- Plant Molecular Biology and Biotechnology, CSIR-IIIM (Branch), Srinagar, India
Introduction: The Kashmir Himalaya, renowned for its rich floristic diversity, harbors a multitude of native and introduced aromatic and medicinal plants. Among these, Salvia rosmarinus (rosemary), a Mediterranean native plant species, known for its culinary and therapeutic properties, is widely being cultivated owing to its local adaptability. Salvia rosmarinus essential oil has been used in folk medicine, pharmaceutical, and cosmetic industries. In our study, we compiled the morphological and chemoprofiling differences of field grown cultivars, wherein populations were grouped into 21 classes. Further, oils from identified accessions were screened for their anti-microbial potential against panel of four priority pathogens.
Methods and results: The characterization was based on phenotypic traits (flower color variability, calyx color, flower size, and leaf morphology) variance across identified genotypes was validated using Chi 2 test. Abundance distribution data displayed polymorphism in evaluated character/traits of rosemary accessions and a total of 21 classes were reported from an underrepresented region. Furthermore, field grown Salvia rosmarinus cultivars in Kashmir Himalaya produced essential oil yield ranging from 0.8% to 1.7% maintaining benchmark constituents. Similarly, variability in chemical constituents using Gas Chromatography Mass Spectrometry (GC/MS), grouped accessions into chemotypes rich in beta-myrcene, 1,8 cineole, and camphor. Antimicrobial assays on the essential oils obtained from different accessions using gram-negative and gram-positive bacteria and one fungal pathogen were conducted to directly evaluate the IC50 (concentration at which there is 50 percent growth inhibition of pathogen) and Minimum Inhibitory concentration (MIC) values. MIC evaluation of the active essential oil was performed using the broth dilution method.
Discussion The data generated in this study emphasizes the use of morphological and chemical characteristics to characterize and conserve elite Salvia rosmarinus cultivars, promoting cultivar R1 (1.7%) in summer season and R14 (0.95%) and R3 (0.93%) in winter season for large-scale cultivation, emphasizing propagation of higher essential oil yielding varieties in Kashmir Himalaya. The diverse rosemary genepool conserved in Kashmir exhibits significant variability in essential oil yield and composition while, certain accessions demonstrate potent antimicrobial properties. The findings of the study are useful for further elaborate studies on the development of natural bioactive compounds to improve human health.
1 Introduction
Globally, Salvia rosmarinus is recognized as a medicinal and aromatic plant species widely used as a fresh or dried herb. Since botanical sources of medicines started garnering attention, Salvia rosmarinus has been cultivated worldwide for its vital role in treatment, therapy, drug development, culinary and ornamental applications (Miguel et al., 2007; Gowda et al., 2024). Although plants thrive under a wide range of environmental conditions (Kadri et al., 2011), ecologically, this shrub plant species is well suited to flourish in dry climates and is predominantly found in the Mediterranean region, despite which it shows vast differences in its distributional range. In Kashmir Himalayan region this plant is an introduced crop considering the importance of the biochemical constituents present in it. Often, abiotic and biotic factors create distinctness in terms of plant taxonomical traits, such as flower color, leaf shape, leaf size, and overall growth habits (Herrera, 2005; Gowda et al., 2024). Such variability in morphological traits such as flower color was previously reported by Nunziata et al. (2019), but with a resurgence of interest and search for bioactive compounds of the plant in healthcare, a recent report illustrated significant differences in taxonomical features such as flowers and leaves in this plant (Gowda et al., 2024). Consequently, these variations in morphological traits, under the influence of abiotic and biotic factors, are increasingly utilized to identify elite Salvia rosmarinus cultivars with superior characteristics. Amongst the known methods of cultivar identification, morphological identification, biochemical identification, identification of signature components in essential oil, nutritional assessment, and molecular marker determination are the most popular techniques to optimize selection of elite cultivars (Sadeh et al., 2019; Gowda et al., 2024).
Quantitative and qualitative screening of essential oils often reveals significant variability and acts as a strong determinant in cultivar screening (Bradley, 2006). Relationships based on the chemical constituents conferring bioactivity to the extract and essential oil remains are also important criteria for cultivar selection (Baratta et al., 1998; Jiang et al., 2011) shaped by environmental factors (Piccaglia et al., 1993). Although specific compound isolation from a particular cultivar, along with its specific activity, remains to be investigated (Santoyo et al., 2005; Ivanovic et al., 2012), Salvia rosmarinus is undergoing large-area expansion cultivation. However, assessment remains a critical aspect for identifying elite cultivars, such as breeding enhancement and germplasm conservation (Paterson et al., 1991, Gowda et al., 2024). Further improvements in the sales of herbal products, and the inherent antimicrobial and antifungal activities of Salvia rosmarinus, has imposed an additional pressure on the identification of cultivars. Moreover, large-scale propagation in India necessitates the assessment of plant genetic diversity across biogeographical regions, especially visa viz underrepresented region like Kashmir Himalaya. Thus, in modern times the characterization and classification of chemotype-based identification and morphometric analysis have been central to similar studies as efficacy, safety, and quality are of utmost importance (Zigene et al., 2022). Zia et al. (2017) emphasized that such studies are important and effective in germplasm conservation. Screening of cultivars based on chemoprofile, such as high levels of 1,8-cineole (Sadeh et al., 2019) and α-pinene, boranyl acetate, myrcene, or verbenone as signature components of Salvia rosmarinus, has been reported previously (Satyal et al., 2017). Despite the above-mentioned important criterion that the plant has different chemotype populations, Salvia rosmarinus is not well characterized in terms of its morphology or chemotypic composition in the study region. To address this knowledge gap, the current study aimed to characterize the adapted population of Salvia rosmarinus cultivars by analyzing their essential oil chemotypic composition and provide a morphometric description of the plant that could ultimately help identify stable and established Salvia rosmarinus cultivars essential for the sustainable use of molecular methods for varietal development.
2 Materials and methods
2.1 Study area and plant material collection
For the present study, Salvia rosmarinus plants were collected from CSIR-IIIM fields in Srinagar in 2023–2024 during summer and autumn seasons, and 21 accessions were tagged and labeled after proper morphometric analysis of the fields based on morphological dissimilarities. The plant material was mounted on an herbarium sheet to obtain accession numbers and for future references. The physical characteristics of the randomly selected 50 individual plants were recorded before they were tagged in the field.
2.2 Essential oil screening
Fresh plant material from morphological distinct tagged accessions of Salvia rosmarinus weighing 300 g was harvested from CSIR-IIIM field/farms. The sample material (300 g approx. of each accession) was chopped finely and subjected to hydro-distillation using Clevenger apparatus (Perfit, India). All samples were processed for extraction of oil for 6 h in triplicates. The essential oil was collected in vials, and excess water was removed either by a micro-syringe or using anhydrous sodium sulfate (HiMedia). The oil obtained was stored in refrigerator at 4°C till Gas Chromatography/Mass Spectrometry downstream analysis was performed. Comparative percentage yield of oil was calculated as volume of oil extracted to the weight of plant material used for extraction of oil (v/w).
2.3 Downstream analysis by GC/MS
The essential oil compounds were identified using GC/MS. A 10 μL sample, prepared with methanol, was subjected to GC/MS to determine the molecular weight and retention time of the compounds. The experiment was performed using an Agilent 7890 A gas chromatograph coupled with an Agilent 5975 C Inert XL MSD mass spectrometer. The data were processed using MassHunter Workstation software (USA) following a method outlined by Manzoor et al. (2021). Helium was used as the carrier gas at a flow rate of 0.5 mL/min. The temperature program was adjusted as 50°C for 1 min, followed by an increase to 250°C at a rate of 50°C/min, maintaining the final temperature for 4 min. The mass spectra were scanned within the range of 50–600 amu, with the ionization energy set at 70 eV and a scan speed of 0.5 s per scan. Full proof of identity of the compounds present in the essential oil was obtained using the Wiley and NIST libraries in conjunction with key compound identification. The peak area percentages were recorded without applying correction factors.
