- 1Key Laboratory of Protein Chemistry and Developmental Biology of Fish of Ministry of Education, Hunan Provincial Key Laboratory of Animal Intestinal Function and Regulation, Hunan International Joint Laboratory of Animal Intestinal Ecology and Health, Hunan Normal University, Changsha, China
- 2Tangrenshen Group Co., Ltd., Zhuzhou, China
- 3CAS Key Laboratory of Agro-ecological Processes in Subtropical Region, Hunan Provincial Key Laboratory of Animal Nutritional Physiology and Metabolic Process, National Engineering Laboratory for Pollution Control and Waste Utilization in Livestock and Poultry Production, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, China
- 4CAS Key Laboratory of Quantitative Engineering Biology, Shenzhen Institute of Synthetic Biology, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
Lactic acid bacteria (LAB) are vital probiotics in the food processing industry, which are widely spread in food additives and products, such as meat, milk, and vegetables. Pediococcus pentosaceus (P. pentosaceus), as a kind of LAB, has numerous probiotic effects, mainly including antioxidant, cholesterol-lowering, and immune effects. Recently, the applications in the probiotic- fermentation products have attracted progressively more attentions. However, it is necessary to screen P. pentosaceus with abundant functions from diverse sources due to the limitation about the source and species of P. pentosaceus. This review summarized the screening methods of P. pentosaceus and the exploration methods of probiotic functions in combination with the case study. The screening methods included primary screening and rescreening including gastric acidity resistance, bile resistance, adhesion, antibacterial effects, etc. The application and development prospects of P. pentosaceus were described in detail, and the shortcomings in the practical application of P. pentosaceus were evaluated to make better application of P. pentosaceus in the future.
Introduction
Owing to the development of antibiotic resistance and the potential damage to human health by chemical food additives, there is a cumulative need for natural food additives (O’Connor et al., 2020). Lactic acid bacteria (LAB) have been used in a wide range of fields for thousands of years as the natural fermentors and producers of probiotic factors, such as food, medicine, and feed. Admittedly, they are a type of food-grade microorganisms non-toxicity and harmless for human (Ouwehand, 1999). Additionally, LABs, with efficient antibacterial, antioxidant, cholesterol-lowering, and immune activity, are available for improving food flavor and enhancing food nutrition in food processing (Erten et al., 2014). Given above advantages, LABs are progressively widespread in the processing of various food, including dairy products, bread, pickles, fruit and vegetable juice, etc. For example, the use of LAB bacteriocins is becoming prominent in meat preservation as the preservatives succedaneum including nitrite (Nanasombat et al., 2017). LABs are a good choice to maintain food flavor and avoid the loss of nutritional components in the products processing (Gutiérrez-Cortés et al., 2018). The genera of Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, and Streptococcus are imperative members of LAB. The genus of Lactobacillus was commonly used as probiotics, since they were believed ideal members of the gut microbiota with a good safety profile in the previous study (Shokryazdan et al., 2017). However, the main focus was frequently on antibacterial activity in most existing studies and LAB were limited in food types and application as a natural food additive. Therefore, there is an accumulative need to find novel probiotics with well-probiotic activities.
Numerous studies confirmed that LAB had potential application value, including Pediococcus pentosaceus (P. pentosaceus). P. pentosaceus belongs to family Streptococcaceae, genus Pediococcus, and is a kind of LAB because it can produce lactic acid (Chen et al., 2020). The individual of P. pentosaceus is spherical in pairs or quadruplets, and an immotile bacterium belonging to facultative anaerobic Gram-positive bacteria. Previous study confirmed that P. pentosaceus was inherent in naturally fermented products and could have active functions in product quality, food safety, and production efficiency by preparing into a mixed fermentor with other bacteria (Balakrishnan and Agrawal, 2014; Jang et al., 2015; Gong and Qi, 2020; Montemurro et al., 2020; Xu et al., 2021). In addition, many studies reported that P. pentosaceus had probiotic functions including anti-inflammation, anti-cancer, antioxidant, detoxification, and cholesterol-lowering (Zhao et al., 2012; Thirabunyanon and Hongwittayakorn, 2013; Sellamani et al., 2016; Asami et al., 2017; Kim et al., 2019). However, the use of LAB poorly supports the diverse demands in food processing. More kinds of probiotic strains are in demand, and P. pentosaceus is the one that may make sense. Given the good application value and probiotic effect of P. pentosaceus, the identification and applications of P. pentosaceus in the food industry have attracted more attention.
To make better use of P. pentosaceus in the food industry and remain probiotic effects in human body to a large extent, several researchers from diverse countries had investigated on the probiotic potentials of P. pentosaceus in local-derived foods. It is known that the diversity of strains may contribute to dissimilar fermentation products with various probiotic functions. For instance, some subtypes of P. pentosaceus can produce common antibacterial substances, such as organic acids and inorganic substances with bacteriostasis effect, and a small number can conduce to the synthesis of substances which could inhibit the growth of other microorganisms, such as proteins, alcohols, and lipids (Shukla and Goyal, 2014; Sellamani et al., 2016; Nanasombat et al., 2017; Ghosh et al., 2019). According to the genomic information from 41 P. pentosaceus strains documented in NCBI, there was no obvious clustering, suggesting that high genetic diversity of the strains and the characteristic of specificity (Jiang et al., 2020). The screening methods for P. pentosaceus must be different and tailored based on individual characteristics and dissimilar product applications (Jiang et al., 2020). However, the limitation about the source and species of P. pentosaceus prompts us to screen strains well-probiotic activities from different sources.
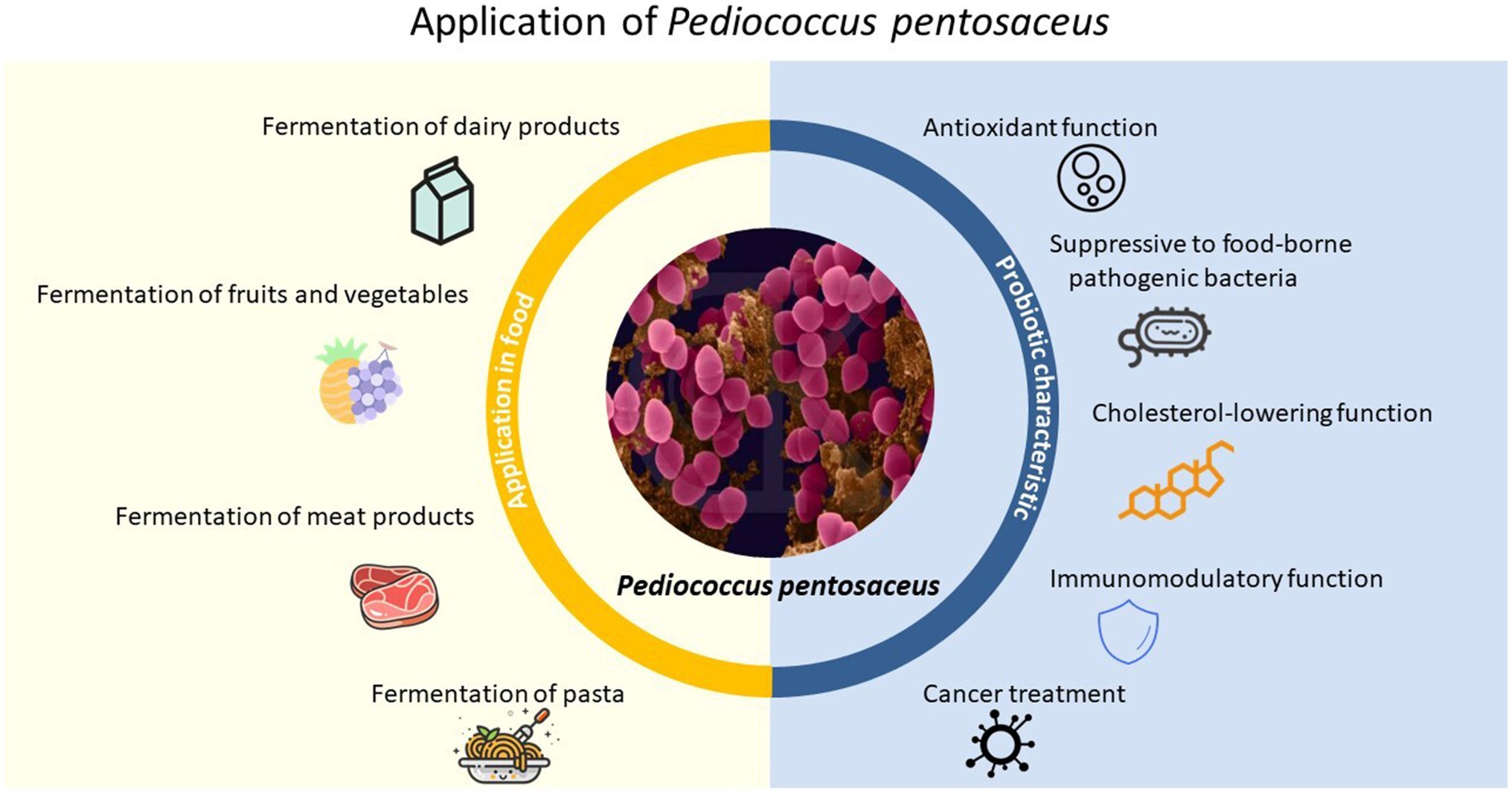
Although more and more studies have found that P. pentosaceus has very important uses, there is still a lack of systematic and pertinent articles on the screening and application of P. pentosaceus. Based on these situations, we have consulted a large number of literature and in order to better apply P. pentosaceus in various aspects and provide a comprehensive and concise description for researchers to conduct further research. In this review, the relevant researches were described in recent years, along with the analysis and summarization of the screening and e probiotic effects of P. pentosaceus involving a series of in vivo and in vitro studies. The obtained excellent strains could provide target strains for production practice and also bring more options for the preparation of new food additives in the future. Applications of P. pentosaceus in the food processing industry and its potential probiotic characteristic were also introduced (Figure 1).
