- 1National Key Laboratory for Development and Utilization of Forest Food Resources, Zhejiang A&F University, Hangzhou, China
- 2Provincial Key Laboratory for Non-wood Forest and Quality Control and Utilization of Its Products, Zhejiang A&F University, Hangzhou, China
Bamboo is widely distributed or cultivated globally, offering significant economic and ecological values. Soil microorganisms are crucial for plant environmental adaptation, playing essential roles in regulating plant growth and development, nutrient absorption, and resistance to environmental stresses. In recent years, substantial progress has been made in the study of bamboo soil microorganisms. This review highlights the scientific challenges in understanding the interactions between bamboo and soil microorganisms, summarizes the research progress, and discusses future research directions. The microbial community composition and diversity in various bamboo soils have been successfully characterized, with some bamboo-associated microorganisms identified and shown to promote plant growth, demonstrating considerable application potential. It has been established that the composition of soil microorganisms in bamboo is influenced by factors such as bamboo species, spatial and temporal distribution, tissue specificity, management practices, and symbiosis with other plants. Future research will likely focus on the functional genomics of bamboo, the screening and identification of bamboo-specific soil microbial communities, the dynamic responses of these microbes to environmental changes, and the molecular mechanisms regulating bamboo growth and environmental adaptation.
Highlights
• Endophytes and rhizosphere microorganisms are crucial in bamboo plant life.
• Bamboo-associated microorganisms have been extensively studied through culture and high-throughput sequencing.
• Microbial composition and function related to bamboo are regulated by species and spatiotemporal factors.
• Research on bamboo-microbe interactions will advance high-quality bamboo forest cultivation.
1 Introduction
Plants provide a rich ecological niche for microorganisms, including bacteria, fungi, protozoa, nematodes, and viruses, which can establish complex interconnections with plants and play crucial roles in promoting plant growth, nutrient acquisition, and stress resistance (Trivedi et al., 2020). Increasing evidence suggested that plant evolution was closely linked to the microbial communities inhabiting their surroundings, with microbes acting as essential symbiotic partners that shaped plant fitness and adaptability (Vandenkoornhuyse et al., 2015). While plant-microbe interactions have been extensively studied in various model and crop plants, the specific mechanisms underlying bamboo-associated microbial communities remain relatively underexplored.
Bamboos, classified under the subfamily Bambusoideae of the Poaceae family, have become one of the most valuable plants in Poaceae family for their wide distribution, huge economic and ecological value. By 2015, they have covered over 30 million hectares worldwide (Lin, 2021). Bamboo is primarily distributed across three major global bamboo regions: the Asia-Pacific, the Americas, and the Africa, with China hosting about half of the world’s bamboo resources (Jiang, 2008). According to the 2021 China Forest and Grass Ecological Comprehensive Monitoring and Evaluation Report, bamboo forests span 7.56 million hectares across 20 provinces in China. In addition, bamboo is valued not only for its economic benefits but also for its role in carbon sequestration, soil and water conservation, and ecosystem restoration (Yang, 2004), underscoring their ecological and economic significance. However, bamboo growth is highly susceptible to environmental stressors such as extreme temperatures, drought, and soil degradation (Wang et al., 2022; Li et al., 2017), and microbes play an important role in coping with these environmental changes. By the end of 2018, 130 genera and 1,700 species of bamboo had been identified globally (Zhong et al., 2019), demonstrating its high species diversity. The microbial communities recruited by different bamboo species are different, which provides a rich research content for the study of bamboo-microbe interaction. Despite increasing recognition of plant-microbe interactions as key regulators of plant adaptation, the role of microbial communities in modulating bamboo resilience to environmental challenges remains insufficiently studied. Research on bamboo—microbe interactions have gained significant attention for its potential to enhance bamboo growth and cultivation conditions.
To address this gap, this review aims to: (1) summarize the current understanding of bamboo—associated microbial communities, including their composition, functions, and ecological roles; (2) examine how environmental and management factors influence bamboo microbiomes; and (3) identify knowledge gaps and propose future research directions in bamboo-microbe interactions. In addition, we discuss how bamboo-microbe interactions fit within the broader framework of plant-microbe symbioses, highlighting their potential contributions to sustainable bamboo forest management and resilience under climate change. By integrating recent findings, we provide a theoretical foundation for advancing research on bamboo-microbe interactions and their applications in sustainable forestry.
2 Research hotspots and progress of soil microorganisms with plant
Soil serves as a fundamental environmental component for plant growth, playing a pivotal role in organic matter decomposing, nutrient cycling, and water retention (Ritz et al., 2009; Sağlam et al., 2015; Zhou et al., 2019). As a dynamic medium of plant-microbe interaction, soil harbors a vast and diverse microbial community, with microbial densities up to 105 per gram of soil. These microorganisms are not only crucial for soil ecosystem stability but also serve as sensitive indicators of environmental changes (Xu et al., 2015; Winding et al., 2005). Beyond their role in carbon cycling, soil microbes drive key biogeochemical processes, including nitrogen fixation, phosphorus solubilization, and organic matter decomposition, directly influencing plant health and productivity (Kong et al., 2023). Therefore, the interactions between plants and soil microorganisms represent a crucial focus for advancing the study of plant—microbe relationships.
Microorganisms can be categorized into two types based on their colonization locations: endophytic microbes and those colonizing the surrounding areas of plant, such as phyllospheric and rhizosphere microorganisms. Endophytes were microorganisms that penetrated and invaded plant interiors (Vandenkoornhuyse et al., 2015), and were found in shoots (Martínez-Arias et al., 2021), leaves (Song et al., 2022), buds (Pirttilä et al., 2005), seeds (Wallace, 2023), and roots (Gaiero et al., 2013). Some endophytes promoted host plant growth by secreting hormones like auxin and participating in metabolic regulation, protecting plants from pathogens through autoimmune defense mechanisms (Ali et al., 2012). Phyllospheric microorganisms, also known as epiphytic or foliar microorganisms, resided on plant leaf surfaces (Beattie and Lindow, 1999; Lindow and Brandl, 2003), impacting leaf function, seed quality, fruit development, and host growth homeostasis (Xu et al., 2022). The rhizosphere, a critical plant-microbe interaction area, spaned 0.5–4 mm on the root surface (Kuzyakov and Razavi, 2019). During plant growth, carbon fixed by photosynthesis was released into the rhizosphere as root exudates, including carbohydrates and organic acids, alongside some enzymes (Mommer et al., 2016; Lakshmanan et al., 2014). These root exudates not only provide a carbon source for the soil microbial communities that colonizes the rhizosphere of plants, but also regulate the species, quantity, and distribution of rhizosphere microbes, thus constructing specific rhizosphere microbial community structures (de la Fuente Cantó et al., 2020; Moe, 2013). The rhizosphere microbiome is therefore termed the “Second genome” of plants (Berendsen et al., 2012). Among which, beneficial rhizosphere microorganisms have potential to enhance plant growth by promoting mineral element absorption (Reis and Teixeira, 2015). In summary, microorganisms in different plant tissue parts or growing spaces play irreplaceable roles in plant growth and development.
Plant species and genotypes affect rhizosphere microorganism and endophyte composition. Meanwhile, plant-related microbial community composition and structure are influenced by research methods (Ren et al., 2025; Chen et al., 2022; Dhondge et al., 2022; Li et al., 2020a; Ma and Xiao, 2004; Gao et al., 2004). Two primary methods for studying plant microbial composition: isolating and quantifying microorganisms using various mediums (Krishnapura and Belur, 2016) and sequencing DNA or RNA under culture-free conditions. The former was convenient for studying isolated strains, only a small proportion of natural microorganisms can be cultured in the laboratory (Pace, 1997; Tholozan et al., 1999; Steen et al., 2019), while the latter, a mainstream method, used metagenomics to assess the full DNA data of environmental microorganisms, allowing analysis of complex microbial communities (Hugenholtz and Tyson, 2008; Handelsman, 2004). Metagenomics is widely applied in microorganism research by using length heterogeneity PCR (lh-PCR), PCR-denaturing gradient gel electrophoresis (PCR-DGGE), terminal restriction fragment length polymorphism (T-RFLP) fingerprinting, and sequencing 16S rRNA gene amplicons, to identify culture-independent bacteria (Chen et al., 2022; Dhondge et al., 2022). However, multiple copies of 16S rRNA genes in bacteria introduced detection deviations (Gulati et al., 2011). High-throughput Illumina sequencing is also widely used to study community structure, diversity, and abundance (Yan et al., 2023). New research techniques and analytical models, such as the use of modular toolkits as DNA barcodes for bacterial strains combined with fluorescent proteins, are being developed to track competition between strains in plant tissues and other microbiome members (Ordon et al., 2024). Most plant-associated microbiome research, especially quantitative studies, primarily focuses on relative quantification methods. However, absolute quantification of specific microbial populations with plant tissues or the rhizosphere represents a key advancement in the field, offering more precise and reliable insights into microbial community structure. Absolute quantification methods, such as quantitative PCR (qPCR) or quantitative microbiome profiling (QMP), can overcome many limitations associated with relative quantification, such as primer bias and variations in sample composition. For instance, QMP has been employed to investigate the effects of unbalanced fertilization on soybean rhizosphere microbiome, demonstrating that QMP provides more accurate measurements of specific microbial abundance compared to relative microbiome profiling (RMP) (Wang M. et al., 2024). This approach allows for a more detailed understanding of microbial population dynamics, which is crucial for elucidating plant-microbe interactions.
In addition to these advances in quantification methods, bioinformatics innovations have propelled research into plant-microbe interaction mechanisms, such as microbial metabolism, signal transduction, and genetic regulation. For example, Schäfer et al. (2023) utilized a metagenomic scale model to explore carbon source utilization and the interactions of specific microbial strains on the Arabidopsis leaf surface, providing valuable insights into microbial resource allocation and adaption in multispecies environments. The integration of multiple “omics”mics integrationften referred to as “Holo-omics,” is gaining attraction in microbial community research (Odriozola et al., 2024). Holo-omics combines data from genomics, transcriptomics, proteomics, and metabolomics to provide a comprehensive view of biological processes at various levels of organization. This holistic approach enables the identification of dynamic interactions between plants and microorganisms by capturing both microbial and plant responses simultaneously. Furthermore, “Genome-Wide Association Studies” (GWAS) is a powerful method used to identify genetic loci linked to specific traits, such as microbial community composition. By associating genetic variations with microbial phenotypes, GWAS can pinpoint host genes that regulate microbial selectivity and composition in plant tissues. For instance, Zhan et al. (2022) leveraged high-throughput techniques and bioinformatics analyses to identify key microbial communities associated with Arabidopsis leaves, and through GWAS and QTLs (Quantitative Trait Locus) mapping, they identified host genes that regulate the microbiota.
Current research on plant-microbe interactions focuses on the relationship between environmental change and microbial community composition and diversity, plant-microbe interaction mechanism, and microbes’ beneficial effects on plants. Studies have examined how diverse elements and temperature changes affects microorganisms (Frey et al., 2023). For instance, long-term nitrogen addition altered the community structure of nitrogen-fixing bacteria in grassland soil without affecting microbial abundance (Frey et al., 2023; Wang et al., 2023). Some studies indicated that microbial responses to warming were weak, with declines in microbial enzyme production, biomass, and other functions based on plant composition analysis (Spinella et al., 2024). Further research by Tao et al. (2024) demonstrated that experimental warming regulated soil priming by altering the active microbial community’s functional structure. Rhizosphere microbiomes are influenced by both soil properties and genes (Xun et al., 2024), emphasizing soil properties’ importance in rhizosphere microorganism composition. Research by Li et al. (2024) showed host plants exhibited strong selectivity for microbes, impacting microbial composition from top to bottom (the effects of plant-driven microbiome assembly), while beneficial microbes played essential roles in plant evolution from bottom to up (microbiome-shaped plant traits). It has been reported that maize-peanut intercropping enriched rhizosphere bacteria associated with secreted iron carriers, increasing iron availability of in intercropping peanut rhizosphere (Wang N. et al., 2024). Beneficial microorganisms promoting plant growth, such as Bacillus and Pseudomonas, were well studied, mainly in crops like rice, wheat, tomato (Zhou et al., 2021; Dasila et al., 2023; He et al., 2019).
3 The diversity and function of microorganisms in bamboos
Like other terrestrial plants, bamboo comprises two primary parts: the aboveground and underground sections. The aboveground part is exposed to the atmosphere, enabling bamboo to interact closely with various substances and microorganisms. The underground part consists of bamboo roots, and in some cases, bamboo rhizomes, which aid in reproduction. The developed bamboo rhizome system provides sample opportunities for microbial colonization. The microbiome in different bamboo tissues and their surrounding environment exhibits specificity at different growth stages under various environmental conditions (Figure 1, generated by Figdraw), significantly affecting the growth, physiological, and ecological characteristics of bamboo. Therefore, it is valuable to study the microbial diversity and function related to bamboo.
