- 1Department of General Studies, Faculty of Health Sciences, Almoosa College, Al Ahsa, Saudi Arabia
- 2School of Allied Health, Faculty of Health, Medicine and Social Care, Anglia Ruskin University, Essex, United Kingdom
Background: Foodborne diseases remain a significant global health concern. Conversely, socioeconomic status represents a crucial predictor of diseases with increased morbidity and mortality rates. This scoping review (ScR) aims to provide an understanding of the impact of socioeconomic status on the occurrence of foodborne illnesses in the Middle East and North Africa (MENA) region.
Methodology: Three databases (Medline [PubMed], Web of Science, and Embase) were searched on 24 August 2024, for articles published in English. The population, concept, and context (PCC) framework was adopted in this review.
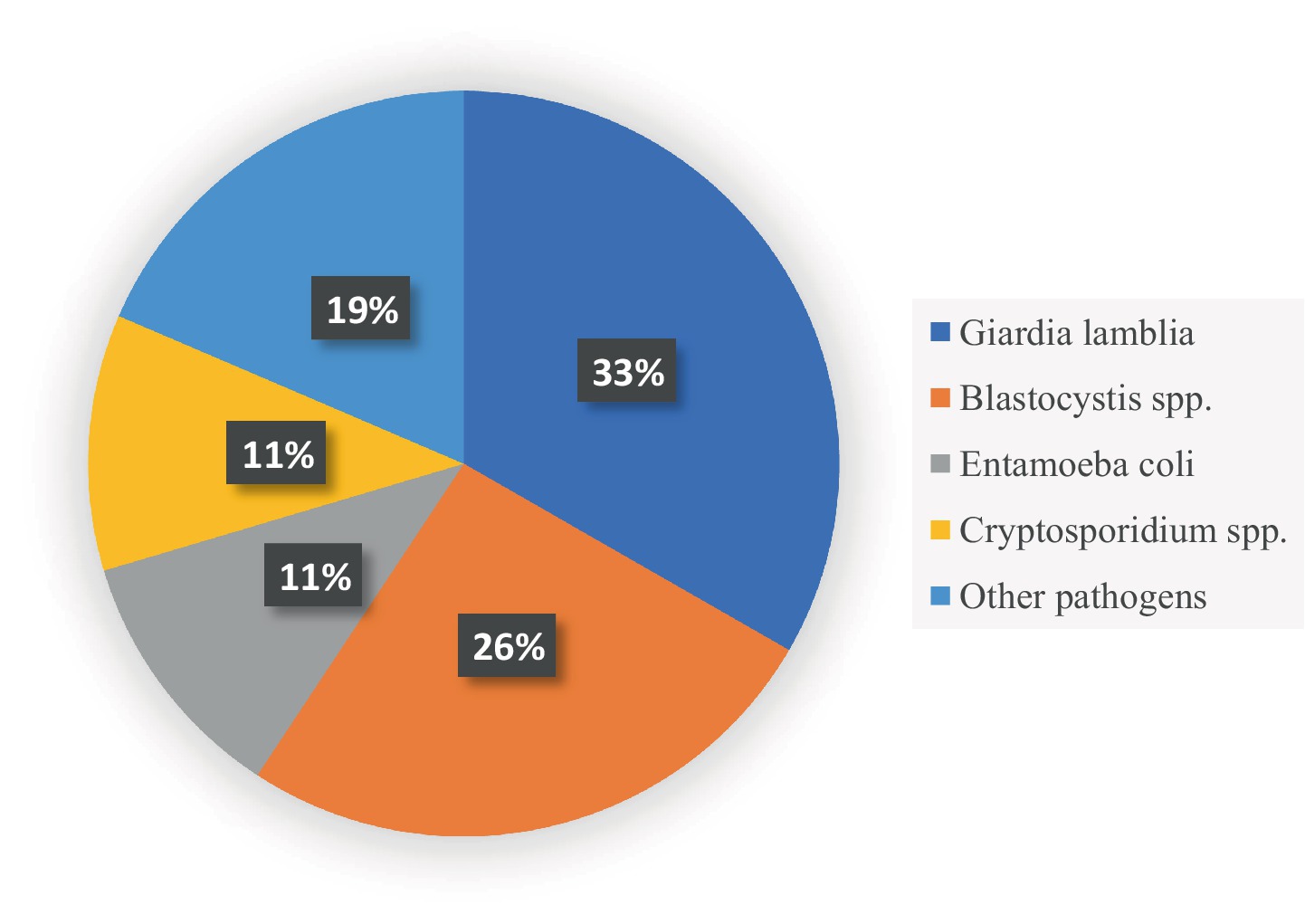
Results: A total of 1,667 records were identified. After removing 530 duplicates, 1,137 records were screened for inclusion. Twelve studies were eligible after excluding records with reasons. Of the 12 studies, 11 were cross-sectional studies and 1 was a case–control in design. The studies were conducted in countries of the MENA region, including Saudi Arabia, Qatar, the United Arab Emirates, Palestine, Lebanon, Egypt, and Iran. Low income was generally associated with higher rates of parasitic infections among populations in Egypt, Palestine, Lebanon, and one study in Iran. The relationship between the level of education and infection rates was divergent. In some studies, individuals with lower education levels have shown higher infection rates, as seen in Egypt, Iran, and Qatar; however, other studies found no significant association. Occupation appeared to be less consistently related to infection rates. Food handlers had the highest rates of infection in the UAE, while studies from other regions did not find significant associations. Giardia lamblia (33%) and Blastocystis hominis (26%) were found to be the predominant intestinal parasites in the included studies.
Conclusion: This scoping review emphasizes discrepancies between studies on the impact of socioeconomic status affects the rate of intestinal infection. Thus, future research should provide clear definitions and indicators of socioeconomic metrics and address the occurrence of foodborne illnesses in terms of cultural factors, healthcare inequality, and food insecurity.
Introduction
Foodborne illness is defined as a disease caused by the consumption of food contaminated with pathogens (bacteria, parasites, or viruses) or toxic substances (World Health Organization, 2024). Accordingly, foodborne diseases (FBDs) are classified, based on the responsible agent, into two major categories: foodborne infections and foodborne poisonings/intoxications (Osaili et al., 2022). During foodborne infections, viable pathogenic organisms are ingested with food and establish an infection. The sources of these pathogenic organisms range from the normal flora of the food to unintentional cross-contamination during food production, processing, or preparation (de Andrade et al., 2019). However, foodborne intoxication is a type of foodborne disease caused by consuming food containing pre-formed toxins produced by bacteria, fungi, or chemical agents (Osaili et al., 2022). In both cases, poor food safety practices, including inadequate washing, storage, cooking, cooling, or freezing, result in pathogen proliferation in the food product, posing a risk to individual health (Augustin et al., 2020). Although improvements in food handling regulations have helped reduce the incidence of some pathogens in food, foodborne diseases (FBDs) remain a global health concern (de Andrade et al., 2019). The consumption of contaminated food can cause a range of illnesses, from mild gastroenteritis to life-threatening conditions such as cancer (Elbehiry et al., 2023). According to the World Health Organization (WHO), around 600 million people fall ill after eating contaminated food each year, resulting in 420,000 deaths and the loss of 33 million healthy life years. In public health, the loss of healthy life is measured using Disability-Adjusted Life Years (DALYs), which reflect the number of years lost due to illness, disability, or premature death (World Health Organization, 2024). In contrast, socioeconomic status (SES) is a crucial predictor of disease. Education level, employment, and income are the three leading indicators used to determine the SES of an individual or community (Newman et al., 2015). Generally, low SES is associated with higher morbidity and mortality rates from chronic diseases such as cardiovascular disease, as well as from some communicable diseases, including tuberculosis and human immunodeficiency virus (HIV; Mtintsilana et al., 2023). However, the association between foodborne illnesses and socioeconomic status (SES) is not well understood, as official reports are often unreliable, especially in low- and middle-income countries, where cases of foodborne illness are frequently neglected or under-reported (Grace et al., 2015). In 2015, foodborne diseases began to receive serious global attention and higher priority when the World Health Organization (WHO) released its first Global Burden of Foodborne Disease Report (Lake et al., 2015). Notably, a study conducted in Portugal showed that financial obstacles significantly impact the amount of money allocated for food consumption, with implications for food safety and food security (Maia et al., 2023). Similarly, a study involving 51 nutrition educators from the New Jersey Expanded Food and Nutrition Education Program and the Food Stamp Nutrition Education Program, which examined the food management practices of program participants revealed that individuals with limited education and resources are at higher risk of engaging in unsafe food handling behaviors such as cutting spoiled part off fruits and vegetables, getting rid of insects and mites from beans and lentils, consuming slimy meat and chicken product, eating others’ leftovers or reheating leftovers several times, all of which increase the risk of foodborne illness occurrence (Kempson et al., 2002). Moreover, equitable access to healthy food is a critical challenge in urban Asia. Wertheim-Heck et al. reported that sub-optimal dietary diversity and reliance on foods sourced through traditional markets, which do not provide formal food safety guarantees, contribute to food safety concerns in Vietnam (Wertheim-Heck et al., 2019). However, some individuals with high socioeconomic status (SES) may also be at increased risk of developing intestinal infections due to the consumption of undercooked foods, raw fish, and rare beef, as higher social class groups often distinguish themselves through specific and sophisticated dining habits (Newman et al., 2015).
