- 1Department of Cell and Molecular Biology, College of the Environment and Life Sciences, University of Rhode Island, Kingston, RI, United States
- 2Biological and Environmental Sciences Graduate Program, University of Rhode Island, Kingston, RI, United States
- 3Department of Geology and Geophysics, Woods Hole Oceanographic Institution, Woods Hole, MA, United States
Coastal acidification could have widespread impact on marine organisms, affecting the ability of calcifying organisms to build shells and skeletons through calcium carbonate precipitation. As an abundant group of calcifying organisms, some protists within the phylum Foraminifera demonstrate potential success under elevated partial pressure of carbon dioxide (pCO2) due to their ability to modulate intracellular pH. However, little is known about their responses under more extreme acidification conditions that are already seen in certain coastal environments. Here we exposed specimens of Haynesina sp., which belongs to a genus that is prevalent in temperate intertidal salt marshes, to moderate (pCO2 = 2386.05+/−97.14 μatm) and high acidification (pCO2 = 4797.64+/−157.82 μatm) conditions through the duration of 28 days. We demonstrate that although this species is capable of withstanding moderate levels of coastal acidification with little impact on overall test thickness, it can experience precipitation deficiency and even dissolution of the calcareous test under highly elevated pCO2. Interestingly, such a deficit was primarily seen among live foraminifera, as compared to dead specimens, throughout the four-week experiment. This study suggests that a combination of environmental stress and the physiological process of test formation (i.e., calcite precipitation) could induce thinning of the test surface. Therefore, with the acceleration of coastal acidification due to anthropogenic production of CO2, benthic foraminifera and other calcifying organisms among coastal ecosystems could reach a tipping point that leads to thinning and dissolution of their calcareous tests, which in turn, will impair their ecological function as a carbon sink.
Introduction
Increasing anthropogenic production of CO2 into the atmosphere has resulted in rising ocean temperatures, causing increases in sea levels and changes in the ocean’s chemistry (Stocker et al., 2014). As excess CO2 dissolves in seawater, the concentration of hydrogen ions increases, leading to acidification and reduced carbonate ion concentration (Leung et al., 2022; Guinotte and Fabry, 2008; Doney et al., 2009). Anthropogenic-driven acidification has resulted in decreasing pH across the world’s oceans, with drops of 0.1 to 0.3 pH units possible in open ocean waters within the next 100 years (Stocker et al., 2014). These changes are exacerbated in coastal areas, examples of which include Narragansett Bay (RI, United States) and Long Island Sound (NY-CT-RI, United States), where low pH (<7.2), high pCO2 (>2,500 μatm), and aragonite undersaturation (Ωaragonite < 1) have been observed periodically in bottom waters (Wallace et al., 2014).
Increased pCO2 may have dire impacts for organisms in the ocean, such as those that produce their own shells, tests, or skeletons by depositing calcium carbonate minerals (i.e., calcite and aragonite). As pCO2 of seawater increases, the aragonite saturation (Ωaragonite) and calcite saturation (Ωcalcite) state of seawater decreases, undermining the shell formation of marine organisms (Stocker et al., 2014; Figuerola et al., 2021; Andersson et al., 2008). However, the impact of ocean acidification on individual calcifying taxa remains difficult to generalize due to the confounding effects of elevated pCO2 and organisms’ own acclimatory responses (Leung et al., 2022; Melzner et al., 2020). Such phenomena call for more studies on specific taxa to gain a more complete picture of their responses to acidification.
The Foraminifera is a phylum of unicellular microeukaryotes prevalent in marine water columns and sedimentary ecosystems, from shallow and intertidal zones to deep water depths. Many foraminifera produce calcareous tests through calcite precipitation (Figuerola et al., 2021; Todd and Blackmon, 1956). Foraminifera account for an estimated 25% of calcium carbonate deposition across the world’s oceans, attesting to their important roles in carbon and mineral cycling (Langer, 2008). The test morphology within certain foraminifera genera varies depending on their reproductive stage (Hottinger, 2000; Lei et al., 2017; Gudina and Levtchuk, 1989; Nigam, 1986; Nigam and Rao, 1987; Alve and Goldstein, 2003; Goldstein, 1999). The sexually reproducing microspheric foraminifera are characterized by a relatively small proloculus (i.e., the first chamber formed during growth) due to cytoplasmic requirements of progeny produced through gametogenesis and fertilization (Hottinger, 2000). In contrast, the asexually reproducing megalospheric foraminifera have a relatively large proloculus and inherit substantial volumes of the parental cytoplasm, including potential symbionts (Hottinger, 2000). Additionally, the microspheric foraminifera tend to form more chambers than their megalospheric counterparts while exhibiting heteromorphic test structure (Lei et al., 2017; Gudina and Levtchuk, 1989).
Despite abundance of calcifying foraminifera in temperate coastal systems, most acidification studies of foraminifera focused on species from reef-associated systems or the open ocean (Supplementary Table S1). A few studies examine the responses of individual foraminifera to coastal acidification (Khanna et al., 2013; Prazeres et al., 2015; McIntyre-Wressnig et al., 2011; Schmidt et al., 2014), but more extreme conditions related to what is seen in coastal habitats (e.g., pCO2 > 2,500 μatm) are rarely considered. Common strategies for measuring foraminifera responses to acidification involve tracking chamber formation rates and surface morphological changes, with prior studies demonstrating variable influences of acidification on different species of foraminifera (Khanna et al., 2013; Prazeres et al., 2015; Schmidt et al., 2014; Keul et al., 2013; Haynert et al., 2014; Kuroyanagi et al., 2021; Manno et al., 2012; Kuroyanagi et al., 2009; Fujita et al., 2011; McIntyre-Wressnig et al., 2013; Reymond et al., 2013; Iwasaki et al., 2019; Sinutok et al., 2011; Bernhard et al., 2021). The direct measurement of test thickness, however, has not been systematically applied to study the acidification responses in calcareous foraminifera.
In this study, laboratory treatments were conducted to examine the response of Haynesina sp., a benthic foraminifer identified from coastal sediments in Rhode Island, United States, to moderate and high acidification conditions. Thicknesses of foraminifera tests were mapped using X-ray tomography, enabling systematic comparisons throughout individual test chambers. The treatments were applied to both live and dead foraminifera, which provides an opportunity for examining the biotic and abiotic responses of foraminifera and their calcareous remains to coastal acidification. We hypothesized that acidification conditions would result in detectable thinning or morphological defects of foraminiferal tests at the end of the experimental period.
Results
Acidification challenges
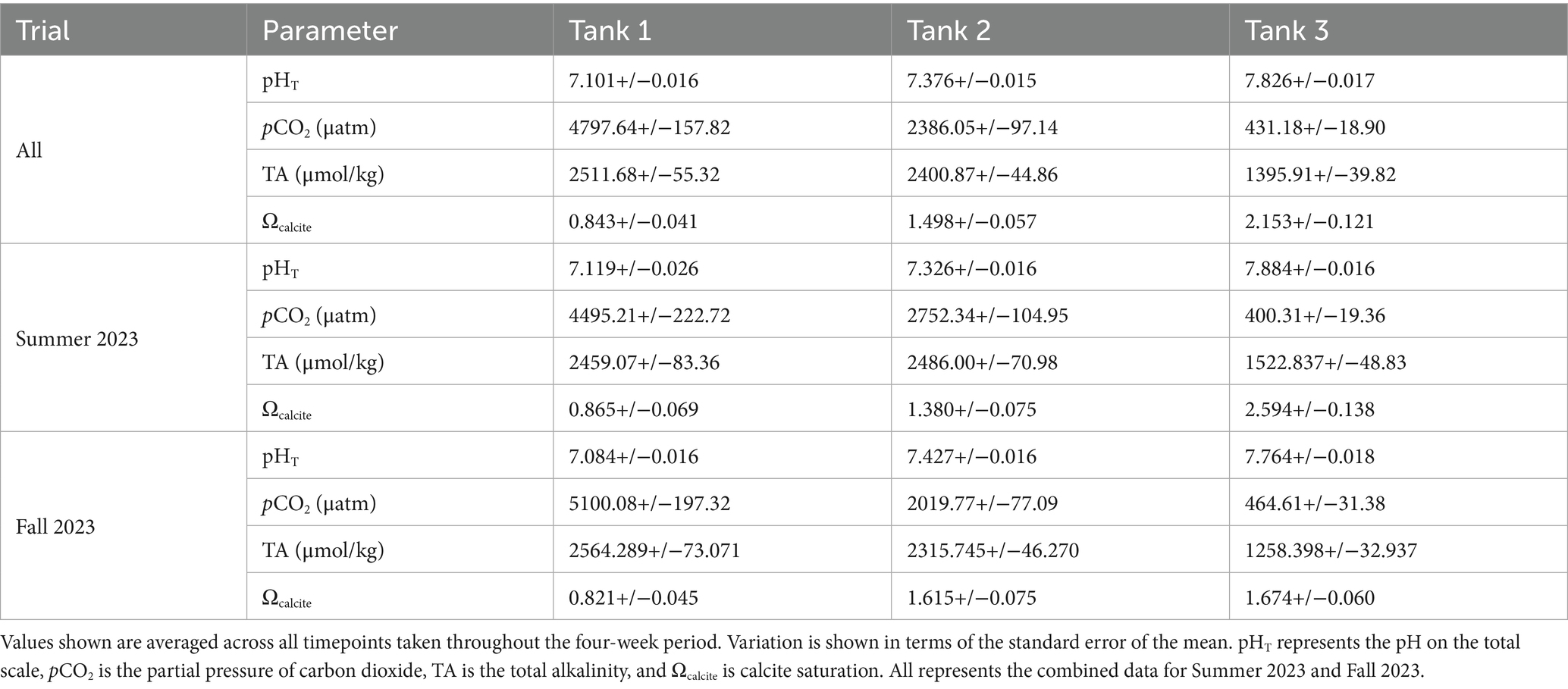
Specimens were obtained from a mudflat associated with the Quonochontaug Salt Marsh (41°20′12.6”N, 71°43′15.9”W) on June 19, 2023 and September 26, 2023 for two replicate pH manipulation experiments (Table 1). Each replicate trial was performed over a period of 28 days with seawater chemistry closely monitored through three distinct pCO2 regimes: no pCO2 manipulation (431.18+/−18.90 μatm), moderately elevated pCO2 (2386.05+/−97.14 μatm), and highly elevated pCO2 (4797.64+/−157.82 μatm) (Figure 1A; Supplementary Table S2). Additionally, untreated specimens were obtained from sediment samples and used as additional controls (Materials and Methods). Measurements of the calcite saturation state indicated supersaturation (Ωcalcite > 1) under both non-elevated (Tank 3) and moderately elevated (Tank 2) pCO2 treatments. However, calcite undersaturation (Ωcalcite < 1) was observed under the highly elevated (Tank 1) pCO2 treatment (Table 1).
