- 1Department of Surgery, The Second Hospital of Jilin University, Changchun, China
- 2Department of Obstetrics and Gynecology, The Second Hospital of Jilin University, Changchun, China
- 3Medical Imaging Technology Program, Jilin Medical University, Jilin, China
Premature ovarian failure is a prevalent gynecological endocrine disorder with an increasing incidence rate each year, impacting women’s physical and mental health. The causes of POF are poorly understood, but genetic, immune, iatrogenic, environmental, and psychological factors are key contributors. Clinically, POF manifests as oligomenorrhea, amenorrhea, elevated follicle-stimulating hormone (FSH) levels, and decreased estrogen levels, leading to infertility in women. POF not only impacts reproductive function but also elevates the risk of cardiovascular diseases, osteoporosis, depression, anxiety, cognitive decline, and neurological disorders, thereby adversely affecting women’s mental health and quality of life over the long term. The gut microbiota (GM) comprises a vast and complex microbial community within the human gastrointestinal tract. GM dysregulation is closely associated with numerous human diseases, including autoimmune diseases, allergic disorders, cardiovascular diseases, cancers, and metabolic disorders. Studies have shown that GMs play a pivotal role in female reproductive health, participating in the pathogenesis of reproductive endocrine disorders through direct or indirect involvement in sex hormone regulation, stimulation of inflammatory cytokine production, modulation of immune function, metabolic homeostasis, and regulation of neurotransmitter synthesis. Recently, advancements in human microbiology have highlighted the significant interest in the connection between POF and the gut microbiome. Researching the molecular mechanisms by which GMs and their metabolites regulate the occurrence of POF opens up a new direction for studying the pathogenesis of POF. This research aims to identify an efficient, non-invasive, and accurate diagnostic method for clinical diagnosis and treatment of POF, providing novel theoretical insights and precise intervention strategies for the clinical prevention and treatment of POF.
1 Introduction
The ovary, one of women’s most vital organs, maintains female fertility and endocrine functions. The ovary is susceptible to various factors, including age, genetics, immunity, medical interventions, environment, dietary structure, and psychosocial factors, which can impair ovarian reserve and function through direct or indirect pathways (Caserta et al., 2013; de Souza et al., 2015; Schuh-Huerta et al., 2012; Várbíró et al., 2022). Ovarian reserve indicates ovarian follicles’ remaining quantity and quality, representing a woman’s reproductive potential (Hussein, 2005). Infertility is a highly prevalent global condition, and the prevention and treatment of female infertility face severe challenges, with no effective means currently available to slow this trend. Despite the rapid advancement of human in vitro fertilization-embryo transfer technology, clinicians face challenges, including low oocyte retrieval, poor embryo quality, high cycle cancelation, and low clinical pregnancy rates. Diminished ovarian function is currently a research hotspot in gynecological reproductive endocrinology (Gulan et al., 2019). POF refers to ovarian dysfunction occurring before age 40 in women. The main clinical manifestations of POF include decreased menstrual flow, prolonged menstrual cycles or even amenorrhea, elevated FSH levels, and decreased estrogen levels (Sullivan et al., 2016). In the 21st century, with the launch of the “Human Microbiome Project,” trillions of microorganisms have received increasing attention, particularly the most complex GM (Cullin et al., 2021; Puschhof et al., 2021). Advances in genome sequencing technology and bioinformatics have elucidated the impact of GM on health and the interaction between microorganisms and their hosts (Sender et al., 2016; Zuo et al., 2023; Figure 1). The GM parasites are on the surface of the gastrointestinal tract, with most not being pathogenic and coexisting with intestinal epithelial cells. The gut microbiota plays crucial roles in host nutrient metabolism, preserving intestinal mucosal barrier integrity, regulating the immune system, and defending against pathogens (Qin et al., 2010). Research findings suggest that GM dysregulation contributes to the onset and progression of multiple reproductive endocrine disorders. The gut microbiota affects seminal fluid microbiota in adult male mice on a high-fat diet, influencing semen quality (Ding et al., 2020). In patients with polycystic ovary syndrome, the composition of the GM is disrupted, and the diversity and abundance of the GM improve significantly after drug treatment (Torres et al., 2019). Consequently, the GM can affect POF occurrence and progression through various pathways and factors. Researching the relationship between the GM and POF holds great potential and value for application, providing new diagnostic and therapeutic approaches for POF.
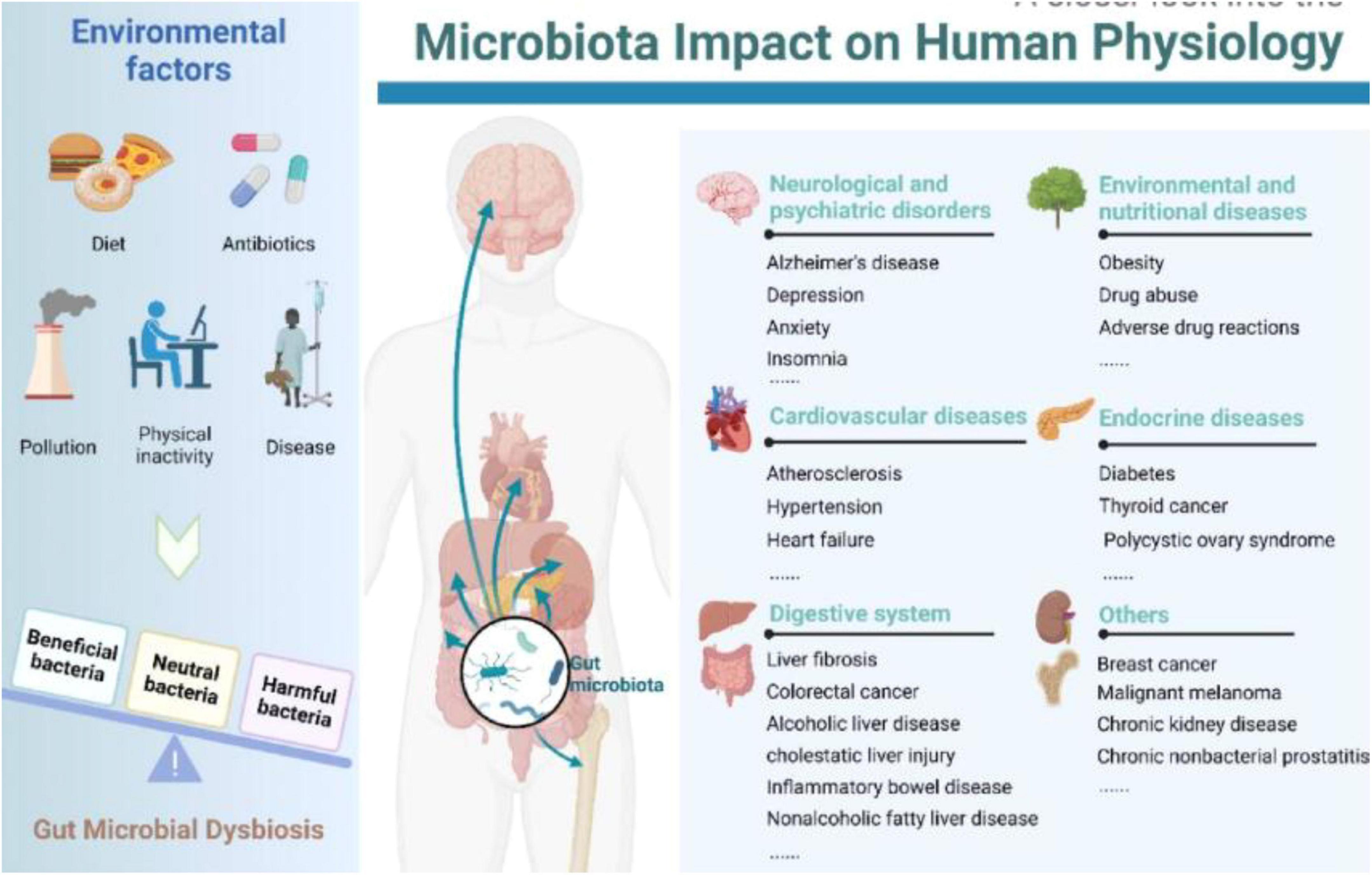
Figure 1. Relationship between gut microbiota and humans (Zuo et al., 2023). Left: Multiple environmental factors, encompassing diet, antibiotics, pollution, physical inactivity, and disease, may engender perturbations in the gut microbiota. Right: Gut microbiota aberrations have been linked to a myriad of morbidities, spanning neuropsychiatric disorders, cardiovascular afflictions, gastrointestinal ailments, diseases arising from environmental and nutritional factors, endocrinopathies, and more.
2 Overview of POF
Premature ovarian failure refers to ovarian dysfunction occurring in women before age 40, accompanied by high gonadotropin (Gn)-induced amenorrhea due to follicular depletion. The main clinical manifestations include oligomenorrhea or amenorrhea, elevated FSH levels, and fluctuating decreases in estrogen levels (Nash and Davies, 2024). Diminished ovarian reserve (DOR) primarily refers to a reduction in the number or quality of oocytes within the ovaries, accompanied by decreased anti-Müllerian hormone levels, decreased antral follicles, and elevated FSH levels (Cedars, 2022). Clinically, this may present as menstrual disorders, reduced flow progressing to amenorrhea, recurrent miscarriages, and infertility, impacting women’s reproductive health and quality of life. Furthermore, it can progress to premature ovarian insufficiency (POI) (Torrealday and Pal, 2015). POI is the early stage of ovarian failure, occurring in women before age 40, characterized by decreased ovarian function with serum hormone changes such as elevated FSH levels and low estrogen levels. The primary features include menstrual disorders, including amenorrhea or frequent menstruation (Shi et al., 2023). Patients often experience Gn-excessive amenorrhea due to estrogen deficiency, accompanied by an increased incidence of osteoporosis, cardiovascular disease, depression, cognitive dysfunction, and other conditions (Eastell, 2003; Roeters van Lennep et al., 2016; Tao et al., 2016).
The postponement of the childbearing age has become a global issue. With the increase in work pressure on women, environmental changes, and various other factors, the incidence of POF has shown an upward trend year by year. It is affecting younger women (American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee, 2014; Yang Q. et al., 2021). The pathogenesis of POF remains unclear, and its etiology is complex and diverse, involving genetic factors, age, iatrogenic factors, autoimmune factors, endocrine disorders, infectious factors, environmental factors, psychosocial factors, and lifestyle habits (D’Avila et al., 2015; Pelosi et al., 2015; Szeliga et al., 2021; Titus et al., 2015). Autoimmune factors account for up to 10%–30% of the causes of POF and are related to the regulation of multiple cytokines (Jagarlamudi et al., 2010). The majority of POF patients experience perimenopausal symptoms such as facial flushing, hot flashes, and hyperhidrosis. POF adversely affects women’s reproductive health and imposes psychological burdens while also elevating the risk of osteoporosis, cardiovascular diseases, and other conditions, thereby impairing patients’ quality of life and overall health (Allshouse et al., 2015; Francucci et al., 2008). Currently, the primary treatment for POF is Hormone Replacement Therapy (HRT). Although HRT can alleviate clinical symptoms such as hot flashes and hyperhidrosis caused by hormone deficiency, it does not fundamentally address the damage to ovarian structure and function. Relapses are common after discontinuation, and long-term hormone use increases the risk of developing diseases such as endometrial cancer, breast cancer, and thrombosis (Lambrinoudaki and Paschou, 2021). Therefore, exploring effective and safe treatment options for POF is an urgent clinical issue (Schover, 2008).
