- 1Department of Urology Surgery, First Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
- 2The First Clinical Medical College, Shanxi Medical University, Taiyuan, Shanxi, China
- 3Department of Urology Surgery, Capital Medical University, Beijing, China
Background: Several reports in recent years have found an association between gut microbiota and upper urinary urolithiasis. However, the causal relationship between them remains to be clarified.
Methods: Genetic variation is used as a tool in Mendelian randomization for inference of whether exposure factors have a causal effect on disease outcomes. We selected summary statistics from a large genome-wide association study of the gut microbiome published by the MiBioGen consortium with a sample size of 18,340 as an exposure factor and upper urinary urolithiasis data from FinnGen GWAS with 4,969 calculi cases and 213,445 controls as a disease outcome. Then, a two-sample Mendelian randomization analysis was performed by applying inverse variance-weighted, MR-Egger, maximum likelihood, and weighted median. In addition, heterogeneity and horizontal pleiotropy were excluded by sensitivity analysis.
Results: IVW results confirmed that class Deltaproteobacteria (OR = 0.814, 95% CI: 0.666–0.995, P = 0.045), order NB1n (OR = 0.833, 95% CI: 0.737–0.940, P = 3.15 × 10−3), family Clostridiaceae1 (OR = 0.729, 95% CI: 0.581–0.916, P = 6.61 × 10−3), genus Barnesiella (OR = 0.695, 95% CI: 0.551–0.877, P = 2.20 × 10−3), genus Clostridium sensu_stricto_1 (OR = 0.777, 95% CI: 0.612–0.986, P = 0.0380), genus Flavonifractor (OR = 0.711, 95% CI: 0.536–0.944, P = 0.0181), genus Hungatella (OR = 0.829, 95% CI: 0.690–0.995, P = 0.0444), and genus Oscillospira (OR = 0.758, 95% CI: 0.577–0.996, P = 0.0464) had a protective effect on upper urinary urolithiasis, while Eubacterium xylanophilum (OR =1.26, 95% CI: 1.010–1.566, P = 0.0423) had the opposite effect. Sensitivity analysis did not find outlier SNPs.
Conclusion: In summary, a causal relationship was found between several genera and upper urinary urolithiasis. However, we still need further randomized controlled trials to validate.
1. Introduction
Urinary stones are one of the most frequent benign diseases with a high incidence of up to 20% worldwide (Hoffman et al., 2021). The most troubled and most studied of urolithiasis is the upper urinary stones, including nephrolithiasis and ureterolithiasis derived from the renal. The prevalence of renal stones is projected to rise further as the growing population with associated diseases including diabetes and hypertension and the changing environmental trends of global warming (Carbone et al., 2018; Johnson et al., 2019). It will likely cause complications including urinary obstruction, infection, pain, and even permanent damage to renal function (Rule et al., 2020). Renal stones are also generally considered to be a lifelong disease with high recurrence (Corbo and Wang, 2019), which has a tremendous influence on individuals and society, and has emerged as a substantial public health issue (Johnson et al., 2019). The mechanism of formation and growth of renal stones is complicated. A variety of processes including supersaturation of urinary stone components, reduction of inhibitors of stone formation (Cicerello et al., 2019), and renal tubular epithelial cell injury (Aggarwal et al., 2013) are involved. Metabolism and inflammation are considered important factors involved in the formation of renal stones (Tian et al., 2022; Capolongo et al., 2023).
The gut microbiota is the largest micro-ecosystem of the human body, participating in and influencing the metabolism of the substance and energy (Anand and Mande, 2022). Crosstalk between the gut microbiome and kidney has been widely documented, for example, intestinal ecological disorders are often found in patients with chronic kidney disease (Voroneanu et al., 2023). The role of gut microbiome in the pathogenesis of kidney stones has also attracted more and more attention. There are significant changes in gut microbiota in patients with and without renal calculi (Siener et al., 2013b; Stern et al., 2016; Tavasoli et al., 2020; Kim et al., 2022). Furthermore, the normal group had a greater abundance of Bifidobacterium (Kim et al., 2022). Stern reported that Bacillus was 3.4 times more abundant in the stone group and Prevotella was 2.8 times more abundant in the non-stone group (Stern et al., 2016). Some microbial producers of short-chain fatty acids deserved our attention, and an observational study found that the proportion of some key taxa responsible for the production of short-chain fatty acids decreased in groups with nephrolithiasis (Liu et al., 2020). Moreover, many studies have focused on Oxalobacter formigenes, Bifidobacterium, and Lactobacillus due to the oxalic acid-degrading ability (Siener et al., 2013a; Tavasoli et al., 2020). Oxalobacter formigenes can stimulate the secretion of oxalate in the colon, thereby reducing oxalic acid levels in the urine (Allison et al., 1985). Some studies found that individuals with oxalate stones had considerably greater urine oxalate excretion and very low levels of Oxalobacter formigenes compared to controls, so it can be assumed that the formation of oxalate stones is related to the lack of colonization by Oxalobacter formigenes (Siener et al., 2013b; Tavasoli et al., 2020). However, clinical supplementation with Oxalobacter reagents did not improve blood and urine oxalate levels in patients with hyperoxaluria in another study (Siener et al., 2013b). Therefore, exploring the causal relationship between gut microbiota and calculi may provide new targets and ideas for the prevention and treatment of upper urinary urolithiasis.
In conclusion, since the gut microbiota is a complex ecosystem, there may be regulatory networks among various types of bacteria as well as the presence of some confounding factors that limit the causal inference between intestinal flora and renal calculi disease. Mendelian Randomization (MR) can be employed to infer the causal link between exposure factors and disease through genetic variation. MR provides a more convenient method for exploration of potential protective and risk factors for disease and has been applied to several research studies on the relationship between gut microbiota and diseases (Freidin et al., 2021; Liu et al., 2022; Luo et al., 2022). The genome-wide association study (GWAS) summary datasets about gut microbiota and renal and ureter stones were applied to this analysis.
