- 1Zhengzhou Tobacco Research Institute of China National Tobacco Corporation, Zhengzhou, China
- 2Inner Mongolia Kunming Cigarettes LLC, Hohhot, China
- 3State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan, China
- 4Institute of Agro-Products Processing and Nuclear Agricultural Technology, Hubei Academy of Agricultural Sciences, Wuhan, China
The combustion of excessive starch in tobacco leaves leads to more harmful substances, adversely affecting the sensory properties of tobacco and posing significant risks to human health. Therefore, there is an urgent need to develop specific amylases targeting tobacco starch to address these issues. In this study, 5 different α-amylase genes were selected for recombinant expression in Bacillus amyloliquefaciens BAX-5, and the amyA(LC) (derived from Bacillus amyloliquefaciens MK10163) was confirmed to be the optimal gene. Then, the α-amylase activity was further increased by screening host bacteria BAX-5 and signal peptides SP003 (derived from the dacB gene of Bacillus subtilis 168). Subsequently, the α-amylase properties were characterized, such as temperature tolerance, pH tolerance and metal ion. Through replacement of culture medium, the recombinant strain BAX-5/PT-17SP003amyA(LC) produced the maximum α-amylase activity of 904.91 IU/mL, which was about 4 times higher than that of the original culture medium. Finally, the α-amylase Amy (LC) was applied to the enzyme treatment of tobacco leaves, and the evaluation results showed that α-amylase Amy (LC) could play a positive role in reducing damage and enhancing quality of cigarettes. This research provides a novel enzymatic resource for the development of amylases, and it has enormous market potential and application value.
1 Introduction
Starch is one kind of abundant biological macromolecules in tobacco leaves, and the content of starch in flue-cured tobacco leaves can reach 40% (Chu et al., 2022). During the roasting of tobacco leaves, the high content of macromolecular substances (e.g., starch) often leads to incomplete combustion, releasing harmful compounds such as aromatic hydrocarbons and aldehydes, which are known to pose significant health risks (Ye et al., 2024; Tian et al., 2023). On the other hand, the high content of macromolecules can reduce the smoke properties and sensory qualities of tobacco, and the starch content have negative correlation with its total sensory evaluation score (Chen et al., 2017), which significantly affect quality of tobacco leaves (Yan et al., 2022). Therefore, reduction of the starch content in the tobacco has become a key issue to reduce the hazard and improve the quality (Yang et al., 2010).
There are many factors that can affect the starch content of tobacco leaf in the growth and cultivation stage. Several methods have been developed to reduce the starch content of tobacco (Yan et al., 2020). Through increase of baking time, the degradation degree of starch changes greatly (Sun et al., 2011). In recent years, application of microbial enzymes to degrade macromolecules has also been explored to improve tobacco quality and reduce the hazards, and the use of α-amylase to treat tobacco leaves has become the focus of the tobacco industry (Xing et al., 2023). However, present α-amylase used in tobacco are from commercial food enzymes, which are aimed to hydrolyze the starch in traditional cereals, and there are no enzymes specifically designed to degrade starch in tobacco leaves. Therefore, it is important to screen and optimize the amylases that target to degrade the starch in tobacco.
At present, Bacillus has become a general industrial microbial chassis cell, and nearly 60% of the enzyme products on the market are derived from Bacillus (Liu et al., 2023). Bacillus expression systems have strong protein secretion ability and high expression efficiency, which can rapidly secrete protease, amylase and lipase, and they also have good compatibility with foreign proteins (Cai et al., 2022). With the development of genetic engineering and molecular biology, Bacillus has been developed as the main expression system for recombinant proteins (Su et al., 2020; Mukherjee and Venkata Mohan, 2021). Signal peptides can guide target proteins into the correct secretory pathway during synthesis, thereby enhancing the efficiency of protein secretion (Kang et al., 2020). After screening signal peptides from 173 Bacillus subtilis strains, the signal peptide SPYpuA was found optimal for α-amylase production, and the fermentation activity was increased by 1.28-fold (Li et al., 2022). Consequently, the optimization of signal peptides has emerged as a crucial strategy for enhancing enzyme production (Miao et al., 2021).
Different Bacillus strains can significantly affect the expression of foreign proteins. Some native proteases secreted by Bacillus can degrade the foreign proteins, thereby interfering or even completely terminating the expression of target proteins. In addition, Bacillus host strains also synthesize a large number of redundant proteins (Yu et al., 2015), which will compete with the secretion of target proteins to reduce the expression level. At present, a variety of protease-deficient Bacillus host strains have been constructed to increase the production of target enzymes, including B. subtilis 168, Bacillus licheniformis WX-02, and B. amyloliquefaciens HZ-12 (Osamura et al., 2023; Wu et al., 1991; Wong et al., 1994; Wei et al., 2015; Chen et al., 2022). This study aims to construct a high-expression engineered strain of amylase through strain screening, gene mining, and optimization of signal peptides and host strains. Furthermore, the enzymatic properties of the amylase were characterized, and its application potential in tobacco leaves was evaluated. This study provides a novel enzymatic resource for the development of amylases, demonstrating significant market potential and application value.
2 Materials and methods
2.1 Enzymes and chemical reagents
In this study, the 1.1 × S4 Fidelity polymerase chain reaction (PCR) Mix was purchased from Beijing Genesand Biotech Co., Ltd. (Beijing, China). T4 DNA ligase, XbaI, BamHI, and SamI were obtained from TransGen Biotech Co., Ltd. (Beijing, China). The ClonExpress II One Step Cloning Kit were get from Nanjing Vazyme Biotech Co., Ltd. (Nanjing, China). The Gel Extraction Kit and Plasmid Mini Kit I were purchased from Omega Bio-tek Co., Ltd. (Norcross, USA). Other chemical reagents were sourced from China National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China).
2.2 Construction of the recombinant expression strains
The methodology for constructing the recombinant expression strain followed established protocols from previous research(Chen et al., 2022). In this study, the construction of recombinant expression strain BAX-5/PT-17amyE(WHC-115) was presented as an example. According to the gene sequence of amyE(WHC-115) in Bacillus subtilis WHC-115, primers amyE-F and amyE-R were designed, and restriction sites of XbaI and BamHI were added at both ends of the primers, respectively. The genomic DNA of B. subtilis WHC-115 was used as a template to amplify the amyE(WHC-115) gene. Then, the recovered PCR product and the PT-17 vector were digested by restriction enzymes BamHI and XbaI. After purification, the digested PCR product and plasmid were linked by T4 DNA ligase at 4°C for 6–12 h. The ligation product was transformed into E. coli DH5α competent cells, and the single colony was verified by PCR amplification and gene sequencing. Finally, the free expression plasmid PT-17amyE(WHC-115) was successfully constructed.
The free expression plasmid PT-17amyE(WHC-115) was mixed with the BAX-5 competent cells. The cells were cultured for 3 h after electro-transformation, and spread on LB solid medium containing 20 μg/mL tetracycline. Finally, the positive single colony was verified by PCR amplification and gene sequencing. The other recombinant expression strains were performed using the same method. All the strains and plasmids constructed in this study were shown in Table 1. All the primers in this study were shown in Supplementary Table S1.
2.3 Fermentation conditions of α-amylase
The strains were inoculated in LB liquid medium (10 g/L NaCl, 10 g/L tryptone, 5 g/L yeast extract, pH 7.2–7.4) as seed culture. The recombinant expression strains was added with 20 μg/mL of tetracycline in LB liquid medium. The seed cultures were incubated at 37°C and 180 r/min for 8–12 h. When the OD600 reached 3.5–4.0, the seed cultures (3%) were transferred into 50 mL fermentation medium (80 g/L tryptone, 25 g/L yeast extract, 5 g/L K2HPO4, and 6 g/L NH4Cl) at 37°C and 180 r/min for 48 h. In addition, the optimized components of the culture medium consisted of 65 g/L corn meal, 70 g/L soybean cake meal, 10 g/L NaCl, and 3 g/L CaCl2 for 52 h at 40°C and pH = 6, The volume of solution medium is 50 mL.
2.4 Detection of α-amylase activity
2.4.1 Standard curve preparation
Glucose standard solutions were prepared at final concentrations of 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8, and 2.0 mg/mL (total volume: 40 μL per concentration). To each solution, 60 μL of 3,5-dinitrosalicylic acid (DNS)(Ghose method) reagent was added, followed by incubation at 99.9°C for 2 min. The samples were then rapidly cooled to 4°C, diluted with 100 μL deionized water in a 96-well plate, and the absorbance at 540 nm was measured. A standard curve was generated by plotting glucose concentration against the corresponding optical density (OD) values.
2.4.2 Enzymatic activity assay
The reaction mixture contained: 10 μL phosphate buffer (45.23 g/L Na₂HPO₄·12H₂O, 8.07 g/L C₆H₈O₇·H₂O), 10 μL 4% (w/v) soluble starch solution and 20 μL crude enzyme extract. The mixture was incubated at 60°C for 5 min in a PCR thermocycler. Subsequently, 60 μL DNS reagent was added, and the samples were heated at 99.9°C for 2 min. After rapid cooling to 4°C, 100 μL deionized water was added, and absorbance was measured at 540 nm. Control: A blank was prepared by replacing the crude enzyme solution with 20 μL heat-inactivated enzyme, with all other steps identical.