2.4 Screening antimicrobial potential of essential oils
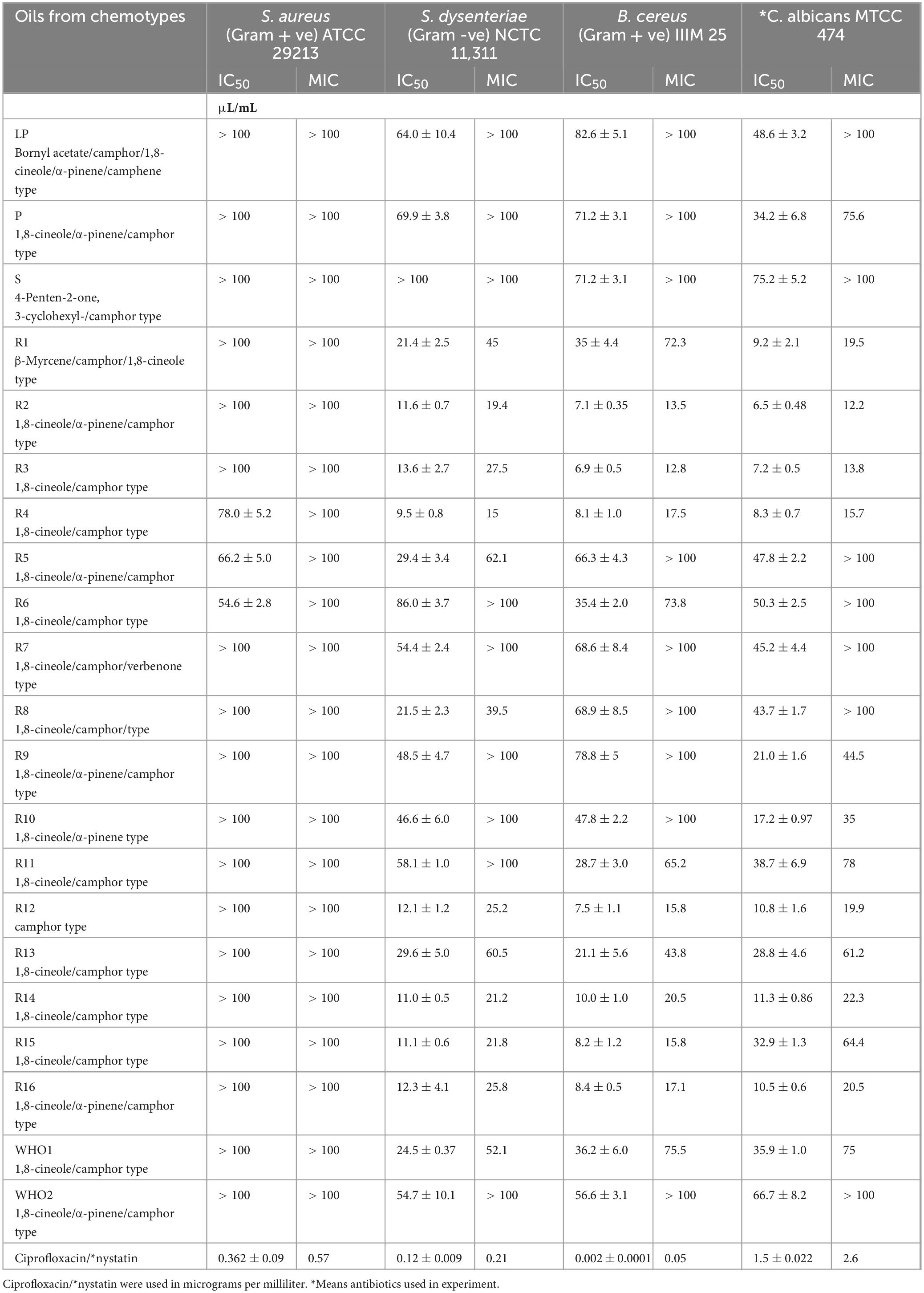
The essential oils obtained from each accession through hydro-distillation were evaluated for their antimicrobial potential. The cultivars with high oil yield (quantitative measure) were evaluated for their bioactive potential against few priority pathogens using the microbroth dilution method. Concentration of essential oil used in evaluation was 1 μL/mL, which was serially diluted. Microtiter plate assay was conducted in triplicates. Bacterial (Gram-positive and negative) pathogens (Staphylococcus aureus, Staphylococcus dysenteriae, and Bacillus cereus) and one fungal pathogen (Candida albicans) were grown in microtiter plates with different concentrations of the oil in Mueller–Hinton broth for bacterial pathogens and Potato Dextrose Broth (PDB) for fungal pathogens (HiMedia, Mumbai, India) at 37°C for 24 h. IC50 and MIC of the oils were calculated (Bashir et al., 2023). Nystatin and ciprofloxacin (HiMedia) were used as positive controls. Oil was used in micrograms per milliliter concentration and antibiotics were used in microliters per milliliter unit.
2.5 Data analysis and presentation
Morphometric analysis of selected plant species was worked out and phenotypic traits were recorded for study accessions. Qualitative traits subjected to Chi-Square (X2) test (p < 0.05) using XL STAT package in MS Excel to determine association between varieties and characters or whether they are independent. Chi square test was evaluated against tabulated values at significance value of 0.05 and calculating degree of freedom from formula df = (rows-1 × column-1). A dendrogram (tree diagram illustrating hierarchical relationships) was constructed using multivariate analysis based on the neighbor-joining method. The linkage analysis was performed using Bray–Curtis similarity to assess the relationships among the accessions. Chemical compound structures were generated using BIOVIA DRAW 2019. Further, the effect of the essential oil on pathogens are presented as means ± standard deviation (SD), based on three independent biological replicates, each containing the same number of technical replicates.
3 Results
3.1 Morphological characterization of accessions
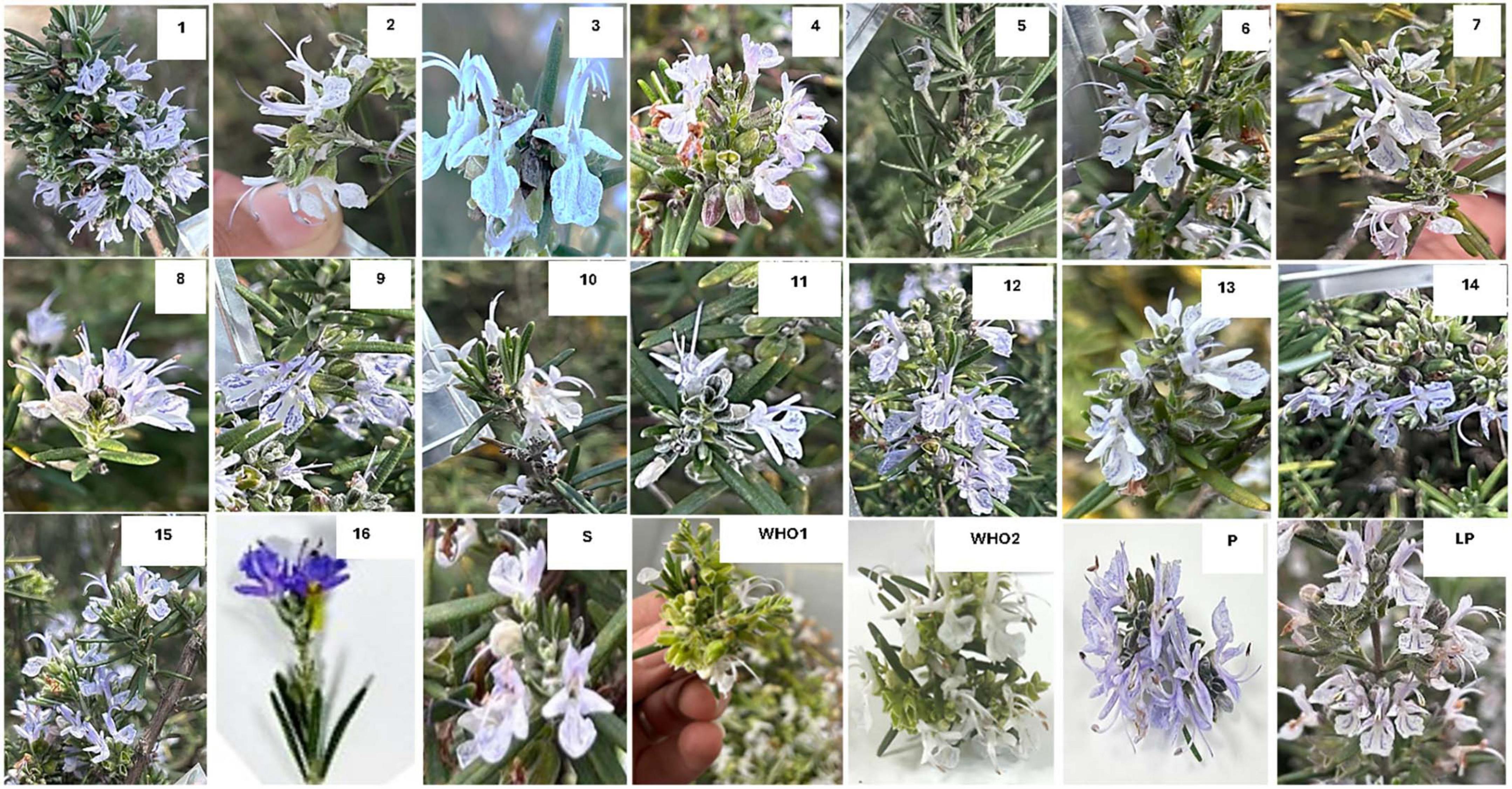
Morphologically, the identified varieties were characterized on five traits including flower petal color, flower size, leaf type, leaf color, and sepal color (Figure 1). Amongst accessions flowers, displayed peculiar characteristics, showing variability in size and color. Perusal of data showed variation in flower color, flowering time, and leaf color. Flowers exhibit six phenotypic classes. Amongst these, prevalence of light blue flowers (Accession R2, R3, R5, R6, R7, R9, R10, R15) was predominant, followed by bluish white (R12), white (R11, R13, R14), purple (R16, P), and light purple (R1, R4) (Supplementary Table 1). Flowers with blue and purple pigments yielded low amounts of essential oils ranging from 0.4% to 0.62% in fall and 0.3 to 1.3% in Summer. In comparison to blue and purple flower plants, light purple (Accessions) plants yielded higher percentage of oil. Plants with light leaf color yielded a higher oil percentage than plants with a dark deep green color (Accessions R1, R3, R6, R9, R12, R15, R16 and L Pur). Supplementary Table 1 provides a detailed description of the traits used for weighing and characterizing the Salvia rosmarinus plants along with ascribed accession names.
