- 1College of Civil Engineering, Hexi University, Zhangye, China
- 2Research Institute of Water Resources Protection and Utilization in Hexi Corridor, Zhangye, China
- 3College of Water Conservancy and Hydropower Engineering, Gansu Agricultural University, Lanzhou, China
- 4College of Agricultural and Ecological Engineering, Hexi University, Zhangye, China
- 5College of Pastoral Agriculture Science and Technology, Lanzhou University, Lanzhou, China
Introduction: Phosphorus reduction in agriculture is crucial for sustainable soil management, yet its interactive effects with microbial fertilizers on soil nutrient dynamics and microbial communities remain poorly understood.
Methods: Here, we evaluated the impacts of phosphorus reduction at four levels [0% (P100), 15% (P85), 30% (P70), and 100% (P0)] combined with two biofertilizers—Bacillus subtilis (BF1) and Bacillus mucilaginosus (BF2)—on soil available nutrients and bacterial community structure.
Results: Our results demonstrated that P85 combined with BF1 significantly enhanced soil microbial diversity, while P85 combined with BF2 notably increased the levels of available phosphorus and potassium, without significant changes in microbial diversity but with a more pronounced shift in community structure. Microbial community analysis revealed that under BF1 treatment, the proportion of Pseudomonadota, which dominates the carbon cycle, significantly increased. Meanwhile, BF2 treatment promoted the enrichment of Acidobacteriota and Planctomycetota, both involved in carbon and nitrogen cycles. Additionally, both biofertilizers significantly increased the abundance of aerobic and biofilm-forming bacteria. Redundancy analysis (RDA) showed that nitrogen cycle-related microbiota under BF1 treatment were the primary drivers of soil nutrient changes, whereas under BF2 treatment, Acidobacteriota, Chloroflexota, and Actinomycetota (involved in carbonnitrogen cycling and organic matter degradation) contributed more to nutrient enhancement. In conclusion, the application of two biofertilizers with P85 can optimize soil nutrient availability and regulate microbial community structure, with BF1 being more beneficial for maintaining microbial diversity and BF2 having a superior effect on enhancing available phosphorus and potassium.
Conclusion: The combined application of biofertilizers with phosphorus reduction demonstrates potential for improving soil health, thereby providing a scientific basis for promoting sustainable agricultural development.
1 Introduction
Global arable land accounts for 24% of the Earth’s terrestrial area (AbdelRahman, 2023). As the fundamental resource for agricultural production, cultivated land plays an irreplaceable role in ensuring food security, maintaining ecological balance, and fostering economic development (McLaughlin and Kinzelbach, 2015; Li C. et al., 2022). Beyond its agricultural production functions, cultivated land ecosystems provide critical ecological services—including carbon sequestration, oxygen release, water conservation, and biodiversity maintenance—that play a pivotal role in mitigating climate change and sustaining regional ecological balance (DeFries et al., 2004; Wang, 2022). Soil, the core of arable land productivity, has multiple aspects of sustainability, including fertility, structure, biological activity, and resistance to degradation (Song et al., 2022). In combination, these characteristics fundamentally determine both the temporal stability of farming outputs and the enduring capacity for sustained crop productivity (Sofo et al., 2022; Jiao et al., 2022).
In agricultural land management practices, scientific fertilization serves as a critical measure for enhancing soil fertility and ensuring high crop yields (Ludwig et al., 2011). Rational fertilization practices not only directly enhance crop productivity but also improve nutrient cycling and use efficiency through optimization of soil microbial community structure (Soman et al., 2017). However, excessive fertilizer use, particularly phosphorus fertilizers, not only raises agricultural production costs but also leads to phosphorus accumulation in the soil, environmental pollution, and ecological imbalances (Li et al., 2015). Studies have shown that a moderate reduction in phosphorus fertilizer use does not decrease crop yield or soil nutrient availability (Duan et al., 2023). Combining moderate phosphorus reduction with biofertilizer can reduce the risk of agricultural non-point source pollution while optimizing soil microbial community structure and improving phosphorus fertilizer efficiency (Liu et al., 2022).
In recent years, biofertilizers have gained widespread attention for their ability to improve the soil microbial environment and enhance plant nutrient absorption (Wang et al., 2020; Liu et al., 2022). Biofertilizers, through the colonization and metabolic activities of functional microorganisms, can enhance the availability of soil nutrients, suppress soil-borne pathogens, and enhance crop resistance to stress (Kapoor et al., 2024). For instance, Bacillus mucilaginosus, a potassium-solubilizing bacterium, is highly effective in potassium release (Basak and Biswas, 2009). Research has shown that functional microorganisms, such as nitrogen-fixing bacteria, phosphorus-solubilizing bacteria, and organic matter-degrading bacteria, can promote the effective utilization of soil nutrients, increase plant nutrient absorption efficiency, and maintain high crop yields and soil health despite reduced chemical fertilizer use (Richardson, 2001). Therefore, exploring the feasibility of reducing phosphorus fertilizer application while combining it with microbial fertilizers (biofertilizers) to maintain soil nutrient balance has become one of the key issues in current sustainable agricultural research (Duan et al., 2023; Liu et al., 2022).
Oases play a critical role in maintaining regional food security (El Janati et al., 2021). In oasis agricultural systems, water scarcity makes the efficient use of soil nutrients particularly important (Xue et al., 2015). Proper fertilization not only promotes crop growth but also optimizes the structure of soil microbial communities, thereby influencing the cycling and utilization of soil nutrients (Li et al., 2021). However, there remains a significant research gap regarding how to effectively enhance nutrient use efficiency under reduced fertilizer input while maintaining soil health and crop productivity in saline-alkaline arid oasis environments. In particular, the synergistic effects of combining specific functional microorganisms—such as B. subtilis and B. mucilaginosus—with phosphorus reduction strategies have not been thoroughly investigated under real-field drip irrigation conditions. To address this knowledge gap, we hypothesize that the co-application of these biofertilizers with moderate phosphorus reduction can not only maintain crop yield but also enhance soil microbial activity and nutrient transformation efficiency in saline-alkaline soils. To test this hypothesis, a field experiment was conducted at the water-saving experimental station in Pingyuanbao Town, Ganzhou District, Zhangye City, a representative arid oasis region. This study integrates drip irrigation and fertigation technology to systematically evaluate the colonization dynamics of functional microbes and their impact on soil nutrient cycling, thereby providing a novel practical strategy and theoretical basis for sustainable phosphorus management and ecological intensification of oasis agriculture in arid regions.
