, using data retrieved from the German Antimicrobial Resistance Surveillance (ARS) database (Noll et al., 2012). ARS is the national laboratory-based surveillance system for antimicrobial resistance in Germany and a priority area for the German Antimicrobial Resistance Strategy (DART). Laboratories that participate on a voluntary basis report data obtained from routine clinical microbiological testing of isolates from patients treated in hospitals and outpatient care clinics. In addition to results of pathogen identification and antimicrobial susceptibility testing, the ARS data include pseudonymized information on medical facilities such as care level, ward type and geographical location, patient characteristics such as age and gender, and type of specimen. High quality data are assured by checking the database for plausibility during data reporting and regularly validating the data for completeness and consistency.
The outcomes of this study were the (i) proportional distribution of pathogens identified in clinical isolates from dental and oral-maxillofacial clinical settings, stratified by setting (i.e., hospital and outpatient care), and (ii) the antibiotic resistance proportions among all tested clinical isolates of the most common pathogens.
Selection of Isolates
In September 2020, we extracted data (2012–2019) on isolates from dental and oral-maxillofacial clinics (outpatient dental practices or hospitals) from the ARS database. We only included the patients’ first isolate per specimen per quarter in order to avoid including multiple isolates of one patient from one infection episode. Isolates from samples labeled as “screening” were excluded. Only isolates from the following specimen materials were included: Swabs (from abscesses, surgery, wound, tongue, not specified sites and other) and biopsies (from tissue, abscesses, joints, not specified sites, and other).
Study Variables and Definitions
Care settings were categorized into hospital care and ambulatory dental practices. The regional origins of isolates were grouped into five major German regions: Northeast (federal states of Mecklenburg-West Pomerania, Brandenburg, Berlin, Saxony-Anhalt), Southeast (Bavaria, Saxony, Thuringia), Southwest (Hesse, Rhineland-Palatinate, Saarland, Baden-Wurttemberg), West (North Rhine-Westphalia) and Northwest (Lower Saxony, Hamburg, Schleswig-Holstein). Patients’ gender was categorized into female and male. Their ages were grouped into the following categories: 0–19, 20–39, 40–64, >65 years; and were also expressed as medians with interquartile ranges (IQRs).
In order to calculate the proportional distributions of pathogens identified in clinical isolates, we included all isolates and categorized them into the ten most common genera. Antimicrobial resistance profiles were analyzed for Streptococcus spp., Staphylococcus aureus, and Klebsiella spp. if the total number of tested isolates per antibiotic was greater than 100 in each care setting.
Based on recommendations from relevant clinical guidelines (Schöfer et al., 2011; Al-Nawas and Karbach, 2017), the following antibiotics were included in the analyses of antimicrobial profiles: Penicillin, aminopenicillins (amoxicillin, ampicillin), markers for methicillin resistance (oxacillin, flucloxacillin), second-generation cephalosporins (cefuroxim), third-generation cephalosporins (ceftriaxon, cefotaxim, ceftazidim), clindamycin, macrolides (erythromycin, clarithromycin), fluoroquinolones (moxifloxacin, levofloxacin), and carbapenems (imipenem, meropenem, ertapenem). An isolate was considered resistant against an antibiotic group if the susceptibility test results were classified by the laboratories as “resistant” for at least one antibiotic of the antibiotic group. In Germany, the majority of laboratories use the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines for interpreting susceptibility testing results (European Committee on Antimicrobial Susceptibility Testing, 2020).
Statistical Analyses
All statistical analyses were performed using R version 3.6.1 (R Core Team, 2013) and the “survey” package (version 4.0) (Lumley, 2020). Estimates of antibiotic resistance proportions are expressed as percentages with 95% confidence intervals (95% CI) accounting for clustering at hospital / dental practice level using the survey package (Ayobami et al., 2020a, b). In order to study potential differences in antibiotic resistance in isolates from dental practices and hospitals as well as temporal changes in antibiotic resistances, multivariable logistic regression analyses were performed. The following predictors were included: Year of sampling, patient gender, patient age group, German region, and care type. All variables were treated as categorical, except year of sampling, which was treated as a continuous variable. The regression analyses also accounted for clustering at hospital / dental practice level (Lumley, 2020).
Results
Baseline Characteristics
In total, 20,645 clinical isolates from dental and oral-maxillofacial settings were included in the study. These isolates were obtained from 299 outpatient dental practices and 34 hospitals. The baseline characteristics of the analyzed isolates are outlined in Table 1. The majority (72.2%) of isolates were derived from patients treated in hospitals, while the remaining 27.8% were derived from dental practices. The isolates were collected from healthcare facilities in all German regions. In dental practices, the majority of clinical isolates were derived from female patients (female/male ratio = 1.14), while in hospitals, isolates were mainly collected from male patients (female/male ratio: 0.67). The majority of isolates were from middle aged (40–64 years) and elderly patients (>65 years).
