- 1Nanjing Drum Tower Hospital Clinical College of Nanjing University of Chinese Medicine, Nanjing, China
- 2Department of Hepatobiliary Surgery, Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, China
- 3Jinan Microecological Biomedicine Shandong Laboratory, Jinan, Shandong, China
Objective: The aims of the present study were to determine the efficacy of edible traditional Chinese medicines (ETCMs) in treating constipation, verify their laxative effects, and conduct preliminary investigations into their mechanisms of action.
Methods: ICR mice were treated with loperamide to induce constipation, and various fecal parameters, including fecal volume, water content, and intestinal transport function, were measured in these constipation model mice to screen for ETCMs with laxative properties. The mechanism of action was preliminarily explored by examining changes in the intestinal mucosal structure, protein expression levels, and alterations in intestinal flora composition.
Results: In ICR mice with loperamide-induced constipation, Elsholtzia ciliata aqueous extract (ECAE) and Hovenia dulcis aqueous extract (HDAE) significantly ameliorated constipation symptoms, mitigated colonic pathological tissue damage, significantly increased the expression levels of proteins associated with the promotion of intestinal peristalsis [Stem Cell Factor Receptor (c-Kit) and Stem Cell Factor (SCF)] and the maintenance of the intestinal barrier [Zonula Occludens-1 (ZO-l), Occludin and Claudin-l], and promoted beneficial intestinal bacterial colonization.
Conclusion: ECAE and HDAE ameliorated constipation in mice, and their mechanism of action may be related to the increased abundance of intestinal bacteria such as Turicibacter, Olsenella, and Odoribacter, which contribute to higher butyrate production. This increase in butyric acid reduces inflammation, improves intestinal barrier function, and increases the abundance of beneficial intestinal bacteria.
1 Introduction
Constipation is a common functional gastrointestinal disorder with symptoms including hard dry stools, inability to pass stools and incomplete bowel movements (Bharucha and Lacy, 2020). The appearance of these symptoms is often closely related to abnormal intestinal function. Studies have shown that the occurrence of constipation may be related to enteric nervous system dysfunction, visceral allergic reactions, abnormal distribution of interstitial Cajal cells and changes in gastrointestinal regulatory peptide levels (King et al., 2010). Because of the difficulty of defecation, constipation patients often experience a loss of appetite, stomach pain, heartburn, acid reflux, heartburn, nausea, vomiting and other gastrointestinal symptoms (Varni et al., 2015). These symptoms not only affect the patient's daily diet and sleep but also cause mood swings and increase psychological stress. Moreover, long-term constipation may also lead to complications such as toxemia, metabolic disorders and even neurasthenia, posing a threat to the physical and mental health of patients (Sumida et al., 2019). The medical community has developed various methods for constipation treatment. Medications represent one of the most used methods. For example, stool softeners can increase stool moisture for easier discharge; osmotic laxatives increase the intestinal osmotic pressure and stimulate intestinal peristalsis by absorbing intestinal water; and stimulant laxatives can directly stimulate the intestinal mucosa and intestinal wall nerve plexus and promote intestinal propulsive peristalsis (Bharucha and Wald, 2019). However, although these drugs can relieve symptoms, they are also associated with side effects such as diarrhea, nausea, abdominal pain and headache, and long-term use can lead to drug dependence and high recurrence rates (Chang et al., 2014). Therefore, there is an urgent need to discover drugs that can safely treat constipation without side effects; ideally, these drugs could be incorporated into daily diets.
Lifestyle, diet, sex, regional differences, and drugs all affect the host's gut flora (Ohkusa et al., 2019; Fan et al., 2022). There are many reports about changes in the intestinal flora during constipation and the recovery of the intestinal flora after treatment (Zhou et al., 2022; Wang R. et al., 2023). The intestinal flora structure of patients with constipation is often disordered (Hofman et al., 2022). Studies have shown that traditional Chinese medicines (TCMs) can effectively relieve constipation by regulating the intestinal microecology, increasing the number of beneficial intestinal bacteria and inhibiting the growth of harmful bacteria (Gao et al., 2021; Zhang et al., 2023). In mechanistic studies, TCMs may indirectly affect the composition and function of the intestinal flora by regulating the inflammatory response, improving intestinal barrier function, and regulating neuroendocrine function (Zhan et al., 2022). The application of 16S rRNA high-throughput sequencing has also facilitated the study of the gut microbiota. The authors employed 16S rRNA sequencing to compare the changes in the intestinal flora before and after modeling in mice (Zhan et al., 2022). The aim of this approach was to investigate the proportion of beneficial bacteria regulated by TCMs that may alleviate constipation.
The pathophysiological mechanisms underlying chronic constipation in humans are highly complex, encompassing not only aberrant colonic motor function, such as increased non-propulsive contractions and prolonged transit time, but also including abnormalities in rectal and anal sphincter function, as well as diminished contractile function of the pelvic floor muscles (Scott et al., 2021; Kilgore and Khlevner, 2024). These factors collectively contribute to difficulties in normal defecation. Loperamide is a commonly used symptomatic drug for the treatment of diarrhea; loperamide mainly delays intestinal peristalsis through multiple mechanisms, such as activating opioid receptors, inhibiting intestinal secretion, increasing endogenous analgesia, and affecting neuroregulation, to reduce the frequency of intestinal emptying (Hughes et al., 1984). Although there are notable differences in the pathophysiology between loperamide-induced constipation and human chronic idiopathic constipation, the clinical manifestations of loperamide-induced constipation are comparable, characterized by reduced bowel movement frequency and quantity, as well as decreased intestinal propulsion rate. Therefore, loperamide hydrochloride was used to establish a constipation model in this study.
According to the information released by China's State Administration for Market Regulation, a total of 110 TCMs have been confirmed to be edible traditional Chinese medicines (ETCMs), which undoubtedly opens up a new way for the application of TCM in modern society. ETCM refers to certain foods and medications that exhibit similar or identical therapeutic effects; thus, health care objectives and disease treatment could be achieved through the consumption of these items in the diet (He et al., 2024). In recent years, ETCMs have gained widespread attention for their unique health and therapeutic effects, which prompted the authors to choose those to regulate constipation through diet. According to ancient Chinese herbal medicine texts such as the herbaceous works of past dynasties as well as modern Chinese Pharmacopeia records, ETCMs such as Illicium verum, Zoacys dhumnades, Citrus aurantium, Ginkgo biloba, Elsholtzia ciliata, Hovenia dulcis, Agkistrodon halys, and Lonicera hypoglauca have shown promising results in treating illnesses unrelated to gastrointestinal function. However, after extensive literature research and studies, no conclusive findings on the laxative effect of these ETCMs have been found.
In this study, by establishing a loperamide-induced constipation model in ICR mice, two ETCMs with potential laxative effects, E. ciliata and H. dulcis, were identified. A preliminary mechanistic study of E. ciliata aqueous extract (ECAE) and H. dulcis aqueous extract (HDAE) was subsequently conducted to provide a theoretical and experimental basis for the development and utilization of ETCMs.
2 Materials and methods
2.1 Materials
2.1.1 Animals experiments and ethics
Female ICR mice, body mass 20–25 g, 6 weeks old, purchased from Yangzhou University Center for Comparative Medicine, Laboratory Animal Production License No.: SCXK2022-0009. Animal experiments were approved by the Animal Ethical and Welfare Committee of NJU (SYXK2019-0056). Housed in isolation cages, free to feed and drink, and started experiments after 3 days of acclimatization rearing.
2.1.2 Reagents and instruments
The E. ciliata and H. dulcis were purchased from Bozhou Jingwan Traditional Chinese Medicine Drinking Tablets Factory, China. Loperamide hydrochloride (Manufacture batch number: L129465), Activated charcoal (Manufacture batch number: C112241), Gum arabic (Manufacture batch number: A108975) were purchased from Shanghai Aladdin Biochemical Science and Technology Co., Ltd, China. ZO-1 Rabbit Polyclonal Antibody, Occludin Rabbit Polyclonal Antibody, Claudin-1 Rabbit Polyclonal Antibody (21773-1-AP, 13409-1-AP, 13050-1-AP), Beta Actin Monoclonal antibody (66009-1-Ig) were purchased from Wuhan Sanying Biotechnology Co., Ltd, China. c-Kit antibody (Bs-10005R) and SCF antibody (Bs-0545R) were purchased from Beijing Biosynthesis Biotechnology Co., Ltd, China. Anti-rabbit IgG, HRP-linked Antibody was purchased from CST (#7074). Citrate repair solution (Manufacture batch number: 09172311), Sheep serum for containment (Manufacture batch number: 10022315), HE staining solution (Manufacture batch number: G1004-100ML) were purchased from Wuhan Servicebio Technology Co., Ltd, China.
2.2 Methods
2.2.1 Extraction of herbs
First and foremost, in accordance with the traditional decoction method, we meticulously extracted and concentrated each Chinese medicinal material to ensure that the levels of its active ingredients met the experimental requirements. One-hundred gram of each of the Chinese herbs I. verum, Z. dhumnades, C. aurantium, G. biloba, E. ciliata, H. dulcis, A. halys, L. hypoglauca were weighed, 10 times the amount of distilled water was added, and the decoction was immersed for 30 min, and then boiled for 2 h, and then 8 times the amount of distilled water was added to the decoction and boiled for 1 h, and then filtered out the medicinal liquid with gauze, and then the medicinal liquids were combined, heated, and then concentrated to obtain the concentrations of raw medicines of 1.43 g·mL−1, 1 g·mL−1, 1.32 g·mL−1, 1 g·mL−1, 1.11 g·mL−1, 1.11 g·mL−1, 1 g·mL−1, 0.91 g·mL−1, all of which were placed in centrifuge tubes for spare use.
To quantify the dry matter components of the Chinese herbs, 1 mL of each decoction was weighed and dried in an oven at 65°C until constant weight was achieved (with intervals exceeding 3 h, and a weight difference of < 0.3 mg after two consecutive dryings). The concentrations of the decoctions were calculated as 0.158 g·mL−1, 0.133 g·mL−1, 0.180 g·mL−1, 0.154 g·mL−1, 0.136 g·mL−1, 0.163 g·mL−1, 0.147 g·mL−1, 0.151 g·mL−1, respectively (based on the mass of the dried substance per milliliter of concentrated liquid).