2 Materials and methods
2.1 Study site
The Experimental Station in Pingyuanbao Town, Ganzhou District, Zhangye City, is located in the central part of the Hexi Corridor, Gansu Province, China, with specific geographic coordinates of 38°32’–39°24’ N and 100°06’–100°52’ E. The average elevation is approximately 1,474 m, and the terrain is flat. It lies within the middle oasis plain area of the Heihe River Basin, a typical agricultural irrigation zone. The annual average temperature ranges from 4.1 °C to 8.3 °C, with significant diurnal temperature variation. Annual precipitation ranges from 112.3 to 354 mm, concentrated in the summer, and the evaporation rate is as high as 2,047 mm. The area enjoys 3,085 h of sunshine annually, providing abundant solar and thermal resources. The frost-free period lasts 138–179 days, making it suitable for crop growth. The predominant soil type is saline-alkaline soil, soil organic matter was 12.4 g kg–1, total nitrogen 0.84 g kg–1, total phosphorus 0.81 g kg–1, and total potassium 17.9 g kg–1.
2.2 Experimental design
The experimental plots were set up with dimensions of 18 m × 4 m. A plastic film cover with a width of 0.7 m was applied to the planting rows, with a plant spacing of 0.25 m and a row spacing of 0.5 m. A 1.0 m observation path was reserved between the plot groups, and a 0.6 m observation path was left between plots to prevent cross-contamination of water and fertilizers. Nine treatments were set (Table 1) with three replications, totaling 27 plots. The base fertilizer was applied as follows: 300 kg ha–1 of triple superphosphate (P2O5, 46%), 150 kg ha–1 of potassium sulfate (K2O, 52%), 75 kg ha–1 of urea (N, 46%), and 22.5 kg ha–1 of zinc sulfate (ZnSO4⋅H2O, 35%). Fertilizers were applied through drip irrigation: the first application at the jointing stage (2024-06-15) consisted of 225 kg ha–1 of soluble nitrogen fertilizer (N, 46%); the second application at the filling stage (2024-07-20) involved 300 kg ha–1 of soluble nitrogen fertilizer (N, 46%); and the third application at the milking stage (2024-08-10) included 225 kg ha–1 of soluble nitrogen fertilizer (N, 46%). All fertilizers were provided by a local fertilizer company, with treatments kept consistent across all plots. Irrigation and weed management were conducted uniformly across the experiment. The biofertilizer was applied at a rate of 75 kg⋅ha–1 during the maize jointing stage (mid-June) using a localized soil incorporation method: the inoculant was placed into excavated soil around plant roots and immediately covered with backfill soil. The microbial blended fertilizer and microbial inoculum were purchased from Gansu Xingshuo Biotechnology Co., Ltd. (the number of viable bacteria ≥ 200 million/g). The solid powder inoculants of B. subtilis and B. mucilaginosus were developed through a standardized process beginning with strain activation on agar slants (NA medium for B. subtilis and silicate medium for B. mucilaginosus) at 30 °C for 48 h, followed by liquid fermentation in specialized media (LB for B. subtilis and low-phosphate medium for B. mucilaginosus) with 5% inoculum under 1:1 vvm aeration at 28 °C –30 °C for 24–36 h until reaching ≥ 109 CFU/mL, where B. subtilis fermentation was supplemented with 0.1% MnSO4 to enhance sporulation while B. mucilaginosus received 1% potassium feldspar powder to stimulate exopolysaccharide production; post-fermentation, the broth was concentrated to ≥ 1011 CFU/mL via disk centrifugation (8,000 rpm, 15 °C), with B. subtilis undergoing additional 0.5% CaCl2 treatment and 40 °C heat shock for 1 h to promote sporulation, after which both concentrated cultures were blended with carrier substrates (B. subtilis: 500 kg peat/humic acid, 200 kg wheat bran, 50 kg diatomite, 30 kg light calcium carbonate, and 5 kg trehalose; B. mucilaginosus: 600 kg peat/humic acid, 150 kg wheat bran, 100 kg diatomite, 30 kg light calcium carbonate, and 10 kg trehalose) in a double-helix mixer at 20 rpm for 30 min, dried via fluidized bed (45 °C inlet air, ≤ 40 °C material temperature) to ≤ 8% moisture, and milled to 80 mesh (180 μm) before vacuum packaging, yielding final products with ≥ 2 × 108 CFU/g viability, ≥ 80% survival after 40 °C/14 days stability testing, ≥ 50 mg/g citric acid secretion (B. mucilaginosus). According to China national bio-organic fertilizer quality standard NY884-2012 (Liu et al., 2019).
2.3 Soil sampling and analysis
After the corn harvest, soil samples from the 0 to 20 cm plow layer were collected from each plot using a five-point sampling method. To avoid fertilization grooves and the edges of plastic film covers, soil samples were taken with a stainless steel soil auger to prevent metal contamination. Soil samples from three replicates of the same treatment were mixed in equal amounts to form biological replicates (n = 3). After removing visible roots and residual plastic film, the samples were sieved through a 2 mm mesh and divided into two parts: one was stored at 4 °C for microbial analysis, and the other was air-dried and ground through a 100-mesh sieve for physicochemical analysis.
Total nitrogen (TN) was determined using the Kjeldahl method, Soil samples (0.5 g) were digested with sulfuric acid-catalyst mixture (420 °C) and distilled, followed by titration with 0.01 mol/L HCl. Total phosphorus (TP) was measured by the sodium hydroxide fusion-molybdenum-antimony colorimetric method, Samples (0.2 g) were fused with NaOH (720 °C) and analyzed via molybdenum-antimony colorimetry (UV-Vis spectrophotometer, 700 nm). Total potassium (TK) was Quantified per using HF-HClO4 digestion and flame photometry (766.5 nm potassium filter). Available nutrients were measured as follows: available phosphorus (SAP) was determined by the molybdenum-antimony colorimetric method. SAP was extracted with 0.5 mol/L NaHCO3 (pH 8.5; modified Olsen method). After shaking (25 °C, 30 min), supernatants were analyzed by molybdenum-blue colorimetry (882 nm). Available potassium (AK) was Extracted with 1 mol/L NH4OAc (pH 7.0) and measured via flame photometry. Soil organic matter (SOM) was determined by K2Cr2O4 oxidation. Samples (0.1 g) were heated (170 °C–180 °C, 5 min) with 0.8 mol/L K2Cr2O7 and concentrated H2SO4, then titrated with 0.2 mol/L FeSO4. Organic carbon content was converted to SOM using the Bemmelen factor (1.724). The analyses of SOM, TN, TP, TK, AP, and AK were based on the standard methods as explained in a previous study (Binkley and Vitousek, 1989; Wilke, 2005).
DNA extraction and amplification: Total DNA was extracted using the PowerSoil® DNA Extraction Kit, and the quality was checked by 1% agarose gel electrophoresis. The bacterial 16S rRNA gene V3–V4 region was amplified using universal primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). High-throughput sequencing: Purified PCR products were sequenced on the Illumina NovaSeq platform with paired-end sequencing (2 × 250 bp). The raw data were quality controlled using Trimmomatic and clustered into OTUs at 97% similarity using USEARCH (v11). Species annotation was performed based on the Silva database (v138).
