- Clinical Laboratory Center, People's Hospital of Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, China
Background: Epidemiological and clinical analyses of brucellosis are crucial for the development of surveillance and case management strategies.
Methods: We analyzed the epidemiological and clinical characteristics of 581 human brucellosis cases in Xinjiang. Demographic characteristics of patients with brucellosis and their clinical manifestations were collected and analyzed.
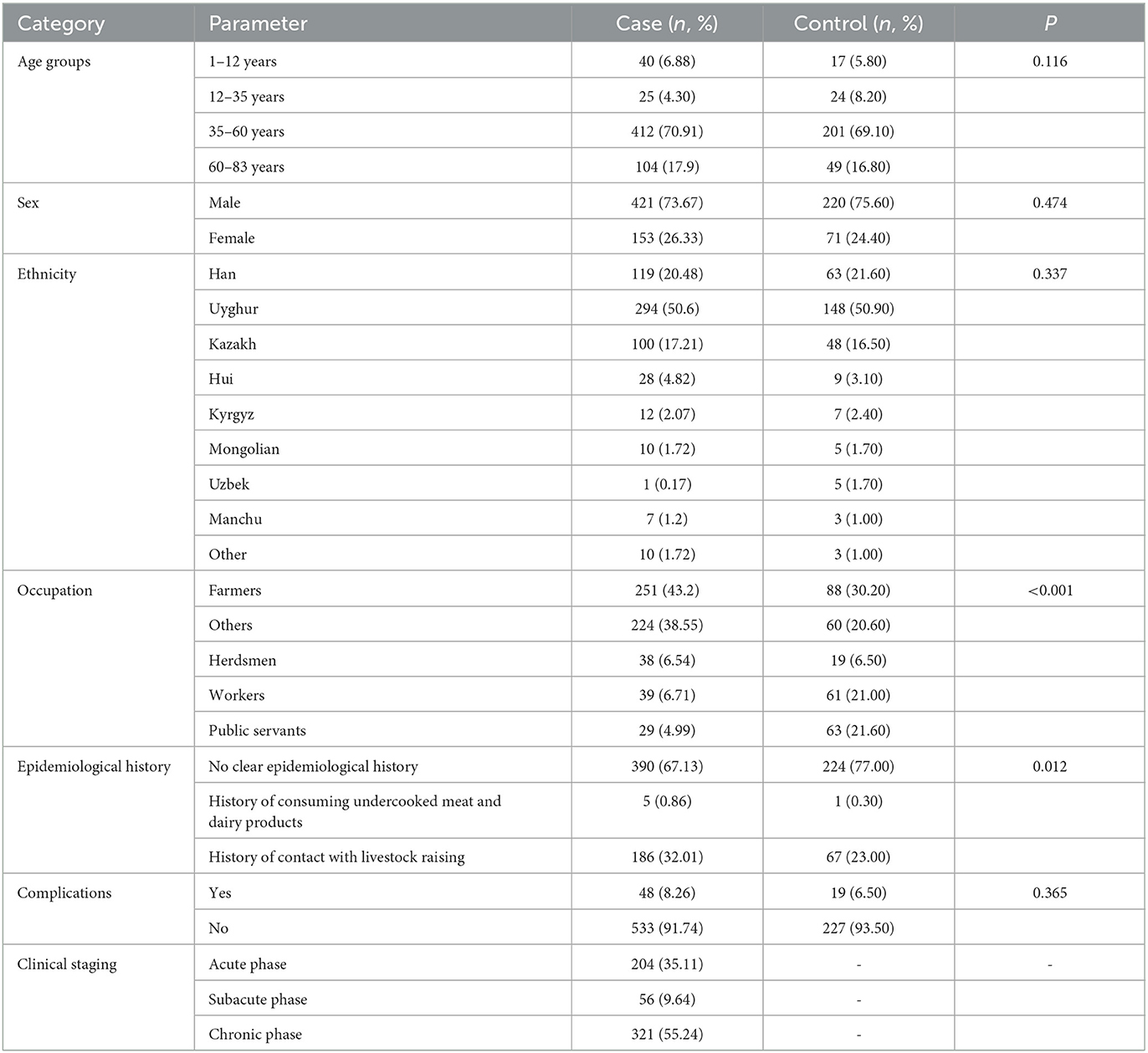
Results: Among the 581 brucellosis patients, the men-to-women ratio was 2.8:1.0 (428:153); the age was (44.41 ± 16.25) years, ranging from 1 to 83 years, mainly concentrated in the 35–60 age group, accounting for 70.91% (412 cases); the ethnic distribution was mainly Uyghur, accounting for 50.60%; the occupational distribution was mainly farmers, accounting for 43.20%. A total of 186 patients had a clear history of contact with cattle and sheep breeding. Clinical staging was mainly chronic stage patients, accounting for 55.24% (321 cases), and there were 48 cases with complications, mainly pain and fatigue, accounting for 8.26%. The most common laboratory examination characteristics were increased erythrocyte sedimentation rate and increased C-reactive protein level, accounting for 29.09% and 23.06%, respectively, and the blood culture detection rate was low (4.48 %).
Conclusion: Patients with brucellosis in the Xinjiang Uyghur Autonomous Region predominantly comprised middle-aged and young men primarily involved in farming. The principal clinical symptoms include pain and fever; however, the positivity rate of Brucella cultures in these patients is low. To minimize the risk of missed diagnoses or misdiagnoses, it is recommended to integrate epidemiological history, clinical manifestations, and laboratory examination results into the diagnostic process to facilitate earlier detection and treatment.
