- 1Department of Clinical Laboratory, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China
- 2Department of Clinical Laboratory, Fujian Medical University Affiliated First Quanzhou hospital, Quanzhou, Fujian, China
- 3Department of Infection Disease, Clinical Medical Research Center for Bacterial and Fungal Infectious Diseases of Fujian province, Fujian Medical University Affiliated First Quanzhou Hospital, Quanzhou, Fujian, China
- 4Key Laboratory of Screening and Control of Infectious Diseases (Quanzhou Medical College), Fujian Provincial University, Quanzhou, Fujian, China
Purpose: To investigate the distribution characteristics and drug resistance of pathogenic bacteria in bloodstream infections, providing a basis for rational clinical treatment.
Patients and methods: Retrospective analysis of 1,282 pathogenic strains isolated from blood cultures in the intensive care unit (ICU) of the Second Affiliated Hospital of Xi’an Jiaotong University from January 1, 2019, to December 31, 2022.
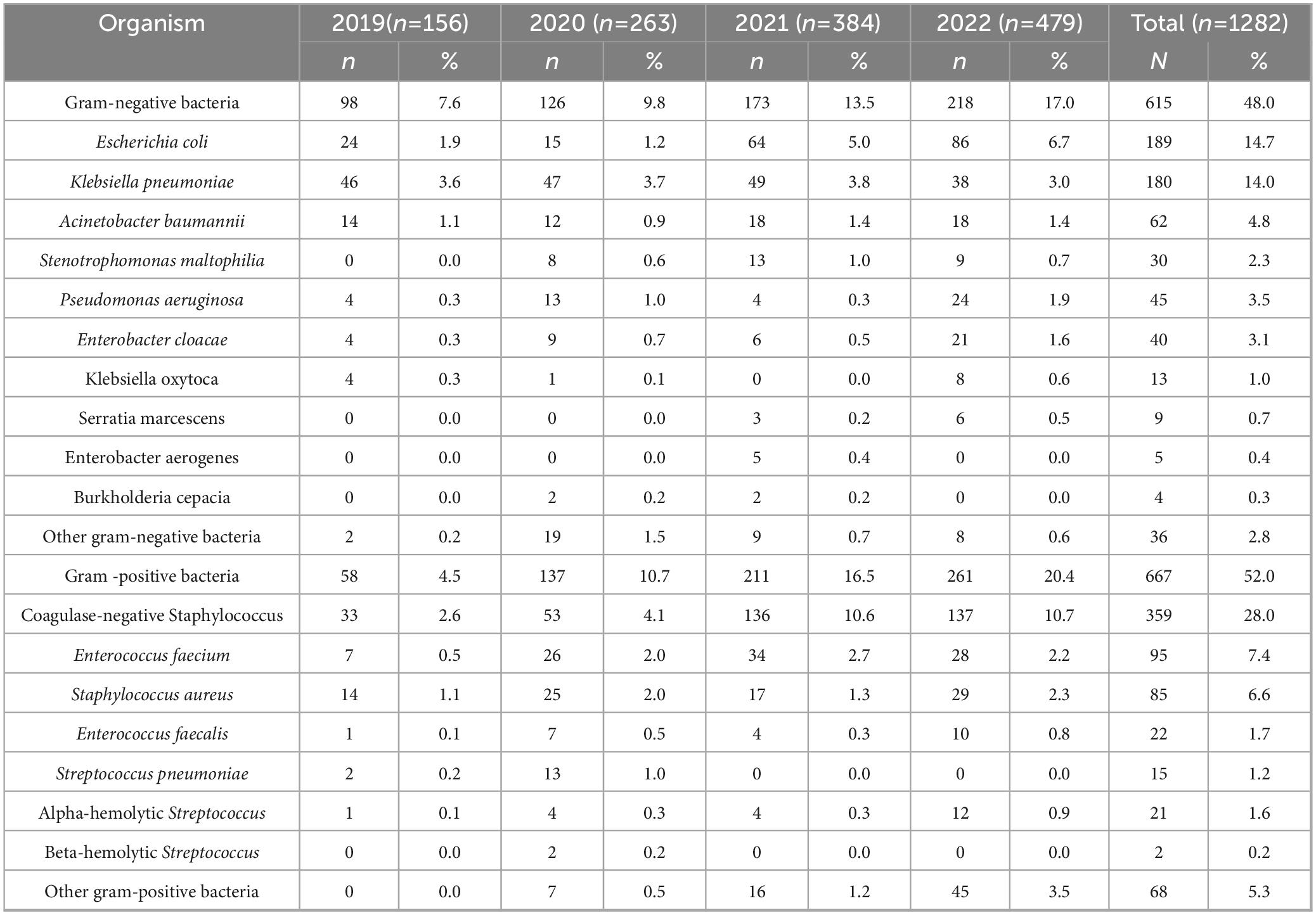
Results: Gram-positive bacteria (52.0%) slightly predominated over gram-negative bacteria (48.0%). The top three gram-positive bacteria were Coagulase-negative Staphylococcus (28.0%), Enterococcus faecium (7.4%), and Staphylococcus aureus (6.6%). Staphylococci exhibited a high resistance rate to penicillin, oxacillin, and erythromycin; no strains resistant to vancomycin or linezolid were found. Among the Enterococci, Enterococcus faecium had a high resistance rate to penicillin, ampicillin, and erythromycin. Two strains of Enterococcus faecalis were resistant to linezolid, but none to vancomycin. The top three gram-negative bacteria were Escherichia coli (14.7%), Klebsiella pneumoniae (14.0%), and Acinetobacter baumannii (4.8%). The resistance rate of Escherichia coli to carbapenems increased from 0.0 to 2.3%. Acinetobacter baumannii reached 100% carbapenem resistance (up from 75.0%), while Klebsiella pneumoniae demonstrated 21.1-80.4% resistance to various carbapenems.
Conclusion: The isolation rate of gram-positive bacteria in patients with bloodstream infection in the ICU of the Second Affiliated Hospital of Xi’an Jiaotong University was slightly higher than that of gram-negative bacteria. The alarming carbapenem resistance among gram-negative pathogens and emerging linezolid resistance in Enterococci demand urgent clinical interventions, including enhanced surveillance, antimicrobial stewardship, and novel therapeutic strategies.
1 Introduction
Bloodstream infection (BSI) is a severe systemic infectious disease characterized by the invasion of pathogenic microorganisms into the body. These microorganisms circulate in the bloodstream, where they undergo transient, intermittent, or continuous reproduction, releasing toxins and metabolic products that trigger the release of cytokines, ultimately resulting in damage to organs. In severe cases, BSI can lead to shock, multiple organ failure, disseminated intravascular coagulation, and death (Tajima et al., 2021; Fabre et al., 2022). Globally, BSIs account for an estimated 20-30% of sepsis cases and are associated with mortality rates exceeding 40% in critically ill populations, particularly in intensive care units (ICUs) (Fleischmann-Struzek et al., 2020). The rising prevalence of antimicrobial resistance (AMR) has further complicated BSI management, with the World Health Organization declaring AMR one of the top 10 global public health threats, projected to cause 10 million annual deaths by 2050 if unchecked (World Health Organization [WHO], 2021).
The diagnoses of BSIs, infective endocarditis, unexplained infections, catheter-related BSIs, arthritis, and bacterial pneumonia rely on blood culture (Gonzalez et al., 2020) to identify the causative pathogens and provide antibiotic susceptibility profiles. These data are critical for guiding evidence-based antibiotic therapy, especially as multidrug-resistant (MDR) pathogens reduce treatment efficacy and increase healthcare costs (Cheng et al., 2020; Mazi et al., 2021). Recent data reveal alarming global shifts: while gram-negative bacteria historically dominated BSI etiology, gram-positive pathogens such as Staphylococcus aureus, coagulase-negative staphylococci, and enterococci now prevail in many regions (Lan et al., 2021). Concurrently, resistance mechanisms like methicillin resistance in S. aureus (MRSA), vancomycin resistance in enterococci (VRE), and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae have escalated, driven by antibiotic overuse in clinical and agricultural settings (Antimicrobial Resistance Collaborators, 2022). For instance, MRSA accounts for > 35% of S. aureus BSIs in high-income countries, while carbapenem-resistant Klebsiella pneumoniae infections in ICUs exceed 60% in some endemic regions (Centers for Disease Control and Prevention [CDC], 2023; European Centre for Disease Prevention and Control [ECDC], 2022).
These trends underscore the urgency of region-specific pathogen surveillance and resistance profiling. This study retrospectively analyzed the distribution of pathogens and their antibiotic resistance in blood culture specimens collected from an intensive care unit (ICU) between 2019 and 2022. By correlating our findings with global antimicrobial resistance dynamics, we aim to establish a scientific foundation for optimizing empirical antibiotic therapy, informing stewardship programs, and mitigating resistance escalation in critical care settings.
2 Materials and methods
2.1 Materials
2.1.1 Strains
Between January 1, 2019, and December 31, 2022, a total of 1,282 bacterial strains were collected from the positive blood cultures of patients in the ICU at the Second Affiliated Hospital of Xi’an Jiaotong University, with duplicate strains from the same patient excluded from the analyses. This study has been approved by the academic committee of the Stem Cell Clinical Research Institute of the Second Affiliated Hospital of Xi’an Jiaotong University and the ethics committee of the same institution (2023414). Informed consent was obtained from all study participants, and the guidelines outlined in the declaration of Helsinki were adhered to.