P. pentosaceus Strain Screening
The Sources of P. pentosaceus
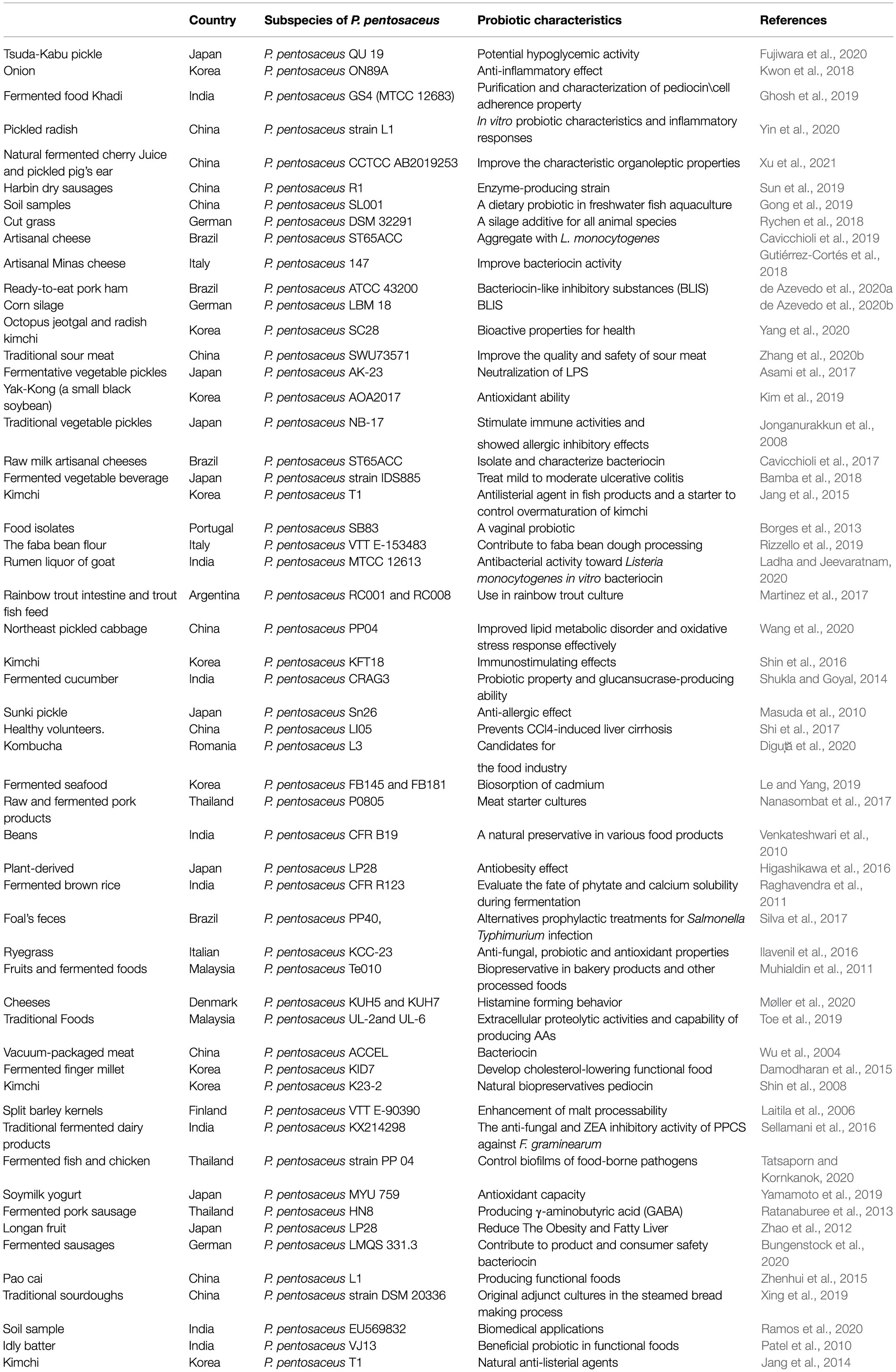
At present, researchers from different countries have isolated P. pentosaceus from different sources. The research area reviewed in this article includes 15 countries, and 65 strains of P. pentosaceus have been isolated and identified, of which the main source of isolation is food (53 strains, 81.6%), especially fermented food (41 strains, 63.1%). And most of the isolated P. pentosaceus were confirmed to have probiotic effects (Table 1). As a preferred source for separation, food-derived P. pentosaceus has the advantage of being safer and more stable. For example, P. pentosaceus in common fermented foods in daily life, such as pickles, dairy products, and sausages, are often used as fermentation initiators. However, the P. pentosaceus derived from human intestines or feces have less been studied, but they are likely to be more adapted to the human intestinal environment and exert better probiotic effects. This suggests that the sources of P. pentosaceus should be more extensive in the future.
Isolation and Culture and Preliminary Screening
The conventional screening method for probiotics is mainly based on different kinds of selective media, with a certain screening factor selected according to the use purpose. For example, P. pentosaceus with pathogen-suppressive effect can be screened by the inhibition zone method using an Oxford Cup, and strains with antioxidant effect can be screened by monitoring the survival outcome after a culture with an addition of H2O2 (Zhang et al., 2020a).
Currently, a combination of conventional screening and molecular biological identification is frequently used to comprehensively determine the type of probiotics from the morphological, biochemical and molecular levels. (Chen et al., 2020) obtained P. pentosaceus with in vitro antibacterial effect from sow milk. They firstly inoculated sheep milk samples into a culture medium, followed by purification of the single-strain isolates using the repeated streaking method; and agar diffusion assay was then conducted to test antibacterial activity; following that, DNA isolation and PCR amplification were performed using the universal primers, and the strains were identified by 16S rDNA gene sequence homology analysis.
For strains that exert probiotic effects by metabolites, additional growth curve and acid production curve analysis should be performed. The typical growth curve of microorganisms is divided into four periods: lag phase (adaptation phase), logarithmic phase, stable phase, and decay phase(Prescott). In the development of general microbial preparations, bacterial culture in the middle and late logarithmic phases and stable phases are often collected. Secondary metabolites, such as bacteriocins and extracellular polysaccharides, are often produced in the stationary phase. By understanding the growth curve of the strain, it is possible to cultivate a bacterial solution with an appropriate growth time. Yanfei et al. (2019) found that the growth rate of P. pentosaceus began to enter the logarithmic growth phase at about 4 h and reached a stable phase after 12 h of culture, and its growth slowed down. Moreover, during the adaptation and logarithmic phases of P. pentosaceus, the pH value in the culture medium has been declining, from 5.64 to 4.14, and stabilized at 12 h. It still maintains stable growth under acidic conditions, indicating that P. pentosaceus has strong acid-tolerant ability and can survive under lower pH conditions.
Secondary Screening
There are some other requirements for probiotics being applicable. A list provided by Food and Agriculture Organization/World Health Organization (FAO/WHO) describes some commonly used in vitro tests for screening and identification of potentially applicable probiotic strains, including analyses in I) anti-gastric acidity; II) activity of bile salt hydrolase (BSH) and resistance to bile salt; III) adhesive ability to mucus and/or human epithelial cells and cell lines to decrease adherence of pathogenic bacteria; IV) antibacterial and antagonistic activity against potential pathogenic bacteria (Shokryazdan et al., 2017). It should be noted that these tests for screening are not specific to P. pentosaceus, but are for probiotics. However, in order to ensure safety and reliability, P. pentosaceus utilized in foods must meet the above requirements, a secondary screening is therefore required to exclude ineligible strains (Figure 2).
Anti-gastric Acidity
A good ability to anti-gastric acid after ingestion is a prerequisite for probiotics to survive and colonize in intestinal tract (Martinez et al., 2017). In normal human body, the gastric acidity ranges from pH 1.5–4.5, which is sufficient to kill most of the ingested microorganisms (Xu et al., 2011). Studies reported that most LAB survived only 1 h in a pH 1.5 environment, while a majority remained to have a high survival ability within 3 h in a pH 2.0–3.0 environment (Jonganurakkun et al., 2008; Martinez et al., 2017; Fujiwara et al., 2020; Yin et al., 2020). Thus, a setting of pH 2.0–3.0 to simulate gastric juice is recommended in anti-acidity test to screen P. pentosaceus strains with acid tolerance.
