- 1Universal Scientific Education and Research Network (USERN), Semnan, Iran
- 2Nervous System Stem Cells Research Center, Semnan University of Medical Sciences, Semnan, Iran
- 3Skull Base Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Student Research Committee, School of Medicine, Shahroud University of Medical Sciences, Shahroud, Iran
- 5Department of Pediatrics, School of Medicine, Semnan University of Medical Sciences, Semnan, Iran
- 6University of Bergen, Bergen, Norway
- 7Department of Clinical and Molecular Medicine, Norwegian University of Science and Technology (NTNU), Trondheim, Norway
- 8Department of Biosciences and Nutrition, Karolinska Institutet, Huddinge, Sweden
- 9Department of Bacteriology and Virology, Faculty of Medicine, Semnan University of Medical Sciences, Semnan, Iran
Dengue virus (DENV), a mosquito-borne Flavivirus, represents a growing global health challenge, particularly in tropical and subtropical regions. The 2009 WHO classification system for dengue categorizes infections into dengue without warning signs, dengue with warning signs, and severe dengue. This framework highlights the diverse clinical presentations and supports more efficient triage and management of cases. The neurological effects of DENV infection, which include direct neuroinvasion, systemic problems, and immune-mediated sequelae, are a less well-studied but nevertheless important consequence. Guillain-Barré syndrome, acute disseminated encephalomyelitis, myelitis, meningitis, and encephalitis are important neurological symptoms. The dengue classification system improves clinical management by dividing cases into dengue without warning signs, dengue with warning signs, and severe dengue. Mild cases show fever with symptoms like headache and rash, while warning signs include abdominal pain, persistent vomiting, bleeding, and lab changes indicating higher risk. Severe dengue is characterized by critical complications such as shock, severe bleeding, or organ failure. Improved diagnostics aid early detection, and new treatments targeting viral replication and inflammation are being explored alongside supportive care. Although there are still challenges in reaching the ideal vaccination coverage, the introduction of potent vaccines like Dengvaxia and Qdenga provide an achievable option for prevention. Thorough study into DENV’s neurological effects and treatment options is essential as the virus’s geographic range is increased by climate change and international travel. Reducing the worldwide burden of dengue-related neurological complications requires addressing the intricate interactions between virological, immunological, and environmental variables.
1 Introduction
Dengue virus (DENV) is a positive-sense RNA virus with a genome size of approximately 10.7 Kb, belonging to the Flaviviridae family (Chambers et al., 1990; Westaway et al., 1985). DENV is primarily transmitted by Aedes aegypti and Aedes albopictus mosquitoes, although it can also be transmitted by Aedes vitattus. The global endemicity of DENV is a result of this widespread vector range, particularly in tropical and subtropical regions (Lee et al., 2018). In 2009, the World Health Organization (WHO) revised its dengue classification system to enhance clinical management and improve case identification, replacing the older 1997 model. The previous approach classified cases into dengue fever, dengue hemorrhagic fever, and dengue shock syndrome. The updated system focuses on identifying warning signs and categorizes dengue into three main groups: dengue without warning signs, dengue with warning signs, and severe dengue. Dengue without warning signs generally refers to febrile illness accompanied by symptoms such as headache, rash, muscle and joint pain, nausea, and mild bleeding. Dengue with warning signs includes additional clinical indicators signaling a greater risk of progression to severe disease. These warning signs may include persistent abdominal pain, continuous vomiting, fluid buildup, mucosal bleeding, lethargy, liver enlargement, and laboratory findings such as a rising hematocrit level coupled with a rapid drop in platelet count. Severe dengue is marked by significant plasma leakage leading to shock or respiratory distress due to fluid accumulation, severe bleeding, or critical organ impairments affecting the liver, central nervous system, or heart. This updated classification aims to facilitate early detection of cases with severe potential, ensuring timely interventions and reducing mortality rates (WHO Guidelines Approved by the Guidelines Review Committee, 2009). Notably, because DENV may enter the central nervous system (CNS) and cause encephalitis, meningitis, and myelitis, neurological involvement has attracted more attention. Neurological symptoms such as altered consciousness, neck rigidity, focal neurological signs, and convulsions are observed in approximately 5% of cases (Verma et al., 2013; Murthy, 2010).
Dengue virus infection progresses through three distinct phases: the febrile phase, the critical phase, and the recovery phase, each characterized by specific virological and immunological dynamics (Martina et al., 2009). The febrile phase typically starts 4–7 days after a mosquito bite and is marked by high fever, headache, muscle pain, and laboratory findings of viremia. Viremia tends to peak early, usually declining by days 4 or 5 of illness as the host’s adaptive immune system activates and seroconversion occurs. Viral RNA is generally cleared from the bloodstream between days 5 and 7 but may persist longer in cerebrospinal fluid or other immune-privileged sites (Soares et al., 2006). The critical phase, overlapping with defervescence (usually around days 4–6), presents the greatest clinical risks. During this period, patients may experience plasma leakage, thrombocytopenia, and hemorrhagic complications. Neurological symptoms can also develop during or shortly after this phase, depending on the underlying mechanisms. Complications such as encephalopathy and encephalitis may arise early in severe cases, likely resulting from direct viral invasion or cytokine-driven neuroinflammation (Solomon et al., 2000). In contrast, delayed immune-mediated conditions like Guillain-Barré syndrome, acute disseminated encephalomyelitis (ADEM), or transverse myelitis generally appear 1–3 weeks following the acute febrile stage, indicative of post-infectious autoimmune responses (Murthy, 2010; Carod-Artal et al., 2013). Additionally, some patients may develop post-acute complications, including prolonged fatigue, cognitive difficulties, or mood disturbances collectively referred to as post-dengue syndrome which can persist for weeks or even months. This underscores the importance of not only acute-phase care but also long-term monitoring for neurological and neuropsychiatric sequelae in those affected (Sigera et al., 2021).
Accurate and timely diagnosis of dengue virus infection plays a critical role in preventing disease progression and associated complications. Current diagnostic strategies include serological tests such as NS1 antigen detection and IgM/IgG ELISA, molecular techniques like RT-PCR, as well as neuroimaging methods like MRI for patients exhibiting neurological symptoms. These tools are particularly vital in atypical presentations, where dengue manifests with conditions such as encephalopathy, stroke, or peripheral neuropathies instead of the typical febrile illness (Hunsperger et al., 2014; Lnu et al., 2022). The global impact of dengue has risen sharply in recent decades due to factors like climate change, urbanization, population expansion, and the broader distribution of Aedes mosquito habitats (Messina et al., 2014). Furthermore, international travel and inadequate vector control measures in endemic areas have contributed to the resurgence and simultaneous circulation of multiple serotypes, often leading to hyperendemicity and heightened risk of severe disease outcomes. Within the diverse clinical spectrum of dengue, neurological complications are increasingly recognized but remain frequently underdiagnosed. Research indicates that up to 21% of severe dengue cases may involve neurological symptoms, ranging from mild issues like headache and dizziness to more serious conditions such as encephalitis, Guillain-Barré syndrome, acute transverse myelitis, and ADEM. This underscores the necessity for integrated diagnostic approaches and surveillance systems that acknowledge the virus’s ability to impact the nervous system (Murthy, 2010).
Despite significant research efforts, no specific antiviral treatment has yet been developed. As a result, managing the disease remains highly dependent on supportive care and preventive measures, with a strong emphasis on vector control and public health interventions (Guzman et al., 2016). In the fight against dengue fever (DF), recent developments in vaccine development have produced two licensed vaccines, Dengvaxia and Qdenga. While Qdenga, a more recent addition, may help prevent diseases across a wider age range and serostatus, Dengvaxia, a live-attenuated tetravalent vaccination, has demonstrated effectiveness in seropositive persons (WHO Guidelines Approved by the Guidelines Review Committee, 2009).
Despite extensive global efforts, no specific antiviral therapy has been approved to treat dengue virus infections to date. As a result, management remains largely supportive. In response, vaccination has taken center stage in dengue prevention strategies, especially in regions where the disease is most prevalent. Two vaccines, Dengvaxia (CYD-TDV) and Qdenga (TAK-003) have emerged as significant breakthroughs in this field, each characterized by unique immunogenicity profiles, efficacy outcomes, and practical limitations. Dengvaxia (CYD-TDV), developed by Sanofi Pasteur, is a live attenuated, recombinant tetravalent vaccine. It is based on a yellow fever virus (YF17D) backbone, incorporating the premembrane (prM) and envelope (E) genes of the four dengue virus serotypes (DENV-1 to DENV-4). Clinical trials have shown that it provides moderate to high efficacy among individuals with prior dengue exposure, particularly against serotypes DENV-3 and DENV-4, though its effectiveness is lower against DENV-1 and DENV-2 (Sridhar et al., 2018). However, post-licensure studies revealed that Dengvaxia poses a risk of antibody-dependent enhancement (ADE) in seronegative individuals upon subsequent natural infections. This phenomenon heightens the likelihood of severe disease and hospitalization (Fatima and Syed, 2018). As a result, the World Health Organization (WHO) and other regulatory authorities recommend pre-vaccination screening to confirm prior dengue exposure. Usage of Dengvaxia is currently limited to individuals aged 9–45 years residing in dengue-endemic areas with a documented history of previous infection (Marti et al., 2024).
To address existing limitations, Qdenga (TAK-003), developed by Takeda Pharmaceuticals, represents a second-generation live attenuated dengue vaccine. It utilizes a DENV-2 backbone, incorporating the prM and E genes of DENV-1, DENV-3, and DENV-4 to achieve tetravalent protection (Hadinegoro et al., 2015). Unlike Dengvaxia, Qdenga has demonstrated protective efficacy in both seropositive and seronegative individuals, making it viable for wider usage without the need for pre-vaccination screening (Hadinegoro et al., 2015; Tricou et al., 2020). Findings from the TIDES study (Tetravalent Immunization against Dengue Efficacy Study) revealed an overall vaccine efficacy of 80.2% against virologically confirmed dengue and 90.4% efficacy in preventing hospitalization over an 18 months follow-up period. Additionally, Qdenga has shown consistent and durable immunogenicity across different age groups and regions, maintaining an acceptable safety profile with no increased risk identified in dengue-naïve populations (Tricou et al., 2020; Flacco et al., 2024). Nevertheless, considerable challenges persist for both vaccines, including variability in efficacy between serotypes, logistical hurdles in deployment within low-resource settings, and the complexity of integrating them into national immunization programs. Dengvaxia suffered a significant loss of public trust due to adverse outcomes in seronegative children, resulting in program suspensions in several countries (Fatima and Syed, 2018). Conversely, Qdenga has recently gained approval from the European Medicines Agency (EMA) and other regulatory bodies, offering hope for expanded global distribution in dengue-endemic regions without the need for prior serological testing. Ongoing real-world monitoring and cost-effectiveness evaluations will be critical to optimizing the worldwide implementation of dengue vaccination strategies (Flacco et al., 2024; Martinez et al., 2015).
