- 1United Graduate School of Agricultural Science, Gifu University, Gifu, Japan
- 2Graduate School of Agriculture, Shizuoka University, Shizuoka, Japan
Introduction: The agricultural pests Liriomyza trifolii (Diptera: Agromyzidae) and Hercinothrips femoralis (Thysanoptera: Thripidae) harbor the endosymbiont Wolbachia, which induces cytoplasmic incompatibility and thelytokous parthenogenesis (asexual reproduction of female offspring without fertilization), respectively. The parasitoid Neochrysocharis formosa (Hymenoptera: Eulophidae), a natural enemy of leaf miners, is infected with Rickettsia, which also induces thelytokous parthenogenesis. Although symbionts can be eliminated in laboratory settings using antibiotics mixed with physical manipulation, the effects of agrochemical antibiotics designed for plant disease control on these insects and their symbionts remain unexplored. This study investigated the effects of MycoShield, a commercially available agrochemical containing 17% oxytetracycline, on symbiont-infected populations of these three insect species.
Methods: MycoShield was applied to kidney bean plants or mixed into honey to expose L. trifolii, H. femoralis, and N. formosa to oxytetracycline. Offspring were screened for symbiont presence using PCR, and infection frequencies were compared across treatment concentrations. Additionally, H. femoralis populations were monitored in caged conditions under continuous exposure to treated plants.
Results: At standard concentrations (1,000-fold dilution), MycoShield eliminated Wolbachia from L. trifolii and H. femoralis, resulting in L. trifolii producing uninfected offspring and H. femoralis producing only uninfected males. Similarly, Rickettsia was eliminated from N. formosa when adults ingested MycoShield-mixed honey. Additionally, N. formosa appeared to ingest the antibiotic indirectly by parasitizing L. trifolii larvae that had fed on treated leaves. Symbiont elimination was dose-dependent. Long-term exposure led to a substantial reduction in H. femoralis populations. Two out of eleven cages experienced complete extinction by day 100, likely due to genetic drift resulting from severe reproductive bottlenecks.
Discussion: These findings demonstrate the potential of agrochemical antibiotics such as MycoShield as insecticidal agents targeting symbiont-mediated reproduction, with possible applications in sterile insect techniques. Further research is required to optimize efficacy and assess feasibility under field conditions.
1 Introduction
The leaf miner Liriomyza trifolii (Burgess) (Diptera: Agromyzidae) is a globally distributed pest that infests various host plants, including vegetables and ornamental flowers (Seal et al., 2002; Masetti et al., 2004). The parasitoid Neochrysocharis formosa (Westwood) (Hymenoptera: Eulophidae), a natural enemy of leaf miners, is native to regions where these pests are prevalent (Arakaki and Kinjo, 1998; Konishi, 1998). N. formosa parasitizes the larvae of L. trifolii, ultimately killing the host. This parasitic behavior enables N. formosa to serve as an effective biological control agent and, in Japan, it is now commercially available for managing Liriomyza species. Another significant pest, Hercinothrips femoralis Reuter (Thysanoptera: Thripidae), commonly known as banded greenhouse thrips, is a polyphagous insect with a cosmopolitan distribution (Houston et al., 1991; Trdan et al., 2007). These three insect species harbor endosymbiotic bacteria such as Wolbachia and Rickettsia in Japan and other regions (Adachi-Hagimori et al., 2008; Tagami et al., 2006b; Kumm and Moritz, 2008).
Wolbachia is a well-studied endosymbiotic bacterium that manipulates host reproduction through various mechanisms, including cytoplasmic incompatibility (Werren, 1997), feminization of genetic males (Bouchon et al., 1998), male killing (Hurst and Jiggins, 2000), and thelytokous parthenogenesis, a form of parthenogenesis in which unfertilized eggs develop into females (Stouthamer et al., 1990; Stouthamer and Kazmer, 1994). In L. trifolii, Wolbachia induces cytoplasmic incompatibility, a reproductive barrier in which crosses between infected males and uninfected females result in embryo mortality, thereby reducing reproductive success (Tagami et al., 2006b; Kumm and Moritz, 2008). However, this mechanism does not affect the sex ratio of the offspring.
In contrast, in H. femoralis, Wolbachia induces thelytokous parthenogenesis, a form of parthenogenesis in which unfertilized eggs develop into females, resulting in strongly female-biased populations (Kumm and Moritz, 2008). Similarly, in N. formosa, the endosymbiont Rickettsia induces thelytokous parthenogenesis, leading to highly female-skewed populations (Hagimori et al., 2006) that contribute to the effectiveness of this species as a biological control agent. Notably, H. femoralis females uninfected with Wolbachia and N. formosa females uninfected with Rickettsia produced only male offspring, and these males do not have a function to reproduce, highlighting the essential role of these endosymbionts in reproduction. In pest species like H. femoralis, disrupting symbiont-mediated reproduction may aid in population suppression.
In contrast, for beneficial parasitoids like N. formosa, which are used as biological control agents against L. trifolii, the loss of symbiont-induced thelytoky parthenogenesis could severely reduce female production and compromise control efficacy. Therefore, it is crucial to assess how agricultural treatments, such as antibiotic applications, might differentially affect symbiont-dependent reproduction in both pests and their natural enemies.
The widespread use of agricultural pesticides, including insect growth regulators, biopesticides targeting Bacillus thuringiensis, and chemical classes such as organophosphates, carbamates, neonicotinoids, organocoppers, and organic sulfur compounds, has been central to pest control strategies (Siddall, 1976; Whalon and Wingerd, 2003; Souto et al., 2021; Sharma et al., 2019; Zhang, 2018; Araújo et al., 2023). Antibiotics are also used in agriculture to manage plant diseases (Stockwell and Duffy, 2012). One such bactericide, MycoShield (Maruwa Biochemical, Tokyo, Japan), contains 17.0% oxytetracycline, a tetracycline derivative used to control bacterial diseases such as fire blight (Erwinia amylovora) and infections caused by Pseudomonas and Xanthomonas species (Copping and Duke, 2007). This antibiotic is also effective against diseases caused by mycoplasma-like organisms (Ishiie et al., 1967) and is applied to stone and pome fruits, as well as turfgrass (Copping and Menn, 2000).
Despite the prevalence of bacterial endosymbionts in many insect species, the effects of antibiotic bactericides on symbionts and their hosts are unclear. Therefore, this study aimed to investigate the effects of MycoShield on L. trifolii, H. femoralis, and N. formosa, with a specific focus on (i) the direct effects of MycoShield on these insects, (ii) the uptake routes of MycoShield in L. trifolii and N. formosa, (iii) the relationship between MycoShield concentration and its effect on infected insects, and (iv) the potential use of MycoShield to eradicate H. femoralis populations.
2 Materials and methods
2.1 Insects
Liriomyza trifolii and N. formosa were collected from kidney bean leaves in Iwata, Shizuoka, Japan, in 1991 and placed in cages (width 30 cm, depth 30 cm, height 40 cm). Each cage contained three kidney bean plants, which served as host plants when L. trifolii adults emerged.
Kidney bean leaves in the field were collected for N. formosa, and N. formosa individuals were selected based on morphological characters when leaf miner parasitoids emerged. N. formosa were reared in cages with L. trifolii larvae as hosts.
Hercinothrips femoralis was collected from a beach lily (Crinum asiaticum) in Iwata, Shizuoka, Japan, in 2005 and reared on kidney bean plants in a cage. When host plants were severely damaged, H. femoralis populations were transferred to new cages containing new kidney bean plants.