2.4.3 Calculations
The amount of reducing sugar released was quantified using a glucose standard curve. Enzyme activity is defined as: under given reaction conditions, 1 mL of enzyme solution reacts to produce the equivalent of 1 μmol of glucose per minute, which is regarded as one unit of enzyme activity, expressed in IU/mL. The formula is as follows: suppose the standard curve is y = kx + b. Then the sample enzyme activity:
Ae - absorbance value of the enzyme solution reaction of the sample.
A0 -absorbance value of blank control.
Df - dilution of the enzyme solution of the sample.
Relative molecular weight of WF-glucose (180.16).
T-enzyme reaction time (5 min).
V - Enzyme volume (20 μL).
2.5 Enzyme characterization
After fermentation for 48 h, the fermentation broth was collected and centrifuged at a rotational speed of 12,000 r/min for 10 min. Then, 5 mL supernatant was taken into the 30 kDa ultrafiltration tube, which was centrifuged at 2000 r/min for 30 min to obtain the α-amylase liquid with higher concentration. The enzyme solution after ultrafiltration will be used for enzymatic properties analysis.
The diluted enzyme solution was subjected to reaction at 40, 50, 60, 70, 80, 90°C, respectively. After that, the enzyme activity of different treatment groups was detected. The enzyme activity was determined by DNS method to determine the optimal reaction temperature, except for the reaction temperature, the reaction system and reaction conditions are the same as the method for determining enzyme activity in 2.4 section.
The enzyme solution after dilution was placed in water bath at 30, 40, 50, 60, 70, 80, 90°C for 30 min. After that, the enzyme activity of different treatment groups were detected. The amylase activity was determined by DNS method, the method is the same as that for determining enzyme activity in 2.4 section in this article.
The enzyme solution was diluted in FeCl2, CaCl2, NaCl, MgCl2, KCl and CuCl2 with a metal ion concentration of 5 mmol/L, and then the diluents were left for 30 min. After that, the enzyme activity of different treatment groups were detected. Then the amylase activity was determined by DNS method (the enzyme diluents without metal ions were used as the control group). The determination method is the same as that for determining enzyme activity in section 2.4 in this article.
The enzyme solution was diluted in phosphate buffers at pH 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, respectively, and placed at room temperature for 30 min. The enzyme activity of the enzyme solutions at different pH buffers were detected by DNS method, the reaction system and reaction conditions are the same as the method for determining enzyme activity in 2.4 part.
The solutions mentioned above used for preparing solutions with different pH gradients are phosphate buffer solutions, they are the same as the phosphate buffer liquid used in the detection of amylase activity by DNS method in section 2.4, and the basic preparation method of this buffer solution has been mentioned in section 2.4 of this article.
2.6 Enzyme treatment and evaluation of tobacco samples
The fermentation broth was concentrated by ultrafiltration and sprayed on B4F tobacco leaves from Chongqing. The concentrated enzyme solution was sprayed with 7 U enzyme activity per gram of tobacco leaf. Then, the tobacco leaves were treated at 45°C and 75% humidity for 48 h, and the tobacco leaves treated with enzymes were inactivated at 100°C in the drying box for 30 s. The treated tobacco leaves were cut into shredded tobacco with a chopper, and the cut tobacco was evenly packed into the 84 mm empty smoke pipe. Then, seven experts authorized by the China National Tobacco Corporation evaluated the sensory quality of cigarettes.
The pyrolysis of tobacco ingredients was used by pyrolysis instrument, gas chromatograph and mass spectrometer (GC–MS). The tobacco leaves treated with enzymes were turned into powder through the blender mechanism, and 1 mg of powder was weighed into the pyrolysis tube. The pyrolysis tube was then sent into a pyrolyzer, each component entered the GC–MS system through the transmission line. The sample was heated to 900°C at a rate of 50°C/s and maintained at this temperature for 5 s. The column model used was DB-WAX capillary column (60 m × 0.25 mm × 0.25 μm), and the carrier gas was helium with a flow rate of 1.5 mL/min. The injection port temperature was 250°C. The column temperature program was as follows: initial temperature of 40°C held for 1 min, and then ramped at 5°C/min to 200°C and the temperature then rose to 250°C at a rate of 10°C/min and remained at this temperature for 20 min.
2.7 Statistical analysis
SPSS 26.0 was used for statistical analysis, including calculation of the mean and deviation values. Origin 2017 and Prism 8.0 was used for data processing and making histograms.
3 Results
3.1 Expression of different α-amylase genes and analysis of the α-amylase Amy (LC)
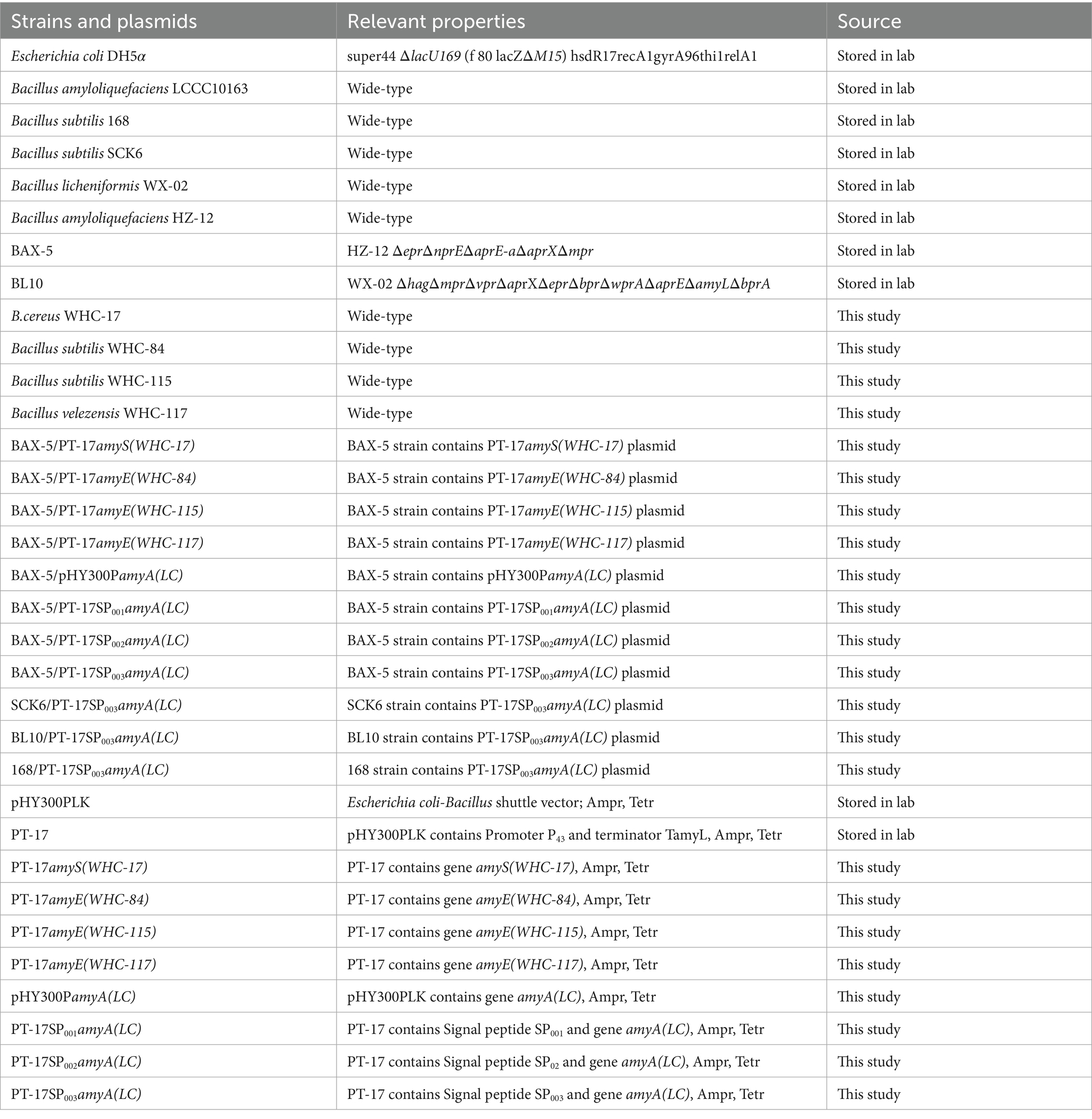
Research has found that Bacillus spp. is an important source of amylase genes (Ye et al., 2024). Therefore, based on the existing Bacillus spp. in the laboratory, we successfully amplified five different alpha-amylase gene fragments from wild strains B. cereus WHC-17, B. subtilis WHC-84, B. subtilis WHC-115, B. velezensis WHC-117, and B. amyloliquefaciens MK10163, respectively. Finally, 5 different α-amylase genes from WHC-17, WHC-84, WHC-115, WHC-117 and MK10163 were successfully amplified (Gel electrophoresis images of gene fragments are shown in the Supplementary Figure S1). The corresponding α-amylase gene fragments were labeled as amyS(WHC-17), amyE(WHC-84), amyE(WHC-115), amyE(WHC-117), and amyA(LC). The gene fragments were further sequenced by Beijing Tsingke Biotechnology Co., Ltd. to obtain the gene sequence information (shown in Supplementary material 1).
