- 1Department of Anatomy, Cell Biology, and Physiological Sciences, Faculty of Medicine, American University of Beirut, Beirut, Lebanon
- 2High Council for Scientific Research and Publication (HCSRP), Islamic University of Lebanon (IUL), Khalde, Lebanon
- 3Department of Biological Sciences, Wayne State University, Detroit, MI, United States
- 4Faculty of Medical Sciences, Lebanese University, Beirut, Lebanon
- 5Department of Pathology and Laboratory Medicine, University of Miami Miller School of Medicine, Miami, FL, United States
Acinetobacter baumannii, a highly adaptive and formidable nosocomial pathogen, has emerged as a symbol of modern medicine's struggle against multidrug resistance (MDR). As a Gram-negative dweller in moist hospital environments, A. baumannii has proven its ability to colonize the most vulnerable—critically ill patients—leaving behind a trail of infections highlighted by high morbidity and mortality and rendering nearly all antibiotics ineffective. This literature review aims to provide an in-depth, comprehensive overview of microbiological features, virulence factors, clinical manifestations, epidemiology, and antibiotic resistance mechanisms of A. baumannii. It also highlights the different diagnostic approaches, possible treatment strategies, and infection control, as well as the profound public health burden this pathogen imposes. The genus Acinetobacter has undergone a pivotal taxonomic journey and categorization. In addition, the intricate virulence mechanisms and factors of A. baumannii, including but not limited to outer membrane components and nutrient acquisition systems, have contributed to its pathogenicity and severe clinical manifestations ranging from respiratory tract infections and meningitis to urinary tract infections, skin infections, and bloodstream infections. This review also describes the epidemiological trend of A. baumannii established by its global prevalence and distribution, risk factors, hospital-acquired vs. community-acquired infections, and its geographical variations. In terms of antibiotic resistance, this pathogen has demonstrated resilience to a wide range of first-line and last-resort antibiotics due to its different evasion mechanisms. The current diagnostic approaches, treatment strategies, and infection control measures are further analyzed in detail, underscoring the need for prompt and precise identification of A. baumannii to guide appropriate therapy and reinforce the optimal approaches to limit its transmission and control outbreaks. Finally, the review addresses the substantial public health implications, reflecting on the hindrance that A. baumannii brings to healthcare systems, and the urgent need for global surveillance, effective infection control protocols, innovative research, and therapeutic approaches to mitigate its global threat.
1 Introduction
In the labyrinth of microbial biology and pathology, Acinetobacter baumannii stands out as a puzzling challenge for modern healthcare systems. Dating back to the early 20th century, A. baumannii is recognized as a strictly aerobic, Gram-negative, non-fermenting, non-fastidious, non-motile, catalase-positive, and oxidase-negative bacterium (Almasaudi, 2018; Jung and Park, 2015). This organism is highly prevalent in moist hospital environments, where it is notorious for its propensity to colonize the skin of hospitalized patients, leading to nosocomial infections that account for over 20% of all hospital-acquired infections (HAIs) in intensive care units (ICUs; Ayobami et al., 2019).
In recent years, A. baumannii has been classified as one of the World Health Organizations' (WHO) critical priority pathogens due to its remarkable ability to develop resistance to multiple last-resort antibiotics. Even during the COVID-19 pandemic, the challenges posed by A. baumannii infections, particularly those resistant to carbapenems, intensified the burden on healthcare systems that were already overwhelmed by the virus outbreak (Kyriakidis et al., 2021; Thoma et al., 2022). Thus, it is imperative to have a comprehensive understanding of A. baumannii's epidemiology, virulence factors, clinical manifestations, and antibiotic resistance mechanisms.
A key factor contributing to the persistence and multidrug resistance (MDR) of A. baumannii is its genetic plasticity. This adaptability enables rapid genetic mutations and the integration of foreign genetic elements, facilitating swift adaptation to hostile hospital environments. Such genetic flexibility enhances its antibiotic resistance, virulence, and environmental resilience, making it particularly challenging to eradicate from healthcare settings (Kyriakidis et al., 2021; Scoffone et al., 2025).
Despite the extensive research on A. baumannii, many studies have taken fragmented approaches, focusing on either epidemiological trend, resistance mechanisms, or clinical manifestations in isolation. This review aims to provide a comprehensive and integrative analysis of A. baumannii, covering its epidemiology, genetic evolution, virulence factors, diagnostic approaches, therapeutic strategies, and infection control measures. By referring to recent findings, this review seeks to bridge existing knowledge gaps and offer a holistic perspective on the burden of A. baumannii on public health.
2 Microbiological features
The genus Acinetobacter has undergone an intriguing taxonomic evolution, with early challenges in classification due to a lack of distinctive morphological and biochemical features. In 1970, advancements in transformation and nutritional studies enabled the formal definition and classification of the family Neisseriaceae (Henriksen, 1976). Initially, Acinetobacter was classified based on a set of distinctive traits: lack of color, non-motility, inability to reduce nitrate, and non-fermentative characteristics (Cowan, 1938). Brisou and Prevot later characterized Acinetobacter as colorless, non-motile, saprophytic Gram-negative bacilli, irrespective of their oxidase activity (Brisou and Prevot, 1954). As research progressed, advancements in the medical field led to classifications based on the bacterium's oxidase activity, distinguishing between oxidase-positive bacteria, referred to as Moraxella, and oxidase-negative bacteria, referred to as Acinetobacter (Gerner-Smidt et al., 1991). By 1971, a clearer understanding of the genus began to emerge, although species were still not well established (Juni, 1972). Prior to the mid-1980s, based on available references, Acinetobacter calcoaceticus (A. calcoaceticus) was the sole recognized species, divided into two subspecies: var. anitratus and var. lwoffii, differentiated by their ability to ferment glucose (Juni, 1972; Bauman et al., 1978). With advancements in bacterial characterization methods, more species have been identified, and the genus classification has been expanded (Shelenkov et al., 2023).
Today, over 50 species of Acinetobacter have been identified, the majority of which are non-pathogenic. Among these, the A. calcoaceticus-baumannii (Acb) complex stands out as a phylogenetically pathogenic species, clinically prevalent in human infections (Touchon et al., 2014). This complex includes five pathogenic species: A. baumannii, Acinetobacter nosocomialis, Acinetobacter pittii, Acinetobacter seifertii, and Acinetobacter dijkshoorniae. along with non-pathogenic species A. calcoaceticus (Bouvet and Grimont, 1986; Nemec et al., 2011, 2015; Cosgaya et al., 2016). The taxonomic complexity within this complex has substantial clinical implications, especially in terms of antimicrobial resistance (AMR) and clinical outcomes. A. baumannii, the most clinically significant species within this complex, is notorious for its MDR, including resistance to last-resort treatments in severe infections (Holmström et al., 2022). The varying AMR profiles across the Acb complex underscore the importance of precise species identification in clinical microbiology. Accurate species-level identification, facilitated by advanced molecular techniques, is crucial for guiding clinicians in selecting the most appropriate therapy. Misidentifying species within the Acb complex can lead to inappropriate treatment, resulting in treatment failures and the further spread of resistant strains (Vijayakumar et al., 2019; Kim and Mun, 2025).
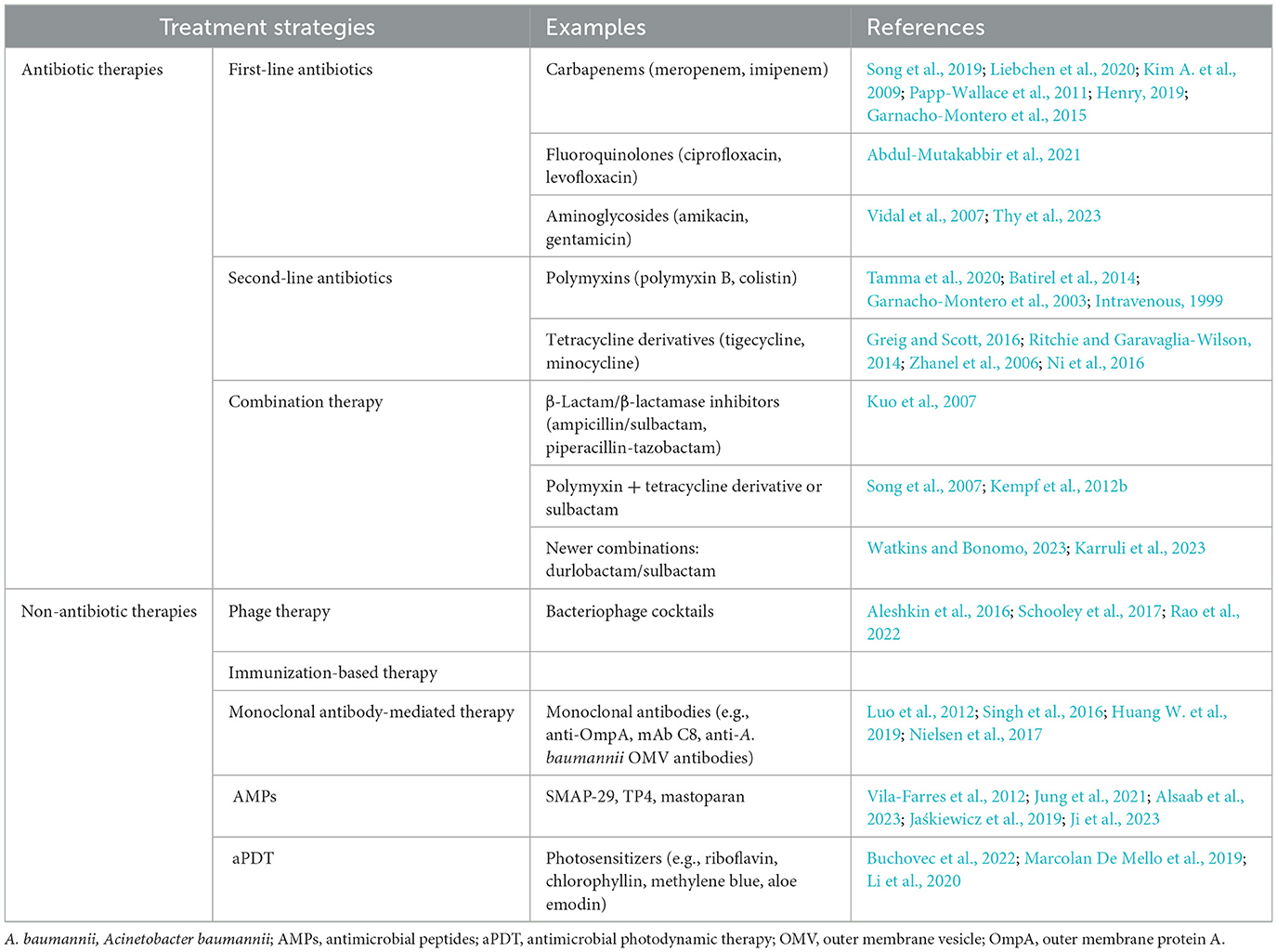
Molecular typing methods, particularly multilocus sequence typing (MLST), have become crucial for understanding the epidemiology of A. baumannii. MLST was proposed in the 20th century as the gold standard technology for Neisseria meningitidis, although this title later shifted to A. baumannii. MLST involves sequencing 400–500 bp internal fragments of typically seven housekeeping genes (gltA, gyrB, gdhB, recA, cpn60, gpi, and rpoD), providing a standardized method for characterizing bacterial isolates. Each species possesses characteristic alleles for each gene, and different combinations of alleles across the seven genes define a sequence type (ST; Bartual et al., 2005; Maiden et al., 2013). The MLST database, PubMLST.org, serves as a global repository for these sequences, offering valuable information for tracking the spread of resistant strains (Jolley et al., 2018).
Two MLST schemes, namely, Pasteur and Oxford schemes, were proposed for A. baumannii (Diancourt et al., 2010; Bartual et al., 2005). The Pasteur scheme includes cpn60, fusA, gltA, pyrG, recA, rplB, and rpoB genes, while the Oxford scheme utilizes gltA, gyrB, gdhB, recA, cpn60, gpi, and rpoD. Although three genes are common to both schemes, the Oxford scheme is more discriminant for closely related isolates, despite facing challenges such as gdhB gene paralogy, recombinations, and technical artifacts (Figure 1). In contrast, the Pasteur scheme appears to be less affected by homologous recombination and is better suited for accurate classification within clonal groups. As on 25 March 2023, the PubMLST database contained 2,262 sequence types for Pasteur profiles and 2,850 for Oxford profiles (Gaiarsa et al., 2019; Hamidian et al., 2017). However, the Oxford scheme remains less discriminative than the pulsed-field gel electrophoresis (PFGE) test, despite its reported significant role in resolving various outbreaks, including identifying the gpi and gyrB genes. Moreover, analysis using the Oxford scheme and PFGE of A. baumannii from different outbreaks and countries revealed sequence types with the same subdivision. In contrast, the Pasteur scheme revealed no variations between outbreaks (Tomaschek et al., 2016).
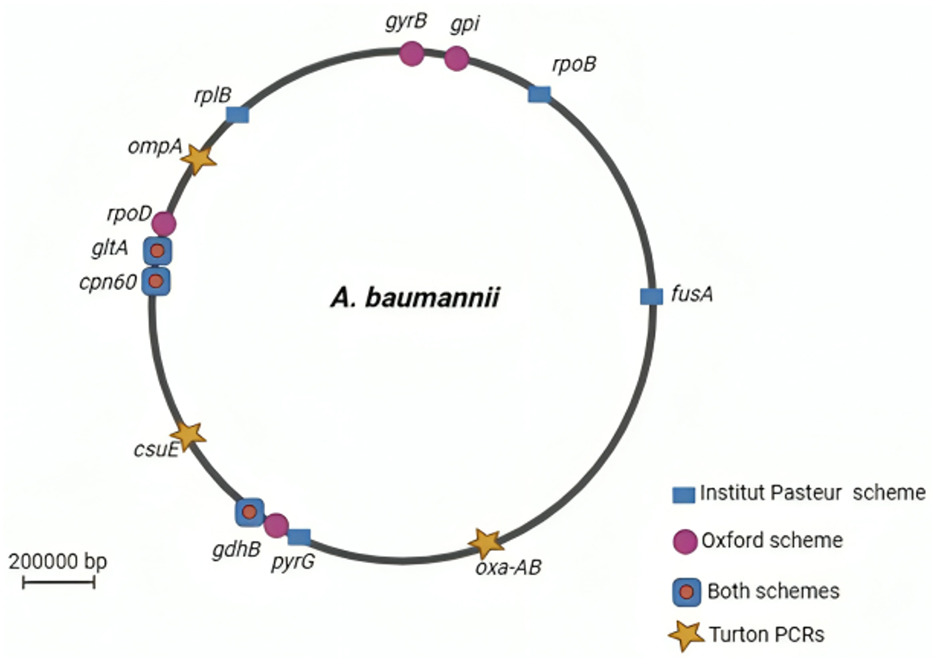
Figure 1. A circular map of the Acinetobacter baumannii chromosome depicting the locations of genes used in the Oxford and Institut Pasteur MLST methods. Stars (Turton PCRs) denote genes found by trilocus typing by PCR. Created in BioRender. ezz, z. (2025) https://BioRender.com/a0dpfdb.
Capsule synthesis loci (K, or KL loci) and lipopolysaccharide outer core loci (OCL) can significantly contribute to isolating typing and classification purposes (Wyres et al., 2020; Arbatsky et al., 2018). The capsule polysaccharide (CPS) gene cluster comprises approximately 30 genes, whereas the OC locus comprises only five genes. Each distinct gene cluster found between the flanking gene clusters is assigned a unique number, designating the locus type. CPS is a valuable epidemiological marker due to its role in bacterial virulence and susceptibility to phages. Similarly, the OCLs exhibit variation in A. baumannii. Like MLST, gene sequences and matching KL and OCL types are stored in a public database (Wyres et al., 2020; Cahill et al., 2022). Additionally, typing methods can be employed using dedicated software online or offline (Lam et al., 2022). This categorization suggests further epidemiological value, especially when combined with other systems like MLST, as there are only a few KL and OCL types for A. baumannii (237 and 22, respectively, as of March 20, 2023), compared to the number of MLST profiles (Tyumentseva et al., 2021).
3 Virulence factors and pathogenesis
3.1 Pathogenesis of A. Baumannii
3.1.1 Primary stage of infection
When discussing the methods and extent of pathogenicity of A. baumannii, emphasis should be placed on establishing contact through adherence or adhesion of the bacterium to the host's surface. Contact is thus a primary step for pathogenicity, preceding the activation of A. baumannii's virulence factors that facilitate substantial colonization (Falagas and Rafailidis, 2007).
Although considerable time may pass before the bacterium contacts its host, time does not pose a threat to A. baumannii, as this organism can survive for up to 5 months on inanimate surfaces (Kramer et al., 2006) and up to 9 days on hospital bed rails after the discharge of infected patients (Fournier and Richet, 2006). These extended survival times may explain the high rates of nosocomial cross-contamination (Pendleton et al., 2013), compensating for the bacterium's reduced capacity to adhere to cells, including mucosal cells, compared to other bacteria, such as Pseudomonas aeruginosa, Neisseria. meningitides, Campylobacter, Yersinia enterocolitica, and Helicobacter pylori (Asif et al., 2018).
Additionally, due to its hydrophobic properties, A. baumannii can increase the likelihood of establishing infection by attaching to foreign materials, such as plastics and catheters. Hydrophobicity refers to the bacterium's ability to adhere to surfaces that repel water. Research has shown that A. baumannii strains isolated from hospitalized patients tend to exhibit greater surface hydrophobicity compared to normal skin flora, which may enhance their ability to adhere to medical devices and cause infections (Peleg et al., 2008). A significant proportion of these organisms in hospitalized patients remain colonized rather than causing active infection. This suggests that A. baumannii strains in colonized patients have less pronounced hydrophobicity, making them less likely to adhere to host tissues or medical devices compared to strains in infected individuals (Fournier and Richet, 2006).
Moreover, once contact has been established, A. baumannii employs a “persist and resist” strategy, transitioning its growth mode from free-swimming to surface-oriented (Mea et al., 2021).
3.1.2 Motility
In the effort for A. baumannii to establish efficient cell-to-cell adhesion and cause disease, the bacterium must be in a free-moving state, employing various techniques to achieve motility, which is essential for targeting (Mea et al., 2021; Josenhans and Suerbaum, 2002). Indeed, a study by Skiebe et al. demonstrated that majority of the A. baumannii organisms exhibit some degree of motility on wet surfaces, which may explain the extensive colonization of the bacterium on hospital equipment (Skiebe et al., 2012).
A. baumannii and other Acinetobacter species can utilize a mode of motility called twitching movement, which involves using type-IV pili (TFP) to cause retraction, thus propelling the bacterial cell forward (Jarrell and McBride, 2008). Moreover, some A. baumannii species have shown both surface-associated movement (swarming motility), enabling movement on semi-solid surfaces (Barker and Maxted, 1975), and twitching motility (Skiebe et al., 2012; Eijkelkamp et al., 2011). Additionally, the swarming motility described by Barker and Maxted in 1975 (Barker and Maxted, 1975) is dependent on the pilT gene, with the loss of this gene resulting in a 54% decrease in organism motility but not abolishing it (Clemmer et al., 2011; Wilharm et al., 2013). Furthermore, among the genes known to constitute pili assembly, only pilR, pilS, pilT, and pilU are involved in twitching (Clemmer et al., 2011).
Furthermore, the extent of motility differs between isolates, where a study by Dahdouh et al. showed an increase in motility in Lebanese isolates compared to Spanish isolates (Dahdouh et al., 2018). Therefore, not all isolates can have the perfect conditions to thrive, and motility can be decreased according to the presence of iron-limiting conditions (Eijkelkamp et al., 2011), mutation transposon insertions in dat and ddc genes (Skiebe et al., 2012), amino acid substitutions in position 520 or 540 of the RpoB protein (Pérez-Varela et al., 2017), light, salinity, LPS, and other extracellular conditions (Mea et al., 2021; Josenhans and Suerbaum, 2002; Corral et al., 2020; Talà et al., 2019).
Collectively, this highlights a promising avenue for researching innovative therapeutic approaches to abolish motility.
3.1.3 Features related to A. baumannii's surface
Once A. baumannii identifies a suitable target, overcoming the repulsive forces between its surface and the target's surface becomes imperative (Mea et al., 2021). This is achieved through the establishment of initially weak and reversible hydrophobic interactions, which become long-lasting over time (Rosenberg et al., 2013).
Therefore, a crucial feature observed in A. baumannii is cell surface hydrophobicity, enabling the bacterium to access hydrocarbon sources (Krasowska and Sigler, 2014) and adapt to new environments (Mea et al., 2021). Furthermore, numerous studies emphasize the role of cell surface hydrophobicity in biofilm formation on medical equipment, thereby enhancing adherence ability (Pour et al., 2011).
A proposed characteristic of A. baumannii's surface is its ability to modulate hydrophobic and hydrophilic interactions according to its requirements, effectively utilizing the repulsive and attractive forces necessary for subsequent adhesion to and invasion of the host (Khelissa et al., 2017; Vij et al., 2020).
3.1.4 The adherence and initiation of an infection
A critical transcriptional change must occur for A. baumannii to tightly adhere to its new habitat, wherein the bacterium downregulates motility-associated genes and upregulates biofilm-forming genes (Rumbo-Feal et al., 2013). This transition is essential for shifting from a free-moving state to a surface-colonizing state (Mea et al., 2021). Several studies have emerged to evaluate the role of pili in the adherence and colonization profile of A. baumannii, prompted by the widespread conservation of pili in all A. baumannii isolates (Moriel et al., 2013; Tomaras et al., 2003).
For this reason, the csuA/B gene has been extensively studied, as it encodes for the CsuA/BABCDE-mediated pilus (Pakharukova et al., 2017), which is not as crucial for the direct attachment of A. baumannii to its target cells, but rather highly essential for biofilm formation on abiotic surfaces (de Breij et al., 2010; Lee et al., 2006).
A biofilm-associated protein (Bap) has been identified in A. baumannii, contributing to biofilm production and differentiation into maturity phases (Brossard and Campagnari, 2012; Loehfelm et al., 2008). There is heterogeneity in this protein among A. baumannii strains, which may account for variations in biofilm formation effectiveness among these strains (De Gregorio et al., 2015). Studies have extensively investigated the role of this gene, revealing that Bap increases cell surface hydrophobicity, thereby enhancing attachment and adherence abilities (Brossard and Campagnari, 2012). Other proteins known as Bap-like proteins (BLP1 and BLP2) have also been isolated from different A. baumannii species and are believed to regulate adherence by reducing its effectiveness when co-expressed with the Bap protein (De Gregorio et al., 2015).
In addition, other factors contribute to the adhesion profile of this organism, such as the β-lactamase gene, which participates in biofilm formation (Lee et al., 2008; Bardbari et al., 2017; Yang et al., 2019), and the autotransporter adhesion (ATA) protein, which mediates bacterial adhesion to extracellular matrix (ECM) proteins (Bentancor et al., 2012a; Weidensdorfer et al., 2015).
It is noteworthy that the ATA protein elicits a severe and intensive immune response, highlighting its significant role in the infection process (Weidensdorfer et al., 2015; Thibau et al., 2020). Indeed, infection with A. baumannii can induce both known types of immune responses: the extensively studied innate immune response and the adaptive immune response, which requires further investigation for a comprehensive understanding (Morris et al., 2019).
During infection initiation, A. baumannii affects various cells of the innate immune system, including neutrophils, macrophages, natural killer (NK) cells, mast cells, and dendritic cells. In the early phase of infection, neutrophils play a crucial role by rapidly phagocytosing bacterial cells, employing defensive mechanisms that can take as little as 20 s to activate (Morris et al., 2019; Nordenfelt and Tapper, 2011). These defense strategies involve immunoglobulin G (IgG)-mediated opsonization, toll-like receptor (TLR) activation, and complement-mediated binding to neutrophil receptors (Nordenfelt and Tapper, 2011). Despite neutrophils' efforts within the first 4–24 h of infection (Bruhn et al., 2015; García-Patiño et al., 2017), A. baumannii can induce neutrophil scattering by binding to neutrophil surfaces in an interleukin-8 (IL-8)-dependent manner (Kamoshida et al., 2016).
Macrophages play a complex role in defense against A. baumannii, exhibiting a slower phagocytic response compared to neutrophils, which may take a minimum of 10 min and occurs at a slower rate (Morris et al., 2019; Qiu et al., 2012; Lázaro-Díez et al., 2017). Despite this, their contribution in the early stages of infection cannot be discounted, as they also play a role in recruiting neutrophils. In an in vivo study utilizing a mouse model of intranasal infection, early depletion of macrophages during infection has been shown to increase disease severity (Qiu et al., 2012).
Furthermore, A. baumannii has developed mechanisms to evade macrophage immunity, including inducing autophagocytosis of macrophages. This evasion strategy is facilitated by the OmpA protein, which acts on several signaling pathways, including the mitogen-activated protein kinase (MAPK)/c-Jun N-terminal kinase (JNK) signaling pathway (An et al., 2019) and the mammalian target of rapamycin (mTOR) pathway (Zhao et al., 2021).
In addition to phagocytic cells, NK cells also play a crucial role, although further investigation is needed to understand their contribution fully. In mice with A. baumannii pneumonia, depletion of NK cells has been shown to increase mortality rates, underscoring their importance (Tsuchiya et al., 2012). Mast cells, in contrast, secrete tissue necrosis factor-α and interleukin-8, which promote the function of neutrophils (Kikuchi-Ueda et al., 2017).
It is suggested that A. baumannii pathogenicity is highly dependent on this mechanism, as the pathogen may modulate OmpA expression to evade the T cell response, particularly T helper 1 cells, and hinder progression toward a more specific adaptive immunity characterized by lymphocyte proliferation and maturation (Lee J. S. et al., 2010).
3.2 Virulence factors of A. Baumannii
A. baumannii is equipped with various virulent factors that enable it to survive in hostile environments and evade the host's immune defenses. These factors contribute to its pathogenicity, making infections difficult to treat and control (Figure 2).
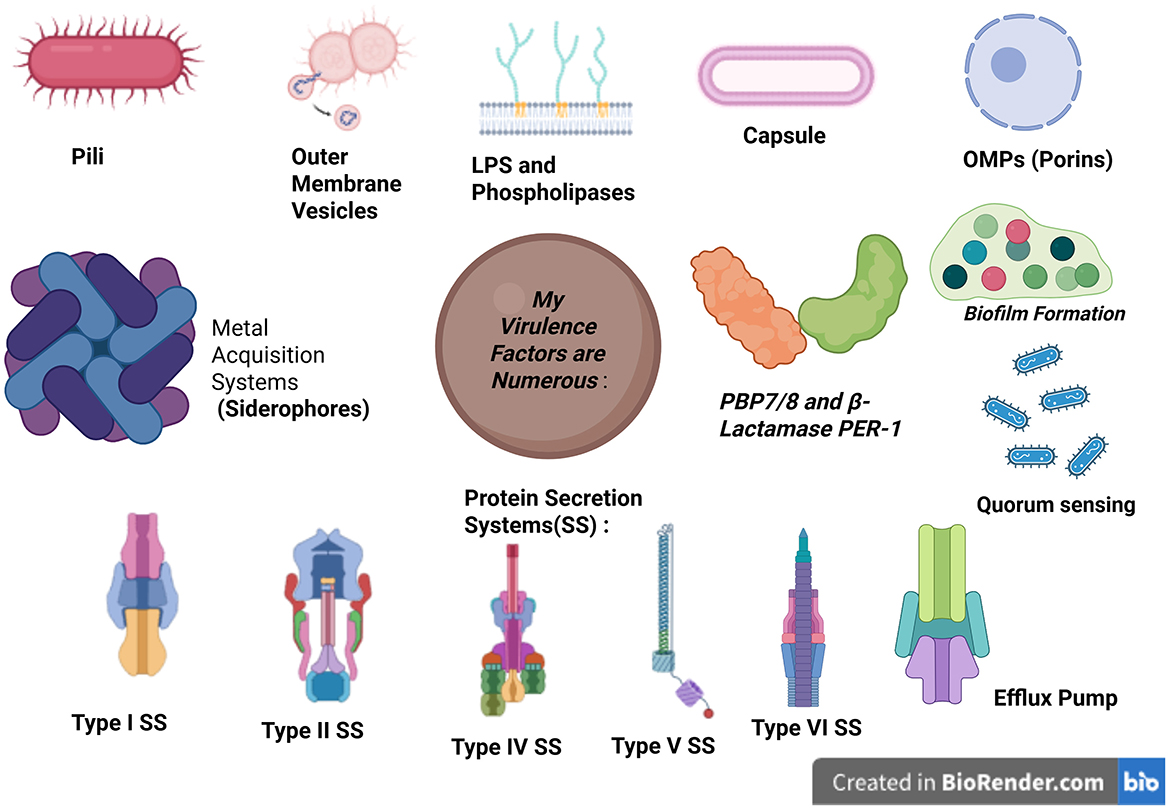
Figure 2. Schematic representation of the major virulence factors of Acinetobacter baumannii. Created in BioRender. b, a. (2025) https://BioRender.com/nflrr8d. OMPs, outer membrane vesicles.
3.2.1 Outer membrane components
3.2.1.1 Outer membrane proteins (porins)
Outer membrane proteins (OMPs), also known as porins, are found on the surface of the outer membrane of A. baumannii (Lee J. S. et al., 2010). These proteins facilitate the transport of solutes across the bacterium's membranes, with the outer membrane enabling for more diffuse transport compared to the inner cytoplasmic membrane. OMPs typically constitute approximately half of the outer membrane (Mea et al., 2021), and are significant virulence factors of A. baumannii, contributing to various aspects of its pathogenicity, including antibiotic resistance.
The first identified and majority of the well-known porin in A. baumannii is OmpA (previously called Omp 38), which is a β-barrel porin with a 2-nm pore diameter (Sugawara and Nikaido, 2012). OmpA exhibits low heterogeneity (Kim et al., 2016) and lower permeability compared to similarly sized porins in other bacteria, such as Escherichia coli, highlighting its significance in antimicrobial resistance (Sugawara and Nikaido, 2012; Kwon et al., 2019).
Furthermore, OmpA enables A. baumannii to attach to epithelial cells of the respiratory tract by binding to and interacting with fibronectin on the surface of lung cells (Smani et al., 2012a,b). Moreover, OmpA constitutes a significant portion of outer membrane vesicles (OMVs), which are another virulence factor in this bacterium, and can induce premature apoptosis of dendritic cells (Lee J. S. et al., 2010).
Additionally, OmpA can induce cytotoxicity in cells by binding to eukaryotic cell surface death receptors (Al Atrouni et al., 2016a). Upon internalization by healthy cells, OmpA translocates to either the mitochondria or the nucleus (Choi et al., 2005; Rumbo et al., 2014), where it activates Bcl-2 family proteins, leading to pro-apoptotic signals responsible for the release of cytochrome C and apoptosis-inducing factor (Choi et al., 2005). Moreover, A. baumannii's OmpA enables serum resistance by neutralizing factor H of the complement pathway, thus avoiding complement-mediated killing (Kim et al., 2016; Schweppe et al., 2015; Kim S. W. et al., 2009).
Furthermore, recently discovered porins, such as Omp34 (previously known as Omp33-36), act as fibronectin-dependent porins (Smani et al., 2012b), activating caspases to attach to and injure epithelial cells in the lungs, leading to apoptosis (Rumbo et al., 2014; Smani et al., 2013).
Another porin, CarO, facilitates carbapenem resistance in A. baumannii species and reduces the expression of nuclear factor-κB (NF-κB) pathway genes, weakening the host's immune response to the bacterium and promoting bacterial persistence (Sato et al., 2017; Zhang et al., 2019).
3.2.1.2 Pili
Pili, also known as fimbriae, play a crucial role in the motility of A. baumannii, as the bacterium lacks flagella. These pili enable the bacterium to perform twitching or surface-associated motility, which is essential for its ability to colonize surfaces and spread within its environment (Yao et al., 2023). Specifically, twitching movement is mediated by type-IV pili, which also play a role in DNA uptake (Corral et al., 2021). Additionally, pili function as virulence factors in A. baumannii. Specific pili, such as photoregulated type-I chaperone–usher pili and Csc pili, are responsible for biofilm formation and adhesion, ultimately inducing apoptosis of epithelial cells (Wood et al., 2018; Chen et al., 2022).
3.2.1.3 Capsule (capsular polysaccharides), lipooligosaccharide, and lipopolysaccharides
Lipopolysaccharides (LPS), lipooligosaccharides (LOS), and the capsule are all synthesized in the cytoplasm and later translocated to the outer bacterial surface (Morris et al., 2019).
LPS consists of three parts: an O-antigen repeat, the carbohydrate core, and a lipid-A anchor (Asif et al., 2018). In contrast, LOS lacks the O-antigen repeat and has an extended carbohydrate core (Whitfield and Trent, 2014; Powers and Trent, 2018).
LPS acts as a chemotactic agent that recruits inflammatory cells and induces them to release cytotoxic materials such as IL-6, TNF-α, IL-1β, and IL-8, primarily due to the presence of lipid A, which is immune-stimulatory (Powers and Trent, 2018). Although LOS biosynthetic genes are essential for A. baumannii's survival and prevention of toxic intermediate accumulation, A. baumannii can survive without the lipid-A component. Strains lacking lipid-A primarily stimulate toll-like receptor 2 rather than toll-like receptor 4, possibly due to increased outer membrane lipoprotein exposure (Moffatt et al., 2010, 2013).
A. baumannii bacteria containing LOS instead of LPS modify the lipid-A moiety to confer antimicrobial resistance (Boll et al., 2015).
The capsule functions as a protective barrier on the extracellular surface, providing protection from cationic antimicrobial peptides and preventing complement activation in about 33% of species with a capsule, thereby delaying phagocytosis (Kaplan et al., 1985). The highly variable capsule loci in A. baumannii's genome allow for flexibility in adaptation (Geisinger and Isberg, 2015; Hu et al., 2013).
The BfmRS two-component regulatory system negatively regulates the formation of the capsule in response to environmental stimuli, including specific antibiotics such as chloramphenicol and erythromycin. Increased expression of the capsule in A. baumannii is associated with higher virulence and pathogenicity, potentially complicating treatment and increasing antimicrobial resistance (Geisinger and Isberg, 2015; Chin et al., 2018).
3.2.1.4 Phospholipase
Phospholipases are enzymes responsible for the hydrolysis of phospholipids. A. baumannii bacteria possess two types of phospholipases: phospholipase C (PLC) and phospholipase D (PLD; Fournier and Richet, 2006).
These virulence factors exhibit different subtypes, with two PLC and three PLD enzymes encoded by the A. baumannii genome (Stahl et al., 2015; Fiester et al., 2016). Both PLC and PLD enzymes affect the stability of the epithelial cell membrane (Lee et al., 2017) and induce erythrocyte hemolysis by exerting cytotoxic effects, aiming to increase the ability to acquire iron from the environment (Morris et al., 2019).
A notable difference between the two types of phospholipases is the conservation of plc genes among different A. baumannii strains (Stahl et al., 2015; Jacobs et al., 2010). Inactivation of any two plc genes leads to a decrease in the cytotoxic effect on host cells (Fiester et al., 2016). On the contrary, the function of pld is more specific to bacterial resistance to antibodies and epithelial cell infectivity (Stahl et al., 2015; Jacobs et al., 2010). Deletion of PLD genes, including the more significant PLD2, only results in a partial decrease in virulence (Stahl et al., 2015).
3.2.1.5 Protein secretion systems
Five secretion systems have been found in A. baumannii, where they function to allow the production of proteins responsible for heterogeneous cellular functions (Figure 3).
Figure 3. Acinetobacter baumannii virulence associated with secretion systems and released effector proteins. The names of the main protein components for each secretion system are indicated, and outer membrane vesicles (OMVs) are also shown. OM, outer membrane; IM, inner membrane. Created in BioRender. ezz, z. (2025) https://BioRender.com/c5l6dl2.
3.2.1.5.1 Type-I secretion system
Type-I secretion system (T1SS) is a tripartite system composed of an inner membrane adenosine 5'-triphosphate (ATP)-binding protein, a periplasmic adaptor, and an outer membrane pore (Yao et al., 2023). This system delivers proteins from the cytosol to the extracellular environment (Morris et al., 2019), particularly the RTX protein and Bap, which play a major role in attachment to epithelial cells of the respiratory and urinary tract, along with biofilm formation and persistence (Noto et al., 2015; Skerniškyte et al., 2019; Harding et al., 2017).
3.2.1.5.2 Type-II secretion system
Since it consists of a complex of proteins, the type-II secretion system (T2SS) is structurally similar to type-IV pili (Korotkov et al., 2012). T2SS is responsible for transporting proteins from the periplasmic space to the outer cell membrane or extracellular space (Korotkov et al., 2012; Harding et al., 2016). This translocation occurs in two steps: first, proteins are moved to the periplasm and then secreted through T2SS (Korotkov et al., 2012). The significance of this secretion system lies in the types of proteins it transports, including lipase A and H, which break down long-chain fatty acids (Harding et al., 2016; Johnson et al., 2015), and the γ-glutamyl transferase enzyme (GGT), which induces apoptosis, inhibits CD4+ T cells, and enhances colonization by resisting antibiotics (Geisinger et al., 2019; Elhosseiny and Attia, 2018; Elhosseiny et al., 2019, 2020).
3.2.1.5.3 Type-IV secretion system
The type-IV secretion system (T4SS) in A. baumannii mediates the conjugative transfer of DNA, plasmids, and additional mobile genetic elements, which is critical for spreading drug-resistant genes among organisms, particularly the OXA-23 gene (Ayoub Moubareck and Hammoudi Halat, 2020; Liu et al., 2014; Smith et al., 2013).
3.2.1.5.4 Type-v secretion system
Type-V secretion system (T5SS) is the most common and basic secretion system in Gram-negative organisms (Henderson et al., 2000). A. baumannii possesses only two of the five known subdivisions of T5SS (Bentancor et al., 2012a,b), namely, the type-Vb and type-Vc secretion systems (Elhosseiny and Attia, 2018; Pérez et al., 2017).
T5bSS includes the proteins AbFhaB, which allows adherence to integrin along with fibronectin, and AbFhaC, which facilitates the recognition and translocation of AbFhaB to the bacterial surface (Elhosseiny and Attia, 2018; Pérez et al., 2017). Moreover, research conducted in murine models indicated that the loss of Vb did not wholly diminish the virulence of A. baumannii (Pérez et al., 2017). T5cSS, which is also known as ATA, forms a trimeric autotransporter that facilitates binding to the ECM and basement membrane proteins, including collagen types I–V and laminin. Studies have revealed that the deletion of Ata reduces Acinetobacter's ability to form biofilms and significantly weakens bacterial virulence (Bentancor et al., 2012a).
3.2.1.5.5 Type-VI secretion system
Type-VI secretion systems (T6SS) are activated in A. baumannii under stress conditions, such as environmental nutrient depletion, bacterial damage, or competition from other bacteria (Yao et al., 2023; Hood et al., 2017). In A. baumannii, the T6SS specifically damages competing bacteria by secreting toxins, which may include peptidoglycan hydrolases, nucleases, or those targeting cell membranes (Morris et al., 2019; Elhosseiny and Attia, 2018; Fitzsimons et al., 2018). Studies have shown that in immunocompromised patients, A. baumannii's T6SS bactericidal activity increases, enabling this opportunistic pathogen to thrive without interference from other bacteria in the low-immunity state (Repizo, 2017).
3.2.1.5.6 Efflux systems
Efflux systems in A. baumannii, composed of bacterial efflux pumps (Morris et al., 2019), function alongside the bacterial capsule to respond to envelope stress (Damier-Piolle et al., 2008) by extruding toxic compounds and providing antibiotic resistance (Du et al., 2018). The inner membrane efflux pumps work in conjunction with OmpA to prevent antibiotics from exerting their effects in the periplasmic space (Smani et al., 2014). Six families of efflux pumps have been described in A. baumannii, including resistance nodulation cell division family (RND), small multidrug-resistance superfamily (SMR), ATP-binding cassette (ABC) family, major facilitator superfamily (MFS), multidrug toxic compound extrusion family (MATE), and Proteobacterial antimicrobial compound efflux family (PACE). Among these, four families of efflux pumps are associated with antimicrobial resistance in A. baumannii (Lin et al., 2014): RND, with the AdeABC efflux pump playing a significant role in resistance, SMR, MFS, and MATE.
