- 1State Key Laboratory of Mariculture Biobreeding and Sustainable Goods, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao, Shandong, China
- 2Laboratory for Marine Fisheries Science and Food Production Processes, Laoshan Laboratory, Qingdao, Shandong, China
- 3Weimi Biotechnology Co., Ltd., Hangzhou, Zhejiang, China
Lysine lactylation (Klac) is a recently discovered post-translational modification (PTM) widespread across species, playing a crucial role in cellular processes and associated with pathological conditions. Photobacterium damselae subsp. damselae, a marine bacterium within the Vibrionaceae family, is a notable pathogen in aquaculture, offering a valuable model for investigating the evolution of pathogenicity from environmental ancestors and assessing the impact of genetic diversity-generating mechanisms on bacterial populations. Therefore, we conducted the first systematic analysis of Klac modification in P. damselae using highly sensitive proteomic techniques. A total of 1,352 Klac modification sites were identified on 486 proteins. The analysis of GO annotations and KEGG pathways for the identified Klac-modified proteins revealed their widespread distribution in subcellular compartments, indicating their involvement in diverse cellular functions and metabolic pathways, particularly in ribosome and protein biosynthesis, as well as central carbon metabolism. Furthermore, 20 highly connected Klac protein clusters were extracted from the global protein-protein interaction (PPI) network, indicating that Klac modification tends to occur on proteins associated with specific functional clusters. These findings enhance our understanding of the functional role of Klac modification and provide a dataset for further exploration of its impact on the physiology and biology of P. damselae.
Introduction
Photobacterium damselae subsp. damselae, formerly designated as Vibrio damsela, is a marine bacterium affiliated with the Vibrionaceae family. Nonetheless, based on DNA-DNA hybridization data and 16S rRNA sequence analysis, it has been taxonomically categorized within the species P. damselae, coexisting with another fish bacterial pathogen, P. damselae subsp. piscicida (formerly identified as Pasteurella piscicida) (Gauthier et al., 1995). It is currently recognized as an important bacterial pathogen in marine aquaculture, similar to various Vibrio species such as V. parahaemolyticus, and has been detected in various aquaculture species in different countries and regions (Osorio et al., 2018). Moreover, due to the ability of most of P. damselae subs. damselae strains to thrive at 37°C (a temperature inhibitory for subsp. piscicida), there is a potential for parasitism and infection in homoiothermic animals (Rivas et al., 2013), potentially culminating in opportunistic infections in humans and evolving into fatal necrotizing fasciitis (Rivas et al., 2013; Yamane et al., 2004). Presently, the virulence factors and pathogenic mechanisms of the bacterium remain incompletely elucidated; however, certain virulence factors have been reported and reviewed in two papers (Dong et al., 2022; Li et al., 2023). The authors of one of the two papers, Osorio et al. (2018), proposed that P. damselae subs. damselae, together with other pathogenic members of the Vibrionaceae family, constitutes an excellent model system for exploring the evolution of pathogenicity from environmental ancestors and the impact of genetic diversity-generating mechanisms on bacterial populations. Despite the shared environmental similarities among all Vibrionaceae bacteria, their dizzying diversity in pathobiology and adaptive gene functions results in each species within the Vibrionaceae family exhibiting its distinctive biological strategy. P. damselae subs. damselae is no exception, and, in fact, it represents a fascinating biological model, the elucidation of whose adaptive molecular mechanisms of biology and pathogenesis is undoubtedly warranted. Consequently, performing proteomic analysis of post-translational modifications in P. damselae subs. damselae will contribute to unveiling its physiological processes.
Protein post-translational modification (PTM), which involves the dynamic and reversible modification of proteins, is considered one of the most effective strategies for altering protein properties, expanding the functional diversity of existing proteins, and enhancing the cellular control of various biological processes and metabolic pathways (Pan et al., 2015). Extensive research on PTMs in both eukaryotic and prokaryotic cells has consistently identified lysine residues in proteins as the most prevalent targets for modification. In recent years, there has been extensive research on lysine modifications in both eukaryotic and prokaryotic cells, with acylation modifications of lysine being a widely identified form of modification. These modifications include methylation (Kme), acetylation (Kac), crotonylation (Kcr), butyrylation (Kbu), glutarylation (Kglu), succinylation (Ksucc), malonylation (Kmal), propionylation (Kpr), ubiquitination (Kub), 2-hydroxyisobutyrylation (Khib) (Pan et al., 2015), and lactylation (Klac or Kla), the latter being a recent discovery made 5 years ago (Zhang et al., 2019).
Recent data indicate that lactate functions not only as a readily accessible fuel and a metabolic buffer bridging glycolysis and oxidative phosphorylation within cells and intracellular compartments, but also as a multifunctional signaling molecule that acts as a coordinator of whole-body metabolism. Lactate can induce various biological effects, such as reducing fat breakdown, modulating the immune system, exerting anti-inflammatory effects, promoting wound healing, and enhancing exercise performance in association with gut microbiota (Lee, 2021). Therefore, exploring protein lactylation not only contributes to elucidating the physiological functions of lactate but, more importantly, by revealing the functions and classifications of proteins modified by lactylation, it facilitates a deeper understanding of the potential regulatory roles of lactylation in physiological functions.
Lactylation at lysine residue (Klac) modification is a novel PTM first discovered on histones in mammalian cells in 2019 (Zhang et al., 2019). It has been observed that Klac modification on histones modulates macrophage polarization and state, and influences cellular metabolic reprogramming in pluripotent stem cells and non-small cell lung cancer, and can even induce tumorigenesis (Dong et al., 2022; Li et al., 2023). Subsequent investigations have shown that Klac modification extends to non-histone proteins in various subcellular compartments and proteins in prokaryotic cells. To date, Klac modification has been extensively identified in various organisms, including humans (Cheng et al., 2024; Hong et al., 2023; Lin et al., 2023; Sung et al., 2023; Wu, 2023; Yang Y. H. et al., 2023; Yang Z. et al., 2023; Yao et al., 2023; Zhang et al., 2019), mice (Hagihara et al., 2021; Yang et al., 2022; Huang et al., 2023), rats (Yao et al., 2023), insects (An et al., 2022), plants [rice (Meng et al., 2021) and wheat (Zhu et al., 2023)], fungi [Botrytis cinerea (Gao et al., 2020)] and Phialophora verrucosa [ Song et al., 2022)], algae [Nannochloropsis oceanica (Wang et al., 2023)], parasites [Toxoplasma gondii (Yin et al., 2022) and Trypanosoma brucei (Zhang et al., 2021)], and bacteria [Escherichia coli (Dong et al., 2022) and Streptococcus mutans (Li et al., 2023)]. These studies collectively demonstrate the evolutionary conservation of Klac modification across diverse organisms. As a reversible PTM, it dynamically regulates protein function, structure, and intracellular interactions, playing crucial roles in various cellular processes such as cellular metabolism, signaling, and gene expression regulation. Moreover, Klac modification is associated with pathological conditions. Currently, the investigation of protein Klac modification represents an active and burgeoning research area.
Accordingly, P. damselae was employed as a representative bacterial strain of the Vibrioideae family, and for the first time, we conducted a systematic identification and analysis of Klac modification in this bacterium using nano-liquid chromatography-tandem mass spectrometry (nano LC-MS/MS) coupled with affinity purification. A total of 1,352 Klac modification sites on 486 proteins were identified, covering various subcellular localizations and diverse biological processes. These findings enhance our understanding of the functional roles of Klac modification and provide a dataset for further exploration of Klac modification in the physiology and biology of P. damselae.
Materials and methods
Bacterial strains and growth conditions
P. damselae subs. damselae was employed in this study. The strain, stored at −80°C, underwent thawing at room temperature. Subsequently, it was activated by inoculating onto Tryptic Soy Broth (TSB) solid agar plates and incubated at 28°C for 24 h. Single colonies obtained were subcultured twice, and PCR analysis was performed on the 16S rDNA and ureC genes to confirm the absence of contamination during activation and subculturing. Once the strain was correctly identified, it was inoculated into 200 mL liquid TSB medium and subjected to shaking at 28°C until the OD600 of the bacterial culture reached approximately 1.0. The bacterial cells were then collected by centrifugation at 8000 rpm for 5 min at 4°C. The collected cells were washed three times with pre-cooled sterile PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) at 4°C. Three independently cultured bacterial samples were used as biological replicates.
