- 1School of Medicine, Henan Provincial Key Laboratory of Nanocomposites and Applications, Institute of Nano-Structured Functional Materials, Huanghe Science and Technology University, Zhengzhou, China
- 2School of Pharmacy, Henan University, Kaifeng, China
- 3Jiangsu Key Laboratory for the Research and Utilization of Plant Resources, Institute of Botany, Jiangsu Province and Chinese Academy of Sciences (Nanjing Botanical Garden, Mem. Sun Yat-Sen), Nanjing, China
Background: Benign prostatic hyperplasia (BPH) significantly compromises patients' quality of life, has detrimental effects on both physical and mental well-being, and poses a substantial economic burden. Current pharmacological interventions primarily focus on symptom relief; nevertheless, their associated adverse effects are evident. Rape bee pollen and maca are both homologous food products, which are not only rich in nutrition, but also have many biological activities. There is no report on the combination of rape bee pollen and maca in benign prostatic hyperplasia therapy, and no report on the molecular mechanism. This study aims to systematically investigate the therapeutic efficacy and underlying molecular mechanisms of a chewable tablet formulation containing rape bee pollen and maca in the management of BPH.
Methods: First, the chewable tablets were prepared from rape bee pollen and maca, and the High Performance Liquid Chromatography (HPLC) fingerprint was established. During a 4-week period, rats were subjected to subcutaneous injections of testosterone propionate solution to establish a BPH model, while simultaneously receiving orally administered chewable tablets for BPH treatment via gavage. We assessed the body weight, prostate tissue index, serum levels of dihydrotestosterone (DHT), testosterone (T), estradiol (E2), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), superoxide dismutase (SOD), malondialdehyde (MDA) and glutathione (GSH) levels in rats. Prostate tissues were collected for histopathological analysis. The protein expression levels of IL-6, phosphorylated Janus kinase 2 (p-JAK2), and phosphorylated signal transducer and activator of transcription 3 (p-STAT3) were determined by Western blotting. Rat feces were collected aseptically for 16S rDNA gene sequencing to assess gut microbiota levels. Additionally, our research findings demonstrate that the combined application of rape pollen and maca exhibits superior therapeutic efficacy compared to the individual use of either rape pollen or maca alone.
Results: We prepared the rape bee pollen and maca chewable tablets, established the HPLC fingerprint map, and quantitatively analyzed four of these main components. We found that the rape bee pollen and maca chewable tablets significantly inhibited DHT, T, E2, E2/T ratio, IL-6, TNF-α, and IL-1β and reduced MDA levels and increased SOD, GSH levels. 16S rDNA gene sequencing revealed that the chewable tablet increased the abundance of the beneficial bacteria Faecalibacterium, Collinsella, Phascolarctobacterium, Kineothrix, and Lactobacillus, and decreased that of the pro-inflammatory bacterium Prevotella. Western blot indicated that the chewable tablet could significantly inhibit the IL-6, p-JAK2, and p-STAT3 protein expression levels.
Conclusion: We found that rape bee pollen and maca chewable tablets had a protective effect on rats with BPH, and also found that the combination of rape bee pollen and maca was better than separate application. The mechanism may be related to the gut microbiota and IL-6/JAK2/STAT3 signaling pathway.
1 Introduction
Benign prostatic hyperplasia (BPH) is a highly prevalent condition in elderly males, with approximately 30%−40% of men affected by age 40 and 70%−80% of men affected by age 85 (Cannarella et al., 2021; Welén and Damber, 2022). This disorder leads to lower urinary tract symptoms (LUTS), including urinary hesitation, intermittent urinary stream, and straining during urination, thereby significantly compromising patients' quality of life and imposing substantial physical, mental, and economic burdens globally (Ho and Habib, 2011; Song et al., 2023). Recent global statistics indicate that over 94 million men worldwide experience BPH-related symptoms as of 2019 (Awedew et al., 2022). The pathophysiology of BPH involves imbalances in epithelial and stromal cell proliferation and apoptosis within the prostate's transition zone, leading to glandular and stromal hyperplasia (Shah et al., 2021). Specifically, epithelial hyperplasia forms larger glandular nodules, while stromal hyperplasia contributes to diffuse extracellular matrix deposition, particularly of type 1 collagen (Hata et al., 2023). These structural changes collectively disrupt urinary function and exacerbate LUTS severity, underscoring the need for effective therapeutic interventions.
Disease development and treatment are closely intertwined with the gut microbiome. Accumulating evidence has established a bidirectional relationship between BPH and gut microbiota dysregulation. For instance, recent studies elucidating the gut–prostate axis have revolutionized our understanding of BPH pathogenesis (Liu et al., 2021; Li et al., 2024). Animal models further revealed that high-fat diet-induced imbalances in the gut microbiome—particularly elevated ratios of Bacteroidetes to Firmicutes—are associated with BPH development (Gu et al., 2021). Therapeutic interventions such as finasteride treatment restored the abundance of beneficial gut bacteria, including Lactobacillus and Acetatifactor, to baseline levels in BPH patients (An et al., 2023). Mechanistically, gut microbiota perturbations may disrupt prostate homeostasis through immune activation and proinflammatory cytokine secretion, thereby promoting pathological inflammation (Russo et al., 2023).
Chronic inflammation has been identified as a key factor in the development and course of certain human illnesses, including prostate cancer and BPH. Interleukin-6 (IL-6) is a cytokine from the gp130 family that mediates the process of inflammation (Staršíchová et al., 2010). The IL-6/JAK2/STAT3 pathway is a key inflammatory signaling pathway. Numerous physiological and pathological processes, including angiogenesis, immunological control, and cell division and proliferation, are influenced by this pathway (Huang et al., 2022). Excessive IL-6 production in response to inflammatory stimulation is an effective stimulator of the JAK/STAT signaling pathway. IL-6 may contribute to inflammation by activating this pathway, which promotes epithelial–mesenchymal transition (Xiao et al., 2017; Singh et al., 2020). Studies have demonstrated that canagliflozin delays the progression of BPH by inhibiting the JAK2/STAT3 pathway (Elbaz et al., 2023).
The primary pharmacological interventions for BPH remain 5α-reductase inhibitors, α1-receptor antagonists, and PDE5 inhibitors (Antoniou et al., 2023). Adverse events associated with 5α-reductase inhibitors encompass erectile dysfunction, ejaculatory disorders, mood disturbances (depression/anxiety), and an elevated risk of prostate cancer progression (Miernik and Gratzke, 2020; Stewart and Lephart, 2023). Meanwhile, α1-receptor blockers are characterized by side effects including orthostatic hypotension, sedation, ejaculatory abnormalities, cognitive impairment, and depressive symptoms. These limitations have catalyzed research interest in complementary/alternative therapies, particularly phytotherapeutic agents (Stewart and Lephart, 2023). Rape bee pollen (Brassica napus L.) emerges as a dual-use agent in traditional Chinese medicine and functional nutrition. Its bioactive profile comprises polyunsaturated fatty acids, flavonoids, and a comprehensive array of vitamins (Chen et al., 2020; Han et al., 2023), manifesting pleiotropic physiological activities including antioxidative, anti-inflammatory, immunomodulatory, antimicrobial, and organoprotective effects (Rodríguez-Pólit et al., 2023). Specifically, its extract modulates gut microbiota composition in BPH rat models by promoting beneficial taxa (Coprococcus and Jeotgalicoccus) while suppressing pathogenic microorganisms (Turicibacter and Clostridiaceae_clostridium) (Qiao et al., 2023). Additionally, rape bee pollen exerts anti-BPH activity through cyclooxygenase-2 (COX-2 pathway inhibition) (Yang et al., 2014). Maca, also known as Peruvian ginseng, was classified as a health food in 2011. Maca root contains numerous bioactive compounds, including macaamide, macaene, glucosinolates, polyphenols, and alkaloids, which can mitigate sexual dysfunction. It also has neuroprotective, memory-enhancing, antidepressant, antioxidant, anti–prostate hyperplasia, anti-inflammatory, and skin-protective properties (Da Silva Leitão Peres et al., 2020). Previous studies have demonstrated that maca can be utilized to treat BPH. Maca can decrease testosterone propionate–induced BPH by preventing dihydrotestosterone conversion (Zou et al., 2017) and can regulate inflammation in patients with BPH by boosting the levels of anti-inflammatory cytokines (IL-4, IFN-γ) and reducing those of pro-inflammatory cytokines (tumor necrosis factor-α) (Vásquez-Velásquez et al., 2020; Ulloa Del Carpio et al., 2024).
In summary, both rape bee pollen and maca exhibit therapeutic potential against BPH. This study formulated chewable tablets using these two ingredients to evaluate their synergistic effects in treating BPH, elucidate the underlying molecular mechanisms, and establish a standardized fingerprint chromatogram for quality control purposes.
2 Materials and methods
2.1 Chemicals and reagents
Testosterone propionate was purchased from Aladdin (Shanghai, China), maca was purchased from Beijing Tongrentang Co., Ltd (Beijing, China), and rape bee pollen was purchased from Henan Mileyuan Beekeeping Specialized Cooperative (Henan, China). Lactose, microcrystalline cellulose and magnesium stearate were purchased from Shaanxi Zhengyi Pharmaceutical Auxiliary Materials Co., Ltd. (Shanxi, China). Citric acid was purchased from Henan Wanbang Chemical Co., Ltd. (Henan, China) and finasteride from HangZhou MSD Pharmaceutical Co., Ltd (Hangzhou China). ELISA kits for the determination of IL-6, TNF-α, and IL-1β in rats were acquired from MultiSciences Lianke Biotech Co., Ltd. (Hangzhou, China). Anti-JAK2, anti–IL-6, anti–phospho-STAT3, anti-STAT3, and anti–phospho-JAK2 were purchased from Chengdu Zhengneng Biotechnology Co., Ltd. (Chengdu, China); Standard products such as rutin, quercetin, kaempferol, Isorhamnetin, etc. were purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China); chromatography-grade phosphoric acid (Tianjin Comio Chemical Reagent Co., Ltd.).
2.2 Chewable tablet preparation
The chewable tablets are prepared using a wet granulation process, with maca water extract and rape bee pollen as principal ingredients, and lactose, microcrystalline cellulose, citric acid, and magnesium stearate as auxiliary components. Each chewable tablet weighs 450 mg and contains the following ingredients: rape bee pollen 136.62 mg, maca 136.62 mg, microcrystalline cellulose 85.545 mg, lactose 83.745 mg, citric acid 2.97 mg, and magnesium stearate 4.5 mg.