Symptoms typical of the infection, including a petechial rash, myalgia, arthralgia, retro-orbital pain, and a severe headache, are frequently present during this febrile phase. The necessity of attentive hydration and supportive care is highlighted by the fact that dehydration in children during this time might increase the risk of neurological disorders, including seizures (WHO Guidelines Approved by the Guidelines Review Committee, 2009). Because DENV infections are systemic, they can impact several organ systems, making early detection and treatment essential to avoiding serious consequences including DHF and DSS. The introduction of vaccines is a complement to current preventive efforts, such as community awareness programs, vector control methods like the use of insecticides, and the elimination of mosquito breeding sites. The integration of these techniques into national immunization programs and achieving optimal vaccine coverage, however, continue to be critical challenges. Reducing the worldwide burden of DF requires ongoing research into the pathophysiology of DENV and improving the effectiveness of vaccines (Henchal and Putnak, 1990).
2 Neurological complications
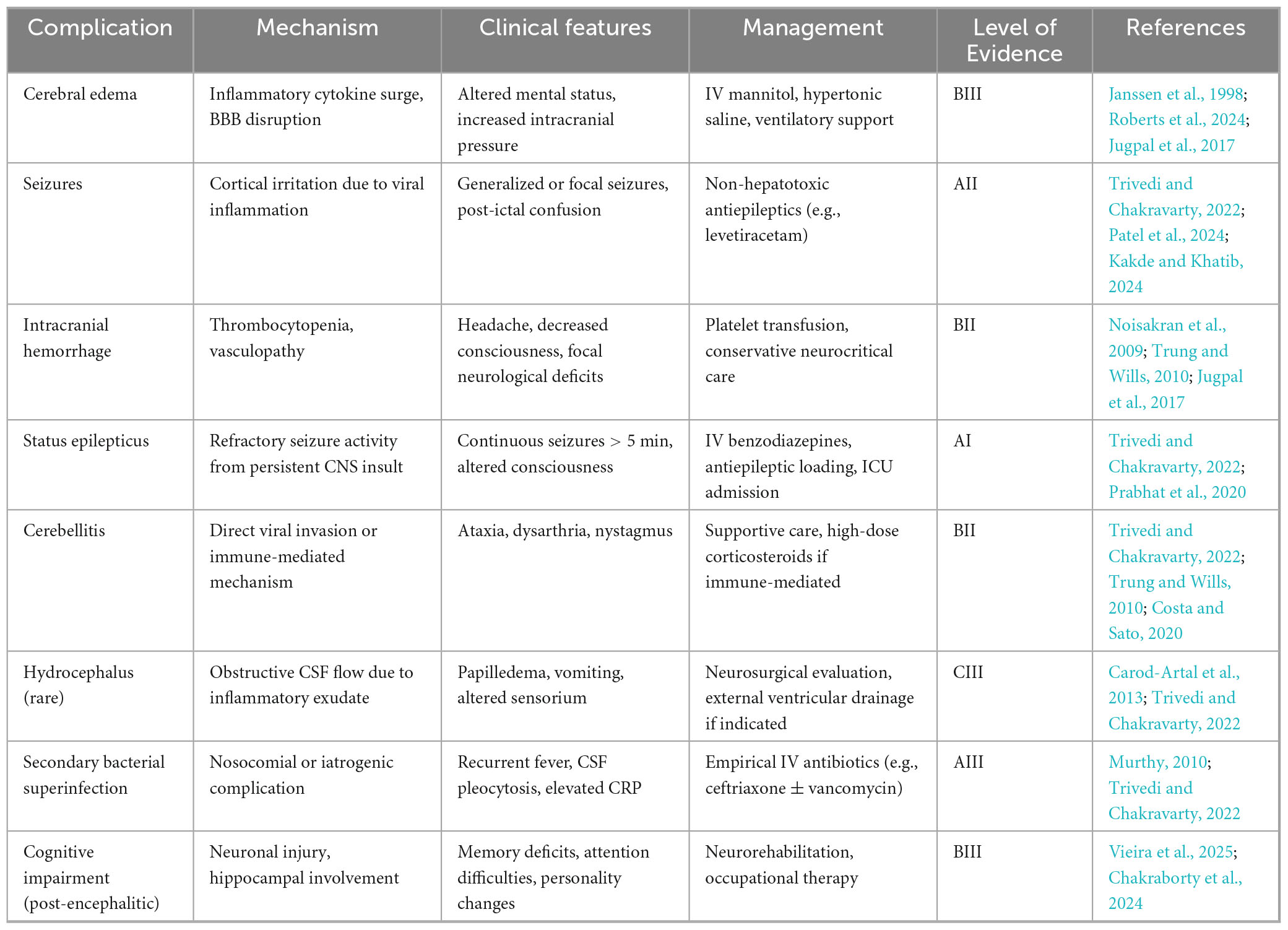
Neurological complications arising from DENV infection can be grouped into three distinct pathophysiological categories based on the underlying mechanism of injury. The first category involves direct neuroinvasion, characterized by viral entry and replication within the central nervous system (Solomon et al., 2000). The second includes systemic complications, which stem from metabolic disturbances, vascular damage, or hemodynamic instability, occurring without direct invasion of the CNS (Murthy, 2010). The third category encompasses immune-mediated complications, driven by post-infectious autoimmune responses (Carod-Artal et al., 2013). The first is direct neuroinvasion, which is caused by the neurotropism of the virus and causes diseases including encephalitis, myelitis, and meningitis. Serotypes of DENV-2 and DENV-3 are commonly linked to serious neurological outcomes (Garg et al., 2015). Systemic consequences, including strokes, hypokalemic paralysis, and encephalopathy, occur in the second group. These are frequently brought on by severe disease progression and subsequent systemic inflammation. Finally, there is growing recognition of immune-mediated post-infectious consequences, such as acute disseminated encephalomyelitis (ADEM), neuromyelitis optica, and Guillain-Barré syndrome (GBS) (Trivedi and Chakravarty, 2022).
Recent research highlights that environmental variables, host immune responses, and virus serotype all affect these consequences, with endemic areas reporting increased incidence. A almost universal symptom of DF, headaches can be quite severe and are frequently accompanied by phonophobia, photophobia, nausea, and vomiting. In certain instances, headaches are linked to symptoms of lymphocytic meningitis, including positive Kernig’s and Brudzinski’s signs and nuchal stiffness. Interestingly, there is not a difference in the intensity of neurological symptoms between primary and secondary infections or between DHF and DF (Sil et al., 2017).
Early identification and categorization of these issues have been made easier by developments in diagnostic methods, such as neuroimaging and cerebrospinal fluid (CSF) analysis. Inflammatory cytokines and viral RNA levels are two examples of biomarkers that are being investigated for their potential to predict the severity and course of disease. In terms of treatment, supportive care is still critical, especially for immune-mediated problems that usually go away in a few weeks to months (Kothur et al., 2016). However, due to the immediate need to reduce the worldwide neurological burden of dengue, experimental therapies that target inflammation and virus replication are being investigated. Urbanization and climate change are contributing factors to the increasing prevalence of DENV infection, which emphasizes the need of public health initiatives. These include improving immediate diagnosis, vector control, and surveillance, especially in endemic regions. There is potential for lowering morbidity and improving patient outcomes with more investigation into the pathophysiology of DENV’s neurological effects and the creation of focused therapies. With dengue’s neurological symptoms accounting for a substantial portion of disease morbidity, a complete approach is essential as the disease continues to emerge as a major worldwide health concern (Enitan et al., 2024) (Table 1).
2.1 Direct neuroinvasion
A form of “direct neuroinvasion” describes the DENV capacity to enter the CNS directly, leading to neurological complications, including encephalitis, meningitis, myelitis, and myositis. These conditions arise from inflammation triggered directly by viral replication within neural tissues, setting them apart from systemic or autoimmune inflammatory responses. The main characteristic of these conditions is inflammation of the brain, spinal cord, and related tissues, which can have severe consequences and, in certain situations, cause irreversible neurological damage. Although the exact processes by which the DENV enters the CNS remain unclear, an increasing amount of data indicates that the virus’s direct invasion of neural tissue plays a major role in the emergence of these conditions. Meningitis, encephalitis, and myelitis are the most often seen neurological symptoms in individuals with direct neuroinvasion of dengue. While encephalitis includes direct inflammation of the brain tissue itself, meningitis is defined by inflammation of the protective membranes surrounding the brain and spinal cord (Farina et al., 2023). Acute transverse myelitis manifests as spinal cord inflammation, while myositis is defined as inflammation of muscle tissue. Cerebellar syndrome and myalgia can also appear; these conditions often show up as issues with coordination and muscular pain, respectively. These clinical manifestations demonstrate the wide range of neurological harm that can arise from a DENV infection of the CNS (Kondziella and Waldemar, 2023).
The DENV capacity to cause CNS infections depends on its capacity to pass across the blood-brain barrier (BBB), a protective barrier that typically keeps pathogens from entering the brain. This is especially significant during the infection’s acute phase, when the majority of neurological problems appear. According to research, the virus can either directly infiltrate neurons or cause immune-mediated inflammation, which has an indirect effect on the CNS. Studies using animal models have shed important light on how the virus causes the production of cytokines that promote inflammation, which may compromise the BBB. When this barrier breaks down, the virus can enter the brain and spinal cord, where it can multiply and intensify the inflammatory reaction (Mustafá et al., 2019). Research involving spinal cord injury models has demonstrated that enhancing autophagy while suppressing necroptosis can reduce neural damage. This indicates that strategic modulation of these pathways might also offer potential for addressing DENV-induced neuroinflammation and central nervous system injury (Chen et al., 2025). The hypothesis of direct neuroinvasion has been strongly supported by recent histological investigations. Histological evidence of encephalitis, including the presence of inflammatory cells in brain tissue, was found during the autopsy of a child who had been diagnosed with dengue encephalitis. Further evidence of the virus’s capacity to impact the CNS comes from the isolation of DENV types 2 and 3 from CSF samples taken from individuals exhibiting neurological symptoms. The theory that the virus can directly infect neural tissue is further supported by the discovery of dengue antigens in the brain tissue of affected individuals, in addition to the virus’s isolation. The pathophysiology of dengue-related encephalitis has been studied by researchers as a result of these outcomes, with an emphasis on the mechanisms that allow the virus to enter the CNS and the following effects it has on brain function (Ludlow et al., 2016).
Numerous cytokines, including interleukins and interferons, have been demonstrated to be activated as part of an immune response triggered by DENV infection in animal models. These cytokines play a key role in modulating the inflammatory response, and the BBB may become more permeable as a result of their high levels in the CSF. This increased permeability could make it easier for the virus to enter the central nervous system, starting the series of events that eventually result in myelitis, encephalitis, and other neurological disorders. Despite being intended to manage the infection, this inflammatory reaction may severe damage the nerve system and leading long-term neurological consequences in affected patients (Guabiraba and Ryffel, 2014).