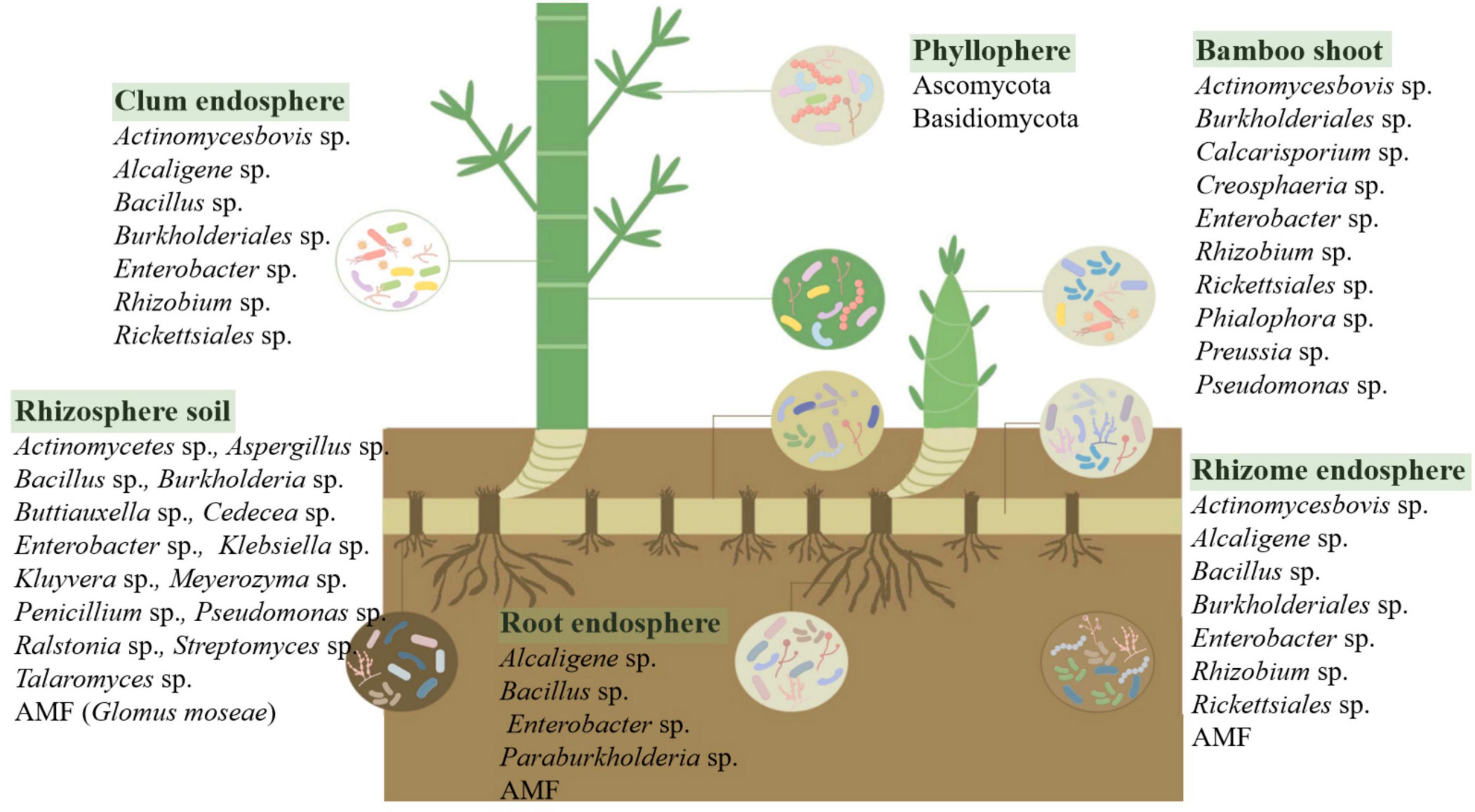
Figure 1. Microbial communities associated with different bamboo niches. It illustrates the distribution of microbial communities across various compartments of the bamboo plant, including the rhizosphere soil, root endosphere, rhizome endosphere, clum endosphere, bamboo shoot, and phyllosphere. The rhizosphere harbors diverse bacteria (Actinomycetes, Bacillus, Burkholderia, Streptomyces) and fungi (Aspergillus, Penicillium, Glomus mosseae), playing crucial roles in nutrient cycling and plant growth promotion. The root and rhizome endospheres contain endophytic bacteria (Alcaligenes, Enterobacter, Rhizobium) and arbuscular mycorrhizal fungi (AMF), contributing to nutrient acquisition and stress resilience. The clum endosphere supports structural integrity, while the bamboo shoot microbiome includes plant growth-promoting bacteria (Pseudomonas, Burkholderiales) and fungi (Calcarisporium, Phialophora). The phyllosphere is dominated by Ascomycota and Basidiomycota, which influence leaf surface homeostasis and disease resistance. Understanding these microbial associations provides insights into plant-microbe interactions, disease prevention, and sustainable bamboo management.
3.1 Composition and function of endophere microorganisms
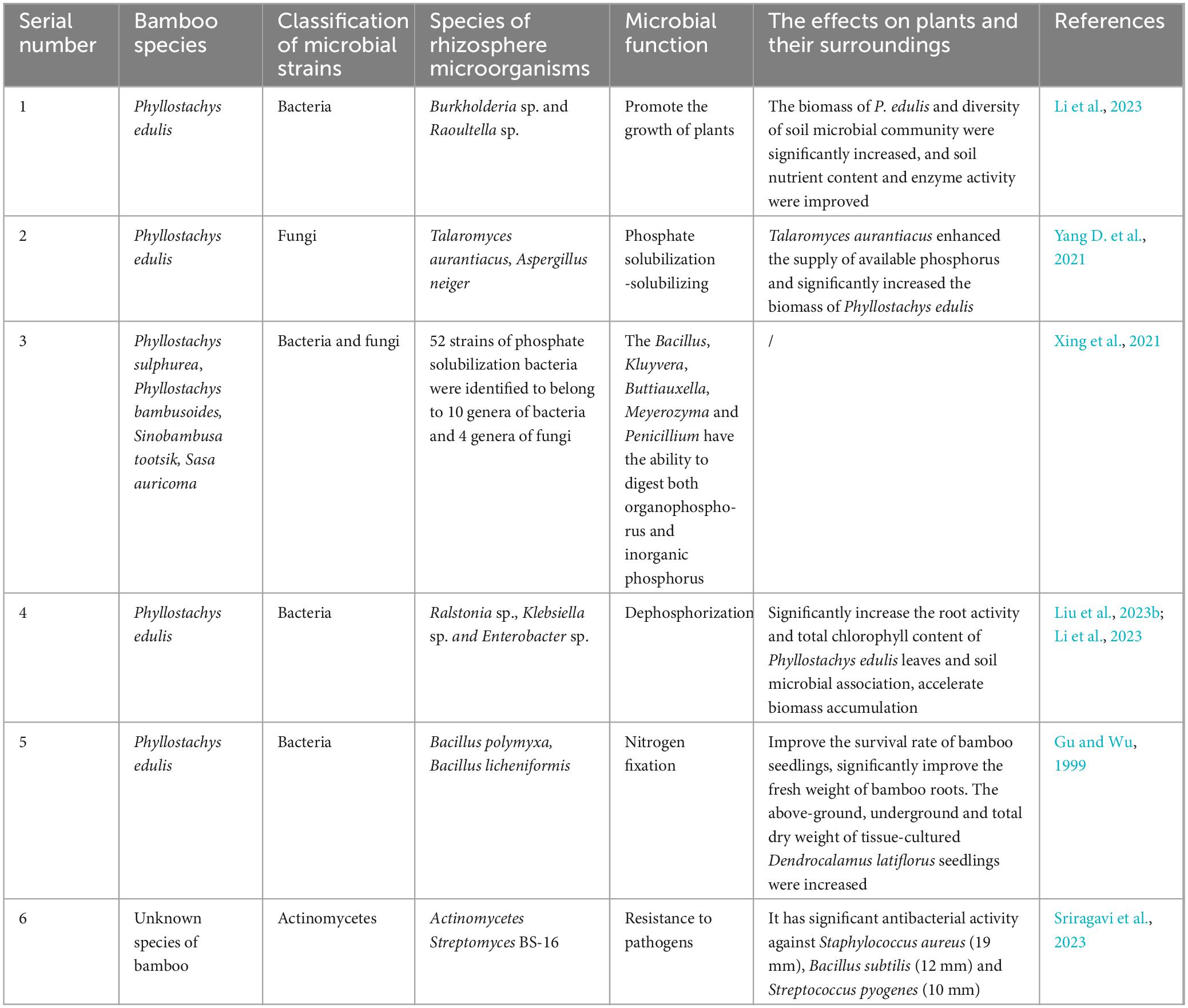
Most land plants are colonized by bacteria (Reinhold-Hurek and Hurek, 2011), fungi (including arbuscular mycorrhizal fungi and other fungi) (Smith and Read, 2008; Vandenkoornhuyse et al., 2007; Streitwolf-Engel et al., 1997), and a few archaea (Sun et al., 2008), with fungi being well studied. Research on the microbial composition in bamboo endophytic tissues mainly focuses on leaves, culms, seeds, and roots. According to previous reports, most known endophytic fungi in bamboo belong to the genus Ascomycota, including Arthrinium, Fusarium, Xylaria, and Ascomycota, and a small variety to Basidiomycota (Wang et al., 2020c). Endophytic bacteria and actinomycetes in bamboos encompass more genera and species, varying in abundance and species across different bamboo varieties and tissues. Studies indicated that endophytes isolated from various bamboo tissues were beneficial for plants growth, exhibiting diverse abilities through functional and genomic analyses, such as promotion plant growth by phosphorus-solubilizing and nitrogen-fixing and inhibiting pathogens. The diversity and function of endophytes in bamboo plants are summarized in Table 1.
3.2 Composition and function of rhizosphere microorganisms
Studies on the rhizosphere microorganisms of bamboo primarily focus on the quantity of rhizosphere bacteria, fungi, and actinomycetes. These studies often target several widely cultivated bamboo species, such as Phyllostachys edulis, Phyllostachys praecox, Fargesia fargesii, Fargesia denudata, and Phyllostachys heterocycla.
Under normal growth conditions, the diversity of bacteria and fungi in rhizosphere soil was significantly higher than that in bulk soil (Meng et al., 2015; Qi and Yang, 2006; Li et al., 2020a). High-throughput sequencing results from the rhizosphere soil of various bamboo species indicated that bacteria were the most abundant among rhizosphere microorganisms (Guo et al., 2013). The abundance and diversity of bacterial communities in rhizosphere soils of Phyllostachys edulis, Phyllostachys praecox, and Phyllostachys vivax f. aureocaulis surpassed those of fungi (Li et al., 2020b). Consequently, research on bamboo rhizosphere bacteria is more extensive compared to fungi and actinomycetes.
For instance, the average count of bacteria in the rhizosphere soil of Drepanostachyum luodianense was 8.32 × 107 CFU/g dry weight, representing the largest number of culturable bacteria in bamboo rhizosphere (Li P. et al., 2016). In contrast, the culturable bacteria in the rhizosphere of Bashania fangiana and F. denudata were 4.3 × 106 CFU/g dry weight and 1.31 × 106 CFU/g dry weight, respectively (Liu et al., 2008; Qi and Yang, 2006). The bacteria count in the rhizosphere soil of Phyllostachys edulis was 7.1 × 105 CFU/g dry weight in Tianmu Mountain and 4.0 × 105 CFU/g dry weight in Jinyun Mountain, both lower than those in Bashania fangiana and Fargesia denudata (Li et al., 2008).
However, fewer studies focus on the count of fungi in bamboo rhizosphere soil, mainly addressing Fargesia denudata and Phyllostachys edulis. Qi and Yang (2006) estimated that the average count of fungi in the rhizosphere soil of Fargesia denudata was 1.02 × 105 CFU/g dry weight. The fungi count in the rhizosphere soil of Drepanostachyum luodianense was 9.19 × 105 CFU/g dry weight, comprising 34 species across 12 genera (Li P. et al., 2016; Chen M. et al., 2021).
The count of actinomycetes in the rhizosphere soil varies significantly between different bamboo species and the same bamboo species in different areas. For example, the actinomycetes count in the rhizosphere soil of Phyllostachys edulis in Tianmu Mountain and Jinyun Mountain were 3.0 × 105 CFU/g dry weight and 3.3 × 103 CFU/g dry weight, respectively (Li et al., 2008). A total of 20 actinomycetes were isolated from the rhizosphere soil of Phyllostachys heterocycla, belonging to 15 species within one genus, with a count of 2.32 × 106 CFU/g dry weight (Qi and Yang, 2006; Chen M. et al., 2021).
The results showed that the diversity of bacteria, fungi and actinomycetes in the rhizosphere soil of bamboo plants had a certain correlation with bamboo species, this may be related to the composition of bamboo root exudates, the growth environment of bamboo species and the soil environment. Bacteria are more abundant than fungi, probably because bamboo plants produce more simple organic compounds in root exudates, and bacteria utilize simple organic compounds more efficiently and compete with fungi for complex compounds, which may contribute to the abundance of bacteria, allowing more bacteria to be recruited (Wang and Kuzyakov, 2024). The number of culturable microorganisms in the rhizosphere of bamboo plants is much less than the total number, which may be due to the fact that most microorganisms cannot be cultured in laboratory conditions away from the natural environment.
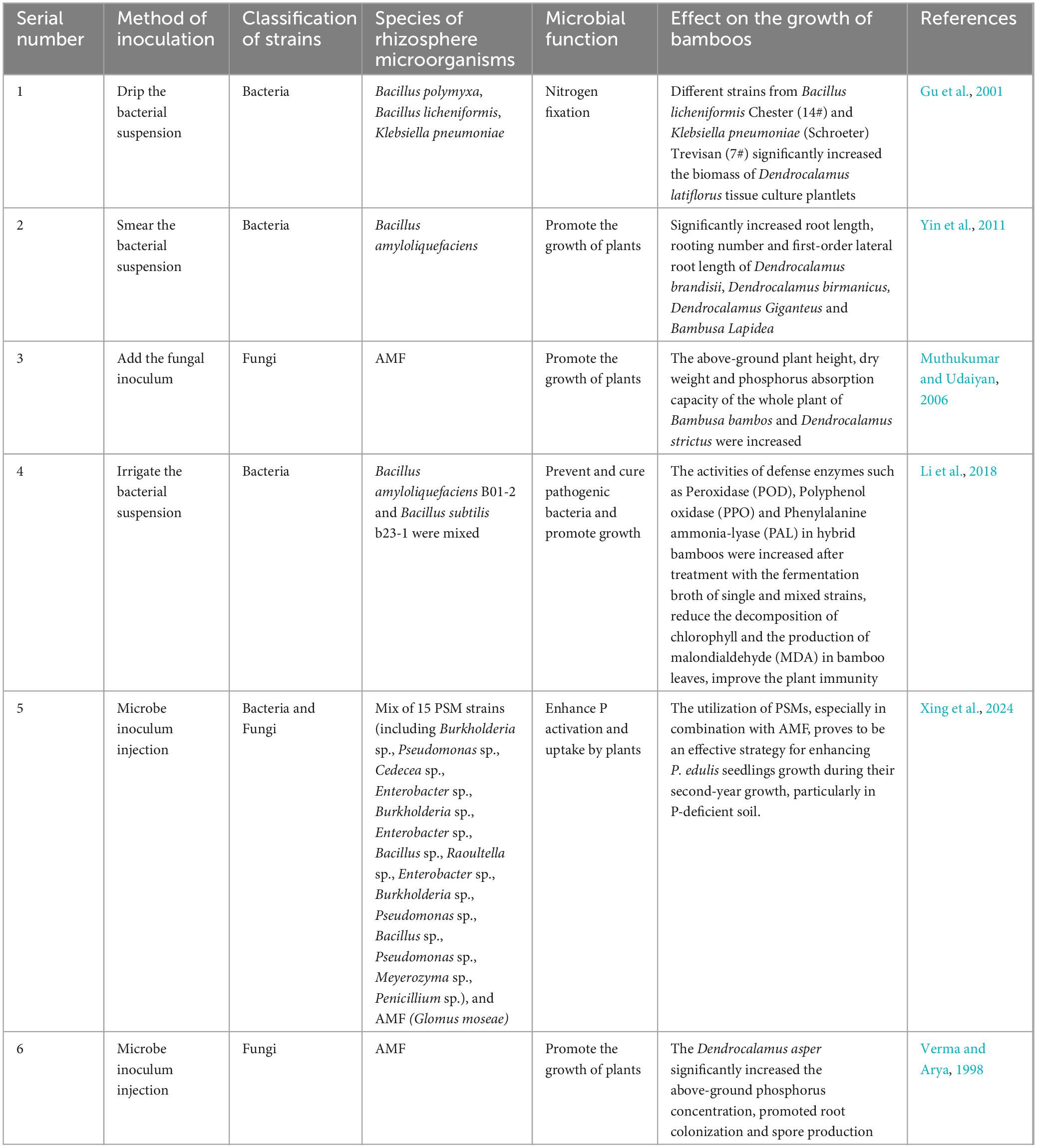
Several rhizosphere microorganisms isolated and cultured from bamboo have demongstrated positive effects on plant growth in experimental studies. These benificial rhizosphere bacteria, collectively known as plant growth-promoting rhizobacteria (PGPR) (Kloepper, 1981), play diverse roles in enhancing bamboo growth. They contribute by increasing biomass accumulation, facilitating nutrient uptake, and suppressing pathogenic infections. Various PGPR species have been identified in the bamboo rhizosphere, each exhibiting distinct mechanisms such as nitrogen fixation, phosphate solubilization, and phytohormone production, as summarized in Table 2.