2. Materials and methods
2.1. Data source
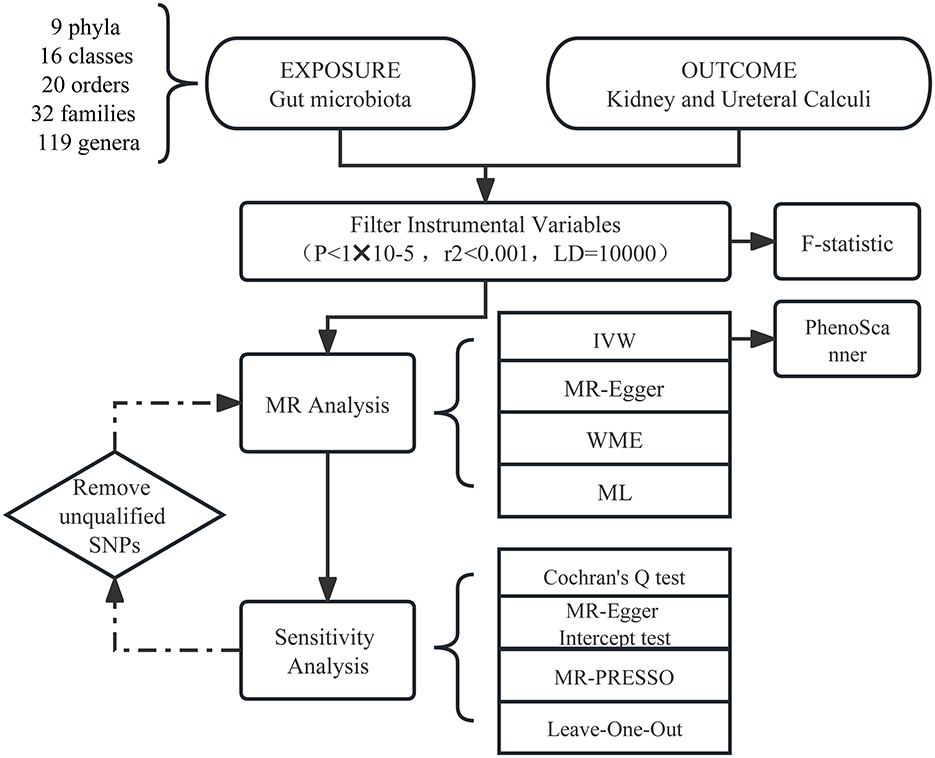
Our workflow diagram is presented in Figure 1. The MiBioGen group released the biggest genome-wide meta-analysis of gut microbiota composition, which included genetic variation data for the gut microbiota (Kurilshikov et al., 2021). The research contains 16SrRNA gene sequencing profiles and genotyping data from 18,340 individuals from the United States, the United Kingdom, Finland, Sweden, Denmark, the Netherlands, and other countries. Nine phyla, 16 classes, 20 orders, 35 families, and 131 genera (Supplementary Table 1) of bacteria were classified in the summary data of this study. Then, we excluded three unknown families and 12 unknown genera. Since genus is the minimal level of bacterial classification, we enrolled nine phyla, 16 classes, 20 orders, 32 families, and 119 genera in the subsequent MR analysis.
Several summary statistics for urolithiasis were available (Supplementary Table 2), and to ensure the credibility of the data, we chose GWAS data with the highest number of SNPs published in 2021 from FinnGen (Kurki et al., 2023). The phenotype “Calculus of renal and ureter” was used, and this GWAS had 218,414 Finnish adult subjects including 4,969 cases and 213,445 controls.
2.2. Filter instrumental variables
Gut microbiota and urolithiasis were exposure factors and outcomes, respectively. Valid IVs must satisfy three key assumptions (Slob and Burgess, 2020): (1) The correlation hypothesis: instrumental variables are strongly correlated with exposure. (2) The exclusivity hypothesis: instrumental variables are independent of the outcome. (3) The independence assumption: instrumental variables are independent of confounding factors. Thus, we made the following criteria. A P-value of < 1*10−5 was chosen as the significance threshold to avoid too few single-nucleotide polymorphisms (SNPs) (Sanna et al., 2019). Linkage disequilibrium (LD) is a phenomenon in which two genes at different seats in a population are inherited at a significantly higher frequency than would be expected at random (Roze, 2023). To avoid LD, we set the chain imbalance threshold r2 <0.001 and the distance to 10,000 kb. Palindromic variation means if the alleles are A and T (or C and G), then the same alleles will appear on the plus and minus chains (Girault and Ménigot, 2022). Thus, Palindromic SNPs were removed to prevent inconsistent SNP orientation in the exposure and outcome. Second, IVs should fulfill a P-value of >1*10−5 in the outcome for the p-value according to assumption (2). The PhenoScanner (Kamat et al., 2019) online tool was used to inquire whether the SNPs were related to confounders of urolithiasis according to the European Association of Urology Guidelines section on urolithiasis (Zeng et al., 2022). Then we excluded the relevant SNPs.
2.3. Statistical analysis
The inverse variance-weighted (IVW) approach was used as the principal analysis method, while three other methods, namely MR-Egger regression, weighted median analysis (WME), and maximum likelihood (ML), were used as secondary references. For exposure factors with individual SNP, the IVW technique provided a consistent estimate when all SNPs were believed to be genuine and the presence of an intercept term was not taken into account. The WME method was premised on the assumption that over half of the SNPs had valid IVs (Bowden et al., 2016). The MR-Egger method assumes that all SNPs were invalid instrumental variables and defaulted to the presence of an intercept term (Bowden et al., 2015). Further sensitivity analysis was carried out only when the IVW results were meaningful.
The F-statistic was utilized to determine the intensity of IVs (F denotes the fraction of variance explained by genetic variation in exposure, n means sample size, and k represents the number of SNPs) (Pierce et al., 2011). When the F-statistic for SNP was more than 10, it was assumed that there was no substantial weak instrumental bias; otherwise, the instrumental variable should be omitted. After removing the corresponding IVs that did not qualify as described above, the MR analysis was rerun to acquire the final MR estimations. When there did not exist heterogeneity and pleiotropy, the IVW results were trustworthy (Bowden et al., 2015). Effect estimates were expressed as odds ratio for binary outcomes.
2.4. Sensitivity analysis
The sensitivity analysis involved a heterogeneity test and a multiplicity of validity test. Cochran's Q-test was performed to confirm IV heterogeneity, with a p-value <0.05 indicating the lack of heterogeneity. MR-PRESSO summed the residuals for each SNP to assess the magnitude of horizontal pleiotropy. The MR-PRESSO outlier test allowed the assessment of outlier SNPs that contributed to the presence of pleiotropy at the overall level. The impact of one outlier on the overall results was assessed by calculating the remaining SNP effects after removing individual SNPs one by the leave-one-out analysis. Both MR-PRESSO and leave-one-out analysis methods could identify and remove SNPs that exhibited pleiotropy or heterogeneity. MR Steiger test was also carried out (Xue and Pan, 2020) to investigate the correctness of the causal direction. Additionally, we performed the reverse Mendelian randomization analysis.
MR analyses were carried out using the R (version 4.1.2) computational environment and the TwoSampleMR (version 0.5.6) and MR-PRESSO packages (version 1.0). The R package “ggplot2” was applied for drawing some figures. For evidence of causal effects, a p-value of <0.05 was judged statistically significant.