2.2.2 Constipation modeling and intervention
Constructed constipation models were performed using loperamide hydrochloride (hereafter referred to as Lop) based on the results of literature surveys (Kim et al., 2019; Yao et al., 2022; Kim et al., 2023; Park et al., 2023; Tuohongerbieke et al., 2024) and pre-experimental results. ICR mice were randomly divided into a normal control group (NC) and a constipation group, and ICR mice in the loperamide-induced constipation group were further divided into a model group (Lop + saline, Lop), I. verum group (Lop + I. verum, Iv), Z. dhumnades group (Lop + Z. dhumnades, Zd), C. aurantium group (Lop + C. aurantium, Ca), G. biloba group (Lop + G. biloba, Gb), E. ciliata group (Lop + E. ciliata, Ec), H. dulcis group (Lop + H. dulcis, Hd), A. halys group (Lop + A. halys, Ah), L. hypoglauca group (Lop + L. hypoglauca, Lh). The ICR mice in the constipation group were injected subcutaneously with 4 mg·kg−1 of Lop twice a day for 4 days, and after a 3-day immobilization period, 8 mg·kg−1 of Lop was injected subcutaneously for 4 days. The fecal pellet morphology and mental activity status of mice in each group were observed. When the rats showed the following symptoms, decreased activity, decreased water content of fecal pellets, hard texture of feces, and decreased volume of fecal pellets, indicating successful modeling. After constipation induction, the 8 treatment groups were gavaged with the same 8 g·kg−1 of aqueous extract of ETCM, while the normal and model groups were given an equal volume of saline by gavage once a day for 7 consecutive days. Standard laboratory food, water and clean cages were provided to all mice.
2.3 Measurement of constipation indicators
The general physiological conditions and fecal conditions of mice in each group were observed daily. On the 7th and 14th days of the experiment, fresh feces were collected from each group of mice for 3 h, the number of pellets was recorded, weighed (wet weight = total weight M1-empty dish M0), and then dried in a dryer at 80°C for 6 h. The feces were again weighed (dry weight = total weight M2-empty dish M0), and the water content of the feces was calculated and statistically analyzed, in order to verify the success of the modeling and the therapeutic efficacy of citrulline. After the last administration, the mice were fasted for 16 h. The mice in each group were kept in a single cage and given 0.2 mL of 10% activated charcoal solution by gavage, and the time was started at the end of the gavage. The time of the first black feces and the number of black feces in the mice within 6 h were recorded and statistically analyzed.
2.4 Measurement of small bowel propulsion rate
After 16 h of fasting without water at the end of the constipation index measurement, mice in each group were given 0.2 mL of 10% activated charcoal solution by gavage. The time was counted from the end of the gavage, and the mice were immediately executed after 20 min. The whole intestine from the pylorus to the cecum was removed by dissection, slowly straightened and placed on a white paper for photographing, and then the total length of the intestine (L1) and the propulsion distance of 10% activated charcoal solution from the pylorus to the cecum (L2) were measured, and the propulsion rate of the small intestine (R) was calculated and statistically analyzed.
2.5 Fecal tissue and colon tissue collection
On the 14th day of the experiment, fecal tissues were collected from each mouse, collected in Eppendorf tubes for liquid nitrogen snap freezing and frozen in −80°C refrigerator for further testing. On the 16th day of the experiment, after the mice were executed by cervical dislocation, the mouse colon tissues were dissected and separated into two parts, one part was sheared, lysed, and milled in preparation for the extraction of total tissue protein, and the other part was fixed in 4% paraformaldehyde for further pathology experiments.
2.6 H&E staining
After paraffin-embedded treatment, the mouse colon tissue was cut into 5 μm—thick paraffin thin slices. The slices were taken into environmentally friendly dewaxing solution, anhydrous ethanol and 75% alcohol for dewaxing and washing, and after repeating the water, the slices were washed and stained with 1% hematoxylin and eosin water (H&E) at 25 ± 2°C, and the slices were blocked with neutral gum. Finally, the morphological changes of the colonic tissues were observed under a light microscope and images were acquired.
2.7 Immunohistochemistry
The paraffin sections of mouse colon were baked in a desiccator at 65°C for 30 min, then dewaxed, dehydrated and washed. In order to improve the specificity and stability of immunohistochemical staining, 2,000 mL of citrate repair solution with a pH of 6.0 was prepared for antigen repair and thus improve the binding efficiency of antigen and antibody, followed by the addition of goat serum for closure. The goat serum was discarded, c-Kit antibody (1:300) and SCF antibody (1:300) were added dropwise, and the sections were incubated for 1 h. The sections were removed and rinsed with PBS-T. The excess liquid on the sections was discarded. Shake off the excess liquid on the sections, add HRP-labeled goat anti-rabbit IgG antibody dropwise, incubate for 30 min, rinse with PBS-T. The sections were treated with DAB solution for color development for 5 min and hematoxylin re-staining for 1 min. The slices were sealed with neutral gum and observed under a light microscope.
2.8 Western blot analysis
Mouse colon tissue was taken, lysate was added and lysed by grinding on ice until homogenized. The supernatant was collected after centrifugation, and it was quantified for protein and adjusted for concentration. A 20 μg protein sample was taken from each well, denatured by boiling and subjected to SDS-PAGE electrophoresis. Then the membrane was immediately transferred by wet transfer method, closed with 5% skimmed milk powder for 1 h. The membrane was washed with TBS-T, followed by the addition of primary antibody (ZO-1 1:5 000, Occludin 1:2 000, Claudin-1 1:1 000) and the internal reference (β-actin 1:60 000) according to molecular weight sizes of the proteins, respectively, and incubated overnight. On the following day, after washing the membrane with TBS-T, HRP-labeled goat anti-rabbit IgG secondary antibody was added and incubated for 1 h. After washing the membrane with TBS-T, protein bands were developed.
2.9 16s rRNA gene sequencing
Total DNA was extracted and analyzed from the fecal samples of four mice per group. The V3-V4 hypervariable region of the 16S rRNA gene was amplified using the Illumina MiSeq PE300 platform with the forward primer 338F (ACTCCTACGGGAGGCAGCAG) and reverse primer 806R (GGACTACHVGGGTWTCTAAT). Raw sequencing reads were obtained, followed by quality control, read assembly, and chimera removal. Subsequently, amplicon sequence variants (ASVs) were identified and taxonomic classification was performed on the processed data. Microbial classification macrogenome sequencing 16S rRNA gene sequencing was done by Shanghai Meiji Bio-pharmaceutical Technology Co. (Contract No.: MJ20240612154). All data analysis was performed on the Meiji Bio Cloud platform (https://cloud.majorbio.com).
2.10 Statistical analysis
All measurement data were analyzed using GraphPad Prism 9.3.0 statistical analysis software. Comparisons between groups were made by t-test, and comparisons between multiple groups were made by one-way ANOVA, with P < 0.05 indicating that the differences between experimental groups were statistically significant.
3 Results
3.1 ECAE and HDAE alleviate constipation in model mice
The overall experimental design is shown in Figure 1A. As shown in Figures 1B–G, compared with those in the normal control (NC) group, the number of black stool samples discharged from the loperamide-treated (Lop) group was significantly greater (P < 0.001), and the number of black feces discharged within 6 h was significantly lower (P < 0.001). Moreover, in the Lop group, the dry weight of the feces was decreased (P < 0.001), the fecal water content also tended to be decreased (P < 0.001), and the intestinal propulsion rate decreased accordingly compared with the NC group (P < 0.001). However, compared with those in the Lop group, the various fecal parameters significantly differed in the ECAE-treated (Ec) group and HDAE-treated (Hd) group. The time to the first black stool was significantly shorter (P < 0.01), the number of black feces excreted within 6 h was greater (P < 0.01), the dry weight and water content of the feces was higher (P < 0.05, P < 0.01 or P < 0.001), and the rate of intestinal propulsion was also significantly higher (P < 0.001) in the Ec and Hd groups than in the Lop group. These findings fully confirmed that ECAE and HDAE improved the defecation status and increased the intestinal motility of the mice in the constipation model.
