- 1Department of Surgical Oncology and General Surgery, First Hospital of China Medical University, Shenyang, China
- 2Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Department of Thoracic Surgery, First Hospital of China Medical University, Shenyang, China
- 4Department of Pathology, The People’s Hospital of Liaoning Province, Shenyang, China
Complex microbial interactions occur on the surfaces within the oral cavity, where biofilms form highly organized ecosystems composed of diverse microbial communities and their metabolic products. These biofilms, typically located on the tooth surface or within the gingival sulcus, play a crucial role in both oral and systemic health. Recent studies have significantly improved our understanding of the mechanisms of biofilm formation, their structural characteristics, and their persistence over time. However, the intricate interactions between biofilms and the host, as well as their contributions to both local (e.g., dental caries and periodontitis) and systemic conditions, remain only partially understood. This mini-review summarizes recent scientific progress on the formation, structural dynamics, and ecological functions of oral biofilms. It also highlights emerging strategies for modulating biofilm composition and activity, the regulatory systems governing these interactions, and potential directions for microbiome-based therapies in future research.
1 Introduction
Oral biofilms are complex microbial consortia that adhere to both hard (e.g., teeth) and soft (e.g., gingiva) surfaces within the oral cavity, forming in response to mechanical forces, salivary flow, nutrient fluctuations, and tissue interfaces (Mirghani et al., 2022; Montelongo-Jauregui and Lopez-Ribot, 2018). Unlike biofilms in other parts of the body, oral biofilms are constantly influenced by mastication, intermittent dietary intake, and host immune factors, creating a highly dynamic microenvironment (Kolenbrander et al., 2010; Jakubovics and Kolenbrander, 2010). These biofilms play central roles in maintaining oral health but can also transition into pathogenic communities associated with caries, gingivitis, and periodontitis (Kinane et al., 2017; Murakami et al., 2018; Bowen et al., 2018; Larsen and Fiehn, 2017). More importantly, dysbiotic oral biofilms are increasingly implicated in systemic diseases, including cardiovascular disease, diabetes, Alzheimer’s disease, and chronic kidney disease, underscoring their broad clinical significance (Kurtzman et al., 2022; Yumoto et al., 2019).
The formation of oral biofilms is a multi-step process involving initial bacterial adhesion, following colonization, interbacterial communication, and maturation into a three-dimensional (3D) structured community (Mirghani et al., 2022; Marsh and Zaura, 2017). The extracellular polymeric substances (EPS)—comprising exopolysaccharides, proteins, lipids, and extracellular DNA (eDNA)—provide structural integrity and mediate metabolic interactions within biofilms (Karygianni et al., 2020; Cugini et al., 2019; Klein et al., 2015). Certain bacteria secrete EPS and surface adhesins to aggregate into polymers, which form a cohesive matrix that promotes attachment, protects against immune clearance, and limits antibiotic penetration. These features also complicate antimicrobial treatment, leading to persistent infections and therapeutic challenges (Mirghani et al., 2022; Kuboniwa and Lamont, 2010; Bjarnsholt et al., 2018; Zijnge et al., 2010; Crabbé et al., 2019).
In response to these challenges, recent research has focused on novel approaches to biofilm regulation, including natural products like plant-derived compounds, biosurfactants, probiotics, and nanomaterials. These agents target specific biofilm components or microbial communication systems (Benoit et al., 2019; Hu et al., 2019; Lee et al., 2020; Campbell et al., 2020). These strategies represent a shift from broad-spectrum antimicrobial eradication to targeted modulation aimed at restoring oral microbial balance.
This review provides a comprehensive and critical synthesis of recent findings on oral biofilm formation, structure, and persistence, along with emerging therapeutic strategies. By bridging structural biology, microbial ecology, and clinical innovation, this work aims to support the development of precise, microbiome-conscious interventions for managing biofilm-associated diseases.
2 Manuscript
2.1 Structural characteristics of oral biofilms
2.1.1 Compositional components of oral biofilms
Oral biofilms comprise diverse microorganisms, with primary and secondary bacterial colonizers sequentially establishing the community structure (Kolenbrander et al., 2006; Marsh, 2006; Huang et al., 2011). These microorganisms secrete EPS contributes to the formation of biofilms. Oral biofilms are embedded in a highly organized EPS matrix, forming a highly organized matrix composed mainly of water, exopolysaccharides, proteins, lipids, inorganic ions, and extracellular DNA (eDNA). Exopolysaccharides, mainly produced by cariogenic bacteria such as Streptococcus mutans, serve as the structural backbone that stabilizes the biofilm and facilitates microbial adhesion and aggregation. Exoproteins contribute to structural integrity, enzymatic activity, and nutrient processing. Lipids are involved in biofilm hydrophobicity and barrier functions, while inorganic ions like calcium and magnesium facilitate cross-link matrix components and regulate mineralization. eDNA, originating from lysed cells, plays multifaceted roles in maintaining structural cohesion, promoting horizontal gene transfer, enhancing antimicrobial resistance through antibiotic chelation, and triggering host immune responses via TLR9 signaling pathways (Panlilio and Rice, 2021; Sharma and Rajpurohit, 2024; Kondo et al., 2022). Collectively, these components create a resilient and dynamic microenvironment that underpins the architecture and pathogenic potential of oral biofilms (Figure 1).
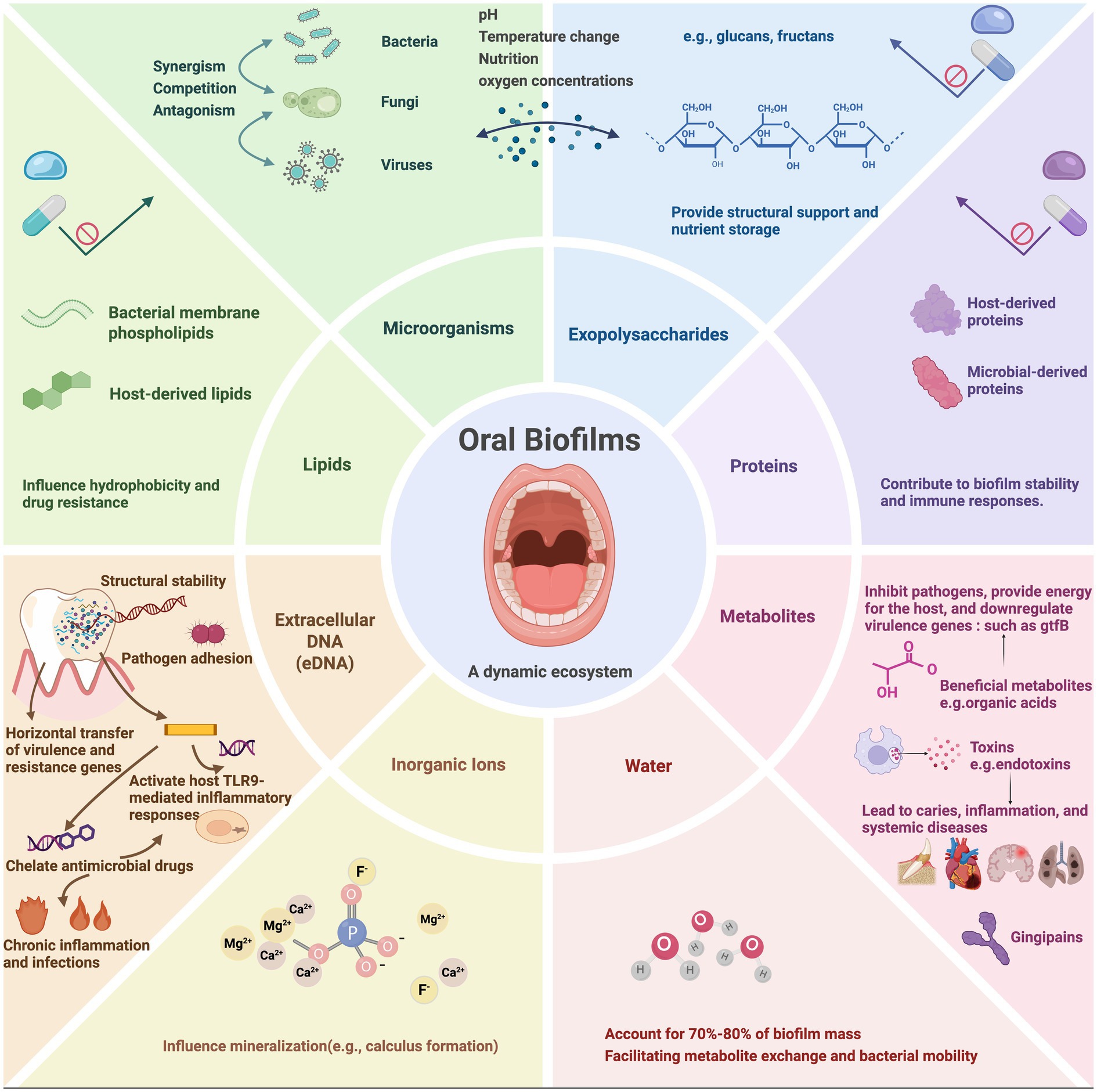
Figure 1. The components of oral biofilms. Oral biofilms are composed of eight elements (microorganisms, extracellular polymeric substances, proteins, metabolites, extracellular DNA, inorganic ions and water). The interactions of these elements contribute to formation and persistence of oral biofilms. Created with BioRender.com.