BSH Activity and Resistance to Bile Salt
In addition to gastric digestion, the digestion and transport by the small intestine is also an indispensable process for foreign bacteria to colonize and exert a role. Liver cell-derived bile salts destabilize cells with their effects on lipid dissolution and emulsifying in the digestive tract (Caggia et al., 2015). In normal people, the concentration of bile salts in the duodenum can reach 0.3–3 g/kg (Fitzgerald et al., 2000). A good tolerance to low pH of gastric juice and to bile salts in intestinal fluid for a certain period of time is one of the important criteria for selecting probiotics (Damodharan et al., 2015). Besides, despite the activity status in the intestinal tract, the tolerance to bile salts also affects the cholesterol-lowering function of probiotics (Argyri et al., 2013). The tolerance to bile salts varies with the type of probiotics, while the bile salt concentration remains to range between 0.1–1.0% (Ilavenil et al., 2016; Silva et al., 2017; Fujiwara et al., 2020; Tatsaporn and Kornkanok, 2020; Yang et al., 2020).
Adhesion
Probiotic strains can adhere to gastrointestinal mucosa to survive and stably colonize and subsequently exert metabolic activities (such as production of digestive enzymes and organic acids). They can compete with the resident flora by producing antibacterial substances, and can also antagonize pathogenic bacteria, which plays a regulatory role in immune response (Yin et al., 2020). Given these characteristics, the adhesion of probiotics in the intestinal tract is generally used to indicate the applicable potential of probiotics.
In adhesion assay, the control strain usually selects the Lactobacillus rhamnosus GG (LGG), which is a commercial probiotic internationally recognized with strong adhesion ability. The colon cancer cell lines Caco-2 and HT-29 are widely used cell models in vitro at present (Kim et al., 2019; Bungenstock et al., 2020). Adhesion assays include: (1) Co-culture of bacteria and cells: i) co-culture of bacteria and cells; ii) washing, and removal of floating bacteria; iii) processing of the cells to blow out the adherent bacteria; and iv) calculating the number of live bacteria to compute adhesion rate. (2) Co-culture of bacteria and cells plus staining process: i) co-culture of bacteria and cells; ii) washing, and removal of floating bacteria; iii) fixation with methanol; iv) Gram’s or Giemsa’s staining; v) microscopic observation and adhesion rate computation (Raghavendra et al., 2010; Shukla and Goyal, 2014; Damodharan et al., 2015; Dubey et al., 2020).
It is reported that the strains with high self-aggregation ability also have high adhesion. In addition, the self-aggregation property could facilitate probiotics to form biofilm and colonize in the host intestinal tract, which could prevent the adhesion and invasion of pathogenic bacteria, showing a possible correlation with pathogen clearance (Rujing et al., 2019). Likewise, higher hydrophobicity also correlates to a higher adhesion ability in the intestinal tract (Jin et al., 2020). Probiotic strains are required to have at least 40% hydrophobicity (Re et al., 2010).
Bacteriostasis
Antibacterial activity is an important indicator that reflects the ability of probiotics to competitively kill or suppress harmful microorganisms in the intestinal tract (de Azevedo et al., 2020a). Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and Salmonella, etc. are used as pathogen indicators in testing the antibacterial activity of probiotics (Ghosh et al., 2019; Ladha and Jeevaratnam, 2020).. Assays include: (1) Oxford Cup method: (i) plate coated by different indicator bacteria and transferred to an Oxford Cup; (ii) addition of the bacterial supernatant and culture; (iii) determination of antibacterial activity according to the diameter of the inhibition zone. (2) Two-layer plating method: (i) addition of bacterial culture medium in a plate; (ii) chlorine gas processing; (iii) spot inoculation of the soft agar (containing indicator bacteria) to the surface of the modified plate; (iv) measurement of the inhibition zone diameter. (3) Agar diffusion method: (i) addition of the soft agar (containing indicator bacteria) to an agar plate, hole punch; (ii) inoculation of the bacterial supernatant or suspension into the holes; (iii) measurement of the inhibition zone diameter after 48 h of culture (Zhenhui et al., 2015; Ilavenil et al., 2016; Soundharrajan et al., 2019; Diguță et al., 2020; Tatsaporn and Kornkanok, 2020).
Testing of Probiotic Characteristics
Considering practical applications, some probiotic characteristics of P. pentosaceus should also be studied, such as antioxidant and cholesterol-lowering capabilities, or effect in immune response, in addition to the characteristics provided by WHO. A combination of in vivo and in vitro study can be a strategy.
Evaluation of the antioxidant capacity is taken as an example. In vitro use DPPH radical scavenging activity, hydroxyl radical scavenging activity, superoxide anion radical scavenging activity, reducing power assay, assay of antioxidant enzyme activity to determine the antioxidation ability (Zhang et al., 2020a). While in vivo study, a murine oxidative damage model was established to study the function and mechanism of oxidative stress resistance in LAB with antioxidant capacity. Some indicators in the tissues (liver, renal, serum, etc.) of the mouse fed LAB (live bacteria or cell extracts), including superoxide dismutase (SOD) activity, glutathione peroxidase (GSH-Px) activity, malondialdehyde (MDA) content, protein amino-compound content, serum endotoxin, structural changes in gut microbial community, were measured and compared with those from the murine oxidative damage model. The significant difference was taken as a reference to evaluate the ability of LAB to intervene oxidative stress in vivo (Wang et al., 2020).
As regards the ability to cholesterol-lowering in vitro, current study commonly uses the phthalaldehyde method to test the ability in vitro and apply the high-fat animal models for evaluation of the ability in vivo (Damodharan et al., 2015). Besides, there are some other methods which can rapidly screen the strains with cholesterol-lowering ability in a large strain pool, such as enzyme precipitation method, calcium carbonate plate method (clear circles) method, and indicator method (Vidhyasagar and Jeevaratnam, 2013).
Requirements in Food Processing
Safety
Safety assessment should be performed for the screened P. pentosaceus strains. Safety is the primary requisite for probiotics (Singla et al., 2018). In vitro tests should cover pathogenicity, toxicity, hemolytic activity, drug sensitivity, antibiotic resistance and so on. The antibiotic resistance of probiotics can be determined by the disk diffusion test (Silva et al., 2017). Hemolysis is the rupture of red blood cells, which may cause sepsis (Thirabunyanon and Hongwittayakorn, 2013). Commonly, blood plate culture is applied to assess hemolysis, where the strains are inoculated onto Columbia blood agar plates using a streaking method and are then cultured at 37°C for 48 h (Diguță et al., 2020).
In vivo tests usually adopt animal models using rats, mice or aquatic products, such as fish and shrimp. In addition, the animals used in tests are fed probiotics to generate the experimental group, and the safety of the probiotics will be evaluated by comparing the resistance to different diseases in control group and experimental group, such as immune diseases and conjunctivitis (Arévalo-Villena et al., 2017).
Stress Tolerance
As P. pentosaceus are prepared for food processing, their stress tolerance and the activity after various industrial processing still needs to be evaluated. Maria de Lourdes Bastos (Rychen et al., 2018) used glucose as a carrier and took diammonium phosphate (5%) and dipotassium phosphate (2.5%) as buffers to standardize P. pentosaceus DSM 32291 to 1×1011 CFU/g. The samples were then preserved in aluminum foil bags at room temperature for 18 months without a significant loss of microbial viability.
Application of P. pentosaceus
Application in Fermentation of Dairy Products
The most common fermented dairy products include cheese, yogurt, cream, etc., which are made from milk (or other milk containing the same milk solids) and fermented by LAB or yeasts. They are nutrient-rich, easy-digestible, palatable, and convenient to storage and are well-received by consumers. P. pentosaceus is inherent in or can be processed into cheese, which actively improves the flavor and accelerates cheese ripening, suggestive of a potential cheese fermenter. (Gerasi et al., 2010) isolated 16 strains of P. pentosaceus from traditional Swiss Manura cheese and found that P. pentosaceus was the only Pediococcus in cheese, which is the same as the study by Ilaria et al. (2015). Gerasi identified that P. pentosaceus was the dominant flora that contributed to flavor formation and accelerated fermentation in Manura cheese, although it was not dominant in number. Ilaria et al. (2015) isolated 29 strains of P. pentosaceus from traditional Italian Marga cheese (TMM) and identified several strains suitable for use in cheese fermentations due to their characteristics, including high-salt tolerance and cryophilic, BSH activity, no biogenic amine (BA) production, rapid acid and conjugated linoleic acid production, and the most important potential in yielding GABA. These characteristics improve the quality and taste of the cheese while being safe and stable, expanding the role of the cheese in promoting consumer health. In the production of yoghourt, P. pentosaceus combined with traditional yoghourt fermentation can get better acid production characteristics and contribute to yoghourt products with higher qualities. The study by Balakrishnan and Agrawal (2014) demonstrated that P. pentosaceus could make an effective use of the nutrients from goat and camel milk, enhance the antioxidant activity and fatty acid content of the fermented milk, and thereby improve the nutritional value of the products.
The safety issue of dairy products is a concern of the customers. The aflatoxin (AFS) is a harmful component in dairy products, and it may pose a serious potential risk to the health of consumers, especially children the most sensitive to the adverse effects of AFS, due to the heavy consumption worldwide. Martínez et al. (2019) screened out microorganisms that could suppress AFS M1AFM1 into the food chain by adsorption/degradation strategies, and the probiotic P. pentosaceus could facilitate the production of metabolites with less toxicity by adsorbing and degrading the AFM1 in milk.