Subsequently, the α-amylase gene fragments of amyS(WHC-17), amyE(WHC-84), amyE(WHC-115), amyE(WHC-117) and amyA(LC) were used to construct 5 expression plasmids, which were transformed into BAX-5 to obtain the recombinant expression strains, including BAX-5/PT-17amyS(WHC-17), BAX-5/PT-17amyE(WHC-84), BAX-5/PT-17amyE(WHC-115), BAX-5/PT-17amyE(WHC-117), and BAX-5/pHY300PamyA(LC). Through further fermentation analysis, the α-amylase activities of the engineered strains were shown in Figure 1B. The α-amylase activity (96.09 IU/mL) of BAX-5/pHY300PamyA(LC) was much higher than that of other engineered strains. The enzyme activity of strain BAX-5/PT-17amyS(WHC-17) and BAX-5/PT-17amyE(WHC-84) were only 34.64 IU/mL and 32.15 IU/mL, respectively, which were slightly higher than that of engineered strain BAX-5/PT-17 (22.85 IU/mL). The enzyme activity of BAX-5/PT-17amyE(WHC-117) was lower than that of BAX-5/PT-17, and strain BAX-5/PT-17amyE(WHC-115) even lost α-amylase activity. Therefore, the α-amylase gene amyA(LC) was selected for further analysis and expression optimization.
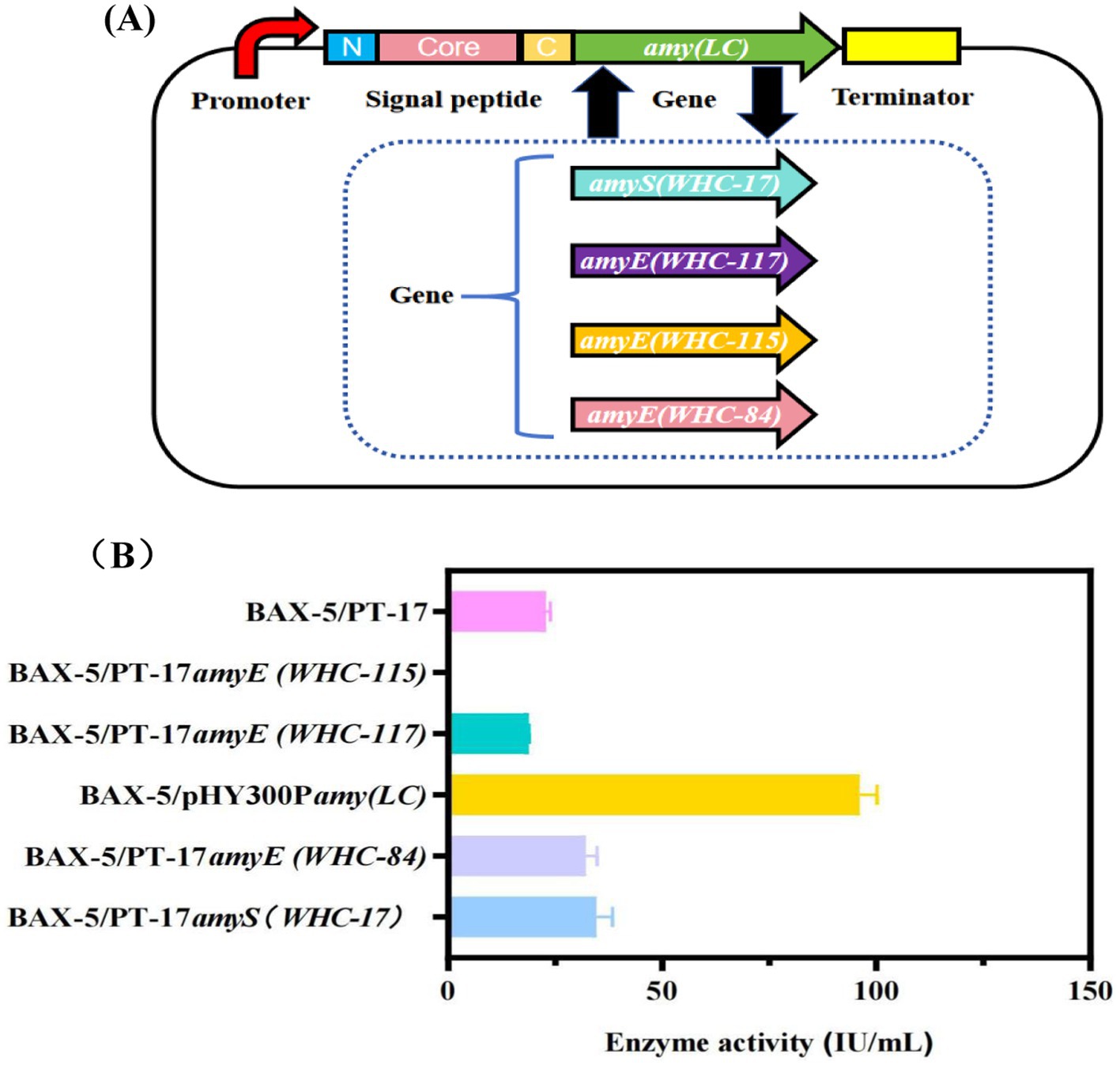
Figure 1. Enzyme activities of different α-amylase gene expression strains and analysis of the α-amylase Amy (LC). (A) Construction of different gene expression vectors. (B) Comparison of extracellular amylase activity of recombinant strains with different amylase genes.
3.2 Effects of different signal peptides on α-amylase expression
Signal peptides are one of the critical elements influencing the level of gene expression, and the signal peptide part of amy (LC) gene was predicted and analyzed by using the signal peptide prediction tool (https://novopro.cn/tools/signalp). The original signal peptide of Amy (LC) amylase belongs to the SP (Sec/SPI) type signal peptide, and the signal peptidase binding site is between 31 and 32 (shown in Supplementary Figure S3). Based on the signal peptide database SPSED (http://www.spsed.com), this study screened the optimal signal peptides for the amylase gene, included three signal peptides SP001, SP002 and SP003. In this study, those signal peptides SP001, SP002, and SP003, were fused with the gene amyA(LC) and the PT17 vector to obtain three recombinant vectors PT-17SP001amyA(LC), PT-17SP002amyA(LC) and PT-17SP003amyA(LC), respectively. All the recombinant plasmids were further transformed into the host strain BAX-5 to obtain the engineering strains BAX-5/PT-17SP001amyA(LC), BAX-5/PT-17SP002amyA(LC), and BAX-5/PT-17SP003amyA(LC), respectively.
The constructed engineered strains containing different signal peptides were fermented for α-amylase analysis, and the fermentation activities were shown in Figure 2B, these three signal peptides led to significantly different α-amylase activities. Therein, the α-amylase activities by SP003 reached 234.52 IU/mL, which was much higher than that of SP001 (40.06 IU/mL) and SP002 (143.18 IU/mL). In summary, the fermentation activity of α-amylase was further improved by optimizing the signal peptide, and the signal peptide SP003 was optimal for mediating the expression of gene amyA(LC).
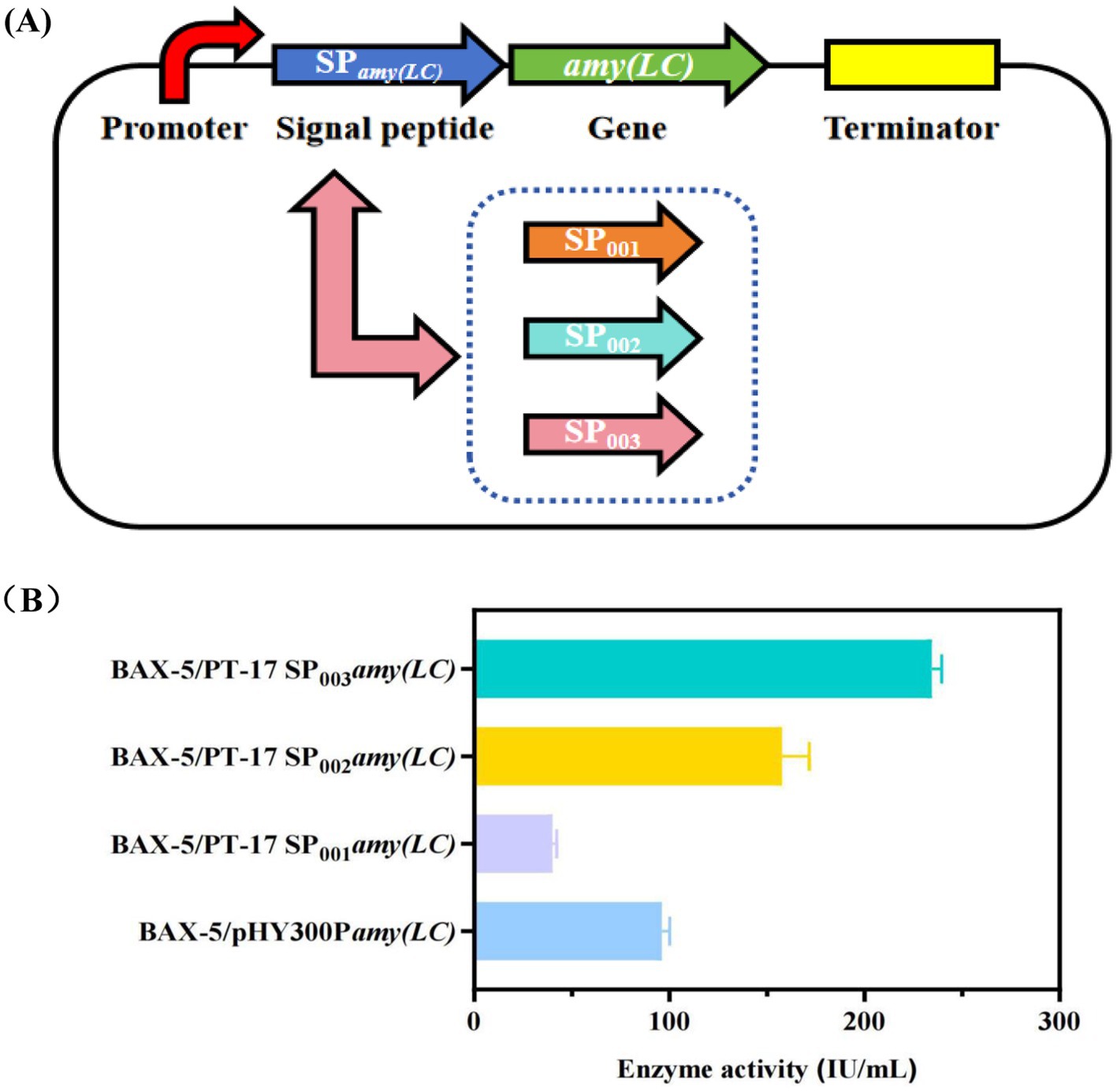
Figure 2. Effects of different signal peptides on the enzyme activities of α-amylase Amy (LC) (A) Construction of different signal peptides expression vectors. (B) Comparison of extracellular amylase activity of recombinant strains with different signal peptides.