1 Introduction
Brucellosis is an important zoonotic disease primarily caused by Brucella species (Seleem et al., 2010). The Brucella species include Bcanis, suis, abortus, mellitensis, ceti, ovis, microti, pinnipedialis, neotomae, vulpis, papionis, and inopinata (Deng et al., 2019; Moriyón et al., 2023; Ullah et al., 2024). In animals, Brucella infection can cause reproductive failure (such as miscarriage, retained placenta, and inflammation of reproductive organs and membranes), arthritis, and bursitis (Sebzda and Kauffman, 2023; Wang et al., 2024; Moradkasani et al., 2024). Patients with brucellosis have symptoms including recurring fever, headaches, migratory joint pain, weakness, muscle pain, fatigue, loss of appetite, sweating, general discomfort, vomiting, and miscarriage (Głowacka et al., 2018). Some complex complications, such as osteomyelitis, sacroiliitis, septic arthritis, spondylodiscitis, and epidural abscesses, occur in clinical practice. In severe cases, they may cause serious diseases, such as meningitis and encephalitis, and even lead to death (Laine et al., 2023). Approximately 2.1 million new human brucellosis cases occur annually, and Asia and Africa account for most of the global risks and cases (Laine et al., 2023). Brucellosis transmission includes direct contact with infected animals, inhalation of airborne agents, consumption of contaminated products, occupational hazards, intrauterine transmission, and other indirect transmissions (Qureshi et al., 2023).
Brucellosis remains a major public health problem in Xinjiang, China (Shi et al., 2018), and is characterized by a high incidence and complex epidemiological situation. Xinjiang is an epidemic area of brucellosis mainly because of its agricultural practices and close contact between people and livestock, especially sheep and goats, which are common sources of infection. Camels and cattle are infected with brucellosis in Xinjiang (Liu et al., 2024a). Brucella melitensis is the most prevalent Brucella species in animals and humans in Xinjiang (Sun et al., 2016).
Epidemiological data have revealed that the disease mainly affects middle-aged individuals, especially those engaged in agricultural and animal husbandry activities. A large proportion of cases occur in individuals who have direct contact with infected animals or eat dairy products without high-temperature disinfection (Lai et al., 2017). Although treatment options are available, the chronic rate of brucellosis remains high, and the risk of recurrence is significant. The failure to eradicate Brucella is common in Xinjiang and its nomadic regions. The lack of cooperation among health officials, veterinary sectors, policymakers, and farmers has been the primary reason why these endemic countries have failed to control and prevent brucellosis in the past decades (Ali et al., 2024). Other factors, including low socioeconomic levels, weak awareness of brucellosis prevention, and difficulty in early diagnosis, could also explain the difficulty in eradicating Brucella (Qureshi et al., 2023). Nevertheless, the clinical management and public health intervention measures must be improved. This study aimed to analyze the epidemiological and clinical characteristics of human brucellosis cases in the Xinjiang region and provide valuable evidence for improving prognosis, reducing the occurrence of brucellosis, and relapse of the disease.
2 Methods
2.1 Patients
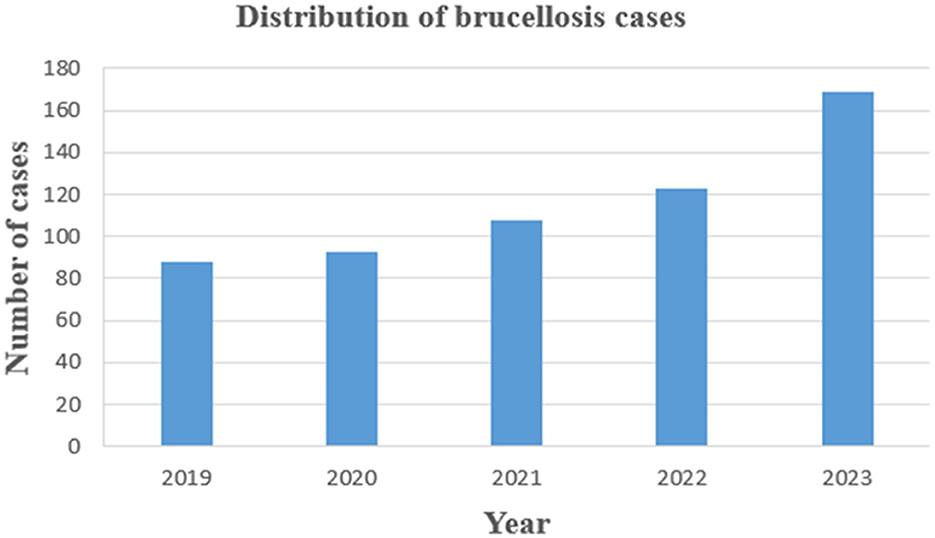
This study conducted a retrospective analysis of 581 patients with brucellosis exported from the Information Management System of the People's Hospital of Xinjiang Uyghur Autonomous Region from 2019 to 2023. Outpatient records of research subjects, laboratory indicator test results, clinical treatment, and other materials were collected, and treatment effects were collected through telephone. The distribution of 581 cases by year of diagnosis from 2019 to 2023 is shown in Figure 1. We also included 291 healthy control people for the identifying the relevant factors associated with brucellosis infection. A map showing the location of Xinjiang in China shows its borders with other endemic areas (Figure 2).
Figure 2. A map showing the location of Xinjiang in China shows its borders with other endemic areas.
2.2 Diagnosis of brucellosis
Brucellosis case diagnosis is based on the Chinese health industry standard “Brucellosis Diagnosis” (WS 269-2019, http://www.nhc.gov.cn/wjw/s9491/201905/b109b71e7a624256985b573944b5d292.shtml). Brucellosis was diagnosed based on a combination of epidemiological history, clinical manifestations, and laboratory tests. The diagnostic criteria for brucellosis were (1) epidemiological history, contact with suspected infected animals, consumption of infected meat, or other. (2) Clinical manifestations: fever, excessive sweating, fatigue, and muscle and joint pain. (3) Brucella detected in the pathogen culture. (4) Serum agglutination test (SAT) titer ≥ 1:50; or the rose Bengal plate agglutination test (RBT) is positive. The diagnosis of Brucella required the presence of criteria (1) or (2), as well as criteria (3) or (4). The clinical staging criteria for brucellosis: 1. acute phase: within 3 months of the course, with a confirmed positive serological reaction; 2. Subacute phase: within 3–6 months of the course, with a confirmed positive serological reaction; 3. Chronic phase: The course exceeds six months and has not healed, with symptoms and signs of brucellosis and a confirmed positive serological reaction. The exclusion criteria were (1) missing personal information or medical records, and (2) positive Brucella-specific antibody reactions due to vaccination against brucellosis.