3 Overview of GM
The microbiome is one of the hottest research areas in biomedicine, related to various life processes in the human body, such as metabolism, immunity, reproduction, neurology, and inflammation. The composition of microorganisms in biological samples is demonstrated through sequencing, classification, and statistical analysis of the microbiome using 16S ribosomal RNA and metagenomic sequencing techniques, revealing its high diversity (Gopalakrishnan et al., 2018; Lee et al., 2024; Yang et al., 2019). GM is a crucial component of human microecology, and due to its vast number and powerful functions, it is known as the “second genome” of humans. The intestinal microecology encompasses the gut microbiome and its environment, where bacteria, fungi, and viruses in the human gastrointestinal tract coevolve and coexist with the host, forming the intestinal microbiota (de Vos et al., 2022). There are over 1,000 known bacterial species in the human gastrointestinal tract, and the number of GM is ten times that of human cells, with genes encoded by GM being 150 times that of the human genome. Through interactions with the environment, GM can influence the body’s physiological metabolism and pathological processes (Makki et al., 2018). GM is distributed segmentally along the digestive tract, with the lowest bacterial count in the stomach and most colonization occurring in the colon and ileum. As an essential component that coexists with the body for a long time, a delicate balance has been formed between GM and the host (Coyte and Rakoff-Nahoum, 2019). GM plays a significant role in the pathophysiological processes of human growth, development, metabolism, immunity, etc., including promoting the maturation of the host’s immune system, inhibiting pathogen overgrowth, influencing host cell proliferation and angiogenesis, regulating intestinal endocrine function, neurotransmitter signaling, energy sources, and vitamin and neurotransmitter synthesis (Cani, 2018; Xu et al., 2024). GM influences the intestinal environment and regulates distant tissues and organs, functioning as a mature endocrine organ.
3.1 Composition of GM
The human GM primarily comprises four phyla: Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, with Bacteroidetes and Firmicutes being the most dominant (Parker et al., 2020; Sun et al., 2021). Firmicutes have immune-regulating effects, while Firmicutes, Bacteroidetes, and Ruminococcus influence the production and metabolism of estrogens. Studies have reported that Verrucomicrobia is a species of significant research value, within which Akkermansia belongs, and is considered to play a crucial role in the human intestinal microbiota, exhibiting probiotic properties (Gómez-Gallego et al., 2016). The Lachnospiraceae family plays a vital role in intestinal health by engaging in the digestion and metabolism of dietary fiber, carbohydrates, and sugar transport. Recent studies have highlighted metabolites such as short-chain fatty acids (SCFAs), trimethylamine N-oxide (TMAO), bile acids, polyphenols, and indoles as being closely linked to host health (Vallianou et al., 2019; Yang X. et al., 2021; Figure 2). TMAO, produced by the metabolism of choline in food by intestinal microorganisms, is a key factor in inducing atherosclerosis. This discovery has advanced research on the relationship between intestinal microbial metabolites and extraintestinal organ damage (Koeth et al., 2013). Changes in these differential microbiotas, such as Firmicutes, Bacteroidetes, Verrucomicrobia, Proteobacteria, Lachnospira, and Ruminococcus, and their compositional structure may be one of the pathogenic mechanisms of ovarian dysfunction (Lin et al., 2019).
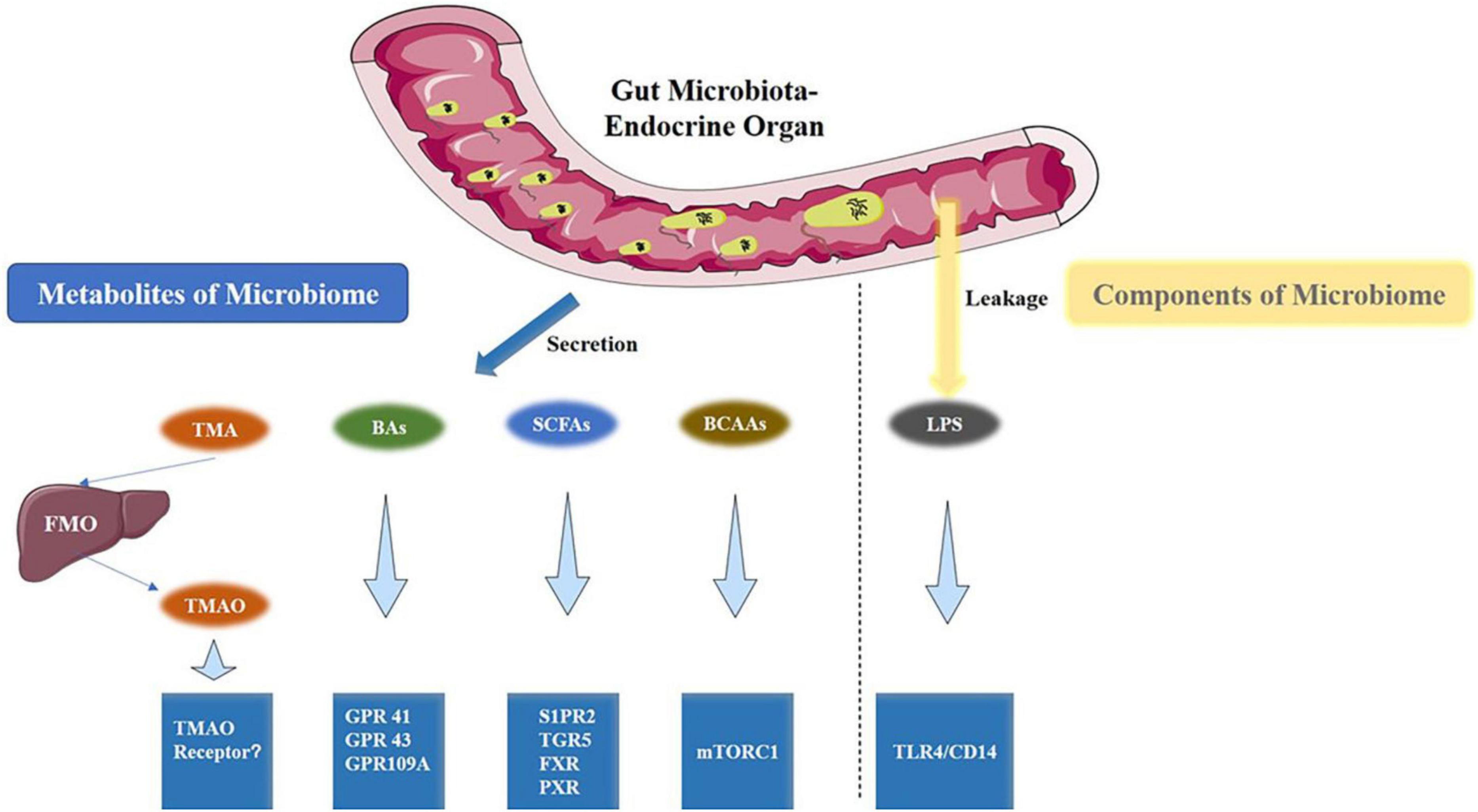
Figure 2. Gut microbiota metabolites and the associated metabolic signaling pathway (Yang Q. et al., 2021). TMAO, BAs, SCFAs, and BCAAs are the metabolites that are produced by the gut microbiota. Conversely, LPS is a component of the cell wall of gut bacteria. All are capable of activating specific signaling pathways. BAs, bile acids; BCAAs, branched-chain amino acids.
3.2 Metabolites of GM–SCFAs
Short-chain fatty acids, mainly acetic, propionic, and butyric acids, constitute over 95% of the total (O’Riordan et al., 2022). As messengers between the GM and the host, SCFAs exert extensive physiological effects on the host (Shen et al., 2019). They preserve intestinal barrier integrity, reduce colon pH, suppress harmful bacterial growth, and are involved in glucose and lipid metabolism (Ji et al., 2019). Lactobacillus and Bifidobacterium, recognized as beneficial bacteria, exert their beneficial effects by producing SCFAs and are widely used in probiotic preparations. SCFAs are essential for immune regulation and promoting inflammatory responses in pathological processes (LeBlanc et al., 2017). They can directly or indirectly inhibit LPS-activated TLR-4/NF-κB pathways, increase the expression of local cellular pro-inflammatory factors, and downregulate IL-8 and MCP-1, thereby achieving localized pro-inflammatory and overall anti-inflammatory effects (Lin et al., 2015; Segain et al., 2000). SCFAs regulate the distribution and localization of zonula occludens-1 and occludin in the intestinal barrier by influencing the endocannabinoid system, reducing the entry of endotoxin into the bloodstream, and thus controlling inflammation, intestinal barrier function, and intestinal peptide secretion (Muccioli et al., 2010). The endocannabinoid system is associated with female infertility, participating in the physiology and pathophysiology of the endometrium and placenta, influencing the successful implantation of blastocysts and the maintenance of early pregnancy, and holding a pivotal position in human reproduction (Maia et al., 2020). SCFAs affect oxidative damage in reproductive organs through autophagy-related pathways, influencing processes such as the origin of oocytes, the formation of ovarian reserve, follicle recruitment, and the selection of dominant follicles, which are crucial for follicular development (Kumariya et al., 2021). SCFAs also influence the hypothalamic-pituitary-ovarian (HPO) axis. In female obese rats, they can notably reverse precocious puberty, inhibit hypothalamic gonadotropin-releasing hormone (GnRH) secretion, and postpone gonadal axis development via the Kiss1-GPR54-PKC-ERK1/2 pathway (Wang et al., 2022).
3.3 The function of GM
Gut microbiota forms a mutualistic symbiotic relationship with the host and participates in various biological processes of the host through multiple mechanisms, including digestion and metabolism of food, development, and immunity (Rudi and Zhao, 2021). Changes in the composition of related microbial species and alterations in internal environmental homeostasis may trigger associated diseases (Wan et al., 2022). The function of the gut microbiota is not limited to the intestine; it can secrete various cytokines, induce inflammation and metabolic changes, and affect the immune system and neuroendocrine system functions (Melamed et al., 2022). The secretion and production of metabolites are the primary ways in which GM participates in the pathophysiological processes of the host. These metabolites can exert local effects in the intestine or enter the blood circulation through the intestinal barrier to affect the functions of multiple extraintestinal organs (Wang et al., 2019). GM can regulate the activity of various enteroendocrine cells and affect the gut-brain axis function. The gut-brain axis can further influence the structure of GM through the immune system (Cheng et al., 2023). GM influences systemic diseases via immune, neuroendocrine, and metabolic pathways (Manor et al., 2020). The human microbiome holds great potential in disease prevention and control: firstly, the composition or functional genes of the human microbiome can be used to predict the occurrence, progression, and outcome of related diseases; secondly, interventions and adjustments can be made to the microbiome to achieve disease prevention and treatment goals (Helmink et al., 2019; Matson et al., 2021; Weersma et al., 2020).
3.4 Balance of GM
Gut microbiota generally maintains a dynamic balance, interacting with the host and influencing the occurrence and progression of diseases by regulating the metabolism of substances such as sugars, lipids, and amino acids. Imbalances in gut microbiota may contribute to the onset of associated diseases (Takeuchi et al., 2023). Like other organs, the normal function of GM depends on its stable composition (Hörmannsperger et al., 2012). GM dysbiosis is primarily driven by environmental and host-related factors, such as antibiotic use, changes in dietary structure, immune activation, and stress states (Janczyk et al., 2007; Mountain, 1988). GM dysbiosis occurs when these factors disrupt the gut microbial ecosystem beyond its resistance and recovery capabilities, with effects ranging from transient to permanent and from benign to harmful (Fassarella et al., 2021). GM dysbiosis is mainly classified into three types: (1) Overproliferation of pathogens. In a healthy gut, pathogens exist at relatively low abundances, but in intestinal infection and inflammation states, Enterobacteriaceae bacteria can overproliferate; (2) Reduce or complete loss of commensal bacteria. This situation can be improved by exogenous supplementation of commensal bacteria. For example, in the case of Clostridium difficile infections, colonization with Clostridium scindens can improve the severity of the disease; (3) Decreased gut microbiota diversity. The impact of GM on reproductive organs is primarily through circulatory transport, where GM products or product-induced endocrine, oxidative stress, and inflammatory factors are transported to the reproductive organs, affecting reproductive function (Dey and Ray Chaudhuri, 2023; Yang et al., 2022).
4 The GM is intricately linked to the onset and progression of reproductive system diseases
The GM is closely related to the occurrence and progression of reproductive system diseases (Figure 3; Zhang et al., 2024). The endocrine levels of the ovaries can indirectly reflect ovarian function. The HPO axis controls hormone secretion. The hypothalamus secretes GnRH to stimulate the pituitary, which then releases FSH. This hormone enhances estrogen secretion by acting on the ovaries. Elevated estrogen levels negatively feedback on GnRH and FSH to maintain hormonal balance. Estrogen and FSH are key indicators for evaluating ovarian function. Estrogen, a type of hormone secreted by mature follicles, promotes the proliferation and differentiation of granulosa cells, regulates their apoptosis, and modulates steroid hormone levels. FSH primarily promotes the differentiation and maturation of granulosa cells in follicles and controls follicle development. Elevated FSH levels can lead to premature follicle maturation and early ovulation, resulting in follicular depletion. When estrogen secretion levels are too low, and FSH secretion levels are too high, it can be used to initially assess ovarian reserve function, which is essential for diagnosing POF (Brown et al., 2010). Corresponding vitamin deficiencies can affect sperm production and follicular development (Salam et al., 2015). Pyridoxine can regulate the synthesis and secretion of estrogens and progesterone, affecting the menstrual cycle and ovarian function. It can prevent preeclampsia and preterm birth and increase fertility rates (Wacker et al., 2000). Vitamin B12 is essential for oocyte maturation and quality, with deficiencies impacting ovarian function and oocyte development (Kim et al., 2020). Lipopolysaccharide (LPS), a key bacterial cell wall component in the gut, is crucial for maintaining intestinal barrier integrity, nutrient absorption, gut microbiota balance, and systemic inflammation (Płóciennikowska et al., 2015). One of the most common causes of reproductive disorders is LPS entering the circulation and triggering systemic inflammation and oxidative stress (Agarwal et al., 2017).