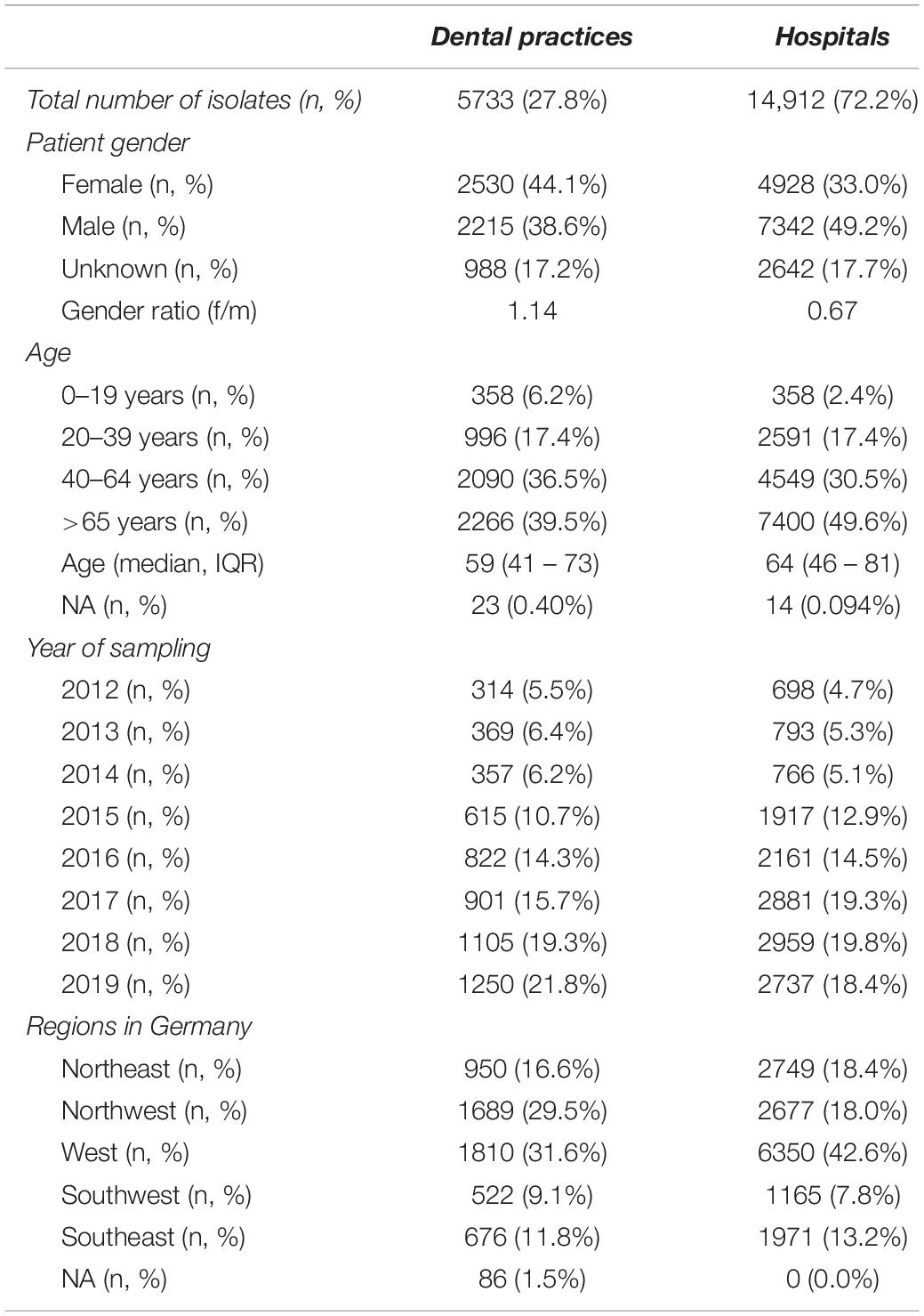
Table 1. Baseline characteristics of clinical isolates from dental and oral-maxillofacial settings in Germany (2012–2019).
Pathogen Spectrum in Dental and Oral-Maxillofacial Medicine
The proportional distributions of the pathogens identified in clinical isolates in dental and oral-maxillofacial settings are shown in Figure 1. A total of 224 different species from 73 genera were found in clinical isolates from dental practices, and 329 different species from 97 genera were identified in isolates from hospital patients.
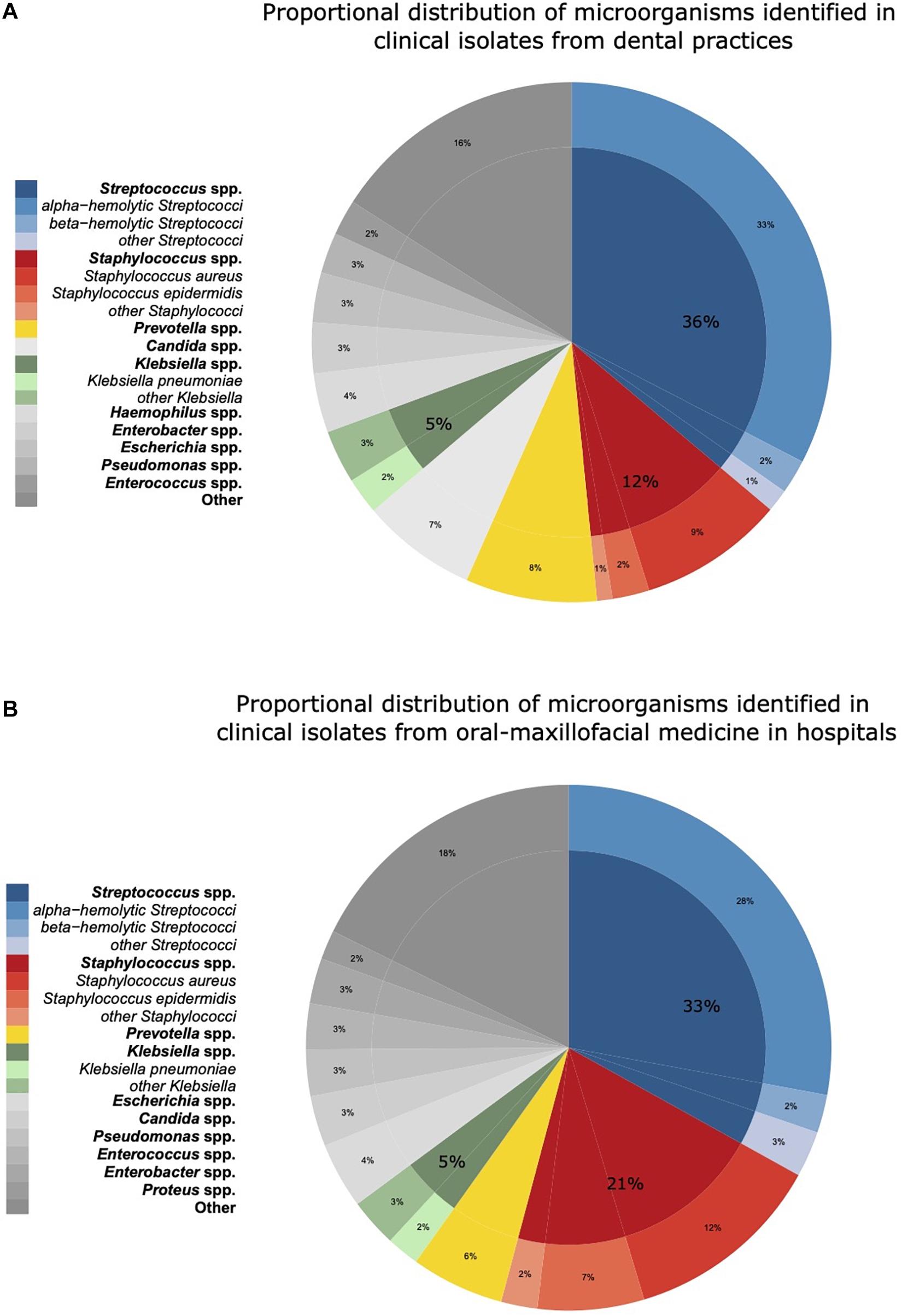
Figure 1. Proportional distribution of genera and species of microorganisms identified in clinical isolates from dental and oral-maxillofacial settings in Germany (2012–2019) stratified by care setting: (A) dental practices and (B) hospitals. Proportional distribution of genera and species among all clinical isolates (dental practices n = 5,733, hospitals n = 14,912) are expressed as mean proportions (%).
