- 1College of Chinese Medicinal Materials, Jilin Agricultural University, Changchun, China
- 2Cultivation Base of State Key Laboratory for Ecological Restoration and Ecosystem Management of Jilin Province, Ministry of Science and Technology, Changchun, China
Introduction: Epimedium koreanum is a traditional Chinese tonic medicine obtained from the wild and cultivation, and its pharmacodynamic composition differs depending on the artificial cultivation method. Rhizosphere microorganisms influence the growth and active component accumulation of medicinal plants; however, the detailed composition, diversity, and connections to soil properties and medicinal herb active components in E. koreanum remain under-researched.
Methods: Illumina NovaSeq technology was used to study the differences in rhizosphere microbial diversity and composition and pharmacodynamic constituents among cultivation methods, including wild tending (WT), bionic cultivated in forest (FP), and simulated habitat cultivation (SC).
Results: Compared with estimates for WT, SC and FP resulted in higher contents of active components in E. koreanum. This cultivation method improves the soil environment by increasing the soil pH, AK, TK, and AP contents. pH, TK, and AP were key factors affecting the bacterial community, while TN, AN, and SOM had significant impacts on the fungal community. Further analyses indicated that the active components of E. koreanum are positively correlated with the abundance of microorganisms, such as Bacillus and Humicola. These microbial communities were significantly enriched in the rhizosphere of FP. In addition, the rhizospheres of FP and WT were enriched with microbial taxa related to plant stress resistance, indicating that different cultivation methods have differential regulatory effects on the plant rhizosphere environment. The proposed FP cultivation method focuses primarily on nitrogen reduction and phosphorus-potassium enhancement along with microbial regulation to synergistically improve both medicinal quality and ecological adaptability.
Conclusion: Soil physicochemical properties and enzymatic activity under different cultivation methods affect soil microbial diversity and composition, thereby impacting plant growth and the synthesis of key components. This work provides a theoretical basis for the scientific and effective cultivation of high-quality E. koreanum.
1 Introduction
Woodlands and grasslands are natural breeding grounds for many medicinal plants. Currently, in China, under the premise of maintaining ecological security and utilizing the advantages of forest and grassland resources, the optimization of ecological cultivation methods to produce safe, efficient, green, and organic high-quality Chinese herbal medicines is necessary to meet demand. China is the modern center of the geographic distribution of Epimedium in the family Berberidaceae. Epimedium koreanum Nakai is the only species in the genus Epimedium distributed in northeastern China, northern Korea, and Japan, and grows naturally in the understory or thickets. E. koreanum, as one of the basal elements of the traditional Chinese medicine Herba Epimedii, is included in the Pharmacopoeia of the People’s Republic of China. The dried leaves are used in medicine and have various functions, including tonifying kidney yang, strengthening muscles and bones, and dispelling wind-dampness. Icariin, epimedin and flavonoids are important indicators for evaluating the quality of medicinal materials (Chinese Pharmacopoeia Commission, 2020). In modern medical clinical practice, it has also applied it to the treatment of Alzheimer’s disease (Wang R. et al., 2023), anti-tumor purposes (Sun et al., 2021) and other diseases. Over 90% of the medicinal material supply of E. koreanum relies on wild resources. However, the destruction of its natural habitats, continuously growing market demand, and excessive harvesting have led to a sharp decline in wild populations (Li Z. et al., 2024). Therefore, it is necessary to develop appropriate cultivation methods, including artificial cultivation, to address the gap between raw material supply and demand and to protect and rationally utilize wild E. koreanum resources.
With the increasing market demand for medicinal plants, many countries have listed medicinal plants as important economic crops. Soil is the basis of medicinal plant cultivation, and there are extremely complex interactions between medicinal plants and soil microorganisms. Specific environmental conditions create specific soil microbiological systems, which have an impact the herbal medicine quality. The plant root system involves the aggregation of microbial communities in the soil micro-ecosystem, providing important ecological niches for interactions between plant roots and microorganisms (Trivedi et al., 2020). The structure and dynamics of rhizosphere microbial communities are important drivers for maintaining a soil micro-ecological balance, measuring soil quality, and maintaining soil fertility and plant stress tolerance (Fitzpatrick et al., 2020; Zhou et al., 2021), and improving the utilization of mineral elements in the soil. Numerous studies have pointed out that the active flora in the rhizosphere soil can greatly promote the enrichment of nutrients in the soil, which has important implications for the production of high-quality medicinal herbs (Feng et al., 2021; Su et al., 2023). The application of the rhizosphere-promoting bacterium Azotobacter chroococcum and Azospirillum brasilense alleviated the adverse effects of drought on Mentha pulegium L. significantly and increased the contents of phenolic compounds, flavonoid, and essential oil (Asghari et al., 2020). Zhang et al. (2021) found that Streptomyces netropsis WLXQSS-4 isolated from the rhizosphere of Clematis manshurica Rupr. contains genes related to the biosynthesis of alloaureothin. Burkholderia promotes the synthesis of indigo, a main active ingredient of Baphicacanthus cusia (NeeS) Bremek (Zeng et al., 2018). Bryobacter and Candidatus Solibacter can indirectly increase the polysaccharide content of Polygonatum cyrtonema Hua (Liu J. et al., 2024). Similar studies have emphasized the ability of rhizosphere microbiota, especially several key taxa, to increase the ginsenoside content in Panax ginseng as well as polysaccharides and flavonoids in Dendrobium officinale (Dong et al., 2019; Zhou et al., 2025).
Despite research on ecological cultivation, such as wild-tending and ecological planting of E. koreanum, the weak seed propagation ability of the species limits the supply of seeds and seedlings, thereby limiting artificial cultivation (Dai et al., 2022; Yan et al., 2021; Zhang et al., 2024). The relationships between the rhizosphere microbial composition, diversity of E. koreanum under different ecological cultivation methods, and soil properties and pharmacodynamic components have not been clearly established. Therefore, this study utilized Illumina NovaSeq technology to investigate the diversity and composition of the rhizosphere soil microbial community of E. koreanum, in addition to analyses of soil physicochemical properties, enzyme activity, pharmacodynamic components, and correlations between these parameters. The results of this study not only deepen our understanding of the rhizosphere microorganisms of E. koreanum but also provide a valuable scientific reference for rational planting and soil cultivation.
2 Materials and methods
2.1 Sample collection and pre-processing
Based on previous research on E. koreanum, late May (the full bloom period) and late August (the late post-fruit growth period) are the key periods for the synthesis and accumulation of pharmacodynamic components (Tang et al., 2007). Plant and rhizosphere soil samples in this study were collected in late May and late August of 2023. Samples of E. koreanum from bionic cultivation in the forest (bionic cultivation in a Larix forest, FP) and cultivation under a simulated habitat (cultivation at logging sites covered with shade nets, SC) were collected from the Epimedium planting base in Tonghua County, Jilin Province, China (125°28.7′E, 41°39.44′N). Wild E. koreanum samples collected from an artificial closure area near the base (WT). All cultivation methods avoided human interference and no exogenous fertilizers were applied. The climatic conditions were the same at each collection site.
The 5-point sampling method was used to collect E. koreanum plants and rhizosphere soil. The whole plant was dug out with soil, the loose soil and impurities were shaken from the roots, and the rhizosphere soil of the fibrous roots was collected using a sterile brush. All samples were placed in a low-temperature storage box and quickly brought back to the laboratory. One soil sample was encapsulated in a sterile EP tube and stored at −80°C for DNA extraction and high-throughput sequencing; one soil sample was dried naturally and sieved through a 20-mesh sieve for the determination of physicochemical properties and enzyme activities. Leaf samples were naturally dried, powdered, and sieved for the determination of major flavonoid contents.
2.2 Determination of the content of the main flavonoid components
Determination was performed with reference to the “Content Determination” method under “Epimedium” of the 2020 edition of Chinese Pharmacopoeia (Chinese Pharmacopoeia Commission, 2020). Determination of icariin, epimedin A, epimedin B, and epimedin C by high-performance liquid chromatography (Agilent 1260 HPLC, Agilent). Meanwhile, the content of total flavonoids was determined using UV spectrophotometry.
2.3 Soil physicochemical properties and enzyme activity tests
Determination of physicochemical properties and enzymatic activities of rhizosphere soils followed relevant standard soil test methods (Bao, 2000). The soil pH value was determined using an international standard 1:5 soil-water ratio while using an ion meter (MP521, Sanxin Instrument Factory, Shanghai, China); the soil organic matter (SOM) content was determined using potassium dichromate oxidation-colorimetry method with water hydration; the total nitrogen (TN) was determined using Kjeldahl nitrogen determination (K9860, Haineng Scientific Instrument Co., Ltd., Shandong, China); determination of ammonia nitrogen (AN) by the alkaline diffusion method; the total phosphorus (TP) was determined using the NaOH melt-molybdenum-antimony anticolorimetric method; determine the available phosphorus (AP) content by the 1/2H2SO4 method; the available potassium (AK) and total potassium (TK) were determined using flame atomic absorption spectrophotometry (AA-6300, Shimadzu Corporation, Japan). Soil saccharase (SSC) activity was determined by colorimetric assay with 3,5-dinitrosalicylic acid; soil urease (SUE) activity was determined by indophenol blue colorimetric assay; soil acid phosphatase (SACP) was determined by colorimetric assay with sodium benzene diphosphate (Solarbio, BC0145); soil catalase (SCAT) was determined by ultraviolet spectrophotometry (Solarbio, BC0105).
2.4 DNA extraction, amplification and sequencing techniques
Soil DNA extraction was performed using the HiPure Soil DNA Extraction Kit (Magen, Guangzhou, China). The purity and concentration of DNA were detected by using a NanoDrop micro-volume spectrophotometer and agarose gel electrophoresis. Amplification of the highly variable V3–V4 region of the bacterial 16SrRNA gene using specific primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 806R (5′-GGACTACHVGGGGTATCTAAT-3′). The transcribed spacer region of the fungal ITS gene was then amplified by PCR using primers ITS3 KYO2 (5′-GATGAAGAACGYAGYRAA-3′) and ITS4 (5′-TCCTCCGCTTATTGATATATGC-3′). PCR amplification conditions: pre-denaturation at 95°C for 5 min; 30 cycles (denaturation at 95°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min); final extension at 72°C for 7 min. Amplification system: 50 μL mixture containing 10 μL of 5 × Q5@ Reaction Buffer, 10 μL of 5 × Q5@ High GC Enhancer, 1.5 μL of 2.5 mM dNTPs, 1.5 μL of each primer (10 μM), 0.2 μL of Q5@ High-Fidelity DNA Polymerase, and 50 ng of template DNA. Related PCR reagents were from New England Biolabs, United States (Supplementary Tables S1, S2). 2% agarose gels were assessed for amplification product quality for integrity. The sequencing library was constructed using the Illumina DNA Prep Kit (Illumina, CA, United States).
Using the NovaSeq 6000 platform, 2 × 250 bp paired-end reads were generated. Filtering, merging, quality control, and organization of raw data were performed to obtain valid data. Chimeric sequences were identified by comparisons against the SILVA (16S, version 138.1) and UNITE (ITS, version 8.3) databases. Taxonomic annotations were performed using RDP annotation software (version 2.2). Soil DNA extraction, amplification, and sequencing were performed by Gene Denovo Corporation (Guangzhou, China).
