- 1College of Pharmaceutical Sciences, Southwest University, Chongqing, China
- 2Department of Pharmacy, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, China
The interplay between microbes and cancer has garnered significant attention in life sciences. Clinically, microbial infections in cancer patients are common complications and one of the major causes of mortality. Cancer patients often experience compromised immune defenses, and conventional therapies—including radiotherapy, chemotherapy, and invasive surgery—further diminish their resistance to pathogens. Emerging evidence indicates that intratumoral microbes and their interactions with the tumor microenvironment exacerbate cancer cell proliferation, drug resistance, metastasis, and poor prognosis. However, complex multidrug regimens increase patient burden and reduce compliance. This necessitates the development of single agents with dual anticancer and antimicrobial properties. Promisingly, naturally derived compounds and synthetic chemicals exhibit such dual functionalities. This review introduces microbial contributions to oncogenesis and analyzes molecular targets of dual-function agents, proposing their potential as novel therapeutics to improve clinical outcomes.
1 Background
Microbial infections constitute a critical complication and leading mortality cause in cancer patients (Sepich-Poore et al., 2021; Helmink et al., 2019). Clinically, specific microbes correlate with malignant tumor behaviors, such as metastasis and drug resistance, and are linked to poor prognosis (Pang et al., 2024; Er et al., 2025). These microbes originate from both environmental sources and endogenous opportunistic pathogens. Their symbiotic relationship with tumor cells weakens immune defenses, exacerbating disease severity and mortality (Jiang et al., 2024; Shen et al., 2024; de Barros et al., 2024). However, the combination application of antibiotics and anticancer drugs currently faces thorny challenges, including dosing complexity, combination toxicity, drug interactions, and reduced compliance (Kubecek et al., 2021; Royo-Cebrecos et al., 2024).
Development of single agents with dual functions of anticancer and antibacterial activities has become an inevitable solution to address cancer complicated with bacterial infection. The agents not only inhibit the proliferation of cancer cells, but also eliminate bacteria at the same concentration, thereby block cancer and infection synergistic control, and simplify the medication regimens (Rai et al., 2024; Xie et al., 2024; Kumari et al., 2024). Preclinical studies demonstrate that such agents achieve long-term relapse-free survival (>700 days) and even complete remission (Wang et al., 2024). This review synthesizes current knowledge on microbial-tumor interactions and highlights advances in dual-functional drug development. This review introduces current knowledge on microbial-tumor interactions, and highlights the advances in the dual-functional drugs at the first time, which would provide a new insight into the development of this new category of drugs.
2 Infection in cancer patients
Immunocompromised cancer patients are highly susceptible to infections. Tumor cells play a pivotal role in undermining immune function. They secrete various immunosuppressive factors, such as transforming growth factor-β(TGF-β), interleukin-10 (IL-10), and prostaglandin E2 (PGE2) (Nduom et al., 2015; Motz and Coukos, 2013; Yaguchi and Kawakami, 2016). As the immune system weakens, the body becomes more susceptible to infections, and the presence of infections could, in turn, exacerbate the stress on the already compromised immune system of cancer patients (Kang et al., 2024). Cancer-associated immune dysfunction, compounded by cytotoxic therapies (chemotherapy, radiation, surgery, transplantation, and corticosteroids), creates a permissive environment for infections (Bastien et al., 2019).
Epidemiological analyses reveal ~20% of cancer patients develop sepsis, with infection-related mortality reaching 40–70% in immunocompromised cohorts (Boucher and Carpenter, 2020; Hensley et al., 2019; Nelmes et al., 2023). Diagnosing infections is challenging due to overlapping symptoms with cancer progression, such as low-grade fever, fatigue, and malaise (Yaegashi et al., 2014). Common pathogens include bacteria (Staphylococcus, Streptococcus, Enterococcus, Pseudomonas aeruginosa, Klebsiella pneumoniae) and fungi (Candida, Aspergillus, Mucormycosis, Cryptococcus, Pneumocystis) (Fentie et al., 2018; Petrikkos et al., 2024; Delgado and Guddati, 2021; Danielsen et al., 2023). To make matters worse, the increasing prevalence of antibiotic-resistant strains has severely complicated treatment, leaving these already vulnerable patients with limited effective treatment options.
3 Intratumoral microbe
Emerging evidence suggests that symbiotic bacteria and fungi reside within malignant tumor cells, as revealed by metagenomics and next-generation sequencing (Gao et al., 2022; Galeano Niño et al., 2022). A study analyzing fungal communities in over 17,000 tissue and blood samples from 35 cancer types detected microbial presence in all samples (Dohlman et al., 2022). Specific microbial communities are associated with gastrointestinal tumors, breast cancer, melanoma, lung cancer, bladder cancer, lymphomas, adenomas, and head and neck paragangliomas (Kim et al., 2024). For instance, Fusobacterium dominates colorectal cancer (CRC) niches, while Malassezia enriches in pancreatic tumors (Zepeda-Rivera et al., 2024; Aykut et al., 2019). Notably, tumor-resident microbes significantly impact therapeutic outcomes—for instance, intratumoral Enterococcus compromises Programmed Death-1 (PD-1) inhibitor efficacy in melanoma models (Matson et al., 2018). Mechanistically, intratumoral microbiota modulate tumor growth, metastasis, and treatment resistance through immune modulation, inflammation induction, and so on (Li and Saxena, 2022; Shi et al., 2024). These findings highlight the critical need for integrating targeted antimicrobial strategies into cancer therapeutic regimens to address microbial contributions to tumor progression and treatment failure.
4 Carcinogenic mechanisms of microbiota
Unlike normal cells, tumor cells could coexist with specific bacteria and fungi, forming a symbiotic microbiota that benefits both tumor and microbial proliferation and drug resistance (Lutsiv et al., 2024). For instance, fungi associated with lung cancer include Aspergillus, Blastomyces, Cryptococcus, Malassezia, Candida, Bacillus, while bacteria such as Staphylococcus, Streptococcus, Pseudomonas aeruginosa (P. aeruginosa), Klebsiella, Escherichia coli (E. coli) contribute to a cancer-promoting microenvironment. The presence of these microbes is strongly associated with elevated mortality rates and unfavorable prognoses in patients.
The carcinogenic effects of bacteria and fungi are mediated through several mechanisms (Figure 1): (1) Chronic inflammation induction; (2) Immunosuppression and immune evasion; (3) Secretion of virulence factors that act as carcinogens; (4) Induction of DNA mutations; (5) Activation of oncogene expression and signaling pathways; (6) Inhibition of tumor cell apoptosis.
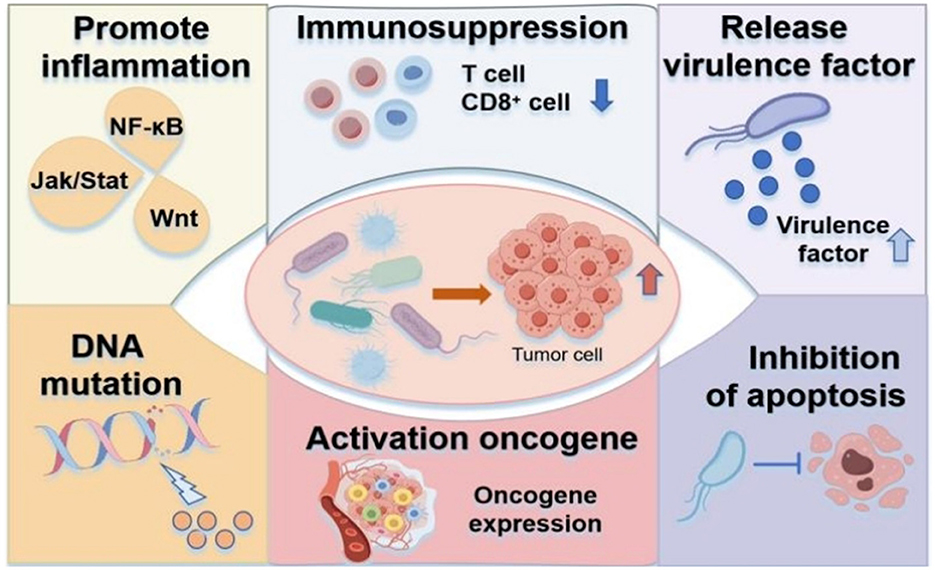
Figure 1. Carcinogenic mechanisms of microbiota. (1) Chronic inflammation from excessive immune activation. (2) Immune evasion via immunosuppressive mechanisms. (3) Virulence factor secretion. (4) DNA damage through mutagenic metabolites. (5) Oncogenic pathway activation. (6) Apoptosis resistance in tumor cells.
4.1 Immunosuppression and inflammatory activation of Porphyromonas gingivalis (P. gingivalis)
P. gingivalis infection enhances programmed death-ligand 1 (PD-L1) expression on dendritic cells within tumor microenvironments and lymph nodes through Akt/STAT3 pathway activation, subsequently impairing CD8+ T cell functionality (Ren et al., 2023). This manifests as significant downregulation of interleukin-2 (IL-2), interferon-γ (IFN-γ), perforin, granzyme B, and CD107a expression in CD8+ T cells. In murine models, P. gingivalis-induced immunosuppression significantly enlarges tumor areas and accelerates oral cancer progression (Luo et al., 2024). Given its prevalence in human gingival microbiota, targeting P. gingivalis proliferation and its molecular signaling pathways could enhance CD8+ T cell activity and improve PD-1/PD-L1 immunotherapy efficacy.
Furthermore, P. gingivalis promotes malignancy in gastrointestinal cancers, including CRC and esophageal cancer. CRC mouse models administered P. gingivalis exhibit increased colorectal tumor burden compared to controls (Wang et al., 2021). Mechanistically, P. gingivalis recruits myeloid immune cells to activate the NLRP3 inflammasome, driving excessive inflammation that facilitates CRC initiation and progression.
