- 1State Key Laboratory of Agricultural and Forestry Biosecurity, College of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou, China
- 2Department of Biological Sciences, University of Illinois, Chicago, IL, United States
- 3XiangYa School of Public Health, Central South University, Changsha, China
- 4Institute of Microbiology, Chinese Academy of Sciences, Beijing, China
- 5Bureau of Fujian Junzifeng National Nature Reserve, Sanming, China
- 6Bureau of Fujian Longqishan National Nature Reserve, Sanming, China
- 7Putian Institute of Agricultural Sciences, Putian, China
- 8College of Pharmacy and Life Sciences, Jiujiang University, Jiujiang, China
- 9College of Bee Science and Biomedicine, Fujian Agriculture and Forestry University, Fuzhou, China
Introduction: Cortinarius are globally distributed mushrooms, all of whom form mycorrhizae, and are characterized by rust-brown to brownish-red spores. The high species richness of the genus results in significant morphological diversity overall, alongside high similarity among closely related species, leading many taxonomists to concentrate their studies on specific infrageneric groups.
Methods: Specimens were noted and collected from Fujian Province, China. Genomic DNA was extracted from specimens and the nucleotide sequences of two loci (ITS AND nr LSU) were determined and used to construct phylogenetic trees. Microscopic features were observed using an optical microscope. Fluorescence reactions were examined using a fluorescence microscope.
Results: We describe two new species of Cortinarius, C. griseoaurantinus (subgenus Leprocybe) and C. yonganensis (subgenus Dermocybe), from Fujian Province, Southern China. Comprehensive morphological descriptions, color photographs of fresh basidiomata, photographs of microscopic characters, and phylogenetic trees are provided.
Discussion: These data identify new species of Cortinarius, enhancing the understanding of the genus and the ecological relationships. The developed keys provide a reference for the further analysis of the evolution and geographic distribution of these fungi.
1 Introduction
The genus Cortinarius (Pers.) Gray (commonly known as cortinar or webcap) belongs to the family Cortinariaceae and the order Agaricales, within Basidiomycota. It is one of the most diverse genera of mushroom-forming fungi, comprising thousands of species found worldwide (Moser et al., 1975; Garnica et al., 2016; Liimatainen et al., 2017; Varga et al., 2019; Liimatainen et al., 2020; Dima et al., 2021). According to previous reports, extracts from certain Cortinarius species are harmful to human health. For example, a type of sterol from Cortinarius xiphidipus has shown cytotoxic activity (Torres et al., 2017); orellanine from Cortinarius orellanus can cause acute nephritis (Nusair et al., 2023); emodin from Cortinarius sanguineus has shown cytotoxic and skin-sensitizing effects (Yli-Öyrä et al., 2024); Cortinarius species have also been reported to cause myocarditis (Balice et al., 2024). Nevertheless, some Cortinarius species are edible, such as Cortinarius purpurascens (Bai et al., 2013) and Cortinarius caerulescens (Song et al., 2022). However, the high morphological similarity between certain species within the genus makes it challenging to distinguish them based solely on appearance. Moreover, the edibility status of the majority of species remains largely undocumented and requires further investigation. Therefore, currently, there is no reliable method to determine the toxicity of Cortinarius species based solely on its morphological characteristics. All members of the genus are ectomycorrhizal fungi, forming symbiotic associations with a wide variety of vascular plants across the majority of ecosystems, from the tropics to the Arctic (Moser et al., 1975). A defining characteristic of Cortinarius species is their rusty-brown spore print, which is a key diagnostic feature for identification. Some species (e.g., C. sanguineus) are notably colorful and have been used as natural sources of pigment dyes (Frøslev et al., 2007; Niskanen et al., 2009; Liimatainen et al., 2014a, b; Zhang et al., 2023; Long et al., 2024). Due to the genus’s extensive species richness, there is considerable morphological diversity overall, along with high similarity between closely related species. Consequently, many taxonomists have focused on specific infrageneric groups in their studies (Brandrud, 1998; Niskanen et al., 2016; Pastor et al., 2019; Soop et al., 2019; Liimatainen et al., 2022; Zhang et al., 2023; Fan et al., 2024; Long et al., 2024).
Historically, Cortinarius was the only genus recognized in the family Cortinariaceae. However, recent phylogenomic studies have divided it into 10 separate genera: Aureonarius Niskanen & Liimat., Austrocortinarius Niskanen & Liimat., Calonarius Niskanen & Liimat., Cortinarius (Pers.) Gray., Cystinarius Niskanen & Liimat., Hygronarius Niskanen & Liimat., Mystinarius Niskanen & Liimat., Phlegmacium Niskanen & Liimat., Thaxterogaster Singer., and Volvanarius Niskanen & Liimat., seven of which—Aureonarius, Austrocortinarius, Calonarius, Cystinarius, Hygronarius, Mystinarius, and Volvanarius—were newly described (Liimatainen et al., 2022). This division has been contested by Gallone et al. (2024), who reanalyzed the phylogenomic dataset and identified substantial conflicts between gene trees and species trees. This latter work showed that the phylogenetic relationships within the genus Cortinarius remain unresolved, with phylogenomic hypotheses displaying short and weakly supported backbone branches. Of the 10 proposed genera, only four—Cortinarius, Phlegmacium, Aureonarius, and Thaxterogaster—were confirmed to be monophyletic, leaving the overall relationships and branching order among groups uncertain (Gallone et al., 2024). To further complicate the issue, within Cortinarius, proposed subgenera include Cortinarius, Camphorati, Dermocybe, Illumini, Infracti, Iodolentes, Leprocybe, Myxacium, Orellani, Paramyxacium, and Telamonia (Liimatainen et al., 2022; Fan et al., 2024).