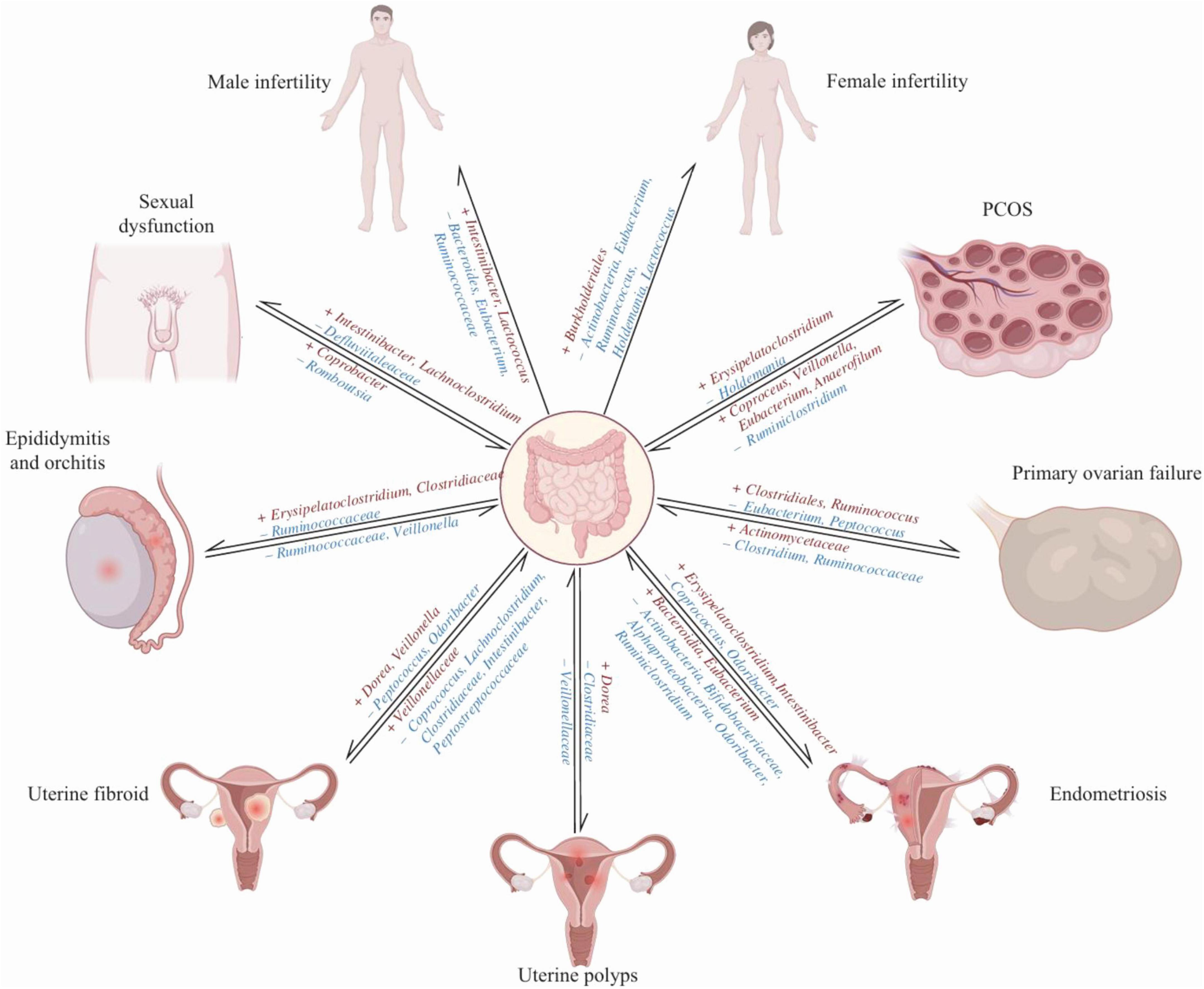
Figure 3. A bidirectional causal relationship between the gut microbiota and infertility, as well as associated diseases (Zhang et al., 2024). The protective bacteria are denoted in blue font (–), whereas the pathogenic bacteria are represented in red font (+).
4.1 The GM influences ovarian function via the HPO axis
Gut microbiota is intrinsically linked to ovarian function. The composition and metabolites of gut microbiota can affect the regulation of the HPO axis, thereby influencing the growth and development of ovarian tissue and oocytes (Hwang et al., 2015). Metabolites like SCFAs and bile acids from GM effectively regulate hypothalamic GnRH neuron function (Liao et al., 2021). GnRH is a key pathway in regulating reproductive function. GnRH secreted by the hypothalamus binds to pituitary receptors, stimulating the release of luteinizing hormone (LH) and FSH, LH and FSH stimulate the gonads to synthesize and secrete steroid hormones like testosterone, estrogen, and progesterone, facilitating the development of target organs. Ovarian aging is closely related to mitochondrial dysfunction, and oxidative stress-induced mitochondrial dysfunction is essential to ovarian aging. Mitochondria are crucial for cellular energy metabolism, the cell cycle, and signaling (May-Panloup et al., 2016). There is a dialogue between GM and mitochondria, and mitochondria can induce granulosa cell apoptosis and autophagy, leading to follicular atresia and premature ovarian failure (Bajpai et al., 2018; Thevaranjan et al., 2017; Yadav et al., 2018).
The gut-brain axis serves as a bridge for bidirectional communication between GM and the central nervous system. GM dysbiosis can alter the release of neuroendocrine hormones and affect neurotransmitter activity, subsequently influencing the central nervous system (Shekarabi et al., 2024). Research indicates that reduced levels of Firmicutes and Bacteroidetes can elevate serum glucagon-like peptide-1 (GLP-1) and its expression, which stimulates GnRH neurons. It modulates neurons and the neurotransmitter gamma-aminobutyric acid (GABA) to regulate GnRH secretion (Outeiriño-Iglesias et al., 2015). GM can produce most neurotransmitters in the brain, such as GABA, dopamine, norepinephrine, acetylcholine, and serotonin (5-HT) 0.5-HT can increase cAMP levels in immature oocytes, promoting oocyte meiotic maturation (Stricker and Smythe, 2001). As a precursor of melatonin, 5-HT can divert tryptophan away from the melatonin pathway through the kynurenine pathway, thereby reducing circulating melatonin levels (Davidson et al., 2022; Fernstrom, 2016). Melatonin may postpone ovarian aging by acting as an antioxidant, preserving telomeres, enhancing SIRT expression and ribosomal function, and decreasing autophagy. It safeguards oocytes from oxidative damage and enhances the expression of genes involved in fatty acid β-oxidation and mitochondrial biogenesis, supplying essential energy for oocyte maturation and embryonic development (Jin et al., 2017; Tamura et al., 2017).
5 POF and GM
5.1 GM characteristics of POF patients
Gut microbiota in patients with POF exhibits significant imbalances, and these alterations are associated with the occurrence and progression of the disease (Huang et al., 2024; Figure 4). Cao et al. (2020) in a POF mouse model, a significant increase in gut microbiota α-diversity was observed compared to normal mice. POF mice exhibited lower Helicobacter, Odoribacter, and Alistipes but higher Clostridium XIVa, Barnesiella, Bacteroides, and Mucispirillum (Cao et al., 2020). A comparison of gut microbiota revealed that healthy women had higher Firmicutes, Ruminococcus, and Caldibacillus levels.
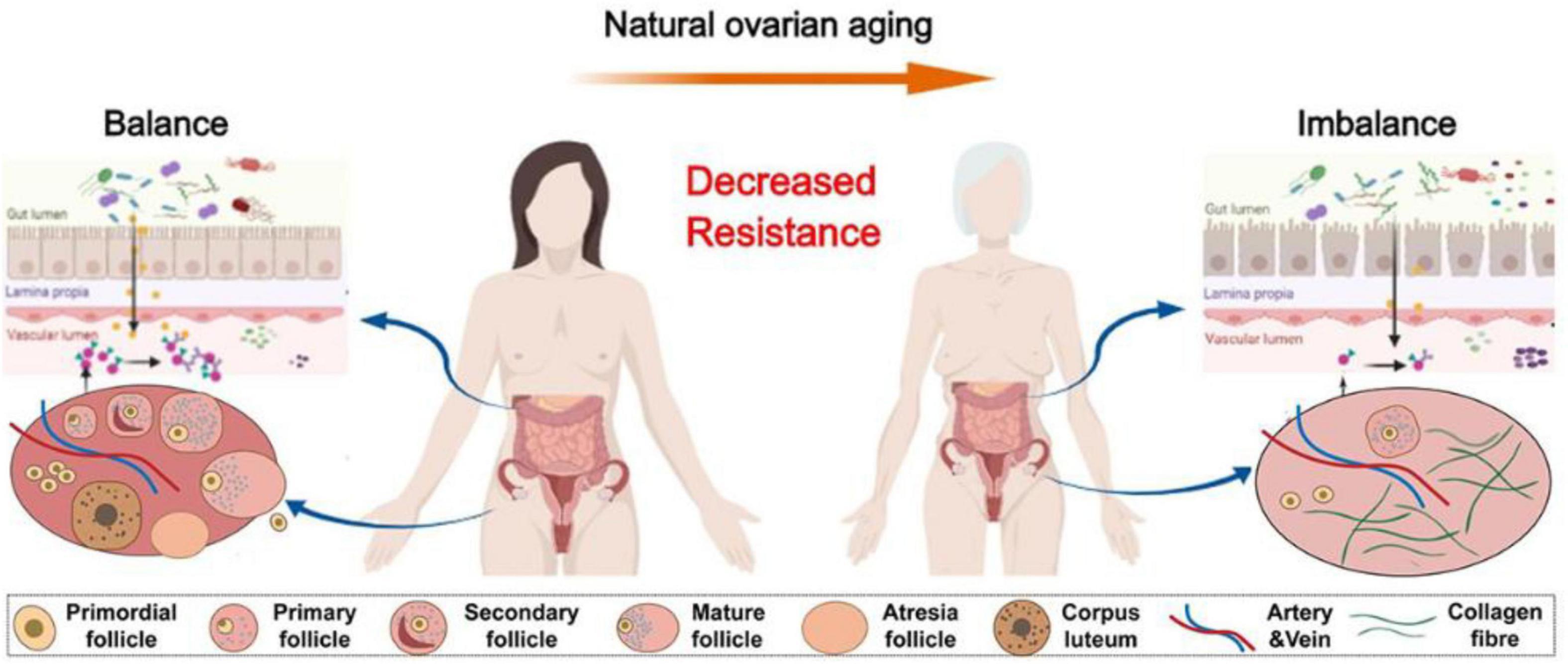
Figure 4. The physiologic succession of gut microbiota across natural ovarian aging (Huang et al., 2024).
In contrast, POF patients exhibited a greater abundance of Bacteroidetes, Butyricimonas, Dorea, Lachnobacterium, and Sutterella (Wu et al., 2021). Differences exist in the gut microbiota of patients with POI compared to a standard control group, with differential bacteria such as Bifidobacterium, Bacillus, and Clostridium being able to produce SCFAs in the human intestine (Wang et al., 2023). Not only does the gut microbiota of POF patients change, but their reproductive tract microbiota also undergoes alterations. Wang et al. (2018) study found notable differences in the diversity and richness of vaginal microbiota between POF patients and healthy women. The relative abundance of lactic acid bacteria was notably lower in POF patients than in postmenopausal women (Wang et al., 2018).
