- 1Guangdong-Hong Kong-Macau Institute of CNS Regeneration, Jinan University, Guangzhou, China
- 2Xin-Huangpu Joint Innovation Institute of Chinese Medicine, Guangzhou, China
- 3Institute of Grand Scientific Devices, Beijing University of Aeronautics and Astronautics, Beijing, China
- 4Modern Traditional Chinese Medicine Haihe Laboratory, Tianjin University of Traditional Chinese Medicine, Tianjin, China
Background: The interaction between the gut microbiota and neuroinflammation plays a crucial role in the pathogenesis of many diseases, particularly neurodegenerative diseases, and has become one of the focal points of research in recent years. Despite the large number of related studies, there is currently a lack of comprehensive analysis and prediction of these data to drive the field forward. This study aims to systematically analyze the clinical practices and research hotspots of the underlying mechanisms in this field using bibliometric and visualization methods, and to explore the future development pathways.
Methods: CiteSpace, VOSviewer, GraphPad Prism and other software were used to analyze 1,404 studies on gut microbiota and neuroinflammation collected by the core of the Web of Science since 2000, to visually present the collaborative network between literatures, structure of authors and countries, co-occurrence of keywords, emerging reference literature, and research hotspots.
Results: From 2000 to 2024, the number of related papers on this topic showed an overall upward trend, and the annual citation peaked in 2020, with significant contributions from China and the United States. Research focused on the relationship between gut microbiota and neuroinflammation, with a particular emphasis on investigating the mechanisms of the microbiota-gut-brain axis through both basic and clinical research. Treatment strategies include probiotic therapy, fecal microbiota transplantation and traditional Chinese medicine.
Conclusion: This study comprehensively reviews the research progress on the association between gut microbiota and neuroinflammation, and discusses the current research focus and frontier directions of this relationship, so as to provide reference for the development of this field.
1 Introduction
The interaction between gut microbiota and their hosts has emerged as a prominent research area in recent years. Mounting evidence suggests that maintaining a balanced composition of gut microbial communities is crucial for the overall health of the host organism. In addition to its role in food digestion and absorption, the gut microbiota also exerts influence on brain function and behavior through the intricate network known as the microbiota-gut-brain axis (MGBA). The gut microbiota communicates with the brain through the “gut-brain axis, “a sophisticated network that not only encompasses the production of microbial metabolites such as short-chain fatty acids (SCFAs) and neurotransmitters, but also orchestrates immune system function to modulate neuroinflammation (Chidambaram et al., 2022).
Recently, the bidirectional effects of the gut microbiota on central nervous system (CNS) inflammation through the MGBA mechanism have been gradually elucidated. Previous studies have demonstrated a close association between dysbiosis of the gut microbiota and the development and occurrence of various neuroinflammatory-related diseases. Specifically, patients with Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS) often exhibit significantly distinct composition and functionality of their gut microbiota compared to healthy individuals (Zhang et al., 2024; Nuzum et al., 2024). The study revealed that dysbiosis of the gut microbiota can influence neuroinflammation through multiple mechanisms, such as but not limited to the disruption of intestinal barrier function, the alteration of metabolic products, and the abnormal activation of the immune system (Kumari et al., 2023). The interactions of these mechanisms might further intensify neuroinflammation, thus facilitating the pathological advancement of neurodegenerative diseases. Additionally, the metabolic products of the gut microbiome, such as SCFAs, have been demonstrated to play a vital role in regulating the host immune response and maintaining the integrity of the gut barrier as well as the blood–brain barrier (BBB) (Ashique et al., 2024; Mathias et al., 2024). SCFAs can regulate the expression of inflammatory factors by binding to receptors on host cells, thereby influencing the development of neuroinflammation. Hence, the equilibrium of the gut microbiome is potentially of great significance in preventing and treating neurodegenerative diseases.
Bibliometric analysis is a quantitative approach for studying scientific literature, which can objectively disclose the potential connections and inherent laws among research objects by excavating a large amount of literature data. Therefore, this study undertakes a bibliometric analysis on the literature related to the gut microbiome and neuroinflammation published since 2000, to reveal the knowledge structure of the field for the first time, identify key research themes and hot topics, disclose the latest advancements and significant findings in these research areas, and analyze the directions where future research might develop. Through this study, we anticipate providing a new perspective on understanding the complex relationship between the gut microbiome and neuroinflammation, and offer a reference for future research endeavors.
2 Methods
2.1 Data sources and cleaning
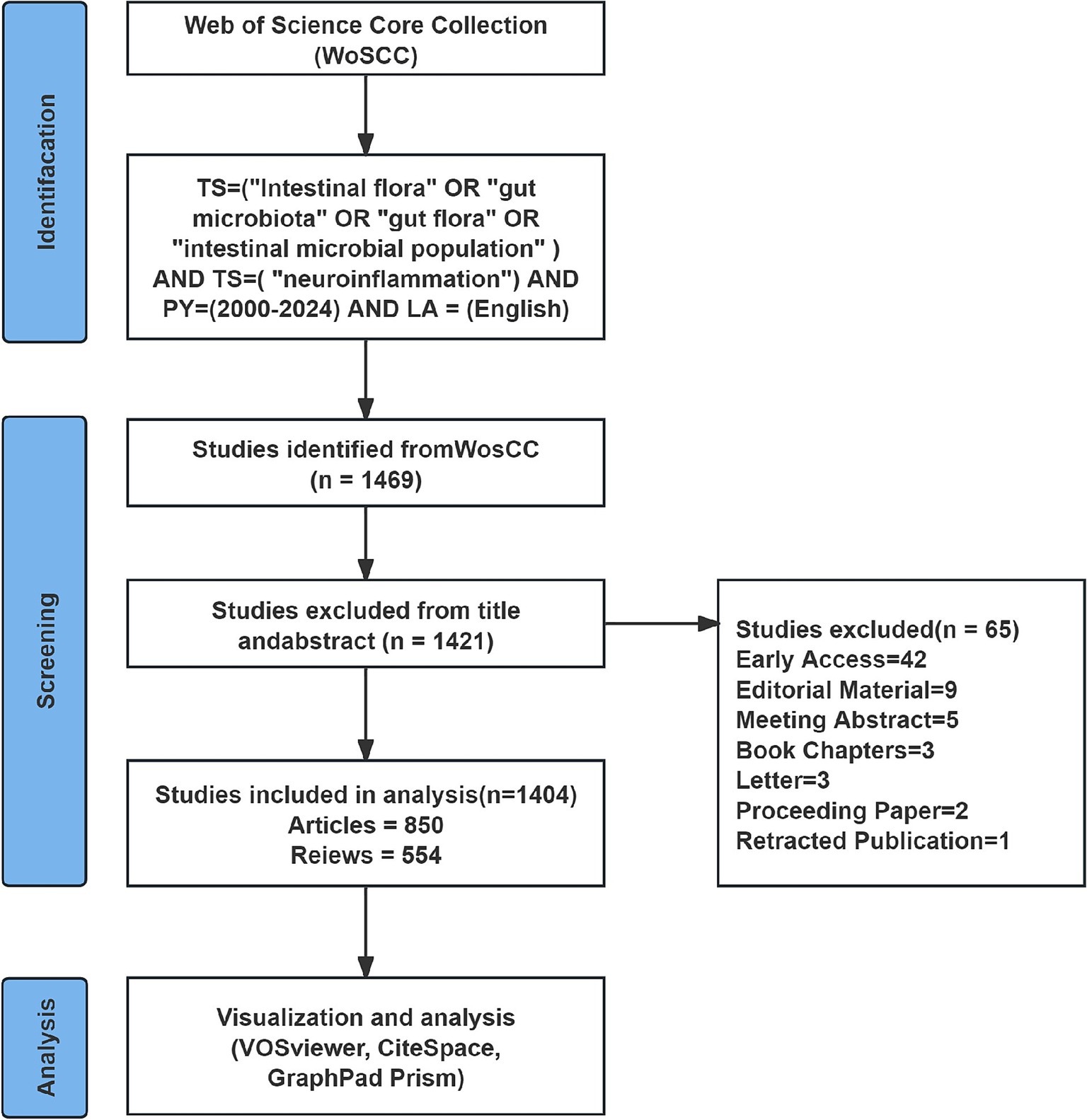
This study used CiteSpace, VOSviewer, and GraphPad Prism software to carry out visual analysis of the selected literature. An exploration of the Web of Science Core Collection (WoSCC) literature database was conducted on September 5, 2024. The search formula was “TS = (“Intestinal flora” OR “gut microbiota” OR “gut flora” OR “intestinal microbial population”) AND TS = (“neuroinflammation”) AND PY = (2000–2024) AND LA = (English),” with the time interval ranging from 2000-01-01 to 2024-09-05. The exported document records consist of titles, authors, institution names, keywords, abstract, journal, publication time, etc. To more precisely target the information requisite for the topic, this study solely incorporates literature of the document types “articles” and “review.” Import the documents into EndNote for preliminary deduplication. Then, two authors (S W and NJ C) manually compare the titles, abstracts, keywords and other contents to eliminate the following literatures: (1) Literatures with duplicated titles and abstracts; (2) Literature that is inconsistent with the target research content; and (3) Literature of types such as conference proceedings, edited materials, letters, conference abstracts, book reviews, corrections, data papers, etc. After data cleaning, all the literature information was exported in plain text format. The process of including literature is presented in Figure 1.
2.2 Data analysis and visualization
This study conducted a visual analysis of the selected literature using CiteSpace, VOSviewer, and GraphPad Prism software. In CiteSpace, the following parameter settings were adopted: (1) The time range was from January 1, 2000 to September 5, 2024, with the analysis time unit set as 1 year; (2) Threshold filtering was applied to select the top n authors with the highest frequency in each time period (n = 50) to highlight important literature; (3) Node types selected for visualization analysis included country, institution, author, and keyword, generating co-occurrence graphs; and (4) Pruning, trimming the functional area to highlight the connection graph of important nodes; other parameters were set to the default values of the system. In VOSviewer, we selected the minimum document quantity of nodes according to the needs of data visualization, and set other documents to the default values. We analyzed the distribution of annual publications and used GraphPad Prism to draw publication data tables and tables of publications by country/region. CiteSpace and VOSviewer generated author, country/region, journal, keyword, and reference collaboration networks, co-occurrence networks, and co-citation graphs of references.
3 Results
3.1 Global trend in publication outputs and citations
A total of 1,404 articles examining the correlation between gut microbiota and neuroinflammation were included, comprising 850 original studies and 554 reviews. Figure 2 illustrates the yearly publication volume and citation frequency of relevant publications from January 2000 to September 2024. Overall, there has been a consistent upward trend in annual publications on gut microbiota and neuroinflammation, with a notable surge since 2019. Annual citations reached their peak in 2020, while the number of publications continued to grow, reaching its zenith in 2023. It is anticipated that by the end of 2024, the number of publications in this field will surpass previous years.
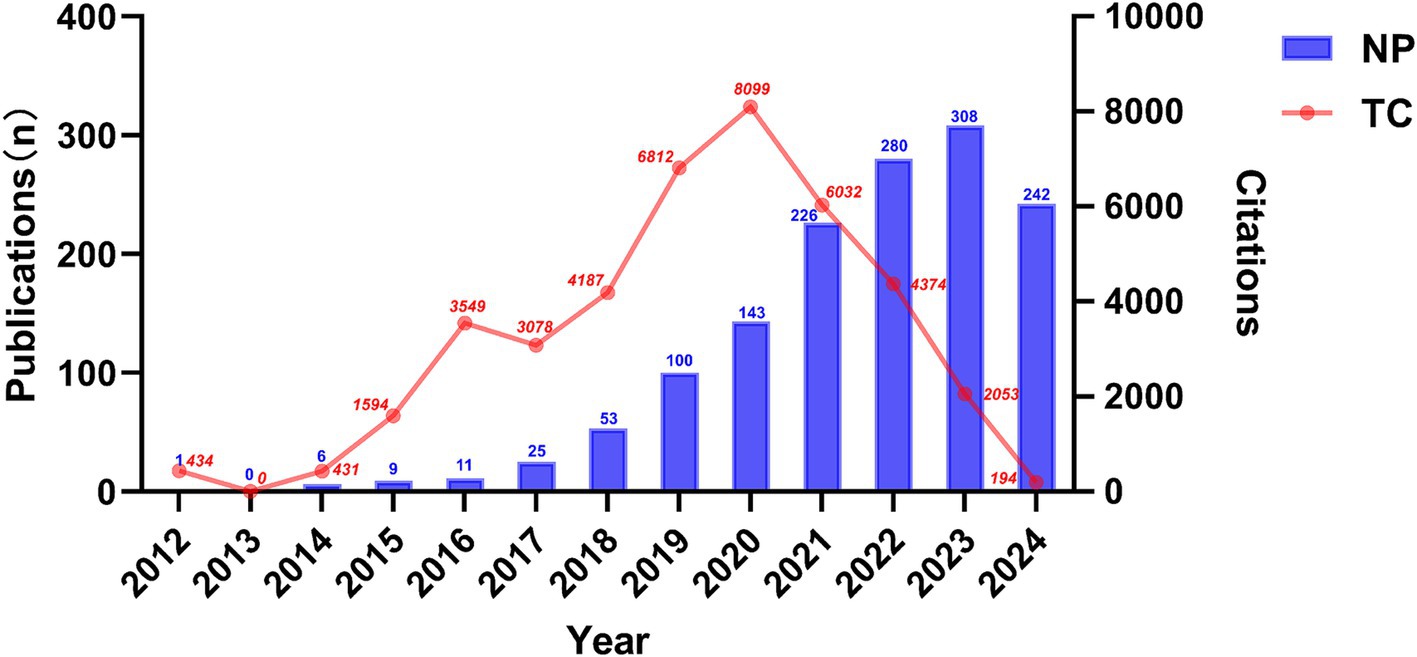
Figure 2. Temporal trends in publications of the association between gut microbiota and neuroinflammation, as indicated by the number of publications (NP) and total citation (TC).