A significant gap in the research literature regarding the impact of socioeconomic status (SES) on the rate of foodborne illnesses in the Middle East and North Africa (MENA) region. A preliminary search of the Medline and Scopus databases revealed no existing or ongoing systematic reviews or scoping reviews (ScR) on the topic. Therefore, this study aims to explore how socioeconomic factors such as income, occupation, and education level affect the occurrence of foodborne illnesses among populations in MENA countries.
Methodology
Research question
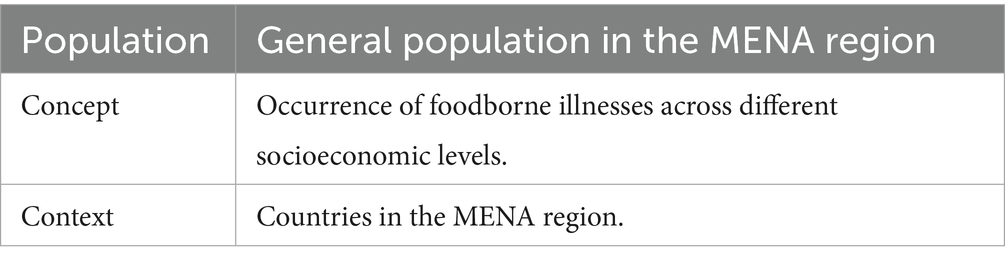
The population, concept, and context (PCC) framework (Table 1) was used to formulate the research question (RQ) of this scoping review (Peters et al., 2015).
RQ: How does socioeconomic status affect the rate for foodborne illness among the general population in the MENA region?
The available literature on the impact of socioeconomic status (SES) on the burden of foodborne illnesses in the MENA region is scattered, underexplored, and fragmented. Moreover, the topic cuts through different methodologies used to examine this interrelationship. Therefore, among the different types of reviews, the scoping review (ScR) was selected. A ScR aims to answer broad research questions (Peters et al., 2015; Rodger et al., 2024; Verdejo et al., 2021; Colquhoun et al., 2014), condense research findings, identify research gaps (Tricco et al., 2018; Munn et al., 2022), and inform proposals for future systematic reviews (Mitton et al., 2009; Mak and Thomas, 2022). The proposed ScR was conducted in accordance with the JBI methodology for scoping reviews (JBI, 2024) and the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) statement (Tricco et al., 2018).
Inclusion and exclusion criteria
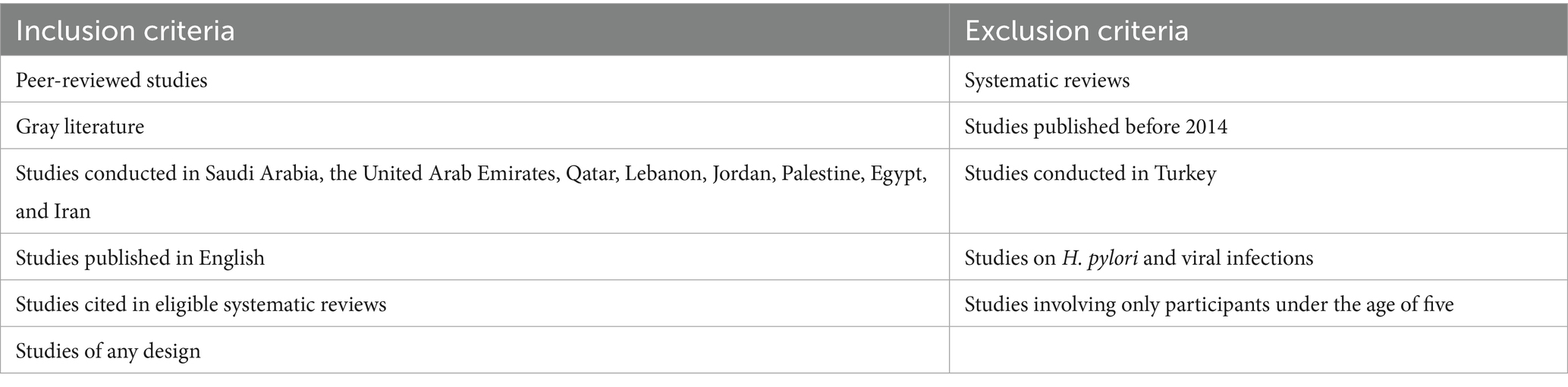
The inclusion and exclusion criteria were determined based on the PCC framework of this scoping review (ScR) and are presented in Table 2. This ScR considered all study designs, peer-reviewed studies, and gray literature. However, systematic reviews that met the inclusion criteria were excluded since they are considered secondary studies, but the papers cited in systematic reviews were eligible. Moreover, all studies published in English within the last 10 years were considered for inclusion. A 10-year cutoff was adopted to ensure that the review focuses on the most recent evidence, as societal conditions can change dramatically over a decade. When it comes to exclusion criteria, studies involving participants under the age of five, studies on foodborne illnesses caused by Helicobacter pylori, and viruses were excluded. Moreover, studies conducted in Turkey were excluded, as the classification of this country as part of the MENA region can vary depending on its geographical, cultural, political, or historical contexts.
Search strategy
The search strategy aimed to identify the published studies and gray literature. In this scoping review, identifying relevant search terms was crucial to ensure the comprehensiveness and relevance of the collected data (Kabir et al., 2024). Therefore, to explore the association between SES and foodborne illnesses in countries of the MENA region, key concepts were considered as follows: “socioeconomic status,” “income,” “food poisoning,” “foodborne pathogens,” “MENA countries,” “Saudi Arabia,” “Jordan”; additional countries in the MENA region; and synonyms and related terms were also included (Table 3). Moreover, Boolean operators (AND, OR, and NOT) were used to refine the search strategy. The Boolean logic narrowed the search by using AND and broadened it by using OR.
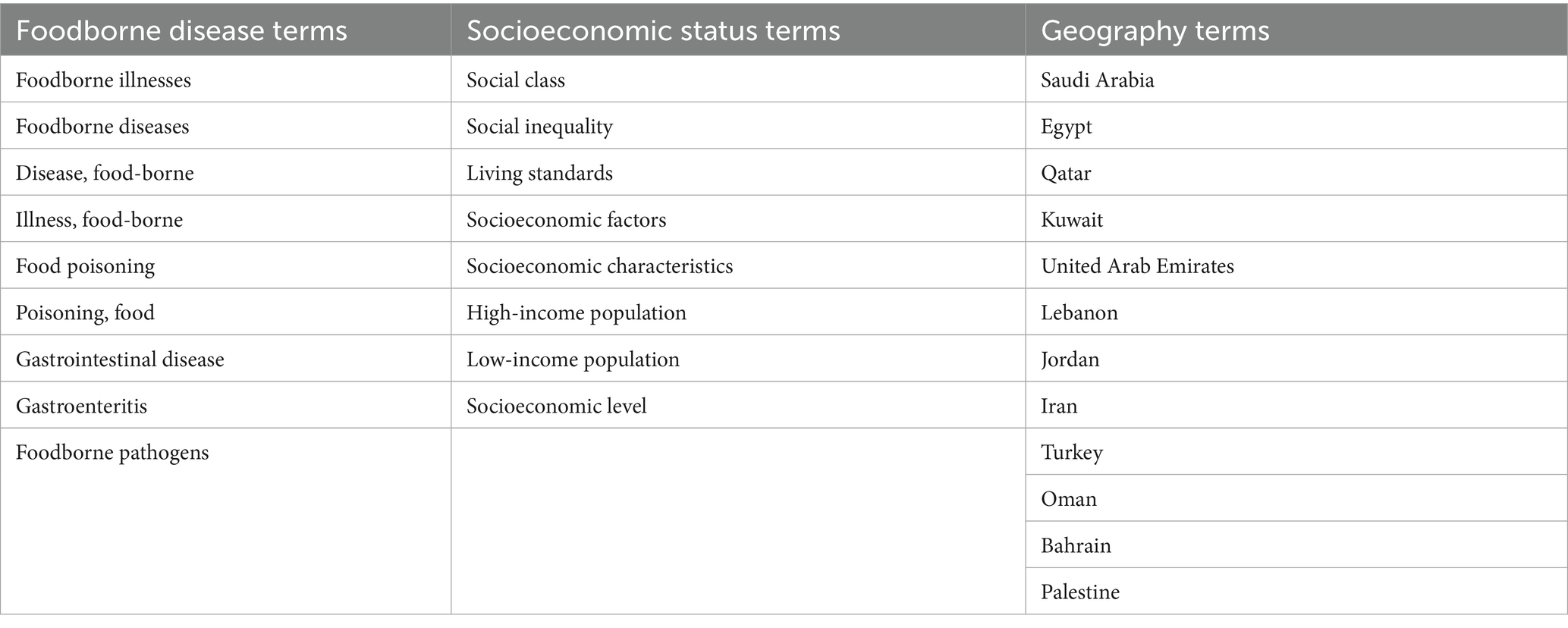
Table 3. MeSH terms and synonyms used in the database search to identify studies on the association between socioeconomic status and foodborne diseases.