Application in Fermentation of Fruits and Vegetables
P. pentosaceus can be used in fermentation of multiple vegetables to improve the sensory characteristics, make the products safer and more stable in quality, such as radishes, cucumbers, and corns (Kati et al., 2020). The most widespread application is seen in kimchi making. Compared with fresh vegetables, kimchi can satisfy a wider range of tastes and is good at appetite-stimulate appetite. Additionally, it can improve the structure of gut microbial communities due to the abundant functional flora (mainly LAB) and is well-received in the diet of people all over the world, especially in Asia. The kimchi produced by LAB fermentation can overcome the defects of the kimchi naturally fermented, including long fermentation period, susceptible to seasonal changes, unstable quality and short shelf life. Jang et al. (2015) found that P. pentosaceus T1 could prevent the overfermentation of kimchi by inhibiting Leuconostoc mesenteroides and Lactobacillus Sakai. In addition, the kimchi fermented by P. pentosaceus T1 could be better accepted in an overall perspective as compared to the kimchi without P. pentosaceus T1, accompanied with higher scores in sour taste, kimchi texture, odor and taste, owing to the control of acidity and the number of bacterial cells by P. pentosaceus. Moreover, Shukla and Goyal (2014) isolated P. pentosaceus CRAG3 from fermented cucumbers and believed that its dextranase and capability of glucan production might have a potential role in functional food applications.
In addition to its host health benefits, P. pentosaceus can also improve the quality of fermented fruit and vegetable juice. Xu et al. (2021) used the P. pentosaceus derived from fermented cherry juice and pickled porcine ears to ferment cauliflower juice. They found that fermentation by P. pentosaceus could change the key odorants and the non-volatile metabolites in cauliflower juice, suggestive of the presence of several metabolic pathways manifested by P. pentosaceus and conducive to enhancing the sensory characteristics of cauliflower juice. They also indicated that P. pentosaceus could help enhance the sensory characteristics of the cauliflower juice products, contributing to more distinct odor and better tastes.
Application in Fermentation of Meat Products
Fermented meat products are produced by the bio-fermentation technology, by which raw meat is exposed to specific microorganisms to induce acid or alcohol-production (reduce pH), and then dehydrated by low-temperature separation method. The fermented meat products always have a low pH value, which can inhibit the growth of spoilage/pathogenic bacteria and the production of toxins, thereby contributing to maintaining the drying of the meat products and prolonging the preservation period. In addition, the microbial fermentation of the meat can remove or reduce the unpleasant smell of the meat, which is readily acceptable by consumers. Nanasombat et al. (2017) used agar diffusion test to screen the LAB with antibacterial activity from raw meat and fermented pork products, in an attempt to find the suitable LAB for meat fermentation. They found that P. pentosaceus P0805 was the best of the 174 isolates. Further experiments revealed that P0805 has some desirable characteristics, such as the ability to produce inhibitory substances against Salmonella typhimurium, catalase to remove the accumulated hydrogen peroxide in fermented products, and nitrate reductase which may facilitate the fermented meat in pinkish. Besides, it does not produce amino acid decarboxylases, and may not result in the accumulation of biogenic amines. These characteristics allow the type of bacterium to be a qualified candidate for good meat fermentation. Sun et al. (2019) purified and analyzed the biochemical characteristics of the P. pentosaceus protease isolated from Harbin dry sausages, and proved that P. pentosaceus can be used as a starter or enzyme-producing strain of Harbin dry sausages. P. pentosaceus can also be combined with other lactic acid bacteria to make a mixed starter to play a better role. Zhang et al. (2020b) prepared the Lactobacillus curvatus LAB26 and P. pentosaceus SWU73571, which were isolated from traditional sour meat, into fermenters for sour meat processing. The prepared fermenters increased the total number of bacteria, LAB, amino nitrogen and free amino acids, and improved the color of the sour meat. Besides, the water activity and pH value were reduced, accompanied by decreased numbers of coliforms, nitrite, biogenic amine, volatile basic nitrogen and malonaldehyde. Compared with natural fermentation, such fermentation significantly improved the quality and safety of fermented sour meat. Ratanaburee et al. (2013) found that fermenters could significantly increase the GABA content in Nham, contributing to a unique Nham product which was low in fat, carbohydrate and energy, and had the best sensory evaluation.
Application in Fermentation of Pasta
The yeast fermentation made by P. pentosaceus is potentially effective in developing fermented pasta. Montemurro et al. (2020) identified P. pentosaceus OA1 and S3N3 from a variety of fermenters according to the acidification growth property and strong proteolytic activity (TFFA increased by up to 80%). Using the strains for bread fermentation, they found that the phytate degradation rate was as high as 58%, and the phenol content and clearance activity were, respectively, increased by 4 times and 2 times, which confirmed the potential of P. pentosaceus as a yeast fermenter for bread fermentation. (Plessas et al., 2020) isolated P. pentosaceus SP2 from kefir grains, and proved its potential as a yeast fermenter for bread fermentation. They noted that the breads fermented by P. pentosaceus SP2 were superior to the yeast breads (wild flora) on market produced under the same conditions as regards acidity, organic acid content and anti-spoilage. Gong and Qi (2020) screened P. pentosaceus from Chinese Laomian, and deeply understood the fermentation effect of P. pentosaceus in Chinese Laomian, which is conducive to the development of traditional Chinese Laomian. Research by Jin et al. (2020) carried out the separation of anti-fungal lactic acid bacteria and yeast strains, and the isolated strains will be used for starter culture to develop preservative-free yeast bread with improved quality. It was discovered that the combination of P. pentosaceus and Saccharomyces cerevisiae is a promising yeast starter for making high-quality preservative-free bread. Xing et al. (2019) evaluated the application potential of lactic acid bacteria from traditional sweet and sour dough in different regions of China as raw materials in the production of steamed bread and found that P. pentosaceus has applications in improving the quality and quality of steamed bread.
Some Other Potential Applications
Despite the above mentioned, P. pentosaceus has some other applications in the food industry. For example, Raghavendra et al. (Raghavendra et al., 2011) isolated P. pentosaceus CFR R123 with phytate degradation ability from fermented grains, and used it in MFSC and soy milk, showing the potential of P. pentosaceus CFR R123 in decreasing phytate level and increasing the bioavailability of some minerals (Calcium, Magnesium) and the potential as a fermenter for development of multiple functional fermented grains. Laitila et al. (2006) found that the fermenter containing P. pentosaceus promoted the growth of yeasts and inhibited the growth of harmful bacteria during malt fermentation. They also noted a positive effect on malt characteristics, such as the reduction of wort viscosity and β-glucan content, and the enhancement of xylanase and microbial β-glucanase activities, suggestive of improved wort-filtering wheat performance. Toe et al. (2019) isolated 8 strains of LAB from Malaysian food and tested their extracellular proteolytic activity and amino acid production capacity. Since amino acids are important in the growth, reproduction and maintenance of organisms, it is significant to find safer food-grade AA producer strains. The extracellular proteolytic activity of LAB has a great potential for producing functional amino acids due to its involvement in the hydrolysis of extracellular protein molecules into free amino acids. Cui Jin Toe et al. found that the P. pentosaceus UL-6 isolated from the 8 strains was highly capable of proline production, and the P. pentosaceus UP-2 could produce a series of amino acids with the highest concentration.
Probiotic Characteristic of P. Pentosaceous
Suppressive to Food-Borne Pathogenic Bacteria
The food-borne diseases caused by food-borne pathogenic bacteria are a global public health problem with growing concerns. Fresh meat and meat products, milk and dairy products, aquatic products and vegetables, etc. are the main bodies with bacterial contamination. P. pentosaceus, which has a good ability to against food-borne pathogenic bacteria, has shown a bright future for applications in food industry. It can be used as a biological preservative in processed food and can be an alternative to replace or reduce the use of chemical preservatives. Ghosh et al. (2019) isolated P. pentosaceus GS4 (MTCC 12683) from a type of Indian fermented food Khadi. They applied bilayer diffusion method to test the antibacterial and antagonistic abilities of P. pentosaceus GS4 on Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Listeria monocytogenes, which identified good suppressive effects on the growth of the tested bacteria. Ladha and Jeevaratnam (2020) isolated P. pentosaceus from rumen fluid of goat and investigated the property of the bacteriocin LJR1. LJR1 is a class IIa bacteriocin, which is heat stable (121°C, 30 min), and has strong killing effect on multiple antibiotic-resistant food-borne pathogenic bacteria. Yin et al. (2020) isolated P. pentosaceus L1 from pickled radishes and found that it could effectively adhere to small intestinal epithelial cells and suppress the adhesion of ETEC, showing a potential in control of ETEC infection. Cavicchioli et al. (2019) isolated P. pentosaceus ST65ACC from raw milk cheese and proved its clearance effect on Listeria monocytogenes by using the co-aggregation assay. Cavicchioli et al. (2019) and Muhialdin et al. (2011) assessed the safety and thermostability of P. pentosaceus ST65ACC and Te010 and confirmed their use in the control of food-borne pathogenic bacteria as beneficial strains.