2.5 Data and statistical analysis
Alpha and beta diversity were analyzed with standardized datasets using the vegan package in R and a nonparametric multivariate analysis of variance (Adonis). Differences in microbial diversity and relative abundance at different microbial classification levels between groups were assessed using one-way ANOVA, t-tests, or Kruskal–Wallis tests using SPSS software. Mantel’s tests, Pearson’s correlation analyses, and structural equation modeling (SEM) were utilized to explore the relationships between medicinal components, soil properties, and microorganisms. Sequencing data were analyzed using the Omicsmart cloud platform.1
3 Results
3.1 Soil physicochemical properties, enzyme activities, and content of medicinal components
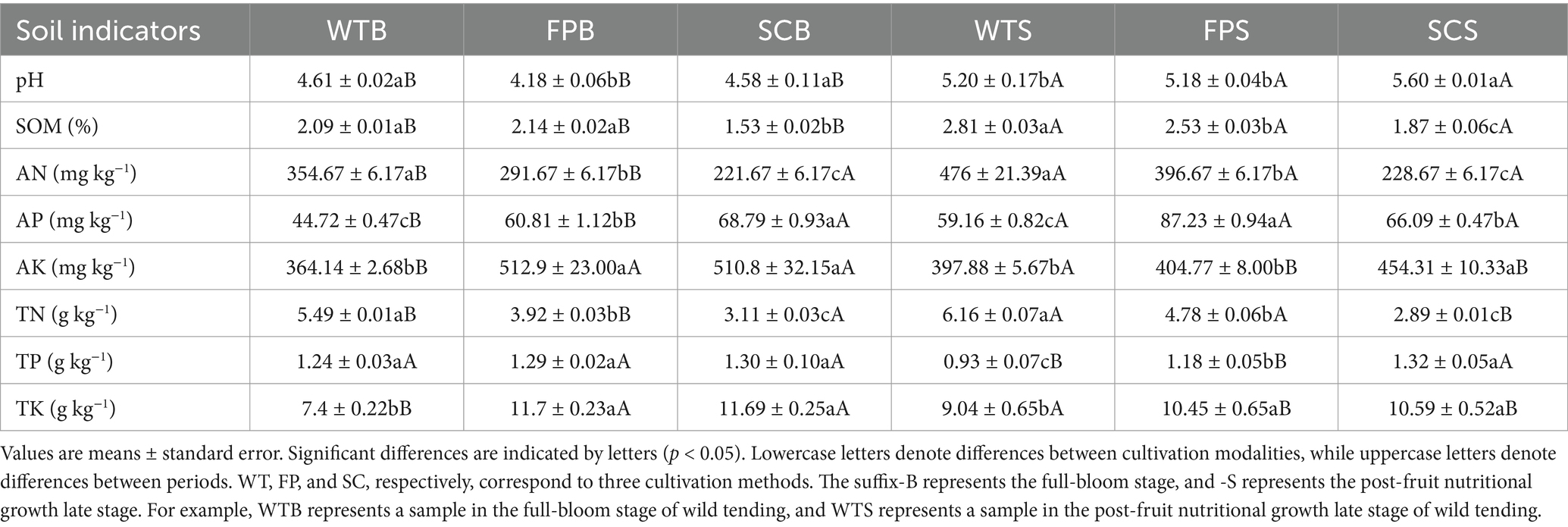
Table 1 shows the physicochemical properties of the soil for different cultivation methods. The soil was generally strongly acidic. The pH value was significantly higher during the late post-fruit nutrient growth period than at the full bloom period. The pH values for FPB were significantly lower than those of WTB and SCB, showing very strong acidity, and the pH of SCS was significantly higher than that of WTS and FPS. The SOM, AN, and TN contents in the rhizosphere soil of SC was significantly lower than that of WT and FP (p < 0.05). The differences in the TP content among cultivation methods were not significant. However, at the late nutritional stage of post-fruit growth, AP contents decreased in the order SC > FP > WT and were significantly lower in WT than in FP and SC. WT samples showed a significantly lower AK and TK contents than that in FP and SC, and WTS > WTB, while FPS < FPB, SCS < SCB. In addition, the AP contents were significantly higher in the late stage of post-fruiting nutrient growth than in the full bloom stage for each cultivation method. The SSC, SACP, and SCAT activity generally decreased in the order of WT > FP > SC. SSC, SACP, and SCAT activity was significantly higher during the late post-fruiting nutrient growth stage than at the full bloom period (Figures 1A,C,D). SUE activity was significantly lower in FPB than in WTB and SCB, whereas FP > WT > SC was observed at the later stages of post-fruiting nutrient growth (Figure 1B). The total contents of medicinal components under different cultivation methods for E. koreanum followed the order SC > FP > WT (Figures 1E–G). The contents of total flavonoids and total flavonol glycosides (icariin, epimedin A, B, and C sum) of WTB, FPB, and SCB, respectively, were 6.33, 0.78%; 7.26, 1.32%; 15.71, 2.94%. The contents of total flavonoids and total flavonol glycosides of WTS, FPS, and SCS were 8.02, 0.71%; 9.30, 0.87%; 9.88, 1.21%, respectively. All values exceeded the standards reported in Chinese Pharmacopoeia.
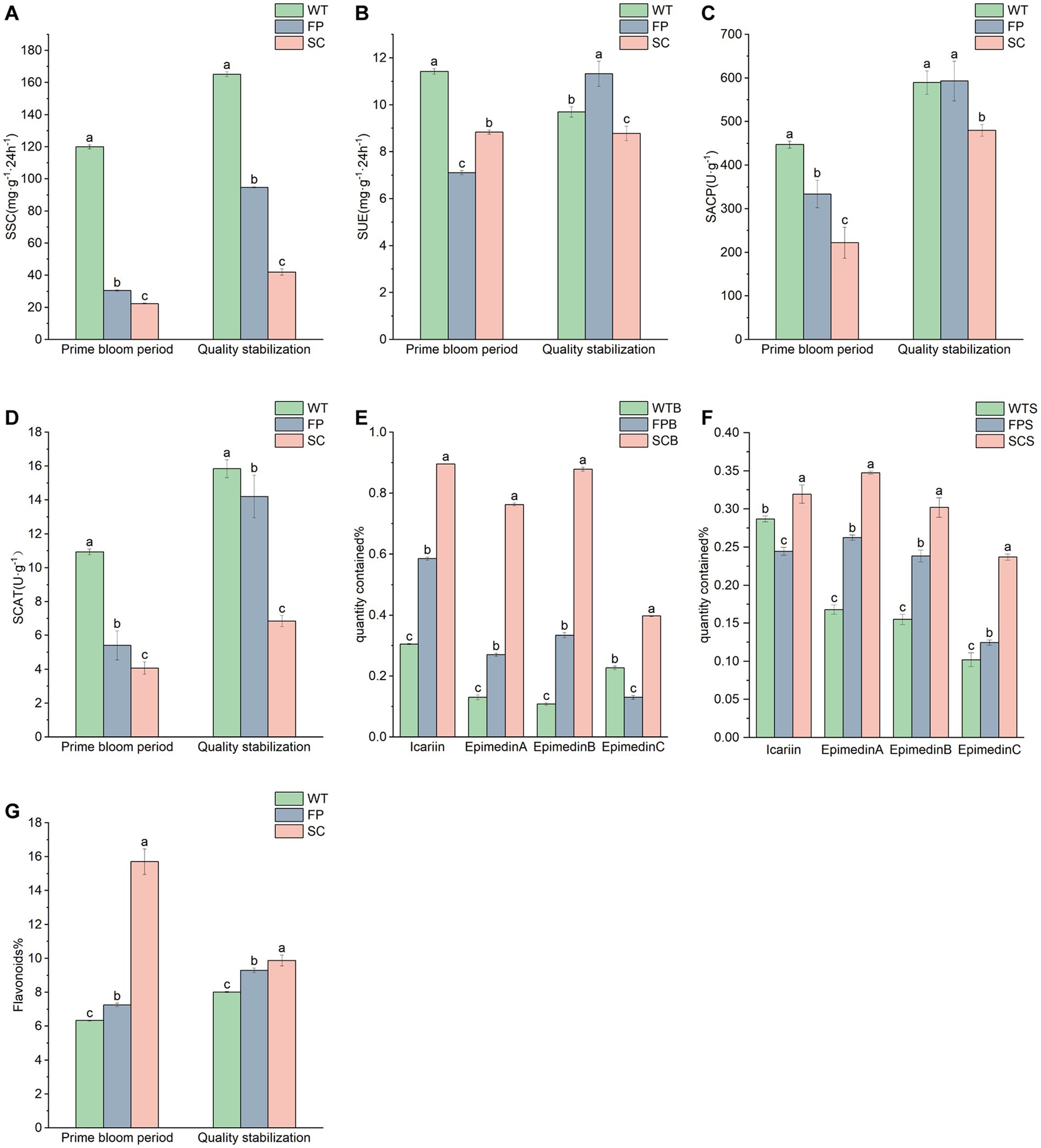
Figure 1. Soil enzyme activities and contents of pharmacodynamic components under different cultivation methods. (A) Soil saccharase. (B) Soil urease. (C) Soil acid phosphatase. (D) Soil catalase; full bloom period. (E) Late post-fruit nutrient growth period. (F) Epimedin A, B, and C contents. (G) Total flavonoid content. The letters “a, b and c” are significant markers, and different letters between treatments indicate significant differences at the significant level of 0.05. WT, FP, and SC, respectively, correspond to three cultivation methods. The suffix-B represents the full-bloom stage, and -S represents the post-fruit nutritional growth late stage. For example, WTB represents a sample in the full-bloom stage of wild tending, and WTS represents a sample in the post-fruit nutritional growth late stage of wild tending.
3.2 Analysis of soil microbial diversity and composition
3.2.1 Analysis of soil microbial community diversity
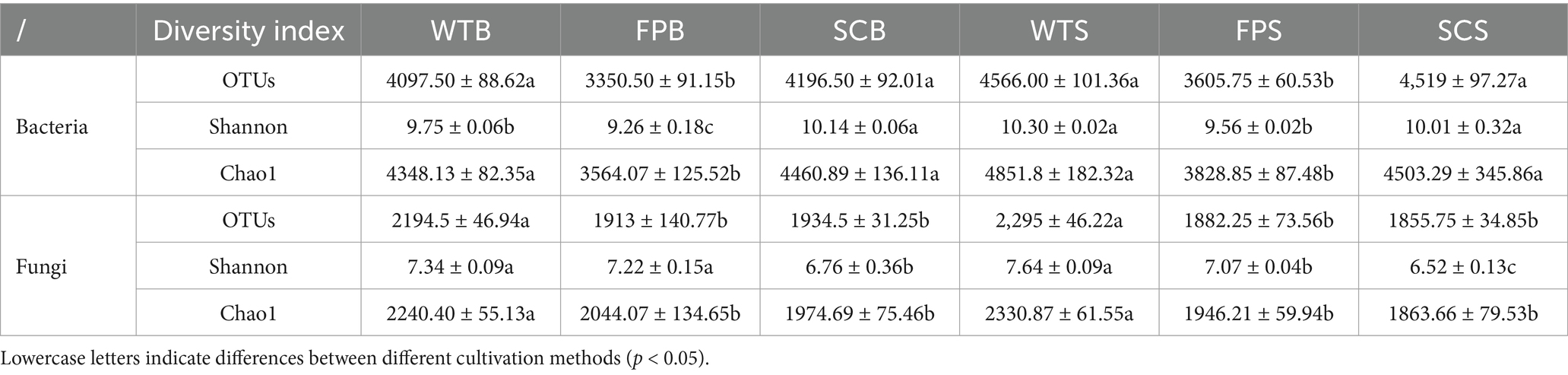
A rarefaction curve analysis showed stabilization with increasing sequencing volumes, indicating that the sequencing depth was sufficient to reflect the structure and composition of bacterial and fungal communities in the rhizosphere soil samples under different cultivation methods (Supplementary Figure S1). In bacteria, there were significantly fewer the OTUs, Shannon, and Chao1 index in FP samples than in WT and SC samples. From the full-bloom to post-fruit nutritional growth late stage, the number of the OTUs, Shannon index, and Chao1 index of WT and FP samples increased significantly; however, the general pattern, WT > FP > SC, indicated that the species richness and diversity of rhizosphere bacteria in WT and SC were significantly higher than those in FP. In terms of fungal diversity, there were significantly more OTUs, Shannon, and Chao1 index during the two periods in SC and FP samples than in other samples during the corresponding periods; rhizosphere fungal diversity and abundance were higher in WT and lower in FP and SC (Table 2). Figure 2 shows the principal coordinates analysis (PCoA) results for soil bacteria and fungi, illustrating the differences in the composition of the rhizosphere soil microbial community between cultivation methods. In an Adonis (PERMANOVA) analysis, as summarized in Figure 2, the R2 value was close to 1, indicating that the block effect has a high explanatory power for sample variation. As shown Figure 2A, the first and second coordinate axes explained 31.14 and 20.39% of the variance in bacterial community composition, respectively. The rhizosphere soil bacterial communities of WT, FP, and SC were clearly separated (R2 = 0.7668, p < 0.001) and could be divided into three distinct groups (Figure 2C). In the PCoA of fungal communities, PCoA1 accounted for 40.20% of the total variance, and PCoA2 accounted for 21.71%. The rhizosphere soil fungal communities under three cultivation methods showed significant differences (R2 = 0.8556, p < 0.001), indicating that different cultivation methods had a significant impact on the structure of soil fungal communities (Figures 2B,D). Therefore, rhizosphere microbial communities of E. koreanum cultivated using different methods have different structures.
