- 1Amity University Rajasthan, Jaipur, India
- 2Department of Chemistry, Shivaji College, University of Delhi, New Delhi, India
- 3Department of Pharmaceutical Sciences, College of Pharmacy, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 4Department of Biology, College of Science, University of Hail, Hail, Saudi Arabia
- 5Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
- 6S.S. Jain Subodh PG College (Autonomous), Jaipur, India
The green synthesis of silver nanoparticles (AgNPs) using Artemisia scoparia (A. scoparia), a plant abundant in bioactive compounds, provides an eco-friendly, cost-effective, and sustainable method for nanoparticle synthesis. Silver ions were successfully reduced using an aqueous extract of A. scoparia, resulting in AgNPs with a characteristic UV–visible absorption peak at 421 nm. Comprehensive characterization using FTIR, TEM, SEM, and DLS confirmed their stability, uniform morphology, and functional groups. The antibacterial activity of the synthesized AgNPs was evaluated against E. faecalis and P. aeruginosa, and their catalytic activity was assessed by reducing aromatic nitro compounds. A. scoparia-derived AgNPs demonstrated potent antibacterial activity, effectively inhibiting the growth of E. faecalis and P. aeruginosa, indicating potential for addressing antibiotic-resistant bacteria. Additionally, these AgNPs exhibited remarkable catalytic efficiency in reducing aromatic nitro compounds, showcasing their potential as eco-friendly catalysts. This dual functionality highlights their significant role in sustainable nanotechnology and environmental remediation efforts.
1 Introduction
Nanotechnology has become a transformative force in science and technology, offering solutions to some of the most pressing challenges in medicine, environmental remediation, and material science (Singh et al., 2024). Silver nanoparticles (AgNPs) are notable among various nanomaterials due to their remarkable characteristics, such as a high surface area-to-volume ratio, adjustable optical and electronic properties, and strong biological activities (Iwuji et al., 2024). AgNPs are widely recognized for their broad-spectrum antimicrobial and catalytic properties. These make them invaluable in diverse applications, ranging from antimicrobial coatings and drug delivery systems to environmental detoxification and renewable energy (More et al., 2023; Singh et al., 2015). Despite their versatility, conventional synthesis methods for AgNPs are often associated with significant drawbacks, including hazardous chemicals, high energy demands, and the generation of toxic byproducts. These limitations underscore the urgent need for environmentally sustainable approaches to nanoparticle production (Nie et al., 2023).
Green synthesis has emerged as an eco-friendly and sustainable alternative, utilizing biological entities such as plants, bacteria, fungi, and algae to facilitate nanoparticle synthesis (Bhardwaj et al., 2020). Among these, plant-based synthesis has garnered particular attention due to its simplicity, cost-effectiveness, and the rich diversity of bioactive metabolites in plant extracts (Gupta et al., 2023). For instance, plants such as Azadirachta indica, Ocimum sanctum, and Artemisia scoparia have been extensively studied for their effectiveness in synthesizing silver nanoparticles (AgNPs), owing to their abundant bioactive compounds (Alharbi et al., 2022). Plant-derived bioactive compounds, including phenols, flavonoids, terpenoids, and alkaloids, act as natural reducing, capping, and stabilizing agents, eliminating the need for external chemical additives (Mutha et al., 2021; Dias et al., 2021). This not only minimizes environmental impact but also enhances the biocompatibility and functionality of the synthesized nanoparticles. Green synthesis minimizes the use of hazardous chemicals, the potential toxicity of AgNPs on human health and the environment remains a critical concern. To ensure their safe application, future research should focus on evaluating the biocompatibility of these nanoparticles through cytotoxicity and genotoxicity assessments (Kirubakaran et al., 2025). Additionally, investigating the environmental fate of AgNPs, including their stability, potential accumulation in ecosystems, and effective disposal methods, is crucial for ensuring their sustainable use. Optimizing AgNP concentration for biomedical and environmental applications can further minimize toxicity risks while maintaining functional efficacy (Nie et al., 2023).
While several plants have been explored for green synthesis, existing methods often suffer from limitations such as low nanoparticle stability, inconsistent particle size distribution, or reduced antimicrobial efficiency. Furthermore, many reported methods require lengthy preparation steps or lack scalability for industrial applications (Ying et al., 2022). In this context, Artemisia scoparia (A. scoparia), a medicinal plant known for its pharmacological potential, emerges as an ideal candidate for green nanoparticle synthesis. A member of the Asteraceae family, A. scoparia is renowned for its diverse bioactive profile, comprising phenols, flavonoids, sesquiterpenes, and essential oils (Ding et al., 2021). These compounds exhibit intrinsic antimicrobial, antioxidant, and anti-inflammatory properties, making the plant a valuable resource for nanoparticle synthesis (Parham et al., 2020; Li et al., 2024). Moreover, A. scoparia's unique phytochemical composition is hypothesized to improve nanoparticle stability, enhance antimicrobial efficacy, and provide better control over particle size distribution, addressing key issues observed in existing plant-based synthesis methods (Ding et al., 2021).
This study focuses on the green synthesis of AgNPs using an aqueous extract of A. scoparia as a natural reducing and stabilizing agent. Comprehensive characterization of the synthesized nanoparticles was performed using a range of advanced analytical techniques, including UV-vis spectroscopy, Dynamic Light Scattering (DLS), Zeta potential analysis, Fourier Transform Infrared (FTIR) spectroscopy, Scanning Electron Microscopy (SEM), and Transmission Electron Microscopy (TEM) (Vladár and Hodoroaba, 2020; Kumari et al., 2023). In parallel, the phytochemical composition of A. scoparia extract was elucidated using confirmatory tests identifying the key bioactive constituents responsible for nanoparticle synthesis and stabilization (Zhang et al., 2024; Ding et al., 2021; Kuerban et al., 2024).
The unique bioactive profile of A. scoparia ensures improved nanoparticle stability compared to conventional plant-based methods, while its synergistic combination of bioactive compounds enhances the antibacterial efficacy of the resulting AgNPs. This eco-friendly method also eliminates the need for toxic reducing agents, ensuring minimal environmental impact and offering a cost-effective solution for sustainable nanoparticle production (Fahim et al., 2024).
The biological and chemical activities of the synthesized AgNPs were also evaluated to explore their potential applications. The AgNPs demonstrated significant antibacterial properties, making them promising candidates for combating microbial infections (Rengarajan et al., 2024; Nie et al., 2023). The catalytic reduction of nitro compounds, a key environmental detoxification process, showed the remarkable chemical efficiency of the nanoparticles (Sassykova et al., 2019).
By integrating green synthesis, advanced characterization, and functional evaluation, this study not only underscores the potential of A. scoparia in the eco-friendly synthesis of AgNPs but also highlights the versatility of these nanoparticles in addressing biomedical and environmental challenges. This research contributes to the growing body of knowledge on plant-mediated nanoparticle synthesis, paving the way for sustainable and multifunctional nanomaterials in the future.
2 Experimental protocol and resources
2.1 Chemicals
All Analytical-grade reagents such as silver nitrate (AgNO3, ≥99.0%), 1-bromo-4-nitrobenzene, 4-nitroaniline, 4-bromo-2-fluoro-1-nitrobenzene, 4-nitrophenol, sodium borohydride (98%), were acquired from Merck (Germany and Mumbai, India), Acros Organics, and Scharlau (Barcelona, Spain), respectively. All solutions were prepared in deionized water, with glassware meticulously cleaned using nitric acid, rinsed in distilled and deionized water and oven-dried to prevent electrolyte interference (Ghojavand et al., 2020).
2.2 Plant sample collection and processing techniques
A. scoparia is an herbaceous plant characterized by its finely dissected leaves and aromatic scent. The plant typically exhibits yellowish-green foliage and produces small, inconspicuous flowers (Boakye et al., 2017). The classification of the plant is as follows:
Family: Asteraceae.
Subfamily: Asteroideae.
Genus: Artemisia.
Species: Scoparia.
Botanical name: A. scoparia.
Common name: Redstem Wormwood.
