- 1Department of Plant Pathology, College of Plant Protection, Agricultural University of Hebei, Biological Control Center for Plant Diseases and Plant Pests of Hebei, Baoding, China
- 2College of Biological Sciences and Engineering, Xingtai University, Xingtai, China
- 3Key Lab of Seed Innovation, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China
- 4Department of Agriculture and Animal Husbandry Engineering, Cangzhou Technical College, Cangzhou, China
- 5Key Laboratory of Plant Resources and China National Botanical Garden, Institute of Botany, Chinese Academy of Sciences, Beijing, China
- 6Dryland Farming Institute, Hebei Academy of Agricultural and Forestry Science, Hengshui, China
As an obligate biotrophic fungus, the leaf rust pathogen Puccinia triticina (Pt) secretes a repertoire of effector proteins into host cells for modulating plant immunity and promoting fungal pathogenesis. Here, we identify the Pt31812 effector and characterize its function in pathogenesis and immune-related activity in plants. In the study, Pt31812 was cloned by PCR, and the expression pattern and structure were analyzed by qRT-PCR and online softwares. Subcellular localization of Pt31812 was analyzed using transient expression on Nicotiana Benthamiana. Further functional analysis was conducted using transient expression and host-induced gene silencing (HIGS). The results showed that Pt31812 encodes candidate effector with a predicted signaling peptide (SP) at the N-terminus, and its expression was highly up-regulated during Pt infection of wheat. Subcellular localization analysis revealed that Pt31812 is localized in cytoplasm and nucleus when expressed in N. Benthamiana. Co-expression of Pt31812 and mammalian BAX protein revealed that Pt31812 inhibited BAX-induced cell death in N. Benthamiana, and the fragment of 22–88 aa from the N-terminus of the effector was important for the inhibiting activity. Interestingly, expression of Pt31812 in a panel of wheat differential lines with different Lr resistance genes showed that Pt31812 specifically triggered cell death in a Lr42-harboring wheat line. Furthermore, transient gene silencing of Pt31812 through BSMV-HIGS approach rendered loss of Lr42-mediated resistance against rust race Pt.-THSN and altered the infection type from resistant to susceptible. Our data reveal that Pt31812, as a candidate effector with immune inhibiting activity, acts as an avirulence determinant factor during Pt infection of Lr42-harboring wheat line. These findings highlight immune-related activity of specific Pt effectors and lay the foundation for further investigation into mechanisms of leaf rust fungal pathogenesis and recognition.
1 Introduction
Plant pathogens secrete a repertoire of effector proteins into host plants to modulate plant immune responses, enabling successful infection and multiplication in plants. Some effectors can either inhibit host immunity and/or sometimes trigger defense responses when recognized by an immune receptors (Zhou and Chai, 2008; Rafiqi et al., 2009). Given their critical roles of effectors in pathogen virulence and in some cases host resistance, the study of pathogen secreted effectors has become a major research focus in the field of plant-pathogen interactions for decades (Lovelace et al., 2023).
Wheat leaf rust, caused by Puccinia triticina (Pt), is a prominent global wheat disease that seriously threatens food security. Yield losses can reach up to 15–40% or even 70% in a suitable environment (Kolmer, 2005; Huerta-Espino et al., 2011; Savary et al., 2019; Abebe, 2021). Pt is a basidiomycete and obligate pathogen with a complex life cycle (Kolmer et al., 2009), encoding hundreds of candidate effector proteins that play key roles in pathogenesis. Many years of studies have identified numerous effectors from rust fungal pathogens, including Uromyces sp., (Kemen et al., 2005; Kemen et al., 2013; Pretsch et al., 2013), Melampsora lini (Catanzariti et al., 2006; Dodds et al., 2006; Upadhyaya et al., 2014; Anderson et al., 2016), and Puccinia graminis sp. tritici (Pgt) (Salcedo et al., 2017; Chen et al., 2017; Upadhyaya et al., 2021; Outram et al., 2024), as well as from Puccinia striiformis sp. tritici (Pst) PS87 (Gu et al., 2011; Tang, 2013; Dagvadorj et al., 2017; Cheng et al., 2017; Liu et al., 2016; Wang et al., 2016), and so on. Previously, some candidate avirulence effector genes have also been identified from Pt, such as Pt27 and Pt3 (Segovia et al., 2016), and potential avirulence effector genes corresponding to Lr20 (Wu et al., 2017). Several M. lini effectors, such as AvrM and AvrL567, are secreted from haustorium and specifically recognized by matching plant R proteins to activate defense response (Catanzariti et al., 2006). Some Pgt effectors such as PGTAUSPE-10-1, AvrSr27, AvrSr35, and AvrSr50 were identified as the avirulence factors corresponding to resistance protein Sr22, Sr27, Sr35, and Sr50, respectively (Upadhyaya et al., 2014; Upadhyaya et al., 2021; Outram et al., 2024; Salcedo et al., 2017; Chen et al., 2017). Heterologous expression system has been used to determine the subcellular localization of many of these effectors (Lorrain et al., 2018). For example, Pst effector proteins Pec6 and PNPi has been shown to localize in the nucleus and cytoplasm in plant cells, with their host targets being identified and co-localized (Liu et al., 2016; Wang et al., 2016).
However, compared with M. lini, Uromyces sp., and other wheat rust fungi, research on the effector proteins of wheat leaf rust has remained in its early stages. There are few reports on the molecular mechanisms underlying the regulation of wheat immunity by Pt effector proteins. For instance, Pt_21 inhibits wheat resistance against leaf rust by interacting with TaTLP1 (Wang et al., 2023). Moreover, some Pt effectors have been found to act as avirulent effectors in wheat lines containing matching Lr genes, such as Pt13024, AvrLr15 and Pt1641 corresponding to TcLr30, TcLr15 and TcLr1, respectively (Qi et al., 2023; Cui et al., 2024; Chang et al., 2024; Wang et al., 2024). Furthermore, Pt1234 modulates wheat immunity by interaction with the TaNAC069 transcription factor through its C subdomain (Geng et al., 2024). Extensive genome and transcriptome data and effective effector identification methods is crucial to accelerate research and advance our understanding of the pathogenic mechanisms of wheat leaf rust.
Previously, a transcriptome library of wheat leaf rust isolates 13-5-72-1(THSN) was constructed, and 635 candidate effectors from different races of Pt were identified through bioinformatics screening by the wheat leaf rust research group at Hebei Agricultural University (HEBAU) (Zhang et al., 2020). Among the 635 candidate effectors, Pt31812 was highly expressed during the haustorial formation stages. We investigated the function of Pt31812 in promoting virulence and immune-related activity in plants. The avirulence activity was examined by expressing Pt31812 in leaves of a panel of wheat differential lines, using BSMV-HIGS approach to further confirm its role as an avirulence determinant factor in a Lr42-harboring wheat line. Our findings lay the foundation for further investigation into mechanisms of leaf rust fungal pathogenesis and pathogen recognition by wheat.
2 Materials and methods
2.1 Plant materials and strains
Wheat differential lines harboring 41 different genes in the Thatcher background, respectively, including TcLr1, TcLr2a, TcLr2c, TcLr3, TcLr9, TcLr16, TcLr24, TcLr26, TcLr3ka, TcLr11, TcLr17, TcLr30, TcLrB, TcLr10, TcLr14a, TcLr18, TcLr21, TcLr28, Lr42, TcLr2b, TcLr3bg, TcLr14b, TcLr15, TcLr19, TcLr20, TcLr23, TcLr25, TcLr29, TcLr27 + 31, TcLr32, TcLr33, TcLr33 + 34, TcLr36, TcLr38, TcLr41, TcLr44, TcLr45, TcLr47, TcLr50, TcLr51, and TcLr53, along with the susceptible line Thatcher and Pt race 13-5-72-1 (THSN) were preserved in our lab at HEBAU.
Agrobacterium tumefaciens strains GV3101 and EHA105, and the recombinant potato X virus vector pGR107 were generously provided by Professor Wenxian Sun of the China Agricultural University. Barley stripe mosaic virus BSMV-VIGS vectors pCaBS-α, pCaBS-β, pCaBS-γbLIC and pCaBS-γbPDS were presented by Professor Dawei Li of China Agricultural University.