3.2.1.5.7 Outer membrane vesicles
OMVs are nano- and sphere-shaped structures ranging from 10 to 300 nm and are produced by all Gram-negative bacteria, including A. baumannii (Roier et al., 2016). These vesicles are enriched with various cytoplasmic components, including proteases, LPS, phospholipids, and OmpA (Kwon et al., 2009), as well as superoxide dismutases, which they transport to host cells (Asif et al., 2018; Ellis and Kuehn, 2010). OMVs play a crucial role in bacterial communication, horizontal gene transfer, host-pathogen interactions, and immune evasion. A significant function of OMVs is their contribution to biofilm formation, which enhances A. baumannii's persistence in hostile environments and facilitates antibiotic resistance. Studies have shown that OMVs contain biofilm-associated proteins and polysaccharides that promote bacterial aggregation and adherence to surfaces, creating a foundation for robust biofilm development. Additionally, OMVs influence the structural integrity of biofilms by delivering extracellular DNA and enzymes that modify the biofilm matrix, enhancing its resilience against antimicrobial agents.
Beyond biofilm formation, OMVs intensify the immune response during A. baumannii infections, leading to exacerbated inflammation and cellular damage in lung tissue (Nho et al., 2015). A study by Li et al. demonstrated that A. baumannii strains producing more OMVs, particularly those containing higher concentrations of virulence factors, induced a stronger immune response and exhibited more significant pathogenic potential (Li et al., 2015). This suggests that OMVs facilitate colonization and contribute significantly to the bacterium's ability to evade immune clearance and establish persistent infections.
3.2.1.6 Penicillin-binding protein (PBP) 7/8 (PBP7/8) and β-lactamase PER-1
The pbpG gene encodes PBP7/8, a virulence factor of A. baumannii. The loss of this gene renders the pathogen vulnerable to the host's immune system in vitro and in vivo (Russo et al., 2009).
Another significant virulence factor is the β-lactamase PER-1, known for its extended spectrum compared to common β-lactamases. Strains producing PER-1 demonstrate strong cell adhesion, a trait lacking in PER-1-negative strains (Sechi et al., 2004). Therefore, PER-1 plays a crucial role in the cell-to-cell adhesion of A. baumannii.
3.2.2 Nutrient (metal) acquisition systems
3.2.2.1 Iron acquisition (mainly by siderophores)
Access to iron in the human body is highly restricted due to the limited solubility of active ferric iron in aerobic environments and chelation by molecules, such as hemoglobin, transferrin, and lactoferrin (Antunes et al., 2011b; Ferreira et al., 2016). To overcome this limitation, A. baumannii employs two iron scavenging mechanisms: direct uptake via receptors and transporters, or secretion of high-affinity iron chelator proteins known as siderophores (Eijkelkamp et al., 2011; De Léséleuc et al., 2014; Figures 4, 5). Among these, A. baumannii produces multiple siderophores, with acinetobactin particularly essential for virulence and infection. The absence of acinetobactin has been shown to impair the growth of A. baumannii, highlighting its critical role in bacterial survival and pathogenicity (Sheldon and Skaar, 2020; Bohac et al., 2019). Additionally, iron-rich environments have been observed to be crucial to promote increased expression of OmpA, a key virulence factor, further emphasizing the importance of iron acquisition in the pathogenesis of A. baumannii (Runci et al., 2019; Liu et al., 2021).
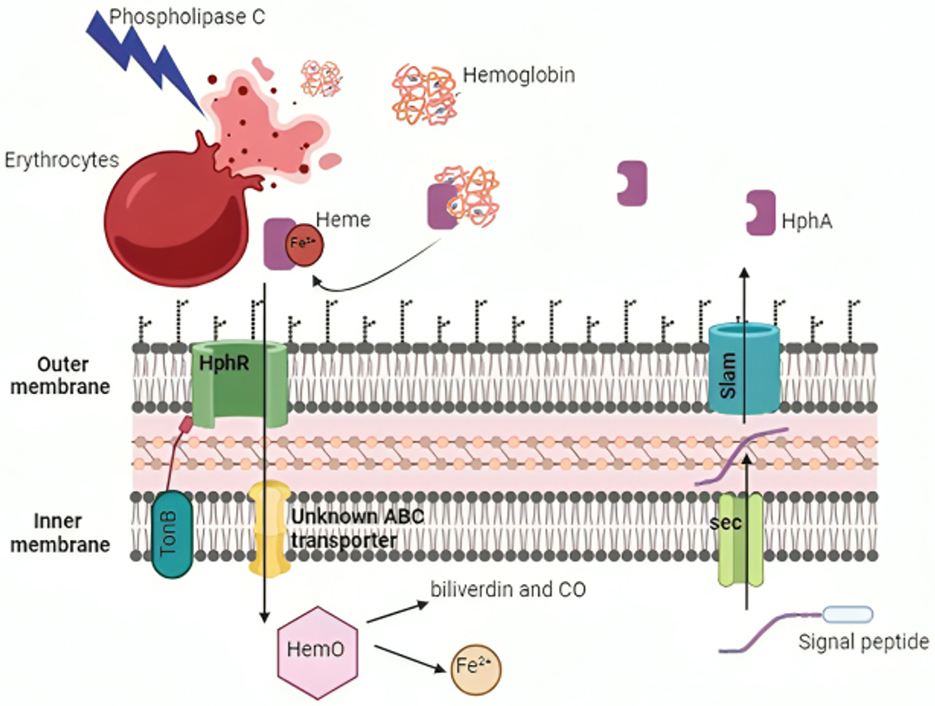
Figure 4. Mechanisms of iron acquisition and utilization by Acinetobacter baumannii. A. baumannii employs various strategies to obtain and utilize iron from the host. HphA is transported across the inner membrane into the periplasm via the Sec translocon and is then secreted into the extracellular medium by Slam (highlighted in blue). The bacterium's phospholipase C lyses host erythrocytes to extract heme, which is transported into the cell through the TonB-dependent HphR outer membrane receptor. The enzyme HemO liberates iron from heme for cellular use. Created with BioRender. Created in BioRender. ezz, z. (2025) https://BioRender.com/ies2m3c.
3.2.2.2 Zinc and manganese acquisition
A. baumannii relies on the import of zinc, crucial for its survival, through the transcription of the zinc uptake regulator (Zur) gene, which activates the ZnuABC transporter and ZigA GTPase. Additionally, the bacterium can export zinc via HutH activation and histidine catabolism, promoting zinc release by the ZigA gene (Mortensen et al., 2014; Nairn et al., 2016).
Similarly, manganese is another essential nutrient for A. baumannii, acquired through analogous mechanisms. Disruption of manganese abundance can impair bacterial colonization in the respiratory tract of the lungs (Juttukonda et al., 2016).
3.2.3 Community interactions
3.2.3.1 Quorum sensing
A. baumannii communicates intercellularly with neighboring cells, including other bacteria, by sensing changes in environmental conditions and responding accordingly (Bose and Ghosh, 2016). This communication is facilitated through the secretion of autoinducers, enabling the bacterium to gauge population density and environmental cues for adaptive responses. A. baumannii employs hormone-like molecules known as acyl-homoserine lactones as signaling molecules for both inter-species and intra-species communication, facilitating efficient biofilm formation through the expression of abaI and abaR genes (Niu et al., 2008), as well as regulating motility (Abraham, 2016; Bhargava et al., 2010; Rutherford and Bassler, 2012).
3.2.3.2 Biofilm formation
Biofilm formation is a critical survival strategy for A. baumannii, enabling it to thrive in harsh environmental conditions and develop resistance to antimicrobial agents (Mea et al., 2021). This protective process occurs in three distinct phases, including early development, matrix formation, and maturation (Hoyle and Costerton, 1991).
• Early Development: Initial adhesion of bacterial cells to surfaces.
• Matrix Formation: Production of extracellular polymeric substances, facilitating the formation of structured communities.
• Maturation: Formation of a fully developed biofilm with embedded bacterial populations, capable of withstanding host immune responses and antimicrobial agents.
Interestingly, A. baumannii strains capable of producing biofilms exhibit twice the survival rate under conditions of desiccation and nutrient depletion (Hoyle and Costerton, 1991). This is particularly concerning in healthcare settings, as biofilms commonly colonize medical devices such as catheters, endotracheal tubes, and prosthetic implants, leading to persistent infections (Gaddy and Actis, 2009). Moreover, biofilm-forming A. baumannii strains exhibit enhanced adherence to biomedical materials such as titanium and polystyrene, complicating infection control measures in clinical settings (Loehfelm et al., 2008).
Multiple factors contribute to biofilm formation, including Csu type-1 chaperone-usher pili, which are essential for early-stage biofilm development, promoting bacterial attachment and aggregation (Tomaras et al., 2003), the ATA, which mediates the tight adhesion of bacterial cells within the biofilm (Choi et al., 2009), and Bap and BLP, which regulate interbacterial interactions and surface attachment (De Gregorio et al., 2015).
One of the primary challenges in treating A. baumannii infections is the limited penetration of antibiotics into biofilms. The dense extracellular matrix acts as a physical barrier, preventing effective drug diffusion, while embedded bacteria remain in a low metabolic state, reducing their susceptibility to antibiotics that target actively growing cells (Greene et al., 2016). Additionally, biofilms provide an immune-privileged niche, shielding A. baumannii from host immune responses and enabling long-term persistence within hospital environments.
Given the pivotal role of biofilm formation in A. baumannii's pathogenicity, understanding its regulatory mechanisms is essential for developing targeted therapeutic interventions, such as biofilm-disrupting agents and anti-adhesive surface coatings for medical devices.
3.3 Metal acquisition
A. baumannii can modify its metabolic and nutritional requirements to adapt to the adverse host environment. A. baumannii, like other organisms, requires nutritional metals for survival. These necessary metals are generally iron, zinc, manganese, copper, magnesium, and nickel. They are co-factors in many basic cellular functions. Furthermore, A. baumannii exhibits broad tissue tropism and has evolved ways for acquiring nutritional metals in a variety of host habitats.
3.3.1 Iron
A. baumannii, like other bacterial pathogens, relies on iron acquisition mechanisms as crucial virulence traits to ensure its survival in a host. Almost all life on Earth depends on iron as a nutrient (Ilbert and Bonnefoy, 2013). It is necessary for the electron transport chain to produce energy as well as other metabolic activities, including enzyme activation of reactions, DNA replication, repair, and gene expression (Caza and Kronstad, 2013). Iron is typically a vital cofactor for bacterial enzymes involved in these processes, such as catalases, succinate dehydrogenases, and cytochromes (Jakubovics and Jenkinson, 2001). Additionally, it is necessary for ribonucleotide reductases that are involved in DNA replication, including NrdAB (Martin and Imlay, 2011). Given that A. baumannii has a nutritional need for iron, acquiring it from the iron-limited host environment is essential for the infection to progress (Frawley and Fang, 2014). This occurs through different mechanisms and strategies, including:
3.3.1.1 The uptake of heme via erythrocyte lysis
Many A. baumannii strains can get heme-bound iron, despite the fact that the majority of the iron in the human host is attached to heme groups inside hemoglobin molecules. Hemolytic proteins allow A. baumannii to reach iron pools within erythrocytes (Figure 4), and are encoded by hemolysin-related genes and two PLC genes (plc1 and plc2; Antunes et al., 2011a; Fiester et al., 2016). In a study by Fiester et al. (2016), horse erythrocytes coincubated with A. baumannii grown in iron-chelated media showed considerable cell lysis and damage to the cell membrane, but not when grown in iron-rich medium. The genes plc1 and plc2 may be implicated in the lysis of erythrocytes to obtain iron, as evidenced by their overexpression and correlation with iron limits. The absence of these genes in non-pathogenic Acinetobacter baylyi ADP1 strains, but their presence in all sequenced A. baumannii genomes, raises the possibility that plc1 and plc2 gene products play a role in virulence (Fiester et al., 2016).
Heme uptake systems are used by A. baumannii to extract iron from heme after lysing erythrocytes (Runyen-Janecky, 2013; Figure 4). Two heme uptake gene clusters were identified by genome analysis of clinical isolates of A. baumannii (Giardina et al., 2019). A periplasmic heme-binding protein, an inner membrane ABC transporter, and a TonB-dependent outer membrane receptor are all encoded by heme uptake cluster 1. A putative heme oxygenase (HemO), an extracytoplasmic function (ECF) sigma factor and its anti-sigma factor, and a TonB-dependent receptor are all encoded by heme uptake cluster 2. HphA, a Slam protein, is a crucial hemophore that the cell secretes throughout the heme import process (Bateman et al., 2021). It has two purposes: it binds hemoglobin and scavenges free heme (Bateman et al., 2021). A two-component receptor system that incorporates hemoglobin-bound iron into A. baumannii cells is formed when HphA transfers these iron sources to the HphR outer membrane receptor. For A. baumannii to reach its maximum virulence, this heme absorption mechanism is necessary (Bateman et al., 2021).
The heme oxygenase (abHemO) has been revealed to be necessary for heme absorption and utilization (Giardina et al., 2019). It has been demonstrated that in other organisms, HemO catalyzes the oxidative cleavage of heme to biliverdin and CO to liberate iron (Ratliff et al., 2001). In contrast to A. baumannii strain ATCC 17,978, which encodes fimsbactin siderophore gene clusters in place of hemO, one study showed that A. baumannii LAC-4, a hypervirulent and hyper-resistant strain, can effectively utilize iron bound to heme (Giardina et al., 2019). Western blotting revealed that LAC-4 was able to express the AbHemO protein and proliferate when given heme as the only iron source. The growth curves of ATCC 17,978 produced with heme as the only iron supply resembled those observed under low-iron circumstances, suggesting that this strain is unable to utilize the extracellular heme (Giardina et al., 2019).
Furthermore, the substrates that HemO can convert to biliverdin were examined in the same study. Extracellular heme served as the only source of iron for A. baumannii cells during growth. Isotope labeling of carbon allowed for the separation of extracellular heme from intracellular heme and for quantifying the relative amounts of each heme type in the medium. The findings supported HemO's uptake and metabolism of extracellular heme, and any intracellular heme discovered in the medium was assumed to be the consequence of heme turnover brought on by the preservation of iron homeostasis (Giardina et al., 2019).
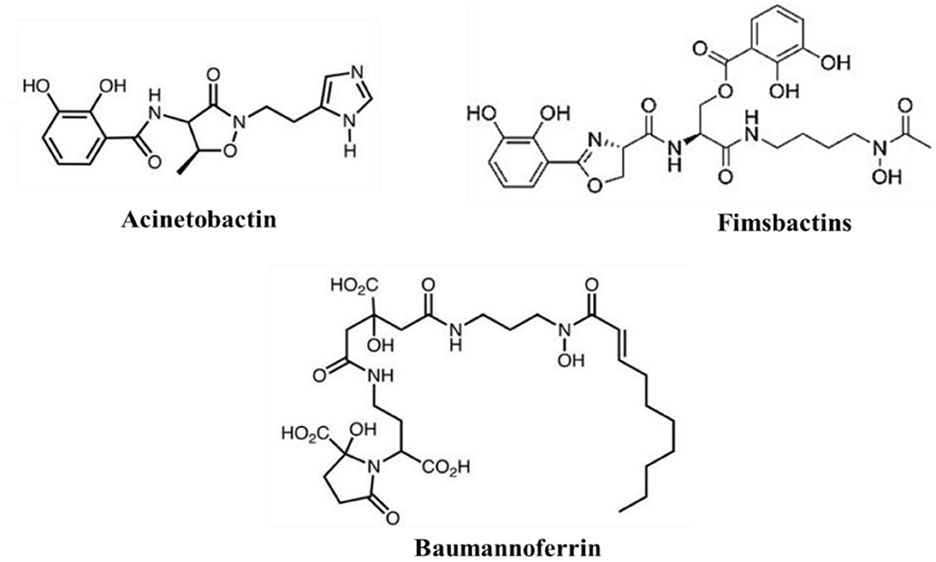
3.3.1.2 Siderophores
Siderophores are secondary metabolites secreted during the iron-acquiring process. They are advantageous because they permit iron to be scavenged extracellularly (Khasheii et al., 2021). Usually, iron-loaded siderophores bind a receptor and gain access to the bacterial cell (Paquelin et al., 2001). After additional processing, the iron is extracted and used as needed for vital physiological functions (Page, 2019). In A. baumannii, iron chelators siderophores are classified into three types: Acinetobactins, fimsbactins, and baumannoferrins (Figure 6; Page, 2019; Song and Kim, 2020). When iron levels are low, all three siderophore gene clusters are elevated, and siderophore synthesis is suppressed when more iron is added to the medium. The increase in gene expression caused by zinc depletion is 10–1,000-fold smaller than that caused by iron depletion; even so, this slight change in expression may indicate that, while siderophores are primarily iron-regulated, zinc can also partially regulate them in situations where metal constraints are sustained (Conde-Pérez et al., 2021; Sheldon and Skaar, 2020). Because studies have shown that deletion of one siderophore system does not significantly alter total siderophore activity in strains with many classes, it is assumed that the various siderophores have some redundancy. Cells can be sufficiently capable of chelating iron in vitro by expressing just one of these siderophore systems (Sheldon and Skaar, 2020).
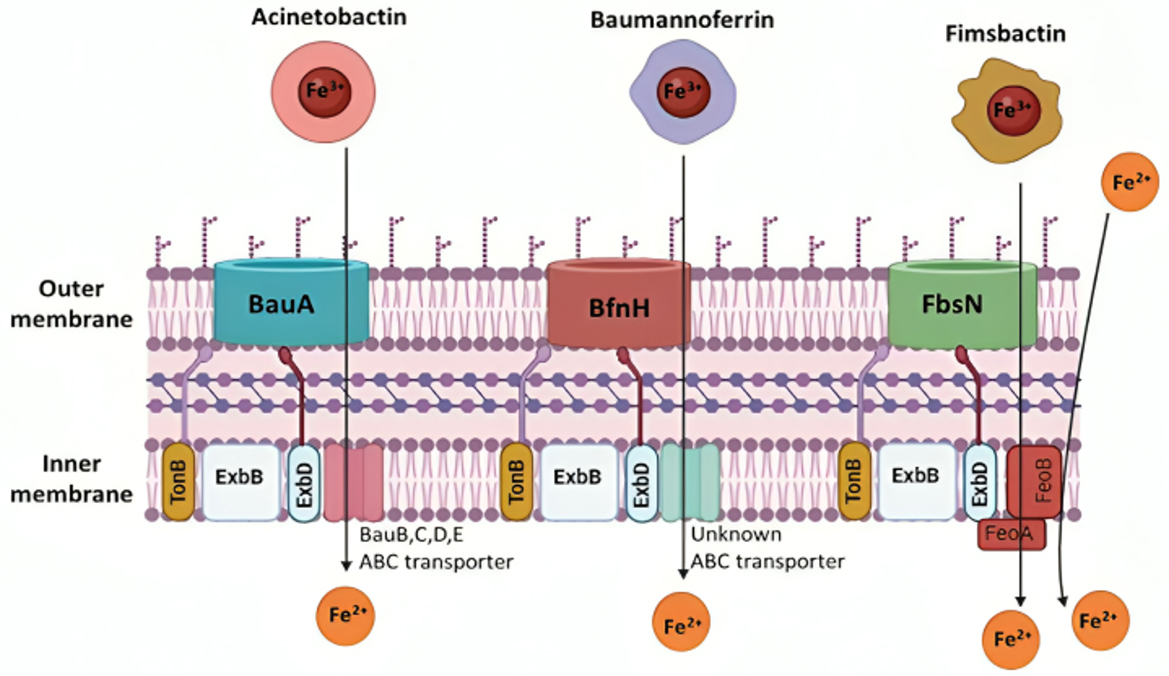
Figure 6. Iron transport mechanisms of Acinetobacter baumannii siderophores. The siderophore Acinetobactin has a high affinity for iron and utilizes an inner membrane ATP-binding cassette (ABC) transporter, consisting of BauB, BauC, BauD, and BauE, along with the BauA TonB-dependent outer membrane receptor, driven by proton-motive force. Baumannoferrin also exhibits a strong affinity for iron, transporting it through an inner membrane ABC transporter and the BfnH TonB-dependent outer membrane receptor, both using proton-motive force. Similarly, Fimsbactin transports iron using the FeoA transporter and the FbsN TonB-dependent outer membrane receptor, with both mechanisms relying on proton-motive force. The cytoplasmic membrane's FeoAB is essential for importing ferrous iron (Sheldon and Skaar, 2020). Created in BioRender. ezz, z. (2025) https://BioRender.com/pels3ql.
3.3.1.2.1 Acinetobactin
Clinical isolates of A. baumannii have a high degree of conservation in the versatile metal siderophore acinetobactin. Two isomers of acinetobactin exist: one is more active at neutral and basic pH levels, while the other is more active at acidic pH levels (Kim et al., 2021). All the genes in the acinetobactin gene cluster were revealed to be substantially elevated in low-iron environments using transcriptional profiling (Eijkelkamp et al., 2011). Research has demonstrated that the acinetobactin siderophore plays a significant role in iron uptake from host serum proteins such as lactoferrin and transferrin (Sheldon and Skaar, 2020). When cultivated in metal-chelated minimum medium containing human serum, mutants missing acinetobactin were seen to develop less rapidly than the fimsbactin and baumannoferrin mutants under the same conditions. The observed decreased growth was comparable to what was observed when the mutants were treated on human transferrin as their only source of iron (Sheldon and Skaar, 2020). A mouse infection model demonstrated the significance of acinetobactin in A. baumannii pathogenesis, as it was the sole siderophore determined to be critical for pathogenesis (Sheldon and Skaar, 2020). Acinetobactin plays a crucial role in the virulence of A. baumannii, as evidenced by the fact that it is the only expressed siderophore system and that it can survive in iron-limiting environments in both vertebrate and invertebrate hosts (Penwell et al., 2012).
Acinetobactin is required for intracellular A. baumannii infection, according to a study that used human alveolar epithelial cells. This was shown by the fact that acinetobactin mutant strains exhibited much lower intracellular persistence as compared to the wild-type ATCC 19,606 (Gaddy et al., 2012). Once more, alveolar epithelial cells were infected with these strains to track the rates of apoptosis in human cells. Compared to the wild type, acinetobactin mutants showed a 2-fold decrease in apoptosis rates, whereas acinetobactin receptor mutants showed a 24-fold decrease in apoptosis rates. This indicates that the acinetobactin siderophore system-expressing A. baumannii causes greater cell damage. The acinetobactin system is necessary for A. baumannii's pathogenicity in Galleria mellonella and mouse models, according to these infection assays (Gaddy et al., 2012).
Certain commensal bacteria may also be inhibited by acinetobactin. Knauf et al. observed that all Staphylococcus species and Corynebacterium striatum growth were considerably suppressed when co-plating A. baumannii ATCC 17,978 with common skin and nose commensals in iron-poor environments (Knauf et al., 2022). Acinetobactin production and transport genes were shown to make up a significant fraction of the genes implicated in the suppression of the Staphylococcus epidermidis library, with the use of a transposon mutant library utilizing A. baumannii AB5075 (Knauf et al., 2022). In comparison to wild-type A. baumannii, acinetobactin mutants also showed decreased suppression of Staphylococcus hominis and C. striatum. Acinetobactin is believed to be the primary siderophore responsible for this reported impact. However, other siderophores may also be involved in commensal suppression through iron competition (Knauf et al., 2022).
3.3.1.2.2 Baumannoferrin
Studying A. baumannii AYE led to the discovery of baumannoferrin. A mutation in entA prevented this strain from producing acinetobactin. Outside of the acinetobactin gene cluster, the entA gene plays a crucial role in the production of 2,3-dihydroxybenzoic acid, which is a prerequisite for acinetobactin. Even though A. baumannii AYE was unable to make acinetobactin; it was still able to thrive in low-iron environments (Gaddy et al., 2012). Upon closer inspection of A. baumannii clinical isolates, three gene clusters encoding siderophores were discovered. Acinetobactin was found to be encoded by one of these gene clusters. In contrast, a hydroxamate siderophore was found to be conserved in all of the isolates that were analyzed, including A. baumannii AYE (Antunes et al., 2011a). When it was discovered that elements of this putative gene cluster were increased in low-iron environments, it was assumed that the cluster—now known as baumannoferrin—was involved in iron acquisition (Eijkelkamp et al., 2011). According to baumannoferrin's characterization, this iron chelator comprises comprises citrate, decenoic acid, 1,3-diaminopropane, 2,4-diaminobutyrate, and α-ketoglutarate make up this iron chelator. It was discovered that baumannoferrin has two isomers: baumannoferrin A and baumannoferrin B, which are only different by one double bond. Genes required for baumannoferrin's production, transport, and intracellular uptake are found in the gene cluster encoding it (Penwell et al., 2012). It is believed that baumannoferrin is the only siderophore that A. baumannii AYE uses, demonstrating that it is adequate on its own for survival in conditions lacking in iron (Penwell et al., 2012). The virulence of A. baumannii AYE in G. mellonella is similar to that of other clinical isolates of A. baumannii, indicating that A. baumannii AYE does not require acinetobactin for pathogenesis. In comparison, A. baumannii ATCC 19,606 encodes all three siderophores, but to be pathogenic against G. mellonella and in mice, it still needs a functional acinetobactin system (Penwell et al., 2012).
3.3.1.2.3 Fimsbactins
A third class of siderophores called fimsbactins is present in fewer than 10% of sequenced A. baumannii strains (Bohac et al., 2019). Among these strains that possess fimsbactin are A. baumannii 6,013,150 and A. baumannii ATCC17978 (Proschak et al., 2013). Fimsbactins consist of one hydroxamate and two catecholates. Their iron-chelating motifs include putrescine and L-serine/threonine in their backbone. The principal siderophore is fimsbactin A, whereas fimsbactins B through F are assumed to be intermediates in the biosynthesis (Bohac et al., 2019). In an investigation aimed at demonstrating the siderophore properties of fimsbactin, A. baumannii ATCC 17,978 was cultured in a low-iron medium, enabling very slow development. It was observed that in a dose- and time-dependent manner, both fimsbactin alone and fimsbactin loaded with ferric iron enhanced cell proliferation (Bohac et al., 2019). Interestingly, this same study found that adding fimsbactin A counteracted the growth improvements brought about by the exogenous acinetobactin siderophore to the media. Pathway competition is a potential explanation for the antagonistic effect found (Bohac et al., 2019). The non-ribosomal peptide synthetase (NRPS) assembly systems are used to create both fimsbactin and acinetobactin. They sharethe precursors of L-threonine and 2,3-dihydroxybenzoic acid (DHBA; Proschak et al., 2013). Furthermore, it was discovered that the BauB periplasmic siderophore-binding protein (Bohac et al., 2019) is directly competed with by these two siderophores for binding. Conde-Pérez et al. investigated the acinetobactin and fimsbactin gene clusters. They discovered that every acinetobactin gene, except entA, has a possible redundant equivalent in the fimsbactin gene cluster (Conde-Pérez et al., 2021). This group postulated that fimsbactin would, therefore, serve as a substitute in the event that acinetobactin is rendered inactive, thereby elucidating the reason for the relative rarity of fimsbactin among isolates of A. baumannii (Conde-Pérez et al., 2021). Further research is necessary to determine the implications of the redundancy between acinetobactin and fimsbactin and any possible connections between baumannoferrin and these two siderophores.
3.3.1.2.4 Siderophores transport
The active process of siderophore- or heme-bound ferric iron transport over the outer membrane and into the periplasmic space is powered by the proton-motive force of the TonB-ExbB-ExbD protein complex (Figure 6; Noinaj et al., 2010). Through a brief transmembrane N-terminal region, TonB binds to ExbB and ExbD (Klebba, 2016). In vitro complex formation between TonB and the outer membrane protein FhuA requires a proline-rich spacer inside the TonB structure (Postle and Larsen, 2007). However, TonB function in vivo is unaffected by the removal of this spacer (Seliger et al., 2001). The presence of this outer membrane protein and the TonB complex in vivo requires more study.
The genomes of A. baumannii have been found to contain about 21 putative TonB-dependent outer membrane transporter genes, some of which are iron-regulated (Antunes et al., 2011b). Three coding genes for the TonB have been found and given the names tonB1, tonB2, and tonB3 (Zimbler et al., 2013). It has been demonstrated that the overexpression of the iron-regulated tonB3, which is overexpressed in iron-chelated as opposed to iron-rich medium (Eijkelkamp et al., 2011), is required for A. baumannii survival in iron-limiting conditions. It is also important for A. baumannii pathogenicity in G. mellonella insect models and mouse mammalian models (Eijkelkamp et al., 2011; Runci et al., 2019). Furthermore, A. baumannii ATCC 19,606 tonB1 tonB2 double mutants exhibit much reduced virulence in comparison to the original strain; yet, virulence remains unaffected by inactivating either tonB1 or tonB2 on its own (Zimbler et al., 2013). All of these results point to the importance of tonB3 in the transfer of iron chelators as opposed to the more minor roles that tonB1 and tonB2 play in this process (Zimbler et al., 2013).
Iron regulation is present in the TonB complex's tonB3 gene because of its Fur-controlled promoter. Fur binds to ferrous iron to form a repressor complex, which prevents transcription of Fur-controlled promoters when iron levels are high enough. When iron is deficient, apo-Fur binds to the promoter, activating transcription (Fillat, 2014). Additionally, it has been discovered that TonB transporters are more abundant in OMVs (Figure 6; Dhurve et al., 2022). All Gram-negative bacteria produce OMVs, which can transport iron and other nutrients (Dhurve et al., 2022). It was discovered that the OMVs of A. baumannii DS002 had 19 distinct TonB transporters. Seven of these transporters had structural characteristics found in other transporters involved in the translocation of siderophore-bound iron, according to an in silico study (Dhurve et al., 2022). This implies that OMVs are engaged in ensnaring siderophores from adjacent bacteria for A. baumannii to receive ferric iron (Dhurve et al., 2022).
Moreover, under iron-limiting circumstances, only tonB3 is upregulated among A. baumannii TonB systems, suggesting an association with iron homeostasis. Compared to the wild-type, tonB1, tonB2, and tonB1 tonB2 mutants of A. baumannii are less efficient at acinetobactin and iron transfer and are unable to grow in iron-starved environments. A tonB3 mutant has not been created, which may indicate that this gene is required for growth (Zimbler et al., 2013). Many bacteria express ferrous iron uptake systems in a reduced ferrous iron environment, most notably FeoAB transporters, which are required for iron acquisition and pathogenesis. FeoAB and its regulator FeoC are putative ferrous iron import systems encoded by A. baumannii, and all sequenced strains have at least one FeoB in addition to FeoA and FeoC (Mortensen and Skaar, 2013). In addition, a study that investigated the entire transcriptional response of A. baumannii to iron deprivation revealed that the main characteristic of this transcriptional response was the upregulation of three siderophore-mediated iron acquisition systems. Given the significant degree of overexpression of these systems in the presence of iron limitation, it is likely that each of them plays a crucial role in facilitating iron uptake and is hence essential for A. baumannii to survive in environments with low iron levels, like human hosts. Significant differences in expression were also seen for several genes related to other functions, including respiration and electron transport. They revealed that 463 genes had transcription levels that were more than 2 times higher under low iron conditions, with 95 of those genes having more than 4 times higher transcription levels. Significantly, there was a significant upregulation of three gene clusters related to siderophore production, including one unique cluster. The ferric uptake regulator (Fur) was found to have binding sites in the promoter regions of numerous upregulated genes, indicating that it plays a significant role in the iron acquisition response of Acinetobacter. Several motility-related genes were among the genes that were downregulated in response to decreased iron availability (Eijkelkamp et al., 2011). A. baumannii also modulates its outer membrane protein composition, with observed changes in proteins like OmpA, to optimize iron uptake. Proteins associated with iron storage (Bfr), energy and metabolic processes (AcnA, AcnB, GlyA, SdhA, and SodB), and lipid biosynthesis are among the many proteins represented by the iron-induced protein spots. Identifying an iron-regulated Hfq ortholog suggests that Fur and short RNAs, as reported in other bacteria, may mediate iron regulation in this bacterium. The reduced capacity of an OmpA isogenic deficient derivative to grow in iron-chelated circumstances indicates that OmpA plays a function in iron metabolism, as suggested by the iron-induced synthesis of this protein (Nwugo et al., 2011).
3.3.1.3 The ferrous transport system FeOAB
In Gram-negative bacteria, the primary mechanism for ferrous iron transport is called Feo (Figure 6) A. baumannii strains retain both the FeoAB import mechanism and its regulator, FeoC. The feo operon codes for the cytosolic transcriptional repressor FeoC, the protein responsible for active ferrous iron transport across the cytoplasmic membrane FeoB, and the cytosolic protein FeoA, which has an unclear function. It is interesting to note that feoB deletion has no effect on A. baumannii growth in iron-poor M9 minimum media, nor does it stop the production of siderophores or reduce A. baumannii pathogenicity. Under these circumstances, ferric iron uptake is thought to be more active than ferrous iron transport. Nonetheless, it appears that the Feo system is required to grow A. baumannii in human serum that has iron chelated to transferrin. When cultured in human serum, mutants with feoB deletion showed a 4-fold decrease in cell density compared to the wild type, indicating that the Feo system may help A. baumannii proliferation. When exposed to the complement systems inside normal human serum, A. baumannii feoB mutant cells exhibited accelerated cellular aging. They died in significantly higher numbers than wild-type cells. Further research is necessary to clarify the contradicting findings indicating that FeoB is essential for growth in iron-chelated human serum but not in iron-poor minimum media. FeoB was also unexpectedly shown to be substantially increased under iron-poor settings (as opposed to iron-supplemented conditions) using reverse transcription-quantitative polymerase chain reaction (RT-qPCR), even though it was not determined to be necessary for A. baumannii growth in minimum medium (Cook-Libin et al., 2022).
Given that putative Fur boxes were found inside the promoters of these genes, the feo operon is iron controlled similar to tonB3. The Feo system's Fur box is located downstream of the feoA promoter's transcriptional start site, further supporting the idea that the Feo system's expression is iron-dependent (Cook-Libin et al., 2022).
3.3.2 Zinc
Although zinc uptake and utilization mechanisms have been studied, non-iron metal homeostasis in A. baumannii is not as well understood. An inner membrane ABC zinc transporter termed ZnuABC is preserved and is encoded by A. baumannii. Numerous bacterial species, including Salmonella, E. coli, and Y. pestis, have been shown to contain ZnuABC systems, which have occasionally been shown to be necessary for virulence (Ammendola et al., 2007). The ZnuABC transporter in A. baumannii is activated during zinc deficiency and A. baumannii pneumonia in mice. The inner membrane permease ZnuB is necessary for both bacterial growth in the mouse pneumonia model and the development under zinc-limiting circumstances. The Fur family Zur, a zinc-sensing repressor that detects a conserved Zur box DNA sequence upstream of target genes when zinc-bound, is also encoded by A. baumannii. Genes implicated in intracellular zinc homeostasis, the TonB/ExbB/ExbD system, and potential outer membrane zinc transporters ZnuD1 and ZnuD2 are among the tentative list of Zur target genes that have been found (Hood et al., 2012). Moreover, when zinc is limited, the expression of znuA, znuB, znuC, znuD1, znuD2, and tonB increases. No TonB system has yet been proved to be directly engaged in zinc acquisition through energy transmission to TonB-dependent receptors, even though TonB-dependent receptors are involved in zinc acquisition. Further investigation is necessary to elucidate the roles of these proteins and their significance in the many in vivo niches of A. baumannii. Ultimately, more research is required to determine and describe the bacterial systems involved in the import and use of additional non-iron metals (Mortensen and Skaar, 2013).
In summary, A. baumannii employs a multifaceted pathogenic strategy, beginning with adhesion to host surfaces and medical devices via hydrophobic interactions, pili, and biofilm-associated proteins. Its ability to survive for months on abiotic surfaces facilitates nosocomial transmission, while motility mechanisms enhance colonization. Biofilm formation, regulated by quorum sensing and extracellular matrix components, provides resistance to antibiotics and immune clearance. Key virulence factors include OMPs that mediate host cell adhesion, immune evasion, and apoptosis induction. Secretion systems contribute to toxin delivery, biofilm formation, and horizontal gene transfer, while efflux pumps enhance antibiotic resistance. Iron acquisition via siderophores and heme uptake systems is critical for survival in host environments, linking nutrient scavenging to virulence. Together, these mechanisms enable A. baumannii to persist in hospitals, evade immune responses, and resist treatment, underscoring its role as a critical opportunistic pathogen.
4 Clinical manifestations
A. baumannii is one of the most severe MDR pathogens, leading to widely variable clinical manifestations and outcomes (Gordon and Wareham, 2010). The mortality rate can vary depending on the site of infection, which may affect various parts of the body, including the respiratory tract, central nervous system (CNS), urinary tract, skin, and bloodstream. A. baumannii is predominantly a nosocomial infection, and multiple studies have identified several risk factors associated with a high risk of infection. These include previous or prolonged stays in the ICU or hospital, advanced age, use of medical devices such as catheters, endotracheal or nasogastric tubes, mechanical ventilation, prior antimicrobial therapy, previous major or invasive procedures, dialysis, prematurity, low birth weight, and prolonged use of parenteral nutrition or intravenous lipids (Djordjevic et al., 2016; Fukuta et al., 2013).
4.1 Respiratory tract infections
A. baumannii is predominantly found in the respiratory tract, particularly among individuals undergoing mechanical ventilation. Distinguishing upper respiratory tract colonization from verifiable pneumonia can be challenging since this pathogen primarily colonizes the respiratory tract, with minimal skin colonization (Peleg et al., 2008). In Lebanon, A. baumannii is most isolated from the respiratory tract (53.1%), followed by the surgical wound (18.8%), blood (15.6%), urine (10.2%), and other sites (2.3%; Kanafani et al., 2018).
As previously discussed, A. baumannii is mainly hospital-acquired, as indicated by a recent study showing a transmission rate of 315.4 cases per 1,000 ICU patient days, with a high mortality rate ranging from 52 to 66% (Huang Y. et al., 2019). Ventilator-associated pneumonia (VAP) acquired from MDR A. baumannii is associated with a high mortality rate in critically ill patients (Jaruratanasirikul et al., 2019). While A. baumannii accounts for 8–14% of VAP cases in the United States and Europe, higher rates are observed in Asia (19% to over 50%), Latin America, and some Middle Eastern countries (Lynch et al., 2017). Nosocomial outbreaks can occur in hospitals due to the colonization of care professionals' hands and inadequate personal hygiene (Peleg et al., 2008). A recent 2-year retrospective study of MDR A. baumannii respiratory infections in critically ill patients in Saudi Arabia found that 6.2% of ICU patients developed respiratory infections, with 93% of these cases progressing to VAP. The study also reported a high mortality rate of 74%, with COVID-19 co-infection leading to worse outcomes (Hafiz et al., 2023).
Although A. baumannii is primarily a nosocomial agent causing hospital-acquired pneumonia (HAP), community-acquired pneumonia (CAP) is also possible. CAP associated with A. baumannii presents an aggressive and sudden course, high bacteremia, and mortality rates, particularly in tropical regions, such as Asia and Australia. CAP is notably linked to the rainy season, excess alcohol consumption (with 10% of individuals being throat carriers), diabetes, smoking, and chronic lung disease (Dexter et al., 2015; Anstey et al., 2002; Wong et al., 2017). A study conducted in China revealed that CAP was linked to a plasmid-encoded bla-OXA-72 with high resistance to carbapenems (Jia et al., 2019).
4.2 Meningitis
Meningitis represents the most severe form of A. baumannii infection, often occurring post-neurosurgery, and is associated with a high mortality rate, reaching up to 70%, particularly in patients with specific risk factors such as ventriculostomy tubes, prior cerebrospinal fistulae, or antimicrobial therapy following surgery (Siegman-Igra et al., 1993). Mortality rates tend to be higher in cases involving individuals over 40 years old, external ventricular drain usage, elevated white blood cell counts in cerebrospinal fluid, diabetes, and hypertension. The extensively drug-resistant (XDR) phenotype of A. baumannii has shown sensitivity only to colistin and tigecycline, as revealed by a large case series in 2019 (Sharma et al., 2019).
A report from China on post-neurosurgical meningitis caused by A. baumannii indicated a prevalence of MDR and XDR strains at 33.64%. These strains exhibited high resistance rates, including 100% resistance to imipenem and meropenem, 98.38% to cefazolin, 100% to ceftazidime, 100% to ceftriaxone, and 98.39% to cefepime. However, they demonstrated sensitivity to polymyxin B (100%), tigecycline (60.66%), and amikacin (49.18%; Pan et al., 2018). A recent single-center retrospective study on A. baumannii meningitis in children found that mechanical ventilation, septic shock, carbapenem-resistant A. baumannii (CRAB), lower peripheral leukocyte counts, along with higher protein levels in the cerebrospinal fluid (CSF) and elevated procalcitonin levels, were significantly associated with failed treatment outcomes (Wang et al., 2024).
