Abstract
Background:
The variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have emerged repeatedly, especially the Omicron strain which is extremely infectious, so early identification of patients who may develop critical illness will aid in delivering proper treatment and optimizing use of resources. We aimed to develop and validate a practical scoring model at hospital admission for predicting which patients with Omicron infection will develop critical illness.
Methods:
A total of 2,459 patients with Omicron infection were enrolled in this retrospective study. Univariate and multivariate logistic regression analysis were performed to evaluate predictors associated with critical illness. Moreover, the area under the receiver operating characteristic curve (AUROC), continuous net reclassification improvement, and integrated discrimination index were assessed.
Results:
The derivation cohort included 1721 patients and the validation cohort included 738 patients. A total of 98 patients developed critical illness. Thirteen variables were independent predictive factors and were included in the risk score: age > 65, C-reactive protein > 10 mg/L, lactate dehydrogenase > 250 U/L, lymphocyte < 0.8*10^9/L, white blood cell > 10*10^9/L, Oxygen saturation < 90%, malignancy, chronic kidney disease, chronic cardiac disease, chronic obstructive pulmonary disease, diabetes, cerebrovascular disease, and non-vaccination. AUROC in the derivation cohort and validation cohort were 0.926 (95% CI, 0.903–0.948) and 0.907 (95% CI, 0.860-0.955), respectively. Moreover, the critical illness risk scoring model had the highest AUROC compared with CURB-65, sequential organ failure assessment (SOFA) and 4C mortality scores, and always obtained more net benefit.
Conclusion:
The risk scoring model based on the characteristics of patients at the time of admission to the hospital may help medical practitioners to identify critically ill patients and take prompt measures.
Introduction
Although two years have passed since the coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbroke, various variants have been identified in different parts of the world (Zhu et al., 2020; Islam et al., 2021). The Omicron variant, in particular, rapidly gained dominance through its ability to fast spread (Petersen et al., 2022). The cumulative diagnosed cases have reached over 600,000 and close to 600 cases have died with or from COVID-19 as of 30 June 2022 since the recent Omicron outbreak in Shanghai (Ye et al., 2022; Yuan et al., 2022). The treatment of patients critically ill with SARS-CoV-2 infection is one of the main challenges facing clinicians and there is a need to adopt reliable predictors of the severity of patients to identify and treat the most severe patients in the early stage.
Previous studies have described the clinical characteristics of SARS-CoV-2 infection and explored the associations of epidemiological, comorbidity factors, imaging with severity and prognosis of COVID-19 (Chen et al., 2020; Huang C. et al., 2020; Liang et al., 2020a; Lacap et al., 2022). A study with 1,590 patients revealed that comorbidities were associated with poorer clinical outcomes (Guan et al., 2020). Older age, male sex, and active cancer also contributed to the increased mortality in a prospective, observational, single-center study (Başaran et al., 2022). Moreover, various studies have gathered evidence on the levels of serum cytokines, and the effect of concentrations of trace elements (Han et al., 2020; Heller et al., 2021). However, studies of COVID-19 prognostic factors have focused on radiological examinations obtained following admission or immunological indicators and other expensive examination indicators. Tools that provide practical, accurate, and low-cost risk estimates are needed, as estimates requiring extensive testing or imaging increase the burden on healthcare systems already operating at capacity. Previous published models tend not to include clinical variables obtained from history and examination carried out on initial assessment, as well as vaccination status. Since many variables of the pandemic have changed, widespread vaccination uptake has affected the incidence of severe COVID-19, and emerging variants of concern also have differing spectra of severity. Predicting the risk of critical illness with COVID-19 could help to identify those patients who require the most urgent help, and it may be of great utility for healthcare professionals to efficiently perform triage of patients, personalize treatment, and monitor clinical progress.
A large-scale outbreak poses significant challenges to the healthcare system, and the ability to identify patients who are most at risk of developing severe disease upon admission is critical for effective triage, management, and discharge decision making. In this study, we aimed to elucidate potential risk factors based on a cohort of Chinese patients with SARS-CoV-2 infection to attempt to identify patients at the time of hospital admission who are probably to develop critical illness. In addition, the accuracy of the novel risk score was compared with CURB-65, sequential organ failure assessment (SOFA), and 4C mortality scores.
Patients and methods
Patients
Between March 23, 2022 to May 26, 2022, 2459 of the 2798 confirmed SARS-CoV-2 Omicron infection patients, who hospitalized in Huashan Hospital of Fudan University were enrolled in the study. SARS-COV-2 infection was confirmed by positive real-time reverse-transcription polymerase-chain-reaction (PCR) assay for nasal and pharyngeal swab specimens. Patients died within 12 h after admission and without sufficient data were excluded. Critical cases were defined according to the World Health Organization as acute respiratory distress syndrome (ARDS), sepsis, septic shock, or other conditions that would normally require the provision of life-sustaining therapies such as mechanical ventilation or vasopressor therapy. The entire cohort was divided into the derivation (n = 1721) cohort and the validation (n = 738) cohort chronologically (Figure 1).