Total protein extraction and in-solution trypsin digestion
The preparation of proteins and in-solution trypsin digestion was conducted following the method described in a previous study (Zhu et al., 2021). Briefly, the bacterial pellets obtained were resuspended in a lysis buffer [8 M urea, 2 mM EDTA, 5 mM DTT, and 1% (v/v) protease inhibitor cocktail III (Calbiochem, United States)], and sonicated on ice water. The protein concentration in the supernatant was then quantified using the 2-D Quant kit (GE Healthcare, United States). Subsequently, the protein sample (3 mg) was reduced with 5 mM dithiothreitol (DTT) at 56°C for 30 min, followed by alkylation with 30 mM iodoacetamide (IAM) in the dark at room temperature for 45 min. Afterward, 5 volumes of pre-cooled methanol at −20°C were added to precipitate the proteins, and the mixture was kept at −20°C overnight. The resulting protein precipitate was subsequently centrifuged at 4°C for 10 min, and the protein pellet obtained was washed twice with pre-cooled methanol and placed at −20°C for 1 h. Finally, the proteins were suspended in 0.1 M triethylammonium bicarbonate (TEAB) buffer and digested with trypsin (Promega, United States) at a 1:50 enzyme/substrate ratio for 15 h at 37°C. The tryptic digestion peptides were desalted using a Strata X C18 SPE column (Phenomenex, United States) and then vacuum-dried.
Affinity enrichment of lysine lactylated peptides
Peptides lactylated at lysine residues were enriched through an immunoprecipitation procedure. Briefly, all of the dried peptides obtained above were redissolved in NETN buffer (100 mM NaCl, 1 mM EDTA, 50 mM Tris-HCl, pH 8.0, and 0.5% NP-40). Subsequently, they were gently rotated overnight at 4°C with anti-lactyl-lysine agarose beads (Micrometer Biotech) at a ratio of 20 μL of beads per milligram of protein. Following enrichment, the supernatant was removed, and the agarose beads were carefully washed three times with NETN buffer and once with double-distilled water. The bound peptides modified with Klac were eluted from the agarose beads with 1% trifluoroacetic acid (TFA), desalted using C18 ZipTips (Millipore, United States), and then vacuum-dried.
Nano HPLC-MS/MS Analysis
The dried Klac-modified peptides were dissolved in buffer A (2% (v/v) acetonitrile (ACN) and 0.1% (v/v) formic acid (FA) in water), followed by nano-LC-MS/MS analysis as previously described.
The dried Klac-modified peptides (see section 2.2 for modification details) were dissolved in buffer A (2% acetonitrile (ACN), 0.1% formic acid (FA), pH 2.5) and transferred entirely to an autosampler vial for nano LC-MS/MS analysis, performed as previously described.
The resulting dried Klac-modified peptides were dissolved in buffer A (2% acetonitrile (ACN) and 0.1% formic acid (FA) in water) and then all samples were used for nano LC-MS/MS analysis following a previously described method (Ye et al., 2021) with slight modifications. Initially, the samples were separated by liquid chromatography (LC) using a reversed-phase C18 analytical column (Thermo Acclaim PepMap RSLC C18 column, 75 μm × 500 mm, 2 μm particles) at a flow rate of 250 nL/min on an UltiMate RSLCnano 3000 system (Thermo Scientific, United States). The gradient was set as follows: 2–10% buffer B (0.1% FA and 80% ACN in water) for 6 min, 10–20% buffer B for 45 min, 20–80% buffer B for 7 min, and held at 80% for 4 min. Subsequently, eluted peptides were ionized and electrosprayed (2.0 kV voltage) into the mass spectrometer (Thermo Scientific Q Exactive HFX, United States) coupled online to the LC system. The mass spectrometric analysis was carried out using a data-dependent mode (DDA) on an Orbitrap-based mass analyzer, with automated switching between full MS scans (350–1,800 m/z) and subsequent MS/MS scans. MS spectra were acquired at a resolution of 60,000 (at m/z 200) with an automatic gain control (AGC) target of 1 × 106. The top 15 most intense precursor ions (charge states ≥ 2 +) were selected for fragmentation by higher-energy collision dissociation (HCD) at 26% normalized collision energy (NCE). MS/MS spectra were acquired at a resolution of 30,000 (at m/z 200) with an AGC target of 1 × 105. Dynamic exclusion was implemented for 30 s to minimize repeated sequencing of abundant precursors. All LC-MS/MS experiments were conducted by Micrometer Biotech Co., Ltd. (Hangzhou, China).
Database searching
All raw data files obtained from mass spectrometry analysis were analyzed using MaxQuant software (v.1.5.2.8). Mass spectra were searched against the protein database of P. damselae (29,932 entries) in UniProt. Carbamidomethylation on cysteine was set as a fixed modification, and oxidation on methionine, lactylation on lysine, and lactylation on protein N-terminal were set as variable modifications. The false discovery rate (FDR) thresholds for peptides, proteins, and modification sites were set at < 1%. Trypsin was set as a cleavage enzyme with up to 4 missing cleavages, and the minimum number of amino acids for peptides was set at 7. The site localization probability for Klac sites was set at > 0.75, and the minimum score for modified peptides was set at > 40. Mass tolerances for precursor ions and fragment ions were set at 10 ppm and 0.02 Da, respectively.
Bioinformatics analysis of lysine lactylated peptides and proteins
To investigate the potential functions of identified Klac-modified peptides and proteins, comprehensive bioinformatics analyses were carried out. Functional classification and enrichment analysis of Gene Ontology (GO) were conducted using the UniProt-GOA database1 (Dimmer et al., 2012). For functional annotation of the Kyoto Encyclopedia of Genes and Genomes (KEGG), the KEGG Automatic Annotation Server (KAAS) tool (v.2.0) was used for pathway annotation, and the online service tool KEGG Mapper was used for mapping (Kanehisa and Goto, 2000). GO term or KEGG pathway enrichment analysis was performed using the DAVID tool (Dennis et al., 2003), and annotation terms with a corrected p-value < 0.05 by Fisher’s exact test were considered significantly enriched. Subcellular location analysis of identified proteins was performed using the WoLF PSORT platform.2 Potential protein-protein interaction relationships among identified proteins were assessed using STRING (v.12),3 and only relationships with a confidence score ≥ 0.7 were considered significant. The protein-protein interaction (PPI) network was constructed and visualized using Cytoscape software (v.3.8) (Shannon et al., 2003), and modules of the PPI network were identified using the Molecular Complex Detection (MCODE) plug-in tool in Cytoscape.
Results and discussion
Global identification of Klac modification in P. damselae
Although Klac modification of proteins was firstly discovered on histones in mammalian cells (Zhang et al., 2019), in recent years, this modification has also been successively found to be present in a wide variety of other organisms, including insects, plants, fungi and bacteria. In bacteria, global identification and characterization of Klac-modified proteins have only been carried out in E. coli (Dong et al., 2022) and S. mutans (Li et al., 2023). A total of 1,047 Klac sites on 478 proteins were identified in the lactylome of E. coli, while in the lactylome of S. mutans, a total of 1,869 Klac sites on 469 proteins were identified under both low- and high-sugar conditions.
P. damselae is a marine bacterium belonging to the Vibrionaceae family, which has been implicated in a variety of fish diseases across its species. Additionally, bacteria in this family possess tremendous metabolic and genetic diversity (Soto, 2022). Therefore, conducting studies on PTM of proteins, including Klac modification, in bacteria of this family contributes to understanding the mechanisms underlying their diversity. In the present study, Klac modifications in P. damselae were identified and systematically analyzed. Total proteins from P. damselae were extracted, and a proteomic research strategy involving affinity enrichment using Klac pan-antibodies and nano LC-MS/MS was employed for Klac-modified protein identification, with three biological replicates performed for verification. After conducting three repeated tests, we identified a total of 1,352 Klac modification sites on 486 proteins. These sites had a localization probability greater than 0.75 in at least one sample across the three replicates (Supplementary Table S1). The identified Klac-modified peptides had scores exceeding 40, and the mass error was within 5 ppm (Figure 1A), meeting the high precision requirements of orbitrap mass spectrometry. This indicates that the mass accuracy of the mass spectrometer is normal and does not adversely affect the qualitative or quantitative analysis of proteins due to excessive mass deviation. Therefore, the identification results meet the requirements for further analysis.