The Cortinarius subgenus Leprocybe was first described by Moser (1969) and was further revised by Niskanen et al. (2009) and Liimatainen et al. (2022). According to the latter, species of the Cortinarius subgenus Leprocybe share a common ancestor; however, some reported nodal support values are low (below 70%). This may be due to different phylogenetic analysis methods (e.g., concatenated analyses and multispecies coalescent model analyses) yielding varying conclusions, which increases uncertainty in classification. Currently, it is not possible to determine the monophyly of the Cortinarius subgenus Leprocybe. Morphologically, members of the Leprocybe subgenus include species with small to medium-sized (occasionally large) basidiocarps, characterized by a typical leprocyboid/dermocyboid or sequestrate appearance, with a dry pileus and stipe, and displaying a yellow, red, or greenish-olive hue. Certain parts of the basidiocarps also possess fluorescent properties (Moser et al., 1975; Liimatainen et al., 2014b; Vellinga et al., 2015; Hong et al., 2024).
The subgenus Dermocybe (common name: skin-heads) within Cortinarius is globally distributed and includes members known for their vibrant and diverse colors, such as the vivid green of C. austrovenetus and the blood-red sheen of the hymenophore of C. semisanguineus. These fungi are also characterized by their non-hygrophanous, dry caps, dry cylindrical non-bulbous stems, and ecological adaptability. The lethal web caps, regarded as some of the world’s most poisonous mushrooms, belong to this group. Members of Dermocybe can be distinguished from other subgenera by their small to medium-sized basidiomata, bright colors, and typically slender stipes (Matheny et al., 2014; Liimatainen et al., 2022). Many species within this group contain unique pigments that have garnered significant attention in ecological studies and biochemical analyses (Matheny et al., 2014; Liimatainen et al., 2022). Anthraquinone is present in the basidiomata of the subgenus Dermocybe and exhibits diverse biological activities. These activities are utilized in crop protection and serve as sustainable, bio-based colorants for textiles, paints, electronics, and cosmetics industries (Fiala et al., 2025; Jetha et al., 2025).
During fieldwork in Fujian Province, China, two potentially new species of Cortinarius were discovered. A detailed morphological examination, along with molecular sequencing and phylogenetic analyses of the nuclear ribosomal internal transcribed spacer (ITS) and nuclear ribosomal large subunit (nrLSU) sequences, confirmed that the specimens represent two new species. They were named Cortinarius griseoaurantinus (subgenus Leprocybe) and Cortinarius yonganensis (subgenus Dermocybe). The macro- and micro-morphological features of the two new species, accompanied by photographs, are provided in this study. These findings enhance our understanding of the diversity within the Cortinarius genus.
2 Materials and methods
2.1 Collection and morphological analyses
Specimens of Cortinarius griseoaurantinus were collected from Mingxi and Mount Wuyi, Fujian Province, in 2023. Specimens of Cortinarius yonganensis were collected from Yong’an City, Fujian Province, in 2023. Images of fresh fruiting bodies were captured using a Canon EOS 6D Mark II camera. The samples were dried at the Fujian Agriculture and Forestry University Fungal Herbarium (HMFAFU) and then deposited in the Fungarium (HMAS) of the Institute of Microbiology, Chinese Academy of Sciences (CAS). Macromorphological descriptions were based on field notes and color photos of basidiocarps. Descriptions of the relevant color codes followed (Kornerup and Wanscher, 1981).
After rehydrating dried tissues in 5% KOH and staining them with a 1% Congo red solution, microscopic features such as basidia and spores were observed using an optical microscope (Leica DM2500). Following the rehydration of dried tissues in 5% KOH, fluorescence reactions were examined using a fluorescence microscope (Carl Zeiss Axio Imager A2). At least 40 basidiospores were analyzed. The dimensions of basidiospores are presented in the notation (a) b-c-d (e). The range b-d includes a minimum of 90% of the measured values. Mean value, i.e., c is provided. Extreme values, i.e., a or e, are provided in parentheses. Q denotes the “length/width ratio” of a basidiospore in a side view; Q indicates the average Q of all basidiospores ± the sample standard deviation.
2.2 DNA extraction, PCR amplification, and sequencing
Genomic DNA was extracted from dry specimens (10–50 mg) using the D3390 Fungal DNA Mini KIT (OMEGA, United States), following the manufacturer’s instructions. To amplify specific genetic regions, the primer pair ITS5/ITS4 (White et al., 1990) was utilized for the internal transcribed spacer (ITS) region of the nuclear ribosomal repeat unit, and the primer pair LROR/LR5 was used for the nuclear ribosomal large subunit (nrLSU) region of the nuclear ribosomal repeat unit. PCR reactions (25 μL total volume) were prepared with 1 μL of DNA solution, 12.5 μL of 2 × PCR mix enzyme, 9.5 μL of sterile deionized water, and 1 μL of each primer.
PCR amplifications were conducted as described. The conditions were set as follows: for ITS amplification, an initial denaturation at 95°C for 3 min, followed by 30 cycles at 95°C for 30 s, 55°C for 45 s, and 72°C for 1 min, and a final extension at 72°C for 10 min (Hong et al., 2024); for LSU amplification, an initial denaturation at 94°C for 1 min, followed by 35 cycles at 94°C for 30 s, 55°C for 1 min, and 72°C for 1.5 min, and a final extension at 72°C for 10 min (Fan et al., 2024). PCR products were subjected to electrophoresis on 1% agarose gel stained with a nucleic acid dye. Subsequently, the samples were sent for sequencing at Fuzhou Biosune Company using the same primers. All newly generated sequences for ITS and nrLSU in this study have been submitted to GenBank.
2.3 Alignment and phylogenetic analyses
Initial BLAST searches1 using the derived ITS and nrLSU sequences confirmed that the isolate is grouped within Cortinarius. Sequences for the final dataset were selected from previously published literature (Fan et al., 2024; Hong et al., 2024) and GenBank (Table 1). The quality of the sequences was considered during the selection process for phylogenetic analyses. Hebeloma cylindrosporum was selected as the outgroup. Sequence alignment and adjustment of ITS and nrLSU sequences were performed separately using MEGA7 software (Kumar et al., 2016). The PhyloSuite software (Zhang et al., 2020) was then employed to concatenate the sequences and conduct Maximum Likelihood (ML) and Bayesian Inference (BI) analyses. Detailed specimen information and GenBank accession numbers used in this study are provided in Table 1. Sequence selection was based on previously described criteria (Fan et al., 2024; Hong et al., 2024).