All three insect species were reared under consistent environmental conditions (25°C, 16 h light:8 h dark photoperiod, relative humidity 70 ± 5%, with light provided by MLR-351, SANYO, Osaka, Japan). Morphological identification of each species was performed based on criteria described previously (Konishi, 1998). The initial Wolbachia and Rickettsia infection status was verified by PCR prior to the experiments.
2.2 Detection of Wolbachia and Rickettsia and distinguishing between sex
Diagnostic PCR detection of bacterial endosymbionts was performed using a thermal cycler (TP600, TAKARA, Japan) as previously described (Tagami et al., 2006b; Hagimori et al., 2006; Tagami et al., 2006a). Wolbachia was detected by amplifying the wsp gene using the primers wsp81F (5′-TGGTCCAATAAGTGATGAAGAAAC-3′) and wsp691R (5′-AAAAATTAAACGCTACTCCA-3′) (Braig et al., 1998). Rickettsia infection was assessed by targeting the bacterial 16S rRNA gene with the primers NforRick1 (5′-AGTGAGTGATGAAGGCCCTA-3′) and NforRick2 (5′-GGAATTCCATCATCCTCTACTAC-3′) (Tagami et al., 2006a). The PCR conditions (reaction components and thermal cycling profiles) followed those established in the cited literature.
Host sex was identified based on adult morphological characteristics, particularly the structure of the sexual organs, in all three species.
2.3 Effect of MycoShield on L. trifolii, H. femoralis, and N. formosa
Prior to MycoShield treatment, the infection status of each insect species was confirmed by PCR using 30 representative individuals from the laboratory-reared lines. All L. trifolii and H. femoralis individuals tested were found to be 100% infected with Wolbachia, and all N. formosa females were 100% infected with Rickettsia.
To assess the effect of short-term antibiotic exposure, adult insects were placed in cages containing MycoShield-treated plants for 24 h. MycoShield was prepared at a standard concentration (1,000-fold dilution with water) and sprayed evenly onto three kidney bean plants, each bearing four leaves. After the 24-h exposure period, three pairs of L. trifolii or H. femoralis were introduced into separate cages and allowed to reproduce. Once the adults of the next generation emerged, six individuals were randomly collected from each cage and screened for Wolbachia infection using PCR. Each experiment was repeated five times.
For N. formosa, five cages were prepared, and a droplet of honey mixed with MycoShield (1,000-fold dilution) was provided as food for 24 h. After this exposure period, three kidney bean plants parasitized by L. trifolii larvae were introduced into each cage. The Rickettsia infection ratio was determined by analyzing 30 adult individuals (six individuals per cage) of N. formosa from the next generation using PCR.
2.4 MycoShield uptake routes of L. trifolii and N. formosa
Understanding the uptake routes of MycoShield by L. trifolii and N. formosa is essential for the application of antibiotic agrochemicals to manage bacteria-infected pest insects and their natural enemies.
2.4.1 MycoShield uptake route of L. trifolii
The uptake route of L. trifolii was initially analyzed. As L. trifolii does not feed directly on the surface of kidney bean leaves, we hypothesized that MycoShield is primarily ingested by larvae feeding on the mesophyll rather than by adults. Two separate experiments were conducted to test this hypothesis.
First, MycoShield was prepared at a standard concentration (diluted 1,000-fold with water) and sprayed onto 12 leaves from three kidney bean plants. Three pairs of L. trifolii adults were introduced into cages containing the treated plants. After one and three days, the adults were transferred to a new cage containing three untreated kidney bean plants. Eggs laid on untreated plants were collected, and the infection status of Wolbachia in the offspring was analyzed. The experiments were conducted under controlled conditions (25°C, 16 h light:8 h dark photoperiod).
Next, three pairs of L. trifolii adults were introduced into a cage containing three untreated kidney bean plants to examine the uptake of MycoShield by the larvae. After 24 h, the plants with L. trifolii eggs were collected. These plants were then sprayed with MycoShield at the standard concentration and placed in a new cage under controlled conditions (25°C, 16 h light:8 h dark photoperiod). Larvae were collected at four, six, and eight days after treatment, and their Wolbachia infection status was analyzed.
2.4.2 MycoShield uptake route of N. formosa
Neochrysocharis formosa does not feed on kidney bean leaves; therefore, it possibly ingests MycoShield through L. trifolii larvae, which consume MycoShield-treated leaves. Adult N. formosa individuals less than 24 h after eclosion were used for these experiments, and all experiments were done at 25°C and a 16 h light:8 h dark photoperiod.
First, it was confirmed that N. formosa adults did not directly ingest MycoShield from the leaves. MycoShields were sprayed onto three unparasitized plants (12 leaves in total) inside a cage, and six N. formosa adults were released. Then these adults were moved to new cages on days one-, three-, five-, and seven-day days later, which introduced untreated kidney bean parasitized by L. trifolii. The infection and male ratios of adult individuals in the next generation were analyzed (Supplementary Figure 1A).
L. trifolii larvae immediately die when parasitized by N. formosa. Second, N. formosa is expected to ingest L. trifolii larvae if L. trifolii larvae die before being sprayed with MycoShield. MycoShield was sprayed after parasitizing N. formosa, and the infection ratio during the pupal stage of N. formosa was determined (Supplementary Figure 1B).
Third, whether N. formosa could ingest MycoShield through adult host feeding was investigated. MycoShield was sprayed onto kidney bean plants that had been oviposited by L. trifolii five days earlier. Three N. formosa individuals were introduced into the cages. N. formosa adults were transferred to new, non-sprayed plants parasitized by L. trifolii on days one, three, five, and seven. Approximately 24 h later, N. formosa eggs were collected, and their infection ratio was analyzed. The experiment was repeated six times (Supplementary Figure 1C).
Fourth, the combined routes of MycoShield uptake by N. formosa were investigated. Five pairs of L. trifolii adults were introduced into the cages containing kidney bean plants. After five days, the oviposited eggs had developed into larvae, and the plants were sprayed with MycoShield before being moved to a new cage. N. formosa adults were introduced in this new cage, where they could only ingest MycoShield through host feeding, whereas N. formosa larvae consumed L. trifolii larvae that had absorbed MycoShield. Emerging N. formosa adults were collected, and their infection and male ratios were analyzed (Supplementary Figure 1D).
2.5 Effect of MycoShield concentration on L. trifolii, H. femoralis, and N. formosa populations
MycoShield solutions were prepared at different concentrations and diluted with water (100-, 1, 000-, 10, 000-, 100, 000-, 1,000,000-fold) with an untreated control. Two kidney bean plants (eight leaves in total) were used in each experiment. Once the plants had grown sufficiently, the MycoShield solutions were sprayed evenly onto their leaves, and these plants were placed inside a cage.
Three pairs of L. trifolii adults were randomly selected and placed in cages. Six H. femoralis and N. formosa adults were introduced into the cage. For N. formosa, two kidney bean plants that had been oviposited with L. trifolii were used. Six adult N. formosa individuals were introduced into the cage when oviposited L. trifolii eggs developed into larvae. The infection and male ratios in the next generation of adults were analyzed.
2.6 Effect of the MycoShield on H. femoralis population dynamics
Twelve kidney bean plants in four pots were prepared, which were sprayed with a standard concentration of MycoShield (diluted 1,000-fold with water) and placed inside a cage. Twenty adult female H. femoralis were introduced into cages. Larvae, adult females, and adult males were counted every 20 days.
After each count, new plants treated with a standard concentration of MycoShield were introduced into the same cage, and all H. femoralis individuals were transferred to new plants. Old plants were removed from the cage, but the soil was not replaced because it contained pupae. The study was conducted over 100 days.