2.1.2 Culture media and antibiotic discs
Mueller-Hinton (MH) agar (Zhengzhou AutoBio Co., Ltd., Zhengzhou, China) was used for disc-diffusion susceptibility testing, and 5% defibrinated sheep blood MH agar was used for streptococci. Culture media were sourced from AutoBio (Co., Ltd., Zhengzhou, China), and the antibiotic discs were acquired from Oxoid, Basingstoke, UK). E-test strips were obtained from Wenzhou Kangtai Biotechnology (Co., Ltd., Wenzhou, China).
2.2 Methods
2.2.1 Bacterial identification and antimicrobial susceptibility test
The fully automated bacterial culture system BacT/ALERT 3D (Marcy l’Etoile, bioMérieux, France) was used to detect blood culture specimens. Bacterial identification and antimicrobial susceptibility tests were conducted using the VITEK 2-Compact bacterial identification system (Marcy l’Etoile, bioMérieux, France), while less common bacteria were identified using the VITEK MS system (Marcy l’Etoile, bioMérieux, France). The interpretation of antimicrobial susceptibility results followed the 2022 performance standards proposed by the Clinical and Laboratory Standards Institute (Clinical and Laboratory Standards Institute [CLSI], 2022). To exclude duplicate strains from the same patient, only the first positive blood culture with a specific pathogen per patient was included in the analysis. Subsequent isolates of the same species from the same patient within 30 days were excluded unless they exhibited distinct antimicrobial susceptibility profiles or were isolated from different anatomical sites, as recommended by international guidelines for bloodstream infection surveillance (Magiorakos et al., 2017).
2.2.2 Phenotype testing for important drug resistance
Carbapenem-resistant Enterobacterales were defined as Enterobacterale specimens resistant to any of the carbapenem antibiotics, specifically imipenem, meropenem, or ertapenem.
2.2.3 Quality control strains
The quality control strains used in this study included Escherichia coli (ATCC 25922 and ATCC 8739), Klebsiella pneumoniae (ATCC 700603), Staphylococcus aureus (ATCC 25923 and ATCC 29213), Pseudomonas aeruginosa (ATCC 27853), Enterococcus faecalis (ATCC 29212), Streptococcus pneumoniae (ATCC 49619), and Haemophilus influenzae (ATCC 49247). These strains were selected based on CLSI recommendations for antimicrobial susceptibility testing (Clinical and Laboratory Standards Institute [CLSI], 2022) and represent common pathogens associated with bloodstream infections. All strains were procured from the American Type Culture Collection (ATCC) to ensure traceability and standardized phenotypic characteristics. Quality control testing was performed weekly alongside clinical isolates, with acceptable ranges defined by CLSI criteria. No deviations from standard protocols were observed during the study period, as confirmed by internal audit records.
2.3 Statistical analysis
The laboratory data analysis and statistical analyses were conducted using WHONET 5.6 software (China).1 SPSS 24.0 software was utilized for statistical analysis. The chi-square test was employed to examine differences in categorical data.
3 Results
3.1 Bacterial distribution
Between January 2019 and December 2022, a total of 1,282 distinct pathogenic strains were isolated from the blood cultures of patients in the ICU (Table 1). Among these, 667 (52.0%) were gram-positive bacteria, while 615 (48.0%) were gram-negative bacteria. The five most prevalent bacterial species were coagulase-negative Staphylococcus (359 strains; 28.0%), Escherichia coli (189 strains; 14.7%), Klebsiella pneumoniae (180 strains; 14.0%), Enterococcus faecium (95 strains; 7.4%), and Staphylococcus aureus (85 strains;6.6%). Other gram-negative bacteria included Raoultella planticola, Citrobacter freundii, Brucella, Haemophilus influenzae, Morganella morganii, and Citrobacter diversus. Other streptococci mainly encompassed Streptococcus viridans, Streptococcus mitis, and Streptococcus agalactiae, while other gram-positive cocci included Enterococcus gallinarum, Streptococcus bovis, Abiotrophia defectiva, and Gemella species.
3.2 Antibiotic resistances of major gram-positive bacteria
3.2.1 Staphylococcus genus
A total of 443 strains of Staphylococcus genus were isolated, comprising 34.6% of all isolated pathogens. Among them, 85 strains were Staphylococcus aureus, and 358 strains were coagulase-negative staphylococci. The detection rates of methicillin-resistant Staphylococcus aureus (MRSA) in 2019, 2020, 2021, and 2022 were 2.3% (10 strains), 1.8% (8 strains), 0.0% (0 strains), and 2.7% (12 strains), respectively (Table 2). With the exception of 2021, the detection rates remained stable. MRSA exhibited a consistent decline in resistance to gentamicin, levofloxacin, moxifloxacin, trimethoprim-sulfamethoxazole, and erythromycin from 2019 to 2022. The resistance rate to gentamicin dropped from 75.0 to 0.0%, while the resistance rate to erythromycin decreased from 100.0 to 41.7%. Methicillin-sensitive Staphylococcus aureus displayed a constant resistance rate to penicillin G from 2019-2022 (100.0%), while the resistance rates to levofloxacin, moxifloxacin, and erythromycin decreased from 2019-2022. Staphylococcus aureus was not resistant to vancomycin, linezolid, or rifampicin.
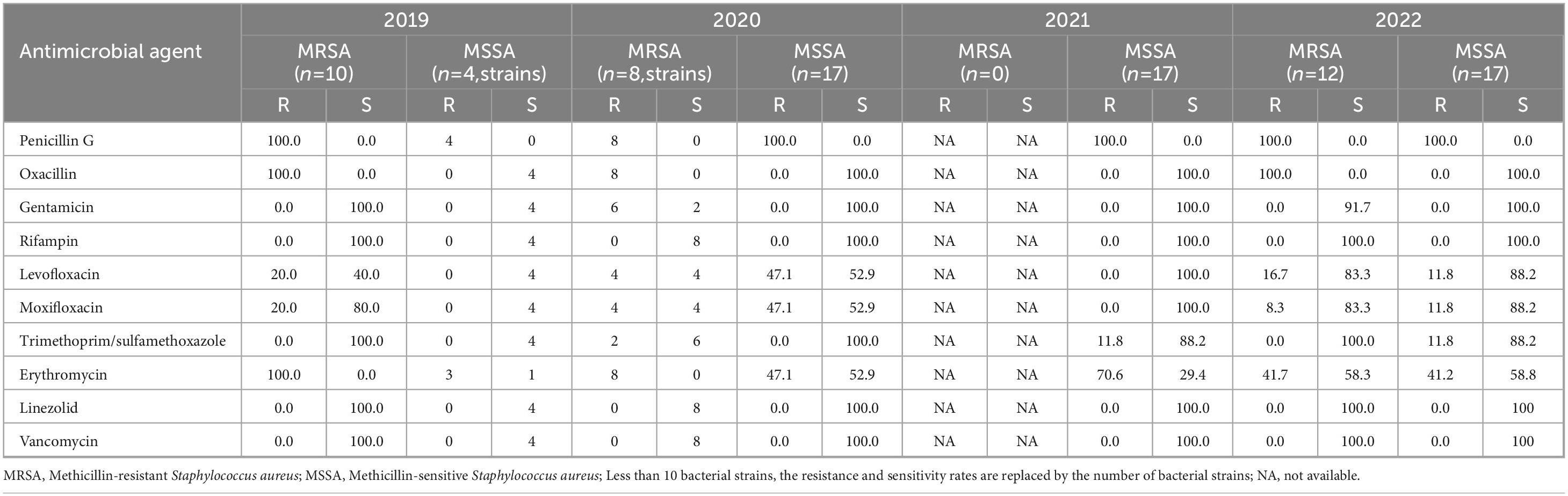
Table 2. Resistance and sensitivity rates of Staphylococcus aureus isolated from blood culture to antimicrobial agents from 2019 to 2022.
Between 2019 and 2022, the detection rates of methicillin-resistant coagulase-negative Staphylococcus (MRCNS) were 7.2% (32 strains), 9.9% (44 strains), 26.2% (116 strains), and 24.8% (110 strains), respectively (Table 3). MRCNS did not show significant changes in resistance rates to gentamicin, rifampicin, levofloxacin, moxifloxacin, or erythromycin. However, the resistance rate to trimethoprim-sulfamethoxazole increased from 27.3% in 2020 to 41.8% in 2022. No instances of resistance to vancomycin or linezolid were detected among coagulase-negative Staphylococcus strains. The resistance to penicillin G and erythromycin remained relatively high among methicillin-sensitive coagulase-negative Staphylococcus strains (MSCNS). The resistance rates of MSCNS to levofloxacin, moxifloxacin, and trimethoprim-sulfamethoxazole decreased from 2019 to 2022.
