- 1State Key Laboratory of Advanced Marine Materials, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 2University of Chinese Academy of Sciences, Beijing, China
- 3Marine Chemistry and Corrosion Research Group, Department of Marine Science, Akwa Ibom State University, Uyo, Nigeria
- 4Department of Biotechnology, Shaheed Benazir Bhutto University, Sheringal, Pakistan
- 5Aquatic Biotechnology, University of Duisburg-Essen, Essen, Germany
- 6Guangxi Key Laboratory of Marine Environmental Science, Institute of Marine Corrosion Protection, Guangxi Academy of Sciences, Nanning, China
Microbially influenced corrosion (MIC) and biomineralization are widely observed in marine, deep-sea, freshwater, and soil ecosystems. Recently, MIC and biomineralization associated with biofouling have significantly impacted marine resources, including deep-sea minerals and organisms. Notably, uncontrolled biomineralization by certain microorganisms, such as barnacles adhering to ship hulls, can lead to structural damage and economic challenges due to biocorrosion. Biomineralization can be categorized into induced mineralization and controlled mineralization. In natural environments, induced biomineralization is the predominant process. The mechanisms of induced biomineralization and MIC in extreme deep-sea environments have attracted significant attention. The factors influencing these processes are highly complex. The microbial-material interfaces serve as the primary sites for key biochemical reactions driving biocorrosion and biomineralization. Within these interfaces, biofilms, their secreted extracellular polymers, and extracellular electron transfer mechanisms play crucial roles in these processes. Thus, a comprehensive understanding of MIC and biomineralization under deep-sea environmental conditions is essential. Investigating the relationship between these phenomena and exploring their underlying mechanisms are critical for both research advancements and industrial applications.
1 Introduction
The deep sea is one of the Earth’s most diverse and species-rich habitats (Saide et al., 2021). As terrestrial resources gradually deplete, there has been an increased effort to explore deep-sea oil and gas, minerals, and biological resources using advanced equipment. However, deep-sea environments pose significant challenges to material durability due to extreme physicochemical conditions such as high hydrostatic pressure, low temperatures, variable salinity, low dissolved oxygen, and fluctuating pH. These factors can significantly accelerate corrosion processes, especially when compounded by microbial activity. Microbiologically influenced corrosion (MIC) and biomineralization are two critical phenomena in deep-sea settings that directly affect the structural integrity of subsea equipment and infrastructure (Wu et al., 2025). Despite technological advancements in deep-sea operations, the role of microorganisms in promoting corrosion or facilitating mineral deposition remains insufficiently understood (Xiao et al., 2025). In particular, the ways in which deep-sea microorganisms survive, adapt, and interact with metal surfaces under such extreme conditions have yet to be fully elucidated. Recent studies have shown that microbial biofilms may enhance localized corrosion or, conversely, induce the formation of protective mineral layers through biomineralization. These processes are thus essential considerations in evaluating material performance and failure mechanisms in deep-sea environments.
Biomineralization processes are widespread in the ocean and play a crucial role in the marine geochemical cycles (He et al., 2023). Biomineralization refers to the differentiation, migration, enrichment, and transformation of inorganic substances under biological influence. It is a process where mineral formation is controlled or influenced by organic matter, converting dissolved ions into inorganic minerals. It occurs through two primary mechanisms: biologically controlled mineralization (BCM), where organisms exert precise control over mineral formation and biologically induced mineralization (BIM), where metabolic byproducts trigger mineralization (Zhang J. et al., 2024). Additionally, Dupraz et al. (2009) introduced biologically influenced mineralization as a third pathway, referring to the passive mineralization of organic matter. These processes not only contribute to the formation of deep-sea minerals, but also have significant engineering applications, including microbial mining and microbial corrosion inhibition (Hamilton, 2003). Biomineralization holds significant importance in various fields. Applications such as self-healing concrete and the fixation of toxic heavy metals have already been widely adopted in aerospace, environmental protection, construction, and energy sectors (Natalio and Maria, 2024; Zhang W. Q. et al., 2023; Park et al., 2022). In addition to biomineralization, MIC, have also been reported to impact the marine environment (Etim et al., 2020; Etim et al., 2018; Lv et al., 2024a).
Microbial activity can alter the processes occurring at the interface, either by accelerating or by slowing down metal corrosion. However, due to the diversity of microorganisms and the immense complexity of their metabolic processes, which are influenced also by environmental factors, MIC still faces challenges in practical applications (Lou et al., 2021a). Early research on MIC focused on the impact of biomineralization processes on metal surfaces and the role of extracellular enzymes within biofilm matrices in influencing electrochemical reactions at the biofilm-metal interface (Beech and Sunner, 2004). Studies suggest that biomineralization plays a key role in the formation of corrosion products through complex electron transfer interactions between microorganisms and metals (Duan et al., 2006). The interactions between biofilms and metal surfaces can lead to MIC, for example, through biomineralization driven by microbial metabolism or by biofilms serving as nucleation sites for mineral precipitation. The layer of corrosion products that forms on a material’s surface is often referred to as a biomineralization film (Borets'ka and Kozlova, 2010; Liu et al., 2022). Many microorganisms exhibit both corrosive and mineralizing properties and their interaction with the same material substrate can result in either mineralization or corrosion, depending on environmental conditions. The dual effect can lead to either corrosion inhibition or corrosion promotion. Understanding the transition from biofilm formation to biomineralization film is crucial for advancing interface studies. Biomineralization can also influence corrosion, providing niches for corrosive microorganisms (Martinez et al., 2022).
Therefore, clarifying the relationship between deep-sea biomineralization and MIC, specifically the mechanisms of corrosion and mineralization, and elucidating microbial-material interfacial corrosion processes from a biomineralization perspective will enhance our understanding of marine and deep-sea environments. This review explores various approaches to studying and improving biogeochemical cycling mechanisms while highlighting how biomineralization in marine environments can contribute to efficient corrosion inhibition and the reduction of corrosion-related losses.
2 Deep-sea environment and microbial mineral types
2.1 Deep-sea environment and related microorganisms
The term deep-sea generally refers to oceans at depths below 200 meters (Danovaro et al., 2020; Li et al., 2023). The deep-sea ecosystem includes hydrothermal vents, cold seeps, whale fall ecosystems, seamounts, oceanic trench ecosystems and abyssal plain (Feng et al., 2022; Simon-Lledó et al., 2023). It also includes incredible volumes of deep-sea water column, as shown in Figure 1B. It is characterized by perpetual darkness, temperatures ranging from −1.0 to 4.0°C or up to 400°C in hydrothermal vents, high pressure, and oligotrophic conditions, making it an extreme environment (Emery, 2001; Feng et al., 2022). Extreme environments are typically characterized by factors such as temperature, pH, pressure, salinity, toxicity, and radiation levels. These environments support a diverse range of microorganisms, known as extremophiles, that have adapted to survive under such conditions (Li et al., 2013). Compared to more familiar environments such as the atmosphere or shallow marine settings, the deep-sea environment has significantly reduced dissolved oxygen levels, which alters corrosion dynamics. Thus, conventional corrosion protection methods are often inadequate to meet the protective requirements of deep-sea equipment (Duan et al., 2023). Figure 1A illustrates representative deep-sea operational equipment, including exploration tools such as remotely operated vehicles and manned submersibles, as well as subsea engineering systems for underwater operations, deep-sea oil and gas production, and mineral resource extraction (Muñoz-Royo et al., 2021; Chen et al., 2024).
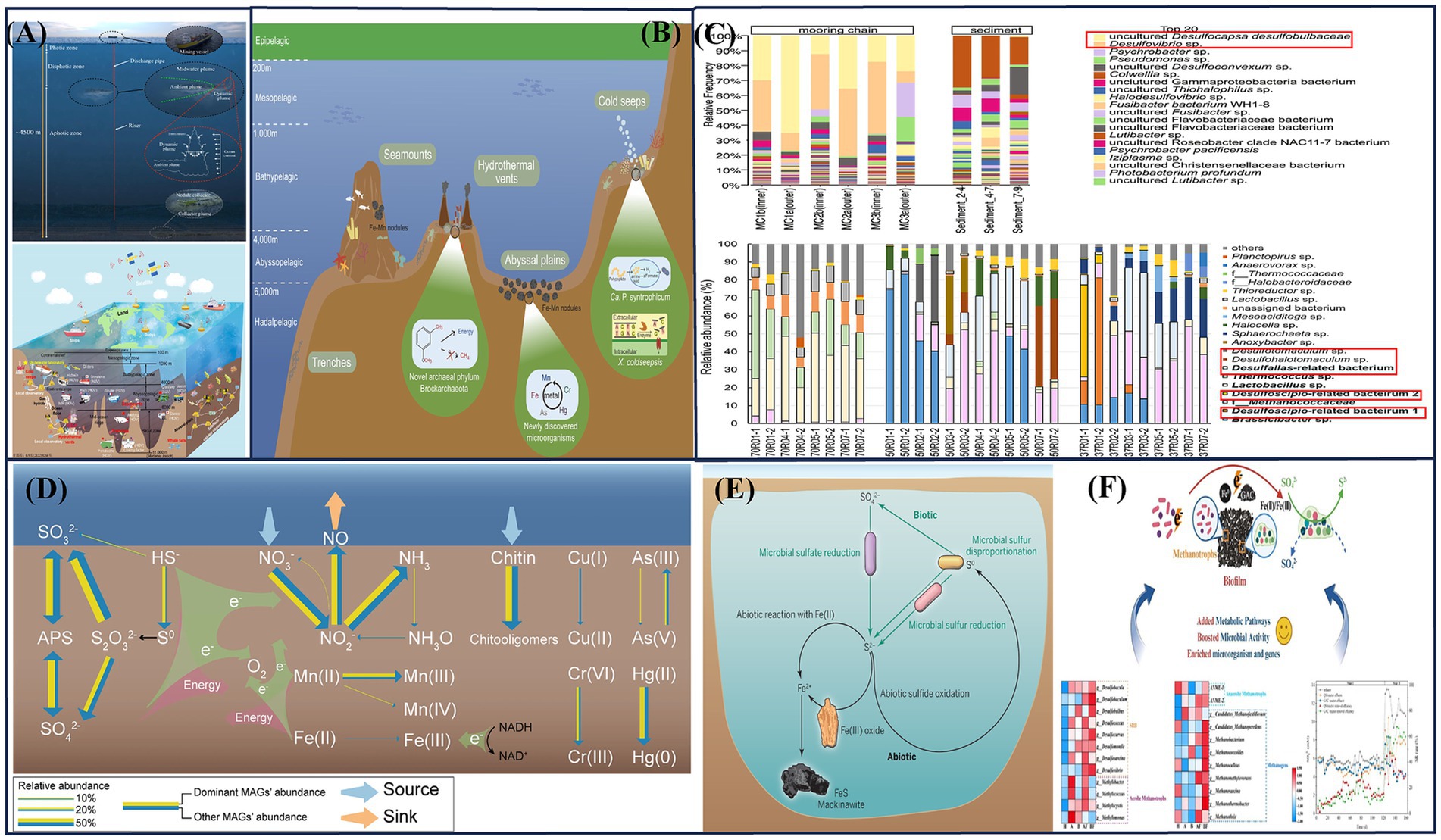
Figure 1. (A) Deep-sea service equipment (Feng et al., 2022; Drazen et al., 2020); (B) Deep-sea ecosystem (Zhang C. et al., 2024); (C) Deep-sea corrosive biotic community (Wakai et al., 2024); (Rajala et al., 2022a); (D) Deep sea iron manganese sulfur cycle diagram (Zhang D. et al., 2023); (E) Schematic diagram of iron sulfur coupling (Friedrich and Finster, 2014); (F) Schematic diagram of methane cycle and sulfur cycle (Liu et al., 2025).
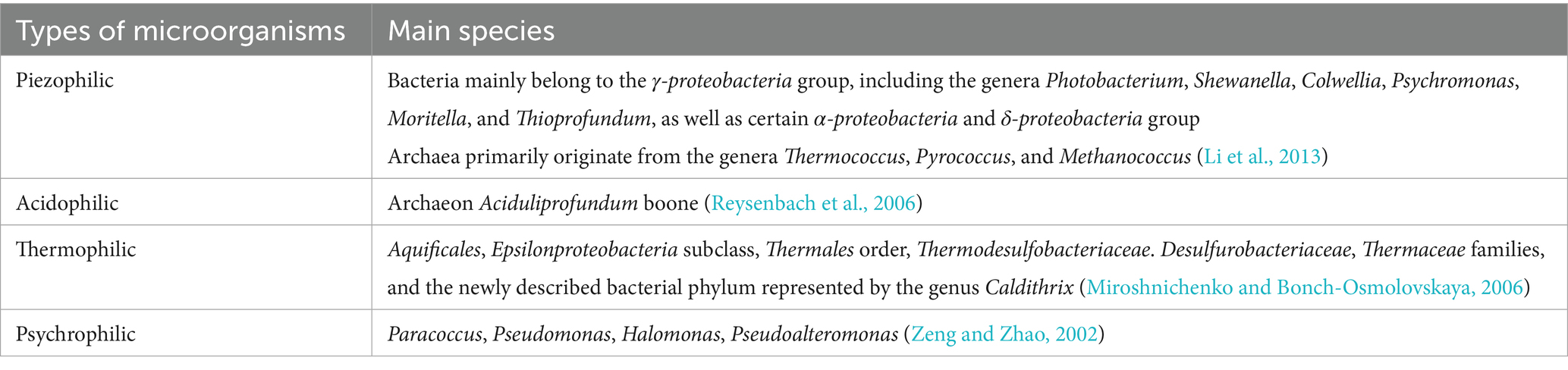
Microorganisms in deep-sea environments are highly specialized and form the foundation of biogeochemical and energy cycles, making them indispensable to deep-sea ecosystems. Exploring the different types of microorganisms in various deep-sea regions, along with their physiological and metabolic characteristics, can provide a theoretical foundation for investigating the corresponding corrosion and mineralization mechanisms. These microorganisms include thermophiles, psychrophiles, acidophiles, alkaliphiles, halophiles, and piezophiles, each adapted to extreme environmental conditions. The different microbial types detected in extreme deep-sea environments are shown in Table 1. Since more than 99% of these microorganisms lack closely related described species, significant challenges remain in understanding their diversity, physiological functional traits, and biogeochemical roles. The establishment of a deep-sea microbial library related to corrosion still requires extensive corrosion coupon experiments. Based on a large amount of genomic data, species-corrosion patterns can be derived. Researchers have successfully discovered, isolated, and cultured microbial species from deep-sea hydrothermal vents, cold seeps, marine sediments, deep-sea water, and oceanic crust. These microorganisms primarily include marine bacteria, archaea, and viruses. Among them, sulfate-reducing prokaryotes (SRP) and iron-oxidizing prokaryotes (IOP) are the most commonly associated with corrosion processes (Barton and Northup, 2007). Guezennec et al. (1998) deployed various materials (such as 316 L stainless steel, titanium, copper-nickel alloy, etc.) in the deep-sea hydrothermal vent area of the Mid-Atlantic Ridge. After 4 days of exposure, a significant accumulation of biomass was observed on all material substrates, with the formation of thick but loosely attached biofilms on the surfaces. Analysis of their lipid extracts showed characteristics of SRP. Rajala et al. (2022a) reported an integrative study of the biofilms growing on the surface of corroding mooring chain links that had been deployed for 10 years at about 2 km depth. Wakai et al. (2024) collected slime-like precipitates composed of corrosion products and microbial communities from a geochemical reactor set on an artificial hydrothermal vent for 14.5 months, and conducted culture-dependent and -independent microbial community analyses with corrosive activity measurements, as shown in Figure 1C. The performance of sulfur-metabolizing microorganisms was prominent. Fe-S-driven element coupling has also become a fundamental basis for investigating corrosion mechanisms, as shown in Figure 1E. The methane cycle in marine sediments was closely linked with the sulfur cycle, as shown in Figure 1F (Friedrich and Finster, 2014). Microorganisms in hydrothermal vent systems are predominantly extremophilic bacteria or archaea, which utilize the abundant sulfides near hydrothermal vents to produce biomass. They form the foundation of the entire hydrothermal vent ecosystem. The deep-sea hydrothermal vent regions exhibit a high microbial diversity due to the chemical complexity and rapid environmental fluctuations in these habitats. In contrast, cold seep systems have higher biomass but lower biodiversity, with archaeal communities being the dominant microbial inhabitants. In deep-sea sediments, microbial abundance is influenced by the organic matter content and proximity to continental plates, with heterotrophic microorganisms prevailing in these regions. The oceanic crust, primarily composed of mafic and ultramafic rocks rich in minerals, hosts chemoautotrophic microorganisms that play a key role in biogeochemical cycles, such as those involving Fe, Mn, and S (Wang et al., 2013). For example, the Figure 1D illustrates a schematic of a microbial-dominated ecological function study in a deep-sea ferromanganese nodule deposition area (Zhang D. et al., 2023).
