- 1Department of Gastroenterology and Hepatology, General Hospital, Tianjin Medical University, Tianjin, China
- 2Tianjin Institute of Digestive Diseases, Tianjin, China
- 3Tianjin Key Laboratory of Digestive Diseases, Tianjin, China
Background: Lactobacillus johnsonii (L. johnsonii) is a lactic acid-producing probiotic, possessing the potential to modulate intestinal microbiota balance, which can enhance immune function, and reduce the risk of intestinal infections. In recent years, increasing studies have demonstrated the positive impact of this strain and its metabolites on the health of multiple systems, including the stomach, intestine, liver, and brain.
Objective: This article aims to systematically review the mechanisms of action and clinical application progress of L. johnsonii in the prevention and treatment of digestive system-related diseases. The focus is on exploring its systemic regulatory role through the “microbiota-gut-organ axis.”
Methodology: By collating and analyzing recent research findings on L. johnsonii, we evaluated its specific mechanisms in regulating intestinal barrier function, immune response, and neuroendocrine signaling pathways. This strain can be isolated and identified through experimental means, and its population abundance can be quantitatively analyzed, providing a basis for studying its biological functions.
Results: Studies have revealed that L. johnsonii exhibits significant interventional potential in various cross-system diseases, including inflammatory bowel disease (IBD), intestinal infections, Helicobacter pylori (H. pylori)-associated gastritis, non-alcoholic fatty liver disease, and neurodegenerative diseases. These findings further validate the important role of this strain in maintaining intestinal microbiota homeostasis, regulating body metabolism, and brain-gut axis function.
Conclusion: In summary, L. johnsonii shows great promise in digestive health by modulating immunity, enhancing the gut barrier and balancing gut microbiota. Future research should further explore its mechanisms of action, aiming to provide a solid theoretical foundation and experimental support for its precise therapeutic reality.
1 Introduction
Digestive diseases are an important public health problem worldwide, affecting millions of people and imposing a huge medical and socio-economic burden. The digestive system, including the oral cavity, esophagus, stomach, intestines and liver, plays a central role in nutrient absorption, immune regulation and maintenance of metabolic homeostasis. In recent years, the incidence of IBD, irritable bowel syndrome, and gastrointestinal infections has continued to rise globally (Molodecky et al., 2012; Peery et al., 2022; Ricciardiello, 2022; Roy and Dhaneshwar, 2023), and there is an urgent need to explore new intervention strategies and therapeutic approaches.
A growing body of evidence suggests that the gut microbiota-a complex ecosystem of diverse microorganisms-plays a critical role in maintaining digestive and systemic health. An imbalance in the composition and function of the microbiota can lead to a variety of diseases including IBD, liver disease, metabolic syndrome and even neuropsychiatric disorders, with mechanisms of action involving immune regulation, epithelial barrier function, and the gut-brain axis (Cryan and Dinan, 2012; Rooks and Garrett, 2016; Fan and Pedersen, 2021). Therefore, strategies to regulate gut microbiota, especially the use of probiotics, have become a hot topic of current research in the prevention and treatment of digestive disorders. Probiotics are defined by the World Health Organization as “active microorganisms that are beneficial to the health of the host when ingested in adequate amounts” (Mu et al., 2018) and have been gradually applied in clinical interventions.
Among many probiotic candidates, L. johnsonii has drawn much attention. This is due to its robust colonization capacity, immunomodulatory potential, and diverse mechanisms of action. It was originally classified as L. acidophilus. L. johnsonii is a Gram-positive, non-bacteriophage-forming, partially anaerobic lactic acid bacterium. It is widely found in the gastrointestinal tract of both humans and animals (Yang et al., 2020). Like other Lactobacillus species such as L. acidophilus, L. johnsonii may contribute to host health in several ways. It can adhere to intestinal epithelial cells. It may enhance the mucosal barrier function. It also produces antimicrobial substances and modulates both innate and adaptive immunity (de Roos and Katan, 2000; Zhang et al., 2021). Specific strains of L. johnsonii have shown anti-inflammatory, antipathogenic, and metabolic-modulating properties. These traits suggest potential therapeutic value in conditions such as IBD, intestinal infections, liver diseases, and gut-brain axis disorders. Advances in histological techniques have deepened our understanding of its molecular mechanisms. Researchers have identified key proteins, such as GroEL and GAPDH, which are crucial in bacterium-host interactions (Xie et al., 2019). However, the functional differences and dose-dependent responses among strains suggest that precise screening and validation are still needed in probiotic development. A comprehensive 2024 review highlighted that probiotics-including specific Lactobacillus strains-modulate the gut-brain axis by regulating neuroinflammation, neurotransmitter production, and cognitive function in human trials involving mild cognitive impairment and dementia. This supports the potential of L. johnsonii and related probiotics in gut-brain disorders (Fekete et al., 2024).
This review systematically summarizes the current research progress of L. johnsonii in a variety of digestive diseases, covering its mechanism of action and potential clinical value in IBD, gastrointestinal infections, gastritis, obesity, hepatopathy, regulation of the gut-brain axis, gut microbiota dysbiosis, and colorectal cancer. We integrated representative results from animal experiments, cellular models and clinical studies (Tables 1–3), focusing on the analysis of its functional properties in regulating the intestinal barrier, metabolic intervention,maintenance of microbiota homeostasis and immune modulation. Currently, there are relatively few studies on the role of the specific strain L. johnsonii in digestive system diseases. This article provides readers with a comprehensive and focused update on the specific strain L. johnsonii, avoiding generalizations. This review not only describes the role of L. johnsonii within the gut but, more importantly, systematically and thoroughly explains how it influences distant organs such as the liver, brain, heart, and lungs through these axises. It systematically covers the research progress of L. johnsonii in almost all major digestive system-related diseases. This review also places significant emphasis on the differing effects of various L. johnsonii strains in specific disease models or clinical contexts. This review aims to provide theoretical support for the targeted use of L. johnsonii. It also lays a foundation for developing future precision probiotic interventions.
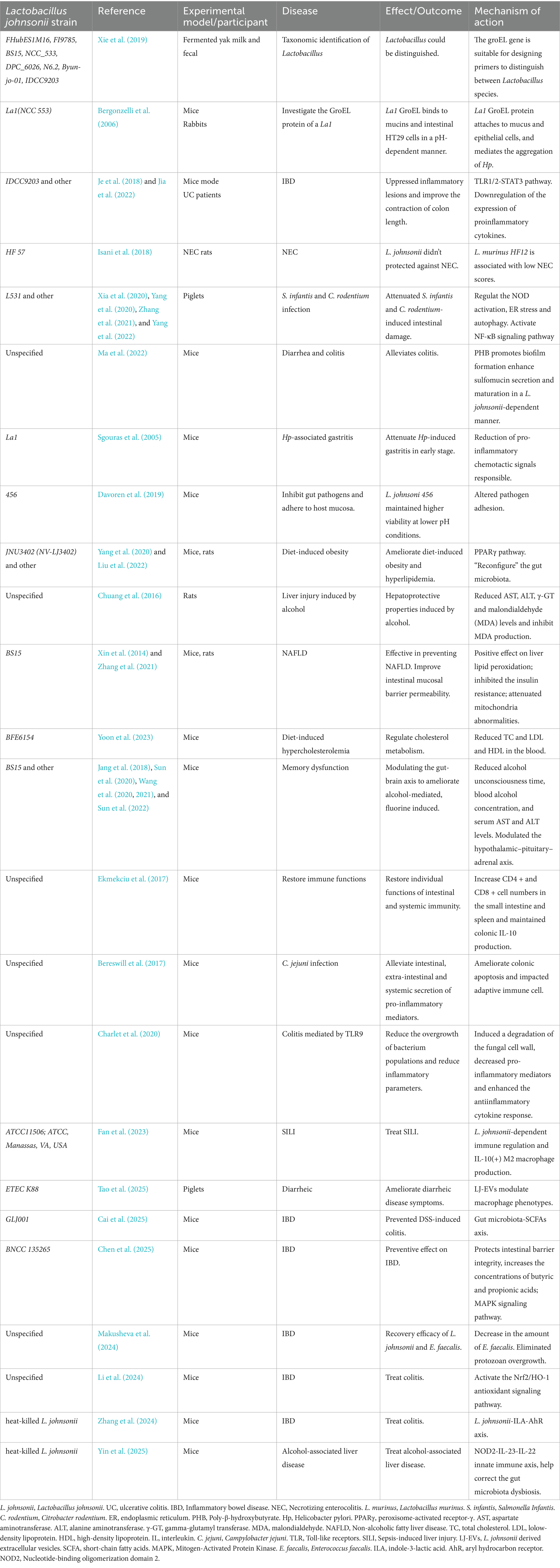
Table 1. Therapeutic efficacy and potential mechanisms of Lactobacillus johnsonii strains in various of the digestive diseases in animal models.
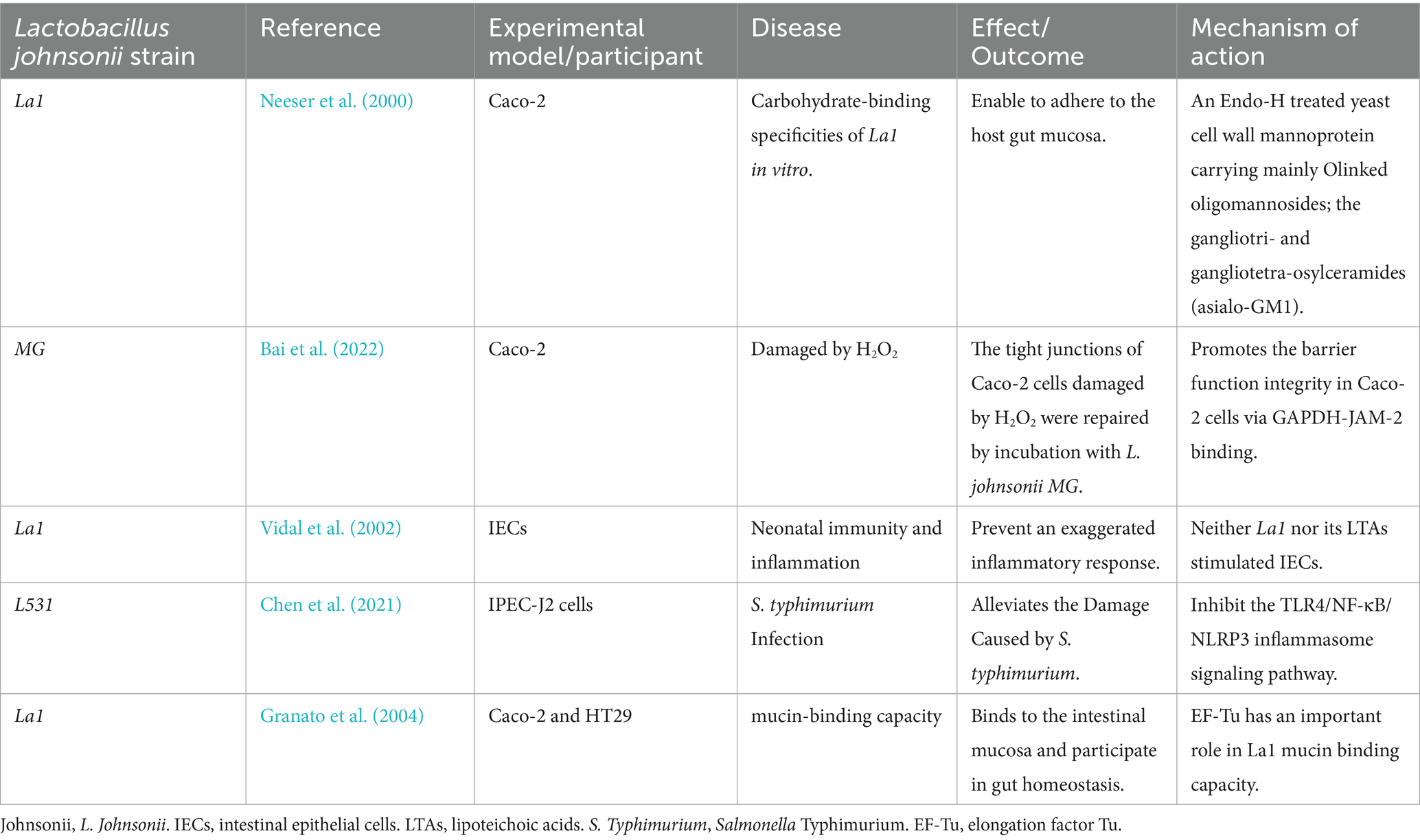
Table 2. Therapeutic efficacy and potential mechanisms of Lactobacillus johnsonii strains in various of the digestive diseases in cell models.
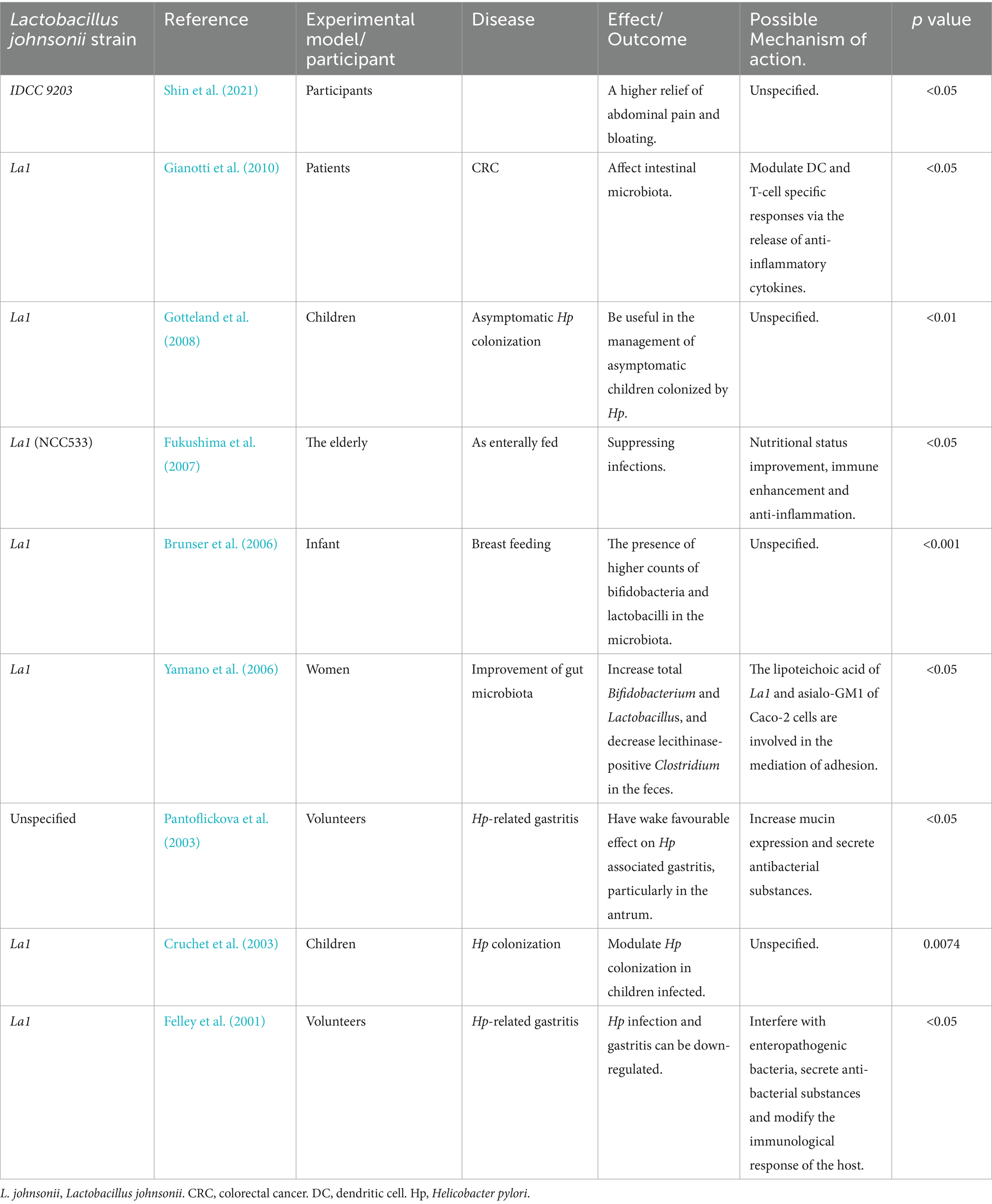
Table 3. Therapeutic efficacy and potential mechanisms of L. johnsonii strains in various diseases of the digestive system in humans.
