- 1College of Life Sciences, Fujian Normal University, Fuzhou, Fujian, China
- 2National Joint Engineering Research Center of Industrial Microbiology and Fermentation Technology, College of Life Sciences, Fujian Normal University, Fuzhou, Fujian, China
Introduction: This study investigated the effects of Bacillus licheniformis on the water quality, growth performance and bacterial community in Penaeus vannamei aquaculture system. The objective was to elucidate the impact of B. licheniformis on P. vannamei aquaculture from a microbial ecological perspective. This research provided valuable theoretical support for the practical application of B. licheniformis in improving aquaculture practices for P. vannamei.
Methods: The design of the aquaculture experiment comprised two groups: a control group (CK) fed a basal diet and a treatment group (PB) was fed the same diet with addition of B. licheniformis (5 × 104 CFU/mL) into the water every 5 days. These groups were systematically evaluated through comprehensive water quality analyses, including pH, ammonia nitrogen, nitrite nitrogen, and Vibrio counts, as well as growth performance assessments such as length, weight, survival rate, yield, and feed conversion ratio (FCR). Additionally, high-throughput sequencing technology was employed to analyze changes in bacterial community structures in both the aquaculture water and the shrimp intestines.
Results: The results demonstrated that B. licheniformis significantly improved water quality, promoted shrimp growth, and altered the bacterial community structure: (1) B. licheniformis significantly reduced the concentrations of ammonia nitrogen, nitrite nitrogen, and pathogenic Vibrio counts in the later stages of cultivation (P < 0.05), while significantly promoting shrimp growth; (2) The addition of B. licheniformis increased the diversity and richness of bacteria both in the water and shrimp intestinal tracts, leading to significant changes in bacterial community structure. It also enhanced beneficial bacterial genera such as Gemmobacter, Paracoccus, and Bacillus in the water, while concurrently reducing the potential pathogenic Flavobacterium in the shrimp intestinal tract; (3) The dominant bacterial populations were significantly affected in both water and shrimp intestinal samples. In water, Aurantimicrobium was the predominant genus in both groups, with the PB group showing a notably lower relative abundance. In the shrimp intestines, the CK group was dominated by Gemmobacter and Fluviicola, while Aurantimicrobium prevailed in the PB group. In conclusion, the study revealed the potential of B. licheniformis in shrimp aquaculture by improving water quality, promoting shrimp growth, and modulating bacterial community structure.
Discussion: This study demonstrated that B. licheniformis significantly improved water quality and shrimp growth performance in P. vannamei aquaculture. It effectively reduced pH, ammonia and nitrite levels, while also decreasing Vibrio counts, which are critical for disease control. B. licheniformis enhanced microbial diversity in both aquaculture water and shrimp intestines, promoting the abundance of beneficial genera, while reducing potential pathogens. The alteration in bacterial community structure suggests that B. licheniformis plays a pivotal role in maintaining a healthy microbial ecosystem, enhancing shrimp health and growth, and providing an environmentally sustainable alternative to antibiotics in aquaculture practices. These findings underscore the potential of B. licheniformis for improving P. vannamei aquaculture systems.
1 Introduction
Penaeus vannamei, native to the Pacific coast of South America (Liao and Chien, 2011), is one of the most economically significant cultured shrimp species globally (FAO, 2024). Recent advancements in living standards and increasing demands for diverse nutritional sources have driven rapid development in shrimp aquaculture (Cabello et al., 2016). However, the high-density intensive shrimp farming model, while economically beneficial, has precipitated several challenges, including the deterioration of water quality (Duan et al., 2018) and the frequent outbreak of diseases (Le et al., 2024). In recent years, bacterial diseases such as acute hepatopancreatic necrosis disease (AHPND), caused by Vibrio parahaemolyticus, have severely disrupted the shrimp industry. In regions affected by AHPND, particularly in Asia and South America, shrimp production has declined by ~ 60%. This disease has resulted in an annual global loss of $1 billion to the shrimp farming industry (Kumar et al., 2018). Consequently, enhancing water quality and bolstering shrimp resistance to pathogenic bacteria are critical for sustaining healthy shrimp aquaculture.
In the aquaculture industry, chemicals such as antibiotics and disinfectants were once widely used in intensive shrimp aquaculture as the preferred means to control the prevalence of diseases and maintain high production (Li et al., 2021). However, the increased use of antibiotics has revealed significant drawbacks: on the one hand, it can lead to the development of antibiotic resistance in pathogenic microorganisms (Cabello, 2006), causing drug accumulation that paralyzes the entire food chain, damages the aquatic environment, and leads to unsustainable development. On the other hand, it can disrupt the balance of the intestinal microbiota in shrimp, negatively affecting the host's immune response and growth performance (Chen et al., 2023). Therefore, there is an urgent need for modern intensive aquaculture to explore better disease prevention and control methods, and to seek safer and more environmentally friendly alternatives to antibiotics, to enhance aquaculture efficiency and safeguard human health.
The use of probiotics has become an alternative approach to antibiotics in aquaculture. Probiotics are bacterial-based products that are eco-friendly, non-polluting, and do not induce antimicrobial resistance. These characteristics make them widely used in shrimp farming as a new tool for promoting health and preventing diseases (Kewcharoen and Srisapoome, 2019; Ringø, 2020). Additionally, the application of non-pathogenic bacteria can enhance dietary safety and environmental performance by modulating host-associated microbiota, which helps control allergic reactions and strengthens immune system function (Van Doan et al., 2020; Llewellyn and Foey, 2017).
Among the various probiotics utilized in aquaculture, Bacillus licheniformis is widely acknowledged for its safety and efficacy in animal feed. This bacterium, a prominent member of the Bacillus genus, is distinguished by its robust spore-forming capability, which ensures its survival in both harsh environmental conditions and the gastrointestinal tract of aquatic organisms (Fan et al., 2021). B. licheniformis has been extensively investigated for its significant role in ecological regulation and its application in aquaculture (Abarike et al., 2018; Chen et al., 2020; Ma et al., 2022; Monier et al., 2023; Vega-Carranza et al., 2024). Currently, related studies primarily focus on the effects of improving aquaculture water quality and increasing production yields. However, in-depth studies investigating the mechanisms of probiotics within P. vannamei aquaculture systems from a microbial ecology perspective remain relatively limited.
In this study, B. licheniformis strain FS051 was added to the aquaculture water of P. vannamei. The effects of B. licheniformis on water quality and shrimp growth performance were comprehensively evaluated. High-throughput sequencing technology was employed to analyze changes in bacterial community structures in both the aquaculture water and the shrimp intestines. The aim was to elucidate the impact of B. licheniformis on P. vannamei aquaculture from a microbial ecological standpoint. This research provided theoretical support for the application of B. licheniformis in P. vannamei aquaculture.
2 Materials and methods
2.1 Experimental materials
2.1.1 Bacterial strain
Bacillus licheniformis strain FS051, was previously isolated from the intestines of healthy Penaeus vannamei in Pingtan, Fujian Province, China, by the laboratory (College of Life Sciences, Fujian Normal University). This strain exhibited both nitrite-degrading ability and antibacterial activity against Micrococcus luteus. The purity of the strain was confirmed through the observation of its morphological and biochemical characteristics, as well as molecular identification of the species.
2.1.2 Shrimp species
The juvenile Penaeus vannamei with an initial weight of 0.80 to 0.90 g and a body length of 3.0 to 4.0 cm, were obtained from an aquaculture farm in Pingtan, Fujian Province, China.
2.2 Experimental set-up and rearing condition
The juvenile P. vannamei were randomly distributed into two groups (in triplicate) in culture tanks (56 cm × 41 cm × 32.5 cm, water depth: 30 cm). Each tank was equipped with an oxygenation device and fitted with a nylon cloth filter of 500 mesh size. The stocking density was set at 30 shrimp per tank. The animals were fed 3–5% of their live body weight for three times (morning, noon, and evening) a day with commercialized feed, primarily composed of fish meal, yeast, peanuts, soybean meal, and others. The commercial feed containing crude protein 43%, crude fat 6%, ash 16%, and water 12%. The experiment lasted for 30 days.
The aquaculture water was prepared by diluting seawater with tap water at a ratio of 11:1.4, maintaining a salinity of ~ 13‰. No water exchange occurred throughout the cultivation process.
Each treatment group were as follows: the CK group did not add any microbial preparations and was managed routinely, the PB group indicated that B. licheniformis was added to the water every 5 days during the aquaculture process at a dosage of 5 × 104 CFU/mL.
2.3 Water quality measurement methods
(1) Determination of water pH : the water pH was directly determined by a pH meter (PHS-3C, Leici, Shanghai, China).