Most cases of myositis associated with dengue fever tend to be self-limiting, resolving effectively with supportive care alone (Dalugama et al., 2017). This condition, marked by inflammation of muscle tissue, typically manifests through symptoms such as muscle tenderness, weakness, and elevated levels of creatine phosphokinase (CPK) (Trivedi and Chakravarty, 2022). Early diagnosis is crucial to avoid severe complications, making CPK assessment and urinalysis key diagnostic measures for dengue patients exhibiting muscle-related symptoms. For cases where dengue myositis persists, corticosteroid therapy has demonstrated notable clinical benefits (Kaur and Singh, 2021).
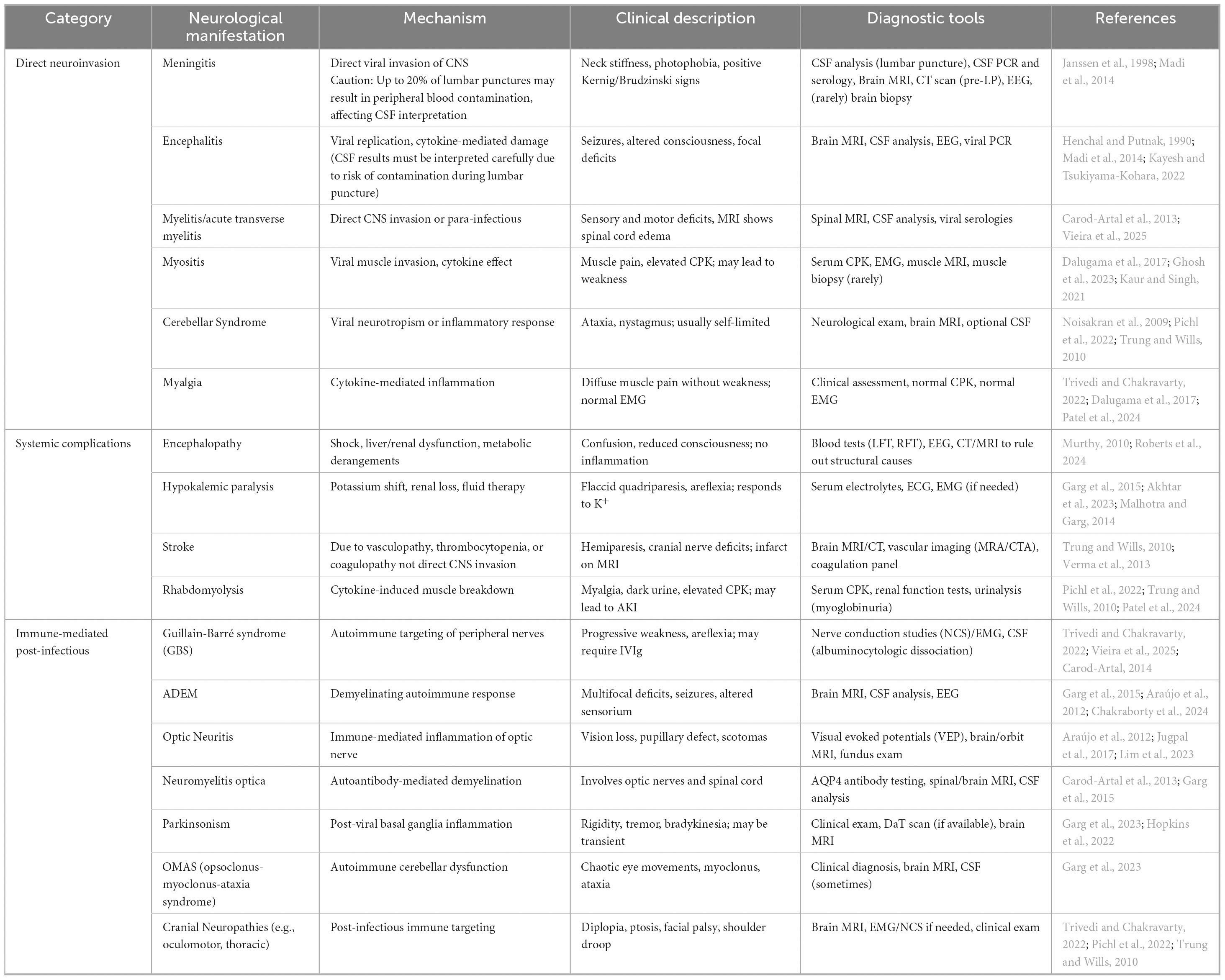
Conversely, myelitis especially acute transverse myelitis is an uncommon but serious neurological complication tied to dengue infection (Carod-Artal et al., 2013). This form of spinal cord inflammation may develop during the active phase of the disease due to direct viral invasion (para-infectious) or in the post-infectious phase as an immune-mediated response (Vieira et al., 2025). Patients with myelitis often present with limb weakness, sensory impairment, and difficulties in bowel or bladder function. Diagnosis typically involves spinal MRI, which may reveal edema or signal changes, alongside CSF analysis that could indicate the presence of viral RNA or intrathecal IgG production (Verma et al., 2013; Vieira et al., 2025; Figure 1).
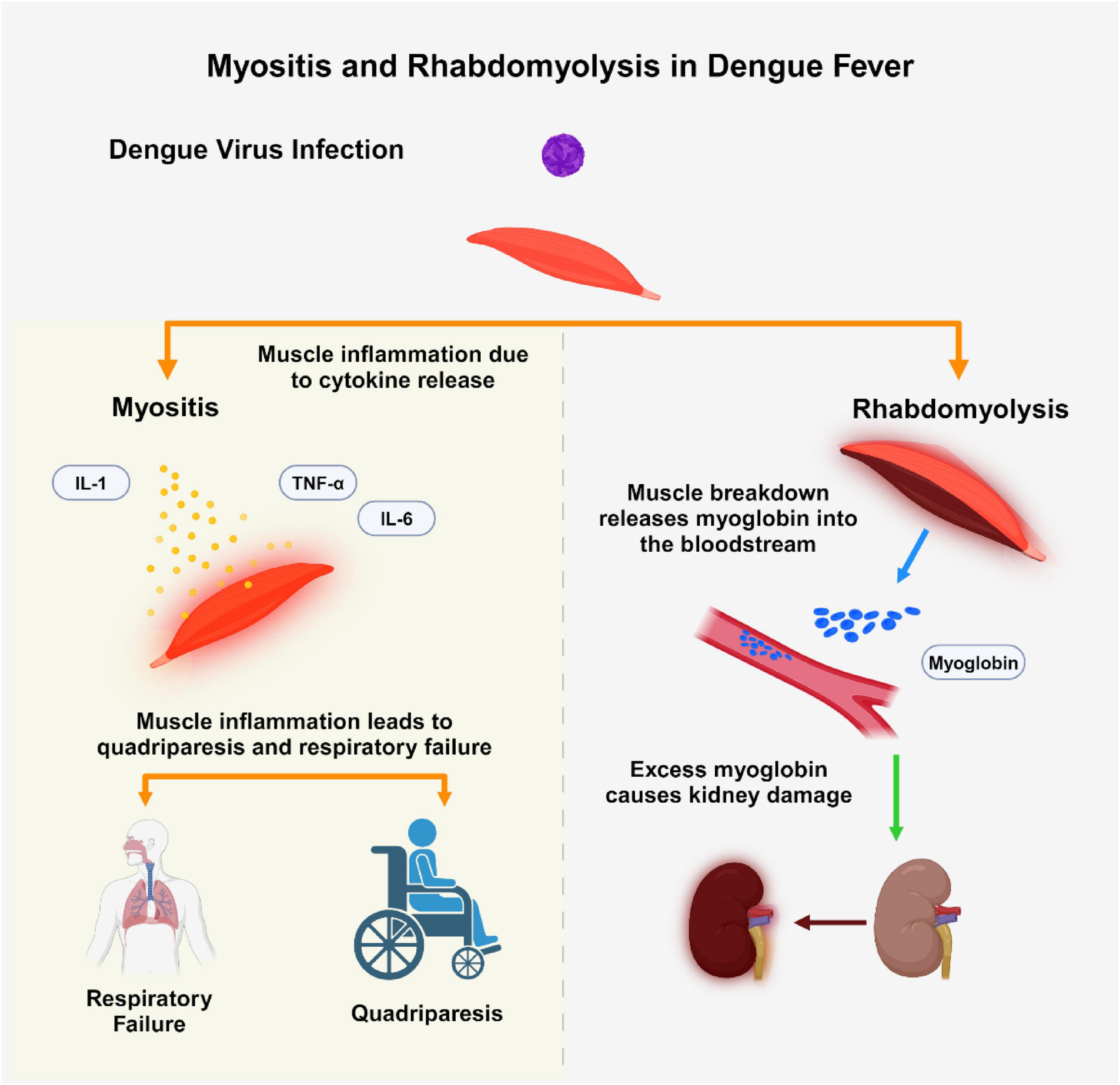
Figure 1. This figure illustrates two muscle-related complications in dengue fever: myositis and rhabdomyolysis. Dengue virus (DENV) infection can induce myositis through cytokine release (IL-1, IL-6, TNF-α), resulting in muscle inflammation, quadriparesis, and respiratory failure. Conversely, rhabdomyolysis is characterized by muscle breakdown, leading to myoglobin release into the bloodstream, potentially causing acute kidney injury (AKI) due to myoglobin accumulation. The Figure was designed using BioRender.com.
The rare and serious neurological side effect of dengue infection is cerebellar involvement. Between 2 days and 2 weeks after the start of the DENV, this disease usually appears (Trung and Wills, 2010). Although they are still not fully understood, the pathophysiological processes generating cerebellar involvement appear to vary among patients. Direct viral invasion of the CNS and an inflammatory reaction brought on by the infection are two hypothesized processes (Noisakran et al., 2009). Viral neurotropism, in which the virus directly damages cerebellar tissues, is considered to be linked to the early development of cerebellar symptoms. In contrast, the inflammatory response, which takes longer to develop, may contribute to delayed symptom presentation. Key clinical features of cerebellar syndrome in DF include ataxia and bilateral nystagmus, which highlight the dysfunction of cerebellar pathways. However, due to the limited characterization of the association between cerebellar syndrome and DENV, it is critical to perform a thorough differential diagnosis to exclude other potential etiologies. Magnetic resonance imaging (MRI) of the brain is often used in diagnostic evaluation, yet findings are typically unremarkable, with normal scans reported in most documented cases. Notably, dengue fever-related cerebellar syndrome often has a benign course. Patients usually fully recover without any lasting neurological impairments, and it is often transient and self-limiting. This good prognosis highlights the significance early detection and supportive care are to symptom management and the best possible recovery results. To further understand the processes, risk factors, and therapeutic care approaches for this uncommon issue, more research is necessary (Pichl et al., 2022).
In the early stages of dengue fever, myalgia is a common and distinct symptom that indicates the disease’s systemic involvement (Trivedi and Chakravarty, 2022). It is believed that both direct viral invasion of muscle tissues and inflammatory alterations caused on by the immune system’s reaction to the infection are the underlying processes causing muscular soreness (Dalugama et al., 2017). The typical symptoms of dengue-associated myalgia are probably caused by this combination. These symptoms cause a great deal of pain and mostly impact the back and proximal muscle groups. Dengue-associated myalgia can be distinguished from other disorders affecting muscle dysfunction because to this differentiation. Despite the severity of the pain, myalgia associated with DF is often transient and self-limiting, with symptoms going away once the disease’s acute phase subsides. This benign course emphasizes the value of supportive care, which aims to reduce pain and guarantee that patients heal without suffering from long-term muscle damage. Deeper understanding of the mechanism and the best ways to treat this prevalent DF presentation may be possible with more study (Patel et al., 2024).
