- 1Faculty of Animal Science and Technology, Yunnan Agricultural University, Kunming, China
- 2Yunnan Provincial Key Laboratory of Animal Nutrition and Feed Science, Kunming, China
- 3Yunnan Center for Animal Disease Control and Prevention, Kunming, China
- 4School of Ethnic Medicine, Yunnan Minzu University, Kunming, China
The widespread misuse of antibiotics in livestock production has raised growing concerns about antimicrobial resistance and residue contamination. These challenges have led to global restrictions on the use of antibiotic growth promoters (AGPs). However, the ban on AGPs has made the management of intestinal inflammation significantly more difficult, highlighting the urgent need for safe and effective alternatives. Lactic acid bacteria (LAB), known for their immunomodulatory properties, have emerged as promising candidates, though their anti-inflammatory mechanisms remain poorly understood. This study investigated the anti-inflammatory effects and underlying molecular mechanisms of a porcine-derived Lactobacillus reuteri (L. reuteri) strain, SBC5-3. Using tumor necrosis factor-alpha (TNF-α)-induced inflammatory models in the HT-29 cells, we employed RNA sequencing (RNA-seq) combined with Western blot analyses to systematically explore the modulation of inflammatory signaling pathways. RNA-seq demonstrated that L. reuteri SBC5-3 downregulated 9 out of 14 TNF-α-induced genes associated with the TNF and/or nuclear factor kappa B (NF-κB) signaling pathways, including BIRC3, PTGS2, CCL20, TNFAIP3, LTB, CXCL1, CXCL10, IL-8, and CSF1. Specifically, L. reuteri SBC5-3 downregulated key genes in the NF-κB pathway, such as TAK1, IKKα, and NFKB1, while upregulating NFKBIA. Concurrent transcriptional suppression occurred in mitogen-activated protein kinase (MAPK) signaling pathway components ERK and JNK. Western blot analysis further confirmed attenuation of phosphorylation involving TAK1, IKKα/β, and IκBα following SBC5-3 treatment. The collective data demonstrate that immunomodulation mediated by L. reuteri SBC5-3 involves inhibiting IκBα degradation, which is known to be essential for the nuclear translocation of the p50/p65 heterodimer, thereby suggesting inhibition of TNF-α-induced NF-κB nuclear translocation. These results position L. reuteri SBC5-3 as a viable therapeutic agent for inflammation modulation through targeted intervention in NF-κB and MAPK signaling pathways.
1 Introduction
For decades, antibiotics have been widely incorporated into livestock feed as AGPs to improve production efficiency, suppress enteric pathogens, and reduce production costs (Liu et al., 2005; Brown et al., 2017). The growth-enhancing mechanism of AGPs primarily involve modulation of intestinal inflammatory responses and reduction of immunological burden (Niewold, 2007). However, with increasing concerns regarding antibiotic residues and resistance stemming from overuse, there has been a growing trend to prohibit AGPs in animal feed in many countries and regions (Miyakawa et al., 2024). While crucial for addressing antibiotic misuse and related complications, it has introduced novel challenges. Restricting AGPs in livestock nutrition exacerbates management of intestinal inflammation induced by pathogens, antinutritional components in feed, and environmental factors, ultimately compromising both animal welfare and farming efficacy (Adedokun and Olojede, 2019). Development of viable antibiotic alternatives now constitutes a priority challenge for sustainable agriculture systems.
LAB, indigenous gut probiotics with multifunctional benefits, represent viable antibiotic alternatives in animal husbandry (Vieco-Saiz et al., 2019). Previous studies has demonstrated LAB-mediated improvements in livestock feed utilization efficiency, suppression of enteropathogen colonization, and maintenance of intestinal redox homeostasis (Wang et al., 2016; Bell et al., 2022; Li et al., 2024). Emerging evidence highlights immunoregulatory properties across numerous Lactobacilli strains. For instance, L. plantarum MTCC 9483 was shown to differentially regulate inflammatory mediators in Caco-2 cells under oxidative stress, lipopolysaccharides (LPS) induction, and pathogen invasion, suppressing pro-inflammatory cytokines (TNF-α, interleukin [IL]-1α, IL-1β, IL-6, and IL-8) while simultaneously upregulating anti-inflammatory genes (TNF-β1, IL-4, and IL-10) (Devi et al., 2018). Experimental evidence (Devi et al., 2018) established that dietary supplementation with L. johnsonii Jlus66 diminished pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) levels in dextran sulfate sodium-induced colitis mice and inhibited the MAPK and NF-κB signaling pathways. Although LAB-mediated anti-inflammatory effects are extensively documented, mechanistic elucidation of these immunomodulatory interactions remains incomplete.
The inflammatory process involves intricate cross-talk among multiple signaling pathways, requiring systematic analysis through modern technological approaches. RNA sequencing (RNA-seq) technology, distinguished by high-throughput capacity, analytical sensitivity, and cost-efficiency, has emerged as an effective methodology for elucidating complex regulatory networks in biological systems (Jackson et al., 2024). Hou et al. (2019) employed RNA-seq to delineate antioxidant pathway activation in L. rhamnosus GG and L. plantarum J26 following hydrogen peroxide (H2O2)-induced conditions in Caco-2 intestinal models. Similarly, Cheng et al. (2024) applied RNA-seq profiling to reveal L. reuteri IMAUJBC1-mediated attenuation of diet-induced hyperlipidemia in murine models through peroxisome proliferator-activated receptor (PPAR) signaling pathway. These investigations collectively validate RNA-seq as an indispensable tool for mechanistic exploration of probiotic mechanisms and host-probiotic interactions.
Our previous investigations identified L. reuteri SBC5-3, a strain isolated from porcine intestinal specimens in Yunnan Province, China, demonstrating significant probiotic characteristics. This strain exhibits key probiotic traits such as gastrointestinal tract tolerance, strong adhesion, and antimicrobial efficacy. Notably, preliminary in vitro experiments indicatedthat co-culture with SBC5-3 substantially reduced TNF-α-stimulated IL-8 secretion in HT-29 cells, implicating its anti-inflammatory capacity (Bao et al., 2023). RNA-seq was employed to profile transcriptional alterations in TNF-α-induced HT-29 cells following co-cultured with L. reuteri SBC5-3, complemented by Western blot analysis to quantify phosphorylation status of critical NF-κB pathway mediators: transforming growth factor-β-activated kinase 1 (TAK1), inhibitor kappa B kinase (IKK) α/β, and inhibitor of kappa B alpha (IκBα). This investigation seeks to elucidate the molecular basis underlying L. reuteri SBC5-3-mediated suppression of TNF-α-induced inflammation in HT-29 cells, thereby providing foundational insights essential for advancing novel anti-inflammatory formulations in animal nutrition.
2 Materials and methods
2.1 Culture and preparation of L. reuteri SBC5-3 suspension
SBC5-3 was originally isolated by our research team from fecal samples of free-range Saba pigs in Chuxiong, Yunnan, China, and cryopreserved at –80°C. Preliminary whole-genome sequencing of strain SBC5-3 revealed that its average nucleotide identity (ANI) values exceeded the 95% species threshold when compared with five representative L. reuteri strains (Supplementary Figure S1), thereby confirming its taxonomic classification as L. reuteri. A sterile inoculation loop was used to transfer a minimal aliquot of the cryostock onto de Man Rogosa Sharpe agar (MRS; Merck, Darmstadt, Germany), followed by aerobic incubated at 37°C for 48 h. Distinct colonies were subcultured into liquid MRS medium, homogenized, and cultured under standard conditions (37°C, 16 h). Subsequently, the culture was augmented by inoculating 1% (v/v) of the previous culture into fresh medium, with subsequent incubation for 8 h under identical parameters. The bacterial cells were harvested by centrifugation at 4,500 rpm for 10 min at 4°C and washed three times with phosphate-buffered saline. The pellet was subsequently resuspended in Roswell Park Memorial Institute-1640 (RPMI-1640) medium (VivaCell, Shanghai, China), and the concentration was adjusted to 1 × 108 colony-forming units (CFU)/mL, as determined by OD600 measurements and a CFU calibration curve.
2.2 Culture of HT-29 cells
The human colon adenocarcinoma HT-29 cell line (Catalog number: KCB200508YJ) was kindly provided by the Kunming Institute of Zoology, Chinese Academy of Sciences. Cells were routinely cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (VivaCell, Shanghai, China), and 1% penicillin-streptomycin (Biological Industries, Kibbutz Beit Haemek, Israel). The cells were maintained in 25-cm2 cell culture flasks. Incubation was performed at 37°C in a humidified 5% CO2 atmosphere, with medium replacement every 48-h and weekly subculturing at 1:3 ratio. Cells from passages 3–5 were utilized for the subsequent experiments.
2.3 Effect of L. reuteri SBC5-3 on TNF-α-induced transcriptomic changes in cells
2.3.1 Experimental groups and treatment
TNF-α (PeproTech, NJ, United States) stock solution (10 μg/mL) was prepared by reconstituting 50 μg of lyophilized powder in 0.5 mL sterile, nuclease-free water with repeated pipetting, followed by PBS dilution to a final volume of 5 mL. The solution was aliquoted into sterile, nuclease-free 1.5mL microcentrifuge tubes and stored at −20°C until use.
HT-29 cells were seeded at 1.5 × 106 cells/well in a six-well plate. After reaching 80% confluence, the medium was discarded, and the cells were washed thrice with pre-warmed RPMI-1640 medium. The cells were subsequently prepared for treatment. The experiment comprised four groups, each containing four biological replicates, as follows:
Control group (CON): Cells were cultured in 2 mL RPMI-1640 medium for 19 h.
TNF-α group (TNF): Cells were cultured in 2 mL of RPMI-1640 medium for 16 h, then treated with 10 μL of 10 μg/mL TNF-α stock solution (final concentration: 50 ng/mL) for 3 h.