3.2 Comprehensive screening of essential oil from accessions
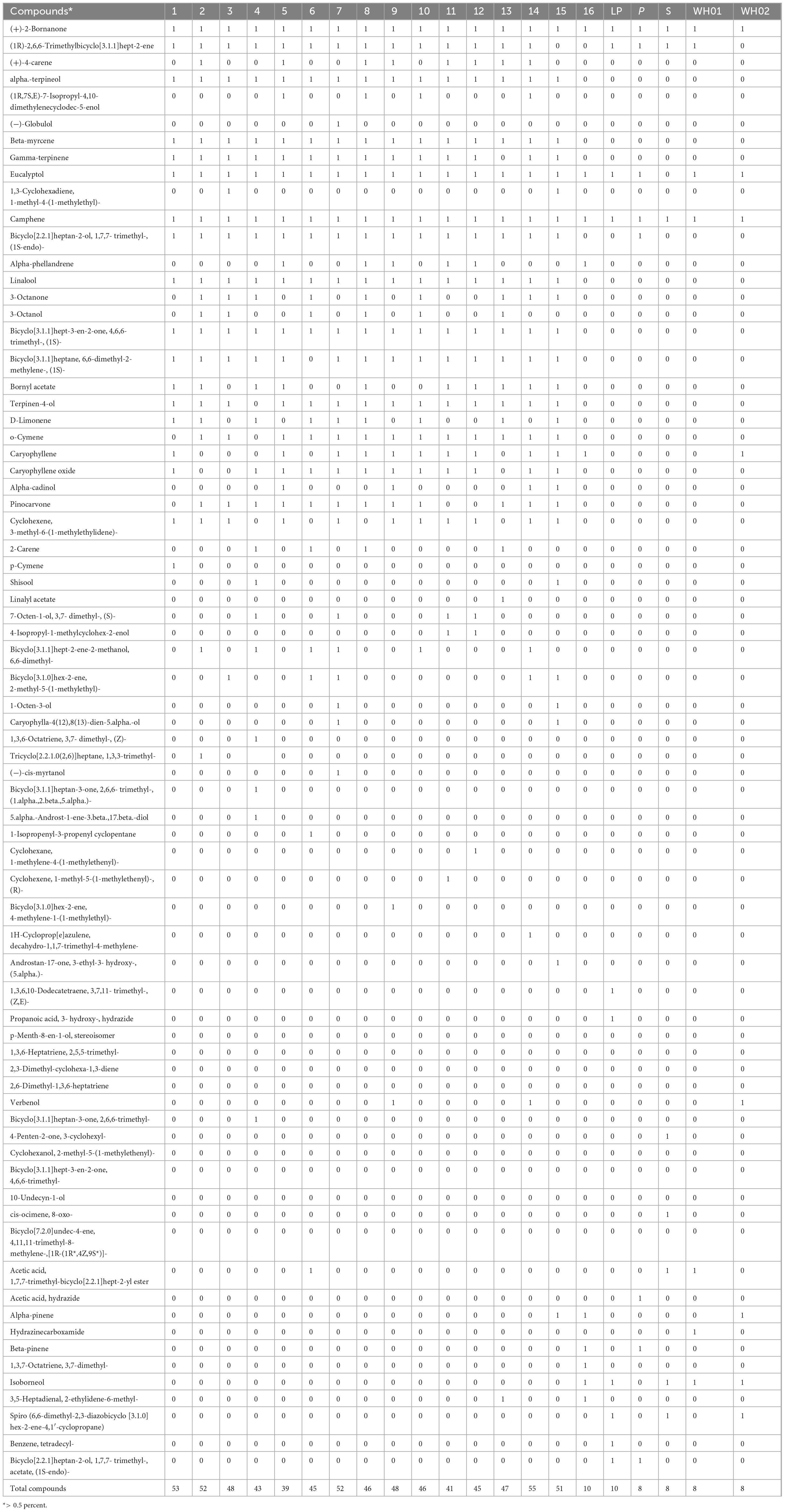
Essential oil yields varied among the cultivars identified and ranged from 0.4% to 1.7% (v/w). Figure 2A illustrates the yields per cultivar during the summer and autumn seasons, while Figure 2B provides a comparative percentage composition of major compounds present in the essential oil across Salvia rosmarinus essential oil accessions. The comparison among different essential oil accessions revealed varying percentages of oil yield, with a marked decline in essential oil content during the winter season. The highest yield was obtained from accession R14 (0.95%), and the lowest from R5 and R6 (0.33% each) in autumn, whereas in peak summer season, R1 yielded the highest amount of essential oil (1.7%) and the lowest yield was reported from R16 (0.3%). The essential oil screened by GC/MS showed 61 compounds abundantly present in the essential oil, and their descriptions and structures are presented in Figure 3 and Table 1. The major components of the oil are monoterpenes, monoterpene alcohols, sesquiterpenes, sesquiterpene alcohols, terpenoid esters, terpene ketones, hydrocarbons, oxygenated hydrocarbons, ketones, esters, alcohols, steroids, and alkaloids (Table 1). The chemical components identified in the essential oils (Table 2) varied across cultivars. Furthermore, Bray–Curtis similarity-based cluster analysis revealed the formation of two major clusters, grouping the accessions according to the compound composition of their essential oils (Figure 4). A comparison of the identified compounds with those reported in previous studies (Chalchat et al., 1993; Aziz et al., 2022) revealed that several accessions contained higher levels of key reference compounds in their essential oils, which includes highest levels of camphor (32.24 area percentage), α-pinene (17.45), β-myrcene (10.1), levoverbenone, l-verbenone, and D-verbenone (10.68) (Figure 4 and Supplementary Table 2).
