- 1Department of Gastroenterology, Beijing Ditan Hospital, Capital Medical University, Beijing, China
- 2Department of Gastroenterology, Beijing Shijitan Hospital, Capital Medical University, Beijing, China
- 3Department of Rheumatology and Immunology, Zhumadian Central Hospital, Zhumadian, Henan, China
- 4Department of Gastroenterology, Peking University Ditan Teaching Hospital, Beijing, China
- 5National Key Laboratory of Intelligent Tracking and Forecasting for Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, China
- 6Beijing Key Laboratory of Emerging Infectious Diseases, Institute of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, China
- 7Beijing Institute of Infectious Diseases, Beijing, China
- 8National Center for Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, China
Background: This study investigates the association between Helicobacter pylori (H. pylori) infection and iron deficiency (ID) as well as its potential link to iron deficiency anemia (IDA) in children.
Methods: As of August 2024, we conducted a comprehensive literature review using the Embase, PubMed, Web of Science, Ovid Medline, and Cochrane databases to compare the risk of IDA in patients with and without H. pylori infection. The Newcastle-Ottawa Quality Assessment Scale was used to assess the methodological quality of the included studies. Odds ratios (ORs) and 95% confidence intervals (CIs) were extracted from the studies, and data were transformed for meta-analysis. Heterogeneity was evaluated using the I2statistic, and homogeneity was tested with the chi-square test. If the I2 value was 50% or greater, a random-effects model was applied; if the I2 value was below 50%, a fixed-effects model was used. The meta-regression was performed to explore the potential sources of heterogeneity. Additionally, a meta-analysis was conducted to assess the changes in hemoglobin and ferritin concentrations before and after H. pylori eradication.
Results: We analyzed data from patients across 13 countries. Our findings reveal that individuals infected with H. pylori have a higher likelihood of developing ID compared to those uninfected, with an OR = 1.52 (p < 0.00001, 95% CI 1.32–1.74, I2 = 44%). The risk of IDA is also higher in patients with H. pylori, with an OR = 1.83 (p = 0.05, 95% CI 1.01–3.33, I2 = 67%). Conversely, the overall effect of H. pylori infection on anemia is minimal, with an OR = 0.94 (p = 0.64, 95% CI 0.73–1.21, I2 = 96%). Meta-regression results suggest that age is the main source of heterogeneity. A meta-analysis of treatment-related randomized controlled trials revealed that combining iron supplementation with H. pylori eradication therapy significantly raised ferritin levels, with an SMD (Standardized Mean Difference) = 0.86 (p = 0.02, 95% CI 0.14–1.57, I2 = 89%). Hemoglobin levels also showed an increase, with an SMD = 0.47 (p = 0.04, 95% CI 0.01–0.93, I2 = 75%).
Conclusion: In children, there is a significant association between H. pylori infection and both ID and IDA. Additionally, the eradication of H. pylori has been shown to lead to an improvement in iron stores.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=426395. PROSPERO ID: CRD42023426395.
1 Introduction
1.1 Helicobacter pylori overview
The gastrointestinal tract is home to millions of microorganisms that collectively form the intestinal microbiota, interacting with the host to maintain homeostasis. Among these microorganisms, Helicobacter pylori (H. pylori) is one of the most prevalent and widely studied bacteria. The World Health Organization (WHO), in 1994, classified H. pylori as a class I carcinogen due to its strong association with gastric cancer (Blanchard and Czinn, 2017). H. pylori is a microaerophilic, gram-negative bacterium that colonizes the gastric mucosa in a spiral configuration. Humans are the exclusive host and reservoir for this bacterium, and its prevalence is particularly high in socioeconomically disadvantaged regions, with infection rates exceeding 80% in some areas (Coelho et al., 2018). H. pylori disrupts the gastric mucosal barrier and produces vacuolating cytotoxin, which contributes to the pathogenesis of various gastrointestinal diseases, including peptic ulcer disease and gastric cancer (Malfertheiner et al., 2012). In recent years, studies have also identified extra-gastrointestinal manifestations of H. pylori infection, such as iron deficiency anemia (IDA) (Kato et al., 2022), vitamin B12 deficiency, and idiopathic thrombocytopenic purpura (Robinson and Atherton, 2021).
1.2 Anemia as a global health concern
Anemia is also recognized by the WHO (Zinebi et al., 2017) as one of the top ten global health issues, affecting approximately one-third of the world’s population, with more than half of these cases attributed to IDA (Lopez et al., 2016). In 2016, anemia affected 41.7% of children under 5 years old, 40.1% of pregnant women, and 32.5% of non-pregnant women globally (WHO, 2022a,b). The WHO has prioritized anemia as a global health issue, aiming to reduce its prevalence among women by 50% by 2025. The clinical manifestations of anemia are primarily due to the reduced capacity of blood to carry oxygen. This reduction is influenced by changes in total blood volume and the respiratory system’s compensatory mechanisms. The most common systemic symptom of anemia is fatigue. Typical signs include pallor of the skin and mucous membranes, dyspnea, loss of appetite, and changes in urine output. Additional manifestations can include pica, alopecia, nail pitting, and atrophic glossitis (Ganz, 2019; Di Angelantonio et al., 2017). Iron deficiency (ID), characterized by reduced systemic iron levels, is the most common micronutrient deficiency worldwide. It is also the primary cause of IDA. Populations at higher risk for ID include children, premenopausal women, and individuals in low-and middle-income countries (GBD 2016 Disease and Injury Incidence and Prevalence Collaborators, 2017; Santos et al., 2020). ID disrupts oxygen transport and impairs enzymatic reactions in various metabolic pathways, leading to decreased hemoglobin levels and, ultimately, anemia.
1.3 Link between Helicobacter pylori and IDA
H. pylori infection and its relationship with IDA involve complex and multifactorial mechanisms (Marignani et al., 1997; Barabino et al., 1999). Firstly, H. pylori infection induces gastric mucosal inflammation and disrupts gastric acid secretion (Sato et al., 2015; Annibale et al., 2020; Kishikawa et al., 2020). Gastric acid is essential for the dissolution and absorption of non-heme iron (Flores et al., 2017; Flores et al., 2015), and H. pylori infection often leads to hypochlorhydria or reduced gastric acid secretion, hindering the conversion of dietary iron into its absorbable form (Capurso et al., 2001; Annibale et al., 2003). In addition to impairing iron absorption, H. pylori itself competes with the host for iron, which is crucial for its growth and survival. In a low-acid environment, H. pylori utilizes specialized iron uptake systems to extract iron from the host (Yokota et al., 2013; Lee et al., 2009). This competition depletes the host’s iron reserves and prompts the host to increase the synthesis of hepcidin, further suppressing iron absorption and transport (Mendoza et al., 2019). However, H. pylori has evolved mechanisms to evade immune surveillance, enabling it to continuously extract iron, further reducing iron bioavailability and eventually leading to the onset of IDA.
Previous studies (Muhsen and Cohen, 2008; Qu et al., 2010; Hudak et al., 2017) have largely focused on the general population and have not conducted in-depth research specifically on the pediatric population. The benefits of H. pylori eradication in children remain controversial. It is well known that active eradication therapy is typically not recommended for children infected with H. pylori. This therapeutic strategy is based on the premise that the natural course of H. pylori infection in children is relatively slow, and most children are able to clear the infection on their own as they grow older. However, this approach may result in some untreated children carrying H. pylori into adulthood, potentially increasing their risk of developing extragastric diseases, such as IDA. Currently, in children, there is a lack of comprehensive and systematic reviews or meta-analyses exploring the relationship between H. pylori infection and IDA. To fill this gap, we conducted a meta-analysis aimed at evaluating the association between H. pylori infection and IDA, exploring potential links, and elucidating the clinical value of early eradication of H. pylori. Therefore, whether eradicating H. pylori can bring benefits to the pediatric population becomes the central focus of our research. Our study emphasizes the widespread health impacts of H. pylori infection, particularly in children, where early intervention and eradication strategies are crucial. Through effective eradication measures, the incidence of systemic diseases, including IDA, may be prevented or significantly reduced, ultimately improving the overall health of children.
2 Materials and methods
Our research encompassed cross-sectional studies, cohort studies, case–control studies, as well as intervention studies and clinical trials. We conducted a meta-analysis in accordance with the PRISMA guidelines for systematic reviews and meta-analysis. Since no original clinical raw data were used in this meta-analysis, ethical approval was not required. Our research protocol (PROSPERO ID: CRD42023426395) is registered with the International Prospective Register of Systematic Reviews.
2.1 Search strategy and inclusion criteria
As of August 9, 2024, a comprehensive search was conducted by four independent authors (WZT, TWT, XHH, and HJL) to identify relevant English-language publications. The search covered the following databases: OVID Medline, Cochrane Library, PubMed, EMBASE, and Web of Science. The focus of the search was on studies reporting the prevalence of H. pylori, IDA, ID and anemia in children and adolescents aged 0 to 20 years. The search was performed using the following keywords: “iron deficiency,” “anemia,” “iron deficiency anemia,” “hemoglobin,” “ferritin,” “iron reserves,” “Helicobacter pylori,” “H. pylori” and “campylobacter pylori,” “children,” and “child.” Examples of the search strategies are provided in Appendix 1–5.
Studies were excluded based on the following criteria: (1) duplicate reports, case reports or series, letters to the editor, comments, or author responses; (2) studies that did not meet the inclusion criteria described below.
To ensure methodological rigor and minimize bias, studies were included if they met the following criteria: (1) data collection based on local hospital surveys; (2) inclusion of general population samples, rather than specific volunteer groups; (3) use of clear, validated blood tests to diagnose ID, IDA and anemia; and (4) confirmation of H. pylori infection through validated diagnostic methods such as carbon-13 breath tests, H. pylori stool tests, or gastric mucosal biopsy specimens.
We excluded factors that could potentially influence the accuracy of H. pylori testing, such as the certain use of proton pump inhibitors (PPIs), opioids, dietary modifications, physical activity, irritable bowel syndrome, gastrointestinal surgery, intestinal motility disorders, diverticulosis, systemic sclerosis, and hypothyroidism. No geographical restrictions were applied to the inclusion criteria.
2.2 Study selection process
As the first step, the database search was filtered to identify publications whose titles were relevant to the study hypothesis. The selected publications were then screened based on their abstracts, followed by a full-text review to assess their eligibility for inclusion. Any discrepancies or disagreements regarding eligibility were resolved through discussion and consensus among the three independent reviewers.