FIGURE 1
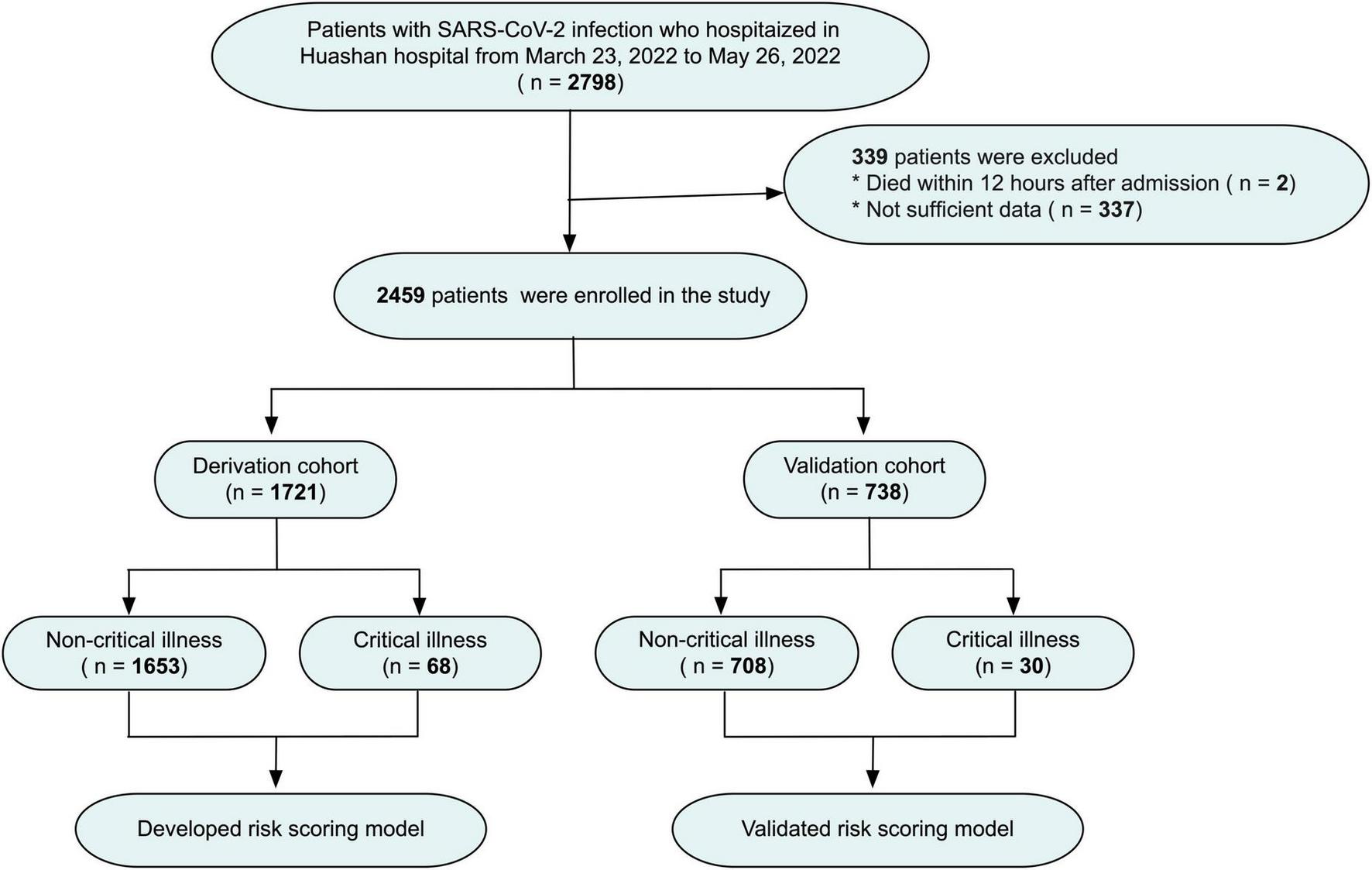
Flow chart of the study design.
This study was approved by the ethics committee of Huashan Hospital of Fudan University. Written informed consent was waived in consideration of the retrospective nature of the study. The clinical diagnosis and treatment complied with the Helsinki declaration.
Data collection
The study data were obtained from electronic medical records and they included demographics (age and gender), vital signs, clinical symptoms on admission, vaccination history, comorbidities, laboratory findings on admission (routine blood analyses, biochemical markers, coagulative function and so on), oxygen support need (high flow oxygen, non-invasive and invasive ventilation), and medications (ritonavir/darunavir, glucocorticoid, heparin, and vasoactive drugs). Chronic cardiac disease is summarized as established diagnoses of coronary artery disease, previous episodes of myocardial infarction and chronic heart failure. Chronic kidney disease was defined as repeatedly measured glomerular filtration rate (GFR) < 60 mL/min prior to present hospitalization.
Statistical analysis
All statistical analyses were performed using SPSS software for Windows (version 26.0; IBM, Endicott, NY, USA). The continuous variables in normal distribution were presented as means ± standard deviations (SDs), and median (interquartile ranges) were used for data that are not normally distributed. The categorical variables were summarized as a proportion (%). Student’s t-test, Wilcoxon’s non-parametric test, and the χ2-test or Fisher’s exact test were applied to normally distributed data, non-normal distribution continuous variables and the categorical variables, respectively. Univariable and multivariable logistic regression analyses were conducted to calculate the parameters predicting the occurrence of critical illness. All variables with p < 0.05 in the univariate analysis were selected for further multivariate analysis to identify independent predictors. Areas under the receiver operating characteristic curve (ROC) were used to estimate the predictive accuracy, and compared using Delong test. Continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated using the package “PredictABEL” in the R statistical computing language (version 3.6.3; R Foundation for Statistical Computing; Auckland University, Auckland, New Zealand). Two-tailed p value < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 2459 patients (out of 2,798 screened) were suitable for inclusion in this study. 29 of 2459 patients had sepsis and/or ARDS on admission. A total of 98 patients eventually developed critical illness and 2,361 did not. The median time from hospitalization to develop critical illness was 6 days. The characteristics of these two groups including age, blood test results, comorbidities, clinical symptom, and COVID-19 vaccination status on admission were shown in Supplementary Figure 1.
A total of 1,721 patients who hospitalized before May 1 constituted the derivation cohort. In the derivation cohort, 23 of 1,721 patients were considered to be severe on admission and a total of 68 patients eventually developed critical illness. A total of 20 patients died during hospitalization. Demographic and clinical characteristics of the derivation cohort were available in Table 1. The age (81 vs. 64, p < 0.001) and proportion of male (63.2% vs. 47.5%, p = 0.011) in critical patients were significantly higher than those in non-critical patients. Critical patients had higher alanine aminotransferase (ALT), aspartate transaminase (AST), total bilirubin (TB), white blood cell (WBC), creatinine, lactate dehydrogenase (LDH), D-dimer, C-reactive protein (CRP), procalcitonin (PCT), Cardiac troponin T (cTnT), and N-terminal forebrain natriuretic peptide (NT-proBNP), but significantly lower lymphocyte count and estimated glomerular filtration rate (eGFR) compared with non-critical patients. Hypertension was the most common comorbidity, followed by diabetes and chronic cardiac disease. Compared with non-critical patients, comorbidities and clinical symptoms (fever, cough, expectoration, pharyngalgia and feeble) were more common in critical patients. There were more patients unvaccinated in critical group than non-critical group (80.9% vs. 38.1%, p < 0.001). In addition, among patients who did develop critical illness, the proportion of using heparin, glucocorticoid, and vasoactive drugs was significantly higher than the patients who did not develop critical illness.