2.2 Wheat inoculation using leaf rust urediniospores
10–14-day-old wheat seedlings (Triticum aestivum cv. ‘Thatcher’, susceptible genotype), was inoculated with fresh urediniospores of Puccinia triticina (Pt) race THSN. Inoculation was performed by evenly dusting spores onto the surface of primary leaves, followed by misting with sterile water. Subsequently, plants were grown under controlled conditions with a diurnal cycle of 16 h light (23°C)/8 h darkness (18°C) at 80% relative humidity.
2.3 Cloning and plasmid construction
Total RNA was extracted from wheat leaves inoculated with Pt race THSN using TaKaRa MiniBEST Plant RNA Extraction Kit, and cDNA was synthesized using a Reverse-Transcription System (Abm, Canada). Pt31812 sequence was amplified from the cDNA with specific primers (Supplementary Table S1), and subcloned into pGR107 vector through restriction enzyme digestion and ligation for agroinfiltration in N. benthamiana or wheat, all candidates confirmed by sequencing.
2.4 Bioinformatics analysis
Signal peptides, mitochondrial/chloroplast targeting signals, and transmembrane domains were predicted using SignalP v4.1,1 TargetP v1.1,2 and TMHMM v2.0 (transmembrane prediction using hidden Markov models),3 respectively. The cysteine residue content was analyzed, the conserved domain was indentified using the Pfam database,4 and de novo motif prediction and analysis of novel sequence motifs using MEME.5 Conserved [Y/F/W]xC motifs were detected using Perl softare.
2.5 Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from wheat leaves at 0, 6, 12, 18, 24, 36, 48, 60, 72, 96, and 120 hpi infected at with Pt race THSN. To assess the transcript level of the Pt31812 gene during Pt infection, qRT-PCR was conducted using the LightCycler® 96 real-time PCR system (Roche) with indicated primer (Supplementary Table S1), and the elongation factor 1-alpha gene (EF1a) serving as the reference gene. Relative gene expression was quantified by comparative 2–ΔΔCt method, with statistical significance determined by Student’s t-test. All experiment were performed with three independent biological replicates.
2.6 Confocal laser scanning microscopy and localization analysis
For subcellular localization analysis, the coding sequences of Pt31812(△SP) was subcloned into vector pGR107 containing green fluorescent protein (GFP) to genrated pGR107-△ Pt31812-GFP. The pGR107-△ Pt31812-GFP and GFP alone (pGR107-GFP) were transformed into A. tumefaciens strain GV3101 and infiltrated into N. benthamiana leaves. The confocol imaging was conducted at 48 h post infiltration, with GFP excitation set at 488 nm. (OLYMPUS BX51, Japan).
2.7 Agrobacterium-mediated transient expression in N. benthamiana
The recombinant vectors pGR107-Pt31812, pGR107-Pt31812-ΔSP, a series of Pt31812 variants and BAX were transformed into A. tumefaciens strain GV3101. Agrobacteria were cultured overnight at 28°C at 220 rpm in LB medium, then resuspended in 10 mM MgCl2 to a final OD600 = 0.5, and incubated in dark at room temperature for 2–3 h. Equal volumes of Pt31812 constructs (or its variants) and BAX were mixed and infiltrated into N. benthamiana leaves (Yuan et al., 2011). The cell death symptoms were observed and photographed at 5 days post-infiltration.
2.8 Agrobacterium-mediated transient expression in wheat
The pGR107-Pt31812 construct was transformed into the A. tumefaciens strain GV3101, and generated Agrobacterium suspension as previously described in 2.7, and infiltrated into second leaf of wheat. Cell death symptoms were observed at 5–7 days post-infiltration.
2.9 Barley stripe mosaic virus-mediated gene silencing in wheat
Barley stripemosaic virus-mediated gene silencing in wheat was modificated with previously described by Cheng et al. (2017). A 261 bp cDNA fragment of Pt31812 was subcloned into pCaBS-γbLIC vector to generated pCaBS-γbLIC-Pt31812. pCaBS-α, pCaBS-β and pCaBS-γbLIC construct were transformed into A. tumefaciens strain EHA105, respectively. The agrobacteria were resuspended in infiltration buffer to OD600 = 1.0 and mixed at 1:1:1 ratio to infiltrate N.benthamiana. After 10 days, N.benthamiana leaf sap was extracted by grounding in 20 mM Na-phosphate buffer (pH 7.2) containing 1% celite. to inoculated two-leaf stage wheat leaves (Yuan et al., 2011). The newly emerged leaves with viral phenotypes were infected with Pt race THSN. The infection types were identificated (Roelfs and Martens, 1988) and recorded at 10 dpi.
2.10 Histological observations
Leaf samples collected at 24, 48, and 120 hpi were stained with Fluorescent Brightener 28 (FB 28) following a modified protocol (Kang et al., 2003). Briefly, samples were first fixed in methanol-chloroform (1:2 v/v) for 6 h, boiled in lactophenol oil-95% ethanol (1:2 v/v) for 1.5 min, and incubated overnight. After sequential washing with 50% ethanol for 30 min and ddH2 O, the samples were treated with 0.5 M sodium hydroxide for 30 min followed by ddH2O rinses. Subsequently, the samples were soaked in 0.1 M Tris-HCl buffer (pH 8.5) for 30 min before staining with 0.1% FB 28 solution for 5 min. Following several ddH2O washes, the stained samples were preserved in 25% glycerol (v/v) for microscopic observation. The hyphal were visualized using a laser scanning confocal microscope (OLYMPUS FV1000, Japan) with excitation at 405 nm and emission and 488 nm (Wang et al., 2014).
3 Results
3.1 Bioinformatics analysis of Pt31812 sequence
Among the 635 candidate effectors of wheat leaf rust fungus, Pt31812 is one of the candidate effectors whose gene expression were highly induced during haustorial stage (Zhang et al., 2020). To further characterize this effector, we amplified the cDNA sequence of Pt31812 from RNA samples derived from the Pt strain THSN, which encodes a candidate effector of 208 amino acids and harbors a 22-aa N-terminal signal peptide (SP), predicted by SignalP v4.1. Further TargetP v2.0 analysis indicated no mitochondria/chloroplast targeting domain, and TMHMM predicted no transmembrane domain, in the effector sequence. Further searching against PfamA and PfamB libraries and NCBI conserved domain database also revealed no known conserved domains, and MEME Suite also did not identify any new motif in Pt31812. Interesting, a conserved [Y/F/W]xC motif characteristic of powdery mildew effectors was identified in Pt31812 by using Perl software (Table 1).
3.2 Pt31812 expression is highly induced during Pt. infection of wheat
To analyze the expression pattern of Pt31812 during infection of wheat by Pt.-THSN isolate, a time-course experiment was performed and data analyzed by qRT-PCR analysis. Expression of Pt31812 was markedly induced at 12 hpi and reached a pick at 36 hpi, and was highly induced later at 96 and 120 hpi (Figure 1).