It is important to emphasize that the effects of probiotics are highly strain-specific. Even among different strains of L. johnsonii, there may be significant differences in their physiological properties, metabolites, host interaction mechanisms, and ultimate health effects. The results of the studies cited in this review are based on strain-specific experimental models or clinical observations unless otherwise stated, and the results cannot be simply extrapolated to other L. johnsonii strains or the species as a whole.
2 Infection-associated intestinal diseases
2.1 The infection of Salmonella
Infection-associated intestinal diseases caused by Salmonella lead to inflammation-induced dysbiosis (Gresse et al., 2017; Gillis et al., 2018). Microbiota-derived short-chain fatty acids (SCFAs) promote the growth of regulatory T cells and have anti-inflammatory effects (Atarashi et al., 2013). Microbiota-derived butyrate works with regulatory T (Treg) cells to activate epithelial cells via peroxisome-activated receptor-γ (PPARγ) signaling (Byndloss et al., 2017). This activation impacts colonocyte metabolism, which is essential for restricting oxygen availability and maintaining the dominance of anaerobic bacteria. In newly weaned piglets, Salmonella infantis infection has a detrimental effect on gut microbiota diversity, leading to enteritis, diarrhea, fever, and stunted growth (Byndloss et al., 2017; Zhang et al., 2017). Salmonella triggers intestinal inflammation, causing an increase in nitrate and oxygen concentrations. This, in turn, depletes SCFAs and creates a favorable environment for Salmonella growth, ultimately resulting in severe diarrhea (Drumo et al., 2015; Byndloss et al., 2017). It has been proven that L. johnsonii N5 and N7 have antagonistic effects against Salmonella with a high survival rate in acidic and bile environments (Wang et al., 2024).
Pretreatment of piglets with L. johnsonii L531 reduces Salmonella-induced diarrhea and limit the overgrowth of intestinal bacteria. As aerotolerant anaerobes, Lactobacillus species, including L. johnsonii L531, can tolerate oxygen briefly and modify gut microbiota composition and SCFA production (Derrien and van Hylckama Vlieg, 2015). Oral administration of L. johnsonii L531 to newly weaned piglets decreases Salmonella colonization in colonic and jejunal contents and accelerates Salmonella clearance in feces after infection. It also ameliorates SCFA depletion caused by S. infantis infection (He et al., 2019). L. johnsonii L531 inhibits ER stress and the production of lipocalin 2 in intestinal mucosa, while promoting CCR6 + T-cell responses. Pretreatment increases the proportion of CD4 + CCR6 + T cells in mesenteric lymph nodes, protecting against intestinal inflammation (Zhang et al., 2017; Yang et al., 2020). A research team found that L. johnsonii L531 ameliorates S. infantis-associated enteritis by regulating inflammasomes and NF-κB-SQSTM1 mitophagy signaling pathway and suppressing mitochondrial damage (Xia et al., 2020). It also attenuates intestinal structural and cellular ultrastructural damage. L. johnsonii L531 protects against S. infantis-induced intestinal damage by regulating NOD activation, ER stress, and autophagy (Yang et al., 2022), while NOD1 and NOD2 activate NF-κB through RIP2, promoting intestinal inflammation (Keestra et al., 2011; Xia et al., 2020; Chen et al., 2021), or inhibiting ER stress correlated with ATG16L1 and IRGM expression (Huang and Brumell, 2014; García-Gil et al., 2018; Yang et al., 2022). The specific mechanism is shown in Figure 1.
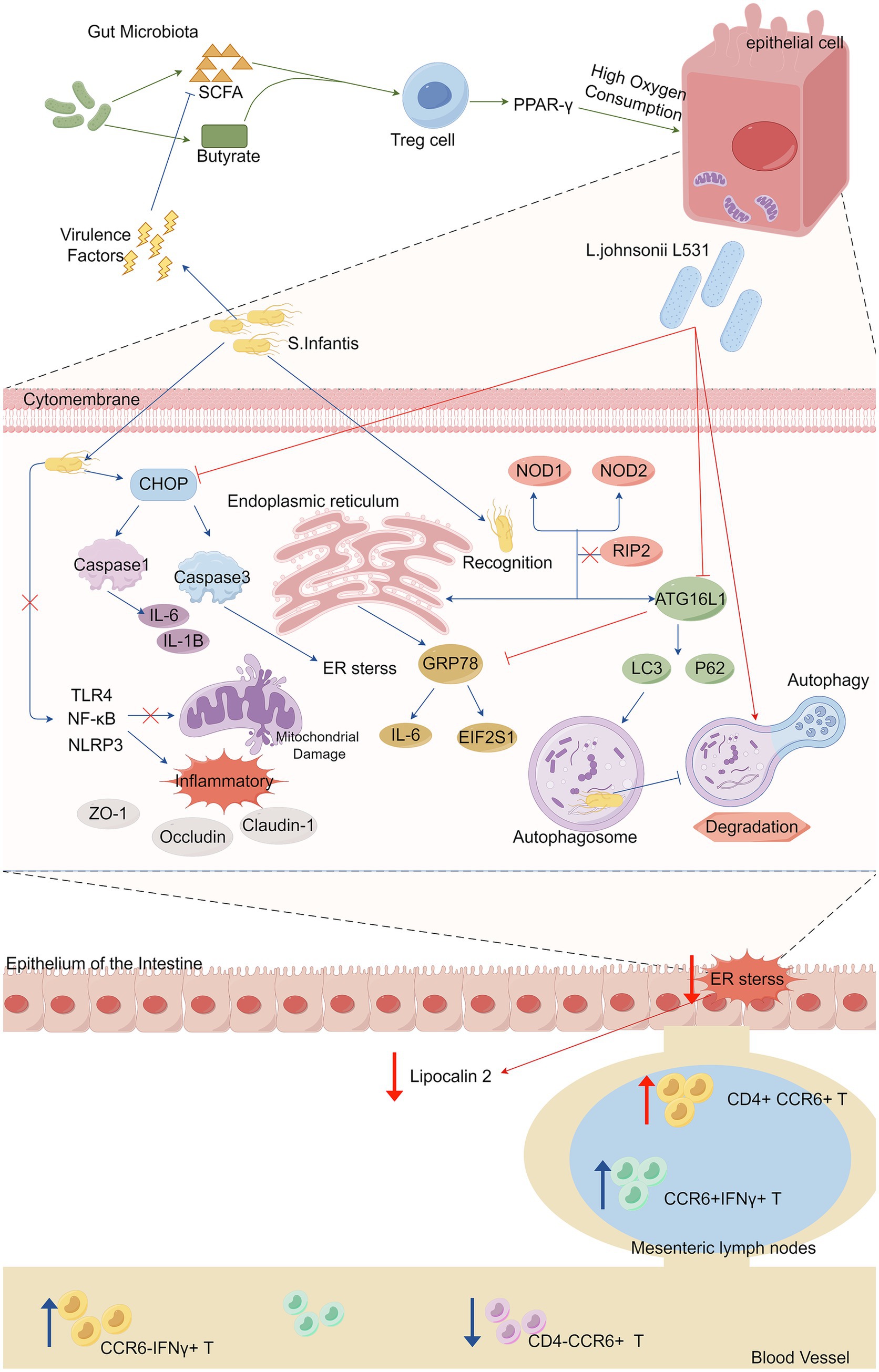
Figure 1. Microbiota-derived short-chain fatty acids (SCFAs) promote the maturation and expansion of Treg cells in the gut and exert profound anti-inflammatory effects, activating intestinal epithelial cells through PPAR-γ signaling to drive high oxygen-consuming metabolism. Salmonella use their virulence factors to trigger intestinal inflammation by depleting the action of commensal bacteria that produce (SCFAs), subsequently reducing the concentration of SCFAs and promoting Salmonella growth. Salmonella infantis infection activated both the NOD pathway and ER stress in vivo and in vitro, and Salmonella typhimurium augmented the inflammatory response by activating the TLR 4/NF-κB/NLRP 3 inflammasome signaling pathway (activation of which has also been linked to the inhibition of mitochondrial damage), which in turn reduced the expression levels of the tight junction proteins ZO-1, Occludin and Claudin-1. Salmonella infantis infection significantly increased the expression of ATG16L1 and IRGM in enterocytes, induced the accumulation of LC3 protein, which promotes autophagy, and utilized autophagy and ER stress to promote their immune escape and multiplication. CHOP is the most important pro-apoptotic transcription factor during endoplasmic reticulum stress because it activates caspase 3. CHOP is a key mediator of the maturation process of IL-1B via key mediator of caspase 1 activation, thereby inducing cell death in a mitochondria-dependent pathway. L. johnsonii modulates IRE 1A/CHOP and ATF 6A/CHOP signaling to abrogate ER stress-associated apoptosis, promotes ER homeostasis and autophagosome degradation, and inhibits overactivation of associated inflammatory signaling pathways, reduces overexpression of inflammatory cytokines, and contributes to the maintenance of tight junction integrity. In mesenteric lymph nodes, the proportion of CCR 6+ IFNγ+ T cells was elevated after stimulation by Salmonella infantis, and L. johnsonii L531 induced an increase in the proportion of CD 4+CCR 6+ T cells. Salmonella infantis attack decreased the percentage of CD 4− CCR 6 + T cells but increased the percentage of CCR 6− IFNγ+ T cells in peripheral blood. L531, Lactobacillus johnsonii L531. SCFA, short-chain fatty acids. Treg cell, regulatory T cell. S, Infantis. Salmonella Infantis. IL-6, Interleukin-6. IL-1B, interleukin-1B. ER stress, endoplasmic reticulum stress. CCR 6, CC-chemokine ligand.
In conclusion, L. johnsonii L531 effectively protects against Salmonella-induced intestinal inflammation by restoring microbiota balance, preserving SCFA levels, and modulating immune responses. Its regulatory role in ER stress, mitophagy, and NOD signaling highlights its therapeutic potential. These findings support L. johnsonii as a promising candidate for preventing infection-associated enteritis.
2.2 The other inflammation of intestine
Lactobacillus johnsonii has demonstrated the ability to inhibit intestinal inflammation instigated by other bacterial infections. For instance, L. johnsonii L531 effectively curbs robust inflammatory responses and diminishes the overexpression of cytokines, thereby safeguarding the integrity of tight junctions that are challenged by Salmonella Typhimurium (S. typhimurium), as shown in Figure 1 (Yang et al., 2022). L. johnsonii N5 promotes the expansion of MHCII+ and CD103 + dendritic cell populations within intestinal Peyer’s patches, augments the number of Treg cells, and mitigates colitis (Yuan et al., 2023). Compared to Escherichia coli (E. coli), L. johnsonii recolonization leads to higher numbers of CD4 + and CD8 + cells in the small intestine and spleen, indicating its potential to restore immune functions (Ekmekciu et al., 2017). Additionally, L. johnsonii NJ13 and L. johnsonii L531 are capable of suppressing the secretion of pro-inflammatory cytokines and inhibiting the activity of the Toll-like receptors 3 (TLR3) inflammasome (Wang et al., 2015; Zou et al., 2020).
The lipoteichoic acids (LTAs) derived from L. johnsonii La1 impede the soluble CD14 (sCD14)-mediated response of intestinal epithelial cells (IECs) to lipopolysaccharide (LPS) (Vidal et al., 2002). In addition, elongation factor Tu (EF-Tu), a novel L. johnsonii La1 bonding factor, plays an important role in the mucin-binding capacity of L. johnsonii La1. EF-Tu recombinant protein induces a proinflammatory response in the presence of soluble CD14 (Granato et al., 2004). L. johnsonii La1 also has probiotic properties enhancing gut microbiota (Yamano et al., 2006). Researches have demonstrated La1 could enhance the nutritional and immunological status of the elderly, suppressing infections (Fukushima et al., 2007), and La1 might be beneficial in preventing bacterial translocation in cirrhosis (Chiva et al., 2002), mainly attributed to the antioxidant properties of vitamin C and glutamate (Soriano et al., 2012). Cloned and expressed Bile-Salt-Hydrolase (BSH) genes from La1 exhibited anti-Giardia activities (Allain et al., 2017). Another finding has demonstrated that BaWeiBaiDuSan can promote the growth of L. johnsonii and its treatment for sepsis-induced liver injury. The underlying mechanism may involve L. johnsonii-mediated immune regulation and the production of interleukin-10 (IL-10)(+) M2 macrophages (Fan et al., 2023).
The regulation of gut microbiota is crucial for combating intestinal bacterial infections and reducing inflammation. Additionally, studies have shown the role of L. johnsonii 6,084 in reducing sepsis mortality by rebalancing gut microbiota (Han et al., 2022). And the beneficial effects of L. johnsonii YH1136 in protecting against intestinal dysfunction in high-altitude environments have been revealed (Wan et al., 2022). Prophylactic L. johnsonii treatment ameliorates Campylobacter jejuni (C. jejuni)-induced colonic apoptosis and alleviates pro-inflammatory mediator secretion (Bereswill et al., 2017). L. johnsonii protects against intestinal inflammation caused by Citrobacter rodentium (C. rodentium) by suppressing immune cell infiltration, cytokine secretion, and ER stress-related apoptosis, and regulates IRE1A/CHOP and ATF6A/CHOP signaling pathways (Bereswill et al., 2017; Zhang et al., 2021). Additionally, L. johnsonii induce fungal cell wall degradation, and inhibit Candida growth (Charlet et al., 2020). In combination with other probiotics, L. johnsonii contributes to enhancing the diversity and composition of the gut microbiota. This results in a reduction in the abundance of harmful bacteria and alleviates diarrhea induced by enterohaemorrhagic Escherichia coli (EHEC) (Hu et al., 2021). Moreover, pre-supplementation of Leuconostoc pseudomesenteroides and L. johnsonii mitigates gut microbiota dysbiosis and diarrhea triggered by enteroinvasive Escherichia coli (EIEC) (Wang et al., 2019). L. johnsonii derived extracellular vesicles (LJ-EVs) might shut down ERK and NLRP3 activation in intestinal epithelial cells in order to activate M2 macrophages which mitigated the adverse impacts induced by ETEC K88 (Tao et al., 2025). These findings suggest the potential of L. johnsonii in regulating gut microbiota homeostasis for intestinal health.