2.2 Systemic complications
This category includes conditions caused by widespread body responses to the viral infection, which indirectly impact CNS function. Examples include encephalopathy (brain dysfunction without inflammation), hypokalemic paralysis (paralysis due to low potassium levels), and stroke. These complications result from metabolic disturbances, dehydration, or other systemic effects of the infection (Murthy, 2010). Encephalopathy is the most frequently observed neurological manifestation of DENV infection. It arises due to systemic complications that occur with severe infection, such as shock, metabolic imbalances, cerebral edema, and organ dysfunction involving the liver or kidneys (Roberts et al., 2024). DENV encephalitis frequently presents with seizures, along with altered mental status, headaches, behavioral changes, and various focal neurological deficits (Araújo et al., 2012; Domingues et al., 2008). Neuroimaging of patients with encephalitis typically reveals T2-weighted and fluid-attenuated inversion recovery (FLAIR) hyper intensities. These hyper intensities are primarily found in deep brain structures, with the thalamus, basal ganglia, and cerebellum being commonly affected (Jugpal et al., 2017).
Intracranial hemorrhagic complications in dengue infection can be attributed to a bleeding diathesis resulting from vasculopathy, thrombocytopenia, and platelet dysfunction (Trung and Wills, 2010). Thrombocytopenia in dengue arises from both reduced production and increased destruction of platelets, with its severity often correlating to the clinical intensity of DHF and activation of the complement system (Noisakran et al., 2009). This impairment in platelet function elevates the risk of vascular fragility and subsequent hemorrhage. Additionally, dengue-related coagulopathy and vasculopathy can contribute to vascular thrombosis and ischemic stroke. Elevated levels of plasminogen activator inhibitor type I (PAI-I), a procoagulant, are also observed in dengue patients, further impacting coagulation dynamics (Trung and Wills, 2010). In a case study, a 68 years-old man with dengue presented with left-sided facial paresis and hemiparesis. Blood tests showed leukocytosis and thrombocytopenia, and dengue NS1 antigen was positive. CSF analysis was normal except for 15 lymphocytes. MRI indicated an acute infarct in the right parietal region. After conservative treatment with low-dose aspirin and physiotherapy, he showed partial improvement in limb strength at a 2 months follow-up (Verma et al., 2013).
Hypokalemic paralysis, a disorder marked by a sudden onset of muscular weakness caused on by critically low blood potassium levels, has been linked to DENV infection. It is difficult to comprehend the pathophysiology of hypokalemia in relation to DF since this electrolyte imbalance is caused by several overlapping pathways. A number of theories provide insight into the possible causes and interactions, even if exact pathways are yet unknown (Garg et al., 2013). The disease known as metabolic alkalosis, in which the blood becomes too alkaline, can result from these fluids. Despite being a well-established physiological reaction, this intracellular shift is especially relevant in dengue because of the intensive fluid resuscitation techniques frequently used to treat severe dehydration or dengue shock syndrome. The condition is made more difficult by the systemic nature of dengue. As part of the more extensive inflammatory and metabolic abnormalities observed in severe cases, the infection may result in a redistribution of potassium between cells and extracellular fluid. These renal abnormalities might be the direct result of the virus’s impact on kidney function or a secondary outcome of changes in vascular permeability and systemic inflammation. Hypokalemia may worsen as a result of increased catecholamine levels stimulating cellular potassium absorption through beta-adrenergic receptors. Although this method inadvertently leads to potassium depletion, it represents the body’s attempt to counteract the systemic stress brought on by the disease. At the molecular level, ion channel-related genetic predispositions have also been linked to hypokalemic paralysis in dengue (Garg et al., 2015). This interaction raises the possibility that certain people are genetically more likely to experience hypokalemia in response to particular stresses, such as dengue illness. The intricate link between systemic inflammation, metabolic disorders, renal dysfunction, and genetic predispositions is highlighted by the multifaceted character of hypokalemia in dengue. Similar to observations in ischemic tissue models, where SNAP29-driven disruptions in autophagic processes and parthanatos exacerbate mitochondrial damage, these mechanisms may likewise play a role in neuronal or muscular injury during severe dengue, potentially driven by oxidative stress and imbalances in cellular homeostasis. It is essential to comprehend these pathways in order to enhance therapeutic management (Yang et al., 2025). Timely intervention, such as cautious fluid control, potassium supplementation, and careful monitoring of renal function and electrolyte balance, is made possible by early detection of hypokalemia and its underlying causes. In order to manage hypokalemic paralysis linked to DENV infection, further study is required to examine the genetic variables and molecular pathways involved. This might lead to the development of individualized treatment plans (Harraz and Delpire, 2024).
Dengue patients complicated with hypokalemic paralysis may present as pure motor weakness of all four limbs (Garg et al., 2015). The quadriparesis can be accompanied by areflexia, hyporeflexia, and hypotonia. Hypokalemic paralysis generally occurs between 2 and 5 days after the onset of fever. A period of 4–24 h of weakness is witnessed. The development of weakness was usually manifested in the phase of defervescence of the febrile stage of dengue. Dengue-associated hypokalemic paralysis must be suspected in patients with motor weakness in dengue-endemic areas. Acute onset of flaccid quadriplegia without any cranial nerve palsy and without any impaired compromise should take our attention to hypokalemic paralysis (Malhotra and Garg, 2014). The serum potassium levels of all suspected Guillain-Barré syndrome patients must be checked. When the serum potassium reaches to 3 mmol/liter or below, the diagnosis of hypokalemic paralysis is indicated. Dengue-associated hypokalemic paralysis is treated completely with adequate potassium supplementation. The patients evolve a rapid recovery without any residual weakness (Akhtar et al., 2023).
2.3 Immunological post-infectious complications
This group comprises complications that emerge after the primary infection has resolved, triggered by the body’s immune response to the virus (Murthy, 2010). Conditions in this category include encephalomyelitis (widespread inflammation of the brain and spinal cord), GBS (an autoimmune disorder causing muscle weakness), optic neuritis (inflammation of the optic nerve), Mononeuropathy, ADEM, Brachial neuritis, Cerebellitis, Opsoclonus–myoclonus–ataxia syndrome, Parkinsonism, neuromyelitis optica (inflammation of the optic nerve and spinal cord), and cranial neuropathies such as oculomotor and long thoracic nerve palsies. These immune-mediated complications typically develop weeks to months after infection and often resolve gradually over time (Trivedi and Chakravarty, 2022).
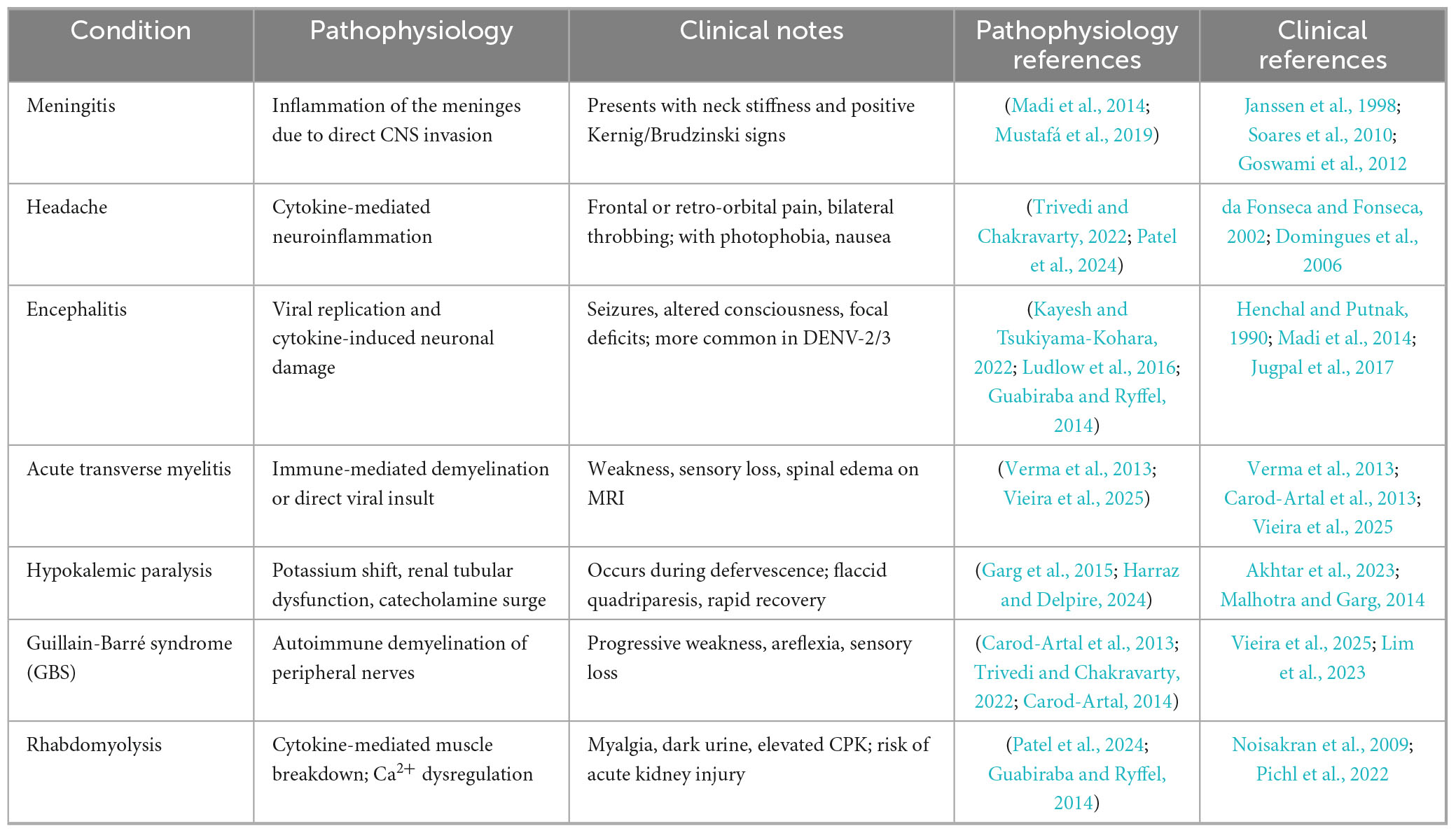
Guillain-Barré syndrome can occur either early or late in the progression of the illness. When immunoglobulins induced by DF interact with components of peripheral nerves that share cross-reactive epitopes, they may trigger an immune response. This response can damage axons or myelin, leading to axonal damage and demyelination, which results in polyneuropathy (Trivedi and Chakravarty, 2022). GBS cases emerge following the acute phase of DF, suggesting an autoimmune mechanism as the underlying cause (Vieira et al., 2025). Both DF and GBS are thought to involve similar pro-inflammatory mediators, potentially triggering the onset of GBS after dengue infection (Sil et al., 2017; Pandey et al., 2018). Key factors in this process include interleukins, tumor necrosis factor-alpha, and complement proteins, all of which may contribute to GBS pathogenesis post-dengue. Additionally, due to a cross-immune response, the body’s immune cells can mistakenly target the myelin and axons of spinal cord roots, leading to nerve damage (Carod-Artal et al., 2013; Figure 2).
