- State Key Laboratory of Plateau Ecology and Agriculture, Academy of Animal Husbandry and Veterinary Sciences, Qinghai University, Xining, China
Introduction: On degraded grasslands, rest-grazing and fertilization measures have been widely applied. In alpine grasslands, numerous studies have examined the impact of rest-grazing and fertilizer application on microbial communities. However, the impact of these measures on the microbial community in Carex tibetikobresia meadows remains largely understudied. Furthermore, the relationship between aboveground vegetation and soil components under these treatments warrants further investigation.
Methods: We conducted a field control experiment in Dawu Town, Maqin County, China, during the winter–spring pasture regreen-up period. The primary treatment consisted of five rest-grazing durations, while the secondary treatment involved nitrogen addition.
Results and discussion: The results indicated that, under rest-grazing treatment, the levels of soil nitrogen can improve and ammonium nitrogen (NH₄+-N) was the primary environmental factor affecting microbial biomass. It showed a significantly negatively correlated with bacteria and gram-negative bacteria (G−), but a positive correlation with the ratio of gram-positive bacteria to gram-negative bacteria (G+:G−). Furthermore, without fertilization treatment, the ratio of fungi to bacteria (F:B) and G+:G− reached a maximum at rest-grazing for 30 days. In contrast, under fertilization treatment, microbial biomass carbon (MBC) became the dominant environmental factor affecting microbial biomass. It was negatively correlated with G−, but positively correlated with the ratio of F:B and G+:G−. Rest-grazing increases soil inorganic nitrogen and promotes actinomycetes growth, providing a viable strategy for restoring inorganic nitrogen levels in degraded grasslands. On the other hand, fertilization reduced the biomass of total phospholipid fatty acids (PLFAs) and all PLFAs groups. Consequently, the recommendation is that fertilization measures should not be utilized on this grassland and that a 30-day rest-grazing durations is preferable. Additionally, we observed inconsistent responses of microbial communities in the Carex tibetikobresia meadow and alpine meadows to rest-grazing and fertilization. These findings offer valuable insights into how fertilization modifies microbial responses to rest-grazing, providing important guidelines for the management of degraded Carex tibetikobresia meadows.
1 Introduction
The Carex tibetikobresia meadow is a significant vegetation type in the three-river source region, serving as an excellent grazing area and an important site for water conservation (Yu et al., 2010). Recently, this meadow ecosystem has experienced significant degradation due to climate warming and unsustainable anthropogenic disturbances, particularly intensive overgrazing, resulting in substantial declines in vegetation productivity and accelerated soil erosion rates (Li et al., 2019). Grassland degradation reduces vegetation cover and diversity, reducing soil nitrogen levels (Teng et al., 2020). These changes profoundly impact soil microbial biomass and community structure (Zhang et al., 2023). Soil nitrogen levels influence the composition of microbial communities and the capacity of microorganisms to acquire carbon (Yang A. et al., 2024). Therefore, studying soil nitrogen levels is crucial for grassland restoration (Yang S. et al., 2024). The health and function of grasslands are closely related to soil quality, and implementing better grazing management systems can support the sustainable development of the grassland ecosystem (Feng et al., 2024). However, the dearth of research on the Carex tibetikobresia meadows in this region is evident.
Soil microorganisms play a vital role in nutrient cycling and utilization, maintaining the function and stability of the grassland ecosystem, which is a crucial component of this environment (Bilen and Turan, 2022). Moreover, soil microorganisms influence plant growth (Zhang et al., 2019). This may be because rest-grazing can reduce vegetative root biomass while increasing soil fungal biomass (Li et al., 2022). The analysis of phospholipid fatty acids (PLFAs) is considered an accurate measure of viable biomass in soil microbial communities, making PLFAs measurements useful indicators for evaluating the effectiveness of vegetation restoration (Hu et al., 2020). Zhao et al. (2017) demonstrated that grazing significantly reduced total microbial, bacterial, and fungal communities, indicating that soil microbial community sizes were significantly influenced by grazing intensity. Consequently, microbial indicators serve as essential biomarkers for evaluating grassland restoration success. Nevertheless, there is a paucity of research on the effects of rest duration on microorganisms.
Rest-grazing is widely recognized as a nature-based restoration method for rehabilitating degraded grasslands throughout the three-river source region to promote the healthy development of grasslands (He et al., 2020). While most studies to date have focused on rest-grazing, rotational grazing, and fenced enclosures, the optimal duration of rest-grazing during the regreen-up period remains unclear (Venter et al., 2019). Fertilization is an effective management strategy for degraded grasslands, improving the stability of vegetation communities (Sheng et al., 2022). However, there are few reports on the combined effects of rest-grazing and fertilization in degraded grasslands (Zong et al., 2021). To address this gap, we conducted experiments on rest-grazing duration and fertilization in alpine meadows during the early growth stage and obtained relevant results (Zhou et al., 2023). Nevertheless, since Carex tibetikobresia meadows possess higher soil nutrient levels than alpine meadows, soil microorganisms may respond differently. In recent years, research on soil microorganisms has primarily focused on various restoration strategies, with limited studies addressing the effects of rest-grazing duration and fertilization on Carex tibetikobresia meadows in the three-river source region (Sadeghi et al., 2023). Considering these ecological impacts, the optimization of grazing regimes and fertilization protocols is essential to facilitate vegetation recovery and improve soil health in degraded grasslands.
To investigate how rest-grazing duration and fertilizer application treatments impact soil microbial communities in Carex tibetikobresia meadows, we formulated the following hypotheses: (1) Rest-grazing can improve the levels of soil nitrogen; (2) After applying nitrogen fertilizer, the ability of microorganisms to acquire carbon will be enhanced; (3) The responses of soil microorganisms in Carex tibetikobresia meadows and alpine meadows to rest-grazing and fertilizer application will differ. To test these hypotheses, we employed PLFAs analysis to evaluate the changes in biomass of each group of soil microorganisms. We identified the primary drivers’ factors influencing these variations under varying rest-grazing durations and fertilization treatments.
2 Materials and methods
2.1 Study area
This research was conducted in the winter–spring pasture of Dawu Town, Guoluo Tibetan Autonomous Prefecture, Qinghai Province, China (34°27′52″N, 100°13′19″E), at an altitude of 3,728 m. This area belongs to the alpine climate of the Qinghai-Tibet Plateau (Mao et al., 2021). The study area exhibits the climate with an average annual temperature of −3.9°C (summer: 9.7°C, winter: −12.6°C), annual precipitation ranging from 513.2 to 542.9 mm predominantly concentrated in June–September, and a permanent frost presence despite having approximately 156 frost-free days supporting vegetation growth (Li et al., 2020). The grassland type is classified as Kobresia tibetica swamp meadow, dominated by Kobresia tibetica, with coexisting species including Blysmus sinocompressus, Carex moorcroftii, Kobresia pygmaea, Deschampsia caespitosa, Elymus nutans, Potentilla anserina, and Trollius pumilus (Tian et al., 2021).
2.2 Experimental design
The primary utilization mode of the Carex tibetikobresia meadow is for the grazing of yaks, where herders have developed various seasonal grazing regimes, including winter–spring, summer, and autumn. In this study, we focused exclusively on the winter–spring grazing regime. Over the past several years, the experimental site has been grazing in winter–spring period. In order to reduce the spatial heterogeneity, the objective was to select grassland with the same stand conditions when choosing sample plots for the experiment. Grazing exclusion through mesh fencing has been implemented to mitigate grassland degradation in the Carex tibetikobresia meadow (Zhao et al., 2016b). In order to evaluate the effects of rest-grazing and fertilizer applicationon the Carex tibetikobresia meadow, we established a primary treatment of five rest-grazing durations and a secondary treatment of fertilization within each primary treatment. The five rest-grazing durations included: (1) rest-grazing for 20 days (20d), (2) rest-grazing for 30 days (30d), (3) rest-grazing for 40 days (40d), (4) rest-grazing for 50 days (50d), and (5) continuous grazing throughout the winter–spring period, serving as the control check group (CK). The grazing intensity in this study was based on the actual grazing intensity (moderate and light grazing) of the herders, which ranged from approximately 0.89 to 1.45 cattle per hectare.
2.3 Treatment applications
On 20 June 2017, a one-time application of approximately 300 kg·ha−1 of nitrogen fertilizer (urea N content was 46%) was made, based on the findings of the study conducted on degraded grassland under consistent conditions (Zong et al., 2014). In the present study, three independent repetitive blocks (A, B, and C) were established as replicates for each treatment, with five plots (CK, 20d, 30d, 40d, and 50d) of 600 m2 (30 m × 20 m) randomly set up within each block. In each plot subjected to the rest-grazing treatments, we set up fertilization treatment as a subplot (15 m × 10 m), which is located in the northeast corner of each plot (Figure 1). A total of 15 experimental plots, with each plot divided into two subplots, resulting in a total of 30 subplots (3 replicate blocks × 5 plots × 2 subplots). Plant sampling involved clipping grass at ground level, sorting by species, and bagging the samples (Wu et al., 2023). Three plant quadrats were established within each subplot to minimize spatial heterogeneity, resulting in 90 plant samples (3 vegetation quadrats × 30 subplots). Soil sampling was conducted by obtaining three randomly distributed samples from each subplot, resulting in 90 individual specimens for laboratory processing. After measuring the plant and soil samples, data for each subplot were expressed as the mean of three samples.