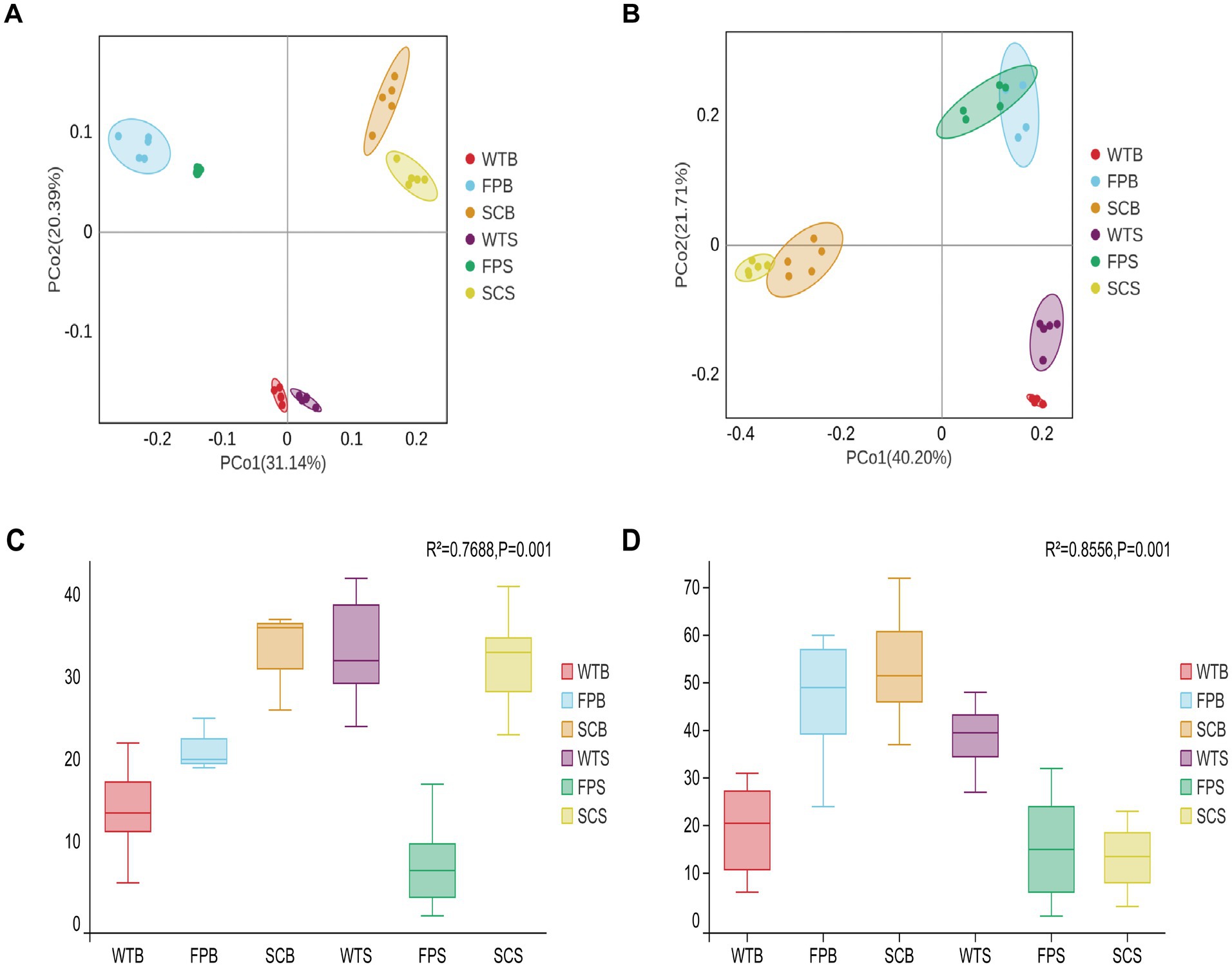
Figure 2. Bacteria (A) and fungi (B) under different cultivation methods principal coordinate analysis (PCoA). Bacterial (C) and fungal (D) Adonis (PERMANOVA) box test plots.
3.2.2 Soil microbial community composition
To analyze the composition of the soil bacterial and fungal communities in-depth, the top 10 dominant phylum in terms of relative abundance as well as the top 15 dominant genera were selected for further analyses (Figure 3). Acidobacteriota was dominant in most of the samples with a relative abundance of 22.99–26.08%. Proteobacteria (12.90–21.85%), Verrucomicrobiota (10.65–12.08%), and Patescibacteria (8.04–13.80%) were relatively abundant bacterial phylum. However, the relative abundance of soil bacterial phyla under different cultivation methods differed significantly (Figure 3A). For example, in WT samples, the relative abundance of Proteobacteria was consistently higher than that of SC and FP samples, whereas the relative abundance of Patescibacteria was consistently lower in WT than in SC and FP. Ascomycota and Basidiomycota were the most abundant fungal phylum, with relative abundances above 81% (Figure 3B). However, the relative abundances of soil fungal phyla differed significantly among cultivation methods. The relative abundances of Patescibacteria, Actinobacteriota, Gemmatimonadota, and other taxa in the FP rhizosphere soil were significantly higher than those in WT and SC. The relative abundance of Chloroflexi and Ascomycota in SC rhizosphere soil was significantly higher than that in WT and FP, while the relative abundance of Basidiomycota was lower. Compared with those in the rhizosphere of WT, the relative abundances of Proteobacteria, Mortierellomycota, and other taxa in the rhizosphere soils of FP and SC were significantly lower.
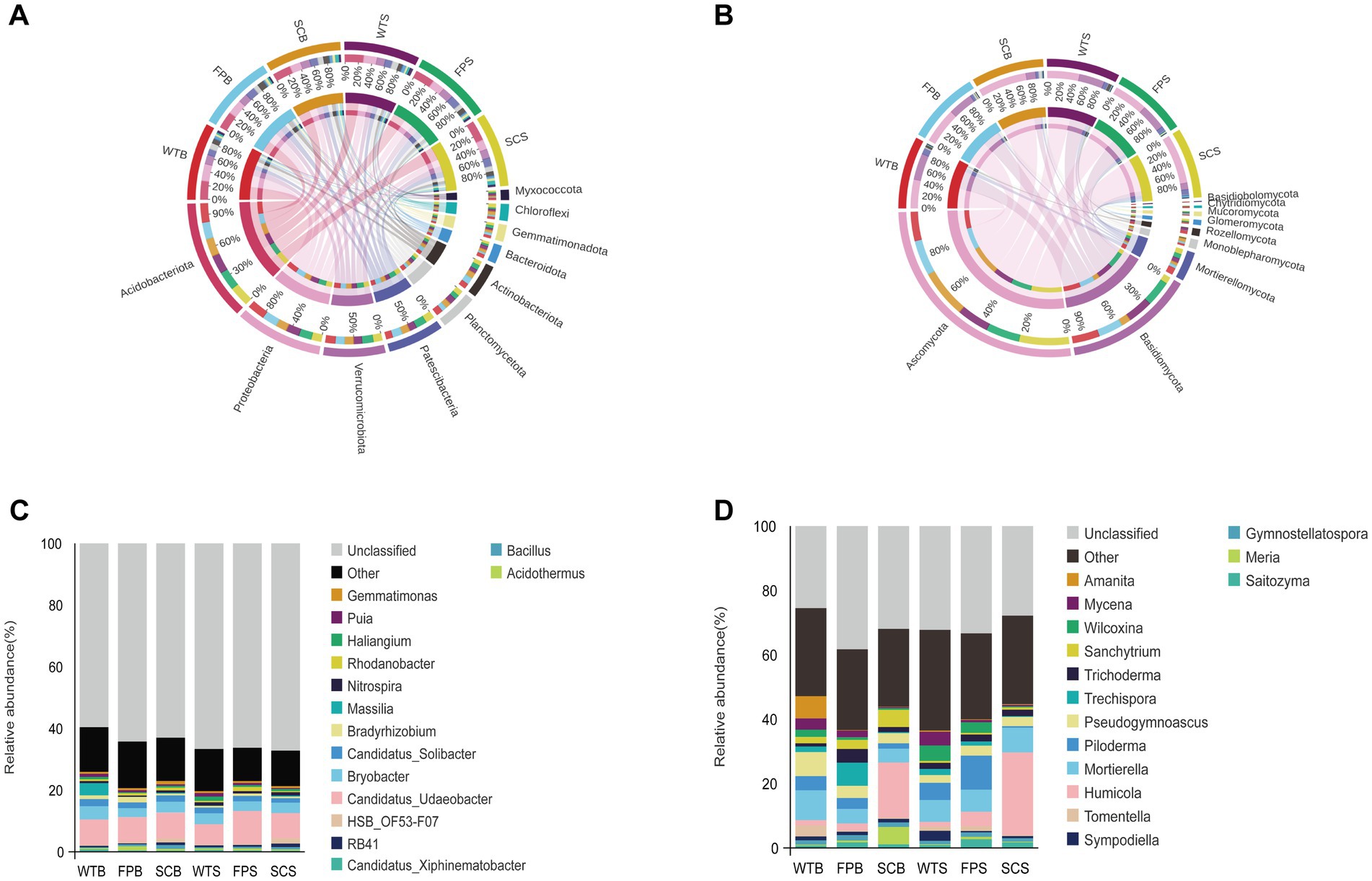
Figure 3. Composition at the level of (A,B) phylum. (C,D) Genus under different cultivation methods. (A,C) Bacteria. (B,D) fungi.
At the genus level, the dominant bacterium Candidatus Udaeobacter showed significant differences during the later stage of post-harvest vegetative growth, decreasing in the order FP > SC > WT (Figure 3C). The abundances of Rhodanobacter and Acidothermus in the rhizosphere soil of FP were significantly higher than those in WT and SC. The abundances of Nitrospira, HSB OF53-F07, RB41 and Bacillus in the rhizosphere soil of SC were significantly higher than those in WT and FP. Among fungi, Humicola was the dominant genus in the SC rhizosphere and had a significantly higher abundance in SC than in WT and FP. The abundance of Mortierella in the rhizosphere soils of WTB and SCS was significantly higher than those in other samples. The abundance of Saitozyma in the rhizosphere of FP was significantly higher than that in WT and SC. In addition, fungi such as Trechispora, Piloderma, and Amanita were significantly more abundant in FP than in other treatments. Other fungi, such as Wilcoxina and Mycena in the soil of WT and Mortierella in the rhizosphere soil of WTB, showed a significantly higher abundance than those in other treatments.
3.2.3 Analysis of soil microbial indicator species
LEfSe (linear discriminant analysis effect size) indicated differences in high-dimensional biomarker taxa in soil bacterial and fungal communities under different cultivation practices (Figure 4; Supplementary Figure S2). The main biomarkers of WT included bacteria such as Massilia and Burkholderiale as well as fungi such as Amanitaceae and Agaricomycetes. The main biomarkers of FP included bacteria such as Subgroup 2, Rhizobiales, and Bacilli and fungi such as Hydnodontaceae, Piloderma, Trechispora. The main biomarkers of SC included bacteria such as Chloroflexi and Acidobacteriales as well as fungi such as Humicola and Helotiales. The number of differential markers in bacterial communities was higher during the late post-fruiting nutrient growth period relative to the bloom period, while fungal biomarkers showed a decrease.
