- 1Food Microbiology Laboratory, Department of Botany, Kurseong College, Kurseong, India
- 2Cell and Molecular Biology Laboratory, Department of Zoology, University of North Bengal, Raja Rammohunpur, India
- 3Food Microbiology Laboratory, Department of Food Technology, University of North Bengal, Raja Rammohunpur, India
Fruits are crucial components of a balanced diet and a good source of natural antioxidants, that have proven efficacy in various chronic illnesses. Various kinds of waste generated from fruit industries are considered a global concern. By utilizing this fruit waste, the international goal of “zero waste” can be achieved by sustainable utilization of these waste materials as a rich source of secondary metabolites. Moreover, to overcome this waste burden, research have focused on recovering the bioactive compounds from fruit industries and obtaining a new strategy to combat certain chronic diseases. The separation of high-value substances from fruit waste, including phytochemicals, dietary fibers, and polysaccharides which can then be used as functional ingredients for long-term health benefits. Several novel extraction technologies like ultrasound-assisted extraction (UAE), pressurized liquid extraction (PLE), and supercritical fluid extraction (SFE) could provide an alternative approach for successful extraction of the valuable bioactives from the fruit waste for their utilization as nutraceuticals, therapeutics, and value-added products. Most of these waste-derived secondary metabolites comprise polyphenols, which have been reported to have anti-inflammatory, insulin resistance-treating, cardiovascular disease-maintaining, probiotics-enhancing, or even anti-microbial and anti-viral capabilities. This review summarizes the current knowledge of fruit waste by-products in pharmacological, biological, and probiotic applications and highlights several methods for identifying efficacious bioactive compounds from fruit wastes.
Introduction
Food is a fundamental element necessary survival and life. Food waste is generated in different phases, i.e., during industrial manufacturing, processing, distribution, and agricultural production. Household activities contribute to about 42% of the total food waste, 39% by the food processing industries, 14% by the food service sector, and 5% during distribution. It was estimated that food waste may rise to 126 million tonnes by 2020 if improvements are not implemented (Mirabella et al., 2014; Baig et al., 2019). India, which has a population of more than 1.4 billion, generates more than 0.5 kg of organic waste per person per day (Paulraj et al., 2019). By separating high-value components such proteins, fibers, phytochemicals, flavor compounds, and polysaccharides, which can then be employed as functional ingredients and nutraceuticals, this prevention can be achieved (Baiano, 2014). There have been a number of initiatives in recent years to create strategies for therapeutically utilizing food (vegetable and fruit) waste. The agro-industrial waste is, however, utilized in huge quantities in the form of animal feed or fertilizers (Rudra et al., 2015).
Some recent reports showed that high-value products were developed by these types of fruit and agro-wastes and used in regular human lifestyles, such as (medicines, food items, and cosmetics) (Rudra et al., 2015; Kandemir et al., 2022). Researchers are looking for natural bioactive substances for treating and preventing several human diseases (Kandemir et al., 2022). These substances efficiently interact with biological molecules, including proteins, DNA, and others, to achieve the desired effects, which are then used to create natural therapeutics (Ajikumar et al., 2008). According to current research, consumers are becoming increasingly interested in food bioactives because of their potential to help people in various ways, such as preventing sickness and promoting good health. To achieve beneficial functional fruit products, it is essential to gather detailed information about the bio-actives (Kumar, 2015). Among these, nutraceuticals are therapeutic foods that significantly improve one’s health, increase immunity, and prevent and cure several diseases. Phytochemicals, on the other hand, have a specific role in showing positive effects on human health (Bansal and Priyadarsini, 2022). Currently, phytochemicals having potential cancer-preventive attributes are prioritized more (Kumar and Kumar, 2015). In recent years, there has been a growing trend in the food industries for the development of functional and nutraceutical products. Due to increasing consumer preference for “healthy” foods, this new category of food products has attracted much attention in the food industry. Hence, finding recent naturally occurring bioactive molecules that can be employed as nutraceuticals, functional food additives, or medicines has become of common interest to the pharmaceutical and food industries (Joana Gil-Chávez et al., 2013; Vilas-Boas et al., 2021). Fruit wastes provide a reliable source of secondary metabolites for the development of possible food additives, functional foods, preservatives, and nutraceuticals (Bhardwaj et al., 2022). Fruits and vegetable wastes represent the simplest form of functional foods because they are highly rich in several bioactive compounds. Fruits waste containing polyphenols and carotenoids showed antioxidant activity and reduce the risk of acquiring certain types of cancers (Day et al., 2009). The isolated bioactive molecules and by-products can be used to develop several functional foods in food processing industries and medicinal or pharmaceutical preparations (Baiano, 2014). Pomaces and other wastes from different fruits keep nutrients and bioactive substances, including phenolic acids, flavonoids, anthocyanins, and carotenoids, with a variety of biological functions (Das et al., 2021; Suri et al., 2022).
Fruit waste is becoming more appealing for research because these residues are a significant source of polyphenols (Lucarini et al., 2021). Fruit peel is the principal waste product in the food processing industries that use fruits as raw material, such as the manufacturing of fruit juices, jams, and dried fruits. Recently, researchers have become increasingly interested in exploring the antioxidant effects of fruit waste, such as peel and pomaces (Arias et al., 2022; Bello et al., 2023). Antioxidants can influence the expression of transcription factors involved in the immune response, reduce pro-inflammatory cytokine expression, and block crucial immune signaling pathways (Yahfoufi et al., 2018). The nuclear factor kappa B (NF-κB) family is one of the most significant signaling pathways that govern immunological responses and inflammation (Lawrence, 2009). The most abundant complex is p65/p50, which regulates gene expression of interleukin (IL)-1, IL-6, IL-8, inducible nitric oxide synthase (iNOS), IFN-γ, tumor necrosis factor (TNF)-α, and cyclooxygenase (COX)-2 (Modak and Bhattacharjee, 2022). Irregular activation of these pathways leads to several inflammatory diseases and even autoimmune diseases like rheumatoid arthritis (RA) and even cancer. Plasma-membrane-bound ligands, including toll-like receptor (TLR) and IL-1, can activate this pathway, resulting in the phosphorylation of IκB and its breakdown. As a result, NF-κB translocates into the nucleus and begins to upregulate transcription factor genes, which regulate cell inflammatory responses and survival (Modak and Bhattacharjee, 2022).
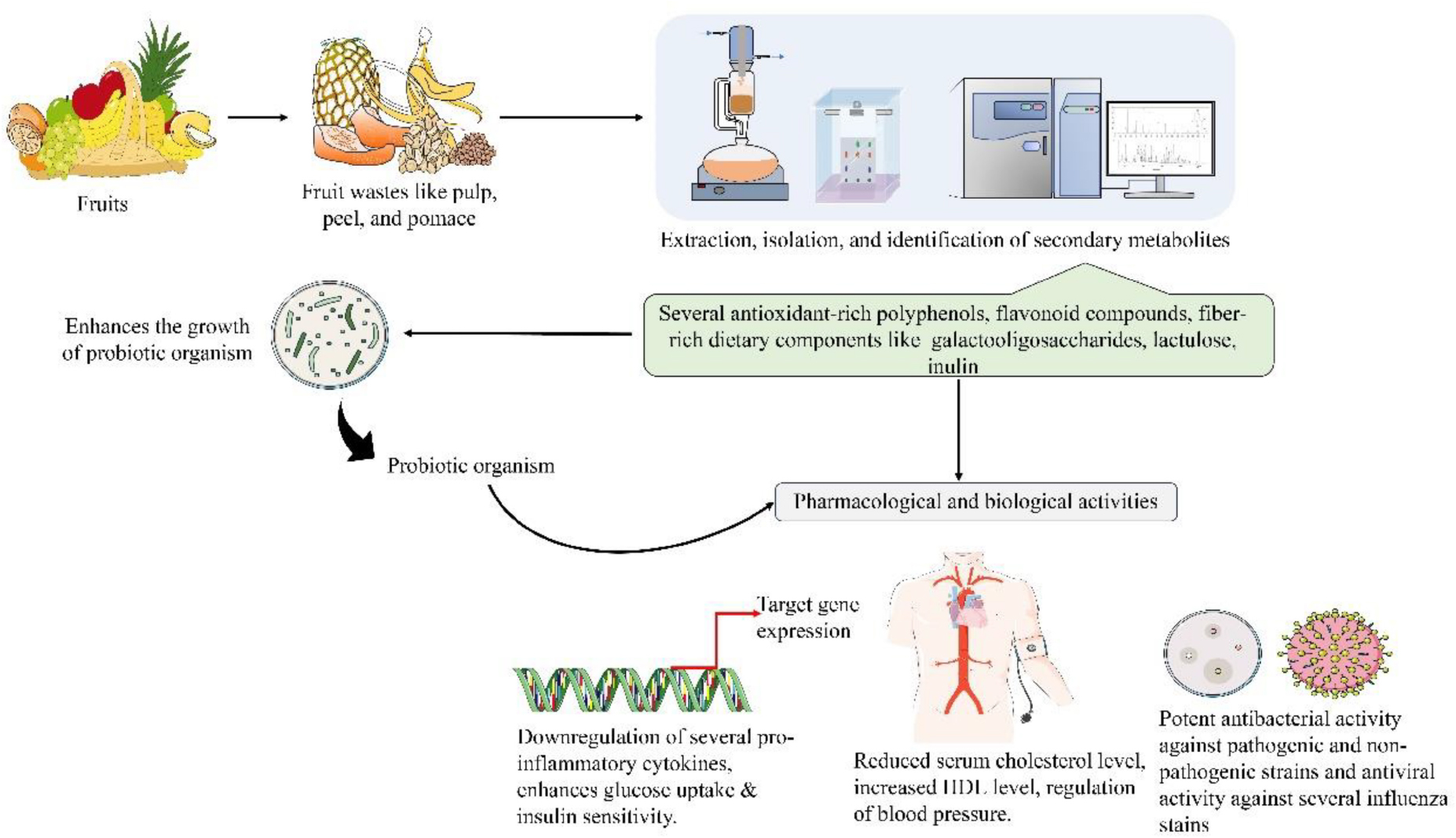
Among the most common non-communicable diseases, cardiovascular disease (CVD) accounts for around 17.7 million deaths worldwide (WHO, 2016). India accounts for around one-fifth of these deaths worldwide (Kumar and Sinha, 2020). CVDs have wide variety of outcomes, including cerebrovascular disease, stroke, atherosclerosis, diabetes, coronary heart disease, obesity, and hypertension (Petrie et al., 2018). High blood cholesterol is also essential in the development of CVD (American Heart Association, 2012). Dietary patterns rich in lipids and cholesterol increase the risk of atherosclerosis, leading to CVD (Stocker and Keaney, 2004; Carson et al., 2020). Atherosclerosis indicates the accumulation of cholesterol within the arterial walls, thereby narrowing the arteries and forming atherosclerotic plaques (Brody, 1999). The disease manifestation of atherosclerosis is mainly due to endothelial damage (Caliceti et al., 2022). Currently, long-term pharmacological therapy is the most common strategy to control CVDs. However, most of these therapeutic approaches are inefficient for all patients and often elicit several side effects (Zhao et al., 2017). So, there is a growing interest in identifying alternative natural resources to combat the increasing incidence of CVD. In this context, natural compounds with potent antioxidant and free radical scavenging activities have drawn increased attention from the scientific community (Fu et al., 2011). Numerous studies support the direct relationship between a diet rich in vegetables and fruits and with low risk of CVDs (Figure 1; Cicero et al., 2017; Marracino et al., 2022).
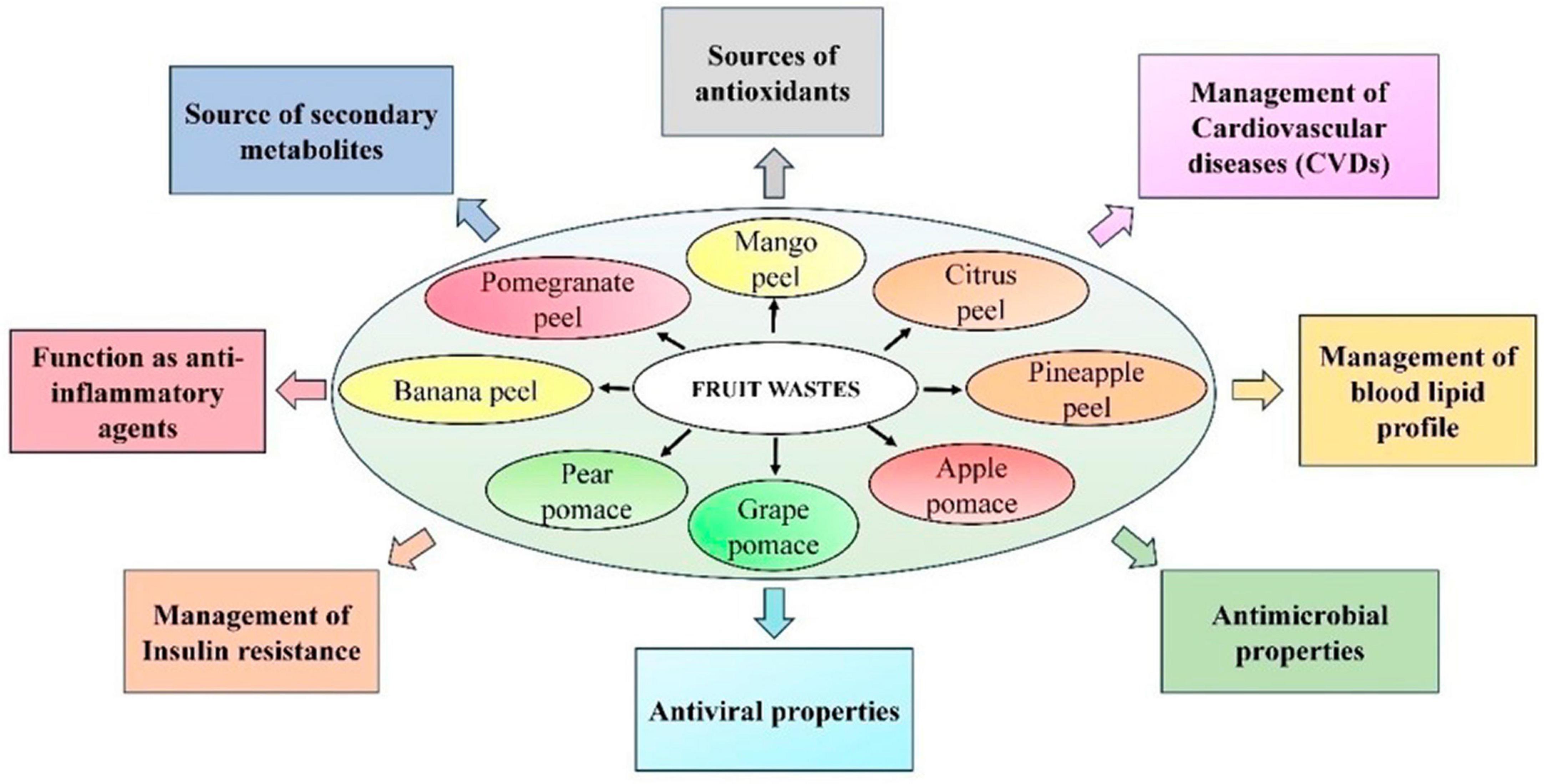
Figure 1. Pharmacological attributes of various edible fruit wastes. Figure generated using Microsoft PowerPoint 2021, 64-bit (Version 2302, Build 16130.20306).
Fruit waste extract also represents a novel strategy for combating harmful bacteria and viruses. Polyphenols are secondary metabolites generated from various portions of edible fruits wastes (apple, citrus, banana, pomegranate, grape, and pear) that include one or more phenolic groups (Gerardi et al., 2021; Ko et al., 2021; Zardo et al., 2021; Hasan et al., 2022). They not only have numerous human health benefits such as anti-diabetic, anti-cancer, antioxidant, and cardioprotective, but additionally, they possess anti-microbial-and antifungal properties (Guo et al., 2020; Saleem and Saeed, 2020; Budiati et al., 2022; Elbandrawy et al., 2022; Ko and Ku, 2022; Lee et al., 2022). Furthermore, there is an increasing prevalence of drug resistance to harmful bacteria, which is a severe threat to humanity. Thus, it is crucial to select the most appropriate antibiotics and employ them properly (Figure 1; Bains and Chawla, 2020). The potential of fruit wastes may be increased by using them as a base for the discovery of bioactive compounds, which could have significant positive effects on the food industry and medicinal fields.