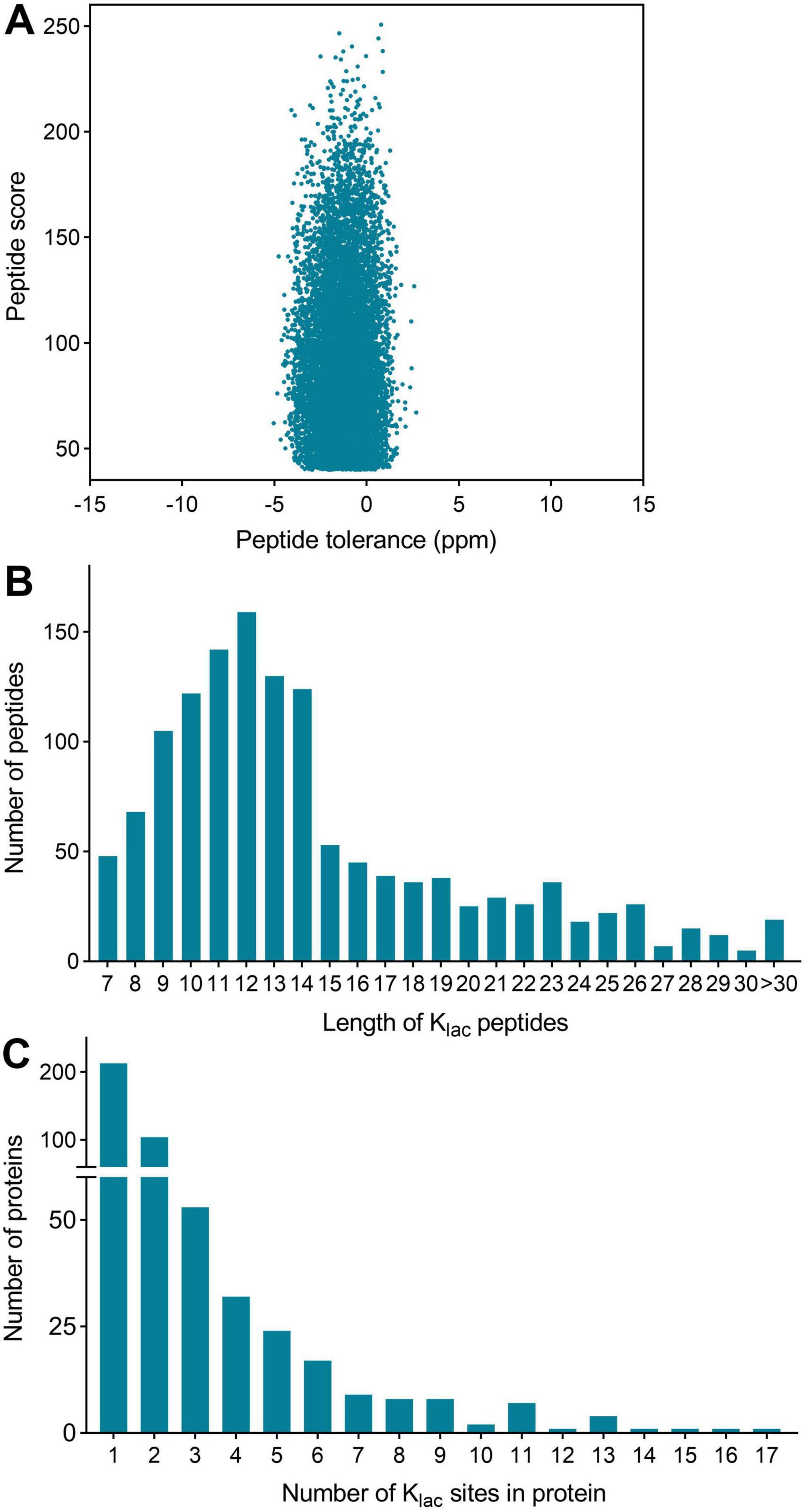
Figure 1. Global analysis of Klac-modified peptides and proteins identified in P. damselae. (A) Peptide tolerance (−5 to 2 ppm) and peptide score (40–264) distribution of all identified Klac-modified peptides. (B) Peptide length distribution of all Klac-modified peptides. (C) Protein distribution of Klac-modified sites contained in each protein.
The length distribution of the Klac-modified peptides was analyzed and found to vary within the range of 7 and 42 amino acid residues, in accordance with the general pattern based on trypsin enzymatic digestion and HCD fragmentation. The majority of these peptides fell within the range of 8–15 residues, constituting 67% of the total identified Klac-modified peptides. Each length category had more than 50 peptides (Figure 1B and Supplementary Table S1). Furthermore, the identified Klac-modified peptides were matched to a total of 486 proteins. The range of Klac modification sites in these proteins varied from 1 to 17, where 44% of proteins had 1 Klac modification site, and proteins with 2–5 Klac modification sites accounted for 21, 11, 7, and 5% respectively. Proteins with more than 5 Klac modification sites constituted 12%, with only 3% having more than 10 sites (Figure 1C and Supplementary Table S1). Notable examples include 50S ribosomal protein L13 with 14 sites (UniProt accession: KFZ67_01775), chaperone protein DnaK with 15 sites (KFZ67_13505), 60 kDa chaperonin with 16 sites (KFZ67_11605), and DNA-directed RNA polymerase subunit β’ with 17 sites (KFZ67_13710) (Supplementary Table S1). The representative MS/MS spectra of Klac-modified peptides at positions K604 and K1133 of the DNA-directed RNA polymerase subunit β’ are shown in Figure 2.
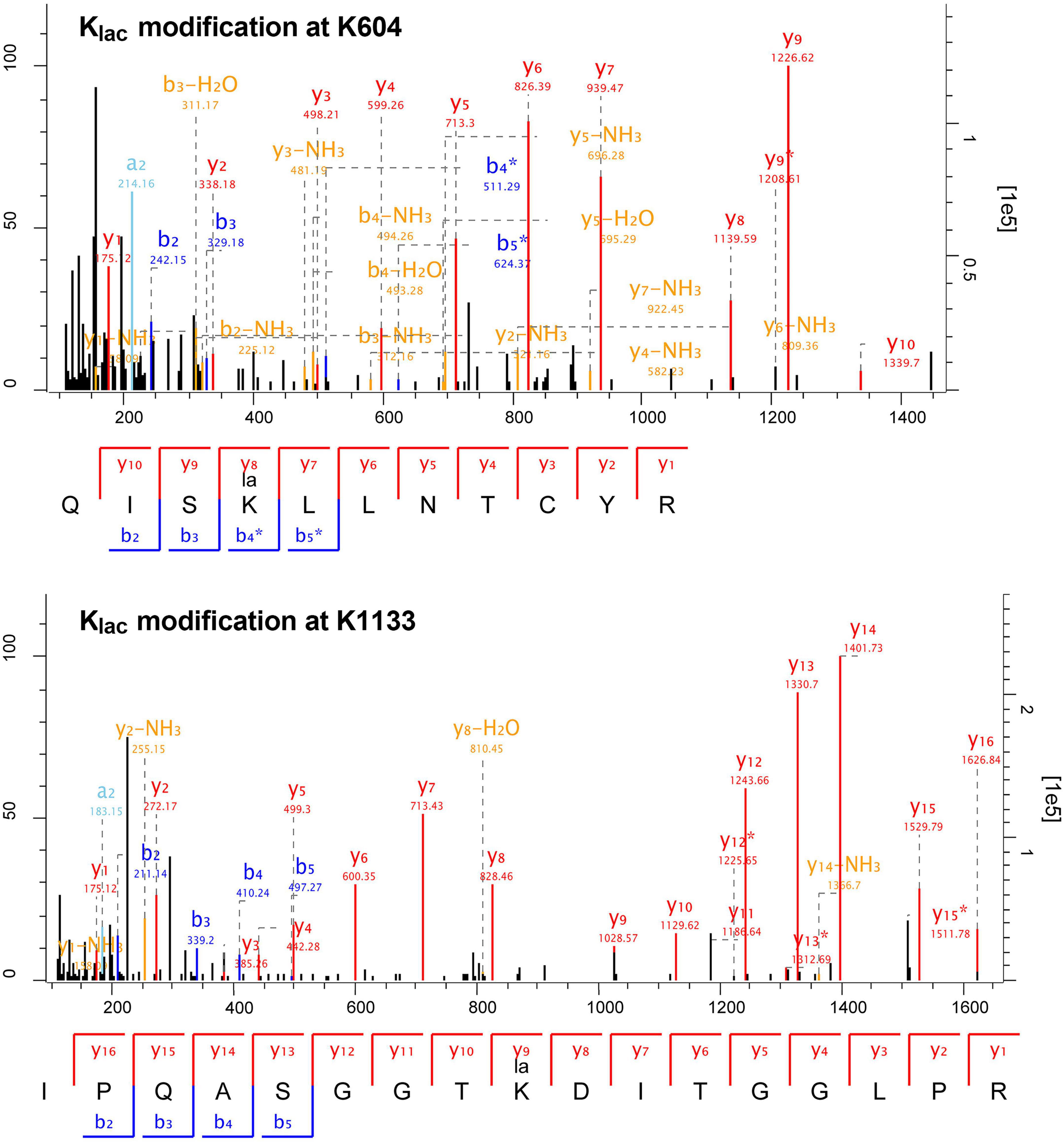
Figure 2. Representative MS/MS spectra of Klac-modified peptides from DNA-directed RNA polymerase subunit β’ (UniProt accession: KFZ67_13710). The fragmentation patterns of the Klac-modified peptides QISKlacLLNTCYR (K604) and IPQASGGTKlacDITGGLPR (K1133) are displayed below their corresponding spectra. Characteristic b and y ions are labeled, with b ions in blue and y ions in red.