2.4 Statistical analysis
All physicochemical data are presented as the mean ± standard error, and one-way analysis of variance (ANOVA) was conducted to compare the effects of different phosphorus fertilizer treatments under identical microbial fertilizer conditions on soil physicochemical properties (organic matter, total nitrogen, total phosphorus, total potassium, available phosphorus, available potassium) and soil bacterial community characteristics (OTU richness, Chao1 index, Shannon-Wiener index, Pielou index), as well as compositional differences at the bacterial phylum level. Tukey’s HSD post hoc test was employed for multiple comparisons. It should be noted that during the one-way ANOVA, the same control group (CK) data were used for both microbial fertilizer treatments combined with different phosphorus fertilizer applications, which are uniformly indicated in gray in the corresponding figures. All statistical analyses described above were performed using SPSS 26.0.
Using the QIIME 2 platform, high-quality sequences were clustered into operational taxonomic units (OTUs) at a 97% similarity threshold to generate an OTU table. The table was then rarefied to an even sequencing depth to minimize the effects of varying sequencing volumes on diversity metrics. Based on the rarefied data, α-diversity was assessed using the following indices: observed OTUs (reflecting species richness), the Chao1 index (estimating total community species richness), the Shannon index (integrating both species richness and evenness), and the Pielou’s evenness index (reflecting the uniformity of species distribution). β-diversity was analyzed to examine differences in microbial community structure using Bray–Curtis distance-based principal coordinate analysis (PCoA). Statistical significance of group differences was tested using PERMANOVA (Adonis) with 999 permutations. All analyses of bacterial α-diversity number β-diversity data was carried out in QIIME2. The relationship between microbial data and environmental factors was analyzed using redundancy analysis (RDA) in Canoco 5, with graphical representations created using Origin 2024.
3 Results
3.1 Effects of combined application of two biofertilizers and P fertilizer on soil nutrients
The application of chemical fertilizer (TSP) at different rates, combined with two biofertilizers (BF1 and BF2) increased soil SOM and TK, though the changes were not significant. The application of BF1 with varying phosphorus levels had no significant effect on soil TN and TP. However, under BF2 combined with P100, both TN and TP in the soil were significantly increased. In contrast, under the reduced phosphorus treatments (P85 and P70), although there was an increase in TN and TP, the changes were not significant. After applying BF1 with P100 and P85, both AP and AK in the soil were significantly enhanced (p < 0.05), with no significant difference observed between P100 and P85 in terms of soil AP and AK.
3.2 Effects of combined application of two biofertilizers and phosphorus fertilizer on soil bacterial communities
After the application of P100 combined with BF1, both the OTU and Chao1 indices of soil bacteria significantly increased, while after combining BF2, only the Chao1 index showed a significant increase (p < 0.05). Shannon-Wiener index of soil bacteria significantly increased after P100 combined with BF1, but both the Shannon-Wiener and Pielou indices significantly decreased after the application of BF2 (p < 0.05). No significant differences in the Shannon-Wiener and Pielou indices of soil bacteria were observed between the P85 and P70 treatments combined with BF2 and the CK treatment. At the phylum level, the application of BF2 combined with different phosphorus fertilizer treatments had a more significant impact on the soil bacterial community structure compared to BF1 (Figure 3).
Compared to the CK treatment, the application of BF1 significantly increased the relative abundance of Acidobacteriota at the phylum level, while the relative abundance of Gemmatimonadota and Bacteroidota significantly decreased (p < 0.05; Figure 4a). Following BF2 application, only the relative abundance of Gemmatimonadota significantly decreased (p < 0.05). However, under phosphorus reduction treatments, particularly the P85 treatment, the relative abundance of Pseudomonadota and Gemmatimonadota significantly decreased, while Acidobacteriota and Planctomycetota significantly increased in the community (p < 0.05; Figures 4a, 5).
Compared to the CK treatment, the application of BF1 significantly increased the relative abundance of Anaerobic and Biofilm-forming functional bacteria in the soil community (Figure 4b). Following BF2 application, the relative abundance of Pathogenic functional bacteria in the soil significantly increased. However, after phosphorus reduction, especially under the P85 treatment, there were no significant differences in the relative abundance of Pathogenic and Stress-tolerant functional bacteria compared to the CK treatment, while the relative abundance of Biofilm-forming functional bacteria significantly increased (Figure 4b).
Redundancy analysis results show that after the application of BF1 combined with different phosphorus treatments (Figure 5), Acidobacteriota, Chloroflexota, and Actinomycetota were the main factors influencing soil TP and TN. In contrast, after the application of BF2 combined with different phosphorus treatments, Patescibacteria and Planctomycetota were the primary factors affecting soil SOM, TN, and AK.
4 Discussion
4.1 Effects of phosphorus reduction combined with two biofertilizers on soil nutrients
Phosphorus is a key nutrient for crop growth and development; however, excessive application can exacerbate soil environmental stress and lead to non-point source pollution (Bindraban et al., 2020). A moderate reduction in phosphorus fertilization, combined with biofertilizers, can improve soil microbial communities and enhance the soil’s self-regulation capacity (Liu et al., 2022). Studies have shown that through scientifically managed phosphorus reduction and biofertilizer application, the levels of total phosphorus (TP) and available phosphorus (AP) in the soil can be maintained at optimal levels, while crop yields remain stable (Withers et al., 2017; Li Z. et al., 2022; Mącik et al., 2022; Liu et al., 2022). This finding is supported by our results, where no significant differences in TP, AP, and available potassium (AK) were observed between the P85 (15% reduced phosphorus with BF2) and P100 (standard phosphorus with BF2) treatments (Figure 1). This can be attributed to two main reasons: (1). B. mucilaginosus (BF2) assimilates some phosphorus during growth to form microbial biomass phosphorus, which, upon the bacteria’s death or dormancy, can be mineralized and released back into the soil, enhancing available phosphorus content. (2). The colonization of B. mucilaginosus enhances the phosphate-solubilizing action of organic acids and protons secreted by plant roots, forming a root-bacteria synergistic effect that further promotes phosphorus availability (Basak and Biswas, 2009). This indicates that reducing phosphorus fertilization by 15% combined with biofertilizer application does not decrease phosphorus availability in the soil. Furthermore, the combination of BF2 led to an increase in AK content, likely due to the ability of B. mucilaginosus to secrete organic acids (such as citric and oxalic acids) and exopolysaccharides that dissolve poorly soluble potassium minerals (e.g., potassium feldspar, mica), converting fixed potassium (K) into a form that plants can absorb, thus enhancing soil’s available potassium content (Basak and Biswas, 2009; Mącik et al., 2022). Additionally, although soil nutrients such as soil organic carbon (SOC), total nitrogen (TN), and TP slightly increased after the application of BF1 with phosphorus reduction treatments, the differences were not significant. This may be attributed to the fact that BF1’s functions are more inclined toward suppressing soilborne pathogens and promoting plant growth.