As a source of secondary metabolites, anti-oxidant, and anti-inflammatory agents
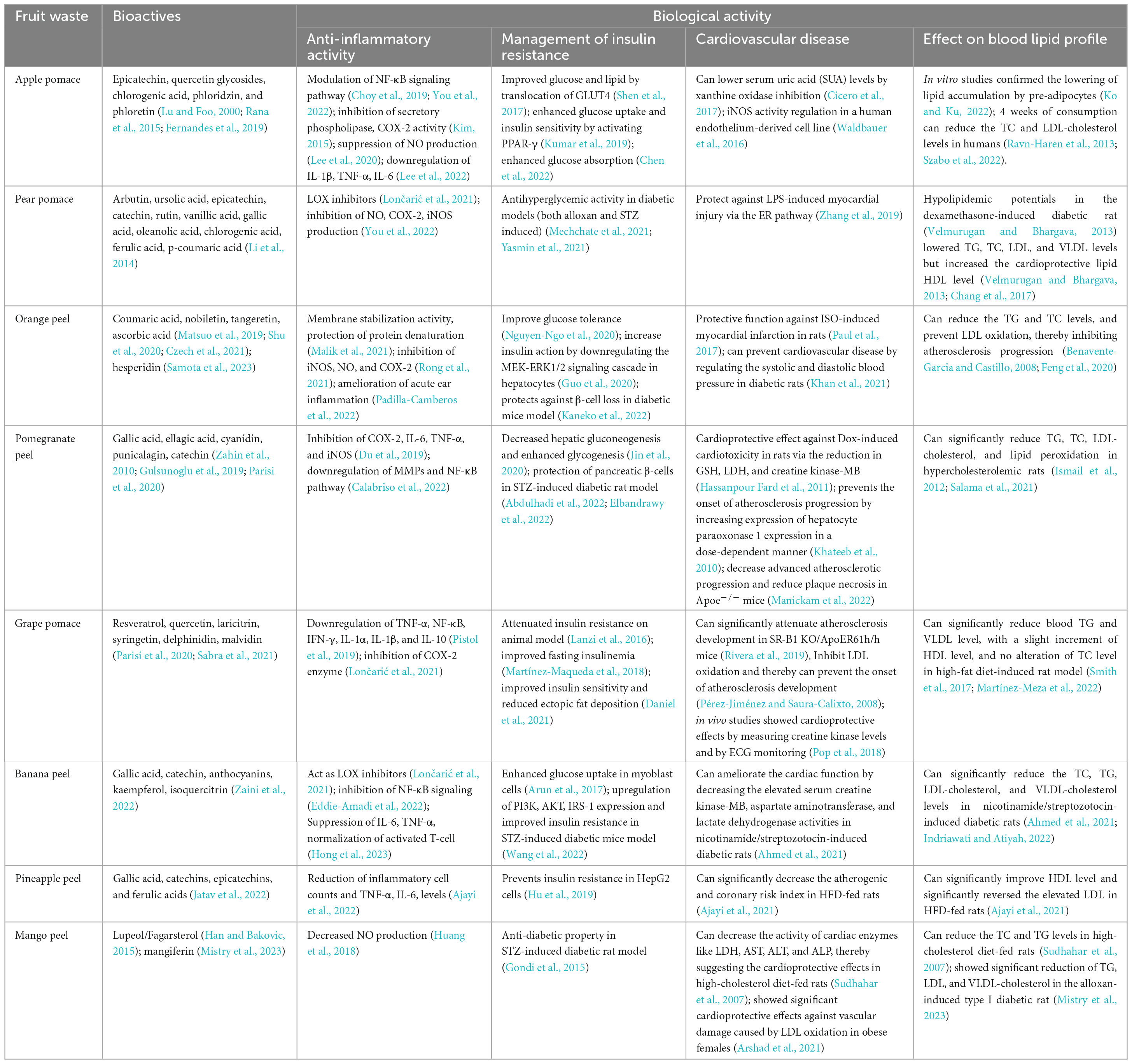
Apple pomace or peel is a by-product of the extraction process used to make apple juice and cider, and it is one of these sources that is considered as having substantial potential as a food ingredient (Kolodziejczyk et al., 2007). It is a solid mass that makes up to 30% of the fruit’s weight and comprises leftover peel, seed, stem, and pulp. Due to the presence of phenolics such as chlorogenic acid, quercetin glycosides, epicatechin, its dimer, phloridzin, and 3-hydroxyphloridzin, apple pomace has high antioxidant capabilities (Lu and Foo, 2000). According to several reports, apple pomace possesses good ferric-reducing ability power (FRAP) activity and 2,2′-diphenyl-1-picrylhydrazyl (DPPH)-scavenging move (Garcia-Montalvo et al., 2022; Llavata et al., 2022). Identification and quantification of significant phenolics by reverse phase-HPLC study show the presence of quercetin, phloretin, and phloridzin in apple pomace (Rana et al., 2015). In a recent survey polyphenolic composition was determined using UHPLC-DAD-ESI-MS and results showed that apple pomace composed of quercetin 3-O-arabinofuranoside (13%), quercetin-3-O-rhamnoside (23%), quercetin-3-O-galactoside (27%), and the dihydrochalcone phloretin-2-O-glucoside (14%) (Fernandes et al., 2019). Various novel extraction techniques like ultrasound-assisted extraction (UAE) ultra-turrax extraction (UTE), are used nowadays to isolate phenolics compounds like phloridzin from fruit wastes. Recent studies showed that the UAE method yields slightly higher phloridzin content (55.86–71.19 μg GAE/g of fresh apple pomace) compared to the UTE method (58.39–64.43 μg/g of fresh apple pomace) (Pollini et al., 2021). However, several other studies have reported slightly lower phloridzin content while extracting polyphenols using the UAE method from various apple pulps, ranging from 11.40 to 40.91 μg/g of fresh pulp (Li et al., 2019). Similarly, when utilizing the UTE method on freeze-dried apple pulps, the reported phloridzin content ranged from 39.9 to 77.0 μg/g of dry weight (Santarelli et al., 2020). The by-products from the processing of pear fruit, called pear pomace, remain rich in bioactive substances, carbohydrates, and fibers that can be used medicinally. According to a study of phenolic compounds in various pear sections, pulps have the lowest phenol concentration containing bioactive molecules, which are after that more abundant in skins and seeds (Kolniak-Ostek and Oszmiański, 2015). Because of this, pear pomace is a rich source of these bioactive components. According to HPLC studies, five phenolic acids (chlorogenic acid, gallic acid, ferulic acid, vanillic acid, and p-coumaric acid), two triterpene compounds (oleanolic acid and ursolic acid), one phenolic glucoside (arbutin), and three significant flavanols (catechin, epicatechin, and rutin) were all detected in pear pomace (Li et al., 2014). According to quantitative studies of the total phenols, triterpenes, and flavonoids content, all components discovered in the peel were 6–20 times more concentrated compared to the pulp (Li et al., 2014).
Several phenolic and flavonoid compounds, including nobiletin, tangerine, and coumaric acid, have been identified and quantified from dried orange peel using HPLC-DAD (Shu et al., 2020). According to Czech et al. (2021), the peel of all citrus fruits had substantially more phenolic components than the pulp and among citrus fruit peel, green grapefruit peel had the highest concentration of ascorbic acid (67.36 mg/100 g) and lemon had the lowest (7.83 mg/100 g). The same study reported that limes, lemons, and mandarins have more DPPH radical scavenging ability than their pulp counterparts (Czech et al., 2021). Lee et al. (2010) successfully extracted polymethoxyflavones (PMFs) like nobiletin and tangeretin from Citrus depressa Hayata peels by supercritical fluid extraction (SFE) technique. Their results showed that these PMFs’ yield increased significantly when 85% aqueous ethanol was used as a modifier with 9.1% supercritical CO2 (Lee et al., 2010; Chien et al., 2022).
Another food product is pomegranate and due to its multiple medicinal properties, pomegranates have been utilized as traditional medicine for decades. In this context, pomegranate peels come into consideration as the peel also contains a mixture of bioactive components, and the synergistic interaction of various components can result in a wide range of physiological actions (Mo et al., 2022). Phytochemical screening showed that pomegranate peels contain high amounts of flavonoids, tannins, alkaloids, carbohydrates, terpenoids, and possess antioxidant activity (Khokar et al., 2021). Studies found that pomegranate peel extracts have the highest soluble phenolic content and higher antioxidant activity than other wastes (Gulsunoglu et al., 2019). The soluble phenolics of pomegranate peel extract contained derivatives of ellagic acid, gallic acid, punicalagin, and cyaniding (Table 1). Punicalagin was shown to be the most hydrolyzable tannin in pomegranate husks, followed by gallic acid, catechin, epicatechin, and ellagic acid (Gulsunoglu et al., 2019). A recent study by Parisi et al. (2020) reported that ellagic acid (ellagitannins) and gallagic acid derivatives (gallagyl esters) are the primary components of pomegranate peel (Parisi et al., 2020). A recent study by García et al. (2021) have successfully extracted punicalagin (17 ± 3.6 mg/g DW) from pomegranate peel extract (PPE) using pressurized liquid extraction (PLE) technique under optimal conditions of 200°C temperature and 77% ethanol (García et al., 2021). The phenolic compound punicic acid was also extracted from pomegranate seed using the SFE technique at optimized temperature (60°C) and pressure (320 bars) (Natolino and Da Porto, 2019). Grape pomace is rich in the phenolic compounds such as resveratrol, anthocyanins (cyanidin, delphinidin, malvidin, and petunidin derivatives), flavonols (quercetin, laricitrin, syringetin, and myricetin glycosides), and flavan-3-ols (catechin/epicatechin and their procyanidin oligomers), as detected in LC-MS/MS analysis (Table 1; Parisi et al., 2020). Duba et al. (2015) have successfully extracted polyphenols from grape peels and seeds using the sub-critical water extraction (SBWE) technique in semi-continuous mode, yielding 44.3–77 and 44–124 mg/g from peels and seeds, respectively (Duba et al., 2015). Other novel extraction methods like UAE and pulsed electric fields were also used to extract anthocyanins and other phenolic compounds from grape peel and pomace (Ghafoor et al., 2011; Maza et al., 2019). Ashraf-Khorassani and Taylor (2004) have successfully removed more than 79% of catechin and epicatechin from grape seeds using SFE with 40% methanol-modified CO2 (Ashraf-Khorassani and Taylor, 2004). Around 29–40% of all pineapple waste is made up of pineapple peel and is abundant in epicatechins, catechins, ferulic acids, and gallic acid, all of which can be used as active antioxidant components (Jatav et al., 2022). This naturally occurring ferulic acid can be extracted using conventional and non-conventional methods. The Soxhlet extraction using methanol and petroleum ether as solvents showed the best results for the extraction of ferulic acid from the pineapple peel powder with a percentage yield of 90.5% mg (Madhumeena et al., 2021). The percent yield of pineapple peel extracts through solvent extraction is 82% mg compared to the product through SFE (79% mg) (Madhumeena et al., 2021). Lun et al. (2014) also found a higher yield of ferulic acid from the autoclaved pineapple wastes (3.65 mg/g) compared to the non-autoclaved pineapple wastes (0.64 mg/g) (Lun et al., 2014).
Due to their synergistic and additive effects, the complex blend of phytochemical components present in fruit-peel extracts is more efficient for preventing inflammation than their components. Polyphenols have shown significant effectiveness in controlling this pathway at various points. The flavonoids like quercetin, kaempferol, myricetin, and apigenin have been shown to inhibit serine/threonine protein kinases (PIK3/AKT) in an antagonistic manner. They can modulate transcription factors such as NF-κB (Choy et al., 2019). Gallic acid, resveratrol, and quercetin can inhibit COX-2 enzymes, whereas lipoxygenase (LOX) inhibitors include kaempferol, ferulic acid, benzoic acid, quercetin, caffeic acid, and catechin (Figure 2; Lončarić et al., 2021). In vitro study demonstrated that the 70% ethanolic apple peel-extract can substantially inhibit secretory phospholipase, COX-1, COX-2, and lipoxygenase activity by up to 53.5 ± 2.3, 13.4 ± 1.8, 64.8 ± 5.4, and 44.4 ± 4.5 (percentage ± SD), respectively (Kim, 2015). Another culture-based study demonstrated that the ethanolic apple peel extract can suppress nitric oxide (NO) production up to 25% at a dose of 500 μg/ml in lipopolysaccharide (LPS)-induced Raw 264.7 macrophage cell line (Lee et al., 2020). This study further confirmed that apple peel extract effectively inhibited LPS-induced production of pro-inflammatory iNOS and COX-2, as well as phosphorylation of NF-κB subunit p65 and further real-time PCR confirmed the expression of pro-inflammatory biomarkers like prostaglandin E synthase 2 (PTGES2), monocyte chemoattractant protein-1 (MCP-1), IL-6, and IL-1β were also significantly downregulated in an in a dose-dependent way (Lee et al., 2020). A more recent study by the same research group revealed that isoquercitrin was the main bioactive extracted from green ball apple peel extract (Lee et al., 2022). Isoquercitrin can inhibit NF-κB, COX-2, iNOS, and p65 protein expressions along with proinflammatory markers like TNF-α, IL-6, and IL-1β in a concentration-dependent fashion in LPS-treated Raw 264.7 macrophage cell line (Figure 2; Lee et al., 2022). A recent study reported that the ethanolic extract of pear pomace can also enhance LPS-induced inflammation in RAW 264.7 cells by decreasing NF-κB expression and NO production (You et al., 2022). Another work by Martins et al. (2017) reported that extracts of grape pomace containing phenolic compounds (in the concentration of 100 μg/ml) can decrease COX-2, prostaglandin (PGE)-2, and IL-8 production in Caco-2 cells pre-treated with leukocyte IL-1. Further HPLC analysis indicated that this extract had a high amount of flavonoids, including catechin, gallic acid, and epicatechin, which may help to modulate PGE-2 and IL-8 release (Martins et al., 2017).
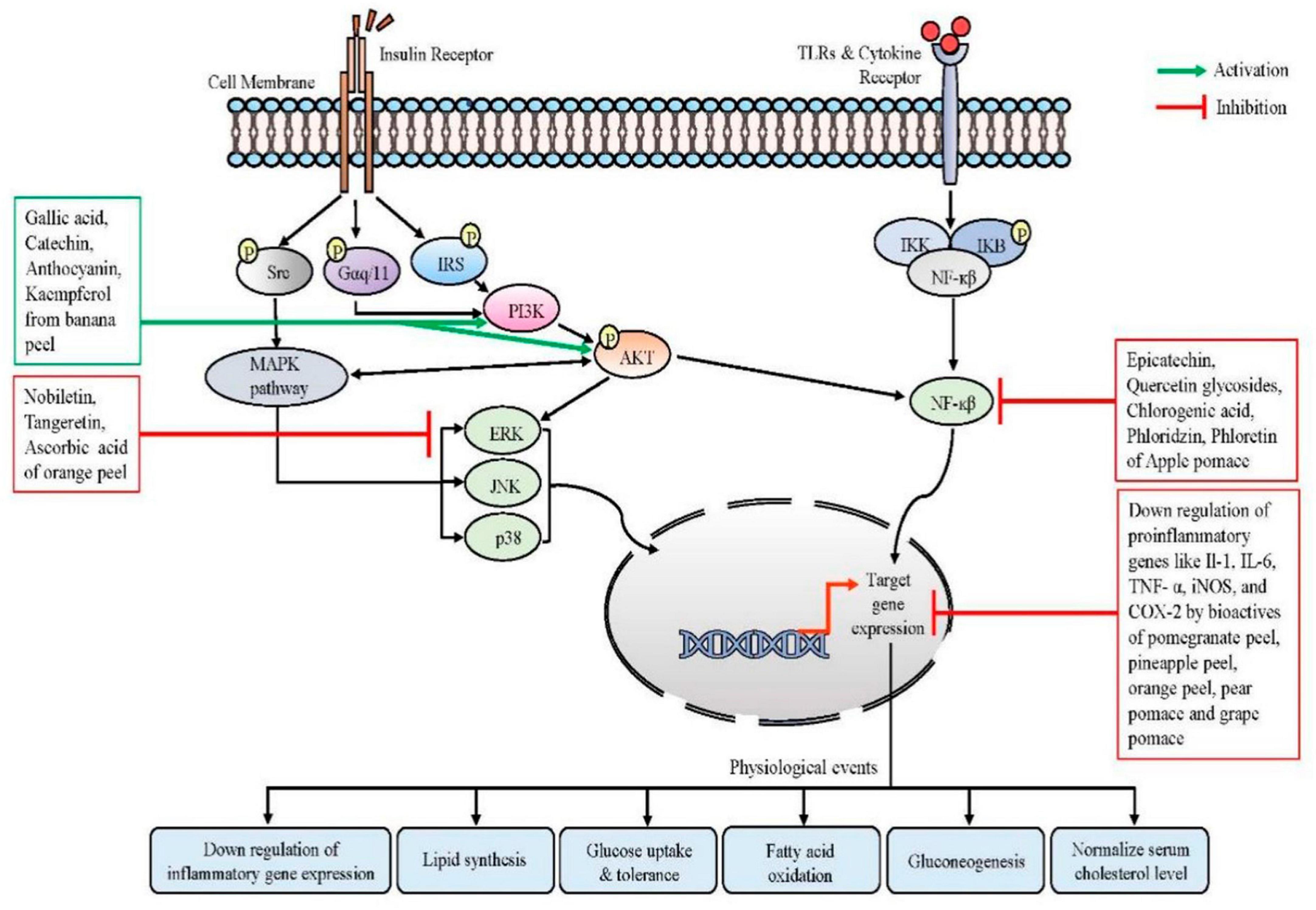
Figure 2. Modulation of intracellular signaling cascades by different edible fruit wastes, their phyto-components, and their physiological events during homeostasis. Binding with insulin receptors, insulin signals stimulate MAPK kinase pathways and activate PI3K, which in turn activates AKT. Phyto-components of banana peel can activate this pathway. AKT also interacts with NF-κβ pathways. Activation of the TLR initiates an inflammatory cascade that leads to the activation of NF-κβ and the production of pro-inflammatory cytokines. Bioactives from several fruit wastes can modulate the NF-κβ signaling pathways, which results in the downregulation of inflammatory biomarkers. Similarly, the MAPK pathway and AKT eventually control the metabolic gene expression, which ultimately restores the normal glucose uptake, promoting fatty acid oxidation and controlling blood cholesterol levels. Src, proto-oncogene tyrosine-protein kinase; TLR, toll-like receptors; Gαq/11, a family of heterotrimeric G protein alpha subunits; PI3K, phosphoinositide 3-kinase; IRS, insulin receptor substrate; AKT, serine-threonine protein kinase; MAPK, mitogen-activated protein kinase; JNK, c-Jun N-terminal kinases; ERK, extracellular signal-regulated kinase; NF-κβ, nuclear factor kappa beta; IKB, inhibitor of NF-κβ; IKK, IKB kinase. Figure generated using Microsoft PowerPoint 2021, 64-bit (Version 2302, Build 16130.20306).
According to Malik et al. (2021), Citrus nobilis peel methanolic extract can protect RBC membrane stabilization by up to 89.67% and can protect the protein denaturation by up to 87.57% at a dose of 200 mg/ml concentration. Most biological proteins lose the three-dimensional structures and their physical function due to heat exposure, which triggers several hypersensitive immune responses and forms chronic inflammatory arthritis (Modak et al., 2021). Due to the presence of sinensetin, nobiletin, and other bioactive components, C. nobilis peel methanolic extract may restore protein integrity by preventing membrane protein rupture by widening the surface-volume ratio and by reducing the release of inflammatory mediators (Malik et al., 2021; Modak et al., 2021). Padilla-Camberos et al. (2022) reported that topical use of a natural essential oil mix of sweet orange peel, allspice, and cumin seeds could ameliorate ear inflammation by 66.67% when compared to the negative control group, and that may be due to the synergistic or additive effect (Padilla-Camberos et al., 2022). Further studies revealed that citrus peel flavonoid nobiletin can exert anti-inflammatory effects by reducing the expression of iNOS, COX-2, and NO production from both the protein and gene level in LPS-treated RAW macrophage 264.7 cell line (Figure 2; Rong et al., 2021).
