- 1Department of Medical Sciences, University of Torino, Torino, Italy
- 2School of Medicine, Tufts University, Boston, MA, United States
- 3Unit of Infectious Diseases, Cardinal Massaia, Asti, Italy
- 4Unit of Infectious Diseases, Department of Medical Sciences, AOU Città della Salute e della Scienza di Torino, University of Turin, Torino, Italy
- 5Department of Oncology, Stem Cell Transplant Center, Città della Salute e della Scienza Hospital, Turin, Italy
- 6Department of Molecular Biotechnologies and Health Sciences, University of Torino, Torino, Italy
- 7Microbiology and Virology Unit, University Hospital Città della Salute e della Scienza di Torino, Torino, Italy
- 8Division of Hematology, AOU “Città della Salute e della Scienza di Torino”, Torino, Italy
Background: Bloodstream infections (BSIs) are a serious threat for patients undergoing allogenic hematopoietic stem cell transplants (a-HSCT). MDR colonization is highly prevalent among a-HSCT patients, due to drug-induced intestinal dysbiosis. Primary outcome of the study was to assess the epidemiology and risk factors for BSIs in the 1st year after a-HSCT. Secondary endpoints were to examine the prevalence of MDR bacterial colonization and factors affecting 1-year post-transplant overall survival (OS).
Methods: In this single-center observational cohort study, all consecutive adult patients undergoing a-HSCT for hematological malignancies between 2012 and 2021 were retrospectively enrolled at the Stem Cell Transplant Center, AOU Città della Salute e della Scienza, Turin (Italy). Cumulative Incidence and risk factors for BSIs were analyzed by Gray and Fine-Gray tests, respectively. OS was evaluated with Kaplan–Meier, while the influence of covariates on OS with Cox regression analysis.
Results: Two hundred and seventy nine patients were enrolled in the study, 43% of which developed BSI within the 1st-year post-transplant. The median onset of BSIs was 10 days after a-HSCT and Gram-negative bacteria were the most common causative agents (58.3%). 20.8% of patients had a positive rectal swab (RS) for MDR bacteria, with extended-spectrum β-lactamases (ESBLs)-producing Enterobacterales being the most common colonizers (60.3% of positive RS), followed by carbapenem-resistant Enterobacteriaceae (CRE) (29.3%). Multivariate competing risks regression analysis showed that colonization by MDR bacteria was associated with a higher incidence of BSIs (SDHR 1.49, 95%CI 1.01–2.20), along with the type of underlying disease (SDHR 0.73, 95%CI 0.58–0.91), donor type (SDHR 1.62, 95%CI 1.02–2.58) and an advanced disease status at the time of transplantation (SDHR 1.57, 95%CI 1.05–2.35). One-year mortality rate was 25.4%. RS colonization was not associated with increased mortality; while BSIs adversely affected OS (SDHR 1.52, 95%CI 1.02–2.26).
Conclusions: BSIs are common complications in a-HSCT, with evidence suggesting a negative impact on OS. Although MDR colonization is not independently linked to increased mortality in a-HSCT, it appears to be associated with an elevated risk of subsequent BSI development. These findings underscore the potential value of pre-transplant surveillance, contact precautions, and early targeted antimicrobial therapy in colonized patients to help mitigate infection-related morbidity and mortality.
1 Introduction
More than 50,000 hematopoietic stem cell transplantations (HSCTs) are performed every year all over the world, both for malignant and non-malignant hematological diseases (Henig and Zuckerman, 2014). In allogeneic HSCT (a-HSCT), donor stem cells, harvested from another individual or from umbilical cord blood units, are used to replace recipient stem cells, previously destroyed by conditioning regimens (Ringdén et al., 2009). Exposure to conditioning regimens themselves, myeloablation and immunosuppressive agents along with the occurrence of acute/chronic graft vs. host disease (GvHD) are some of the factors predisposing a-HSCT patients to develop serious, often life-threatening, infections (Sahin et al., 2016).
In the broad spectrum of infections that patients may develop following a-HSCT, bloodstream infections (BSIs) are a major threat, especially in the pre-engraftment phase (Mikulska et al., 2009). Historically, the mainly involved pathogens were Gram-positive bacteria with a rising rate of resistance to penicillins and fluoroquinolones (Collin et al., 2001). Epidemiology of BSIs in a-HSCT has recently changed with a remarkable increase in Gram-negative bacilli (Macesic et al., 2014) and the problem of multidrug-resistant (MDR) bacteria is an issue that clinicians face in daily clinical practice (Sievert et al., 2013). In recent decades, there has been a significant rise in BSIs caused by Extended Spectrum Beta-lactamases (ESBL)-producing and carbapenem-resistant Enterobacteriaceae (CRE). This increase parallels the prevalence of MDR Pseudomonas aeruginosa and, among Gram-positive bacteria, vancomycin-resistant Enterococci (VRE) (Satlin et al., 2014; Satlin and Walsh, 2017).
Overall, BSIs have been demonstrated to exert an unfavorable prognostic impact on the outcome of hematological patients undergoing a-HSCT (Trecarichi et al., 2023; Girmenia et al., 2017). This seems to be especially true in the case of infections sustained by MDR pathogens (Girmenia et al., 2017; Trecarichi et al., 2019, 2016). In a large multicenter study conducted in 15 Italian hematological wards, resistance to 3rd generation cephalosporins among Escherichia coli, identified in the 25.7% of the isolates, was a significant 30-day mortality predictor, together with acute hepatic failure, septic shock, male sex, refractory/relapsed malignancy (Trecarichi et al., 2019). Moreover, resistance to carbapenems dramatically increases the overall mortality among patients suffering from hematological malignancies with BSIs caused by Klebsiella pneumoniae as described by (Trecarichi et al. 2016), and inadequate initial therapy is another crucial risk factor for increased mortality.
Since MDR colonization is a recognized risk factor for development of MDR-BSI in the general population (Giannella et al., 2014), some studies have started to focus on epidemiology and implications of MDR bacterial colonization among hematological patients (Henig and Zuckerman, 2014; Cattaneo et al., 2018). Indeed, patients undergoing a-HSCT are particularly prone to acquiring MDR rectal colonization due to infection control measures, local epidemiology, intestinal dysbiosis favored by chemotherapy and extensive antibiotic use (Chong et al., 2017). The emergence of MDR bacteria in the setting of a-HSCT recipients, may raise critical concerns about transplant eligibility of colonized patients and potential for infection outbreaks within the transplant center. In this scenario, the relationship between colonization and the development of infections, particularly BSIs, has also been investigated (Trecarichi et al., 2023; De Rosa et al., 2017).
The aim of this study was to analyze epidemiology and risk factors for BSIs during the 1st-year post-a-HSCT. We also aimed to describe the epidemiology of MDR colonization in a-HSCT patients and to identify factors affecting overall survival (OS) within the first year after a-HSCT.
2 Materials and methods
2.1 Study design
We performed a single-center observational retrospective cohort study including all consecutive adults (≥18-year-old) undergoing a-HSCT for hematological malignancy between August 2012 and October 2021 at the Stem Cell Transplant Center, AOU Città della Salute e della Scienza, Turin (Italy).
The primary endpoint was to report the epidemiology and risk factors for BSI occurring in the 1st year post-HSCT. Secondary endpoints were to describe the epidemiology of MDR-bacteria intestinal colonization during hospitalization for a-HSCT and its relationship with the development of BSI and defining risk factors for the OS during the 1st year after a-HSCT.
The study was conducted according to the principles of the Declaration of Helsinki and the Declaration of Istanbul and was approved by our Institutional Ethics Committee (resolution no. 506/2021). All the participants signed a written consent for transplant procedure as previewed by clinical practice and for the retrospective use of anonymous medical data for research purpose.