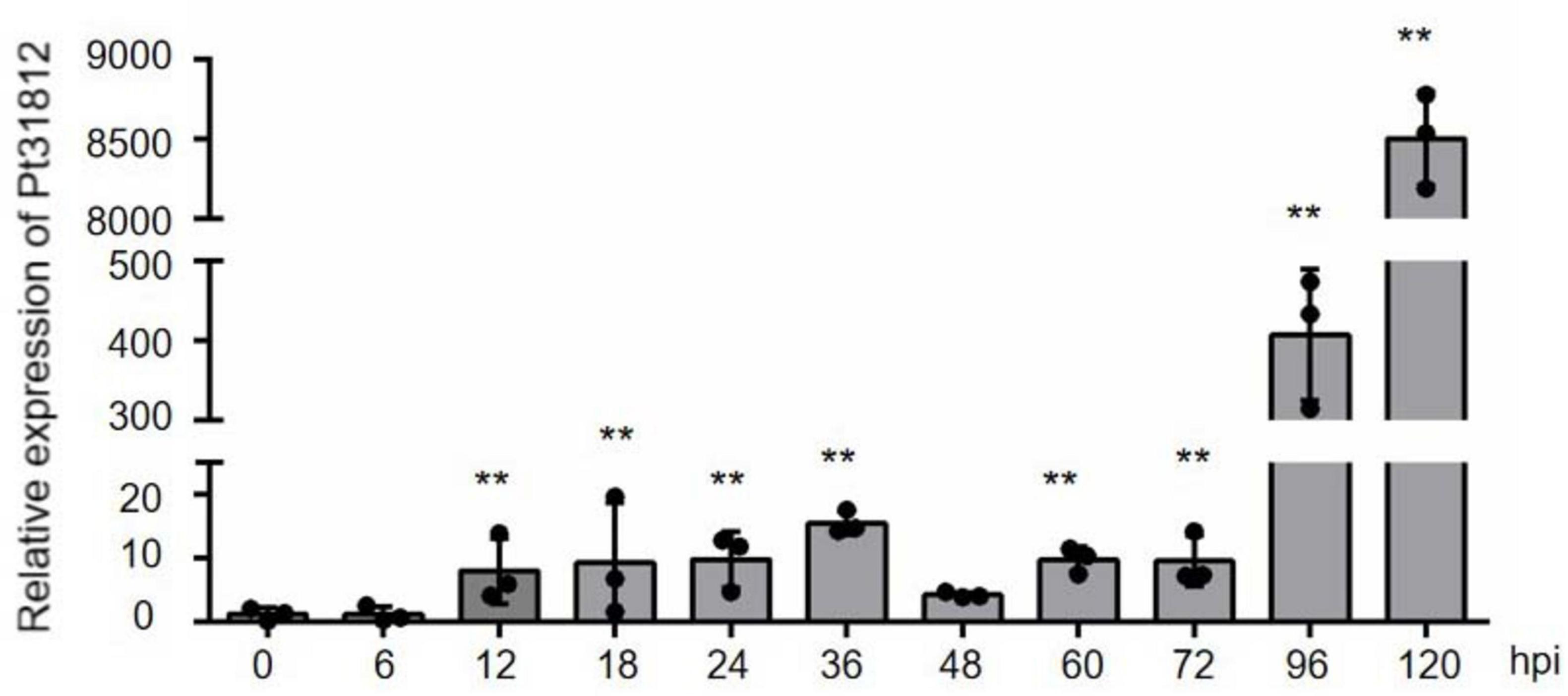
Figure 1. Pt31812 expression is highly up-regulated during Pt. infection of wheat. Pt31812 transcript level were analyzed by qRT-PCR analysis during Pt. infection of leaves of wheat cv. Thatcher from 0 to 120 hpi. Relative expression was calculated by the comparative Ct method with EF1 as a reference gene. Error bars = ± SD (n = 3 for biological replicates). ** for P < 0.01; Statistic analysis was performed by Student’s t-test.
3.3 Pt31812 is localized to the cytoplasm and nucleus in N. benthamiana
To examine the subcellular localization of Pt31812, we generated construct of GFP fusion of Pt31812 (lacking SP). Pt31812(△SP)-GFP and GFP alone were individually expressed in N. benthamiana by Agrobacterium-infiltration. Confocal imaging showed that Pt31812(△SP)-GFP was distributed in the cytoplasm and nucleus, similar to the control GFP (Figure 2), indicating Pt31812 is localized to both the cytoplasm and nucleus in plant cells.
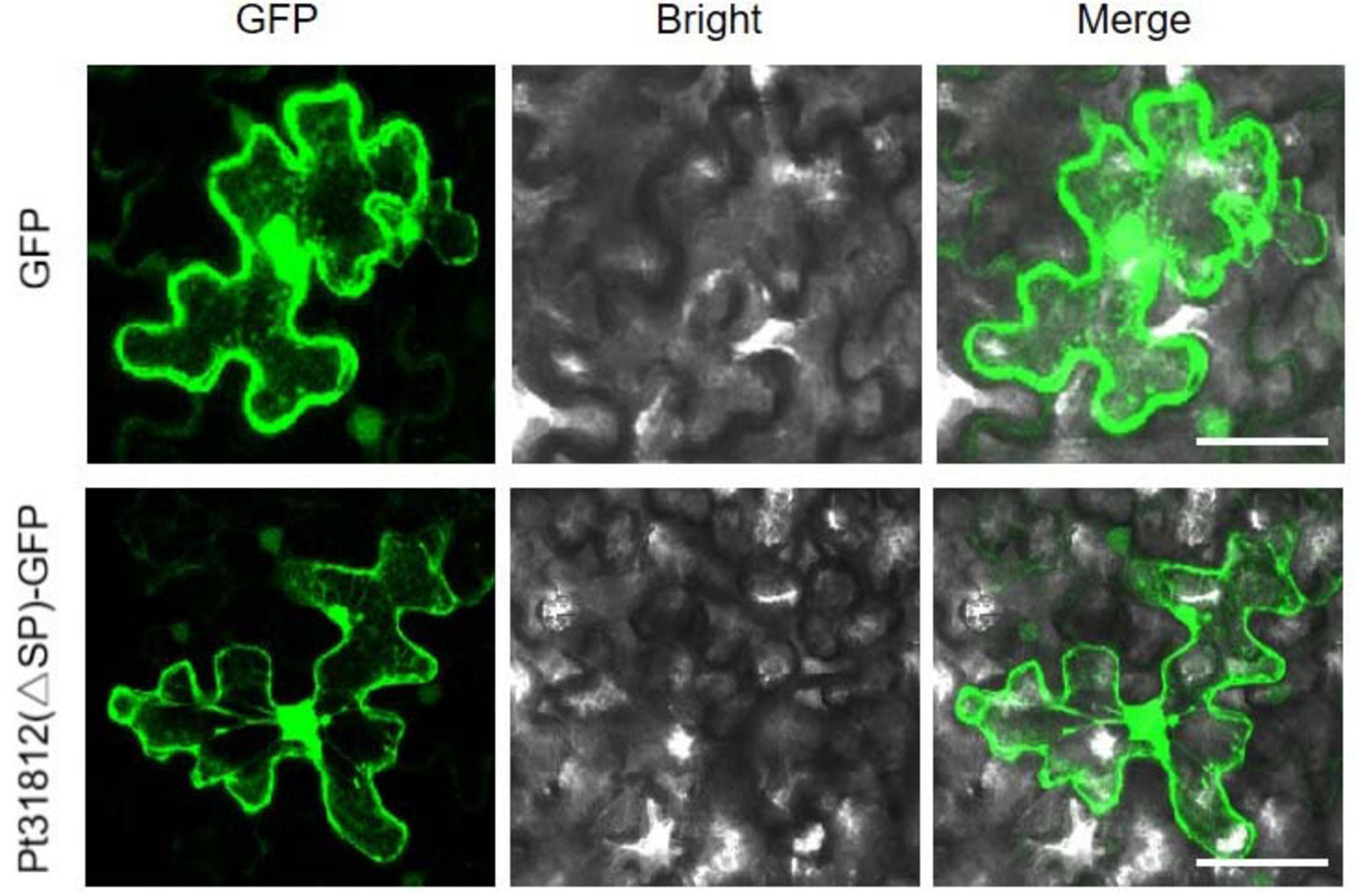
Figure 2. Pt31812 is localized in cytoplasm and nucleus in N. benthamiana. GFP and Pt31812(△SP)-GFP fusion proteins were transiently expressed in N. benthamiana via Agrobacterium-infiltration. Confocal imaging was done at 48 hpi. Scale bar = 5 μm.
3.4 Pt31812 inhibits BAX-induced cell death in N. benthamiana
BAX is a pro-apoptotic protein of the Bcl-2 family in mouse that can trigger hypersensitive responsive (HR)-like cell death in plants (Lacomme and Santa, 1999). To examine the activity of Pt31812 in inducing cell-death or inhibiting BAX-induced cell death in plants, we performed Agrobacterium-mediated transient gene expression in N. benthamiana according to the scheme (Figure 3A). Leaves of N. benthaminana expressing Pt31812-GFP alone or co-expressing Pt31812-GFP and BAX were observed at 5-days after infiltration. As shown in Figure 3B, BAX alone induced obvious cell death, whereas co-expression of Pt31812(FL)-GFP and BAX resulted in complete suppression of cell death, and co-expression of GFP and BAX led to strong cell death. Similarly, co-expression of Pt31812(△SP)-GFP and BAX also led to complete suppression of cell death (Figure 3C). These results suggest that Pt31812 can effectively inhibit BAX-induced cell death in plants.