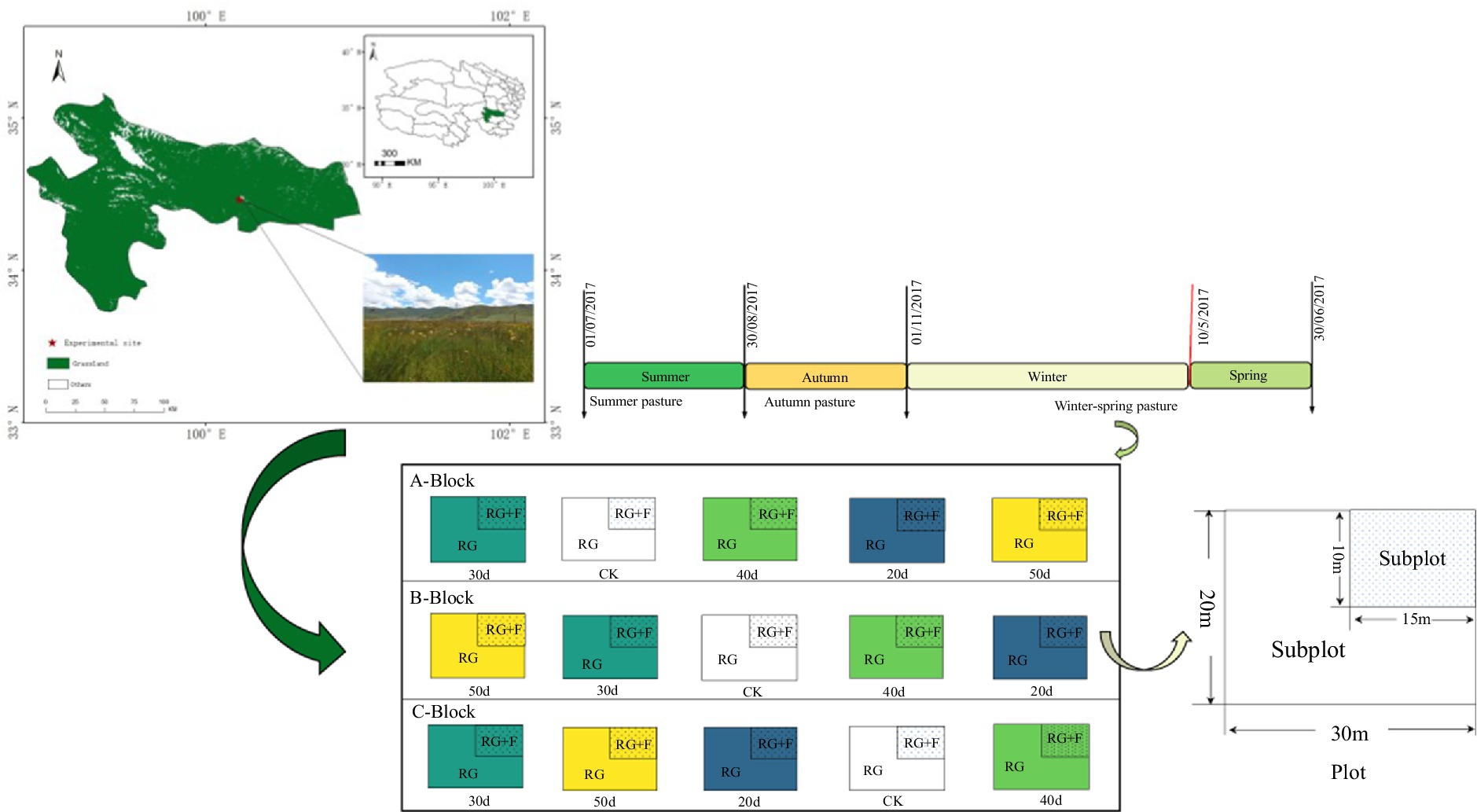
Figure 1. Layout of the field control experiment. Winter–spring pasture was grazing in winter and spring but rest-grazing in summer and autumn. A-Block, B-Block and C-Block: three independent replicate blocks; CK, control check group; 20d, rest-grazing from June10th to June 30th; 30d, rest-grazing from May 30th to June 30th; 40d, rest-grazing from May 20th to June 30th; 50d, rest-grazing May 10th to June 30th; RG, rest-grazing treatment; RG + F, rest-grazing with fertilization treatment; there were 2 subplots in each plot and there were 5 plots in each block.
2.4 Plant investigation and analysis
2.4.1 Plant quadrat surveys
August 2017, we assessed the coverage, height, and aboveground biomass of all species in each vegetation quadrat. The calculation of the aboveground biomass (g·m−2) was based on the dry weight of the plant samples. The above-ground parts of the plants were collected, and subjected to a drying process at 65°C for a period of 48 h, following which they were analyzed.
2.4.2 Characteristics analysis of vegetation community
The Shannon-Wiener index (H), Simpson index (D), and Pielou evenness index (J) were calculated using plant importance values (Pi):
Shannon-Weiner index (H) = −∑Pi * ln Pi.
Simpson index (D) = 1 − ∑Pi2.
Pielou index (J) = H/ln S.
Where Pi represents the important value of each species, and S represents the number of species in the plant quadrat.
2.5 Soil sampling and analysis
2.5.1 Collection and storage of soil
Soil samples were collected and processed following a structured protocol. A soil sample is constituted by the combination of five soil cores extracted at a depth of 0 to 15 cm, using a soil auger with an inner diameter of 3.5 cm. Each sample was sieved through a 2 mm mesh and divided into three parts upon returning to the laboratory (Joshi and Garkoti, 2023). One portion of the fresh soil was stored at 4°C for microbial biomass analysis (carbon and nitrogen), inorganic nitrogen (NH₄+-N and NO3−-N), and available phosphorus (AP). The second portion was air-dried indoors to determine soil physicochemical properties (Zhao et al., 2018). The third portion was stored in a refrigerator at −80°C for PLFAs analysis.
2.5.2 Determination of soil characteristics
Soil factors included soil moisture (SM), the potential of hydrogen (pH), soil organic carbon (SOC), total nitrogen (TN), ammonia nitrogen and nitrate nitrogen (NH₄+-N and NO3−-N), total phosphorus (TP), AP, total potassium (TK), and microbial biomass carbon and nitrogen (MBC, MBN). SM (%) was determined by the drying method. Soil pH was determined in a 1:5 soil-to-water suspension using a pH meter (METTER TOLEDO) (Su et al., 2005). SOC (g·kg−1) was quantified using potassium dichromate (K₂Cr₂O₇) oxidation followed by ferrous sulfate (FeSO₄) titration. TN (g·kg−1) was determined by means of the Kjeldahl method. NH₄+-N (g·kg−1) and NO3−-N (g·kg−1) were determined by potassium chloride leaching method. TP (g·kg−1) was determined by the molybdenum antimony colorimetric method and AP (g·kg−1) by the sodium bicarbonate extraction-molybdenum-antimony anti-colorimetric method. TK was assessed using a flame photometer (Huang et al., 2014). Finally, MBC (g·kg−1) and MBN (g·kg−1) were determined using chloroform fumigation extraction (Brookes et al., 1985).
2.6 Determination of phospholipid fatty acids
Microbial PLFAs were analyzed based on the method described by Bossio and Scow to elucidate the effects of rest-grazing and fertilization on the structure of the soil microbial community in the Carex tibetikobresia meadow (Bossio and Scow, 1998). Eight grams of lyophilised soil sample were used for the purposes of this analysis. The specific steps of the experiment were adapted from Zhou et al. (2023). The PLFA analysis method is based on phospholipids, which are an important component of microbial cell membranes (White et al., 1979). The classification is based on the observation that gram-positive bacteria (G+) have a thick peptidoglycan layer dominated by branched-chain saturated fatty acids, whereas gram-negative bacteria (G−) have thin cell membranes dominated by monounsaturated fatty acids (Ji et al., 2020). Fungal cell membranes have been found to contain complex lipids, such as ergocalciferol, and are dominated by long-chain polyunsaturated fatty acids (Ji et al., 2020). The fatty acid synthesis pathway in Actinomycetes (Act) adds a methyl group to the fatty acids’10th carbon (Ji et al., 2020). The following classification has been proposed for this. Arbuscular mycorrhizal fungi (AMF) were identified by the presence of the fatty acid 16:1ω5c (Bossio and Scow, 1998). Act were represented by the fatty acids 10Me-16:0, 10Me-17:0, and 10Me-18:0 (Kroppenstedt, 1985). Fungi (F) were characterized by 18:2ω6, 9c and 18:1ω9c (Frostegard et al., 1993). The values of i14:0, i15:0, a15:0, i16:0, a17:0, and i17:0 were used to represent G+, while 16:1ω7c, cy-17:0, 18:1ω5c, 18:1ω7c, and cy-19:0 were used to represent G− (Zhang et al., 2022). Bacteria (B) were characterized by the following fatty acids: 14:0, i14:0, 15:0, i15:0, a15:0, 16:0, i16:0, 16:1ω7c, 17:0, a17:0, i17:0, cy-17:0, 18:0, 18:1ω5c, 18:1ω7c, and cy-19:0 (White et al., 1979). Additionally, the ratio of fungi to bacteria (F:B) and the ratio of gram-positive bacteria to gram-negative bacteria (G+:G−) were calculated (Sadeghi et al., 2023). Bacterial stress indicators (cy:pre) were also calculated as the ratio of (cy-17:0 + cy-19:0) to (16:1ω7c + 18:1ω7c) (Fierer et al., 2003). The total PLFAs was calculated by summing the PLFAs in each group.
2.7 Statistical analysis
Initially, raw data were summarized and collated using MS Excel and data were tested and analyzed using Statistical Package for Social Sciences 25.0. Comparisons between the five rest-grazing durations were made using one-way ANOVA and Least Significant Difference Tests, and independent samples t-tests were used to test for differences between fertilization and non-fertilization treatments. Differences between microbial communities in the rest-grazing were tested by Nonmetric multidimensional scaling (NMDS), and linear regression was used to test the relationship between microbial community composition and plant and soil properties. Finally, redundancy analysis (RDA) was used to screen the effects of environmental factors on microbial communities. Scatter charts and histograms were generated using Origin 2021 and RDA analysis was performed using CANOCO 5.0. In all tests, significance levels were set at p < 0.05 or p < 0.01.