Necrotizing enterocolitis (NEC), a severe intestinal inflammatory disorder in preterm infants, significantly contributes to neonatal morbidity and mortality (Hull et al., 2014; Gupta and Paria, 2016). A study explored the potential of three Lactobacilli species, namely L. murinus, L. acidophilus, and L. johnsonii, in relation to NEC. The findings revealed that L. johnsonii did not offer protection against NEC (Isani et al., 2018). Another research indicated that the supplementation with Lactobacillus rhamnosus and L. johnsonii failed to elicit predictable alterations in the fecal microbiota or the overall growth of children afflicted with short bowel syndrome (SBS) (Piper et al., 2020).
Lactobacillus johnsonii exhibits strong potential in mitigating intestinal inflammation caused by diverse pathogenic infections through barrier protection, immune modulation and microbiota regulation. However, its variable efficacy in diseases like NEC and SBS highlights the need for more targeted investigations. Future research should focus on elucidating strain-specific mechanisms, understanding host-dependent responses, and characterizing active metabolites such as SCFAs and extracellular vesicles. Integrating multi-omics technologies will help uncover precise host–microbe interactions, while well-designed clinical trials are essential to validate its safety and therapeutic efficacy. A deeper mechanistic understanding will be key to translating the experimental promise of L. johnsonii into therapeutic reality.
Considerable variability among strains further complicates the interpretation of L. johnsonii’s role in NEC prevention. The article highlights that different Lactobacillus species or even strains within the same genus exhibit markedly distinct effects. For example, while L. murinus HF12 was associated with reduced NEC severity, L. johnsonii itself failed to demonstrate comparable protective benefits in similar experimental models. These findings suggest that significant functional differences exist at the strain level, which have not been fully addressed or standardized in clinical practice.
Collectively, these inconsistencies underscore the urgent need for future studies to implement precise strain identification and selection, alongside comprehensive characterization of probiotic properties. Furthermore, clinical trials should incorporate rigorous strain-level distinctions and consider host-specific factors to clarify the true therapeutic potential of L. johnsonii in NEC management and to reconcile the observed gap between experimental data and clinical outcomes.
2.3 Colorectal cancer
Several studies have examined the correlation between L. johnsonii and colorectal cancer (CRC), but findings may be limited. In a particular experimental study, the co-transplantation of L. johnsonii and Bifidobacterium animalis in ApcMin/+ mice that were concurrently administered 5-fluorouracil (5-FU) resulted in a discernible reduction in polyp burden (Yin et al., 2022). Another study showed that strain L. johnsonii La1, not BB536, adhered to colonic mucosa, reducing pathogen concentration and modulating local immunity via gut microbiota (Gianotti et al., 2010). The supplementation with either L. johnsonii or protocatechuic acid (PCA), a metabolite produced by L. johnsonii, effectively arrested the progression of chronic stress-induced CRC. This was accomplished by downregulating the expression of β-catenin. Moreover, PCA activated the cGMP pathway, thereby abrogating the detrimental impact of chronic stress on CRC (Cao et al., 2024). Despite these encouraging findings, more research is needed to confirm L. johnsonii’s preventive and treatment potential in CRC.
3 Inflammatory bowel disease
Changes in the gut microbiota can arise from shifts in bacterial community diversity or microbiota-host interactions, and these changes are directly linked to various diseases, particularly IBD, which encompasses Ulcerative Colitis (UC) and Crohn’s Disease (CD) (Hoarau et al., 2016). Numerous hypotheses have been put forward regarding the effects of probiotics on IBD: (1) Probiotics modulate the intestinal immune system through regulating the expression of pro-inflammatory cytokines (Liu et al., 2011). (2) Probiotics regulate the microbial balance of the gastrointestinal tract by inhibiting the growth of pathogens (Chen et al., 2013). (3) Probiotics enhance the function of the intestinal barrier by increasing the expression of tight junction proteins (Bai et al., 2022). Additionally, glycoconjugates from the Lactobacillus cell wall act as antigens that may serve as novel diagnostic biomarkers for IBD (Paściak et al., 2017). Biosynthesized poly-β-hydroxybutyrate (PHB) alleviates inflammation by regulating fucose residues to promote goblet cell differentiation and sulfomucin maturation, providing a favorable environment for L. johnsonii biofilm formation as seen in Figure 2 (Ma et al., 2022). The supernatant of L. johnsonii culture alleviated colitis and remodeled gut microbiota, with increased SCFA production inhibiting the MAPK signaling pathway and M1 macrophage polarization (Wu et al., 2023). L. johnsonii GLJ001 prevented dextran sulfate sodium (DSS)-induced colitis in mice by inhibiting M1 macrophage polarization via gut microbiota-SCFAs axis (Cai et al., 2025). Findings suggest that Butyrolactone-I (BTL-1) regulates intestinal flora, promotes the proliferation of L. johnsonii, safeguards the integrity of the intestinal barrier, increases the concentrations of butyric and propionic acids, and ultimately inhibits the activation of the MAPK signaling pathway in mice, thus alleviating IBD (Chen et al., 2025). In Muc2(−/−) mice, L. johnsonii demonstrates rapid growth, recovers to the initial population level and reduces the amount of Enterococcus faecalis (E. faecalis) (Makusheva et al., 2024). LJ-EVs can be directly internalized by intestinal epithelial cells and activate the Nrf2/HO-1 antioxidant signaling pathway, reducing endotoxin damage to cells and maintaining intestinal barrier homeostasis, which in turn alleviates the intestinal inflammation response (Li et al., 2024). There are fewer studies on LJ-EVs available. Future studies need to use sterile animal models or purified metabolite compartments to differentiate between the direct effects of Lactobacillus strains themselves and their metabolites or their potential synergistic effects. Additionally, research has revealed that β-Glucan ameliorates colitis via the L. johnsonii-indole-3-lactic acid (ILA)-aryl hydrocarbon receptor (AhR) axis (Zhang et al., 2024). Other studies have indicated that endoplasmic reticulum (ER) stress-related apoptosis is a crucial biological pathway in the pathophysiology of IBD (Cao et al., 2013; Ma et al., 2017). As shown in Figure 3. It has been demonstrated that L. johnsonii attenuated ER stress-related cell death (Zhang et al., 2021).
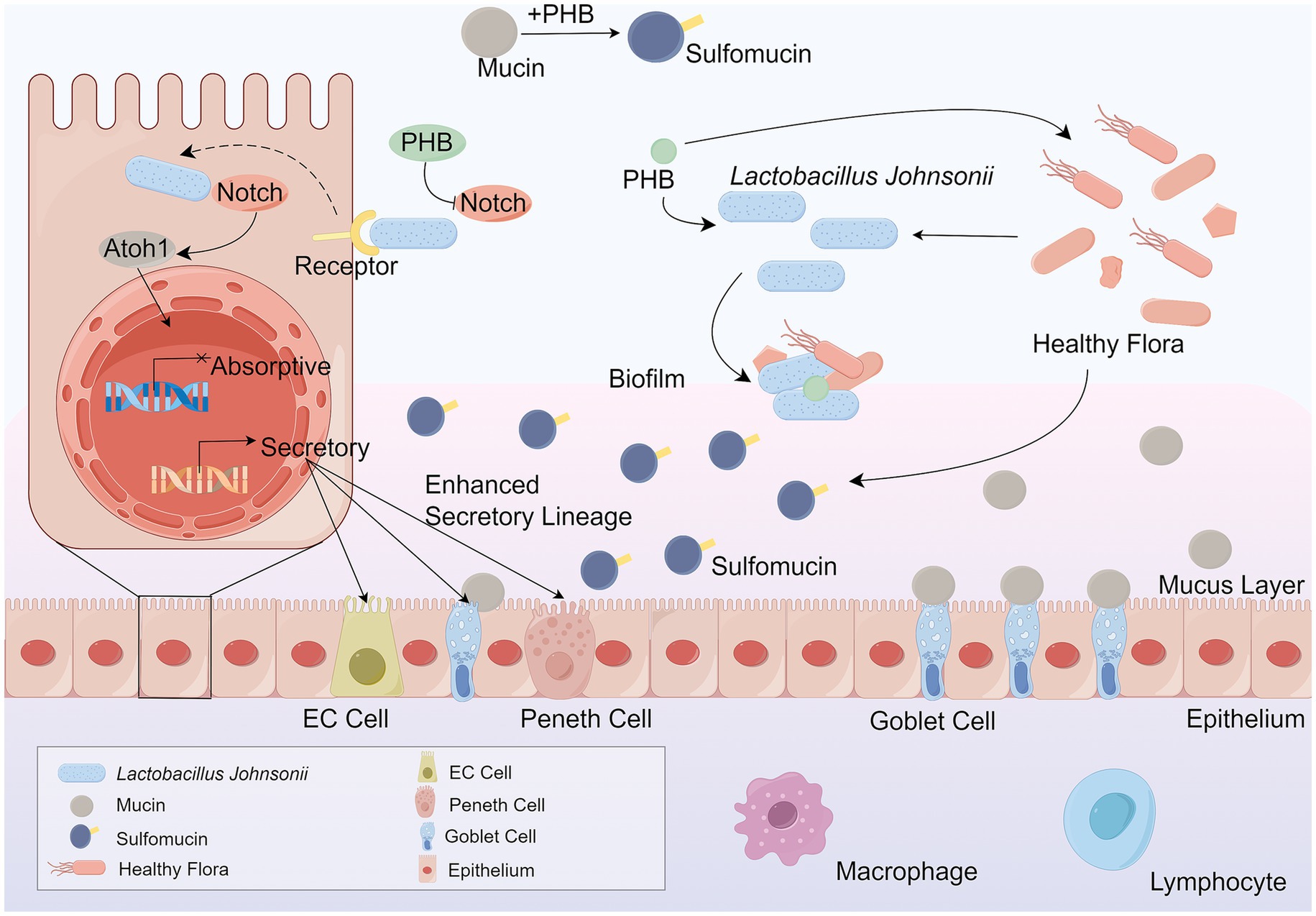
Figure 2. PHB mitigates diarrhea and colitis-associated inflammation by promoting the differentiation of goblet cells and enhancing sulfomucin production. This process is mediated by L. johnsonii, which utilizes PHB to establish a favorable ecological niche that supports biofilm formation and stabilizes its colonization in the intestinal mucosa. The biofilm formed by L. johnsonii enhances gut resilience by regulating fucose residue metabolism, thereby promoting goblet cell differentiation and sulfomucin maturation. Sulfomucins, as essential components of the mucus barrier, play a critical role in maintaining mucosal integrity and limiting pathogen invasion. Importantly, the anti-inflammatory effect of PHB is dependent on the presence of L. johnsonii, highlighting the synergistic relationship between microbial metabolites and beneficial bacteria in maintaining intestinal homeostasis. Through this pathway, PHB indirectly strengthens the mucosal immune barrier, attenuates colitis symptoms, and contributes to the host’s defense against gut inflammation. L. johnsonii, Lactobacillus johnsonii. PHB, poly-β-hydroxybutyrate. EC cell, enteroendocrine cell.
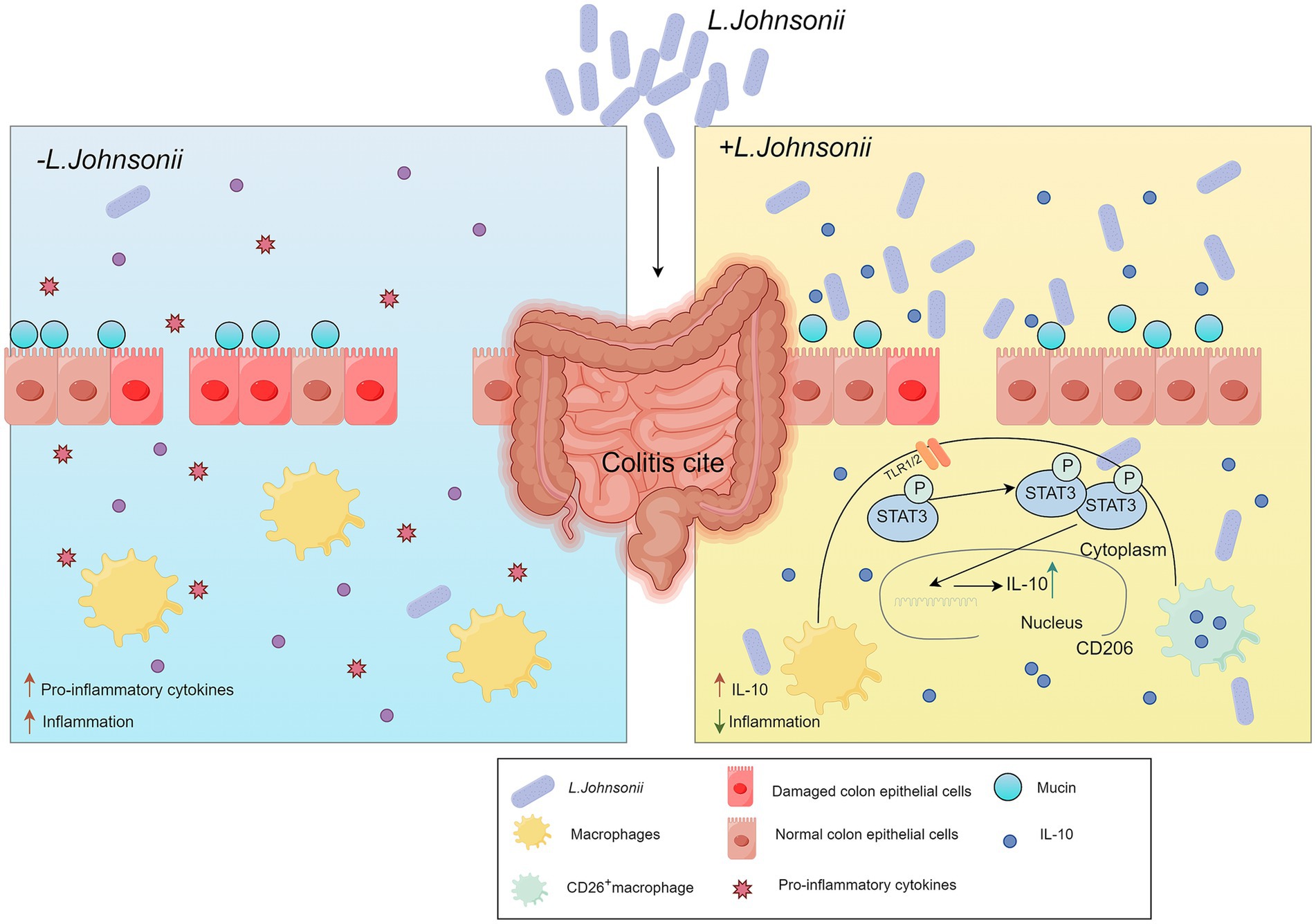
Figure 3. Schematic representation illustrating how resident L. johnsonii activates intestinal macrophages and promotes their polarization into CD206+ anti-inflammatory macrophages via the TLR1/2-STAT3 signaling pathway. This macrophage reprogramming enhances the secretion of IL-10, a key anti-inflammatory cytokine, thereby alleviating experimental colitis. L. johnsonii, Lactobacillus johnsonii. IL-10, interleukin-10.