2.3 Data collection
Each brucellosis case was comprehensively assessed, including detailed epidemiological contact history, physical examination, and biochemical analysis (blood cell count, routine biochemical parameters, and routine urine analysis). Laboratory values reported in the text and tables refer to the results of the patient's first examination. Patients with complications underwent additional imaging examinations, such as ultrasound, CT, MRI, echocardiography, and scrotal Doppler ultrasound. Relapse was defined as the reappearance of symptoms or a fourfold increase in the SAT titer within 6 months after treatment. All patients with brucellosis received treatments for 6–9 weeks and were followed up for 6 months. For healthy control, the following information was collected: age, sex, ethnicity, occupation, epidemiological history, and complications.
2.4 Ethics
The study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the People's Hospital of the Xinjiang Uyghur Autonomous Region. Informed consent was obtained from all patients and patient data were anonymized.
2.5 Statistical analysis
The continuous variables were shown as mean ± standard deviations and the categorical variables were presented as number and proportion (%). The t-test and chi-square test were used to analyze differences. Chi-square or Fisher's exact tests were used to analyze the differences in categorical variables; T test, One-way ANOVA, and nonparametric tests were used to compare the differences in continuous variables. Statistical significance was set at P < 0.05. Data were analyzed using SPSS software (version 21.0, SPSS Inc., Chicago, Ill., USA).
3 Results
3.1 Demographic and epidemiological characteristics
Among the 581 brucellosis patients, there were 428 men and 153 women, accounting for 73.67% and 26.33%, respectively, with a men-to-women ratio of 2.8:1.0. The age of the participants was 44.41 ± 16.25 years, ranging from 1 to 83 years, mainly concentrated in the 35–60 age group, with 412 cases accounting for 70.91%; followed by elderly patients (60–83 years old), with 104 cases accounting for 17.90%. The ethnic distribution was mainly Uyghur, accounting for 50.60% of the population. No significant differences were found for age, sex, and ethnic distribution between brucellosis group and healthy control group (P > 0.05). Brucellosis is an occupational disease affecting farmers and veterinarians and other people (Nedjma et al., 2022). The occupational distribution of this disorder was primarily farming (251 cases, 43.2 %) that was significantly higher than the control group (30.20%, P < 0.001).
Of the 390 patients with brucellosis (Table 1), 186 had a relatively clear history of contact with cattle and sheep breeding, and 5 had a history of consuming undercooked meat and dairy products, accounting for 67.13%, 32.01%, and 0.86%, respectively. The ratios were 77.0%, 0.30% and 23.0 in the control group, which was significantly lower than the case group (P = 0.012). In terms of clinical staging, chronic stage patients were the main group, with 204 cases (55.24 %); acute and subacute phase patients comprised 321 and 56 (35.11% and 9.64 %, respectively). Complications occurred in 48 (8.26%) patients and 19 (6.50%) in the control group, and there was no significance (P = 0.365).
3.2 Clinical symptoms and signs
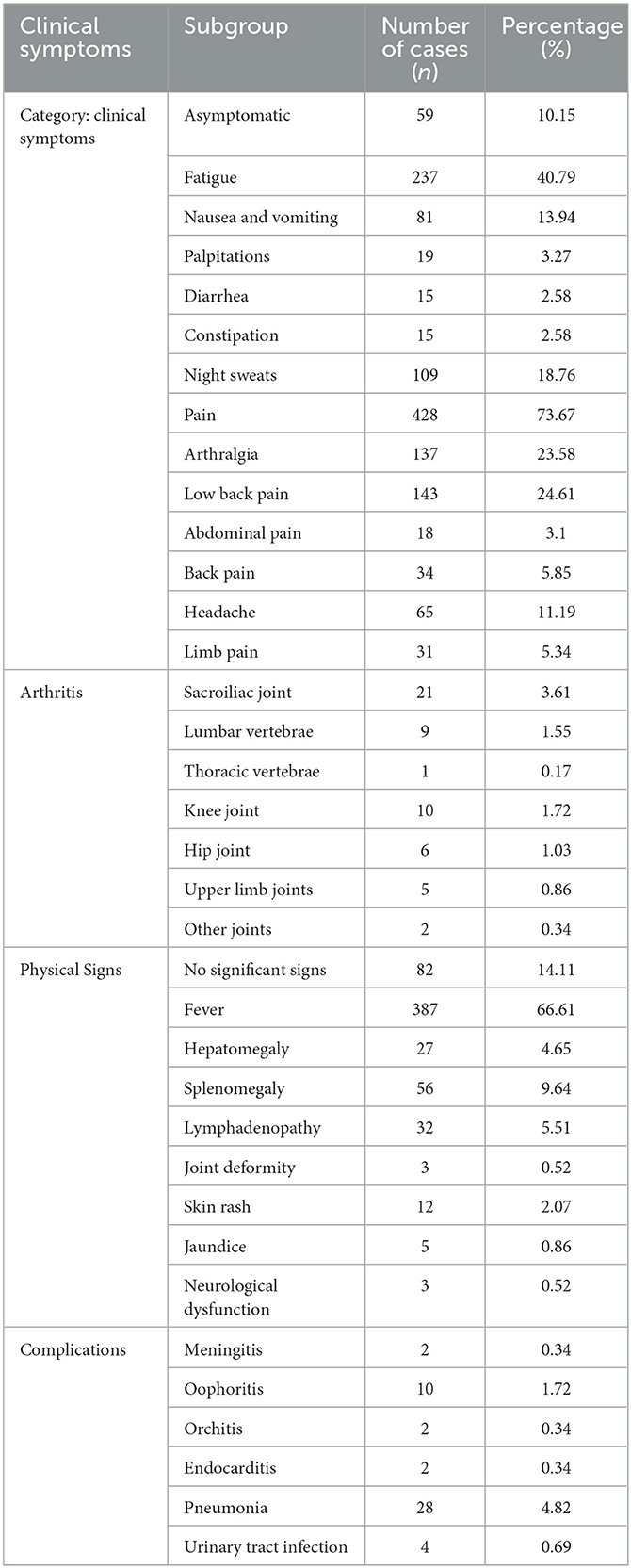
Among the 581 brucellosis patients, the main clinical symptom was pain, with 428 cases (73.67%), mainly lower back pain and joint pain, with 143 (24.61%) and 137 cases (23.58%), respectively; followed by fatigue with 237 cases (40.79%), night sweats with 109 cases (18.76%), nausea and vomiting with 81 cases (13.94%), arthritis with 54 cases (9.29%), palpitations with 19 cases (3.27%), and diarrhea and constipation with 15 cases each (2.58%); the other was asymptomatic, with 59 cases, accounting for 10.15%. There were 387 patients with fever, accounting for 66.61%; there were no significant signs in 82 cases (14.11 %), splenomegaly (56 cases, 9.64 %), lymph node enlargement (32 cases, 5.51 %), hepatomegaly (n = 27, 4.65%), skin rash (12 cases, 2.07 %; jaundice, 5 cases, 0.86 %), or neurological dysfunction, as shown in Table 2.