The number of Klac-modified proteins we identified in P. damselae is comparable to that in two other bacteria, E. coli (478) (Dong et al., 2022) and S. mutans (469) (Li et al., 2023). However, there is a significant difference in the number of Klac modification sites, with 1,352, 1,047, and 1,869 in P. damselae, E. coli, and S. mutans, respectively. This indicates a distinct distribution of Klac modification sites on individual proteins among these three strains. The variation in Klac modification site numbers may reflect inherent differences in the lactylation levels of proteins among different bacterial species. However, it cannot be excluded that such differences are result from separate identifications conducted by different laboratories, despite the adoption of similar proteomic identification strategies.
The identification of so many Klac-modified sites and proteins in these bacteria not only indicates that Klac modification is also an abundant and complex PTM in prokaryotic cells but also underscores its crucial role in regulating protein functions in these bacteria. Particularly noteworthy is the widespread Klac modification observed in ribosomal proteins and RNA polymerase. Additionally, Pan et al. (2015) previously identified extensive succinylation modification on these two types of proteins in V. parahaemolyticus, suggesting a stringent control of protein translation in bacteria. In a word, this study represents the first investigation into Klac modification in P. damselae, and the obtained Klac-modified proteomic data will contribute to further elucidating the regulatory role of Klac modification in the physiological functions of this bacterium.
Gene Ontology (GO) functional annotation and subcellular localization distribution of the Klac-modified proteins in P. damselae
To gain a comprehensive understanding of the Klac-modified proteins identified in P. damselae, we conducted a thorough analysis of these proteins in terms of gene ontology (GO) annotation and subcellular localization. This is important because Klac-modified protein plays a vital role in regulating cellular functions (Pan et al., 2015). GO serves as a crucial bioinformatics analysis method and tool, employed to describe various attributes of genes and gene products (Dimmer et al., 2012). GO annotations comprise three dimensions: biological process, cellular component, and molecular function, offering diverse perspectives on the biological roles of proteins.
The results of the GO functional classification analysis are shown in Figure 2A and Supplementary Table S2. In the classification of biological processes, Klac-modified proteins are mainly associated with cellular processes and metabolic processes, with protein numbers of 257 and 236, respectively, significantly exceeding those ranked 3-5 in response to stimulus, biological regulation, growth and localization with 73, 68, 40 and 36 proteins, respectively (Figure 3A, green bars). Classification by molecular function revealed that the majority of Klac-modified proteins are associated with catalytic activity and binding, with 180 proteins for both categories, which was also significantly higher than the proteins with other functions (Figure 3A, purple bars), consistent with the classification of biological processes. These results suggest that Klac modification may play a critical role in cellular processes and metabolic processes. In the cellular component classification, Klac-modified proteins are mainly categorized into cell and intracellular (Figure 3A, orange bars).
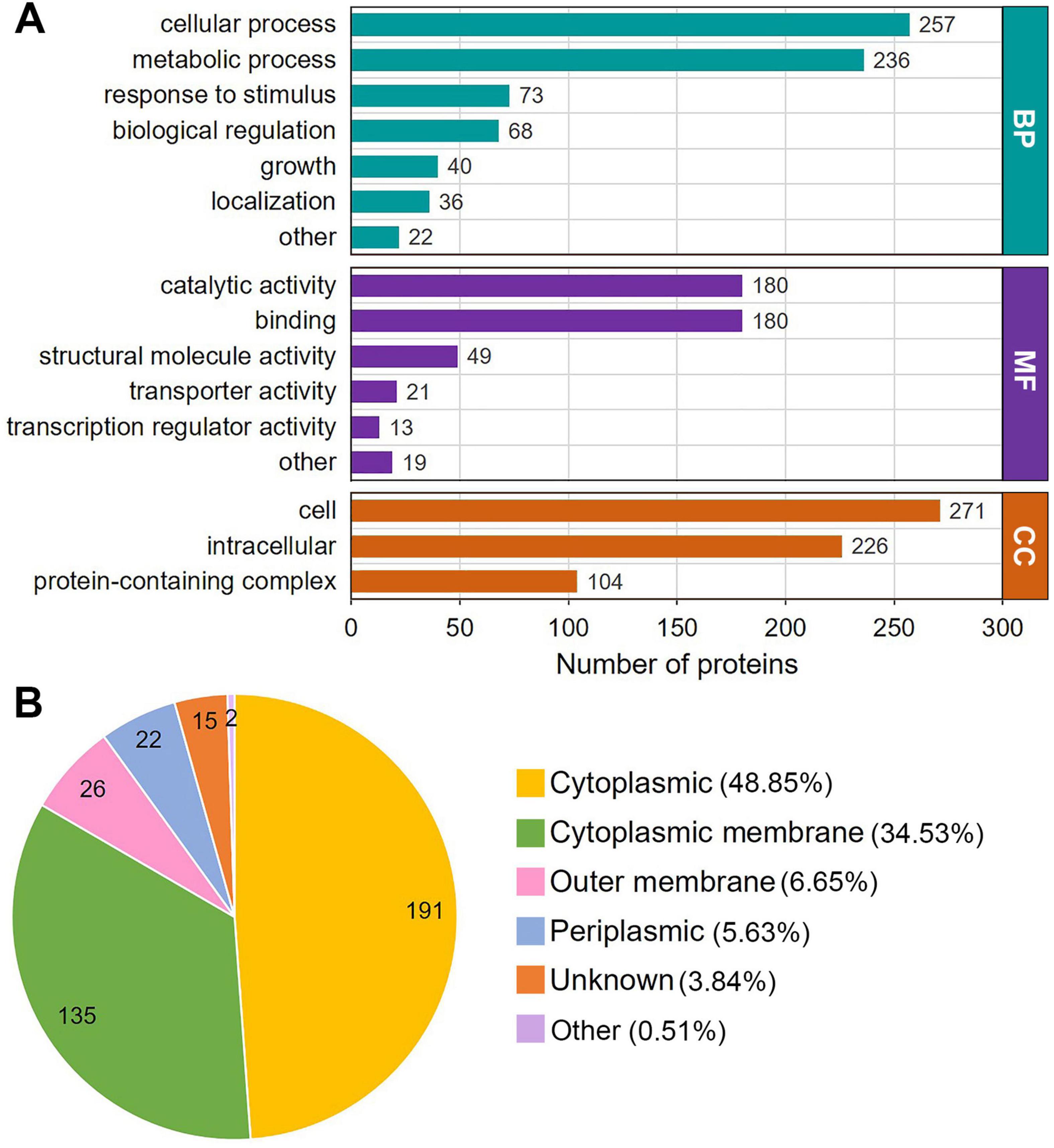
Figure 3. Gene Ontology (GO) functional annotation and subcellular localization distribution of the Klac-modified proteins in P. damselae. (A) GO annotation based on biological process (BP), cellular component (CC) and molecular function (MF). The number of proteins in each category is indicated. (B) Subcellular localization distribution of Klac–modified proteins.