5.2 The mechanism of GM disorder participating in POF
Current research suggests that the relationship between GM and POF is mainly reflected in autoimmune responses, participation in sex hormone regulation, oxidative stress, inflammatory reactions, and ovulation disorders (Sharif et al., 2019). The GM acts as a messenger in the interaction between the host and the external environment, not only maintaining normal intestinal barrier function, resisting the invasion of pathogenic bacteria, participating in the digestion and absorption of nutrients to provide energy for the host, but also producing important metabolites and promoting the maturation of immune cells (Schluter et al., 2020). The GM can mitigate POF by modulating the expression of immune-related cytokines, including regulatory T cells (Treg), interferon-γ (IFN-γ), and T helper 17 cells (Th17) (Colliou et al., 2018; Motedayyen et al., 2018; Yin et al., 2018b). The GM is also broadly involved in hormone metabolism, bone density, and central nervous system regulation, all of which are closely related to the etiology and clinical symptoms of POF. GM’s development prospects and research space are vast, and an in-depth investigation of the relationship between GM and POF holds significant clinical value.
5.2.1 GM and humoral immunity, cellular immunity
Autoimmune abnormalities are one of the crucial factors contributing to the development of POF. A systemic pro-inflammatory state affects the ovarian environment, and autoimmunity can attack ovarian tissue, impacting follicular growth and development, causing follicular atresia and ovarian atrophy, directly leading to decreased ovarian function. Disruptions in gut microbiota can influence immune cytokines, producing primary ovarian failure POF (Kirshenbaum and Orvieto, 2019). Gomez de Agüero et al. (2016) the study observed transient colonization of the gut microbiota in pregnant rats, identifying it as a key factor in stimulating immune system development in their offspring, thereby suggesting that maternal gut microbiota influences the offspring’s immune system. In the host immune system, GM plays a crucial role. If GM is disrupted, the immune surveillance function of the body decreases, leading to immune escape and disease occurrence. Studies have found an imbalance in the expression of Th1/Th2 cytokines in T lymphocyte subpopulations in POF patients (Lu X. et al., 2019). Some scholars believe that the immune cells CD4 + T/CD8 + T may be related to Th and Treg cells. An increased or decreased CD4 + T/CD8 + T ratio in POI patients reflects a close correlation between POI pathogenesis and T-cell subpopulation imbalance and immune suppression (Kobayashi et al., 2019).
Gut microbiota can affect Treg, IFN-γ, and Th17, and there is a correlation between POF and these immune components (Britton et al., 2019). The potential connections between POF, GM, and immune cytokines include a correlation involving GM, POF, and Treg cells (Song et al., 2018). GM enhances Treg cell expression and differentiation, facilitating their role in anti-inflammatory responses and influencing immune and metabolic homeostasis. Clinically, post-treatment POF patients exhibit Treg numbers and enhanced immune regulation alterations. GM, POF, and IFN-γ are correlated. GM treatment may influence serum IFN-γ levels, which, along with genetic stimulation, can enhance MHC class II antigen expression. This process might trigger autoimmune responses, resulting in follicular atresia and the development of POF (Coulam and Stern, 1991). A correlation exists among GM, POF, and Th17. The study observed that hMSC transplantation in POF mice led to a reduction in Th17/Tc17 and Th17/Treg cell ratios, lower serum IL-17 levels, and decreased granulosa cell apoptosis compared to POF mice treated with a PI3K/Akt inhibitor. This suggests that the PI3K/Akt signaling pathway might play a role in restoring ovarian function by balancing Th17/Tc17 and Th17/Treg cells (Yin et al., 2018a). Therefore, decreased ovarian function is closely related to immune cytokines.
5.2.2 GM participates in hormone metabolism
5.2.2.1 The effect of GM on estrogen
Estrogens influence the gut microbiota’s structure and impact estrogen levels, playing a role in estrogen-regulated diseases through the estrogen-GM axis (Salliss et al., 2021; Figure 5). GM influences ovarian function through estrogen levels (Bates, 2018; García-Peñarrubia et al., 2020). Estrogens are crucial for female reproductive development and maintenance. Estrogens are major regulators of the GM, which is influenced by estrogens and positively affects estrogen levels. The GM capable of metabolizing estrogens possesses a gene pool known as the “estrogenome” (Baker et al., 2017). James and other scholars suggest that gut microbiota contributes to the development of estrogen-dependent diseases by introducing the “estrogen-gut microbiota axis,” which influences estrogen levels via β-glucuronidase secretion. β-Glucuronidase deconjugates estrogens, allowing them to bind to estrogen receptors and trigger physiological effects (Noguera-Julian et al., 2016). Altered gut microbiota composition and reduced diversity diminish β-glucuronidase activity, resulting in lower active estrogen levels (Plottel and Blaser, 2011). Reduced circulating estrogens change the activation of estrogen receptors, further contributing to various reproductive endocrine disorders. Estrogens promote follicular development directly and influence ovarian function indirectly by exerting negative feedback on Gn release through the HPO axis. Decreased estrogen levels are one of the essential factors in ovarian function decline. Recent studies indicate alterations in the gut microbiota of POI patients, accompanied by reduced estrogen levels. After adjusting for body mass index and performing Pearson correlation analysis, correlations were found between specific gut microbiota and serum estradiol, FSH, LH, and anti-Müllerian hormone levels (Wu et al., 2021).
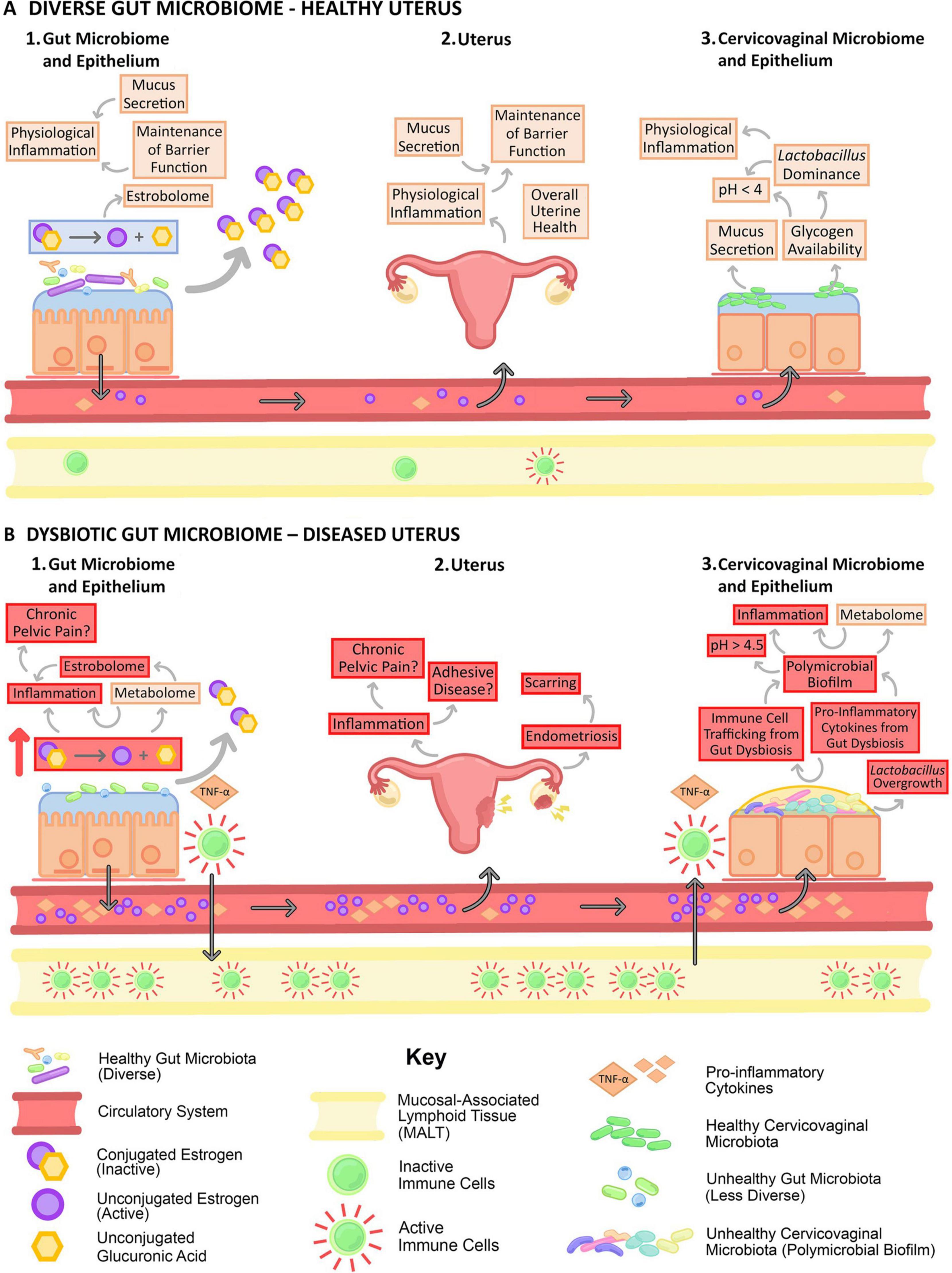
Figure 5. Gut dysbiosis negatively impacts the estrobolome (Salliss et al., 2021). Gut dysbiosis negatively impacts the estrobolome and the female reproductive tract by altering circulating estrogen levels, increasing systemic inflammation, and immune cell trafficking.
Studies have shown that altering GM through antibiotic use can lead to contraceptive failure and unintended pregnancies among women using oral contraceptives (Adlercreutz et al., 1984). Consuming yogurt to improve GM, primarily by increasing GABA and isoflavone levels, is beneficial for health (Fayed, 2015). Isoflavones are the most common “plant estrogens,” structurally and functionally similar to human estrogens. Research results indicate that altering the GM of ovariectomized rats leads to menopause-related symptoms due to decreased estrogen levels (Jeong et al., 2017). Prolonged intake of a symbiotic formulation with Lactobacillus fermentum and β-glucan can alleviate menopause symptoms due to estrogen deficiency in rats. The possible mechanisms of action are: ① β-Glucan can comprehensively stimulate the immune system, promote the production of immunoglobulin M antibodies, and enhance humoral immunity. β-Glucan positively influences gastrointestinal microecology by fostering beneficial intestinal bacteria growth and removing harmful substances. It also contributes to lowering cholesterol and low-density lipoprotein levels while enhancing high-density lipoprotein levels (Lewis-Barned et al., 2000).
5.2.2.2 The effect of GM on androgens
Androgen levels are one of the fundamental prerequisites for women’s health (McHenry et al., 2014). Androgen deficiency can cause sexual dysfunction symptoms, including reduced libido and diminished sexual response (Davis and Wahlin-Jacobsen, 2015). Women with POI not only lack estrogens but may also experience reduced ovarian androgen production due to ovarian cortical atrophy (Gleicher et al., 2013). A meta-analysis reveals that women with POI or POF are at risk of reduced levels of total testosterone, dehydroepiandrosterone sulfate, androstenedione (Soman et al., 2019). Androgens can improve the early stages of follicular development and effectively enhance ovarian reserve in women with DOR (Gleicher et al., 2011). Studies have found that low testosterone levels are essential steps involved in the occurrence of endometriosis combined with POI (Ono et al., 2014). In women with DOR, baseline testosterone levels are positively correlated with pregnancy outcomes following in vitro fertilization (Lu et al., 2014). There is a close relationship between the GM’s composition and circulating testosterone levels. Prenatal exposure of female rats to testosterone cypionate results in decreased abundances of Akkermansia, Bacteroides, Lactobacillus, and Clostridium in adult female offspring, suggesting an interaction between androgens and the gut microbiota (Sherman et al., 2018; Yurkovetskiy et al., 2013). The exact role of gut dysbiosis in the pathogenesis of androgen-dependent metabolic disorders deserves further investigation (Markle et al., 2013; Mayneris-Perxachs et al., 2020).