Ellagic acid, one of the primary bioactive of pomegranate peel extract, can inhibit the expression of pro-inflammatory cytokines like IL-6, TNF-α and IL-1, as well as down-regulate the COX-2 and iNOS expression in macrophage Raw 264.7 cell line (Du et al., 2019). This study further reported that polyphenols from pomegranate peel can inhibit the mRNA and protein production of TLR4 and could inhibit p65 nuclear translocation by inhibiting NF-κB activation by preventing LPS-induced phosphorylation, ubiquitination, and degradation of IκB (Figure 2; Du et al., 2019). Another recent study by Calabriso et al. (2022) showed the anti-inflammatory effects of red grape pomace on LPS and TNF-induced inflammation in Caco-2 cells and HMEC-1 cells. According to their study, they found that treatment with grape pomace reduced the levels of IL-6 and MCP-1 (monocyte chemoattractant protein-1) as well as the production of MMP-9 and MMP-2 in a dose dependant manner in HMEC-1 cell line. Furthermore, they noted that treatment with the red grape pomace also downregulated the NF-κB pathway which ultimately inhibited the gene expression of several pro-inflammatory markers like COX-2, TNF-α, IL-1β, and macrophage colony-stimulating factor (M-CSF) (Calabriso et al., 2022). The peels of pineapple and banana has been reported to possess several phenolic compounds like gallic acid, catechin, epicatechin, anthocyanins, kaempferol, and isoquercitrin (Jatav et al., 2022; Zaini et al., 2022). These phenolic compounds and flavonoids can be successfully extracted using UAE method at optimal extraction conditions. Using UAE technique at optimal parameters (30°C, 5 min, 150 W), 1 g of Musa cavendish peel can yield up to 23.49 mg of phenolic compounds, 13.11 mg of proanthocyanidins, and 39.46 mg of flavonoids (Vu et al., 2017). The presence of such phenolic-rich constituents in banana peel extract has been reported to exert an anti-inflammatory role by inhibiting the NF-κB pathway (Eddie-Amadi et al., 2022). Another recent study found that banana peel can inhibit the expression of IL-6 production in the LPS-stimulated RAW264.7 cells (Table 1). According to the in vivo study in mice model, the banana peel extract has been reported to lower the elevated serum TNF-α, IL-6 levels, and also normalized the activated T-cell population (Hong et al., 2023). Parisi et al. (2020) reported the anti-inflammatory activity of herbal formulation comprising of propolis, pomegranate peel, and aglianico grape pomace extracts (4:1:1) in collagen-induced arthritis (CIA) model (Parisi et al., 2020). According to their study, early treatment with the herbal dose (150 mg/kg body weight) can significantly ameliorated the paw swelling and also reduced the number of affected paws by 60% when compared with negative control mice. Furthermore, the downregulation of IL-17 and IL-1β cytokines at protein levels in the treatment group, also confirmed the anti-rheumatoid activity of the herbal formulation (Table 1; Parisi et al., 2020).
Management of insulin resistance
Insulin resistance (IR) is one of the major symptoms of a number of human diseases, including CVD, obesity, type 2 diabetes, polycystic ovary syndrome, metabolic dysfunction-associated fatty liver disease and others (James et al., 2021; Li et al., 2022). Current understanding states that adipose tissue could have an essential role in the development of IR through the generation of lipids and other circulating substances that induce IR, as well as in the insulin-mediated regulation of glucose metabolism in other organs (Li et al., 2022). In healthy individuals, the transport of the glucose transporter solute carrier family 2 protein facilitated GLUT-4 to the cell surface and promotes cellular glucose absorption from the circulation into tissues (James et al., 2021; Li et al., 2022). This procedure eliminates high postprandial glucose from the blood, however, a habitually high glycemic index diet results in excessive insulin production and increased binding to the insulin receptor, leading to fatty acid synthesis in addition to the typical glucose uptake. The PI3K and the MAP kinase pathway are the two primary pathways that are activated as a result of all of these activities (Figure 2; Li et al., 2022). Any change in these two pathways hindered glucose re-uptake in both the adipose tissues as well as the skeletal muscle tissues, contributing to the phenomenon of IR. Several studies have shown that the high anti-oxidant activity of fruit waste can help to improve and manage IR and type 2 diabetes through their synergic effects. In differentiated adipocytes, phloretin and phloridzin, two of the primary active components of apple peel, stimulate peroxisome proliferator-activated receptor (PPAR)-γ and inhibit cyclin-dependent kinase (Cdk)-5 to enhance glucose uptake and insulin sensitivity (Kumar et al., 2019). Phloretin reduces IR in vitro and improves the glucose and lipid metabolism in streptozotocin-induced diabetes rats by translocation of GLUT4 (Table 1; Shen et al., 2017). According to Chen et al. (2022), phloridzin derivatives are a potential therapy for type 2 diabetes patients who have IR. In untreated C2C12 myotubes, docosahexaenoic acid-acylated phloridzin, a new polyphenol fatty acid ester derivative, enhanced glucose absorption and mitochondrial function via enhancing AMPK activity (Chen et al., 2022). A novel formulation that has the potential to be an important replacement for conventional medications is validated by the synergistic combination of pear pomace, another rich source of bioactive (Li et al., 2014). The anti-diabetic formulation containing flavonoids such as catechin, epicatechin, and rutin showed substantial anti-hyperglycemic activity at 10 mg/kg body weight dose in the alloxan-induced diabetic mice model (Mechchate et al., 2021). Ferulic acid, another important polyphenol from pear pomace also improved hyperglycemia significantly in STZ-nicotinamide-induced diabetic rat model (Yasmin et al., 2021). Nobiletin and tangeretin were important flavonoids identified from the orange peel that also possess anti-diabetic activity (Table 1). Nguyen-Ngo et al. (2020), reported that nobiletin treatment can substantially boost TNF-related glucose uptake in human skeletal muscle. Additionally, it was observed to downregulate the expression of pro-inflammatory markers at both the mRNA and protein levels in human placenta and visceral adipose tissue. Furthermore, the study conducted on gestational diabetes mellitus mice model revealed that, nobiletin at 50 mg/kg body weight dose can significantly improve glucose tolerance and can inhibit Akt activation in the placenta (Nguyen-Ngo et al., 2020). The intragastric injection of tangeretin at a dose of 50 mg/kg body weight was found to improve glucose homeostasis and hepatic insulin sensitivity in diabetic mice with genetically altered leptin receptors. The results suggest that tangeretin may have the ability to increase insulin action by downregulating the MEK-ERK1/2 pathways in hepatocytes (Figure 2; Guo et al., 2020). According to Kaneko et al. (2022), the subcutaneous administration of nobiletin led to a significant reduction in blood glucose levels in non-fasting condition and during an OGT test in male diabetic db/db mice model. Indeed, the study further confirmed that continuous administration of nobiletin to db/db mice resulted in a larger β-cell mass and higher insulin content compared to the vehicle control group. These findings suggest that suggesting nobiletin not only improves IR but also offers protection against β-cell loss in the type 2 diabetic db/db mice model (Kaneko et al., 2022). As per other studies, ellagic acid, cyanidin, and punicalagin are potentially promising supplements for lowering diabetes-related insulitis. Treatment with ellagic acid 50 mg/kg can enhance the islets of Langerhans in STZ-induced diabetic rats by downregulating TNF-α and IL-6-induced systematic inflammation (Elbandrawy et al., 2022). Jin et al. (2020) reported that punicalagin treatments activated the PI3K/AKT signaling pathway, which decreased hepatic gluconeogenesis and enhanced glycogenesis, both in glucosamine-induced HepG2 cell line and STZ-induced mice model (Figure 2). In another study, it was found that punicalagin exhibited protective effects on β-cells in the pancreas of STZ-induced diabetic Wistar rats. Additionally, punicalagin was observed to reduce oxidative stress levels and enhance antioxidant levels in diabetic rat model (Abdulhadi et al., 2022). Punicalagin’s effects on the liver and kidney, resulting in hypoglycemic and anti-inflammatory effects, were primarily mediated by a decrease in gluconeogenesis and an increase in glycogenesis, as well as by activating the PI3K/AKT signaling pathway, controlling the inflammatory signaling network and by regulating gut microbiota homeostasis (Jin et al., 2020; Cao et al., 2021). Cyanidin, a different phenolic compound from pomegranate peel, can also ameliorate metabolic IR. A recent study by Geng et al. (2022) reported that cyanidin-3-O-glucoside supplementation improves metabolic IR in STZ-induced mice models by downregulating inflammatory cytokines and TLR4/NF-κB inhibitor alpha (IκBα) activation and restoring the suppressed AKTt/eNOS signaling pathway (Figure 2). Such research continues to be beneficial in revealing a novel alternative method for treating IR in diabetic complications, which has a lot of potential in the future that involves turning fruit waste into a distinctive source of antioxidant-rich by-products.
Management of cardiovascular diseases and blood lipid profile
Polyphenols extracted from apple pomace were known to exhibit cardiovascular protective effects by lowering serum uric acid (SUA) levels (by xanthine oxidase inhibition) and improving endothelial reactivity (ER) (Cicero et al., 2017). The triterpenic acids from methanol/water extracts of apple pomace can modulate the eNOS (endothelial nitric oxide synthase) activity in a cell line derived from human endothelium (Table 1; Waldbauer et al., 2016). Apple skin contains phloridzin, chlorogenic acid, and quercetin, which have the potential to regulate glucose absorption and can reduce postprandial glycemia (blood glucose levels after a meal) and insulinemia (insulin levels in the blood) (Makarova et al., 2015; Cicero et al., 2017). Various in vitro studies also confirmed the anti-obesity properties of apple peel extract by lowering lipid accumulation by pre-adipocytes (Ko and Ku, 2022). In healthy human volunteers, a daily uptake of apple, apple pomace, or apple juice for a duration of 4 weeks has been shown to lead to a reduction in both total cholesterol (TC) and LDL-cholesterol levels (Ravn-Haren et al., 2013). Phloridzin, chlorogenic acid, and catechin found in apple pomace have the potential to lower serum triglycerides (TG) and LDL-cholesterol levels while also helping in regulating glycemia (blood sugar levels) (Table 1; Szabo et al., 2022).
The biomolecules obtained from pear and its extracts has also many cardioprotective functions. The extracts of Pyrus, commonly known as Himalayan pear have rich content of arbutin, catechin, and chlorogenic acid and were known to inhibit the COX-2 activities and reduce IL-6 and TNF-α expression in LPS-stimulated RAW macrophages (Figure 2; Om et al., 2022). The arbutin treatment could also protect against LPS-induced myocardial injury via ER pathway in an in vivo model system through the modulation of TNF-α and IL-6 levels (Zhang et al., 2019). Pear and pear by-product extracts also showed hypoglycemic (blood-sugar lowering) and hypolipidemic (cholesterol lowering) potentials in the dexamethasone-induced diabetic rat (Velmurugan and Bhargava, 2013). This extract not only lowered TG, TC, LDL, and VLDL levels but also increased the cardioprotective lipid HDL level (Velmurugan and Bhargava, 2013). Chang et al. (2017) found out that the pear insoluble dietary fiber (IDF) can decrease the LDL-cholesterol and TC levels in high-fat-induced rats (Table 1; Chang et al., 2017). Therefore, the pear and its by-product extracts have the potency to prevent the formation of CVDs like atherosclerosis and coronary heart disease.
The extracts obtained from both the peel and pulp of the wild orange, Citrus macroptera, has been found to have cardioprotective effects. These extracts showed notable protective capabilities against isoproterenol (ISO)-induced myocardial infarction (MI) in rats (Paul et al., 2017). Citrus fruits’ peel, pulp, and seeds are rich in flavanone glycosides, with hesperidin being one of the prominent compounds in high amounts (Samota et al., 2023). Hesperidin (at a dose of 100 mg/kg) was found to control blood glucose levels effectively and showed cardioprotective functions in isoproterenol-induced MI in STZ-nicotinamide-induced diabetic rats (Kakadiya et al., 2010). Hesperidin also has blood pressure-reducing effects, and studies have confirmed its ability to prevent and treat CVDs by regulating both systolic and diastolic blood pressure in diabetic rats (Table 1; Khan et al., 2021). Various flavanone derivatives, such as hesperidin and naringin, in the juices of Citrus fruits has been verified to exhibit cholesterol-lowering activity in human subjects. The essential oils from the lemon and citrus peel can also reduce triglycerides and TC levels, and prevent LDL oxidation, thereby inhibiting atherosclerosis progression (Figure 2; Benavente-Garcia and Castillo, 2008; Feng et al., 2020).
Pomegranate, Punica sp.’s peels, and juice have various therapeutic potentials related to cardiovascular complications. Almost 92% of the antioxidant property of the fruit is mainly due to the presence of multiple flavonoids (such as anthocyanins and catechins) and tannins (such as gallic acid, ellagic acid, punicalin, and punicalagin) concentrated in the peels of pomegranate (Zahin et al., 2010). The hydroethanolic extract obtained from pomegranate peel, rich in polyphenols (such as punicalagin), showed a significant reduction of plaque necrosis and advanced atherosclerotic progression in Apoe–/– mice via the suppression of MerTK cleavage and elevation of lesional macrophage efferocytosis (Table 1; Manickam et al., 2022). The water extract derived from pomegranate aril, rind, and seeds has been found to possess a cardioprotective effect against doxorubicin (Dox)-induced cardiotoxicity in rats via the reduction in glutathione, lactate dehydrogenase (LDH), and creatine kinase-MB (Hassanpour Fard et al., 2011). The pomegranate peel extract, owing to its rich antioxidant content, can enhance the hepatocyte paraoxonase one expression in a dose-dependent manner, thereby preventing the onset of atherosclerosis progression (Khateeb et al., 2010). Another study in hypercholesterolemic mice showed elevated eNOS expression and inhibition of atherosclerosis progression by uptake of punicalagin-enriched pomegranate juice (De Nigris et al., 2007). Studies in human subjects also suggest the importance of daily uptake of pomegranate juice as it can attenuate atherosclerosis and prevents hypertension (Wang et al., 2018). The long-term consumption of pomegranate peel powder can significantly reduce TC, TG, LDL-cholesterol levels, and lipid peroxidation in hypercholesterolemic rats (Figure 2; Ismail et al., 2012; Salama et al., 2021).
The bioactives obtained from grape extract (Vitis sp.) also possess many cardioprotective functions. Bioactive molecules like resveratrol and quercetin have been effective as anti-hypertensive and treatment of cardiac damage (Theodotou et al., 2017; Sabra et al., 2021). Grape pomace (GP) is a by-product of the winery industry that contains a high concentration of polyphenols and dietary fiber (Taladrid et al., 2022). Studies have suggested the in vivo cardioprotective effects of the pomace extract by measuring creatine kinase levels and ECG monitoring (Pop et al., 2018). Recent studies have demonstrated that red wine grape pomace can significantly attenuate atherosclerosis progression in mice with SR-B1 KO/ApoER61h/h (Rivera et al., 2019). The polyphenolics from grape extracts inhibit LDL oxidation and thereby can prevent the onset of atherosclerosis development (Pérez-Jiménez and Saura-Calixto, 2008). Grape pomace can also regulate blood lipid profile. Studies on the high-fat diet model (AIN-93G-induced) for ten weeks showed that with the increasing concentration of grape pomace, there was a noteworthy reduction in blood TG and VLDL level, with a slight increment of HDL level and no alteration of TC level (Table 1; Smith et al., 2017).
Lupeol (also known as Fagarsterol) is a naturally occurring pentacyclic triterpene predominantly found in high concentrations in mango peels (Han and Bakovic, 2015). Lupeol can be extracted from the mango peel using the UAE method as it enhances the extraction efficiency by shortening time and increasing yield (Ruiz-Montañez et al., 2014). Due to these pentacyclic triterpenoids, mango peel may possess cardioprotective functions. Studies in high-cholesterol-fed rats found that consumption of lupeol and its derivatives can reduce the TC triglyceride, and decrease the activity of cardiac enzymes like LDH, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP), thereby suggesting the cardioprotective effects of triterpenoids (Sudhahar et al., 2007). Mango peel powder has shown significant cardioprotective effects in obese females by protecting against vascular damage caused by LDL oxidation (Arshad et al., 2021). Both mango peel extract and mangiferin have been found to produce significant reductions in TG, LDL, and VLDL-cholesterol in the alloxan-induced type I diabetic rat (Table 1; Mistry et al., 2023).
Antimicrobial and antiviral properties of fruit waste products
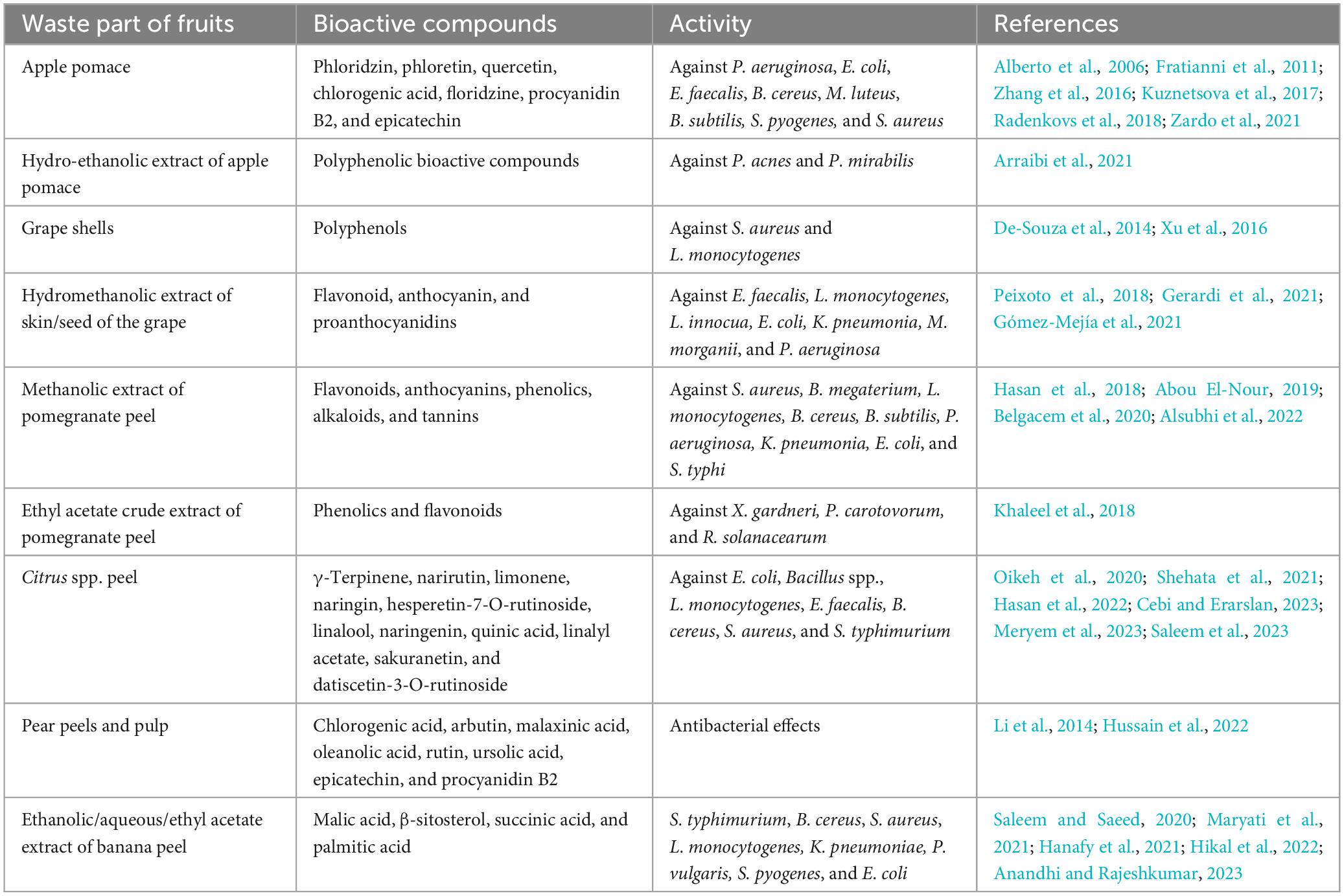
Apple pomace contains flavonoids, phenolic acids, carotenoids, and anthocyanins that have various anti-microbial and anti-viral activities (Kuznetsova et al., 2017; Barreira et al., 2019; Zardo et al., 2021). Polyphenolic bioactives from apple pomace showed antimicrobial activity against Paenibacillus larva (American foulbrood in honeybees) (Giménez-Martínez et al., 2020). Lipophilic compounds from different species of Malus (apple pomace) consist of saturated, unsaturated, and polyunsaturated fatty acids, along with polyphenols, phytosterols, and four homologs of tocopherol were found to exhibit inhibitory effects on the growth of a few Gram-positive and Gram-negative bacterial cultures such as Escherichia coli, Pseudomonas aeruginosa, Enterococcus faecalis, Streptococcus pyogenes, Bacillus cereus, Micrococcus luteus, Bacillus subtilis, and Staphylococcus aureus (Table 2; Alberto et al., 2006; Fratianni et al., 2011; Zhang et al., 2016; Kuznetsova et al., 2017; Radenkovs et al., 2018; Zardo et al., 2021). Phenolic compounds isolated from different extraction solvent varies in antimicrobial activities (Zhang et al., 2016; Zardo et al., 2021; Santos et al., 2022). Bioactive compounds such as phloridzin, phloretin, epicatechin, floridzine, procyanidin B2, chlorogenic acid, and quercetin were found to possess significant anti-bacterial activities against S. aureus and E. coli (Zhang et al., 2016; Zardo et al., 2021). Hydro-ethanolic extract of apple pomace also showed antibacterial activity against Gram-negative bacteria Propionibacterium acnes and Proteus mirabilis with minimal inhibition concentration (MIC) of 2.5 and 10 mg/ml, respectively (Table 2; Arraibi et al., 2021). However, in another study, it was shown that volatile components from apple pomace have anti-bacterial potential against S. aureus, Salmonella typhimurium, and E. coli with MIC of >12.5 mg/ml for the tested bacterial strains (Carpes et al., 2021).
