- 1Pathogenic Yeast Research Group, Department of Microbiology and Biochemistry, University of the Free State, Bloemfontein, South Africa
- 2Department of Microbiology, Faculty of Biological Sciences, Abia State University, Uturu, Nigeria
Background: The impact of climate change and increasing global temperature is contributing to the emergence of unknown and the reemergence of known fungal pathogens.
Main body: In the process of adapting to the increasing global temperatures, some fungi have evolved multidrug resistance traits thus narrowing the therapeutic options in our antifungal arsenal. Interestingly, all emerging fungal pathogens and known emergent fungi are multidrug and pandrug resistant, suggesting that the drug resistance traits observed are partly due to thermal stress-induced cross stress-responses. This paper argues that the acquired drug resistance traits may also be driving increased virulence and adaptation to infection-related conditions, resulting in the outbreak of community and hospital infections.
Conclusion: Continued surveillance and more research are required to enhance our understanding of the impact of heat-induced evolution of antifungal resistance in this rapidly evolving area of research that may define a new era in medical mycology.
Introduction
For the vast majority of fungi, the inability to grow at elevated temperatures limits their capacity to infect human. Fungal pathogens common to immunologically competent human were rather those associated with superficial mycoses—tinea, oral thrush and (vulvovaginal candidiasis) VVC—which are non-fatal and amenable to antifungal therapies (Seagle et al., 2021). However, invasive human mycoses became common in the past three decades because of diseases that weaken the immune system such as diabetes and advanced medical interventions (Casadevall, 2018). Examples include the use of indwelling devices, and cancer therapies that impair the body’s immunity.
Currently, global warming is giving rise to extensive variation in temperatures that can alter cellular metabolism and genetic makeup of an organism (Seidel et al., 2024). These temperatures are higher (by about 2°C) than in the past two centuries with significantly hotter days that can act as selection events for the survival of certain organisms, altering membrane fluidity, mobilize heat shock proteins (Hsps), and cell surface remodeling enzymes and induce rapid mutagenesis leading to thermotolerance, thus eliminate environmental temperature difference with endotherms (Gong et al., 2017; Heilmann et al., 2013; Huang et al., 2024). This has remained increasingly worrisome.
Opinion
The emergence of multidrug resistant Candidozyma auris has been a scientific mystery, especially, its independent occurrence in three continents where the fungus caused candidiasis (Lockhart et al., 2017). Though the mechanism(s) responsible for this is yet understood, many scholars have offered opinions on this phenomenon. While some of these opinions may differ, for example those providing evidence for (Casadevall et al., 2019) or against (Money, 2024) thermal stress as a contributing factor in the emergence and reemergence of fungal pathogens, they offer areas of potential continuing research. Data implicating thermal stress is compelling indicating that, climatic (Mora et al., 2022) or endothermic (Huang et al., 2024; Schwiesow et al., 2024; Casadevall, 2023) thermal stress adaptation is at least impacting the expression of virulence traits whether novel or latent in otherwise benign fungi. What we do not also understand yet is the extent to which this is occurring, and evidence suggests at a rather worrisome rate. An interesting argument is that emergent fungi acquire resistance traits in their evolutionary path which may entirely be environmentally induced (thermotolerance) before the opportunity to cause outbreaks through cross-stress advantages (Figure 1). For example, C. auris strains with fluconazole minimum inhibitory concentration (MIC) of >256 μg/mL, and amphotericin B MIC of 2–4 μg/mL have been isolated in the Andaman Island, India, in habitats without any human activities indicating its prior existence as a drug resistant environmental fungus (Arora et al., 2021). The survival of C. auris in wetland shows its tolerance to harsh environment (Casadevall et al., 2019). Microorganisms thermal stress adaptation alone may not fully explain the emergence of fungal pathogens. A combination of factors including the impact of thermal stress on host immune response which remains to be understood may also be at play. However, the ability to overcome the core human body temperature is important both in the development of drug resistance and expression of virulence traits (novel or latent) and in the ability to infect sufficiently immunocompromised hosts.