(2) Determination of ammonia nitrogen in water: samples were taken from each tank at a depth of 10 cm, and after centrifugation at 8,000 r/min for 10 min, the concentration of ammonia in the water samples was determined using the Nessler's reagent colorimetric method (Wu and Cao, 2013), with an ultraviolet spectrophotometer (UV-1800, Shimadzu, Suzhou, China).
(3) Determination of nitrite nitrogen in water: the concentration of nitrite nitrogen was determined by the diazotization-azo coupling colorimetric method.
(4) Determination of Vibrio counts in water: after diluting the culture water to 10−1 and 10−2 gradient, 0.1 mL of the original solution and the 10−1 and 10−2 gradient solutions were plated onto TCBS solid culture medium. Colony counts between 30 and 300 were selected and expressed using the CFU counting method.
2.4 Measurement of shrimp-related indicators
(1) Measurement of shrimp length and weight: shrimp length was measured with a straightedge with an accuracy of 0.01 cm, and weight was measured with an electronic balance with an accuracy of 0.01 g.
(2) Measurement of shrimp yield, feed conversion ratio (FCR) and survival rate: shrimp yield was expressed as the total fresh weight of all shrimp at the end of the experiment. The shrimp survival rate was determined as the ratio of the final number of shrimp to the initial number of shrimp. The FCR was calculated using the formula:
2.5 Samples collection
At the end of the trial, the shrimp and the environment were sampled. Six shrimp were randomly taken from each tank. The shrimp were placed in a −20°C refrigerator to freeze for 20 min to death and then transferred to a 4°C refrigerator for cold storage. Then they were soaked in 75% alcohol for 30 s and rinsed with sterile water. In a clean bench, the shrimp intestinal tracts were extracted using sterile tweezers and packed into sterile centrifuge tubes, sealed, and stored at −80°C.
A sterile sampler was used to take a sample of 500 mL at a water depth of 10 cm in each culture tank, which was first filtered with a 500-mesh nylon cloth for primary filtration, and after the primary filtration, it was filtered with a 0.22 μm filter membrane for secondary filtration, and after filtration, the membrane was put into a 50 mL sterile centrifuge tube and stored at −80 °C.
Subsequently, the shrimp intestinal tract samples and the water samples were subjected to high-throughput sequencing.
2.6 DNA extraction and PCR amplification
After extracting the DNA of the samples by CTAB, the quality and purity of DNA were assessed through 1% agarose gel electrophoresis. The concentration of DNA was diluted to 1 ng·μL−1 to serve as a template for PCR amplification. The PCR reaction was performed using Phusion® High-Fidelity PCR Master Mix with GC Buffer, along with high-performance, high-fidelity enzymes, and the prokaryotic universal primers 341F and 806R. The primers and sequences utilized for PCR amplification were as described below:
341F (5'-CCTAYGGGRBGCASCAG-3')
806R (5'-GGACTACNNGGGTATCTAAT-3')
The amplified products were analyzed using 1% agarose gel electrophoresis to determine the sizes of the amplified bands. Subsequently, the PCR products were purified using the Agencourt AMPure XP nucleic acid purification kit.
2.7 High-throughput sequencing and sequence data processing
The library was constructed using the Ion Plus Fragment Library Kit 48rxns from Thermo Fisher. After library construction, quantification was performed using Qubit, and libraries that passed quality control were sequenced on the Thermo Fisher Life Ion S5™ system.
The original sequences were analyzed using the QIIME software, with end-splicing accomplished by FLASH. Preliminary quality control was conducted through UPARSE, removing low-quality sequences, followed by clustering of all effective tags into OTUs and species annotation of representative OTU sequences. For the top 20 genera in abundance, heatmaps were generated using R language. The QIIME package was used to calculate alpha diversity for each sample, and principal component analysis (PCA) and other graphical representations were generated using the VennDiagram package.
2.8 Statistical analysis
The experimental data were presented as the mean ± standard error. The independent-samples t-test and one-way analysis of variance (ANOVA) were used to identify significant differences using SPSS software. A level of P < 0.05 was accepted as statistically significant. The data processing was conducted using Excel 2016. Graphs were generated using Origin 2018 software.
3 Results and analysis
3.1 Effects of B. licheniformis on the water quality
3.1.1 Changes in pH in the culture water
P. vannamei exhibits strong adaptability to its environment, with its optimal growth pH range is between 7.5 and 8.5 (Chen and Chen, 2003). During the culture process, the pH was measured every 5 days. With the extension of the culture time, the CK group and the PB group showed a decreasing trend in pH, and both of them had basically the same trend of change, with the pH maintained between 7.3 and 8.1 (Figure 1A).
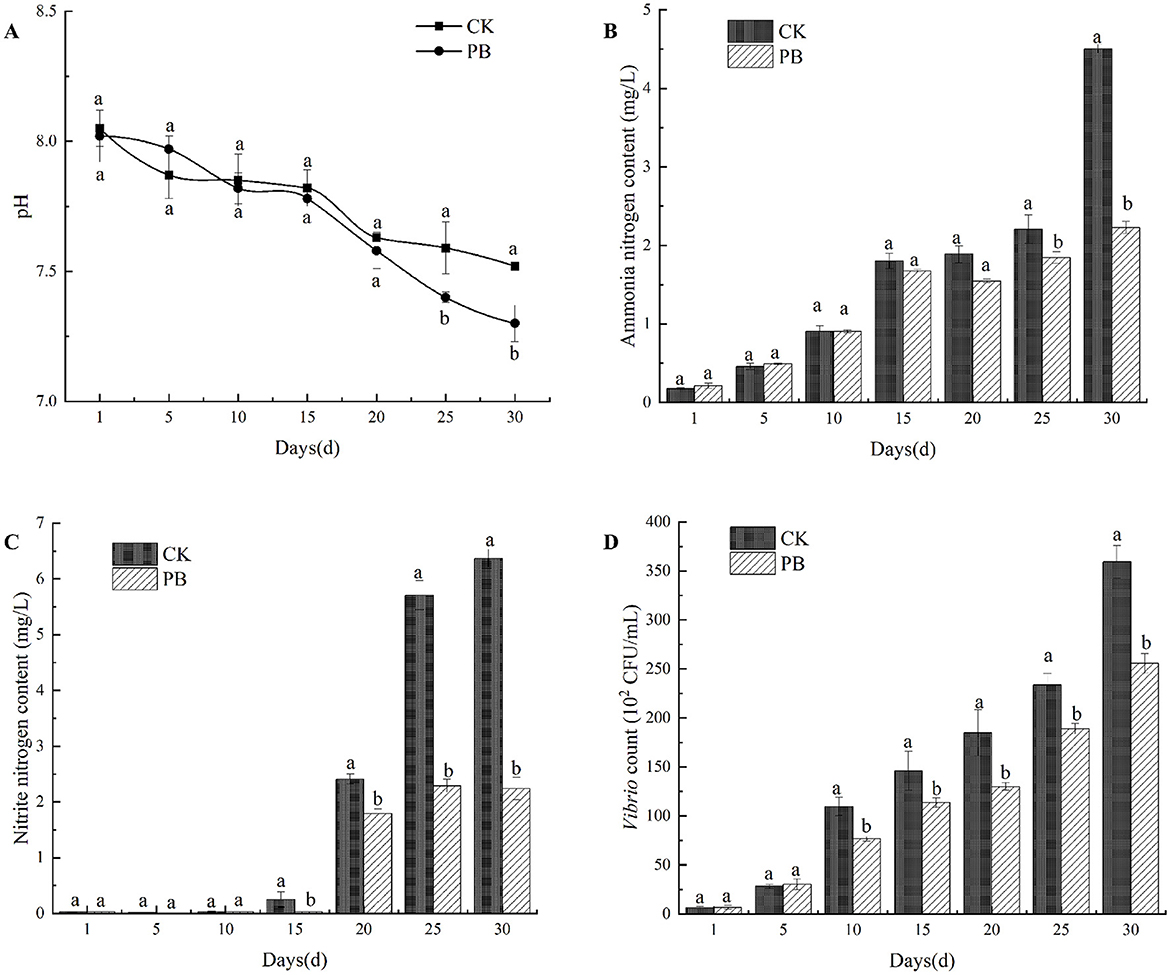
Figure 1. Effects of Bacillus licheniformis on the water quality. (A) pH variations in the culture water of CK and PB groups. (B) Changes of ammonia nitrogen content in different treatment groups. (C) Changes of nitrite nitrogen content in different treatment groups. (D) Changes of Vibrio count in different treatment groups. CK: no probiotic added; PB: B. licheniformis added to the water. Different letters within the same time point indicate statistically significant differences (P <0.05). Error bars represent standard deviations.
The experimental results indicated that compared to the CK group, B. licheniformis lowered water pH for P. vannamei, but it remained within the optimal range for shrimp growth. There was no significant difference between the two groups in the early stages, while in the later stages, the pH in the PB group was significantly lower than that in the CK group (P < 0.05).