5.2.3 GM and oxidative stress and inflammatory responses
Gut microbiota dysbiosis may contribute to DOR development by inducing oxidative stress and chronic inflammation. Studies have confirmed that one of the reasons for increased oxidative stress in the body is GM dysbiosis, and oxidative stress has also been proven to be associated with DOR (Zou et al., 2020). In women with DOR, total granulosa cells are induced by oxidative stress to undergo increased apoptosis, leading to poorer ovarian response and reduced oocytes (Fan et al., 2019). Reactive oxygen species accumulation in oocytes leads to mitochondrial dysfunction, endoplasmic reticulum stress, and meiotic maturation disorders, ultimately impairing oocyte maturation and quality (Jungheim et al., 2010; Wu et al., 2010). The imbalance between elevated pro-inflammatory cytokines (TNF-α, IL-6) and reduced anti-inflammatory cytokines (IL-4, IL-10) contributes to POI (Huang et al., 2019). Studies have found that some ovarian tissue pathological biopsies from patients with POF show follicular inflammatory cell infiltration (Sun et al., 2018). Prebiotics help restore gut microbiota balance, decrease oxidative stress, and enhance the expression of anti-inflammatory and antioxidant molecules (Vasquez et al., 2019).
5.2.4 GM dysbiosis and ovulatory dysfunction
Hiroyuki et al. found that the relative abundance of butyrate-producing bacteria decreased in women with chronic anovulation. In contrast, the relative abundance of Prevotella increased, suggesting that GM dysbiosis could contribute to ovulatory dysfunction (Sasaki et al., 2019). Allobaculum can utilize carbohydrates to produce butyrate, increase SCFAs, enhance glucuronidase activity, reduce estrogen excretion, regulate hypothalamic neurons, and improve ovarian function (Flores et al., 2012). The abundance of Hungatella bacteria is positively correlated with taurine levels in the blood, and after the increase of Hungatella, taurine levels in the blood return to normal (Li et al., 2020). Taurine may activate the TGR5 protein-mediated AKT signaling pathway, promote ovarian tissue repair, and protect ovarian tissue (Lu M. et al., 2019). Fecal microbiota transplantation from women with ovulatory dysfunction to normal mice resulted in ovarian dysfunction and impaired fertility (Qi et al., 2021). GM dysbiosis can lead to oocyte developmental disorders, disruption of estrus cycles, and abnormal ovulation, and in the future, improving fertility may be achieved by regulating GM.
5.3 GM and complications of POF
5.3.1 GM and bone metabolism
The incidence of osteoporosis in women is generally higher than in men (Gregson et al., 2022). The prevalence of osteoporosis in women is primarily attributed to ovarian aging, diminishing follicular reserves, reduced oocyte quality, and lower serum estrogen levels (Touraine, 2020). Estrogen influences bone remodeling by altering cytokines and growth factors within the bone marrow and bone cells (Warden et al., 2010). Recent bone microbiota studies have also linked changes in bone phenotypes to changes in the GM. The hypothesis that a “healthy” microbiota is necessary to prevent sex hormone deficiency-induced bone loss is supported by evidence showing that supplementing the diet of ovariectomized mice with probiotics can reverse the pathogenic process of osteoporosis (Lyte, 2011). Additionally, several studies assessing the bacterial quantity and diversity in the intestines of patients with osteoporosis indicate that adult osteoporosis reduces biodiversity, such as species within the genera Fusobacterium, Faecalibacterium, and Bacteroides (Gustafsson et al., 2006; Yan and Charles, 2017).
5.3.2 GM and mental psychology
Psychological factors are one of the main contributors to POF. As a difficult-to-treat gynecological disease, POF not only causes abnormal menstruation, hot flashes, vaginal dryness, and other discomforting symptoms but may also induce psychological issues such as depression, anxiety, tension, and decreased self-confidence (Haller-Kikkatalo et al., 2015). Research has demonstrated that maintaining gut microbiota balance is essential for regulating brain behavior and cognitive function (Cossu et al., 2021). The interaction between gut microbiota and the central nervous system is crucial in developing mental disorders. The GM participates in basic neurobiological activities such as blood-brain barrier formation, myelination, neurotransmitter transmission, and glial cell maturation and regulates various animal behaviors (Li et al., 2022). Symbiotic bacteria, probiotics, and pathogens in the digestive tract, as initiators of a mild inflammatory state, can stimulate central nervous system pathways and signaling systems, causing dysfunction in the “microbe-gut-brain axis” and thus exacerbating the neuropsychiatric problems of POF patients (Kohn et al., 2021).
5.4 Chemotherapy-induced premature ovarian failure
Chemotherapy-induced premature ovarian failure refers to ovarian failure occurring after chemotherapy treatment (Molina et al., 2005). The ovarian damage caused by chemotherapy is a common treatment complication among female cancer patients and affects their quality of life (Chen and Manson, 2006). Discovering ovarian protective agents with minimal side effects for clinical use could dramatically enhance the quality of life for female cancer patients undergoing chemotherapy, offering substantial social and academic advantages. Studies have indicated that GM influences the host’s responsiveness to chemotherapy drugs, enhancing efficacy and neutralizing drug toxicity. This suggests that GM will be a key aspect of individualized treatment for cancer patients (Zhang et al., 2016). Gori et al. (2019) found that antibiotics against Gram-positive bacteria can reduce the therapeutic effect of platinum-based chemotherapy drugs; Perales-Puchalt et al. (2018) discovered that fecal microbiota transplantation from healthy mice could improve Cis-platinum-induced intestinal mucosa damage. Research indicates that cyclophosphamide-induced POF in mice leads to notable alterations in gut microbiota, characterized by reduced Akkermansia abundance and increased Lactobacillus levels. Fisetin mitigates CTX-induced ovarian damage by modulating the GM to decrease CD4 + T lymphocytes and IL-12 levels (Lin et al., 2020). Targeting the GM could be a “breakthrough” in studying the pathogenesis of CIPOF and developing new preventive and therapeutic drugs.
6 Conclusion
Premature ovarian failure is closely related to GM dysfunction, which not only affects disease progression but also exacerbates complications such as mental and psychological problems, decreased bone density, and autoimmune diseases (Table 1). Although the importance of GM and its metabolites in the pathogenesis of POF is increasingly recognized, current research still faces many challenges. These include the lack of long-term human studies, insufficient understanding of GM functional changes, and the wide variation in microbial profiles due to the diverse etiologies of POF. Future research should focus on longitudinal microbiome analysis in POF patients to uncover the deep connections and potential causal mechanisms between GM and POF. Integrating microbiome analysis into POF’s diagnostic and therapeutic strategies is essential for more precise and personalized treatments. Given that current studies have only demonstrated an association between GM and POF, further validation and exploration of their causal relationship are needed through large-scale samples, multicenter clinical trials, and animal experiments. Future research directions include using liquid chromatography-mass spectrometry (LC-MS) to analyze metabolites and their pathways in the serum and fecal samples of POF patients and healthy controls; integrating multi-omics data from microbiomics, genomics, and transcriptomics to construct comprehensive models that reveal the complex relationship between GM dysbiosis and POF, and identify new biomarkers and therapeutic targets; and conducting cell culture and animal model experiments to verify the impact of GM metabolites on ovarian and granulosa cell functions, thereby understanding the interaction mechanisms between GM and POF at the molecular level. These research directions and methods will provide new ideas and strategies for diagnosing and treating POF.
Author contributions
ZL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MW: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. YL: Conceptualization, Data curation, Investigation, Writing – review & editing. KX: Conceptualization, Data curation, Formal Analysis, Writing – review & editing. LF: Conceptualization, Data curation, Validation, Project administration, Validation, Visualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of Jilin Province (YDZJ202501ZYTS669) and the Jilin Provincial Department of Finance’s Medical and Health Talent Project (2024WSZX-B17).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
POF, premature ovarian failure; FSH, follicle-stimulating hormone; GM, gut microbiota; Gn, gonadotropin; DOR, diminished ovarian reserve; POI, premature ovarian insufficiency; HRT, hormone replacement therapy; SCFAs, short-chain fatty acids; TMAO, trimethylamine N-oxide; HPO, hypothalamic-pituitary-ovarian; GnRH, gonadotropin-releasing hormone; LPS, lipopolysaccharide; LH, luteinizing hormone; GLP-1, glucagon-like peptide-1; GABA, gamma-aminobutyric acid; 5-HT, serotonin; Treg, regulatory T cells; IFN-γ, interferon-γ; Th17, T helper 17 cells; CIPOF, Chemotherapy-induced premature ovarian failure; BAs, bile acids; BCAAs, branched-chain amino acids.
References
Adlercreutz, H., Pulkkinen, M., Hämäläinen, E., and Korpela, J. (1984). Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J. Steroid. Biochem. 20, 217–229. doi: 10.1016/0022-4731(84)90208-5
Agarwal, A., Roychoudhury, S., Sharma, R., Gupta, S., Majzoub, A., and Sabanegh, E. (2017). Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: Clinical utility in male factor infertility. Reprod. Biomed. Online 34, 48–57. doi: 10.1016/j.rbmo.2016.10.008
Allshouse, A., Semple, A., and Santoro, N. (2015). Evidence for prolonged and unique amenorrhea-related symptoms in women with premature ovarian failure/primary ovarian insufficiency. Menopause 22, 166–174. doi: 10.1097/GME.0000000000000286
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee (2014). Female age-related fertility decline. committee opinion No. 589. Fertil. Steril. 101, 633–634. doi: 10.1016/j.fertnstert.2013.12.032
Bajpai, P., Darra, A., and Agrawal, A. (2018). Microbe-mitochondrion crosstalk and health: An emerging paradigm. Mitochondrion 39, 20–25. doi: 10.1016/j.mito.2017.08.008
Baker, J., Al-Nakkash, L., and Herbst-Kralovetz, M. (2017). Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 103, 45–53. doi: 10.1016/j.maturitas.2017.06.025
Bates, G. (2018). Is hope on the horizon for premature ovarian insufficiency? Fertil. Steril. 109, 800–801. doi: 10.1016/j.fertnstert.2018.02.129
Britton, G., Contijoch, E., Mogno, I., Vennaro, O., Llewellyn, S., Ng, R., et al. (2019). Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity 50, 212–224.e4. doi: 10.1016/j.immuni.2018.12.015
Brown, C., LaRocca, J., Pietruska, J., Ota, M., Anderson, L., Smith, S., et al. (2010). Subfertility caused by altered follicular development and oocyte growth in female mice lacking PKB alpha/Akt1. Biol. Reprod. 82, 246–256. doi: 10.1095/biolreprod.109.077925
Cani, P. (2018). Human gut microbiome: Hopes, threats and promises. Gut 67, 1716–1725. doi: 10.1136/gutjnl-2018-316723
Cao, L., Leung, C., Law, P., Lv, Y., Ng, C., Liu, H., et al. (2020). Systemic changes in a mouse model of VCD-induced premature ovarian failure. Life Sci. 262:118543. doi: 10.1016/j.lfs.2020.118543
Caserta, D., Bordi, G., Di Segni, N., D’Ambrosio, A., Mallozzi, M., and Moscarini, M. (2013). The influence of cigarette smoking on a population of infertile men and women. Arch. Gynecol. Obstet. 287, 813–818. doi: 10.1007/s00404-012-2643-5
Cedars, M. (2022). Managing poor ovarian response in the patient with diminished ovarian reserve. Fertil. Steril. 117, 655–656. doi: 10.1016/j.fertnstert.2022.02.026
Chen, W., and Manson, J. (2006). Premature ovarian failure in cancer survivors: New insights, looming concerns. J. Natl. Cancer Inst. 98, 880–881. doi: 10.1093/jnci/djj276