3. Results
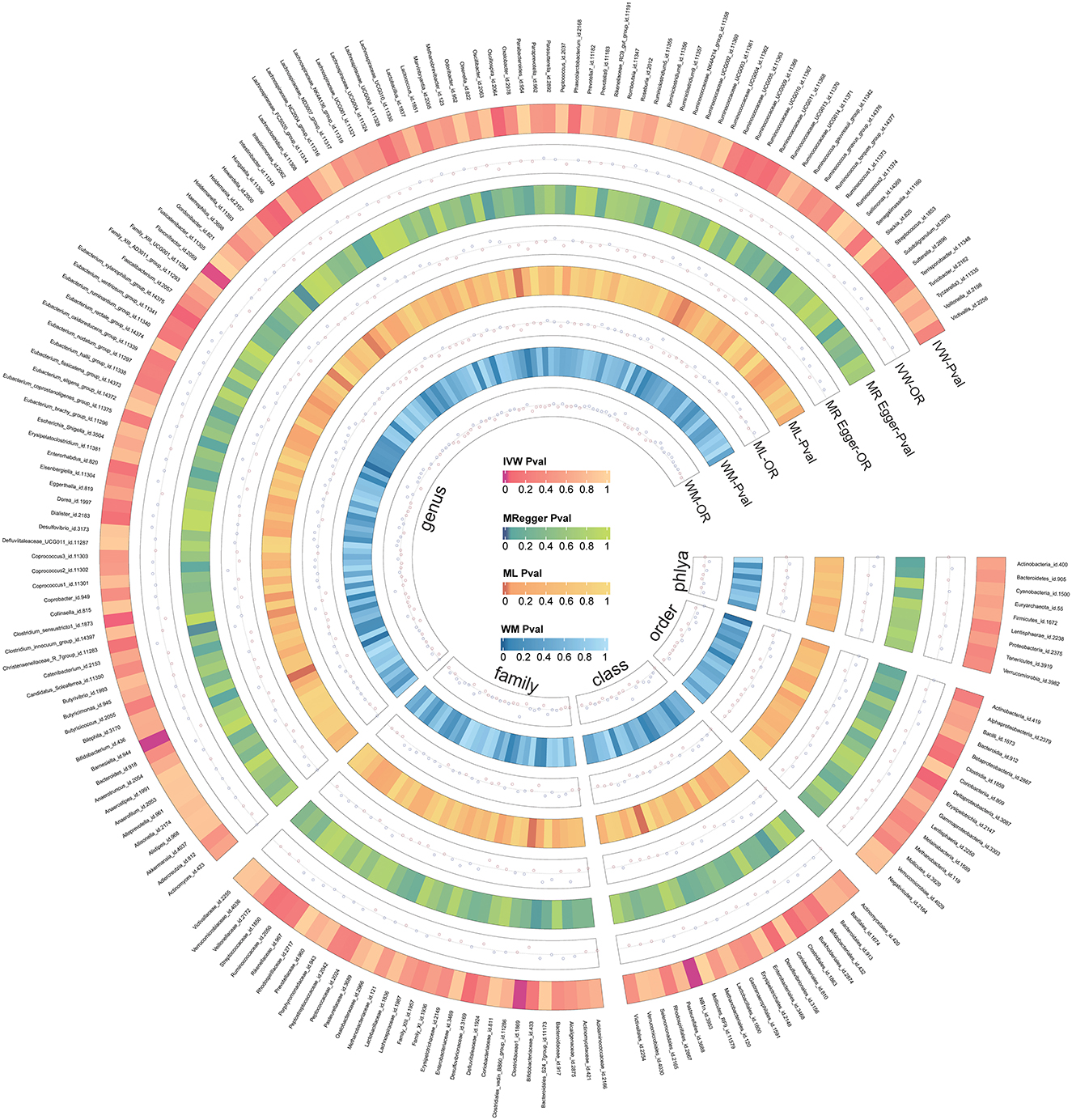
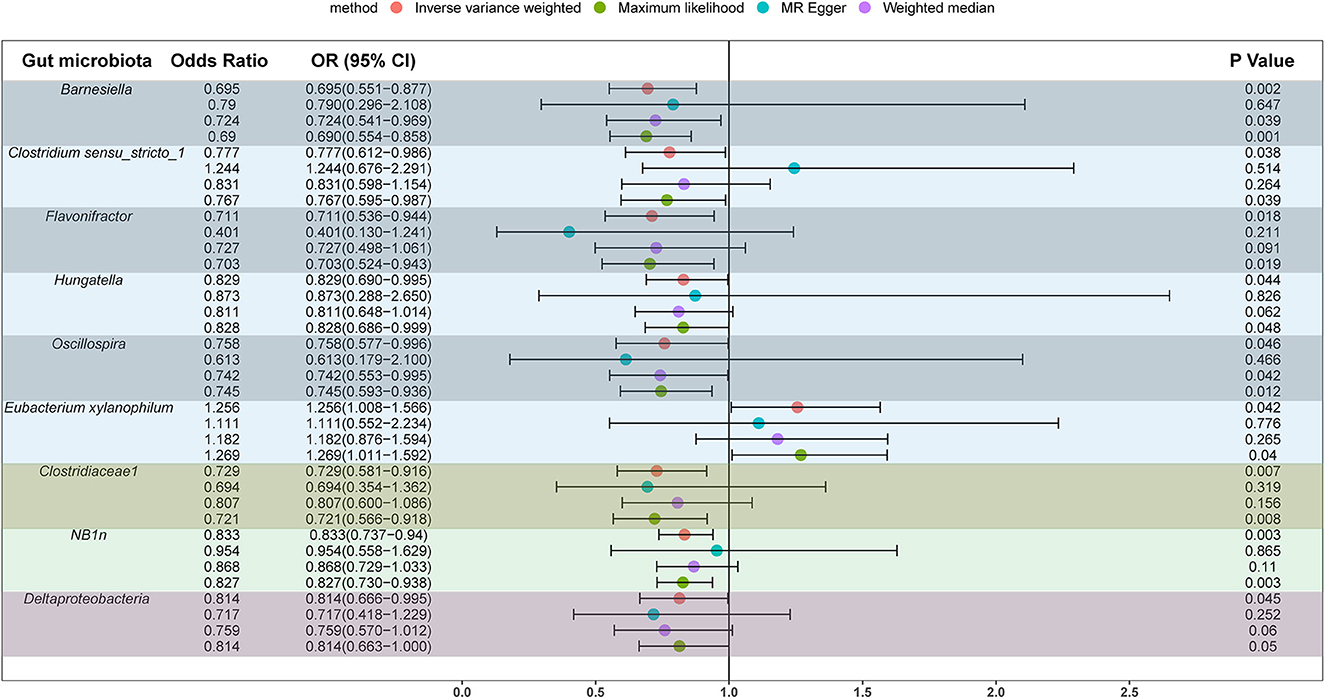
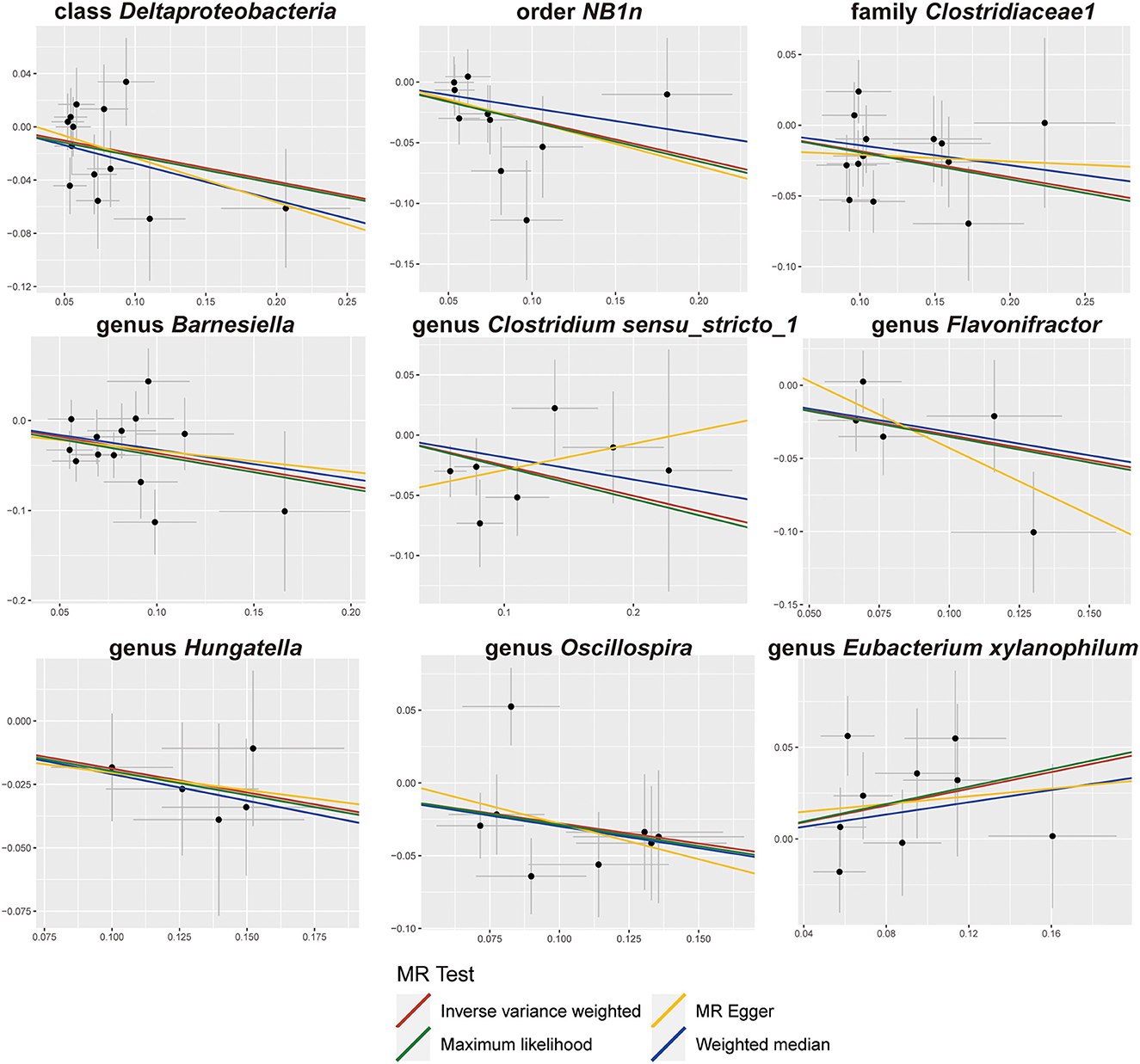
We screened 2,104 SNPs as instrumental variables from 196 gut microbiota. F-statistics for all instrumental variables were calculated. Two SNPs (rs17074066 and rs2835874) not satisfying F-statistic > 10 were excluded (Supplementary Table 3). The results of the MR analysis for IVs are shown in a circus plot (Figure 2) and detailed in Supplementary Table 4. We queried the aforementioned SNPs for positive findings in PhenoScanner and found no SNPs associated with the aforementioned confounders (Supplementary Table 5). Finally, one class, one order, one family, and six genera showing significant results for IVW analysis (Figure 3) were class Deltaproteobacteria (OR = 0.814, 95% CI: 0.666–0.995, P = 0.045), order NB1n (OR = 0.833, 95% CI: 0.737–0.940, P = 3.15 × 10−3), family Clostridiaceae1 (OR = 0.729, 95% CI: 0.581–0.916, P = 6.61 × 10−3), genus Barnesiella (OR = 0.695, 95% CI: 0.551–0.877, P = 2.20 × 10−3), genus Clostridium sensu_stricto_1 (OR = 0.777, 95% CI: 0.612–0.986, P = 0.0380), genus Flavonifractor (OR = 0.711, 95% CI: 0.536–0.944, P = 0.0181), genus Hungatella (OR = 0.829, 95% CI: 0.690–0.995, P = 0.0444), genus Oscillospira (OR = 0.758, 95% CI: 0.577–0.996, P = 0.0464), and genus Eubacterium xylanophilum (OR =1.26, 95% CI: 1.010–1.566, P = 0.0423).
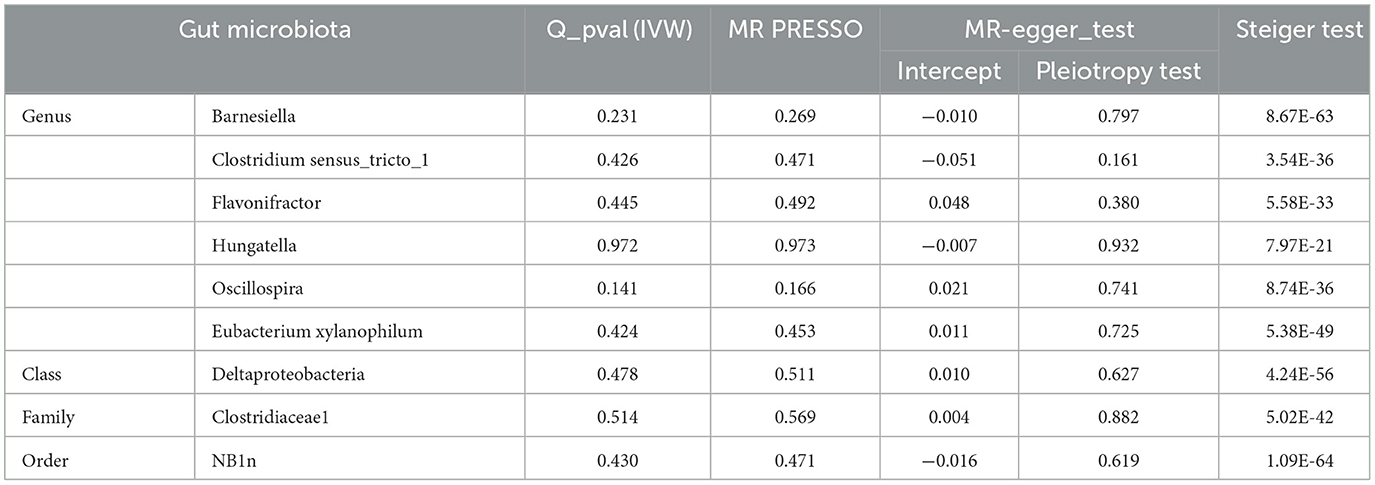
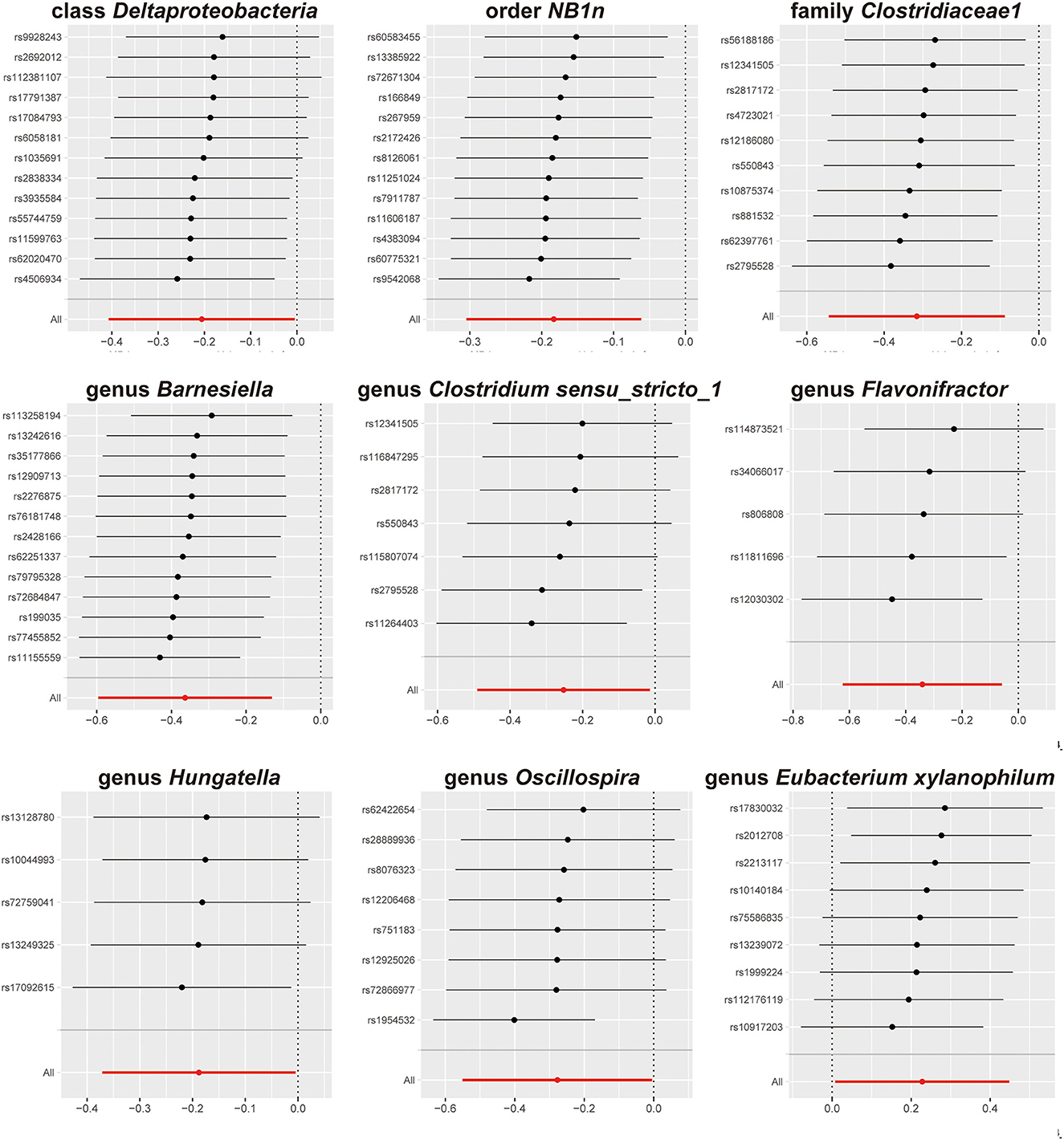