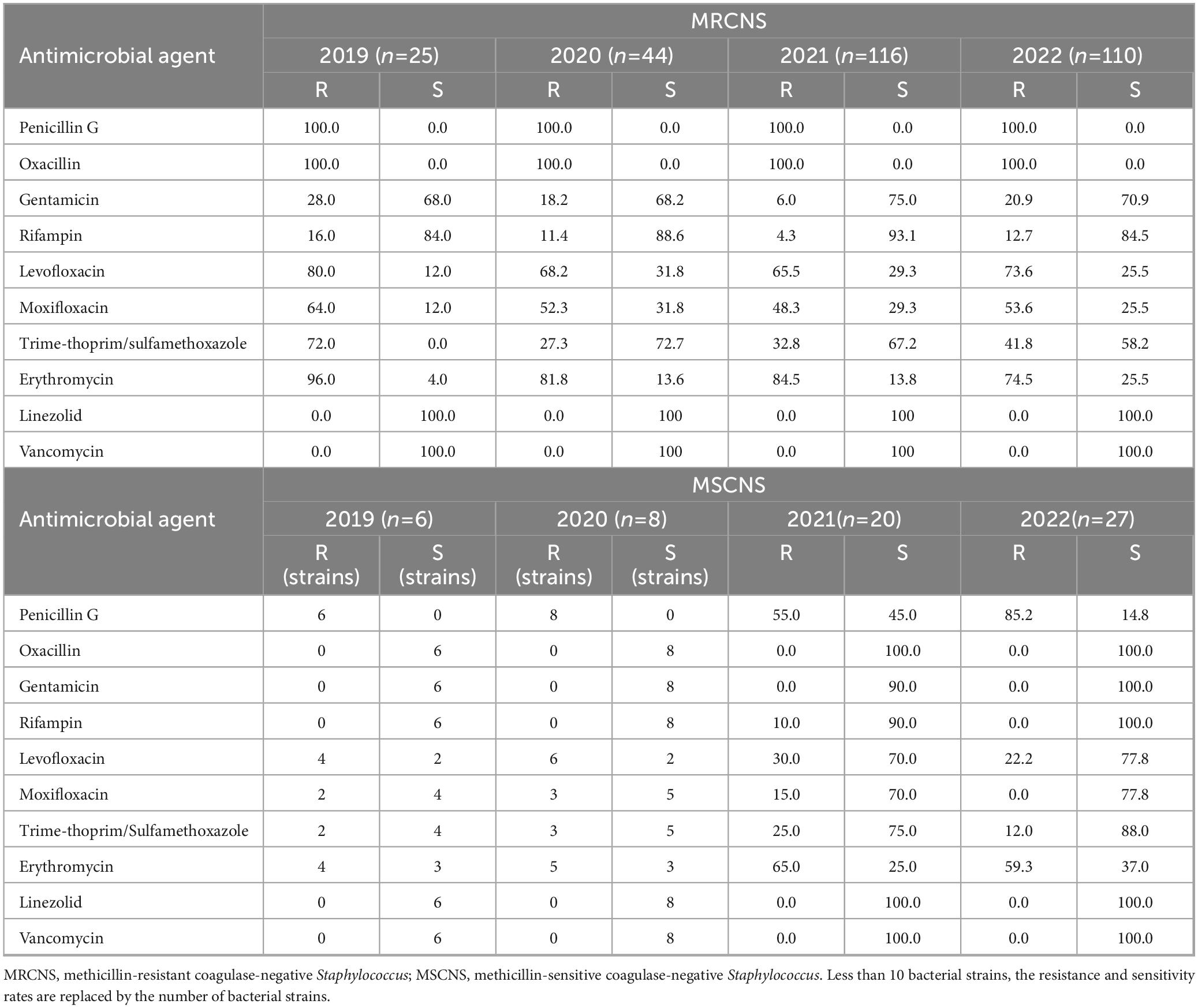
Table 3. Resistance and sensitivity rates of coagulase-negative Staphylococcus isolated from blood culture to antimicrobial agents from 2019 to 2022.
3.2.2 Enterococcus genus
A total of 132 strains from the Enterococcus genus were isolated, including 22 strains of Enterococcus faecalis, 95 strains of Enterococcus faecium, and 15 strains of other Enterococcus species. Enterococcus faecium exhibited > 80.0% resistance to ampicillin, though Enterococcus faecalis was sensitive to the drug (Table 4). Enterococcus faecium displayed significantly higher antibiotic resistance rates than those displayed by Enterococcus faecalis. Enterococcus faecium demonstrated a resistance rate > 90% to penicillin, although its resistance rate to erythromycin declined from 2019-2022. In contrast, Enterococcus faecalis exhibited resistance rates of 20.0% and 40.0% to penicillin and erythromycin, respectively. Neither Enterococcus faecium nor Enterococcus faecalis were resistant to vancomycin, though two strains of Enterococcus faecium were found to be resistant to linezolid.
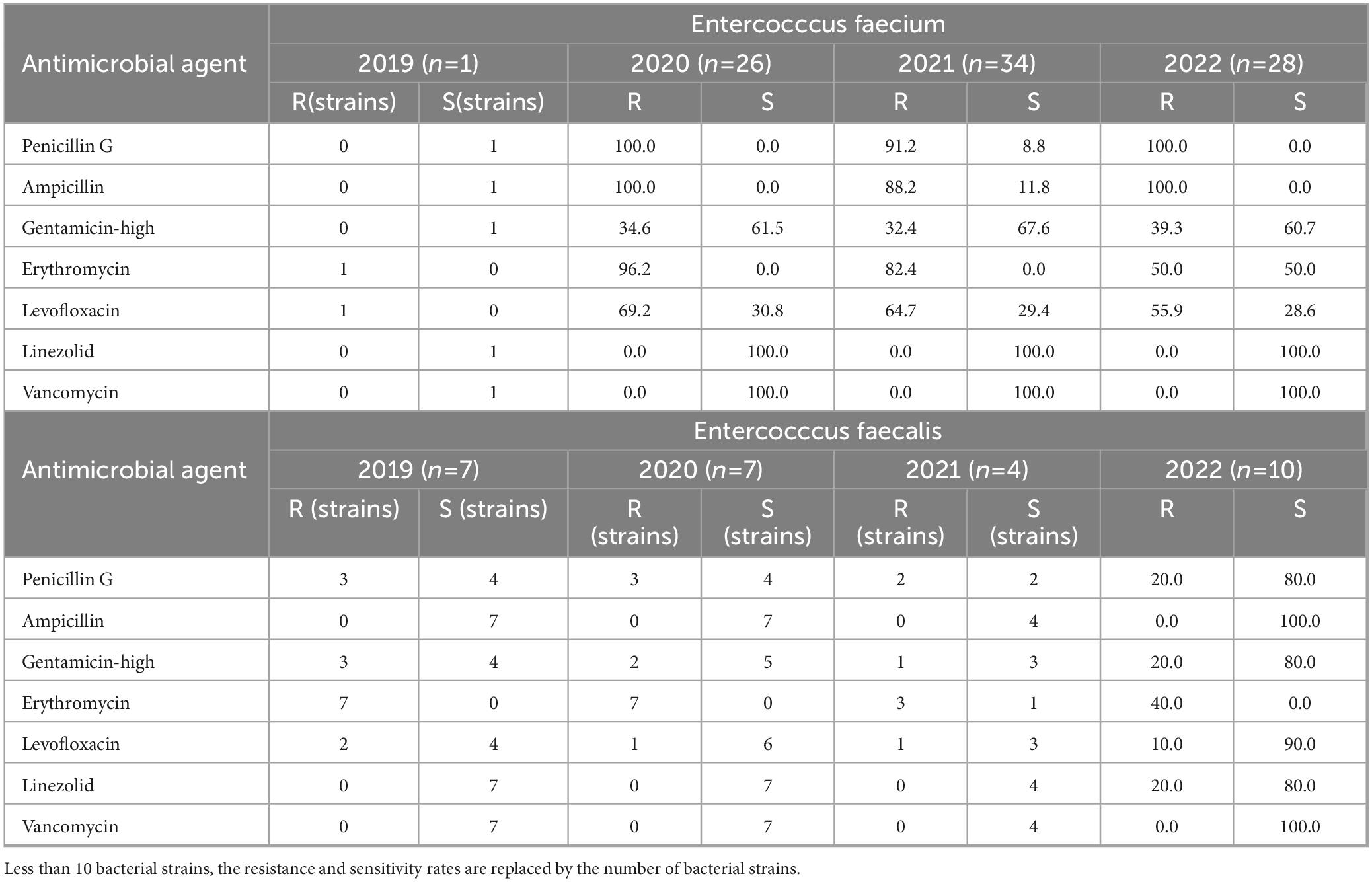
Table 4. Resistance and sensitivity rates of Enterococcus spp. isolated from blood culture to antimicrobial agents from 2019 to 2022.
3.3 Antibiotic resistances of enterobacterales
3.3.1 Escherichia coli
Escherichia coli exhibited increased carbapenem resistance rising from 0.0% in 2019 to 2.3% in 2022 (Table 5). The resistance rates of Escherichia coli to cefazolin, ceftazidime, and ceftriaxone exceeded 20.0%, with resistance to cefazolin and ceftriaxone increasing each year. However, resistance rates to amikacin and piperacillin-tazobactam remained relatively low. No strains were found resistant to imipenem, meropenem, tigecycline, or polymyxin B. Resistance to ampicillin-Sulbactam, Cefazolin, Cefepime, Levofloxacin, and Trime-thoprim/sulfamethoxazole varied significantly each year, demonstrating statistical significance.