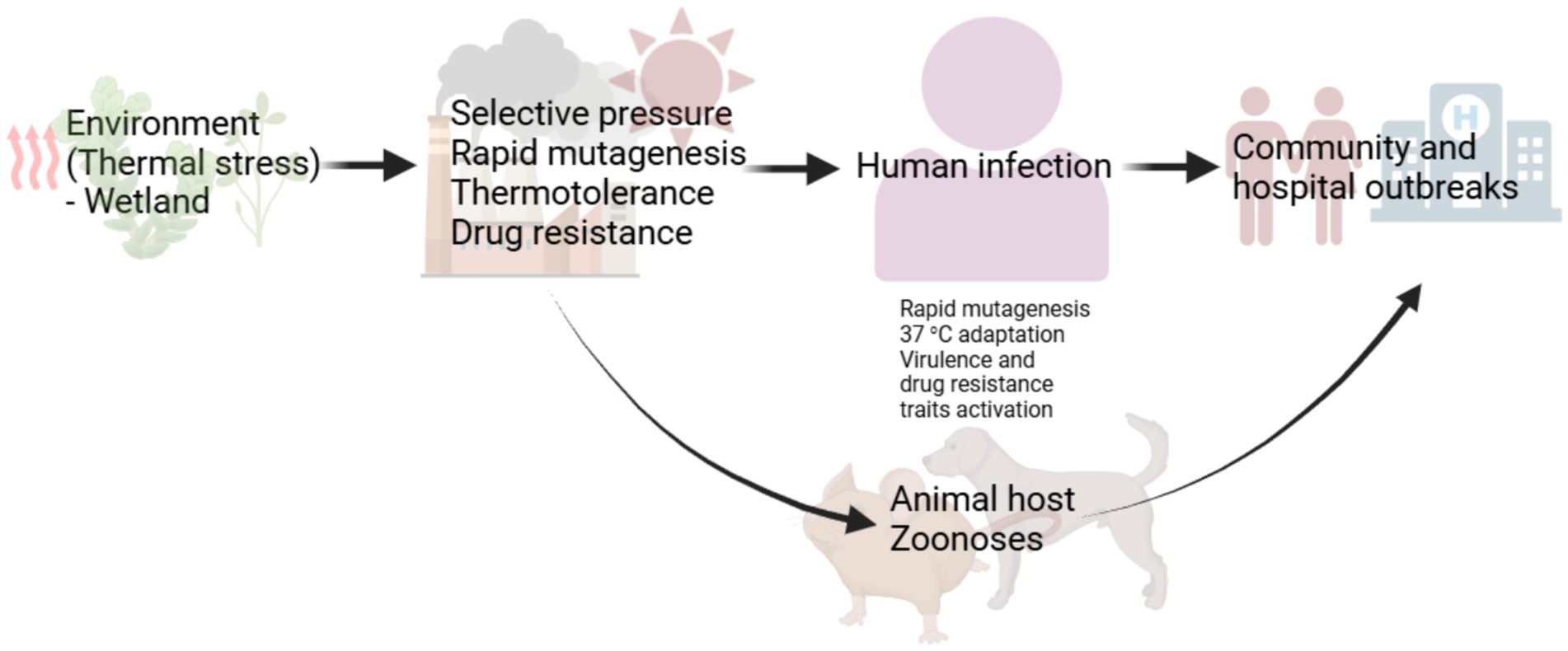
Figure 1. A proposed evolutionary path for emerging fungal pathogens. Emergent fungi become drug resistant before emerging in the community or causing hospital outbreaks. Thermal stress responses including the activation of heat shock proteins recruit transcription factors regulating salvage response pathways involved in drug tolerance and drug resistance. This may account for the emergence of drug resistant Candidozyma auris, Rhodosporidiobulus fluvialis and Candida khanbhai. The development of drug resistance may also follow the accumulation of mutation, due to thermal stress, that confer drug resistance through cross-stress advantages. Cross-stress responses can ultimately trigger the expression of latent or novel pathogenicity traits in the course of infection. Initial endothermic stress adaptation can induce hypervirulence and multidrug resistance traits development and expression.