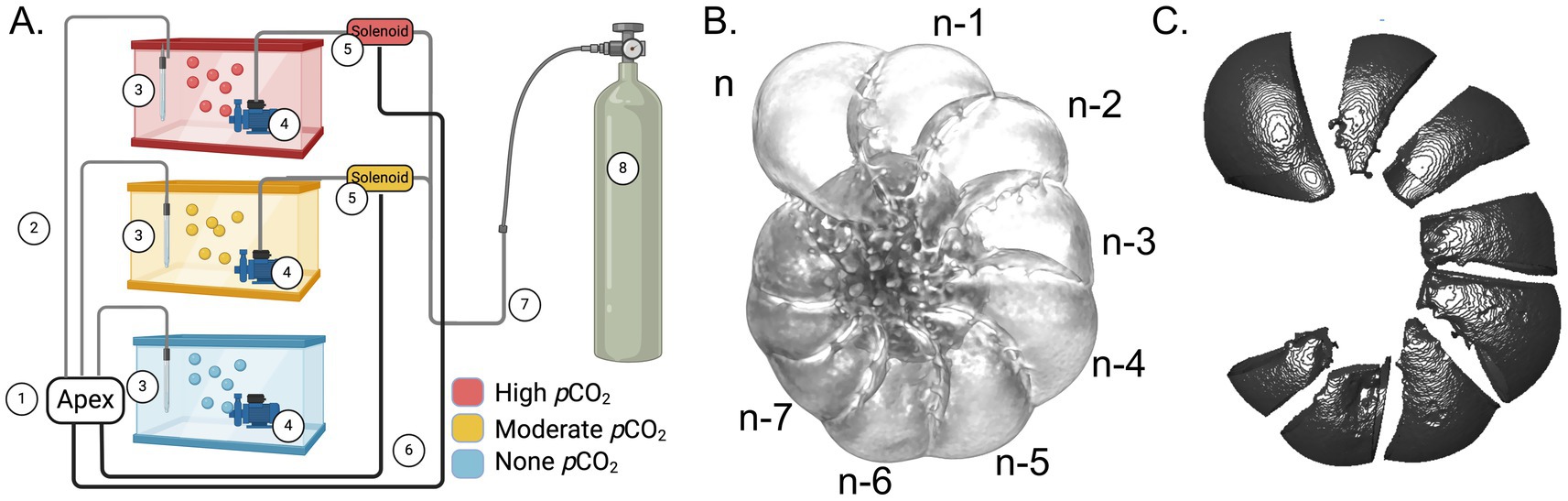
Figure 1. (A) Schematic representation of the experimental setup for pCO2 manipulation. Components of the diagram are as follows: ① the Apex controller system, ② wires connecting pH probe to APEX controller, ③ pH probe, ④ water pumps with Venturi injector, ⑤ solenoids controlling gas flow for the elevated pCO2 treatments, ⑥ wires connecting the apex controller to the solenoids, ⑦ gas tubing connecting the CO2 gas supply to the treatment tanks, ⑧ CO2 gas supply. (B) A foraminifera test with labels showing the 8 newest chambers. (C) An example of isolated exteriorly facing test areas from each chamber for test-thickness analysis. Created with BioRender.com.
Microscopy and three-dimensional test reconstruction
Three-dimensional (3D) reconstruction of foraminifera tests was achieved with a voxel size of 0.57 μm (resolution around 1 μm) using microCT scanning (Figure 1B). Individual chambers were extracted during image processing following the 3D reconstruction, and the thicknesses of exteriorly facing test areas were measured (Figure 1C, Materials and Methods). From each replicate trial, we initially collected at least six live and six dead specimens from each treatment tank, and at least eight specimens as untreated controls. Some tests were lost during handling, and some others were damaged when mounted for microCT scanning due to their delicate nature. These structurally damaged tests were found among all treatment conditions and were not included in further analyses. In total, 76 specimens (28 live treated, 33 dead treated, and 15 untreated) were included in the final collection of the microCT scanning data (Supplementary Table S3).
Assignment of test morphology
Foraminifera specimens collected from each treatment were classified into two distinct groups, microspheric and megalospheric, based on their heteromorphic test geometry (Materials and Methods). A total of 18 megalospheric and 58 microspheric specimens were identified across all treatments based on a bimodal distribution of proloculus sizes (Supplementary Figure S1). These assignments were independently verified by examining the number of chambers for all assigned tests (Figure 2A), as the proloculus size and number of chambers are both known to vary greatly between sexual and asexual reproductive stages (Gudina and Levtchuk, 1989; Alve and Goldstein, 2003).
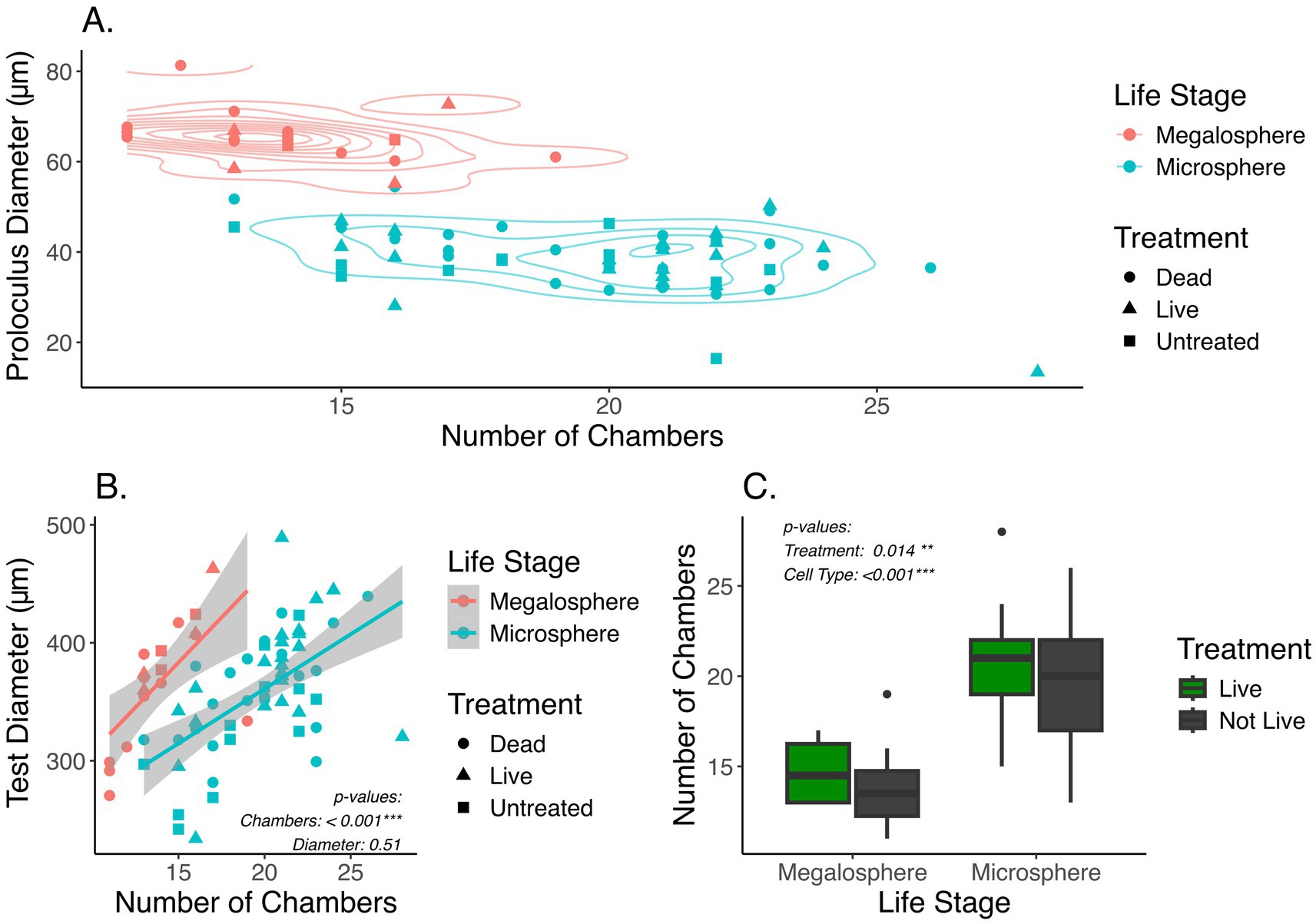
Figure 2. (A) Plot showing the number of chambers against the proloculus diameter. Each point represents a foraminifer. Color represents the assignment of two life stages, microsphere or megalosphere, based on the diameter of proloculus. Symbol shape represents different treatment groups, including untreated. (B) Comparison of the number of chambers and the test diameter between two life stages. p-values are based on one-way ANOVA accounting for different life stages (Materials and Methods). (C) Box and whisker plot showing distribution of the number of chambers in live and not-live foraminifera between the two life stages. The “not-live” specimens include both untreated and dead treated samples. p-values are based on two-way ANOVA accounting for life stages and live vs. not-live treatment groups (Materials and Methods).