In hospitals, Streptococcus spp. was identified as the most abundant pathogen (33.1%), followed by Staphylococcus spp. (21.1%), Prevotella spp. (5.7%), and Klebsiella spp. (5.0%). Within the Streptococcus genus, alpha-hemolytic Streptococci were most frequent in hospitals (27.9%). In contrast, Staphylococcus spp. was less frequently found in dental practices than in hospitals (12.3 vs. 21.1%). Notably, Candida spp. was more frequently identified in isolates from dental practices (8.2 vs. 3.1%). Apart from Staphylococcus spp. and Candida spp., the spectrum of pathogens was similar between isolates from hospitals and dental practices. A comprehensive overview of the pathogen identification results is provided in Supplementary Table 1. Over the course of the study period, the distribution of the major pathogen groups did not systemically change with the exception of Staphylococcus spp. in dental practices. The proportion of Staphylococcus spp. isolated among all isolated pathogens from patients treated in dental practices continuously increased from 9% in 2012–2013 to 15% in 2018–2019.
Antimicrobial Resistance Profiles in Dental and Oral-Maxillofacial Settings
The resistance patterns of Streptococcus spp. isolates from dental and oral-maxillofacial settings for various antibiotics are represented in Figure 2. In Streptococcus spp. isolates from hospital patients, penicillin and aminopenicillin resistance proportions were 8.0% (95%CI 4.7–14.9%) and 6.9% (95%CI 4.7–9.9%), respectively. Substantially lower penicillin and aminopenicillin resistance proportions were observed in Streptococcus spp. isolates from dental practices [2.6% (95%CI 1.4–4.7%) and 2.1% (95%CI 1.1–4.0%), respectively]. A multivariable analysis adjusting for some factors (Supplementary Tables 2, 3) that could influence antimicrobial resistance confirmed that Streptococcus spp. isolates from hospitals have a greater likelihood of exhibiting penicillin and aminopenicillin resistance than isolates from dental practices [adjusted odds ratios: 3.35 (95%CI 1.46–7.72), p = 0.00503 and 3.85 (95%CI 1.82–8.16), p < 0.001].
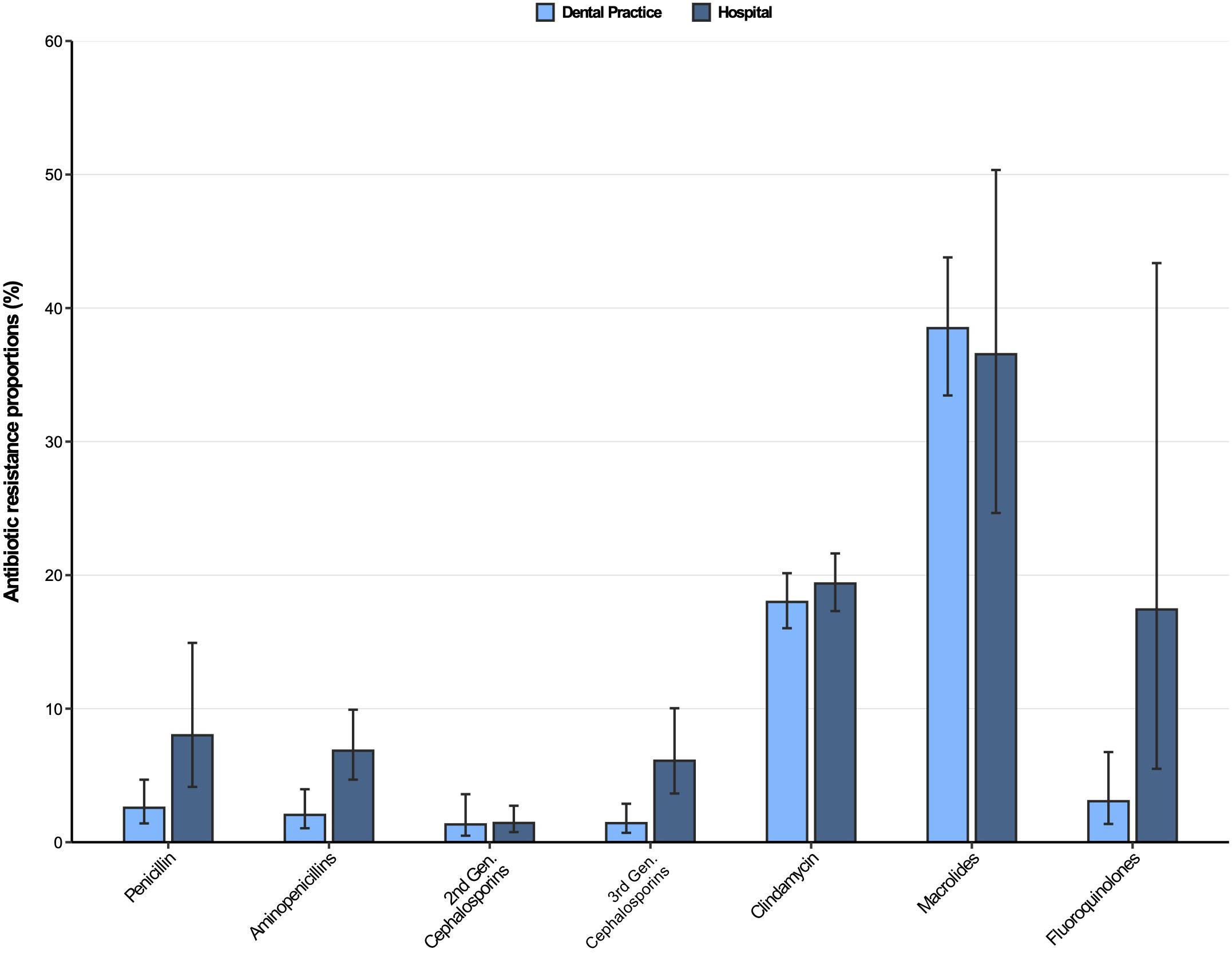
Figure 2. Antibiotic resistance proportions in clinical Streptococcus spp. isolates from dental and oral-maxillofacial settings in Germany (2012–2019) stratified by care setting: Dental practices and hospitals. Resistance proportions with corresponding 95% confidence intervals are expressed as proportions (%) of isolates tested as “resistant” among all isolates that were tested against the respective antibiotic. Total numbers of tested isolates per care setting: dental practice: penicillin: n = 2,016, aminopenicillins: n = 2,004, second-generation cephalosporins: n = 525, third-generation cephalosporins: n = 1,752, clindamycin: n = 2,034, macrolides: n = 1,130, fluoroquinolones: n = 456; hospitals: penicillin: n = 4,883, aminopenicillins: n = 4,099, second-generation cephalosporins: n = 2,496, third-generation cephalosporins: n = 4,491, clindamycin: n = 4,831, macrolides: n = 1,754, fluoroquinolones: n = 1,639.