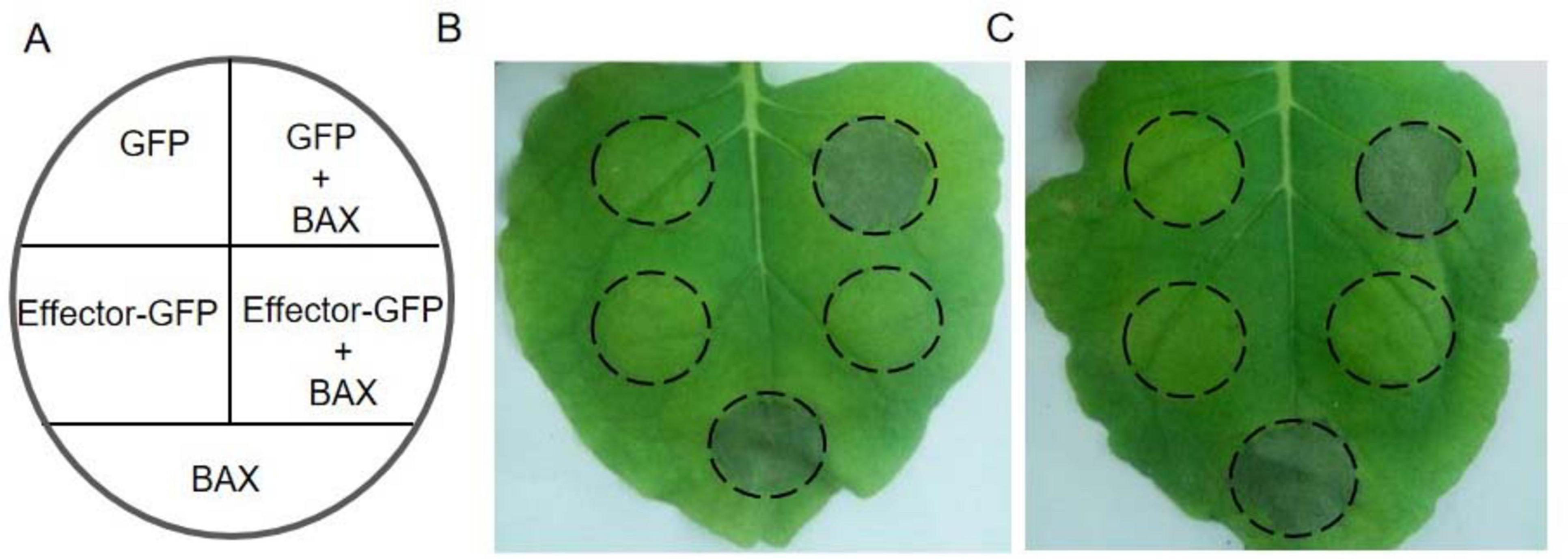
Figure 3. Pt31812 suppresses BAX-induced cell death in N. benthamiana. (A) Scheme of Agrobacterium-infiltration. (B,C) Expression of GFP or effector-GFP fusion alone (left half), or coexpression with BAX (right half) in leaves of N. benthamiana, and Effector-GFP fusion represents Pt31812(FL)-GFP (B) or Pt31812(△SP)-GFP (C). The cell death phenotype was photographed at 5 d post infiltration.
3.5 A portion of Pt31812 is important for cell death suppression in plants
To further identify the region required for cell-death suppression in Pt31812, a series of deletion mutants of the effector was constructed (Figure 4A). Transient expression of full-length or deletion variants of Pt31812 indicated that Pt31812-FL or Pt31812 variants does not induce cell death in N. benthamiana (Figures 4A,B). However, co-expression of full-length or deletion variants of Pt31812 with BAX in N. benthaminana resulted cell-death suppression, as compared with co-expression of GFP with BAX (Figure 4C). The shortest fragment we tested is Pt31812 (22–88 aa) that could consistently suppress BAX-induced cell death. These results suggest that the activity of Pt31812 in suppressing BAX-induced cell death relies on the 22–88 aa region, and the SP is not essential for Pt31812 cell-death suppression in plant cells.
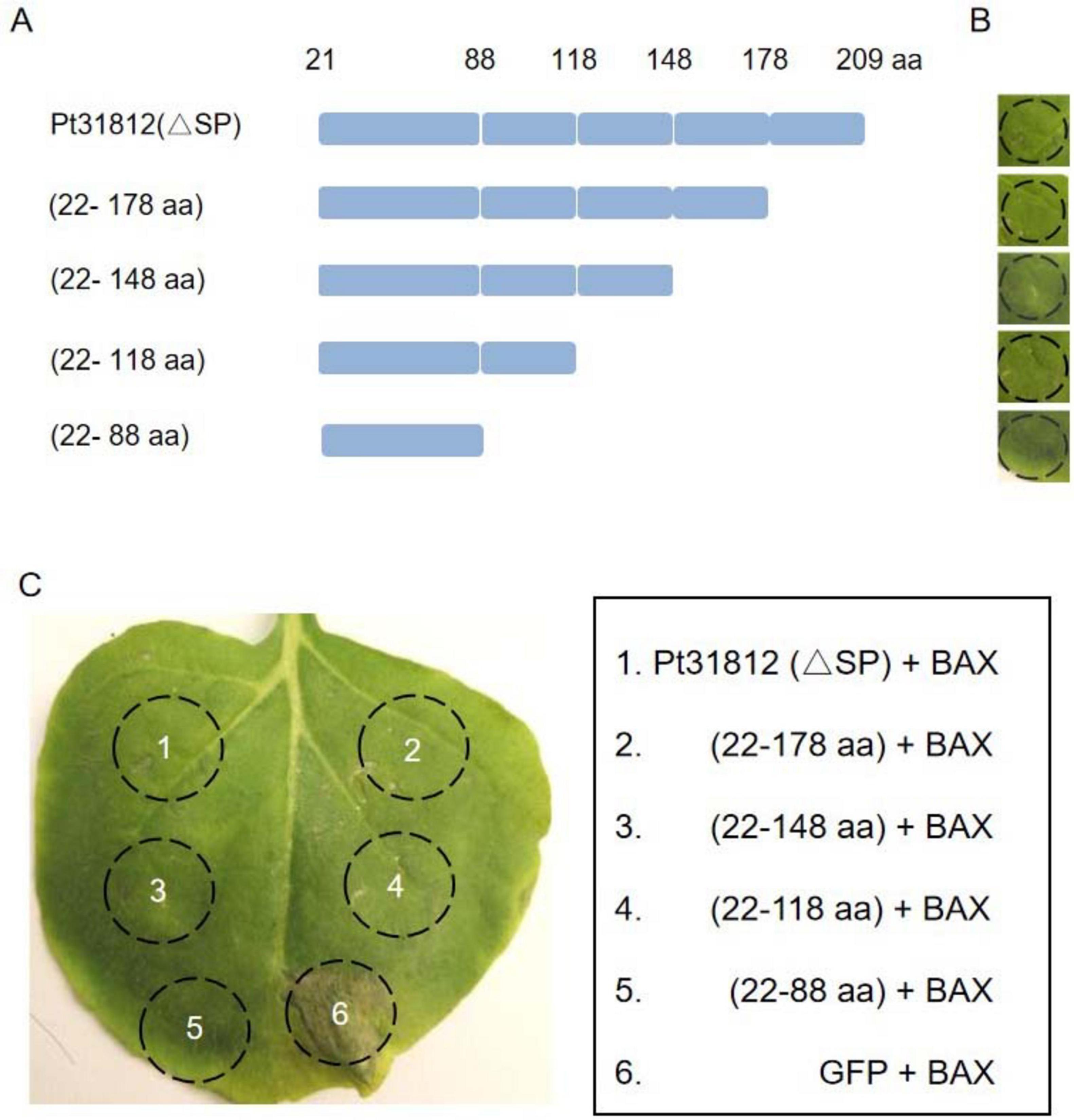
Figure 4. The region of 22–88 aa in Pt31812 is important for suppressing BAX-induced cell death. (A) Schematic representation of Pt31812 deletion variants. (B) expression of Pt31812 deletion variants did not induce cell death upon transient expression in N. benthamiana via Agrobacterium-infiltration. (C) Co-expression of Pt31812 deletion variants with BAX in N. benthamiana leaves, with GFP as a control. Images were taken at 5 dpi.
3.6 Pt31812 induces cell death in Lr42-containing wheat line
To investigate the role of Pt31812 in relation to wheat immunity and/or rust pathogen virulence, we expressed Pt31812 in a panel of wheat differential lines via Agrobacterium-infiltration, each of the wheat lines harboring at least a differential leaf rust resistant gene (Qi et al., 2023). Interestingly, among the 41 differential lines we tested, expression of Pt31812 triggered apparent cell-death phenotype in leaves of the Lr42-harboring wheat line (Figure 5; Supplementary Figure S2). Moreover, this Pt31812-induced cell-death phenotype was reproducible in leaves of the Lr42-harboring wheat line, which was not observed for the empty vector (EV), at 7 days post infiltration (Figure 5). These results suggest that Pt31812 may specifically induce cell death in Lr42-harboring wheat line.