2.2 Demographic and HSCT data
Demographic and clinical data were retrieved through the revision of a computerized database of prospectively collected data, updated until October 2023. Indication for a-HSCT were hematologic malignancies including Acute Myeloid Leukemia (AML), Myelodysplastic syndromes (MDS), Acute Lymphoblastic Leukemia (ALL), Lymphomas, Chronic Myeloid Leukemia (CML) and Multiple Myeloma (MM). We excluded transplants performed for non-hematologic malignancies or for solid tumors. Patients receiving multiple a-HSCT during the study period were censored at the time of second or third a-HSCT and each single transplant was analyzed independently from the others. Both acute-Graft-vs.-host disease (a-GVHD) and chronic-Graft-vs.-host disease (c-GVHD) were diagnosed based on clinical symptoms and/or biopsies according to standard criteria (Malard et al., 2023; Shulman et al., 2015). Neutropenia was defined as an absolute neutrophil count < 500/mm3. Transplant risk for each patient was calculated using the Hematopoietic Cell Transplantation-Specific Comorbidity Index (HSCT-CI) (Sorror et al., 2005). Patients with febrile neutropenia were treated in accordance with internal protocols (i.e., piperacillin/tazobactam or cefepime). In instances of fever or febrile neutropenia without septic shock, an additional drug with possible KPC action, such as tigecycline and/or an aminoglycoside, was included into the empirical antibiotic regimen for febrile neutropenia. In the event of septic shock or clinical deterioration, an empirical therapy incorporating an anti-KPC agent (e.g., ceftazidime/avibactam, meropenem/vaborbactam, imipenem/cilastatin/relebactam, cefiderocol) was included into the therapeutic regimen. Moreover, patients at risk with confirmed rectal ESBL presenting with fever or febrile neutropenia, without septic shock, had tigecycline and/or an aminoglycoside included into their empirical antibiotic regimen. In the event of septic shock or clinical deterioration, an empirical therapy including a high-dose carbapenem and aminoglycosides was incorporated into the therapeutic regimen. Moreover, patient care was provided adhering to infection prevention and control standards, including proper use of PPE, contact precautions, barrier nursing, and environmental cleaning.
2.3 Bloodstream infections definitions and diagnostic technique
BSI was defined as the isolation of one or more bacterial or fungal pathogens in a blood culture, excluding organisms listed as common commensals by the NHSN (Centers for Disease Control and Prevention, 2025). Coagulase-negative staphylococci (CoNS) and other typical skin contaminants were considered significant if the patient showed clinical signs of infection—such as fever, low blood pressure, chills, or rigors—and the same organism was isolated in at least two separate blood cultures. BSIs were defined as “polymicrobial” if more than one bacterial microorganism was detected on the 1st day of a BSI episode. Only BSI occurring in the 1st year from transplantation were considered and, in case of multiple and relapsing bacterial BSI, only the first episode was included in the analysis. Incidence data were based on the rate of BSI per the yearly number of transplants. Cultures were analyzed with the BD BACTECTM FX system (Becton Dickinson) according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoint tables (European Committee on Antimicrobial Susceptibility Testing, 2025). The identification of microorganisms was conducted with matrix-assisted laser desorption ionization time-of-flight mass spectrome- try and VITEK®, whereas susceptibility to antibiotic molecules was tested using VITEK 2 (VITEK® according to EUCAST breakpoint tables).
2.4 MDR colonization sampling
Patients were screened with a rectal swab to detect ESBL-producers and CR Enterobacteriaceae, as well as MDR non-fermenting GNB. Carbapenemases have been considered as KPC, the most common carbapenemase based on local epidemiology, unless otherwise specified. Biomolecular test for detection of ESBL strains on RS was introduced in our center in 2017, so it was not possible to assess the prevalence of these rectal colonizers before 2017. Gram negative bacteria were considered MDR if they produced ESBL or carbapenemases, and in all cases of resistance to 1 agent in 3 therapeutically relevant classes of antibiotics. Our screening policy did not cover vancomycin-resistant Enterococcus faecium colonization. Screening was performed from the admission for transplant induction and then weekly until discharge.
2.5 Statistical analysis
Primary endpoint was the cumulative incidence (CI) for BSI at 1–3–6–12 months from the day of a-HSCT (main event); competing event was the relapse/death without BSI, alive patients were censored at the date of last contact. The CI function was compared across groups by the Gray-test, while the competing risks regression model was used to estimate the role of risk factors on BSI occurrence by the Fine-Gray test. The following covariates were selected by investigator consensus and tested as BSI occurrence determinants: recipient age (>60 vs. 40–60 vs. < 40 years), recipient gender (female vs. male), diagnosis (AML/MDS vs. ALL vs. other hematological malignancies), disease status at transplant (advanced vs. early disease), Hematopoietic cell transplantation comorbidity index (HCT-CI, ≥3 vs. 0–2), donor type (haploidentical vs. matched unrelated donor vs. matched related donor), stem cell source (peripheral blood vs. bone marrow), conditioning regimen (reduced intensity vs. myeloablative), anti-thymocyte globulin (ATG) administration as GvHD prophylaxis (yes vs. no), antibiotic prophylaxis (yes vs. no), BSI occurrence (yes vs. no) and etiology, RS (positive vs. negative), acute (grade II–IV vs. 0–I), and chronic GVHD (moderate/severe vs. no/mild) occurrence. Secondary endpoint was OS, defined as the time from transplant to death from any cause. Survival curves were estimated by the Kaplan–Meier method and compared across groups by the log-rank test. The effect on OS of the same set of covariates was analyzed by the Cox proportional hazards regression model, comparing the two arms by the Wald test, and calculating 95% confidence intervals (95%CI). Patient characteristics were tested using Fisher's exact test for categorical variables and the Mann–Whitney and Kruskal–Wallis tests for continuous ones; continuous variables were described as median (Inter Quartile Range-IQR). All reported p-values were obtained by the two-sided exact method at the conventional 5% significance level. Data were analyzed as of October 2024 by R 4.4.2 (R Foundation for Statistical Computing, Vienna-A, http://www.R-project.org, accessed on November 21, 2024).
3 Results
3.1 Patients' characteristics
Two hundreds and seventy-nine patients undergoing a-HSCT were included in the study and clinical and microbiological characteristics were summarized in Table 1. A slight prevalence of male (N = 153, 54.8%) was found and median age was 52 years (range 18–69). AML/MDS were the most common malignancies (63.6%) leading to a-HSCT in our population. Most of the patients (82.1%) were in complete remission (CR) or partial remission (PR) at the time of transplantation, while 17.9% had an advanced stage of disease. More than a quarter of the patients had an HCT-CI above 3 (N = 82, 29.4%).
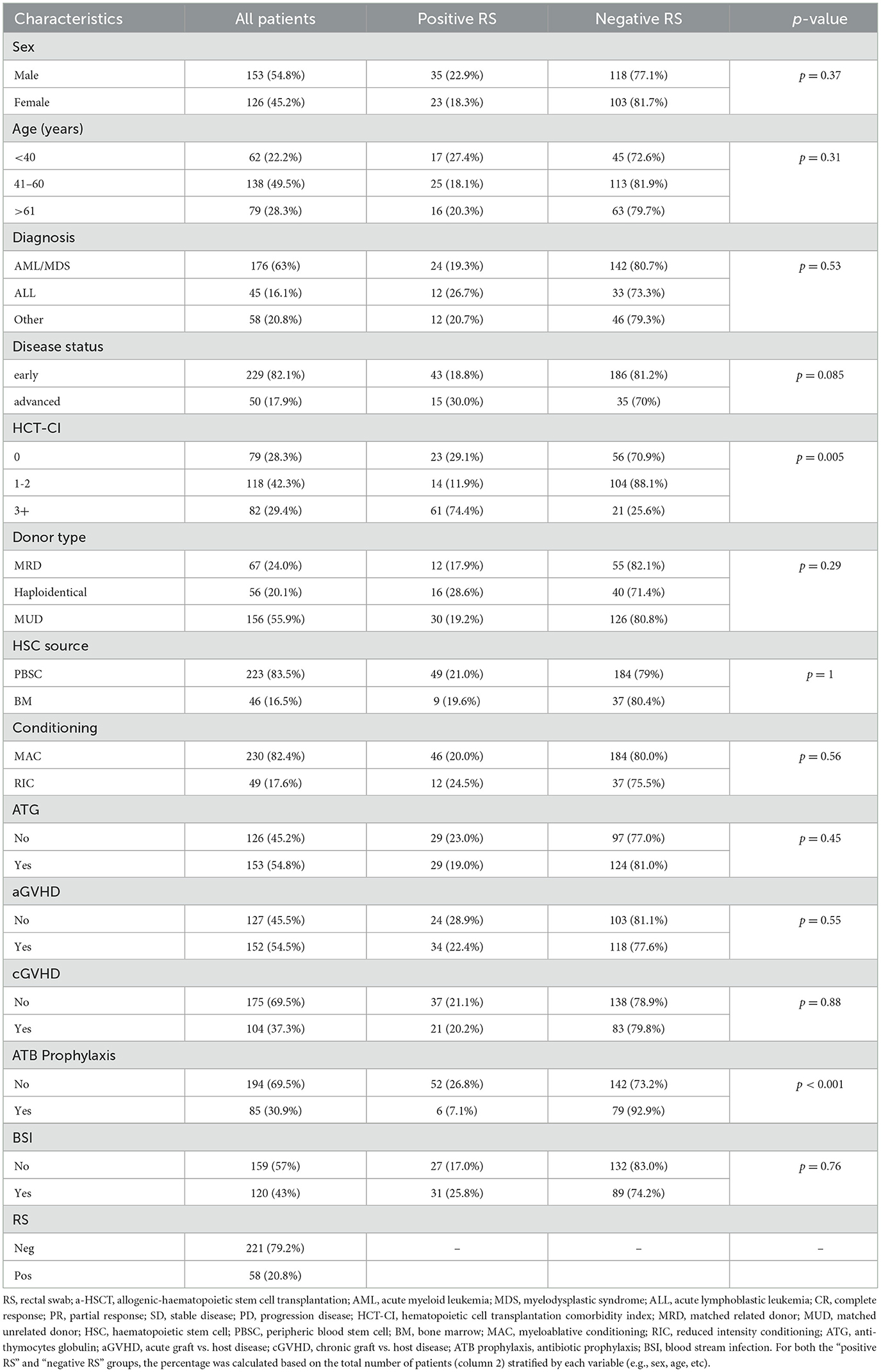
Table 1. Clinical and microbiological characteristics of the whole cohort and stratified by colonization status.