3 Results
3.1 Responses of plant and soil properties to rest-grazing duration and fertilization
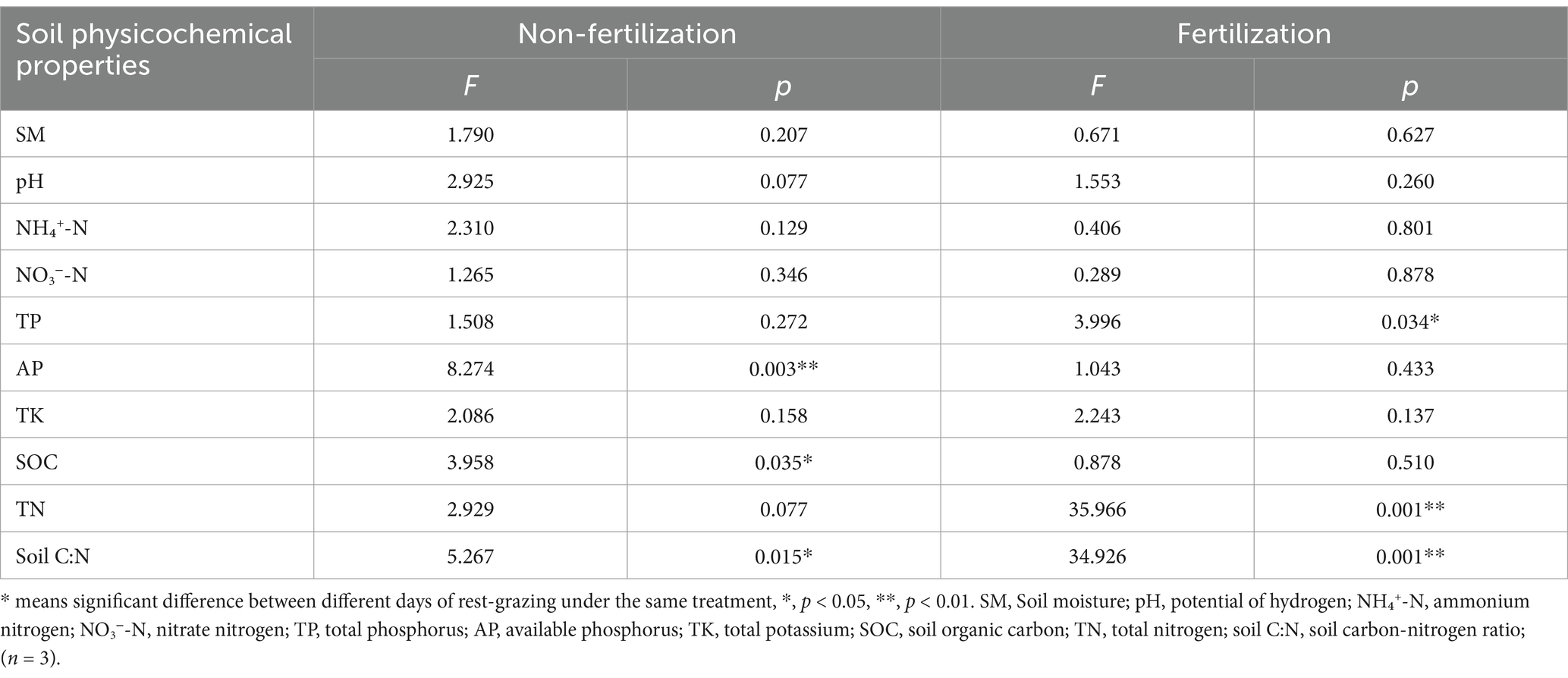
Implementation of rest-grazing and fertilization treatments induced significant modifications in both plant community traits and soil physicochemical properties (Figure 2; Table 1). Rest-grazing alone did not significantly impact the aboveground vegetation characteristics; however, fertilization under rest-grazing conditions significantly modified the aboveground biomass and Shannon-Wiener index (Figure 2). AP and SOC were significantly influenced by rest-grazing measures (p = 0.003, p = 0.015) (Table 1). In contrast, fertilization significantly affected soil C:N ratio TP, TN, and the (p = 0.001, p = 0.034 and p = 0.001) (Table 1). Compared to non-fertilized conditions, fertilization significantly increased SOC, TN, and TK but decreased the soil C:N ratio, AP, and NO3−-N. When comparing non-fertilized and fertilized conditions, fertilization increased SOC, TN, and TK while decreasing the soil C:N ratio, AP and NO3−-N (Supplementary Table S1).
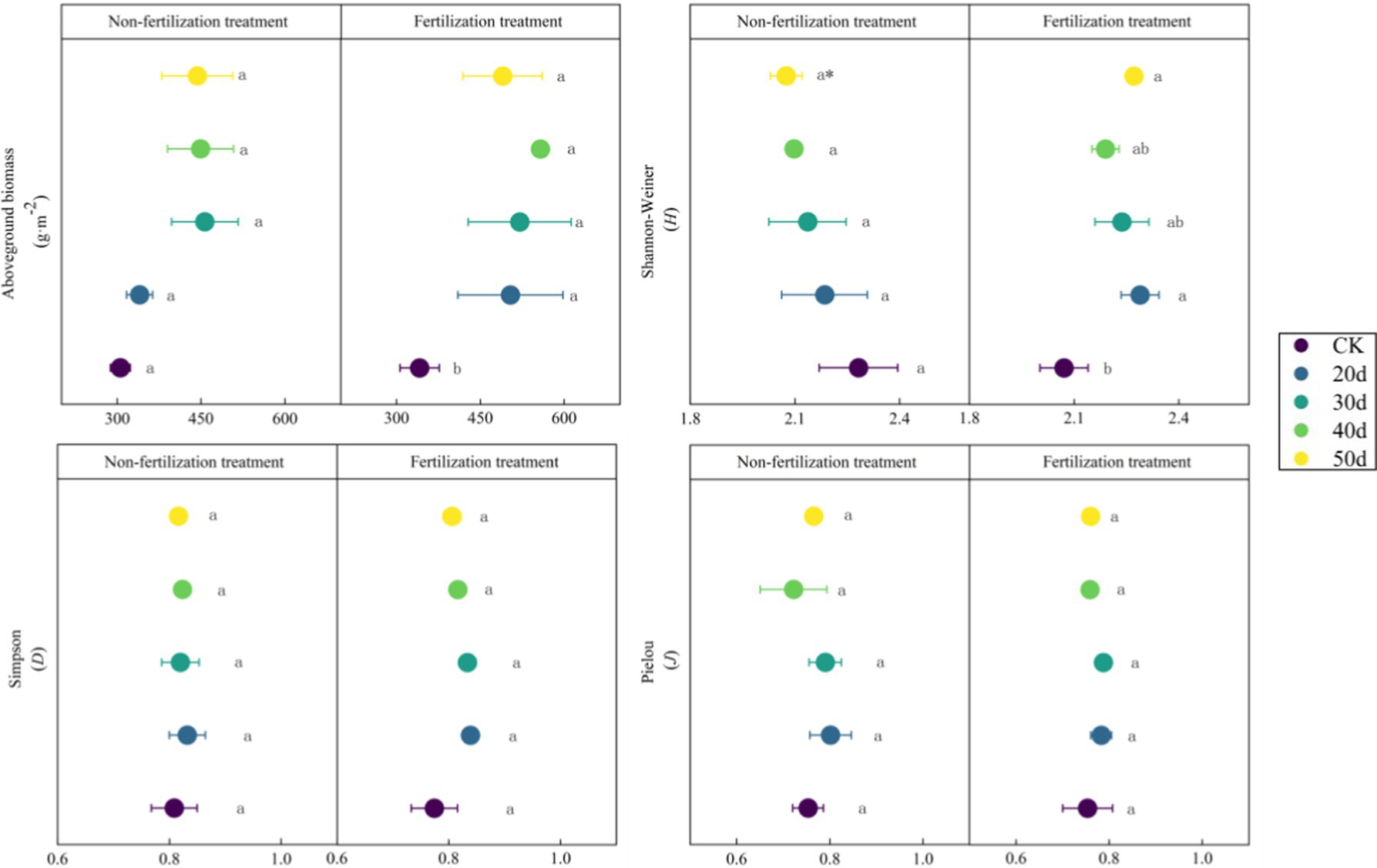
Figure 2. The biomass of aboveground vegetation and plant communities’ diversity index. These scatter diagrams show the biomass of aboveground vegetation and plant communities’ diversity index (Shannon-Weiner, Simpson, Pielou) under rest-grazing and fertilization treatments. Different lowercase letters in the same picture indicate significant differences between different days of rest-grazing under the same treatment; * means significant difference between non- fertilization and fertilization under the same number of rest-grazing days, *, p < 0.05. CK, control check group; 20d, rest-grazing from June10th to June 30th; 30d, rest-grazing from May 30th to June 30th; 40d, rest-grazing from May 20th to June 30th; 50d, rest-grazing May 10th to June 30th, (n = 3).
3.2 Responses of microbial community composition to rest-grazing duration and fertilization
The soil MBC, MBN, and the MBC:MBN ratio were altered following rest-grazing and fertilization (Figure 3). Without fertilization, there were significant differences between the CK and rest-grazing groups for three metrics: the MBC:MBN ratio, MBC and MBN. At this time, from 20d to 50d of rest-grazing, MBC showed an inverted V-shaped change and reached its highest point at 40d, while MBN showed a V-shaped change and reached its lowest point at 40d (Figure 3). However, with fertilization, from 20d to 50d of rest-grazing, MBC and MBN showed an inverted V-shaped variation, reaching a maximum on 30d, which was significantly higher than in the other groups (Figure 3). Under both non-fertilizer and fertilizer treatments, maximum PLFAs were observed after 50d. Notably, fertilization reduced the biomass of total PLFAs and all PLFAs groups. After 40d, AMF, Bacteria and Act, values of non-fertilization treatment were significantly higher than those of the fertilization treatment (p = 0.018, p = 0.001 and p = 0.019) (Figure 4). Without fertilization, from 20d to 50d of rest-grazing, G+ showed an inverted V-shaped variation, reaching a maximum on 30d, while G− showed a linear upward trend, reaching its maximum value after 50 days (Figure 4). However, with fertilization, from 20d to 50d of rest-grazing, G + showed an increasing and then decreasing trend, reaching a minimum at 40d. G- showed a V-shaped trend, also reaching a minimum at 40 d (Figure 4).