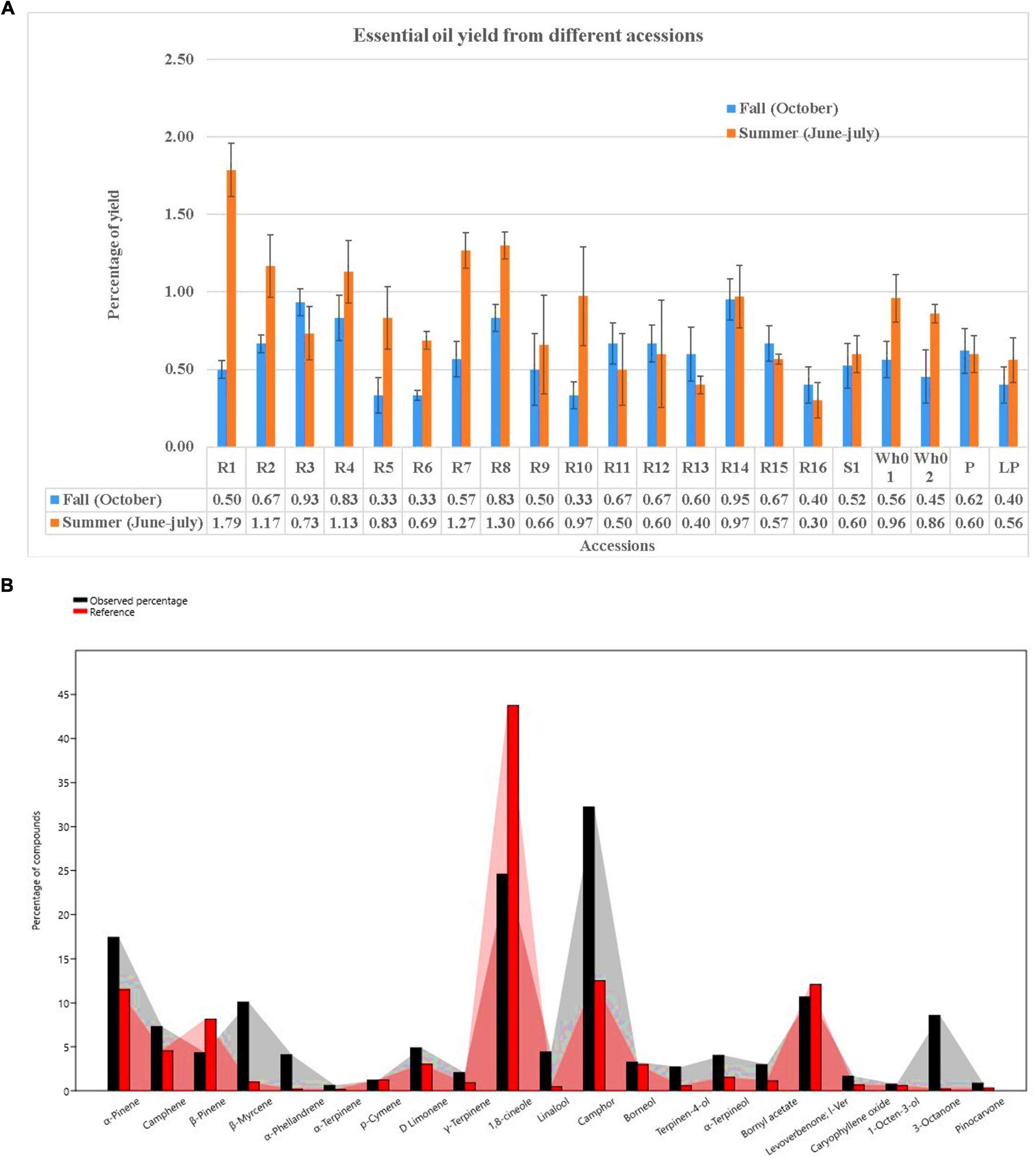
Figure 2. (A) Seasonal comparison of essential oil yield in accessions during Fall and Summer. (B) Thorough chemoprofiling based on reference compounds.
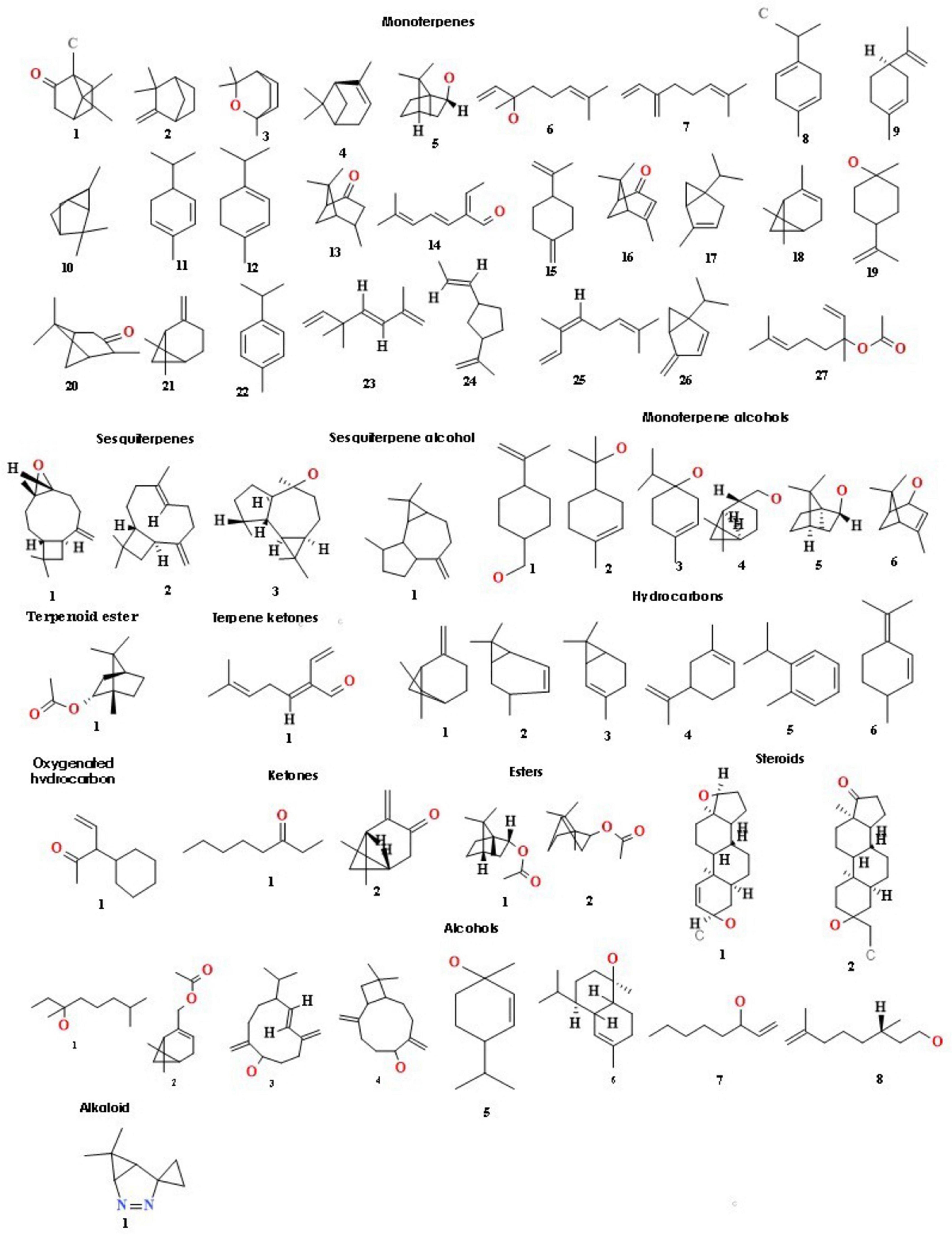
Figure 3. Structures of key volatile compounds present in the essential oil of Rosemary, including: monoterpenes (1–27), sesquiterpenes (1–3), a sesquiterpene alcohol (1), monoterpene alcohols (6), terpenoid esters (1–2), a terpene ketone (1), hydrocarbons (6), an oxygenated hydrocarbon (1), ketones (1–2), esters (1–2), steroids (1–2), alcohols (8), and an alkaloid (1).