2.3 Preparation of the standard solution and sample solution
Take the appropriate amount of each control product, add methanol to make the mixed control solution of rutin, quercetin, kaempferol and Isorhamnetin quality concentration of 12.74 μg/mL, 34.12 μg/mL, 76.56 μg/mL, and 10.23 μg/mL, respectively. Shake well and set aside.
The 10 batches of rape bee pollen maca masticable tablets were prepared according to the “2.2” method. Each sample was weighed 1.0 g, placed in 100 mL round bottom flask, add 50 mL of methanol and 10 mL of 25% hydrochloric acid solution, weighed, heat reflux for 60 min, cool, weigh the mass with methanol, shake well, filter 0.22 μm microporous filter membrane, take the continued filtrate, and set aside.
2.4 Instruments and chromatographic conditions
Chromatographic conditions: Chromatographic column: Agilent ZORBAX SB-C18 column (4.6 × 250 mm, 5 μm), mobile phase: methanol (A)-0.4% phosphoric acid water (B), gradient elution: 0–30 min (A: 30%−50%), 30–50 min (A: 50%−55%), 50–51 min (A: 55%−30%), 51–60 min (A: 30%−30%), detection wavelength: 368 nm, The flow rate was 1 mL/min, the injection volume was 10 μL, and the column temperature was 30°C.
2.5 Animals experiment
After a week-long acclimation period, male Sprague—Dawley rats were randomized into 8 groups with 10 rats per group using a weight-based stratified random sampling technique, which guaranteed that the average weight of rats in each group was the same. Rats were divided into the following 8 groups: control group, model group, finasteride group (0.52 mg/kg), rape bee pollen group (630.0 mg/kg), maca group (630.0 mg/kg), and Chewable Tablets at 3 different dosages groups, namely, low (518.7 mg/kg), middle (1,037.5 mg/kg), and high (2,075.0 mg/kg) dosages. BPH was induced in rats in the model and drug-administered groups using a subcutaneous injection of testosterone propionate (5 mg/kg/day dissolved in olive oil) for 28 consecutive days. Only olive oil was administered subcutaneously for 28 days to rats in the control group. Along with the testosterone propionate injection, rats in the drug-administered groups received daily gavage for 28 days, whereas those in the control group received distilled water following the same protocol. Fecal samples were obtained from each group of rats on the day before the experiment ended. After overnight fasting, blood was drawn from the abdominal aorta under anesthesia with 2% sodium pentobarbital (50 mg). The blood samples were centrifuged at 4,000 rpm for 15 min to obtain the serum. After prostate tissues were imaged for morphological evaluation, they were quickly separated and weighed. The serum was collected and stored at −80°C for ELISA. Rat feces were stored at −80°C until they were used for gut microbiome identification. A part of the prostate tissues was fixed in 4% paraformaldehyde for further histological evaluation. The remaining prostate tissues were promptly stored at −80°C for western blotting.
2.6 Oxidative stress
Serum superoxide dismutase (SOD), malondialdehyde (MDA) and glutathione (GSH) levels were measured using commercial reagents (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's recommendations.
2.7 Histopathological analysis
H&E staining was used to determine the morphological characteristics of rat prostate glands that were obtained after treatment with testosterone propionate. The epithelial thickness was determined using ImageJ software.
2.8 Enzyme-linked immunosorbent assay
Three types of pro-inflammatory cytokines, i.e., IL-1β, IL-6, and TNF-α, were measured in serum supernatant using the rat ELISA Commercial Kit (MultiSciences Lianke Biotech, Hangzhou, China) according to the manufacturer's recommendations.
2.9 Gut microbiota analysis
The feces of rats in the control group, model group, chewable tablets middle dosages group, and finasteride group were used as test samples. The gut microbiome samples were sent to BGI Co., Ltd, China (Shenzhen, China) for DNA extraction and sequencing of 16S rRNA gene. Total genomic DNA of the gut microbiome was extracted using E.Z.N.A.® Bacterial DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to manufacturer's instruction. The V4 of 16S rRNA gene from the single gut microbiome sample was amplified. After genome DNA was normalized to 30 ng per PCR reaction, V4 dual-index fusion PCR primer cocktail and PCR master mix were added, then to run PCR. The melting temperature was 56°C and PCR cycle was 30. The PCR products were purified with AmpureXP beads to remove the unspecific products. The resulting library was used for sequencing on Illumina HiSeq 2500 platform following the standard pipelines of Illumina, and generating 2 × 250 bp paired-end reads (Sinclair et al., 2015).
To obtain clean reads, the raw data were filtered to eliminate the adapter pollution and low-quality reads by an in-house procedure as following (Fadrosh et al., 2014): The clean paired-end reads with overlap were merged to tags using FLASH (fast length adjustment of short reads, v1.2.11) (Magoč and Salzberg, 2011). Then, the tags were clustered to OTUs at 97% sequence similarity by scripts of software USEARCH (v9.1.13) (Edgar, 2013). Taxonomic ranks were assigned to OTUs representative sequence using Greengene (v201305, https://docs.qiime2.org/2024.2/data-resources/#greengenes) (Cole et al., 2014). At last, alpha diversity, beta diversity and the different species screening were analyzed based on OTUs and taxonomic ranks using mother (v1.31.2), software R (v3.1.1), QIIME (v1.80), or metastats (https://huttenhower.sph.harvard.edu/metastats/) (Schloss et al., 2009; White et al., 2009).
2.10 Western blot analysis
The total protein was isolated from prostate tissue homogenate by Radioimmunoprecipitation assay buffer. The protein concentration was then determined using the BCA assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Protein samples were separated on a 10% SDS-PAGE gel and transferred to a 0.45 μm polyvinylidene fluoride (PVDF) membrane, and then the PVDF membrane was blocked with 5% skim milk or 5% bovine serum albumin for 2 h. The blocked PVDF membrane were incubated with the following primary antibodies; p-JAK2 (1:1000), JAK2 (1:1000), p-STAT3 (1:1000), STAT3 (1:1000), β-Actin (1:5000) diluted with 5% bovine serum albumin overnight at 4°C, The PVDF membrane was washed 3 times with 1 × TBST on a shaker, 10 min each time. The membrane was then incubated with HRP-labeled secondary antibodies for 2 h at room temperature, and the immunobands were visualized using chemiluminescent reagents in the Chemi-Doc XRS system, and the gray value of the protein bands was analyzed by Image J software. All the antibodies listed in this section were obtained from Chengdu Zhengneng Biotechnology Co., Ltd. (Chengdu, China).
2.11 Statistical analysis
Data computations and graphical representations were executed using GraphPad Prism software (version 9.5; San Diego, CA, United States). Intergroup disparities were examined using independent sample t-test, one-way analysis of variance, and Kruskal—Wallis test. Correlation was determined using the Spearman rank correlation coefficient (with a significance threshold of p < 0.05) in the corrplot software package of R (v3.4.1). GraphPad Prism v9.5 and R were used for analyses and figure generation. p < 0.05 was considered to be statistically significant. The results were expressed as mean ± standard deviations.
3 Results and discussion
3.1 HPLC fingerprint
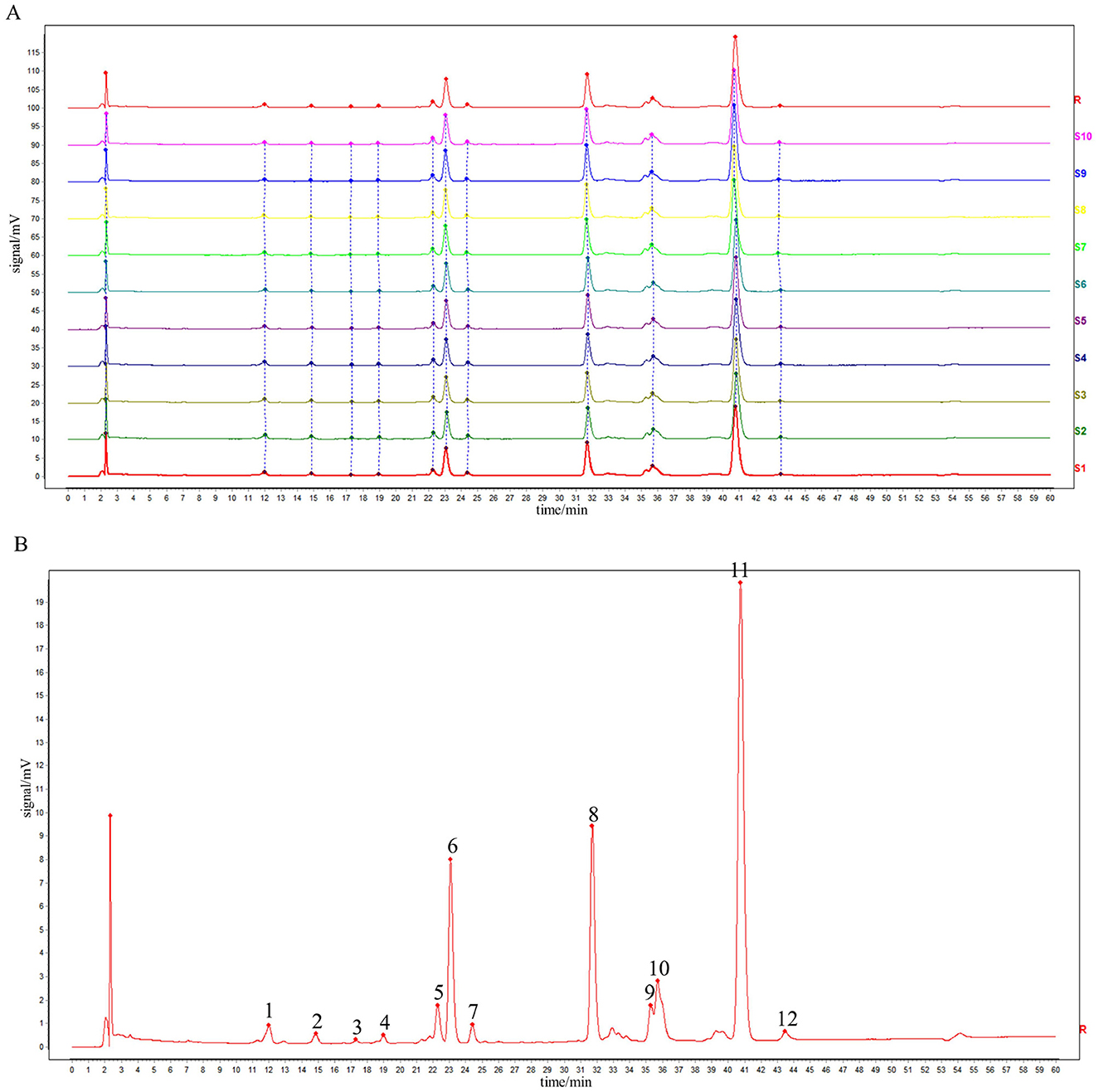
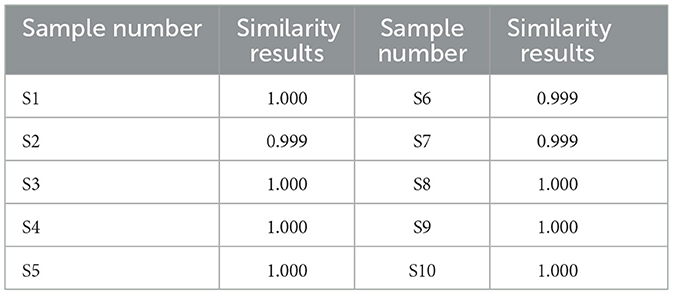
Prepare 10 batches of rape bee pollen maca chewable tablet test solution (numbered S1~S10), measure according to the chromatographic conditions under item “2.4”, and record the chromatogram for 60 min. “Chinese traditional medicine chromatographic fingerprint similarity evaluation system (2012, 1 Edition)” was used to evaluate 10 batches of AIA data of sample to be tested. The chromatographic fingerprint of sample S1 was designated as the reference standard. Following multi-point calibrated full-spectrum peak alignment, a consensus fingerprint was generated using the median method, resulting in the identification of 12 characteristic common peaks. Among these, four peaks were structurally authenticated: Peak 4 (rutin), Peak 8 (quercetin), Peak 11 (kaempferol), and Peak 12 (isorhamnetin). Similarity analysis between the reference fingerprint and those of 10 batches of rape pollen-maca chewable tablets demonstrated high consistency, with similarity indices ranging from 0.999 to 1.000. As shown in Figures 1A, B and Table 1.