2.7 Statistical analyses
All statistical analyses were performed using R software [version 4.4.2; (R Core Team, 2024)]. Logistic regression analysis was performed using the glm function (family = binomial) in R version 4.4.2 to model the relationship between elapsed days and infection probability. Data normality was assessed using the Shapiro–Wilk test. Nonparametric tests were applied if normality was violated (p < 0.05). All statistical tests were two-tailed, and significance was set at α = 0.05. Data visualization was performed using ggplot2 in R. Fisher’s exact test was used to evaluate the differences in infection rates among the experimental groups. For post hoc analysis, the Dunn–Bonferroni test was used. A logit transformation was performed prior to statistical analysis as the sex ratio data were proportional. The Kruskal–Wallis test was used for group comparisons, followed by the Dunn–Bonferroni test for post hoc analysis. The number of larvae was analyzed using the nonparametric Friedman test to assess temporal differences (p < 0.01). The Dunn–Bonferroni test was conducted as a post hoc analysis when significant differences were detected. A repeated-measures analysis of variance was performed to evaluate temporal changes in the number of H. femoralis adults. Mauchly’s sphericity test was applied, and the Greenhouse–Geisser correction was used if the assumption of sphericity was violated (p < 0.05).
3 Results
3.1 Reduction of Wolbachia and Rickettsia infection rates in adult offspring under standard MycoShield treatment
Following a 24-h exposure of adult insects to MycoShield treatment at the standard concentration (1,000-fold dilution), Wolbachia infection rates among adult offspring were 6.67% (2/30 individuals) in L. trifolii and 3.33% (1/30 individuals) in H. femoralis. For N. formosa, the Rickettsia infection rate was 0% (0/30 individuals) after 24 h of exposure to a honey droplet containing MycoShield at the same concentration.
3.2 MycoShield ingestion mechanisms of L. trifolii and N. formosa
3.2.1 Liriomyza trifolii ingests MycoShield through leaf feeding at the adult and larval stages
The next-generation Wolbachia infection ratio decreased to 60% after one day of exposure when adult L. trifolii were fed by leaves sprayed with MycoShield and allowed to oviposit on fresh leaves. Additionally, the infection rate significantly declined to 23.3% by day three and 16.7% by day five, compared to that before treatment (Figure 1A). The relationship between elapsed days and infection probability was modeled as:
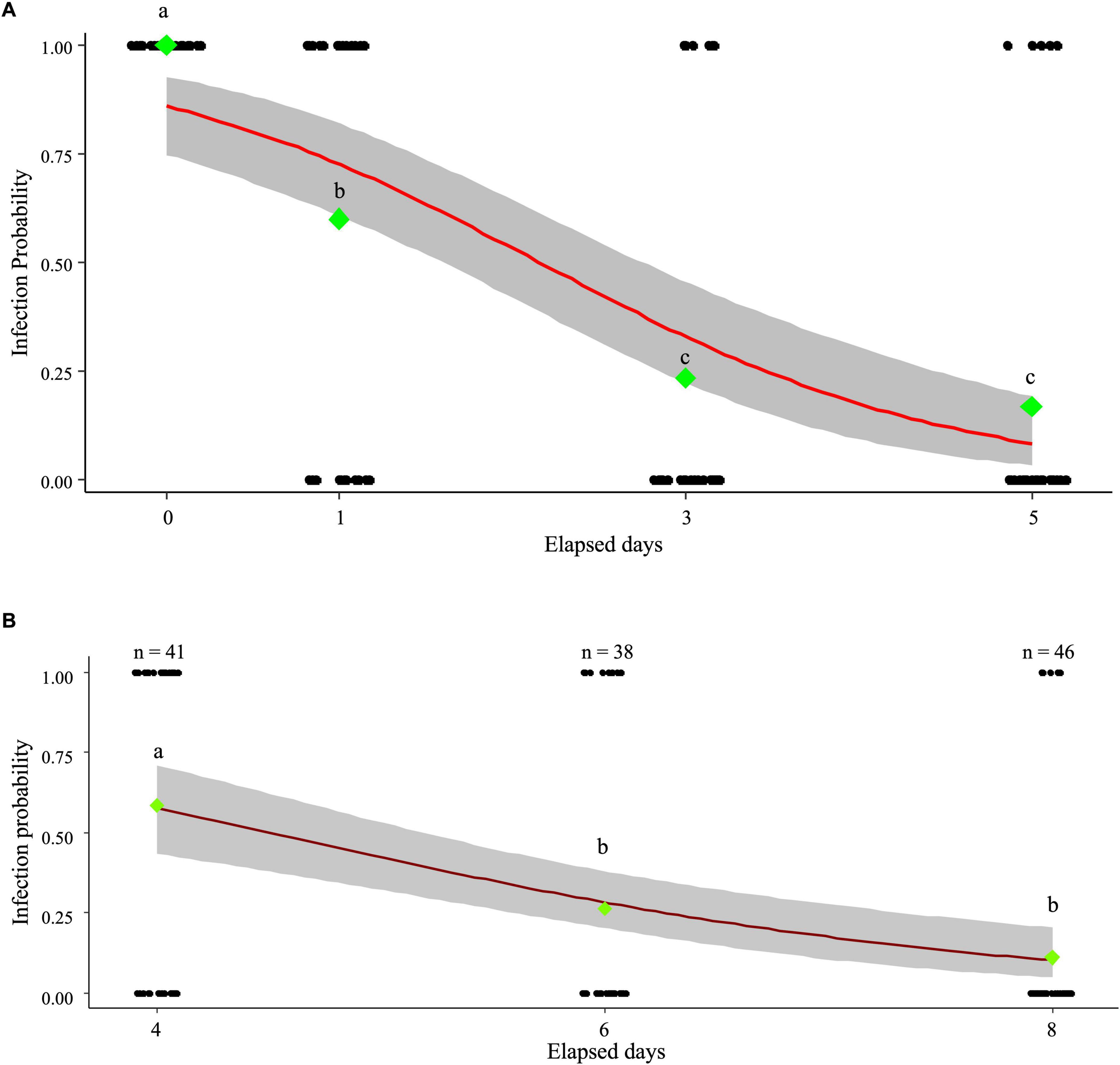
Figure 1. MycoShield uptake by Liriomyza trifolii adults and larvae from treated leaves. The data for each individual, with infected individuals represented as one and uninfected individuals as zero, are shown as black-jitter plots. Green diamonds indicate the infection rates for each experimental group. The data were logit-transformed into actual values before statistical analysis, as the infection ratio is proportional to the data. Fisher’s exact test indicated a significant difference (p < 0.01), with different letters representing statistical significance (a, b, and c). (A) MycoShield uptake experiment results in L. trifolii adults. Liriomyza trifolii adults were provided with MycoShield-treated leaves for either one or three days. They were then transferred to fresh leaves, and the Wolbachia infection rate in the eggs laid on these leaves was assessed. The red line represents the logistic regression curve [, p < 0.01], whereas the gray area denotes the confidence interval. (B) MycoShield uptake experiment results in L. trifolii larvae. MycoShields were applied to the leaves on which eggs had been laid, and larvae were collected at four, six-, and eight days post-application to assess infection. The red line represents the logistic regression curve [, p < 0.01], whereas the gray area denotes the confidence interval.
where P(x) is the probability of infection at time x. The effect of time was significant (p = 5.93 × 10–9), indicating a decrease in infection probability over time.
When MycoShield was applied to leaves containing L. trifolii larvae, the Wolbachia infection rate in L. trifolii gradually declined, reaching 10.9% on day eight (Figure 1B). The relationship between elapsed days and infection probability was modeled as:
where P(x) is the probability of infection at time x. The effect of time was significant (p = 9.24 × 10–9), indicating a decrease in infection probability over time.