Male patients may also have orchitis, while female patients may show oophoritis. Acute phase patients can have a variety of rashes, some patients can have jaundice, and chronic phase patients show damage to the bone and joint systems.
3.3 Laboratory examinations
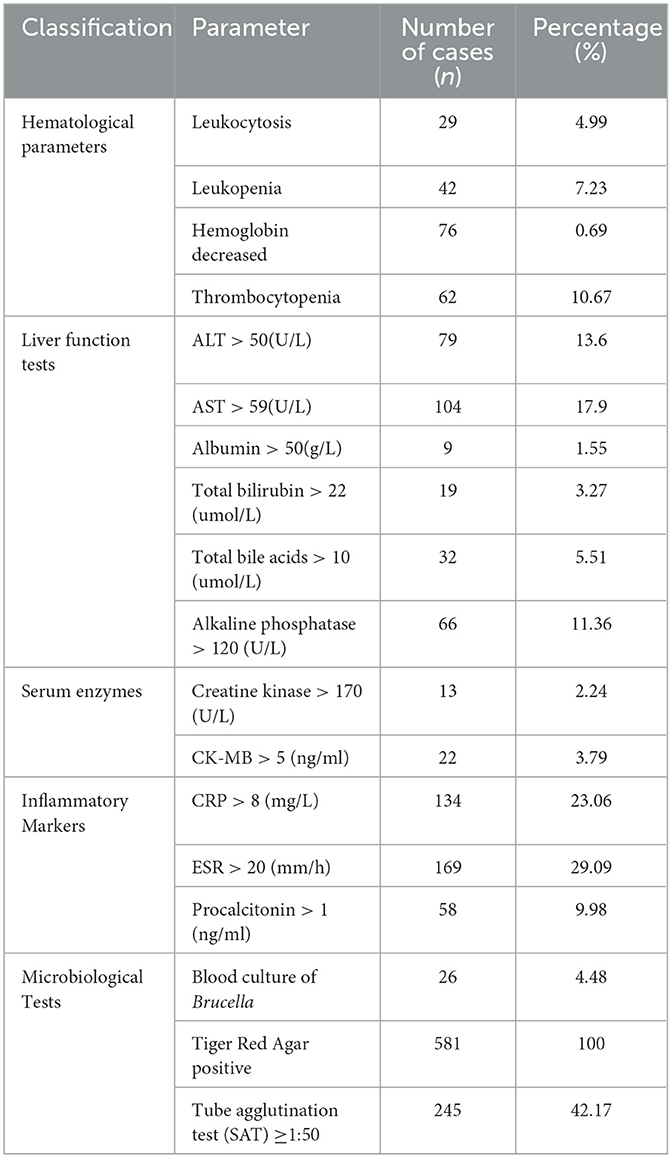
Among patients with brucellosis, the most significant changes after brucellosis infection were increased erythrocyte sedimentation rate (ESR) and increased C-reactive protein (CRP) levels, with 169 cases (29.09%) and 134 cases (23.06%), respectively. 87 cases (14.99%) had an increased proportion of monocytes/macrophages, leukopenia (42 patients, 7.23%), thrombocytopenia (62 patients, 10.67%), and decreased hemoglobin levels (76 patients, 0.69%). Biochemical examinations revealed an increase in glutamic-pyruvic transaminase and glutamic-oxaloacetic transaminase levels, with 79 cases (13.6%) and 104 cases (17.9%), respectively, and 66 cases (11.36%) with increased alkaline phosphatase levels, with other biochemical projects changing below 10%. Among the 581 patients, only 26 were infected with Brucella species, and all of them were infected with Brucella melitensis, while the positivity rates of SAT and Tiger Red Agar were high. The specific content could be seen in Table 3.
3.4 Treatment status and results
Of the 581 patients, 379 received first-line treatment (doxycycline combined with rifampicin), 174 received second-line treatment (levofloxacin, rifampicin, or doxycycline), and 28 received third-line treatment (ceftriaxone plus doxycycline or ceftriaxone sodium). The most commonly used treatment plans were levofloxacin, rifampicin, doxycycline, and amoxicillin clavulanate potassium, with 574 cases achieving clinical cure or improvement and seven cases of treatment failure (five patients had drug resistance and two patients did not comply with treatment protocols). During the follow-up period after treatment, 45 patients experienced relapse.