The mean proloculus diameter of the megalospheric tests (65.47+/−5.77 μm) was approximately 70% larger than that of the microspheric tests (38.78+/−7.12 μm), consistent with morphological features known for these two life stages (Supplementary Table S4). The number of chambers per microspheric test was significantly higher than per megalospheric test (One-Way ANOVA: F1,74 = 50.28, p < 0.001). However, the overall test diameter was comparable between the microspheric and megalospheric specimens (Figure 2B). A significant increase in the number of chambers was seen when live versus not-live (including untreated and dead treated) specimens were compared (Two-Way ANOVA: F1,72 = 6.319, p = 0.0014) (Figure 2C). This difference in the number of chambers likely resulted from growth of live foraminifera through the duration of the four-week incubation period, while the not-live specimens were not expected to grow because they were bleached prior to experimentation. For microspheric foraminifera, the average number of chambers in the live and not-live groups are 20 and 19, respectively. For megalospheric foraminifera, the average number of chambers in the live and not-live groups are 15 and 14, respectively. Therefore, a putative growth of about one chamber was expected among the live foraminifera compared to their untreated or dead counterpart (Supplementary Table S4). However, the TukeyHSD comparison of live and not-live foraminifera did not appear to support statistical significance within each of the two different life stages (pmegalosphere = 0.93, pmicrosphere = 0.63). This indicates a high level of variability in the number of chambers among individual foraminifera.
Variation of test thicknesses across different treatments
The test morphology was not assigned until after the experimental period due to challenges in keeping foraminifera alive following microCT scanning, which involves bleaching to remove soft tissues and the exposure to high X-rays through extended scanning period during imaging (Materials and Methods). According to our post-experimental assignment of test morphology, an uneven number of megalospheric and microspheric specimens were included in the different experimental treatments. Due to the sparsity of megalospheric samples in multiple treatment conditions (Supplementary Table S3), statistical comparison of test thicknesses across different treatment groups (e.g., treated versus untreated, different pCO2 treatment conditions, or live versus dead treatments) were performed only with the microspheric specimens.
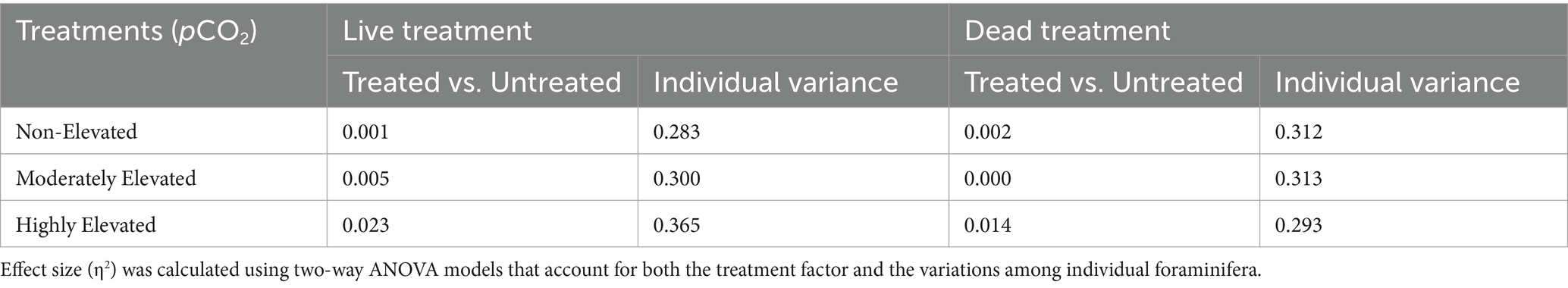
Comparisons of test thicknesses indicated substantial variations among individual foraminifera. The effect size (η2) related to the individual variance in two-way ANOVA analyses was around 0.283–0.365, which is 1–2 orders of magnitude higher than what was seen in the effect of experimental treatments (Table 2). Although a relatively small effect was seen in the factor that compared different treatments to the no-treatment control, they revealed variable responses. The non-elevated and moderately elevated pCO2 treatments had negligible effect sizes (η2 ≤ 0.01) compared to the untreated control. In contrast, specimens in the highly elevated pCO2 treatments had thinner tests compared to the untreated control, with effect sizes of 0.023 and 0.014, respectively, for the live and dead foraminifera (Table 2).
Significant differences in test thicknesses were observed for both live and dead specimens across the different pCO2 treatments, where a slightly higher effect size was observed among the live (η2 = 0.024) than the dead treatments (η2 = 0.011) (Figures 3A,B). Specifically, thinner tests were observed in the live cell treatments under highly and moderately elevated pCO2 compared to the non-elevated pCO2 (Figure 3A). Differential responses between live and dead treatments were also observed in the distribution of test thicknesses. The largest effect was seen in the highly elevated pCO2 treatment (η2 = 0.072), showing the thinning of tests in live compared to dead treated foraminifera (Figure 3C), while the non-elevated and moderately elevated pCO2 treatments had little evidence of thinning when comparing the live and dead treatments (Figures 3D,E). Comparisons on each of the eight newest chambers also revealed thinner tests among the live compared to the dead foraminifera, particularly, under the high pCO2 treatment. Statistical comparison of test thicknesses between live and dead treated specimens were visualized for each chamber by their η2 values (Figure 4). Interestingly, higher effect sizes (η2 > 0.1) were observed in the six newest chambers (from n to n−5), while a lower effect (η2 < 0.1) was seen in chambers n−6 and n−7 among the high pCO2 treatment of live versus dead specimens (Figure 4A).
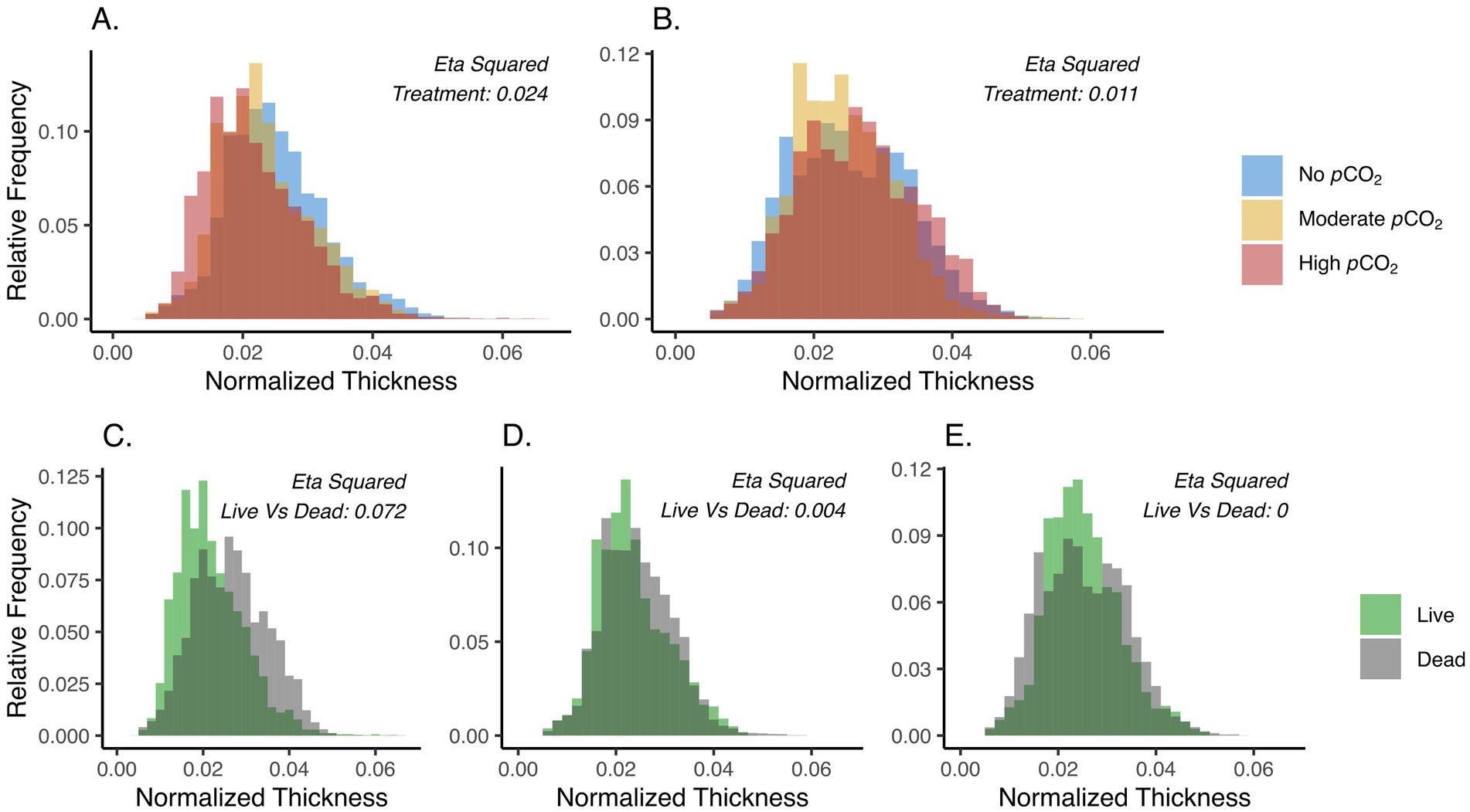
Figure 3. (A,B) Distribution of normalized test thickness across the highly- (red), moderately- (gold), and no- (Blue) elevated pCO2 conditions among the live (A) and dead (B) specimens. (C–E) Distribution of the normalized test thickness between the live (green) and dead (gray) foraminifera at the highly elevated pCO2 (C), moderately elevated pCO2 (D), and no elevated pCO2 (E) treatments. Only microspheric foraminifera were used in this analysis (Materials and Methods). Untreated specimens were not included in this comparison. The η2 values are effect sizes derived from two-way ANOVA (Materials and Methods).