The results of sensitivity analyses are presented in Table 1. Cochran's Q-test results revealed no significant values for any of these gut microbiota, indicating that none of the IVs were heterogeneous. MR-PRESSO results did not show the presence of outliers. MR-Egger's intercept analysis had no meaningful results and demonstrated that there was no horizontal pleiotropy (Figure 4) and the directions calculated by each method were consistent except for the genus Clostridium sensu_stricto_1. The effect value calculated using the MR-Egger method for Clostridia was not consistent with the other three methods. Considering that the MR-Egger method assumed that all IVs were invalid, which weakened the statistical power making the results less precise. Therefore, we primarily used it to assess horizontal pleiotropy. As shown in Figure 5, the results of the leave-one-out technique were really robust to the outcomes of this MR analysis, where no matter which SNP was removed, it did not have a fundamental effect on the results.
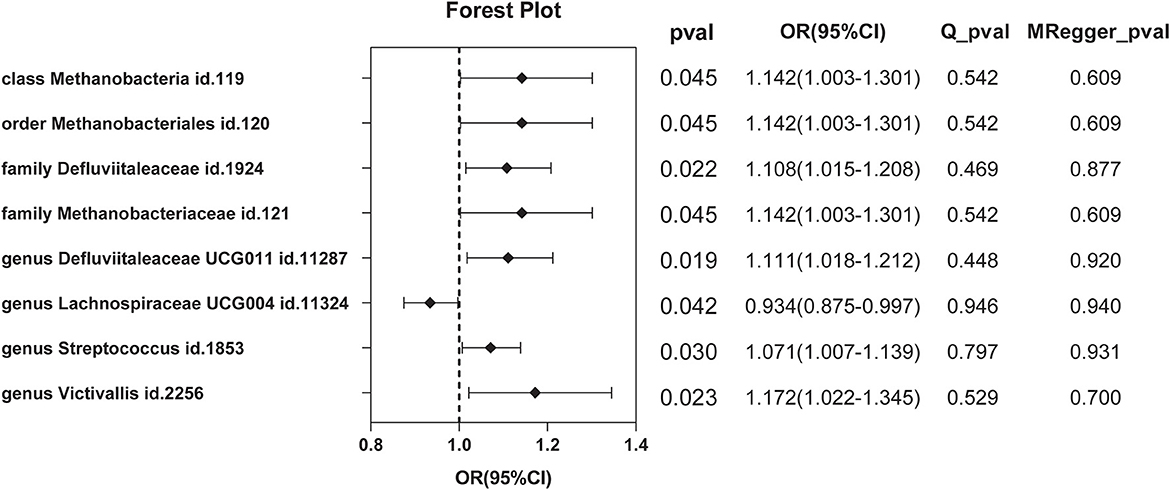
A total of nine SNPs were used as instrumental variables for each reverse Mendelian randomization (Supplementary Table 6). Significant results of the IVW method (Figure 6) showed class Methanobacteria (OR = 1.142, 95% CI: 1.003–1.301, P = 0.045), order Methanobacteriales (OR = 1.142, 95% CI: 1.003–1.301, P = 0.045), family Methanobacteriaceae (OR = 1.142, 95% CI: 1.003–1.301, P = 0.045), family Defluviitaleaceae (OR =1.108, 95% CI: 1.015–1.208, P = 0.022), genus Defluviitaleaceae UCG001 (OR =1.111, 95% CI: 1.018–1.212, P = 0.019), genus Lachnospiraceae UCG004 (OR = 0.934, 95% CI: 0.875–0.997, P = 0.042), genus Streptococcus (OR =1.071, 95% CI: 1.007–1.139, P = 0.030), and genus Victivallis (OR = 1.172, 95% CI: 1.022–1.345, P = 0.023).
4. Discussion
The gut microbiome is associated with several diseases in humans. Since the theory of the kidney gut axis became available, several clinical and animal model studies have confirmed the association of the gut microbiome with kidney diseases, especially chronic kidney disease (Cigarran Guldris et al., 2017; Yang et al., 2018). The relationship between intestinal flora and renal and ureter stones has also been gradually recognized in recent years. However, the correct direction of causality could not be inferred from observational studies. Our study was the first to confirm a causal relationship between gut microbiota and upper urinary tract stones using an MR analysis.
In this study, we used summary information on the gut microbiota from the largest GWAS meta-analysis conducted by the MiBioGen Consortium and summary statistics on upper urinary urolithiasis released by FinnGen R8 to investigate the causal link. Mendelian randomization and sensitivity analysis were performed on the filtered qualifying instrumental variables, and we found a causal relationship between several gut microbiota for upper urinary urolithiasis, with high levels of the genera Barnesiella, Clostridium sensu_stricto_1, Flavonifractor, Hungatella, Oscillospira, family Clostridiaceae1, class Deltaproteobacteria, and order NB1n, reducing the risk of upper urinary tract stones (OR <1), whereas the genus Eubacterium xylanophilum (OR > 1) had the opposite effect.