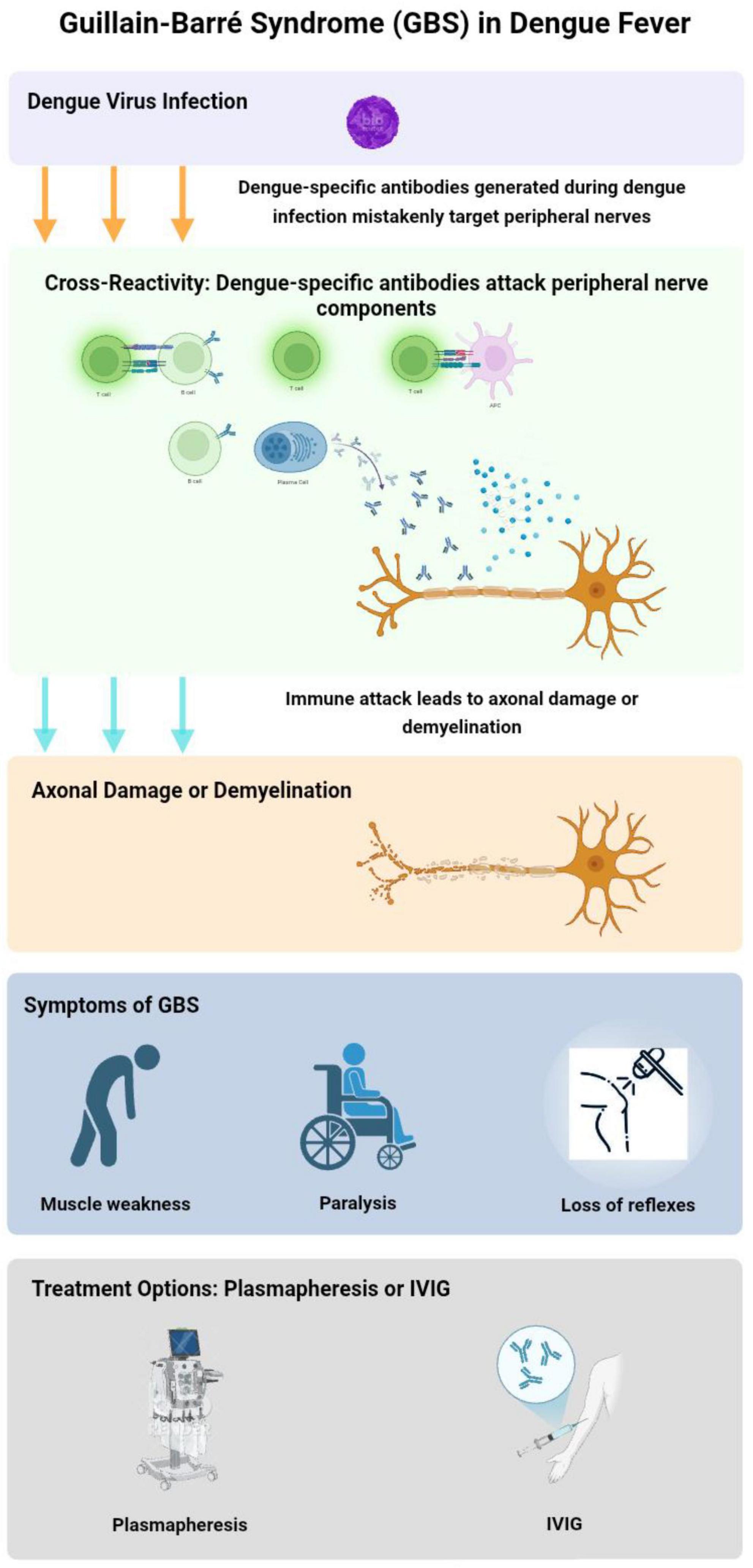
Figure 2. Pathogenesis of Guillain-Barré syndrome (GBS) in dengue fever. This flowchart illustrates the development of GBS during dengue fever. The process begins with dengue virus (DENV) infection, leading to immune cross-reactivity where viral antibodies attack peripheral nerve components. This results in axonal damage or demyelination, manifesting as muscle weakness, paralysis, and loss of reflexes Treatment options include plasmapheresis and intravenous immunoglobulin (IVIg) therapy to reduce immune-mediated nerve damage. The Figure was designed using BioRender.com.
A case reported by Lim et al. (2023) in June 2023 involved a 49 years-old man with a known history of type 2 diabetes and dyslipidemia, who presented with symmetrical weakness of the lower limbs just 2 days after the onset of DF (febrile phase). This presentation is uncommon, as other case studies indicate that GBS typically occurs 1 or 2 weeks after the onset of DF (Lim et al., 2023).
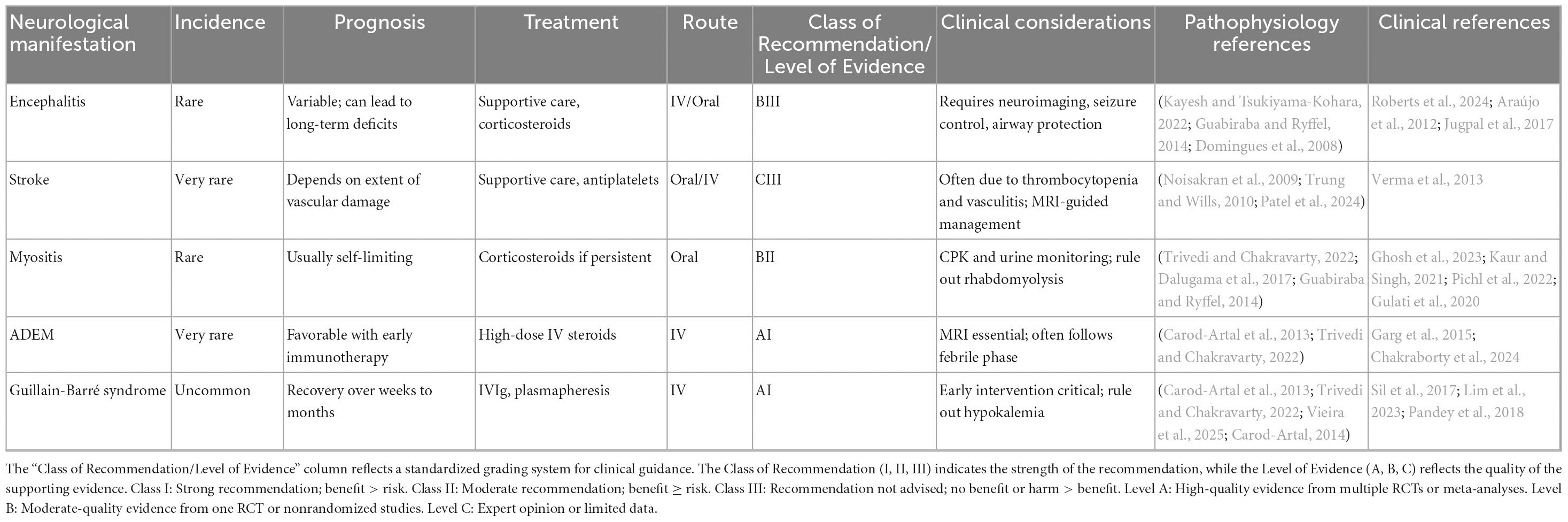
Cranial nerve involvement includes oculomotor nerve palsy, optic neuritis, long thoracic neuropathy and isolated palsies of sixth and seventh and phrenic nerves (Trivedi and Chakravarty, 2022; Trung and Wills, 2010). Optic neuritis may occur post-dengue fever, with symptoms like decreased vision, weakened color perception, pupillary defects, and scotomas. Although optic neuritis typically affects both eyes, some patients may experience unilateral symptoms (Noisakran et al., 2009). The exact cause of post dengue optic neuritis after is unclear, but it may involve complex vascular leakage (Araújo et al., 2012). The condition may result from direct viral infection or immune-mediated responses following the illness (Garg et al., 2015). The delayed onset of visual symptoms likely favors an immune-mediated process over direct viral infection. However, the precise pathogenesis is still undetermined. Affected patients generally present with blurred vision, weakened color perception, pupillary defects, and scotomas. Both eyes are usually involved, but some patients may show unilateral symptoms. Early recognition is essential to prevent permanent ocular complications (Jugpal et al., 2017; Lim et al., 2023). Differentiating between localized inflammation caused by direct viral replication in the central nervous system, such as in encephalitis or meningitis, and systemic or immune-mediated inflammation, seen in conditions like encephalopathy or Guillain-Barré Syndrome, is crucial to prevent diagnostic confusion. This distinction highlights their unique underlying mechanisms and plays a vital role in guiding clinical decisions (Ellul and Solomon, 2018). Ocular manifestations of dengue are managed symptomatically, often involving the use of anti-inflammatory and immunosuppressive agents. However, it remains uncertain whether observed improvements are due to therapeutic interventions or the natural course of recovery. The primary focus of treatment is supportive care, with corticosteroids showing potential benefits in the early stages of the disease (Wang et al., 2024; Figure 3).