UC is a subtype of IBD characterized by persistent, nonspecific inflammation of the rectum and colon (Danese and Fiocchi, 2011; Ordás et al., 2012). Mounting evidence has revealed a significant correlation between L. johnsonii and the severity of UC (He et al., 2022). Research indicates that L. johnsonii activates resident macrophages, reprogramming them into CD206 + macrophages via the TLR1/2-the Signal transducer and activator of Transcription3 (STAT3) signaling pathway. This activation elicits the release of IL-10, effectively alleviating experimental colitis (Jia et al., 2022). The product ID-JPL934 comprises L. johnsonii IDCC9203 (isolated from infant feces), which demonstrated the ability to the model for UC, including the constriction of the length of the colon, immune cell infiltration in the mucosa and submucosa, severe crypt damage, and loss of goblet and epithelial cells as observed in histological analysis (Je et al., 2018).
CD is another IBD that has seen a significant increase in incidence worldwide in recent years (Ng et al., 2017; Shouval and Rufo, 2017). Unlike UC, CD can affect the entire digestive tract, with transmural and segmental inflammation most commonly found in the terminal ileum and colon (Torres et al., 2017). Evidence suggests that the pathogenesis of CD is a result of gut inflammation caused by a combination of genetic and microbial factors, leading to abnormal immune regulation in the host (Ramos and Papadakis, 2019; Roda et al., 2020). Existing research suggests that the impact of L. johnsonii in the management of CD appears to be rather limited. The elemental diet (ED), widely utilized as an enteral nutritional intervention for CD, leads to a decrease in the population of L. johnsonii. This reduction is accompanied by a decline in the quantity and diversity of the intestinal microbiota, as well as a decrease in proinflammatory cytokines. Such changes are likely a consequence of alterations in the composition of lactic acid bacteria (Kajiura et al., 2009). A randomized, double-blind, placebo-controlled study, which spanned over 6 months, concluded that L. johnsonii LA1 was not significantly effective, if at all, in preventing endoscopic recurrence of CD (Marteau et al., 2006). Another multicenter randomized-controlled clinical trial had the similar result, which failed to prevent early endoscopic recurrence 12 weeks after ileo-colorectal resection (Van Gossum et al., 2007). The reasons for the ineffectiveness of L. johnsonii LA1 need to be further investigated. The potential influence of inter-individual differences in the patient’s gut microbiota may be a contributing factor.
The discrepancy between clinical and basic research findings may be influenced by multiple factors. First, strain-level variability should not be overlooked. Within the L. johnsonii species, substantial genomic diversity exists, leading to significant differences among strains in terms of metabolite production, colonization capacity, and immunomodulatory properties. Therefore, the anti-inflammatory effects demonstrated by certain strains in animal models or in vitro experiments may not be directly applicable to all clinical settings (Arzola-Martínez et al., 2024). Second, host-related factors also play a critical role. Individual differences among CD patients, including genetic background, gut microbiota composition, baseline inflammation levels, and postoperative recovery status, can affect the colonization efficiency and functional expression of L. johnsonii, thereby contributing to inconsistent intervention outcomes. Moreover, limitations in the design of existing clinical trials—such as short intervention durations, limited follow-up periods, and the lack of standardized dosages and delivery methods—may further obscure the potential long-term benefits of L. johnsonii.
In this context, future research should focus on precise strain selection, taking into account host-specific characteristics, and optimizing clinical trial protocols. Such efforts are essential to comprehensively evaluate the true therapeutic potential of L. johnsonii in CD management and to elucidate the fundamental reasons underlying the gap between mechanistic studies and therapeutic reality.
Psoriasis and IBD exhibit resemblances in terms of their commensal microbial communities and immune mechanisms. A prior investigation demonstrated that in the imiquimod (IMQ) mouse model (van der Fits et al., 2009; Van Belle et al., 2012; Terhorst et al., 2015), which is a murine model for psoriasis, the gut microbiome undergoes perturbations with reduced L. johnsonii and Lactobacillus reuteri (L. reuteri) populations (Kiyohara et al., 2019). This finding implies a potential connection between the skin microbiota and the gut microbiota in the context of psoriasis.
In summary, L. johnsonii exerts multifaceted regulatory effects on intestinal homeostasis in IBD, particularly by reshaping gut microbiota, strengthening barrier function, modulating immune responses, and alleviating inflammation through SCFA production and signaling pathway regulation. Despite promising results in UC models, its efficacy in Crohn’s disease and therapeutic reality s remains inconclusive. Future studies should prioritize clarifying strain-specific mechanisms, exploring microbial metabolite-host interactions, and investigating the role of L. johnsonii in extraintestinal diseases linked to gut dysbiosis, such as psoriasis.
4 Helicobacter pylori associated gastritis
H. pylori is a Gram-negative pathogen with spiral motility and ability to colonize acidic stomach. Chronic infection causes diseases, including chronic active gastritis, chronic atrophic gastritis, peptic ulcer and cancer. Previous studies have indicated that probiotics may hold potential in the treatment of H. pylori infection (Malfertheiner et al., 2002). L. johnsonii intake has a weak but favorable effect on associated gastritis (Pantoflickova et al., 2003), like L. johnsonii La1 strain reducing colonization in children (Felley et al., 2001; Cruchet et al., 2003; Gotteland et al., 2008).
Regarding the mechanism of L. johnsonii inhibition of H. pylori associated gastritis, the current study did the following. As mentioned earlier, the surface GroEL protein of L. johnsonii La1 has been identified to possess activities contributing to its probiotic characteristics. These activities include attachment to mucus and epithelial cells, stimulation of cytokine secretion from macrophages and epithelial cells, and the capacity to mediate the aggregation of the gastric pathogen H. pylori. L. johnsonii La1 GroEL binds to mucins and intestinal HT29 cells in a pH-dependent manner: while a strong binding capacity was observed at pH 5.0, no binding occurred at pH 7.2. The recombinant GroEL (rGroEL) protein of L. johnsonii La1 can induce the aggregation of H. pylori (Bergonzelli et al., 2006). An acidic pH environment induces stress-sensitive GroEL expression and increases L. johnsonii La1 death (Bergonzelli et al., 2006). In the early infection stages of H. pylori, L. johnsonii La1 can reduce gastritis reducing macrophage inflammatory protein 2 (MIP-2) and keratinocyte-derived cytokine (KC) in serum and mucosa. Neutralized L. johnsonii La1 spent culture supernatant reduces IL-8 secretion by human adenocarcinoma AGS cells, which may be through a reduction in pro-inflammatory chemotactic signals that recruit lymphocytes and neutrophils in the lamina propria (Sgouras et al., 2005). Both live and lyophilised strains of L. johnsonii No. 1088 (HK-LJ88) can inhibit H. pylori growth in mice, not through co-aggregation but an unknown mechanism involving cell surface molecules (Aiba et al., 2015; Aiba et al., 2017). Additionally, HK-LJ88 can inhibit gastric acid secretion by reducing gastrin-positive cells (Aiba et al., 2015). The GroEL protein of L. johnsonii La1 is a core effector for its gastric colonization and anti-Hp, which is distinct from other strains of Enterobacteriaceae such as L531 and BS15 in terms of molecular targeting. For example, LJ-EVs are important in activating the Nrf2/HO-1 antioxidant pathway induction, and M2 macrophage polarization. L531 alters the composition of the intestinal microbiota and the production of SCFA, which attenuates intestinal infections. However, the specific adhesion mechanism with intestinal epithelial cells has not been clearly elucidated. BS15, on the other hand, possesses major functions in regulating metabolism and strengthening the intestinal mucosal barrier. Future studies could focus on their differences in molecular targets or mechanisms of action.
Studies also showed that L. johnsonii 456 has higher viability at lower pH compared to other strains (Davoren et al., 2019), and proton pump inhibitors (PPIs) can reduce L. johnsonii and exacerbate indomethacin-induced small intestinal damage (Nadatani et al., 2019). Lactic acid from L. johnsonii plays a protective role in NSAID-induced small bowel injury. The discrepancy observed in these research outcomes can be ascribed to a diverse range of factors. For example, significant differences exist in assay results when examining the gut microbiota of mice versus humans. Additionally, variations in the bacterial homeostasis of the gastrointestinal mucosa among different individuals contribute to these discrepancies. These factors highlight the urgent need for further in-depth exploration, especially in identifying elements that foster the survival of gut microbiota and their adhesion to the gastrointestinal tract.
Based on the above, L. johnsonii exhibits strain-specific potential in mitigating H. pylori-induced gastritis by modulating host immune responses, interfering with pathogen adhesion, and influencing gastric environment via factors like GroEL protein and lactic acid production. While strains such as L. johnsonii La1 and No. 1088 have shown efficacy in reducing H. pylori colonization and associated inflammation, variations in outcomes across studies underscore the complexity of host–microbiota–pathogen interactions. Future research should focus on elucidating the molecular pathways underpinning these probiotic effects, optimizing strain viability under gastric conditions, and identifying host-specific factors influencing probiotic colonization and therapeutic efficacy. Personalized approaches integrating microbial and host biomarkers may ultimately improve probiotic strategies against H. pylori.
5 Liver disease
5.1 Alcoholic liver disease
Excessive alcohol consumption has long been recognized as a firmly established risk factor for the development of chronic liver disease (Cojocariu et al., 2014). Oxidative stress and lipid peroxidation are pivotal pathogenic mechanisms underpinning the formation of fatty liver (Wu and Cederbaum, 2003; Albano, 2006). Malondialdehyde (MDA) is a biomarker for oxidative stress. A study found that heat-killed L. johnsonii is capable of countering the elevation of alanine transaminase (ALT), aspartate transaminase (AST), gamma - glutamyl transferase (γ-GT), MDA, and triglyceride (TG) within the HepG2 cell line, which represents a human hepatocellular carcinoma cell line. In animal experiments, L. johnsonii effectively reduced γ-GT levels but had no significant impact on AST and ALT (Chuang et al., 2016). Heat-killed L. johnsonii upregulated intestinal lysozyme expression. This enhanced the production of immunoregulatory substances by gut bacteria, activating the Nucleotide-binding oligomerization domain 2 (NOD2)-interleukin (IL)-23-IL-22 innate immune axis. Elevated IL-22 increased antimicrobial peptide synthesis for intestinal homeostasis and activated STAT3 pathway in the liver, promoting hepatic injury repair (Yin et al., 2025).
5.2 Metabolic dysfunction-associated fatty liver disease
Non-alcoholic fatty liver disease (NAFLD), now re-designated as metabolic dysfunction-associated fatty liver disease (MAFLD) (Eslam et al., 2020), is a liver disorder of global prevalence that exhibited a robust association with obesity (Lazo et al., 2013). L. johnsonii BS15 (BS15), isolated from yogurt, has manifested beneficial effects in murine models. It can bring about a reduction in body weight, liver weight, and fat content. In hyperlipidemic mice, BS15 safeguards against hepatic steatosis and apoptosis. In MAFLD mice, BS15 mitigates mitochondrial abnormalities by diminishing the levels of uncoupling protein-2 and cytochrome c. Additionally, BS15 modulates intestinal permeability and the composition of the gut microbiota, thereby resulting in a decline in serum lipopolysaccharide and inflammatory factors (Xin et al., 2014). Another research investigation indicates that the combination of BS15 and abdominal massage treatment in MAFLD model rats can decrease cholesterol levels and liver injury indices, enhance intestinal permeability, fortify the intestinal mucosal barrier, and facilitate the repair of hepatocyte biofilms (Zhang et al., 2021).
Given the above, L. johnsonii exhibits promising hepatoprotective effects in both alcoholic and MAFLD. In alcoholic liver injury, heat-killed L. johnsonii can alleviate oxidative stress and modulate immune responses through the NOD2–IL-23–IL-22 axis, contributing to intestinal homeostasis and hepatic repair. In MAFLD models, the BS15 strain demonstrates metabolic benefits by reducing lipid accumulation, improving mitochondrial function, and strengthening the gut barrier. These findings underscore the strain-specific and multi-targeted potential of L. johnsonii in liver disease management. Future research should focus on clinical validation and exploring personalized probiotic interventions that account for host metabolic and microbial variability.
Notably, MAFLD often coexists with diabetes mellitus, and both share the core pathologic basis of insulin resistance and intestinal dysbiosis. Although the present study primarily focused on the role of L. johnsonii in a simple MAFLD model, its demonstrated ability to improve insulin sensitivity, regulate lipid metabolism, and repair the intestinal barrier and flora suggests its potential value as an adjunctive means to intervene in diabetes mellitus complicating MAFLD. However, the assessment of specific effects and microbiota regulatory capacity in this complex co-morbid state is uniquely challenging and requires subsequent specialized studies.
6 Obesity and lipid metabolism
Obesity and related metabolic disorders result from excessive caloric intake and insufficient physical activity, leading to fat accumulation and various diseases (Xu et al., 2003). Evidence shows probiotics, including Lactobacillus, help restore metabolic and immune function (Dahiya et al., 2017; Park et al., 2018, 2019). L. johnsonii, from human and animal intestines, has strains with probiotic properties (Fukushima et al., 2007; Buhnik-Rosenblau et al., 2012; Fonseca et al., 2017; Zheng et al., 2020). Its progress in obesity metabolism and fatty liver disease is as follows.
Lactobacillus johnsonii has anti-obesity and cholesterol-lowering effects. L. johnsonii JNU3402 (LJ3402), from Korean infant feces, is bile and acid resistant. In high-fat diet mice, non-viable LJ3402 was observed to reduce liver weight, as well as epididymal white adipose tissue (WAT), regulate triglyceride levels, and slightly improve insulin resistance. The anti - obesity effect of LJ3402 is mediated via the PPARγ pathway. This pathway not only enhances mitochondrial function within WAT but also promotes thermogenesis (Yang et al., 2020). Phycobiliproteins (derived from arthrospira platensis) bioactive peptide extracts (PPE) can increase L. johnsonii abundance and is a potential supplement for obesity (Liu et al., 2022). Another study linked hypercholesterolemia to liver cholesterol metabolism. L. johnsonii BFE6154 exerts its cholesterol - lowering effect by downregulating the expression of the Niemann-Pick C1-like 1 (NPC1L1) gene. It achieves this by activating the liver X receptor (LXR) in liver cells, thereby reducing cholesterol uptake. Furthermore, the metabolite of L. johnsonii BFE6154 stimulates the expression of the low density lipoprotein receptor (LDLR) gene in hepatocytes, facilitating enhanced cholesterol clearance. In the intestine, L. johnsonii BFE6154 upregulates the ABCG5 gene while downregulating the ABCG8 and NPC1L1 genes, effectively curbing intestinal cholesterol absorption. These findings have substantially enhanced our understanding of the complex cholesterol-lowering mechanisms of L. johnsonii (Yoon et al., 2023).
The high degree of co-morbidity between diabetes mellitus and MAFLD and their common pathophysiologic basis (insulin resistance, chronic low-grade inflammation, intestinal flora dysbiosis, intestinal barrier damage, and gut-hepatic axis disturbances). This overlapping of multiple pathologies creates more complex stresses on host metabolism and the intestinal microenvironment, challenging traditional monotherapies. L. johnsonii has shown potential in modulating obesity and related metabolic disorders such as insulin resistance, dyslipidemia (e.g., strain JNU3402, BFE6154). These metabolic benefits, together with its potential hepatoprotective effects, make it one of the candidate probiotics for exploring interventions in the obesity-diabetes-fatty liver “metabolic co-morbidity syndrome.” Strains like L. johnsonii with potential to modulate metabolism and the gut environment serve as potential adjunctive intervention strategies. Future studies need to deeply explore their effects and mechanisms in the superimposed state of multiple metabolic disorders.