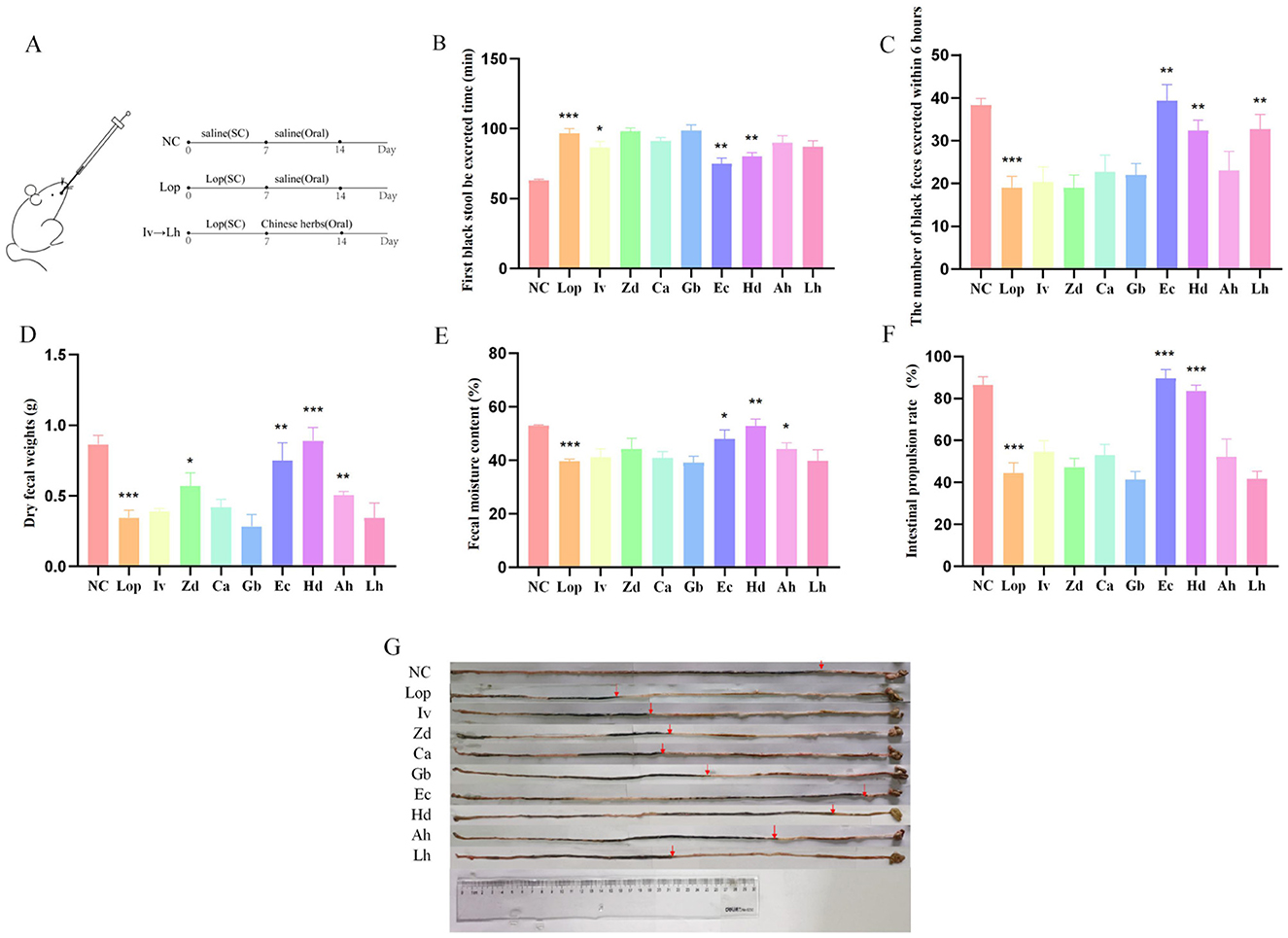
Figure 1. Effects of ECAE and HDAE on defecation parameters in a mouse model of constipation. (A) Animal model and treatment flow chart; (B–F) Fecal parameters. Compared with the NC group, ***P < 0.001; compared with the Lop group, *P < 0.05, **P < 0.01, ***P < 0.001; (G) Schematic representation of the intestinal propulsion rate. ECAE, E. ciliata aqueous extract; HDAE, H. dulcis aqueous extract; Lop, loperamide-treated; NC, normal control.
3.2 ECAE and HDAE ameliorate the intestinal tissue structural and morphological damage caused by loperamide hydrochloride in mice
To determine the histological changes in the colons of Ec and Hd group mice, hematoxylin and eosin (H&E) staining was performed, as shown in Figure 2. The NC group presented a healthy and intact colonic histological structure with dense and well-arranged epithelial cells of the mucous layer and a separating layer between the mucosa and the submucosa, with morphologically normal goblet cells and intestinal crypts with regular surfaces. In contrast, the Lop group exhibited severe histological damage, with thinning of the muscularis propria, breakage and disappearance of the epithelial cells of the mucus layer, depletion and fracture of the goblet cells, atrophy and distortion of the crypt structure, and scattered inflammatory cell infiltration in the submucosa. However, the intestinal pathomorphological damage in the Ec and Hd groups was ameliorated, as indicated by intact and well-arranged epithelial cells in the mucous layer, increased numbers of goblet cells, and the restoration of the crypt structure to normal. These findings suggest that ECAE and HDAE can repair pathological damage to the colon in constipation model mice.
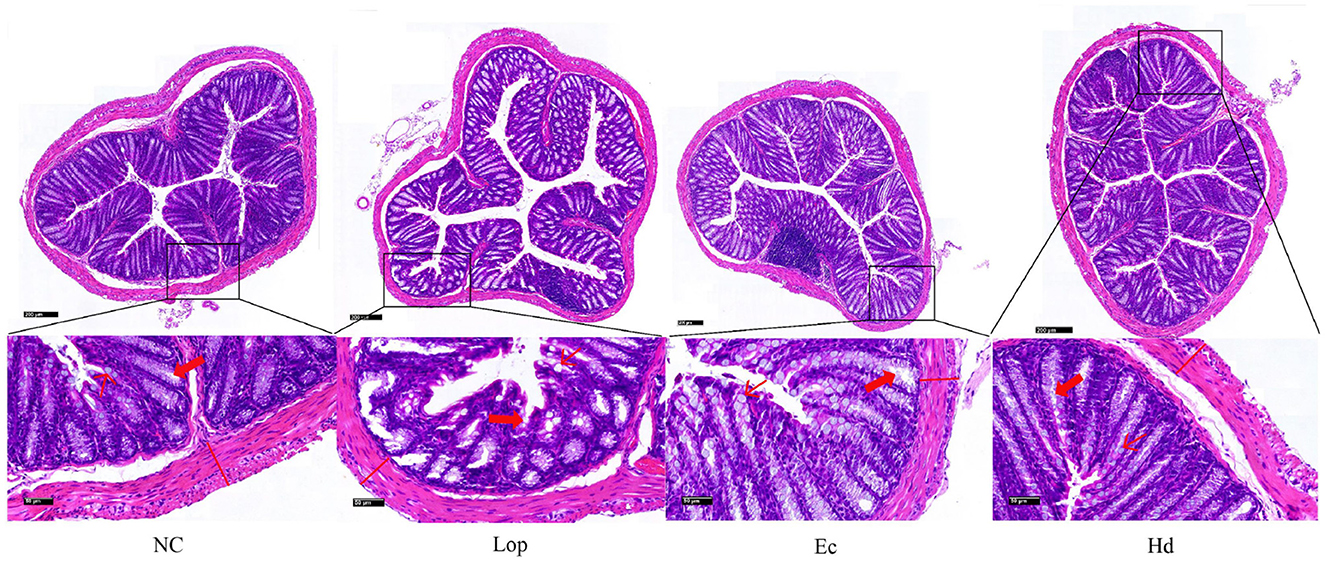
Figure 2. Effects of ECAE and HDAE on intestinal tissue structure and cell morphology induced by loperamide hydrochloride in mice. H&E staining was used to observe colon tissue sections from the NC group, the Lop group, the Ec group and the Hd group via light microscopy, with scale bars of 200 μm and 50 μm.  , Goblet cells;
, Goblet cells;  , Intestinal crypts;
, Intestinal crypts;  , Muscularis externa. ECAE, E. ciliata aqueous extract; HDAE, H. dulcis aqueous extract; NC, normal control; Lop, loperamide-treated; Ec, ECAE-treated; Hd, HDAE-treated.
, Muscularis externa. ECAE, E. ciliata aqueous extract; HDAE, H. dulcis aqueous extract; NC, normal control; Lop, loperamide-treated; Ec, ECAE-treated; Hd, HDAE-treated.
3.3 ECAE and HDAE increase the number of c-Kit- and SCF-positive cells in the colon of constipation model mice
The immunoreactivity of Stem Cell Factor Receptor (c-Kit) on the surface of interstitial cells of Cajal (ICCs) with its Stem Cell Factor (SCF) ligand was determined by immunohistochemistry. As shown in Figures 3A, B, analysis of the diaminobenzidine (DAB) staining results revealed that the expression of c-Kit and SCF in the intestinal tissues after loperamide treatment was significantly lower and sparsely distributed than that in the NC group. After treatment with ECAE or HDAE, the number of c-Kit- and SCF-positive cells significantly rebounded, and these cells were densely distributed, which was consistent with the results of the quantitative analysis (P < 0.05 or P < 0.01) as shown in Figure 3C. These findings suggest that loperamide treatment leads to a reduction in the number of or the dysfunction of ICCs in the mouse colon and that ECAE and HDAE may rescue this consequence by activating the c-Kit/SCF pathway, which in turn coordinates intestinal smooth muscle contraction and promotes intestinal peristalsis.