3.2 Country and institution analysis
A total of 78 countries and 1,000 academic institutions were involved in the research on the association between gut microbiota and neuroinflammation. China (n = 630) made the greatest contribution to the number of publications, followed by the United States (n = 308) and Italy (n = 102). By filtering and visualizing the 78 countries based on the number of publications equal to or greater than 2, and constructing a collaboration network based on the number of published papers and relationships in each country. There was close collaboration among different countries (Figure 3A). For instance, the United States collaborated closely with China, Brazil, Italy, and Canada, while China collaborated closely with the United States and Australia.
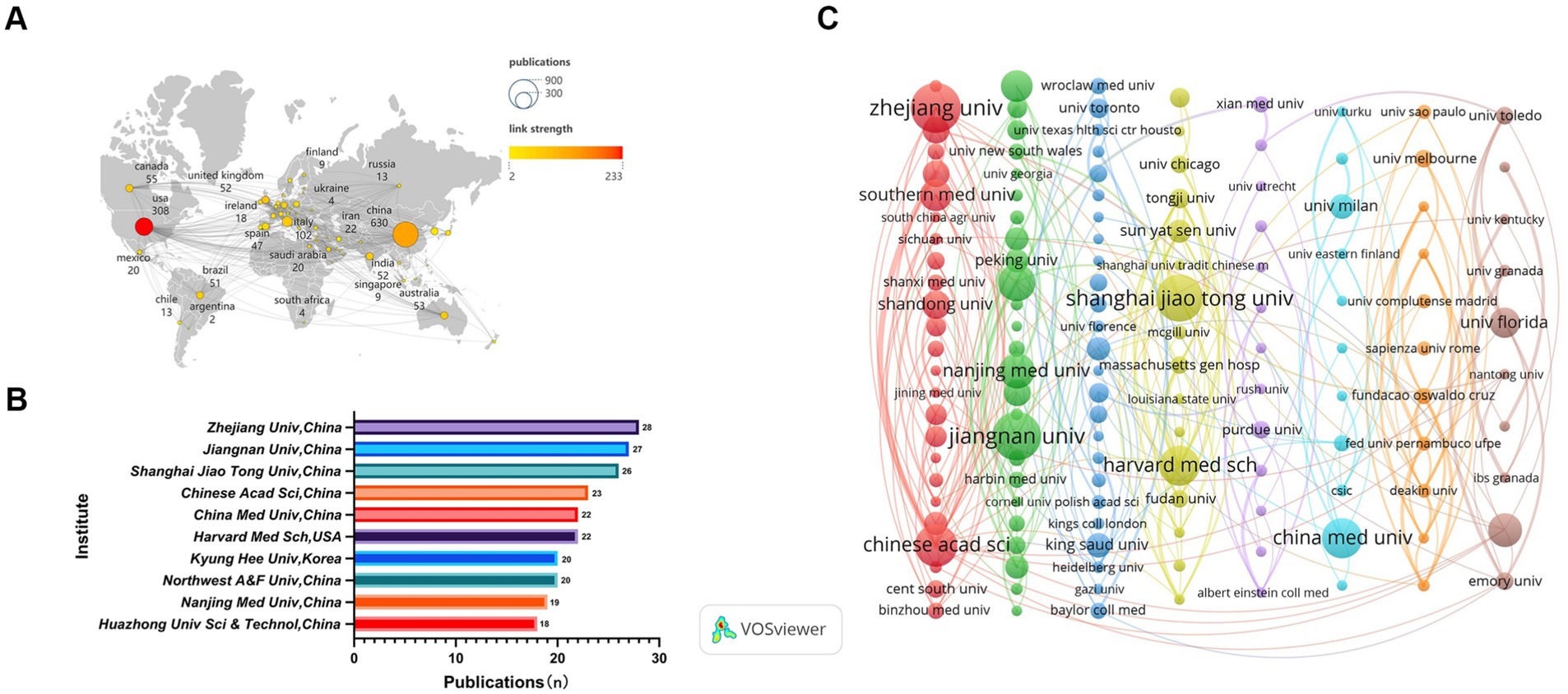
Figure 3. Analysis of country/region. (A) Visual clustering analysis of the national collaboration network. (B) The top 10 institutions by publication volume. (C) Visual clustering analysis of inter-institutional collaboration relations. Nodes represent countries/institutions, the size reflects the number of publications, the connecting lines indicate cooperation, the thickness represents the degree of closeness, and different colors represent different clusters, which can reflect the size and composition of the clusters.
Among the top 10 academic institutions worldwide in terms of the number of publications regarding the relationship between gut microbiota and neuroinflammation, three countries are represented. Eight of them are from China, while the remaining two are from the United States and South Korea. The top three academic institutions with the greatest number of publications on this subject are Zhejiang University (n = 28), Jiangnan University (n = 27), and Shanghai JiaoTong University (n = 26) (Figure 3B). Subsequently, 151 academic institutions were selected based on the criterion that the minimum number of publications was equal to 5, and they were visualized based on the number of publications and relationships. The collaboration among Chinese Academy of Sciences, University of Chinese Academy of Sciences and South China Agricultural University was highly intense, and the same gone for Harvard University, Tongji University and Sun Yat-Sen University (Figure 3C).
3.3 Journals and co-cited journals
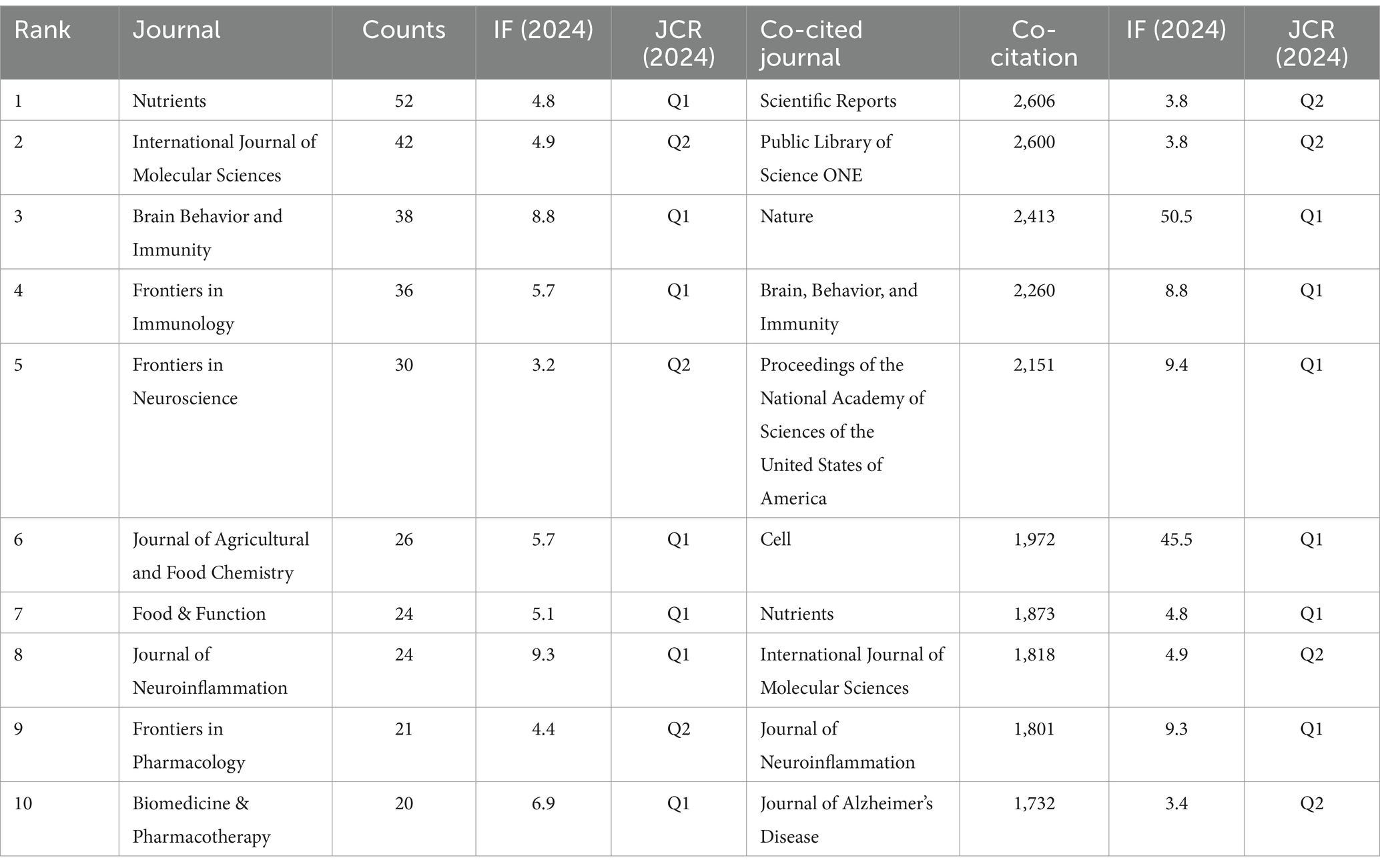
Research on the association between gut microbes and neuroinflammation has been published in 443 journals. The most published papers are Nutrients (n = 52, 11.74%), followed by International Journal of Molecular Sciences (n = 42, 9.48%), Brain Behavior and Immunity (n = 38, 8.58%). Among them, the highest impact factor was found in the Journal of Neuroinflammation (IF: 9.3). This was followed by Brain Behavior and Immunity (IF: 8.8) and Biomedicine & Pharmacotherapy (IF: 6.9). Of the top 10 journals cited, five were cited more than 2,000 times, with Scientific Reports (2,606 times) being the most cited journal, followed by Plos One (2,600 times) (Table 1).
Subsequently, we selected 59 journals based on the principle of a minimum co-cited publication number of 6 and created a journal network diagram. Figures 4A,B reveal that the citation relationships between Nutrients and International Journal of Molecular Sciences, as well as Brain Behavior and Immunity, are vigorous. Among the co-cited journals, the highest impact factor belongs to Nature (IF: 50.5), followed by Cell (IF: 45.5). Then, we chose the journals with a maximum co-cited number of more than 500 times and drew a co-cited network diagram. Figure 4C indicates that the co-cited relationships between Plos one and Nature, Scientific Reports, Cell, and Proceedings of the National Academy of Sciences of the United States of America are dynamic.