Evidence supporting the independent emergence of drug resistance
Drug resistance can emerge in fungal pathogens without prior exposure to antifungal drugs or their analogues. Data has shown no correlation with azole agricultural use and the emergence of multidrug resistant C. auris; also, the emergence of azole-resistance in Candida spp. predates the emergence of the ‘auris’ species. Azole and polyene resistance in C. haemolunii var. vulnera a close relative of C. auris, without prior antifungal exposure, was shown to likely be due to Erg11 variant (Muro et al., 2012; Rodrigues et al., 2020). In addition, a survey of antimicrobial susceptibility profile of 1,698 reference fungal strains showed that fungal species recovered from fruits and insects already are resistant to major classes of antifungals with several showing pandrug resistance traits (Desnos-Ollivier et al., 2012). This is consistent with the data from the semi-arid Eastern Karoo climate region of South Africa, where C. auris isolated from the gut of Locustana pardalina (locust) were fluconazole resistant (32 μg/mL), survived growth at 50°C and tolerated 15% NaCl. The interesting thing here is that the locusts were collected from a remote rural area consisting of large private grazing with limited human activity suggesting that metabolic activities of thermotolerant insects can select for drug resistance traits in fungi.
Evidence implicating thermal stress response genes and pathways in drug resistance and virulence
It has been argued that acquisition of drug resistance can occur independent of virulence, because they are controlled by different cell properties and simultaneous occurrence can lead to fitness defects (Vincent et al., 2013). Studies have shown that Hsps play a role in adaptation to heat stress. Heat stress responses by Hsps are generally regulated by an essential transcription factor, Hsf1. Hsf1 and transcription factors regulating cell surface stress-activated protein kinase pathways: Hog1, Mkc1 and Cek1, are thought to be Hsps (Hsp90) client proteins; with all potentially involved in thermotolerance in Candida spp. (Ibe and Munro, 2021). It can be argued that this heat shock-stress response regulatory circuit partly explains the emergence of a drug resistant fungal pathogen. The evolutionarily conserved Hog1 pathway linked to virulence in Cryptococcus neoformans has been shown to promote stress response and virulence in C. auris (Day et al., 2018), indicating a link between adaptation and virulence. In Aspergillus fumigatus, it has been hypothesised that antifungal resistance can drive virulence and adaptation to infection-related stress (Paul et al., 2019; Hagiwara et al., 2017). In addition, deletion of Hsps genes has shown altered traditional drug tolerance phenotype and attenuated virulence in Candida spp (Gong et al., 2017).
Increasing temperature adaptation alters virulence and drug resistance traits
In a current study, Schwiesow et al. (2024) used Ftc239-1, an environmental isolate of C. neoformans strains with mild and severe growth defects at 30°C and 37°C, respectively, to understand the impact of adaptation to increasing temperatures on pathogenicity traits and drug resistance. To test tolerance, Ftc239-1 strains were evolved through 38 passages every 4–6 days with 0.5–1°C temperature increases to 30°C and 35°C. The evolved strains grew well at 35°C and even more substantially at 37°C than their parent strain and showed phenotypic heterogeneity. For example, the capsule sizes and capsule production were similar to the parent strain with one of the evolved strains producing substantially more titan cells (important in pathogenesis). The evolved strains had fitness advantages at high temperatures with genotypic heterogeneity. Interestingly, frameshift and point mutations were observed in SSK2 and PBS2, respectively, which are key genes in Hog1 pathway highlighting the importance of the pathway in yeast stress response. Ftc239-1 had a fluconazole MIC of 8 μg/mL while the evolved strains MICs ranged from 3 to 16 μg/mL. MIC of the evolved strains showed that heat stress can have a complex effect on microorganisms however, MIC of 16 μg/mL indicates potential cross-stress advantages including reduced drug susceptibility. Strains with impaired ability to produce titan cells carried frameshift mutation in LMP1, the gene required for the production of titan cells, suggesting possible loss of function.