4.2 Induction of chronic inflammation by Candida albicans (C. albicans)
C. albicans promotes carcinogenesis through chronic inflammation by orchestrating a cascade of molecular and immunological disruptions. Studies have demonstrated that C. albicans is closely associated with oral malignancies. Recent research has elucidated several mechanisms underlying this association, including disruption of the epithelial barrier, production of carcinogenic substances (e.g., nitrosamines and acetaldehyde), and induction of chronic inflammation (Liao et al., 2020). Concurrently, C. albicans disrupts mucosal barriers via hydrolases (e.g., proteases), enabling persistent microbial invasion and chronic antigen exposure. This triggers sustained activation of NF-κB and STAT3 pathways, driving the production of proinflammatory cytokines (IL-6, TNF-α, and IL-1β) that fuel hyperproliferation and apoptosis resistance in epithelial tissues (Wang X. et al., 2023).
The interplay between dysregulated immune responses and fungal persistence further exacerbates oncogenic processes. In genetically susceptible hosts, such as those with chronic mucocutaneous candidiasis, defective IFN pathways amplify inflammatory damage and epithelial dysplasia (Smeekens et al., 2013). Additionally, C. albicans synergizes with bacterial pathogens (e.g., Streptococcus) to activate TLR2/4 and NLRP3 inflammasomes, amplifying IL-1β-driven inflammation and reactive oxygen species (ROS) generation, both implicated in malignant transformation (Wang X. et al., 2023). Animal models of oral carcinogenesis confirm these mechanisms, where antifungal therapy reduces squamous hyperplasia and carcinoma incidence, directly linking C. albicans to inflammation-mediated oncogenesis (Sano et al., 2009).
4.3 Promotion of tumor cell immune evasion by Malassezia
Malassezia, an opportunistic pathogenic fungus commensal on human skin, exhibits conditional pathogenicity contingent upon host immune status. Emerging evidence indicates that immunosuppression facilitates fungal translocation into internal organs, thereby potentiating tumorigenesis and malignant progression (Spatz and Richard, 2020). Notably, Malassezia demonstrates tissue tropism for multiple malignancies, including pancreatic ductal adenocarcinoma (PDA), breast cancer, gastric cancer, and prostate cancer. Quantitative analysis reveals a remarkable 3,000-fold enrichment of fungal biomass in PDA tumor tissues compared to adjacent normal parenchyma, a phenomenon conserved across human and murine model (Aykut et al., 2019). In breast cancer patients, fungal detection in tumor specimens correlates with significantly reduced overall survival rates compared to Malassezia-negative cohorts (Liu et al., 2024).
The mechanistic underpinnings involve immunomodulatory pathways mediated by Malassezia. Notably, gastric cancer specimens with fungal colonization exhibit concomitant upregulation of PD-L1 expression, suggesting fungal-mediated immune evasion through checkpoint protein induction (Zhang et al., 2022). This immunoregulatory axis may explain the observed acceleration of tumor progression in colonized malignancies.
4.4 Release of virulence factors from Helicobacter pylori (H. pylori)
H. pylori is a well-established driver of gastric carcinogenesis, implicated in ~90% of gastric cancer cases (Engelsberger et al., 2024). It also associates with malignancies such as colon cancer, pancreatic cancer, and gastric mucosa-associated lymphoid tissue lymphoma. Notably, 80% of infected individuals remain asymptomatic despite severe potential consequences.
The procarcinogenic activity of H. pylori primarily stems from virulence factor secretion. Cytotoxin-associated gene A (CagA), a key virulence factor, undergoes phosphorylation by host c-Src/c-Abl kinases, activating oncogenic signaling pathways that induce gastric epithelial inflammation, precancerous lesions, and adenocarcinoma (Zhao et al., 2024). Additional virulence factors include vacuolating cytotoxin A (VacA), which triggers immune cell apoptosis, and γ-glutamyl transpeptidase (γ-GT), which disrupts gastric epithelial integrity and promotes immune tolerance (Wang B. et al., 2023; Baskerville et al., 2023). Targeting H. pylori infection may thus complement existing cancer therapies.
4.5 Induction of tumor cell DNA mutation by polyketide-non-ribosomal peptide synthase operon (pks+) E. coli
The genotoxic pks+ E. coli contributes to colorectal carcinogenesis through a mutagenic mechanism mediated by its secondary metabolite, colibactin (Pleguezuelos-Manzano et al., 2020). Pks+ E. coli produces colibactin, a secondary metabolite synthesized by the pks genomic island. Colibactin acts as a crosslinking agent, forming covalent interstrand crosslinks between adenine residues in double-stranded DNA. Colibactin induces DNA interstrand crosslinks, primarily targeting adenine residues, which obstruct replication forks and lead to double-strand breaks during S phase. This damage triggers error-prone repair processes, such as non-homologous end joining, resulting in characteristic mutational signatures. These mutations preferentially affect cancer driver genes (e.g., APC and KRAS) and promote chromosomal instability through chromothripsis-like rearrangements. Clinically, pks+ E. coli colonization correlates with elevated mutation burden and adverse prognosis, highlighting its role as a microbial driver of oncogenic mutagenesis in colorectal cancer.
4.6 Activation of oncogenic signal pathways by Fusobacterium nucleatum (F. nucleatum)
Intestinal microbiota such as F. nucleatum is strongly linked to CRC pathogenesis (Garrett, 2019). F. nucleatum exerts potent procancer effects in vitro and in vivo, notably enhancing CRC tumor growth in murine models. Its primary carcinogenic mechanism involves FadA adhesion protein-mediated activation of the Wnt/β-catenin pathway via E-cadherin binding (Cohn et al., 2023; Rubinstein et al., 2013). Additionally, F. nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling (TLR4) to nuclear factor -κB (NF-κB), and up-regulating expression of microRNA-21 (Yang et al., 2017). Moreover, F. nucleatum colonization accelerates breast cancer metastasis, highlighting its systemic oncogenic potential (Fu et al., 2022).
5 Progress of single agents for anticancer and antibacterial activities
The interdependent relationship between tumor cells and microbes presents a formidable obstacle, making it challenging to entirely halt tumor growth and recurrence through the exclusive use of anticancer treatments or antibacterial medications (Kapoor et al., 2022; Ma et al., 2023). In clinical settings, established anticancer interventions, such as chemotherapy, radiotherapy, and invasive surgical methods, typically succeed in restraining tumor cell proliferation. However, their effectiveness against the bacteria and fungi associated with tumors remains notably inadequate (Inamura, 2023; Sheng et al., 2024). Given these difficulties, single agents exhibiting broad-spectrum antimicrobial and anticancer capabilities are surfacing as promising prospects for both preclinical and clinical exploration (Rohilla et al., 2014; Yadav et al., 2023).
5.1 Overall mechanism of single agents with anticancer and antimicrobial effects
Natural products, clinically established antimicrobial and antitumor agents, and newly synthesized single compounds have been identified as pivotal sources for developing novel antimicrobial and antitumor therapeutics. Diverse phytochemicals, such asalkaloids, sesquiterpenes, flavonoids, and saponins, demonstrate broad-spectrum bioactivities encompassing antioxidant, anti-inflammatory, antimicrobial, and antitumor properties. Furthermore, clinically validated dual-activity drugs and novel synthetic bifunctional compounds exhibit multimodal biological effects, including antibacterial, antifungal, and antitumor activities. These agents execute their pharmacological actions via diverse mechanisms of action (Table 1), ranging from microbial membrane disruption to tumor cell apoptosis induction. These findings underscore the concurrent antimicrobial and antitumor capacities inherent in both natural and synthetic compounds, highlighting substantial potential for designing multifunctional therapeutic agents targeting the concurrent management of infectious diseases and malignancies.
Dual-functional agents eliminate microbes and cancer cells through multiple mechanisms (Figure 2): (1) Cell cycle arrest: Targeting the uncontrolled proliferation shared by microbes and tumor cells. (2) Regulation of cell energy metabolism. (3)Immune modulation: Enhancing immune surveillance and defense capabilities. (4) Membrane permeabilization: Increasing cell membrane permeability to induce cytotoxicity. (5) ROS induction: Promoting localized ROS overproduction to eradicate pathogens and tumor cells.
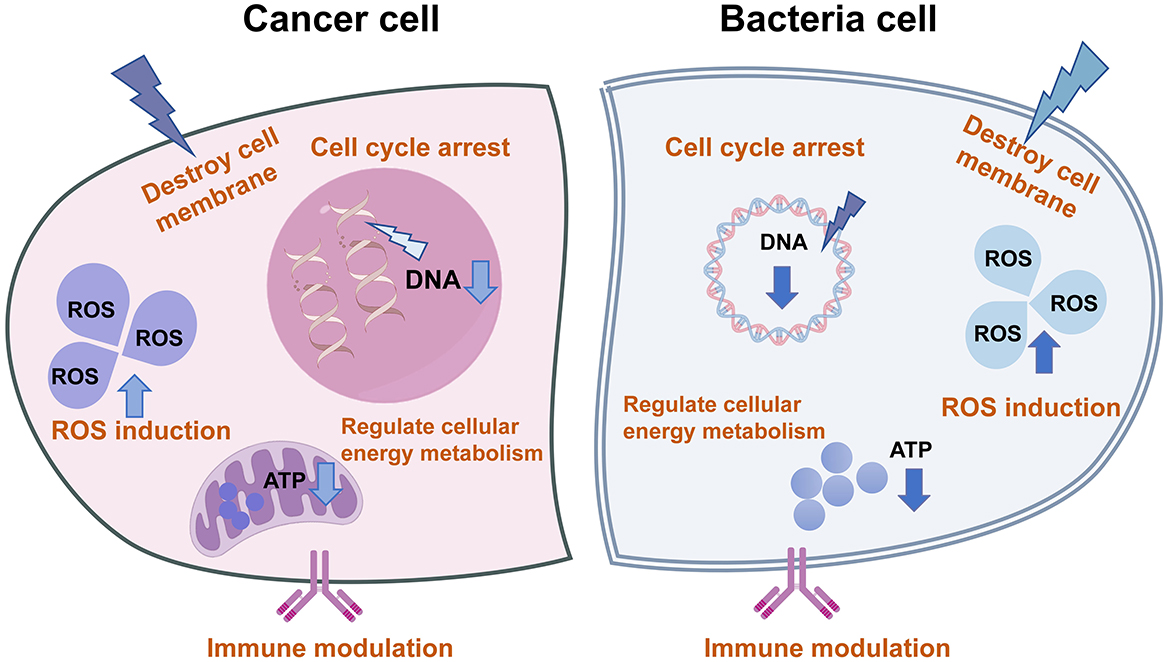
Figure 2. The molecular mechanism of single agents against cancer and microbia. These agents typically exert dual inhibitory effects on both cancer and bacterial pathogens through multifaceted mechanisms, including cell cycle arrest, modulation of energy metabolism pathways, immunoregulatory functions, enhancement of membrane permeability, and induction of ROS generation.