SBC5-3 + TNF-α group (SBC + TNF): Cells were pre-incubated for 16 h in 2 mL of RPMI-1640 medium containing L. reuteri SBC5-3 (1 × 108 CFU/mL); followed by treatment with 10 μL of 10 μg/mL TNF-α stock solution (final concentration: 50 ng/mL) for 3 h.
SBC5-3 group (SBC): Cells were cultured in 2 mL of RPMI-1640 medium containing L. reuteri SBC5-3 (1 × 108 CFU/mL) for 19 h.
All incubations were conducted at 37°C in a humidified 5% CO2 incubator. Post-treatments, total RNA was extracted using the RNA Simple Total RNA Extraction Kit (DP419, Tiangen Biotech, Beijing, China) following manufacturer’s instructions. RNA purity was assessed with a Microvolume Spectrophotometer (IMPLEN, Munich, Germany), while agarose gel electrophoresis was used to verify RNA quality. The extracted RNA was subsequently stored at –80°C for further analysis.
2.3.2 RNA Sequencing and differential expression analysis
Total RNA samples stored at –80°C were processed for ribosomal RNA (rRNA) depletion and strand-specific library construction. After the quality control of the libraries, transcriptomic sequencing was performed utilizing the Illumina NovaSeq™ 6000 platform (Illumina, San Diego, CA, United States). Raw sequences were processed using Cutadapt to eliminate low-quality reads, resulting in high-quality data. The resultant clean data were aligned to the human reference genome utilizing HISAT2, and location-specific information was annotated for genes or genomic regions, along with sequence characteristics unique to the sequencing samples.
Gene expression was quantified by calculating the fragments per kilobase of transcript per million mapped reads (FPKM) for each gene. The number of reads mapped to each gene was counted. Differential expression analysis was conducted to calculate the fold changes and assess statistical significance. Differentially expressed genes (DEGs) were identified using the criteria of false discovery rate (FDR)-adjusted p-value (q-value) < 0.05 and Log2 (fold change) > 1. These DEGs underwent subsequent Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis using the same q-value < 0.05 for statistical significance.
2.4 Real-time quantitative polymerase chain reaction (RT-qPCR) validation of DEGs
A subset of significant DEGs identified in the RNA-seq were selected for RT-qPCR validation to validate the reliability of the RNA-seq data. RT-qPCR was used to measure mRNA abundance of the following genes: ACTB, IL-8, IL-1β, CCL20, CXCL10, NFKB1, NFKBIA, PTGS2, and TNFAIP3. Shanghai Generay Biotechnology Co., Ltd. synthesized primers for RT-qPCR, as detailed in Supplementary Table S1.
PCR amplification was performed under the following thermal cycling conditions: initial denaturation at 94°C for 5 min, followed by 40 cycles of 94°C for 15 s, 60°C for 45 s, and a final elongation step at 54°C for 10 min. A negative control without the target gene was included in the PCR reactions. Fluorescence was measured at 60°C during extension phases, followed by melting curve analysis to confirm the specificity of amplification. The 2−ΔΔCt method (Livak and Schmittgen, 2001) was used for data analysis.
2.5 Effect of L. reuteri SBC5-3 on TNF-α-induced activation of the NF-κB inflammatory signaling pathway in cells
According to the grouping method outlined in section 2.3.1, four groups (CON, TNF-α, SBC5-3, and SBC5-3 + TNF-α) were cultured for 16 h. Following the initial culture period, the cells were subjected to specific treatments based on their group assignments.
In the CON and SBC5-3 groups, cells were further cultured without any additional treatment for 15, 30, 45, and 60 min.
In the TNF-α and SBC5-3 + TNF-α groups, 10 μL of 10 μg/mL TNF-α was added to achieve a final concentration of 50 ng/mL. The cultures were then maintained for 15, 30, 45, and 60 min.
After the designated treatment times, cells were harvested for protein extraction. The cells were lysed in a buffer containing RIPA lysis buffer (Solarbio, Beijing, China) and protease inhibitors, homogenized through pipetting, supplemented with 10% sodium dodecyl sulfate, and incubated at 4°C for 20 min. The lysates were heated in boiling water for 15 min, followed by centrifugation at 12,000 rpm for 5 min at 4°C, after which supernatants were collected. Protein concentration was measured with a bicinchoninic acid assay kit (Thermo Fisher Scientific, MA, United States). Equal protein quantities (10 μg) underwent Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis separation before transfer onto nitrocellulose membranes (GE Healthcare, IL, United States). Membrane blocking was performed for 1 h at room temperature using 5% non-fat dry milk (BD Difco) in Tris-buffered saline with Tween 20. Subsequent to blocking, membranes were subjected to overnight incubation at 4°C with primary antibodies targeting TAK1 and p-TAK1 (Biorbyt, Cambridge, United Kingdom); IKKα/β (Abcam, Cambridge, United Kingdom); p-IKKα/β and p-IκBα (Cell Signaling Technology, MA, United States); IκBα and β-actin (PeproTech, NJ, United States). Following washing procedures, membranes were treated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (PeproTech, NJ, United States). Visualization of immunoreactive bands was achieved using a gel imaging system (Bio-Rad Laboratories, CA, United States) and quantified with ImageJ software (NIH, Bethesda, MD, United States).
2.6 Data analysis
Protein expression data were calculated as the ratio of the intensity of the target protein band to that of the housekeeping protein band. Initial data organization was performed using Excel 2019 (Microsoft Corporation, Redmond, WA, United States), followed by statistical analysis using SPSS 25.0 software (IBM, Armonk, NY, United States). Statistical comparisons were made via Student’s t-test, with results reported as the mean ± standard deviation. Statistical significance was set at P < 0.05.
3 Results
3.1 Effect of L. reuteri SBC5-3 on the transcriptome of TNF-α-induced cells
3.1.1 Statistical analysis of transcriptome sequencing data
Figures 1A–D illustrate the expression of significant DEGs between the comparison groups. In the TNF-α versus CON group, 27 DEGs were detected, comprising 24 upregulated and 3 downregulated genes. The SBC5-3 versus CON comparison revealed 6,132 DEGs. with 2,863 upregulated and 3,269 downregulated transcripts. For the SBC5-3 + TNF-α versus TNF-α comparison, 6,961 DEGs were identified, including 3,276 upregulated and 3,685 downregulated genes.
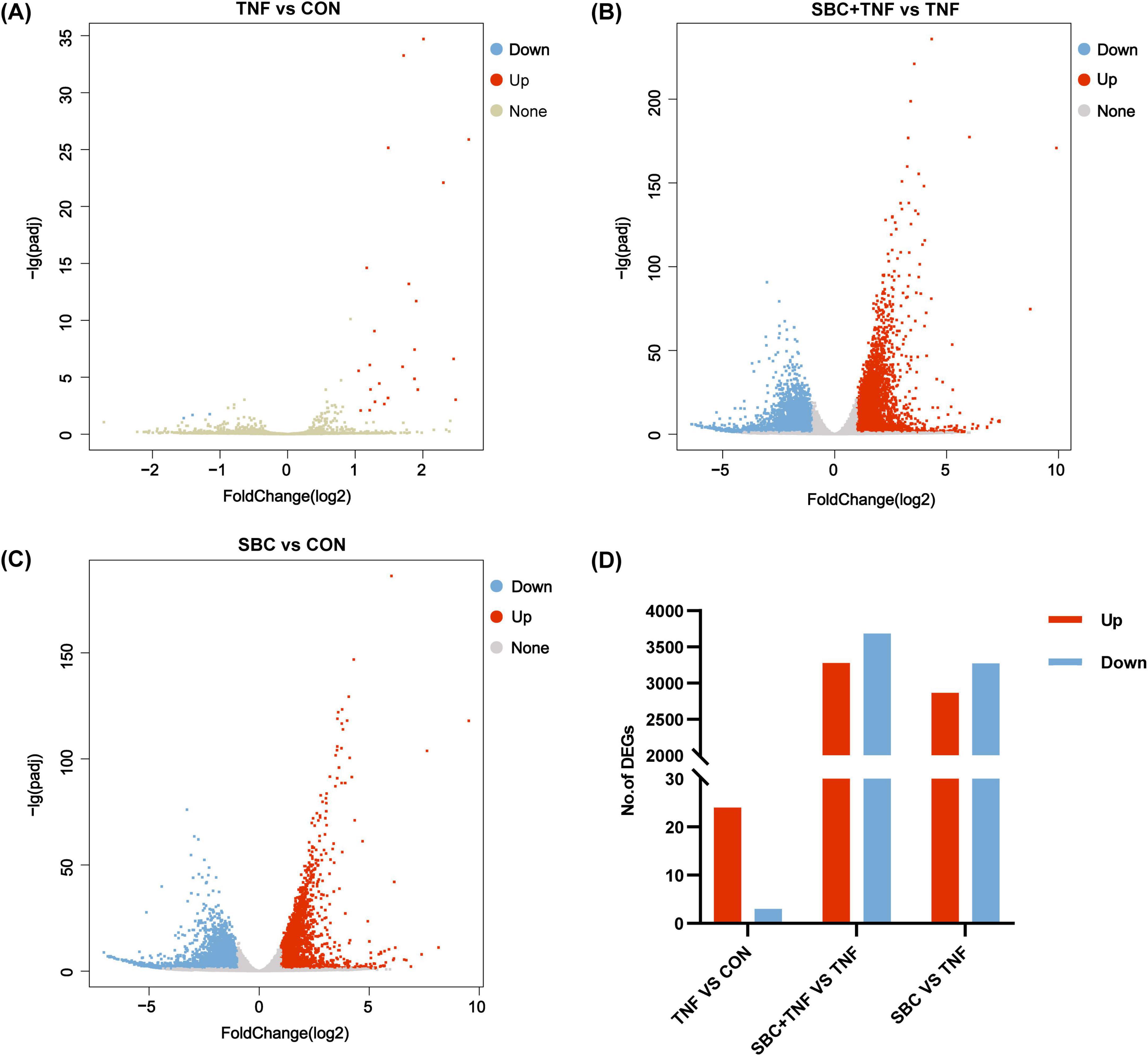
Figure 1. Volcano plots of DEGs between groups: (A) TNF versus CON; (B) SBC + TNF versus TNF. (C) SBC versus CON. (D) Summary of DEGs. Red represents upregulated DEGs, blue represents downregulated DEGs, and gray represents DEGs without significant changes. n = 4 replicates/group.
