- 1Department of Agricultural Science and Technology, Kenyatta University, Nairobi, Kenya
- 2One CGIAR, Impact Area Platform on Environmental Health and Biodiversity, Nairobi, Kenya
- 3International Institute of Tropical Agriculture (IITA), Dar es Salaam, Tanzania
Elucidating the diversity of native arbuscular mycorrhizal (AM) fungi is essential for the sustainable management of semi-arid land ecosystems. This is because they significantly improve plant nutrient uptake and decrease the stress caused by biotic and abiotic factors. In this study, we examined the AM fungal communities and the key drivers influencing their diversity and occurrence in the smallholder farming systems of Eastern Kenya. Soils samples were collected from 34 diverse agricultural fields and AM fungal spores were extracted using wet-sieving and decantation techniques. The spores were quantified, and AM fungal communities were identified based on their morphological characteristics. Statistical data analyses, including relative abundance, the Shannon-Wiener index, analysis of variance (ANOVA), and principal component analysis (PCA), were performed using R software 4.4.0. The results revealed that two AM fungal families dominated the agricultural fields, namely Gigasporaceae (61.0%) and Acaulosporaceae (39.0%). These fungal families comprised a total of five genera, with the following relative abundances: Acaulospora (39.0%), Gigaspora (35.05%), Scutellospora (23.92%), Dentiscutata (1.32%), and Rococetra (0.72%). The AM fungal morpho-species were ranked from 1 to 26 across the five genera. Acaulospora denticulata ranked the highest, with a proportion of 25.19%. The Shannon-Wiener diversity index revealed a higher diversity of AM fungi in agricultural fields with greater spore richness. The PCA showed that the composition of AM fungal communities was strongly related to soil physiochemical characteristics. Dryland farming systems also played a role in AM fungal composition. Overall, the distribution of AM fungal communities across the agricultural fields was lower, implying the need to adopt sustainable dryland farming systems to enhance native AM fungal communities and support the development of context-specific biofertilizers.
1 Introduction
Semi-arid lands (SALs) cover ~40% of the terrestrial land surface and account for ~40 % of global net primary productivity (Wang et al., 2012). In East Africa, over 250 million people depend on SALs for their livelihoods (De Leeuw, 2014). In Kenya, SALs are estimated to occupy 89–90% of the total terrestrial surface area (Amwata et al., 2016). These areas receive erratic rainfall between 300 and 600 mm annually (Vohland and Barry, 2009). SALs are synonymous with ecological degradation, such as soil erosion (Bishaw et al., 2013), which limits agricultural production and rural settlement (AbdelRahman et al., 2023). These regions are continually undergoing massive land use changes due to an increasing population, leading to the transformation of natural ecosystems into crop and animal farmlands (Mganga et al., 2024). Climate change remains a key challenge (Aguilar et al., 2024; Ntinyari and Gweyi-Onyango, 2020), and anthropogenic activities worsen the challenges faced in SALs, making them more severe and unpredictable (Coleine et al., 2024).
SALs host a diverse array of microorganisms that are either free-living or symbiotic (Coleine et al., 2024). One such key microbiome is arbuscular mycorrhizal (AM) fungi, which form mutualistic relationships with more than 80% of terrestrial plant species, ranging from bryophytes to tracheophytes (Smith and Read, 2010). AM fungi are obligate symbionts (Brundrett and Tedersoo, 2018). They create a healthy soil environment (Gupta, 2020) and provides several other benefits, including enhanced nutrient uptake, notably phosphorus and nitrogen (Smith and Read, 2008). This fungi help plants mitigate abiotic stresses, such as drought (Begum et al., 2019), and protect plant roots from soil-borne pathogen attacks (Del Fabbro and Prati, 2014). This mutual association often increases crop yield (Sakha et al., 2019; Buczkowska and Sałata, 2020) and improves the survival rate of vitro-grown plantlets to 100% (Dushimimana et al., 2022). Furthermore, AM fungi contribute indirectly to soil carbon sequestration by promoting soil aggregation (Leifheit et al., 2014). The exudates released by the extraradical hyphae of AM fungi bind soil particles and enhance soil aggregate stability (Wang et al., 2022). Consequently, understanding the dynamics and variables driving changes in AM fungal communities is necessary for applying AM fungi's biological functions in various fields (Yu et al., 2023).
Various environmental factors influence the AM fungal community, and it is clear that the soil environment imposes a strong selection pressure on resident AM fungi (Helgason and Fitter, 2009). Soil chemistry predominantly influences the dynamics of AM fungal spore abundance, root colonization, distribution, and diversity (Song et al., 2019; Šmilauer et al., 2020). The most pronounced soil factor determining the structure of AM fungal communities is pH (Dumbrell et al., 2010; Melo et al., 2017). Other factors include nitrogen (Silvana et al., 2020), phosphorus (Zhao et al., 2017; Ezeokoli et al., 2020), organic matter (Wang et al., 2015), and soil moisture (Veresoglou et al., 2013; Deepika and Kothamasi, 2015). Soil type is also another major factor shaping AM fungal communities. Van Geel et al. (2018) noted that there is a difference between AM fungal communities in acidic and calcareous grasslands. Climate change, particularly increased aridity, can reduce AM fungal diversity and abundance by limiting soil carbon and nitrogen storage due to primary production constraints (Delgado-Baquerizo et al., 2016). The community dynamics of AM fungi also depend on dispersal limitations, abiotic filtering, and biotic interactions (Vályi et al., 2016; Zhang et al., 2021).
One of the principal pillars of agroecology is soil health (HLPE, 2019), which is in line with the Kunming-Montreal Global Biodiversity Framework. This framework aims to ensure that at least 30% of degraded terrestrial land areas are effectively restoredby 2030 (Global Biodiversity Framework, 2022). Therefore, it is fundamental to continuously monitor natural microflora and characterize their exact roles in addressing the degradation of ecosystems and fostering resilience since native microorganisms are effective at colonizing soils and providing ecosystem services, especially in poor soils (Jiang et al., 2023). The main goal of this study was to examine the diversity and structure of native AM fungal communities in the semi-arid lands of Eastern Kenya. Despite their roles in SALs, limited studies have investigated these communities. We hypothesized that (i) the abundance and diversity of AM fungal spores differ between agricultural fields and (ii) AM fungal communities are related to edaphic and climatic factors. We anticipate that our study will support the adoption of appropriate dryland farming systems and the need for agricultural field restoration in SALs to maintain their ecological functions through the rational application of AM fungal inoculum.
2 Materials and methods
2.1 Study area
This study was conducted in Makueni County, Mbooni sub-county, Eastern Kenya (Figure 1). Makueni is situated at altitudes ranging from 600 m to 1,280 m above sea level (Saiz et al., 2016). The region receives an annual rainfall between 300 and 800 mm, with annual temperatures varying between 20°C and 36°C. Makueni County covers an area of ~8, 035 km2 and has a population of 987,550, according to the 2019 National Census (KNBs, 2019). The area is characterized by smallholder mixed farming, with farmers engaging in both livestock and crop farming. The major crops cultivated include maize (Zea mays), pigeon peas (Cajanus cajan), sorghum (Sorghum bicolor), cowpeas (Vigna unguiculata), millet (Eleusine coracana), common beans (Phaseolus vulgaris), green grams (Vigna radiata), and mangoes (Mangifera indica) (Makueni County Integrated Development Plan, 2023).
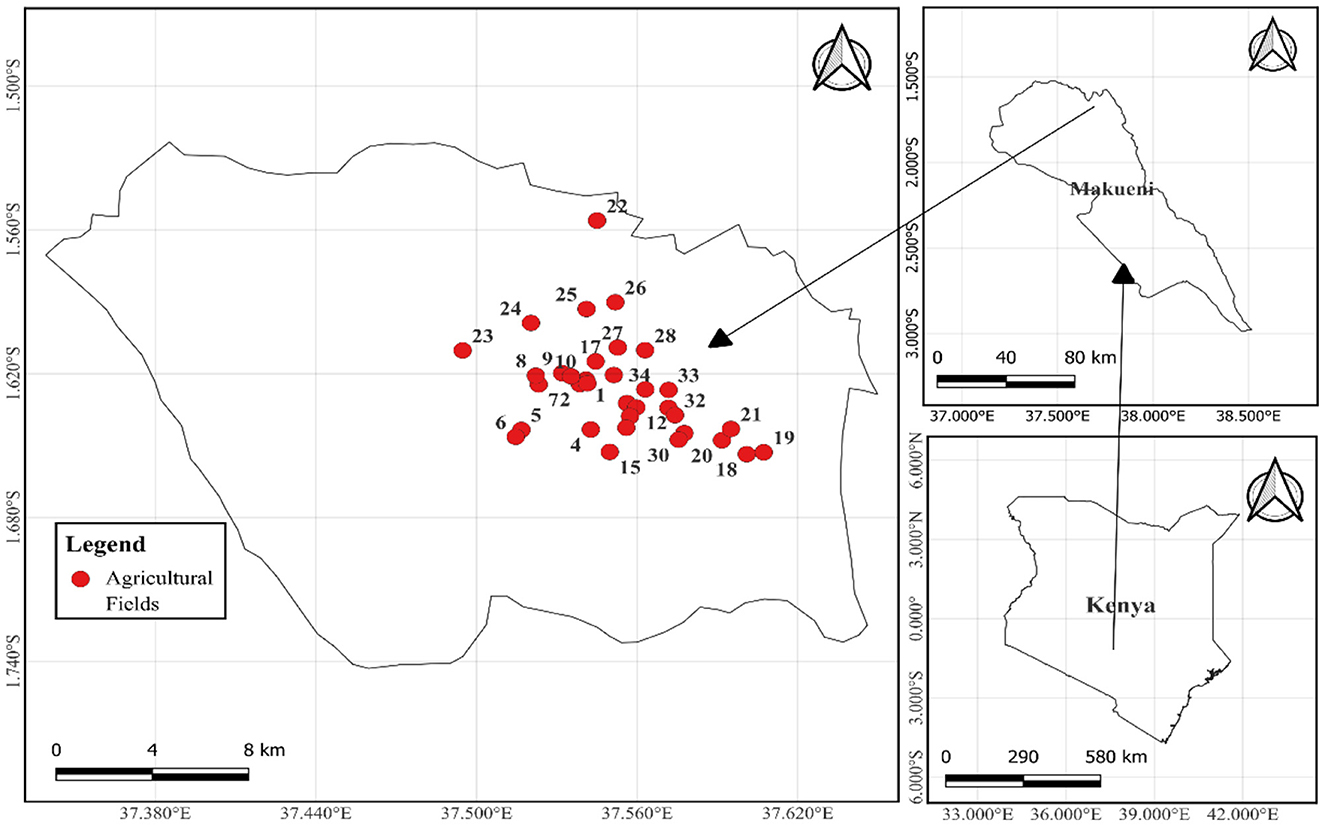
Figure 1. Map of Makueni County showing the location of the sampled agricultural fields. The numbers 1 to 34 marked in red indicate each of the sampled agricultural fields.
2.2 Soil sampling and handling
Soil samples, from which AM fungal spores were isolated, were collected from 34 farmer-managed agricultural fields (Figure 1), representing a diversity of farms in this Agroecological Living Landscapes (ALLs). The owners were selected during a co-design workshop implemented by a team of experts from the Agroecology Initiative of Consultative Group on International Agricultural Research (CGIAR) (Fuchs et al., 2023). The selected fields were primarily used for growing a variety of crops, including maize, common beans, cowpeas, pigeon peas, and green grams. Soil amendments used by the farmers included organic fertilizers, inorganic fertilizers, or a combination of the two (Table 1). Baseline soil samples were collected during the dry season of late September 2023 when root activities had declined and AM fungal spores were expected to be highly sporulating (Jefwa et al., 2006).