2.2 Deep-sea minerals and biomineralization layers
Deep-sea minerals encompass a variety of mineral resources located on the seafloor, primarily distributed across seamounts, ridges, and basins. Common deep-sea mineral deposits include manganese nodules, seafloor cobalt crusts, and hydrothermal deposits, which polymetallic resources formed naturally under deep-sea conditions. Interestingly, recent research suggests that metal nodules in deep sea may have the potential to produce oxygen (Sweetman et al., 2024). Oceanic ferromanganese nodules, a significant underwater metal resource, are widely distributed on the modern seafloor and are rich in Fe, Mn, Ni, Cu, Co, Mo, Li, and rare earth elements. These nodules generally have a core surrounded by concentric mineral layers and are typically found at depths greater than 4,000 m. The primary minerals include Fe-rich birnessite, amorphous ferrihydrite, and various detrital minerals like magnetite, hematite, quartz, calcite feldspar, clay minerals and mica (Hein et al., 2013).
Minerals influence many important microbial activities, including energy production, nutrient acquisition, cell adhesion, and biofilm formation (Hochella, 2002). For example FeMn crust and phosphorite substrates in the Southern California Borderland support enhanced trophic biodiversity and specific trophic niches (Pereira et al., 2024). Interestingly, a recent study found that minerals not only serve as a nutritional source for microorganisms but also act as signaling molecules that regulate microbial adaptation to the environment. Minerals function as “information transmitters” in ecosystems, and the rational use of mineral signals may become a new direction (Zhang et al., 2025).
Most organic carbon mineralization in marine sediments is associated with dissimilatory sulfate reduction (DSR), as shown in Figure 1D (Qian et al., 2019). A wide range of marine organisms and microorganisms have been identified for their role in mineralization. Examples include marine organisms such as sponges and tunicates as well as marine microorganisms that facilitate the mineralization of elements like Fe, S, Mg, Ca, Mn, and Cu, and U. Since these elements exist in multiple valence states, the mineralization layers formed under different environmental and biological conditions exhibit significant diversity.
3 Mechanisms and factors influencing biomineralization
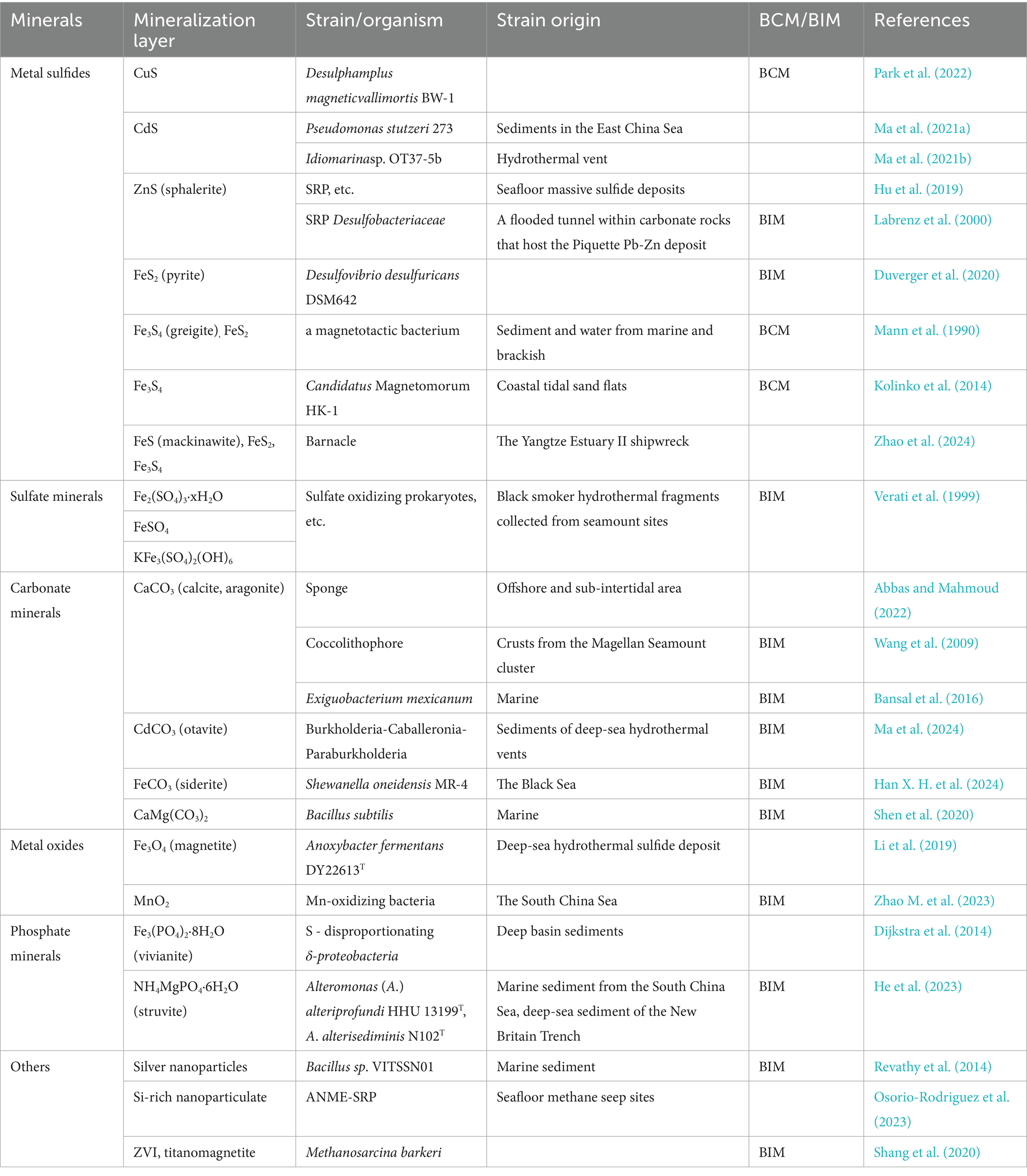
Mineralization refers to the process by which microorganisms degrade organic matter into CO2, H2O, and inorganic substances. As mentioned in the introduction, biomineralization specifically involves microbial generation of minerals through BCM, BIM and biologically influenced mineralization pathways, each with distinct mechanisms. Figure 2 illustrates the different types of mineralization associated with both biological and abiotic factors. Most microbial mineralization processes can induce mineral formation (Dupraz et al., 2009; Weiner and Dove, 2003). Notably, studies have reported that Pd ions on filamentous viruses can undergo spontaneous biomineralization under environmental conditions, forming ligand-free Pd nanowires (Kim et al., 2017). In marine and deep-sea environments, there is growing interest in the formation of minerals such as metal sulfides, sulfate minerals and carbonate minerals. Some of the key biomineralization studies are summarized in Table 2.
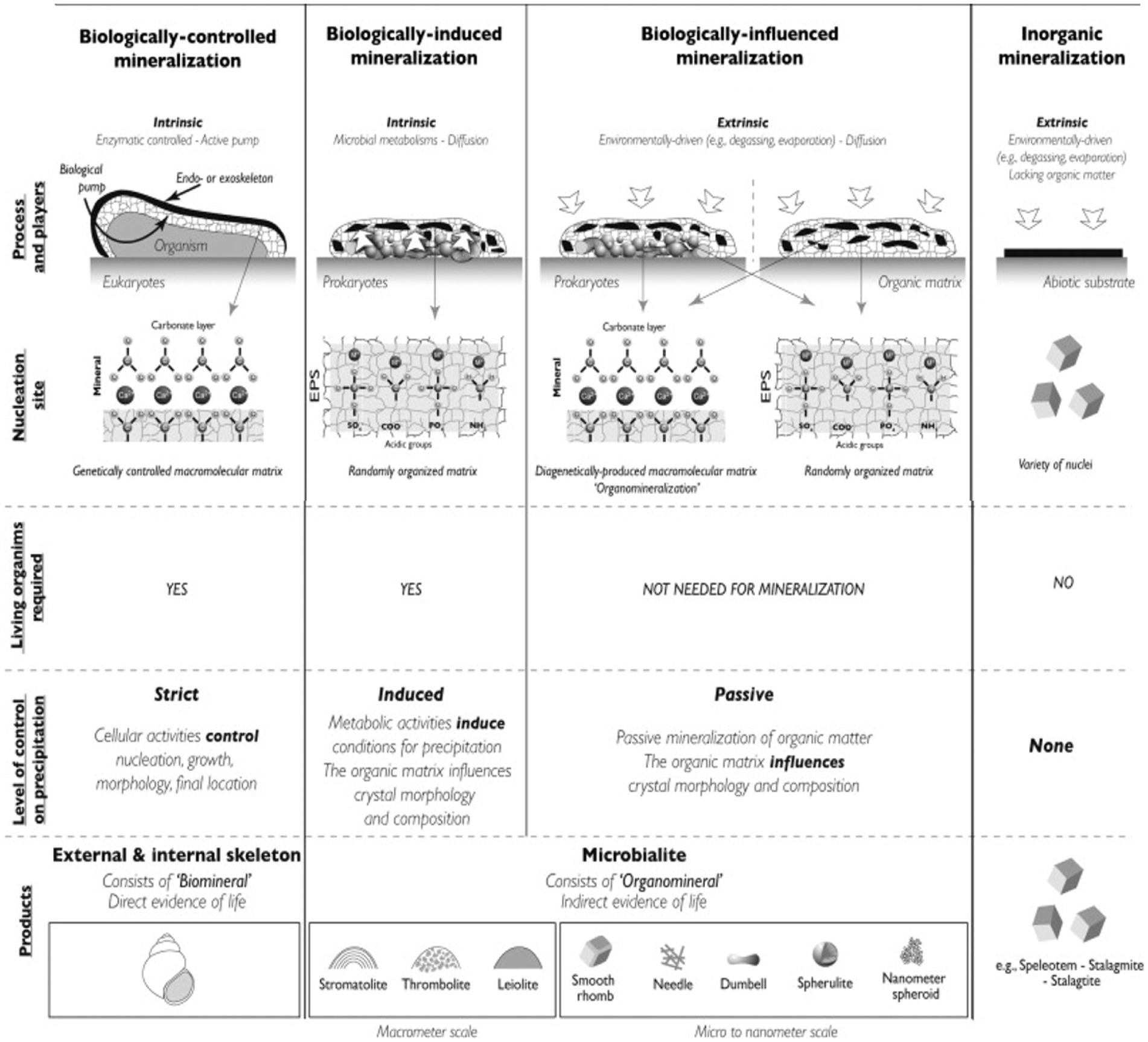
Figure 2. Different types of mineralization associated with both biological and abiotic factors (Dupraz et al., 2009).
3.1 Biologically induced mineralization
BIM is a process in which organisms modify their local microenvironment through metabolic activities, creating physicochemical conditions that favor mineral precipitation. However, the resulting products often lack specific biological functions, as illustrated in Figures 2, 3A. BIM is widespread in nature. For example, microbial mineralization contributes to the formation of carbonates, moon milk, silicates, clays, iron and manganese oxides, sulfur, and saltpeter in caves (Northup and Lavoie, 2001). Some microorganisms can directly or indirectly induce the decomposition, hydration, dissolution, or formation of secondary minerals (Banfield et al., 1999). Biomineralization is a two-step process: initially, metal ions electrostatically bind to the anionic surfaces of the cell wall and the surrounding EPS, which then as nucleation sites for crystal growth (Konhauser, 1998). Induced mineralization involves interactions between bacterial surfaces and metal ions in colloidal ionic form. Specific organic and inorganic interactions allow biomineralization to play a potential role in forming high-grade mineral deposits (Leguey et al., 2010). Further studies by He et al. (2023) on deep-sea strains Alteromonas alteriprofundi HHU 13199T and Alteromonas alterisediminis N102T have provided insights into the primary mechanism underlying BIM.
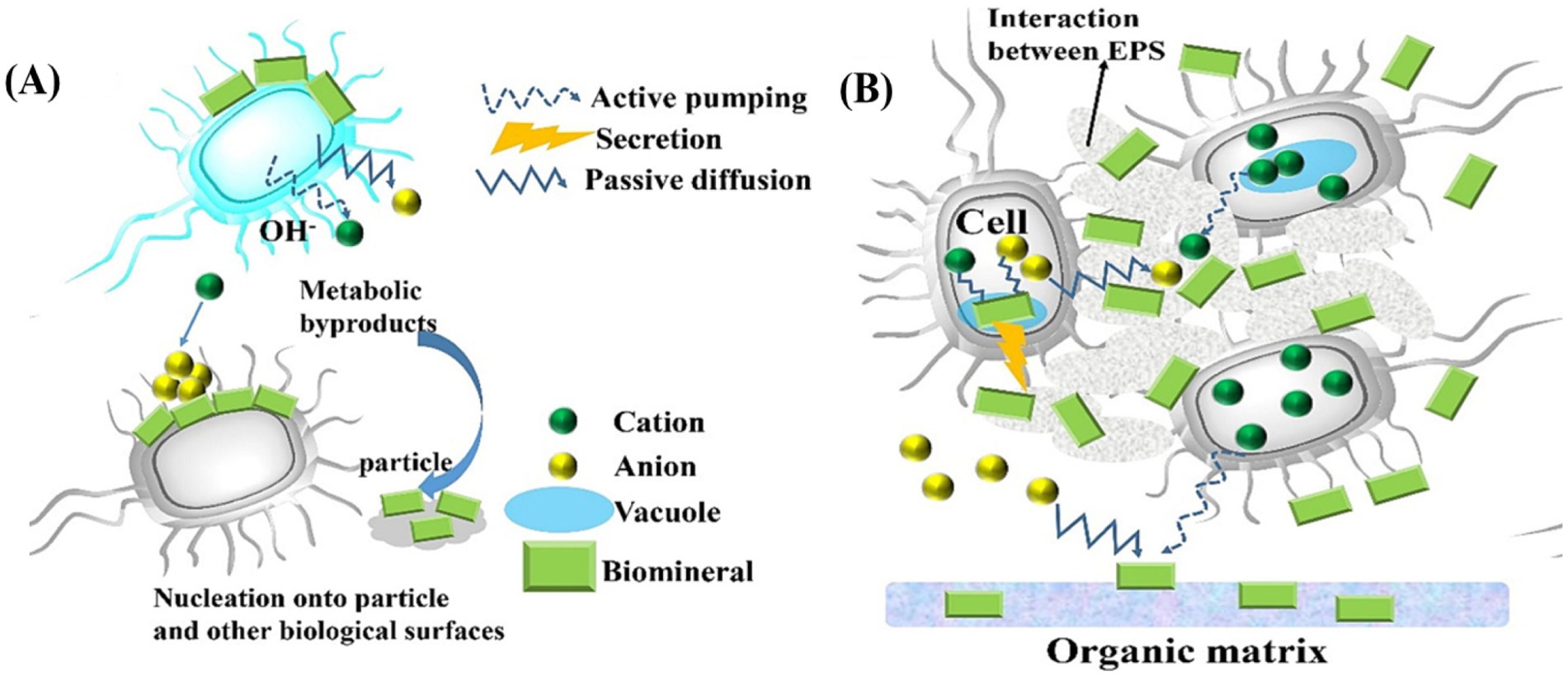
Figure 3. (A) Schematic of BIM; (B) Schematics of biologically controlled intracellular mineralization shows that nucleation occurs within the cell in a specialized vesicle (Zhang J. et al., 2024).