Cheng, L., Wu, H., Chen, Z., Hao, H., and Zheng, X. (2023). Gut microbiome at the crossroad of genetic variants and behavior disorders. Gut Microbes 15, 2201156. doi: 10.1080/19490976.2023.2201156
Colliou, N., Ge, Y., Gong, M., Zadeh, M., Li, J., Alonzo, F., et al. (2018). Regulation of Th17 cells by P. UF1 against systemic Listeria monocytogenes infection. Gut Microbes 9, 279–287. doi: 10.1080/19490976.2017.1417731
Cossu, D., Watson, R., and Farina, C. (2021). Editorial: A microbial view of central nervous system disorders: Interplay between microorganisms, neuroinflammation and behaviour. Front. Immunol. 12:816227. doi: 10.3389/fimmu.2021.816227
Coulam, C., and Stern, J. (1991). Immunology of ovarian failure. Am. J. Reprod. Immunol. 25, 169–174. doi: 10.1111/j.1600-0897.1991.tb01089.x
Coyte, K., and Rakoff-Nahoum, S. (2019). Understanding competition and cooperation within the mammalian gut microbiome. Curr. Biol. 29, R538–R544. doi: 10.1016/j.cub.2019.04.017
Cross, T., Simpson, A., Lin, C., Hottmann, N., Bhatt, A., Pellock, S., et al. (2024). Gut microbiome responds to alteration in female sex hormone status and exacerbates metabolic dysfunction. Gut Microbes 16:2295429. doi: 10.1080/19490976.2023.2295429
Cullin, N., Azevedo Antunes, C., Straussman, R., Stein-Thoeringer, C., and Elinav, E. (2021). Microbiome and cancer. Cancer Cell. 39, 1317–1341. doi: 10.1016/j.ccell.2021.08.006
Davidson, M., Rashidi, N., Nurgali, K., and Apostolopoulos, V. (2022). The role of tryptophan metabolites in neuropsychiatric disorders. Int. J. Mol. Sci. 23:9968. doi: 10.3390/ijms23179968
D’Avila, ÂM., Biolchi, V., Capp, E., and Corleta, H. V. (2015). Age, anti-müllerian hormone, antral follicles count to predict amenorrhea or oligomenorrhea after chemotherapy with cyclophosphamide. J. Ovarian Res. 8:82. doi: 10.1186/s13048-015-0209-4
Davis, S., and Wahlin-Jacobsen, S. (2015). Testosterone in women–the clinical significance. Lancet Diabetes Endocrinol. 3, 980–992. doi: 10.1016/S2213-8587(15)00284-3
de Souza, F., da Silva, C., Yamakami, L., dos Santos Trindade Viana, V., Bonfá, E., and Shinjo, S. K. (2015). Reduced ovarian reserve in patients with adult polymyositis. Clin. Rheumatol. 34, 1795–1799. doi: 10.1007/s10067-015-3064-1
de Vos, W., Tilg, H., Van Hul, M., and Cani, P. (2022). Gut microbiome and health: Mechanistic insights. Gut 71, 1020–1032. doi: 10.1136/gutjnl-2021-326789
Dey, P., and Ray Chaudhuri, S. (2023). The opportunistic nature of gut commensal microbiota. Crit. Rev. Microbiol. 49, 739–763. doi: 10.1080/1040841X.2022.2133987
Ding, N., Zhang, X., Zhang, X., Jing, J., Liu, S., Mu, Y., et al. (2020). Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 69, 1608–1619. doi: 10.1136/gutjnl-2019-319127
Eastell, R. (2003). Management of osteoporosis due to ovarian failure. Med. Pediatr. Oncol. 41, 222–227. doi: 10.1002/mpo.10341
Fan, Y., Chang, Y., Wei, L., Chen, J., Li, J., Goldsmith, S., et al. (2019). Apoptosis of mural granulosa cells is increased in women with diminished ovarian reserve. J. Assist. Reprod. Genet. 36, 1225–1235. doi: 10.1007/s10815-019-01446-5
Fassarella, M., Blaak, E., Penders, J., Nauta, A., Smidt, H., and Zoetendal, E. (2021). Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 70, 595–605. doi: 10.1136/gutjnl-2020-321747
Fayed, A. (2015). Review article: Health benefits of some physiologically active ingredients and their suitability as yoghurt fortifiers. J. Food Sci. Technol. 52, 2512–2521. doi: 10.1007/s13197-014-1393-8
Fernstrom, J. D. (2016). A perspective on the safety of supplemental tryptophan based on its metabolic fates. J. Nutr. 146, 2601S–2608S. doi: 10.3945/jn.115.228643
Flores, R., Shi, J., Fuhrman, B., Xu, X., Veenstra, T., Gail, M., et al. (2012). Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 10:253. doi: 10.1186/1479-5876-10-253
Francucci, C., Romagni, P., Camilletti, A., Fiscaletti, P., Amoroso, L., Cenci, G., et al. (2008). Effect of natural early menopause on bone mineral density. Maturitas 59, 323–328. doi: 10.1016/j.maturitas.2008.03.008
García-Peñarrubia, P., Ruiz-Alcaraz, A., Martínez-Esparza, M., Marín, P., and Machado-Linde, F. (2020). Hypothetical roadmap towards endometriosis: Prenatal endocrine-disrupting chemical pollutant exposure, anogenital distance, gut-genital microbiota and subclinical infections. Hum. Reprod. Update 26, 214–246. doi: 10.1093/humupd/dmz044
Gleicher, N., Kim, A., Weghofer, A., Kushnir, V., Shohat-Tal, A., Lazzaroni, E., et al. (2013). Hypoandrogenism in association with diminished functional ovarian reserve. Hum. Reprod. 28, 1084–1091. doi: 10.1093/humrep/det033
Gleicher, N., Weghofer, A., and Barad, D. (2011). The role of androgens in follicle maturation and ovulation induction: Friend or foe of infertility treatment? Reprod. Biol. Endocrinol. 9:116. doi: 10.1186/1477-7827-9-116
Gomez de Agüero, M., Ganal-Vonarburg, S. C., Fuhrer, T., Rupp, S., Uchimura, Y., Li, H., et al. (2016). The maternal microbiota drives early postnatal innate immune development. Science 351, 1296–1302. doi: 10.1126/science.aad2571
Gómez-Gallego, C., Pohl, S., Salminen, S., De Vos, W., and Kneifel, W. (2016). Akkermansia muciniphila: A novel functional microbe with probiotic properties. Benef. Microbes 7, 571–584. doi: 10.3920/BM2016.0009
Gopalakrishnan, V., Helmink, B., Spencer, C., Reuben, A., and Wargo, J. (2018). The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 33, 570–580. doi: 10.1016/j.ccell.2018.03.015
Gori, S., Inno, A., Belluomini, L., Bocus, P., Bisoffi, Z., Russo, A., et al. (2019). Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit. Rev. Oncol. Hematol. 143, 139–147. doi: 10.1016/j.critrevonc.2019.09.003
Gregson, C., Armstrong, D., Bowden, J., Cooper, C., Edwards, J., Gittoes, N., et al. (2022). UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 17:58. doi: 10.1007/s11657-022-01061-5
Gulan, T., Yeernuer, T., Sui, S., and Mayinuer, N. A. (2019). Rat model of maternal polycystic ovary syndrome shows that exposure to androgens in utero results in dysbiosis of the intestinal microbiota and metabolic disorders of the newborn rat. Med. Sci. Monit. 25, 9377–9391. doi: 10.12659/MSM.918600
Gustafsson, B., Thommesen, L., Stunes, A., Tommeras, K., Westbroek, I., Waldum, H., et al. (2006). Serotonin and fluoxetine modulate bone cell function in vitro. J. Cell. Biochem. 98, 139–151. doi: 10.1002/jcb.20734
Haller-Kikkatalo, K., Uibo, R., Kurg, A., and Salumets, A. (2015). The prevalence and phenotypic characteristics of spontaneous premature ovarian failure: A general population registry-based study. Hum. Reprod. 30, 1229–1238. doi: 10.1093/humrep/dev021
Helmink, B., Khan, M., Hermann, A., Gopalakrishnan, V., and Wargo, J. (2019). The microbiome, cancer, and cancer therapy. Nat. Med. 25, 377–388. doi: 10.1038/s41591-019-0377-7
Hörmannsperger, G., Clavel, T., and Haller, D. (2012). Gut matters: Microbe-host interactions in allergic diseases. J. Allergy Clin. Immunol. 129, 1452–1459. doi: 10.1016/j.jaci.2011.12.993
Huang, F., Cao, Y., Liang, J., Tang, R., Wu, S., Zhang, P., et al. (2024). The influence of the gut microbiome on ovarian aging. Gut Microbes 16:2295394. doi: 10.1080/19490976.2023.2295394
Huang, Y., Hu, C., Ye, H., Luo, R., Fu, X., Li, X., et al. (2019). Inflamm-aging: A new mechanism affecting premature ovarian insufficiency. J. Immunol. Res. 2019:8069898. doi: 10.1155/2019/8069898
Hussein, M. (2005). Apoptosis in the ovary: Molecular mechanisms. Hum. Reprod. Update 11, 162–177. doi: 10.1093/humupd/dmi001
Hwang, I., Park, Y., Kim, Y., Kim, Y., Ka, S., Lee, H., et al. (2015). Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J. 29, 2397–2411. doi: 10.1096/fj.14-265983
Jagarlamudi, K., Reddy, P., Adhikari, D., and Liu, K. (2010). Genetically modified mouse models for premature ovarian failure (POF). Mol. Cell. Endocrinol. 315, 1–10. doi: 10.1016/j.mce.2009.07.016
Janczyk, P., Pieper, R., Souffrant, W., Bimczok, D., Rothkötter, H., and Smidt, H. (2007). Parenteral long-acting amoxicillin reduces intestinal bacterial community diversity in piglets even 5 weeks after the administration. ISME J. 1, 180–183. doi: 10.1038/ismej.2007.29
Jeong, S., Kang, S., Hua, C., Ting, Z., and Park, S. (2017). Synbiotic effects of β-glucans from cauliflower mushroom and Lactobacillus fermentum on metabolic changes and gut microbiome in estrogen-deficient rats. Genes Nutr. 12:31. doi: 10.1186/s12263-017-0585-z
Ji, J., Ge, X., Chen, Y., Zhu, B., Wu, Q., Zhang, J., et al. (2019). Daphnetin ameliorates experimental colitis by modulating microbiota composition and Treg/Th17 balance. FASEB J. 33, 9308–9322. doi: 10.1096/fj.201802659RR
Jiang, L., Fei, H., Tong, J., Zhou, J., Zhu, J., Jin, X., et al. (2021). Hormone replacement therapy reverses gut microbiome and serum metabolome alterations in premature ovarian insufficiency. Front. Endocrinol. 12:794496. doi: 10.3389/fendo.2021.794496
Jin, J., Lee, S., Taweechaipaisankul, A., Kim, G., and Lee, B. (2017). Melatonin regulates lipid metabolism in porcine oocytes. J. Pineal Res. 62, doi: 10.1111/jpi.12388
Jungheim, E., Schoeller, E., Marquard, K., Louden, Schaffer, J., and Moley, K. (2010). Diet-induced obesity model: Abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology 151, 4039–4046. doi: 10.1210/en.2010-0098
Kim, K., Mills, J., Michels, K., Chaljub, E., Wactawski-Wende, J., Plowden, T., et al. (2020). Dietary intakes of vitamin B-2 (Riboflavin), vitamin B-6, and vitamin B-12 and ovarian cycle function among premenopausal women. J. Acad. Nutr. Diet. 120, 885–892. doi: 10.1016/j.jand.2019.10.013
Kirshenbaum, M., and Orvieto, R. (2019). Premature ovarian insufficiency (POI) and autoimmunity-an update appraisal. J. Assist. Reprod. Genet. 36, 2207–2215. doi: 10.1007/s10815-019-01572-0
Kobayashi, M., Nakashima, A., Yoshino, O., Yoshie, M., Ushijima, A., Ito, M., et al. (2019). Decreased effector regulatory T cells and increased activated CD4+ T cells in premature ovarian insufficiency. Am. J. Reprod. Immunol. 81:e13125. doi: 10.1111/aji.13125
Koeth, R., Wang, Z., Levison, B., Buffa, J., Org, E., Sheehy, B., et al. (2013). Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585. doi: 10.1038/nm.3145
Kohn, N., Szopinska-Tokov, J., Llera Arenas, A., Beckmann, C., Arias-Vasquez, A., and Aarts, E. (2021). Multivariate associative patterns between the gut microbiota and large-scale brain network connectivity. Gut Microbes 13:2006586. doi: 10.1080/19490976.2021.2006586
Kumariya, S., Ubba, V., Jha, R., and Gayen, J. (2021). Autophagy in ovary and polycystic ovary syndrome: Role, dispute and future perspective. Autophagy 17, 2706–2733. doi: 10.1080/15548627.2021.1938914
Lambrinoudaki, I., and Paschou, S. (2021). Hormone therapy for menopause and premature ovarian insufficiency. Best. Pract. Res. Clin. Endocrinol. Metab. 35:101597. doi: 10.1016/j.beem.2021.101597
LeBlanc, J., Chain, F., Martín, R., Bermúdez-Humarán, L., Courau, S., and Langella, P. (2017). Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell. Fact. 16:79. doi: 10.1186/s12934-017-0691-z
Lee, J., Menon, N., and Lim, C. (2024). Dissecting gut-microbial community interactions using a gut microbiome-on-a-chip. Adv. Sci. 11:e2302113. doi: 10.1002/advs.202302113
Lewis-Barned, N., Sutherland, W., Walker, R., de Jong, S., Walker, H., Edwards, E., et al. (2000). Plasma cholesteryl ester fatty acid composition, insulin sensitivity, the menopause and hormone replacement therapy. J. Endocrinol. 165, 649–655. doi: 10.1677/joe.0.1650649
Li, H., Xu, H., Li, Y., Jiang, Y., Hu, Y., Liu, T., et al. (2020). Alterations of gut microbiota contribute to the progression of unruptured intracranial aneurysms. Nat. Commun. 11:3218. doi: 10.1038/s41467-020-16990-3
Li, X., Chen, L., Kumar, G., Zhang, S., Zhong, Q., Zhang, H., et al. (2022). Therapeutic interventions of gut-brain axis as novel strategies for treatment of alcohol use disorder associated cognitive and mood dysfunction. Front. Neurosci. 16:820106. doi: 10.3389/fnins.2022.820106
Liao, B., Qiao, J., and Pang, Y. (2021). Central regulation of PCOS: Abnormal neuronal-reproductive-metabolic circuits in PCOS pathophysiology. Front. Endocrinol. 12:667422. doi: 10.3389/fendo.2021.667422
Lin, J., Nie, X., Xiong, Y., Gong, Z., Chen, J., Chen, C., et al. (2020). Fisetin regulates gut microbiota to decrease CCR9+/CXCR3+/CD4+ T-lymphocyte count and IL-12 secretion to alleviate premature ovarian failure in mice. Am. J. Transl. Res. 12, 203–247.