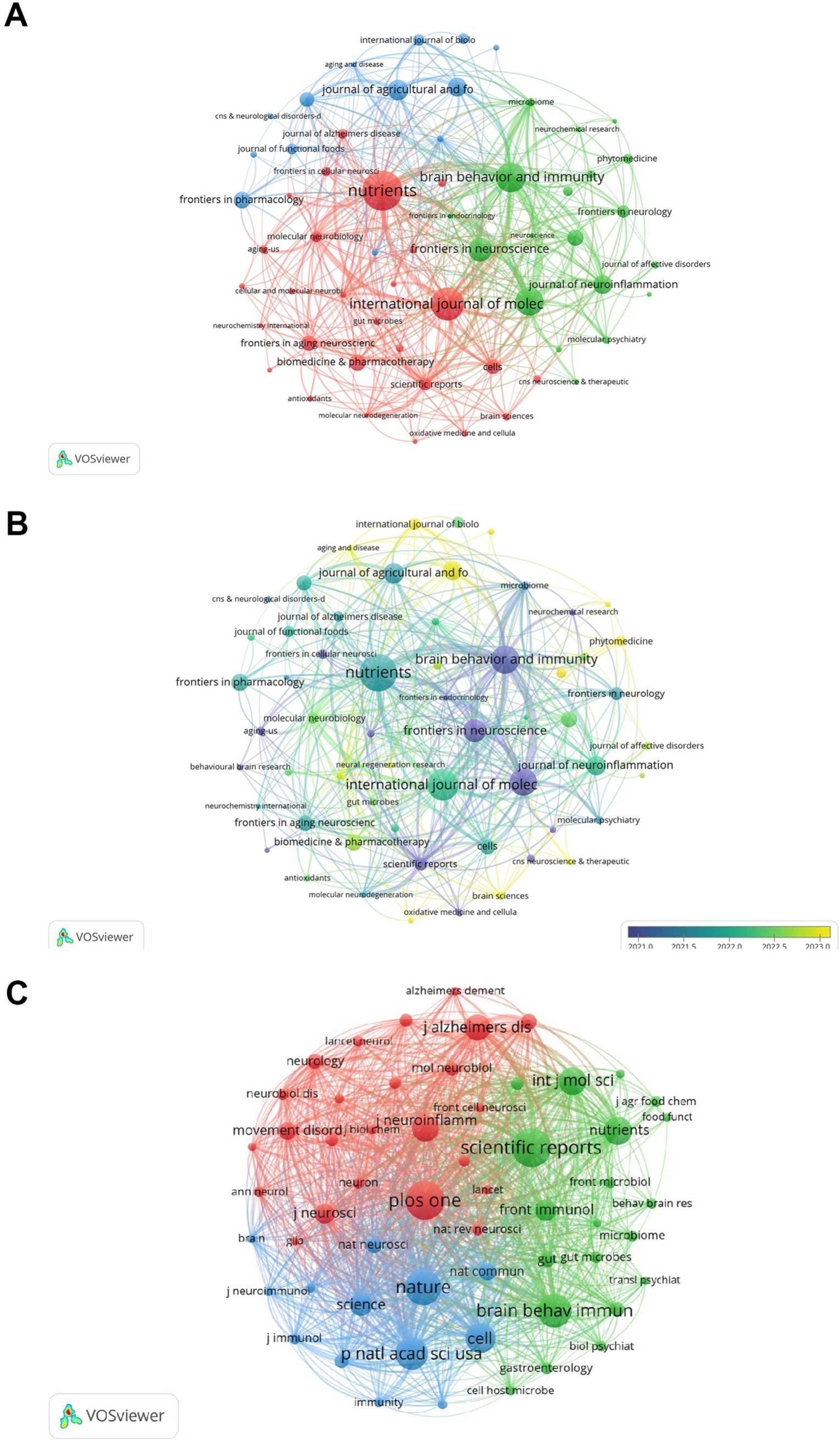
Figure 4. Analysis of journals. (A) Visualization of journal collaboration network. (B) Visualization of journal collaboration network with overlaid time. (C) Co-citation network visualization. The different colors in the collaborative network diagram represent different clusters, which can reflect the size and composition of the clusters. In the superimposed temporal collaborative network diagram, the time axis from purple to yellow indicates the emergence time and development sequence of these hotspots. The same applies to Figures 5–7.
3.4 Author productivity and co-citation analysis
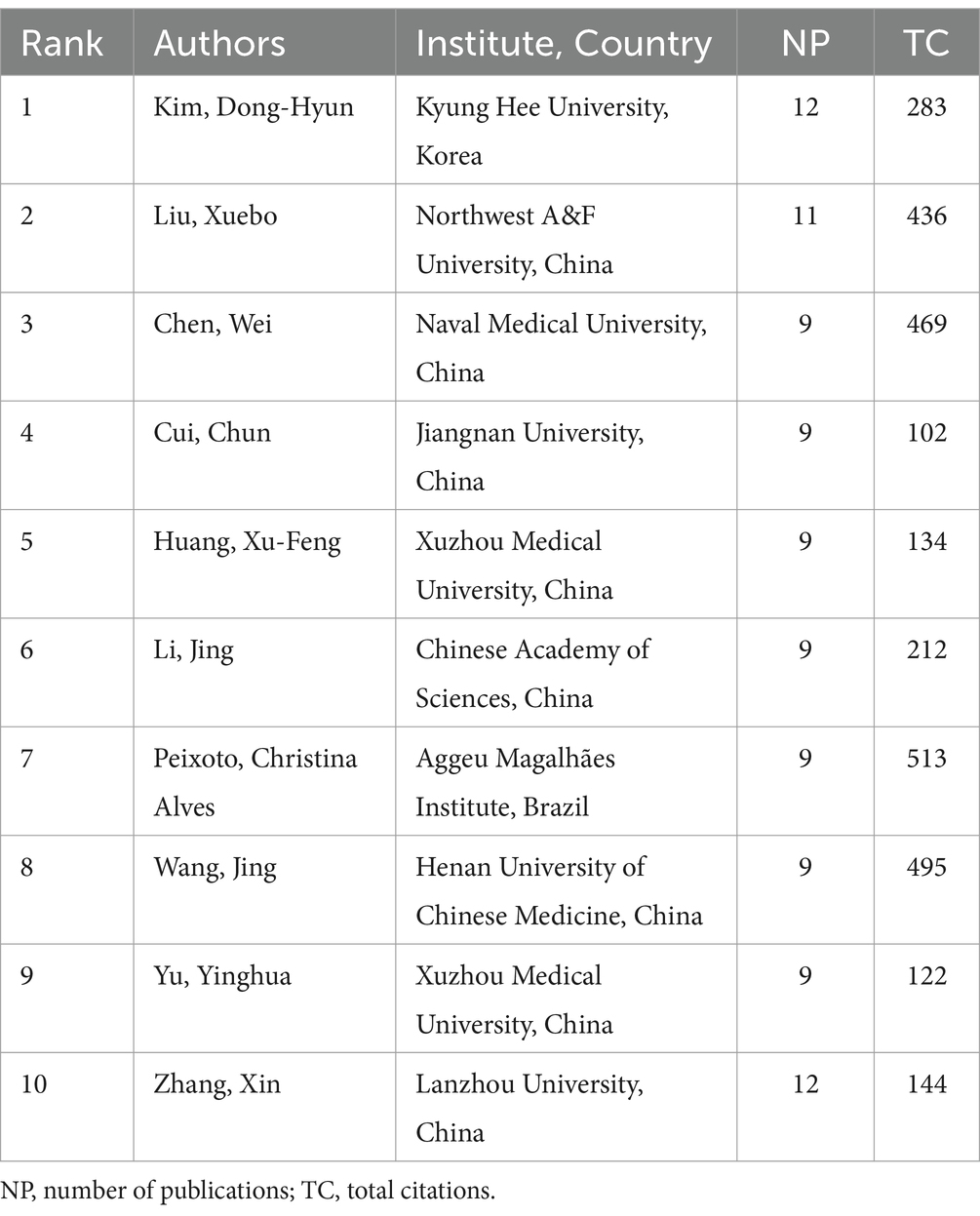
A total of 8,194 authors engaged in research on the relationship between gut microbiota and neuroinflammation. Among the top 10 authors, two of them each published over 10 papers (Table 2). We established a cooperation network based on the criterion that authors had published at least 6 papers in this field (Figures 5A,B). Three small cooperation networks were formed around Cui Chun, Li Jing, and Yu Yinghua, with relatively fewer researchers collaborating with others, indicating that the current cooperation network structure in this area is relatively scattered and has not yet formed a large-scale cooperation network.
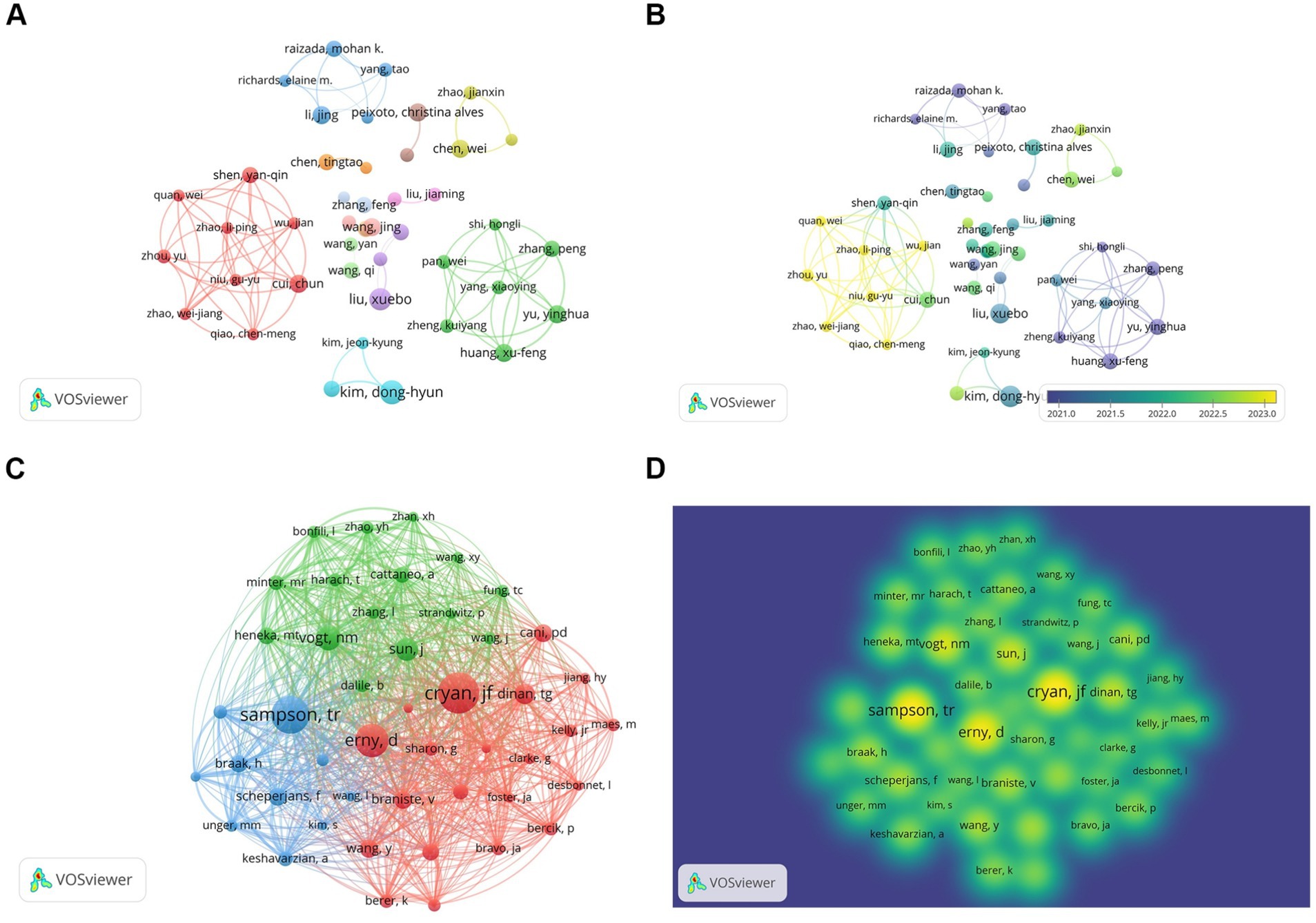
Figure 5. Author and co-cited author analysis. (A) Author collaboration network visualization. (B) Author collaboration network visualization with overlaid time. Colors represent average time. (C) Co-cited author network diagram. (D) Co-cited author density map.
Among the 58,786 co-cited authors, those with a minimum co-citation count of 100 or above were selected, and a co-cited network was plotted (Figures 5C,D). There were three authors whose co-citation counts exceeded 300, namely Cryan, JF (n = 424), Sampson, TR (n = 394), and Erny, D (n = 345), and they exhibited close co-cited relationships.