In addition to PGPR, arbuscular mycorrhizal fungi (AMF), have been found to establish symbiotic associations with bamboo roots. AMF, which colonized the roots of approximately 80% of land plants (Vierheilig et al., 1998), play a crucial role in improving nutrient acquisition and stress tolerance. Studies have indicated that AMF inoculation enhanced bamboo growth by increasing phosphorus uptake, improving root system development, and potentially boosting resistance to abiotic stresses (Verma and Arya, 1998). Despite limited research on AMF-bamboo interactions, available findings highlight their significant contribution to bamboo health and productivity.
Although some microorganism strains are not originally isolated from the rhizosphere of bamboos, the growth of bamboos can be significantly improved by introducing these strains into the rhizosphere through liquid medium. This method has been shown to increase bamboo biomass and root length. Details of these reported strains and their effects are summarized in Table 3.
3.3 Composition and function of microorganisms in phyllosphere
The systematic and thorough study of plant microecology is conducive to understanding the relationship between microorganisms and plants. Phyllospheric microorganisms are an important part of plant microecology. It is significant to enrich microbial sources and develop new applications in the control of plant diseases by intensive research. Compared to rhizosphere microbes, research on the composition and diversity of the microbes in bamboo leaves is less extensive. Using ITS1 amplification and metagenomic sequencing, Kang et al. (2022) analyzed the diversity and function of phyllosphere fungi in three main bamboo species preferred by panda: Arundinaria spanostachya, Yushania lineolata, and Fargesia Ferax. The results showed that the predominant fungi were Ascomycota and Basidiomycota, and their relative abundance did not significantly differ among the three bamboo species.
As the sole food source for giant panda, bamboo is high in cellulose. However, giant pandas lack the genome-encoding enzyme necessary to digest cellulose (Hu et al., 2017), suggesting that microbial degradation plays a significant role in bamboo digestion (Li et al., 2010). Some bacterial phyla such as Proteobacteria, Acidobacteria, and Bacteroidetes in bamboo phyllospheres are dominant (Kang et al., 2022). These microbes may contribute to the breakdown of complex plant materials and improve digestion. Additionally, these microbiota play important biological roles in promoting plants growth and development, such as regulating root growth, promoting nutrient absorption, balancing plant hormones, and preventing disease invasion (Xu et al., 2018). An imbalance in the panda’s gut flora can lead to gastrointestinal diseases, which are the most common cause of death in pandas (Tun et al., 2014). As an important food source for giant pandas, bamboo leaves have different nutrient and microbial compositions as compared with bamboo shoots, bamboo stems, and branches (Wei et al., 2015; Wu et al., 2017; Long et al., 2021). Thus, the microbes on the bamboo leaves may influence the composition and changes in the panda’s gut flora after consuming bamboo leaves. Therefore, studying the composition and abundance of microbes in the phyllosphere of bamboo plants, especially those species consumed by giant panda, is crucial for maintaining the normal growth and population stability of giant panda. Moreover, further research is needed to identify microbial communities in bamboo foliage that benefit both giant panda digestion and bamboo plant growth.
4 Factors affecting the microbial composition of bamboos
According to previous researches, the main factors affecting the microbial composition of bamboo plants include bamboo species, tissue specificity, spatial and temporal distribution, soil properties, management measures, and the expansion of bamboo forests (Figure 2, generated by Figdraw).
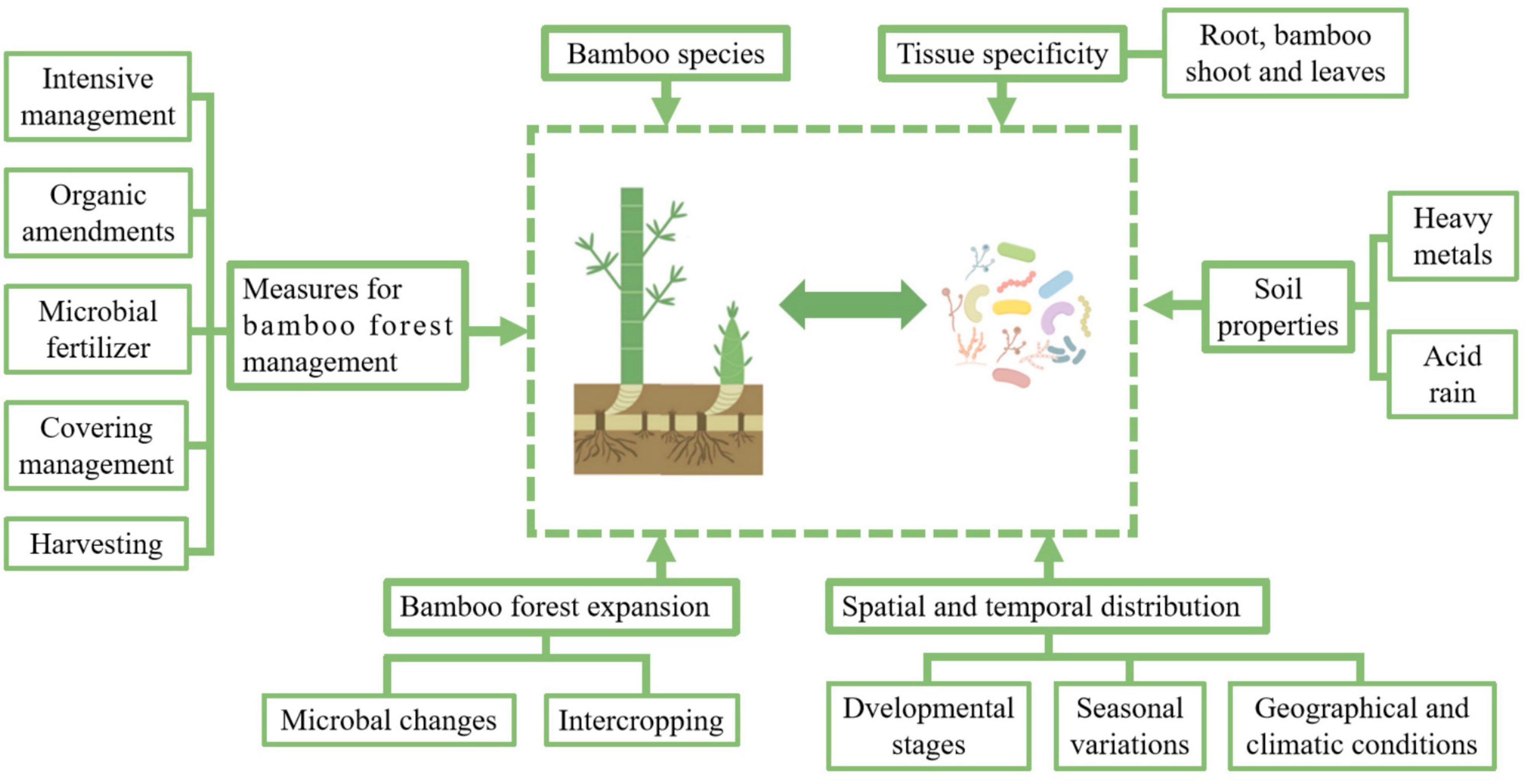
Figure 2. Key factors influencing the bamboo-associated microbiome. This figure illustrates the diverse factors shaping the composition and dynamics of the bamboo-associated microbiome. The microbiome is influenced by bamboo species and tissue specificity, with distinct microbial communities colonizing roots, shoots, and leaves. Soil properties, including heavy metal contamination and acid rain, impose selective pressures that alter microbial diversity and function. The spatial and temporal distribution of microbes varies with developmental stages, seasonal changes, and geographical and climatic conditions, reflecting dynamic shifts in plant-microbe interactions. The expansion of bamboo forests, whether through natural spread or intercropping, affects microbial composition by modifying soil environments and plant-microbe relationships. Additionally, management practices, such as intensive cultivation, organic amendments, microbial fertilizers, covering techniques, and harvesting methods, play a crucial role in structuring microbial communities. These practices can enhance soil health, promote beneficial microbes, and improve bamboo resilience. Understanding these interconnected factors provides insights into microbiome regulation and offers strategies for optimizing bamboo forest management to support sustainable growth and ecosystem stability.
4.1 Bamboo species
Plants recruit soil microorganisms to colonize the rhizosphere through root exudates, resulting in variations in rhizosphere microbe communities among different plants (Xia et al., 2023; Dawson et al., 2017). Similar to crop studies, the bacterial communities at the phylum level with high relative abundance in the rhizosphere of rice are Proteobacteria, Actinobacteria, Gemmatimonadetes, and Acidobacteria (Zhang Z. et al., 2015), while those in wheat are Proteobacteria, Bacteroidetes, Acidophilus and Chloroflexi (Wang et al., 2019), demonstrating that there are different from each other in the bacterial communities.
High-throughput sequencing studies on the rhizosphere microbial composition of common bamboo species in the genus Phyllostachys revealed differences in dominant microbial populations (Li et al., 2020a; Fu et al., 2022). The rhizosphere soils of several bamboo species, including Phyllostachys edulis, Phyllostachys glauca, Phyllostachys vivax f. aureocaulis, and Phyllostachys iridescens, contained dominant bacteria such as Proteobacteria, Chloroflexi, Actinobacteria, and Acidobacteria (Li et al., 2020b; Fu et al., 2022). Although these dominant bacterial phyla are consistent across species, their relative abundance varies. For instance, the relative abundance of the four dominant bacterial phyla, including Proteobacteria, Chloroflexi, Actinobacteria, and Acidobacteria, in the rhizosphere of Phyllostachys edulis is similar, in contrast, Proteobacteria and Acidobacteria are most abundant in Phyllostachys praecox and Phyllostachys vivax f. aureocaulis, respectively. The highest relative abundance of Actinomycetes is found in the rhizosphere soils of Phyllostachys glauca and Phyllostachys iridescens.
At the class level, Alphaproteobacteria and Acidobacteria dominate the rhizosphere soils of Phyllostachys edulis, Phyllostachys praecox, and Phyllostachys vivax f. aureocaulis, whereas Actinobacteria dominate Phyllostachys glauca and Phyllostachys iridescins, indicating significant differences in relative abundance among microbial classes in rhizosphere soils of different bamboo species. For example, the relative abundances of Thermoleophilia in the rhizosphere soils of Phyllostachys edulis, Phyllostachys glauca, and Phyllostachys iridescens are significantly different, with the highest abundance in Phyllostachys glauca and the lowest in Phyllostachys edulis. Similarly, the relative abundances of Gammaproteobacteria, Bacteroidia and Bacilli exhibit extremely significant difference, with Gammaproteobacteria being most abundant in Phyllostachys edulis and least abundant in Phyllostachys glauca. At the order level, Rhizobiales is dominant in the rhizosphere soil of Phyllostachys edulis, while both Rhizobiales and Acidobacteriales dominate in the rhizosphere soils of Phyllostachys praecox and Phyllostachys vivax f. aureocaulis. Both Phyllostachys edulis and Phyllostachys praecox need to absorb large amounts of water during their growth, otherwise they may wilt. Previous study reported that when exposed to drought stress, rhizobia, as beneficial bacteria, were enriched in the rhizosphere to improve drought resistance by uptaking nutrients and synthesizing plant hormones (Chen et al., 2024), inferring that may be the reason why Rhizobiales are the dominant class in the rhizosphere soil of these two bamboo species. Rhizosphere fungal communities of Phyllostachys edulis, Phyllostachys glauca, and Phyllostachys iridescens are primarily composed of Ascomycota and Basidiomycota, with no significant differences at the phyla level. However, at the class level, Sordariomycetes is more abundant in Phyllostachys glauca, Leotiomycetes in Phyllostachys iridescens, and Eurotiomycetes in Phyllostachys edulis. Additionally, unique differences at the order level were detected, such as the presence of the unique fungi family Helotiaceae in the rhizosphere soil of Phyllostachys edulis (Fu et al., 2022).
Moreover, the composition of endophytic fungi and the abundance of soil microorganisms vary among bamboo species. For instance, Pleioblastus amarus had the highest abundance of endophytic fungi, followed by Phyllostachys edulis (Yan et al., 2023). The abundance of soil bacteria in the three bamboo forests followed the order Phyllostachys edulis > Bambusa pervariabilis × Dendrocalamopsis daii > Bambusa emeiensis (Xu et al., 2022).
4.2 Tissue specificity
The microbial community structure in different tissues of bamboo exhibits significant variation, with distinct dominant phyla and diversity levels across tissues such as leaves, roots, rhizosphere soil, and non-rhizosphere soil. Comparative analyses of these microbial communities reveal marked differences in both bacterial and fungal populations. By studying the changes of microbial communities in different tissues under various growth conditions, strains actively responsive to stress can be screened and made into microbial fertilizers to regulate the growth of bamboo plants, improving the ability to cope with environmental stress.
4.2.1 Microbial community structure in different tissues
Studies using principal coordinate analysis (PCoA) and hierarchical cluster analysis (HCA) based on the Bray-Curtis difference matrix demonstrated significant differences in bacterial diversity and composition between the surface of leaves and roots in various bamboo species. These differences were apparent in β diversity but not in α diversity, indicating variations in community composition rather than richness (Zheng and Lin, 2020).