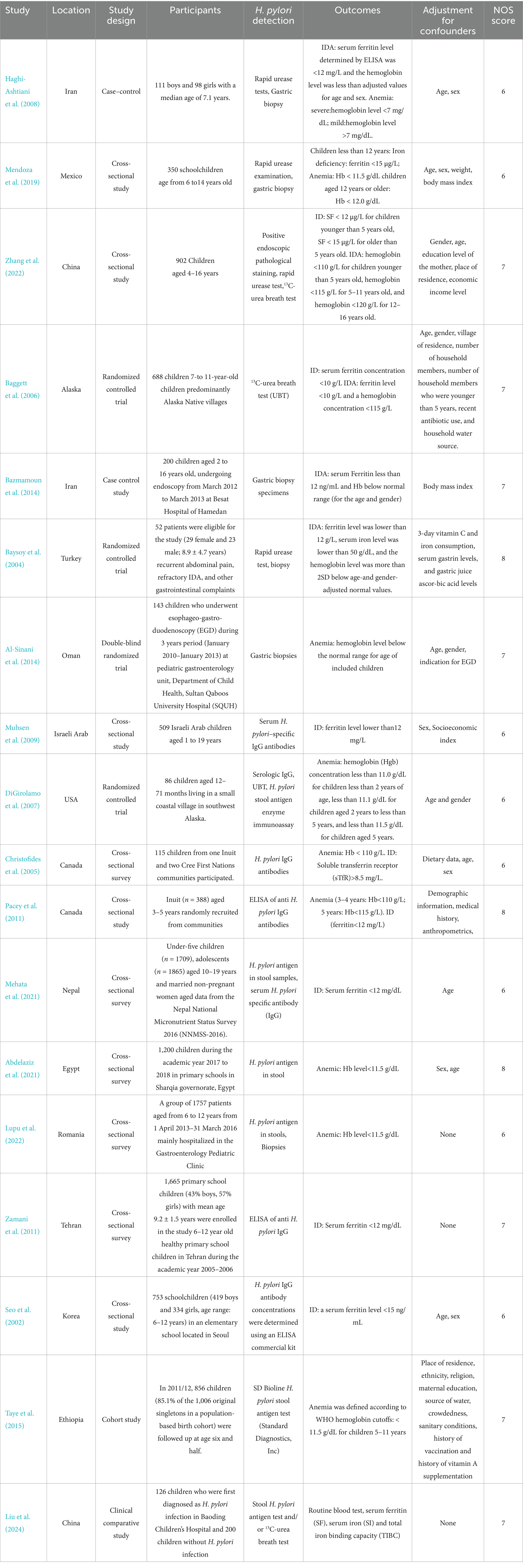
2.3 Data extraction
We developed Tables 1, 2 to systematically summarize the data from each study, including the study title, author names, publication date, participant demographics, study location, H. pylori infection testing methods, iron status markers, and the classifications for ID, IDA, and anemia. Additionally, we recorded the results and any adjustments made for confounding factors. For each study, we extracted the number of individuals infected with H. pylori, as well as the number of cases of IDA, ID and anemia. For studies reporting relative risk (RR) or odds ratio (ORs), along with their 95% confidence intervals (CIs) or p-values, we used these data directly from the original articles. When these statistics were not provided, we applied appropriate statistical methods to calculate them. In randomized controlled trials (RCTs), we extracted data on the number of participants in both the intervention and control groups, and recorded the mean and standard deviation (SD) of ferritin and hemoglobin levels at both baseline and follow-up.
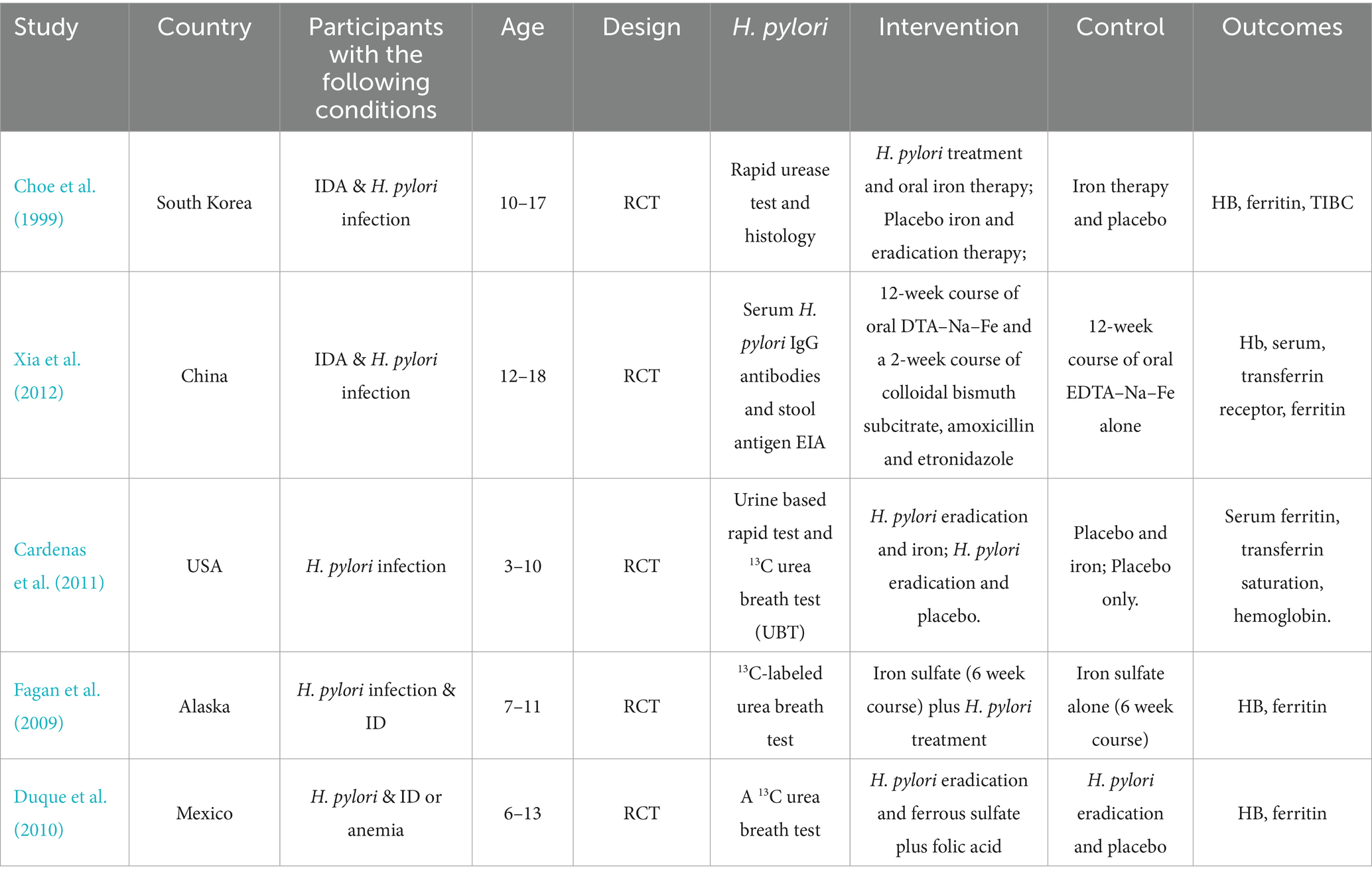
Table 2. Clinical trials assessing the impact of anti-H. pylori therapy on hemoglobin and iron biomarkers.
2.4 Statistical analysis
This meta-analysis includes cross-sectional, cohort, and case–control studies. Eligible studies are summarized, and relevant data are presented in tables. We assessed heterogeneity using the I2statistic, with statistical significance of heterogeneity evaluated through a chi-square test (p < 0.05). The I2 values were interpreted as follows: 0–25% indicated no significant heterogeneity, 26–50% indicated low heterogeneity, 51–75% indicated moderate heterogeneity, and values greater than 75% suggested high heterogeneity. A fixed-effects model was used when I2 was less than 50%, and a random-effects model was applied when I2 exceeded 50%. To further explore potential sources of heterogeneity, we conducted meta-regression analyses. This allowed us to examine how study-level characteristics (e.g., study design, sample size, geographical location, or other relevant covariates) might explain variation in effect sizes across studies. Publication bias was assessed using a funnel plot, and subgroup analyses were conducted by study design (i.e., cross-sectional, cohort, and case–control studies). Sensitivity analyses were performed by excluding individual studies to assess the stability of the results and the impact on heterogeneity. In the meta-analysis of RCTs, we compared the effect sizes of changes in ferritin and hemoglobin levels. We assumed a correlation of 0.5 between baseline and follow-up ferritin and hemoglobin levels. Participants were grouped according to their treatment regimen: those who received H. pylori eradication therapy combined with iron supplementation were compared with those who received H. pylori eradication therapy alone. Additionally, participants who received both anti-H. pylori therapy and iron supplementation were compared with those who received iron supplementation alone. The standardized mean difference (SMD) was calculated using a random-effects model. All statistical analyses were performed using RevMan 5 (version 5.1, Cochrane Collaboration) and STATA (version 17.0, United States).
2.5 Risk of bias
Three independent researchers (WZT, TWT, and HJL) assessed the quality of the included studies using the Newcastle-Ottawa Quality Assessment Scale (NOS). Different criteria were applied depending on the type of study. For cohort studies, the NOS evaluation focused on several aspects, including: (1) the representativeness of the exposed cohort, (2) the appropriate selection of the non-exposed cohort, (3) the suitability of the exposure factor, (4) the clarity of outcome measures (which did not need to be observed at the study’s onset), (5) the comparability of the exposed and non-exposed groups in terms of design and statistical analysis, (6) the adequacy of outcome evaluation, (7) the duration of follow-up after the outcome, and (8) the adequacy of follow-up for both exposed and non-exposed groups. For case–control studies, the NOS evaluation included: (1) the appropriateness and representativeness of the cases, (2) the selection and appropriateness of the control group, (3) the comparability of cases and controls in terms of design and statistical analysis, (4) the accuracy of exposure factor determination, (5) the consistency of methods used to assess exposure in both cases and controls, and (6) ensuring that the non-response rate was similar between the case and control groups. The maximum score on the NOS scale was 9, with scores of 6–8 indicating good quality. For RCTs, we applied the quality assessment framework proposed by the Cochrane Collaboration. All relevant data from the included studies were systematically compiled into Tables 1, 2. Potential biases identified during the quality assessment process are also highlighted in these tables.