Community-acquired meningitis caused by A. baumannii is rare and predominantly affects healthy individuals in hot climates, with low rates of drug resistance (Chang et al., 2000). Conversely, nosocomial cases have been associated with the administration of intrathecally contaminated methotrexate and the use of suctioning equipment in neurosurgery units (Kelkar et al., 1989). Clinical features of A. baumannii meningitis typically include fever and meningeal signs, with or without seizures. Lumbar puncture often reveals pleocytosis with high neutrophil count, elevated protein levels, and a decreased CSF to serum glucose ratio (Rodríguez Guardado et al., 2001).
4.3 Bloodstream infection
VAP and bloodstream infections are prominent clinical manifestations of A. baumannii infections. In a study from the United States, A. baumannii ranked as the 10th most common cause of all monomicrobial hospital-acquired bloodstream infections (Wisplinghoff et al., 2004). ICU-acquired bloodstream infections were more prevalent than non-ICU infections, with mortality rates ranging from 34 to 43.4% in ICU settings and 16.3% in non-ICU settings. A. baumannii is noted for having the third highest mortality rate in ICU settings, following P. aeruginosa and Candida. It is noteworthy that A. baumannii bloodstream infections typically occur after a mean duration of 26 days, making it one of the latest-onset bloodstream infections acquired in the hospital (Wisplinghoff et al., 2004).
Bloodstream infections with A. baumannii are frequently associated with vascular catheters and the respiratory tract, with less common associations with skin injuries or urinary tract infections. Mortality rates are higher in pneumonia cases compared to catheter-related infections (Chen et al., 2005; Seifert et al., 1995). Various risk factors contribute to these infections, including cancer, immunocompromised states, burns, prolonged hospital stays, and invasive procedures (Chen et al., 2005; Seifert et al., 1995; Wisplinghoff et al., 2000; Cisneros and Rodríguez-Baño, 2002; García-Garmendia et al., 2001; Gómez et al., 1999; Tilley and Roberts, 1994). Notably, a Brazilian study revealed that 68% of bacteremia cases were linked to cancer patients, with a high mortality rate attributed more to bacterial control than cancer-related effects like low white blood cell count (Freire et al., 2016). In severely burned patients, A. baumannii was identified as the most common cause of bacteremia over 4 years, exhibiting 100% resistance to all antibiotics except for low resistance to polymyxin B and minocycline (Gong et al., 2016). Additionally, 102 patients presented with bacteremia among American military personnel injured in Iraq and Afghanistan (Centers for Disease Control Prevention, 2004). Septic shock was reported in one-third of individuals with bacteremia (Seifert et al., 1995; Cisneros et al., 1996).
4.4 Urinary tract infection
Urinary tract infections (UTIs) caused by A. baumannii are relatively rare, accounting for only 1.6% of all ICU-acquired UTIs (Gaynes and Edwards, 2005). Among these cases, 95% are associated with catheter infections or colonization (Gaynes and Edwards, 2005). Another study has shown that more than 50% of A. baumannii strains isolated from urine samples come from catheterized patients, emphasizing its strong association with device-related infections (Mohamed et al., 2022). Moreover, Di Venanzio et al. analyzed Acinetobacter isolates from over 19,000 cases in the BJC Healthcare System between 2007 and 2017. The study showed that 17.1% of the cases came from the urinary tract, and only 2% of UTIs are caused by this pathogen (Di Venanzio et al., 2019).
The challenge in managing A. baumannii-associated UTIs is compounded by its high resistance to commonly used antibiotics. A retrospective study analyzing 749 urine samples to determine the development of susceptibility to 10 antibiotics and the antibiotic consumption of patients with suspicion of UTI found that A. baumannii isolates exhibited high resistance to carbapenems and piperacillin-tazobactam, rendering these agents unsuitable for empirical therapy. Among the tested antibiotics, colistin remained the most active in vitro. The study also reported a marked increase in piperacillin-tazobactam consumption, despite its declining efficacy against A. baumannii (Jiménez-Guerra et al., 2018). Community-acquired UTIs involving A. baumannii are even rarer. In a study conducted across 10 hospitals in Korea, 55.6% of A. baumannii strains were implicated in UTIs, with 19.8% exhibiting resistance to imipenem, 25% to meropenem, 13.5% to polymyxin B, and 17.7% demonstrating resistance to colistin (Park et al., 2010).
4.5 Skin infections
Skin injuries, burns, and wounds are susceptible to A. baumannii infections, particularly in military or disaster settings (Ayoub Moubareck and Hammoudi Halat, 2020). This has been notably reported in retrospective studies of the US military, primarily in Iraq and Afghanistan (Johnson et al., 2007). Injuries infected with A. baumannii are often associated with using prosthetic materials, and larval therapy may be considered. While community-acquired or hospital-acquired skin infections caused by A. baumannii are rare, they can manifest as cellulitis, necrotizing fasciitis, abscesses, and folliculitis (Glew et al., 1977; Chiang et al., 2003; Ng et al., 2004; Adler et al., 2014). Although more common in war injuries and military service, A. baumannii is also implicated in burn units, presenting challenges for elimination (Trottier et al., 2007). It accounts for 2.1% of skin infections occurring in the ICU (Gaynes and Edwards, 2005). In a group of victims with open tibial fractures, Acinetobacter was the most common agent found. Still, its pathogenicity appeared low, as it was not associated with any amputation when not detected in follow-up cultures (Johnson et al., 2007). In contrast, infections in burned military patients were more severe, leading to longer hospital stays and higher mortality rates (Albrecht et al., 2006). Osteomyelitis can also manifest as a complication of skin wound infections caused by A. baumannii (Davis et al., 2005). When A. baumannii infects a skin wound, it typically presents as cellulitis initially, appearing as a red, edematous lesion resembling peau d'orange. Subsequently, it may progress to a sandpaper-like lesion with multiple vesicles, which can evolve into hemorrhagic bullae (Sebeny et al., 2008). A fatal case study described a 41-year-old morbidly obese man with a history of alcoholic liver disease who developed cellulitis caused by MDR A. baumannii. Despite early antibiotic intervention, the infection progressed to extensive myositis and fat necrosis, requiring multiple surgical debridements (Ali et al., 2014). The patient ultimately succumbed to multiorgan failure despite aggressive treatment. This case underscores the challenges in managing MDR A. baumannii skin infections, emphasizing the importance of early recognition, appropriate antibiotic selection, and timely surgical intervention to improve patient outcomes.
4.6 Others
Rare manifestations of A. baumannii infections include endocarditis, often associated with prosthetic valve replacement (Menon et al., 2006; Olut and Erkek, 2005; Rizos et al., 2007; Starakis et al., 2006; Valero et al., 1999). Colonization of contact lenses by A. baumannii can lead to endophthalmitis, keratitis, corneal ulcers, and periorbital cellulitis, which may also occur following eye surgery or penetrating trauma. Acinetobacter ranks as the third most common cause of corneal ulcers, accounting for 7% of cases in one study (Corrigan et al., 2001; Wang et al., 1998; Mahajan, 1984; Mark and Gaynon, 1983; Miller, 2005; Mathews et al., 2005).
Patients undergoing mechanical ventilation in the ICU may develop sinusitis due to Acinetobacter infection, which can subsequently progress to pneumonia (Bert and Lambert-Zechovsky, 1996; Pneumatikos et al., 2006). Peritonitis can also occur during peritoneal dialysis, presenting symptoms such as abdominal pain and cloudy dialysate (Dandecha and Sangthawan, 2002; Galvao et al., 1989; Valdez et al., 1991).
5 Epidemiology
5.1 Global prevalence and distribution
Acinetobacter spp. are widespread organisms, with the first isolate, initially identified as Micrococcus calcoaceticus in 1911 by the Dutch microbiologist Beijerinck, originating from soil (Beijerinck, 1911). These highly ubiquitous organisms have diverse natural reservoirs that are found in nearly all environmental niches, ranging from water bodies, soils, crude oil, and solid surfaces to raw food products, wild animals, plants, humans, and even nectar (Al Atrouni et al., 2016b). In contrast, the natural habitats of A. baumannii are not well-defined, as it is primarily isolated from hospital environments, particularly in ICUs and communities with close contact (Antunes et al., 2014). Growing evidence suggests that a similar pattern is observed in veterinary medicine, with infection and colonization occurring in seriously ill animals in veterinary clinics and ICUs (Zordan et al., 2011). Numerous countries worldwide have reported the presence of A. baumannii in human body lice (La Scola and Raoult, 2004; Kempf et al., 2012a; Bouvresse et al., 2011). For instance, the highly antibiotic-susceptible A. baumannii strain SDF, which causes community-acquired infections, was initially isolated from the interior of body lice collected from homeless individuals in France (Fournier et al., 2006). Additionally, traces of A. baumannii have been identified in environmental soil and water body samples around the world (Ma and McClean, 2021). An early study found that over a third of soil samples from Hong Kong contained Acinetobacter spp., with A. baumannii making up 14.7% (n = 34; Houang et al., 2001). Later, Hrenovic et al. isolated an antibiotic-resistant A. baumannii strain from acid paleosol in Croatia (Hrenovic et al., 2014). A. baumannii colonization has also been noted in livestock, particularly in cattle and poultry. It has also been reported in horses, pigs, donkeys, mules, goats, rabbits, dogs, and cats, as well as in food products from these animals, with a considerable variability in prevalence among birds (Ma and McClean, 2021). A. baumannii is considered a nosocomial bacterium with high potential to exhibit MDR and virulence (Rice, 2008). The antimicrobial resistance of A. baumannii is of significant concern worldwide (World Health Organization, 2024). Clinical isolates of A. baumannii strains often show varying degrees of resistance to commonly prescribed antibiotics, including carbapenems, such as imipenem and meropenem. Being carbapenem resistant is considered a key marker for extensively resistant bacteria, as it signifies a broad range of co-resistance to unrelated classes of antibiotics (Tacconelli et al., 2018). Antimicrobial-resistant A. baumannii is found worldwide, across all six inhabited continents, but in varying percentages among countries. A study mapping the global prevalence of A. baumannii measures the percentage of carbapenem-resistant isolates among all clinically isolated A. baumannii strains documented over the past decade (Ma and McClean, 2021). A recent meta-analysis study reveals the widespread occurrence of CRAB across Asia (76.2% resistance rate) and the Americas (69.4 % resistance rate), where it has become a significant issue in the majority of the countries, with lower prevalence rates for Mongolia, Japan, and Canada (Boyd et al., 2019; Beig et al., 2024). This resistant strain is also widespread in South Africa, the Ivory Coast, and regions surrounding the Mediterranean, including Southern Europe, the Middle East, and North Africa (Ma and McClean, 2021). Carbapenem resistance was remarkably low in Ireland at just 4%, whereas the Philippines reported an alarmingly high rate of 96.1% (Beig et al., 2024). In Latin America, carbapenem resistance among A. baumannii is among the highest in the world, with rates exceeding 50% in many countries and reaching up to 90% in some regions (Chagas et al., 2024; Table 1). A multicountry cohort study in Oceania has found an alarmingly high prevalence of the carbapenem-resistant A. baumannii (CRAB) in Fiji and Samoa (Baleivanualala et al., 2023). The study also indicated the existence of an Oceania outbreak strain among hospitalized patients in New Zealand and Australia (Baleivanualala et al., 2023). Another systematic review in 2023 has shown a significant prevalence of the CRAB in the sub-Saharan Africa region (Arowolo et al., 2023). This leaves Canada, Mongolia, Japan, Western Europe, and the Nordic region with the lowest prevalence of CRAB so far (Table 1).
5.2 Geographical variations and prevalence of multidrug-resistant strains
The distribution of MDR A. baumannii varies across different regions worldwide due to varying selective environmental pressures, differences in hospital infection control practices, and the presence of endemic strains in certain hospitals. A study in the Asia–Pacific region has shown a significant geographic variation in the carbapenem resistance rates, ranging from <5% in Japan to 88% in South Korea (Lee et al., 2023). Carbapenem resistance in A. baumannii is primarily attributed to carbapenem-hydrolyzing class-D β-lactamase genes, including blaOXA−51−like, blaOXA−23−like, blaOXA−24/40−like, and blaOXA−58−like (Lee et al., 2012a). The blaOXA−23−like, blaOXA−24/40−like, and blaOXA−58−like genes are the three primary classes driving the global CR-AB epidemics (Hamidian and Nigro, 2019). Additionally, these gene classes exhibit different geographic distributions worldwide (Peleg et al., 2008). Geographical differences are also observed in hospital-acquired and community-acquired infections. For instance, Central America, Latin America, and the Caribbean have the highest prevalence of HAP and VAP caused by A. baumannii, whereas Eastern Asia has the lowest prevalence (Mohd Sazlly Lim et al., 2019). Although rare, the majority of the cases of CAP have been reported during the summer months in tropical and subtropical climates, highlighting the role of environmental factors in the geographic variation of infection (Falagas and Rafailidis, 2007).
Regional variations in the proportion of A. baumannii among all clinically isolated aerobic and facultative Gram-negative pathogens have been documented, with rates ranging from 0.7% in North America to 4.6% in the Middle East (Lob et al., 2016). Also, the prevalence of colonization of A. baumannii among livestock varies by the geographic location of the farms (Ma and McClean, 2021). In contrast, the connection between A. baumannii and infections following injuries in conflict zones, such as those in Iraq, Afghanistan, Gaza, and Ukraine (Joly-Guillou, 2005; Granata et al., 2024; Moussally et al., 2023) or after natural disasters like the 6 February 2023 earthquake in central and southeast Turkey (Eryilmaz-Eren et al., 2024) has been recently viewed as a possible phenomenon affecting the geographical distribution of the pathogen. There is some evidence suggesting that morphine use, which is common after battlefield or crush injuries, may exacerbate A. baumannii infections, potentially due to its immunosuppressive effects (Breslow et al., 2011). Table 1 summarizes the distribution of CARB worldwide.
Furthermore, a systematic review addressing A. baumannii's regional heterogeneity and antimicrobial susceptibility reported higher antibiotic resistance rates in ICUs compared to non-ICU floors. However, MDR rates were high in both settings, especially in Europe and the Middle East (Lob et al., 2016). Similarly, MDR rates ranged from 77 to 87% in Africa, Asia, and Latin America, while they spanned 47% in North America and over 93% in the Middle East and Europe (Lob et al., 2016). Additionally, a recent 2021 review on the global prevalence of A. baumannii identified the Middle East as one of the heavily afflicted areas (Ma and McClean, 2021).
Despite its ubiquity, A. baumannii infections are not uniformly distributed worldwide; they vary geographically due to changing environmental conditions, differences in hospital infection control practices, and different strains across different countries.
5.3 Risk factors for infection
A. baumannii is a significant opportunistic pathogen responsible for healthcare-associated infections, particularly in hospitalized patients, including those in ICUs. It can cause a wide range of infections, such as VAP, bloodstream infections, urinary tract infections, and meningitis (Antunes et al., 2014). The established risk factors associated with A. baumannii infections include invasive procedures, prior hospitalization, host-specific factors, extended ICU stays, and previous broad-spectrum antibiotic use (Lin and Lan, 2014). Notably, patients with A. baumannii infections experience longer ICU stays and higher mortality rates, with the ICU mortality rate for VAP caused by MDR A. baumannii reaching up to 84.3% (Inchai et al., 2015). Mechanical ventilation is one of the primary contributors to the development of nosocomial pneumonia caused by MDR A. baumannii. A retrospective incidence study revealed that mechanical ventilation was associated with a 2.5-fold increased risk of acquiring nosocomial infections with MDR bacteria (Fang et al., 2016). Additionally, mechanical ventilation significantly increases the risk of developing healthcare-associated infections with MDR A. baumannii, further highlighting its role as a critical risk factor in ICU settings (Michalopoulos et al., 2011). Benaissa et al. performed a multivariate analysis, which concluded that mechanical ventilation, use of probabilistic antibiotic therapy for more than 4 days, prolonged invasive procedures, and neoplastic pathologies are independent risk factors for A. baumannii infection (Benaissa et al., 2023). The study also shows that prior exposure to carbapenem antibiotics, namely, imipenem and colistin, significantly increased the risk of A. baumannii infection by eighteen and 5 times, respectively (Benaissa et al., 2023). Also, several studies have identified prior exposure to antibiotics as a risk factor for MDR bacterial infections (Ang and Sun, 2018), highlighting the importance of rational use of antibiotics. Finally, host-specific factors such as underlying medical conditions or compromised immunity are regarded as risk factors for A. baumannii infection. For instance, neoplastic pathology increased the risk of A. baumannii infection by 5.7 times compared to the control group (Benaissa et al., 2023). Notably, during the COVID-19 pandemic, hospital-acquired co- or secondary infections caused by A. baumannii in COVID-19 patients were reported globally (Lai et al., 2020; Sharifipour et al., 2020). It has been reported that 85% of patients with hospital-acquired A. baumannii infections who developed VAP had co-infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; Perez et al., 2020). In conclusion, patients admitted to intensive care units, those with indwelling medical devices, and individuals with immunocompromised conditions are particularly susceptible to A. baumannii infections.
5.4 Hospital-acquired vs. community-acquired infections
A. baumannii is commonly found in hospitals and often shows resistance. It has been isolated in intensive care units and detected on various reusable medical equipment, such as ventilators, humidifiers, blood pressure monitors, stethoscopes, laryngoscopes, curtains, mattresses, pillows, urinals, sinks, door handles, and even the gowns and gloves of hospital staff (Ahuatzin-Flores et al., 2024). Additionally, it has been found in bronchial and oropharyngeal secretions, as well as in the digestive tract of hospitalized patients (Almasaudi, 2018; Jung and Park, 2015). A. baumannii is a significant hospital pathogen, responsible for 2–10% of all Gram-negative HAIs (Ayobami et al., 2019; Fahy et al., 2023). In ICUs, it accounts for 20.9% of HAIs, with CRAB representing 13.6% of these infections (Ayobami et al., 2019). Hospitals across North America saw a sharp rise in A. baumannii resistance, escalating from 1.0% in 2003 to 58.0% in 2008. Meanwhile, the findings from the European Antimicrobial Resistance Surveillance Network (EARS-Net) reveal that carbapenem-resistant Acinetobacter species pose a significant public health risk in Europe. This issue is particularly severe in Southern and Eastern Europe, where over 70% of invasive isolates are resistant to carbapenems (Nguyen and Joshi, 2021). Its spread within healthcare premises is facilitated by its ability to survive in both dry and humid environments, with an ability to tolerate such conditions for long periods (Ahuatzin-Flores et al., 2024). Moreover, A. baumannii's resistance to disinfectants and antibiotics, combined with its ability to form biofilms, allows it to colonize inert surfaces and medical devices. This characteristic is ascribed to the presence of capsular polysaccharides, enabling A. baumannii to survive in nutrient-limiting conditions (Ramirez et al., 2020; Anane et al., 2019). Community-acquired A. baumannii infections are rarely reported, but they are more common in certain tropical or subtropical regions, suggesting that the pathogen may be acquired from sources outside of hospitals (Ahuatzin-Flores et al., 2024). The presence of A. baumannii in out-of-hospital environments remains debatable. There is a lack of definitive evidence concerning its natural habitat beyond hospitals, how it is introduced into hospital settings, and its potential re-emergence in the environment (Ahuatzin-Flores et al., 2024). Nonetheless, several studies have identified A. baumannii in multiple non-hospital settings, including aquatic environments (Galarde-López et al., 2022), livestock (Ghaffoori Kanaan et al., 2020), soil (Suresh et al., 2022), food (Bitar et al., 2019; Cho et al., 2018), produce (Berlau et al., 1999; Malta et al., 2020), domestic pets (Kimura et al., 2018; Lysitsas et al., 2023; Wareth et al., 2019), wildlife (Deng et al., 2021), and even human body lice (Ly et al., 2020). In contrast to healthcare-associated infections, A. baumannii strains responsible for community-acquired infections tend to be more susceptible to antibiotics. Farrugia et al. sequenced the entire genome of a community strain, D1279779, and discovered that it lacked the antibiotic resistance island AbaR, which is commonly found in nosocomial strains (Farrugia et al., 2013). Although community-acquired A. baumannii infections are relatively rare, they often present with a severe and rapidly progressive course, including septic shock, respiratory failure, severe sepsis, and pneumonia, with a considerably high mortality rate (Fang et al., 2016; Farrugia et al., 2013; Brigo et al., 2022).
6 Antibiotic resistance
Among the six pathogens contributing most significantly to the burden of deaths due to antimicrobial resistance in 2019, CRAB emerged as the fourth most burdensome globally (Antimicrobial Resistance Collaborators, 2022). A. baumannii is classified among the MDR ESKAPE organisms (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumannii, P. aeruginosa, and Enterobacter species), recognized as critical priority pathogens by the WHO. These pathogens, designated as Priority 1 tiers, have developed resistance to a wide range of first-line and last-resort antibiotics, including carbapenems and third-generation cephalosporins (WHO, 2017). Consequently, patients infected with carbapenem-resistant pathogens, including A. baumannii, face heightened risks of morbidity and mortality, with bloodstream infections contributing to poor outcomes and limited therapeutic options (van Duin et al., 2013). Furthermore, the widespread dissemination of MDR A. baumannii strains has been exacerbated by the overuse of antibiotics and inadequate antibiotic usage (van Duin et al., 2013; Endale et al., 2023). Consequently, A. baumannii has been classified based on its antibiotic sensitivity into MDR, XDR, and pan-drug-resistant (PDR) phenotypes (Vrancianu et al., 2020).
6.1 Mechanisms of resistance to antibiotics
To counteract the effects of antibiotics, A. baumannii employs a diverse array of antibiotic resistance mechanisms, each targeting specific types of drugs. These mechanisms include enzymatic degradation, reduced membrane permeability, increased efflux mechanisms, target site modification, and genetic mechanisms of resistance (Vrancianu et al., 2020; Ibrahim et al., 2021).
6.1.1 Enzymatic warfare
A. baumannii can evade the effects of some antibiotics by producing certain enzymes. Consequently, the bacteria can hydrolyze antibiotics or modify the antibiotic's structure by transferring functional groups (Blair et al., 2015). An example of these enzymes is the β-lactamases, among the oldest known enzymes that cleave the β-lactam ring structure (Pollock, 1967). β-Lactam antibiotics, which include penicillins, cephalosporins, carbapenems, monobactams, and β-lactamase inhibitors, target the penicillin-binding proteins (PBPs) of bacteria (Pandey and Cascella, 2024). The key feature of β-lactamase enzymes is their ability to render ineffective a broad range of β-lactams, including some of the most potent carbapenems and extended-spectrum cephalosporins, thus augmenting and amplifying A. baumannii's resistance to β-lactam antibiotics (Aurilio et al., 2022). Furthermore, enzymes can inactivate antibiotics by transferring chemical groups onto the antibiotic molecules, preventing them from binding to their target proteins. For instance, A. baumannii can produce aminoglycoside-modifying enzymes (AMEs) such as acetyltransferases, nucleotidyltransferases, or phosphotransferases, leading to enzymatic modification of aminoglycoside antibiotics. Consequently, aminoglycosides will no longer be able to exert their effects on the bacteria. Through mutagenesis and the transferability of encoding genes at the molecular level, AMEs enable the acquisition of heightened aminoglycoside resistance (Ramirez and Tolmasky, 2010).
6.1.2 Reduced membrane permeability
Gram-negative bacteria possess a complex outer membrane composed of phospholipids, lipopolysaccharides, lipoproteins, and β-barrel porins, forming a permeability barrier. This intrinsic complexity renders them less permeable to many antibiotics through the regulation of porins or substitution with more-selective channels (Blair et al., 2015; Choi and Lee, 2019; Maher and Hassan, 2023). Previously, limited knowledge about A. baumannii included sparse data on its outer membrane proteins and permeability (Vila et al., 2007). However, the primary and most abundant OM protein, outer membrane protein A (OmpAb), a 40 kDa porin, plays a crucial role in reducing membrane permeability (Jyothisri et al., 1999). Further studies, including one by Nikaido and Sugawara, confirmed that OmpAb forms a low permeability channel, serving as the “principal non-specific slow porin” of A. baumannii (Sugawara and Nikaido, 2012).
6.1.3 Increased efflux mechanisms
Efflux mechanisms represent a fundamental strategy bacteria employ to develop resistance to drugs, including antibiotics. These cell-membrane-lodged efflux pumps enable bacteria to regulate their inner environment by transporting molecules, such as antibiotics, outside of the cell (Pearson et al., 1999). As previously noted, several principal categories of efflux pumps have been identified in A. baumannii: MFS, RND, SMR, MATE, ABC, and PACE (Lin et al., 2014), with MFS, RND, SMR, and MATE families being the most frequently correlated to resistance.
MFS efflux pumps, including Tet(A) and Tet(B), confer resistance to tetracyclines in A. baumannii (Martí et al., 2006). Recently, Gaona et al. identified a novel MFS efflux pump SxtP as well as its associated activator LysR-type transcriptional regulator (LTTR) SxtR, both of which are involved in mediating resistance to sulfamethoxazole/trimethoprim (Gaona et al., 2024).
The AdeABC pump, belonging to the RND superfamily, was the first pump identified in A. baumannii and is associated with decreased susceptibility to various antibiotics, including aminoglycosides, tetracycline, erythromycin, chloramphenicol, fluoroquinolones, and β-lactams (Fournier et al., 2006; Vila et al., 2007; Magnet et al., 2001). In a recent study tackling the resistance role of RND efflux pumps, A. baumannii isolates recovered from tertiary care hospitals of three western Balkan countries revealed that the overexpression of RND efflux pumps mediates resistance to tigecycline. This further accentuates the significant role of such pumps in antibiotic resistance (Novović et al., 2025).
AbeS, a central efflux pump of the SMR family, is widespread in MDR strains of A. baumannii, contributing to antibiotic resistance (Srinivasan et al., 2009). MATE efflux pumps represent another family of pumps that function as antiporters by pumping out cations for hydrogen or sodium ions. For instance, the AbeM efflux pump belonging to MATE has proved to have increased resistance to norfloxacin, ciprofloxacin, and ofloxacin (Zack et al., 2024). Additionally, the A1S_3371 MATE efflux pump enhances the virulence of A. baumannii (Pérez-Varela et al., 2019).
The ATP-binding cassettes, such as the MacAB-TolC system, are overexpressed in mature biofilms of A. baumannii, aiding in adaptation to deleterious conditions (Robin et al., 2021). Furthermore, the PACE family proteins, such as Acel, assist in resisting chlorhexidine, a widely used antiseptic (Hassan et al., 2015).
In summary, A. baumannii employs various efflux mechanisms to transport drugs out of the cell, reducing drug concentration inside and compromising treatment efficacy.
6.1.4 Target site modification
When administered, antibiotics usually bind tightly to target sites within bacteria, halting bacterial growth and activity. However, once these target sites undergo changes, the antibiotic loses its capacity to bind effectively, leading to resistance (Blair et al., 2015). One method by which resistance is conferred is via transformation, which involves the uptake of DNA from the environment to form mosaic genes, enabling modification of target proteins (Blair et al., 2015). A. baumannii can develop resistance to various antibiotics through such target site modifications, either by genetic mutations in specific sites or by post-transcriptional modifications in diverse proteins (Kyriakidis et al., 2021; Singh et al., 2013). Target site modification fosters resistance against a plentiful array of antimicrobials, such as β-lactams, macrolides, fluoroquinolones, aminoglycosides, including polymyxin resistance, namely, colistin (Martínez-Trejo et al., 2022). For instance, imipenem, a carbapenem antibiotic, inhibits the activity of its target site, penicillin-binding protein 2 (PBP2). Over time, A. baumannii has developed molecular adaptations by reducing the affinity of PBP2 for Imipenem, enabling it to resist the effects of this antibiotic (Gehrlein et al., 1991). Similarly, A. baumannii exhibits fluoroquinolone resistance through mutations in genes such as gyrA, gyrB, and parC, which encode type-II and type-IV topoisomerases, the targets of fluoroquinolones (Vrancianu et al., 2020; Redgrave et al., 2014). In addition, for the antibiotic Linezolid, mutations by base substitutions in the V domain of the 23S rRNA and the presence of a transmissible Cfr(B) rRNA 23S methyltransferase can occur as a target site modification, while being the most prevalent mechanism of resistance of A. baumannii to this antibiotic (Martínez-Trejo et al., 2022). Colistin has long been used for MDR A. baumannii, and resistance to colistin has been described in several in vitro and in vivo studies. In A. baumannii, this mechanism is governed by the mutation of the pmrA and/or prmB protein genes along with the constitutive expression of prmA, leading to the positive regulation of the pmrCAB operon and the addition of phosphoethanolamine to the phosphate of LPSs (Martínez-Trejo et al., 2022).
6.1.5 Genetic mechanisms of resistance
Resistance can be acquired by emerging mobile genetic elements (MGEs) in A. baumannii. Transposons, plasmids, and integrons facilitate the spread of MDR strains by enabling the bacteria to acquire new resistance genes or transfer existing ones to other bacteria (Ibrahim et al., 2021).
Transposons facilitate antibiotic resistance in A. baumannii through various mechanisms, including enhanced expression of resistance genes by potent promoter sequences, disruption of expression due to promoter disruption by transposons, and direct gene activation through insertion within coding sequences (Noel et al., 2022). For instance, insertion sequence (IS) elements promote A. baumannii clinical resistance to carbapenems by furnishing strong promoter sequences to silence bla genes encoding β-lactamase enzymes (Noel et al., 2022). By downregulating gene expression, IS can also confer resistance. A study done by Palmer et al. identified a suppressor mutation (ISAba11 transposition) that reduced the expression of the isoprenoid biosynthesis gene ispB, thus establishing resistance to different antibiotics, including meropenem, imipenem, and gentamicin (Palmer et al., 2020). Plasmids are another mobile genetic element utilized by A. baumannii to acquire resistance. They can be transferred between bacteria either vertically or horizontally. Vertically transmitted plasmids are those disseminated from a parent cell to the progeny, while horizontally transmitted plasmids are conveyed by conjugation (Partridge et al., 2018). For example, XerCD-dif site-specific recombination systems are flanked by antibiotic-resistance genes on plasmids, creating a potential mechanism for acquiring and disseminating resistance genes (Balalovski and Grainge, 2020). To understand how different types of plasmids contribute to the spread of AMR, another recent study conducted by Lam and Hamidian analyzed the distribution of varying plasmid types and antimicrobial resistance genes on A. baumannii plasmids. They showed that many RP-T1 and R3-T2 types, and rep-less pNDM plasmids can spread various carbapenem resistance genes, noting that carbapenems are often among the last-resort antibiotics for MDR A. baumannii (Lam and Hamidian, 2024).
Integrons, which Acinetobacter species harbor, are aligned with antibiotic resistance acquisition and serve as a significant source of resistance genes in Gram-negative bacteria, including A. baumannii (Nemec et al., 2004). High prevalence of integron class 1 in A. baumannii isolates has been observed, contributing to resistance to a wide range of antibiotics through the transmission of resistance genes (Ghazalibina et al., 2019). Another study by Shetty et al. identified the presence of class-1 integrons in A. baumannii and detected new variants of resistance genes within the strain AB34: AAC(6′)-Ib-CatB8-ANT(3″)-Ia-AadA1, which code for amikacin, chloramphenicol, and streptomycin. In addition, integrons of the strains AB36 and AB41 contained a rare gene cassette CmlA5-Arr2 coding for chloramphenicol and rifampin (Shetty et al., 2024). In summary, A. baumannii can evade antibiotics by acquiring and disseminating various resistance genes in its genome through mobile genetic elements.
6.2 Impact of antibiotic resistance of A. baumannii on clinical outcomes and treatment options
Bacteria resistant to antibiotics render these drugs ineffective, making infections difficult to treat and leading to a notable escalation in mortality rates and deterioration in clinical outcomes (WHO, 2023). Compared to non-infected patients and those infected with susceptible strains of A. baumannii, infections with MDR A. baumannii are associated with prolonged hospital and ICU stays and more unfavorable clinical outcomes (Sunenshine et al., 2007). Among hospitalized patients with MDR A. baumannii bacteremia, higher mortality rates and medical financial burden and costs have been observed compared to non-MDR A. baumannii bacteremia. Remarkably, MDR A. baumannii bacteremia resulted in 13.4 days of supplementary hospitalization and US$3,758 of additional costs (Lee et al., 2007).
During the COVID-19 pandemic, patients affected by both MDR A. baumannii and COVID-19 had an augmented mortality risk, illustrating the pronounced impact of simultaneous infections with MDR A. baumannii on clinical outcomes (Alenazi et al., 2023). Additionally, a recent Lebanese study demonstrated the perturbing mortality rate of A. baumannii infections, especially in patients where improper initial antimicrobial treatment was initiated with existing antimicrobial resistance (Itani et al., 2023). Another “16-year retrospective cohort study” displayed the characteristics of carbapenem-resistant Gram-negative bacterial meningitis associated with A. baumannii and concluded that it is associated with prominent in-hospital mortality rates (Xu et al., 2024).
Regarding the impact of A. baumannii antibiotic resistance on treatment options, numerous studies have shown an increasing rate of resistance against carbapenems and colistin, especially in Asia, Latin America, Europe, and Australia (Kumar et al., 2021). Over time, A. baumannii has developed resistance to colistin via two main mechanisms: the complete loss of the target lipopolysaccharide or its modification (Novović and Jovčić, 2023). As a result, combined therapy with synergic drugs is endorsed when all conventional treatments become ineffective against MDR A. baumannii and may even be the exclusive therapeutic option for PDR A. baumannii (Kumar et al., 2021; Karakonstantis et al., 2021).
6.3 Current drugs with resistance
Before the 1970s, ampicillin, carbenicillin, gentamicin, and nalidixic acid, whether given alone or in combination therapy, were considered optimal for treating Acinetobacter infections. However, after 1975, resistance levels soared significantly (Manchanda et al., 2010). Consequently, A. baumannii has developed resistance to several current drugs.
6.3.1 β-lactams
As previously discussed, β-lactam antibiotics (penicillins, cephalosporins, carbapenems, monobactams, and β-lactamase inhibitors) share a common β-lactam ring structure and target bacterial PBPs (Pandey and Cascella, 2024). A. baumannii has developed resistance to β-lactam antibiotics through various mechanisms, including hydrolysis by β-lactamases, increased expression and activity of efflux pumps, decreased membrane permeability leading to reduced influx, and modification of target sites (Kyriakidis et al., 2021). In Gram-negative bacteria like A. baumannii, the primary mechanism of β-lactam resistance is the synthesis of β-lactamase enzymes (Matthew and Harris, 1976).
β-Lactamases are classified according to the Ambler classification, which groups these enzymes into four classes (A, B, C, and D) based on molecular analysis and amino acid sequences. Classes A, C, and D enzymes utilize a serine amino acid for β-lactam hydrolysis, forming an acyl enzyme intermediate, while class-B β-lactamases are zinc-dependent metalloenzymes (metallo-β-lactamases, MBLs) that require at least one active-site zinc ion for substrate hydrolysis (Ambler, 1980; Bush and Jacoby, 2010).
According to the findings summarized in Kyriakidis et al.'s review, Class A β-lactamases, including SHV, CTX M, and KPC, confer resistance to penicillins, cephalosporins, monobactams, and carbapenems (Kyriakidis et al., 2021; Tooke et al., 2019). Catalysis by class-B β-lactamases (VIM, IMP, SPM, GIM, and NDM) extends to the breakdown and hydrolysis of almost all β-lactams, except monobactams (Kyriakidis et al., 2021; Kateete et al., 2016).
Ambler class-C β-lactamases, also known as chromosomally encoded Acinetobacter-derived cephalosporinases (ADCs), contribute to resistance against cephalosporin antibiotics through an ISAba1 insertion sequence close to the resistance genes (Kyriakidis et al., 2021).
Class-D β-lactamases, also known as oxicillinases (OXA) or carbapenem-hydrolyzing class D-lactamases (CHDLs), particularly those of the OXA-10 family, can render all β-lactam antibiotics inactive, including carbapenems (Kyriakidis et al., 2021). Over 400 OXA enzymes have been identified, with the largest being the OXA-51-like β-lactamases identified to date (Monem et al., 2020; Evans and Amyes, 2014).
Resistance to carbapenems often depends on β-lactamases of classes B (MBLs) and D (OXA-type carbapenemases; Ibrahim, 2019). For instance, the OXA-23 enzyme, one of the Ambler class-D β-lactamases, is sufficient to confer resistance to carbapenem antibiotics (Evans and Amyes, 2014).
The other classification of β-lactamases, proposed by Bush, Jacoby, and Medeiros, was based on the enzymes' ability to cleave specific β-lactam classes and their susceptibility to β-lactam inhibitors (Bush and Jacoby, 2010; Bush et al., 1995). According to this classification, β-lactamases were grouped into three categories: group-1 cephalosporinases, group-2 serine β-lactamases, and group-3 metallo-β-lactamases (Bush and Jacoby, 2010).
In ICUs, CRAB is emerging as a major cause of healthcare-associated infections (Tomczyk et al., 2019). Designated by the WHO as a critical-priority bacterium, CRAB ranks among the top 12 pathogens exhibiting resistance, posing significant public health challenges and necessitating urgent action (Tacconelli et al., 2018). One subset of CRAB, known as carbapenemase gene-positive CRAB (CP-CRAB), carries antibiotic resistance genes encoded on mobile genetic elements and produces carbapenemase enzymes. These enzymes confer remarkable resistance by degrading the majority of the β-lactam antibiotics, including carbapenems, making CP-CRAB infections particularly concerning.
In a recent scientific review on carbapenem resistance in A. baumannii, Nguyen and Joshi summarized the mechanisms into four groups: alterations in penicillin-binding proteins, membrane impermeability due to loss of OMPs, overexpression of efflux pumps, and carbapenem inactivation through synthesis of carbapenemases (Nguyen and Joshi, 2021).
6.3.2 Fluoroquinolones
Fluoroquinolones, derivatives of quinolones with added fluorine atoms, belong to the family of broad-spectrum antibiotics effective against a wide range of aerobic Gram-positive and Gram-negative organisms. These antibiotics disrupt bacterial mRNA synthesis and DNA replication by inhibiting type-II and type-IV DNA topoisomerases. Common fluoroquinolone drugs include ciprofloxacin, Gemifloxacin, Levofloxacin, Moxifloxacin, Norfloxacin, and Ofloxacin (National Institute of Diabetes and Digestive and Kidney Diseases, 2012).
A. baumannii commonly develops resistance to fluoroquinolones through various mechanisms, with target site mutation being the most prevalent. Mutations in the gyrA, gyrB, and parC genes, which encode gyrase and topoisomerase IV, allow bacteria to acquire resistance. For example, clinical isolates of A. baumannii from Mansoura University hospitals in Egypt showed resistance to ciprofloxacin primarily through gyrA and parC mutations, with combined mutations accounting for 85.5% of cases (Zaki et al., 2018). Similarly, studies have demonstrated a strong association between fluoroquinolone resistance and mutations in the gyrA, gyrB, and parC genes (Park et al., 2011). Recent research using EUCAST antimicrobial susceptibility testing has highlighted the increasing resistance of A. baumannii to ciprofloxacin, surpassing EUCAST breakpoints and reducing its efficacy against the bacterium (Darby et al., 2024).
In addition to target site mutations, fluoroquinolone resistance in A. baumannii can also occur through plasmidic and chromosomal efflux mechanisms, reduced membrane permeability, and transmissible mechanisms mediated by plasmids (plasmid-mediated quinolone resistance genes; Redgrave et al., 2014).
6.3.3 Aminoglycosides
Aminoglycosides, such as gentamicin, amikacin, tobramycin, neomycin, and streptomycin, are broad-spectrum bactericidal antibiotics that inhibit protein synthesis in Gram-negative pathogens (Germovsek et al., 2017; Brewer, 1977; Krause et al., 2016).
In A. baumannii, resistance to aminoglycosides can occur through three distinct mechanisms, with the most prevalent being the alteration of hydroxyl or amino groups of these antibiotics by AMEs, which reduces their binding capacity. Other mechanisms include efflux mechanisms and target site modification (ribosomal binding protein; Kyriakidis et al., 2021; Asif et al., 2018).
AMEs, such as acetyltransferases, nucleotidyltranferases, or phosphotransferases, enhance A. baumannii's ability to develop aminoglycoside resistance through gene transfer and mutagenesis (Ramirez and Tolmasky, 2010). For example, a 2019 study analyzing 1,200 bacterial isolates from ICU patients found high levels of resistance, with various types of aminoglycoside-modifying genes detected in A. baumannii isolates, including aadB, aphA6, aadA1, and aacC1 (Rizk and Abou El-Khier, 2019).
Efflux mechanisms also contribute significantly to aminoglycoside resistance in A. baumannii. Research has identified seven gene products in A. baumannii involved in successful aminoglycoside removal through efflux mechanisms, including two-component systems, pumps, permeases, and periplasmic adaptors (Kyriakidis et al., 2021; De Silva and Kumar, 2019).