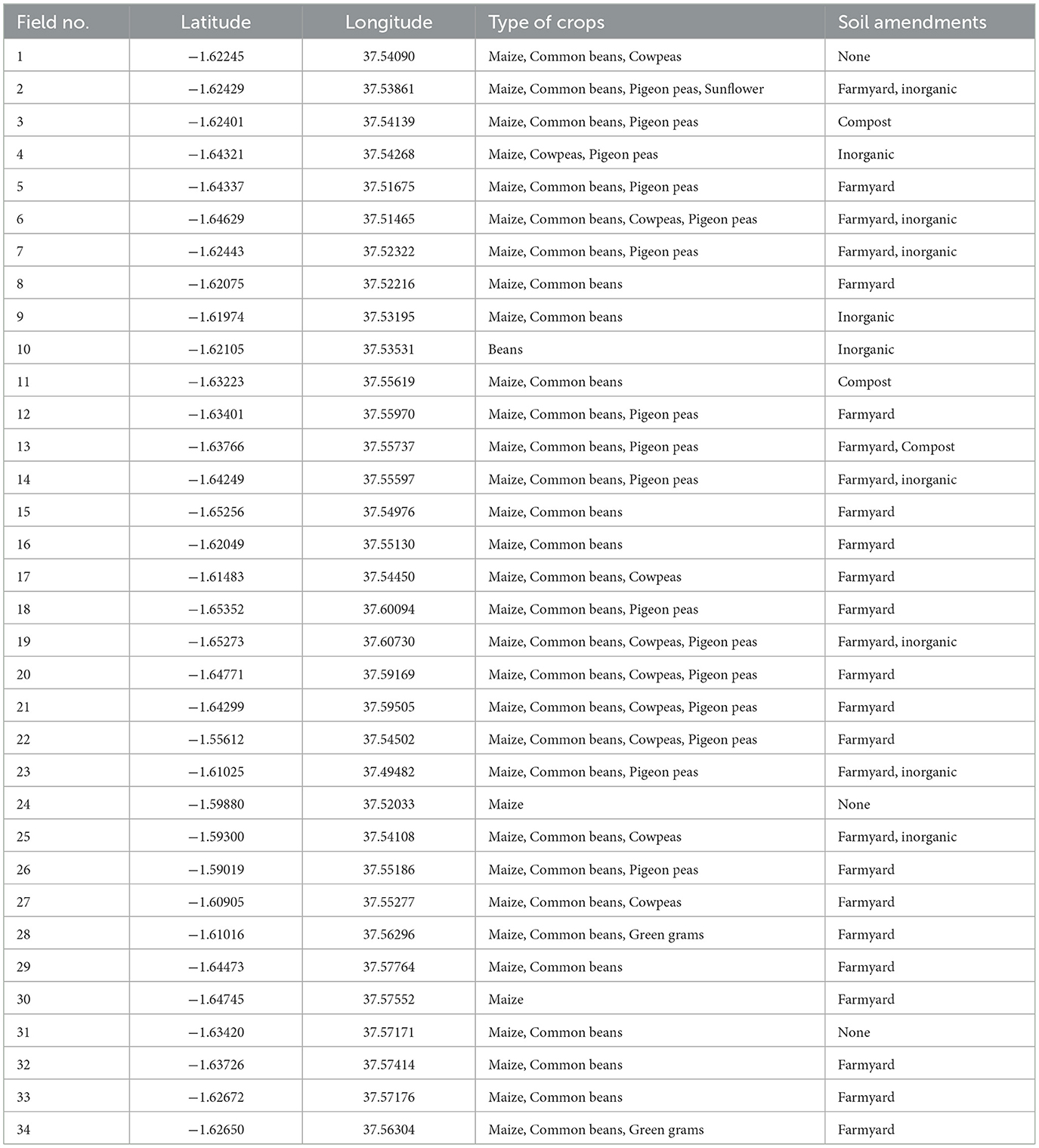
Table 1. GPS location, cropping system, and types of soil amendments in the agricultural fields where soil samples for AM fungal spore isolation were collected.
One soil sampling plot, measuring 12 m by 10 m, was delineated in each of the selected agricultural fields. Thereafter, nine sub-samples (5 cm in diameter and 20 cm in depth) were collected from each plot, following the adapted steps described in Figure 2 (Society for the Protection of Underground Networks, 2023). After removing any loose litter, the soil sampling procedure proceeded by collecting the first sample from the central point (number 5). In addition, four sampling points were established, with 3 m measured in the cardinal directions 2 and 8 from the central point and 2.5 m measured in the cardinal directions 4 and 6 from the central point. A sample was collected at each of these points, resulting in a total of four samples. Furthermore, from the projected four corners (1, 3, 7, and 9), four soil samples were collected. The soil sub-samples from each plot were manually homogenized by mixing them in a clean bucket. A 1 kg sample was drawn from the composite sample, stored in a plastic bag, and labeled with the owner's name, GPS coordinates, field history, and sampling date. Finally, 34 soil samples were collected.
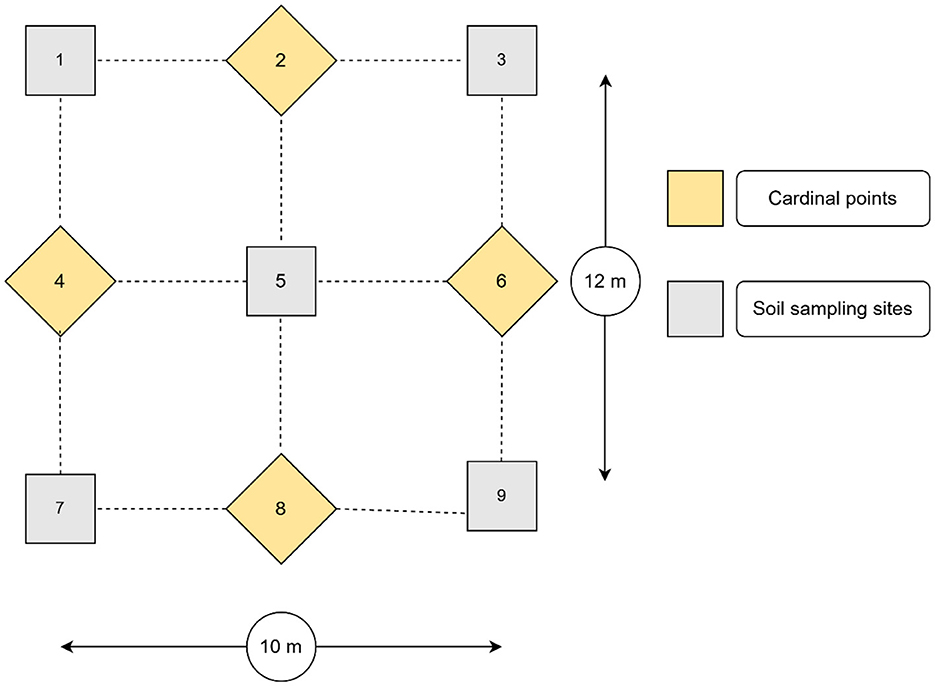
Figure 2. Overall soil sampling scheme for AM fungal spore isolation from a plot. The method was adapted from the Society for the Protection of Underground Networks (2023). The numbers 1, 3, 5, 7, and 9 represent the soil sampling points, while the numbers 2, 4, 6, and 8 represent the cardinal directions.
2.3 Spore isolation and morphological characterization
The collected soils were wet-sieved and sucrose-centrifuged to extract AM fungal spores, following the method described by Gerdemann and Nicolson (1963) with modifications made by Ingleby (2007). In summary, 50 g of rhizosphere soil was suspended in 500 mL tap water and stirred for 1 min. The solutions were then sequentially sieved through mesh sizes of 710, 400, 200, 100, and 45μm under flowing tap water to separate the spores by size. The soil fraction from each sieve was collected into a beaker. Then, the spore suspensions were transferred to 50 mL centrifugation tubes and centrifuged with a sucrose-water solution (20% and 60% w/v) for 5 min at 2,700 rpm (Sakha et al., 2024). The supernatant was decanted into a 45-μm sieve, washed, and transferred to Petri dishes for quantification under a stereomicroscope. The spores were enumerated and distinguished according to their morphological characteristics (such as spore size, color, surface appearance, and hyphal attachment).
Some spore morphotypes were mounted on slides in polyvinyl–lactic acid–glycerine (PVLG) and a mixture of PVLG with Melzer's reagent (1:1; v/v) (Morton, 1991) to observe wall structures and other specific attributes using a compound microscope at 40 × magnification (Zeiss standard microscope). Then, the spores were identified to the species level or as a specific morphotype using descriptions and identification criteria based on the method described by Schenck and Perez-Collins (1990), online references of species descriptions from the International Culture Collection of Vesicular–Arbuscular Mycorrhizal Fungi website (INVAM) (http://fungi.invam.wvu.edu/the-fungi/species-descriptions.html), and descriptions in the literature.
2.4 Diversity measures
2.4.1 Shannon-Wiener diversity index
The Shannon-Wiener index is a method used to measure the diversity of species in a community. This index is calculated by taking the ratio of the number of species to their importance values (e.g., biomass or productivity) within a trophic level or community, as indicated in the equation below:
Where H' is the Shannon-Wiener diversity index, s is the number of species in a sample, N is the total number of species, and ni is the number of individuals within a species. The higher the value of H', the higher the diversity of species in a particular community. A value of H' = 0 indicates a community with only one species.
2.4.2 Spore abundance, relative abundance, and species richness
The spores were counted in three replicates under a stereomicroscope at 40 × magnification, and spore abundance was expressed as the number of spores per 50 g of soil.
Relative abundance (RA %) was estimated using the formula: (number of spores of individuals of a given species)/(the total number of individuals in a community) × 100 (Kumar and Ghose, 2008).
Species richness was measured as the number of AM fungal species present in the soil sample.
2.5 Soil analysis
The samples were scanned using a Bruker Tracer 5i Portable X-ray Fluorescence (pXRF) instrument to determine the total elemental concentrations of magnesium (Mg), aluminum (Al), phosphorous (P), sulfur (S), potassium (K), calcium (Ca), manganese (Mn), iron (Fe), copper (Cu), and zinc (Zn). The samples were predicted using the ICRAF's global soil models. The models were fitted using the Bayesian regularization for feed-forward neural networks (BRNN) and random forest (RF) algorithms. The performance of the model was evaluated using a 30% hold-out validation set. The predicted soil properties included soil pH, exchangeable Al, Ca, Cu, Fe, K, Mg, Mn, CEC, and S, phosphorus sorption index (PSI), soil organic carbon (SOC), and total nitrogen (TN). The soil pH was determined using a pH meter at a soil-to-water ratio of 1:2.5 (w/v) (Bao, 2000). Exchangeable Al, Ca, Cu, Fe, K, Mg, Mn, CEC, and S were determined using the Mehlich 3 extraction method (Mehlich, 1984). The phosphorus sorption index (PSI) was calculated based on the molar ratio of P to Al or Al plus Fe (Dari et al., 2015). Soil organic carbon (SOC) was determined using K2Cr2O7 digestion (Walkley and Black, 1934), and total nitrogen (TN) was analyzed using both Kjeldahl and HClO4-H2SO4 methods (Yang et al., 2023).