Freshly collected leaves and stems, from Amity University Rajasthan in Jaipur and thoroughly washed with distilled water to remove contaminants. The cleaned leaves and stems were dried in an oven at 60°C. For extract preparation, 10 g of the dried leaves and stems were ground into a fine powder (Figure 1) and mixed with 100 mL of deionized water in a 250 mL beaker. The mixture was boiled for 30 min and cooled to room temperature. The extract was filtered through Whatman No. 1 filter paper (pore size 25 μm), and centrifuged at 5,000 rpm for 15 min, the supernatant was filtered and further refined with 0.4 μm syringe filters. The final aqueous extract was stored in a clean bottle at 4°C for future synthesis applications.

Figure 1. This figure shows the processing steps of A. scoparia for the preparation of its powder. The first image shows the fresh plant of A. scoparia in its natural habitat followed by the dried plant material in the second image. The final image displays the powdered form of A. scoparia, which is obtained after drying and grinding the plant material.
2.3 Phytochemicals screening
2.3.1 Confirmatory tests
Phytochemical screening is an essential qualitative test in herbal medicine and natural product research. It identifies key bioactive compounds, such as Phenols, Tannins, Flavonoids, Saponins, and Carbohydrates that play a vital role in plants' medicinal effects (Labulo et al., 2022).
2.3.2 Test for phenols and tannins
Ferric Chloride Test: Take 1–2 mL of the plant extract in a test tube. Add 2–3 drops of 5% ferric chloride (FeCl3) solution to the extract. Mix gently and observe the color change.
2.3.3 Test for flavonoids
Alkaline Test: Take 1–2 mL of the plant extract in a test tube. Add a few drops of 10% sodium hydroxide (NaOH) solution. Observe the color change. Add a few drops of dilute hydrochloric acid (HCl) to the mixture and observe again.
2.3.4 Test for saponins
Foam Test: Take 1–2 mL of the plant extract in a test tube. Add 2–3 mL of distilled water. Shake the solution vigorously for about 1 min.
2.3.5 Test for carbohydrates
Molisch Test: Take 2 ml of the plant extract in a test tube, add 2–3 drops of Molisch's reagent (a-naphthol), and then add concentrated sulfuric acid carefully along the side of the test tube.
2.4 Green synthesis of silver nanoparticles
To synthesize AgNPs through a green method, 5 mL of fresh plant extract was slowly added to 95 mL of a 3 mM AgNO3 solution in an Erlenmeyer flask. The mixture was gently heated to 45°C and continuously stirred. The formation of nanoparticles was indicated by a color change from pale yellowish to reddish brown (Figure 2, Scheme 1). After allowing the reaction to proceed for 24 h or until reaching room temperature, the mixture was centrifuged at 5,000 rpm for 30 min to isolate the nanoparticles. The supernatant was carefully discarded, and the nanoparticles were dried at 25°C before being stored in labeled vials. UV-vis spectroscopy was used to confirm the successful synthesis of the AgNPs (Fahim et al., 2024).

Figure 2. (A) Solution of silver nitrate (AgNO3), (B) the plant extract, and (C) synthesized 3 mM AgNPs.
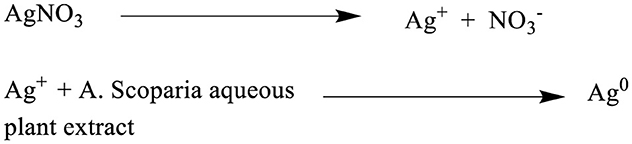
Scheme 1. Schematic representation of the synthesis of AgNPs (Ag°) using A. scoparia aqueous plant extract. As shown in Scheme 1, the process involves the dissociation of AgNO3 into silver ions (Ag+) and nitrate ions (), followed by the reduction of Ag+ to elemental silver (Ag°) through the reducing and stabilizing action of phytochemicals present in the plant extract, culminating in the formation of AgNPs.
3 Characterizations of synthesized silver nanoparticles (AgNPs)
3.1 UV–visible spectrum analysis (UV-VIS)
The formation of AgNPs through the bioreduction of silver ions (Ag+) was confirmed by UV-visible spectrophotometry Thermo Scientific (Multiskan Go) with a 1 nm resolution over a wavelength range of 200–800 nm. Spectral measurements were taken at room temperature at various intervals, using distilled water as a baseline reference, and a 2 mL sample pipetted into the cuvette. The analysis of absorption peaks in this range verified the presence and stability of AgNPs in the colloidal solution (Labulo et al., 2022).
3.2 Fourier transform infrared analysis (FTIR)
The purified AgNPs analysis was conducted using Fourier Transform Infrared (FTIR) spectroscopy in ATR mode to determine the contributions of phytochemicals from the plant extracts in modifying their surfaces. Using a PerkinElmer Spectrum FTIR spectrometer at room temperature, the spectra were recorded over a 4,000–400 cm−1 wavelength range. This investigation identified significant interactions between silver and bioactive molecules, as evidenced by distinct peaks that indicate the formation and stabilization of AgNPs. The FTIR results for AgNPs were compared to those of the plant extract to highlight the key biomolecules in reducing silver ions (Ghojavand et al., 2020).
3.3 Transmission electron microscopic analysis (TEM)
The AgNPs' morphological characteristics and size distribution were examined using TEM analysis with a Tecnai G2 20 S-TWIN [FEI] at 200 kV. Sample preparation involved dilution, sonication, filtration, and placing a drop onto carbon-coated copper grids for air-drying (Sreelekha et al., 2021).
3.4 Field emission scanning electron microscopy analysis (FESEM)
The AgNPs' surface morphology, shape, size, crystalline structure, and elemental composition were analyzed using FESEM on TESCAN MIRA-LMS. Samples were prepared as thin films on carbon-coated copper grids, dried, and examined confirming the silver presence and other elemental compositions (Labulo et al., 2022).
3.5 DLS zeta analysis (particle analyzer)
Dynamic light scattering (DLS) and zeta potential analysis on an Anton Paar Litesizer 500 were used to determine the synthesized AgNPs' size distribution, surface charge, and stability, providing a detailed profile of their suspension stability (Mahiuddin et al., 2020).
4 Biological applications and chemical applications
4.1 Antibacterial activity
The antibacterial activity of AgNPs was evaluated using the agar disc diffusion method against two bacterial strains: Enterococcus faecalis (E. faecalis) (Ali et al., 2021) and Pseudomonas aeruginosa (P. aeruginosa) (Muddassir et al., 2022), obtained from the Amity Institute of Biotechnology at Amity University Rajasthan, Jaipur. Bacterial cultures were grown in Luria-Bertani Broth (LBB) for 12 h at 37°C in an orbital shaker set at 180 rpm to ensure proper aeration (Kim et al., 2020). For testing, fresh Luria-Bertani Agar (LBA) plates were prepared, and 6 mm sterile filter paper discs were impregnated with 3 mM AgNPs and the corresponding plant extract. The discs were then placed on the surface of agar plates inoculated with the bacterial strains. Ciprofloxacin (CIP) and Kanamycin (KAN) were used as positive controls, while distilled water was the negative control. The plates were incubated for 24 h at 37°C, allowing bacterial growth (Hidayat et al., 2023). After incubation, the zones of inhibition around the discs were measured in millimeters to assess the antibacterial effectiveness of the AgNPs. The antimicrobial efficacy of the AgNPs was further quantified by determining the minimum inhibitory concentration (MIC), where applicable.
4.2 Catalytic reduction of nitro compounds using synthesized AgNPs
The catalytic reduction activity of AgNPs synthesized using A. scoparia extract was evaluated on four nitroaromatic compounds: 1-bromo-4-nitrobenzene, 4-bromo-2-fluoro-1-nitrobenzene, 4-nitroaniline, and 4-nitrophenol (Singh et al., 2018). Each nitro compound was subjected to a reduction reaction with AgNPs as a catalyst. Sodium borohydride (NaBH4), served as the reducing agent, facilitating electron transfer and hydrogenation of the nitro groups to their respective amine forms (Gondwal et al., 2023). The reaction was monitored via UV-vis spectroscopy, tracking the decrease in absorbance at the characteristic wavelengths of the nitro compounds as they were converted into their reduced products. This reduction was indicated by the progressive disappearance of the nitro peak and the emergence of a new peak corresponding to the amine product.