3.1.2 Changes in ammonia and nitrite levels in the culture water
During the culture process, the concentrations of ammonia and nitrite in the water of each treatment group were measured every 5 days. In the first 10 days of aquaculture, the ammonia concentration maintained at low levels (Figure 1B), consistently below 1 mg/L, with no significant difference between the CK group and the PB group. However, from day 10 to day 20, the ammonia levels began to rise sharply, peaking at 4.50 mg/L on day 30. Notably, on the 25th day and the 30th day, the ammonia levels in the PB group were significantly lower than those in the CK group (P < 0.05), indicating that B. licheniformis significantly reduced the ammonia concentration in water in the later stages of culture.
The nitrite concentration maintained at low levels during the first 5 days of aquaculture (Figure 1C), not exceeding 0.5 mg/L. After the 15th day, the nitrite levels in both the CK group and the PB group began to increase, reaching their highest levels on day 30. The nitrite concentration in the PB group was significantly lower than that in the CK group after the 15th day (P < 0.05), indicating that B. licheniformis significantly reduced the nitrite concentration in the water during the later stages of aquaculture.
3.1.3 Variations of Vibrio counts in the culture water
Vibrio is a bacterial pathogen in P. vannamei aquaculture that can cause red leg disease, with diseased shrimp displaying symptoms such as reddened appendages, slow movement, and anorexia (Sudheesh and Xu, 2001). Vibrio counts in the water were measured every 5 days (Figure 1D). The counts were determined by plating diluted water samples onto TCBS solid culture medium and counting the CFU. The data showed a gradual increase in Vibrio counts for both the CK and PB groups throughout the culture process. In the first 10 days, Vibrio counts remained below 100 × 102 CFU/mL, with no significant difference between the CK group and PB group. After day 10, Vibrio counts increased, peaking on day 30 at 3.60 × 104 CFU/mL for the CK group and 2.56 × 104 CFU/mL for the PB group. From day 10 to 30, Vibrio counts in the PB group were significantly lower than that in the CK group (P < 0.05), demonstrating that B. licheniformis significantly reduced the number of Vibrio in the aquaculture water during the later stages of P. vannamei aquaculture.
3.2 Impacts of B. licheniformis on the growth of P. vannamei
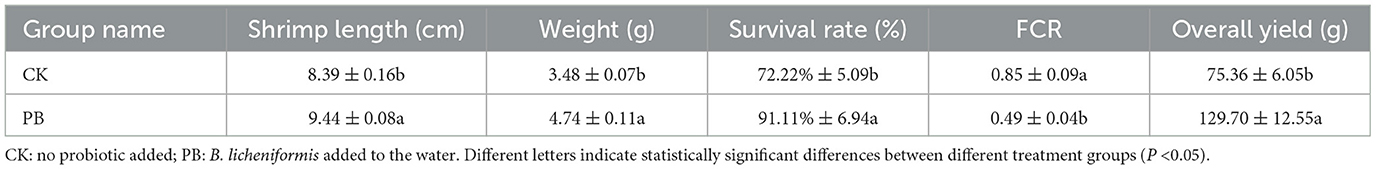
The growth parameters of shrimp were measured (Table 1). The data clearly indicated that B. licheniformis significantly enhanced growth indicators (P < 0.05), including shrimp length, weight, survival rate, and overall yield compared to the CK group. FCR represents the feed required to cultivate 1 kg of shrimp, and serves as a crucial efficiency indicator. A lower FCR indicates better feed conversion. As shown in the table, the PB group's FCR was significantly lower than the CK group's. In summary, B. licheniformis promoted shrimp growth and improved feed conversion.
3.3 Influences of B. licheniformis on bacterial diversity in culture water and shrimp intestinal tract
3.3.1 Bacterial diversity indices of the culture water and the shrimp intestinal tracts in different treatment groups
Alpha diversity analysis was conducted on bacterial communities in the culture water and the shrimp intestinal tracts using high-throughput sequencing technology. By comparing the diversity indices of different treatment groups (Table 2), the numbers of OTUs were 195, 230, 194, and 231, respectively. The library coverage was 100%, indicating a high detection rate and reliable reflection of actual bacterial communities in the aquaculture water and shrimp intestines. In the water samples, the W_PB group had higher Chao1, Shannon, and Simpson indices than the W_CK group. Similarly, the S_PB group had higher indices than the S_CK group in shrimp intestinal tract samples. Therefore, B. licheniformis enhanced bacterial community richness in both the water and shrimp intestinal tract samples.
3.3.2 The OTU distribution among different treatment groups
Using Venn diagrams to compare the shared and unique OTUs among different treatment groups (Figure 2), it was evident that in the water samples, the W_CK group and the W_PB group possessed 53 and 88 unique OTUs, respectively, with 85 OTUs shared between them. In the shrimp intestinal tract samples, both the S_CK and S_PB groups had 73 unique OTUs each, with 139 OTUs shared between them. These results indicated that B. licheniformis altered the microbial composition in the aquaculture water and the shrimp intestines.
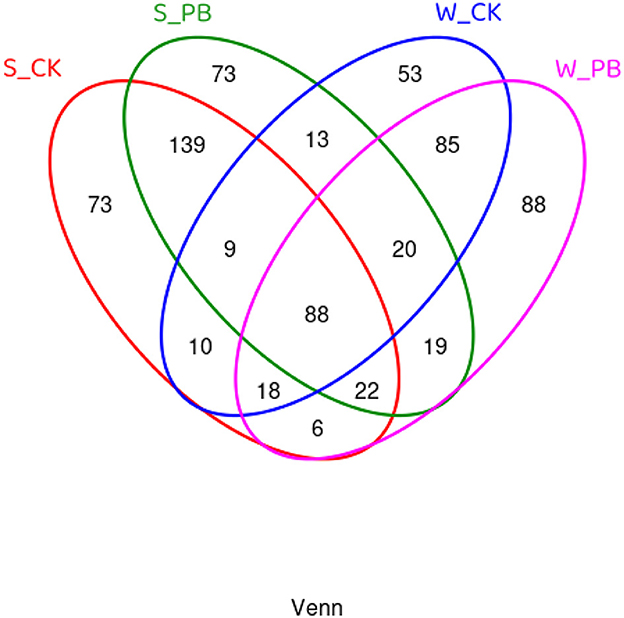
Figure 2. Venn diagram of OTU distribution, showing shared and unique OTU among different treatment groups. W, water; S, shrimp. Where W_CK; S_CK represents samples obtained from water and shrimp intestine samples obtained from untreated group and W_PB; S_PB probiotic treated group.
3.4 Influences of B. licheniformis on the composition of bacterial community in the culture water and shrimp intestine
3.4.1 Bacterial community composition at the phylum level in the aquaculture water
Analyzing the bacterial community composition at the phylum level in the water samples (Figure 3A). The top 10 phyla accounted for 99.98% to 99.99% of the total relative abundance. These phyla included Proteobacteria, Bacteroidota, Actinobacteriota, Bdellovibrionota, Desulfobacterota, Firmicutes, Verrucomicrobiota, Patescibacteria, Cyanobacteria, and Planctomycetota. Of which Proteobacteria, Bacteroidota, Actinobacteriota, and Bdellovibrionota were the dominant phyla, accounting for 98.68% to 99.42% of the total. Compared to the W_CK group, the W_PB group exhibited a decrease in the relative abundance of Proteobacteria, Bacteroidota, and Bdellovibrionota, while Actinobacteriota increased. This indicated that the B. licheniformis altered the bacterial community structure in the aquaculture water.