The bacterial communities in the rhizosphere soil and endophytic tissues of Phyllostachys edulis differ significantly from those in non-rhizosphere soil, rhizome soil, and root endophytic tissues (Yuan et al., 2021). For example, endophytic bacterial communities in bamboo shoots and culms showed distinct compositions, emphasizing tissue-specific microbial associations (Liu et al., 2017).
Recent high-throughput sequencing analyses of Phyllostachys edulis revealed that Proteobacteria dominated in bamboo shoot and rhizome samples, while Acidobacteria were prevalent in rhizosphere and forest soil samples. The main genera in rhizome samples included Acidothermus, Bradyrhizobium, and Acidobacterium, whereas soil samples were dominated by Acidothermus and Acidobacterium (Yuan et al., 2023). This indicates a clear distinction in microbial communities between soil-associated and plant tissue-associated niches.
4.2.2 Differences in dominant microbial phyla
The dominant phyla in various tissues of bamboo also show significant variation. For instance, the abundance of Proteobacteria and Actinomycetes was notably lower in the root endosphere of Fargesia spathacea compared to the rhizosphere and root zone. Conversely, the dominant fungi, Ascomycetes and Basidiomycetes, exhibited higher abundances in the root endosphere compared to the rhizosphere and root zone (Zhang et al., 2023). This contrast highlights the influence of tissue-specific environments on microbial community structure.
4.3 Spatial and temporal distribution
Microbes play crucial roles in plant growth and development, and the feedback regulation by plants can significantly alter microbial community structures and functions. Consequently, microbial communities in bamboo plants exhibit significant variation over different spatial and temporal scales. These changes are influenced by factors such as the developmental stages of bamboo, seasonal variations, and geographical conditions.
4.3.1 Developmental stages of bamboo
The composition of microbial communities in the rhizosphere of bamboo changes with the age of the bamboo. For instance, in Fargesia denudata, the total number of bacteria, fungi, and other microorganisms increased with bamboo age up to the 5-year-old and then decreased from 7- to 13-year-old (Qi and Yang, 2006). Similarly, in Phyllostachys edulis, the bacteria count was significantly higher in the rhizosphere soil of young bamboo compared to mature and old bamboo, while the fungi count was the highest in the rhizosphere soil of mature bamboo (Meng et al., 2015). However, the count of endophyte in bamboo culms increased with bamboo growth (Liu et al., 2017).
Changes in soil microbial communities are also observed with increasing forest age. In a study of Phyllostachys edulis forests at 19, 37, and 64 years, the relative abundances of Alphaproteobacteria, Gammaproteobacteria and Bacteroidetes increased significantly with forest age, while the relative abundances of Acidobacteria and Verrucomicrobia decreased (Yang Y. et al., 2021). Using phospholipid fatty acid (PLFA) analysis, it was found that the microbial community structure of II degree bamboo in rhizosphere soil differed significantly different from that of other ages (Zhang W. et al., 2015). Both flowering and non-flowering Phyllostachys edulis rhizosphere soils contain Proteobacteria and Acidobacteria strains, but the ratio of Firmicutes in flowering Phyllostachys edulis rhizosphere is slightly higher than in non-flowering Phyllostachys edulis rhizosphere, while the counts of Rokubacteria, Latescibacteria and Chlorofexi decrease significantly. This may be due to the stress resistance, different nutrient condition adaptions and plant immune responses (Yuan et al., 2021). At the genus level, the abundance of denitrifying bacteria Stenotrophomonas in the rhizosphere soil of non-flowering Phyllostachys edulis is significantly higher than in flowering Phyllostachys edulis; after flowering, the abundance of Flavobacterium, which can decompose organic matter, is much lower in the rhizosphere soil than in non-flowering Phyllostachys edulis, while the abundance of Bacillus responding to nitrogen change is over seven times higher than in non-flowering Phyllostachys edulis.
4.3.2 Seasonal variations
Seasonal changes, closely related to temperature, have a profound effect on the microbial communities in bamboo rhizospheres. Tao et al. (2024) demonstrated that simulated warming could alter the functional structure of the active microbial community to regulate the soil excitation effect. Cephalostachyum pingbianense, an endemic bamboo species found in southern Yunnan, is currently the only bamboo species known to produce shoots year-round. Li et al. (2022) showed that there was a positive correlation between the shoot yield of Cephalostachyum pingbianense and microbial communities involved in carbon and nitrogen cycling. Although seasonal variation has no significant effect on the α-diversity of rhizobacteria in Cephalostachyum pingbianense, it strongly impacts the community structure of bacteria and fungi, as well as the total abundance of fungi. During the four seasons, bacteria genera such as Acidotherus sp., Roseiarcus sp., and Bradyrhizobium sp., and fungi genera such as Saitozyma sp. Mortierella sp., and Trichoderma sp. are dominant in rhizosphere soil of Cephalostachyum pingbianense.
In Phyllostachys reticulata, unique bacterial strains are isolated in each season. The bacterial richness index was highest in September, and diversity indices (Simpson, Shannon-Wiener, and evenness) increased from April to September (Yin et al., 2016). Similarly, in Phyllostachys edulis forests, the Shannon diversity of rhizosphere microbiome varies significantly between on-year and off-year periods during defoliation and growing seasons (Wang Y. et al., 2024). Seasonal studies of various bamboo species, such as Bambusa emeiensis and Bambusa pervariabilis × Dendrocalamopsis daii, showed that bacteria and actinomycetes were most abundant in summer and least in winter, while fungi peaked in summer and increased slightly in winter (Xu et al., 2015).
4.3.3 Geographical and climatic conditions
Geographical and climatic conditions also affect the rhizosphere microbial composition of bamboo. Comparing P. edulis rhizosphere microbial communities in Tianmu Mountain and Jinyun Mountain, the diversity and evenness were higher in Tianmu Mountain. Unique bacterial strains such as Deinococcus were found in Tianmu Mountain, while Microbacterium was exclusive to Jinyun Mountain. Tianmu Mountain has a subtropical climate with a more humid climate and more solar radiation than Jinyun mountain area, which may result in the various microbial community composition. Subdominant genera also differed, with Streptomyces in Tianmu Mountain and Acinetobacter in Jinyun Mountain (Li et al., 2008).
Slope direction and altitude significantly influence soil microorganisms in bamboo. In Dendrocalamus giganteus, bacterial diversity, fungal diversity, and bacterial abundance were higher on sunny slopes of semi-natural forests than in other plots (Liu et al., 2020). Microbial community studies at different altitudes (600–1,800 m) in Phyllostachys edulis showed that Acidobacteria and Proteobacteria were dominant across altitudes, but their relative abundances varied. The microbial diversity was highest at mid-altitudes (1,000 –1,200 m) (Lin et al., 2015).
4.4 Soil properties
The distribution of bamboo forests across diverse geographical and climatic regions results in a variety of soil types and properties. This diversity influences the composition and structure of soil microorganisms and endophytic microbial communities in bamboo. Conversely, soil microorganisms can alter the physical and chemical properties of their surrounding soil, highlighting a bidirectional relationship between soil properties and microorganisms (Philippot et al., 2024).
4.4.1 Influence of soil properties on microbial communities
The rhizobacteria community diversity and structure in Cephalostachyum pingbianense are mainly affected by soil temperature, water and organic matter, while the fungal community structure is also influenced by soil temperature and water, with available phosphorus in soil having the greatest effect on fungi diversity (Li et al., 2022). It may be caused by the decrease of available phosphorus in soil, which promotes the symbiosis between plants and mycorrhizal fungi, thereby increasing the phosphorus uptake by plants (Shi et al., 2021), inferring that the enrichment of mycorrhizal fungi may improve the fungal community diversity. In Phyllostachys praecox, soil pH, total organic carbon (TOC), total nitrogen (TN), total phosphorus (TP), available potassium (AK), and TOC/TP ratio were highly correlated with the bacterial community composition of rhizosphere soil and root endophytes (Zhang et al., 2022a). Intensive management of Phyllostachys praecox also showed significant correlations between available nitrogen (AN), available phosphorus (AP), AK, TOC, and the composition of endophytic bacterial communities (Zhang et al., 2019a). Specifically, TOC, AN, AP, and pH are closely related to bacterial community composition, while AK and pH significantly influence fungal community composition. In conclusion, the high correlation between microbes and soil properties provides the possibility to modify bamboo bacterial community composition of rhizosphere soil and root endophytes by regulating soil properties.
4.4.2 Impact of heavy metals
High levels of heavy metals in soil can significantly alter the composition of rhizosphere microorganisms. There is a negative correlation between the Shannon index for bacteria and fungi and the presence of chromium (Cr) in the soil. The community composition of bacteria and fungi in the rhizosphere soil of Phyllostachys praecox is significantly correlated with soil Cr, pH, TOC, AP and AN (Zhang et al., 2020). In cases of heavy metal pollution, such as with total chromium (TCR) and extractable chromium (ACR), there is a negative correlation with the soil bacterial α-diversity indices (Chao1 and Shannon). Interestingly, this negative correlation is not observed for fungi, indicating that fungi are more resistant to chromium contamination (Zhang et al., 2021).
4.4.3 Effects of acid rain
Changes in external conditions, such as acid rain, can affect soil microbial composition by changing soil physical and chemical properties, notably soil pH. Under simulated acid rain conditions with a pH 2.5, the diversity indices of fungal community in Phyllostachys edulis soil increases, while the diversity and abundance of soil bacteria decrease (Wang et al., 2020a). Simulated acid rain also affects the structure of soil fungal community, changing the relative abundance of genera such as Bifiguratu, Geminibasidium, Purpureocillium, and Oidiodendron (Wang et al., 2020b).
4.5 Measures for bamboo forest management
Effective bamboo forest management involves various practices aimed at increasing forest yield per unit area. These measures primarily include intensive management and covering management. Each approach influences the composition and structure of microbial communities in different ways.
4.5.1 Intensive Management
Intensive forest management includes practices such as fertilization, removal of understory vegetation, and deep ploughing (Xu et al., 2008). For monopodial bamboo, which absorbs nutrients from the soil through an extensive underground system, these practices can significantly impact the microbial communities in the rhizosphere. For instance, the count of bacteria in rhizosphere soil decreased with intensive management post-deep ploughing and fertilization in Phyllostachys edulis plantations. However, there is no significant difference among treatments of deep plowing with biennial fertilization, quadrennial fertilization, and no-tillage with no fertilization. Intensive management also significantly reduces soil fungi populations, and increases the bacterial-to-fungal ratio (Ni et al., 2021). There was also a study revealed that intensive management measures like deep ploughing, fertilization, and organic mulching changed soil properties in Phyllostachys edulis forests, reducing Shannon index of microbial communities in both soil and rhizome, though they had no significant effect on community composition (Zheng et al., 2022).
Additionally, duration of intensive management also affects the associated microbiome of bamboos. Over 5, 10, 15, and 20 years, intensive management increases the relative abundance of Firmicutes and Bacteroidetes and decreases Proteobacteria. However, relative abundance trends vary over these periods, with significant differences in some phyla. For instance, Acidobacteriota abundance in Phyllostachys praecox plantation roots under 15 years of intensive management is not significantly different from normal conditions, whereas it increases or decreases significantly at other times (Zhang et al., 2019a). Concomitantly, intensive management increases the relative abundance of Actinobacteria and Crenarchaeota and decreases Acidobacteria in bacterial community of Phyllostachys edulis forest soil (Li Q. et al., 2016). It also alters fungal communities, increasing Basidiomycota abundances and Mortierellomycota while decreasing Ascomycota and Rozellomycota (Zhang et al., 2022b).
Furthermore, intensive management can facilitate pathogen invasion. For instance, intensive management conferred to a decrease in the soil microbial diversity of Phyllostachys edulis during pathogenic Escherichia coli invasion, indicating that intensive management benefits pathogen invasion but is detrimental to maintain soil microbial diversity (Qin et al., 2023). According to Niu et al. (2017), long-term extensive management resulted in a gradual decrease in soil microbial richness and diversity in Phyllostachys edulis forest. Abandoned farmland also affected soil microbial communities in bamboo forest, with soil bacterial community indices (e.g., Shannon, Simpson, and Chao1) increasing and then decreasing over time (Jin et al., 2023).
4.5.2 Organic amendments and microbial fertilizer application
Organic amendments significantly increase the relative abundance of Proteobacteria and decrease Acidobacteria, Bacteroidota, and Verrucomicrobia in rhizosphere and endophytic microbial communities of Phyllostachys praecox plantations (Zhang et al., 2022a). In contrast to untreated P. praecox roots, organic amendments increase endophytic Proteobacteria and Acidobacteria while decreasing Actinobacteria and Firmicutes. Proteobacteria and Acidobacteria dominate the rhizosphere microbiome, suggesting they could be indicators of root-related microbiome response to organic amendments. Lack of organic amendments increases the complexity of the microbial network of root endophytes but decreases that of rhizosphere soil. Combining organic fertilizer with Bacillus amyloliquefaciens bio-fertilizer or Bacillus mucilaginosus Krassilnikov bio-fertilizer significantly affects soil bacterial species’ relative abundance (Li et al., 2023). Organic amendments and bio-fertilizers significantly improve soil microflora in various bamboo species (Singh et al., 2020).
4.5.3 Covering management
Covering management regimes like mulching is generally used in bamboo forests, especially in Phyllostachys praecox, to enhance bamboo shoot production, affecting soil microbial composition (Zhao et al., 2015). Mulching initially increases soil bacteria and actinomycetes, followed by a decrease, while fungi populations significantly increase over time (Zhai et al., 2017). Furtherly, Mulching affects the population of ammoniacal bacteria but not nitrogen-fixing bacteria (Luo et al., 2016). Addition, mulching time and materials significantly impact bacterial community composition and diversity. For instance, a 4-month covering treatment with wheat straw and chicken manure reduces α-diversity of soil bacterial community in Phyllostachys praecox forest (Wu et al., 2023). For Phyllostachys edulis, mulching alters Chao1 and Simpson indices (Sun et al., 2023a).
4.5.4 Harvesting
Rational harvesting and management practices, such as strip cutting, can improve productivity and ecological balance in bamboo forests (Zhao et al., 2016). Strip cutting creates forest gaps and influences the bamboo forest ecosystem (Amir and Duke, 2019). Cutting width significantly affects soil bacterial phyla abundance. A 3-m cutting width results in the highest bacterial community abundance and uniform species distribution (Wang S. et al., 2021). Increasing cutting width decreases bacterial community abundance and species evenness while increasing Proteobacteria and decreasing Acidobacterium and Campylobacter (Wang S. et al., 2021; Wang et al., 2022).