In a word, L. johnsonii exhibits notable potential in combating obesity and related metabolic disorders through multiple mechanisms. Specific strains such as LJ3402 demonstrate anti-obesity effects by enhancing mitochondrial function and promoting thermogenesis via the PPARγ pathway, while BFE6154 exerts cholesterol-lowering activity by modulating key hepatic and intestinal genes involved in lipid absorption and metabolism. Additionally, dietary interventions like phycobiliprotein-derived peptides may further augment L. johnsonii abundance, offering a synergistic approach to metabolic regulation. These findings highlight the multifaceted metabolic benefits of L. johnsonii, warranting further investigation into its clinical efficacy and application in personalized metabolic therapies.
7 Other organs
7.1 Brain-gut axis
The gut microbiota is crucial for regulating brain function, especially in emotional processing and behavior (Dinan and Cryan, 2012; Steenbergen et al., 2015; Bharwani et al., 2016). There is growing evidence for a “microbe-gut-brain axis, “where gut microbiota influence central nervous system function by modulating neuroimmune function, neuronal signaling, and metabolic activity (Fang et al., 2020). The relationship between gut microbiota, including L. johnsonii, and brain function offers new approaches and potential for gut microbiota-based therapies for brain-related diseases.
Psychological stress can impair cognitive function (Sandi and Pinelo-Nava, 2007; Mo et al., 2013; Guo et al., 2017). A study shows BS15 can address stress-induced memory disorders via the brain-gut axis. BS15 pretreated group had increased trypsin and lipase activity, more cupped cells, and improved jejunum and ileum development. BS15 further led to a reduction in cytokine levels within the small intestine and regulated the expression of memory-related functional proteins. BS15 also restored antioxidant capacity and protected against mitochondria-mediated apoptosis in the hippocampus (Wang et al., 2020). It enhanced memory test performance under stress and regulated the hypothalamic–pituitary–adrenal axis (Wang et al., 2021). Excessive fluoride intake can cause chronic fluorosis and neurotoxicity (Queste et al., 2001). In mice exposed to high levels of fluoride, BS15 increased the number of goblet cells, decreased the secretion of sIgA, and protected against fluoride-induced intestinal damage and memory deficits (Sun et al., 2020). Chronic alcohol consumption disrupts intestinal microbes and impairs brain memory (Stärkel and Schnabl, 2016; Neyrinck et al., 2017; Scholey et al., 2019). BS15 increased EtOH metabolism, shortened the duration of alcohol-induced coma and blood alcohol concentration, and alleviated memory impairment (Sun et al., 2022). As illustrated in Figure 4. Furthermore, BS15 attenuated 2,4,6-trinitrobenzenesulfonic acid (TNBS) and E. coli-induced memory impairment and colitis and restored gut microbiota balance. Treatment with L. johnsonii reduced LPS levels and restored disrupted gut microbiota (Jang et al., 2018). Taken together, these findings strongly suggest that BS15 has the potential to ameliorate stress-related memory disorders and holds great promise for the treatment of diseases associated with memory impairment.
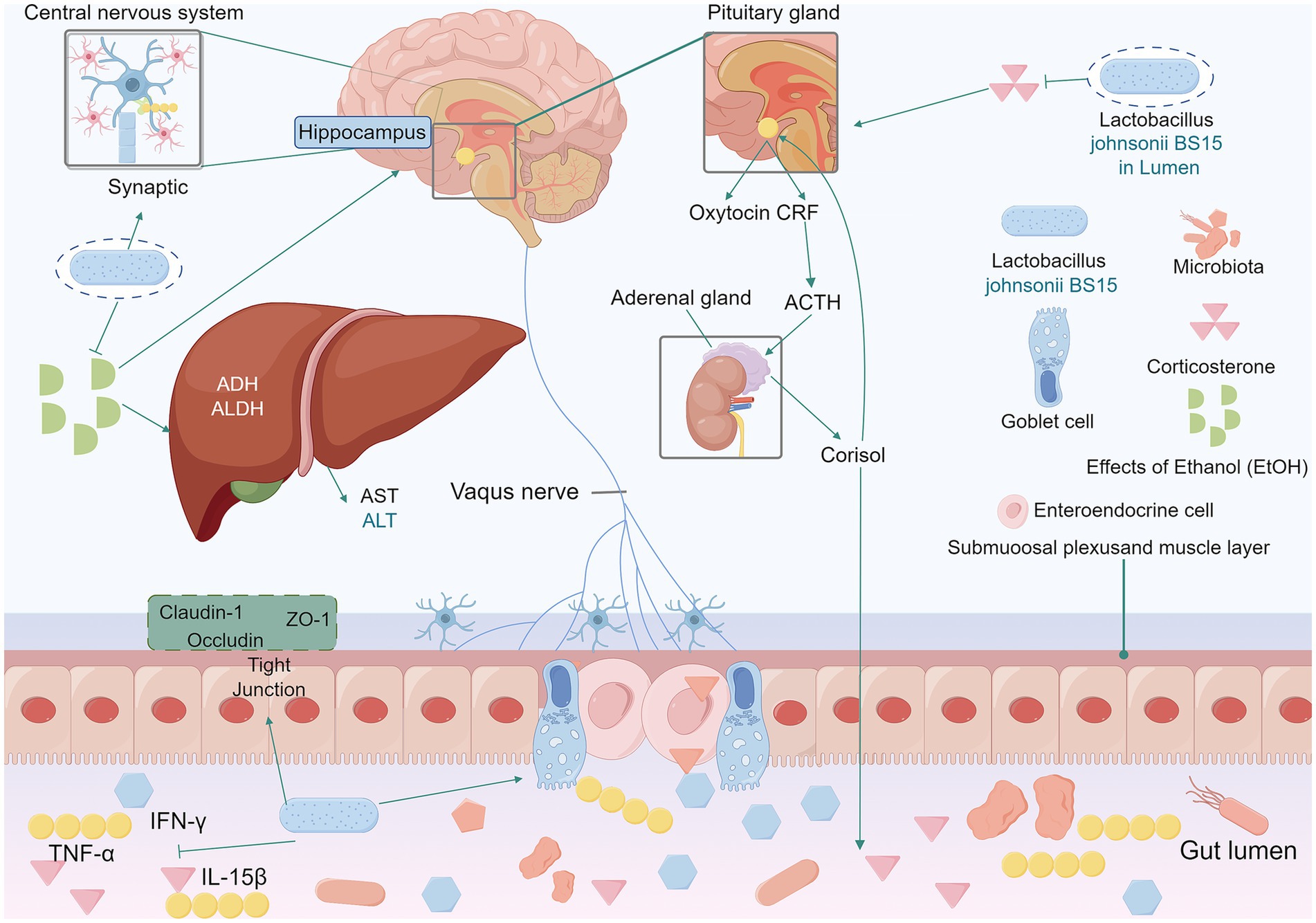
Figure 4. In mice exposed to high levels of fluoride, L. johnsonii BS15 increased the number of small intestinal cuprocytes and constituted an effective intestinal barrier against fluoride-induced intestinal injury. BS15 positively regulated the hypothalamo-pituitary–adrenal axis by lowering the serum corticosterone level. BS15 also reduced the levels of TNF-α, IFN-γ and IL-15β in the mouse small intestine. Enhanced gut development, modulat mRNA expression levels of the tight junction proteins claudin-1, occludin, and ZO-1. Lower gut microbiota, and blood LPS levelsIn the intestine, BS15 prevents intestinal barrier damage in mice by increasing mRNA levels of tight junction proteins and exerts a beneficial effect on reducing pro-inflammatory cytokine levels. BS15 improves the duration of ethanol resistance and the activities of ADH and aldehyde dehydrogenase ALDH in the liver of acute alcohol-induced mice. Increases ethanol metabolism levels to ameliorate alcohol-mediated memory impairment. L. johnsonii BS15, Lactobacillus johnsonii BS15. ADH, alcohol dehydrogenase. EtOH, effects of ethanol. AST, aspartate aminotransferase. ALT, alanine aminotransferase. ADH, alcohol dehydrogenase. ALDH, aldehyde dehydrogenase. TNF-α, tumor necrosis factor-α. IFN-γ, interferon gamma. IL-15β, Interleukin-15β.
There are studies on the specific serotonergic pathways within the brain-gut axis, vagus nerve-mediated mechanisms, etc. still lacking. Future studies should focus on finding more direct evidence of molecular interactions, such as changes in receptor expression or neurotransmitter levels.
7.2 Heart and lung
In addition to the brain-gut axis, L. johnsonii also plays a role in regulating the functions of other organs. The “gut-heart axis” is a novel concept that provides insights into the complex mechanisms of acute myocardial infarction (AMI) (Zununi Vahed et al., 2018). The intestinal barrier is a pivotal component in the communication network between the gut microbiota and the heart (Lewis and Taylor, 2020). A specific study has shown that a 28-day intervention with L. johnsonii effectively preserved cardiac function, retarded the development of cardiac pathological changes, suppressed the production of cytokines associated with myocardial injury, and enhanced the integrity of the intestinal barrier. Notably, this treatment also reshaped the gut microbiota, leading to a significant increase in the relative abundance of L. johnsonii (Zhong et al., 2023).
Accumulating experimental and epidemiological data highlight a strong connection between the gut microbiota and the lungs, which is commonly referred to as the “gut-lung axis” (Dang and Marsland, 2019). Published research has demonstrated that intranasal administration of L. johnsonii can confer protection against hyperoxia - induced lung injury and modulate the composition of the gut microbiota, suggesting a potential therapeutic strategy for lung-related disorders (Chen et al., 2023).
To sum up, L. johnsonii exerts beneficial effects beyond the gut, notably through the brain-gut, gut-heart, and gut-lung axes. Strain BS15 has demonstrated the ability to alleviate stress-induced memory impairment, counteract neurotoxicity from fluoride and alcohol exposure, and restore cognitive function by modulating the hypothalamic–pituitary–adrenal axis, enhancing insstestinal barrier integrity, and improving antioxidant defenses in the hippocampus. Additionally, L. johnsonii supports cardiovascular health by reinforcing the gut barrier, reducing myocardial inflammation, and preserving cardiac function post-AMI. In respiratory contexts, intranasal L. johnsonii administration mitigates hyperoxia-induced lung injury while positively shaping gut microbial composition. Collectively, these findings underscore the systemic regulatory capacity of L. johnsonii, suggesting promising therapeutic applications in neuroprotection, cardioprotection, and pulmonary health via microbiota-mediated pathways.
8 Summary and outlook
Lactobacillus johnsonii plays a critical role in gastrointestinal (GI) health through immunomodulatory mechanisms, reinforcement of intestinal barrier functionality, and attenuation of inflammatory responses. Although the specific effects vary depending on the strain and application context, this review reveals that the potential common mechanisms underlying the probiotic functions of L. johnsonii primarily include: regulating the composition and function of the gut microbiota (e.g., increasing beneficial bacteria and inhibiting pathogenic bacteria); enhancing or repairing the intestinal barrier (e.g., promoting the expression of tight junction proteins and increasing mucus secretion); modulating host immune responses (e.g., regulating cytokine secretion and influencing immune cell differentiation); producing bioactive metabolites (SCFAs, ILA, PCA, etc.); and mediating distant organ effects. However, translating preclinical and clinical findings into robust applications faces significant hurdles, including strain-specific variability in probiotic efficacy (e.g., divergent outcomes between L. johnsonii La1 and BS15 in IBD management), inconsistent clinical trial results (such as the failure of L. johnsonii La1 to prevent CD recurrence), and fragmented regulatory frameworks for validating health claims. These challenges underscore the need for strain-specific characterization, precised strain identification and functional validation, personalized probiotic strategies aligned with individual microbiota profiles, and harmonized safety and efficacy standards across jurisdictions. In summary, despite significant differences in the actions of different strains of L. johnsonii, the available research strongly suggests that the strain as a whole holds core effects on great potential to improve digestive health and associated systemic diseases through the multiple mechanistic modules mentioned above (flora modulation, barrier repair, immunomodulation, metabolite production, other organs) Potential. The positive effects (e.g., anti-infective, anti-inflammatory, metabolic regulation, neuroprotection) demonstrated by many strains in specific models or clinical situations provide an important basis and direction for further research and development of this strain.
Despite established anti-inflammatory mechanisms mediated through TLR/STAT3 signaling and ER stress modulation, critical gaps persist in elucidating the bioactive metabolites (e.g., propionic acid, bacteriocins) and host–microbe interactions underlying L. johnsonii’s effects. Current research is further constrained by overreliance on animal models and small-scale human trials, which may fail to recapitulate human pathophysiology or account for microbiome heterogeneity. Further, the potential impact of host genetic factors (e.g., polymorphisms in NOD2, TLR4) on strain efficacy remains worth exploring. In case the results of the study may be biased. Additionally, the interplay between L. johnsonii and comorbid conditions, such as metabolic disorders or mental health issues, remains underexplored, necessitating advanced multi-omics approaches to identify predictive biomarkers.
Lactobacillus johnsonii also has an interaction network with other major symbiotic bacteria, such as Bifidobacterium or Bifidobacterium. Charlet et al. (2020) found that L. johnsonii can co-secrete chitinase and mannosidase with Bacteroides thetaiotaomicron to degrade fungal cell walls. This significantly inhibits the overgrowth of Candida species and reduces the abundance of pathogenic bacteria such as E. coli and Enterococcus, thereby alleviating colitis symptoms in mice (Charlet et al., 2020). In addition to its local effects, L. johnsonii can also influence systemic diseases through microbiota remodeling. For example, in a rat model of AMI, L. johnsonii can increase the abundance of Muribaculaceae and Lactobacillus while decreasing the levels of potential pathogens such as Romboutsia and Clostridia, thereby improving cardiac function and demonstrating its potential to modulate the function of distal organs through the “gut-heart axis” (Zhong et al., 2023). In terms of competitive colonization, L. johnsonii can bind tightly to host epithelial cells via surface proteins, which may affect the ecological niches occupied by other microorganisms. The surface “moonlighting protein” GAPDH of L. johnsonii can bind to the tight junction protein JAM-2 of intestinal epithelial cells, thereby enhancing intestinal barrier function and providing an advantage for its stable colonization (Bai et al., 2022). In addition, this strain exhibits potent antimicrobial capabilities, inhibiting a variety of pathogenic microorganisms, including H.pylori, E. coli, Salmonella, and Clostridium difficile, thereby further altering the gut microbiota structure (Aiba et al., 2015).
Future research priorities include large-scale, double-blind randomized controlled trials (RCTs) to evaluate strain- and disease-specific efficacy in IBD subtypes, H. pylori eradication, and metabolic syndrome. Meanwhile, future therapeutic reality of L. johnsonii necessitate meticulous strain selection, as distinct strains (e.g., L. johnsonii La1, L531, BS15) exhibit divergent therapeutic effects across digestive diseases, underscoring the importance of strain-specific mechanistic studies and rigorous clinical validation to optimize probiotic efficacy and safety. Mechanistic studies should integrate genomics, proteomics, and metabolomics to map strain-specific pathways, such as GroEL-mediated pathogen aggregation or GAPDH-JAM-2 interactions in epithelial barrier repair. Concurrent efforts to optimize industrial-scale production through culture condition refinement or genetic engineering could enhance probiotic viability and functionality. Safety assessments in vulnerable populations (e.g., immunocompromised individuals, neonates) are imperative to address risks like horizontal gene transfer or opportunistic infections. Finally, interdisciplinary collaboration is essential to develop standardized guidelines for probiotic use, validate functional food formulations, and establish regulatory pathways that balance innovation with public health safeguards. By addressing these challenges, L. johnsonii may transition from a promising probiotic to a cornerstone of precision medicine in gastrointestinal and systemic health management.