3.5 Co-cited references analysis
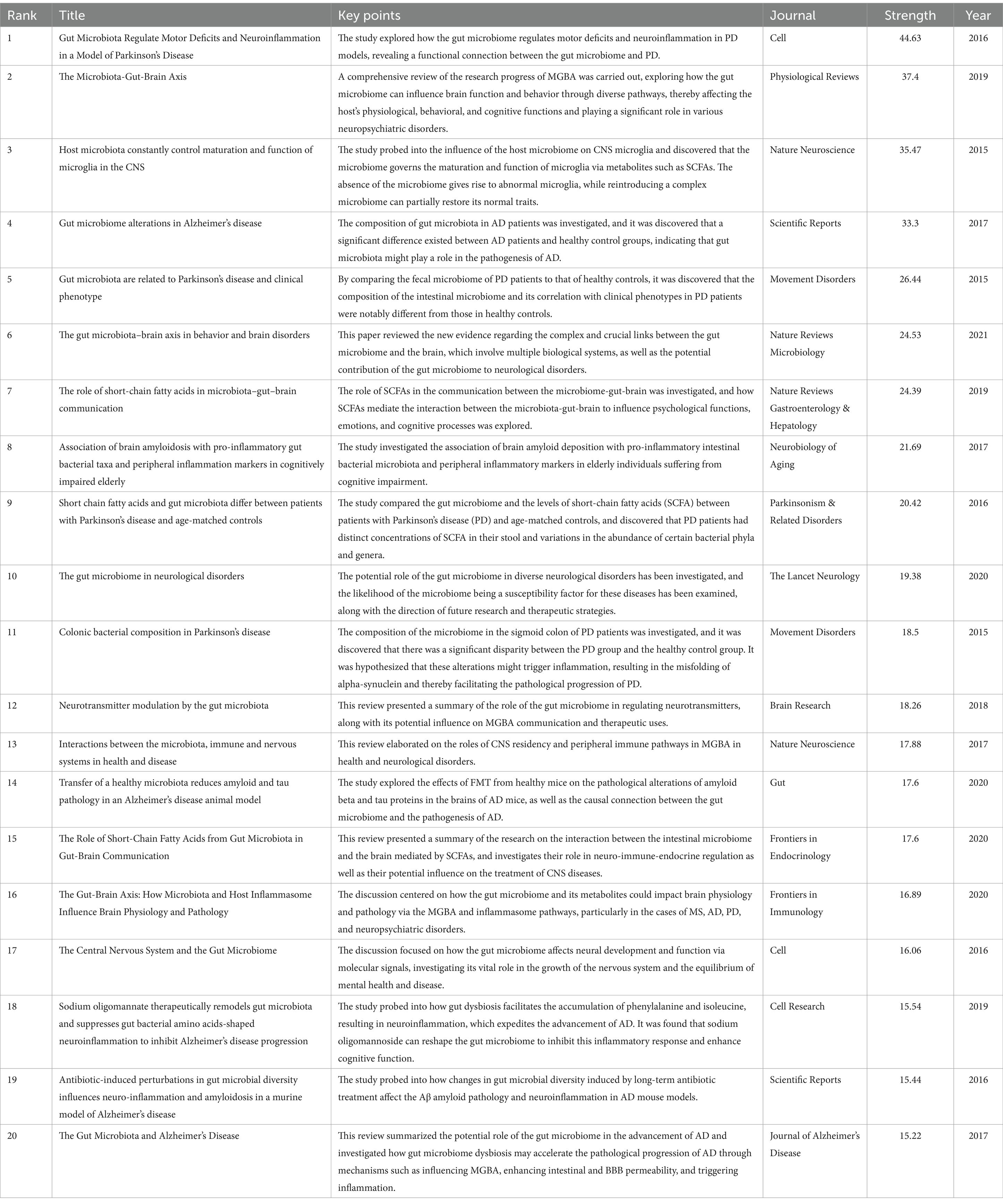
Over the past 20 years, a total of 87,577 citations have been made to research on the role of gut microbiota in neuroinflammation. Among the top 10 most highly cited papers, at least 128 citations have been received, with three papers being cited over 200 times. We constructed a co-citation network graph using papers with a co-citation count of at least 60. Sampson (2016) in Cell, Vol. 167, P1469, Erny et al. (2015) in Nat Neurosci, Vol. 18, P965, Braniste (2014) in Sci Transl Med, Vol. 6, and Vogt (2017) in Sci Rep-UK, Vol. 7, have a considerable number of co-citations, indicating that these three papers are frequently cited together (Figures 6A,B).
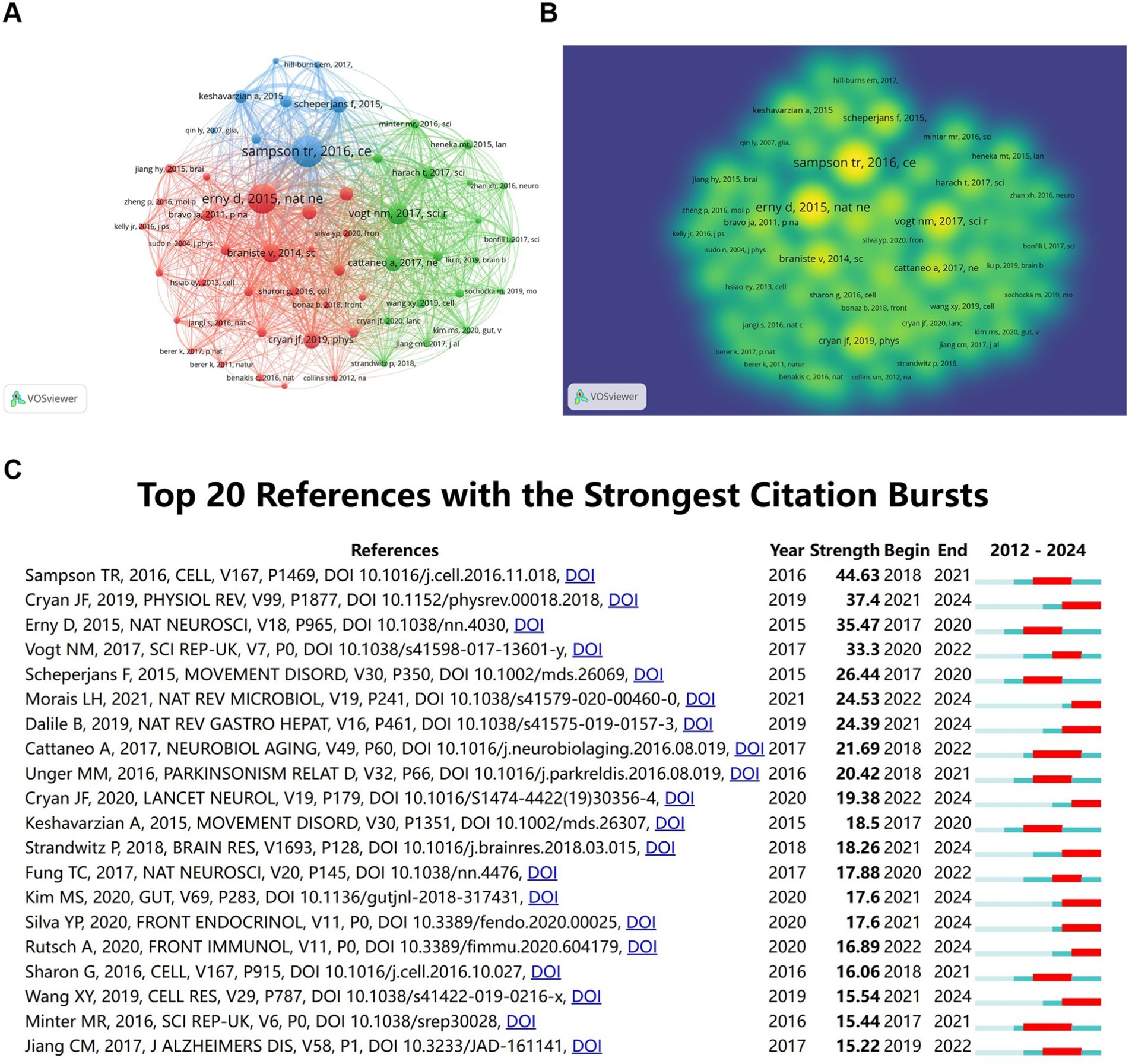
Figure 6. Co-cited references analysis. (A) Co-cited references network. (B) Co-cited references density map. (C) Top 20 references with the strongest citation bursts.
The citation co-occurrence map (Figure 6C) generated by CiteSpace reveals that the reference with the most intense citation burst (intensity = 44.63) is “Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease” by Sampson et al. (2016). The period of citation burst is from 2018 to 2021. The second most intense citation burst (intensity = 37.4) is “The Microbiota-Gut-Brain Axis” by Cryan JF et al., whose citation burst period ranges from 2021 to 2024 in Physiological Reviews. We have summarized the main research contents of the top 20 most-cited papers with the strongest citation bursts in Table 3.
3.6 Keywords analysis of research hotspots
3.6.1 Co-occurrence analysis of keywords
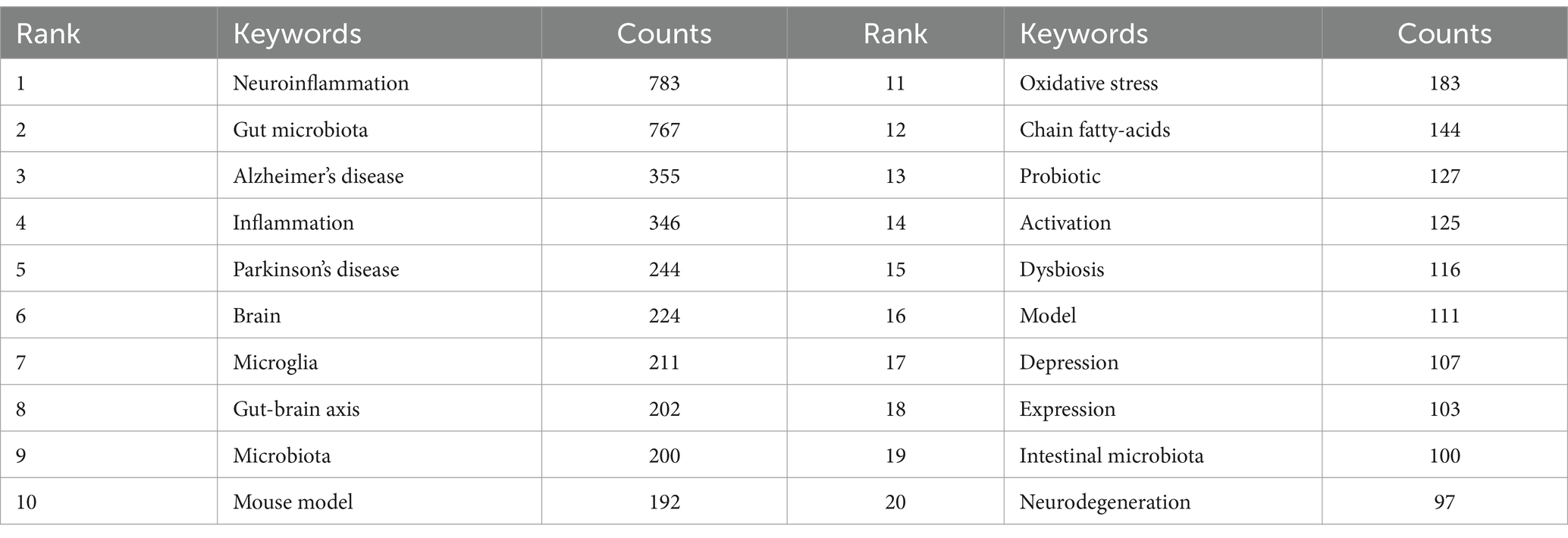
By conducting co-occurrence analysis on keywords, it becomes feasible to promptly identify the research hotspots in a specific field. In the case of the research regarding the role of gut microbiota in neuroinflammation, there are a total of 4,867 keywords. Table 4 presents the top 20 keywords with the highest frequency. Besides the theme words, Alzheimer’s disease, inflammation, and Parkinson’s disease occur most frequently, representing the main research directions in studies related to gut microbiota and neuroinflammation.
We chose keywords that appeared at least 50 times and carried out a clustering analysis using VOSviewer (Figure 7A). The larger the node was, the more frequently the keyword occurred, and the more it signified a research hotspot. The lines between nodes indicated the strength of association, and the thicker the line was, the stronger the connection between the keywords. As depicted in Figure 7A, we obtained a total of three clusters, representing three research directions. The keywords in the red cluster encompassed neuroinflammation, gut microbiota, and inflammation, among others. The keywords in the green cluster included Parkinson’s disease, gut-brain axis, and chain fatty-acids, among others. The keywords in the blue cluster comprised Alzheimer’s disease, mouse model, and mouse model, among others.