3 Results
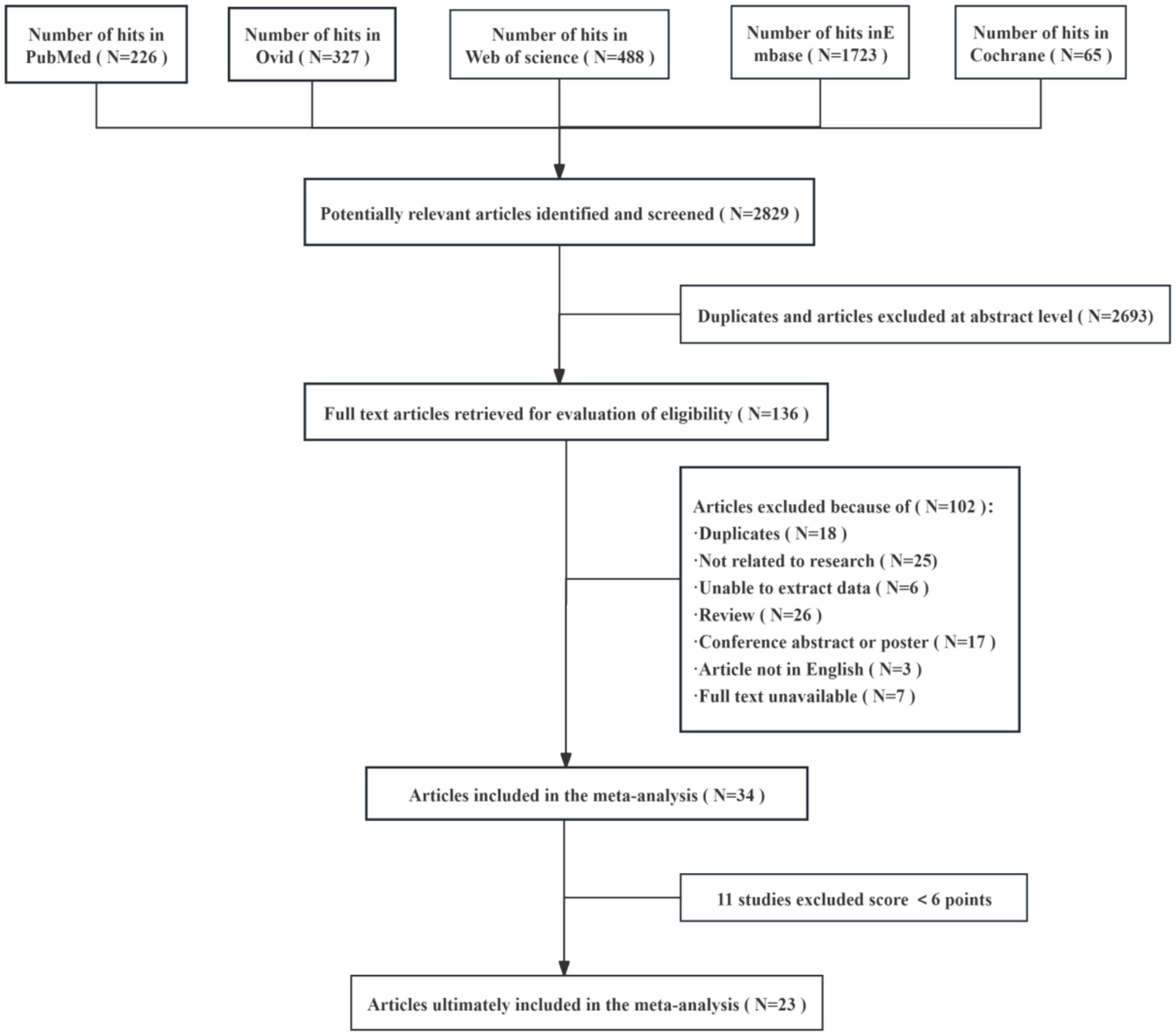
In this study, a total of 2,829 relevant articles were retrieved from several databases, including PubMed, Ovid, Web of Science, Embase, and Cochrane. After automatic de-duplication and screening of titles and abstracts, 2,693 duplicate or irrelevant papers were excluded, leaving 136 articles for full-text review and further screening. During the full-text screening phase, 102 articles were excluded for the following reasons: duplicates, unrelated to the research topic, unextractable data, review articles, conference abstracts or posters, non-English language, and unavailable full text. Ultimately, 34 articles were included in the meta-analysis. Following a quality assessment, 11 studies were excluded for scoring below 6 points, resulting in 23 studies being included in the final meta-analysis. This rigorous screening process ensured the scientific validity of the included studies and the reliability of the data (Figure 1).
3.1 Helicobacter pylori infection and ID
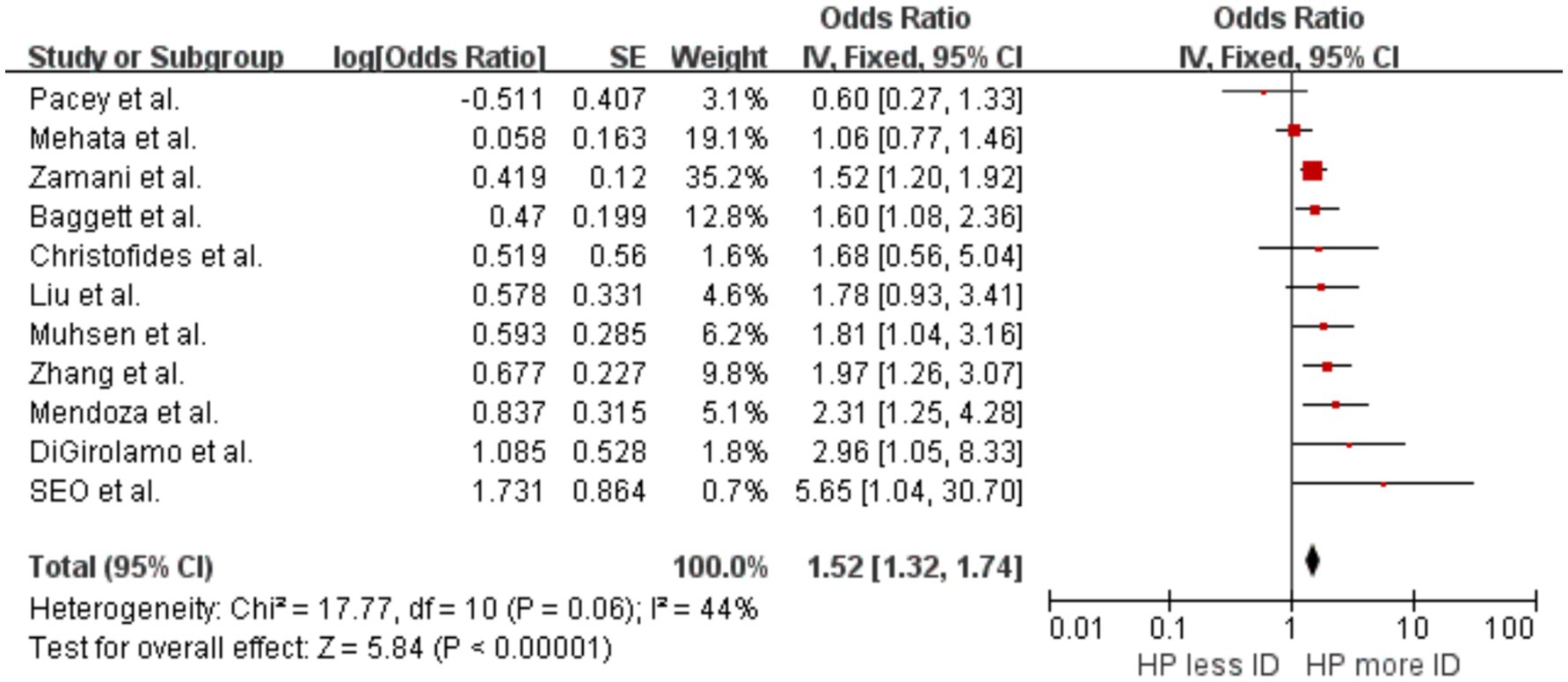
Our meta-analysis included eight cross-sectional studies (Mendoza et al., 2019; Zhang et al., 2022; Muhsen et al., 2009; Christofides et al., 2005; Pacey et al., 2011; Mehata et al., 2021; Zamani et al., 2011; Seo et al., 2002) and three clinical and interventional trial studies (Baggett et al., 2006; DiGirolamo et al., 2007; Liu et al., 2024). The results showed an increased likelihood of developing ID in individuals with evidence of H. pylori infection compared with uninfected individuals, with OR = 1.52 (p < 0.00001, 95% CI 1.32–1.74, I2 = 44%) (Figure 2). There was significant heterogeneity between studies, with moderate heterogeneity between studies. Sensitivity analyses suggest that the study by Mehata et al. (2021) led to this heterogeneity. Analysis excluding these studies showed no significant heterogeneity, with OR = 1.65 (p < 0.00001, 95% CI 1.41–1.93, I2 = 24%).
3.2 Helicobacter pylori infection and IDA
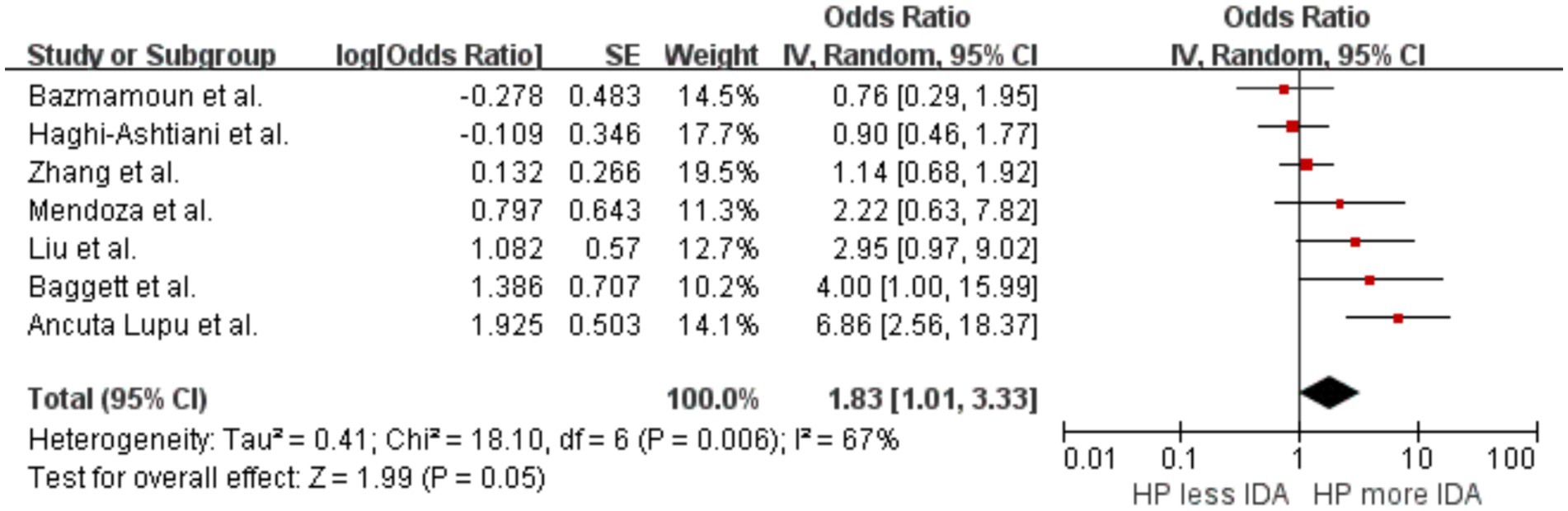
A summary analysis was conducted on seven studies (Mendoza et al., 2019; Zhang et al., 2022; Baggett et al., 2006; Liu et al., 2024; Haghi-Ashtiani et al., 2008; Bazmamoun et al., 2014; Lupu et al., 2022) to explore the correlation between H. pylori infection and IDA. The results indicated that individuals infected with H. pylori had a higher likelihood of developing IDA compared to those not infected, with an OR of 1.83 (p = 0.05, 95% CI 1.01–3.33, I2 = 67%) (Figure 3). The initial analysis showed significant heterogeneity between studies. After excluding the study by Lupu et al. (2022), heterogeneity decreased, yielding an I2of 37%.