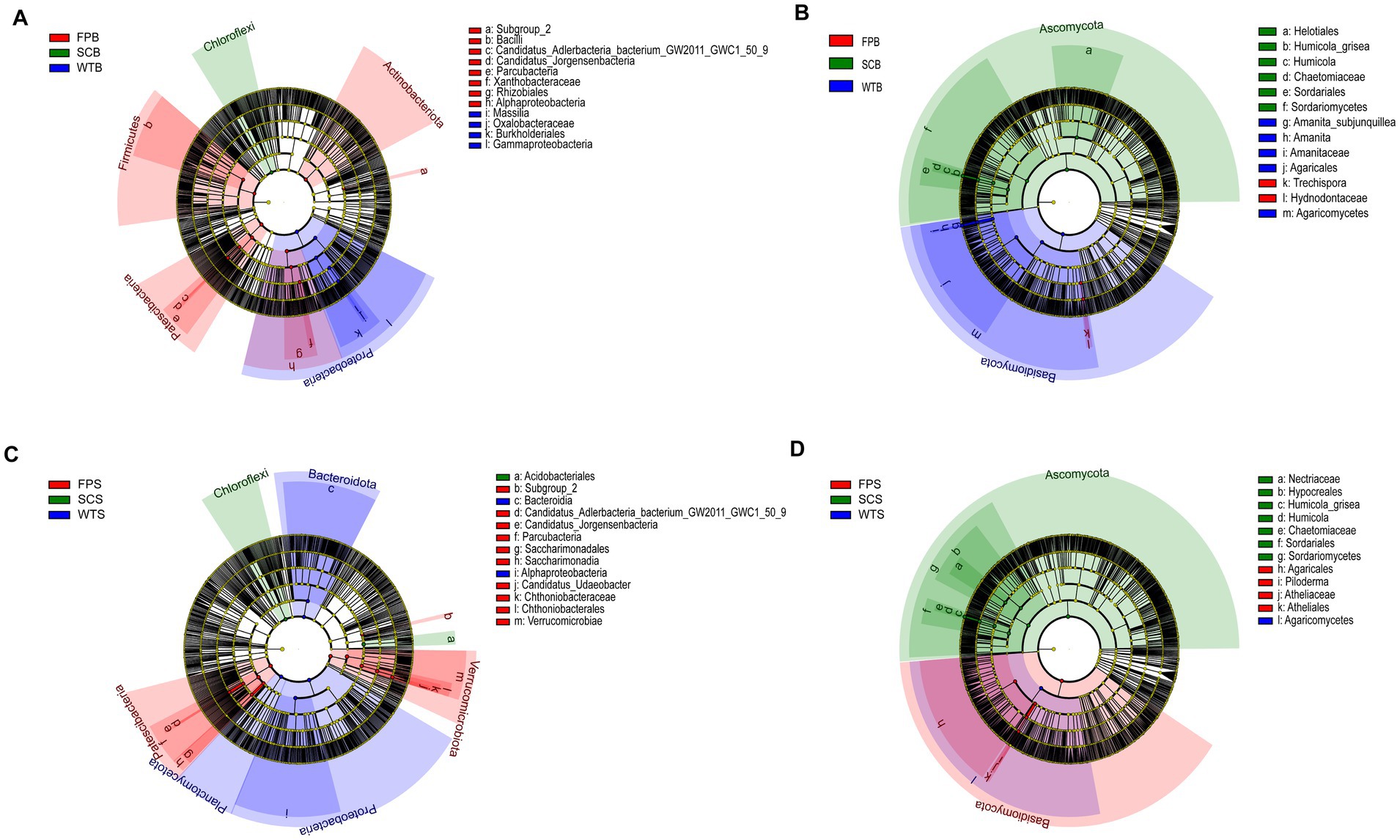
Figure 4. Linear discriminant analysis (LEfSe) plots of different cultivation methods (A,C) bacterial and (B,D) fungal. Bacteria: LDA ≥4.0; Fungi: LDA ≥4.5. The dendrograms were categorized at the level from phylum to genus. Nodes of different colors indicate microorganisms that were significantly enriched in the corresponding group and had a significant effect on the differences between groups (p < 0.05). Light yellow nodes indicate microorganisms that were not significantly different in any of the different groups (p > 0.05).
3.3 Relationships among soil physicochemical properties, enzyme activities, and microbial communities
Using Mantel analysis, found that the diversity of rhizosphere microbial species composition was significantly correlated with soil physicochemical properties (Figure 5A). pH, TK, AP, and SSC had highly significant effects (p < 0.01) on bacterial communities, while TN, AN, SOM, and SSC had highly significant effects on fungal communities (p < 0.001). To further explore the response of the microbial community to environmental factors, are redundancy analysis (RDA) of the dominant genera in the top 15 for each of the bacterial and fungal abundances with soil properties and enzyme activities was performed. The results showed that the RDA1 and RDA2 axes explained a total of 56.70% of the variance in the composition of soil bacterial communities and 49.94% of the variance in the composition of fungal communities, clarifying the main factors that had a significant effect on the presence of bacterial and fungal communities (Figures 5B,C). Nitrospira and RB41 showed a highly significant positive correlation with pH (p < 0.01). HSB OF53-F07, Bacillus, Gemmatimona, and other bacteria, and Humicola, Meria, and Sanchytrum fungi were significantly and positively correlated with AP, AK, and TK contents, in addition, Candidatus Udaeobacter was also significantly and positively correlated with AP, TP, and SUE contents (p < 0.05). Bryobacter, Puia, Piloderma, Candidatus Xiphinematobacter, Candidatus Solibacter, Bradyrhizobium, Wilcoxina, and Massilia were significantly and positively correlated (p < 0.05) with soil AN, SOM, SSC, SACP, and SCAT content.
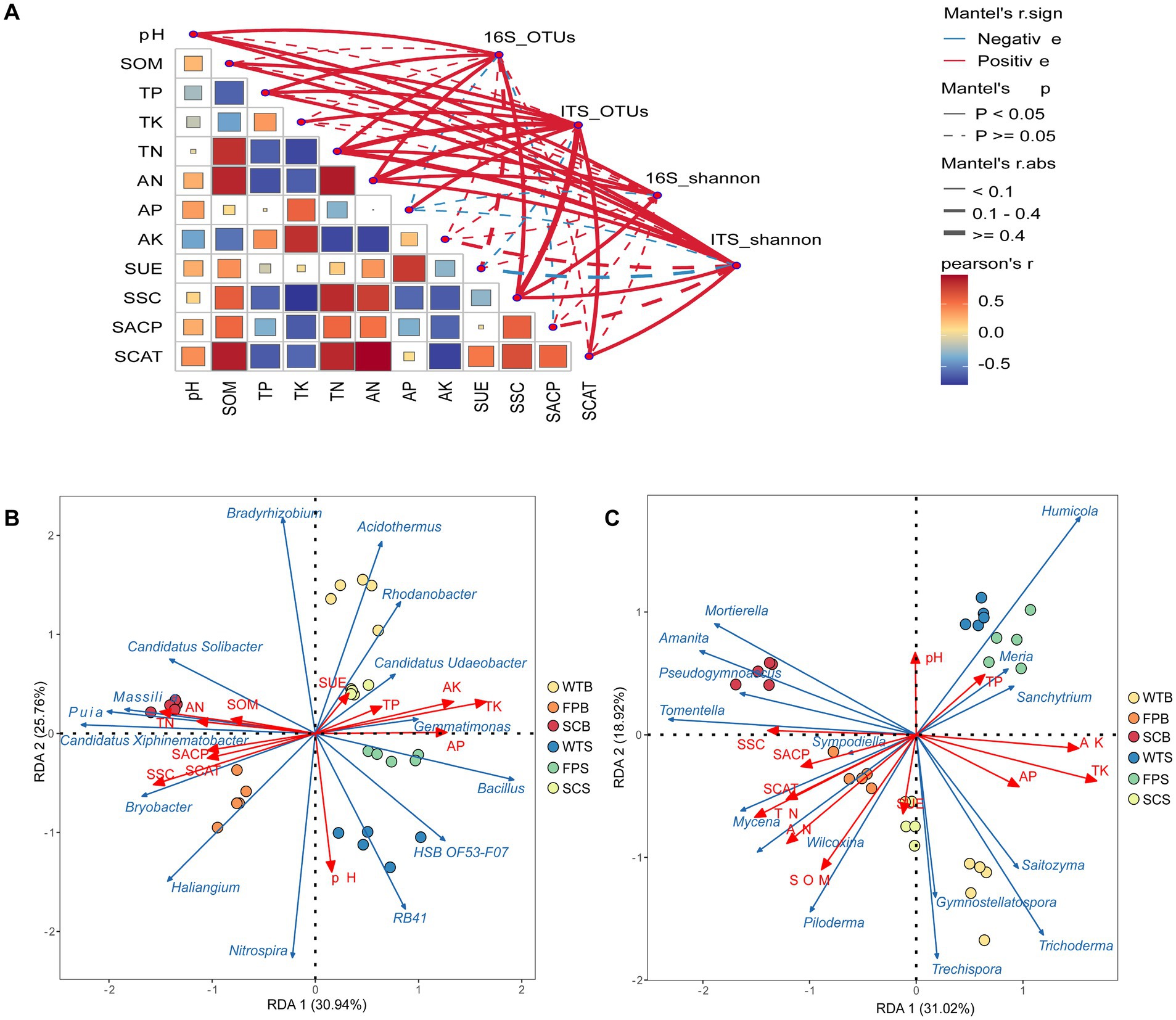
Figure 5. Soil properties and microbial community composition Mantel analysis (A), line width corresponds to Mantel’s r-value, color represents significance, Pearson’s correlation coefficients are shown by line color gradient; soil properties with soil bacterial (B) and fungal (C) RDA analyses, with red arrows indicating the relative position of their physicochemical properties and enzyme activities on the horizontal plane. Blue arrows indicate the distribution of species at the genus level, with longer arrows indicating a greater impact on the species. The strength of the correlation varies when the angle between the arrow and the sorting axis is different. The smaller the angle, the stronger the correlation, and the longer the length of the arrow, the greater the influence of environmental factors.
3.4 Relationship of medicinal components to soil properties, enzyme activities, and microbial communities
Based on Pearson correlation coefficients, there were correlations between the medicinal components of E. koreanum and the nature of the rhizosphere soil, enzyme activity, and abundances of bacterial and fungal genera under different cultivation methods (Figure 6). The flavonol glycoside and total flavonoid content of E. koreanum were extremely significantly positively correlated with AK, TK, TP, Bacillus, Gemmatimonas, Humicola, Sanchytrium (p < 0.01). RB41 and HSB OF53-F07 was significantly positively correlated with total flavonoids and epimedin content. The above pharmacologically active components were extremely significantly negatively correlated with soil SOM, TN, AN and most fungal genera, such as Puia, Piloderma, Wilcoxina, and Tomentella (p < 0.01). The influence of characteristic microorganisms on the quality of E. koreanum was further explored through SEM (Figure 7). The overall goodness-of-fit of the model was significant (R2 = 0.98), indicating that the three-level path of cultivation method-soil-microorganisms can explain 98% of the variation in medicinal material quality. Gemmatimonas, Bacillus, and Humicola had a significant impact on the quality of E. koreanum. Overall, AP and Bacillus had the greatest impact on quality (standardized total coefficients is 0.515, 0.489). TK and SSC was also positively correlated with the abundance of Gemmatimonas. The significance of the critical pathway (such as AP → Bacillus → quality) further supports the rationality of the “soil property changes-microbial colonization-component accumulation” process.