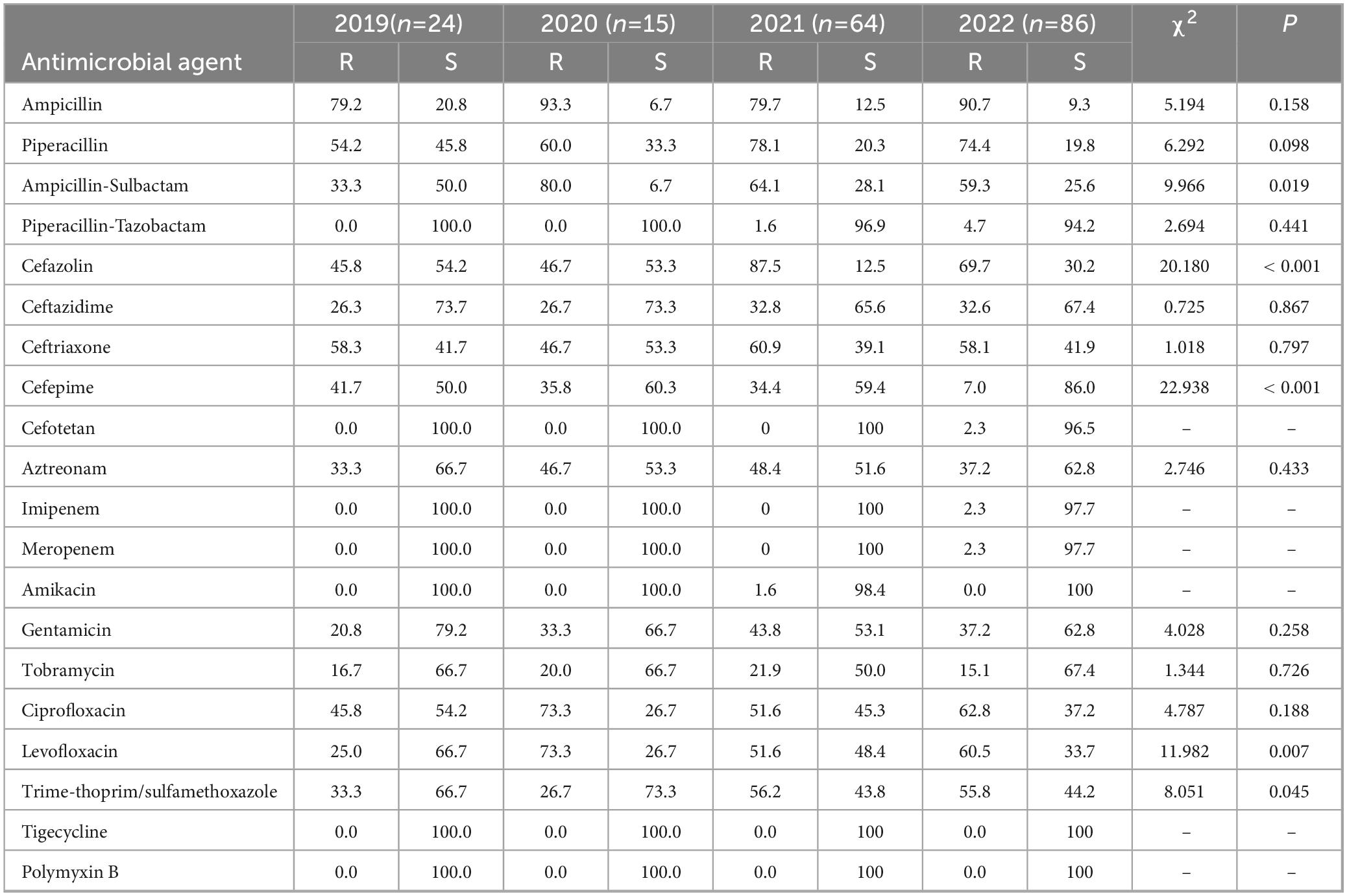
Table 5. Resistance and sensitivity rates of Escherichia Coli isolated from blood culture to antimicrobial agents from 2019 to 2022.
3.3.2 Klebsiella pneumoniae
Klebsiella pneumoniae demonstrated resistance rates to carbapenem antibiotics that were consistently exceeding 20.0% from 2019 to 2022 (Table 6). The resistance rates of cefazolin, ceftazidime, ceftriaxone, and cefepime exceeded 20.0%. Klebsiella pneumoniae demonstrated slightly higher resistance rates than Escherichia coli. Both bacteria exhibited their highest resistance rates in 2021, with some declines observed in 2022. Significant annual variations in resistance rates were observed for all tested antibiotics (p < 0.05).
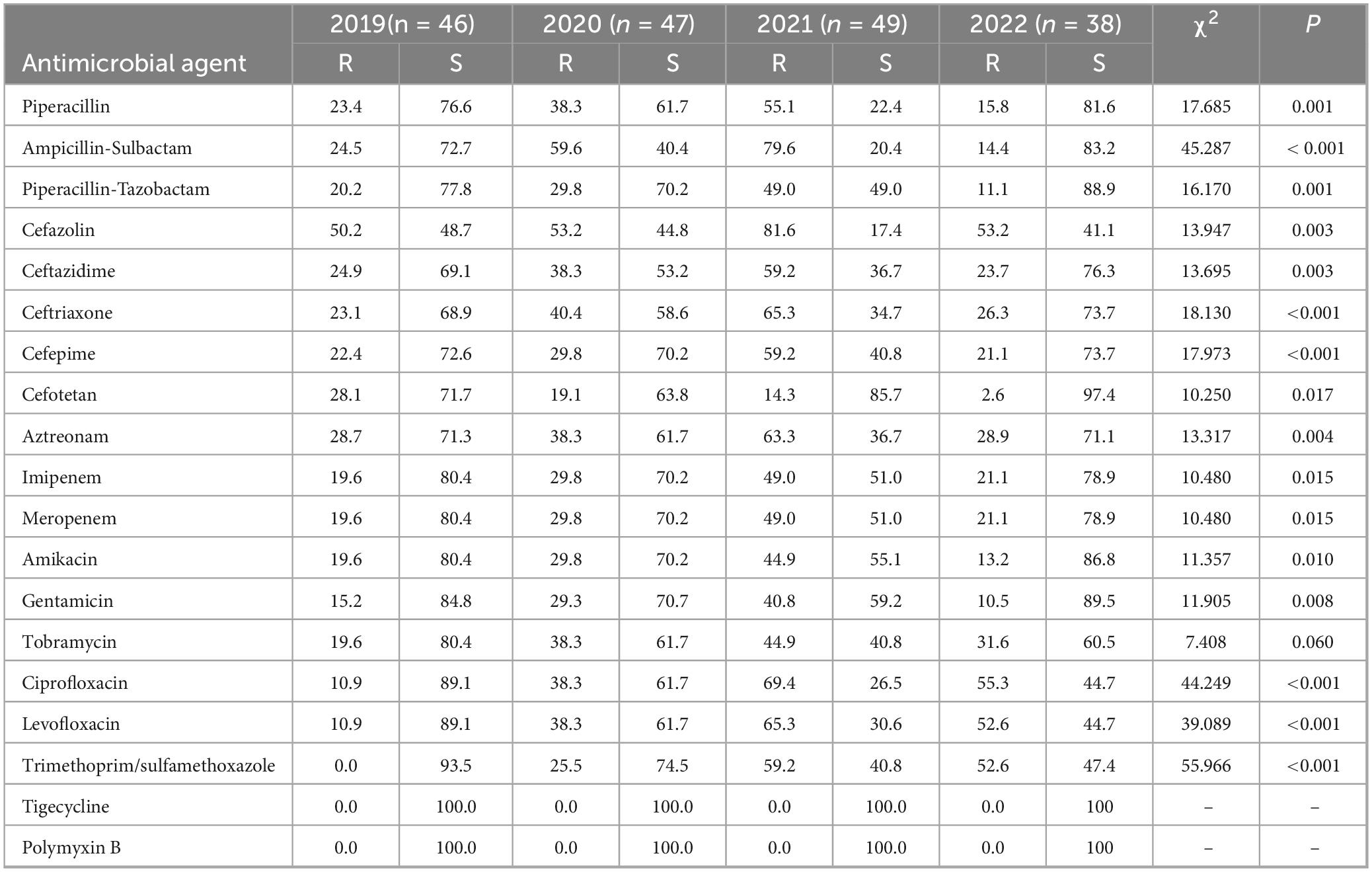
Table 6. Resistance and sensitivity rates of Klebsiella pneumoniae isolated from blood culture to antimicrobial agents from 2019 to 2022.
The comparison of antibiotic resistance rates between E. coli and K. pneumoniae from 2019 to 2022 is shown in Figure 1.