The association between these florae in our findings and upper urinary tract stones has rarely been reported. However, risk-associated flora is often reported to be associated with some inflammation-related diseases. For instance, Barnesiella is anti-inflammatory and protective in animal models and is associated with several immunomodulatory cells. Higher levels of Barnesiella in the colon are correlated with the intestinal environment less prone to inflammation (Berry and Reinisch, 2013). The main products of Barnesiella derivation are butyric acid and isobutyric acid (Sakamoto et al., 2009), and butyrate stimulates GFR receptors, which limit the production of inflammatory proteins such as IL-6, IL-1, and NF-B, reducing inflammation (Chen et al., 2018; Clemente et al., 2018). Clostridium sensu_stricto_1 belonging to the family Clostridiaceae is one of the most important anaerobic bacteria in the intestine (Guo et al., 2020) and has positive effects on short-chain fatty acid production and immune regulation through the production of butyrate through fermentation (Vital et al., 2014; Li et al., 2019). Bioinformatics analysis of the phylum Firmicutes (Rawat et al., 2022) showed that their genomes, particularly the genus Hungatella, are a rich source of glycosaminoglycan-specific catabolic enzymes and that the interaction of glycosaminoglycans with many ligands is relevant to the biological function of inflammation. Li found that feeding Hungatella to a mouse model ameliorated inflammation and extracellular matrix remodeling (He et al., 2016). Flavonifractors have been suggested in several articles to affect inflammation and obesity through multiple mechanisms (Kasai et al., 2015; Mikami et al., 2020; Ogita et al., 2020). Animal experiments by Tasuku (Mikami et al., 2020) have demonstrated that this genus suppresses allergen-specific IgE synthesis and may contribute to the relief of antigen-specific immunological responses in a Th2-dominated environment. Another study (Ogita et al., 2020) found that oral treatment of Flavonifractor preparations reduced the inflammatory response in the adipose tissue of obese mice, raising the possibility that it is involved in the suppression of TNF expression in an inflammatory environment. Mice treated with Hungatella exhibited reduced cytokine release and NF-κB activation in dendritic cells (Rossi et al., 2016). Oscillospira, a genus capable of synthesizing short-chain fatty acids like butyrate, has been linked to inflammation-related disorders, such as inflammatory bowel disease, non-alcoholic fatty liver disease, and aging processes distinguished through increased levels of circulating inflammatory mediators (Chierico et al., 2017; Lima and Longman, 2021; Xu et al., 2021), and is strongly negatively associated with pro-inflammatory monocyte chemoattractant protein-1 (Buford, 2017). Deltaproteobacteria were found to be negatively related to antineutrophil cytoplasmic antibody-associated vasculitis (AAV) with kidney injury (Yu et al., 2022). The herbal tea ingredient Rabdosia serra acts as an anti-inflammatory agent by boosting the number of helpful bacteria, such as Lactobacillus, as well as reducing the number of harmful bacteria including Eubacterium xylanophilum, thus alleviating artificially induced colitis in mice (Li et al., 2022).
It is believed that in the prevalent explanation of kidney stone production, the inflammatory immunological response contributes to the creation of Randall's plaques and calcium stones. Crystal deposition in mouse kidneys has been linked to reactive oxygen species generation, inflammatory vesicle activation, and the increased expression of molecules involved in the inflammatory cascade response (Khan et al., 2021). The renal—intestinal axis theory suggests that inflammatory cells, cytokines, soluble urokinase produced in our intestines promote renal inflammation via the circulation and metabolites of the microbiota entering the circulation may also have an impact on the kidney (Ticinesi et al., 2018). This is laterally supported by the higher prevalence of urinary stones in the inflammatory bowel population than in the general population (Dimke et al., 2021). Genera with protective effects usually exhibit anti-inflammatory actions in this study. Because this anti-inflammatory effect may depend on butyrate, a kind of SCFs, we hypothesized that the gut microbiota may influence the development of kidney stones by altering the level of inflammation in the body.
Reverse MR analysis revealed that several gut microbiota had the propensity to colonize in the gut of patients with kidney and ureteral stones. This may be related to the dietary habits and antibiotic usage of the patients. For example, increased abundances of Methanobacteria and Victivallis were found in mice fed with a high-fat diet (Mathur et al., 2013; Rodriguez et al., 2020) and a high sugar diet-induced changes in Lachnospiraceae UCG004 (Han et al., 2022). An epidemiological survey revealed a greater predilection for a western-style diet (a diet high in fat, calories, and animal protein and low in fiber and plant-based proteins) among the population with kidney stones (Kohjimoto et al., 2013).
The major strength of our study was that MR analysis results were unlikely to be biased by confounders and reverse causation compared with conventional observational studies; thus, our results provided more convincing evidence to support the causality of gut microbiota and upper urinary tract stones. In addition, the data of both gut microbiota and upper urinary tract stones were obtained from a large sample population, which could greatly improve the MR analysis power based on the pooled data.
This study had some limitations. First, our study was conducted in a European population only and may not be applicable to other populations. Second, because the minimal taxonomic level was genus, we could not further explore the causal relationship between gut microbiota and upper urinary urolithiasis at the species level. Furthermore, summary statistics lacked grouping information for stone composition, such as calcium oxalate stones or uric acid stones; therefore, we were unable to perform subgroup analyses.
5. Conclusion
Finally, a causal relationship was established between upper urinary urolithiasis and the gut microbiota through two-sample MR. Deltaproteobacteria, NB1n, Clostridiaceae1, Barnesiella, Clostridium sensu_stricto_1, Flavonifractor, Hungatella, Oscillospira, and Eubacterium xylanophilum were identified. These strains may develop into new biomarkers and provide potential direction for the treatment and prevention of urinary stones. In addition, the mechanism and role of the inflammatory response in the formation of upper urinary tract stones deserve our attention.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RZhang and XL were responsible for the concept and design. XL, WZ, RZhao, and YZhao assisted with carrying out the analyses. YZhang assisted in revising the manuscript. RZhang and XL drafted the early version of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors express their appreciation to the FinnGen study's participants and investigators. The authors also thank the MiBioGen consortium for making the gut microbiota GWAS summary statistics available.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1170793/full#supplementary-material