Source of evidence selection
A three-step search strategy was adopted for selecting sources of evidence. In the first step, the search was conducted in Medline and Scopus databases, since they provide comprehensive coverage of relevant literature and support a thorough exploration of research gaps. The initial search was broad, identifying words and phrases found in the titles, abstracts, and indexes of papers that were used in the final search strategy. Then, further search was performed using terms identified in the initial search to find additional databases and gray literature sites. The list from references of the initial and further search papers was screened to retrieve additional studies. This last step, known as “snowballing,” was time-consuming; however, it was a crucial step to ensure that as many relevant papers as possible were retrieved. Accordingly, the selected databases in this ScR (Medline [PubMed], Web of Science, and Embase) were accessed to develop a complete search strategy using all possible MeSH terms, keywords, and Emtree combinations for each database (Table 4). Additional articles and gray literature were retrieved from Google Scholar and Google search to be included in the PRISMA-ScR flow chart (Tricco et al., 2018). After completing the search, all identified relevant citations were exported and uploaded into Zotero 6.0.37, followed by the removal of duplicates. Then, titles and abstracts of the identified articles were screened to select articles aligned with the inclusion criteria of the scoping review. The full text of relevant sources was retrieved and further assessed to check in detail whether they align with the review’s inclusion criteria to end up with the eligible articles to be considered in this scoping review. Moreover, the reasons for excluding sources of evidence at each stage were reported and presented in a PRISMA-ScR flow diagram.

Table 4. MeSH terms, keywords, and Emtree combination using Boolean logic for databases’ full search strategy.
Data extraction and charting
Once the study inclusion was confirmed, the key data points were identified for extraction from the eligible studies and then charted. The abstraction tool included the authors’ names, year of publication, region, aim of the study, study design, sampling techniques, sample size, key findings, type of pathogens, and limitations. Article selection, screening, data extraction, and charting were completed by 24 August 2024.
Data synthesis
The synthesis in this study was qualitative due to the broad nature of ScR. A descriptive narrative synthesis was adopted in this review, and the abstracted information was grouped by income, level of education, and employment of study participants. No critical appraisal or formal assessment of study quality was conducted as per ScR guidelines (Tricco et al., 2018; JBI, 2024).
Results
A total of 1,667 records were identified through database search; Medline [PubMed], Web of Science, and Embase (n = 1,315) and through Google Scholar and Google Search (n = 352); after removal of 530 duplicates across databases using Zotero, 1,137 records were screened for inclusion. In the screening stage of the title and abstract, 662 records were excluded as irrelevant.
Actually, the term “Gastrointestinal disease” led to the irrelevant 662 records that are not related to foodborne illness. These records were related to: Inflammatory Bowel Disease (IBD), Inflammatory Bowel Syndrome (IBS), Lactose intolerance, celiac disease, peptic ulcers, pancreatic and liver diseases, and rectal cancer, which are not relevant to the scope of my professional project and were excluded.
After a full-text review of 475 records for eligibility, we excluded 463 records as follows: articles not related to MENA region (n = 165), articles not addressing the research question (n = 233), articles related to H. pylori and viral infections (n = 23), articles involving participants under 5 years of age (n = 22) and articles before 2014 (n = 20; Figure 1). Therefore, 12 studies published in English were included in this review (Table 5).
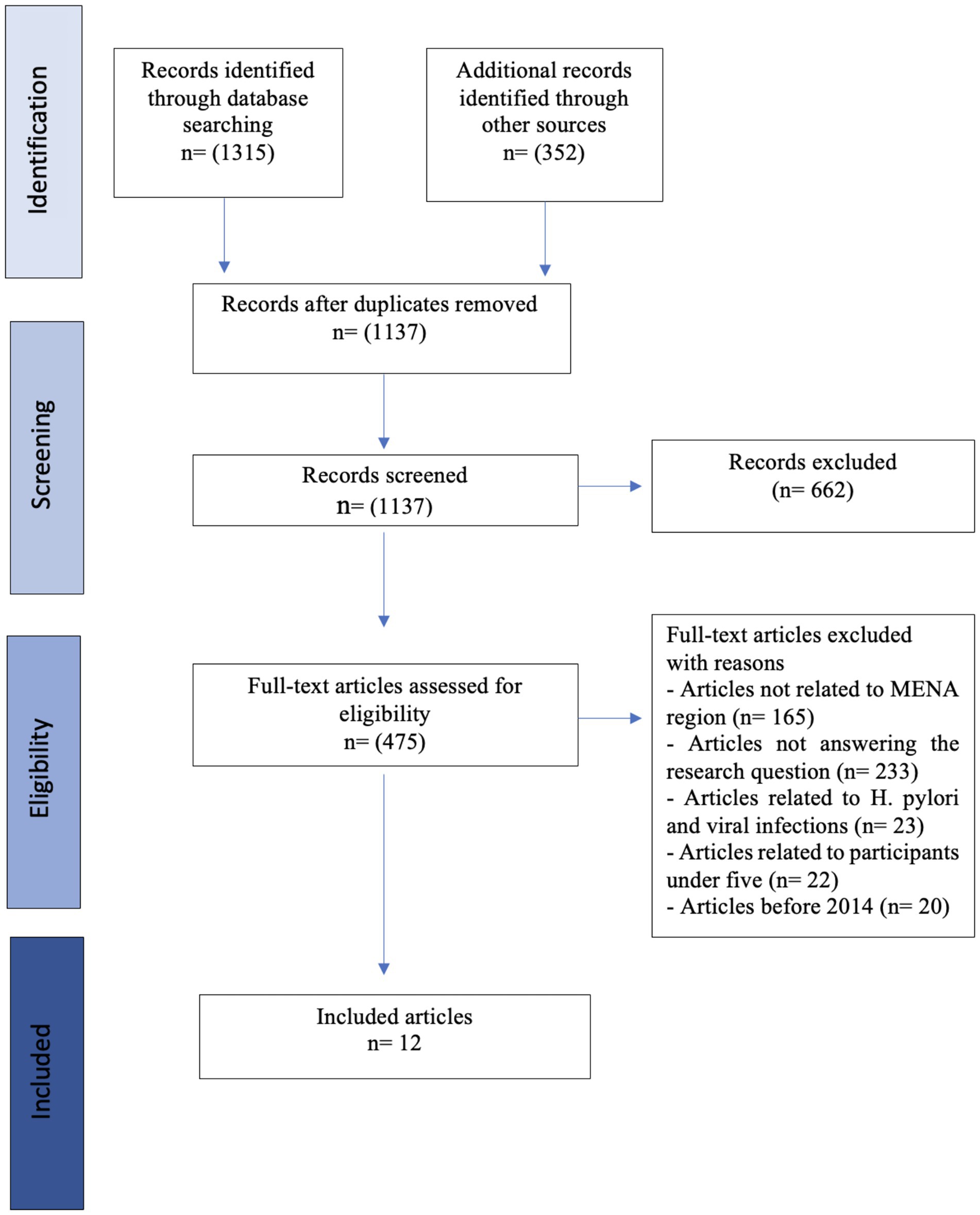
Figure 1. PRISMA-ScR flow chart. Source: https://doi.org/10.7326/M18-0850 (Tricco et al., 2018).