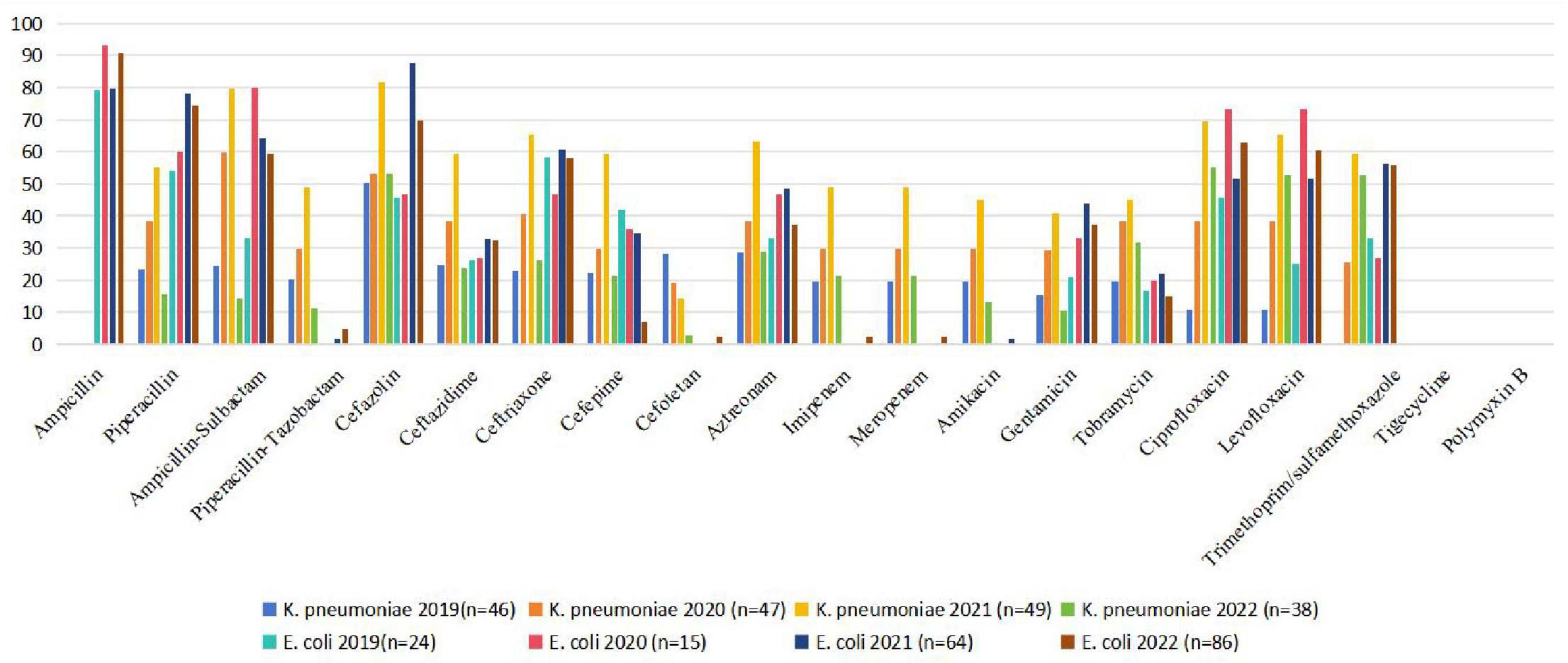
Figure 1. Comparison of antibiotic resistance rates of E. coli and K. pneumoniae (2019–2022). E. coli, Escherichia coli; K. pneumoniae, Klebsiella pneumoniae.
3.3.3 Enterobacter cloacae
Enterobacter cloacae exhibited a rising trend in resistance rates to carbapenem antibiotics, increasing from 0.0% in 2019 to 19.0% in 2022 (Table 7). The resistance rates to piperacillin, cefotiam, and ceftriaxone all exceeded 80.0%. The resistance rate to piperacillin-tazobactam decreased from 75.0 to 38.1%, and the resistance rate to amikacin decreased from 100 to 52.4%. There were no significant changes in resistance rates to gentamicin, levofloxacin, and ciprofloxacin. However, the resistance rate to trimethoprim-sulfamethoxazole increased from 25.0 to 61.9%.
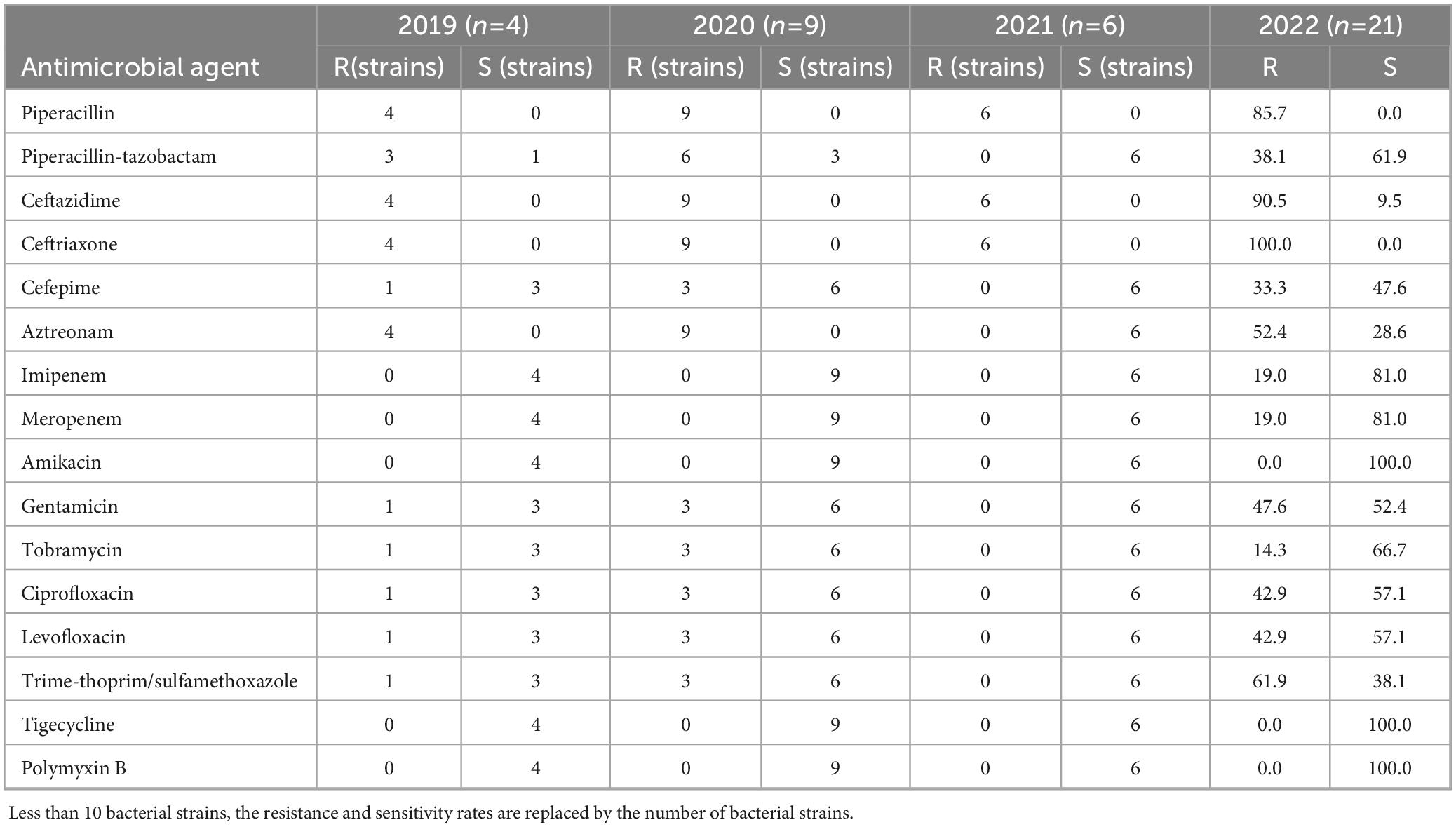
Table 7. Resistance and sensitivity rates of Enterobacter cloacae isolated from blood culture to antimicrobial agents from 2019 to 2022.
3.4 Antibiotic resistances of non-fermenting gram-negative bacteria
3.4.1 Pseudomonas aeruginosa
The resistance rates of Pseudomonas aeruginosa to imipenem and meropenem ranged from 73.7 to 84.6%, with a noticeable decrease from 2019 to 2022 (Table 8). The resistance rates to piperacillin-tazobactam, cefotiam, cefepime, amikacin, and tobramycin were steady at approximately 10.0%. The resistance rate to ciprofloxacin decreased from 84.6% in 2019 to 0.0% in 2022, as did the resistance rate to levofloxacin, which decreased from 84.6% in 2019 to 29.2% in 2022. Pseudomonas aeruginosa did not display any resistance to polymyxin B.