5.2 Naturally-originating single compounds with antibacterial and anticancer effects
Compounds with anticancer and antimicrobial properties, such as alkaloids, sesquiterpenes, flavonoids, and saponins, are widely distributed in nature. Natural products and their derivatives with antibacterial and anticancer activities, including rapamycin, curcumin, berberine, xanthoxylin, allicin, quercetin, solanine, quinalizarin, and evodiamine, have been intensively investigated (Fan and Yao, 2022; Vuong, 2021). These natural compounds have shown remarkable potential in both combating cancer cells and inhibiting the growth of various microbes. For instance, rapamycin, initially discovered as an antifungal agent, has now been extensively studied for its anti-cancer properties (Ganesh and Subathra, 2023). It functions by inhibiting the mammalian target of rapamycin (mTOR) pathway, which plays a crucial role in cell growth, proliferation, and survival, thus impeding the development of tumors. Curcumin, a major component of turmeric, has potent antioxidant and anti-inflammatory properties (Abd El-Hack et al., 2021). In cancer treatment, it could induce apoptosis in cancer cells, modulate multiple signaling pathways, and also exhibits antibacterial activity against a wide range of bacteria, including both Gram-positive and Gram-negative strains (Weng and Goel, 2022). Berberine, found in plants like Coptis chinensis, has been shown to have antibacterial effects by intBerberineerfering with bacterial DNA replication and cell membrane integrity (Khan et al., 2022). In the context of cancer, it could suppress tumor cell growth, invasion, and metastasis through various mechanisms such as regulating gene expression and cell cycle progression (Xiong et al., 2022).
Antimicrobial peptides (AMPs), such as LL-37, Human β-defensin, Buforin II, Peptide-P2, demonstrate dual antimicrobial and antitumor activities through diverse mechanisms (Wu et al., 2021; Baindara et al., 2018; Min et al., 2024; Girdhar et al., 2024). Membrane disruption is a common antibacterial strategy: cationic AMPs bind to negatively charged bacterial membranes via electrostatic interactions, forming pores or dissolving lipid bilayers, leading to cell lysis (Pérez-Peinado et al., 2018; Dong et al., 2014). Similarly, lactoferrin-derived peptides disrupts microbial membranes while modulating immune responses to enhance pathogen clearance (Gruden and Poklar, 2021).
In antitumor activity, AMPs exploit cancer cells' altered membrane properties (e.g., phosphatidylserine exposure). Melectin penetrates cancer cell membranes and binds intracellular DNA or RNA, inhibiting replication and inducing apoptosis (Liang et al., 2021). Defensins may trigger mitochondrial dysfunction or activate death receptors in tumors, akin to Bogorol B-JX's ROS-mediated apoptosis (Jiang et al., 2017). These mechanisms, combined with low resistance development, underscore AMPs as versatile therapeutic candidates.
5.3 New function of clinical antibiotics and anticancer drugs
Dactinomycin, also known as actinomycin D, is an antibiotic isolated from the bacterium Streptomyces parvulus. It has long been recognized for its potent antibacterial properties, but in recent years, its anticancer activity has attracted significant attention. It is a key component in the treatment of Wilms' tumor, a common kidney cancer in children (Green et al., 1993; Armstrong et al., 2025). It is also used in the treatment of rhabdomyosarcoma, a type of soft-tissue sarcoma, and orther soft tissue sarcomas (Koscielniak et al., 2024). The primary mechanism by which dactinomycin exerts its anticancer effects is through its ability to intercalate into DNA. It specifically binds to the guanine-cytosine (G-C) base pairs in the DNA double helix. This binding disrupts the normal function of DNA-dependent RNA polymerase, thereby inhibiting transcription (Passaquin et al., 1998). Moreover, dactinomycin could also induce DNA strand breaks, leading to genomic instability and ultimately triggering apoptosis in cancer cells.
Furthermore, antifungal agents have also been found to possess anticancer activity. Thiabendazole (TBZ) is a widely used generic antifungal drug with over 50 years of clinical application and high safety. Its antifungal mechanism lies in inhibiting the formation of microtubules during fungal mitosis. Later, it was serendipitously discovered that TBZ has potential anticancer activity (Garge et al., 2021; Hu et al., 2022). In a mouse model of fibrosarcoma (a connective-tissue tumor with a rich vascular network), TBZ reduces angiogenesis in the tumor by more than 50%, thereby delaying tumor growth and metastasis. The vascular damage caused by TBZ is related to a decrease in microtubule protein, which is similar to its antifungal mechanism.
Natamycin (NAT) is a polyene zwitterionic macrolide antibiotic that exerts antifungal effects by binding to ergosterol on fungal cell membranes. Recently, An et al. reported that NAT could induce liver cancer cell apoptosis both in vitro and in vivo. The mechanism is closely associated with the down-regulation of the expression of peroxisome protein 1, which leads to a significant increase in ROS accumulation (An et al., 2021). In addition, the blockade of the AKT/mTOR signal pathway is also involved in the anticancer activity of NAT. This blockade induces cell-protective autophagy and reduces the acquired drug resistance of cancer cells.
Itraconazole is a commonly used antifungal medication in clinical. It is found that has the ability to significantly increase the overall survival rate of tumor-bearing animalst as combination with antitumor drugst (Kast, 2024). In clinical trials (NCT02749513), itraconazole is orally administered to patients with esophageal cancer, including adenocarcinoma (EAC) and squamous cell carcinoma (ESCC), and the results show that itraconazole has potent antitumor properties, partially through blockade of HER2/AKT signaling pathway (Zhang W. et al., 2021). Moreover, another antifungal agent, clotrimazole, can inhibit cancer cell division and proliferation by blocking the entry of calcium and potassium ions into the cells (Cortat and Zobi, 2023).
The third-generation EGFR inhibitor osimertinib (OSI) has a dual function. In addition to being an anticancer drug, it can also be used in the treatment of fungal infections. OSI could target the host EGFR receptor to inhibit the endocytosis of Candida albicans into host cells, thereby protecting the host cells from fungal invasion (Liu et al., 2022). Additionally, OSI could bind to the drug efflux pump Pdr5 of C. albicans and suppress the efflux of the antifungal drug fluconazole (FLC). This action increases the accumulation of FLC in cells and improves the antifungal efficacy of FLC. Thus, OSI combined with FLC has broad-spectrum antifungal efficacy against multiple fungal resistant strains.
Certain antibiotics, such as thiabendazole, as well as anticancer drugs like actinomycin D, exhibit dual functionalities of antibacterial and anticancer activities, enabling the simultaneous treatment of infections and cancer. These medications have been extensively applied in clinical practice, with their safety and efficacy well-established. Their mechanisms of these drugs, which include inhibiting microtubule formation or inducing DNA strand breaks, have been intensively investigated. However, the relatively low antibacterial or anticancer activity of these drugs restricts their widespread application. Moreover, some drugs exhibit a narrow therapeutic window, manifested as dose-limiting toxicity.
5.4 Development of single compounds with antibacterial and anticancer activities
Based on the interplay between tumor cells and microbial infections, diverse single compounds exhibiting both antimicrobial and anticancer properties have been synthesized. The mechanisms of action of these drugs include inhibiting the cell cycle, inducing the generation of ROS, regulating the immune system, and inhibiting energy metabolism, etc.
5.4.1 Inhibition of cell cycle
Minor groove binders (MGBs) exhibit antitumor and antibacterial properties. These agents generally induce either permanent DNA damage or temporary inactivation of DNA (Msallam et al., 2025). Upon binding, MGBs disrupt interactions between critical proteins (e.g., transcription factors and polymerases) and DNA in tumor cells through allosteric perturbation, thereby inhibiting replication or transcription processes (Alniss et al., 2024). Notably, MGBs such as MGB28 and MGB32 demonstrate potent inhibitory effects against Gram-positive bacteria and Gram-negative bacteria. Their antibacterial mechanism involves binding to the minor groove of bacterial DNA, which interferes with DNA unwinding or impairs nuclease functionality.
5.4.2 Induction of ROS generation
In recent years, several biomaterials have been synthesized that exhibit both anticancer and antibacterial activities. The underlying mechanism of these biomaterials against tumors and bacteria primarily involves increasing intracellular ROS levels. This leads to irreparable oxidative damage to bacterial cell membranes and other functional biomolecules such as DNA, enzymes, and fatty acids, thereby exerting cytotoxic effects on both bacteria and tumor cells. These bifunctional nanomaterials encompass inorganic nanomaterials, organic polymer materials, and photosensitizers. Notably, inorganic nano antibacterial agents are a type of anticancer and antibacterial material that capitalizes on the properties of inorganic metal ions (Shi et al., 2018; Tehrani Nejad et al., 2022). Once these nanomaterials enter cells, they could promote bacteria to generate ROS, causing cell deformation, collapse, and ultimately cell death (Jing et al., 2024).