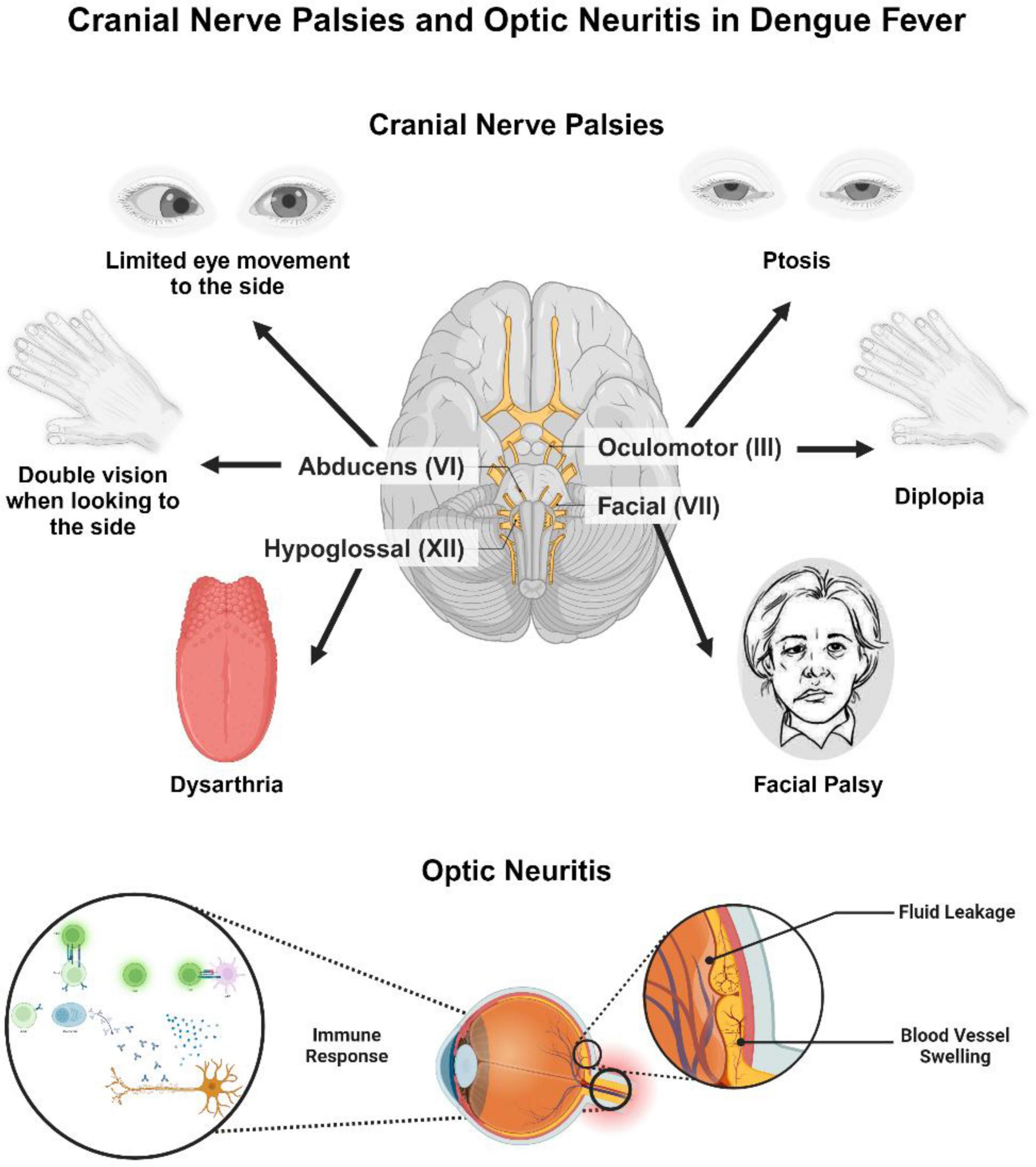
Figure 3. Cranial nerve palsies and optic neuritis in dengue fever. This figure illustrates cranial nerve involvement and optic neuritis as complications of dengue fever. Affected cranial nerves include the oculomotor nerve (III), abducens nerve (VI), facial nerve (VII), and hypoglossal nerve (XII), leading to symptoms such as ptosis, diplopia, limited lateral eye movement, facial palsy, and dysarthria. The lower section depicts optic neuritis, with immune-mediated inflammation of the optic nerve, leading to vascular leakage and vasculitis. The immune response contributing to this inflammation is also depicted, indicating the underlying pathophysiological mechanisms. The Figure was designed using BioRender.com.
2.4 Acute disseminated encephalomyelitis (ADEM)
Acute disseminated encephalomyelitis is an uncommon inflammatory demyelinating disease of the CNS that usually progresses in a monophasic course and is characterized by multifocal white matter involvement. ADEM frequently appears during the recovery stage of a dengue disease. Seizures, focal neurological abnormalities, and altered awareness are possible clinical manifestations; they often happen after the febrile phase has passed (Yip et al., 2012). The underlying pathophysiology is a temporary autoimmune response that targets unknown self-antigens or myelin. Diagnostic techniques like CSF analysis and brain MRI can yield important information. Methylprednisolone administered by pulse intravenously has shown therapeutic effectiveness during the active period of ADEM. Two weeks after an uncomplicated dengue episode, a 38 years-old male developed ADEM, according to a noteworthy case documented by Chakraborty et al. (2024). This uncommon condition was characterized by lower limb weakness and numbness, bowel and bladder incontinence, and a band-like feeling in the T4 dermatome. Importantly, no indications of cerebral involvement were seen. The brain and spinal cord have demyelinating lesions, according to imaging scans. Intravenous corticosteroids were used to treat the patient with success, highlighting the need of quick action in treating this uncommon dengue-related disease (Chakraborty et al., 2024).
Bradykinesia, tremor, postural instability, and stiffness are the four cardinal signs of Parkinsonism that are present after viral infections. Inflammation-induced disruption of dopaminergic transmission is thought to be the etiology of viral parkinsonism. The development of parkinsonism during or after the course of dengue disease and clinical evidence of dengue infection verified by laboratory testing are diagnostic criteria for dengue-associated parkinsonism. The majority of individuals with this condition are men, according to a research, and they exhibit lymphocytosis, speech problems, and an emotionless facial expression (Hopkins et al., 2022). Opsoclonus-myoclonus ataxia syndrome (OMAS) is a neurological disorder characterized by involuntary eye movements and muscle spasms. Its pathophysiology involves autoimmune dysfunction targeting Purkinje cells in the cerebellar dorsal vermis. This leads to motor control disruption by disinhibiting the oculomotor fastigial region and impairing omnipause cells in the pontine raphe nucleus. Neurological complications of dengue-related OMAS can be categorized into direct neurotropic effects, systemic neurological effects due to metabolic disturbances, and immune-mediated effects such as ADEM, GBS, and OMAS itself. Treatment often includes high-dose intravenous methylprednisolone followed by tapered oral prednisolone (Garg et al., 2023). Numerous viruses, such as the measles, dengue, Epstein-Barr virus, and varicella-zoster virus, can produce cerebellitis, a consequence of viral encephalitis. Ataxia may be the outcome of immune-mediated cerebellar dysfunction or direct viral invasion. There have only been five cases of dengue-associated cerebellitis documented in 2018. With the exception of one instance, which had T2 hyperintensity in the cerebellum, the majority of patients had normal brain MRIs. Another report noted a “bright MCP sign” with T2-weighted hyperintensities in the cerebellar peduncle, successfully treated with intravenous methylprednisolone (Costa and Sato, 2020). Diagnostic and imaging tools for neurological manifestations shown in Table 2. The neurological manifestations of DENV infection are shown in Figure 4.
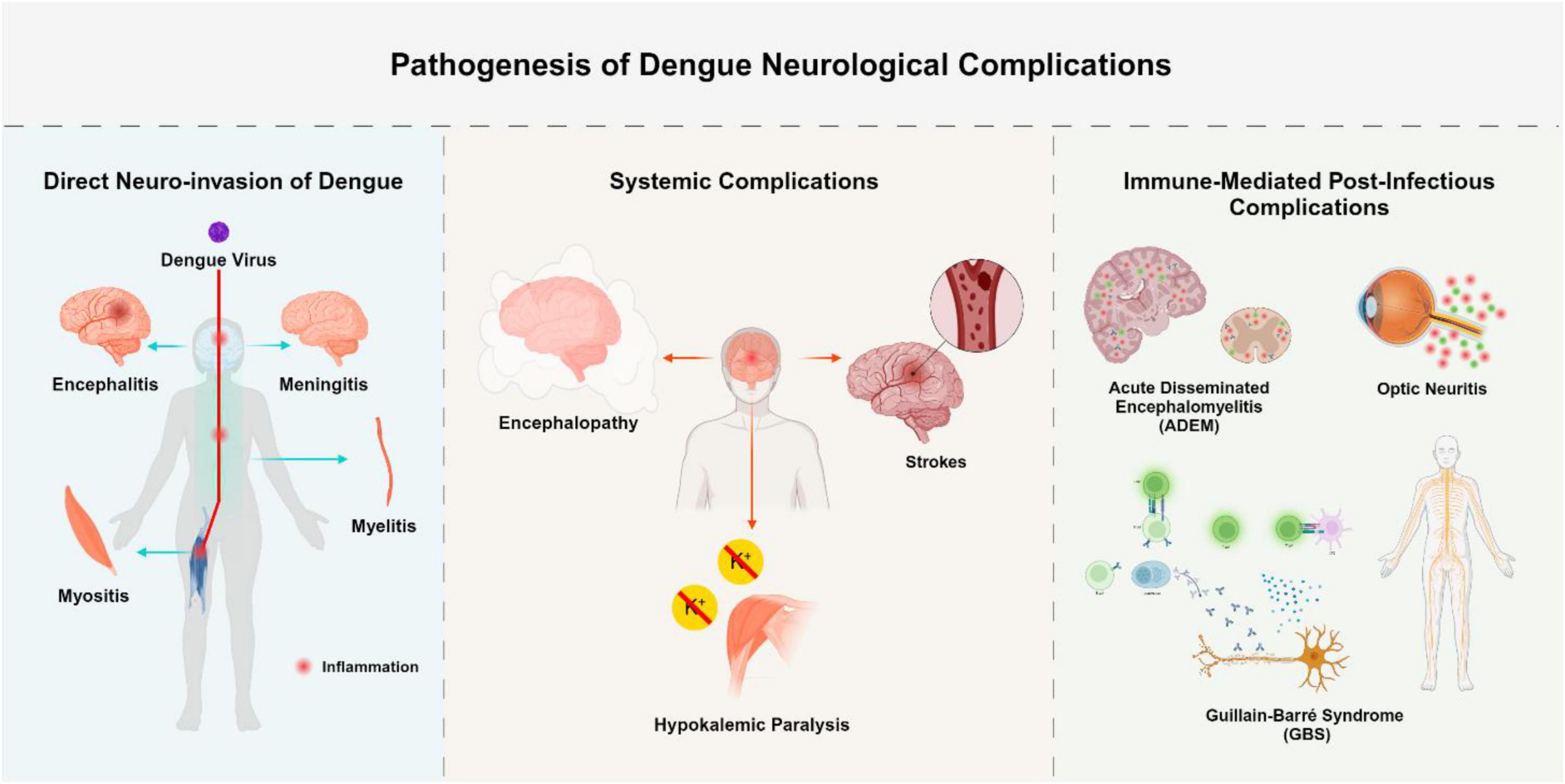
Figure 4. Pathogenesis of dengue neurological complications. This figure summarizes the neurological complications of dengue virus (DENV) based on three pathophysiological pathways: Direct Neuroinvasion [via viral entry and localized central nervous system (CNS) inflammation due to viral replication]; Systemic Complications (via indirect mechanisms such as vasculopathy, coagulopathy, or metabolic disturbance); Immune-Mediated Complications (via post-infectious immune responses leading to demyelination or neuronal injury). The Figure was designed using BioRender.com.