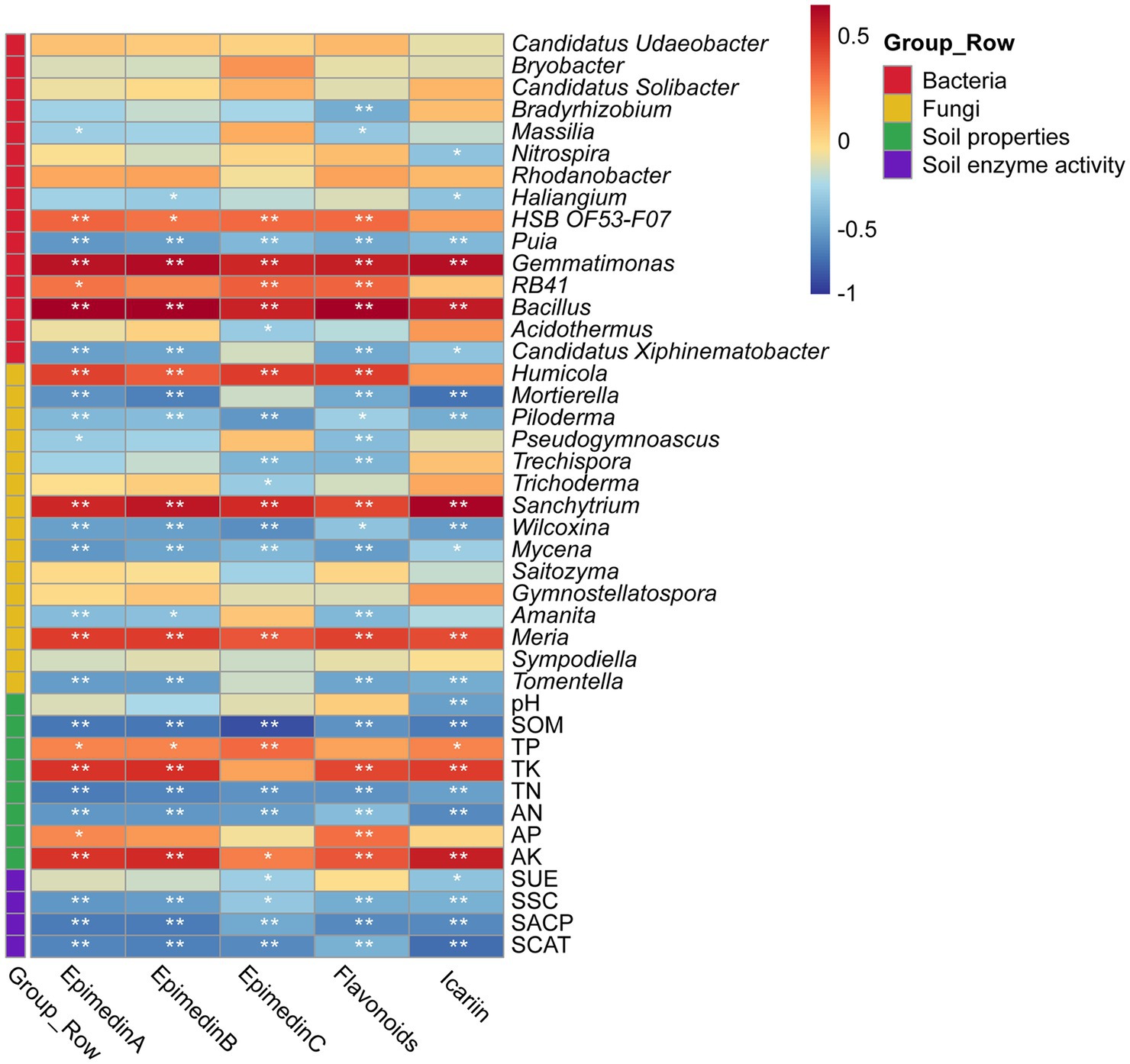
Figure 6. Heat map of correlation between the pharmacodynamic components of E. koreanum and soil environmental factors, bacteria and fungi genus levels. “*” Indicates a significant correlation at the 0.05 level, “**” indicates a highly significant correlation at the 0.01 level.
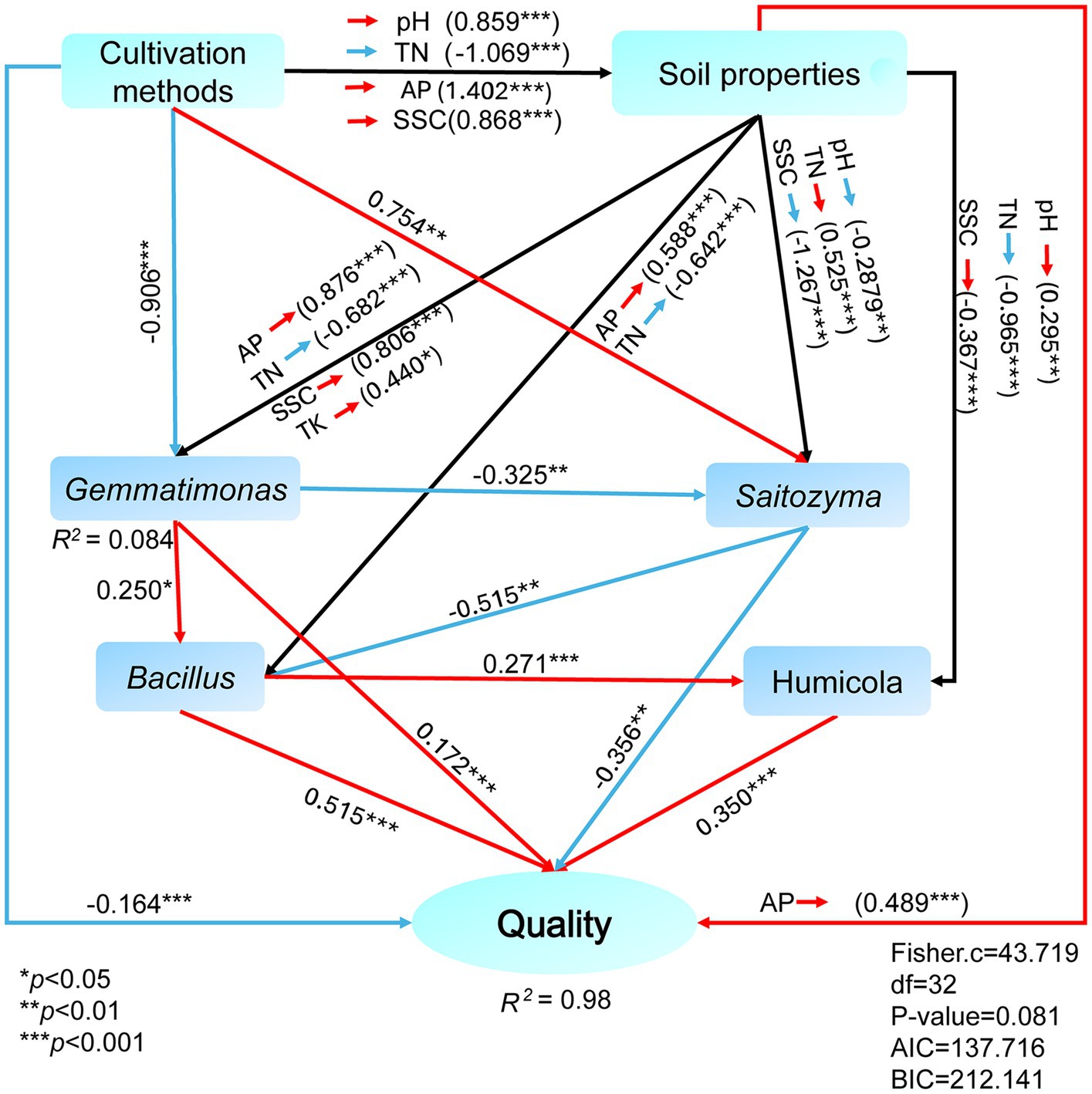
Figure 7. SEM analysis of the correlation between active components of E. koreanum, soil environmental factors, and characteristic microorganisms. The red lines represent positive correlations, the blue lines represent negative correlations, and the black lines represent both positive and negative correlations.
3.5 Prediction of soil microbial community function
The function of the soil bacterial community based on KEGG metabolic pathways during mass stabilization was analyzed using the TAX4Fun prediction method (Figure 8A). Cell motility and endocrine system-related bacterial communities were more enriched in WT than in FP and SC. Membrane transport-related communities in FP were also characterized by significant enrichment. In SC, the abundance of metabolism of other amino acids—related groups was higher than that in WT. Of note, the functional groups did not show significant differences among the three cultivation methods (p > 0.05).
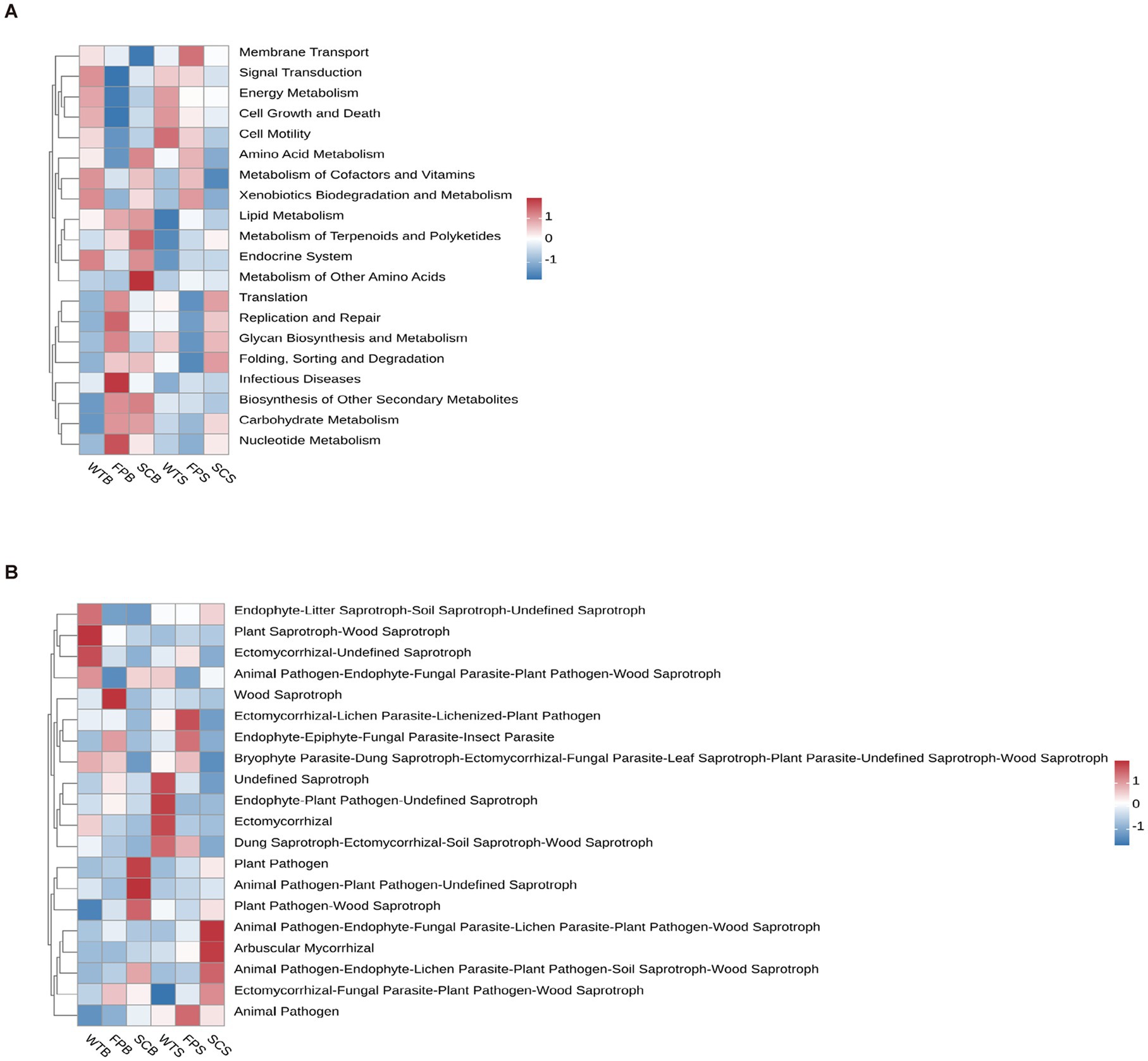
Figure 8. Prediction of functional genes of soil bacterial metabolic pathways KEGG (A). Prediction of functional genes of soil fungal metabolic pathways (B).
