- 1UK Centre for Astrobiology, School of Physics and Astronomy, University of Edinburgh, Edinburgh, United Kingdom
- 2Eastchem and School of Chemistry, University of Edinburgh, Edinburgh, United Kingdom
Fullerenes of extra-terrestrial origin may have been accessible as carbon sources for anaerobic microorganisms on the early Earth. Very little is known about how anaerobic microorganisms respond to and use fullerenes and their soluble derivatives. We present an investigation into the effects of fullerenes C60 and C70 and their hydroxylated fullerol derivatives on an environmentally relevant anaerobic community and a microbial isolate. Fullerenes and fullerols irradiated with 254 nm UV radiation for 2 weeks in the absence of oxygen to simulate UV irradiation under anoxia on early Earth were also assessed. The anaerobic community could grow using glucose in the presence of C60 up to 500 mg/mL without inhibitory effects on growth. Concentrations of C70 of 500 mg/ml were inhibitory. We attribute these results to the different chemical reactivity and photophysical properties of the fullerenes. The experiments suggest the potential for the use of C60 as a sole carbon source. Both C60 and C70 fullerols were inhibitory to growth in the presence of glucose, especially when exposed to light. When we exposed C60 fullerol suspensions to 254 nm UV radiation under an anoxic atmosphere, they become significantly more inhibitory to both the community and the isolate, but only if the cultures are grown under ambient light exposure. The anaerobic isolate was unable to grow on C60 alone, but after UV radiation exposure, the C60 photodegradation products served as a potentially accessible carbon source. Our data show that fullerenes and their derivatives are biologically active and capable of influencing growth in anoxic environments such as those that would have been prevalent on early Earth or in modern-day anoxic soils. Our results show that carbon sources such as these can be both beneficial or deleterious to life depending on their concentrations and environmental processing.
1 Introduction
There was likely a significant amount of extra-terrestrial organic carbon present on the early Earth when microbial life first emerged. Investigating the impact of this carbon on primitive microorganisms is crucial for comprehending the origins of early life on Earth, as well as for understanding the potential for life on other young planets.
Carbonaceous chondrites are composed of approximately 3–5% carbon, the majority of which is in the form of insoluble macromolecules (Botta and Bada, 2002; Chyba et al., 1990). An estimated total of 1020kg of extra-terrestrial organic material has been accreted by the Earth, at least 109kg of which was deposited during the Late Heavy Bombardment (Chyba et al., 1990; Ehrenfreund et al., 2011). Life is likely to have originated sufficiently early that it would have interacted with this Late Heavy Bombardment reservoir of extra-terrestrial organic material. Although the nature of these interactions is not yet understood, it is possible that the high concentration of organics provided an energy source for the growth of early microorganisms either in fermentative metabolism or as electron donors for other anaerobic metabolic processes (Pasek and Lauretta, 2008; Schönheit et al., 2016).
Fullerenes are a class of compounds characterized by their carbon cage “buckyball” structure which exist in the interstellar medium (Maier and Campbell, 2017) and organic-rich meteorites in amounts ranging from 15 to 28 ppm (Sabbah et al., 2022). Very little is known about how modern microorganisms interact with fullerenes. The limited number of studies that do address this usually do so from an ecological standpoint stemming from the risk fullerenes and related carbon nanomaterials pose to the environment as contaminants (Liu et al., 2023; Zuo et al., 2024; Leroy et al., 2024). Fullerene C60, the most stable and therefore most abundant and well-studied fullerene, is widely reported to be bactericidal under aerobic conditions (Chae et al., 2014; Duncan et al., 2008; Fang et al., 2007; Fortner et al., 2005; Hancock et al., 2012; Lyon et al., 2006; Tsao et al., 2002). However, it does not appear to be inhibitory to anaerobic microorganisms in anoxic environments as the presence of C60 is not found to significantly alter gas production or community composition in anaerobic wastewater reactors (Nyberg et al., 2008; Zhao et al., 2018).
Although C60 appears to be resistant to mineralization in soils over long periods (Avanasi et al., 2014), there is some evidence to suggest it may be susceptible to microbial degradation under certain conditions (Chae et al., 2014). In 2020, it was reported that the aerobic bacterial species Labrys. sp. WJW could degrade C60 by excreting a siderophore to facilitate a Fenton-like biodegradation reaction to break the fullerene cage structure (Wang et al., 2020). To the best of our knowledge at the time of this paper, there is no report of biodegradation of any fullerene by an anaerobic microorganism. This paucity of investigations could be because most environments where the effects of fullerene contamination are of concern are aerobic, and therefore fewer studies have focused on anaerobic species.
Fullerene C60 has very low water solubility in its native form (Avanasi et al., 2014; Lyon et al., 2006; Ruoff et al., 1993). On contact with water, it forms stable colloidal aggregates, known as nC60, which possess different chemical properties to bulk C60 (Avanasi et al., 2014; Duncan et al., 2008; Lyon et al., 2006). Therefore, it is these nC60 aggregates that microorganisms interact with in aqueous media, both in the laboratory and the environment. Furthermore, C60 is readily altered by exposure to environmental conditions, therefore it is highly likely that certain factors on the early Earth would have impacted the chemistry of the fullerenes present, and therefore changed their accessibility to, and effects on, early microorganisms.
In addition to fullerenes, another class of molecules of potential interest on early Earth are the fullerols. Fullerol C60 is a derivative of fullerene C60 characterized by the attachment of many hydroxyl functional groups (Semenov et al., 2016). Fullerol C60 is highly photosensitive and soluble in water (Semenov et al., 2016). Although it still tends to form spherical aggregates in aqueous solutions, this is to a lesser extent than native C60 (Brant et al., 2007; Hotze et al., 2009). Fullerol C60 is much more bioavailable in soils than its fullerene counterpart, as it is readily mineralized to CO2, albeit at a much slower rate than highly bioavailable carbon sources such as glucose (Navarro et al., 2015). Additionally, C60 fullerol is readily taken up and converted into microbial biomass by a range of microorganisms in soils under conditions where pristine C60 is not mineralized in any detectable amount (Berry et al., 2017), indicating this particular fullerene derivative may be much more accessible as a carbon source for microbes.
Yet another set of derivative chemical compounds are produced by irradiation of the aforementioned molecules. Aqueous C60 is readily degraded to produce water-soluble intermediates when exposed to solar-wavelength light in the presence of oxygen (Hou et al., 2010; Hou and Jafvert, 2009), and these degradation products are much more readily degraded by soil bacteria than unaltered C60 (Berry et al., 2016). On the early Earth, any aqueous C60 would have been in a largely anoxic environment and surface ultraviolet (UV) radiation levels would have been much higher with wavelengths down to 200 nm likely reaching the surface of the planet (Cnossen et al., 2007; Cockell, 1998). C60 degraded under UV radiation at 254 nm forms products that are significantly less antibacterial to aerobic bacteria than unaltered C60 (Lee et al., 2009). Conversely, C60 fullerol is highly photosensitive and produces a range of reactive oxygen species such as singlet oxygen and hydroxyl radicals when exposed to UV radiation (Brunet et al., 2009; Chae et al., 2009; Ma et al., 2020), which has been found to induce lipid and protein oxidation in membranes (Kamat et al., 2000), potentially indicating an increase in antimicrobial activity upon exposure to light.
It is thought that fullerene C60 in organic solvents still degrades to some extent under UV radiation when no oxygen is present (Taylor et al., 1991). Very little is known about how C60 and fullerol C60 behave in anoxic aqueous media when exposed to UV radiation. Any degradation products, including reactive oxygen species, formed under these conditions are likely to be most similar to those that would have been produced on the early Earth, just as any antibacterial effects produced by photoactivated fullerol likely represent what may have occurred in early microbial systems.
In addition to the C60 fullerenes and fullerols, there are larger structures that could be biologically relevant. Fullerene C70 is the second most stable high molecular weight fullerene and is found in amounts of a similar magnitude to C60 (Kroto, 1988). There are very few reported studies concerning the effect of C70 on microorganisms, either as an inhibitory species or a subject of biodegradation. It is reported that C70 derivatives exert antimicrobial activity toward both Gram-positive and Gram-negative bacteria (Huang et al., 2013), likely due to the release of reactive oxygen species from the functionalized C70 particles which attack bacterial cell walls (Huang et al., 2014, 2013; Ouyang et al., 2016). However, there is little literature available to support this.
In this study, the effects of native and photodegraded fullerenes C60 and C70 and their fullerol derivatives on anaerobic microorganisms were examined. An anaerobic community that was previously selected to grow using raw meteorite material as a carbon source by Waajen et al. (2022) was used in these experiments as an analog of early heterotrophic microbial life. An isolate obtained from this community was used in additional experiments to compare the effects of fullerenes on a community with a single related organism. Aqueous C60 and C70 were irradiated with 254 nm UV radiation under anoxic conditions and the degradation products and their effects on both the anaerobic community and isolate were characterized and compared with the corresponding native fullerenes. We discuss these results in the context of early life on Earth. These results have implications for the modern challenge of understanding the effects of human fabricated carbon nanomaterials in the environment.
2 Materials and methods
2.1 Fullerene suspensions
Powdered fullerene C60 (99.5%+ purity) and C70 (98%+ purity) were acquired from IOLITEC GmbH (Germany) and MST (Latvia), respectively. One hundred mg/L aqueous suspensions of fullerene C60 were prepared by the following method: powdered fullerene was added to sterile M9 minimal media and placed in a sonicator bath (Elma S 60 H Elmasonic) for 6 h until no large aggregates were visible, and the medium appeared dark gray and cloudy. The sonicator bath temperature was kept below 45°C by adding ice water every 30 min.
Fullerol C60 and C70 were synthesized and characterized according to a procedure adapted from Kokubo et al. (2011). Firstly, C60 (150 mg) was dissolved in 150 mL toluene and then 25 mL of 30% H2O2 was added slowly, followed by 0.8 mL of 40% aqueous solution of Tetrabutylammonium hydroxide (Bu4NOH). The mixture was stirred (750–1,000 rpm) at 60 °C until complete decolourization of the organic layer was observed (10–15 h). The toluene and aqueous layers were then separated. The evaporation of water/H2O2 (55–60°C; < 20 mbar) was continued until the consistency of the residue resembled soft wax. The residue was diluted with 30 mL of isopropyl alcohol (iPrOH) and the solution was acidified with 0.125 mL of concentrated HCl (for complete neutralization of Bu4NOH). Precipitation of a light-yellow solid started immediately and was completed within 2–3 h. The solid material was separated by centrifugation and then vacuum dried. A total of 149 mg of material was collected. This material was characterized by FT-IR (Fourier Transform Infra Red) spectroscopy, solid-state 13C NMR (Nuclear Magnetic Resonance) spectroscopy and thermal gravimetric analysis (Supplementary Figures 1–3). The synthesized material was concluded to have the brutto chemical formula C60(OH)44(6–8)H2O. A similar procedure was followed for C70 giving the brutto chemical formula C70(OH)50(14–16)H2O.
To make aqueous fullerol suspensions, fullerol powder was added to sterile M9 minimal media and dissolved by stirring. All fullerene and fullerol suspensions were stored in the dark before use. To prepare fullerene and fullerol suspensions for UV irradiation, 100 mg/L of C60, C70 or their respective fullerols were added to sterile M9 media and dispersed using the methods previously described, as appropriate. The resulting suspensions were purged under nitrogen and sealed inside quartz Erlenmeyer flasks inside an anaerobic chamber using rubber butyl stoppers and parafilm. The flasks were then removed from the chamber and placed 10 cm below a suspended UV lamp within a dark container. Suspensions were irradiated at 254 nm for 2 weeks to obtain the UV-irradiated suspensions used in subsequent culture experiments. UV-vis spectra of the suspensions were taken before and after irradiation and compared.
2.2 Fullerene aggregate sizing
Dynamic light scattering (DLS: Malvern ALV/LSE-5004) was used to determine the hydrodynamic diameters of the suspensions in distilled water and sterile M9 media. The results were also compared to diffusion measurements using differential dynamic microscopy (DDM). The influence of UV irradiation on the measured size distributions after 2 weeks, using conditions identical to those above, and after a further 2 weeks was determined.
2.3 Glassware
Fifteen-milliliter glass serum bottles and airtight butyl rubber stoppers were used for all microcosm culturing. To ensure all glassware and stoppers were sterile and free of organic contaminants, an adaptation of the protocol described by Eaton and Franson (2005) was used. Briefly, all glassware was washed with detergent (Decon90, 2% solution), rinsed three times with deionized water and soaked in 0.1 M HCl for a minimum of 12 h. The glassware was then rinsed again three times with deionized water, air dried, capped with aluminum foil and heated in a furnace at 550°C for 6 h. The butyl rubber stoppers were washed with detergent, boiled three times for 5 min in deionized water and air dried under a laminar flow hood.
2.4 Microorganisms
An environmental microbial community sample was collected from anaerobic pond sediment (Blackford pond, Edinburgh) and selectively cultured over three rounds of sub-culturing to grow on raw meteorite material by Waajen et al. (2022). The resulting anaerobic community was used in these experiments. An isolate from this community was obtained from a single colony, itself obtained by swabbing the surface of an M9 agar plate with the community described above supplemented with glucose. The selected isolate was sub-cultured three times to obtain a pure culture with uniform colonies.
2.5 Culture preparation
Microcosm volumes of 5 mL were used throughout the experiment and all conditions and controls were prepared in triplicate. All positive controls consisted of 5 mL of M9 minimal media supplemented with 0.4% (w/v) glucose. All conditions with fullerenes, fullerols or their derivatives were made with 5 mL of the suspension as described above either with or without the addition of 0.4% (w/v) glucose as specified. Negative controls were prepared as described above, but without microbial inoculation.
All microcosms were prepared aerobically under sterile conditions and then purged with N2 for 15 min to remove dissolved oxygen. After purging, the microcosms were moved into an anaerobic chamber (COY Laboratory Products Inc., vinyl anaerobic chamber 2% hydrogen 98% nitrogen) for the duration of the experiment.
All microcosms except negative controls were inoculated with 50 μL from a subculture of the anaerobic community or isolate in M9 minimal media with 0.4% (w/v) glucose.
2.6 Cell counting
Over a 28-day growth period, 100 μL aliquots of each microcosm were taken for direct cell counting measurements, beginning on day 0 immediately after inoculation and on subsequent days, as specified. Forty-five microliters of each aliquot was added to 5 mL of SYBR gold nucleic acid stain (Invitrogen) to a final working solution (diluted 1:1,000 in sterile deionized water) according to the manufacturer’s instructions for 5 min at room temperature. Ten microliters was pipetted into the chamber of a hemocytometer (Blaubrand Neubauer Bright-Line 0.100 mm × 0.0025 mm2). Cell counting was carried out under a 20x magnification microscope lens with a fluorescent light source (Leica DM 4000 B, Leica Kübler CODIX) and cell density was calculated.