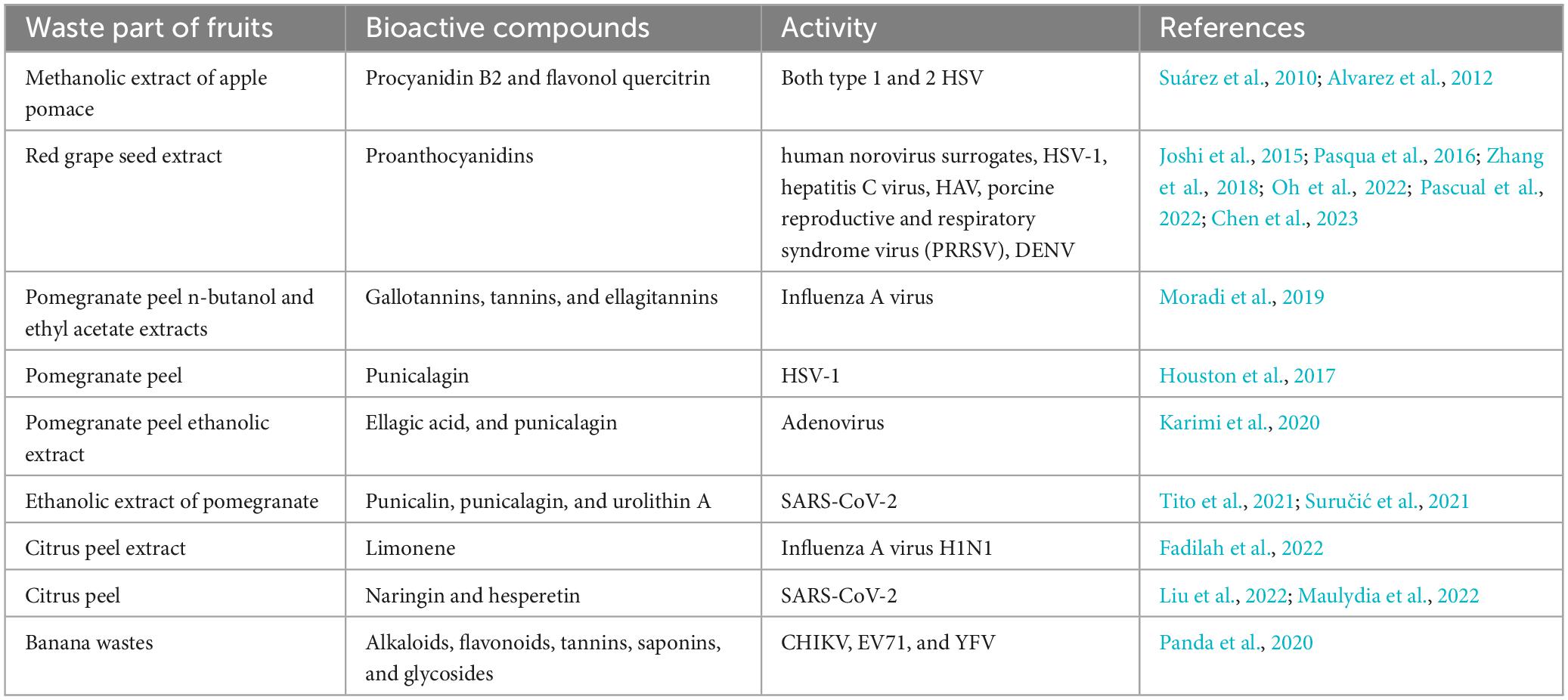
In vitro studies reported that polyphenols from the methanolic extract of apple pomace can inhibit both type 1 and 2 of herpes simplex virus (HSV) viral replication at the concentration of 1,200 μg/ml (Table 3; Suárez et al., 2010). Phytochemicals such as quercitrin and procyanidin B2 were the most important compounds to have a role in the inhibition of HSV viruses by inactivating extracellular virions or inhibiting early viral replication events (Alvarez et al., 2012; Figure 3).
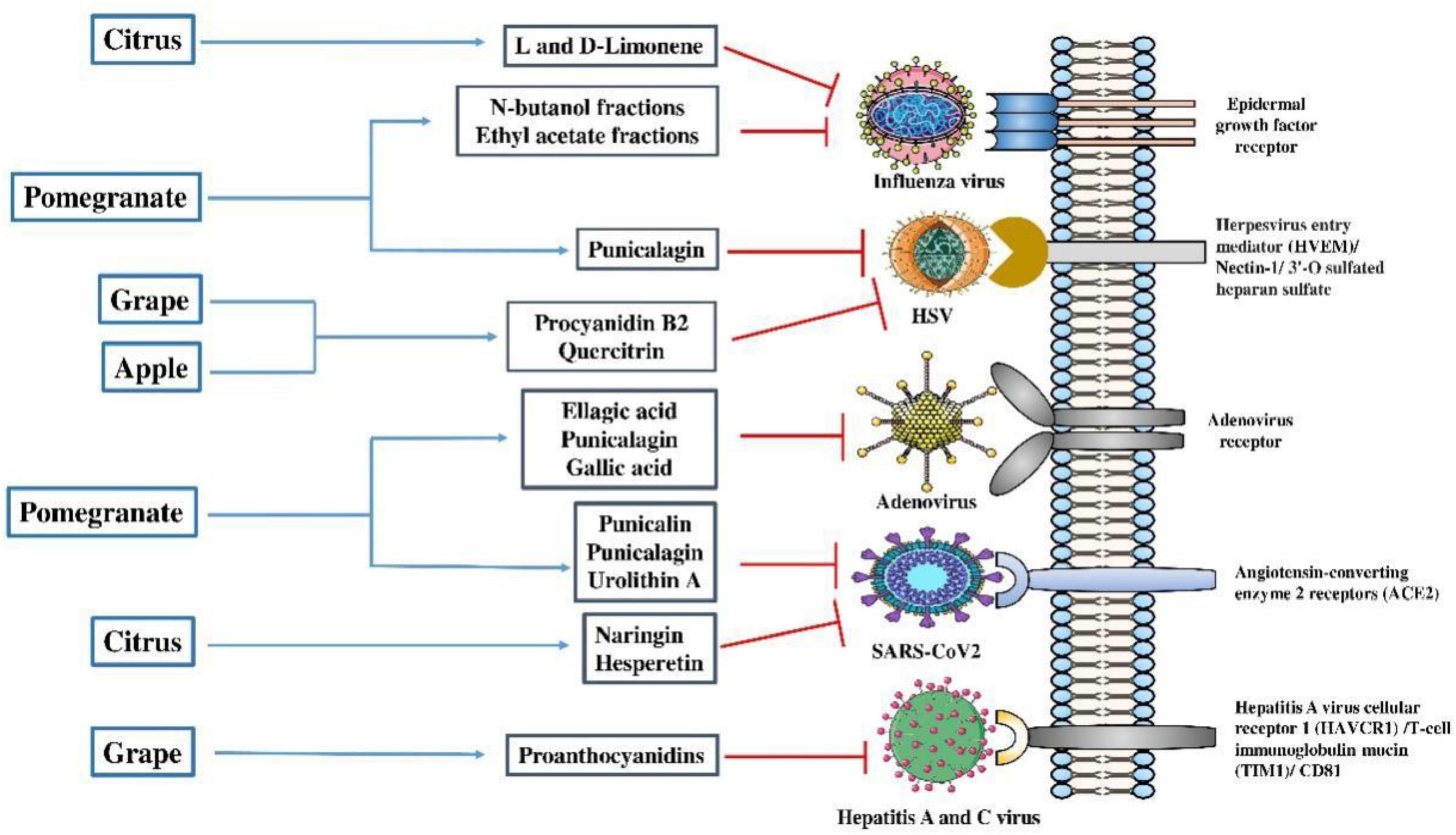
Figure 3. Mode of action of antiviral properties of secondary metabolites extracted from edible fruit wastes. Secondary metabolites like procyanidin B2 and quercitrin extracted from apple and grape wastes can inhibit the entry and replication of herpesvirus and hepatitis A and C virus. L and D limonene, naringin, and hesperetin extracted from wastes of citrus fruit showed anti-influenza and anti-SARS-CoV-2 virus action. Antiviral effects of ellagic acid, punicalagin, and gallic acid from pomegranate peel extracts were found against adenoviruses. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HSV, herpes simplex virus. Parts of the figure were drawn using pictures from Servier Medical Art and generated using Microsoft PowerPoint 2021, 64-bit (Version 2302, Build 16130.20306).
Polyphenol extracts from muscadine grape shells, as well as seeds, had a significant bactericidal effect against Gram-positive bacteria like S. aureus and Listeria monocytogenes in vitro, but only an inhibitory effect against Salmonella enteritidis (Gram-negative) (De-Souza et al., 2014; Xu et al., 2016). The variations of antibacterial and bactericidal activities depend on not only the concentration of polyphenolic compounds but also the presence of specific components with different combinations that may result from the synergistic effect those components (Xu et al., 2014). Recent studies reported that hydroethanolic extract of skin/seed or skin-seed mixture has antibacterial activities against both the Gram-positive and harmful bacterial strains such as E. faecalis, L. monocytogenes, Listeria innocua, E. coli, P. aeruginosa, Klebsiella pneumoniae, Morganella morganii with MIC ranges from 5 mg/ml to 20+ mg/ml (Peixoto et al., 2018; Gerardi et al., 2021; Gómez-Mejía et al., 2021). Both the seed and pomace extract of grape showed a more potent impact on Gram-positive bacteria (S. aureus, L. monocytogenes, and E. faecalis) than Gram-negative ones (Table 2; Pfukwa et al., 2019; Gerardi et al., 2021). This is due to the lipopolysaccharide cell wall, which may prevent polyphenols from entering the cell effectively (Gerardi et al., 2021). The inhibitory effect of grape pomace or seed extract substantially correlates with flavonoid, anthocyanin, and proanthocyanidins, potent bioactives of the grape extract (Pfukwa et al., 2019).
Whole grape extracts have been reported to inhibit different enteric viruses and type 1 of HSV (Figure 3; Pascual et al., 2022). Studies reported that grape seed extract had potent antiviral activities against human norovirus surrogates and hepatitis A virus (HAV) (Table 3; Joshi et al., 2015; Oh et al., 2022). Another report suggested that the active compounds from the grape seed extract can block the hepatitis C virus (HCV) replication in HepG2 cells in vitro (Pasqua et al., 2016). An in vitro study reported that proanthocyanidin, a bioactives from grape seed extract, had anti-viral activity against respiratory syndrome virus and porcine reproductive virus infection in cell line (Zhang et al., 2018). Recently, Chen et al. (2023) demonstrated that grape seed proanthocyanidins extract inhibits dengue virus (DENV) replication by reducing COX-2 expression by modulating NF-κB translocation and ERK/P38 MAPK signaling pathways (Table 3; Chen et al., 2023).
Several bioactive phytochemicals, such flavonoids, phenolics, alkaloids, anthocyanins, and tannins are abundant in pomegranates. It shows a broad spectrum of anti-microbial activity against various pathogenic and multidrug-resistant bacterial strains. Several studies reported that pomegranate peel methanolic extracts had antimicrobial activity against several pathogenic and foodborne bacterial strains like S. aureus, B. cereus, L. monocytogenes, P. aeruginosa, K. pneumonia, E. coli, Salmonella typhi Bacillus megaterium, and B. subtilis (Table 2; Hasan et al., 2018; Abou El-Nour, 2019; Belgacem et al., 2020; Alsubhi et al., 2022). Whereas, ethyl acetate crude extract exhibited antibacterial activity in plant pathogenic bacteria (Xanthomonas gardneri, Pectobacterium carotovorum, and Ralstonia solanacearum) (Khaleel et al., 2018).
In the Madin-Darby Canine Kidney (MDCK) cell line-based experiment, pomegranate peels n-butanol and ethyl acetate extract showed an inhibitory action against influenza A virus having IC50 value that ranges from 5 to 6 μg/ml (Figure 3; Moradi et al., 2019). According to Houston et al. (2017), punicalagin, a bioactives of pomegranate peel extract, has potent anti-viral activity against HSV-1 (Table 3; Houston et al., 2017). Ethanolic fractions of pomegranate peel containing ellagic acid, punicalagin, and gallic acid were proven effective in anti-adenoviral activity, having IC50 values of 2.16 mg/ml through inhibiting adsorption and post-adsorption phases of viral replication in human epithelial (Hep-2) cell lines (Karimi et al., 2020). Phenolic compounds and tannins from pomegranate peel extract significantly reduce the number of human noroviruses (HuNoV) particles, the causative agent of viral gastroenteritis in different food components (Živković et al., 2021). A recent in silico and in vitro study reported that ethanolic extract of pomegranate containing punicalin, punicalagin, and urolithin A showed effective inhibition of SARS-CoV-2 through both (Table 3; Tito et al., 2021). The synergistic effects of bioactive substances prevent SARS-CoV-2 spike protein from interacting with human angiotensin-converting (ACE)-2 receptors and limit the viral protease’s activity in vitro, ultimately preventing viral multiplication and infection (Figure 3; Suručić et al., 2021).
Disc diffusion test confirmed that the essential oils such as linalool, linalyl acetate, γ-terpinene, narirutin, naringin, limonene, hesperetin-7-o-rutinoside, naringenin, quinic acid, and sakuranetin, identified from Citrus spp. showed potent inhibition of Bacillus spp., E. coli, E. faecalis, L. monocytogenes, B. cereus, S. typhimurium, and S. aureus. Limonene is the pre-dominant component contributing to the anti-bacterial potentiality (Table 2; Oikeh et al., 2020; Shehata et al., 2021; Hasan et al., 2022; Cebi and Erarslan, 2023; Meryem et al., 2023; Saleem et al., 2023). Another study reported that in disc diffusion test, the highest inhibitory activity of orange peel oil was demonstrated against Gram-positive S. aureus, compared to Gram-negative bacterial strains like E. coli (Table 2; Kamel et al., 2022).
Citrus peel extract containing essential oils and limonene (both L and D-limonene) exhibited virucidal activity against influenza A virus H1N1 (Figure 3; Fadilah et al., 2022). Different inflammatory disease conditions, such as SARS-CoV-2 infection was shown to be controlled by the reduction of pro-inflammatory cytokines iNOS, IL-1β, IL-6, and COX-2 expression, with the exposure of citrus peel bioactive compounds (active compound – naringin and hesperetin) both in vitro and in vivo (Figure 2; Liu et al., 2022). These phytochemicals are the most potent compounds targeting ACE2 receptor for the blockage of SARS-CoV-2, which was further validated through an in silico molecular docking approach (Table 3; Maulydia et al., 2022). Moreover, the peels and pulp of Citrus contain arbutin, oleanolic acid, malaxinic acid, ursolic acid, chlorogenic acid, epicatechin, and procyanidin B2 that exhibited antibacterial effects (Li et al., 2014; Hussain et al., 2022).
Ethanolic banana peel extract was reported to prevent the growth of S. typhimurium, B. cereus, K. pneumoniae, S. aureus, L. monocytogenes, Proteus vulgaris, S. pyogenes, and E. coli in vitro (Table 2; Saleem and Saeed, 2020; Hanafy et al., 2021; Maryati et al., 2021; Anandhi and Rajeshkumar, 2023; Singh et al., 2023). β-Sitosterol, malic acid, succinic acid, and palmitic acid from banana peel extract (aqueous/ethyl acetate) showed anti-bacterial activity against food poisoning bacterial strains like S. aureus, B. subtilis, B. cereus, S. enteritidis, and E. coli (Hikal et al., 2022). Studies also reported that extracts of banana waste products in different solvents, including hexane, acetone, ethanol, and water, showed in vitro antiviral activity against the chikungunya virus (CHIKV), enterovirus 71 (EV71), and yellow fever virus (YFV) (Panda et al., 2020).