Overall 67 patients (24.0%) received a-HSCT from a matched related donor, (MRD) while 212 patients (76%) were grafted from alternative donors, including a matched unrelated donor (MUD) in 156 cases and haploidentical donors in 56 cases. Myeloablative conditioning (MAC) regimen was used in 82.4% of the transplants, while for the remainder (17.6%) a reduced-intensity conditioning (RIC) was preferred. Peripheral blood was the graft source in 83.5% of the patients. GVHD prophylaxis included ATG in 54.8% of the cases. Overall, 85 patients (31%) received antibacterial prophylaxis with levofloxacin (most of them were grafted before 2017). Acute-GVHD occurred in 54.5% of patients, while cGVHD was reported in 37.3% of the patients.
3.2. Epidemiology of BSIs
Overall, 120 patients (43%) developed a BSI during hospitalization. The CI of BSI at 1, 3, 6, and 12 months after a-HSCT was 30.8%, 37.3%, 39.1%, and 43.0%, respectively. In Table 2 was reported CI of bacterial BSI following a-HSCT according to study population characteristics.
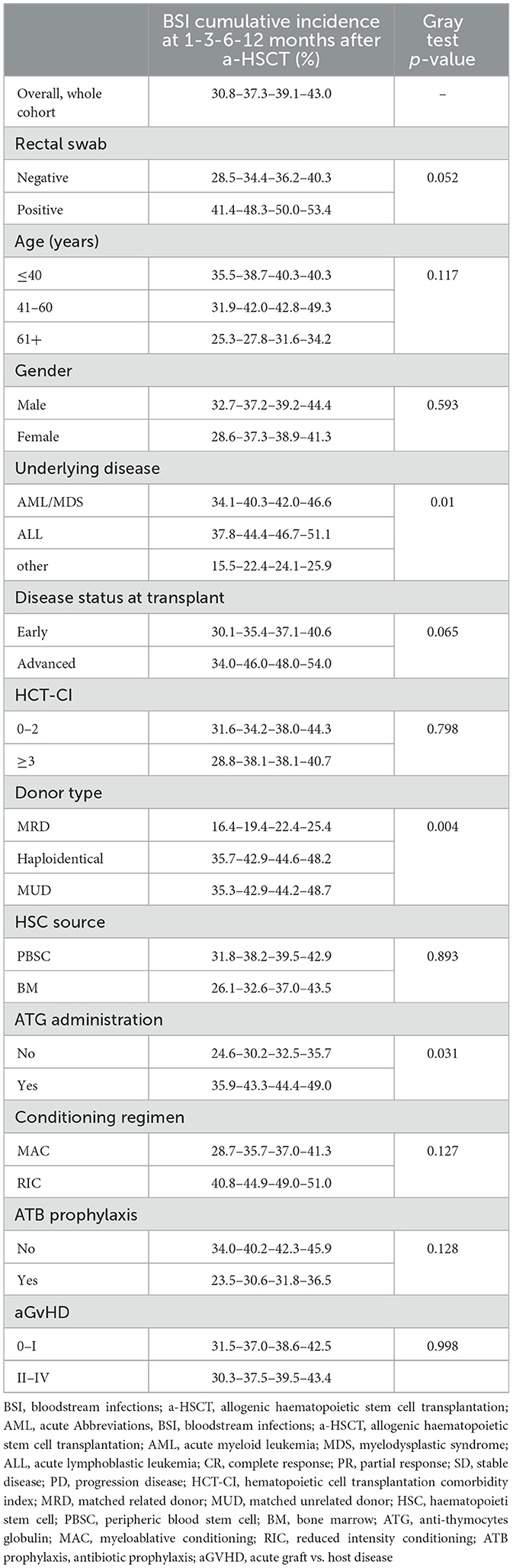
Table 2. Bloodstream infections cumulative incidence according to clinical and microbiological factors.
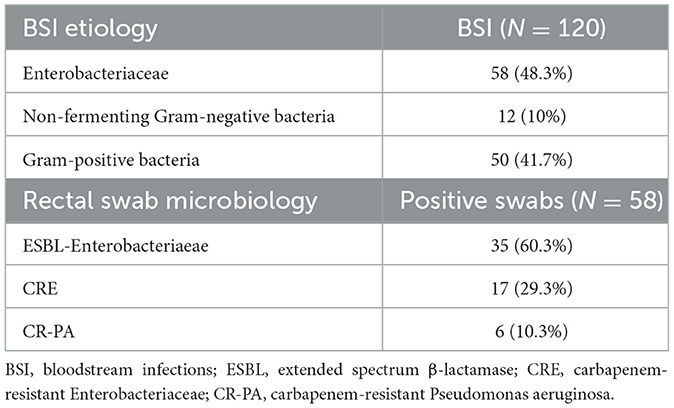
Gram-negative bacteria were responsible for BSI in the majority of cases (58.3%). Among these, 58 (48.3%) were Enterobacteriaceae, with E. coli and K. pneumoniae being the most frequently isolated. Conversely, non-fermenting Gram-negative bacteria accounted for 10% of cases, with P. aeruginosa being the predominant species within this group. Gram-positive bacteria were isolated in 41.7% (50 cases) of instances, with methicillin-resistant S. epidermidis (MRSE) and other coagulase-negative staphylococci being the most prevalent (refer to Table 3).
3.3 Epidemiology of MDR bacterial colonization
MDR bacterial colonization screening was conducted on the entire population using RS. Of the 58 positive swabs (20.8%), 32 (55.2%) were collected on pre-transplant days and 26 (44.8%) after transplant. As shown in Table 3, ESBLs-producing Enterobacteriaceae were confirmed to be common colonizers (60.3% of positive swabs), followed by carbapenem-resistant Enterobacteriaceae (CRE) (29.3%). Only 6 patients were colonized by carbapenems-resistant P. aeruginosa (CRPA). Table 1 shows that the colonization rate in age groups up to 40, 41–60 and 61 years and older were 27.4%, 18.1%, and 20.3%, respectively. When stratifying colonization prevalence based on the underlying hematological condition, RS positivity was found in 26.7% of patients with ALL and 19.3% of those with AML/MDS. Although not statistically significant, a higher colonization rate (30% vs. 18.8%, p = 0.085) was observed in patients with advanced disease compared to those in CR/PR. Additionally, a greater colonization rate was noted in patients with a higher HCT-CI (≥3) (74.4%, vs. 25.6% p = 0.005). Only 7.1% of patients on fluoroquinolone prophylaxis were colonized, compared to 26.8% of patients who did not take FQ prophylaxis.