2.7 Transmission electron microscopy
To obtain transmission electron microscopy (TEM) images of C60 cultures and controls, microcosms were prepared and grown following the previously described method. One milliliter of each culture was washed three times with 1x PBS buffer and resuspended in 1x PBS. Samples were fixed in 3% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.3) for 2 h and then washed three times in 0.1 M sodium cacodylate for 10 min. Each sample was post-fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate for 45 min then washed three times in 0.1 M sodium cacodylate for 10 min. Samples were then dehydrated in 50, 70, 90, and 100% ethanol for 15 min each, then twice with propylene oxide for 10 min, before being embedded in TAAB 812 resin. One millimeter thick sections were cut on a Leica Ultracut ultramicrotome and stained with toluidine blue. Suitable areas for investigation were selected by viewing under a light microscope, and these areas were further cut into 60 nm ultrathin sections and stained with uranyl acetate and lead citrate. Samples were viewed with a JEOL JEM-1400 Plus TEM and images were collected on a GATAN OneView camera. The material observed in the TEM images was confirmed with Raman spectroscopy (Renishaw) to be intact C60 cages using the same ultrathin section samples (785 nm, 1 mW, 10 s exposure time) (Supplementary Figure 5).
2.8 Microbial growth analysis
For each microcosm, the final cell density counted on day 28 was converted to a percentage increase relative to the initial day 0 cell count. This was done to allow direct comparison of cell densities between conditions while accounting for the inevitable discrepancy in initial cell number immediately after inoculation. All subsequent analysis was performed on the cell density percentage increase values, rather than the raw cell density. The average cell density percentage increase was used for graphical representation.
To compare each condition to the control or another condition, an F-test was performed to determine if the variances between the two conditions were equal or unequal. t-tests assuming unequal or equal variances, as appropriate, were performed assuming a significance level of α = 0.05. In all graphical representations, a “*” is used to denote a significant result while “ns” represents a non-significant result, usually when compared to the positive control, unless otherwise specified. All error bars represent the standard error on the mean for each condition.
2.9 DNA sequencing and microbial identification
Cultures were prepared for DNA extraction by inoculating 10 mL of anoxic M9 media and 0.4% (w/v) glucose with either the anaerobic community or the isolate from single plated colonies. These were grown anaerobically for 28 days as previously described. DNA was extracted from each culture using an extraction kit (DNeasy PowerSoil Pro, QIAGEN) per the manufacturer’s instructions and quantified using a Qubit 3 fluorometer (Invitrogen). DNA samples were sent to Novogene, United Kingdom, for Illumina 16S rDNA sequencing and bioinformatics analysis. The following methods were carried out by Novogene. The 16S rDNA variable region (V3-4) region was amplified via PCR using the primers CCTAYGGGRBGCASCAG and GGACTACNNGGGTATCTAAT. The resulting amplicons were sequenced on an Illumina NovaSeq 6000 paired-end platform to generate 250 bp reads. The raw amplicon data was cleaned using DADA2 (RRID:SCR_023519) software to remove noise and obtain non-duplicated amplicon sequence variants (ASVs). QIIME2 (RRID:SCR_021258) software was used to annotate each ASV based on the Silva 138.1 open source database. From this data, species abundance tables were generated at the level of kingdom down to genus for the anaerobic microbial community.
3 Results
3.1 Growth of anaerobic community in the presence of fullerenes and derivatives
The anaerobic community was grown for 28 days with two different concentrations of C60 or C70; a concentration of 100 mg/L or a higher one of 500 mg/L (Figure 1). The community was grown with either C60 or C70 as the sole carbon source or with additional glucose to test whether either fullerene would have an inhibitory effect on the growth of the bacteria on other carbon sources. The results were compared to the positive control using an unpaired t-test with a significance level of 0.05, and the p-values obtained are listed in Table 1. The results indicate that a concentration of 100 mg/L C60 did not significantly change the growth of the community compared to the positive control, regardless of whether glucose was present or not. There was no significant difference between the C60 (100 mg/L) cultures with or without glucose (p = 0.094) which suggests that in this experiment, C60 (100 mg/L) in the presence of glucose did not increase the amount of growth, but in the absence of glucose, the C60 alone could be used as a carbon source.
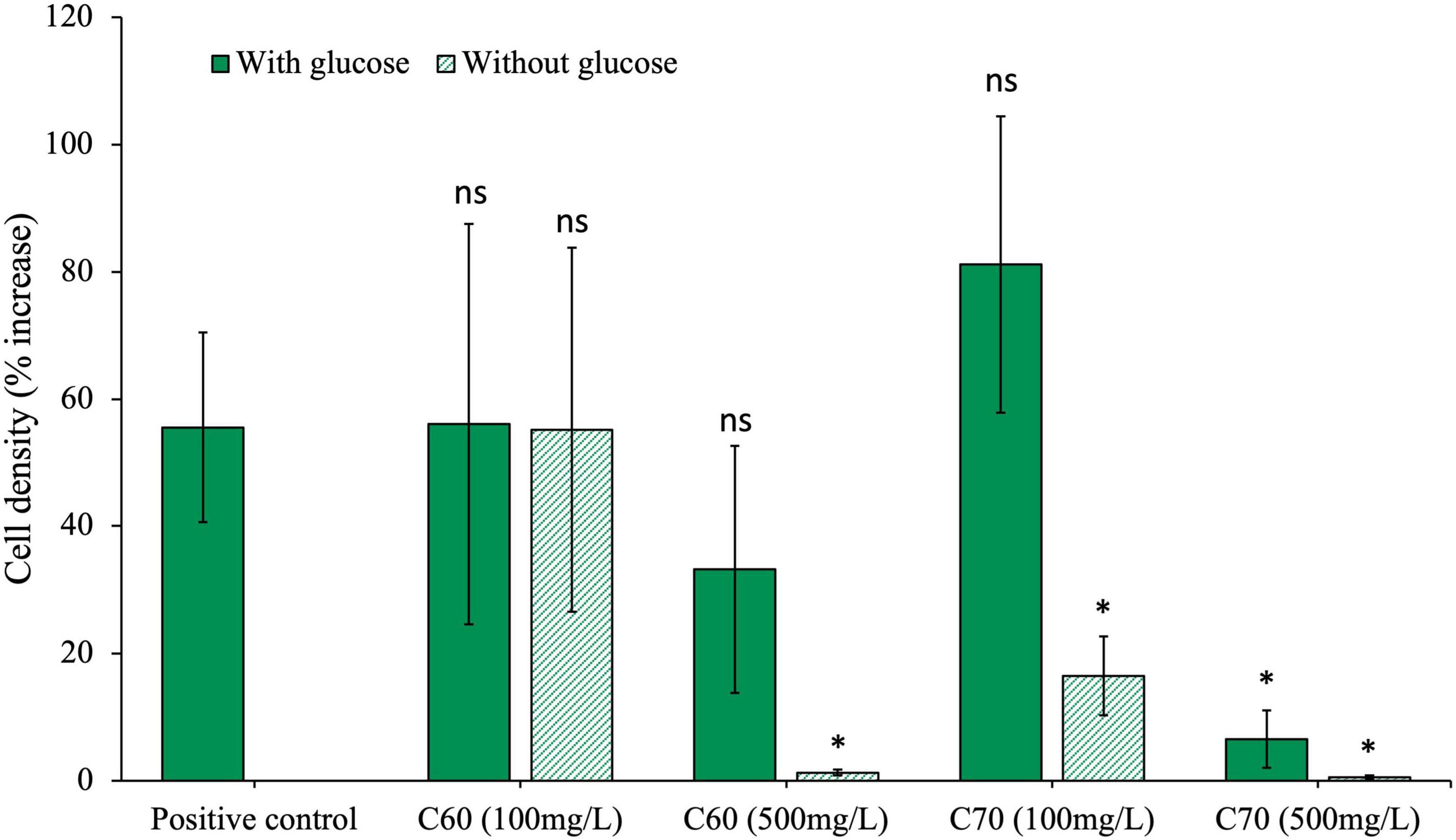
Figure 1. Effects of C60 and C70 on anaerobic community growth. The community was cultured with either a low (100 mg/L) or high (500 mg/L) concentration of C60 or C70, with and without additional glucose. Final cell density counted on day 28 of growth and expressed as a percentage increase from day 0 post-inoculation. Error bars represent the standard error on the mean for each condition. All statistics are carried out with either paired or unpaired t-tests (dependent on the significance of an F-test) comparing each condition to the positive control where p > 0.05 is considered significant.
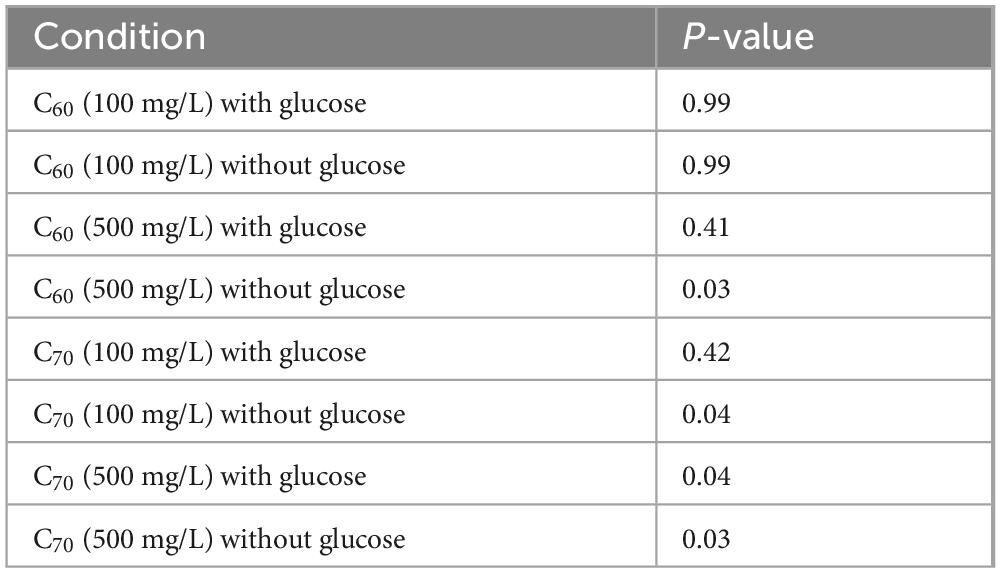
Table 1. Significance of the effects of anaerobic community growth with C60 and C70 at 100 mg/L or 500 mg/L, with and without glucose, compared to the positive control (glucose).
At a concentration of 500 mg/L, C60 did not significantly affect community growth compared with the positive control, indicating that it was not inhibitory at this concentration provided there was an additional carbon source. However, when there was no glucose, there was no notable growth (cell density was indistinguishable from the negative control), which suggests that at the higher concentration, C60 could no longer be used as a carbon source.
Community growth with C70 at 100 mg/L was not significantly different from the positive control if glucose was available, however, if no glucose was present, the cell density was much lower. At 500 mg/L, community growth with C70 was significantly reduced both with and without glucose, indicating that at the higher concentration, C70 could not be used as a carbon source and the low level of growth recorded in the presence of glucose compared to the positive control suggests that it was inhibitory to growth.
We then sought to test the reproducibility of the result which indicates the community could use C60 as a carbon source. We did this by conducting four different growth experiments in which the community was cultured with C60 (100 mg/L) as a sole carbon source. The results are summarized in Supplementary Figure 4. For each experiment, the cell density (% increase) of the C60 condition on day 28 was compared to the corresponding positive control. In experiments 1–3, growth with C60 as a sole carbon source was significantly lower than the positive controls (p = 0.021, 0.02 and 0.05 for experiments 1–3, respectively), which suggests that although growth was reduced compared with the positive control, there was a small amount of C60 use occurring. Conversely, in experiment 4, there was no significant difference in cell density between the C60 condition and the positive control (p = 0.99), indicating growth was very similar with C60 as a carbon source compared with the glucose in the positive control.
Figure 2 summarizes all the data from experiments in which the community was cultured with C60 (100 mg/L) as a sole carbon source or with additional glucose. The box and whisker style plot gives an indication of how the final cell densities of each condition overlap as well as allowing for comparison of the mean and average spread of the data. The C60 with glucose condition consistently overlapped with the positive control, showing that when glucose was available, the presence of C60 had little influence on growth. Growth with C60 as a sole carbon source was lower compared to the positive control, except for two outliers in which the final cell densities were as high. However, some growth was observed, which suggests it can be used as a carbon source.