Resistance proportions in Streptococcus spp. isolates for second-generation and third-generation cephalosporins, as well as fluoroquinolones, were generally low (<7%), except for fluoroquinolone resistance proportion in hospitals [17.4% (95%CI 5.5–43.4%)]. Importantly, third-generation cephalosporins resistance proportions were higher in Streptococcus spp. isolates from patients treated in hospitals compared to those treated in dental practices [6.1% (95%CI 3.7–10.0%) vs. 1.4% (95%CI 0.70–2.9%)], supported by a multivariable analysis [adjusted odds ratio: 6.45 (95%CI 2.91–14.28), p ≤ 0.001] (Supplementary Table 4). Importantly, time trend analyses showed that third-generation as well as second-generation cephalosporin resistances in Streptococcus spp. isolates increased over the course of the study period [adjusted odds ratios: 1.34 (95%CI 1.02–1.76), p = 0.037 and 1.37 (95%CI 1.17–1.61), p < 0.001]. Our findings indicate that there was a relatively high resistance against clindamycin in both hospital and dental practices [19.4% (95%CI 17.3–21.6%) and 18.0% (95%CI 16.0–20.2%)]. High resistance proportions among clinical Streptococcus spp. isolates from hospitals and dental practices were observed for macrolides [36.6% (95%CI 24.7–50.3%) and 38.5% (95%CI 33.5–43.8%)].
Antibiotic resistance profiles for Staphylococcus aureus isolates from dental and oral-maxillofacial settings are displayed in Figure 3. As expected, penicillin and aminopenicillin resistance proportions were very high (>65%) in S. aureus isolates from hospitals and dental practices. The methicillin resistance proportion (i.e., resistance to oxacillin / flucloxacillin) among S. aureus isolates were significantly higher in isolates from patients treated in hospitals compared to those treated in dental practices [12.0% (95%CI 9.7–14.8%) vs. 5.8% (95%CI 4.1–8.1%)]. This finding is also confirmed by a multivariable analysis that shows that S. aureus isolates from hospitals had a greater likelihood to exhibit methicillin resistance compared to isolates from dental practices [adjusted odds ratio: 2.48 (95%CI 1.58–3.90), p < 0.001] (Supplementary Table 5). For clindamycin and macrolides, resistance proportions were relatively high (>17%) in S. aureus isolates from both hospitals and dental practices. Importantly, compared to isolates from hospital patients, fluoroquinolone resistance proportions were much lower in isolates from dental practices [16.6% (95%CI 13.3–20.6%) vs. 9.4% (95%CI 6.2–13.8%)], which is also supported by a multivariable analysis [adjusted odds ratio: 2.28 (95%CI 1.56–3.31), p < 0.001] (Supplementary Table 6). Time trend analyses revealed that fluoroquinolone resistances in S. aureus isolates decreased between 2012 and 2019 [adjusted odds ratio: 0.91 (95%CI 0.86–0.97), p = 0.004].
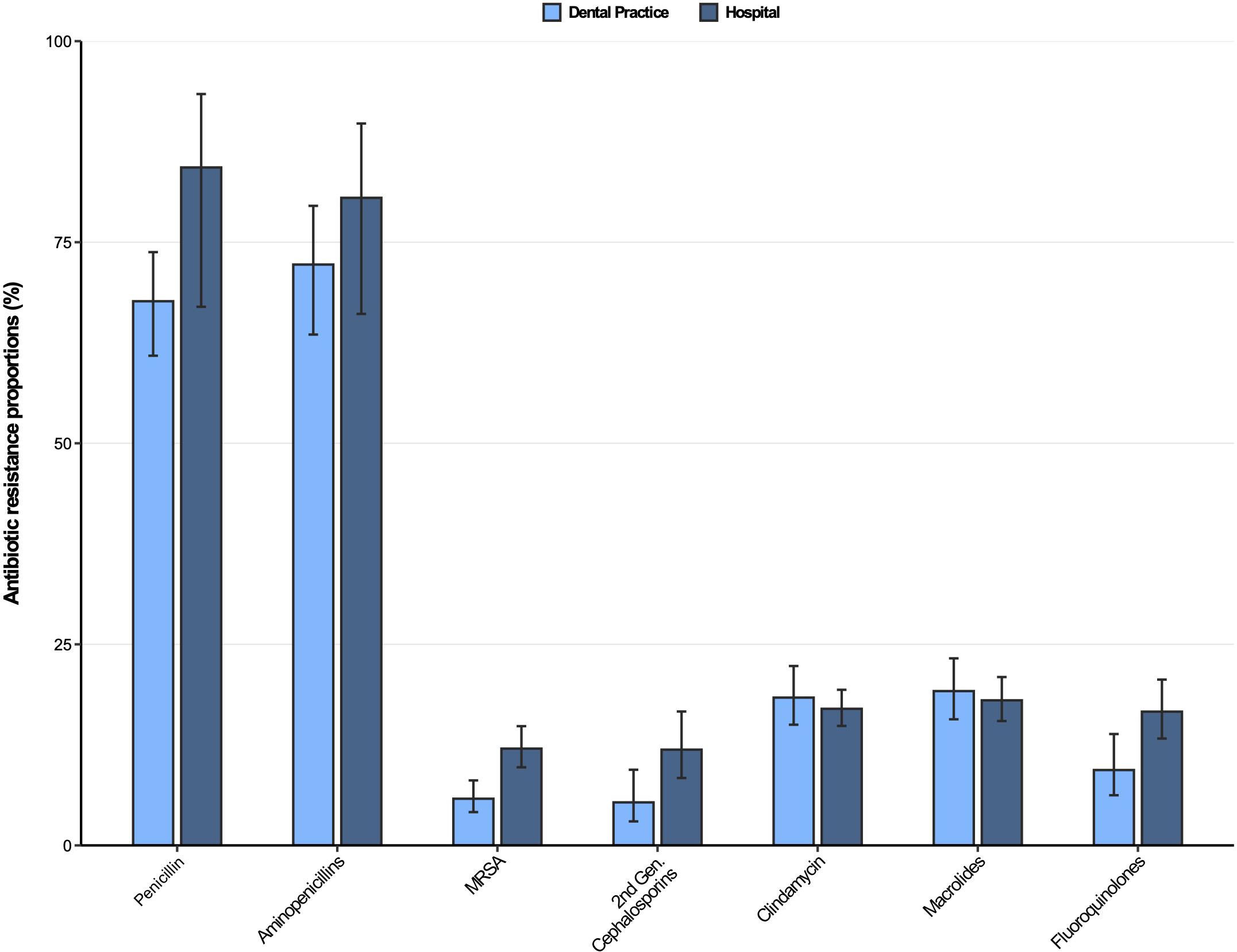
Figure 3. Antibiotic resistance proportions in clinical Staphylococcus aureus isolates from dental and oral-maxillofacial settings in Germany (2012–2019) stratified by care setting: Dental practices and hospitals. Resistance proportions with corresponding 95% confidence intervals are expressed as proportions (%) of isolates tested as “resistant” among all isolates that were tested against the respective antibiotic. Total numbers of tested isolates per care setting: dental practice: Penicillin: n = 470, aminopenicillins: n = 198, methicillin-resistant Staphylococcus aureus: n = 517, second-generation cephalosporins: n = 243, clindamycin: n = 517, macrolides: n = 516, fluoroquinolones: n = 491; hospitals: penicillin: n = 1,751, aminopenicillins: n = 657, methicillin-resistant Staphylococcus aureus: n = 1,828, second-generation cephalosporins: n = 1,008, clindamycin: n = 1,831, macrolides: n = 1,824, fluoroquinolones: n = 1,828.