3 Management
DF continues to lack a specific antiviral treatment, necessitating primarily supportive care strategies (Trivedi and Chakravarty, 2022). These approaches aim to stabilize the patient by focusing on meticulous fluid management, blood pressure monitoring, and fever reduction. Vigilant hematological surveillance is essential to identify complications, such as thrombocytopenia or hemoconcentration, which are characteristic of severe cases. For patients displaying neurological manifestations, a thorough clinical evaluation is crucial to discern potential contributing factors, including electrolyte disturbances, intracranial hemorrhages, and organ failures such as hepatic or renal insufficiency (Patel et al., 2024). Management of neurological symptoms associated with DF involves airway protection in patients with altered mental status or seizures, ensuring adequate hydration and nutrition, and careful observation of consciousness levels. Because non-hepatotoxic anti-epileptic drugs reduce the risk of liver damage in a weakened hepatic environment, their usage is recommended for the management of seizures (Kakde and Khatib, 2024). The effectiveness of corticosteroids and antiviral drugs for severe neurological complications like dengue encephalopathy or encephalitis is still unknown, especially in critical care settings when these treatments have not demonstrated clear advantages (Prabhat et al., 2020). Both direct invasion of the CNS and ADE, which increases the immune response, are ways that the DENV causes neurological damage. Supportive treatments, such as careful monitoring of awareness, airway protection, and seizure management, are therefore designed to meet the particular neurological signs. Appropriate measures must be taken when there is high intracranial pressure. While there is currently little evidence, IVIg with pulsed steroids may provide therapeutic advantages for immune-mediated disorders such as GBS or ADEM. Treatments for GBS include plasmapheresis and high-dose IVIg, which are usually given at a dosage of 2 mg/kg to enhance recovery (Carod-Artal et al., 2013; Lim et al., 2023). Neurorehabilitation is crucial for the post-acute management of patients experiencing neurological complications related to dengue, especially when residual motor, cognitive, or sensory deficits are present. Employing strategies such as physical therapy, occupational therapy, and cognitive rehabilitation can greatly improve functional recovery and overall quality of life. While the acute treatment phase mainly focuses on supportive care, seizure management, and immunotherapy, incorporating structured rehabilitation into long-term care plans is essential. This approach is particularly important for addressing conditions like Guillain-Barré syndrome, post-encephalitic cognitive impairment, and ataxia resulting from cerebellitis (Khan and Amatya, 2012; Castell et al., 2025). Management and treatment strategies of neurological manifestation shown in Table 3, and complications of dengue-associated viral encephalitis and their management shown in Table 4.
4 Conclusion
With its complex pathophysiology and neurological consequences that significantly increase disease morbidity, DENV poses a serious public health risk. Through complex processes including direct viral neuroinvasion, cytokine dysregulation, and changes in vascular permeability, the virus can cause neurological symptoms including encephalitis, meningitis, and immune-mediated disorders like GBS. Pathophysiologically, the development of neurological problems depends mainly on DENV’s interactions with the blood-brain barrier, immune response regulation, and systemic inflammation. Although CSF analyses and neuroinflammatory markers have improved our knowledge of these processes, the exact molecular mechanisms are still not well-understood. Effective intervention is limited by the lack of specific antiviral treatments, even with advancements in diagnostics and supportive care techniques. Neurological outcomes further emphasize the critical need for comprehensive methods that include the development of immunomodulatory treatments, new diagnostic tools, and public health measures. Reducing the worldwide neurological burden of DENV and improving patient outcomes will require understanding the complex processes underlying the neurotropism and immunological interactions of the diseases.
Author contributions
RamN: Investigation, Resources, Writing – original draft. EP: Data curation, Software, Writing – original draft. AM: Data curation, Investigation, Writing – original draft. SK: Conceptualization, Investigation, Methodology, Writing – original draft. AH: Investigation, Methodology, Resources, Writing – original draft. RayN: Data curation, Formal analysis, Writing – review & editing. SH: Investigation, Methodology, Visualization, Writing – original draft. VO: Conceptualization, Data curation, Funding acquisition, Project administration, Supervision, Writing – review & editing. ME: Conceptualization, Project administration, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank to Cancer Research Center, Semnan University of Medical Sciences, Semnan, Iran.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akhtar, U., Mushtaq, M., Nisar, S., Naeem, A., and Naim, F. (2023). Dengue and Hypokalemic paralysis: A rare association. Cureus 15:e44705. doi: 10.7759/cureus.44705
Araújo, F., Nogueira, R., Araújo Mde, S., Perdigão, A., Cavalcanti, L., Brilhante, R., et al. (2012). Dengue in patients with central nervous system manifestations, Brazil. Emerg. Infect. Dis. 18, 677–679. doi: 10.3201/eid1804.111552
Carod-Artal, F. (2014). Neurological manifestations of dengue viral infection. Res. Rep. Trop. Med. 5, 95–104. doi: 10.2147/RRTM.S55372
Carod-Artal, F., Wichmann, O., Farrar, J., and Gascón, J. (2013). Neurological complications of dengue virus infection. Lancet Neurol. 12, 906–919. doi: 10.1016/S1474-4422(13)70150-9
Castell, H., Hilton, O., Ellerington, A., Michael, B. D., and Easton, A. (2025). Nursing patients with encephalitis. Br. J. Neurosci. Nurs. 21, 88–94. doi: 10.12968/bjnn.2023.0029
Chakraborty, S., Narayan, A., Chakraborty, D., Ukil, B., and Singh, S. (2024). Postdengue acute disseminated encephalomyelitis. J. Assoc. Phys. India 72, 94–96. doi: 10.59556/japi.72.0555
Chambers, T., Hahn, C., Galler, R., and Rice, C. (1990). Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44, 649–688. doi: 10.1146/annurev.mi.44.100190.003245
Chen, Y., Liu, R., Cai, W., Jiang, L., Chen, K., Shi, J., et al. (2025). Davunetide promotes structural and functional recovery of the injured spinal cord by promoting autophagy. Neural Regen. Res. 58, 1–11. doi: 10.4103/NRR.NRR-D-24-00154
Costa, B., and Sato, D. (2020). Viral encephalitis: A practical review on diagnostic approach and treatment. J. Pediatr. 96, 12–19. doi: 10.1016/j.jped.2019.07.006
da Fonseca, B., and Fonseca, S. (2002). Dengue virus infections. Curr. Opin. Pediatr. 14, 67–71. doi: 10.1097/00008480-200202000-00012
Dalugama, C., Ralapanawa, U., and Jayalath, T. (2017). Dengue myositis and review of literature. Clin. Case Rep. Res. Trials 2, 16–18. doi: 10.1186/s13256-017-1510-1
Domingues, R., Kuster, G., Onuki de Castro, F. L., Souza, V. A., Levi, J. E., and Pannuti, C. S. (2006). Headache features in patients with dengue virus infection. Cephalalgia 26, 879–882. doi: 10.1111/j.1468-2982.2006.01100.x
Domingues, R., Kuster, G., Onuki-Castro, F., Souza, V., Levi, J., and Pannuti, C. (2008). Involvement of the central nervous system in patients with dengue virus infection. J. Neurol. Sci. 267, 36–40. doi: 10.1016/j.jns.2007.09.040
Ellul, M., and Solomon, T. (2018). Acute encephalitis - diagnosis and management. Clin. Med. 18, 155–159. doi: 10.7861/clinmedicine.18-2-155
Enitan, S. S., Abbas, K., Elrufal, R., Umukoro, S., Tsague, C., Laura, M., et al. (2024). Advancing dengue fever preparedness in Africa: Challenges, resilience, and contributions to global health. Acta Elit Salutis 9, 1–22. doi: 10.48075/aes.v9i1.33267
Farina, A., Villagrán-García, M., and Honnorat, J. (2023). Neurological adverse events of immune checkpoint inhibitors: An update of clinical presentations, diagnosis, and management. Rev. Neurol. 179, 506–515. doi: 10.1016/j.neurol.2023.03.003
Fatima, K., and Syed, N. (2018). Dengvaxia controversy: Impact on vaccine hesitancy. J. Glob. Health 8:010312. doi: 10.7189/jogh.08.020312
Flacco, M., Bianconi, A., Cioni, G., Fiore, M., Calò, G., Imperiali, G., et al. (2024). Immunogenicity, safety and efficacy of the dengue vaccine TAK-003: A meta-analysis. Vaccines 12:70. doi: 10.3390/vaccines12070770
Garg, D., Gupta, A., Tiwari, S., and Sharma, S. (2023). Rapid lingual tremor and parkinsonism in dengue encephalitis. Ann. Indian Acad. Neurol. 26, 586–587. doi: 10.4103/aian.aian_184_23
Garg, R., Malhotra, H., Jain, A., and Malhotra, K. (2015). Dengue-associated neuromuscular complications. Neurol. India 63, 497–516. doi: 10.4103/0028-3886.161990
Garg, R., Malhotra, H., Verma, R., Sharma, P., and Singh, M. (2013). Etiological spectrum of hypokalemic paralysis: A retrospective analysis of 29 patients. Ann. Indian Acad. Neurol. 16, 365–370. doi: 10.4103/0972-2327.116934
Ghosh, A., Sinha, S., and Chowdhury, N. (2023). Acute dengue myositis in a pediatric patient—an uncommon complication of a common disease: A case report. Indian J. Med. Biochem. 27, 58. doi: 10.5005/jp-journals-10054-0224
Goswami, R., Mukherjee, A., Biswas, T., Karmakar, P., and Ghosh, A. (2012). Two cases of dengue meningitis: A rare first presentation. J. Infect. Dev. Ctries 6, 208–211. doi: 10.3855/jidc.2241
Guabiraba, R., and Ryffel, B. (2014). Dengue virus infection: Current concepts in immune mechanisms and lessons from murine models. Immunology 141, 143–156. doi: 10.1111/imm.12188
Gulati, K., Pasi, R., Gupta, A., and Ravi, K. (2020). Dengue fever presenting with severe myositis-an unusual presentation. J. Fam. Med. Prim. Care 9, 6285–6287. doi: 10.4103/jfmpc.jfmpc_1680_20
Guzman, M., Gubler, D., Izquierdo, A., Martinez, E., and Halstead, S. (2016). Dengue infection. Nat. Rev. Dis. Prim. 2:16055. doi: 10.1038/nrdp.2016.55
Hadinegoro, S., Arredondo-García, J., Capeding, M., Deseda, C., Chotpitayasunondh, T., Dietze, R., et al. (2015). Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 373, 1195–1206. doi: 10.1056/NEJMoa1506223
Harraz, O., and Delpire, E. (2024). Recent insights into channelopathies. Physiol. Rev. 104, 23–31. doi: 10.1152/physrev.00022.2023
Henchal, E., and Putnak, J. (1990). The dengue viruses. Clin. Microbiol. Rev. 3, 376–396. doi: 10.1128/CMR.3.4.376
Hopkins, H., Traverse, E., and Barr, K. (2022). Viral parkinsonism: An underdiagnosed neurological complication of Dengue virus infection. PLoS Negl. Trop. Dis. 16:e0010118. doi: 10.1371/journal.pntd.0010118
Hunsperger, E., Yoksan, S., Buchy, P., Nguyen, V., Sekaran, S., Enria, D., et al. (2014). Evaluation of commercially available diagnostic tests for the detection of dengue virus NS1 antigen and anti-dengue virus IgM antibody. PLoS Negl. Trop. Dis. 8:e3171. doi: 10.1371/journal.pntd.0003171
Janssen, H., Bienfait, H., Jansen, C., van Duinen, S., Vriesendorp, R., Schimsheimer, R., et al. (1998). Fatal cerebral oedema associated with primary dengue infection. J. Infect. 36, 344–346. doi: 10.1016/s0163-4453(98)94783-1
Jugpal, T., Dixit, R., Garg, A., Gupta, S., Jain, V., Patel, R., et al. (2017). Spectrum of findings on magnetic resonance imaging of the brain in patients with neurological manifestations of dengue fever. Radiol. Bras. 50, 285–290. doi: 10.1590/0100-3984.2016.0048
Kakde, U., and Khatib, M. (2024). Neurological Complications in dengue among males of the adult age group. Cureus 16:e51586. doi: 10.7759/cureus.51586
Kaur, M., and Singh, P. (2021). Acute dengue myositis: A case report. AMEIs Curr. Trends Diagn. Treat. 5, 102–103. doi: 10.5005/jp-journals-10055-0125
Kayesh, M., and Tsukiyama-Kohara, K. (2022). Mammalian animal models for dengue virus infection: A recent overview. Arch. Virol. 167, 31–44. doi: 10.1007/s00705-021-05298-2
Khan, F., and Amatya, B. (2012). Rehabilitation interventions in patients with acute demyelinating inflammatory polyneuropathy: A systematic review. Eur. J. Phys. Rehabil. Med. 48, 507–522.