TABLE 1
| Total | Non-critical illness | Critical illness | p value | |
| Total (n) | 1721 | 1,653 | 68 | |
| Male | 829 (48.2%) | 786 (47.5%) | 43 (63.2%) | 0.011 |
| Age (years) | 64.0 (49.0-73.0) | 64.0 (48.0-73.0) | 81.0 (71.0-90.0) | <0.001 |
| Laboratory findings on admission | ||||
| ALT (U/L) | 16.0 (11.0-25.0) | 16.0 (11.0-24.0) | 19.5 (11.0-36.5) | <0.001 |
| AST (U/L) | 20.0 (16.0-26.0) | 20.0 (15.0-26.0) | 28.0 (19.0-48.5) | <0.001 |
| TB(umol/L) | 8.0 (5.6-11.4) | 7.9 (5.6-11.3) | 9.9 (7.1-14.7) | <0.001 |
| WBC (*10^9/L) | 5.2 (3.9-6.8) | 5.1 (3.9-6.7) | 7.5 (5.6-11.7) | <0.001 |
| Lymphocyte (*10^9/L) | 1.2 (0.8-1.7) | 1.3 (0.9-1.7) | 0.6 (0.5-1.0) | <0.001 |
| Creatinine (umol/L) | 76.0 (61.0-103.0) | 75.0 (60.0-99.0) | 126.5 (70.8-346.0) | 0.003 |
| eGFR (mL/min) | 84.1 (57.8-104.1) | 84.9 (61.9-104.5) | 44.6 (14.2-86.1) | <0.001 |
| LDH (U/L) | 195.0 (166.0-232.5) | 193.0 (165.0-229.0) | 294.0 (219.5-398.0) | <0.001 |
| D-dimer (FEUmg/L) | 0.6 (0.3-1.1) | 0.6 (0.3-1.0) | 2.4 (1.1-4.6) | <0.001 |
| CRP (mg/L) | 8.0 (5.0-26.1) | 6.9 (5.0-21.9) | 72.4 (35.6-111.0) | <0.001 |
| PCT (ng/ml) | 0.1 (0.1-0.5) | 0.1 (0.1-0.4) | 0.6 (0.2-2.8) | <0.001 |
| cTnT (ng/ml) | 0.010 (0.007-0.033) | 0.012 (0.007-0.028) | 0.079 (0.035-0.179) | <0.001 |
| NT-proBNP (pg/ml) | 137.0 (47.5-877.5) | 124.0 (45.1-599.0) | 2724.5 (1052.5-19420.3) | <0.001 |
| Comorbidities, N (%) | ||||
| Hypertension | 805 (46.8%) | 762 (46.1%) | 43 (63.2%) | 0.006 |
| Diabetes | 433 (25.2%) | 401 (24.3%) | 32 (47.1%) | <0.001 |
| Chronic cardiac disease | 276 (16.0%) | 247 (14.9%) | 29 (42.6%) | <0.001 |
| Cerebrovascular disease | 201 (11.7%) | 182 (11.0%) | 19 (27.9%) | <0.001 |
| Chronic kidney disease | 260 (15.1%) | 234 (14.2%) | 26 (38.2%) | <0.001 |
| COPD | 66 (3.8%) | 60 (3.6%) | 6 (8.8%) | 0.029 |
| Liver disease | 44 (2.6%) | 39 (2.4%) | 5 (7.4%) | 0.011 |
| Malignancy | 131 (7.6%) | 119 (7.2%) | 12 (17.6%) | 0.001 |
| Symptoms, N (%) | ||||
| Fever | 367 (21.3%) | 342 (20.7%) | 25 (36.8%) | 0.002 |
| Cough and expectoration | 503 (29.2%) | 470 (28.4%) | 33 (48.5%) | < 0.001 |
| Pharyngalgia | 54 (3.1%) | 48 (2.9%) | 6 (8.8%) | 0.006 |
| Feeble | 210 (12.2%) | 196 (11.9%) | 14 (20.6%) | 0.031 |
| Runny nose | 33 (1.9%) | 30 (1.8%) | 3 (4.4%) | 0.126 |
| Vaccination status, N (%) | ||||
| Unvaccinated | 685 (39.8%) | 630 (38.1%) | 55 (80.9%) | < 0.001 |
| Partially vaccinated | 53 (3.1%) | 52 (3.1%) | 1 (1.5%) | 0.433 |
| Fully vaccinated | 455 (26.4%) | 452 (27.3%) | 3 (4.4%) | 0.001 |
| Booster vaccination | 528 (30.7%) | 519 (31.4%) | 9 (13.2%) | 0.001 |
| Oxygen support need, N (%) | ||||
| Non-invasive ventilation | 333 (19.3%) | 307 (18.6%) | 26 (38.2%) | <0.001 |
| High flow oxygen | 35 (2.0%) | 20 (1.2%) | 15 (22.1%) | <0.001 |
| Mechanical ventilation | 28 (1.6%) | 0 (0%) | 28 (41.2%) | <0.001 |
| Medications, N (%) | ||||
| Ritonavir/Darunavir | 620 (36.0%) | 591 (35.8%) | 29 (42.6%) | 0.246 |
| Heparin | 419 (24.3%) | 381 (23.0%) | 38 (55.9%) | <0.001 |
| Glucocorticoid | 100 (5.8%) | 75 (4.5%) | 25 (36.8%) | <0.001 |
| Vasoactive drugs | 38 (2.2%) | 0 (0%) | 38 (55.9%) | <0.001 |
Demographics and clinical characteristics of patients in the derivation cohort.
Values are expressed as median (IQR) or number of patients (%). ALT, alanine aminotransferase; AST, aspartate transaminase; TB, total bilirubin; WBC, white blood cell; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; CRP, C-reactive protein; PCT, procalcitonin; cTnT, cardiac troponin T; NT-proBNP, N-terminal forebrain natriuretic peptide; COPD, chronic obstructive pulmonary disease.
Predictors of critical illness risk in patients with omicron infection
The preliminary univariate and multivariate logistic regression analyses were used to identify predictors of critical illness with laboratory parameters and oxygen saturation at admission. The results of the univariate analyses showed that ALT, AST, TB, WBC, lymphocyte, creatinine, LDH, D-dimer, CRP, PCT, cTnT, NT-proBNP, and oxygen saturation were associated with critical illness. Multivariate analyses indicated that the WBC count (odds ration [OR]: 1.149; 95% confidence interval [CI]: 1.046-1.261; p = 0.004), lymphocyte count (OR: 0.297; 95% CI: 0.139-0.637; p = 0.002), LDH (OR: 1.008; 95% CI: 1.004-1.011; p < 0.001), CRP (OR: 1.007; 95% CI: 1.001-1.014; p = 0.020), and oxygen saturation on room air (OR: 0.955; 95% CI: 0.922-0.990; p = 0.011) were significant independent predictors of critical illness (Table 2).