3.3 Effects of different host strains on α-amylase expression
In addition, this study further screened the host strains, including B. subtilis SCK6, B. subtilis 168, and B. licheniformis BL10 (deficient in 10 genes on the basis of Bacillus licheniformis WX-02)(Wei et al., 2015). Accordingly, three new engineered strains were obtained, named as SCK6/PT-17SP003amyA(LC), 168/PT-17SP003amyA(LC) and BL10/PT-17SP003amyA(LC). As it showed in Figure 3B. The result showed that the enzyme activity of engineered strain BAX-5/PT-17SP003amyA(LC) was the highest, which was significantly higher than that of the other engineered strains. Therefore, the B. amyloliquefaciens BAX-5 was the optimal host bacteria.
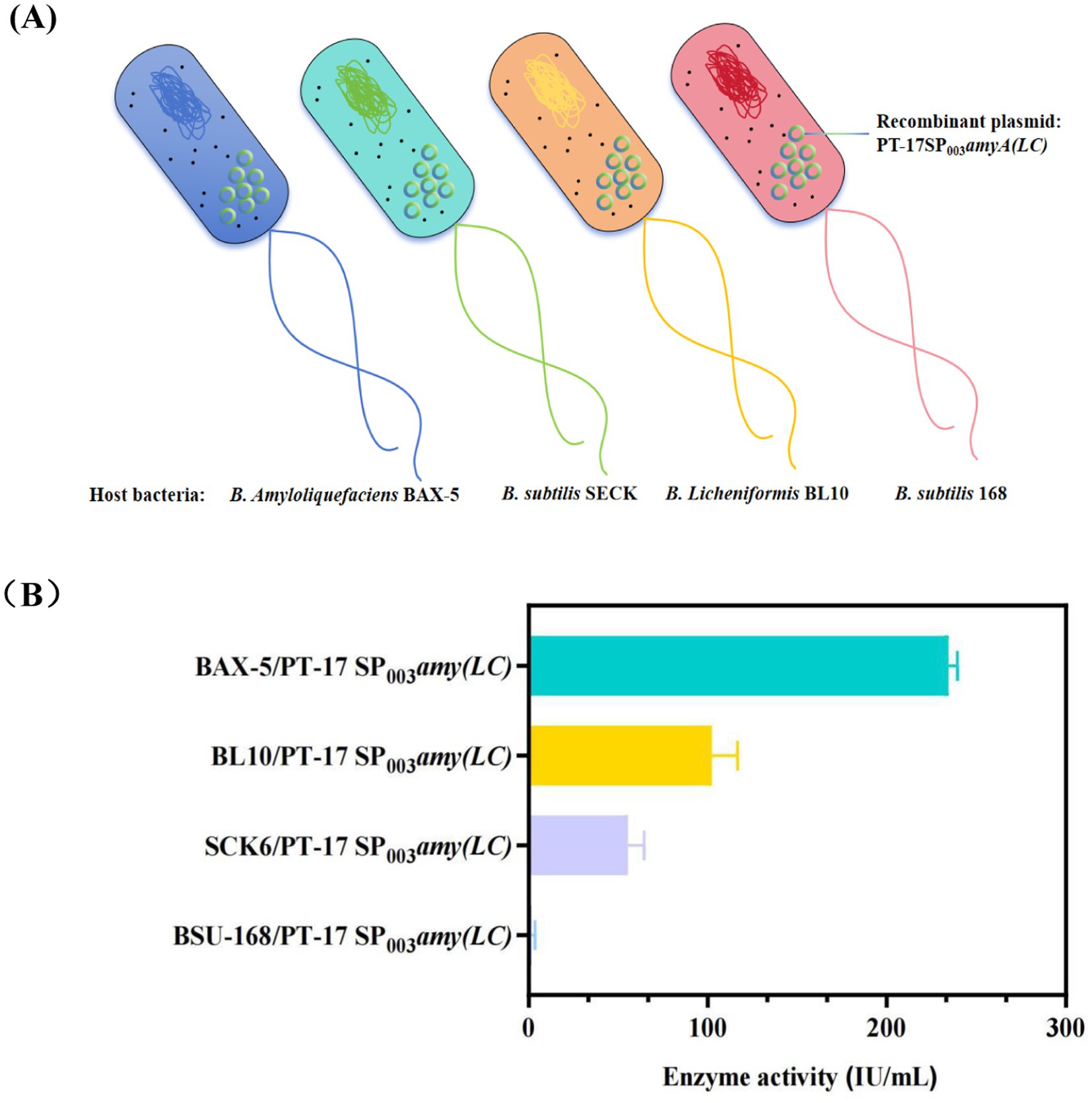
Figure 3. Effects of different host bacteria on the enzyme activities of α-amylase Amy (LC) (A) Construction of recombinant strains with different host bacteria. (B) Comparison of extracellular amylase activity of recombinant strains with different host bacteria.
3.4 Characterization of enzymatic characterization of recombinant expression strain
In the previous stage, this study obtained the optimal Bacillus expression system for the target enzyme genes. In order to further understand the characteristics of the amylase, the amylase activity at different reaction temperatures, temperature tolerance, pH tolerance, metal ion tolerance and organic solvent tolerance were analyzed. Firstly, the amylase reaction temperature was adjusted to 30°C, 40°C, 50°C, 60°C, 70°C 80°C and 90°C, respectively, and then the amylase activities of different temperature groups were measured by DNS method. The results are shown in Figure 4A, the amylase activity exhibited its highest activity at 40°C, reaching 314.37 IU/mL. Then, the amylase activity values gradually decreased as the temperature gradually increased above 40°C. When the reaction temperature reached 90°C, the amylase activity was only 59.65 IU/mL. Subsequently, the results of the temperature tolerance analysis indicated a decreasing trend with the increase of temperature at the range of 30°C to 90°C (Figure 4B). Among them, the highest amylase activity reached 261.39 IU/mL after being placed at 30°C for 30 min. When the enzyme solution was placed at 70°C, 80°C or 90°C, almost no amylase activity was detected. Therefore, α-amylase Amy (LC) does not possess high temperature tolerance.