Certain microorganisms, such as SRP, are well known for their role in biomineralization. SRP are among the primary strains studied in marine MIC and biomineralization research. For example, discovered study found that dissimilatory SRP, particularly those from the genera Desulfovibrio and Desulfotomaculum, could deposit electron dense FeS particles within their cells when cultured in an Fe rich medium (Jones et al., 1976). In modern sedimentary environments, SRP are the main producers of sulfides. The mineral composition resulting from SRP metabolic activity is highly dependent on the source of iron. Microorganisms play a key role in the formation of iron sulfides, particularly pyrite (FeS2) (Duverger et al., 2020). The application of BIM in engineering, such as self-healing concrete and nanoparticle synthesis, has become a significant research focus.
Microbially-induced calcium carbonate precipitation (MICP) is a naturally occurring process that has gained considerable attention in recent decades (Li et al., 2024). BIM, primarily observed in various Bacillus strains, plays a key role in concrete self-healing. Among them, Bacillus pasteurii’s demonstrated excellent mineralization capability, making it a focal point of interest in geotechnical engineering (Debnath and Sen, 2024; Natalio and Maria, 2024). Recently, filamentous fungi have emerged as promising candidates for biomineralization-based self-healing concrete. Their hyphae networks form an interwoven three-dimensional structure, providing nucleation sites for CaCO3 precipitation to repair cracks (Van Wylick et al., 2021). Recent advances in fungal-mediated self-healing concrete have further confirmed their potential in sustainable construction materials. Beyond construction applications, biomineralization has significant implications for nanoparticle synthesis. The interactions between proteins and nanoparticles at mineral interfaces have garnered significant attention in the development of novel biomaterial. However, the precise role of proteins in microbial transformation and BIM remains unclear (Liu et al., 2021). Park et al. (2022) described that periplasmic proteins, such as DegP-like proteins and heavy metal-binding proteins, may be involved in the biomineralization process. Additionally, Mn has recently been identified as an accelerator of nitrogen metabolism. Biomineralized MnOx is now believed to mediate ammonia oxidation, offering a potential alternative to conventional nitrogen removal processes (Liu et al., 2024).
3.2 Biologically controlled mineralization
BCM is a process in which biological macromolecules and cells actively regulate the reaction of ingested metal ions and anions through various physicochemical mechanisms, leading to the formations of biominerals with specific assembly forms and advanced structures (Weiner and Dove, 2003). The resulting mineralized products often provide organism with specific physiological functions. The BCM process can occur extracellularly, intercellularly, or intracellularly, with the mineralization sites depending on the specific cells involved in the process (Weiner and Dove, 2003). Figure 3B demonstrates an example of intracellular BCM. Almost all BCM processes take place within isolated microenvironment. During mineralization, vesicles often serve as confined microenvironment, guiding biominerals nucleation within microorganisms while controlling the composition and morphology of the resulting biominerals.
A typical representative of BCM is magnetotactic bacteria (MTB), which are Gram-negative bacteria capable of moving along magnetic field lines and oxygen concentration gradients. They are primarily isolated from hypoxic conditions in aquatic niches and rarely from soil (Mistry et al., 2024). MTB can synthesize intracellular nanoscale minerals of magnetite (Fe3O4) and/or greigite (Fe3S4), forming magnetosomes that enable magnetotactic orientation. As a result, they serve as an excellent model system for studying biomineralization (Lin et al., 2014). Magnetosomes are generally 20–120 nm in length, consist of highly pure magnetite or greigite and are arranged in chains within MTB. These structures have been regarded as magnetic fossils of life, serving as important clues for exploring early earth conditions and potential extraterrestrial life activities. In some marine protozoa, magnetotactic ability is acquired through the uptake of extracellular symbiotic bacteria, Wherein the protozoa ingest MTB along with their magnetosome chains, internalizing the bacteria (Mandal, 2023). Metal sulfides are a common type of extracellular bacterial biominerals, but intracellular biomineralization has also been observed, such as the formation of greigite in MTB (Park et al., 2022). Numerous MTBs may significantly influence the iron cycle in oxic-anoxic transition zones and anoxic zones, facilitating the deposition of fine-grained magnetite and greigite. Consequently, they enhance the remanent magnetization of sediments (Bazylizinki et al., 1993).
3.3 Biologically influenced mineralization
Biologically influenced mineralization is a passive process that does not directly result from microbial activities but rather from interactions between extracellular biopolymers and the geochemical environment (Decho, 2010). In biologically influenced mineralization, external, environmental factors, such as increased alkalinity, play a crucial role in creating conditions favorable for mineral precipitation, rather than microbial metabolism itself. However, an organic matrix is involved in biologically influenced mineralization, influencing the morphology and composition of the resulting crystals. This occurs through interactions between the forming mineral and the organic matter, which serves as a template for precipitation (Dupraz et al., 2009).
3.4 Factors influencing biomineralization
3.4.1 EPS and biofilm
The 1988 glossary of the Berlin Dahlem Conference on biofilm structure and function described EPS as “microbially derived organic polymers,” responsible for binding cells and particulate matter together in biofilm systems. Polysaccharides, proteins, nucleic acids, and lipids are the primary components of EPS. The EPS matrix plays a crucial role in biofilms by keeping bacteria in proximity, expanding interactions, capturing and enriching metal ions, and enhancing mechanical strength. Additionally, EPS contributes to biofilm resilience by increasing resistance to antimicrobials and environmental stresses (Flemming, 2016). For example, spores coated with EPS have been shown to enhance biomineralization capabilities (Debnath and Sen, 2024).
Biofilm is an aggregation of microorganisms, in which cells are typically embedded in a self-produced EPS matrix and attached to surfaces (Vert et al., 2012). Bacterial biofilms are formed through a complex yet orderly process involving intra- or inter-cellular communication, environmentally responsive gene expression, and secretion of EPS (Fang et al., 2020). Cultivable microbial populations are more abundant in biofilm samples than in those exposed to natural weathering (Zhang Y. et al., 2023). Bacteria with strong biofilm-forming abilities can adhere to the surfaces, such as carbon steel, forming dense biofilms that may induce biomineralization (Hu et al., 2023). In marine environments, biofilms, along with iron and manganese oxides, are closely associated with biomineralization and biocorrosion caused by iron bacteria in marine environments (Zhang K. et al., 2024). Shift in bacterial populations, including SRP, denitrifying bacteria, and iron-reducing bacteria, are observed within biofilms. Notably, various exogenous factors, such as riboflavin and quorum-sensing compounds, including autoinducer-1 [acyl-homoserine lactone (AHL)] and autoinducer-2, can influence biofilm formation, ultimately affecting mineralization processes (Hao et al., 2024; Zhao J. et al., 2023; Mukherjee and Bossier, 2019).
3.4.2 Biochemical parameters
Biochemical parameters significantly influence microbial community distribution and biomineralization capacity (Zhang Y. et al., 2023). These parameters include pH, temperature, humidity, cell concentration, and enzyme activities. It is generally believed that the morphology of mature magnetite crystals formed by MTB is largely unaffected by environmental conditions. However, Moisescu et al. (2014) reviewed various studies and found that changes in environmental factors such as temperature, pH, external iron concentration, external magnetic field, static or dynamic fluid conditions, and nutrient availability or concentration could affect the biomineralization processes. Research indicates that pressure release, temperature reduction, and alkali metal loss can drive carbonated rare-earth mineralization. Additionally, the coupling of alkali metals and sulfates under high-temperature and high-pressure conditions promotes the formation of sulfate-enriched hydrothermal fluids, which efficiently transport rare-earth elements (Wan et al., 2023).
3.4.3 Metal ions concentration and composition
EPS readily binds various metal ions under certain chemical conditions, facilitating minerals formation. Various studies indicate that metal binding and biomineralization in nature may be influenced directly, through microbial type, energy metabolism, and variations in cell surface and EPS chemistry, and indirectly, via microbial impacts on solution Eh and pH (McLean et al., 1996). For example, Mo, a commonly used metal in steel, has been studied using low-alloyed steels with varying Mo concentrations. It was found that a low concentration of Mo (e.g., 0.6 wt.%) significantly enhanced biofilm formation and mineralization. Mechanistically, Mo ions have been demonstrated to act as chemoattractants for Pseudoalteromonas lipolytica, by activating chemotactic pathways (Guo et al., 2019).
3.4.4 Other factors
Other influencing factors include pressure, magnetic fields, and voltage. Debnath and Sen (2024) demonstrated that under pressure conditions, the biomineralization rate of encapsulated microorganisms increased. Similarly, Nan and Pei (2006) produced titanium oxide films under an applied voltage of 250–550 V and found that the biomineralization rate increased with higher voltage resulting in morphological differences among the samples. The structure and composition of these films significantly influenced their biomineralization behavior. Additionally, Liu H. et al. (2016) investigated the effects of magnetic fields (MF) on the biomineralization and corrosion behavior of Q235 steel. Their findings revealed that MF altered the morphology of the biomineralized films, leading to the formation of denser mineralized films.
4 MIC and microbe-material interfacial interactions
In deep-sea sediments and similar environments, microorganisms (including bacteria, archaea, fungi, and protozoa) can accelerate corrosion even under conditions typically unfavorable to the process (Rajala et al., 2022b). Understanding biomineralization and MIC processes requires a detailed examination of the interaction between microorganisms and materials. The uniqueness of bacteria and the electrical structure of mineral surfaces impact important processes (Conners et al., 2022). The interactions between biofilms and metal surfaces can lead to two biologically induced material degradation processes: MIC and bioleaching (Parra et al., 2021). Biofilm is a dynamic process, and chemical changes at the metal-biofilm interface can trigger corrosion (Knisz et al., 2023; Anusha and Mulky, 2023; Etim et al., 2025).
4.1 Overview of MIC mechanisms
Knisz et al. (2023) described the primary mechanisms of MIC, including oxygen gradient corrosion under sediments, crevice corrosion, metabolite-induced corrosion, and electrochemical microbially influenced corrosion (EMIC). EMIC is classified into two types: direct and indirect. Direct EMIC occurs when microorganisms facilitate extracellular electron transfer (EET) by directly contacting metal surfaces leading to corrosion. Indirect EMIC involves the release of soluble electron mediators by microbes, which act as electron transfer shuttle, and accelerate corrosion of metals, as shown in Figures 4A,B. Biofilms further create additional crevices for various corrosion-related bacteria. Microbial activities within a biofilm can also enhance the influence of abiotic metallurgical conditions, promoting localized corrosion. During pipeline operations, factors such as pressure, stray currents, cathodic protection, and environmental variations can interact synergistically with microorganisms. These combined factors can lead to microbial-induced stress corrosion cracking (SCC), microbially-induced hydrogen-induced cracking (HIC), or crevice corrosion and under-deposit corrosion, where microbially-induced biomineralization occurs at specific locations. Such processes pose a severe risk to pipeline operational safety (Xiong et al., 2018).
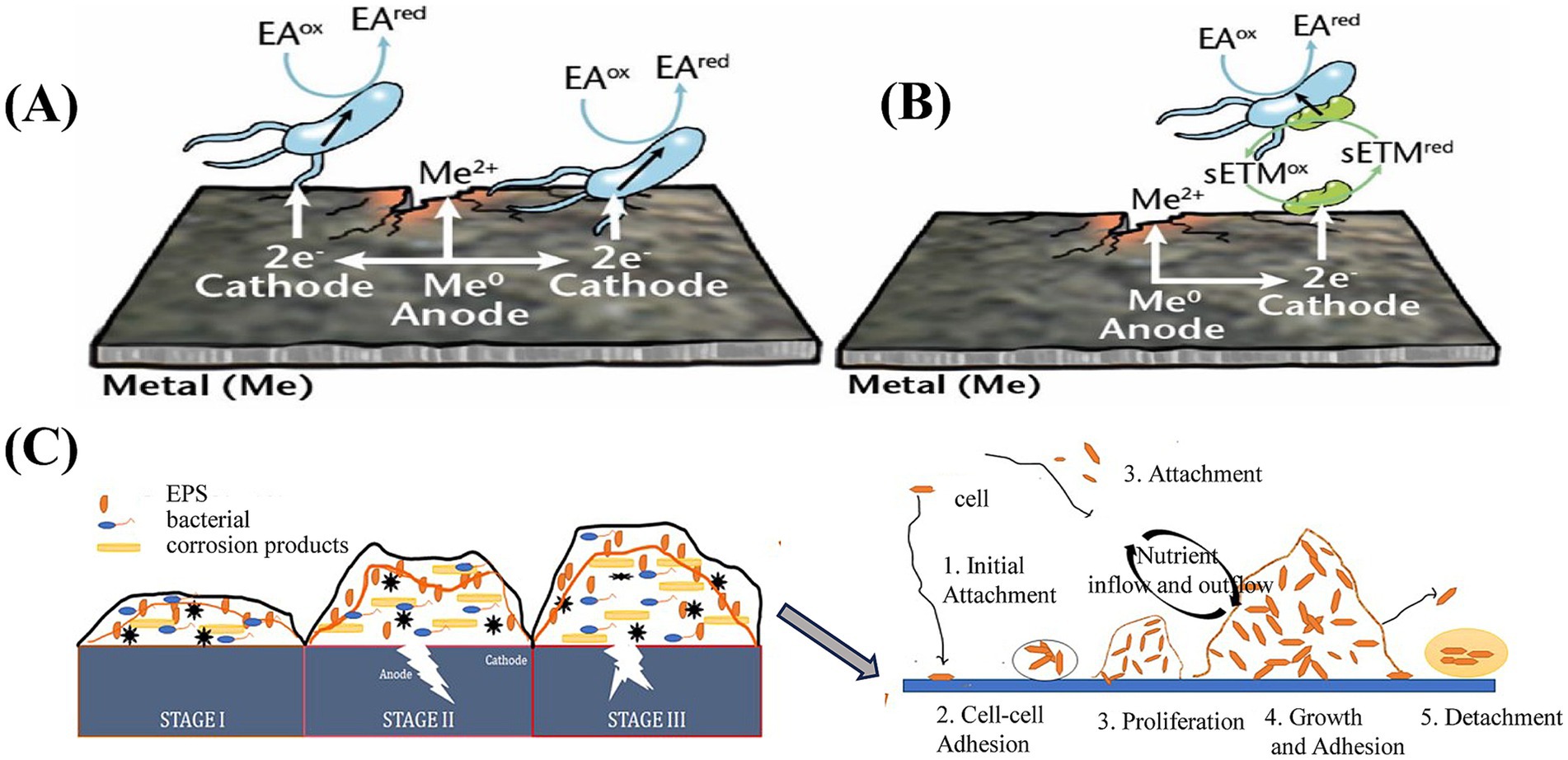
Figure 4. (A) Direct EMIC of metals; (B) Indirect EMIC of metals, EA: electron acceptor, ED: electron donor; ox: oxidized; red: reduced; sETM: soluble electron transfer mediator (Knisz et al., 2023); (C) Biofilm stages and the five-step model of its development (Anusha and Mulky, 2023).
4.2 Microbe-material (mineral) interfacial interactions
Interfacial processes include early cell adhesion, biofilm development, and the formation of passive layers on mineral surfaces (Safar et al., 2020). For example, microorganisms can catalyze metal accumulation through microfossilization, which is induced by microbe-metal interactions (Henne et al., 2021). When materials are immersed in seawater, a conditioning film rapidly forms on their surface. This film is a surface coating formed by the adsorption of biomolecules from the surrounding environment, altering the material’s inherent surface properties and facilitating microbial attachment (Bhagwat et al., 2021). Subsequently, microorganisms undergo the biofilm development process, which includes adhesion, intercellular interactions, proliferation, maturation, and dispersion (Costerton et al., 1999), as illustrated in Figure 4C (Anusha and Mulky, 2023). A deeper understanding of the interactions between microorganisms and metallic minerals requires analytical tools with high chemical sensitivity and spatial resolution. In recent years, the growing application of X-ray techniques, ranging from nanoscale to microscale, as well as large-scale synchrotron accelerators has provided new insights into microbial growth, mineral dissolution, redox transformations, and biomineralization processes (Templeton and Knowles, 2009).