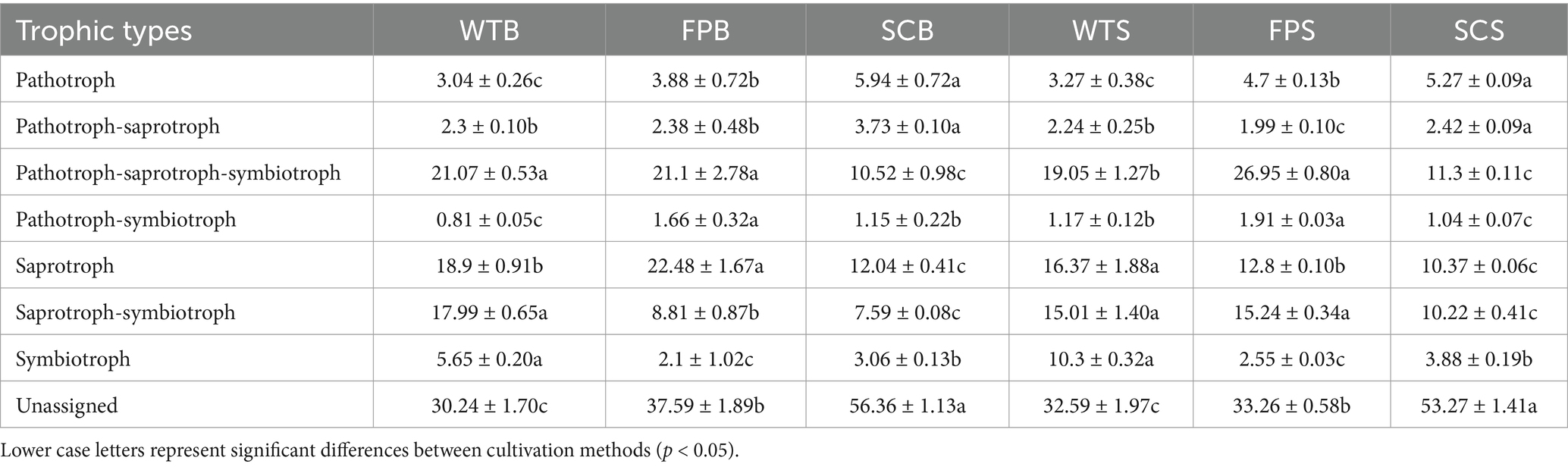
A predictive analysis of fungal functions using FUNGuild showed that fungal taxa with unknown functions were dominant and differed significantly in abundance between cultivation methods. The abundances of pathotroph-saprotroph-symbiotroph and saprotroph-symbiotroph fungi were significantly lower in SC than in WT, with decreases reaching 59.25 and 60.59% at the full bloom period, respectively, and 39.75 and 29.93% in the later stages of post-fruit growth and nutrition (Table 3). The abundance of pathotroph-saprotroph in SC was significantly higher than that in WT and was significantly lower during the later stages of post-fruiting nutrient growth. The relative abundance of saprotroph in FP increased by 20.79% at full bloom and decreased by 21.02% at the later stages of post-fruit growth and nutrition compared with those in WT. The relative abundance of bryophytes, endophyte-litter saprotroph-soil saprotroph-undefined saprotropin in WT rhizosphere soil was significantly higher than that in SC. Wood saprotrophs had a significantly higher relative abundance in FP than in WT and FP (Figure 8B).
4 Discussion
The quality of forest and grass medicinal materials is affected by the medicinal plant species, cultivation methods, ecological conditions, and other factors, and the content of medicinal components reflects the quality of medicinal plants (Yang et al., 2016). Rhizosphere microbial communities are directly or indirectly involved in the growth and development of medicinal plants and the synthesis and accumulation of medicinal components through a variety of pathways (Berendsen et al., 2012). Previous studies have shown that there are significant differences in the main active components of medicinal plants under different cultivation methods (Song et al., 2023). Yang et al. (2024) found that the cultivation method affects the content of medicinal components of Scutellaria baicalensis, where cultivated S. baicalensis showed significantly higher content of most flavonoids than those of wild populations. In this study, the FP and SC cultivation method significantly enhanced the synthesis and accumulation of the active pharmaceutical components in E. koreanum, exceeding the standards of the Chinese Pharmacopoeia. Therefore, appropriate cultivation methods can effectively improve the quality of E. koreanum.
Differences in cultivation affect soil physicochemical properties, and microorganisms respond to environmental changes through potential adaptive evolution (Li J. et al., 2024; Wang Z. et al., 2024). Dong et al. (2023) found that the rhizosphere microbial community of wild Fritillaria pallidiflora Schrenk tended to be dominated by fungal taxa, whereas the rhizosphere microbial community of cultivated Fritillaria pallidiflora Schrenk tended to be dominated by bacterial taxa. In this study, the rhizosphere soil of SC also exhibited higher bacterial diversity, while the rhizosphere community of the bionic cultivated in forest FP tended to include more fungal taxa, which may be related to the relatively similar environment to that of WT under the larch forest. The rhizosphere microbial communities of SC and FP exhibit significant functional advantages. The rhizosphere soil of SC is dominated by phyla such as Chloroflexi and Ascomycota, which significantly enhance the utilization rate of organic elements by participating in plant nutrient cycling and energy transformation (Challacombe et al., 2019; Narsing Rao et al., 2022). The FP rhizosphere was enriched with Verrucomicrobiota and Patescibacteria, related to stress tolerance and nutrient cycling, further contributing to plant adaptability to abiotic stresses (Trivedi et al., 2020; Ye et al., 2024). In contrast, the rhizosphere of WT was dominated by Proteobacteria and Basidiomycota. The reduced abundance of Basidiomycota might be related to the inhibitory effect of the cultivation environment on soil microorganisms, consistent with the results of previous studies showing that cultivation reduces the abundance of Basidiomycota in Zea mays L. (Li Y. et al., 2022). At the genus level, compared with levels in WT, a large number of functional microorganisms related to nutrient absorption and the synthesis of pharmacologically active compounds accumulated in the rhizosphere of SC and FP. For example, microorganisms such as HSB OF53-F07, Bacillus, and Humicola can convert nutrients from ineffective and slow-acting states to effective and fast-acting states, thus enhancing nutrient uptake by plant roots (Lou et al., 2018; Turner et al., 2013). Gemmatimonas help plants efficiently utilize soil nutrients by promoting nutrient uptake, which in turn promotes plant growth and development (Zhou et al., 2022); its enrichment in the FP rhizosphere also suggests the recruitment of beneficial microorganisms. In addition, the abundance of rhizosphere Piloderma was higher in WT and late post-fruiting nutrient growth of FP than in SC, and the chemicals released from this species may have adverse effects on plants (Li M. et al., 2023), there by influencing the lower levels of key compounds in WT and FP than in SC and reducing the utilization of nutrients by the plant. Enrichment of WT rhizosphere Amanita may affect ecological interactions with host plants through the dynamic evolution of toxin genes in the colony, with implications for plant growth and development (Drott et al., 2023). WT rhizospheres showed the aggregation of Mortierella (Val-Torregrosa et al., 2022), Bryobacter (Liu Z. et al., 2020) and other microbiota associated with plant stress tolerance. In conclusion, different cultivation methods result in differences in the abundance of characteristic rhizosphere microbiota of E. koreanum.
Soil properties and enzyme activity levels are important indicators of the natural environment and are highly correlated with microbial activity (Krause et al., 2017; Ma et al., 2021). Bacterial communities in this study were influenced by TK and AP. Bacillus, Humicola, and HSB OF53-F07 were positively correlated with TK and AP, and a correlation study on Dipterocarpus yunnanens is also showed that the role of AP and AK in shaping bacterial communities (Wang C. et al., 2023). Soil enzyme activities were also related to bacterial communities, and RDA results showed a positive correlation between Bryobacter and Candidatus Udaeobacter abundance and SSC activity. Candidatus Udaeobacter had the highest relative abundance among bacterial genera under different cultivation methods. It can utilize limited carbon sources and participate in the metabolism of amino acids and polysaccharides (Brewer et al., 2016), revealing the role of energy transformation in the association between soil enzyme activity and the microbial community. pH can indirectly and directly regulate bacterial communities. Studies have shown that pH has a significant impact on the aggregation of bacterial communities in tobacco (Jiang et al., 2023; Lammel et al., 2018), consistent with the results of this study. The soil fungal community was less affected by changes in soil pH and had a closer relationship with soil nutrients (Lauber et al., 2008; Rousk et al., 2010), similar to the results of this study. As evaluated using the Mantel test, TN, AN, SOM, and SSC were key factors affecting fungal communities, in line with Wu et al. (2020) and Yang et al. (2019). AN and TN was positively correlated with the abundance of Piloderma and Wilcoxina and negatively correlated with the abundance of Humicola, further demonstrating the impact of nitrogen on specific fungal groups. In addition, the significant associations of Pseudogymnoascus, Tmentella and other taxa with SSC activity corroborate the regulatory effect of SSC on fungal communities in a study of the rhizosphere microbiota of Angelica sinensis (Oliv.) Diels, indicating that enzyme activity may influence fungal functional networks through carbon source metabolic pathways (Xie et al., 2023). These findings suggest that soil properties and enzyme activity levels play important roles in shaping the plant microbiome, in turn influencing the diversity and composition of rhizosphere microorganisms under different cultivation practices.
Ecological conditions are closely related to the growth, development, and quality of forest and grassland medicinal materials (Zhang et al., 2020). There is a significant relationship between the accumulation of pharmacologically active components in E. koreanum and rhizosphere microbial community. Studies have shown that Icariin and epimedin content are significantly positively correlated with AK, AP, TK, TP and microorganisms, such as Bacillus, Humicola, Gemmatimonas and negatively correlated with SOM, AN, soil enzyme activities and most fungi. Additionally, higher phosphorus and potassium content and low levels of nitrogen in the soil are conducive to the accumulation of flavonoid components in medicinal plants (Kong et al., 2010; Li Q. et al., 2023; Liu et al., 2021). Bacillus is an important component of plant growth-promoting rhizobacteria; in most studies related to medicinal plants, it has been shown to promote the synthesis and accumulation of pharmacologically active compounds (Feng et al., 2021; Guo et al., 2025). Bacillus may influence the redox state of the host, helping to overcome critical periods of development and seedling establishment (Pitzschke, 2016), which could also explain why Bacillus was more abundant in the full bloom period. HSB OF53-F07 participates in the metabolism of nutrients in the soil, improves the soil environment, and promotes plant growth (Yang et al., 2021). Gemmatimonas not only promotes plant growth but also increases plant tolerance to abiotic stresses (Li P. et al., 2022). Humicola can degrade organic molecules in the soil, promote the dissolution of phosphates, increase the availability of soil phosphorus to plants, and enhance the accumulation of active ingredients in medicinal plants (Yang et al., 2014). The growth of most fungi is negatively correlated with the accumulation of active pharmaceutical ingredients in E. koreanum. For example, microorganisms such as Piloderma and Wilcoxina, enriched in the rhizosphere of WT plants, compete for nutrients and inhibit the activity of beneficial microorganisms, thereby affecting plant quality and leading to root rot (Liu Y. et al., 2024; Wang L. et al., 2024). The accumulation of rhizosphere pathogens induces an increase in the abundance of antagonistic rhizosphere pathogens (Bakker et al., 2018); for example, Mortierella regulates root rot in plants and induces P. ginseng resistance to black spot disease (Ji et al., 2024; Liu L. et al., 2020). Moderate dual-source stress can promote the accumulation of active pharmaceutical ingredients (Zhuang et al., 2023); however, in this study, the synergistic effect of the high nitrogen content in WT soil and pathogens inhibited the accumulation of active pharmaceutical ingredients. These results indicate that differences in soil physicochemical properties and enzyme activity among cultivation methods affect the community composition of rhizosphere microorganisms, which ultimately and indirectly contribute to plant growth and the synthesis of pharmacodynamic components.