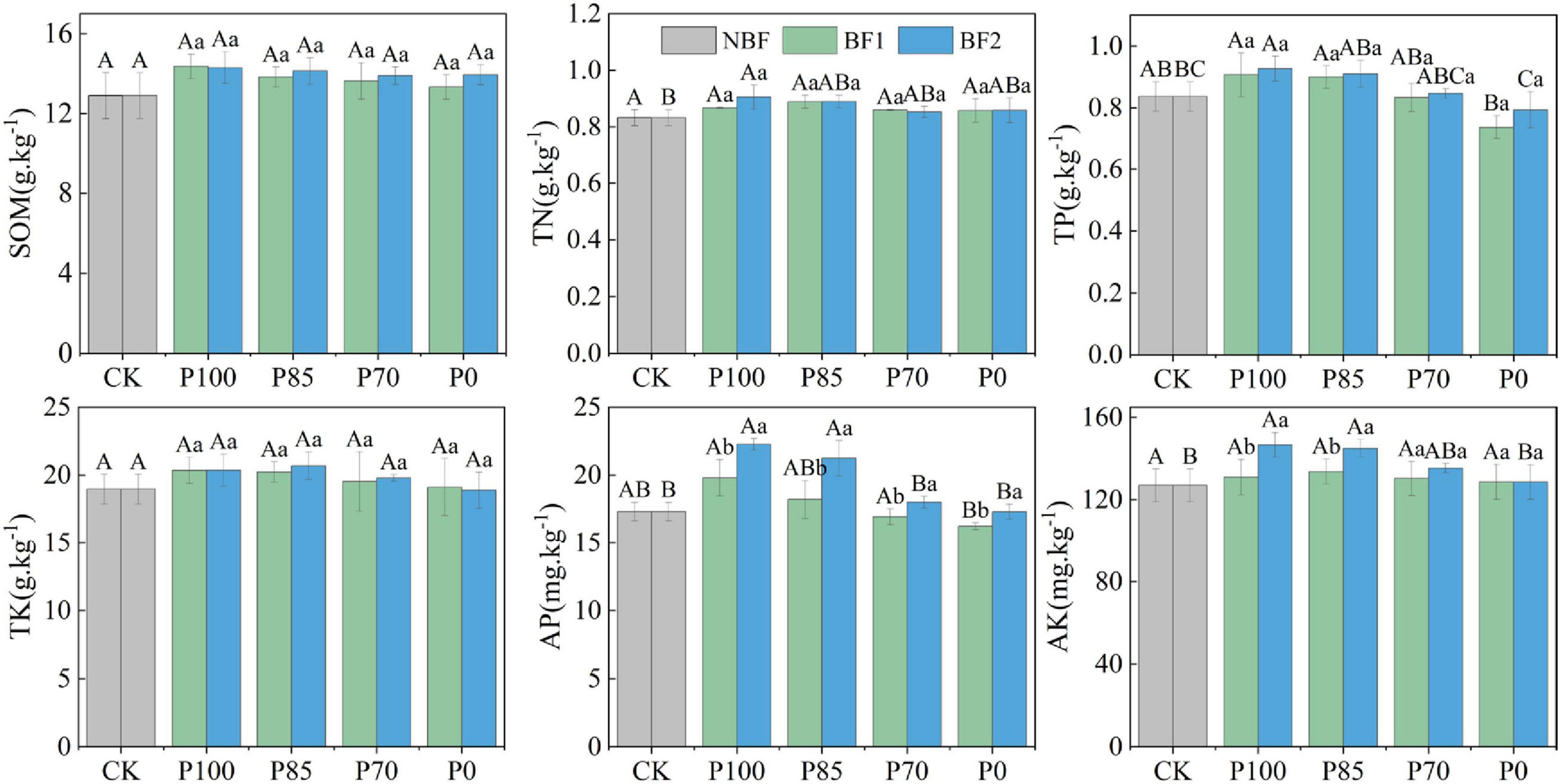
Figure 1. Effects of combined application of two biofertilizers and phosphorus fertilizer on soil organic matter (SOM), total nitrogen (TN), total phosphorus (TP), total potassium (TK), available phosphorus (AP), and available potassium (AK). Different uppercase letters in the figure indicate significant differences among different phosphorus fertilizer treatments under the same microbial fertilizer treatment, while lowercase letters denote significant differences among various microbial fertilizer treatments under the same phosphorus fertilizer application. BF1 and BF2 indicate Bacillus subtilis-based and Bacillus mucilaginosus-based biofertilizers, respectively.
4.2 Effects of phosphorus reduction combined with two biofertilizers on soil bacteria
Soil microorganisms are the core driving force behind maintaining soil ecosystem functions. Changes in microbial community structure directly impact key processes such as soil nutrient cycling, organic matter stability, and pollutant degradation, thereby influencing soil health and productivity (Schmidt et al., 2019; Prasad et al., 2021). Our results show that after the application of BF1, the OTU, Chao1, and Shannon-Wiener indices of soil bacteria significantly increased. This is likely due to B. subtilis (BF1) breaking down organic matter to release nutrients, suppressing pathogenic bacteria to reduce competitive pressure, and optimizing the microenvironment, which synergistically drives increased bacterial diversity and abundance (Kovács, 2019; Liu et al., 2021).
In contrast, after the application of BF2, no significant differences in OTU or Chao1 indices were observed, but the Shannon-Wiener and Pielou indices of soil bacteria significantly decreased (Figure 2). This could be because B. mucilaginosus (BF2) selectively releases mineral nutrients and metabolic products, enriching oligotrophic functional bacterial communities, thereby intensifying interspecies resource competition. This results in a higher dominance of certain species and lower evenness in the soil microbial community, maintaining stability in species richness (OTU, Chao1) but significantly reducing diversity indices (Shannon/Pielou) (Liu et al., 2021). Moreover, community structure analysis of soil bacteria also indicates that BF2 application leads to more significant changes in bacterial community structure compared to BF1 (Figure 3).

Figure 2. Effects of combined application of two biofertilizers and phosphorus fertilizer on soil bacterial diversity. Different uppercase letters in the figure indicate significant differences among different phosphorus fertilizer treatments under the same microbial fertilizer treatment, while lowercase letters denote significant differences among various microbial fertilizer treatments under the same phosphorus fertilizer application. BF1 and BF2 indicate Bacillus subtilis-based and Bacillus mucilaginosus-based biofertilizers, respectively.