The antibiotic resistance proportions for Klebsiella spp. isolates from dental and oral-maxillofacial settings are displayed in Figure 4. Moderately high resistance proportions (9–16%) were found for second-generation cephalosporins, while low resistance proportions (<8%) were observed for third-generation cephalosporins, as well as fluoroquinolones in isolates from both hospitals and dental practices. However, an increase of fluoroquinolone resistance in Klebsiella spp. isolates was observed over the course of the study period [adjusted odds ratio: 1.14 (95%CI 1.02–1.27), p = 0.026]. Importantly, carbapenem resistance among Klebsiella spp. isolates was very rare (dental practices: 0%, hospitals 0.13%).
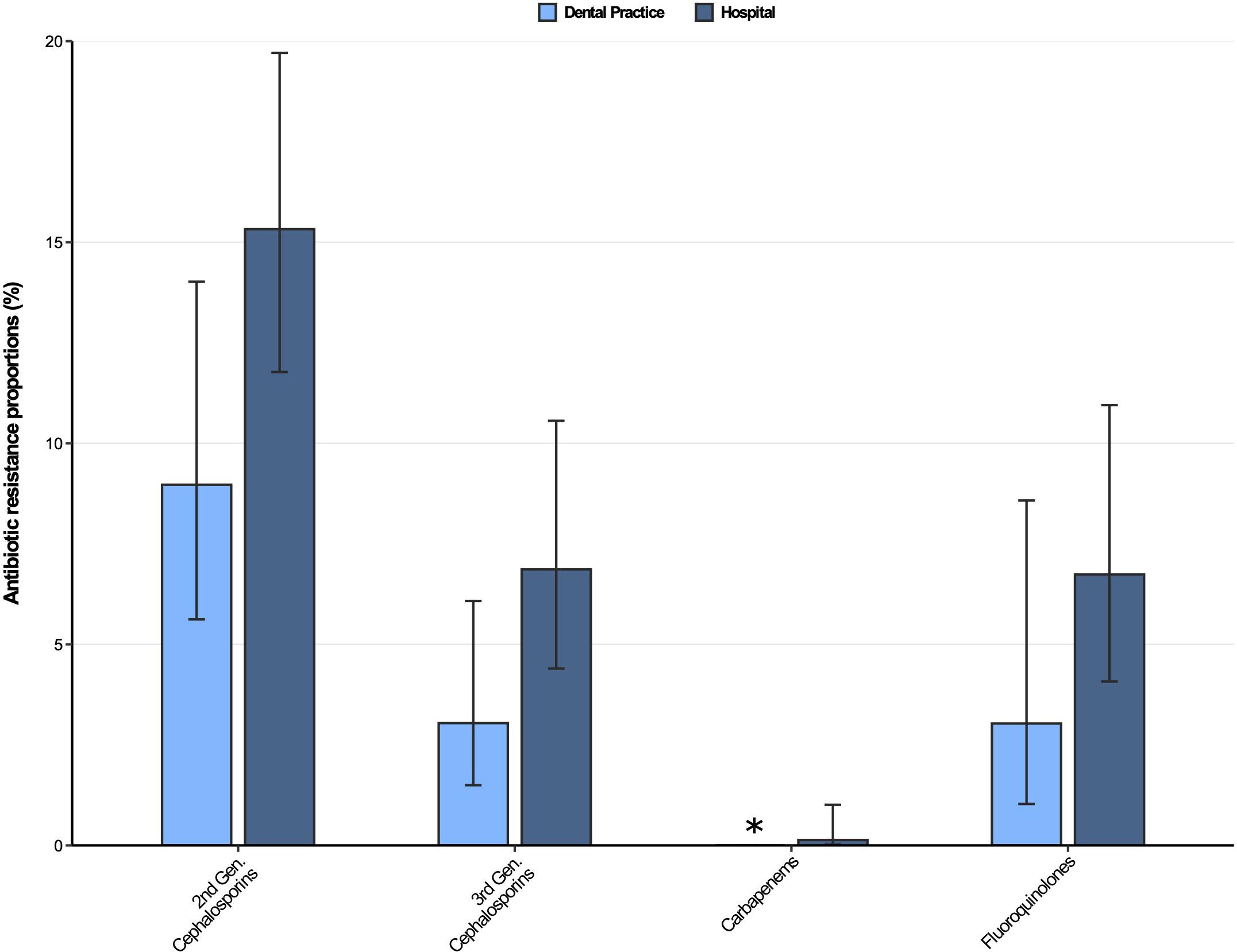
Figure 4. Antibiotic resistance proportions in clinical Klebsiella spp. isolates from dental and oral-maxillofacial settings in Germany (2012–2019) stratified by care setting: Dental practices and hospitals. Resistance proportions with corresponding 95% confidence intervals are expressed as proportions (%) of isolates tested as “resistant” among all isolates that were tested against the respective antibiotic. Total number of tested isolates per care setting: dental practice: second-generation cephalosporins: n = 223, third-generation cephalosporins: n = 296, carbapenems: n = 298, fluoroquinolones: n = 264; hospitals: second-generation cephalosporins: n = 633, third-generation cephalosporins: n = 743, carbapenems: n = 744, fluoroquinolones: n = 727. ∗ All isolates were tested sensible.