In conclusion, L. johnsonii emerges as a clinically significant probiotic strain that demonstrates critical efficacy in human health maintenance, particularly in the context of gastrointestinal disorder management, while exhibiting substantial translational potential for therapeutic applications in digestive tract pathologies.
Further research should prioritize elucidating strain-dependent molecular pathways and conducting large-scale trials to establish tailored therapeutic strategies for targeted disease interventions.
Author contributions
JZ: Visualization, Writing – original draft. SM: Visualization, Writing – original draft. ZH: Visualization, Writing – original draft. QY: Conceptualization, Writing – review & editing. ZY: Conceptualization, Writing – review & editing. JC: Conceptualization, Writing – review & editing. LY: Conceptualization, Writing – review & editing. LZ: Conceptualization, Supervision, Writing – review & editing. XC: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Science and Technology Program of Tianjin (No. 21JCQNJC00990).
Acknowledgments
The authors would like to thank Home for Researchers for providing the Figdraw drawing platform for our schematic production.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aiba, Y., Ishikawa, H., Tokunaga, M., and Komatsu, Y. (2017). Anti-Helicobacter pylori activity of non-living, heat-killed form of lactobacilli including Lactobacillus johnsonii no. 1088. FEMS Microbiol. Lett. 364:102. doi: 10.1093/femsle/fnx102
Aiba, Y., Nakano, Y., Koga, Y., Takahashi, K., and Komatsu, Y. (2015). A highly acid-resistant novel strain of Lactobacillus johnsonii no. 1088 has antibacterial activity, including that against helicobacter pylori, and inhibits gastrin-mediated acid production in mice. Microbiology 4, 465–474. doi: 10.1002/mbo3.252
Albano, E. (2006). Alcohol, oxidative stress and free radical damage. Proc. Nutr. Soc. 65, 278–290. doi: 10.1079/PNS2006496
Allain, T., Chaouch, S., Thomas, M., Vallée, I., Buret, A. G., Langella, P., et al. (2017). Bile-salt-hydrolases from the probiotic strain Lactobacillus johnsonii La1 mediate anti-giardial activity in vitro and in vivo. Front. Microbiol. :82707. doi: 10.3389/fmicb.2017.02707
Arzola-Martínez, L., Ravi, K., Huffnagle, G. B., Lukacs, N. W., and Fonseca, W. (2024). Lactobacillus johnsonii and host communication insight into modulatory mechanisms during health and disease. Front. Microbiol. 2:330. doi: 10.3389/frmbi.2023.1345330
Atarashi, K., Tanoue, T., Oshima, K., Suda, W., Nagano, Y., Nishikawa, H., et al. (2013). Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236. doi: 10.1038/nature12331
Bai, Y., Lyu, M., Fukunaga, M., Watanabe, S., Iwatani, S., Miyanaga, K., et al. (2022). Lactobacillus johnsonii enhances the gut barrier integrity via the interaction between GAPDH and the mouse tight junction protein JAM-2. Food Funct. 13, 11021–11033. doi: 10.1039/D2FO00886F
Bereswill, S., Ekmekciu, I., Escher, U., Fiebiger, U., Stingl, K., and Heimesaat, M. M. (2017). Lactobacillus johnsonii ameliorates intestinal, extra-intestinal and systemic pro-inflammatory immune responses following murine Campylobacter jejuni infection. Sci. Rep. 7:2138. doi: 10.1038/s41598-017-02436-2
Bergonzelli, G. E., Granato, D., Pridmore, R. D., Marvin-Guy, L. F., Donnicola, D., and Corthésy-Theulaz, I. E. (2006). Gro EL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect. Immun. 74, 425–434. doi: 10.1128/IAI.74.1.425-434.2006
Bharwani, A., Mian, M. F., Foster, J. A., Surette, M. G., Bienenstock, J., and Forsythe, P. (2016). Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 63, 63217–63227. doi: 10.1016/j.psyneuen.2015.10.001
Brunser, O., Figueroa, G., Gotteland, M., Haschke-Becher, E., Magliola, C., Rochat, F., et al. (2006). Effects of probiotic or prebiotic supplemented milk formulas on fecal microbiota composition of infants. Asia Pac. J. Clin. Nutr. 15, 368–376. doi: 10.1016/j.appet.2005.10.003
Buhnik-Rosenblau, K., Matsko-Efimov, V., Jung, M., Shin, H., Danin-Poleg, Y., and Kashi, Y. (2012). Indication for co-evolution of Lactobacillus johnsonii with its hosts. BMC Microbiol. :12149. doi: 10.1186/1471-2180-12-149
Byndloss, M. X., Olsan, E. E., Rivera-Chávez, F., Tiffany, C. R., Cevallos, S. A., Lokken, K. L., et al. (2017). Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575. doi: 10.1126/science.aam9949
Cai, Y., Huang, Y., Wang, Y., Lin, C., Qiu, L., and Wei, H. (2025). Lactobacillus johnsonii GLJ001 prevents DSS-induced colitis in mice by inhibiting M1 macrophage polarization via gut microbiota-SCFAs axis. Int. Immunopharmacol. 144:113671. doi: 10.1016/j.intimp.2024.113671
Cao, Q., Zhao, M., Su, Y., Liu, S., Lin, Y., Da, H., et al. (2024). Chronic stress dampens Lactobacillus johnsonii-mediated tumor suppression to enhance colorectal cancer progression. Cancer Res. 84:705. doi: 10.1158/0008-5472.CAN-22-3705
Cao, S. S., Zimmermann, E. M., Chuang, B. M., Song, B., Nwokoye, A., Wilkinson, J. E., et al. (2013). The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology 144, 989–1000.e6. doi: 10.1053/j.gastro.2013.01.023
Charlet, R., Bortolus, C., Sendid, B., and Jawhara, S. (2020). Bacteroides thetaiotaomicron and Lactobacillus johnsonii modulate intestinal inflammation and eliminate fungi via enzymatic hydrolysis of the fungal cell wall. Sci. Rep. 10:11510. doi: 10.1038/s41598-020-68214-9
Chen, S., Li, Y., Chu, B., Yuan, L., Liu, N., Zhu, Y., et al. (2021). Lactobacillus johnsonii L531 alleviates the damage caused by Salmonella Typhimurium via inhibiting TLR4, NF-κB, and NLRP3 Inflammasome signaling pathways. Microorganisms 9:1983. doi: 10.3390/microorganisms9091983
Chen, S., Niu, X., Zhang, Y., Wen, J., Bao, M., Li, Y., et al. (2025). Butyrolactone-I from marine fungi alleviates intestinal barrier damage caused by DSS through regulating lactobacillus johnsonii and its metabolites in the intestine of mice. J. Nutr. Biochem. 135:109786. doi: 10.1016/j.jnutbio.2024.109786
Chen, C. M., Yang, Y., Chou, H. C., Yang, Y.-C. S. H., and Lin, S. (2023). Intranasal administration of Lactobacillus johnsonii attenuates hyperoxia-induced lung injury by modulating gut microbiota in neonatal mice. J. Biomed. Sci. 30:57. doi: 10.1186/s12929-023-00958-8
Chen, L. L., Zou, Y. Y., Lu, F. G., Li, F. J., and Lian, G. H. (2013). Efficacy profiles for different concentrations of Lactobacillus acidophilus in experimental colitis. World J. Gastroenterol. 19, 5347–5356. doi: 10.3748/wjg.v19.i32.5347
Chiva, M., Soriano, G., Rochat, I., Peralta, C., Rochat, F., Llovet, T., et al. (2002). Effect of Lactobacillus johnsonii La1 and antioxidants on intestinal flora and bacterial translocation in rats with experimental cirrhosis. J. Hepatol. 37, 456–462. doi: 10.1016/S0168-8278(02)00142-3
Chuang, C. H., Tsai, C. C., Lin, E. S., Huang, C. S., Lin, Y. Y., Lan, C. C., et al. (2016). Heat-killed Lactobacillus salivarius and Lactobacillus johnsonii reduce liver injury induced by alcohol in vitro and in vivo. Molecules 21:456. doi: 10.3390/molecules21111456
Cojocariu, C. E., Trifan, A. V., Gîrleanu, I., and Stanciu, C. (2014). Alcoholic liver disease--epidemiology and risk factors. Rev. Med. Chir. Soc. Med. Nat. Iasi. 118, 910–917
Cruchet, S., Obregon, M. C., Salazar, G., Diaz, E., and Gotteland, M. (2003). Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition 19, 716–721. doi: 10.1016/S0899-9007(03)00109-6
Cryan, J. F., and Dinan, T. G. (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712. doi: 10.1038/nrn3346
Dahiya, D. K., Renuka,, Puniya, M., Shandilya, U. K., Dhewa, T., Kumar, N., et al. (2017). Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: a review. Front. Microbiol. :8563. doi: 10.3389/fmicb.2017.00563
Danese, S., and Fiocchi, C. (2011). Ulcerative colitis. N. Engl. J. Med. 365, 1713–1725. doi: 10.1056/NEJMra1102942
Dang, A. T., and Marsland, B. J. (2019). Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 12, 843–850. doi: 10.1038/s41385-019-0160-6
Davoren, M. J., Liu, J., Castellanos, J., Rodríguez-Malavé, N. I., and Schiestl, R. H. (2019). A novel probiotic, Lactobacillus johnsonii 456, resists acid and can persist in the human gut beyond the initial ingestion period. Gut Microbes 10, 458–480. doi: 10.1080/19490976.2018.1547612
de Roos, N. M., and Katan, M. B. (2000). Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. Am. J. Clin. Nutr. 71, 405–411. doi: 10.1093/ajcn/71.2.405
Derrien, M., and van Hylckama Vlieg, J. E. (2015). Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 23, 354–366. doi: 10.1016/j.tim.2015.03.002
Dinan, T. G., and Cryan, J. F. (2012). Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 37, 1369–1378. doi: 10.1016/j.psyneuen.2012.03.007
Drumo, R., Pesciaroli, M., Ruggeri, J., Tarantino, M., Chirullo, B., Pistoia, C., et al. (2015). Salmonella enterica serovar typhimurium exploits inflammation to modify swine intestinal microbiota. Front. Cell. Infect. Microbiol. :5106. doi: 10.3389/fcimb.2015.00106
Ekmekciu, I., von Klitzing, E., Neumann, C., Bacher, P., Scheffold, A., Bereswill, S., et al. (2017). Fecal microbiota transplantation, commensal Escherichia coli and Lactobacillus johnsonii strains differentially restore intestinal and systemic adaptive immune cell populations following broad-spectrum antibiotic treatment. Front. Microbiol. :82430. doi: 10.3389/fmicb.2017.02430
Eslam, M., Newsome, P. N., Sarin, S. K., Anstee, Q. M., Targher, G., Romero-Gomez, M., et al. (2020). A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J. Hepatol. 73, 202–209. doi: 10.1016/j.jhep.2020.03.039
Fan, X., Mai, C., Zuo, L., Huang, J., Xie, C., Jiang, Z., et al. (2023). Herbal formula BaWeiBaiDuSan alleviates polymicrobial sepsis-induced liver injury via increasing the gut microbiota Lactobacillus johnsonii and regulating macrophage anti-inflammatory activity in mice. Acta Pharm. Sin. B 13, 1164–1179. doi: 10.1016/j.apsb.2022.10.016
Fan, Y., and Pedersen, O. (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71. doi: 10.1038/s41579-020-0433-9
Fang, P., Kazmi, S. A., Jameson, K. G., and Hsiao, E. Y. (2020). The microbiome as a modifier of neurodegenerative disease risk. Cell Host Microbe 28, 201–222. doi: 10.1016/j.chom.2020.06.008
Fekete, M., Lehoczki, A., Major, D., Fazekas-Pongor, V., Csípő, T., Tarantini, S., et al. (2024). Exploring the influence of gut-brain Axis modulation on cognitive health: a comprehensive review of prebiotics, probiotics, and Symbiotics. Nutrients 16:789. doi: 10.3390/nu16060789
Felley, C. P., Corthésy-Theulaz, I., Rivero, J. L., Rivero, J.-L. B., Sipponen, P., Kaufmann, M., et al. (2001). Favourable effect of an acidified milk (LC-1) on Helicobacter pylori gastritis in man. Eur. J. Gastroenterol. Hepatol. 13, 25–29. doi: 10.1097/00042737-200101000-00005
Fonseca, W., Lucey, K., Jang, S., Fujimura, K. E., Rasky, A., Ting, H. A., et al. (2017). Lactobacillus johnsonii supplementation attenuates respiratory viral infection via metabolic reprogramming and immune cell modulation. Mucosal Immunol. 10, 1569–1580. doi: 10.1038/mi.2017.13
Fukushima, Y., Miyaguchi, S., Yamano, T., Kaburagi, T., Iino, H., Ushida, K., et al. (2007). Improvement of nutritional status and incidence of infection in hospitalised, enterally fed elderly by feeding of fermented milk containing probiotic Lactobacillus johnsonii La1 (NCC533). Br. J. Nutr. 98, 969–977. doi: 10.1017/S0007114507764723
García-Gil, A., Galán-Enríquez, C. S., Pérez-López, A., Nava, P., Alpuche-Aranda, C., and Ortiz-Navarrete, V. (2018). SopB activates the Akt-YAP pathway to promote Salmonella survival within B cells. Virulence 9, 1390–1402. doi: 10.1080/21505594.2018.1509664
Gianotti, L., Morelli, L., Galbiati, F., Rocchetti, S., Coppola, S., Beneduce, A., et al. (2010). A randomized double-blind trial on perioperative administration of probiotics in colorectal cancer patients. World J. Gastroenterol. 16, 167–175. doi: 10.3748/wjg.v16.i2.167
Gillis, C. C., Hughes, E. R., Spiga, L., Winter, M. G., Zhu, W., Furtado de Carvalho, T., et al. (2018). Dysbiosis-associated change in host metabolism generates lactate to support Salmonella growth. Cell Host Microbe 23, 54–64.e6. doi: 10.1016/j.chom.2017.11.006
Gotteland, M., Andrews, M., Toledo, M., Muñoz, L., Caceres, P., Anziani, A., et al. (2008). Modulation of Helicobacter pylori colonization with cranberry juice and Lactobacillus johnsonii La1 in children. Nutrition 24, 421–426. doi: 10.1016/j.nut.2008.01.007
Granato, D., Bergonzelli, G. E., Pridmore, R. D., Marvin, L., Rouvet, M., and Corthésy-Theulaz, I. E. (2004). Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins. Infect. Immun. 72, 2160–2169. doi: 10.1128/IAI.72.4.2160-2169.2004
Gresse, R., Chaucheyras-Durand, F., Fleury, M. A., van de Wiele, T., Forano, E., and Blanquet-Diot, S. (2017). Gut microbiota Dysbiosis in Postweaning piglets: understanding the keys to health. Trends Microbiol. 25, 851–873. doi: 10.1016/j.tim.2017.05.004