2.1.2 3D architecture and functional properties of oral biofilms
Bacteria in the oral cavity form biofilms with complex 3D structures embedded within the EPS matrix (Zijnge et al., 2010). Confocal laser scanning microscopy has revealed that these biofilms comprise multiple layers, with different bacteria species occupying distinct ecological niches—enabling interspecies interactions and competition (Reichhardt and Parsek, 2019). Within this architecture, bacteria organize into diverse spatial arrangements, multilayered communities that give rise to microenvironments with varying nutrient gradients, oxygen levels, and metabolic activities. Early colonizers, such as Streptococcus spp., consume oxygen and reduce local oxygen tension, creating anaerobic niches that support obligate anaerobes, including pathogenic species implicated in periodontal disease. This ecological succession—from aerobic to anaerobic conditions—is thought to contribute to the maturation and pathogenic potential of oral biofilms. For instance, under aerobic conditions, the formation of S. mutans biofilms is inhibited, and alterations in surface glycosylation enzyme expression impact its virulence potential (Ahn et al., 2007). This architectural complexity confers mechanical stability and enhances resistance to antibiotics and immune defenses (Xiao et al., 2012). Spatial heterogeneity also influences bacterial gene expression and metabolism, promoting interspecies interactions and facilitating the shift from commensal to pathogenic communities characterized by increased virulence, immune evasion, and pro-inflammatory activity. As such, biofilm architecture represents a critical therapeutic target in managing biofilm-associated oral diseases (Zijnge et al., 2010; Bell et al., 2024).
2.1.3 Bacterial interactions within oral biofilms
Bacterial interactions within biofilms involve synergistic, antagonistic, and neutral mechanisms, collectively influencing biofilm development, persistence, and pathogenicity (Marsh and Zaura, 2017; Maier, 2021). For example, Porphyromonas gingivalis and Treponema denticola mutually promote each other’s growth and virulence within periodontal biofilms, whereas S. mutans inhibit Streptococcus sanguinis via bacteriocins production— an example of niche competition, where one species suppresses another occupying a similar ecological niche (Ng et al., 2019; Kreth et al., 2005). Cooperative interactions can also promote antibiotic resistance through synergistic protective mechanisms. Beyond physical and metabolic interactions, quorum-sensing (QS) system plays a pivotal role in regulating collective bacterial behaviors. QS system is a cell-to-cell communication system in which bacteria detect population density via signaling molecules, thereby regulating biofilm formation, virulence expression, and resistance development. A well-known example is the use of N-acyl-homoserine lactones (AHLs) in Gram-negative bacteria to regulate biofilm maturation and pathogenic gene expression (Kumar et al., 2022; Asahi et al., 2010).
2.2 Process of oral biofilm formation
2.2.1 Initial stage: initial adhesion and colonization
The initial stage of oral biofilm formation begins with pioneer species such as S. mutans adhering to the salivary pellicle, a unique oral structure composed of proteins (e.g., statherin, proline-rich proteins) and carbohydrates that selectively bind oral microorganisms. This attachment is usually mediated by adhesion factors, known as adhesins and biofilm-associated proteins such as Streptococcus gordonii SspB and S. mutans SpaP, which bacteria use to bind host-derived substances (Mirghani et al., 2022; Álvarez et al., 2022). Adhesion progresses from weak, reversible interactions (e.g., van der Waals forces) to stronger, irreversible binding as bacteria produce EPS, particularly glucans and fructans, which facilitate further colonization. Environmental factors such as pH, temperature, nutrient availability, and mechanical shear from salivary flow can also influence adhesion stability (Álvarez et al., 2022; Whittaker et al., 1996). Moreover, early colonizers can facilitate the recruitment of secondary species by exposing hidden receptors via sialidase and releasing signaling molecules such as competence-stimulating peptide (CSP) and autoinducer-2 (AI-2), thereby promoting increasingly community complexity (Jakubovics, 2015).
2.2.2 Developmental stage: formation and diversity of bacterial communities
Following initial adhesion, biofilms undergo substantial structural and compositional changes as early colonizers proliferate and new microbial species are incorporated, increasing complexity and heterogeneity. Bacteria secrete EPS, which provide structural scaffold that facilitates interspecies interactions, including nutrient sharing and metabolic cross-feeding, thereby promoting microbial diversity (Cugini et al., 2019; Serrage et al., 2021; Koo et al., 2009). Pioneer colonizers like Streptococcus and Actinomyces contribute to this architecture by producing EPS and surface adhesions, enabling the attachment of secondary colonizers (Klein et al., 2015; Dige et al., 2009; de Oliveira et al., 2020). Environmental factors, including salivary composition and dietary habits, influence community development and diversity (Simon-Soro et al., 2022). For instance, short-term juice consumption has been reported to negatively affect the microbiota (Sardaro et al., 2025).
Traditional culture-based methods detect only a fraction of oral bacteria, many of which are non-culturable under standard conditions. Culture-independent techniques, such as 16S rRNA sequencing and metagenomics, have revealed a much broader microbial landscape, uncovering previously unknown phyla with potential roles in health and disease. This distinction between culturable and unculturable taxa highlights the complexity of oral microbiome and underscores the need for comprehensive analytical approaches.
As biofilms mature and become diverse, their intricate 3D architecture and protective EPS matrix confer increased resistance to antimicrobial agents and host immunity. Notably, microbial diversity itself has been associated with clinical outcomes—higher oral diversity correlates with significantly reduced all-cause mortality (Yu et al., 2024; Rudney et al., 2003). Interspecies interactions, including both cooperation and competition, further biofilms development and function. Certain probiotic strains, such as Lactobacillus plantarum and Streptococcus salivarius K12, play regulatory roles by modulating microbial composition and behavior. For example, S. salivarius K12, suppresses cariogenic biofilm formation by downregulating S. mutans glucosyltransferase genes (gtfB, gtfC, gtfD), thereby interfering with EPS synthesis and biofilm stability (Lee et al., 2021; Kim and Yoo, 2023; Ahn et al., 2018).
2.2.3 Stable stage: stability and maintenance of the physiological state of oral biofilms
The stable stage marks the maturation of oral biofilms into structurally resilient, metabolically active communities capable of enduring environmental stress while supporting diverse microbial populations within spatially distinct microenvironments (Maier, 2021). This stability is maintained through synergistic interactions among key factors, including the EPS matrix, microbial metabolism, and intercellular signaling pathways (Cugini et al., 2019; Panlilio and Rice, 2021). The dense EPS framework serves as a physical barrier that impedes antibiotic penetration and promotes antimicrobial tolerance (Cugini et al., 2019; Panlilio and Rice, 2021). Concurrently, bacterial coordination—through QS and stress response systems—enhances structural integrity and collective resistance. A deeper understanding of the mechanisms governing oral biofilm stability and resilience is essential for developing targeted strategies to prevent and manage biofilm-associated oral diseases.