Microbial function prediction provides insight into the metabolic functions of the flora (Bell et al., 2023). The functional prediction results of this study showed that the terms membrane transport and lipid metabolism were more abundant in SC and FP than in WT. Membrane transport related functions improve diffusion and transport efficiency, thus enhancing plant adaptation (Martínez et al., 2022). Lipids metabolism are an essential component of microbial biofilms and play a crucial role in the response to environmental stress and survival (Wu et al., 2018). This phenomenon explained the enrichment of nutrient-cycling microorganisms, such as Bacillus and Humicola in the rhizosphere of SC and FP in this study. Fungal functional prediction indicated that the abundance of saprotrophic fungi was significantly lower in the SC rhizosphere than in WT. Studies have shown that saprotrophic fungi are readily enriched in decaying and diseased plants and are more likely to cause plant diseases (Zhang et al., 2022), further suggesting that the aggregation of pathogenic bacteria, such as Piloderma, Wilcoxina, in the WT rhizosphere compared with SC and FP may pose an obstacle to root nutrient cycling and the accumulation of key components. The enrichment of pathotroph-saprotroph-symbiotroph fungi resulted in greater resistance and nutrient utilization in the rhizosphere of FP, conferring the potential for E. koreanum growth in harsh environments (Shi et al., 2016). The functional prediction results further confirmed the enrichment of the SC rhizosphere for microorganisms related to nutrient accumulation and enrichment of the FP rhizosphere with resistant microorganisms.
From a practical production point of view, although the simulated habitat cultivation samples had higher content of medicinal components, this approach is limited by the smaller number of plots to choose from, large economic investment required, and potential impact on the return of forests, which is not conducive to large-scale production. Therefore, against the backdrop of strengthening the conservation of wild E. koreanum resources, the bionic cultivated in forest is preliminarily recommended. During soil management, a “nitrogen control and phosphorus-potassium increase” strategy should be implemented, and the quality of E. koreanum can be improved by increasing the abundance of bacterial groups, such as Bacillus, Gemmatimonas and Humicola, in the soil.
Data availability statement
The data presented in the study are deposited in the NCBI repository (https://www.ncbi.nlm.nih.gov/), accession number PRJNA1202879 and PRJNA1202845.
Author contributions
YZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. HH: Data curation, Methodology, Software, Writing – original draft, Writing – review & editing, Visualization. YL: Conceptualization, Supervision, Writing – review & editing. KL: Conceptualization, Supervision, Writing – review & editing. HL: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Modern Agricultural Industrial Technology System Fund Project (Grant Number: CARS-21), Jilin Province Science and Technology Innovation Special Fund Project (2022YY04) and the National Key R&D Program of China (2024YFD1501505).
Acknowledgments
The authors thank the Omicsmart tools at http://www.omicsmart.com for providing the bioinformatic analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fmicb.2025.1634518.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1556173/full#supplementary-material
Footnotes
References
Asghari, B., Khademian, R., and Sedaghati, B. (2020). Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Sci. Hortic. 263:109132. doi: 10.1016/j.scienta.2019.109132
Bakker, P. A. H. M., Pieterse, C. M. J., de Jonge, R., and Berendsen, R. L. (2018). The soil-borne legacy. Cell 172, 1178–1180. doi: 10.1016/j.cell.2018.02.024
Bell, H. N., Huber, A. K., Singhal, R., Korimerla, N., Rebernick, R. J., Kumar, R., et al. (2023). Microenvironmental ammonia enhances T cell exhaustion in colorectal cancer. Cell Metab. 35, 134–149.e6. doi: 10.1016/j.cmet.2022.11.013
Berendsen, R. L., Pieterse, C. M. J., and Bakker, P. A. H. M. (2012). The rhizosphere microbiome and plant health. Trends Plant Sci. 17, 478–486. doi: 10.1016/j.tplants.2012.04.001
Brewer, T. E., Handley, K. M., Carini, P., Gilbert, J. A., and Fierer, N. (2016). Genome reduction in an abundant and ubiquitous soil bacterium ‘Candidatus Udaeobacter copiosus’. Nat. Microbiol. 2:16198. doi: 10.1038/nmicrobiol.2016.198
Challacombe, J. F., Hesse, C. N., Bramer, L. M., McCue, L. A., Lipton, M., Purvine, S., et al. (2019). Genomes and secretomes of ascomycota fungi reveal diverse functions in plant biomass decomposition and pathogenesis. BMC Genomics 20:976. doi: 10.1186/s12864-019-6358-x
Chinese Pharmacopoeia Commission (2020). Pharmacopoeia of People’s Republic of China. Beijing: Chinese Medical Science and Technology Press, 340–342.
Dai, A., Zhang, Y., Ai, Q., Teng, X., and Yang, L. (2022). Effects of different light qualities on growth, physiological characteristics and flavonoids accumulation of Epimedium koreanum. J. Agric. Sci. Technol. 24, 85–92. doi: 10.13304/j.nykjdb.2021.0676
Dong, L., Li, Y., Xu, J., Yang, J., Wei, G., Shen, L., et al. (2019). Biofertilizers regulate the soil microbial community and enhance Panax ginseng yields. Chin. Med. 14:20. doi: 10.1186/s13020-019-0241-1
Dong, J., Zhao, W., Shi, P., Zhou, M., Liu, Z., and Wang, Y. (2023). Soil differentiation and soil comprehensive evaluation of in wild and cultivated Fritillaria pallidiflora schrenk. Sci. Total Environ. 872:162049. doi: 10.1016/j.scitotenv.2023.162049
Drott, M. T., Park, S. C., Wang, Y.-W., Harrow, L., Keller, N. P., and Pringle, A. (2023). Pangenomics of the death cap mushroom amanita phalloides, and of agaricales, reveals dynamic evolution of toxin genes in an invasive range. ISME J. 17, 1236–1246. doi: 10.1038/s41396-023-01432-x
Feng, W. M., Liu, P., Yan, H., Zhang, S., Shang, E. X., Yu, G., et al. (2021). Impact of bacillus on phthalides accumulation in Angelica sinensis (Oliv.) by stoichiometry and microbial diversity analysis. Front. Microbiol. 11:611143. doi: 10.3389/fmicb.2020.611143
Fitzpatrick, C. R., Salas-González, I., Conway, J. M., Finkel, O. M., Gilbert, S., Russ, D., et al. (2020). The plant microbiome: from ecology to reductionism and beyond. Ann. Rev. Microbiol. 74, 81–100. doi: 10.1146/annurev-micro-022620-014327
Guo, H., Wei, T., Cui, W., Shi, H., Mao, F., Gu, X., et al. (2025). Effect of different phosphorus application on morphological traits, active ingredients and rhizosphere soil microbial community of Polygala tenuifolia. China J. Chin. Mater. Med., 1–15. doi: 10.19540/j.cnki.cjcmm.20250308.101
Ji, X. Y., Ye, C., Kang, W., Luan, W., Liu, Y., He, X., et al. (2024). Interspecific allelopathic interaction primes direct and indirect resistance in neighboring plants within agroforestry systems. Plant Commun. 6:101173. doi: 10.1016/j.xplc.2024.101173
Jiang, Q., Yu, J., Wang, J., Liu, D., Gong, J., Jiang, L., et al. (2023). Soil properties affect bacterial community assembly and co-occurrence network in tobacco rhizosphere. Acta Microbiol Sin. 63, 1168–1184. doi: 10.13343/j.cnki.wsxb.20220540
Kong, L., Li, Y., Quan, Q., and Zhang, L. (2010). Total flavonoids and icariin contents of Epimedium pubescens in different types of communities and their relationships with soil factors. Chin. J. Appl. Ecol. 21, 2517–2522. doi: 10.13287/j.1001-9332.2010.0367
Krause, S. M. B., Johnson, T., Samadhi Karunaratne, Y., Fu, Y., Beck, D. A. C., Chistoserdova, L., et al. (2017). Lanthanide-dependent cross-feeding of methane-derived carbon is linked by microbial community interactions. Proc. Natl. Acad. Sci. U.S.A. 114, 358–363. doi: 10.1073/pnas.1619871114
Lammel, D. R., Barth, G., Ovaskainen, O., Cruz, L. M., Zanatta, J. A., Ryo, M., et al. (2018). Direct and indirect effects of a pH gradient bring insights into the mechanisms driving prokaryotic community structures. Microbiome 6:106. doi: 10.1186/s40168-018-0482-8
Lauber, C. L., Strickland, M. S., Bradford, M. A., and Fierer, N. (2008). The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 40, 2407–2415. doi: 10.1016/j.soilbio.2008.05.021
Li, Q., Han, F., Cao, R., Tan, Q., and Ren, M. (2023). Community and diversity of rhizosphere microorganisms and its correlation with active ingredients of Epimedium sagittatum in different growth stages. Chin. Tradit. Herb. Drug 54, 641–651. doi: 10.7501/j.issn.0253-2670.2023.02.032
Li, Z., Li, S., and Liu, Z. (2024). Study on the reproductive characteristics of Epimedium koreanum. J. Chin. Med. Mater. 47, 1106–1109. doi: 10.13863/j.issn1001-4454.2024.05.006
Li, P., Liu, J., Saleem, M., Li, G., Luan, L., Wu, M., et al. (2022). Reduced chemodiversity suppresses rhizosphere microbiome functioning in the mono-cropped agroecosystems. Microbiome 10:108. doi: 10.1186/s40168-022-01287-y
Li, M., Miao, N., and Liu, S. (2023). Habitats shape root-associated fungal and bacterial communities of Minjiang fir saplings. J. For. Res. 34, 1491–1502. doi: 10.1007/s11676-023-01609-2
Li, Y., Qu, Z., Xu, W., Chen, W., Hu, Y., and Wang, Z. (2022). Maize (Zea mays L.) genotypes induce the changes of rhizosphere microbial communities. Arch. Microbiol. 204:321. doi: 10.1007/s00203-022-02934-6
Li, J., Yang, W., Li, Y., Han, L., Li, G., and Zhang, A. (2024). Integration of transcriptome, metabolome and high-throughput amplicon sequencing to compare the performance of wild and cultivated psammosilene tunicoides to reveal the beneficial plant-microbe interactions for domestication. Environ. Exp. Bot. 218:105587. doi: 10.1016/j.envexpbot.2023.105587
Liu, Y., Du, Y., Li, Y., Li, C., Zhong, S., Xu, Z., et al. (2024). Does Bidens pilosa L. affect carbon and nitrogen contents, enzymatic activities, and bacterial communities in soil treated with different forms of nitrogen deposition? Microorganisms 12:1624. doi: 10.3390/microorganisms12081624
Liu, L., Huang, X., Zhang, J., Cai, Z., Jiang, K., and Chang, Y. (2020). Deciphering the relative importance of soil and plant traits on the development of rhizosphere microbial communities. Soil Biol. Biochem. 148:107909. doi: 10.1016/j.soilbio.2020.107909