4.6 Effects of bamboo forest expansion on soil microorganisms
The expansion of bamboo forests, particularly Phyllostachys edulis, can significantly affect soil microorganisms. The rhizome system of scattered bamboos enables lateral growth, allowing them to invade other natural and secondary forests, thereby altering the rhizosphere and soil microbial composition of native plants.
4.6.1 Impact on rhizosphere soil microorganisms
For instance, the invasion of Phyllostachys edulis increases the abundance and diversity of denitrifying bacteria in the rhizosphere soil of Robinia pseudoacacia, including Shewanella, Chitinophaga, and Achromobacter. The higher the level of invasion, the more denitrifying bacteria are present in the forest soil. This invasion changes the nitrogen cycle in the settled habitat by altering the population of denitrifying bacteria (Cao et al., 2020). Shewanella species, often isolated from water, are capable of bioremediation of metal contaminants and can naturally break down harmful compounds (Lemaire et al., 2020). Consequently, the content of harmful compounds in the soil may be reduced post-invasion, indirectly enhancing the stress resistance of native tree species.
4.6.2 Changes in soil microbial diversity and biomass
The transitioning from broad-leaved forests to Phyllostachys edulis forests results in a significant reduction in soil bacterial richness and diversity, with minimal impact on bacterial evenness. In contrast, fungal diversity shows a marked increase under Phyllostachys edulis dominance. Specifically, the soil microbial richness index is significantly lower in Phyllostachys edulis forests compared to both bamboo-broad-leaved and broad-leaved forest ecosystems. Additionally, microbial diversity indices, including the ACE index and Shannon index, are notably reduced in Phyllostachys edulis and mixed forests relative to broad-leaved forests (Ma et al., 2022). The significant increase in fungal diversity when Phyllostachys edulis was used as the dominant position in invasion may be due to the increased secretion of complex components of root exudates of other plants affected by Phyllostachys edulis, attracting fungi for utilization (Wang and Kuzyakov, 2024). In terms of microbial biomass, Phyllostachys edulis invasion significantly enhances soil microbial biomass nitrogen, fungal ACE diversity, fungal biomass, and bacterial diversity. This expansion also alters the microbial community structure by reducing the Gram-positive to Gram-negative bacteria ratio, alongside a decrease in the biomass of Gram-positive bacteria (Luo et al., 2024). Furthermore, Phyllostachys edulis invasion increases the operational taxonomic units (OTUs) of fungi in both coniferous and broad-leaved forest soils, indicating a higher fungal species diversity in these ecosystems. However, the invasion slightly decreases bacterial OTUs and the ACE index (a measure of microbial species richness) in broad-leaved forest soils, suggesting a shift toward a more specialized microbial community under bamboo dominance (Wu et al., 2024). These results highlight the complex, multifaceted influence of bamboo invasion on soil microbial dynamics, which may have important implications for forest management and ecosystem function.
4.6.3 Relative abundance of soil fungi and bacteria
The relative abundance of fungal families Hyaloscyphaceae (Ascomycota), Clavulinaceae, Hydnangiaceae, Inocybaceae, Russulaceae, Sebacinaceae, Thelephoraceae (Basidiomycota) were higher in broad-leaved forest soil compared to Phyllostachys edulis forest soil. Conversely, the relative abundance of families Herpotrichiellaceae (Ascomycota), Entolomataceae, Hydnodontaceae, Hygrophoraceae were more abundant in Phyllostachys edulis forest soil, indicating significant changes in the fungal community post-invasion (Chen Z. et al., 2021). There were also studies showing that that the expansion of bamboo forests can significantly influence the composition and diversity of soil bacterial communities (Tian et al., 2020). This impact is primarily attributed to changes in soil properties such as pH, organic matter content, and nutrient availability, all of which are affected by bamboo growth and litter input. For instance, Zhang (2023) investigated soil bacterial communities in a cedar plantation invaded by Moso bamboo and found significant shifts in bacterial composition, highlighting alterations in soil microbial dynamics.
4.6.4 Effects on arbuscular mycorrhizal fungi
Phyllostachys edulis invasion alters the arbuscular mycorrhizal fungi (AMF) community structure in both broad-leaved and coniferous forests. The AMF richness (Chao1) is significantly higher in mixed bamboo-broadleaved forests than in pure bamboo forests. The relative abundance of Acaulosporaceae and Archaeosporaceae is lower in bamboo forest soil compared to broad-leaved forest soil (Qin et al., 2017). The AMF community composition varies significantly across Cedrus deodara, Phyllostachys edulis-Cedrus deodara and Phyllostachys edulis mixed forests, with decreasing relative abundance of Glomerales and increasing Rhizophagus with higher invasion degrees, though AMF diversity remains unaffected (Zou et al., 2023).
4.6.5 Soil colony composition and structure
In invaded secondary forests by bamboo, the phospholipid fatty acid (PLFA, quantitatively reflecting the biomass and total biomass of different groups of living microorganisms in the sample) ratio of bacterial to fungal increases, while bacterial PLFA content in the organic layer decreases markedly (Wang et al., 2016). Comparing soil microbial communities in Phyllostachys edulis forests, nearby cedar plantations, and the transitional zone of bamboo invasion, Phyllostachys edulis invasion increases soil bacterial community diversity, with dominant bacteria remaining the same but differing in relative abundance (Lin et al., 2014).
The invasion also significantly alters bacterial and fungal community structures in Cedrus deodara plantation, reducing the proportion of Gram-positive and Gram-negative bacteria in total PLFA (Chang and Chiu, 2015). Post-invasion, the microbial community structure in subtropical broad-leaved forests shifts towards a lower fungi-to-bacteria ratio (F:B, the higher the ratio, the healthier the soil) (Zhao et al., 2021), increasing the diversity index (OTU abundance and Shannon index) of bacteria and fungi, and altering their community composition. The invasion of Actinobacteria and Basidiomycota decreases, while Ascomycota and Mortierellomycota increase (Liu C. et al., 2023). The abundance of Acidobacteria bacterium and Acidobacteria bacterium 13_2_20CM_58_27, and Verrucomicrobia bacterium decreases with lower Phyllostachys edulis invasion, while the abundance of Alphaproteobacteria, Actinobacteria bacterium, Trebonia kvetii, and Bradyrhizobium erythrophlei increase (Sun et al., 2023b).
4.6.6 Intercropping effects
Due to the fast growth of bamboo, there is sufficient space under the forest for intercropping other plants. Intercropping other plants in bamboo undergrowth can alter the rhizosphere microbial composition and abundance. The rhizosphere soil of Phyllostachys edulis was studied after 2 years of intercropping with medicinal plants Tetrastigma hemsleyanum, Paris polyphylla, and Bletilla striata (Zhang et al., 2019b). The result showed that the Chao1, Ace, and Shannon indices of bamboo rhizosphere bacteria are significantly higher with Paris polyphylla intercropping compared to control Phyllostachys edulis. However, the α-diversity index of rhizosphere bacteria significantly decreases with Bletilla striata intercropping. In the fungal community, richness decreases when the medicinal plants are intercropped under Phyllostachys edulis forest. Intercropping with Paris polyphylla significantly reduces the relative abundance of Actinomycetes and Mucoromycota and increases the relative abundance of Gemmatimonadetes in Phyllostachys edulis rhizosphere soil. Compared to control, intercropping with Tetrastigma hemsleyanum significantly increases the relative abundance of Acidobacteria and Blastomonas in Phyllostachys edulis rhizosphere. Intercropping with Bletilla striata significantly increases the relative abundance of Acidobacteria and WPS-2 in Phyllostachys edulis rhizosphere. Intercropping with Tetrastigma hemsleyanum and Bletilla striata significantly reduces the abundance of Mortierellomycota in Phyllostachys edulis rhizosphere.
5 Perspective
The bamboo industry plays a crucial role in economic development, especially in subtropical regions like India, Japan, and south China. Compared to woody plants, bamboo grows rapidly and can supply raw materials to the industry at a higher frequency, thus accelerating product production. Rich bamboo germplasm resources and extensive cultivation areas provide a solid foundation for the development of the bamboo industry. However, promoting the productivity of bamboo forests and ensuring their healthy growth are inevitable challenges for the long-term sustainability of the bamboo industry.
Recent advances in bamboo genomics research have laid the groundwork for a deeper understanding of bamboo growth mechanisms and interactions with microorganisms. With new technologies, research on the relationships between bamboo plants and microorganisms has become more comprehensive. However, bamboo growth is somewhat limited by climate and other unfavorable factors. Therefore, exploring the interaction mechanisms between microbes and bamboo, and facilitating bamboo yield and growth, holds significant importance. Future research should delve into the following scientific and technical aspects of bamboo-microbe interactions:
Current research on bamboo-associated microorganisms is limited to a few bamboo species. It is essential to broaden the research scope through identifying bamboo plant-specific microbial strains and communities and developing their utilization value. For instance, the discovery of four new fungi genera in the Phyllostachys edulis seeds indicates the presence of bamboo-specific microorganisms. Exploring their functions, mechanisms, and application values is crucial.
The composition and structure of microbial communities can alter under certain treatments or environmental conditions. The functions of these communities and the regulatory mechanisms are not clearly and deeply analyzed beyond the observed changes. Additionally, current research methods restrict study of microbial composition and diversity. For instance, primer constraints during sequencing lead to limited microbial composition and fail to identify specific strains. Developing new methods to perform relative research according to the specific characteristics of genes from microorganisms is essential. Whole-genome sequencing of plants is a prerequisite for accurate metagenomic sequencing of endophytic bacteria. Currently, only a few bamboo species have completed whole-genome sequencing, limiting the widespread use of metagenomic sequencing to detect endophyte composition. Sequencing the whole genomes of various bamboo species will facilitate understanding of the gene constitution of bamboo plants and enhance the use of metagenomic sequencing to study microorganisms.
Further exploration is needed on how microbes regulate bamboo growth. Key questions include which secretions of bamboo plants recruit microbes to colonize the rhizosphere and surrounding soil, the gene expression patterns related to plant growth after microbial colonization, and whether there is a specific recruitment relationship between bamboo root exudates and microbes.
Growth-promoting bacteria play a positive role in crop (such as rice) growth and abiotic stress responses, have been widely used as bio-bacterial fertilizers in crops. However, similar studies on bamboo are scarce. Developing and studying growth-promoting bacteria isolated from bamboo, their colonization and proliferation characteristics, and their functions in alleviation stresses may provide new methods and insights for creating bio-bacterial fertilizers to enhance bamboo growth efficiency.
At last, these areas of research will not only contribute to the sustainable development of the bamboo industry but also offer new insights into plant-microbe interactions, potentially leading to innovative agricultural practices and products.
Author contributions
YW: Conceptualization, Formal Analysis, Visualization, Writing – original draft. HR: Funding acquisition, Writing – original draft, Writing – review editing. YZ: Writing – original draft. RS: Investigation, Visualization, Writing – original draft. SJ: Investigation, Resources, Writing – original draft. ML: Investigation, Writing – original draft. YS: Investigation, Writing – original draft. SL: Conceptualization, Supervision, Writing – review & editing. WS: Conceptualization, Writing – review & editing. GQ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the State Key Laboratory of Subtropical Silviculture (grant no. SKLSS-KF2022-08) and the National Key R&D Program for Young Scientists (grant no. 2021YFD2200900).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali, S., Charles, T. C., and Glick, B. R. (2012). Delay of flower senescence by bacterial endophytes expressing 1-aminocyclopropane-1-carboxylate deaminase. J. Appl. Microbiol. 113, 1139–1144. doi: 10.1111/j.1365-2672.2012.05409.x
Amir, A. A., and Duke, N. C. (2019). Distinct characteristics of canopy gaps in the subtropical mangroves of Moreton Bay, Australia. Estuarine Coastal Shelf Sci. 222, 66–80. doi: 10.1016/j.ecss.2019.04.007
Beattie, G. A., and Lindow, S. E. (1999). Bacterial colonization of leaves: A spectrum of strategies. Phytopathology 89, 353–359. doi: 10.1094/PHYTO.1999.89.5.353
Berendsen, R. L., Pieterse, C. M., and Bakker, P. A. (2012). The rhizosphere microbiome and plant health. Trends Plant Sci. 17, 478–486. doi: 10.1016/j.tplants.2012.04.001
Cao, L., Yu, X., Liu, C., Liu, M., Chen, J., Qin, H., et al. (2020). Alteration of soil nitrifiers and denitrifiers and their driving factors during intensive management of Moso bamboo (Phyllostachys pubescens). Sci. Total Environ. 705:135236. doi: 10.1016/j.scitotenv.2019.135236
Chang, E., and Chiu, C. (2015). Changes in soil microbial community structure and activity in a cedar plantation invaded by moso bamboo. Appl. Soil Ecol. 91, 1–7. doi: 10.1016/j.apsoil.2015.02.001
Chen, L., Fang, R., Wu, J., and Zhang, L. (2022). Research progress in the detection methods of endophytic bacteria. Microbiol. China 49, 1105–1119. doi: 10.13344/j.microbiol.china.210695
Chen, M., Chen, J., Liu, J., Wu, M., Yan, Q., and Li, P. (2021). Diversity analysis of rhizosphere soil fungi and endophytic fungi in Ampelocalamus luodianensis. Acta Ecol. Sinica 41, 4120–4230.