2.2.4 Dispersion stage: biofilm dispersal and initiation of new colonization
The dispersion stage marks the final phase of the oral biofilm lifecycle, during which bacterial detach from mature biofilms and colonize new surfaces, initiating subsequent rounds of biofilm formation (Rumbaugh and Sauer, 2020). This stage is crucial for the propagation and persistence of microbial communities and plays a central role in the recurrence of oral infections.
Biofilm dispersion is triggered by various environmental and endogenous cues, including nutrient depletion, pH changes, accumulation of metabolic waste, and QS signals (Teschler et al., 2022). To facilitate release from the matrix, bacteria deploy mechanisms such as enzymatic degradation of EPS (e.g., glycoside hydrolases, DNases, and proteases), altered expression of adhesion molecules, and enhanced motility (Wang et al., 2023). Notably, dispersed cells often display increased virulence and antibiotic tolerance, posing significant challenges for therapeutic intervention.
As a biological transition between biofilm stability and renewed colonization, dispersion completes the biofilm lifecycle. Elucidating the regulatory mechanisms governing this process not only deepens our understanding of oral microbial ecology but also offers potential targets for disrupting biofilm persistence and transmission.
2.3 Persistence of oral biofilms
The mechanisms underlying biofilm formation—such as EPS production, spatial structuring, and interspecies communications—are not only essential for initial community establishment, but also play a pivotal role in the long-term persistence and resistance of oral biofilms. Understanding this continuum from formation to persistence is critical for identifying points of therapeutic vulnerability.
2.3.1 Adaptability of oral biofilms to environmental changes
Oral biofilms exhibit remarkable adaptability to the ever-changing conditions of the oral cavity through metabolic reprogramming, gene regulation, and interspecies communication. S.mutans, a key cariogenic species, responds to oxidative stress via ActA-mediated acetylation of PykF, which reconfigures central carbon metabolism and enhances bacterial survival. In acidic environments, S. mutans upregulates proton pumps and aciduric enzymes to maintain intracellular pH homeostasis (Ma et al., 2024; Dashper and Reynolds, 1992; Sekiya et al., 2019). Conversely, S. gordonii produces hydrogen peroxide to suppress competitors while simultaneously enhancing its own antioxidant defenses (Zheng et al., 2011; Jakubovics et al., 2002).
Under nutrient-limited conditions, S. mutans modulates its phosphotransferase system to optimize carbohydrate uptake and activates gluconeogenesis to sustain energy production and promote exopolysaccharide synthesis (Vadeboncoeur and Pelletier, 1997; Zeng et al., 2022). Interspecies signaling further reinforces collective resilience: QS coordinates population-wide gene expression in response to the environmental stimuli, while cyclic dinucleotide signaling (e.g., c-di-AMP) regulates stress-response pathways and promotes biofilm stability (Peng et al., 2016; Gürsoy et al., 2017).
These adaptive mechanisms are interconnected—metabolic and transcriptional shifts support bacterial survival while simultaneously enhancing extracellular matrix production, which reinforces biofilm architecture and buffers against environmental stressors (Costa et al., 2023). Collectively, these mechanisms clarify how oral biofilms maintain persistence and structural integrity under environmental challenges.
2.3.2 Interactions between oral biofilms and host immune system
Interactions between oral biofilms and the host immune system significantly affect oral health (Lang et al., 2010). Commensal bacteria within biofilms, such as S. sanguinis, a key health-associated colonizer, contribute to immune homeostasis by eliciting minimal pro-inflammatory responses and suppressing inflammation induced by periodontal pathogens (Nobbs and Kreth, 2019). In contrast, pathogenic species such as P. gingivalis and F. nucleatum can impair host immunity. P. gingivalis secretes gingipains that degrade a wide range of host proteins, including cytokines and complement proteins, thereby impairing immune surveillance. F. nucleatum releases outer membrane vesicles that activate pro-inflammatory signaling pathway, exacerbating immune responses (Hočevar et al., 2020; Chen et al., 2022). The extracellular matrix acts as a physical barrier, protecting embedded bacteria from immune cell attacks (Karygianni et al., 2020). Additionally, certain bacteria can evade immune detection; for example, T. denticola can alter its surface antigens and actively suppress host immune responses to avoid detection and clearance (Jo et al., 2014; Hajishengallis, 2014; Dashper et al., 2011). These intricate interactions, shaped by biofilm composition and host immune status, influence biofilm stability and persistence. A better understanding of these mechanisms is crucial for developing innovative therapies that selectively target pathogenic biofilms while preserving beneficial components of the oral microbiota.
2.3.3 Antibiotic resistance mechanisms of biofilms
The antibiotic resistance of oral biofilms presents a significant challenge in the treatment of periodontal diseases and dental caries. Beyond serving as a structural scaffold, the EPS matrix functions as a physical and chemical barrier, limiting antibiotic diffusion and reducing antimicrobial efficacy (Cugini et al., 2019; Panlilio and Rice, 2021; Simon-Soro et al., 2022). For instance, the penetration of chlorhexidine into S. mutans biofilms is significantly reduced; even following apparent disinfection, residual biofilm structure facilitates secondary adhesion and reformation (Takenaka et al., 2016). Certain bacteria, such as Streptococcus anginosus and Lactobacillus salivarius, can reversibly enter a metabolically dormant state, enhancing survival under stress and reducing antibiotic susceptibility (Chávez de Paz et al., 2008; Suppiger et al., 2020). Furthermore, in response to antimicrobial exposure, bacteria within biofilms may also undergo phenotypic shifts, including the upregulation of efflux pumps that actively expel antibiotics and reduce intracellular concentrations (Alav et al., 2018; Sionov and Steinberg, 2022; Cieplik et al., 2019). Moreover, intercellular communications, particularly via QS system, plays a crucial role in resistance. S. mutans, for instance, activates the CSP-ComDE system to promote the formation of multidrug-resistant persister cells, thereby enhancing biofilm persistence and resistance (Leung et al., 2015).
2.4 Oral biofilms in health and diseases
2.4.1 Association between oral biofilms and oral health
Biofilms play a dual role in oral health. They are not inherently pathogenic but function as dynamic ecological communities essential for maintaining oral homeostasis. When dominated by commensal bacteria in healthy individuals, oral biofilms act as a barrier against external pathogens and stimuli, thereby contributing to a stable oral ecosystem. In this state, they modulate host immune responses, promote immune tolerance, and prevent excessive inflammation. This beneficial relationship reflects the concept of oral eubiosis, wherein balanced microbial interactions support tissue integrity and resist microbial dysbiosis (Santacroce et al., 2023; Zanetta et al., 2025). Beneficial species such as Weissella cibaria and members of genus Lactobacillus contribute to oral health by producing antimicrobial compounds, downregulating virulence genes such as gtf B, and preventing the adhesion of pathogens like S. mutans (Zanetta et al., 2025; Kang et al., 2023; Giordani et al., 2021). However, under certain conditions, this balanced environment may shift. When pathogenic bacteria become dominant, biofilms can transition into a dysbiotic state, promoting oral diseases, contributing to systemic disorders, and fostering antimicrobial resistance (Kurtzman et al., 2022; Yumoto et al., 2019).
2.4.2 Association between oral biofilms and systemic diseases
Numerous studies have demonstrated a strong association between oral health, particularly the oral microenvironment, and overall systemic health. Bacteria involved in oral diseases can translocate into the bloodstream, triggering systemic inflammation and contributing to the onset of various related conditions (Kurtzman et al., 2022). For instance, Xiong et al. confirmed in animal models that gingipains produced by P. gingivalis induce insulin resistance by proteolytically degrading insulin receptors, promoting diabetes development (Liu et al., 2024). P. gingivalis has also been implicated in the pathogenesis of Alzheimer’s disease by inducing tau degradation (leading to impaired microtubule stability and neuronal dysfunction) and promoting Aβ1–42 deposition (a key component of amyloid plaques) via gingipains, thereby driving neurodegeneration (Dominy et al., 2019; Loughman et al., 2023).