Fruit wastes as a source of prebiotic
As per FAO/WHO (2002), probiotics are beneficial bacterial strains that improve the host’s health when taken in sufficient amounts. Several studies have been done to increase the growth of probiotics in food and nutritional items by supplementing them with prebiotics, which may be an approach to obtain health advantages. A prebiotic is a selective elements that permits particular changes in the gastrointestinal (GI) tract microflora’s composition and activity, both of which are advantageous to the host’s well-being and health (Gibson et al., 2004). Prebiotic properties are typically present in non-digestible polysaccharides and oligosaccharides, such as galactooligosaccharides, fructooligosaccharides, resistant starch, lactulose, and inulin, that are obtainable from a variety of sources, including fruits and vegetables (Figure 4; Thammarutwasik et al., 2009). A few of them, such as inulin, galactooligosaccharides, and fructooligosaccharides, are major prebiotics marketed for industrial use. A synbiotic product is created when probiotics and prebiotics are combined into a single substrate (Donkor et al., 2007; Sah et al., 2016). Probiotics are consortia of yeasts, molds, or lactic acid bacteria (Sha et al., 2018, 2019). In recent days, industrial microbiologists and food biotechnologists have been very much interested in exploring and introducing new prebiotic compounds with added functional properties, such as fiber-rich fractions from grains, fruits, and vegetables, for a variety of reasons, including industrial significance, and health benefits. Many research works have been conducted on prebiotics to boost the development of probiotic microbes by fortification or enrichment of galactooligosaccharides, fructooligosaccharides, rich herbal fractions (Chowdhury et al., 2008), cereals (Vasiljevic et al., 2007), and passion, banana, apple processing fruit wastes or by-products (Espírito Santo et al., 2012).
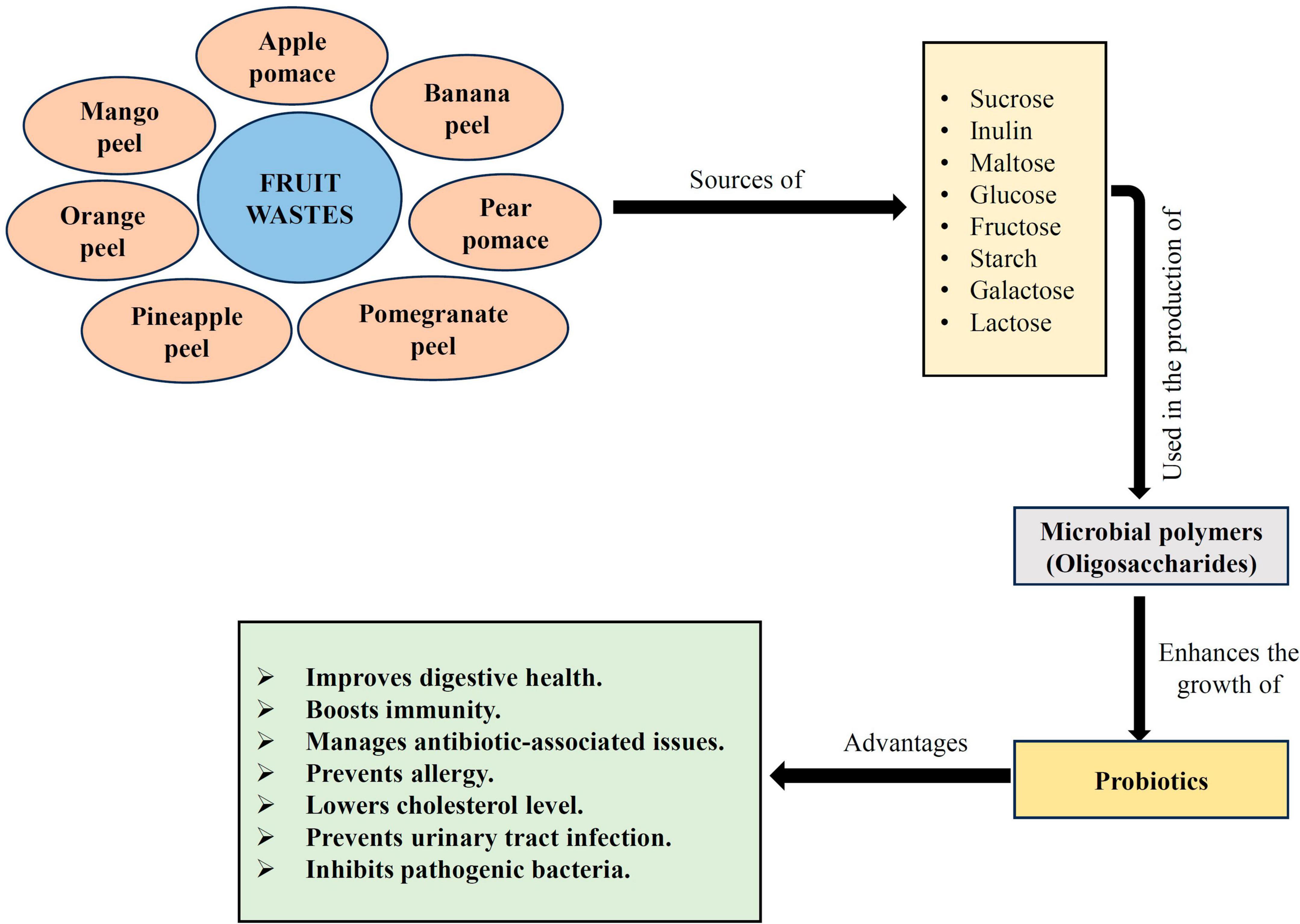
Figure 4. An approach for enhancement of probiotic growth by prebiotic supplementation. Figure generated using Microsoft PowerPoint 2021, 64-bit (Version 2302, Build 16130.20306).
Value addition to fruit wastes
Different types of fruit-waste converted into value-added products, including nutritional foods, bioplastics, biosurfactants, bioenergy, biofertilizers, and single-cell proteins, have potential biotechnological significance. A ground-breaking method of decreasing waste and creating new economic opportunities is the value addition of fruit waste through the creation of value-added products based on their bioactive ingredients (Figure 5). These bioactive substances provide numerous health advantages, including polyphenols, vitamins, minerals, and prebiotics and raise the value of the products (Vilas-Boas et al., 2021). It is feasible to produce rich and functional food components, cosmetics, and nutritional supplements using fruit waste’s capacity to extract these substances (Figure 5). This strategy intends to encourage the development of a circular economy while reducing fruit waste and generating new sources of income. Future fruit-waste valorization must consider this waste’s availability throughout time, its techno-economic potential, and the environmental evaluation of benefits and costs based on its life cycle to be both environmentally and economically sustainable (Caldeira et al., 2020). There are various approaches for converting and recycling fruit waste into value-added products for the betterment of human beings (Caldeira et al., 2020).
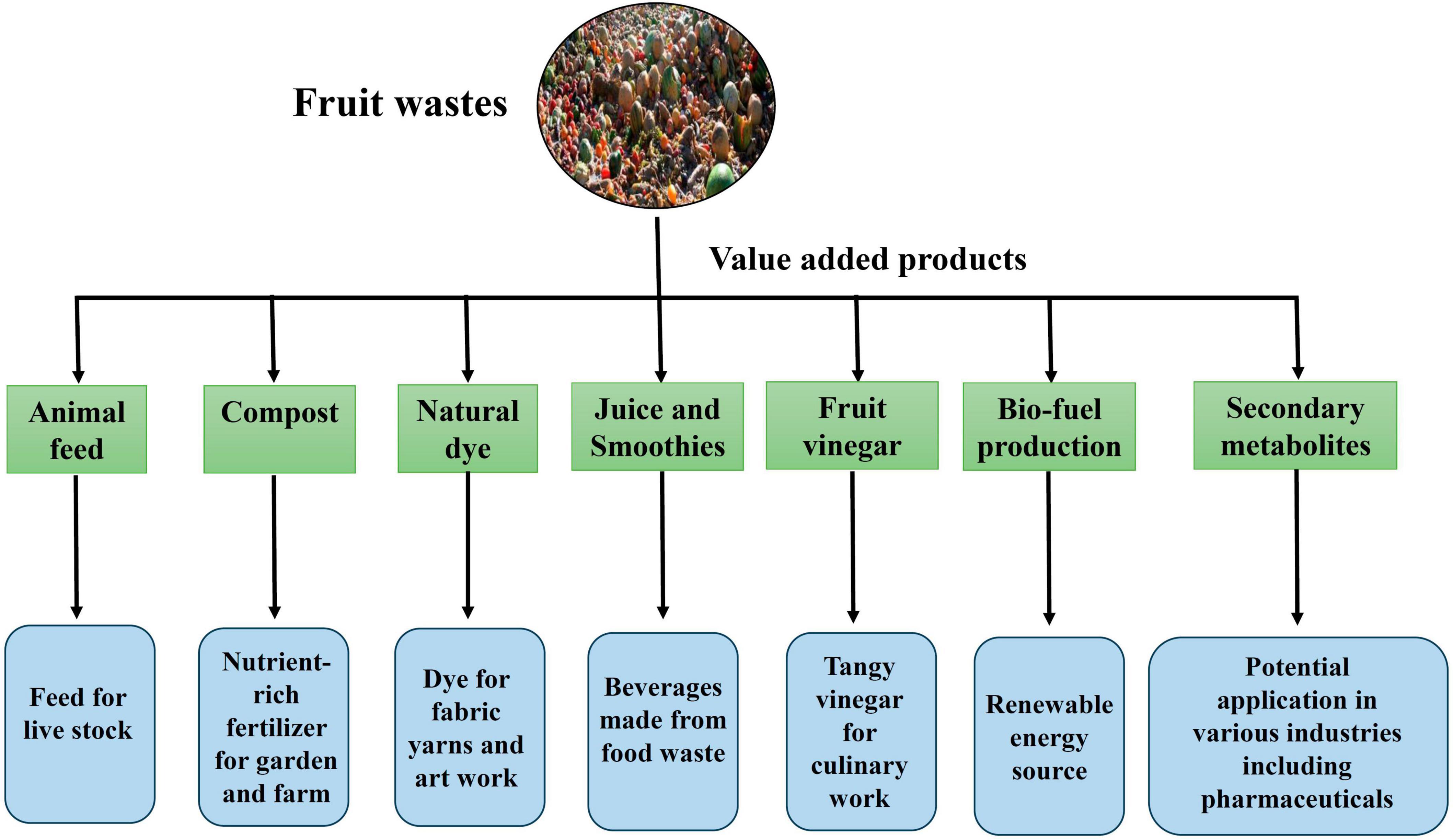
Figure 5. Value addition of various fruit wastes to food, animal feed, energy generation, and medicines production. Figure generated using Microsoft PowerPoint 2021, 64-bit (Version 2302, Build 16130.20306).
Conclusion
The fruit wastes operate as enormous bioactive reservoirs with various health-promoting functions. It has been shown that fruit waste and its bioactives act as potent anti-inflammatory agents and help prevent several cardiovascular disorders by modulating serum cholesterol. Additionally, the secondary byproducts demonstrated their synergistic role in combating hypoglycemic activity and regulate IR. Moreover, this review also supports the anti-viral and anti-bacterial potency of various secondary metabolites derived from fruit waste as well as these fruit waste contains galactooligosaccharides, fructooligosaccharides in rich fractions, regarded as prebiotics that promotes growth of probiotic consortia of human gut and hence, provides various health benefits. Value addition of various fruit wastes into various products like food, animal feed, energy generation, and medicines production can be done for human welfare. Due to the field’s rapid evolution, this review may not fully reflect the most recent developments or newly emerging research on therapeutic applications of fruit waste-derived secondary metabolites in human health. However, this comprehensive review summarizes the current achievements and will enlighten the reuse of various fruit wastes toward sustainable use of these neglected bioactive chemicals and by-products in diverse biological and pharmacological applications that might help the world attain its “zero waste” objective and human welfare.
Author contributions
SSh: Conceptualization, Writing – review and editing, Supervision. SB: Conceptualization, Writing – review and editing. KG: Conceptualization, Writing – review and editing. DM: Validation, Writing – review and editing. SS: Writing – review and editing. SSa: Validation, Writing – review and editing. SR: Formal analysis, Writing – review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. SSh gratefully acknowledges the financial support of Science and Engineering Research Board (SERB) Department of Science and Technology, Government of India for granting research project “SRG/2021/002035,” 30 December 2021. Co-authors also acknowledge the Council of Scientific and Industrial Research (CSIR), Human Resource Development group and University of North Bengal for granting the Junior Research Fellowship to SS [CSIR-JRF sanction no. 09/285(0094)/2019-EMR-I, 3 March 2020]; SR [CSIR-JRF sanction no. 09/285(0088)/2019-EMR-I, 7 October 2019], and Institutional Fellowship to DM [University fellowship no. 259/R-2018, 5 July 2018].
Acknowledgments
The authors acknowledged Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 Unported License.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdulhadi, H. L., Dabdoub, B. R., Ali, L. H., Othman, A. I., Amer, M. E., and El-Missiry, M. A. (2022). Punicalagin protects against pancreatic injury and insulitis in rats with induced T1DM by reducing inflammation and oxidative stress. Mol. Cell. Biochem. 477, 2817–2828. doi: 10.1007/s11010-022-04478-1
Abou El-Nour, M. M. (2019). Functional properties and medical benefits of pomegranate (Punica granatum L.) peels as agro-industrial wastes. Egypt. J. Exp. Biol. 15, 377–392. doi: 10.5455/egyjebb.20191130124643
Ahmed, O. M., Abd El-Twab, S. M., Al-Muzafar, H. M., Adel Amin, K., Abdel Aziz, S. M., and Abdel-Gabbar, M. (2021). Musa paradisiaca L. leaf and fruit peel hydroethanolic extracts improved the lipid profile, glycemic index and oxidative stress in nicotinamide/streptozotocin-induced diabetic rats. Vet. Med. Sci. 7, 500–511. doi: 10.1002/vms3.389
Ajayi, A. M., Coker, A. I., Oyebanjo, O. T., Adebanjo, I. M., and Ademowo, O. G. (2022). Ananas comosus (L) Merrill (pineapple) fruit peel extract demonstrates antimalarial, anti-nociceptive, and anti-inflammatory activities in experimental models. J. Ethnopharmacol. 282:114576. doi: 10.1016/j.jep.2021.114576
Ajayi, A. M., John, K. A., Emmanuel, I. B., Chidebe, E. O., and Adedapo, A. D. (2021). High-fat diet-induced memory impairment and anxiety-like behavior in rats attenuated by peel extract of Ananas comosus fruit via atheroprotective, antioxidant, and anti-inflammatory actions. Metabol. Open. 9:100077. doi: 10.1016/j.metop.2021.100077
Ajikumar, P., Tyo, K., Carlsen, S., Mucha, O., Phon, T., and Stephanopoulos, G. (2008). Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol Pharm. 5, 167–190.