Discussion
This study analyzed the spectrum of pathogens and antibiotic resistance profiles in both the outpatient (i.e., dental practices) and hospital sector of dentistry using data from the German Antibiotic-Resistance-Surveillance database. Our study identified 360 individual species from more than 106 different genera in clinical samples from patients with dental and oral-maxillofacial infections, reflecting the highly diverse microbiome of the oral cavity. Oral-maxillofacial infections are often characterized by a mixed growth of anaerobic and aerobic bacteria (Bahl et al., 2014). Although some genera / species were exclusively isolated in outpatient dental practices or hospitals, in more than 85% of the included isolates, genera / species were identified that were found in both healthcare settings. Our results show that the most frequently found pathogens were gram-positive cocci, especially Streptococcus spp. and Staphylococcus spp. Alpha-hemolytic Streptococci, which also include the viridans group of Streptococci (Facklam, 2002), are the largest individual group of pathogens to be detected in clinical samples from the oral cavity. In line with our findings, studies from other parts of the world also found that Streptococci, especially species from the viridans group, are frequent causes of odontogenic infections (Farmahan et al., 2014; Al-Nawas and Karbach, 2017; Heim et al., 2017; Haque et al., 2019). Streptococci are commensals typically found in the oral cavity, but they are also commonly found in intestinal tract and genital area in healthy individuals. However, under conditions where the microbiota balance is disrupted, Streptococci can cause infections, including dental and oral-maxillofacial infections.
The second most frequent pathogen group found in this study were Staphylococci. Until recently, the Staphylococcus species were not considered member of the oral flora. Heim et al. (2017) investigated head and neck space infections of odontogenic origin in 2018 and found that Staphylococcus species are one of the four most commonly isolated bacteria. Smith and colleagues (Smith et al., 2001) noted that the Staphylococcus species are a more frequent colonizer of the oral cavity than previously thought. Nevertheless, its role as a transient member of the oral microbiome or a possible pathogen is not fully understood yet (Koukos et al., 2015).
Interestingly, while the Candida spp. were not prominently encountered in clinical samples from hospital patients with odontogenic infections, these fungal pathogens were found in 6% of all clinical samples from patients treated in outpatient dental practices, underlining their importance in community settings. Although oral candidiasis is not harmful to otherwise healthy people, it can be more severe and difficult to control in people who are immune-compromised (Ostrosky-Zeichner and Pappas, 2006).
Our analyses of antibiotic resistance profiles shows that clinical Streptococcus spp. and S. aureus isolates from both hospital and dental practices show relatively high proportions of clindamycin resistance (17–19%). This finding is of clinical importance, since clindamycin is one of the most frequently prescribed antibiotics by dental practitioners in Germany (Halling et al., 2017). Notably, the German guidelines on odontogenic infections recommends penicillin and amoxicillin for empiric antibiotic therapy, while clindamycin is only recommended in cases of penicillin allergy (Al-Nawas and Karbach, 2017). In line with our findings, Heim et al. (2017) and Poeschl et al. (2010) reported similar clindamycin resistance rates for Streptococci and S. aureus in Germany and Austria. It is encouraging that resistance proportions against the recommended first-line antibiotics penicillin and aminopenicillins (including amoxicillin) are very low (<3%) in clinical Streptococcus spp. isolates from dental practices.
Although significantly higher resistance rates were found in Streptococcus spp. isolates from hospitals, penicillin and aminopenicillin resistance proportions remain moderate (∼7–8%), but continuous efforts in antibiotic stewardship are needed to preserve the clinical effectiveness of these antibiotics. As expected, S. aureus shows very high resistance proportions against penicillin and aminopenicillin (>65%). Together with its relatively high clindamycin and macrolide resistance proportions (>17%), treatment options are very limited, which is concerning since our results indicate that S. aureus is frequently found in odontogenic infections. Importantly, in our study on dental and oral-maxillofacial infections, the proportion of β-lactam penicillinase resistance (i.e., methicillin resistance) in S. aureus isolates from dental practices (6%) and hospitals (12%) are similar to MRSA proportions observed in 2018 in other clinical samples from outpatient and hospital settings in Germany. In general, there has been a decrease in the MRSA proportion in all S. aureus isolates from all specimen materials from 2010 to 2018. In hospitals, the MRSA proportion declined from 23.8 to 13.3%, and in the outpatient sector from 13.0 to 7.7% (Layer et al., 2019).
Both Streptococcus spp. and S. aureus show relatively high resistance proportions (>18%) against macrolides in hospitals as well as in dental practices. These findings are somewhat contrary to other studies, which only found resistance proportions up to 13% in the hospital setting (Poeschl et al., 2010) for Streptococcus species.
Although our dataset on antibiotic resistance profiles is limited for Klebsiella spp. isolates, our data indicate moderate to low resistance proportions for cephalosporins and fluoroquinolones. Importantly, only one carbapenem-resistant isolate was found in a hospital sample, which is in line with findings from Koppe et al. (2018), who found that less than 1% of all Klebsiella spp. isolates from German hospitals were non-susceptible to carbapenems. In line with these results, low carbapenem resistance rates were also found for other Gram-negative bacteria in Germany, such as Acinetobacter baumannii (Said et al., 2021) and E. coli (ECDC, 2020).
Our results demonstrate that frequent Gram-positive pathogens isolated from clinical odontogenic samples show substantial antibiotic resistance against important antibiotics. Moreover, although resistances against third and secondary cephalosporins in Streptococcus spp. were relatively low, it is worrying that resistances against these antibiotic classes increased over the study period, which underlines the importance of continuous efforts in antibiotic stewardship. In Germany, about 10% of all antibiotics are prescribed by dentists (Halling et al., 2017). It is estimated that approximately one-third of all outpatient antibiotic prescriptions are unnecessary (Fleming-Dutra et al., 2016) and thereby contribute to the development of antibiotic resistance. The potential overuse of antibiotics (e.g., in antibiotic prophylaxis) is rarely addressed in dentistry, but a recent study by Löffler and Böhmer (2017) showed that a combination of audit and feedback and education on antibiotics could help as an intervention in hospital dental care and outpatient dental settings.
When interpreting our data, it is important to consider that microbiological sampling is not routinely performed in the management of patients with clinical infections in outpatient dental practices. It is likely that the included isolates from dental practices represent infections episodes with higher severity, chronic progressions and/or complications, such as treatment failure in first-line antibiotic therapy. Therefore, the observed pathogen spectrum and associated antibiotic resistances may not be generalizable for all clinical infections treated in dental practices. In addition, information on any antibiotic treatment before sampling is lacking, which can also bias the data.
Strengths and Limitations
This study is based on data from the ARS database, which is the largest and most representative surveillance system for pathogen identifications and antibiotic resistance in Germany (Noll et al., 2012). To our knowledge, this multicenter study is the most comprehensive study on the spectrum of pathogens and antibiotic resistance profiles in Europe, and includes more than 20,700 clinical isolates from more than 12,400 patients with dental and oral-maxillofacial infections. In contrast to many previous studies, we included data from patients treated in hospital and dental practices.
However, it is also important to consider the limitations of the study. Firstly, participation in ARS is voluntary and therefore laboratories and hospitals are not evenly distributed in Germany, which may limit the representativeness of the data. However, isolates were from all major regions in Germany, without disproportionate under-representation in any particular region.