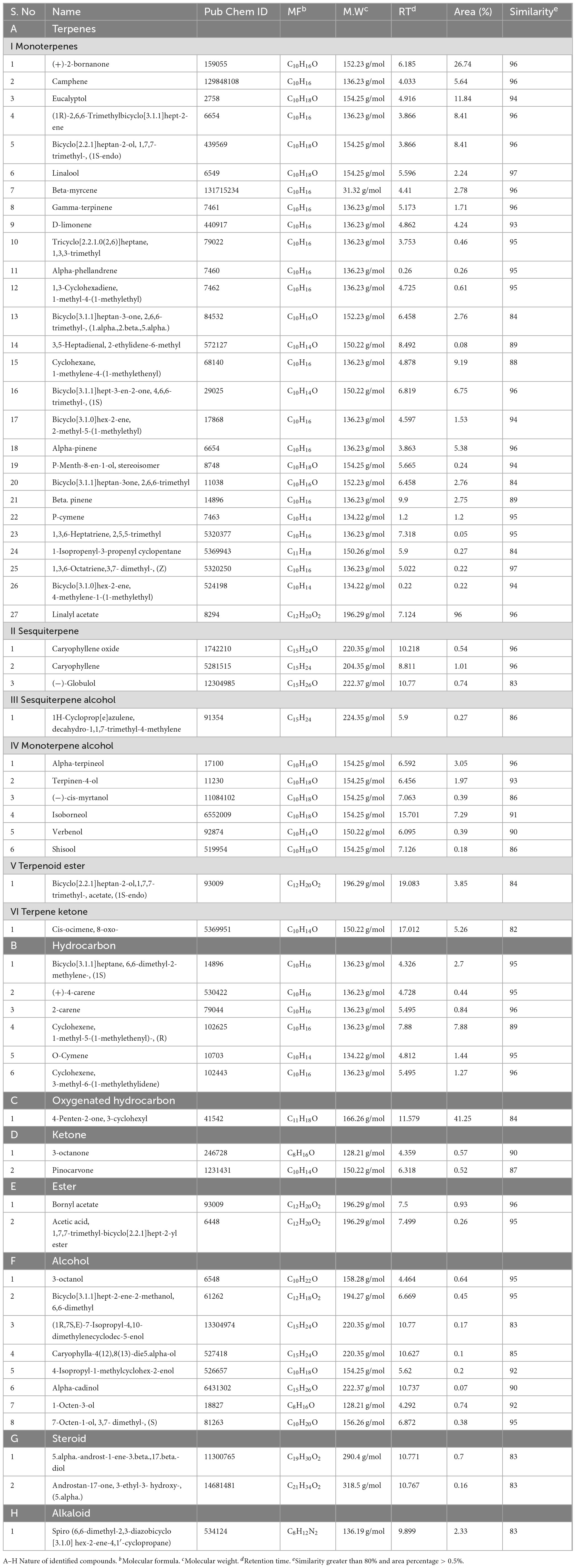
Table 1. Characterization of major identified compounds across accession at > 80% similarity with retention time and area percentage using GC/MS.
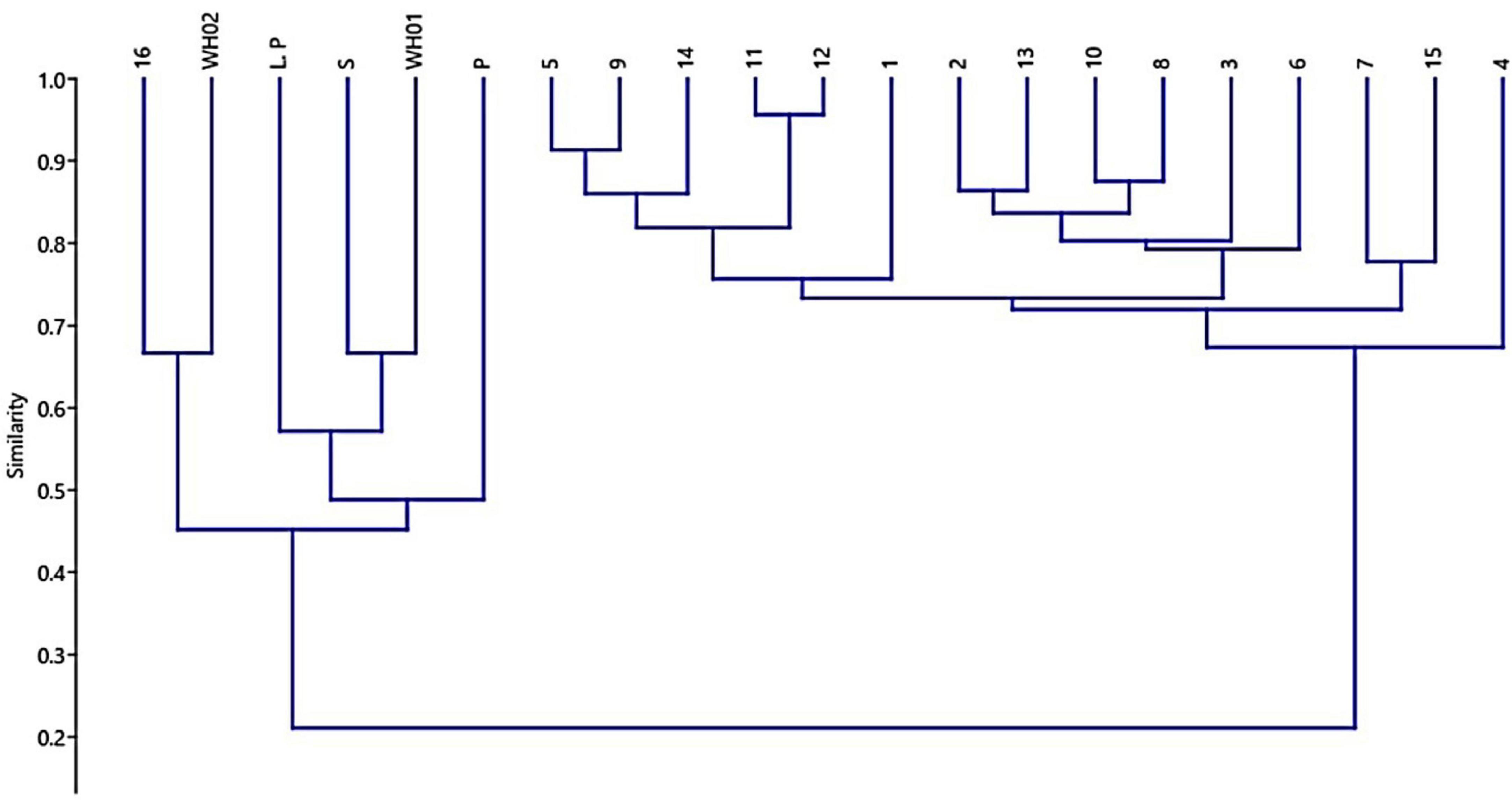
Figure 4. Dendrogram generated using Bray-Curtis similarity to depict the chemoprofile-based relationships among 21 accessions based on their essential oil composition 14.
A variable number of compounds were obtained from the accessions (Figure 3). Higher number of volatiles were reported from accessions R1, R2, R3, R7, R9, R10, R12, R13, R14, and R15. Using GC/MS analyses, 138 volatiles were identified as constituents of the investigated essential oils across the 21 accessions (Supplementary Table 1). In-depth analysis revealed the chemoprofiles of cineole-camphor, verbenone, linalool, and borneol chemotypes. The 1,8-cineole and camphor content were higher in summer and winter for all accessions. Overall, accessions growing in this region exhibited higher concentrations of monoterpenes i.e., 27 compounds out 138 compounds were monoterpenes (Table 1). In autumn, the essential oil constituents displayed the presence of 33 major compounds as compared to the average of 100 compounds in summer.
3.3 Antimicrobial effect of volatiles
The effect of the essential oils on pathogen inhibition resulted in variable degrees of activity. This variation, detected through IC50 and MIC values, exhibited differences in the quality of oils from different source accessions. Oil extract from different accessions inhibited three of the four pathogens tested [including S. aureus, S. dysenteriae, B. cereus (bacterial pathogens), and C. albicans (a fungal pathogen)] with IC50 of < 50 μl/ml. While most oils displayed substantial inhibition of S. dysenteriae, B. cereus, and C. albicans growth, all sample oils showed minimal inhibitory effects against S. aureus. C. albicans showed potential inhibition by oil extract from accessions R1, R2, R3, R4 R9, R10, R14, R13, and R16 with an IC50 ranging from 6.5 to 48.6 μl/ml (Table 3). The oil samples from Accessions R3, R2, R11, R12, R13, R14, R15, R16, and WHO1 were effective against Gram-positive bacterium B. cereus with IC50 ranging from 6.9 to 36.2 μl/ml (Table 3). However, the Gram-positive bacterium S. aureus was only slightly inhibited by oil accessions R4, R5, and R6, whereas Gram-negative bacterium S. dysenteriae was inhibited by oil samples with IC50 ranging from 9.5 to 48.5 μL ml–1. Furthermore, the antifungal activity against C. albicans reported MIC of 12.2 μl/mL. Similarly, the antibacterial activity against Gram-positive (B. cereus) and Gram-negative pathogen (S. dysenteriae) showed susceptibility to the MIC of Salvia rosmarinus essential oil at 8 and 15 μl/mL concentrations, respectively. Our findings show that oils from accessions R4, R5, and R6 possess antimicrobial properties that are effective in controlling pathogen development against all four test pathogens, albeit with erratic IC50 values. However, R1, R2, R3, R8, R12, R14, R15, and R16 and the most effective R4 were found to have inhibitory effects on gram-negative bacteria. Moreover, R1, R2, R3, R4, R6, R11, R12, R13, R14, R15, R16, and WH01 exhibited maximum inhibition of gram-positive bacteria.