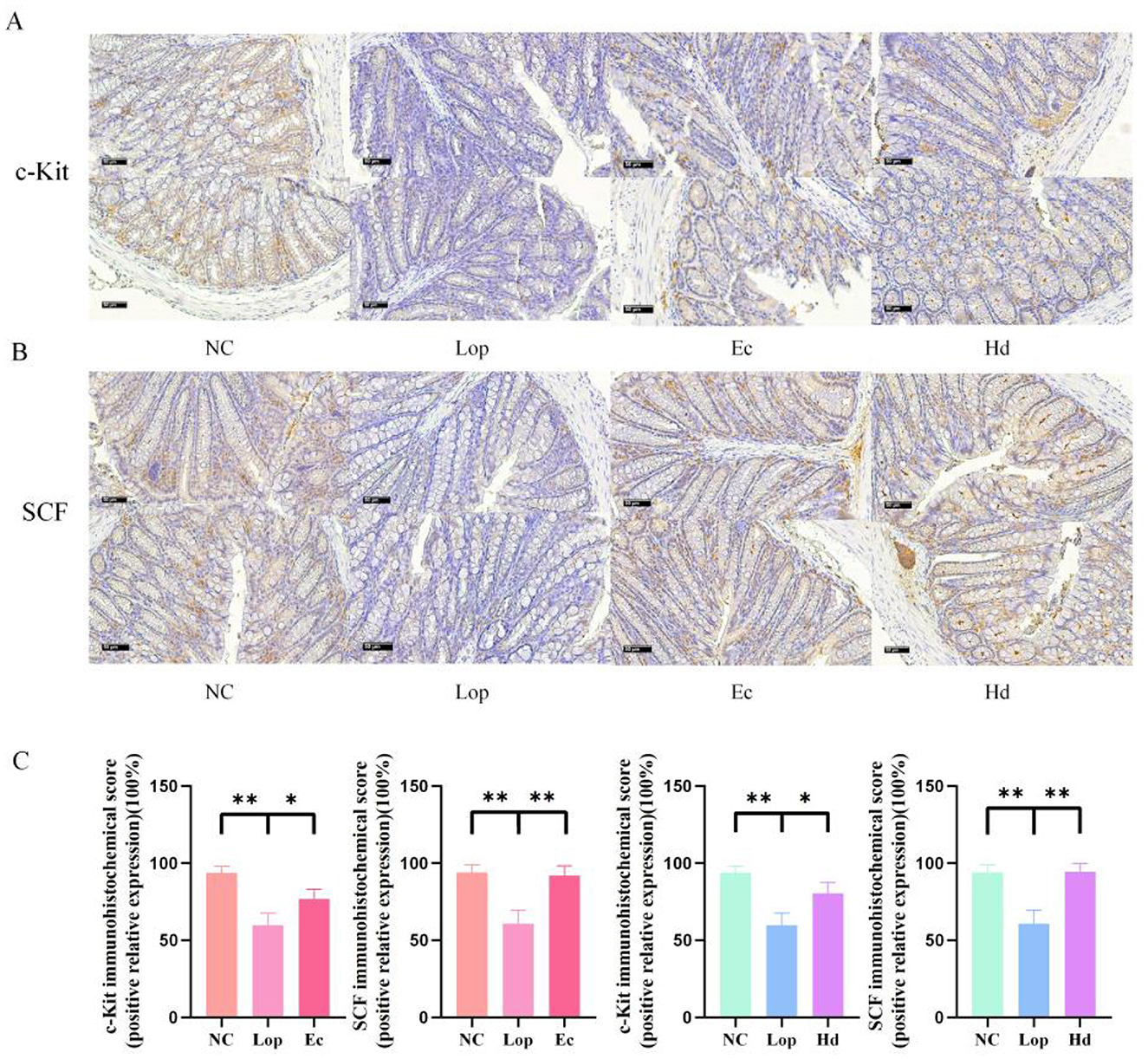
Figure 3. Regulation of c-Kit and SCF in the intestinal tissue of constipation model mice by ECAE and HDAE. (A) IHC images of c-Kit; (B) IHC images of SCF; Results of IHC were scored and expressed as shown in (C). Compared with the NC group, **P < 0.01; compared with the Lop group, *P < 0.05, **P < 0.01. ECAE, E. ciliata aqueous extract; HDAE, H. dulcis aqueous extract; NC, normal control; Lop, loperamide-treated; Ec, ECAE-treated; Hd, HDAE-treated; IHC, immunohistochemistry; c-Kit, Stem Cell Factor Receptor; SCF, Stem Cell Factor.
3.4 ECAE and HDAE increase the expression levels of ZO-1, Occludin and Claudin-1 in the colons of constipation model mice
The authors further investigated the effects of ECAE and HDAE on the expression levels of three intestinal tight junction proteins, Zonula Occludens-1 (ZO-l), Occludin and Claudin-l, in the colonic tissues of constipation model mice. The immunoblotting results are shown in Figures 4A, B. Statistical analysis of the gray values of the bands revealed that the expression levels of tight junction proteins in the colons of mice in the Lop group were significantly lower than those in the colons of mice in the NC group (P < 0.01 or P < 0.001). However, the expression levels of tight junction proteins in the colons of the mice in the Ec and Hd groups were significantly greater (P < 0.05 or P < 0.01) than those in the colons of mice in the Lop group. These findings suggest that ECAE and HDAE can repair intestinal barrier damage in mice with loperamide-induced constipation.
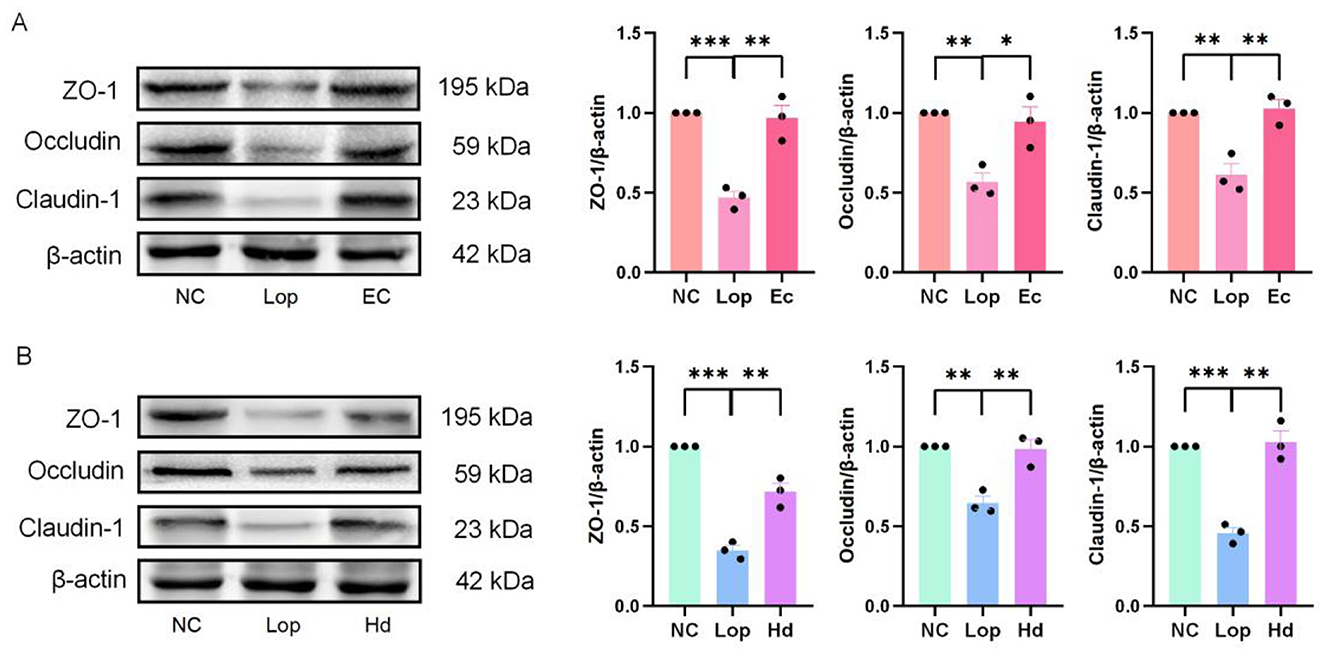
Figure 4. Effects of ECAE and HDAE on ZO-1, Occludin and Claudin-1 levels in the colons of constipation model mice. (A, B) Protein expression levels of ZO-1, Occludin and Claudin-1 were evaluated via western blot analysis, and the results of the quantitative analysis of gray intensity were calculated. Compared with the NC group, **P < 0.01, ***P < 0.001; compared with the Lop group, *P < 0.05, **P < 0.01. ECAE, E. ciliata aqueous extract; HDAE, H. dulcis aqueous extract; NC, normal control; Lop, loperamide-treated; Ec, ECAE-treated; Hd, HDAE-treated.
3.5 Effects of ECAE and HDAE on the diversity of the intestinal flora in constipation model mice
The authors used 16S rRNA sequencing to detect changes in the intestinal flora composition in mice in the NC group, Lop group, Ec group and Hd group. To assess β diversity, the authors performed principal coordinate analysis (PCoA) and nonmetric multidimensional scaling analysis (NMDS) of the intestinal samples. The closer the sample points in the graph were, the more similar the species composition of the samples was. PCoA and NMDS revealed two significant clustering groups: the Ec group (pressure = 0.067) and the Hd group (pressure = 0.05; pressure < 0.2 indicates the validity of NMDS; Figures 5A, B). The results obtained from the β diversity analysis showed that the different treatments had significant effects on the intestinal flora of each group of mice. By comparing the sequences of the bacteria in the intestinal samples, the authors found that the microbiota compositions of both the Ec group and the Hd group were different from those of the NC and Lop groups. A total of 102 genera were common between the Ec group and the NC and Lop groups, and g__Psychrobacter, g__Kocuria, g__unclassified_f__Lactobacillaceae, g__Rodentibacter, and g__Globicatella were the species specific to the Ec group. A total of 101 genera were common between the Hd group and the NC and Lop groups, and g__Eubacterium_ruminantium_group, g__norank_f__norank_o__Bacteroidales, g__Olsenella, g__Fusicatenibacter, g__Tyzzerella, g__Ruminiclostridium, g__norank_f__norank_o__Izemoplasmatales, g__unclassified_f__Marinifilaceae, g__Bilophila, and Ruminococcaceae_UCG-002 were species unique to the Hd group (Figure 5C). As shown in Figure 5D, at the phylum level, the relative abundances of Firmicutes and Bacteroidota were high in all groups. The ratio of Firmicutes to Bacteroidota in the Lop group was significantly lower than that in the NC group, and the ratios of Firmicutes to Bacteroidota in the Ec group and Hd group were significantly higher than that in the Lop group. As shown in Figure 5E, at the genus level, the relative abundances of norank_f__Muribaculaceae and Lactobacillus were high. Compared with that in the NC group, the ratio of norank_f__Muribaculaceae to Lactobacillus in the Lop group was significantly lower. Compared with that in the Lop group, the ratios of norank_f__Muribaculaceae to Lactobacillus in the Ec group and Hd group were significantly higher. The relative abundances of major bacterial genera in the NC, Lop, Ec and Hd groups are shown in heatmaps in Figure 5F. These results fully demonstrated that ECAE and HDAE could partially restore many aspects of loperamide-induced gut microbial dysbiosis, such as microbial diversity, community structure, and species composition.