3.1.2 KEGG pathway enrichment analysis of DEGs
Functional annotation of relevant sequences was performed utilizing KEGG analysis. As shown in Figure 2A, eight signaling and immune-related pathways demonstrated significant enrichment in the TNF versus CON comparison, particularly NF-κB, TNF, and IL-17 signaling pathways. Notably, the NF-κB signaling pathway displayed the highest enrichment rate, indicating that TNF-α may induce the inflammatory response in HT-29 cells through NF-κB signaling. In contrast, the SBC5-3 + TNF versus CON comparison revealed enrichment of 20 signaling and immune-related pathways, including TNF, sphingolipid, VEGF, and NF-κB signaling pathways (Figure 2B). Similarly, nine pathways were enriched in the SBC versus CON comparison (Figure 2C), including phosphatidylinositol signaling, TNF, B cell receptor, and NF-κB signaling.
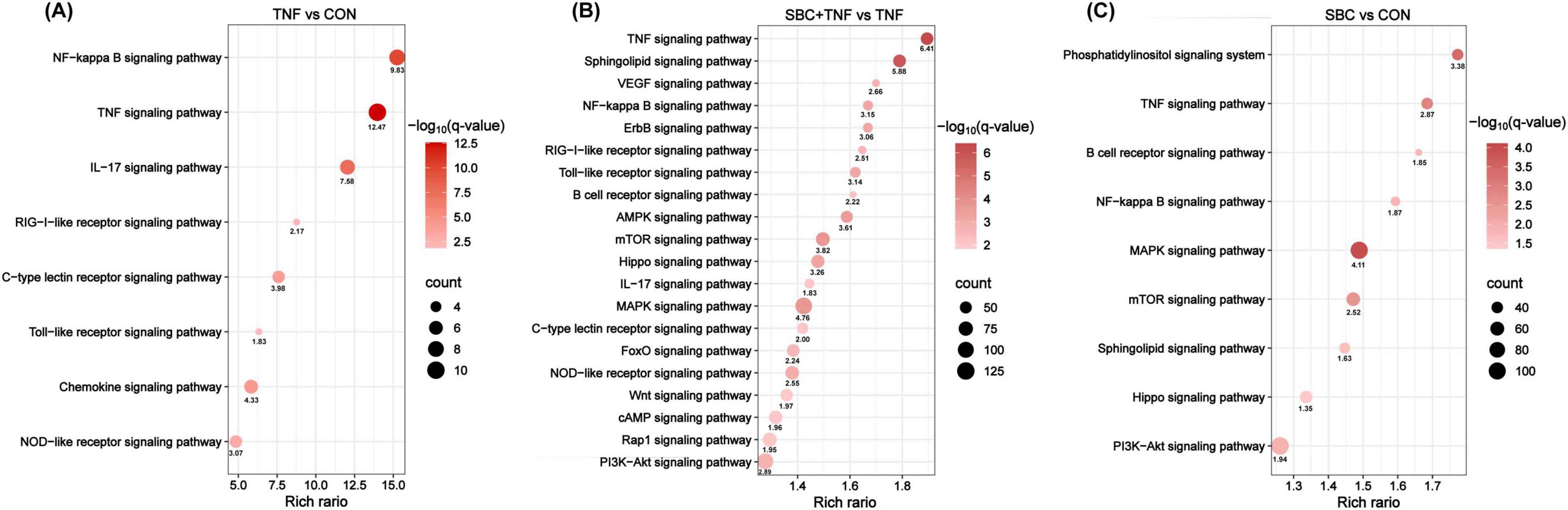
Figure 2. KEGG pathway enrichment analysis of signaling and immune-related pathways between groups: (A) TNF versus CON. (B) SBC + TNF versus TNF. (C) SBC versus CON. n = 4 replicates/group.
Analysis of pathway enrichment revealed significant involvement of both NF-κB and TNF signaling pathways across all three experimental comparisons, suggesting potential modulation of TNF-α-induced inflammatory responses by L. reuteri SBC5-3 through these molecular pathways. In the TNF versus CON group, DEGs analysis identified 27 significantly altered genes in HT-29 cells following TNF-α treatment, comprising 24 upregulated and 3 downregulated transcripts (Figure 3A). Among these 27 DEGs, 14 were functionally associated with TNF and/or NF-κB signaling pathways, all exhibiting upregulated expression, which confirms successful induction of inflammatory response by TNF-α in HT-29 cells. Importantly, administration of L. reuteri SBC5-3 resulted in downregulation of 9 out of these 14 inflammation-related genes, namely C-X-C motif chemokine ligand (CXCL) 1, CXCL10, IL-8, C-C motif chemokine ligand 20 (CCL20), baculoviral IAP repeat containing 3 (BIRC3), prostaglandin-endoperoxide synthase 2 (PTGS2), tumor necrosis factor alpha induced protein 3 (TNFAIP3), lymphotoxin beta (LTB), and colony stimulating factor 1 (CSF1), with IL-8, CXCL10, BIRC3, and PTGS2 exhibited significant downregulation (P < 0.05) (Figure 3B). Functional analysis identified predominant involvement of these genes in the NF-κB signaling pathway, where multiple components function as downstream effectors. This observation implies that L. reuteri SBC5-3 potentially regulates inflammation responses through modulation of NF-κB signaling cascade. This finding justify additional research to elucidate the precise mechanisms by which SBC5-3 influences gene expression within the NF-κB signaling pathway.
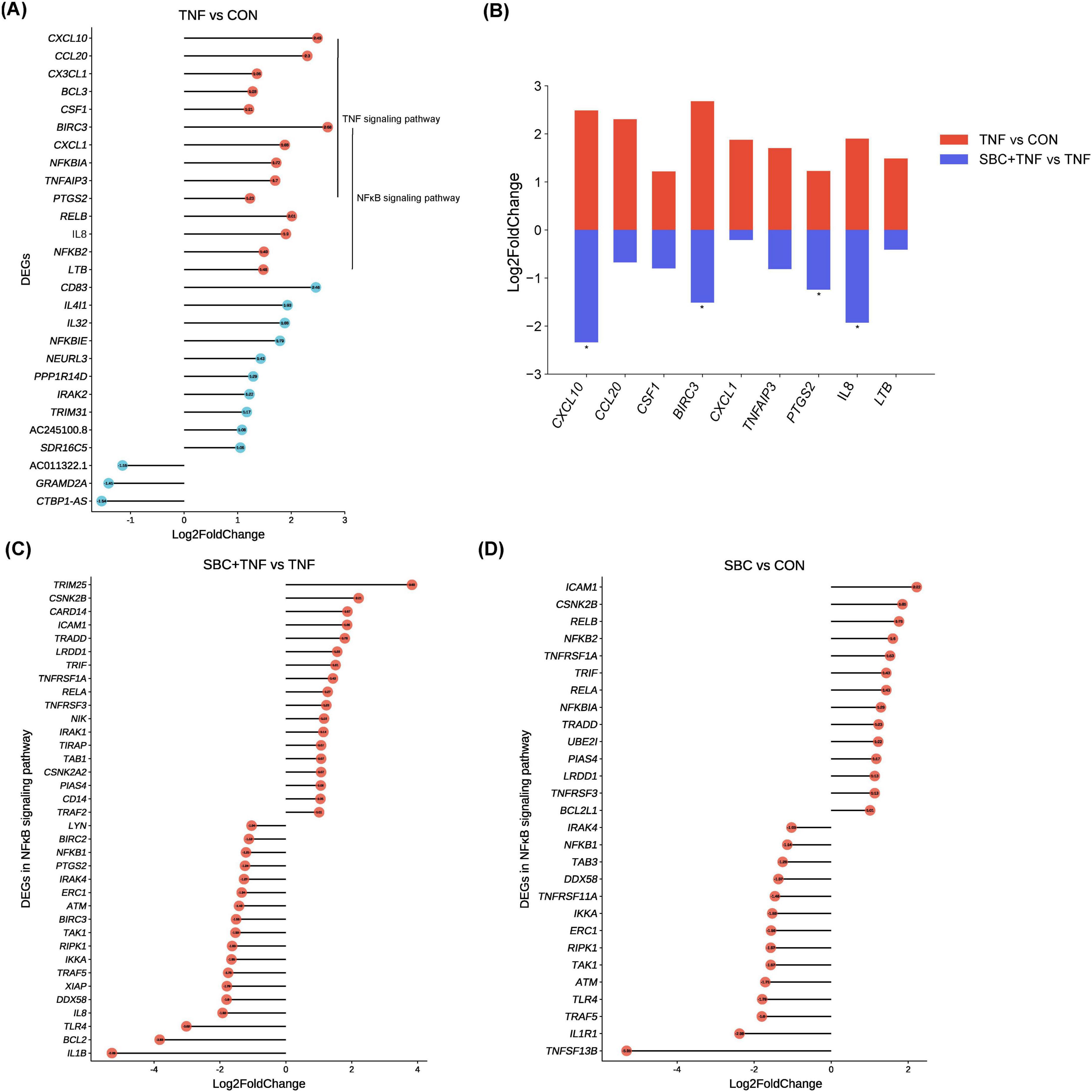
Figure 3. Changes in DEGs between groups: (A) Changes in all DEGs in the TNF versus CON group. (B) Changes in gene expression induced by TNF-α in HT-29 cells co-cultured with L. reuteri SBC5-3. (C) Changes in DEGs in the NF-κB signaling pathway in the SBC + TNF versus TNF group. (D) Changes in DEGs in the NF-κB signaling pathway in the SBC versus CON group. n = 4 replicates/group; In panel (B), all genes shown in red for the TNF vs. CON comparison are DEGs, while for the SBC + TNF vs. TNF comparison, the genes marked with an asterisk “*” are DEGs.