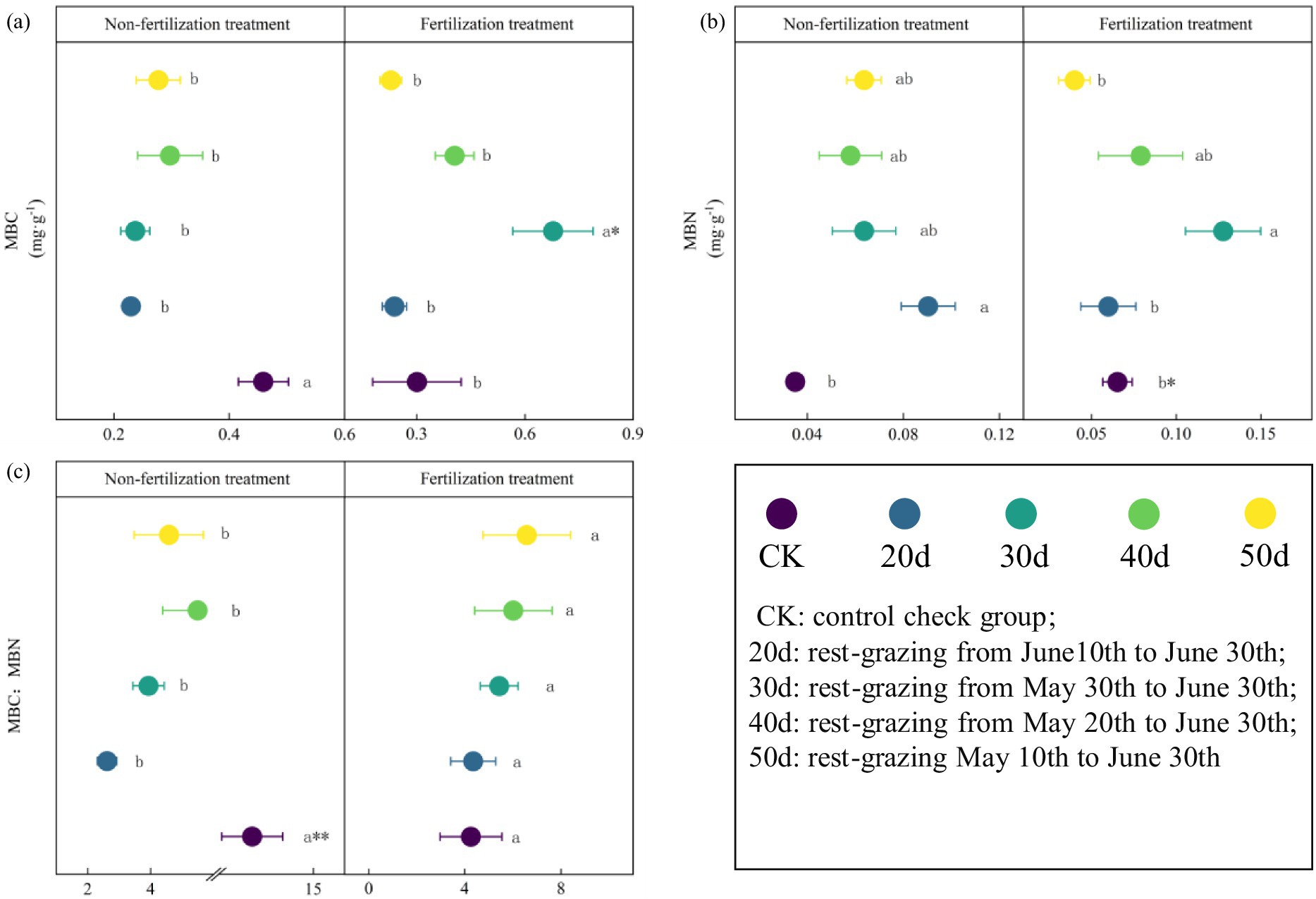
Figure 3. The biomass of soil microbial biomass carbon (MBC), nitrogen(N) and the ratio of MBC: MBN. These scatter diagrams show the biomass of soil microbial biomass carbon (MBC), soil microbial biomass nitrogen (MBN) and the ratio of MBC: MBN under rest-grazing and fertilization treatments. (a) The biomass of soil microbial biomass carbon; (b) the biomass of soil microbial biomass nitrogen; (c) the ratio of MBC: MBN. Different lowercase letters in the same picture indicate significant differences between different days of rest-grazing under the same treatment; * means significant difference between non- fertilization and fertilization under the same number of rest-grazing days, *, p < 0.05. CK: control check group; 20d: rest-grazing from June10th to June 30th; 30d: rest-grazing from May 30th to June 30th; 40d: rest-grazing from May 20th to June 30th; 50d: rest-grazing May 10th to June 30th, (n = 3).
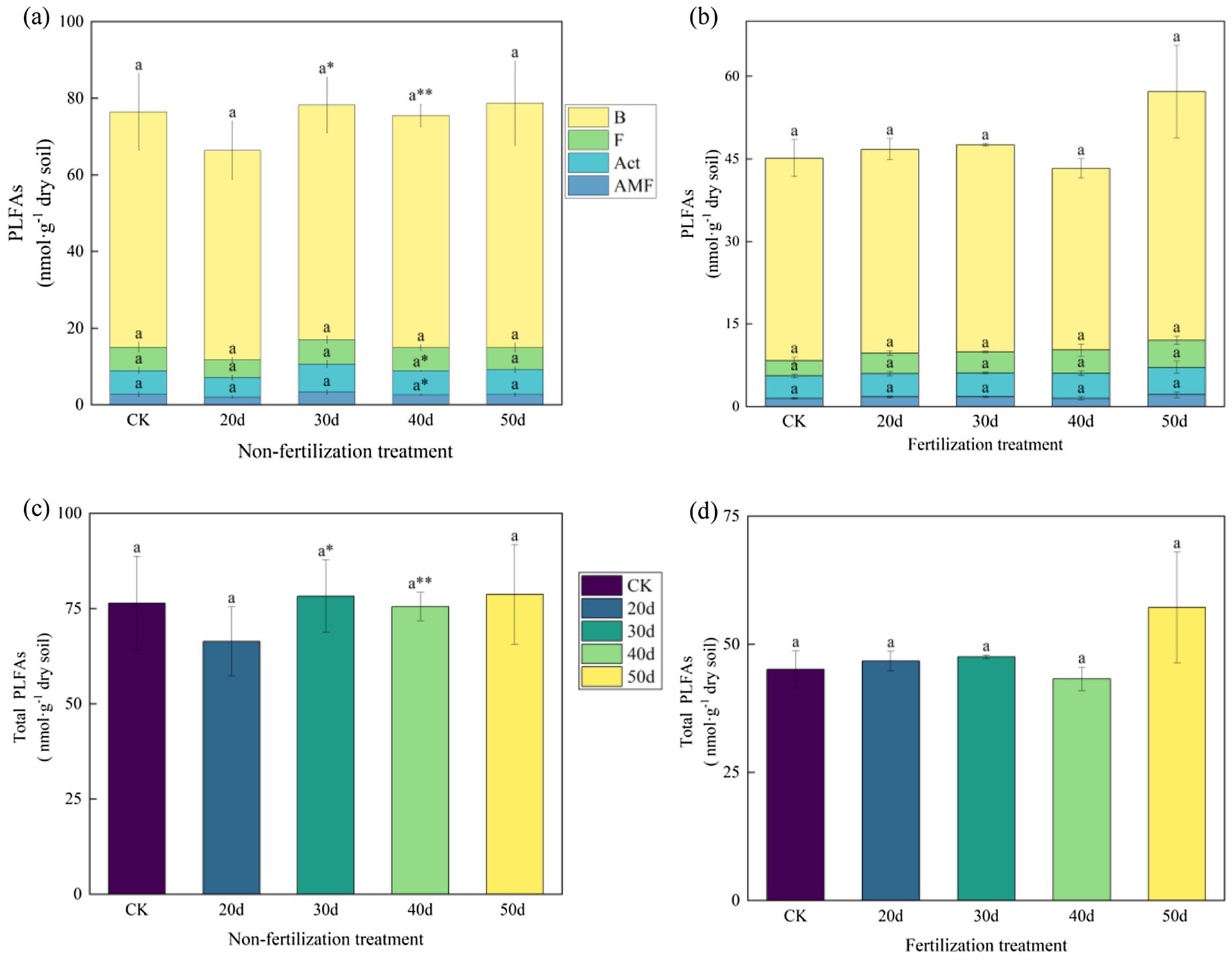
Figure 4. The content of phospholipid fatty acids in soil microorganisms. These histograms show the content of phospholipid fatty acids in soil microorganisms under rest-grazing and fertilization. (a) Contents of PLFAs groups under non-fertilization treatment; (b) contents of PLFAs groups under fertilization treatment; (c) contents of total PLFAs under non-fertilization treatment; (d) contents of total PLFAs under fertilization treatment. Different lowercase letters in the same picture indicate significant differences between different days of rest-grazing under the same treatment; * means significant difference between non- fertilization and fertilization under the same number of rest-grazing days, *, p < 0.05, **, p < 0.01. PLFAs: phospholipid fatty acids; B: total bacteria PLFAs; F: fungi PLFAs; Act: actinomyces PLFAs; AMF: arbuscular mycorrhizal fungi PLFAs; Total PLFAs: the sum of the PLFAs of each group of microorganisms. CK: control check group; 20d: rest-grazing from June10th to June 30th; 30d: rest-grazing from May 30th to June 30th; 40d: rest-grazing from May 20th to June 30th; 50d: rest-grazing May 10th to June 30th, (n = 3).