The process by which bacteria adhere to mineral surfaces is not yet fully understood. Some studies suggest a relationship between bacterial cells and mineral surfaces. For example, Pseudomonas aeruginosa PAO1 tends to adhere to mineral surfaces using its flagella and/or pili. Surface roughness is a critical factor that may hinder attachment (Han M. et al., 2024). To better understand the underlying molecular processes and mechanisms, Yan et al. (2016) analyzed the adhesion of Shewanella oneidensis MR-1 to goethite using flow-cell attenuated total reflection (ATR), Fourier-transform infrared (FTIR) spectroscopy, combined with two-dimensional correlation spectroscopy (2D-COS) analysis. Other studies have described that interactions between goethite and bacteria can damage bacterial cells by piercing the cell wall, causing bacterial aggregation at the liquid-air interface Ma et al. (2017). Analyzing the physical interfacial properties between cell envelopes, outer membrane cytochromes responsible for interfacial electron transfer, and complex mineral particles remains challenging. Models explaining how microorganisms acquire electrons from solid donors are multifaceted, and include electron transfer via redox mediators such as H₂ or direct contact through membrane proteins (Liu J. et al., 2016; Mei et al., 2024). In recent years, molecular mechanisms and gene regulation have also become key areas of interest in studying microbe-mineral interactions. For instance, studies have shown that extracellular DNA promotes EET via pyocyanin in P. aeruginosa biofilms (Xiao et al., 2014; Saunders et al., 2020).
Microorganisms utilize EET and external minerals during their growth, a process accompanied by the conversion of chemical energy (Tian et al., 2019). Electroactive microorganisms can establish electrical contacts with other cells and minerals on their outer-surface or reduce soluble extracellular redox-active molecules such as flavins and humic substances. Cytochromes, conductive protein filaments, soluble electron shuttles, and abiotic conductive materials can significantly extend the electronic reach of microorganisms beyond the cell surface (Lovley and Holmes, 2021). Enzymes such as hydrogenases, which catalyze the oxidation of molecular hydrogen, can mediate electron transfer to metals and may facilitate the formation of minerals at the bacteria-mineral interface. Additionally, research has shown that some methanogens are electrogenic and corrosion-causing microbes. Many methanogens involved in MIC can thrive extensively at abiotic-biotic interfaces (Mei et al., 2024). Scheller et al. (2016) studied EET between ANME and sulfate reduction, they lent support to the hypothesis that interspecies extracellular electron transfer is the syntrophic mechanism for ANME.
4.3 MIC research in deep-sea environments
Due to the unique environmental characteristics of the deep sea, such as high hydrostatic pressure and oligotrophic conditions, corrosion of materials in deep-sea environments differs from that in shallow seawater. Rajala et al. (2022b) conducted a deep-sea hanging plate experiment and detected significant MIC on the material surface after 10 years. Numerous microorganisms adhered to and extensively colonized the mooring chain surface, greatly accelerating the metal’s corrosion rate. The corrosion mechanism of mooring chains in deep-sea environments is illustrated in Figure 5A.
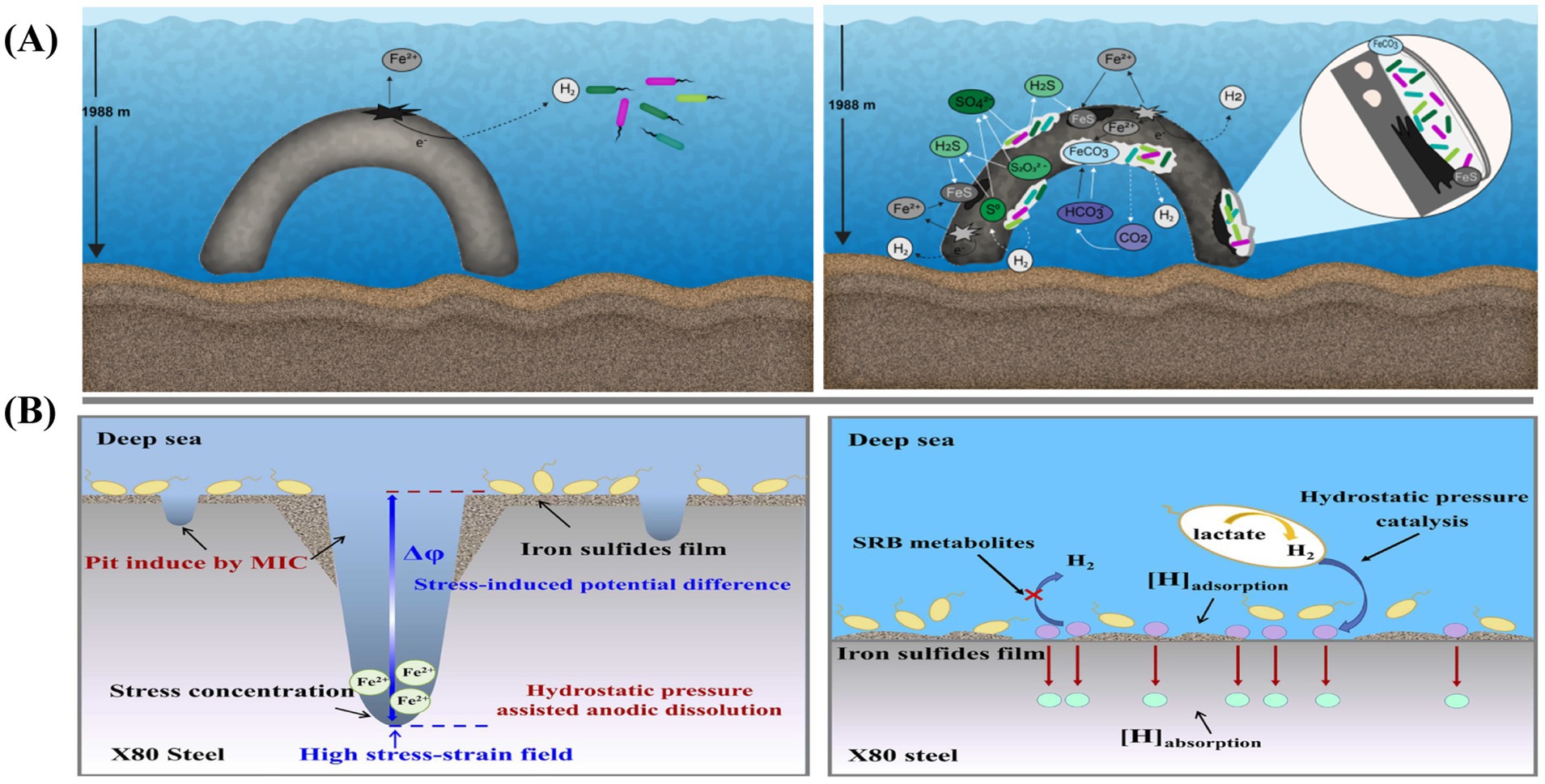
Figure 5. (A) Schematic model of abiotic (left panel) and biotic (right panel) processes inducing corrosion of steel on the mooring chain under deep-sea conditions (Rajala et al., 2022b); (B) Accelerated SCC mechanisms of X80 pipeline steel under the combined effects of SRB and hydrostatic pressure (Li J. et al., 2025).
Currently, due to the challenges in simulating the extreme conditions of the deep sea, research on the impact of deep-sea environmental factors on microbial corrosion remains limited. Laboratory simulations must account for factors such as high hydrostatic pressure, oligotrophic conditions, seawater flow dynamics, and temperature. Li J. et al. (2025) investigated the accelerated SCC mechanism of X80 steel under the combined influence of SRP and hydrostatic pressure in a high-pressure reactor. By varying the hydrostatic pressure to simulate deep-sea conditions, they observed that hydrostatic pressure promoted MIC, as shown in Figure 5B. In this case, hydrostatic pressure and iron sulfide facilitated the hydrogen evolution reaction, further accelerating corrosion.
4.4 Corrosion data
Corrosion data are continuously collected across various industries, including petroleum, natural gas, shipping, and nuclear sectors, for risk assessment, component life prediction, and corrosion control (Li et al., 2015). Establishing and sharing corrosion data is an important topic at present. Multiple factors, such as material composition, microstructure, design parameters, as well as oxygen content, humidity, salinity, pH, temperature, and microbial communities, all influence the degradation of materials like pipelines. By integrating artificial intelligence, real-time corrosion monitoring can be achieved. For instance, Yao et al. (2019) proposed a method for detecting corrosion damage and other surface structural damages on hull structures based on convolutional neural networks. This method accelerates the application of artificial intelligence technologies in the field of shipbuilding and marine engineering, providing practical value for reasonably mitigating corrosion loss.
Currently, quantifiable data of MIC primarily manifest in corrosion weight loss and biofilm formation detection. Specifically, this includes corrosion rate, biofilm quantity, thickness, distribution, and the properties of corrosion products. Most theoretical and practical studies use methods such as SEM, CLSM, and electrochemical techniques to characterize biofilms and corrosion products. Additionally, many studies now employ various sensors, such as probes for the microenvironment within biofilms, to detect the microenvironment and determine their impact on biofilm-specific processes. For example, quantitative biofilm measurements and the detection of pH, polysaccharides, proteins, eDNA, and cytochrome C which is related to electron transfer mediators, are commonly used (Legner et al., 2020; Hunter Ryan and Beveridge Terry, 2005; Eddenden and Nitz, 2022; Jennings et al., 2015). The corrosion rate calculated using the formula shown below, where vcorr is the corrosion rate (mm/y), m0 is the initial weight of the coupon before weight loss testing (g), m1 is the weight of the coupon after testing and the corrosion products are removed (g). K is the unit constant (87,600), A is the working area of the coupon (cm2), t is the immersion period of the coupon (h), and ρ is the density of the coupon (g/cm3).
5 MIC-biomineralization relationship in marine environments and prospects
The primary food source for deep-sea biota is the precipitation of particulate organic polymers (Dang et al., 2024). The progression from the formation of the conditioning film to the maturation of a biofilm on material surfaces can lead to MIC (Qian et al., 2022). Additionally, the transition from biofilm formation to the development of a biomineralized film can further influence MIC. In deep-sea environments microbial mineralization layers may either promote or inhibit corrosion. For example, the minerals formed through biomineralization can accelerate crevice corrosion and under-deposit corrosion.
The microbe-mineral interface is the primary site for biochemical MIC reactions, making its understanding crucial for deciphering MIC and biomineralization mechanisms. Under varying environmental conditions, such as different carbon source availability, the same microorganism may induce both corrosion and mineralization on the same material substratum, as illustrated in Figure 6. In addition, microorganisms can generate dense mineral films through biomineralization, which may exhibit protective characteristics and inhibit corrosion. Furthermore, interactions at the minerals-bacteria interface significantly influence microbial behavior (Gong et al., 2018). The stability of minerals and their morphologies, formed through mechanisms like EET at the microbe-material interface, is not always guaranteed. For instance, SRP can facilitate the transformation of metal oxides into metal sulfides, potentially accelerating corrosion (Duan et al., 2006). It is important to acknowledge that once corrosion occurs, the subsequent formation of a mineralized protective layer may not necessarily provide effective long-term protection (Mao et al., 2022).
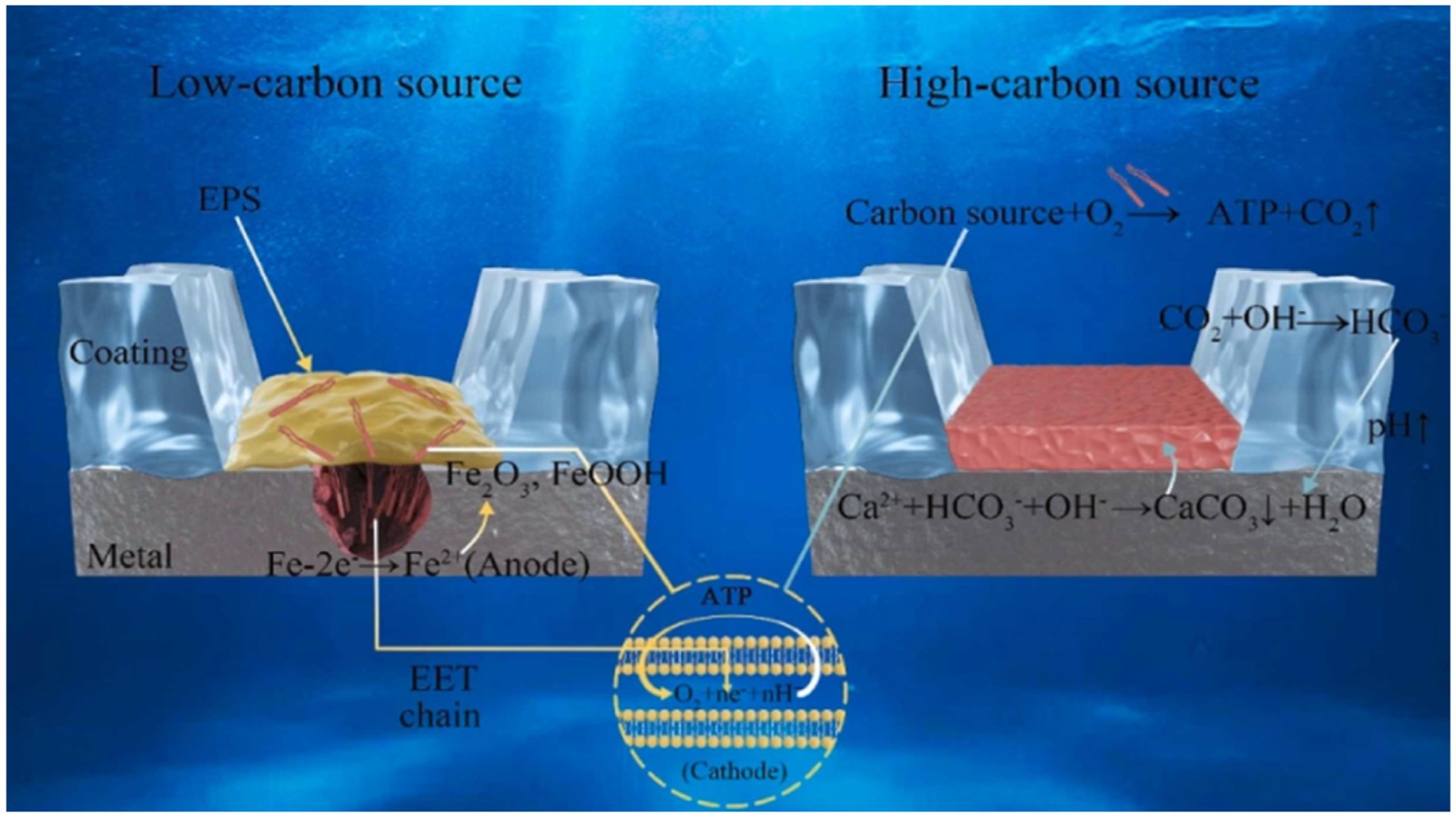
Figure 6. A schematic illustration describing the mechanism of the effect of carbon sources in media on biomineralization and corrosion processes (Hao et al., 2024).
5.1 Corrosion inhibition through biomineralization
The structure of biomineralized films plays a crucial role in determining the corrosion behavior of metals (Liu et al., 2022). The critical transition from biofilm to a biomineralized film is essential for ensuring long-term anti-corrosion properties, as it addresses the inherent instability of biofilm protection against corrosion (Liu et al., 2018). Microorganisms can induce the formation of biomineralized films, which may possess properties such as super hydrophobicity to reduce biological adhesion or enhanced density to achieve corrosion inhibition. Given the complex competitive, symbiotic, and cooperative interactions among different microorganisms, the synergistic effects of various microbial communities in corrosion inhibition have garnered significant attention.