The spirochete T. denticola has been shown to contribute directly to the progression of cardiovascular diseases, such as atherosclerosis (Chukkapalli et al., 2014). Additionally, bacteria present in oral biofilms including Streptococcus pneumoniae, Prevotella spp., and Veillonella spp. can reach the lower respiratory tract through microaspiration or inhalation, especially in elderly or immunocompromised individuals, thereby increasing the risk of pneumonia and chronic obstructive pulmonary disease (Pu et al., 2020; Mammen et al., 2020; Imai et al., 2021). Furthermore, T. denticola, Tannerella forsythia, and Prevotella intermedia have been shown association with chronic kidney disease by inducing the release of pro-inflammatory factors (e.g., IL-1β, TNF-α, IL-6, IL-17), which exacerbate renal inflammation, immune dysregulation, and renal endothelial damage (Li et al., 2021; Pontes and Chikte, 2020).
2.5 Emerging strategies for oral diseases based on oral biofilms
The emergence of antibiotic resistance in oral diseases has prompted the development of alternative treatment strategies. Recent studies have highlighted various promising approaches—including natural products, probiotics, biosurfactants, nanomaterials, enzymes, and biomolecules—that primarily function through three key mechanisms: direct anti-biofilm activity, biological immunomodulation, and oral microbiome balance regulation. These strategies offer comprehensive solutions for biofilm-associated oral diseases.
2.5.1 Direct anti-biofilm activity
Antibiofilm strategies have become an active area of research, with diverse agents—such as antimicrobial peptides, natural compounds, biosurfactants, and nanoparticles—targeting different stages of biofilm development or specific components like EPS and extracellular nucleic acids. Recent studies have identified extracellular RNA (eRNA) as a key component of eDNA networks within biofilms formed by pathogens such as Pseudomonas aeruginosa, streptococci, and Staphylococcus aureus (Lee et al., 2020; Chiba et al., 2022; Mugunthan et al., 2023). Although eRNA is generally unstable and its structural role remains incompletely understood, emerging evidence suggests that it may serve as a promising target for disrupting oral biofilm formation (Mugunthan et al., 2023). For example, Aronia melanocarpa extracts significantly inhibit initial biofilm development by degrading eRNA in oral streptococcal biofilms (Lee et al., 2020).
Traditional Chinese medicine also exhibits anti-biofilm activity. For example, Paeoniae Radix Alba exhibits inhibitory effects against S. mutans biofilms, with albiflorin identified as its active constituent (Liu et al., 2025). Additionally, natural products such as stem extracts of Rhamnus prinoides (gesho) have been found to prevent biofilm formation in co-culture of S. mutans and Candida albicans (Campbell et al., 2020). New biosurfactants like rhamnolipids have demonstrated efficacy against biofilms formed by pathogenic bacteria such as Aggregatibacter actinomycetemcomitans Y4 (Yamasaki et al., 2020). Furthermore, nanoparticle-mediated treatments are emerging as innovative approaches, capable of generating of reactive oxygen species under acidic conditions to degrade biofilm matrix and eliminate pathogens like S. mutans (Benoit et al., 2019; Hu et al., 2019).
2.5.2 Biological immunomodulation
Biological immunomodulation represents another emerging therapeutic strategy for oral diseases, functioning through multiple mechanisms to inhibit biofilm formation and restore oral homeostasis. Weissella cibaria has been shown to effectively suppress biofilm formation by various species, likely through competitive inhibition and downregulation of pro-inflammatory pathways (Kang et al., 2023). Similarly, probiotic bacteria such as Lactobacillus and Bifidobacterium spp. contribute to oral health by enhancing the production of anti-inflammatory cytokines (Mahdizade Ari et al., 2024). Beyond probiotics, certain immunomodulatory agents, such as D-galactose, have demonstrated potential in preventing pathogenic bacterial biofilms while promoting the growth of commensal streptococci (Ryu et al., 2020). Incorporating immunomodulators into oral healthcare strategies holds significant promise for the prevention and management of biofilm-associated oral diseases.
2.5.3 Oral microbiome balance regulation
Oral biofilms are dynamic ecosystems that can exert both beneficial and detrimental effects; therefore, complete eradication is neither feasible nor desirable. Instead, modulating the oral microbiome toward a more health-promoting state is a promising strategy. For example, probiotics, like Lactobacillus species, can effectively suppress inflammation-associated pathogenic biofilms while promoting oral microbial balance (Ng et al., 2019). Although excessive level of short-chain fatty acids (SCFAs)—metabolic byproducts of the oral microbiota—are usually associated with oral dysbiosis, growing evidence highlights their beneficial roles. SCFAs can inhibit pathogens like S. gordonii by disrupting biofilm formation and suppressing virulence expression, and they also help alleviate chronic inflammation and contribute to a more balanced microbiome (Park et al., 2021; Leonov et al., 2023).
Natural compounds such as xylitol, Aronia melanocarpa, and Rhamnus prinoides have also demonstrated the potential to support beneficial bacterial growth and enhance the oral microbiome homeostasis (Lee et al., 2020; Campbell et al., 2020; Teixeira Essenfelder et al., 2019). Furthermore, targeted interference with biofilm signaling molecules, such as AHLs in QS system, offers a means of selectively modulating microbial composition and suppressing pathogenic biofilm formation (Ahn et al., 2018; Parga et al., 2023). Collectively, these approaches facilitate oral microbial re-equilibrium and provide innovative strategies for the prevention and long-term management of biofilm-associated oral diseases (Figure 2).
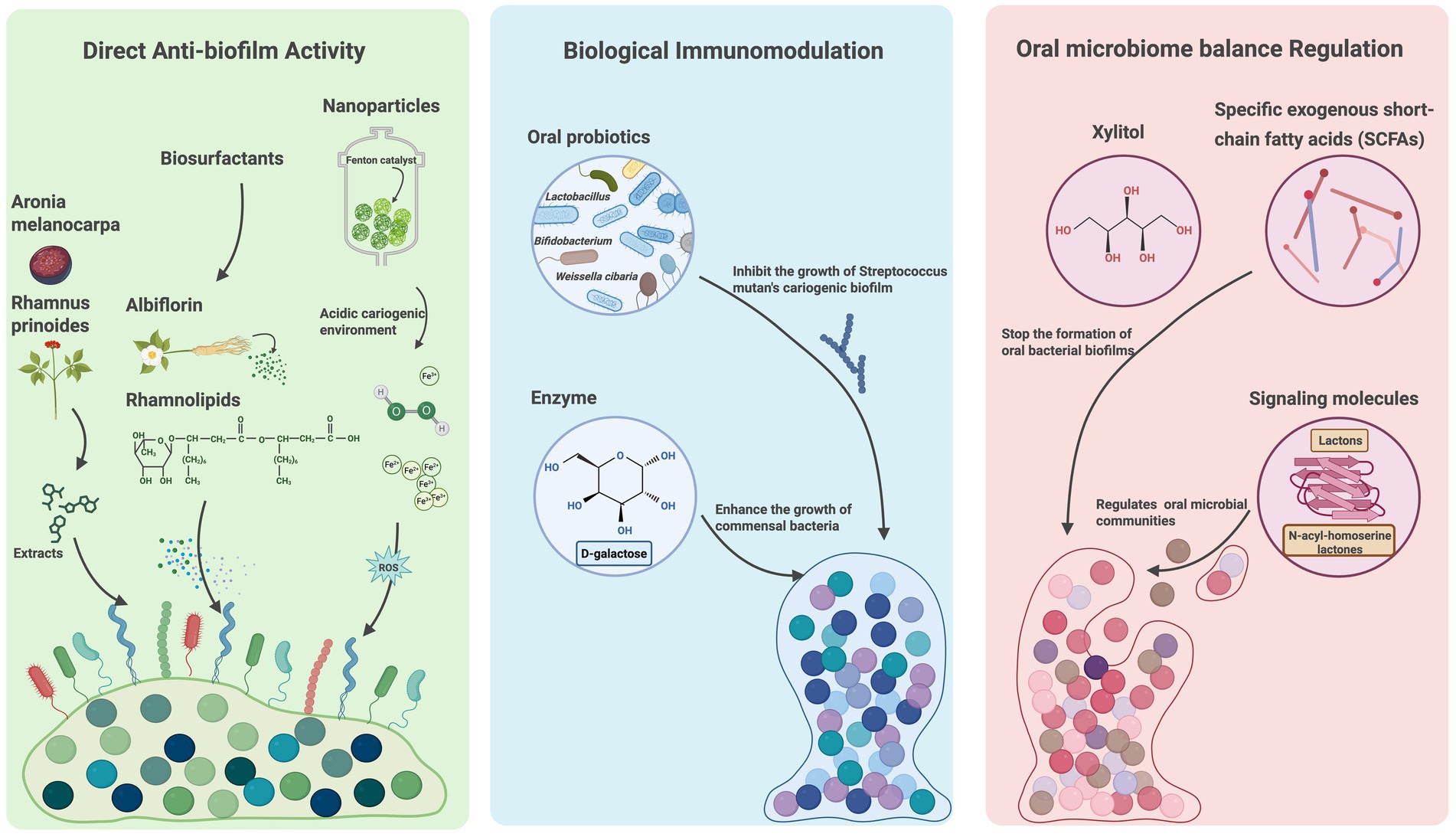
Figure 2. Emerging strategies for oral diseases based on oral biofilms. Emerging treatment strategies targeting oral biofilms are classified into direct anti-biofilm activity, biological immunomodulation, oral microbiome balance regulation based on the mechanisms. The treatment approaches include natural products, probiotics, biosurfactants, nanomaterials, enzymes and biomolecules. Created with BioRender.com.