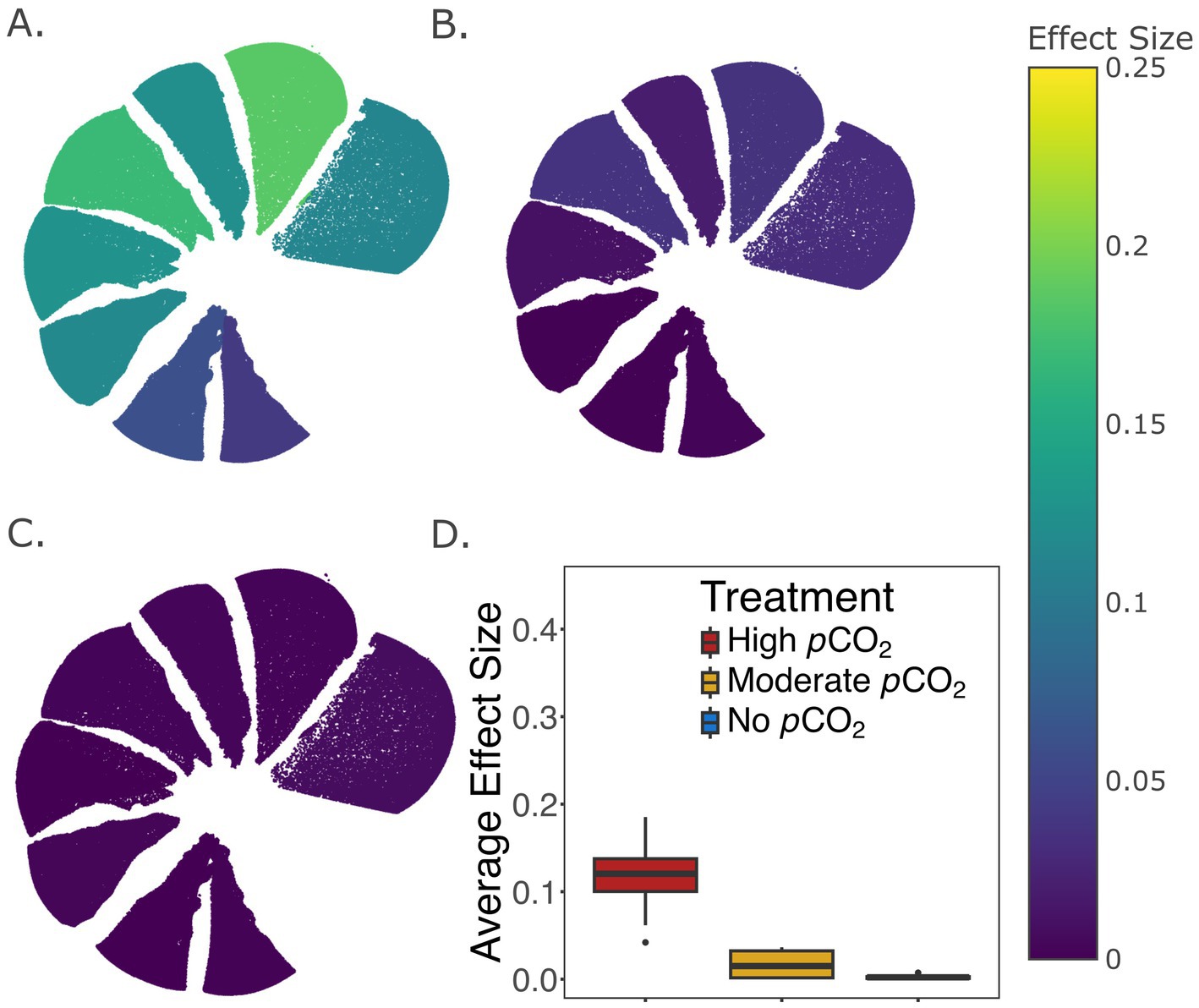
Figure 4. (A–C) Comparison of test thicknesses between live and dead treated specimens within each of the 8 newest chambers under the highly elevated pCO2 (A), moderately elevated pCO2 (B), and no elevated pCO2 (C) treatment conditions. The color of each chamber represents the effect size (η2) of the live vs. dead factor in a two-way ANOVA that accounts for variations among individual foraminifera. (D) Box and whisker plot showing the median, the first and third quartiles, and the minimum and maximum of effect sizes across all 8 chambers for each treatment. Only microspheric foraminifera were used in this analysis (Materials and Methods). The total number of specimens in each treatment group is documented in Supplementary Table S3.
Discussion
Calcareous foraminifera serve as carbon sinks across the global ocean by incorporating calcium carbonate to their tests, sequestering carbon from the surrounding seawater. Benthic foraminifera play important roles in the worldwide carbon budget with an estimated production of 200 million tons of calcium carbonate per year (Langer, 2008). However, calcium carbonate production by foraminifera could be negatively impacted by the anthropogenic production of excess CO2, which causes ocean and coastal acidification, subsequently decreasing the saturation of the carbonate system in the marine environment.
Ocean and coastal acidification could have mixed impacts on foraminifera, with studies noting that some foraminifera species can survive in moderate elevation of pCO2 (790–1865 μatm) without major growth defects (Schmidt et al., 2014; Haynert et al., 2014) or even showing increased growth rates (Fujita et al., 2011). However, the majority of studies indicate either decreased growth rate or defects in morphology at decreased pH (7.4–7.9) or at moderate to highly elevated pCO2 (e.g., up to 3,247 μatm) (Khanna et al., 2013; Prazeres et al., 2015; Schmidt et al., 2014; Keul et al., 2013; Haynert et al., 2014; Kuroyanagi et al., 2021; Manno et al., 2012; Kuroyanagi et al., 2009; Fujita et al., 2011; McIntyre-Wressnig et al., 2013; Reymond et al., 2013; Iwasaki et al., 2019; Sinutok et al., 2011). Study of Haynesina germanica, a temperate salt marsh foraminifer closely related to the Haynesina sp. examined in this study, suggests their feeding-related test ornamentation can be deformed during prolonged treatments (36 weeks) of moderately elevated pCO2 (380–1,000 ppm) (Khanna et al., 2013). However, morphological alteration has not been systematically documented throughout the entire test. Further, with the projected increases of ocean pCO2, more extreme acidification conditions, such as those observed in porewaters of estuarine mudflat sediments (Fouet et al., 2024), will become more impactful to coastal foraminifera.
To our knowledge, this is the first study that differentiates the two alternative generations of the foraminifera lifecycle, microsphere and megalosphere, in examining foraminifera responses to acidification. This distinction could be crucial as varied test structures have been observed between the two life stages of foraminifera, such as those documented in some Elphidiids (Gudina and Levtchuk, 1989). This variability is shown in our experimental data, where microspheric and megalospheric foraminifera had varied test thickness distributions and different levels of sensitivity to laboratory treatments (Supplementary Figure S2). Our current technology, however, supports the identification of life stages only after experimental treatments because of the destructive nature of extended exposure to high X-rays during MicroCT scanning (Materials and Methods). As a result, an insufficient number (n < 3) of megalospheres was included in some treatment conditions (Supplementary Table S3), and the test-thickness analyses were performed only on microspheres. Therefore, the response of megalospheres to coastal acidification remains unknown, which could be a topic for future investigations, for example, by increasing the sample size of each treatment condition to increase the chance of capturing a sufficient number of both micro- and megalospheric specimens.