Furthermore, under rest-grazing measures, the mean of F:B ratios, G+:G−, and cy:pre ratios were highest after 30d. In contrast, with fertilization, these indicators peaked after 40d (Figure 5). NMDS results indicated significant differences in microbial community compositions across different rest-grazing durations. Specifically, under rest-grazing, NMDS2 was significantly higher at 30d than at 20d (p = 0.019) (Figure 6a). When considering fertilization under rest-grazing measures, NMDS2 was significantly higher at 50d than at CK (p = 0.024) (Figure 6b). A comparative analysis between non-fertilization and fertilization treatments revealed significantly greater differentiation in microbial community composition following fertilization (Figure 6c).
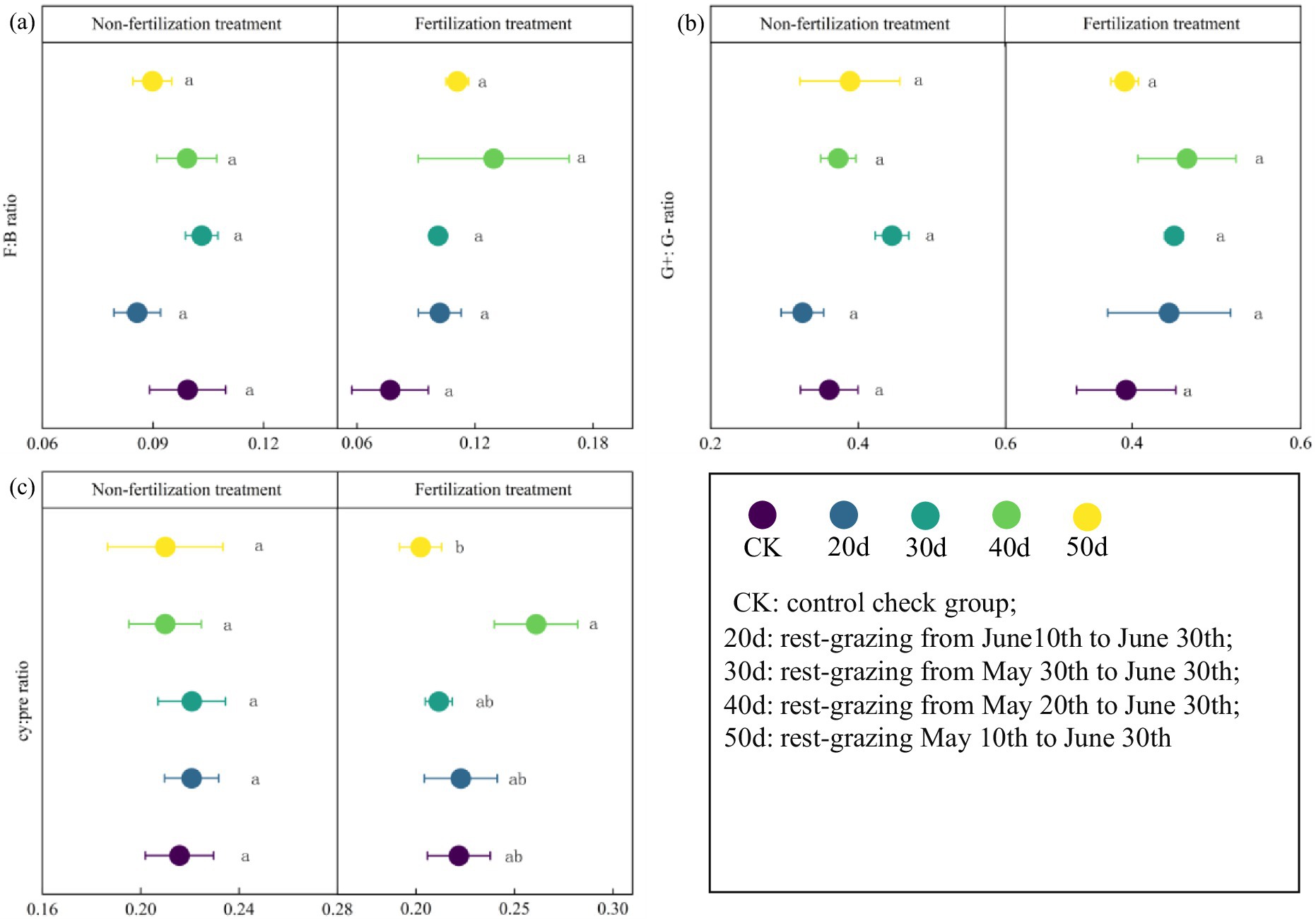
Figure 5. The stress indicators of phospholipid fatty acids in soil microorganisms. These scatter diagrams show the stress indicators of phospholipid fatty acids in soil microorganisms under rest-grazing and fertilization treatments. (a) The ratio of fungi to bacteria PLFAs; (b) the ratio of gram-positive to gram-negative bacteria PLFAs; (c) the ratio of (cy-17:0 + cy-19:0) to (16:1 ω7c + 18:1ω7c). Different lowercase letters in the same picture indicate significant differences between different days of rest-grazing under the same treatment. F: B ratio: the ratio of fungi to bacteria PLFAs; G+:G− ratio: the ratio of gram-positive to gram-negative bacteria PLFAs; cy: pre ratio: the ratio of (cy-17:0 + cy-19:0) to (16:1 ω7c + 18:1ω7c). CK: control check group; 20d: rest-grazing from June10th to June 30th; 30d: rest-grazing from May 30th to June 30th; 40d: rest-grazing from May 20th to June 30th; 50d: rest-grazing May 10th to June 30th, (n = 3).
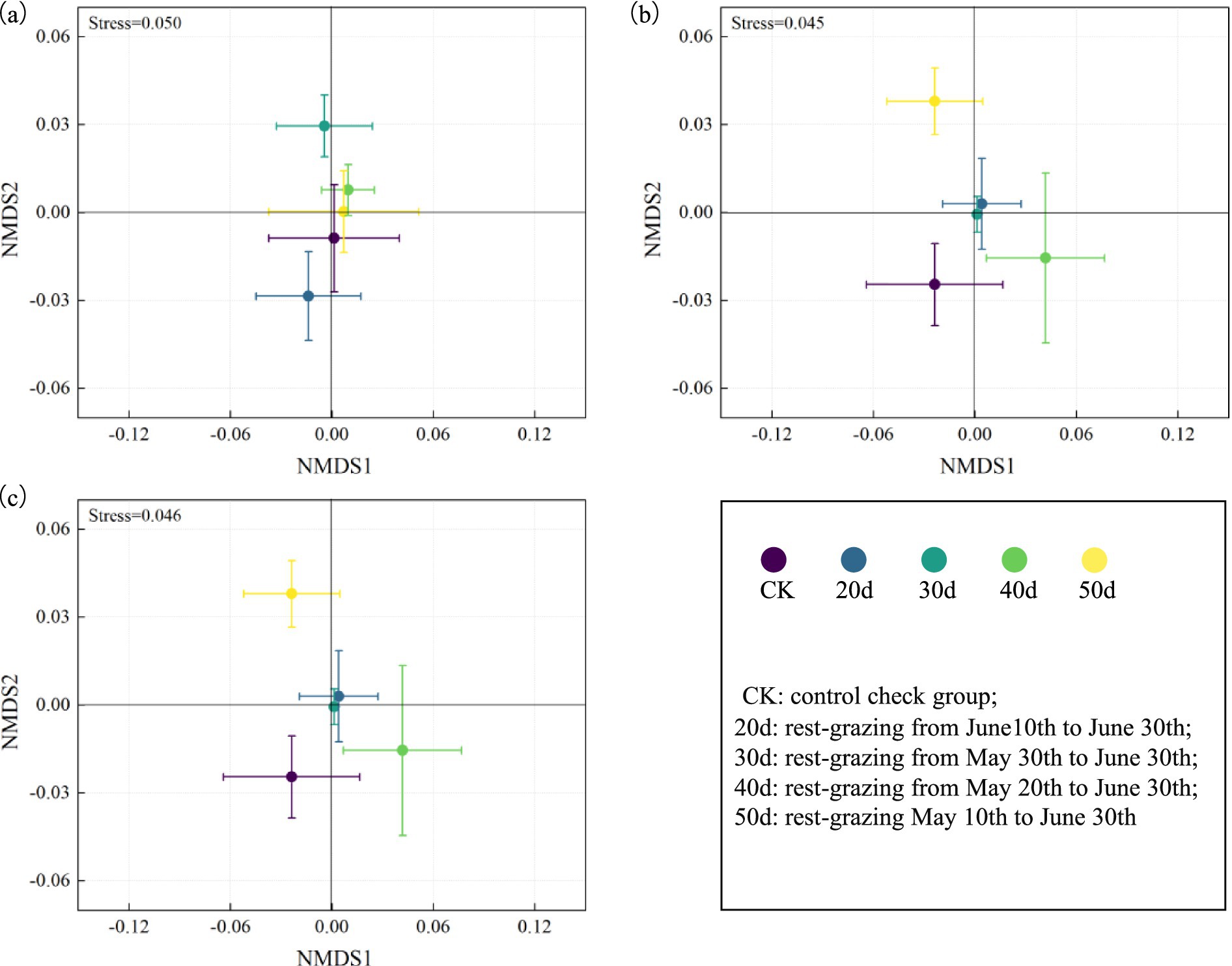
Figure 6. The microbial community compositions were analyzed by Nonmetric multidimensional scaling. These scatter diagrams show the microbial community compositions were analyzed by Nonmetric multidimensional scaling under rest-grazing and fertilization treatments. (a) Non-fertilization treatment; (b) fertilization treatment; (c) combined treatment of fertilization and non-fertilization. CK, control check group; 20d, rest-grazing from June10th to June 30th; 30d, rest-grazing from May 30th to June 30th; 40d, rest-grazing from May 20th to June 30th; 50d, rest-grazing May 10th to June 30th, (n = 3).