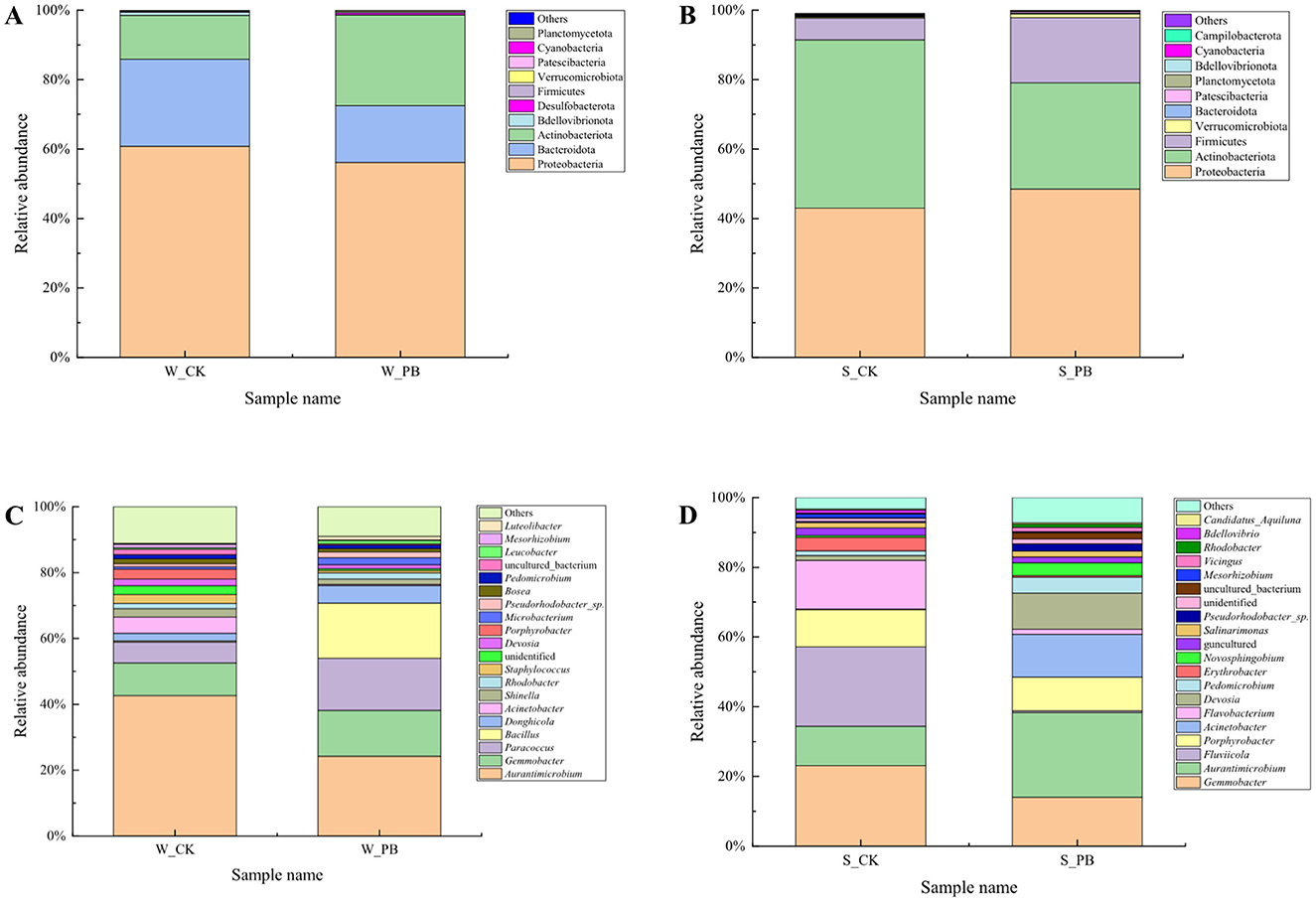
Figure 3. Bacterial community composition in different treatment groups. (A) Phylum-level composition in culture water, W_CK: the untreated group of water samples; W_PB: the probiotic treated group of water samples. (B) Phylum-level composition in shrimp intestinal tract, S_CK: the untreated group of shrimp intestinal tract samples; S_PB: the probiotic treated group of shrimp intestinal tract samples. (C) Genus-level composition in culture water, W_CK: the untreated group of water samples; W_PB: the probiotic treated group of water samples. (D) Genus-level composition in shrimp intestinal tract, S_CK: the untreated group of shrimp intestinal tract samples; S_PB: the probiotic treated group of shrimp intestinal tract samples.
3.4.2 Bacterial community composition at the phylum level in shrimp intestinal tracts
Analyzing the bacterial community composition at the phylum level in shrimp intestinal tract samples (Figure 3B). The top 10 phyla in terms of relative abundance accounted for 99.77% to 99.90% of the total. These phyla included Proteobacteria, Actinobacteriota, Firmicutes, Verrucomicrobiota, Bacteroidota, Patescibacteria, Planctomycetota, Bdellovibrionota, Cyanobacteria, and Campilobacterota. Among these, Proteobacteria, Actinobacteriota, Firmicutes, and Verrucomicrobiota were the dominant phyla, comprising more than 98% of the community. Compared to the S_CK group, the S_PB group exhibited an increase in the relative abundance of Proteobacteria, Firmicutes, and Verrucomicrobiota, while Actinobacteriota decreased. This indicated that B. licheniformis altered the composition of the bacterial community in the shrimp intestinal tracts.
3.4.3 Bacterial community composition at the genus level in the aquaculture water
Analyzing the bacterial community composition at the genus level in the water samples (Figure 3C). The dominant genera, Aurantimicrobium, Gemmobacter, Paracoccus, Bacillus, Donghicola, and Acinetobacter, had relative abundances ranging from 66.49% to 76.41%. Compared to the W_CK group, the W_PB group showed increased relative abundances of Gemmobacter, Paracoccus, Bacillus, and Donghicola, while Aurantimicrobium and Acinetobacter decreased. This indicated that B. licheniformis altered the relative abundances of the dominant bacterial genera in the aquaculture water, changing the structure of the bacterial community within the aquaculture environment.
3.4.4 Bacterial community composition at the genus level in shrimp intestinal tracts
Analyzing the bacterial community composition at the genus level in shrimp intestinal tract samples (Figure 3D). The dominant genera, Gemmobacter, Aurantimicrobium, Fluviicola, Porphyrobacter, Acinetobacter, Flavobacterium, Devosia, and Pedomicrobium, accounted for 77.13% to 84.72% of the total, indicating their predominance in the shrimp intestinal tracts. Compared to the S_CK group, the S_PB group exhibited an increase in the relative abundance of Aurantimicrobium, Acinetobacter, Devosia, and Pedomicrobium, while Gemmobacter, Fluviicola, Porphyrobacter, and Flavobacterium decreased. This indicated that B. licheniformis also altered the relative abundances of the dominant bacterial genera in the shrimp intestinal tracts, changing the bacterial community composition at the genus level.
3.4.5 Effects of B. licheniformis on the distribution of bacterial genera in the aquaculture water and the shrimp intestinal tracts
The top 20 genera were plotted as heatmaps using the R language in the culture water and shrimp intestinal tract samples (Figure 4). Heatmap analysis can further explore the variability of the dominant genera under different treatment conditions in the aquaculture water and shrimp intestinal tracts. Darker colors indicated greater relative abundance of the genus. Aurantimicrobium was the absolute dominant genus in both W_CK and W_PB groups. Gemmobacter and Fluviicola were dominant in the S_CK group, while Aurantimicrobium was dominant in the S_PB group. It was obvious that in the aquaculture water, both the W_CK and W_PB groups shared the same dominant bacterial genera, though their relative abundances differed, with the W_CK group exhibiting higher relative abundance. In the shrimp intestinal tracts, the dominant genera differed between the S_CK and S_PB groups, with variations in relative abundance. This indicated that B. licheniformis altered the microbial living environment in both the aquaculture water and the shrimp intestinal tracts, subsequently changing their bacterial composition.