3.2 The content of four components in the sample
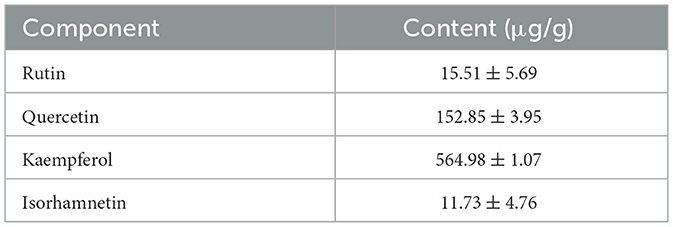
The content of rape bee pollen maca chewable tablets was analyzed using HPLC method. Quantitative analysis was performed on chromatographic peaks 4 (rutin), 8 (quercetin), 11 (kaempferol), and 12 (isorhamnetin) in the fingerprint spectrum. As illustrated in Figure 2B, these four components constitute the primary constituents of the rape bee pollen maca chewable tablets, with quercetin and kaempferol being particularly prominent. HPLC determination was performed on the sample of rape bee pollen maca chewable tablets and the mixed reference solution separately. The results are shown in Figures 2A, B. In addition, Figure 2C shows the structural formulas of each compound. Simultaneously settle the content of each component, as shown in Table 2.
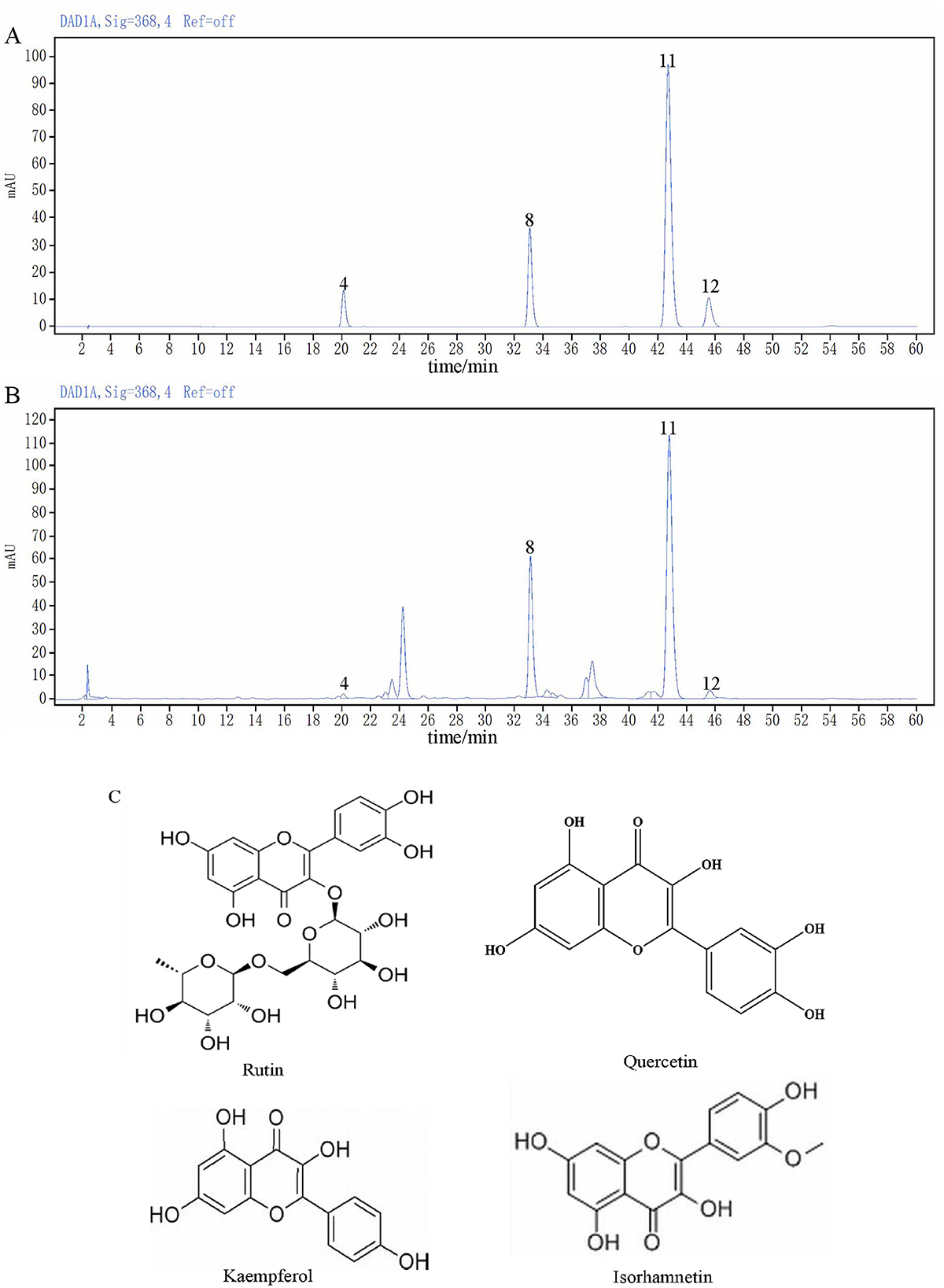
Figure 2. The chromatogram of the mixed reference substance (A), the chromatogram of sample (B) and Chemical structures of four components (C).
3.3 Effect of chewable tablets containing rape bee pollen and maca in rats with BPH
To confirm whether rape bee pollen maca chewable tablets decreased BPH, a rat model of BPH was induced by testosterone propionate. Prostate tissues from the control group were light pink in color; they had intact morphology, did not adhere to the surrounding tissues, and had no nodules (Figure 3A). However, prostate tissues from the model group were dark red, had a nonsmooth surface, and showed obvious nodules. Tissues from the finasteride, pollen, and maca groups were lighter red in color and showed no obvious nodules. Tissues from the low-dose group of chewable tablets appeared dark red and showed obvious hyperplastic nodules, whereas tissues from the middle- and high-dose groups from chewable tablets were lighter red in color, had a smooth surface, and showed no obvious nodules. The body weight of rats in the model group was significantly lower (p < 0.01) than that of rats in the control group. Intriguingly, we observed that rats in the middle- and high-dose groups of the rape pollen maca chewable tablets exhibited greater body weight compared to those in the rape bee pollen and maca groups, though the difference did not reach statistical significance (Figure 3B). The model group showed a significant increase (p < 0.01) in both prostate index and prostate wet weight compared with the control group. The prostate index and prostate wet weight in the finasteride group (p < 0.01), pollen group (p < 0.01), maca group (p < 0.01), and the administration of varying doses of chewable tablets group (p < 0.01) were significantly lower than those in the model group (Figures 3C, D). Furthermore, our findings revealed that the middle- and high-dose groups of the rape bee pollen maca chewable tablets demonstrated superior efficacy compared to the rape bee pollen and maca treatment groups (Figures 3C, D).