Chen, M., Xing, Y., Chen, C., and Wang, Z. (2024). Enhancing sugarcane’s drought resilience: The influence of Streptomycetales and Rhizobiales. Front. Plant Sci. 15:1471044. doi: 10.3389/fpls.2024.1471044
Chen, Z., Li, Y., Chang, S. X., Xu, Q., Li, Y., Ma, Z., et al. (2021). Linking enhanced soil nitrogen mineralization to increased fungal decomposition capacity with Moso bamboo invasion of broadleaf forests. Sci. Total Environ. 771:144779. doi: 10.1016/j.scitotenv.2020.144779
Dasila, H., Sah, V. K., Jaggi, V., Kumar, A., Tewari, L., Taj, G., et al. (2023). Cold-tolerant phosphate-solubilizing Pseudomonas strains promote wheat growth and yield by improving soil phosphorous (P) nutrition status. Front. Microbiol. 14:1135693. doi: 10.3389/fmicb.2023.1135693
Dawson, W., Hör, J., Egert, M., van Kleunen, M., and Pester, M. A. (2017). Small number of low-abundance bacteria dominate plant species-specific responses during rhizosphere colonization. Front. Microbiol. 8:975. doi: 10.3389/fmicb.2017.00975
de la Fuente Cantó, C., Simonin, M., King, E., Moulin, L., Bennett, M. J., Castrillo, G., et al. (2020). An extended root phenotype: The rhizosphere, its formation and impacts on plant fitness. Plant J. 103, 951–964. doi: 10.1111/tpj.14781
Dhondge, H. V., Barvkar, V. T., Paul, D., Dastager, S. G., Pable, A. A., and Nadaf, A. B. (2022). Exploring the core microbiota in scented rice (Oryza sativa L.) rhizosphere through metagenomics approach. Microbiol. Res. 263:127157. doi: 10.1016/j.micres.2022.127157
Frey, B., Moser, B., Tytgat, B., Zimmermann, S., Alberti, S., Biederman, L. A., et al. (2023). Long-term N-addition alters the community structure of functionally important N-cycling soil microorganisms across global grasslands. Soil Biol. Biochem. 176:108887. doi: 10.1016/j.soilbio.2022.108887
Fu, H., Zeng, X., Song, Z., Lan, S., and Huang, W. (2022). Soli microbial community structures under threee urban landscape bamboo forests. Soils 54, 1165–1174. doi: 10.13758/j.cnki.tr.2022.06.010
Gaiero, J. R., McCall, C. A., Thompson, K. A., Day, N. J., Best, A. S., and Dunfield, K. E. (2013). Inside the root microbiome: Bacterial root endophytes and plant growth promotion. Am. J. Bot. 100, 1738–1750. doi: 10.3732/ajb.1200572
Gao, Z., Zhuang, J., Chen, J., Liu, X., Tang, S., et al. (2004). Population of entophytic bacteria in maize roots and its dynamic analysis. Ying Yong Sheng Tai Xue Bao 15, 1344–1348. doi: 10.3321/j.issn:1001-9332.2004.08.009
Gu, X., and Wu, X. (1999). Effects of inoculating associated nitrogen fixing bacteria to Moso bamboo seedlings growth. For. Res. 12, 7–12. doi: 10.3321/j.issn:1001-1498.1999.01.002
Gu, X., Wu, X., and Wang, Y. (2001). Study on associated nitrogen fixation of several sympodial bamboo species. For. Res. 14, 28–34. doi: 10.13275/j.cnki.lykxyj.2001.01.005
Gulati, A., Sood, S., Rahi, P., Thakur, R., Chauhan, S., and Chawla, I. (2011). Diversity analysis of diazotrophic bacteria associated with the roots of tea (Camellia sinensis (L.) O. Kuntze). J. Microbiol. Biotechnol. 21, 545–555. doi: 10.4014/JMB.1012.12022
Guo, Z., Yu, W., Chen, S., Li, Y., and Yang, Q. (2013). Influence of mulching management on soil microbe and its relationship with soil nutrient in Phyllostachys praecox stand. Acta Ecol. Sinica 33, 5623–5630. doi: 10.5846/stxb201305040920
Handelsman, J. (2004). Metagenomics: Application of genomics to uncultured microorganisms. Microbiology and Molecular Biology Reviews. 68, 669–685. doi: 10.1128/mmbr.68.4.669-685.2004
He, Y., Pantigoso, H. A., Wu, Z., and Vivanco, J. M. (2019). Co-inoculation of Bacillus sp. and Pseudomonas putida at different development stages acts as a biostimulant to promote growth, yield and nutrient uptake of tomato. J. Appl. Microbiol. 127, 196–207. doi: 10.1111/jam.14273
Hu, Y., Wu, Q., Ma, S., Ma, T., Shan, L., Wang, X., et al. (2017). Comparative genomics reveals convergent evolution between the bamboo-eating giant and red pandas. Proc. Natl. Acad. Sci. U. S. A. 114, 1081–1086. doi: 10.1073/pnas.1613870114
Jin, J., Ye, L., Wang, Z., Fu, W., Lin, H., and Wu, J. (2023). Abandonment of moso bamboo (Phyllostachys pubescens) plantations could lead to stand structural complications and changes in soil properties and microbial community. J. Soil Sci. Plant Nutrit. 23, 6670–6680. doi: 10.1007/s42729-023-01518-7
Kang, L., Luo, W., Dai, Q., Zhou, H., Wei, W., Tang, J., et al. (2022). Giant pandas’ staple food bamboo phyllosphere fungal community and its influencing factors. Front. Microbiol. 13:1009588. doi: 10.3389/fmicb.2022.1009588
Kloepper, J. W. (1981). Relationship of in vitro antibiosis of plant growth-promoting rhizobacteria to plant growth and the displacement of root microflora. Phytopathology 71, 1020–1024. doi: 10.1094/PHYTO-71-1020
Kong, Y., Qin, H., Zhu, C., Tian, W., Zhu, X., Yu, Y., et al. (2023). Research progress on the mechanism by which soil microorganisms affect soil health. Acta Pedol. Sin. 61, 331–347. doi: 10.11766/trxb202301200448
Krishnapura, P. R., and Belur, P. D. (2016). Isolation and screening of endophytes from the rhizomes of some Zingiberaceae plants for L-asparaginase production. Prep. Biochem. Biotechnol. 46, 281–287. doi: 10.1080/10826068.2015.1031385
Kuzyakov, Y., and Razavi, B. S. (2019). Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil Biol. Biochem. 135, 343–360. doi: 10.1016/j.soilbio.2019.05.011
Lakshmanan, V., Selvaraj, G., and Bais, H. P. (2014). Functional soil microbiome: Belowground solutions to an aboveground problem. Plant Physiol. 166, 689–700. doi: 10.1104/pp.114.245811
Lemaire, O. N., Méjean, V., and Iobbi-Nivol, C. (2020). The Shewanella genus: Ubiquitous organisms sustaining and preserving aquatic ecosystems. FEMS Microbiol. Rev. 44, 155–170. doi: 10.1093/femsre/fuz031
Li, F., Liu, H., Tian, S., and Liu, Y. (2018). Biological control effect of compounded bacillus on wilt disease of Bambusa pervariabilis × Dendrocalamopsis daii. J. f Beijing Foerestry Univ. 40, 76–84. doi: 10.13332/j.1000-1522.20170320
Li, L., Liu, M., Yang, S., Liu, L., Miao, K., Yang, K., et al. (2008). [Cultivable microbial diversity at the rhizosphere of Phyllostachys pubescens]. Wei Sheng Wu Xue Bao 48, 772–779. doi: 10.13343/j.cnki.wsxb.2008.06.007
Li, L., Song, S., Fang, X., Yang, L., Shao, S., Ying, Y., et al. (2017). Protection enzymes and lipid peroxidation in Phyllostachys edulis seedlings with temperature and water stresses. J. Zhejiang A F Univ. 34, 268–275. doi: 10.11833/j.issn.2095-0756.2017.02.010
Li, L., Xia, T., and Yang, H. (2022). Seasonal patterns of rhizosphere microorganisms suggest carbohydrate-degrading and nitrogen-fixing microbes contribute to the attribute of full-year shooting in woody bamboo Cephalostachyum pingbianense. Front. Microbiol. 13:1033293. doi: 10.3389/fmicb.2022.1033293
Li, N., Wen, J., Wu, R., Hu, D., Zhang, L., Zhang, W., et al. (2023). Dual effects of plant growth-promoting rhizobacteria (PGPR) on the Moso bamboo-soil system: Plant growth promotion and microbial community stability. Indus. Crops Products 203:117151. doi: 10.1016/j.indcrop.2023.117151
Li, P., Liu, J., Wen, A., and Gao, P. Wang J., and Mo, H. (2016). Diversity of culturable bacteria in rhizosphere soil of Drepanostachyum luodianense. Jiangsu Agricult. Sci. 44, 223–227. doi: 10.15889/j.issn.1002-1302.2016.07.062
Li, Q., Song, X., Gu, H., and Gao, F. (2016). Nitrogen deposition and management practices increase soil microbial biomass carbon but decrease diversity in Moso bamboo plantations. Sci. Rep. 6:28235. doi: 10.1038/srep28235
Li, R., Fan, W., Tian, G., Zhu, H., He, L., Cai, J., et al. (2010). The sequence and de novo assembly of the giant panda genome. Nature 463, 311–317. doi: 10.1038/nature08696
Li, S., Fan, W., Xu, G., Cao, Y., Zhao, X., Hao, S., et al. (2023). Bio-organic fertilizers improve Dendrocalamus farinosus growth by remolding the soil microbiome and metabolome. Front. Microbiol. 14:1117355. doi: 10.3389/fmicb.2023.1117355
Li, X., Zheng, X., Yadav, N., Saha, S., Salama, E. S., Li, X., et al. (2024). Rational management of the plant microbiome for the second green revolution. Plant Commun. 5:100812. doi: 10.1016/j.xplc.2024.100812
Li, Y., Cheng, P., Yu, L., Kuang, X., Zeng, Q., and Huang, S. (2020a). Microbial community structure in rhizosphere soil of 3 bamboo species of Phyllostachys. World Bamboo Rattan 18, 32–37.
Li, Y., Yu, L., Cheng, P., Guo, X., Zeng, Q., Huang, S., et al. (2020b). Analysis of soil microbial community structure in rhizosphere and non-rhizosphere soil of Phyllostachys vivax f. aureocaulis. South China Forestry Sci. 48, 40–44. doi: 10.16259/j.cnki.36-1342/s.2020.06.009
Lin, T. (2021). Global assessment of bamboo and rattan resources (Gabar). World Bamboo Rattan Commun. 19, 72–73.
Lin, Y. T., Tang, S. L., Pai, C. W., Whitman, W. B., Coleman, D. C., and Chiu, C. Y. (2014). Changes in the soil bacterial communities in a cedar plantation invaded by moso bamboo. Microb. Ecol. 67, 421–429. doi: 10.1007/s00248-013-0291-3
Lin, Y. T., Whitman, W. B., Coleman, D. C., Shi, S. Y., Tang, S. L., and Chiu, C. Y. (2015). Changes of soil bacterial communities in bamboo plantations at different elevations. FEMS Microbiol. Ecol. 91:fiv033. doi: 10.1093/femsec/fiv033
Lindow, S. E., and Brandl, M. T. (2003). Microbiology of the phyllosphere. Appl. Environ. Microbiol. 69, 1875–1883. doi: 10.1128/AEM.69.4.1875-1883.2003
Liu, C., Zheng, C., Wang, L., Zhang, J., Wang, Q., Shao, S., et al. (2023). Moso bamboo invasion changes the assembly process and interactive relationship of soil microbial communities in a subtropical broadleaf forest. For. Ecol. Manag. 536:120901. doi: 10.1016/j.foreco.2023.120901
Liu, F., Yuan, Z., Zhang, X., Zhang, G., and Xie, B. (2017). Characteristics and diversity of endophytic bacteria in moso bamboo (Phyllostachys edulis) based on 16S rDNA sequencing. Arch. Microbiol. 199, 1259–1266. doi: 10.1007/s00203-017-1397-7
Liu, M., Li, L., Yang, K., Han, J., Zhu, B., Peng, Z., et al. (2008). Culturable bacterial diversity in rhizosphere of Bashania fangiana. Biodivers. Sci. 16, 91–95. doi: 10.3724/SP.J.1003.2008.07239
Liu, W., Wang, F., Sun, Y., Yang, L., Chen, H., Liu, W., et al. (2020). Influence of dragon bamboo with different planting patterns on microbial community and physicochemical property of soil on sunny and shady slopes. J. Microbiol. 58, 906–914. doi: 10.1007/s12275-020-0082-8
Liu, Y., Nessa, A., Zheng, Q., Hu, D., Zhang, W., Zhang, M., et al. (2023). Inoculations of phosphate-solubilizing bacteria alter soil microbial community and improve phosphorus bioavailability for moso bamboo (Phyllostachys edulis) growth. Appl. Soil Ecol. 189:104911. doi: 10.1016/j.apsoil.2023.104911
Long, J., Luo, W., Xie, J., Yuan, Y., Wang, J., Kang, L., et al. (2021). Environmental factors influencing phyllosphere bacterial communities in giant pandas’ staple food bamboos. Front. Microbiol. 12:748141. doi: 10.3389/fmicb.2021.748141
Luo, W., Zhang, Q., Wang, P., Luo, J., She, C., Guo, X., et al. (2024). Unveiling the impacts moso bamboo invasion on litter and soil properties: A meta-analysis. Sci. Total Environ. 909:168532. doi: 10.1016/j.scitotenv.2023.168532
Luo, Z., Liu, Z., Chen, Y., Chen, C., Wang, Z., Lai, Y., et al. (2016). Effect of soil surface covering on soil microorganism and enzyme of Phyllostachys parecon. Hubei For. Sci. Technol. 45, 11–14. doi: 10.3969/j.issn.1004-3020.2016.03.006
Ma, G., and Xiao, C. (2004). Population dynamics of endophytic bacteria in symptom-free tobacco plants. J. Microbiol. 24, 7–11. doi: 10.3969/j.issn.1005-7021.2004.04.004
Ma, X. R., Zheng, X. L., Zheng, C. Y., Hu, Y. T., Qin, H., Chen, J. H., et al. (2022). [Effects of moso bamboo (Phyllostachys edulis) expansion on soil microbial community in evergreen broad-leaved forest]. Ying Yong Sheng Tai Xue Bao 33, 1091–1098. doi: 10.13287/j.1001-9332.202204.030
Martínez-Arias, C., Sobrino-Plata, J., Medel, D., Gil, L., Martín, J. A., and Rodríguez-Calcerrada, J. (2021). Stem endophytes increase root development, photosynthesis, and survival of elm plantlets (Ulmus minor Mill.). J. Plant Physiol. 261:153420. doi: 10.1016/j.jplph.2021.153420
Meng, Y., Ai, W., Li, M., Yang, M., Hu, W., and Tu, J. (2015). Variation of soil microbial populations and relationship between microbial factors and soil nutrients in rhizome root zone of Phyllostachys edulis. Hun. For. Sci. Technol. 42, 1–5. doi: 10.3969/j.issn.1003-5710.2015.05.001
Moe, L. A. (2013). Amino acids in the rhizosphere: From plants to microbes. Am. J. Bot. 100, 1692–1705. doi: 10.3732/ajb.1300033
Mommer, L., Kirkegaard, J., and van Ruijven, J. (2016). Root-Root interactions: Towards a rhizosphere framework. Trends Plant Sci. 21, 209–217. doi: 10.1016/j.tplants.2016.01.009
Muthukumar, T., and Udaiyan, K. (2006). Growth of nursery-grown bamboo inoculated with Arbuscular Mycorrhizal fungi and plant growth promoting rhizobacteria in two tropical soil types with and without fertilizer application. New For. 31, 469–485. doi: 10.1007/s11056-005-1380-z
Ni, H., Su, W., Fan, S., and Chu, H. (2021). Effects of intensive management practices on rhizosphere soil properties, root growth, and nutrient uptake in Moso bamboo plantations in subtropical China. For. Ecol. Manag. 493:119083. doi: 10.1016/j.foreco.2021.119083
Niu, L., Miao, L. J., Chris, P., Xu, Q., Wu, Q., and Qin, H. (2017). Variation patterns of soil microbial community of Phyllostachys edulis stands under long-term extensive management. For. Res. 30, 285–292. doi: 10.13275/j.cnki.lykxyj.2017.02.014
Odriozola, I., Rasmussen, J. A., Gilbert, M. T. P., Limborg, M. T., and Alberdi, A. (2024). A practical introduction to holo-omics. Cell Rep. Methods 4:100820. doi: 10.1016/j.crmeth.2024.100820
Ordon, J., Thouin, J., Nakano, R. T., Ma, K. W., Zhang, P., Huettel, B., et al. (2024). Chromosomal barcodes for simultaneous tracking of near-isogenic bacterial strains in plant microbiota. Nat. Microbiol. 9, 1117–1129. doi: 10.1038/s41564-024-01619-8
Pace, N. R. (1997). A molecular view of microbial diversity and the biosphere. Science 276, 734–740. doi: 10.1126/science.276.5313.734
Philippot, L., Chenu, C., Kappler, A., Rillig, M. C., and Fierer, N. (2024). The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 22, 226–239. doi: 10.1038/s41579-023-00980-5
Pirttilä, A. M., Pospiech, H., Laukkanen, H., Myllylä, R., and Hohtola, A. (2005). Seasonal variations in location and population structure of endophytes in buds of Scots pine. Tree Physiol. 25, 289–297. doi: 10.1093/treephys/25.3.289
Qi, Z., and Yang, W. (2006). Microbial quantity and enzyme activity in Fargesia denudate rhizosphere soil of western Sichuan. Chin. J. Ecol. 25, 1370–1374.