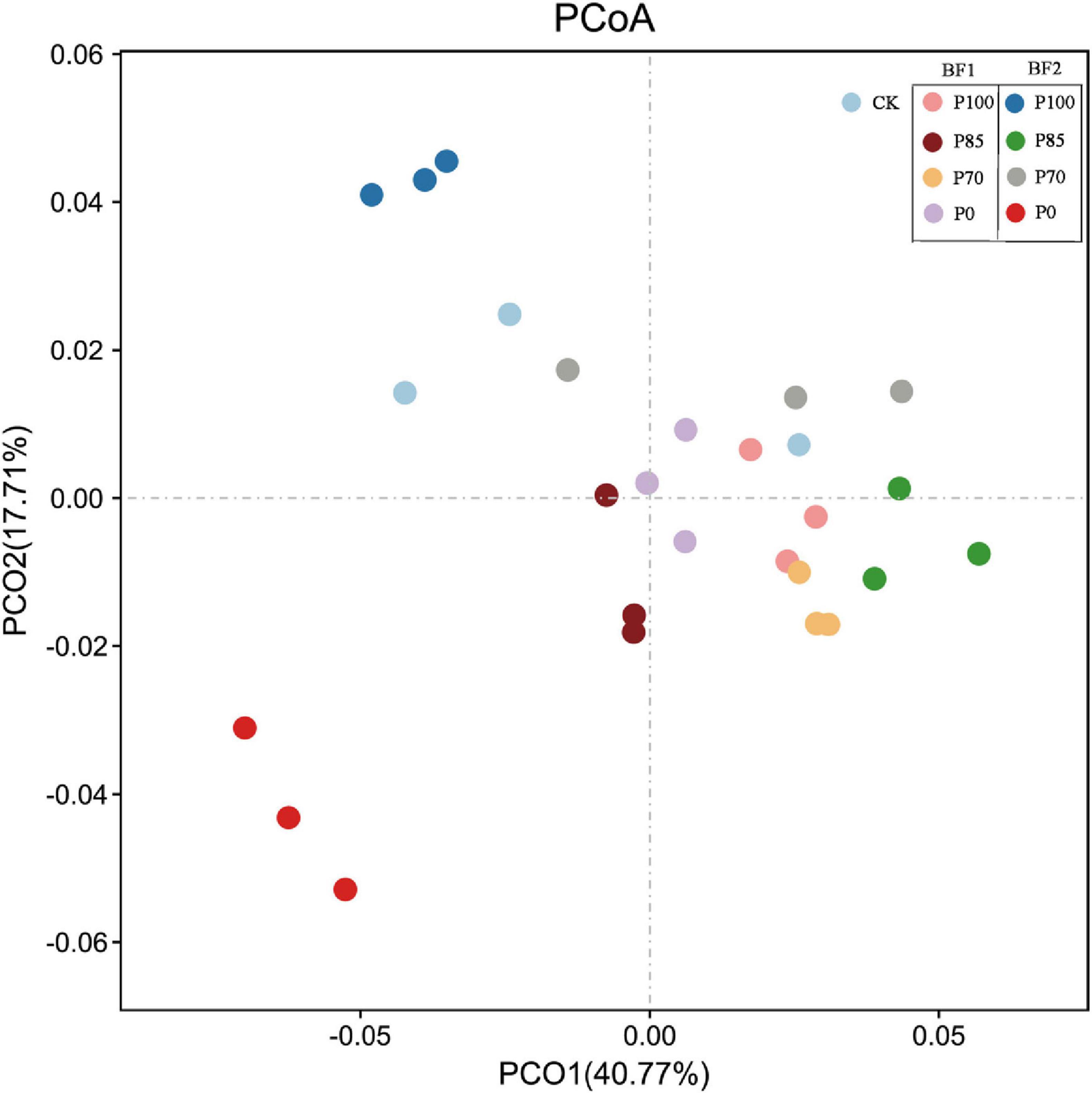
Figure 3. Effects of combined application of two biofertilizers and phosphorus fertilizer on bacterial community structure. BF1 and BF2 indicate Bacillus subtilis-based and Bacillus mucilaginosus-based biofertilizers, respectively.
After reducing phosphorus fertilization by 15%, bacterial OUT, Chao1 indices and Shannon-Wiener index under the BF2 treatment did not show significant changes (p > 0.05), but under the BF1 treatment, the diversity index significantly increased (Figure 2, p < 0.05). This increase in diversity under low-phosphorus conditions is likely due to B. mucilaginosus solubilizing and releasing phosphorus from insoluble compounds, which enhances microbial niche differentiation and functional redundancy, while phosphorus addition reduces resource competition and cooperative interaction demands (Kovács, 2019; Liu et al., 2022). Furthermore, under the P70 treatment, where phosphorus was reduced by 30%, there were decreases in total nitrogen, total phosphorus, available phosphorus, and available potassium in the soil (Figure 1), which could negatively affect crop yield, this may be due to phosphorus fertilizer reduction directly decreases the soil available phosphorus pool. As phosphorus serves as both an energy carrier and a critical component of nucleic acids, its deficiency may inhibit microbial organic nitrogen mineralization capacity, consequently leading to reduced soil total nitrogen accumulation (Liu et al., 2022). Furthermore, the synergistic phosphorus-potassium co-uptake effect becomes weakened under these conditions (Mącik et al., 2022; Liu et al., 2022). These results suggest that a 15% reduction in phosphorus fertilization (P85) combined with biofertilizer application does not negatively affect soil bacterial diversity or nutrient availability, and the biofertilizer facilitates nutrient cycling (Billah et al., 2019).
After the application of biofertilizers, functional microorganisms drive the rapid turnover of dominant species through mechanisms such as niche competition (where metabolites preempt resources) and allelopathic inhibition (targeted antagonism of competing microbial communities) (Kovács, 2019; Liu et al., 2022). Therefore, functional microorganisms play a direct and leading role in community construction. Our results indicate that after the application of BF1 (Bacillus subtilis), the relative abundance of Acidobacteriota at the phylum level significantly increased, while Gemmatimonadota and Bacteroidota significantly decreased. This phenomenon can be attributed to B. subtilis-mediated soil acidification through organic acid secretion and carbon source availability modification, which selectively enriches acidophilic Acidobacteriota while suppressing neutrophilic Gemmatimonadota and Bacteroidota populations, ultimately leading to microbial community restructuring (Yongling Hongjuan Zhang et al., 2024).
Following the application of BF2 (B. mucilaginosus), only the relative abundance of Gemmatimonadota decreased significantly, likely due to the production of organic acids during the metabolic processes of B. mucilaginosus, which lowers the soil pH. This acidic environment may inhibit the growth of Bacteroidota, thus reducing their proportion in the community (Sun et al., 2015). However, under the phosphorus reduction treatments (e.g., P85), the relative abundance of Pseudomonadota and Gemmatimonadota in the microbial community significantly decreased, while Acidobacteriota and Planctomycetota increased significantly (Figures 4a, 5). This is likely because phosphorus is a key element for microbial growth, and phosphorus reduction may limit the growth of microorganisms such as Pseudomonadota and Bacteroidota, which require sufficient phosphorus for growth, leading to a decrease in their abundance (Glick, 2012).