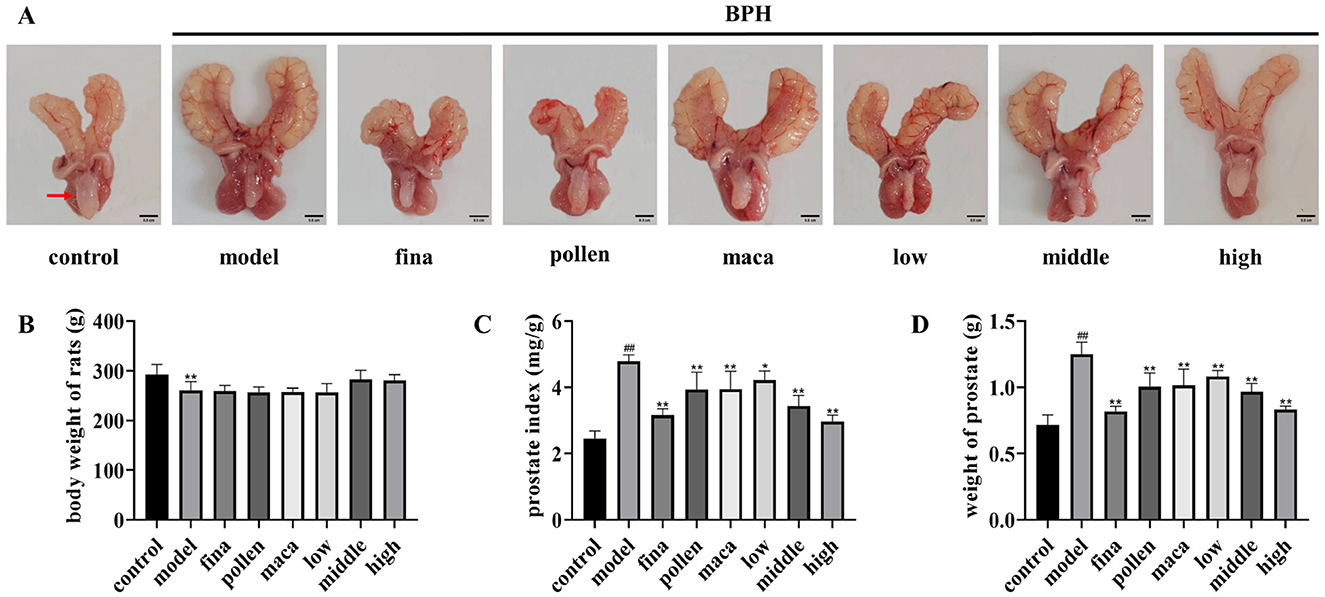
Figure 3. (A) Representative images of the prostate from each group. Scale bar = 0.5 cm. (Red arrows indicate rat prostate tissue.) (B) Body weight. (C) Prostate weight. (D) Prostate weight (PW) to body weight (BW) ratio. Data are presented as mean ± standard deviation. ##p < 0.01 vs. rats that did not receive TP injection, *p < 0.05, **p < 0.01 vs. rats injected with TP. Low, middle, and high in the figure represent the low, middle, and high the rape pollen maca chewable tablets groups, respectively.
3.4 Effect of chewable tablets containing rape bee pollen and maca on serum sex hormone levels in rats
Using ELISA, we looked at the variations in serum hormone levels in each group of rats. As demonstrated in Figures 4A–D, serum levels of T, DHT, and E2 were significantly elevated in the model group compared to the control group, along with an increased E2/T ratio (p < 0.01). After treatment with different doses of chewable tablets, the levels of T, DHT, and E2 significantly decreased (p < 0.01), as well as the serum E2/T ratio (p < 0.01), but did not fully recover to normal levels (vs control). The collective data indicate that chewable tablets containing rape bee pollen and maca can reduce serum hormone levels in rats with BPH, and the chewable tablet is more efficacious than the use of rape bee pollen and maca as standalone agents.
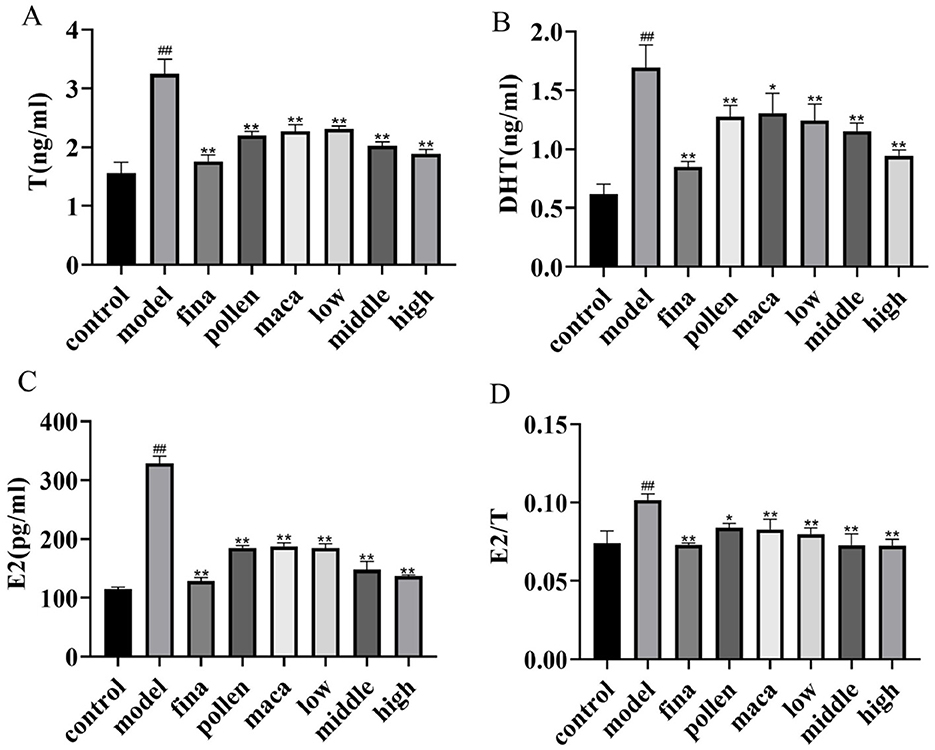
Figure 4. (A–C) Serum T, DHT, and E2 levels in rats determined using ELISA. (D) The serum E2/T ratio. Data are presented as mean ± standard deviation. ##p < 0.01 vs. rats that did not receive TP injection, *p < 0.05, **p < 0.01 vs. rats injected with TP. Low, middle, and high in the figure represent the low, middle, and high chewable tablets groups, respectively.
3.5 Effect of chewable tablets containing rape bee pollen and maca on serum oxidative stress levels in rats
Using the kit, we observed alterations in serum oxidative stress levels across all groups of rats. As shown in Figures 5A–C, compared with the control group, the model group rats exhibited significantly decreased serum levels of SOD and GSH along with markedly elevated MDA levels (p < 0.01), demonstrating the occurrence of severe redox imbalance in these experimental animals. After treatment with different doses of chewable tablets, the levels of SOD and GSH significantly increased (p < 0.01), Meanwhile, the MDA levels were significantly reduced (p < 0.01). Furthermore, we found that the combined application of rape pollen and maca demonstrated superior efficacy compared to the individual use of either rape bee pollen or maca alone.
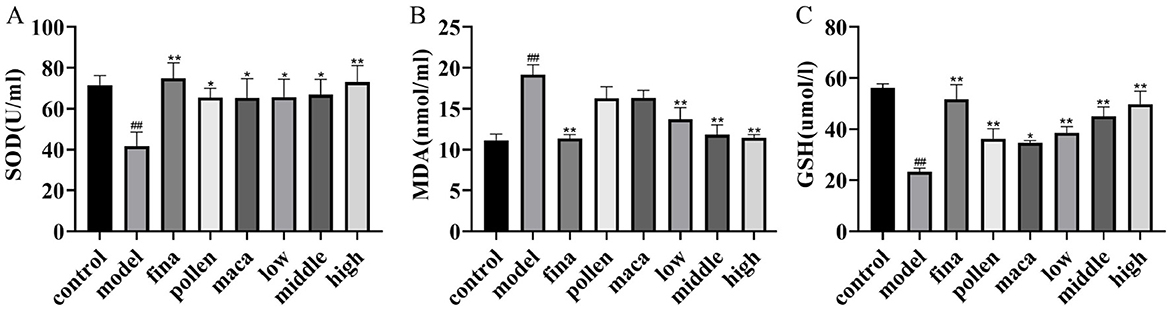
Figure 5. (A–C) Serum SOD, MDA and GSH levels in rats determined using Kit. Data are presented as mean ± standard deviation. ##p < 0.01 vs. rats that did not receive TP injection, *p < 0.05, **p < 0.01 vs. rats injected with TP. Low, middle, and high in the figure represent the low, middle, and high chewable tablets groups, respectively.
3.6 Effect of chewable tablets containing rape bee pollen and maca on the histopathology and serum inflammatory factor levels in rats
Figure 6A reflects the histopathologic changes in prostate tissues stained with hematoxylin & eosin (H&E). Prostate tissues were distinctly ordered, had a smooth surface, and exhibited fewer acinar epithelial folds, and the epithelial cells were firmly packed in a single column in the control group. In contrast, prostate tissues from model rats exhibited considerable hyperplasia compared with that from control rats. Furthermore, the epithelial cells displayed a multilayer arrangement with more rough folds and increased epithelial cell thickness (p < 0.01). In addition, rats in the pollen group, maca group, and low-dose group of chewable tablets showed a significant reduction in prostate epithelial cell thickness (p < 0.01) and moderate hyperplasia of the prostate gland, whereas rats in the middle-dose group showed mild hyperplasia of the prostate gland and a substantial reduction in epithelial cell thickness (p < 0.01) compared with those in rats in the model group. On the other hand, rats in the high-dose group and finasteride group showed normalization of the prostate gland structure and a significant reduction in epithelial cell thickness (p < 0.01) (Figure 6B). Enzyme-linked immunosorbent assay (ELISA) was used to determine variations in serum inflammatory factor levels in each group of rats. The chewable tablet significantly decreased IL-6, TNF-α, and IL-1β levels in the blood (p < 0.01) (Figures 6C–E). The collective data indicate that chewable tablets containing rape bee pollen and maca could impede the progression of BPH and indicated chewable tablets to be more efficacious than rape bee pollen or maca used singly.