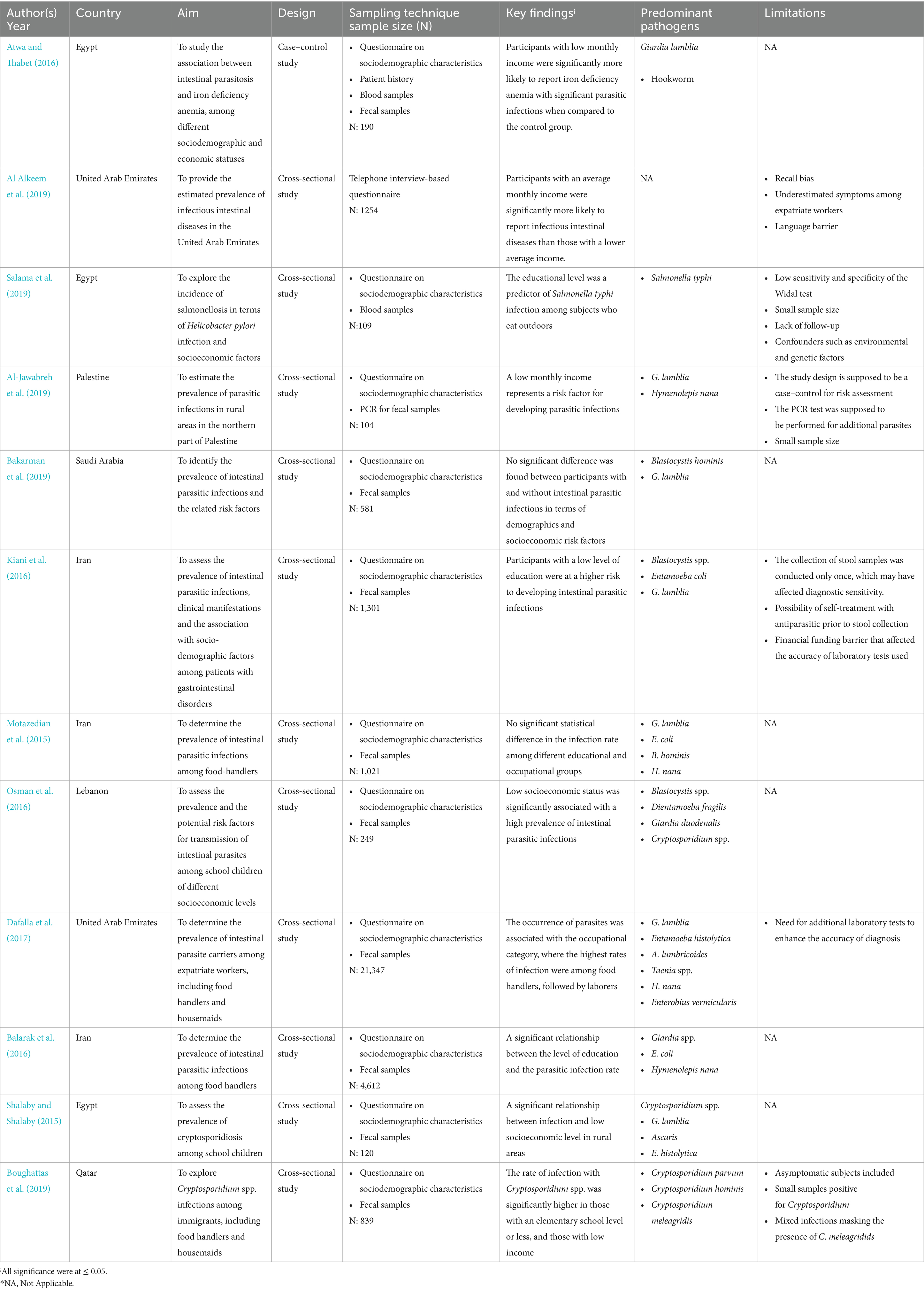
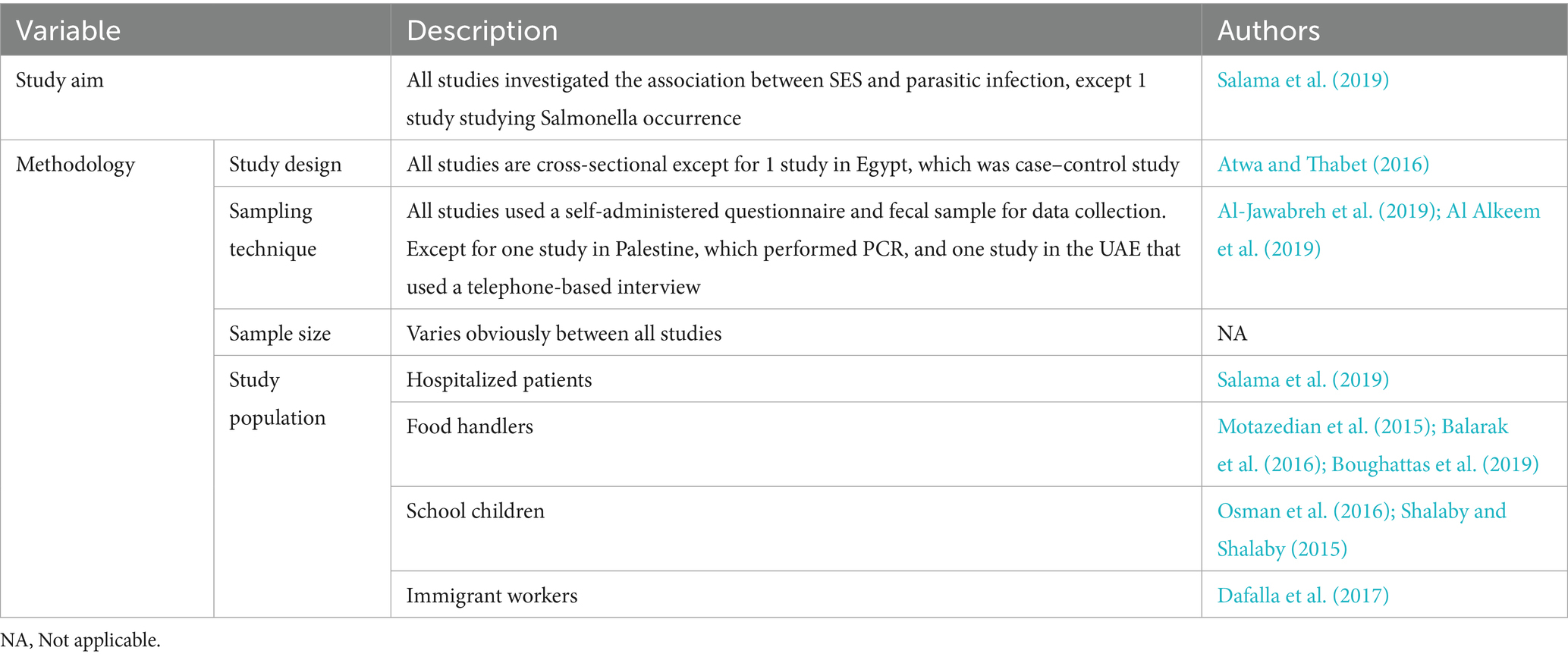
Out of 12 eligible studies, 11 were cross-sectional studies (Al Alkeem et al., 2019; Salama et al., 2019; Al-Jawabreh et al., 2019; Bakarman et al., 2019; Kiani et al., 2016; Motazedian et al., 2015; Osman et al., 2016; Dafalla et al., 2017; Balarak et al., 2016; Shalaby and Shalaby, 2015; Boughattas et al., 2019) and 1 was a case–control study (Atwa and Thabet, 2016). Most of the data were collected within 7 months or fewer, except for Al-Jawabreh et al., Osman et al., Dafalla et al., and Balarak et al., whose data were collected over 1 year (Al-Jawabreh et al., 2019; Osman et al., 2016; Dafalla et al., 2017; Balarak et al., 2016). Most importantly, Shalaby et al. and Boughattas et al. did not report the periods over which the data were collected in their studies (Shalaby and Shalaby, 2015; Boughattas et al., 2019). The eligible studies were conducted in MENA region countries, namely, the United Arab Emirates (Al Alkeem et al., 2019; Dafalla et al., 2017), Saudi Arabia (Bakarman et al., 2019), Qatar (Boughattas et al., 2019), Palestine (Al-Jawabreh et al., 2019), Egypt (Salama et al., 2019; Shalaby and Shalaby, 2015; Atwa and Thabet, 2016), Lebanon (Osman et al., 2016), and Iran (Kiani et al., 2016; Motazedian et al., 2015; Balarak et al., 2016). The sampling technique in the cross-sectional studies was convenience; the majority of the studies collected data using structured questionnaires to obtain sociodemographic characteristics, in addition to microscopic examination of fecal samples to detect parasites (Bakarman et al., 2019; Kiani et al., 2016; Motazedian et al., 2015; Osman et al., 2016; Dafalla et al., 2017; Balarak et al., 2016; Shalaby and Shalaby, 2015; Boughattas et al., 2019). One study collected data through a 15-min interview, followed by fecal sample collection to be tested by polymerase chain reaction (PCR; Al-Jawabreh et al., 2019). Another study used a questionnaire and collected blood samples (Salama et al., 2019), and one study collected data via a telephone interview-based questionnaire on sociodemographic characteristics and the prevalence of intestinal infectious diseases without any human sample collection (Al Alkeem et al., 2019). When it comes to the case–control, an age-matching technique was adopted, and the data included patient history in addition to blood and stool sample collection (Atwa and Thabet, 2016). The largest sample size was 21,347 participants (Dafalla et al., 2017), followed by 4,612 (Balarak et al., 2016); the remaining studies had a sample size varying between thousands (Al Alkeem et al., 2019; Kiani et al., 2016; Motazedian et al., 2015) to hundreds of participants (25, 26, 27, 30, 33, 34, 35).
The three themes identified in this ScR were income, level of education, and occupational group, which are represented in Figure 2. The narrative synthesis of the included studies shows varying relationships between socioeconomic factors (income, education, and occupation) and the risk of developing intestinal infections among the population in the MENA region. In general, some studies declared that low income and low levels of education are associated with higher rates of infections, which is the case in countries, such as Egypt, Palestine, and Qatar (Atwa and Thabet, 2016; Al-Jawabreh et al., 2019; Boughattas et al., 2019). However, studies in Saudi Arabia have shown conflicting findings, where income and education levels were not significantly associated with infection rates. Very few studies investigated the impact of occupation on foodborne illness occurrence, with no consistent patterns, although food handlers have been found to be at higher risk in some cases. While the synthesis reveals some common trends, the robustness of these associations is limited by methodological differences (cross-sectional vs. case–control), sample sizes (ranging between 100 and 20,000), and population demographics. This suggests that the association between socioeconomic status and foodborne infections is complex, multifaceted, and context-dependent.
Figure 2. Key themes emerging from the scoping review of impact of socioeconomic status on the occurrence of foodborne infection.
Key findings in terms of the PCC framework
Population
School children
• Studies in Saudi Arabia and other countries showed mixed results, with no significant relationship in Saudi children, but a higher rate of infection in Lebanese children with low SES (particularly G. duodenalis) and in Egypt and Palestine where a significant association between low family income and high rates of parasitic infections such as G. lamblia and E. histolytica was detected.
Hospitalized patients
• In one study on hospitalized patients, low income was strongly associated with Salmonella spp. infections, with 92.9% of participants being from low socioeconomic backgrounds.
Immigrant workers
• A study in Qatar showed that low-educated immigrant workers were associated with higher rates of intestinal infections.