TABLE 2
| Predictors | Univariate analysis | Multivariate analysis | ||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| ALT (U/L) | 1.004 (1.002-1.006) | <0.001 | 1.003 (0.990-1.015) | 0.126 |
| AST (U/L) | 1.005 (1.003-1.008) | <0.001 | 1.001 (0.991-1.011) | 0.836 |
| TB (umol/L) | 1.009 (1.002-1.016) | 0.011 | 1.004 (0.989-1.019) | 0.629 |
| WBC (*10^9/L) | 1.117 (1.078-1.158) | <0.001 | 1.149 (1.046-1.261) | 0.004 |
| Lymphocyte (*10^9/L) | 0.066 (0.037-0.119) | <0.001 | 0.297 (0.139-0.637) | 0.002 |
| Creatinine (umol/L) | 1.006 (1.002-1.011) | 0.004 | 1.006 (1.001-1.010) | 0.062 |
| LDH (U/L) | 1.007 (1.005-1.009) | <0.001 | 1.008 (1.004-1.011) | < 0.001 |
| D-dimer (FEUmg/L) | 1.074 (1.045-1.105) | <0.001 | 1.006 (0.958-1.057) | 0.800 |
| CRP (mg/L) | 1.020 (1.016-1.024) | <0.001 | 1.007 (1.001-1.014) | 0.020 |
| PCT (ng/ml) | 1.024 (1.007-1.041) | 0.006 | 1.025 (1.008-1.042) | 0.093 |
| Cardiac troponin T (ng/ml) | 2.793 (1.271-6.136) | 0.011 | 2.363 (0.670-8.329) | 0.181 |
| NT-proBNP (per 100 pg/ml increase) | 1.006 (1.005-1.008) | <0.001 | 1.002 (0.998-1.006) | 0.436 |
| Oxygen saturation on room air | 0.888 (0.860-0.917) | <0.001 | 0.955 (0.922-0.990) | 0.011 |
Univariate and multivariate analysis of laboratory parameters and oxygen saturation at admission predicting critical illness.
CI, confidence interval; OR, odds ration; ALT, alanine aminotransferase; AST, aspartate transaminase; TB, total bilirubin; WBC, white blood cell; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; CRP, C-reactive protein; PCT, procalcitonin; NT-proBNP, N-terminal forebrain natriuretic peptide.
Development of a practical scoring model for critical illness
In order to simplify the score and increase its reproducibility and applicability in other hospitals, the values of the five independent predictors mentioned above were transformed into categorical variables, and put into a multivariate logistic analysis including demographics, comorbidities and COVID-19 vaccination history. The result of multivariate analyses showed that history of malignancy was a significant predictor (OR: 6.084; 95% CI: 2.832-13.069; p < 0.001), and oxygen saturation < 90% on room air was a secondary positive predictor of critical illness (OR: 5.213; 95% CI: 2.839-9.573; p < 0.001). Other independent predictors for critical illness were chronic cardiac disease (OR: 3.288; 95% CI: 1.933-5.593; p < 0.001), CRP > 10 mg/L (OR: 3.259; 95% CI: 1.491-7.126; p = 0.003), chronic kidney disease (OR: 3.200; 95% CI: 1.863-5.498; p < 0.001), LDH > 250 U/L (OR: 3.113; 95% CI: 1.833-5.288; p < 0.001), COPD (OR: 3.040; 95% CI: 1.209-7.642; p = 0.018), age > 65 (OR: 2.917; 95% CI: 1.471-5.786; p = 0.002), non-vaccination (OR: 2.671; 95% CI: 1.356-5.259; p = 0.005), lymphocyte < 0.8*10^9/L (OR: 2.659; 95% CI: 1.570-4.504; p < 0.001), diabetes (OR: 2.451; 95% CI: 1.439-4.173; p = 0.001), cerebrovascular disease (OR: 2.036; 95% CI: 1.093-3.796; p = 0.025), and WBC > 10*10^9/L (OR: 1.933; 95% CI: 1.038-3.599; p = 0.038) (Table 3).
TABLE 3
| Predictors | Univariate analysis | Multivariate analysis | ||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age > 65 | 6.603 (3.727-11.698) | <0.001 | 2.917 (1.471-5.786) | 0.002 |
| Male sex | 1.895 (1.247-2.882) | 0.003 | 1.013 (0.613-1.674) | 0.742 |
| WBC > 10 (*10^9/L) | 6.032 (3.826-9.511) | <0.001 | 1.933 (1.038-3.599) | 0.038 |
| Lymphocyte < 0.8 (*10^9/L) | 6.368 (4.180-9.703) | <0.001 | 2.659 (1.570-4.504) | <0.001 |
| LDH > 250 (U/L) | 8.925 (5.840-13.640) | <0.001 | 3.113 (1.833-5.288) | <0.001 |
| CRP > 10 (mg/L) | 12.816 (6.424-25.568) | <0.001 | 3.259 (1.491-7.126) | 0.003 |
| Oxygen saturation < 90% on room air | 9.835 (6.175-15.665) | <0.001 | 5.213 (2.839-9.573) | <0.001 |
| Hypertension | 1.627 (1.076-2.458) | 0.021 | 1.090 (0.536-1.978) | 0.930 |
| Diabetes | 4.574 (3.034-6.895) | <0.001 | 2.451 (1.439-4.173) | 0.001 |
| Chronic cardiac disease | 8.049 (5.302-12.219) | <0.001 | 3.288 (1.933-5.593) | <0.001 |
| Chronic kidney disease | 6.369 (4.193-9.673) | <0.001 | 3.200 (1.863-5.498) | <0.001 |
| COPD | 3.666 (1.763-7.622) | <0.001 | 3.040 (1.209-7.642) | 0.018 |
| Liver disease | 2.585 (1.085-6.158) | 0.032 | 1.844 (0.522-6.515) | 0.342 |
| Cerebrovascular disease | 4.512 (2.838-7.174) | <0.001 | 2.036 (1.093-3.796) | 0.025 |
| Malignancy | 3.470 (1.969-6.114) | <0.001 | 6.084 (2.832-13.069) | <0.001 |
| Non-vaccination | 2.837 (1.875-4.294) | <0.001 | 2.671 (1.356-5.259) | 0.005 |
Univariate and multivariate analysis of factors predicting critical illness.
CI, confidence interval; OR, odds ration; WBC, white blood cell; LDH, lactate dehydrogenase; CRP, C-reactive protein; COPD, chronic obstructive pulmonary disease.
In order to develop a useful clinical predicting tool, relative weights were assigned according to the regression coefficient of each categorical variable (Figure 2), and each patient had a prognostic score of critical illness. The sum of scores from each of the thirteen predictors ranged from 0 to 36 in this study, and patients were divided into high-risk and low-risk groups with the cut-off value of 13. The area under the ROC (AUROC) of the derivation cohort was 0.926 (95% CI, 0.903–0.948), with sensitivity of 81.2% and specificity of 89.8% (Figure 3A).