Compared to the untreated group, the experimental treatment of both live and dead microspheric foraminifera had a larger effect size (η2 > 0.01) in highly elevated pCO2 relative to the little to no effect (η2 ≤ 0.005) in non- or moderately elevated pCO2 (Table 2). Most calcareous foraminifera form tests that are mainly composed of calcite, which is structurally more stable (Erez, 2003) and less prone to dissolution (Sulpis et al., 2022) than the calcium carbonate polymorph aragonite. Given that calcite oversaturation was measured in the moderate treatment (Ωcalcite = 1.498+/−0.057), it is unsurprising that Haynesina test thickness exhibited little to no change in the moderately elevated pCO2. In contrast, the highly elevated pCO2 treatment exhibited calcite undersaturation (Ωcalcite = 0.843+/−0.041), consistent with the observation of test thinning in both live and dead specimens (Tables 1, 2). It is worth noting that the treatment period of our study was 4 weeks, significantly shorter than the long-term treatment (36 weeks) performed on Haynesina germanica (Khanna et al., 2013). This shorter time frame was chosen because it better represents the environmental condition of the Haynesina foraminifera used in our study, where only short episodes of extreme low pH occur during summer months (Wallace et al., 2014). Future studies are required to examine acidification responses through extended periods under both moderately and highly elevated pCO2, especially as such prolonged exposure becomes relevant to the coastal benthic environment.
Typically, new chambers in foraminifera precipitate via multiple steps: (1) formation of an outer organic layer, which is a protective cytoplasmic envelope that defines the bound of the new chamber; (2) construction of the primary organic sheet, which forms under the protective envelope; and (3) calcification around the organic sheet (Erez, 2003; Sliter, 1970; Toyofuku et al., 2017; De Nooijer et al., 2009; Nagai et al., 2018). The calcification relies on the maintenance of a local environment within the protective envelope with conditions favorable for calcium carbonate precipitation (Toyofuku et al., 2017; De Nooijer et al., 2009; Bentov and Erez, 2006; Bentov et al., 2009). This process could be facilitated by vacuolar ATPases, which transport protons from the calcification site to vesicles that are then exported to the extracellular space (Ujiié et al., 2023). Therefore, maintenance of calcification-promoting conditions in foraminifera could involve potential energetic expenses due to consumption of ATP for proton export.
Comparing the acidification treatment of live or dead specimens demonstrated differences in their responses to acidification, with the highest effect observed under the high pCO2 condition (Figure 3). This indicates that thinning of foraminifera tests could be driven not only by calcite undersaturation, but also by the physiological activity of live foraminifera, likely related to the formation of new chambers (Figure 2C). The chamber-specific comparison of live and dead specimens has further emphasized the significant effect of foraminifera physiology on test chamber thickness under highly elevated pCO2. In particular, a more substantial effect size (η2 from 0.11 to 0.19) was observed in each of the six newest chambers (n to n−5) compared to chambers n-6 (η2 = 0.06) or n−7 (η2 = 0.04) (Figure 4), suggesting potential effects of new chamber formation in exacerbating test thinning in high pCO2 systems, likely due to the export of protons mediated by vacuolar ATPases.
Proton release during the formation of new test chamber can lead to increased proton concentration (Figure 5A), subsequently lowering the pH in the microenvironment that surrounds the foraminifera test (Toyofuku et al., 2017). The decreasing pH alters calcite saturation (Ωcalcite), which in turn can lead to potential dissolution of the test surface (Figure 5B). Under the no-elevated and moderately elevated pCO2 treatments performed in this study, Ωcalcite is relatively high, and hence the decrease of pH caused by calcification could have less effect. However, Ωcalcite in the highly elevated pCO2 was close to the value of 1 (Figure 5C), below which dissolution is expected due to calcite undersaturation. Therefore, even a slight decrease of pH could have significant effects on the foraminiferal test, not only increasing the energy demands in promoting calcification and new chamber formation, but also resulting in the dissolution of existing test surfaces.
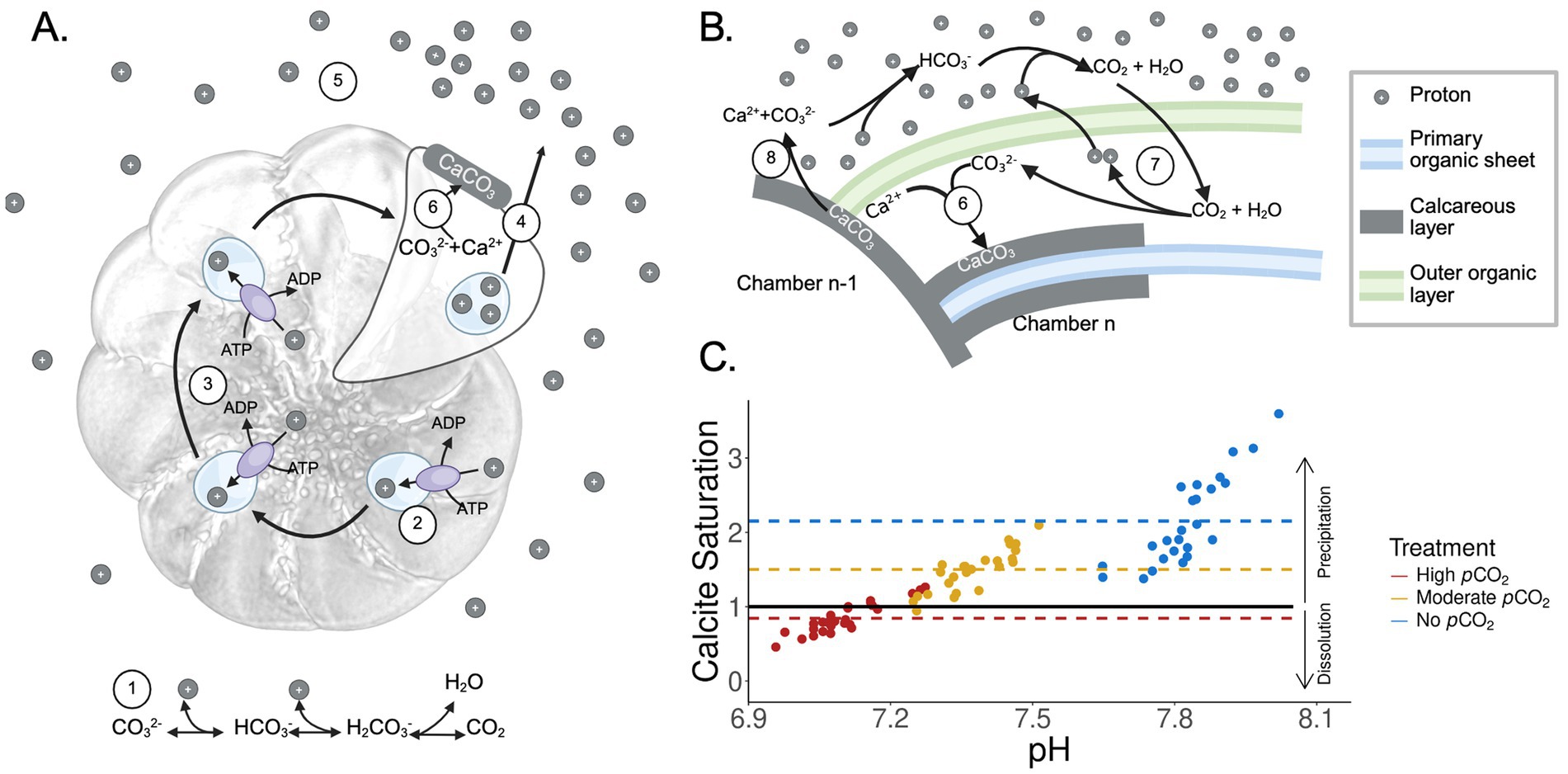
Figure 5. (A) Schematic of chamber formation in foraminifera. Components are as follows: ① the system of reactions dictating that increased CO2 results in increased proton concentration, ② vacuolar ATPases facilitate the export of protons by collecting them into vacuoles, as reported by Ujiié et al. (2023), ③ proton vacuoles are moved throughout the cytoplasm to coordinate exocytosis, ④ protons are released through exocytosis, ⑤ protons diffuse outward and around the test, lowering pH in the microenvironment surrounding the actively growing foraminifera cell (Toyofuku et al., 2017), ⑥ The proton-depleted environment allows for calcium carbonate precipitation. (B) Carbon chemistry during foraminiferal test formation. ⑦ Foraminifera promote calcification through proton export, ⑧ test surface dissolution driven by acidification. (C) Calcite saturation state predicted based on tri-weekly experimental measurements acquired from this study. Each dot represents a measurement data point. Red represents the highly elevated pCO2 treatment, gold represents the moderately elevated pCO2 treatment, and blue represents the no elevated pCO2 treatment. The black horizontal line represents a calcite saturation of 1. Dashed lines represent the mean calcite saturation values of each treatment. Arrows on the right indicate the effect of calcite saturation state on the dissolution or precipitation of calcareous tests. Created with BioRender.com.
We suggest that newer chambers could be more sensitive to acidification than the older chambers, as the physiologically driven pH reduction is likely initiated in the extracellular space near the site of calcium carbonate precipitation of the new chamber (Figure 5). Our experimental observations of the Haynesina sp. (Figure 4A) support models of foraminifera calcification previously described in other studies (Toyofuku et al., 2017; Ujiié et al., 2023) and are consistent with observations from another foraminifera, Ammonia sp., where the lowest extracellular pH in its surrounding microenvironment was measured near the newest chamber (Toyofuku et al., 2017; Glas et al., 2012).