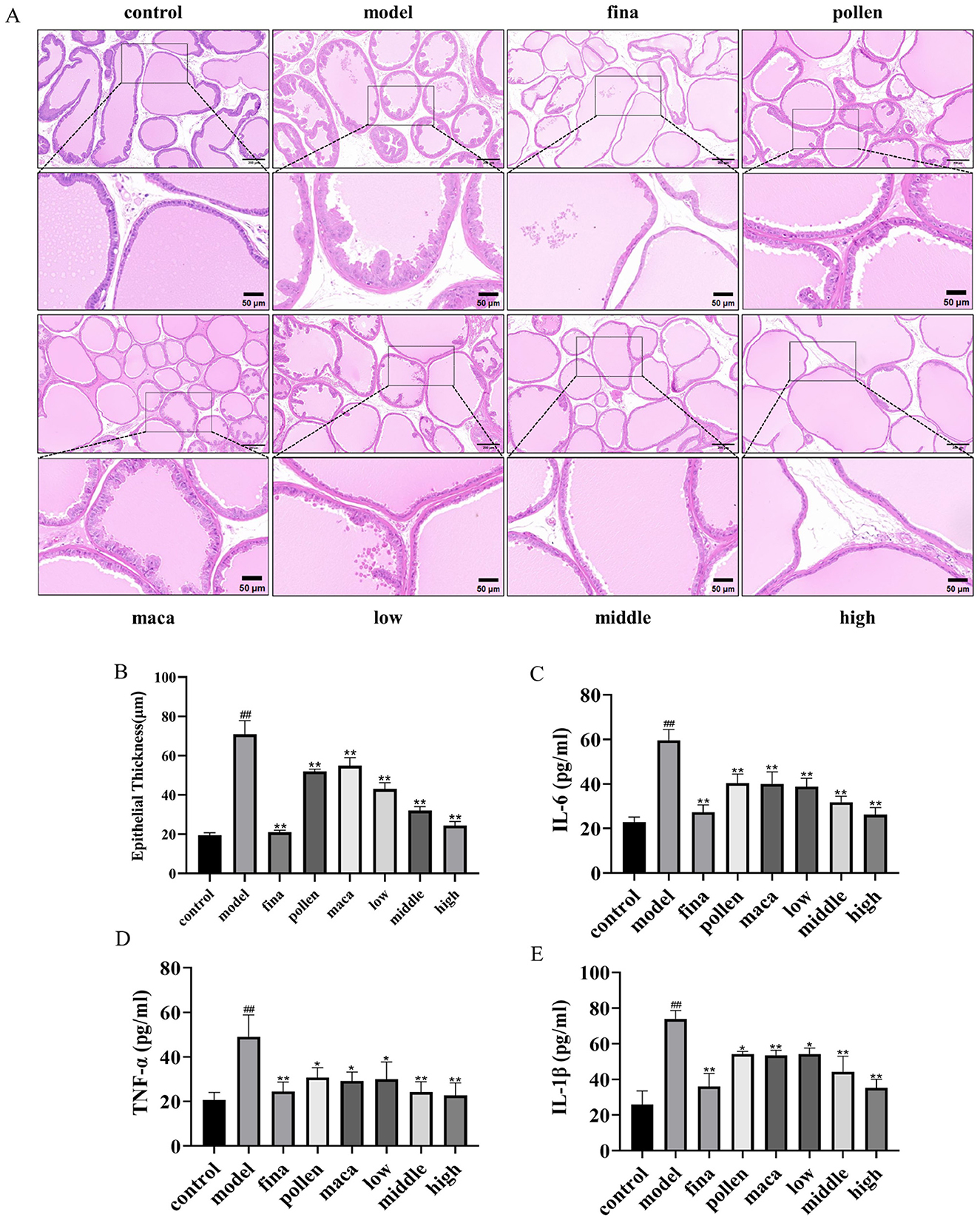
Figure 6. (A) H&E staining scale bar = 200 μm. Scale bar = 50 μm. (B) Quantification of epithelial thickness. (C–E) Serum IL-6, TNF-α, and IL-1β levels in rats determined using ELISA. Data are presented as mean ± standard deviation. ##p < 0.01 vs. rats that did not receive TP injection; *p < 0.05, **p < 0.01 vs. rats injected with TP. Low, middle, and high in the figure represent the low, middle, and high chewable tablets groups, respectively.
3.7 Effect of chewable tablets containing rape bee pollen and maca on the diversity of gut microbiota of rats
To investigate the effects of rape pollen maca chewable tablets on the gut microbiota in rats with BPH, we selected the following groups as study subjects: the control group, model group, finasteride group, and middle-dose rape bee pollen maca chewable tablet group. Operational taxonomic unit (OTU) data revealed differences in the gut microbiota among the four groups. A total of 941 OTUs were identified by grouping the pristine reads using a 97% threshold as shown in the Venn diagram (Figure 7A). The model, middle-dose chewable tablet group, finasteride, and control groups shared 798 OTUs. A total of 25 unique OTUs in the control group, 46 in the model group, 49 in the middle-dose chewable tablet group, and 23 in the finasteride group were identified. The Chao and Simpson indices, representing the gut microbiota alpha diversity, are commonly used to assess species richness and evenness. The data revealed that the differences in the Chao index among the groups were not statistically significant (p > 0.05). Nevertheless, the Chao index demonstrated an increase in the model and treatment groups compared with the control group, implying a higher abundance of intestinal flora compared with that in the control group (Figure 7B). A comparison of the Simpson index of the intestinal flora in the model group with that of the control group revealed a decrease in the former (p > 0.05) (Figure 7C). The dissimilarity in microbial communities between samples or populations is referred to as beta diversity. Principal component analysis and partial least squares discriminant analysis revealed variations in gut microbiota between the model group and other groups (Figures 7D, E). Principal coordinates analysis derived from unweighted Unifrac distances revealed that the model group grouped independently, whereas the other groups exhibited partial cross-aggregation. These findings demonstrated that the intestinal flora composition of rats in the model group differed from that of control rats. Furthermore, treatment with finasteride or the middle-dose chewable tablet led to changes in the intestinal flora composition in rats with BPH (Figure 7F).
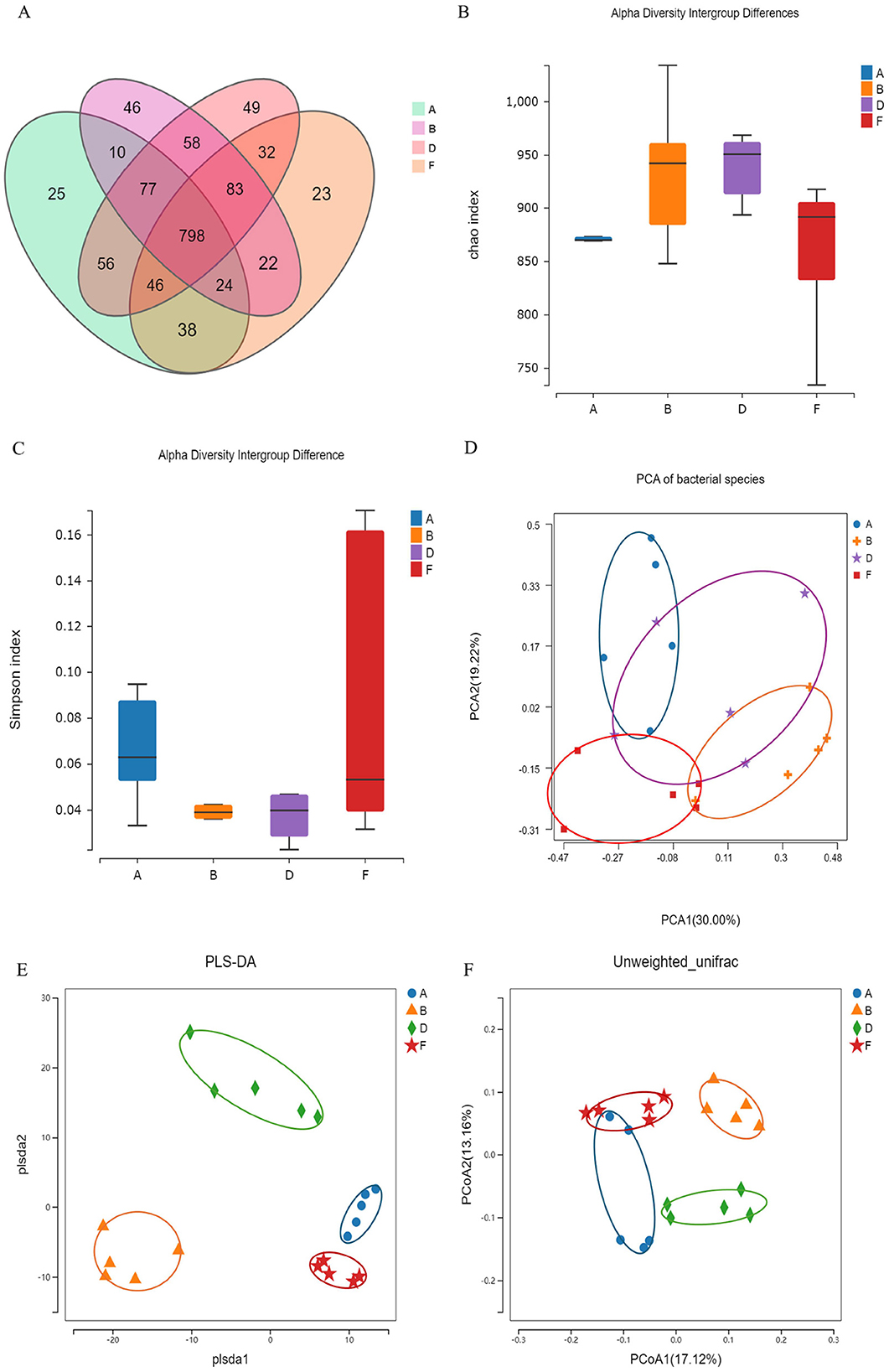
Figure 7. (A) Overview of OTUs in diverse groups. (B) Chao index. (C) Simpson index. Differences were assessed by the Kruskal-Wallis test. (D) Principal components analysis (PCA). (E) Partial least squares discrimination analysis (PLS-DA). (F) Principal Coordinates Analysis (PCoA). (A, B, D, F) In the figure represent the control, model, middle-dose chewable tablet, and finasteride groups, respectively.
3.8 Effect of chewable tablets containing rape bee pollen and maca on the composition of gut microbiota
Species difference maps were used to show the constitution of the main gut microbiota in different intervention groups; identify significant changes in the gut microbe at the phylum, and genus levels; analyze the reasons for the differences in the diversity of the gut microbiota in different groups; and explain changes in the structure of the intestinal microbiota. A significant increase in Bacteroidia (p < 0.01) and a significant decrease in Bacillus (p < 0.05) were noted at the phylum level in the model group compared with that in the control group (Figures 8A, C, D). At the genus level, significant increases were observed in the abundance of Segatella and Prevotellamassilia, but substantial decreases in the abundance of Lactobacillus (p < 0.01), Phascolarctobacterium, Kineothrix, Collinsella, and Faecalibacterium were reported in the model group relative to the control group (Figures 8B, E). The middle-dose chewable tablet group showed a reversal in this trend at various taxonomic levels compared with that in the model group.