FIGURE 2
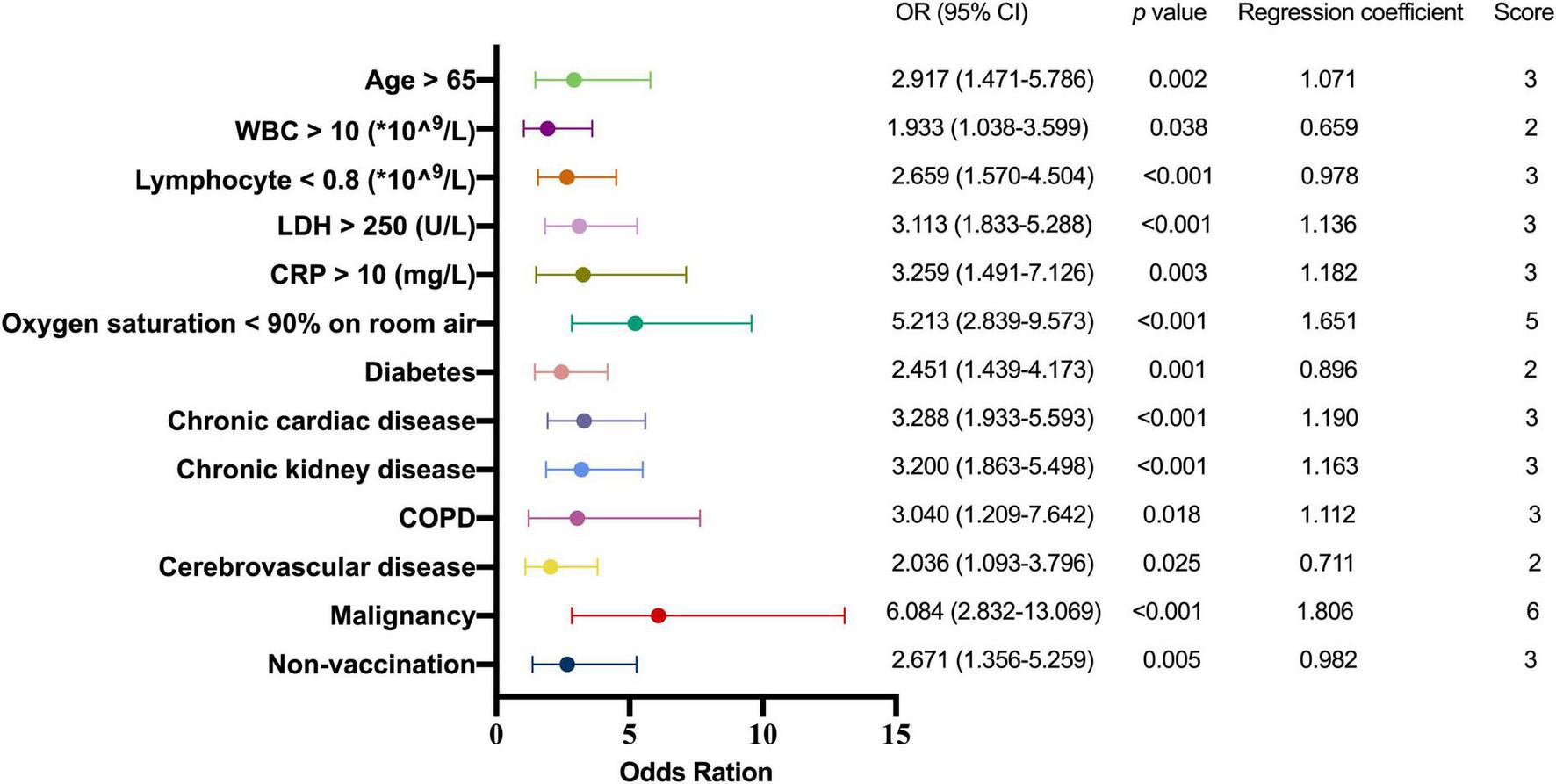
Multivariate logistic regression analysis of factors predicting critical illness. OR, odds ration; WBC, white blood cell; LDH, lactate dehydrogenase; CRP, C-reactive protein; COPD, chronic obstructive pulmonary disease.
FIGURE 3
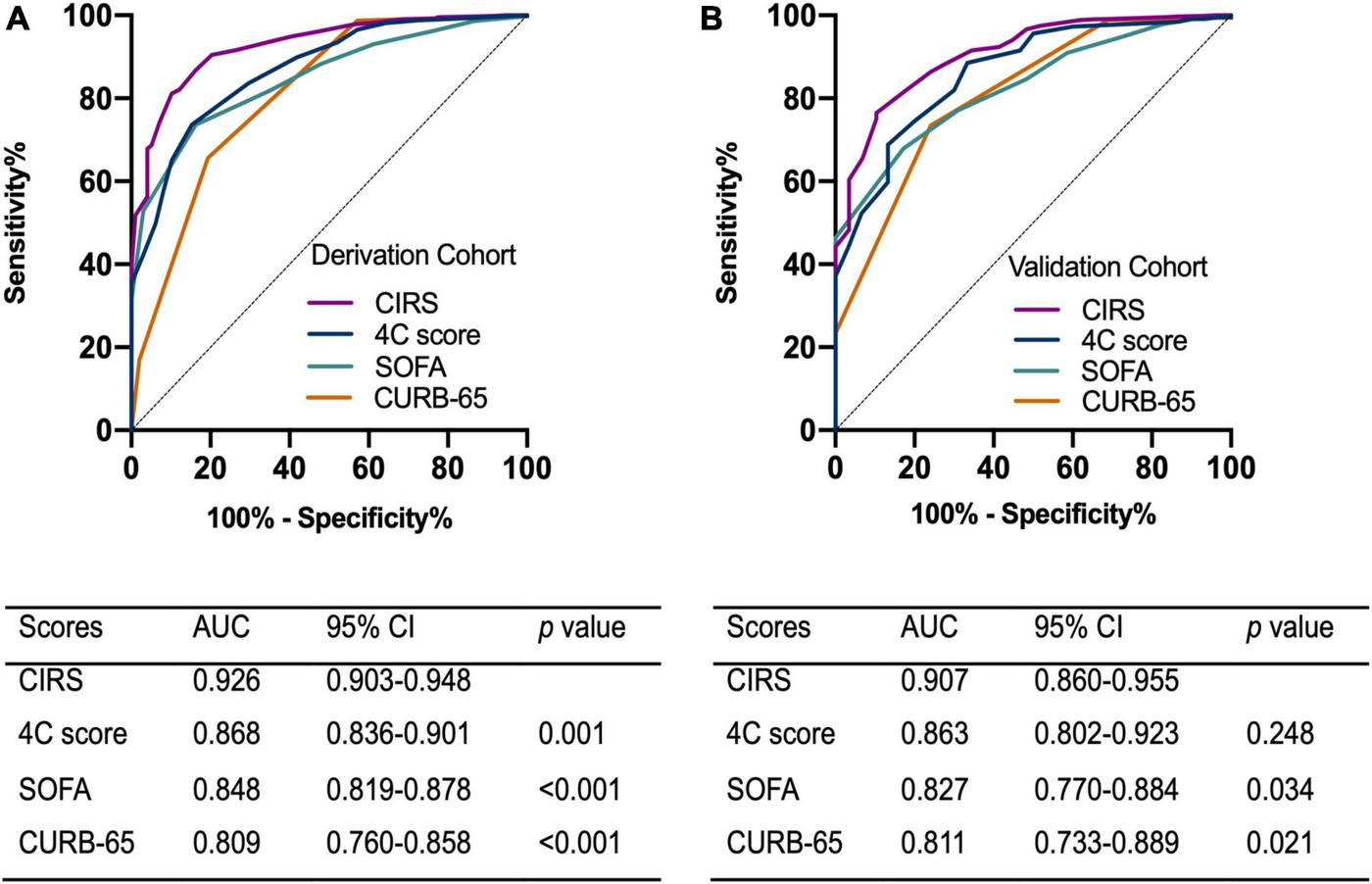
Receiver operating characteristic curve of the scoring models on the prediction of critical illness in (A) the derivation cohort (B) and the validation cohort. AUC, area under curve; CI, confidence interval; CIRS, critical illness risk score; SOFA, sequential organ failure assessment.