5 Result and discussion
5.1 Bioactive compounds in the plant extract confirmatory tests
Table 1 shows the phytochemical composition of the plant extract. The aqueous extract of A. scoparia confirmed the presence of phenols, tannins, flavonoids, Saponins, and Carbohydrates.
5.2 Characterizations of silver nanoparticles (AgNPs)
In this section, the structural, morphological, and compositional properties of AgNPs synthesized using A. scoparia extract were characterized using various advanced techniques. The results from each analytical method are detailed as follows.
5.2.1 UV–vis spectrum
The UV–vis spectra of AgNO3, the plant extract, the synthesized AgNPs and colloidal solutions are presented in Figure 3. The sharp absorption bands confirm the characteristic peaks at 303 nm for AgNO3, 290 nm for the plant extract, and 421 nm for the AgNPs, respectively, corroborating successful nanoparticle synthesis.
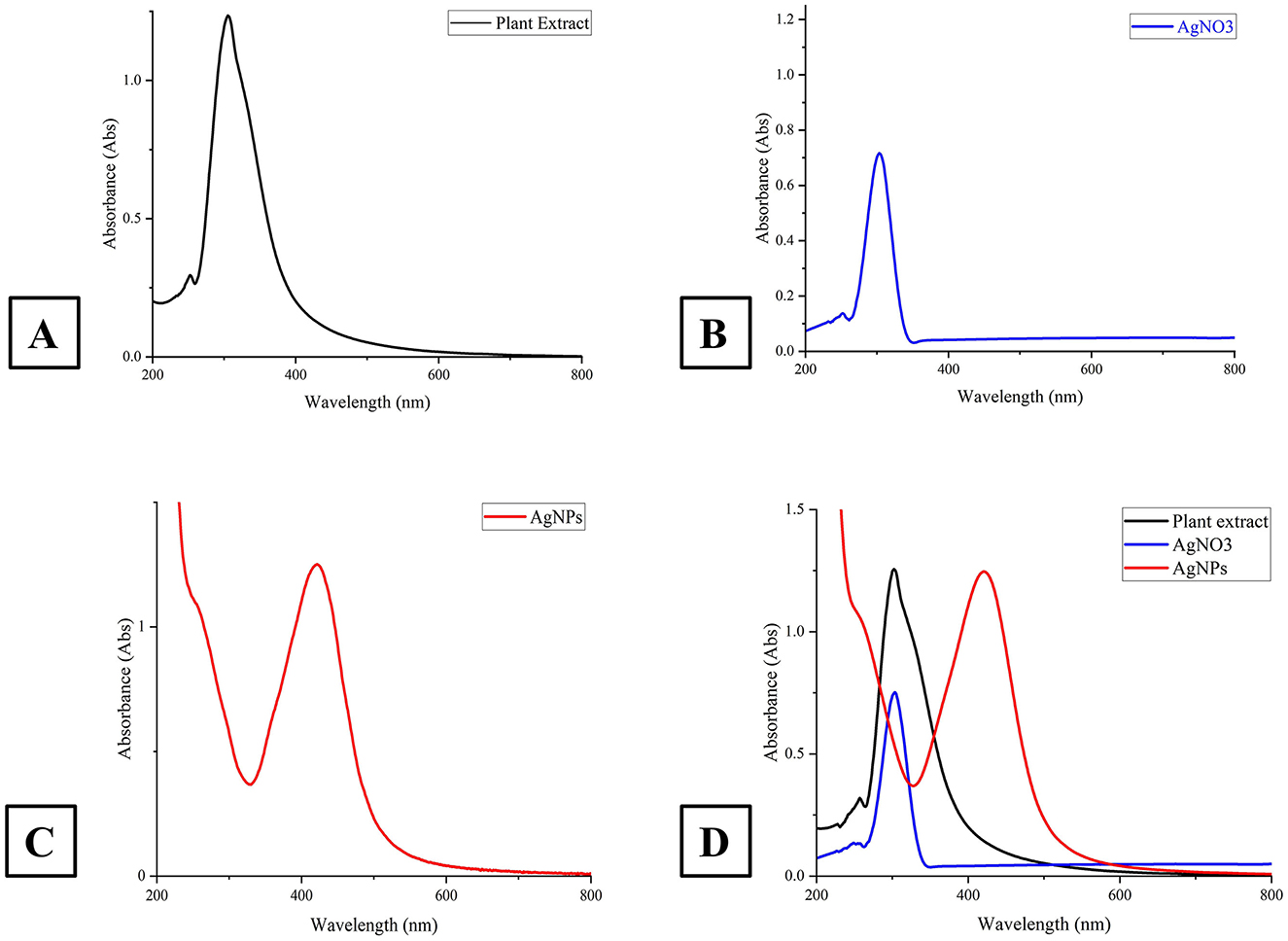
Figure 3. This figure presents the UV-Vis absorption spectra of A. scoparia plant extract, silver nitrate (AgNO3) solution, and AgNPs. (A) spectrum of the plant extract; (B) shows the spectrum of the AgNO3 solution; (C) depicts the absorption spectrum of the synthesized AgNPs at 421 nm, indicating successful nanoparticle formation and (D) compares the spectra of the plant extract (black), AgNO3 solution (blue), and AgNPs (green), emphasizing the shift in the absorption peak as the synthesis progresses.
5.2.2 FTIR
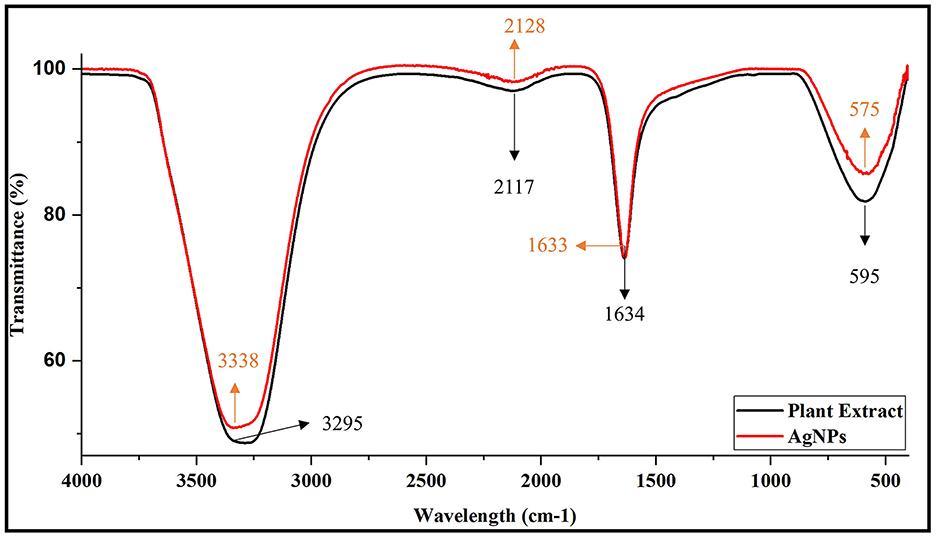
The FTIR spectra of the plant extract and the synthesized AgNPs reveal important functional groups and their interactions during nanoparticle synthesis. In the plant extract, prominent peaks are observed at 3,295 cm−1 (hydroxyl, –OH group), 2,117 cm−1 (nitrile, C=N group), 1,634 cm−1 (amide carbonyl, C=O group), and 595 cm−1 (likely aromatic C–H out-of-plane bending). The FTIR spectrum of the synthesized AgNPs shows slight shifts in these peaks: the hydroxyl group peak moves to 3,338 cm−1, the nitrile group peak shifts to 2,128 cm−1, and the amide carbonyl peak appears at 1,633 cm−1, suggesting interactions with the AgNPs. Additionally, the peak at 595 cm−1 in the plant extract shifts to 575 cm−1 in the AgNPs, likely due to the formation of silver-oxygen (Ag–O) bonds. These spectral changes confirm that the bioactive compounds in the plant extract play a crucial role in both reducing and stabilizing the AgNPs (Figure 4).