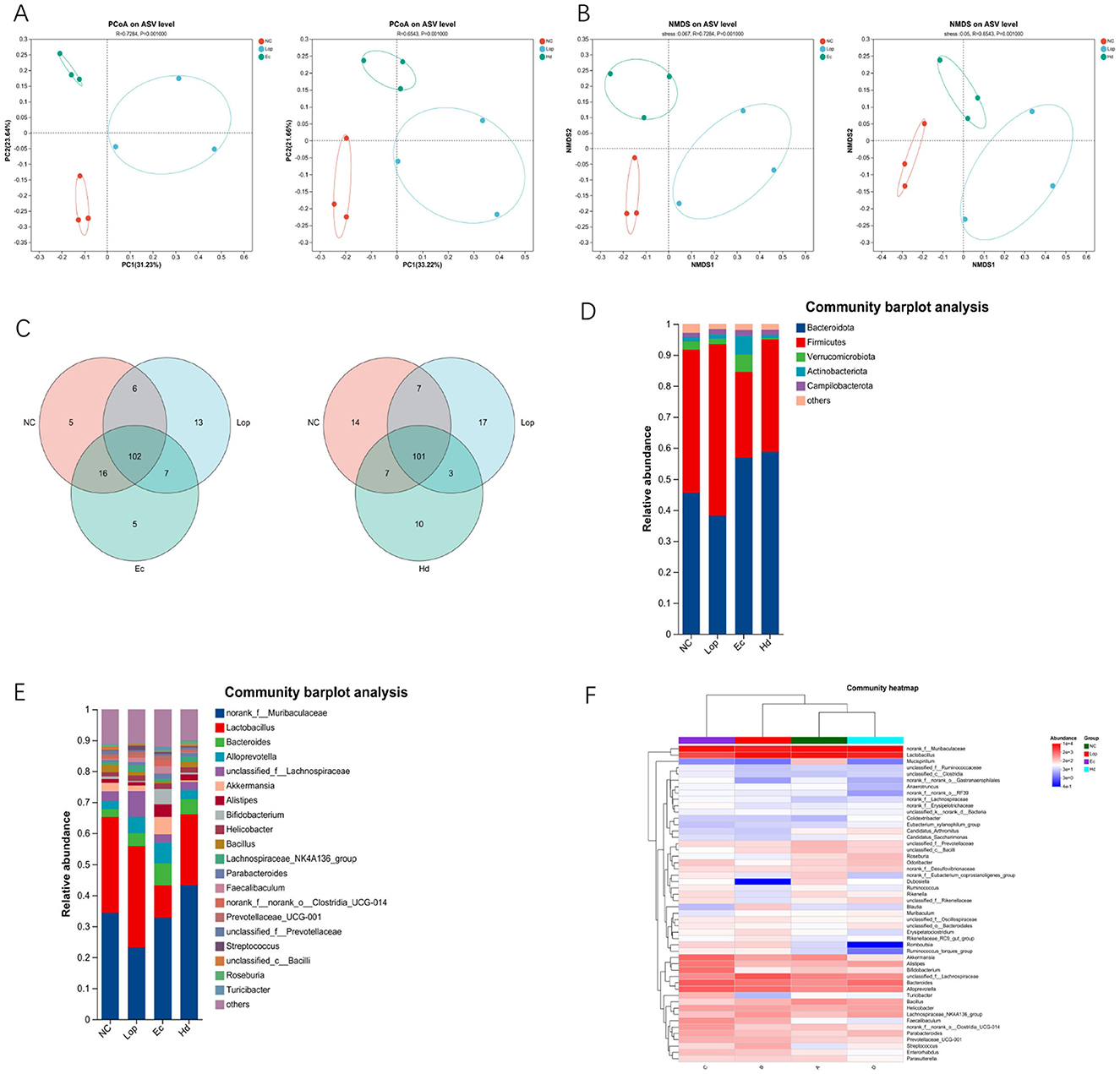
Figure 5. Effects of ECAE and HDAE on intestinal flora diversity in constipation model mice. (A) PCoA of the β diversity of the Ec and Hd groups; (B) NMDS of the β diversity of the Ec and Hd groups; (C) Venn diagram of bacterial sequences in the Ec and Hd groups compared with those in the NC and Lop groups; (D) Cluster bar map at the phylum level; (E) Cluster bar map at the genus level; (F) Cluster heatmap. ECAE, E. ciliata aqueous extract; HDAE, H. dulcis aqueous extract; NC, normal control; Lop, loperamide-treated; Ec, ECAE-treated; Hd, HDAE-treated; PCoA, principal component analysis; NMDS, nonmetric multidimensional scaling analysis.
3.6 Changes in the intestinal flora of constipation model mice after ECAE and HDAE treatment
The authors used LEfSe (linear discriminant analysis effect size) analysis to identify biomarkers unique to the three groups at the phylum, order, order, family, genus, and species levels, setting the linear discriminant analysis (LDA) threshold at 2. As shown in Figures 6A, B, the dominant intestinal flora of the NC, Lop, and Ec groups were o__Bacillales, p__Firmicutes, and f__Bacteroidaceae, respectively. The dominant intestinal flora of the mice in the NC, Lop and Hd groups were g__Dubosiella, o__Lachnospirales, and g__norank_f__Muribaculaceae, respectively. After loperamide treatment and ECAE and HDAE treatment, the composition of the intestinal flora of the mice significantly changed. LEfSe multilevel species discrimination revealed that ECAE or HDAE treatment produced beneficial bacteria. The authors subsequently conducted a comparative analysis of the two groups, as shown in Figure 6C. Compared with the NC group, the abundances of uncultured_bacterium_g__Dubosiella, unclassified_g__Ruminococcus, and uncultured_Muribaculaceae_bacterium were significantly lower in in the intestinal flora of mice in the Lop group (P < 0.05). As shown in Figure 6D, compared with the Lop group, the abundances of uncultured_bacterium_g__Turicibacter, unultured_bacterium_g__norank_f__norank_o__Gastranaerophilales, unultured_bacterium_g__norank_f__norank_o__Clostridia_vadinBB60_group, and unultured_Erysipelotrichales_bacterium were significantly higher (P < 0.05), and the abundances of uncultured_organism_g__norank_f__Muribaculaceae, unultured_bacterium_g__Candidatus_Stoquefichus were significantly lower (P < 0.05), in the intestinal flora of the Ec group. As shown in Figure 6E, compared with the Lop group, the abundances of uncultured_bacterium_g__Odoribacter, uncultured_Muribaculaceae_bacterium, uncultured_bacterium_g__norank_f__Rs-E47_termite_group, uncultured_bacterium_g__Rikenella, and uncultured_bacterium_g__norank_f__norank_o__Clostridia_vadinBB60_group were significantly higher (P < 0.05), and the abundances of Adlercreutzia_mucosicola, uncultured_bacterium_g__Anaerotruncus, and uncultured_bacterium_g__Candidatus_Stoquefichus were significantly lower (P < 0.05), in the intestinal flora of Hd group mice. In summary, after treatment with ECAE and HDAE, the abundances of beneficial bacteria in the intestinal tract of constipation model mice increased significantly, which may have alleviated the symptoms of constipation by maintaining the balance of the intestinal flora, promoting the digestion and absorption of food, reducing the accumulation of harmful substances, and reducing the inflammatory response.
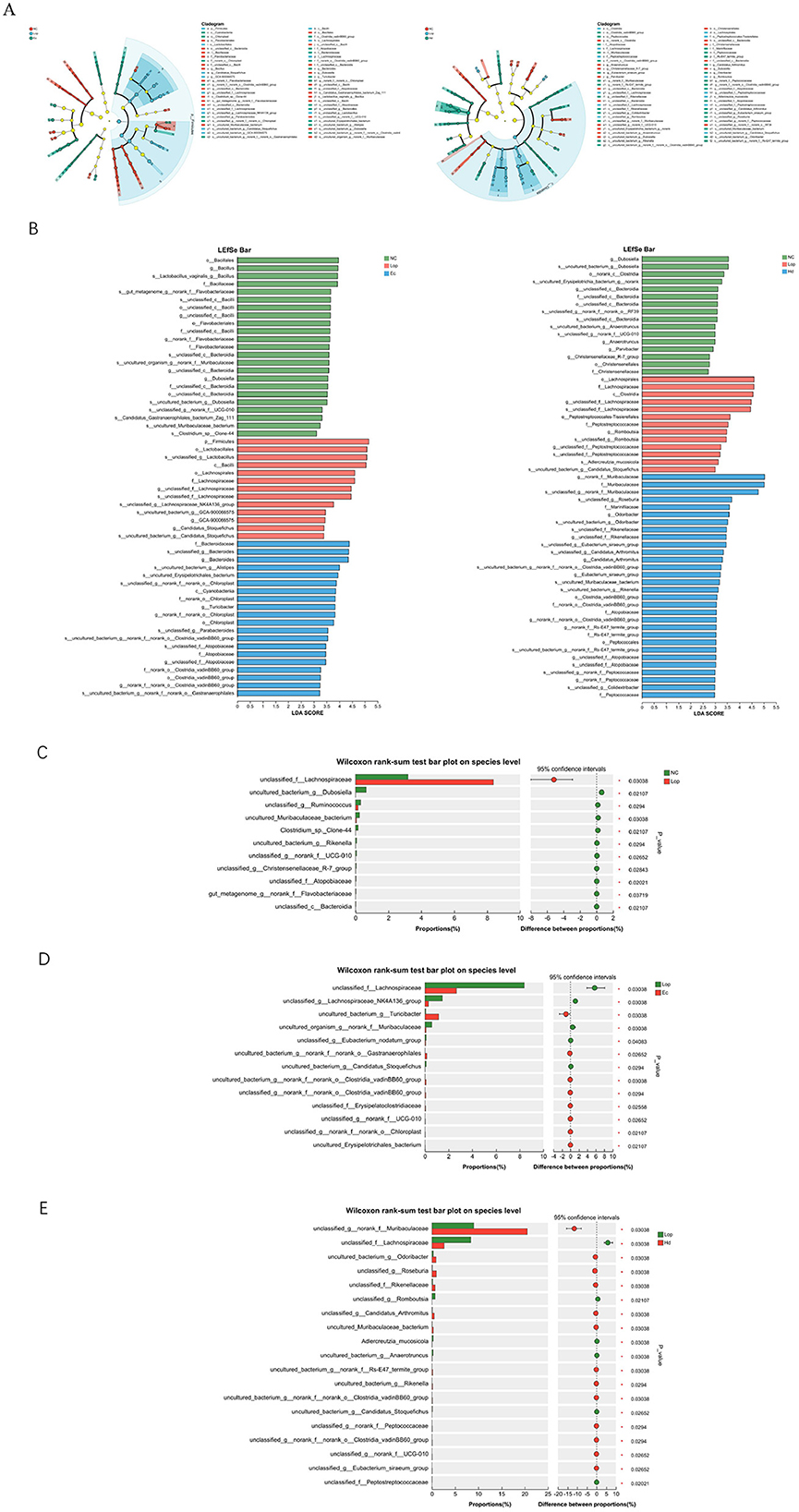
Figure 6. Changes in the intestinal flora in constipation model mice after ECAE and HDAE treatment. (A) Differences in species at different taxonomic levels presented as developmental dendrograms, which visually reflect the different species at different hierarchical levels among different subgroups; (B) LDA discriminant bar charts, with bars showing the LDA values of the various differential species, which visually demonstrate the magnitude of the influence of the unique species identified among different groups on the effect of the differences; the bars show the difference in the abundance of the same species among different groups in terms of the mean relative abundance differences between different groups, with labels indicating whether the differences are significant or not, visualizing the mean relative abundance differences of the same species between different groups. *P < 0.05. (C) NC group vs. Lop group; (D) Lop group vs. Ec group; (E) Lop group vs. Hd group. ECAE, E. ciliata aqueous extract; HDAE, H. dulcis aqueous extract; NC, normal control; Lop, loperamide-treated; Ec, ECAE-treated; Hd, HDAE-treated; LDA, linear discriminant analysis.