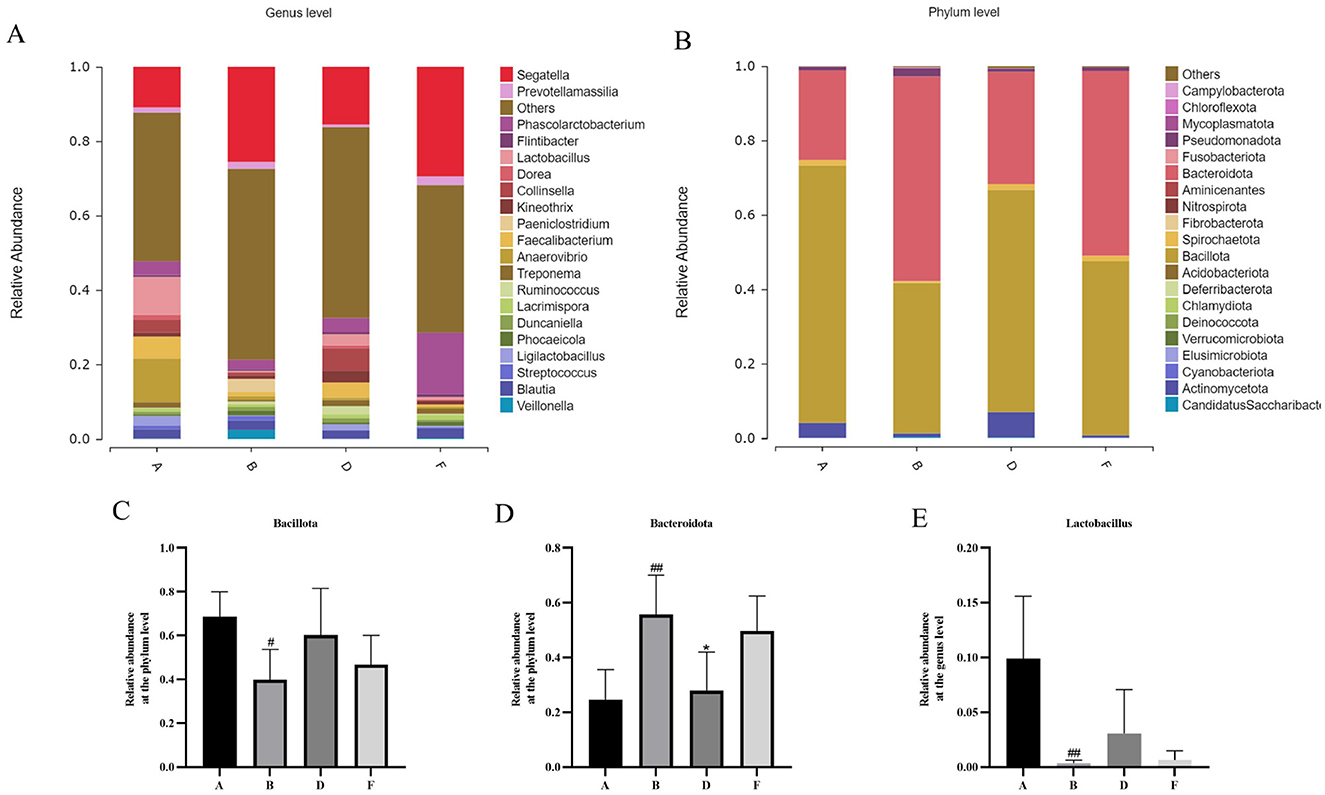
Figure 8. (A) Histogram showing the relative abundance of gut bacteria at the phylum level. (B) The bar chart shows the relative abundance of intestinal microbes at the genus level. (C) relative abundance of bacillota at the phylum level. (D) relative abundance of bacteroidota at the phylum level. (E) relative abundance of lactobacillus at the genus level. (A, B, D, F) in the figure represent the control, model, middle-dose chewable tablet, and finasteride groups, respectively.
3.9 Effect of chewable tablets containing rape bee pollen and maca on the IL-6/JAK2/STAT3 signaling pathway
To further investigate the mechanism of action of rape bee pollen and maca chewable tablets on BPH, we employed Western blotting to detect the expression levels of key proteins in the IL-6/JAK2/STAT3 signaling pathway within prostatic tissues. As shown in Figure 9, compared with the control group, the protein expression levels of IL-6, p-JAK2, and p-STAT3 in the prostatic tissues of model group rats were significantly increased (p < 0.01). After drug administration, a marked decrease in the expression levels of IL-6, p-JAK2, and p-STAT3 was observed in the prostate tissues of the treated groups compared to the model group (p < 0.01). Our findings indicate that the therapeutic mechanism of rape pollen maca chewable tablets against BPH involves the regulation of the IL-6/JAK2/STAT3 signaling pathway.
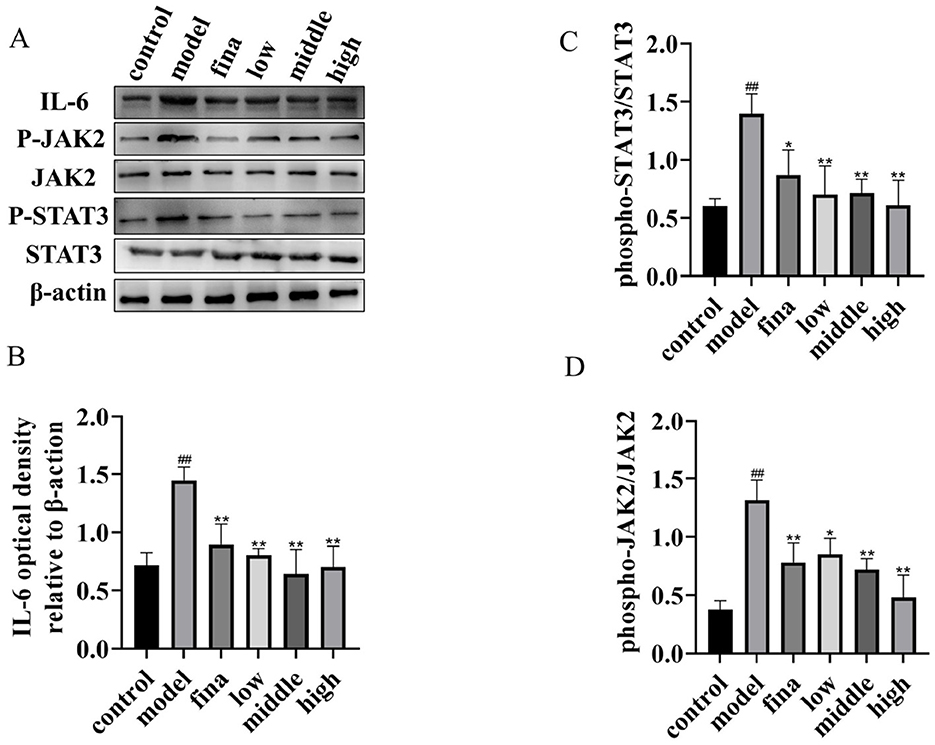
Figure 9. (A) Total protein and phosphorylated protein of STAT3 and JAK2, IL-6 proteins. One representative blot was selected from 3 independent experiments. (B–D) The relative band intensity was normalized to the relative actin intensity compared with the control. ##p < 0.01 compared with rats not injected with TP; *p < 0.05, **p < 0.01 compared with the model group.
4 Discussion
This study established a fingerprint of rape bee pollen maca chewable tablets using HPLC, and 12 common peaks were identified as characteristic peaks. Four known components were identified by comparison with the fingerprint of the mixed control solution, Peak 4 was rutin, peak 8 was quercetin, peak 11 was kaempferol, and peak 12 was isorhamnetin. Their amounts were 15.51 ± 5.69 μg/g, 155.85 ± 3.95 μg/g, 564.98 ± 1.07 μg/g, and 11.73 ± 4.76 μg/g, respectively. The fingerprint spectrum of rape bee pollen maca chewable tablets established in this study is simple, stable, and has good repeatability. It can comprehensively reflect the effective chemical components of rape bee pollen maca chewable tablets, which is conducive to the comprehensive analysis of the quality of rape bee pollen maca chewable tablets. Additionally, studies have reported that quercetin and kaempferol exhibit therapeutic effects on benign prostatic hyperplasia induced by testosterone propionate solution (Wang et al., 2021; Fu et al., 2022).
Our findings revealed that the chewable tablet of rape bee pollen and maca showed better efficacy compared with the use of rape bee pollen or maca alone (Figures 3–5). According to clinical medication observations, excessive consumption of rape bee pollen may lead to gastrointestinal reactions such as nausea, vomiting, and loose stools. Additionally, a small number of patients may develop allergic skin reactions, including rash or pruritus (Hemmer et al., 2004). Therefore, the combined application of rape pollen and maca allows for a reduction in the dosage of rape pollen required, thereby mitigating the occurrence of certain adverse reactions. Since both rape pollen and maca serve dual purposes as medicinal agents and food-grade ingredients, they exhibit a low risk of adverse effects and minimal contamination during production processes. Thus, the chewable tablet of rape bee pollen and maca is more widely available than the chemically synthesized drug finasteride. Therefore, this study conducted a pharmacodynamic evaluation of rape pollen maca chewable tablets on benign prostatic hyperplasia, investigated their impact on gut microbiota, and further explored the IL-6/JAK2/STAT3 signaling pathway in prostatic tissues.
It has been established that BPH is influenced by both estrogens and androgens. The prostate's response to estrogens is linked to several processes, such as aromatase expression, apoptosis, and paracrine regulation through prostaglandin E2. E2 to T ratio rises in BPH and malignant prostate tissues due to aberrant expression of aromatase (Wang et al., 2024). 5α reductase (5-AR) converts T, the major circulating androgen in males, to DHT. DHT enters the nucleus by attaching to the AR at the androgen binding site. The DHT/AR complex molecule then attaches to the ARE in the promoter of the target gene, encouraging the growth and survival of prostate epithelial cells (Park et al., 2022; Gong et al., 2023).
In this study, a rat model of testosterone propionate induced BPH was used to determine the efficacy of chewable tablets containing rape bee pollen and maca. Our findings demonstrated that chewable tablets could lead to a notable reduction in prostate volume, epithelial thickness, and serum levels of IL-6, TNF-α, and IL-1β. Inflammation is an important factor in the process of BPH (Cai et al., 2020). Chronic inflammation leads to the overproduction of pro-inflammatory factors, including IL-6, IL-1β, and TNF-α, which induces the overproliferation of prostate epithelial and stromal cells and activates fibrosis to promote signaling and malignant transformation (Cai et al., 2020). We found that chewable tablets group exerted anti-inflammatory activity by significantly inhibiting the IL-6/JAK2/STAT3 signaling pathway. The IL-6/JAK2/STAT3 signaling pathway plays an important role in the development of BPH. IL-6 binds to the IL-6 receptor on the cell membrane and activates intracellular JAK2 and phosphorylating it. Phosphorylated JAK2 further activates STAT3. After dimerization of phosphorylated STAT3, the downstream nuclear transcription factors are activated, which promotes the production and release of IL-6 and exacerbates the inflammatory response (Bowman et al., 2000; Kim et al., 2016; Zhang et al., 2018).