The ability of foraminifera to use proton pumping to manipulate carbonate chemistry is a competitive advantage against ocean and coastal acidification, as it enables the organism to decouple calcium carbonate precipitation from the chemistry of the surrounding seawater (Toyofuku et al., 2017; De Nooijer et al., 2009; Bentov et al., 2009; Ujiié et al., 2023; Glas et al., 2012). However, our results suggest that in conditions near the borderline of calcite undersaturation, foraminifera could reach a tipping point that exacerbates the risk of test dissolution. Further, the energetic cost of proton pumping could increase with any continued rise of pCO2 (De Nooijer et al., 2009), as foraminifera must overcome stronger concentration gradients to achieve an optimal calcification rate (Riebesell and Tortell, 2011). This is notable, as the pCO2 conditions tested in this study have already been observed in coastal systems (Wallace et al., 2014). Therefore, coastal benthic foraminifera are likely experiencing acidification stress that impairs new chamber formation and dissolves already formed test surfaces. With continued anthropogenic production of CO2, coastal acidification will accelerate in intensity and duration (Findlay and Turley, 2021), leaving the ecological function of foraminifera as a carbon sink at greater risk.
Conclusion
This study used laboratory experiments to examine the morphological response of specimens belonging to the foraminiferal genus Haynesina to increasing acidification. The impact of moderate and high acidification conditions on the thickness of foraminifera tests were quantified, demonstrating potential resilience to moderate acidification but deficiency when experiencing high acidification. Contrasting responses of live versus dead foraminifera specimens further documented the combined effects of acidification and physiological processes in the thinning of test surfaces. These observations suggest that increasing ocean and coastal acidification will likely aggravate the precipitation deficiency of foraminifera and presumably other calcifying organisms.
Materials and methods
Field sampling and sample preparation
Surface sediments were collected into 125 mL high density polyethylene Nalgene containers using a plastic scoop. Collected samples were sieved with USA standard sieves 120 (Thermo Fisher Scientific 039988.ON) and 40 (Thermo Fisher Scientific 039984.ON) to select for the size fraction between 125 μm and 425 μm. Isolated sediments were subsequently picked for approximately 600 specimens of Haynesina sp. using 50 μL calibrated pipettes (Drummond Scientific Company 2-000-050), which were pulled to a thin point over a bunsen burner, to isolate individual foraminifera while visualizing with a trinocular stereo microscope under 10–25x with maximum brightness (VanGuard 1372ZL). A subsample of 60 individuals were placed in 2 mL 6% sodium hypochlorite solution (Fisher Scientific NC1796686) for 12 h to remove organic material from the test through bleaching. After bleaching, specimens were rinsed twice for five minutes with Milli-Q H2O (Type I H2O purified with EMD Millipore MilliQ EQ-7008). Eight of the bleached specimens were collected as a no-treatment control (i.e., untreated) and were retained in 100% ethanol at 4°C until microscopic imaging. The rest of the bleached specimens were used in the dead treatment and were stored in Milli-Q H2O at 4°C until experimental manipulation. The rest of the picked foraminifera were kept alive under room temperature in artificial seawater composed of Milli-Q water and Reef Pro Mix (Fritz Aquatics 80,243) made at a salinity of 35 ppt until being used as live treatment in experimental manipulation.
Experimental pCO2 manipulation
Experimental pCO2 manipulation was performed in three 75-liter glass treatment tanks with target pH maintained at 7.2 (Tank 1), 7.6 (Tanks 2), and 8.1 (Tank 3), respectively. All treatment aquaria were maintained with artificial seawater. Replicate foraminifera samples were introduced to treatment tanks in six-well plates sealed with 60-μm nylon mesh (Amazon ASIN#B092D8TJDQ). Each tank had 4 replicate six-well plates, with each plate contained 35 live foraminifera in one well and 3 bleached (dead) foraminifera in a separate well. The acidification treatments were designed following prior examples (Putnam et al., 2016), with pCO2 levels controlled using an A3 Apex Aquarium Controller System (Bulk Reef Supply, SKU 251246). The Apex system measures pH and temperature (°C) every 10 s and adjusts the pH to a target value by injecting CO2 gas using controls of solenoid valves (Figure 1A). Three times per week (Monday, Wednesday, and Friday), 200 mL of tank water from each glass tank were filtered through 0.2 μm surfactant-free cellulose acetate (SFCA) syringe filters (Thermo Scientific 723–2,520). This filtered seawater was stored at −20°C for stability before being used for carbonate system analysis (Mos et al., 2021). At each time point, pH was measured using a calibrated pH meter (OHAUS Aquasearcher 30,589,830), salinity was measured using a refractometer (Amazon ASIN#B018LRO1SU), and temperature was measured using the Apex controller temperature probe (Bulk Reef Supply, SKU 207517). Each Friday, the OHAUS pH meter was calibrated through examination of temperature and voltage correlation, and replicate wells of live foraminifera specimens were fed with Skeletonema dohrnii clone PA 250716_D1 isolated from Narragansett Bay and obtained from Dr. Tatiana Rynearson’s lab at the University of Rhode Island Graduate School of Oceanography. The S. dohrnii was cultured in F/2 medium (Guillard and Ryther, 1962) under 12 h light and 12 h dark cycles. At the time of feeding, concentration of live S. dohrnii culture was quantified with a hemocytometer (Fisher Scientific 02-671-6) to determine the volume used for feeding live foraminifera. An average of 124 μL S. dohrnii culture was used in each feeding to add approximately 25,000 cells to each treatment.
At the end of the experimental period (28 days), a subset of the specimens from both treatments (nlive = 6–8, ndead = 10–12) were randomly collected and prepared for MicroCT scanning. Samples of experimentally treated live specimens were bleached in 6% sodium hypochlorite solution (Fisher Scientific NC1796686) for 12 h to remove organic material from the test. The bleached tests from both live and dead cell treatments were washed twice with Milli-Q water, followed by subsequent washing with 50, 80, and 100% ethanol to rinse any remaining debris and dehydrate the tests in preparation for microscopic imaging (Supplementary Figure S3). All the live and dead treatment specimens were stored in 100% ethanol at 4°C until microCT scanning, which facilitated air drying the tests for mounting.
Seawater carbonate chemistry
Filtered tank water stored at −20°C was used for carbonate-system analysis. Quality control for pH data was assessed three times per week with Tris standard (Dickson Lab Tris Standard Batch 205) and handheld conductivity probes used for discrete measurements were calibrated once per week. Total alkalinity (TA) was measured using an open-cell titration (Dickson et al., 2007) with certified HCl titrant (~0.1 mol kg−1, ~0.6 mol kg−1 NaCl; Dickson Lab) and TA measurements identified < 1% error when compared against certified reference materials (Dickson Lab CO2 CRM Batch 196). Seawater chemistry was completed following Guide to Best Practices (Dickson et al., 2007). Tri-weekly measurements were used to calculate carbonate system parameters (Table 1), using the SEACARB package (Gattuso et al., 2015) in R v3.5.1.
Imaging of foraminifera tests with microCT scanning
Foraminifera tests (untreated, dead treated, and live treated) preserved in 100% ethanol were allowed to air dry completely before mounted with Bondic resin (Bondic, CECOMINOD032561) on a flat surface and cured under UV light. Coordinates of mounted tests were identified through a prescan with a Zeiss Xradia Versa 610 X-Ray microscope under the 0.4x objective using the following parameters: 50 kV voltage, 4.5 W power, 401 projections. Identified tests were individually imaged with the following imaging parameters under the 4x objective: 80 kV voltage, 10 W power, 2,401 projections. Stacked TIFF images were produced based on automatic reconstruction settings during the imaging. The resulting image stacks were imported into the Dragonfly image analysis software (ORS systems Core dll version 2022.2.0.1399, Montreal, CA), which creates a 3D-reconstruction for each foraminifera test. The 8 newest chambers in the 3D-reconstruction of each test were manually isolated through the graphical interface of the Dragonfly software by extracting a region of interest (ROI) containing test areas that are visible from the outside and deleting any undesired regions (e.g., the sutures or air bubbles introduced by the mounting process) (Figures 1B,C). Voxels with an intensity lower than 32,000 were filtered out from each chamber, preserving regions that contained the calcium carbonate test, but excluding voxels that imaged the resin or most air bubbles. The extracted ROIs were then used to calculate a thickness mesh using the “generate thickness mesh” function in Dragonfly, where thicknesses throughout the test were calculated by fitting spheres between the outer and inner test surfaces. The thickness mesh of each test chamber was individually exported to a csv file and used for statistical analysis. The number of thickness measurement data points exported ranged between 49,721 and 700,539 per chamber, covering the entire ROI of each chamber.
Classification of microspheric and megalospheric foraminifera
Diameter of the proloculus and the overall test were determined by fitting a smallest possible sphere over their corresponding outer surfaces using the Dragonfly image analysis software, where the radius of the fitted sphere was reported and used for calculating the diameter of its corresponding proloculus or test. The number of chambers present in each foraminifera was manually counted based on an internal slice projection that included all chambers. During analysis of the 3D-reconstruction of foraminifera tests, a bimodal distribution of proloculus diameters were observed, resulting in two populations: (1) megalospheric, tests with proloculus diameter greater than or equal to 55 μm, (2) microspheric, tests with proloculus diameter less than 55 μm (Supplementary Figure S1). Correspondingly, these two populations had distinct distributions of the number of chambers (Figure 2B).