References
Aggarwal, K. P., Narula, S., Kakkar, M., and Tandon, C. (2013). Nephrolithiasis: molecular mechanism of renal stone formation and the critical role played by modulators. Biomed. Res. Int. 2013, 292953. doi: 10.1155/2013/292953
Allison, M. J., Dawson, K. A., Mayberry, W. R., and Foss, J. G. (1985). Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch. Microbiol. 141, 1–7. doi: 10.1007/BF00446731
Anand, S., and Mande, S. S. (2022). Host-microbiome interactions: gut-liver axis and its connection with other organs. NPJ Biofilms Microbiomes 8, 89. doi: 10.1038/s41522-022-00352-6
Berry, D., and Reinisch, W. (2013). Intestinal microbiota: a source of novel biomarkers in inflammatory bowel diseases. Best Pract. Res. Clin. Gastroenterol. 27, 47–58. doi: 10.1016/j.bpg.2013.03.005
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525. doi: 10.1093/ije/dyv080
Bowden, J., Smith, G. D., Haycock, P. C., and Burgess, S. (2016). Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. doi: 10.1002/gepi.21965
Buford, T. W. (2017). (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome 5, 80. doi: 10.1186/s40168-017-0296-0
Capolongo, G., Ferraro, P. M., and Unwin, R. (2023). Inflammation and kidney stones: cause and effect. Curr. Opin. Urol. 33, 129–135. doi: 10.1097/MOU.0000000000001066
Carbone, A., Al Salhi, Y., Tasca, A., Palleschi, G., Fuschi, A., De Nunzio, C., et al. (2018). Obesity and kidney stone disease: a systematic review. Minerva Urol. Nefrol. 70, 393–400. doi: 10.23736/S0393-2249.18.03113-2
Chen, G., Ran, X., Li, B., Li, Y., He, D., Huang, B., et al. (2018). Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine 30, 317–325. doi: 10.1016/j.ebiom.2018.03.030
Chierico, F. D., Nobili, V., Vernocchi, P., Russo, A., De Stefanis, C., Gnani, D., et al. (2017). Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 65, 451–464. doi: 10.1002/hep.28572
Cicerello, E., Ciaccia, M., Cova, G., and Mangano, M. (2019). The impact of potassium citrate therapy in the natural course of Medullary Sponge Kidney with associated nephrolithiasis. Arch. Ital. Urol. Androl. 91. doi: 10.4081/aiua.2019.2.102
Cigarran Guldris, S., González Parra, E., and Cases Amenós, A. (2017). Gut microbiota in chronic kidney disease. Nefrologia 37, 9–19. doi: 10.1016/j.nefroe.2017.01.017
Clemente, J. C., Manasson, J., and Scher, J. U. (2018). The role of the gut microbiome in systemic inflammatory disease. BMJ 360, j5145. doi: 10.1136/bmj.j5145
Corbo, J., and Wang, J. (2019). Kidney and ureteral stones. Emerg. Med. Clin. North Am. 37, 637–648. doi: 10.1016/j.emc.2019.07.004
Dimke, H., Winther-Jensen, M., Allin, K. H., Lund, L., and Jess, T. (2021). Risk of urolithiasis in patients with inflammatory bowel disease: a nationwide Danish cohort study 1977-2018. Clin. Gastroenterol. Hepatol. 19, 2532–2540.e2. doi: 10.1016/j.cgh.2020.09.049
Freidin, M. B., Stalteri, M. A., Wells, P. M., Lachance, G., Baleanu, A. F., Bowyer, R. C. E., et al. (2021). An association between chronic widespread pain and the gut microbiome. Rheumatology 60, 3727–3737. doi: 10.1093/rheumatology/keaa847
Girault, J. M., and Ménigot, S. (2022). Palindromic vectors, symmetropy and symmentropy as symmetry descriptors of binary data. Entropy 24. doi: 10.3390/e24010082
Guo, P., Zhang, K., Ma, X., and He, P. (2020). Clostridium species as probiotics: potentials and challenges. J. Anim. Sci. Biotechnol. 11, 24. doi: 10.1186/s40104-019-0402-1
Han, X., Feng, Z., Chen, Y., Zhu, L., Li, X., Wang, X., et al. (2022). Effects of high-fructose corn syrup on bone health and gastrointestinal microbiota in growing male mice. Front. Nutr. 9, 829396. doi: 10.3389/fnut.2022.829396
He, Z., Shao, T., Li, H., Xie, Z., and Wen, C. (2016). Alterations of the gut microbiome in Chinese patients with systemic lupus erythematosus. Gut Pathog. 8, 64. doi: 10.1186/s13099-016-0146-9
Hoffman, A., Braun, M. M., and Khayat, M. (2021). Kidney disease: kidney stones. FP Essent. 509, 33–38.
Johnson, R. J., Sánchez-Lozada, L. G., Newman, L. S., Lanaspa, M. A., Diaz, H. F., Lemery, J., et al. (2019). Climate change and the kidney. Ann. Nutr. Metab. 74 (Suppl. 3), 38–44. doi: 10.1159/000500344
Kamat, M. A., Blackshaw, J. A., Young, R., Surendran, P., Burgess, S., Danesh, J., et al. (2019). PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 35, 4851–4853. doi: 10.1093/bioinformatics/btz469
Kasai, C., Sugimoto, K., Moritani, I., Tanaka, J., Oya, Y., Inoue, H., et al. (2015). Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 15, 100. doi: 10.1186/s12876-015-0330-2
Khan, S. R., Canales, B. K., and Dominguez-Gutierrez, P. R. (2021). Randall's plaque and calcium oxalate stone formation: role for immunity and inflammation. Nat. Rev. Nephrol. 17, 417–433. doi: 10.1038/s41581-020-00392-1
Kim, H.-.N, Kim, J. H., Chang, Y., Yang, D., Joo, K. J., et al. (2022). Gut microbiota and the prevalence and incidence of renal stones. Sci. Rep. 12, 3732. doi: 10.1038/s41598-022-07796-y
Kohjimoto, Y., Sasaki, Y., Iguchi, M., Matsumura, N., Inagaki, T., Hara, I., et al. (2013). Association of metabolic syndrome traits and severity of kidney stones: results from a nationwide survey on urolithiasis in Japan. Am. J. Kidney Dis. 61, 923–929. doi: 10.1053/j.ajkd.2012.12.028
Kurilshikov, A., Medina-Gomez, C., Bacigalupe, R., Radjabzadeh, D., Wang, J., Demirkan, A., et al. (2021). Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 53, 156–165. doi: 10.1038/s41588-020-00763-1
Kurki, M. I., Karjalainen, J., Palta, P., Sipil,ä, T. P., Kristiansson, K., Donner, K., et al. (2023). FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 613, 508–518. doi: 10.1038/s41586-022-05473-8
Li, H., Wang, Y., Shao, S., Yu, H., Wang, D., Li, C., et al. (2022). Rabdosia serra alleviates dextran sulfate sodium salt-induced colitis in mice through anti-inflammation, regulating Th17/Treg balance, maintaining intestinal barrier integrity, and modulating gut microbiota. J. Pharm. Anal. 12, 824–838. doi: 10.1016/j.jpha.2022.08.001
Li, J.-.W, Fang, B., Pang, G-, F., Zhang, M., Ren, F.-., et al. (2019). Age- and diet-specific effects of chronic exposure to chlorpyrifos on hormones, inflammation and gut microbiota in rats. Pestic. Biochem. Physiol. 159, 68–79. doi: 10.1016/j.pestbp.2019.05.018
Lima, S., and Longman, R. S. A. (2021). Diamond in the rough: IgA-Seq signatures stratify new onset IBD. Cell Host Microbe 29, 10–12. doi: 10.1016/j.chom.2020.12.014
Liu, K., Zou, J., Fan, H., Hu, H., and You, Z. (2022). Causal effects of gut microbiota on diabetic retinopathy: a Mendelian randomization study. Front. Immunol. 13, 930318. doi: 10.3389/fimmu.2022.930318