3.3 Soil and vegetation drivers of microbial community composition
Both edaphic properties and vegetative characteristics collectively drive structural reorganizations in microbial communities (Figure 7). RDA indicated that, under rest-grazing measures, the microbial community was primarily influenced by NH₄+-N, Simpson index (D), and SM. After fertilization, MBC, MBN, and SOC mainly influenced the microbial community. Specifically, under rest-grazing conditions, NH₄+-N accounted for 22.80% of the variation (p = 0.042) (Supplementary Table S2); B and G− decreased with increasing NH₄+-N, while G+:G− increased (Figures 8a–c). Following fertilization, MBC explained 18.10% of the variation (p = 0.028) (Supplementary Table S2), with F:B and G+:G− increasing alongside MBC, whereas G− decreased (Figures 8d–f).
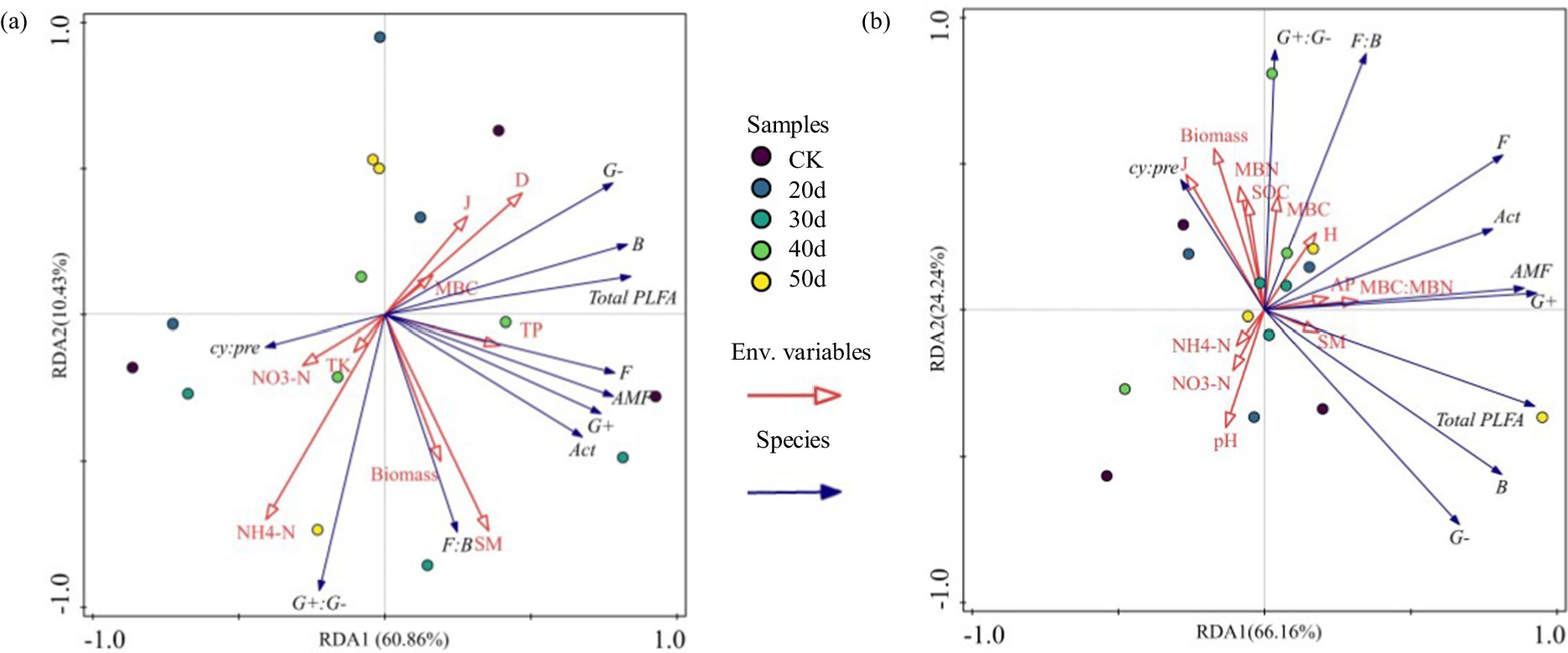
Figure 7. Redundancy analysis of soil physicochemical properties and environmental factors. (a) No fertilization treatment; (b) fertilization treatment; CK, control check group; 20d, rest-grazing from June10th to June 30th; 30d, rest-grazing from May 30th to June 30th; 40d, rest-grazing from May 20th to June 30th; 50d, rest-grazing May 10th to June 30th; Env. Variables, plant and soil properties namely environmental variables; Species, all PLFAs groups namely species variables; Act, actinomyces PLFAs; AMF, arbuscular mycorrhizal fungi PLFAs; B, total bacterial PLFAs; cy:pre, the ratio of (cy-17:0 + cy-19:0) to (16:1 ω7c + 18:1ω7c); F, fungal PLFAs; F:B, the ratio of fungal to bacterial PLFAs; G−, gram-negative bacterial PLFAs; G+, gram-positive bacterial PLFAs; G+:G−, the ratio of gram-positive to gram-negative bacterial PLFAs; Total PLFAs, the sum of the PLFAs of each group of microorganisms; D, Simpson index; SM, soil moisture; NH₄+-N, ammonium nitrogen; TP, total phosphorus; J, Pielou index; TK, total potassium; NO3—N, nitrate nitrogen; MBC, soil microbial biomass carbon; Biomass, the biomass of aboveground vegetation; H, Shannon-Weiner index; MBC:MBN, the ratio of soil microbial biomass carbon to soil microbial biomass nitrogen; AP, available phosphorus; pH, potential of hydrogen; MBN, soil microbial biomass nitrogen; SOC, soil organic carbon; (n = 3).
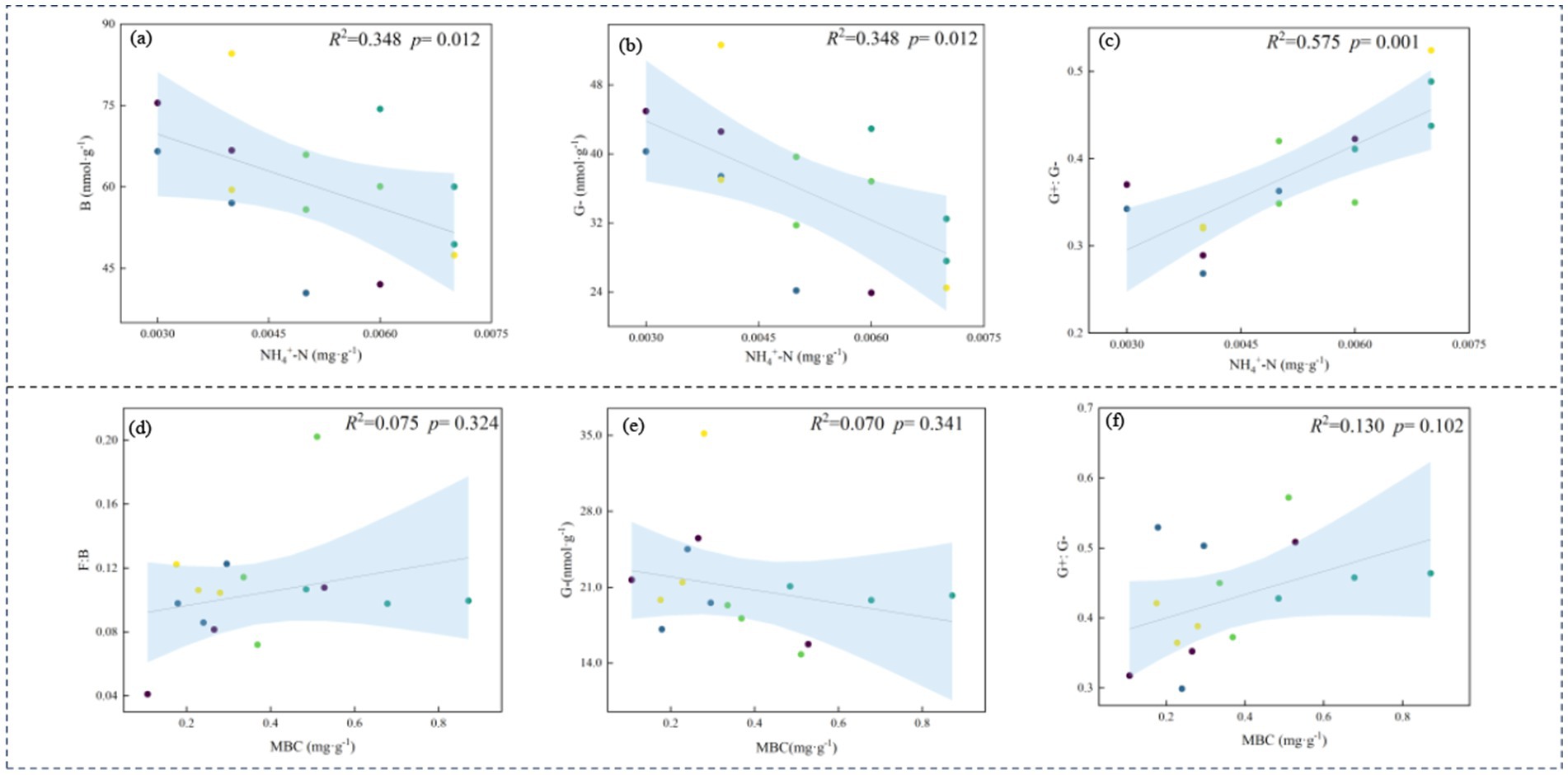
Figure 8. Relationships between the soil physicochemical properties and microbial properties. No fertilization treatment (a–c); fertilization treatment (d–f); NH₄+-N, ammonium nitrogen; MBC, soil microbial biomass carbon; B, total bacteria PLFAs; G−, gram-negative bacteria PLFAs; G+:G−, the ratio of gram-positive to gram-negative bacteria PLFAs; F:B, the ratio of fungi to bacteria PLFAs, (n = 3).
4 Discussion
4.1 Fertilization will change the effects of rest-grazing on plant and soil properties, and microorganisms
Rest-grazing and fertilization measures can benefit degraded grasslands and alter soil microbial communities (Clegg, 2006). This study uniquely examines how rest-grazing duration and fertilization regimes collectively structure soil microbial communities in the three-river source region. Our findings offer implementable solutions for rehabilitating degraded Carex tibetikobresia meadows and optimizing rotational grazing systems in fragile pastures.