Data analysis
All statistical analysis was performed in R v4.2.3 using the sjstats package version 0.19.0 (Lüdecke, 2024) and the stats package version 4.2.3. Results were visualized in R v4.2.3 using ggplot2 version 3.4.1 and plotly version 4.10.4 (Ginestet, 2011; Plotly Technologies Inc, 2015). The number of chambers per test and the test diameters were compared between microspheric and megalospheric foraminifera using one-way analysis of variance (ANOVA) (Figure 2B). Growth of live foraminifera throughout the treatment period was approximated by comparing their number of chambers to the pool of dead treated and untreated specimens (referred to as “not-live”) using two-way ANOVA that accounted for differences in microspheric and megalospheric samples, followed by the Tukey’s honestly significant difference test (TukeyHSD) (Figure 2C). Data normality was confirmed through inspection of quantile-quantile plots before the application of ANOVA models.
Due to the low abundance of megalospheric specimens in several treatments (Supplementary Table S3), all the statistical analyses related to test thicknesses were performed with only the microspheric foraminifera. To normalize the thickness measurements from microCT scanning, test thicknesses were divided by the diameter of each corresponding test. The normalized thickness values were compared using two-way ANOVA that accounted for a treatment factor (e.g., treated versus untreated, different pCO2 conditions, or live versus dead treatment) and a second factor that accounted for variations of individual foraminifera. To account for the large number of thickness measurement data points from each specimen, all ANOVA analyses that showed statistical significance (p < 0.05) were followed by the calculation of effect size (η2) measures (Table 2; Figures 3, 4). The effect size ranges from 0 to 1 and is representative of the proportion of variance in the model explained by a given factor.
Specifically, test thickness differences between experimentally treated and untreated foraminifera were examined separately with live or dead specimens and across the three pCO2 treatments (Table 2). Variation of test thicknesses across different pCO2 conditions were compared separately for the live or the dead treatments (Figures 3A,B), and the variation between live and dead foraminifera were compared separately for the different pCO2 conditions (Figures 3C–E). Finally, test-thickness variations between live and dead specimens were examined within each of the eight newest chambers (from n to n−7) to assess their differential responses to the different pCO2 conditions (Figure 4).
Data availability statement
Data files including water chemistry data and test thickness measurements are available on figshare at https://figshare.com/s/4464cc33548faf92e211. All scripts used for analysis are available at https://doi.org/10.5281/zenodo.15653269.
Author contributions
CP: Conceptualization, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing, Data curation, Investigation, Software, Visualization. AP: Data curation, Investigation, Methodology, Writing – review & editing. AZ: Data curation, Investigation, Methodology, Writing – review & editing. KH: Data curation, Investigation, Methodology, Writing – review & editing. MG: Data curation, Investigation, Methodology, Writing – review & editing. JB: Investigation, Methodology, Writing – review & editing, Formal analysis, Validation. YZ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by the National Science Foundation Office of Integrative Activities, award #1929078 and an Undergraduate Research Award from the University of Rhode Island (Fall 2022). The MicroCT data was acquired at the RI Consortium for Nanoscience and Nanotechnology, a URI College of Engineering core facility partially funded by the National Science Foundation EPSCoR, Cooperative Agreement #OIA-1655221.
Acknowledgments
We thank Dr. Tatiana Rynearson’s laboratory for providing culture of S. dohrnii PA 250716_D1 for the feeding of live foraminifera during acidification treatments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1570629/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | (A) Cross section of a microspheric test. (B) Cross section of a megalospheric test. Each scale bar represents 100 μm. (C) Histogram of proloculus diameters showing a bimodal distribution.
SUPPLEMENTARY FIGURE S2 | (A–E) Distribution of normalized test thickness between microspheric and megalospheric specimens within each experimental treatment.
SUPPLEMENTARY FIGURE S3 | Schematic of experimental workflow as detailed in materials and methods. Created with BioRender.com.
SUPPLEMENTARY TABLE S1 | Collection of previous ocean and coastal acidification studies of foraminifera, including reference information for the paper, condition of acidification treatments (i.e., pH, pCO2), and a summary of key findings.
SUPPLEMENTARY TABLE S2 | Tri-weekly water chemistry data from each treatment tank of both the Summer 2023 and Fall 2023 experiments. Parameters shown in the table were calculated using the SEACARB R package V3.5.1 with the exception of pH, temperature, salinity, and total alkalinity (TA), which were experimentally measured.
SUPPLEMENTARY TABLE S3 | Number of megalospheric and microspheric specimens collected from the Summer 2023 and Fall 2023 acidification experiments across each treatment. Total indicates the sum of numbers from both replicate experiments.
SUPPLEMENTARY TABLE S4 | Measurements of foraminifera test morphology for all specimens analyzed in this study. Data includes the trial number (OA2 or OA3), treatment tank, specimen ID, measurements of test radius/diameter, proloculus radius/diameter, number of chambers, and assignment of foraminifera life stage.
References
Alve, E., and Goldstein, S. T. (2003). Propagule transport as a key method of dispersal in benthic foraminifera (Protista). Limnol. Oceanogr. 48, 2163–2170. doi: 10.4319/lo.2003.48.6.2163
Andersson, A. J., Mackenzie, F. T., and Bates, N. R. (2008). Life on the margin: implications of ocean acidification on mg-calcite, high latitude and cold-water marine calcifiers. Mar. Ecol. Prog. Ser. 373, 265–273. doi: 10.3354/meps07639
Bentov, S., Brownlee, C., and Erez, J. (2009). The role of seawater endocytosis in the biomineralization process in calcareous foraminifera. Proc. Natl. Acad. Sci. USA 106, 21500–21504. doi: 10.1073/pnas.0906636106
Bentov, S., and Erez, J. (2006). Impact of biomineralization processes on the mg content of foraminiferal shells: a biological perspective. Geochem. Geophys. Geosyst. 7, 1–11. doi: 10.1029/2005gc001015
Bernhard, J. M., Wit, J. C., Starczak, V. R., Beaudoin, D. J., Phalen, W. G., and McCorkle, D. C. (2021). Impacts of multiple stressors on a benthic foraminiferal community: a long-term experiment assessing response to ocean acidification, hypoxia and warming. Front. Mar. Sci. 8:643339. doi: 10.3389/fmars.2021.643339
De Nooijer, L. J., Toyofuku, T., and Kitazato, H. (2009). Foraminifera promote calcification by elevating their intracellular pH. Proc. Natl. Acad. Sci. USA 106, 15374–15378. doi: 10.1073/pnas.0904306106
Dickson, A. G., Sabine, C. L., and Christian, J. R., “SOP 3b: Determination of total alkalinity in sea water using an open-cell titration ” (2007). Available online at: https://www.ncei.noaa.gov/access/ocean-carbon-acidification-data-system/oceans/Handbook_2007.html (Accessed February 9, 2023).
Doney, S. C., Fabry, V. J., Feely, R. A., and Kleypas, J. A. (2009). Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192. doi: 10.1146/annurev.marine.010908.163834
Erez, J. (2003). The source of ions for biomineralization in foraminifera and their implications for Paleoceanographic proxies. Rev. Mineral. Geochem. 54, 115–149. doi: 10.2113/0540115
Figuerola, B., Hancock, A. M., Bax, N., Cummings, V. J., Downey, R., Griffiths, H. J., et al. (2021). A review and Meta-analysis of potential impacts of ocean acidification on marine Calcifiers from the Southern Ocean. Front. Mar. Sci. 8:584445. doi: 10.3389/fmars.2021.584445
Findlay, H. S., and Turley, C. (2021). “Chapter 13 – ocean acidification and climate change” in Climate change. ed. T. M. Letcher. Third edn. (Amsterdam, Netherlands: Elsevier), 251–279.
Fouet, M., Daviray, M., Geslin, E., Metzger, E., and Jorissen, F. (2024). Foraminiferal test dissolution reveals severe sediment acidification in estuarine mudflats: new perspectives for present and historical assessment. Compt. Rendus Geosci. 356, 83–96. doi: 10.5802/crgeos.269
Fujita, K., Hikami, M., Suzuki, A., Kuroyanagi, A., and Kawahata, H. (2011). Effects of ocean acidification on calcification of symbiont-bearing reef foraminifers. Biogeosci. Discuss. 8, 1809–1829. doi: 10.5194/bg-8-2089-2011
Gattuso, J.-P., Epitalon, J.-M., Lavigne, H., Orr, J., Gentili, B., Hagens, M., et al. (2015). Others, package “seacarb”. Available online at: http://cran.r-project.org/package=seacarb (Accessed August 22, 2023).
Ginestet, C. (2011). ggplot2: elegant graphics for data analysis. J. R. Stat. Soc. Ser. A 174, 245–246. doi: 10.1111/j.1467-985X.2010.00676_9.x
Glas, M. S., Langer, G., and Keul, N. (2012). Calcification acidifies the microenvironment of a benthic foraminifer (Ammonia sp.). J. Exp. Mar. Biol. Ecol. 424–425, 53–58. doi: 10.1016/j.jembe.2012.05.006
Goldstein, S. T. (1999). “Foraminifera: A biological overview.” In: Modern Foraminifera, (ed.) B. K. Sen Gupta, pp. 37–55. (Netherlands, Dordrecht: Springer) doi: 10.1007/0-306-48104-9_3
Gudina, V. I., and Levtchuk, L. K. (1989). Fossil and modern elphidiids of arctic and boreal regions; morphology and taxonomic classification. J. Foraminifer. Res. 19, 20–37. doi: 10.2113/gsjfr.19.1.20
Guillard, R. R., and Ryther, J. H. (1962). Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) gran. Can. J. Microbiol. 8, 229–239. doi: 10.1139/m62-029
Guinotte, J. M., and Fabry, V. J. (2008). Ocean acidification and its potential effects on marine ecosystems. Ann. N. Y. Acad. Sci. 1134, 320–342. doi: 10.1196/annals.1439.013
Haynert, K., Schönfeld, J., Schiebel, R., Wilson, B., and Thomsen, J. (2014). Response of benthic foraminifera to ocean acidification in their natural sediment environment: a long-term culturing experiment. Biogeosciences 11, 1581–1597. doi: 10.5194/bg-11-1581-2014
Hottinger, L. C. (2000). Functional morphology of benthic foraminiferal shells, envelopes of cells beyond measure. Micropaleontology 46, 57–86.