Finally, emerging resistance of A. baumannii to clinically useful aminoglycosides, such as gentamicin, tobramycin, and amikacin, can occur through target site modification via methylation of 16S ribosomal RNA (Doi and Arakawa, 2007).
6.3.4 Tetracyclines
Tetracyclines, including doxycycline, minocycline, and tigecycline, are broad-spectrum antibiotics that inhibit bacterial growth by targeting the 30S ribosomal subunit, thereby preventing protein synthesis (Shutter and Akhondi, 2024).
In A. baumannii, resistance to tetracyclines is achieved through various mechanisms, including ATP-driven efflux mechanisms mediated by RND pumps (AdeABC and AdeIJK) and MFS pumps (TetA and TetB), drug enzymatic inactivation, and ribosomal protection proteins (RPPs), such as TetM, TetW, TetO, and TetSf (Kyriakidis et al., 2021; Warburton et al., 2016).
Recent evidence suggests promising developments in overcoming tetracycline resistance with aminomethylcyclines KBP-7072 and omadacycline, third-generation tetracyclines. These compounds have demonstrated the ability to circumvent efflux and RPPs tetracycline resistance mechanisms in vitro, offering potential avenues for the development of “anti-resistant” tetracycline-based antibiotics (Huband et al., 2020).
However, the effectiveness of tigecycline against A. baumannii has been compromised by the emergence of plasmid-mediated tet(X) mono-oxygenase gene variants. These bacterial mono-oxygenases have the capacity to render all tetracyclines, including tigecycline, eravacycline, and omadacycline, inactive (Kyriakidis et al., 2021). He et al. found that tet(X3) and tet(X4) increased the tigecycline minimal inhibitory concentration values for E. coli, K. pneumoniae, and A. baumannii and notably compromised tigecycline in in vivo infection models (He et al., 2019). Consequently, a novel, plasmid-mediated tigecycline resistance tet(X) gene variant, tet(X5), was detected in a clinical A. baumannii isolate from China in 2017. Whole-genome sequencing confirmed the sequence of tet(X) from AB17H194, where it conferred resistance to tigecycline, eravacycline, and omadacycline (Wang et al., 2019).
6.3.5 Polymyxins
Polymyxins, comprising polymyxin B and polymyxin E (colistin), serve as antibiotics for treating Gram-negative MDR infections, including those caused by A. baumannii. They function by altering and destabilizing the LPS of the outer bacterial membrane (Shatri and Tadi, 2024).
As polymyxins, particularly colistin, emerge as the last line of defense against MDR A. baumannii infections, the rise of colistin-resistant A. baumannii strains presents significant public health implications. The escalating prevalence of these strains, particularly in healthcare settings, can be attributed to selective pressure on bacteria and the extensive use of polymyxins, even against carbapenem-resistant Gram-negative bacteria (Lima et al., 2020).
A. baumannii's resistance to polymyxins can occur through two primary mechanistic modalities: the complete loss or modification of the target LPS. This resistance can be chromosomally or plasmid mediated. Mechanisms include mutations in genes responsible for synthesizing lipid-A and LPS cofactors, alterations in the outer membrane structure through modifications of lipid A, metabolic shifts affecting amino acids, and the upregulation of efflux pumps (Lima et al., 2018).
6.3.6 Combination therapy
While combination therapies are often employed to combat MDRA. baumannii infections, it is important to note that resistance mechanisms can also develop against these combinations. A review of 12 studies involving 1,040 patients with MDR Acinetobacter infections reported variable mortality rates, ranging from 27 to 57.1% in the combination therapy groups. Although some combinations, such as carbapenem with colistin and carbapenem with ampicillin/sulbactam, showed improved outcomes, the benefit was inconsistent across all studies. Furthermore, resistance emergence was observed with off-label tigecycline-based regimens, underscoring the adaptability of A. baumannii to combination treatments (Poulikakos et al., 2014). Additionally, a systematic review of 84 studies evaluating 818 A. baumannii isolates resistant to all components of combination therapy found that while specific double and triple polymyxin-based regimens exhibited synergy at clinically relevant concentrations, no combination was universally effective against all strains. Some combinations showed synergy only at concentrations unlikely to be clinically achievable (Karakonstantis et al., 2021).
This adaptation poses challenges in combating A. baumannii infections and underscores the need for effective antimicrobial stewardship to preserve the efficacy of polymyxins in clinical settings.
7 Diagnostic approaches
Rapid and accurate identification of A. baumannii is clinically crucial, as it enables timely and precise diagnosis, which has significant therapeutic implications, enabling healthcare providers to implement targeted and effective treatment strategies for managing severe infections caused by this bacterium. For the diagnosis of A. baumannii, many tests can be used, either phenotypic or molecular, and they are used primarily nowadays (Tables 2, 3).
7.1 Laboratory methods
7.1.1 Traditional and automated phenotypic methods
In cases where A. baumannii is suspected, a specimen is typically obtained and cultured on blood agar, chromogenic agar, and MacConkey agar (Thermo Fisher Scientific, Basingstoke, UK). These cultural methods aid in identifying bacterial growth based on Gram stain and colony morphology (Khan et al., 2015). These traditional methods are commonly used in clinical settings, although they are time-consuming and may have limited sensitivity for detecting A. baumannii in low-density infections (Ayoub Moubareck and Hammoudi Halat, 2020).
Additionally, the antibiogram, an antibiotic sensitivity test, offers another means of detecting A. baumannii, primarily utilizing the disk diffusion method. This method, outlined in the Clinical and Laboratory Standards Institute (CLSI) guidelines, specifically measures resistance to colistin using the agar dilution method (Performance, 2016). It involves impregnating bacteria with specific antibiotics and measuring the diameter of the antibiotic zone of inhibition. This method is widely used but can be slow and limited in its ability to detect all resistance mechanisms. Adewoyin et al. reported that A. baumannii demonstrates resistance to various antibiotics, including piperacillin-tazobactam (11.2%), ceftazidime (12%), cefotaxime (18.8%), cefepime (8.8%), imipenem (2.7%), meropenem (4.15%), amikacin (2.4%), gentamicin (8.8%), tetracycline (16.8%), ciprofloxacin (11%), and trimethoprim/sulfamethoxazole (20.5%). Furthermore, this bacterium is known for its MDR (Adewoyin et al., 2021). matrix-assisted laser desorption–ionization time-of-flight mass spectrometry (MALDI-TOF MS) is able to identify A. baumanii with 100% specificity when combined with chemometric tools. It detects β-lactamase activity by analyzing antibiotic hydrolysis (e.g., imipenem degradation by carbapenemases), Nonetheless, it is limited to β-lactamase-mediated resistance; efflux pumps or porin mutations require supplementary testing. This method is beneficial because of its ability to directly detecting resistance in 2.5–4 h from blood cultures. MALDI-TOF MS is highly costly, but it reduces long-term labor and consumable costs (Bagudo et al., 2020).
Moreover, MCDA-LFP can simultaneously identify A. baumannii (via pgaD) and carbapenem resistance (via bla_OXA-23-like) with 100% specificity and in <1.5 h. To note that it is a cost-effective method as it eliminates the need for thermocyclers, reducing infrastructure costs (Hu et al., 2019).
CarbAcineto NP test is beneficial as it detects all carbapenemase types (OXA, GES, metallo-β-lactamases) with 94.7% sensitivity and 100% specificity, by outperforming traditional biochemical assays, which fail to detect non-MBL carbapenemases. This method is cost-effective as it uses affordable reagents, gives rapid results (in 2 h), and requires minimal training, rendering it suitable for resource-limited settings (Dortet et al., 2014).
A notable phenotypic characteristic of A. baumannii is its ability to form biofilms, which are considered virulence factors enabling the bacteria to persist on both biotic and abiotic surfaces (Khalawetektook and Tektook, 2018; Goodman, 1988). Biofilm formation also contributes to increased resistance to antibiotics by facilitating the production of capsular polysaccharides (Gallego, 2016). Biofilms can be detected using the polystyrene blue assay, which relies on crystal violet staining. This method is in use but can be less sensitive and is generally more labor-intensive.
Statistical analysis by Yang et al. revealed that the ompA and bap genes play a significant role in A. baumannii biofilm formation and antimicrobial resistance (Yang et al., 2019). The study found that the ompA gene was prevalent in 68.8% of the A. baumannii isolates and plays a crucial role in biofilm formation and antimicrobial resistance. ompA aids in surface adhesion and contributes to biofilm initiation, which aligns with its role in enhancing bacterial survival and evading immune responses. In contrast, the bap gene, found in 79.2% of the isolates, was similarly linked to biofilm formation and antimicrobial resistance. It is essential for biofilm maturation, promoting bacterial aggregation and stabilizing the biofilm structure, which in turn contributes to reduced antibiotic penetration and increased bacterial persistence (Yang et al., 2019).
In colistin resistance, detecting resistance in A. baumannii poses significant challenges due to the complexity of resistance mechanisms, particularly the presence of mcr genes and modifications in the pmrCAB operon. Phenotypic methods like broth microdilution (BMD) are considered the gold standard. Still, they are time-consuming, while molecular techniques such as PCR offer precise detection of mcr genes but require specialized resources (Novović and Jovčić, 2023). The most thorough detection strategy is a hybrid approach that combines the two techniques. BMD and PCR are advised for accurate detection in high-resource environments, whereas disk elution and other more straightforward phenotypic tests are more useful in low-resource environments (Arroyo et al., 2005). Molecular epidemiology relies on PCR for tracking resistance patterns and outbreaks. Additionally, mechanisms such as the loss of LPS production contribute to colistin resistance, highlighting the need for diverse diagnostic approaches (Moffatt et al., 2010).
7.1.2 Conventional molecular methods
Currently, the definitive diagnosis and identification of A. baumannii are primarily accomplished through molecular methods, particularly PCR, which is used extensively for confirmation of A. baumannii and the detection of resistance genes to carbapenems (Turton et al., 2006). This method, also known as random amplified polymorphic DNA (RAPD), can be employed in investigations to trace local spread during epidemics (Pourcel et al., 2011). PCR is widely used due to its sensitivity and specificity, but it can be time-consuming and requires laboratory infrastructure for reliable results.
In addition to traditional PCR, several other techniques, such as PCR replicon typing, multiplex PCR, and multiplex RT-PCR, offer accurate and efficient screening methods for various bacterial strains and genetic targets. Polymerase chain reaction-electrospray ionization mass spectrometry (PCR-ESI/MS) provides broad-based capabilities for detecting samples, specifically identifying silent mutations and genetic rearrangements of target genes (Wolk et al., 2012). While promising, PCR-ESI/MS is still under development and not widely used in clinical settings, and the majority are currently available only for research use. It requires specialized equipment and is relatively expensive and time-consuming compared to traditional PCR (Wolk et al., 2012).
Moreover, repetitive sequence-based PCR (rep-PCR) and multiple locus variable number of tandem repeat analysis (MLVA) enable the detection of repetitive sequences in the bacterial genome and tandem repeats, respectively (Rafei et al., 2014). While rep-PCR is mainly used for genotyping rather than routine clinical detection of A. baumannii, MLVA has been applied in clinical settings for outbreak surveillance and epidemiological studies due to its high discriminatory power. However, MLVA's use in routine diagnostics is limited by the need for specialized analysis tools and potential variability in repeat numbers, which may impact reproducibility (Anwer, 2024). PFGE is a method for studying the clonality of environmental and clinical isolates (Goodman, 1988). Widely regarded as the gold standard in epidemiological investigations, PFGE is instrumental in understanding the spread of pathogens (Zarrilli et al., 2013). However, it is time-consuming and requires specialized equipment, making it less practical for routine use in clinical diagnostics.
7.1.3 Emerging and novel tools
Various typing methods are employed in large-scale epidemiological studies, including ribotyping and amplified fragment length polymorphism (AFLP). Sequence-based approaches, such as single-locus typing (e.g., adeB, rpoB, blaOXA-51-like, and recA) or multilocus sequencing (e.g., 3-LST, trilocus sequencing), are also utilized to characterize A. baumannii isolates (Rafei et al., 2014). These methods offer high precision but are more complex and require advanced infrastructure, limiting their widespread application in clinical diagnostics (Anwer, 2024).
Furthermore, loop-mediated isothermal amplification (LAMP) has emerged as a rapid and highly sensitive tool for detecting carbapenemase genes associated with A. baumannii resistance, notably ISAba1-blaOXA-51-like. LAMP offers advantages such as cost-effectiveness and fast processing, requiring only 21 min for the entire procedure (Mu et al., 2016). This method enables amplification without the prior purification of the pathogen's DNA and allows for sensitive detection of genes, such as recA and the carbapenemase OXA-23. LAMP is practical, inexpensive, and faster than many PCR-based methods, making it a strong candidate for use in resource-limited settings. Additionally, multiplex LAMP can be employed for the simultaneous detection of multiple genes, further enhancing its practicality for rapid, comprehensive diagnostics (Yang et al., 2018).
The lab-on-a-disk (LOAD) specimen method is a sample-to-answer diagnostic approach for bacterial infections. It offers simplicity and automation across all its steps, including DNA extraction and purification from bacteria, targeted genetic marker amplification via LAMP reaction, and signal detection. The LOAD platform demonstrates high specificity and rapidity, providing results within 2 h. This makes it particularly valuable for critically ill patients at risk of shock or sepsis, facilitating early administration of appropriate treatment. Notably, its microfluidic setup enables operation independent of fluid composition (Loo et al., 2017). The rapid turnaround time of LOAD makes it a promising method for point-of-care settings. However, it is still under development and may not be widely available in clinical practice.
Fatmawati et al. emphasized the efficacy of a multidisciplinary approach, combining various tests such as antibiograms, genetic profiling of OXA-23 genes, and random amplified polymorphism DNA polymerase chain reaction (RAPD-PCR), in surveillance systems. Particularly beneficial in hospitals with limited microbiology laboratories, this cost-effective strategy enables early assessment of MDR A. baumannii. Furthermore, it holds promise for extending detection to other MDR bacteria (Fatmawati et al., 2023).
7.2 Challenges in accurate diagnosis
Several challenges hinder the accurate diagnosis of A. baumannii infections, foremost among them being the incomplete understanding of its virulence mechanisms. Furthermore, the intricate interplay between virulence factors and antimicrobial resistance complicates diagnostic efforts (Wong et al., 2017).
For instance, drug-resistant strains pose a significant challenge in lateral flow immunoassays (LFIA), a technique reliant on antibodies targeting specific bacterial antigens. LFIA offers practicality and rapidity in detection (Wang et al., 2022, 2023b). In the case of A. baumannii, monoclonal antibodies targeting genes such as OXA-28 and OXA-40 can aid in assessing the bacterium's carbapenem resistance clinically (Mertins et al., 2023). However, drug-resistant strains may exhibit antigenic variation or altered expression of resistance markers, reducing monoclonal antibodies (mAbs) binding efficiency and leading to false negatives in LFIA tests. Additionally, some carbapenemase-producing strains express diverse OXA variants, which may not be recognized by the antibodies used in standard LFIA assays. These challenges are particularly relevant in clinical settings, where timely and accurate detection of resistant strains is crucial for appropriate treatment. Implementation of an LFIA test in the clinical setting has to take into account the local epidemiological context in terms of the prevalence of resistance mechanisms (Boutal et al., 2022).
Furthermore, traditional DNA sequencing methods are time-consuming, costly, and labor-intensive, particularly when analyzing different strains. An innovative approach called PCR-restriction fragment length polymorphism (PCR-RFLP) has emerged to address this. Mismatched PCR-RFLP assays, for instance, have been developed to detect specific genes associated with A. baumannii's resistance to fluoroquinolones, offering rapid and accurate differentiation from other Acinetobacter strains (Kakuta et al., 2020).
Regarding PCR-ESI/MS, as mentioned earlier, several potential challenges exist, including the risk of contamination and limitations in identifying mixed infections due to the need to analyze multiple factors. Also, detecting colony-forming units as low as 1–10/mL may pose a limitation. Moreover, PCR-ESI/MS tends to be costlier than existing molecular diagnostic tools (Wolk et al., 2012).
Furthermore, specific molecular techniques for A. baumannii detection, such as PCR, DNA sequencing, and MALDI-TOF-MS, have their own limitations, particularly in resource-limited regions. These include high costs and the requirement for highly trained professionals (Wang et al., 2023a).
7.3 Novel diagnostic tools and technologies
Traditionally, the DNA–DNA hybridization assay was utilized, but it has since been supplanted by WGS methods and single-nucleotide polymorphism (SNP) determination. Currently, Albert et al. have demonstrated that a multiplex gyrB PCR assay can be employed for easy A. baumannii identification (Albert et al., 2022). While PCR is highly sensitive and specific, it requires specialized equipment and trained personnel, making it less cost-effective in resource-limited settings compared to newer technologies. Moreover, NALFA, short for nucleic acid amplification lateral flow assay, offers minimal development costs and easy production, making it highly cost-effective. It boasts a short immunoassay reaction time of <20 min and can detect various drug-resistant bacteria on the same paper without amplifying specific antibodies. NALFA is advantageous in its simplicity, lower cost, and rapid turnaround, but it may not match the sensitivity of PCR or mass spectrometry methods (Boutal et al., 2022).
On the contrary, the LAMP technique, the most developed isothermal amplification method, is cost-effective and simple, but requires specialized personnel for result interpretation and may take longer than an hour. Hence, a novel technology combining both methods has been innovated, specifically for detecting A. baumannii and CRAB. This method offers a quick and particular diagnostic test that can be completed in <43 min, combining the low cost and ease of NALFA with the sensitivity of LAMP. It also eliminates the need for specialized personnel, thus enhancing its practicality in diverse healthcare settings (Wang et al., 2023a).
In addition, MALDI-TOF-MS is another method capable of identifying and confirming bacterial species, making it suitable for detecting A. baumannii (Owusu et al., 2023). With a sensitivity of 100% for A. baumannii detection, it exhibits a specificity ranging from 91.7 to 99% (Vijayakumar et al., 2019). While MALDI-TOF-MS provides rapid results (typically within minutes) and offers high specificity and sensitivity, it requires a significant initial investment in equipment and regular maintenance, which might not be feasible for low-resource hospitals. As the technology continues to develop and costs decrease, it is expected that these methods will become more widespread and accessible (Jacobs et al., 2021).
Furthermore, the Vitek2 Compact system, utilizing ID-GNs cards, has demonstrated a 99% accuracy rate in diagnosing A. baumannii compared to traditional methods (Manfi Ahmed et al., 2022). This system is efficient and offers rapid results, but its higher cost, limited ability to distinguish between species within the A. baumannii complex, and the need for trained personnel limit its applicability (Lee et al., 2014).
Finally, in artificial intelligence (AI), a machine learning system has been developed and proven effective in the early detection of A. baumannii infection and/or colonization. This system utilizes data from laboratory tests and time series chest X-rays to aid in administering individualized treatments and achieving better outcomes by estimating risks. While promising, this technique requires further validation through additional prospective large-scale trials and studies, as suggested by Zeng et al. (2024). The main advantage of AI systems lies in their potential for personalized treatment, although current limitations include the need for extensive data and further refinement in accuracy.
8 Treatment strategies for A. Baumannii infections
Despite advancements in antimicrobial therapy, A. baumannii remains an essential but challenging-to-treat pathogen, owing to its tendency to develop resistance and to cause severe clinical complications. The antibiotic options for treating A. baumannii are remarkably restricted due to high rates of resistance. Nonetheless, different antibiotic treatment modalities, including empirical and targeted therapies and alternative non-antibiotic treatments, are opted for the optimal management of A. baumannii infection (Table 4).
8.1 Antibiotic therapies
In the absence of definitive microbiological pathogen identification and susceptibility testing, the initial antimicrobial regimen involves starting with empirical antimicrobial therapy (EAT). The latter aims to cover potential pathogens based on the patient's clinical history, risk factors, and local epidemiology, thereby halting further complications (Boyd and Nailor, 2011; Luo et al., 2023). Empirical therapy for A. baumannii infections necessitates broad-spectrum antibiotics that are active against Gram-negative bacteria. EAT against A. baumannii includes first-line and second-line antibiotics.
First-line antibiotics are prescribed for infections caused by relatively antimicrobial-susceptible A. baumannii isolates. β-Lactam antibiotics, including carbapenems and β-lactam/β-lactamase inhibitor combinations, are often adopted as first-line treatment. In ICU settings with lower prevalence of CRAB, carbapenems, such as meropenem and imipenem, are usually considered the drugs of choice due to their broad-spectrum potency, safety, and tolerability profile compared to other antibiotics within the same family (Song et al., 2019; Liebchen et al., 2020; Kim A. et al., 2009; Papp-Wallace et al., 2011; Henry, 2019; Garnacho-Montero et al., 2015). Additional empirical strategies include utilizing sulbactam, such as in ampicillin/sulbactam combination modality, which relies on the irreversible intrinsic activity of sulbactam against A. baumannii (Smolyakov et al., 2003). Other modalities may involve the use of carbapenems in combination with a β-lactam and a β-lactamase inhibitor, such as piperacillin-tazobactam, to enhance gram-negative coverage and prevent resistance (Kuo et al., 2007). For mild infections, fluoroquinolones, such as ciprofloxacin and levofloxacin, and third-generation cephalosporins may also be considered based on local susceptibility data, despite higher resistance rates (Abdul-Mutakabbir et al., 2021). Aminoglycosides, including amikacin and gentamicin, may sometimes be used in combination with other antibiotics (Vidal et al., 2007; Thy et al., 2023).
Second-line agents are employed in the setting of a high suspicion of CRAB or resistance to the other first-line agents, second-line agents are employed. These agents mainly include polymyxins and tetracycline derivatives, with sulbactam remaining an efficient option (Tamma et al., 2020). Polymyxins, such as polymyxin B and polymyxin E, are reserved for serious MDR infections due to their nephrotoxicity and neurotoxicity profile (Tamma et al., 2020; Batirel et al., 2014; Garnacho-Montero et al., 2003; Intravenous, 1999). Tetracycline derivatives, such as tigecycline and minocycline, are used for their broad-spectrum activity against resistant strains, although tigecycline's low serum concentration limits its use in bloodstream infections (Greig and Scott, 2016; Ritchie and Garavaglia-Wilson, 2014; Zhanel et al., 2006; Ni et al., 2016). Polymyxin-based combination therapy is often recommended for CRAB cases to enhance efficacy and prevent further resistance. The rationale for combination therapy lies in the synergistic or additive effects between agents, enabling for more effective eradication of the infecting pathogen. However, selecting appropriate combinations requires careful consideration of antimicrobial susceptibilities, potential drug interactions, and the risk of toxicity. The common combination regimens include polymyxin with a tetracycline derivative or sulbactam (Kengkla et al., 2018). For instance, colistin or polymyxin B combined with tigecycline or minocycline, or with sulbactam, showed effective selections in vitro (Song et al., 2007; Kempf et al., 2012b). Newer sulbactam combinations, such as durlobactam/sulbactam, have emerged as a newer and a step forward treatment for CRAB infections (Watkins and Bonomo, 2023; Karruli et al., 2023).
Despite the efficacy of empirical therapy, it is essential to recognize that over-reliance on these regimens can increase the risk of developing resistance and lead to suboptimal outcomes (Luo et al., 2023). Thus, once the diagnostic testing identifies the causative pathogen, switching from broad-spectrum empirical antibiotics to narrower-spectrum targeted agents is essential to ensure satisfactory outcomes and mitigate the risk of further resistance. Employing targeted antibiotic therapy effectively minimizes side effects and damages to the patient's microbiota and reduces the selective pressure for antimicrobial resistance. By tailoring the treatment to the specific pathogen and its resistance profile, targeted and personalized therapy not only enhances the efficacy of the treatment but also minimizes antibiotic abuse.
Epidemiological data highlight significant geographical variations in MDR/XDR A. baumannii prevalence, directly influencing the appropriate antimicrobial treatment selection, especially for those used as empirical therapies. A recent meta-analysis underscores these geographical disparities, revealing notably high carbapenem resistance in Asia, with an incidence of 76.2%, while America reports the lowest incidence at 69.4% (Beig et al., 2024). A more detailed study from the Asia–Pacific region, conducted from 2012 to 2019, revealed that carbapenem resistance rates were as low as 2.8% in Japan and 6.5% in Australia, but reached as high as 88% in South Korea and 87.2% in India. Among the carbapenem-resistant isolates, 96.6% harbored at least one carbapenemase gene, with the blaOXA-23 gene being the most prevalent. The study also reported significant resistance to other antibiotics, including amikacin, ampicillin/sulbactam, and cefepime, highlighting the ongoing challenge of managing A. baumannii infections in regions with high resistance rates (Lee et al., 2023). This geographical variation underscores the importance of understanding local resistance patterns and the limited treatment options.
To guide evidence-based clinical decision-making, several authoritative organizations, including the Infectious Diseases Society of America (IDSA) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), provide treatment recommendations for A. baumannii infections. For CRAB infections, IDSA recommends combining sulbactam-durlobactam with either meropenem or imipenem-cilastatin as the preferred regimen. High-dose ampicillin-sulbactam, in combination with at least one additional agent, has been downgraded from a preferred to an alternative option if sulbactam-durlobactam is unavailable. The suggested dosing for high-dose ampicillin-sulbactam is 27 grams daily (18 g of ampicillin and 9 g of sulbactam; Tamma et al., 2024). In contrast, ESCMID recommends polymyxin-meropenem combination therapy (high certainty of evidence) and polymyxin-rifampin combination therapy (moderate certainty of evidence) for all patients with CRAB infections. For patients with severe or high-risk CRAB infections, ESCMID suggests combination therapy using two in vitro active antibiotics from available options (polymyxin, aminoglycoside, tigecycline, or sulbactam combinations). However, this recommendation is conditional with very low certainty of evidence. For patients with CRAB infections and a meropenem minimum inhibitory concentration (MIC) <8 mg/L, carbapenem combination therapy with high-dose extended-infusion carbapenem dosing is considered good clinical practice (Paul et al., 2022). Consulting local guidelines ensures that treatment strategies align with the regional resistance patterns and available therapeutic options.
8.2 Alternative non-antibiotic therapies
To counteract the rising antibiotic resistance and the challenges to discovering and synthesizing new antibiotics, alternative treatment strategies are being explored for managing the emergence and dissemination of MDR A. baumannii. These mainly include non-antibiotic therapies such as bacteriophage therapy, immunization-based therapy, monoclonal antibodies, and adjunctive therapies such as antimicrobial peptides and antimicrobial photodynamic therapy.
8.2.1 Phage therapy
Bacteriophages are natural bacterial killers used in practical applications since the early 20th century. In the post-antibiotic era, phagebiotics have received special attention as an alternative treatment option to fight bacterial infections (Wei et al., 2020; Gordillo Altamirano and Barr, 2019). The main advantage of phage therapy over traditional antibiotic treatments lies in the high specificity of phages to their targets, effectively killing pathogenic bacteria without affecting the normal microflora (Rai and Kumar, 2022). Aleshkin et al. conducted a small-scale clinical trial to evaluate the therapeutic efficacy of a phage cocktail in an ICU emergency caused by multiresistant bacterial strains, including A. baumannii. After 3 days of administering phage therapy, the infectious contamination level in 14 patients dropped from 79 to 21%, indicating clinical improvement (Aleshkin et al., 2016). Additionally, an 88-year-old patient diagnosed with CRAB-caused HAP was treated with personalized phage lysis preparation in combination with tigecycline and polymyxin E. The treatment was well tolerated and resulted in the clearance of the pathogen and improvement in the patient's lung function (Clemmer et al., 2011). Another study showed that administering A. baumannii phage cocktails to a patient with necrotizing pancreatitis, diabetes mellitus, and MDR A. baumannii infection gradually improved overall clinical symptoms (Schooley et al., 2017). Indeed, compared with single phage administration, phage–antibiotic combination therapy is a promising treatment option. In a critically ill patient with MDR A. baumannii pneumonia, intravenous phage treatment co-administered with antibiotics significantly improved the patient's condition compared to single administrations (Rao et al., 2022).
While phage therapy is gaining prominence as an antibiotic alternative, its clinical implementation faces several challenges. While it is advantageous in reducing off-target effects, the high specificity of bacteriophages necessitates individualized treatment plans that may not be feasible for widespread clinical application. Bacterial resistance to phages and the potential for immune system recognition and neutralization remain significant concerns. Large-scale clinical trials are required to establish standardized protocols, determine optimal delivery methods, and assess long-term safety and efficacy (Pires et al., 2020; Ogungbe et al., 2024).
8.2.2 Immunization-based therapy
Vaccine development targeting antibiotic resistance-associated proteins and virulence factors has gained interest as a proactive approach to preventing infections rather than treating them after occurrence (Micoli et al., 2021). Vaccine candidates against A. baumannii have demonstrated promising preclinical efficacy. For instance, vaccination of diabetic mice with recombinant OmpA (rOmpA) markedly improved survival, reduced tissue bacterial burden, and induced high titers of anti-OmpA, which are correlated with better survival in mice (Luo et al., 2012). Another study conducted an in silico analysis of the A. baumannii ATCC 19606 proteome, predicting that FilF, a conserved OMP, could be a potential vaccine candidate. The study demonstrated that FilF provided immunoprotective efficacy in mice, indicated by increased antibody titers, reduced inflammation, and decreased severity of infection and bacterial burden (Singh et al., 2016). In combination therapy, Huang et al. utilized a mouse sepsis model to examine the effect of delivering anti-A. baumannii OMV antibodies through both active and passive immunization strategies. The results indicated significant improvements in susceptibility to quinolone antibiotics, higher survival rates, and reduced bacterial loads in the organs (Huang W. et al., 2019). In another pneumonia model, the combination of serum anti-A. baumannii OMV and levofloxacin enhanced antibiotic sensitivity and significantly reduced bacterial loads in the lungs and spleen compared to single treatments (Huang W. et al., 2019).
Despite promising preclinical results, developing an effective A. baumannii vaccine faces several challenges. The genetic diversity of A. baumannii strains complicates the identification of universally protective antigens. Moreover, immunization strategies must balance efficacy with potential adverse effects, particularly in immunocompromised patients. The design and execution of clinical trials for such vaccines are further complicated by the need to select appropriate diseases and target populations, particularly without established immune markers. Further research is essential to optimize antigen selection, evaluate long-term immune responses, and conduct comprehensive clinical trials to assess the effectiveness (Brazzoli et al., 2023; Rumata et al., 2023).
8.2.3 Monoclonal antibody-mediated therapy
Beyond vaccination, mAbs are gaining significant momentum as a viable therapeutic option for infections caused by antibiotic-resistant bacteria. Nielsen et al. reported that a mAb, C8, raised against A. baumannii capsule showed a protective effect in bloodstream and lung models of A. baumannii infection, including an XDR clinical isolate. This mAb enhanced bacterial clearance, prevented the progression to septic shock, and worked synergistically when combined with colistin (Nielsen et al., 2017). Building on this, recent advances have led to the development of mAb 65, which offers broader strain coverage than C8 and significantly improves survival rates in murine models of bacteremic sepsis and aspiration pneumonia (Nielsen et al., 2017). Notably, mAb 65 enhances bacterial clearance, modulates inflammatory responses, and exhibits synergy with colistin, improving overall treatment outcomes (Nielsen et al., 2017). In another study, utilizing a human immune repertoire mouse model, researchers identified 297 antibodies targeting clinically relevant A. baumannii strains and analyzed 26 for functional potential. Among these, mAb1416, which specifically targets the KL49 capsular polysaccharide, demonstrated prophylactic efficacy in vivo against CRAB lineages associated with neonatal sepsis mortality (Baker et al., 2024). Additionally, Yeganeh et al. developed novel mAbs against OmpA of A. baumannii, with 1G1-E7 showing strong bacterial reactivity and significantly enhancing opsonophagocytic killing of a clinical isolate. These findings highlight OmpA as a promising target for antibody-mediated therapies against A. baumannii infections (Yeganeh et al., 2021).
Monoclonal antibody therapy offers a targeted approach to combating MDR A. baumannii infections, but high production costs and complex manufacturing processes may limit accessibility. Variations in bacterial surface antigens can reduce efficacy across different strains, while potential immunogenicity and the need for repeated dosing present additional challenges. Addressing these limitations requires advances in biotechnology, optimized delivery strategies, and rigorous clinical research to ensure safety, efficacy, and cost-effectiveness (Luo et al., 2025).
8.2.4 Antimicrobial peptides
Antimicrobial peptides (AMPs) emerge as promising new antimicrobial agents with significant potential for treating A. baumannii infections. These peptides, also known as host defense peptides, are naturally produced by nearly all living organisms as part of their innate immune system to combat pathogens (Fan et al., 2016; Bucataru and Ciobanasu, 2024). Numerous AMPs are currently under investigation to determine their therapeutic efficacy against A. baumannii strains, and various in vitro and in vivo studies have demonstrated that several AMPs exhibit antimicrobial activity against MDR A. baumannii (Vila-Farres et al., 2012; Jung et al., 2021; Alsaab et al., 2023; Jaśkiewicz et al., 2019; Ji et al., 2023). For instance, an in vivo study using a mouse model identified two AMPs, SMAP-29 and TP4, as having prophylactic effects that prevented mortality in mice with A. baumannii-induced pneumonia. Additionally, two derivatives of TP4, dN4 and dC4, showed therapeutic efficacy in pneumonia mouse models when administered peritoneally or intravenously. These derivatives also effectively inhibited and/or eradicated A. baumannii biofilms at higher doses (Jung et al., 2021). Moreover, research by Vila-Farres et al. examined the activity of 15 peptides against both colistin-susceptible and colistin-resistant A. baumannii strains in vitro. Among the AMPs tested, mastoparan displayed notable bactericidal activity at a concentration 8 times the minimum inhibitory concentration (MIC x8) against both strains, indicating its potential as an alternative treatment for colistin-resistant A. baumannii infections (Vila-Farres et al., 2012). Interestingly, when mastoparan was co-administered with colistin in another study, a 100% synergistic effect was observed against 24 XDR A. baumannii strains (Kim et al., 2023). Despite their promising antimicrobial potential, AMPs face several challenges that hinder their clinical translation. High production costs, susceptibility to enzymatic degradation, and potential toxicity at therapeutic concentrations limit their widespread use. Additionally, the complex and lengthy drug development process, coupled with the incomplete understanding of their mechanism of action, poses further barriers to their optimization and regulatory approval. As a result, only a limited number of AMPs have progressed through clinical trials, with even fewer receiving FDA approval for clinical use (Bucataru and Ciobanasu, 2024; Mishra et al., 2025).
8.2.5 Antimicrobial photodynamic therapy
Antimicrobial photodynamic therapy (aPDT) offers a promising alternative and complementary approach to battle MDR A. baumannii (Bustamante and Palavecino, 2023). This technique combines a photosensitizer, light, and oxygen to produce reactive oxygen species (ROS), which exert bactericidal or bacteriostatic effects by inducing photooxidative stress on bacterial structures. This method is beneficial for localized infections and minimizes damage to surrounding healthy tissues (da Fonseca et al., 2021; Piksa et al., 2023; Rajesh et al., 2011). One study evaluated the efficacy of aPDT based on natural photosensitizers, riboflavin- and chlorophyllin, using near-ultraviolet or blue light on A. baumannii biofilms. The study found that the antibacterial effect of riboflavin-based aPDT depends on photoactivated riboflavin's ability to generate intracellular ROS. Additionally, chlorophyllin-based aPDT's effectiveness was linked to the sensitivity of A. baumannii biofilms to light exposure. The results significantly impacted bacterial viability, structural stability, and ROS generation (Buchovec et al., 2022). Another study evaluated the effect of aPDT against carbapenem-susceptible and CRAB strains, using methylene blue and a low-level laser. The results showed a decline in A. baumannii cells across all isolates tested, with reductions ranging from 63 to 88% for susceptible isolates and 26–97% for resistant ones (Marcolan De Mello et al., 2019). Further research evaluated the photodynamic inactivation (PDI) efficacy mediated by aloe emodin (AE), a natural compound isolated from Aloe vera and Rheum palmatum, against MDR A. baumannii isolates in vitro. AE exhibited no significant dark toxicity and effectively inactivated clinical isolates in a concentration -and light-dose-dependent manner. The study revealed that AE-mediated aPDT caused genomic DNA damage and significant bacterial membrane disruption, as evidenced by the LIVE/DEAD ratio. These findings suggest that AE is a promising photosensitizer for aPDT treatment of infections caused by MDR A. baumannii (Li et al., 2020).
Despite its potential, aPDT faces several limitations, including limited penetration depth of light, potential damage to host tissues, and the need for precise dosing to avoid cytotoxic effects. The efficacy of aPDT is also influenced by bacterial resistance mechanisms and variations in light absorption properties among different strains. More extensive clinical research is required to determine optimal photosensitizers, refine light delivery techniques, and assess long-term safety for use in human infections (Bustamante and Palavecino, 2023).
Though these alternative treatments hold great promise, further research is needed to establish their efficacy, safety, and clinical applicability in the management of A. baumannii infections. Rigorous clinical trials and studies are essential to understand these therapies' full potential and limitations, ensuring they can be effectively integrated into clinical practice to improve patient outcomes and combat the growing threat of antibiotic resistance.
9 Infection control and prevention
9.1 Strategies for preventing transmission in healthcare settings
9.1.1 Antimicrobial stewardship
Antimicrobial stewardship is a comprehensive program that entails antibiotic restriction policies aimed at optimizing antimicrobial use, reducing the incidence of infection, improving patients' clinical outcomes, ensuring minimum unintended consequences, and saving on unnecessary healthcare costs (Garnacho-Montero et al., 2015; WHO, 2019). It has been suggested as a way to manage A. baumannii-related outbreaks, as the proportion of patients in the ICU who are infected has decreased (Cheon et al., 2016). The intention is to stop circulating strains of A. baumannii from developing resistance in the future (Bianco et al., 2016). For a better adoption of its maximum interventions, basic continuous education of healthcare workers (HCWs) is necessary to improve the prescribing behaviors (Orok et al., 2025). Prospective or retrospective auditing, which provides feedback about the prescribed antibiotic treatment and de-escalation, which shifts away from broad-spectrum antibiotics to more targeted options, are key feedback interventions in this program that minimize the risks associated with inappropriate antibiotic use (WHO, 2019; Akpan et al., 2016). As part of this stewardship, a restricted list of authorized drugs for colistin, tigecycline, carbapenems, and fluoroquinolones is enforced to reduce antibiotic use and improve antibiotic prescribing practices (Meschiari et al., 2021).
9.1.2 Education
Adequate knowledge of infection prevention and control is crucial for preventing HAIs, which affect up to 37% of ICU patients. Despite knowledge, compliance with hand hygiene and personal protective equipment (PPE) use varied widely, so regular education and training should be implemented (Alhumaid et al., 2021). Training sessions were held for hospital staff to raise their compliance with hand hygiene protocols and to enhance their knowledge about why it is crucial to prevent the spread of A. baumannii (Cheon et al., 2016). The importance of following standard precautions was highlighted through educational sessions and reminders, which were integrated into a training program that also included a sociological component (Tanguy et al., 2017). To ensure that this measure is implemented, screensavers with hand hygiene guidelines downloaded from the WHO website were installed on all computers in the hospital (Cheon et al., 2016).
9.1.3 Hand hygiene and contact precautions
It is imperative for healthcare providers to rigorously adhere to standard and contact precautions that include hand hygiene before and after glove removal, as well as disposable gown and glove removal before exiting the room, to effectively suppress the transmission of infections (Tanguy et al., 2017; WHO, 2022). To facilitate proper hand hygiene in the ICUs, alcohol-based hand rub was made readily accessible at the bedside for all patients. Additionally, a non-alcoholic hand sanitizer was provided to any HCW who reported allergies to alcohol. Patients who tested positive for A. baumannii were included in a cohort in a designated area within each unit, and contact precautions were enforced (Cheon et al., 2016).
Moreover, in ICUs lacking sufficient isolation rooms or where the proximity of the beds presents a concern, patients were separated using transparent plastic curtains (Cheon et al., 2016). It aims to prevent cross-transmission between these patients along with the use of specialized personal protective equipment such as long-sleeved gowns, FFP3 masks, and face visors (Warde et al., 2019). However, antimicrobial curtains impregnated with silver or treated with quaternary ammonium chlorides were able to reduce the contamination by a significant margin compared to standard hospital ones (Luk et al., 2019). As an alternative to curtains, thick red lines painted on the floor can function as “virtual walls,” marking the boundaries of each patient's personal space, where passing through it in either direction means the performance of hand hygiene. This was associated with a decrease in the risk of acquiring resistant pathogens (Ben-Chetrit et al., 2018).