Concept
Income
Low income was generally associated with higher rates of intestinal infections, particularly parasitic infections. This was especially evident in populations in Egypt, Palestine, Lebanon, and one study in Iran.
Education
The relationship between the level of education and infection rates was divergent. In some studies, individuals with lower education levels have shown higher infection rates, such as in Egypt, Iran, and Qatar, while other studies found no significant association.
Occupation
Occupation appeared to be less consistently related to infection rates. Food handlers had the highest rates of infection in the UAE, while studies from other regions did not find significant associations.
Context
Geographic differences
Studies from urban areas in high-income countries of the MENA region, such as Qatar and Saudi Arabia, did not show significant associations between income and infection rates, possibly due to good healthcare services and sanitation. Moreover, larger sample sizes with robust designs, such as those from the UAE, yielded more reliable findings, whereas studies with smaller sample sizes and convenience sampling may have had less conclusive results.
Impact of income on intestinal infection rate
In this scoping review, four studies did not explore the impact of participants’ monthly income on the rate of developing intestinal infections (Kiani et al., 2016; Motazedian et al., 2015; Dafalla et al., 2017; Balarak et al., 2016). Al-jawabreh et al. showed a significant difference among participants in terms of monthly income, where participants with low income were at higher risk of developing intestinal parasitic infections (IPIs; Al-Jawabreh et al., 2019). Moreover, Salama and his colleagues declared that 92.9% of hospitalized participants with Salmonella spp. infection was associated with a low socioeconomic level (Salama et al., 2019). Surprisingly, the average income was significantly associated with a higher rate of infectious intestinal disease among study participants compared to those with either a higher or lower income (Al Alkeem et al., 2019). When it comes to studies recruiting school children, Atwa et al. showed that the rate of infection with intestinal parasites increases significantly when monthly family income decreases (Atwa and Thabet, 2016). Moreover, another study reported that living in a rural area with low SES was significantly associated with a higher rate of parasitic infection, where 67.7% of study participants reported infection with Cryptosporidium spp. (Shalaby and Shalaby, 2015). Boughattas et al. declared that among 29 participants, no one showed parasitic infection; thus, the top-earning category in his study showed significantly lower rate of infections (Boughattas et al., 2019). However, the two studies conducted in Saudi Arabia and Lebanon showed findings that conflicted with those mentioned previously, since the difference between children with and without IPIs was not significant in terms of the monthly income of the Saudi family (Bakarman et al., 2019). Although the rate of parasitic infection was higher among Lebanese school children with low SES than those with higher SES (Osman et al., 2016), the difference was not statistically significant except for G. duodenalis, where the rate of infection was 36.6% compared to those with high SES 15% (CI 3.2 [1.6–6.1]).
Impact of level of education on intestinal infection rate
Out of 12 studies, three studies did not assess the association between the level of education and the prevalence of intestinal infection among the study participants (Osman et al., 2016; Dafalla et al., 2017; Shalaby and Shalaby, 2015). Similarly, Al-jawabreh et al. and Al Alkeem et al. reported that the level of education of participants was not significantly associated with the rate of intestinal infection either among the study participants (Al Alkeem et al., 2019) or their children (Al-Jawabreh et al., 2019). These findings are similar to those reported by the studies conducted in Egypt (Atwa and Thabet, 2016) and Saudi Arabia (Bakarman et al., 2019). Moreover, the rate of Salmonella spp. infection was not correlated with the education level of the hospitalized patients in the study conducted by Salama and his colleagues (Salama et al., 2019). When it comes to the two studies that assessed the prevalence of IPIs among food-handlers in Iran, the level of education was not associated with the rate of parasitic infections among all participants (Motazedian et al., 2015; Balarak et al., 2016). However, Kiani et al. proved that the participants with a low level of education were at a higher risk of developing IPIs, which act as a key driver of gastrointestinal disorders such as diarrhea, dysentery, and abdominal pain (Kiani et al., 2016). Most importantly, a study conducted in Qatar to explore the sociodemographic risk factors for developing IPIs among immigrants found that the level of IPIs was heterogeneous across the five levels of education, with the rate of infection significantly higher in those with the elementary school level and lower in those with higher levels (Boughattas et al., 2019).
Impact of occupation on intestinal infections rate
Among the three key components of socioeconomic factors, occupation was the least assessed factor in the eligible records, since only 6 out of 12 studies explored the association between occupation categories and intestinal infections. Moreover, only one study found that the rate of intestinal infections was significantly associated with the category of occupation, where food handlers showed the highest rate of infection at 52% followed by a rate that decreased to 16.4% (Dafalla et al., 2017). In contrast, profession type had no association with the rate of IPIs among hospitalized patients or food handlers in Iran (Kiani et al., 2016; Balarak et al., 2016). The rate of infections varied between different occupations in a study conducted by Motazedian et al. The highest rate was recorded among participants working as herbal sellers (16%), while the lowest rate was recorded among those working as office servers; howevre, this difference was not statistically significant in terms of occupational groups (Motazedian et al., 2015). Identical results were reported after exploring the rate of intestinal infections among immigrant workers in Qatar (Boughattas et al., 2019). In addition, the study recruiting school children found that the difference between children with and without IPIs was not significant in terms of parental occupation (paternal or maternal; Bakarman et al., 2019).
Prevalence and distribution of intestinal pathogens
The predominant pathogens explored in the eligible studies were classified as follows: one study conducted in the United Arab Emirates, which reported a prevalence of intestinal infectious diseases (IIDs) of 4.2%, but did not report the causative pathogens (Al Alkeem et al., 2019). On the other hand, out of 12 records, only one study was related to bacterial foodborne diseases. This study was conducted in Egypt, and the findings showed that the proportion of Salmonella-infected subjects was 33.9% among H. pylori-negative patients (Salama et al., 2019). Two studies exclusively explored the prevalence of protozoan Cryptosporidium spp. among participants, with results recorded in prevalence rates of 13.5 (Shalaby and Shalaby, 2015) and 4.5% (Boughattas et al., 2019) among school children and immigrant workers, respectively. Regarding the other studies, the prevalence of parasitic pathogens was heterogeneous. After fecal examination of the collected samples from participants, the prevalence ranged between 5 and 85% (26–32, 35), and the infections included single, double or multi-parasitic infectious intestinal disease. Moreover, G. lamblia, E. histolytica and E. coli were found to be the predominant intestinal parasites in the studies conducted in the UAE, Iran, Palestine, and Egypt (Al-Jawabreh et al., 2019; Motazedian et al., 2015; Dafalla et al., 2017; Balarak et al., 2016; Atwa and Thabet, 2016). However, Osman et al., Kiani et al., and Bakarman et al. reported that the predominant isolated protozoan was Blastocystis sp., followed by E. coli, G. lamblia, and Cryptosporidium spp. (Bakarman et al., 2019; Kiani et al., 2016; Osman et al., 2016). Helminth infections were less frequent and included A. lumbricoides, Hookworm Trichuris trichiura, Taenia spp., with very few cases caused by H. nana. (Al-Jawabreh et al., 2019; Dafalla et al., 2017; Atwa and Thabet, 2016). Although the prevalence and causative agents varied significantly between studies, similar findings were reported in terms of symptoms such as abdominal pain, diarrhea, and vomiting among study participants (Bakarman et al., 2019; Kiani et al., 2016; Osman et al., 2016).