5.2.3 TEM
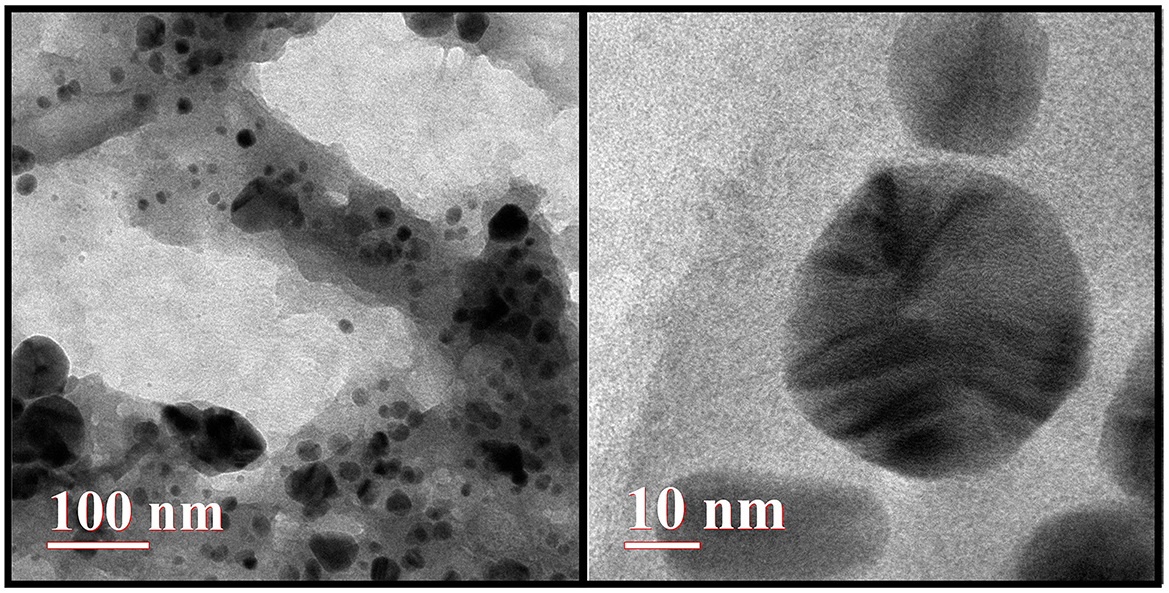
As shown in Figure 5, the transmission electron microscopy (TEM) analysis revealed that the synthesized AgNPs were predominantly spherical with a uniform morphology. Minimal agglomeration was observed, and the TEM images highlight the successful synthesis and stability of the AgNPs.
5.2.4 FESEM
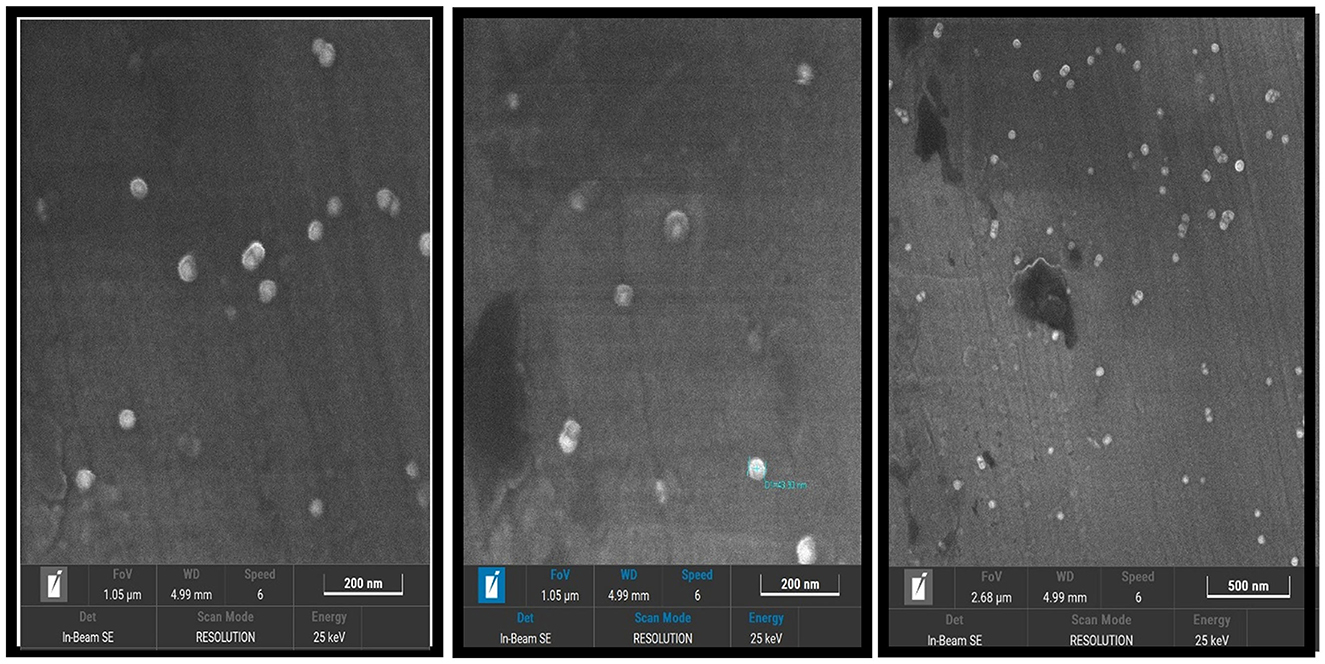
The field emission scanning electron microscopy (FESEM) analysis confirmed the formation of AgNPs with predominantly spherical shapes and smooth surfaces. The nanoparticles were uniformly distributed, with an average size of ~43.30 nm. Minimal agglomeration observed in the FESEM images indicates the stability of the synthesized AgNPs, further validating the success of the synthesis method (Figure 6).
5.2.5 DLS zeta
The dynamic light scattering (DLS) and zeta potential analysis provided valuable insights into the size distribution and surface charge of the synthesized AgNPs. The DLS results confirmed a uniform size distribution with an average hydrodynamic diameter of ~232.9 nm, indicating the formation of nanoparticles within the desired range. The relatively narrow polydispersity index (PDI) value of 0.148 demonstrated the homogeneity of the synthesized AgNPs.
The zeta potential measurements revealed a surface charge of −25.3 mV, indicating significant electrostatic stability of the nanoparticles in the colloidal solution. The high zeta potential value suggests strong repulsive forces between particles, preventing aggregation and ensuring dispersion stability. These findings collectively confirm the successful synthesis of stable and uniformly dispersed AgNPs (Figure 7).
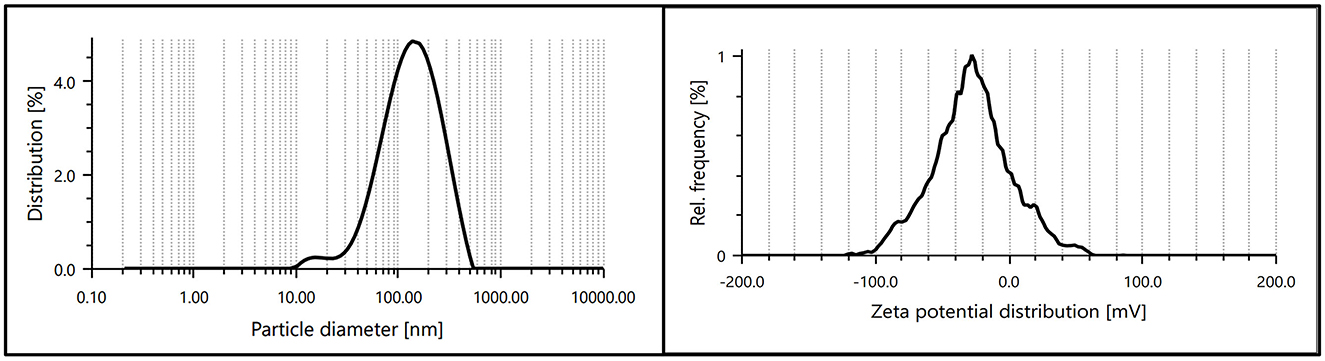
Figure 7. Particle size distribution and zeta potential of synthesized AgNPs, indicating uniform size and high colloidal stability.