Validation of the clinical scoring model
The validation cohort included 738 patients with a median age of 66 years. Critical illness eventually developed in 30 of these patients and six died. The clinical and laboratory characteristics of the derivation and validation cohorts are listed in Supplementary Table 1. The two cohorts differed on CRP, history of hypertension, symptoms on admission including fever, cough and expectoration. Compared with derivation cohort, there were more patients with a history of hypertension (52.4% vs. 46.8%, p = 0.010), while fewer patients had fever (14.1% vs. 21.3%, p < 0.001), cough and expectoration (23.3% vs. 29.2%, p = 0.003) on admission in the validation cohort. The potency of the clinical scoring model to predict critical illness in patients with Omicron infection was assessed using the area under the ROC in the validation cohort. The AUROC of the validation cohort was 0.907 (95% CI, 0.860-0.955) (Figure 3B). The critical illness risk score (CIRS) when compared with the CURB-65, SOFA, and 4C mortality scores displayed an AUROC that was significantly higher in both cohorts. Furthermore, the better performance of the CIRS was further demonstrated by significant improvements in reclassification as assessed by continuous NRI and IDI in both cohorts (Supplementary Table 2).
All 2459 patients in this study were pooled to further assess the correlation between the clinical scoring model and the rate of critical illness events. The score was categorized into two groups with high risk (score > 13) and low risk (score < 13) of critical illness. Among patients with score of 0-6, 0.1% had developed critical illness, as compared to 1.0% of patients with score of 7-13, 9.7% of patients with score of 14-20, 48.1% and 86.7% of patients with score of 21-26 and > 26, respectively (Figure 4). For the summed clinical scores of 0-13 and 14-36, the critical illness event was 0.1-1.0% and 9.7%-86.7%, respectively.
FIGURE 4
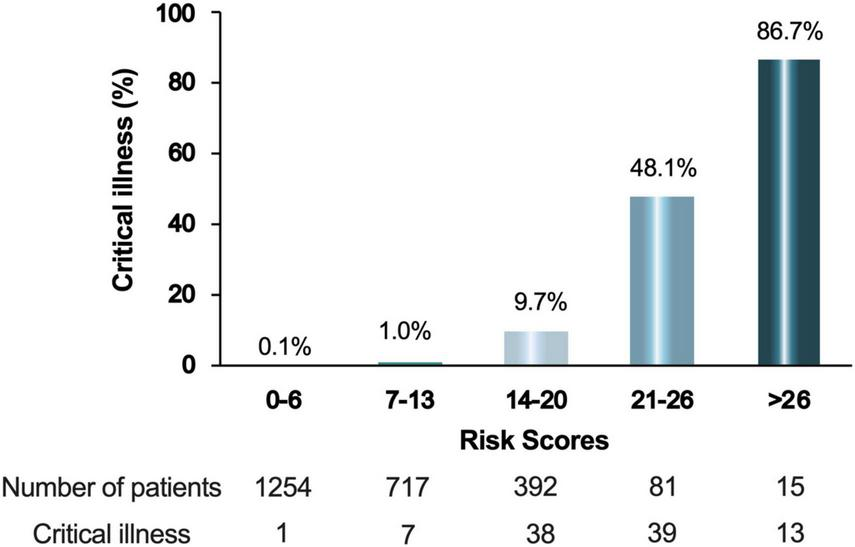
Percentage of critical ill patients according to the score. The integer scores were converted by rounding the odds ratios of the predictors and the final score was the sum of these values. Patients were divided into 5 groups according to their scores.
Discussion
In this study, we tested the extent to which demographic, routine laboratory parameters, and comorbidities predicted the critical illness events. We found that age, LDH, CRP, WBC count, lymphocyte count, oxygen saturation, the history of malignancy, COPD, diabetes, chronic cardiac disease, chronic kidney disease, cerebrovascular disease, and non-vaccination were all independent predictors for critical illness. Furthermore, we developed and validated a clinical scoring model to predict the development of critical illness among hospitalized SARS-CoV-2 infected patients. The performance of this risk scoring model was satisfactory with accuracy based on AUROCs in both the derivation and validation cohorts.
COVID-19 is a heterogeneous disease of varying severity and prognosis (Samadizadeh et al., 2021). An increasing number of studies have analyzed the associated factors in patients with COVID-19. Previous studies have shown that the predictors of severe prognosis in patients with COVID-19 included age, sex, CRP, LDH, lymphocyte count, D-dimer, and comorbidity (including hypertension, diabetes, cardiovascular disease, respiratory disease) (Huang H. et al., 2020; Liang et al., 2020b; Wynants et al., 2020). These predictors coincide with most of those we have observed in the multivariable analysis and included in the predictive models. Some studies have shown that the increase of CRP in critical illness may be related to cytokine storms, which produce a series of inflammatory responses and cause disorders in peripheral WBCs (Chen et al., 2020), while the decrease in lymphocytes indicated that coronavirus consumed many immune cells and inhibited the body’s cellular immune function (Liu et al., 2017). LDH may be related to respiratory function and the increase of LDH will cause greater disease severity (Poggiali et al., 2020). In addition, some other studies have suggested that radiological findings, immunological index, tobacco and comorbidities as obesity may also be associated with a worse outcome (Huang Y. et al., 2020; Li et al., 2020; Ruch et al., 2020; Zheng et al., 2020; Surme et al., 2021). We could not assess the effect of the variables such as body mass index and smoking history since the data from electronic medical records were incomplete.