The antibacterial mechanisms of P. pentosaceus mainly include four aspects: (1) Secrete bacteriocin to damage cell wall or directly kill pathogenic bacteria; (2) The secreted organic acids can infiltrate the cell membrane of pathogenic bacteria and reduce the intracellular pH value to suppress metabolism, while the decrease of pH value can lead to inhibited gene expression of virulence factors; (3) Compete for the adhesion sites on intestinal epithelial cells with pathogenic bacteria to suppress the adhesion of pathogenic bacteria; (4) To aggregate with pathogenic bacteria to make them unable to exert a role.
Antioxidant Function
Oxidative stress and injury are closely related to accelerated aging and the development of a variety of systemic and metabolic diseases in the body, such as cancer, diabetes, hypertension, and atherosclerosis (Yamamoto et al., 2019). Since the health is growing important and receiving increasing attention, antioxidant studies are prominent in fields like Food Science, Medicine, and Life science. There are some studies reporting that P. pentosaceus with antioxidant activity helps improve the antioxidant function of fermented products. Additionally, research believed the application of fermenter YK provides a new functional food, which can decrease the risk of cardiovascular disease caused by oxidative stress (Kim et al., 2019). (Yang et al., 2020) evaluated the antioxidant capacity of Black Gamju extract fermented by P. pentosaceus by ABTS radical scavenging assay and β-carotene bleaching assay, and found that ABTS radical scavenging effect was significant. Huang et al., (2020) found that P. pentosaceus B49 alleviated oxidative stress in constipated mice by reducing serum malondialdehyde (MDA) levels. Wang et al. (2020) found P. pentosaceus PP04 effectively improved oxidative stress induced by high-fat diet by activating Nrf2/CYP2E1 signaling pathway. The signal pathway can be enhanced superoxide dismutase (SOD) and glutathione peroxidase (GSH-px) and antioxidant activity.
The antioxidant mechanisms of P. pentosaceus mainly fall into four aspects: (1) Clearance of radicals, including DPPH, hydroxyl radical, and superoxide anions. (2) Tolerance to oxidative stress, mainly O2 or H2O2 at a certain concentration. (3) Ability to anti-lipid peroxidation, which is mainly reflected by the inhibition rate of linoleic acid peroxidation or the content of malondialdehyde (MDA), a product of lipid peroxidation. (4) Equipped with enzymatic and non-enzymatic antioxidant defense systems which can produce antioxidant substances, such as superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPx), and glutathione S-transferase (GST).
Cholesterol-Lowering Function
Cholesterol is an important component of the body tissue characterized by a variety of functions, yet an increase of blood cholesterol is the main risk factor of coronary heart disease (Kumar et al., 2012). Studies have shown that P. pentosaceus can be used in development of cholesterol-lowering functional foods after appropriate clinical trials in human beings. Damodharan et al. (2015) established an atherosclerosis diet-induced hyperlipidemia model in male mice (C57BL/6 J) and provided 3 × 108 CFU P. pentosaceus KID7 by oral gavage after 28 days, once a day for 32 consecutive days. They found that there was a significant decrease of total cholesterol, low-density fatty acids, and alanine aminotransferase in serum, and total cholesterol in liver, along with a significant increase of free cholic acid content in feces. Wang et al. (2020) fed C57BL/6 N mice with high-fat diet and treated with P. pentosaceus PP04. The results showed that the weight gain of total cholesterol (TC), triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C) in mice was significantly reduced, and similar phenomenon was also found (Zhao et al., 2012).
The mechanisms about the cholesterol-lowering function of P. pentosaceus may include: (1) The co-precipitation of free cholate and cholesterol; (2) Deconjugation effect on cholate; (3) Cholesterol assimilation to reduce cholesterol content; (4) Simultaneous co-precipitation and absorption.
Immunomodulatory Function
Owing to the wide applications in the food industry, P. pentosaceus plays a certain role in improving people’s health and life quality via exerting regulatory functions in immunity. It is reported that P. pentosaceus is important in the improvement of immune function and anti-infection ability of the body. Shin et al. (2016) isolated P. pentosaceus KFT18 (PE-EPS) from Korean kimchi and found that its extracellular polysaccharide could stimulate IFN-γ to activate macrophages and primary splenocytes, leading to activation of immune response and improvement of the immunosuppression induced by cyclophosphamide. Jonganurakkun et al. (2008) found that there was a significant increase of IFN-γ and IL-12p70 secretion level and a suppression on IL-4 production by the presence of P. pentosaceus in spleen cells of mice sensitized with ovalbumin (OVA). It suggested that P. pentosaceus could effectively stimulate immune activity and be suppressive to anergy.
The mechanisms of P. pentosaceus in immune regulation could be described by (1) the enhanced phagocytosis and natural killer cell activity; and (2) regulating the expression and secretion of cytokines produced by immune organs or cells.
Cancer Treatment
Cancer is the disease with the highest mortality in the world. Studies have shown that P. pentosaceus plays an anti-tumor effect by significantly inhibiting the proliferation of cancer cells. Byung Chull An et al. (2019) developed a gene expression box that can induce a large amount of P8 protein secreted by P. pentosaceus SL4 (PP). Through studies, it was found that P8 protein can be used as a therapeutic anti-cancer molecule for colorectal treatment. It can inhibit cell proliferation to a certain extent and reduce the total amount of Cyclin B1/ CDK1 p21 in a P8 dose-dependent manner. Furthermore, Shukla and Goyal (2013) showed that the dextran isolated from P. pentosaceus CRAG3 has anti-cancer properties, and has reduced activity on both HeLa and HT29 cell lines, which may be related to the adhesion disorder caused by the ability of dextran to modify tumor cell membrane surface proteins. Thirabunyanon and Hongwittayakorn (2013) isolated a strain of P. pentosaceus FP3 and found its inhibitory effect on the proliferation of colon cancer cells. Meanwhile, it was speculated that P. pentosaceus FP3 adheres to colon cancer cells and induces the biological production of short-chain fatty acids (SCFAs), thereby inducing the apoptosis of colon cancer cells is an important mechanism.
The specific mechanism remains an open issue. Speculations include: (1) Induce tumor cell apoptosis by secreting organic acids; (2) Inhibit cell spreading and metastasis via producing glucan, which can modify membrane surface proteins leading to decreased adhesion of tumor cells. (3) Induce P. pentosaceus to synthesize P8 protein and acting on cyclin to inhibit cell proliferation.
Summary and Conclusion
As for P. pentosaceus, there are still many problems which need to be solved. For example, the strains documented in NCBI are mostly isolated from fermented foods or animals and plants (Jiang et al., 2020). There is a notion that probiotic bacterial strains should be derived from the human gut microbiome, which may help easier intestinal colonization and improve more specific applications. According to the complex interactions between the gut microbiome and their multi-faceted effect on the host, the isolation of P. pentosaceus from the human gastrointestinal tract is also worthy of being studied in the future. Besides, studies devoted to the interactions of P. pentosaceus with the host intestinal microbiome are a few, leading to the functions related to gut microecological balance regulation less evidenced (Suez et al., 2019).
Another problem is that, there is a deficiency in in vivo experiments for the probiotic P. pentosaceus. Most of the studies only covered the screening of available bacterial strains and identification of the probiotic characteristics, without genomics research or further in vivo study. Besides, the mechanisms about the probiotic effect of P. pentosaceus have not been thoroughly studied. For instance, some strains in murine diarrhea models were found to aggravate the inflammatory response by promoting the production of pro-inflammatory factors, suggestive of high strain specificity which requires further in vivo verification (B. et al., 2017). There are some studies reporting that the P. pentosaceus derived from food may also potentially risky. Møller et al. (2020) found that the P. pentosaceus isolated from cheese were capable of producing histamine. This was the first report on P. pentosaceus as a histamine-producing bacterium. High concentrations of histamine may cause symptoms, such as urticaria, rash, hypertension, and headache.
To sum up, the current studies on P. pentosaceus are not perfect. P. pentosaceus as a normal flora in gut microbiota plays a role in antioxidation, cholesterol-lowering, immune regulation, and cancer treatment. Besides, P. pentosaceus can be a promising natural additive in food processing, which can help improve product quality and safety while assisting fermentation. It also indicates that P. pentosaceus, as a potential probiotic bacterium prevalent in traditional food, is promising for future applications. In the future, a thorough understanding of the functions and related mechanisms of P. pentosaceus at the molecular level is required, in an attempt to make it further promoted in a variety of fields and play a more active role.
Author Contributions
JY and YQ: conceptualization. LH: methodology. YZ: resources. YQ: writing – original draft preparation. LH, YZ, JiX, and JuX: writing – review and editing. WL and DZ: supervision. JY, QT, and DD: project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31700004), Shandong Key Research and Development Program (2019JZZY010724), Construction of Innovative Provinces in Hunan Province (2019RS3022), and the National Students Platform for Innovation and Entrepreneurship Training Program (2020056). The funding bodies had no contribution in the study design, data collection, interpretation, or preparation of the manuscript.
Conflict of Interest
JiX and DD are employed by Tangrenshen Group Co., Ltd., China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
An, B. C., Ryu, Y., Yoon, Y. S., Choi, O., Park, H. J., Kimv, T. Y., et al. (2019). Colorectal Cancer Therapy Using a Pediococcus pentosaceus SL4 Drug Delivery System Secreting Lactic Acid Bacteria-Derived Protein p8. Mol. Cells 42.