Guo, Y., Sun, J., Li, T., Zhang, Q., Bu, S., Wang, Q., et al. (2017). Melatonin ameliorates restraint stress-induced oxidative stress and apoptosis in testicular cells via NF-κB/iNOS and Nrf2/HO-1 signaling pathway. Sci. Rep. 7:9599. doi: 10.1038/s41598-017-09943-2
Gupta, A., and Paria, A. (2016). Etiology and medical management of NEC. Early Hum. Dev. 97, 17–23. doi: 10.1016/j.earlhumdev.2016.03.008
Han, S., Zheng, H., Han, F., Zhang, X., Zhang, G., Ma, S., et al. (2022). Lactobacillus johnsonii 6084 alleviated sepsis-induced organ injury by modulating gut microbiota. Food Sci. Nutr. 10, 3931–3941. doi: 10.1002/fsn3.2989
He, C., Gao, M., Zhang, X., Lei, P., Yang, H., Qing, Y., et al. (2022). The protective effect of Sulforaphane on dextran sulfate sodium-induced colitis depends on gut microbial and Nrf2-related mechanism. Front. Nutr. 9:893344. doi: 10.3389/fnut.2022.893344
He, T., Zhu, Y. H., Yu, J., Xia, B., Liu, X., Yang, G. Y., et al. (2019). Lactobacillus johnsonii L531 reduces pathogen load and helps maintain short-chain fatty acid levels in the intestines of pigs challenged with Salmonella enterica Infantis. Vet. Microbiol. 230, 187–194. doi: 10.1016/j.vetmic.2019.02.003
Hoarau, G., Mukherjee, P. K., Gower-Rousseau, C., Hager, C., Chandra, J., Retuerto, M. A., et al. (2016). Bacteriome and Mycobiome interactions underscore microbial Dysbiosis in familial Crohn's disease. MBio 7:16. doi: 10.1128/mBio.01250-16
Hu, Y., Zhao, M., Lu, Z., Lv, F., Zhao, H., and Bie, X. (2021). L. johnsonii, L. plantarum, and L. rhamnosus alleviated Enterohaemorrhagic Escherichia coli-induced diarrhoea in mice by regulating gut microbiota. Microb. Pathog. 154:104856. doi: 10.1016/j.micpath.2021.104856
Huang, J., and Brumell, J. H. (2014). Bacteria-autophagy interplay: a battle for survival. Nat. Rev. Microbiol. 12, 101–114. doi: 10.1038/nrmicro3160
Hull, M. A., Fisher, J. G., Gutierrez, I. M., Jones, B. A., Kang, K. H., Kenny, M., et al. (2014). Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: a prospective cohort study. J. Am. Coll. Surg. 218, 1148–1155. doi: 10.1016/j.jamcollsurg.2013.11.015
Isani, M., Bell, B. A., Delaplain, P. T., Bowling, J. D., Golden, J. M., Elizee, M., et al. (2018). Lactobacillus murinus HF12 colonizes neonatal gut and protects rats from necrotizing enterocolitis. PLoS One 13:e0196710. doi: 10.1371/journal.pone.0196710
Jang, S. E., Lim, S. M., Jeong, J. J., Jang, H. M., Lee, H. J., Han, M. J., et al. (2018). Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal Immunol. 11, 369–379. doi: 10.1038/mi.2017.49
Je, I. G., Lee, D. G., Jeong, D. G., Hong, D., Yoon, J. M., Moon, J. S., et al. (2018). The probiotic, ID-JPL934, attenuates dextran sulfate sodium-induced colitis in mice through inhibition of Proinflammatory cytokines expression. J. Med. Food 21, 858–865. doi: 10.1089/jmf.2017.4152
Jia, D. J., Wang, Q. W., Hu, Y. Y., Cheng, J. D., He, J.-M., Ge, Q.-W., et al. (2022). Lactobacillus johnsonii alleviates colitis by TLR1/2-STAT3 mediated CD206(+) macrophages (IL-10) activation. Gut Microbes 14:2145843. doi: 10.1080/19490976.2022.2145843
Kajiura, T., Takeda, T., Sakata, S., Sakamoto, M., Hashimoto, M., Suzuki, H., et al. (2009). Change of intestinal microbiota with elemental diet and its impact on therapeutic effects in a murine model of chronic colitis. Dig. Dis. Sci. 54, 1892–1900. doi: 10.1007/s10620-008-0574-6
Keestra, A. M., Winter, M. G., Klein-Douwel, D., Xavier, M. N., Winter, S. E., Kim, A., et al. (2011). A Salmonella virulence factor activates the NOD1/NOD2 signaling pathway. MBio 2:266. doi: 10.1128/mBio.00266-11
Kiyohara, H., Sujino, T., Teratani, T., Miyamoto, K., Arai, M. M., Nomura, E., et al. (2019). Toll-like receptor 7 agonist-induced dermatitis causes severe dextran sulfate sodium colitis by altering the gut microbiome and immune cells. Cell. Mol. Gastroenterol. Hepatol. 7, 135–156. doi: 10.1016/j.jcmgh.2018.09.010
Lazo, M., Hernaez, R., Eberhardt, M. S., Bonekamp, S., Kamel, I., Guallar, E., et al. (2013). Prevalence of nonalcoholic fatty liver disease in the United States: the third National Health and nutrition examination survey, 1988-1994. Am. J. Epidemiol. 178, 38–45. doi: 10.1093/aje/kws448
Lewis, C. V., and Taylor, W. R. (2020). Intestinal barrier dysfunction as a therapeutic target for cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 319, H1227–H1233. doi: 10.1152/ajpheart.00612.2020
Li, Z., Li, M., Fang, X., Yu, D., and Hu, X. (2024). Dietary Lactobacillus johnsonii-derived extracellular vesicles ameliorate acute colitis by regulating gut microbiota and maintaining intestinal barrier homeostasis. Food Funct. 15, 11757–11779. doi: 10.1039/d4fo04194a
Liu, Y. W., Su, Y. W., Ong, W. K., Cheng, T. H., and Tsai, Y. C. (2011). Oral administration of Lactobacillus plantarum K68 ameliorates DSS-induced ulcerative colitis in BALB/c mice via the anti-inflammatory and immunomodulatory activities. Int. Immunopharmacol. 11, 2159–2166. doi: 10.1016/j.intimp.2011.09.013
Liu, J., Zhen, D., Hu, C., Liu, Y., Shen, X., Fu, P., et al. (2022). Reconfiguration of gut microbiota and reprogramming of liver metabolism with phycobiliproteins bioactive peptides to rehabilitate obese rats. Nutrients 14:635. doi: 10.3390/nu14173635
Ma, X., Dai, Z., Sun, K., Zhang, Y., Chen, J., Yang, Y., et al. (2017). Intestinal epithelial cell endoplasmic reticulum stress and inflammatory bowel disease pathogenesis: an update review. Front. Immunol. 8:81271. doi: 10.3389/fimmu.2017.01271
Ma, N., Guo, P., Chen, J., Qi, Z., Liu, C., Shen, J., et al. (2022). Poly-β-hydroxybutyrate alleviated diarrhea and colitis via Lactobacillus johnsonii biofilm-mediated maturation of sulfomucin. Sci. China Life Sci. 66:2213. doi: 10.1007/s11427-022-2213-6
Makusheva, Y., Goncharova, E., Bets, V., Korel, A., Arzhanova, E., and Litvinova, E. (2024). Restoration of Lactobacillus johnsonii and Enterococcus faecalis caused the elimination of Tritrichomonas sp. in a model of antibiotic-induced dysbiosis. Int. J. Mol. Sci. 25:5090. doi: 10.3390/ijms25105090
Malfertheiner, P., Mégraud, F., O'Morain, C., Hungin, A. P. S., Jones, R., Axon, A., et al. (2002). Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 consensus report. Aliment. Pharmacol. Ther. 16, 167–180. doi: 10.1046/j.1365-2036.2002.01169.x
Marteau, P., Lémann, M., Seksik, P., Laharie, D., Colombel, J. F., Bouhnik, Y., et al. (2006). Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn's disease: a randomised, double blind, placebo controlled GETAID trial. Gut 55, 842–847. doi: 10.1136/gut.2005.076604
Mo, C., Renoir, T., Pang, T. Y., Pang, T. Y. C., and Hannan, A. J. (2013). Short-term memory acquisition in female Huntington's disease mice is vulnerable to acute stress. Behav. Brain Res. 253:253318-22. doi: 10.1016/j.bbr.2013.07.041
Molodecky, N. A., Soon, I. S., Rabi, D. M., Ghali, W. A., Ferris, M., Chernoff, G., et al. (2012). Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001
Mu, Q., Tavella, V. J., and Luo, X. M. (2018). Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 9:9757. doi: 10.3389/fmicb.2018.00757
Nadatani, Y., Watanabe, T., Suda, W., Nakata, A., Matsumoto, Y., Kosaka, S., et al. (2019). Gastric acid inhibitor aggravates indomethacin-induced small intestinal injury via reducing Lactobacillus johnsonii. Sci. Rep. 9:17490. doi: 10.1038/s41598-019-53559-7
Neeser, J. R., Granato, D., Rouvet, M., Servin, A., Teneberg, S., and Karlsson, K. A. (2000). Lactobacillus johnsonii La1 shares carbohydrate-binding specificities with several enteropathogenic bacteria. Glycobiology 10, 1193–1199. doi: 10.1093/glycob/10.11.1193
Neyrinck, A. M., Etxeberria, U., Taminiau, B., Daube, G., van Hul, M., Everard, A., et al. (2017). Rhubarb extract prevents hepatic inflammation induced by acute alcohol intake, an effect related to the modulation of the gut microbiota. Mol. Nutr. Food Res. 61:899. doi: 10.1002/mnfr.201500899
Ng, S. C., Shi, H. Y., Hamidi, N., Underwood, F. E., Tang, W., Benchimol, E. I., et al. (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778. doi: 10.1016/S0140-6736(17)32448-0
Ordás, I., Eckmann, L., Talamini, M., Baumgart, D. C., and Sandborn, W. J. (2012). Ulcerative colitis. Lancet 380, 1606–1619. doi: 10.1016/S0140-6736(12)60150-0
Pantoflickova, D., Corthésy-Theulaz, I., Dorta, G., Stolte, M., Isler, P., Rochat, F., et al. (2003). Favourable effect of regular intake of fermented milk containing Lactobacillus johnsonii on Helicobacter pylori associated gastritis. Aliment. Pharmacol. Ther. 18, 805–813. doi: 10.1046/j.1365-2036.2003.01675.x
Park, S. S., Lee, Y. J., Kang, H., Yang, G., Hong, E. J., Lim, J. Y., et al. (2019). Lactobacillus amylovorus KU4 ameliorates diet-induced obesity in mice by promoting adipose browning through PPARγ signaling. Sci. Rep. 9:20152. doi: 10.1038/s41598-019-56817-w
Park, S. S., Lee, Y. J., Song, S., Kim, B., Kang, H., Oh, S., et al. (2018). Lactobacillus acidophilus NS1 attenuates diet-induced obesity and fatty liver. J. Endocrinol. 237, 87–100. doi: 10.1530/JOE-17-0592
Paściak, M., Górska, S., Jawiarczyk, N., and Gamian, A. (2017). Lactobacillus johnsonii glycolipids, their structure and immunoreactivity with sera from inflammatory bowel disease patients. Microb. Biotechnol. 10, 456–468. doi: 10.1111/1751-7915.12424
Peery, A. F., Crockett, S. D., Murphy, C. C., Jensen, E. T., Kim, H. P., Egberg, M. D., et al. (2022). Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2021. Gastroenterology 162, 621–644. doi: 10.1053/j.gastro.2021.10.017
Piper, H. G., Coughlin, L. A., Hussain, S., Nguyen, V., Channabasappa, N., and Koh, A. Y. (2020). The impact of Lactobacillus probiotics on the gut microbiota in children with short bowel syndrome. J. Surg. Res. 251, 112–118. doi: 10.1016/j.jss.2020.01.024
Queste, A., Lacombe, M., Hellmeier, W., Hillermann, F., Bortulussi, B., Kaup, M., et al. (2001). High concentrations of fluoride and boron in drinking water wells in the muenster region--results of a preliminary investigation. Int. J. Hyg. Environ. Health 203, 221–224. doi: 10.1078/S1438-4639(04)70032-2
Ramos, G. P., and Papadakis, K. A. (2019). Mechanisms of disease: inflammatory bowel diseases. Mayo Clin. Proc. 94, 155–165. doi: 10.1016/j.mayocp.2018.09.013
Ricciardiello, L. (2022). Digestive diseases: big burden, low funding? Results of the new united European gastroenterology white book on digestive diseases. United European Gastroenterol J 10, 627–628. doi: 10.1002/ueg2.12297
Roda, G., Chien Ng, S., Kotze, P. G., Argollo, M., Panaccione, R., Spinelli, A., et al. (2020). Crohn's disease. Nat. Rev. Dis. Primers 6:22. doi: 10.1038/s41572-020-0156-2
Rooks, M. G., and Garrett, W. S. (2016). Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352. doi: 10.1038/nri.2016.42
Roy, S., and Dhaneshwar, S. (2023). Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: current perspectives. World J. Gastroenterol. 29, 2078–2100. doi: 10.3748/wjg.v29.i14.2078
Sandi, C., and Pinelo-Nava, M. T. (2007). Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast. 2007, 1–20. doi: 10.1155/2007/78970
Scholey, A., Benson, S., Kaufman, J., Terpstra, C., Ayre, E., Verster, J. C., et al. (2019). Effects of alcohol hangover on cognitive performance: findings from a field/internet mixed methodology study. J. Clin. Med. 8:440. doi: 10.3390/jcm8040440
Sgouras, D. N., Panayotopoulou, E. G., Martinez-Gonzalez, B., Petraki, K., Michopoulos, S., and Mentis, A. (2005). Lactobacillus johnsonii La1 attenuates Helicobacter pylori-associated gastritis and reduces levels of proinflammatory chemokines in C57BL/6 mice. Clin. Diagn. Lab. Immunol. 12, 1378–1386. doi: 10.1128/CDLI.12.12.1378-1386.2005
Shin, C. M., Choi, Y. J., Lee, D. H., Moon, J. S., Kim, T. Y., Kim, Y. K., et al. (2021). Validity and safety of ID-JPL934 in lower gastrointestinal symptom improvement. Sci. Rep. 11:13046. doi: 10.1038/s41598-021-92007-3
Shouval, D. S., and Rufo, P. A. (2017). The role of environmental factors in the pathogenesis of inflammatory bowel diseases: a review. JAMA Pediatr. 171, 999–1005. doi: 10.1001/jamapediatrics.2017.2571
Soriano, G., Sánchez, E., Guarner, C., and Schiffrin, E. J. (2012). Lactobacillus johnsonii La1 without antioxidants does not decrease bacterial translocation in rats with carbon tetrachloride-induced cirrhosis. J. Hepatol. 57, 1395–1396. doi: 10.1016/j.jhep.2012.07.019
Stärkel, P., and Schnabl, B. (2016). Bidirectional communication between liver and gut during alcoholic liver disease. Semin. Liver Dis. 36, 331–339. doi: 10.1055/s-0036-1593882
Steenbergen, L., Sellaro, R., van Hemert, S., Bosch, J. A., and Colzato, L. S. (2015). A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 48, 258–264. doi: 10.1016/j.bbi.2015.04.003