In addition, in our study, the finding was expected that age and complications were important predictors for critical illness. An analysis using propensity score matching (PSM) was further conducted to reduce the effects of age and comorbidities. Based on a caliper set as 0.02, a 1:1match was achieved using the nearest neighbor-matching algorithm. After age-, sex- and comorbidities-matching, 196 patients (98 patients each for critical illness and non-critical illness groups) were analyzed to identify independent predictors of critical illness. Demographics and clinical characteristics between the two groups were shown in Supplementary Table 3. The result showed that WBC > 10*10^9/L, CRP > 10 mg/L, LDH > 250 U/L, lymphocyte < 0.8*10^9/L, Oxygen saturation < 90%, and non-vaccination were still independent predictors for critical illness (Supplementary Table 4). Therefore, patients with abnormal WBC, lymphocyte, LDH, CRP, Oxygen saturation, and non-vaccination were at a higher risk of developing critical illness than the general population, under the same age, sex, and presence of comorbidities.
Although most patients have mild or moderate disease, Omicron infection can progress to severe disease and result in acute respiratory distress syndrome, multiorgan failure and death (Wu and McGoogan, 2020). Since early intervention was helpful to improve the prognosis (Garcia-Del-Barco et al., 2021; Qian et al., 2022), early stratifying of patients based on disease severity is vital. CURB-65 is a commonly applied severity score in community-acquired pneumonia management, and it has also been previously validated in different populations of COVID-19 positive patients (Nguyen et al., 2020; Artero et al., 2021; Elmoheen et al., 2021). The SOFA score has been proven to have a high predictive value for intensive care unit (ICU) mortality in severely ill patients (Vincent et al., 1996; Liu et al., 2020). The 4C mortality score is a well-established model that has been validated internally and externally to predict the outcome of COVID-19 (Knight et al., 2020). In our study, we compared the predictive ability of the proposed CIRS model with the SOFA, CURB-65 and 4C mortality scores, based on the AUROC. We found that the CIRS performed best. Moreover, the IDI and NRI indices also supported this conclusion.
Compared with the original and the Delta strains, the pathogenicity of Omicron greatly weakened, while it has become extremely infectious (He et al., 2021). Our risk scoring model can divide patients into high-risk and low-risk groups at admission. The stratified analysis of patients in the high-risk group found that patients with higher scores had higher critical illness rate. These findings indicated that when managing COVID-19 patients, special attention should be paid to those patients with higher risk score.
This study had several limitations. First, this is a single-center and retrospective study. Second, only 98 patients who have developed critical illness in our study, and it is likely that the AUROC overestimates the predictive power of the model. Third, although in our study, patients were divided into derivation cohort and validation cohort, the risk scoring model has a certain predictive value, the model was not been verified in the external validation cohort. Moreover, a large-scale multicenter prospective study is warranted to validate the risk model in the future.
In summary, we developed a clinical scoring model to estimate the risk of developing critical illness among patients with Omicron infection based on thirteen variables commonly measured on admission to the hospital. We believe that the results from this study will help clinicians stratify risk and provide opportunity for early intervention.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Huashan Hospital, Fudan University. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
YZ, JH, FS, YuG, YiG, HaZ, FL, ZX, SM, HuZ, ZG, JY, YoZ, LQ, KM, RM, and JZ contributed to study concept and design. YZ and JH accessed and verified all the data and did the statistical analysis. YZ drafted the manuscript. YZ, JH, FS, YuG, YiG, and HaZ conducted the collection of clinical data. FL, ZX, SM, HuZ, ZG, JY, YoZ, and LQ contributed to the data interpretation. KM and RM did critical revision of manuscript for important intellectual content. JZ supervised the whole project. All authors have read and approved the final version of the manuscript.
Funding
This study was financially supported by Shanghai Municipal Science and Technology Major Project (ZD2021CY001) and Shanghai Key Clinical Specialty Construction Program (ZK2019B24).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1031231/full#supplementary-material
References
1
Artero A. Madrazo M. Fernández-Garcés M. Muiño Miguez A. González García A. Crestelo Vieitez A. et al (2021). Severity scores in COVID-19 pneumonia: A multicenter. Retrospective, cohort Study.J. Gen. Intern. Med.361338–1345. 10.1007/s11606-021-06626-7
2
Başaran N. Ç Özdede M. Uyaroğlu O. A. Şahin T. K. Özcan B. Oral H. et al (2022). Independent predictors of in-hospital mortality and the need for intensive care in hospitalized non-critical COVID-19 patients: A prospective cohort study.Intern. Emerg. Med.171413–1424. 10.1007/s11739-022-02962-6
3
Chen N. Zhou M. Dong X. Qu J. Gong F. Han Y. et al (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan. China: A descriptive study.Lancet395507–513. 10.1016/S0140-6736(20)30211-7
4
Elmoheen A. Abdelhafez I. Salem W. Bahgat M. Elkandow A. Tarig A. et al (2021). External validation and recalibration of the CURB-65 and PSI for predicting 30-day mortality and critical care intervention in multiethnic patients with COVID-19.Int. J. Infect. Dis.111108–116. 10.1016/j.ijid.2021.08.027
5
Garcia-Del-Barco D. Risco-Acevedo D. Berlanga-Acosta J. Martos-Benítez F. D. Guillén-Nieto G. (2021). Revisiting pleiotropic effects of type I interferons: Rationale for Its prophylactic and therapeutic use against SARS-CoV-2.Front. Immunol.12:655528. 10.3389/fimmu.2021.655528
6
Guan W. J. Liang W. H. Zhao Y. Liang H. R. Chen Z. S. Li Y. M. et al (2020). Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis.Eur. Respir. J.55:2000547. 10.1183/13993003.00547-2020
7
Han H. Ma Q. Li C. Liu R. Zhao L. Wang W. et al (2020). Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors.Emerg. Microbes Infect.91123–1130. 10.1080/22221751.2020.1770129
8
He X. Hong W. Pan X. Lu G. Wei X. (2021). SARS-CoV-2 Omicron variant: Characteristics and prevention.Med. Comm.2838–845. 10.1002/mco2.110
9