4 Discussion
This study retrospectively analyzed 581 human brucellosis cases in Xinjiang between 2019 and 2023 to elucidate the epidemiological characteristics, clinical features, and laboratory findings associated with brucellosis. The results of this study differ from those of previous research (Megged et al., 2016), revealing a higher proportion of chronic cases, which may be linked to the challenges of seeking medical treatment exacerbated by the COVID-19 pandemic. Moreover, restrictions on population mobility may have hindered the treatment of patients in the acute phase Brucellosis is a major zoonotic disorder with high prevalence in animals and humans, especially in the Middle East, Asia, Africa, and South America (Olsen and Palmer, 2014). Brucellosis is endemic in Asian countries. In the Middle East, brucellosis is highly endemic in the majority of countries (Kadir and Gülseren, 2023; Majdil et al., 2024). A study from Iran showed that the incidence rates ranged from 12.07/100,000 to 25.89/100,000 between 2009 and 2017 (Norouzinezhad et al., 2021). The Bekaa District in Lebanon had the highest human brucellosis incidence with 300/100,000 in 2019 (Hassan et al., 2020). The incidence rates in Palestine and Kuwait were 15.32/100,000 and 12.25/100,000, respectively, in 2018. In Saudi Arabia, the incidence rate was 14.17/100,000 in 2017 (Liu et al., 2024b). Among Central Asian countries, data indicated that the incidence rates of human brucellosis in Kazakhstan were 0.038/100,000 in 2019, 12.45/100,000 in Kyrgyzstan in 2018, 2.39/100,000 in Uzbekistan in 2018, 9.71/100,000 in Tajikistan in 2015, and 7.82/100,000 in Turkmenistan in 2016 (Liu et al., 2024b). This disease is also endemic to Pakistan (Tariq et al., 2024); however, brucellosis is highly misdiagnosed and underreported (Jamil et al., 2021). Xinjiang is located on the border with Kyrgyzstan, which is the most endemic region of brucellosis in animals and humans among the five central Asian countries (Kydyshov et al., 2022a,b). Although China is an important trading partner of Kyrgyzstan, the import and export of meat and animals are underdeveloped in both countries (Kydyshov et al., 2022b). A Chinese study reported an upward trend in the incidence of human brucellosis between 2004 and 2021 (Wen et al., 2024). Between 2016 and 2019, the average annual incidence of human brucellosis in China was 3.0/100, 000 individuals (Tao et al., 2021). According to the data from China's National Notifiable Disease Reporting System (Tao et al., 2021), the prevalence of Brucellosis in Xinjiang was 16.3/100,000 in 2019, ranking third in the country. The prevalence rates in Inner Mongolia and Ningxia were 54.4/100,000 and 31.96/100,000, respectively, in 2019 (Wang et al., 2021), which were higher than those in Xinjiang. The annual incidences in the northern region of China ranged from 3.1/100,000 to 28.2/100,000, while the incidence of human brucellosis was lower than 1.0/100,000 in the southern region of China (Tao et al., 2021). The incidence in northern China is significantly higher than that in southern China because animals such as cattle, goats, and sheep are more common in northern China (Lai et al., 2017). Human brucellosis is an ongoing epidemic in the above-mentioned countries, and one undeniable reason is that animal owners are directly involved in animal care and management.
Brucellosis is primarily associated with occupational exposure, dietary habits, and living conditions. Infected animals, along with contaminated food, water sources, and soil, can serve as vectors for the transmission of Brucella to humans (Qureshi et al., 2023). The infection rate of human brucellosis is closely correlated with the extent of contact between the sources of infection and the transmission vectors. The occupational risk associated with brucellosis is significant, particularly among individuals who handle sick animals, with farmers representing a major occupational group. Susceptibility to Brucella primarily depends on the frequency of contact opportunities (Tao et al., 2021). Brucellosis is an occupational disease that affects farmers, veterinarians, and other people. In this study, 390 patients with brucellosis had no clear epidemiological history. The percentage of patients with brucellosis without a clear contact history with animals infected with Brucella was very high. The reasons may be as follows. One, some cases indeed have a contact history with animals infected with Brucella, but the brucellosis patients did not realize this, which underestimates the actual percentage of animal contact history. Two, among 390 cases, their occupations were mostly workers, public servants, and others without a history of direct contact with animals infected with Brucella in this study. Besides, dairy products are also common of the source of contagion. In the Mediterranean and Latin America, infections have also occurred from consumption of unsterilized goat and camel milk products (Alhussain et al., 2022; Dianelys et al., 2022).
The incidence of Brucella infection is higher in males than in females, because men in Xinjiang are more likely to engage in livestock care activities. In our study, 213/428 men and 57/153 women engaged in occupations related to animals. The sex distribution in this study was consistent with that of previous studies (Jia et al., 2017; Shi et al., 2018; Abbas et al., 2025). Young and middle-aged individuals, who constitute the main labor force and frequently come into contact with sick animals, exhibit higher infection rates than those in other age groups. Furthermore, the infection rate of brucellosis is higher in pastoral areas than in urban regions because individuals in pastoral settings interact more frequently with livestock, thereby increasing their exposure to infection. In urban areas, cases are predominantly found among workers in fur, milk, and meat-processing enterprises (Abbas et al., 2025). Among the Brucella species, Brucella melitensis is the most pathogenic, with sick sheep being the primary source of infection in endemic regions (Shi et al., 2018). Brucella melitensis biovars 1, 2, and 3 are highly invasive and pathogenic to humans and animals, easily causing outbreaks and epidemics of human and animal brucellosis, with most presenting as typical clinical symptoms and signs (DelVecchio et al., 2002). This study found that the clinical symptoms and signs of brucellosis primarily include fever, night sweats, fatigue, joint pain, and hepatosplenomegaly [17]. Following infection, Brucella can affect multiple organs in the body, leading to corresponding symptoms and signs. Although involvement of the cardiovascular system occurs at a low incidence, it can cause brucellosis-related deaths [18]. In this study, liver function abnormalities were more prevalent among brucellosis patients, with a notable frequency of liver involvement. Patients may exhibit varying degrees of liver function abnormalities, and in some cases, develop liver abscesses. Notably, some patients present with liver function abnormalities as an initial manifestation when seeking medical attention, ultimately leading to a diagnosis of brucellosis.