Lin, M., de Zoete, M., van Putten, J., and Strijbis, K. (2015). Redirection of epithelial immune responses by short-chain fatty acids through inhibition of histone deacetylases. Front. Immunol. 6:554. doi: 10.3389/fimmu.2015.00554
Lin, S., Wang, Z., Lam, K., Zeng, S., Tan, B., and Hu, J. (2019). Role of intestinal microecology in the regulation of energy metabolism by dietary polyphenols and their metabolites. Food Nutr. Res. 63:63. doi: 10.29219/fnr.v63.1518
Lu, M., Hartmann, D., Braren, R., Gupta, A., Wang, B., Wang, Y., et al. (2019). Oncogenic Akt-FOXO3 loop favors tumor-promoting modes and enhances oxidative damage-associated hepatocellular carcinogenesis. BMC Cancer 19:887. doi: 10.1186/s12885-019-6110-6
Lu, Q., Shen, H., Li, Y., Zhang, C., Wang, C., Chen, X., et al. (2014). Low testosterone levels in women with diminished ovarian reserve impair embryo implantation rate: A retrospective case-control study. J. Assist. Reprod. Genet. 31, 485–491. doi: 10.1007/s10815-014-0186-3
Lu, X., Cui, J., Cui, L., Luo, Q., Cao, Q., Yuan, W., et al. (2019). The effects of human umbilical cord-derived mesenchymal stem cell transplantation on endometrial receptivity are associated with Th1/Th2 balance change and uNK cell expression of uterine in autoimmune premature ovarian failure mice. Stem. Cell. Res. Ther. 10:214. doi: 10.1186/s13287-019-1313-y
Lyte, M. (2011). Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 33, 574–581. doi: 10.1002/bies.201100024
Maia, J., Fonseca, B., Teixeira, N., and Correia-da-Silva, G. (2020). The fundamental role of the endocannabinoid system in endometrium and placenta: Implications in pathophysiological aspects of uterine and pregnancy disorders. Hum. Reprod. Update 26, 586–602. doi: 10.1093/humupd/dmaa005
Makki, K., Deehan, E., Walter, J., and Bäckhed, F. (2018). The impact of dietary fiber on gut microbiota in host health and disease. Cell. Host. Microbe 23, 705–715. doi: 10.1016/j.chom.2018.05.012
Manor, O., Dai, C., Kornilov, S., Smith, B., Price, N., Lovejoy, J., et al. (2020). Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 11:5206. doi: 10.1038/s41467-020-18871-1
Markle, J., Frank, D., Mortin-Toth, S., Robertson, C., Feazel, L., Rolle-Kampczyk, U., et al. (2013). Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088. doi: 10.1126/science.1233521
Matson, V., Chervin, C., and Gajewski, T. (2021). Cancer and the microbiome-influence of the commensal microbiota on cancer, immune responses, and immunotherapy. Gastroenterology 160, 600–613. doi: 10.1053/j.gastro.2020.11.041
Mayneris-Perxachs, J., Arnoriaga-Rodríguez, M., Luque-Córdoba, D., Priego-Capote, F., Pérez-Brocal, V., Moya, A., et al. (2020). Gut microbiota steroid sexual dimorphism and its impact on gonadal steroids: Influences of obesity and menopausal status. Microbiome 8:136. doi: 10.1186/s40168-020-00913-x
May-Panloup, P., Boucret, L., Chao de la Barca, J. M., Desquiret-Dumas, V., Ferré-L’Hotellier, V., Morinière, C., et al. (2016). Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 22, 725–743. doi: 10.1093/humupd/dmw028
McHenry, J., Carrier, N., Hull, E., and Kabbaj, M. (2014). Sex differences in anxiety and depression: Role of testosterone. Front. Neuroendocrinol. 35:42–57. doi: 10.1016/j.yfrne.2013.09.001
Melamed, J., LeBlanc, G., and Constantinides, M. (2022). Gut microbiota gestalt. Cell. Host. Microbe 30, 899–901. doi: 10.1016/j.chom.2022.06.009
Molina, J., Barton, D., and Loprinzi, C. (2005). Chemotherapy-induced ovarian failure: Manifestations and management. Drug Saf. 28, 401–416. doi: 10.2165/00002018-200528050-00004
Motedayyen, H., Zarnani, A., Tajik, N., Ghotloo, S., and Rezaei, A. (2018). Immunomodulatory effects of human amniotic epithelial cells on naive CD4+ T cells from women with unexplained recurrent spontaneous abortion. Placenta 71, 31–40. doi: 10.1016/j.placenta.2018.06.008
Mountain, C. F. (1988). Prognostic implications of the international staging system for lung cancer. Semin. Oncol. 15, 236–245.
Muccioli, G., Naslain, D., Bäckhed, F., Reigstad, C., Lambert, D., Delzenne, N., et al. (2010). The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 6:392. doi: 10.1038/msb.2010.46
Nash, Z., and Davies, M. (2024). Premature ovarian insufficiency. BMJ 384:e077469. doi: 10.1136/bmj-2023-077469
Noguera-Julian, M., Rocafort, M., Guillén, Y., Rivera, J., Casadellà, M., Nowak, P., et al. (2016). Gut microbiota linked to sexual preference and HIV infection. EBioMedicine 5, 135–146. doi: 10.1016/j.ebiom.2016.01.032
Ono, Y., Tanabe, A., Nakamura, Y., Yamamoto, H., Hayashi, A., Tanaka, T., et al. (2014). A low-testosterone state associated with endometrioma leads to the apoptosis of granulosa cells. PLoS One 9:e115618. doi: 10.1371/journal.pone.0115618
O’Riordan, K., Collins, M., Moloney, G., Knox, E., Aburto, M., Fülling, C., et al. (2022). Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 546:111572. doi: 10.1016/j.mce.2022.111572
Outeiriño-Iglesias, V., Romaní-Pérez, M., González-Matías, L., Vigo, E., and Mallo, F. G. (2015). LP-1 increases preovulatory LH Source and the number of mature follicles, as well as synchronizing the onset of puberty in female rats. Endocrinology 156, 4226–4237. doi: 10.1210/en.2014-1978
Parker, B., Wearsch, P., Veloo, A., and Rodriguez-Palacios, A. (2020). The genus alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 11:906. doi: 10.3389/fimmu.2020.00906
Pelosi, E., Forabosco, A., and Schlessinger, D. (2015). Genetics of the ovarian reserve. Front. Genet. 6:308. doi: 10.3389/fgene.2015.00308
Perales-Puchalt, A., Perez-Sanz, J., Payne, K., Svoronos, N., Allegrezza, M., Chaurio, R., et al. (2018). Frontline science: Microbiota reconstitution restores intestinal integrity after cisplatin therapy. J. Leukoc. Biol. 103, 799–805. doi: 10.1002/JLB.5HI1117-446RR
Płóciennikowska, A., Hromada-Judycka, A., Borzęcka, K., and Kwiatkowska, K. (2015). Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 72, 557–581. doi: 10.1007/s00018-014-1762-5
Plottel, C., and Blaser, M. (2011). Microbiome and malignancy. Cell. Host. Microbe 10, 324–335. doi: 10.1016/j.chom.2011.10.003
Puschhof, J., Pleguezuelos-Manzano, C., and Clevers, H. (2021). Organoids and organs-on-chips: Insights into human gut-microbe interactions. Cell. Host. Microbe 29, 867–878. doi: 10.1016/j.chom.2021.04.002
Qi, X., Yun, C., Pang, Y., and Qiao, J. (2021). The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 13, 1–21. doi: 10.1080/19490976.2021.1894070
Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K., Manichanh, C., et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. doi: 10.1038/nature08821
Roeters van Lennep, J. E., Heida, K. Y., Bots, M. L., and Hoek, A. (2016). Cardiovascular disease risk in women with premature ovarian insufficiency: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 23, 178–186. doi: 10.1177/2047487314556004
Rudi, K., and Zhao, L. (2021). Grand challenges in understanding gut microbes. Front. Microbiol. 12:752829. doi: 10.3389/fmicb.2021.752829
Salam, R., Zuberi, N., and Bhutta, Z. (2015). Pyridoxine (vitamin B6) supplementation during pregnancy or labour for maternal and neonatal outcomes. Cochrane Database Syst. Rev. 2015:CD000179. doi: 10.1002/14651858.CD000179.pub3
Salliss, M., Farland, L., Mahnert, N., and Herbst-Kralovetz, M. (2021). The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum. Reprod. Update 28, 92–131. doi: 10.1093/humupd/dmab035
Sasaki, H., Kawamura, K., Kawamura, T., Odamaki, T., Katsumata, N., Xiao, J., et al. (2019). Distinctive subpopulations of the intestinal microbiota are present in women with unexplained chronic anovulation. Reprod. Biomed. Online 38, 570–578. doi: 10.1016/j.rbmo.2018.12.026
Schluter, J., Peled, J., Taylor, B., Markey, K., Smith, M., Taur, Y., et al. (2020). The gut microbiota is associated with immune cell dynamics in humans. Nature 588, 303–307. doi: 10.1038/s41586-020-2971-8
Schover, L. (2008). Premature ovarian failure and its consequences: Vasomotor symptoms, sexuality, and fertility. J. Clin. Oncol. 26, 753–758. doi: 10.1200/JCO.2007.14.1655
Schuh-Huerta, S., Johnson, N., Rosen, M., Sternfeld, B., Cedars, M., and Reijo Pera, R. (2012). Genetic variants and environmental factors associated with hormonal markers of ovarian reserve in Caucasian and African American women. Hum. Reprod. 27, 594–608. doi: 10.1093/humrep/der391
Segain, J., Raingeard de la Blétière, D., Bourreille, A., Leray, V., Gervois, N., Rosales, C., et al. (2000). Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut 47, 397–403. doi: 10.1136/gut.47.3.397
Sender, R., Fuchs, S., and Milo, R. (2016). Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14:e1002533. doi: 10.1371/journal.pbio.1002533
Sharif, K., Watad, A., Bridgewood, C., Kanduc, D., Amital, H., and Shoenfeld, Y. (2019). Insights into the autoimmune aspect of premature ovarian insufficiency. Best Pract. Res. Clin. Endocrinol. Metab. 33:101323. doi: 10.1016/j.beem.2019.101323
Shekarabi, A., Qureishy, I., Puglisi, C., Dalseth, M., and Vuong, H. (2024). Host-microbe interactions: Communication in the microbiota-gut-brain axis. Curr. Opin. Microbiol. 80:102494. doi: 10.1016/j.mib.2024.102494
Shen, B., Hu, J., Song, H., Wang, Z., Fan J., Sun, Y., et al. (2019). Antibiotics exacerbated colitis by affecting the microbiota, Treg cells and SCFAs in IL10-deficient mice. Biomed. Pharmacother. 114:108849. doi: 10.1016/j.biopha.2019.108849
Sherman, S., Sarsour, N., Salehi, M., Schroering, A., Mell, B., Joe, B., et al. (2018). Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes 9, 400–421. doi: 10.1080/19490976.2018.1441664
Shi, Y., Zhu, X., Zhang, S., Ma, Y., Han, Y., Jiang, Y., et al. (2023). Premature ovarian insufficiency: A review on the role of oxidative stress and the application of antioxidants. Front. Endocrinol. 14:1172481. doi: 10.3389/fendo.2023.1172481
Soman, M., Huang, L., Cai, W., Xu, J., Chen, J., He, R., et al. (2019). Serum androgen profiles in women with premature ovarian insufficiency: A systematic review and meta-analysis. Menopause 26, 78–93. doi: 10.1097/GME.0000000000001161
Song, K., Cai, H., Zhang, D., Huang, R., Sun, D., and He, Y. (2018). Effects of human adipose-derived mesenchymal stem cells combined with estrogen on regulatory T cells in patients with premature ovarian insufficiency. Int. Immunopharmacol. 55, 257–262.