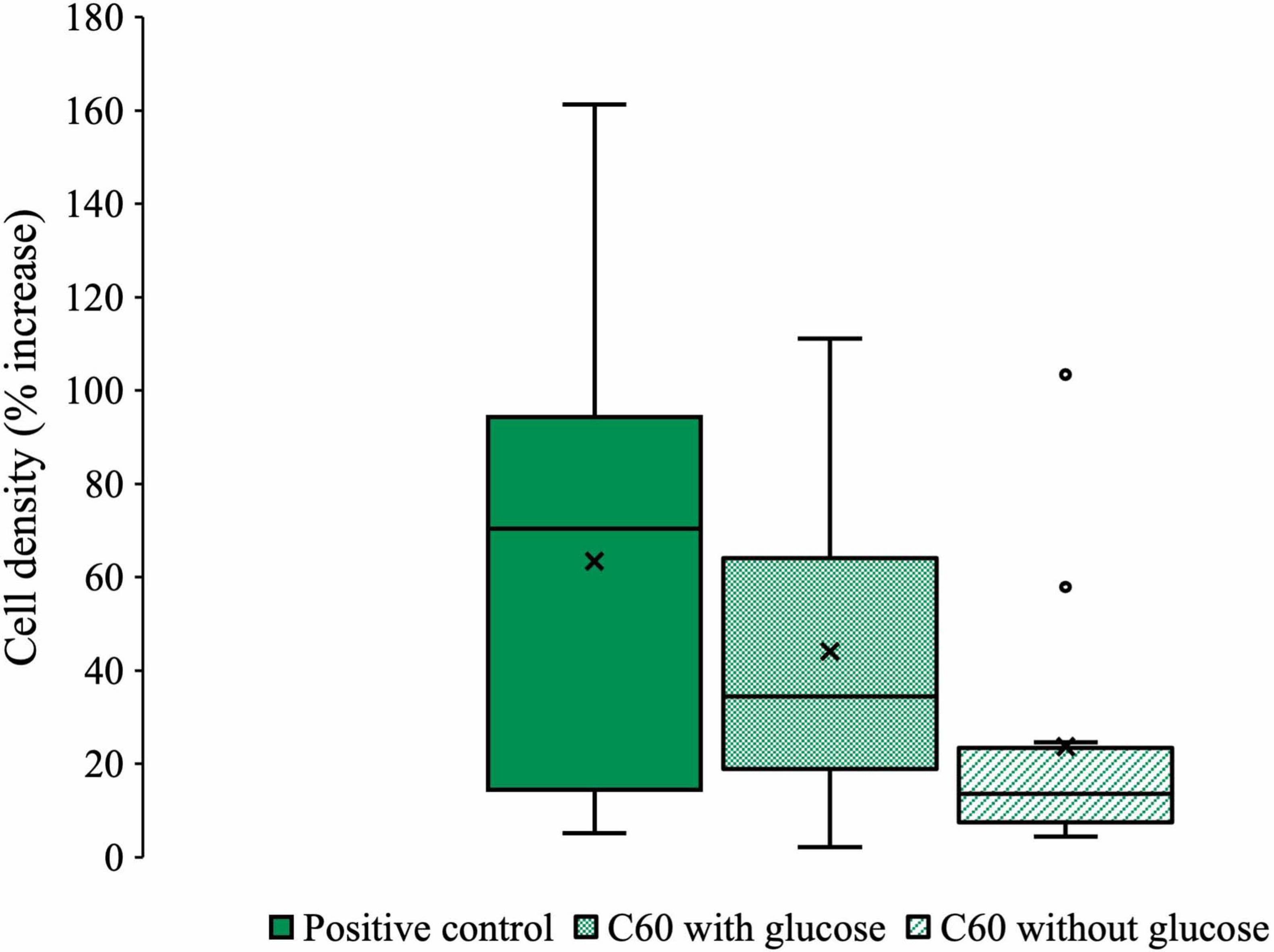
Figure 2. Collected microbial community growth data from experiments with C60. Box and whisker plot compiling all the pooled data from each experiment where the anaerobic community was cultured with C60 (100 mg/L) with or without additional glucose. Plot whiskers describe the range of data points for each condition, the outer bounds of the solid boxes represent the lower and upper quartiles, the middle line is the median and X markers represent the mean. Outliers are displayed as open circles.
The anaerobic community was cultured with 100 mg/L C60 with or without additional glucose and TEM images were obtained of these cultures on day 28 of growth (Figure 3). Figure 3A shows images of the community grown with C60 as a sole carbon source, where the aggregated material was confirmed by Raman spectroscopy to be C60 with possibly some evidence for cage oxidation at the surface (Supplementary Figure 5), in agreement with previous observations of surface oxidation of C60 aggregates (Hwang and Li, 2010; Zygouri et al., 2020). In these images, small black deposits were observed on the surface of some of the bacterial cells. Figure 3B shows the community grown with C60 and additional carbon. In these images, aggregated C60 was observed but the small black cell surface deposits were not, suggesting these only occur in cultures in which C60 is the sole carbon source. Images of the negative controls (100 mg/L C60 with no bacteria) are shown in Figure 3C, where aggregated C60 was observed, and no bacterial cells were present.
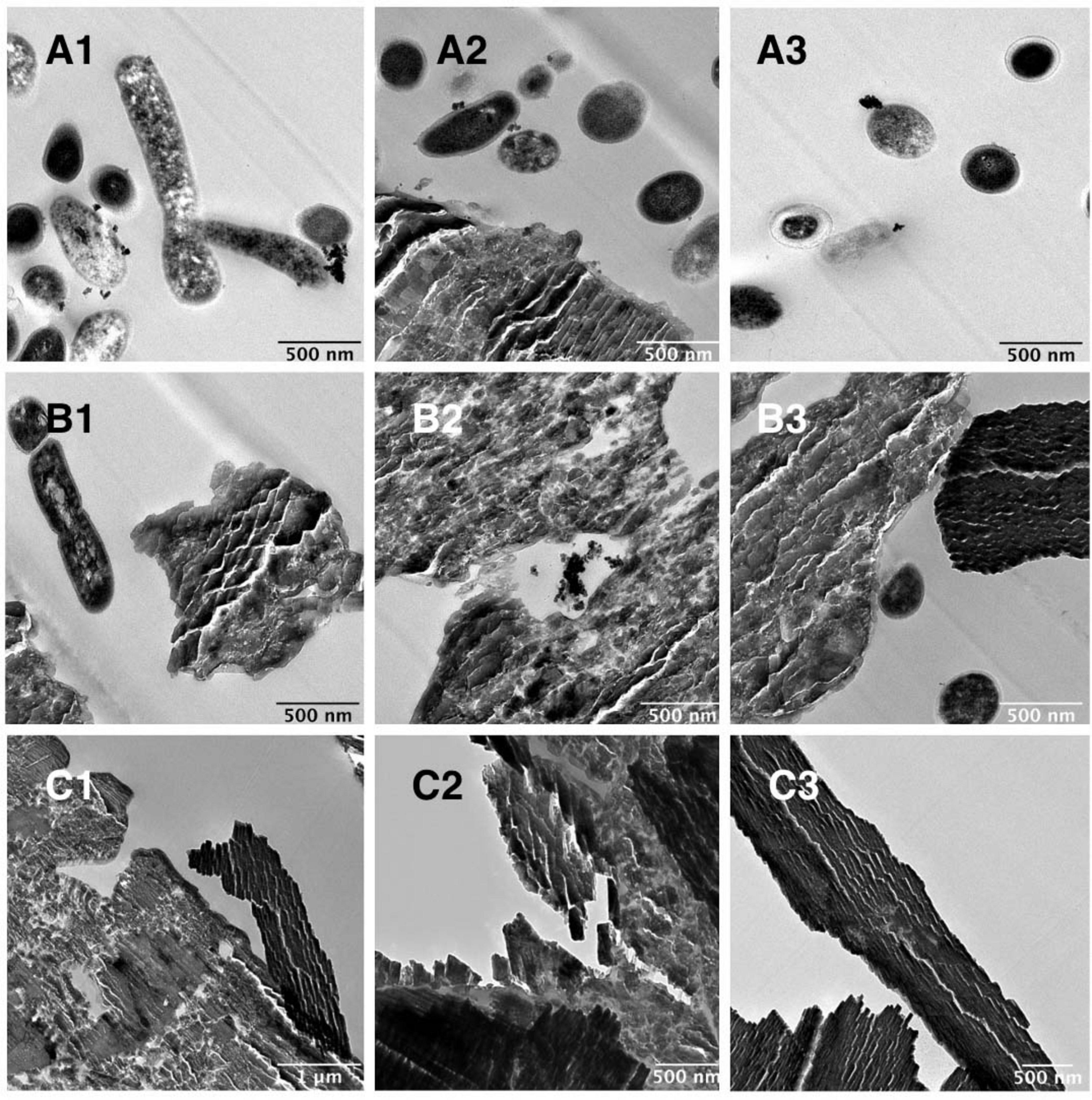
Figure 3. TEM images of anaerobic community cultures grown with 100 mg/L C60 as a sole carbon source or with glucose. (A) Community grown with 100 mg/L C60 as a sole carbon source, (B) community grown with 100 mg/L C60 with additional glucose, (C) 100 mg/L C60 negative controls (not inoculated).
DNA was extracted from the community and the 16S rDNA gene was amplified and sequenced to obtain the relative abundances of each taxon present in the community after incubation with C60 as the sole carbon source. [Supplementary Figure 6 shows the relative abundances of each taxonomic family detected. The majority of the species present belong to the Enterobacteriacaea family (82%)].
In addition to C60, we sought to investigate the effects of soluble fullerene derivatives on the anaerobic community. We achieved this by first culturing the community with 100 mg/L of the water-soluble C60 derivative, C60 fullerol, with and without additional glucose (Figure 4).
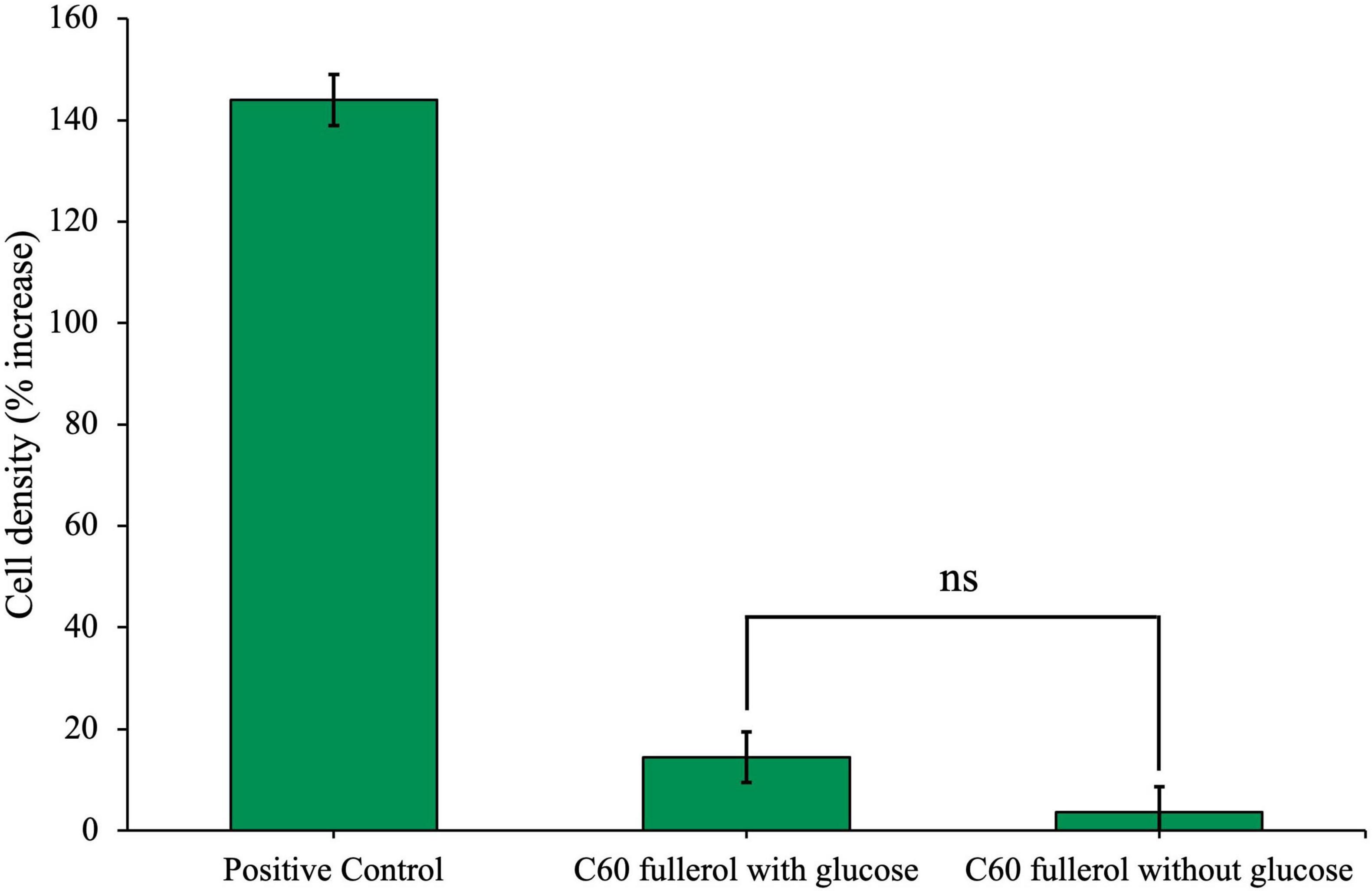
Figure 4. Effects of C60 fullerol on anaerobic microbial growth. Anaerobic community cultured for 28 days with 100 mg/L C60 fullerol, with or without glucose. Final cell density counted on day 28 of growth and expressed as a percentage increase from day 0 post-inoculation.
Community cell density with C60 fullerol was significantly reduced compared with the positive control on day 28, both with and without glucose (p = 0.046 and 0.042), and there was no significant difference between the C60 fullerol conditions with or without glucose (p = 0.092). The growth reduction in the presence of glucose compared to the positive control suggests that exposure to C60 fullerol at 100 mg/L under ambient conditions was inhibitory to the community. The small amount of growth without glucose could be attributable to use of the fullerol as a sole carbon source (although this could be potentially caused by carbon carry over as discussed later).
C60 fullerol at a concentration of 100 mg/L was exposed to 254 nm UV radiation for 2 weeks before being used as a substrate for microbial inoculation. The community cultures were incubated for 28 days with C60 fullerol, both irradiated and non-irradiated, without glucose, under ambient lab light conditions as well as in the dark. The resulting cell densities are presented in Figure 5.