Key similarities and differences between studies
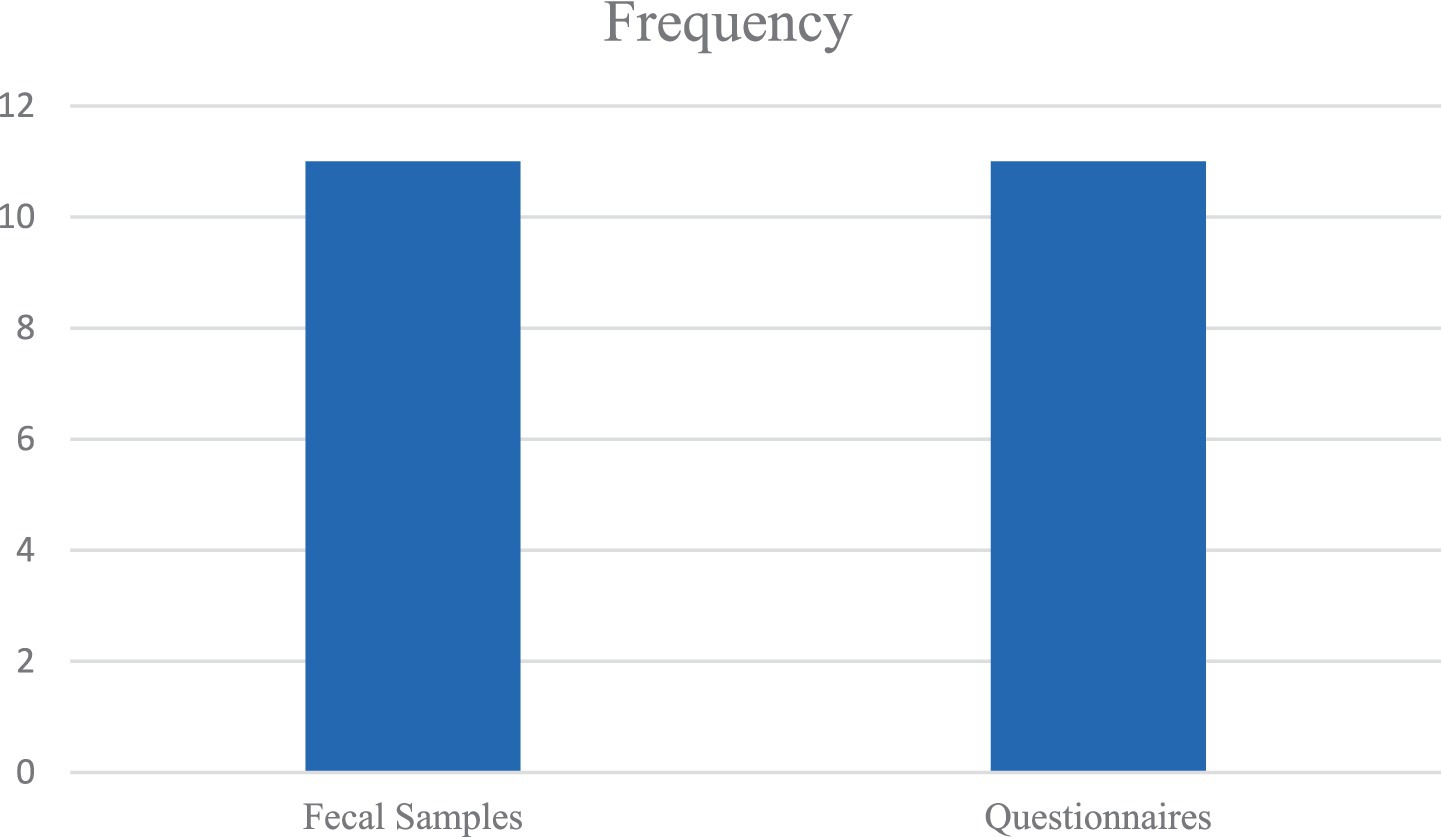
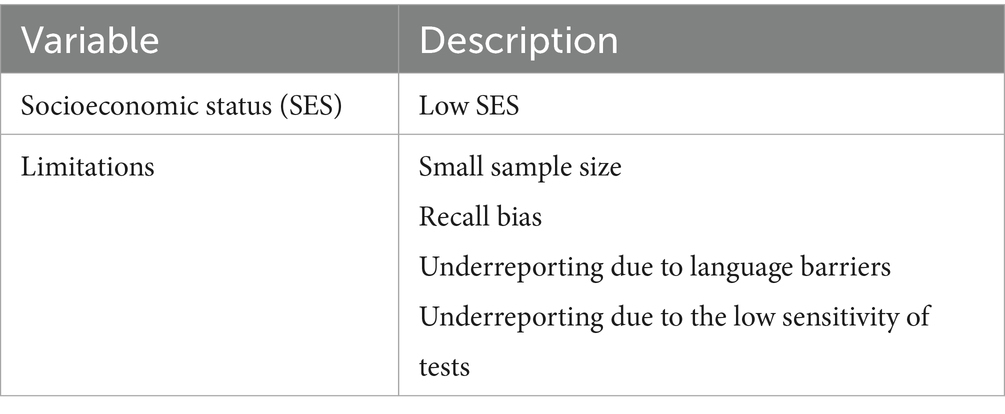
In this review, the similarities lie in the most common types of pathogens (Figure 3), sampling techniques (Figure 4), socioeconomic status, and limitations (Table 6). In contrast, the most important differences are presented in Table 7.
Discussion
Exploring the association between living standard and the burden of intestinal infections
The literature showed high discrepancies regarding the impact of income/social class on the occurrence of intestinal diseases. In this scoping review, Dafalla et al. Motazedia et al., Balarak et al., and Kiani et al. did not study the association between income/social class and the occurrence of intestinal infections. However, it has been proven that a higher rate of intestinal infections is significantly associated with low monthly income and/or living in rural areas (Salama et al., 2019; Al-Jawabreh et al., 2019; Shalaby and Shalaby, 2015; Boughattas et al., 2019). These findings were in accordance with other studies showing that low-income families may consume food from traditional sources, drink improperly treated water, and have poor access to healthcare services, which puts them at higher risk of developing infectious diseases (Quandt et al., 2004; Gulliford, 2002; Goh et al., 2004). Moreover, Atwa et al. declared that family size was a crucial predictor of parasitic infection, since a large number of persons per household with low income negatively impacts their health in terms of intestinal infection rates (Atwa and Thabet, 2016). These findings were in accordance with other studies that showed a direct impact of poverty on Cryptosporidium spp. infection rate, since these studies suggested more immediate links between household food insecurity and the risk of developing parasitic intestinal infections (Katona and Katona-Apte, 2008). Food inadequacy reduces the nutritional status of the host and their immune response, which in turn increases their susceptibility to parasitic infection (Katona and Katona-Apte, 2008; Weigel et al., 2007). On the other hand, households with inadequate access to food due to low income are more likely to consume food products from traditional sources where food safety and sanitation regulations could be violated (Quandt et al., 2004). Moreover, populations with low income have poor access to healthcare, which makes them at higher risk of developing infectious diseases (Gulliford, 2002). According to Goh et al., the source of drinking water is an additional risk factor for low-income households in developing countries, which sometimes lack an efficient treatment system (Goh et al., 2004). On the other hand, two studies in this scoping review proved no correlation between income and the rate of intestinal infection (Bakarman et al., 2019; Osman et al., 2016), these findings were in accordance with the study by Becker et al., who found a difference in the odds of seropositivity between individuals in households close to the poverty threshold and those in households with an income three times above the poverty line; however, this association was not significant (Becker et al., 2015). Conversely, other studies showed that in some high-income countries, populations with high living standards are at higher risk of developing intestinal parasitic infections (Lake et al., 2007). Some studies link this greater risk to the fact that individuals living with high living standards engage in recreational activities such as traveling (McLaughlin et al., 2000), using swimming pools, walking in the countryside with animals (Kavanagh et al., 2005) in addition to consuming fresh vegetables and fruits (Hunter et al., 2004).
Exploring the educational and occupational consequences of the burden of intestinal infections
Out of the 12 eligible studies, 3 studies did not assess the association between the level of education of participants and the occurrence of intestinal diseases (Osman et al., 2016; Dafalla et al., 2017; Shalaby and Shalaby, 2015). Seven studies have proven that the rate of parasitic (Al Alkeem et al., 2019; Al-Jawabreh et al., 2019; Bakarman et al., 2019; Motazedian et al., 2015; Balarak et al., 2016; Atwa and Thabet, 2016) and bacterial (Salama et al., 2019) intestinal infections is significantly higher in those with low-level of education. These findings were in accordance with other studies that have shown that parasitic infection is more prevalent in uneducated people (Becker et al., 2015; Schmidt et al., 2009; Ali et al., 2014). According to these studies, individuals lack adequate access to media and educational resources related to hand hygiene (Schmidt et al., 2009; Ali et al., 2014). Moreover, Sarkari et al. and Moragaa et al. declared that educated individuals showed better knowledge, attitudes, and practices toward parasitic infection transmission (Sarkari et al., 2016; Moragaa et al., 2024). Therefore, health education enhances good personal hygiene and sanitary practices, as well as improves the implementation of prevention and control measures for parasitic diseases (Choy et al., 2014). Conversely, the eligible studies conducted in Iran and Qatar showed no significant association between intestinal infections and level of education (Kiani et al., 2016; Boughattas et al., 2019). This finding aligns with Alqarni et al. who demonstrated that the intestinal parasite infections were detected among food handlers with a high level of education (Alqarni et al., 2023). This may bring up a suggestion that the two socioeconomic factors education and occupation may interfere, and the type of occupation may pose the individual at higher risk of parasitic infection regardless of the education level, this hypothesis was proved by Dafalla et al., who reported that food handlers had the highest rate of infection compared to all other occupational groups studied (Dafalla et al., 2017). This finding was in accordance with other studies, which shows that farmers (Fuhrimann et al., 2016) and agricultural workers (Lengerich et al., 1993) are more likely to be infected by hookworms and are highly exposed to parasites such as Cryptosporidium spp. when compared to other groups. This could be explained by the fact that these workers are in direct contact with soil and water contaminated with livestock excrement (Becker et al., 2015).
Limitations and strength
This scoping review has some limitations that should be mentioned. First, not all 21 countries in the MENA region were included in search terms to align with the Achievable and Time-bound principles of specific, measurable, achievable, relevant, and time-bound (SMART) criteria. Moreover, some low-income countries in the MENA region have not addressed the topic under investigation, with no relevant studies. Second, the inclusion criteria could exclude important studies not published in the English language, studies with participants under five, and reporting viral infections. Additionally, 1,137 articles were retrieved, and after deduplication, only 12 studies were ultimately included. This reduction was partly due to the exclusion of studies conducted in Turkey at a certain stage of the screening process. These articles were removed after it was realized that not all sources classified Turkey as part of the MENA region. As a result, the number of included studies dropped significantly. Third, the lack of rigorous methodology across the included studies; the sample sizes in several of the included studies were relatively small, which may limit the generalizability of the findings to broader populations. Studies with small samples are more susceptible to statistical variability and may not adequately represent the diversity of the target population. Fourth, several studies relied on self-administered questionnaires or telephone-based data collection methods, which introduce potential biases such as recall bias, social desirability bias, and misreporting. These methodological limitations may have influenced the accuracy and reliability of the reported outcomes. Fifth, the majority of the retrieved papers were related to parasitic infections. This may be due to the heterogeneity in keyword selection and the potential influence of pathogen type on associated risk factors. Parasites and bacteria differ significantly in their modes of transmission, environmental resilience, and infection dynamics. For instance, parasitic infections such as those caused by protozoa or helminths often require specific environmental conditions (presence of intermediate hosts or contaminated soil), whereas bacterial infections may spread more easily through direct person-to-person contact or contaminated food and water. These differences could partly explain the observed variation in risk factors across different pathogens in our study. These limitations should be addressed in future research. However, this scoping review has strengths that could be acknowledged. This scoping review mapped the available literature and provided a comprehensive overview of the topic under investigation, which gives clear insights into existing literature as well as the gap in knowledge to be addressed in future studies.