Secondly, underlying diagnoses are not collected in ARS, so we can only assume that clinical specimens represent infectious diseases. We excluded all isolates labeled as screening samples, but it is possible that some of the analyzed isolates actually represent screening samples that were not assigned as such by the hospital or laboratory. Importantly, the identified pathogens in the clinical samples may not be the actual infectious agents, but may represent commensals that “contaminated” the microbiological samples drawn from the actual infection site. In addition, about 50% of the species of the oral flora cannot be grown in vitro and therefore cannot be detected in standard microbiological analyses and their potential role in infectious disease remains unknown (Siqueira and Rôças, 2013).
Importantly, microbiological sampling procedures (i.e., aerobic or aerobic sampling) also impact the observed the pathogen spectrum. Although oral-maxillofacial infections are often associated with a mixed growth of anaerobic and aerobic bacteria, anaerobic bacteria are largely absent in our data set, with the exception of Prevotella spp., which might be explained by aerobic sampling methods in routine diagnostics. No information on the microbiological sampling method is available in the ARS database.
Conclusion
Our study shows that dental and oral-maxillofacial infections in Germany are associated with a wide range of different pathogens, and that Streptococci (especially alpha-hemolytic Streptococci) and Staphylococci (especially S. aureus) are the most frequently identified pathogens in hospitals and dental practices. Both Streptococcus spp. and S. aureus show substantial resistance against important antibiotics, which calls for strengthened efforts in antibiotic stewardship and infection prevention and control measures in both hospitals and dental practices.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
AM, AR, TE, and RM were responsible for conceptualization of the study and formulation of the research goals and aims. AM, NW, and RM developed the statistical methodology and models. IN and MF established and maintain the ARS database and continuously validate the data. AM and RM performed the statistical analysis. AM, AR, BA-N, and RM wrote the original draft. All authors reviewed, commented the draft and gave input on editing, read, and approved the final manuscript.
Funding
This work was supported by the Robert Koch Institute’s internal funds. The institute is a Federal Institute within the portfolio of the German Ministry of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the laboratories and hospitals for contributing data to this analysis. We thank our colleagues at the Robert Koch Institute for their input during this study, namely, Angelina Taylor and Hans-Peter Blank.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.676108/full#supplementary-material
References
Aas, J. A., Paster, B. J., Stokes, L. N., Olsen, I., and Dewhirst, F. E. (2005). Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 43, 5721–5732. doi: 10.1128/jcm.43.11.5721-5732.2005
Al-Nawas, B., and Karbach, J. (2017). S3 Leitlinie: Odontogene Infektionen Online verfügbar unter. Available Online at: https://wwwawmforg/uploads/tx_szleitlinien/007-006l_S3_Odontogene_Infektionen_2017-12pdf.
Ayobami, O., Willrich, N., Reuss, A., Eckmanns, T., and Markwart, R. (2020a). The ongoing challenge of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Europe: an epidemiological analysis of bloodstream infections. Emerging Microb. Infect. 9, 1180–1193. doi: 10.1080/22221751.2020.1769500
Ayobami, O., Willrich, N., Suwono, B., Eckmanns, T., and Markwart, R. (2020b). The epidemiology of carbapenem-non-susceptible Acinetobacter species in Europe: analysis of EARS-Net data from 2013 to 2017. Antimicrob. Resist. Infect. Control. 9, 1–10.
Bahl, R., Sandhu, S., Singh, K., Sahai, N., and Gupta, M. (2014). Odontogenic infections: microbiology and management. Contemporary Clin. Dentist. 5:307. doi: 10.4103/0976-237x.137921
Cassini, A., Högberg, L. D., Plachouras, D., Quattrocchi, A., Hoxha, A., Simonsen, G. S., et al. (2019). Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect. Dis. 19, 56–66.
CDC. (2019). Antibiotic Resistance Threats in the United States. In: U.S. Department of Health and Human Services. Available Online at: www.cdc.gov/DrugResistance/Biggest-Threats.html2019
Desvarieux, M., Demmer, R. T., Jacobs, D. R. Jr., Rundek, T., Boden-Albala, B., Sacco, R. L., et al. (2010). Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J. Hypertension. 28, 1413–1421. doi: 10.1097/hjh.0b013e328338cd36
Dewhirst, F. E., Chen, T., Izard, J., Paster, B. J., Tanner, A. C., Yu, W. H., et al. (2010). The human oral microbiome. J. Bacteriol. 192, 5002–5017.
Diekema, D. J., Hsueh, P. R., Mendes, R. E., Pfaller, M. A., Rolston, K. V., Sader, H. S., et al. (2019). The microbiology of bloodstream infection: 20-Year trends from the SENTRY antimicrobial surveillance program. Antimicrob. Agents chemother. 63:e00355–19.
Døving, M., Handal, T., and Galteland, P. (2020). Bacterial odontogenic infections. Tidsskrift Den Norske Legeforening 140:778. doi: 10.4045/tidsskr.19.0778
ECDC. (2019). Surveillance of antimicrobial resistance in Europe 2018. Stockholm: European Centre for Disease Prevention and Control.
ECDC. (2020). Antimicrobial resistance in the EU/EEA (EARS-Net) - Anual Epidemiological Report 2019. Stockholm: European Centre for Disease Prevention and Control.
El Kholy, K., Genco, R. J., and Van Dyke, T. E. (2015). Oral infections and cardiovascular disease. Trends Endocrinol. Metabol. 26, 315–321.
European Committee on Antimicrobial Susceptibility Testing. (2020). Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0. Available Online at: http://www.eucast.org. http://www.eucast.org2020
Facklam, R. (2002). What happened to the Streptococci: overview of taxonomic and nomenclature changes. Clin. Microbiol. Rev. 15, 613–630. doi: 10.1128/cmr.15.4.613-630.2002
Farmahan, S., Tuopar, D., and Ameerally, P. J. (2014). The clinical relevance of microbiology specimens in head and neck space infections of odontogenic origin. Br. J. Oral Maxillofacial Surg. 52, 629–631. doi: 10.1016/j.bjoms.2014.02.027
Fleming-Dutra, K. E., Hersh, A. L., Shapiro, D. J., Bartoces, M., Enns, E. A., and File, T. M. Jr., et al. (2016). Prevalence of inappropriate antibiotic prescriptions among US Ambulatory care visits, 2010-2011. JAMA 315, 1864–1873. doi: 10.1001/jama.2016.4151
Halling, F., Neff, A., Heymann, P., and Ziebart, T. (2017). Trends in antibiotic prescribing by dental practitioners in Germany. J. Cranio-Maxillofacial Surg. 45, 1854–1859. doi: 10.1016/j.jcms.2017.08.010
Han, Y., and Wang, X. (2013). Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J. Dental Res. 92, 485–491. doi: 10.1177/0022034513487559
Haque, M., Sartelli, M., and Haque, S. Z. (2019). Dental infection and resistance—global health consequences. Dentistry J. 7:22. doi: 10.3390/dj7010022
Heim, N., Faron, A., Wiedemeyer, V., Reich, R., and Martini, M. (2017). Microbiology and antibiotic sensitivity of head and neck space infections of odontogenic origin. Differences in inpatient and outpatient management. J. Cranio-Maxillofacial Surg. 45, 1731–1735. doi: 10.1016/j.jcms.2017.07.013
Klingeberg, A., Noll, I., Willrich, N., Feig, M., Emrich, D., Zill, E., et al. (2018). Antibiotic-Resistant E. coli in uncomplicated community-acquired urinary tract infection. Dtsch Arztebl Int. 115, 494–500.