Liu, Z., Liu, J., Yu, Z., Yao, Q., Li, Y., Liang, A., et al. (2020). Long-term continuous cropping of soybean is comparable to crop rotation in mediating microbial abundance, diversity and community composition. Soil Tillage Res. 197:104503. doi: 10.1016/j.still.2019.104503
Liu, J., Qian, Y., Yang, W., Yang, M., Zhang, Y., Duan, B., et al. (2024). Elucidating the interaction of rhizosphere microorganisms and environmental factors influencing the quality of Polygonatum kingianum Coll. et Hemsl. Sci. Rep. 14:19092. doi: 10.1038/s41598-024-69673-0
Liu, Z., Zhang, S., Dai, A., Han, M., Yang, L., and Zhang, Y. (2021). Effects of soil factors on effective ingredients in Epimedium koreanum Nakai. J. Northeast For. Univ. 49, 40–44. doi: 10.13759/j.cnki.dlxb.2021.05.007
Lou, Y., Guo, Q., Peng, C., Shi, M.-D., Li, H.-Y., Li, X., et al. (2018). Effects of three bacillus strains on growth promoting and rhizosphere soil microflora of tomato. Chin. J. Appl. Ecol. 29, 260–268. doi: 10.13287/j.1001-9332.201801.040
Ma, S., Chen, G., Tang, W., Xing, A., Chen, X., Xiao, W., et al. (2021). Inconsistent responses of soil microbial community structure and enzyme activity to nitrogen and phosphorus additions in two tropical forests. Plant Soil 460, 453–468. doi: 10.1007/s11104-020-04805-9
Martínez, P. D. F., Morgante, V., and González-Pastor, J. E. (2022). Isolation of novel cold-tolerance genes from rhizosphere microorganisms of Antarctic plants by functional metagenomics. Front. Microbiol. 13:1026463. doi: 10.3389/fmicb.2022.1026463
Narsing Rao, M. P., Luo, Z. H., Dong, Z. Y., Li, Q., Liu, B. B., Guo, S. X., et al. (2022). Metagenomic analysis further extends the role of chloroflexi in fundamental biogeochemical cycles. Environ. Res. 209:112888. doi: 10.1016/j.envres.2022.112888
Pitzschke, A. (2016). Developmental peculiarities and seed-borne endophytes in quinoa: omnipresent, robust bacilli contribute to plant fitness. Front. Microbiol. 7:2. doi: 10.3389/fmicb.2016.00002
Rousk, J., Bååth, E., Brookes, P. C., Lauber, C. L., Lozupone, C., Caporaso, J. G., et al. (2010). Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 4, 1340–1351. doi: 10.1038/ismej.2010.58
Shi, S., Nuccio, E. E., Shi, Z. J., He, Z., Zhou, J., and Firestone, M. K. (2016). The interconnected rhizosphere: high network complexity dominates rhizosphere assemblages. Ecol. Lett. 19, 926–936. doi: 10.1111/ele.12630
Song, P., Liu, J., Huang, P., Han, Z., Wang, D., and Sun, N. (2023). Diversity and structural analysis of rhizosphere soil microbial communities in wild and cultivated Rhizoma Atractylodis Macrocephalae and their effects on the accumulation of active components. PeerJ 11:e14841. doi: 10.7717/peerj.14841
Su, J., Wang, Y., Bai, M., Peng, T., Li, H., Xu, H., et al. (2023). Soil conditions and the plant microbiome boost the accumulation of monoterpenes in the fruit of citrus reticulata ‘chachi’. Microbiome 11:61. doi: 10.1186/s40168-023-01504-2
Sun, Y., Pang, B., Wang, Y., Xiao, J., and Jiang, D. (2021). Baohuoside I inhibits the proliferation of hepatocellular carcinoma cells via apoptosis signaling and NF-kB pathway. Chem. Biodivers. 18:e2100063. doi: 10.1002/cbdv.202100063
Tang, C., Huang, W., Pang, Y. T., and Lam, K. P. (2007). The influence of growing season changes on flavonoid components in Epimedium koreanum. China J. Chin. Mater. Med. 32:2438–2440. doi: 10.3321/j.issn:1001-5302.2007.22.033
Trivedi, P., Leach, J. E., Tringe, S. G., Sa, T., and Singh, B. K. (2020). Plant–microbiome interactions: from community assembly to plant health. Nat. Rev. Microbiol. 18, 607–621. doi: 10.1038/s41579-020-0412-1
Turner, T., Ramakrishnan, K., Walshaw, J., Heavens, D., Alston, M., Swarbreck, D., et al. (2013). Comparative metatranscriptomics reveals kingdom level changes in the rhizosphere microbiome of plants. ISME J. 7, 2248–2258. doi: 10.1038/ismej.2013.119
Val-Torregrosa, B., Bundó, M., Martín-Cardoso, H., Bach-Pages, M., Chiou, T.-J., Flors, V., et al. (2022). Phosphate-induced resistance to pathogen infection in Arabidopsis. Plant J. 110, 452–469. doi: 10.1111/tpj.15680
Wang, R., Bai, Y., Shi, H., Hou, W., and Li, Y. (2023). Effects of icariin on scopolamine-induced cognitive impairment and neuronal excitability in mice. Chin. Tradit. Pat. Med. 45, 1114–1119. doi: 10.3969/j.issn.1001-1528.2023.04.013
Wang, Z., Dai, Q., Su, D., Zhang, Z., Tian, Y., Tong, J., et al. (2024). Comparative analysis of the microbiomes of strawberry wild species Fragaria nilgerrensis and cultivated variety Akihime using amplicon-based next-generation sequencing. Front. Microbiol. 15:1377782. doi: 10.3389/fmicb.2024.1377782
Wang, L., Liu, Y., Ni, H., Zuo, W., Shi, H., Liao, W., et al. (2024). Systematic characterization of plant-associated bacteria that can degrade indole-3-acetic acid. PLoS Biol. 22:e3002921. doi: 10.1371/journal.pbio.3002921
Wang, C., Peng, C., Yang, Q., Yang, Y., Xin, W., and Tao, Y. (2023). Effects of hot pepper stalks on rhizosphere microflora structure of Polygonatum kingianum. Microbiol. China 50, 486–502. doi: 10.13344/j.microbiol.china.220436
Wu, L., Wang, J., Wu, H., Chen, J., Xiao, Z., Qin, X., et al. (2018). Comparative metagenomic analysis of rhizosphere microbial community composition and functional potentials under Rehmannia glutinosa consecutive monoculture. Int. J. Mol. Sci. 19:2394. doi: 10.3390/ijms19082394
Wu, J., Yang, R., Wang, Y., Qu, S., Liu, Z., and Hou, C. (2020). Community and diversity of rhizosphere soil fungi in three different vegetation types in caohai basin, Guizhou province. Mycosystema 39, 1250–1262. doi: 10.13346/j.mycosystema.190458
Xie, T., Zhang, J., Liu, N., Liu, Y., Kou, L., Qu, X., et al. (2023). Changes in rhizospheresoil enzymatic activities and microbial communities across growth stages of Angelica sinensis. hinese. J. Soil Sci. 54, 138–150. doi: 10.19336/j.cnki.trtb.2021120902
Yan, L., Liu, Y., Wang, Z., Li, J., Wu, Y., Bai, Y., et al. (2021). Study on the effects of cultivation conditions on physiological characteristics and quality of medicinal herbs of Epimedium koreanum Nakai. Lishizhen Med. Mater. Med. 32, 2258–2262. doi: 10.3969/j.issn.1008-0805.2021.09.59
Yang, C. H., Lin, M. J., Su, H. J., and Ko, W. H. (2014). Multiple resistance-activating substances produced by Humicola phialophoroides isolated from soil for control of phytophthora blight of pepper. Bot. Stud. 55:40. doi: 10.1186/1999-3110-55-40
Yang, L., Sui, X., Wei, D., Cui, F., Zhu, D., and Ni, H. (2019). Fungal diversity in the brown coniferous forest soils of Daxing'anling Mountains, Northeast China. Chin. J. Appl. Ecol. 30, 3411–3418. doi: 10.13287/j.1001-9332.201910.030
Yang, Y., Tian, K., and Bai, G. (2016). Empirical study on factors affecting the quality of Chinese herbal medicine. J. Chin. Med. Mater. 39, 1251–1256. doi: 10.13863/j.issn1001-4454.2016.06.012
Yang, S., Xiao, J., Liang, T., He, W., and Tan, H. (2021). Response of soil biological properties and bacterial diversity to different levels of nitrogen application in sugarcane fields. AMB Express 11:172. doi: 10.1186/s13568-021-01331-4
Yang, K., Zheng, Y., Sun, K., Sun, X., Xiao, P., and He, C. (2024). Regulation of rhizosphere microorganisms on the quality of Scutellaria baicalensis: from wild and cultivated perspectives. Ind. Crop. Prod. 222:119917. doi: 10.1016/j.indcrop.2024.119917
Ye, Y., Xiang, G., Guo, X., Min, W., and Guo, H. (2024). Regulation effect of biochar on bacterial community in cotton field soil under saline water drip irrigation. Chin. Agric. Sci. Bull. 40, 91–100. doi: 10.11924/j.issn.1000-6850.casb2023-0236
Zeng, M., Zhong, Y., Cai, S., and Diao, Y. (2018). Deciphering the bacterial composition in the rhizosphere of Baphicacanthus cusia (NeeS) Bremek. Sci. Rep. 8:15831. doi: 10.1038/s41598-018-34177-1
Zhang, W., Wang, T., Guo, Q., Zou, Q., Yang, F., Lu, D., et al. (2020). Effect of soil moisture regimes in the early flowering stage on inflorescence morphology and medicinal ingredients of Chrysanthemum morifolium Ramat. Cv. ‘Hangju’. Sci. Hortic. 260:108849. doi: 10.1016/j.scienta.2019.108849
Zhang, Y., Wang, D., Wu, F., Huang, X., Chai, X., and Yang, L. (2024). Transcriptome analysis on the quality of Epimedium koreanumin different soil moisture conditions at harvesting stage. Genes 15:528. doi: 10.3390/genes15050528
Zhang, J., Yao, Z., Chen, Y., Zhang, J., Luo, S., Tian, C., et al. (2022). Study of rhizosphere microbial community structures of Asian wild and cultivated rice showed that cultivated rice had decreased and enriched some functional microorganisms in the process of domestication. Diversity 14:67. doi: 10.3390/d14020067
Zhang, S., Zhang, L., Zhu, J., Chen, H., Chen, Z., Si, T., et al. (2021). Genomic and metabolomic investigation of a rhizosphere isolate Streptomyces netropsis WLXQSS-4 associated with a traditional Chinese medicine. Molecules 26:2147. doi: 10.3390/molecules26082147
Zhou, W., Duan, Y., Zhang, Y., Wang, H., Huang, D., and Zhang, M. (2021). Effects of foliar selenium application on growth and rhizospheric soil micro-ecological environment of Atractylodes macrocephala Koidz. S. Afr. J. Bot. 137, 98–109. doi: 10.1016/j.sajb.2020.09.032
Zhou, D. Y., Hou, C., Leng, C. Y., Li, R. J., Xing, Y. M., and Chen, J. (2025). Deciphering the root microbiome and its relationship with active compound accumulation in medicinal Dendrobium officinale (Orchidaceae) from different regions. Ind. Crops Prod. 226:120692. doi: 10.1016/J.INDCROP.2025.120692
Zhou, X., Wang, J., Liu, F., Liang, J., Zhao, P., Tsui, C. K. M., et al. (2022). Cross-kingdom synthetic microbiota supports tomato suppression of Fusarium wilt disease. Nat. Commun. 13:7890. doi: 10.1038/s41467-022-35452-6
Keywords: Epimedium koreanum Nakai, rhizosphere microbial, pharmacodynamic components, soil properties, soil enzyme activity
Citation: Zhang Y, Hou H, Li Y, Li K and Lin H (2025) Differential analysis of the quality and soil microhabitat of Epimedium koreanum Nakai under different cultivation methods. Front. Microbiol. 16:1556173. doi: 10.3389/fmicb.2025.1556173
Edited by:
Jesús Navas-Castillo, CSIC, SpainCopyright © 2025 Zhang, Hou, Li, Li and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongmei Lin, aG9uZ21laWxAamxhdS5lZHUuY24=
†These authors share first authorship
 Yonggang Zhang1,2†
Yonggang Zhang1,2† Huiling Hou
Huiling Hou Kexin Li
Kexin Li