Liu, Y., Jin, X., Hong, H. G., Xiang, L., Jiang, Q., Ma, Y., et al. (2020). The relationship between gut microbiota and short chain fatty acids in the renal calcium oxalate stones disease. FASEB J. 34, 11200–11214. doi: 10.1096/fj.202000786R
Luo, Q., Hu, Y., Chen, X., Luo, Y., Chen, J., Wang, H., et al. (2022). Effects of gut microbiota and metabolites on heart failure and its risk factors: a two-sample Mendelian randomization study. Front. Nutr. 9, 899746. doi: 10.3389/fnut.2022.899746
Mathur, R., Kim, G., Morales, W., Sung, J., Rooks, E., Pokkunuri, V., et al. (2013). Intestinal Methanobrevibacter smithii but not total bacteria is related to diet-induced weight gain in rats. Obesity 21, 748–754. doi: 10.1002/oby.20277
Mikami, A., Ogita, T., Namai, F., Shigemori, S., Sato, T., Shimosato, T., et al. (2020). Oral administration of Flavonifractor plautii attenuates inflammatory responses in obese adipose tissue. Mol. Biol. Rep. 47, 6717–6725. doi: 10.1007/s11033-020-05727-6
Ogita, T., Yamamoto, Y., Mikami, A., Shigemori, S., Sato, T., Shimosato, T., et al. (2020). Oral administration of Flavonifractor plautii strongly suppresses Th2 immune responses in mice. Front. Immunol. 11, 379. doi: 10.3389/fimmu.2020.00379
Pierce, B. L., Ahsan, H., and Vanderweele, T. J. (2011). Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 40, 740–752. doi: 10.1093/ije/dyq151
Rawat, P. S., Li, Y., Zhang, W., Meng, X., and Liu, W. (2022). Hungatella hathewayi, an efficient glycosaminoglycan-degrading firmicutes from human gut and its chondroitin ABC exolyase with high activity and broad substrate specificity. Appl. Environ. Microbiol. 88, e0154622. doi: 10.1128/aem.01546-22
Rodriguez, J., Hiel, S., Neyrinck, A. M., Roy, T. L., Pötgens, S. A., Leyrolle, Q., et al. (2020). Discovery of the gut microbial signature driving the efficacy of prebiotic intervention in obese patients. Gut 69, 1975–1987. doi: 10.1136/gutjnl-2019-319726
Rossi, O., van Berkel, L. A., Chain, F., Khan, M. T., Taverne, N., Sokol, H., et al. (2016). Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci. Rep. 6, 18507. doi: 10.1038/srep18507
Roze, D. (2023). Causes and consequences of linkage disequilibrium among transposable elements within eukaryotic genomes. Genetics. doi: 10.1093/genetics/iyad058
Rule, A. D., Lieske, J. C., and Pais, V. M. Jr. (2020). Management of kidney stones in 2020. JAMA 323, 1961–1962. doi: 10.1001/jama.2020.0662
Sakamoto, M., Takagaki, A., Matsumoto, K., Kato, Y., Goto, K., Benno, Y., et al. (2009). Butyricimonas synergistica gen. nov., sp. nov. and Butyricimonas virosa sp. nov., butyric acid-producing bacteria in the family 'Porphyromonadaceae' isolated from rat faeces. Int. J. Syst. Evol. Microbiol. 59, 1748–1753. doi: 10.1099/ijs.0.007674-0
Sanna, S., van Zuydam, N. R., Mahajan, A., Kurilshikov, A., Vila, A. V., Võsa, U., et al. (2019). Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 51, 600–605. doi: 10.1038/s41588-019-0350-x
Siener, R., Bade, D. J., Hesse, A., and Hoppe, B. (2013a). Dietary hyperoxaluria is not reduced by treatment with lactic acid bacteria. J. Transl. Med. 11, 306. doi: 10.1186/1479-5876-11-306
Siener, R., Bangen, U., Sidhu, H., Hönow, R., von Unruh, G., Hesse, A., et al. (2013b). The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int. 83, 1144–1149. doi: 10.1038/ki.2013.104
Slob, E., and Burgess, S. A. (2020). comparison of robust Mendelian randomization methods using summary data. Genet. Epidemiol. 44, 313–329. doi: 10.1002/gepi.22295
Stern, J. M., Moazami, S., Qiu, Y., Kurland, I., Chen, Z., Agalliu, I., et al. (2016). Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis 44, 399–407. doi: 10.1007/s00240-016-0882-9
Tavasoli, S., Alebouyeh, M., Naji, M., Majd, G. S., Nashtaei, M. S., Broumandnia, N., et al. (2020). Association of intestinal oxalate-degrading bacteria with recurrent calcium kidney stone formation and hyperoxaluria: a case-control study. BJU Int. 125, 133–143. doi: 10.1111/bju.14840
Tian, L., Liu, Y., Xu, X., Jiao, P., Hu, G., Cui, Y., et al. (2022). Lactiplantibacillus plantarum J-15 reduced calcium oxalate kidney stones by regulating intestinal microbiota, metabolism, and inflammation in rats. FASEB J. 36, e22340. doi: 10.1096/fj.202101972RR
Ticinesi, A., Milani, C., Guerra, A., Allegri, F., Lauretani, F., Nouvenne, A., et al. (2018). Understanding the gut-kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut 67, 2097–2106. doi: 10.1136/gutjnl-2017-315734
Vital, M., Howe, A. C., and Tiedje, J. M. (2014). Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio 5, e00889. doi: 10.1128/mBio.00889-14
Voroneanu, L., Burlacu, A., Brinza, C., Covic, A., Balan, G. G., Nistor, I., et al. (2023). Gut microbiota in chronic kidney disease: from composition to modulation towards better outcomes-a systematic review. J. Clin. Med. 12, 1948. doi: 10.3390/jcm12051948
Xu, N., Bai, X., Cao, X., Yue, W., Jiang, W., Yu, Z., et al. (2021). Changes in intestinal microbiota and correlation with TLRs in ulcerative colitis in the coastal area of northern China. Microb. Pathog. 150, 104707. doi: 10.1016/j.micpath.2020.104707
Xue, H., and Pan, W. (2020). Inferring causal direction between two traits in the presence of horizontal pleiotropy with GWAS summary data. PLoS Genet. 16, e1009105. doi: 10.1371/journal.pgen.1009105
Yang, T., Richards, E. M., Pepine, C. J., and Raizada, M. K. (2018). The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 14, 442–456. doi: 10.1038/s41581-018-0018-2
Yu, M., Li, L., Ren, Q., Feng, H., Tao, S., Cheng, L., et al. (2022). Understanding the gut-kidney axis in antineutrophil cytoplasmic antibody-associated vasculitis: an analysis of gut microbiota composition. Front. Pharmacol. 13, 783679. doi: 10.3389/fphar.2022.783679
Zeng, G., Zhao, Z., Mazzon, G., Pearle, M., Choong, S., Skolarikos, A., et al. (2022). European association of urology section of urolithiasis and international alliance of urolithiasis joint consensus on retrograde intrarenal surgery for the management of renal stones. Eur. Urol. Focus 8, 1461–1468. doi: 10.1016/j.euf.2021.10.011
Keywords: upper urinary urolithiasis, renal stones, gut microbiota, Mendelian randomization, causal relationship, inverse variance weighted
Citation: Zhang R, Zhao W, Zhao R, Zhao Y, Zhang Y and Liang X (2023) Causal relationship in gut microbiota and upper urinary urolithiasis using Mendelian randomization. Front. Microbiol. 14:1170793. doi: 10.3389/fmicb.2023.1170793
Received: 21 February 2023; Accepted: 24 April 2023;
Published: 18 May 2023.
Edited by:
Yingnan Hou, Shanghai Jiao Tong University, ChinaReviewed by:
Astghik Zaveni Pepoyan, Armenian National Agrarian University, ArmeniaZhengrui Li, Shanghai Jiao Tong University, China
Copyright © 2023 Zhang, Zhao, Zhao, Zhao, Zhang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuezhi Liang, bGlhbmd4ejIwMDhAc2luYS5jb20=
 Ruiqiao Zhang
Ruiqiao Zhang Weijie Zhao2
Weijie Zhao2 Yanlong Zhang
Yanlong Zhang