Photodynamic therapy (PDT) is an outstanding technique for treating tumors and microbial infections. It utilizes photosensitive agents and laser activation to generate ROS (Xu et al., 2024; Law et al., 2024). This technique works by injecting a photosensitizer into animals and then irradiating the lesion site with a specific wavelength. This process facilitates the selective accumulation of the photosensitizer in the lesion, triggering a photochemical reaction to generate excessive ROS and destroy tumors and microbes (Zhang J. et al., 2024). Notably, aggregation-induced emission (AIE) photosensitizers in the PDT technique have been extensively employed in photodynamic anticancer and antibacterial therapy (Xu et al., 2025). Among the reported organic photosensitizers, AIE photosensitizers have unique aggregation-induced enhancement effects on ROS production. They have been successfully applied in microbial and tumor detection, identification, and treatment (Yang et al., 2021).
5.4.3 Improvement of immune surveillance and defense ability
Tumor resident intracellular microbiota (TRIM) has recently garnered significant attention as a crucial factor in carcinogenic processes (Fu et al., 2022; Ma et al., 2021). The immunosuppressive tumor microenvironment (TME) serves as a haven for TRIM. Accumulating evidence indicates that TRIM can not only reduce the efficacy of chemotherapy but also enhance drug toxicity to normal tissues, while simultaneously promoting the pro-inflammatory response and proliferation of tumor cells (Geller et al., 2017; Sears et al., 2014).
In this regard, Yuanlin Wan et al. proposed a solution to the issues of immunosuppression and over-growth in TRIM-infected cancers by incorporating TRIM-targeted antimicrobials into anti-tumor therapy (Wang et al., 2024). To enable simultaneous antimicrobial and anti-tumor therapy with a single drug, they designed Ag2Se shell-coated Au nanoparticles whose surface was modified with folic acid (Au@Ag2Se-FA NPs). FA on the surface of Au@Ag2Se-FA is a pivotal targeting moiety. Given that FA has a high affinity for folate receptors, which are overexpressed on a wide range of cancer cells, this specific binding enables Au@Ag2Se-FA to be selectively internalized by tumor cells. Once inside, it could initiate a series of events that impact cellular immunity. For instance, it may enhance the recognition of tumor-associated antigens by T cells, promoting their activation and proliferation. This activation leads to a more robust and targeted attack on cancer cells. Moreover, The Ag ions released from the nanoparticle possess potent antibacterial and anticancer capabilities. These ions can disrupt the cellular structures and functions of both bacteria and cancer cells, effectively eliminating them.
Au@Ag2Se-FA restores cellular immunity in both anticancer and antibacterial contexts. By targeting cancer cells, releasing antigens, and promoting T-cell activation, it rejuvenates the antitumor immune response. Simultaneously, its antibacterial action helps maintain a healthy immune environment, enabling better-coordinated cellular immune responses against both tumors and bacteria, which ultimately contributes to improved patient outcomes and the potential for long-term relapse-free survival.
5.4.4 Regulation of cell energy metabolism
Mitochondria are the key organelles for cellular energy metabolism. According to the endosymbiotic theory, mitochondria might originate from ancient bacteria. Due to the high dependence of tumor and bacterial cells on energy metabolism for maintaining the cell proliferation, targeted damage of mitochondria might have dual anticancer and antibacterial effects (Tehrani Nejad et al., 2022).
Hemiprotonic bisphenanthroline (ph-ph+) possesses practical antimicrobial and anticancer activities, partially through disrupting the energy metabolism of bacteria and tumor cells (Zhao et al., 2022a,b). Antibacterial experiments demonstrate that ph-ph+ has an excellent inhibitory effect on both Gram-positive and Gram-negative bacteria, exhibiting broad-spectrum antibacterial activity against S. aureus, E. coli, S. pyogenes, S. pneumoniae, C. nucleatum, P. mirabilis, and B. fragilis. In addition, ph-ph+ could induce apoptosis in tumor cells such as H22, B16, U251MG, and SH-SY5Y at the same concentration used for antibacterial activity. Although the anticancer and antibacterial mechanisms of ph-ph+ are not fully understood, preliminary studies suggest that they may be related to the inhibition of energy generation in eukaryotic cell mitochondria and bacteria.
5.4.5 Others
Recently, thanks to the rapid advancement of chemical synthesis technology, emerging studies highlight novel strategies for dual-functional drug development. These innovative approaches leverage advances in synthetic biology and targeted delivery to address the complex tumor-microbe interplay. For instance, antibiotic-antibody conjugates (AACs) combine tumor-targeting antibodies with antimicrobial payloads, enabling selective delivery to infection-primed tumor microenvironments (Nurkesh et al., 2019). A study demonstrated that a ciprofloxacin-conjugated anti-EGFR antibody effectively eradicated Pseudomonas aeruginosa-infected colorectal tumors while sparing normal tissues. Small-molecule inhibitors such as quinoline-5-sulfonamides norfloxacin analogs, and zelkovamycin analogs possess anticancer and antibacterial activities (Zieba et al., 2024; Qurban et al., 2024). BET-HDAC dual inhibitors can prevent the proliferation of cancer cells and C. albicans (Huang et al., 2023). Moreover, polymersomes and carbon nanodots can function as drug carriers against cancers, fungi, and bacteria simultaneously (Nurkesh et al., 2019; Wang et al., 2022).
These compounds have diverse structures and can simultaneously combat tumors and potential infections, achieving a synergistic therapeutic effect. The synthesized compounds allow for precise chemical modifications to enhance potency and selectivity. They may act on multiple targets simultaneously or interfere with the physiological functions of cells through a completely new mechanism of action. This multi-target or new-mechanism mode of action increases the difficulty of drug resistance and is expected to delay or overcome the problem of drug resistance to a certain extent. Generally, these drugs exhibit strong antibacterial and anti-tumor activities. However, these results were obtained from experimental animals and need to be verified in clinical settings. Although these compounds possess antibacterial and anti-cancer activities, their molecular targets have not been fully elucidated. This means that we are not clear about which specific molecules the drugs interact with inside cells to exert their effects. This uncertainty makes it difficult to further optimize the drugs, predict drug responses, and understand potential side effects.
6 Conclusion and future perspectives
The intricate symbiosis between microorganisms and tumors presents both challenges and opportunities in modern oncology. Here we review the effects of microorganisms on cancer cell proliferation, infiltration, metastasis, drug resistance, and highlight the significance of single agents against both bacteria and cancer cells. The dual functional agents can simultaneously target tumor proliferation pathways and key active sites of pathogens, reducing clinical treatment complexity and minimizing the risk of multi-drug application. They also would achieve synergistic effects by decreasing the mutual promotion between pathogens and cancer cells.
Regarding the predictive biomarkers for the dual-functional therapies in patients, it may be considered to use the factors as predictive biomarkers of patient response to the therapies because bacterial infection and tumor infiltration are accompanied by the alteration in inflammation and immune factors (Zhang X. et al., 2024). In addition, 16S rRNA gene sequencing and genomic identification could achieve the comprehensive analysis of bacterial flora in tumor-bearing animals, which would be another method to evaluate the effects of the dual-functional therapies (Roje et al., 2024; Zeng et al., 2025).
Due to the persistent problem of concurrent infections in cancer patients, single agents with antibacterial and anticancer activities hold great promise. This innovative therapeutic strategy possesses transformative potential for oncology by employing a dual-targeting mechanism that simultaneously disrupts tumor-microbe symbiosis and modulates tumor-associated microbiota.
Author contributions
KZ: Project administration, Writing – review & editing. ZY: Software, Visualization, Writing – review & editing. JL: Software, Visualization, Writing – review & editing. AF: Writing – original draft, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Hubei Province (grant number JCZRLH202501013); Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science (grant number 2024YJ06A).