In C. deneoformans, data showed that the rate of mutation is higher at 37°C (with an 8–25 fold increase) than at 30°C. Interestingly, heat induced mobile genetic elements were shown to be the major drivers of microevolution and rapid adaptation to heat stress compared to point mutations and small insertions/deletions (Gusa et al., 2023). These genetic element mutations are the major source of stress induced genetic variation.
Endothermic stress responses confer pandrug resistance and hypervirulence traits on yeast
The work of Casadevall in 2015 showed a negative correlation between thermal stress and antifungal resistance, however, a strong correlation existed between caspofungin resistance and basidiomycetes (Robert et al., 2015). Ten years after, data now show that ascomycetes can evolve drug resistance traits linked to thermal stress and may emerge as a human pathogen due to cross stress adaptation or the acquisition of new virulence traits or activation of latent ones (Money, 2024). For example, the first description of C. auris (amphotericin B: MIC, 0.5–32 mg/mL; fluconazole: MIC, 2–128 mg/mL) (Kim et al., 2009), was in an infection of human ear which is a cooler environment than the gut; current bloodstream infections may indicate successful adaptation to 37°C which induced virulence and mutagenesis that further conferred pandrug resistance traits on the fungus. This may partly explain the emergence of multidrug resistant C. auris and has recently been demonstrated in Rhodosporidiobulus fluvialis, a previously undescribed fungal pathogen (Huang et al., 2024), as well observed in Candida khanbhai a recently characterised member of the C. haemulonii species complex (De Jong et al., 2023).
Huang et al. (2024) showed that temperature-dependent mutagenesis can drive the development of virulence and intrinsic resistance to most antifungal therapies. Their research is the outcome of China’s surveillance to monitor the epidemiology and drug resistance patterns of bloodstream yeast pathogens through the CHIF-NET—China hospital invasive fungal surveillance net, programme. The programme identified infection with two clinical strains: NJ103 and TZ579, of R. fluvialis. R. fluvialis was first described in aquatic environment, vegetation and soil in different countries as a benign fungus before being isolated in two immunocompromised patients in China with 100% fatality. The authors used mouse-model of invasive fungal infection to show that only NJ103 and TZ579 of the genus Rhodosporidiobulus and the environmental yeast R. nylandii were able to adapt to 37°C and cause disease.
Intriguingly, in the course of mice infection with NJ103 and TZ579, part of their cell population was able to transition and grow in a more virulent psuedohyphae form which also evaded macrophage internalization than the yeast form causing mice mortality. Growth at 37°C induced the morphological transition (significant in pathogenesis); it also led to the accumulation of intracellular reactive oxygen species resulting in oxidative DNA damage and rapid acquisition of single nucleotide polymorphisms. NJ103 and TZ579 were resistant to fluconazole and echinocandins. However, the elevated mutation rates induced by growth at 37°C resulted in rapid emergence of resistance to amphotericin B and 5-fluorocytosine. While this is a worrisome outcome, making the clinical strains unusual but perhaps not unique, it showed that human body heat adaptation can drive rapid evolution of pandrug resistance in yeasts. The molecular basis of drug resistance in the pathogens is yet understood, however, whole genome sequencing showed duplicate copy of ERG11 which has been implicated in fluconazole-resistance. Deletion of one ERG11 copy did not affect the drug resistance phenotype. This indicates a complex drug resistance mechanism or the presence of a genetically unusual ERG11 homologue in the fungus. Heterologously expressing the ERG11 copy in C. neoformans, conferred fluconazole-resistance.
The R. nylandii strain was intrinsically resistant to caspofungin and fluconazole. However, adaptation to mammalian core body temperature induced amphotericin B resistance (Huang et al., 2024) leading to pandrug resistance. This further showed that temperature induced rapid mutagenesis can drive evolution of traits in fungi. R. nylandii is yet to cause any human disease but has the traits of known fungal pathogens.