Qin, H., Cai, R., Wang, Y., Deng, X., Chen, J., and Xing, J. (2023). Intensive management facilitates bacterial invasion on soil microbial community. J. Environ. Manage 340:117963. doi: 10.1016/j.jenvman.2023.117963
Qin, H., Niu, L., Wu, Q., Chen, J., Li, Y., Liang, C., et al. (2017). Bamboo forest expansion increases soil organic carbon through its effect on soil arbuscular mycorrhizal fungal community and abundance. Plant Soil 420, 407–421. doi: 10.1007/s11104-017-3415-6
Reinhold-Hurek, B., and Hurek, T. (2011). Living inside plants: Bacterial endophytes. Curr. Opin. Plant Biol. 14, 435–443. doi: 10.1016/j.pbi.2011.04.004
Reis, V. M., and Teixeira, K. R. (2015). Nitrogen fixing bacteria in the family Acetobacteraceae and their role in agriculture. J. Basic Microbiol. 55, 931–949. doi: 10.1002/jobm.201400898
Ren, H., Hong, H., Zha, B., Lamlom, S. F., Qiu, H., Cao, Y., et al. (2025). Soybean productivity can be enhanced by understanding rhizosphere microbiota: Evidence from metagenomics analysis from diverse agroecosystems. Microbiome 13:105. doi: 10.1186/s40168-025-02104-y
Ritz, K., Black, H., Campbell, C. D., Harris, J. A., and Wood, C. (2009). Selecting biological indicators for monitoring soils: A framework for balancing scientific and technical opinion to assist policy development. Ecol. Indic. 9, 1212–1221. doi: 10.1016/j.ecolind.2009.02.009
Sağlam, M., Dengiz, O., and Saygın, F. (2015). Assessment of horizantal and vertical variabilities of soil quality using multivariate statistics and geostatistical methods. Commun. Soil Sci. Plant Anal. 46, 1677–1697. doi: 10.1080/00103624.2015.1045596
Schäfer, M., Pacheco, A. R., Künzler, R., Bortfeld-Miller, M., Field, C. M., Vayena, E., et al. (2023). Metabolic interaction models recapitulate leaf microbiota ecology. Science 381:eadf5121. doi: 10.1126/science.adf5121
Shen, X. Y., Cheng, Y. L., Cai, C. J., Fan, L., Gao, J., and Hou, C. L. (2014). Diversity and antimicrobial activity of culturable endophytic fungi isolated from moso bamboo seeds. PLoS One 9:e95838. doi: 10.1371/journal.pone.0095838
Shi, J., Zhao, B., Zheng, S., Zhang, X., Wang, X., Dong, W., et al. (2021). A phosphate starvation response-centered network regulates mycorrhizal symbiosis. Cell 184, 5527–5540.e18. doi: 10.1016/j.cell.2021.09.030.
Singh, L., Sridharan, S., Thul, S. T., Kokate, P., Kumar, K., Kumar, S., et al. (2020). Eco-rejuvenation of degraded land by microbe assisted bamboo plantation. Indus. Crops Products 155:112795. doi: 10.1016/j.indcrop.2020.112795
Smith, S. E., and Read, D. (2008). Mycorrhizal symbiosis, 3rd Edn. Cambridge: Academic Press, doi: 10.1016/B978-0-12-370526-6.X5001-6
Song, M., Sun, B., Li, R., Zhang, Z., Bai, Z., and Zhuang, X. (2022). Dynamic succession patterns and interactions of phyllospheric microorganisms during NOx exposure. J. Hazard Mater. 430:128371. doi: 10.1016/j.jhazmat.2022.128371
Spinella, S., Classen, A. T., Sanders, N. J., and McLaren, J. R. (2024). Context dependence of warming-induced shifts in montane soil microbial functions. Funct. Ecol. 38, 1199–1209. doi: 10.1111/1365-2435.14538
Sriragavi, G., Sangeetha, M., Santhakumar, M., Lokesh, E., Nithyalakshmi, M., Saleel, C. A., et al. (2023). Exploring antibacterial properties of bioactive compounds isolated from Streptomyces sp. in bamboo rhizosphere soil. ACS Omega 8, 36333–36343. doi: 10.1021/acsomega.3c04954
Steen, A. D., Crits-Christoph, A., Carini, P., DeAngelis, K. M., Fierer, N., Lloyd, K. G., et al. (2019). High proportions of bacteria and archaea across most biomes remain uncultured. ISME J. 13, 3126–3130. doi: 10.1038/s41396-019-0484-y
Streitwolf-Engel, R., Boller, T., Wiemken, A., and Sanders, I. R. (1997). Clonal growth traits of two Prunella species are determined by co-occurring arbuscular mycorrhizal fungi from a calcareous grassland. J. Ecol. 85, 181–191. doi: 10.2307/2960650
Sun, H., Cao, Z., Wu, Z., Fang, M., Zhang, R., Liu, J., et al. (2023a). Effects of mulching on soil nutrients, enzyme activities and microbial community in Phyllostachys edulis forest. Non-wood For. Res. 41, 23–33. doi: 10.14067/j.cnki.1003-8981.2023.02.023
Sun, H., Hu, W., Dai, Y., Ai, L., Wu, M., Hu, J., et al. (2023b). Moso bamboo (Phyllostachys edulis (Carrière) J. Houzeau) invasion affects soil microbial communities in adjacent planted forests in the Lijiang River basin, China. Front. Microbiol. 14:1111498. doi: 10.3389/fmicb.2023.1111498
Sun, L., Qiu, F., Zhang, X., Dai, X., Dong, X., and Song, W. (2008). Endophytic bacterial diversity in rice (Oryza sativa L.) roots estimated by 16S rDNA sequence analysis. Microb. Ecol. 55, 415–424. doi: 10.1007/s00248-007-9287-1
Tao, X., Yang, Z., Feng, J., Jian, S., Yang, Y., Bates, C. T., et al. (2024). Experimental warming accelerates positive soil priming in a temperate grassland ecosystem. Nat. Commun. 15:1178. doi: 10.1038/s41467-024-45277-0
Tholozan, J. L., Cappelier, J. M., Tissier, J. P., Delattre, G., and Federighi, M. (1999). Physiological characterization of viable-but-nonculturable Campylobacter jejuni cells. Appl. Environ. Microbiol. 65, 1110–1116. doi: 10.1128/AEM.65.3.1110-1116.1999
Tian, X., Wang, M., Meng, P., Zhang, J. S., Zhou, B.-Z., and Ge, X.-G. (2020). Native bamboo invasions into subtropical forests alter microbial communities in litter and soil. Forests 11:314. doi: 10.3390/f11030314
Trivedi, P., Leach, J. E., Tringe, S. G., Sa, T., and Singh, B. K. (2020). Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 18, 607–621. doi: 10.1038/s41579-020-0412-1
Tun, H. M., Mauroo, N. F., Yuen, C. S., Ho, J. C., Wong, M. T., and Leung, F. C. (2014). Microbial diversity and evidence of novel homoacetogens in the gut of both geriatric and adult giant pandas (Ailuropoda melanoleuca). PLoS One 9:e79902. doi: 10.1371/journal.pone.0079902
Vandenkoornhuyse, P., Mahé, S., Ineson, P., Staddon, P., Ostle, N., Cliquet, J. B., et al. (2007). Active root-inhabiting microbes identified by rapid incorporation of plant-derived carbon into RNA. Proc. Natl. Acad. Sci. U. S. A. 104, 16970–16975. doi: 10.1073/pnas.0705902104
Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A., and Dufresne, A. (2015). The importance of the microbiome of the plant holobiont. New Phytol. 206, 1196–1206. doi: 10.1111/nph.13312
Verma, R., and Arya, I. (1998). Effect of arbuscular mycorrhizal fungal isolates and organic manure on growth and mycorrhization of micropropagated Dendrocalamus asper plantlets and on spore production in their rhizosphere. Mycorrhiza 8, 113–116. doi: 10.1007/s005720050221
Vierheilig, H., Bago, B., Albrecht, C., Poulin, M. J., and Piché, Y. (1998). Flavonoids and arbuscular-mycorrhizal fungi. Adv. Exp. Med. Biol. 439, 9–33. doi: 10.1007/978-1-4615-5335-9_2
Wallace, J. G. (2023). Maize seed endophytes. Mol. Plant Pathol. 24, 801–810. doi: 10.1111/mpp.13278
Wang, C., and Kuzyakov, Y. (2024). Mechanisms and implications of bacterial-fungal competition for soil resources. ISME J. 18:wrae073. doi: 10.1093/ismejo/wrae073
Wang, K., Wu, Y., Ye, M., Yang, Y., Asiegbu, F. O., Overmyer, K., et al. (2021). Comparative genomics reveals potential mechanisms of plant beneficial effects of a novel bamboo-endophytic bacterial isolate Paraburkholderia sacchari Suichang626. Front. Microbiol. 12:686998. doi: 10.3389/fmicb.2021.686998
Wang, M., Ge, A. H., Ma, X., Wang, X., Xie, Q., Wang, L., et al. (2024). Dynamic root microbiome sustains soybean productivity under unbalanced fertilization. Nat. Commun. 15:1668. doi: 10.1038/s41467-024-45925-5
Wang, N., Pan, X., and Bai, S. (2020a). Effects of simulated acid rain on soil respiration and microbial diversity in Moso bamboo forest in subtropical China. Acta Ecol. Sin. 40, 3420–3430. doi: 10.5846/stxb201905241073
Wang, N., Pan, X.-C., Wang, C.-K., and Bai, S.-B. (2020b). [Effects of simulated acid rain on soil fungi diversity in the transition zone of moso bamboo and broadleaf forest]. Huan Jing Ke Xue 41, 2476–2484. doi: 10.13227/j.hjkx.201910180
Wang, N., Wang, T., Chen, Y., Wang, M., Lu, Q., Wang, K., et al. (2024). Microbiome convergence enables siderophore-secreting-rhizobacteria to improve iron nutrition and yield of peanut intercropped with maize. Nat. Commun. 15:839. doi: 10.1038/s41467-024-45207-0
Wang, S., Wang, B., Fan, S., Xiao, X., Xia, W., and Guan, F. (2022). Short-term influence of strip cutting on soil physical and chemical properties and dominant flora in Phyllostachys edulis stands. J. Northeast For. Univ. 50, 46–51. doi: 10.13759/j.cnki.dlxb.2022.01.014
Wang, S., Wang, B., Fan, S., Xiao, X., Xia, W., Guan, F., et al. (2021). Influence of strip cutting management on soil bacterial community structure and diversity in Phyllostachys edulis stands. J. Nanjing For. Univ. 45, 61–68. doi: 10.12302/j.issn.1000-2006.202004038
Wang, X., Chen, R., Jing, Z., Feng, Y., Yao, T., and Lin, X. (2019). Comparative Study on rhizosphere effects and bcterial community characteristics of of rice and wheat. Acta Pedol. Sinica 56, 443–453. doi: 10.11766/trxb201806020042
Wang, X., Feng, J., Ao, G., Qin, W., Han, M., Shen, Y., et al. (2023). Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol. Biochem. 179:108982. doi: 10.1016/j.soilbio.2023.108982
Wang, X., Li, L., and Wang, T. (2020c). Research progress of endophytes in bamboo. J. Bamboo Res. 39, 34–39. doi: 10.19560/j.cnki.issn1000-6567.2020.04.004
Wang, X., Sasaki, A., Toda, M., and Nakatsubo, T. (2016). Changes in soil microbial community and activity in warm temperate forests invaded by moso bamboo (Phyllostachys pubescens). J. For. Res. 21, 235–243. doi: 10.1007/s10310-016-0533-6
Wang, Y., Wang, B., Chen, J., Sun, L., Hou, Y., Wang, Y., et al. (2024). Dynamics of rhizosphere microbial structure and function associated with the biennial bearing of moso bamboo. J. Environ. Manage 351:119977. doi: 10.1016/j.jenvman.2023.119977
Wei, F., Hu, Y., Yan, L., Nie, Y., Wu, Q., and Zhang, Z. (2015). Giant pandas are not an evolutionary cul-de-sac: Evidence from multidisciplinary research. Mol. Biol. Evol. 32, 4–12. doi: 10.1093/molbev/msu278
Winding, A., Hund-Rinke, K., and Rutgers, M. (2005). The use of microorganisms in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf. 62, 230–248. doi: 10.1016/j.ecoenv.2005.03.026
Wu, F., Wu, N., Zhang, L., Li, Z., Pei, N., Jin, C., et al. (2023). Winter mulching practice alters soil bacterial communities and networks in lei bamboo (Phyllostachys praecox) forests. Land Degrad. Dev. 34, 2535–2547. doi: 10.1002/ldr.4626
Wu, Q., Wang, X., Ding, Y., Hu, Y., Nie, Y., Wei, W., et al. (2017). Seasonal variation in nutrient utilization shapes gut microbiome structure and function in wild giant pandas. Proc. Biol. Sci. 284:20170955. doi: 10.1098/rspb.2017.0955
Wu, Y., Guo, J., Tang, Z., Wang, T., Li, W., Wang, X., et al. (2024). Moso bamboo (Phyllostachys edulis) expansion enhances soil pH and alters soil nutrients and microbial communities. Sci. Total Environ. 912:169346. doi: 10.1016/j.scitotenv.2023.169346