4 Discussion
Among eight ETCMs, ECAE and HDAE significantly ameliorated the symptoms of loperamide-induced constipation in ICR mice, ameliorated pathological tissue damage in the colons of constipation model mice, significantly increased the expression levels of intestinal peristalsis-associated proteins (c-kit and SCF) and intestinal barrier maintenance, significantly increased the expression levels of proteins related to the promotion of intestinal motility (c-Kit and SCF) and the maintenance of the intestinal barrier (ZO-1, Occludin and Claudin-1), and promoted the colonization of beneficial intestinal bacteria in constipation model mice.
Constipation is characterized by reduced intestinal peristalsis and difficulty defecating and is often accompanied by abdominal pain, bloating, and other discomfort (Black and Ford, 2018). The concept of ETCM underscores the commonalities of these symptoms; thus, the use of ETCMs may be an innovative approach for treating constipation. In this context, the authors conducted a literature review. They systematically examined 110 ETCMs recognized by the Ministry of Health as homologous to both medicine and food as of 2023 within the 2020 edition of the Chinese Pharmacopeia, among which 35 ETCMs, such as Dioscorea oppositifolia L., Cannabis sativa L., and Amomum villosum Lour., had primary therapeutic functions related to gastrointestinal health. These functions include tonifying the spleen and nourishing the stomach, moistening the intestines to alleviate laxity, and warming the spleen to mitigate diarrhea, among others. In modern research, the key words “constipation” and “laxative” were used to search the “Web of Science,” “PubMed,” “China Knowledge” and other scientific databases. The remaining 75 ETCMs were screened, and the roles of I. verum, Z. dhumnades, C. aurantium, G. biloba, E. ciliata, H. dulcis, A. halys, and L. hypoglauca in ameliorating constipation or promoting defecation have not been reported thus far. ETCM has emerged as a prominent research focus in numerous countries, highlighting the potential for managing diseases safely and effectively through dietary interventions rather than relying solely on pharmacological or surgical treatments. Research is being conducted on the efficacy of various ETCMs in the treatment of chronic conditions such as obesity, cancer, diabetes, and hypertension (Zheng et al., 2022; Liu Z. et al., 2023). By employing appropriate dietary combinations, some components can mimic the therapeutic effects of medications, thereby effectively controlling or even reversing disease progression. The evidence suggests that the Mediterranean diet offers significant cardiovascular protection and plays a crucial role in preventing and managing hypertension, hyperlipidemia, and related disorders (Wang et al., 2018). Additionally, a ketogenic diet has shown marked effectiveness in controlling seizures while also leading to positive outcomes in tumor treatment (Husari and Cervenka, 2021; Yang et al., 2024).
Fecal quantity, fecal water content and intestinal function are considered important factors in assessing the severity of constipation and drug efficacy (Vriesman et al., 2020). The authors initially assessed and documented the fecal parameters of experimental mice and discovered that ECAE and HDAE facilitated the expulsion of intestinal contents. The intestinal propulsion rates across all the groups were subsequently evaluated through mouse experiments, with visual data further substantiating that ECAE and HDAE improved defecation function by promoting intestinal peristalsis. To gain deeper insight into the impact of the herbal extracts on the intestinal microstructure, H&E staining was used to compare and analyze intestinal tissue sections obtained from NC, Lop, Ec and Hd group mice. The findings indicated that ECAE and HDAE treatment ameliorated damage to the intestinal mucosa and restored the structural integrity of intestinal layers to normal. This evidence may serve as a morphological basis for improving digestive and absorptive functions while alleviating constipation. These results align with previous reports indicating that certain drug extracts have therapeutic effects on constipation (Wang L. et al., 2023; Zhang et al., 2024).
In the gastrointestinal tract, Cajal interstitial cells (ICCs) constitute a distinct class of interstitial cells that form an intricate network between the enteric nervous system and smooth muscle (Fan et al., 2014). This network is essential for coordinating gastrointestinal motility and secretion. The c-Kit/SCF signaling pathway plays a pivotal role in this process by regulating ICC development, proliferation, and survival, thereby ensuring the proper functioning of the network (Satoshi et al., 2020). Immunohistochemical protein expression assays revealed that ECAE and HDAE regulated the expression levels of proteins closely related to intestinal peristalsis, such as SCF and c-Kit, which explains, at the molecular level, how these two aqueous extracts can regulate intestinal motility and improve intestinal function. Prunus persica (L.) Batsch blossom soluble dietary fiber synergia polyphenol was demonstrated to increase the protein and mRNA expressions of SCF and c-Kit in the c-Kit/SCF signaling pathway to speed up gut movement (Liang et al., 2024). Suaeda salsa (L.) Pall was found to upregulate c-Kit and SCF protein levels, promote the proliferation of the main intestinal rhythm generators, ICCs, and thus increase intestinal peristalsis (Zhang et al., 2024). Furthermore, the efficacy of constipation treatment can be inhibited by blocking the SCF/c-Kit signaling pathways (Zhang et al., 2021).
Alterations in mouse intestinal pathological morphology reflect the importance of intestinal mucosal barrier function in the treatment of constipation. Short-chain fatty acids (SCFAs) play a crucial role in maintaining the integrity of tight junctions between intestinal epithelial cells (Guo et al., 2021). For instance, butyric acid, as a representative SCFA, enhances the mechanical function of the intestinal mucosal barrier by upregulating the expression of ZO-1 and Occludin, and by modulating the distribution of Claudin-1 on the cell surface (Soret et al., 2010; Rekha et al., 2024). Western blot analysis provided quantitative evidence for the specific effects of ECAE and HDAE on the expression levels of the intestinal proteins ZO-1, Occludin, and Claudin-1, confirming their positive roles in protecting the mucosal barrier. This insight offers a novel approach to investigating the intestinal microbiota in constipated mice, particularly focusing on butyrate-producing bacteria. Cymbopogon citratus (DC.) Stapf aqueous extract has been shown to restore loperamide-induced impaired barrier function and maintain intestinal homeostasis by increasing the mRNA expression levels of mechanical barrier proteins (Occludin and ZO-1; Gao et al., 2022). The water extract of Cannabis sativa L. was found to significantly reverse the reduction in the expression of gut barrier-related molecules, including Claudin-1 and Occludin, at the mRNA level in constipation model mice (Li et al., 2022).
The ratio of Firmicutes to Bacteroidetes is an important indicator of the balance of the intestinal flora (Wu et al., 2021). 16S rRNA sequencing revealed that, at the phylum level, Firmicutes and Bacteroidetes were the dominant intestinal flora in all groups of mice. Consistent with the authors' study, a decrease in the proportion of Firmicutes and an increase in the proportion of Bacteroidetes was observed in patients with functional constipation (Hu et al., 2019). ECAE and HDAE reversed the decrease in the ratio of Firmicutes to Bacteroidetes caused by loperamide, increased the proportion of Firmicutes, and restored the imbalanced structure of the intestinal flora. Many bacteria within the Firmicutes phylum are beneficial, such as Lactobacillus, which not only promotes intestinal peristaltic secretion but also inhibits the growth of harmful bacteria. This action helps restore the balance of the intestinal flora and alleviates constipation. Additionally, Firmicutes are the primary producers of butyrate, a compound that plays a crucial role in the gastrointestinal tract. As an important SCFA, butyrate plays a crucial role in maintaining intestinal microecological balance. Butyrate is particularly important for repairing damaged intestinal wall cells, improving intestinal barrier function (Zheng et al., 2017), alleviating the intestinal inflammatory response (Lee et al., 2016) and alleviating constipation symptoms by stimulating water and electrolyte absorption in the colon, thereby reducing stool volume. More importantly, butyric bacteria and probiotics that colonize the intestine have synergistic effects on the growth of harmful bacteria (Ye et al., 2018). Paeniclostridium, Coriobacteriales, Macrococcus, Faecalitalea and other pathogenic bacteria disappeared from the endemic flora of the Lop group after ECAE treatment. Compared with those in the Lop group, the abundances of butyrate-producing bacteria such as Turicibacter (Zeng et al., 2024), Clostridia_vadinBB60_group (Huang et al., 2024), and Erysipelotrichales (Li et al., 2020) in the Ec group were significantly higher. After HDAE treatment, Enterococcus, Coriobacteriales, Peptostreptococcales-Tissierellales, Paeniclostridium, Macrococcus, Faecalitalea and other pathogenic bacteria from the Lop group of the unique bacterial groups disappeared from the Lop group. Compared with the Lop group, the Hd group exhibited new butyrate-producing bacteria, including Olsenella (Shi et al., 2022), Fusicatenibacter (Zeng et al., 2024), Ruminiclostridium (Liu et al., 2021), Tyzzerella (Xu et al., 2022), and Ruminococcaceae_UCG-002 (Liao et al., 2021), after treatment. HDAE promoted the growth of butyrate-producing bacteria of Odoribacter (Liu et al., 2021), Muribaculaceae (Liu P. et al., 2023), Rikenella (Shi et al., 2017), Clostridia_vadinBB60_group (Huang et al., 2024), etc. Studies have shown that some Chinese herbs can play a “prebiotic” role after entering the intestine, enriching beneficial butyrate-producing bacteria. For example, a water insoluble polysaccharide (WIP) was isolated and identified from Poria cocos, and the abundances of the mouse cecal butyrate-producing bacteria Lachnospiracea and Clostridium increased after treatment (Sun et al., 2019). Gegen Qinlian Decoction (GQD) is a TCM formula that enriches the rat gut with many butyrate-producing bacteria, including Faecalibacterium and Roseburia, thereby attenuating intestinal inflammation and lowering glucose (Xu et al., 2020). In summary, ECAE and HDAE can increase the diversity of the intestinal flora, increase the proportion of beneficial bacteria, reduce the proportion of harmful bacteria, and improve the intestinal microecological environment, all of which may be important ways to alleviate constipation.