Another example of emergent fungi developing drug resistance first in their evolutionary path is in the C. haemulonii species complex—all members are closely related to C. auris (all clustered in Metschnikowiaceae clade)—which has attracted attention because of their emerging multidrug resistance (Chen et al., 2022), high transmissibility in clinical settings and increasing worldwide emergence (Cao et al., 2023). Azole resistance in the species has been linked to ERG11 substitutions and amplification suggesting the act of evolutionary pressure (Gade et al., 2020). A newly characterised member of the complex, Candida khanbhai, was recently identified from the nostrils of a Kuwaiti patient in 2019 and bloodstream of a Malaysian patient in 2014 as an emerging fungal pathogen (De Jong et al., 2023). This suggests that the fungus emergence and adaptation to 37°C may have begun by initially infecting cooler areas in humans just like C. auris. The impact of endothermic stress adaptation on drug resistance was also reflected in the strains drug susceptibility profile. Though both strains had elevated amphotericin B MIC values: 2–4μg/mL, the bloodstream strain showed a strong increase in MIC values: 4–16 μg/mL for flucytosine and moderately elevated MICs for anidulafungin (0.5–1 μg/mL) and micafungin (0.25–0.5 μg/mL) indicating that endothermic stress adaptation can further amplify drug resistance traits. Generally, both strains showed reduced susceptibility to multiple antifungals tested, however, an interesting observation by the authors, using C. auris breakpoint, was the clinical impact of trailing effect after 48 h which showed multidrug resistance patterns. This report is similar to that of C. auris, emerging from geographically distinct locations with multidrug resistance traits.
Impact on antifungal therapy
Antifungal resistance (AFR) has become a serious global problem partly because antimicrobial resistance programmes have traditionally removed antifungals due to fungi being widely neglected as a public health threat. For example, the joint programme initiative on antimicrobial resistance (JPIAMR) consortium only included AFR in their agenda for strategic research and innovation on antimicrobial resistance in 2021 (Fisher et al., 2022). Despite the limited funding in antifungal research, our current antifungal arsenal, still has some very potent antifungals such as the echinocandin that very few yeasts including Nakaseomyces glabratus are intrinsically resistant to. However, emerging (e.g., C. auris) (Jacobs et al., 2022) and emergent (e.g., R. fluvialis) fungi (Huang et al., 2024) now show intrinsic resistance to echinocandin and to all the classes of available antifungals.
Acquisition and emergence of AFR which may originate from the environment (Huang et al., 2024) or the host (Shields et al., 2012) is fundamentally an evolutionary response to the selective pressures exerted by a drug. Mechanistically, AFR is usually acquired due to changes that directly or indirectly affect the drug–target interaction. These include genetic changes to the target, altering the drug concentration through drug efflux activity for azole (Robbins et al., 2017) or for flucytosine (Edlind and Katiyar, 2010) inhibition of prodrug activity, and overexpressing the amount of the target available. However, data have now shown the possibility, in the absence of any drug selective pressures, of temperature-induced rapid mutagenesis that results in multidrug and pandrug resistance in fungi (Huang et al., 2024). It is possible that the drug resistance traits in these fungi are products of unintended cross-stress advantages situated by thermotolerance and perhaps by other yet unknown factors. Data is yet to show if the accumulation of these kinds of mutations come at a fitness cost in the fungi in the future. However, it is currently negatively impacting therapeutic outcomes in fungal disease management (Takoutsing et al., 2023).
Conclusion
Since most, if not all, emerging pathogenic yeasts emerged as multidrug resistant, it can be argued that their virulence traits may partly be due to cross-thermal-stress adaptation responses of evolved drug resistance traits which are further amplified upon infection. The direct impact of thermal stress adaptation on the evolution of fungal pathogens is yet understood though the risks remain high. Surveillance remains the cornerstone for controlling emerging fungal pathogens (Liao et al., 2024). in Asia, one of notable surveillance efforts is CHIF-NET through which 9,713 yeasts have been grown (8,829 Candida and 884 non-Candida yeast) with several rare or uncommon yeasts described, including Trichosporon dohaense, Diutina catenulata (Candida catenulata), Yarrowia lipolytica (Candida lipolytica) and Kodamaea ohmeri, and members of the Metschnikowiaceae clade (Bottery and Denning, 2024).