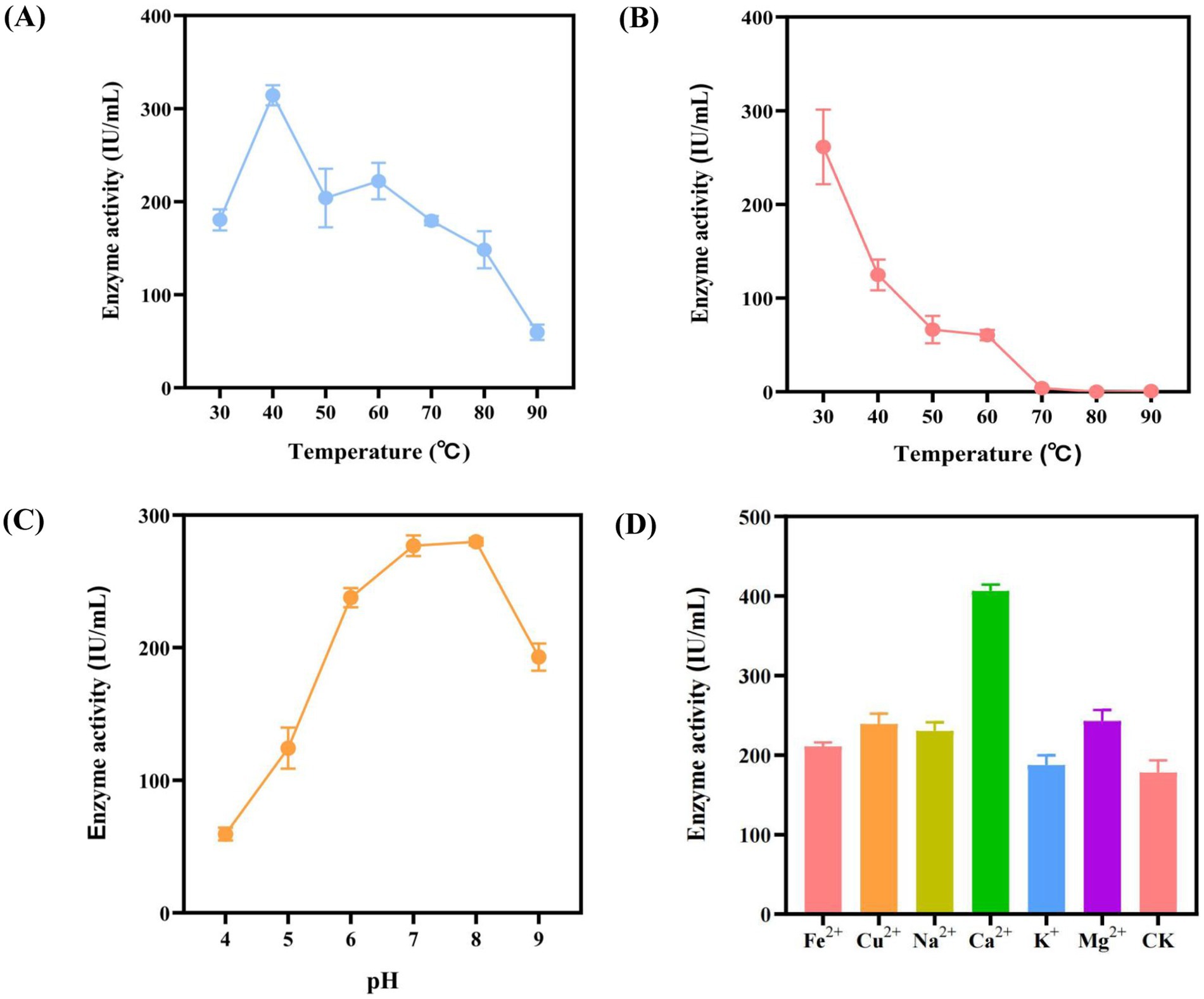
Figure 4. Enzyme characterization. (A) Reaction temperature: The ability of the enzyme to degrade substrates at different reaction temperatures were evaluated. (B) Thermal stability: The tolerance of the enzyme to different temperature environments was evaluated. (C) pH stability: The tolerance of the enzyme to different pH environments was evaluated. (D) Effects of metal ions on α-amylase activity. CK: negative control, no metal ions added.
Furthermore, this study investigated the effect of pH on amylase activity. The enzyme solution was exposed to different pH for 30 min, and the results of enzyme activity are shown in Figure 4C. The enzyme activity reached the peak of 279.92 IU/mL under pH 8. As the pH increased or decreased to both sides, the enzyme activity gradually decreased. As shown in Figure 4D, the performance of enzyme activity varies greatly under different metal ion environments. Compared with the control group, the k+ had almost no effect on the amylase activity, while the Fe2+, Na2+, Cu2+, Mg2+ metal ions leading to a slight increase in the amylase activity. In addition, the strongest effect on the enzyme activity was observed in the Ca2+, which nearly doubled the amylase activity compared with the control group.
3.5 Replacement of culture medium for recombinant strain
To better understand the fermentation status of the engineered strain BAX-5/PT-17SP003amyA(LC) during the fermentation period, this study determined the amylase activity of the BAX-5/PT-17SP003amyA(LC) at different time over the fermentation period. As shown in Figure 5A, the amylase activity gradually grew form 0–48 h, and the enzyme activity reached its peak at 238.91 IU/mL at 48 h. Subsequently, the amylase activity began to decline after 48 h. Therefore, the optimal fermentation duration for the amylase in this study was determined to be 48 h.
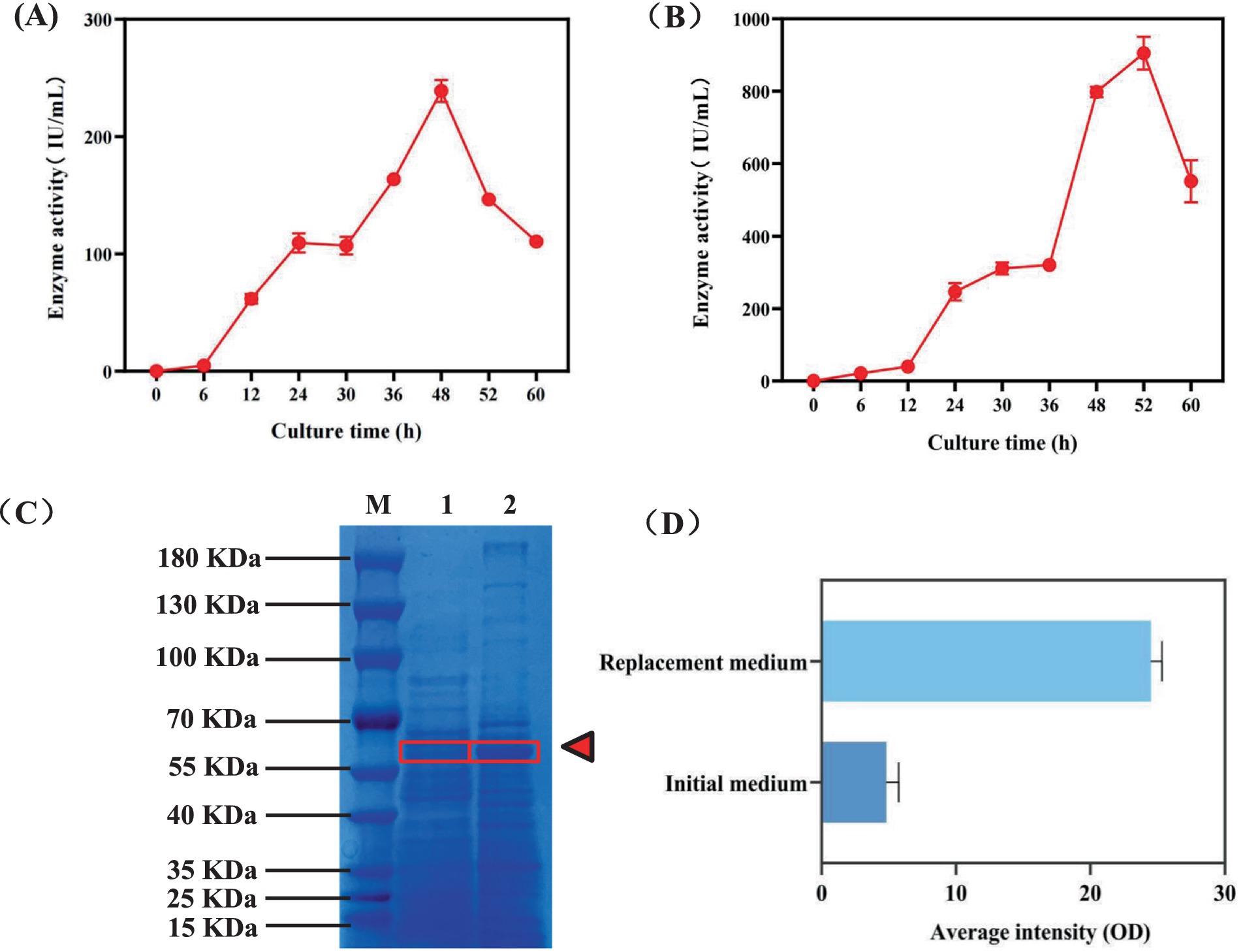
Figure 5. Comparison of the expression ability of α-amylase Amy (LC) before and after medium replacement. (A) Changes in enzyme activity over time when α-amylase Amy (LC) was fermented in the original medium. (B) The change of enzyme activity with time when α-amylase Amy (LC) was fermented in replaced medium. (C) Changes in α-amylase Amy (LC) expression before and after medium replacement. Sample 1: Expression of α-amylase Amy (LC) after fermentation of the previous medium. Sample 2: Expression of α-amylase Amy (LC) after fermentation with replaced medium.
Then, this study attempted to further enhance the α-amylase activity by replacing the culture medium. The strain BAX-5/PT-17SP003amy(LC) was used to fermentation with a known medium (The mixture consisted of 65 g/L corn meal, 70 g/L soybean cake meal, 10 g/L NaCl, and 3 g/L CaCl2 for 52 h at 40°C and pH 6, The volume of solution medium is 50 mL) that highly expressed α-amylase of engineered Bacillus. As shown in Figure 5B, the α-amylase activity increased with the extension of fermentation time. The optimal fermentation time was 52 h, and the α-amylase activity reached 904.91 IU/mL, which was nearly 4 times as much as that of the original culture medium. Then, the SDS-PAGE result showed that the α-amylase expression level was significantly improved after replacement (Figure 5C). The expression intensity of α-amylase Amy (LC) in SDS-PAGE gel map was analyzed. As shown in Figure 5D, the average intensity of α-amylase in the initial medium was 4.81, and the average intensity of α-amylase was 24.49 after replacement of culture medium, which was 5.09 times than that of the original medium. This result suggests that the increase in α-amylase activity may be due to the enhanced expression level of α-amylase.