Stricker, S., and Smythe, T. (2001). 5-HT causes an increase in cAMP that stimulates, rather than inhibits, oocyte maturation in marine nemertean worms. Development 128, 1415–1427. doi: 10.1242/dev.128.8.1415
Sullivan, S., Sarrel, P., and Nelson, L. (2016). Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil. Steril. 106, 1588–1599. doi: 10.1016/j.fertnstert.2016.09.046
Sun, D., Bai, R., Zhou, W., Yao, Z., Liu, Y., Tang, S., et al. (2021). Angiogenin maintains gut microbe homeostasis by balancing α-Proteobacteria and Lachnospiraceae. Gut 70, 666–676. doi: 10.1136/gutjnl-2019-320135
Sun, S., Chen, H., Zheng, X., Ma, C., and Yue, R. (2018). Analysis on the level of IL-6, IL-21, AMH in patients with auto-immunity premature ovarian failure and study of correlation. Exp. Ther. Med. 16, 3395–3398. doi: 10.3892/etm.2018.6592
Szeliga, A., Calik-Ksepka, A., Maciejewska-Jeske, M., Grymowicz, M., Smolarczyk, K., Kostrzak, A., et al. (2021). Autoimmune diseases in patients with premature ovarian insufficiency-our current state of knowledge. Int. J. Mol. Sci. 22:2594.
Takeuchi, T., Kameyama, K., Miyauchi, E., Nakanishi, Y., Kanaya, T., Fujii, T., et al. (2023). Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell. Metab. 35, 361–375.e9. doi: 10.1016/j.cmet.2022.12.013
Tamura, H., Kawamoto, M., Sato, S., Tamura, I., Maekawa, R., Taketani, T., et al. (2017). Long-term melatonin treatment delays ovarian aging. J. Pineal Res. 62, doi: 10.1111/jpi.12381
Tao, X., Zuo, A., Wang, J., and Tao, F. (2016). Effect of primary ovarian insufficiency and early natural menopause on mortality: A meta-analysis. Climacteric 19, 27–36. doi: 10.3109/13697137.2015.1094784
Thevaranjan, N., Puchta, A., Schulz, C., Naidoo, A., Szamosi, J., Verschoor, C., et al. (2017). Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell. Host. Microbe 21, 455–466.e4. doi: 10.1016/j.chom.2017.03.002
Titus, S., Stobezki, R., and Oktay, K. (2015). Impaired DNA repair as a mechanism for oocyte aging: Is it epigenetically determined? Semin. Reprod. Med. 33, 384–388. doi: 10.1055/s-0035-1567824
Torrealday, S., and Pal, L. (2015). Premature menopause. Endocrinol. Metab. Clin. North Am. 44, 543–557. doi: 10.1016/j.ecl.2015.05.004
Torres, P., Ho, B., Arroyo, P., Sau, L., Chen, A., Kelley, S., et al. (2019). Exposure to a healthy gut microbiome protects against reproductive and metabolic dysregulation in a PCOS mouse model. Endocrinology 160, 1193–1204. doi: 10.1210/en.2019-00050
Touraine, P. (2020). Premature ovarian insufficiency: Step-by-step genetics bring new insights. Fertil. Steril. 113, 767–768. doi: 10.1016/j.fertnstert.2019.12.032
Vallianou, N., Stratigou, T., Christodoulatos, G., and Dalamaga, M. (2019). Understanding the role of the gut microbiome and microbial metabolites in obesity and obesity-associated metabolic disorders: Current evidence and perspectives. Curr. Obes. Rep. 8, 317–332. doi: 10.1007/s13679-019-00352-2
Várbíró, S., Takács, I., Tűű, L., Nas, K., Sziva, R., Hetthéssy, J., et al. (2022). Effects of vitamin D on fertility, pregnancy and polycystic ovary syndrome-A review. Nutrients 14:1649. doi: 10.3390/nu14081649
Vasquez, E., Pereira, T., Peotta, V., Baldo, M., and Campos-Toimil, M. (2019). Probiotics as beneficial dietary supplements to prevent and treat cardiovascular diseases: Uncovering their impact on oxidative stress. Oxid. Med. Cell. Longev. 2019:3086270. doi: 10.1155/2019/3086270
Wacker, J., Frühauf, J., Schulz, M., Chiwora, F., Volz, J., and Becker, K. (2000). Riboflavin deficiency and preeclampsia. Obstet. Gynecol. 96, 38–44. doi: 10.1016/s0029-7844(00)00847-4
Wan, Y., Zuo, T., Xu, Z., Zhang, F., Zhan, H., Chan, D., et al. (2022). Underdevelopment of the gut microbiota and bacteria species as non-invasive markers of prediction in children with autism spectrum disorder. Gut 71, 910–918. doi: 10.1136/gutjnl-2020-324015
Wang, J., Luo, R., Zhao, X., Xia, D., Liu, Y., Shen, T., et al. (2023). Association between gut microbiota and primary ovarian insufficiency: A bidirectional two-sample Mendelian randomization study. Front. Endocrinol. 14:1183219. doi: 10.3389/fendo.2023.1183219
Wang, L., Ravichandran, V., Yin, Y., Yin, J., and Zhang, Y. (2019). Natural products from mammalian gut microbiota. Trends Biotechnol. 37, 492–504. doi: 10.1016/j.tibtech.2018.10.003
Wang, L., Xu, H., Tan, B., Yi, Q., Liu, H., Deng, H., et al. (2022). Gut microbiota and its derived SCFAs regulate the HPGA to reverse obesity-induced precocious puberty in female rats. Front. Endocrinol. 13:1051797. doi: 10.3389/fendo.2022.1051797
Wang, P., Lu, Y., Chen, S., Chen, Y., Hu, C., and Zuo, Y. (2018). Protective function of Bu Shen Huo Xue formula on the immunity of B6AF1 mice with experimental autoimmune premature ovarian failure. Exp. Ther. Med. 15, 3302–3310. doi: 10.3892/etm.2018.5804
Warden, S., Robling, A., Haney, E., Turner, C., and Bliziotes, M. (2010). The emerging role of serotonin (5-hydroxytryptamine) in the skeleton and its mediation of the skeletal effects of low-density lipoprotein receptor-related protein 5 (LRP5). Bone 46, 4–12. doi: 10.1016/j.bone.2009.06.029
Weersma, R., Zhernakova, A., and Fu, J. (2020). Interaction between drugs and the gut microbiome. Gut 69, 1510–1519. doi: 10.1136/gutjnl-2019-320204
Wu, J., Zhuo, Y., Liu, Y., Chen, Y., Ning, Y., and Yao, J. (2021). Association between premature ovarian insufficiency and gut microbiota. BMC Pregnancy Childbirth. 21:418. doi: 10.1186/s12884-021-03855-w
Wu, L., Dunning, K., Yang, X., Russell, D., Lane, M., Norman, R., et al. (2010). High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology 151, 5438–5445. doi: 10.1210/en.2010-0551
Xu, M., Pan, F., Peng, L., and Yang, Y. (2024). Advances in the isolation, cultivation, and identification of gut microbes. Mil. Med. Res. 11:34. doi: 10.1186/s40779-024-00534-7
Yadav, P., Tiwari, M., Gupta, A., Sharma, A., Prasad, S., Pandey, A., et al. (2018). Germ cell depletion from mammalian ovary: Possible involvement of apoptosis and autophagy. J. Biomed. Sci. 25:36. doi: 10.1186/s12929-018-0438-0
Yan, J., and Charles, J. (2017). Gut microbiome and bone: To build, destroy, or both? Curr. Osteoporos. Rep. 15, 376–384. doi: 10.1007/s11914-017-0382-z
Yang, J., Hou, L., Wang, J., Xiao, L., Zhang, J., Yin, N., et al. (2022). Unfavourable intrauterine environment contributes to abnormal gut microbiome and metabolome in twins. Gut 71, 2451–2462. doi: 10.1136/gutjnl-2021-326482
Yang, Q., Mumusoglu, S., Qin, Y., Sun, Y., and Hsueh, A. J. A. (2021). kaleidoscopic view of ovarian genes associated with premature ovarian insufficiency and senescence. FASEB J. 35:e21753. doi: 10.1096/fj.202100756R
Yang, X., Zhang, X., Yang, W., Yu, H., He, Q., Xu, H., et al. (2021). Gut microbiota in adipose tissue dysfunction induced cardiovascular disease: Role as a metabolic organ. Front. Endocrinol. 12:749125. doi: 10.3389/fendo.2021.749125
Yang, Y., Misra, B., Liang, L., Bi, D., Weng, W., Wu, W., et al. (2019). Integrated microbiome and metabolome analysis reveals a novel interplay between commensal bacteria and metabolites in colorectal cancer. Theranostics 9, 4101–4114. doi: 10.7150/thno.35186
Yin, N., Wang, Y., Lu, X., Liu, R., Zhang, L., Zhao, W., et al. (2018a). hPMSC transplantation restoring ovarian function in premature ovarian failure mice is associated with change of Th17/Tc17 and Th17/Treg cell ratios through the PI3K/Akt signal pathway. Stem. Cell. Res. Ther. 9:37. doi: 10.1186/s13287-018-0772-x
Yin, N., Zhao, W., Luo, Q., Yuan, W., Luan, X., and Zhang, H. (2018b). Restoring ovarian function with human placenta-derived mesenchymal stem cells in autoimmune-induced premature ovarian failure mice mediated by treg cells and associated cytokines. Reprod. Sci. 25, 1073–1082. doi: 10.1177/1933719117732156
Yurkovetskiy, L., Burrows, M., Khan, A., Graham, L., Volchkov, P., Becker, L., et al. (2013). Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400–412. doi: 10.1016/j.immuni.2013.08.013
Zhang, J., Li, Q., Wang, X., Zhang, C., Chen, S., Liu, X., et al. (2024). Causal link between gut microbiota and infertility: A two-sample bidirectional mendelian randomization study. Curr. Med. Sci. 44, 1312–1324. doi: 10.1007/s11596-024-2931-x
Zhang, T., Yan, D., Yang, Y., Ma, A., Li, L., Wang, Z., et al. (2016). The comparison of animal models for premature ovarian failure established by several different source of inducers. Regul. Toxicol. Pharmacol. 81, 223–232. doi: 10.1016/j.yrtph.2016.09.002
Zou, W., Wang, Z., Xia, J., and Yang, J. (2020). Retinol-binding protein 4 (RBP4) and high sensitivity C-reactive protein (hs-CRP) levels in patients with diminished ovarian reserve (DOR): A cross-sectional study. Reprod. Biol. Endocrinol. 18:111. doi: 10.1186/s12958-020-00670-4
Keywords: gut microbiota, premature ovarian failure, premature ovarian insufficiency, infertility, biomarkers, therapeutics
Citation: Liu Z, Wang M, Lei Y, Xu K and Fan L (2025) Gut microbiota: emerging biomarkers and potential therapeutics for premature ovarian failure. Front. Microbiol. 16:1606001. doi: 10.3389/fmicb.2025.1606001
Received: 04 April 2025; Accepted: 23 June 2025;
Published: 15 July 2025.
Edited by:
Anusorn Cherdthong, Khon Kaen University, ThailandReviewed by:
Chandrima Gain, University of California, Los Angeles, United StatesZhaoBo Luo, Harvard Medical School, United States
Copyright © 2025 Liu, Wang, Lei, Xu and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Limei Fan, ZmFubGltZWkxOTcxQDEyNi5jb20=
 Zongyu Liu1,2
Zongyu Liu1,2 Min Wang
Min Wang