Moreover, the subcellular localization of all identified Klac-modified proteins was analyzed using the WoLF PSORT software, which showed that about half of the proteins (48.85%) are located in the cytoplasm, while the percentage of proteins located in the cytoplasmic membrane is also high at 34.53%, and in contrast, proteins in the outer membrane and periplasmic space constitute only a small fraction, accounting for 6.65 and 5.63%, respectively (Figure 3B and Supplementary Table S3). Taken together, these findings suggest that Klac-modified proteins are widely distributed throughout the cell, and are involved in a diverse variety of functions and consequently participating in various biological processes.
Enrichment analysis of Klac-modified proteins in P. damselae
To better understand the functions of Klac-modified proteins in P. damselae and to investigate whether Klac modification shows significant enrichment in specific functional categories, we performed further enrichment analysis on the identified Klac-modified proteins in terms of GO annotation, KEGG pathways, and protein domains. The results demonstrate a significant enrichment of Klac-modified proteins in these categories (Figure 4 and Supplementary Tables S4–S6). The molecular function-based GO enrichment analysis reveals significant enrichment in functions such as small ribosomal subunit, rRNA binding, structural constituent of ribosome, structural molecule activity, mRNA binding, and aminoacyl-tRNA ligase activity (Figure 4A, orange bars, and Supplementary Figure S1). Interestingly, these significantly enriched molecular functions, with the exception of structural molecule activity and ligase activity (forming carbon-oxygen bonds), are all related to ribosome and protein biosynthesis. The GO enrichment analysis of cellular components reveals that the top 4 highly enriched cellular components, including ribosomal subunit, cytosolic ribosome, ribosome, and ribonucleoprotein complex, are also closely associated with ribosome and protein biosynthesis. However, other cellular components, such as intracellular non-membrane-bounded organelle, intracellular organelle, organelle, and non-membrane-bounded organelle, also exhibit a certain degree of enrichment (Figure 3A, purple bars, and Supplementary Figure S1). Consistent with these observation, in the biological process-based GO enrichment analysis, Klac-modified proteins are significantly enriched in processes related to ribosome and protein synthesis, including the top 3 highly enriched processes: ribosome assembly, ribonucleoprotein complex subunit organization, and peptide biosynthetic process (Figure 4A, green bars, and Supplementary Figure S1). The remaining enriched processes mainly involve nucleic acid metabolism (such as ribose phosphate biosynthetic process, assembly purine-containing compound biosynthetic process, ribose phosphate metabolic process, and nucleoside phosphate metabolic process) and protein metabolism (including peptide metabolic process, cellular protein-containing complex assembly, and cellular protein metabolic process, etc.). These GO enrichment analysis results suggest that proteins associated with ribosome, protein biosynthesis, and metabolism are more prone to undergo Klac modification in P. damselae, indicating that Klac modification plays a more crucial regulatory role in these physiological functions compared to other functions.
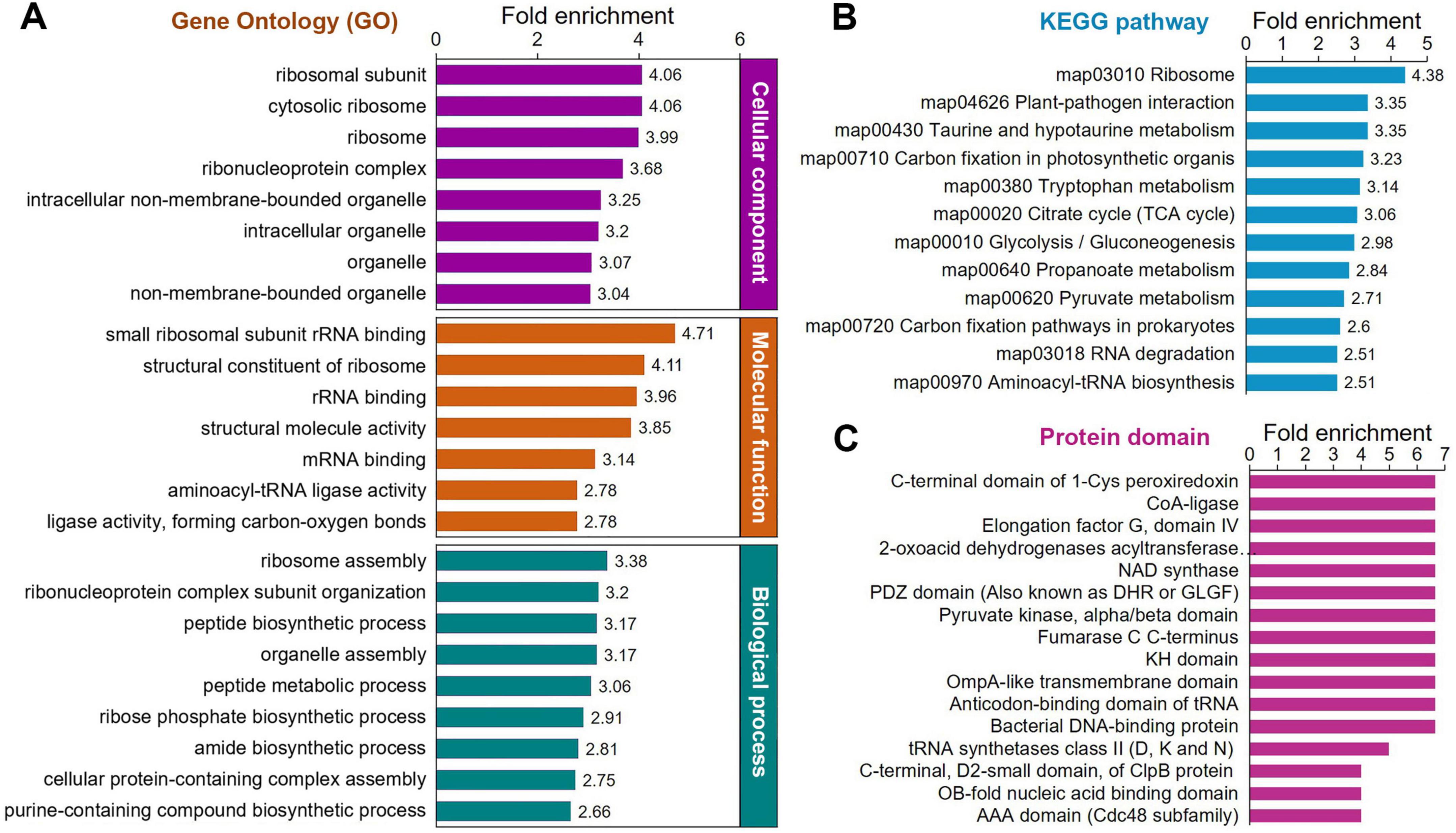
Figure 4. Protein enrichment analysis of the Klac-modified proteins in P. damselae. (A) GO enrichment for biological process (P < 10–3), molecular function (P < 10–7) and cellular components (P < 10–11). (B) KEGG pathway enrichment (P < 10–3). (C) Enrichment for protein domains (P < 10–3).
Enrichment analysis of KEGG pathways indicates significant enrichment in several pathways related to ribosome, protein biosynthesis, and metabolism (Figure 4B, Supplementary Figure S2, and Supplementary Table S5). Among these enriched pathways, ribosome, and aminoacyl-tRNA biosynthesis are associated with ribosome and protein biosynthesis (Klac-modified proteins in the ribosome are shown in Figure 5C, and Klac-modified proteins in the aminoacyl-tRNA biosynthesis pathway are shown in Supplementary Figure S3). Additionally, pathways involved in central carbon metabolism, such as TCA cycle, glycolysis/gluconeogenesis, pentose phosphate pathway, pyruvate metabolism, and glyoxylate and dicarboxylate metabolism, exhibit significant enrichment (Klac-modified proteins in glycolysis/gluconeogenesis and TCA are shown in Figure 5A, and Klac-modified proteins in pyruvate metabolism are presented in Figure 5B). Other enriched pathways also mostly involve metabolic processes, such as amino acid metabolism and nucleotide metabolism (Supplementary Figure S2). These findings are largely consistent with the aforementioned GO enrichment results.