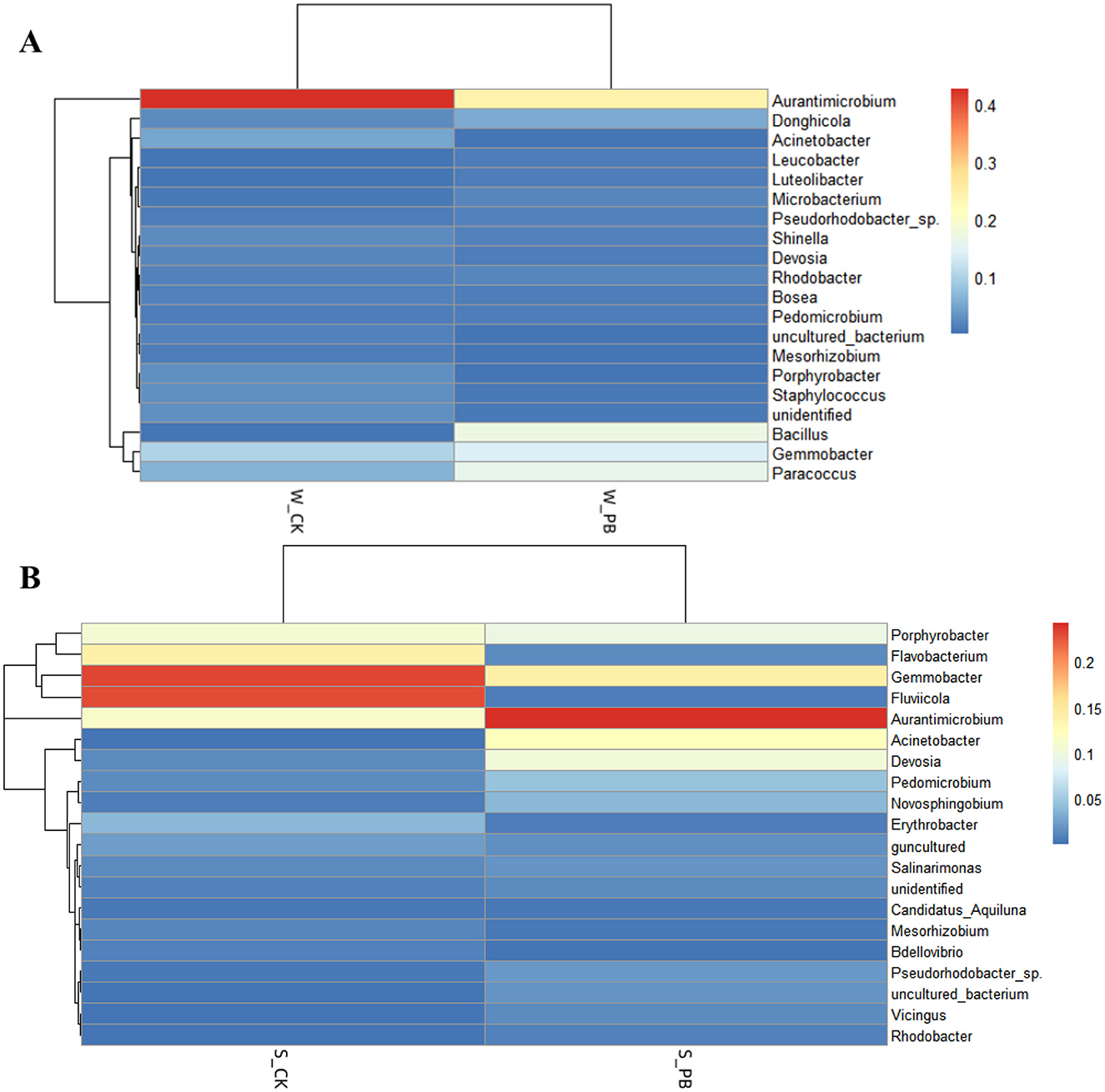
Figure 4. (A) Heatmap of different treatment groups in aquaculture water. W_CK: the untreated group of water samples; W_PB: the probiotic treated group of water samples. (B) Heatmap of different treatment groups in shrimp intestinal tract. S_CK: the untreated group of shrimp intestinal tract samples; S_PB: the probiotic treated group of shrimp intestinal tract samples.
3.4.6 Impacts of B. licheniformis on the principal components of the bacterial communities in the aquaculture water and the shrimp intestinal tracts
PCA was conducted on the aquaculture water and the shrimp intestinal tract samples based on OTUs (Figure 5), effectively illustrating differences in bacterial communities following the application of B. licheniformis. In the aquaculture water samples, the inter-parallel distances between the W_CK1, W_CK2, W_CK3 groups, as well as between the W_PB1, W_PB2, W_PB3 groups are close, indicating similarity in bacterial communities among each parallel groups. However, the distance between the W_CK and W_PB groups is considerable, suggesting significant differences between their bacterial communities. Similarly, in the shrimp intestinal tract samples, the inter-parallel distances between the S_CK1, S_CK2, S_CK3 groups, as well as between the S_PB1, S_PB2, S_PB3 groups are close, indicating similarity in bacterial communities among each parallel groups. However, the distance between the S_CK and S_PB groups is considerable, suggesting significant differences between their bacterial communities. In summary, B. licheniformis altered the bacterial community structure in both the aquaculture water and the shrimp intestinal tracts.
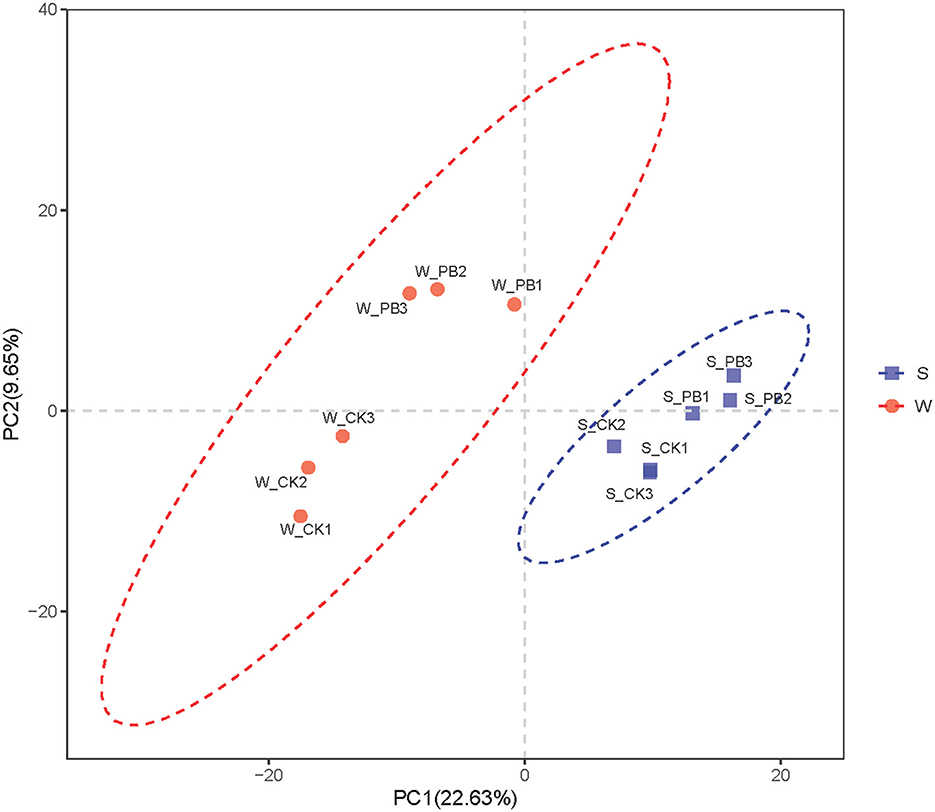
Figure 5. PCA of different treatment groups. The red section represents the water samples, while the blue section represents the shrimp intestinal tract samples. Each point in the graph corresponds to replicates of different treatment groups, and the greater the distance between points, the more significant the difference in bacterial communities. The numbers 1, 2, and 3 represent the three replicate samples. W, water; S, shrimp. Where W_CK; S_CK represents samples obtained from water and shrimp intestine samples obtained from untreated group and W_PB; S_PB probiotic treated group.
4 Discussion
4.1 B. licheniformis improved the water quality and promoted the shrimp growth
In the present study, water pH gradually decreased in both the CK and PB groups. Initially, there was no significant difference between the two groups. However, in the later stages, the pH in the PB group was significantly lower than that in the CK group (P < 0.05). A reduction in pH over time can be attributed to microbial processes such as organic matter degradation, nitrification, and the resultant increase in carbonic acid (Ebeling et al., 2006). Nimrat et al. (2012) found that the addition of Bacillus probiotics to Penaeus vannamei resulted in a significant reduction in pH. Hassan et al. (2022) observed a significant decrease in the pH of the ponds after probiotics treatment (P < 0.05) compared to pre-treatment levels. These reports were consistent with the results of this study.
In the later stages of aquaculture, the concentrations of ammonia and nitrite in the water of both the CK and PB groups increased considerably. This was attributed to the accumulation of shrimp excreta and uneaten feed in the culture tanks. The proteins and amino acids in these substances contributed to the elevated levels of organic nitrogen in the water, thereby stimulating the production of microbial metabolic byproducts, including -N, -N, and -N (Jackson et al., 2003; Zhou and Boyd, 2015). However, the concentrations of ammonia and nitrite in the PB group were significantly lower than those in the CK group (P < 0.05). Microorganisms play diverse ecological roles as producers, consumers, and decomposers, and participate in various nitrogen cycling processes, such as nitrogen fixation (Yousuf et al., 2017), nitrification (Rout et al., 2017), ammonification (Hui et al., 2019), and denitrification (Verbaendert et al., 2011), etc. The microbial preparations used in this experiment were Bacillus strains. Monier et al. (2023) found that the application of Bacillus species probiotics (B. subtilis and B. licheniformis) to shrimp aquaculture lowered total ammonia nitrogen (TAN) and NH3 in the pond water; Nimrat et al. (2020) found that the addition of Bacillus probiotics in the treatment group was associated with remarkable reductions in ammonia-nitrogen and nitrite-nitrogen concentrations in the water than the control group; Wang et al. (2022) similarly found that adding Bacillus to the P. vannamei culture process significantly reduced ammonia and nitrite contents in the water; These findings illustrated that microecological preparations dominated by Bacillus bacteria played a significant role in reducing ammonia and nitrite in water, consistent with this study's results.