Acknowledgments
We thank Dr. Muhammad Samee Mubarik to edit the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd El-Hack, M. E., El-Saadony, M. T., Swelum, A. A., Arif, M., Abo Ghanima, M. M., Shukry, M., et al. (2021). Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 101, 5747–5762. doi: 10.1002/jsfa.11372
Adyns, L., Proost, P., and Struyf, S. (2023). Role of defensins in tumor biology. Int. J. Mol. Sci. 24:5268. doi: 10.3390/ijms24065268
Alniss, H. Y., Al-Jubeh, H. M., Msallam, Y. A., Siddiqui, R., Makhlouf, Z., Ravi, A., et al. (2024). Structure-based drug design of DNA minor groove binders and evaluation of their antibacterial and anticancer properties. Eur. J. Med. Chem. 271:116440. doi: 10.1016/j.ejmech.2024.116440
An, Y., Jiang, J., Zhou, L., Shi, J., Jin, P., Li, L., et al. (2021). Peroxiredoxin 1 is essential for natamycin-triggered apoptosis and protective autophagy in hepatocellular carcinoma. Cancer Lett. 521, 210–223. doi: 10.1016/j.canlet.2021.08.023
Armstrong, A. E., Daw, N. C., Renfro, L. A., Geller, J. I., Kalapurakal, J. A., Khanna, G., et al. (2025). Treatment of focal anaplastic Wilms tumor: a report from the Children's Oncology Group AREN0321 and AREN03B2 studies. Cancer 131:e35713. doi: 10.1002/cncr.35713
Aykut, B., Pushalkar, S., Chen, R., Li, Q., Abengozar, R., Kim, J. I., et al. (2019). The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574, 264–267. doi: 10.1038/s41586-019-1608-2
Bai, R. F., Wang, Y. W., Yang, S. S., Zhang, Y. H., Zhou, B. H., Zhou, C. W., et al. (2024). Design, synthesis and bioactivity of benzyl propiolates with broad-spectrum inhibition activity on phytopathogenic fungi. J. Agric. Food Chem. 72, 27053–27061. doi: 10.1021/acs.jafc.4c06770
Baindara, P., Korpole, S., and Grover, V. (2018). Bacteriocins: perspective for the development of novel anticancer drugs. Appl. Microbiol. Biotechnol. 102, 10393–10408. doi: 10.1007/s00253-018-9420-8
Baskerville, M. J., Kovalyova, Y., Mejías-Luque, R., Gerhard, M., and Hatzios, S. K. (2023). Isotope tracing reveals bacterial catabolism of host-derived glutathione during Helicobacter pylori infection. PLoS Pathog. 19:e1011526. doi: 10.1371/journal.ppat.1011526
Bastien, J. P., Minguy, A., Dave, V., and Roy, D. C. (2019). Cellular therapy approaches harnessing the power of the immune system for personalized cancer treatment. Semin. Immunol. 42:101306. doi: 10.1016/j.smim.2019.101306
Bhattacharjya, S., Zhang, Z., and Ramamoorthy, A. (2024). LL-37: structures, antimicrobial activity, and influence on amyloid-related diseases. Biomolecules 14:320. doi: 10.3390/biom14030320
Bian, P., Hu, W., Liu, C., and Li, L. (2020). Resveratrol potentiates the anti-tumor effects of rapamycin in papillary thyroid cancer: PI3K/AKT/mTOR pathway involved. Arch. Biochem. Biophys. 689:108461. doi: 10.1016/j.abb.2020.108461
Boucher, J. E., and Carpenter, D. (2020). Sepsis: symptoms, assessment, diagnosis, and the hour-1 bundle in patients with cancer. Clin. J. Oncol. Nurs. 24, 99–102. doi: 10.1188/20.CJON.99-102
Bui, P. H., Cao, T. N. M., Tran, T. T., Matsumoto, T., Akada, J., and Yamaoka, Y. (2025). Identification of genetic determinants of antibiotic resistance in Helicobacter pylori isolates in Vietnam by high-throughput sequencing. BMC Microbiol. 25:264. doi: 10.1186/s12866-025-03990-w
Cao, P., Zhang, Z. W., Leng, D. J., Li, X. Y., and Li, Y. (2016). [Progress of antibacterial activity and antibacterial mechanism of isoquinoline alkaloids]. Zhongguo Zhong Yao Za Zhi 41, 2600–2606. doi: 10.4268/cjcmm20161406
Chen, Y., Li, H., Liu, N., Feng, D., Wu, W., Gu, K., et al. (2024). Multi-mechanism antitumor/antibacterial effects of Cu-EGCG self-assembling nanocomposite in tumor nanotherapy and drug-resistant bacterial wound infections. J. Colloid Interface Sci. 671, 751–769. doi: 10.1016/j.jcis.2024.05.080
Cohn, D. E., Forder, A., Marshall, E. A., Vucic, E. A., Stewart, G. L., Noureddine, K., et al. (2023). Delineating spatial cell-cell interactions in the solid tumour microenvironment through the lens of highly multiplexed imaging. Front. Immunol. 14:1275890. doi: 10.3389/fimmu.2023.1275890
Cortat, Y., and Zobi, F. (2023). Resurgence and repurposing of antifungal azoles by transition metal coordination for drug discovery. Pharmaceutics 15:2398. doi: 10.3390/pharmaceutics15102398
Danielsen, A. S., Franconeri, L., Page, S., Myhre, A. E., Tornes, R. A., Kacelnik, O., et al. (2023). Clinical outcomes of antimicrobial resistance in cancer patients: a systematic review of multivariable models. BMC Infect. Dis. 23:247. doi: 10.1186/s12879-023-08182-3
de Barros, G. M., Borges, I. N., Ravetti, C. G., Diniz, P. H., Ferreira, S. R., De Mori, L. H., et al. (2024). Significant drop in serum C-reactive protein in patients with solid neoplasia and bacterial infection is associated with a better prognosis and identifies candidates for short-course antibiotic therapy. BMC Infect. Dis. 24:974. doi: 10.1186/s12879-024-09544-1
Delgado, A., and Guddati, A. K. (2021). Infections in hospitalized cancer patients. World J. Oncol. 12, 195–205. doi: 10.14740/wjon1410
Dohlman, A. B., Klug, J., Mesko, M., Gao, I. H., Lipkin, S. M., Shen, X., et al. (2022). A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 185, 3807–3822. doi: 10.1016/j.cell.2022.09.015
Dong, L., Qin, J., Tai, L., Mou, K., Liao, X., Chen, F., et al. (2022). Inactivation of bacillus subtilis by curcumin-mediated photodynamic technology through inducing oxidative stress response. Microorganisms 10:802. doi: 10.3390/microorganisms10040802
Dong, N., Zhu, X., Lv, Y. F., Ma, Q. Q., Jiang, J. G., and Shan, A. S. (2014). Cell specificity and molecular mechanism of antibacterial and antitumor activities of carboxyl-terminal RWL-tagged antimicrobial peptides. Amino Acids 46, 2137–2154. doi: 10.1007/s00726-014-1761-8
Engelsberger, V., Gerhard, M., and Mejias-Luque, R. (2024). Effects of Helicobacter pylori infection on intestinal microbiota, immunity and colorectal cancer risk. Front. Cell. Infect. Microbiol. 14:1339750. doi: 10.3389/fcimb.2024.1339750
Er, A. G., Aslan, A. T., Mikulska, M., and Akova, M. (2025). Prevention and treatment of bacterial infections in patients with haematological cancers and haematopoietic stem cell transplantation: headways and shortcomings. Clin. Microbiol. Infect. 31, 24–28. doi: 10.1016/j.cmi.2024.09.015
Fan, M., and Yao, L. (2022). The synthesis, structural modification and mode of anticancer action of evodiamine: a review. Recent Pat. Anticancer. Drug Discov. 17, 284–296. doi: 10.2174/1574892817666211221165739
Fentie, A., Wondimeneh, Y., Balcha, A., Amsalu, A., and Adankie, B. T. (2018). Bacterial profile, antibiotic resistance pattern and associated factors among cancer patients at University of Gondar Hospital, Northwest Ethiopia. Infect. Drug Resist. 11, 2169–2178. doi: 10.2147/IDR.S183283
Fu, A., Yao, B., Dong, T., Chen, Y., Yao, J., Liu, Y., et al. (2022). Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 185, 1356–1372. doi: 10.1016/j.cell.2022.02.027
Galeano Niño, J. L., Wu, H., LaCourse, K. D., Kempchinsky, A. G., Baryiames, A., Barber, B., et al. (2022). Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 611, 810–817. doi: 10.1038/s41586-022-05435-0
Ganesh, S. K., and Subathra, D. C. (2023). Molecular and therapeutic insights of rapamycin: a multi-faceted drug from Streptomyces hygroscopicus. Mol. Biol. Rep. 50, 3815–3833. doi: 10.1007/s11033-023-08283-x
Gao, F., Yu, B., Rao, B., Sun, Y., Yu, J., Wang, D., et al. (2022). The effect of the intratumoral microbiome on tumor occurrence, progression, prognosis and treatment. Front. Immunol. 13:1051987. doi: 10.3389/fimmu.2022.1051987
Garge, R. K., Cha, H. J., Lee, C., Gollihar, J. D., Kachroo, A. H., Wallingford, J. B., et al. (2021). Discovery of new vascular disrupting agents based on evolutionarily conserved drug action, pesticide resistance mutations, and humanized yeast. Genetics 219:iyab101. doi: 10.1093/genetics/iyab101
Garrett, W. S. (2019). The gut microbiota and colon cancer. Science 364, 1133–1135. doi: 10.1126/science.aaw2367
Geller, L. T., Barzily-Rokni, M., Danino, T., Jonas, O. H., Shental, N., Nejman, D., et al. (2017). Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160. doi: 10.1126/science.aah5043
Girdhar, M., Sen, A., Nigam, A., Oswalia, J., Kumar, S., and Gupta, R. (2024). Antimicrobial peptide-based strategies to overcome antimicrobial resistance. Arch. Microbiol. 206:411. doi: 10.1007/s00203-024-04133-x
Green, D. M., Breslow, N. E., and D‘Angio, G. J. (1993). The treatment of children with unilateral Wilms' tumor. J. Clin. Oncol. 11, 1009–1010. doi: 10.1200/JCO.1993.11.6.1009