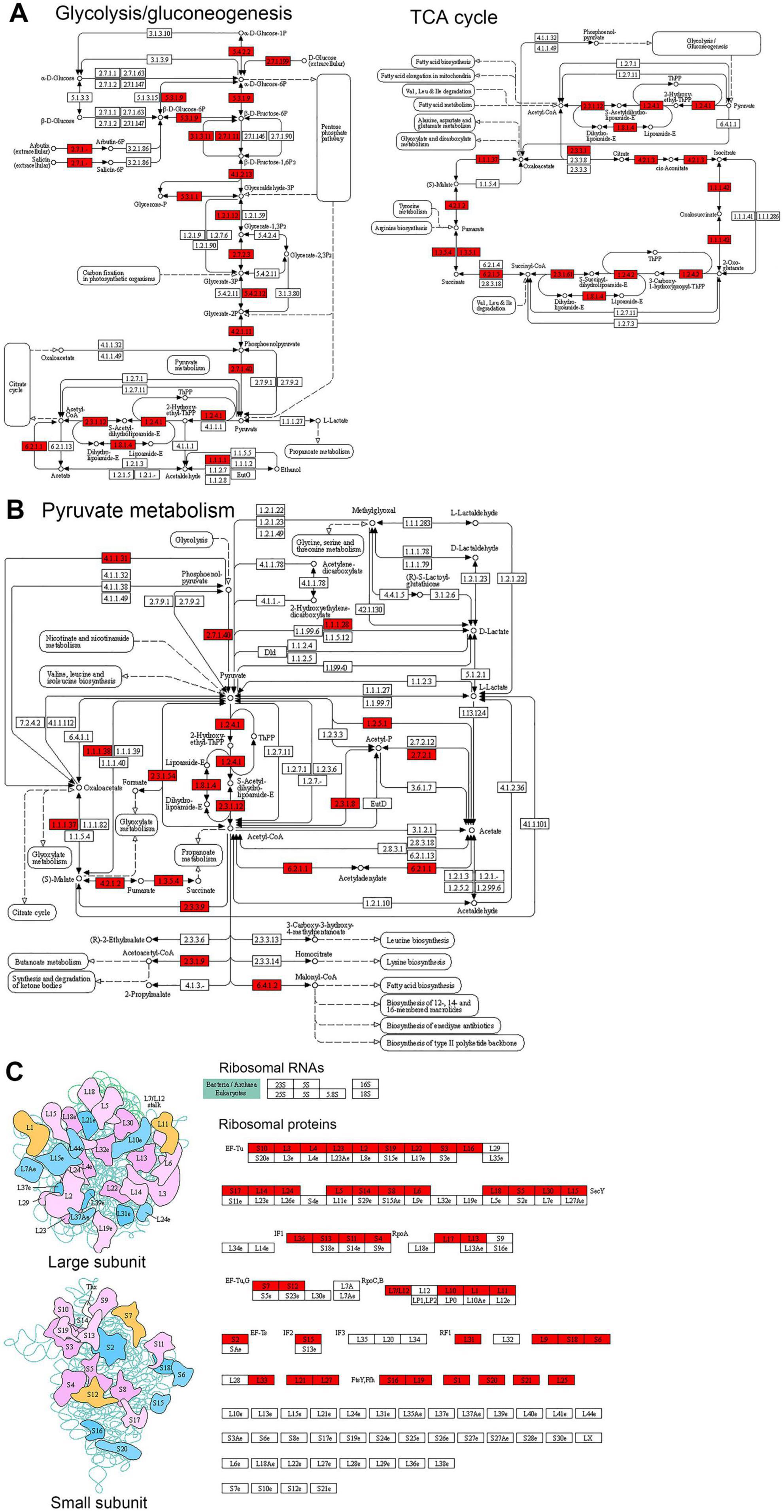
Figure 5. Representative KEGG pathways showing significant enrichment of Klac-modified proteins in P. damselae. (A) Central metabolism, including glycolysis/gluconeogenesis, and TCA cycle and oxidative phosphorylation. (B) Pyruvate metabolism. (C) Ribosome. The Klac-modified enzymes are indicated by the red background.
Furthermore, we performed enrichment analysis on the protein domains of Klac-modified proteins. Protein domains refer to certain structural elements recurring in different proteins, often having similar sequence, structure and function, and they serve as the evolutionary units of proteins. As shown in Figure 4C, Supplementary Figure S2, and Supplementary Table S6, the enrichment results further confirm that proteins involved in protein biosynthesis and metabolism are more prone to be Klac-modified. Therefore, these diverse enrichment analysis results mentioned above collectively suggest that Klac modification plays a crucial regulatory role in physiological functions related to protein biosynthesis and metabolism in P. damselae.
It is noteworthy that GO enrichment analysis of identified Klac-modified proteins in S. mutans suggests that these proteins are mainly enriched in biological processes related to translation, peptide biosynthesis, and amide biosynthesis (Li et al., 2023). The molecular functions of Klac-modified proteins are enriched in structural molecular activity, and structural composition of ribosomes. KEGG pathway analysis indicates Klac-modified proteins closely associated with ribosome, aminoacyl-tRNA synthesis, and glycolysis/gluconeogenesis pathways (Li et al., 2023). In E. coli, proteins related to metabolism, translation, ribosome assembly, and biosynthesis are significantly enriched (Dong et al., 2022). The enrichment analysis results for Klac-modified proteins in these two bacteria are remarkably similar to the results obtained in this study for P. damselae. Combined with the available data for Klac-modified proteins in these three different bacteria, it is reasonable to suggest that Klac modification of proteins associated with protein biosynthesis and central carbon metabolism may represent an important regulatory pattern in the control of physiological functions.
Analysis of protein interaction networks of Klac-modified proteins in P. damselae
We conducted a protein-protein interaction network (PPI) analysis of all identified Klac-modified proteins using the STRING database and Cytoscape software (Smoot et al., 2011) for a better understanding of the cellular processes regulated by Klac modification in P. damselae. As a result, 283 Klac-modified proteins were identified as network nodes, and 4868 interactions with their scores of ≥ 0.90 were obtained from the STRING database (Supplementary Table S7). The global PPI network diagram for these interactions is shown in Figure 6, and detailed interaction information can be found in Supplementary Figure S4 and Supplementary Table S7. Furthermore, 20 PPI clusters were extracted from the global PPI network by a clustering algorithm executed using the MCODE plug-in toolkit in Cytoscape (Supplementary Table S7), including highly connected clusters related to ribosomes, glycolysis/gluconeogenesis, purine and pyrimidine metabolism, aminoacyl-tRNA biosynthesis, and amino acid metabolism, each represented by a different color in Figure 6. These results suggest that Klac modification tends to occur on proteins associated with specific functional clusters. Notably, the extracted clusters are consistent with GO annotations and KEGG pathway enrichment analysis.