Analysis revealed 36 DEGs (18 upregulated and 18 downregulated) in the NF-κB signaling pathway when comparing SBC + TNF versus TNF conditions (Figure 3C). Similarly, the SBC versus CON comparison showed 29 DEGs (14 upregulated and 15 downregulated) within the same pathway (Figure 3D). Notably, TAK1, IKKa, and NFKB1 expression exhibited significant downregulation in both experimental comparisons, representing key components of the NF-κB signaling cascade. Additionally, the SBC + TNF versus TNF comparison demonstrated reduced expression of toll-like receptor 4 (TLR4) along with critical MAPK signaling pathway components, specifically extracellular regulated protein kinases (ERK) and c-Jun N-terminal kinase (JNK) (Supplementary Table S2). Furthermore, the SBC versus CON comparison revealed upregulated expression of NFKBIA, a negative regulator of NF-κB signaling. These observations indicate that L. reuteri SBC5-3 may differentially modulate TNF-α-induced inflammatory response in HT-29 cells through distinct molecular mechanisms.
3.1.3 Validation of transcriptome results by RT-qPCR
For validation of transcriptomic findings, eight genes from the NF-κB signaling pathway (IL-8, IL-1β, CXCL10, CCL20, NFKB1, NFKBIA, TNFAIP3, and PTGS2) were selected for RT-qPCR analysis. Figure 4 illustrate that the expression patterns of these genes observed in the DEG analysis were consistent with the trends detected by RT-qPCR. This correlation confirms the reliability of the transcriptome data.
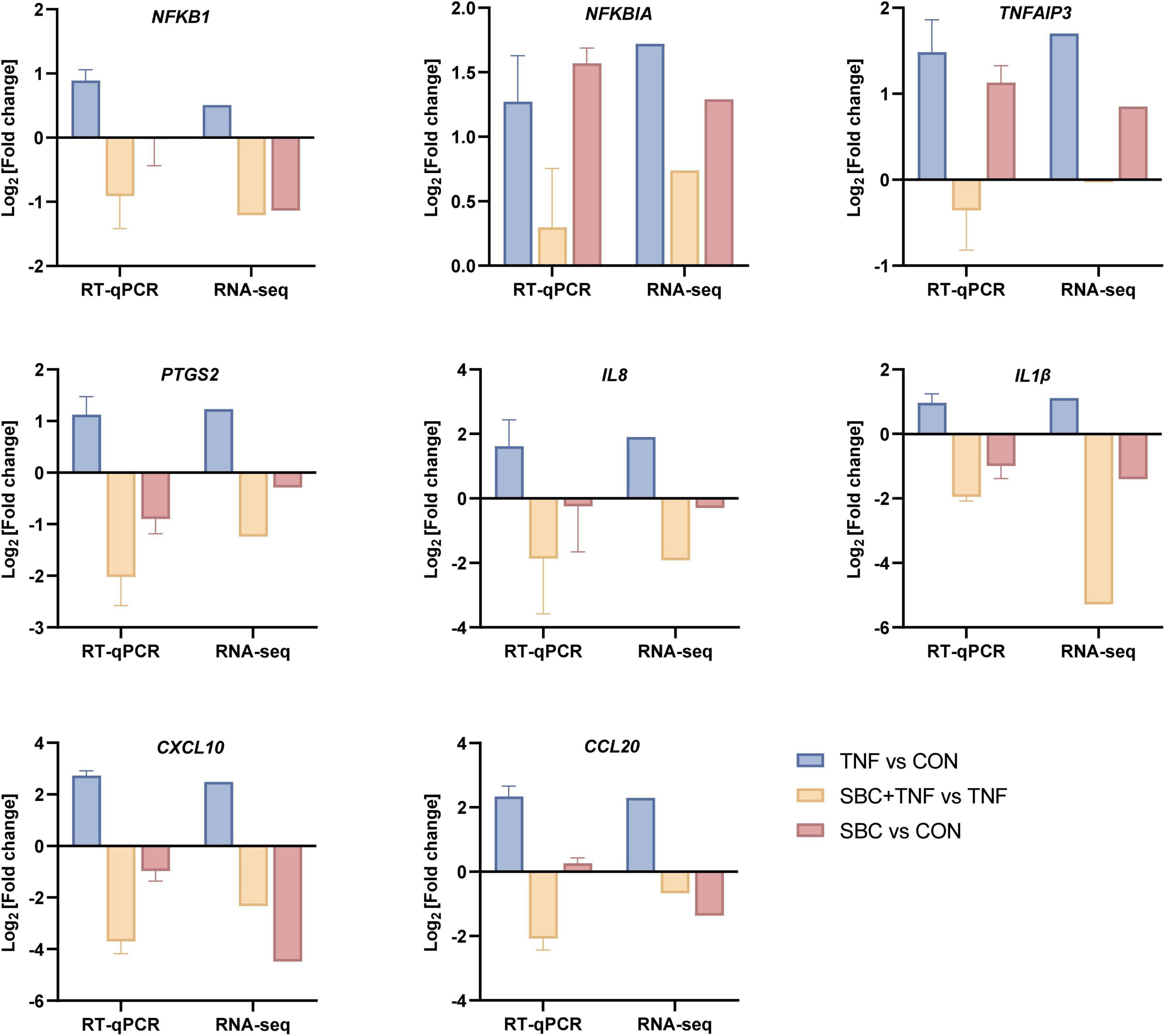
Figure 4. RT-qPCR validation of transcriptome DEG results. RT-qPCR data are presented as the mean ± SD. n = 4 replicates/group.
3.2 L. reuteri SBC5-3 modulates NF-κB signaling pathway activation in TNF-α-induced cells
3.2.1 Effect of L. reuteri SBC5-3 on phosphorylation of upstream kinases in the NF-κB Pathway in TNF-α-induced cells
Quantitative analysis revealed an increased p-TAK1/TAK1 ratio in HT-29 cells at 5 and 15 min after TNF-α treatment compared to CON group (P > 0.05; Figure 5). In contrast, the SBC5-3 group showed no significant difference in the p-TAK1/TAK1 ratio at these time points (P > 0.05). When compared to the TNF-α group, the p-TAK1/TAK1 ratio in the SBC5-3 + TNF-α group decreased at 5 min (P > 0.05) and was significantly reduced at 15 min (P < 0.05). These data indicate that 15-min TNF-α exposure enhances TAK1 phosphorylation in HT-29 cells, while 16-h pre-incubation with L. reuteri SBC5-3 effectively mitigates this TNF-α-induced TAK1 phosphorylation response.
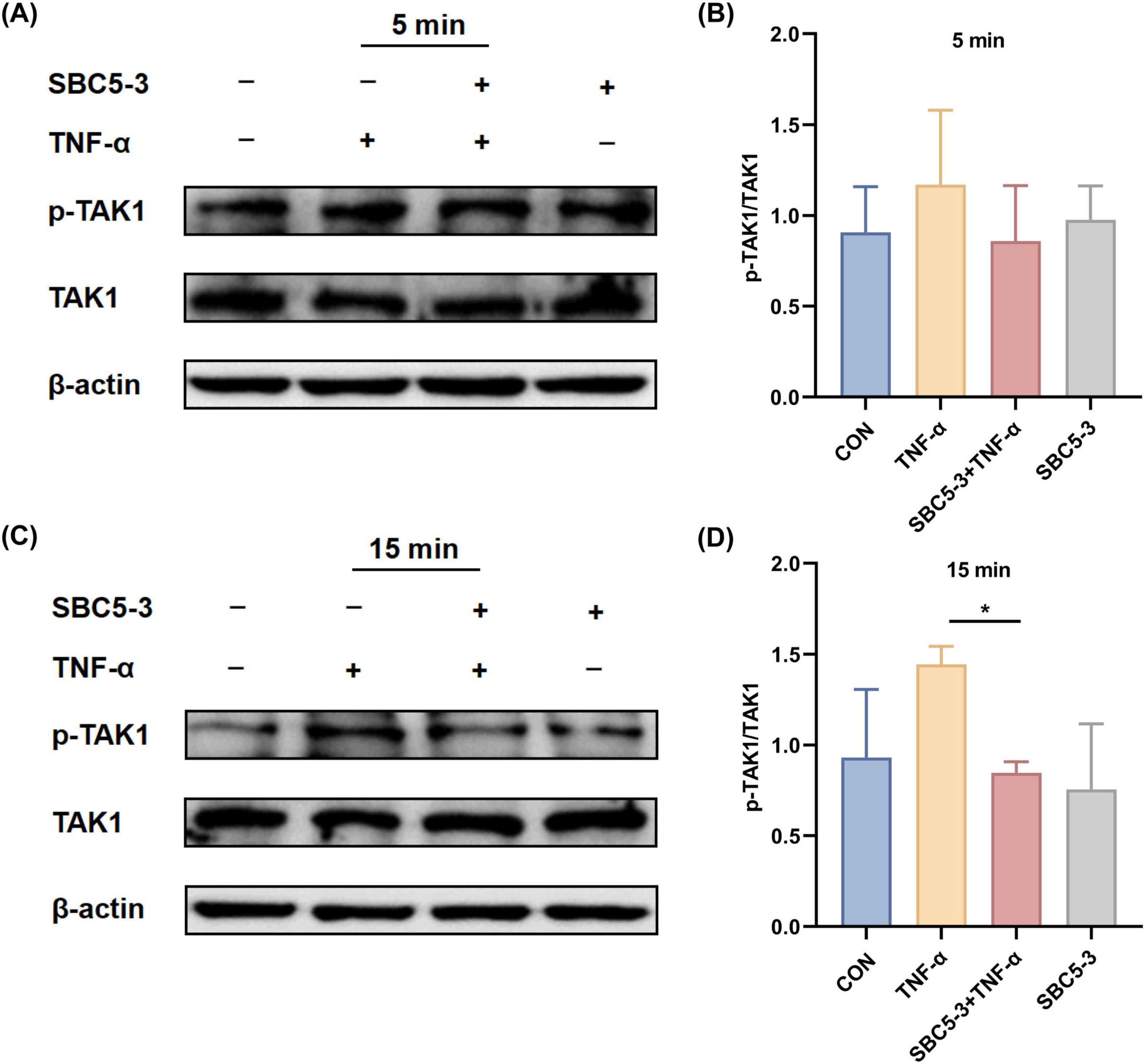
Figure 5. Effect of L. reuteri SBC5-3 on TNF-α-induced TAK1 phosphorylation in HT-29 cells. (A,B) TNF-α treatment for 5 min. (C,D) TNF-α treatment for 15 min. The “*” indicates a significant difference (P < 0.05); n = 4 replicates/group.