Alberto, M. R., Rinsdahl Canavosio, M. A., and Manca de Nadra, M. C. (2006). Antimicrobial effect of polyphenols from apple skins on human bacterial pathogens. Electron. J. Biotechnol. 9, 205–209. doi: 10.2225/vol9-issue3-fulltext-1
Alsubhi, N. H., Al-Quwaie, D. A., Alrefaei, G. I., Alharbi, M., Binothman, N., Aljadani, M., et al. (2022). Pomegranate Pomace Extract with Antioxidant, Anticancer, Antimicrobial, and Antiviral Activity Enhances the Quality of Strawberry-Yogurt Smoothie. Bioengineering 9:735. doi: 10.3390/bioengineering9120735
Alvarez, A. L., Melón, S., Dalton, K. P., Nicieza, I., Roque, A., Suárez, B., et al. (2012). Apple pomace, a by-product from the Asturian cider industry, inhibits herpes simplex virus types 1 and 2 in vitro replication: study of its mechanisms of action. J. Med. Food. 15, 581–587. doi: 10.1089/jmf.2011.0308
American Heart Association. (2012). Heart disease and stroke statistics – 2012 update: A report from the American Heart Association. Circulation 125, e2–e220. doi: 10.1161/CIR.0b013e31823ac046
Anandhi, P., and Rajeshkumar, S. (2023). Banana peel extract has antimicrobial, antioxidant, anti-inflammatory, and wound healing potential-A review. J. Surv. Fish. 10, 39–48. doi: 10.17762/sfs.v10i1S.149
Arias, A., Feijoo, G., and Moreira, M. T. (2022). Exploring the potential of antioxidants from fruits and vegetables and strategies for their recovery. Innov. Food Sci. Emerg. Technol. 77:102974. doi: 10.1016/j.ifset.2022.102974
Arraibi, A. A., Liberal, Â, Dias, M. I., Alves, M. J., Ferreira, I. C., Barros, L., et al. (2021). Chemical and bioactive characterization of Spanish and Belgian apple pomace for its potential use as a novel dermocosmetic formulation. Foods 10:1949. doi: 10.3390/foods10081949
Arshad, F., Umbreen, H., Aslam, I., Hameed, A., Aftab, K., Al-Qahtani, W. H., et al. (2021). Therapeutic role of mango peels in management of dyslipidemia and oxidative stress in obese females. BioMed Res. Int. 2021, 1–8. doi: 10.1155/2021/3094571
Arun, K. B., Thomas, S., Reshmitha, T. R., Akhil, G. C., and Nisha, P. (2017). Dietary fibre and phenolic-rich extracts from Musa paradisiaca inflorescence ameliorates type 2 diabetes and associated cardiovascular risks. J. Funct. Foods 31, 198–207. doi: 10.1016/j.jff.2017.02.001
Ashraf-Khorassani, M., and Taylor, L. T. (2004). Sequential fractionation of grape seeds into oils, polyphenols, and procyanidins via a single system employing CO2-based fluids. J. Agric. Food Chem. 52, 2440–2444. doi: 10.1021/jf030510n
Baiano, A. (2014). Recovery of biomolecules from food wastes-a review. Molecules 19, 14821–14842. doi: 10.3390/molecules190914821
Baig, M. B., Al-Zahrani, K. H., Schneider, F., Straquadine, G. S., and Mourad, M. (2019). Food waste posing a serious threat to sustainability in the Kingdom of Saudi Arabia–A systematic review. Saudi J. Biol. Sci. 26, 1743–1752. doi: 10.1016/j.sjbs.2018.06.004
Bains, A., and Chawla, P. (2020). In vitro bioactivity, antimicrobial and anti-inflammatory efficacy of modified solvent evaporation assisted Trametes versicolor extract. 3 Biotech 10:404. doi: 10.1007/s13205-020-02397-w
Bansal, A., and Priyadarsini, C. (2022). Medicinal Properties of Phytochemicals and Their Production. London: IntechOpen, doi: 10.5772/intechopen.98888
Barreira, J. C., Arraibi, A. A., and Ferreira, I. C. (2019). Bioactive and functional compounds in apple pomace from juice and cider manufacturing: Potential use in dermal formulations. Trends Food Sci Technol. 90, 76–87. doi: 10.1016/j.tifs.2019.05.014
Belgacem, I., Schena, L., Teixidó, N., Romeo, F. V., Ballistreri, G., and Abadias, M. (2020). Effectiveness of a pomegranate peel extract (PGE) in reducing Listeria monocytogenes in vitro and on fresh-cut pear, apple and melon. Eur. Food Res. Technol. 246, 1765–1772. doi: 10.1007/s00217-020-03529-5
Bello, U., Amran, N. A., and Ruslan, M. S. H. (2023). Improving antioxidant scavenging effect of fruit peel waste extracts and their applicability in biodiesel stability enhancement. J. Saudi Chem. Soc. 27:101653. doi: 10.1016/j.jscs.2023.101653
Benavente-Garcia, O., and Castillo, J. (2008). Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 56, 6185–6205. doi: 10.1021/jf8006568
Bhardwaj, K., Najda, A., Sharma, R., Nurzyńska-Wierdak, R., Dhanjal, D. S., Sharma, R., et al. (2022). Fruit and vegetable peel-enriched functional foods: potential avenues and health perspectives. Evid. Based Complement. Alternat. Med. 2022:8543881. doi: 10.1155/2022/8543881
Budiati, T., Suryaningsih, W., and Bethiana, T. N. (2022). Antimicrobial of tropical fruit and vegetable waste extract for food-borne pathogenic bacteria. Ital. J. Food Saf. 11:10510. doi: 10.4081/ijfs.2022.10510
Calabriso, N., Massaro, M., Scoditti, E., Verri, T., Barca, A., Gerardi, C., et al. (2022). Grape pomace extract attenuates inflammatory response in intestinal epithelial and endothelial cells: Potential health-promoting properties in bowel inflammation. Nutrients 14:1175. doi: 10.3390/nu14061175
Caldeira, C., Vlysidis, A., Fiore, G., De Laurentiis, V., Vignali, G., and Sala, S. (2020). Sustainability of food waste biorefifinery: A review on valorisation pathways, techno-economic constraints, and environmental assessment. Bioresour. Technol. 312:123575. doi: 10.1016/j.biortech.2020.123575
Caliceti, C., Malaguti, M., Marracino, L., Barbalace, M. C., Rizzo, P., and Hrelia, S. (2022). Agri-food waste from apple, pear, and sugar beet as a source of protective bioactive molecules for endothelial dysfunction and its major complications. Antioxidants 11:1786. doi: 10.3390/antiox11091786
Cao, Y., Ren, G., Zhang, Y., Qin, H., An, X., Long, Y., et al. (2021). A new way for punicalagin to alleviate insulin resistance: regulating gut microbiota and autophagy. Food Nutr. Res. 65:5689. doi: 10.29219/fnr.v65.5689
Carpes, S. T., Bertotto, C., Bilck, A. P., Yamashita, F., Anjos, O., Siddique, M. A. B., et al. (2021). Bio-based films prepared with apple pomace: Volatiles compound composition and mechanical, antioxidant and antibacterial properties. Lwt 144:111241. doi: 10.1016/j.lwt.2021.111241
Carson, J. A. S., Lichtenstein, A. H., Anderson, C. A., Appel, L. J., Kris-Etherton, P. M., Meyer, K. A., et al. (2020). Dietary cholesterol and cardiovascular risk: a science advisory from the American Heart Association. Circulation 141, e39–e53. doi: 10.1161/cir.0000000000000743
Cebi, N., and Erarslan, A. (2023). Determination of the antifungal, antibacterial activity and volatile compound composition of Citrus bergamia peel essential oil. Foods 12:203. doi: 10.3390/foods12010203
Chang, S., Cui, X., Guo, M., Tian, Y., Xu, W., Huang, K., et al. (2017). Insoluble dietary fiber from pear pomace can prevent high-fat diet-induced obesity in rats mainly by improving the structure of the gut microbiota. J. Microbiol. Biotechnol. 27, 856–867. doi: 10.4014/jmb.1610.10058
Chen, J., Wu, Z., Wang, J., Si, X., Zhang, R., Sun, T., et al. (2022). Docosahexaenoic acid ester of phloridzin reduces inflammation and insulin resistance via AMPK. Curr. Pharm. Des. 28, 1854–1862. doi: 10.2174/1381612828666220518102440
Chen, W. C., Hossen, M., Liu, W., Yen, C. H., Huang, C. H., Hsu, Y. C., et al. (2023). Grape seed proanthocyanidins inhibit replication of the dengue virus by targeting NF-kB and MAPK-Mediated Cyclooxygenase-2 Expression. Viruses 15:884. doi: 10.3390/v15040884
Chien, W. J., Saputri, D. S., and Lin, H. Y. (2022). Valorization of Taiwan’s Citrus depressa Hayata peels as a source of nobiletin and tangeretin using simple ultrasonic-assisted extraction. Curr. Res. Nutr. Food Sci. 5, 278–287. doi: 10.1016/j.crfs.2022.01.013
Chowdhury, B., Chakraborty, R., and Raychaudhuri, U. (2008). Study on β-galactosidase enzymatic activity of herbal yogurt. Int. J. Food Sci. Nutr. 59, 116–122. doi: 10.1080/09637480701447787
Choy, K. W., Murugan, D., Leong, X. F., Abas, R., Alias, A., and Mustafa, M. R. (2019). Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: a mini review. Front. Pharmacol. 10:1295. doi: 10.3389/fphar.2019.01295
Cicero, A. F. G., Caliceti, C., Fogacci, F., Giovannini, M., Calabria, D., Colletti, A., et al. (2017). Effect of apple polyphenols on vascular oxidative stress and endothelium function: A translational study. Mol. Nutr. Food Res. 61:1700373. doi: 10.1002/mnfr.201700373
Czech, A., Malik, A., Sosnowska, B., and Domaradzki, P. (2021). Bioactive substances, heavy metals, and antioxidant activity in whole fruit, peel, and pulp of citrus fruits. Int. J. Food Sci. 2021, 1–14. doi: 10.1155/2021/6662259
Daniel, T., Ben-Shachar, M., Drori, E., Hamad, S., Permyakova, A., Ben-Cnaan, E., et al. (2021). Grape pomace reduces the severity of non-alcoholic hepatic steatosis and the development of steatohepatitis by improving insulin sensitivity and reducing ectopic fat deposition in mice. J. Nutr. Biochem. 98:108867. doi: 10.1016/j.jnutbio.2021.108867
Das, Q., Tang, J., Yin, X., Ross, K., Warriner, K., Marcone, M. F., et al. (2021). Organic cranberry pomace and its ethanolic extractives as feed supplement in broiler: impacts on serum Ig titers, liver and bursal immunity. Poult. Sci. 100, 517–526.
Day, L., Seymour, R., Pitts, K., Konczak, I., and Lundin, L. (2009). Incorporation of functional ingredients into foods. Trends Food Sci. Technol. 20, 388–395. doi: 10.1016/j.tifs.2008.05.002
De Nigris, F., Williams-Ignarro, S., Sica, V., Lerman, L. O., D’armiento, F. P., Byrns, R. E., et al. (2007). Effects of a pomegranate fruit extract rich in punicalagin on oxidation-sensitive genes and eNOS activity at sites of perturbed shear stress and atherogenesis. Cardiovasc. Res. 73, 414–423. doi: 10.1016/j.cardiores.2006.08.021
De-Souza, V. B., Fujita, A., Thomazini, M., da Silva, E. R., Lucon, J. F. Jr., and Genovese, M. I. (2014). Functional properties and stability of spray-dried pigments from Bordo grape (Vitis labrusca) winemaking pomace. Food Chem. 164, 380–386. doi: 10.1016/j.foodchem.2014.05.049
Donkor, O., Nilmini, S., Stolic, P., Vasiljevic, T., and Shah, N. (2007). Survival and activity of selected probiotic organisms in set-type yoghurt during cold storage. Int. Dairy J. 17, 657–665. doi: 10.1016/j.idairyj.2006.08.006
Du, L., Li, J., Zhang, X., Wang, L., Zhang, W., Yang, M., et al. (2019). Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264. 7 macrophages via the suppression of TLR4/NF-κB pathway activation. Food Nutr. Res. 63:3392. doi: 10.29219/fnr.v63.3392
Duba, K. S., Casazza, A. A., Mohamed, H. B., Perego, P., and Fiori, L. (2015). Extraction of polyphenols from grape skins and defatted grape seeds using subcritical water: experiments and modeling. Food Bioprod. Process. 94, 29–38. doi: 10.1016/j.fbp.2015.01.001
Eddie-Amadi, B. F., Ezejiofor, A. N., Orish, C. N., Rovira, J., Allison, T. A., and Orisakwe, O. E. (2022). Banana peel ameliorated hepato-renal damage and exerted anti-inflammatory and anti-apoptotic effects in metal mixture mediated hepatic nephropathy by activation of Nrf2/Hmox-1 and inhibition of Nfkb pathway. Food Chem. Toxicol. 170:113471. doi: 10.1016/j.fct.2022.113471
Elbandrawy, M. M., Sweef, O., Elgamal, D., Mohamed, T. M., and Elgharabawy, R. M. (2022). Ellagic acid regulates hyperglycemic state through modulation of pancreatic IL-6 and TNF-α immunoexpression. Saudi J. Biol. Sci. 29, 3871–3880. doi: 10.1016/j.sjbs.2022.03.016
Espírito Santo, A., Cartolano, N., Silva, T., Soares, F., Gioielli, L., Perego, P., et al. (2012). Fibers from fruit by-products enhance probiotic viability and fatty acid profile and increase CLA content in yoghurts. Int. J. Food Microbiol. 154, 135–144. doi: 10.1016/j.ijfoodmicro.2011.12.025
Fadilah, N. Q., Jittmittraphap, A., Leaungwutiwong, P., Pripdeevech, P., Dhanushka, D., Mahidol, C., et al. (2022). Virucidal Activity of Essential Oils From Citrus x aurantium L. Against Influenza A Virus H 1 N 1: Limonene as a Potential Household Disinfectant Against Virus. Nat. Prod. Commun. 17:1934578X211072713. doi: 10.1177/1934578X211072713
Feng, K., Zhu, X., Liu, G., Kan, Q., Chen, T., Chen, Y., et al. (2020). Dietary citrus peel essential oil ameliorates hypercholesterolemia and hepatic steatosis by modulating lipid and cholesterol homeostasis. Food Funct. 11, 7217–7230. doi: 10.1039/D0FO00810A
Fernandes, P. A., Ferreira, S. S., Bastos, R., Ferreira, I., Cruz, M. T., Pinto, A., et al. (2019). Apple pomace extract as a sustainable food ingredient. Antioxidants 8:189. doi: 10.3390/antiox8060189
Fratianni, F., Coppola, R., and Nazzaro, F. (2011). Phenolic composition and antimicrobial and antiquorum sensing activity of an ethanolic extract of peels from the apple cultivar Annurca. J. Med. Food. 14, 957–963. doi: 10.1089/jmf.2010.0170
Fu, L., Xu, B. T., Xu, X. R., Gan, R. Y., Zhang, Y., Xia, E. Q., et al. (2011). Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 129, 345–350. doi: 10.1016/j.foodchem.2011.04.079
García, P., Fredes, C., Cea, I., Lozano-Sánchez, J., Leyva-Jiménez, F. J., Robert, P., et al. (2021). Recovery of bioactive compounds from pomegranate (Punica granatum L.) peel using pressurized liquid extraction. Foods 10:203. doi: 10.3390/foods10020203
Garcia-Montalvo, J., Garcia-Martín, A., Ibañez Bujan, J., Santos Mazorra, V. E., Yustos Cuesta, P., Bolivar, J. M., et al. (2022). Extraction of antioxidants from grape and apple pomace: Solvent selection and process kinetics. Appl. Sci. 12:4901. doi: 10.3390/app12104901
Geng, X., Ji, J., Liu, Y., Li, X., Chen, Y., Su, L., et al. (2022). Cyanidin-3-O-Glucoside Supplementation Ameliorates Metabolic Insulin Resistance via Restoration of Nitric Oxide-Mediated Endothelial Insulin Transport. Mol. Nutr. Food Res. 66:2100742. doi: 10.1002/mnfr.202100742
Gerardi, C., Pinto, L., Baruzzi, F., and Giovinazzo, G. (2021). Comparison of Antibacterial and Antioxidant Properties of Red (cv. Negramaro) and White (cv. Fiano) Skin Pomace Extracts. Molecules 26:5918. doi: 10.3390/molecules26195918
Ghafoor, K., Hui, T., and Choi, Y. H. (2011). Optimization of ultrasonic-assisted extraction of total anthocyanins from grape peel using response surface methodology. J. Food Biochem. 35, 735–746. doi: 10.1111/j.1745-4514.2010.00413.x
Gibson, G., Probert, H., Van Loo, J., Rastall, R., and Roberfroid, M. (2004). Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 17, 259–275. doi: 10.1079/NRR200479
Giménez-Martínez, P., Ramírez-Ambrosi, M., Rosa, M. A. S., Gallo, B., Luis, A. B., Maggi, M., et al. (2020). Antimicrobial Activity of Phenolic Extract of Apple Pomace against and its Toxicity on Apis mellifera. J. Apic. Sci. 64, 199–208. doi: 10.2478/jas-2020-0016
Gómez-Mejía, E., Roriz, C. L., Heleno, S. A., Calhelha, R., Dias, M. I., Pinela, J., et al. (2021). Valorisation of black mulberry and grape seeds: Chemical characterization and bioactive potential. Food Chem. 337:127998. doi: 10.1016/j.foodchem.2020.127998
Gondi, M., Basha, S. A., Bhaskar, J. J., Salimath, P. V., and Prasada Rao, U. J. (2015). Anti-diabetic effect of dietary mango (Mangifera indica L.) peel in streptozotocin-induced diabetic rats. J. Sci. Food Agric. 95, 991–999. doi: 10.1002/jsfa.6778
Gulsunoglu, Z., Karbancioglu-Guler, F., Raes, K., and Kilic-Akyilmaz, M. (2019). Soluble and insoluble-bound phenolics and antioxidant activity of various industrial plant wastes. Int. J. Food Prop. 22, 1501–1510. doi: 10.1080/10942912.2019.1656233
Guo, J., Chen, J., Ren, W., Zhu, Y., Zhao, Q., Zhang, K., et al. (2020). Citrus flavone tangeretin is a potential insulin sensitizer targeting hepatocytes through suppressing MEK-ERK1/2 pathway. Biochem. Biophys. Res. Commun. 529, 277–282. doi: 10.1016/j.bbrc.2020.05.212
Han, N., and Bakovic, M. (2015). Biologically active triterpenoids and their cardioprotective and anti-inflammatory effects. J. Bioanal. Biomed. 12, 1948–1955. doi: 10.4172/1948-593X.S12-005
Hanafy, S. M., Abd El-Shafea, Y. M., Saleh, W. D., and Fathy, H. M. (2021). Chemical profiling, in vitro antimicrobial and antioxidant activities of pomegranate, orange and banana peel-extracts against pathogenic microorganisms. J. Genet. Eng. Biotechnol. 19:80. doi: 10.1186/s43141-021-00151-0
Hasan, A. M., Redha, A. A., and Mandeel, Q. (2018). Phytochemical investigations of pomegranate (Punica granatum) rind and aril extracts and their antioxidant, antidiabetic and antibacterial activity. Nat. Prod. Chem. Res. 6:332. doi: 10.4172/2329-6836.1000332
Hasan, M. M., Roy, P., Alam, M., Hoque, M. M., and Zzaman, W. (2022). Antimicrobial activity of peels and physicochemical properties of juice prepared from indigenous citrus fruits of Sylhet region, Bangladesh. Heliyon 8, e09948. doi: 10.1016/j.heliyon.2022.e09948
Hassanpour Fard, M., Ghule, A. E., Bodhankar, S. L., and Dikshit, M. (2011). Cardioprotective effect of whole fruit extract of pomegranate on doxorubicin-induced toxicity in rat. Pharm. Biol. 49, 377–382. doi: 10.3109/13880209.2010.517758
Hikal, W. M., Ahl, S. A., Hussein, A. H., Bratovcic, A., Tkachenko, K. G., Sharifi-Rad, J., et al. (2022). Banana peels: A waste treasure for human being. Evid. Based Complementary Altern. Med. 2022:7616452. doi: 10.1155/2022/7616452
Hong, Y. H., Kao, C., Chang, C. C., Chang, F. K., Song, T. Y., Houng, J. Y., et al. (2023). Anti-Inflammatory and T-Cell Immunomodulatory Effects of Banana Peel Extracts and Selected Bioactive Components in LPS-Challenged In Vitro and In Vivo Models. Agriculture 13:451. doi: 10.3390/agriculture13020451
Houston, D. M., Bugert, J. J., Denyer, S. P., and Heard, C. M. (2017). Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against Herpes simplex virus (HSV) when co-administered with zinc (II) ions, and antiviral activity of PRE against HSV and aciclovir-resistant HSV. PLoS One 12:e0179291. doi: 10.1371/journal.pone.0179291
Hu, H., Zhao, Q., Xie, J., and Sun, D. (2019). Polysaccharides from pineapple pomace: new insight into ultrasonic-cellulase synergistic extraction and hypoglycemic activities. Int. J. Biol. Macromol. 121, 1213–1226. doi: 10.1016/j.ijbiomac.2018.10.054
Huang, C. Y., Kuo, C. H., Wu, C. H., Kuan, A. W., Guo, H. R., Lin, Y. H., et al. (2018). Free radical-scavenging, anti-inflammatory, and antibacterial activities of water and ethanol extracts prepared from compressional-puffing pretreated mango (Mangifera indica L.) peels. J. Food Qual. 2018, 1–13. doi: 10.1155/2018/1025387
Hussain, H., Mamadalieva, N. Z., Hussain, A., Hassan, U., Rabnawaz, A., Ahmed, I., et al. (2022). Fruit peels: Food waste as a valuable source of bioactive natural products for drug discovery. Curr. Issues Mol. Biol. 44, 1960–1994. doi: 10.3390/cimb44050134
Indriawati, R., and Atiyah, F. U. (2022). Antihyperglycemic and Hypolipidemic Potential of Kepok Banana Peel in Diabetic Rats. IOP Conf. Ser.: Earth Environ. Sci. 985:012040. doi: 10.1088/1755-1315/985/1/012040
Ismail, T., Sestili, P., and Akhtar, S. (2012). Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. J Ethnopharmacol. 143, 397–405. doi: 10.1016/j.jep.2012.07.004
James, D. E., Stöckli, J., and Birnbaum, M. J. (2021). The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 22, 751–771. doi: 10.1038/s41580-021-00390-6
Jatav, J., Tarafdar, A., Saravanan, C., and Bhattacharya, B. (2022). Assessment of antioxidant and antimicrobial property of polyphenol-rich chitosan-pineapple peel film. J. Food Qual. 2022:8064114. doi: 10.1155/2022/8064114
Jin, D., Zhang, B., Li, Q., Tu, J., and Zhou, B. (2020). Effect of punicalagin on multiple targets in streptozotocin/high-fat diet-induced diabetic mice. Food Funct. 11, 10617–10634. doi: 10.1039/D0FO01275K
Joana Gil-Chávez, G., Villa, J., Fernando Ayala-Zavala, J., Basilio Heredia, J., Sepulveda, D., Yahia, E., et al. (2013). Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr. Rev. Food Sci. Food Saf. 12, 5–23.