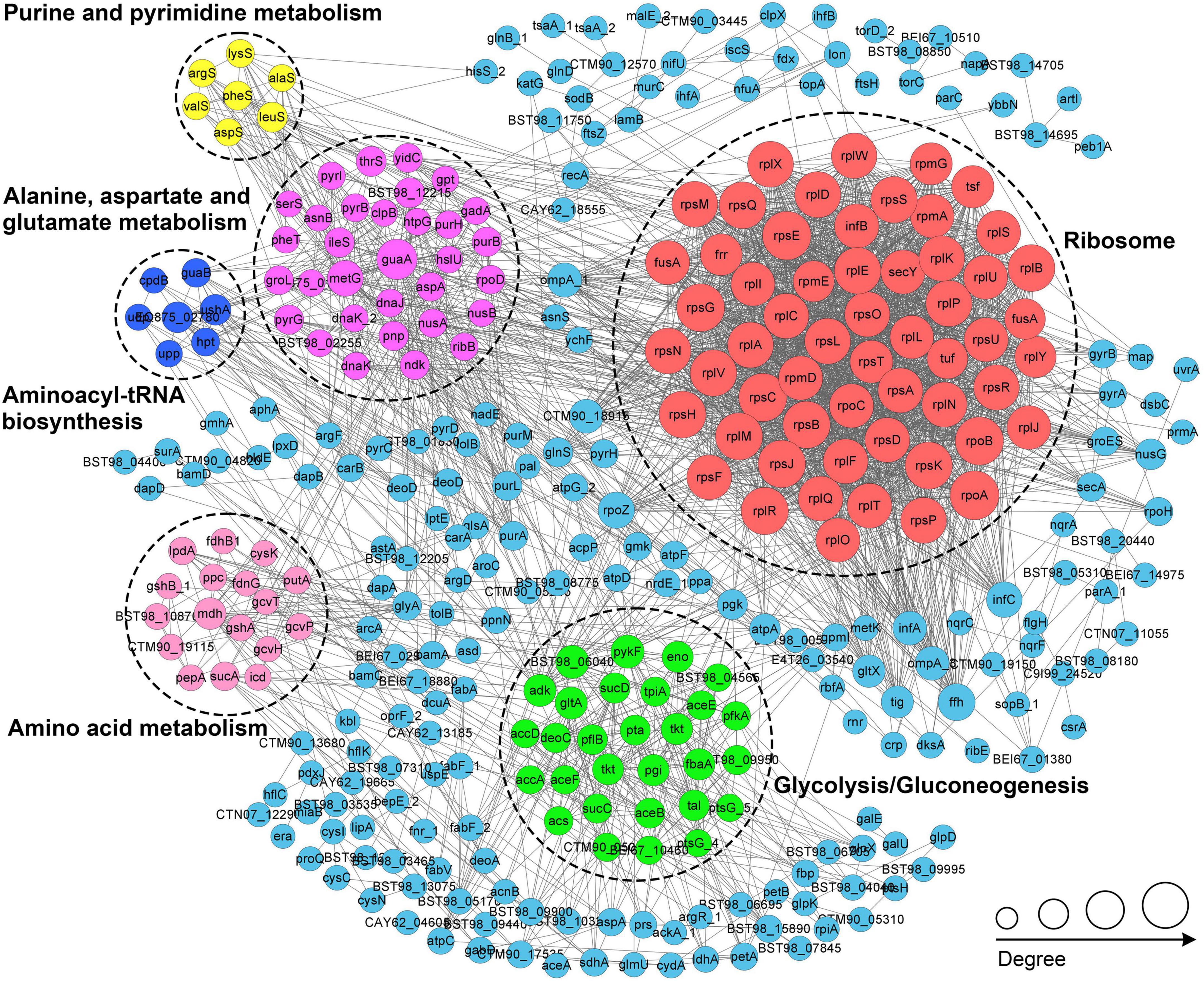
Figure 6. PPI network analysis of the Klac-modified proteins in P. damselae. Representatives of the six PPI clusters enriched in ribosomes (red), glycolysis/glycogenesis (green), alanine, aspartate, and glutamate metabolism (purple), amino acid metabolism (pink), aminoacyl-tRNA biosynthesis (blue), and purine and pyrimidine metabolism (yellow), respectively, are shown in different colors.
In S. mutans, the PPI network of Klac-modified proteins was also analyzed, where the top 5 clusters were mainly enriched for ribosomes, glycolysis/gluconeogenesis, PTS, sulfur metabolism, and aminoacyl-tRNA synthesis (Li et al., 2023). Among these clusters, those involving ribosomes, glycolysis/gluconeogenesis, and aminoacyl-tRNA synthesis were also found to be enriched in the present study. In E. coli, despite the identification of the lactylome, the Klac-modified proteins were not subjected to PPI network analysis. However, given the high homology (68.9%) between CobB, the enzyme identified as the primary regulator of Klac modification in E. coli (Li et al., 2023), and CobB in P. damselae (Supplementary Figure S5), it is highly probable that PPI network analysis would result in predominantly enriched clusters related to ribosome/protein biosynthesis and central metabolism. Since PTMs with dynamic and reversible properties can impact the PPI network (Rao et al., 2014), the results of these PPI analyses suggest that Klac modifications may regulate these relevant physiological processes and metabolic pathways by influencing PPIs, thereby modulating cellular physiological functions.
Conclusion
In this study, we present the first systematic analysis of Klac modification in P. damselae using a highly sensitive proteomic approach. A total of 1,352 Klac-modified sites corresponding to 486 proteins were identified in P. damselae, and analysis of the GO annotation, KEGG pathway and PPI network of Klac-modified proteins indicates their involvement in various cellular functions and metabolic pathways, particularly in ribosome and protein biosynthesis, and central carbon metabolism. This suggests that Klac modification is an abundant and conserved PTM in P. damselae, similar to the other two bacteria or eukaryotic organisms that have undergone global Klac identification. Although the potential regulatory roles and mechanisms of Klac modification in P. damselae remain to be elucidated, our Klac-modified protein dataset will help to better reveal the role of Klac modification in regulating the physiological functions of P. damselae.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
YY: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. HL: Data curation, Formal Analysis, Methodology, Writing – original draft. CW: Data curation, Formal Analysis, Investigation, Writing – original draft. YW: Data curation, Formal Analysis, Investigation, Writing – original draft. XR: Data curation, Formal Analysis, Investigation, Writing – original draft. ML: Data curation, Formal Analysis, Investigation, Writing – original draft. BL: Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. XY: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. ZZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key Research and Development Program of China (2023YFD2400704), Natural Science Foundation of Shandong Province (ZR2020QC216), Central Public-interest Scientific Institution Basal Research Fund, YSFRl, CAFS (20603022025012), and Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD29).
Acknowledgments
The authors thank Micrometer Biotechnology (Hangzhou, China) for conducting the LC-MS/MS analyses.