3.2.2 Effect of L. reuteri SBC5-3 on TNF-α-induced NF-κB pathway IκB kinase activation in cells
The effect of L. reuteri SBC5-3 on TNF-α-induced phosphorylation of IκB kinase IKKα/β in HT-29 cells is as follows. Compared to the CON group, TNF-α stimulation induced an increasing trend in the p-IKKα/β to IKKα/β ratio at 5 and 15 min (P > 0.05; Figure 6). In the SBC5-3 group, no significant changes were observed in the p-IKKα/β to IKKα/β ratio at these time points (P > 0.05). Notably, co-treatment with SBC5-3 + TNF-α demonstrated a decreasing trend in the p-IKKα/β to IKKα/β ratio at 5 and 15 min (P > 0.05). These results indicate that TNF-α promotes IκB kinase activation through enhanced IKKα/β phosphorylation, while 16-h pre-incubation with L. reuteri SBC5-3 suppresses this TNF-α-induced IκB kinase activation and reduces IKKα/β phosphorylation.
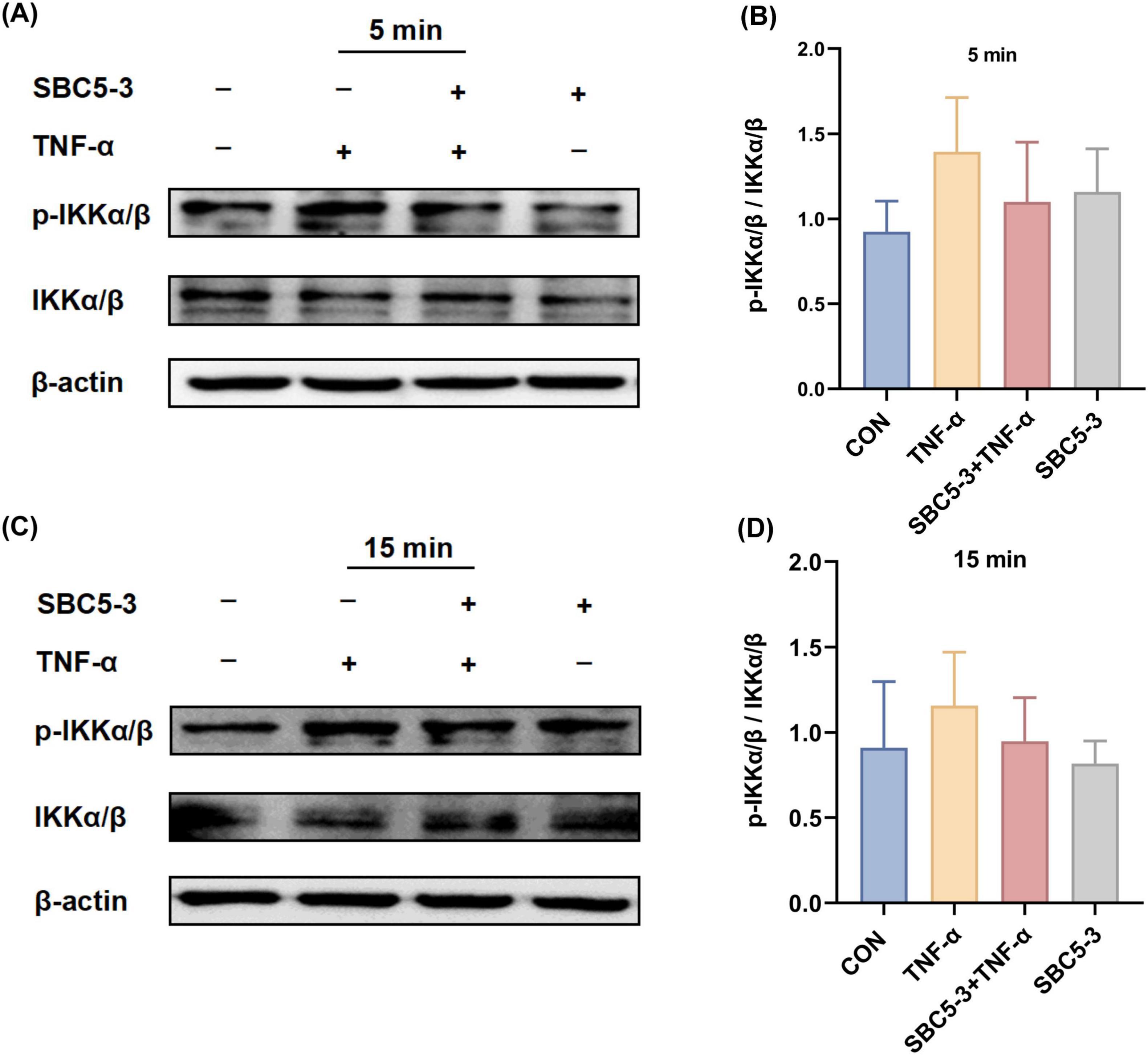
Figure 6. Effect of L. reuteri SBC5-3 on TNF-α-induced phosphorylation of IKKα/β in HT-29 cells. (A,B) TNF-α treatment for 5 min. (C,D) TNF-α treatment for 15 min. n = 4 replicates/group.
3.2.3 Effect of L. reuteri SBC5-3 on phosphorylation and degradation of NF-κB inhibitor IκBα in TNF-α-induced cells
The effect of L. reuteri SBC5-3 on TNF-α-induced degradation and phosphorylation of the NF-κB inhibitor IκBα in HT-29 cells is as follows. TNF-α stimulation significantly elevated the p-IκBα/IκBα ratio relative to CON at all examined timepoints (5, 15, 30, and 60 min; P < 0.05; Figure 7). In contrast, the SBC5-3 group alone did not significantly alter the p-IκBα/IκBα ratio at these timepoints (P > 0.05). Co-treatment with SBC5-3 + TNF-α group exhibited a decrease in the p-IκBα/IκBα ratio compared to TNF-α alone at all-time points (5, 15, 30, and 60 min), with statistically significant suppression observed at 30 min and 60 min (P < 0.05). These data demonstrate that TNF-α induces both IκBα degradation and phosphorylation in HT-29 cells, while 16-h pre-incubation with L. reuteri SBC5-3 attenuates these effects by preserving IκBα expression and decreasing its phosphorylation level.
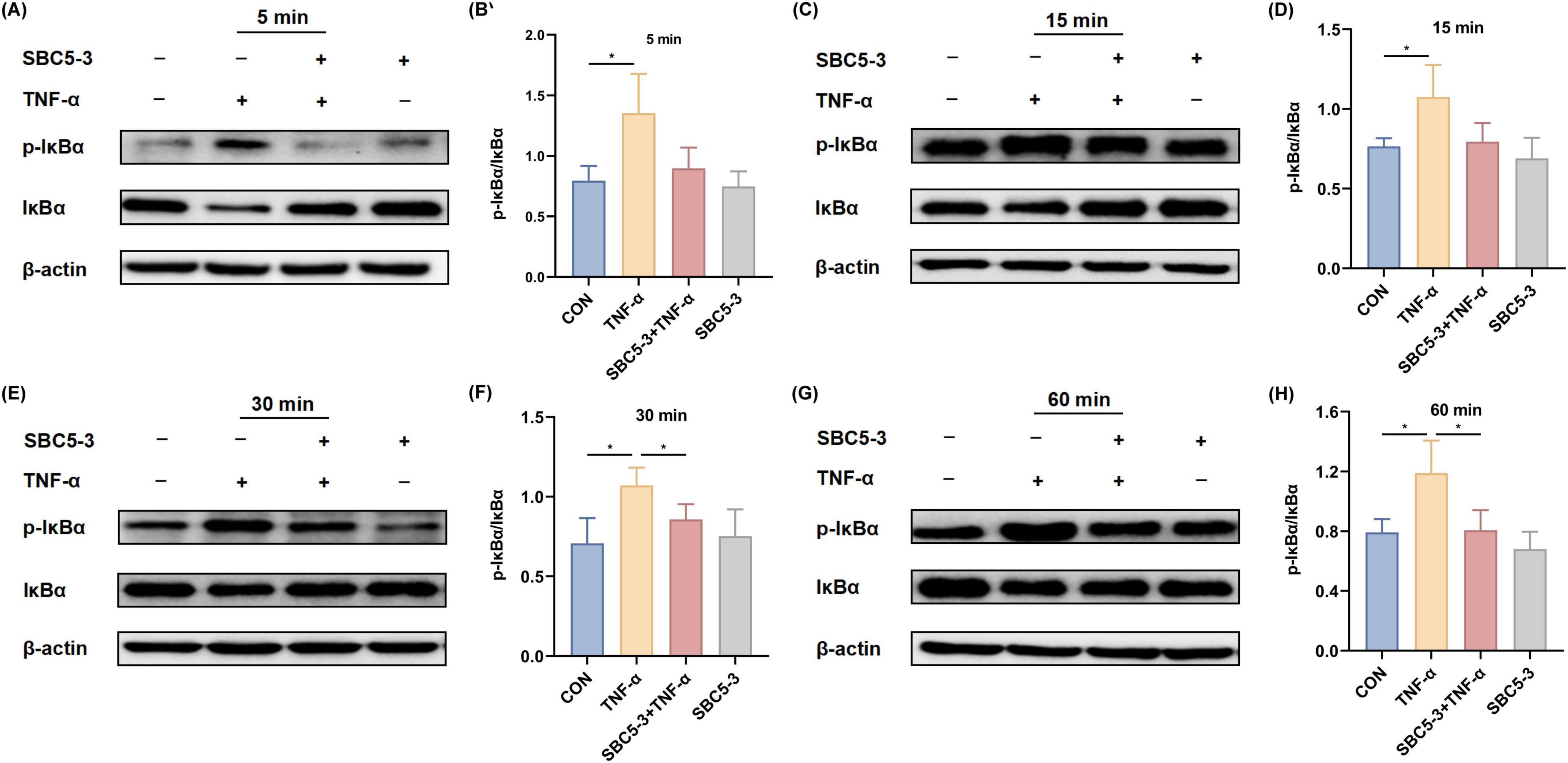
Figure 7. Effect of L. reuteri SBC5-3 on TNF-α-induced phosphorylation of IκBα in HT-29 cells. (A,B) TNF-α treatment for 5 min. (C,D) TNF-α treatment for 15 min. (E,F) TNF-α treatment for 30 min. (G,H) TNF-α treatment for 60 min. The “*” indicates a significant difference (P < 0.05); n = 4 replicates/group.