2.6 Statistical analysis
All data were checked for normality and homogeneity of variance, and the data with a non-normal distribution were log (x + 1) transformed to ensure a normal distribution. Spore relative abundance and the Shannon-Wiener index of diversity (H) were calculated. To understand the relationship between AM fungal community structure and soil physicochemical parameters, principal component analysis (PCA) was performed. To compare AMF diversity between agricultural fields, a one-way analysis of variance (ANOVA) was performed, and Tukey's test was used to analyze the differences in spore abundance at a p-value of < 0.05 using R software (version 4.4.0).
3 Results
3.1 Presence of native arbuscular mycorrhizal fungi in cultivated soils
The study revealed the presence of two native AM fungal families, distinguished based on spore morphological features such as spore color, spore size, spore ornamentation, spore shape, and hyphal attachments on the spore (INVAM and Schenck and Perez-Collins, 1990). The relative abundances of the two families isolated from the rhizosphere soil primarily revealed the dominance of Gigasporaceae and Acaulosporaceae (Figure 3).
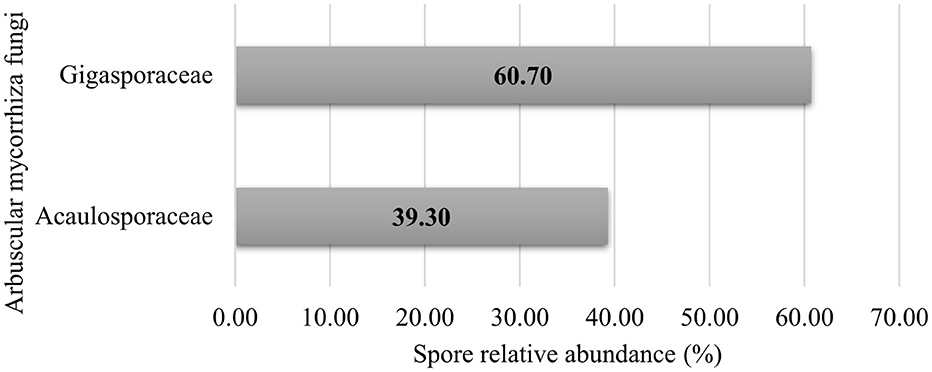
Figure 3. Family-wise taxonomic profile composition of native arbuscular mycorrhizal fungi communities in cultivated soils of Makueni County.
Based on the morphological appearances, five genera from the sub-family Glomeromycota were identified (Figure 4). The analysis of spore relative abundance showed that the most common genera, in order of superiority, were Acaulospora, Gigaspora, Scutellospora, Dentiscutata, and Rococetra. Of the two AM fungal families (Figure 4), Gigasporaceae consisted of two genera, namely Gigaspora and Scutellospora, while Acaulosporaceae comprised three genera, namely Acaulospora, Dentiscutata, and Rococetra.
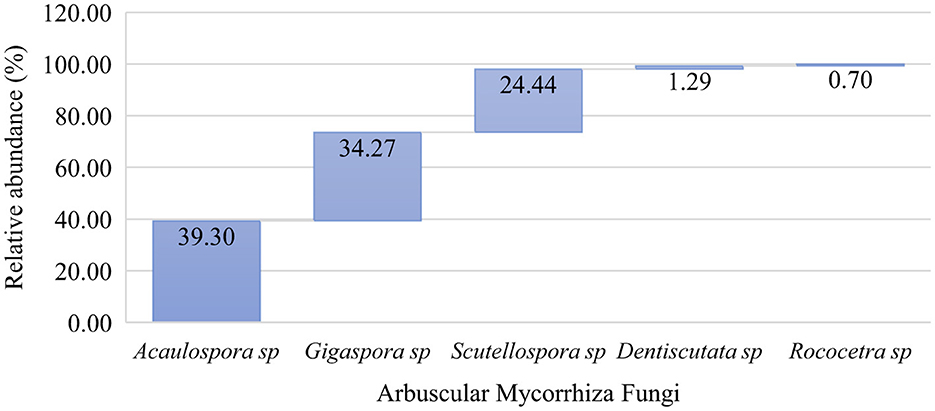
Figure 4. Genus-wise taxonomic profile composition of native arbuscular mycorrhizal fungal communities in the cultivated soils of Makueni County.
The AM fungal morpho-species isolated from the SAL farming systems exhibited variations in spore abundance per 200 g of soil. A total of 26 morpho-species were isolated (Table 2). While all morpho-species were recorded in the area, they differed significantly in spore abundance, with the highest count being 198 spores and the lowest being 1 spore per 200 g of soil. However, this is very low since it was a cumulative measure from the 34 agricultural fields. The analysis of spore abundance indicated that Acaulospora denticulata had the highest rank, accounting for a proportion of 24.57%. Based on the rankings, three species—Acaulospora denticulata, Gigaspora margarita, and Scutellospora sp. 5—recorded the largest proportion, accounting for ~54.09%, which is slightly higher than half of the total proportion. In addition, nine spores could not be identified at the species level because they lacked enough distinct features.
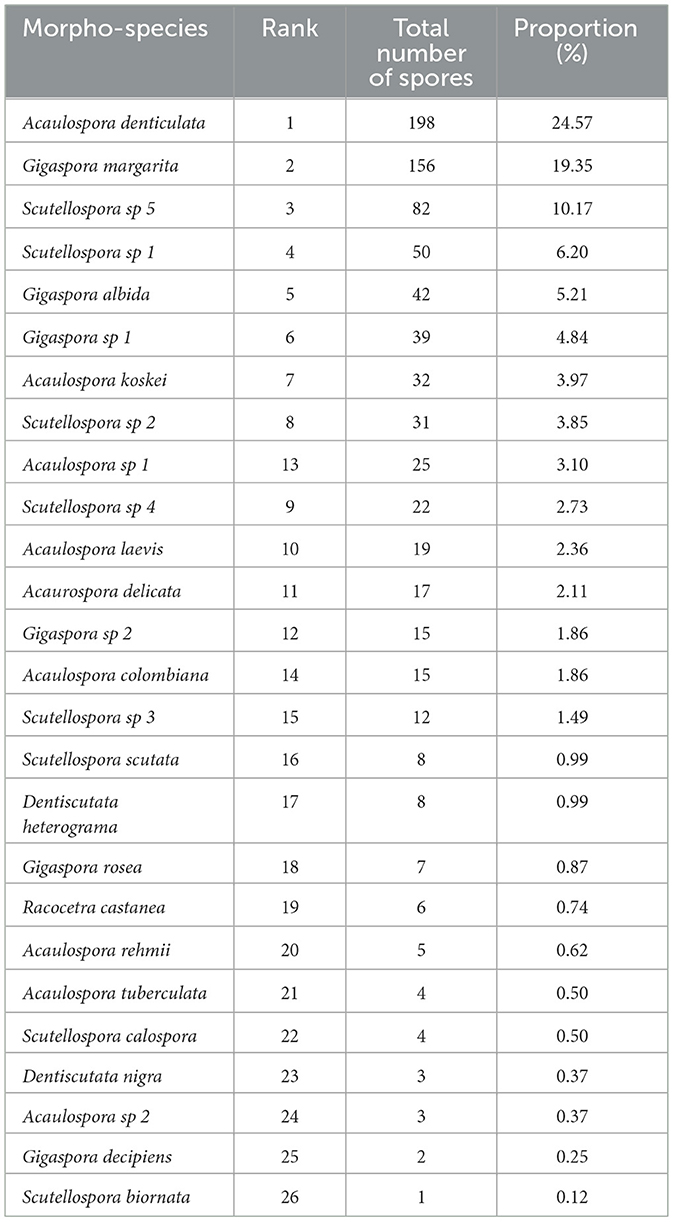
Table 2. Rank of the total number of arbuscular mycorrhizal fungal spores per species and their proportions in 200 g−1 of air-dried soil.
3.2 Genus-wise taxonomic profile composition of the native arbuscular mycorrhizal fungal communities in the study fields
The genus-wise taxonomic profile showed that Gigaspora sp. dominated in 20 agricultural fields (Figure 5). Furthermore, eight fields had 100% Gigaspora sp., and four had 100% Acaulopora sp. Two fields had 100% Scutellopora sp, two fields each recorded 100% Rococetra sp., and four fields recorded 100% Dentiscutata sp., Acaulospora sp., and Scutellospora sp. or Gigaspora sp. Scutellospora sp., together with Gigaspora sp., occurred in five fields. In addition, Dentiscutata sp., along with Scutellospora sp. or Gigaspora sp., was isolated in one field each. However, only one field did not record any AM fungal species. Overall, Gigaspora sp. was found in 20 fields, followed by Acaulospora sp., and Scutellospora sp., which was found in 14 fields each.
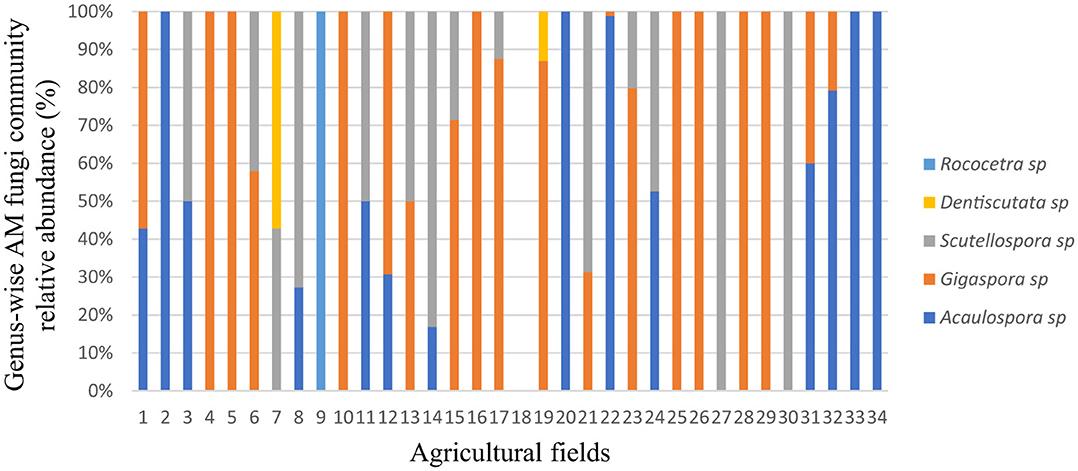
Figure 5. Genus-wise taxonomic profile composition of native arbuscular mycorrhizal fungal communities' relative abundance in different agricultural fields.