5.1.1 MIC and biomineralization in marine engineering cases
There have been some projects and experimental experiments exploring or applying biomineralization to suppress corrosion in relevant marine environments, especially in interesting works such as corrosion research on offshore structures, pipelines, and historical sunken ships.
In the study of historical sunken ships made of wood and steel materials, black corrosive substances have been commonly found. Zhao et al. (2024) discovered a distinct black corrosion pattern primarily concentrated in the interface region where barnacles attach to the wooden shipwrecks. This corrosion is mainly composed of FeS, FeS2, and Fe3S4, and exhibits a significant trend of extending inward along the surface of the wood. The origin of this black corrosion is intricately linked to barnacle cement, its role in biological corrosion, and the subsequent biomineralization process. The “black shell” formed on the gold coins found in the wreck of the SS Central America in 1857 played a key role in preserving them in an almost pristine state. In the years following the shipwreck, a large amount of steel on the wreck produced fine-grained iron mineral layers as geochemical precipitates on the coins. This coating served to protect the coins from future chemical or biological attacks (Melchiorre et al., 2019). The protection of a large number of cultural relics discovered in sunken ships is also an important issue, and many research works have been carried out (Pan et al., 2023; Yanagida et al., 2024; Grousset et al., 2016). In addition, a large number of studies have analyzed the attached microbial communities of underwater structures and conducted numerous studies on the mechanisms of single bacterial influence under laboratory conditions (Mugge et al., 2019; Lv et al., 2024b).
5.1.2 Protective role of biomineralized layers on materials
Biomineralization products are primarily composed of inorganic–organic hybrid compounds. Compared to biofilms, the biomineralization layer formed between biofilms and metal ions exhibits higher stability and stronger barrier properties. Furthermore, alterations in biochemical parameters, microbial activity, and oxygen depletion collectively contribute to the protective function of the biomineralized layer. Biomineralization can provide protective effects by altering the surface properties of coatings, limiting the diffusion of dissolved oxygen, isolating corrosive ions in seawater. or disrupting electrical contact. Inspired by the nacre of seashells, Zhang et al. (2022) achieved the micro- or nanoscale surface roughness necessary for a superhydrophobic coating through Bacillus subtilis- induced mineralization, as shown in Figure 7A. Olesen et al. (2000) observed that when MnO₂ was directly bio-deposited on low-carbon steel, the accumulation of corrosion products on the steel surface hindered electrical contact between manganese oxide and the underlying metal, thereby preventing an increase in the corrosion rate, as shown in Figure 7B. Marques et al. (2024) indicates that under natural environmental conditions, the surface of Al-Mg alloys exposed to sunlight forms a mineralized layer composed of EPS and Ca-Mg, which hinders Cl− and thereby protects the metal substrate, as shown in Figure 7C. Another study found that the biomineralization of Mn increased the open circuit potential of the 316 L stainless steel with deposited MnOOH and MnO2 (Shi et al., 2002). As discussed in Section 3.4.2, key biochemical parameters (e.g., pH) significantly influence the formation of the biomineralized layer. Using pH variation as an example, Guo et al. reported that synergistic biomineralization by P. lipolytica and Bacillus subtilis elevated the medium’s pH and induced carbonic anhydrase secretion, leading to the formation of a denser and more uniform biomineralized film (Guo N. et al., 2021). At the microbial level, while earlier studies hypothesized that aerobic and facultative microorganisms in the upper biomineralized layer could create localized anaerobic conditions favoring SRP growth, Wang et al. revealed a competitive relationship between mineralizing bacteria and SOB. Their findings showed that biomineralized film formation significantly reduced both the total and relative abundance of SRB communities and the proportion of SOB, while also suppressing functional genes involved in the sulfur cycle (Wang et al., 2025).
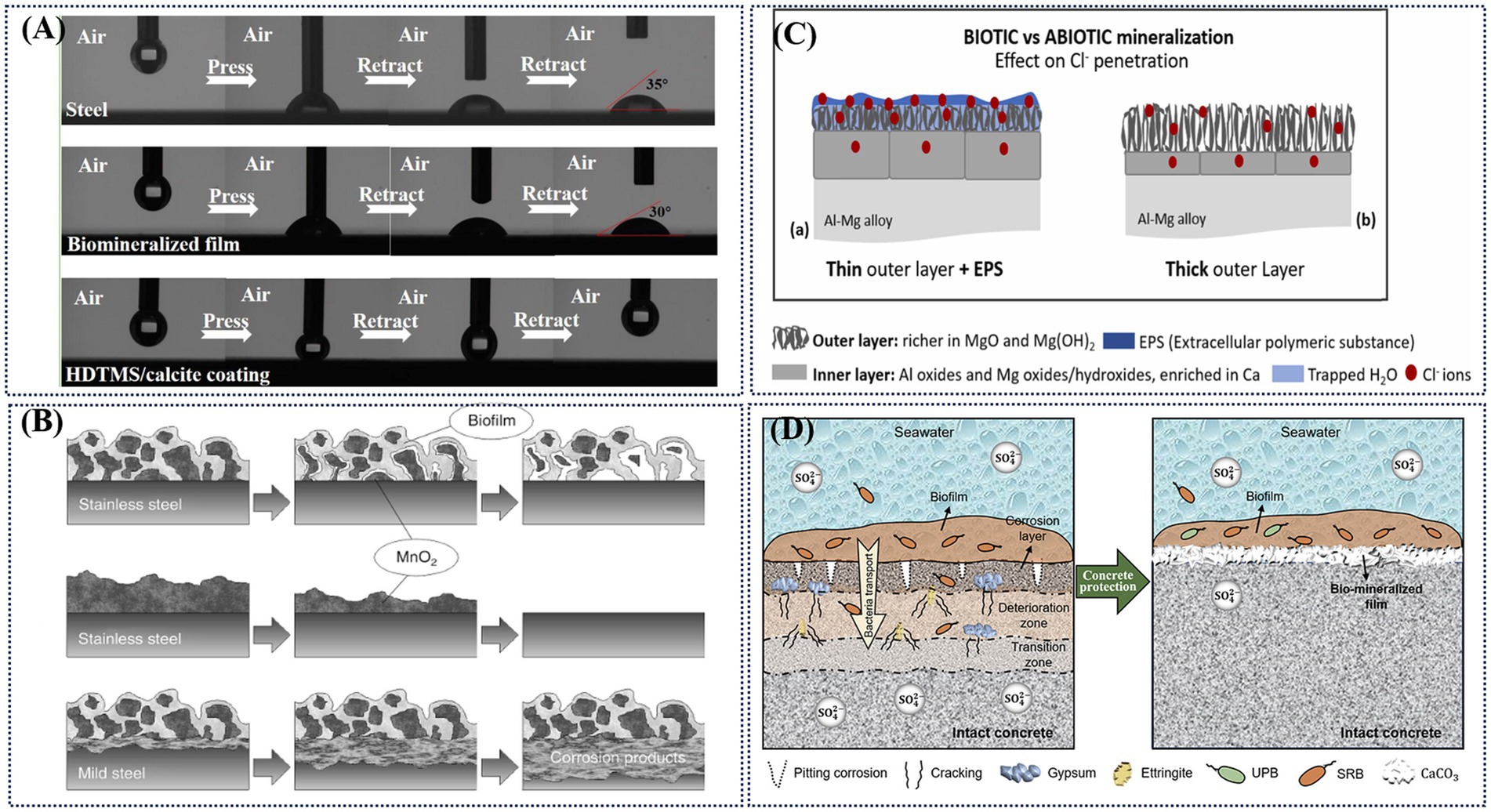
Figure 7. (A) Images of adhesion behavior of water droplets on steel, biomineralized film, and HDTMS/calcite coating (Zhang et al., 2022); (B) Corrosion products electrically insulated the biologically deposited minerals from the underlying metal (Olesen et al., 2000); (C) Cross-section schematic representation of Cl- ion penetration through surface layers on Al-Mg immersion in biotic/light side (a) and abiotic (b) conditions. (D) Biomineralization for corrosion inhibition (Sun et al., 2023).
Consequently, protecting steel surfaces with biomineralized films requires a careful selection of bacteria capable of effective mineralization. Moreover, existing methods for mitigating metal biocorrosion are often costly and environmentally unfriendly. To address this problem, researchers have conducted have explored various strategies. Liu et al. (2018) proposed the formation of biofilms by the marine bacterium P. lipolytica, which subsequently developed into an organic–inorganic hybrid membrane. This hybrid membrane, consisting of multilayered calcite and EPS, demonstrated high and stable barrier protection efficiency while also providing in-situ self-healing activity. Lou et al. (2021b) investigated corrosion inhibition with Q235 carbon steel and found that Shewanella putrefaciens utilized its cell wall as nucleation site to induce the formation of a protective biomineralized layer containing calcite and extracellular polymers. This biomineralized layer exhibited wear resistance and could the ability to self-repair after mechanical damage. For other materials, such as Al alloys, Cu-Al alloys and Ti alloys, biomineralization has also been explored as a corrosion inhibition strategy. However, further research is required to elucidate additional mechanisms (Shen et al., 2020; George et al., 2016; Marques et al., 2024.; Zhu et al., 2020).
5.1.3 Research on typical marine biomineralization and synergistic corrosion inhibition
SRP and methanogenic archaea have been extensively studied in the fields of corrosion and mineralization. Biomineralized films have been shown to reduce both the total amount and relative abundance of SRP. These films act as protective layers to control sulfate diffusion by isolating concrete from corrosive SRP communities, as shown in Figure 7D (Sun et al., 2023). Methanogenic archaea can also reduce iron from Fe (III) to Fe (II). Shang et al. (2020) demonstrated that in anaerobic environments rich in organic matter and Fe (III), Methanosarcina barkeri can similarly reduce Fe (III) to Fe (II) and Zerovalent Iron. This finding suggests significant potential applications in protecting iron materials from corrosion during sediment diagenesis.
Different bacterial groups, when present together, can exhibit synergistic mechanisms. Research on microbial communities in real-world environments can provide new perspectives for the future applications of biomineralization in natural marine and deep-sea settings. ANME frequently form cell consortia with SRP from the Deltaproteobacteria family. Chen et al. (2014) proposed a biomineralization mechanism mediated by anaerobic methane oxidation (AOM). They speculated that ANME cell consortia could interact with other microorganisms and their substrates via a silica shell. This silica accumulation mechanism contributes to the formation of clay minerals in marine sedimentary environments.
5.2 Promotion of corrosion by biomineralization
While the formation of biomineralized layers can inhibit uniform corrosion, it may also exacerbate localized corrosion processes such as pitting and crevice corrosion. For example, corrosion pits may form due to the presence of small anodes, and pitting corrosion can occur under the biomineralized films due to pigments secreted by microorganisms (Guo Z. et al., 2021; Liu et al., 2022). Interestingly, biomineralization can affect the visual appearance of corrosion by forming bubbles, these may create ecological niches for microorganisms that ultimately contribute to MIC (Martinez et al., 2022). Additionally, as described in Section 5, microbial-material interfacial interactions can lead to instability in mineral formation and their morphology, potentially promote corrosion.
5.3 Emerging technologies
Researchers can currently construct strains with enhanced mineralization and extracellular polymer secretion capabilities through microbial genetic engineering, thereby promoting the formation of denser, more uniform mineralized layers on metal surfaces to inhibit corrosion. Alternatively, through biofilm management technology, the porous, highly adhesive, and low-cost characteristics of EPS can be combined with existing corrosion inhibitors to develop multifunctional, versatile bio-coatings that sustainably enhance metal corrosion resistance. Li Z. et al. (2025) genetically engineered a mutant strain (Shewanella oneidensis PCA) capable of overproducing the electron shuttle phenazine-1-carboxylic acid (PCA). By integrating high-resolution passivation film characterization with molecular biology techniques, they systematically elucidated a novel microbial corrosion mechanism involving synergistic direct–indirect electron transfer that accelerates passive film dissolution. This work highlights the dual significance of microbial genetic engineering in both fundamental research and practical applications. Li X. et al. (2025) first utilized the biofilm formed by corrosion-protective microorganisms (Tenacibaculum mesophilum D-6) as a scaffold to construct a novel “smooth liquid-injected porous surface” (SLIPS) coating (TM@SLIPS). The biofilm’s high adhesion, flexibility, and porous structure provide an ideal framework for the uniform distribution of lubricants, thereby enhancing the coating’s durability. It is produced using bio-based materials through controlled cultivation, enabling scalable production at low cost and easy scalability.
5.4 Environmental and ethical considerations of deep-sea MIC and biomineralization processes
Corrosion is not only related to economic losses, but its impact on the environment should also not be underestimated. Over 3,800 World War II sunken ships containing thousands of tons of oil are at risk of structural collapse due to continuous corrosion, posing a threat to the marine environment (Carter et al., 2021). As deep-sea engineering and exploration advance, the environmental consequences of MIC and biomineralization have become increasingly relevant. These microbial processes, while naturally occurring, can be exacerbated by anthropogenic materials and interventions, leading to long-term disruptions of fragile and poorly understood ecosystems. This section explores key areas of concern—including microbial biodiversity, toxic byproducts, long-term ecological consequences, mitigation strategies, and ethical considerations—and outlines approaches to reduce ecological impact while promoting sustainable deep-sea operations.
5.4.1 Impacts on biodiversity and community structure
MIC and biomineralization processes can significantly reshape microbial community structures by creating chemically selective microenvironments on metal surfaces. These conditions often favor metabolically aggressive groups such as SRP, methanogens, and IOP, which may displace native, slow-growing microbial taxa. The loss of microbial diversity and functional redundancy can impair key ecosystem services such as methane oxidation, nitrogen cycling, and trophic support for benthic macrofauna (Shu and Huang, 2022).
To reduce these impacts, several mitigation strategies can be employed. Material selection plays a crucial role: inert substrates such as titanium alloys or ceramics discourage biofilm formation, while surface modification techniques—like nanopatterning or antifouling coatings—can further inhibit microbial adhesion. Another approach involves preconditioning surfaces with benign microbial consortia to outcompete corrosive taxa, though care must be taken to avoid non-native species introduction. Site selection also matters; locating infrastructure away from ecologically sensitive areas such as cold seeps or hydrothermal vents minimizes disturbance. Finally, building microbial baseline datasets and performing community-level monitoring allow researchers to detect shifts in biodiversity and assess the ecological footprint of engineering activities.
5.4.2 Toxic byproducts and metal mobilization
The metabolic byproducts of MIC, including hydrogen sulfide, organic acids, and ammonia, are not only corrosive but can be toxic to surrounding organisms. In confined environments such as crevices or sediment-buried structures, these compounds may accumulate to harmful levels. At the same time, microbial activity can enhance the mobilization of metal ions (Fe2+, Mn2+, Ni2+, Cr3+) from structural materials, with potentially toxic effects due to their high bioavailability in nanoparticulate or reduced forms (Mallick et al., 2025).
Mitigating these effects requires a multi-pronged approach. Corrosion-resistant materials, such as high-alloy stainless steels with molybdenum or titanium, can limit metal ion release. Where biomineralization is used intentionally, controlling the precipitation of low-solubility minerals like calcite or magnetite under stable geochemical conditions helps immobilize harmful metals. The addition of stabilizing agents (e.g., phosphate, silicate) may enhance long-term mineral stability. In situ monitoring systems—including pH sensors, redox probes, an d electrochemical detectors—should be integrated to detect toxic compound buildup in real time, enabling timely responses. Hydrodynamic design considerations, such as ensuring adequate current flow, can also help disperse harmful byproducts, reducing localized toxicity.
5.4.3 Long-term environmental consequences
Deep-sea ecosystems are characterized by low metabolic rates and long recovery times. Persistent MIC and biomineralization processes can irreversibly alter substrate characteristics, disrupt microbial recolonization, and reduce habitat suitability for benthic species. Accumulated mineral layers or biofilms can inhibit larval settlement or shift faunal community structures. Over time, these changes may shift ecological baselines and suppress resilience to other anthropogenic pressures, such as mining or climate-driven changes (Levin et al., 2016).