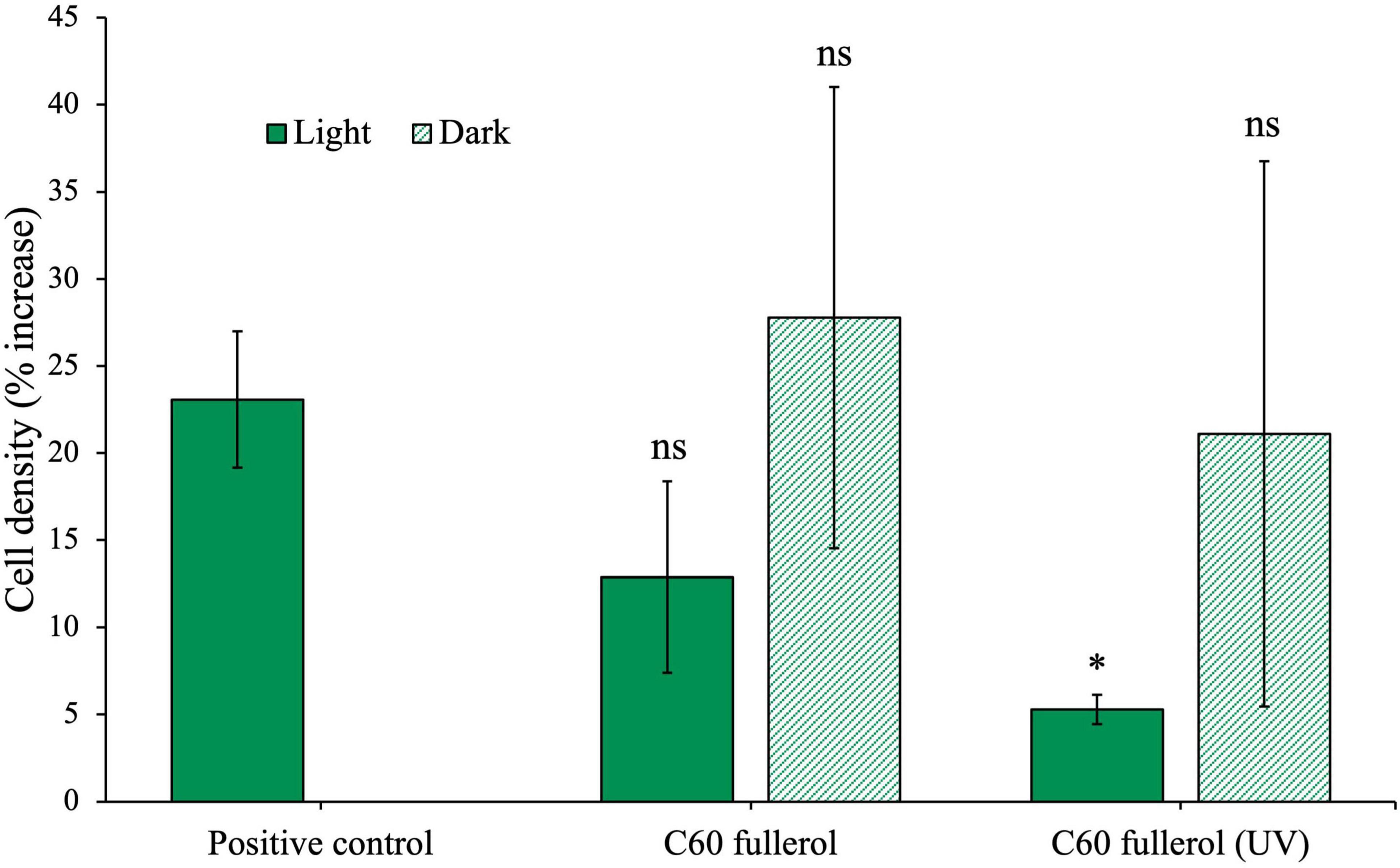
Figure 5. Effects of fullerols on the growth of the anaerobic community with or without prior UV exposure, in ambient light or dark growth conditions without glucose. Anaerobic community cultured for 28 days with 100 mg/L C60 fullerol. Final cell density counted on day 28 of growth and expressed as a percentage increase from day 0 post-inoculation. In the case of the positive control, since there was no effect of light, the growth data are compared to the light control, shown here.
On day 28 of this experiment, we found that the non-irradiated C60 fullerol experiment (light or dark) did not have different cell densities compared to the positive control (light and dark conditions with p-values of 0.17 and 0.75, respectively). Note the difference in this result compared to the growth in fullerols in the experiment reported in Figure 4 in which cell concentrations were significantly lower compared to the positive control without glucose. We found no significant difference between the cultures incubated in the dark with UV-irradiated C60 fullerol and the positive control (p = 0.59). However, when the UV-irradiated C60 fullerol cultures were exposed to light, their growth was significantly reduced compared to the positive control (p = 0.005). Based on this, we infer that C60 fullerol is most toxic to the community when it has been irradiated with UV and is then subject to continued ambient light exposure after inoculation.
3.2 Growth of anaerobic isolate in the presence of fullerenes and derivatives
To determine if similar results would be obtained with an individual species, we repeated some of the community experiments with an anaerobic isolate obtained from the anaerobic community. The DNA sequencing results indicate that this isolate is from the family Enterobacteriaceae, however, due to difficulties with DNA extraction and sequencing we are unable to conclusively determine genus-level taxonomic identification.
The isolate was grown for 28 days with 100 mg/L of native C60 or C70 or UV-irradiated C60 or C70, with or without additional glucose, the results of which are shown in Figure 6. Unpaired t-tests were performed to compare each condition to the positive control on day 28, the results of which are summarized in Table 2.
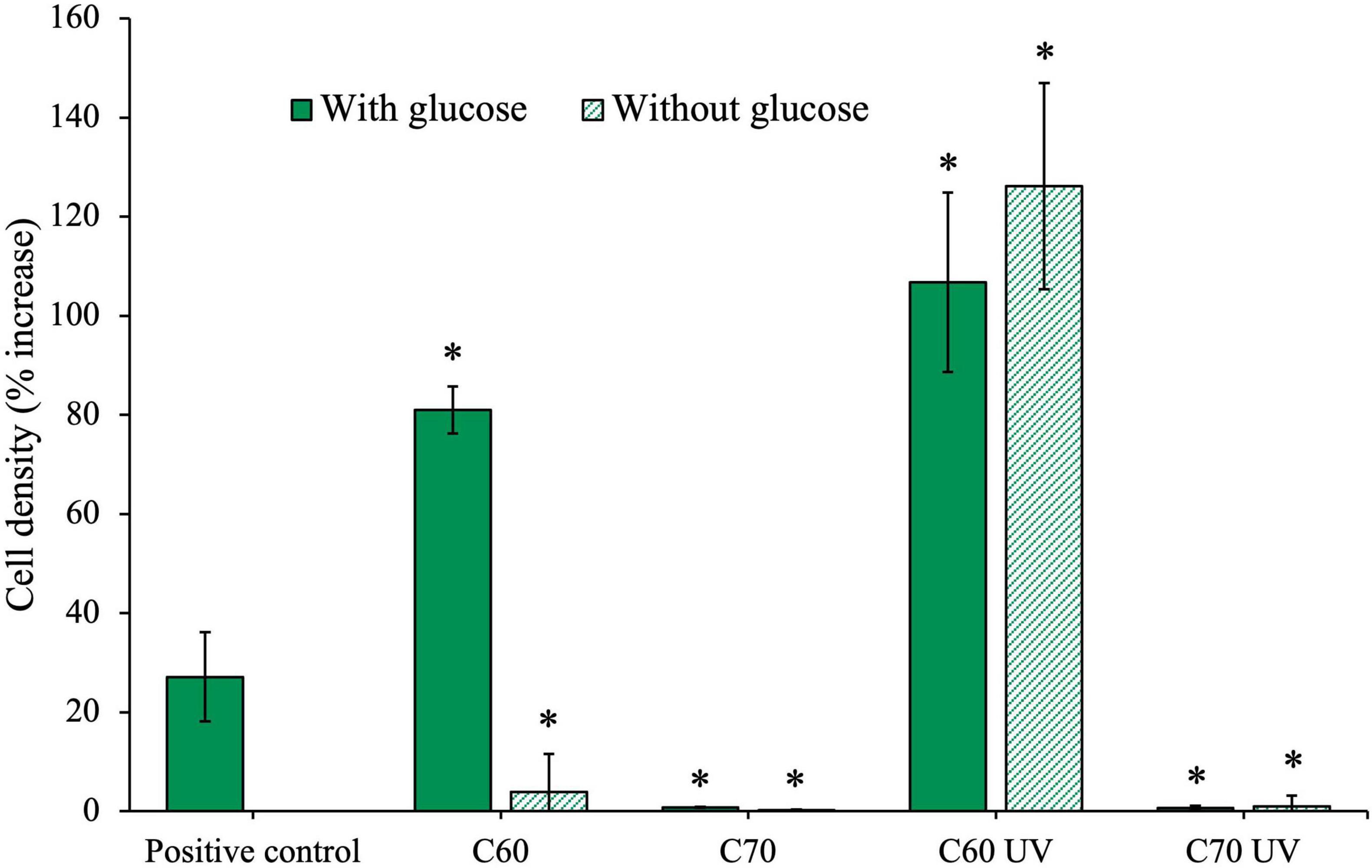
Figure 6. Effects of irradiated and non-irradiated fullerenes on growth of an anaerobic isolate. The isolate was cultured with C60 and C70 either in the native form or after UV irradiation, with and without additional glucose. Cell density (%) increase was measured on day 28 of growth. C60 and C70 UV were irradiated for 2 weeks before inoculation.
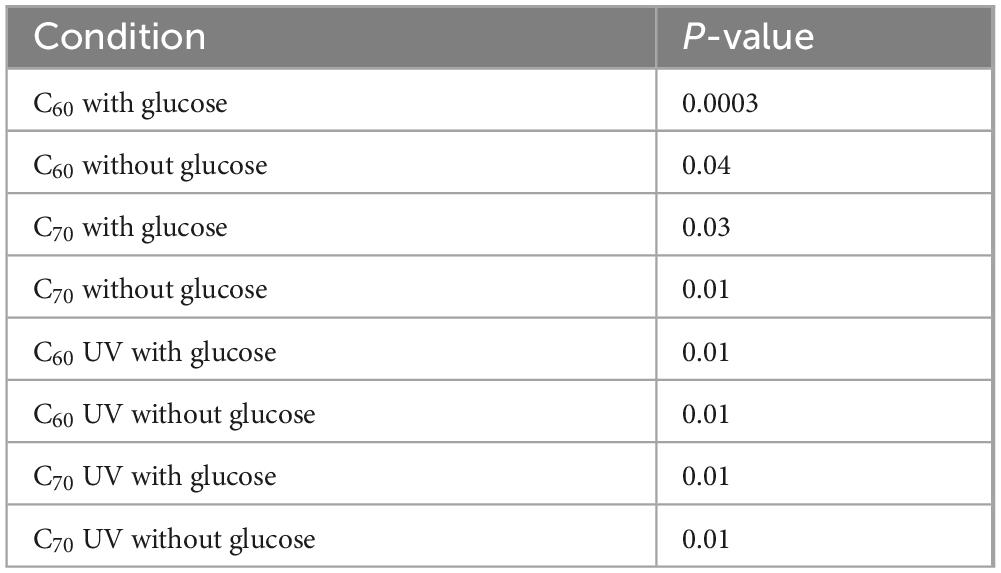
Table 2. Significance of anaerobic isolate growth with C60, C70 and their UV-degradation products, with and without glucose, compared to the positive control (glucose).
The final cell density was increased compared to the positive control when the isolate was grown with C60 and additional glucose. This could suggest that C60 was being used in addition to glucose, resulting in the observed increase in growth. However, when glucose was not available, the isolate cell density was much lower and cannot be concludively attributed to use of the compound as a sole carbon source. Conversely, in cultures grown with UV-irradiated C60, we observed significant growth, greater than the positive control, even when no additional glucose is available. This suggests that the C60 UV degradation products produced after 2 weeks of irradiation were available as a carbon source.
The isolate did not grow in the presence of C70 at 100 mg/mL either with or without glucose, indicating that not only is the C70 not used, but it was also highly inhibitory. Similarly, isolate growth was severely inhibited in both C70 UV conditions, indicating the UV degradation products of C70 were also highly inhibitory to growth in the isolate.
As we did with the anaerobic community, we next investigated the growth of the isolate with fullerol derivatives. The isolate was cultured for 28 days with 100 mg/L C60 fullerol under two different conditions: in ambient lab light or the dark, and this was compared to growth with the C60 fullerene (100 mg/L) and a mixture of C60 fullerene and fullerol in a total combined concentration 100 mg/L (Figure 7). All cultures were given additional glucose. The results show that when grown with C60 fullerol in the light, the cell densities on day 28 were significantly lower compared to the positive control (p = 0.043). However, when grown in the dark, there was no significant difference from the positive control (p = 0.47). These findings suggest that C60 fullerol was more inhibitory to the isolate when exposed to light, qualitatively similar to the results obtained with the community.
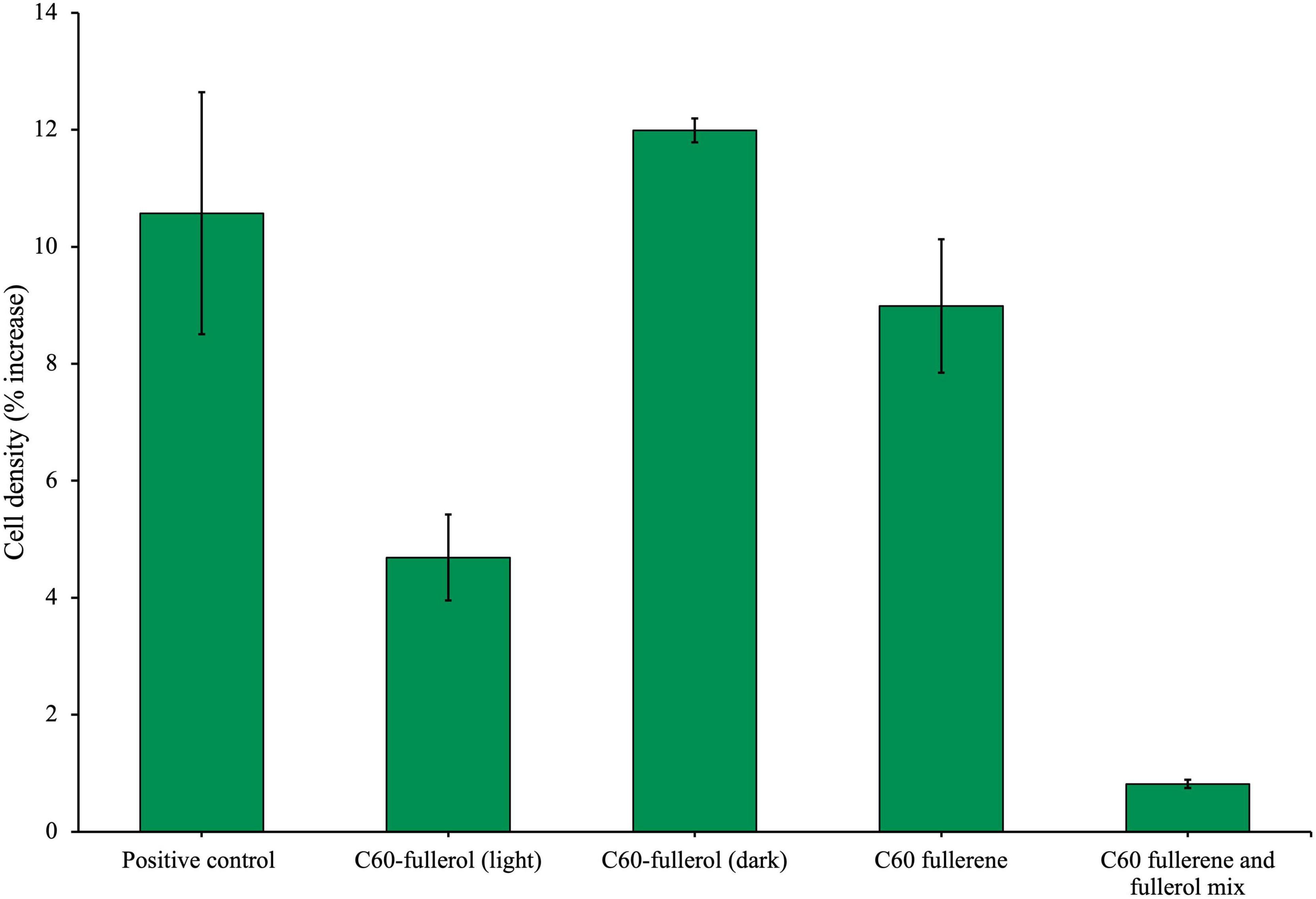
Figure 7. Effects of fullerols and fullerene/fullerol mixture on the growth of an anaerobic isolate. The isolate was cultured with either C60 fullerol (100 mg/L) in the light or dark, C60 fullerene (100 mg/L) or a 100 mg/L equal mix of C60 fullerene and fullerol. All conditions were supplemented with glucose. Cell density (%) increase was measured on day 28 of growth. C60 and C70 UV were irradiated for 2 weeks before inoculation.