9.2 Environmental disinfection
9.2.1 Daily and post-discharge cleaning
Routine cleaning of rooms and surfaces was conducted daily, and after the discharge of every patient. Environmental cleaning using a chlorine-based disinfectants (10% sodium hypochlorite) was done at least twice per day in addition to hydrogen peroxide wipes for cleaning the medical devices (Meschiari et al., 2021; Tanguy et al., 2017; Warde et al., 2019). For sensitive electronic equipment, quaternary ammonium-containing wipes were employed (Ben-Chetrit et al., 2018). The concentration of disinfectants was carefully controlled to ensure effective cleaning, and surfaces are left to dry completely before being used again (Chmielarczyk et al., 2012).
9.2.2 Patient relocation and skin disinfection
Infected patients were temporarily moved to a transitory unit while their original rooms were disinfected. In this unit, the patient's skin was disinfected with 2% leave-on chlorhexidine disposable cloths or decolonized with octenidine-containing products, which have been shown to reduce the number of positive cases. Afterward, the patient was transferred back to the cleaned unit (Meschiari et al., 2021).
9.2.3 Terminal disinfection
When conventional cleaning approaches proved insufficient in preventing the spread of A. baumannii, a cutting-edge technique was employed. It utilized vaporized hydrogen peroxide (VHP) for terminal disinfection. VHP is a powerful disinfectant that eliminates a wide range of hospital-acquired pathogens while being safe for use, as it ultimately breaks down into harmless oxygen and water (Chmielarczyk et al., 2012). The disinfection was carried out using the Nocospray® (Oxy'Pharm, Champigny-sur-Marne, France) system, with the room sealed and emptied for the process to be effective (Meschiari et al., 2021; Tanguy et al., 2017). Moreover, ultraviolet-C (UV-C) of 222-nm wavelength, which damages the genetic material, was used for high-touch surfaces to avoid the possible deterioration of the material from long-term alcohol wipes. The effectiveness of UV-C light might depend on factors such as exposure time, dose, and UV intensity for each material, which necessitates further research to evaluate its potential applications fully and to assess the optimal exposure conditions (Huang et al., 2024).
9.2.4 Contamination control measures
Hospital rooms gained advantages from the use of portable HEPA filters, as they have been shown to lower airborne A. baumannii levels significantly (Barbut et al., 2013). To lessen the environmental contamination, closed-suction systems were also used for patients whose respiratory secretions contained A. baumannii. However, new protocols for aseptic methods during open suctioning were implemented for patients not requiring mechanical ventilation (Choi et al., 2010). The results of environmental control measures were reported to ICU head nurses, and subsequent cleaning efforts were focused on areas that yielded positive results for A. baumannii and near-patient ones (Cheon et al., 2016). Additionally, the Microflow α microbiological air sampler can be used to analyze the surrounding air, while fluorescent spray and UV torch were used to examine surfaces for the sake of assessing the thoroughness of terminal cleaning (Bianco et al., 2016; Meschiari et al., 2021).
Various techniques are under investigation to reduce A. baumannii prevalence in the ICU, although some may be harmful to people or surface materials. Owing to its resistance to the majority of sanitizers and antibiotics, phage φAB2 is recommended as an alternative for environmental decontamination, with close attention to concentration and incubation time (Chen et al., 2013).
9.3 Surveillance and outbreak management
The bacteria (culture and sensitivity) were identified using the agar diffusion method, and the VITEK® system (bioMérieux, Marcy-l'Étoile, France), while identification was performed using the MALDI-TOF-MS system (Tanguy et al., 2017; Sousa et al., 2014).
All ICU patients were tested for A. baumannii when admitted and continued to have weekly surveillance cultures until they were discharged or had three negative results. Samples collected for patient screening included swabs from the nose, groin, and throat, wound swabs, urine samples, a sputum sample, and potentially a stool sample. If the patient was intubated or had a tracheostomy, tracheal aspirate was obtained. Staff screening involved taking swabs from their nasal passages and skin lesions to monitor potential colonization. This was particularly important in identifying temporary colonization due to their frequent interactions with ventilators and other equipment used in patient care. Furthermore, a study was conducted among staff members to identify any temporary colonization due to their frequent interactions with ventilators and other equipment used in patient care (Cheon et al., 2016; Warde et al., 2019).
Testing was accomplished to evaluate the surroundings of patients who were colonized or infected with A. baumannii. Samples from a variety of surfaces, including bed rails, bedside tables, infusion pumps, blood pressure cuffs, supply carts, stethoscopes, notes trolleys, keyboards, shower handles, bathtub walls, floors, suction equipment, pillows, mattresses, sinks, washbasins, water taps, ventilator control panels, and external surfaces of patients' endotracheal tubes on mechanical ventilation, were collected both before and after the rooms were properly cleaned. A pre-moistened, sterile cotton swab can be used to test the environmental and hand cultures. After that, the swab is inoculated onto MacConkey agar plates (Cheon et al., 2016; Barbut et al., 2013; Choi et al., 2010; Alfandari et al., 2014).
An outbreak control team was put together during the outbreak and consisted of infectious disease physicians, a microbiologist, an infection control nurse, and senior nursing staff from the ICU. The first strategy for controlling the outbreak was to halt its transmission through strict contact precautions and intensified infection control measures. The second approach involved minimizing environmental contamination through sterilization and disinfection. Using a closed-suction system was the third strategy (Choi et al., 2010). Furthermore, meetings were conducted with HCWs to offer training periods and to address any potential flaws in the practices (Bianco et al., 2016). Proactive isolation of all new patients until they test negative may be required if standard isolation measures fail to stop the outbreak; if not, the ICU may need to close temporarily (Meschiari et al., 2021; Barbut et al., 2013). Modern means of communication, such as electronic alert systems utilizing GLIMS software (MIPS, a CliniSys Group company, Ghent, Belgium), can be beneficial by notifying whenever a positive sample is confirmed by the bacteriology laboratory, making work easier (Tanguy et al., 2017).
9.4 Role of infection control
Implementing comprehensive infection control measures is a critical intervention for effectively managing outbreaks related to A. baumannii. These measures included screening patients, separating affected individuals, promoting hand hygiene compliance, putting programs in place to prolong the effectiveness of antibiotics, and improving environmental cleaning and disinfection (Garnacho-Montero et al., 2015; Barbut et al., 2013). Applying these strategies simultaneously led to achieving success in reducing hospital-acquired infection rates and containing outbreaks of A. baumannii. Additionally, surveillance and monitoring help identify outbreaks early, enabling prompt intervention to contain the spread of the bacteria.
In summary, preventing and managing A. baumannii infections in healthcare settings requires a comprehensive approach, including antimicrobial stewardship, infection control measures, and environmental disinfection. Key strategies include optimizing antibiotic use, promoting hand hygiene and contact precautions, and implementing effective cleaning protocols. Surveillance and outbreak management, regular patient and staff screening, and electronic alert systems are crucial for early detection and intervention. Future research should start helping staff practice creating realistic outbreak scenarios, using virtual reality technology and AI-powered robots for real-time disinfection, to prevent widespread outbreaks.
10 Public health implications
10.1 Economic burden of A. Baumannii infections
A. baumannii infections impose a substantial financial burden on healthcare systems worldwide. These infections frequently result in extended hospital stays, larger number of ICU admissions, more need for invasive procedures, and extensive use of broad-spectrum antibiotics, all of which significantly drive up healthcare costs.
A study by Lee et al. on 252 patients with monomicrobial A. baumannii infections revealed that the overall 14-day mortality rate in cases of bacteremia was as high as 29.8% (Lee et al., 2012b). More recent studies have confirmed these findings, with A. baumannii-related infections continuing to be associated with high mortality rates and economic costs (Alrahmany et al., 2022; Appaneal et al., 2022). The financial burden is particularly true in cases of MDR strains of A. baumannii. Compared with non-MDR bacteremia, patients with MDR A. baumannii bacteremia experience a significantly higher severity of illness at onset, extended hospitalization (mean duration: 29 days vs. 22.5 days), and increased bacteremia-related mortality (56.2% vs. 4.7%; Zhou et al., 2019).
In addition to direct healthcare expenditures, A. baumannii infections also result in substantial indirect costs, such as productivity losses due to prolonged illness, long-term disability, and increased caregiver burden. For example, according to a study by the Public Health Computational and Operations Research (PHICOR) group at the University of Pittsburgh, Pittsburgh, PA, the mean cost to a hospital of each A. baumannii case was US$8,246 ± US$4,472 while the costs to the hospital ranged from US$7.4–26.1 million during the period 2006–2007 (Lee B. Y. et al., 2010). The need for expensive last-line antibiotics and heightened infection control measures further adds to the financial strain on healthcare systems (Antunes et al., 2014).
10.2 Impact on healthcare systems and patient outcomes: mortality and morbidity impact
The impact of A. baumannii infections on the healthcare systems worldwide extends beyond economic costs to profoundly affect patient outcomes and overall quality of care provided. Infections caused by MDR A. baumannii often occur in critically ill patients, particularly those in ICUs, causing severe complications, such as VAP, bloodstream infections, and meningitis, as previously discussed (Kanafani et al., 2018; Huang Y. et al., 2019; Jaruratanasirikul et al., 2019; Lynch et al., 2017; Sharma et al., 2019; Pan et al., 2018; Chang et al., 2000; Kelkar et al., 1989; Rodríguez Guardado et al., 2001; Diep et al., 2023). Recent epidemiological data indicate that MDR A. baumannii infections have case fatality rates ranging from 29 to 73%, depending on the site of infection and underlying patient conditions, and exceeding 90% in patients with septic shock (Appaneal et al., 2022; Corcione et al., 2024; Russo et al., 2018; Shorr et al., 2014; Tokur et al., 2022).
A significant factor contributing to the poor prognosis of A. baumannii infections is its high level of antimicrobial resistance, limiting the treatment options and necessitating last-resort antibiotics such as carbapenems and colistin, which are associated with significant side effects. This situation complicates the management of A. baumannii infections and eventually strains healthcare resources and imposing rigorous infection control measures (Peleg et al., 2008). Therefore, it is essential to emphasize the significance of global surveillance and the implementation of effective infection control measures to curb the spread of A. baumannii (Falagas and Karveli, 2007). Recent data from global surveillance programs indicate an alarming rise in XDR and PDR A. baumannii isolates, complicating treatment efforts even further (Quraini et al., 2023).
The increasing prevalence of drug-resistant A. baumannii strains underscores the urgent need for novel therapeutic approaches to overcome the issue of antibiotic resistance, including bacteriophage therapy, antimicrobial peptides, and combination antibiotic regimens, some of which are currently under clinical investigation (Li et al., 2024; Choi and Kim, 2024; Oladipo et al., 2025).
10.3 Future directions for research and intervention
Due to its ability to persist in healthcare environments and survive on surfaces for extended periods, A. baumannii presents a significant infection control challenge. Stringent measures are required to limit its spread, particularly in hospitals, nursing homes, rehabilitation centers, outpatient clinics, dialysis centers, urgent care centers, hospices, and military medical facilities, which is crucial to prevent the spread of A. baumannii. This includes implementing strict hand hygiene practices, which were found to be the most effective intervention (Doan et al., 2016), environmental cleaning, although it is challenging to eradicate A. baumannii even after terminal cleaning (Manian et al., 2011; Russotto et al., 2015), and active surveillance for MDR A. baumannii colonization by monitoring adherence to infection control guidelines. Nevertheless, infection control still presents a challenge, where even after four rounds of terminal cleaning and disinfection, Manian et al. reported that 26.6% of rooms newly vacated by patients with MDR A. baumannii had at least one positive culture site (Manian et al., 2011). International collaboration between researchers, healthcare providers, and public health authorities is essential to address this issue. Data sharing and molecular surveillance programs, such as the A. baumannii database hosted by PubMLST.org, facilitate tracking of resistance patterns and outbreak investigations (Bartual et al., 2005; Jolley et al., 2018; Diancourt et al., 2010; Gaiarsa et al., 2019).
11 Conclusion
Substantial strides have been attained in our understanding of A. baumannii, yet many perplexing mysteries abide. As a successful nosocomial pathogen, A. baumannii infection can be hospital-acquired, community-acquired, or even war-acquired. This literature review sheds light on this bacterium's infamy for the association with severe infections, antibiotic resistance, and high mortality and morbidity, underscoring the urgency of early recognition through advancements of diagnostic techniques and effective management strategies. As expounded upon previously, A. baumannii can achieve antibiotic resistance through almost all bacterial resistance mechanisms, mainly via enzymatic warfare, reduced membrane permeability, increased efflux mechanisms, target site modification, and genetic mechanisms of resistance. Moreover, a deeper understanding of A. baumannii's virulence factors and pathogenicity promotes future potential targets for therapeutic alternatives and even vaccine development, which is of exceptional importance to control such a pathogen. Particularly noteworthy are the strategies for preventing transmission in healthcare settings, including antibiotic stewardship and surveillance. Ultimately, A. baumannii is an affliction for both the economic and healthcare sectors, which makes this understanding essential for hospitals to invest more in controlling the spread of this infection, especially in the ICUs.
In conclusion, A. baumannii remains a formidable pathogen, with its ability to acquire AMR posing a significant threat to healthcare and public health systems globally. The evolving resistance mechanisms, particularly to carbapenems and colistin, underscore the urgent need to develop new therapeutic strategies. This review highlights the critical gaps in current treatment options and the importance of exploring novel approaches, such as antibiotic combinations, immunotherapies, and vaccines, which offer promising alternatives to traditional antibiotics.
The findings of this review should inform health policy decisions, particularly in implementing stricter infection control practices and antibiotic stewardship programs in healthcare settings, especially in ICUs, where A. baumannii poses the greatest threat. Moreover, as resistance rates continue to rise, investment in research for new drugs and diagnostic tools must be prioritized at the global level.
Given the clinical and global public health significance of A. baumannii infections, efforts to combat this pathogen must be intensified. This requires the collaboration of healthcare providers and researchers to drive innovative solutions. The urgency of addressing AMR in A. baumannii cannot be overstated. Only through international cooperation and the development of cutting-edge therapies can we hope to effectively manage and ultimately control the spread of this highly resistant pathogen.
Author contributions
AY: Writing – original draft. ZE: Writing – original draft. MC: Writing – original draft. ZD: Writing – original draft. MB: Writing – original draft. AB: Writing – original draft. LN: Writing – original draft. NN: Writing – original draft. MY: Writing – original draft. HB: Writing – original draft, Writing – review & editing. GG: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Figures 1–3, 5 were created with BioRender.com. BioRender reserves all rights and ownership of BioRender content. BioRender content included in the completed graphic is not licensed for commercial uses beyond publication in a journal. For any commercial use of this figure, users may, if allowed, recreate it in BioRender under an Industry BioRender Plan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdul-Mutakabbir, J. C., Griffith, N. C., Shields, R. K., Tverdek, F. P., and Escobar, Z. K. (2021). Contemporary perspective on the treatment of Acinetobacter baumannii infections: insights from the society of infectious diseases pharmacists. Infect. Dis. Ther. 10, 1–26. doi: 10.1007/s40121-021-00541-4
Abouelfetouh, A., Mattock, J., Turner, D., Li, E., and Evans, B. A. (2022). Diversity of carbapenem-resistant Acinetobacter baumannii and bacteriophage-mediated spread of the Oxa23 carbapenemase. Microb. Genom. 8:000752. doi: 10.1099/mgen.0.000752
Abraham, W. R. (2016). Going beyond the control of quorum-sensing to combat biofilm infections. Antibiotics 5:3. doi: 10.3390/antibiotics5010003
Adewoyin, M. A., Ebomah, K. E., and Okoh, A. I. (2021). Antibiogram profile of Acinetobacterbaumannii recovered from selected freshwater resources in the Eastern Cape Province, South Africa. Pathogens 10:1110. doi: 10.3390/pathogens10091110
Adler, B. L., Krausz, A., and Friedman, A. J. (2014). Acinetobacter baumannii emerging as a multidrug-resistant skin and soft-tissue pathogen: parallels to methicillin-resistant Staphylococcus aureus. JAMA Dermatol. 150, 905–906. doi: 10.1001/jamadermatol.2013.8855
Ahuatzin-Flores, O. E., Torres, E., and Chávez-Bravo, E. (2024). Acinetobacter baumannii, a multidrug-resistant opportunistic pathogen in new habitats: a systematic review. Microorganisms 12:644. doi: 10.3390/microorganisms12040644
Akeda, Y. (2021). Current situation of carbapenem-resistant Enterobacteriaceae and Acinetobacter in Japan and Southeast Asia. Microbiol. Immunol. 65, 229–237. doi: 10.1111/1348-0421.12887
Akpan, M. R., Ahmad, R., Shebl, N. A., and Ashiru-Oredope, D. (2016). A review of quality measures for assessing the impact of antimicrobial stewardship programs in hospitals. Antibiotics 5:5. doi: 10.3390/antibiotics5010005
Al Atrouni, A., Hamze, M., Rafei, R., Eveillard, M., Joly-Guillou, M. L., Kempf, M., et al. (2016a). Diversity of Acinetobacter species isolated from different environments in Lebanon: a nationwide study. Future Microbiol. 11, 1147–1156. doi: 10.2217/fmb-2016-0082
Al Atrouni, A., Joly-Guillou, M. L., Hamze, M., and Kempf, M. (2016b). Reservoirs of non-baumannii Acinetobacter species. Front. Microbiol. 7:49. doi: 10.3389/fmicb.2016.00049
Albert, M. J., Al-Hashem, G., and Rotimi, V. O. (2022). Multiplex gyrB PCR assay for identification of Acinetobacter baumannii is validated by whole genome sequence-based assays. Med. Princ. Pract. 31, 493–496. doi: 10.1159/000526402
Albrecht, M. C., Griffith, M. E., Murray, C. K., Chung, K. K., Horvath, E. E., Ward, J. A., et al. (2006). Impact of Acinetobacter infection on the mortality of burn patients. J. Am. Coll. Surg. 203, 546–550. doi: 10.1016/j.jamcollsurg.2006.06.013
Alenazi, T. A., Shaman, M. S. B., Suliman, D. M., Alanazi, T. A., Altawalbeh, S. M., Alshareef, H., et al. (2023). The impact of multidrug-resistant Acinetobacter baumannii infection in critically ill patients with or without COVID-19 infection. Healthcare 11:487. doi: 10.3390/healthcare11040487
Aleshkin, A. V., Ershova, O. N., Volozhantsev, N. V., Svetoch, E. A., Popova, A. V., Rubalskii, E. O., et al. (2016). Phagebiotics in treatment and prophylaxis of healthcare-associated infections. Bacteriophage 6:e1251379. doi: 10.1080/21597081.2016.1251379
Alfandari, S., Gois, J., Delannoy, P.-Y., Georges, H., Boussekey, N., Chiche, A., et al. (2014). Management and control of a carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit. Med. Mal. Infect 44, 229–231. doi: 10.1016/j.medmal.2014.03.005
Alhumaid, S., Al Mutair, A., Al Alawi, Z., Alsuliman, M., Ahmed, G. Y., Rabaan, A. A., et al. (2021). Knowledge of infection prevention and control among healthcare workers and factors influencing compliance: a systematic review. Antimicrob. Resist. Infect. Control. 10:86. doi: 10.1186/s13756-021-00957-0
Ali, A., Botha, J., and Tiruvoipati, R. (2014). Fatal skin and soft tissue infection of multidrug resistant Acinetobacter baumannii: a case report. Int. J. Surg. Case Rep. 5, 532–536. doi: 10.1016/j.ijscr.2014.04.019
Almasaudi, S. B. (2018). Acinetobacter spp. as nosocomial pathogens: epidemiology and resistance features. Saudi J. Biol. Sci. 25, 586–596. doi: 10.1016/j.sjbs.2016.02.009
Alrahmany, D., Omar, A. F., Alreesi, A., Harb, G., and Ghazi, I. M. (2022). Acinetobacter baumannii infection-related mortality in hospitalized patients: risk factors and potential targets for clinical and antimicrobial stewardship interventions. Antibiotics 11:1086. doi: 10.3390/antibiotics11081086
Al-Rashed, N., Bindayna, K. M., Shahid, M., Saeed, N. K., Darwish, A., Joji, R. M., et al. (2023). Prevalence of carbapenemases in carbapenem-resistant Acinetobacter baumannii isolates from the Kingdom of Bahrain. Antibiotics 12:1198. doi: 10.3390/antibiotics12071198
Alsaab, F. M., Dean, S. N., Bobde, S., Ascoli, G. G., and van Hoek, M. L. (2023). Computationally Designed AMPs with Antibacterial and Antibiofilm Activity against MDR Acinetobacter baumannii. Antibiotics 12:1396. doi: 10.3390/antibiotics12091396
Ambler, R. P. (1980). The structure of beta-lactamases. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 289, 321–331. doi: 10.1098/rstb.1980.0049
Ammendola, S., Pasquali, P., Pistoia, C., Petrucci, P., Petrarca, P., Rotilio, G., et al. (2007). High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect. Immun. 75, 5867–5876. doi: 10.1128/IAI.00559-07
An, Z., Huang, X., Zheng, C., and Ding, W. (2019). Acinetobacter baumannii outer membrane protein A induces HeLa cell autophagy via MAPK/JNK signaling pathway. Int. J. Med. Microbiol. 309, 97–107. doi: 10.1016/j.ijmm.2018.12.004
Anane, A. Y., Apalata, T., Vasaikar, S., Okuthe, G. E., and Songca, S. (2019). Prevalence and molecular analysis of multidrug-resistant Acinetobacter baumannii in the extra-hospital environment in Mthatha, South Africa. Braz J. Infect. Dis. 23, 371–380. doi: 10.1016/j.bjid.2019.09.004
Ang, H., and Sun, X. (2018). Risk factors for multidrug-resistant gram-negative bacteria infection in intensive care units: a meta-analysis. Int. J. Nurs. Pract. 24:e12644. doi: 10.1111/ijn.12644
Anstey, N. M., Currie, B. J., Hassell, M., Palmer, D., Dwyer, B., Seifert, H., et al. (2002). Community-acquired bacteremic Acinetobacter pneumonia in tropical Australia is caused by diverse strains of Acinetobacter baumannii, with carriage in the throat in at-risk groups. J. Clin. Microbiol. 40, 685–686. doi: 10.1128/JCM.40.2.685-686.2002
Antimicrobial Resistance Collaborators (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655. doi: 10.1016/S0140-6736(21)02724-0
Antunes, L. C., Imperi, F., Carattoli, A., and Visca, P. (2011a). Deciphering the multifactorial nature of Acinetobacter baumannii pathogenicity. PLoS ONE 6:e22674. doi: 10.1371/journal.pone.0022674
Antunes, L. C., Imperi, F., Towner, K. J., and Visca, P. (2011b). Genome-assisted identification of putative iron-utilization genes in Acinetobacter baumannii and their distribution among a genotypically diverse collection of clinical isolates. Res. Microbiol. 162, 279–284. doi: 10.1016/j.resmic.2010.10.010
Antunes, L. C. S., Visca, P., and Towner, K. J. (2014). Acinetobacter baumannii: evolution of a global pathogen. Pathog. Dis. 71, 292–301. doi: 10.1111/2049-632X.12125
Anwer, R. (2024). Molecular epidemiology and molecular typing methods of Acinetobacter baumannii: an updated review. Saudi Med. J. 45:458. doi: 10.15537/smj.2024.45.5.20230886
Appaneal, H. J., Lopes, V. V., and K.L. LaPlante, A.R. Caffrey. (2022). Treatment, clinical outcomes, and predictors of mortality among a national cohort of admitted patients with Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 66:e01975-21. doi: 10.1128/aac.01975-21
Arbatsky, N. P., Shneider, M. M., Dmitrenok, A. S., Popova, A. V., Shagin, D. A., Shelenkov, A. A., et al. (2018). Structure and gene cluster of the K125 capsular polysaccharide from Acinetobacter baumannii MAR13-1452. Int. J. Biol. Macromol. 117, 1195–1199. doi: 10.1016/j.ijbiomac.2018.06.029
Arowolo, M. T., Orababa, O. Q., Olaitan, M. O., Osibeluwo, B. V., Essiet, U. U., Batholomew, O. H., et al. (2023). Prevalence of carbapenem resistance in Acinetobacter baumannii and Pseudomonas aeruginosa in sub-Saharan Africa: a systematic review and meta-analysis. PLoS ONE 18:e0287762. doi: 10.1371/journal.pone.0287762
Arroyo, L., Garcia-Curiel, A., Pachon-Ibanez, M., Llanos, A., Ruiz, M., Pachon, J., et al. (2005). Reliability of the E-test method for detection of colistin resistance in clinical isolates of Acinetobacter baumannii. J. Clin. Microbiol. 43, 903–905. doi: 10.1128/JCM.43.2.903-905.2005
Asif, M., Alvi, I. A., and Rehman, S. U. (2018). Insight into Acinetobacter baumannii: pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect. Drug. Resist. 11, 1249–1260. doi: 10.2147/IDR.S166750
Aurilio, C., Sansone, P., Barbarisi, M., Pota, V., Giaccari, L. G., Coppolino, F., et al. (2022). Mechanisms of action of carbapenem resistance. Antibiotics 11:421. doi: 10.3390/antibiotics11030421
Ayobami, O., Willrich, N., Harder, T., Okeke, I. N., Eckmanns, T., Markwart, R., et al. (2019). The incidence and prevalence of hospital-acquired (carbapenem-resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: a systematic review and meta-analysis. Emerg. Microbes. Infect. 8, 1747–1759. doi: 10.1080/22221751.2019.1698273
Ayobami, O., Willrich, N., Suwono, B., Eckmanns, T., and Markwart, R. (2020). The epidemiology of carbapenem-non-susceptible Acinetobacter species in Europe: analysis of EARS-Net data from 2013 to 2017. Antimicrob Resist. Infect. Control 9, 1–10. doi: 10.1186/s13756-020-00750-5
Ayoub Moubareck, C., and Hammoudi Halat, D. (2020). Insights into Acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics 9:119. doi: 10.3390/antibiotics9030119
Bagudo, A. I., Obande, G. A., Harun, A., and Singh, K. K. B. (2020). Advances in automated techniques to identify Acinetobacter calcoaceticus–Acinetobacter baumannii complex. Asian Biomed. 14:177. doi: 10.1515/abm-2020-0026
Baker, S., Krishna, A., Higham, S., Naydenova, P., O'Leary, S., Scott, J. B., et al. (2024). Exploiting human immune repertoire transgenic mice for protective monoclonal antibodies against antimicrobial resistant Acinetobacter baumannii. Nat. Commun. 15:7979. doi: 10.1038/s41467-024-52357-8
Balalovski, P., and Grainge, I. (2020). Mobilization of pdif modules in Acinetobacter: a novel mechanism for antibiotic resistance gene shuffling? Mol. Microbiol. 114, 699–709. doi: 10.1111/mmi.14563
Baleivanualala, S. C., Isaia, L., Devi, S. V., Howden, B., Gorrie, C. L., Matanitobua, S., et al. (2023). Molecular and clinical epidemiology of carbapenem resistant Acinetobacter baumannii ST2 in Oceania: a multicountry cohort study. Lancet Reg. Health West Pac. 40:100896. doi: 10.1016/j.lanwpc.2023.100896
Barbut, F., Yezli, S., Mimoun, M., Pham, J., Chaouat, M., Otter, J. A., et al. (2013). Reducing the spread of Acinetobacter baumannii and methicillin-resistant Staphylococcus aureus on a burns unit through the intervention of an infection control bundle. Burns 39, 395–403. doi: 10.1016/j.burns.2012.07.007
Bardbari, A. M., Arabestani, M. R., Karami, M., Keramat, F., Alikhani, M. Y., Bagheri, K. P., et al. (2017). Correlation between ability of biofilm formation with their responsible genes and MDR patterns in clinical and environmental Acinetobacter baumannii isolates. Microb. Pathog. 108, 122–128. doi: 10.1016/j.micpath.2017.04.039
Barker, J., and Maxted, H. (1975). Observations on the growth and movement of Acinetobacter on semi-solid media. J. Med. Microbiol. 8, 443–446. doi: 10.1099/00222615-8-3-443
Bartual, S. G., Seifert, H., Hippler, C., Luzon, M. A., Wisplinghoff, H., Rodríguez-Valera, F., et al. (2005). Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J. Clin. Microbiol. 43, 4382–4390. doi: 10.1128/JCM.43.9.4382-4390.2005
Bateman, T. J., Shah, M., Ho, T. P., Shin, H. E., Pan, C., Harris, G., et al. (2021). A Slam-dependent hemophore contributes to heme acquisition in the bacterial pathogen Acinetobacter baumannii. Nat. Commun. 12:6270. doi: 10.1038/s41467-021-26545-9
Batirel, A., Balkan, I., Karabay, O., Agalar, C., Akalin, S., Alici, O., et al. (2014). Comparison of colistin–carbapenem, colistin–sulbactam, and colistin plus other antibacterial agents for the treatment of extremely drug-resistant Acinetobacter baumannii bloodstream infections. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1311–1322. doi: 10.1007/s10096-014-2070-6
Bauman, V. K., Bogoslovskii, N. A., Kisel'nikova, T. A., Shakhova, M. K., and Valinietse, M. (1978). [1 alpha-hydroxyvitamin D3: chemical synthesis and biological effect]. Prikl. Biokhim. Mikrobiol. 14, 243–252.
Beig, M., Parvizi, E., Navidifar, T., Bostanghadiri, N., Mofid, M., Golab, N., et al. (2024). Geographical mapping and temporal trends of Acinetobacter baumannii carbapenem resistance: a comprehensive meta-analysis. PLoS ONE 19:e0311124. doi: 10.1371/journal.pone.0311124
Beijerinck, M. W. (1911). Pigments as products of oxidation by bacterial action. Proc. K. Ned. Akad. Wet. 13, 1910–1911.
Benaissa, E., Belouad, E., Maleb, A., and Elouennass, M. (2023). Risk factors for acquiring Acinetobacter baumannii infection in the intensive care unit: experience from a Moroccan hospital. Access Microbiol. 5:acmi000637.v3. doi: 10.1099/acmi.0.000637.v3
Ben-Chetrit, E., Wiener-Well, Y., Lesho, E., Kopuit, P., Broyer, C., Bier, L., et al. (2018). An intervention to control an ICU outbreak of carbapenem-resistant Acinetobacter baumannii: long-term impact for the ICU and hospital. Crit. Care 22, 1–10. doi: 10.1186/s13054-018-2247-y
Bentancor, L. V., Camacho-Peiro, A., Bozkurt-Guzel, C., Pier, G. B., and Maira-Litrán, T. (2012a). Identification of ATA, a multifunctional trimeric autotransporter of Acinetobacter baumannii. J. Bacteriol. 194, 3950–3960. doi: 10.1128/JB.06769-11
Bentancor, L. V., Routray, A., Bozkurt-Guzel, C., Camacho-Peiro, A., Pier, G. B., Maira-Litrán, T., et al. (2012b). Evaluation of the trimeric autotransporter Ata as a vaccine candidate against Acinetobacter baumannii infections. Infect. Immun. 80, 3381–3388. doi: 10.1128/IAI.06096-11
Berlau, J., Aucken, H. M., Houang, E., and Pitt, T. L. (1999). Isolation of Acinetobacter spp. including A. baumannii from vegetables: implications for hospital-acquired infections. J. Hosp. Infect 42, 201–204. doi: 10.1053/jhin.1999.0602
Bert, F., and Lambert-Zechovsky, N. (1996). Sinusitis in mechanically ventilated patients and its role in the pathogenesis of nosocomial pneumonia. Eur J. Clin. Microbiol. Infect. Dis 15, 533–544. doi: 10.1007/BF01709360
Bhargava, N., Sharma, P., and Capalash, N. (2010). Quorum sensing in Acinetobacter: an emerging pathogen. Crit. Rev. Microbiol. 36, 349–360. doi: 10.3109/1040841X.2010.512269
Bianco, A., Quirino, A., Giordano, M., Marano, V., Rizzo, C., Liberto, M. C., et al. (2016). Control of carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit of a teaching hospital in Southern Italy. BMC Infect. Dis. 16, 1–7. doi: 10.1186/s12879-016-2036-7
Bitar, I., Medvecky, M., Gelbicova, T., Jakubu, V., Hrabak, J., Zemlickova, H., et al. (2019). Complete nucleotide sequences of mcr-4.3-carrying plasmids in Acinetobacter baumannii sequence type 345 of human and food origin from the Czech Republic, the first case in Europe. Antimicrob. Agents Chemother. 63:e01166-19. doi: 10.1128/AAC.01166-19
Blair, J. M., Webber, M. A., Baylay, A. J., Ogbolu, D. O., and Piddock, L. J. (2015). Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 13, 42–51. doi: 10.1038/nrmicro3380
Bohac, T. J., Fang, L., Giblin, D. E., and Wencewicz, T. A. (2019). Fimsbactin and acinetobactin compete for the periplasmic siderophore binding protein BauB in pathogenic Acinetobacter baumannii. ACS Chem. Biol. 14, 674–687. doi: 10.1021/acschembio.8b01051
Boll, J. M., Tucker, A. T., Klein, D. R., Beltran, A. M., Brodbelt, J. S., Davies, B. W., et al. (2015). Reinforcing lipid A acylation on the cell surface of Acinetobacter baumannii promotes cationic antimicrobial peptide resistance and desiccation survival. mBio 6:e00478-15. doi: 10.1128/mBio.00478-15
Bose, S., and Ghosh, A. K. (2016). Understanding of quorum–sensing: a possible solution for drug resistance in bacteria. Int. J. Curr. Microbiol. App. Sci. 5, 540–546. doi: 10.20546/ijcmas.2016.502.061
Boutal, H., Moguet, C., Pommiès, L., Simon, S., Naas, T., Volland, H., et al. (2022). The revolution of lateral flow assay in the field of AMR detection. Diagnostics 12:1744. doi: 10.3390/diagnostics12071744
Bouvet, P. J. M., and Grimont, P. A. D. (1986). Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., and Acinetobacter junii sp. nov. and emended descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int. J. Syst. Evol. Microbiol. 36, 228–240. doi: 10.1099/00207713-36-2-228
Bouvresse, S., Socolovshi, C., Berdjane, Z., Durand, R., Izri, A., Raoult, D., et al. (2011). No evidence of Bartonella quintana but detection of Acinetobacter baumannii in head lice from elementary schoolchildren in Paris. Comp. Immunol. Microbiol. Infect. Dis. 34, 475–477. doi: 10.1016/j.cimid.2011.08.007
Boyd, D. A., Mataseje, L. F., Pelude, L., Mitchell, R., Bryce, E., Roscoe, D., et al. (2019). Results from the Canadian nosocomial infection surveillance program for detection of carbapenemase-producing Acinetobacter spp. in Canadian hospitals, 2010–16. J. Antimicrob. Chemother. 74, 315–320. doi: 10.1093/jac/dky416
Boyd, N., and Nailor, M. D. (2011). Combination antibiotic therapy for empiric and definitive treatment of gram-negative infections: insights from the society of infectious diseases pharmacists. Pharmacotherapy 31, 1073–1084. doi: 10.1592/phco.31.11.1073
Brazzoli, M., Piccioli, D., and Marchetti, F. (2023). Challenges in development of vaccines directed toward antimicrobial resistant bacterial species. Hum. Vaccin. Immunother. 19:2228669. doi: 10.1080/21645515.2023.2228669
Breslow, J. M., Monroy, M. A., Daly, J. M., Meissler, J. J., Gaughan, J., Adler, M. W., et al. (2011). Morphine, but not trauma, sensitizes to systemic Acinetobacter baumannii infection. J. Neuroimmune Pharmacol. 6, 551–565. doi: 10.1007/s11481-011-9303-6
Brewer, N. S. (1977). Antimicrobial agents–Part II. The aminoglycosides: streptomycin, kanamycin, gentamicin, tobramycin, amikacin, neomycin. Mayo Clin. Proc. 52, 675–679.
Brigo, I. R., Yamamoto, L. R., and Molina, R. J. (2022). Community-acquired Acinetobacter baumannii pneumonia: a rare case in Brazil. Rev. Soc. Bras. Med. Trop. 55:e03012022. doi: 10.1590/0037-8682-0301-2022
Brisou, J., and Prevot, A. R. (1954). [Studies on bacterial taxonomy. X. The revision of species under acromobacter group]. Ann. Inst. Pasteur. 86, 722–728.
Brossard, K. A., and Campagnari, A. A. (2012). The Acinetobacter baumannii biofilm-associated protein plays a role in adherence to human epithelial cells. Infect. Immun. 80, 228–233. doi: 10.1128/IAI.05913-11
Bruhn, K. W., Pantapalangkoor, P., Nielsen, T., Tan, B., Junus, J., Hujer, K. M., et al. (2015). Host fate is rapidly determined by innate effector-microbial interactions during Acinetobacter baumannii bacteremia. J. Infect. Dis. 211, 1296–305. doi: 10.1093/infdis/jiu593
Bucataru, C., and Ciobanasu, C. (2024). Antimicrobial peptides: opportunities and challenges in overcoming resistance. Microbiol. Res. 286:127822. doi: 10.1016/j.micres.2024.127822
Buchovec, I., Vyčaite, E., Badokas, K., SuŽiedeliene, E., and Bagdonas, S. (2022). Application of antimicrobial photodynamic therapy for inactivation of Acinetobacter baumannii biofilms. Int. J. Mol. Sci. 24:722. doi: 10.3390/ijms24010722
Bulens, S. N., Campbell, D., McKay, S. L., Vlachos, N., Burgin, A., Burroughs, M., et al. (2024). Carbapenem-resistant Acinetobacter baumannii complex in the United States-an epidemiological and molecular description of isolates collected through the Emerging Infections Program, 2019. Am. J. Infect. Control 52, 1035–1042. doi: 10.1016/j.ajic.2024.04.184
Bush, K., and Jacoby, G. A. (2010). Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 54, 969–976. doi: 10.1128/AAC.01009-09
Bush, K., Jacoby, G. A., and Medeiros, A. A. (1995). A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39, 1211–1233. doi: 10.1128/AAC.39.6.1211
Bustamante, V., and Palavecino, C. (2023). Effect of photodynamic therapy on multidrug-resistant Acinetobacter baumannii: a scoping review. Photodiagn. Photodyn. Ther. 43:103709. doi: 10.1016/j.pdpdt.2023.103709
Cahill, S. M., Hall, R. M., and Kenyon, J. J. (2022). An update to the database for Acinetobacter baumannii capsular polysaccharide locus typing extends the extensive and diverse repertoire of genes found at and outside the K locus. Microb. Genom. 8:e000878. doi: 10.1099/mgen.0.000878
Castillo Bejarano J. I. Llaca Díaz J. E la O Cavazos M. E. Sánchez Alanís H. Mascareñas de los Santos A. H. Espinosa-Villaseñor F. . (2023). Carbapenem-resistant Acinetobacter baumannii infection in children from a third-level hospital in Mexico: clinical characteristics and molecular epidemiology. J. Pediatric Infect. Dis. Soc. 12, 431–435. doi: 10.1093/jpids/piad046
Caza, M., and Kronstad, J. W. (2013). Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans. Front. Cell. Infect. Microbiol. 3:80. doi: 10.3389/fcimb.2013.00080
Centers for Disease Control and Prevention (2004). Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002–2004. MMWR Morb. Mortal. Wkly. Rep. 53, 1063–1066.
Chagas, T. P. G., Rangel, K., and De-Simone, S. G. (2024). “Carbapenem-resistant Acinetobacter baumannii in Latin America,” in Acinetobacter baumannii-The Rise of a Resistant Pathogen (London: IntechOpen). doi: 10.5772/intechopen.1003713
Chang, W. N., Lu, C. H., Huang, C. R., and Chuang, Y. C. (2000). Community-acquired Acinetobacter meningitis in adults. Infection 28, 395–397. doi: 10.1007/s150100070013
Chen, C. L., Dudek, A., Liang, Y. H., Janapatla, R. P., Lee, H. Y., Hsu, L., et al. (2022). d-mannose-sensitive pilus of Acinetobacter baumannii is linked to biofilm formation and adherence onto respiratory tract epithelial cells. J. Microbiol. Immunol. Infect 55, 69–79. doi: 10.1016/j.jmii.2021.01.008
Chen, H. P., Chen, T. L., Lai, C. H., Fung, C. P., Wong, W. W., Yu, K. W., et al. (2005). Predictors of mortality in Acinetobacter baumannii bacteremia. J. Microbiol. Immunol. Infect 38, 127–136.