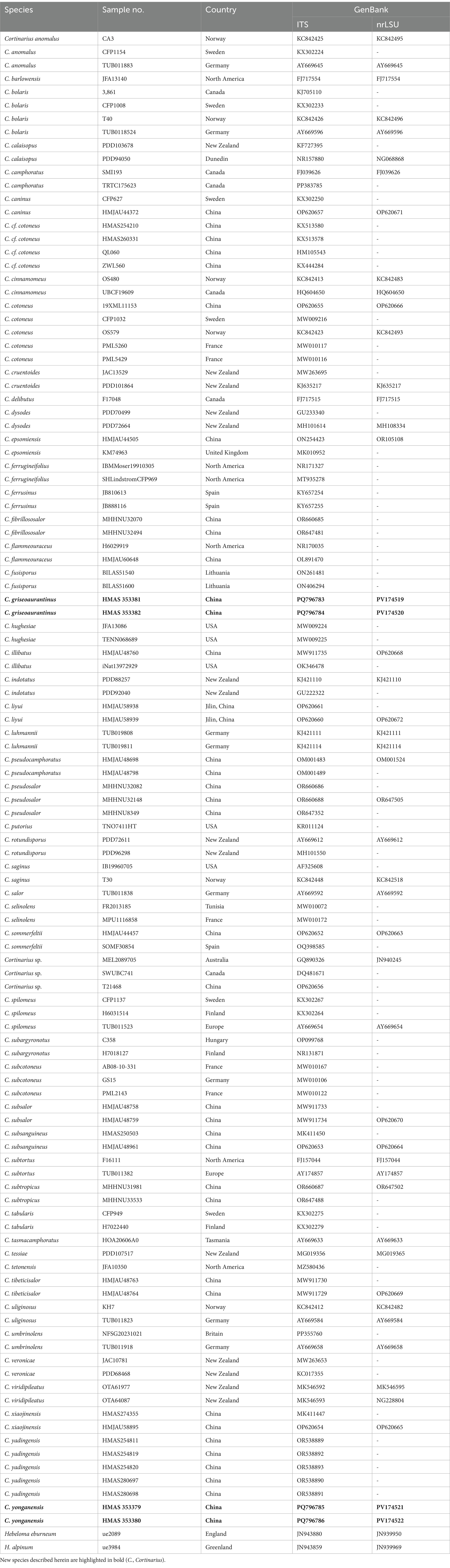
Table 1. Species and specimens of Cortinarius and Hebeloma used for the molecular phylogenetic analyses.
3 Results
3.1 Phylogenetic analyses
Four sequences were generated from the specimens, with two corresponding to the ITS locus and two to the nrLSU locus, and these were deposited in GenBank (see accession numbers in Table 1). To construct a robust phylogenetic tree, homologs were identified through initial BLAST searches, leading to a final DNA sequence alignment comprising a dataset of 114 specimens. After removing ambiguously aligned regions and gaps, the final combined sequence data matrix comprised 3,967 characters. Sequences from two specimens of Hebeloma cylindrosporum (IK-H0467 and HKAS134461) served as the outgroup. The tree topology derived from Maximum Likelihood (ML) analysis was similar to that obtained from Bayesian Inference (BI) analysis (refer to Figure 1). The best scoring maximum-likelihood (ML; ILn = −16999.896), based on RAxML analysis of the combined ITS-nrLSU dataset, is presented.
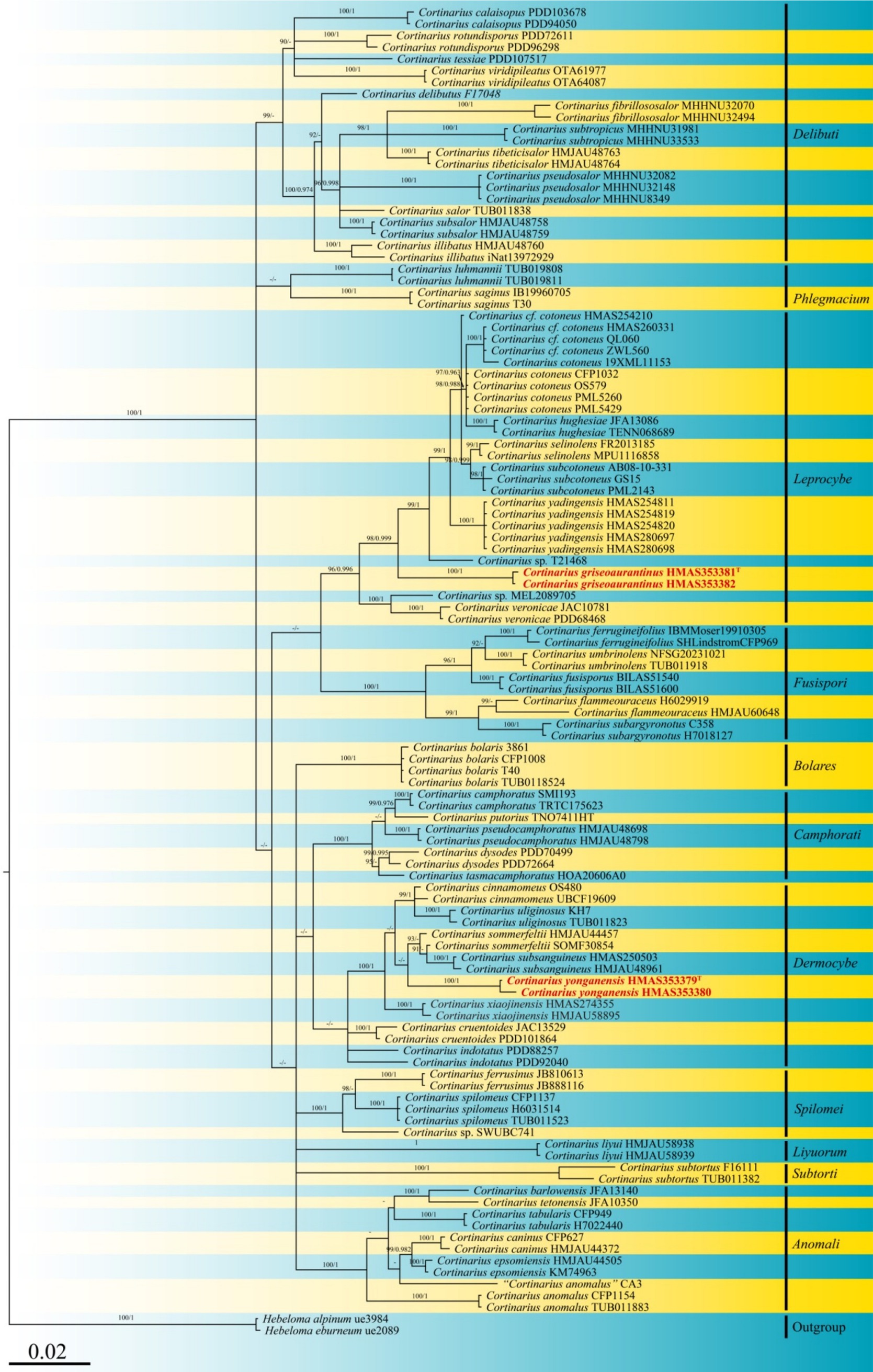
Figure 1. Phylogeny inferred from ITS and nrLSU sequences using Bayesian analysis. Bootstrap values (ML/BI) above 90/0.95 are indicated above the individual branches. The scale bar represents the number of nucleotide substitutions per site. New species are highlighted in red and bold. Arrows indicate the support values at the branching points. The superscript “T” denotes the type strain of the new species.