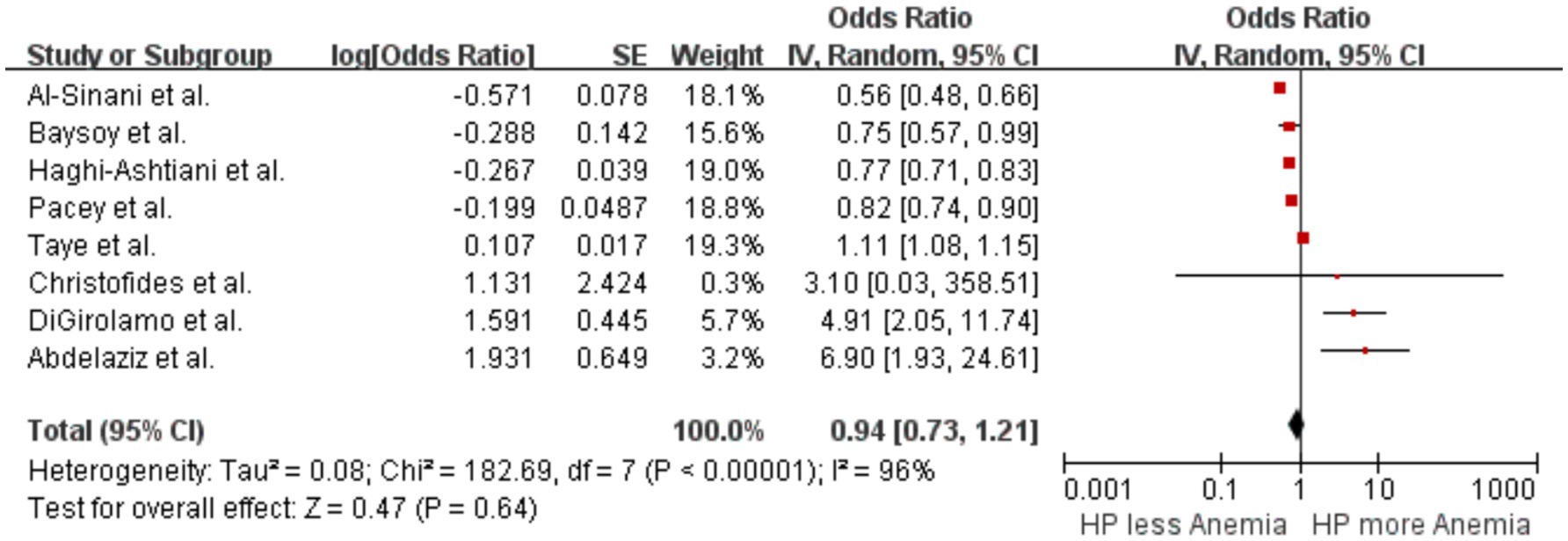
3.3 Helicobacter pylori infection and anemia
A pooled analysis of eight studies (Christofides et al., 2005; Pacey et al., 2011; DiGirolamo et al., 2007; Haghi-Ashtiani et al., 2008; Baysoy et al., 2004; Al-Sinani et al., 2014; Abdelaziz et al., 2021; Taye et al., 2015) examined the association between H. pylori infection and the occurrence of anemia. The results showed no significant difference in the likelihood of anemia between patients with H. pylori infection and those without, with an OR of 0.94 (p = 0.64, 95% CI 0.73–1.21, I2 = 96%) (Figure 4). There was significant heterogeneity among the studies.
3.4 Heterogeneity analysis
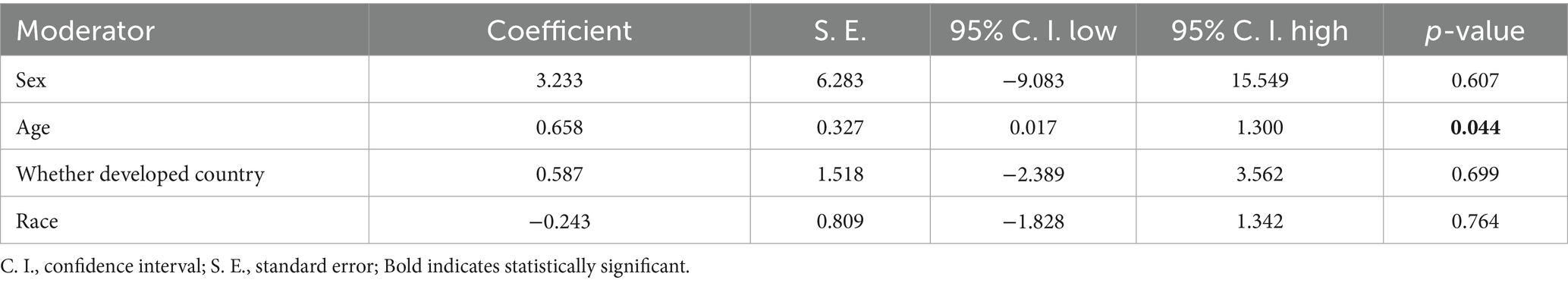
To identify the sources of heterogeneity in the association between H. pylori infection and IDA, we conducted a meta-regression analysis considering factors such as age, gender, geographical region (developed vs. developing), and ethnicity. The results of the meta-regression indicated that age was the primary source of heterogeneity (p = 0.044) (Table 3). Specifically, younger individuals infected with H. pylori were more likely to develop IDA. This finding aligns with the objectives of our study and further supports the conclusions drawn from our analysis.
3.5 Clinical trials
Currently, the commonly used methods for eradicating H. pylori include quadruple therapy, which consists of a PPI, two antibiotics, and a gastric mucosal protective agent. In some regions, simpler triple therapy is also employed, along with the latest medications such as Potassium-Competitive Acid Blocker. The success rate of H. pylori eradication is influenced by various factors, including patient resistance, medication compliance, and regional differences. Typically, the eradication rate falls within the range of 80 to 90%. Our meta-analysis additionally evaluates the impact of H. pylori eradication therapy on iron reserves in children. Children constitute the primary focus of our research, and the included patients are those diagnosed with ID, IDA or anemia. Our study ultimately included five RCTs (Fagan et al., 2009; Duque et al., 2010; Cardenas et al., 2011; Xia et al., 2012; Choe et al., 1999). We compare the changes in hemoglobin concentration and ferritin concentration to reflect whether receiving treatment to eradicate H. pylori affects the patient’s iron reserves. Participants were classified based on their treatment regimen: those who received both H. pylori eradication therapy and iron supplementation were compared with those who received only H. pylori eradication therapy. Furthermore, participants who received both anti-H. pylori therapy and iron supplementation were compared with those who received iron supplementation alone.
Summary analysis indicated that, compared with the H. pylori eradication group alone, the ferritin level in the combined H. pylori eradication and iron supplementation group was slightly increased, SMD = 1.04 (p = 0.17, 95% CI −0.45–2.54, I2 = 94%). Significant heterogeneity was observed between the groups (Figure 5). However, when comparing the group receiving both iron therapy and H. pylori eradication to the subsidized treatment group alone, significant increase in ferritin levels was found, SMD = 0.57 (p < 0.0001, 95% CI 0.29–0.86, I2 = 0%). Additionally, when the two treatments were combined, there was a statistically significant difference in ferritin levels, with SMD of 0.86 (p = 0.02, 95% CI 0.14–1.57, I2 = 89%).
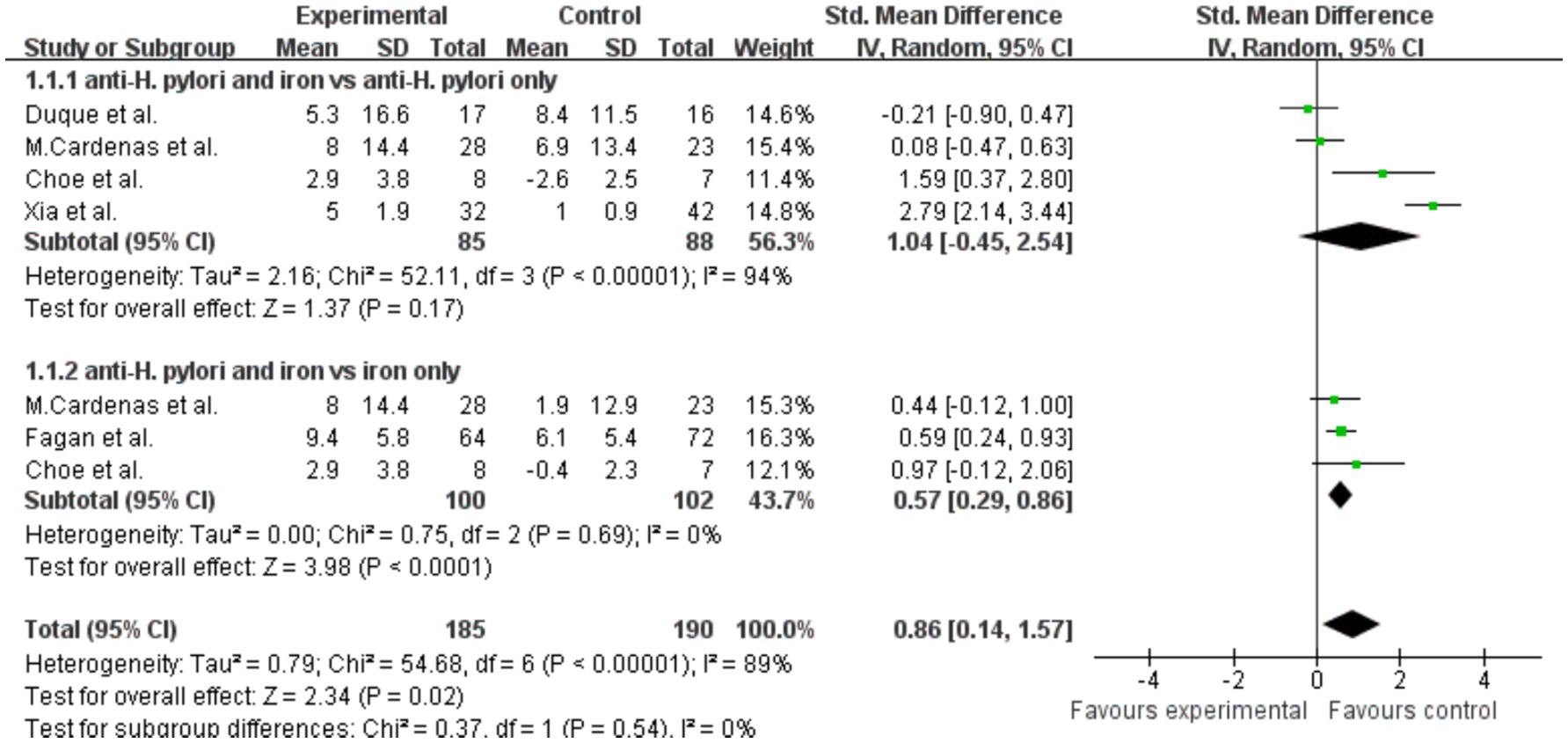
Figure 5. The effect of anti-H. pylori therapy plus iron supplementation compared to iron supplementation alone on serum ferritin levels, meta-analysis of fixed controlled trials.