Chen, L.-K., Liu, Y.-L., Hu, A., Chang, K.-C., Lin, N.-T., Lai, M.-J., et al. (2013). Potential of bacteriophage ΦAB2 as an environmental biocontrol agent for the control of multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 13, 1–10. doi: 10.1186/1471-2180-13-154
Cheon, S., Kim, M.-J., Yun, S.-J., Moon, J. Y., and Kim, Y.-S. (2016). Controlling endemic multidrug-resistant Acinetobacter baumannii in intensive care units using antimicrobial stewardship and infection control. Korean J. Intern. Med. 31:367. doi: 10.3904/kjim.2015.178
Chiang, W. C., Su, C. P., Hsu, C. Y., Chen, S. Y., Chen, Y. C., Chang, S. C., et al. (2003). Community-acquired bacteremic cellulitis caused by Acinetobacter baumannii. J. Formos. Med. Assoc. 102, 650–652. Available online at: https://hub.tmu.edu.tw/zh/publications/community-acquired-bacteremic-cellulitis-caused-by-acinetobacter-
Chin, C. Y., Tipton, K. A., Farokhyfar, M., Burd, E. M., Weiss, D. S., Rather, P. N., et al. (2018). A high-frequency phenotypic switch links bacterial virulence and environmental survival in Acinetobacter baumannii. Nat. Microbiol. 3, 563–569. doi: 10.1038/s41564-018-0151-5
Chmielarczyk, A., Higgins, P., Wojkowska-Mach, J., Synowiec, E., Zander, E., Romaniszyn, D., et al. (2012). Control of an outbreak of Acinetobacter baumannii infections using vaporized hydrogen peroxide. J. Hosp. Infect. 81, 239–245. doi: 10.1016/j.jhin.2012.05.010
Cho, G. S., Li, B., Rostalsky, A., Fiedler, G., Rösch, N., Igbinosa, E., et al. (2018). Diversity and antibiotic susceptibility of Acinetobacter strains from milk powder produced in Germany. Front. Microbiol. 9:536. doi: 10.3389/fmicb.2018.00536
Choi, A. H., Slamti, L., Avci, F. Y., Pier, G. B., and Maira-Litrán, T. (2009). The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J. Bacteriol. 191, 5953–5963. doi: 10.1128/JB.00647-09
Choi, C. H., Lee, E. Y., Lee, Y. C., Park, T. I., Kim, H. J., Hyun, S. H., et al. (2005). Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 7, 1127–1138. doi: 10.1111/j.1462-5822.2005.00538.x
Choi, S. J., and Kim, E. S. (2024). Optimizing treatment for carbapenem-resistant Acinetobacter baumannii complex infections: a review of current evidence. Infect. Chemother. 56, 171–187. doi: 10.3947/ic.2024.0055
Choi, U., and Lee, C. R. (2019). Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front. Microbiol. 10:953. doi: 10.3389/fmicb.2019.00953
Choi, W. S., Kim, S. H., Jeon, E. G., Son, M. H., Yoon, Y. K., Kim, J.-Y., et al. (2010). Nosocomial outbreak of carbapenem-resistant Acinetobacter baumannii in intensive care units and successful outbreak control program. J. Korean Med. Sci. 25, 999–1004. doi: 10.3346/jkms.2010.25.7.999
Cisneros, J. M., Reyes, M. J., Pachón, J., Becerril, B., Caballero, F. J., García-Garmendía, J. L., et al. (1996). Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin. Infect. Dis. 22, 1026–1032. doi: 10.1093/clinids/22.6.1026
Cisneros, J. M., and Rodríguez-Baño, J. (2002). Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin. Microbiol. Infect. 8, 687–693. doi: 10.1046/j.1469-0691.2002.00487.x
Clemmer, K. M., Bonomo, R. A., and Rather, P. N. (2011). Genetic analysis of surface motility in Acinetobacter baumannii. Microbiology 157, 2534–2544. doi: 10.1099/mic.0.049791-0
Conde-Pérez, K., Vázquez-Ucha, J. C., Álvarez-Fraga, L., Ageitos, L., Rumbo-Feal, S., Martínez-Guitián, M., et al. (2021). In-depth analysis of the role of the acinetobactin cluster in the virulence of Acinetobacter baumannii. Front. Microbiol. 12:752070. doi: 10.3389/fmicb.2021.752070
Cook-Libin, S., Sykes, E. M., Kornelsen, V., and Kumar, A. (2022). Iron acquisition mechanisms and their role in the virulence of Acinetobacter baumannii. Infect. Immun. 90:e00223-22. doi: 10.1128/iai.00223-22
Corcione, S., Longo, B. M., Scabini, S., Pivetta, E., Curtoni, A., Shbaklo, N., et al. (2024). Risk factors for mortality in Acinetobacter baumannii bloodstream infections and development of a predictive mortality model. J. Glob. Antimicrob. Resist. 38, 317–326. doi: 10.1016/j.jgar.2024.06.010
Corral, J., Pérez-Varela, M., Sánchez-Osuna, M., Cortés, P., Barbé, J., Aranda, J., et al. (2021). Importance of twitching and surface-associated motility in the virulence of Acinetobacter baumannii. Virulence 12, 2201–2213. doi: 10.1080/21505594.2021.1950268
Corral, J., Sebastià, P., Coll, N. S., Barbé, J., Aranda, J., Valls, M., et al. (2020). Twitching and swimming motility play a role in ralstonia solanacearum pathogenicity. mSphere 5:e00740-19. doi: 10.1128/mSphere.00740-19
Corrigan, K. M., Harmis, N. Y., and Willcox, M. D. (2001). Association of acinetobacter species with contact lens-induced adverse responses. Cornea 20, 463–466. doi: 10.1097/00003226-200107000-00004
Cosgaya, C., Marí-Almirall, M., Van Assche, A., Fernández-Orth, D., Mosqueda, N., Telli, M., et al. (2016). Acinetobacter dijkshoorniae sp. nov., a member of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex mainly recovered from clinical samples in different countries. Int. J. Syst. Evol. Microbiol. 66, 4105–4111. doi: 10.1099/ijsem.0.001318
Cowan, S. T. (1938). Unusual infections following cerebral operations. Lancet 232, 1052–1054. doi: 10.1016/S0140-6736(00)41786-1
da Fonseca, A. d. S., Mencalha, A. L., and de Paoli, F. (2021). Antimicrobial photodynamic therapy against Acinetobacter baumannii. Photodiagn. Photodyn. Ther. 35:102430. doi: 10.1016/j.pdpdt.2021.102430
Dahdouh, E. A., Suarez Rodriguez, M., and Daoud, Z. (2018). Comparison of virulence determinants among Acinetobacter baumannii Clinical isolates obtained from Spain and Lebanon. J. Infect. Dev. Ctries. 12:15S. doi: 10.3855/jidc.9956
Damier-Piolle, L., Magnet, S., Brémont, S., Lambert, T., and Courvalin, P. (2008). AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob. Agents Chemother. 52, 557–562. doi: 10.1128/AAC.00732-07
Dandecha, P., and Sangthawan, P. (2002). Peritonitis in acute peritoneal dialysis in a university hospital. J. Med. Assoc. Thai. 85, 477–481.
Darby, E. M., Moran, R. A., Holden, E., Morris, T., Harrison, F., Clough, B., et al. (2024). Differential development of antibiotic resistance and virulence between Acinetobacter species. mSphere 9:e0010924. doi: 10.1128/mSphere.00109-24
Davis, K. A., Moran, K. A., McAllister, C. K., and Gray, P. J. (2005). Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg. Infect. Dis. 11, 1218–1224. doi: 10.3201/1108.050103
de Breij, A., Dijkshoorn, L., Lagendijk, E., van der Meer, J., Koster, A., Bloemberg, G., et al. (2010). Do biofilm formation and interactions with human cells explain the clinical success of Acinetobacter baumannii? PLoS ONE 5:e10732. doi: 10.1371/journal.pone.0010732
De Gregorio, E., Del Franco, M., Martinucci, M., Roscetto, E., Zarrilli, R., Di Nocera, P. P., et al. (2015). Biofilm-associated proteins: news from Acinetobacter. BMC Genomics 16:933. doi: 10.1186/s12864-015-2136-6
De Léséleuc, L., Harris, G., KuoLee, R., Xu, H. H., and Chen, W. (2014). Serum resistance, gallium nitrate tolerance and extrapulmonary dissemination are linked to heme consumption in a bacteremic strain of Acinetobacter baumannii. Int. J. Med. Microbiol. 304, 360–369. doi: 10.1016/j.ijmm.2013.12.002
De Silva, P. M., and Kumar, A. (2019). Signal transduction proteins in Acinetobacter baumannii: role in antibiotic resistance, virulence, and potential as drug targets. Front. Microbiol. 10:49. doi: 10.3389/fmicb.2019.00049
Deng, Y., Du, H., Tang, M., Wang, Q., Huang, Q., He, Y., et al. (2021). Biosafety assessment of Acinetobacter strains isolated from the three gorges reservoir region in nematode Caenorhabditis elegans. Sci. Rep. 11:19721. doi: 10.1038/s41598-021-99274-0
Dexter, C., Murray, G. L., Paulsen, I. T., and Peleg, A. Y. (2015). Community-acquired Acinetobacter baumannii: clinical characteristics, epidemiology and pathogenesis. Expert Rev. Anti. Infect. Ther. 13, 567–573. doi: 10.1586/14787210.2015.1025055
Dhurve, G., Madikonda, A. K., Jagannadham, M. V., and Siddavattam, D. (2022). Outer membrane vesicles of Acinetobacter baumannii DS002 are selectively enriched with TonB-dependent transporters and play a key role in iron acquisition. Microbiol. Spectr. 10:e00293-22. doi: 10.1128/spectrum.00293-22
Di Venanzio, G., Flores-Mireles, A. L., Calix, J. J., Haurat, M. F., Scott, N. E., Palmer, L. D., et al. (2019). Urinary tract colonization is enhanced by a plasmid that regulates uropathogenic Acinetobacter baumannii chromosomal genes. Nat. Commun. 10:2763. doi: 10.1038/s41467-019-10706-y
Diancourt, L., Passet, V., Nemec, A., Dijkshoorn, L., and Brisse, S. (2010). The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE 5:e10034. doi: 10.1371/journal.pone.0010034
Diep, D. T. H., Tuan, H. M., Ngoc, K. M., Vinh, C., Dung, T. T. N., Phat, V. V., et al. (2023). The clinical features and genomic epidemiology of carbapenem-resistant Acinetobacter baumannii infections at a tertiary hospital in Vietnam. J. Glob. Antimicrob. Resist. 33, 267–275. doi: 10.1016/j.jgar.2023.04.007
Djordjevic, Z. M., Folic, M. M., Folic, N. D., Gajovic, N., Gajovic, O., Jankovic, S. M., et al. (2016). Risk factors for hospital infections caused by carbapanem-resistant Acinetobacter baumannii. J. Infect. Dev. Ctries. 10, 1073–1080. doi: 10.3855/jidc.8231
Doan, T. N., Kong, D. C., Marshall, C., Kirkpatrick, C. M., and McBryde, E. S. (2016). Modeling the impact of interventions against Acinetobacter baumannii transmission in intensive care units. Virulence 7, 141–152. doi: 10.1080/21505594.2015.1076615
Doi, Y., and Arakawa, Y. (2007). 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 45, 88–94. doi: 10.1086/518605
Dortet, L., Poirel, L., Errera, C., and Nordmann, P. (2014). CarbAcineto NP test for rapid detection of carbapenemase-producing Acinetobacter spp. J. Clin. Microbiol. 52, 2359–2364. doi: 10.1128/JCM.00594-14
Du, D., Wang-Kan, X., Neuberger, A., van Veen, H. W., Pos, K. M., Piddock, L. J. V., et al. (2018). Multidrug efflux pumps: structure, function and regulation. Nat. Rev. Microbiol. 16, 523–539. doi: 10.1038/s41579-018-0048-6
Eijkelkamp, B. A., Hassan, K. A., Paulsen, I. T., and Brown, M. H. (2011). Investigation of the human pathogen Acinetobacter baumannii under iron limiting conditions. BMC Genomics 12:126. doi: 10.1186/1471-2164-12-126
Elhosseiny, N. M., and Attia, A. S. (2018). Acinetobacter: an emerging pathogen with a versatile secretome. Emerg. Microbes. Infect. 7:33. doi: 10.1038/s41426-018-0030-4
Elhosseiny, N. M., Elhezawy, N. B., and Attia, A. S. (2019). Comparative proteomics analyses of Acinetobacter baumannii strains ATCC 17978 and AB5075 reveal the differential role of type II secretion system secretomes in lung colonization and ciprofloxacin resistance. Microb. Pathog. 128, 20–27. doi: 10.1016/j.micpath.2018.12.039
Elhosseiny, N. M., Elhezawy, N. B., Sayed, R. M., Khattab, M. S., El Far, M. Y., Attia, A. S., et al. (2020). γ-Glutamyltransferase as a novel virulence factor of Acinetobacter baumannii inducing alveolar wall destruction and renal damage in systemic disease. J. Infect. Dis. 222, 871–879. doi: 10.1093/infdis/jiaa262
Ellis, T. N., and Kuehn, M. J. (2010). Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74, 81–94. doi: 10.1128/MMBR.00031-09
Endale, H., Mathewos, M., and Abdeta, D. (2023). Potential causes of spread of antimicrobial resistance and preventive measures in one health perspective-a review. Infect. Drug Resist. 16, 7515–7545. doi: 10.2147/IDR.S428837
Eryilmaz-Eren, E., Yalcin, S., Ozan, F., Saatci, E., Suzuk-Yildiz, S., Ture, Z., et al. (2024). An outbreak analysis of wound infection due to Acinetobacter baumannii in earthquake-trauma patients. Am. J. Infect. Control 52, 599–604. doi: 10.1016/j.ajic.2023.12.005
Evans, B. A., and Amyes, S. G. (2014). OXA β-lactamases. Clin. Microbiol. Rev. 27, 241–263. doi: 10.1128/CMR.00117-13
Fahy, S., O'Connor, J., Lucey, B., and Sleator, R. (2023). Hospital reservoirs of multidrug resistant Acinetobacter species—the elephant in the room! Br. J. Biomed. Sci. 80:11098. doi: 10.3389/bjbs.2023.11098
Falagas, M. E., and Karveli, E. A. (2007). The changing global epidemiology of Acinetobacter baumannii infections: a development with major public health implications. Clin. Microbiol. Infect. 13, 117–119. doi: 10.1111/j.1469-0691.2006.01596.x
Falagas, M. E., and Rafailidis, P. I. (2007). Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit. Care 11:134. doi: 10.1186/cc5911
Fan, L., Sun, J., Zhou, M., Zhou, J., Lao, X., Zheng, H., et al. (2016). DRAMP: a comprehensive data repository of antimicrobial peptides. Sci. Rep. 6:24482. doi: 10.1038/srep24482
Fang, C., Chen, X., and Zhou, M. (2016). Epidemiology and cytokine levels among children with nosocomial multidrug-resistant Acinetobacter baumannii complex in a tertiary hospital of Eastern China. PLoS ONE 11:e0161690. doi: 10.1371/journal.pone.0161690
Farrugia, D. N., Elbourne, L. D., Hassan, K. A., Eijkelkamp, B. A., Tetu, S. G., Brown, M. H., et al. (2013). The complete genome and phenome of a community-acquired Acinetobacter baumannii. PLoS ONE 8:e58628. doi: 10.1371/journal.pone.0058628
Fatmawati, N. N. D., Suwardana, G. N. R., Dharmika, I., Tarini, N. M. A., Sujaya, I. N., Suranadi, I. W., et al. (2023). Early detection of a possible multidrug-resistant Acinetobacter baumannii outbreak in the local hospital setting by using random amplified polymorphism DNA-polymerase chain reaction (RAPD-PCR), oxacillinase gene profiles, and antibiograms. Iran J. Microbiol. 15, 642–653. doi: 10.18502/ijm.v15i5.13870
Ferreira, D., Seca, A. M. L., Diana, C. G. A., and Silva, A. M. S. (2016). Targeting human pathogenic bacteria by siderophores: a proteomics review. J. Proteomics 145, 153–166. doi: 10.1016/j.jprot.2016.04.006
Fiester, S. E., Arivett, B. A., Schmidt, R. E., Beckett, A. C., Ticak, T., Carrier, M. V., et al. (2016). Iron-regulated phospholipase C activity contributes to the cytolytic activity and virulence of Acinetobacter baumannii. PLoS ONE 11:e0167068. doi: 10.1371/journal.pone.0167068
Fillat, M. F. (2014). The FUR (ferric uptake regulator) superfamily: diversity and versatility of key transcriptional regulators. Arch. Biochem. Biophys. 546, 41–52. doi: 10.1016/j.abb.2014.01.029
Fitzsimons, T. C., Lewis, J. M., Wright, A., Kleifeld, O., Schittenhelm, R. B., Powell, D., et al. (2018). Identification of novel Acinetobacter baumannii type VI secretion system antibacterial effector and immunity pairs. Infect. Immun. 86:e00297-18. doi: 10.1128/IAI.00297-18
Fournier, P. E., and Richet, H. (2006). The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 42, 692–699. doi: 10.1086/500202
Fournier, P. E., Vallenet, D., Barbe, V., Audic, S., Ogata, H., Poirel, L., et al. (2006). Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet 2:e7. doi: 10.1371/journal.pgen.0020007
Frawley, E. R., and Fang, F. C. (2014). The ins and outs of bacterial iron metabolism. Mol. Microbiol. 93, 609–616. doi: 10.1111/mmi.12709
Freire, M. P., de Oliveira Garcia, D., Garcia, C. P., Campagnari Bueno, M. F., Camargo, C. H., Kono Magri, A. S. G., et al. (2016). Bloodstream infection caused by extensively drug-resistant Acinetobacter baumannii in cancer patients: high mortality associated with delayed treatment rather than with the degree of neutropenia. Clin. Microbiol. Infect. 22, 352–358. doi: 10.1016/j.cmi.2015.12.010
Fukuta, Y., Muder, R. R., Agha, M. E., Clarke, L. G., Wagener, M. M., Hensler, A. M., et al. (2013). Risk factors for acquisition of multidrug-resistant Acinetobacter baumannii among cancer patients. Am. J. Infect. Control 41, 1249–1252. doi: 10.1016/j.ajic.2013.04.003
Gaddy, J. A., and Actis, L. A. (2009). Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 4, 273–278. doi: 10.2217/fmb.09.5
Gaddy, J. A., Arivett, B. A., McConnell, M. J., López-Rojas, R., Pachón, J., Actis, L. A., et al. (2012). Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, galleria mellonella caterpillars, and mice. Infect. Immun. 80, 1015–1024. doi: 10.1128/IAI.06279-11
Gaiarsa, S., Batisti Biffignandi, G., Esposito, E. P., Castelli, M., Jolley, K. A., Brisse, S., et al. (2019). Comparative analysis of the two Acinetobacter baumannii multilocus sequence typing (MLST) schemes. Front. Microbiol. 10:930. doi: 10.3389/fmicb.2019.00930
Galarde-López, M., Velazquez-Meza, M. E., Bobadilla-Del-Valle, M., Cornejo-Juárez, P., Carrillo-Quiroz, B. A. A., Ponce-de-León, A., et al. (2022). Alpuche-aranda, antimicrobial resistance patterns and clonal distribution of E. coli, Enterobacter spp. and Acinetobacter spp. strains isolated from two hospital wastewater plants. Antibiotics 11:601. doi: 10.3390/antibiotics11050601
Gallego, L. (2016). Acinetobacter baumannii: factors involved in its high adaptability to adverse environmental conditions. J. Microbiol. Exp. 3:85. doi: 10.15406/jmen.2016.03.00085
Galvao, C., Swartz, R., Rocher, L., Reynolds, J., Starmann, B., Wilson, D., et al. (1989). Acinetobacter peritonitis during chronic peritoneal dialysis. Am. J. Kidney Dis. 14, 101–104. doi: 10.1016/S0272-6386(89)80184-2
Gaona, M., Corral, J., Campoy, S., Barbé, J., Pérez– Varela, M., and Aranda, J. (2024). The novel MFS efflux pump SxtP, regulated by the LysR-type transcriptional activator SxtR, is involved in the susceptibility to sulfamethoxazole/trimethoprim (SXT) and the pathogenesis of Acinetobacter baumannii. Antimicrob. Agents Chemother. 68:e00712-24. doi: 10.1128/aac.00712-24
García-Garmendia, J. L., Ortiz-Leyba, C., Garnacho-Montero, J., Jiménez-Jiménez, F. J., Pérez-Paredes, C., Barrero-Almodóvar, A. E., et al. (2001). Risk factors for Acinetobacter baumannii nosocomial bacteremia in critically ill patients: a cohort study. Clin. Infect. Dis. 33, 939–946. doi: 10.1086/322584
García-Patiño, M. G., García-Contreras, R., and Licona-Limón, P. (2017). The immune response against Acinetobacter baumannii, an emerging pathogen in nosocomial infections. Front. Immunol. 8:441. doi: 10.3389/fimmu.2017.00441
Garnacho-Montero, J., Dimopoulos, G., Poulakou, G., Akova, M., Cisneros, J. M., de Waele, J., et al. (2015). Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med. 41, 2057–2075. doi: 10.1007/s00134-015-4079-4
Garnacho-Montero, J., Ortiz-Leyba, C., Jimenez-Jimenez, F., Barrero-Almodovar, A., Garcia-Garmendia, J., Bernabeu-Wittell, M., et al. (2003). Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin. Infect. Dis. 36, 1111–1118. doi: 10.1086/374337
Gaynes, R., and Edwards, J. R. (2005). Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41, 848–854. doi: 10.1086/432803
Gehrlein, M., Leying, H., Cullmann, W., Wendt, S., and Opferkuch, W. (1991). Imipenem resistance in Acinetobacter baumanii is due to altered penicillin-binding proteins. Chemotherapy 37, 405–412. doi: 10.1159/000238887
Geisinger, E., Huo, W., Hernandez-Bird, J., and Isberg, R. R. (2019). Acinetobacter baumannii: envelope determinants that control drug resistance, virulence, and surface variability. Annu. Rev. Microbiol. 73, 481–506. doi: 10.1146/annurev-micro-020518-115714
Geisinger, E., and Isberg, R. R. (2015). Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 11:e1004691. doi: 10.1371/journal.ppat.1004691
Germovsek, E., Barker, C. I., and Sharland, M. (2017). What do I need to know about aminoglycoside antibiotics? Arch. Dis. Child Educ. Pract. Ed. 102, 89–93. doi: 10.1136/archdischild-2015-309069
Gerner-Smidt, P., Tjernberg, I., and Ursing, J. (1991). Reliability of phenotypic tests for identification of Acinetobacter species. J. Clin. Microbiol. 29, 277–282. doi: 10.1128/jcm.29.2.277-282.1991
Ghaffoori Kanaan, M. H., Al-Shadeedi, S. M. J., Al-Massody, A. J., and Ghasemian, A. (2020). Drug resistance and virulence traits of Acinetobacter baumannii from Turkey and chicken raw meat. Comp. Immunol. Microbiol. Infect. Dis. 70:101451. doi: 10.1016/j.cimid.2020.101451
Ghazalibina, M., Mortazavi, H., Babadi, M., Rahimi, M., Khaledi, A., Teymouri, M., et al. (2019). Prevalence of integrons and antibiotic resistance pattern in Acinetobacter baumannii isolated from clinical samples of Iranian patients: a systematic review and meta-analysis. Ethiop. J. Health Sci. 29, 639–648. doi: 10.4314/ejhs.v29i5.15
Giardina, B. J., Shahzad, S., Huang, W., and Wilks, A. (2019). Heme uptake and utilization by hypervirulent Acinetobacter baumannii LAC-4 is dependent on a canonical heme oxygenase (abHemO). Arch. Biochem. Biophys. 672:108066. doi: 10.1016/j.abb.2019.108066
Glew, R. H., Moellering, R. C. Jr., and Kunz, L. J. (1977). Infections with Acinetobacter calcoaceticus (Herellea vaginicola): clinical and laboratory studies. Medicine 56, 79–97. doi: 10.1097/00005792-197703000-00001
Gómez, J., Simarro, E., Baños, V., Requena, L., Ruiz, J., García, F., et al. (1999). Six-year prospective study of risk and prognostic factors in patients with nosocomial sepsis caused by Acinetobacter baumannii. Eur J. Clin. Microbiol. Infect. Dis 18, 358–361. doi: 10.1007/PL00015019
Gong, Y. L., Yang, Z. C., Yin, S. P., Liu, M. X., Zhang, C., Luo, X. Q., et al. (2016). [Analysis of the pathogenic characteristics of 162 severely burned patients with bloodstream infection]. Zhonghua Shao Shang Za Zhi 32, 529–535. doi: 10.3760/cma.j.issn.1009-2587.2016.09.004
Goodman, T. (1988). Grafts and flaps in plastic surgery. An overview. Aorn J. 48, 658–663. doi: 10.1016/S0001-2092(07)69122-4
Gordillo Altamirano, F. L., and Barr, J. J. (2019). Phage therapy in the postantibiotic era. Clin. Microbiol. Rev. 32:e00066-18. doi: 10.1128/CMR.00066-18
Gordon, N. C., and Wareham, D. W. (2010). Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int. J. Antimicrob. Agents 35, 219–226. doi: 10.1016/j.ijantimicag.2009.10.024
Granata, G., Petersen, E., Capone, A., Donati, D., Andriolo, B., Gross, M., et al. (2024). The impact of armed conflict on the development and global spread of antibiotic resistance: a systematic review. Clin. Microbiol. Infect. 30, 858–865. doi: 10.1016/j.cmi.2024.03.029
Greene, C., Vadlamudi, G., Newton, D., Foxman, B., and Xi, C. (2016). The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am. J. Infect. Control 44, e65–e71. doi: 10.1016/j.ajic.2015.12.012
Greig, S. L., and Scott, L. J. (2016). Intravenous minocycline: a review in Acinetobacter infections. Drugs 76, 1467–1476. doi: 10.1007/s40265-016-0636-6
Gurung, A., Napit, R., Shrestha, B., and Lekhak, B. (2024). Carbapenem resistance in Acinetobacter calcoaceticus-baumannii complex isolates from Kathmandu Model Hospital, Nepal, is attributed to the presence of blaOXA-23-like and blaNDM-1 Genes. BioMed Res. Int. 2024:8842625. doi: 10.1155/2024/8842625
Hafiz, T. A., Alghamdi, S. S., Mubaraki, M. A., Alghamdi, S. S. M., Alothaybi, A., Aldawood, E., et al. (2023). A two-year retrospective study of multidrug-resistant Acinetobacter baumannii respiratory infections in critically Ill patients: clinical and microbiological findings. J. Infect. Public Health. 16, 313–319. doi: 10.1016/j.jiph.2023.01.004
Hamidian, M., and Nigro, S. J. (2019). Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb. Genom. 5:e000306. doi: 10.1099/mgen.0.000306
Hamidian, M., Nigro, S. J., and Hall, R. M. (2017). Problems with the oxford multilocus sequence typing scheme for Acinetobacter baumannii: do sequence type 92 (ST92) and ST109 exist? J. Clin. Microbiol. 55, 2287–2289. doi: 10.1128/JCM.00533-17
Harding, C. M., Kinsella, R. L., Palmer, L. D., Skaar, E. P., and Feldman, M. F. (2016). Medically relevant Acinetobacter species require a type II secretion system and specific membrane-associated chaperones for the export of multiple substrates and full virulence. PLoS Pathog. 12:e1005391. doi: 10.1371/journal.ppat.1005391
Harding, C. M., Pulido, M. R., Di Venanzio, G., Kinsella, R. L., Webb, A. I., Scott, N. E., et al. (2017). Pathogenic Acinetobacter species have a functional type I secretion system and contact-dependent inhibition systems. J. Biol. Chem. 292, 9075–9087. doi: 10.1074/jbc.M117.781575
Hassan, K. A., Liu, Q., Henderson, P. J., and Paulsen, I. T. (2015). Homologs of the Acinetobacter baumannii AceI transporter represent a new family of bacterial multidrug efflux systems. mBio 6:e01982-14. doi: 10.1128/mBio.01982-14
He, T., Wang, R., Liu, D., Walsh, T. R., Zhang, R., Lv, Y., et al. (2019). Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 4, 1450–1456. doi: 10.1038/s41564-019-0445-2
Henderson, I. R., Cappello, R., and Nataro, J. P. (2000). Autotransporter proteins, evolution and redefining protein secretion. Trends Microbiol. 8, 529–532. doi: 10.1016/S0966-842X(00)01853-9
Henriksen, S. D. (1976). Moraxella, neisseria, branhamella, and acinetobacter. Annu. Rev. Microbiol. 30, 63–83. doi: 10.1146/annurev.mi.30.100176.000431
Henry, R. (2019). Etymologia: carbapenem. Emerg. Infect. Dis. 25, 1393–1393. doi: 10.3201/eid2507.ET2507
Holmström, T. C., David, L. A., Motta, C. C., Rocha-de-Souza, C. M., Maboni, G, Coelho, I. S., et al. (2022). Acinetobacter calcoaceticus-Acinetobacter baumannii complex in animals: identification and antimicrobial resistance profile. Pesquisa Veterinária Brasileira 42:e07043. doi: 10.1590/1678-5150-pvb-7043
Hood, M. I., Mortensen, B. L., Moore, J. L., Zhang, Y., Kehl-Fie, T. E., Sugitani, N., et al. (2012). Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog. 8:e1003068. doi: 10.1371/journal.ppat.1003068
Hood, R. D., Peterson, S. B., and Mougous, J. D. (2017). From striking out to striking gold: discovering that type VI secretion targets bacteria. Cell Host Microbe 21, 286–289. doi: 10.1016/j.chom.2017.02.001
Houang, E. T., Chu, Y. W., Leung, C. M., Chu, K. Y., Berlau, J., Ng, K. C., et al. (2001). Epidemiology and infection control implications of Acinetobacter spp. in Hong Kong. J. Clin. Microbiol. 39, 228–234. doi: 10.1128/JCM.39.1.228-234.2001
Hoyle, B. D., and Costerton, J. W. (1991). Bacterial resistance to antibiotics: the role of biofilms. Prog. Drug Res. 37, 91–105. doi: 10.1007/978-3-0348-7139-6_2
Hrenovic, J., Durn, G., Goic-Barisic, I., and Kovacic, A. (2014). Occurrence of an environmental Acinetobacter baumannii strain similar to a clinical isolate in paleosol from Croatia. Appl. Environ. Microbiol. 80, 2860–2866. doi: 10.1128/AEM.00312-14
Hu, D., Liu, B., Dijkshoorn, L., Wang, L., and Reeves, P. R. (2013). Diversity in the major polysaccharide antigen of Acinetobacter baumannii assessed by DNA sequencing, and development of a molecular serotyping scheme. PLoS ONE 8:e70329. doi: 10.1371/journal.pone.0070329
Hu, S., Niu, L., Zhao, F., Yan, L., Nong, J., Wang, C., et al. (2019). Identification of Acinetobacter baumannii and its carbapenem-resistant gene bla OXA-23-like by multiple cross displacement amplification combined with lateral flow biosensor. Sci. Rep. 9:17888. doi: 10.1038/s41598-019-54465-8
Huang, J.-R., Yang, T.-W., Hsiao, Y.-I., Fan, H.-M., Kuo, H.-Y., Hung, K.-H., et al. (2024). Far-UVC light (222 nm) efficiently inactivates clinically significant antibiotic-resistant bacteria on diverse material surfaces. Microbiol. Spectr. 12:e04251-23. doi: 10.1128/spectrum.04251-23
Huang, W., Zhang, Q., Li, W., Chen, Y., Shu, C., Li, Q., et al. (2019). Anti-outer membrane vesicle antibodies increase antibiotic sensitivity of pan-drug-resistant Acinetobacter baumannii. Front. Microbiol. 10:1379. doi: 10.3389/fmicb.2019.01379
Huang, Y., Zhou, Q., Wang, W., Huang, Q., Liao, J., Li, J., et al. (2019). Acinetobacter baumannii ventilator-associated pneumonia: clinical efficacy of combined antimicrobial therapy and in vitro drug sensitivity test results. Front. Pharmacol. 10:92. doi: 10.3389/fphar.2019.00092
Huband, M. D., Mendes, R. E., Pfaller, M. A., Lindley, J. M., Strand, G. J., Benn, V. J., et al. (2020). In vitro activity of KBP-7072, a novel third-generation tetracycline, against 531 recent geographically diverse and molecularly characterized Acinetobacter baumannii species complex isolates. Antimicrob. Agents Chemother. 64:e02375-19. doi: 10.1128/AAC.02375-19
Ibrahim, M. E. (2019). Prevalence of Acinetobacter baumannii in Saudi Arabia: risk factors, antimicrobial resistance patterns and mechanisms of carbapenem resistance. Ann. Clin. Microbiol. Antimicrob. 18:1. doi: 10.1186/s12941-018-0301-x
Ibrahim, S., Al-Saryi, N., Al-Kadmy, I. M. S., and Aziz, S. N. (2021). Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol. Biol. Rep. 48, 6987–6998. doi: 10.1007/s11033-021-06690-6
Ilbert, M., and Bonnefoy, V. (2013). Insight into the evolution of the iron oxidation pathways. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1827, 161–175. doi: 10.1016/j.bbabio.2012.10.001
Inchai, J., Pothirat, C., Bumroongkit, C., Limsukon, A., Khositsakulchai, W., Liwsrisakun, C., et al. (2015). Prognostic factors associated with mortality of drug-resistant Acinetobacter baumannii ventilator-associated pneumonia. J. Intensive Care 3, 1–8. doi: 10.1186/s40560-015-0077-4
Intravenous, L. A. S. (1999). colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin. Infect. Dis. 28, 2008–2011. doi: 10.1086/514732
Itani, R., Khojah, H. M. J., Karout, S., Rahme, D., Hammoud, L., Awad, R., et al. (2023). Acinetobacter baumannii: assessing susceptibility patterns, management practices, and mortality predictors in a tertiary teaching hospital in Lebanon. Antimicrob. Resist. Infect. Control 12:136. doi: 10.1186/s13756-023-01343-8
Jacobs, A. C., Hood, I., Boyd, K. L., Olson, P. D., Morrison, J. M., Carson, S., et al. (2010). Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect. Immun. 78, 1952–1962. doi: 10.1128/IAI.00889-09
Jacobs, M., Colson, J., and Rhoads, D. (2021). Recent advances in rapid antimicrobial susceptibility testing systems. Expert Rev. Mol. Diagn. 21, 563–557. doi: 10.1080/14737159.2021.1924679
Jakubovics, N. S., and Jenkinson, H. F. (2001). Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology 147, 1709–1718. doi: 10.1099/00221287-147-7-1709
Jarrell, K. F., and McBride, M. J. (2008). The surprisingly diverse ways that prokaryotes move. Nat. Rev. Microbiol. 6, 466–476. doi: 10.1038/nrmicro1900
Jaruratanasirikul, S., Nitchot, W., Wongpoowarak, W., Samaeng, M., and Nawakitrangsan, M. (2019). Population pharmacokinetics and Monte Carlo simulations of sulbactam to optimize dosage regimens in patients with ventilator-associated pneumonia caused by Acinetobacter baumannii. Eur. J. Pharm. Sci. 136:104940. doi: 10.1016/j.ejps.2019.05.018
Jaśkiewicz, M., Neubauer, D., Kazor, K., Bartoszewska, S., and Kamysz, W. (2019). Antimicrobial activity of selected antimicrobial peptides against planktonic culture and biofilm of Acinetobacter baumannii. Probiotics Antimicrob. Prot. 11, 317–324. doi: 10.1007/s12602-018-9444-5
Ji, F., Tian, G., Shang, D., and Jiang, F. (2023). Antimicrobial peptide 2K4L disrupts the membrane of multidrug-resistant Acinetobacter baumannii and protects mice against sepsis. Front. Microbiol. 14:1258469. doi: 10.3389/fmicb.2023.1258469
Jia, H., Sun, Q., Ruan, Z., and Xie, X. (2019). Characterization of a small plasmid carrying the carbapenem resistance gene bla (OXA-72) from community-acquired Acinetobacter baumannii sequence type 880 in China. Infect. Drug. Resist. 12, 1545–1553. doi: 10.2147/IDR.S202803
Jiménez-Guerra, G., Heras-Cañas, V., Gutiérrez-Soto, M., Del Pilar, M., Aznarte-Padial, M., Expósito-Ruiz, J. M., et al. (2018). Urinary tract infection by Acinetobacter baumannii and Pseudomonas aeruginosa: evolution of antimicrobial resistance and therapeutic alternatives. J. Med. Microbiol. 67, 790–797. doi: 10.1099/jmm.0.000742
Johnson, E. N., Burns, T. C., Hayda, R. A., Hospenthal, D. R., and Murray, C. K. (2007). Infectious complications of open type III tibial fractures among combat casualties. Clin. Infect. Dis. 45, 409–415. doi: 10.1086/520029
Johnson, T. L., Waack, U., Smith, S., Mobley, H., and Sandkvist, M. (2015). Acinetobacter baumannii is dependent on the type II secretion system and its substrate LipA for lipid utilization and in vivo fitness. J. Bacteriol. 198, 711–719. doi: 10.1128/JB.00622-15
Jolley, K. A., Bray, J. E., and Maiden, M. C. J. (2018). Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 3:124. doi: 10.12688/wellcomeopenres.14826.1
Joly-Guillou, M. L. (2005). Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 11, 868–873. doi: 10.1111/j.1469-0691.2005.01227.x
Josenhans, C., and Suerbaum, S. (2002). The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291, 605–614. doi: 10.1078/1438-4221-00173
Joshi, P. R., Acharya, M., Kakshapati, T., Leungtongkam, U., Thummeepak, R., and Sitthisak, S. (2017). Co-existence of bla OXA-23 and bla NDM-1 genes of Acinetobacter baumannii isolated from Nepal: antimicrobial resistance and clinical significance. Antimicrob. Resist. Infect. Control 6, 1–7. doi: 10.1186/s13756-017-0180-5
Jung, C.-J., Liao, Y.-D., Hsu, C.-C., Huang, T.-Y., Chuang, Y.-C., Chen, J.-W., et al. (2021). Identification of potential therapeutic antimicrobial peptides against Acinetobacter baumannii in a mouse model of pneumonia. Sci. Rep. 11:7318. doi: 10.1038/s41598-021-86844-5
Jung, J., and Park, W. (2015). Acinetobacter species as model microorganisms in environmental microbiology: current state and perspectives. Appl. Microbiol. Biotechnol. 99, 2533–2548. doi: 10.1007/s00253-015-6439-y
Juni, E. (1972). Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J. Bacteriol. 112, 917–931. doi: 10.1128/jb.112.2.917-931.1972
Juttukonda, L. J., Chazin, W. J., and Skaar, E. P. (2016). Acinetobacter baumannii coordinates urea metabolism with metal import to resist host-mediated metal limitation. mBio 7:e01475-16. doi: 10.1128/mBio.01475-16
Jyothisri, K., Deepak, V., and Rajeswari, M. R. (1999). Purification and characterization of a major 40 kDa outer membrane protein of Acinetobacter baumannii. FEBS Lett. 443, 57–60. doi: 10.1016/S0014-5793(98)01679-2
Kakuta, N., Nakano, R., Nakano, A., Suzuki, Y., Tanouchi, A., Masui, T., et al. (2020). A novel mismatched PCR-restriction fragment length polymorphism assay for rapid detection of gyrA and parC mutations associated with fluoroquinolone resistance in Acinetobacter baumannii. Ann. Lab. Med. 40, 27–32. doi: 10.3343/alm.2020.40.1.27
Kamoshida, G., Tansho-Nagakawa, S., Kikuchi-Ueda, T., Nakano, R., Hikosaka, K., Nishida, S., et al. (2016). A novel bacterial transport mechanism of Acinetobacter baumannii via activated human neutrophils through interleukin-8. J. Leukoc. Biol. 100, 1405–1412. doi: 10.1189/jlb.4AB0116-023RR
Kanafani, Z. A., Zahreddine, N., Tayyar, R., Sfeir, J., Araj, G. F., Matar, G. M., et al. (2018). Multi-drug resistant Acinetobacter species: a seven-year experience from a tertiary care center in Lebanon. Antimicrob. Resist. Infect. Control 7:9. doi: 10.1186/s13756-017-0297-6
Kaplan, N., Rosenberg, E., Jann, B., and Jann, K. (1985). Structural studies of the capsular polysaccharide of Acinetobacter calcoaceticus BD4. Eur. J. Biochem. 152, 453–458. doi: 10.1111/j.1432-1033.1985.tb09218.x