Gruden, S., and Poklar, U. N. (2021). Diverse mechanisms of antimicrobial activities of lactoferrins, lactoferricins, and other lactoferrin-derived peptides. Int. J. Mol. Sci. 22:11264. doi: 10.3390/ijms222011264
Hanaoka, Y., Yamaguchi, Y., Yamamoto, H., Ishii, M., Nagase, T., Kurihara, H., et al. (2016). In vitro and in vivo anticancer activity of human beta-defensin-3 and its mouse homolog. Anticancer Res. 36, 5999–6004. doi: 10.21873/anticanres.11188
Helmink, B. A., Khan, M. A. W., Hermann, A., Gopalakrishnan, V., and Wargo, J. A. (2019). The microbiome, cancer, and cancer therapy. Nat. Med. 25, 377–388. doi: 10.1038/s41591-019-0377-7
Hensley, M. K., Donnelly, J. P., Carlton, E. F., and Prescott, H. C. (2019). Epidemiology and outcomes of cancer-related versus non-cancer-related sepsis hospitalizations. Crit. Care Med. 47, 1310–1316. doi: 10.1097/CCM.0000000000003896
Hu, Y., Zhou, W., Xue, Z., Liu, X., Feng, Z., Zhang, Y., et al. (2022). Thiabendazole inhibits glioblastoma cell proliferation and invasion targeting mini-chromosome maintenance protein 2. J. Pharmacol. Exp. Ther. 380, 63–75. doi: 10.1124/jpet.121.000852
Huang, Y., Liu, N., Pan, Z., Li, Z., and Sheng, C. (2023). BET-HDAC dual inhibitors for combinational treatment of breast cancer and concurrent candidiasis. J. Med. Chem. 66, 1239–1253. doi: 10.1021/acs.jmedchem.2c01191
Hujber, Z., Petovári, G., Szoboszlai, N., Dankó, T., Nagy, N., Kriston, C., et al. (2017). Rapamycin (mTORC1 inhibitor) reduces the production of lactate and 2-hydroxyglutarate oncometabolites in IDH1 mutant fibrosarcoma cells. J Exp Clin Cancer Res 36:74. doi: 10.1186/s13046-017-0544-y
Inamura, K. (2023). Beyond bacteria: fungi in the tumor microbiome. Cancers 15:572. doi: 10.3390/cancers15030572
Jiang, H., Ji, C., Sui, J., Sa, R., Wang, X., Liu, X., et al. (2017). Antibacterial and antitumor activity of Bogorol B-JX isolated from Brevibacillus laterosporus JX-5. World J. Microbiol. Biotechnol. 33:177. doi: 10.1007/s11274-017-2337-z
Jiang, H., Li, L., Bao, Y., Cao, X., and Ma, L. (2024). Microbiota in tumors: new factor influencing cancer development. Cancer Gene Ther. 31, 1773–1785. doi: 10.1038/s41417-024-00833-0
Jiang, H., Rao, Y., Mei, L., and Wang, Y. (2021). Antifungal activity of rapamycin on Botryosphaeria dothidea and its effect against Chinese hickory canker. Pest Manag. Sci. 77, 425–431. doi: 10.1002/ps.6035
Jing, L., Zhuang, F., Feng, W., Huang, H., Chen, Y., and Huang, B. (2024). Doping-engineered piezoelectric ultrathin nanosheets for synergistically piezo-chemocatalytic antitumor and antibacterial therapies against cutaneous melanoma. Small 20:e2401171. doi: 10.1002/smll.202401171
Kang, K., Lin, X., Chen, P., Liu, H., Liu, F., Xiong, W., et al. (2024). T cell exhaustion in human cancers. Biochim. Biophys. Acta Rev. Cancer 1879:189162. doi: 10.1016/j.bbcan.2024.189162
Kang, M., Wu, Y., Shi, Q., Wang, Z., Zhang, Y., Chen, K., et al. (2025). Antifungal susceptibility and clinical efficacy of chlorhexidine combined with topical ophthalmic medications against Fusarium species isolated from corneal samples. Front. Cell Infect. Microbiol. 15:1532289. doi: 10.3389/fcimb.2025.1532289
Kapoor, R., Saini, A., and Sharma, D. (2022). Indispensable role of microbes in anticancer drugs and discovery trends. Appl. Microbiol. Biotechnol. 106, 4885–4906. doi: 10.1007/s00253-022-12046-2
Kast, R. E. (2024). IC regimen: delaying resistance to lorlatinib in ALK driven cancers by adding repurposed itraconazole and cilostazol. Cells. (2024) 13:1175. doi: 10.3390/cells13141175
Khan, S., Hussain, A., Attar, F., Bloukh, S. H., Edis, Z., Sharifi, M., et al. (2022). A review of the berberine natural polysaccharide nanostructures as potential anticancer and antibacterial agents. Biomed. Pharmacother. 146:112531. doi: 10.1016/j.biopha.2021.112531
Kim, J. H., Kim, J. S., Choi, N., Koh, J., Jeon, Y. K., Chang, J. H., et al. (2024). Higher microbial abundance and diversity in bronchus-associated lymphoid tissue (BALT) lymphomas than in non-cancerous lung tissues. Cancer Res. Treat. 57, 580–589. doi: 10.4143/crt.2024.689
Koscielniak, E., Ljungman, G., Kazanowska, B., Niggli, F., Sparber-Sauer, M., Handgretinger, R., et al. (2024). Maintenance therapy with trofosfamide, idarubicin and etoposide in patients with rhabdomyosarcoma and other high-risk soft tissue sarcomas (CWS-2007-HR): a multicentre, open-label, randomised controlled phase 3 trial. EClinicalMedicine 78:102957. doi: 10.1016/j.eclinm.2024.102957
Kubecek, O., Paterova, P., and Novosadova, M. (2021). Risk factors for infections, antibiotic therapy, and its impact on cancer therapy outcomes for patients with solid tumors. Life 11:1387. doi: 10.3390/life11121387
Kulkarni, A. M., Gayam, P. K. R., Baby, B. T., and Aranjani, J. M. (2025). Epithelial-mesenchymal transition in cancer: a focus on itraconazole, a Hedgehog inhibitor. Biochim. Biophys. Acta Rev. Cancer 1880:189279. doi: 10.1016/j.bbcan.2025.189279
Kumari, S., Kumari, A., Dhiman, A., Mihooliya, K. N., Raje, M., Prasad, G. S., et al. (2024). Unveiling the potential of novel Metschnikowia yeast biosurfactants: triggering oxidative stress for promising antifungal and anticancer activity. Microb. Cell Fact. 23:245. doi: 10.1186/s12934-024-02489-9
Law, S. K., Liu, C. W. C., Tong, C. W. S., and Au, D. C. T. (2024). Potential of resveratrol to combine with hydrogel for photodynamic therapy against bacteria and cancer-a review. Biomedicines 12:2095. doi: 10.3390/biomedicines12092095
Lee, H. S., Park, C. B., Kim, J. M., Jang, S. A., Park, I. Y., Kim, M. S., et al. (2008). Mechanism of anticancer activity of buforin IIb, a histone H2A-derived peptide. Cancer Lett. 271, 47–55. doi: 10.1016/j.canlet.2008.05.041
Lee, J. K., Chang, S. W., Perinpanayagam, H., Lim, S. M., Park, Y. J., Han, S. H., et al. (2013). Antibacterial efficacy of a human beta-defensin-3 peptide on multispecies biofilms. J. Endod. 39, 1625–1629. doi: 10.1016/j.joen.2013.07.035
Li, X., and Saxena, D. (2022). The tumor mycobiome: a paradigm shift in cancer pathogenesis. Cell 185, 3648–3651. doi: 10.1016/j.cell.2022.09.013
Li, Y., Chen, L., Chen, Y., Shi, H., Yu, S., Funmilayo, A., et al. (2025). Exosome-decorated bio-heterojunctions reduce heat and ROS transfer distance for boosted antibacterial and tumor therapy. Biomaterials 315:122921. doi: 10.1016/j.biomaterials.2024.122921
Liang, X., Yan, J., Lu, Y., Liu, S., and Chai, X. (2021). The antimicrobial peptide melectin shows both antimicrobial and antitumor activity via membrane interference and DNA binding. Drug Des. Devel. Ther. 15, 1261–1273. doi: 10.2147/DDDT.S288219
Liao, M., Cheng, L., Zhou, X. D., and Ren, B. (2020). [Research progress of Candida albicans on malignant transformation of oral mucosal diseases]. Hua Xi Kou Qiang Yi Xue Za Zhi 38, 431–437. doi: 10.7518/hxkq.2020.04.014
Liu, M.-M., Zhu, H.-H., Bai, J., Tian, Z.-Y., Zhao, Y.-J., Boekhout, T., et al. (2024). Breast cancer colonization by Malassezia globosa accelerates tumor growth. Mbio 15:e199324. doi: 10.1128/mbio.01993-24
Liu, N. N., Zhou, J., Jiang, T., Tarsio, M., Yu, F., Zheng, X., et al. (2022). A dual action small molecule enhances azoles and overcomes resistance through co-targeting Pdr5 and Vma1. Transl. Res. 247, 39–57. doi: 10.1016/j.trsl.2022.04.002
Luo, Z., Lv, S., Lou, F., Yan, L., Xu, J., Kang, N., et al. (2024). Roles of intralesional bacteria in the initiation and progression of oral squamous cell carcinoma. Cancer Med. 13:e70209. doi: 10.1002/cam4.70209
Lutsiv, T., Hussan, H., and Thompson, H. J. (2024). Ecosystemic approach to understanding gut microbiome-mediated prevention of colorectal cancer. Cancer J. 30, 329–344. doi: 10.1097/PPO.0000000000000743
Ma, J., Huang, L., Hu, D., Zeng, S., Han, Y., and Shen, H. (2021). The role of the tumor microbe microenvironment in the tumor immune microenvironment: bystander, activator, or inhibitor? J. Exp. Clin. Cancer Res. 40:327. doi: 10.1186/s13046-021-02128-w
Ma, J., Wei, Q., Cheng, X., Zhang, J., Zhang, Z., and Su, J. (2023). Potential role of gut microbes in the efficacy and toxicity of immune checkpoints inhibitors. Front. Pharmacol. 14:1170591. doi: 10.3389/fphar.2023.1170591
Matson, V., Fessler, J., Bao, R., Chongsuwat, T., Zha, Y., Alegre, M. L., et al. (2018). The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108. doi: 10.1126/science.aao3290
Min, K. H., Kim, K. H., Ki, M. R., and Pack, S. P. (2024). Antimicrobial peptides and their biomedical applications: a review. Antibiotics 13:794. doi: 10.3390/antibiotics13090794
Motz, G. T., and Coukos, G. (2013). Deciphering and reversing tumor immune suppression. Immunity 39, 61–73. doi: 10.1016/j.immuni.2013.07.005