This study indicated that compared to the CK group, B. licheniformis significantly improved shrimp growth indicators, including length, weight, survival rate, and yield (P < 0.05). Furthermore, the feed conversion ratio was significantly reduced (P < 0.05). B. licheniformis can promote intestinal health in shrimp, enhance the immune system, directly secrete digestive enzymes, and increase the activity of shrimp digestive enzymes, thereby improving feed utilization and promoting shrimp growth and survival. This has been confirmed in many studies and practices. For instance, Mirbakhsh et al. (2021) found that Bacillus subtilis administration improved growth and enhanced the immune response of Penaeus vannamei during the early hatchery period, thus increasing resistance to bacterial pathogens. Ranjit Kumar et al. (2013) found that dietary supplementation with Bacillus licheniformis inhibited pathogenic bacteria in the gut of Macrobrachium rosenbergii, enhanced growth and feed efficiency, and improved immune response, thereby increasing protection against bacterial infections. Ziaei-Nejad et al. (2006) also concluded that the use of Bacillus at different growth stages of shrimp promoted digestive enzyme activity, and enhanced survival rates and yield. These findings support the idea that B. licheniformis promotes shrimp growth and improves survival rates.
4.2 B. licheniformis increased the diversity of the microbial community in the shrimp aquaculture system, and promoted shrimp growth
The results indicated that B. licheniformis increased the number of OTUs, and the Chao1, Shannon, and Simpson indices of the microbial communities in both the aquaculture water and shrimp intestinal tracts, compared to the CK group. This suggested that B. licheniformis enhanced bacterial diversity within shrimp farming systems. As a probiotic, B. licheniformis, has the ability to improve the aquaculture water quality, promote nutrient cycling and other roles, thus increasing bacterial diversity. The diversity of microbial communities plays a crucial role in maintaining ecological functions, low diversity may indicate poor functional stability of microbial communities, increasing the risk of disease in organisms. The diversity indices of aquaculture water are positively correlated with shrimp health, and variations in intestinal bacterial structure and diversity distinguish between healthy and diseased shrimp. Numerous studies confirmed this: Wu et al. (2016) analyzed the intestinal bacterial community of healthy and diseased shrimp by high-throughput sequencing, the results showed that the bacterial diversity was significantly lower in diseased shrimps than in healthy ones. Yao et al. (2018) and Dai et al. (2018) found that the bacterial Shannon index in the intestinal tract of diseased shrimp was significantly lower than that of healthy shrimp. DU Shi-cong et al. (2019) found that planktonic bacteria in Penaeus vannamei culture ponds before the disease emergence exhibited a significantly lower Shannon index than healthy ponds and suggested that a decrease in Shannon index could be a sign of divergence in shrimp health. Reyes et al. (2022) compared the microbiome of Penaeus vannamei with high and low survival in shrimp hatchery tanks, the Shannon diversity index was significantly lower at the low-survival tanks.
B. licheniformis altered the microbial diversity, bacterial abundance, and community structure in the aquaculture water and the shrimp intestinal tracts, however, the impacts differed between the two environments. In the present study, B. licheniformis resulted in changes in the OTUs of bacteria in the aquaculture water and the shrimp intestinal tracts. Notably, in the shrimp intestinal tract samples, the S_PB group shared more OTUs with the S_CK group, indicating a lesser impact of B. licheniformis on the shrimp intestinal tract. Compared to the aquaculture water, the microbial community in the shrimp intestines was more stable. Luo et al. (2006) demonstrated that external environmental factors could influence the microbial community structure in the shrimp intestinal tract. However, as shrimp cultivation progressed to a certain stage, the intestinal bacterial community became relatively stable and mature, becoming less susceptible to environmental influences. This finding aligned with the results of this study.
4.3 B. licheniformis altered bacterial community structure in the culture water and the shrimp intestinal tracts
B. licheniformis increased the relative abundance of beneficial bacterial genera. It can enhance synergistic interactions among microorganisms, such as stimulating the growth of other probiotics through the production of bioactive substances, or by establishing symbiotic relationships to participate together in certain biochemical processes. In the culture water samples, it was found that after the addition of B. licheniformis, the relative abundance of the genera Bacillus, Gemmobacter, and Paracoccus was higher than in the CK group. Bacillus can inhibit the growth of pathogenic bacteria, as a biological barrier to compete with pathogenic bacteria for nutrients (Tran et al., 2023); The genera Gemmobacter (Liu et al., 2021) and Paracoccus (Si et al., 2020) have strong denitrification capabilities and effectively decompose organic matter in water, belonging to the beneficial bacteria in shrimp aquaculture process.
B. licheniformis reduced the relative abundance of potential pathogens. As a probiotic, B. licheniformis competes with potential pathogens for nutrients and living space, and it can produce a broad spectrum of antimicrobial substances. The present study found that B. licheniformis, decreased the relative abundance of Flavobacterium, a potential pathogen, in shrimp intestinal tracts (Dai et al., 2018). It was observed that Vibrio counts in the culture water significantly decreased following the addition of B. licheniformis (P < 0.05), during the cultivation period between day 10 and day 30. Vibrio, a prevalent pathogen in aquatic animal farming environments, is a primary pathogen during the shrimp larval stage and can lead to shrimp mortality (Zhou et al., 2012). The increase of pathogenic bacteria can lead to the occurrence of disease, which has been confirmed in many studies: Rahardjo et al. (2023) investigated microbial composition in rearing media of Penaeus vannamei infected by the white feces disease (WFD) and healthy Penaeus vannamei. They found that Vibrio vulnificus dominated in WFD-infected shrimp pond water. Oxley et al. (2002), Abraham et al. (2004) and Phung et al. (2020) found introducing Bacillus into shrimp cultivation significantly reduced the number of Vibrio. These findings were consistent with the idea that B. licheniformis could increase the relative abundance of beneficial bacterial genera and decrease the relative abundance of potential pathogens.
Some studies have identified the genera such as Rhodobacter, Pseudomonas, and Paracoccus are the dominant bacterial groups in aquaculture water (Wu, 2016). In the present study, these genera were detectable in the aquaculture water, but at relatively low percentages. The genus Aurantimicrobium was found to be the most predominant in the aquaculture water, accounting for the largest proportion of the bacterial community. Numerous studies have found that Gammaproteobacteria is the absolutely dominant bacterial phylum in shrimp intestines, with Vibrio and Aeromonas being the absolutely dominant genera (Beardsley et al., 2011; Liu et al., 2010). However, in this study, the dominant bacterial genera in shrimp intestines were Gemmobacter, Fluviicola, and Aurantimicrobium. It is hypothesized that the aforementioned changes might be related to the fact that the present study was conducted in a small-scale farming setting. Microorganisms respond sensitively to environmental changes, and shifts in microbial genera are influenced by variations in the environment, with specific genera changing across different aquaculture environments (Quero et al., 2020). For example, Al-Masqari et al. (2022) cultured Penaeus vannamei under different temperature conditions and found that temperature changes significantly influenced the composition of bacterial communities. Furthermore, variations in probiotic species, strains, and application dosages can lead to differences in microbial community composition.
5 Conclusion
The results of this study demonstrated that B. licheniformis could improve the water quality in P. vannamei aquaculture, enhance shrimp growth performance and disease resistance, and regulate the microbial communities in both the cultivation water and the shrimp intestinal tract. Specifically, the abundance of potentially harmful bacteria was reduced, while the abundance of beneficial bacteria increased. These findings indicated that B. licheniformis has great potential for practical application in the large-scale cultivation of P. vannamei.