Karakonstantis, S., Ioannou, P., Samonis, G., and Kofteridis, D. P. (2021). Systematic review of antimicrobial combination options for pandrug-resistant Acinetobacter baumannii. Antibiotics 10:3144. doi: 10.3390/antibiotics10111344
Karruli, A., Migliaccio, A., Pournaras, S., Durante-Mangoni, E., and Zarrilli, R. (2023). Cefiderocol and sulbactam-durlobactam against carbapenem-resistant Acinetobacter baumannii. Antibiotics 12:1729. doi: 10.3390/antibiotics12121729
Kateete, D. P., Nakanjako, R., Namugenyi, J., Erume, J., Joloba, M. L., Najjuka, C. F., et al. (2016). Carbapenem resistant Pseudomonas aeruginosa and Acinetobacter baumannii at Mulago Hospital in Kampala, Uganda (2007–2009). Springerplus 5:1308. doi: 10.1186/s40064-016-2986-7
Kelkar, R., Gordon, S. M., Giri, N., Rao, K., Ramakrishnan, G., Saikia, T., et al. (1989). and et al., Epidemic iatrogenic Acinetobacter spp. meningitis following administration of intrathecal methotrexate. J. Hosp. Infect 14, 233–243. doi: 10.1016/0195-6701(89)90040-6
Kempf, M., Abdissa, A., Diatta, G., Trape, J. F., Angelakis, E., Mediannikov, O., et al. (2012a). Detection of Acinetobacter baumannii in human head and body lice from Ethiopia and identification of new genotypes. Int J. Infect. Dis. 16:e680-3. doi: 10.1016/j.ijid.2012.05.1024
Kempf, M., Djouhri-Bouktab, L., Brunel, J.-M., Raoult, D., and Rolain, J.-M. (2012b). Synergistic activity of sulbactam combined with colistin against colistin-resistant Acinetobacter baumannii. Int. J. Antimicrob. Agents 39:180. doi: 10.1016/j.ijantimicag.2011.10.001
Kengkla, K., Kongpakwattana, K., Saokaew, S., Apisarnthanarak, A., and Chaiyakunapruk, N. (2018). Comparative efficacy and safety of treatment options for MDR and XDR Acinetobacter baumannii infections: a systematic review and network meta-analysis. J. Antimicrob. Chemother. 73, 22–32. doi: 10.1093/jac/dkx368
Khalawetektook, N., and Tektook, N. (2018). Study the virulence factors and patterns of antibiotics resistance in acinetobacter baumannii isolated from hospitalized patients in Baghdad City. Pak. J. Biotechnol. 15, 19–23. Retrieved from: https://pjbt.org/index.php/pjbt/article/view/100
Khan, S., Priti, S., and Ankit, S. (2015). Bacteria etiological agents causing lower respiratory tract infections and their resistance patterns. Iran Biomed. J. 19, 240–246. doi: 10.7508/ibj.2015.04.008
Khasheii, B., Mahmoodi, P., and Mohammadzadeh, A. (2021). Siderophores: importance in bacterial pathogenesis and applications in medicine and industry. Microbiol. Res. 250:126790. doi: 10.1016/j.micres.2021.126790
Khelissa, S. O., Jama, C., Abdallah, M., Boukherroub, R., Faille, C., Chihib, N. E., et al. (2017). Effect of incubation duration, growth temperature, and abiotic surface type on cell surface properties, adhesion and pathogenicity of biofilm-detached Staphylococcus aureus cells. AMB Express 7:191. doi: 10.1186/s13568-017-0492-0
Kikuchi-Ueda, T., Kamoshida, G., Ubagai, T., Nakano, R., Nakano, A., Akuta, T., et al. (2017). The TNF-α of mast cells induces pro-inflammatory responses during infection with Acinetobacter baumannii. Immunobiology 222, 1025–1034. doi: 10.1016/j.imbio.2017.05.015
Kim, A., Kuti, J. L., and Nicolau, D. P. (2009). Probability of pharmacodynamic target attainment with standard and prolonged-infusion antibiotic regimens for empiric therapy in adults with hospital-acquired pneumonia. Clin. Ther. 31, 2765–2778. doi: 10.1016/j.clinthera.2009.11.026
Kim, C., Lee, S., Ko, Y., Kang, S., Park, G., Jang, S., et al. (2023). Synergistic peptide-antibiotic approach to combat multidrug-resistant Acinetobacter baumannii. Jundishapur J. Microbiol. 16:e136712. doi: 10.5812/jjm-136712
Kim, M., Kim, D. Y., Song, W. Y., Park, S. E., Harrison, S. A., Chazin, W. J., et al. (2021). Distinctive roles of two acinetobactin isomers in challenging host nutritional immunity. MBio 12:e02248-21. doi: 10.1128/mBio.02248-21
Kim, S.-H., and Mun, S. J. (2025). Comparative analysis of clinical characteristics and antimicrobial resistance between Acinetobacter baumannii and other Acinetobacter species. Pathogens 14:46. doi: 10.3390/pathogens14010046
Kim, S. W., Choi, C. H., Moon, D. C., Jin, J. S., Lee, J. H., Shin, J. H., et al. (2009). Serum resistance of Acinetobacter baumannii through the binding of factor H to outer membrane proteins. FEMS Microbiol. Lett. 301, 224–231. doi: 10.1111/j.1574-6968.2009.01820.x
Kim, S. W., Oh, M. H., Jun, S. H., Jeon, H., Kim, S. I., Kim, K., et al. (2016). Outer membrane protein A plays a role in pathogenesis of Acinetobacter nosocomialis. Virulence 7, 413–426. doi: 10.1080/21505594.2016.1140298
Kimura, Y., Harada, K., Shimizu, T., Sato, T., Kajino, A., Usui, M., et al. (2018). Species distribution, virulence factors, and antimicrobial resistance of Acinetobacter spp. isolates from dogs and cats: a preliminary study. Microbiol. Immunol. 62, 462–466. doi: 10.1111/1348-0421.12601
Klebba, P. E. (2016). ROSET model of TonB action in gram-negative bacterial iron acquisition. J. Bacteriol. 198, 1013–1021. doi: 10.1128/JB.00823-15
Knauf, G. A., Powers, M. J., Herrera, C. M., Trent, M. S., and Davies, B. W. (2022). Acinetobactin-mediated inhibition of commensal bacteria by Acinetobacter baumannii. MSphere 7:e00016-22. doi: 10.1128/mSphere.00016-22
Korotkov, K. V., Sandkvist, M., and Hol, W. G. (2012). The type II secretion system: biogenesis, molecular architecture and mechanism. Nat. Rev. Microbiol. 10, 336–351. doi: 10.1038/nrmicro2762
Kramer, A., Schwebke, I., and Kampf, G. (2006). How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 6:130. doi: 10.1186/1471-2334-6-130
Krasowska, A., and Sigler, K. (2014). How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell Infect. Microbiol. 4:112. doi: 10.3389/fcimb.2014.00112
Krause, K. M., Serio, A. W., Kane, T. R., and Connolly, L. E. (2016). Aminoglycosides: an overview. Cold Spring Harb. Perspect. Med. 6:a027029. doi: 10.1101/cshperspect.a027029
Kumar, S., Anwer, R., and Azzi, A. (2021). Virulence potential and treatment options of multidrug-resistant (MDR) Acinetobacter baumannii. Microorganisms 9:2104. doi: 10.3390/microorganisms9102104
Kuo, L.-C., Lai, C.-C., Liao, C.-H., Hsu, C.-K., Chang, Y.-L., Chang, C.-Y., et al. (2007). Multidrug-resistant Acinetobacter baumannii bacteraemia: clinical features, antimicrobial therapy and outcome. Clin. Microbiol. Infect. 3, 196–198. doi: 10.1111/j.1469-0691.2006.01601.x
Kwon, H. I., Kim, S., Oh, M. H., Shin, M., and Lee, J. C. (2019). Distinct role of outer membrane protein A in the intrinsic resistance of Acinetobacter baumannii and Acinetobacter nosocomialis. Infect. Genet. Evol. 67, 33–37. doi: 10.1016/j.meegid.2018.10.022
Kwon, S. O., Gho, Y. S., Lee, J. C., and Kim, S. I. (2009). Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiol. Lett. 297, 150–156. doi: 10.1111/j.1574-6968.2009.01669.x
Kyriakidis, I., Vasileiou, E., Pana, Z. D., and Tragiannidis, A. (2021). Acinetobacter baumannii antibiotic resistance mechanisms. Pathogens 10:373. doi: 10.3390/pathogens10030373
La Scola, B., and Raoult, D. (2004). Acinetobacter baumannii in human body louse. Emerg. Infect. Dis. 10, 1671–1673. doi: 10.3201/eid1009.040242
Lai, C. C., Wang, C. Y., and Hsueh, P. R. (2020). Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect 53, 505–512. doi: 10.1016/j.jmii.2020.05.013
Lam, M. M. C., and Hamidian, M. (2024). Examining the role of Acinetobacter baumannii plasmid types in disseminating antimicrobial resistance. NPJ Antimicrob. Resist. 2:1. doi: 10.1038/s44259-023-00019-y
Lam, M. M. C., Wick, R. R., Judd, L. M., Holt, K. E., and Wyres, K. L. (2022). Kaptive 2.0: updated capsule and lipopolysaccharide locus typing for the Klebsiella pneumoniae species complex. Microb. Genom. 8:e000800. doi: 10.1099/mgen.0.000800
Lázaro-Díez, M., Chapartegui-González, I., Redondo-Salvo, S., Leigh, C., Merino, D., Segundo, D. S., et al. (2017). Human neutrophils phagocytose and kill Acinetobacter baumannii and A. pittii. Sci. Rep. 7:4571. doi: 10.1038/s41598-017-04870-8
Lee, B. Y., McGlone, S. M., Doi, Y., Bailey, R. R., and Harrison, L. H. (2010). Economic impact of Acinetobacter baumannii infection in the intensive care unit. Infect. Control Hosp. Epidemiol. 31, 1087–1089. doi: 10.1086/656378
Lee, C. R., Lee, J. H., Park, M., Park, K. S., Bae, I. K., Kim, Y. B., et al. (2017). Biology of Acinetobacter baumannii: pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell Infect. Microbiol. 7:55. doi: 10.3389/fcimb.2017.00055
Lee, H. W., Koh, Y. M., Kim, J., Lee, J. C., Lee, Y. C., Seol, S. Y., et al. (2008). Capacity of multidrug-resistant clinical isolates of Acinetobacter baumannii to form biofilm and adhere to epithelial cell surfaces. Clin. Microbiol. Infect. 14, 49–54. doi: 10.1111/j.1469-0691.2007.01842.x
Lee, J. C., Koerten, H., van den Broek, P., Beekhuizen, H., Wolterbeek, R., van den Barselaar, M., et al. (2006). Adherence of Acinetobacter baumannii strains to human bronchial epithelial cells. Res. Microbiol. 157, 360–366. doi: 10.1016/j.resmic.2005.09.011
Lee, J. S., Choi, C. H., Kim, J. W., and Lee, J. C. (2010). Acinetobacter baumannii outer membrane protein A induces dendritic cell death through mitochondrial targeting. J. Microbiol. 48, 387–392. doi: 10.1007/s12275-010-0155-1
Lee, M. J., Jang, S. J., Li, X. M., Park, G., Kook, J.-K., Kim, M. J., et al. (2014). Comparison of rpoB gene sequencing, 16S rRNA gene sequencing, gyrB multiplex PCR, and the VITEK2 system for identification of Acinetobacter clinical isolates. Diagn. Microbiol. Infect. Dis 78, 29–34. doi: 10.1016/j.diagmicrobio.2013.07.013
Lee, N. Y., Lee, H. C., Ko, N. Y., Chang, C. M., Shih, H. I., Wu, C. J., et al. (2007). Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect. Control Hosp. Epidemiol. 28, 713–719. doi: 10.1086/517954
Lee, Y. L., Ko, W. C., and Hsueh, P. R. (2023). Geographic patterns of Acinetobacter baumannii and carbapenem resistance in the Asia-Pacific region: results from the antimicrobial testing leadership and surveillance (ATLAS) program, 2012-2019. Int J. Infect. Dis. 127, 48–55. doi: 10.1016/j.ijid.2022.12.010
Lee, Y. T., Kuo, S. C., Chiang, M. C., Yang, S. P., Chen, C. P., Chen, T. L., et al. (2012a). Emergence of carbapenem-resistant non-baumannii species of Acinetobacter harboring a blaOXA-51-like gene that is intrinsic to A. baumannii. Antimicrob. Agents Chemother. 56, 1124–1127. doi: 10.1128/AAC.00622-11
Lee, Y. T., Kuo, S. C., Yang, S. P., Lin, Y. T., Tseng, F. C., Chen, T. L., et al. (2012b). Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin. Infect. Dis. 55, 209–215. doi: 10.1093/cid/cis385
Li, J., Qin, M., Liu, C., Ma, W., Zeng, X., Ji, Y., et al. (2020). Antimicrobial photodynamic therapy against multidrug-resistant Acinetobacter baumannii clinical isolates mediated by aloe-emodin: an in vitro study. Photodiagn. Photodyn. Ther. 29:101632. doi: 10.1016/j.pdpdt.2019.101632
Li, S., Wei, B., Xu, L., Cong, C., Murtaza, B., Wang, L., et al. (2024). In vivo efficacy of phage cocktails against carbapenem resistance Acinetobacter baumannii in the rat pneumonia model. J. Virol. 98:e0046724. doi: 10.1128/jvi.00467-24
Li, Z. T., Zhang, R. L., Bi, X. G., Xu, L., Fan, M., Xie, D., et al. (2015). Outer membrane vesicles isolated from two clinical Acinetobacter baumannii strains exhibit different toxicity and proteome characteristics. Microb. Pathog. 81, 46–52. doi: 10.1016/j.micpath.2015.03.009
Liebchen, U., Paal, M., Jung, J., Schroeder, I., Frey, L., Zoller, M., et al. (2020). Therapeutic drug monitoring-guided high dose meropenem therapy of a multidrug resistant Acinetobacter baumannii-A case report. Respir. Med. Case Rep. 29:100966. doi: 10.1016/j.rmcr.2019.100966
Lima, W. G., Alves, M. C., Cruz, W. S., and Paiva, M. C. (2018). Chromosomally encoded and plasmid-mediated polymyxins resistance in Acinetobacter baumannii: a huge public health threat. Eur. J. Clin. Microbiol. Infect. Dis. 37, 1009–1019. doi: 10.1007/s10096-018-3223-9
Lima, W. G., Brito, J. C. M., Cardoso, B. G., Cardoso, V. N., de Paiva, M. C., de Lima, M. E., et al. (2020). Rate of polymyxin resistance among Acinetobacter baumannii recovered from hospitalized patients: a systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 39, 1427–1438. doi: 10.1007/s10096-020-03876-x
Lin, M.-F., and Lan, C.-Y. (2014). Antimicrobial resistance in Acinetobacter baumannii: from bench to bedside. World J. Clin. Cases 2:787. doi: 10.12998/wjcc.v2.i12.787
Lin, M. F., Lin, Y. Y., Yeh, H. W., and Lan, C. Y. (2014). Role of the BaeSR two-component system in the regulation of Acinetobacter baumannii adeAB genes and its correlation with tigecycline susceptibility. BMC Microbiol. 14:119. doi: 10.1186/1471-2180-14-119
Liu, C. C., Kuo, H. Y., Tang, C. Y., Chang, K. C., and Liou, M. L. (2014). Prevalence and mapping of a plasmid encoding a type IV secretion system in Acinetobacter baumannii. Genomics 104, 215–223. doi: 10.1016/j.ygeno.2014.07.011
Liu, H., Cao, C. Y., Qiu, F. L., Huang, H. N., Xie, H., Dong, R., et al. (2021). Iron-rich conditions induce OmpA and virulence changes of Acinetobacter baumannii. Front. Microbiol. 12:725194. doi: 10.3389/fmicb.2021.725194
Lob, S. H., Hoban, D. J., Sahm, D. F., and Badal, R. E. (2016). Regional differences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int. J. Antimicrob. Agents 47, 317–323. doi: 10.1016/j.ijantimicag.2016.01.015
Loehfelm, T. W., Luke, N. R., and Campagnari, A. A. (2008). Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J. Bacteriol. 190, 1036–1044. doi: 10.1128/JB.01416-07
Loo, J., Kwok, H. C., Leung, C. C. H., Wu, S. Y., Law, I. L. G., Cheung, Y. K., et al. (2017). Sample-to-answer on molecular diagnosis of bacterial infection using integrated lab–on–a–disc. Biosens. Bioelectron. 93, 212–219. doi: 10.1016/j.bios.2016.09.001
Luk, S., Chow, V. C. Y., Yu, K. C. H., Hsu, E. K., Tsang, N. C., Chuang, V. W. M., et al. (2019). Effectiveness of antimicrobial hospital curtains on reducing bacterial contamination—a multicenter study. Infect. Control Hosp. Epidemiol. 40, 164–170. doi: 10.1017/ice.2018.315
Luo, G., Lin, L., Ibrahim, A. S., Baquir, B., Pantapalangkoor, P., Bonomo, R. A., et al. (2012). Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS ONE 7:e29446. doi: 10.1371/journal.pone.0029446
Luo, L., Li, Q., Xing, C., Li, C., Pan, Y., Sun, H., et al. (2025). Antibody-based therapy: An alternative for antimicrobial treatment in the post-antibiotic era. Microbiol. Res. 290:127974. doi: 10.1016/j.micres.2024.127974
Luo, Y., Guo, Z., Li, Y., Ouyang, H., Huang, S., Chen, Y., et al. (2023). Appropriateness of empirical antibiotic therapy in hospitalized patients with bacterial infection: a retrospective cohort study. Infect. Drug. Resist. 16, 4555–4568. doi: 10.2147/IDR.S402172
Ly, T. D. A., Amanzougaghene, N., Hoang, V. T., Dao, T. L., Louni, M., Mediannikov, O., et al. (2020). Molecular evidence of bacteria in clothes lice collected from homeless people living in shelters in Marseille. Vector Borne Zoonotic Dis. 20, 872–874. doi: 10.1089/vbz.2019.2603
Lynch, J. P. III., Zhanel, G. G., and Clark, N. M. (2017). Infections due to Acinetobacter baumannii in the ICU: treatment options. Semin. Respir. Crit. Care Med. 38, 311–325. doi: 10.1055/s-0037-1599225
Lysitsas, M., Triantafillou, E., Chatzipanagiotidou, I., Antoniou, K., and Valiakos, G. (2023). Antimicrobial susceptibility profiles of Acinetobacter baumannii strains, isolated from clinical cases of companion animals in Greece. Vet. Sci. 10:635. doi: 10.3390/vetsci10110635
Ma, C., and McClean, S. (2021). Mapping global prevalence of Acinetobacter baumannii and recent vaccine development to tackle it. Vaccines 9:570. doi: 10.3390/vaccines9060570
Magnet, S., Courvalin, P., and Lambert, T. (2001). Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 45, 3375–3380. doi: 10.1128/AAC.45.12.3375-3380.2001
Mahajan, V. M. (1984). Postoperative ocular infections: an analysis of laboratory data on 750 cases. Ann. Ophthalmol. 16, 847–848.
Maher, C., and Hassan, K. A. (2023). The gram-negative permeability barrier: tipping the balance of the in and the out. mBio 14:e0120523. doi: 10.1128/mbio.01205-23
Maiden, M. C., Jansen, M. J., van Rensburg, J. E., Bray, S. G., Earle, S. A., Ford, K. A., et al. (2013). MLST revisited: the gene-by-gene approach to bacterial genomics. Nat. Rev. Microbiol. 11, 728–736. doi: 10.1038/nrmicro3093
Malta, R. C. R., Ramos, G., and Nascimento, J. D. S. (2020). From food to hospital: we need to talk about Acinetobacter spp. Germs 10, 210–217. doi: 10.18683/germs.2020.1207
Manchanda, V., Sanchaita, S., and Singh, N. (2010). Multidrug resistant acinetobacter. J. Glob. Infect. Dis. 2, 291–304. doi: 10.4103/0974-777X.68538
Manenzhe, R. I., Zar, H. J., Nicol, M. P., and Kaba, M. (2015). The spread of carbapenemase-producing bacteria in Africa: a systematic review. J. Antimicrob. Chemother. 70, 23–40. doi: 10.1093/jac/dku356
Manfi Ahmed, S., Hashim Yaseen, K., and Mohammed Mahmood, M. (2022). Immunological Evaluation of Individuals Infected with Acinetobacter baumannii. Arch Razi Inst 77, 1813–1819.
Manian, F. A., Griesenauer, S., Senkel, D., Setzer, J. M., Doll, S. A., Perry, A. M., et al. (2011). Isolation of Acinetobacter baumannii complex and methicillin-resistant Staphylococcus aureus from hospital rooms following terminal cleaning and disinfection: can we do better? Infect. Control Hosp. Epidemiol. 32, 667–672. doi: 10.1086/660357
Marcolan De Mello, M., de Barros, P. P., de Cassia Bernardes, R., Alves, S. R., Ramanzini, N. P., Figueiredo-Godoi, L. M. A., et al. (2019). Antimicrobial photodynamic therapy against clinical isolates of carbapenem-susceptible and carbapenem-resistant Acinetobacter baumannii. Lasers Med. Sci. 34, 1755–1761. doi: 10.1007/s10103-019-02773-w
Mark, D. B., and Gaynon, M. W. (1983). Trauma-induced endophthalmitis caused by Acinetobacter anitratus. Br. J. Ophthalmol. 67, 124–126. doi: 10.1136/bjo.67.2.124
Martí, S., Fernández-Cuenca, F., Pascual, A., Ribera, A., Rodríguez-Baño, J., Bou, G., et al. (2006). [Prevalence of the tetA and tetB genes as mechanisms of resistance to tetracycline and minocycline in Acinetobacter baumannii clinical isolates]. Enferm. Infecc. Microbiol. Clin. 24, 77–80. doi: 10.1157/13085012
Martin, J. E., and Imlay, J. A. (2011). The alternative aerobic ribonucleotide reductase of Escherichia coli, NrdEF, is a manganese-dependent enzyme that enables cell replication during periods of iron starvation. Mol. Microbiol. 80, 319–334. doi: 10.1111/j.1365-2958.2011.07593.x
Martínez-Trejo, A., Ruiz-Ruiz, J. M., Gonzalez-Avila, L. U., Saldaña-Padilla, A., Hernández-Cortez, C., Loyola-Cruz, M. A., et al. (2022). Evasion of antimicrobial activity in acinetobacter baumannii by target site modifications: an effective resistance mechanism. Int. J. Mol. Sci. 23:6582. doi: 10.37247/PAMOL3ED.3.22.32
Mathews, D., Mathews, J. P., Kwartz, J., and Inkster, C. (2005). Preseptal cellulitis caused by Acinetobacter lwoffi. Indian J. Ophthalmol. 53, 213–214. doi: 10.4103/0301-4738.16692
Matthew, M., and Harris, A. M. (1976). Identification of beta-lactamases by analytical isoelectric focusing: correlation with bacterial taxonomy. J. Gen. Microbiol. 94, 55–67. doi: 10.1099/00221287-94-1-55
Mea, H. J., Yong, P. V. C., and Wong, E. H. (2021). An overview of Acinetobacter baumannii pathogenesis: motility, adherence and biofilm formation. Microbiol. Res. 247:126722. doi: 10.1016/j.micres.2021.126722
Menon, T., Shanmugasundaram, S., Nandhakumar, B., Nalina, K., and Balasubramaniam. (2006). Infective endocarditis due to Acinetobacter baumannii complex–a case report. Indian J. Pathol. Microbiol. 49, 576–578.
Mertins, S., Higgins, P. G., Thunissen, C., Magein, H., Gilleman, Q., Mertens, P., et al. (2023). Development of an immunochromatographic lateral flow assay to rapidly detect OXA-23-, OXA-40-, OXA-58- and NDM-mediated carbapenem resistance determinants in Acinetobacter baumannii. J. Med. Microbiol. 72:1681. doi: 10.1099/jmm.0.001681
Meschiari, M., Lòpez-Lozano, J.-M., Di Pilato, V., Gimenez-Esparza, C., Vecchi, E., Bacca, E., et al. (2021). A five-component infection control bundle to permanently eliminate a carbapenem-resistant Acinetobacter baumannii spreading in an intensive care unit. Antimicrob. Resist. Infect. Control. 10, 1–13. doi: 10.1186/s13756-021-00990-z
Michalopoulos, A., Falagas, M. E., Karatza, D. C., Alexandropoulou, P., Papadakis, E., Gregorakos, L., et al. (2011). Epidemiologic, clinical characteristics, and risk factors for adverse outcome in multiresistant gram-negative primary bacteremia of critically ill patients. Am. J. Infect. Control 39, 396–400. doi: 10.1016/j.ajic.2010.06.017
Micoli, F., Bagnoli, F., Rappuoli, R., and Serruto, D. (2021). The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 19, 287–302. doi: 10.1038/s41579-020-00506-3
Miller, J. (2005). Acinetobacter as a causative agent in preseptal cellulitis. Optometry 76, 176–180. doi: 10.1016/S1529-1839(05)70282-7
Mishra, S. K., Akter, T., Urmi, U. L., Enninful, G., Sara, M., Shen, J., et al. (2025). Harnessing non-antibiotic strategies to counter multidrug-resistant clinical pathogens with special reference to antimicrobial peptides and their coatings. Antibiotics 14:57. doi: 10.3390/antibiotics14010057
Moffatt, J. H., Harper, M., Harrison, P., Hale, J. D., Vinogradov, E., Seemann, T., et al. (2010). Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 54, 4971–4977. doi: 10.1128/AAC.00834-10
Moffatt, J. H., Harper, M., Mansell, A., Crane, B., Fitzsimons, T. C., Nation, R. L., et al. (2013). Lipopolysaccharide-deficient Acinetobacter baumannii shows altered signaling through host toll-like receptors and increased susceptibility to the host antimicrobial peptide LL-37. Infect. Immun. 81, 684–689. doi: 10.1128/IAI.01362-12
Mohamed, A. H., Sheikh Omar, N. M., Osman, M. M., Mohamud, H. A., Eraslan, A., Gur, M., et al. (2022). Antimicrobial resistance and predisposing factors associated with catheter-associated UTI caused by uropathogens exhibiting multidrug-resistant patterns: a 3-year retrospective study at a tertiary hospital in Mogadishu, Somalia. Trop. Med. Infect. Dis. 7:42. doi: 10.3390/tropicalmed7030042
Mohd Sazlly Lim, S., Zainal Abidin, A., Liew, S. M., Roberts, J. A., and Sime, F. B. (2019). The global prevalence of multidrug-resistance among Acinetobacter baumannii causing hospital-acquired and ventilator-associated pneumonia and its associated mortality: a systematic review and meta-analysis. J. Infect 79, 593–600. doi: 10.1016/j.jinf.2019.09.012
Monem, S., Furmanek-Blaszk, B., Łupkowska, A., Kuczyńska-Wiśnik, D., Stojowska-Swedrzyńska, K., Laskowska, E., et al. (2020). Mechanisms protecting Acinetobacter baumannii against multiple stresses triggered by the host immune response, antibiotics and outside-host environment. Int. J. Mol. Sci. 21:5498. doi: 10.3390/ijms21155498
Moriel, D. G., Beatson, S. A., Wurpel, D. J., Lipman, J., Nimmo, G. R., Paterson, D. L., et al. (2013). Identification of novel vaccine candidates against multidrug-resistant Acinetobacter baumannii. PLoS ONE 8:e77631. doi: 10.1371/journal.pone.0077631
Morris, F. C., Dexter, C., Kostoulias, X., Uddin, M. I., and Peleg, A. Y. (2019). The mechanisms of disease caused by Acinetobacter baumannii. Front. Microbiol. 10:1601. doi: 10.3389/fmicb.2019.01601
Mortensen, B. L., Rathi, S., Chazin, W. J., and Skaar, E. P. (2014). Acinetobacter baumannii response to host-mediated zinc limitation requires the transcriptional regulator Zur. J. Bacteriol. 196, 2616–2626. doi: 10.1128/JB.01650-14
Mortensen, B. L., and Skaar, E. P. (2013). The contribution of nutrient metal acquisition and metabolism to Acinetobacter baumannii survival within the host. Front. Cell. Infect. Microbiol. 3:95. doi: 10.3389/fcimb.2013.00095
Moussally, K., Abu-Sittah, G., Gomez, F. G., Fayad, A. A., and Farra, A. (2023). Antimicrobial resistance in the ongoing Gaza war: a silent threat. Lancet 402, 1972–1973. doi: 10.1016/S0140-6736(23)02508-4
Mu, X., Nakano, R., Nakano, A., Ubagai, T., Kikuchi-Ueda, T., Tansho-Nagakawa, S., et al. (2016). Loop-mediated isothermal amplification: rapid and sensitive detection of the antibiotic resistance gene ISAba1-blaOXA-51-like in Acinetobacter baumannii. J. Microbiol. Methods 121, 36–40. doi: 10.1016/j.mimet.2015.12.011
Nairn, B. L., Lonergan, Z. R., Wang, J., Braymer, J. J., Zhang, Y., Calcutt, M. W., et al. (2016). The Response of Acinetobacter baumannii to zinc starvation. Cell Host Microbe 19, 826–836. doi: 10.1016/j.chom.2016.05.007
National Institute of Diabetes and Digestive and Kidney Diseases (2012). Fluoroquinolones, LiverTox: Clinical and Research Information on Drug-Induced Liver Injury, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda (MD).
Nemec, A., Dolzani, L., Brisse, S., van den Broek, P., and Dijkshoorn, L. (2004). Diversity of aminoglycoside-resistance genes and their association with class 1 integrons among strains of pan-European Acinetobacter baumannii clones. J. Med. Microbiol. 53, 1233–1240. doi: 10.1099/jmm.0.45716-0
Nemec, A., Krizova, L., Maixnerova, M., Sedo, O., Brisse, S., Higgins, P. G., et al. (2015). Acinetobacter seifertii sp. nov., a member of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex isolated from human clinical specimens. Int. J. Syst. Evol. Microbiol. 65, 934–942. doi: 10.1099/ijs.0.000043
Nemec, A., Krizova, L., Maixnerova, M., van der Reijden, T. J., Deschaght, P., Passet, V., et al. (2011). Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov. (formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov. (formerly Acinetobacter genomic species 13TU). Res. Microbiol. 162, 393–404. doi: 10.1016/j.resmic.2011.02.006
Ng, G., Sharma, B. K., and Fox, G. F. (2004). Acinetobacter skin abscess in a neonate. J. Perinatol. 24, 526–527. doi: 10.1038/sj.jp.7211133
Nguyen, M., and Joshi, S. (2021). Carbapenem resistance in Acinetobacter baumannii, and their importance in hospital-acquired infections: a scientific review. J. Appl. Microbiol. 131, 2715–2738. doi: 10.1111/jam.15130
Nho, J. S., Jun, S. H., Oh, M. H., Park, T. I., Choi, C. W., Kim, S. I., et al. (2015). Acinetobacter nosocomialis secretes outer membrane vesicles that induce epithelial cell death and host inflammatory responses. Microb. Pathog. 81, 39–45. doi: 10.1016/j.micpath.2015.03.012
Ni, W., Han, Y., Zhao, J., Wei, C., Cui, J., Wang, R., et al. (2016). Tigecycline treatment experience against multidrug-resistant Acinetobacter baumannii infections: a systematic review and meta-analysis. Int. J. Antimicrob. Agents. 47, 107–116. doi: 10.1016/j.ijantimicag.2015.11.011
Nielsen, T. B., Pantapalangkoor, P., Luna, B. M., Bruhn, K. W., Yan, J., Dekitani, K., et al. (2017). Monoclonal antibody protects against Acinetobacter baumannii infection by enhancing bacterial clearance and evading sepsis. J. Infect. Dis. 216, 489–501. doi: 10.1093/infdis/jix315
Niu, C., Clemmer, K. M., Bonomo, R. A., and Rather, P. N. (2008). Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J. Bacteriol. 190, 3386–3392. doi: 10.1128/JB.01929-07
Noel, H. R., Petrey, J. R., and Palmer, L. D. (2022). Mobile genetic elements in Acinetobacter antibiotic-resistance acquisition and dissemination. Ann. NY Acad. Sci. 1518, 166–182. doi: 10.1111/nyas.14918
Noinaj, N., Guillier, M., Barnard, T. J., and Buchanan, S. K. (2010). TonB-dependent transporters: regulation, structure, and function. Annu. Rev. Microbiol. 64, 43–60. doi: 10.1146/annurev.micro.112408.134247
Nordenfelt, P., and Tapper, H. (2011). Phagosome dynamics during phagocytosis by neutrophils. J. Leukoc. Biol. 90, 271–284. doi: 10.1189/jlb.0810457
Noto, M. J., Boyd, K. L., Burns, W. J., Varga, M. G., Peek, R. M. Jr., and Skaar, E. P. (2015). Toll-Like Receptor 9 Contributes to Defense against Acinetobacter baumannii Infection. Infect. Immun. 83, 4134–4141. doi: 10.1128/IAI.00410-15
Novović, K., and Jovčić, B. (2023). Colistin Resistance in Acinetobacter baumannii: molecular mechanisms and epidemiology. Antibiotics 12:516. doi: 10.3390/antibiotics12030516
Novović, K., Radovanović, M., Gajić, I., Vasiljević, Z., Malešević, M., Šapić, K., et al. (2025). AdeABC, AdeFGH, and AdeIJK efflux pumps as key factors in tigecycline resistance of Acinetobacter baumannii: a study from Western Balkan hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 44, 129–142. doi: 10.1007/s10096-024-04974-w
Nwugo, C. C., Gaddy, J. A., Zimbler, D. L., and Actis, L. A. (2011). Deciphering the iron response in Acinetobacter baumannii: a proteomics approach. J. Proteomics 74, 44–58. doi: 10.1016/j.jprot.2010.07.010
Ogungbe, B. A., Awoniyi, S. O., Bolarinde, B. F., and Awotimiro, O. E. (2024). Progress of phage therapy research as an alternative to antibiotics: current status, challenges, and the future of phage therapeutics. J. Med. Surg. Public Health 2:100042. doi: 10.1016/j.glmedi.2023.100042
Oladipo, E. K., Adeyemo, S. F., Oluwasanya, G. J., Adaramola, E. O., Akintola, S. B., Afolabi, V. O., et al. (2025). Novel antibacterial agents and emerging therapies in the fight against multidrug-resistant Acinetobacter baumannii. Microb. Pathog. 200:107361. doi: 10.1016/j.micpath.2025.107361
Olut, A. I., and Erkek, E. (2005). Early prosthetic valve endocarditis due to Acinetobacter baumannii: a case report and brief review of the literature. Scand J. Infect. Dis. 37, 919–921. doi: 10.1080/00365540500262567
Orok, E., Ikpe, F., Williams, T., and Ekada, I. (2025). Impact of educational intervention on knowledge of antimicrobial resistance and antibiotic use patterns among healthcare students: a pre-and post-intervention study. BMC Med. Educ. 25:283. doi: 10.1186/s12909-025-06856-x
Owusu, F. A., Obeng-Nkrumah, N., Gyinae, E., Kodom, S., Tagoe, R., Tabi, B. K. A., et al. (2023). Occurrence of carbapenemases, extended-spectrum beta-lactamases and AmpCs among beta-lactamase-producing gram-negative bacteria from clinical sources in Accra, Ghana. Antibiotics 12:1016. doi: 10.3390/antibiotics12061016
Page, M. G. (2019). The role of iron and siderophores in infection, and the development of siderophore antibiotics. Clin. Infect. Dis. 69, S529–S537. doi: 10.1093/cid/ciz825
Pakharukova, N., Tuittila, M., Paavilainen, S., and Zavialov, A. (2017). Methylation, crystallization and SAD phasing of the Csu pilus CsuC-CsuE chaperone-adhesin subunit pre-assembly complex from Acinetobacter baumannii. Acta. Crystallogr. F. Struct. Biol. Commun. 73, 450–454. doi: 10.1107/S2053230X17009566
Palmer, L. D., Minor, K. E., Mettlach, J. A., Rivera, E. S., Boyd, K. L., Caprioli, R. M., et al. (2020). Modulating isoprenoid biosynthesis increases lipooligosaccharides and restores Acinetobacter baumannii resistance to host and antibiotic stress. Cell Rep. 32:108129. doi: 10.1016/j.celrep.2020.108129
Pan, S., Huang, X., Wang, Y., Li, L., Zhao, C., Yao, Z., et al. (2018). Efficacy of intravenous plus intrathecal/intracerebral ventricle injection of polymyxin B for post-neurosurgical intracranial infections due to MDR/XDR Acinectobacter baumannii: a retrospective cohort study. Antimicrob. Resist. Infect. Control 7:8. doi: 10.1186/s13756-018-0305-5
Pandey, N., and Cascella, M. (2024). Beta-Lactam Antibiotics, StatPearls, StatPearls Publishing. Copyright © 2024, StatPearls Publishing LLC., Treasure Island (FL).
Papp-Wallace, K. M., Endimiani, A., Taracila, M. A., and Bonomo, R. A. (2011). Carbapenems: past, present, and future. Antimicrob. Agents Chemother. 55, 4943–4960. doi: 10.1128/AAC.00296-11
Paquelin, A., Ghigo, J. M., Bertin, S., and Wandersman, C. (2001). Characterization of HasB, a Serratia marcescens TonB-like protein specifically involved in the haemophore-dependent haem acquisition system. Mol. Microbiol. 42, 995–1005. doi: 10.1046/j.1365-2958.2001.02628.x
Park, S., Lee, K. M., Yoo, Y. S., Yoo, J. S., Yoo, J. I., Kim, H. S., et al. (2011). Alterations of gyrA, gyrB, and parC and activity of efflux pump in fluoroquinolone-resistant Acinetobacter baumannii. Osong. Public Health Res. Perspect. 2, 164–170. doi: 10.1016/j.phrp.2011.11.040
Park, Y. K., Lee, G. H., Baek, J. Y., Chung, D. R., Peck, K. R., Song, J. H., et al. (2010). A single clone of Acinetobacter baumannii, ST22, is responsible for high antimicrobial resistance rates of Acinetobacter spp. isolates that cause bacteremia and urinary tract infections in Korea. Microb. Drug Resist. 16, 143–149. doi: 10.1089/mdr.2009.0088
Partridge, S. R., Kwong, S. M., Firth, N., and Jensen, S. O. (2018). Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 32:108129. doi: 10.1128/CMR.00088-17
Paul, M., Carrara, E., Retamar, P., Tängdén, T., Bitterman, R., Bonomo, R. A., et al. (2022). European society of clinical microbiology and infectious diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 28, 521–547. doi: 10.1016/j.cmi.2021.11.025
Pearson, J. P., Van Delden, C., and Iglewski, B. H. (1999). Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181, 1203–1210. doi: 10.1128/JB.181.4.1203-1210.1999
Peleg, A. Y., Seifert, H., and Paterson, D. L. (2008). Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21, 538–582. doi: 10.1128/CMR.00058-07
Pendleton, J. N., Gorman, S. P., and Gilmore, B. F. (2013). Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti. Infect. Ther. 11, 297–308. doi: 10.1586/eri.13.12
Penwell, W. F., Arivett, B. A., and Actis, L. A. (2012). The Acinetobacter baumannii entA gene located outside the acinetobactin cluster is critical for siderophore production, iron acquisition and virulence. PLoS ONE 7:e36493. doi: 10.1371/journal.pone.0036493
Pérez, A., Merino, M., Rumbo-Feal, S., Álvarez-Fraga, L., Vallejo, J. A., Beceiro, A., et al. (2017). The FhaB/FhaC two-partner secretion system is involved in adhesion of Acinetobacter baumannii AbH12O-A2 strain. Virulence 8, 959–974. doi: 10.1080/21505594.2016.1262313
Perez, S., Innes, G. K., Walters, M. S., Mehr, J., Arias, J., Greeley, R., et al. (2020). Increase in hospital-acquired carbapenem-resistant Acinetobacter baumannii infection and colonization in an acute care hospital during a surge in COVID-19 admissions - New Jersey, February-July 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 1827–1831. doi: 10.15585/mmwr.mm6948e1
Pérez-Varela, M., Corral, J., Aranda, J., and Barbé, J. (2019). Roles of efflux pumps from different superfamilies in the surface-associated motility and virulence of Acinetobacter baumannii ATCC 17978. Antimicrob. Agents Chemother. 63:e02190-18. doi: 10.1128/AAC.02190-18
Pérez-Varela, M., Corral, J., Vallejo, J. A., Rumbo-Feal, S., Bou, G., Aranda, J., et al. (2017). Mutations in the β-subunit of the RNA polymerase impair the surface-associated motility and virulence of Acinetobacter baumannii. Infect. Immun. 85:e00327-17. doi: 10.1128/IAI.00327-17
Performance, C. (2016). Standards for Antimicrobial Susceptibility Testing, CLSI Supplement M100S. Wayne, PA: Clinical and Laboratory Standards Institute.