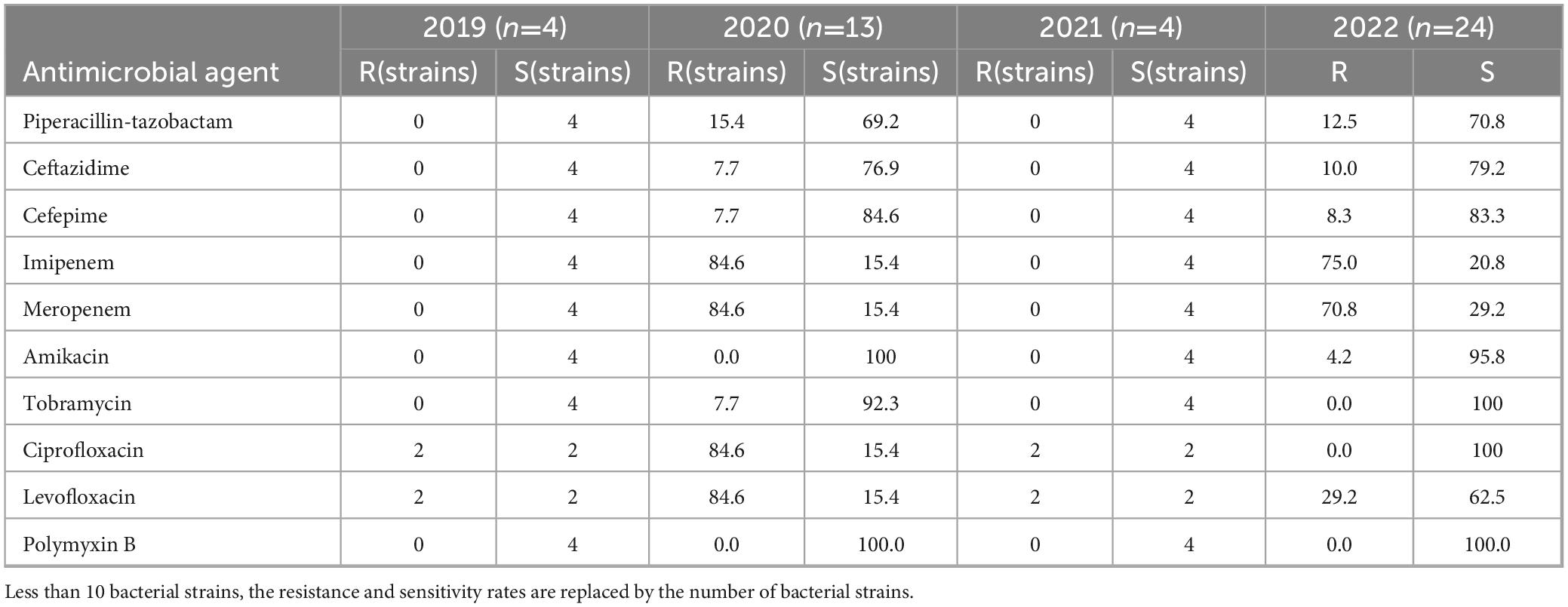
Table 8. Resistance and sensitivity rates of Pseudomonas aeruginosa isolated from blood culture to antimicrobial agents from 2019 to 2022.
3.4.2 Acinetobacter baumannii
Acinetobacter baumannii displayed a notable resistance to multiple antibiotics (Table 9). The resistance rate to amikacin increased from 16.7 to 72.2% in 2022, while resistance rates to imipenem and meropenem were consistently > 75.0%. The resistance rates to other antibiotics also exhibited a rising trend from 2019 to 2022. Acinetobacter baumannii did not display any resistance to tigecycline or polymyxin B. Resistance to ampicillin-Sulbactam, piperacillin-tazobactam, imipenem, meropenem, amikacin, tobramycin and Trime-thoprim/sulfamethoxazole varied significantly each year, demonstrating statistical significance (p < 0.05).
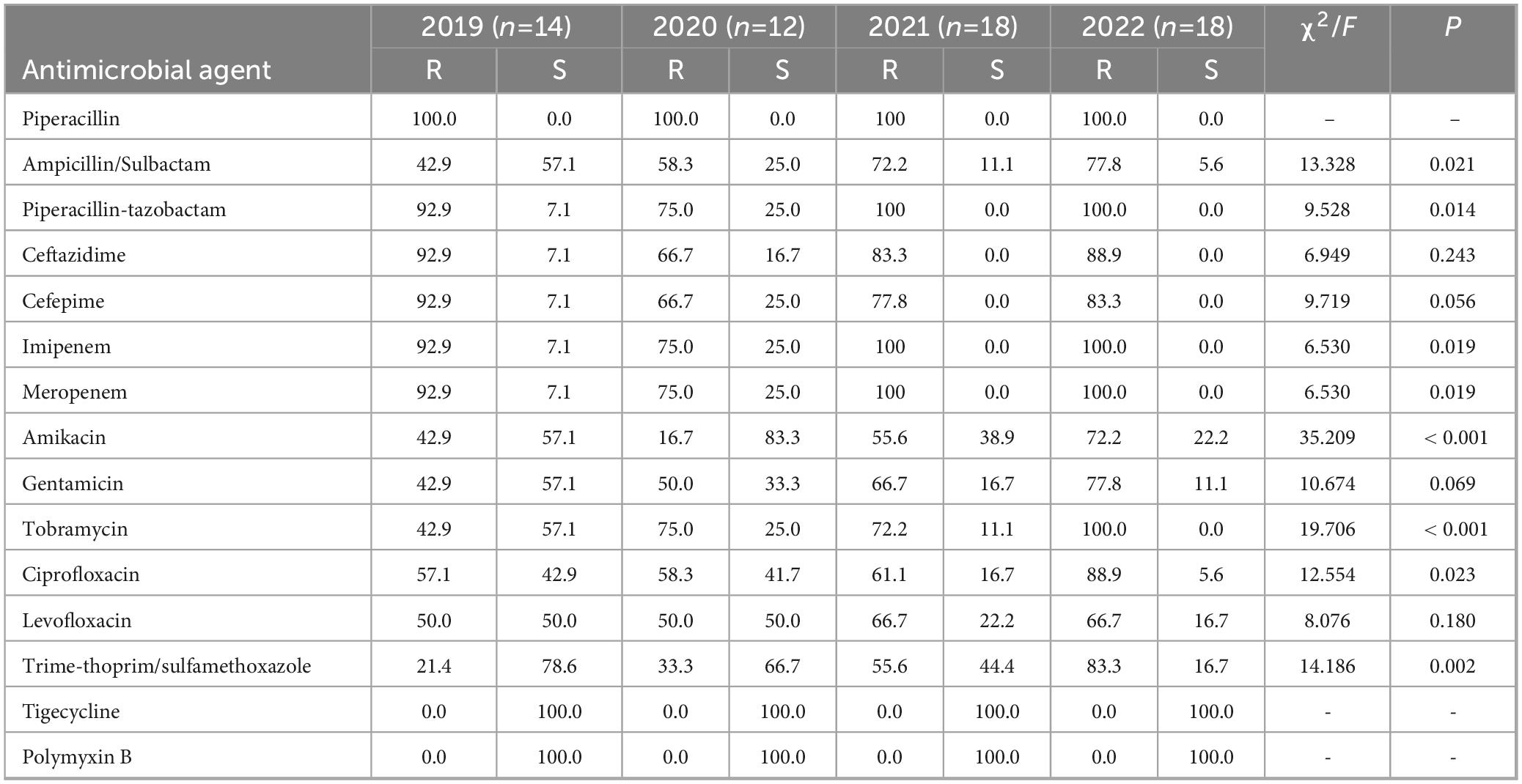
Table 9. Resistance and sensitivity rates of Acinetobacter baumannii isolated from blood culture to antimicrobial agents from 2019 to 2022.
3.5 Carbapenem-resistant gram-negative bacilli
Between 2019 and 2022, the detection rates of carbapenem-resistant organisms (CRO) among gram-negative bacilli varied (Table 10). In 2019, CRO accounted for 4.1% (52 strains) of gram-negative bacilli, including 37 strains of Klebsiella pneumoniae, 2 strains of Pseudomonas aeruginosa, and 13 strains of Acinetobacter baumannii. In 2020, CRO accounted for 3.1% (40 strains) of gram-negative bacilli, including 14 strains of Klebsiella pneumoniae, 6 strains of Raoultella planticola, 11 strains of Pseudomonas aeruginosa, and 9 strains of Acinetobacter baumannii. In 2021, the CRO detection rate was 3.3% (42 strains), including 24 strains of Klebsiella pneumoniae and 18 strains of Acinetobacter baumannii. In 2022, the CRO detection was 3.8% (49 strains), including 2 strains of Escherichia coli, 8 strains of Klebsiella pneumoniae, 4 strains of Enterobacter cloacae, 17 strains of Pseudomonas aeruginosa, and 18 strains of Acinetobacter baumannii. Carbapenem-resistant Klebsiella pneumoniae (CRKP) and Carbapenem-resistant Acinetobacter baumannii (CRAB) were consistently identified from 2019 to 2022. CRKP showed a decreasing resistance to aminoglycosides but an increasing resistance to trimethoprim-sulfamethoxazole. Conversely, CRAB exhibited increasing resistance rates to amikacin and gentamicin, from 16.7% in 2019 to 77.8% in 2022 and from 66.7% in 2019 to 94.4% in 2022, respectively. The resistance to trimethoprim-sulfamethoxazole increased from 61.5% in 2019 to 69.1% in 2022, while resistance to levofloxacin decreased from 100.0% in 2019 to 66.7% in 2022. Carbapenem-resistant Enterobacter cloacae (CREC) was only detected in 2022 and was sensitive to tigecycline, polymyxin B, and aminoglycoside antibiotics. Carbapenem-resistant Pseudomonas aeruginosa (CRPA) was identified in 2019, 2020, and 2022, with resistance rates to ceftazidime and cefepime increasing from 0.0% in 2019 to 70.6% in 2022 and from 0.0% in 2019 to 52.7% in 2022, respectively. The resistance rates of CRPA to ciprofloxacin and levofloxacin decreased from 100.0% in 2019 to 41.2% and 52.9% in 2022, respectively.