Analysis of hemoglobin results showed that, compared with the H. pylori eradication group alone, there was no significant change in hemoglobin levels in the combined H. pylori eradication and iron supplementation group, SMD = 0.53 (p = 0.22, 95% CI −0.31–1.36, I2 = 84%). High heterogeneity was observed within the groups (Figure 6). Similarly, there was no significant increase in hemoglobin levels in the group receiving both iron therapy and H. pylori eradication compared with the group receiving iron supplementation alone, SMD = 0.31 (p = 0.11, 95% CI −0.07–0.69, I2 = 29%). Additionally, significant difference in hemoglobin levels was observed when the effects of both treatments were combined, SMD = 0.47 (p = 0.04, 95% CI 0.01–0.93, I2 = 75%).
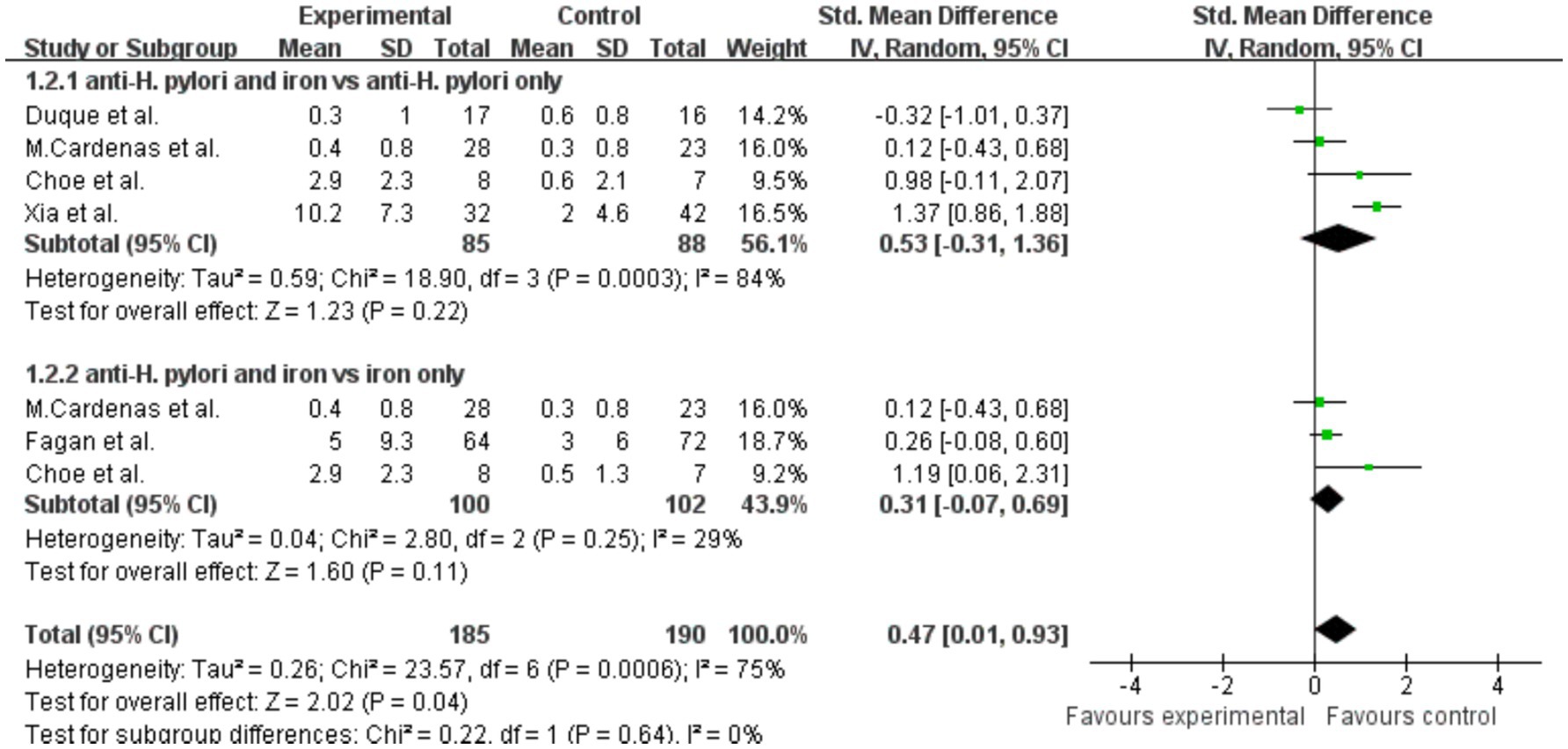
Figure 6. The effect of anti-H. pylori therapy plus iron supplementation compared to iron supplementation alone on hemoglobin levels, meta-analysis of randomized controlled trials.
3.6 Assessment of publication bias
Funnels were constructed based on effect estimates and accuracy for each study to assess the presence of publication bias (Figures 7, 8). The graph is relatively symmetrical, but there are still partly favorable factors for publication.
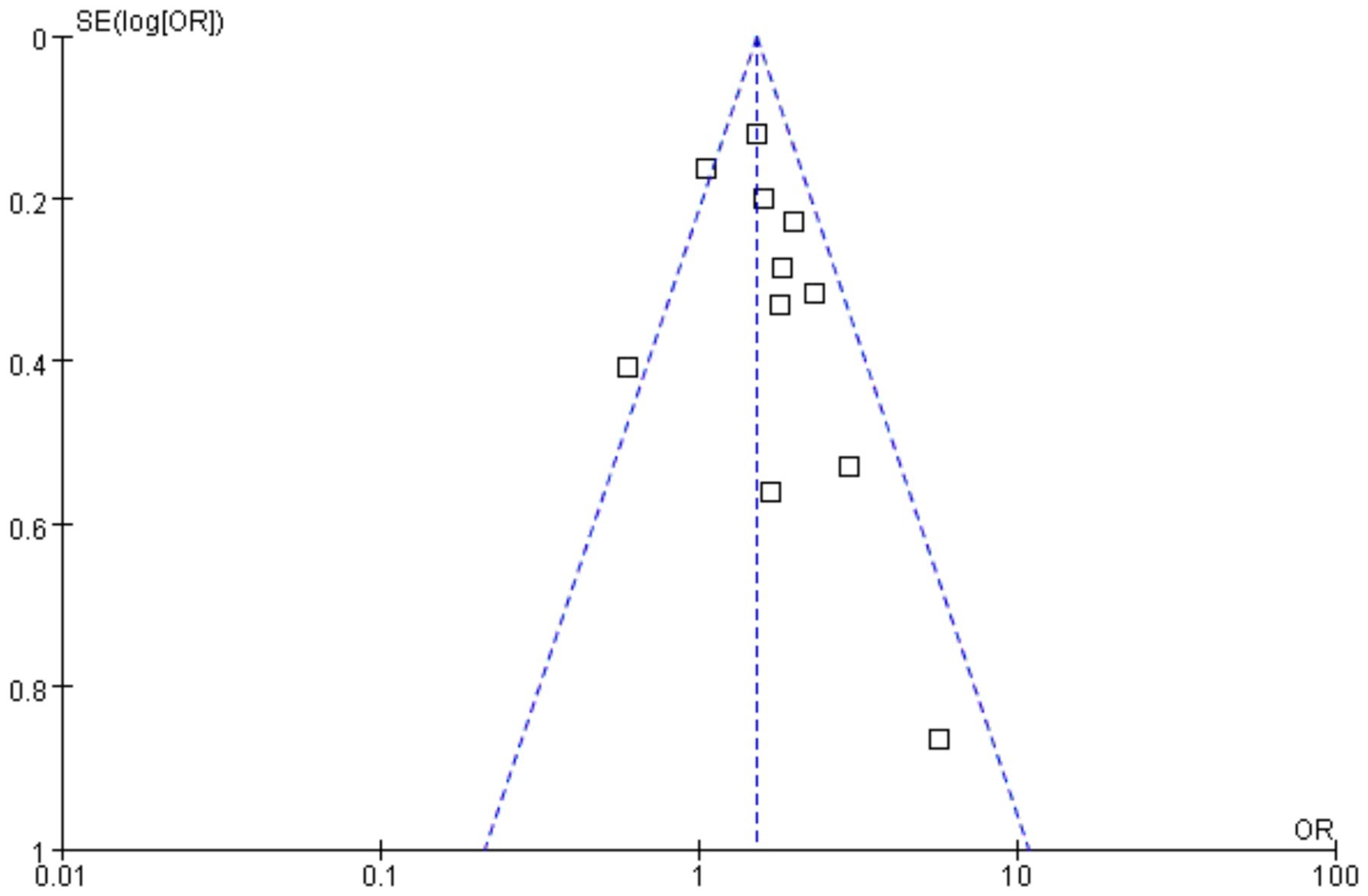
Figure 7. Funnel plot for event rate in the meta-analysis on H. pylori infection and iron deficiency.