Phylogenetically, Cortinarius griseoaurantinus and Cortinarius yonganensis were separated into individual lineages with strong statistical support and were distinct from their closest taxa. C. griseoaurantinus nested within a clade of the subgenus Leprocybe, clustering alongside C. veronicae and C. yadingensis. C. yonganensis nested within a clade of the subgenus Dermocybe, clustering alongside C. subsanguineus and C. sommerfeltii.
3.2 Taxonomy
Cortinarius griseoaurantinus M. J. Zhu & Jun Z. Qiu, sp. nov. (Figures 1, 2 and 3)
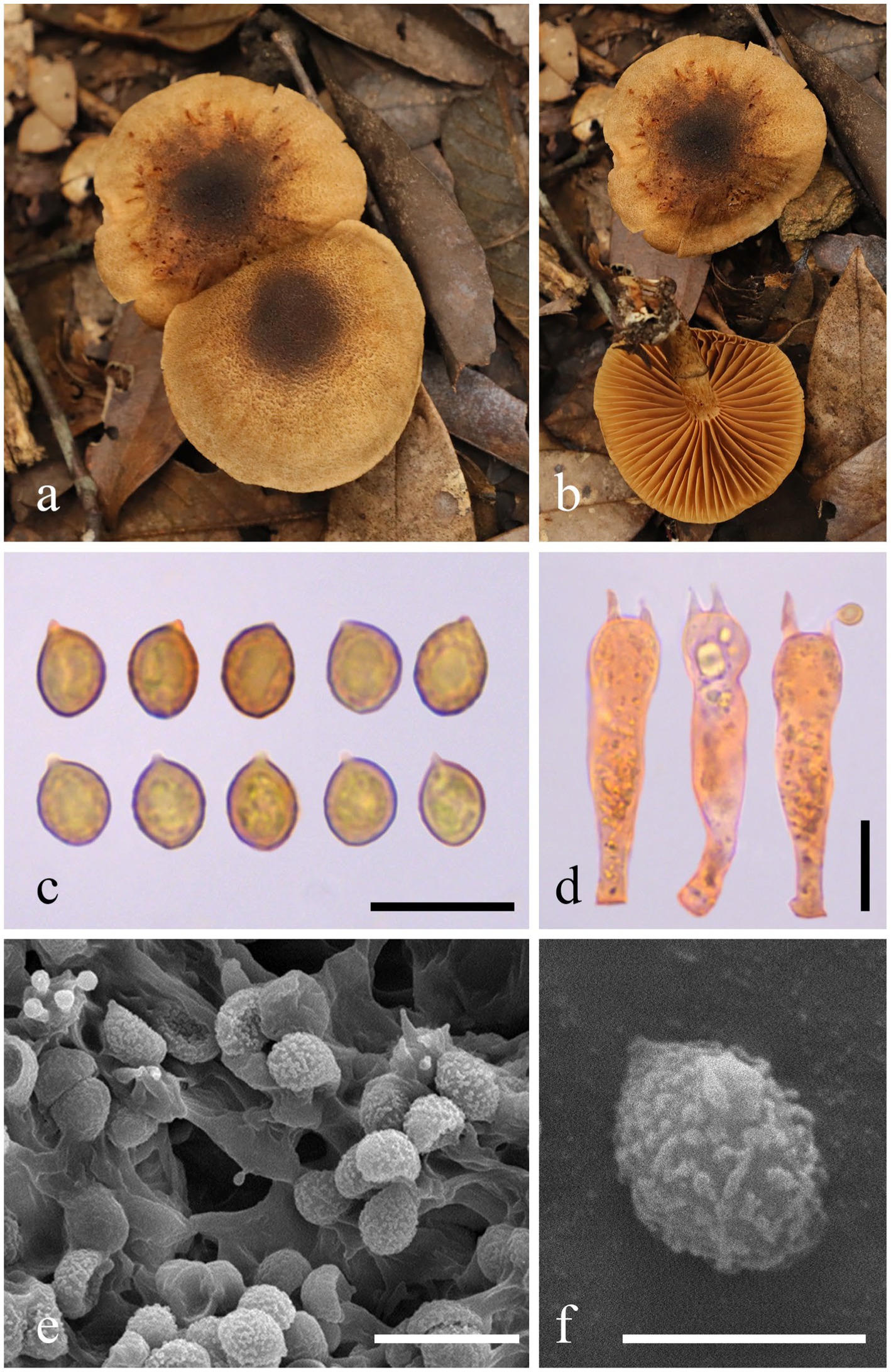
Figure 2. Morphological characteristics of Cortinarius griseoaurantinus (HMAS 353381). (a,b) Basidiomata; (c) Basidiospores in Congo Red reagent; (d) Basidium in Congo Red reagent; (e,f) SEM images of basidiospores. Bars: (c,d,e) = 10 μm; (f) = 5 μm.