We found that when C60 fullerol and fullerene were mixed, the mixture was inhibitory to the isolate in the presence of glucose (Figure 7), as it significantly reduced growth compared with the positive control (p = 0.0095). For comparison, the cultures grown with C60 in the presence of glucose were not significantly different to the positive control (p = 0.68), which suggests it is the presence of the fullerol in this mixture which caused it to be inhibitory.
Our next objective was to investigate how UV radiation exposure impacts the effect of C60 fullerol on the anaerobic isolate. To achieve this, we exposed C60 fullerol suspensions to 254 nm UV radiation for 2 weeks. We then cultured the isolate with the irradiated fullerol under two conditions: in the dark and under ambient light, both with and without glucose (Figure 8).
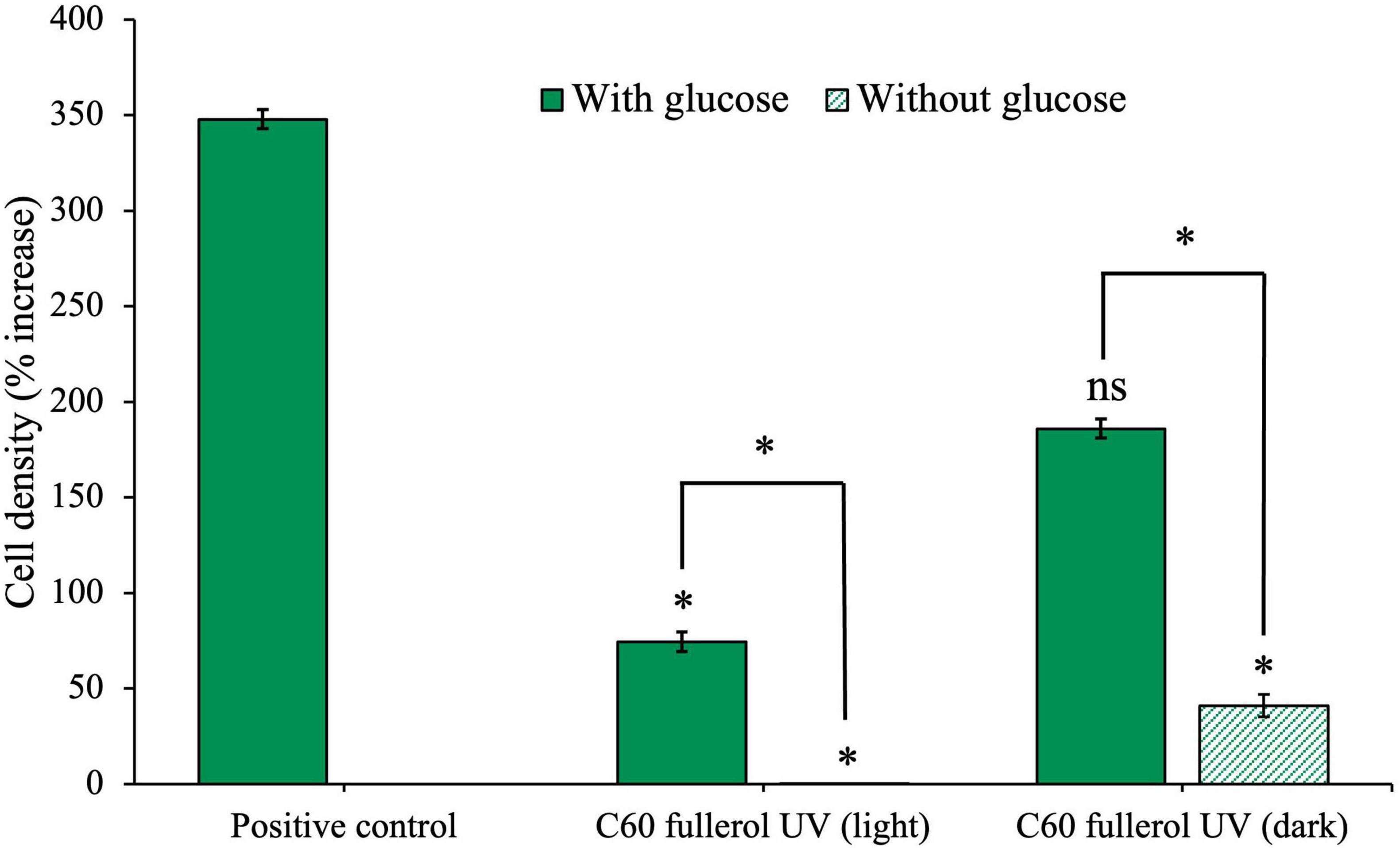
Figure 8. Effects of UV-irradiated C60-fullerol on the growth of an anaerobic isolate. Cell density after 28 days of growth with UV-irradiated C60 fullerol in light or dark growth conditions, with and without supplementary glucose is shown.
The culture with glucose and UV-irradiated C60 fullerol in ambient light (Figure 8) had significantly reduced growth compared to the positive control (p = 0.05) indicating that the C60 fullerol was inhibitory after UV irradiation and with sustained ambient light exposure. Without glucose, growth was negligible (p = 0.04 compared to the positive control), which may be attributable to the lack of a carbon source for growth (both a lack of glucose and the inability to use UV-irradiated C60 fullerol as a carbon source). There was a significant difference between the light conditions with and without glucose (p = 0.0073), indicating that although the UV-irradiated C60 fullerol was inhibitory in the light, there was still growth occurring when glucose was available.
In contrast, under dark conditions (Figure 8), we observed that if glucose was available, isolate growth with UV-irradiated C60 fullerol was not significantly different to the positive control (p = 0.1) suggesting that the UV-irradiated C60 fullerol was not inhibitory in the dark provided it was kept in the dark. However, there was significantly reduced growth when no glucose was present (p = 0.045) which could suggest some use of the UV-irradiated products of C60 fullerol as a sole carbon source, noting the caveats discussed later. These results suggest that the highest toxicity of UV-irradiated C60 fullerol was linked to prolonged light exposure.
We next sought to investigate how light conditions could influence the growth of the anaerobic isolate in the presence of the larger C70 fullerol. The isolate was grown with 100 mg/L C70 fullerol in either ambient light or dark conditions, as a sole carbon source or with additional glucose (Figure 9). In this experiment, the positive control was also kept in either light or dark conditions, however because there was no significant difference between growth in the light or dark (p = 0.5), all statistical comparisons were made using the cell density of the light condition.
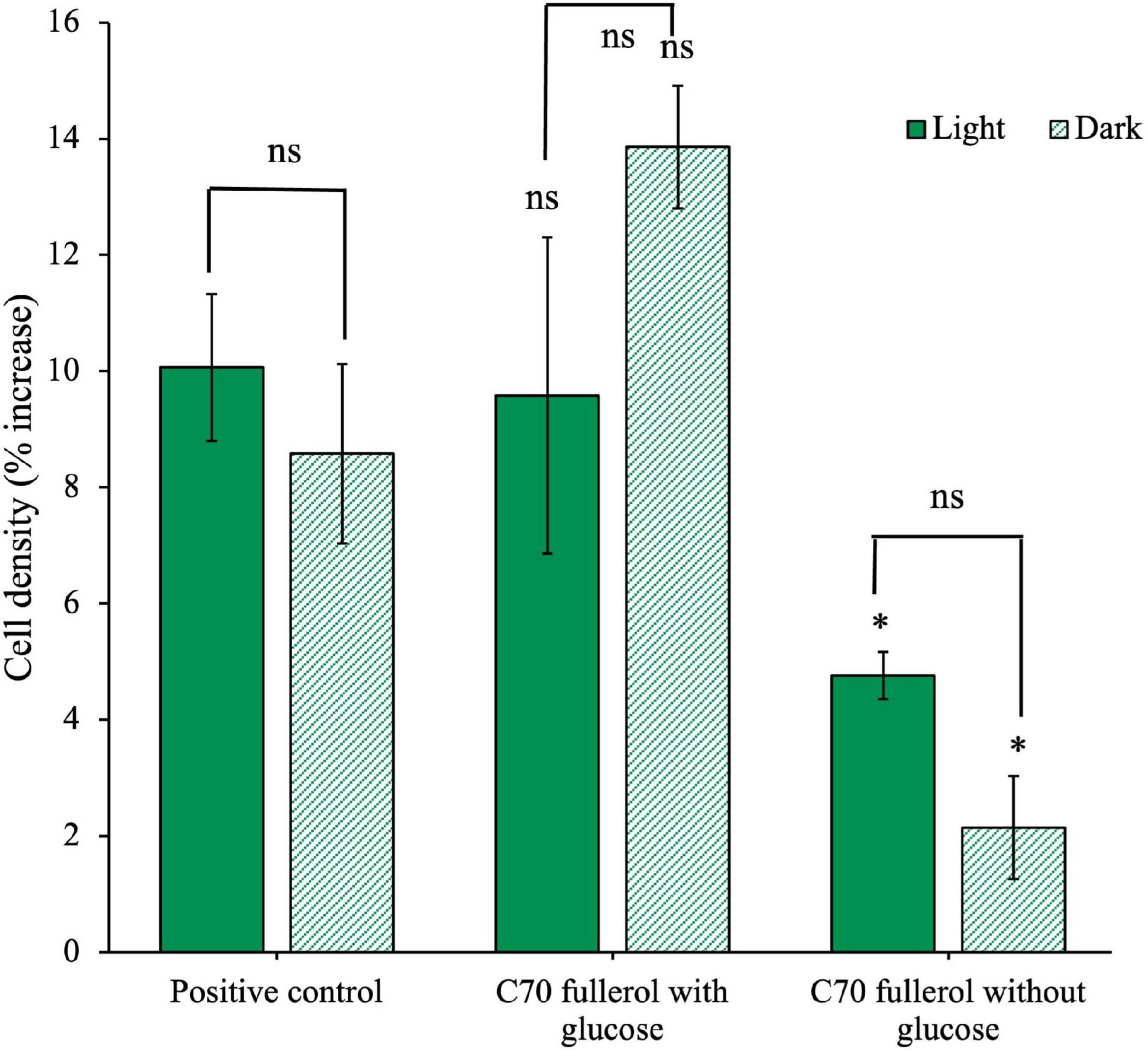
Figure 9. Effects of C70 fullerol on the growth of an anaerobic isolate. Cell density after 28 days of growth with C70-fullerol, with and without glucose under light or dark growth conditions.
We found no significant difference between either of the C70 fullerol conditions with glucose and the positive control (p = 0.88 and 0.82 for the light and dark conditions, respectively). Additionally, we found there was no significant difference between the C70 fullerol with glucose in light and dark conditions (p = 0.22), all of which indicate that as long as glucose is available, the C70 fullerol was not inhibitory and that exposure to light made no significant difference. When glucose was not available, however, growth in both the light and dark C70 fullerol conditions was significantly reduced compared to the positive control (p = 0.029 and 0.0068 for light and dark, respectively). Without glucose, growth was significantly less than the positive control. In this experiment, overall growth in all conditions was small (not exceeding 15% of cell starting concentrations) and thus should be treated with caution.
3.3 Fullerene particle sizing
UV-vis spectra and DLS measurements were taken of C60 and C70 suspensions in either distilled water or M9 media just after preparation, after 2 weeks of UV irradiation and after 4 weeks of UV irradiation. The results of the DLS measurements can be seen in Figure 10 where the limits indicate the spread of size distributions for each condition. DLS measurements indicate the presence of large aggregates, particularly in the M9 media, which confirms the observations made in the TEM images in Figure 3. In our suspensions, there was a greater propensity for large aggregate formation in M9 media compared to distilled water for both C60 and C70, which is in agreement with previous studies (Kyzyma et al., 2019). The initial aggregate sizes of both C60 and C70 in freshly prepared H2O suspensions (measurement series 1) were very similar (approximately 180 ± 100 nm hydrodynamic radius). In M9, the aggregate sizes are also similar to each other with 300 ± 70 nm and 330 ± 180 nm for C60 and C70, respectively.