Msallam, Y. A., Ramadan, W. S., Al-Jubeh, H., Ravi, A., Alhamidi, R. S., Yener Ilce, B., et al. (2025). Design, synthesis, and anticancer evaluation of novel MGBs with alkyne-linked thiazole moieties. J. Med. Chem. 68, 15065–15084. doi: 10.1021/acs.jmedchem.5c01216
Nagaoka, I., Tamura, H., and Reich, J. (2020). Therapeutic potential of cathelicidin peptide LL-37, an antimicrobial agent, in a murine sepsis model. Int. J. Mol. Sci. 21:5973. doi: 10.3390/ijms21175973
Nduom, E. K., Weller, M., and Heimberger, A. B. (2015). Immunosuppressive mechanisms in glioblastoma. Neuro Oncol. 17(Suppl. 7), vii9–vii14. doi: 10.1093/neuonc/nov151
Nelmes, E., Edwards, L., Jhanji, S., Antcliffe, D. B., and Tatham, K. C. (2023). Patients with cancer and sepsis trials: an unfair representation?. Clin. Med. 23, 635–636. doi: 10.7861/clinmed.2023-0408
Nurkesh, A. A., Sun, Q., Fan, H., Dukenbayev, K., Tsoy, A., Altaikyzy, A., et al. (2019). Date pit carbon dots induce acidic inhibition of peroxidase and disrupt DNA repair in antibacteria resistance. Glob. Chall. 3:1900042. doi: 10.1002/gch2.201900042
Olender, A., Bogut, A., Dabrowski, W., Pietrzak, D. J., Szukała, M., Wójtowicz-Bobin, M., et al. (2025). Analysis of antifungal drug resistance among Candida Spp. and other pathogenic yeasts isolated from patients in eastern poland: diagnostic problems. Infect. Drug Resist. 18, 2187–2199. doi: 10.2147/IDR.S504516
Pang, S. W., Armon, S., Chook, J. B., Chew, J., Peh, K. B., Lim, W. W., et al. (2024). Association of Fusobacterium nucleatum infection with the clinicopathological characteristics in colorectal cancer patients. Mol. Biol. Rep. 51:124. doi: 10.1007/s11033-023-09150-5
Passaquin, A. C., Lhote, P., and Ruegg, U. T. (1998). Calcium influx inhibition by steroids and analogs in C2C12 skeletal muscle cells. Br. J. Pharmacol. 124, 1751–1759. doi: 10.1038/sj.bjp.0702036
Patel, S. S., Acharya, A., Ray, R. S., Agrawal, R., Raghuwanshi, R., and Jain, P. (2020). Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 60, 887–939. doi: 10.1080/10408398.2018.1552244
Perez, J., Cifuentes, J., Cuellar, M., Suarez-Arnedo, A., Cruz, J. C., and Muñoz-Camargo, C. (2019). Cell-penetrating and antibacterial BUF-II nanobioconjugates: enhanced potency via immobilization on polyetheramine-modified magnetite nanoparticles. Int. J. Nanomed. 14, 8483–8497. doi: 10.2147/IJN.S224286
Pérez-Peinado, C., Dias, S. A., Domingues, M. M., Benfield, A. H., Freire, J. M., Rádis-Baptista, G., et al. (2018). Mechanisms of bacterial membrane permeabilization by crotalicidin (Ctn) and its fragment Ctn(15-34), antimicrobial peptides from rattlesnake venom. J. Biol. Chem. 293, 1536–1549. doi: 10.1074/jbc.RA117.000125
Petrikkos, L., Kourti, M., Antoniadi, K., Tziola, T. S., Sfetsiori, A. E., Antari, V., et al. (2024). Central nervous system fungal diseases in children with malignancies: a 16-year study from the infection working group of the hellenic society of pediatric hematology oncology. J. Fungi 10:654. doi: 10.3390/jof10090654
Pleguezuelos-Manzano, C., Puschhof, J., Rosendahl Huber, A., van Hoeck, A., Wood, H. M., Nomburg, J., et al. (2020). Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature 580, 269–273. doi: 10.1038/s41586-020-2080-8
Porrello, A., Postiglione, A., Badalamenti, N., Bruno, M., Basile, A., Capasso, L., et al. (2025). Investigating the antiproliferative and antioxidant potential of xanthoxylin and of essential oil isolated from Pulicaria incisa (Lam.) DC. herbal medicine. Fitoterapia 180:106344. doi: 10.1016/j.fitote.2024.106344
Qurban, F., Shahzad, S. A., Khaskheli, M. S., Khan, S. U., Khan, S. A., Rauf, W., et al. (2024). Design, synthesis and evaluation of novel norfloxacin analogs as potent anticancer and antioxidant agents. Fut. Med. Chem. 16, 1777–1789. doi: 10.1080/17568919.2024.2383165
Rai, N., Tiwari, R. T., Sahu, A., Verma, E., Rathore, S., Patil, S., et al. (2024). Exploring tryptophan-based short peptides: promising candidate for anticancer and antimicrobial therapies. Anticancer. Agents Med. Chem. 25, 124–133. doi: 10.2174/0118715206260662240613054521
Ren, J., Han, X., Lohner, H., Hoyle, R. G., Li, J., Liang, S., et al. (2023). P. gingivalis infection upregulates PD-L1 expression on dendritic cells, suppresses CD8+ T-cell responses, and aggravates oral cancer. Cancer Immunol. Res. 11, 290–305. doi: 10.1158/2326-6066.CIR-22-0541
Rohilla, P., Deep, A., Kamra, M., Narasimhan, B., Ramasamy, K., Mani, V., et al. (2014). Synthesis, antimicrobial and anticancer evaluation of N'-(substituted benzylidene)-2-(benzo[d]oxazol-3(2H)-yl)acetohydrazide derivatives. Drug Res. 64, 505–509. doi: 10.1055/s-0034-1368720
Roje, B., Zhang, B., Mastrorilli, E., Kovačić, A., Sušak, L., Ljubenkov, I., et al. (2024). Gut microbiota carcinogen metabolism causes distal tissue tumours. Nature 632, 1137–1144. doi: 10.1038/s41586-024-07754-w
Royo-Cebrecos, C., Laporte-Amargós, J., Peña, M., Ruiz-Camps, I., Garcia-Vidal, C., Abdala, E., et al. (2024). Pseudomonas aeruginosa bloodstream infections presenting with septic shock in neutropenic cancer patients: impact of empirical antibiotic therapy. Microorganisms 12:705. doi: 10.3390/microorganisms12040705
Rubinstein, M. R., Wang, X., Liu, W., Hao, Y., Cai, G., and Han, Y. W. (2013). Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206. doi: 10.1016/j.chom.2013.07.012
Sano, T., Ozaki, K., Kodama, Y., Matsuura, T., and Narama, I. (2009). Effects of the antifungal agent itraconazole on proliferative changes of the forestomach mucosa in alloxan-induced diabetic rats. Toxicol. Pathol. 37, 790–798. doi: 10.1177/0192623309344204
Sears, C. L., Geis, A. L., and Housseau, F. (2014). Bacteroides fragilis subverts mucosal biology: from symbiont to colon carcinogenesis. J. Clin. Invest. 124, 4166–4172. doi: 10.1172/JCI72334
Sepich-Poore, G. D., Zitvogel, L., Straussman, R., Hasty, J., Wargo, J. A., and Knight, R. (2021). The microbiome and human cancer. Science 371:eabc4552. doi: 10.1126/science.abc4552
Shen, S., Yu, S., Yao, D., Wu, H., and Qian, Y. (2024). Special tissue microbiota such as Cyanobacteria are associated with the immune microenvironment of lung adenocarcinoma. Transl. Cancer Res. 13, 4408–4419. doi: 10.21037/tcr-24-107
Sheng, D., Jin, C., Yue, K., Yue, M., Liang, Y., Xue, X., et al. (2024). Pan-cancer atlas of tumor-resident microbiome, immunity and prognosis. Cancer Lett. 598:217077. doi: 10.1016/j.canlet.2024.217077
Shi, T., Sun, X., and He, Q. Y. (2018). Cytotoxicity of silver nanoparticles against bacteria and tumor cells. Curr. Protein Pept. Sci. 19, 525–536. doi: 10.2174/1389203718666161108092149
Shi, Z., Li, Z., and Zhang, M. (2024). Emerging roles of intratumor microbiota in cancer: tumorigenesis and management strategies. J. Transl. Med. 22:837. doi: 10.1186/s12967-024-05640-7
Singh, A., Sridhar, A., Poddar, A., Dimou, A., Parikh, K., Shanshal, M., et al. (2025). Combination of lurbinectedin and osimertinib for treatment of EGFR-mutated transformed SCLC: a brief report. JTO Clin. Res. Rep. 6:100807. doi: 10.1016/j.jtocrr.2025.100807
Smeekens, S. P., Ng, A., Kumar, V., Johnson, M. D., Plantinga, T. S., van Diemen, C., et al. (2013). Functional genomics identifies type I interferon pathway as central for host defense against Candida albicans. Nat. Commun. 4:1342. doi: 10.1038/ncomms2343
Spatz, M., and Richard, M. L. (2020). Overview of the potential role of malassezia in gut health and disease. Front. Cell. Infect. Microbiol. 10:201. doi: 10.3389/fcimb.2020.00201
Stakheev, D., Taborska, P., Kalkusova, K., Bartunkova, J., and Smrz, D. (2022). LL-37 as a powerful molecular tool for boosting the performance of ex vivo-produced human dendritic cells for cancer immunotherapy. Pharmaceutics 14:2747. doi: 10.3390/pharmaceutics14122747
Takeda, A., Takano, N., Kokuba, H., Hino, H., Moriya, S., Abe, A., et al. (2020). Macrolide antibiotics enhance the antitumor effect of lansoprazole resulting in lysosomal membrane permeabilization-associated cell death. Int. J. Oncol. 57, 1280–1292. doi: 10.3892/ijo.2020.5138
Tao, Z., Geng, D., Tao, J., Wang, J., Liu, S., Wang, Q., et al. (2023). Synergistic Antibacterial effect and mechanism of allicin and an Enterobacter cloacae bacteriophage. Microbiol. Spectr. 11:e315522. doi: 10.1128/spectrum.03155-22
Tehrani Nejad, S., Rahimi, R., Rabbani, M., and Rostamnia, S. (2022). Zn (II)-porphyrin-based photochemically green synthesis of novel ZnTPP/Cu nanocomposites with antibacterial activities and cytotoxic features against breast cancer cells. Sci. Rep. 12:17121. doi: 10.1038/s41598-022-21446-3