Recent studies have shown that appropriate grazing pressure and nitrogen fertilizer application effectively enhance grassland productivity and maintain soil microbial community stability (Qi et al., 2023). According to results from NMDS, fertilization enhances the effects of rest-grazing duration on microbial communities (Figure 6). This may occur because fertilization disrupts the balance of the soil–plant system, thereby affecting the composition and abundance of microbial communities (Coonan et al., 2020). Additionally, we found that the fertilization treatment decreased the biomass of all PLFAs groups and total PLFAs (Figure 4). This finding is consistent with previous research indicating an average biomass decrease of 15% for the entire microbial community under nitrogen fertilization (Treseder, 2008). Possible explanations for this phenomenon include nitrogen application inhibiting the growth or ligninase activity of white rot fungi (Waldrop and Zak, 2006) or the production of brown, recalcitrant compounds induced by nitrogen application, leading to the accumulation of compounds toxic to microorganisms (Fog, 1988). This may also explain why the effect of NH₄+-N on microbial biomass after fertilization was insignificant. It is also possible that soil acidification subsequent to fertilization exerts an effect on microbial metabolic demand, which in turn leads to a decline in microbial biomass (Bardgett et al., 1999). While some studies indicate that nitrogen fertilizer increases microbial biomass, a few reports suggest a decrease in microbial biomass post-fertilization (Zheng et al., 2023). The differing ecosystem biomes and experimental durations may account for these inconsistencies (Jia et al., 2020).
However, in alpine meadows, fertilization under rest-grazing can promote the growth of nitrogen-rich plant species and increases aboveground vegetative litter, thereby enhancing microbial biomass (Zhou et al., 2023). This inconsistency may arise from differences in the fundamental conditions (soil texture, organic matter, moisture) and dominant species of the two grassland types (Li et al., 2016). The Carex tibetikobresia is the dominant species in swamping meadows, while Carex parvula predominates in alpine meadows (Zheng et al., 2000). The Carex tibetikobresia is a resource-harvesting plant characterized by a high specific root length, specific root area, and root nitrogen content, enabling rapid acquisition of water and nutrients (Laliberté, 2017). In contrast, Carex parvula is a resource-conservative plant with a low specific root length and high root tissue density, acquiring resources continuously with low metabolic intensity and long root life (Laliberté, 2017). Consequently, soil nutrients in the Carex tibetikobresia meadow are more abundant than in alpine meadows (Edwards et al., 2004), leading to a negative effect of fertilization treatment in the former. The implementation of fertilization and rest-grazing exhibited divergent impacts on plant community composition across distinct grassland ecosystems, a pattern corroborated by our experimental findings (Socher et al., 2013). Furthermore, research has demonstrated that the impact of rest-grazing on ecosystems is primarily influenced by the type of vegetation involved (Zhao et al., 2016a). Thus, the different responses of the two grasslands to rest-grazing and fertilization can be attributed to changes in vegetation type and effectiveness of soil properties.
4.2 The relationship between soil microbial community characteristics and potential drivers
Changes in soil microbial community composition occur along environmental gradients, primarily due to the characteristics of plants and soil (Dong et al., 2023). Therefore, we analyzed the correlation between environmental factors and microbial composition (Figures 7, 8). Our results indicate that, under rest-grazing measures, soil microbial community was significantly related to NH₄+-N, which may be attributed to several factors. First, NH₄+-N levels increased with longer rest-grazing duration, leading to a corresponding increase in the abundance of Act (Figure 4). This can be explained by the fact that rest-grazing promotes inorganic nitrogen availability by enhancing belowground primary productivity, thereby increasing organic matter input into the soil (Dong et al., 2020). NH₄+-N is incorporated in organic nitrogen, contributing to the long-term nutrient supply of the soil (Ren et al., 2024). Consequently, as inorganic nitrogen levels increase with rest-grazing, less NH4+-N is converted to nitrification, reducing nitrogen losses (Lv et al., 2024). This further suggests that rest-grazing enhances the retention of NH₄+-N. Second, NH₄+-N inhibited the growth of B and G− bacteria (Figure 8), thereby altering the soil microbial community structure (LeBauer and Treseder, 2008). Additionally, NH₄+-N significantly influenced the supply level of organic matter, as indicated by the G+:G− ratio (Figure 8). Recent research demonstrates that optimal nitrogen (N) inputs synergistically improve microbial community structure and functional dynamics via nutrient coupling effects, a finding that aligns with our experimental observations (Xue et al., 2025). This alteration in nutrient availability may impose stress on the microbial community, further contributing to changes in microbial composition (Chen et al., 2023).
Moreover, our research revealed that, the G+:G− ratio was higher in the fertilization treatment than in the non-fertilization treatment (Figure 5). This suggests that soil microorganisms experience poor nutrient supply under fertilization, which may be detrimental to the sustainable functioning of the entire ecosystem (Lopes and Fernandes, 2020). A possible explanation for this observation is that the MBC content increased following fertilization, promoting an increase in the G+:G− ratio (Figure 8). This finding is consistent with research indicating that increased nitrogen fertilizer enhances soil MBC (Bargali et al., 2018). This effect is primarily attributed to nitrogen fertilizer applications improving soil conditions, which allows microorganisms to utilize carbon sources more effectively (Tuo et al., 2023). Additionally, fertilizer application may increase soil enzyme activity, which has a significant positive correlation with MBC (Tuo et al., 2024). Recent research demonstrates that nitrogen fertilizer application alters soil biochemical processes, subsequently enhancing hydrolase enzymatic activity (Shi et al., 2025). Studies have demonstrated that urease, sucrase, and alkaline phosphatase activities positively correlate with MBC (Raiesi and Beheshti, 2015). Therefore, MBC is the predominant factor influencing microbial biomass following fertilization.
5 Conclusion
Our findings demonstrated that microbial communities were strongly associated with MBC and NH₄+-N. Compared to grazing, rest-grazing increased inorganic nitrogen levels, increasing Act and the ratio of gram-positive bacteria to gram-negative bacteria. Rest-grazing is an effective means of enhancing inorganic nitrogen availability in degraded grassland soils. Furthermore, without fertilization treatment, the ratio of fungi to bacteria and gram-positive bacteria to gram-negative bacteria reached a maximum at rest-grazing for 30 days. Fertilization mitigated the impact of NH₄+-N on microbial communities, with soil MBC emerging as a primary influencing factor. Fertilization reduced the biomass of total phospholipid fatty acids (PLFAs) and all PLFAs groups. Furthermore, microbial communities exhibited divergent responses to rest-grazing and fertilization between Carex tibetikobresia meadows and alpine meadows. This study assessed microbial community compositions, plant and soil properties, and their correlation with rest-grazing duration and fertilization. Given the above considerations, it is recommended that fertilization measures are not utilized on Carex tibetikobresia meadows, and that a 30-day rest-grazing duration is preferable. The results of the study highlight the importance of rest-grazing duration on microbial communities and provide a theoretical basis for grazing systems in degraded Carex tibetikobresia meadows.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XZ: Data curation, Investigation, Writing – original draft. XW: Conceptualization, Project administration, Writing – review & editing. YusM: Funding acquisition, Project administration, Writing – review & editing. YW: Investigation, Resources, Writing – review & editing. YuaM: Methodology, Resources, Writing – review & editing. LX: Investigation, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by National Natural Science Foundation of China (32230068 & 32260327) and the Chief Scientist Program of Qinghai Province (2024-SF-101).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1608011/full#supplementary-material
References
Bardgett, R. D., Lovell, R. D., Hobbs, P. J., and Jarvis, S. C. (1999). Seasonal changes in soil microbial communities along a fertility gradient of temperate grasslands. Soil Biol. Biochem. 31, 1021–1030. doi: 10.1016/S0038-0717(99)00016-4
Bargali, K., Manral, V., Padalia, K., Bargali, S., and Upadhyay, V. (2018). Effect of vegetation type and season on microbial biomass carbon in central Himalayan forest soils, India. CATENA 171, 125–135. doi: 10.1016/j.catena.2018.07.001
Bilen, S., and Turan, V. (2022). “Enzymatic analyses in soils” in Practical handbook on agricultural microbiology. eds. N. Amaresan, P. Patel, and D. Amin (New York, NY: Springer), 377–385.