The limitations of this study include the lack of determination of the optimal dose and route of administration for ECAE and HDAE, which currently precludes an assessment of their effective application in clinical settings. Consequently, in future research, multiple dosing groups and various routes of administration should be established on the basis of the findings from this study to ascertain the optimal dosing and administration parameters for ECAE and HDAE. Additionally, subsequent investigations could be focused on refining the isolation and purification processes for the active ingredients in ECAE and HDAE, on validating the critical role of specific intestinal flora in constipation development through Fecal Microbiota Transplantation (FMT), providing a theoretical foundation for personalized extract treatments, as well as on evaluating the mechanisms of action and clinical effects of ECAE and HDAE in humans.
In this study, for the first time, evidence is presented regarding the laxative effects and mechanisms of action associated with non-beneficial gastrointestinal function related to ETCMs. The results indicate that ECAE and HDAE have significant potential in treating constipation by increasing intestinal propulsion rates, reducing defecation times, improving the intestinal mucosal structure, regulating the expression of proteins linked to intestinal barrier integrity, and optimizing the composition of the gut microbiota. This research transcends traditional pharmaceutical or surgical treatment modalities by laying a foundation for integrating dietary modifications with lifestyle changes; such an approach may provide a safer and more effective treatment strategy for patients suffering from constipation.
Data availability statement
The data presented in the study are deposited in the NCBI repository, the accession number(s) is PRJNA1256912.
Ethics statement
Animal experiments were approved by the Animal Ethical and Welfare Committee of NJU (SYXK2019-0056). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JW: Conceptualization, Investigation, Writing – original draft. QX: Data curation, Writing – original draft. SW: Formal analysis, Writing – original draft. XZ: Methodology, Writing – original draft. CJ: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This was funded by Shandong Provincial Laboratory Project (2022 Shandong Provincial Laboratory Project No. SYS202202) and the Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-202219B, JNL-202204A, and JNL-2023017D).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bharucha, A. E., and Lacy, B. E. (2020). Mechanisms, evaluation, and management of chronic constipation. Gastroenterology 158, 1232–1249.e1233. doi: 10.1053/j.gastro.2019.12.034
Bharucha, A. E., and Wald, A. (2019). Chronic constipation. Mayo. Clin. Proc. 94, 2340–2357. doi: 10.1016/j.mayocp.2019.01.031
Black, C. J., and Ford, A. C. (2018). Chronic idiopathic constipation in adults: epidemiology, pathophysiology, diagnosis and clinical management. Med. J. Aust. 209, 86–91. doi: 10.5694/mja18.00241
Chang L. Lembo A. and S. Sultan (2014). American gastroenterological association institute technical review on the pharmacological management of irritable bowel syndrome. Gastroenterology 147, 1149–1172.e1142. doi: 10.1053/j.gastro.2014.09.002
Fan, Y., Wu, S., Yin, Z., and Fu, B. B. (2014). Cellular and molecular mechanism study of declined intestinal transit function in the cholesterol gallstone formation process of the guinea pig. Exp. Ther. Med. 8, 1518–1522. doi: 10.3892/etm.2014.1943
Fan, Y., Xu, C., Xie, L., Wang, Y., Zhu, S., An, J., et al. (2022). Abnormal bile acid metabolism is an important feature of gut microbiota and fecal metabolites in patients with slow transit constipation. Front. Cell Infect. Microbiol. 12:956528. doi: 10.3389/fcimb.2022.956528
Gao, C. C., Li, G. W., Wang, T. T., Gao, L., Wang, F. F., Shang, H. W., et al. (2021). Rhubarb extract relieves constipation by stimulating mucus production in the colon and altering the intestinal flora. Biomed. Pharmacother. 138:111479. doi: 10.1016/j.biopha.2021.111479
Gao, X., Hu, Y., Tao, Y., Liu, S., Chen, H., Li, J., et al. (2022). Cymbopogon citratus (DC.) Stapf aqueous extract ameliorates loperamide-induced constipation in mice by promoting gastrointestinal motility and regulating the gut microbiota. Front. Microbiol. 13:1017804. doi: 10.3389/fmicb.2022.1017804
Guo, Y., Yu, Y., Li, H., Ding, X., Li, X., Jing, X., et al. (2021). Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in Uox-knockout mice. Eur. J. Nutr. 60, 2217–2230. doi: 10.1007/s00394-020-02414-x
He, X. Q., Zou, H. D., Liu, Y., Chen, X. J., Atanasov, A. G., Wang, X. L., et al. (2024). Discovery of curcuminoids as pancreatic lipase inhibitors from medicine-and-food homology plants. Nutrients 16:2566. doi: 10.3390/nu16152566
Hofman, D., Kudla, U., Miqdady, M., Nguyen, T. V. H., Morán-Ramos, S., and Vandenplas, Y. (2022). Faecal microbiota in infants and young children with functional gastrointestinal disorders: a systematic review. Nutrients 14:974. doi: 10.3390/nu14050974
Hu, T. G., Wen, P., Fu, H. Z., Lin, G. Y., Liao, S. T., and Zou, Y. X. (2019). Protective effect of mulberry (Morus atropurpurea) fruit against diphenoxylate-induced constipation in mice through the modulation of gut microbiota. Food Funct. 10, 1513–1528. doi: 10.1039/C9FO00132H
Huang, B., Yin, T., Fu, S., Liu, L., Yang, C., Zhou, L., et al. (2024). Inflammation-oriented montmorillonite adjuvant enhanced oral delivery of anti-TNF-α nanobody against inflammatory bowel disease. Proc. Natl. Acad. Sci. U S A 121:e2320482121. doi: 10.1073/pnas.2320482121
Hughes, S., Higgs, N. B., and Turnberg, L. A. (1984). Loperamide has antisecretory activity in the human jejunum in vivo. Gut 25, 931–935. doi: 10.1136/gut.25.9.931
Husari, K. S., and Cervenka, M. C. (2021). Ketogenic diet therapy for the treatment of post-encephalitic and autoimmune-associated epilepsies. Front. Neurol. 12:624202. doi: 10.3389/fneur.2021.624202
Kilgore, A., and Khlevner, J. (2024). Functional constipation: pathophysiology, evaluation, and management. Aliment. Pharmacol. Ther. 60, s20–s29. doi: 10.1111/apt.17852
Kim, H., Jeong, E. J., Park, C., Lee, J. S., Kim, W. J., Yu, K. W., et al. (2023). Modulation of gut microbiota ecosystem by a glucan-rich snail mucin heteropolysaccharide attenuates loperamide-induced constipation. Int. J. Biol. Macromol. 253:126560. doi: 10.1016/j.ijbiomac.2023.126560
Kim, J. E., Park, J. W., Kang, M. J., Choi, H. J., Bae, S. J., Choi, Y., et al. (2019). Laxative effect of spicatoside A by cholinergic regulation of enteric nerve in loperamide-induced constipation: ICR mice model. Molecules 24:896. doi: 10.3390/molecules24050896
King, S. K., Sutcliffe, J. R., Ong, S. Y., Lee, M., Koh, T. L., Wong, S. Q., et al. (2010). Substance P and vasoactive intestinal peptide are reduced in right transverse colon in pediatric slow-transit constipation. Neurogastroenterol. Motil. 22, 883–892.e234. doi: 10.1111/j.1365-2982.2010.01524.x
Lee, J., Jang, Y. S., Han, M. J., Kim, J. Y., and Lee, S. Y. (2016). Deciphering clostridium tyrobutyricum metabolism based on the whole-genome sequence and proteome analyses. mBio 7:e00743-16. doi: 10.1128/mBio.00743-16
Li, J., Hu, Q., Xiao-Yu, D., Zhu, L., Miao, Y. F., Kang, H. X., et al. (2020). Effect of Sheng-Jiang powder on gut microbiota in high-fat diet-induced NAFLD. Evid. Based Complement Alternat. Med. 2020:6697638. doi: 10.1155/2020/6697638
Li, R., Li, M., Li, B., Chen, W. H., and Liu, Z. (2022). Cannabis sativa L. alleviates loperamide-induced constipation by modulating the composition of gut microbiota in mice. Front. Pharmacol. 13:1033069. doi: 10.3389/fphar.2022.1033069
Liang, S., He, Z., Liang, Z., Wang, K., Du, B., Guo, R., et al. (2024). Prunus persica. (L.) batsch blossom soluble dietary fiber synergia polyphenol improving loperamide-induced constipation in mice via regulating stem cell factor/C-kit, NF-κB signaling pathway and gut microbiota. Food Res. Int. 192:114761. doi: 10.1016/j.foodres.2024.114761
Liao, R., Xie, X., Lv, Y., Dai, J., Lin, Y., and Zhu, L. (2021). Ages of weaning influence the gut microbiota diversity and function in Chongming white goats. Appl. Microbiol. Biotechnol. 105, 3649–3658. doi: 10.1007/s00253-021-11301-2
Liu, P., Zhang, M., Liu, T., Mo, R., Wang, H., Zhang, G., et al. (2023). Avenanthramide improves colonic damage induced by food allergies in mice through altering gut microbiota and regulating Hsp70-NF-κB signaling. Nutrients 15:992. doi: 10.3390/nu15040992