Arévalo-Villena, M., Fernandez-Pacheco, P., Castillo, N., Bevilacqua, A., and Pérez, A. (2017). Probiotic capability in yeasts: set-up of a screening method. LWT Food Sci. Technol. 89, 657–665. doi: 10.1016/j.lwt.2017.11.047
Argyri, A. A., Zoumpopoulou, G., Karatzas, K., Tsakalidou, E., Nychas, G., Panagou, E. Z., et al. (2013). Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol. 33, 282–291. doi: 10.1016/j.fm.2012.10.005
Asami, K., Kondo, A., Suda, Y., Shimoyamada, M., and Kanauchi, M. (2017). Neutralization of lipopolysaccharide by heat shock protein in Pediococcus pentosaceus AK-23. J. Food Sci. 82, 1657–1663. doi: 10.1111/1750-3841.13679
Balakrishnan, G., and Agrawal, R. (2014). Antioxidant activity and fatty acid profile of fermented milk prepared by Pediococcus pentosaceus. J. Food Sci. Technol. 51, 4138–4142. doi: 10.1007/s13197-012-0891-9
Bamba, S., Takahashi, K., Imaeda, H., Nishida, A., Kawahara, M., Inatomi, O., et al. (2018). Effect of fermented vegetable beverage containing Pediococcus pentosaceus in patients with mild to moderate ulcerative colitis. Biomed. Rep. 9, 74–80. doi: 10.3892/br.2018.1099
Borges, S., Barbosa, J., Silva, J., and Teixeira, P. (2013). Evaluation of characteristics of Pediococcus spp. to be used as a vaginal probiotic. J. Appl. Microbiol. 115, 527–538. doi: 10.1111/jam.12232
Bungenstock, L., Abdulmawjood, A., and Reich, F. (2020). Evaluation of antibacterial properties of lactic acid bacteria from traditionally and industrially produced fermented sausages from Germany. PLoS One 15:e0230345. doi: 10.1371/journal.pone.0230345
Caggia, C., De Angelis, M., Pitino, I., Pino, A., and Randazzo, C. L. (2015). Probiotic features of lactobacillus strains isolated from Ragusano and pecorino Siciliano cheeses. Food Microbiol. 50, 109–117. doi: 10.1016/j.fm.2015.03.010
Cavicchioli, V. Q., Camargo, A. C., Todorov, S. D., and Nero, L. A. (2017). Novel bacteriocinogenic enterococcus hirae and Pediococcus pentosaceus strains with antilisterial activity isolated from Brazilian artisanal cheese. J. Dairy Sci. 100, 2526–2535. doi: 10.3168/jds.2016-12049
Cavicchioli, V. Q., Camargo, A. C., Todorov, S. D., and Nero, L. A. (2019). Potential control of listeria monocytogenes by Bacteriocinogenic enterococcus hirae ST57ACC and Pediococcus pentosaceus ST65ACC strains isolated From artisanal cheese. Probio. Antimicrob. Proteins 11, 696–704. doi: 10.1007/s12602-018-9449-0
Chen, T., Wang, L., Li, Q., Long, Y., Lin, Y., Yin, J., et al. (2020). Functional probiotics of lactic acid bacteria from Hu sheep milk. BMC Microbiol. 20:228. doi: 10.1186/s12866-020-01920-6
Damodharan, K., Lee, Y. S., Palaniyandi, S. A., Yang, S. H., and Suh, J. W. (2015). Preliminary probiotic and technological characterization of Pediococcus pentosaceus strain KID7 and in vivo assessment of its cholesterol-lowering activity. Front. Microbiol. 6:768. doi: 10.3389/fmicb.2015.00768
de Azevedo, P. O. D. S., Mendonça, C. M. N., Moreno, A. C. R., Bueno, A. V. I., de Almeida, S. R. Y., Seibert, L., et al. (2020a). Antibacterial and antifungal activity of crude and freeze-dried bacteriocin-like inhibitory substance produced by Pediococcus pentosaceus. Sci. Rep. 10:12291. doi: 10.1038/s41598-020-68922-2
de Azevedo, P. O. S., Mendonça, C. M. N., Seibert, L., Domínguez, J. M., Converti, A., Gierus, M., et al. (2020b). Bacteriocin-like inhibitory substance of Pediococcus pentosaceus as a biopreservative for listeria sp. control in ready-to-eat pork ham. Braz. J. Microbiol. 51, 949–956. doi: 10.1007/s42770-020-00245-w
Diguță, C. F., Nițoi, G. D., Matei, F., Luță, G., and Cornea, C. P. (2020). The biotechnological potential of Pediococcus spp. Isolated from Kombucha Microbial Consortium. Foods 9:1780. doi: 10.3390/foods9121780
Dubey, V., Mishra, A. K., and Ghosh, A. R. (2020). Cell adherence efficacy of probiotic Pediococcus pentosaceus GS4 (MTCC 12683) and demonstrable role of its surface layer protein (Slp). J. Proteome 226:103894. doi: 10.1016/j.jprot.2020.103894
Erten, H., Agirman, B., Guenduez, C. P. B., Carsanba, E., Sert, S., Bircan, S., et al. (2014). Importance of Yeasts and Lactic Acid Bacteria in Food Processing. Food Process. Strategies 351–378. doi: 10.1007/978-1-4939-1378-7_14
Fitzgerald, M. L., Moore, K. J., Freeman, M. W., and Reed, G. L. (2000). Lipopolysaccharide induces scavenger receptor A expression in mouse macrophages: A divergent response relative to human THP-1 monocyte/macrophages. J. Immunol. 164, 2692–2700. doi: 10.4049/jimmunol.164.5.2692
Fujiwara, M., Kuwahara, D., Hayashi, M., Zendo, T., Sato, M., Nakayama, J., et al. (2020). Lowering effect of viable Pediococcus pentosaceus QU 19 on the rise in postprandial glucose. Biosci. Microflora 39, 57–64. doi: 10.12938/bmfh.19-041
Gerasi, E., Litopoulo-Tzanetaki, E., and Tzanetakis, N. (2010). Microbiological study of Manura, a hard cheese made from raw ovine milk in the Greek island Sifnos. Int. J. Dairy Technol. 56, 117–122. doi: 10.1046/j.1471-0307.2003.00085.x
Ghosh, B., Sukumar, G., and Ghosh, A. R. (2019). Purification and characterization of pediocin from probiotic Pediococcus pentosaceus GS4, MTCC 12683. Folia Microbiol. (Praha) 64, 765–778. doi: 10.1007/s12223-019-00689-0
Gong, L., He, H., Li, D., Cao, L., Khan, T. A., Li, Y., et al. (2019). A new isolate of Pediococcus pentosaceus (SL001) With antibacterial activity Against fish pathogens and potency in facilitating the immunity and growth performance of grass carps. Front. Microbiol. 10:1384. doi: 10.3389/fmicb.2019.01384
Gong, Y., and Qi, X. (2020). A study revealing volatile aroma produced by Pediococcus pentosaceus in dough fermentation. Food Sci. Nutr. 8, 5077–5085. doi: 10.1002/fsn3.1807
Gutiérrez-Cortés, C., Suarez, H., Buitrago, G., Nero, L. A., and Todorov, S. D. (2018). Enhanced Bacteriocin production by Pediococcus pentosaceus 147 in co-culture With lactobacillus plantarum LE27 on cheese whey broth. Front. Microbiol. 9:2952. doi: 10.3389/fmicb.2018.02952
Higashikawa, F., Noda, M., Awaya, T., Danshiitsoodol, N., Matoba, Y., Kumagai, T., et al. (2016). Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: a randomized, double-blind, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 70, 582–587. doi: 10.1038/ejcn.2016.17
Ilaria, C., Tiziana, N., Roberto, L., Roberto, V., Kieran, T., and Elena, F. (2015). Identification and characterization of wild lactobacilli and pediococci from spontaneously fermented mountain cheese. Food Microbiol. 48, 123–132. doi: 10.1016/j.fm.2014.12.003
Ilavenil, S., Vijayakumar, M., Kim, D. H., Valan Arasu, M., Park, H. S., Ravikumar, S., et al. (2016). Assessment of probiotic, antifungal and cholesterol lowering properties of Pediococcus pentosaceus KCC-23 isolated from Italian ryegrass. J. Sci. Food Agric. 96, 593–601. doi: 10.1002/jsfa.7128
Jang, S., Lee, D., Jang, I. S., Choi, H. S., and Suh, H. J. (2015). The culture of Pediococcus pentosaceus T1 inhibits listeria proliferation in Salmon fillets and controls maturation of Kimchi. Food Technol. Biotechnol. 53, 29–37. doi: 10.17113/ftb.53.01.15.3754
Jang, S., Lee, J., Jung, U., Choi, H. S., and Suh, H. J. (2014). Identification of an anti-listerial domain from Pediococcus pentosaceus T1 derived from Kimchi, a traditional fermented vegetable. Food Control 43, 42–48. doi: 10.1016/j.foodcont.2014.02.040
Jiang, J., Yang, B., Ross, R. P., Stanton, C., and Chen, W. (2020). Comparative genomics of Pediococcus pentosaceus isolated From different niches reveals genetic diversity in carbohydrate metabolism and immune system. Front. Microbiol. 11:253. doi: 10.3389/fmicb.2020.00253