Conclusion and recommendations
The assessment of the SES impact on foodborne disease is an under-explored topic with a degree of complexity, which makes a scoping review a valuable approach to identify and map the available literature. Moreover, a scoping review emphasizes the gap in evidence in the literature to be addressed in future studies rather than critically appraising the quality of included papers. In the present scoping review, we have summarized the relevant papers published between 2014 and 2024 based on predefined inclusion criteria. We found, in most of the studies, that individuals with low income, low level of education, and/or being a food handler have a greater risk of developing intestinal infections. The pathogenic organisms varied between protozoa and hookworms, resulting in mono- or multiple-infections. In contrast, some studies proved that some parameters of SES are not related to the occurrence of intestinal infection among the study participants. Interestingly, we identified several gaps. First, studies highlighting an association between foodborne illnesses and populations with high socioeconomic status (SES) were lacking. For instance, individuals with high living standards have special dining and cultural habits such as eating rare meat, raw fish, raw milk, and cheese, which put them at a similar risk as the population with low living standards or those experiencing food insecurity. Second, SES metrics in the available literature lack a clear definition and indicators, which makes it difficult to compare different SES levels. Thus, for future research, it is highly recommended to explore the impact of SES on the occurrence of foodborne illnesses in terms of cultural factors, dining habits, geographical disparities, healthcare inequality, and food insecurity. Therefore, to decrease the risk of intestinal infections among low socioeconomic groups, public health professionals should address key drivers of foodborne diseases such as food cross-contamination with biological and/or industrial contaminants, poor food safety practices, poor sanitation, and unsafe water. Then, some practical applications may improve public health outcomes. These interventions may include tailored community-based health education on hygiene and food safety practices, improving access to safe and clean water, increasing surveillance on foodborne illness trends, particularly in underserved countries, and advocating for policies that address income and educational disparities.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AK: Methodology, Data curation, Visualization, Project administration, Validation, Conceptualization, Writing – original draft, Software, Investigation, Formal analysis, Resources. RK: Formal analysis, Project administration, Supervision, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Almoosa College of Health Sciences, which covered the article processing Charges (APC). The funder has no role in the Data the study’s design, Data collection, analysis, manuscript preparation or decision to publish.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al Alkeem, F., Loney, T., Aziz, F., Blair, I., Sonnevend, Á., and Sheek-Hussein, M. (2019). Prevalence and factors associated with infectious intestinal diseases in Ras Al Khaimah, United Arab Emirates, 2017: a population-based cross-sectional study. Int. J. Infect. Dis. 85, 188–194. doi: 10.1016/j.ijid.2019.06.004
Ali, M. M., Verrill, L., and Zhang, Y. (2014). Self-reported hand washing behaviors and foodborne illness: a propensity score matching approach. J. Food Prot. 77, 352–358. doi: 10.4315/0362-028X.JFP-13-286
Al-Jawabreh, A., Ereqat, S., Dumaidi, K., Al-Jawabreh, H., Abdeen, Z., and Nasereddin, A. (2019). Prevalence of selected intestinal protozoan infections in marginalized rural communities in Palestine. BMC Public Health 19:1. doi: 10.1186/s12889-019-8024-2.
Alqarni, A. S., Wakid, M. H., and Gattan, H. S. (2023). Hygiene practices and factors influencing intestinal parasites among food handlers in the province of Belgarn, Saudi Arabia. PeerJ. 11:e14700. doi: 10.7717/peerj.14700
Atwa, Z. T., and Thabet, M. M. (2016). Intestinal parasitic infection in Egyptian children: could it be a risk factor for Iron deficiency Anemia? J. Egypt. Soc. Parasitol. 46, 533–540
Augustin, J. C., Kooh, P., Bayeux, T., Guillier, L., Meyer, T., Jourdan-Da Silva, N., et al. (2020). Anses working group on consumer information on foodborne biological risks. Contribution of foods and poor food-handling practices to the burden of foodborne infectious diseases in France. Foods. 9, 16–44. doi: 10.3390/foods9111644
Bakarman, M. A., Hegazi, M. A., and Butt, N. S. (2019). Prevalence, characteristics, risk factors, and impact of intestinal parasitic infections on school children in Jeddah, Western Saudi Arabia. J. Epidemiol. Global Health. 9, 81–87. doi: 10.2991/jegh.k.190219.001
Balarak, D., Modrek, M. J., Bazrafshan, E., Ansari, H., and Kord, M. F. (2016). Prevalence of intestinal parasitic infection among food handlers in Northwest Iran. J. Parasitol. Res. 2016, 1–6. doi: 10.1155/2016/8461965
Becker, D. J., Oloya, J., and Ezeamama, A. E. (2015). Household socioeconomic and demographic correlates of Cryptosporidium seropositivity in the United States. PLoS Negl. Trop. Dis. 9:e0004080. doi: 10.1371/journal.pntd.0004080
Boughattas, S., Behnke, J. M., Al-Sadeq, D., Ismail, A., and Abu-Madi, M. (2019). Cryptosporidium spp., prevalence, molecular characterisation and socio-demographic risk factors among immigrants in Qatar. PLoS Negl. Trop. Dis. 13:e0007750. doi: 10.1371/journal.pntd.0007750
Choy, S. H., Al-Mekhlafi, H. M., Mahdy, M. A., Nasr, N. N., Sulaiman, M., Lim, Y. A., et al. (2014). Prevalence and associated risk factors of Giardia infection among indigenous communities in rural Malaysia. Sci. Rep. 4:6909. doi: 10.1038/srep06909
Colquhoun, H. L., Levac, D., O’Brien, K. K., Straus, S., Tricco, A. C., Perrier, L., et al. (2014). Scoping reviews: time for clarity in definition, methods, and reporting. J. Clin. Epidemiol. 67, 1291–1294. doi: 10.1016/j.jclinepi.2014.03.013
Dafalla, A. I., Almuhairi, S. A., AlHosani, M. H., Mohamed, M. Y., Alkous, M. I., AlAzzawi, M. A., et al. (2017). Intestinal parasitic infections among expatriate workers in various occupations in Sharjah, United Arab Emirates. Rev. Inst. Med. Trop. Sao Paulo 59:e82. doi: 10.1590/S1678-9946201759082
de Andrade, M. L., Rodrigues, R. R., Antongiovanni, N., and da Cunha, D. T. (2019). Knowledge and risk perceptions of foodborne disease by consumers and food handlers at restaurants with different food safety profiles. Food Res. Int. 121, 845–853. doi: 10.1016/j.foodres.2019.01.006
Elbehiry, A., Abalkhail, A., Marzouk, E., Elmanssury, A. E., Almuzaini, A. M., Alfheeaid, H., et al. (2023). An overview of the public health challenges in diagnosing and controlling human foodborne pathogens. Vaccine 11:725. doi: 10.3390/vaccines11040725
Fuhrimann, S., Winkler, M. S., Pham-Duc, P., Do-Trung, D., Schindler, C., Utzinger, J., et al. (2016). Intestinal parasite infections and associated risk factors in communities exposed to wastewater in urban and peri-urban transition zones in Hanoi, Vietnam. Parasit. Vectors 9, 1–4. doi: 10.1186/s13071-016-1809-6
Goh, S., Reacher, M., Casemore, D. P., Verlander, N. Q., Chalmers, R., Knowles, M., et al. (2004). Sporadic cryptosporidiosis, North Cumbria, England, 1996–2000. Emerg. Infect. Dis. 10, 1007–1015. doi: 10.3201/10.3201/eid1006.030325
Grace, D., Mahuku, G., Hoffmann, V., Atherstone, C., Upadhyaya, H. D., and Bandyopadhyay, R. (2015). International agricultural research to reduce food risks: case studies on aflatoxins. Food Secur. 7, 569–582. doi: 10.1007/s12571-015-0469-2
Gulliford, M. C. (2002). Availability of primary care doctors and population health in England: is there an association? J. Public Health 24, 252–254. doi: 10.1093/pubmed/24.4.252
Hunter, P. R., Hughes, S., Woodhouse, S., Syed, Q., Verlander, N. Q., Chalmers, R. M., et al. (2004). Sporadic cryptosporidiosis case-control study with genotyping. Emerg. Infect. Dis. 10, 1241–1249. doi: 10.3201/eid1007.030582
JBI. JBI manual for evidence synthesis: scoping reviews chapter. Jbi.global. (2024). Available online at: https://jbi.global/scoping-review-network/resources (Accessed on 2024 July 18)
Kabir, R., Syed, H. Z., Hayhoe, R., Parsa, A. D., Sivasubramanian, M., Mohammadnezhad, M., et al. (2024). Meta-analysis using SPSS: a simple guide for clinicians, public health, and allied health specialists. Evidence. 2, 6–9. doi: 10.62377/j544ed47
Katona, P., and Katona-Apte, J. (2008). The interaction between nutrition and infection. Clin. Infect. Dis. 46, 1582–1588. doi: 10.1086/587658
Kavanagh, A. M., Goller, J. L., King, T., Jolley, D., Crawford, D., and Turrell, G. (2005). Urban area disadvantage and physical activity: a multilevel study in Melbourne, Australia. J. Epidemiol. Community Health 59, 934–940. doi: 10.1136/jech.2005.035931
Kempson, K. M., Keenan, D. P., Sadani, P. S., Ridlen, S., and Rosato, N. S. (2002). Food management practices used by people with limited resources to maintain food sufficiency as reported by nutrition educators. J. Am. Diet. Assoc. 102, 1795–1799. doi: 10.1016/S0002-8223(02)90385-8
Kiani, H., Haghighi, A., Rostami, A., Azargashb, E., Tabaei, S. J., Solgi, A., et al. (2016). Prevalence, risk factors and symptoms associated to intestinal parasite infections among patients with gastrointestinal disorders in Nahavand, Western Iran. Rev. Inst. Med. Trop. Sao Paulo 58:42. doi: 10.1590/S1678-9946201658042
Lake, R. J., Devleesschauwer, B., Nasinyama, G., Havelaar, A. H., Kuchenmüller, T., Haagsma, J. A., et al. (2015). National studies as a component of the World Health Organization initiative to estimate the global and regional burden of foodborne disease. PLoS One 10, 140–319. doi: 10.1371/journal.pone.0140319
Lake, I. R., Harrison, F. C., Chalmers, R. M., Bentham, G., Nichols, G., Hunter, P. R., et al. (2007). Case-control study of environmental and social factors influencing cryptosporidiosis. Eur. J. Epidemiol. 22, 805–811. doi: 10.1007/s10654-007-9179-1
Lengerich, E. J., Addiss, D. G., Marx, J. J., Ungar, B. L., and Juranek, D. D. (1993). Increased exposure to cryptosporidia among dairy farmers in Wisconsin. J. Infect. Dis. 167, 1252–1255.