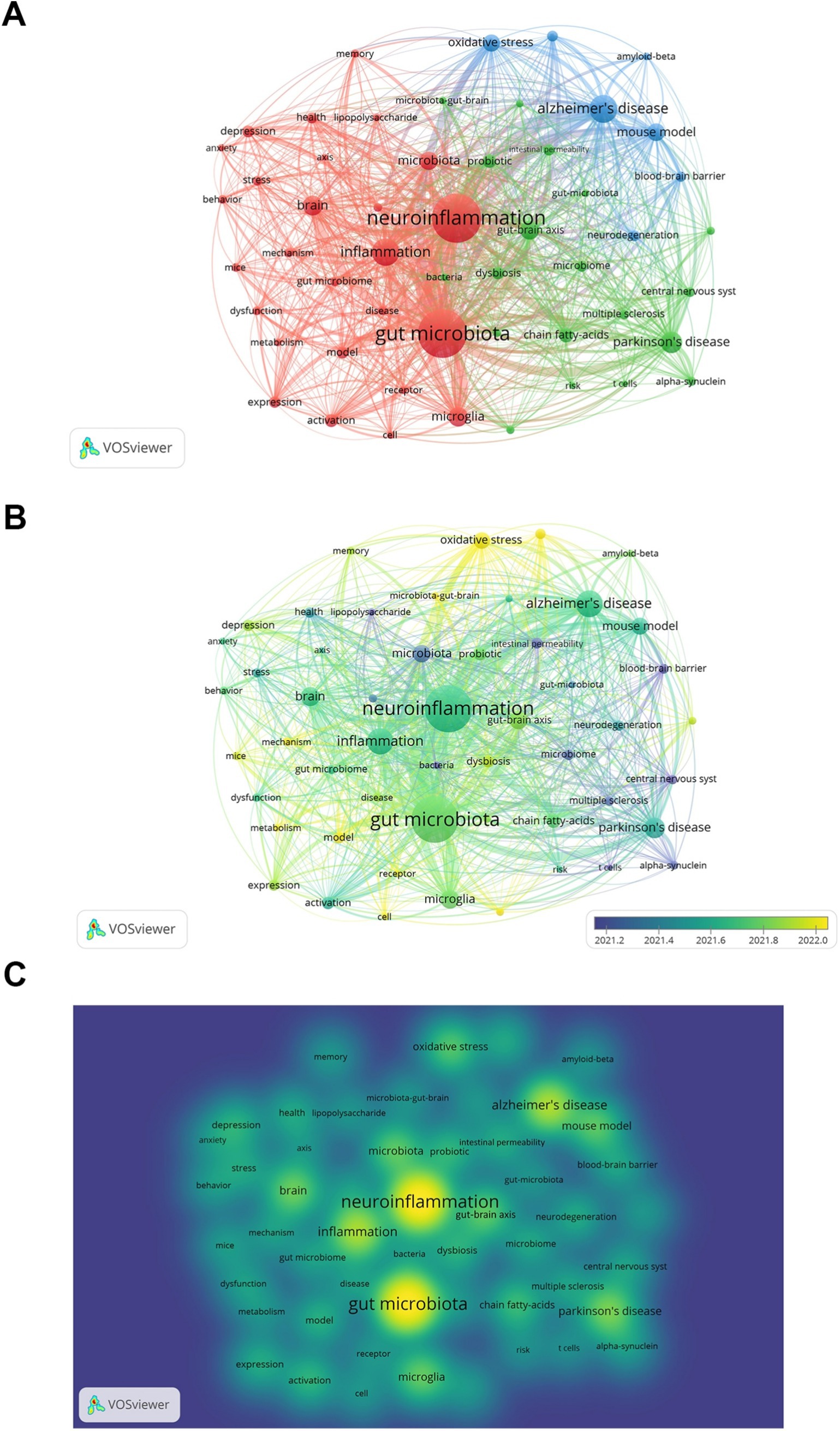
Figure 7. Keyword co-occurrence analysis. (A) Keywords network visualization. (B) Keywords network visualization with overlaid time. Color represents average time. (C) Keywords network density map.
3.6.2 Keywords clustering and timeline graph analysis
Keyword clustering and temporal line analysis are the prominent features of CiteSpace allowing us to have a comprehensive understanding of the hotspots and trends in related research fields. The software parameter settings are as follows: Select the top N (N = 50) threshold value setting method set the temporal slice to 1 year cluster high-frequency keywords and choose the log-likelihood ratio (LLR) method for clustering marking to generate a clustering view of high-frequency keywords. The statistical results indicate that the Q value is 0.8464 and the S value is 0.9414. With the Q value exceeding 0.3 and the S value surpassing 0.7 it shows that the visualized data is valid and reliable.
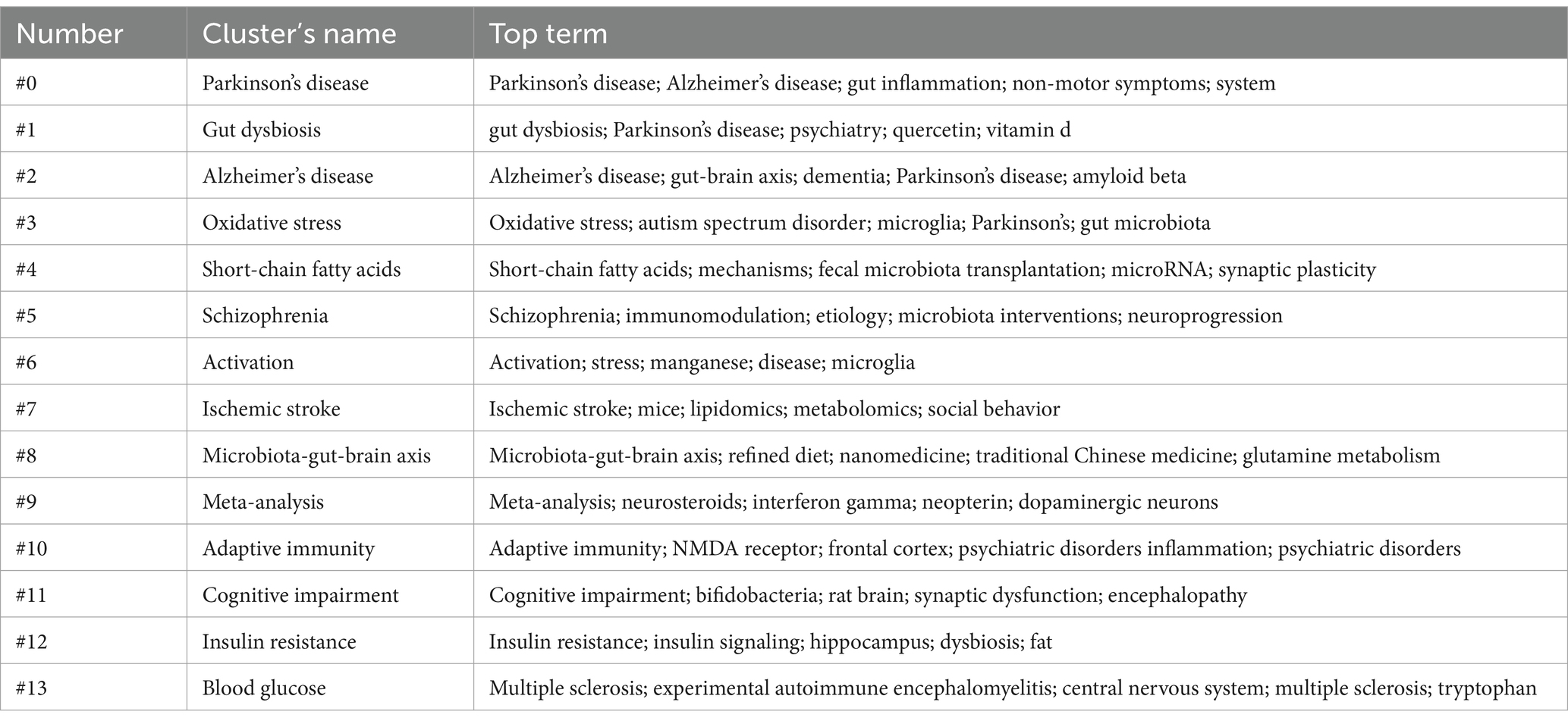
Using CiteSpace, a network of keyword co-occurrence was constructed consisting of 445 nodes and 1,608 links (Figure 8A). The software parameters were configured as follows. Time slices: ranging from 2000 to 2024, with each slice representing 1 year. Selection criteria: the top 50 terms with the highest frequency in each slice were selected. Eventually, 13 high-frequency keyword clusters were obtained (Figure 8A; Table 5).
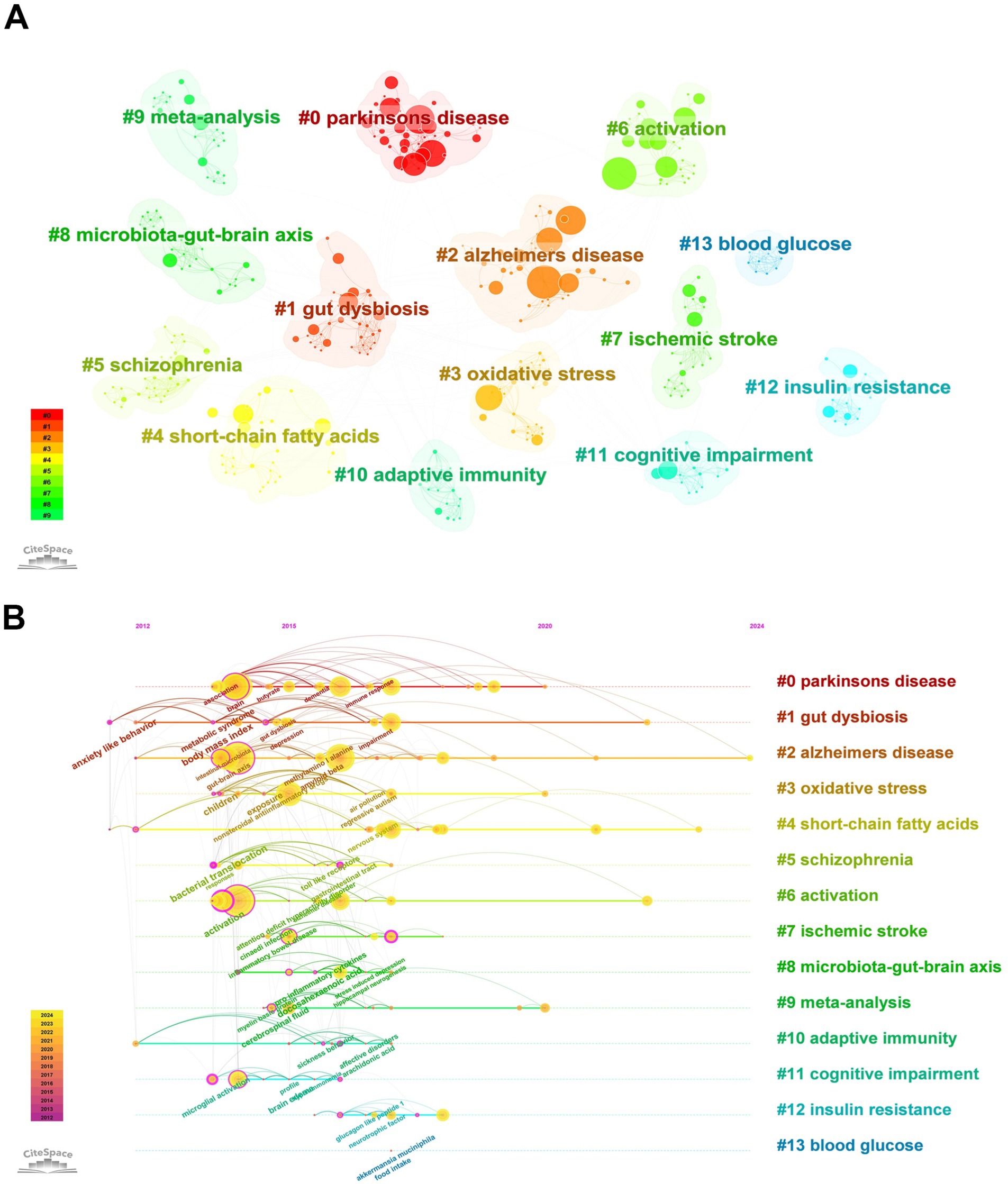
Figure 8. Keywords clustering and timeline graph analysis. (A) Cluster analysis of keywords. (B) Timeline view of keywords.