3.2.2 Neochrysocharis formosa does not ingest MycoShield from treated leaves
The Rickettsia infection rate in N. formosa remained 100% on days one (n = 43), three (n = 31), five (n = 28), and seven (n = 45) after exposure to MycoShield-treated leaves, with no males observed. An examination of N. formosa pupae revealed a Rickettsia infection rate of 100% (n = 48) when MycoShield was applied to leaves containing L. trifolii parasitized by N. formosa. The infection ratio remained unchanged, regardless of the number of days after N. formosa was fed dead L. trifolii larvae.
3.2.3 Neochrysocharis formosa ingests MycoShield from L. trifolii larvae through host feeding and parasitism
The Rickettsia infection rate in the next generation of N. formosa adults on day seven after MycoShield exposure declined to 53% (Figure 2A). N. formosa adults acquired MycoShield through host feeding, and the Rickettsia infection ratio declined with time. The Rickettsia infection rate gradually decreased and reached 0% on day five when N. formosa parasitized L. trifolii larvae that had ingested MycoShield. The male ratio gradually increased, reaching 100% after five days (Figure 2B).
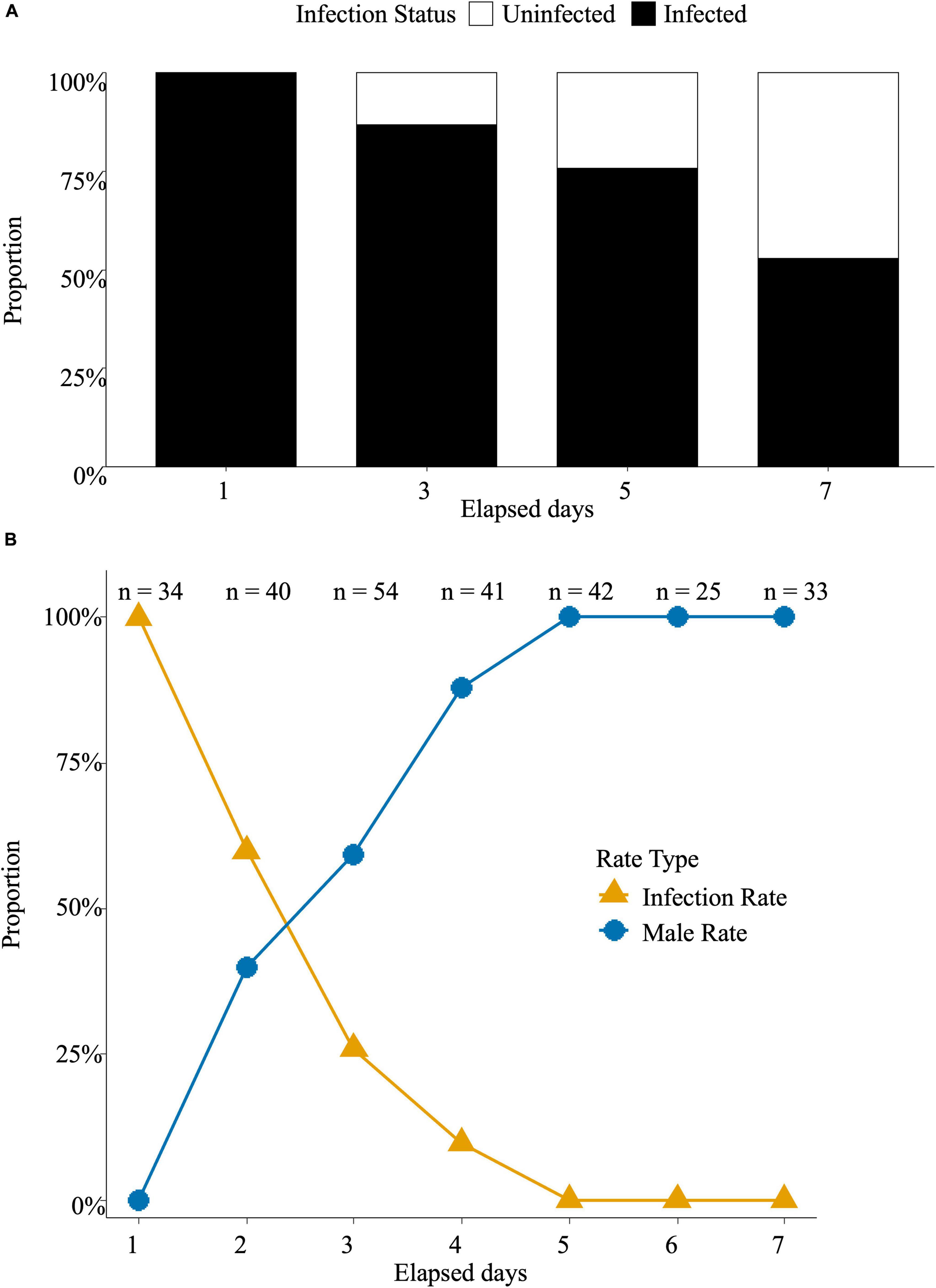
Figure 2. Neochrysocharis formosa acquires antibiotics from its host, L. trifolii larvae. (A) Neochrysocharis formosa was introduced and maintained for one, three, five, and seven days after spraying MycoShield on leaves containing L. trifolii larvae. They were then transferred to fresh, untreated leaves for oviposition, and whether the collected eggs were infected was determined. Black boxes represent the proportion of infected individuals, whereas white boxes represent the proportion of uninfected individuals. (B) Liriomyza trifolii adults were allowed to oviposit on MycoShield-treated leaves for five days. When the larvae emerged, N. formosa was introduced and allowed to oviposit. Rickettsia infection in the next-generation adults was determined. Blue circular plots represent the male sex ratio, whereas orange triangular plots indicate the Rickettsia infection rate. The sample size for each time point was denoted as n = X.
3.3 Effect of MycoShield concentrations
The effects of various MycoShield concentrations on L. trifolii, H. femoralis, and N. formosa are presented in Table 1. At a 100-fold dilution with water, only one N. formosa individual was infected, and all tested L. trifolii and H. femoralis individuals were uninfected. At the standard concentration (diluted 1,000-fold with water), Wolbachia was removed from almost all individuals (1.4–2.8% remaining). The infection ratio was increased at low concentrations. The male ratio of L. trifolii was not affected by MycoShield, regardless of the concentration. However, for H. femoralis and N. formosa, the male proportion gradually increased as the concentration decreased.