3.6 Application of amylase in tobacco leaves
Based on the aforementioned research findings, the strain BAX-5/PT-17SP003amy(LC) was further applied to the treatment of tobacco leaves, and the quality of cigarettes made from treated tobacco leaves was evaluated. This study determined the performance of each group of the tobacco leaves treated with enzymes after preparation into cigarettes, and the degradation of harmful substances in tobacco by α-amylase was studied by Pyrolysis Gas Chromatography–Mass Spectrometry (Py-GC/MS). As shown in Figure 6A, the starch content in fermented tobacco leaves decreased from 5.27 to 4.43% compared with the control group. Subsequently, the application potential of the amylase was further evaluated.
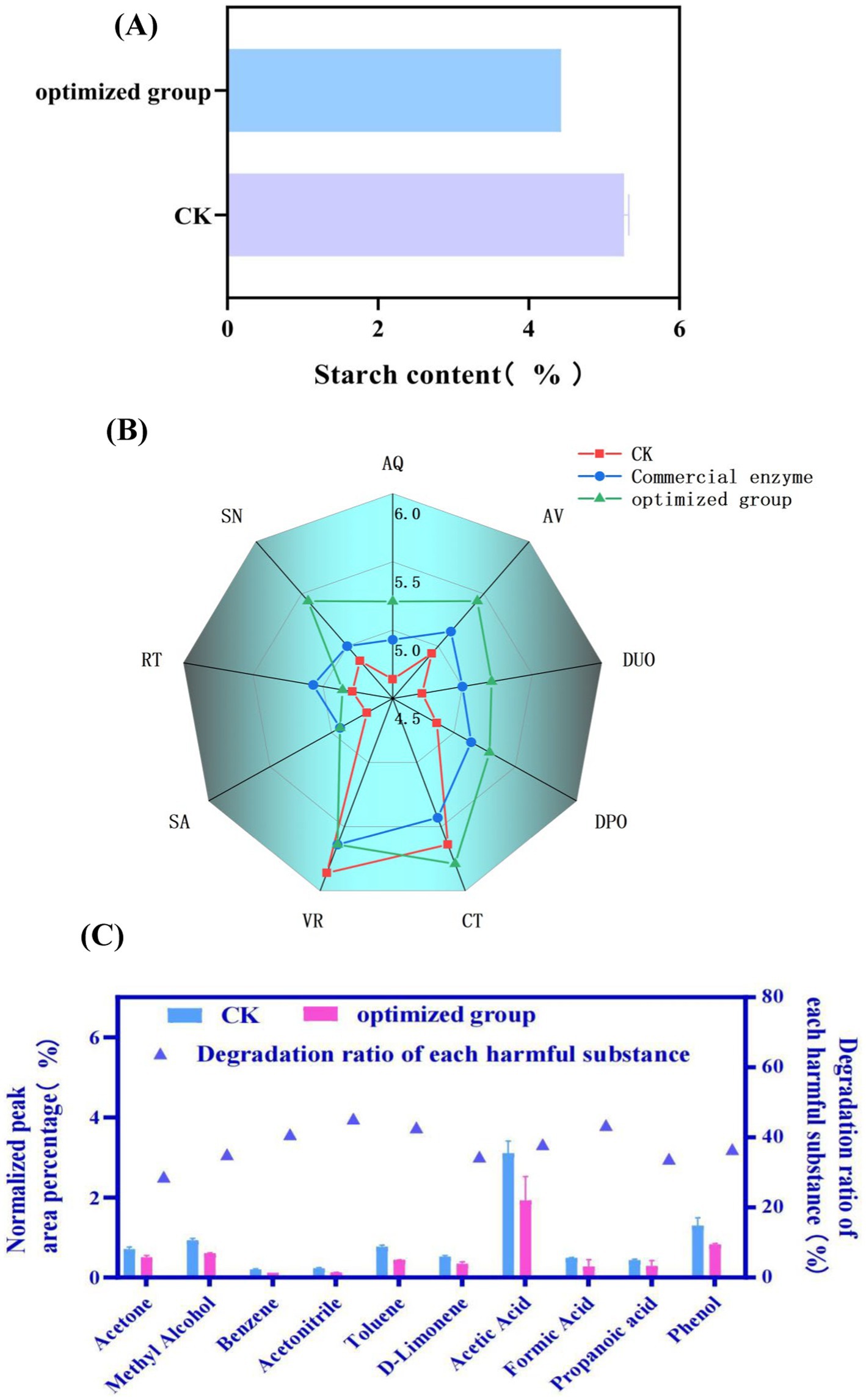
Figure 6. Quality assessment of tobacco treated with amylase amy (LC). (A) The effect of enzyme treatment on starch content. CK: control group. (B) Sensory evaluation of tobacco smoking during combustion. (Evaluation is conducted from 9 different dimensions. AQ, aroma quality; AV, aroma volume; DUO, decrease of unpleasant odor; DPO, decrease of pungent odor; CT, concentration; VR, vigorous; SA, sweete aroma; RT, remaining taste; SN, softness). (C) The content of harmful substances released during the pyrolysis process of tobacco leaves. The content of harmful substances is depicted by a bar chart (left y-axis), while the percentage reduction of these substances in the enzyme-treated group compared to the control group is represented by a blue triangle (right y-axis). CK: control group.
Next, the tobacco leaves treated with enzymes are prepared into cigarettes. The prepared cigarettes were used for sensory evaluation. As shown in Figure 6B, most of the indexes of the cigarettes fermented by the enzyme solution produced by the engineered bacteria BAX-5/PT-17SP003 amy(LC) (the optimized group) were better than those of the control group and the commercial enzyme group. The improvement of aroma quality was more significant than that in the control group and commercial enzyme group. The index of the optimized group was 5.21, while the score of the control group and commercial enzyme group was 4.64 and 5.07, respectively. The performance of the optimized group was better than that of the commercial enzyme group and the control group in terms of aroma quantity, softness degree, decrease of unpleasant odor, decrease of pungent odor, concentration but the vigorous score of the optimized group was lower than that of the control group. Although the sweet aroma score of the optimized group was higher than that of the control group, the performance was the same as that of the commercial enzyme group. The remaining taste index of the optimized group was higher than that of the control group, but lower than that of the commercial enzyme group.
Finally, the tobacco powder prepared from treated tobacco leaves was tested for Py/GC–MS experiment. The method simulates the environment of tobacco combustion, and the ratio of the peak area of each substance to the total peak area was used to characterize the change of substance content. As shown in Figure 6C, the peak area proportion of ten common harmful substances in the fermented tobacco leaves decreased compared with the blank sample, and the reduction rates for all ten substances ranged from 28.74 to 45.93%. Among them, the reduction rate of benzene, acetonitrile, toluene and formic acid is more than 40%, the reduction rates of phenol, propionic acid, D-limonene, acetic acid and methanol were between 30 and 40%. The degradation rate of acetone was the lowest, only 28.74%.
In summary, the α-amylase Amy (LC) enzyme liquid obtained by the engineered strain BAX-5/PT-17SP003amyA (LC) can not only reduce the starch content in tobacco leaves, but also significantly improve the sensory quality of cigarettes and it can reduce the proportion of ten harmful substances in the total substances during cigarette combustion. Therefore, the engineered strain BAX-5/PT-17SP003amyA (LC) has great significance for the application of tobacco leaf enzyme treatment in future industrial production.
4 Discussion
The starch content of tobacco plays a crucial role in the harmful substances and quality of tobacco. In order to reduce the influence of starch content on the tobacco and improve the quality of tobacco leaves, this study aimed to develop a novel α-amylase for tobacco starch degradation through gene mining, signal peptide and host strain optimization.
Firstly, the α-amylase gene fragments of 5 strains were amplified and sequenced. The repetitive α-amylase genes were removed by sequence alignment, and 5 α-amylase genes with significantly different sequences were successfully excavated. Then, above 5 α-amylase genes were used as target genes to construct different free expression vectors, which were further transformed into the host strain BAX-5 to construct the recombinant expression strains. The α-amylase fermentation activities were analyzed. It is worth noting that BAX-5/PT-17amyE(WHC-115) and BAX-5/PT-17amyE(WHC-117) showed lower enzyme activity than the control group. This is because, although the gene sequences of different α -amylases are similar, even very small differences in base sequences can affect the three-dimensional structure of the enzymes, especially when key amino acids change, it often significantly affects the activity of enzymes. Li et al. also reported similar findings: when the Ala269 residue in sucrose-6-phosphate hydrolase (SacA) was mutated to Thr269, the enzymatic activity of the Ala269Thr mutant increased by approximately 50%. This demonstrates that even a single amino acid mutation can significantly alter enzyme activity (Li et al., 2025). Due to the best enzymatic activity performance, the gene amyA(LC) was finally determined as the optimal α-amylase gene.