5.3 Biological applications and chemical applications
5.3.1 Antibacterial activity
The antibacterial activity of the synthesized AgNPs was evaluated against P. aeruginosa (Gram-negative) and E. faecalis (Gram-positive) using the agar disc diffusion method. The positive control (antibiotic) showed the largest inhibition zones, while the negative control (distilled water) displayed no antibacterial activity (Figure 8). AgNPs (3 mM) demonstrated strong antibacterial effects, with inhibition zones of 8 ± 0.577 mm for E. faecalis and 12 ± 1.155 mm for P. aeruginosa, which were larger than those observed with the plant extract alone. All experiments were conducted in triplicate to ensure reliability, and the results are presented as mean ± standard error (SE) (Figure 9).
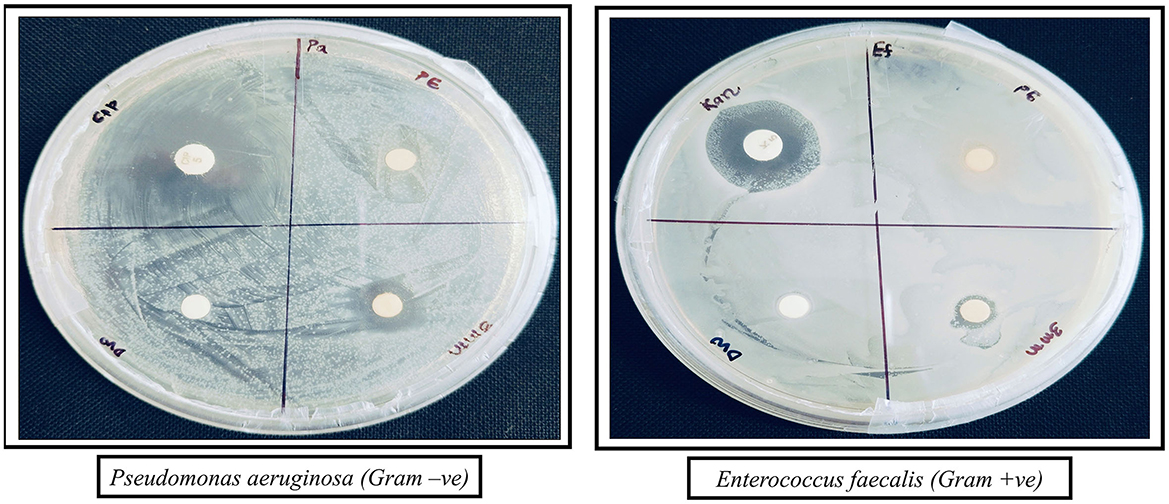
Figure 8. Antibacterial activity of AgNPs against P. aeruginosa (Gram-negative) and E. faecalis (Gram-positive). The zones of inhibition show the effectiveness of different treatments: Antibiotic (positive control), DW (negative control), PE (plant extract), and AgNPs (silver nanoparticles).
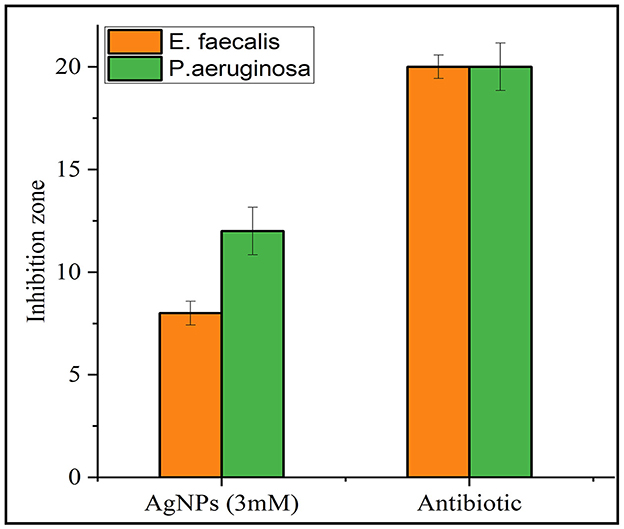
Figure 9. Comparative inhibition zones (in mm) for E. faecalis and P. aeruginosa treated with AgNPs and a standard antibiotic. Orange bars represent the inhibition zones for E. faecalis, while green bars represent those for P. aeruginosa. The results demonstrate antimicrobial activity for both AgNPs and the antibiotic against the tested strains.
The SE was calculated using the formula:
where:
SD: represents the standard deviation.
n: represents the number of replicates.
These results demonstrate that biosynthesized AgNPs are effective antimicrobial agents, likely due to their small size and large surface area.
5.3.2 Catalytic reduction of nitro compounds using synthesized AgNPs
The catalytic potential of the synthesized AgNPs was comprehensively demonstrated through the reduction of various nitroaromatic compounds using NaBH4, monitored via UV-vis spectroscopy. The reduction of 1-bromo-4-nitrobenzene (1-Br-4-NB) to 4-bromoaniline (4-BrA) was evidenced by the reduction of the 365 nm peak and the emergence of a new peak at 300 nm, achieving a 44.22% reduction within 20 min and accompanied by a visible color change (Figure 10A, Scheme 2). Similarly, the reduction of 4-bromo-2-fluoro-1-nitrobenzene (4-Br-2-F-1-NB) to 4-bromo-2-fluoroaniline (4-Br-2-F-An) showed a decline of the 401 nm peak and the appearance of a 288 nm peak, with a 61.20% reduction in 30 min (Figure 10B, Scheme 3). The reduction of 4-nitroaniline (4-NA) to 4-phenylenediamine (4-PDA) was marked by the disappearance of the 381 nm peak and the emergence of a 305 nm peak, achieving a 92.91% reduction within 25 min, accompanied by a color change (Figure 10C, Scheme 4). Finally, the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) was characterized by an initial shift of the 316 nm peak to 400 nm upon the addition of NaBH4, followed by a reduction to 299 nm, achieving a 90.90% reduction within 15 min, along with a yellowish-green color change indicating the formation of 4-nitrophenolate ions (Figure 10D, Scheme 5). In all cases, negligible changes were observed in the absence of AgNPs, underscoring the kinetic barriers of the uncatalyzed reactions and highlighting the remarkable catalytic efficiency of AgNPs in facilitating these transformations (Figure 11).
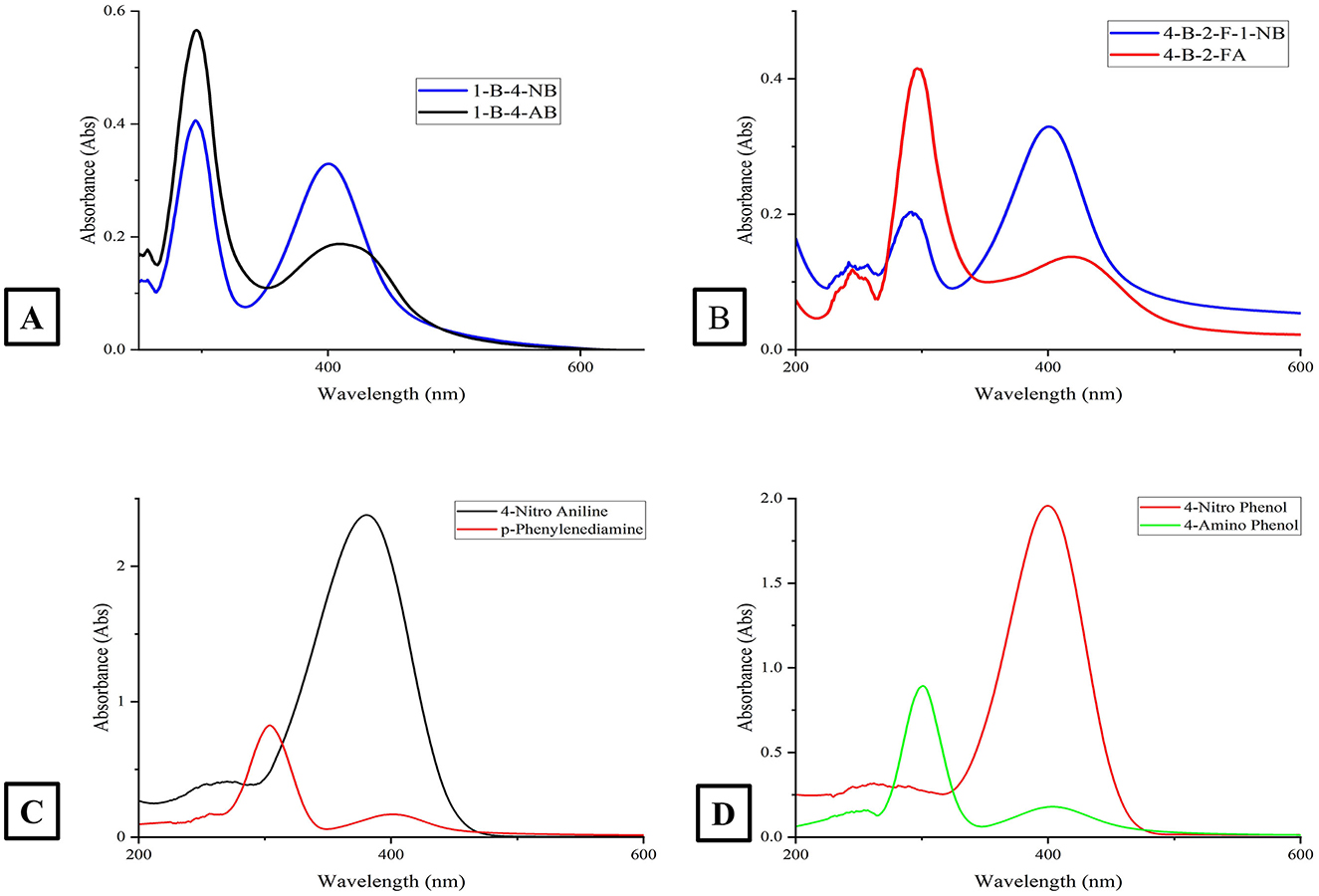
Figure 10. This figure presents four absorption spectra plots (labeled A–D) for various chemical compounds. (A) Displays the absorption spectra for the compounds 1-Br-4-NB and 1-Br-4-AB; (B) shows the absorption spectra for the compounds 4-Br-2-F-1-NB and 4-Br-2-F-1-A; (C) presents the absorption spectra for 4-Nitro Aniline and p-phenylenediamine and (D) depicts the absorption spectra for 4-Nitro Phenol and 4-Amino Phenol. The x-axis in all subplots represents the wavelength in nanometers (nm), and the y-axis shows the absorbance. Each plot identifies the specific compounds being displayed.