Maia, I., Oliveira, A., and Santos, A. C. (2023). Food insecurity is associated with an unhealthy lifestyle score in middle-and older-aged adults: findings from the EPIPorto cohort. Food Secur. 15, 661–671. doi: 10.1007/s12571-023-01366-4
Mak, S., and Thomas, A. (2022). Steps for conducting a scoping review. J. Grad. Med. Educ. 14, 565–567. doi: 10.4300/JGME-D-22-00621.1
McLaughlin, J., Amar, C., Pedraza-diaz, S., and Nichols, G. L. (2000). Molecular epidemiological analysis of cryptosporidium spp. in the United Kingdom: results of genotyping Cryptosporidium spp. in 1,705 fecal samples from humans and 105 fecal samples from livestock animals. J. Clin. Microbiol. 38, 3984–3990. doi: 10.1128/JCM.38.11.3984-3990.2000
Mitton, C., Smith, N., Peacock, S., Evoy, B., and Abelson, J. (2009). Public participation in health care priority setting: a scoping review. Health Policy 91, 219–228. doi: 10.1016/j.healthpol.2009.01.005
Moragaa, I. E., Soliman, F. E., Abd Rabo, R. A., and Abou Asha, N. I. (2024). The effect of self learning regarding food–borne diseases on knowledge and practices of primary school teachers. Tanta Scientific Nurs. J. 32, 12–29. doi: 10.21608/tsnj.2024.340820
Motazedian, M. H., Najjari, M., Ebrahimipour, M., Asgari, Q., Mojtabavi, S., and Mansouri, M. (2015). Prevalence of intestinal parasites among food-handlers in shiraz, Iran. Iran. J. Parasitol. 10:652.
Mtintsilana, A., Craig, A., Mapanga, W., Dlamini, S. N., and Norris, S. A. (2023). Association between socio-economic status and non-communicable disease risk in young adults from Kenya, South Africa, and the United Kingdom. Sci. Rep. 13:728. doi: 10.1038/s41598-023-28013-4
Munn, Z., Pollock, D., Khalil, H., Alexander, L., Mclnerney, P., Godfrey, C. M., et al. (2022). What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis. JBI Evidence Synthesis. 20, 950–952. doi: 10.11124/JBIES-21-00483
Newman, K. L., Leon, J. S., Rebolledo, P. A., and Scallan, E. (2015). The impact of socioeconomic status on foodborne illness in high-income countries: a systematic review. Epidemiol. Infect. 143, 2473–2485. doi: 10.1017/S0950268814003847
Osaili, T. M., Saeed, B. Q., Taha, S., Omar Adrees, A., and Hasan, F. (2022). Knowledge, practices, and risk perception associated with foodborne illnesses among females living in Dubai, United Arab Emirates. Foods. 11:290. doi: 10.3390/foods11030290
Osman, M., El Safadi, D., Cian, A., Benamrouz, S., Nourrisson, C., Poirier, P., et al. (2016). Correction: prevalence and risk factors for intestinal protozoan infections with Cryptosporidium, Giardia, Blastocystis and Dientamoeba among school children in Tripoli, Lebanon. PLoS Negl. Trop. Dis. 10:e0004643. doi: 10.1371/journal.pntd.0004643
Peters, M. D., Godfrey, C. M., Khalil, H., McInerney, P., Parker, D., and Soares, C. B. (2015). Guidance for conducting systematic scoping reviews. JBI Evidence Implementation. 13, 141–146. doi: 10.1097/XEB.0000000000000050
Quandt, S. A., Arcury, T. A., Early, J., Tapia, J., and Davis, J. D. (2004). Household food security among migrant and seasonal Latino farmworkers in North Carolina. Public Health Rep. 119, 568–576. doi: 10.1016/j.phr.2004.09.006
Rodger, D., Admani, A., and Thomas, M. (2024). What is a scoping review? Evid Based Nurs 27, 84–85. doi: 10.1136/ebnurs-2024-103969
Salama, R. I., Emara, M. H., Mostafa, H. M., Abd-Elsalam, S., Alnabawy, S. M., Elshweikh, S. A., et al. (2019). Helicobacter pylori infection and risk of salmonella infection. Medicine 98:e14335. doi: 10.1097/MD.0000000000014335
Sarkari, B., Hosseini, G., Motazedian, M. H., Fararouei, M., and Moshfe, A. (2016). Prevalence and risk factors of intestinal protozoan infections: a population-based study in rural areas of Boyer-Ahmad district, southwestern Iran. BMC Infect. Dis. 16, 1–5. doi: 10.1186/s12879-016-2047-4
Schmidt, W. P., Aunger, R., Coombes, Y., Maina, P. M., Matiko, C. N., Biran, A., et al. (2009). Determinants of handwashing practices in Kenya: the role of media exposure, poverty and infrastructure. Trop. Med. Int. Health 14, 1534–1541. doi: 10.1111/j.1365-3156.2009.02404.x
Shalaby, N., and Shalaby, M. (2015). Cryptosporidium pavum infection among Egyptian scholl children. J. Egypt. Soc. Parasitol. 45, 125–131. doi: 10.12816/0010858
Tricco, A. C., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA extension for scoping reviews (PRISMAScR): checklist and explanation. Ann. Intern. Med. 169, 467–473. doi: 10.7326/M18-0850
Verdejo, C., Tapia-Benavente, L., Schuller-Martínez, B., Vergara-Merino, L., Vargas-Peirano, M., and Silva-Dreyer, A. M. (2021). What you need to know about scoping reviews. Medwave. 21:e8144. doi: 10.5867/medwave.2021.02.8144
Weigel, M. M., Armijos, R. X., Hall, Y. P., Ramirez, Y., and Orozco, R. (2007). The household food insecurity and health outcomes of US–Mexico border migrant and seasonal farmworkers. J. Immigr. Minor. Health 9, 157–169. doi: 10.1007/s10903-006-9026-6
Wertheim-Heck, S., Raneri, J. E., and Oosterveer, P. (2019). Food safety and nutrition for low-income urbanites: exploring a social justice dilemma in consumption policy. Environ. Urban. 31, 397–420. doi: 10.1177/0956247819858019
World Health Organization. Food safety. WHO. (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/food-safety (Accessed on 2024 Oct 18)
Keywords: foodborne disease, socioeconomic factors, burden, MENA region (Middle East and North Africa) region, foodborne pathogen
Citation: Al Khatib A and Kabir R (2025) The impact of socioeconomic status on the burden of foodborne illnesses: a scoping review in the Middle East and North African region. Front. Microbiol. 16:1606382. doi: 10.3389/fmicb.2025.1606382
Edited by:
Marta Laranjo, University of Evora, PortugalReviewed by:
Ricardo Assunção, National Health Institute Doutor Ricardo Jorge (INSA), PortugalPedro Henriques, University of Evora, Portugal
Copyright © 2025 Al Khatib and Kabir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alissar Al Khatib, YS5raGF0aWJAYWxtb29zYWNvbGxlZ2UuZWR1LnNh
 Alissar Al Khatib
Alissar Al Khatib Russell Kabir
Russell Kabir