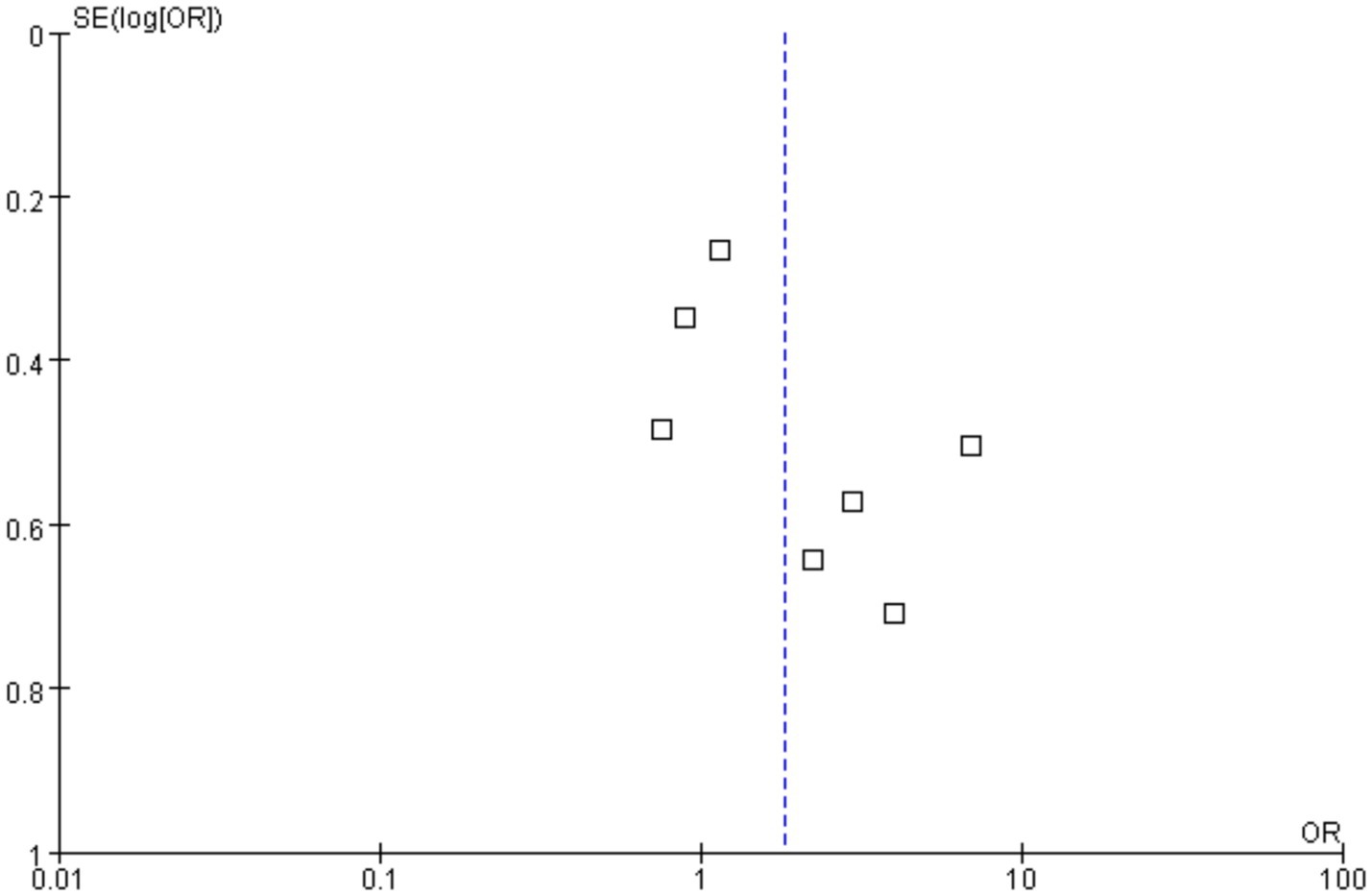
Figure 8. Funnel plot for event rate in the meta-analysis on H. pylori infection and iron deficiency anemia.
4 Discussion
4.1 Main findings
This study systematically integrated data including a total of 13,573 pediatric patients from 16 regions, representing three ethnic groups, with ages under 20. Our core findings reveal a significant positive association between H. pylori infection and both ID and IDA, while no statistically significant association was found with the overall incidence of anemia. These results provide new evidence to better understand the systemic impact of H. pylori infection, particularly its role in iron metabolism and deficiency. In the meta-analysis of ID, the combined OR = 1.52 (95% CI 1.32–1.74), indicating a 52% increased risk of ID in individuals infected with H. pylori compared to uninfected individuals. Although moderate heterogeneity (I2 = 44%) was observed, sensitivity analysis showed that excluding the study by Mehata et al. (2021) significantly reduced the heterogeneity to I2 = 24%, while strengthening the effect size to OR = 1.65, further confirming the robustness of the original findings. Regarding the association with IDA, the combined OR = 1.83 (95% CI 1.01–3.33), demonstrating a stronger effect. This finding aligns with previous mechanistic studies, which suggest that H. pylori may exacerbate ID through multiple pathways, including impaired iron absorption due to decreased gastric acid secretion, elevated hepcidin levels induced by chronic inflammation, and bacterial iron acquisition mechanisms that deplete host iron stores. Importantly, our study found no significant association between H. pylori infection and overall anemia (OR = 0.94, p = 0.64), despite high heterogeneity (I2 = 96%). This apparent contradiction may stem from differences in the types of anemia included in the studies. Specifically, in non-IDA, the anemia-inducing effect of H. pylori may be overshadowed by other underlying causes. This highlights the importance of precise classification in anemia etiology research and suggests that the anemia-inducing mechanisms of H. pylori are specific to iron metabolism. In conclusion, H. pylori infection is significantly positively associated with IDA in children, with the effect mediated through multiple mechanisms impacting iron metabolism.
In addition, our study observed that H. pylori infection exerts a more indirect or less significant influence on anemia. This result is consistent with our initial expectations. We believe this is due to the varying underlying causes of anemia. Chronic anemia, for instance, is frequently associated with systemic chronic inflammation and immune responses rather than solely with disorders of iron absorption and utilization (Gallagher, 2022). In such cases, inflammatory cytokines, including tumor necrosis factor-alpha and interleukin-6, stimulate hepatic iron regulation, thereby reducing iron release and utilization (Raj, 2009). This leads to iron “sequestration” within the body, where iron reserves remain adequate, yet the iron cannot be efficiently transported to the bone marrow, ultimately resulting in anemia. Aplastic anemia (Young, 2018), on the other hand, typically arises from insufficient bone marrow hematopoietic function or abnormalities in hematopoietic stem cells. The direct relationship between local gastric inflammation caused by H. pylori infection and bone marrow hematopoietic function appears to be minimal, rendering H. pylori infection’s impact on this type of anemia relatively insignificant. Additionally, H. pylori infection may indirectly impair iron metabolism by altering the gut microbiota composition (Chen et al., 2021). Disruptions to intestinal health, particularly to the integrity of the intestinal barrier, could further hinder iron absorption. However, the extent of this effect on non-IDA, such as chronic anemia, remains uncertain and warrants further investigation. In summary, while H. pylori infection plays a critical role in the pathogenesis of IDA, its effects on other forms of anemia are more complex and often indirect. Non-iron-deficiency anemias, such as chronic anemia or aplastic anemia, frequently involve broader systemic immune responses or bone marrow dysfunction, processes that H. pylori infection influences only under specific conditions. Further studies are required to elucidate these nuanced interactions and better understand the broader implications of H. pylori infection in anemia subtypes.
To further investigate the sources of heterogeneity in the association between H. pylori infection and IDA, we conducted a meta-regression analysis that included potential confounders such as age, gender, region (developed vs. developing), and ethnicity. Our regression analysis revealed that age is a significant factor contributing to the observed heterogeneity. This finding aligns with our research objectives and provides further support for the strong association between H. pylori infection and IDA, particularly in children (Milman et al., 1998). Furthermore, our study suggests that younger patients may be more prone to developing IDA following H. pylori infection. There are several potential reasons for this observed relationship. First, the gastrointestinal system of young children is still developing and not yet fully matured. As such, it is more susceptible to gastric mucosal inflammation and reduced gastric acid secretion caused by H. pylori infection (Kostaki et al., 2003). Since iron absorption predominantly occurs in the small intestine and gastric acid plays a crucial role in the dissolution and absorption of iron, young children are at a higher risk of developing IDA due to the impaired absorption of iron (Chen et al., 2021). Second, compared to adults, children—especially infants and toddlers—have lower iron reserves. Consequently, any factors that impair iron absorption or increase iron loss will have a more pronounced effect on their iron status, making them more vulnerable to IDA (Queiroz et al., 2024). Third, the typical diet of young children, which is often primarily based on breast milk or formula, may not provide sufficient iron to meet their needs. This can further exacerbate ID (Goddard et al., 2011). Taken together, these factors suggest that young children are particularly vulnerable to the negative effects of H. pylori infection on iron absorption. Their developing immune and gastrointestinal systems, coupled with lower iron stores, increase their susceptibility to IDA.
Notably, our meta-analysis revealed no significant associations between H. pylori-IDA interactions and gender, geographic region, or ethnicity. The relationship between H. pylori infection and IDA exhibits regional variations influenced by socioeconomic and environmental factors. In developing countries, higher H. pylori prevalence (attributed to inadequate sanitation, contaminated water sources, and overcrowded living conditions) interacts synergistically with malnutrition and low dietary iron intake, exacerbating iron absorption impairments and IDA risk (Li et al., 2023). Conversely, developed nations demonstrate lower infection rates and better nutritional status, potentially mitigating H. pylori’s hematological consequences (Sitas et al., 1992). However, our findings do not align with the aforementioned conclusion. This apparent homogeneity may stem from three methodological considerations: First, the predominance of studies from developed regions created sampling bias, potentially underrepresenting socioeconomic heterogeneity within developing nations. Second, while infection rates remain elevated in low-income populations across all regions, this persistent exposure may diminish observable geographic variation. Third, confounding variables such as age-related physiological changes and comorbidities might overshadow regional effects. These findings underscore the necessity for population-specific interventions. Further research employing stratified sampling across socioeconomic subgroups is required to elucidate the complex interplay between environmental determinants and host factors in H. pylori-associated iron metabolism disorders.
Finally, we conducted a summary analysis of changes in ferritin and hemoglobin concentrations following the eradication of H. pylori. The results demonstrated a significant increase in ferritin levels after eradication, indicating an improvement in iron stores. However, the change in hemoglobin concentrations did not reach statistical significance, suggesting that while H. pylori eradication improves iron reserves, its direct impact on hemoglobin levels may be more limited. Several factors may explain this discrepancy. First, ferritin serves as the primary storage form of iron in the body and reflects the level of available iron reserves. After H. pylori eradication, improved iron absorption leads to a rapid restoration of iron stores, as evidenced by the increase in ferritin levels (Mahroum et al., 2022). However, the restoration of hemoglobin concentrations likely requires a longer period of iron supplementation or replenishment, especially in individuals with a history of long-term IDA (Shesh and Connor, 2023). Second, chronic gastritis resulting from H. pylori infection can have lasting effects on iron absorption and utilization. Even after the bacterium is eradicated, the inflammatory state of the gastric mucosa may persist for some time (Homan et al., 2024), potentially accompanied by other gastrointestinal dysfunctions. This ongoing inflammation can continue to impair the effective absorption of iron, thereby limiting hemoglobin synthesis. Finally, while ferritin levels reflect iron storage, the distribution and utilization of iron in the body are regulated by complex mechanisms, including hepcidin, a key regulator of iron homeostasis (Yanatori et al., 2023). After H. pylori eradication, iron absorption may improve rapidly, but the redistribution and utilization of iron for processes like hemoglobin synthesis could take longer to fully reflect. This delayed redistribution may explain why hemoglobin concentrations take more time to respond to the improved iron availability (Zhang et al., 2021; Sharndama and Mba, 2022; Carabotti et al., 2021). In summary, following the eradication of H. pylori, ferritin levels showed a significant increase, which provides further evidence that early eradication of H. pylori can help improve iron stores in the body, particularly in children. This finding suggests that addressing H. pylori infection early may play a crucial role in restoring iron reserve levels and mitigating the risk of IDA in affected populations.
4.2 Advantages of the meta-analysis
This paper has several notable strengths. It provides a comprehensive review of the relationship between H. pylori infection and ID, IDA, and other types of anemia, systematically analyzing potential mechanisms and impacts. By focusing on the pediatric population, it offers valuable insights into an important yet underexplored group. Additionally, the inclusion of studies from diverse regions enhances the generalizability of the findings and highlights differences in healthcare systems and nutritional statuses across populations. From a mechanistic perspective, the paper explores how H. pylori infection contributes to ID and IDA through its effects on gastric acid secretion, iron absorption, iron metabolism, and gastric mucosal damage. This in-depth analysis advances the understanding of its pathogenic mechanisms. Moreover, the study underscores the importance of early diagnosis and intervention, providing practical guidance for clinical practice and a foundation for future prevention and treatment strategies.