Koppe, U., von Laer, A., Kroll, L. E., Noll, I., Feig, M., Schneider, M., et al. (2018). Carbapenem non-susceptibility of Klebsiella pneumoniae isolates in hospitals from 2011 to 2016, data from the German Antimicrobial Resistance Surveillance (ARS). Antimicrob. Resist. Infect. Control. 7:71.
Koren, O., Spor, A., Felin, J., Fåk, F., Stombaugh, J., Tremaroli, V., et al. (2011). Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. 108, 4592–4598. doi: 10.1073/pnas.1011383107
Koukos, G., Sakellari, D., Arsenakis, M., Tsalikis, L., Slini, T., and Konstantinidis, A. (2015). Prevalence of Staphylococcus aureus and methicillin resistant Staphylococcus aureus (MRSA) in the oral cavity. Arch. Oral Biol. 60, 1410–1415.
Layer, F., Strommenger, B., Cuny, C., Noll, I., Eckmanns, T., and Werner, G. (2019). Häufigkeit, eigenschaften und Verbreitung von MRSA in deutschland–Zur situation 2017/2018. Epid. Bull. 42, 437–442.
Löffler, C., and Böhmer, F. (2017). The effect of interventions aiming to optimise the prescription of antibiotics in dental care—A systematic review. PLoS One. 12:e0188061. doi: 10.1371/journal.pone.0188061
Markwart, R., Willrich, N., Haller, S., Noll, I., Koppe, U., Werner, G., et al. (2019). The rise in vancomycin-resistant Enterococcus faecium in Germany: data from the German Antimicrobial Resistance Surveillance (ARS). Antimicrob. Resist. Infect. Control. 8:147.
Noll, I., Schweickert, B., Abu, M. S., Feig, M., Claus, H., and Eckmanns, T. (2012). Antimicrobial resistance in Germany. Four years of antimicrobial resistance surveillance (ARS). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 55, 1370–1376. doi: 10.1007/s00103-012-1559-3
Ostrosky-Zeichner, L., and Pappas, P. G. (2006). Invasive candidiasis in the intensive care unit. Critical Care Med. 34, 857–863. doi: 10.1097/01.ccm.0000201897.78123.44
Poeschl, P. W., Spusta, L., Russmueller, G., Seemann, R., Hirschl, A., Poeschl, E., et al. (2010). Antibiotic susceptibility and resistance of the odontogenic microbiological spectrum and its clinical impact on severe deep space head and neck infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 110, 151–156. doi: 10.1016/j.tripleo.2009.12.039
R Core Team. (2013). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Said, D., Willrich, N., Ayobami, O., Noll, I., Eckmanns, T., and Markwart, R. (2021). The epidemiology of carbapenem resistance in Acinetobacter baumannii complex in Germany (2014–2018): an analysis of data from the national Antimicrobial Resistance Surveillance system. Antimicrob. Resist. Infec. Control. 10, 1–13.
Scannapieco, F. A. (2013). The oral microbiome: its role in health and in oral and systemic infections. Clin. Microbiol. Newslett. 35, 163–169. doi: 10.1016/j.clinmicnews.2013.09.003
Schöfer, H., Bruns, R., Effendy, I., Hartmann, M., Jappe, U., Plettenberg, A., et al. (2011). S2k+ IDA Leitlinie: Diagnostik und Therapie Staphylococcus aureus bedingter Infektionen der Haut und Schleimhäute. Staphylococcus aureus bedingte Infektionen. 23:2015.
Singh, A., Verma, R., Murari, A., and Agrawal, A. (2014). Oral candidiasis: an overview. J. Oral Maxillofacial Pathol. 18:S81.
Siqueira, J. F. Jr., and Rôças, I. N. (2013). As-yet-uncultivated oral bacteria: breadth and association with oral and extra-oral diseases. J. Oral Microbiol. 5:21077. doi: 10.3402/jom.v5i0.21077
Smith, A., Jackson, M., and Bagg, J. (2001). The ecology of Staphylococci in the oral cavity: a review. J. Med. Microbiol. 50, 940–946.
Tacconelli, E., Carrara, E., Savoldi, A., Harbarth, S., Mendelson, M., Monnet, D. L., et al. (2018). Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18, 318–327.
Tandogdu, Z., and Wagenlehner, F. M. (2016). Global epidemiology of urinary tract infections. Curr. Opin. Infect. Dis. 29, 73–79. doi: 10.1097/qco.0000000000000228
Weiner, L. M., Webb, A. K., Limbago, B., Dudeck, M. A., Patel, J., Kallen, A. J., et al. (2016). Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention, 2011-2014. Infect. Control Hospital Epidemiol. 37, 1288–1301. doi: 10.1017/ice.2016.174
Keywords: antimicrobial resistance, AMR, surveillance, pathogen spectrum, odontogenic infections, dental care, AMR in oral-maxillofacial infections
Citation: Meinen A, Reuss A, Willrich N, Feig M, Noll I, Eckmanns T, Al-Nawas B and Markwart R (2021) Antimicrobial Resistance and the Spectrum of Pathogens in Dental and Oral-Maxillofacial Infections in Hospitals and Dental Practices in Germany. Front. Microbiol. 12:676108. doi: 10.3389/fmicb.2021.676108
Received: 04 March 2021; Accepted: 10 May 2021;
Published: 02 June 2021.
Edited by:
Kristina Kadlec, Independent Researcher, Wunstorf, GermanyReviewed by:
Mehmet Demirci, Kırklareli University, TurkeySueli Fumie Yamada-Ogatta, State University of Londrina, Brazil
Copyright © 2021 Meinen, Reuss, Willrich, Feig, Noll, Eckmanns, Al-Nawas and Markwart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Annika Meinen, bWVpbmVuYW5AcmtpLmRl
 Annika Meinen1*
Annika Meinen1* Niklas Willrich
Niklas Willrich