4 Discussion
The gastrointestinal tract serves as the principal immunological interface in vertebrates, critically regulating systemic immune homeostasis (Vancamelbeke and Vermeire, 2017). Modern livestock production systems, characterized by high stocking densities and frequent pathogen exposure, compromise intestinal immune competence, with consequent reductions in growth performance and economic returns from meat and egg production (Celi et al., 2017). The global restriction on AGPs has exacerbated this challenge, as existing nutritional strategies show insufficient capacity to alleviate diet-induced enteric inflammation and microbial dysbiosis (Shao et al., 2021; Wang et al., 2023). This immunological dysfunction triggers a pathogenic cascade marked by persistent mucosal inflammation, elevated pro-inflammatory cytokine expression, and progressive impairment of intestinal barrier function. These pathophysiological changes collectively undermine both production efficiency and animal welfare standards (Small et al., 2013; Teodorowicz et al., 2018). Current research reveals distinct strain-specific immunomodulatory properties of LAB, demonstrating functional divergence between pro-inflammatory and anti-inflammatory activities (Vincenzi et al., 2021). Certain strains enhance immune responses through cytokine induction, suggesting potential therapeutic applications for immunodeficiency disorders (Rocha-Ramírez et al., 2017). Conversely, other LAB strains predominantly mediate immunosuppressive effects through multiple pathways. Specifically, L. plantarum Lp01 exhibited bifunctional immunoregulation in Salmonella-infected murine models, concurrently inhibiting pro-inflammatory cytokines (including TNF-α, IL-6) while stimulating IL-10 production (Liu et al., 2023). Analogously, L. rhamnosus CY12 ameliorated intestinal inflammation through modulation of the TLR4-MyD88-NF-κB signaling pathway, leading to elevated IL-10 levels accompanied by marked decreases in IL-1β and TNF-α levels (Zheng et al., 2024). These findings highlight the importance of strain-level characterization of LAB–epithelial interactions, which proves essential for designing targeted microbiota therapies with defined immunoregulatory outcomes.
TNF-α, a pivotal pro-inflammatory cytokine, mediates both cell proliferation or induces apoptosis, playing crucial roles in inflammation and immunity (Zelová and Hošek, 2013). In HT-29 cells, TNF-α stimulation markedly elevates IL-8 production, a well-established biomarker for inflammatory model development (Sharma et al., 2007; Noh et al., 2013). Previous study (Bao et al., 2023) demonstrated that L. reuteri SBC5-3 co-culturing HT-29 cells substantially decreases TNF-α-induced IL-8 gene expression, revealing its immunomodulatory capacity. To further elucidate the underlying mechanisms, RNA-seq was performed to analyze gene expression changes in HT-29 cells following TNF-α stimulation, both in the presence and absence of L. reuteri SBC5-3 co-culture, thereby elucidating how L. reuteri SBC5-3 modulates this inflammatory response.
In the absence of TNF-α stimulation, L. reuteri SBC5-3 fails to trigger pro-inflammatory gene expression in HT-29 cells. However, TNF-α treatment induces differential expression of 27 genes in HT-29 cells, including upregulation of 14 genes functionally linked to TNF and NF-κB signaling pathways. Following L. reuteri SBC5-3 intervention, nine of these upregulated genes (BIRC3, PTGS2, CCL20, TNFAIP3, LTB, CXCL1, CXCL10, IL-8, and CSF1) exhibited significant downregulation. This observation aligns with previous studies. For instance, a study (Lin et al., 2016) reported that treating HT-29 cells with L. helveticus-fermented soybean extract decreased the expression of genes including CXCL1, CXCL2, CXCL3, LTB, IL-8, CSF1, and BIRC3, which TNF-α induced. Consistent with these results, L. fermentum 664 was shown to downregulate expression of TNF-α, CXCL6, CXCL1β, and PTGS2 in LPS-stimulated RAW264.7 macrophages (Hao et al., 2024). Chemokines including CXCL1, CXCL10, CCL20, and IL-8 demonstrate characteristic upregulation during inflammation, facilitating the recruitment of neutrophils and T cells to the affected tissues (Laing and Secombes, 2004). CSF1 stimulates monocyte and macrophage proliferation and differentiation, thereby amplifying immune response (Sehgal et al., 2021). PTGS2 catalyzes prostaglandins synthesis, generating lipid mediators that contribute substantially to inflammatory processes (Ricciotti and FitzGerald, 2011). LTB functions as a potent inflammatory mediator through binding to the lymphotoxin-beta receptor, which triggers NF-κB signaling pathwayactivation and subsequent pro-inflammatory genes transcription (Dejardin et al., 2002; Li et al., 2020). The observed downregulation of these critical inflammatory markers indicates that L. reuteri SBC5-3 potentially attenuates TNF-α-induced cellular inflammation via three distinct mechanisms: modulating immune cell recruitment and activation, reducing prostaglandin synthesis, and suppressing NF-κB signaling pathways. Notably, TNFAIP3 (A20) and BIRC3, NF-κB-responsive genes encoding negative feedback regulators, exhibited downregulation following SBC5-3 treatment. Although this observation appears paradoxical given their anti-inflammatory functions, the reduced expression indicates diminished NF-κB activation, as sustained pathway activity is necessary to maintain their transcription levels (Momtazi et al., 2019; Frazzi, 2021). This finding correlates with the observation of suppressed IκBα phosphorylation, thereby confirming NF-κB signaling inhibition.
The NF-κB signaling pathway represents a pivotal regulator of inflammation responses due to its central role in controlling inflammation-related gene expression, rapid activation kinetics, extensive biological effects, and cross-talk with other signaling pathways (Liu et al., 2017). Treatment with L. reuteri SBC5-3 downregulated multiple established direct targets of the NF-κB, including IL-8, CXCL10, BIRC3, TNFAIP3, and PTGS2. Additional genes affected by this treatment may modulate NF-κB signaling through indirect mechanisms. KEGG enrichment analysis revealed significant enrichment of the NF-κB signaling pathway across all comparison groups, indicating that L. reuteri SBC5-3 may regulate the NF-κB pathway through unique mechanisms.
The canonical NF-κB pathway is primarily regulated through a core signaling axis comprising TAK1, IKKβ, and IκBα. Initial phosphorylation of TAK1 at Thr184/187 triggers a sequential activation cascade leading to IKKβ activation phosphorylated at Ser177/181 and eventual IκBα degradation (Karin, 1999). Within TNFR1 signaling, ligand binding induces the formation of a RIPK1-TRAF2/5 complex, which mediates K63-linked polyubiquitination of RIPK1. This ubiquitin scaffold recruits the TAK1-TAB2/3 complex, enabling TAK1 autophosphorylation and kinase activation (Hacker and Karin, 2006). The activated TAK1 directly phosphorylates IKKβ, which mediates IκBα phosphorylation at Ser32/36. This phosphorylation event targets IκBα for K48-linked ubiquitination and proteasomal degradation, ultimately liberating the p50/p65 heterodimer for nuclear translocation and transcriptional activation of pro-inflammatory mediators (Hayden and Ghosh, 2004).
The current study revealed that L. reuteri SBC5-3 treatment markedly reduced expression levels of RIPK1, TRAF5, TAK1, IKKα, and NFKB1 in TNF-α-stimulated HT-29 cells. These observations indicate potential interference with TRAF2/5-RIPK1 signaling complex formation at the upstream region of the NF-κB pathway, resulting in inhibition of K63-linked polyubiquitination, an essential step for TAK1 phosphorylation and activation. Such disruption led to subsequent suppression of IKKβ phosphorylation and IκBα degradation, thereby enabling the blockade of nuclear translocation of the p50/p65 heterodimer. Furthermore, SBC5-3-mediated transcriptional downregulation of TAK1, IKKα, and NFKB1 may promote prolonged pathway suppression through depletion of these critical signaling components.
Western blot analysis revealed that L. reuteri SBC5-3 significantly suppressed phosphorylation of TAK1, IKKα/β, and IκBα in TNF-α-induced HT-29 cells, consequently mitigating the inflammatory response induced by TNF-α. The current observations align with previously reported findings. Specifically, Li et al. (2022) documented that L. plantarum 17-5 mitigated the inflammation induced by Escherichia coli in mammary epithelial cells by inhibiting IκBα phosphorylation. Similarly, Lee et al. (2015) observed that L. sakei OK67 alleviated collagen-induced arthritis in murine models via suppression of both TAK1 phosphorylation and NF-κB activation. Additionally, Chen et al. (2021) establishedthat L. plantarum KLDS 1.0344 diminished inflammatory cytokine expression in LPS-challenged mouse mammary epithelial cells by blocking p65 and IκBα phosphorylation. Our research demonstrates that immune regulation mediated by L. reuteri SBC5-3 involves the inhibition of IκBα degradation, a process essential for the nuclear translocation of p50/p65 and subsequent DNA binding (Ferreiro and Komives, 2010). This finding suggests that SBC5-3 may inhibit the nuclear translocation of the p50/p65 dimer, thereby modulating the inflammatory response. However, this hypothesis lacks direct experimental support. Future studies will employ direct methods, such as p65 immunofluorescence, to verify this mechanism.