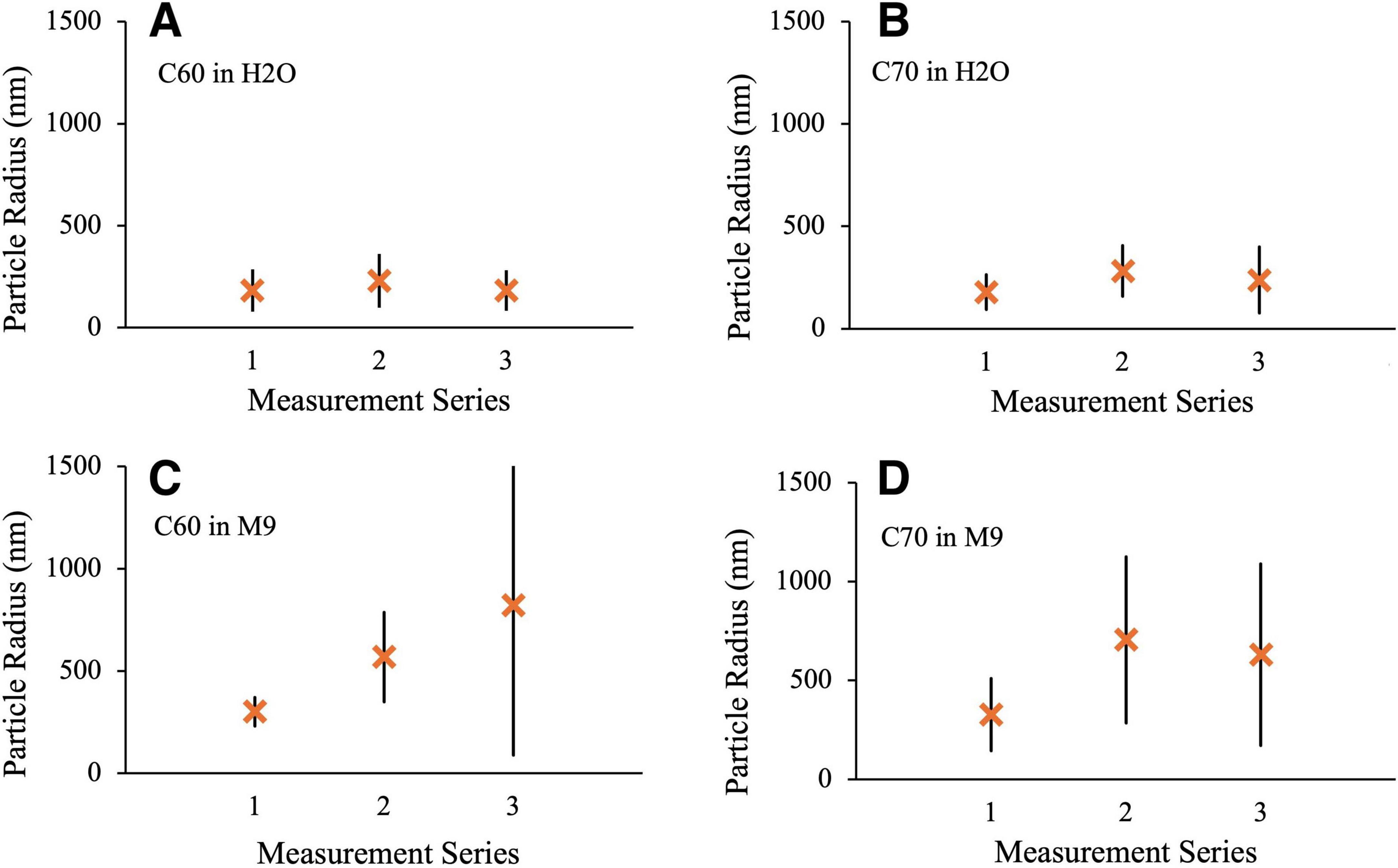
Figure 10. DLS measurements of aggregate radius. Series 1: fresh suspension, Series 2: After 2 weeks UV irradiation, Series 3: after 4 weeks UV irradiation. (A) 100 mg/L C60 in distilled H2O (B) 100 mg/L C70 in distilled H2O (C) 100 mg/L C60 in M9 (D) 100 mg/L C70 in M9.
Measurement series 2 was obtained after 2 weeks of UV exposure under conditions identical to those used for the microbial growth studies. In all cases, there was an increase in the size of the aggregates after UV irradiation, most notably for C60 and C70 in M9 where the mean radii increase to 570 ± 220 and 700 ± 420 nm, respectively. Doubling the UV exposure time to 4 weeks (measurement series 3) made very little difference to the particle radii and distributions for either H2O suspension or for C70 in M9, if anything slightly reducing the values. For C60 in M9, however, the mean radius and size range continued to increase with longer UV exposure to 820 ± 720 nm. Corresponding results for the C60 fullerol in M9 are shown in Figure 11. Here, the aggregate sizes in M9 before UV irradiation were somewhat smaller than for the non-functionalized fullerenes, 240 ± 160 nm with a smaller increase in aggregate size on irradiation to 330 ± 150 nm and 420 ± 160 nm after 2 and 4 weeks, respectively.
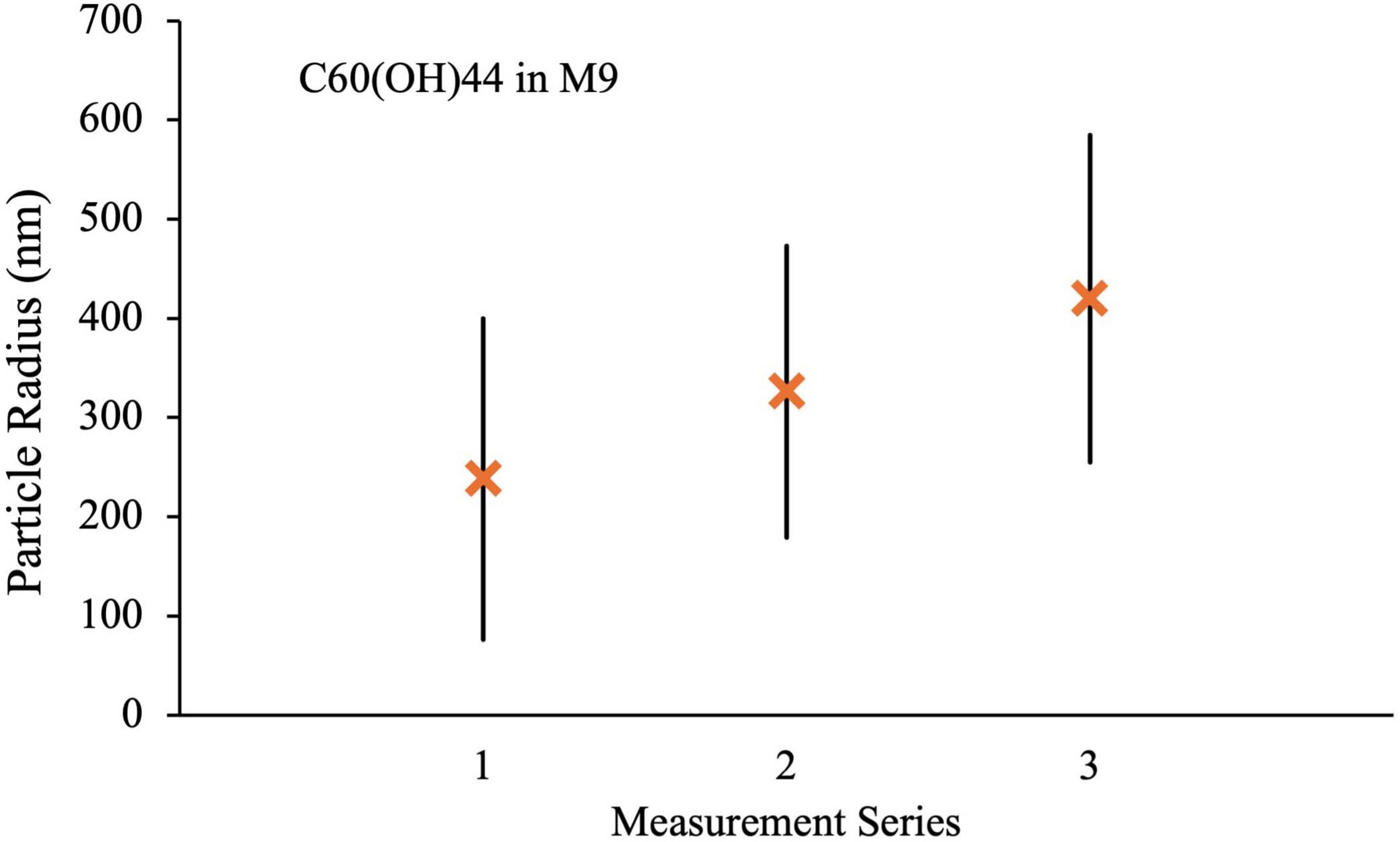
Figure 11. DLS measurements for C60(OH)44 in M9 media. Series 1: fresh suspension, Series 2: After 2 weeks UV irradiation, Series 3: after 4 weeks UV irradiation.
4 Discussion
4.1 Growth of C60 fullerenes with anaerobic microorganisms
With this study, we aimed to evaluate the interactions of fullerenes and their derivatives with modern anaerobic microorganisms for a better understanding of how these materials may have been used as a carbon source by early anaerobic life on Earth and other young planets receiving an extraterrestrial influx of organic materials.
Our initial experiments were conducted using an anaerobic community that had already been selected to grow on powdered meteorite containing a representative sample of the types of organics likely to have been present on the early Earth (Waajen et al., 2022). The community, originally derived from anaerobic pond sediment, was chosen for its environmental relevance as well as its predisposition to dealing with complex organic mixtures. In the original study by Waajen et al., a reduction in diversity was observed after the community was grown with powdered meteorite material, indicating that habitat filtering was taking place to produce a more specialized community. Growth with meteorite material created a niche that selected for certain species present in the original community, favoring species from the Deltaproteobacteria, Geobacteraceae and Desulfuromonadaceae families which are known to use sulfur and iron as electron acceptors (Waajen et al., 2022). In our experiments, we observed a further decrease in diversity when this community was grown with fullerene C60 as the carbon source. This is in line with the habitat filtering observed by Waajen et al., as by limiting the carbon source to C60, we created an even narrower niche for microbes to grow, increasing habitat selection pressure which results in fewer species present. Interestingly, we found that the majority of the species present in the community after growth with C60 belong to the Enterobacteriaceae family. Although this is a large family consisting of over 30 genera such as Escherichia, Klebsiella and Shigella, most are only facultative anaerobes. Other families present in the C60-adapted community were from the taxonomic families Pleomorphomonadaceae, Cellulomonadaceae, Xanthomonadaceae, Microbacteriacea and Rhizobiaceae, all of which are made up primarily of environmentally associated species found in soils.
We observed the anaerobic community to grow in the presence of only C60, suggesting that it may use this as a sole carbon source. However, in our four non-simultaneous growth experiments where the community was given 100 mg/L C60 as the sole carbon source, we found that the degree to which the C60 cultures grew compared to the positive controls varied between these seemingly identical experiments. There was consistently at least some degree of growth observed with C60 as the sole carbon source, and in some cases, growth was comparable to that of the community with glucose. We suggest that this discrepancy between experiment repeats might be caused by a shift in community composition over time arising from its complex microbial structure, even though starting conditions were similar. Similar variations in growth compared to positive controls were observed between ostensibly identical conditions in experiments involving fullerols.
With respect to growth on C60, we cannot completely rule out trace carbon in reagents that allowed for the growth of cells in C60. However, both Raman and FTIR spectroscopic analysis of dried M9 medium preparations did not reveal any contaminant carbon (data not shown) and glassware was acid washed and baked (see Materials and Methods). There is the potential for some carry over of organic carbon from the inoculum and we cannot rule out intracellular carbon reserves in cells which could allow for growth. However, given that growth with C60 (100 mg/L) in some of the experiments was not significantly different from positive control growth with glucose, we attribute these results to the potential use of C60 as a carbon source. Nevertheless, in all our experiments where low levels of growth were observed in fullerenes or fullerols and their derivatives in the absence of glucose, caution should be excercised in interpreting the use of these compounds as sole carbon sources. Isotopically labeled tracer experiments, microcalorimetry or “omics” approaches could be used to more directly investigate metabolic use of the compounds.
At the higher C60 concentration of 500 mg/L, although culture growth was the same as the positive control if supplementary glucose was available, it was significantly reduced when this additional carbon source was removed, and much lower than the growth obtained under similar conditions with 100 mg/L C60 (without glucose) suggesting that at these higher concentrations an inhibitory effect may be occurring which can be overcome if glucose is provided as a substrate. This result might be consistent with previous studies in which C60 was shown to reduce the growth of Pseudomonas fluorescens proportionally to the concentration of C60, meaning higher concentrations were more inhibitory (Riding et al., 2012).
Previous studies that have assessed the effect of C60 on anaerobic microorganisms are very limited. However, what literature is available is consistent with our observation that C60 is not inhibitory as it has not been found to negatively affect the growth, gas production or composition of microbial wastewater communities, although the exact microorganisms present in these communities are not specified (Nyberg et al., 2008; Zhao et al., 2018).
Our results contrast with some previous data. It has previously been found that C60 at 2.5 mg/L or higher is inhibitory to both Escherichia coli and Bacillus subtilis when grown both aerobically and anaerobically (Fortner et al., 2005). This concentration is significantly lower than that used in our experiments, and yet we do not observe this reported biotoxicity. However, Fortner et al. conducted their experiments with two species in pure cultures. In a community, however, we hypothesize that the diversity of species allows for some redundancy. It is more likely that even if some species are susceptible to C60 toxicity, others are better adapted to withstand it and will therefore become more dominant and able to grow, which may explain why we do not observe any inhibition. Interestingly, Auwerter et al. (2017) found that although lower concentrations of C60 did not affect the anaerobic community in their study when these microbes were exposed to higher concentrations, the number of microbes was initially reduced. However, after prolonged exposure, certain species possessing higher resistance to C60 were selected for and began to significantly increase in abundance (Auwerter et al., 2017). This is consistent with our proposal that microbial communities possess redundancy that makes them better able to resist fullerene biotoxicity. Certain species in the community used in our study may have been better equipped to use C60 and therefore when these species become more dominant through compositional shift, we observe the community as a whole to be growing on C60 as a sole carbon source.
We obtained TEM images of the anaerobic community after growth with C60 as the sole carbon source. We observed that some of the C60 in these cultures and non-biological controls formed flake-like structures in the M9 media, probably due to the salt content causing larger aggregates to form (Lyon et al., 2005) but confirmed with Raman spectroscopy that these still consisted of C60. The particle sizes in these images are consistent with the hydrodynamic particle radii we obtained from DLS measurements of the fullerene suspensions. Some of the C60 did remain in smaller aggregates that were similar in size and appearance to those observed under TEM by Lyon et al. (2006).
A particularly interesting feature present in our TEM images is the small black spherical clusters on the surface of the bacterial cells consistently visible when the anaerobic community was cultured with C60 as the sole carbon source. Similar structures have been previously observed under atomic force microscopy of E. coli after incubation with water-soluble fullerene derivatives, where it was proposed that these clusters were evidence of the fullerene derivative penetrating the microbial cells (Deryabin et al., 2014). Although in this case, the derivatives were antibacterial and it seemed this penetration resulted in detrimental electrostatic binding to cellular structures, the similarities between these and our TEM images suggest that membrane penetration of C60 clusters might be involved in the mechanism of penetration into the cells of the anaerobic community.
The anaerobic isolate, unlike the community, did not seem to be able to use C60 as a sole carbon source, although it was not inhibited as long as glucose was available. There are two possible reasons for this discrepancy. First, the community may be capable of degrading C60 only via a collaborative mechanism that relies on the combined biodegradation processes from multiple different metabolically diverse species. Syntrophic degradation of hydrocarbons, including PAHs (polycyclic aromatic hydrocarbons), has been reported previously (Gieg et al., 2014; Harindintwali et al., 2023) and it could be that different organisms can contribute different enzymatic capabilities that together enhance the degradation of fullerenes. Second, it may be that only a select few species present in the community are capable of using C60 and that the single species we isolated and cultured independently did not possess this ability. This would be consistent with the suggestion that the complexity of the microbial community results in a certain degree of redundancy that proves to be essential for the degradation of complex organics such as fullerenes.