Data availability statement
The data presented in the study are deposited in the NCBI Short Read Archive (SRA), accession numbers SRX28901524-SRX28901535.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
YZ: Writing – original draft, Writing – review & editing. YW: Writing – original draft, Writing – review & editing. ST: Writing – review & editing. JL: Writing – review & editing. LF: Writing – review & editing. WH: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Fujian Province regional development project (Nos. 2017N3015) and Demonstration Project on Innovative Development of Marine Economy in the 13th Five-Year Plan of the State Oceanic Administration (Fuzhou, China; Nos. FZHJ03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abarike, E. D., Cai, J., Lu, Y., Yu, H., Chen, L., Jian, J., et al. (2018). Effects of a commercial probiotic BS containing Bacillus subtilis and Bacillus licheniformis on growth, immune response and disease resistance in nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 82, 229–238. doi: 10.1016/j.fsi.2018.08.037
Abraham, T. J., Ghosh, S., Nagesh, T., and Sasmal, D. (2004). Distribution of bacteria involved in nitrogen and sulphur cycles in shrimp culture systems of West Bengal, India. Aquaculture 239, 275–288. doi: 10.1016/j.aquaculture.2004.06.023
Al-Masqari, Z. A., Guo, H., Wang, R., Yan, H., Dong, P., Wang, G., et al. (2022). Effects of high temperature on water quality, growth performance, enzyme activity and the gut bacterial community of shrimp (Litopenaeus vannamei). Aquac. Res. 53, 3283–3296. doi: 10.1111/are.15836
Beardsley, C., Moss, S., Malfatti, F., and Azam, F. (2011). Quantitative role of shrimp fecal bacteria in organic matter fluxes in a recirculating shrimp aquaculture system. FEMS Microbiol. Ecol. 77, 134–145. doi: 10.1111/j.1574-6941.2011.01094.x
Cabello, F. C. (2006). Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ. Microbiol. 8, 1137–1144. doi: 10.1111/j.1462-2920.2006.01054.x
Cabello, F. C., Godfrey, H. P., Buschmann, A. H., and Dölz, H. J. (2016). Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect. Dis. 16, e127-e133. doi: 10.1016/S1473-3099(16)00100-6
Chen, M., Chen, X.-Q., Tian, L.-X., Liu, Y.-J., and Niu, J. (2020). Enhanced intestinal health, immune responses and ammonia resistance in pacific white shrimp (Litopenaeus vannamei) fed dietary hydrolyzed yeast (Rhodotorula mucilaginosa) and Bacillus licheniformis. Aquac. Rep. 17:100385. doi: 10.1016/j.aqrep.2020.100385
Chen, S.-M., and Chen, J.-C. (2003). Effects of pH on survival, growth, molting and feeding of giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 218, 613–623. doi: 10.1016/S0044-8486(02)00265-X
Chen, Y., Zhou, L., Yu, Q., Li, E., and Xie, J. (2023). Effects of sulfamethoxazole and florfenicol on growth, antioxidant capacity, immune responses and intestinal microbiota in pacific white shrimp Litopenaeus vannamei at low salinity. Antibiotics 12:575. doi: 10.3390/antibiotics12030575
Dai, W., Yu, W., Xuan, L., Tao, Z., and Xiong, J. (2018). Integrating molecular and ecological approaches to identify potential polymicrobial pathogens over a shrimp disease progression. Appl. Microbiol. Biotechnol. 102:3755–3764. doi: 10.1007/s00253-018-8891-y,
Du Shi-cong, H. L., Yang, K.-J., Yao, Z.-Y., Chen, H.-P, and Zhang, D. M. (2019). The bacterioplankton variation of Litopenaeus vannamei culture before and after the disease emergence. Chin. J. Ecol. 38:2456. doi: 10.13292/j.1000-4890.201908.025
Duan, Y., Liu, Q., Wang, Y., Zhang, J., and Xiong, D. (2018). Impairment of the intestine barrier function in Litopenaeus vannamei exposed to ammonia and nitrite stress. Fish Shellfish Immunol. 78, 279–288. doi: 10.1016/j.fsi.2018.04.050
Ebeling, J. M., Timmons, M. B., and Bisogni, J. (2006). Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia-nitrogen in aquaculture systems. Aquaculture 257, 346–358. doi: 10.1016/j.aquaculture.2006.03.019
Fan, Y., Wang, X., Wang, Y., Ye, H., Yu, X., Wang, S., et al. (2021). Effect of dietary Bacillus licheniformis on growth, intestinal health, and resistance to nitrite stress in pacific white shrimp Litopenaeus vannamei. Aquac. Int. 29, 2555–2573. doi: 10.1007/s10499-021-00764-9
FAO. (2024). The State of World Fisheries and Aquaculture 2024 - Blue Transformation in Action. Food and Agriculture Organization of the United Nations, Rome. Report No. ISBN 978-92-5-138763-4.
Hassan, M. A., Fathallah, M. A., Elzoghby, M. A., Salem, M. G., and Helmy, M. S. (2022). Influence of probiotics on water quality in intensified Litopenaeus vannamei ponds under minimum-water exchange. AMB Express 12:22. doi: 10.1186/s13568-022-01370-5
Hui, C., Wei, R., Jiang, H., Zhao, Y., and Xu, L. (2019). Characterization of the ammonification, the relevant protease production and activity in a high-efficiency ammonifier Bacillus amyloliquefaciens DT. Int. Biodeterior. Biodegrad. 142, 11–17. doi: 10.1016/j.ibiod.2019.04.009
Jackson, C., Preston, N., Thompson, P. J., and Burford, M. (2003). Nitrogen budget and effluent nitrogen components at an intensive shrimp farm. Aquaculture 218, 397–411. doi: 10.1016/S0044-8486(03)00014-0
Kewcharoen, W., and Srisapoome, P. (2019). Probiotic effects of Bacillus spp. from pacific white shrimp (Litopenaeus vannamei) on water quality and shrimp growth, immune responses, and resistance to Vibrio parahaemolyticus (AHPND strains). Fish Shellfish Immunol. 94, 175–189. doi: 10.1016/j.fsi.2019.09.013
Kumar, V., Baruah, K., Nguyen, D. V., Smagghe, G., Vossen, E., and Bossier, P. (2018). Phloroglucinol-mediated HSP70 production in crustaceans: protection against Vibrio parahaemolyticus in Artemia franciscana and Macrobrachium rosenbergii. Front. Immunol. 9:1091. doi: 10.3389/fimmu.2018.01091
Le, N. T. T., Armstrong, C. W., Brækkan, E. H., and Eide, A. (2024). Climatic events and disease occurrence in intensive Litopenaeus vannamei shrimp farming in the mekong area of vietnam. Aquaculture 587:740867. doi: 10.1016/j.aquaculture.2024.740867
Li, F., Huang, J., Wang, M., Chen, L., and Xiao, Y. (2021). Sources, distribution and dynamics of antibiotics in Litopenaeus vannamei farming environment. Aquaculture 545:737200. doi: 10.1016/j.aquaculture.2021.737200
Liao, I. C., and Chien, Y.-H. (2011). “The pacific white shrimp, Litopenaeus vannamei, in Asia: The world's most widely cultured alien crustacean,” in The Wrong Place – Alien Marine Crustaceans: Distribution, Biology and Impacts. Invading Nature – Springer Series in Invasion Ecology, Vol 6., eds. B. Galil, P. Clark, and J. Carlton (Dordrecht: Springer), 489–519. doi: 10.1007/978-94-007-0591-3_17
Liu, H., Liu, M., Wang, B., Jiang, K., Jiang, S., Sun, S., et al. (2010). Pcr-dgge analysis of intestinal bacteria and effect of Bacillus spp. on intestinal microbial diversity in kuruma shrimp (Marsupenaeus japonicus). Chin. J. Oceanol. Limnol. 28, 808–814. doi: 10.1007/s00343-010-9101-7
Liu, L., Li, N., Tao, C., Zhao, Y., Gao, J., Huang, Z., et al. (2021). Nitrogen removal performance and bacterial communities in zeolite trickling filter under different influent c/n ratios. Environ. Sci. Pollut. Res. 28, 15909–15922. doi: 10.1007/s11356-020-11776-y
Llewellyn, A., and Foey, A. (2017). Probiotic modulation of innate cell pathogen sensing and signaling events. Nutrients 9:1156. doi: 10.3390/nu9101156
Luo, P., Hu, C., Xie, Z., Zhang, L., Ren, C., and Xu, Y. (2006). PCR-DGGE analysis of bacterial community composition in brackish water Litopenaeus vannamei culture system. J. Trop. Oceanogr. 25, 49–53. doi: 10.3969/j.issn.1009-5470.2006.02.009
Ma, S., Yu, D., Liu, Q., Zhao, M., Xu, C., and Yu, J. (2022). Relationship between immune performance and the dominant intestinal microflora of turbot fed with different Bacillus species. Aquaculture 549:737625. doi: 10.1016/j.aquaculture.2021.737625
Mirbakhsh, M., Mahjoub, M., Afsharnasab, M., Kakoolaki, S., Sayyadi, M., and Hosseinzadeh, S. (2021). Effects of Bacillus subtilis on the water quality, stress tolerance, digestive enzymes, growth performance, immune gene expression, and disease resistance of white shrimp (Litopenaeus vannamei) during the early hatchery period. Aquac. Int. 29:2489–2506. doi: 10.1007/s10499-021-00758-7