In Africa, several laboratories, are optimising protocols using culture-based and non-culture-based techniques in pilot studies for the surveillance of pathogenic yeasts in wastewater (Baker et al., 2025). Data so far suggests that the development of azole-resistance in yeasts is not necessarily through the selective pressure of environmental azoles but could be through adaptive responses that unknowingly confer drug resistance. Part of the surveillance efforts at the University of the Free State, South Africa, also include sampling freshwater plastisphere to increase our understanding of their yeast community which can be a vehicle for multidrug resistant pathogenic yeasts distribution and transmission (Baker et al., 2024). Further work is required in this rapidly evolving area of research.
Author contributions
CI: Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. CP: Formal analysis, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research is cofunded by the UK Department of Health and Social Care (DHSC) as part of the Global AMR Innovation Fund (GAMRIF). This is a One Health UK aid fund that supports research and development around the world to reduce the threat of antimicrobial resistant (AMR) in humans, animals and the environment for the benefits of people in low-and middle-income countries (LMICs). The views expressed in this publication are those of the author(s) and not necessarily those of the UK DHSC.
Acknowledgments
The authors thank the University of the Free State for support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arora, P., et al. (2021). Environmental isolation of candida auris from the coastal wetlands of Andaman islands, India. mBio 12, e03181–e03120. doi: 10.1128/mBio.03181-20
Baker, T., Bester, A., Sebolai, O., Albertyn, J., and Pohl, C. (2024). Biofilms on urban aquatic plastic pollution as a reservoir for pathogenic yeasts. J. Water Health 22, 1826–1842. doi: 10.2166/wh.2024.133
Baker, T., Bester, P. A., Sebolai, O. M., Albertyn, J., and Pohl, C. H. (2025). Culture-dependent and -independent wastewater surveillance for multiple pathogenic yeasts. J. Fungi 11:86. doi: 10.3390/jof11020086
Bottery, M. J., and Denning, D. W. (2024). Body heat drives antifungal resistance. Nat. Microbiol. 9, 1638–1639. doi: 10.1038/s41564-024-01738-2
Cao, C., Bing, J., Liao, G., Nobile, C. J., and Huang, G. (2023). Candida haemulonii species complex: emerging fungal pathogens of the Metschnikowiaceae clade. Zoonoses 3:43. doi: 10.15212/ZOONOSES-2023-0021
Casadevall, A. (2018). Fungal diseases in the 21st century: the near and far horizons. Pathog. Immun. 3, 183–196. doi: 10.20411/pai.v3i2.249
Casadevall, A. (2023). Global warming could drive the emergence of new fungal pathogens. Nat. Microbiol. 8, 2217–2219. doi: 10.1038/s41564-023-01512-w
Casadevall, A., Kontoyiannis, D. P., and Robert, V. (2019). On the emergence of candida auris: climate change, azoles, swamps, and birds. MBio 10, e01397–e01319. doi: 10.1128/mBio.01397-19
Chen, X. F., et al. (2022). First two fungemia cases caused by Candida haemulonii var. vulnera in China with emerged antifungal resistance. Front. Microbiol. 13:1036351. doi: 10.3389/fmicb.2022.1036351
Day, A. M., McNiff, M. M., da Silva Dantas, A., Gow, N. A. R., and Quinn, J. (2018). Hog1 regulates stress tolerance and virulence in the emerging fungal pathogen Candida auris. mSphere 3, e00506–e00518. doi: 10.1128/mSphere.00506-18
De Jong, A. W., et al. (2023). Candida khanbhai sp. nov., a new clinically relevant yeast within the Candida haemulonii species complex. Med. Mycol. 61:myad009. doi: 10.1093/mmy/myad009