3.3 Arbuscular mycorrhizal fungal diversity indices and spore abundance in agricultural fields
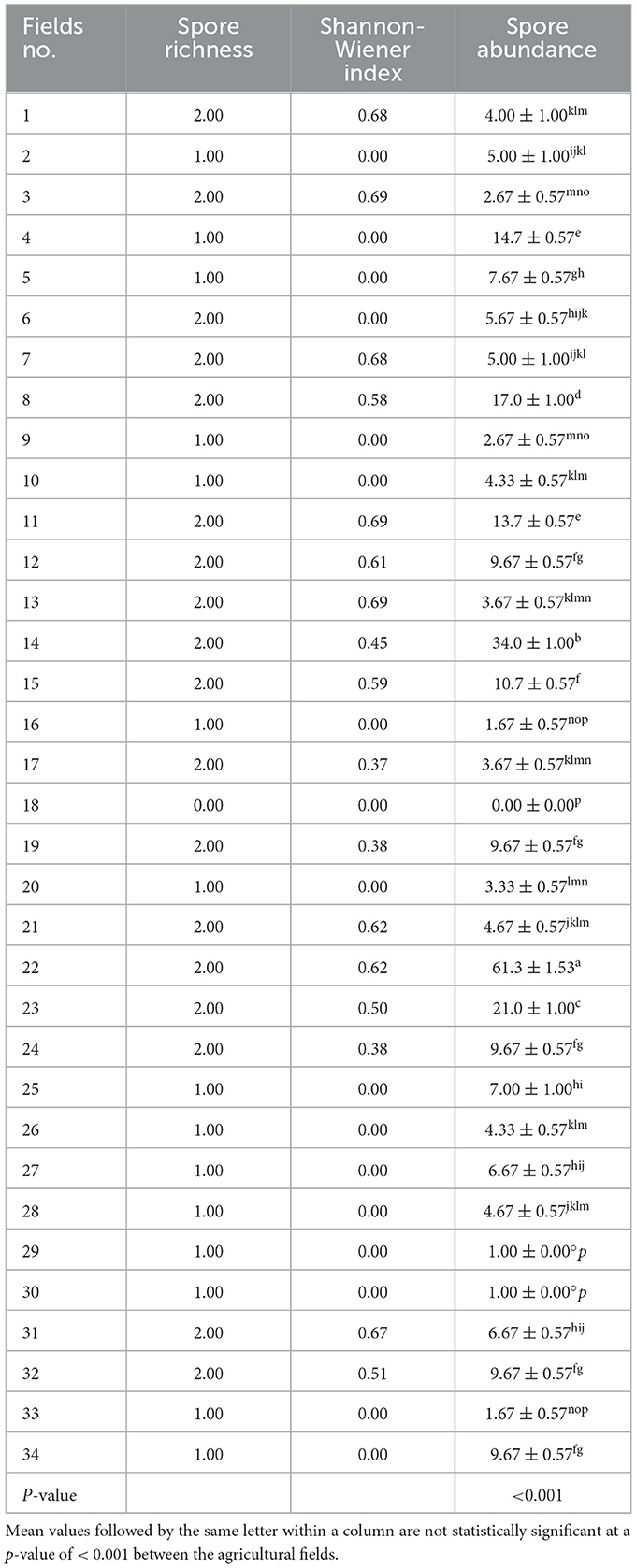
Approximately 18 fields recorded a spore richness of 2, while the remaining fields had a spore richness of 1, where AM fungi were present (Table 3). The Shannon-Wiener diversity index revealed that AM fungal diversity was higher in the fields with greater spore richness, with only six fields having an approximate index of 0.6. Spore abundance was significantly different between the fields (Table 3). The average number of AM fungal spores ranged from 0 to 61.30 per 50 g of dry soil−1 in the area. The highest spore abundance, 61 spores, was recorded in field number 22, followed by 34 spores in field number 14 (Table 3). The remarkable difference in the AM fungal spore abundance and communities observed within the agricultural fields could be due to several factors, including the types of cropping systems and soil amendments (Table 1). The field that exhibited high AM fungal spore abundance previously had maize, beans, cowpeas, and pigeon peas as intercrops, with farmyard manure as a soil amendment. This demonstrates that dryland farming systems affect AM fungal spore abundance and composition, mainly due to the interactions between plant species.
3.4 The relationship between AMF spore abundance, elevation, and latitude
We found that AM fungal spore abundance had a moderate positive correlation with latitude (0.482**), although it had a very weak positive correlation of 0.025 with elevation (Figure 6).
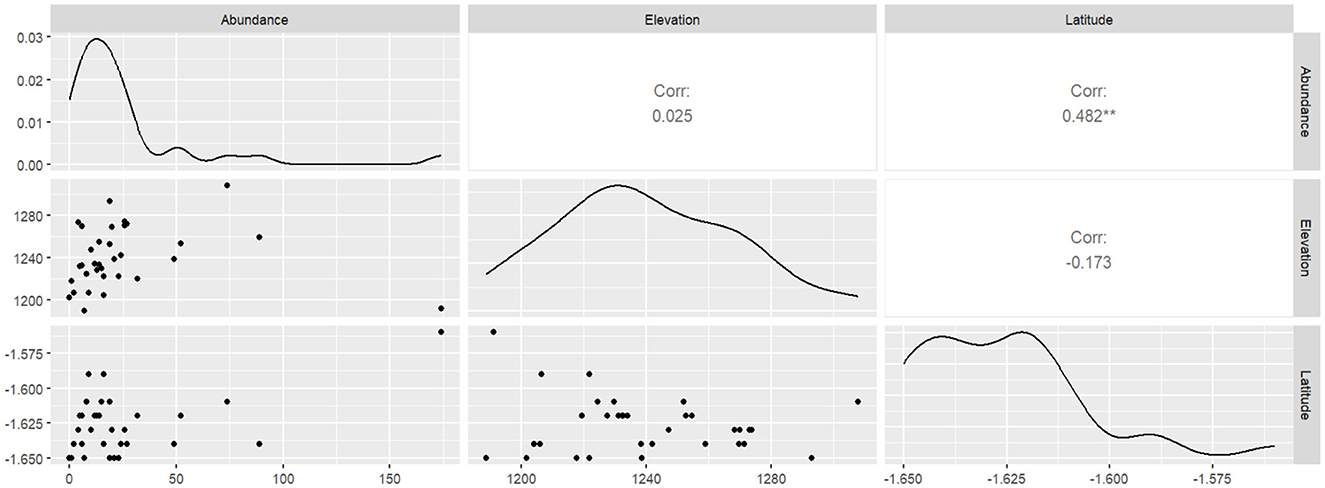
Figure 6. Pearson correlation analysis between arbuscular mycorrhiza fungal abundance and geographic coordinates.
3.5 The relationship between the AMF spore abundance, genera, and soil physicochemical properties
The correlation between the AM fungal community structure and soil properties is presented in Figures 7, 8. In the PCA plots, the length of the arrows represents the relative importance affecting the community, while the angle between the variables indicates the degree to which factors are correlated, with a smaller angle reflecting a higher correlation. The resulting PCA analysis of the soil physical properties, AMF spore abundance, and AM fungal communities is presented in Figure 7. The first two latent variables (Dim-1 and Dim-2) accounted for more than 52% of the variation in AM fungal genera and soil physical properties. The total spore abundance, as well as the sand and clay contents, showed very high variance, whereas the spore abundance of the genera Gigaspora and Dentiscutata showed low variance in relation to sand and clay contents. The spore abundance and AM fungal genera showed a weak correlation with clay, sand, and silt.
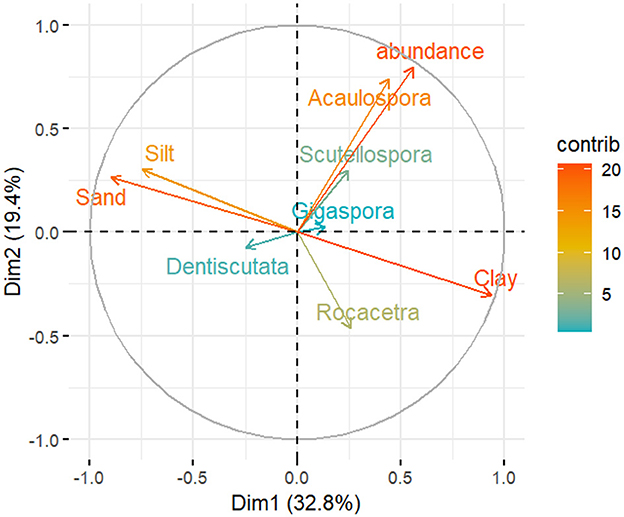
Figure 7. Principal component analysis (PCA) of AM fungal spore abundance, AM fungal communities, and soil physical properties across the 34 sampling sites in the dryland farming system (with the mean values). The correlation circle (correlation = 1) shows the relationship between the variables and dimensions (Dim-1 and Dim-2). Variable points away from the origin are well represented on the factor map.
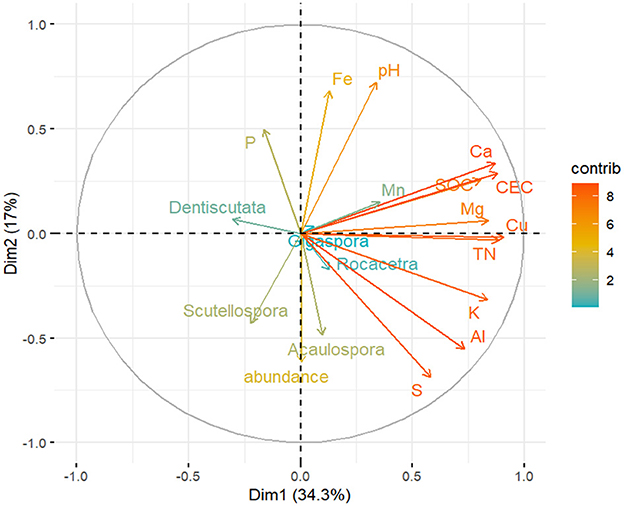
Figure 8. Principle component analysis (PCA) of the AM fungal spore abundance, AM fungal community genera, and soil chemical properties across the 34 sampling sites in the dryland farming system (with the mean values). The correlation circle (correlation = 1) shows the relationship between the variables and dimensions (Dim-1 and Dim-2). Variable points away from the origin are well represented on the factor map.
The PCA of soil chemical properties revealed that S, Al, K, Cu, TN, SOC, CEC, and Ca were the key factors that shaped the AM fungal community (Figure 8). The first two latent variables, Dim-1 and Dim-2, accounted for 34.3% and 17% of the variability, respectively. The spore abundance of AM fungi and the genus Acaulospora were positively correlated with S, Al, K, Cu, and TN. However, these elements negatively influenced the genera Dentiscutata and Gigaspora. Spore abundance, Acaulospora, and Scutellospora were negatively correlated with P, Fe, and pH.
The results of the redundancy analysis are presented using the spore abundances of the different AM fungal genera. Integrating the above results, a comprehensive model was established to identify the factors that jointly affect the distribution of AM fungal communities at a finer spatial scale in dryland agroecosystems (Figure 9).