Recent studies have documented that LAB can modulate host immune response through multiple mechanisms (Vincenzi et al., 2021). In the present investigation, L. reuteri SBC5-3 treatment markedly reduced JNK and ERK genes expression in TNF-α-induced inflammation model using HT-29 cells. As critical kinases within the MAPK pathway, JNK and ERK serve crucial functions in transducing signals that lead to the activation and nuclear translocation of transcription factors responsible for pro-inflammatory cytokine gene expression (Papa et al., 2019). These findings suggest that L. reuteri SBC5-3 potentially modulates immune response in HT-29 cells through suppression of these kinase genes.
As demonstrated in this study, L. reuteri SBC5-3 effectively blocked TNF-α-induced TAK1 phosphorylation. TAK1 functions as an upstream kinase that activates both NF-κB and MAPK signaling pathways upon phosphorylation. The observed inhibition of TAK1 phosphorylation may interfere with the upstream activation of the MAPK pathway, thereby suppressing the downstream phosphorylation of MAPK family members, including ERK, JNK, and p38 (Mihaly et al., 2014). Such inhibition may ultimately decrease pro-inflammatory cytokines transcription, alleviating inflammation and cellular damage triggered by external stimuli. Additionally, analysis revealed significant downregulation of TLR4 gene expression in both SBC5-3 + TNF-α versus TNF-α and SBC5-3 versus CON comparison groups. Similar effects were reported for Bifidobacterium infantis ATCC 15697 (Meng et al., 2016) and L. rhamnosus CCFM1120 (Niu et al., 2024). TLR4 serves as a core innate immune system receptor primarily responsible for recognizing LPS from Gram-negative bacteria. Upon activation, this receptor initiates downstream NF-κB and MAPK signaling cascades (Zamyatina and Heine, 2020). The observed reduction in TLR4 expression suggests potential therapeutic benefits for modulating Gram-negative bacteria-induced inflammatory responses.
The immunomodulatory effects of L. reuteri SBC5-3 may be associated with metabolites such as lipoteichoic acid, acetate, and histamine. Current studies indicate that lipoteichoic acid from L. paracasei 6-1 suppresses LPS-induced inflammatory responses in RAW264.7 cells by inhibiting the TLR4-MyD88-MAPK and NF-κB signaling pathways (Zhang et al., 2023). Acetate can inhibit LPS-induced NF-κB activation in RAW264.7 cells and reduce the production of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 (Liu et al., 2012). Histamine produced by L. reuteri ATCC PTA 6475 can suppress TNF production in a Pam3Cys-SKKKK-induced THP-1 cell inflammation model by modulating PKA and ERK signaling (Thomas et al., 2012). Genomic analysis of L. reuteri SBC5-3 reveals the absence of critical histamine biosynthesis genes, specifically the hdc gene cluster (hdcA, hdcB, and hdcP) (unpublished data), suggesting that its anti-inflammatory properties may operate through histamine-independent mechanisms. These findings highlight the need for subsequent investigations to evaluate the immunomodulatory potential of L. reuteri SBC5-3-derived lipoteichoic acid and metabolic byproducts, which could elucidate the strain-specific anti-inflammatory pathways.
5 Conclusion
The anti-inflammatory mechanism of L. reuteri SBC5-3 primarily involves transcriptional suppression of key regulatory genes in the NF-κB signaling pathway, including TAK1, IKKα, and NFKB1. This regulatory effect extends to phosphorylation inhibition of TAK1, IKKα/β, and IκBα, consequently disrupting the NF-κB signaling cascade by preventing both IκBα degradation. Furthermore, L. reuteri SBC5-3 appears to suppress ERK and JNK transcription in the MAPK signaling pathway while reducing TAK1 phosphorylation, thereby potentially interfering with the p38/ERK/JNK-mediated MAPK cascade. These findings provide a theoretical foundation for developing novel microbiome-based therapeutics utilizing L. reuteri SBC5-3.
Data availability statement
The raw data associated with this study are accessible through the NCBI Sequence Read Archive (SRA) database under BioProject ID PRJNA1221070. The whole-genome sequence data of Lactobacillus reuteri SBC5-3 are also accessible through the NCBI database under BioProject ID PRJNA1233652.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
SC: Formal Analysis, Methodology, Visualization, Writing – original draft. TH: Conceptualization, Data curation, Methodology, Writing – original draft. LX: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. JL: Conceptualization, Data curation, Formal Analysis, Validation, Writing – review & editing. CL: Data curation, Formal Analysis, Validation, Writing – review & editing. BZ: Data curation, Formal Analysis, Methodology, Writing – review & editing. QL: Project administration, Resources, Supervision, Writing – review & editing. ZC: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science foundation of China (Grant No. 32160809), Yunnan Provincial Reserve Talents Program for Young and Middle-Aged Academic and Technical Leaders (Grant No. 202305AC160040), Yunnan Provincial Major Science and Technology Special Project (Grant No. 202202AE090005), and Yunnan Provincial Academician Expert Workstation (Grant No. 202305AF150128).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1573479/full#supplementary-material
References
Adedokun, S. A., and Olojede, O. C. (2019). Optimizing gastrointestinal integrity in poultry: The role of nutrients and feed additives. Front. Vet. Sci. 5:348. doi: 10.3389/fvets.2018.00348
Bao, G., Hang, Y., Wang, X., Ye, P., Ye, P., Hu, T., et al. (2023). Isolation, identification and probiotic characteristics of lactic acid bacteria derived from free range local pigs in yunnan province. Chin. J. Anim. Nutr. 35, 5418–5429. doi: 10.12418/CJAN2023.499
Bell, H. N., Rebernick, R. J., Goyert, J., Singhal, R., Kuljanin, M., Kerk, S. A., et al. (2022). Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell 40, 185–200.e6. doi: 10.1016/j.ccell.2021.12.001.
Brown, K., Uwiera, R. R., Kalmokoff, M. L., and Brooks, S. P. (2017). Antimicrobial growth promoter use in livestock: A requirement to understand their modes of action to develop effective alternatives. Int. J. Antimicrob. Agents 49, 12–24. doi: 10.1016/j.ijantimicag.2016.08.006
Celi, P., Cowieson, A., Fru-Nji, F., Steinert, R., Kluenter, A.-M., and Verlhac, V. J. (2017). Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed Sci. Technol. 234, 88–100. doi: 10.1016/j.anifeedsci.2017.09.012
Chen, Q., Wang, S., Guo, J., Xie, Q., Evivie, S. E., Song, Y., et al. (2021). The protective effects of Lactobacillus plantarum KLDS 1.0344 on LPS-induced mastitis in vitro and in vivo. Front. Immunol. 12:770822. doi: 10.3389/fimmu.2021.770822
Cheng, F., Zhang, K., Yang, X., Shi, L., Wei, Y., Wang, D., et al. (2024). Multi-omics analysis reveals the mechanism of Lacticaseibacillus paracasei IMAUJBC1 in alleviating hyperlipidemia. J. Funct. Foods 114:106079. doi: 10.1016/j.jff.2024.106079
Dejardin, E., Droin, N. M., Delhase, M., Haas, E., Cao, Y., Makris, C., et al. (2002). The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity 17, 525–535. doi: 10.1016/S1074-7613(02)00423-5
Devi, S. M., Kurrey, N. K., and Halami, P. M. (2018). In vitro anti-inflammatory activity among probiotic Lactobacillus species isolated from fermented foods. J. Funct. Foods 47, 19–27. doi: 10.1016/j.jff.2018.05.036
Ferreiro, D. U., and Komives, E. A. (2010). Molecular mechanisms of system control of NF-κB signaling by IκBα. Biochemistry 49, 1560–1567. doi: 10.1021/bi901948j
Frazzi, R. J. (2021). BIRC3 and BIRC5: Multi- faceted inhibitors in cancer. Cell Biosci. 11:8. doi: 10.1186/s13578-020-00521-0
Hacker, H., and Karin, M. J. (2006). Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006:re13. doi: 10.1126/stke.3572006re13
Hao, H., Nie, Z., Wu, Y., Liu, Z., Luo, F., Deng, F., et al. (2024). Probiotic characteristics and anti-inflammatory effects of Limosilactobacillus fermentum 664 isolated from chinese fermented pickles. Antioxidants 13:703. doi: 10.3390/antiox13060703
Hayden, M. S., and Ghosh, S. J. (2004). Signaling to NF-κB. Genes Dev. 18, 2195–2224. doi: 10.1101/gad.1228704
Hou, Y., Li, X., Liu, X., Zhang, Y., Zhang, W., Man, C., et al. (2019). Transcriptomic responses of Caco-2 cells to Lactobacillus rhamnosus GG and Lactobacillus plantarum J26 against oxidative stress. J. Dairy Sci. 102, 7684–7696. doi: 10.3168/jds.2019-16332
Jackson, D. J., Cerveau, N., and Posnien, N. J. (2024). De novo assembly of transcriptomes and differential gene expression analysis using short-read data from emerging model organisms–a brief guide. Front. Zool. 21:17. doi: 10.1186/s12983-024-00538-y
Karin, M. (1999). The beginning of the end: IκB kinase (IKK) and NF-κB activation. J. Biol. Chem. 274, 27339–27342. doi: 10.1074/jbc.274.39.27339