Desnos-Ollivier, M., Robert, V., Raoux-Barbot, D., Groenewald, M., and Dromer, F. (2012). Antifungal susceptibility profiles of 1698 yeast reference strains revealing potential emerging human pathogens. PLoS One 7:e32278. doi: 10.1371/journal.pone.0032278
Edlind, T. D., and Katiyar, S. K. (2010). Mutational analysis of flucytosine resistance in Candida glabrata. Antimicrob. Agents Chemother. 54, 4733–4738. doi: 10.1128/AAC.00605-10
Fisher, M. C., Alastruey-Izquierdo, A., Berman, J., Bicanic, T., Bignell, E. M., Bowyer, P., et al. (2022). Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 20, 557–571. doi: 10.1038/s41579-022-00720-1
Gade, L., Muñoz, J. F., Sheth, M., Wagner, D., Berkow, E. L., Forsberg, K., et al. (2020). Understanding the emergence of multidrug-resistant Candida: using whole-genome sequencing to describe the population structure of Candida haemulonii species complex. Front. Genet. 11:554. doi: 10.3389/fgene.2020.00554
Gong, Y., Li, T., Yu, C., and Sun, S. (2017). Candida albicans heat shock proteins and Hsps-associated signaling pathways as potential antifungal targets. Front. Cell. Infect. Microbiol. 7:520. doi: 10.3389/fcimb.2017.00520
Gusa, A., et al. (2023). Genome-wide analysis of heat stress-stimulated transposon mobility in the human fungal pathogen Cryptococcus deneoformans. Proc. Natl. Acad. Sci. U.S.A 120:e2209831120. doi: 10.1073/pnas.2209831120
Hagiwara, D., et al. (2017). A novel Zn2-Cys6 transcription factor AtrR plays a key role in an azole resistance mechanism of Aspergillus fumigatus by co-regulating cyp51A and cdr1B expressions. PLoS Pathog. 13:e1006096. doi: 10.1371/journal.ppat.1006096
Heilmann, C. J., et al. (2013). Surface stress induces a conserved cell wall stress response in the pathogenic fungus Candida albicans. Eukaryot. Cell 12, 254–264. doi: 10.1128/EC.00278-12
Huang, J., Hu, P., Ye, L., Shen, Z., Chen, X., Liu, F., et al. (2024). Pan-drug resistance and hypervirulence in a human fungal pathogen are enabled by mutagenesis induced by mammalian body temperature. Nat. Microbiol. 9, 1686–1699. doi: 10.1038/s41564-024-01720-y
Ibe, C., and Munro, C. A. (2021). Fungal cell wall proteins and signaling pathways form a cytoprotective network to combat stresses. J. Fungi 7:739. doi: 10.3390/jof7090739
Jacobs, S. E., Jacobs, J. L., Dennis, E. K., Taimur, S., Rana, M., Patel, D., et al. (2022). Candida auris Pan-drug-resistant to four classes of antifungal agents. Antimicrob. Agents Chemother. 66:e0005322. doi: 10.1128/aac.00053-22
Kim, M. N., Shin, J. H., Sung, H., Lee, K., Kim, E. C., Ryoo, N., et al. (2009). Candida haemulonii and closely related species at 5 university hospitals in Korea: identification, antifungal susceptibility, and clinical features. Clin. Infect. Dis. 48, e57–e61. doi: 10.1086/597108
Liao, H., Lyon, C. J., Ying, B., and Hu, T. (2024). Climate change, its impact on emerging infectious diseases and new technologies to combat the challenge. Emerging Microbes Infect. 13:2356143. doi: 10.1080/22221751.2024.2356143
Lockhart, S. R., Etienne, K. A., Vallabhaneni, S., Farooqi, J., Chowdhary, A., Govender, N. P., et al. (2017). Simultaneous emergence of multidrug-resistant candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 64, 134–140. doi: 10.1093/cid/ciw691
Money, N. P. (2024). Fungal thermotolerance revisited and why climate change is unlikely to be supercharging pathogenic fungi (yet). Fungal Biol. 128, 1638–1641. doi: 10.1016/j.funbio.2024.01.005
Mora, C., et al. (2022). Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Chang. 12, 869–875. doi: 10.1038/s41558-022-01426-1
Muro, M. D., Motta, F. D. A., Burger, M., Melo, A. S. D. A., and Dalla-Costa, L. M. (2012). Echinocandin resistance in two Candida haemulonii isolates from pediatric patients. J. Clin. Microbiol. 50, 3783–3785. doi: 10.1128/JCM.01136-12.