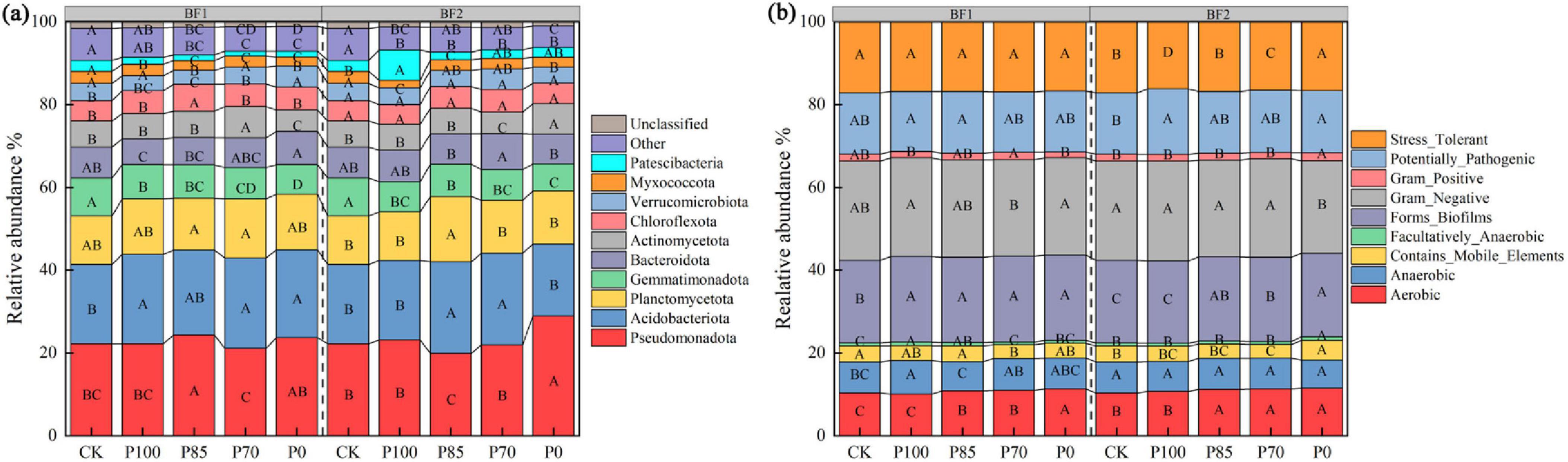
Figure 4. Effects of combined application of two biofertilizers and phosphorus fertilizer on bacterial community composition (a) and phenotypic classification (b). Different uppercase letters in the figure indicate significant differences among different phosphorus fertilizer treatments under the same microbial fertilizer treatment. BF1 and BF2 indicate Bacillus subtilis-based and Bacillus mucilaginosus-based biofertilizers, respectively.
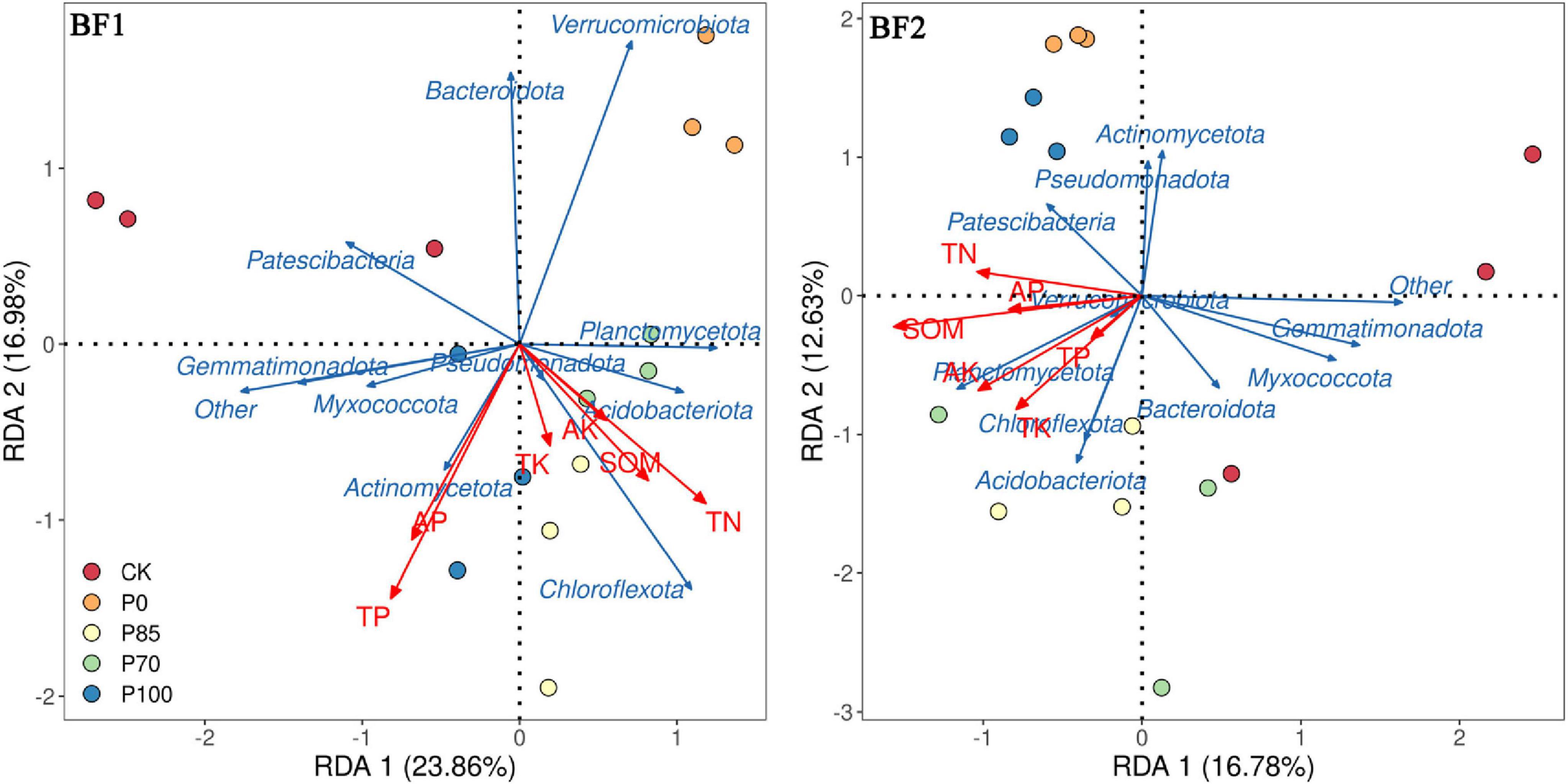
Figure 5. The relationship between bacterial community structure and soil nutrients following the application of biofertilizers combined with phosphorus fertilizers. BF1 and BF2 indicate Bacillus subtilis-based and Bacillus mucilaginosus-based biofertilizers, respectively.