The clinical manifestations of brucellosis are nonspecific; thus, if clinicians do not prioritize brucellosis in their differential diagnosis and fail to conduct relevant laboratory tests [19], misdiagnosis may occur, resulting in delayed diagnosis and treatment, which can prolong the disease course [20]. In this study, increased erythrocyte sedimentation rate, elevated proportions of monocytes/macrophages, elevated levels of CRP, decreased hemoglobin, and reduced platelet counts were all relatively common laboratory manifestations observed in patients with brucellosis. Several studies have investigated the role of ESR and CRP levels in the diagnosis of brucellosis. Halil et al. showed that the AUCs of CRP and ESR for the diagnosis of brucellosis were 0.635 (sensitivity=57.6, specificity=65.7) and (sensitivity=68.3, specificity=62.9), respectively (Halil and Süleyman, 2020). The cut-off values of CRP and ESR for diagnosing brucellosis complications were > 5.4 mg/L (sensitivity 73.4% and specificity 51.9%) and > 25 mm/h (sensitivity 47.9% and specificity 71.1%) (Shi et al., 2024). A Chinese study of 2,041 human brucellosis cases by Shi et al. revealed that CRP and ESR were not risk factors for an unfavorable prognosis for brucellosis patients (Shi et al., 2018). In their study, the percentages of increased ESR and CRP levels were 69% and 39%, respectively (Shi et al., 2018) which were higher than those observed in our study (CRP=23.06%, ESR = 23.09%). We believe that the higher percentage of acute brucellosis in our study could explain the higher rates of increased CRP levels and ESR observed in the study by Shi et al. (2018). In summary, CRP and ESR are important markers to diagnose brucellosis; however, their sensitivity and specificity are not sufficiently large, which requires other laboratory diagnostic indicators and diagnostic methods to compensate for their shortcomings in diagnosing brucellosis. Currently, the primary basis for laboratory diagnoses relies on various biological agglutination tests. Although bacterial culture positivity is considered the gold standard for the diagnosis of brucellosis, the positive rate was notably low. A diagnosis of brucellosis should be made in conjunction with the patient's epidemiological contact history, clinical manifestations, and laboratory findings for a comprehensive assessment. Notably, other more sensitive alternatives, such as molecular diagnostic methods (e.g., PCR and NGS), were not used in this study, which is a limitation of this study. As this study was retrospective, we could not present the PCR or NGS data. Notably, molecular diagnostic methods (e.g., PCR and NGS) show high sensitivity and specificity for the diagnosis of brucellosis according to data from our hospital in 2024 (data not shown here). Hererin, we recommend that it is necessary to use these molecular diagnostic methods combined with other diagnostic methods to comprehensively diagnose brucellosis. Special attention is warranted when differentiating patients with a history of residing in Brucella-endemic areas. In this study, doxycycline combined with rifampicin remained the primary first-line treatment. However, previous studies have indicated that, compared to doxycycline combined with streptomycin, the combination of doxycycline and rifampicin is more prone to treatment failure and relapse (Reza et al., 2012; Solis and Solera, 2012). This may explain the 7.75% (45/581) recurrence rate observed during the six-month follow-up period. Numerous clinical studies have demonstrated that the efficacy of doxycycline combined with streptomycin is comparable to that of doxycycline combined with gentamicin, suggesting that the treatment regimen could be adjusted accordingly for recurrent cases (Roushan et al., 2010; Hasanjani et al., 2006). The combined use of antimicrobial drugs remains the primary treatment method for brucellosis, and an adequate treatment duration is essential to enhance the cure rate and minimize the risk of relapse (Majzoobi et al., 2022).
This study had some limitations. One, the sample size of this study was not very large. Two crucial indices and data were not presented in this study. Third, we address how the characteristics of Xinjiang shed light on regions with similar socioeconomic structures worldwide. Fourth, the challenges and limitations of generalizing our study findings to other populations should be discussed more explicitly. Different levels of government management, socioeconomic status, and educational level may restrict Xinjiang's experience in other regions.
5 Conclusion
Given the high incidence of brucellosis in Xinjiang, specific public health measures such as educational campaigns and vaccination programs have been implemented by the government. Although human brucellosis prevention and control have progressed in recent years, brucellosis remains a public health burden in Xinjiang. Central Asian countries surrounding Xinjiang have taken similar health measures to control brucellosis. Continued efforts are essential to enhance public health response measures, improve clinical management, promote vaccination programs including the vaccination of the livestock, and increase community awareness through educational campaigns (Marvi et al., 2018; Laine et al., 2023) to effectively prevent and control this zoonotic disease in these regions. Besides, the primary hosts of brucellosis are cattle (Brucella abortus), camel and goats (Brucella melitensis), and pigs (Brucella suis). etc. For these animal hosts, targeted vaccination is an important measure to effectively prevent brucellosis infection. Implementing surveillance and brucellosis testing is crucial for early detection of this disorder. In Kazakhstan, the incidence of human brucellosis decreased from 2007 to 2019 owing to the implementation of a vaccination program (Liu et al., 2024b). Overall, human brucellosis in these regions is endemic, and brucellosis control and eradication are still difficult because livestock farming may be their main job and only source of livelihood for these brucellosis patients. Their religious beliefs about animals, lifestyle (such as eating habits), and low socioeconomic levels are the key reasons for this (Hikal et al., 2023).