Joshi, S. S., Su, X., and D’Souza, D. H. (2015). Antiviral effects of grape seed extract against feline calicivirus, murine norovirus, and hepatitis A virus in model food systems and under gastric conditions. Food Microbiol. 52, 1–10. doi: 10.1016/j.fm.2015.05.011
Kakadiya, J., Mulani, H., and Shah, N. (2010). Protective effect of hesperidin on cardiovascular complication in experimentally induced myocardial infarction in diabetes in rats. J. Basic Clin. Pharm. 1, 85–91.
Kamel, F., Sabir, S., Mahal, A., and Wei, X. (2022). In vitro Antibacterial Activity of Orange Peel Oil Extract from Citrus sinensis Fruit in Erbil. Egypt. J. Chem. 65, 157–160. doi: 10.21608/ejchem.2021.93484.4416
Kandemir, K., Piskin, E., Xiao, J., Tomas, M., and Capanoglu, E. (2022). Fruit juice industry wastes as a source of bioactives. J. Agric. Food Chem. 70, 6805–6832. doi: 10.1021/acs.jafc.2c00756
Kaneko, Y. K., Tara, Y., Ihim, S. A., Yamamoto, M., Kaji, M., and Ishikawa, T. (2022). Nobiletin ameliorates glucose tolerance by protecting against β-cell loss in type-2 diabetic db/db mice. Phytomed. Plus 2:100367. doi: 10.1016/j.phyplu.2022.100367
Karimi, A., Moradi, M. T., Rabiei, M., and Alidadi, S. (2020). In vitro anti-adenoviral activities of ethanol extract, fractions, and main phenolic compounds of pomegranate (Punica granatum L.) peel. Antivir. Chem. Chemother. 28:2040206620916571. doi: 10.1177/2040206620916571
Khaleel, A., Sijam, K., and Rashid, T. (2018). Determination of antibacterial compounds of Punica granatum peel extract by TLC direct bio-autography and GCMS analysis. Biochem. Cell. Arch. 18, 379–384.
Khan, U. M., Sameen, A., Aadil, R. M., Shahid, M., Sezen, S., Zarrabi, A., et al. (2021). Citrus genus and its waste utilization: a review on health-promoting activities and industrial application. Evid. Based Complementary Altern. Med. 2021, 1–17. doi: 10.1155/2021/2488804
Khateeb, J., Gantman, A., Kreitenberg, A. J., Aviram, M., and Fuhrman, B. (2010). Paraoxonase 1 (PON1) expression in hepatocytes is upregulated by pomegranate polyphenols: a role for PPAR-gamma pathway. Atherosclerosis 208, 119–125. doi: 10.1016/j.atherosclerosis.2009.08.051
Khokar, R., Hachani, K., Hasan, M., Othmani, F., Essam, M., Al Mamari, A., et al. (2021). Anti-Alzheimer potential of a waste by-product (peel) of Omani pomegranate fruits: Quantification of phenolic compounds, in-vitro antioxidant, anti-cholinesterase and in-silico studies. Biocatal. Agric. Biotechnol. 38:102223. doi: 10.1016/j.bcab.2021.102223
Kim, I. (2015). Inhibitory effects of apple peel extract on inflammatory enzymes. Korean J. Food Sci. 47, 534–538. doi: 10.9721/KJFST.2015.47.4.534
Ko, D. Y., and Ku, K. M. (2022). Effect of Anti-Obesity and Antioxidant Activity through the Additional Consumption of Peel from ‘Fuji’. Pre-Washed Apple. Foods 11:497. doi: 10.3390/foods11040497
Ko, K., Dadmohammadi, Y., and Abbaspourrad, A. (2021). Nutritional and bioactive components of pomegranate waste used in food and cosmetic applications: A review. Foods 10:657.
Kolniak-Ostek, J., and Oszmiański, J. (2015). Characterization of phenolic compounds in different anatomical pear (Pyrus communis L.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS). Int. J. Mass Spectrom. 392, 154–163. doi: 10.1016/j.ijms.2015.10.004
Kolodziejczyk, K., Markowski, J., Kosmala, M., Król, B., and Plocharski, W. (2007). Apple pomace as a potential source of nutraceutical products. Polish J. Food Nutr. Sci. 57, 291–295.
Kumar, A. S., and Sinha, N. (2020). Cardiovascular disease in India: a 360-degree overview. Med. J. Armed Forces India. 76, 1–3. doi: 10.1016/j.mjafi.2019.12.005
Kumar, K. (2015). Role of edible mushrooms as functional foods—a review. South Asian J. Food Technol. Environ. 1, 211–218.
Kumar, K., and Kumar, S. (2015). Role of nutraceuticals in health and disease prevention: a review. South Asian J.Food Technol. Environ. 1, 116–121.
Kumar, S., Sinha, K., Sharma, R., Purohit, R., and Padwad, Y. (2019). Phloretin and phloridzin improve insulin sensitivity and enhance glucose uptake by subverting PPARγ/Cdk5 interaction in differentiated adipocytes. Exp. Cell Res. 383:111480. doi: 10.1016/j.yexcr.2019.06.025
Kuznetsova, E., Emelyanov, A., Klimova, E., Bychkova, T., Vinokurov, A., Selifonova, N., et al. (2017). Antioxidant, antimicrobial activity and mineral composition of low-temperature fractioning products of Malus domestica Borkh (common Antonovka). Potravinarstvo. Potr. S. J. F. Sci. 11, 658–663. doi: 10.5219/820
Lanzi, C. R., Perdicaro, D. J., Antoniolli, A., Fontana, A. R., Miatello, R. M., Bottini, R., et al. (2016). Grape pomace and grape pomace extract improve insulin signaling in high-fat-fructose fed rat-induced metabolic syndrome. Food Funct. 7, 1544–1553. doi: 10.1039/C5FO01065A
Lawrence, T. (2009). The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1:a001651. doi: 10.1101/cshperspect.a001651
Lee, E. H., Park, H. J., Jung, H. Y., Kang, I. K., Kim, B. O., and Cho, Y. J. (2022). Isoquercitrin isolated from newly bred Green ball apple peel in lipopolysaccharide-stimulated macrophage regulates NF-κB inflammatory pathways and cytokines. 3 Biotech 12:100. doi: 10.1007/s13205-022-03118-1
Lee, E. H., Park, H. J., Kim, B. O., Choi, H. W., Park, K. I., Kang, I. K., et al. (2020). Anti-inflammatory effect of Malus domestica cv. Green ball apple peel extract on Raw 264.7 macrophages. J. Appl. Biol. Chem. 63, 117–123. doi: 10.3839/jabc.2020.016
Lee, Y. H., Charles, A. L., Kung, H. F., Ho, C. T., and Huang, T. C. (2010). Extraction of nobiletin and tangeretin from Citrus depressa Hayata by supercritical carbon dioxide with ethanol as modifier. Ind. Crops Prod. 31, 59–64. doi: 10.1016/j.indcrop.2009.09.003
Li, M., Chi, X., Wang, Y., Setrerrahmane, S., Xie, W., and Xu, H. (2022). Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct. Target Ther. 7:216. doi: 10.1038/s41392-022-01073-0
Li, X., Wang, T., Zhou, B., Gao, W., Cao, J., and Huang, L. (2014). Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 152, 531–538. doi: 10.1016/j.foodchem.2013.12.010
Li, X., Wu, X., Bi, J., Liu, X., Li, X., and Guo, C. (2019). Polyphenols accumulation effects on surface color variation in apple slices hot air drying process. LWT-Food Sci. Technol. 108, 421–428. doi: 10.1016/j.lwt.2019.03.098
Liu, W., Zheng, W., Cheng, L., Li, M., Huang, J., Bao, S., et al. (2022). Citrus fruits are rich in flavonoids for immunoregulation and potential targeting ACE2. Nat. Prod. Bioprospecting. 12:4. doi: 10.1007/s13659-022-00325-4
Llavata, B., Picinelli, A., Simal, S., and Cárcel, J. A. (2022). Cider apple pomace as a source of nutrients: Evaluation of the polyphenolic profile, antioxidant and fiber properties after drying process at different temperatures. Food Chem. 15:100403. doi: 10.1016/j.fochx.2022.100403
Lončarić, M., Strelec, I., Moslavac, T., Šubarić, D., Pavić, V., and Molnar, M. (2021). Lipoxygenase inhibition by plant extracts. Biomolecules 11:152. doi: 10.3390/biom11020152
Lu, Y., and Foo, L. Y. (2000). Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 68, 81–8512.
Lucarini, M., Durazzo, A., Bernini, R., Campo, M., Vita, C., Souto, E. B., et al. (2021). Fruit wastes as a valuable source of value-added compounds: A collaborative perspective. Molecules 26:6338. doi: 10.3390/molecules26216338
Lun, O. K., Wai, T. B., and Ling, L. S. (2014). Pineapple cannery waste as a potential substrate for microbial biotranformation to produce vanillic acid and vanillin. Int. Food Res. J. 21, 953–958.
Madhumeena, S., Preetha, R., and Prasad, S. (2021). Effective utilization of pineapple waste. J. Phys. Conf. Ser. 1979:012001. doi: 10.1088/1742-6596/1979/1/012001
Makarova, E., Górna’s, P., Konrade, I., Tirzite, D., Cirule, H., Gulbe, A., et al. (2015). Acute antihyperglycaemic effects of an unripe apple preparation containing phlorizin in healthy volunteers: A preliminary study. J. Sci. Food Agric. 95, 560–568. doi: 10.1002/jsfa.6779
Malik, A., Najda, A., Bains, A., Nurzyńska-Wierdak, R., and Chawla, P. (2021). Characterization of Citrus nobilis peel methanolic extract for antioxidant, antimicrobial, and anti-inflammatory activity. Molecules 26:4310. doi: 10.3390/molecules26144310
Manickam, V., Dhawan, U. K., Singh, D., Gupta, M., and Subramanian, M. (2022). Pomegranate Peel Extract Decreases Plaque Necrosis and Advanced Atherosclerosis Progression in Apoe-/- Mice. Front. Pharmacol. 13:888300. doi: 10.3389/fphar.2022.888300
Marracino, L., Punzo, A., Severi, P., Nganwouo Tchoutang, R., Vargas-De-la-Cruz, C., Fortini, F., et al. (2022). Fermentation of Vaccinium floribundum Berries with Lactiplantibacillus plantarum Reduces oxidative stress in endothelial cells and modulates macrophages function. Nutrients 14:1560. doi: 10.3390/nu14081560
Martínez-Maqueda, D., Zapatera, B., Gallego-Narbón, A., Vaquero, M. P., Saura-Calixto, F., and Pérez-Jiménez, J. (2018). A 6-week supplementation with grape pomace to subjects at cardiometabolic risk ameliorates insulin sensitivity, without affecting other metabolic syndrome markers. Food Funct. 9, 6010–6019. doi: 10.1039/C8FO01323C
Martínez-Meza, Y., Pérez-Jiménez, J., Salgado-Rodríguez, L. M., Castellanos-Jiménez, A. K., and Reynoso-Camacho, R. (2022). In Vivo Evaluation of the Cardiometabolic Potential of Grape Pomace: Effect of Applying Instant Controlled Pressure Drop. Foods 11:3537. doi: 10.3390/foods11213537
Martins, I. M., Macedo, G. A., Macedo, J. A., Roberto, B. S., Chen, Q., Blumberg, J. B., et al. (2017). Tannase enhances the anti-inflammatory effect of grape pomace in Caco-2 cells treated with IL-1β. J. Funct. Foods. 29, 69–76. doi: 10.1016/j.jff.2016.12.011
Maryati, T., Nugroho, T., Bachruddin, Z., and Pertiwiningrum, A. (2021). Antibacterial effects of Kepok Banana bunch (Musa paradisiaca L.) against Staphylococcus aureus. IOP Conf. Ser.: Earth Environ. Sci. 637:012046. doi: 10.1088/1755-1315/637/1/012046
Matsuo, Y., Miura, L. A., Araki, T., and Yoshie-Stark, Y. (2019). Proximate composition and profiles of free amino acids, fatty acids, minerals and aroma compounds in Citrus natsudaidai peel. Food Chem. 279, 356–363. doi: 10.1016/j.foodchem.2018.11.146
Maulydia, N. B., Tallei, T. E., Ginting, B., Idroes, R., and Faradilla, M. (2022). Analysis of flavonoid compounds of Orange (Citrus sp.) peel as anti-main protease of SARS-CoV-2: A molecular docking study. IOP Conf. Ser. Earth Environ. Sci. 951:012078. doi: 10.1088/1755-1315/951/1/012078
Maza, M., Álvarez, I., and Raso, J. (2019). Thermal and non-thermal physical methods for improving polyphenol extraction in red winemaking. Beverages 5:47. doi: 10.3390/beverages5030047
Mechchate, H., Es-Safi, I., Haddad, H., Bekkari, H., Grafov, A., and Bousta, D. (2021). Combination of Catechin, Epicatechin, and Rutin: Optimization of a novel complete antidiabetic formulation using a mixture design approach. J. Nutr. Biochem. 88:108520. doi: 10.1016/j.jnutbio.2020.108520
Meryem, S., Mohamed, D., Nour-eddine, C., and Faouzi, E. (2023). Chemical composition, antibacterial and antioxidant properties of three Moroccan citrus peel essential oils. Sci. Afr. 20:e01592. doi: 10.1016/j.sciaf.2023.e01592
Mirabella, N., Castellani, V., and Sala, S. (2014). Current options for the valorization of food manufacturing waste: A review. J Clean Prod 65, 28–41.
Mistry, J., Biswas, M., Sarkar, S., and Ghosh, S. (2023). Antidiabetic activity of mango peel extract and mangiferin in alloxan-induced diabetic rats. Future J. Pharm. Sci. 9, 1–13. doi: 10.1186/s43094-023-00472-6
Mo, Y., Ma, J., Gao, W., Zhang, L., Li, J., Li, J., et al. (2022). Pomegranate peel as a source of bioactive compounds: A mini review on their physiological functions. Front. Nutr. 9:887113. doi: 10.3389/fnut.2022.887113
Modak, D., and Bhattacharjee, S. (2022). A review of fibroblast-like synoviocytes in the pathogenesis of Rheumatoid arthritis: Their activation and the inhibition of their apoptosis. Biomed. Res. Ther. 9, 5394–5409. doi: 10.15419/bmrat.v9i11.779
Modak, D., Paul, S., Sarkar, S., Thakur, S., and Bhattacharjee, S. (2021). Validating potent anti-inflammatory and anti-rheumatoid properties of Drynaria quercifolia rhizome methanolic extract through in vitro, in vivo, in silico and GC-MS-based profiling. BMC Complement Altern. Med. 21:89. doi: 10.1186/s12906-021-03265-7
Moradi, M. T., Karimi, A., Shahrani, M., Hashemi, L., and Ghaffari-Goosheh, M. S. (2019). Anti-influenza virus activity and phenolic content of pomegranate (Punica granatum L.) peel extract and fractions. Avicenna J. Med. Biotechnol. 11, 285.
Natolino, A., and Da Porto, C. (2019). Supercritical carbon dioxide extraction of pomegranate (Punica granatum L.) seed oil: Kinetic modelling and solubility evaluation. J. Supercrit. Fluids. 151, 30–39. doi: 10.1016/j.supflu.2019.05.002
Nguyen-Ngo, C., Salomon, C., Quak, S., Lai, A., Willcox, J. C., and Lappas, M. (2020). Nobiletin exerts anti-diabetic and anti-inflammatory effects in an in vitro human model and in vivo murine model of gestational diabetes. Clin. Sci. 134, 571–592. doi: 10.1042/CS20191099
Oh, C., Chowdhury, R., Samineni, L., Shisler, J. L., Kumar, M., and Nguyen, T. H. (2022). Inactivation mechanism and efficacy of grape seed extract for Human Norovirus surrogate. Appl. Environ. Microbiol. 88, e2247–e2221. doi: 10.1128/aem.02247-21
Oikeh, E. I., Oviasogie, F. E., and Omoregie, E. S. (2020). Quantitative phytochemical analysis and antimicrobial activities of fresh and dry ethanol extracts of Citrus sinensis (L.) Osbeck (sweet Orange) peels. Clin. Phytoscience. 6, 1–6. doi: 10.1186/s40816-020-00193-w
Om, P., Gopinath, M. S., Madan Kumar, P., Muthu Kumar, S. P., and Kudachikar, V. B. (2022). Ethanolic extract of Pyrus pashia buch ham ex. D. Don (Kainth): A bioaccessible source of polyphenols with anti-inflammatory activity in vitro and in vivo. J. Ethnopharmacol. 282:114628. doi: 10.1016/j.jep.2021.114628
Padilla-Camberos, E., Sanchez-Hernandez, I. M., Torres-Gonzalez, O. R., del Rosario Gallegos-Ortiz, M., Méndez-Mona, A. L., Baez-Moratilla, P., et al. (2022). Natural essential oil mix of sweet orange peel, cumin, and allspice elicits anti-inflammatory activity and pharmacological safety similar to non-steroidal anti-inflammatory drugs. Saudi J. Biol. Sci. 29, 3830–3837. doi: 10.1016/j.sjbs.2022.03.002
Panda, S. K., Castro, A. H. F., Jouneghani, R. S., Leyssen, P., Neyts, J., Swennen, R., et al. (2020). Antiviral and cytotoxic activity of different plant parts of banana (Musa spp.). Viruses 12:549. doi: 10.3390/v12050549
Parisi, V., Vassallo, A., Pisano, C., Signorino, G., Cardile, F., Sorrentino, M., et al. (2020). A herbal mixture from propolis, pomegranate, and grape pomace endowed with anti-inflammatory activity in an in vivo rheumatoid arthritis model. Molecules 25:2255. doi: 10.3390/molecules25092255
Pascual, G., López, M. D., Vargas, M., Aranda, M., and Cañumir, J. A. (2022). Next generation ingredients based on winemaking by-products and an approaching to antiviral properties. Foods 11:1604. doi: 10.3390/foods11111604
Pasqua, G., Simonetti, G., and Rodriguez, J. M. L. (2016). Antimicrobial and antiviral activities of grape seed extracts. Grape seeds: nutrient content, antioxidant properties and health benefits. New York, NY: Nova Science Publishers, 211–224.