3.4 Risk factors for BSI
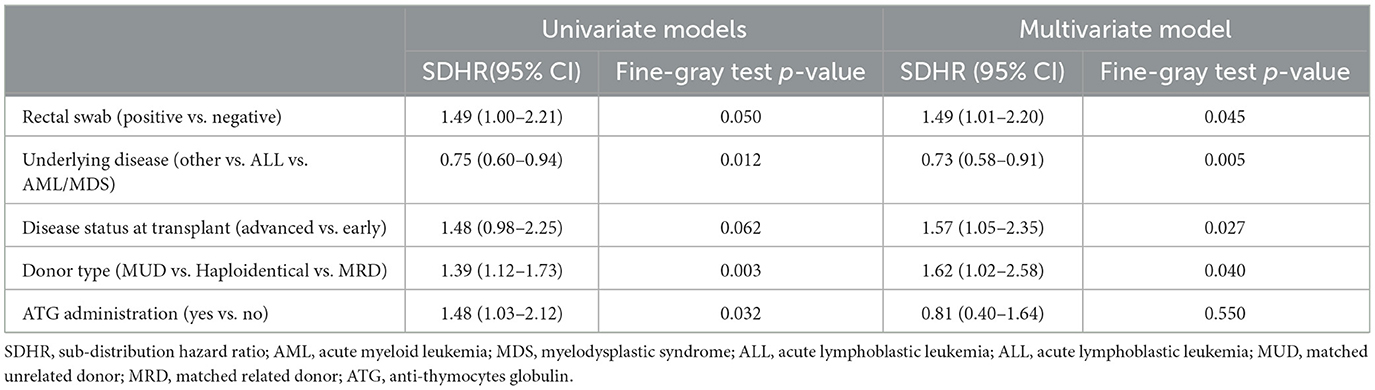
In Table 4, we reported the univariate and multivariate competing risks regression analyses for bacterial BSI after a-HSCT. A positive RS (SDHR 1.49, 95%CI 1.01–2.20), underlying disease (other vs. ALL vs. AML/MDS, SDHR 0.73, 95%CI 0.58–0.91), donor type (MUD vs. haploidentical vs. MRD, SDHR 1.62, 95%CI 1.02–2.58) and an advanced disease status at time of transplant (advanced vs. early, SDHR 1.57, 95%CI 1.05–2.35), were associated with a higher CI of bacterial BSIs at multivariate analysis.
Moreover, we reported in Figure 1 the CI of bacterial BSI after a-HSCT in the 12-months following transplant (CI BSI at 1/3/6/12 months: 30.8%/37.3%/39.1%/43.0%, while Figure 2 differentiates CI based on RS result (CI BSI with negative swab at 1/3/6/12 months: 28.1%/34.4%/36.2%/40.3%; CI BSI with positive swab at 1/3/6/12 months: 41.4%/48.3%/50.0%/53.4%. Although BSI incidence was higher in patients with a positive RS, it was marginally significant (p = 0.052). In 17/31(54%) of the BSIs in MDR-colonized patients, the causative agent was the same bacterium isolated on rectal swab. Moreover, the rate of MDR pattern in G-negative BSIs was higher in colonized patients than in non-colonized patients (61.9% vs. 17.4% p = 0.0003).
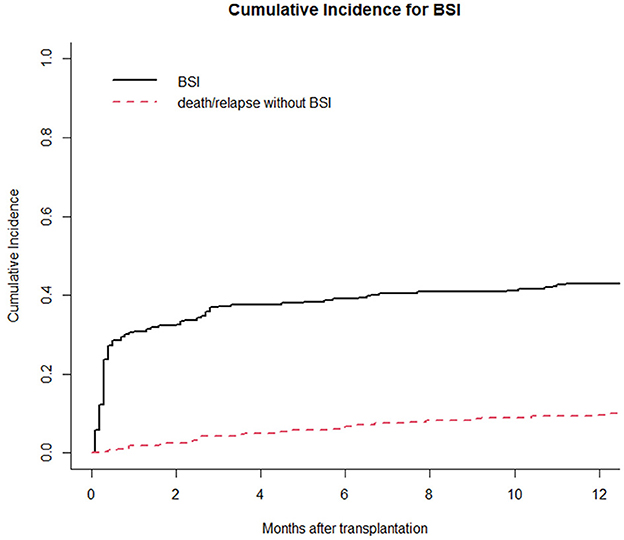
Figure 1. Cumulative incidence of bacterial BSI in the 1st year after a-HSCT. Death/relapse without BSIs is considered the competing event.
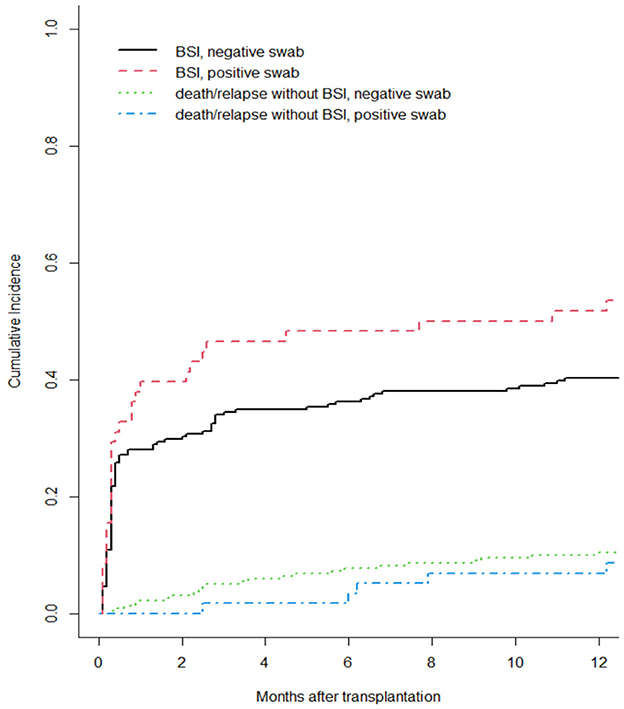
Figure 2. Cumulative incidence of bacterial BSI after a-HSCT in the 1st year after a-HSCT, stratified by colonization status. Death/relapse without BSIs were considered the competing event.
3.5 Mortality and outcome
In our cohort, 208 patients (74.6%) were alive at 1-year follow-up. One-year overall mortality was 25.4% (71 patients). The occurrence of BSI within 1 year post-transplantation had a significant impact on OS (p = 0.005), with a notably lower OS observed in patients experiencing BSI (Figure 3). Conversely, as shown in Figure 4, no consistent differences in OS were observed when comparing patients with positive RS with those with negative RS (p = 0.77).
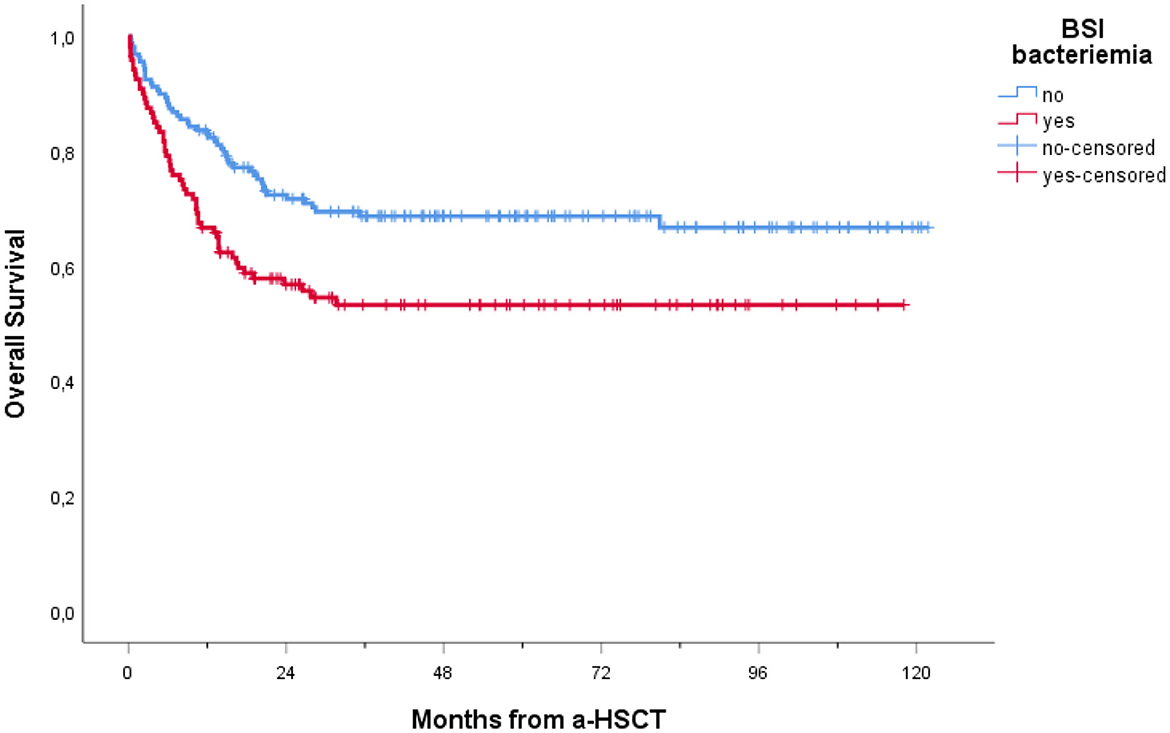
Figure 3. Overall survival of patients receiving a-HSCT stratified by BSI occurrence. Ticks on probability lines indicate dates of censoring at the last follow-up.