Figure 5. Expression of Pt31812 induces cell death in a Lr42-harboring wheat line. Transient expression of Pt31812 or empty vector (EV) in leave of Lr42-harboring wheat via Agrobacterium infiltration. Representative images were photographed 7 dpi. The two black lines mark the Agrobacterium-infiltrated region in the leaf.
3.7 Pt31812 acts as an avirulence factor during Pt.-THSN infection of Lr42-wheat line
To further understand the nature of Lr42-mediated resistance to Pt.-THSN, we performed silencing of Pt31812 by BSMV-HIGS approach in Lr42-harboring wheat line followed by inoculation with Pt.-THSN isolate (Figure 6A). The expression of Pt31812 was significantly reduced at 24, 48, and 120 hpi in the Lr42-harboring wheat line upon treatment with BSMV:Pt31812, as compared to the BSMV:00 control (Figure 6A). Remarkably, BSMV-HIGS of Pt31812 altered the infection phenotype of Pt.-THSN on Lr42-barboring wheat line, resulting in the formation of Pt. pustule on leaf surface of the wheat line, as compared to BSMV:00-treated and other control plants (Figure 6B). Silencing of Pt31812 therefore rendered the loss of Lr42-mediated resistance to Pt.-THSN, suggesting a possibility that Pt31812 serves as an avirulence determining factor for Lr42-mediated resistance in wheat.
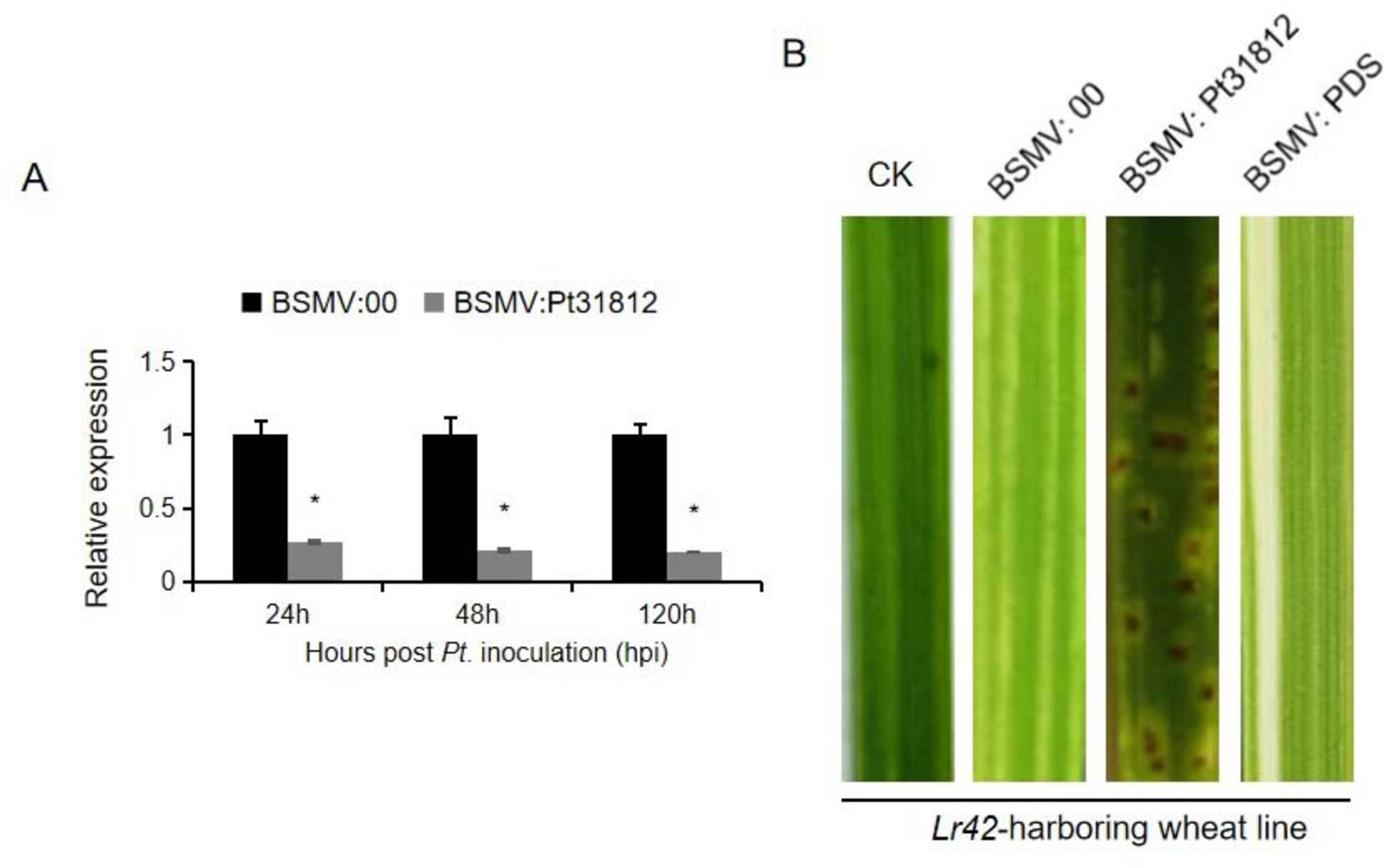
Figure 6. Silencing of Pt31812 converts avirulent Pt.-THSN to a virulent isolate in Lr42-harboring wheat line. (A) Transcript levels of Pt31812 were determined by qRT-PCR at 24, 48, and 120 hpi of Pt. isolate in BSMV:00 or BSMV:Pt31812 treated leaves of a Lr42-harboring wheat line. * for P < 0.05. (B) Disease phenotypes of Pt.-THSN infected leaves upon BSMV treatment. The second leaves of Lr42-harboring wheat line were treated by sodium phosphate buffer (CK), or BSMV-HIGS vector (BSMV:Pt31812), or empty vector control (BSMV:00). Images were taken at 10 dpi.
We further followed the growth and development of the Pt. fungus in Lr42-harboring wheat leaves treated with BSMV:00 or BSMV:Pt31812. After inoculated with uredospores of Pt.-THSN isolate, formation of rust appressorium, substomatal vesicle, infection hypha, and haustorial mother cell were observed already at 24 hpi in leaves of both BSMV:00 and BSMV:Pt31812 treated plants (Figures 7A,B), however, necrosis was only observed around the haustorial mother cells in BSMV:00 but not BSMV:Pt31812 treated wheat leaves (Figures 7A,B). Later at 48 hpi, necrosis was persistent and expanded in BSMV:00 treat wheat leaves, along with fewer fungal structures in infected area (Figure 7C). By contrast, necrosis was rarely observed in BSMV:Pt31812 treated wheat leaves at 48 hpi, but formation of fungal structures was significantly increased with development of more fungal mycelia (Figure 7D). These data demonstrated that Lr42-harboring wheat line confer resistance to Pt.-THSN isolate, and silencing of Pt31812 rendered loss of Lr42-mediated resistance thus converted an Lr42-specific avirulent isolate to a virulent isolate.