2.6 Future direction of treatment strategies
In recent years, emerging therapeutic approaches increasingly emphasize selective targeting of pathogenic biofilms while preserving beneficial commensals—a paradigm shift from traditional broad-spectrum antimicrobials, which often disrupt oral microbial homeostasis and potentially leading to secondary infections or the emergence of resistant strains.
To address this, novel approaches have been proposed. For example, certain natural compounds and antimicrobial peptides can specifically inhibit pathogenic bacteria by recognizing distinct surface structures or metabolic features or downregulating the expression of gtf genes in S. mutans, thereby disrupting its extracellular polysaccharide synthesis and biofilm formation without affecting non-cariogenic streptococci (Xu et al., 2012). Similarly, probiotics such as S. salivarius K12 exhibit selective suppression of S. mutans colonization through competitive exclusion and modulating local microenvironment, indirectly supporting the growth of beneficial species (Begić et al., 2023). Additionally, QS-based interventions further enable disruption of pathogenic signaling networks without exerting bactericidal pressure, offering a potential route to disarm pathogenic biofilms while maintaining microbial homeostasis. These strategies emphasize regulation over eradication, aiming to restore a balanced and health-associated oral microbiome.
3 Conclusion
Oral biofilms, complex assemblages of microorganisms and their byproducts, play a pivotal role in both oral and systemic health. The formation and maturation of oral biofilms is a dynamic, multistage process, resulting in structurally intricate ecosystems that enable microbial survival, interaction, and adaptation. Consequently, oral biofilms can exert either protective or pathogenic effects. Their persistence and resistance to the environmental stressors have long challenged researchers and clinicians. Traditional strategies aimed at non-selective eradication are increasingly regarded as suboptimal. In contrast, emerging approaches emphasize modulation—shifting the composition and activity of biofilms toward a beneficial, health-promoting state. Advances in microbial and molecular technologies have deepened our understanding of oral microbial ecology, paving the way for more precise and sustainable interventions. As research in this field accelerates, growing attention is paid to the dualistic roles of biofilms in maintaining homeostasis and promoting disease. Future efforts, guided by multidisciplinary insights, should prioritize the targeted regulation of biofilms to foster eubiosis, enabling effective prevention and management of oral diseases while preserving microbial balance.
Author contributions
CL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. ZW: Conceptualization, Writing - review & editing. ZL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. XS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. MX: Writing – original draft, Writing – review & editing, Formal analysis, Validation, Funding acquisition. YX: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82072733), the Central Government Guides Local Science and Technology Development Fund of Liaoning Province (2023JH6/100100012), and the Joint Funding Program of the Department of Science and Technology of Liaoning Province (2023-MSLH-136).
Acknowledgments
We thank BioRender (https://biorender.com/) for providing the tool to create schematic figures in this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CSP, Competence-stimulating peptide; AI-2, Autoinducer-2; AHLs, N-acyl-homoserine lactones; EPS, Extracellular polymeric substances; 3D, Three-dimensional; eRNA, Extracellular RNA; eDNA, Extracellular DNA; QS, Quorum-sensing.
References
Ahn, K. B., Baik, J. E., Park, O. J., Yun, C. H., and Han, S. H. (2018). Lactobacillus plantarum lipoteichoic acid inhibits biofilm formation of Streptococcus mutans. PLoS One 13:e0192694. doi: 10.1371/journal.pone.0192694
Ahn, S. J., Wen, Z. T., and Burne, R. A. (2007). Effects of oxygen on virulence traits of Streptococcus mutans. J. Bacteriol. 189, 8519–8527. doi: 10.1128/JB.01180-07
Alav, I., Sutton, J. M., and Rahman, K. M. (2018). Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 73, 2003–2020. doi: 10.1093/jac/dky042
Álvarez, S., Leiva-Sabadini, C., Schuh, C. M. A. P., and Aguayo, S. (2022). Bacterial adhesion to collagens: implications for biofilm formation and disease progression in the oral cavity. Crit. Rev. Microbiol. 48, 83–95. doi: 10.1080/1040841X.2021.1944054
Asahi, Y., Noiri, Y., Igarashi, J., Asai, H., Suga, H., and Ebisu, S. (2010). Effects of N-acyl homoserine lactone analogues on Porphyromonas gingivalis biofilm formation. J. Periodontal Res. 45, 255–261. doi: 10.1111/j.1600-0765.2009.01228.x
Begić, G., Badovinac, I. J., Karleuša, L., Kralik, K., Cvijanovic Peloza, O., Kuiš, D., et al. (2023). Streptococcus salivarius as an important factor in dental biofilm homeostasis: influence on Streptococcus mutans and Aggregatibacter actinomycetemcomitans in mixed biofilm. IJMS 24:7249. doi: 10.3390/ijms24087249
Bell, R. D., Cann, E. A., Mishra, B., Valencia, M., Zhang, Q., Huang, M., et al. (2024). Staphyloccocus aureus biofilm, in absence of planktonic bacteria, produces factors that activate counterbalancing inflammatory and immune-suppressive genes in human monocytes. J. Orthop. Res. 42, 2582–2592. doi: 10.1002/jor.25919
Benoit, D. S. W., Sims, K. R., and Fraser, D. (2019). Nanoparticles for oral biofilm treatments. ACS Nano 13, 4869–4875. doi: 10.1021/acsnano.9b02816
Bjarnsholt, T., Buhlin, K., Dufrêne, Y. F., Gomelsky, M., Moroni, A., Ramstedt, M., et al. (2018). Biofilm formation – what we can learn from recent developments. J. Intern. Med. 284, 332–345. doi: 10.1111/joim.12782
Bowen, W. H., Burne, R. A., Wu, H., and Koo, H. (2018). Oral biofilms: pathogens, matrix and polymicrobial interactions in microenvironments. Trends Microbiol. 26, 229–242. doi: 10.1016/j.tim.2017.09.008
Campbell, M., Fathi, R., Cheng, S. Y., Ho, A., and Gilbert, E. S. (2020). Rhamnus prinoides (gesho) stem extract prevents co-culture biofilm formation by Streptococcus mutans and Candida albicans. Lett. Appl. Microbiol. 71, 294–302. doi: 10.1111/lam.13307
Chávez de Paz, L. E., Hamilton, I. R., and Svensäter, G. (2008). Oral bacteria in biofilms exhibit slow reactivation from nutrient deprivation. Microbiology 154, 1927–1938. doi: 10.1099/mic.0.2008/016576-0
Chen, G., Sun, Q., Cai, Q., and Zhou, H. (2022). Outer membrane vesicles from Fusobacterium nucleatum switch M0-like macrophages toward the M1 phenotype to destroy periodontal tissues in mice. Front. Microbiol. 13:815638. doi: 10.3389/fmicb.2022.815638