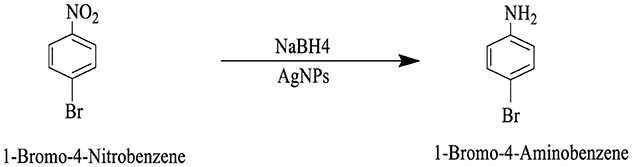
Scheme 2. This reaction scheme depicts the reduction of 1-bromo-4-nitrobenzene to 1-bromo-4-aminobenzene using sodium borohydride (NaBH4), and AgNPs as reducing agents. The nitro group (NO2), on the starting material is converted to an amino group (NH2), in the product.
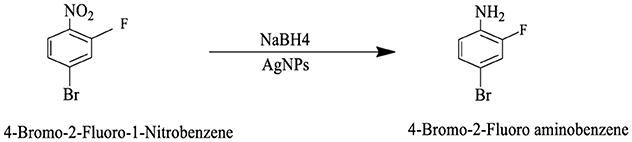
Scheme 3. This reaction scheme shows the reduction of 4-bromo-2-fluoro-1-nitrobenzene to 4-bromo-2-fluoroaniline using sodium borohydride (NaBH4), and AgNPs as reducing agents. The nitro group (NO2), is converted to an amino group (NH2), in the product.
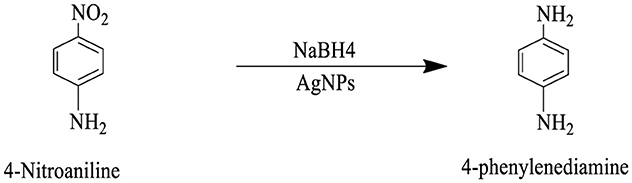
Scheme 4. This reaction scheme represents the reduction of 4-nitroaniline to 4-phenylenediamine using sodium borohydride (NaBH4), and AgNPs as reducing agents. The nitro group (NO2), is converted to an amino group (NH2), in the final product.
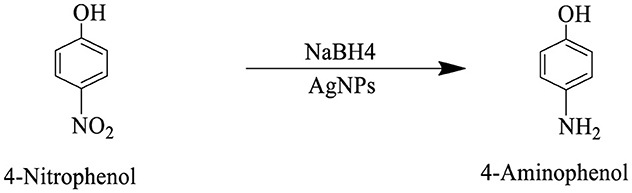
Scheme 5. The reaction scheme represents the chemical reduction of 4-nitrophenol to 4-aminophenol, utilizing sodium borohydride (NaBH4), and AgNPs. The nitro group (NO2), is successfully reduced to an amino group (NH2).
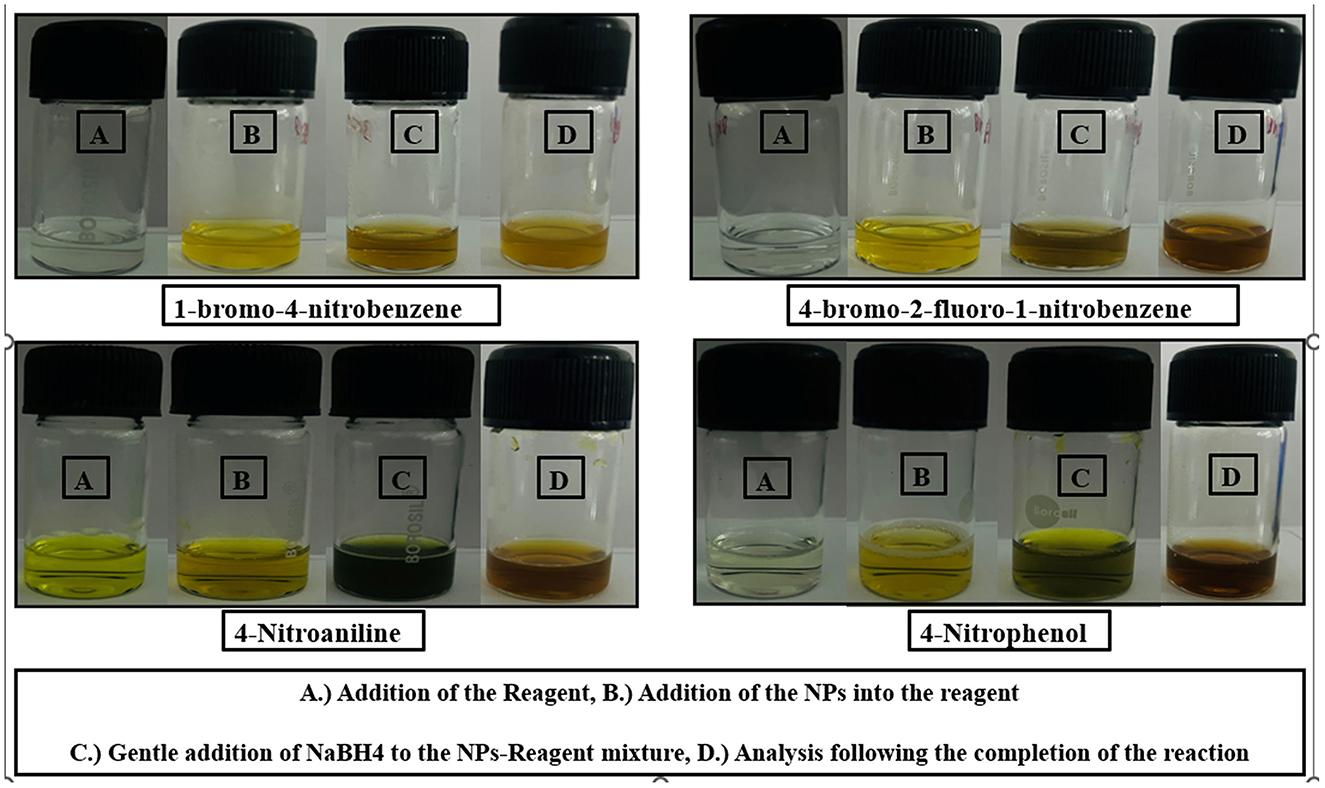
Figure 11. This figure presents a series of images showcasing the visual changes observed during the reaction processes for four different chemical compounds: 1-bromo-4-nitrobenzene, 4-bromo-2-fluoro-1-nitrobenzene, 4-nitroaniline, and 4-nitrophenol. (A) Addition of the reagent, (B) Addition of the NPs into the reagent, (C) Gentle addition of NaBH4 to the Nps-reagent mixture, (D) Analysis following the completion of the reaction.