Table 10. Resistance rate and sensitivity rate of carbapenem resistant gram-negative bacteria isolated from blood culture to antimicrobial agents from 2019 to 2022.
4 Discussion
Blood culture remains the gold standard for diagnosing bloodstream infections (BSIs) due to its accessibility, clinical utility, and ability to guide antibiotic susceptibility testing (Bai et al., 2022). While emerging molecular diagnostics (e.g., PCR-based assays) offer rapid pathogen identification, blood culture remains indispensable for capturing viable pathogens and resistance profiles, particularly in critically ill ICU patients requiring timely targeted therapy (El Haddad et al., 2018). The isolation of clinically-relevant pathogens via blood culture indicates that the defense mechanisms of the host and/or prior clinical interventions were unsuccessful in eradicating the infecting pathogens at the primary infection site. Moreover, the specific types of pathogens identified via blood culture offer important prognostic insights (El Haddad et al., 2018; Wildenthal et al., 2023). When multidrug-resistant organisms are identified in blood cultures, the patient mortality rate is as high as 35% (Abu-Saleh et al., 2018; GBD 2019 Antimicrobial Resistance Collaborators, 2022).
From 2019 to 2022, a total of 1,282 distinct strains were isolated from the positive blood cultures of patients in the ICU at the Second Affiliated Hospital of Xi’an Jiaotong University. gram-positive bacteria (52.0%) slightly outnumbered gram-negative isolates (48.0%), aligning with global ICU trends (Li et al., 2022; Van An et al., 2023). However, the data in this study may be biased due to a lower number of strains in 2021. Among the gram-positive bacteria, coagulase-negative staphylococci were the most common, followed by Enterococcus faecalis and Staphylococcus aureus. MRSA displayed a decreasing resistance to gentamicin, levofloxacin, moxifloxacin, trimethoprim-sulfamethoxazole, and erythromycin from 2019 to 2022. No resistance to vancomycin, linezolid, or rifampicin was observed in Staphylococcus aureus. However, coagulase-negative staphylococci (CoNS) accounted for 28.0% of isolates, raising questions about their clinical significance. While CoNS are frequent blood culture contaminants due to improper skin disinfection (Wang et al., 2023), they may represent true pathogens in immunocompromised patients or those with indwelling devices (Heilmann et al., 2019). In our cohort, standardized blood culture collection were followed, yet persistent CoNS isolation underscores the need for rigorous clinical correlation to distinguish contamination from true infection. The resistance rates of MRCNS to gentamicin, rifampicin, levofloxacin, moxifloxacin, and erythromycin did not change significantly throughout the study period, though the resistance of MRCNS to trimethoprim-sulfamethoxazole increased slightly. MRCNS were not resistant to vancomycin or linezolid; therefore, these are the preferred antibiotics for clinical Staphylococcus infections. The Enterococcus genus constituted 11.0% of the isolated strains in our hospital, with Enterococcus faecium displaying higher resistance rates than Enterococcus faecalis. More specifically, Enterococcus faecium exhibited high resistance to ampicillin, while Enterococcus faecalis was sensitive to ampicillin. The resistance of Enterococcus faecium decreased from 2019 to 2022, while Enterococcus faecalis was slightly resistant to penicillin and erythromycin. Neither Enterococcus faecium nor Enterococcus faecalis displayed resistance to vancomycin, though two Enterococcus faecium strains were resistant to linezolid.
The rising carbapenem resistance in Enterobacterales is alarming. Escherichia coli exhibited a carbapenem resistance increase from 0% (2019) to 2.3% (2022), while Klebsiella pneumoniae maintained resistance rates exceeding 20% throughout the study period. Nevertheless, the overall resistance rates decreased from 2019 to 2022. According to the 2023 CHINET China Bacterial Resistance Surveillance data, Klebsiella pneumoniae had resistance rates of 26.2 and 27.1% to imipenem and meropenem, respectively, which are similar to the national resistance levels (Hu et al., 2022). These trends are likely driven by horizontal gene transfer of blaKPC carbapenemases (Han et al., 2021) and prolonged carbapenem use in critically ill patients. Enterobacter cloacae demonstrated an increase in resistance rates to carbapenem antibiotics from 2019 to 2022. No resistance to tigecycline or polymyxin B was observed among bacteria in Enterobacterales. Therefore, it is imperative to prioritize the identification and management of risk factors associated with carbapenemase-induced nosocomial infections.
Among non-fermenting gram-negative bacteria, Pseudomonas aeruginosa displayed relatively high sensitivity to piperacillin-tazobactam, cefotiam, cefepime, amikacin, and tobramycin. However, its resistance rates to imipenem and meropenem decreased during the study period. Pseudomonas aeruginosa did not display resistance to polymyxin B. In contrast, Acinetobacter baumannii remained sensitive to tigecycline and polymyxin B. However, resistance rates to imipenem and meropenem were high from 2019 to 2022. The rise in carbapenem resistance aligns with global trends (GBD 2019 Antimicrobial Resistance Collaborators, 2022), particularly in A. baumannii (100% resistance in 2022), surpassing national averages reported by CHINET (26od.%) (Hu et al., 2022). It is likely due to various factors, including compromised immunity in patients in the ICU and prolonged use of broad-spectrum antibiotics (Teerawattanapong et al., 2018; Kaye et al., 2023). This phenomenon may also be linked to the production of carbapenemase hydrolytic enzymes, decreased outer membrane permeability or loss of porins, reduced affinity of penicillin-binding proteins, and overexpression of efflux pumps (Abdi et al., 2020; Somily et al., 2022).
The ICU is a high-incidence area of multidrug-resistant organisms and carries a considerable disease burden. Research shows that the six leading pathogens for deaths associated with resistance-related deaths—Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa—were responsible for approximately 929,000 (660,000–1,270,000) deaths ascribed to AMR and 3.57 million (2.62–4.78) deaths associated with AMR in 2019. Due to various factors, the number of detected strains in individual years is relatively small, and some drug resistance rates may be skewed. Clinical implications of resistance patterns demand urgent action. For gram-positive infections, vancomycin and linezolid remain effective against staphylococci and enterococci, though two Enterococcus faecium linezolid-resistant strains highlight emerging threats. For gram-negative infections, carbapenem-sparing regimens (e.g., ceftazidime-avibactam for K. pneumoniae) should be prioritized where susceptibility permits, while polymyxins and tigecycline serve as last-resort options. For critically ill patients in the ICU, who often undergo invasive medical procedures and have multiple underlying conditions and compromised immune function, the detection of CRO in blood specimens is associated with an increased risk of mortality, emphasizing the need for prompt, effective, and precise treatment (Yi and Kim, 2021; Martínez et al., 2023).
Study limitations include its single-center, retrospective design and small annual sample sizes (e.g., 2021), which may skew resistance rates due to stochastic variation. To mitigate this, we aggregated data across the 4-year period to identify overarching trends. Additionally, infection control measures (e.g., enhanced environmental decontamination, antimicrobial stewardship programs) were implemented during the study period, potentially influencing resistance dynamics. Future multicenter studies with larger cohorts are needed to validate these findings.
5 Conclusion
The pathogens responsible for BSI and their antimicrobial resistance profiles are constantly changing. Timely surveillance of pathogen distribution and resistance trends in blood cultures remain indispensable for guiding empirical antibiotic choices in ICU patients with infections. The resistance patterns reported here offer actionable insights to optimize treatment regimens and inform antimicrobial stewardship efforts in critical care settings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
ZL: Data curation, Funding acquisition, Writing – original draft, Writing – review & editing. HC: Methodology, Writing – review & editing. JL: Data curation, Formal Analysis, Writing – review & editing. XZ: Formal Analysis, Writing – original draft. JY: Data curation, Writing – review & editing. YZ: Funding acquisition, Writing – review & editing. XY: Funding acquisition, Validation, Writing – review & editing. YG: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Key Research and Development Plan of Shaanxi Province (2024SF-YBXM-160), the Key Research and Development Program of Shaanxi Province (2020SF-173), the National Natural Science Foundation of China (82370604), and Natural Science Foundation of Fujian Province (2023J01239).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CRAB, Carbapenem-resistant Acinetobacter baumannii; CREC, Carbapenem-resistant Escherichia coli; CRKP, Carbapenem-resistant Klebsiella pneumoniae; CRO, Carbapenem-resistant organisms; CRPA, Carbapenem-resistant Pseudomonas aeruginosa; ICU, Intensive care unit; KPC, Klebsiella pneumoniae carbapenemases; MH, Mueller-Hinton; MRCNS, Methicillin-resistant coagulase-negative Staphylococcus; MRSA, Methicillin-resistant Staphylococcus aureus; MSCNS, Methicillin-sensitive coagulase-negative Staphylococcus; MSSA, Methicillin-sensitive Staphylococcus aureus; KPN, Klebsiella pneumonia; KPL, Raoultella planticola; PAE, Pseudomonas aeruginosa; ABA, Acinetobacter baumannii; ECO, Escherichia coli; ECL, Enterobacter cloacae; NA, not available.