Mycobank No.: 857170
Etymology: “griseo-” comes from “griseus,” meaning gray; “aurantinus” means orange, which is the color of the fungal pileus.
Diagnosis: The diagnosis indicates that this species, related to C. yadingensis, mainly differs in having a bright orange to brownish-orange pileus.
Holotype: CHINA, Fujian Province, Mingxi, Xiafang Township, Gaoyang Village, in a mixed forest at 26°33′43″N, 116°53′38″E, at an elevation of 499 m, collected on 3 September 2023 (holotype HMAS353381).
Description: Basidiomata are medium-sized. The pileus measures 33–55 mm in diameter, initially convex, expanding to become applanate at maturity, dry, and bright orange (5A5) to brownish orange (5C5), with a lighter color appearing as pale yellowish white (4A2) toward the margin; the disc ranges from slightly to more strongly brownish, featuring light brown (5D6) to brown (5F6); the surface is light brown (5D6) and ciliated; the margin is slightly striate with a light brown (5D6) hue; the context is 1–3 mm thick above the stipe. Lamellae are adnate to slightly adnexed, sub-sparse, and forking near the margin, presenting a light pastel yellow (3A4) to rotgold brownish orange (6C6) coloration. The stipe measures 3.5–5 × 0.6–0.8 cm, central, equal to slightly tapering upwards, with a base measuring 1–1.2 cm in diameter, dry, and displaying the same light pastel yellow (3A4) to rotgold brownish orange (6C6) background color as the lamellae. It is covered with light brown (5D6) to brown (5F6) fibrillose squamules, featuring an annular zone from the partial veil, remaining unchanging when bruised, hollow in the center, and soft. The spore print is brown. The odor is indistinct. The fluorescence reaction exhibits a bright green light under ultraviolet light.
Basidiospores (5.9) 6.1–6.6–7.5 (8.2) × (4.6) 4.9–5.3–5.9 (6.1) μm, Q = 1.23 ± 0.07 from a sample size of 40; they are subglobose, yellow-brown, and distinctly verrucose, often appearing punctate or aligned in lines. Basidia (30.7) 31.4-34.1-37.9 (42.0) × (6.9) 7.2-8.1-9.3(10.1) μm, Q = 4.26 ± 0.39 based on 20 samples, clavate, thin-walled, mostly subhyaline, with four sterigmata measuring (1.8) 2.2–3.1–4.4 (4.4) μm in length. The edges of the lamella display heterogeneity, comprising sterile hyphae that measure 10–20 × 6–8 μm, which are clavate, subhyaline, and thin-walled. Pleurocystidia are not present. The pileipellis features a well-developed epicutis, hyphae 6–10 μm wide, subcylindrical, colorless to brownish, thin-walled, and smooth; the hypodermium is also present, consisting of hyphae 13–20 μm in width, presenting irregularities in 5% KOH. Clamp connections are also present.
Additional specimens examined: China, Fujian Province, Huangxizhou, Wuyishan National Park, in a mixed forest located at 27°42′40″N, 117°45′36″E, at an elevation of 689 m, on 7 September 2023 (paratype HMAS 353382).
Habitat: This species is solitary in coniferous and broadleaf mixed forests. It was collected in September 2023 and occurs at an altitude range of 450–700 m, growing on the ground near Fagaceae trees.
Note: Cortinarius griseoaurantinus can be distinguished from other species of subgenus Leprocybe by its bright orange to brownish-orange pileus, with the disc varying from slightly to deeply brownish, and by its distinctly verrucose basidiospores (6.1–7.5 × 4.9–5.9 μm).
Cortinarius yonganensis M. J. Zhu & Jun Z. Qiu, sp. nov. (Figures 1, 3 and 4)

Figure 3. Fluorescence reactions when exposed to ultraviolet light. (a) Cortinarius griseoaurantinus (HMAS 353381); (b) Cortinarius yonganensis (HMAS 353379).

Figure 4. Morphological characteristics of Cortinarius yonganensis (HMAS 353379). (a,b) Basidiomata; (c) Basidiospores in Congo Red reagent; (d) Basidium in Congo Red reagent; (e,f) SEM images of basidiospores. Scale bars: (c,d) = 10 μm; (e,f) = 5 μm.
Mycobank No.: 857171
Etymology: The term “yonganensis” is derived from Chinese and refers to Yong’an City in Fujian Province, China, which is the locality of the type collection.
Diagnosis: Its diagnosis differs from that of the related species C. subsanguineus primarily due to the presence of punctate basidiospores and a pileus featuring a prominent violet-brown umbo at the center, along with striations along the margin.
Holotype: CHINA, Fujian Province, Sanming City, Yong’an City, Nanxi, discovered in a mixed forest at coordinates 25°31′48″N, 117°13′48″E, at an elevation of 1,031 m, on 24 June 2023 (holotype HMAS 353379).
Description: Basidiomata are small to medium-sized. The pileus measures 20–40 mm in diameter, initially hemispherical, then convex, and finally applanate with a conspicuous umbo at the center; the margin is decurrent to straight, and the surface ranges from reddish-pink (9B5) to deep jasper red (9B7), featuring persistent violet-brown (10F6) minute floccose squamules from the universal veil; the violet-brown (10F6) umbo at the center is notable, and the margin is pale pink with orange to graurot (7B4) and antiviolent brown (10E7) striations; the context is 0.5–2 mm thick above the stipe. Lamellae are adnate to emarginate-adnate, sub-sparse, and forking with small branches near the margin, with color ranging from reddish pink (9B5) to deep jasper red (9B7). The stipe measures 2.0–5.0 × 0.3–0.5 cm, central, cylindrical, dry, and rust-red, covered with rust-red floccules from the universal veil; it is rough, solid to soft, and hollow in the center. The spore print is not recorded. The odor is indistinct. The fluorescence reaction under ultraviolet light is pale green.