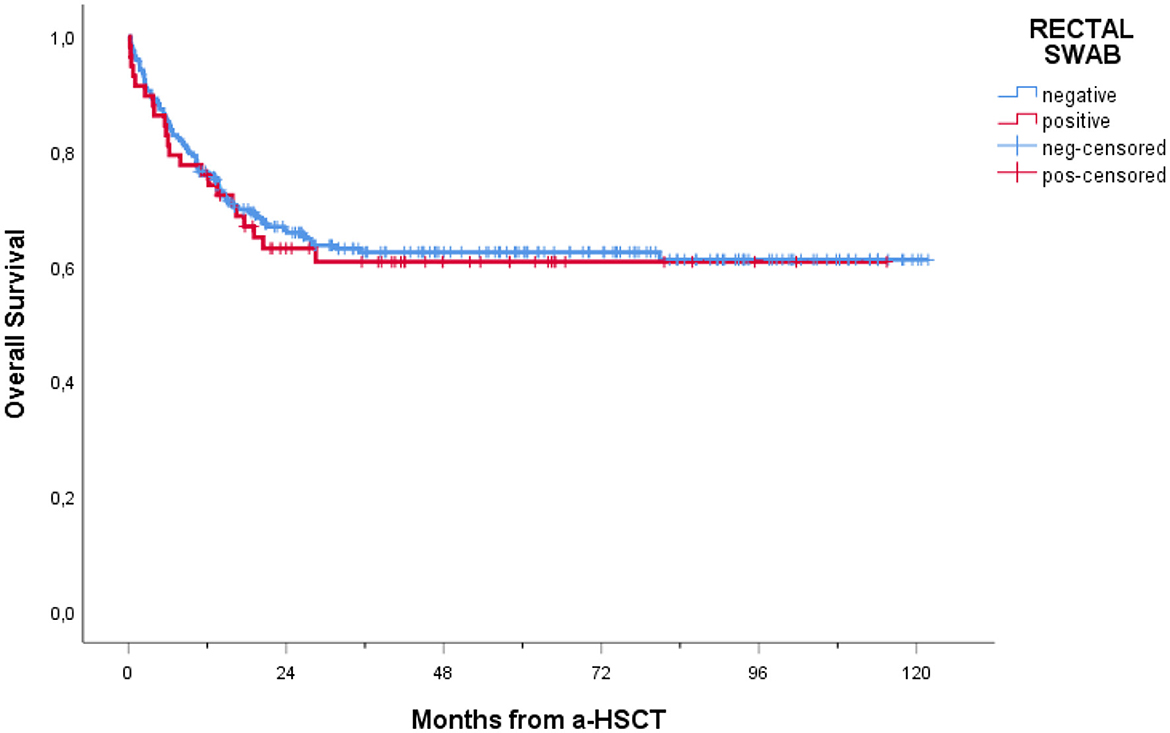
Figure 4. Overall survival of patients receiving a-HSCT stratified by colonization status. Ticks on probability lines indicate dates of censoring at the last follow-up.
Furthermore, lower OS was recorded among patients with BSI vs. non-BSI patients, regardless of whether the RS was positive or negative (Figure 5).
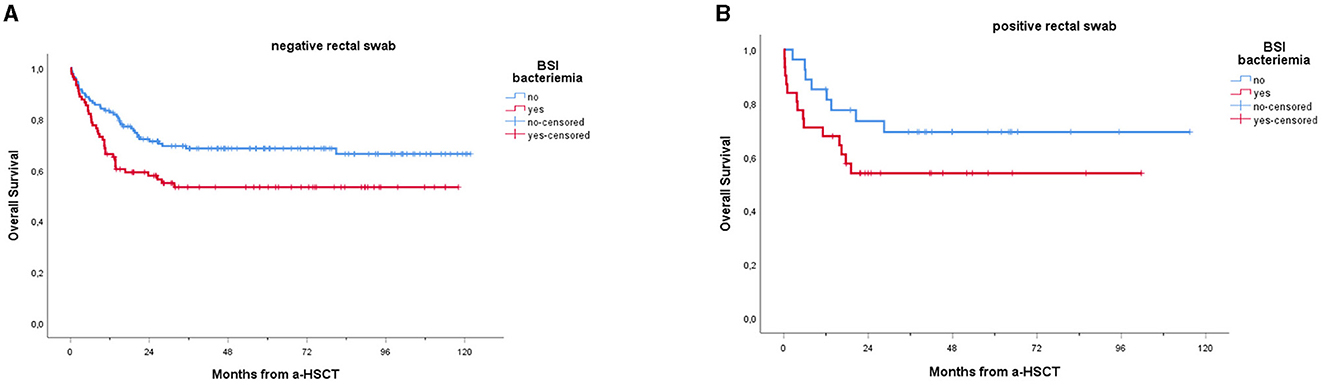
Figure 5. Overall survival of patients receiving a-HSCT stratified by BSI: a comparison of non-carriers (A) vs. carriers (B). Ticks on probability lines indicate dates of censoring at the last follow-up. A trend toward reduced OS can be observed among patients experiencing BSI, compared to non-BSI patients; this trend is confirmed for both carriers (A, p = 0.018) and non-carriers (p = 0.045), with no significant difference between the two groups.