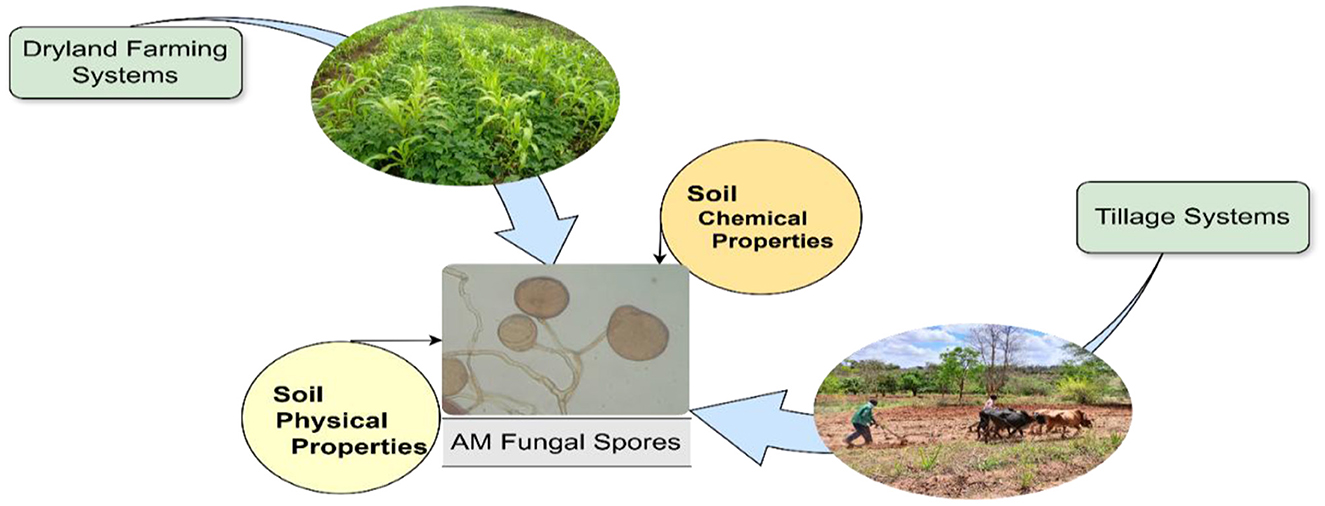
Figure 9. Conceptual representation of factors that affect arbuscular mycorrhizal fungal community structure at a finer spatial scale in drylands farming systems. The arrows with greater thickness indicate the main above-ground agricultural management practices, such as cropping systems and tillage systems, while the thin arrows indicate the below-ground soil properties, such as soil physiochemical properties, that influence AM fungal community structure in an agricultural field.
4 Discussion
Few studies have explored AM fungal communities and their drivers in low-input dryland farming systems. This study, therefore, is a significant contribution to our understanding of these organisms' ecological preferences at each site. It is recommended that morphological and molecular characteristics be combined for AM fungal identification at the species level because both methods provide unique and crucial information (Overby et al., 2015; Säle et al., 2015). Ideally, both methods have their respective advantages and limitations (Oehl et al., 2017). This study used the morphological characterization due to some limitations, although we banked on its advantages and the fact that it can provide evidence unaffected by potential biases introduced during PCR steps. However, its limitations include challenges in identifying cryptic species or the underrepresentation of non-sporulating taxa. Based on the most reliable morphological features and through direct AM fungal spore isolation from the rhizosphere soil, two AM fungal families, Gigasporaceae and Acaulosporaceae, were unexpectedly distinguished. This denotes that these two are the most dominant families associated with low-input SAL farming systems. Egerton-Warburton et al. (2007) observed a higher abundance of Gigasporaceae and Acaulosporaceae in typically mesic environments than in semiarid ecosystems, although no such comparison was performed in the present study.
SALs host many endemic species, including AM fungi, which exhibit unique adaptations to harsh dryland conditions (Kamalvanshi et al., 2012). Currently, the primary driving factor of AM fungal communities is habitat conditions (Vasar et al., 2022), and these AM fungal communities respond to these conditions with lower diversity and less variability. The distribution of AM fungal genera occurred in patches, with some restrictions to specific agricultural fields, probably due to habitat filtering. This implies a finer-scale spatial selection of AM fungi species, particularly by tillage practices, cropping systems, and edaphic factors. Our findings suggested that the AM fungal communities in the soil responded spatially to the SAL farming systems. The main cropping system within the agricultural field was intercropping, but the intercrops varied from one agricultural field to another. In addition, different soil amendments were used for growing the crops. This provided clear evidence that SAL farming systems affected the AM fungal spore abundance and composition mainly due to the interactions between plant species. Our results are consistent with those of Yang et al. (2012), who observed that AM fungal community composition was significantly correlated with the plant community among the ecosystems. Similarly, Egbokaa et al. (2022) concluded that the cropping system significantly influenced the population density of AM fungi. For instance, monocropping records lower AM fungal diversity due to the limited diversity of hosts (Burrows and Pfleger, 2002; Oehl et al., 2003). Moreover, Liu et al. (2012) demonstrated that the types of host plant species grown have a strong and significant impact on AM fungal diversity and distribution. Furthermore, Säle et al. (2015) reported that the type of fertilization significantly affects AM fungal spore populations.
Among all the AM fungi species, the Gigasporaceae family cumulatively had more species than Acaulosporaceae. Members of the Gigasporaceae family are known for their competitive life strategy, characterized by increased hyphal production, higher carbon demand, and enhanced phosphorus absorption from the soil. These traits are important in environments with low levels of soil nutrients (Maherali and Klironomos, 2007; Chagnon et al., 2013). Notably, Hart and Reader (2002) revealed that this family produces more extraradical mycelia in the soil than intraradical mycelia within root systems. Mycelia provide improved opportunities for soil exploration, ensuring a better nutritional contribution to the hosts. In addition, through increased mycelial production, the species in the family may help stabilize the growth media and contribute to increased soil aggregation (Vieira et al., 2020).
The family Gigasporaceae consisted of two genera, Gigaspora and Scutellospora, while Acaulosporaceae comprised three genera, namely Acaulospora, Dentiscutata, and Rococetra. The genus Acaulospora showed a high relative spore abundance and was more frequent (Figures 3, 4). Studies have shown that this genus sporulates more rapidly than Scutellospora and Gigaspora in the same environment (Jefwa et al., 2012; Oehl et al., 2009). At the same time, our findings closely align with those of Cuenca et al. (1998) and Boddington and Dodd (2000), who detailed the superiority of Acaulospora species in disturbed soils, one of the characteristics of the agricultural fields in the study area. This observation aligns with those of De Pontes et al. (2024), who reported the predominance of Acaulospora species in dry soils of the Brazilian Cerrado, transitional areas toward the Caatinga, and the Atlantic Forest, possibly due to their adaptation to moisture conditions. Accordingly, Xavier Martins and Rodrigues (2020) revealed that Acaulospora species are associated with low-input farming systems and forest and grassland soils. Acaulospora species are considered facultative symbionts adapted to a broad range of soils and host species (Sieverding et al., 1991; Shepherd et al., 1996; Straker et al., 2010).
The diversity indices and the spore abundance of AM fungi were lower and varied significantly across the agricultural fields, with each field presenting one or two genera (Table 3). This could be due to the farming systems. Boddington and Dodd (2000) showed that soil disturbance caused by agricultural activities directly affects AM fungal propagule availability as richness and spore density are reduced, along with the length of AM fungal extraradical mycelium. Furthermore, the AM fungal diversity index showed a trend toward the dominance of AM fungal diversity in fields with high spore richness; however, this did not necessarily result in higher spore abundance. The diversity reported in the current study was particularly low, considering that soil sampling was performed at a single point in time. Sampling other land use types beyond cropland or across different seasons could have likely revealed greater spore diversity and density, as spores represent the dormant stage of AM fungi. Their production in natural systems varies seasonally (Bever et al., 2001), which likely impacted the number of species detected in our study. Inadequate exploration of seasonal variation in AM fungal spore production and diversity has been conducted in drylands with biannual precipitation patterns. We collected soils samples in September during the dry season, and as a result, certain AM fungal taxa were not captured because they may preferentially sporulate during the wet season or do not sporulate at all.
Elevation lays the foundation for investigating the driving forces that lead to species aggregation since different elevation gradients provide a unique opportunity to explore AM fungal community distribution and diversity (Wagg et al., 2011). In this study, we found that AM fungal abundance had a weak positive correlation with elevation, while spore abundance showed a moderate positive correlation with latitude (Figure 6). This could be due to the fact that the sampled agricultural fields were located within the same elevation and latitude zones, thereby limiting the influence of these factors on spore diversity and abundance. The finding is consistent with those of landscape-scale studies by Hazard et al. (2013), Xiang et al. (2014), and De Beenhouwer et al. (2015), which revealed that AM fungal communities are primarily structured by local abiotic conditions rather than geographic distance. This finding is an indicator that elevation and latitude play a moderate role in determining the assembly of contemporary AM fungal communities at a local level.
Our study provides direct evidence of environmental filtering by edaphic factors in SAL farming systems. The results showed that spore abundance and AM fungal communities were highly influenced by soil textural characteristics, such as clay, sand, and silt, although their correlation was weak (Figure 7). This could be because of the general composition of soil textural classes in the present study; for instance, loam soil was not detected in the agricultural fields. In support of this, soil texture has been identified as a factor that affects mycorrhizal efficiency (Joshi and Singh, 1995) such as root colonization (Carrenho et al., 2007) and AM fungal community differences (Moebius-Clune et al., 2013). Furthermore, evidence indicates that AM fungal community composition differs greatly between soil types in the field. The genus Acaulospora showed a slight positive correlation with sand (Mukhongo et al., 2023), the family Gigasporaceae was more abundant in sandy soil, and the family Glomeraceae was more abundant in clay soil (Lekberg et al. (2007). These findings were observed under conditions where confounding factors, such as host plants, were minimized. Since the family Gigasporaceae was more abundant in the region, it may have had a competitive advantage in the sand.
Regarding soil chemical properties, the results of this study indicated that the AM fungal genera differed in their sensitivity to these properties, with some species being specifically associated with certain elements. Indeed, previous studies have demonstrated the influence of soil chemical properties on the composition of AM fungal communities (Jansa et al., 2014; Xiang et al., 2016). The PCA revealed that the genus Acaulospora was closely associated with S, Al, K, and Cu. Soil P, pH, and Fe were significantly and negatively correlated with spore abundance and the genera Acaulospora and Scutellospora. In contrast, the genus Dentiscutata appeared to be more tolerant (Figure 8). Changes in the AM fungal community composition have been examined in relation to P. Notably, Amballa and Bhumi (2011) and Johnson et al. (2015) concluded that available soil P suppressed AM fungi root colonization and spore density. Specifically, Qin et al. (2020) observed that high soil fertility, especially available soil P status, reduced AM fungal development in intensively managed agricultural systems. In this context, Johnson et al. (2013) reported that high concentrations of P were associated with lower diversity of AMF due to optimal resource allocation and biotic interactions. Accordingly, Gomes et al. (2018) revealed a high abundance of Glomeromycota when the P content was low, with a prevalence of the genera Gigaspora, Scutellospora, and Rococetra in the root samples of maize. Zhang et al. (2022) highlighted the possibility of pH affecting the formation and development of AM fungal spores. According to Davison et al. (2021), soil pH is one of the most important abiotic factors regulating microbial communities, particularly AM fungi (Jansa et al., 2014; Da Silva et al., 2014). In this study, we observed specifically that the family Acaulosporaceae was among the leading families, and this corroborates with Chagnon et al. (2013) and Li et al. (2020), who reported the presence of this family in environments with low pH. Mukhongo et al. (2023) suggested that the favorable soil pH range supporting AM fungal spore sporulation is from 5.85 to 6.64, Dobo et al. (2016) reported a range of 6.18 to 6.28, and Ketchiemo et al. (2024) reported a range of 6.33 to 6.67—values that are close to neutral. In the current study, the pH value ranged from 5.84 to 7.36. This evidence shows that AM fungi species vary in their response to soil pH, not only at the local scale but also at the landscape level. Since the genus Acaulospora was strongly negatively correlated with soil pH (Figure 8) compared to other genera, this may indicate the coexistence of acid-tolerant and acid-sensitive fungi within the SAL ecosystem. For instance, the family Acaulosporaceae is reported to be tolerant of acidic tropical soils (Bagyaraj, 2014; Temegne et al., 2017), and low pH levels (Melo et al., 2019). Other studies have found Fe among the distinct soil chemical factors that influence AM fungi negatively (Vieira et al., 2018; Rodríguez-Rodríguez et al., 2021).