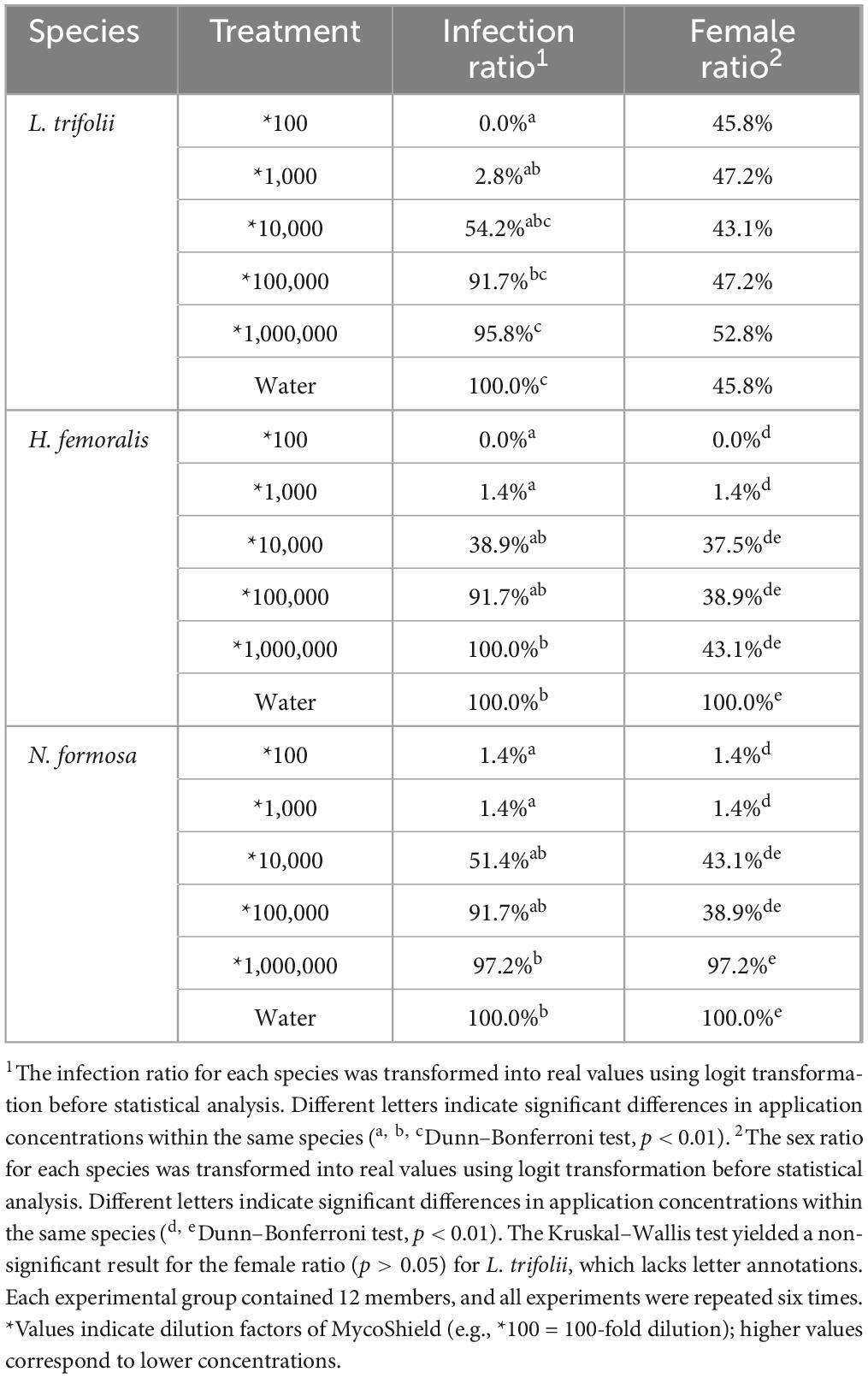
Table 1. Symbiont infection rates and female ratios in the next generation of three insect species following MycoShield treatment (Wolbachia for L. trifolii and H. femoralis; Rickettsia for N. formosa).
3.4 Extinction of H. femoralis
Whether the application of MycoShield affects the population dynamics of H. femoralis, in which Wolbachia induces parthenogenesis, was investigated. Larval numbers were initially high on day 20 but showed a sharp decline by day 40 and remained low thereafter (Figure 3A). The proportion of adult females was nearly 100% on day 20 but dropped dramatically by day 40, remaining consistently at approximately 20% or lower through day 100 (Figure 3B).
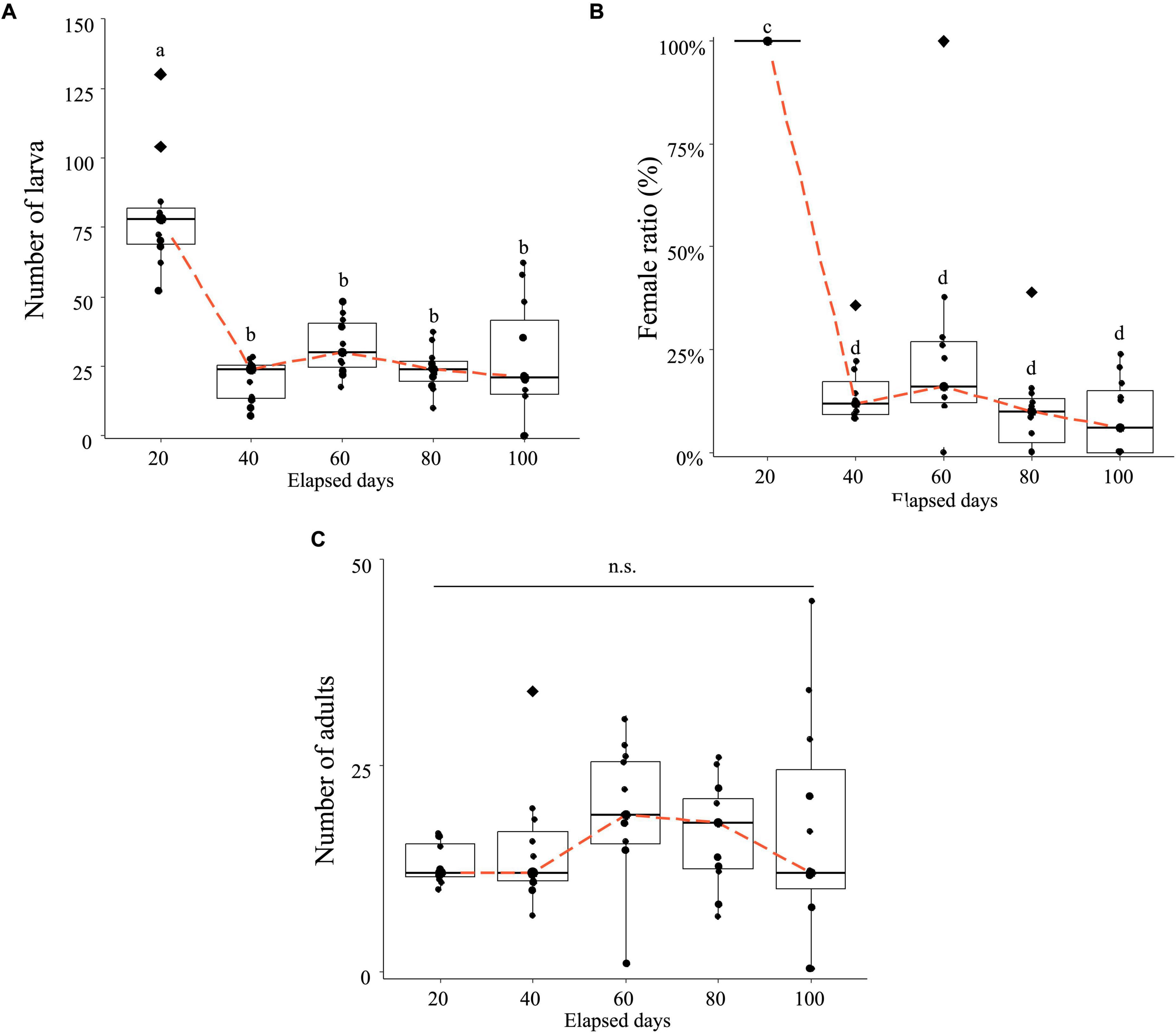
Figure 3. Hercinothrips femoralis exhibits a decrease in the female ratio and population size under prolonged MycoShield application. The data obtained from the 11 rearing cages are plotted along with box plots. The orange dashed line connects the median values for each time point, and diamonds represent the outliers. (A) Changes in larval count over 100 days of rearing after MycoShield application. Post hoc analysis was performed using the Dunn–Bonferroni test, and significantly different groups are indicated by different letters (a and b). (B) Changes in the female ratio over 100 days of rearing after MycoShield application. Because the female ratio is proportional to the data, it was logit-transformed into real values before statistical analysis. Post hoc analysis was conducted using the Dunn–Bonferroni test and significantly different groups are indicated by different letters (c and d). (C) Changes in adult counts after MycoShield application. Repeated measures analysis of variance with this correction revealed no significant differences among the time points (n.s.: p = 0.388).