Chiba, A., Seki, M., Suzuki, Y., Kinjo, Y., Mizunoe, Y., and Sugimoto, S. (2022). Staphylococcus aureus utilizes environmental RNA as a building material in specific polysaccharide-dependent biofilms. npj Biofilms Microbiomes 8, 1–10. doi: 10.1038/s41522-022-00278-z
Chukkapalli, S. S., Rivera, M. F., Velsko, I. M., Lee, J. Y., Chen, H., Zheng, D., et al. (2014). Invasion of oral and aortic tissues by oral spirochete Treponema denticola in Apo E−/− mice causally links periodontal disease and atherosclerosis. Infect. Immun. 82, 1959–1967. doi: 10.1128/IAI.01511-14
Cieplik, F., Jakubovics, N. S., Buchalla, W., Maisch, T., Hellwig, E., and Al-Ahmad, A. (2019). Resistance toward chlorhexidine in Oral Bacteria-is there cause for concern? Front. Microbiol. 10:587. doi: 10.3389/fmicb.2019.00587
Costa, R. C., Bertolini, M., Oliveira, B. E. C., Nagay, B. E., Dini, C., Benso, B., et al. (2023). Polymicrobial biofilms related to dental implant diseases: unravelling the critical role of extracellular biofilm matrix. Crit. Rev. Microbiol. 49, 370–390. doi: 10.1080/1040841X.2022.2062219
Crabbé, A., Jensen, P. Ø., Bjarnsholt, T., and Coenye, T. (2019). Antimicrobial tolerance and metabolic adaptations in microbial biofilms. Trends Microbiol. 27, 850–863. doi: 10.1016/j.tim.2019.05.003
Cugini, C., Shanmugam, M., Landge, N., and Ramasubbu, N. (2019). The role of exopolysaccharides in Oral biofilms. J. Dent. Res. 98, 739–745. doi: 10.1177/0022034519845001
Dashper, S. G., and Reynolds, E. C. (1992). pH regulation by Streptococcus mutans. J. Dent. Res. 71, 1159–1165. doi: 10.1177/00220345920710050601
Dashper, S. G., Seers, C. A., Tan, K. H., and Reynolds, E. C. (2011). Virulence factors of the Oral spirochete Treponema denticola. J. Dent. Res. 90, 691–703. doi: 10.1177/0022034510385242
de Oliveira, R. V. D., Bonafé, F. S. S., Spolidorio, D. M. P., Koga-Ito, C. Y., de Farias, A. L., Kirker, K. R., et al. (2020). Streptococcus mutans and Actinomyces naeslundii interaction in dual-species biofilm. Microorganisms 8:194. doi: 10.3390/microorganisms8020194
Dige, I., Raarup, M. K., Nyengaard, J. R., Kilian, M., and Nyvad, B. (2009). Actinomyces naeslundii in initial dental biofilm formation. Microbiology (Reading, Engl.) 155, 2116–2126. doi: 10.1099/mic.0.027706-0
Dominy, S. S., Lynch, C., Ermini, F., Benedyk, M., Marczyk, A., Konradi, A., et al. (2019). Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5:eaau3333. doi: 10.1126/sciadv.aau3333
Giordani, B., Parolin, C., and Vitali, B. (2021). Lactobacilli as anti-biofilm strategy in Oral infectious diseases: a Mini-review. Front. Med. Technol. 3:769172. doi: 10.3389/fmedt.2021.769172
Gürsoy, U. K., Gürsoy, M., Könönen, E., and Sintim, H. O. (2017). Cyclic dinucleotides in oral bacteria and in oral biofilms. Front. Cell. Infect. Microbiol. 7:273. doi: 10.3389/fcimb.2017.00273
Hajishengallis, G. (2014). Immuno-microbial pathogenesis of periodontitis: keystones, pathobionts, and the host response. Trends Immunol. 35, 3–11. doi: 10.1016/j.it.2013.09.001
Hočevar, K., Vizovišek, M., Wong, A., Kozieł, J., Fonović, M., Potempa, B., et al. (2020). Proteolysis of gingival keratinocyte cell surface proteins by Gingipains secreted from Porphyromonas gingivalis – proteomic insights into mechanisms behind tissue damage in the diseased gingiva. Front. Microbiol. 11:722. doi: 10.3389/fmicb.2020.00722
Hu, C., Wang, L., Lin, Y., Liang, H., Zhou, S., Zheng, F., et al. (2019). Nanoparticles for the treatment of oral biofilms: current state, mechanisms, influencing factors, and prospects. Adv. Healthc. Mater. 8:1901301. doi: 10.1002/adhm.201901301
Huang, R., Li, M., and Gregory, R. L. (2011). Bacterial interactions in dental biofilm. Virulence 2, 435–444. doi: 10.4161/viru.2.5.16140
Imai, K., Iinuma, T., and Sato, S. (2021). Relationship between the oral cavity and respiratory diseases: aspiration of oral bacteria possibly contributes to the progression of lower airway inflammation. Jpn. Dent. Sci. Rev. 57, 224–230. doi: 10.1016/j.jdsr.2021.10.003
Jakubovics, N. S. (2015). Intermicrobial interactions as a driver for community composition and stratification of Oral biofilms. J. Mol. Biol. 427, 3662–3675. doi: 10.1016/j.jmb.2015.09.022
Jakubovics, N., and Kolenbrander, P. (2010). The road to ruin: the formation of disease-associated oral biofilms. Oral Dis. 16, 729–739. doi: 10.1111/j.1601-0825.2010.01701.x
Jakubovics, N. S., Smith, A. W., and Jenkinson, H. F. (2002). Oxidative stress tolerance is manganese (Mn2+) regulated in Streptococcus gordonii. Microbiology 148, 3255–3263. doi: 10.1099/00221287-148-10-3255
Jo, A., Baek, K. J., Shin, J. E., and Choi, Y. (2014). Mechanisms of IL-8 suppression by Treponema denticola in gingival epithelial cells. Immunol. Cell Biol. 92, 139–147. doi: 10.1038/icb.2013.80
Kang, C. E., Park, Y. J., Kim, J. H., Lee, N. K., and Paik, H. D. (2023). Probiotic Weissella cibaria displays antibacterial and anti-biofilm effect against cavity-causing Streptococcus mutans. Microb. Pathog. 180:106151. doi: 10.1016/j.micpath.2023.106151
Karygianni, L., Ren, Z., Koo, H., and Thurnheer, T. (2020). Biofilm Matrixome: extracellular components in structured microbial communities. Trends Microbiol. 28, 668–681. doi: 10.1016/j.tim.2020.03.016
Kim, H. J., and Yoo, H. J. (2023). Inhibitory effects of Streptococcus salivarius K12 on formation of cariogenic biofilm. J. Dent. Sci. 18, 65–72. doi: 10.1016/j.jds.2022.07.011
Kinane, D. F., Stathopoulou, P. G., and Papapanou, P. N. (2017). Periodontal diseases. Nat. Rev. Dis. Primers 3:17038. doi: 10.1038/nrdp.2017.38
Klein, M. I., Hwang, G., Santos, P. H. S., Campanella, O. H., and Koo, H. (2015). Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front. Cell. Infect. Microbiol. 5:10. doi: 10.3389/fcimb.2015.00010
Kolenbrander, P. E., Palmer, R. J., Periasamy, S., and Jakubovics, N. S. (2010). Oral multispecies biofilm development and the key role of cell–cell distance. Nat. Rev. Microbiol. 8, 471–480. doi: 10.1038/nrmicro2381
Kolenbrander, P. E., Palmer, R. J., Rickard, A. H., Jakubovics, N. S., Chalmers, N. I., and Diaz, P. I. (2006). Bacterial interactions and successions during plaque development. Periodontol 42, 47–79. doi: 10.1111/j.1600-0757.2006.00187.x
Kondo, T., Okawa, H., Hokugo, A., Shokeen, B., Sundberg, O., Zheng, Y., et al. (2022). Oral microbial extracellular DNA initiates periodontitis through gingival degradation by fibroblast-derived cathepsin K in mice. Commun. Biol. 5, 1–14. doi: 10.1038/s42003-022-03896-7
Koo, H., Xiao, J., and Klein, M. I. (2009). Extracellular polysaccharides matrix — An often forgotten virulence factor in Oral biofilm research. Int. J. Oral Sci. 1, 229–234. doi: 10.4248/IJOS.09086