4.3 Limitations of the meta-analysis
This study highlights a link between H. pylori infection and IDA but has several limitations that warrant further investigation. First, it primarily focuses on the pediatric population, leaving the effects on adults, the elderly, and individuals with chronic conditions underexplored. Second, the absence of bone marrow puncture biopsy, the gold standard for diagnosing ID, may have affected diagnostic accuracy due to its invasive nature. Future studies should address these gaps by increasing sample diversity, utilizing more sensitive and non-invasive diagnostic methods, and considering multi-dimensional influencing factors. Additionally, the moderate quality of included studies, as indicated by NOS scores, underscores the need for well-designed RCTs. These should assess the long-term efficacy and potential risks of H. pylori eradication therapy for IDA. Placebo-controlled trials could clarify the direct impact of eradication on IDA recovery, but ethical concerns must be considered, as H. pylori infection is strongly linked to severe gastrointestinal diseases. Balancing ethical considerations with the need for robust evidence is crucial for advancing our understanding of H. pylori and its role in IDA and related conditions.
5 Conclusion
We conducted a meta-analysis using data from over 10,000 children under the age of 20 from 13 countries and 3 ethnicities to assess the relationship between H. pylori infection and ID as well as anemia. The results show that individuals infected with H. pylori have a significantly higher likelihood of developing ID compared to those uninfected. Additionally, there is a significant association between H. pylori infection and the occurrence of IDA, with an OR of 1.83 (p = 0.05, 95% CI 1.01–3.33, I2 = 67%). However, the overall effect of H. pylori infection on anemia is minimal. Age is the main source of heterogeneity. In the meta-analysis of treatment-related RCTs, the combination of iron supplementation and H. pylori eradication therapy significantly increased ferritin levels. Although hemoglobin levels also showed an increase, the increase was not as significant as that in ferritin levels. These results indicate a significant association between H. pylori infection and both ID and IDA, and that H. pylori eradication therapy has a certain effect on improving iron stores.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
ZW: Data curation, Formal analysis, Investigation, Software, Supervision, Writing – original draft, Writing – review & editing. WT: Data curation, Formal analysis, Software, Supervision, Writing – original draft, Writing – review & editing. HX: Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Supervision. JH: Writing – original draft, Writing – review & editing. HeW: Supervision, Writing – review & editing. ML: Supervision, Writing – review & editing. JL: Investigation, Software, Supervision, Writing – review & editing. WA: Supervision, Validation, Writing – review & editing. LH: Supervision, Writing – review & editing. JM: Supervision, Writing – review & editing. FX: Supervision, Writing – review & editing. HongW: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1541011/full#supplementary-material
References
Abdelaziz, T. A., Almalky, M., Hanna, D., and Baz, E. G. (2021). Prevalence of Helicobacter pylori infection among anemic school-age children in Egypt: a cross-sectional population-based study. J. Child Sci. 11:e317. doi: 10.1055/s-0041-1740465
Al-Sinani, S., Sharef, S. W., Al-Naamani, K., and Al-Sharji, H. (2014). Helicobacter pylori infection in Omani children. Helicobacter 19, 306–311. doi: 10.1111/hel.12134
Annibale, B., Capurso, G., Lahner, E., Passi, S., Ricci, R., Maggio, F., et al. (2003). Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut 52, 496–501. doi: 10.1136/gut.52.4.496
Annibale, B., Esposito, G., and Lahner, E. (2020). A current clinical overview of atrophic gastritis. Expert Rev. Gastroenterol. Hepatol. 14, 93–102. doi: 10.1080/17474124.2020.1718491
Baggett, H. C., Parkinson, A. J., Muth, P. T., Gold, B. D., and Gessner, B. D. (2006). Endemic iron deficiency associated with Helicobacter pylori infection among school-aged children in Alaska. Pediatrics 117, e396–e404. doi: 10.1542/peds.2005-1129
Barabino, A., Dufour, C., Marino, C. E., Claudiani, F., and De Alessandri, A. (1999). Unexplained refractory iron-deficiency anemia associated with Helicobacter pylori gastric infection in children: further clinical evidence. J. Pediatr. Gastroenterol. Nutr. 28, 116–119. doi: 10.1097/00005176-199901000-00027
Baysoy, G., Ertem, D., Ademoğlu, E., Kotiloğlu, E., Keskin, S., and Pehlivanoğlu, E. (2004). Gastric histopathology, iron status and iron deficiency anemia in children with Helicobacter pylori infection. J. Pediatr. Gastroenterol. Nutr. 38, 146–151. doi: 10.1097/00005176-200402000-00008
Bazmamoun, H., Razavi, Z., Esfahani, H., and Arefian, M. S. (2014). Evaluation of iron deficiency anemia and BMI in children suffering from Helicobacter pylori infection. Iran. J. Ped. Hematol. Oncol. 4, 167–171.
Blanchard, T. G., and Czinn, S. J. (2017). Identification of Helicobacter pylori and the evolution of an efficacious childhood vaccine to protect against gastritis and peptic ulcer disease. Pediatr. Res. 81, 170–176. doi: 10.1038/pr.2016.199
Capurso, G., Lahner, E., Marcheggiano, A., Caruana, P., Carnuccio, A., Bordi, C., et al. (2001). Involvement of the corporal mucosa and related changes in gastric acid secretion characterize patients with iron deficiency anaemia associated with Helicobacter pylori infection. Aliment. Pharmacol. Ther. 15, 1753–1761. doi: 10.1046/j.1365-2036.2001.01101.x
Carabotti, M., Annibale, B., and Lahner, E. (2021). Common pitfalls in the Management of Patients with micronutrient deficiency: keep in mind the stomach. Nutrients 13:208. doi: 10.3390/nu13010208
Cardenas, V. M., Prieto-Jimenez, C. A., Mulla, Z. D., Rivera, J. O., Dominguez, D. C., Graham, D. Y., et al. (2011). Helicobacter pylori eradication and change in markers of iron stores among non-iron-deficient children in El Paso, Texas: an etiologic intervention study. J. Pediatr. Gastroenterol. Nutr. 52, 326–332. doi: 10.1097/MPG.0b013e3182054123
Chen, C. C., Liou, J. M., Lee, Y. C., Hong, T. C., El-Omar, E. M., and Wu, M. S. (2021). The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes 13, 1–22. doi: 10.1080/19490976.2021.1909459
Choe, Y. H., Kim, S. K., Son, B. K., Lee, D. H., Hong, Y. C., and Pai, S. H. (1999). Randomized placebo-controlled trial of Helicobacter pylori eradication for iron-deficiency anemia in preadolescent children and adolescents. Helicobacter 4, 135–139. doi: 10.1046/j.1523-5378.1999.98066.x
Christofides, A., Schauer, C., and Zlotkin, S. H. (2005). Iron deficiency and anemia prevalence and associated etiologic risk factors in first nations and Inuit communities in northern Ontario and Nunavut. Can. J. Public Health 96, 304–307. doi: 10.1007/BF03405171.16625803; PMCID: PMC6975618
Coelho, L. G. V., Marinho, J. R., Genta, R., Ribeiro, L. T., Passos, M. D. C. F., Zaterka, S., et al. (2018). Ivth Brazilian consensus conference on Helicobacter pylori infection. Arq. Gastroenterol. 55, 97–121. doi: 10.1590/S0004-2803.201800000-20
Di Angelantonio, E., Thompson, S. G., Kaptoge, S., Moore, C., Walker, M., Armitage, J., et al. (2017). Efficiency and safety of varying the frequency of whole blood donation (INTERVAL): a randomised trial of 45 000 donors. Lancet 390, 2360–2371. doi: 10.1016/S0140-6736(17)31928-1
DiGirolamo, A. M., Perry, G. S., Gold, B. D., Parkinson, A., Provost, E. M., Parvanta, I., et al. (2007). Helicobacter pylori, anemia, and iron deficiency: relationships explored among Alaska native children. Pediatr. Infect. Dis. J. 26, 927–934. doi: 10.1097/INF.0b013e31812e52cd
Duque, X., Moran, S., Mera, R., Medina, M., Martinez, H., Mendoza, M. E., et al. (2010). Effect of eradication of Helicobacter pylori and iron supplementation on the iron status of children with iron deficiency. Arch. Med. Res. 41, 38–45. doi: 10.1016/j.arcmed.2009.11.006
Fagan, R. P., Dunaway, C. E., Bruden, D. L., Parkinson, A. J., and Gessner, B. D. (2009). Controlled, household-randomized, open-label trial of the effect of treatment of Helicobacter pylori infection on iron deficiency among children in rural Alaska: results at 40 months. J. Infect. Dis. 199, 652–660. doi: 10.1086/596659
Flores, S. E., Aitchison, A., Day, A. S., and Keenan, J. I. (2017). Helicobacter pylori infection perturbs iron homeostasis in gastric epithelial cells. PLoS One 12:e0184026. doi: 10.1371/journal.pone.0184026
Flores, S. E., Day, A. S., and Keenan, J. I. (2015). Measurement of total iron in Helicobacter pylori-infected gastric epithelial cells. Biometals 28, 143–150. doi: 10.1007/s10534-014-9810-z
Gallagher, P. G. (2022). Anemia in the pediatric patient. Blood 140, 571–593. doi: 10.1182/blood.2020006479
Ganz, T. (2019). Anemia of inflammation. N. Engl. J. Med. 381, 1148–1157. doi: 10.1056/NEJMra1804281
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet 390, 1211–1259. doi: 10.1016/S0140-6736(17)32154-2
Goddard, A. F., James, M. W., McIntyre, A. S., and Scott, B. B.British Society of Gastroenterology (2011). Guidelines for the management of iron deficiency anaemia. Gut 60, 1309–1316. doi: 10.1136/gut.2010.228874
Haghi-Ashtiani, M. T., Monajemzadeh, M., Motamed, F., Mahjoub, F., Sharifan, M., Shahsiah, R., et al. (2008). Anemia in children with and without Helicobacter pylori infection. Arch. Med. Res. 39, 536–540. doi: 10.1016/j.arcmed.2008.04.005