4 Discussion
The Kashmir Himalayan region is a non-native region for aromatic herbs such as Salvia rosmarinus. The region is now showing increased cultivation practices for this aromatic plant on a larger scale. A reference database for identifying elite genotypes based on 21 accessions growing in the Kashmir Himalayan region during summer and autumn was documented in this study. A total of 50 individuals randomly selected from different accessions were screened from densely growing populations. This study classified entire accessions of rosemary (Salvia rosmarinus) based on a comprehensive evaluation of morphometric traits, including flower color and size, leaf size, color, and type, as well as sepal pigmentation (presence or absence of anthocyanins). These traits hold practical significance for breeding programs aimed at selecting desired phenotypes. A substantial degree of morphological divergence was observed among the accessions. Notably, quantitative traits were predominantly used to estimate genetic variability within the gene pool (Bonny et al., 2019; Zigene et al., 2022), as these traits are primarily influenced by allelic differences and less so by environmental pressures (Briggs and Knowles, 1967; Van Hintum et al., 2000; Zigene et al., 2022). Morphological trait analyses have been pivotal in identifying genetic diversity, thereby aiding the selection and enhancement of cultivars. Among the observed growth habits, upright growth was most prevalent, followed by prostrate and semi-erect forms, aligning with earlier findings by Carrubba et al. (2020) and Zigene et al. (2022), who also documented these three growth types in S. rosmarinus. Prostrate forms are often cultivated for higher leaf biomass, whereas upright forms, which yield less foliage and essential oil, are generally favored for ornamental use. Consequently, leaf characteristics such as size and color significantly influence breeding targets aimed at enhancing essential oil yield.
Lattoo et al. (2008) advocated for the use of integrated morphological, phytochemical, and molecular markers to identify elite germplasm. Their findings demonstrated a wide spectrum of flower colors—blue, white, and purple—whose variation was attributable to genetic factors, developmental stages, pigment composition, and environmental variables, including soil pH and UV exposure. For example, in Xanthoceras sorbifolium, petal bases shift from yellow to purple during maturation due to anthocyanin accumulation (Lu et al., 2022). Similarly, in Antirrhinum majus, petal color varies with pigment composition chlorophylls, carotenoids, and flavonoids which are modulated through different developmental phases (Nabipour Sanjbod et al., 2023). UV light also plays a role, as differing exposure levels can enhance photopigment accumulation, affecting visual cues for pollinators sensitive to UV wavelengths (Valenta et al., 2020). In addition, the study documented leaf size variations among different S. rosmarinus cultivars, with lengths ranging from 2 to 4 cm and widths between 2 and 5 mm (World Health Organization [WHO], 1999). Despite these size differences, no major divergence was observed in other morphological features such as leaf texture (leathery), shape (linear to lanceolate), and presence of a distinct aroma (World Health Organization [WHO], 1999). Such size variation may reflect the interplay of genetic regulation and environmental influence (Tisné et al., 2013). Light-responsive pigment regulation contributes to leaf color, aiding photosynthesis, and stress protection (Biswal et al., 2011). Furthermore, environmental shifts, such as soil composition or UV exposure, can induce adaptive changes in leaf morphology and pigmentation (Singh et al., 2006; Goslee et al., 2009; Zhao et al., 2016). The study also assessed seasonal variability in essential oil yield across accessions maintained at CSIR-IIIM, Srinagar, using a standardized extraction protocol under controlled experimental conditions. Significant variation in yield was recorded. Accession R1 exhibited the highest yield (1.7%) in the summer, while accessions R14 (0.95%) and R3 (0.93%) showed relatively higher yields in winter. These results are consistent with the findings of Salido et al. (2003), who reported yields ranging from 1% to 1.8%, with peak production occurring during summer. The elevated yields in warmer months may be attributed to enhanced volatile compound synthesis driven by environmental variables such as temperature and humidity. The seasonal variation in essential oil content was also accompanied by differences in chemical composition, supporting the notion that oil yield is strongly influenced by both chemotype and morphological traits, including leaf and flower color (da Silva et al., 2003; Sajjadi and Ghannadi, 2012). Lower yields were noted during full flowering and toward the end of the growing season, a pattern similarly noted by Salido et al. (2003), though contrary to earlier reports by Moretti et al. (1998). This study revealed notable differences in the chemical composition of essential oils from various S. rosmarinus accessions, influenced both by seasonal changes and inherent genetic variability. Major constituents included camphor (32.24%), 1,8-cineole (24.59%), α-pinene (17.45%), verbenone (10.68%), β-myrcene (10.1%), 3-octanone (8.58%), linalool (4.4%), and borneol (3.25%). This chemotypic profile aligns with previous studies identifying camphor as a dominant compound (Daferera et al., 2000). These profiles directly influence the oil’s therapeutic and aromatic properties, enhancing its utility in traditional medicine and aromatherapy. Although morphological traits showing significant differences between varieties but alone are insufficient to identify elite cultivars, a significant correlation was found between chemoprofile and flower/leaf color. Cluster analysis based on Bray–Curtis similarity grouped accessions into two distinct clusters, indicating underlying genetic variation even among accessions sourced from the same region. Several minor yet significant compounds—3-octanone, endo-borneol, alpha-phellandrene, 3-octanol, fenchol, pinocarvone, caryophyllene oxide, and bornyl acetate—were present in higher concentrations than reported in earlier literature (Supplementary Table 2), suggesting potential chemical enrichment. Among the 21 major constituents commonly identified in rosemary essential oil, the following were notable: alpha-ocimene, cyclofenchene, isopinocamphone, 1-androstenediol, cyclopentane derivatives, spathulenol, bergamiol, and globulol, among others (Dellacassa et al., 1999; Daferera et al., 2000; Celiktas et al., 2007; Laborda et al., 2013; Jordán et al., 2013). These findings support the classification of S. rosmarinus essential oils into cineoliferum, camphoriferum, and verbenoniferum chemotypes (Napoli et al., 2010). Notably, the composition of the present samples closely resembled Moroccan rosemary oil, which is rich in 1,8-cineole (43.5%–57.7%), differing from French and Spanish variants typically high in α-pinene (19.4%–35.1%) (Chalchat et al., 2011). The essential oils from different accessions exhibited potent antimicrobial activity, assessed through MIC and IC50 values against four pathogenic bacteria. Oils from most accessions inhibited three out of four test organisms effectively, primarily due to their bioactive constituents. Consistent with findings by Kabotso et al. (2024), the principal antimicrobial components included eucalyptol, camphor, and endo-borneol. MIC and MBC values ranged from 3.13 to 6.25 mg/mL and 3.12 to 12.5 mg/mL, respectively, indicating promising antibacterial potential. Accession R1, characterized by a β-myrcene/camphor/1,8-cineole profile, showed inhibitory effects at concentrations of 21.4, 35, and 9.2 μL/mL against specific pathogens. Similarly, accessions R2 and R3 (1,8-cineole/α-pinene/camphor type) demonstrated robust antimicrobial properties, as did R12 (camphor type). These observations are in line with earlier reports attributing such activity to phenolic and flavonoid compounds (Meccatti et al., 2021). The antibacterial effects of rosemary oil against S. aureus, B. cereus, E. coli, and Pseudomonas aeruginosa have also been documented (Jafarı-Sales and Pashazadeh, 2020; Abdul Rahman, 2015). In addition, the tested essential oils were effective against Shigella dysenteriae, further supporting their broad-spectrum antibacterial properties. While preliminary results suggest high antimicrobial efficacy, further studies are necessary to evaluate therapeutic applications. The present study focused on MIC and IC50 evaluations as preliminary indicators of antimicrobial activity. In future studies, the therapeutic potential will be further assessed through comprehensive analyses, including time-kill kinetics, synergy assays, cytotoxicity profiling, and benchmarking against clinical reference strains.