Conflict of interest
XY was employed by the Weimi Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1539893/full#supplementary-material
Footnotes
References
An, D., Song, L., Li, Y., Shen, L., Miao, P., Wang, Y., et al. (2022). Comprehensive analysis of lysine lactylation in Frankliniella occidentalis. Front. Genet. 13:1014225. doi: 10.3389/fgene.2022.1014225
Cheng, Z., Huang, H., Li, M., and Chen, Y. (2024). Proteomic analysis identifies PFKP lactylation in SW480 colon cancer cells. iScience 27:108645. doi: 10.1016/j.isci.2023.108645
Dennis, G., Sherman, B. T., Hosack, D. A., Yang, J., Gao, W., Lane, H. C., et al. (2003). DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 4:3. doi: 10.1186/gb-2003-4-5-p3
Dimmer, E. C., Huntley, R. P., Alam-Faruque, Y., Sawford, T., O’Donovan, C., Martin, M. J., et al. (2012). The uniprot-GO annotation database in 2011. Nucleic Acids Res. 40, D565–D570. doi: 10.1093/nar/gkr1048
Dong, H., Zhang, J., Zhang, H., Han, Y., Lu, C., Chen, C., et al. (2022). YiaC and CobB regulate lysine lactylation in Escherichia coli. Nat. Commun. 13:6628. doi: 10.1038/s41467-022-34399-y
Gao, M., Zhang, N., and Liang, W. (2020). Systematic analysis of lysine lactylation in the plant fungal pathogen Botrytis cinerea. Front. Microbiol. 11:594743. doi: 10.3389/fmicb.2020.594743
Gauthier, G., Lafay, B., Ruimy, R., Breittmayer, V., Nicolas, J. L., Gauthier, M., et al. (1995). Small-subunit rRNA sequences and whole DNA relatedness concur for the reassignment of Pasteurella piscicida (Snieszko et al.) Janssen and Surgalla to the genus Photobacterium as Photobacterium damsela subsp. piscicida comb. nov. Int. J. Syst. Bacteriol. 45, 139–144. doi: 10.1099/00207713-45-1-139
Hagihara, H., Shoji, H., Otabi, H., Toyoda, A., Katoh, K., Namihira, M., et al. (2021). Protein lactylation induced by neural excitation. Cell Rep. 37:109820. doi: 10.1016/j.celrep.2021.109820
Hong, H., Chen, X., Wang, H., Gu, X., Yuan, Y., and Zhang, Z. (2023). Global profiling of protein lysine lactylation and potential target modified protein analysis in hepatocellular carcinoma. Proteomics 23:e2200432. doi: 10.1002/pmic.202200432
Huang, W., Su, J., Chen, X., Li, Y., Xing, Z., Guo, L., et al. (2023). High-Intensity interval training induces protein lactylation in different tissues of mice with specificity and time dependence. Metabolites 13:647. doi: 10.3390/metabo13050647
Kanehisa, M., and Goto, S. K. E. G. G. (2000). kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. doi: 10.1093/nar/28.1.27
Lee, T. Y. (2021). Lactate: A multifunctional signaling molecule. Yeungnam Univ. J. Med. 38, 183–193. doi: 10.12701/yujm.2020.00892
Li, Z., Gong, T., Wu, Q., Zhang, Y., Zheng, X., Li, Y., et al. (2023). Lysine lactylation regulates metabolic pathways and biofilm formation in Streptococcus mutans. Sci. Signal. 16:eadg1849. doi: 10.1126/scisignal.adg1849
Lin, Y., Chen, M., Wang, D., Yu, Y., Chen, R., Zhang, M., et al. (2023). Multi-Proteomic analysis reveals the effect of protein lactylation on matrix and cholesterol metabolism in tendinopathy. J. Proteome Res. 22, 1712–1722. doi: 10.1021/acs.jproteome.2c00756
Meng, X., Baine, J. M., Yan, T., and Wang, S. (2021). Comprehensive analysis of lysine lactylation in rice (Oryza sativa) Grains. J. Agric. Food Chem. 69, 8287–8297. doi: 10.1021/acs.jafc.1c00760
Osorio, C. R., Vences, A., Matanza, X. M., and Terceti, M. S. (2018). Photobacterium damselae subsp. damselae, a generalist pathogen with unique virulence factors and high genetic diversity. J. Bacteriol. 200:e00002-18. doi: 10.1128/JB.00002-18
Pan, J., Chen, R., Li, C., Li, W., and Ye, Z. (2015). Global analysis of protein lysine succinylation profiles and their overlap with lysine acetylation in the marine bacterium Vibrio parahemolyticus. J. Proteome Res. 14, 4309–4318. doi: 10.1021/acs.jproteome.5b00485
Rao, V. S., Srinivas, K., Sujini, G. N., and Kumar, G. N. (2014). Protein-protein interaction detection: Methods and analysis. Int. J. Proteom. 2014:147648. doi: 10.1155/2014/147648
Rivas, A. J., Lemos, M. L., and Osorio, C. R. (2013). Photobacterium damselae subsp. damselae, a bacterium pathogenic for marine animals and humans. Front. Microbiol. 4:283. doi: 10.3389/fmicb.2013.00283
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. doi: 10.1101/gr.1239303
Smoot, M. E., Ono, K., Ruscheinski, J., Wang, P. L., and Ideker, T. (2011). Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics 27, 431–432. doi: 10.1093/bioinformatics/btq675
Song, Y., Liu, X., Stielow, J. B., de Hoog, S., and Li, R. (2022). Post-translational changes in Phialophora verrucosa via lysine lactylation during prolonged presence in a patient with a CARD9-related immune disorder. Front. Immunol. 13:966457. doi: 10.3389/fimmu.2022.966457
Soto, W. (2022). Emerging research topics in the vibrionaceae and the squid- vibrio symbiosis. Microorganisms 10:1946. doi: 10.3390/microorganisms10101946
Sung, E., Sim, H., Cho, Y. C., Lee, W., Bae, J. S., Tan, M., et al. (2023). Global profiling of lysine acetylation and lactylation in kupffer cells. J. Proteome Res. 22, 3683–3691. doi: 10.1021/acs.jproteome.3c00156
Wang, J., Ouyang, L., and Wei, L. (2023). novel insight of nitrogen deprivation affected lipid accumulation by genome-wide lactylation in Nannochloropsis oceanica. J. Agric. Food Chem. 71, 10107–10123. doi: 10.1021/acs.jafc.3c00122
Wu, X. (2023). In-depth discovery of protein lactylation in hepatocellular carcinoma. Proteomics 23:e2300003. doi: 10.1002/pmic.202300003
Yamane, K., Asato, J., Kawade, N., Takahashi, H., Kimura, B., and Arakawa, Y. (2004). Two cases of fatal necrotizing fasciitis caused by Photobacterium damsela in Japan. J. Clin. Microbiol. 42, 1370–1372. doi: 10.1128/JCM.42.3.1370-1372.2004
Yang, K., Fan, M., Wang, X., Xu, J., Wang, Y., Tu, F., et al. (2022). Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 29, 133–146. doi: 10.1038/s41418-021-00841-9
Yang, Y. H., Wang, Q. C., Kong, J., Yang, J. T., and Liu, J. F. (2023). Global profiling of lysine lactylation in human lungs. Proteomics 23:e2200437. doi: 10.1002/pmic.202200437
Yang, Z., Yan, C., Ma, J., Peng, P., Ren, X., Cai, S., et al. (2023). Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat. Metab. 5, 61–79. doi: 10.1038/s42255-022-00710-w
Yao, Y., Bade, R., Li, G., Zhang, A., Zhao, H., Fan, L., et al. (2023). Global-Scale profiling of differential expressed lysine-lactylated proteins in the cerebral endothelium of cerebral ischemia-reperfusion injury rats. Cell Mol. Neurobiol. 43, 1989–2004. doi: 10.1007/s10571-022-01277-6
Ye, C., Ge, Y., Zhang, Y., Zhou, L., Chen, W., Zhu, X., et al. (2021). Deletion of vp0057, a gene encoding a Ser/Thr protein kinase, impacts the proteome and promotes iron uptake and competitive advantage in Vibrio parahaemolyticus. J. Proteome Res. 20, 250–260. doi: 10.1021/acs.jproteome.0c00361
Yin, D., Jiang, N., Cheng, C., Sang, X., Feng, Y., Chen, R., et al. (2022). Protein lactylation and metabolic regulation of the zoonotic parasite Toxoplasma gondii. Genom. Proteom. Bioinform. 21, 1163–1181. doi: 10.1016/j.gpb.2022.09.010
Zhang, D., Tang, Z., Huang, H., Zhou, G., Cui, C., Weng, Y., et al. (2019). Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580. doi: 10.1038/s41586-019-1678-1
Zhang, N., Jiang, N., Yu, L., Guan, T., Sang, X., Feng, Y., et al. (2021). Protein lactylation critically regulates energy metabolism in the protozoan parasite Trypanosoma brucei. Front. Cell Dev. Biol. 9:719720. doi: 10.3389/fcell.2021.719720
Zhu, J., Guo, W., and Lan, Y. (2023). Global analysis of lysine lactylation of germinated seeds in wheat. Int. J. Mol. Sci. 24:16195. doi: 10.3390/ijms242216195
Keywords: Photobacterium damselae subsp. damselae, post-translational modification (PTM), lysine lactylation (Klac), proteomics, protein-protein interaction (PPI) network
Citation: Yu Y, Liu H, Wang C, Wang Y, Rong X, Liao M, Li B, Yi X and Zhang Z (2025) Global analysis of protein lysine lactylation profiles in the marine bacterium Photobacterium damselae subsp. damselae. Front. Microbiol. 16:1539893. doi: 10.3389/fmicb.2025.1539893
Received: 05 December 2024; Accepted: 20 May 2025;
Published: 17 June 2025.
Edited by:
Marla Trindade, University of the Western Cape, South AfricaReviewed by:
Fei Fang, Michigan State University, United StatesMateus De Souza Terceti, University of Santiago de Compostela, Spain
Copyright © 2025 Yu, Liu, Wang, Wang, Rong, Liao, Li, Yi and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Zhang, emhhbmd6aGVuZ0B5c2ZyaS5hYy5jbg==
 Yongxiang Yu
Yongxiang Yu Haozhe Liu1
Haozhe Liu1 Meijie Liao
Meijie Liao Zheng Zhang
Zheng Zhang