Although adult numbers increased between days 20 and 60, no significant difference was observed across the cages during the entire observation period (Figure 3C). By day 80, three cages exhibited a female ratio of 0%. Among these, two cages (Rep. 5 and Rep. 10) experienced complete extinction by day 100, with both larvae and adults absent (Figure 3C and Table 2). Notably, the cages in which larvae had disappeared were identical to those in which adult populations ultimately became extinct.
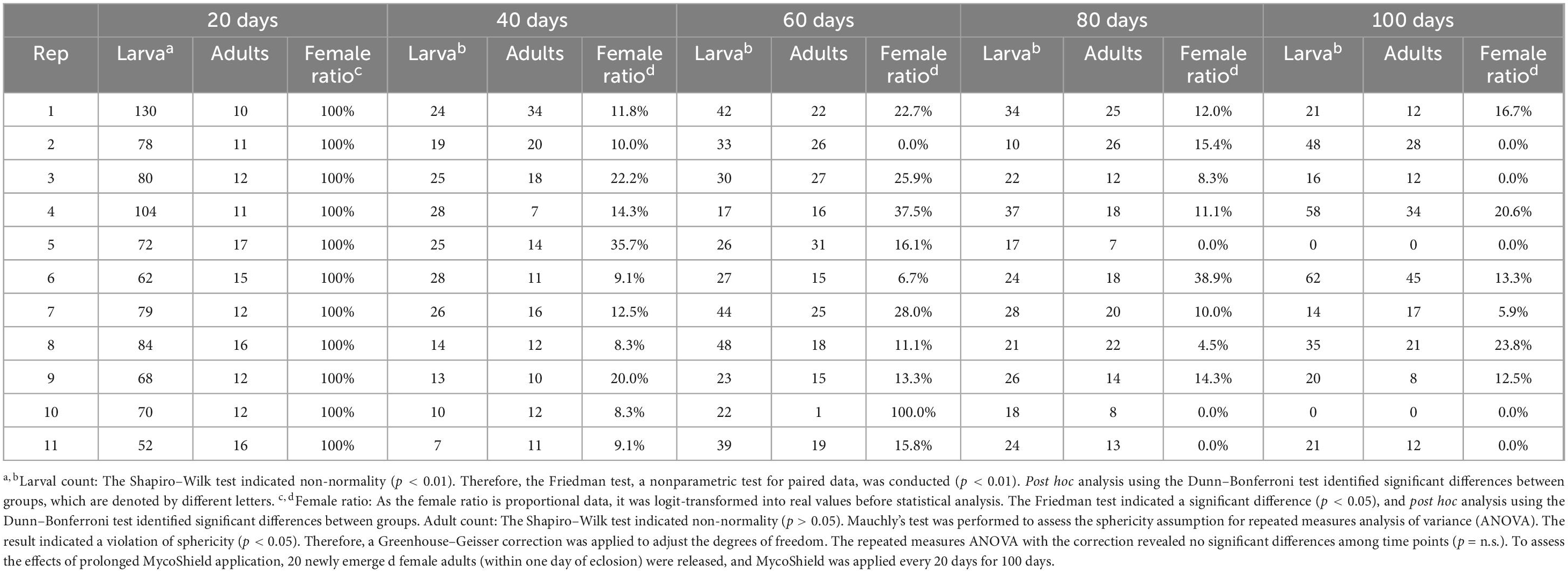
Table 2. Effects of prolonged MycoShield application on female ratio and population size for Hercinothrips femoralis.
These results indicate that MycoShield treatment drastically reduced the reproductive potential and population size of H. femoralis under caged conditions. Complete extinction occurred in two out of eleven cages within 100 days, likely due to genetic drift following severe population bottlenecks.
4 Discussion
Wolbachia was almost eliminated from L. trifolii and H. femoralis when MycoShield was sprayed at the standard concentrations on kidney bean leaves. Additionally, Rickettsia was completely removed (Table 1) when N. formosa was fed MycoShield with honey. Although only one agrochemical antibiotic was tested, two species of endosymbionts were successfully removed. A wide variety of agrochemical antibiotics exist globally, and Wolbachia and Rickettsia infect 52% and 24% of arthropod species, respectively (Weinert et al., 2015; Zug and Hammerstein, 2012), with numerous other symbionts also present in many species (Douglas, 1998; Kikuchi et al., 2007; Rupawate et al., 2023). These findings suggest that agrochemical antibiotics can be used as effective tools to manage agricultural pests.
The application of MycoShield to leaf surfaces resulted in a significant reduction in Wolbachia infection rates of L. trifolii and H. femoralis adults, suggesting that Wolbachia was eliminated through the gradual uptake of the antibiotic by adults (Figure 1A and Table 1). Although L. trifolii larvae burrow into the leaves, the application of MycoShield to the leaf surface also reduced the Wolbachia infection rate (Figure 1B). This finding suggests that Wolbachia were eliminated as a result of larval ingestion of MycoShield, which penetrated the leaf tissue.
The route of MycoShield uptake by N. formosa, which parasitizes L. trifolii, was also investigated. N. formosa did not ingest antibiotics upon contact with its leaves. However, when its mouthparts came into contact with L. trifolii exposed to MycoShield or when it fed on the host’s body fluids or internal tissues, antibiotic absorption occurred, leading to a reduced infection rate (Figures 2A, B). These findings suggest that antibiotic uptake by N. formosa is indirect, occurring through exposure to an antibiotic-treated host. Key stages of this process include feeding on the host and larval consumption of infected tissues.
In agricultural settings, MycoShield is typically diluted 1,000-fold with water before application. At this concentration, the Wolbachia infection rate in L. trifolii decreases to 2.8%, the Wolbachia infection rate in H. femoralis decreases to 1.4%, and the Rickettsia infection rate in N. formosa decreases to 1.4% (Table 1). These results indicate that MycoShield application in agricultural settings can effectively eliminate symbiotic bacteria from insects.
When N. formosa was exposed to MycoShield diluted 1,000-fold, Rickettsia was eliminated, resulting in the loss of thelytoky parthenogenesis and the production of male offspring (Figure 2B). This finding suggests that MycoShield may also influence N. formosa populations in agricultural settings, particularly in controlling leaf miners. Some parasitoid wasps are commercially available as biological control agents, playing a crucial role in pest population management via parasitism (van Lenteren, 2012). As female wasps are essential for successful biological control, careful selection of pest management methods is necessary to avoid compromising parasitoid populations.
Although selective elimination of Wolbachia without affecting Rickettsia would be ideal for preserving the biological control capacity of N. formosa, currently no antibiotics are known that can achieve such specificity. This is because Wolbachia and Rickettsia are both members of the Alphaproteobacteria (Dunning Hotopp et al., 2006) and share many fundamental molecular targets, such as ribosomal components and replication machinery (Andersson et al., 1998; Ioannidis et al., 2007; Zug and Hammerstein, 2015). Consequently, the application of broad-spectrum antibiotics like tetracycline inevitably impacts both symbionts (Nguyen et al., 2014). However, previous studies in aphids have demonstrated selective removal of specific endosymbionts through dose-dependent antibiotic treatments—e.g., Serratia via ampicillin and Buchnera via rifampicin (Koga et al., 2007). These findings suggest that with careful optimization of antibiotic type and concentration, selective targeting of symbionts like Wolbachia and Rickettsia may be achievable in other insect systems. Further research to explore such possibilities could significantly improve the integration of antibiotic-based approaches with biological control programs.