5 Conclusion
This study documented the morphological and chemical variability of Salvia rosmarinus cultivated in the Kashmir Himalayas, with morphological characterization serving as an first and foremost classification approach which can be combined with chemical profiles of essential oil to describe the varieties growing in the region. Field-grown Salvia rosmarinus (syn. Rosmarinus officinalis) in Kashmir’s temperate Himalaya produced essential oil yields (0.88%–1.70%) that exceeded the 0.8%–1.5% range reported for its Mediterranean native region, while maintaining the benchmark camphor/1,8-cineole chemotype. Oil dominantly showed presence of Monoterpenes, Sesquiterpene, and Monoterpene alcohol. The study identified few potential cultivars with better essential oil yield and chemoprofiles. In nutshell, field grown cultivars also yielded satisfactory amount of essential oil under unfavorable winter conditions. A subset of Kashmir accessions shows markedly stronger antibacterial activity (MIC = 4% v/v against Staphylococcus aureus) than many commercial reference oils, positioning these high-altitude cultivars as a globally competitive, climate-resilient source of pharmaceutical and flavor grade rosemary oil. This result confirms potential use of essential oil against priority pathogens. Evidently, essential oil rich in β-myrcene, camphor, and 1,8-cineole, exhibited the strongest antimicrobial activity against three test pathogens. While 1,8-cineole/α-pinene/camphor type and a camphor-type, also displayed antimicrobial effects against these pathogens. Moreover, tested oil samples exerted minimal effect inhibitory activity against on Staphylococcus aureus. Our findings from this study highlight the potential of Salvia rosmarinus essential oils cultivars, as natural antimicrobial agents. Considering that the identified accessions have a significant inhibitory role in pathogenesis, these accessions require the identification of active compounds for further investigation into their mechanisms of action against other priority pathogens.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
NK: Conceptualization, Formal Analysis, Writing – original draft, Writing – review and editing. AB: Validation, Writing – review and editing. KG: Visualization, Writing – review and editing. SS: Data curation, Writing – review and editing. RN: Visualization, Writing – review and editing. PS: Writing – review and editing. QH: Supervision, Validation, Visualization, Writing – review and editing.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The research was conducted with support from CSIR Aroma Mission Phase-III and represents a revised version of the Institutional Manuscript No. CSIR-IIIM/IPR/00900.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1579383/full#supplementary-material
References
Abdul Rahman, M. (2015). Measurement of minimum inhibitory concentration (MIC) of individual and combinations of essential oil volatiles in food: A thesis presented in partial fulfilment of the requirements for the degree of Doctor of Philosophy in Food Technology at Massey University, Palmerston North, New Zealand. PhD disseration, Massey University: New Zealand.
Aziz, E., Batool, R., Akhtar, W., Shahzad, T., Malik, A., Shah, M. A., et al. (2022). Salvia rosmarinus species: A review of phytochemicals, bioactivities and industrial applications. South Afr. J. Botany 151, 3–18. doi: 10.1016/j.sajb.2021.09.026
Baratta, M. T., Damien Dorman, H. J., Deans, S. G., Biondi, D. M., and Ruberto, G. (1998). Chemical composition, antimicrobial and antioxidative activity of laurel, sage, rosemary, oregano and coriander essential oils. J. Essent. Oil Res. 10, 618–627. doi: 10.1080/10412905.1998.9700989
Bashir, A., Manzoor, M. M., Ahmad, T., Farooq, S., Sultan, P., Gupta, A. P., et al. (2023). Endophytic fungal community of Rosa damascena Mill. as a promising source of indigenous biostimulants: Elucidating its spatial distribution, chemical diversity, and ecological functions. Microbiol. Res. 276:127479. doi: 10.1016/j.micres.2023.127479
Biswal, B., Joshi, P. N., Raval, M. K., and Biswal, U. C. (2011). Photosynthesis, a global sensor of environmental stress in green plants: Stress signalling and adaptation. Curr. Sci. 101, 47–56.
Bonny, B. S., Adjoumani, K., Seka, D., Koffi, K. G., Kouonon, L. C., Koffi, K. K., et al. (2019). Agromorphological divergence among four agro-ecological populations of Bambara groundnut (Vigna subterranea (L.) Verdc.) in Côte d’Ivoire. Ann. Agricult. Sci. 64, 103–111. doi: 10.1016/j.aoas.2019.04.001
Bradley, P. (2006). British herbal compendium: A handbook of scientific information of widely used plant drugs. Bournemouth: British Herbal Medicine Association.
Carrubba, A., Abbate, L., Sarno, M., Sunseri, F., Mauceri, A., Lupini, A., et al. (2020). Characterization of Sicilian rosemary (Rosmarinus officinalis L.) germplasm through a multidisciplinary approach. Planta 251:37. doi: 10.1007/s00425-019-03327-8
Celiktas, O. Y., Hames Kocabas, E. E., Bedir, E., Vardar Sukan, F., Ozek, T., and Baser, K. H. C. (2007). Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 100, 553–559. doi: 10.1016/j.foodchem.2005.10.011
Chalchat, J. C., Garry, R. P., Michet, A., Benjilali, B., and Chabart, J. L. (1993). Essential oils of rosemary (Rosmarinus officinalis L.). the chemical composition of oils of various origins (Morocco, Spain, France). J. Essent. Oil Res. 5, 613–618.
Chalchat, J. C., Özcan, M. M., and Figueredo, G. (2011). The composition of essential oils of different parts of Laurel, Mountain Tea, Sage and Ajowan. J. Food Biochem. 35, 484–499. doi: 10.1111/j.1745-4514.2010.00397.x
da Silva, J. D., Luz, A. I. R., da Silva, M. H. L., Andrade, E. H. A., Zoghbi, M. D. G. B., and Maia, J. G. S. (2003). Essential oils of the leaves and stems of four Psidium spp. Flav. Fragrance J. 18, 240–243. doi: 10.1002/ffj.1219
Daferera, D. J., Ziogas, B. N., and Polissiou, M. G. (2000). GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. Food Chem. 48, 2576–2581. doi: 10.1021/jf990835x
Dellacassa, E., Lorenzo, D., Moyna, P., Frizzo, C. D., Serafini, L. A., and Dugo, P. (1999). Rosmarinus officinalis L.(Labiatae) essential oils from the South of Brazil and uruguay. J. Essent. Oil Res. 11, 27–30. doi: 10.1080/10412905.1999.9701061
Goslee, S., Sanderson, M., and Gonet, J. (2009). No persistent changes in pasture vegetation or seed bank composition after fallowing. Agronomy J. 101, 1168–1174. doi: 10.2134/agronj2008.0243
Gowda, M. R. S., Arpitha, K., Gamyashree, K., Prabhu, K. N., Niranjana Kumar, A., Satya Srinivas, K. V. N., et al. (2024). Chemical and molecular diversity of rosemary (Salvia rosmarinus L.) clones. Genet. Resources Crop Evol. 71, 2003–2018. doi: 10.1007/s10722-023-01758-7
Herrera, C. M. (2005). Plant generalization on pollinators: Species property or local phenomenon? Am. J. Bot. 92, 13–20. doi: 10.3732/ajb.92.1.13
Ivanovic, J., Misic, D., Zizovic, I., and Ristic, M. (2012). In vitro control of multiplication of some food-associated bacteria by thyme, rosemary and sage isolates. Food Control 25, 110–116. doi: 10.1016/j.foodcont.2011.10.019
Jafarı-Sales, A., and Pashazadeh, M. (2020). Study of chemical composition and antimicrobial properties of Rosemary (Rosmarinus officinalis) essential oil on Staphylococcus aureus and Escherichia coli in vitro. Int. J. Life Sci. Biotechnol. 3, 62–69. doi: 10.38001/ijlsb.693371
Jiang, Y., Wu, N., Fu, Y. J., Wang, W., Luo, M., Zhao, C. J., et al. (2011). Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 32, 63–68. doi: 10.1016/j.etap.2011.03.011
Jordán, M. J., Lax, V., Rota, M. C., Lorán, S., and Sotomayor, J. A. (2013). Effect of the phenological stage on the chemical composition, and antimicrobial and antioxidant properties of Rosmarinus officinalis L essential oil and its polyphenolic extract. Indust. Crops Prod. 48, 144–152. doi: 10.1016/j.indcrop.2013.04.031
Kabotso, D. E. K., Neglo, D., Gaba, S. E., Danyo, E. K., Dayie, A. D., Asantewaa, A. A., et al. (2024). In vitro evaluation of rosemary essential oil: Gc-ms profiling, antibacterial synergy, and biofilm inhibition. Pharmaceuticals (Basel) 17:1653. doi: 10.3390/ph17121653
Kadri, A., Zarai, Z., Ben Chobba, I., Bekir, A., Gharsallah, N., Damak, M., et al. (2011). Chemical constituents and antioxidant properties of Rosmarinus officinalis L. essential oil cultivated from South-Western Tunisia. J. Med. Plants Res. 5, 5999–6004.