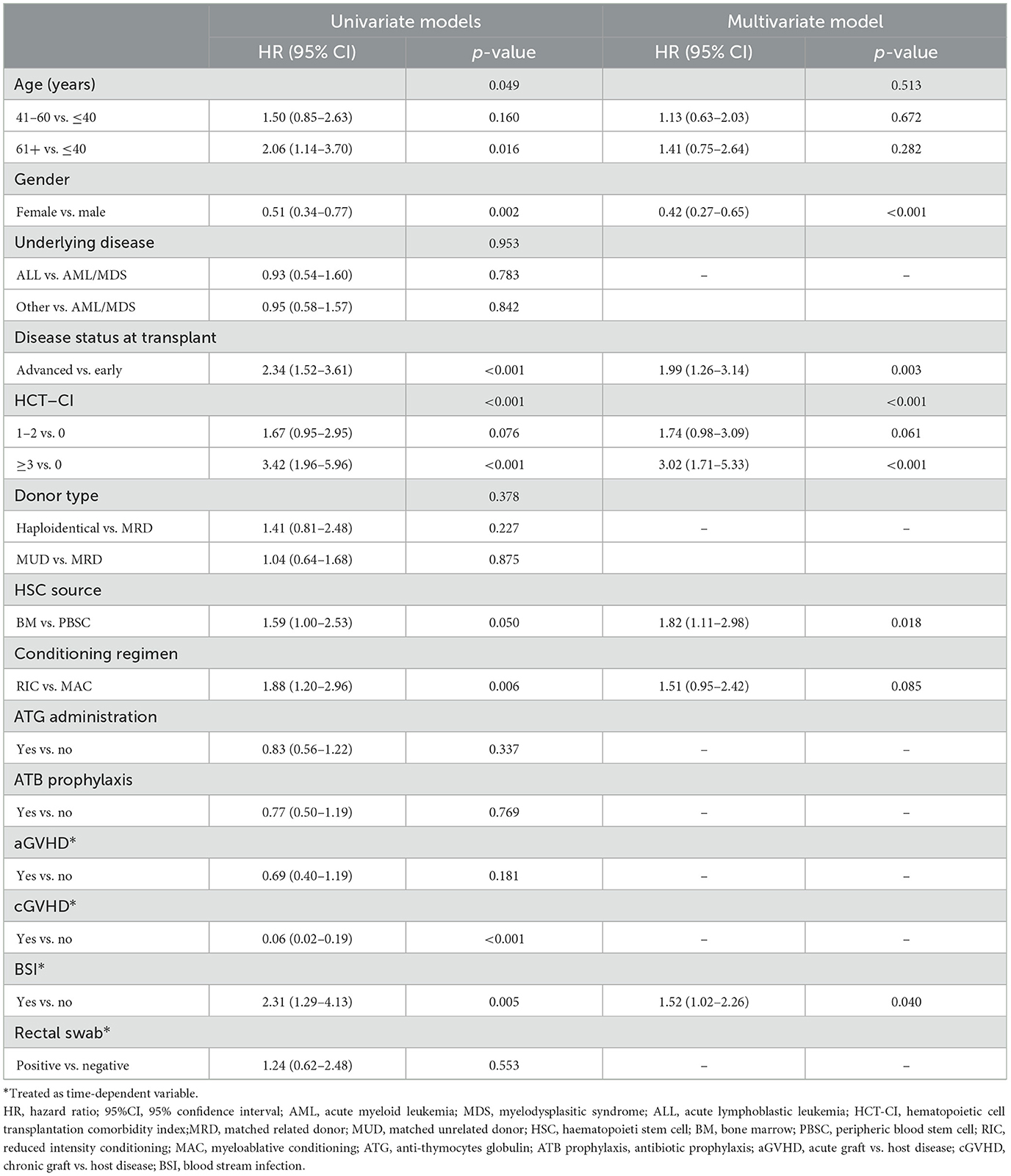
Moreover, Table 5 shows univariate and multivariate Cox proportional hazard regression for OS after a-HSCT. At univariate analysis, factors significantly associated with OS were age at a-HSCT (HR 2.06, p = 0.016), gender (HR 0.51, p = 0.002), disease status at transplant (HR 2.34, p < 0.001), HCT-CI (HR 3.42, p < 0.001), stem cell source (HR 1.59, p = 0.050), conditioning regimen (HR 1.88, p = 0.006) and BSI (HR 2.31, p = 0.005). Multivariate analysis confirmed that male gender (HR 0.42, p < 0.001), advanced disease status at transplant (HR 1.99, p = 0.003), HCT-CI ≥ 3 (HR 3.02, p < 0.001), use of BM as stem cell source (HR 1.82, p = 0.018), and BSI (HR 1.52, p = 0.040) had an adverse significant impact on the OS of patients.
4 Discussion
In this single-center study, we outlined the epidemiology of BSIs and clinical outcomes of 279 patients who underwent a-HSCT, focusing on the colonization of the rectal swabs by MDR pathogens. We also kept track of any BSIs that occurred within the 1st year after transplant, and identified risk factors that might have influenced infection and death.
In recent decades, the a-HSCT procedure has gained increasing approval and now represents a pivotal treatment option with an acceptable complication rate, even with HLA-haploidentical donors. Regrettably, infectious complications, particularly BSIs, still have a major impact on the survival of a-HSCT recipients, especially with the worldwide spread of MDR bacteria (De Rosa et al., 2017; Sorror et al., 2005).
In our cohort, 43% of patients developed BSIs in the post-transplant year, with median time onset of 10 days after a-HSCT. Likewise, previous works have reported similar average time onset, ranging between 4.5 and 15.5 days post-transplantation (Niyazi et al., 2023). During the observation period, we recorded an increasing trend in BSIs sustained by Gram-negative bacteria, which represented the main etiological agents. Previous studies have extensively described the role of Enterobacteriaceae and P. aeruginosa in BSIs occurring after a-HSCT (Macesic et al., 2014; Younes et al., 2017), representing a major issue for hematological patients. Nevertheless, Gram-positive bacteria are still a common cause of BSIs in hospitalized fragile patients and were involved in 50/120 (41.7%) BSIs in our study.
Patients undergoing HSCT are at high risk of intestinal mucosal damage due to intensive chemotherapy or radiation treatments. This explains the selection of MDR colonizers, particularly in cases of broad spectrum-antibiotic use, as they eliminate the beneficial bacteria in the gut that do not require oxygen, leading to intestinal dysbiosis (Chong and Koh, 2020). We identified a positive RS in 58 cases (20.8%). ESBL bacteria were the most common colonizers in our cohort, followed by CRE. (Forcina et al. 2018) described a comparable colonization rate, with ~17% of a-HSCT patients in their cohort exhibiting rectal colonization by MDR Gram-negative bacteria. Another Italian study reported that MDR Gram-negative bacteria colonized ~5% of HSCT recipients (Patriarca et al., 2017). Overall, carbapenem-resistant bacteria colonized 8.4% of our patients, a slightly lower percentage than the one described in a Turkish study on HSCT recipients (Demiraslan et al., 2017). The majority of positive RS were detected pre-transplant (55.2%). Nevertheless, the rate of colonization observed post-transplant remains significantly high (45%), underscoring the need for continued surveillance beyond the pre-transplant phase, rather than relying solely on pre-transplant screening to assess colonization risk.
According to the published data (Ferreira et al., 2018; Liss et al., 2012; Cao et al., 2022), our study confirmed high incidence of MDR-BSI was found among patients with MDR colonization. This finding aligns with a recent prospective multicenter cohort survey in Italy on hematological patients, revealing an independent association between MDR-positive RS and the occurrence of MDR Gram-negative bacteria BSIs (Trecarichi et al., 2023). It has also been shown that BSIs caused by CR-K. pneumoniae and P. aeruginosa have a worse outcome when compared to those caused by pathogens that are sensitive to carbapenems (Girmenia et al., 2017). Therefore, in this setting, we should prioritize interventions to reduce colonization, along with individualized, prompt, and active therapy for septic MDR-colonized patients. Researchers have recently developed a model that predicts the risk of a subsequent BSI in patients with hematologic malignancies who have CRE colonization (Liu et al., 2023).
In addition to rectal colonization, type of underlying disease, donor type, and disease status at the time of transplantation can also affect the risk of developing BSIs (Trecarichi et al., 2023; Forcina et al., 2018; Gill et al., 2023). Our analysis showed that patients with AML/MDS, those receiving transplants from MUD, and those in SD-PD had an increased risk of BSIs. This is likely due to the fact that these conditions are associated to a significant immune system impairment, increasing overall infectious risk. In particular, as pointed out by (Forcina et al. 2018), LAM/MDS is characterized by profound neutropenia and requires intensive conditioning regimens, which exacerbate the risk for microbial translocation and bloodstream infections. Additionally, the genetic difference between MUD donors and other types of donors increases the risk of GVHD, necessitating stronger immunosuppression and a longer period for immune reconstitution. Similarly, patients with SD-PD have higher infection risks compared to those in CR-PR due to severe immune function impairment secondary to previous aggressive treatments.
Furthermore, BSIs had a negative impact on OS in our cohort, adding to other better-known variables predicting poor outcomes in a-HSCT (e.g., disease not in remission at the time of transplant and GVHD). In line with previous research (Mikulska et al. 2009); (Stoma et al. 2016) and (Ustun et al. 2019) have recently confirmed, that BSIs negatively impact the OS of a-HSCT patients, regardless of whether the BSI occurs before or after engraftment. Also, Girmenia et al. found that GNB BSIs were a predictor of poor outcome in HSCT patients, being the main cause of death in 39.1% of a-HSCT recipients and 36.4% of auto-HSCT recipients who died before engraftment (Girmenia et al., 2017).