Footnotes
References
Abdi, S. N., Ghotaslou, R., Ganbarov, K., Mobed, A., Tanomand, A., Yousefi, M., et al. (2020). Acinetobacter baumannii efflux pumps and antibiotic resistance. Infect. Drug Resist. 13, 423–434. doi: 10.2147/IDR.S228089
Abu-Saleh, R., Nitzan, O., Saliba, W., Colodner, R., Keness, Y., Yanovskay, A., et al. (2018). Bloodstream infections caused by contaminants: Epidemiology and risk factors: A 10-year surveillance. Isr. Med. Assoc. J. 20, 433–437.
Antimicrobial Resistance Collaborators (2022). Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 399, 629–655. doi: 10.1016/S0140-6736(21)02724-0
Bai, A. D., Lo, C. K. L., Komorowski, A. S., Suresh, M., Guo, K., Garg, A., et al. (2022). Staphylococcus aureus bacteraemia mortality: A systematic review and meta-analysis. Clin. Microbiol. Infect. 28, 1076–1084. doi: 10.1016/j.cmi.2022.03.015
Centers for Disease Control and Prevention [CDC] (2023). Antibiotic resistance threats in the United States. Atlanta, GA: Centers for Disease Control and Prevention.
Cheng, M. P., Stenstrom, R., Paquette, K., Yansouni, C., and Sweet, D. (2020). Blood culture results before and after antimicrobial administration. Ann. Intern. Med. 172, 440–441. doi: 10.7326/L19-0796
Clinical and Laboratory Standards Institute [CLSI] (2022). Performance standards for antimicrobial susceptibility testing. Wayne, PA: CLSI.
El Haddad, H., Chaftari, A. M., Hachem, R., Chaftari, P., and Raad, I. I. (2018). Biomarkers of sepsis and bloodstream infections: The role of procalcitonin and proadrenomedullin with emphasis in patients with cancer. Clin. Infect. Dis. 67, 971–977. doi: 10.1093/cid/ciy331
European Centre for Disease Prevention and Control [ECDC] (2022). Surveillance of antimicrobial resistance in Europe. Sweden: European Centre for Disease Prevention and Control.
Fabre, V., Carroll, K. C., and Cosgrove, S. E. (2022). Blood culture utilization in the hospital setting: A call for diagnostic stewardship. J. Clin. Microbiol. 60:e0100521. doi: 10.1128/JCM.01005-21
Fleischmann-Struzek, C., Mellhammar, L., Rose, N., Cassini, A., Rudd, K. E., Schlattmann, P., et al. (2020). Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 46, 1552–1562. doi: 10.1007/s00134-020-06151-x
GBD 2019 Antimicrobial Resistance Collaborators (2022). Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 400, 2221–2248. doi: 10.1016/S0140-6736(22)02185-7
Gonzalez, M. D., Chao, T., and Pettengill, M. A. (2020). Modern blood culture: Management decisions and method options. Clin. Lab. Med. 40, 379–392. doi: 10.1016/j.cll.2020.07.001
Han, R. R., Guo, Y., Peng, M. J., Shi, Q., Wu, S., Yang, Y., et al. (2021). Evaluation of the immunochromatographic NG-test carba 5, RESIST-5 O.O.K.N.V., and IMP K-SeT for rapid detection of KPC-, NDM-, IMP-, VIM-type, and OXA-48-like carbapenemase among Enterobacterales. Front. Microbiol. 11:609856. doi: 10.3389/fmicb.2020.609856
Heilmann, C., Ziebuhr, W., and Becker, K. (2019). Are coagulase-negative staphylococci virulent? Clin. Microbiol. Infect. 25, 1071–1080. doi: 10.1016/j.cmi.2018.11.012
Hu, F., Yuan, L., Yang, Y., Xu, Y., Huang, Y., Hu, Y., et al. (2022). A multicenter investigation of 2,773 cases of bloodstream infections based on China antimicrobial surveillance network (CHINET). Front. Cell. Infect. Microbiol. 12:1075185. doi: 10.3389/fcimb.2022.1075185
Kaye, K. S., Shorr, A. F., Wunderink, R. G., Du, B., Poirier, G. E., Rana, K., et al. (2023). Efficacy and safety of sulbactam-durlobactam versus colistin for the treatment of patients with serious infections caused by Acinetobacter baumannii-calcoaceticus complex: A multicentre, randomised, active-controlled, phase 3, non-inferiority clinical trial (ATTACK). Lancet Infect. Dis. 23, 1072–1084. doi: 10.1016/S1473-3099(23)00184-6
Lan, P., Jiang, Y., Zhou, J., and Yu, Y. (2021). A global perspective on the convergence of hypervirulence and carbapenem resistance in Klebsiella pneumoniae. J. Glob. Antimicrob. Resist. 25, 26–34. doi: 10.1016/j.jgar.2021.02.020
Li, J., Jiang, F., Xie, A., and Jiang, Y. (2022). Analysis of the distribution and drug resistance of pathogens in patients with urinary tract infection in the eastern Chongming area of shanghai from 2018 to 2020. Infect. Drug Resist. 15, 6413–6422. doi: 10.2147/IDR.S384515
Magiorakos, A. P., Burns, K., Rodríguez-Baño, J., Borg, M., Daikos, G., Dumpis, U., et al. (2017). Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: Guidance from the European Centre for Disease Prevention and Control. Antimicrob. Resist. Infect. Control. 6:113. doi: 10.1186/s13756-017-0259-z
Martínez, D. A., Cai, J., Lin, G., Goodman, K. E., Paul, R., Lessler, J., et al. (2023). Modelling interventions and contact networks to reduce the spread of carbapenem-resistant organisms between individuals in the ICU. J. Hosp. Infect. 136, 1–7. doi: 10.1016/j.jhin.2023.02.016
Mazi, W. A., Abdulwahab, M. H., Alashqar, M. A., Aldecoa, Y. S., Bahat, Z. R., Suaking, J. L., et al. (2021). Sustained low incidence rates of central line-associated blood stream infections in the intensive care unit. Infect. Drug Resist. 14, 889–894. doi: 10.2147/IDR.S290791
Somily, A., Balkhy, H. H., Enani, M. A. S., Althawadi, S. I., Alawi, M., Al Johani, S. M., et al. (2022). Antimicrobial resistance trends of non-fermenter Gram negative bacteria in Saudi Arabia: A six-year national study. J. Infect. Public Heal. 14, 1144–1150. doi: 10.1016/j.jiph.2021.07.007
Tajima, T., Asai, Y., Endo, M., Suzuki, T., Matsunaga, N., Tsuzuki, S., et al. (2021). Rate of blood culture submissions in Japan as an indicator of bloodstream infections. J. Infect. Chemother. 27, 1270–1272. doi: 10.1016/j.jiac.2021.04.019
Teerawattanapong, N., Panich, P., Kulpokin, D., Na Ranong, S., Kongpakwattana, K., Saksinanon, A., et al. (2018). A systematic review of the burden of multidrug-resistant healthcare-associated infections among intensive care unit patients in Southeast Asia:the rise of multidrug-resistant Acinetobacter baumannii. Infect. Control Hosp. Epidemiol. 39, 525–533. doi: 10.1017/ice.2018.58
Van An, N., Hoang, L. H., Le, H. H. L., Thai Son, N., Hong, L. T., Viet, T. T., et al. (2023). Distribution and antibiotic resistance characteristics of bacteria isolated from blood culture in a teaching hospital in Vietnam during 2014–2021. Infect. Drug Resist. 16, 1677–1692. doi: 10.2147/IDR.S402278
Wang, S., Song, Y., Shi, N., Yin, D., Kang, J., Cai, W., et al. (2023). Characteristics, outcomes, and clinical indicators of bloodstream infections in neutropenic patients with hematological malignancies: A 7-year retrospective study. Infect. Drug Resist. 16, 4471–4487. doi: 10.2147/IDR.S413454
Wildenthal, J. A., Atkinson, A., Lewis, S., Sayood, S., Nolan, N. S., Cabrera, N. L., et al. (2023). Outcomes of partial oral antibiotic treatment for complicated Staphylococcus aureus bacteremia in people who inject drugs. Clin. Infect. Dis. 76, 487–496. doi: 10.1093/cid/ciac714
World Health Organization [WHO] (2021). Antimicrobial resistance: Global report on surveillance. Geneva: WHO.
Keywords: blood culture, drug resistance, pathogens, intensive care unit, antimicrobial susceptibility test
Citation: Liu Z, Cai H, Lei J, Zhang X, Yin J, Zhang Y, Yu X and Geng Y (2025) Distribution and analysis of the resistance profiles of bacteria isolated from blood cultures in the intensive care unit. Front. Microbiol. 16:1464573. doi: 10.3389/fmicb.2025.1464573
Received: 14 July 2024; Accepted: 19 June 2025;
Published: 14 July 2025.
Edited by:
Ana P. Tedim, Institute of Health Sciences Studies of Castilla y León (IECSCYL), SpainReviewed by:
Susana Patricia Costa, International Iberian Nanotechnology Laboratory (INL), PortugalTripti Nair, University of Southern California, United States
Copyright © 2025 Liu, Cai, Lei, Zhang, Yin, Zhang, Yu and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueping Yu, eHB5dTE1QGZ1ZGFuLmVkdS5jbg==; Yan Geng, d3N3ODc2NzkzNThAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Zeshi Liu
Zeshi Liu Hehui Cai2†
Hehui Cai2† Yanping Zhang
Yanping Zhang Xueping Yu
Xueping Yu Yan Geng
Yan Geng