The current situation of human brucellosis in Xinjiang illustrates the intricate interplay between epidemiological, clinical, and socioeconomic factors. As Xinjiang has a high incidence of brucellosis, timely detection and management of the disease are imperative. This study summarizes the clinical and laboratory characteristics of brucellosis in Xinjiang to elucidate its epidemiology, clinical symptoms, and signs in this region. This study aimed to enhance the understanding of brucellosis among clinical and laboratory physicians, reduce instances of misdiagnosis and mistreatment, and provide a foundation for optimizing diagnosis and treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the People's Hospital of Xinjiang Uyghur Autonomous Region. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
BL: Writing – review & editing, Data curation, Resources, Validation. QW: Writing – original draft. SY: Writing – original draft. XS: Writing – original draft. ZL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Outstanding Youth Science Fund of Xinjiang Uygur Autonomous Regional Natural Science Foundation (2022D01E30), Xinjiang Uygur Autonomous Regional “Tianshan” Talent Program (2022TSYCCX0102), Xinjiang Uygur Autonomous Regional Science and Technology Support Program (2022E02118), Natural Science Foundation of Xinjiang Uygur Autonomous Region (2023D01C83, 2023D01C84, 2024D01C86), and Karamay Central Hospital In-Hospital Project (20240103). The funders did not play any role in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, F., Ali, S., Muhammad, A., Azam, A., Moawad, A. A., Ejaz, M., et al. (2025). Human brucellosis in the rural and urban population of Pakistan: seroprevalence, risk factors, and clinical manifestations. Curr. Microbiol. 82:80. doi: 10.1007/s00284-025-04063-x
Alhussain, H., Zughaier, S., Gawish, A., Mahmoud, M., Yassine, H., Al, T., et al. (2022). Seroprevalence of camel brucellosis in Qatar. Trop. Anim. Health. Prod. 54:351. doi: 10.1007/s11250-022-03335-z
Ali, S., Mushtaq, A., Hassan, L., Syed, M. A., Foster, J. T., and Dadar, M. (2024). Molecular epidemiology of brucellosis in Asia: insights from genotyping analyses. Vet. Res. Commun. 48, 3533–3550. doi: 10.1007/s11259-024-10519-5
DelVecchio, V. G., Kapatral, V., Elzer, P., Patra, G., and Mujer, C. V. (2002). The genome of Brucella melitensis. Vet. Microbiol. 90, 587–592. doi: 10.1016/S0378-1135(02)00238-9
Deng, Y., Liu, X., Duan, K., and Peng, Q. (2019). Research progress on brucellosis. Curr. Med. Chem. 26, 5598–5608. doi: 10.2174/0929867325666180510125009
Dianelys, S., Ricardo, G., Magda, C., Beatriz, A., Patricia, T., and Carlos, R. (2022). Evaluation of the fluorescence polarization assay for the diagnosis of brucellosis in goat milk. Vet. Sci. 9:303. doi: 10.3390/vetsci9060303
Głowacka, P., Żakowska, D., Naylor, K., Niemcewicz, M., and Bielawska-Drózd, A. (2018). Brucella – virulence factors, pathogenesis and treatment. Pol. J. Microbiol. 67, 151–161. doi: 10.21307/pjm-2018-029
Halil, K., and Süleyman, G. (2020). Investigation of the sensitivity and specificity of laboratory tests used in differential diagnosis of childhood brucellosis. Cureus 12:e6756. doi: 10.7759/cureus.6756
Hasanjani, R., Mohraz, M., Hajiahmadi, M., Ramzani, A., and Valayati, A. (2006). Efficacy of gentamicin plus doxycycline vs. streptomycin plus doxycycline in the treatment of brucellosis in humans. Clin. Infect. Dis. 42, 1075–1080. doi: 10.1086/501359
Hassan, H., Salami, A., Ghssein, G., El-Hage, J., Nehme, N., and Awada, R. (2020). Seroprevalence of Brucella abortus in cattle in Southern Lebanon using different diagnostic tests. Vet. World. 13, 2234–2242. doi: 10.14202/vetworld.2020.2234-2242
Hikal, A. F., Gamal, W., and Ashraf, K. (2023). Brucellosis: Why is it eradicated from domestic livestock in the United States but not in the Nile River Basin countries? Ger. J. Microbiol. 3, 19–25. doi: 10.51585/gjm.2023.2.0026
Jamil, T., Khan, A. U., Saqib, M., Hussain, M. H., Melzer, F., Rehman, A., et al. (2021). Animal and human brucellosis in Pakistan. Front. Public Health. 9:660508. doi: 10.3389/fpubh.2021.660508
Jia, B., Zhang, F., Lu, Y., Zhang, W., Li, J., Zhang, Y., et al. (2017). The clinical features of 590 patients with brucellosis in Xinjiang, China with the emphasis on the treatment of complications. PLOS Negl. Trop. Dis. 11:e0005577. doi: 10.1371/journal.pntd.0005577
Kadir, A., and Gülseren, Y. Ö. (2023). The first report of Brucella melitensis biovar 2 strain isolated from cattle in Turkey. Ger. J. Vet. Res. 3, 11–15. doi: 10.51585/gjvr.2023.2.0053
Kydyshov, K., Usenbaev, N., Berdiev, S., Dzhaparova, A., Abidova, A., Kebekbaeva, N., et al. (2022a). First record of the human infection of Brucella melitensis in Kyrgyzstan: evidence from whole-genome sequencing-based analysis. Infect. Dis. Poverty. 11:120. doi: 10.1186/s40249-022-01044-1
Kydyshov, K., Usenbaev, N., Sharshenbekov, A., Aitkuluev, N., Abdyraev, M., Chegirov, S., et al. (2022b). Brucellosis in humans and animals in Kyrgyzstan. Microorganisms 10:1293. doi: 10.3390/microorganisms10071293
Lai, S., Zhou, H., Xiong, W., Gilbert, M., Huang, Z., Yu, J., et al. (2017). Changing epidemiology of human brucellosis, China, 1955–2014. Emerg. Infect. Dis. 23, 184–194. doi: 10.3201/eid2302.151710
Laine, C. G., Johnson, V. E., Scott, H. M., and Arenas-Gamboa, A. M. (2023). Global estimate of human brucellosis incidence. Emerg. Infect. Dis. 29, 1789–1797. doi: 10.3201/eid2909.230052
Liu, X., Shi, G., Li, L., Zhang, R., and Qiao, J. (2024a). Detection and molecular typing of epidemic Brucella strains among camels, sheep, and cattle in Xinjiang, China. PLoS ONE. 19:e0311933. doi: 10.1371/journal.pone.0311933
Liu, Z., Gao, L., Wang, M., Yuan, M., and Li, Z. (2024b). Long ignored but making a comeback: a worldwide epidemiological evolution of human brucellosis. Emerg. Microbes Infect. 13:2290839. doi: 10.1080/22221751.2023.2290839