Sun, N., Ni, X., Wang, H., Xin, J., Zhao, Y., Pan, K., et al. (2020). Probiotic Lactobacillus johnsonii BS15 prevents memory dysfunction induced by chronic high-fluorine intake through modulating intestinal environment and improving gut development. Probiotics Antimicrob. Proteins 12, 1420–1438. doi: 10.1007/s12602-020-09644-9
Sun, N., Zhu, B., Xin, J., Li, L., Gan, B., Cao, X., et al. (2022). Psychoactive effects of Lactobacillus johnsonii BS15 on preventing memory dysfunction induced by acute ethanol exposure through modulating intestinal microenvironment and improving alcohol metabolic level. Front. Microbiol. 13:13847468. doi: 10.3389/fmicb.2022.847468
Tao, S., Fan, J., Li, J., Wu, Z., Yao, Y., Wang, Z., et al. (2025). Extracellular vesicles derived from Lactobacillus johnsonii promote gut barrier homeostasis by enhancing M2 macrophage polarization. J. Adv. Res. 69, 545–563. doi: 10.1016/j.jare.2024.03.011
Terhorst, D., Chelbi, R., Wohn, C., Malosse, C., Tamoutounour, S., Jorquera, A., et al. (2015). Dynamics and transcriptomics of skin dendritic cells and macrophages in an Imiquimod-induced, biphasic mouse model of psoriasis. J. Immunol. 195, 4953–4961. doi: 10.4049/jimmunol.1500551
Torres, J., Mehandru, S., Colombel, J. F., and Peyrin-Biroulet, L. (2017). Crohn's disease. Lancet 389, 1741–1755. doi: 10.1016/S0140-6736(16)31711-1
Van Belle, A. B., de Heusch, M., Lemaire, M. M., Hendrickx, E., Warnier, G., Dunussi-Joannopoulos, K., et al. (2012). IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J. Immunol. 188, 462–469. doi: 10.4049/jimmunol.1102224
van der Fits, L., Mourits, S., Voerman, J. S., Kant, M., Boon, L., Laman, J. D., et al. (2009). Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845. doi: 10.4049/jimmunol.0802999
Van Gossum, A., Dewit, O., Louis, E., de Hertogh, G., Baert, F., Fontaine, F., et al. (2007). Multicenter randomized-controlled clinical trial of probiotics (Lactobacillus johnsonii, LA1) on early endoscopic recurrence of Crohn's disease after lleo-caecal resection. Inflamm. Bowel Dis. 13, 135–142. doi: 10.1002/ibd.20063
Vidal, K., Donnet-Hughes, A., and Granato, D. (2002). Lipoteichoic acids from Lactobacillus johnsonii strain La1 and Lactobacillus acidophilus strain La10 antagonize the responsiveness of human intestinal epithelial HT29 cells to lipopolysaccharide and gram-negative bacteria. Infect. Immun. 70, 2057–2064. doi: 10.1128/IAI.70.4.2057-2064.2002
Wan, Z., Zhang, X., Jia, X., Qin, Y., Sun, N., Xin, J., et al. (2022). Lactobacillus johnsonii YH1136 plays a protective role against endogenous pathogenic bacteria induced intestinal dysfunction by reconstructing gut microbiota in mice exposed at high altitude. Front. Immunol. 13:131007737. doi: 10.3389/fimmu.2022.1007737
Wang, H., He, S., Xin, J., Zhang, T., Sun, N., Li, L., et al. (2021). Psychoactive effects of Lactobacillus johnsonii against restraint stress-induced memory dysfunction in mice through modulating intestinal inflammation and permeability-a study based on the gut-brain Axis hypothesis. Front. Pharmacol. 12:12662148. doi: 10.3389/fphar.2021.662148
Wang, S. Y., Ho, Y. F., Chen, Y. P., and Chen, M. J. (2015). Effects of a novel encapsulating technique on the temperature tolerance and anti-colitis activity of the probiotic bacterium Lactobacillus kefiranofaciens M1. Food Microbiol. 46, 494–500. doi: 10.1016/j.fm.2014.09.015
Wang, Y., Li, A., Liu, J., Mehmood, K., Wangdui, B., Shi, H., et al. (2019). L. Pseudomesenteroides and L. johnsonii isolated from yaks in Tibet modulate gut microbiota in mice to ameliorate enteroinvasive Escherichia coli-induced diarrhea. Microb. Pathog., 1321–1329. doi: 10.1016/j.micpath.2019.04.020
Wang, H., Sun, Y., Xin, J., Zhang, T., Sun, N., Ni, X., et al. (2020). Lactobacillus johnsonii BS15 prevents psychological stress-induced memory dysfunction in mice by modulating the gut-brain Axis. Front. Microbiol. 11:111941. doi: 10.3389/fmicb.2020.01941
Wang, K., Wang, Y., Gu, L., Yu, J., Liu, Q., Zhang, R., et al. (2024). Characterization of probiotic properties and whole-genome analysis of Lactobacillus johnsonii N5 and N7 isolated from swine. Microorganisms 12:672. doi: 10.3390/microorganisms12040672
Wu, D., and Cederbaum, A. I. (2003). Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health 27, 277–284
Wu, Z., He, J., Zhang, Z., Li, J., Zou, H., Tan, X., et al. (2023). Propionic acid driven by the Lactobacillus johnsonii culture supernatant alleviates colitis by inhibiting M1 macrophage polarization by modulating the MAPK pathway in mice. J. Agric. Food Chem. 71, 14951–14966. doi: 10.1021/acs.jafc.3c00278
Xia, B., Yu, J., He, T., Liu, X., Su, J., Wang, M., et al. (2020). Lactobacillus johnsonii L531 ameliorates enteritis via elimination of damaged mitochondria and suppression of SQSTM1-dependent mitophagy in a Salmonella infantis model of piglet diarrhea. FASEB J. 34, 2821–2839. doi: 10.1096/fj.201901445RRR
Xie, M., Pan, M., Jiang, Y., Liu, X., Lu, W., Zhao, J., et al. (2019). Gro EL gene-based phylogenetic analysis of Lactobacillus species by high-throughput sequencing. Genes 10:530. doi: 10.3390/genes10070530
Xin, J., Zeng, D., Wang, H., Ni, X., Yi, D., Pan, K., et al. (2014). Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl. Microbiol. Biotechnol. 98, 6817–6829. doi: 10.1007/s00253-014-5752-1
Xu, H., Barnes, G. T., Yang, Q., Tan, G., Yang, D., Chou, C. J., et al. (2003). Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830. doi: 10.1172/JCI200319451
Yamano, T., Iino, H., Takada, M., Blum, S., Rochat, F., and Fukushima, Y. (2006). Improvement of the human intestinal flora by ingestion of the probiotic strain Lactobacillus johnsonii La1. Br. J. Nutr. 95, 303–312. doi: 10.1079/BJN20051507
Yang, G., Hong, E., Oh, S., and Kim, E. (2020). Non-viable Lactobacillus johnsonii JNU3402 protects against diet-induced obesity. Food Secur. 9:494. doi: 10.3390/foods9101494
Yang, L., Wang, J. F., Liu, N., Wang, X., Wang, J., Yang, G. H., et al. (2022). Lactobacillusjohnsonii L531 protects against Salmonella Infantis-induced intestinal damage by regulating the NOD activation, endoplasmic reticulum stress, and autophagy. Int. J. Mol. Sci. 23:395. doi: 10.3390/ijms231810395
Yang, G. Y., Xia, B., Su, J. H., He, T., Liu, X., Guo, L., et al. (2020). Anti-inflammatory effects of Lactobacillus johnsonii L531 in a pig model of Salmonella Infantis infection involves modulation of CCR6(+) T cell responses and ER stress. Vet. Res. 51:26. doi: 10.1186/s13567-020-00754-4
Yin, L., Huang, G., Khan, I., Su, L., Xia, W., Law, B. Y. K., et al. (2022). Poria cocos polysaccharides exert prebiotic function to attenuate the adverse effects and improve the therapeutic outcome of 5-FU in Apc (min/+) mice. Chin. Med. 17:116. doi: 10.1186/s13020-022-00667-8
Yin, R., Wang, T., Sun, J., Dai, H., Zhang, Y., Liu, N., et al. (2025). Postbiotics from Lactobacillus johnsonii activates gut innate immunity to mitigate alcohol-associated liver disease. Adv. Sci. 12:e2405781. doi: 10.1002/advs.202405781
Yoon, H., Lee, Y., Park, H., Kang, H. J., Ji, Y., and Holzapfel, W. H. (2023). Lactobacillus johnsonii BFE6154 ameliorates diet-induced hypercholesterolemia. Probiotics Antimicrob. Proteins 15, 451–459. doi: 10.1007/s12602-021-09859-4
Yuan, L., Zhu, C., Gu, F., Zhu, M., Yao, J., Zhu, C., et al. (2023). Lactobacillus johnsonii N5 from heat stress-resistant pigs improves gut mucosal immunity and barrier in dextran sodium sulfate-induced colitis. Anim. Nutr. 15, 210–224. doi: 10.1016/j.aninu.2023.04.012
Zhang, W., Li, H., Zhao, N., Luo, X., Liu, S., Bao,, et al. (2021). Lactobacillus johnsonii BS15 combined with abdominal massage on intestinal permeability in rats with nonalcoholic fatty liver and cell biofilm repair. Bioengineered 12, 6354–6363. doi: 10.1080/21655979.2021.1954134
Zhang, Y., Mu, T., Yang, Y., Zhang, J., Ren, F., and Wu, Z. (2021). Lactobacillus johnsonii attenuates Citrobacter rodentium-induced colitis by regulating inflammatory responses and endoplasmic reticulum stress in mice. J. Nutr. 151, 3391–3399. doi: 10.1093/jn/nxab250
Zhang, S., Nie, Q., Sun, Y., Zuo, S., Chen, C., Li, S., et al. (2024). Bacteroides uniformis degrades β-glucan to promote Lactobacillus johnsonii improving indole-3-lactic acid levels in alleviating colitis. Microbiome 12:177. doi: 10.1186/s40168-024-01896-9
Zhang, W., Zhu, Y. H., Yang, G. Y., Liu, X., Xia, B., Hu, X., et al. (2017). Lactobacillus rhamnosus GG affects microbiota and suppresses autophagy in the intestines of pigs challenged with Salmonella Infantis. Front. Microbiol. :82705. doi: 10.3389/fmicb.2017.02705
Zheng, J., Wittouck, S., Salvetti, E., Franz, C. M. A. P., Harris, H. M. B., Mattarelli, P., et al. (2020). A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 70, 2782–2858. doi: 10.1099/ijsem.0.004107
Zhong, X., Zhao, Y., Huang, L., Liu, J., Wang, K., Gao, X., et al. (2023). Remodeling of the gut microbiome by Lactobacillus johnsonii alleviates the development of acute myocardial infarction. Front. Microbiol. 14:141140498. doi: 10.3389/fmicb.2023.1140498
Zou, Y. J., Xu, J. J., Wang, X., Zhu, Y. H., Wu, Q., and Wang, J. F. (2020). Lactobacillus johnsonii L531 ameliorates Escherichia coli-induced cell damage via inhibiting NLRP3 Inflammasome activity and promoting ATG5/ATG16L1-mediated autophagy in porcine mammary epithelial cells. Vet. Sci. 7:112. doi: 10.3390/vetsci7030112
Zununi Vahed, S., Barzegari, A., Zuluaga, M., Letourneur, D., and Pavon-Djavid, G. (2018). Myocardial infarction and gut microbiota: an incidental connection. Pharmacol. Res. 129:129308-17. doi: 10.1016/j.phrs.2017.11.008
Glossary
5-FU - 5-fluorouracil
AhR - aryl hydrocarbon receptor
ALT - alanine transaminase
AMI - acute myocardial infarction
AST - aspartate transaminase
BS15 - L. johnsonii BS15
BSH - Bile-Salt-Hydrolase
BTL-1 - Butyrolactone-I
C. jejuni - Campylobacter jejuni
C. rodentium - Citrobacter rodentium
CD - Crohn’s Disease
CRC - colorectal cancer
DSS - dextran sulfate sodium
E. coli - Escherichia coli
E. faecalis - Enterococcus faecalis
EF-Tu - elongation factor Tu
ED - elemental diet
EHEC - enterohaemorrhagic Escherichia coli
EIEC - enteroinvasive Escherichia coli
ER - endoplasmic reticulum
GAPDH - glycerol-3-phosphate dehydrogenase
GI - gastrointestinal
H. pylori - Helicobacter pylori
HK-LJ88 - L. johnsonii No. 1088
IBD - inflammatory bowel disease
IECs - intestinal epithelial cells
IL - interleukin
ILA - indole-3-lactic acid
IMQ - imiquimod
KC - keratinocyte-derived cytokine
L. acidophilus - Lactobacillus acidophilus
L. johnsonii - Lactobacillus johnsonii
L. reuteri - Lactobacillus reuteri
LDLR - low density lipoprotein receptor
LJ3402 - L. johnsonii JNU3402
LJ-EVs - L. johnsonii derived extracellular vesicles
LPS - lipopolysaccharide
LTAs - lipoteichoic acids
LXR - liver X receptor
MAFLD - metabolic dysfunction-associated fatty liver disease
MAPK - Mitogen-Activated Protein Kinase
MDA - Malondialdehyde
MIP-2 - macrophage inflammatory protein 2
NAFLD - Non-alcoholic fatty liver disease
NEC - Necrotizing enterocolitis
NOD2 - Nucleotide-binding oligomerization domain 2
NPC1L1 - Niemann-Pick C1-like 1
PCA - protocatechuic acid
PHB - poly-β-hydroxybutyrate
PPARγ - peroxisome-activated receptor-γ
PPE - peptide extracts
PPIs - proton pump inhibitors
RCTs - randomized controlled trials
rGroEL - recombinant GroEL
S. typhimurium - Salmonella Typhimurium
SBS - short bowel syndrome
sCD14 - soluble CD14
SCFAs - short-chain fatty acids
STAT3 - the Signal transducer and activator of Transcription3
TG - triglyceride
TLR3 - Toll-like receptors 3
TNBS - 2,4,6-trinitrobenzenesulfonic acid
Treg - regulatory T
UC - Ulcerative Colitis
WAT - white adipose tissue
γ-GT - gamma - glutamyl transferase
Keywords: Lactobacillus johnsonii , digestive diseases, intestinal infections, gut microbiota, inflammatory bowel disease, brain-gut axis
Citation: Zhou J, Ma S, Huang Z, Yao Q, Yu Z, Chen J, Yao L, Zhu L and Chen X (2025) Unveiling the potential of Lactobacillus johnsonii in digestive diseases: a comprehensive review. Front. Microbiol. 16:1508382. doi: 10.3389/fmicb.2025.1508382
Edited by:
Renpeng Du, Heilongjiang University, ChinaReviewed by:
Anjani Devi Chintagunta, Vignan’s Foundation for Science, Technology and Research, IndiaSheng Song, University of North Carolina at Chapel Hill, United States
Iftikhar Younis Mallhi, Minhaj University Lahore, Pakistan
Guoli Chang, Zhejiang Gongshang University, China
Copyright © 2025 Zhou, Ma, Huang, Yao, Yu, Chen, Yao, Zhu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lanping Zhu, emh1bHBAdG11LmVkdS5jbg==; Xin Chen, eGNoZW4wM0B0bXUuZWR1LmNu
†These authors have contributed equally to this work
 Jinjie Zhou1,2,3†
Jinjie Zhou1,2,3† Zhuo Huang
Zhuo Huang Zihan Yu
Zihan Yu Liuqing Yao
Liuqing Yao Xin Chen
Xin Chen