Jin, B., Shuyang, W., Qiaoqiao, L., Fuqiang, X., and Miaoyin, D. (2020). Screening of lactic acid bacteria against Helicobecter pylori. China Brewing 39, 45–48. doi: 10.11882/j.issn.0254-5071.2020.04.009
Jonganurakkun, B., Wang, Q., Xu, S. H., Tada, Y., Minamida, K., Yasokawa, D., et al. (2008). Pediococcus pentosaceus NB-17 for probiotic use. J. Biosci. Bioeng. 106, 69–73. doi: 10.1263/jbb.106.69
Kati, V., Jessica, H., Anna-Inkeri, S., Patricia, S., Gloria, D., Atte von, W., et al. (2020). Effect of different starter cultures on the sensory properties and microbiological quality of Atole agrio, a fermented maize product. Food Control 109:106907. doi: 10.1016/j.foodcont.2019.106907
Kim, J. S., Kim, J. H., Palaniyandi, S. A., Lee, C. C., You, J. W., Yang, H., et al. (2019). Yak-Kong soybean (Glycine max) fermented by a novel Pediococcus pentosaceus inhibits the oxidative stress-induced monocyte-endothelial cell adhesion. Nutrients 11:1380. doi: 10.3390/nu11061380
Kumar, M., Nagpal, R., Kumar, R., Hemalatha, R., Verma, V., Kumar, A., et al. (2012). Cholesterol-Lowering Probiotics as Potential Biotherapeutics for Metabolic Diseases. J. Diabetes Research 902917. doi: 10.1155/2012/902917
Kwon, H. K., Song, M. J., Lee, H. J., Park, T. S., Kim, M. I., and Park, H. J. (2018). Pediococcus pentosaceus-fermented Cordyceps militaris inhibits inflammatory reactions and alleviates contact dermatitis. Int. J. Mol. Sci. 19:3504. doi: 10.3390/ijms19113504
Ladha, G., and Jeevaratnam, K. (2020). Characterization of purified antimicrobial peptide produced by Pediococcus pentosaceus LJR1, and its application in preservation of white leg shrimp. World J. Microbiol. Biotechnol. 36:72. doi: 10.1007/s11274-020-02847-w
Laitila, A., Sweins, H., Vilpola, A., Kotaviita, E., Olkku, J., Home, S., et al. (2006). Lactobacillus plantarum and Pediococcus pentosaceus starter cultures as a tool for microflora management in malting and for enhancement of malt processability. J. Agric. Food Chem. 54, 3840–3851. doi: 10.1021/jf052979j
Le, B., and Yang, S. H. (2019). Biosorption of cadmium by potential probiotic Pediococcus pentosaceus using in vitro digestion model. Biotechnol. Appl. Biochem. 66, 673–680. doi: 10.1002/bab.1783
Martinez, M. P., Gonzalez Pereyra, M. L., Pena, G. A., Poloni, V., Fernandez Juri, G., and Cavaglieri, L. R. (2017). Pediococcus acidolactici and Pediococcus pentosaceus isolated from a rainbow trout ecosystem have probiotic and ABF1 adsorbing/degrading abilities in vitro. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 34, 2118–2130. doi: 10.1080/19440049.2017.1371854
Martínez, M. P., Magnoli, A. P., González Pereyra, M. L., and Cavaglieri, L. (2019). Probiotic bacteria and yeasts adsorb aflatoxin M(1) in milk and degrade it to less toxic AFM(1)-metabolites. Toxicon 172, 1–7. doi: 10.1016/j.toxicon.2019.10.001
Masuda, T., Kimura, M., Okada, S., and Yasui, H. (2010). Pediococcus pentosaceus Sn26 inhibits IgE production and the occurrence of ovalbumin-induced allergic diarrhea in mice. Biosci. Biotechnol. Biochem. 74, 329–335. doi: 10.1271/bbb.90656
Møller, C. O. A., Ücok, E. F., and Rattray, F. P. (2020). Histamine forming behaviour of bacterial isolates from aged cheese. Food Res. Int. 128:108719. doi: 10.1016/j.foodres.2019.108719
Montemurro, M., Celano, G., De Angelis, M., Gobbetti, M., Rizzello, C. G., and Pontonio, E. (2020). Selection of non-lactobacillus strains to be used as starters for sourdough fermentation. Food Microbiol. 90:103491. doi: 10.1016/j.fm.2020.103491
Muhialdin, B. J., Hassan, Z., and Sadon, S. (2011). Antifungal activity of lactobacillus fermentum Te007, Pediococcus pentosaceus Te010, lactobacillus pentosus G004, and L. paracasi D5 on selected foods. J. Food Sci. 76, M493–M499. doi: 10.1111/j.1750-3841.2011.02292.x
Nanasombat, S., Treebavonkusol, P., Kittisrisopit, S., Jaichalad, T., Phunpruch, S., Kootmas, A., et al. (2017). Lactic acid bacteria isolated from raw and fermented pork products: identification and characterization of catalase-producing Pediococcus pentosaceus. Food Sci. Biotechnol. 26, 173–179. doi: 10.1007/s10068-017-0023-4
O’Connor, P. M., Kuniyoshi, T. M., Oliveira, R. P., Hill, C., and Cotter, P. D. (2020). Antimicrobials for food and feed; a bacteriocin perspective. Curr. Opin. Biotechnol. 61, 160–167. doi: 10.1016/j.copbio.2019.12.023
Ouwehand, A. C. (1999). Probiotics: mechanisms and established effects. Int. Dairy J. 9, 43–52. doi: 10.1016/S0958-6946(99)00043-6
Patel, S., Kasoju, N., Bora, U., and Goyal, A. (2010). Structural analysis and biomedical applications of dextran produced by a new isolate Pediococcus pentosaceus screened from biodiversity hot spot Assam. Bioresour. Technol. 101, 6852–6855. doi: 10.1016/j.biortech.2010.03.063
Plessas, S., Mantzourani, I., and Bekatorou, A. (2020). Evaluation of Pediococcus pentosaceus SP2 as Starter Culture on Sourdough Bread Making. Foods 9. doi: 10.3390/foods9010077
Raghavendra, P., Rao, T. S., and Halami, P. M. (2010). Evaluation of beneficial attributes for phytate-degrading Pediococcus pentosaceus CFR R123. Benef. Microbes. 1, 259–264. doi: 10.3920/bm2009.0042
Raghavendra, P., Ushakumari, S. R., and Halami, P. M. (2011). Phytate-degrading Pediococcus pentosaceus CFR R123 for application in functional foods. Benef Microbes 2, 57–61. doi: 10.3920/bm2010.0031
Ramos, B., Brandão, T. R. S., Teixeira, P., and Silva, C. L. M. (2020). Biopreservation approaches to reduce listeria monocytogenes in fresh vegetables. Food Microbiol. 85:103282. doi: 10.1016/j.fm.2019.103282
Ratanaburee, A., Kantachote, D., Charernjiratrakul, W., and Sukhoom, A. (2013). Enhancement of γ-aminobutyric acid (GABA) in Nham (Thai fermented pork sausage) using starter cultures of lactobacillus namurensis NH2 and Pediococcus pentosaceus HN8. Int. J. Food Microbiol. 167, 170–176. doi: 10.1016/j.ijfoodmicro.2013.09.014
Re, B. D., Sgorbati, B., Miglioli, M., and Palenzona, D. (2010). Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 31, 438–442. doi: 10.1046/j.1365-2672.2000.00845.x
Rizzello, C. G., Coda, R., Wang, Y., Verni, M., Kajala, I., Katina, K., et al. (2019). Characterization of indigenous Pediococcus pentosaceus, Leuconostoc kimchii, Weissella cibaria and Weissella confusa for faba bean bioprocessing. Int. J. Food Microbiol. 302, 24–34. doi: 10.1016/j.ijfoodmicro.2018.08.014
Rujing, Z., Yafan, H., Dan, H., Mengna, Z., Zhaoxin, L., Fengxia, L., et al. (2019). Screening and identification of probiotic lactobacillus strains inhibiting intestinal pathogens and adhering to Caco-2 cells. Sci. Technol. Food Ind. 40, 133–139, 153. doi: 10.13386/j.issn1002-0306.2019.20.022
Rychen, G., Aquilina, G., Azimonti, G., Bampidis, V., Bastos, M. L., Bories, G., et al. (2018). Safety and efficacy of Pediococcus pentosaceus DSM 32291 as a silage additive for all animal species. EFSA J. 16:e05202. doi: 10.2903/j.efsa.2018.5202
Sellamani, M., Kalagatur, N. K., Siddaiah, C., Mudili, V., Krishna, K., Natarajan, G., et al. (2016). Antifungal and Zearalenone inhibitory activity of Pediococcus pentosaceus isolated from dairy products on Fusarium graminearum. Front. Microbiol. 7:890. doi: 10.3389/fmicb.2016.00890
Shi, D., Lv, L., Fang, D., Wu, W., Hu, C., Xu, L., et al. (2017). Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 prevents CCl(4)-induced liver cirrhosis by protecting the intestinal barrier in rats. Sci. Rep. 7:6927. doi: 10.1038/s41598-017-07091-1