Monier, M. N., Kabary, H., Elfeky, A., Saadony, S., El-Hamed, N. N. A., Eissa, M. E., et al. (2023). The effects of Bacillus species probiotics (Bacillus subtilis and B. licheniformis) on the water quality, immune responses, and resistance of whiteleg shrimp (Litopenaeus vannamei) against Fusarium solani infection. Aquac. Int. 31, 3437–3455. doi: 10.1007/s10499-023-01136-1
Nimrat, S., Khaopong, W., Sangsong, J., Boonthai, T., and Vuthiphandchai, V. (2020). Improvement of growth performance, water quality and disease resistance against Vibrio harveyi of postlarval whiteleg shrimp (Litopenaeus vannamei) by administration of mixed microencapsulated Bacillus probiotics. Aquac. Nutr. 26, 1407–1418. doi: 10.1111/anu.13028
Nimrat, S., Suksawat, S., Boonthai, T., and Vuthiphandchai, V. (2012). Potential Bacillus probiotics enhance bacterial numbers, water quality and growth during early development of white shrimp (Litopenaeus vannamei). Vet. Microbiol. 159, 443–450. doi: 10.1016/j.vetmic.2012.04.029
Oxley, A., Shipton, W., Owens, L., and McKay, D. (2002). Bacterial flora from the gut of the wild and cultured banana prawn, Penaeus merguiensis. J. Appl. Microbiol. 93, 214–223. doi: 10.1046/j.1365-2672.2002.01673.x
Phung, L., Phung, N., Phuong, T., Nicolas, M., Vincent, M., Sandra, A., et al. (2020). “Effect of Bacillus sp. as probiotic on the treatment of environment in brackish water shrimp aquaculture,” in IOP Conference Series: Materials Science and Engineering, Vol. 991 (IOP Publishing), 012052. doi: 10.1088/1757-899X/991/1/012052
Quero, G. M., Ape, F., Manini, E., Mirto, S., and Luna, G. M. (2020). Temporal changes in microbial communities beneath fish farm sediments are related to organic enrichment and fish biomass over a production cycle. Front. Mar. Sci. 7:524. doi: 10.3389/fmars.2020.00524
Rahardjo, K. K. E., Satyantini, W. H., and Amin, M. (2023). Profiling of microbial community in rearing water of white shrimp (Litopenaeus vannamei) infected with white feces disease syndrome. J. Aquac. Fish Health 12, 216–225. doi: 10.20473/jafh.v12i2.35023
Ranjit Kumar, N., Raman, R. P., Jadhao, S. B., Brahmchari, R. K., Kumar, K., and Dash, G. (2013). Effect of dietary supplementation of Bacillus licheniformis on gut microbiota, growth and immune response in giant freshwater prawn, Macrobrachium rosenbergii (De Man, 1879). Aquac. Int. 21, 387–403. doi: 10.1007/s10499-012-9567-8
Reyes, G., Betancourt, I., Andrade, B., Panchana, F., Román, R., Sorroza, L., et al. (2022). Microbiome of Penaeus vannamei larvae and potential biomarkers associated with high and low survival in shrimp hatchery tanks affected by acute hepatopancreatic necrosis disease. Front. Microbiol. 13:838640. doi: 10.3389/fmicb.2022.838640
Ringø, E. (2020). Probiotics in shellfish aquaculture. Aquac. Fish. 5, 1–27. doi: 10.1016/j.aaf.2019.12.001
Rout, P. R., Bhunia, P., and Dash, R. R. (2017). Simultaneous removal of nitrogen and phosphorous from domestic wastewater using Bacillus cereus GS-5 strain exhibiting heterotrophic nitrification, aerobic denitrification and denitrifying phosphorous removal. Bioresour. Technol. 244, 484–495. doi: 10.1016/j.biortech.2017.07.186
Si, Y., Wang, L., Zhang, Z., Lin, J., Chen, X., and Hu, Y. (2020). Effect of organic carbon source on the nitrogen removal of Paracoccus denitrificans. Technol. Water Treat. 46, 52–55. doi: 10.16796/j.cnki.1000-3770.2020.04.011
Sudheesh, P., and Xu, H.-S. (2001). Pathogenicity of Vibrio parahaemolyticus in tiger prawn Penaeus monodon fabricius: possible role of extracellular proteases. Aquaculture 196, 37–46. doi: 10.1016/S0044-8486(00)00575-5
Tran, C., Horyanto, D., Stanley, D., Cock, I. E., Chen, X., and Feng, Y. (2023). Antimicrobial properties of Bacillus probiotics as animal growth promoters. Antibiotics 12:407. doi: 10.3390/antibiotics12020407
Van Doan, H., Hoseinifar, S. H., Tapingkae, W., Seel-Audom, M., Jaturasitha, S., Dawood, M. A., et al. (2020). Boosted growth performance, mucosal and serum immunity, and disease resistance nile tilapia (Oreochromis niloticus) fingerlings using corncob-derived xylooligosaccharide and Lactobacillus plantarum CR1T5. Probiotics Antimicrob. Proteins 12, 400–411. doi: 10.1007/s12602-019-09554-5
Vega-Carranza, A. S., Escamilla-Montes, R., Fierro-Coronado, J. A., Diarte-Plata, G., Guo, X., García-Gutiérrez, C., et al. (2024). Investigating the effect of Bacilli and lactic acid bacteria on water quality, growth, survival, immune response, and intestinal microbiota of cultured Litopenaeus vannamei. Animals 14:2676. doi: 10.3390/ani14182676
Verbaendert, I., Boon, N., De Vos, P., and Heylen, K. (2011). Denitrification is a common feature among members of the genus Bacillus. Syst. Appl. Microbiol. 34, 385–391. doi: 10.1016/j.syapm.2011.02.003
Wang, M., Liu, Y., Luo, K., Li, T., Liu, Q., and Tian, X. (2022). Effects of Bacillus pumilus BP-171 and carbon sources on the growth performance of shrimp, water quality and bacterial community in Penaeus vannamei culture system. Water 14:4037. doi: 10.3390/w14244037
Wu, D. (2016). Studies of Effects on Litopenaeus vannamei Bacterial Ecology Caused by Probiotics Application and Mechanism of Interaction Between Microalgae and Probiotics. Phd thesis, Huazhong Agricultural University.
Wu, H. Z., and Cao, A. (2013). Preparation and adding methods of nessler's reagent having effects on determination of water quality ammonia nitrogen. Adv. Mater. Res. 726, 1362–1366. doi: 10.4028/www.scientific.net/AMR.726-731.1362
Wu, J., Xion, J., Wang, X., Qiu, Q., Zheng, J., and Zhang, D. (2016). Intestinal bacterial community is indicative for the healthy status of Litopenaeus vannamei. Chin. J. Appl. Ecol. 27, 611–621. doi: 10.13287/j.1001-9332.201602.039
Yao, Z., Yang, K., Huang, L., Huang, X., Qiuqian, L., Wang, K., et al. (2018). Disease outbreak accompanies the dispersive structure of shrimp gut bacterial community with a simple core microbiota. AMB Express 8, 1–10. doi: 10.1186/s13568-018-0644-x
Yousuf, J., Thajudeen, J., Rahiman, M., Krishnankutty, S. P, Alikunj, A., Abdulla, M. H. A., et al. (2017). Nitrogen fixing potential of various heterotrophic Bacillus strains from a tropical estuary and adjacent coastal regions. J. Basic Microbiol. 57, 922–932. doi: 10.1002/jobm.201700072
Zhou, J., Fang, W., Yang, X., Zhou, S., Hu, L., Li, X., et al. (2012). A nonluminescent and highly virulent Vibrio harveyi strain is associated with “bacterial white tail disease” of Litopenaeus vannamei shrimp. PLoS ONE 7:e29961. doi: 10.1371/journal.pone.0029961
Zhou, L., and Boyd, C. E. (2015). An assessment of total ammonia nitrogen concentration in alabama (USA) ictalurid catfish ponds and the possible risk of ammonia toxicity. Aquaculture 437, 263–269. doi: 10.1016/j.aquaculture.2014.12.001
Ziaei-Nejad, S., Rezaei, M. H., Takami, G. A., Lovett, D. L., Mirvaghefi, A.-R., and Shakouri, M. (2006). The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the indian white shrimp Fenneropenaeus indicus. Aquaculture 252, 516–524. doi: 10.1016/j.aquaculture.2005.07.021
Keywords: Penaeus vannamei aquaculture, Bacillus licheniformis, high-throughput sequencing, bacterial community analysis, water quality, growth performance
Citation: Zheng Y, Wu Y, Tang S, Li J, Fan L and He W (2025) Effects of Bacillus licheniformis on the water quality, growth performance and bacterial community in Penaeus vannamei aquaculture system. Front. Microbiol. 16:1595680. doi: 10.3389/fmicb.2025.1595680
Received: 18 March 2025; Accepted: 30 April 2025;
Published: 02 June 2025.
Edited by:
Haitham Mohammed, Texas A and M University, United StatesReviewed by:
Wilawan Thongda, National Center for Genetic Engineering and Biotechnology (BIOTEC), ThailandXiaolei Wang, Ocean University of China, China
Copyright © 2025 Zheng, Wu, Tang, Li, Fan and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Zheng, ZXlpemhlbmdAZmpudS5lZHUuY24=
†These authors have contributed equally to this work
 Yi Zheng
Yi Zheng Yingzi Wu
Yingzi Wu Shuling Tang1
Shuling Tang1