To evaluate long-term suppression, we conducted a 100-day experiment in which MycoShield was applied every 20 days at a field-relevant concentration (1,000-fold dilution). In theory, eliminating thelytoky parthenogenesis-inducing Wolbachia should result in all-male offspring, leading to population collapse (Table 1, female ratio: 1.4%). This outcome was observed in two plots where both larvae and adults were absent by day 100 (Figure 3C). However, in most cages, female production persisted and complete suppression was not achieved, likely due to partial clearance of symbionts. Although Table 1 shows a sharp decline in the female ratio among next-generation adults following treatment, Table 2 indicates that female ratios remained at 10–20% in most cages during the 40–100 day period. This discrepancy can be explained by several biological and methodological factors.
First, the life cycle of H. femoralis progresses from egg to adult in approximately 18–20 days at 27°C (Laughlin, 1971). Since insect development generally slows down at lower temperatures, the cycle at our experimental condition (25°C) is expected to be slightly longer. Therefore, the 100% female ratio observed on day 20 likely reflects surviving F0 females rather than newly emerged F1 individuals. Second, although larvae likely ingest antibiotics during feeding, the efficacy of symbiont elimination depends on both the timing and cumulative dose of exposure (Koga et al., 2007; Shan et al., 2016). Since plants were replaced every 20 days, all larval stages likely encountered treated leaves; however, variability in ingestion among instars may have led to insufficient antibiotic uptake in some individuals. Moreover, as H. femoralis pupates on the soil surface, prepupae and pupae may have escaped continued exposure, especially if they entered these protected stages shortly after initial ingestion. This partial exposure scenario may explain why endosymbionts persisted in some individuals.
Interestingly, five cages had only male adults by day 100, suggesting that eradication may occur over a longer timeframe. These male-only populations were effectively reproductively sterile, as no functional females remained to sustain reproduction. This condition reflects a severe reproductive bottleneck, where antibiotic-induced elimination of symbionts disrupts thelytokous parthenogenesis. In such small, male-biased groups, genetic drift further impedes the chance of symbiont persistence or spontaneous recovery, ultimately leading to local extinction. These findings highlight the importance of optimizing treatment timing and frequency to achieve consistent disruption of symbiont-mediated reproduction. However, field conditions may introduce additional variables—such as environmental fluctuation and immigration—that could buffer or counteract this process, warranting further investigation in open settings.
The diverse beneficial roles of symbiotic microorganisms in insect hosts are well-documented. The partial or complete suppression of these microorganisms can result in nutrient deficiencies, weakened immunity, impaired plant defense mechanisms, and increased susceptibility to pathogens, parasites, and predators. Such disruptions may contribute to the partial or complete elimination of pest populations in the field by disrupting essential host functions (Rupawate et al., 2023; Gonella and Alma, 2023). Consequently, targeting symbiotic microorganisms is a promising strategy for integrated pest management.
This study used MycoShield (17.0% oxytetracycline) as the antibiotic treatment; however, numerous antibiotic agrochemicals are commercially available worldwide (McManus et al., 2002). Given the diversity of these compounds, alternative antibiotic agrochemicals may exert broader or more potent antibacterial effects, not only against obligate endosymbionts but also against gut-associated microbiota and mycetocyte-residing microorganisms in infected insects. Additionally, their effects on insect physiology, feeding behavior, and population dynamics warrant further investigation. Investigating the differential effects of various agrochemical antibiotics on insect pests and their associated microbiota is essential for optimizing symbiont-targeted pest control strategies.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
YO: Funding acquisition, Resources, Validation, Formal Analysis, Writing – original draft, Project administration, Conceptualization, Data curation, Investigation, Writing – review and editing. YT: Writing – review and editing, Funding acquisition, Investigation, Supervision, Resources, Project administration.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study was funded by JSPS KAKENHI (Grant Number JP419K06069) and JST SPRING “Interdisciplinary Frontier Next-Generation Researcher Program of the Tokai Higher Education and Research System” (Grant Number JPMJSP2125).
Acknowledgments
Special gratitude is extended to Yuki Ishihara for maintaining insects and plants. We would like to thank Editage (www.editage.jp) for the English language editing. We gratefully acknowledge the work of the past and present members of our laboratory.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1605308/full#supplementary-material
References
Adachi-Hagimori, T., Miura, K., and Stouthamer, R. (2008). A new cytogenetic mechanism for bacterial endosymbiont-induced parthenogenesis in Hymenoptera. Proc. Biol. Sci. 275, 2667–2673. doi: 10.1098/rspb.2008.0792
Andersson, S., Zomorodipour, A., Andersson, J., Sicheritz-Pontén, T., Alsmark, U., Podowski, R., et al. (1998). The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396, 133–140. doi: 10.1038/24094
Arakaki, N., and Kinjo, K. (1998). Notes on the parasitoid fauna of the serpentine leafminer Liriomyza trifolii (Burgess) (Diptera: Agromyzidae) in Okinawa, southern Japan. Japan Appl. Entomol. Zool. 33, 577–581. doi: 10.1303/aez.33.577
Araújo, M., Castanheira, E., and Sousa, S. (2023). The buzz on insecticides: A review of uses, molecular structures, targets, adverse effects, and alternatives. Molecules 28:3641. doi: 10.3390/molecules28083641
Bouchon, D., Rigaud, T., and Juchault, P. (1998). Evidence for widespread Wolbachia infection in isopod crustaceans: Molecular identification and host feminization. Proc. Biol. Sci. 265, 1081–1090. doi: 10.1098/rspb.1998.0402
Braig, H., Zhou, W., Dobson, S., and O’Neill, S. (1998). Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J. Bacteriol. 180, 2373–2378. doi: 10.1128/JB.180.9.2373-2378.1998
Copping, L., and Duke, S. (2007). Natural products that have been used commercially as crop protection agents. Pest Manag. Sci. 63, 524–554. doi: 10.1002/ps.1378
Copping, L., and Menn, J. (2000). Biopesticides: A review of their action, applications and efficacy. Pest Manag. Sci. 56, 651–676. doi: 10.1002/1526-4998(200008)56:8<651::aid-ps201<3.0.co;2-u
Douglas, A. (1998). Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43, 17–37. doi: 10.1146/annurev.ento.43.1.17
Dunning Hotopp, J., Lin, M., Madupu, R., Crabtree, J., Angiuoli, S., Eisen, J., et al. (2006). Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2:e21. doi: 10.1371/journal.pgen.0020021