Basidiospores (5.0) 5.3–6.0–6.6 (7.4) × (3.9) 4.1–4.3–4.6 (4.7) μm, Q=1.41 ± 0.11 from a sample size of 40, subglobose, yellow, and punctate. Basidia (22.1) 24.3–26.5–29.2 (30.0) × (7.0) 7.0–8.2–9.3 (9.9) μm, Q=3.27 ± 0.27 from a sample size of 20, clavate, thin-walled, mostly subhyaline, with four sterigmata, measuring (2.2) 2.4–3.1–3.6 (3.7) μm in length. The lamella edges exhibit heterogeneity, characterized by sterile hyphae measuring between 14–21×5.5–7 μm, which are clavate, subhyaline, and thin-walled. Pleurocystidia are absent. The pileipellis features a well-developed epicutis with hyphae measuring 5.5–7.5 μm in width, subcylindrical, colorless to brownish, thin-walled, and smooth. The hypodermium is present, with irregular hyphae that are 18.5–40 μm wide in 5% KOH. Clamp connections are also present.
Additional specimens examined: China, Fujian Province, Sanming City, Yong’an City, Nanxi, located in a mixed forest at 25°31′43″N, 117°13′46″E, at an elevation of 1,020 m, on 24th June 2023 (paratype HMAS 353380).
Habitat: This species is solitary in mixed forests. It was collected during the summer months (June to August) and is found at altitudes above 1,000 m, growing on rocky cliffs in mixed forests.
Note: Cortinarius yonganensis can be distinguished from other species of subgenus Dermocybe by its reddish-pink to deep jasper red pileus, which features a prominent violet-brown umbo at the center and a margin with violet-brown striations. Additionally, the basidiospores (measuring 5.3–6.6 × 4.1–4.6 μm) appear punctate.
4 Discussion
In this study, we identified two new taxa of Cortinarius: C. griseoaurantinus and C. yonganensis. The identification of these species was based on molecular phylogenetic analyses of the ITS and nrLSU loci, which demonstrated the separation of C. griseoaurantinus and C. yonganensis from their closest relatives, along with their distinct morphological characteristics. Additionally, we utilized electron microscopy to capture photographs of the surface ornamentation of spores, providing further features for differentiating between the species. The morphology of the two new species aligns well with the subgenus concept. For example, the basidiomata of C. griseoaurantinus are medium-sized, with a distinctly orange pileus. In contrast, C. yonganensis features a brightly colored pileus ranging from reddish-pink to deep jasper red, accompanied by a relatively slender stipe measuring 2.0–5.0 × 0.3–0.5 cm.
According to the phylogram, C. griseoaurantinus lies within the subgenus Leprocybe and clusters with C. veronicae and C. yadingensis. All three species produce medium-sized basidiomata, with pileus diameters ranging from 5 to 6 cm (C. veronicae), 2 to 6 cm (C. yadingensis), and 3.3 to 5.5 cm (C. griseoaurantinus), and stipes of comparable lengths (3.5 to 8 cm for C. yadingensis and 3.5 to 5 cm for C. griseoaurantinus). However, C. yadingensis has a pale brown to rust brown pileus with fibrillose squamules, while C. veronicae features a red-orange to brick-red pileus and lamellae (Soop, 1998). In contrast, as mentioned, C. griseoaurantinus has a bright orange to brownish-orange pileus with the disc slightly to more strongly brownish. Furthermore, C. yadingensis has a brownish stipe with fibrillose squamules, whereas C. veronicae has a pink to yellowish-pink stipe with cinnabar-orange girdles, and C. griseoaurantinus has a pastel yellow to rot-gold brownish-orange stipe (Hong et al., 2024).
Cortinarius yonganensis was found to cluster within the subgenus Dermocybe, being related to C. subsanguineus and C. sommerfeltii. In particular, C. subsanguineus is both morphologically and phylogenetically closely related to C. yonganensis. However, C. subsanguineus generally exhibits larger, ellipsoid, verrucose basidiospores (6.5–8 × 4.5–5.5 μm), whereas C. yonganensis has smaller, subglobose, punctate basidiospores (5.3–6.6 × 4.1–4.6 μm). Both species have similar pileus colors, but C. yonganensis differs from C. subsanguineus by having a conspicuous violet-brown umbonate at the center and a margin with violet-brown striations (Yuan et al., 2020). Morphologically, C. sommerfeltii also differs from C. yonganensis with its deep reddish-brown to ochraceous-brown pileus, while C. yonganensis has a reddish-pink to deep jasper red pileus with a conspicuous violet-brown umbo at the center and a margin marked by violet-brown striations and punctate details. Additionally, C. sommerfeltii is commonly found in coniferous forests (Høiland, 1983), whereas C. yonganensis is found on rocky cliffs in mixed forests.
In summary, we describe two new species of Cortinarius through the integration of morphological traits, molecular phylogenetic analysis, and ecological information. The identification of Cortinarius griseoaurantinus (subgenus Leprocybe) and Cortinarius yonganensis (subgenus Dermocybe) enriches our understanding of the genus Cortinarius and its ecological relationships. As species of Cortinarius have been rarely discovered or characterized in China, our data suggest the potential for additional discoveries and contribute to a better understanding of the evolution, origin, and geographical distribution of this important group of mushrooms.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
YD: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. MeZ: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. NK: Conceptualization, Data curation, Formal analysis, Writing – review & editing. ZW: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. CheQ: Conceptualization, Data curation, Formal analysis, Writing – original draft. JX: Data curation, Formal analysis, Methodology, Writing – original draft. HP: Data curation, Investigation, Methodology, Resources, Writing – original draft. LL: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. CX: Data curation, Formal analysis, Investigation, Resources, Writing – original draft. ZZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. MiZ: Data curation, Formal analysis, Methodology, Writing – original draft. TM: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing. YL: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Writing – original draft. YH: Data curation, Formal analysis, Resources, Writing – original draft. XY: Data curation, Formal analysis, Methodology, Resources, Writing – original draft. ChuQ: Data curation, Formal analysis, Methodology, Writing – original draft. XJ: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – review & editing. JQ: Conceptualization, Data curation, Funding acquisition, Investigation, Writing – review & editing. YC: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was financed by the National Natural Science Foundation of China (nos. 32270029, U1803232, and 31670026), the National Key R&D Program of China (no. 2017YFE0122000), a Social Service Team Support Program Project (no. 11899170165), the Science and Technology Innovation Special Fund (nos. KFB23084, CXZX2019059S, and CXZX2019060G) of Fujian Agriculture and Forestry University, a Fujian Provincial Major Science and Technology Project (no. 2022NZ029017), the Investigation and Evaluation of Biodiversity in the Jiulong River Basin (no. 082·232591515), the Macrofungal and Microbial Resource Investigation Project in Longqishan Nature Reserve (no. SMLH2024(TP)-JL003#), and the Investigation of Macrofungal Diversity in Junzifeng National Nature Reserve, Fujian Province (no. Min Qianyu Sanming Recruitment 2024-23).