Shin, M. S., Han, S. K., Ryu, J. S., Kim, K. S., and Lee, W. K. (2008). Isolation and partial characterization of a bacteriocin produced by Pediococcus pentosaceus K23-2 isolated from Kimchi. J. Appl. Microbiol. 105, 331–339. doi: 10.1111/j.1365-2672.2008.03770.x
Shin, J. S., Jung, J. Y., Lee, S. G., Shin, K. S., Rhee, Y. K., Lee, M. K., et al. (2016). Exopolysaccharide fraction from Pediococcus pentosaceus KFT18 induces immunostimulatory activity in macrophages and immunosuppressed mice. J. Appl. Microbiol. 120, 1390–1402. doi: 10.1111/jam.13099
Shokryazdan, P., Jahromi, M. F., Liang, J. B., and Ho, Y. W. (2017). Probiotics: From isolation to application. J. Am. Coll. Nutr. 36, 666–676. doi: 10.1080/07315724.2017.1337529
Shukla, R., and Goyal, A. (2013). Novel dextran from Pediococcus pentosaceus CRAG3 isolated from fermented cucumber with anti-cancer properties. Int. J. Biol. Macromol. 62, 352–357. doi: 10.1016/j.ijbiomac.2013.09.043
Shukla, R., and Goyal, A. (2014). Probiotic potential of Pediococcus pentosaceus CRAG3: A new isolate from fermented cucumber. Probiotics Antimicrob Proteins 6, 11–21. doi: 10.1007/s12602-013-9149-8
Silva, B. C., Sandes, S. H., Alvim, L. B., Bomfim, M. R., Nicoli, J. R., Neumann, E., et al. (2017). Selection of a candidate probiotic strain of Pediococcus pentosaceus from the faecal microbiota of horses by in vitro testing and health claims in a mouse model of salmonella infection. J. Appl. Microbiol. 122, 225–238. doi: 10.1111/jam.13339
Singla, V., Mandal, S., Sharma, P., Anand, S., and Tomar, S. K. (2018). Antibiotic susceptibility profile of Pediococcus spp. from diverse sources. 3. Biotech 8:489. doi: 10.1007/s13205-018-1514-6
Soundharrajan, I., Kim, D., Kuppusamy, P., Muthusamy, K., Lee, H. J., and Choi, K. C. (2019). Probiotic and triticale silage fermentation potential of Pediococcus pentosaceus and lactobacillus brevis and their impacts on pathogenic bacteria. Microorganisms 7:318. doi: 10.3390/microorganisms7090318
Suez, J., Zmora, N., Segal, E., and Elinav, E. (2019). The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729. doi: 10.1038/s41591-019-0439-x
Sun, F., Hu, Y., Chen, Q., Kong, B., and Liu, Q. (2019). Purification and biochemical characteristics of the extracellular protease from Pediococcus pentosaceus isolated from Harbin dry sausages. Meat Sci. 156, 156–165. doi: 10.1016/j.meatsci.2019.05.030
Tatsaporn, T., and Kornkanok, K. (2020). Using potential lactic acid bacteria biofilms and their compounds to control biofilms of foodborne pathogens. Biotechnol. Rep. (Amst.) 26:e00477. doi: 10.1016/j.btre.2020.e00477
Thirabunyanon, M., and Hongwittayakorn, P. (2013). Potential probiotic lactic acid bacteria of human origin induce Antiproliferation of colon cancer cells via synergic actions in adhesion to cancer cells and short-chain fatty acid bioproduction. Appl. Biochem. Biotech. 169, 511–525. doi: 10.1007/s12010-012-9995-y
Toe, C. J., Foo, H. L., Loh, T. C., Mohamad, R., Abdul Rahim, R., and Idrus, Z. (2019). Extracellular Proteolytic activity and amino acid production by lactic acid bacteria isolated from Malaysian foods. Int. J. Mol. Sci. 20. doi: 10.3390/ijms20071777
Venkateshwari, S., Halami, P. M., and Vijayendra, S. V. (2010). Characterisation of the heat-stable bacteriocin-producing and vancomycin-sensitive Pediococcus pentosaceus CFR B19 isolated from beans. Benef. Microbes 1, 159–164. doi: 10.3920/bm2009.0032
Vidhyasagar, V., and Jeevaratnam, K. (2013). Evaluation of Pediococcus pentosaceus strains isolated from idly batter for probiotic properties in vitro. J. Funct. Foods 5, 235–243. doi: 10.1016/j.jff.2012.10.012
Wang, Y., You, Y., Tian, Y., Sun, H., Li, X., Wang, X., et al. (2020). Pediococcus pentosaceus PP04 ameliorates high-fat diet-induced hyperlipidemia by regulating lipid metabolism in C57BL/6N mice. J. Agric. Food Chem. 68, 15154–15163. doi: 10.1021/acs.jafc.0c05060
Wu, C. W., Yin, L. J., and Jiang, S. T. (2004). Purification and characterization of bacteriocin from Pediococcus pentosaceus ACCEL. J. Agric. Food Chem. 52, 1146–1151. doi: 10.1021/jf035100d
Xing, X., Suo, B., Yang, Y., Li, Z., Nie, W., and Ai, Z. (2019). Application of lactobacillus as adjunct cultures in wheat dough fermentation. J. Food Sci. 84, 842–847. doi: 10.1111/1750-3841.14496
Xu, X., Bi, S., Lao, F., Chen, F., Liao, X., and Wu, J. (2021). Comprehensive investigation on volatile and non-volatile metabolites in broccoli juices fermented by animal- and plant-derived Pediococcus pentosaceus. Food Chem. 341:128118. doi: 10.1016/j.foodchem.2020.128118
Xu, R., Shang, N., and Li, P. (2011). Invitro and invivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe 17, 226–231. doi: 10.1016/j.anaerobe.2011.07.010
Yamamoto, N., Shoji, M., Hoshigami, H., Watanabe, K., Watanabe, K., Takatsuzu, T., et al. (2019). Antioxidant capacity of soymilk yogurt and exopolysaccharides produced by lactic acid bacteria. Biosci. Microbiota. Food Health 38, 97–104. doi: 10.12938/bmfh.18-017
Yanfei, S., Rui, C., and Lu, W. (2019). Surface properties of Pediococcus pentosaceus and antibacterial effect. J. Food Sci. Technol. 37, 54–61. doi: 10.3969/j.issn.2095-6002.2019.01.009
Yang, S. J., Kim, K. T., Kim, T. Y., and Paik, H. D. (2020). Probiotic properties and antioxidant activities of Pediococcus pentosaceus SC28 and Levilactobacillus brevis KU15151 in fermented black Gamju. Foods 9:1154. doi: 10.3390/foods9091154
Yin, H., Ye, P., Lei, Q., Cheng, Y., Yu, H., Du, J., et al. (2020). In vitro probiotic properties of Pediococcus pentosaceus L1 and its effects on enterotoxigenic Escherichia coli-induced inflammatory responses in porcine intestinal epithelial cells. Microb. Pathog. 144:104163. doi: 10.1016/j.micpath.2020.104163
Zhang, Y., Hu, P., Xie, Y., and Wang, X. (2020b). Co-fermentation with lactobacillus curvatus LAB26 and Pediococcus pentosaceus SWU73571 for improving quality and safety of sour meat. Meat Sci. 170:108240. doi: 10.1016/j.meatsci.2020.108240
Zhang, H., Wang, Q., Liu, H., Kong, B., and Chen, Q. (2020a). In vitro growth performance, antioxidant activity and cell surface physiological characteristics of Pediococcus pentosaceus R1 and lactobacillus fermentum R6 stressed at different NaCl concentrations. Food Funct. 11, 6376–6386. doi: 10.1039/c9fo02309g
Zhao, X., Higashikawa, F., Noda, M., Kawamura, Y., Matoba, Y., Kumagai, T., et al. (2012). The obesity and fatty liver are reduced by plant-derived Pediococcus pentosaceus LP28 in high fat diet-induced obese mice. PLoS One 7:e30696. doi: 10.1371/journal.pone.0030696
Keywords: Pediococcus pentosaceus, probiotics, food additives, antimicrobial activity, cell surface characteristics, resistance
Citation: Qi Y, Huang L, Zeng Y, Li W, Zhou D, Xie J, Xie J, Tu Q, Deng D and Yin J (2021) Pediococcus pentosaceus: Screening and Application as Probiotics in Food Processing. Front. Microbiol. 12:762467. doi: 10.3389/fmicb.2021.762467
Edited by:
Andreas Ernst Schwiertz, MVZ Institute of Microecology, GermanyReviewed by:
Neti Yuliana, Lampung University, IndonesiaMahdi Rohani, Pasteur Institute of Iran (PII), Iran
Copyright © 2021 Qi, Huang, Zeng, Li, Zhou, Xie, Xie, Tu, Deng and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Tu, cWlhbmcudHVAc2lhdC5hYy5jbg==; Dun Deng, ZGQteWZAdHJzZ3JvdXAuY24=; Jia Yin, amlheWluQGh1bm51LmVkdS5jbg==
 Yining Qi1
Yining Qi1 Yan Zeng
Yan Zeng Junyan Xie
Junyan Xie Jia Yin
Jia Yin