To calculate the Efficiency (%), rate constant (k) and half-life (t1/2), we use the following first-order kinetic equations:
Efficiency (%):
where:
a0: Initial absorbance at the characteristic wavelength of the nitro compound.
at: Absorbance at the same wavelength after a specific time.
Rate constant k:
where:
A0: The initial absorbance of the peak.
At: The final absorbance of the peak after time t (reaction time).
k: The rate constant for the reaction, specific to the reaction conditions such as temperature.
t: The time elapsed since the start of the reaction.
Half-life t1/2:
where:
0.693 (ln), a constant.
k: The first-order rate constant (in min−1).
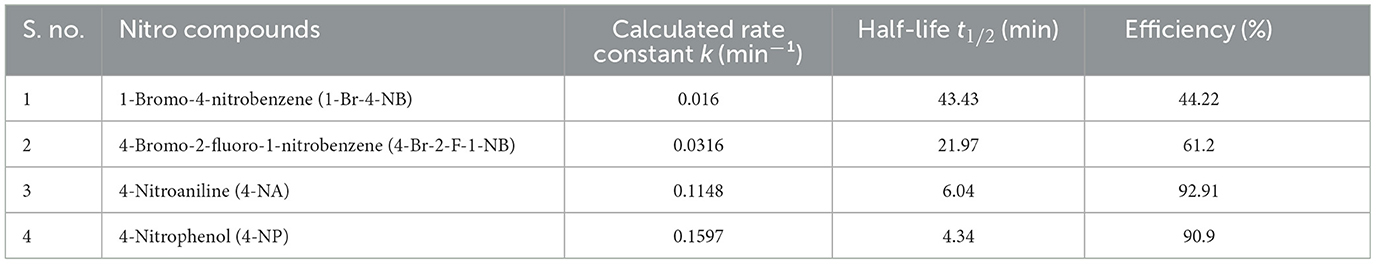
Based on the calculated rate constants (k) for the reduction of the nitroaromatic compounds, the rate of reduction follows the order:
4-Nitrophenol (4-NP) > 4-Nitroaniline (4-NA) > 4-Bromo-2-fluoro-1-nitrobenzene (4-Br-2-F-1-NB) > 1-Bromo-4-nitrobenzene (1-Br-4-NB) shows in Table 2 (Gondwal et al., 2023).
The figure is divided into four rows, with each row representing a different compound. Within each row, there are four labeled vials (A, B, C, D) depicting the visual changes at various stages of the reaction, as described in the caption:
(A) Addition of the Reagent.
(B) Addition of the NPs into the reagent.
(C) Gentle addition of NaBH4 to the NPs-Reagent mixture.
(D) Analysis following the completion of the reaction.
The images provide a clear visual representation of the reaction processes and the resulting changes in the sample appearance for the four compounds under investigation.
6 Conclusion
This study demonstrates the successful green synthesis of silver nanoparticles (AgNPs) using Artemisia scoparia (A. scoparia) extract, offering an eco-friendly and sustainable approach to nanoparticle production. Phytochemical screening of A. scoparia revealed the presence of phenols, tannins, flavonoids, saponins, and carbohydrates, which acted as natural reducing and capping agents during synthesis. Comprehensive characterization through UV-vis spectroscopy, FTIR, FE-SEM, TEM, and DLS Zeta analysis confirmed the stable morphology of the AgNPs. The synthesized AgNPs exhibited significant antibacterial activity against both Gram-positive (E. faecalis) and Gram-negative (P. aeruginosa) bacteria, highlighting their potential as effective antimicrobial agents. Additionally, their catalytic efficiency in the reduction of nitro compounds demonstrated their suitability for industrial and environmental applications. Overall, the use of A. scoparia-derived phytochemicals for AgNP synthesis not only reduces environmental impact but also enhances the nanoparticles' functional properties, opening avenues for their application in biomedical, catalytic, and environmental fields. These findings pave the way for future research focused on expanding their applications in drug delivery, advanced catalysis, and sustainable environmental solutions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
PB: Writing – review & editing, Conceptualization. RT: Writing – review & editing. SK: Writing – review & editing, Formal analysis. KR: Writing – review & editing, Formal analysis. NL: Writing – review & editing, Formal analysis. YK: Writing – review & editing. RB: Writing – review & editing, Formal analysis. MS: Writing – review & editing. SO: Writing – review & editing. SS: Writing – review & editing. KS: Supervision, Writing – review & editing. MC: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R304), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia also the authors are thankful to the Deanship of Research and Graduate Studies, King Khalid University, Abha, Saudi Arabia, for financially supporting this work through the Large Research Group Project under Grant no. R.G.P.2/514/45.
Acknowledgments
We deeply thank the Department of Science and Technology (DST) under the Promotion of University Research and Scientific Excellence (PURSE) program, New Delhi, India, for their generous support and funding. This work was made possible through the DST PURSE Grant/Award Number: (SR/PURSE/2021/77), which has been instrumental in facilitating our research endeavors and advancing scientific progress in this field. We also acknowledge the collaborative efforts and technical support of MNIT Jaipur and its Materials Research Center (MRC), whose contributions were invaluable to this work. RB, MS, and SO would like to express their sincere gratitude to Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia, for providing financial support for this research project (PNURSP2025R304). Additionally, the authors appreciate the financial support provided by the Deanship of Research and Graduate Studies, King Khalid University, Abha, Saudi Arabia, through the Large Research Group Project Grant (R.G.P.2/514/45). The authors, KR and YK, would like to acknowledge and express their sincere appreciation to the Principal of Shivaji College, University of Delhi, for providing valuable moral support. Additionally, they gratefully acknowledge the financial assistance provided by the College Research & Innovation Cell, Shivaji College (University of Delhi), through Project No. MRP/2024-2025/00012.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alharbi, N. S., Alsubhi, N. S., and Felimban, A. I. (2022). Green synthesis of silver nanoparticles using medicinal plants: characterization and application. J. Radiat. Res. Appl. Sci. 15, 109–124. doi: 10.1016/j.jrras.2022.06.012
Ali, A. R., Anani, H. A. A., and Selim, F. M. (2021). Biologically formed silver nanoparticles and in vitro study of their antimicrobial activities on resistant pathogens. Iran. J. Microbiol. 13:848. doi: 10.18502/ijm.v13i6.8090
Bhardwaj, B., Singh, P., Kumar, A., Kumar, S., and Budhwar, V. (2020). Eco-friendly greener synthesis of nanoparticles. Adv. Pharm. Bull. 10:566. doi: 10.34172/apb.2020.067
Boakye, Y. D., Shaheen, S., Nawaz, H., Nisar, S., and Azeem, M. W. (2017). Artemisia scoparia: a review on traditional uses, phytochemistry and pharmacological properties. Int. J. Chem. Biochem. Sci. 12, 92–97.