Iwasaki, S., Kimoto, K., Sasaki, O., Kano, H., and Uchida, H. (2019). Sensitivity of planktic foraminiferal test bulk density to ocean acidification. Sci. Rep. 9:9803. doi: 10.1038/s41598-019-46041-x
Keul, N., Langer, G., De Nooijer, L. J., and Bijma, J. (2013). Effect of ocean acidification on the benthic foraminifera Ammonia sp. is caused by a decrease in carbonate ion concentration. Biogeosci. Discuss. 10, 1147–1176. doi: 10.5194/bg-10-6185-2013
Khanna, N., Godbold, J. A., Austin, W. E. N., and Paterson, D. M. (2013). The impact of ocean acidification on the functional morphology of foraminifera. PLoS One 8:e83118. doi: 10.1371/journal.pone.0083118
Kuroyanagi, A., Irie, T., Kinoshita, S., Kawahata, H., Suzuki, A., Nishi, H., et al. (2021). Decrease in volume and density of foraminiferal shells with progressing ocean acidification. Sci. Rep. 11:19988. doi: 10.1038/s41598-021-99427-1
Kuroyanagi, A., Kawahata, H., Suzuki, A., Fujita, K., and Irie, T. (2009). Impacts of ocean acidification on large benthic foraminifers: results from laboratory experiments. Mar. Micropaleontol. 73, 190–195. doi: 10.1016/j.marmicro.2009.09.003
Langer, M. R. (2008). Assessing the contribution of foraminiferan protists to global ocean carbonate production. J. Eukaryot. Microbiol. 55, 163–169. doi: 10.1111/j.1550-7408.2008.00321.x
Lei, Y., Li, T., Nigam, R., Holzmann, M., and Lyu, M. (2017). Environmental significance of morphological variations in the foraminifer Ammonia aomoriensis (Asano, 1951) and its molecular identification: a study from the Yellow Sea and East China Sea, PR China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 483, 49–57. doi: 10.1016/j.palaeo.2016.05.010
Leung, J. Y. S., Zhang, S., and Connell, S. D. (2022). Is ocean acidification really a threat to marine calcifiers? A systematic review and meta-analysis of 980+ studies spanning two decades. Small 18:e2107407. doi: 10.1002/smll.202107407
Lüdecke, D. (2024). Sjstats: statistical functions for regression models. Available online at: https://CRAN.R-project.org/package=sjstats (Accessed August 1, 2023).
Manno, C., Morata, N., and Bellerby, R. (2012). Effect of ocean acidification and temperature increase on the planktonic foraminifer Neogloboquadrina pachyderma (sinistral). Polar Biol. 35, 1311–1319. doi: 10.1007/s00300-012-1174-7
McIntyre-Wressnig, A., Bernhard, J. M., McCorkle, D. C., and Hallock, P. (2011). Non-lethal effects of ocean acidification on two symbiont-bearing benthic foraminiferal species. Biogeosci. Discuss. 8, 9165–9200. doi: 10.5194/bgd-8-9165-2011
McIntyre-Wressnig, A., Bernhard, J. M., McCorkle, D. C., and Hallock, P. (2013). Non-lethal effects of ocean acidification on the symbiont-bearing benthic foraminifer Amphistegina gibbosa. Mar. Ecol. Prog. Ser. 472, 45–60. doi: 10.3354/meps09918
Melzner, F., Mark, F. C., Seibel, B. A., and Tomanek, L. (2020). Ocean acidification and coastal marine invertebrates: tracking CO2 effects from seawater to the cell. Annu. Rev. Mar. Sci. 12, 499–523. doi: 10.1146/annurev-marine-010419-010658
Mos, B., Holloway, C., Kelaher, B. P., Santos, I. R., and Dworjanyn, S. A. (2021). Alkalinity of diverse water samples can be altered by mercury preservation and borosilicate vial storage. Sci. Rep. 11:9961. doi: 10.1038/s41598-021-89110-w
Nagai, Y., Uematsu, K., Wani, R., and Toyofuku, T. (2018). Reading the fine print: ultra-microstructures of foraminiferal calcification revealed using focused ion beam microscopy. Front. Mar. Sci. 5:67. doi: 10.3389/fmars.2018.00067
Nigam, R. (1986). Dimorphic forms of recent foraminifera: an additional tool in paleoclimatic studies. Palaeogeogr. Palaeoclimatol. Palaeoecol. 53, 239–244. doi: 10.1016/0031-0182(86)90060-X
Nigam, R., and Rao, A. S. (1987). Proloculus size variation in recent benthic foraminifera: implications for paleoclimatic studies. Estuar. Coast. Shelf Sci. 24, 649–655. doi: 10.1016/0272-7714(87)90104-1
Prazeres, M., Uthicke, S., and Pandolfi, J. M. (2015). Ocean acidification induces biochemical and morphological changes in the calcification process of large benthic foraminifera. Proc. Biol. Sci. 282:20142782. doi: 10.1098/rspb.2014.2782
Putnam, H. M., Davidson, J. M., and Gates, R. D. (2016). Ocean acidification influences host DNA methylation and phenotypic plasticity in environmentally susceptible corals. Evol. Appl. 9, 1165–1178. doi: 10.1111/eva.12408
Reymond, C. E., Lloyd, A., Kline, D. I., Dove, S. G., and Pandolfi, J. M. (2013). Decline in growth of foraminifer Marginopora rossi under eutrophication and ocean acidification scenarios. Glob. Chang. Biol. 19, 291–302. doi: 10.1111/gcb.12035
Riebesell, U., and Tortell, P. D. (2011). “Effects of Ocean Acidification on Pelagic Organisms and Ecosystems” in Ocean Acidification. (eds). J.-P. Gattuso and L. Hansson, (Oxford, 2011; online edn, Oxford Academic, 12 Nov. 2020), doi: 10.1093/oso/9780199591091.003.0011
Schmidt, C., Kucera, M., and Uthicke, S. (2014). Combined effects of warming and ocean acidification on coral reef foraminifera Marginopora vertebralis and Heterostegina depressa. Coral Reefs 33, 805–818. doi: 10.1007/s00338-014-1151-4
Sinutok, S., Hill, R., Doblin, M. A., Wuhrer, R., and Ralph, P. J. (2011). Warmer more acidic conditions cause decreased productivity and calcification in subtropical coral reef sediment-dwelling calcifiers. Limnol. Oceanogr. 56, 1200–1212. doi: 10.4319/lo.2011.56.4.1200
Sliter, W. V. (1970). Bolivina doniezi Cushman and Wickenden in clone culture. Cushman Found. Foram. Res. Contr. 21, 87–99.
Stocker, T. F., Qin, D., Plattner, G.-K., Tignor, M. M. B., Allen, S. K., Boschung, J., et al. (2014). Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of IPCC the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Sulpis, O., Agrawal, P., Wolthers, M., Munhoven, G., Walker, M., and Middelburg, J. J. (2022). Aragonite dissolution protects calcite at the seafloor. Nat. Commun. 13:1104. doi: 10.1038/s41467-022-28711-z
Todd, R., and Blackmon, P. (1956). Calcite and Aragonite in Foraminifera. J. Paleontol. 30, 217–219.
Toyofuku, T., Matsuo, M. Y., De Nooijer, L. J., Nagai, Y., Kawada, S., Fujita, K., et al. (2017). Proton pumping accompanies calcification in foraminifera. Nat. Commun. 8:14145. doi: 10.1038/ncomms14145
Ujiié, Y., Ishitani, Y., Nagai, Y., Takaki, Y., Toyofuku, T., and Ishii, S. (2023). Unique evolution of foraminiferal calcification to survive global changes. Sci. Adv. 9:eadd3584. doi: 10.1126/sciadv.add3584
Keywords: foraminifera, ocean acidification, Haynesina , pCO2, calcification
Citation: Powers C, Paz A, Zyck A, Harri K, Geraci M, Bernhard JM and Zhang Y (2025) Morphological responses of a temperate intertidal foraminifer, Haynesina sp., to coastal acidification. Front. Microbiol. 16:1570629. doi: 10.3389/fmicb.2025.1570629
Edited by:
Jin-e Liu, Nanjing Normal University, ChinaReviewed by:
Veronica Rossi, University of Bologna, ItalyYvonne Milker, University of Hamburg, Germany
Copyright © 2025 Powers, Paz, Zyck, Harri, Geraci, Bernhard and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Zhang, eWluZ3poYW5nQHVyaS5lZHU=
 Christopher Powers
Christopher Powers Alberto Paz
Alberto Paz Amaelia Zyck
Amaelia Zyck Kaylee Harri1
Kaylee Harri1 Madison Geraci
Madison Geraci Joan M. Bernhard
Joan M. Bernhard Ying Zhang
Ying Zhang