Finally, of the 26 AM fungi species reported in the current study, two species are considered rare (species that occurred in one or two farms). Assis et al. (2014) noted that natural ecosystems contain a higher number of rare species compared to agricultural ones and observed that some species may have disappeared in cultivated soils. Similarly, De Carvalho et al. (2012) found high species richness (49 species) in plant species-rich areas with high plant endemism in rocky fields within the Cerrado savanna, indicating that natural vegetation sites in tropical regions generally support high AM fungal diversity. According to Pagano et al. (2012), semi-arid regions have favorable conditions for the growth of AM fungi. The low diversity of species in agricultural fields in the present study underscores the importance of adopting biodiversity-friendly farming practices. One potential solution is the use of microbial inoculants, particularly AM fungi, which can serve as an eco-friendly alternative to traditional fertilizers and pesticides (Ghorui et al., 2025). Accordingly, Martínez et al. (2024) identified that historical legacies significantly influence the persistence of AM fungi in the field by preserving natural habitats that act as safe place for vulnerable species. Therefore, maintaining natural habitats around agricultural fields can further promote soil AM fungal diversity for future generations.
5 Conclusion
Our findings revealed the sensitivity of AM fungal community structure to agricultural farming practices and environmental factors in drylands. This community sensitivity has a substantial impact on their function, which subsequently affects their ecological functions. It was also evident that the spore abundance was lower across all farms, implying the need to adopt sustainable biodiversity-friendly farming practices such as reducing soil disturbance, planting a diverse range of species, avoiding the overuse of fertilizers and chemicals, and incorporating organic matter to support and intensify native AM fungi in the soil. Furthermore, the results prompt a discussion regarding the importance of developing context-specific, highly efficient native AM fungal strains that account for natural adaptations in dryland farming systems. Future research should expand the scope to explore AM fungal dynamics across an entire landscape to achieve vibrant, diverse, and healthy landscapes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MS: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JG-O: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. CM: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. FB: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Agroecology Initiative of CGIAR through the International Institute of Tropical Agriculture (IITA).
Acknowledgments
This study was conducted in collaboration with partner and host organizations and farmers. We are grateful for the support, team effort, and commitment of all those involved. We would like to thank our partners, the Drylands Natural Resources Centre (DNRC), a locally registered non-government organization (NGO) dedicated to promoting sustainable resource development in the dryland regions of Kenya.
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
AbdelRahman, M. A. (2023). An overview of land degradation, desertification and sustainable land management using GIS and remote sensing applications. Rendiconti Lincei Scienze Fisiche Naturali 34, 767–808. doi: 10.1007/s12210-023-01155-3
Aguilar, R. A. C., Diaz-Bonilla, C., Fujs, T., Lakner, C., Nguyen, M. C., Viveros, M., et al. (2024). March 2024 Global Poverty Update From the World Bank: First Estimates of Global Poverty Until 2022 From Survey Data. Washington, DC: World Bank Blogs.
Amballa, H., and Bhumi, N. R. (2011). Occurrence and distribution of arbuscular mycorrhizal fungi and microbial flora in the rhizosphere soils of mungbean [Vigna radiata (L.) wilczek] and soybean [Glycine max (L.) Merr.] from Adilabad, Nizamabad and Karimnagar districts of Andhra Pradesh State, India. Adv. Biosci. Biotechnol. 275–286. doi: 10.4236/abb.2011.24040
Amwata, D. A., Nyariki, D. M., and Musimba, N. R. (2016). Factors influencing pastoral and agropastoral household vulnerability to food insecurity in the drylands of Kenya: a case study of Kajiado and Makueni Counties. J. Int. Dev. 28, 771–787. doi: 10.1002/jid.3123
Assis, P. C. R., Saggin-Júnior, O., Paulino, H. B., Sturmer, L., Siqueira, J. O., and Carneiro, M. A. C. (2014). Fungos micorrízicos arbusculares em campos de Murundus após a conversão para sistemas agrícolas no cerrado. Rev. Bras. Cienc. Solo. 38, 1703–1711. doi: 10.1590/S0100-06832014000600005
Bagyaraj, D. J. (2014). Ecology of arbuscular mycorrhizal fungi. Microb. Diversity Biotechnol. Food Secur. 133–146. doi: 10.1007/978-81-322-1801-2_10
Bao, S. D. (2000). Soil and Agricultural Chemistry Analysis. Beijing: Agriculture Publication (in Chinese).
Begum, N., Qin, C., Ahanger, M. A., Raza, S., Khan, M. I., Ashraf, M., et al. (2019). Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front. Plant Sci. 10:1068. doi: 10.3389/fpls.2019.01068
Bever, J. D., Schultz, P. A., Pringle, A., and Morton, J. B. (2001). Arbuscular mycorrhizal fungi: more diverse than meets the eye, and the ecological tale of why: the high diversity of ecologically distinct species of arbuscular mycorrhizal fungi within a single community has broad implications for plant ecology. Bioscience 51, 923–931. doi: 10.1641/0006-3568(2001)051[0923:AMFMDT]2.0.CO;2
Bishaw, B., Neufeldt, H., Mowo, J., Abdelkadir, A., Muriuki, J., Dalle, G., et al. (2013). Farmers' strategies for adapting to and mitigating climate variability and change through agroforestry in Ethiopia and Kenya. Available online at: https://ir.library.oregonstate.edu/concern/defaults/5999n3901
Boddington, C. L., and Dodd, J. C. (2000). The effect of agricultural practices on the development of indigenous arbuscular mycorrhizal fungi. I. Field studies in an Indonesian ultisol. Plant Soil 218, 137–144. doi: 10.1023/A:1014966801446
Brundrett, M. C., and Tedersoo, L. (2018). Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 220, 1108–1115. doi: 10.1111/nph.14976
Buczkowska, H. B., and Sałata, A. (2020). Inoculation with arbuscular mycorrhizal fungi (AMF) and plant irrigation with yield–forming factors in organic sweet pepper (Capsicum annuum L.) cultivation. Acta Sci. Pol. Hortorum Cultus 19, 125–138. doi: 10.24326/asphc.2020.6.11
Burrows, R. L., and Pfleger, F. L. (2002). Arbuscular mycorrhizal fungi respond to increasing plant diversity. Can. J. Bot. 80, 120–130. doi: 10.1139/b01-138
Carrenho, R., Trufem, S. F. B., Bononi, V. L. R., and Silva, E. S. (2007). The effect of different soil properties on arbuscular mycorrhizal colonization of peanuts, sorghum and maize. Acta Bot. Bras. 21, 723–730. doi: 10.1590/S0102-33062007000300018
Chagnon, P. L., Bradley, R. L., Maherali, H., and Klironomos, J. N. (2013). A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 18, 484–491. doi: 10.1016/j.tplants.2013.05.001
Coleine, C., Delgado-Baquerizo, M., DiRuggiero, J., Guirado, E., Harfouche, A. L., Perez-Fernandez, C., et al. (2024). Dryland microbiomes reveal community adaptations to desertification and climate change. ISME J. 18:wrae056. doi: 10.1093/ismejo/wrae056
Cuenca, G., De Andrade, Z., and Escalante, G. (1998). Diversity of Glomalean spores from natural, disturbed and revegetated communities growing on nutrient-poor tropical soils. Soil Biol. Biochem. 30, 711–719. doi: 10.1016/S0038-0717(97)00191-0
Da Silva, I. R., de Mello, C. M. A., Neto, R. A. F., da Silva, D. K. A., de Melo, A. L., Oehl, F., et al. (2014). Diversity of arbuscular mycorrhizal fungi along an environmental gradient in the Brazilian semiarid. Appl. Soil Ecol. 84, 166–175. doi: 10.1016/j.apsoil.2014.07.008
Dari, B., Nair, V. D., Colee, J., Harris, W. G., and Mylavarapu, R. (2015). Estimation of phosphorus isotherm parameters: a simple and cost-effective procedure. Front. Env. Sci. 3:70. doi: 10.3389/fenvs.2015.00070
Davison, J., Moora, M., Semchenko, M., Adenan, S. B., Ahmed, T., Akhmetzhanova, A. A., et al. (2021). Temperature and pH define the realised niche space of arbuscular mycorrhizal fungi. New Phytol. 231, 763–776. doi: 10.1111/nph.17240
De Beenhouwer, M., Van Geel, M., Ceulemans, T., Muleta, D., Lievens, B., and Honnay, O. (2015). Changing soil characteristics alter the arbuscular mycorrhizal fungi communities of Arabica coffee (Coffea arabica) in Ethiopia across a management intensity gradient. Soil Biol. Biochem. 91, 133–139. doi: 10.1016/j.soilbio.2015.08.037
De Carvalho, F., De Souza, F. A., Carrenho, R., de Souza Moreira, F. M., da Conçeição Jesus, E., and Fernandes, G. W. (2012). The mosaic of habitats in the high-altitude Brazilian rupestrian fields is a hotspot for arbuscular mycorrhizal fungi. Appl. Soil Ecol. 52, 9–19. doi: 10.1016/j.apsoil.2011.10.001
De Leeuw, J. (2014). Treesilience: An assessment of the resilience provided by trees in the drylands of Eastern Africa Edited. Nairobi, Kenya: World Agroforestry Center (ICRAF).
De Pontes, J. S., Oehl, F., Pereira, C. D., de Toledo Machado, C. T., Coyne, D., da Silva, D. K. A., et al. (2024). Heterogeneity in arbuscular mycorrhizal fungi and plant communities of the brazilian Cerrado, transitional areas toward the Caatinga, and the Atlantic Forest. Microb. Ecol. 87:29. doi: 10.1007/s00248-023-02337-0
Deepika, S., and Kothamasi, D. (2015). Soil moisture-a regulator of arbuscular mycorrhizal fungal community assembly and symbiotic phosphorus uptake. Mycorrhiza 25, 67–75. doi: 10.1007/s00572-014-0596-1
Del Fabbro, C., and Prati, D. (2014). Early responses of wild plant seedlings to arbuscular mycorrhizal fungi and pathogens. Basic Appl. Ecol. 15, 534–542. doi: 10.1016/j.baae.2014.08.004
Delgado-Baquerizo, M., Maestre, F. T., Reich, P. B., Jeffries, T. C., Gaitan, J. J., Encinar, D., et al. (2016). Microbial diversity drives multifunctionality in terrestrial ecosystems. Nature Commun. 7:10541. doi: 10.1038/ncomms10541
Dobo, B., Asefa, F., and Asfaw, Z. (2016). Diversity of arbuscular mycorrhizal fungi of different plant species grown in three land use types in Wensho and Shebidino Districts of Sidama in Southern Ethiopia. Adv. Biosci. Bioeng. 4, 25–34. doi: 10.11648/j.abb.20160404.11
Dumbrell, A. J., Nelson, M., Helgason, T., Dytham, C., and Fitter, A. H. (2010). Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J. 4, 337–345. doi: 10.1038/ismej.2009.122
Dushimimana, C., Sakha, M. A., Korir, M. J., Jefwa, J. M., Vandenabeele, J., Magomere, T., et al. (2022). Early growth performance of in vitro raised Melia volkensii Gürke plantlets in response to beneficial microorganisms under semi-arid conditions. Plants 11:1300. doi: 10.3390/plants11101300
Egbokaa, N. T., Agima, L. C., Okona, M. A., Okolia, N. H., Afangidea, A. I., and Okonjob, P. N. (2022). Population density of arbuscular mycorrhizal fungi and physico-chemical properties of soils as affected by cropping systems. J. Clean WAS 6, 27–32. doi: 10.26480/jcleanwas.01.2022.27.32
Egerton-Warburton, L. M., Johnson, N. C., and Allen, E. B. (2007). Mycorrhizal community dynamics following nitrogen fertilization: a cross-site test in five grasslands. Ecol. Monogr. 77, 527–544. doi: 10.1890/06-1772.1
Ezeokoli, O. T., Mashigo, S. K., Maboeta, M. S., Bezuidenhout, C. C., Khasa, D. P., and Adeleke, R. A. (2020). Arbuscular mycorrhizal fungal community differentiation along a post-coal mining reclamation chronosequence in South Africa: a potential indicator of ecosystem recovery. Appl. Soil Ecol. 147:103429. doi: 10.1016/j.apsoil.2019.103429
Fuchs, L. E., Korir, H., Adoyo, B., Bolo, P., Kuria, A., Sakha, M., et al. (2023). Co-designing on-farm innovations in the Agroecological Living Landscapes (ALLs) in Kenya. Agroecology Initiative Technical Report.