In order to more clearly identify the development context of the research on gut microbiota and neuroinflammation, the keywords were arranged in a chronological sequence and a timeline graph of the keywords was drawn (Figure 8B). Each node represents a distinct research keyword. The size of the node indicates the frequency of the keywords’ occurrence, and the lines represent co-occurrence. The results of keywords clustering from the literature offer us some clues: there are multiple research hotspots in the field of neuroinflammation related to the gut microbiome, such as “Parkinson’s disease,” “Alzheimer’s disease,” “oxidative stress,” “short-chain fatty acids,” and “schizophrenia.”
3.6.3 Keywords burst analysis
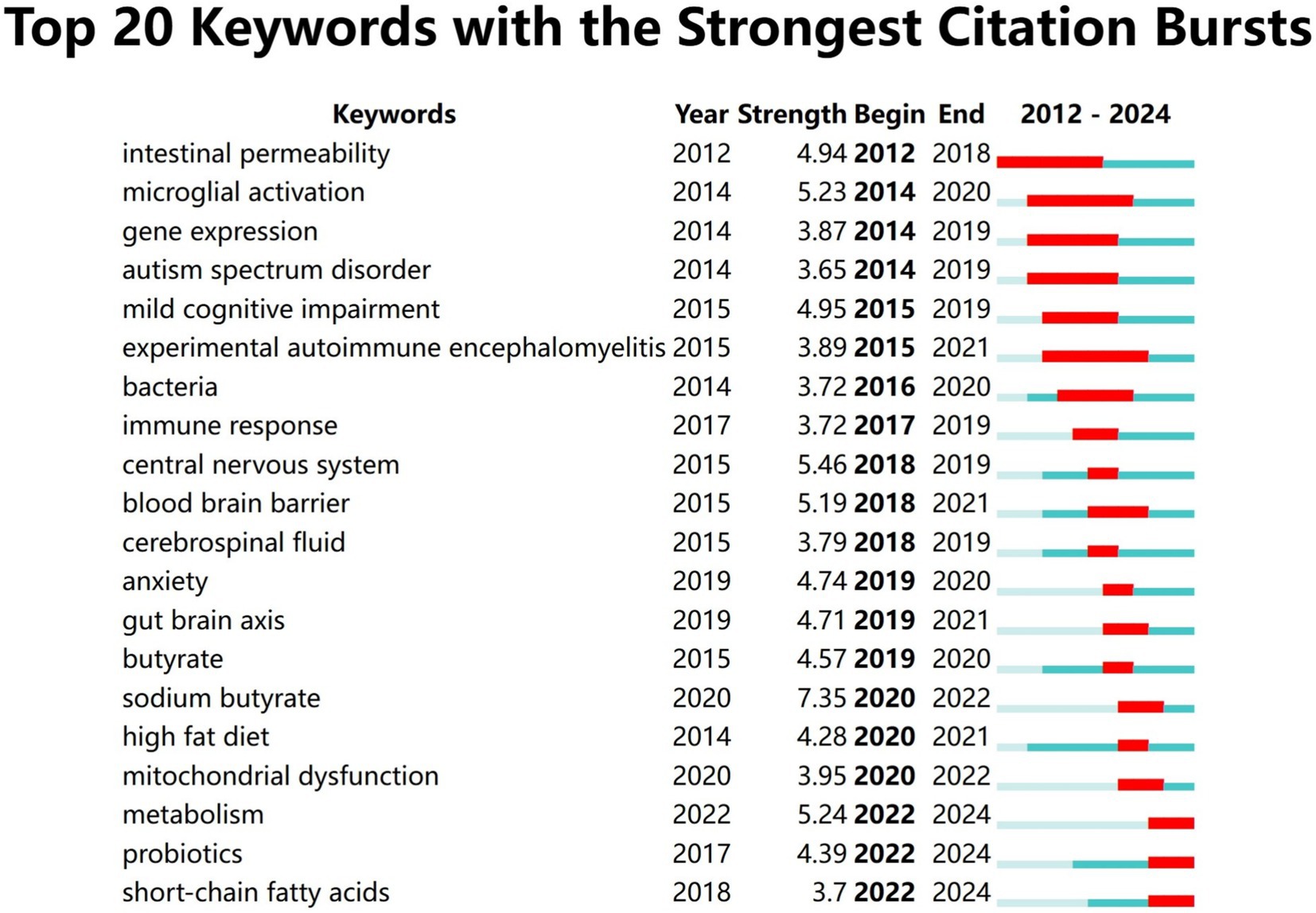
Keywords burst analysis can assist us in comprehending the rapidly escalating research interests or emerging research subjects in a specific field within a particular period and might indicate future research tendencies. We selected the top 20 burst keywords based on burst intensity (Figure 9). The keyword with the highest burst intensity is nitric oxide synthase. The earliest and latest years of keyword bursts are 2012 and 2022, respectively. The keyword with the highest ranking is “sodium butyrate (7.34)” which received extensive attention in 2020 and persisted until 2022. This reflects the researchers’ enthusiasm for exploring the potential role of sodium butyrate in the gut microbiota in neuroinflammation research during that period. The most recent keywords are metabolism (5.29) probiotics (4.43) and short-chain fatty acids (3.73) among which probiotics garnered widespread concern as soon as they emerged which may suggest future research directions.
4 Discussion
4.1 General information
Since 2000, the number of studies relating gut microbiota to neuroinflammation has presented a consistent upward trend, suggesting that this topic has garnered substantial researcher attention and is in a continuous state of development. Our study offers a comprehensive overview of the increasing interest in this area and identifies the most significant contributors across countries, institutions, journals, and authors. Figure 1 reveals that the number of citations for publications in 2020 peaked, followed by a rapid growth in the number of publications. From the perspective of citation burst analysis, this might be related to the fact that several authoritative journals published systematic reviews in 2020. These representative works integrated the progress in the field and provided theoretical frameworks for subsequent research, significantly increasing the citation volume of the literature that year. For instance, The Lancet Neurology published “The gut microbiome in neurological disorders,” which summarized the potential role of the gut microbiome in multiple neurological disorders over the past decade and pointed out the direction for future research and treatment strategies. The upsurge in research activities since 2020 can be attributed to an enhanced awareness of the role of gut microbiota in CNS-related diseases, which also represents a new milestone of research achievements in this domain.
China and the United States have made remarkable contributions to the research output in this field. China has published a greater number of papers, while the United States has engaged in more extensive inter-institutional collaboration. This might be related to various factors. In terms of policy orientation, China’s “Healthy China 2030 Program Outline” explicitly supports cutting-edge fields such as brain science and microbiome.1 The National Institutes of Health (NIH) of the United States has long funded interdisciplinary research on gut microbiota and neuroscience through the “Human Microbiome Project” (HMP) and the “Brain Research through Advancing Innovative Neurotechnologies” (BRAIN Initiative). In terms of the scientific research system, China’s scientific research system is good at concentrating resources to quickly produce results, for example, by accumulating data through large-scale sequencing projects (such as the “Ten Thousand Microbial Genomes Project”), promoting the growth of the number of papers. The American scientific research system, on the other hand, emphasizes cross-institutional collaboration, such as the “National Microbiome Initiative” (NMI) integrating resources from multiple universities, national laboratories, and enterprises. Regarding the authors, Kim, Dong-Hyun (n = 12) and Liu, Xuebo (n = 11) have published the largest number of papers, indicating their significant contributions to the field. Cryan, JF (n = 424), Sampson, TR (n = 394), and Erny, D (n = 345) are the most highly cited authors, serving as bridges in the field and exerting considerable academic influence. Although many authors have made substantial contributions to this research area, more efforts are necessary to enhance international and domestic institutional cooperation to expedite the solution of major scientific problems and achieve breakthrough progress jointly.
Regarding journals, the top 10 ones accounted for 22.29% of the total number of publications. Over 70% of the publications were presented in 433 periodicals, suggesting that the research topic goes beyond a single focus area and offers a broad perspective and resource selection. This includes top-tier journals like Cell and Nature, demonstrating the innovativeness and academic value of the research topic.
4.2 Hotspots and frontiers
Highly frequent co-citation references and keywords often reflect the research focus and direction in a specific field, providing valuable insights into important findings. We used key co-occurrence analysis to identify the main research focus and emerging trends in the study of the association between gut microbiota and neuroinflammation, and to clarify the evolution and dynamics of its subject structure.
4.2.1 Relationship between gut microbiota metabolism and neuroinflammation
According to Table 3, a study published by Sampson et al. (2016) in Cell in 2016 possesses the highest citation burst intensity, signifying its significant breakthrough and receiving substantial attention from relevant researchers. The study indicated that the gut microbiome regulates neuroinflammation and α-synuclein aggregation via a pathway that affects the nervous system, modulating neuroinflammation in PD models and uncovering the crucial relationship between the gut microbiome and the neuroinflammation pathology of Parkinson’s disease. The review article titled “THE MICROBIOTA-GUT-BRAIN AXIS” written by John F. Cryan et al. comprehensively reviews the research progress of MGBA. It discusses how the gut microbiota affects brain functions and behaviors through multiple pathways, thereby influencing the host’s physiological, behavioral and cognitive functions, and playing a significant role in various neuropsychiatric disorders (Cryan et al., 2019). This study not only provides a theoretical basis for understanding how the gut microbiota affects neuropsychiatric health, but also offers a new perspective for future research directions. The paper titled “Host microbiota constantly control maturation and function of microglia in the CNS” published by Daniel Erny in Nature Neuroscience investigated the influence of the host microbiota on microglia in the CNS. It was discovered that the microbiota regulates the maturation and function of microglia through metabolites like SCFAs. The absence of the microbiota would result in abnormal microglia, while reintroducing a complex microbiota could partially restore their normal characteristics.
MGBA plays a vital role in facilitating bidirectional communication between the gut microbiome and the brain, being capable of maintaining and promoting beneficial microorganisms, which are indispensable for maintaining normal functions (Grosso, 2021; Margolis et al., 2021). MGBA involves complex interactions between multiple systems, including the immune system related to the gut (Minaya et al., 2024), the enteric nervous system (Luesma et al., 2024), the neuroendocrine system (Yu et al., 2024), and the gut microbiome itself. These interactions are promoted by the gut microbiome, which generates crucial compounds such as neurotransmitters (Wu et al., 2024), tryptophan (Gu et al., 2024), and SCFAs (Shen et al., 2024), etc. These compounds simultaneously exert influences on the functions of both the gut and the brain. Furthermore, the metabolites of the gut microbiome, for instance, SCFAs, have been verified to be capable of traversing the BBB and thereby influencing the neurochemical milieu of the brain (Mann et al., 2024; Uceda et al., 2023). The gut microbiome assumes a key role in preserving the integrity of the intestinal barrier, facilitating the prevention of the infiltration of pathogens and toxins and thereby forestalling the occurrence of systemic inflammation. Nevertheless, compromised intestinal barrier function can result in augmented intestinal permeability, namely the so-called “leaky gut, “enabling harmful substances to enter the bloodstream and subsequently impact brain health (Aburto and Cryan, 2024).
These findings further substantiate the notion that the gut microbiome plays a crucial role in regulating neuroinflammation. Hence, a more profound comprehension of the interactions between the gut microbiome and neuroinflammation is of paramount importance for developing novel therapeutic strategies and preventive measures.
4.2.2 Neuroinflammation and other disorders related to the gut microbiota
Modern research has disclosed that alterations in the composition of the gut microbiome might influence neuroinflammation through diverse mechanisms, thereby facilitating the advancement of diseases such as AD, PD, and MS. From the results of cluster analysis, it can be seen that there are some common mechanisms underlying the association between gut microbiota and the pathological manifestations of neuroinflammation in these diseases. For instance, there are disorders of intestinal barrier function, the cross-action of oxidative stress (OS), and the bidirectional regulation of short-chain fatty acids (SCFAs). Some researchers have compared the fecal microbiome of AD patients to that of healthy controls and discovered substantial differences between the two. The dysregulation of MGBA could result in neuropathological alterations and cognitive dysfunction in AD patients, encompassing neuroinflammation, increased BBB permeability, and exacerbated Aβ deposition (Jin et al., 2023; Krishaa et al., 2023). It was further confirmed the connection between the abundance of gut bacteria and the markers identified in the cerebrospinal fluid of AD patients (Xie et al., 2023). The transplantation of the fecal microbiota from normal mice into AD model mice can ameliorate the cognitive function, Aβ pathology and hippocampal inflammation of these mice (Shen et al., 2020), further suggesting that the disorder of gut microbiota in AD patients is associated with the increased generation of cerebral amyloid beta proteins.