4.2 Fullerene degradation under UV radiation and microbial growth
C60 aggregates in water, in the presence of oxygen, degrade readily when exposed to UV radiation, and the degradation products are less aggregated and have increased water stability (Hou et al., 2010; Hou and Jafvert, 2009; Lee et al., 2009; Sanchís et al., 2018). In our studies, the average size of the aggregates was seen to grow in M9 media when exposed to UVC under anoxic conditions for two weeks, although the total amount of carbon incorporated within these aggregates reduced with time. We also observed a distinct color change after irradiation that is consistent with the larger aggregate size. In the absence of oxygen, UV irradiation is known to induce photopolymerization of C60 by a 2 + 2 cycloaddition reaction via the triplet excited state (Zhou et al., 1993), however, we saw no evidence for polymerization products in the Raman spectra of the irradiated supernatant or precipitated material. The UVC irradiation of H2O is known to produce hydrogen peroxide and radical species such as HO2 and OH that can react with the fullerene cage to produce oxidized species, similar to the reaction products found in the presence of oxygen (Ou et al., 2019; Piskarev et al., 2018). These reactions are found to be enhanced for fullerenes in the triplet photoexcited state (Ou et al., 2019). Raman spectra of the irradiated fullerenes show that the cage structure remains intact but indicate the presence of oxidation by the small shift to lower wavenumbers and broadening of peaks (Supplementary Figure 7).
The anaerobic isolate was cultured with the irradiation products from C60, both with and without glucose, and we found the resulting cell densities to be statistically comparable to the positive control in both cases. This suggests that not only are the C60 UV irradiation products used by the isolate as a sole carbon source, but they also appear to be equally as accessible as glucose. This does seem to be consistent with results from previous studies; it is known that the combination of photodegradation and biodegradation significantly increases the rate of C60 mineralization in soils (Peng et al., 2020), and this enhanced degradation of UV-irradiated C60 has been observed in soil microcosms (Berry et al., 2016).
4.3 The effect of fullerene size on microbial growth and inhibition
The effect of C70 on both the community and the isolate deviated from what we observe with C60. The community, while able to utilize C60, did not seem to utilize C70 as a carbon source. If glucose was available, then the community was still able to grow normally with C70 at 100 mg/L, but at the higher concentration of 500 mg/L it becomes significantly inhibitory, even with glucose. A significant difference between the two fullerene species is their photoreactivity. Although triplet-mediated singlet oxygen production (the main source of production of reactive oxygen species and resulting toxicity in the presence of oxygen) is not relevant under the anoxic conditions of relevance here, there could still be triplet mediated processes in the complex M9 environment that lead to the observed differences.
Similarly to the results observed with C60, we observed differences between the community and the isolate. The isolate was not able to grow with C70, with or without glucose. This susceptibility to C70 toxicity could be a reflection of a species specific effect which is not seen in the community because of the redundancy provided by the community, in which some species may be better adapted to resist complete inhibition, while the isolate is evidently not. These results again underscore the importance in understanding community-level effects of these compounds on total community function. Results using isolated strains of microorganisms, although important, may not represent how whole communities respond to these compounds in natural environments.
4.4 The effect of fullerols on microbial growth and inhibition
The soluble fullerol derivatives of C60 and C70 exhibit significantly different effects on the anaerobic microbes in this study compared with their underivatised counterparts. While C60 seemed biologically accessible to the anaerobic community, C60 fullerol is inhibitory under identical growth conditions, even with supplementary glucose, although this inhibition seems to be reduced when cultures were grown in the dark. This trend is also observed with the anaerobic isolate, which is significantly inhibited by C60 fullerol in ambient light but not in the dark. When we exposed C60 fullerol suspensions to UV radiation under an anoxic atmosphere, they became significantly more inhibitory to both the community and the isolate, but only if the cultures were grown under prolonged ambient light exposure after inoculation. Interestingly, while C70 fullerene was inhibitory to both the community and isolate to varying degrees, C70 fullerol has no effect on the growth of either, provided additional glucose is available, but the lack of available literature on the effects of C70 and its fullerol derivative makes it difficult to form any consensus on the reproducibility of this observation. The difference between light and dark conditions indicates that the photoreactivity is key. Infrared spectra show that after prolonged exposure to light there was a reduction in the number of OH ligands attached to the cage (Supplementary Material, Figure 8). The OH radical is highly oxidizing in nature and the release of this ROS could be the reason for the inhibitory nature of the fullerol species in these experiments.
Several previous studies have found that C60 fullerol does not exhibit antibacterial activity despite its photoactivity (Chiang et al., 1995; Li et al., 2008), and is even used by soil microbes as a carbon source (Berry et al., 2017). This is contradictory to our results; however, a major difference is that these previous studies were conducted aerobically and ours was carried out with anaerobic species in an anoxic environment. ROS are generated intracellularly as a product of aerobic metabolism, and therefore aerobic bacteria possess mechanisms to counteract the effects of oxidation and can tolerate low levels of ROS (Nasim and Hamblin, 2017). Anaerobes, on the other hand, can escape oxidative damage by living in anoxic habitats and therefore some may not have mechanisms to mitigate the damage of reactive oxygen (Seixas et al., 2022). It is therefore possible that the aerobic species from previous studies are less susceptible to fullerol biotoxicity than the anaerobes used in our experiments as they are better equipped to deal with the oxidative stress fullerols exert.
C60 fullerol produces reactive oxygen species (ROS) when exposed to both UV radiation and visible light (Pickering and Wiesner, 2005). ROS are generated at a much higher rate under UV radiation (Pickering and Wiesner, 2005), which may explain why the greatest biotoxicity we observed was from irradiated C60 fullerol which was kept under prolonged visible light exposure during culturing as this condition likely produces the highest ROS concentration. Additionally, ROS tend to be short-lived due to their high reactivity, therefore those produced under UV radiation are likely neutralized quickly within the suspension and therefore do not have a chance to exert oxidative effects on the microbes when incubated in the dark. When under constant visible light exposure, however, this supply of ROS may be continually generated, leading to the greater antibacterial activity we observed in the cultures kept in ambient light. This effect is important from an early Earth perspective as constant UV radiation exposure would presumably increase the biotoxicity of any fullerols in the environment.
If ROS generation is indeed the cause of the biotoxicity we observed when microorganisms are cultured with fullerols, then we might expect to find the same inhibition in the C60 suspensions as previous studies claim to have detected ROS production in photosensitized C60 suspensions (Kamat et al., 2000). However, Lyon et al. (2008) did not observe any ROS-mediated inhibition to the growth of E. coli or B. subtilis after exposure to C60, and neither did they detect any ROS production in the suspensions (Lyon et al., 2008). After further investigation, these authors propose that C60 itself reacts with the dyes used in ROS colorimetric assays, giving false positive results, and that in actuality fullerene suspensions do not produce ROS in any detectable amount (Lyon et al., 2008). Hotze et al. (2008) similarly went on to explain the absence of ROS in UV-irradiated C60 suspensions by comparing the density of fullerene aggregates to their fullerol derivatives: more densely aggregated C60 is less likely to produce singlet oxygen than the more loosely packed fullerol derivative (Hotze et al., 2008). The less densely-packed fullerols have a larger fullerol-water interface, favoring ROS production, as well as a longer-lived triplet state which leads to a higher generation of singlet oxygen (Hotze et al., 2008).
The microbial accessibility of UV-degraded C60 is consistent with the conjecture that microbes on the early Earth could have used extra-terrestrial fullerenes as a carbon source, but the toxicity of their soluble derivatives may counteract this to some extent. Before speculating on the significance of fullerol toxicity on the early Earth, it is important to consider whether or not fullerols would even have been present in this environment. A recent study by Sanchís et al. (2018) concluded that fullerols are not produced from UV exposure in fullerene water suspensions, and so if this is the case, it is possible that fullerols might not have been present on the early Earth in high enough quantities to make them a significant component of the various fullerenes to which early organisms were exposed.
5 Conclusion
Our findings indicate that fullerene C60 is biologically accessible for utilization as a carbon source by an anaerobic microbial community. We do not observe this when a single species from this community is grown in isolation, which indicates either C60 degradation is a collaborative mechanism, or the isolate selected does not possess the individual ability to metabolize fullerenes as well as other species in the community. When C60 is exposed to environmental conditions relevant to the early Earth, namely short-wave UV radiation exposure in anoxic media, the degradation products provide an accessible carbon source for the anaerobic isolate, which is more indicative of how fullerenes may have supported early heterotrophic growth. C60 fullerol is highly inhibitory to both the community and the isolate, particularly when exposed to UV radiation and kept under ambient light. However, it is possible that fullerols are not produced from fullerene suspensions under environmental conditions in any amount sufficient to inhibit growth, and therefore their biotoxicity may not be as relevant when considering early microbes and their response to extra-terrestrial fullerenes. C70 fullerene is not accessible as a carbon source for the anaerobic microorganisms in this study, and at sufficient concentrations can be inhibitory. The difference in the behavior of C60 and C70 may be attributable to the difference in photophysical properties and chemical reactivity.
Our work shows how fullerenes and their derivatives could potentially have interacted with anaerobic life on a young Earth. Although the presence of a carbon source is assumed to be good for habitability, we show that microbial interactions with these molecules under anoxic conditions are complex, providing a food source or being inhibitory depending on environmental conditions. These results also have implications for the environmental impact of these industrially-produced compounds in anoxic environments on Earth in the present day.
Data availability statement
The data presented in the study are deposited in the Datashare repository, accession: https://datashare.ed.ac.uk/handle/10283/8828.
Author contributions
EB: Conceptualization, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. AG: Investigation, Methodology, Writing – original draft, Writing – review & editing. EC: Formal Analysis, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. CC: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by a Leverhulme Trust Research Project Grant, RPG-397 Cosmic Chemistry as Food for Life. CC was funded by the Science and Technology Facilities Council (STFC) (grant ST/Y001788/1).
Acknowledgments
We would like to thank Stephen Mitchell from the University of Edinburgh, for preparing samples and overseeing TEM imaging. Additionally, we acknowledge Novogene UK for performing the 16S rDNA sequencing detailed in this study and Dr. Jochen Arlt, Dept of Physics, University of Edinburgh for carrying out DLS and DDM measurements. We also thank Annemiek Waajen for providing the original microbial community sample. The article is based upon work from COST Action CA 21126 Carbon molecular nanostructures in space (NanoSpace), supported by COST (European Cooperation in Science and Technology).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1511842/full#supplementary-material
References
Auwerter, L. C. C., Ouki, S. K., Asaadi, M., and Shana, A. (2017). Effects of nanosized titanium dioxide (TiO2) and fullerene (C60) on wastewater microorganisms activity. J. Water Process Eng. 16, 35–40. doi: 10.1016/j.jwpe.2016.12.006
Avanasi, R., Jackson, W. A., Sherwin, B., Mudge, J. F., and Anderson, T. A. (2014). C60 fullerene soil sorption, biodegradation, and plant uptake. Environ. Sci. Technol. 48, 2792–2797. doi: 10.1021/es405306w
Berry, T. D., Clavijo, A. P., Zhao, Y., Jafvert, C. T., Turco, R. F., and Filley, T. R. (2016). Soil microbial response to photo-degraded C60 fullerenes. Environ. Pollut. 211, 338–345. doi: 10.1016/j.envpol.2015.12.025
Berry, T. D., Filley, T. R., Clavijo, A. P., Bischoff Gray, M., and Turco, R. (2017). Degradation and microbial uptake of C60 fullerols in contrasting agricultural soils. Environ. Sci. Technol. 51, 1387–1394. doi: 10.1021/acs.est.6b04637
Botta, O., and Bada, J. L. (2002). Extraterrestrial organic compounds in meteorites. Surv. Geophys. 23, 411–467. doi: 10.1023/A:1020139302770
Brant, J. A., Labille, J., Robichaud, C. O., and Wiesner, M. (2007). Fullerol cluster formation in aqueous solutions: Implications for environmental release. J. Colloid Interface Sci. 314, 281–288. doi: 10.1016/J.JCIS.2007.05.020
Brunet, L., Lyon, D. Y., Hotze, E. M., Alvarez, P. J. J., and Wiesner, M. R. (2009). Comparative photoactivity and antibacterial properties of C60 fullerenes and titanium dioxide nanoparticles. Environ. Sci. Technol. 43, 4355–4360. doi: 10.1021/ES803093T
Chae, S.-R., Hotze, E. M., and Wiesner, M. R. (2009). Evaluation of the oxidation of organic compounds by aqueous suspensions of photosensitized hydroxylated-C60 fullerene aggregates. Environ. Sci. Technol. 43, 6208–6213. doi: 10.1021/ES901165Q
Chae, S. R., Hunt, D. E., Ikuma, K., Yang, S., Cho, J., Gunsch, C. K., et al. (2014). Aging of fullerene C60 nanoparticle suspensions in the presence of microbes. Water Res. 65, 282–289. doi: 10.1016/j.watres.2014.07.038
Chiang, L. Y., Lu, F. J., and Lin, J. T. (1995). Free radical scavenging activity of water-soluble fullerenols. J. Chem. Soc. Chem. Commun. 0, 1283–1284. doi: 10.1039/C39950001283
Chyba, C. F., Thomas, P. J., Brookshaw, L., and Sagan, C. (1990). Cometary delivery of organic molecules to the early earth. Science 249, 366–373. doi: 10.1126/science.11538074
Cnossen, I., Sanz-Forcada, J., Favata, F., Witasse, O., Zegers, T., and Arnold, N. F. (2007). Habitat of early life: Solar X-ray and UV radiation at Earth’s surface 4–3.5 billion years ago. J. Geophys. Res. Planets 112:2008. doi: 10.1029/2006JE002784
Cockell, C. S. (1998). Biological effects of high ultraviolet radiation on rarly Earth-a theoretical evaluation. J. Theor. Biol. 193, 717–729. doi: 10.1006/jtbi.1998.0738
Deryabin, D. G., Davydova, O. K., Yankina, Z. Z., Vasilchenko, A. S., Miroshnikov, S. A., Kornev, A. B., et al. (2014). The activity of [60]fullerene derivatives bearing amine and carboxylic solubilizing groups against Escherichia coli: A comparative study. J. Nanomater. 2014. doi: 10.1155/2014/907435
Duncan, L. K., Jinschek, J. R., and Vikesland, P. J. (2008). C60 colloid formation in aqueous systems: Effects of preparation method on size, structure, and surface charge. Environ. Sci. Technol. 42, 173–178. doi: 10.1021/es071248s
Eaton, A. D., and Franson, M. A. H. (2005). Standard Methods for the Examination of Water and Wastewater, 21st Edn. Washington, DC: American Public Health Association.