Liu, Y., Xie, C., Zhai, Z., Deng, Z. Y., Jonge, H. R. De., Wu, X., et al. (2021). Uridine attenuates obesity, ameliorates hepatic lipid accumulation and modifies the gut microbiota composition in mice fed with a high-fat diet. Food Funct. 12, 1829–1840. doi: 10.1039/D0FO02533J
Liu, Z., Cheng, Y., and Chao, Z. (2023). A comprehensive quality analysis of different colors of medicinal and edible honeysuckle. Foods 12:3126. doi: 10.3390/foods12163126
Ohkusa, T., Koido, S., Nishikawa, Y., and Sato, N. (2019). Gut microbiota and chronic constipation: a review and update. Front Med. 6:19. doi: 10.3389/fmed.2019.00019
Park, C. W., Lee, J., Hong, Y. H., Kim, Y. S., Suh, H. J., and Ahn, Y. (2023). Coadministration of lactulose with probiotics ameliorates loperamide-induced constipation in mice. Prev. Nutr. Food Sci. 28, 427–435. doi: 10.3746/pnf.2023.28.4.427
Rekha, K., Venkidasamy, B., Samynathan, R., Nagella, P., Rebezov, M., Khayrullin, M., et al. (2024). Short-chain fatty acid: an updated review on signaling, metabolism, and therapeutic effects. Crit. Rev. Food Sci. Nutr. 64, 2461–2489. doi: 10.1080/10408398.2022.2124231
Satoshi, I., Kazuhide, H., and Satomi, H. (2020). c-Kit-stem cell factor signal-independent development of interstitial cells of Cajal in murine small intestine. Cell Tissue Res. 379, 121–129. doi: 10.1007/s00441-019-03120-9
Scott, S. M., Simrén, M., Farmer, A. D., Dinning, P. G., Carrington, E. V., Benninga, M. A., et al. (2021). Chronic constipation in adults: contemporary perspectives and clinical challenges. 1: Epidemiology, diagnosis, clinical associations, pathophysiology and investigation. Neurogastroenterol. Motil. 33:e14050. doi: 10.1111/nmo.14050
Shi, C., Liu, Y., Wu, Y., Han, D., Ma, J., Li, K., et al. (2022). Target and enhance ethanol and butyrate production from anaerobic fermentation via the pH and organic loading rate combined strategy. Appl. Biochem. Biotechnol. 194, 6367–6385. doi: 10.1007/s12010-021-03729-z
Shi, H., Chang, Y., Gao, Y., Wang, X., Chen, X., Wang, Y., et al. (2017). Dietary fucoidan of Acaudina molpadioides alters gut microbiota and mitigates intestinal mucosal injury induced by cyclophosphamide. Food Funct 8, 3383–3393. doi: 10.1039/C7FO00932A
Soret, R., Chevalier, J., Coppet, P. De., Poupeau, G., Derkinderen, P., Segain, J. P., et al. (2010). Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 138, 1772–1782. doi: 10.1053/j.gastro.2010.01.053
Sumida, K., Molnar, M. Z., Potukuchi, P. K., Thomas, F., Lu, J. L., Yamagata, K., et al. (2019). Constipation and risk of death and cardiovascular events. Atherosclerosis 281, 114–120. doi: 10.1016/j.atherosclerosis.2018.12.021
Sun, S. S., Wang, K., Ma, K., Bao, L., and Liu, H. W. (2019). An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 17, 3–14. doi: 10.1016/S1875-5364(19)30003-2
Tuohongerbieke, A., Wang, H., Wu, J., Wang, Z., Dong, T., Huang, Y., et al. (2024). Xiao Cheng Qi decoction, an ancient Chinese herbal mixture, relieves loperamide-induced slow-transit constipation in mice: an action mediated by gut microbiota. Pharmaceuticals 17:153. doi: 10.3390/ph17020153
Varni, J. W., Nurko, S., Shulman, R. J., Self, M. M., Saps, M., Bendo, C. B., et al. (2015). Pediatric functional constipation gastrointestinal symptom profile compared with healthy controls. J. Pediatr. Gastroenterol. Nutr. 61, 424–430. doi: 10.1097/MPG.0000000000000869
Vriesman, M. H., Koppen, I. J. N., Camilleri, M., Lorenzo, C. Di., and Benninga, M. A. (2020). Management of functional constipation in children and adults. Nat. Rev. Gastroenterol. Hepatol. 17, 21–39. doi: 10.1038/s41575-019-0222-y
Wang, D. D., Zheng, Y., Toledo, E., Razquin, C., Ruiz-Canela, M., Guasch-Ferré, M., et al. (2018). Lipid metabolic networks, mediterranean diet and cardiovascular disease in the PREDIMED trial. Int J Epidemiol. 47, 1830–1845. doi: 10.1093/ije/dyy198
Wang, L., Xie, S., Jiang, X., Xu, C., Wang, Y., Feng, J., et al. (2023). Therapeutic effects of bombax ceiba flower aqueous extracts against loperamide-induced constipation in mice. Pharm. Biol. 61, 125–134. doi: 10.1080/13880209.2022.2157841
Wang, R., Lu, X., Zhao, L., Zhang, W., and Zhang, S. (2023). Houpo paiqi mixture promotes intestinal motility in constipated rats by modulating gut microbiota and activating 5-HT-cAMP-PKA signal pathway. J. Appl. Microbiol. 134:lxad153. doi: 10.1093/jambio/lxad153
Wu, Y., Wu, Y., Wu, H., Wu, C., Ji, E., Xu, J., et al. (2021). Systematic survey of the alteration of the faecal microbiota in rats with gastrointestinal disorder and modulation by multicomponent drugs. Front. Pharmacol. 12:670335. doi: 10.3389/fphar.2021.670335
Xu, X., Gao, Z., Yang, F., Yang, Y., Chen, L., Han, L., et al. (2020). Antidiabetic effects of gegen qinlian decoction via the gut microbiota are attributable to its key ingredient berberine genomics proteomics. Bioinformatics 18, 721–736. doi: 10.1016/j.gpb.2019.09.007
Xu, Y., Yang, Y., Li, B., Xie, Y., Shi, Y., and Le, G. (2022). Dietary methionine restriction improves gut microbiota composition and prevents cognitive impairment in D-galactose-induced aging mice. Food Funct. 13, 12896–12914. doi: 10.1039/D2FO03366F
Yang, H., Zingaro, V. A., Lincoff, J., Tom, H., Oikawa, S., Oses-Prieto, J. A., et al. (2024). Remodelling of the translatome controls diet and its impact on tumorigenesis. Nature 633, 189–197. doi: 10.1038/s41586-024-07781-7
Yao, Z., Fu, S., Ren, B., Ma, L., and Sun, D. (2022). Based on network pharmacology and gut microbiota analysis to investigate the mechanism of the laxative effect of pterostilbene on loperamide-induced slow transit constipation in mice. Front. Pharmacol. 13:913420. doi: 10.3389/fphar.2022.913420
Ye, J., Lv, L., Wu, W., Li, Y., Shi, D., Fang, D., et al. (2018). Butyrate protects mice against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis by improving gut barrier function, attenuating inflammation and reducing endotoxin levels. Front. Microbiol. 9:1967. doi: 10.3389/fmicb.2018.01967
Zeng, X., Chen, L., and Zheng, B. (2024). Extrusion and chlorogenic acid treatment increase the ordered structure and resistant starch levels in rice starch with amelioration of gut lipid metabolism in obese rats. Food Funct. 15, 5224–5237. doi: 10.1039/D3FO05416K
Zhan, Y., Wen, Y., Du, L. J., Wang, X. X., Tang, S. Y., Kong, P. F., et al. (2022). Effects of maren pills on the intestinal microflora and short-chain fatty acid profile in drug-induced slow transit constipation model rats. Front. Pharmacol. 13:804723. doi: 10.3389/fphar.2022.804723
Zhang, M. M., Gong, Z. C., Zhao, Q., Xu, D. Q., Fu, R. J., Tang, Y. P., et al. (2023). Time-dependent laxative effect of sennoside A, the core functional component of rhubarb, is attributed to gut microbiota and aquaporins. J. Ethnopharmacol. 311:116431. doi: 10.1016/j.jep.2023.116431
Zhang, W., Wang, X., Yin, S., Wang, Y., and Li, Y. (2024). Improvement of functional dyspepsia with Suaeda salsa. (L.) Pall via regulating brain-gut peptide and gut microbiota structure. Eur. J. Nutr. 63, 1929–1944. doi: 10.1007/s00394-024-03401-2
Zhang, X., Zheng, F. J., and Zhang, Z. (2021). Therapeutic effect of Cistanche deserticola on defecation in senile constipation rat model through stem cell factor/C-kit signaling pathway. World J. Gastroenterol. 27, 5392–5403. doi: 10.3748/wjg.v27.i32.5392
Zheng, H., Han, L., Shi, W., Fang, X., Hong, Y., and Cao, Y. (2022). Research advances in lotus leaf as chinese dietary herbal medicine. Am. J. Chin. Med. 50, 1423–1445. doi: 10.1142/S0192415X22500616
Zheng, L., Kelly, C. J., Battista, K. D., Schaefer, R., Lanis, J. M., Alexeev, E. E., et al. (2017). Microbial-Derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of claudin-2. J. Immunol. 199, 2976–2984. doi: 10.4049/jimmunol.1700105
Keywords: constipation, Elsholtzia ciliata, Hovenia dulcis, gut microbiota, loperamide, edible traditional Chinese medicine (ETCM)
Citation: Wu J, Xin Q, Wang S, Zhang X and Jiang C (2025) Aqueous extracts of Elsholtzia ciliata and Hovenia dulcis ameliorate loperamide-induced constipation in mice by promoting intestinal peristalsis and barrier function and the abundance of intestinal beneficial bacteria. Front. Microbiol. 16:1531232. doi: 10.3389/fmicb.2025.1531232
Received: 20 November 2024; Accepted: 15 April 2025;
Published: 13 May 2025.
Edited by:
Valentina Caputi, University College Cork, IrelandCopyright © 2025 Wu, Xin, Wang, Zhang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunping Jiang, Y2h1bnBpbmdqaWFuZ0BuanUuZWR1LmNu
 Junnan Wu1,2
Junnan Wu1,2 Chunping Jiang
Chunping Jiang