Studies have demonstrated that neuroinflammation and Oxidative Stress (OS) play a significant role in the neuronal damage of PD patients. The content of metabolites produced by gut microbiota can vary, which can influence neuroinflammation and OS through the MGBA and be involved in the progression of PD. For instance, one of the common endotoxins in gut microbiota, LPS, may enhance its penetration in the intestinal wall. This may be associated with neuroinflammation and OS in PD patients and may further exacerbate neuronal damage (Massaro Cenere et al., 2024). Additionally, certain gut bacteria can produce SCFAs, which play a crucial role in regulating immune responses and maintaining the integrity of the gut barrier (Silva et al., 2020). Changes in SCFAs levels may be associated with the neuroinflammatory state in PD patients, as they can affect the differentiation and function of T cells, thereby influencing the regulation of neuroinflammation (Gao et al., 2024).
MS is a chronic inflammatory disease that demyelinates the CNS (Kalia et al., 2024). In common animal models of MS, alterations in the microbiome have also been witnessed (Jangi et al., 2016). The intestinal microenvironment possesses the capacity to regulate the activation and differentiation of self-reactive T cells and direct them towards the CNS (Miljković et al., 2021). When the permeability of the intestine varies, the quantity of harmful or immunogenic antigens passing through the mucosa rises, which might result in persistent neuroimmune dysregulation (Camara-Lemarroy et al., 2018). Numerous clinical and experimental research findings indicate that intestinal microbial dysregulation might be involved in the occurrence of MS through multiple means, such as regulating the differentiation of T lymphocytes, enhancing the permeability of the BBB, and modulating immune factors (Fettig et al., 2024). At present, further fundamental research is still required to clarify the mechanisms underlying these correlations.
As the research advances, the influence of the gut microbiome and neuroinflammation on related diseases becomes more evident, offering a potential for the development of novel treatment strategies. Future research is required to further clarify the specific interaction mechanisms between the gut microbiome and neuroinflammation, as well as how to improve the clinical symptoms and prognosis of neurodegenerative diseases through intervention in the gut microbiome.
4.2.3 Treatments related to neuroinflammation and gut microbiota
The imbalance of specific microbiota may intensify the inflammatory response in the CNS, and subsequently facilitate the development of neurodegenerative changes (Wei et al., 2024). Probiotics are live microorganisms that are beneficial to the human body and have been proven to play a crucial role in treating autoimmune diseases and regulating dysbiosis in the gut. For instance, supplementing with probiotics and dietary fiber has demonstrated potential anti-inflammatory and neuroprotective effects. Research has discovered that interventions utilizing specific strains of lactic acid bacteria and bifidobacteria have shown great prospects in correcting ecological imbalances, reducing neuroinflammation, and enhancing motor function in PD models (Domínguez Rojo et al., 2024).
Probiotic therapy also has a positive effect on regulating the relationship between stress, neuroinflammation, and irritable bowel syndrome (Bruce-Keller et al., 2018). These drugs include hormones such as serotonin, gastrin, and progesterone, ANS regulators such as beta-receptor blockers, lipid-lowering drugs such as statins, and gut microbiome regulators such as probiotics and antibiotics, which target different inflammation pathways in the brain and gut after trauma to reduce neuroinflammation (Azarfarin et al., 2024). These therapeutic drugs have a promising future in reducing inflammatory responses in the MBGA and improving neurocognitive abilities in patients with traumatic brain injury. The gut microbiome also plays a regulatory role in the motor deficits and neuroinflammation in PD models, with SCFAs playing a key role. The research results suggest that changes in the gut microbiome may represent a new risk factor for PD, and future possible treatment options may include targeting immune activation through the microbiome (Wang et al., 2024).
Fecal microbiota transplant (FMT) can improve the diversity and function of the gut microbiome. This therapeutic approach has shown positive effects in treating certain intestinal diseases, such as refractory Crohn’s disease (Kao et al., 2024) and recurrent Clostridioides Difficile Infections (CDI) (Ghani et al., 2024).
In the context of neuroinflammation, FMT may indirectly have a positive impact on neuroinflammation by affecting the host’s immune system and metabolic pathways. Research has found that SCFAs have significant differences before and after FMT treatment for CDI, which can significantly improve cognitive function, emphasizing the need for clinicians to be aware of the impact of FMT on MGBA and its potential as a treatment for dementia patients (Park et al., 2021). Additionally, Zhao et al. (2021) have discovered that FMT is capable of alleviating the damage of the BBB and suppressing neuroinflammation in the substantia nigra, thereby diminishing the injury of dopaminergic neurons. A double-blind, placebo-controlled, randomized clinical trial result shows that fecal microbiota transplantation (FMT) is safe for patients with Parkinson’s disease, but it does not provide clinically significant improvements (Scheperjans et al., 2024). Probiotics and FMT have shown potential in neuroinflammatory-related diseases, but current evidence mostly comes from small-scale trials and animal studies. Their application is still at the initial stage of research, and it is necessary to solve issues such as strain specificity, individualized responses, and long-term safety before they can be translated to clinical practice.
In recent years, the advantages of traditional Chinese medicine in regulating intestinal microbiota to improve neuroinflammation have gradually become apparent. In the field of research on single herbs, it has been found that low-fructose polygalactoside from Morinda officinalis can increase the abundance, diversity, and stability of beneficial bacteria, thereby influencing the shape of the intestine, the production of mucus, and the permeability of the intestinal mucosa. Additionally, it can improve OS state and downregulate the expression of Aβ1-42, thereby helping to slow down the pathological progression of AD (Chen et al., 2017). Rhein is capable of treating cognitive dysfunction in high-fat diet-induced mouse models of hyperlipidemia by regulating the homeostasis of the microbiome, inhibiting the accumulation of macrophages, counteracting neuroinflammation, and promoting the expression of BDNF (Wang et al., 2016). There are also studies that show that quercetin can significantly improve cognitive function when vitamin D levels are low, reduce the accumulation of Aβ, tau protein phosphorylation, and neuroinflammation, and this improvement may be related to enhancing the diversity and richness of the intestinal microbiome (Lv et al., 2018). In terms of compound research, traditional Chinese medicine, centering on the latest pathogenic mechanism theory of intestinal microbiota and neuroinflammation, has put forward traditional Chinese medicine compounds with multi-pathway and multi-target therapeutic effects, such as Jiedu Huayu Decoction (Zhang et al., 2019), Chaihu Shugan Powder (Hu et al., 2020), Danggui Shaoyao Powder (Pan et al., 2022) and Xiaoyao Powder (Hao et al., 2024). By regulating the microecology of the digestive tract, they directly or indirectly suppress inflammatory responses and activate the immune response in the brain, thereby improving neuroinflammation. Despite the potential of TCM in treating neuroinflammation, its mechanism of action is complex, involving multiple signaling pathways and multiple targets. Therefore, future research needs to further investigate the specific effects of TCM on the gut microbiome, as well as how these effects translate into therapeutic effects against neuroinflammation.
In conclusion, the relevant therapeutic modalities of the gut microbiome and neuroinflammation not only facilitate a deeper comprehension of the mechanisms of neuroinflammation but also offer novel strategies and targets for the prevention and treatment of neurodegenerative disorders. Future studies need to explore in depth the specific mechanisms between the microbiome and neuroinflammation and develop targeted microbiome modulation therapeutics, personalized medicine and precise nutrition will emerge as significant directions in the treatment of neurodegenerative diseases. Nevertheless, this research has certain limitations. For instance, the selection of a single WoSCC database and the time constraint might result in some information bias. Future research needs to break through the limitations of a single database and integrate PubMed, Scopus, clinical cohort data, and multi-omics (metagenomics, metabolomics, proteomics) information to comprehensively analyze the molecular network of microbiota-host interaction. At the same time, innovative research methods should be introduced, such as the incorporation of machine learning, complex network modeling, and causal inference methods, to reveal the causal relationship between the phased characteristics of neuroinflammation and the evolution of microbiota.
5 Conclusion
This study offers a visual overview of the research on the association between gut microbiota and neuroinflammation through bibliometric analysis, delving into their relationship, the mechanisms influencing the pathogenesis of related diseases, and the potential value of corresponding therapeutic approaches. This provides a rather comprehensive perspective for understanding the connection between gut microbiota and neuroinflammation and supplies ideas and references for future in-depth studies. The future should break through the limitations of a single database and introduce more innovative research methods to gain a deeper understanding of the interaction between gut microbiota and neuroinflammation, as well as their potential applications in disease treatment, thereby providing more precise guidance for clinical treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SW: Writing – original draft, Writing – review & editing. NC: Software, Visualization, Writing – review & editing. CW: Funding acquisition, Resources, Writing – review & editing. K-FS: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Special Project of the State Key Laboratory of TCM Dampness Syndrome Co-built by the Ministry and the Province (SZ2021ZZ01): Research on the origin and development of theory of dampness syndrome, Principal Investigator: HJ; National Funding Program for Postdoctoral Researchers (GZC20231929): Development of a Coronary Heart Disease Early Warning System Based on Tongue Sublingual Meridian Microcirculation Detection, Principal Investigator: CW; General Funding of China Postdoctoral Science Foundation (2024M762389): Construction of a Coronary Heart Disease Risk Prediction Model Based on Dynamic Sequential Signals of Tongue Sublingual and Nailfold Microvessels, Principal Investigator: CW; and Basic Research Business Fund for Outstanding Young Scientists of China Academy of Traditional Chinese Medicine (ZZ15-YQ-069): Research on Characteristics and Diagnostic Techniques of the Syndrome of Phlegm-Stasis Interlocking in Coronary Heart Disease, Principal Investigator: CW.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer WH declared a shared affiliation with the authors SW and K-FS to the handling editor at the time of review.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Aburto, M. R., and Cryan, J. F. (2024). Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota-gut-brain axis. Nat. Rev. Gastroenterol. Hepatol. 21, 222–247. doi: 10.1038/s41575-023-00890-0
Ashique, S., Mohanto, S., Ahmed, M. G., Mishra, N., Garg, A., Chellappan, D. K., et al. (2024). Gut-brain axis: a cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 10:e34092. doi: 10.1016/j.heliyon.2024.e34092
Azarfarin, M., Moradikor, N., Matin, S., and Dadkhah, M. (2024). Association between stress, Neuroinflammation, and irritable bowel syndrome: the positive effects of probiotic therapy. Cell Biochem. Funct. 42:e70009. doi: 10.1002/cbf.70009