Interestingly, in our cohort, the negative impact of BSIs on OS seems to be independent of rectal colonization (refer to Figure 5). This aligns with a recent study by (Forcina et al. 2018), who found no difference in 14-day attributed mortality after MDR Gram-negative bacteria BSIs between non-carriers and carriers, despite the fact that in carriers, BSIs were mainly sustained by CR-Klebsiella pneumoniae or CR-PA, which are known to be associated with higher mortality (Girmenia et al., 2017). This might be related to the fact that in patients with known rectal colonization, the available microbiological data guide the choice and timing of antibiotic therapy, leading to the prompt initiation of targeted first-line therapy tailored to the RS colonizer.
In line with this, in our study, RS colonization did not have a significant impact on OS. The literature on the topic is controversial, and uniform evidence is currently lacking. (Heidenreich et al. 2017) found no clear and significant correlation between MDR colonization and an increase in mortality if prompt, effective antibiotic therapy is administered in cases of infective complications. In contrast, other research found that MDR bacteria rectal colonization lowers OS by raising the risk of transplant-related death and infectious complications after a-HSCT, even when antibiotics are used to target colonizing pathogens (Bilinski et al., 2016). For these reasons, considering MDR colonization as a relative contraindication to allogenic transplant is still a matter of debate (Patriarca et al., 2017).
Our study presents some limitations, such as those intrinsic in its retrospective, single-center nature, which limits the generalizability of our findings. Furthermore, the inclusion of patients undergoing multiple transplants may introduce selection bias, as these individuals present distinct clinical characteristics which could act as confounding factors. The decision was based on the clinical relevance of capturing the full spectrum of HSCT recipients encountered in real-world practice. In future analyses, we plan to explore the impact of transplant sequence more explicitly and to analyze these subgroups separately to further clarify their specific risk profiles. Additionally, in our center antibiotic prophylaxis as a preventive measure was withdrawn in 2016, midway through our observation period. The decision was driven by concerns about microbiota disruption, potentially increasing the risk of GVHD and antibiotic resistance. This change in internal protocol could have impacted local epidemiology, as suggested by a recent Italian perspective survey, reporting a favorable shift in the resistance patterns of Gram negative bacteria isolates following fluoroquinolone prophylaxis discontinuation (Trecarichi et al., 2023). We did not evaluate this effect in our study, potentially introducing a source of bias. Nevertheless, the cost-effectiveness of this strategy was analyzed on the same cohort in a separate study (Shbaklo et al., 2023).
Despite these limits, our study outlines that BSIs are still frequent complications in a-HSCT, with a significant impact on OS. This is especially true in the pre-engraftment period, when aplasia is still a major issue. Conversely, despite increasing the risk for BSI occurrence, MDR colonization itself is not associated with increased mortality. This finding may support the idea that MDR colonization is not per se a risk factor for mortality, although pre-transplant active surveillance, along with contact precautions and the early initiation of targeted combined therapy in carriers are crucial to minimize the risk of BSIs development and their associated adverse outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by AOU Città della Salute e della Scienza (Turin, Italy) Institutional Ethics Committee (resolution no. 506/2021). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SC: Writing – review & editing, Conceptualization, Methodology, Supervision. BL: Writing – original draft, Data curation. WR: Writing – original draft, Data curation, Formal analysis. ID: Writing – review & editing, Supervision. TL: Conceptualization, Writing – original draft. NS: Writing – original draft, Data curation, Formal analysis. SZ: Writing – review & editing. JG: Writing – review & editing. AC: Methodology, Writing – original draft. RP: Methodology, Writing – original draft, Data curation, Formal analysis. AB: Supervision, Writing – review & editing. BB: Writing – review & editing, Validation. FD: Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AK declared a past co-authorship with the author(s) SC and FD to the handling editor.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bilinski, J., Robak, K., Peric, Z., Marchel, H., Karakulska-Prystupiuk, E., Halaburda, K., et al. (2016). Impact of gut colonization by antibiotic-resistant bacteria on the outcomes of allogeneic hematopoietic stem cell transplantation: a retrospective, single-center study. Biol. Blood Marrow Transplant. 22, 1087–1093. doi: 10.1016/j.bbmt.2016.02.009
Cao, W., Zhang, J., Bian, Z., Li, L., Zhang, S., Qin, Y., et al. (2022). Active screening of intestinal colonization of carbapenem-resistant enterobacteriaceae for subsequent bloodstream infection in allogeneic hematopoietic stem cell transplantation. Infect. Drug Resist. 15, 5993–6006. doi: 10.2147/IDR.S387615
Cattaneo, C., Di Blasi, R., Skert, C., Candoni, A., Martino, B., Di Renzo, N., et al. (2018). Bloodstream infections in haematological cancer patients colonized by multidrug-resistant bacteria. Ann. Hematol. 97, 1717–1726. doi: 10.1007/s00277-018-3341-6
Centers for Disease Control and Prevention (2025). NHSN Organism List.Atlanta, GA: Centers for Disease Control and Prevention. Available online at: http://www.cdc.gov/nhsn/xls/master-organism-com-commensals-lists.xlsx (Accessed April 14, 2025).
Chong, P. P., and Koh, A. Y. (2020). The gut microbiota in transplant patients. Blood Rev. 39:100614. doi: 10.1016/j.blre.2019.100614
Chong, Y., Shimoda, S., Miyake, N., Aoki, T., Ito, Y., Kamimura, T., et al. (2017). Incomplete recovery of the fecal flora of hematological patients with neutropenia and repeated fluoroquinolone prophylaxis. Infect. Drug Resist. 10, 193–199. doi: 10.2147/IDR.S133333
Collin, B. A., Leather, H. L., Wingard, J. R., and Ramphal, R. (2001). Evolution, incidence, and susceptibility of bacterial bloodstream isolates from 519 bone marrow transplant patients. Clin. Infect. Dis. 33, 947–953. doi: 10.1086/322604
De Rosa, F. G., Corcione, S., Raviolo, S., Bruno, B., and Busca, A. (2017). Management of carbapenem-resistant K. pneumoniae in allogenic stem cell transplant recipients: the Turin bundle. New Microbiologica 40, 143–145.
Demiraslan, H., Cevahir, F., Berk, E., Metan, G., Cetin, M., Alp, E., et al. (2017). Is surveillance for colonization of carbapenem-resistant gram-negative bacteria important in adult bone marrow transplantation units? Am. J. Infect. Control. 45, 735–739. doi: 10.1016/j.ajic.2017.01.006
European Committee on Antimicrobial Susceptibility Testing (2025). Clinical Breakpoints and Dosing of Antibiotics. Available online at: https://www.eucast.org/clinical_breakpoints (Accessed June 8, 2025).