Lactobacillus has anti-inflammatory effects, stimulates brain functions, modulates metabolism, and antagonizes intestinal pathogens (Cai et al., 2020). It can metabolize organic acids such as lactic acid, formic acid, acetic acid, and butyric acid, which reduce the intestinal pH and inhibit the growth of acid-intolerant genera in the host intestine (Petrova et al., 2017; Tramontano et al., 2018). Faecalibacterium has been demonstrated to confer protection in several studies owing to its anti-inflammatory and symbiotic characteristics. It plays a role in metabolizing acetate into butyrate, which serves as a primary source of energy for colonocytes and functions as an anti-inflammatory agent (Sha et al., 2020; Maioli et al., 2021; Martín et al., 2023). Collinsella is responsible for the production of butyric acid and reducing inflammatory diseases (Jeong et al., 2019; Qin et al., 2019). The excessive accumulation of succinic acid in the gut can lead to diarrhea, and Phascolarctobacterium can use succinic acid to produce propionic acid (Wu et al., 2017). Kineothrix can produce butyrate, which plays a role in modulating inflammation (Choi et al., 2023). Prevotella has virulence factors, including adhesins, pili, hemolysins, nucleases, proteases, lipopolysaccharides, exopolysaccharides, and other enzymes, which promote its pathogenicity and survival in the host (Sharma et al., 2022). The overgrowth of Prevotella or its translocation across the mucosal barrier enhances the immune response and leads to inflammatory diseases (Iljazovic et al., 2021). The genus Prevotella exhibited abnormally elevated abundance in the model group, while the SCFA-producing gut microbiota including Lactobacillus, Faecalibacterium, Collinsella, Phascolarctobacterium, and Kineothrix were significantly reduced. This imbalance led to an overproduction of pro-inflammatory factors and facilitated the development of BPH. In contrast, chewable tablets group demonstrated an increase in the abundance of beneficial bacteria, including Faecalibacterium, Collinsella, Phascolarctobacterium, Kineothrix, and Lactobacillus, while concurrently demonstrating a decrease in the abundance of the pro-inflammatory bacterial Prevotella. The finasteride group demonstrated an increase in the levels of beneficial bacteria, specifically Phascolarctobacterium and Lactobacillus, while concurrently demonstrating a decrease in the abundance of the pro-inflammatory bacterial Prevotella.
5 Conclusion
In conclusion, this study demonstrates that the chewable tablet of rape bee pollen and maca ameliorates testosterone propionate-induced BPH in rats. The protective effects, mediated through the IL-6/JAK2/STAT3 signaling pathway, are associated with reduced inflammation, oxidative stress, and hormonal imbalance, alongside a beneficial modulation of the gut microbiota. In addition, we also established an HPLC fingerprint of rape bee pollen maca chewable tablets. Our findings indicate these chewable tablets containing rape bee pollen and maca as a promising natural therapeutic strategy for BPH management.
Data availability statement
The data presented in the study are deposited in the NCBI Sequence Read Archive (SRA) repository, accession number PRJNA1353094.
Ethics statement
The animal study was approved by the Animal Ethical Committee of Huanghe Science & Technology University approved the Animal Experiments (NO: 2023-011) (Zhengzhou, China). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XH: Funding acquisition, Investigation, Writing – original draft. GR: Investigation, Writing – original draft. HT: Data curation, Writing – review & editing. PZ: Writing – review & editing, Formal analysis. HZ: Formal analysis, Writing – review & editing. SZ: Formal analysis, Validation, Writing – review & editing. SW: Writing – review & editing, Data curation. AJ: Writing – review & editing, Conceptualization, Funding acquisition. WZ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Postgraduate Education Reform and Quality Improvement Project of Henan Province (YJS2023JD68); Henan Province Science and Technology Research Project (242102311266, 242400410217, 142102310146); Zhengzhou Science and Technology Research project (2024TLZDJH017); Open Research Project of Henan Provincial Key Laboratory of Nanocomposites and Applications (HKD20240902).
Acknowledgments
We gratefully acknowledge MJEditor (www.mjeditor.com) for providing valuable language support during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
An, J., Song, Y., Kim, S., Kong, H., and Kim, K. (2023). Alteration of gut microbes in benign prostatic hyperplasia model and finasteride treatment model. IJMS 24:5904. doi: 10.3390/ijms24065904
Antoniou, V., Gauhar, V., Modi, S., and Somani, B. K. (2023). Role of phytotherapy in the management of BPH: a summary of the literature. J. Clin. Med. 12:1899. doi: 10.3390/jcm12051899
Awedew, A. F., Han, H., Abbasi, B., Abbasi-Kangevari, M., Ahmed, M. B., Almidani, O., et al. (2022). The global, regional, and national burden of benign prostatic hyperplasia in 204 countries and territories from 2000 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Healthy Longev. 3, e754–e776. doi: 10.1016/S2666-7568(22)00213-6
Bowman, T., Garcia, R., Turkson, J., and Jove, R. (2000). STATs in oncogenesis. Oncogene 19, 2474–2488. doi: 10.1038/sj.onc.1203527
Cai, Y., Folkerts, J., Folkerts, G., Maurer, M., and Braber, S. (2020). Microbiota-dependent and -independent effects of dietary fibre on human health. Br. J. Pharmacol. 177, 1363–1381. doi: 10.1111/bph.14871
Cannarella, R., Condorelli, R. A., Barbagallo, F., La Vignera, S., and Calogero, A. E. (2021). Endocrinology of the aging prostate: current concepts. Front. Endocrinol. 12:554078. doi: 10.3389/fendo.2021.554078
Chen, X., Wu, R., Ren, Z., Tong, Y., Chen, S., Yang, F., et al. (2020). Regulation of microRNAs by rape bee pollen on benign prostate hyperplasia in rats. Andrologia 52:e13386. doi: 10.1111/and.13386
Choi, K. J., Yoon, M. Y., Kim, J.-E., and Yoon, S. S. (2023). Gut commensal Kineothrix alysoides mitigates liver dysfunction by restoring lipid metabolism and gut microbial balance. Sci. Rep. 13:14668. doi: 10.1038/s41598-023-41160-y
Cole, J. R., Wang, Q., Fish, J. A., Chai, B., McGarrell, D. M., Sun, Y., et al. (2014). Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 42, D633–D642. doi: 10.1093/nar/gkt1244
Da Silva Leitão Peres, N., Cabrera Parra Bortoluzzi, L., Medeiros Marques, L. L., Formigoni, M., Fuchs, R. H. B., Droval, A. A., et al. (2020). Medicinal effects of Peruvian maca (Lepidium meyenii): a review. Food Funct. 11, 83–92. doi: 10.1039/C9FO02732G
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Elbaz, E. M., Darwish, A., Gad, A. M., Abdel Rahman, A. A. S., and Safwat, M. H. (2023). Canagliflozin alleviates experimentally induced benign prostate hyperplasia in a rat model: exploring potential mechanisms involving mir-128b/EGFR/EGF and JAK2/STAT3 signaling pathways through in silico and in vivo investigations. Eur. J. Pharmacol. 957:175993. doi: 10.1016/j.ejphar.2023.175993
Fadrosh, D. W., Ma, B., Gajer, P., Sengamalay, N., Ott, S., Brotman, R. M., et al. (2014). An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2:6. doi: 10.1186/2049-2618-2-6
Fu, W., Chen, S., Zhang, Z., Chen, Y., You, X., and Li, Q. (2022). Quercetin in Tonglong Qibi decoction ameliorates testosterone-induced benign prostatic hyperplasia in rats by regulating Nrf2 signalling pathways and oxidative stress. Andrologia 54:e14502. doi: 10.1111/and.14502
Gong, G.-Y., Xi, S.-Y., Li, C.-C., Tang, W.-L., Fu, X.-M., and Huang, Y.-P. (2023). Bushen Tongluo formula ameliorated testosterone propionate-induced benign prostatic hyperplasia in rats. Phytomedicine 120:155048. doi: 10.1016/j.phymed.2023.155048
Gu, M., Liu, C., Yang, T., Zhan, M., Cai, Z., Chen, Y., et al. (2021). High-fat diet induced gut microbiota alterations associating with Ghrelin/Jak2/Stat3 up-regulation to promote benign prostatic hyperplasia development. Front. Cell Dev. Biol. 9:615928. doi: 10.3389/fcell.2021.615928
Han, Y. Y., Zhang, Q. H., Chen, W. S., Li, Z. L., Xie, D., Zhang, S. L., et al. (2023). Fermented rape pollen powder can alleviate benign prostatic hyperplasia in rats by reducing hormone content and changing gut microbiota. Benef. Microbes 14, 503–524. doi: 10.1163/18762891-20230039
Hata, J., Harigane, Y., Matsuoka, K., Akaihata, H., Yaginuma, K., Meguro, S., et al. (2023). Mechanism of androgen-independent stromal proliferation in benign prostatic hyperplasia. IJMS 24:11634. doi: 10.3390/ijms241411634
Hemmer, W., Focke, M., Kolarich, D., Dalik, I., Götz, M., and Jarisch, R. (2004). Identification by immunoblot of venom glycoproteins displaying immunoglobulin E-binding N-glycans as cross-reactive allergens in honeybee and yellow jacket venom. Clin. Exp. Allergy 34, 460–469. doi: 10.1111/j.1365-2222.2004.01897.x
Ho, C. K. M., and Habib, F. K. (2011). Estrogen and androgen signaling in the pathogenesis of BPH. Nat. Rev. Urol. 8, 29–41. doi: 10.1038/nrurol.2010.207