Acknowledgments
We would like to thank Sen Liu, Weibin Zhang, Jinhui Chen, and Chenjie Yang at Fujian Agriculture and Forestry University, China, for their assistance in collecting samples. The authors would also like to thank China General Microbiological Culture Collection Center and Fungarium (HMAS), Institute of Microbiology, CAS, for their support in strain and sample storage.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Bai, M. S., Wang, C., Zong, S. C., Lei, M., and Gao, J. M. (2013). Antioxidant polyketide phenolic metabolites from the edible mushroom Cortinarius purpurascens. Food Chem. 141, 3424–3427. doi: 10.1016/j.foodchem.2013.05.099
Balice, G., Boksebeld, M., Barrier, Q., Boccalini, S., Kassai-Koupai, B., Paret, N., et al. (2024). Mushroom poisoning-related cardiac toxicity: a case report and systematic review. Toxins (Basel) 16:265. doi: 10.3390/toxins16060265
Brandrud, T. E. (1998). Cortinarius subgenus Phlegmacium section Phlegmacioides (= Variecolores) in Europe. Edinb. J. Bot. 55, 65–156. doi: 10.1017/S0960428600004364
Dima, B., Liimatainen, K., Niskanen, T., Bojantchev, D., Harrower, E., Papp, V., et al. (2021). Type studies and fourteen new north American species of Cortinarius section Anomali reveal high continental species diversity. Mycol. Prog. 20, 1399–1439. doi: 10.1007/s11557-021-01738-0
Fan, L., Zhong, X., Ma, T., Zhou, H., Wang, B., and Ji, X. (2024). Four new species of Cortinariaceae (Agaricales) from northwestern China. Front. Microbiol. 15:1454736. doi: 10.3389/fmicb.2024.1454736
Fiala, J., Battlogg, M., Bösking, J., Buchauer, K., May, T. W., Pannwitz, A., et al. (2025). Photoantimicrobial anthraquinones in Australian fungi of the genus Cortinarius. Fitoterapia 182:106402. doi: 10.1016/j.fitote.2025.106402
Frøslev, T. G., Jeppesen, T. S., Laessøe, T., and Kjøller, R. (2007). Molecular phylogenetics and delimitation of species in Cortinarius section Calochroi (Basidiomycota, Agaricales) in Europe. Mol. Phylogenet. Evol. 44, 217–227. doi: 10.1016/j.ympev.2006.11.013
Gallone, B., Kuyper, T. W., and Nuytinck, J. (2024). The genus Cortinarius should not (yet) be split. IMA Fungus 15:24. doi: 10.1186/s43008-024-00159-4
Garnica, S., Schön, M. E., Abarenkov, K., Riess, K., Liimatainen, K., Niskanen, T., et al. (2016). Determining threshold values for barcoding fungi: lessons from Cortinarius (Basidiomycota), a highly diverse and widespread ectomycorrhizal genus. FEMS Microbiol. Ecol. 92:fiw045. doi: 10.1093/femsec/fiw045
Høiland, K. (1983). “Cortinarius subgenus dermocybe” in Opera Botanica 71 (Council for Nordic Publications in Botany).
Hong, P., Wang, K., Du, Z., Zhao, M. J., Xie, M. L., Liu, D., et al. (2024). Two new Cortinarius species in subgenus Leprocybe from Southwest China. PeerJ 12:e17599. doi: 10.7717/peerj.17599
Jetha, P., Mojzita, D., Maiorova, N., De Ruijter, J. C., Maaheimo, H., Hilditch, S., et al. (2025). Discovery of Cortinarius O-methyltransferases for the heterologous production of dermolutein and physcion. Biotechnol. Biofuels Bioprod. 18:25. doi: 10.1186/s13068-025-02625-6
Kornerup, A., and Wanscher, J. H. (1981). Taschenlexikon der Farben, 3rd edn. Muster-Schmidt Verlag, Zürich und Göttingen.
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Liimatainen, K., Carteret, X., Dima, B., Kytovuori, I., Bidaud, A., Reumaux, P., et al. (2017). Cortinarius section Bicolores and section Saturnini (Basidiomycota, Agaricales), a morphogenetic overview of European and north American species. Persoonia 39, 175–200. doi: 10.3767/persoonia.2017.39.08
Liimatainen, K., Kim, J. T., Pokorny, L., Kirk, P. M., Dentinger, B., and Niskanen, T. (2022). Taming the beast: a revised classification of Cortinariaceae based on genomic data. Fungal Divers. 112, 89–170. doi: 10.1007/s13225-022-00499-9
Liimatainen, K., Niskanen, T., Ammirati, J. F., Kytövuori, I., and Dima, B. (2014a). Cortinarius, subgenus Telamonia, section Disjungendi, cryptic species in North America and Europe. Mycol. Prog. 14:1016. doi: 10.1007/s11557-014-1016-9
Liimatainen, K., Niskanen, T., Dima, B., Ammirati, J. F., Kirk, P. M., and Kytövuori, I. (2020). Mission impossible completed: unlocking the nomenclature of the largest and most complicated subgenus of Cortinarius, Telamonia. Fungal Divers. 104, 291–331. doi: 10.1007/s13225-020-00459-1
Liimatainen, K., Niskanen, T., Dima, B., Kytövuori, I., Ammirati, J. F., and Frøslev, T. G. (2014b). The largest type study of Agaricales species to date: bringing identification and nomenclature of Phlegmacium (Cortinarius) into the DNA era. Persoonia 33, 98–140. doi: 10.3767/003158514X684681
Long, P., Zhou, S. Y., Li, S. N., Liu, F. F., and Chen, Z. H. (2024). three new species of Cortinarius section Delibuti (Cortinariaceae, Agaricales) from China. MycoKeys 101, 143–162. doi: 10.3897/mycokeys.101.114705
Matheny, P., Moreau, P.-A., Vizzini, A., Harrower, E., Haan, A., Marco, C., et al. (2014). Crassisporium and Romagnesiella: two new genera of dark-spored Agaricales. Syst. Biodivers. 13, 28–41. doi: 10.1080/14772000.2014.967823
Moser, M. M. (1969). CortinariusFr. Untergattung Leprocybe sungen. nov. Die Rauhköpfe Zeitschrift für Pilzkunde. 35:213–248.