Gerdemann, J. W., and Nicolson, T. H. (1963). Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 46, 235–244. doi: 10.1016/S0007-1536(63)80079-0
Ghorui, M., Chowdhury, S., and Burla, S. (2025). Recent advances in the commercial formulation of arbuscular mycorrhizal inoculants. Front. Ind. Microbiol. 3:1553472. doi: 10.3389/finmi.2025.1553472
Global Biodiversity Framework (2022). Kunming-Montreal Global Biodiversity Framework. Convention Biological Diversity.
Gomes, E. A., Lana, U. G., Quensen, J. F, de Sousa, S. M., Oliveira, C. A., Guo, J., et al. (2018). Root-associated microbiome of maize genotypes with contrasting phosphours use efficiency. Phytobiomes 2, 129–137. doi: 10.1094/PBIOMES-03-18-0012-R
Gupta, M. M. (2020). Arbuscular mycorrhizal fungi: the potential soil health indicators. Soil Health 183–195. doi: 10.1007/978-3-030-44364-1_11
Hart, M. M., and Reader, R. J. (2002). Does percent root length colonization and soil hyphal length reflect the extent of colonization for all AMF?. Mycorrhiza 12, 297–301. doi: 10.1007/s00572-002-0186-5
Hazard, C., Gosling, P., Van Der Gast, C. J., Mitchell, D. T., Doohan, F. M., and Bending, G. D. (2013). The role of local environment and geographical distance in determining community composition of arbuscular mycorrhizal fungi at the landscape scale. ISME J. 7, 498–508. doi: 10.1038/ismej.2012.127
Helgason, T., and Fitter, A. H. (2009). Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota). J. Exp. Bot. 60, 2465–2480. doi: 10.1093/jxb/erp144
HLPE (2019). Agroecological and other innovative approaches for sustainable agriculture and food systems that enhance food security and nutrition. A report by the High-Level Panel of Experts on Food Security and Nutrition of the Committee on World Food Security. Rome: FAO.
Ingleby, K. (2007). Mycorrhizal training manual assessment of mycorrhizal diversity in soils and roots and nursery inoculation to improve the survival and growth of seedlings. Penicuik: Centre for Ecology and Hydrology, 1–43.
Jansa, J., Erb, A., Oberholzer, H. R., Šmilauer, P., and Egli, S. (2014). Soil and geography are more important determinants of indigenous arbuscular mycorrhizal communities than management practices in Swiss agricultural soils. Molecul. Ecol. 23, 2118–2135. doi: 10.1111/mec.12706
Jefwa, J. M., Okoth, S., Wachira, P., Karanja, N., Kahindi, J., Njuguini, S., et al. (2012). Impact of land use types and farming practices on occurrence of arbuscular mycorrhizal fungi (AMF) Taita-Taveta district in Kenya. Agric. Ecosyst. Env. 157, 32–39. doi: 10.1016/j.agee.2012.04.009
Jefwa, J. M., Sinclair, R., and Maghembe, J. A. (2006). Diversity of glomale mycorrhizal fungi in maize/sesbania intercrops and maize monocrop systems in southern Malawi. Agrofor. Syst. 67, 107–114. doi: 10.1007/s10457-004-2370-4
Jiang, M., Delgado-Baquerizo, M., Yuan, M. M., Ding, J., Yergeau, E., Zhou, J., et al. (2023). Home-based microbial solution to boost crop growth in low-fertility soil. New Phytol. 239, 752–765. doi: 10.1111/nph.18943
Johnson, N. C., Angelard, C., Sanders, I. R., and Kiers, E. T. (2013). Predicting community and ecosystem outcomes of mycorrhizal responses to global change. Ecol. Lett. 16, 140–153. doi: 10.1111/ele.12085
Johnson, N. C., Wilson, G. W. T., Wilson, J. A., Miller, R. M., and Bowker, M. A. (2015). Mycorrhizal phenotypes and the law of the minimum. New Phytol. 205, 1473–1484. doi: 10.1111/nph.13172
Joshi, K. C., and Singh, H. P. (1995). Interrelationships among vesicular-arbuscular mycorrhizae population, soil properties and root colonization capacity of soil. J. Ind. Soc. Soil Sci. 43, 204–207.
Kamalvanshi, M., Kumar, A., Jha, A., and Dhyani, S. K. (2012). Occurrence of arbuscular mycorrhizal fungi in rhizosphere of Jatropha curcas L. in arid and semi-arid regions of India. Ind. J. Microbiol. 52:4924494. doi: 10.1007/s12088-011-0224-0
Ketchiemo, F. T., Fotso, B., Djabou, A. S. M., Evina, V. J. E., Essambita, J. Y., Tang, F. M. E., et al. (2024). Soil physico-chemical properties and different altitudes affect arbuscular mycorrhizal fungi abundance and colonization in cacao plantations of Cameroon. American J. Plant Sci. 15, 57–82. doi: 10.4236/ajps.2024.152005
KNBs (2019). 2019 Kenya population and housing census. Analytical report on population dynamics volume III. Available online at: https://www.knbs.or.ke/wp-content/uploads/2023/09/2019-Kenya-population-and-Housing-Census-Analytical-Report-on-Population-Dynamics.pdf (accessed January 12, 2025).
Kumar, T., and Ghose, M. (2008). Status of arbuscular mycorrhizal fungi (AMF) in the Sundarbans of India in relation to tidal inundation and chemical properties of soil. Wetl. Ecol. Manag. 16, 471–483. doi: 10.1007/s11273-008-9085-7
Leifheit, E. F., Veresoglou, S. D., Lehmann, A., Morris, E. K., and Rillig, M. C. (2014). Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation-a meta-analysis. Plant Soil, 374, 523–537. doi: 10.1007/s11104-013-1899-2
Lekberg, Y. L. V. A., Koide, R. T., Rohr, J. R., Aldrich-Wolfe, L. A. U. R. A., and Morton, J. B. (2007). Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. J. Ecol. 95, 95–105. doi: 10.1111/j.1365-2745.2006.01193.x
Li, X., Xu, M., Li, X., Christie, P., Wagg, C., and Zhang, J. (2020). Linkages between changes in plant and mycorrhizal fungal community composition at high versus low elevation in alpine ecosystems. Environ. Microb. Rep. 12, 229–240. doi: 10.1111/1758-2229.12827
Liu, Y., Shi, G., Mao, L., Cheng, G., Jiang, S., Ma, X., et al. (2012). Direct and indirect influences of 8 yr of nitrogen and phosphorus fertilization on Glomeromycota in an alpine meadow ecosystem. New Phytol. 194, 523–535. doi: 10.1111/j.1469-8137.2012.04050.x
Maherali, H., and Klironomos, J. N. (2007). Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316, 1746–1748. doi: 10.1126/science.1143082
Makueni County Integrated Development Plan (2023). Makueni County Integrated Development Plan 2023-2027. CIDP THEME: A resilient economy for sustainable development.
Martínez, O. Z., Hiiesalu, I., Sepp, S. K., Koorem, K., Vasar, M., Wipulasena, A. A. P., et al. (2024). Arbuscular mycorrhizal fungal diversity in agricultural fields is explained by the historical proximity to natural habitats. Soil Biol. Biochem. 199:109591. doi: 10.1016/j.soilbio.2024.109591
Mehlich, A. (1984). Mehlich 3 soil test extractant: a modifica-tion of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 15, 1409–1416. doi: 10.1080/00103628409367568
Melo, C. D., Luna, S., Krüger, C., Walker, C., Mendonça, D., Fonseca, H. M., et al. (2017). Arbuscular mycorrhizal fungal community composition associated with Juniperus brevifolia in native Azorean forest. Acta Oecologica 79, 48–61. doi: 10.1016/j.actao.2016.12.006
Melo, C. D., Walker, C., Krüger, C., Borges, P. A., Luna, S., Mendonça, D., et al. (2019). Environmental factors driving arbuscular mycorrhizal fungal communities associated with endemic woody plant Picconiaazorica on native forest of Azores. Annals Microbiol. 69, 1309–1327. doi: 10.1007/s13213-019-01535-x
Mganga, K. Z., Rolando, J., Kalu, S., and Karhu, K. (2024). Microbial soil quality indicators depending on land use and soil type in a semi-arid dryland in Kenya. Eur. J. Soil Biol. 121:103626. doi: 10.1016/j.ejsobi.2024.103626
Moebius-Clune, D. J., Moebius-Clune, B. N, van Es, H. M., and Pawlowska, T. E. (2013). Arbuscular Mycorrhizal fungi associated with a single agronomic plant host across the landscape: community differentiation along a soil textural gradient. Soil Biol. Biochem. 64, 191–199. doi: 10.1016/j.soilbio.2012.10.043
Mukhongo, R. W., Ebanyat, P., Masso, C., and Tumuhairwe, J. B. (2023). Composition and spore abundance of arbuscular mycorrhizal fungi in sweet potato producing areas in Uganda. Front. Soil Sci. 3:1152524. doi: 10.3389/fsoil.2023.1152524
Ntinyari, W., and Gweyi-Onyango, J. P. (2020). “Greenhouse gases emissions in agricultural systems and climate change effects in Sub-Saharan Africa,” in African Handbook of Climate Change Adaptation (Cham: Springer International Publishing), 1–25.
Oehl, F., Laczko, E., Oberholzer, H. R., Jansa, J., and Egli, S. (2017). Diversity and biogeography of arbuscular mycorrhizal fungi in agricultural soils. Biol Fertil. Soils 53, 777–797. doi: 10.1007/s00374-017-1217-x
Oehl, F., Sieverding, E., Ineichen, K., Mader, P., Boller, T., and Wiemken, A. (2003). Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of central Europe. Appl. Environ. Microbiol. 69, 2816–2824. doi: 10.1128/AEM.69.5.2816-2824.2003
Oehl, F., Sieverding, E., Ineichen, K., Maeder, P., Wiemken, A., and Boller, T. (2009). Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agric. Ecosyst. Env. 134, 257–268. doi: 10.1016/j.agee.2009.07.008
Overby, S. T., Owen, S. M., Hart, S. C., Neary, D. G., and Johnson, N. C. (2015). Soil microbial community resilience with tree thinning in a 40-year-old experimental ponderosa pine forest. Appl. Soil Ecol. 93, 1–10. doi: 10.1016/j.apsoil.2015.03.012
Pagano, M. C., Scotti, M. R., and Cabello, M. N. (2012). “Mycorrhizas, an important component in Semiarid sites,” in Mycorrhiza: Occurrence in Natural and Restored Environments, ed. M. Pagano (New York: Nova Science Publishers), 127–146.