Ehrenfreund, P., Spaans, M., and Holm, N. G. (2011). The evolution of organic matter in space. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 369, 538–554. doi: 10.1098/rsta.2010.0231
Fang, J., Lyon, D. Y., Wiesner, M. R., Dong, J., and Alvarez, P. J. J. (2007). Effect of a fullerene water suspension on bacterial phospholipids and membrane phase behavior. Environ. Sci. Technol. 41, 2636–2642. doi: 10.1021/es062181w
Fortner, J. D., Lyon, D. Y., Sayes, C. M., Boyd, A. M., Falkner, J. C., Hotze, E. M., et al. (2005). C60 in water: Nanocrystal formation and microbial response. Environ. Sci. Technol. 39, 4307–4316. doi: 10.1021/es048099n
Gieg, L. M., Fowler, S. J., and Berdugo-Clavijo, C. (2014). Syntrophic biodegradation of hydrocarbon contaminants. Curr. Opin. Biotech. 27, 21–29. doi: 10.1016/j.copbio.2013.09.002
Hancock, D. E., Indest, K. J., Gust, K. A., and Kennedy, A. J. (2012). Effects of C60 on the Salmonella typhimurium TA100 transcriptome expression: Insights into C60-mediated growth inhibition and mutagenicity. Environ. Toxicol. Chem. 31, 1438–1444. doi: 10.1002/etc.1848
Harindintwali, J. D., Xiang, L., Wang, F., Chang, S. X., Zhao, Z., Mei, Z., et al. (2023). Syntrophy of bacteria and archaea in the anaerobic catabolism of hydrocarbon contaminants. Crit. Rev. Environ. Sci. Techn. 53, 1331–1357. doi: 10.1080/10643389.2022.2134702
Hotze, E. M., Badireddy, A. R., Chellam, S., and Wiesner, M. R. (2009). Mechanisms of bacteriophage inactivation via singlet oxygen generation in UV illuminated fullerol suspensions. Environ. Sci. Technol. 43, 6639–6645. doi: 10.1021/ES901110M
Hotze, E. M., Labille, J., Alvarez, P., and Wiesner, M. R. (2008). Mechanisms of photochemistry and reactive oxygen production by fullerene suspensions in water. Environ. Sci. Technol. 42, 4175–4180. doi: 10.1021/ES702172W
Hou, W. C., and Jafvert, C. T. (2009). Photochemical transformation of aqueous C clusters in sunlight. Environ. Sci. Technol. 43, 362–367. doi: 10.1021/es802465z
Hou, W. C., Kong, L., Wepasnick, K. A., Zepp, R. G., Fairbrother, D. H., and Jafvert, C. T. (2010). Photochemistry of aqueous C60 clusters: Wavelength dependency and product characterization. Environ. Sci. Technol. 44, 8121–8127. doi: 10.1021/es101230q
Huang, L., Wang, M., Dai, T., Sperandio, F. F., Huang, Y. Y., Xuan, Y., et al. (2014). Antimicrobial photodynamic therapy with decacationic monoadducts and bisadducts of [70]fullerene: In vitro and in vivo studies. Nanomed. 9, 253–266. doi: 10.2217/NNM.13.22/SUPPL_FILE/SUPPL_INFORMATION.DOCX
Huang, L., Wang, M., Sharma, S., Sperandio, F., Maragani, S., Nayka, S., et al. (2013). Decacationic [70]Fullerene approach for efficient photokilling of infectious bacteria and cancer cells. ECS Trans. 45, 65–73. doi: 10.1149/04520.0065ECST/XML
Hwang, Y. S., and Li, Q. (2010). Characterizing photochemical transformation of aqueous nC60 under environmentally relevant conditions. Environ. Sci. Technol. 44, 3008–3013. doi: 10.1021/es903713j
Kamat, J. P., Devasagayam, T. P. A., Priyadarsini, K. I., and Mohan, H. (2000). Reactive oxygen species mediated membrane damage induced by fullerene derivatives and its possible biological implications. Toxicology 155, 55–61. doi: 10.1016/S0300-483X(00)00277-8
Kokubo, K., Shirakawa, S., Kobayashi, N., Aoshima, H., and Oshima, T. (2011). Facile and scalable synthesis of a highly hydroxylated water-soluble fullerenol as a single nanoparticle. Nano Res. 4, 204–215. doi: 10.1007/s12274-010-0071-z
Kroto, H. (1988). Space, stars, C60, and soot. Science 242, 1139–1145. doi: 10.1126/science.242.4882.1139
Kyzyma, O. A., Avdeev, M. V., Bolshakova, O. I., Melentev, P., Sarantseva, S. V., Ivankov, O. I., et al. (2019). State of aggregation and toxicity of aqueous fullerene solutions. Appl. Surf. Sci. 483, 69–75. doi: 10.1016/j.apsusc.2019.03.167
Lee, J., Cho, M., Fortner, J. D., Hughes, J. B., and Kim, J. H. (2009). Transformation of aggregated C60 in the aqueous phase by UV irradiation. Environ. Sci. Technol. 43, 4878–4883. doi: 10.1021/es8035972
Leroy, M., Jassey, V. E., Silvestre, J., Barret, M., Flahaut, E., and Larue, C. (2024). Carbon nanotubes alter agrosystem multifunctionality. Enviro. Sci. 11, 4126–4137. doi: 10.1039/D4EN00195H
Li, Q., Mahendra, S., Lyon, D. Y., Brunet, L., Liga, M. V., Li, D., et al. (2008). Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 42, 4591–4602. doi: 10.1016/J.WATRES.2008.08.015
Liu, W., Wang, Z., Chai, G., and Deng, W. (2023). Effect of carbon nanomaterials on functional diversity and structure of soil microbial community under single and repeated exposures. Environ. Sci. Poll. Res. 30, 115896–115906. doi: 10.1007/s11356-023-30653-y
Lyon, D. Y., Adams, L. K., Falkner, J. C., and Alvarez, P. J. J. (2006). Antibacterial activity of fullerene water suspensions: Effects of preparation method and particle size. Environ. Sci. Technol. 40, 4360–4366. doi: 10.1021/es0603655
Lyon, D. Y., Brunet, L., Hinkal, G. W., Wiesner, M. R., and Alvarez, P. J. J. (2008). Antibacterial activity of fullerene water suspensions (nC60) is not due to ROS-mediated damage. Nano Lett. 8, 1539–1543. doi: 10.1021/NL0726398
Lyon, D. Y., Fortner, J. D., Sayes, C. M., Colvin, V. L., and Hughes, J. B. (2005). Bacterial cell association and antimicrobial activity of a C60 water suspension. Environ. Toxicol. Chem. 24, 2757–2762. doi: 10.1897/04-649R.1
Ma, J., Guo, R., and Tan, X. (2020). Aqueous photochemistry of fullerol revisited: Energy transfer vs. electron transfer processes probed by Rhodamine B degradation. J. Photochem. Photobiol. Chem. 397:112600. doi: 10.1016/J.JPHOTOCHEM.2020.112600
Maier, J. P., and Campbell, E. K. (2017). Fullerenes in space. Angew. Chem. Int. Ed. 56, 4920–4929. doi: 10.1002/anie.201612117
Nasim, K., and Hamblin, M. R. (2017). Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 31:31. doi: 10.1016/j.drup.2017.07.003
Navarro, D. A., Kookana, R. S., McLaughlin, M. J., and Kirby, J. K. (2015). Fullerol as a potential pathway for mineralization of fullerene nanoparticles in biosolid-amended soils. Environ. Sci. Technol. Lett. 3, 7–12. doi: 10.1021/ACS.ESTLETT.5B00292
Nyberg, L., Turco, R. F., and Nies, L. (2008). Assessing the impact of nanomaterials on anaerobic microbial communities. Environ. Sci. Technol. 42, 1938–1943. doi: 10.1021/es072018g
Ou, Y., Wu, J., Meyer, J. R., Foston, M., Fortner, J. D., and Li, W. (2019). Photoenhanced oxidation of nC60 in water: Exploring H2O2 and hydroxyl radical based reactions. Chem. Eng. J. 360, 665–672. doi: 10.1016/j.cej.2018.12.035
Ouyang, K., Dai, K., Walker, S. L., Huang, Q., Yin, X., and Cai, P. (2016). Efficient photocatalytic disinfection of Escherichia coli O157:H7 using C70-TiO2 hybrid under visible light irradiation. Nat. Publ. Group 6:25702. doi: 10.1038/srep25702
Pasek, M., and Lauretta, D. (2008). Extraterrestrial flux of potentially prebiotic C, N, and P to the early earth. Orig. Life Evol. Biospheres 38, 5–21. doi: 10.1007/s11084-007-9110-5
Peng, Z., Liu, X., Zhang, W., Zeng, Z., Liu, Z., Zhang, C., et al. (2020). Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 134:105298. doi: 10.1016/j.envint.2019.105298
Pickering, K. D., and Wiesner, M. R. (2005). Fullerol-sensitized production of reactive oxygen species in aqueous solution. Environ. Sci. Technol. 39, 1359–1365. doi: 10.1021/ES048940X
Piskarev, I. M., Astaf’eva, K. A., and Ivanova, I. P. (2018). The UV-C radiation mechanism to form HO2 radicals from water. J. Phys. Conf. Ser. 1094:012024. doi: 10.1088/1742-6596/1094/1/012024
Riding, M. J., Martin, F. L., Trevisan, J., Llabjani, V., Patel, I. I., Jones, K. C., et al. (2012). Concentration-dependent effects of carbon nanoparticles in Gram-negative bacteria determined by infrared spectroscopy with multivariate analysis. Environ. Pollut. 163, 226–234. doi: 10.1016/j.envpol.2011.12.027
Ruoff, R. S., Tse, D. S., Malhotra, R., and Lorents, D. C. (1993). Solubility of C60 in a variety of solvents. J. Phys. Chem. 97, 3379–3383. doi: 10.1021/j100115a049
Sabbah, H., Carlos, M., Jenniskens, P., Shaddad, M. H., Duprat, J., Goodrich, C. A., et al. (2022). Detection of cosmic fullerenes in the Almahata Sitta Meteorite: Are they an interstellar heritage? Astrophys. J. 931:91. doi: 10.3847/1538-4357/ac69dd
Sanchís, J., Aminot, Y., Abad, E., Jha, A. N., Readman, J. W., and Farré, M. (2018). Transformation of C60 fullerene aggregates suspended and weathered under realistic environmental conditions. Carbon 128, 54–62. doi: 10.1016/j.carbon.2017.11.060
Schönheit, P., Buckel, W., and Martin, W. F. (2016). On the origin of heterotrophy. Trends Microbiol. 24, 12–25. doi: 10.1016/j.tim.2015.10.003
Seixas, A. F., Quendera, A. P., Sousa, J. P., Silva, A. F. Q., Arraiano, C. M., and Andrade, J. M. (2022). Bacterial response to oxidative stress and RNA oxidation. Front. Genet. 12:821535. doi: 10.3389/fgene.2021.821535
Semenov, K. N., Charykov, N. A., Postnov, V. N., Sharoyko, V. V., Vorotyntsev, I. V., Galagudza, M. M., et al. (2016). Fullerenols: Physicochemical properties and applications. Prog. Solid State Chem. 44, 59–74. doi: 10.1016/j.progsolidstchem.2016.04.002
Taylor, R., Parsons, J. P., Avent, A. G., Rannard, S. P., Dennis, T. J., Hare, J. P., et al. (1991). Degradation of C60 by light. Nature 351:277. doi: 10.1038/351277A0
Tsao, N., Luh, T. Y., Chou, C. K., Chang, T. Y., Wu, J. J., Liu, C. C., et al. (2002). In vitro action of carboxyfullerene. J. Antimicrob. Chemother. 49, 641–649. doi: 10.1093/jac/49.4.641
Waajen, A. C., Prescott, R., and Cockell, C. S. (2022). Meteorites as food source on early Earth: Growth, selection, and inhibition of a microbial community on a carbonaceous chondrite. Astrobiology 22, 495–508. doi: 10.1089/ast.2021.0089
Wang, J., Ma, Q., Zhang, Z., Diko, C., and Qu, Y. (2020). Biogenic Fenton-like reaction involvement in anaerobic degradation of C60 by Labrys sp. WJW. Environ. Pollut. 272:115300. doi: 10.1016/j.envpol.2020.115300
Zhao, L., Ji, Y., Sun, P., Li, R., Xiang, F., Wang, H., et al. (2018). Effects of individual and complex ciprofloxacin, fullerene C60, and ZnO nanoparticles on sludge digestion: Methane production, metabolism, and microbial community. Bioresour. Technol. 267, 46–53. doi: 10.1016/j.biortech.2018.07.024
Zhou, P., Dong, Z.-H., Rao, A. M., and Eklund, P. C. (1993). Reaction mechanism for the photopolymerization of solid fullerene C60. Chem. Phys. Lett. 211, 337–340. doi: 10.1016/0009-2614(93)87069-F
Zuo, Y., Zeng, W., and Huang, J. (2024). Effects of exposure to carbon nanomaterials on soil microbial communities: A global meta-analysis. Land Degrad. Develop. 35, 238–248. doi: 10.1002/ldr.4912
Keywords: fullerene, fullerol, C60, C70, anaerobic, microorganism, bacteria, early Earth
Citation: Bethune E, Gromov A, Campbell EEB and Cockell CS (2025) Harnessing cosmic carbon: anaerobic microbial responses to fullerenes under early Earth conditions. Front. Microbiol. 16:1511842. doi: 10.3389/fmicb.2025.1511842
Received: 15 October 2024; Accepted: 12 June 2025;
Published: 04 August 2025.
Edited by:
Philippe M. Oger, UMR5240 Microbiologie, Adaptation et Pathogenie (MAP), FranceReviewed by:
Timothy Berry, University of Wisconsin-Madison, United StatesJinsong Liang, Hebei University of Technology, China
Copyright © 2025 Bethune, Gromov, Campbell and Cockell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charles S. Cockell, Yy5zLmNvY2tlbGxAZWQuYWMudWs=
 Elle Bethune
Elle Bethune Andrey Gromov
Andrey Gromov Eleanor E. B. Campbell
Eleanor E. B. Campbell Charles S. Cockell
Charles S. Cockell