Tsubura, A., Lai, Y. C., Kuwata, M., Uehara, N., and Yoshizawa, K. (2011). Anticancer effects of garlic and garlic-derived compounds for breast cancer control. Anticancer Agents Med. Chem. 11, 249–253. doi: 10.2174/187152011795347441
Vallee, A., Lecarpentier, Y., and Vallee, J. N. (2019). Curcumin: a therapeutic strategy in cancers by inhibiting the canonical WNT/beta-catenin pathway. J. Exp. Clin. Cancer Res. 38:323. doi: 10.1186/s13046-019-1320-y
Vuong, T. V. (2021). Natural products and their derivatives with antibacterial, antioxidant and anticancer activities. Antibiotics 10:70. doi: 10.3390/antibiotics10010070
Wang, B., Gan, Q., Tong, Y., Qiao, Y., Han, M., Zhang, R., et al. (2023). A visual diagnostic detection of Helicobacter pylori and the gastric carcinoma-related virulence genes (cagA and vacA) by a fluorescent loop-mediated isothermal amplification (LAMP). Talanta 256:124260. doi: 10.1016/j.talanta.2023.124260
Wang, J., Peng, Y., Liu, Y., Yang, J., Ding, N., and Tan, W. (2015). Berberine, a natural compound, suppresses Hedgehog signaling pathway activity and cancer growth. BMC Cancer 15:595. doi: 10.1186/s12885-015-1596-z
Wang, T., Qin, J., Cheng, J., Li, C., and Du, J. (2022). Intelligent design of polymersomes for antibacterial and anticancer applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 14:e1822. doi: 10.1002/wnan.1822
Wang, X., Jia, Y., Wen, L., Mu, W., Wu, X., Liu, T., et al. (2021). Porphyromonas gingivalis promotes colorectal carcinoma by activating the hematopoietic NLRP3 inflammasome. Cancer Res. 81, 2745–2759. doi: 10.1158/0008-5472.CAN-20-3827
Wang, X., Zhang, W., Wu, W., Wu, S., Young, A., and Yan, Z. (2023). Is Candida albicans a contributor to cancer? A critical review based on the current evidence. Microbiol. Res. 272:127370. doi: 10.1016/j.micres.2023.127370
Wang, Y., Han, Y., Yang, C., Bai, T., Zhang, C., Wang, Z., et al. (2024). Long-term relapse-free survival enabled by integrating targeted antibacteria in antitumor treatment. Nat. Commun. 15:4194. doi: 10.1038/s41467-024-48662-x
Weng, W., and Goel, A. (2022). Curcumin and colorectal cancer: an update and current perspective on this natural medicine. Semin. Cancer Biol. 80, 73–86. doi: 10.1016/j.semcancer.2020.02.011
Wong, S. C., Kamarudin, M., and Naidu, R. (2021). Anticancer mechanism of curcumin on human glioblastoma. Nutrients 13:950. doi: 10.3390/nu13030950
Wu, R., Patocka, J., Nepovimova, E., Oleksak, P., Valis, M., Wu, W., et al. (2021). Marine invertebrate peptides: antimicrobial peptides. Front. Microbiol. 12:785085. doi: 10.3389/fmicb.2021.785085
Xie, X., Huang, H., Jaiswal, Y. S., Su, S., Yang, L., Fan, Y., et al. (2024). Synthesis and anticancer activity assessment of zelkovamycin analogues. Molecules 29:4483. doi: 10.3390/molecules29184483
Xiong, R. G., Huang, S. Y., Wu, S. X., Zhou, D. D., Yang, Z. J., Saimaiti, A., et al. (2022). Anticancer effects and mechanisms of berberine from medicinal herbs: an update review. Molecules 27:4523. doi: 10.3390/molecules27144523
Xu, X., Lu, W., Zhang, H., Wang, X., Huang, C., Huang, Q., et al. (2024). Hepatoma-targeting and ROS-responsive polymeric micelle-based chemotherapy combined with photodynamic therapy for hepatoma treatment. Int. J. Nanomed. 19, 9613–9635. doi: 10.2147/IJN.S475531
Xu, Y., Qiang, X., Xing, L., Wang, H., Zhang, J., Zhang, F., et al. (2018). A fusion antitumor peptide regulates proliferation and apoptosis of endothelial cells. Amino Acids 50, 1121–1129. doi: 10.1007/s00726-018-2589-4
Xu, Y., Zhang, J., Wang, Z., Zhang, P., Zhang, Z., Yang, Z., et al. (2025). Water-soluble AIE photosensitizer in short-wave infrared region for albumin-enhanced and self-reporting phototheranostics. Biomaterials 314:122847. doi: 10.1016/j.biomaterials.2024.122847
Yadav, P., Kaushik, C. P., Yadav, A., Yadav, J., and Singh, D. (2023). Piperazine-1,2,3-triazole scaffolds: design, synthesis, anticancer and antimicrobial evaluation. Fut. Med. Chem. 15, 679–697. doi: 10.4155/fmc-2022-0316
Yadav, S., Narasimhan, B., and Kaur, H. (2016). Perspectives of benzimidazole derivatives as anticancer agents in the New Era. Anticancer Agents Med. Chem. 16, 1403–1425. doi: 10.2174/1871520616666151103113412
Yaegashi, H., Izumi, K., Kitagawa, Y., Kadono, Y., Konaka, H., Mizokami, A., et al. (2014). Differential diagnosis between bacterial infection and neoplastic fever in patients with advanced urological cancer: the role of procalcitonin. Int. J. Urol. 21, 104–106. doi: 10.1111/iju.12178
Yaguchi, T., and Kawakami, Y. (2016). Cancer-induced heterogeneous immunosuppressive tumor microenvironments and their personalized modulation. Int. Immunol. 28, 393–399. doi: 10.1093/intimm/dxw030
Yang, K., Zhou, Y., Wang, Y., Zhao, S., Wu, X., Peng, X., et al. (2021). An iridium complex as an AIE-active photosensitizer for image-guided photodynamic therapy. Chem. Asian J. 16, 1780–1785. doi: 10.1002/asia.202100291
Yang, Y., Weng, W., Peng, J., Hong, L., Yang, L., Toiyama, Y., et al. (2017). Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-kappaB, and up-regulating expression of microRNA-21. Gastroenterology 152, 851–866. doi: 10.1053/j.gastro.2016.11.018
Zeng, W., Wang, J., Chen, Z., Yang, J., Zhu, A., Zheng, Y., et al. (2025). Efficient predictor for immunotherapy efficacy: detecting pan-clones effector tumor antigen-specific T cells in blood by nanoparticles loading whole tumor antigens. Adv. Sci. 12:e2409913. doi: 10.1002/advs.202409913
Zepeda-Rivera, M., Minot, S. S., Bouzek, H., Wu, H., Blanco-Míguez, A., Manghi, P., et al. (2024). A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 628, 424–432. doi: 10.1038/s41586-024-07182-w
Zhang, J., Xu, J., Zhang, J., Lin, Y., Li, J., Chen, D., et al. (2024). Poly(photosensitizer-prodrug) unimolecular micelles for chemo-photodynamic synergistic therapy of antitumor and antibacteria. Langmuir 40, 14908–14921. doi: 10.1021/acs.langmuir.4c00950
Zhang, Q., Yang, N., Mao, R., Hao, Y., Ma, X., Teng, D., et al. (2021). A recombinant fungal defensin-like peptide-P2 combats Streptococcus dysgalactiae and biofilms. Appl. Microbiol. Biotechnol. 105, 1489–1504. doi: 10.1007/s00253-021-11135-y
Zhang, W., Bhagwath, A. S., Ramzan, Z., Williams, T. A., Subramaniyan, I., Edpuganti, V., et al. (2021). Itraconazole exerts its antitumor effect in esophageal cancer by suppressing the HER2/AKT signaling pathway. Mol. Cancer Ther. 20, 1904–1915. doi: 10.1158/1535-7163.MCT-20-0638
Zhang, X., Shao, S., Song, N., Yang, B., Liu, F., Tong, Z., et al. (2024). Integrated omics characterization reveals reduced cancer indicators and elevated inflammatory factors after thermal ablation in non-small cell lung cancer patients. Respir. Res. 25:309. doi: 10.1186/s12931-024-02917-9
Zhang, Y., Luo, Z., Wang, D., He, F., and Li, D. (2014). Phytochemical profiles and antioxidant and antimicrobial activities of the leaves of Zanthoxylum bungeanum. Sci. World J. 2014:181072. doi: 10.1155/2014/181072
Zhang, Z., Qiu, Y., Feng, H., Huang, D., Weng, B., Xu, Z., et al. (2022). Identification of Malassezia globosa as a gastric fungus associated with PD-L1 expression and overall survival of patients with gastric cancer. J. Immunol. Res. 2022:2430759. doi: 10.1155/2022/2430759
Zhao, H., Chen, S., Bai, X., Zhang, J., Liu, S., Sun, Z., et al. (2024). GRB7-mediated enhancement of cell malignant characteristics induced by Helicobacter pylori infection. Front. Microbiol. 15:1469953. doi: 10.3389/fmicb.2024.1469953
Zhao, Z., Fu, C., Zhang, Y., Zhang, Y., Yang, X., and Fu, A. (2022a). Inhibitory effect of protonic bis(5-amino-1,10-phenanthroline) on proliferation of hepatocellular carcinoma and its molecular mechanism. Arab. J. Chem. 15:103982. doi: 10.1016/j.arabjc.2022.103982
Zhao, Z., Li, X., Cui, Z., Tong, T., Zhang, Y., Zhang, Y., et al. (2022b). Synthesis of hemiprotonic phenanthroline-phenanthroline(+) compounds with both antitumor and antimicrobial activity. J. Med. Chem. 65, 2532–2547. doi: 10.1021/acs.jmedchem.1c01982
Keywords: infection, anticancer, antibacterial, targets, drug development
Citation: Zhu K, Yuan Z, Li J and Fu A (2025) Microbial infection and treatment strategies in cancer patients. Front. Microbiol. 16:1582382. doi: 10.3389/fmicb.2025.1582382
Received: 24 February 2025; Accepted: 29 July 2025;
Published: 13 October 2025.
Edited by:
Francesca Montagnani, University of Siena, ItalyReviewed by:
Sanpreet Singh, University of Pittsburgh, United StatesVahideh Tarhriz, Tabriz University of Medical Sciences, Iran
Copyright © 2025 Zhu, Yuan, Li and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ailing Fu, ZmFsQHN3dS5lZHUuY24=
†These authors have contributed equally to this work
 Kejing Zhu
Kejing Zhu Zhibo Yuan1†
Zhibo Yuan1† Ailing Fu
Ailing Fu