Moser, M., Horak, E., and Gruber, I. (1975). Cortinarius Fr. und nahe verwandte Gattungen in Südamerika. Mycologia 67:1078.
Niskanen, T., Kytövuori, I., and Liimatainen, K. (2009). Cortinarius sect. Brunnei (Basidiomycota, Agaricales) in North Europe. Mycol. Res. 113, 182–206. doi: 10.1016/j.mycres.2008.10.006
Niskanen, T., Liimatainen, K., Kytövuori, I., Lindström, H., Dentinger, B. T. M., and Ammirati, J. F. (2016). Cortinarius subgenus Callistei in North America and Europe—type studies, diversity, and distribution of species. Mycologia 108, 1018–1027. doi: 10.3852/16-033
Nusair, S. D., Abandah, B., Al-Share, Q. Y., Abu-Qatouseh, L., and Ahmad, M. I. A. (2023). Toxicity induced by orellanine from the mushroom Cortinarius orellanus in primary renal tubular proximal epithelial cells (RPTEC): novel mechanisms of action. Toxicon 235:107312. doi: 10.1016/j.toxicon.2023.107312
Pastor, N., Chiapella, J., Kuhar, F., Mujic, A. B., Crespo, E. M., and Nouhra, E. R. (2019). Unveiling new sequestrate Cortinarius species from northern Patagonian Nothofagaceae forests based on molecular and morphological data. Mycologia 111, 103–117. doi: 10.1080/00275514.2018.1537350
Song, C., Wu, M., Zhang, Y., Li, J., Yang, J., Wei, D., et al. (2022). Correction to bioactive monomer and polymer polyketides from edible mushroom Cortinarius caerulescens as glutamate dehydrogenase inhibitors and antioxidants. J. Agric. Food Chem. 70:1747. doi: 10.1021/acs.jafc.2c00554
Soop, K. (1998). Notes Et Observations Sur Les Champignons Cortinarioides De Nouvelle-Zelande. Documents Mycologiques 28: 13–25.
Soop, K., Dima, B., Cooper, J. A., Park, D., and Oertel, B. (2019). A phylogenetic approach to a global supraspecific taxonomy of Cortinarius (Agaricales) with an emphasis on the southern mycota. Persoonia 42, 261–290. doi: 10.3767/persoonia.2019.42.10
Torres, S., Cajas, D., Palfner, G., Astuya, A., Aballay, A., Pérez, C., et al. (2017). Steroidal composition and cytotoxic activity from fruiting body of Cortinarius xiphidipus. Nat. Prod. Res. 31, 473–476. doi: 10.1080/14786419.2016.1185717
Varga, T., Krizsán, K., Földi, C., Dima, B., Sánchez-García, M., Sánchez-Ramírez, S., et al. (2019). Megaphylogeny resolves global patterns of mushroom evolution. Nat Ecol Evol 3, 668–678. doi: 10.1038/s41559-019-0834-1
Vellinga, E. C., Kuyper, T. W., Ammirati, J., Desjardin, D. E., Halling, R. E., Justo, A., et al. (2015). Six simple guidelines for introducing new genera of fungi. IMA Fungus 6, A65–A68. doi: 10.1007/BF03449356
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols, 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Yli-Öyrä, J., Herrala, M., Kovakoski, H., Huuskonen, E., Toukola, P., Räisänen, R., et al. (2024). In vitro toxicity assessment of Cortinarius sanguineus Anthraquinone Aglycone extract. J Fungi 10:369. doi: 10.3390/jof10060369
Yuan, H. S., Lu, X., Dai, Y. C., Hyde, K. D., Kan, Y. H., Kušan, I., et al. (2020). Fungal diversity notes 1277–1386: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 104, 1–266. doi: 10.1007/s13225-020-00461-7
Zhang, D., Gao, F., Jakovlić, I., Zou, H., Zhang, J., Li, W. X., et al. (2020). PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 20, 348–355. doi: 10.1111/1755-0998.13096
Keywords: Agaricales, morphological, new species, phylogenetic analyses, taxonomy
Citation: Dang Y, Zhu M, Keyhani NO, Wu Z, Qiu C, Xiong J, Pu H, Lin L, Xiong C, Zhao Z, Zheng M, Mu T, Lin Y, Huang Y, Yuan X, Qiu C, Ji X, Qiu J and Chen Y (2025) Discovery of two new Cortinarius species in Southern China. Front. Microbiol. 16:1558935. doi: 10.3389/fmicb.2025.1558935
Edited by:
Wen-Jun Li, Sun Yat-sen University, ChinaReviewed by:
Jing Si, Beijing Forestry University, ChinaMing Zhang, Guangdong Academy of Science, China
Copyright © 2025 Dang, Zhu, Keyhani, Wu, Qiu, Xiong, Pu, Lin, Xiong, Zhao, Zheng, Mu, Lin, Huang, Yuan, Qiu, Ji, Qiu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohong Ji, eGhqaTIwMTBAMTYzLmNvbQ==; Junzhi Qiu, anVuemhpcWl1QDEyNi5jb20=; Yuxi Chen, bGllc2xleXVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yuxiao Dang
Yuxiao Dang Mengjia Zhu
Mengjia Zhu Nemat O. Keyhani2
Nemat O. Keyhani2 Longbing Lin
Longbing Lin Taichang Mu
Taichang Mu Xiaohong Ji
Xiaohong Ji Junzhi Qiu
Junzhi Qiu