The signal peptide is a 5–30 amino acid part at the front of a protein, which is important for extracellular secretion of target protein (Smets et al., 2022). Various signal peptides from different strains usually resulted in different expression levels for target protein (Liu et al., 2022), and replacement of signal peptides has been an important optimization strategy to enhance protein expression. Since α-amylase is extracellular protein, the signal peptide will play an important role in its secretion. To further improve the expression of α-amylase, the signal peptides of α-amylase were optimized, and the DNA sequences of signal peptides were mined from the signal peptide library. The original signal peptide coding region of gene amyA(LC) was replaced, and the signal peptide with the strongest effect on α-amylase was screened. Both the α -amylases mediated by SP002 and SP003 showed high enzymatic activities, among which SP003 exhibited the highest α-amylase activity. These three signal peptides (SP001, SP002 and SP003) were derived from the gene tag1 of Lactobacillus plantarum (Mathiesen et al., 2009), genes bglS and dacB of B. subtilis (Yao et al., 2019; Liu et al., 2019), respectively. It indicated that the signal peptides from Bacillus were more beneficial for expression of gene amyA(LC). Ultimately, the signal peptide SP003, derived from the dacB gene of B. subtilis 168 (Liu et al., 2019) was confirmed to be the optimal signal peptide for mediating the expression of gene amyA(LC).
Different Bacillus host strains can significantly affect the expression of foreign proteins (Osamura et al., 2023; Wu et al., 1991; Wong et al., 1994; Wei et al., 2015). Therefore, the plasmid PT-17SP003amyA (LC) was further transformed into several host strains, including B. amyloliquefaciens BAX-5, B. subtilis SCK6, B. subtilis 168, and B. licheniformis BL10. Among them, the BAX-5/PT-17SP003amyA(LC) showed the highest α-amylase activity. Therefore, B. amyloliquefaciens BAX-5 is considered to be the most suitable host strain for amyA (LC) expression. B. amyloliquefaciens BAX-5 was obtained by knocking out five protease genes mpr, epr, nprE, aprE and aprX of wild-type Bacillus amyloliquefaciens HZ-12. This five genes were classified as important intracellular or extracellular protease genes in strain HZ-12. The deletion of these five protease genes might reduce the secretory pressure by the corresponding proteins on the host bacteria B. amyloliquefaciens BAX-5 (Chen et al., 2022), thereby increasing the secretion of exogenous α-amylase.
The enzymatic properties of biological enzymes can better reflect their characteristics. In this study, through enzymatic property analysis experiments, we found that amylase Amy (LC) was a medium temperature neutral amylase, and its tolerance to weak acidic environment was stronger than that to weak alkali environment, and calcium ion environment helped to enhance its enzymatic activity. This may be attributed to the presence of numerous binding sites within the structure of α-amylase that can interact with calcium ions. By binding with calcium ions, the structural stability of α-helix in the three-dimensional structure of α-amylase can be increased, so as to further improve the stability of the entire enzyme structure and stabilize the α-amylase activity (Abedi et al., 2024).
We further enhanced the activity of α-amylase Amy (LC) by using a laboratory-available medium formulation. The replaced medium contains soybean cake flour, a nitrogen source commonly used in industrial fermentation, and corn flour, a carbon source commonly used, as well as two inorganic salts: NaCl and CaCl2. Compared with the new medium, the main components of initial medium were yeast extract, tryptone, NH4Cl and K2HPO4, in which yeast extract, and tryptone acted as organic nitrogen sources, and NH4Cl was inorganic nitrogen sources. Although the first two substances (tryptone and yeast extract) have a small amount of carbon source components, the medium still lacks components that can be used as the main carbon source. Therefore, the main nutrients provided by the replaced medium are more comprehensive than the initial medium, which may be the main reason why the new medium is better than the initial medium. In addition, starch and other carbon sources contained in corn meal can induce the synthesis of amylase (Mishra and Behera, 2008; Ensari et al., 1995). The initial medium could not continuously improve the enzyme production efficiency through this induction mechanism. Moreover, the calcium ions in the inorganic salt can stabilize the enzyme activity in the optimized medium (Zhao et al., 2024). Finally, the cost of each component in the replaced medium is lower than the initial medium, which means that the new medium is more suitable for large-scale applications in industrial production in the future.
After medium replacement, we used the enzyme liquid after ultrafiltration to deal with tobacco leaves and evaluated the results of treated tobacco leaves. The results of sensory evaluation showed that most of the sensory indexes of cigarettes prepared by amylase Amy(LC) treated tobacco were better than those prepared by commercial enzyme fermented tobacco and control group. Reducing sugar is an important product of starch hydrolysis. Along with the degradation of starch, the content of reducing sugar increases accordingly, and sugar has a significant impact on the quality of tobacco leaves. On the one hand, sugar can be transformed into aromatic substances through reactions such as the Maillard reaction and caramelization reaction. On the other hand, an increase in sugar content can improve sweetness. This might explain the improvement in indicators such as the aroma quality, aroma quantity and sweetness of cigarettes prepared from enzyme-treated tobacco leaves in the sensory evaluation results (Yang et al., 2014). In addition, the amylase preparation from B. amyloliquefaciens strain isolated from tobacco was used to prepare flue-cured tobacco, and the large molecular substances such as starch in the flue-cured tobacco also showed a significant reduction. At the same time, the aroma quality and aroma quantity of the flue-cured tobacco were enhanced (Gong et al., 2023). This is consistent with the results of this study.
Since the incomplete combustion of starch in tobacco will also affect the release of harmful substances. Therefore, we also analyzed the release of harmful substances when tobacco was burned after enzyme treatment through thermal cracking experiments. The result indicated that the proportion of these ten harmful substances had decreased. Although there are limited reports on how these harmful substances are formed, But the 10 substances reduced after enzyme treatment were on the list of 149 harmful substances produced during the burning of tobacco (Rodgman and Green, 2003). All of these substances are toxic, carcinogenic and addictive to some degree. It can be seen that cigarettes made from tobacco leaves treated by Amy (LC) enzyme have certain positive effects on improving sensory quality and reducing harmful substances.
The above results and conclusions preliminarily prove that the engineered strain BAX-5/PT-17SP003amyA(LC) has great significance for reducing the damage and increasing the quality of tobacco leaves. It has great application potential in the enzyme treatment step of tobacco production.
Data availability statement
The data presented in this study have been deposited in the NCBI repository, with accession numbers: PV799417 (The gene sequence of amyS (WHC-17)), PV799418 (The gene sequence of amyE (WHC-84)), PV799419 (The gene sequence of amyE (WHC-115)), PV799420 (The gene sequence of amyE (WHC-117)), PV799421 (The gene sequence of amyA (LC)).
Author contributions
ZH: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JH: Funding acquisition, Investigation, Resources, Supervision, Writing – review & editing. DZ: Methodology, Software, Supervision, Visualization, Writing – review & editing. ZS: Data curation, Formal analysis, Methodology, Writing – review & editing. ZZ: Data curation, Formal analysis, Methodology, Writing – review & editing. CN: Methodology, Supervision, Writing – review & editing. QL: Investigation, Methodology, Supervision, Writing – review & editing. KH: Methodology, Supervision, Writing – review & editing. CY: Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing. XW: Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Key Research and Development Projects of China National Tobacco Corporation (No. 110202102019 and No. 110202202023), the National Natural Science Foundation of China (No. 32001758), the Key Research and Development Program of Hubei Province (No. 2022BBA0040).