Heller R. A. Sun Q. Hackler J. Seelig J. Seibert L. Cherkezov A. et al (2021). Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker.Redox Biol.38:101764. 10.1016/j.redox.2020.101764
10
Huang C. Wang Y. Li X. Ren L. Zhao J. Hu Y. et al (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China.Lancet395497–506. 10.1016/S0140-6736(20)30183-5
11
Huang H. Cai S. Li Y. Li Y. Fan Y. Li L. et al (2020). Prognostic factors for COVID-19 pneumonia progression to severe symptoms based on earlier clinical features: A retrospective analysis.Front. Med. (Lausanne)7:557453. 10.3389/fmed.2020.557453
12
Huang Y. Lu Y. Huang Y. M. Wang M. Ling W. Sui Y. et al (2020). Obesity in patients with COVID-19: A systematic review and meta-analysis.Metabolism113:154378. 10.1016/j.metabol.2020.154378
13
Islam O. K. Al-Emran H. M. Hasan M. S. Anwar A. Jahid M. Hossain M. A. (2021). Emergence of European and North American mutant variants of SARS-CoV-2 in South-East Asia.Transbound. Emerg. Dis.68824–832. 10.1111/tbed.13748
14
Knight S. R. Ho A. Pius R. Buchan I. Carson G. Drake T. M. et al (2020). Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: Development and validation of the 4C Mortality Score.BMJ370:m3339. 10.1136/bmj.m3339
15
Lacap E. M. Jr. Varghese A. Khamis F. Al Bahrani M. Al Naamani H. Kolamban S. et al (2022). Development and validation of the CoV19-OM intensive care unit score: An early ICU identification for laboratory-confirmed SARS-CoV-2 patients from a retrospective cohort study in Oman.Int. J. Infect. Dis.117241–246. 10.1016/j.ijid.2021.04.069
16
Li Q. Zhang J. Ling Y. Li W. Zhang X. Lu H. et al (2020). A simple algorithm helps early identification of SARS-CoV-2 infection patients with severe progression tendency.Infection48577–584. 10.1007/s15010-020-01446-z
17
Liang W. Liang H. Ou L. Chen B. Chen A. Li C. et al (2020a). Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19.JAMA Intern. Med.1801081–1089. 10.1001/jamainternmed.2020.2033
18
Liang W. Yao J. Chen A. Lv Q. Zanin M. Liu J. et al (2020b). Early triage of critically ill COVID-19 patients using deep learning.Nat. Commun.11:3543. 10.1038/s41467-020-17280-8
19
Liu S. Yao N. Qiu Y. He C. (2020). Predictive performance of SOFA and qSOFA for in-hospital mortality in severe novel coronavirus disease.Am. J. Emerg. Med.382074–2080. 10.1016/j.ajem.2020.07.019
20
Liu W. J. Zhao M. Liu K. Xu K. Wong G. Tan W. et al (2017). T-cell immunity of SARS-CoV: Implications for vaccine development against MERS-CoV.Antiviral. Res.13782–92. 10.1016/j.antiviral.2016.11.006
21
Nguyen Y. Corre F. Honsel V. Curac S. Zarrouk V. Fantin B. et al (2020). Applicability of the CURB-65 pneumonia severity score for outpatient treatment of COVID-19.J. Infect.81e96–e98. 10.1016/j.jinf.2020.05.049
22
Petersen M. S. Í Kongsstovu S. Eliasen E. H. Larsen S. Hansen J. L. Vest N. et al (2022). Clinical characteristics of the Omicron variant-results from a nationwide symptoms survey in the faroe Islands.Int. J. Infect. Dis.122636–643. 10.1016/j.ijid.2022.07.005
23
Poggiali E. Zaino D. Immovilli P. Rovero L. Losi G. Dacrema A. et al (2020). Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in CoVID-19 patients.Clin. Chim. Acta509135–138. 10.1016/j.cca.2020.06.012
24
Qian J. Wang M. You H. Luo J. Li S. Chen S. et al (2022). Early renal-replacement therapy may reduce the all-cause mortality of severe COVID-19: An observational cohort study.Blood Purif.511006–1014. 10.1159/000524229
25
Ruch Y. Kaeuffer C. Ohana M. Labani A. Fabacher T. Bilbault P. et al (2020). CT lung lesions as predictors of early death or ICU admission in COVID-19 patients.Clin. Microbiol. Infect.26.e5–.e8. 10.1016/j.cmi.2020.07.030
26
Samadizadeh S. Masoudi M. Rastegar M. Salimi V. Shahbaz M. B. Tahamtan A. (2021). COVID-19: Why does disease severity vary among individuals?Respir. Med.180:106356. 10.1016/j.rmed.2021.106356
27
Surme S. Buyukyazgan A. Bayramlar O. F. Cinar A. K. Copur B. Zerdali E. et al (2021). Predictors of intensive care unit admission or mortality in patients with coronavirus disease 2019 pneumonia in Istanbul. Turkey.Jpn. J. Infect. Dis.74458–464. 10.7883/yoken.JJID.2020.1065
28
Vincent J. L. Moreno R. Takala J. Willatts S. De Mendonça A. Bruining H. et al (1996). The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine.Intensive Care Med.22707–710. 10.1007/BF01709751
29
Wu Z. McGoogan J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention.JAMA3231239–1242. 10.1001/jama.2020.2648
30
Wynants L. Van Calster B. Collins G. S. Riley R. D. Heinze G. Schuit E. et al (2020). Prediction models for diagnosis and prognosis of covid-19: Systematic review and critical appraisal.BMJ369:m1328. 10.1136/bmj.m1328
31
Ye L. Li W. F. Shao J. Xu Z. Ju J. Xu H. (2022). Fighting omicron epidemic in China: Real-world big data from Fangcang shelter hospital during the outbreak in Shanghai 2022.J. Infect.85436–480. 10.1016/j.jinf.2022.07.006
32
Yuan W. Hou Y. Lin Q. Chen L. Ren T. (2022). How China responds to Omicron.J. Infect.8590–122. 10.1016/j.jinf.2022.04.017
33
Zheng Z. Peng F. Xu B. Zhao J. Liu H. Peng J. et al (2020). Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis.J. Infect.81e16–e25. 10.1016/j.jinf.2020.04.021
34
Zhu N. Zhang D. Wang W. Li X. Yang B. Song J. et al (2020). A novel coronavirus from patients with pneumonia in China, 2019.N. Engl. J. Med.382727–733. 10.1056/NEJMoa2001017
Summary
Keywords
COVID-19, severe acute respiratory syndrome coronavirus 2, omicron, critical illness, risk factors
Citation
Zhang Y, Han J, Sun F, Guo Y, Guo Y, Zhu H, Long F, Xia Z, Mao S, Zhao H, Ge Z, Yu J, Zhang Y, Qin L, Ma K, Mao R and Zhang J (2022) A practical scoring model to predict the occurrence of critical illness in hospitalized patients with SARS-CoV-2 omicron infection. Front. Microbiol. 13:1031231. doi: 10.3389/fmicb.2022.1031231
Received
29 August 2022
Accepted
05 December 2022
Published
19 December 2022
Volume
13 - 2022
Edited by
Matthaios Papadimitriou-Olivgeris, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland
Reviewed by
Jiezuan Yang, Zhejiang University, China; Ingeborg D. Welters, University of Liverpool, United Kingdom
Updates
Copyright
© 2022 Zhang, Han, Sun, Guo, Guo, Zhu, Long, Xia, Mao, Zhao, Ge, Yu, Zhang, Qin, Ma, Mao and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke Ma, KMA@163.comRicheng Mao, njxiaomao@163.comJiming Zhang, jmzhang@fudan.edu.cn
†These authors have contributed equally to this work and share first authorship
This article was submitted to Virology, a section of the journal Frontiers in Microbiology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.