To prevent long-term degradation, engineering solutions must incorporate predictive models of corrosion and material aging to anticipate the timing and severity of biological interactions. Substrate designs should favor modular components that can be maintained, cleaned, or replaced to prevent chronic ecological disruption. Where disturbance has occurred, pilot efforts in ecological rehabilitation—such as reseeding native microbes or deploying biodegradable structures—can help initiate recovery, though such approaches remain experimental.
5.4.4 Mitigation and monitoring strategies
Sustaining ecological balance in deep-sea environments affected by MIC and biomineralization requires systemic, lifecycle-based strategies. Because infrastructure often remains on the seafloor for decades, proactive environmental integration is vital. First, predictive corrosion modeling under high-pressure, low-temperature conditions should inform design and material selection, minimizing long-term reactivity and emissions. Second, dynamic environmental monitoring—using autonomous platforms equipped with microbial samplers, chemical sensors, and imaging systems—should track both corrosion processes and ecological responses.
Importantly, these monitoring systems should not only serve engineering goals but also detect shifts in ecosystem health, such as biodiversity loss, metabolite accumulation, or habitat degradation. Feedback from this data must guide adaptive management actions, including component replacement, intervention pause, or spatial redesign of deployments. In cases of severe or cumulative impact, localized remediation—such as surface neutralization or selective module recovery—may be warranted. Moreover, international protocols for post-deployment ecological assessment and data transparency will be essential, particularly in regions beyond national jurisdiction.
Ultimately, integrating engineering resilience with ecological foresight will be key to minimizing long-term environmental harm and promoting a dynamic balance between technological use and deep-sea ecosystem function.
5.4.5 Ethical considerations in microbial interventions
The application of microbial approaches for deep-sea corrosion control—such as engineered biomineralization or microbial inhibition—raises significant ethical considerations. Deep-sea ecosystems remain among the most poorly understood on the planet, and human interventions may carry unpredictable or potentially irreversible consequences. Actions like introducing microbial consortia, altering substrate chemistry, or influencing microbial succession risk disturbing critical ecological processes or destabilizing host–microbe interactions.
Ethical practice in this context requires strict adherence to the precautionary principle, supported by comprehensive risk assessments and sustained monitoring both prior to and following any intervention. In regions beyond national jurisdiction, where governance is limited, international cooperation, transparent data sharing, and adherence to environmental impact protocols are particularly vital. Above all, ethical stewardship demands that deep-sea microbiomes be regarded not merely as tools for engineering solutions but as vital components of delicate, interconnected ecosystems that warrant respect and protection.
6 Conclusion
Deep-sea MIC and biomineralization is an interdisciplinary field that integrates materials science, microbiology, geochemistry, and oceanography. Multidisciplinary approaches and collaborative research teams are essential for understanding how individual microbial cells and microbial communities interact with their surroundings on the nanoscale or microscale. Emerging methods and technologies, such as bio-electrochemostasis for exploring the impact of flow dynamics on corrosive biofilms under simulated deep-sea high-pressure conditions (Ivanovich et al., 2025), are needed to characterize and elucidate molecular-level interactions, including bonding, electron transfer, and various physiological activities at the microbial-material interface. Advanced techniques, including multi-omics analysis, machine learning, bioinformatics, gene editing, real-time spectroscopy and computational simulations, can help identify key microbial drivers in real-world environments and explain their mechanisms of action. Investigating the mechanisms of deep-sea corrosion and biomineralization can lead to sustainable strategies for corrosion prevention in deep-sea settings.
Author contributions
YG: Data curation, Writing – original draft, Writing – review & editing, Investigation. CW: Data curation, Writing – original draft, Writing – review & editing, Investigation. I-IE: Writing – review & editing. SK: Writing – review & editing. CL: Formal analysis, Writing – original draft. LY: Writing – review & editing. JiaL: Data curation, Writing – original draft. PY: Data curation, Writing – original draft. JiazL: Writing – original draft. WS: Writing – review & editing. RZ: Conceptualization, Writing – review & editing, Investigation, Supervision, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by the National Key R&D Program of China (2024YFF0510100).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, S., and Mahmoud, H. (2022). Identification of sponge-associated bacteria from the coast of Kuwait and their potential biotechnological applications. Front. Microbiol. 13:896718. doi: 10.3389/fmicb.2022.896718
Anusha, Y. G., and Mulky, L. (2023). Biofilms and beyond: a comprehensive review of the impact of Sulphate reducing Bacteria on steel corrosion. Biofouling 39, 897–915. doi: 10.1080/08927014.2023.2284316
Banfield, J. F., Barker, W. W., Welch, S. A., and Taunton, A. (1999). Biological impact on mineral dissolution: application of the lichen model to understanding mineral weathering in the rhizosphere. Proc. Natl. Acad. Sci. USA 96, 3404–3411. doi: 10.1073/pnas.96.7.3404
Bansal, R., Dhami, N. K., Mukherjee, A., and Reddy, M. S. (2016). Biocalcification by halophilic bacteria for remediation of concrete structures in marine environment. J. Ind. Microbiol. Biotechnol. 43, 1497–1505. doi: 10.1007/s10295-016-1835-6
Barton, H. A., and Northup, D. E. (2007). Geomicrobiology in cave environments: past, current and future perspectives. J. Cave Karst Stud. 69, 163–178.
Bazylizinki, D. A., Heywood, B. R., Mann, S., and Frankel, R. B. (1993). Fe3O4 and Fe3S4 in a bacterium. Nature 366:218. doi: 10.1038/366218a0
Beech, W. B., and Sunner, J. (2004). Biocorrosion: towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol. 15, 181–186. doi: 10.1016/j.copbio.2004.05.001
Bhagwat, G., O'Connor, W., Grainge, I., and Palanisami, T. (2021). Understanding the fundamental basis for biofilm formation on plastic surfaces: role of conditioning films. Front. Microbiol. 12:687118. doi: 10.3389/fmicb.2021.687118
Borets'ka, M. O., and Kozlova, I. P. (2010). Biofilm on a metal surface as a factor of microbial corrosion. Mikrobiolohichnyi zhurnal (Kiev, Ukraine: 1993) 72, 57–65.
Carter, M., Goodsir, F., Cundall, P., Devlin, M., Fuller, S., Jeffery, B., et al. (2021). Ticking ecological time bombs: risk characterisation and management of oil polluting world war II shipwrecks in the Pacific Ocean. Mar. Pollut. Bull. 164:112087. doi: 10.1016/j.marpolbul.2021.112087
Chen, X., Kou, H., Niu, X., Wang, C., Zhang, L., and Li, H. (2024). Development of deep-sea underwater technology and equipment. Strategic Study CAE 26, 1–14. doi: 10.15302/J-SSCAE-2024.02.002
Chen, Y., Li, Y.-L., Zhou, G.-T., Li, H., Lin, Y.-T., Xiao, X., et al. (2014). Biomineralization mediated by anaerobic methane-consuming cell consortia. Sci. Rep. 4:5696. doi: 10.1038/srep05696
Conners, E. M., Rengasamy, K., and Bose, A. (2022). Electroactive biofilms: how microbial electron transfer enables bioelectrochemical applications. J. Ind. Microbiol. Biotechnol. 49:kuac012. doi: 10.1093/jimb/kuac012
Costerton, J. W., Stewart, P. S., and Greenberg, E. P. (1999). Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322. doi: 10.1126/science.284.5418.1318
Dang, Y.-R., Cha, Q.-Q., Liu, S.-S., Wang, S.-Y., Li, P.-Y., Li, C.-Y., et al. (2024). Phytoplankton-derived polysaccharides and microbial peptidoglycans are key nutrients for deep-sea microbes in the Mariana trench. Microbiome 12:77. doi: 10.1186/s40168-024-01789-x
Danovaro, R., Fanelli, E., Aguzzi, J., Billett, D., Carugati, L., Corinaldesi, C., et al. (2020). Ecological variables for developing a global deep-ocean monitoring and conservation strategy. Nat. Ecol. Evol. 4, 181–192. doi: 10.1038/s41559-019-1091-z
Debnath, A., and Sen, R. (2024). Construction nanobiotechnology approach for performance enhancement of microbially induced biomineralization (MIB) using a biopolymer encapsulated spore-based system. Appl. Environ. Microbiol. 90:e0140724. doi: 10.1128/aem.01407-24
Decho, A. W. (2010). Overview of biopolymer-induced mineralization: what goes on in biofilms? Ecol. Eng. 36, 137–144. doi: 10.1016/j.ecoleng.2009.01.003
Dijkstra, N., Kraal, P., Kuypers, M. M. M., Schnetger, B., and Slomp, C. P. (2014). Are Iron-phosphate minerals a sink for phosphorus in anoxic Black Sea sediments? PLoS One 9:e101139. doi: 10.1371/journal.pone.0101139
Drazen, J. C., Smith, C. R., Gjerde, K. M., Haddock, S. H. D., Carter, G. S., Choy, C. A., et al. (2020). Midwater ecosystems must be considered when evaluating environmental risks of deep-sea mining. Proc. Natl. Acad. Sci. USA 117, 17455–17460. doi: 10.1073/pnas.2011914117
Duan, J., Hou, B., and Yu, Z. (2006). Characteristics of sulfide corrosion products on 316L stainless steel surfaces in the presence of sulfate-reducing bacteria. Mater. Sci. Eng. C 26, 624–629. doi: 10.11902/1005.4537.2022.102
Duan, T., Li, Z., Peng, W., Zhang, P., Ding, K., Guo, W., et al. (2023). Corrosion characteristics of 5A06 Al-alloy exposed in natural deep-sea environment. J. Chin. Soc. Corros. Prot. 43, 352–358.
Dupraz, C., Reid, R. P., Braissant, O., Decho, A. W., Norman, R. S., and Visscher, P. T. (2009). Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 96, 141–162. doi: 10.1016/j.earscirev.2008.10.005
Duverger, A., Berg, J. S., Busigny, V., Guyot, F., Bernard, S., and Miot, J. (2020). Mechanisms of pyrite formation promoted by sulfate-reducing bacteria in pure culture. Front. Earth Sci. 8:588310. doi: 10.3389/feart.2020.588310
Eddenden, A., and Nitz, M. (2022). Applications of an inactive Dispersin B probe to monitor biofilm polysaccharide production. Methods Enzymol. 665, 209–231. doi: 10.1016/bs.mie.2021.11.006
Etim, I.-I. N., Dong, J., Wei, J., Nan, C., Daniel, E. F., Subedi, D. B., et al. (2020). Mitigation of sulphate-reducing bacteria attack on the corrosion of 20SiMn steel rebar in sulphoaluminate concrete using organic silicon quaternary ammonium salt. Constr. Build. Mater. 257:119047. doi: 10.1016/j.conbuildmat.2020.119047
Etim, I.-I. N., Ekerenam, O. O., Ikeuba, A. I., Njoku, C. N., Emori, W., Zhang, R., et al. (2025). Navigating the blue economy: a comprehensive review of marine pollution and sustainable approaches. J. Oceanol. Limnol. doi: 10.1007/s00343-024-4129-2
Etim, I.-I. N., Wei, J., Dong, J., Xu, D., Chen, N., Wei, X., et al. (2018). Mitigation of the corrosion-causing Desulfovibrio desulfuricans biofilm using an organic silicon quaternary ammonium salt in alkaline media simulated concrete pore solutions. Biofouling 34, 1121–1137. doi: 10.1080/08927014.2018.1547377
Fang, K., Park, O.-J., and Hong, S. H. (2020). Controlling biofilms using synthetic biology approaches. Biotechnol. Adv. 40:107518. doi: 10.1016/j.biotechadv.2020.107518
Feng, J. C., Liang, J. Z., Cai, Y. P., Zhang, S., Xue, J. C., and Yang, Z. F. (2022). Deep-sea organisms research oriented by deep-sea technologies development. Sci. Bull. 67, 1802–1816. doi: 10.1016/j.scib.2022.07.016
Friedrich, M. W., and Finster, K. W. (2014). How sulfur beats iron. Science 344, 974–975. doi: 10.1126/science.1255442
George, R. P., Mudali, U. K., and Raj, B. (2016). Characterizing biofilms for biofouling and microbial corrosion control in cooling water systems. Anti-Corros. Methods Mater. 63, 477–489. doi: 10.1108/acmm-07-2014-1401
Gong, B. N., Wu, P. X., Ruan, B., Zhang, Y. T., Lai, X. L., Yu, L. F., et al. (2018). Differential regulation of phenanthrene biodegradation process by kaolinite and quartz and the underlying mechanism. J. Hazard. Mater. 349, 51–59. doi: 10.1016/j.jhazmat.2018.01.046
Grousset, S., Bayle, M., Dauzeres, A., Crusset, D., Deydier, V., Linard, Y., et al. (2016). Study of iron sulphides in long-term iron corrosion processes: characterisations of archaeological artefacts. Corros. Sci. 112, 264–275. doi: 10.1016/j.corsci.2016.07.022
Guezennec, J., Ortega-Morales, O., Raguenes, G., and Geesey, G. (1998). Bacterial colonization of artificial substrate in the vicinity of deep-sea hydrothermal vents. FEMS Microbiol. Ecol. 26, 89–99. doi: 10.1016/s0168-6496(98)00022-1
Guo, Z., Hui, X., Zhao, Q., Guo, N., Yin, Y., and Liu, T. (2021). Pigmented Pseudoalteromonas piscicida exhibited dual effects on steel corrosion: inhibition of uniform corrosion and induction of pitting corrosion. Corros. Sci. 190:109687. doi: 10.1016/j.corsci.2021.109687
Guo, Z., Wang, W., Guo, N., Zeng, Z., Liu, T., and Wang, X. (2019). Molybdenum-mediated chemotaxis of Pseudoalteromonas lipolytica enhances biofilm-induced mineralization on low alloy steel surface. Corros. Sci. 159:108123. doi: 10.1016/j.corsci.2019.108123
Guo, N., Wang, Y., Hui, X., Zhao, Q., Zeng, Z., Pan, S., et al. (2021). Marine bacteria inhibit corrosion of steel via synergistic biomineralization. J. Mater. Sci. Technol. 66, 82–90. doi: 10.1016/j.jmst.2020.03.089
Hamilton, W. A. (2003). Microbially influenced corrosion as a model system for the study of metal microbe interactions: a unifying electron transfer hypothesis. Biofouling 19, 65–76. doi: 10.1080/0892701021000041078
Han, X. H., Wang, F. X., Zheng, S. L., Qiu, H., Liu, Y., Wang, J., et al. (2024). Morphological, microstructural, and in situ chemical characteristics of siderite produced by iron-reducing bacteria. Environ. Sci. Technol. 58, 11016–11026. doi: 10.1021/acs.est.3c10988
Han, M., Zhu, X. Y., Ruan, C. J., Wu, H. Q., Chen, G. W., Zhu, K., et al. (2024). Micro-biophysical interactions at bacterium-mineral interfaces determine potassium dissolution. Environ. Technol. Innov. 33:103524. doi: 10.1016/j.eti.2023.103524
Hao, X., Qian, Y., Bai, Y., Wang, N., Qian, H., Lou, Y., et al. (2024). Effects of extracellular electron transfer on biomineralization by Shewanella putrefaciens in damaged organic coatings. Corros. Sci. 229:111881. doi: 10.1016/j.corsci.2024.111881
He, W., Xue, H. P., Liu, C., Zhang, A. H., Huang, J. K., and Zhang, D. F. (2023). Biomineralization of struvite induced by indigenous marine bacteria of the genus Alteromonas. Front. Mar. Sci. 10:1085345. doi: 10.3389/fmars.2023.1085345
Hein, J. R., Mizell, K., Koschinsky, A., and Conrad, T. A. (2013). Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: comparison with land-based resources. Ore Geol. Rev. 51, 1–14. doi: 10.1016/j.oregeorev.2012.12.001
Henne, A., Craw, D., Gagen, E., and Southam, G. (2021). Biologically facilitated precipitation of metals in low-Fe waters at the sulphidic mount Chalmers mine, Queensland, Australia. Ore Geol. Rev. 136:104238. doi: 10.1016/j.oregeorev.2021.104238
Hochella, M. F. (2002). Sustaining earth: thoughts on the present and future roles of mineralogy in environmental science. Mineral. Mag. 66, 627–652. doi: 10.1180/0026461026650053
Hu, S. Y., Barnes, S. J., Pagès, A., Parr, J., Binns, R., Grice, K., et al. (2019). Biomineralization in modern seafloor hydrothermal chimneys: implications for microbe-metal interactions in ancient VMS deposits. In Proceedings of the 15th SGA Biennial Meeting on Life with Ore Deposits on Earth, pp. 1421–1424.