Laborda, R., Manzano, I., Gamon, M., Gavidia, I., Perez-Bermudez, P., and Boluda, R. (2013). Effects of Rosmarinus officinalis and Salvia officinalis essential oils on Tetranychus urticae Koch (Acari: Tetranychidae). Industrial Crops Prod. 48, 106–110. doi: 10.1016/j.indcrop.2013.04.011
Lattoo, S. K., Dhar, R. S., Khan, S., Bamotra, S., Bhan, M. K., Dhar, A. K., et al. (2008). Comparative analysis of genetic diversity using molecular and morphometric markers in Andrographis paniculata (Burm. f.) Nees. Genet. Resources Crop Evol. 55, 33–43. doi: 10.1007/s10722-007-9212-y
Lu, Y., Wang, H., Liu, Z., Zhang, T., Li, Z., Cao, L., et al. (2022). A naturally-occurring phenomenon of flower color change during flower development in Xanthoceras sorbifolium. Front. Plant Sci. 13:1072185. doi: 10.3389/fpls.2022.1072185
Manzoor, M. M., Goyal, P., Pandotra, P., Dar, M. S., Dar, M. J., Misra, P., et al. (2021). Transcriptome-wide identification of squalene epoxidase genes from Glycyrrhiza glabra L.: Expression analysis and heterologous expression of GgSQE1 suggest important role in terpenoid biosynthesis. Protoplasma 258, 991–1007. doi: 10.1007/s00709-021-01616-2
Meccatti, V. M., Oliveira, J. R., Figueira, L. W., Lagareiro Netto, A. A., Zamarioli, L. S., Marcucci, M. C., et al. (2021). Rosmarinus officinalis L. (rosemary) extract has antibiofilm effect similar to the antifungal nystatin on Candida samples. An Acad. Bras. Cienc. 93:e20190366. doi: 10.1590/0001-3765202120190366
Miguel, M. G., Guerrero, C., Rodrigues, H., Brito, J., Duarte, F., Venâncio, F., et al. (2007). “Essential oils of Rosmarinus officinalis L., effect of harvesting dates, growing media and fertilizers,” in Int. Conf. on Energy, Environment, Ecosystem and Sustainable Development, (Greece: Agios Nikolaos), 24–26.
Moretti, M. D. L., Peana, A. T., Passino, G. S., and Solinas, V. (1998). Effects of soil properties on yield and composition of Rosmarinus officinalis essential oil. J. Essent. Oil Res. 10, 261–267. doi: 10.1080/10412905.1998.9700898
Nabipour Sanjbod, R., Chamani, E., Pourbeyrami Hir, Y., and Estaji, A. (2023). Autophagic and phytochemical aspects of color changes in white petals of snapdragon flower during development and senescence. Physiol. Mol. Biol. Plants 29, 695–707. doi: 10.1007/s12298-023-01323-7
Napoli, E. M., Curcuruto, G., and Ruberto, G. (2010). Screening of the essential oil composition of wild Sicilian rosemary. Biochem. Syst. Ecol. 38, 659–670. doi: 10.1016/j.bse.2010.04.001
Nunziata, A., De Benedetti, L., Marchioni, I., and Cervelli, C. (2019). High throughput measure of diversity in cytoplasmic and nuclear traits for unravelling geographic distribution of rosemary. Ecol. Evol. 9, 3728–3739. doi: 10.1002/ece3.4998
Paterson, A. H., Tanksley, S. D., and Sorrells, M. E. (1991). DNA markers in plant improvement. Adv. Agronomy 46, 39–90. doi: 10.1016/S0065-2113(08)60578-7
Piccaglia, R., Marotti, M., Giovanelli, E., Deans, S. G., and Eaglesham, E. (1993). Antibacterial and antioxidant properties of Mediterranean aromatic plants. Industrial Crops Prod. 2, 47–50. doi: 10.1016/0926-6690(93)90010-7
Sadeh, D., Nitzan, N., Chaimovitsh, D., Shachter, A., Ghanim, M., and Dudai, N. (2019). Interactive effects of genotype, seasonality and extraction method on chemical compositions and yield of essential oil from (Rosmarinus officinalis L.). Indust. Crops Prod. 138:111419. doi: 10.1016/j.indcrop.2019.05.068
Sajjadi, S. E., and Ghannadi, A. (2012). Analysis of the essential oil of Lamium amplexicaule L. from Northeastern Iran. J. Essent. Oil-Bear. Plants. 15, 577–581. doi: 10.1080/0972060X.2012.10644091
Salido, S., Altarejos, J., Nogueras, M., Saánchez, A., and Luque, P. (2003). Chemical composition and seasonal variations of rosemary oil from southern Spain. J. Essent. Oil Res. 15, 10–14. doi: 10.1080/10412905.2003.9712248
Santoyo, S., Cavero, S., Jaime, L., Ibañez, E., Señoráns, F. J., and Reglero, G. (2005). Chemical composition and antimicrobial activity of Rosmarinus officinalis L. essential oil obtained via supercritical fluid extraction. J. Food Prot. 68, 790–795. doi: 10.4315/0362-028x-68.4.790
Satyal, P., Jones, T. H., Lopez, E. M., McFeeters, R. L., Ali, N. A., Mansi, I., et al. (2017). Chemotypic characterization and biological activity of Rosmarinus officinalis. Foods 6:20. doi: 10.3390/foods6030020
Singh, S. S., Kumar, P., and Ashwani, K. R. A. (2006). “Ultraviolet radiation stress: Molecular and physiological adaptations in trees,” in Abiotic stress tolerance in plants, eds A. K. Rai and Takabe T. Dordrecht, and Springer Netherlands.
Tisné, S., Serrand, Y., Bach, L., Gilbault, E., Ben Ameur, R., Balasse, H., et al. (2013). Phenoscope: An automated large-scale phenotyping platform offering high spatial homogeneity. Plant J. 74, 534–544. doi: 10.1111/tpj.12131
Valenta, K., Dimac-Stohl, K., Baines, F., Smith, T., Piotrowski, G., Hill, N., et al. (2020). Ultraviolet radiation changes plant color. BMC Plant Biol. 20:253. doi: 10.1186/s12870-020-02471-8
Van Hintum, J. L., Brown, A. H. D., and Spillane, C. (2000). Core collections of plant genetic resources. Rome: Bioversity International.
World Health Organization [WHO] (1999). WHO monographs on selected medicinal plants. Geneva: World Health Organization.
Zhao, L.-L., Zhang, Y., Wang, P.-C., Luo, T.-Q., Zhang, W., and Chen, J. (2016). Morphological and genetic variations of Sophora davidii populations originating from different altitudes in the mountains of southwestern China. Flora 224, 1–6. doi: 10.1016/j.flora.2016.06.002
Zia-Ul-Haq, M., Ahmad, M., Iqbal, S., Ahmad, S., and Ali, H. (2007). Characterization and compositional studies of oil from seeds of desi chickpea (Cicer arietinum L.) cultivars grown in pakistan. J. Am. Oil. Chem. Soc. 84, 1143–1148. doi: 10.1007/s11746-007-1136-3
Keywords: Salvia rosmarinus, secondary metabolites, anti-microbial, phenotypic traits, gene pool
Citation: Khan NF, Bashir A, Ganaie KA, Shah SQ, Nazir R, Sultan P and Hassan QP (2025) Chemical composition and anti-microbial potential of essential oils from morphologically distinct Salvia rosmarinus (Spenn.) cultivars from Kashmir, India. Front. Microbiol. 16:1579383. doi: 10.3389/fmicb.2025.1579383
Received: 21 March 2025; Accepted: 03 June 2025;
Published: 02 July 2025.
Edited by:
Amira Zairi, University of Sousse, TunisiaReviewed by:
Vidyullatha Peddireddy, Central University of Haryana, IndiaAidé Sáenz-Galindo, Autonomous University of Coahuila, Mexico
Copyright © 2025 Khan, Bashir, Ganaie, Shah, Nazir, Sultan and Hassan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nafeesa Farooq Khan, a2hhbm5hZmVlc2Fjc2lyaWlpbUBnbWFpbC5jb20=; Qazi Parvaiz Hassan, cXBoYXNzYW5AaWlpbS5hYy5pbg==
 Nafeesa Farooq Khan
Nafeesa Farooq Khan Abid Bashir
Abid Bashir Khursheed Ahmad Ganaie
Khursheed Ahmad Ganaie