Majdil, I., Marie, H., and Gamal, W. (2024). Brucellosis in Iraq: a comprehensive overview of public health and agricultural challenges. Ger. J. Microbiol. 4, 10–20. doi: 10.51585/gjm.2024.3.0039
Majzoobi, M. M., Hashmi, S. H., Emami, K., and Soltanian, A. R. (2022). Combination of doxycycline, streptomycin and hydroxychloroquine for short-course treatment of brucellosis: a single-blind randomized clinical trial. Infection 50, 1267–1271. doi: 10.1007/s15010-022-01806-x
Marvi, A., Asadi-Aliabadi, M., Darabi, M., Abedi, G., Siamian, H., and Rostami-Maskopaee, F. (2018). Trend analysis and affecting components of human brucellosis incidence during 2006 to 2016. Med. Arch. 72, 17–21. doi: 10.5455/medarh.2018.72.17-21
Megged, O., Chazan, B., Ganem, A., Ayoub, A., Yanovskay, A., Sakran, W., et al. (2016). Brucellosis outbreak in children and adults in two areas in Israel. Am. J. Trop. Med. Hyg. 95, 31–34. doi: 10.4269/ajtmh.16-0116
Moradkasani, S., Goodarzi, F., Beig, M., Tadi, D. A., and Sholeh, M. (2024). Prevalence of Brucella melitensis and Brucella abortus aminoglycoside-resistant isolates: a systematic review and meta-analysis. Braz. J. Microbiol. 55, 429–439. doi: 10.1007/s42770-023-01233-6
Moriyón, I., Blasco, J. M., Letesson, J. J., De Massis, F., and Moreno, E. (2023). Brucellosis and one health: inherited and future challenges. Microorganisms 11:2070. doi: 10.3390/microorganisms11082070
Nedjma, L., Djamila, Y., Dihya, T., and Safia, Z. (2022). A survey on the occupational exposure of veterinarians to brucellosis in Algeria. Ger. J. Microbiol. 2, 28–35. doi: 10.51585/gjm.2022.2.0017
Norouzinezhad, F., Erfani, H., Norouzinejad, A., Ghaffari, F., and Kaveh, F. (2021). Epidemiological characteristics and trend in the incidence of human brucellosis in Iran from 2009 to 2017. J. Res. Health Sci. 21:e00535. doi: 10.34172/jrhs.2021.70
Olsen, S. C., and Palmer, M. V. (2014). Advancement of knowledge of Brucella over the past 50 years. Vet. Pathol. 51, 1076–1089. doi: 10.1177/0300985814540545
Qureshi, K. A., Parvez, A., Fahmy, N. A., Abdel Hady, B. H., Kumar, S., Ganguly, A., et al. (2023). Brucellosis: epidemiology, pathogenesis, diagnosis and treatment-a comprehensive review. Ann. Med. 55:2295398. doi: 10.1080/07853890.2023.2295398
Reza, Y., Sameh, M., Mehdi, M., and Parham, S. (2012). Antibiotics for treating human brucellosis. Cochrane Database Syst. Rev. 10:CD007179. doi: 10.1002/14651858.CD007179.pub2
Roushan, M., Amiri, M., Janmohammadi, N., Hadad, M., Javanian, M., Baiani, M., et al. (2010). Comparison of the efficacy of gentamicin for 5 days plus doxycycline for 8 weeks vs. streptomycin for 2 weeks plus doxycycline for 45 days in the treatment of human brucellosis: a randomized clinical trial. J. Antimicrob. Chemother. 65, 1028–1035. doi: 10.1093/jac/dkq064
Sebzda, M. K., and Kauffman, L. K. (2023). Update on Brucella canis: understanding the past and preparing for the future. Vet. Clin. North Am. Small Anim. Pract. 53, 1047–1062. doi: 10.1016/j.cvsm.2023.05.002
Seleem, M. N., Boyle, S. M., and Sriranganathan, N. (2010). Brucellosis: a re-emerging zoonosis. Vet. Microbiol. 140, 392–398. doi: 10.1016/j.vetmic.2009.06.021
Shi, Q. N., Qin, H. J., Lu, Q. S., Li, S., Tao, Z. F., Fan, M. G., et al. (2024). Incidence and warning signs for complications of human brucellosis: a multi-center observational study from China. Infect. Dis. Poverty. 13:18. doi: 10.1186/s40249-024-01186-4
Shi, Y., Gao, H., Pappas, G., Chen, Q., Li, M., Xu, J., et al. (2018). Clinical features of 2041 human brucellosis cases in China. PLoS ONE. 13:e0205500. doi: 10.1371/journal.pone.0205500
Solis, G., and Solera, J. (2012). Systematic review and meta-analysis of randomized clinical trials in the treatment of human brucellosis. PLoS ONE. 7:e32090. doi: 10.1371/journal.pone.0032090
Sun, M. J., Di, D. D., Li, Y., Zhang, Z. C., Yan, H., Tian, L. L., et al. (2016). Genotyping of Brucella melitensis and Brucella abortus strains currently circulating in Xinjiang, China. Infect. Genet. Evol. 44, 522–529. doi: 10.1016/j.meegid.2016.07.025
Tao, Z., Chen, Q., Chen, Y., Li, Y., Mu, D., Yang, H., et al. (2021). Epidemiological characteristics of human brucellosis – China, 2016–2019. China CDC Wkly. 3, 114–119. doi: 10.46234/ccdcw2021.030
Tariq, J., Sana, I., and Vassilios, S. (2024). Exploring in vivo and in vitro infection models in brucellosis research: a mini-review. Ger. J. Vet. Res. 4, 32–38. doi: 10.51585/gjvr.2024.1.0072
Ullah, I., Naz, S., Khattak, U. S., Saeed, M., Akbar, N. U., and Rauf, S. (2024). Molecular prevalence, phylogenetic analysis, and PCR-based detection of Brucella melitensis in humans and cattle in Southern Khyber Pakhtunkhwa, Pakistan. Comp. Immunol. Microbiol. Infect. Dis. 115, 102262. doi: 10.1016/j.cimid.2024.102262
Wang, Y., Vallée, E., Heuer, C., Wang, Y., Guo, A., Zhang, Z., et al. (2024). A scoping review on the epidemiology and public significance of Brucella abortus in Chinese dairy cattle and humans. One Health 18:100683. doi: 10.1016/j.onehlt.2024.100683
Wang, Z., Lin, S., Liu, X., Yu, A., Muhtar, H., Bayidawulieti, J., et al. (2021). Brucellosis knowledge and personal protective equipment usage among high-risk populations in brucellosis-endemic areas – China, 2019–2020. China CDC Wkly. 3, 106–109. doi: 10.46234/ccdcw2021.028
Keywords: brucellosis, Xinjiang, epidemiology, clinical characteristics, laboratory characteristics
Citation: Luo B, Wang Q, Yang S, Song X and Li Z (2025) Epidemiological, clinical, and laboratory characteristics of 581 human brucellosis cases in Xinjiang, China. Front. Microbiol. 16:1541277. doi: 10.3389/fmicb.2025.1541277
Received: 11 December 2024; Accepted: 15 April 2025;
Published: 07 May 2025.
Edited by:
Eduardo H. Gotuzzo, Universidad Peruana Cayetano Heredia, PeruReviewed by:
Gamal Wareth, Friedrich Loeffler Institut, GermanyLauren Wood Stranahan, Texas A and M University, United States
Julián Solís García Del Pozo, Complejo Hospitalario Universitario de Albacete, Spain
Copyright © 2025 Luo, Wang, Yang, Song and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiwei Li, MTM4OTk5OTQ0NTVAMTYzLmNvbQ==
 Bin Luo
Bin Luo Qian Wang
Qian Wang Zhiwei Li
Zhiwei Li