Dias, M. C., Pinto, D. C. G. A., and Silva, A. M. S. (2021). Plant flavonoids: chemical characteristics and biological activity. Molecules. 26:5377. doi: 10.3390/molecules26175377
Ding, J., Wang, L., He, C., Zhao, J., Si, L., Huang, H., et al. (2021). Artemisia scoparia: traditional uses, active constituents and pharmacological effects. J. Ethnopharmacol. 273:113960. doi: 10.1016/j.jep.2021.113960
Fahim, M., Shahzaib, A., Nishat, N., Jahan, A., Bhat, T. A., Inam, A., et al. (2024). Green synthesis of silver nanoparticles: a comprehensive review of methods, influencing factors, and applications. JCIS Open. 16:100125. doi: 10.1016/j.jciso.2024.100125
Ghojavand, S., Madani, M., and Karimi, J. (2020). Green synthesis, characterization and antifungal activity of silver nanoparticles using stems and flowers of felty germander. J. Inorg. Organomet. Polym. Mater. 30, 2987–2997. doi: 10.1007/s10904-020-01449-1
Gondwal, M., Sharma, N., Joshi nee Pant, G., Pratap Singh Gautam, B., Singh, S., Tumba, K., et al. (2023). Bioactivity and catalytic reduction of aryl nitro-compounds by biosynthesized silver nanoparticles using skimmiaanquetilia. ChemistrySelect. 8:e202203782. doi: 10.1002/slct.202203782
Gupta, D., Boora, A., Thakur, A., and Gupta, T. K. (2023). Green and sustainable synthesis of nanomaterials: recent advancements and limitations. Environ. Res. 231:116316. doi: 10.1016/j.envres.2023.116316
Hidayat, M. T., Koentjoro, M. P., and Prasetyo, E. N. (2023). Antimicrobial activity test of medicinal plant extract using antimicrobial disc and filter paper. Bioscientist : Jurnal Ilmiah Biologi. 11:456. doi: 10.33394/bioscientist.v11i1.7544
Iwuji, C., Saha, H., Ghann, W., Dotson, D., Bhuiya, M. d. A. K., Parvez, M. d. S., et al. (2024). Synthesis and characterization of silver nanoparticles and their promising antimicrobial effects. Chem. Phys. Impact. 9:100758. doi: 10.1016/j.chphi.2024.100758
Kim, M. A., Rosa, V., and Min, K. S. (2020). Characterization of Enterococcus faecalis in different culture conditions. Sci. Rep. 10, 1–8. doi: 10.1038/s41598-020-78998-5
Kirubakaran, D., Wahid, J. B. A., Karmegam, N., Jeevika, R., Sellapillai, L., Rajkumar, M., et al. (2025). A comprehensive review on the green synthesis of nanoparticles: advancements in biomedical and environmental applications. Biomed. Mat. Dev. 2025, 1–26. doi: 10.1007/s44174-025-00295-4
Kuerban, G., Turak, A., Begmatov, N. B., Zhao, J., and Aisa, H. A. (2024). Chemical composition of Artemisia Scoparia and their bioactivities. Chem. Biodivers. 21:e202400414. doi: 10.1002/cbdv.202400414
Kumari, S., Raturi, S., Kulshrestha, S., Chauhan, K., Dhingra, S., András, K., et al. (2023). A comprehensive review on various techniques used for synthesizing nanoparticles. J. Mat. Res. Technol. 27, 1739–63. doi: 10.1016/j.jmrt.2023.09.291
Labulo, A. H., David, O. A., and Terna, A. D. (2022). Green synthesis and characterization of silver nanoparticles using Morinda lucida leaf extract and evaluation of its antioxidant and antimicrobial activity. Chem. Zvesti. 76:7313. doi: 10.1007/s11696-022-02392-w
Li, S., Jiang, S., Jia, W., Guo, T., Wang, F., Li, J., et al. (2024). Natural antimicrobials from plants: Recent advances and future prospects. Food Chem. 432:137231. doi: 10.1016/j.foodchem.2023.137231
Mahiuddin, M., Saha, P., and Ochiai, B. (2020). Green synthesis and catalytic activity of silver nanoparticles based on piper chaba stem extracts. Nanomaterials. 10, 1–15. doi: 10.3390/nano10091777
More, P. R., Pandit, S., De Filippis, A., Franci, G., Mijakovic, I., Galdiero, M., et al. (2023). Silver nanoparticles: bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms. 11:369. doi: 10.3390/microorganisms11020369
Muddassir, M., Raza, A., Munir, S., Basirat, A., Ahmed, M., Butt, M. S., et al. (2022). Antibacterial efficacy of silver nanoparticles (AgNPs) against metallo-β-lactamase and extended spectrum β-lactamase producing clinically procured isolates of Pseudomonas aeruginosa. Sci. Rep. 12:20685. doi: 10.1038/s41598-022-24531-9
Mutha, R. E., Tatiya, A. U., and Surana, S. J. (2021). Flavonoids as natural phenolic compounds and their role in therapeutics: an overview. Futur. J. Pharmaceut. Sci. 7, 1–13. doi: 10.1186/s43094-020-00161-8
Nie, P., Zhao, Y., and Xu, H. (2023). Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: a review. Ecotoxicol. Environ. Saf. 253:114636. doi: 10.1016/j.ecoenv.2023.114636
Parham, S., Kharazi, A. Z., Bakhsheshi-Rad, H. R., Nur, H., Ismail, A. F., Sharif, S., et al. (2020). Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants. 9:1309. doi: 10.3390/antiox9121309
Rengarajan, S., Sivalingam, A. M., Pandian, A., and Chaurasia, P. K. (2024). Nanomaterial (AgNPs) Synthesis using calotropis gigantea extract, characterization and biological application in antioxidant and antibacterial activity. J. Inorg. Organomet. Polym. Mater. 34, 4005–4021. doi: 10.1007/s10904-024-03058-8
Sassykova, L. R., Aubakirov, Y. A., Sendilvelan, S., Tashmukhambetova, Z., Zhakirova, N. K., Faizullaeva, M. F., et al. (2019). Studying the mechanisms of nitro compounds reduction (A-Review). Orient. J. Chem. 35, 22–38. doi: 10.13005/ojc/350103
Singh, J., Kaur, N., and Rawat, M. (2018). Eco-friendly approach for synthesis of AgNPs and their catalytic application toward 4-nitrophenol to 4-aminophenol reduction. Micro. Nano. Lett. 13, 1600–1603. doi: 10.1049/mnl.2018.5139
Singh, N. B., Kumar, B., Usman, U. L., and Susan, M. A. B. H. (2024). Nano revolution: exploring the frontiers of nanomaterials in science, technology, and society. Nano-Struct. Nano-Objects. 39:101299. doi: 10.1016/j.nanoso.2024.101299
Singh, R., Shedbalkar, U. U., Wadhwani, S. A., and Chopade, B. A. (2015). Bacteriagenic silver nanoparticles: synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 99, 4579–93. doi: 10.1007/s00253-015-6622-1
Sreelekha, E., George, B., Shyam, A., Sajina, N., and Mathew, B. A. (2021). Comparative study on the synthesis, characterization, and antioxidant activity of green and chemically synthesized silver nanoparticles. Bionanoscience. 11, 489–496. doi: 10.1007/s12668-021-00824-7
Vladár, A. E., and Hodoroaba, V. D. (2020). Characterization of nanoparticles by scanning electron microscopy. Characteriz. Nanopart. Measure. Process. Nanopart. 2020, 7–27. doi: 10.1016/B978-0-12-814182-3.00002-X
Ying, S., Guan, Z., Ofoegbu, P. C., Clubb, P., Rico, C., He, F., et al. (2022). Green synthesis of nanoparticles: current developments and limitations. Environ. Technol. Innov. 26:102336. doi: 10.1016/j.eti.2022.102336
Keywords: Artemisia scoparia, Silver nanoparticles (AgNPs), phytochemical screening, nanoparticle characterization, antibacterial activity, catalytic reduction
Citation: Bhati P, Tiwari R, Kothari SL, Ray K, Lamba NP, Kumar Y, Binsuwaidan R, Saeed M, Obaidur S, Srivastava SC, Sambhav K and Chauhan MS (2025) Green synthesis of silver NPs using aqueous extract of Artemisia scoparia for hydrogenation of aromatic nitro compounds and their biological activity. Front. Microbiol. 16:1584066. doi: 10.3389/fmicb.2025.1584066
Received: 26 February 2025; Accepted: 24 March 2025;
Published: 25 April 2025.
Edited by:
Abhijeet Mishra, University of Delhi, IndiaReviewed by:
Bhasha Sharma, Netaji Subhas University of Technology, IndiaAnil Singh, Mahatma Gandhi Central University, India
Copyright © 2025 Bhati, Tiwari, Kothari, Ray, Lamba, Kumar, Binsuwaidan, Saeed, Obaidur, Srivastava, Sambhav and Chauhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manmohan Singh Chauhan, bXM4cmVzZWFyY2hAZ21haWwuY29t; Kumar Sambhav, a3VtYXJzYW1iaGF2MjlAZ21haWwuY29t
 Prashant Bhati1
Prashant Bhati1 Manmohan Singh Chauhan
Manmohan Singh Chauhan