Hu, Y., Chen, C., Liu, S., Zhou, Y., Jia, W., and Cao, Y. (2023). Biofilm-induced corrosion inhibition of Q235 carbon steel by anaerobic Bacillus cereus inoculum in simulated cooling water. Environ. Sci. Pollut. Res. 30, 20833–20848. doi: 10.1007/s11356-022-23561-0
Hunter Ryan, C., and Beveridge Terry, J. (2005). Application of a pH-sensitive fluoroprobe (C-SNARF-4) for pH microenvironment analysis in Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 71, 2501–2510. doi: 10.1128/AEM.71.5.2501-2510.2005
Ivanovich, N., Marsili, E., Shen, X., Messinese, E., Marcos,, Rajala, P., et al. (2025). Exploring the impact of flow dynamics on corrosive biofilms under simulated deep-sea high-pressure conditions using bio-electrochemostasis. Front. Microbiol. 16:1540664. doi: 10.3389/fmicb.2025.1540664
Jennings, L. K., Storek, K. M., Ledvina, H. E., Coulon, C., Marmont, L. S., Sadovskaya, I., et al. (2015). Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 112, 11353–11358. doi: 10.1073/pnas.1503058112
Jones, H. E., Trudinger, P. A., Chambers, L. A., and Pyliotis, N. A. (1976). Metal accumulation by bacteria with particular reference to dissimilatory sulfate-reducing bacteria. Z. Allg. Mikrobiol. 16, 425–435. doi: 10.1002/jobm.3630160603
Kim, I., Kang, K., Oh, M. H., Yang, M. Y., Park, I., and Nam, Y. S. (2017). Virus-templated self-mineralization of ligand-free colloidal palladium nanostructures for high surface activity and stability. Adv. Funct. Mater. 27:1703262. doi: 10.1002/adfm.201703262
Knisz, J., Eckert, R., Gieg, L. M., Koerdt, A., Lee, J. S., Silva, E. R., et al. (2023). Microbiologically influenced corrosion—more than just microorganisms. FEMS Microbiol. Rev. 47:fuad041. doi: 10.1093/femsre/fuad041
Kolinko, S., Richter, M., Glöckner, F. O., Brachmann, A., and Schüler, D. (2014). Single-cell genomics reveals potential for magnetite and greigite biomineralization in an uncultivated multicellular magnetotactic prokaryote. Environ. Microbiol. Rep. 6, 524–531. doi: 10.1111/1758-2229.12198
Konhauser, K. O. (1998). Diversity of bacterial iron mineralization. Earth-Sci. Rev. 43, 91–121. doi: 10.1016/s0012-8252(97)00036-6
Labrenz, M., Druschel, G. K., Thomsen-Ebert, T., Gilbert, B., Welch, S. A., Kemner, K. M., et al. (2000). Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science 290, 1744–1747. doi: 10.1126/science.290.5497.1744
Legner, M., Jonkman, J., and Swift, D. (2020). Evaluating the effects of disinfectants on bacterial biofilms using a microfluidics flow cell and time-lapse fluorescence microscopy. Microorganisms 8:1837. doi: 10.3390/microorganisms8111837
Leguey, S., de Leon, D. R., Ruiz, A. I., and Cuevas, J. (2010). The role of biomineralization in the origin of sepiolite and dolomite. Am. J. Sci. 310, 165–193. doi: 10.2475/03.2010.02
Levin, L. A., Mengerink, K., Gjerde, K. M., Rowden, A. A., Van Dover, C. L., Clark, M. R., et al. (2016). Defining "serious harm" to the marine environment in the context of deep-seabed mining. Mar. Policy 74, 245–259. doi: 10.1016/j.marpol.2016.09.032
Li, Z. F., Liu, A. Z., Sun, C. H., Li, H. T., Kong, Z., and Zhai, H. R. (2024). Biomineralization process of CaCO3 precipitation induced by Bacillus mucilaginous and its potential application in microbial self-healing concrete. Appl. Biochem. Biotechnol. 196, 1896–1920. doi: 10.1007/s12010-023-04634-3
Li, Z., Wang, J., Li, F., Li, Z. T., Zhou, E. Z., Fan, Y. Q., et al. (2025). Accelerated destruction of passive film and microbial corrosion of 316 L stainless steel via extracellular electron transfer. Angew. Chem. Int. Ed. 64:e202425220. doi: 10.1002/anie.202425220
Li, G., Wong, T.-W., Shih, B., Guo, C., Wang, L., Liu, J., et al. (2023). Bioinspired soft robots for deep-sea exploration. Nat. Commun. 14:7097. doi: 10.1038/s41467-023-42882-3
Li, X., Xu, J., and Xiao, X. (2013). High pressure adaptation of deep-sea microorganisms and biogeochemical cycles. Microbiology 40, 59–70. doi: 10.13344/j.microbiol.china.2013.01.012
Li, X., Zeng, X., Qiu, D. H., Zhang, Z., Chen, J. X., and Shao, Z. Z. (2019). Dissimilatory iron Fe(III) reduction by a novel fermentative, piezophilic bacterium Anoxybacter fermentans DY22613T isolated from East Pacific rise hydrothermal sulfides. Geomicrobiol J. 36, 291–302. doi: 10.1080/01490451.2018.1526985
Li, X., Zhang, D., Liu, Z., Li, Z., Du, C., and Dong, C. (2015). Materials science: share corrosion data. Nature 527, 441–442. doi: 10.1038/527441a
Li, X., Zhang, R., Zhang, J., Li, Q., Yu, Z., Zhou, Z., et al. (2025). Harnessing biofilm scaffold for structurally Adaptative slippery surfaces with integrated antifouling and anticorrosion properties. Angew. Chem. Int. Edi. 64:e202503295. doi: 10.1002/anie.202503295
Li, J., Zhou, E., Xie, F., Li, Z., Wang, F., and Xu, D. (2025). Accelerated stress corrosion cracking of X80 pipeline steel under the combined effects of sulfate-reducing bacteria and hydrostatic pressure. Corros. Sci. 243:112593. doi: 10.1016/j.corsci.2024.112593
Lin, W., Bazylinski, D. A., Xiao, T., Wu, L.-F., and Pan, Y. (2014). Life with compass: diversity and biogeography of magnetotactic bacteria. Environ. Microbiol. 16, 2646–2658. doi: 10.1111/1462-2920.12313
Liu, H., Chen, W., Tan, Y., Meng, G., Liu, H., and Cheng, Y. (2022). Characterizations of the biomineralization film caused by marine Pseudomonas stutzeri and its mechanistic effects on X80 pipeline steel corrosion. J. Mater. Sci. Technol. 125, 15–28. doi: 10.1016/j.jmst.2022.02.033
Liu, H., Gu, T., Zhang, G., Cheng, Y., Wang, H., and Liu, H. (2016). The effect of magneticfield on biomineralization and corrosion behavior of carbon steel induced by iron-oxidizing bacteria. Corros. Sci. 102, 93–102. doi: 10.1016/j.corsci.2015.09.023
Liu, T., Guo, Z., Zeng, Z., Guo, N., Lei, Y., Liu, T., et al. (2018). Marine Bacteria provide lasting anticorrosion activity for steel via biofilm-induced mineralization. ACS Appl. Mater. Interfaces 10, 40317–40327. doi: 10.1021/acsami.8b14991
Liu, J., Pearce, C. I., Shi, L., Wang, Z. M., Shi, Z., Arenholz, E., et al. (2016). Particle size effect and the mechanism of hematite reduction by the outer membrane cytochrome OmcA of Shewanella oneidensis MR-1. Geochim. Cosmochim. Acta 193, 160–175. doi: 10.1016/j.gca.2016.08.022
Liu, F. X., Shah, D. S., and Gadd, G. M. (2021). Role of protein in fungal biomineralization of copper carbonate nanoparticles. Curr. Biol. 31:358. doi: 10.1016/j.cub.2020.10.044
Liu, Y., Song, X., Xu, Z., Wang, Y., Hou, X., Wang, Y., et al. (2024). Biomineralized manganese oxide mediated nitrogen-contained wastewater treatment. Bioresour. Technol. 400:130689. doi: 10.1016/j.biortech.2024.130689
Liu, Q., Yang, Q., Li, Y., Liu, D., and Li, J. (2025). Metagenomics reveals the hidden sulfur cycle mediated by methanotrophs. J. Environ. Chem. Eng. 13:116743. doi: 10.1016/j.jece.2025.116743
Lou, Y., Chang, W., Cui, T., Qian, H., Huang, L., Ma, L., et al. (2021a). Microbiologically influenced corrosion inhibition of carbon steel via biomineralization induced by Shewanella putrefaciens. NPJ Mater. Degrad. 5:59. doi: 10.1038/s41529-021-00206-0
Lou, Y., Chang, W., Cui, T., Wang, J., Qian, H., Ma, L., et al. (2021b). Microbiologically influenced corrosion inhibition mechanisms in corrosion protection: a review. Bioelectrochemistry 141:107883. doi: 10.1016/j.bioelechem.2021.107883
Lovley, D. R., and Holmes, D. E. (2021). Electromicrobiology: the ecophysiology of phylogenetically diverse electroactive microorganisms. Nat. Rev. Microbiol. 20, 5–19. doi: 10.1038/s41579-021-00597-6
Lv, X., Wang, C., Liu, J., Sand, W., Etim, I.-I. N., Zhang, Y., et al. (2024). The microbiologically influenced corrosion and protection of pipelines: a detailed review. Materials 17:4996. doi: 10.3390/ma17204996
Ma, N., Cai, R., and Sun, C. (2021a). Threonine dehydratase enhances bacterial cadmium resistance via driving cysteine desulfuration and biomineralization of cadmium sulfide nanocrystals. J. Hazard. Mater. 417:126102. doi: 10.1016/j.jhazmat.2021.126102
Ma, R., Feng, Y., Li, H., Liu, M., Cui, Y., Wang, J., et al. (2024). Deep-sea microorganisms-driven V5+ and Cd2+ removal from vanadium smelting wastewater: bacterial screening, performance and mechanism. Environ. Pollut. 360:124599. doi: 10.1016/j.envpol.2024.124599
Ma, W. T., Peng, D. H., Walker, S. L., Cao, B., Gao, C. H., Huang, Q. Y., et al. (2017). Bacillus subtilis biofilm development in the presence of soil clay minerals and iron oxides. NPJ Biofilms Microbiomes 3:4. doi: 10.1038/s41522-017-0013-6
Ma, N., Sha, Z. L., and Sun, C. M. (2021b). Formation of cadmium sulfide nanoparticles mediates cadmium resistance and light utilization of the deep-sea bacterium Idiomarina sp.OT37-5b. Environ. Microbiol. 23, 934–948. doi: 10.1111/1462-2920.15205
Mallick, S., Pradhan, T., and Das, S. (2025). Bacterial biomineralization of heavy metals and its influencing factors for metal bioremediation. J. Environ. Manag. 373:123977. doi: 10.1016/j.jenvman.2024.123977
Mandal, F. B. (2023). Interaction between marine protists and bacteria results in magnetotaxis and iron recycling. Isr. J. Ecol. Evol. 69, 10–18. doi: 10.1163/22244662-bja10042
Mann, S., Sparks, N. H. C., Frankel, R. B., Bazylinski, D. A., and Jannasch, H. W. (1990). Biomineralization of ferrimagnetic greigite (Fe3S4) and iron pyrite (FeS2) in a magnetotactic bacterium. Nature 343, 258–261. doi: 10.1038/343258a0
Mao, X., Liu, T., Guo, N., Guo, Z., and Dong, L. (2022). Corrosion behavior of marine low alloy steel under the condition of multi-factor coupling in simulated Arctic route. Surf. Technol. 51, 36–47. doi: 10.16490/j.cnki.issn.1001-3660.2022.06.004
Marques, M. J. F., Jaume, J., Mercier, D., Seyeux, A., Zanna, S., Basseguy, R., et al. (2024). The positive impact of biomineralization for marine corrosion protection of AA5083 alloy. Corros. Sci. 233:112053. doi: 10.1016/j.corsci.2024.112053
Martinez, S., Ilhan-Sungur, E., Cansever, N., and Khoshnaw, F. (2022). A comparative analysis of perforation and blister features on internally corroded aged water pipeline wall. Mater. Corros. 73, 1193–1204. doi: 10.1002/maco.202213088
McLean, R. J. C., Fortin, D., and Brown, D. A. (1996). Microbial metal-binding mechanisms and their relation to nuclear waste disposal. Can. J. Microbiol. 42, 392–400. doi: 10.1139/m96-055
Mei, N., Tremblay, P.-L., Wu, Y., and Zhang, T. (2024). Proposed mechanisms of electron uptake in metal-corroding methanogens and their potential for CO2 bioconversion applications. Sci. Total Environ. 923:171384. doi: 10.1016/j.scitotenv.2024.171384
Melchiorre, E. B., Seymour, B. H., and Evans, R. D. (2019). The interpretation of biogeochemical growths on gold coins from the SS Central America shipwreck: applications for biogeochemistry and geoarchaeology. J. Mar. Sci. Eng. 7:209. doi: 10.3390/jmse7070209
Miroshnichenko, M. L., and Bonch-Osmolovskaya, E. A. (2006). Recent developments in the thermophilic microbiology of deep-sea hydrothermal vents. Extremophiles 10, 85–96. doi: 10.1007/s00792-005-0489-5
Mistry, K., Markande, A. R., Patel, J. K., and Parekh, K. (2024). Screening, isolation and characterization of aerobic Magnetotactic Bacteria from Western Ghats Forest soil. Indian J. Microbiol. 64, 1257–1265. doi: 10.1007/s12088-024-01316-4
Moisescu, C., Ardelean, I. I., and Benning, L. G. (2014). The effect and role of environmental conditions on magnetosome synthesis. Front. Microbiol. 5:49. doi: 10.3389/fmicb.2014.00049
Mugge, R. L., Brock, M. L., Salerno, J. L., Damour, M., Church, R. A., Lee, J. S., et al. (2019). Deep-sea biofilms, historic shipwreck preservation and the Deepwater horizon spill. Front. Mar. Sci. 6:48. doi: 10.3389/fmars.2019.00048
Mukherjee, S., and Bossier, B. L. (2019). Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 17, 371–382. doi: 10.1038/s41579-019-0186-5
Muñoz-Royo, C., Peacock, T., Alford, M. H., Smith, J. A., Le Boyer, A., Kulkarni, C. S., et al. (2021). Extent of impact of deep-sea nodule mining midwater plumes is influenced by sediment loading, turbulence and thresholds. Commun. Earth Environ. 2:148. doi: 10.1038/s43247-021-00213-8
Nan, K. H., and Pei, G. X. (2006). “Mineralization behavior of bioactive ceramic coatings formed by micro-arc oxidation on titanium” in Proceedings of the 19th international symposium on ceramics in medicine, Key Engineering Materials, 629.