Gonella, E., and Alma, A. (2023). The role of symbiont-targeted strategies in the management of pentatomidae and tephritidae pests under an integrated vision. Agronomy 13:868. doi: 10.3390/agronomy13030868
Hagimori, T., Abe, Y., Date, S., and Miura, K. (2006). The first finding of a Rickettsia bacterium associated with parthenogenesis induction among insects. Curr. Microbiol. 52, 97–101. doi: 10.1007/s00284-005-0092-0
Houston, K., Mound, L., and Palmer, J. (1991). Two pest Thrips (Thysanoptera) new to Australia, with notes on the distribution and structural variation of other species. Aust. J. Entomol. 30, 231–232. doi: 10.1111/j.1440-6055.1991.tb00419.x
Hurst, G., and Jiggins, F. (2000). Male-killing bacteria in insects: Mechanisms, incidence, and implications. Emerg. Infect. Dis. 6, 329–336. doi: 10.3201/eid0604.000402
Ioannidis, P., Dunning Hotopp, J., Sapountzis, P., Siozios, S., Tsiamis, G., Bordenstein, S., et al. (2007). New criteria for selecting the origin of DNA replication in Wolbachia and closely related bacteria. BMC Genom. 8:182. doi: 10.1186/1471-2164-8-182
Ishiie, T., Doi, Y., Yora, K., and Asuyama, H. (1967). Suppressive effects of antibiotics of tetracycline group on symptom development of mulberry dwarf disease. Nippon Shokubutsu Byori Gakkaiho 33, 267–275. doi: 10.3186/jjphytopath.33.267
Kikuchi, Y., Hosokawa, T., and Fukatsu, T. (2007). Insect-microbe mutualism without vertical transmission: A stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl. Environ. Microbiol. 73, 4308–4316. doi: 10.1128/AEM.00067-07
Koga, R., Tsuchida, T., Sakurai, M., and Fukatsu, T. (2007). Selective elimination of aphid endosymbionts: Effects of antibiotic dose and host genotype, and fitness consequences. FEMS Microbiol. Ecol. 60, 229–239. doi: 10.1111/j.1574-6941.2007.00284.x
Konishi, K. (1998). An illustrated key to the Hymenopterous parasitoids of Liriomyza trifolii in Japan. Jpn. Misc. Publ. Natl. Inst. Agro Environ. Sci. 22, 27–76. doi: 10.24514/00008644
Kumm, S., and Moritz, G. (2008). First detection of Wolbachia in arrhenotokous populations of thrips species (Thysanoptera: Thripidae and Phlaeothripidae) and its role in reproduction. Environ. Entomol. 37, 1422–1428. doi: 10.1603/0046-225x-37.6.1422
Laughlin, R. (1971). A culture method for Hercinothrips femoralis (Reuter) (Thysanoptera). Aust. J. Entomol. 10, 301–303. doi: 10.1111/j.1440-6055.1971.tb00047.x
Masetti, A., Lanzoni, A., Burgio, G., and Süss, L. (2004). Faunistic study of the Agromyzidae (Diptera) on weeds of marginal areas in northern Italy agroecosystems. Ann. Entomol. Soc. Am. 97, 1252–1262. doi: 10.1603/0013-87462004097[1252:fsotad]2.0.co;2
McManus, P., Stockwell, V., Sundin, G., and Jones, A. (2002). Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 40, 443–465. doi: 10.1146/annurev.phyto.40.120301.093927
Nguyen, F., Starosta, A., Arenz, S., Sohmen, D., Dönhöfer, A., and Wilson, D. (2014). Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 395, 559–575. doi: 10.1515/hsz-2013-0292
R Core Team (2024). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Rupawate, P., Roylawar, P., Khandagale, K., Gawande, S., Ade, A., Jaiswal, D., et al. (2023). Role of gut symbionts of insect pests: A novel target for insect-pest control. Front. Microbiol. 14:1146390. doi: 10.3389/fmicb.2023.1146390
Seal, D., Betancourt, R., and Sabines, C. (2002). Control of Liriomyza trifolii (Burgess) (Diptera: Agromyzidae) using various insecticides. Proc. Fla. State Hort. Soc. 115, 308–314. doi: 10.1653/024.099.0405.full
Shan, H., Zhang, C., Yan, T., Tang, H., Wang, X., Liu, S., et al. (2016). Temporal changes of symbiont density and host fitness after rifampicin treatment in a whitefly of the Bemisia tabaci species complex. Insect Sci. 23, 200–214. doi: 10.1111/1744-7917.12276
Sharma, A., Kumar, V., Shahzad, B., Tanveer, M., Sidhu, G., Handa, N., et al. (2019). Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 1:1446. doi: 10.1007/s42452-019-1485-1
Siddall, J. (1976). Insect growth regulators and insect control: A critical appraisal. Environ. Health Perspect. 14, 119–126. doi: 10.1289/ehp.7614119
Souto, A., Sylvestre, M., Tölke, Tavares, J., Barbosa-Filho, J., and Cebrián-Torrejón, G. (2021). Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules 26:4835. doi: 10.3390/molecules26164835
Stockwell, V., and Duffy, B. (2012). Use of antibiotics in plant agriculture. Rev. Sci. Tech. 31, 199–210. doi: 10.20506/rst.31.1.2104
Stouthamer, R., and Kazmer, D. (1994). Cytogenetics of microbe-associated parthenogenesis and its consequences for gene flow in Trichogramma wasps. Heredity 73, 317–327. doi: 10.1038/hdy.1994.139
Stouthamer, R., Luck, R., and Hamilton, W. (1990). Antibiotics cause parthenogenetic Trichogramma (Hymenoptera/Trichogrammatidae) to revert to sex. Proc. Natl. Acad. Sci. U.S.A. 87, 2424–2427. doi: 10.1073/pnas.87.7.2424
Tagami, Y., Doi, M., Sugiyama, K., Tatara, A., and Saito, T. (2006b). Wolbachia-induced cytoplasmic incompatibility in Liriomyza trifolii and its possible use as a tool in insect pest control. Biol. Control 38, 205–209. doi: 10.1016/j.biocontrol.2006.03.008
Tagami, Y., Doi, M., Sugiyama, K., Tatara, A., and Saito, T. (2006a). Survey of leafminers and their parasitoids to find endosymbionts for improvement of biological control. Biol. Control 38, 210–216. doi: 10.1016/j.biocontrol.2006.01.015
Trdan, S., KuŽNik, L., and Vidrih, M. (2007). First results concerning the efficacy of entomopathogenic nematodes against Hercinothrips femoralis (Reuter). Acta Agric. Slov. 89:2. doi: 10.2478/v10014-007-0001-2
van Lenteren, J. (2012). The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. Biocontrol (Dordrecht) 57, 1–20. doi: 10.1007/s10526-011-9395-1
Weinert, L., Araujo-Jnr, E., Ahmed, M., and Welch, J. (2015). The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. Biol. Sci. 282:20150249. doi: 10.1098/rspb.2015.0249
Werren, J. (1997). Biology of Wolbachia. Annu. Rev. Entomol. 42, 587–609. doi: 10.1146/annurev.ento.42.1.587
Whalon, M., and Wingerd, B. (2003). Bt: Mode of action and use. Arch. Insect. Biochem. Physiol. 54, 200–211. doi: 10.1002/arch.10117
Zhang, W. (2018). Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 8, 1–27.
Zug, R., and Hammerstein, P. (2012). Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One 7:e38544. doi: 10.1371/journal.pone.0038544
Keywords: endosymbiont, antibiotic agrochemical, pest control, Wolbachia, natural enemy insect
Citation: Ohata Y and Tagami Y (2025) Antibiotic agrochemical treatment reduces endosymbiont infections and alters population dynamics in leafminers, thrips, and parasitoid wasps. Front. Microbiol. 16:1605308. doi: 10.3389/fmicb.2025.1605308
Received: 03 April 2025; Accepted: 16 May 2025;
Published: 10 June 2025.
Edited by:
Toshiyuki Harumoto, Kyoto University, JapanReviewed by:
Monica Rosenblueth, National Autonomous University of Mexico, MexicoDaisuke Kageyama, National Agriculture and Food Research Organization (NARO), Japan
Chizu Sanjoba, The University of Tokyo, Japan
Copyright © 2025 Ohata and Tagami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuta Ohata, b2hhdGEueXV0YUBnbWFpbC5jb20=; Yohsuke Tagami, dGFnYW1peUBnbWFpbC5jb20=
 Yuta Ohata
Yuta Ohata Yohsuke Tagami
Yohsuke Tagami