Paul, S., Das, S., Tanvir, E. M., Hossen, M. S., Saha, M., Afroz, R., et al. (2017). Protective effects of ethanolic peel and pulp extracts of Citrus macroptera fruit against isoproterenol-induced myocardial infarction in rats. Biomed. Pharmacother. 94, 256–264. doi: 10.1016/j.biopha.2017.07.080
Paulraj, C. R. K. J., Bernard, M. A., Raju, J., and Abdulmajid, M. (2019). Sustainable waste management through waste to energy technologies in India-opportunities and environmental impacts. Int. J. Renew. Energy Re.s 9, 309–342. doi: 10.20508/ijrer.v9i1.9017.g7586
Peixoto, C. M., Dias, M. I., Alves, M. J., Calhelha, R. C., Barros, L., Pinho, S. P., et al. (2018). Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 253, 132–138. doi: 10.1016/j.foodchem.2018.01.163
Pérez-Jiménez, J., and Saura-Calixto, F. (2008). Grape products and cardiovascular disease risk factors. Nutr. Res. Rev. 21, 158–173. doi: 10.1017/S0954422408125124
Petrie, J. R., Guzik, T. J., and Touyz, R. M. (2018). Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J Cardiol. 34, 575–584. doi: 10.1016/j.cjca.2017.12.005
Pfukwa, T. M., Fawole, O. A., Manley, M., Gouws, P. A., Opara, U. L., and Mapiye, C. (2019). Food preservative capabilities of grape (Vitis vinifera) and clementine mandarin (Citrus reticulata) by-products extracts in South Africa. Sustainability 11:1746. doi: 10.3390/su11061746
Pistol, G. C., Marin, D. E., Dragomir, C., and Taranu, I. (2019). Synbiotic combination of prebiotic grape pomace extract and probiotic Lactobacillus sp. reduced important intestinal inflammatory markers and in-depth signalling mediators in lipopolysaccharide-treated Caco-2 cells. Br. J. Nutr. 121, 291–305. doi: 10.1017/S0007114518003410
Pollini, L., Cossignani, L., Juan, C., and Mañes, J. (2021). Extraction of phenolic compounds from fresh apple pomace by different non-conventional techniques. Molecules 26:4272. doi: 10.3390/molecules26144272
Pop, R. M., Popolo, A., Trifa, A. P., and Stanciu, L. A. (2018). Phytochemicals in cardiovascular and respiratory diseases: Evidence in oxidative stress and inflammation. Oxid. Med. Cell. 2018:1603872. doi: 10.1155/2018/1603872
Radenkovs, V., Kviesis, J., Juhnevica-Radenkova, K., Valdovska, A., Püssa, T., Klavins, M., et al. (2018). Valorization of wild apple (Malus spp.) by-products as a source of essential fatty acids, tocopherols and phytosterols with antimicrobial activity. Plants 7:90. doi: 10.3390/plants7040090
Rana, S., Gupta, S., Rana, A., and Bhushan, S. (2015). Functional properties, phenolic constituents and antioxidant potential of industrial apple pomace for utilization as active food ingredient. Food Sci. Hum. Wellness 4, 180–187. doi: 10.1016/j.fshw.2015.10.001
Ravn-Haren, G., Dragsted, L. O., Buch-Andersen, T., Jensen, E. N., Jensen, R. I., Németh-Balogh, M., et al. (2013). Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur. J. Nutr. 52, 1875–1889. doi: 10.1007/s00394-012-0489-z
Rivera, K., Salas-Pérez, F., Echeverría, G., Urquiaga, I., Dicenta, S., Pérez, D., et al. (2019). Red wine grape pomace attenuates atherosclerosis and myocardial damage and increases survival in association with improved plasma antioxidant activity in a murine model of lethal ischemic heart disease. Nutrients 11:2135. doi: 10.3390/nu11092135
Rong, X., Xu, J., Jiang, Y., Li, F., Chen, Y., Dou, Q. P., et al. (2021). Citrus peel flavonoid nobiletin alleviates lipopolysaccharide-induced inflammation by activating IL-6/STAT3/FOXO3a-mediated autophagy. Food Funct. 12, 1305–1317. doi: 10.1039/D0FO02141E
Rudra, S., Nishad, J., Jakhar, N., and Kaur, C. (2015). Food industry waste: mine of nutraceuticals. Int. J. Sci. Environ. Technol. 4, 205–229.
Ruiz-Montañez, G., Ragazzo-Sánchez, J. A., Calderón-Santoyo, M., Velazquez-De La Cruz, G., De León, J. R., and Navarro-Ocaña, A. (2014). Evaluation of extraction methods for preparative scale obtention of mangiferin and lupeol from mango peels (Mangifera indica L.). Food Chem. 159, 267–272. doi: 10.1016/j.foodchem.2014.03.009
Sabra, A., Netticadan, T., and Wijekoon, C. (2021). Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem. 12:100149. doi: 10.1016/j.fochx.2021.100149
Sah, B., Vasiljevic, T., McKechnie, S., and Donkor, O. (2016). Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT Food Sci. Technol. 65, 978–986. doi: 10.1016/j.lwt.2015.09.027
Salama, A. A., Ismael, N. M., and Bedewy, M. (2021). The anti-inflammatory and antiatherogenic in vivo effects of pomegranate peel powder: from waste to medicinal food. J. Med. Food. 24, 145–150. doi: 10.1089/jmf.2019.0269
Saleem, M., Durani, A. I., Asari, A., Ahmed, M., Ahmad, M., Yousaf, N., et al. (2023). Investigation of antioxidant and antibacterial effects of citrus fruits peels extracts using different extracting agents: Phytochemical analysis with in silico studies. Heliyon 9, e15433. doi: 10.1016/j.heliyon.2023.e15433
Saleem, M., and Saeed, M. T. (2020). Potential application of waste fruit peels (orange, yellow lemon and banana) as wide range natural antimicrobial agent. J. King Saud Univ. Sci. 32, 805–810. doi: 10.1016/j.jksus.2019.02.013
Samota, M. K., Kaur, M., Sharma, M., Krishnan, V., Thakur, J., Rawat, M., et al. (2023). Hesperidin from citrus peel waste: extraction and its health implications. Qual. Assur. Saf. Crops Foods 15, 71–99. doi: 10.15586/qas.v15i2.1256
Santarelli, V., Neri, L., Sacchetti, G., Di Mattia, C. D., Mastrocola, D., and Pittia, P. (2020). Response of organic and conventional apples to freezing and freezing pre-treatments: Focus on polyphenols content and antioxidant activity. Food Chem. 308:125570. doi: 10.1016/j.foodchem.2019.125570
Santos, L. F. D., Lopes, S. T., Nazari, M. T., Biduski, B., Pinto, V. Z., Santos, J. S. D., et al. (2022). Fruit pomace as a promising source to obtain biocompounds with antibacterial activity. Crit. Rev. Food Sci. Nutr. doi: 10.1080/10408398.2022.2103510 [Epub ahead of print].
Sha, S. P., Suryavanshi, M. V., Jani, K., Sharma, A., Shouche, Y. S., and Tamang, J. P. (2018). Diversity of yeasts and molds by culture-dependent and culture-independent methods for mycobiome surveillance of traditionally prepared dried starters for the production of Indian alcoholic beverages. Front. Microbiol. 9:2237. doi: 10.3389/fmicb.2018.02237
Sha, S. P., Suryavanshi, M. V., and Tamang, J. P. (2019). Mycobiome diversity in traditionally prepared starters for alcoholic beverages in India by high-throughput sequencing method. Front. Microbiol. 10:348. doi: 10.3389/fmicb.2019.00348
Shehata, M. G., Awad, T. S., Asker, D., El Sohaimy, S. A., Abd El-Aziz, N. M., and Youssef, M. M. (2021). Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Nutr. Food Sci. 4, 326–335. doi: 10.1016/j.crfs.2021.05.001
Shen, X., Zhou, N., Mi, L., Hu, Z., Wang, L., Liu, X., et al. (2017). Phloretin exerts hypoglycemic effect in streptozotocin-induced diabetic rats and improves insulin resistance in vitro. Drug Des. Dev. Ther. 11, 313–324. doi: 10.2147/DDDT.S127010
Shu, B., Wu, G., Wang, Z., Wang, J., Huang, F., Dong, L., et al. (2020). The effect of microwave vacuum drying process on citrus: drying kinetics, physicochemical composition and antioxidant activity of dried citrus (Citrus reticulata Blanco) peel. J. Food Meas. Charact. 14, 2443–2452. doi: 10.1007/s11694-020-00492-3
Singh, R., Saati, A. A., Faidah, H., Bantun, F., Jalal, N. A., Haque, S., et al. (2023). Prospects of microbial cellulase production using banana peels wastes for antimicrobial applications. Int. J. Food Microbiol. 388:110069. doi: 10.1016/j.ijfoodmicro.2022.110069
Smith, I., Yu, J., Hurley, S. L., and Hanner, T. (2017). Impact of diet containing grape pomace on growth performance and blood lipid profile of young rats. J. Med. Food. 20, 550–556. doi: 10.1089/jmf.2016.0117
Stocker, R., and Keaney, J. F. Jr. (2004). Role of oxidative modifications in atherosclerosis. Physiol. Rev. 84, 1381–1478. doi: 10.1152/physrev.00047.2003
Suárez, B., Álvarez, ÁL., García, Y. D., del Barrio, G., Lobo, A. P., and Parra, F. (2010). Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 120, 339–342. doi: 10.1016/j.foodchem.2009.09.073
Sudhahar, V., Kumar, S. A., Sudharsan, P. T., and Varalakshmi, P. (2007). Protective effect of lupeol and its ester on cardiac abnormalities in experimental hypercholesterolemia. Vascul. Pharmacol. 46, 412–418. doi: 10.1016/j.vph.2006.12.005
Suri, S., Singh, A., and Nema, P. K. (2022). Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2:100050. doi: 10.1016/j.afres.2022.100050
Suručić, R., Travar, M., Petković, M., Tubić, B., Stojiljković, M. P., Grabež, M., et al. (2021). Pomegranate peel extract polyphenols attenuate the SARS-CoV-2 S-glycoprotein binding ability to ACE2 Receptor: In silico and in vitro studies. Bioorg. Chem. 114:105145. doi: 10.1016/j.bioorg.2021.105145
Szabo, K., Mitrea, L., Călinoiu, L. F., Teleky, B. E., Martău, G. A., Plamada, D., et al. (2022). Natural Polyphenol Recovery from Apple-, Cereal-, and Tomato-Processing By-Products and Related Health-Promoting Properties. Molecules 27:7977. doi: 10.3390/molecules27227977
Taladrid, D., de Celis, M., Belda, I., Bartolomé, B., and Moreno-Arribas, M. V. (2022). Hypertension-and glycaemia-lowering effects of a grape-pomace-derived seasoning in high-cardiovascular risk and healthy subjects. Interplay with the gut microbiome. Food Funct. 13, 2068–2082. doi: 10.1039/D1FO03942C
Thammarutwasik, P., Hongpattarakere, T., Chantachum, S., Kijroongrojana, K., Itharat, A., Reanmongkol, W., et al. (2009). Prebiotics – a review. Songklanakarin J Sci Technol. 31, 401–408.
Theodotou, M., Fokianos, K., Mouzouridou, A., Konstantinou, C., Aristotelous, A., Prodromou, D., et al. (2017). The effect of resveratrol on hypertension: A clinical trial. Exp. Ther. Med. 13, 295–301. doi: 10.3892/etm.2016.3958
Tito, A., Colantuono, A., Pirone, L., Pedone, E., Intartaglia, D., Giamundo, G., et al. (2021). Pomegranate peel extract as an inhibitor of SARS-CoV-2 spike binding to human ACE2 receptor (in vitro): a promising source of novel antiviral drugs. Front. Chem. 9:638187. doi: 10.3389/fchem.2021.638187
Vasiljevic, T., Kealy, T., and Mishra, V. (2007). Effects of β-glucan addition to a probiotic containing yogurt. J. Food Sci. 72, C405–C411. doi: 10.1111/j.1750-3841.2007.00454.x
Velmurugan, C., and Bhargava, A. (2013). Anti-diabetic and hypolipidemic activity of fruits of Pyrus communis L. in hyperglycemic rats. Asian J. Pharm. Clin. Res. 6, 108–111.
Vilas-Boas, A. A., Pintado, M., and Oliveira, A. L. (2021). Natural bioactive compounds from food waste: Toxicity and safety concerns. Foods 10:1564. doi: 10.3390/foods10071564
Vu, H. T., Scarlett, C. J., and Vuong, Q. V. (2017). Optimization of ultrasound-assisted extraction conditions for recovery of phenolic compounds and antioxidant capacity from banana (Musa cavendish) peel. J. Food Process. Preserv. 41, e13148. doi: 10.1111/jfpp.13148
Waldbauer, K., Seiringer, G., Nguyen, D. L., Winkler, J., Blaschke, M., McKinnon, R., et al. (2016). Triterpenoic Acids from Apple Pomace Enhance the Activity of the Endothelial Nitric Oxide Synthase (eNOS). J. Agric. Food Chem. 64, 185–194. doi: 10.1021/acs.jafc.5b05061
Wang, D., Özen, C., Abu-Reidah, I. M., Chigurupati, S., Patra, J. K., Horbanczuk, J. O., et al. (2018). Vasculoprotective effects of pomegranate (Punica granatum L.). Front. Pharm. 9:544. doi: 10.3389/fphar.2018.00544
Wang, M., Yang, F., Yan, X., Chao, X., Zhang, W., Yuan, C., et al. (2022). Anti-diabetic effect of banana peel dietary fibers on type 2 diabetic mellitus mice induced by streptozotocin and high-sugar and high-fat diet. J. Food Biochem. 46, e14275. doi: 10.1111/jfbc.14275
WHO (2016). World Health Organization; Geneva: Global Health Estimates 2015: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2015. Geneva: WHO.
Xu, C., Yagiz, Y., Hsu, W. Y., Simonne, A., Lu, J., and Marshall, M. R. (2014). Antioxidant, antibacterial, and antibiofilm properties of polyphenols from muscadine grape (Vitis rotundifolia Michx.) pomace against selected foodborne pathogens. J. Agric. Food Chem. 62, 6640–6649. doi: 10.1021/jf501073q
Xu, Y., Burton, S., Kim, C., and Sismour, E. (2016). Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four Virginia-grown grape varieties. Food Sci. Nutr. 4, 125–133. doi: 10.1002/fsn3.264
Yahfoufi, N., Alsadi, N., Jambi, M., and Matar, C. (2018). The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 10:1618. doi: 10.3390/nu10111618
Yasmin, S., Cerchia, C., Badavath, V. N., Laghezza, A., Dal Piaz, F., Mondal, S. K., et al. (2021). A series of ferulic acid amides reveals unexpected peroxiredoxin 1 inhibitory activity with in vivo antidiabetic and hypolipidemic effects. Chem. Med. Chem. 16, 484–498. doi: 10.1002/cmdc.202000564
You, M., Wang, Z., Kim, H. J., Lee, Y. H., and Kim, H. A. (2022). Pear pomace alleviated atopic dermatitis in NC/Nga mice and inhibited LPS-induced inflammation in RAW 264.7 macrophages. Nutr. Res. Pract. 16, 577–588. doi: 10.4162/nrp.2022.16.5.577
Zahin, M., Aqil, F., and Ahmad, I. (2010). Broad spectrum antimutagenic activity of antioxidant active fraction of Punica granatum L. peel extracts. Mutat. Res. 703, 99–107. doi: 10.1016/j.mrgentox.2010.08.001
Zaini, H. M., Roslan, J., Saallah, S., Munsu, E., Sulaiman, N. S., and Pindi, W. (2022). Banana peels as a bioactive ingredient and its potential application in the food industry. J. Funct. Foods. 92:105054. doi: 10.1016/j.jff.2022.105054
Zardo, D. M., Alberti, A., Zielinski, A. A. F., Prestes, A. A., Esmerino, L. A., and Nogueira, A. (2021). Influence of solvents in the extraction of phenolic compounds with antibacterial activity from apple pomace. Sep. Sci. Technol. 56, 903–911. doi: 10.1080/01496395.2020.1744652
Zhang, B., Zeng, M., Li, B., Wang, Y., Kan, Y., Wang, S., et al. (2019). Inhibition of oxidative stress and autophagy by arbutin in lipopolysaccharide-induced myocardial injury. Pharmacogn. Mag. 15, 507–513. doi: 10.4103/pm.pm_552_18
Zhang, M., Wu, Q., Chen, Y., Duan, M., Tian, G., Deng, X., et al. (2018). Inhibition of proanthocyanidin A2 on porcine reproductive and respiratory syndrome virus replication in vitro. PLoS One 13:e0193309. doi: 10.1371/journal.pone.0193309
Zhang, T., Wei, X., Miao, Z., Hassan, H., Song, Y., and Fan, M. (2016). Screening for antioxidant and antibacterial activities of phenolics from Golden Delicious apple pomace. Chem. Cent. J. 10, 1–9. doi: 10.1186/s13065-016-0195-7
Zhao, C. N., Meng, X., Li, Y., Li, S., Liu, Q., Tang, G. Y., et al. (2017). Fruits for prevention and treatment of cardiovascular diseases. Nutrients 9:598. doi: 10.3390/nu9060598
Keywords: fruit wastes, bioactive compounds, nutraceuticals, diseases, secondary metabolites, pharmacological potential
Citation: Sha SP, Modak D, Sarkar S, Roy SK, Sah SP, Ghatani K and Bhattacharjee S (2023) Fruit waste: a current perspective for the sustainable production of pharmacological, nutraceutical, and bioactive resources. Front. Microbiol. 14:1260071. doi: 10.3389/fmicb.2023.1260071
Received: 17 July 2023; Accepted: 09 October 2023;
Published: 24 October 2023.
Edited by:
Amit Kumar Rai, Institute of Bioresources and Sustainable Development, Tadong, Gangtok, IndiaReviewed by:
Vinod Kumar, Indian Institute of Integrative Medicine (CSIR), IndiaAntonio Ferrante, University of Milan, Italy
Copyright © 2023 Sha, Modak, Sarkar, Roy, Sah, Ghatani and Bhattacharjee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shankar Prasad Sha, c2hhbmthcnByYXNhZHNoYUBnbWFpbC5jb20=; Soumen Bhattacharjee, c291bWVuYkBuYnUuYWMuaW4=
†These authors share first authorship
 Shankar Prasad Sha
Shankar Prasad Sha Debabrata Modak
Debabrata Modak Sourav Sarkar
Sourav Sarkar Sudipta Kumar Roy
Sudipta Kumar Roy Sumit Prasad Sah
Sumit Prasad Sah Kriti Ghatani
Kriti Ghatani Soumen Bhattacharjee
Soumen Bhattacharjee