Braniste, V., Al-Asmakh, M., Kowal, C., Anuar, F., Abbaspour, A., Tóth, M., et al. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 6:263ra158. doi: 10.1126/scitranslmed.3009759
Bruce-Keller, A. J., Salbaum, J. M., and Berthoud, H. R. (2018). Harnessing gut microbes for mental health: getting from Here to there. Biol. Psychiatry 83, 214–223. doi: 10.1016/j.biopsych.2017.08.014
Camara-Lemarroy, C. R., Metz, L., Meddings, J. B., Sharkey, K. A., and Wee, Y. V. (2018). The intestinal barrier in multiple sclerosis: implications for pathophysiology and therapeutics. Brain 141, 1900–1916. doi: 10.1093/brain/awy131
Chen, D., Yang, X., Yang, J., Lai, G., Yong, T., Tang, X., et al. (2017). Prebiotic effect of Fructooligosaccharides from Morinda officinalis on Alzheimer's disease in rodent models by targeting the microbiota-gut-brain Axis. Front. Aging Neurosci. 9:403. doi: 10.3389/fnagi.2017.00403
Chidambaram, S. B., Essa, M. M., Rathipriya, A. G., Bishir, M., Ray, B., Mahalakshmi, A. M., et al. (2022). Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle. Pharmacol. Ther. 231:107988. doi: 10.1016/j.pharmthera.2021.107988
Cryan, J. F., O'Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The microbiota-gut-brain Axis. Physiol. Rev. 99, 1877–2013. doi: 10.1152/physrev.00018.2018
Domínguez Rojo, N., Blanco Benítez, M., Cava, R., Fuentes, J. M., Canales Cortés, S., and González Polo, R. A. (2024). Convergence of Neuroinflammation, microbiota, and Parkinson's disease: therapeutic insights and prospects. Int. J. Mol. Sci. 25:11629. doi: 10.3390/ijms252111629
Erny, D., Hrabě de Angelis, A. L., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., et al. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 18, 965–77. doi: 10.1038/nn.4030
Fettig, N. M., Pu, A., Osborne, L. C., and Gommerman, J. L. (2024). The influence of aging and the microbiome in multiple sclerosis and other neurologic diseases. Immunol. Rev. 325, 166–189. doi: 10.1111/imr.13361
Gao, X., Fu, S., Wen, J., Yan, A., Yang, S., Zhang, Y., et al. (2024). Orally administered Ginkgolide C alleviates MPTP-induced neurodegeneration by suppressing Neuroinflammation and oxidative stress through microbiota-gut-brain Axis in mice. J. Agric. Food Chem. 72, 22115–22131. doi: 10.1021/acs.jafc.4c03783
Ghani, R., Chrysostomou, D., Roberts, L. A., Pandiaraja, M., Marchesi, J. R., and Mullish, B. H. (2024). Faecal (or intestinal) microbiota transplant: a tool for repairing the gut microbiome. Gut Microbes 16:2423026. doi: 10.1080/19490976.2024.2423026
Grosso, G. (2021). Nutritional psychiatry: how diet affects brain through gut microbiota. Nutrients 13:1282. doi: 10.3390/nu13041282
Gu, X., Fan, M., Zhou, Y., Zhang, Y., Wang, L., Gao, W., et al. (2024). Intestinal endogenous metabolites affect neuroinflammation in 5×FAD mice by mediating "gut-brain" axis and the intervention with Chinese medicine. Alzheimers Res. Ther. 16:222. doi: 10.1186/s13195-024-01587-5
Hao, W., Ma, Q., Wang, L., Yuan, N., Gan, H., He, L., et al. (2024). Gut dysbiosis induces the development of depression-like behavior through abnormal synapse pruning in microglia-mediated by complement C3. Microbiome 12:34. doi: 10.1186/s40168-024-01756-6
Hu, D., Liu, Y. Y., and Sheng, L. (2020). The effects of Chaihu Shugan powder on BDNF/Trk B signaling pathway and inflammatory markers in rats with post-stroke depression. Jiangsu J. Tradit. Chin. Med. 52, 78–81. doi: 10.19844/j.cnki.1672-397X.2020.08.028
Jangi, S., Gandhi, R., Cox, L. M., Li, N., von Glehn, F., Yan, R., et al. (2016). Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7:12015. doi: 10.1038/ncomms12015
Jin, J., Xu, Z., Zhang, L., Zhang, C., Zhao, X., Mao, Y., et al. (2023). Gut-derived β-amyloid: likely a centerpiece of the gut-brain axis contributing to Alzheimer's pathogenesis. Gut Microbes 15:2167172. doi: 10.1080/19490976.2023.2167172
Kalia, L. V., Asis, A., Arbour, N., Bar-Or, A., Bove, R., Di Luca, D. G., et al. (2024). Disease-modifying therapies for Parkinson disease: lessons from multiple sclerosis. Nat. Rev. Neurol. 20, 724–737. doi: 10.1038/s41582-024-01023-0
Kao, D., Wong, K., Jijon, H., Moayyedi, P., Franz, R., McDougall, C., et al. (2024). Preliminary results from a multicenter, randomized trial using fecal microbial transplantation to induce remission in patients with mild to moderate Crohn's disease. Am. J. Gastroenterol. doi: 10.14309/ajg.0000000000003196
Krishaa, L., Ng, T. K. S., Wee, H. N., and Ching, J. (2023). Gut-brain axis through the lens of gut microbiota and their relationships with Alzheimer's disease pathology: review and recommendations. Mech. Ageing Dev. 211:111787. doi: 10.1016/j.mad.2023.111787
Kumari, S., Dhapola, R., Sharma, P., Singh, S. K., and Reddy, D. H. (2023). Implicative role of cytokines in Neuroinflammation mediated AD and associated signaling pathways: current Progress in molecular signaling and therapeutics. Ageing Res. Rev. 28:102098. doi: 10.1016/j.arr.2023.102098
Luesma, M. J., López-Marco, L., Monzón, M., and Santander, S. (2024). Enteric nervous system and its relationship with neurological diseases. J. Clin. Med. 13:5579. doi: 10.3390/jcm13185579
Lv, M., Yang, S., Cai, L., Qin, L. Q., Li, B. Y., and Wan, Z. (2018). Effects of quercetin intervention on cognition function in APP/PS1 mice was affected by vitamin D status. Mol. Nutr. Food Res. 62:e1800621. doi: 10.1002/mnfr.201800621
Mann, E. R., Lam, Y. K., and Uhlig, H. H. (2024). Short-chain fatty acids: linking diet, the microbiome and immunity. Nat. Rev. Immunol. 24, 577–595. doi: 10.1038/s41577-024-01014-8
Margolis, K. G., Cryan, J. F., and Mayer, E. A. (2021). The microbiota-gut-brain Axis: from motility to mood. Gastroenterology 160, 1486–1501. doi: 10.1053/j.gastro.2020.10.066
Massaro Cenere, M., Tiberi, M., Paldino, E., D'Addario, S. L., Federici, M., Giacomet, C., et al. (2024). Systemic inflammation accelerates neurodegeneration in a rat model of Parkinson's disease overexpressing human alpha synuclein. NPJ Parkinsons Dis. 10:213. doi: 10.1038/s41531-024-00824-w
Mathias, K., Machado, R. S., Stork, S., Martins, C. D., Dos Santos, D., Lippert, F. W., et al. (2024). Short-chain fatty acid on blood-brain barrier and glial function in ischemic stroke. Life Sci. 354:122979. doi: 10.1016/j.lfs.2024.122979
Miljković, Đ., Jevtić, B., Stojanović, I., and Dimitrijević, M. (2021). ILC3, a central innate immune component of the gut-brain Axis in multiple sclerosis. Front. Immunol. 12:657622. doi: 10.3389/fimmu.2021.657622
Minaya, D. M., Kim, J. S., Kirkland, R., Allen, J., Cullinan, S., Maclang, N., et al. (2024). Transfer of microbiota from lean donors in combination with prebiotics prevents excessive weight gain and improves gut-brain vagal signaling in obese rats. Gut Microbes 16:2421581. doi: 10.1080/19490976.2024.2421581
Nuzum, N. D., Deady, C., Kittel-Schneider, S., Cryan, J. F., O'Mahony, S. M., and Clarke, G. (2024). More than just a number: the gut microbiota and brain function across the extremes of life. Gut Microbes 16:2418988. doi: 10.1080/19490976.2024.2418988
Pan, Y. F., Jia, X. T., Qu, M. Y., Fang, Y., and Ying, X. P. (2022). The impact of Danggui Shaoyao powder on learning and memory in SAMP8 mice through modulation of the IL-1β signaling pathway. Chin. J. Integr. Med. Cardio-Cerebrovasc. Dis. 20, 1969–1973. doi: 10.12102/j.issn.1672-1349.2022.11.009
Park, S. H., Lee, J. H., Shin, J., Kim, J. S., Cha, B., Lee, S., et al. (2021). Cognitive function improvement after fecal microbiota transplantation in Alzheimer's dementia patient: a case report. Curr. Med. Res. Opin. 237, 1739–1744. doi: 10.1080/03007995.2021.1957807
Sampson, T. R., Debelius, J. W., Thron, T., Janssen, S., Shastri, G. G., Ilhan, Z. E., et al. (2016). Gut microbiota regulate motor deficits and Neuroinflammation in a model of Parkinson's disease. Cell 167, 1469–1480.e12. doi: 10.1016/j.cell.2016.11.018
Scheperjans, F., Levo, R., Bosch, B., Lääperi, M., Pereira, P. A. B., Smolander, O. P., et al. (2024). Fecal microbiota transplantation for treatment of Parkinson disease: a randomized clinical trial. JAMA Neurol. 81, 925–938. doi: 10.1001/jamaneurol.2024.2305
Shen, H., Guan, Q., Zhang, X., Yuan, C., Tan, Z., Zhai, L., et al. (2020). New mechanism of neuroinflammation in Alzheimer's disease: the activation of NLRP3 inflammasome mediated by gut microbiota. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 100:109884. doi: 10.1016/j.pnpbp.2020.109884
Shen, H., Zhang, C., Zhang, Q., Lv, Q., Liu, H., Yuan, H., et al. (2024). Gut microbiota modulates depressive-like behaviors induced by chronic ethanol exposure through short-chain fatty acids. J. Neuroinflammation 21:290. doi: 10.1186/s12974-024-03282-6
Silva, Y. P., Bernardi, A., and Frozza, R. L. (2020). The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. (Lausanne) 11:25. doi: 10.3389/fendo.2020.00025
The Central People's Government of the People's Republic of China. The Central Committee of the Communist Party of China and the State Council have issued the "Healthy China 2030" Planning Outline. (2016). Available online at: https://www.gov.cn/zhengce/2016-10/25/content_5124174.htm (Accessed April 03, 2025).
Uceda, S., Echeverry-Alzate, V., Reiriz-Rojas, M., Martínez-Miguel, E., Pérez-Curiel, A., Gómez-Senent, S., et al. (2023). Gut microbial metabolome and Dysbiosis in neurodegenerative diseases: Psychobiotics and fecal microbiota transplantation as a therapeutic approach-a comprehensive narrative review. Int. J. Mol. Sci. 24:13294. doi: 10.3390/ijms241713294
Vogt, N. M., Kerby, R. L., Dill-McFarland, K. A., Harding, S. J., Merluzzi, A. P., Johnson, S. C., et al. (2017). Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 7:13537. doi: 10.1038/s41598-017-13601-y
Wang, S., Huang, X. F., Zhang, P., Wang, H., Zhang, Q., Yu, S., et al. (2016). Chronic rhein treatment improves recognition memory in high-fat diet-induced obese male mice. J. Nutr. Biochem. 36, 42–50. doi: 10.1016/j.jnutbio.2016.07.008
Wang, C., Yang, M., Liu, D., and Zheng, C. (2024). Metabolic rescue of α-synuclein-induced neurodegeneration through propionate supplementation and intestine-neuron signaling in C.Elegans. Cell Rep. 43:113865. doi: 10.1016/j.celrep.2024.113865
Wei, F., Jiang, H., Zhu, C., Zhong, L., Lin, Z., Wu, Y., et al. (2024). The co-fermentation of whole-grain black barley and quinoa improves murine cognitive impairment induced by a high-fat diet via altering gut microbial ecology and suppressing neuroinflammation. Food Funct. 15, 11667–11685. doi: 10.1039/D4FO02704C
Wu, Q., He, Q., Zhang, X., Chen, S., and Xue, X. (2024). Systemic modulators: potential mechanism for the 5-HT system to mediate exercise amelioration in Alzheimer's disease. Aging Dis. doi: 10.14336/AD.2024.0834
Xie, J., Bruggeman, A., De Nolf, C., Vandendriessche, C., Van Imschoot, G., Van Jieduhuayu, E., et al. (2023). Gut microbiota regulates blood-cerebrospinal fluid barrier function and Aβ pathology. EMBO J. 42:e111515. doi: 10.15252/embj.2022111515
Yu, W., Zhu, Z., and Tang, F. (2024). Emerging insights into postoperative neurocognitive disorders: the role of signaling across the gut-brain Axis. Mol. Neurobiol. 61, 10861–10882. doi: 10.1007/s12035-024-04228-y
Zhang, Y. L., Liu, Y., Xu, P. Y., and Wen, L. (2019). Investigating the mechanism of Jieduhuayu decoction in enhancing cognitive function in Alzheimer's disease mice through modulation of the brain-gut Axis. Chin. J. Pharmacol. Toxicol. 33:672.
Zhang, Y., Wang, H., Sang, Y., Liu, M., Wang, Q., Yang, H., et al. (2024, 2020). Gut microbiota in health and disease: advances and future prospects. MedComm 5:e70012. doi: 10.1002/mco2.70012
Zhao, Z., Ning, J., Bao, X. Q., Shang, M., Ma, J., Li, G., et al. (2021). Fecal microbiota transplantation protects rotenone-induced Parkinson's disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome 9:226. doi: 10.1186/s40168-021-01107-9
Keywords: gut microbiota, neuroinflammation, bibliometric analysis, research trends, neurodegenerative
Citation: Wu S, Chen N, Wang C and So K-F (2025) Research focus and trends of the association between gut microbiota and neuroinflammation. Front. Microbiol. 16:1564717. doi: 10.3389/fmicb.2025.1564717
Edited by:
Isaias Glezer, Federal University of São Paulo, BrazilReviewed by:
Iraj Alipourfard, Polish Academy of Sciences, PolandWenzhi Hao, Jinan University, China
Ziyun Li, Nanjing University of Chinese Medicine, China
Copyright © 2025 Wu, Chen, Wang and So. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kwok-Fai So, aHJtYXNrZkBoa3VjYy5oa3UuaGs=
†These authors have contributed equally to this work
 Shan Wu
Shan Wu Nanjie Chen
Nanjie Chen Chuanchi Wang2,4
Chuanchi Wang2,4 Kwok-Fai So
Kwok-Fai So