Homan, M., Jones, N. L., Bontems, P., Carroll, M. W., Czinn, S. J., Gold, B. D., et al. (2024). Updated joint ESPGHAN/NASPGHAN guidelines for management of Helicobacter pylori infection in children and adolescents (2023). J. Pediatr. Gastroenterol. Nutr. 79, 758–785. doi: 10.1002/jpn3.12314
Hudak, L., Jaraisy, A., Haj, S., and Muhsen, K. (2017). An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter 22:e12330. doi: 10.1111/hel.12330
Kato, S., Gold, B. D., and Kato, A. (2022). Helicobacter pylori-associated Iron deficiency Anemia in childhood and adolescence-pathogenesis and clinical management strategy. J. Clin. Med. 11:7351. doi: 10.3390/jcm11247351
Kishikawa, H., Ojiro, K., Nakamura, K., Katayama, T., Arahata, K., Takarabe, S., et al. (2020). Previous Helicobacter pylori infection-induced atrophic gastritis: a distinct disease entity in an understudied population without a history of eradication. Helicobacter 25:e12669. doi: 10.1111/hel.12669
Kostaki, M., Fessatou, S., and Karpathios, T. (2003). Refractory iron-deficiency anaemia due to silent Helicobacter pylori gastritis in children. Eur. J. Pediatr. 162, 177–179. doi: 10.1007/s00431-002-1139-x
Lee, J. H., Choe, Y. H., and Choi, Y. O. (2009). The expression of iron-repressible outer membrane proteins in Helicobacter pylori and its association with iron deficiency anemia. Helicobacter 14, 36–39. doi: 10.1111/j.1523-5378.2009.00658.x
Li, Y., Choi, H., Leung, K., Jiang, F., Graham, D. Y., and Leung, W. K. (2023). Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 8, 553–564. doi: 10.1016/S2468-1253(23)00070-5
Liu, X., Zhang, Y., Dong, Q., Deng, H., and Bi, J. (2024). Correlation of iron deficiency indexes with eradication and recurrence of Helicobacter Pylori infection in children. Pak. J. Med. Sci. 40, 674–678. doi: 10.12669/pjms.40.4.7816
Lopez, A., Cacoub, P., Macdougall, I. C., and Peyrin-Biroulet, L. (2016). Iron deficiency anaemia. Lancet 387, 907–916. doi: 10.1016/S0140-6736(15)60865-0
Lupu, A., Miron, I. C., Cianga, A. L., Cernomaz, A. T., Lupu, V. V., Munteanu, D., et al. (2022). The relationship between Anemia and Helicobacter Pylori infection in children. Children (Basel) 9:1324. doi: 10.3390/children9091324
Mahroum, N., Alghory, A., Kiyak, Z., Alwani, A., Seida, R., Alrais, M., et al. (2022). Ferritin – from iron, through inflammation and autoimmunity, to COVID-19. J. Autoimmun. 126:102778. doi: 10.1016/j.jaut.2021.102778
Malfertheiner, P., Megraud, F., O'Morain, C. A., Atherton, J., Axon, A. T., Bazzoli, F., et al. (2012). Management of Helicobacter pylori infection--the Maastricht IV/Florence consensus report. Gut 61, 646–664. doi: 10.1136/gutjnl-2012-302084
Marignani, M., Angeletti, S., Bordi, C., Malagnino, F., Mancino, C., Delle Fave, G., et al. (1997). Reversal of long-standing iron deficiency anaemia after eradication of Helicobacter pylori infection. Scand. J. Gastroenterol. 32, 617–622. doi: 10.3109/00365529709025109
Mehata, S., Parajuli, K. R., Pant, N. D., Rayamajhee, B., Yadav, U. N., Mehta, R. K., et al. (2021). Prevalence and correlates of Helicobacter pylori infection among under-five children, adolescent and non-pregnant women in Nepal: further analysis of Nepal national micronutrient status survey 2016. PLoS Negl. Trop. Dis. 15:e0009510. doi: 10.1371/journal.pntd.0009510
Mendoza, E., Duque, X., Hernández Franco, J. I., Reyes Maldonado, E., Morán, S., Martínez, G., et al. (2019). Association between active H. pylori infection and Iron deficiency assessed by serum Hepcidin levels in school-age children. Nutrients 11:2141. doi: 10.3390/nu11092141
Milman, N., Rosenstock, S., Andersen, L., Jørgensen, T., and Bonnevie, O. (1998). Serum ferritin, hemoglobin, and Helicobacter pylori infection: a seroepidemiologic survey comprising 2794 Danish adults. Gastroenterology 115, 268–274. doi: 10.1016/S0016-5085(98)70192-1
Muhsen, K., Barak, M., Shifnaidel, L., Nir, A., Bassal, R., and Cohen, D. (2009). Helicobacter pylori infection is associated with low serum ferritin levels in Israeli Arab children: a seroepidemiologic study. J. Pediatr. Gastroenterol. Nutr. 49, 262–264. doi: 10.1097/MPG.0b013e31818f0a0d
Muhsen, K., and Cohen, D. (2008). Helicobacter pylori infection and iron stores: a systematic review and meta-analysis. Helicobacter 13, 323–340. doi: 10.1111/j.1523-5378.2008.00617.x
Pacey, A., Weiler, H., and Egeland, G. M. (2011). Low prevalence of iron-deficiency anaemia among Inuit preschool children: Nunavut Inuit child health survey, 2007-2008. Public Health Nutr. 14, 1415–1423. doi: 10.1017/S1368980010002429
Qu, X. H., Huang, X. L., Xiong, P., Zhu, C. Y., Huang, Y. L., Lu, L. G., et al. (2010). Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J. Gastroenterol. 16, 886–896. doi: 10.3748/wjg.v16.i7.886
Queiroz, D. M. M., Braga, L. L. C., Rocha, G. A., Batista, S. A., Rocha, A. M. C., Saito, M., et al. (2024). Infection by Helicobacter pylori cytotoxin-A-associated antigen-positive strains is associated with iron deficiency anemia in a longitudinal birth cohort in Brazil. Haematologica 109, 4112–4115. doi: 10.3324/haematol.2024.285446
Raj, D. S. (2009). Role of interleukin-6 in the anemia of chronic disease. Semin. Arthritis Rheum. 38, 382–388. doi: 10.1016/j.semarthrit.2008.01.006
Robinson, K., and Atherton, J. C. (2021). The spectrum of Helicobacter-mediated diseases. Annu. Rev. Pathol. 16, 123–144. doi: 10.1146/annurev-pathol-032520-024949
Santos, M. L. C., de Brito, B. B., da Silva, F. A. F., Sampaio, M. M., Marques, H. S., Oliveira E Silva, N., et al. (2020). Helicobacter pylori infection: beyond gastric manifestations. World J. Gastroenterol. 26, 4076–4093. doi: 10.3748/wjg.v26.i28.4076
Sato, Y., Yoneyama, O., Azumaya, M., Takeuchi, M., Sasaki, S. Y., Yokoyama, J., et al. (2015). The relationship between iron deficiency in patients with Helicobacter pylori-infected nodular gastritis and the serum prohepcidin level. Helicobacter 20, 11–18. doi: 10.1111/hel.12170
Seo, J. K., Ko, J. S., and Choi, K. D. (2002). Serum ferritin and Helicobacter pylori infection in children: a sero-epidemiologic study in Korea. J. Gastroenterol. Hepatol. 17, 754–757. doi: 10.1046/j.1440-1746.2002.02797.x
Sharndama, H. C., and Mba, I. E. (2022). Helicobacter pylori: an up-to-date overview on the virulence and pathogenesis mechanisms. Braz. J. Microbiol. 53, 33–50. doi: 10.1007/s42770-021-00675-0
Shesh, B. P., and Connor, J. R. (2023). A novel view of ferritin in cancer. Biochim. Biophys. Acta Rev. Cancer 1878:188917. doi: 10.1016/j.bbcan.2023.188917
Sitas, F., Yarnell, J., and Forman, D. (1992). Helicobacter pylori infection rates in relation to age and social class in a population of welsh men. Gut 33:1582. doi: 10.1136/gut.33.11.1582
Taye, B., Enquselassie, F., Tsegaye, A., Amberbir, A., Medhin, G., Fogarty, A., et al. (2015). Effect of early and current Helicobacter pylori infection on the risk of anaemia in 6.5-year-old Ethiopian children. BMC Infect. Dis. 15:270. doi: 10.1186/s12879-015-1012-y
WHO. (2022a) Anaemia in children <5 years Estimates by WHO region. Available online at: http://apps.who.int/gho/data/view.main.ANEMIACHILDRENREGv?lang=en (accessed April 01, 2022).
WHO. (2022b) Anaemia in women of reproductive age. Available online at: https://www.who.int/data/gho/data/themes/topics/indicator-groups/indicator-group-details/GHO/prevalence-of-anaemia-in-women-of-reproductive-age (accessed April 01, 2022).
Xia, W., Zhang, X., Wang, J., Sun, C., and Wu, L. (2012). Survey of anaemia and Helicobacter pylori infection in adolescent girls in Suihua, China and enhancement of iron intervention effects by H. pylori eradication. Br. J. Nutr. 108, 357–362. doi: 10.1017/S0007114511005666
Yanatori, I., Nishina, S., Kishi, F., and Hino, K. (2023). Newly uncovered biochemical and functional aspects of ferritin. FASEB J. 37:e23095. doi: 10.1096/fj.202300918R
Yokota, S., Toita, N., Yamamoto, S., Fujii, N., and Konno, M. (2013). Positive relationship between a polymorphism in Helicobacter pylori neutrophil-activating protein a gene and iron-deficiency anemia. Helicobacter 18, 112–116. doi: 10.1111/hel.12011
Zamani, A., Shariat, M., Oloomi Yazdi, Z., Bahremand, S., Akbari Asbagh, P., and Dejakam, A. (2011). Relationship between Helicobacter pylori infection and serum ferritin level in primary school children in Tehran-Iran. Acta Med. Iran. 49, 314–318.
Zhang, Y., Bi, J., Wang, M., Deng, H., and Yang, W. (2022). Correlation between Helicobacter pylori infection and iron deficiency in children. Pak. J. Med. Sci. 38, 1188–1192. doi: 10.12669/pjms.38.5.5175
Zhang, N., Yu, X., Xie, J., and Xu, H. (2021). New insights into the role of ferritin in Iron homeostasis and neurodegenerative diseases. Mol. Neurobiol. 58, 2812–2823. doi: 10.1007/s12035-020-02277-7
Keywords: Helicobacter pylori, iron-deficiency anemia, children, iron deficiency, iron store
Citation: Wang Z, Tan W, Xiong H, Huang J, Wei H, Li M, Luo J, An W, He L, Ma J, Xiao F and Wei H (2025) Impact of Helicobacter pylori infection on iron deficiency anemia in children: a systematic review and meta-analysis with early intervention implications. Front. Microbiol. 16:1541011. doi: 10.3389/fmicb.2025.1541011
Edited by:
Silvia Turroni, University of Bologna, ItalyReviewed by:
Alaullah Sheikh, Washington University in St. Louis, United StatesAugustina Jankauskiene, Vilnius University Hospital Santaros Clinics, Lithuania
Copyright © 2025 Wang, Tan, Xiong, Huang, Wei, Li, Luo, An, He, Ma, Xiao and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongshan Wei, ZHJ3ZWlAY2NtdS5lZHUuY24=
 Ziteng Wang
Ziteng Wang Wentao Tan
Wentao Tan Huanhuan Xiong
Huanhuan Xiong Jiali Huang2
Jiali Huang2 Jing Luo
Jing Luo Fan Xiao
Fan Xiao Hongshan Wei
Hongshan Wei