Conflict of interest
JH, ZS and ZZ were employed by the Inner Mongolia Kunming Cigarettes LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1603337/full#supplementary-material
References
Abedi, E., Kaveh, S., and Hashemi, S. M. B. (2024). Structure-based modification of a-amylase by conventional and emerging technologies: comparative study on the secondary structure, activity, thermal stability and amylolysis efficiency. Food Chem. 437:137903. doi: 10.1016/j.foodchem.2023.137903
Cai, M.-Z., Kee, P. E., Ng, H. S., and Chen, P.-T. (2022). Development of Bacillus subtilis self-inducible expression system for keratinase production using piggery wastewater. J. Taiwan Inst. Chem. Eng. 137:104218. doi: 10.1016/j.jtice.2022.104218
Chen, W., Li, L., Ye, C., Zhao, Z., Huang, K., Zou, D., et al. (2022). Efficient production of extracellular alkaline protease in Bacillus amyloliquefaciens by host strain construction. Lwt 163:113620. doi: 10.1016/j.lwt.2022.113620
Chen, X., Liu, L., Zhang, Y., Zhou, X., Lin, T., Song, Y., et al. (2017). Acetylsalicylic acid application decreased tobacco-specific nitrosamines and its precursors but maintained quality of air-cured burley tobacco (Nicotiana tabacum L.). Ind. Crop. Prod. 104, 221–228. doi: 10.1016/j.indcrop.2017.04.031
Chu, K. L., Koley, S., Jenkins, L. M., Bailey, S. R., Kambhampati, S., Foley, K., et al. (2022). Metabolic flux analysis of the non-transitory starch tradeoff for lipid production in mature tobacco leaves. Metab. Eng. 69, 231–248. doi: 10.1016/j.ymben.2021.12.003
Ensari, A. N. Y., Otludil, B., and Aytekin, A. M. C. (1995). Effect of starch induced bacterial growth and amylase production in Bacillus subtilis. Starch 47, 315–321. doi: 10.1002/star.19950470807
Gong, Y., Li, J., Deng, X., Chen, Y., Chen, S., Huang, H., et al. (2023). Application of starch degrading bacteria from tobacco leaves in improving the flavor of flue-cured tobacco. Front. Microbiol. 14:1211936. doi: 10.3389/fmicb.2023.1211936
Kang, X.-M., Cai, X., Huang, Z.-H., Liu, Z.-Q., and Zheng, Y.-G. (2020). Construction of a highly active secretory expression system in Bacillus subtilis of a recombinant amidase by promoter and signal peptide engineering. Int. J. Biol. Macromol. 143, 833–841. doi: 10.1016/j.ijbiomac.2019.09.144
Li, X., Shi, S., Hao, Y., Zhai, Z., Zhao, Z., Feng, X., et al. (2025). Surface hydrophilic amino acids of sucrose-6-phosphate hydrolase SacA play a key role in high acid production rates in Lacticaseibacillus casei. Lwt 218:117465. doi: 10.1016/j.lwt.2025.117465
Li, H., Yao, D., Ying, J., Han, X., Zhang, X., Fang, X., et al. (2022). Enhanced extracellular raw starch-degrading α-amylase production in Bacillus subtilis through signal peptide and translation efficiency optimization. Biochem. Eng. J. 189:108718. doi: 10.1016/j.bej.2022.108718
Liu, P., Guo, J., Miao, L., and Liu, H. (2022). Enhancing the secretion of a feruloyl esterase in Bacillus subtilis by signal peptide screening and rational design. Protein Expr. Purif. 200:106165. doi: 10.1016/j.pep.2022.106165
Liu, Q., Li, R., Shi, H., Yang, R., Shen, Q., Cui, Q., et al. (2023). A recombineering system for Bacillus subtilis based on the native phage recombinase pair YqaJ/YqaK. Eng. Microbiol. 3:100099. doi: 10.1016/j.engmic.2023.100099
Liu, Y., Shi, C., Li, D., Chen, X., Li, J., Zhang, Y., et al. (2019). Engineering a highly efficient expression system to produce Bcapro protease in Bacillus subtilis by an optimized promoter and signal peptide. Int. J. Biol. Macromol. 138, 903–911. doi: 10.1016/j.ijbiomac.2019.07.175
Mathiesen, G., Sveen, A., Brurberg, M. B., Fredriksen, L., Axelsson, L., and Eijsink, V. G. (2009). Genome-wide analysis of signal peptide functionality in Lactobacillus plantarum Wcfs1. BMC Genomics 10, 1–13. doi: 10.1186/1471-2164-10-425
Miao, H., Jiang, R., Han, N., Ma, Y., Wu, Q., Mu, Y., et al. (2021). Enhanced extracellular expression of α-amylase Dl3-4-1 in Bacillus subtilis via systematic screening of optimal signal peptides. Process Biochem. 108, 176–184. doi: 10.1016/j.procbio.2021.06.018
Mishra, S., and Behera, N. (2008). Amylase activity of a starch degrading bacteria isolated from soil receiving kitchen wastes. Afr. J. Biotechnol. 7, 3326–3331. doi: 10.20546/ijcmas.2019.804.071
Mukherjee, T., and Venkata Mohan, S. (2021). Metabolic flux of Bacillus subtilis under poised potential in electrofermentation system: gene expression vs product formation. Bioresour. Technol. 342:125854. doi: 10.1016/j.biortech.2021.125854
Osamura, T., Takahashi, F., Endo, K., Okuda, M., and Takimura, Y. (2023). Autolysis-induced extracellular production of intracellular carboxylesterase EstGtA2 using multiple-protease-deficient Bacillus subtilis strains. Biochem. Eng. J. 198:108996. doi: 10.1016/j.bej.2023.108996
Rodgman, A., and Green, C. R. (2003). Toxic chemicals in cigarette mainstream smoke - hazard and hoopla. Contributions Tobacco Nicotine Research 20, 481–545. doi: 10.2478/cttr-2013-0764
Smets, D., Smit, J., Xu, Y., Karamanou, S., and Economou, A. (2022). Signal peptide-rheostat dynamics delay secretory Preprotein folding. J. Mol. Biol. 434:167790. doi: 10.1016/j.jmb.2022.167790
Su, Y., Liu, C., Fang, H., and Zhang, D. (2020). Bacillus subtilis: a universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Factories 19:173. doi: 10.1186/s12934-020-01436-8
Sun, J.-G., He, J.-W., Wu, F.-G., Tu, S.-X., Yan, T.-J., Si, H., et al. (2011). Comparative analysis on chemical components and sensory quality of aging flue-cured tobacco from four main tobacco areas of China. Agric. Sci. China 10, 1222–1231. doi: 10.1016/S1671-2927(11)60113-2
Tian, D., Huang, L., Zhang, Z., Tian, Z., Ge, S., Wang, C., et al. (2023). A novel approach for quantitative determination of cellulose content in tobacco via 2D Hsqc Nmr spectroscopy. Carbohydr. Res. 526:108790. doi: 10.1016/j.carres.2023.108790
Wei, X., Zhou, Y., Chen, J., Cai, D., Wang, D., Qi, G., et al. (2015). Efficient expression of nattokinase in Bacillus licheniformis: host strain construction and signal peptide optimization. J. Ind. Microbiol. Biotechnol. 42, 287–295. doi: 10.1007/s10295-014-1559-4
Wong, S. L., Ye, R. Q., and Nathoo, S. S. (1994). Engineering and production of streptokinase in a Bacillus subtilis expression-secretion system. Appl. Environ. Microbiol. 60, 517–523. doi: 10.1128/aem.60.2.517-523.1994
Wu, X. C., Lee, W., Tran, L., and Wong, S. L. (1991). Engineering a Bacillus subtilis expression-secretion system with a strain deficient in six extracellular proteases. J. Bacteriol. 173, 4952–4958. doi: 10.1128/jb.173.16.4952-4958.1991
Xing, L., Zhang, M., Liu, L., Hu, X., Liu, J., Zhou, X., et al. (2023). Multiomics provides insights into the succession of microbiota and metabolite during plant leaf fermentation. Environ. Res. 221:115304. doi: 10.1016/j.envres.2023.115304
Yan, S., Ren, T., Wan Mahari, W. A., Feng, H., Xu, C., Yun, F., et al. (2022). Soil carbon supplementation: improvement of root-surrounding soil bacterial communities, sugar and starch content in tobacco (N. tabacum). Sci. Total Environ. 802:149835. doi: 10.1016/j.scitotenv.2021.149835
Yan, S., Zhao, J., Ren, T., and Liu, G. (2020). Correlation between soil microbial communities and tobacco aroma in the presence of different fertilizers. Ind. Crop. Prod. 151:112454. doi: 10.1016/j.indcrop.2020.112454
Yang, Y., Wang, J.-J., Wang, C.-X., Li, Q., and Yang, G.-H. (2010). Awareness of tobacco-related health hazards among adults in China. Biomed. Environ. Sci. 23, 437–444. doi: 10.1016/S0895-3988(11)60004-4
Yang, C., Wu, W., Wu, S. C., and Liu, H.-B. (2014). Aroma types of flue-cured tobacco in China: spatial distribution and association with climatic factors. Theor. Appl. Climatol. 115, 541–549. doi: 10.1007/s00704-013-0914-0
Yao, D., Su, L., Li, N., and Wu, J. (2019). Enhanced extracellular expression of Bacillus stearothermophilus α-amylase in Bacillus subtilis through signal peptide optimization, chaperone overexpression and α-amylase mutant selection. Microb. Cell Factories 18:69. doi: 10.1186/s12934-019-1119-8
Ye, C., Zhao, W., Liu, D., Yang, R., Cui, Z., Zou, D., et al. (2024). Screening, identification, engineering, and characterization of Bacillus-derived α-amylase for effective tobacco starch degradation. Int. J. Biol. Macromol. 282:137364. doi: 10.1016/j.ijbiomac.2024.137364
Yu, J.-F., Chen, Q.-L., Ren, J., Yang, Y.-L., Wang, J.-H., and Sun, X. (2015). Analysis of the multi-copied genes and the impact of the redundant protein coding sequences on gene annotation in prokaryotic genomes. J. Theor. Biol. 376, 8–14. doi: 10.1016/j.jtbi.2015.04.002
Keywords: tobacco starch, α-amylase, Bacillus amyloliquefaciens, expression optimization, application evaluation
Citation: Han Z, Hao J, Zou D, Sun Z, Zhang Z, Niu C, Lu Q, Huang K, Ye C and Wei X (2025) Efficient expression of a novel α-amylase for reduction of tobacco starch and smoke hazard. Front. Microbiol. 16:1603337. doi: 10.3389/fmicb.2025.1603337
Edited by:
Kunal R. Jain, Sardar Patel University, IndiaReviewed by:
Abdallah M. A. Hassane, Al-Azhar University, EgyptDessy Natalia, Bandung Institute of Technology, Indonesia
Copyright © 2025 Han, Hao, Zou, Sun, Zhang, Niu, Lu, Huang, Ye and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changwen Ye, eWVjdHNyY0AxNjMuY29t; Xuetuan Wei, d2VpeHVldHVhbkBtYWlsLmh6YXUuZWR1LmNu
†These authors have contributed equally to this work
 Zongchen Han1†
Zongchen Han1† Zhikang Sun
Zhikang Sun Kuo Huang
Kuo Huang Changwen Ye
Changwen Ye Xuetuan Wei
Xuetuan Wei