Kreth, J., Merritt, J., Shi, W., and Qi, F. (2005). Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 187, 7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005
Kuboniwa, M., and Lamont, R. J. (2010). Subgingival biofilm formation. Periodontol. 52, 38–52. doi: 10.1111/j.1600-0757.2009.00311.x
Kumar, L., Patel, S. K. S., Kharga, K., Kumar, R., Kumar, P., Pandohee, J., et al. (2022). Molecular mechanisms and applications of N-acyl Homoserine lactone-mediated quorum sensing in Bacteria. Molecules 27:7584. doi: 10.3390/molecules27217584
Kurtzman, G. M., Horowitz, R. A., Johnson, R., Prestiano, R. A., and Klein, B. I. (2022). The systemic oral health connection: biofilms. Medicine 101:e30517. doi: 10.1097/MD.0000000000030517
Lang, M. L., Zhu, L., and Kreth, J. (2010). Keeping the bad bacteria in check: interactions of the host immune system with oral cavity biofilms. Endodontic Topics 22, 17–32. doi: 10.1111/j.1601-1546.2012.00278.x
Larsen, T., and Fiehn, N. E. (2017). Dental biofilm infections - an update. APMIS 125, 376–384. doi: 10.1111/apm.12688
Lee, D., Im, J., Park, D. H., Jeong, S., Park, M., Yoon, S., et al. (2021). Lactobacillus plantarum Lipoteichoic acids possess strain-specific regulatory effects on the biofilm formation of dental pathogenic Bacteria. Front. Microbiol. 12:758161. doi: 10.3389/fmicb.2021.758161
Lee, H., Oh, S. Y., and Hong, S. (2020). Inhibition of streptococcal biofilm formation by Aronia by extracellular RNA degradation. J. Sci. Food Agric. 100, 1806–1811. doi: 10.1002/jsfa.10223
Leonov, G. E., Varaeva, Y. R., Livantsova, E. N., and Starodubova, A. V. (2023). The complicated relationship of short-chain fatty acids and Oral microbiome: a narrative review. Biomedicines 11:2749. doi: 10.3390/biomedicines11102749
Leung, V., Ajdic, D., Koyanagi, S., and Lévesque, C. M. (2015). The formation of Streptococcus mutans Persisters induced by the quorum-sensing peptide pheromone is affected by the LexA regulator. Armitage JP, editor. J. Bacteriol. 197, 1083–1094. doi: 10.1128/JB.02496-14
Li, L., Zhang, Y. L., Liu, X. Y., Meng, X., Zhao, R. Q., Ou, L. L., et al. (2021). Periodontitis exacerbates and promotes the progression of chronic kidney disease through Oral Flora, cytokines, and oxidative stress. Front. Microbiol. 12:656372. doi: 10.3389/fmicb.2021.656372
Liu, X., Yu, Y., Jiang, H., Wang, Y., Gao, Y., Xiao, L., et al. (2025). Screening of anti-biofilm compounds from Paeoniae Radix Alba based on oral biofilm biochromatography. Biomed. Chromatogr. 39:e70019. doi: 10.1002/bmc.70019
Liu, F., Zhu, B., An, Y., Zhou, Z., Xiong, P., Li, X., et al. (2024). Gingipain from Porphyromonas gingivalis causes insulin resistance by degrading insulin receptors through direct proteolytic effects. Int. J. Oral Sci. 16, 1–14. doi: 10.1038/s41368-024-00313-z
Loughman, A., Adler, C. J., and Macpherson, H. (2023). Unlocking modifiable risk factors for Alzheimer’s disease: does the Oral microbiome hold some of the keys? JAD 92, 1111–1129. doi: 10.3233/JAD-220760
Ma, Q., Li, J., Yu, S., Liu, Y., Zhou, J., Wang, X., et al. (2024). Act A-mediated Pyk F acetylation negatively regulates oxidative stress adaptability of Streptococcus mutans. MBio 15:e0183924. doi: 10.1128/mbio.01839-24
Mahdizade Ari, M., Mirkalantari, S., Darban-Sarokhalil, D., Darbandi, A., Razavi, S., and Talebi, M. (2024). Investigating the antimicrobial and anti-inflammatory effects of Lactobacillus and Bifidobacterium spp. on cariogenic and periodontitis pathogens. Front. Microbiol. 15:1383959. doi: 10.3389/fmicb.2024.1383959
Maier, B. (2021). How physical interactions shape bacterial biofilms. Annu. Rev. Biophys. 50, 401–417. doi: 10.1146/annurev-biophys-062920-063646
Mammen, M. J., Scannapieco, F. A., and Sethi, S. (2020). Oral-lung microbiome interactions in lung diseases. Periodontol. 83, 234–241. doi: 10.1111/prd.12301
Marsh, P. D. (2006). Dental plaque as a biofilm and a microbial community – implications for health and disease. BMC Oral Health 6:S14. doi: 10.1186/1472-6831-6-S1-S14
Marsh, P. D., and Zaura, E. (2017). Dental biofilm: ecological interactions in health and disease. J. Clin. Periodontol. 44, S12–S22. doi: 10.1111/jcpe.12679
Mirghani, R., Saba, T., Khaliq, H., Mitchell, J., Do, L., Chambi, L., et al. (2022). Biofilms: formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 8, 239–277. doi: 10.3934/microbiol.2022019
Montelongo-Jauregui, D., and Lopez-Ribot, J. L. (2018). Candida interactions with the Oral bacterial microbiota. JoF 4:122. doi: 10.3390/jof4040122
Mugunthan, S., Wong, L. L., Winnerdy, F. R., Summers, S., Bin Ismail, M. H., Foo, Y. H., et al. (2023). RNA is a key component of extracellular DNA networks in Pseudomonas aeruginosa biofilms. Nat. Commun. 14:7772. doi: 10.1038/s41467-023-43533-3
Murakami, S., Mealey, B. L., Mariotti, A., and Chapple, I. L. C. (2018). Dental plaque-induced gingival conditions. J. Periodontol. 89, S17–S27. doi: 10.1002/JPER.17-0095
Ng, H. M., Slakeski, N., Butler, C. A., Veith, P. D., Chen, Y. Y., Liu, S. W., et al. (2019). The role of Treponema denticola motility in synergistic biofilm formation with Porphyromonas gingivalis. Front. Cell. Infect. Microbiol. 9:432. doi: 10.3389/fcimb.2019.00432
Nobbs, A., and Kreth, J. (2019). Genetics of sanguinis-group streptococci in health and disease Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Braunstein M, Rood JI, editors. Microbiol. Spectr. 7:7.1.05. doi: 10.1128/microbiolspec.GPP3-0052-2018
Panlilio, H., and Rice, C. V. (2021). The role of extracellular DNA in the formation, architecture, stability, and treatment of bacterial biofilms. Biotechnol. Bioeng. 118, 2129–2141. doi: 10.1002/bit.27760
Parga, A., Muras, A., Otero-Casal, P., Arredondo, A., Soler-Ollé, A., Àlvarez, G., et al. (2023). The quorum quenching enzyme Aii 20J modifies in vitro periodontal biofilm formation. Front. Cell. Infect. Microbiol. 13:1118630. doi: 10.3389/fcimb.2023.1118630
Park, T., Im, J., Kim, A. R., Lee, D., Jeong, S., Yun, C. H., et al. (2021). Short-chain fatty acids inhibit the biofilm formation of Streptococcus gordonii through negative regulation of competence-stimulating peptide signaling pathway. J. Microbiol. 59, 1142–1149. doi: 10.1007/s12275-021-1576-8
Peng, X., Zhang, Y., Bai, G., Zhou, X., and Wu, H. (2016). Cyclic di-AMP mediates biofilm formation. Mol. Microbiol. 99, 945–959. doi: 10.1111/mmi.13277
Pontes, C. C., and Chikte, U. M. (2020). How oral infections can influence chronic kidney disease – a review of the literature. AJN 23, 130–139. doi: 10.21804/23-1-4110
Pu, C. Y., Seshadri, M., Manuballa, S., and Yendamuri, S. (2020). The oral microbiome and lung diseases. Curr. Oral Health Rep. 7, 79–86. doi: 10.1007/s40496-020-00259-1