Xia, Z., He, Y., Korpelainen, H., and Niinemets, Ü, andLi, C. (2023). Allelochemicals and soil microorganisms jointly mediate sex-specific belowground interactions in dioecious Populus cathayana. New Phytol. 240, 1519–1533. doi: 10.1111/nph.19224
Xing, Y., Shi, W., Zhu, Y., Wang, F., Wu, H., and Ying, Y. (2021). Screening and activity assessing of phosphorus availability improving microorganisms associated with bamboo rhizosphere in subtropical China. Environ. Microbiol. 23, 6074–6088. doi: 10.1111/1462-2920.15633
Xing, Y., Wang, F., Yu, S., Zhu, Y., Ying, Y., Shi, W., et al. (2024). Enhancing Phyllostachys edulis seedling growth in phosphorus-deficient soil: Complementing the role of phosphate-solubilizing microorganisms with arbuscular mycorrhizal fungi. Plant Soil 497, 449–466. doi: 10.1007/s11104-023-06406-8
Xu, J., Zhang, Y., Zhang, P., Trivedi, P., Riera, N., Wang, Y., et al. (2018). The structure and function of the global citrus rhizosphere microbiome. Nat. Commun. 9:4894. doi: 10.1038/s41467-018-07343-2
Xu, N., Zhao, Q., Zhang, Z., Zhang, Q., Wang, Y., Qin, G., et al. (2022). Phyllosphere microorganisms: Sources, drivers, and their interactions with plant hosts. J. Agric Food Chem. 70, 4860–4870. doi: 10.1021/acs.jafc.2c01113
Xu, Q., Jiang, P., and Xu, Z. (2008). Soil microbial functional diversity under intensively managed bamboo plantations in southern China. J. Soils Sediments 8, 177–183. doi: 10.1007/s11368-008-0007-3
Xu, X., Yang, C., Liu, Y., Xia, H., and Bai, Y. (2015). Characteristics of soil microorganisms in three bamboo ecosystems. J. Northeast For. Univ. 43, 51–54. doi: 10.13759/j.cnki.dlxb.20141224.003
Xun, W., Liu, Y., Ma, A., Yan, H., Miao, Y., Shao, J., et al. (2024). Dissection of rhizosphere microbiome and exploiting strategies for sustainable agriculture. New Phytol. 242, 2401–2410. doi: 10.1111/nph.19697
Yan, K., Zhang, J., Cai, Y., Cao, G., Meng, L., Soaud, S. A., et al. (2023). Comparative analysis of endophytic fungal communities in bamboo species Phyllostachys edulis, Bambusa rigida, and Pleioblastus amarus. Sci. Rep. 13:20910. doi: 10.1038/s41598-023-48187-1
Yang, C., Zhang, X., Ni, H., Gai, X., Huang, Z., Du, X., et al. (2021). Soil carbon and associated bacterial community shifts driven by fine root traits along a chronosequence of Moso bamboo (Phyllostachys edulis) plantations in subtropical China. Sci. Total Environ. 752:142333. doi: 10.1016/j.scitotenv.2020.142333
Yang, D., Shi, F., Wan, S., and Li, G. Q. (2021). Influence of Talaromyces aurantiacus on the soil phosphorus fraction and biomass of Moso bamboo seedling. For. Res. 34, 145–151. doi: 10.13275/j.cnki.lykxyj.2021.03.016
Yang, Q. (2004). Reviews on the capability of soil and water conservation of Phyllostachys pubescens forests. J. Bmboo Res. 23, 1–3. doi: 10.3969/j.issn.1000-6567.2004.04.001
Yang, Y., Lou, M., Li, Z., Chen, J., Wu, F., Huang, X., et al. (2021). Isolation of endophytic fungi from Phyllostachys praecox f. prevelnalis and screening and identification of functional strains. Biol. Disaster Sci. 44, 271–277. doi: 10.3969/j.issn.2095-3704.2021.03.46
Yin, T., Wang, G., Yan, Y., Gao, X., and Wen, H. (2016). Seasonal variation of bacteria community in the rhizosphere of Phyllostachys bambusoides forest. J. Jiangsu Normal Univ. 34, 56–59. doi: 10.3969/j.issn.2095-4298.2016.02.013
Yin, Z., Li, L., Wang, K., and Guo, Q. (2011). Effects of Bacillus amyloliquefaciens and maternal bamboo age on seedlings rooting abilities of four bamboo species with by burying culm method. J. Bmboo Res. 30, 28–33. doi: 10.3969/j.issn.1000-6567.2011.02.006
Yuan, Z. S., Liu, F., and Zhang, G. F. (2015). Characteristics and biodiversity of endophytic phosphorus- and potassium-solubilizing bacteria in Moso Bamboo (Phyllostachys edulis). Acta Biol. Hung. 66, 449–459. doi: 10.1556/018.66.2015.4.9
Yuan, Z. S., Liu, F., Liu, Z. Y., Huang, Q. L., Zhang, G. F., and Pan, H. (2021). Structural variability and differentiation of niches in the rhizosphere and endosphere bacterial microbiome of moso bamboo (Phyllostachys edulis). Sci. Rep. 11:1574. doi: 10.1038/s41598-021-80971-9
Yuan, Z. S., Liu, F., Xie, B. G., and Zhang, G. F. (2018). The growth-promoting effects of endophytic bacteria on Phyllostachys edulis. Arch. Microbiol. 200, 921–927. doi: 10.1007/s00203-018-1500-8
Yuan, Z., Liu, F., Zeng, Z. H., and Pan, H. (2023). Community structure and diversity of rhizosphere soil and endophytic bacteria during the period of growth of moso bamboo shoots. Front. Biosci. 28:329. doi: 10.31083/j.fbl2812329
Zhai, W., Zhong, Z., Gao, G., and Yang, H. (2017). Influence of mulching management on soil bacterial structure and diversity in Phyllostachys praecox stands. Sci. Silvae Sin. 53, 133–142. doi: 10.11707/j.1001-7488.20170916
Zhan, C., Matsumoto, H. H., Liu, Y., and Wang, M. (2022). Pathways to engineering the phyllosphere microbiome for sustainable crop production. Nat. Food 3, 997–1004. doi: 10.1038/s43016-022-00636-2
Zhang, L. (2023). “Bamboo expansion and soil microbial communities,” in Bamboo expansion: Processes, impacts, and management, (Singapore: Springer), 281–304. doi: 10.1007/978-981-99-4113-1_12
Zhang, N. N., Chen, X. X., Liang, J., Zhao, C., Xiang, J., Luo, L., et al. (2023). Rhizocompartmental microbiomes of arrow bamboo (Fargesia nitida) and their relation to soil properties in subalpine coniferous forests. PeerJ 11:e16488. doi: 10.7717/peerj.16488
Zhang, W., Sheng, K., Fan, C., Liu, S., Chen, M., Zhong, S., et al. (2015). Phospholipid fatty acid analysis of microbial community structure in rhizosphere soil of Phyllostachys edulis in Gannan. Acta Agricult. Univ. Jiangxiensis 37, 475–483. doi: 10.13836/j.jjau.2015074
Zhang, X., Bian, F., Zhong, Z., Gai, X., and Yang, C. (2020). Deciphering the rhizosphere microbiome of a bamboo plant in response to different chromium contamination levels. J. Hazard Mater. 399:123107. doi: 10.1016/j.jhazmat.2020.123107
Zhang, X., Gai, X., Zhong, Z., Bian, F., Yang, C., Li, Y., et al. (2021). Understanding variations in soil properties and microbial communities in bamboo plantation soils along a chromium pollution gradient. Ecotoxicol. Environ. Saf. 222:112507. doi: 10.1016/j.ecoenv.2021.112507
Zhang, X., Gao, G., Wu, Z., Wen, X., Zhong, H., Zhong, Z., et al. (2019b). Agroforestry alters the rhizosphere soil bacterial and fungal communities of moso bamboo plantations in subtropical China. Appl. Soil Ecol. 143, 192–200. doi: 10.1016/j.apsoil.2019.07.019
Zhang, X., Huang, Z., Zhong, Z., Li, Q., Bian, F., Gao, G., et al. (2022a). Evaluating the rhizosphere and endophytic microbiomes of a bamboo plant in response to the long-term application of heavy organic amendment. Plants 11:2129. doi: 10.3390/plants11162129
Zhang, X., Li, Q., Zhong, Z., Huang, Z., Bian, F., Yang, C., et al. (2022b). Changes in soil organic carbon fractions and fungal communities, subsequent to different management practices in moso bamboo plantations. J. Fungi (Basel) 8:640. doi: 10.3390/jof8060640
Zhang, X., Zhong, Z., Gai, X., Du, X., Bian, F., Yang, C., et al. (2019a). Changes of root endophytic bacterial community along a chronosequence of intensively managed lei bamboo (Phyllostachys praecox) forests in subtropical China. Microorganisms 7:616. doi: 10.3390/microorganisms7120616
Zhang, Z., Lin, Y., Dai, P., Li, Z., Li, Z., and Li, W. (2015). Ecological characteristics of rice rhizosphere of dry-raised seedlings. Chinese J. Eco-Agricult. 23, 1552–1561. doi: 10.13930/j.cnki.cjea.150642
Zhao, A., Ding, X., Huang, M., and Cheng, Y. (2023). Bioprospecting plant-growth-promoting endophytic bacteria isolated from moso bamboo (Phyllostachys edulis) shoots. Forests 14:2061. doi: 10.3390/f14102061
Zhao, J. C., Su, W. H., Fan, S. H., Cai, C. J., Zhu, X. W., and Liu, G. (2016). Effects of various fertilization depths on ammonia volatilization in Moso bamboo (Phyllostachys edulis) forests. Plant Soil Environ. 62, 128–134. doi: 10.11707/j.1001-7488.20161107
Zhao, L., Zhong, Z., Yang, H., Liu, Z., Yu, Q., and Bai, R. (2015). The effects of mulch management on soil acidification process in the Phyllostachys praecox forests. J. Bamboo Res. 34, 19–24. doi: 10.3969/j.issn.1000-6567.2015.03.004
Zhao, Y., Liang, C., Shao, S., Chen, J., Qin, H., Xu, Q., et al. (2021). Linkages of litter and soil C:N:P stoichiometry with soil microbial resource limitation and community structure in a subtropical broadleaf forest invaded by Moso bamboo. Plant Soil 456, 473–490. doi: 10.1007/s11104-021-05028-2
Zheng, Y., and Lin, X. (2020). Niche specialization and functional overlap of bamboo leaf and root microbiota. Front. Microbiol. 11:571159. doi: 10.3389/fmicb.2020.571159
Zheng, Y., Liu, X., Cai, Y., Shao, Q., Zhu, W., and Lin, X. (2022). Combined intensive management of fertilization, tillage, and organic material mulching regulate soil bacterial communities and functional capacities by altering soil potassium and pH in a Moso bamboo forest. Front. Microbiol. 13:944874. doi: 10.3389/fmicb.2022.944874
Zhong, Y., Wu, C., Hu, Y., Mi, Y., Yu, Z., and Cao, F. (2019). Update of the world bamboo list. Subtrop. Plant Sci. 48, 176–180. doi: 10.3969/j.issn.1009-7791.2019.02.013
Zhou, H., Zhang, D., Jiang, Z., Sun, P., Xiao, H., Yuxin, W., et al. (2019). Changes in the soil microbial communities of alpine steppe at Qinghai-Tibetan Plateau under different degradation levels. Sci. Total Environ. 651(Pt 2), 2281–2291. doi: 10.1016/j.scitotenv.2018.09.336
Zhou, X., Liu, X., Zhao, J., Guan, F., Yao, D., Wu, N., et al. (2021). The endophytic bacterium Bacillus koreensis 181-22 promotes rice growth and alleviates cadmium stress under cadmium exposure. Appl. Microbiol. Biotechnol. 105, 8517–8529. doi: 10.1007/s00253-021-11613-3
Zhou, Y. K., Shen, X. Y., and Hou, C. L. (2017). Diversity and antimicrobial activity of culturable fungi from fishscale bamboo (Phyllostachys heteroclada) in China. World J. Microbiol. Biotechnol. 33:104. doi: 10.1007/s11274-017-2267-9
Keywords: bamboo, endophytes, rhizosphere microorganisms, microbial composition, influence factors
Citation: Wang Y, Ren H, Zhong Y, Song R, Jiang S, Lai M, Shen Y, Liu S, Shi W and Qi G (2025) Microbial diversity and function in bamboo ecosystems. Front. Microbiol. 16:1533061. doi: 10.3389/fmicb.2025.1533061
Received: 23 November 2024; Accepted: 27 May 2025;
Published: 25 June 2025.
Edited by:
Sabine Dagmar Zimmermann, IPSiM Institute of Plant Science in Montpellier CNRS UMR5004, FranceReviewed by:
Anandham Rangasamy, Tamil Nadu Agricultural University, IndiaMuthusamy Ramakrishnan, Nanjing Forestry University, China
Lal Singh, National Environmental Engineering Research Institute (CSIR), India
Zishan Ahmad, Nanjing Forestry University, China
Copyright © 2025 Wang, Ren, Zhong, Song, Jiang, Lai, Shen, Liu, Shi and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shenkui Liu, c2hlbmt1aWxpdUBuZWZ1LmVkdS5jbg==; Wenhui Shi, c2hpd2VuaHVpMjAwOEAxNjMuY29t; Guoning Qi, Z25xaUB6YWZ1LmVkdS5jbg==
†These authors have contributed equally to this work
 Yexuan Wang1†
Yexuan Wang1† Yue Zhong
Yue Zhong Shenkui Liu
Shenkui Liu Guoning Qi
Guoning Qi