Huang, B., Lang, X., and Li, X. (2022). The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 12:1023177. doi: 10.3389/fonc.2022.1023177
Iljazovic, A., Amend, L., Galvez, E. J. C., De Oliveira, R., and Strowig, T. (2021). Modulation of inflammatory responses by gastrointestinal Prevotella spp. – From associations to functional studies. Int. J. Med. Microbiol. 311:151472. doi: 10.1016/j.ijmm.2021.151472
Jeong, Y., Kim, J.-W., You, H. J., Park, S.-J., Lee, J., Ju, J. H., et al. (2019). Gut microbial composition and function are altered in patients with early rheumatoid arthritis. JCM 8:693. doi: 10.3390/jcm8050693
Kim, B.-H., Yi, E. H., and Ye, S.-K. (2016). Signal transducer and activator of transcription 3 as a therapeutic target for cancer and the tumor microenvironment. Arch. Pharm. Res. 39, 1085–1099. doi: 10.1007/s12272-016-0795-8
Li, J., Li, Y., Zhou, L., Li, C., Liu, J., Liu, D., et al. (2024). The human microbiome and benign prostatic hyperplasia: current understandings and clinical implications. Microbiol. Res. 281:127596. doi: 10.1016/j.micres.2023.127596
Liu, Y., Yang, C., Zhang, Z., and Jiang, H. (2021). Gut microbiota dysbiosis accelerates prostate cancer progression through increased LPCAT1 expression and enhanced DNA repair pathways. Front. Oncol. 11:679712. doi: 10.3389/fonc.2021.679712
Magoč, T., and Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Maioli, T. U., Borras-Nogues, E., Torres, L., Barbosa, S. C., Martins, V. D., Langella, P., et al. (2021). Possible benefits of Faecalibacterium prausnitzii for obesity-associated gut disorders. Front. Pharmacol. 12:740636. doi: 10.3389/fphar.2021.740636
Martín, R., Rios-Covian, D., Huillet, E., Auger, S., Khazaal, S., Bermúdez-Humarán, L. G., et al. (2023). Faecalibacterium: a bacterial genus with promising human health applications. FEMS Microbiol. Rev. 47:fuad039. doi: 10.1093/femsre/fuad039
Miernik, A., and Gratzke, C. (2020). Current treatment for benign prostatic hyperplasia. Dtsch. Arztebl. Int. 117, 843–854. doi: 10.3238/arztebl.2020.0843
Park, W. Y., Song, G., Park, J. Y., Ahn, K. S., Kwak, H. J., Park, J., et al. (2022). Ellagic acid improves benign prostate hyperplasia by regulating androgen signaling and STAT3. Cell Death Dis. 13:554. doi: 10.1038/s41419-022-04995-3
Petrova, M. I., Reid, G., Vaneechoutte, M., and Lebeer, S. (2017). Lactobacillus iners: friend or foe? Trends Microbiol. 25, 182–191. doi: 10.1016/j.tim.2016.11.007
Qiao, J., Xiao, X., Wang, K., Haubruge, E., Dong, J., and Zhang, H. (2023). Rapeseed bee pollen alleviates chronic non-bacterial prostatitis via regulating gut microbiota. J. Sci. Food Agric. 103, 7896–7904. doi: 10.1002/jsfa.12878
Qin, P., Zou, Y., Dai, Y., Luo, G., Zhang, X., and Xiao, L. (2019). Characterization a novel butyric acid-producing bacterium Collinsella aerofaciens subsp. shenzhenensis subsp. nov. Microorganisms 7:78. doi: 10.3390/microorganisms7030078
Rodríguez-Pólit, C., Gonzalez-Pastor, R., Heredia-Moya, J., Carrera-Pacheco, S. E., Castillo-Solis, F., Vallejo-Imbaquingo, R., et al. (2023). Chemical properties and biological activity of bee pollen. Molecules 28:7768. doi: 10.3390/molecules28237768
Russo, G. I., Bongiorno, D., Bonomo, C., Musso, N., Stefani, S., Sokolakis, I., et al. (2023). The relationship between the gut microbiota, benign prostatic hyperplasia, and erectile dysfunction. Int. J. Impot. Res. 35, 350–355. doi: 10.1038/s41443-022-00569-1
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Sha, S., Ni, L., Stefil, M., Dixon, M., and Mouraviev, V. (2020). The human gastrointestinal microbiota and prostate cancer development and treatment. Investig. Clin. Urol. 61, S43–S52. doi: 10.4111/icu.2020.61.S1.S43
Shah, A., Shah, A. A., K, N., and Lobo, R. (2021). Mechanistic targets for BPH and prostate cancer-a review. Rev. Environ. Health 36, 261–270. doi: 10.1515/reveh-2020-0051
Sharma, G., Garg, N., Hasan, S., and Shirodkar, S. (2022). Prevotella: an insight into its characteristics and associated virulence factors. Microb. Pathog. 169:105673. doi: 10.1016/j.micpath.2022.105673
Sinclair, L., Osman, O. A., Bertilsson, S., and Eiler, A. (2015). Microbial community composition and diversity via 16S rRNA gene amplicons: evaluating the illumina platform. PLoS ONE 10:e0116955. doi: 10.1371/journal.pone.0116955
Singh, A. K., Bhadauria, A. S., Kumar, U., Raj, V., Rai, A., Kumar, P., et al. (2020). Author correction: novel indole-fused benzo-oxazepines (IFBOs) inhibit invasion of hepatocellular carcinoma by targeting IL-6 mediated JAK2/STAT3 oncogenic signals. Sci. Rep. 10:2391. doi: 10.1038/s41598-020-59134-9
Song, G., Tong, J., Wang, Y., Li, Y., Liao, Z., Fan, D., et al. (2023). Nrf2-mediated macrophage function in benign prostatic hyperplasia: novel molecular insights and implications. Biomed. Pharmacother. 167:115566. doi: 10.1016/j.biopha.2023.115566
Staršíchová, A., Lincová, E., Pernicová, Z., Kozubík, A., and Souček, K. (2010). TGF-β1 suppresses IL-6-induced STAT3 activation through regulation of Jak2 expression in prostate epithelial cells. Cell. Signal. 22, 1734–1744. doi: 10.1016/j.cellsig.2010.06.014
Stewart, K. L., and Lephart, E. D. (2023). Overview of BPH: symptom relief with dietary polyphenols, vitamins and phytochemicals by nutraceutical supplements with implications to the prostate microbiome. Int. J. Mol. Sci. 24:5486. doi: 10.3390/ijms24065486
Tramontano, M., Andrejev, S., Pruteanu, M., Klünemann, M., Kuhn, M., Galardini, M., et al. (2018). Nutritional preferences of human gut bacteria reveal their metabolic idiosyncrasies. Nat. Microbiol. 3, 514–522. doi: 10.1038/s41564-018-0123-9
Ulloa Del Carpio, N., Alvarado-Corella, D., Quiñones-Laveriano, D. M., Araya-Sibaja, A., Vega-Baudrit, J., Monagas-Juan, M., et al. (2024). Exploring the chemical and pharmacological variability of Lepidium meyenii: a comprehensive review of the effects of maca. Front. Pharmacol. 15:1360422. doi: 10.3389/fphar.2024.1360422
Vásquez-Velásquez, C., Gasco, M., Fano-Sizgorich, D., and Gonzales, G. F. (2020). Inflammatory pathway employed by red maca to treat induced benign prostatic hyperplasia in rats. Andrologia 52:e13516. doi: 10.1111/and.13516
Wang, F., Ma, D., Yang, J., Lyu, D., Gao, Q., Li, C., et al. (2024). Mechanisms and efficacy of Chinese herbal medicines in benign prostatic hyperplasia. Chin. J. Integr. Med. doi: 10.1007/s11655-024-3916-0
Wang, X., Zhu, J., Yan, H., Shi, M., Zheng, Q., Wang, Y., et al. (2021). Kaempferol inhibits benign prostatic hyperplasia by resisting the action of androgen. Eur. J. Pharmacol. 907:174251. doi: 10.1016/j.ejphar.2021.174251
Welén, K., and Damber, J.-E. (2022). Androgens, aging, and prostate health. Rev. Endocr. Metab. Disord. 23, 1221–1231. doi: 10.1007/s11154-022-09730-z
White, J. R., Nagarajan, N., and Pop, M. (2009). Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol. 5:e1000352. doi: 10.1371/journal.pcbi.1000352
Wu, F., Guo, X., Zhang, J., Zhang, M., Ou, Z., and Peng, Y. (2017). Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp. Ther. Med. 14, 3122–3126. doi: 10.3892/etm.2017.4878
Xiao, J., Gong, Y., Chen, Y., Yu, D., Wang, X., Zhang, X., et al. (2017). IL-6 promotes epithelial-to-mesenchymal transition of human peritoneal mesothelial cells possibly through the JAK2/STAT3 signaling pathway. Am. J. Physiol. Renal Physiol. 313, F310–F318. doi: 10.1152/ajprenal.00428.2016
Yang, B.-C., Jin, L.-L., Yang, Y.-F., Li, K., and Peng, D.-M. (2014). Inhibitory effect of rape pollen supercritical CO2 fluid extract against testosterone-induced benign prostatic hyperplasia in rats. Exp. Ther. Med. 8, 31–37. doi: 10.3892/etm.2014.1680
Zhang, X., Lu, H., Hong, W., Liu, L., Wang, S., Zhou, M., et al. (2018). Tyrphostin B42 attenuates trichostatin A-mediated resistance in pancreatic cancer cells by antagonizing IL-6/JAK2/STAT3 signaling. Oncol. Rep. 39, 1876–1884. doi: 10.3892/or.2018.6241
Keywords: benign prostatic hyperplasia, rape bee pollen, maca, gut microbiota, IL-6/JAK2/STAT3 signaling pathway
Citation: Huang X, Ru G, Tian H, Zhang P, Zhao H, Zhang S, Wang S, Jia A and Zhao W (2025) Chewable tablets containing rape bee pollen and maca attenuate testosterone propionate–induced benign prostatic hyperplasia in Sprague–Dawley rats by regulating the gut microbiota and modulating the IL-6/JAK2/STAT3 signaling pathway. Front. Microbiol. 16:1547724. doi: 10.3389/fmicb.2025.1547724
Received: 14 January 2025; Accepted: 24 October 2025;
Published: 18 November 2025.
Edited by:
Mohammad Altamimi, An-Najah National University, PalestineReviewed by:
Shalaka Naidu, Symbiosis International University, IndiaGbenga Alabi, Citrus Research International, South Africa
Copyright © 2025 Huang, Ru, Tian, Zhang, Zhao, Zhang, Wang, Jia and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: An Jia, MjAxMjA4MTIwQGhoc3R1LmVkdS5jbg==; Wanli Zhao, emhhb3dhbmxpdGNtQDEyNi5jb20=
†These authors have contributed equally to this work
 Xiaoqiang Huang1†
Xiaoqiang Huang1† Hongchang Zhao
Hongchang Zhao Wanli Zhao
Wanli Zhao