After the application of BF1 (B. subtilis), the relative abundance of anaerobic bacteria and biofilm-forming bacteria significantly increased. This may be due to the metabolic activity of B. subtilis in the soil, which breaks down organic matter and releases nutrients that other microorganisms can utilize. These organic materials provide carbon sources for anaerobic bacteria, promoting their growth (Kovács, 2019). Following the application of BF2 (B. mucilaginosus), the relative abundance of pathogenic bacteria significantly increased, while the abundance of stress-tolerant bacteria remained largely unchanged. Additionally, biofilm-forming bacteria significantly increased in abundance (Basak and Biswas, 2009). The application of BF2 significantly increased the relative abundance of pathogenic bacteria and biofilm-forming bacteria, while showing no notable effect on stress-tolerant bacteria. This phenomenon may be attributed to: (1) nutrient release through BF2-mediated solubilization promoting pathogenic bacterial proliferation; (2) potential suppression of pathogen-antagonistic microorganisms by BF2 metabolites; (3) provision of extracellular polysaccharides (EPS) as a matrix for biofilm formation. The stability of stress-tolerant bacteria could result from unaltered soil stress conditions or functional redundancy within microbial communities (Basak and Biswas, 2009; Li Z. et al., 2022).
Under phosphorus reduction treatments, such as P85, there were no significant changes in the relative abundance of pathogenic and stress-tolerant functional bacteria, but the abundance of biofilm-forming functional bacteria increased significantly. This suggests that moderate phosphorus reduction benefits the growth of beneficial bacterial functional groups and improves soil fertility and plant health (Liu et al., 2022; Mącik et al., 2022).
Bacteria influence soil nutrient transformation, cycling, and availability through various mechanisms, thereby improving the soil nutrient status (Gao et al., 2022; Liu et al., 2022). RDA (Redundancy Analysis) results indicate that after the application of BF1 combined with different phosphorus fertilizers, Acidobacteriota, Chloroflexota, and Actinomycetota were the primary factors influencing soil total phosphorus (TP) and total nitrogen (TN). In contrast, after the application of BF2 with different phosphorus fertilizers, Patescibacteria and Planctomycetota were the major factors affecting soil soil organic matter (SOM), TN, and available potassium (AK) (Figure 5). This suggests that different biofertilizers, by modulating microbial communities, can optimize soil nutrient cycling and enhance soil quality (Liu et al., 2022).
5 Conclusion
Our findings demonstrate that a 15% phosphorus reduction combined with biofertilizer application (BF1/BF2) effectively improves soil nutrient status while differentially altering bacterial communities: BF1 significantly enhanced microbial diversity and Pseudomonadota (carbon cycle), whereas BF2 markedly increased available phosphorus/potassium and enriched Acidobacteriota/Planctomycetota (carbon-nitrogen cycling), with both biofertilizers promoting aerobic and biofilm-forming bacteria. RDA revealed Chloroflexota (nitrogen cycling) as the primary driver of nutrient dynamics under BF1, while BF2 effects were mediated by coordinated action of Acidobacteriota, Chloroflexota, and Actinomycetota (organic matter degradation). This optimized phosphorus-reduction strategy with biofertilizers enhances nutrient availability, improves microbial diversity, and represents a sustainable approach for maintaining soil health and agricultural productivity.
Data availability statement
The raw sequencing data supporting the findings of this study have been deposited in the NCBI Sequence Read Archive (SRA) under the BioProject accession number PRJNA1330030.
Author contributions
YLZ: Data curation, Investigation, Writing – original draft. RX: Conceptualization, Data curation, Formal analysis, Project administration, Writing – original draft, Writing – review & editing. YCZ: Data curation, Writing – review & editing. TL: Investigation, Writing – original draft. HYC: Data curation, Writing – original draft. HJZ: Data curation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (41867010), Gansu Provincial Key Research and Development Program (22YF7NG128), Hexi University Innovation Team Project (CXTD2023002), and the College Student Innovation and Entrepreneurship Training Program (202310740029, S202310740052).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fmicb.2025.1724784.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
AbdelRahman, M. A. (2023). An overview of land degradation, desertification and sustainable land management using GIS and remote sensing applications. Rendiconti Lincei. Scienze Fisiche e Naturali 34, 767–808. doi: 10.1007/s12210-023-01155-3
Basak, B. B., and Biswas, D. R. (2009). Influence of potassium solubilizing microorganism (Bacillus mucilaginosus) and waste mica on potassium uptake dynamics by sudan grass (Sorghum vulgare Pers.) grown under two Alfisols. Plant Soil 317, 235–255. doi: 10.1007/s11104-008-9805-z
Billah, M., Khan, M., Bano, A., Hassan, T. U., Munir, A., and Gurmani, A. R. (2019). Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 36, 904–916. doi: 10.1080/01490451.2019.1654043
Bindraban, P. S., Dimkpa, C. O., and Pandey, R. (2020). Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertility Soils 56, 299–317. doi: 10.1007/s00374-019-01430-2
Binkley, D., and Vitousek, P. (1989). Soil nutrient availability[M]//Plant physiological ecology: field methods and instrumentation. Dordrecht: Springer Netherlands, 75–96.