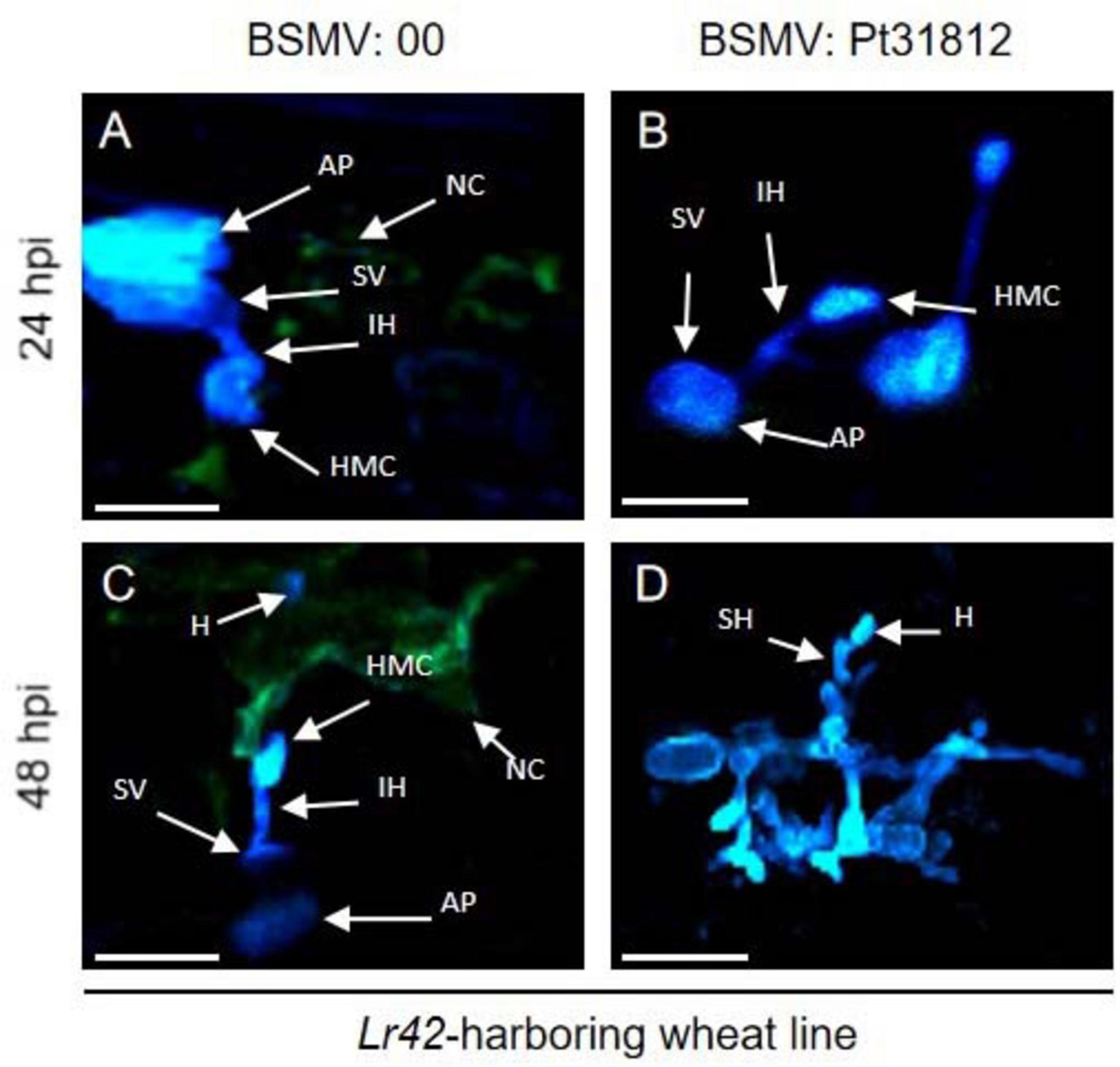
Figure 7. Silencing of Pt31812 results in loss of Lr42-mediated cell death in wheat. (A–D): Confocal imaging of Pt.-THSN infected leaf cells of Lr42-harboring wheat line at 24 hpi and 48 hpi. Wheat leaves were treated with BSMV empty vector (BSMV:00) or silencing vector (BSMV: Pt31812) followed by inoculation of Pt.-THSN uredospores. AP: appresorium; IH: infection hypha; SV: substomatal vesicle; HMC: haustorial mother cell; SH: second hypha; H: haustorium; NC: necrotic cell. Scale bar = 30 μm.
Taken together, these results suggest that Pt31812 play an avirulence determinant role during Pt.-THSN infection of Lr42-harboring wheat line.
4 Discussion
4.1 Pt31812 may be an intracellular effector protein
Pathogen effectors can be broadly categorized into apoplastic effectors functioning in the extracellular space and cytoplasmic effectors delivered into host cells. Among these, intracellular effectors have been more extensively documented for their functions (Dou et al., 2008). For example, the avirulence effectors AvrL567 and AvrM of M. lini can be transferred into host cells to exert functions independent of pathogen presence (Rafiqi et al., 2010). Previous studies showed that effectors are localized in plant cell membrane (Lewis et al., 2014), cytoplasm (Zhang et al., 2015) and nucleus (Escoll et al., 2016). In this study, Agrobacterium-mediated transient expression in N. benthamian also revealed that Pt31812 is located to cytoplasm and nucleus in plant cell. Thus, we conclude that Pt31812 is an intracellular effector protein that is transported into the cell via a complex mechanism and functions within the host cell.
The transport mechanism of intracellular effector proteins has been the focal point of effector protein research. The RXLR motif is known to be responsible for transporting effectors into host cells in Oomycetes (Whisson et al., 2007), while [Y/F/W]xC motif of Powdery Mildew is characterized as a putative motif required for host intracellular localization (Godfrey et al., 2010). In this study, no RXLR motif was identified in Pt31812, wheras a [Y/F/W]xC motif was detected at its N-terminus. Notably, similar [Y/F/W]xC-containing effectors in Pst have been reported, and point mutation analysis has revealed that the [Y/F/W]xC motif cannot be responsible for translocation of effectors (Cheng, 2015). In this study, Pt31812 contained the YxC motif, and its potential role in the transport of effector proteins in wheat leaf rust requires further investigation.
4.2 Pt31812 plays an avirulent role during the infection of Lr42-harboring wheat line by THSN
Identifying new avirulence genes and investigating the interaction mechanisms between avirulence and resistance genes are crucial for developing new disease resistance strategies (Gururani et al., 2012). The discovery of AvrSr35 (Salcedo et al., 2017) and AvrSr50 (Chen et al., 2017) in Pgt provides new research insights. We constructed a recombinant expression vector based on published Lr1, Lr10, and Lr21 sequences and co-expressed with Pt31812 in N. benthemmian. However, co-expression of Pt31812 with Lr1, Lr10 or Lr21 failed to induce cell death, indicating that Pt31812 is not an avirulent gene for Lr1, Lr10, or Lr21. As most wheat leaf rust resistance genes have not yet been cloned, the co-expression method used for AvrSr35 (Salcedo et al., 2017) and AvrSr50 (Chen et al., 2017) cannot currently be applied to explore the recognition between resistance genes and effectors. Therefore, we transiently expressed Pt31812 in 41 near-isogenic wheat lines (single-gene lines) with a Thatcher genetic background to determine whether it could be recognized by the corresponding resistance genes and induce hypersensitive cell death. The results demonstrated that Pt31812 induced HR response in the Lr42 single-gene line. Furthermore, upon infection of Lr42-harboring wheat with Pt, silencing of Pt31812 enhanced the virulence of Pt. race THSN. Collectively, these results indicating that Pt31812 may be an avirulence gene for Lr42.
Previous studies have found that two unrelated Type III effect genes (Avr Rpm1 and Avr B) of bacteria P. syringae can also be recognized and interacted by the same Arabidopsis disease-resistant gene (RPM1), while studies have found that the plant protein that directly interacts with Avr Rpm1 and Avr B protein is RIN4 instead of RPM1 (Mackey et al., 2003). Studies indicate that RIN4 is a guardee protein, targeted by Avr Rpm1 and Avr B, and is guarded by RIN4 (Mackey et al., 2003; Axtell and Staskawicz, 2003). This supports the “guard model” for the interaction between avirulence proteins and resistance proteins (Van der Biezen and Jones, 1998). RIN4 is also guarded by another R protein, RPS2, enabling the recognition of other distinct bacterial effector proteins (Mackey et al., 2003). In our study, Pt31812 can induce HR-like cell necrosis in Lr42-harboring wheat line, so we speculate that there is a guard protein similar to RIN4 in wheat, which can be targeted by the effector protein Pt31812 and protected by Lr42 disease-resistant protein.
The avirulent function of Pt31812 was demonstrated when the infection type of the Lr42-harboring wheat line inoculated with THSN shifted from low to high after silencing. The leaf rust resistance gene Lr42 was identified from accession TA2450 in a collection of the wheat wild relative Aegilops tauschii Coss. (DD, 2n = 14), the diploid D-genome donor for hexaploid bread wheat (Triticum aestivum L., AABBDD, 2n = 42) (Cox et al., 1994). Lr42 confers all-stage resistance to leaf rust. Since the Lr42 gene has been cloned, we can verify the avirulent function of the effector protein Pt31812 through co-expression in the future, and then determine whether Pt31812 is an avirulence gene for Lr42 or not.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JYL: Methodology, Writing – original draft, Writing – review & editing. JLL: Data curation, Writing – review & editing. JW: Data curation, Writing – original draft. LL: Software, Writing – review & editing. YQ: Data curation, Writing – review & editing. YZ: Methodology, Writing – review & editing. WY: Methodology, Project administration, Supervision, Writing – review & editing. Q-HS: Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key R&D Program of China (2022YFD1400802), National Natural Science Foundation of China (31571956, 301871915, 32172367), National Key R&D Research Program of China (No. 2017YFD020170700), Modern Agricultural Industry System of the Wheat Industry in Hebei Province (No. HBCT2023010205), Xingtai Innovation Ability Improvement Plan Project (No. 2023ZZ080), Science and technology plan self-financing project of Cangzhou (222001003), and Science and Technology Research Projects for Higher Education Institutions in Hebei Province (QN2024111).