Paul, S., et al. (2019). AtrR is an essential determinant of azole resistance in Aspergillus fumigatus. MBio 10, e02563–e02518. doi: 10.1128/mBio.02563-18
Robbins, N., Caplan, T., and Cowen, L. E. (2017). Molecular evolution of antifungal drug resistance. Ann. Rev. Microbiol. 71, 753–775. doi: 10.1146/annurev-micro-030117-020345
Robert, V., Cardinali, G., and Casadevall, A. (2015). Distribution and impact of yeast thermal tolerance permissive for mammalian infection. BMC Biol. 13:18. doi: 10.1186/s12915-015-0127-3
Rodrigues, L. S., Gazara, R. K., Passarelli-Araujo, H., Valengo, A. E., Pontes, P. V. M., Nunes-da-Fonseca, R., et al. (2020). First genome sequences of two multidrug-resistant Candida haemulonii var. vulnera isolates from pediatric patients with Candidemia. Front. Microbiol. 11:1535. doi: 10.3389/fmicb.2020.01535
Schwiesow, M. J. W., Elde, N. C., and Hilbert, Z. A. (2024). Distinct routes to thermotolerance in the fungal pathogen Cryptococcus neoformans. bioRxiv. Available online at: https://doi.org/10.1101/2024.04.08.588590. [Epub ahead of preprint]
Seagle, E. E., Williams, S. L., and Chiller, T. M. (2021). Recent trends in the epidemiology of fungal infections. Infect. Dis. Clin. N. Am. 35, 237–260. doi: 10.1016/j.idc.2021.03.001
Seidel, D., Wurster, S., Jenks, J. D., Sati, H., Gangneux, J. P., Egger, M., et al. (2024). Impact of climate change and natural disasters on fungal infections. Lancet Microbe 5, e594–e605. doi: 10.1016/S2666-5247(24)00039-9
Shields, R. K., Nguyen, M. H., Press, E. G., Kwa, A. L., Cheng, S., du, C., et al. (2012). The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob. Agents Chemother. 56, 4862–4869. doi: 10.1128/AAC.00027-12
Takoutsing, B. D., Ooi, S. Z. Y., Egu, C. B., Gillespie, C. S., Bandyopadhyay, S., Dada, O. E., et al. (2023). Management and outcomes of intracranial fungal infections in children and adults in Africa: a scoping review protocol. BMJ Open 13:e065943. doi: 10.1136/bmjopen-2022-065943
Keywords: temperature increases, emerging and emergent fungi, AFR, virulence, stress
Citation: Ibe C and Pohl CH (2025) Climatic and endothermic thermal stress adaptation may be a major driver of emerging and emergent multidrug resistant fungi. Front. Microbiol. 16:1575755. doi: 10.3389/fmicb.2025.1575755
Edited by:
Amanda Claire Brown, Tarleton State University, United StatesReviewed by:
Renátó Kovács, University of Debrecen, HungarySanjay Kumar Rohaun, University of Illinois at Urbana-Champaign, United States
Copyright © 2025 Ibe and Pohl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chibuike Ibe, Y2hpYnVpa2VfaWJlQHlhaG9vLmNvLnVr
 Chibuike Ibe
Chibuike Ibe Carolina Henritta Pohl1
Carolina Henritta Pohl1