DeFries, R. S., Foley, J. A., and Asner, G. P. (2004). Land-use choices: Balancing human needs and ecosystem function. Front. Ecol. Environ. 2:249–257. doi: 10.1890/1540-92952004002
Duan, X., Zou, C., Jiang, Y., Yu, X., and Ye, X. (2023). Effects of reduced phosphate fertilizer and increased Trichoderma application on the growth, yield, and quality of pepper. Plants 12:2998. doi: 10.3390/plants12162998
El Janati, M., Akkal-Corfini, N., Bouaziz, A., Oukarroum, A., Robin, P., Sabri, A., et al. (2021). Benefits of circular agriculture for cropoing systems and soil fertility in oases. Sustainability 13:4713. doi: 10.3390/su13094713
Gao, Y., Song, X., Zheng, W., Wu, L., Chen, Q., Yu, X., et al. (2022). The controlled-release nitrogen fertilizer driving the symbiosis of microbial communities to improve wheat productivity and soil fertility. Field Crops Res. 289, 108712. doi: 10.1016/j.fcr.2022.108712
Glick, B. R. (2012). Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 963401. doi: 10.6064/2012/963401
Jiao, S., Qi, J. J., Jin, C. C., Liu, Y., Wang, Y., Pan, H., et al. (2022). Core phylotypes enhance the resistance of soil microbiome to environmental changes to maintain multifunctionality in agricultural ecosystems. Glob. Change Biol. 28, 6653–6664. doi: 10.1111/gcb.16387
Kapoor, D., Sharma, P., Sharma, M. M. M., Yadav, S., and Husen, A. (2024). Exploring soil microbiota and their role in plant growth, stress tolerance, disease control and nutrient immobilizer. Biocatalysis Agric. Biotechnol. 61:103358. doi: 10.1016/j.bcab.2024.103358
Kovács, ÁT. (2019). Bacillus subtilis. Trends Microbiol. 27, 724–725. doi: 10.1016/j.bcab.2024.103358
Li, C., Li, Y., Ma, J., Wang, Y., Wang, Z., and Liu, Y. (2021). Microbial community variation and its relationship with soil carbon accumulation during long-term oasis formation. Appl. Soil Ecol. 168:104126. doi: 10.1016/j.apsoil.2021.104126
Li, C., Wang, X., Ji, Z., Li, L., and Guan, X. (2022). Optimizing the use of cultivated land in China’s main grain-producing areas from the dual perspective of ecological security and leading-function zoning. Int. J. Environ. Res. Public Health 19:13630. doi: 10.3390/ijerph192013630
Li, H., Liu, J., Li, G., Shen, J., Bergström, L., and Zhang, F. (2015). Past, present, and future use of phosphorus in Chinese agriculture and its influence on phosphorus losses. Ambio 44, 274–285. doi: 10.1007/s13280-015-0633-0
Li, Z., Alami, M. M., Tang, H., Zhao, J. S., Nie, Z. N., Hu, J. L., et al. (2022). Applications of Streptomyces jingyangensis T. and Bacillus mucilaginosus A. improve soil health and mitigate the continuous cropping obstacles for Pinellia ternata (Thunb.). Breit 180:114691. doi: 10.1016/j.indcrop.2022.114691
Liu, B., Chen, Q. Q., Wang, J. P., Ruan, C. Q., Chen, Y. P., and Xia, J. P. (2019). Proposition, development and application of the integrated microbiome agent. Sci. Agric. Sin. 52, 2450–2467. doi: 10.3864/j.issn.0578-1752.2019.14.006
Liu, H., Li, S., Qiang, R., Lu, E., Li, C., Zhang, J., et al. (2022). Response of soil microbial community structure to phosphate fertilizer reduction and combinations of microbial fertilizer. Front. Environ. Sci. 10:899727. doi: 10.3389/fenvs.2022.899727
Liu, J., Shu, A., Song, W., Shi, W., Li, M., Zhang, W., et al. (2021). Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 404:115287. doi: 10.1016/j.geoderma.2021.115287
Ludwig, B., Geisseler, D., Michel, K., Joergensen, R. G., Schulz, E., Merbach, I., et al. (2011). Effects of fertilization and soil management on crop yields and carbon stabilization in soils. A review. Agron. Sustainable Dev. 31, 361–372. doi: 10.1051/agro/2010030
Mącik, M., Gryta, A., Sas-Paszt, L., and Frąc, M. (2022). Composition, activity and diversity of bacterial and fungal communities responses to inputs of phosphorus fertilizer enriched with beneficial microbes in degraded Brunic Arenosol. Land Degradation Dev. 33, 844–865. doi: 10.1002/ldr.4179
McLaughlin, D., and Kinzelbach, W. (2015). Food security and sustainable resource management. Water Resour. Res. 51, 4966–4985. doi: 10.1002/2015WR017053
Prasad, S., Malav, L. C., Choudhary, J., Kannojiya, S., Kundu, M., Kumar, S., et al. (2021). “Soil microbiomes for healthy nutrient recycling,” in Current trends in microbial biotechnology for sustainable agriculture. environmental and microbial biotechnology, eds A. N. Yadav, J. Singh, C. Singh, and N. Yadav (Singapore: Springer), doi: 10.1007/978-981-15-6949-4_1
Richardson, A. E. (2001). Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plant Biol. 28, 897–906. doi: 10.1071/PP01093
Schmidt, J. E., Kent, A. D., Brisson, V. L., and Gaudin, A. C. (2019). Agricultural management and plant selection interactively affect rhizosphere microbial community structure and nitrogen cycling. Microbiome 7, 1–18. doi: 10.1186/s40168-019-0756-9
Sofo, A., Zanella, A., and Ponge, J. F. (2022). Soil quality and fertility in sustainable agriculture, with a contribution to the biological classification of agricultural soils. Soil Use Manag. 38, 1085–1112. doi: 10.1111/sum.12702
Soman, C., Li, D., Wander, M. M., and Kent, A. D. (2017). Long-term fertilizer and crop-rotation treatments differentially affect soil bacterial community structure. Plant Soil 413, 145–159. doi: 10.1007/s11104-016-3083-y
Song, W., Zhang, H., Zhao, R., Wu, K., Li, X., Niu, B., et al. (2022). Study on cultivated land quality evaluation from the perspective of farmland ecosystems. Ecol. Indicators 139:108959. doi: 10.1016/j.ecolind.2022.108959
Sun, D., Meng, J., Liang, H., Yang, E., Huang, Y., Chen, W., et al. (2015). Effect of volatile organic compounds absorbed to fresh biochar on survival of Bacillus mucilaginosus and structure of soil microbial communities. J. Soils Sediments 15, 271–281. doi: 10.1007/s11368-014-0996-z
Wang, J., Li, R., Zhang, H., Wei, G., and Li, Z. (2020). Beneficial bacteria activate nutrients and promote wheat growth under conditions of reduced fertilizer application. BMC Microbiol. 20:38. doi: 10.1186/s12866-020-1708-z
Wang, X. (2022). Managing land carrying capacity: Key to achieving sustainable production systems for food security. Land 11:484. doi: 10.3390/land11040484
Wilke, B.-M. (2005). Determination of chemical and physical soil properties. Monitoring and assessing soil bioremediation. Berlin: Springer Berlin Heidelberg, 47–95.
Withers, P. J., Hodgkinson, R. A., Rollett, A., Dyer, C., Dils, R., Collins, A. L., et al. (2017). Reducing soil phosphorus fertility brings potential long-term environmental gains: A UK analysis. Environ. Res. Lett. 12:063001. doi: 10.1088/1748-9326/aa69fc
Xue, X., Liao, J., Hsing, Y., Huang, C., and Liu, F. (2015). Policies, land use, and water resource management in an arid oasis ecosystem. Environ. Manag. 55, 1036–1051. doi: 10.1007/s00267-015-0451-y
Keywords: phosphorus reduction, biofertilizers, soil available nutrients, microbial diversity, carbon-nitrogen cycling, sustainable agriculture
Citation: Zhang YL, Xiao R, Zhao YC, Li T, Cheng HY and Zhang HJ (2025) Impact of phosphorus reduction combined with biofertilizer application on soil nutrients and microbial communities in arid oasis agricultural areas. Front. Microbiol. 16:1606813. doi: 10.3389/fmicb.2025.1606813
Received: 07 April 2025; Accepted: 03 September 2025;
Published: 03 October 2025;
Corrected: 27 October 2025.
Edited by:
Jincai Ma, Jilin University, ChinaReviewed by:
Sumera Yasmin, National Institute for Biotechnology and Genetic Engineering, PakistanArup Sen, Bidhan Chandra Krishi Viswavidyalaya, India
Copyright © 2025 Zhang, Xiao, Zhao, Li, Cheng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rang Xiao, eGlhb3Jhbmc5OTlAMTYzLmNvbQ==
 Yong Ling Zhang1,2
Yong Ling Zhang1,2 Rang Xiao
Rang Xiao