Ferreira, A. M., Moreira, F., Guimaraes, T., Spadão, F., Ramos, J. F., Batista, M. V., et al. (2018). Epidemiology, risk factors and outcomes of multi-drug-resistant bloodstream infections in haematopoietic stem cell transplant recipients: importance of previous gut colonization. J. Hosp. Infect. 100, 83–91. doi: 10.1016/j.jhin.2018.03.004
Forcina, A., Lorentino, F., Marasco, V., Oltolini, C., Marcatti, M., Greco, R., et al. (2018). Clinical impact of pretransplant multidrug-resistant gram-negative colonization in autologous and allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 24, 1476–1482. doi: 10.1016/j.bbmt.2018.02.021
Giannella, M., Trecarichi, E. M., De Rosa, F. G., Del Bono, V., Bassetti, M., Lewis, R. E., et al. (2014). Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: a prospective observational multicentre study. Clin. Microbiol. Infect. 20, 1357–1362. doi: 10.1111/1469-0691.12747
Gill, J., Busca, A., Cinatti, N., Passera, R., Dellacasa, C. M., Giaccone, L., et al. (2023). Bacterial bloodstream infections after allogeneic hematopoietic stem cell transplantation: etiology, risk factors and outcome in a single-center study. Microorganisms 11:742. doi: 10.3390/microorganisms11030742
Girmenia, C., Bertaina, A., Piciocchi, A., Perruccio, K., Algarotti, A., Busca, A., et al. (2017). Incidence, risk factors and outcome of pre-engraftment gram-negative bacteremia after allogeneic and autologous hematopoietic stem cell transplantation: an Italian prospective multicenter survey. Clin. Infect. Dis. 65, 1884–1896. doi: 10.1093/cid/cix690
Heidenreich, D., Kreil, S., Nolte, F., Hofmann, W. K., Miethke, T., Klein, S. A., et al. (2017). Multidrug-resistant organisms in allogeneic hematopoietic cell transplantation. Eur. J. Haematol. 98, 485–492. doi: 10.1111/ejh.12859
Henig, I., and Zuckerman, T. (2014). Hematopoietic stem cell transplantation-50 years of evolution and future perspectives. Rambam Maimonides Med. J. 5:e0028. doi: 10.5041/RMMJ.10162
Liss, B. J., Vehreschild, J. J., Cornely, O. A., Hallek, M., Fätkenheuer, G., Wisplinghoff, H., et al. (2012). Intestinal colonisation and blood stream infections due to vancomycin-resistant enterococci (VRE) and extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBLE) in patients with haematological and oncological malignancies. Infection. 40, 613–619. doi: 10.1007/s15010-012-0269-y
Liu, J., Zhang, H., Feng, D., Wang, J., Wang, M., Shen, B., et al. (2023). Development of a risk prediction model of subsequent bloodstream infection after carbapenem-resistant enterobacteriaceae isolated from perianal swabs in hematological patients. IDR. 16, 1297–1312. doi: 10.2147/IDR.S400939
Macesic, N., Morrissey, C. O., Cheng, A. C., Spencer, A., and Peleg, A. Y. (2014). Changing microbial epidemiology in hematopoietic stem cell transplant recipients: increasing resistance over a 9-year period. Transplant. Infect. Dis. 16, 887–896. doi: 10.1111/tid.12298
Malard, F., Holler, E., Sandmaier, B. M., Huang, H., and Mohty, M. (2023). Acute graft-vs.-host disease. Nat. Rev. Dis. Prim. 9, 1–18. doi: 10.1038/s41572-023-00438-1
Mikulska, M., Del Bono, V., Raiola, A. M., Bruno, B., Gualandi, F., Occhini, D., et al. (2009). Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of gram-negative rods and increasing antibiotic resistance. Biol. Blood Marrow Transplant. 15, 47–53. doi: 10.1016/j.bbmt.2008.10.024
Niyazi, D., Micheva, I., Dokova, K., and Stoeva, T. (2023). Incidence, risk factors and outcome of bloodstream infections in patients after hematopoietic stem-cell transplantation: a single center study. Indian J. Hematol. Blood Transfus. 39, 1–5. doi: 10.1007/s12288-023-01645-2
Patriarca, F., Cigana, C., Massimo, D., Lazzarotto, D., Geromin, A., Isola, M., et al. (2017). Risk factors and outcomes of infections by multidrug-resistant gram-negative bacteria in patients undergoing hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 23, 333–339. doi: 10.1016/j.bbmt.2016.11.005
Ringdén, O., Karlsson, H., Olsson, R., Omazic, B., and Uhlin, M. (2009). The allogeneic graft-vs.-cancer effect. Br. J. Haematol. 147, 614–633. doi: 10.1111/j.1365-2141.2009.07886.x
Sahin, U., Toprak, S. K., Atilla, P. A., Atilla, E., and Demirer, T. (2016). An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J. Infect. Chemother. 22, 505–514. doi: 10.1016/j.jiac.2016.05.006
Satlin, M. J., Jenkins, S. G., and Walsh, T. J. (2014). The global challenge of carbapenem-resistant enterobacteriaceae in transplant recipients and patients with hematologic malignancies. Clin. Infect. Dis. 58, 1274–1283. doi: 10.1093/cid/ciu052
Satlin, M. J., and Walsh, T. J. (2017). Multidrug-resistant enterobacteriaceae, pseudomonas aeruginosa, and vancomycin-resistant enterococci: three major threats to hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 19:10.e12762. doi: 10.1111/tid.12762
Shbaklo, N., Vicentini, C., Busca, A., Giaccone, L., Dellacasa, C., Dogliotti, I., et al. (2023). Cost-effectiveness of targeted prophylaxis among allogenic stem cell transplant recipients. Pharmaceuticals. 16:466. doi: 10.3390/ph16030466
Shulman, H. M., Cardona, D. M., Greenson, J. K., Hingorani, S., Horn, T., Huber, E., et al. (2015). Histopathologic diagnosis of chronic graft vs. host disease. Biol. Blood Marrow Transplant. 21, 589–603. doi: 10.1016/j.bbmt.2014.12.031
Sievert, D. M., Ricks, P., Edwards, J. R., Schneider, A., Patel, J., Srinivasan, A., et al. (2013). Antimicrobial-resistant pathogens associated with healthcare-associated infections summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 34, 1–14. doi: 10.1086/668770
Sorror, M. L., Maris, M. B., Storb, R., Baron, F., Sandmaier, B. M., Maloney, D. G., et al. (2005). Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 106, 2912–2919. doi: 10.1182/blood-2005-05-2004
Stoma, I., Karpov, I., Milanovich, N., Uss, A., and Iskrov, I. (2016). Risk factors for mortality in patients with bloodstream infections during the pre-engraftment period after hematopoietic stem cell transplantation. Blood Res. 51, 102–106. doi: 10.5045/br.2016.51.2.102
Trecarichi, E. M., Giuliano, G., Cattaneo, C., Ballanti, S., Criscuolo, M., Candoni, A., et al. (2019). Bloodstream infections caused by Escherichia coli in onco-haematological patients: risk factors and mortality in an Italian prospective survey. PLoS One. 14:e0224465. doi: 10.1371/journal.pone.0224465
Trecarichi, E. M., Giuliano, G., Cattaneo, C., Ballanti, S., Criscuolo, M., Candoni, A., et al. (2023). Bloodstream infections due to Gram-negative bacteria in patients with hematologic malignancies: updated epidemiology and risk factors for multidrug-resistant strains in an Italian perspective survey. Int. J. Antimicrobial. Agents. 61:106806. doi: 10.1016/j.ijantimicag.2023.106806
Trecarichi, E. M., Pagano, L., Martino, B., Candoni, A., Di Blasi, R., Nadali, G., et al. (2016). Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am. J. Hematol. 91, 1076–1081. doi: 10.1002/ajh.24489
Ustun, C., Young, J. A. H., Papanicolaou, G. A., Kim, S., Ahn, K. W., Chen, M., et al. (2019). Bacterial blood stream infections (BSI), particularly post-engraftment bsi, are associated with increased mortality after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 54, 1254–1265. doi: 10.1038/s41409-018-0401-4
Keywords: HSCT, bloodstream infections, MDR, hematology, colonization
Citation: Corcione S, Longo BM, Rugge W, De Benedetto I, Lupia T, Shbaklo N, Zompi S, Gill J, Curtoni A, Passera R, Busca A, Bruno B and De Rosa FG (2025) Bloodstream infections epidemiology and clinical outcomes after 1 year post allogeneic hematogenous stem cell transplantation. Front. Microbiol. 16:1596900. doi: 10.3389/fmicb.2025.1596900
Received: 20 March 2025; Accepted: 23 July 2025;
Published: 07 August 2025.
Edited by:
Alessandra Mularoni, Mediterranean Institute for Transplantation and Highly Specialized Therapies (ISMETT), ItalyReviewed by:
Esma Eryilmaz-Eren, Republic of Turkey Ministry of Health Sciences, TürkiyeArta Karruli, University Medical Center Mother Teresa (QSUT), Albania
Emanuela Zappulo, University of Naples Federico II, Italy
Patricia Cornejo Juarez, National Institute of Cancerology (INCAN), Mexico
Temenuga Stoeva, Medical University of Varna, Bulgaria
Copyright © 2025 Corcione, Longo, Rugge, De Benedetto, Lupia, Shbaklo, Zompi, Gill, Curtoni, Passera, Busca, Bruno and De Rosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bianca Maria Longo, YmlhbmNhLmxvbmdvMjNAZ21haWwuY29t
 Silvia Corcione
Silvia Corcione Bianca Maria Longo
Bianca Maria Longo Walter Rugge1
Walter Rugge1 Tommaso Lupia
Tommaso Lupia Jessica Gill
Jessica Gill Antonio Curtoni
Antonio Curtoni Roberto Passera
Roberto Passera Benedetto Bruno
Benedetto Bruno Francesco Giuseppe De Rosa
Francesco Giuseppe De Rosa