Piksa, M., Lian, C., Samuel, I. C., Pawlik, K. J., Samuel, I. D., Matczyszyn, K., et al. (2023). The role of the light source in antimicrobial photodynamic therapy. Chem. Soc. Rev. 52, 1697–1722. doi: 10.1039/D0CS01051K
Piotrowski, M., Alekseeva, I., Arnet, U., and Yücel, E. (2024). Insights into the rising threat of carbapenem-resistant Enterobacterales and Pseudomonas aeruginosa epidemic infections in Eastern Europe: a systematic literature review. Antibiotics 13:978. doi: 10.3390/antibiotics13100978
Pires, D. P., Costa, A. R., Pinto, G., Meneses, L., and Azeredo, J. (2020). Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 44, 684–700. doi: 10.1093/femsre/fuaa017
Pneumatikos, I., Konstantonis, D., Tsagaris, I., Theodorou, V., Vretzakis, G., Danielides, V., et al. (2006). Prevention of nosocomial maxillary sinusitis in the ICU: the effects of topically applied alpha-adrenergic agonists and corticosteroids. Intensive Care Med. 32, 532–537. doi: 10.1007/s00134-006-0078-9
Pollock, M. R. (1967). Origin and function of penicillinase: a problem in biochemical evolution. Br. Med. J. 4, 71–77. doi: 10.1136/bmj.4.5571.71
Postle, K., and Larsen, R. A. (2007). TonB-dependent energy transduction between outer and cytoplasmic membranes. Biometals 20, 453–465. doi: 10.1007/s10534-006-9071-6
Poulikakos, P., Tansarli, G. S., and Falagas, M. E. (2014). Combination antibiotic treatment versus monotherapy for multidrug-resistant, extensively drug-resistant, and pandrug-resistant Acinetobacter infections: a systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1675–1685. doi: 10.1007/s10096-014-2124-9
Pour, N. K., Dusane, D. H., Dhakephalkar, P. K., Zamin, F. R., Zinjarde, S. S., Chopade, B. A., et al. (2011). Biofilm formation by Acinetobacter baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol. Med. Microbiol. 62, 328–338. doi: 10.1111/j.1574-695X.2011.00818.x
Pourcel, C., Minandri, F., Hauck, Y., D'Arezzo, S., Imperi, F., Vergnaud, G., et al. (2011). Identification of variable-number tandem-repeat (VNTR) sequences in Acinetobacter baumannii and interlaboratory validation of an optimized multiple-locus VNTR analysis typing scheme. J. Clin. Microbiol. 49, 539–548. doi: 10.1128/JCM.02003-10
Powers, M. J., and Trent, M. S. (2018). Expanding the paradigm for the outer membrane: Acinetobacter baumannii in the absence of endotoxin. Mol. Microbiol. 107, 47–56. doi: 10.1111/mmi.13872
Proschak, A., Lubuta, P., Grün, P., Löhr, F., Wilharm, G., de Berardinis, V., et al. (2013). Structure and biosynthesis of fimsbactins A–F, siderophores from Acinetobacter baumannii and Acinetobacter baylyi. ChemBioChem 14, 633–638. doi: 10.1002/cbic.201200764
Qiu, H., KuoLee, R., Harris, G., Van Rooijen, N., Patel, G. B., and Chen, W. (2012). Role of macrophages in early host resistance to respiratory Acinetobacter baumannii infection. PLoS ONE 7:e40019. doi: 10.1371/journal.pone.0040019
Quraini, M. A., Jabri, Z. A., Sami, H., Mahindroo, J., Taneja, N., Muharrmi, Z. A., et al. (2023). Exploring synergistic combinations in extended and pan-drug resistant (XDR and PDR) whole genome sequenced Acinetobacter baumannii. Microorganisms 11:1409. doi: 10.3390/microorganisms11061409
Rafei, R., Kempf, M., Eveillard, M., Dabboussi, F., Hamze, M., Joly-Guillou, M. L., et al. (2014). Current molecular methods in epidemiological typing of Acinetobacter baumannii. Future Microbiol. 9, 1179–1194. doi: 10.2217/fmb.14.63
Rai, S., and Kumar, A. (2022). Bacteriophage therapeutics to confront multidrug-resistant Acinetobacter baumannii-a global health menace. Environ. Microbiol. Rep. 14, 347–364. doi: 10.1111/1758-2229.12988
Rajesh, S., Koshi, E., Philip, K., and Mohan, A. (2011). Antimicrobial photodynamic therapy: an overview. J. Indian Soc. Periodontol. 15, 323–327. doi: 10.4103/0972-124X.92563
Ramirez, M. S., Bonomo, R. A., and Tolmasky, M. E. (2020). Carbapenemases: transforming Acinetobacter baumannii into a yet more dangerous menace. Biomolecules 10:720. doi: 10.3390/biom10050720
Ramirez, M. S., and Tolmasky, M. E. (2010). Aminoglycoside modifying enzymes. Drug Resist. Updat. 13, 151–171. doi: 10.1016/j.drup.2010.08.003
Rao, S., Betancourt-Garcia, M., Kare-Opaneye, Y. O., Swierczewski, B. E., Bennett, J. W., Horne, B. A., et al. (2022). Critically ill patient with multidrug-resistant Acinetobacter baumannii respiratory infection successfully treated with intravenous and nebulized bacteriophage therapy. Antimicrob. Agents Chemother. 66:e00824-21. doi: 10.1128/AAC.00824-21
Ratliff, M., Zhu, W., Deshmukh, R., Wilks, A., and Stojiljkovic, I. (2001). Homologues of neisserial heme oxygenase in gram-negative bacteria: degradation of heme by the product of the pigA gene of Pseudomonas aeruginosa. J. Bacteriol. 183, 6394–6403. doi: 10.1128/JB.183.21.6394-6403.2001
Redgrave, L. S., Sutton, S. B., Webber, M. A., and Piddock, L. J. (2014). Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 22, 438–445. doi: 10.1016/j.tim.2014.04.007
Repizo, G. D. (2017). Prevalence of Acinetobacter baumannii strains expressing the Type 6 secretion system in patients with bacteremia. Virulence 8, 1099–1101. doi: 10.1080/21505594.2017.1346768
Rice, L. B. (2008). Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 197, 1079–1081. doi: 10.1086/533452
Ritchie, D. J., and Garavaglia-Wilson, A. (2014). A review of intravenous minocycline for treatment of multidrug-resistant Acinetobacter infections. Clin. Infect. Dis. 59, S374–S380. doi: 10.1093/cid/ciu613
Rizk, M. A., and Abou El-Khier, N. T. (2019). Aminoglycoside resistance genes in Acinetobacter baumannii clinical isolates. Clin. Lab. 65, 1285–1291. doi: 10.7754/Clin.Lab.2019.190103
Rizos, I., Tsiodras, S., Papathanasiou, S., Rigopoulos, A., Barbetseas, J., Stefanadis, C., et al. (2007). Prosthetic valve endocarditis due to Acinetobacter spp: a rare case and literature review. Am. J. Med. Sci. 333, 197–199. doi: 10.1097/MAJ.0b013e31803193c4
Robin, B., Nicol, M., Le, H., Tahrioui, A., Schaumann, A., Vuillemenot, J. B., et al. (2021). MacAB-TolC contributes to the development of Acinetobacter baumannii biofilm at the solid-liquid interface. Front. Microbiol. 12:785161. doi: 10.3389/fmicb.2021.785161
Rodríguez Guardado, A., Maradona, J. A., Asensi, V., Cartón, J. A., Pérez, F., Blanco, A., et al. (2001). [Postsurgical meningitis caused by Acinetobacter baumannii: study of 22 cases and review of the literature]. Rev. Clin. Esp 201, 497–500. doi: 10.1016/S0014-2565(01)70895-8
Rodríguez, C. H., Nastro, M., and Famiglietti, A. (2018). Carbapenemasas en Acinetobacter baumannii: revisión de su diseminación en América Latina. Rev. Argent. Microbiol. 50, 327–333. doi: 10.1016/j.ram.2017.10.006
Roier, S., Zingl, F. G., Cakar, F., Durakovic, S., Kohl, P., Eichmann, T. O., et al. (2016). A novel mechanism for the biogenesis of outer membrane vesicles in gram-negative bacteria. Nat. Commun. 7:10515. doi: 10.1038/ncomms10515
Rosenberg, E., DeLong, E. F., Thompson, F., Lory, S., and Stackebrandt, E. (2013). The Prokaryotes: Human Microbiology. Springer: New York. doi: 10.1007/978-3-642-30141-4
Rumata, N. R., Djide, N. J. N., Frediansyah, A., Nurul, A. M., Mutair, A., Alhumaid, S., et al. (2023). Progress and challenges in antimicrobial resistance and bacterial vaccines. Biointerface Res. Appl. Chem. 13:489. doi: 10.33263/BRIAC135.489
Rumbo, C., Tomás, M., Fernández Moreira, E., Soares, N. C., Carvajal, M., Santillana, E., et al. (2014). The Acinetobacter baumannii Omp33-36 porin is a virulence factor that induces apoptosis and modulates autophagy in human cells. Infect. Immun. 82, 4666–4680. doi: 10.1128/IAI.02034-14
Rumbo-Feal, S., Gómez, M. J., Gayoso, C., Álvarez-Fraga, L., Cabral, M. P., Aransay, A. M., et al. (2013). Whole transcriptome analysis of Acinetobacter baumannii assessed by RNA-sequencing reveals different mRNA expression profiles in biofilm compared to planktonic cells. PLoS ONE 8:e72968. doi: 10.1371/journal.pone.0072968
Runci, F., Gentile, V., Frangipani, E., Rampioni, G., Leoni, L., Lucidi, M., et al. (2019). Contribution of active iron uptake to Acinetobacter baumannii pathogenicity. Infect. Immun. 87:e00755-18. doi: 10.1128/IAI.00755-18
Runyen-Janecky, L. J. (2013). Role and regulation of heme iron acquisition in gram-negative pathogens. Front. Cell. Infect. Microbiol. 3:55. doi: 10.3389/fcimb.2013.00055
Russo, A., Giuliano, S., Ceccarelli, G., Alessandri, F., Giordano, A., Brunetti, G., et al. (2018). Comparison of septic shock due to multidrug-resistant Acinetobacter baumannii or Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in intensive care unit patients. Antimicrob. Agents Chemother.62:e02562-17. doi: 10.1128/AAC.02562-17
Russo, T. A., MacDonald, U., Beanan, J. M., Olson, R., MacDonald, I. J., Sauberan, S. L., et al. (2009). Penicillin-binding protein 7/8 contributes to the survival of Acinetobacter baumannii in vitro and in vivo. J. Infect. Dis. 199, 513–521. doi: 10.1086/596317
Russotto, V., Cortegiani, A., Raineri, S. M., and Giarratano, A. (2015). Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J. Intensive Care 3:54. doi: 10.1186/s40560-015-0120-5
Rutherford, S. T., and Bassler, B. L. (2012). Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2:a012427. doi: 10.1101/cshperspect.a012427
Sato, Y., Unno, Y., Kawakami, S., Ubagai, T., and Ono, Y. (2017). Virulence characteristics of Acinetobacter baumannii clinical isolates vary with the expression levels of omps. J. Med. Microbiol. 66, 203–212. doi: 10.1099/jmm.0.000394
Schooley, R. T., Biswas, B., Gill, J. J., Hernandez-Morales, A., Lancaster, J., Lessor, L., et al. (2017). Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 61:e00954-17. doi: 10.1128/AAC.00954-17
Schweppe, D. K., Harding, C., Chavez, J. D., Wu, X., Ramage, E., Singh, P. K., et al. (2015). Host-microbe protein interactions during bacterial infection. Chem. Biol. 22, 1521–1530. doi: 10.1016/j.chembiol.2015.09.015
Scoffone, V. C., Trespidi, G., Barbieri, G., Arshad, A., Israyilova, A., Buroni, S., et al. (2025). The evolution of antimicrobial resistance in Acinetobacter baumannii and new strategies to fight it. Antibiotics 14:85. doi: 10.3390/antibiotics14010085
Sebeny, P. J., Riddle, M. S., and Petersen, K. (2008). Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin. Infect. Dis. 47, 444–449. doi: 10.1086/590568
Sechi, L. A., Karadenizli, A., Deriu, A., Zanetti, S., Kolayli, F., Balikci, E., et al. (2004). PER-1 type beta-lactamase production in Acinetobacter baumannii is related to cell adhesion. Med. Sci. Monit. 10, Br180-4. Available online at: https://medscimonit.com/abstract/index/idArt/11673
Seifert, H., Strate, A., and Pulverer, G. (1995). Nosocomial bacteremia due to Acinetobacter baumannii. Clinical features, epidemiology, and predictors of mortality. Medicine 74, 340–349. doi: 10.1097/00005792-199511000-00004
Seliger, S. S., Mey, A. R., Valle, A. M., and Payne, S. M. (2001). The two TonB systems of vibrio cholerae: redundant and specific functions. Mol. Microbiol. 39, 801–812. doi: 10.1046/j.1365-2958.2001.02273.x
Sharifipour, E., Shams, S., Esmkhani, M., Khodadadi, J., Fotouhi-Ardakani, R., Koohpaei, A., et al. (2020). Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect. Dis. 20:646. doi: 10.1186/s12879-020-05374-z
Sharma, R., Goda, R., Borkar, S. A., Katiyar, V., Agarwal, S., Kumar, A., et al. (2019). Outcome following postneurosurgical Acinetobacter meningitis: an institutional experience of 72 cases. Neurosurg. Focus 47:E8. doi: 10.3171/2019.5.FOCUS19278
Shatri, G., and Tadi, P. (2024). Polymyxin, StatPearls, StatPearls Publishing. Copyright © 2024, StatPearls Publishing LLC., Treasure Island (FL).
Sheldon, J. R., and Skaar, E. P. (2020). Acinetobacter baumannii can use multiple siderophores for iron acquisition, but only acinetobactin is required for virulence. PLoS Pathog. 16:e1008995. doi: 10.1371/journal.ppat.1008995
Shelenkov, A., Akimkin, V., and Mikhaylova, Y. (2023). International clones of high risk of Acinetobacter baumannii-definitions, history, properties and perspectives. Microorganisms 11:2115. doi: 10.3390/microorganisms11082115
Shetty, V. P., Rodrigues, C., and Deekshit, V. K. (2024). Detection of multidrug-resistant integrons associated with Acinetobacter baumannii isolated from clinical and environmental samples. J. Pure Appl. Microbiol. 18, 605–613. doi: 10.22207/JPAM.18.1.44
Shorr, A. F., Zilberberg, M. D., Micek, S. T., and Kollef, M. H. (2014). Predictors of hospital mortality among septic ICU patients with Acinetobacter spp.bacteremia: a cohort study. BMC Infect. Dis. 14:572. doi: 10.1186/PREACCEPT-9162747951401206
Shutter, M. C., and Akhondi, H. (2024). Tetracycline, StatPearls, StatPearls Publishing. Copyright © 2024, StatPearls Publishing LLC., Treasure Island (FL).
Siegman-Igra, Y., Bar-Yosef, S., Gorea, A., and Avram, J. (1993). Nosocomial acinetobacter meningitis secondary to invasive procedures: report of 25 cases and review. Clin. Infect. Dis. 17, 843–849. doi: 10.1093/clinids/17.5.843
Singh, H., Thangaraj, P., and Chakrabarti, A. (2013). Acinetobacter baumannii: a brief account of mechanisms of multidrug resistance and current and future therapeutic management. J. Clin. Diagn. Res. 7, 2602–2605. doi: 10.7860/JCDR/2013/6337.3626
Singh, R., Garg, N., Shukla, G., Capalash, N., and Sharma, P. (2016). Immunoprotective efficacy of Acinetobacter baumannii outer membrane protein, FilF, predicted in silico as a potential vaccine candidate. Front. Microbiol. 7:158. doi: 10.3389/fmicb.2016.00158
Skerniškyte, J., Karazijaite, E., Deschamps, J., Krasauskas, R., Armalyte, J., Briandet, R., et al. (2019). Blp1 protein shows virulence-associated features and elicits protective immunity to Acinetobacter baumannii infection. BMC Microbiol. 19:259. doi: 10.1186/s12866-019-1615-3
Skiebe, E., de Berardinis, V., Morczinek, P., Kerrinnes, T., Faber, F., Lepka, D., et al. (2012). Surface-associated motility, a common trait of clinical isolates of Acinetobacter baumannii, depends on 1,3-diaminopropane. Int. J. Med. Microbiol. 302, 117–128. doi: 10.1016/j.ijmm.2012.03.003
Smani, Y., Docobo-Pérez, F., López-Rojas, R., Domínguez-Herrera, J., Ibáñez-Martínez, J., Pachón, J., et al. (2012a). Platelet-activating factor receptor initiates contact of Acinetobacter baumannii expressing phosphorylcholine with host cells. J. Biol. Chem. 287, 26901–26910. doi: 10.1074/jbc.M112.344556
Smani, Y., Dominguez-Herrera, J., and Pachón, J. (2013). Association of the outer membrane protein Omp33 with fitness and virulence of Acinetobacter baumannii. J. Infect. Dis. 208, 1561–1570. doi: 10.1093/infdis/jit386
Smani, Y., Fàbrega, A., Roca, I., Sánchez-Encinales, V., Vila, J., Pachón, J., et al. (2014). Role of OmpA in the multidrug resistance phenotype of Acinetobacter baumannii. Antimicrob. Agents Chemother. 58, 1806–1808. doi: 10.1128/AAC.02101-13
Smani, Y., McConnell, M. J., and Pachón, J. (2012b). Role of fibronectin in the adhesion of Acinetobacter baumannii to host cells. PLoS ONE 7:e33073. doi: 10.1371/journal.pone.0033073
Smith, C. A., Antunes, N. T., Stewart, N. K., Toth, M., Kumarasiri, M., Chang, M., et al. (2013). Structural basis for carbapenemase activity of the OXA-23 β-lactamase from Acinetobacter baumannii. Chem. Biol. 20, 1107–1115. doi: 10.1016/j.chembiol.2013.07.015
Smolyakov, R., Borer, A., Riesenberg, K., Schlaeffer, F., Alkan, M., Porath, A., et al. (2003). Nosocomial multi-drug resistant Acinetobacter baumannii bloodstream infection: risk factors and outcome with ampicillin-sulbactam treatment. J. Hosp. Infect. 54, 32–38. doi: 10.1016/S0195-6701(03)00046-X
Song, J. Y., Kee, S. Y., Hwang, I. S., Seo, Y. B., Jeong, H. W., Kim, W. J., et al. (2007). In vitro activities of carbapenem/sulbactam combination, colistin, colistin/rifampicin combination and tigecycline against carbapenem-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 60, 317–322. doi: 10.1093/jac/dkm136
Song, W. Y., and Kim, H. J. (2020). Current biochemical understanding regarding the metabolism of acinetobactin, the major siderophore of the human pathogen Acinetobacter baumannii, and outlook for discovery of novel anti-infectious agents based thereon. Nat. Prod. Rep. 37, 477–487. doi: 10.1039/C9NP00046A
Song, X., Wu, Y., Cao, L., Yao, D., and Long, M. (2019). Is meropenem as a monotherapy truly incompetent for meropenem-nonsusceptible bacterial strains? A pharmacokinetic/pharmacodynamic modeling with Monte Carlo simulation. Front. Microbiol. 10:2777. doi: 10.3389/fmicb.2019.02777
Sousa, C., Botelho, J., Silva, L., Grosso, F., Nemec, A., Lopes, J., et al. (2014). MALDI-TOF MS and chemometric based identification of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex species. Int. J. Med. Microbiol. 304, 669–677. doi: 10.1016/j.ijmm.2014.04.014
Srinivasan, V. B., Rajamohan, G., and Gebreyes, W. A. (2009). Role of AbeS, a novel efflux pump of the SMR family of transporters, in resistance to antimicrobial agents in Acinetobacter baumannii. Antimicrob. Agents Chemother. 53, 5312–5316. doi: 10.1128/AAC.00748-09
Stahl, J., Bergmann, H., Göttig, S., Ebersberger, I., and Averhoff, B. (2015). Acinetobacter baumannii virulence is mediated by the concerted action of three phospholipases D. PLoS ONE 10:e0138360. doi: 10.1371/journal.pone.0138360
Starakis, I., Blikas, A., Siagris, D., Marangos, M., Karatza, C., Bassaris, H., et al. (2006). Prosthetic valve endocarditis caused by Acinetobacter lwoffi: a case report and review. Cardiol. Rev. 14, 45–49. doi: 10.1097/01.crd.0000163801.67781.a2
Sugawara, E., and Nikaido, H. (2012). OmpA is the principal nonspecific slow porin of Acinetobacter baumannii. J. Bacteriol. 194, 4089–4096. doi: 10.1128/JB.00435-12
Sunenshine, R. H., Wright, M. O., Maragakis, L. L., Harris, A. D., Song, X., Hebden, J., et al. (2007). Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg. Infect. Dis. 13, 97–103. doi: 10.3201/eid1301.060716
Suresh, S., Aditya, V., Deekshit, V. K., Manipura, R., and Premanath, R. (2022). A rare occurrence of multidrug-resistant environmental Acinetobacter baumannii strains from the soil of Mangaluru, India. Arch. Microbiol. 204:422. doi: 10.1007/s00203-022-03035-0
Tacconelli, E., Carrara, E., Savoldi, A., Harbarth, S., Mendelson, M., Monnet, D. L., et al. (2018). Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18, 318–327. doi: 10.1016/S1473-3099(17)30753-3
Talà, L., Fineberg, A., Kukura, P., and Persat, A. (2019). Pseudomonas aeruginosa orchestrates twitching motility by sequential control of type IV pili movements. Nat. Microbiol. 4, 774–780. doi: 10.1038/s41564-019-0378-9
Tamma, P. D., Aitken, S. L., Bonomo, R. A., Mathers, A. J., van Duin, D., Clancy, C., et al. (2020). Infectious diseases society of America antimicrobial resistant treatment guidance: gram-negative bacterial infections. Practice 6. doi: 10.1093/cid/ciad428
Tamma, P. D., Heil, E. L., Justo, J. A., Mathers, A. J., Satlin, M. J., Bonomo, R. A., et al. (2024). Infectious diseases society of America 2024 guidance on the treatment of antimicrobial-resistant gram-negative infections. Clin. Infect. Dis. ciae403. doi: 10.1093/cid/ciae403
Tanguy, M., Kouatchet, A., Tanguy, B., Pichard, É., Fanello, S., Joly-Guillou, M.-L., et al. (2017). Management of an Acinetobacter baumannii outbreak in an intensive care unit. Med. Mal. Infect. 47, 409–414. doi: 10.1016/j.medmal.2017.06.003
Thibau, A., Dichter, A. A., Vaca, D. J., Linke, D., Goldman, A., Kempf, V. A. J., et al. (2020). Immunogenicity of trimeric autotransporter adhesins and their potential as vaccine targets. Med. Microbiol. Immunol. 209, 243–263. doi: 10.1007/s00430-019-00649-y
Thoma, R., Seneghini, M., Seiffert, S. N., Vuichard Gysin, D., Scanferla, G., Haller, S., et al. (2022). The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob. Resist. Infect. Control 11:12. doi: 10.1186/s13756-022-01052-8
Thy, M., Timsit, J.-F., and de Montmollin, E. (2023). Aminoglycosides for the treatment of severe infection due to resistant gram-negative pathogens. Antibiotics 12:860. doi: 10.3390/antibiotics12050860
Tilley, P. A., and Roberts, F. J. (1994). Bacteremia with Acinetobacter species: risk factors and prognosis in different clinical settings. Clin. Infect. Dis. 18, 896–900. doi: 10.1093/clinids/18.6.896
Tokur, M. E., Korkmaz, P., Alkan, S., Yildiz, E., Arik, Ö., Renders, D. P., et al. (2022). Mortality predictors on the day of healthcare-associated Acinetobacter baumanni bacteremia in intensive care unit. J. Infect. Dev. Ctries. 16, 1473–1481. doi: 10.3855/jidc.16902
Tomaras, A. P., Dorsey, C. W., Edelmann, R. E., and Actis, L. A. (2003). Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology 149, 3473–3484. doi: 10.1099/mic.0.26541-0
Tomaschek, F., Higgins, P. G., Stefanik, D., Wisplinghoff, H., and Seifert, H. (2016). Head-to-head comparison of two multi-locus sequence typing (MLST) schemes for characterization of Acinetobacter baumannii outbreak and sporadic isolates. PLoS ONE 11:e0153014. doi: 10.1371/journal.pone.0153014
Tomczyk, S., Zanichelli, V., Grayson, M. L., Twyman, A., Abbas, M., Pires, D., et al. (2019). Control of carbapenem-resistant enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa in healthcare facilities: a systematic review and reanalysis of quasi-experimental studies. Clin. Infect. Dis. 68, 873–884. doi: 10.1093/cid/ciy752
Tooke, C. L., Hinchliffe, P., Bragginton, E. C., Colenso, C. K., Hirvonen, V. H. A., Takebayashi, Y., et al. (2019). β-lactamases and β-lactamase inhibitors in the 21st century. J. Mol. Biol. 431, 3472–3500. doi: 10.1016/j.jmb.2019.04.002
Touchon, M., Cury, J., Yoon, E. J., Krizova, L., Cerqueira, G. C., Murphy, C., et al. (2014). The genomic diversification of the whole Acinetobacter genus: origins, mechanisms, and consequences. Genome. Biol. Evol. 6, 2866–2882. doi: 10.1093/gbe/evu225
Trottier, V., Segura, P. G., Namias, N., King, D., Pizano, L. R., Schulman, C. I., et al. (2007). Outcomes of Acinetobacter baumannii infection in critically ill burned patients. J. Burn Care Res. 28, 248–254. doi: 10.1097/BCR.0B013E318031A20F
Tsuchiya, T., Nakao, N., Yamamoto, S., Hirai, Y., Miyamoto, K., Tsujibo, H., et al. (2012). NK1.1(+) cells regulate neutrophil migration in mice with Acinetobacter baumannii pneumonia. Microbiol. Immunol. 56, 107–116. doi: 10.1111/j.1348-0421.2011.00402.x
Turton, J. F., Woodford, N., Glover, J., Yarde, S., Kaufmann, M. E., Pitt, T. L., et al. (2006). Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J. Clin. Microbiol. 44, 2974–2976. doi: 10.1128/JCM.01021-06
Tyumentseva, M., Mikhaylova, Y., Prelovskaya, A., Tyumentsev, A., Petrova, L., Fomina, V., et al. (2021). Genomic and phenotypic analysis of multidrug-resistant Acinetobacter baumannii clinical isolates carrying different types of CRISPR/CAS systems. Pathogens 10:205. doi: 10.3390/pathogens10020205
Valdez, J. M., Asperilla, M. O., and Smego, R. A. Jr. (1991). Acinetobacter peritonitis in patients receiving continuous ambulatory peritoneal dialysis. South Med. J. 84, 607–610.
Valero, C., Fariñas, M. C., García Palomo, D., Mazarrasa, J. C., and González Macías, J. (1999). Endocarditis due to Acinetobacter lwoffi on native mitral valve. Int. J. Cardiol. 69, 97–99. doi: 10.1016/S0167-5273(99)00006-6
van Duin, D., Kaye, K. S., Neuner, E. A., and Bonomo, R. A. (2013). Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn. Microbiol. Infect. Dis. 75, 115–120. doi: 10.1016/j.diagmicrobio.2012.11.009
Vidal, L., Gafter-Gvili, A., Borok, S., Fraser, A., Leibovici, L., Paul, M., et al. (2007). Efficacy and safety of aminoglycoside monotherapy: systematic review and meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 60, 247–257. doi: 10.1093/jac/dkm193
Vij, R., Danchik, C., Crawford, C., Dragotakes, Q., and Casadevall, A. (2020). Variation in cell surface hydrophobicity among cryptococcus neoformans strains influences interactions with amoebas. mSphere 5:e00310-20. doi: 10.1128/mSphere.00310-20
Vijayakumar, S., Biswas, I., and Veeraraghavan, B. (2019). Accurate identification of clinically important Acinetobacter spp.: an update. Future Sci. OA 5:Fso395. doi: 10.2144/fsoa-2018-0127
Vila, J., Martí, S., and Sánchez-Céspedes, J. (2007). Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 59, 1210–1215. doi: 10.1093/jac/dkl509
Vila-Farres, X., de la Maria Garcia, C., López-Rojas, R., Pachón, J., Giralt, E., and Vila, J. (2012). In vitro activity of several antimicrobial peptides against colistin-susceptible and colistin-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 18, 383–387. doi: 10.1111/j.1469-0691.2011.03581.x
Vrancianu, C. O., Gheorghe, I., Czobor, I. B., and Chifiriuc, M. C. (2020). Antibiotic resistance profiles, molecular mechanisms and innovative treatment strategies of Acinetobacter baumannii. Microorganisms 8:935. doi: 10.3390/microorganisms8060935
Wang, A. G., Wu, C. C., and Liu, J. H. (1998). Bacterial corneal ulcer: a multivariate study. Ophthalmologica 212, 126–132. doi: 10.1159/000027291
Wang, C., Wang, C., Li, J., Tu, Z., Gu, B., Wang, S., et al. (2022). Ultrasensitive and multiplex detection of four pathogenic bacteria on a bi-channel lateral flow immunoassay strip with three-dimensional membrane-like SERS nanostickers. Biosens. Bioelectron. 214:114525. doi: 10.1016/j.bios.2022.114525
Wang, L., Liu, D., Lv, Y., Cui, L., Li, Y., Li, T., et al. (2019). Novel plasmid-mediated tet (X5) gene conferring resistance to tigecycline, eravacycline, and omadacycline in a clinical Acinetobacter baumannii isolate. Antimicrob. Agents Chemother. 64:e01326-19. doi: 10.1128/AAC.01326-19
Wang, Q., Zheng, S., Liu, Y., Wang, C., Gu, B., Long, Z., et al. (2023a). Isothermal amplification and hypersensitive fluorescence dual-enhancement nucleic acid lateral flow assay for rapid detection of Acinetobacter baumannii and its drug resistance. Biosensors 13:945. doi: 10.3390/bios13100945
Wang, W., Yang, X., Rong, Z., Tu, Z., Zhang, X., Gu, B., et al. (2023b). Introduction of graphene oxide-supported multilayer-quantum dots nanofilm into multiplex lateral flow immunoassay: a rapid and ultrasensitive point-of-care testing technique for multiple respiratory viruses. Nano. Res. 16, 3063–3073. doi: 10.1007/s12274-022-5043-6
Wang, Z., Ye, L., Fu, P., Wu, X., Xu, J., Ye, Y., et al. (2024). Clinical outcomes and risk factors of Acinetobacter baumannii meningitis in pediatric patients at a tertiary hospital in China. Front. Cell Infect. Microbiol. 14:1408959. doi: 10.3389/fcimb.2024.1408959
Warburton, P. J., Amodeo, N., and Roberts, A. P. (2016). Mosaic tetracycline resistance genes encoding ribosomal protection proteins. J. Antimicrob. Chemother. 71, 3333–3339. doi: 10.1093/jac/dkw304
Warde, E., Davies, E., and Ward, A. (2019). Control of a multidrug-resistant Acinetobacter baumannii outbreak. Br. J. Nurs. 28, 242–248. doi: 10.12968/bjon.2019.28.4.242
Wareth, G., Abdel-Glil, M. Y., Schmoock, G., Steinacker, U., Kaspar, H., Neubauer, H., et al. (2019). Draft genome sequence of an Acinetobacter baumannii isolate recovered from a horse with conjunctivitis in Germany. Microbiol. Resour. Announc. 8:e01128-19. doi: 10.1128/MRA.01128-19
Watkins, R. R., and Bonomo, R. A. (2023). Sulbactam-durlobactam: a step forward in treating carbapenem-resistant Acinetobacter baumannii (CRAB) infections. Clin. Infect. Dis. 76, S163–S165. doi: 10.1093/cid/ciad093
Wei, J., Peng, N., Liang, Y., Li, K., and Li, Y. (2020). Phage therapy: consider the past, embrace the future. Appl. Sci. 10:7654. doi: 10.3390/app10217654
Weidensdorfer, M., Chae, J. I., Makobe, C., Stahl, J., Averhoff, B., Müller, V., et al. (2015). Analysis of endothelial adherence of Bartonella henselae and Acinetobacter baumannii using a dynamic human ex vivo infection model. Infect. Immun. 84, 711–722. doi: 10.1128/IAI.01502-15
Whitfield, C., and Trent, M. S. (2014). Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 83, 99–128. doi: 10.1146/annurev-biochem-060713-035600
WHO (2019). Antimicrobial Stewardship Programmes in Health-Care Facilities in Low-and Middle-Income Countries: A WHO Practical Toolkit. Geneva.
Wilharm, G., Piesker, J., Laue, M., and Skiebe, E. (2013). DNA uptake by the nosocomial pathogen Acinetobacter baumannii occurs during movement along wet surfaces. J. Bacteriol. 195, 4146–4153. doi: 10.1128/JB.00754-13
Wisplinghoff, H., Bischoff, T., Tallent, S. M., Seifert, H., Wenzel, R. P., Edmond, M. B., et al. (2004). Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39, 309–317. doi: 10.1086/421946
Wisplinghoff, H., Edmond, M. B., Pfaller, M. A., Jones, R. N., Wenzel, R. P., Seifert, H., et al. (2000). Nosocomial bloodstream infections caused by Acinetobacter species in United States hospitals: clinical features, molecular epidemiology, and antimicrobial susceptibility. Clin. Infect. Dis. 31, 690–697. doi: 10.1086/314040
Wolk, D. M., Kaleta, E. J., and Wysocki, V. H. (2012). PCR-electrospray ionization mass spectrometry: the potential to change infectious disease diagnostics in clinical and public health laboratories. J. Mol. Diagn. 14, 295–304. doi: 10.1016/j.jmoldx.2012.02.005
Wong, D., Nielsen, T. B., Bonomo, R. A., Pantapalangkoor, P., Luna, B., Spellberg, B., et al. (2017). Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin. Microbiol. Rev. 30, 409–447. doi: 10.1128/CMR.00058-16
Wood, C. R., Ohneck, E. J., Edelmann, R. E., and Actis, L. A. (2018). A light-regulated type I pilus contributes to Acinetobacter baumannii biofilm, motility, and virulence functions. Infect. Immun. 86:e00442-18. doi: 10.1128/IAI.00442-18
World Health Organization (2024). Who Updates List of Drug-Resistant Bacteria Most Threatening to Human Health. Available online at: https://www.who.int/news/item/17-05-2024-who-updates-list-of-drug-resistant-bacteria-most-threatening-to-human-health (accessed April 16, 2025).
Wyres, K. L., Cahill, S. M., Holt, K. E., Hall, R. M., and Kenyon, J. J. (2020). Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb. Genom. 6:e000339. doi: 10.1099/mgen.0.000339
Xu, J., Du, X., Li, D., Li, P., Guo, Q., Xu, X., et al. (2024). Clinical characteristics and antimicrobial therapy of healthcare-associated carbapenem-non-susceptible gram-negative bacterial meningitis: a 16-year retrospective cohort study. BMC Infect. Dis. 24:368. doi: 10.1186/s12879-024-09237-9
Yang, C. H., Su, P. W., Moi, S. H., and Chuang, L. Y. (2019). Biofilm formation in Acinetobacter baumannii: genotype-phenotype correlation. Molecules 24:1849. doi: 10.3390/molecules24101849
Yang, R., Zhang, H., Li, X., Ye, L., Gong, M., Yang, J., et al. (2018). A multiplex loop-mediated isothermal amplification assay for rapid screening of Acinetobacter baumannii and D carbapenemase OXA-23 gene. Biosci. Rep. 38:BSR20180425. doi: 10.1042/BSR20180425
Yao, Y., Chen, Q., and Zhou, H. (2023). Virulence factors and pathogenicity mechanisms of Acinetobacter baumannii in respiratory infectious diseases. Antibiotics 12:1749. doi: 10.3390/antibiotics12121749
Yeganeh, O., Shabani, M., Pakzad, P., Mosaffa, N., and Hashemi, A. (2021). Production and characterization of novel monoclonal antibodies against outer membrane protein A (OmpA) of Acinetobacter baumannii. J. Immunol. Methods 499:113169. doi: 10.1016/j.jim.2021.113169
Zack, K. M., Sorenson, T., and Joshi, S. G. (2024). Types and mechanisms of efflux pump systems and the potential of efflux pump inhibitors in the restoration of antimicrobial susceptibility, with a special reference to Acinetobacter baumannii. Pathogens 13:197. doi: 10.3390/pathogens13030197
Zaki, M. E. S., Abou ElKheir, N., and Mofreh, M. (2018). Molecular study of quinolone resistance determining regions of gyrA gene and parC genes in clinical isolates of Acintobacter baumannii resistant to fluoroquinolone. Open Microbiol. J. 12, 116–122. doi: 10.2174/1874285801812010116
Zarrilli, R., Pournaras, S., Giannouli, M., and Tsakris, A. (2013). Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int. J. Antimicrob. Agents 41, 11–19. doi: 10.1016/j.ijantimicag.2012.09.008
Zeng, Z., Wu, J., Qin, G., Yu, D., He, Z., Zeng, W., et al. (2024). Using time-series chest radiographs and laboratory data by machine learning for identifying pulmonary infection and colonization of Acinetobacter baumannii. Respir. Res. 25:2. doi: 10.1186/s12931-023-02624-x
Zhanel, G. G., Karlowsky, J. A., Rubinstein, E., and Hoban, D. J. (2006). Tigecycline: a novel glycylcycline antibiotic. Expert Rev. Anti Infect. Ther. 4, 9–25. doi: 10.1586/14787210.4.1.9
Zhang, L., Liang, S.-g., Xu, J., Mei, Y.-y., Di, H.-H., Lan, Y., et al. (2019). CarO promotes adhesion and colonization of acinetobacter baumannii through inhibiting NF-κB pathways. Int. J. Clin. Exp. Med. 12, 2518–2524. Available online at: https://e-century.us/files/ijcem/12/3/ijcem0083388.pdf
Zhao, D., Li, Y., Peng, C., Lin, J., Yu, F., Zhao, Y., et al. (2021). Outer membrane protein a in Acinetobacter baumannii induces autophagy through mTOR signalling pathways in the lung of SD rats. Biomed. Pharmacother. 135:111034. doi: 10.1016/j.biopha.2020.111034
Zhou, H., Yao, Y., Zhu, B., Ren, D., Yang, Q., Fu, Y., et al. (2019). Risk factors for acquisition and mortality of multidrug-resistant Acinetobacter baumannii bacteremia: a retrospective study from a Chinese hospital. Medicine 98:e14937. doi: 10.1097/MD.0000000000014937
Zimbler, D. L., Arivett, B. A., Beckett, A. C., Menke, S. M., and Actis, L. A. (2013). Functional features of TonB energy transduction systems of Acinetobacter baumannii. Infect. Immun. 81, 3382–3394. doi: 10.1128/IAI.00540-13
Keywords: Acinetobacter baumannii, multidrug-resistant, nosocomial, pathogen, review
Citation: Yehya A, Ezzeddine Z, Chakkour M, Dhaini Z, Bou Saba MS, Bou Saba AS, Nohra L, Nassar NB, Yassine M, Bahmad HF and Ghssein G (2025) The intricacies of Acinetobacter baumannii: a multifaceted comprehensive review of a multidrug-resistant pathogen and its clinical significance and implications. Front. Microbiol. 16:1565965. doi: 10.3389/fmicb.2025.1565965
Received: 24 January 2025; Accepted: 07 April 2025;
Published: 12 May 2025.
Edited by:
Jens Andre Hammerl, Bundesinstitut für Risikobewertung, GermanyReviewed by:
Pedro José Alcolea, Spanish National Research Council (CSIC), SpainMaría Dolores Alcántar-Curiel, Universidad Nacional Autónoma de México, Mexico
Copyright © 2025 Yehya, Ezzeddine, Chakkour, Dhaini, Bou Saba, Bou Saba, Nohra, Nassar, Yassine, Bahmad and Ghssein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ghassan Ghssein, Z2hhc3Nhbi5naHNzZWluQGl1bC5lZHUubGI=; Hisham F. Bahmad, aHhiNTkyQG1pYW1pLmVkdQ==
†These authors have contributed equally to this work
‡ORCID: Miriama S. Bou Saba orcid.org/0009-0004-2037-1391
 Amani Yehya
Amani Yehya Zeinab Ezzeddine
Zeinab Ezzeddine Mohamed Chakkour
Mohamed Chakkour Zahraa Dhaini4†
Zahraa Dhaini4† Miriama S. Bou Saba
Miriama S. Bou Saba Anthony S. Bou Saba
Anthony S. Bou Saba Hisham F. Bahmad
Hisham F. Bahmad Ghassan Ghssein
Ghassan Ghssein