Qin, Z., Zhang, H., Feng, G., Christie, P., Zhang, J., Li, X., et al. (2020). Soil phosphorus availability modifies the relationship between AM fungal diversity and mycorrhizal benefits to maize in an agricultural soil. Soil Biol. Biochem. 144:107790. doi: 10.1016/j.soilbio.2020.107790
Rodríguez-Rodríguez, R. M., Kemmelmeier, K., de Fátima Pedroso, D., Pinto, F. A., dos Santos, J. V., Gastauer, M., et al. (2021). Native arbuscular mycorrhizal fungi respond to rehabilitation in iron ore mining areas from the Eastern Brazilian Amazon. Pedobiologia 89:150768. doi: 10.1016/j.pedobi.2021.150768
Saiz, G., Wandera, F. M., Pelster, D. E., Ngetich, W., Okalebo, J. R., Rufino, M. C., et al. (2016). Long-term assessment of soil and water conservation measures (Fanya-juu terraces) on soil organic matter in southeastern Kenya. Geoderma 274, 1–9. doi: 10.1016/j.geoderma.2016.03.022
Sakha, M., Jefwa, J., Mutua, L., Egesa, A. O., Gumo, P., Otieno, E., et al. (2024). Most probable number bioassays and trap culture techniques are promising in estimating quantitative and qualitative arbuscular mycorrhizal fungi. IJBS 11, 1–14. doi: 10.30954/2347-9655.01.2024.1
Sakha, M. A., Jefwa, J., and Gweyi-Onyango, J. P. (2019). Effects of arbuscular mycorrhizal fungal inoculation on growth and yield of two sweet potato varieties. J. Agric. Ecol. Res. Int. 18, 1–8. doi: 10.9734/jaeri/2019/v18i330063
Säle, V., Aguilera, P., Laczko, E., Mäder, P., Berner, A., Zihlmann, U., et al. (2015). Impact of conservation tillage and organic farming on the diversity ofarbuscular mycorrhizal fungi. Soil Biol. Biochem. 84, 38–52. doi: 10.1016/j.soilbio.2015.02.005
Schenck, N. C., and Perez-Collins, Y. (1990). Manual for the Identification of VA Mycorrhizal Fungi, 3rd Edn. Gainesville, Fla: Synergistic publications (1990).
Shepherd, K. D., Jefwa, J., Wilson, J., Ndufa, J. K., Ingleby, K., and Mbuthia, K. W. (1996). Infection potential of farm soils as mycorrhizal inocula for Leucaena leucocephala. Biol. Fertil. Soils 22, 16–21. doi: 10.1007/BF00384427
Sieverding, E., Friedrichsen, J., and Suden, W. (1991). Vesicular-Arbuscular Mycorrhiza Management In Tropical Agrosystems (No. 224). Schriftenreihe der GTZ.
Silvana, V. M., Carlos, F. J., Lucía, A. C., Natalia, A., and Marta, C. (2020). Colonization dynamics of arbuscular mycorrhizal fungi (AMF) in Ilex paraguariensis crops: seasonality and influence of management practices. J. King Saud Univ. Sci. 32, 183–188. doi: 10.1016/j.jksus.2018.03.017
Šmilauer, P., Košnar, J., Kotilínek, M., and Šmilauerová, M. (2020). Contrasting effects of host identity, plant community, and local species pool on the composition and colonization levels of arbuscular mycorrhizal fungal community in a temperate grassland. New Phytol. 225, 461–473. doi: 10.1111/nph.16112
Society for the Protection of Underground Networks (2023). Soil Sampling Protocol. Available online at: https://www.spun.earth/expeditions/sampling-protocol (accessed August 20, 2023).
Song, J., Han, Y., Bai, B., Jin, S., He, Q., and Ren, J. (2019). Diversity of arbuscular mycorrhizal fungi in rhizosphere soils of the Chinese medicinal herb Sophora flavescens Ait. Soil Tillage Res. 195:104423. doi: 10.1016/j.still.2019.104423
Straker, C. J., Hilditch, A. J., and Rey, M. E. C. (2010). Arbuscular mycorrhizal fungi associated with cassava (Manihot esculenta Crantz) in South Africa. South Afr. J. Bot. 76, 102–111. doi: 10.1016/j.sajb.2009.09.005
Temegne, N. C., Wakem, G. A., Taffouo, V. D., Mbogne, T. J., Onguene, A. N., Youmbi, E., et al. (2017). Effect of phosphorus fertilization on arbuscular mycorrhizal fungi in the Bambara groundnut rhizosphere. Afr. J. Microbiol. Res. 11, 1399–1410. doi: 10.5897/AJMR2017.8680
Vályi, K., Mardhiah, U., Rillig, M. C., and Hempel, S. (2016). Community assembly and coexistence in communities of arbuscular mycorrhizal fungi. ISME J. 10, 2341–2351. doi: 10.1038/ismej.2016.46
Van Geel, M., Jacquemyn, H., Plue, J., Saar, L., Kasari, L., Peeters, G., et al. (2018). Abiotic rather than biotic filtering shapes the arbuscular mycorrhizal fungal communities of European seminatural grasslands. New Phytol. 220, 1262–1272. doi: 10.1111/nph.14947
Vasar, M., Davison, J., Sepp, S. K., Oja, J., Al-Quraishy, S., Bueno, C. G., et al. (2022). Global taxonomic and phylogenetic assembly of AM fungi. Mycorrhiza, 32, 135–144. doi: 10.1007/s00572-022-01072-7
Veresoglou, S. D., Caruso, T., and Rilling, M. C. (2013). Modelling the environment and soil factors that shape the niches of two common arbuscular mycorrhizal fungal families. Plant Soil 368, 507–518. doi: 10.1007/s11104-012-1531-x
Vieira, C. K., Marascalchi, M. N., Rodrigues, A. V., de Armas, R. D., and Stürmer, S. L. (2018). Morphological and molecular diversity of arbuscular mycorrhizal fungi in revegetated iron-mining site has the same magnitude of adjacent pristine ecosystems. J. Env. Sci. 67, 330–343. doi: 10.1016/j.jes.2017.08.019
Vieira, L. C., Silva, D. K. A. D., Escobar, I. E. C., Silva, J. M. D., Moura, I. A. D., Oehl, F., et al. (2020). Changes in an arbuscular mycorrhizal fungi community along an environmental gradient. Plants 9:52. doi: 10.3390/plants9010052
Vohland, K., and Barry, B. (2009). A review of in situ rainwater harvesting (RWH) practices modifying landscape functions in African drylands. Agric. Ecosyst. Env. 131, 119–127. doi: 10.1016/j.agee.2009.01.010
Wagg, C., Husband, B. C., Green, D. S., Massicotte, H. B., and Peterson, R. L. (2011). Soil microbial communities from an elevational cline differ in their effect on conifer seedling growth. Plant Soil. 340, 491–504. doi: 10.1007/s11104-010-0621-x
Walkley, A., and Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
Wang, C., Gu, Z., Cui, H., Zhu, H., Fu, S., and Yao, Q. (2015). Differences in arbuscular mycorrhizal fungal community composition in soils of three land use types in subtropical hilly area of Southern China. PLoS ONE 10:e0130983. doi: 10.1371/journal.pone.0130983
Wang, F., Zhan, L., Zhou, J., Rengel, Z., George, T. S., and Feng, G. (2022). Exploring the secrets of hyphosphere of arbuscular mycorrhizal fungi: processes and ecological functions. Plant Soil 481, 1–22. doi: 10.1007/s11104-022-05621-z
Wang, L., d'Odorico, P., Evans, J. P., Eldridge, D. J., McCabe, M. F., Caylor, K. K., et al. (2012). Dryland ecohydrology and climate change: critical issues and technical advances. Hydrol. Earth Syst. Sci. 16, 2585–2603. doi: 10.5194/hess-16-2585-2012
Xavier Martins, W. F., and Rodrigues, B. F. (2020). Identification of dominant arbuscular mycorrhizal fungi in different rice ecosystems. Agric. Res. 9, 46–55. doi: 10.1007/s40003-019-00404-y
Xiang, D., Verbruggen, E., Hu, Y., Veresoglou, S. D., Rillig, M. C., Zhou, W., et al. (2014). Land use influences arbuscular mycorrhizal fungal communities in the farming–pastoral ecotone of northern China. New Phytol. 204, 968–978. doi: 10.1111/nph.12961
Xiang, X., Gibbons, S. M., He, J. S., Wang, C., He, D., Li, Q., et al. (2016). Rapid response of arbuscular mycorrhizal fungal communities to short-term fertilization in an alpine grassland on the Qinghai-Tibet Plateau. PeerJ 4:e2226. doi: 10.7717/peerj.2226
Yang, H., Zang, Y., Yuan, Y., Tang, J., and Chen, X. (2012). Selectivity by host plants affects the distribution of arbuscular mycorrhizal fungi: evidence from ITS rDNA sequence metadata. BMC Evol. Biol. 12, 1–13. doi: 10.1186/1471-2148-12-50
Yang, L., Du, L., Li, W., Wang, R., and Guo, S. (2023). Divergent responses of phoD-and pqqC-harbouring bacterial communities across soil aggregates to long fertilization practices. Soil Till. Res. 228:105634. doi: 10.1016/j.still.2023.105634
Yu, L., Zhang, Z., Zhou, L., and Huang, K. (2023). Effects of altitude and continuous cropping on arbuscular mycorrhizal fungi community in Siraitia grosvenorii rhizosphere. Agriculture 13:1548. doi: 10.3390/agriculture13081548
Zhang, J., Quan, C., Ma, L., Chu, G., Liu, Z., and Tang, X. (2021). Plant community and soil properties drive arbuscular mycorrhizal fungal diversity: A case study in tropical forests. Soil Ecol. Lett. 3, 52–62. doi: 10.1007/s42832-020-0049-z
Zhang, M. G., Shi, Z. Y., Xu, X. F., and Wang, X. G. (2022). Arbuscular mycorrhizal fungi associated with roots reveal high diversity levels at different elevations in tropical montane rainforests. Diversity 14:587. doi: 10.3390/d14080587
Keywords: microbial diversity, native microbiome, spore morphological analysis, ecosystem services, sustainable farming
Citation: Sakha M, Gweyi-Onyango JP, Masso C and Baijukya FP (2025) Diversity, characteristics, and abundance of native arbuscular mycorrhizal fungi in the semi-arid lands of Eastern Kenya. Front. Microbiol. 16:1582476. doi: 10.3389/fmicb.2025.1582476
Received: 24 February 2025; Accepted: 28 April 2025;
Published: 21 May 2025.
Edited by:
Agnieszka Kuźniar, The John Paul II Catholic University of Lublin, PolandReviewed by:
Jadson Belem De Moura, Evangelical School of Goianésia, BrazilLin Zhang, China Agricultural University, China
Copyright © 2025 Sakha, Gweyi-Onyango, Masso and Baijukya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Sakha, c2FraGFtaWNoYWVsMUBnbWFpbC5jb20=
 Michael Sakha
Michael Sakha Joseph P. Gweyi-Onyango
Joseph P. Gweyi-Onyango Cargele Masso
Cargele Masso Frederick P. Baijukya
Frederick P. Baijukya