Acknowledgments
We would like to thank Wenxian Sun (China Agricultural University) for providing Agrobacterium tumefaciens strains GV3101 and EHA105 and Dawei Li (China Agricultural University) for providing the BSMV silencing vector.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1570072/full#supplementary-material
Footnotes
1. ^http://www.cbs.dtu.dk/services/SignalP/
2. ^http://www.cbs.dtu.dk/services/TargetP/
References
Abebe, W. (2021). Wheat leaf rust disease management: A review. J. Plant Pathol. Microbiol. 8, 1–14. doi: 10.35248/2157-7471.21.12.554
Anderson, C., Khan, M. A., Catanzariti, A. M., Jack, C. A., Nemri, A., Lawrence, G. J., et al. (2016). Genome analysis and avirulence gene cloning using a high-density RADseq linkage map of the flax rust fungus, Melampsora lini. BMC Genom. 17:667. doi: 10.1186/s12864-016-3011-9
Axtell, M. J., and Staskawicz, B. J. (2003). Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112, 369–377. doi: 10.1016/s0092-8674(03)00036-9
Catanzariti, A. M., Dodds, P. N., Lawrence, G. J., Ayliffe, M. A., and Ellis, J. G. (2006). Haustorially expressed secreted proteins from flax rust are highly enriched for avirulence elicitors. Plant Cell 18, 243–256. doi: 10.1105/tpc.105.035980
Chang, J., Mapuranga, J., Li, R., Zhang, Y., Shi, J., Yan, H., et al. (2024). Wheat leaf rust fungus effector protein Pt1641 is avirulent to TcLr1. Plants (Basel) 13:2255. doi: 10.3390/plants13162255
Chen, J., Upadhyaya, N. M., Ortiz, D., Sperschneider, J., Li, F., Bouton, C., et al. (2017). Loss of AvrSr50 by somatic exchange in stem rust leads to virulence for Sr50 resistance in wheat. Science 358, 1607–1610. doi: 10.1126/science.aao4810
Cheng, Y., Wu, K., Yao, J., Li, S., Wang, X., Huang, L., et al. (2017). PSTha5a23, a candidate effector from the obligate biotrophic pathogen Puccinia striiformis f. sp. tritici, is involved in plant defense suppression and rust pathogenicity. Environ. Microbiol. 19, 1717–1729. doi: 10.1111/1462-2920.13610
Cheng, Y. L. (2015). Identification and functional analysis of pathogenicity-related genes of Puccinia striiformis f.sp. tritici. Shanxi: Northwest A & F University.
Cox, T. S., Raupp, W. J., and Gill, B. S. (1994). Leaf rust-resistance genes Lr41, Lr42, and Lr43 transferred from Triticum tauschii to common wheat. Crop. Sci. 34, 339–343. doi: 10.2135/cropsci1994.0011183X003400020005x
Cui, Z., Shen, S., Meng, L., Sun, X., Jin, Y., Liu, Y., et al. (2024). Evasion of wheat resistance gene Lr15 recognition by the leaf rust fungus is attributed to the coincidence of natural mutations and deletion in AvrLr15 gene. Mol. Plant Pathol. 25:e13490. doi: 10.1111/mpp.13490
Dagvadorj, B., Ozketen, A. C., Andac, A., Duggan, C., Bozkurt, T. O., and Akkaya, M. S. (2017). A Puccinia striiformis f. sp. tritici secreted protein activates plant immunity at the cell surface. Sci. Rep. 7:1141. doi: 10.1038/s41598-017-01100-z
Dodds, P. N., Lawrence, G. J., Catanzariti, A. M., Teh, T., Wang, C. I., Ayliffe, M. A., et al. (2006). Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc. Natl. Acad. Sci. U. S. A. 103, 8888–8893. doi: 10.1073/pnas.0602577103
Dou, D., Kale, S. D., Wang, X., Chen, Y., Wang, Q., Wang, X., et al. (2008). Conserved C-terminal motifs required for avirulence and suppression of cell death by Phytophthora sojae effector Avr1b. Plant Cell 20, 1118–1133. doi: 10.1105/tpc.107.057067
Escoll, P., Mondino, S., Rolando, M., and Buchrieser, C. (2016). Targeting of host organelles by pathogenic bacteria: A sophisticated subversion strategy. Nat. Rev. Microbiol. 14, 5–19. doi: 10.1038/nrmicro.2015.1
Geng, H. M., Zhang, Y. J., Qin, Z., Wang, S., Liu, C., Cui, Z., et al. (2024). Puccinia triticina effector Pt-1234 modulates wheat immunity by targeting transcription factor TaNAC069 via its C subdomain. Crop J. 13, 69–78. doi: 10.1016/j.cj.2024.07.013
Godfrey, D., Böhlenius, H., Pedersen, C., Zhang, Z., Emmersen, J., and Thordal-Christensen, H. (2010). Powdery mildew fungal effector candidates share N-terminal Y/F/WxC-motif. BMC Genom. 11:317. doi: 10.1186/1471-2164-11-317
Gu, B., Kale, S. D., Wang, Q., Wang, D., Pan, Q., Cao, H., et al. (2011). Rust secreted protein Ps87 is conserved in diverse fungal pathogens and contains a RXLR-like motif sufficient for translocation into plant cells. PLoS One 6:e27217. doi: 10.1371/journal.pone.0027217
Gururani, M. A., Venkatesh, J., Upadhyaya, C. P., Nookaraju, A., Kumar Pandey, S., Won Park, S., et al. (2012). Plant disease resistance genes: Current status and future directions. Physiol. Mol. Plant Pathol. 78, 51–65. doi: 10.1016/j.pmpp.2012.01.002
Huerta-Espino, J., Singh, R. P., Germán, S., McCallum, B. D., Park, R. F., Chen, W. Q., et al. (2011). Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 179, 143–160. doi: 10.1007/s10681-011-0361-x
Kang, Z. S., Wang, Y., Huang, L. L., Wei, G. R., and Zhao, J. (2003). Histology and ultrastructure of incompatible combination between Puccinia striiformis and wheat cultivars with low reaction type resistance. J. Integr. Agr. 2, 1102–1113. doi: CNKI:SUN:ZGNX.0.2003-10-006.
Kemen, E., Kemen, A., Ehlers, A., Voegele, R., and Mendgen, K. (2013). A novel structural effector from rust fungi is capable of fibril formation. Plant J. 75, 760–780. doi: 10.1111/tpj.12237
Kemen, E., Kemen, A. C., Rafiqi, M., Hempel, U., Mendgen, K., Hahn, M., et al. (2005). Identification of a protein from rust fungi transferred from haustoria into infected plant cells. Mol. Plant Microbe Interact. 18, 1130–1139. doi: 10.1094/MPMI-18-1130
Kolmer, J. A. (2005). Tracking wheat rust on a continental scale. Curr. Opin. Plant Biol. 8, 441–449. doi: 10.1016/j.pbi.2005.05.001
Kolmer, J., Ordoñez, M., and Groth, J. (2009). “The rust fungi,” in Encyclopedia of Life Sciences (ELS). Chichester: John Wiley & Sons Ltd.
Lacomme, C., and Santa Cruz, S. (1999). Bax-induced cell death in tobacco is similar to the hypersensitive response. Proc. Natl. Acad. Sci. U. S. A. 96, 7956–7961. doi: 10.1073/pnas.96.14.7956
Lewis, J. D., Wilton, M., Mott, G. A., Lu, W., Hassan, J. A., Guttman, D. S., et al. (2014). Immunomodulation by the Pseudomonas syringae HopZ type III effector family in Arabidopsis. PLoS One 9:e116152. doi: 10.1371/journal.pone.0116152
Liu, C., Pedersen, C., Schultz-Larsen, T., Aguilar, G. B., Madriz-Ordeñana, K., Hovmøller, M. S., et al. (2016). The stripe rust fungal effector PEC6 suppresses pattern-triggered immunity in a host species-independent manner and interacts with adenosine kinases. N. Phytol. doi: 10.1111/nph.14034 [Epub ahead of print].