Natalio, F., and Maria, R. (2024). Microbial biomineralization of alkaline earth metal carbonates on 3D-printed surfaces. ACS Appl. Mater. Interfaces 16, 6327–6336. doi: 10.1021/acsami.3c13665
Northup, D. E., and Lavoie, K. H. (2001). Geomicrobiology of caves: a review. Geomicrobiol J. 18, 199–222. doi: 10.1080/01490450152467750
Olesen, B. H., Nielsen, P. H., and Lewandowski, Z. (2000). Effect of biomineralized manganese on the corrosion behavior of C1008 mild steel. Corrosion 56, 80–89. doi: 10.5006/1.3280525
Osorio-Rodriguez, D., Metcalfe, K. S., McGlynn, S. E., Yu, H., Dekas, A. E., Ellisman, M., et al. (2023). Microbially induced precipitation of silica by anaerobic methane- oxidizing consortia and implications for microbial fossil preservation. Proc. Natl. Acad. Sci. USA 120:e2302156120. doi: 10.1073/pnas.2302156120
Pan, J., Han, Y., Wang, C., Du, J., Wang, Y., Chen, Y., et al. (2023). Analysis of microbial community and biodeterioration of maritime cultural relics (ironware, porcelain, axes, hull wood) from the Nanhai No. 1 shipwreck. Ann. Microbiol. 73:8. doi: 10.1186/s13213-022-01705-4
Park, Y., Eyal, Z., Pekker, P., Chevrier, D. M., Lefèvre, C. T., Arnoux, P., et al. (2022). Periplasmic bacterial biomineralization of copper sulfide nanoparticles. Adv. Sci. 9:e2203444. doi: 10.1002/advs.202203444
Parra, C., Aristizabal, J., Arce, B., Montero-Silva, F., Lascano, S., Henriquez, R., et al. (2021). Graphene coating as an effective barrier to prevent bacteria-mediated dissolution of gold. Metals 11:147. doi: 10.3390/met11010147
Pereira, O. S., Vlach, D., Bradley, A., Gonzalez, J., Mizell, K., and Levin, L. A. (2024). Invertebrate trophic structure on marine ferromanganese and phosphorite hardgrounds. Limnol. Oceanogr. 69, 1636–1650. doi: 10.1002/lno.12612
Qian, P. Y., Cheng, A. F., Wang, R. J., and Zhang, R. (2022). Marine biofilms: diversity, interactions and biofouling. Nat. Rev. Microbiol. 20, 671–684. doi: 10.1038/s41579-022-00744-7
Qian, Z., Tianwei, H., Mackey, H. R., van Loosdrecht, M. C. M., and Guanghao, C. (2019). Recent advances in dissimilatory sulfate reduction: from metabolic study to application. Water Res. 150, 162–181. doi: 10.1016/j.watres.2018.11.018
Rajala, P., Cheng, D.-Q., Rice, S. A., and Lauro, F. M. (2022). Sulfate-dependant microbially induced corrosion of mild steel in the deep sea: a 10-year microbiome study. Microbiome 10:4. doi: 10.1186/s40168-021-01196-6
Revathy, T., Saranya, R., Jayasri, M. A., Saurav, K., and Suthindhiran, K. (2014). Morphological alterations in erythrocytes treated with silver nanoparticles biomineralized by marine sediment-derived Bacillus sp VITSSN01. Ann. Microbiol. 64, 1291–1299. doi: 10.1007/s13213-013-0773-z
Reysenbach, A. L., Liu, Y. T., Banta, A. B., Beveridge, T. J., Kirshtein, J. D., Schouten, S., et al. (2006). A ubiquitous thermoacidophilic archaeon from deep-sea hydrothermal vents. Nature 442, 444–447. doi: 10.1038/nature04921
Safar, C., Castro, C., and Donati, E. (2020). Importance of initial interfacial steps during chalcopyrite bioleaching by a Thermoacidophilic archaeon. Microorganisms 8:1009. doi: 10.3390/microorganisms8071009
Saide, A., Lauritano, C., and Ianora, A. (2021). A treasure of bioactive compounds from the Deep Sea. Biomedicines 9:1556. doi: 10.3390/biomedicines9111556
Saunders, S. H., Tse, E. C. M., Yates, M. D., Otero, F. J., Trammell, S. A., Stemp, E. D. A., et al. (2020). Extracellular DNA promotes efficient extracellular Electron transfer by Pyocyanin in Pseudomonas aeruginosa biofilms. Cell 182, 919–932.e19. doi: 10.1016/j.cell.2020.07.006
Scheller, S., Yu, H., Chadwick, G. L., McGlynn, S. E., and Orphan, V. J. (2016). Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction. Science 351, 703–707. doi: 10.1126/science.aad7154
Shang, H., Daye, M., Sivan, O., Borlina, C. S., Tamura, N., Weiss, B. P., et al. (2020). Formation of Zerovalent Iron in Iron-reducing cultures of Methanosarcina barkeri. Environ. Sci. Technol. 54, 7354–7365. doi: 10.1021/acs.est.0c01595
Shen, Y., Dong, Y., Dong, L., and Yin, Y. (2020). Corrosion inhibition effect of microorganism on 5754 Al alloy in seawater. Acta Metall. Sin. 56, 1681–1689. doi: 10.11900/0412.1961.2020.00129
Shi, X. M., Avci, R., and Lewandowski, Z. (2002). Electrochemistry of passive metals modified by manganese oxides deposited by Leptothrix discophora: two-step model verified by ToF-SIMS. Corros. Sci. 44, 1027–1045. doi: 10.1016/s0010-938x(01)00104-4
Shu, W.-S., and Huang, L.-N. (2022). Microbial diversity in extreme environments. Nat. Rev. Microbiol. 20, 219–235. doi: 10.1038/s41579-021-00648-y
Simon-Lledó, E., Amon, D. J., Bribiesca-Contreras, G., Cuvelier, D., Durden, J. M., Ramalho, S. P., et al. (2023). Carbonate compensation depth drives abyssal biogeography in the Northeast Pacific. Nat. Ecol. Evol. 7, 1388–1397. doi: 10.1038/s41559-023-02122-9
Sun, X., Wai, O. W. H., Xie, J., and Li, X. (2023). Biomineralization to prevent microbially induced corrosion on concrete for sustainable marine infrastructure. Environ. Sci. Technol. 58, 522–533. doi: 10.1021/acs.est.3c04680
Sweetman, A. K., Smith, A. J., de Jonge, D. S. W., Hahn, T., Schroedl, P., Silverstein, M., et al. (2024). Evidence of dark oxygen production at the abyssal seafloor. Nat. Geosci. 17, 737–739. doi: 10.1038/s41561-024-01480-8
Templeton, A., and Knowles, E. (2009). Microbial transformations of minerals and metals: recent advances in geomicrobiology derived from synchrotron-based X-ray spectroscopy and X-ray microscopy. Annu. Rev. Earth Planet. Sci. 37, 367–391. doi: 10.1146/annurev.earth.36.031207.124346
Tian, X. C., Wu, X. E., Zhan, D. P., Zhao, F., Jiang, Y. X., and Sun, S. G. (2019). Research on electron transfer in the microenvironment of the biofilm by scanning electrochemical microscopy. Acta Phys. -Chim. Sin. 35, 22–27. doi: 10.3866/pku.Whxb201801085
Van Wylick, A., Monclaro, A. V., Elsacker, E., Vandelook, S., Rahier, H., De Laet, L., et al. (2021). A review on the potential of filamentous fungi for microbial self-healing of concrete. Fungal Biol. Biotechnol. 8:16. doi: 10.1186/s40694-021-00122-7
Verati, C., de Donato, P., Prieur, D., and Lancelot, J. (1999). Evidence of bacterial activity from micrometer-scale layer analyses of black-smoker sulfide structures (Pito seamount site, Easter microplate). Chem. Geol. 158, 257–269. doi: 10.1016/s0009-2541(99)00054-6
Vert, M., Doi, Y., Hellwich, K. H., Hess, M., Hodge, P., Kubisa, P., et al. (2012). Terminology for biorelated polymers and applications (IUPAC recommendations 2012). Pure Appl. Chem. 84, 377–408. doi: 10.1351/pac-rec-10-12-04
Wakai, S., Sakai, S., Nozaki, T., Watanabe, M., and Takai, K. (2024). Accelerated Iron corrosion by microbial consortia enriched from slime-like precipitates from a corroded metal apparatus deployed in a deep-sea hydrothermal system. Microbes Environ. 39:n/a. doi: 10.1264/jsme2.ME23089
Wan, Y., Chou, I. M., Wang, X. L., Wang, R. H., and Li, X. C. (2023). Hydrothermal sulfate surges promote rare earth element transport and mineralization. Geology 51, 449–453. doi: 10.1130/g50848.1
Wang, X. H., Schlossmacher, U., Natalio, F., Schröder, H. C., Wolf, S. E., Tremel, W., et al. (2009). Evidence for biogenic processes during formation of ferromanganese crusts from the Pacific Ocean: implications of biologically induced mineralization. Micron 40, 526–535. doi: 10.1016/j.micron.2009.04.005
Wang, H., Sun, X., Wang, Y., Shi, W., Wu, L., and Miao, L. (2025). Marine steel protection based on biomineralization for sustainable development of coastal cities. Bioresour. Technol. 428:132404. doi: 10.1016/j.biortech.2025.132404
Wang, F., Zhou, Y., Zhang, X., and Xiao, X. (2013). Biodiversity of deep-sea microorganisms. Biodivers. Sci. 21, 445–455. doi: 10.3724/SP.J.1003.2013.11094
Weiner, S., and Dove, P. M. (2003). An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 54, 1–29. doi: 10.2113/0540001
Wu, Y., Zhao, W., and Wang, L. (2025). State of the art and current trends on the metal corrosion and protection strategies in deep sea. J. Mater. Sci. Technol. 215, 192–213. doi: 10.1016/j.jmst.2024.07.026
Xiao, L. L., Hao, J. C., Wang, W. Y., Lian, B., Shang, G. D., Yang, Y. W., et al. (2014). The up-regulation of carbonic anhydrase genes of Bacillus mucilaginosus under soluble Ca2+ deficiency and the heterologously expressed enzyme promotes calcite dissolution. Geomicrobiol J. 31, 632–641. doi: 10.1080/01490451.2014.884195
Xiao, X., Zhao, W., Song, Z., Qi, Q., Wang, B., Zhu, J., et al. (2025). Microbial ecosystems and ecological driving forces in the deepest ocean sediments. Cell 188, 1363–1377.e9. doi: 10.1016/j.cell.2024.12.036
Xiong, F., Wang, J., Abbas, A. F., Fu, C., Gu, T., Hu, Y., et al. (2018). Research progress of sulfate-reducing bacteria induced SCC. Corrosion Sci. Protect. Technol. 30, 213–221. doi: 10.11903/1002.6495.2017.142
Yan, W., Wang, H. B., and Jing, C. Y. (2016). Adhesion of Shewanella oneidensis MR-1 to goethite: a two-dimensional correlation spectroscopic study. Environ. Sci. Technol. 50, 4343–4349. doi: 10.1021/acs.est.6b00066
Yanagida, A., Wakiya, S., Kigawa, R., Sato, Y., Shiga, S., Koizumi, Y., et al. (2024). What kind of corrosion products are “black spots”? —effects of reduced sulfur compounds on corrosion of bronze artefacts. J. Cult. Herit. 68, 307–315. doi: 10.1016/j.culher.2024.06.010
Yao, Y., Yang, Y., Wang, Y., and Zhao, X. (2019). Artificial intelligence-based hull structural plate corrosion damage detection and recognition using convolutional neural network. Appl. Ocean Res. 90:101823. doi: 10.1016/j.apor.2019.05.008
Zeng, R., and Zhao, J. (2002). Molecular identification and classification of deep sea psychrophilic bacteria from east-pacific sediment. Microbiology 29, 12–16. doi: 10.13344/j.microbiol.china.2002.06.004
Zhang, J., Deng, J., He, Y., Wu, J., Simoes, M. F., Liu, B., et al. (2024). A review of biomineralization in healing concrete: mechanism, biodiversity, and application. Sci. Total Environ. 917:170445. doi: 10.1016/j.scitotenv.2024.170445
Zhang, D., Li, X., Wu, Y., Xu, X., Liu, Y., Shi, B., et al. (2023). Microbe-driven elemental cycling enables microbial adaptation to deep-sea ferromanganese nodule sediment fields. Microbiome 11:160. doi: 10.1186/s40168-023-01601-2
Zhang, Y., Liu, T., Kang, J., Guo, N., Guo, Z., Chen, J., et al. (2022). Design of Multi-Functional Superhydrophobic Coating via bacterium-induced hierarchically structured minerals on steel surface. Front. Microbiol. 13:934966. doi: 10.3389/fmicb.2022.934966
Zhang, W. Q., Liu, H. X., Ying, J., and Liu, H. W. (2023). Preparation of nickel-iron sulfide/oxide nanocomposites by biomineralization of sulfate-reducing bacterium for efficient oxygen evolution. Chem. Eng. J. 475:146211. doi: 10.1016/j.cej.2023.146211
Zhang, C., Peng, Y., Liu, X., Wang, J., and Dong, X. (2024). Deep-sea microbial genetic resources: new frontiers for bioprospecting. Trends Microbiol. 32, 321–324. doi: 10.1016/j.tim.2024.01.002
Zhang, Y., Su, M., Wu, F. S., Gu, J. D., Li, J., He, D. P., et al. (2023). Diversity and composition of culturable microorganisms and their biodeterioration potentials in the sandstone of Beishiku Temple, China. Microorganisms 11:429. doi: 10.3390/microorganisms11020429
Zhang, J., Wang, B., Qu, S., Zhu, X., Wang, Y., and Teng, H. H. (2025). Minerals function as signals to modulate microbial adaptation. Commun. Earth Environ. 6:464. doi: 10.1038/s43247-025-02429-4
Zhang, K., Zhou, W., Zhao, J., Zhao, M., and Luo, H. (2024). Research on the corrosion mechanism of ancient celadons from the Dalian Island no. 1 shipwreck. Stud. Conserv. 70, 1–15. doi: 10.1080/00393630.2024.2365524
Zhao, J., Li, F., Kong, S., Chen, T., Song, H., and Wang, Z. (2023). Elongated riboflavin-producing Shewanella neidensis in a hybrid biofilm boosts extracellular Electron transfer. Adv. Sci. 10:e2206622. doi: 10.1002/advs.202206622
Zhao, M., Zhai, Y., Zhao, J., Zhao, L., Zhang, K., Ge, Y., et al. (2024). Barnacle attachment and its corrosion effects on the surface of the Yangtze estuary II shipwreck. J. Cult. Herit. 67, 73–79. doi: 10.1016/j.culher.2024.02.008
Zhao, M., Zhai, Y., Zhao, J., Zhou, W. H., Zhao, L., Ge, Y., et al. (2023). Microbial corrosion on underwater pottery relics with typical biological condensation disease. Herit. Sci. 11:260. doi: 10.1186/s40494-023-01111-2
Keywords: biomineralization, microbially influenced corrosion, deep-sea, interfacial interaction, marine environment
Citation: Ge Y, Wang C, Etim I-IN, Khan S, Li C, Yang L, Liu J, Yi P, Liu J, Sand W and Zhang R (2025) Deep-sea microbially influenced corrosion and biomineralization. Front. Microbiol. 16:1605909. doi: 10.3389/fmicb.2025.1605909
Edited by:
Drew Gorman-Lewis, University of Washington, United StatesReviewed by:
Matthew Saxton, Miami University, United StatesOlivier Habimana, Guangdong Technion-Israel Institute of Technology (GTIIT), China
Copyright © 2025 Ge, Wang, Etim, Khan, Li, Yang, Liu, Yi, Liu, Sand and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruiyong Zhang, cnVpeW9uZy56aGFuZ0BxZGlvLmFjLmNu
†These authors have contributed equally to this work
 Yanchen Ge
Yanchen Ge Can Wang
Can Wang Ini-Ibehe Nabuk Etim
Ini-Ibehe Nabuk Etim Sikandar Khan
Sikandar Khan Chengpeng Li1,2
Chengpeng Li1,2 Wolfgang Sand
Wolfgang Sand Ruiyong Zhang
Ruiyong Zhang