Laing, K. J., and Secombes, C. J. (2004). Chemokines. Dev. Comp. Immunol. 28, 443–460. doi: 10.1016/j.dci.2003.09.006
Lee, S.-Y., Jeong, J.-J., Kim, K.-A., and Kim, D.-H. (2015). Lactobacillus sakei OK67 ameliorates collagen-induced arthritis in mice by inhibiting NF-κB activation and restoring Th17/Treg cell balance. J. Funct. Foods 18, 501–511. doi: 10.1016/j.jff.2015.08.006
Li, K., Yang, M., Tian, M., Jia, L., Du, J., Wu, Y., et al. (2022). Lactobacillus plantarum 17–5 attenuates Escherichia coli-induced inflammatory responses via inhibiting the activation of the NF-κB and MAPK signalling pathways in bovine mammary epithelial cells. BMC Vet. Res. 18:250. doi: 10.1186/s12917-022-03355-9
Li, Q. S., Tian, C., and Hinds, D. (2020). Genome-wide association studies of antidepressant class response and treatment-resistant depression. Transl. Psychiatry 10:360. doi: 10.1038/s41398-020-01035-6
Li, X., Li, W., Zhao, L., Li, Y., He, W., Ding, K., et al. (2024). Characterization and assessment of native lactic acid bacteria from broiler intestines for potential probiotic properties. Microorganisms 12:749. doi: 10.3390/microorganisms12040749
Lin, Q., Mathieu, O., Tompkins, T. A., Buckley, N. D., and Green-Johnson, J. M. (2016). Modulation of the TNFα-induced gene expression profile of intestinal epithelial cells by soy fermented with lactic acid bacteria. J. Funct. Foods 23, 400–411. doi: 10.1016/j.jff.2016.02.047
Liu, R.-H., Sun, A.-Q., Liao, Y., Tang, Z.-X., Zhang, S.-H., Shan, X., et al. (2023). Lactiplantibacillus plantarum regulated intestinal microbial community and cytokines to inhibit Salmonella typhimurium infection. Probiot. Antimicrob. Proteins 15, 1355–1370. doi: 10.1007/s12602-022-09987-5
Liu, T., Li, J., Liu, Y., Xiao, N., Suo, H., Xie, K., et al. (2012). Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264. 7 cells. Inflammation 35, 1676–1684. doi: 10.1007/s10753-012-9484-z
Liu, T., Zhang, L., Joo, D., and Sun, S.-C. (2017). NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2:17023. doi: 10.1038/sigtrans.2017.23
Liu, X., Miller, G. Y., and McNamara, P. E. (2005). Do antibiotics reduce production risk for US pork producers? J. Agric. Appl. Econ. 37, 565–575. doi: 10.1017/S1074070800027085
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Meng, D., Zhu, W., Ganguli, K., Shi, H. N., and Walker, W. (2016). Anti-inflammatory effects of Bifidobacterium longum subsp infantis secretions on fetal human enterocytes are mediated by TLR-4 receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G744–G753. doi: 10.1152/ajpgi.00090.2016
Mihaly, S., Ninomiya-Tsuji, J., and Morioka, S. (2014). TAK1 control of cell death. Cell Death Differ. 21, 1667–1676. doi: 10.1038/cdd.2014.123
Miyakawa, M. E. F., Casanova, N. A., and Kogut, M. H. (2024). How did antibiotic growth promoters increase growth and feed efficiency in poultry? Poult. Sci. 103:103278. doi: 10.1016/j.psj.2023.103278
Momtazi, G., Lambrecht, B. N., Naranjo, J. R., and Schock, B. C. (2019). Regulators of A20 (TNFAIP3): New drug-able targets in inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 316, L456–L469. doi: 10.1152/ajplung.00335.2018
Niewold, T. A. (2007). The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult. Sci. 86, 605–609. doi: 10.1093/ps/86.4.605
Niu, B., Feng, Y., Cheng, X., Xiao, Y., Zhao, J., Lu, W., et al. (2024). The alleviative effects of viable and inactive Lactobacillus paracasei CCFM1120 against alcoholic liver disease via modulation of gut microbiota and the Nrf2/HO-1 and TLR4/MyD88/NF-κB pathways. Food Funct. 15, 8797–8809. doi: 10.1039/D4FO02592J
Noh, M. K., Jung, M., Kim, S. H., Lee, S. R., Park, K. H., Kim, D. H., et al. (2013). Assessment of IL-6, IL-8 and TNF-α levels in the gingival tissue of patients with periodontitis. Exp. Ther. Med. 6, 847–851. doi: 10.3892/etm.2013.1222
Papa, S., Choy, P. M., and Bubici, C. (2019). The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 38, 2223–2240. doi: 10.1038/s41388-018-0582-8
Ricciotti, E., and FitzGerald, G. A. (2011). Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 986–100000. doi: 10.1161/ATVBAHA.110.207449
Rocha-Ramírez, L., Pérez-Solano, R., Castañón-Alonso, S., Moreno Guerrero, S., Ramírez Pacheco, A., García Garibay, M., et al. (2017). Probiotic Lactobacillus strains stimulate the inflammatory response and activate human macrophages. J. Immunol. Res. 2017:4607491. doi: 10.1155/2017/4607491
Sehgal, A., Irvine, K. M., and Hume, D. A. (2021). Functions of macrophage colony-stimulating factor (CSF1) in development, homeostasis, and tissue repair. Semin. Immunol. 54:101509. doi: 10.1016/j.smim.2021.101509
Shao, Y., Wang, Y., Yuan, Y., and Xie, Y. (2021). A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 798:149205. doi: 10.1016/j.scitotenv.2021.149205
Sharma, A., Satyam, A., and Sharma, J. B. (2007). Leptin, IL- 10 and inflammatory markers (TNF-α, IL- 6 and IL- 8) in pre- eclamptic, normotensive pregnant and healthy non- pregnant women. Am. J. Reprod. Immunol. 58, 21–30. doi: 10.1111/j.1600-0897.2007.00486.x
Small, C.-L. N., Reid-Yu, S. A., McPhee, J. B., and Coombes, B. K. (2013). Persistent infection with Crohn’s disease-associated adherent-invasive Escherichia coli leads to chronic inflammation and intestinal fibrosis. Nat. Commun. 4:1957. doi: 10.1038/ncomms2957
Teodorowicz, M., Hendriks, W. H., Wichers, H. J., and Savelkoul, H. F. J. (2018). Immunomodulation by processed animal feed: The role of maillard reaction products and advanced glycation end-products (AGEs). Front. Immunol. 9:2088. doi: 10.3389/fimmu.2018.02088
Thomas, C. M., Hong, T., Van Pijkeren, J. P., Hemarajata, P., Trinh, D. V., Hu, W., et al. (2012). Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 7:e31951. doi: 10.1371/journal.pone.0031951
Vancamelbeke, M., and Vermeire, S. (2017). The intestinal barrier: A fundamental role in health and disease. Exp. Rev. Gastroenterol. Hepatol. 11, 821–834. doi: 10.1080/17474124.2017.1343143
Vieco-Saiz, N., Belguesmia, Y., Raspoet, R., Auclair, E., Gancel, F., Kempf, I., et al. (2019). Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Immunol. 10:57. doi: 10.3389/fmicb.2019.00057
Vincenzi, A., Goettert, M. I., and de Souza, C. F. V. (2021). An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-α) signaling and gene expression. Cytokine Growth Factor Rev. 57, 27–38. doi: 10.1016/j.cytogfr.2020.10.004
Wang, L., Li, W., Xin, S., Wu, S., Peng, C., Ding, H., et al. (2023). Soybean glycinin and β-conglycinin damage the intestinal barrier by triggering oxidative stress and inflammatory response in weaned piglets. Eur. J. Nutr. 62, 2841–2854. doi: 10.1007/s00394-023-03188-8
Wang, L., Zhou, H., He, R., Xu, W., Mai, K., and He, G. (2016). Effects of soybean meal fermentation by Lactobacillus plantarum P8 on growth, immune responses, and intestinal morphology in juvenile turbot (Scophthalmus maximus L.). Aquaculture 464, 87–94. doi: 10.1016/j.aquaculture.2016.06.026
Zamyatina, A., and Heine, H. J. (2020). Lipopolysaccharide recognition in the crossroads of TLR4 and caspase-4/11 mediated inflammatory pathways. Front. Immunol. 11:585146. doi: 10.3389/fimmu.2020.585146
Zelová, H., and Hošek, J. (2013). TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 62, 641–651. doi: 10.1007/s00011-013-0633-0
Zhang, L., Liu, J., Kong, S., Chen, N., Hung, W.-L., Zhao, W., et al. (2023). Lipoteichoic acid obtained from Lactobacillus paracasei via low-temperature pasteurization alleviates the macrophage inflammatory response by downregulating the NF-κB signaling pathway. J. Funct. Foods 107:105673. doi: 10.1016/j.jff.2023.105673
Zheng, J., Ahmad, A. A., Yang, C., Liang, Z., Shen, W., Liu, J., et al. (2024). Orally administered Lactobacillus rhamnosus CY12 alleviates DSS-Induced colitis in mice by restoring the intestinal barrier and inhibiting the TLR4-MyD88-NF-κB pathway via intestinal microbiota modulation. J. Agric. Food Chem. 72, 9102–9116. doi: 10.1021/acs.jafc.3c07279
Keywords: Lactobacillus reuteri, nuclear factor-κB signaling, tumor necrosis factor-alpha, anti-inflammatory, intestinal epithelial cells
Citation: Chen S, Hu T, Xu L, Li J, Liu C, Zeng B, Lin Q and Cao Z (2025) Lactobacillus reuteri SBC5-3 suppresses TNF-α-induced inflammatory responses via NF-κB pathway inhibition in intestinal epithelial cells. Front. Microbiol. 16:1573479. doi: 10.3389/fmicb.2025.1573479
Received: 10 February 2025; Accepted: 10 June 2025;
Published: 08 July 2025.
Edited by:
Sabina Fijan, University of Maribor, SloveniaReviewed by:
Yafei Duan, South China Sea Fisheries Research Institute, ChinaJinhui Li, Saint Louis University, United States
Copyright © 2025 Chen, Hu, Xu, Li, Liu, Zeng, Lin and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuye Lin, bGlucWl1eWVAMTI2LmNvbQ==; Zhenhui Cao, Y3pobHF5QDEyNi5jb20=
†These authors have contributed equally to this work
 Shiyu Chen
Shiyu Chen Tiannian Hu1†
Tiannian Hu1† Chen Liu
Chen Liu