Reichhardt, C., and Parsek, M. R. (2019). Confocal laser scanning microscopy for analysis of Pseudomonas aeruginosa biofilm architecture and matrix localization. Front. Microbiol. 10:677. doi: 10.3389/fmicb.2019.00677
Rudney, J. D., Pan, Y., and Chen, R. (2003). Streptococcal diversity in oral biofilms with respect to salivary function. Arch. Oral Biol. 48, 475–493. doi: 10.1016/S0003-9969(03)00043-8
Rumbaugh, K. P., and Sauer, K. (2020). Biofilm dispersion. Nat. Rev. Microbiol. 18, 571–586. doi: 10.1038/s41579-020-0385-0
Ryu, E. J., An, S. J., Sim, J., Sim, J., Lee, J., and Choi, B. K. (2020). Use of d-galactose to regulate biofilm growth of oral streptococci. Arch. Oral Biol. 111:104666. doi: 10.1016/j.archoralbio.2020.104666
Santacroce, L., Passarelli, P. C., Azzolino, D., Bottalico, L., Charitos, I. A., Cazzolla, A. P., et al. (2023). Oral microbiota in human health and disease: a perspective. Exp. Biol. Med. (Maywood) 248, 1288–1301. doi: 10.1177/15353702231187645
Sardaro, M. L. S., Grote, V., Baik, J., Atallah, M., Amato, K. R., and Ring, M. (2025). Effects of vegetable and fruit juicing on gut and Oral microbiome composition. Nutrients 17:458. doi: 10.3390/nu17030458
Sekiya, M., Izumisawa, S., Iwamoto-Kihara, A., Fan, Y., Shimoyama, Y., Sasaki, M., et al. (2019). Proton-pumping F-ATPase plays an important role in Streptococcus mutans under acidic conditions. Arch. Biochem. Biophys. 666, 46–51. doi: 10.1016/j.abb.2019.03.014
Serrage, H. J., Jepson, M. A., Rostami, N., Jakubovics, N. S., and Nobbs, A. H. (2021). Understanding the matrix: the role of extracellular DNA in Oral biofilms. Front. Oral Health 2:640129. doi: 10.3389/froh.2021.640129
Sharma, D. K., and Rajpurohit, Y. S. (2024). Multitasking functions of bacterial extracellular DNA in biofilms. J. Bacteriol. 206:e0000624. doi: 10.1128/jb.00006-24
Simon-Soro, A., Ren, Z., Krom, B. P., Hoogenkamp, M. A., Cabello-Yeves, P. J., Daniel, S. G., et al. (2022). Polymicrobial aggregates in human saliva build the Oral biofilm. MBio 13:e0013122. doi: 10.1128/mbio.00131-22
Sionov, R. V., and Steinberg, D. (2022). Targeting the holy triangle of quorum sensing, biofilm formation, and antibiotic resistance in pathogenic Bacteria. Microorganisms 10:1239. doi: 10.3390/microorganisms10061239
Suppiger, S., Astasov-Frauenhoffer, M., Schweizer, I., Waltimo, T., and Kulik, E. M. (2020). Tolerance and Persister formation in Oral streptococci. Antibiotics (Basel) 9:167. doi: 10.3390/antibiotics9040167
Takenaka, S., Oda, M., Domon, H., Wakamatsu, R., Ohsumi, T., Terao, Y., et al. Adverse influences of antimicrobial strategy against mature oral biofilm. In: Microbial biofilms-importance and applications [Internet]. Intech Open; (2016). Available online at: https://www.intechopen.com/chapters/50850
Teixeira Essenfelder, L., Gomes, A. A., Miquelutti, D., Da Silva, G. F., and Magalhães, M. L. B. (2019). Effect of xylitol on salivary β-glucosidase in humans. Eur. J. Oral Sci. 127, 472–475. doi: 10.1111/eos.12649
Teschler, J. K., Nadell, C. D., Drescher, K., and Yildiz, F. H. (2022). Mechanisms underlying Vibrio cholerae biofilm formation and dispersion. Ann. Rev. Microbiol. 76, 503–532. doi: 10.1146/annurev-micro-111021-053553
Vadeboncoeur, C., and Pelletier, M. (1997). The phosphoenolpyruvate: sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol. Rev. 19, 187–207. doi: 10.1111/j.1574-6976.1997.tb00297.x
Wang, S., Zhao, Y., Breslawec, A. P., Liang, T., Deng, Z., Kuperman, L. L., et al. (2023). Strategy to combat biofilms: a focus on biofilm dispersal enzymes. NPJ Biofilms Microbiomes 9:63. doi: 10.1038/s41522-023-00427-y
Whittaker, C. J., Klier, C. M., and Kolenbrander, P. E. (1996). Mechanisms of adhesion by oral bacteria. Ann. Rev. Microbiol. 50, 513–552. doi: 10.1146/annurev.micro.50.1.513
Xiao, J., Klein, M. I., Falsetta, M. L., Lu, B., Delahunty, C. M., Yates, J. R., et al. (2012). The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 8:e1002623. doi: 10.1371/journal.ppat.1002623
Xu, X., Zhou, X. D., and Wu, C. D. (2012). Tea catechin epigallocatechin gallate inhibits Streptococcus mutans biofilm formation by suppressing gtf genes. Arch. Oral Biol. 57, 678–683. doi: 10.1016/j.archoralbio.2011.10.021
Yamasaki, R., Kawano, A., Yoshioka, Y., and Ariyoshi, W. (2020). Rhamnolipids and surfactin inhibit the growth or formation of oral bacterial biofilm. BMC Microbiol. 20:358. doi: 10.1186/s12866-020-02034-9
Yu, J., Lin, B., Zhang, Z., Chen, W., Lv, W., and Zheng, L. (2024). Association between oral microbiome diversity and all-cause mortality: a longitudinal study of NHANES, 2009–2012. BMJ Open 14:e087288. doi: 10.1136/bmjopen-2024-087288
Yumoto, H., Hirota, K., Hirao, K., Ninomiya, M., Murakami, K., Fujii, H., et al. (2019). The pathogenic factors from Oral streptococci for systemic diseases. Int. J. Mol. Sci. 20:4571. doi: 10.3390/ijms20184571
Zanetta, P., De Giorgis, V., Barberis, E., Manfredi, M., Amoruso, A., Pane, M., et al. (2025). Lactobacillus probiotic cell-free supernatants and vitamin D influence interleukin-6 production and mitigate oral periodontopathogens-induced cytotoxicity in FaDu cells. Front. Microbiol. 16:1578267. doi: 10.3389/fmicb.2025.1578267
Zeng, L., Walker, A. R., Burne, R. A., and Taylor, Z. A. (2022). Glucose PTS modulates pyruvate metabolism, bacterial fitness, and microbial ecology in oral streptococci. bioRxiv. doi: 10.1101/2022.09.21.508965
Zheng, L., Itzek, A., Chen, Z., and Kreth, J. (2011). Environmental influences on competitive hydrogen peroxide production in Streptococcus gordonii. Appl. Environ. Microbiol. 77, 4318–4328. doi: 10.1128/AEM.00309-11
Keywords: oral biofilm, oral disease, microbiome, colony, structure, persistence, bacterial adhesion, regulation
Citation: Lv C, Wang Z, Li Z, Shi X, Xiao M and Xu Y (2025) Formation, architecture, and persistence of oral biofilms: recent scientific discoveries and new strategies for their regulation. Front. Microbiol. 16:1602962. doi: 10.3389/fmicb.2025.1602962
Edited by:
George Grant, Independent Researcher, Aberdeen, United KingdomReviewed by:
Marwan Mansoor Ali Mohammed, University of Sharjah, United Arab EmiratesBrandon W. Peterson, University Medical Center Groningen, Netherlands
Ali Al-Ahmad, University of Freiburg Medical Center, Germany
Dr. Vijetha Vishwanath, The University of Hong Kong, Hong KongSAR China
Copyright © 2025 Lv, Wang, Li, Shi, Xiao and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingming Xiao, eGlhb19taW5nX21pbmdAb3V0bG9vay5jb20=; Yan Xu, eWFueHVAY211LmVkdS5jbg==
†These authors have contributed equally to this work
 Chengyuan Lv1†
Chengyuan Lv1† Ziyi Wang
Ziyi Wang Zehui Li
Zehui Li