Kondziella, D., and Waldemar, G. (2023). Clinical History and Neuroanatomy:“Where Is the Lesion?”. Neurology at the Bedside. Cham: Springer, 5–83.
Kothur, K., Wienholt, L., Brilot, F., and Dale, R. C. (2016). CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: A systematic review. Cytokine 77, 227–237. doi: 10.1016/j.cyto.2015.10.001
Lee, I., Bos, S., Li, G., Wang, S., Gadea, G., Desprès, P., et al. (2018). Probing Molecular insights into zika virus-host interactions. Viruses 10:233. doi: 10.3390/v10050233
Lim, C., Kaisbain, N., and Lim, W. J. (2023). A rare combination: Dengue fever complicated with Guillain-Barre syndrome. Cureus 15:e40957. doi: 10.7759/cureus.40957
Lnu, P., Sehgal, V., Bhalla Sehgal, L., Gulati, N., and Kapila, S. (2022). The spectrum of MRI findings in dengue encephalitis. Cureus 14:e29048. doi: 10.7759/cureus.29048
Ludlow, M., Kortekaas, J., Herden, C., Hoffmann, B., Tappe, D., Trebst, C., et al. (2016). Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 131, 159–184. doi: 10.1007/s00401-015-1511-3
Madi, D., Achappa, B., Ramapuram, J., Chowta, N., Laxman, M., and Mahalingam, S. (2014). Dengue encephalitis-A rare manifestation of dengue fever. Asian Pac. J. Trop. Biomed. 4, S70–S72. doi: 10.12980/APJTB.4.2014C1006
Malhotra, H., and Garg, R. (2014). Dengue-associated hypokalemic paralysis: Causal or incidental? J. Neurol. Sci. 340, 19–25. doi: 10.1016/j.jns.2014.03.016
Marti, M., Nohynek, H., Duclos, P., O’Brien, K., and Hombach, J. (2024). The strategic advisory group of experts (SAGE) on immunization-past, present and future. Vaccines 12:1402. doi: 10.3390/vaccines12121402
Martina, B., Koraka, P., and Osterhaus, A. (2009). Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 22, 564–581. doi: 10.1128/CMR.00035-09
Martinez, L., Lin, L., Blaylock, J., Lyons, A., Bauer, K., De La Barrera, R., et al. (2015). Safety and immunogenicity of a dengue virus serotype-1 purified-inactivated vaccine: Results of a phase 1 clinical trial. Am. J. Trop. Med. Hyg. 93, 454–460. doi: 10.4269/ajtmh.14-0819
Messina, J., Brady, O., Scott, T., Zou, C., Pigott, D., Duda, K., et al. (2014). Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 22, 138–146. doi: 10.1016/j.tim.2013.12.011
Murthy, J. (2010). Neurological complication of dengue infection. Neurol. India 58, 581–584. doi: 10.4103/0028-3886.68654
Mustafá, Y., Meuren, L., Coelho, S., and de Arruda, L. (2019). Pathways exploited by flaviviruses to counteract the blood-brain barrier and invade the central nervous system. Front. Microbiol. 10:525. doi: 10.3389/fmicb.2019.00525
Noisakran, S., Chokephaibulkit, K., Songprakhon, P., Onlamoon, N., Hsiao, H., Villinger, F., et al. (2009). A re-evaluation of the mechanisms leading to dengue hemorrhagic fever. Ann. N. Y. Acad. Sci. 1171, E24–E35. doi: 10.1111/j.1749-6632.2009.05050.x
Pandey, S., Garg, R., Malhotra, H., Kumar, N., and Uniyal, R. (2018). Simultaneous occurrence of axonal guillain-barré syndrome in two siblings following dengue infection. Ann. Indian Acad. Neurol. 21, 315–317. doi: 10.4103/aian.AIAN_454_17
Patel, J., Saiyed, F., and Hardaswani, D. (2024). Dengue Fever Accompanied by Neurological Manifestations: Challenges and Treatment. Cureus 16:e60961. doi: 10.7759/cureus.60961
Pichl, T., Wedderburn, C., Hoskote, C., Turtle, L., and Bharucha, T. A. (2022). systematic review of brain imaging findings in neurological infection with Japanese encephalitis virus compared with Dengue virus. Int. J. Infect. Dis. 119, 102–110. doi: 10.1016/j.ijid.2022.03.010
Prabhat, N., Ray, S., Chakravarty, K., Kathuria, H., Saravana, S., Singh, D., et al. (2020). Atypical neurological manifestations of dengue fever: A case series and mini review. Postgrad. Med. J. 96, 759–765. doi: 10.1136/postgradmedj-2020-137533
Roberts, J., Kapadia, R., Pastula, D., and Thakur, K. (2024). Public health trends in neurologically relevant infections: A global perspective. Ther. Adv. Infect. Dis. 11:20499361241274206. doi: 10.1177/20499361241274206
Sigera, P., Rajapakse, S., Weeratunga, P., De Silva, N., Gomes, L., Malavige, G., et al. (2021). Dengue and post-infection fatigue: Findings from a prospective cohort-the Colombo Dengue Study. Trans. R. Soc. Trop. Med. Hyg. 115, 669–676. doi: 10.1093/trstmh/traa110
Sil, A., Biswas, T., Samanta, M., Konar, M., De, A., and Chaudhuri, J. (2017). Neurological manifestations in children with dengue fever: An Indian perspective. Trop. Doct. 47, 145–149. doi: 10.1177/0049475516679788
Soares, C., Cabral-Castro, M., Peralta, J., Freitas, M., and Puccioni-Sohler, M. (2010). Meningitis determined by oligosymptomatic dengue virus type 3 infection: Report of a case. Int. J. Infect. Dis. 14, e150–e152. doi: 10.1016/j.ijid.2009.03.016
Soares, C., Faria, L., Peralta, J., de Freitas, M., and Puccioni-Sohler, M. (2006). Dengue infection: Neurological manifestations and cerebrospinal fluid (CSF) analysis. J. Neurol. Sci. 249, 19–24. doi: 10.1016/j.jns.2006.05.068
Solomon, T., Dung, N., Vaughn, D., Kneen, R., Thao, L., Raengsakulrach, B., et al. (2000). Neurological manifestations of dengue infection. Lancet 355, 1053–1059. doi: 10.1016/S0140-6736(00)02036-5
Sridhar, S., Luedtke, A., Langevin, E., Zhu, M., Bonaparte, M., Machabert, T., et al. (2018). Effect of dengue serostatus on dengue vaccine safety and efficacy. N. Engl. J. Med. 379, 327–340. doi: 10.1056/NEJMoa1800820
Tricou, V., Sáez-Llorens, X., Yu, D., Rivera, L., Jimeno, J., Villarreal, A., et al. (2020). Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2-17 years: A randomised, placebo-controlled, phase 2 trial. Lancet 395, 1434–1443. doi: 10.1016/S0140-6736(20)30556-0
Trivedi, S., and Chakravarty, A. (2022). Neurological complications of dengue fever. Curr. Neurol. Neurosci. Rep. 22, 515–529. doi: 10.1007/s11910-022-01213-7
Trung, D., and Wills, B. (2010). Systemic vascular leakage associated with dengue infections –the clinical perspective. Curr. Top. Microbiol. Immunol. 338, 57–66. doi: 10.1007/978-3-642-02215-9_5
Verma, R., Sahu, R., Singh, A., and Atam, V. (2013). Dengue infection presenting as ischemic stroke: An uncommon neurological manifestation. Neurol. India 61, 317–318. doi: 10.4103/0028-3886.115083
Vieira, G., Campos, M., de Souza, F., Bandeira, I., Breis, L., Parolin, L., et al. (2025). Spectrum of neuroimmunological manifestations of dengue fever. Ann. Neurosci. 32, 75–77. doi: 10.1177/09727531241288313
Wang, C., Castillo, A., Cortes-Bejarano, F., Lopez, E., de Souza, E., and Wu, L. (2024). An update on the ocular manifestations of dengue. Taiwan J. Ophthalmol. 14, 540–547. doi: 10.4103/tjo.TJO-D-23-00106
Westaway, E., Brinton, M., Gaidamovich, S. Y., Horzinek, M. C., Igarashi, A., Kääriäinen, L., et al. (1985). Flaviviridae. Intervirology 24, 183–192. doi: 10.1159/000149642
WHO Guidelines Approved by the Guidelines Review Committee (2009). Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization.
Yang, N., Lai, Y., Yu, G., Zhang, X., Shi, J., Xiang, L., et al. (2025). METTL3-dependent m6A modification of SNAP29 induces “autophagy-mitochondrial crisis” in the ischemic microenvironment after soft tissue transplantation. Autophagy 52, 1–24. doi: 10.1080/15548627.2025.2493455
Keywords: dengue, DENV, encephalitis, neuroinvasion, neurological disorder
Citation: Naderian R, Paraandavaji E, Maddah AH, Keshavarzi S, Habibian A, Naderian R, Hosseini SM, Oksenych V and Eslami M (2025) Pathophysiology and clinical implications of dengue-associated neurological disorders. Front. Microbiol. 16:1536955. doi: 10.3389/fmicb.2025.1536955
Received: 29 November 2024; Accepted: 31 August 2025;
Published: 12 September 2025.
Edited by:
Kok Keng Tee, Kunming Medical University, ChinaReviewed by:
Hong Yien Tan, Xiamen University Malaysia, MalaysiaKristen Funk, University of North Carolina at Charlotte, United States
Copyright © 2025 Naderian, Paraandavaji, Maddah, Keshavarzi, Habibian, Naderian, Hosseini, Oksenych and Eslami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Majid Eslami, bS5lc2xhbWlAc2VtdW1zLmFjLmly; Valentyn Oksenych, dmFsZW50eW4ub2tzZW55Y2hAdWliLm5v
 Ramtin Naderian
Ramtin Naderian Elham Paraandavaji3
Elham Paraandavaji3 Valentyn Oksenych
Valentyn Oksenych Majid Eslami
Majid Eslami