Lorrain, C., Petre, B., and Duplessis, S. (2018). Show me the way: Rust effector targets in heterologous plant systems. Curr. Opin. Microbiol. 46, 19–25. doi: 10.1016/j.mib.2018.01.016
Lovelace, A. H., Dorhmi, S., Hulin, M. T., Li, Y., Mansfield, J. W., and Ma, W. (2023). Effector identification in plant pathogens. Phytopathology 113, 637–650. doi: 10.1094/PHYTO-09-22-0337-KD
Mackey, D., Belkhadir, Y., Alonso, J. M., Ecker, J. R., and Dangl, J. L. (2003). Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112, 379–389. doi: 10.1016/s0092-8674(03)00040-0
Outram, M. A., Chen, J., Broderick, S., Li, Z., Aditya, S., Tasneem, N., et al. (2024). AvrSr27 is a zinc-bound effector with a modular structure important for immune recognition. N. Phytol. 243, 314–329. doi: 10.1111/nph.19801
Pretsch, K., Kemen, A., Kemen, E., Geiger, M., Mendgen, K., and Voegele, R. (2013). The rust transferred proteins-a new family of effector proteins exhibiting protease inhibitor function. Mol. Plant Pathol. 14, 96–107. doi: 10.1111/j.1364-3703.2012.00832.x
Qi, Y., Li, J., Mapuranga, J., Zhang, N., Chang, J., Shen, Q., et al. (2023). Wheat leaf rust fungus effector Pt13024 is avirulent to TcLr30. Front. Plant Sci. 13:1098549. doi: 10.3389/fpls.2022.1098549
Rafiqi, M., Bernoux, M., Ellis, J. G., and Dodds, P. N. (2009). In the trenches of plant pathogen recognition: Role of NB-LRR proteins. Semin. Cell Dev. Biol. 20, 1017–1024. doi: 10.1016/j.semcdb.2009.04.010
Rafiqi, M., Gan, P. H., Ravensdale, M., Lawrence, G. J., Ellis, J. G., Jones, D. A., et al. (2010). Internalization of flax rust avirulence proteins into flax and tobacco cells can occur in the absence of the pathogen. Plant Cell 22, 2017–2032. doi: 10.1105/tpc.109.072983
Roelfs, A. P., and Martens, J. W. (1988). An international system of nomenclature for Puccinia striiformis f.sp. tritici. Phytopathology 78, 526–533. doi: 10.1094/Phyto-78-526
Salcedo, A., Rutter, W., Wang, S., Akhunova, A., Bolus, S., Chao, S., et al. (2017). Variation in the AvrSr35 gene determines Sr35 resistance against wheat stem rust race Ug99. Science 358, 1604–1606. doi: 10.1126/science.aao7294
Savary, S., Willocquet, L., Pethybridge, S. J., Esker, P., McRoberts, N., and Nelson, A. (2019). The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 3, 430–439. doi: 10.1038/s41559-018-0793-y
Segovia, V., Bruce, M., Rupp, J., Huang, L., Bakkeren, G., Trick, H. N., et al. (2016). Two small secreted proteins from Puccinia triticina induce reduction of ß-glucoronidase transient expression in wheat isolines containing Lr9, Lr24 and Lr26. Can. J. Plant Pathol. 38, 91–102. doi: 10.1080/07060661.2016.1150884
Tang, C. L. (2013). Characterization and functional analyses of effectors and host cell death inducing genes in wheat and Puccinia striiformis interactions. PhD thesis, Northwest A & F University: Shanxi.
Upadhyaya, N. M., Mago, R., Panwar, V., Hewitt, T., Luo, M., Chen, J., et al. (2021). Genomics accelerated isolation of a new stem rust avirulence gene-wheat resistance gene pair. Nat. Plants 7, 1220–1228. doi: 10.1038/s41477-021-00971-5
Upadhyaya, N. M., Mago, R., Staskawicz, B. J., Ayliffe, M. A., Ellis, J. G., and Dodds, P. N. (2014). A bacterial type III secretion assay for delivery of fungal effector proteins into wheat. Mol. Plant Microbe Interact. 27, 255–226. doi: 10.1094/MPMI-07-13-0187-FI
Van der Biezen, E. A., and Jones, J. D. (1998). Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem. Sci. 23, 454–456. doi: 10.1016/s0968-0004(98)01311-5
Wang, B., Chang, J., Mapuranga, J., Zhao, C., Wu, Y., Qi, Y., et al. (2024). Effector Pt9226 from Puccinia triticina presents a virulence role in wheat line TcLr15. Microorganisms 12:1723. doi: 10.3390/microorganisms12081723
Wang, F., Shen, S. S., Cui, Z. C., Yuan, S., Qu, C., Jia, H., et al. (2023). Puccinia triticina effector protein Pt_21 interacts with wheat thaumatin-like protein TaTLP1 to inhibit its antifungal activity and suppress wheat apoplast immunity. Crop J. 11, 1431–1440. doi: 10.1016/j.cj.2023.04.006
Wang, X., Wang, X., Deng, L., Chang, H., Dubcovsky, J., Feng, H., et al. (2014). Wheat TaNPSN SNARE homologues are involved in vesicle-mediated resistance to stripe rust (Puccinia striiformis f. sp. tritici). J. Exp. Bot. 65, 4807–4820. doi: 10.1093/jxb/eru241
Wang, X., Yang, B., Li, K., Kang, Z., Cantu, D., and Dubcovsky, J. (2016). A conserved Puccinia striiformis protein interacts with wheat NPR1 and reduces induction of pathogenesis-related genes in response to pathogens. Mol. Plant Microbe Interact. 29, 977–989. doi: 10.1094/MPMI-10-16-0207-R
Whisson, S. C., Boevink, P. C., Moleleki, L., Avrova, A. O., Morales, J. G., Gilroy, E. M., et al. (2007). A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature 450, 115–118. doi: 10.1038/nature06203
Wu, J. Q., Sakthikumar, S., Dong, C., Zhang, P., Cuomo, C. A., and Park, R. F. (2017). Comparative genomics integrated with association analysis identifies candidate effector genes corresponding to Lr20 in phenotype-paired Puccinia triticina isolates from Australia. Front. Plant Sci. 8:148. doi: 10.3389/fpls.2017.00148
Yuan, C., Li, C., Yan, L., Jackson, A. O., Liu, Z., Han, C., et al. (2011). A high throughput barley stripe mosaic virus vector for virus induced gene silencing in monocots and dicots. PLoS One 6:e26468. doi: 10.1371/journal.pone.0026468
Zhang, M., Li, Q., Liu, T., Liu, L., Shen, D., Zhu, Y., et al. (2015). Two cytoplasmic effectors of Phytophthora sojae regulate plant cell death via interactions with plant catalases. Plant Physiol. 167, 164–175. doi: 10.1104/pp.114.252437
Zhang, Y., Wei, J., Qi, Y., Li, J., Amin, R., Yang, W., et al. (2020). Predicating the effector proteins secreted by Puccinia triticina through transcriptomic analysis and multiple prediction approaches. Front. Microbiol. 11:538032. doi: 10.3389/fmicb.2020.538032
Keywords: wheat leaf rust, effector, Pt31812, avirulence gene, pathogenesis, Lr42
Citation: Li J, Li J, Wei J, Li L, Qi Y, Zhang Y, Yang W and Shen Q-H (2025) Effector Pt31812 from Puccinia triticina acts as avirulence factor for Lr42-mediated resistance in wheat. Front. Microbiol. 16:1570072. doi: 10.3389/fmicb.2025.1570072
Received: 02 February 2025; Accepted: 23 June 2025;
Published: 18 July 2025.
Edited by:
Xiaofeng Su, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Gregor Langen, University of Cologne, GermanySatinder Kaur, Punjab Agricultural University, India
Copyright © 2025 Li, Li, Wei, Li, Qi, Zhang, Yang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxiang Yang, d2VueGlhbmd5YW5nMjAwM0AxNjMuY29t; Qian-Hua Shen, cWhzaGVuQGdlbmV0aWNzLmFjLmNu
 Jianyuan Li
Jianyuan Li Jiali Li3
Jiali Li3 Jie Wei
Jie Wei Wenxiang Yang
Wenxiang Yang Qian-Hua Shen
Qian-Hua Shen