Bossio, D., and Scow, K. (1998). Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 35, 265–278. doi: 10.1007/s002489900082
Brookes, P., Landman, A., Pruden, G., and Jenkinson, D. (1985). Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 17, 837–842. doi: 10.1016/0038-0717(85)90144-0
Chen, K., Huo, T., Zhang, Y., Guo, T., and Liang, J. (2023). Response of soil organic carbon decomposition to intensified water variability co-determined by the microbial community and aggregate changes in a temperate grassland soil of northern China. Soil Biol. Biochem. 176:108875. doi: 10.1016/j.soilbio.2022.108875
Clegg, C. D. (2006). Impact of cattle grazing and inorganic fertiliser additions to managed grasslands on the microbial community composition of soils. Appl. Soil Ecol. 31, 73–82. doi: 10.1016/j.apsoil.2005.04.003
Coonan, E. C., Kirkby, C. A., Kirkegaard, J. A., Amidy, M. R., Strong, C. L., and Richardson, A. E. (2020). Microorganisms and nutrient stoichiometry as mediators of soil organic matter dynamics. Nutr. Cycl. Agroecosyst. 117, 273–298. doi: 10.1007/s10705-020-10076-8
Dong, S., Li, Y., Ganjurjav, H., Gao, Q., Gao, X., Zhang, J., et al. (2020). Grazing promoted soil microbial functional genes for regulating C and N cycling in alpine meadow of the Qinghai-Tibetan plateau. Agric. Ecosyst. Environ. 303:107111. doi: 10.1016/j.agee.2020.107111
Dong, R., Wang, X., Wang, Y., Ma, Y., Yang, S., Zhang, L., et al. (2023). Differences in soil microbial communities with successional stage depend on vegetation coverage and soil substrates in alpine desert shrublands. Plant Soil 485, 549–568. doi: 10.1007/s11104-022-05849-9
Edwards, E. J., Benham, D. G., Marland, L. A., and Fitter, A. H. (2004). Root production is determined by radiation flux in a temperate grassland community. Glob. Change Biol. 10, 209–227. doi: 10.1111/j.1365-2486.2004.00729.x
Feng, B., Liu, Y., Liu, W., Lv, W., Sun, C., Yang, Z., et al. (2024). Soil physicochemical properties and plant functional traits regulate ecosystem multifunctionality of alpine grassland under different livestock grazing assemblies. Agric. Ecosyst. Environ. 366:108947. doi: 10.1016/j.agee.2024.108947
Fierer, N., Schimel, J. P., and Holden, P. A. (2003). Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 35, 167–176. doi: 10.1016/S0038-0717(02)00251-1
Fog, K. (1988). The effect of added nitrogen on the rate of decomposition of organic matter. Biol. Rev. 63, 433–462. doi: 10.1111/j.1469-185X.1988.tb00725.x
Frostegard, A., Baath, E., and Tunlio, A. (1993). Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 25, 723–730. doi: 10.1016/0038-0717(93)90113-P
He, J., Bu, H., Hu, X., Feng, Y., Li, S., Zhu, J., et al. (2020). Close-to-nature restoration of degraded alpine grasslands: theoretical basis and technical approach. Chin. Sci. Bull. 65, 3898–3908. doi: 10.1360/TB-2020-0405
Hu, P., Xiao, J., Zhang, W., Xiao, L., Yang, R., Xiao, D., et al. (2020). Response of soil microbial communities to natural and managed vegetation restoration in a subtropical karst region. CATENA 195:104849. doi: 10.1016/j.catena.2020.104849
Huang, X., Liu, S., Wang, H., Hu, Z., Li, Z., and You, Y. (2014). Changes of soil microbial biomass carbon and community composition through mixing nitrogen-fixing species with Eucalyptus urophylla in subtropical China. Soil Biol. Biochem. 73, 42–48. doi: 10.1016/j.soilbio.2014.01.021
Ji, L., Yang, Y., Yang, L., and Zhang, D. (2020). Effect of land uses on soil microbial community structures among different soil depths in northeastern China. Eur. J. Soil Biol. 99:103205. doi: 10.1016/j.ejsobi.2020.103205
Jia, X., Zhong, Y., Liu, J., Zhu, G., Shangguan, Z., and Yan, W. (2020). Effects of nitrogen enrichment on soil microbial characteristics: from biomass to enzyme activities. Geoderma 366:114256. doi: 10.1016/j.geoderma.2020.114256
Joshi, R. K., and Garkoti, S. C. (2023). Influence of vegetation types on soil physical and chemical properties, microbial biomass and stoichiometry in the central Himalaya. CATENA 222:106835. doi: 10.1016/j.catena.2022.106835
Kroppenstedt, R. (1985). “Fatty acid and menaquinone analysis of actinomycetes and related organisms” in Chemical methods in bacterial systematics, SAB Technical Series 20. eds. M. Goodfellow and D. Minnikin (London: Academic Press), 173–199.
Laliberté, E. (2017). Below-ground frontiers in trait-based plant ecology. New Phytol. 213, 1597–1603. doi: 10.1111/nph.14247
LeBauer, D., and Treseder, K. K. (2008). Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89, 371–379. doi: 10.1890/06-2057.1
Li, N., Chang, R., Jiang, H., Tariq, A., Sardans, J., Peñuelas, J., et al. (2022). Combined livestock grazing-exclusion and global warming decreases nitrogen mineralization by changing soil microbial community in a Tibetan alpine meadow. CATENA 219:106589. doi: 10.1016/j.catena.2022.106589
Li, Y., Li, J., Are, K. S., Huang, Z., Yu, H., and Zhang, Q. (2019). Livestock grazing significantly accelerates soil erosion more than climate change in Qinghai-Tibet plateau: evidenced from 137Cs and 210Pbex measurements. Agric. Ecosyst. Environ. 285:106643. doi: 10.1016/j.agee.2019.106643
Li, Q., Lu, H., Shen, C., Zhao, Y., and Ge, Q. (2016). Vegetation successions in response to Holocene climate changes in the central Tibetan plateau. J. Arid Environ. 125, 136–144. doi: 10.1016/j.jaridenv.2015.07.010
Li, F., Yang, G., Peng, Y., Wang, G., Qin, S., Song, Y., et al. (2020). Warming effects on methane fluxes differ between two alpine grasslands with contrasting soil water status. Agric. For. Meteorol. 290:107988. doi: 10.1016/j.agrformet.2020.107988
Lopes, L. D., and Fernandes, M. F. (2020). Changes in microbial community structure and physiological profile in a kaolinitic tropical soil under different conservation agricultural practices. Appl. Soil Ecol. 152:103545. doi: 10.1016/j.apsoil.2020.103545
Lv, P., Sun, S., Li, Y., Zhao, S., Zhang, J., Hu, Y., et al. (2024). Plant composition change mediates climate drought, nitrogen addition, and grazing effects on soil net nitrogen mineralization in a semi-arid grassland in North China. Sci. Total Environ. 908:168282. doi: 10.1016/j.scitotenv.2023.168282
Mao, Y., Huang, J., Cao, W., Fu, Y., Zhao, Z., and Bao, N. (2021). Visible-NIR spectral characterization and grade inversion modelling study of the Derni copper deposit. Infrared Phys. Technol. 115:103717. doi: 10.1016/j.infrared.2021.103717
Qi, L., Zhang, M., Yin, J., Ren, W., Sun, S., Chen, Z., et al. (2023). The interactive effect of grazing and fertilizer application on soil properties and bacterial community structures in a typical grassland in the Central Inner Mongolia plateau. Front. Ecol. Evol. 11:1174866. doi: 10.3389/fevo.2023.1174866
Raiesi, F., and Beheshti, A. (2015). Microbiological indicators of soil quality and degradation following conversion of native forests to continuous croplands. Ecol. Indic. 50, 173–185. doi: 10.1016/j.ecolind.2014.11.008
Ren, S., Huo, T., Wang, D., and Liang, J. (2024). Losses of native mineral-associated organic nitrogen through microbial mineralization and gaseous emissions induced by ammonium and nitrate addition. Soil Biol. Biochem. 193:109420. doi: 10.1016/j.soilbio.2024.109420
Sadeghi, S., Petermann, B. J., Steffan, J. J., Brevik, E. C., and Gedeon, C. (2023). Predicting microbial responses to changes in soil physical and chemical properties under different land management. Appl. Soil Ecol. 188:104878. doi: 10.1016/j.apsoil.2023.104878
Sheng, J., Zhou, M., Guo, Y., Yuan, Y., Li, X., Zhang, W.-H., et al. (2022). Aboveground productivity and community stability tend to keep stable under long-term fencing and nitrogen fertilization on restoration of degraded grassland. Ecol. Indic. 140:108971. doi: 10.1016/j.ecolind.2022.108971
Shi, J., Rahman, M. K. U., Ma, R., Li, Q., Huang, Y., and Li, G. (2025). Effects of nitrogen enrichment on soil enzyme activities in grassland ecosystems in China: a multilevel meta-analysis. Pedosphere 35, 84–96. doi: 10.1016/j.pedsph.2024.10.006
Socher, S. A., Prati, D., Boch, S., Müller, J., Baumbach, H., Gockel, S., et al. (2013). Interacting effects of fertilization, mowing and grazing on plant species diversity of 1500 grasslands in Germany differ between regions. Basic Appl. Ecol. 14, 126–136. doi: 10.1016/j.baae.2012.12.003
Su, Y., Li, Y., Cui, J., and Zhao, W. (2005). Influences of continuous grazing and livestock exclusion on soil properties in a degraded sandy grassland, Inner Mongolia, northern China. CATENA 59, 267–278. doi: 10.1016/j.catena.2004.09.001
Teng, Y., Zhan, J., Agyemang, F. B., and Sun, Y. (2020). The effects of degradation on alpine grassland resilience: a study based on meta-analysis data. Glob. Ecol. Conserv. 24:e01336. doi: 10.1016/j.gecco.2020.e01336
Tian, L., Bai, Y., Wang, W., Qu, G., Deng, Z., Li, R., et al. (2021). Warm-and cold-season grazing affect plant diversity and soil carbon and nitrogen sequestration differently in Tibetan alpine swamp meadows. Plant Soil 458, 151–164. doi: 10.1007/s11104-020-04573-6
Treseder, K. K. (2008). Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol. Lett. 11, 1111–1120. doi: 10.1111/j.1461-0248.2008.01230.x
Tuo, Y., Tan, H., Liang, J., Li, J., Xiang, P., Yang, Q., et al. (2024). Optimization of water and fertilizer management of Panax pseudoginseng based on changes in soil microbial biomass carbon and nitrogen and enzyme activities. Appl. Soil Ecol. 196:105282. doi: 10.1016/j.apsoil.2024.105282
Tuo, Y., Wang, Z., Zheng, Y., Shi, X., Liu, X., Ding, M., et al. (2023). Effect of water and fertilizer regulation on the soil microbial biomass carbon and nitrogen, enzyme activity, and saponin content of Panax notoginseng. Agric. Water Manag. 278:108145. doi: 10.1016/j.agwat.2023.108145
Venter, Z. S., Cramer, M. D., and Hawkins, H.-J. (2019). Rotational grazing management has little effect on remotely-sensed vegetation characteristics across farm fence-line contrasts. Agric. Ecosyst. Environ. 282, 40–48. doi: 10.1016/j.agee.2019.05.019
Waldrop, M. P., and Zak, D. R. (2006). Response of oxidative enzyme activities to nitrogen deposition affects soil concentrations of dissolved organic carbon. Ecosystems 9, 921–933. doi: 10.1007/s10021-004-0149-0
White, D., Davis, W., Nickels, J., King, J., and Bobbie, R. (1979). Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 40, 51–62. doi: 10.1007/BF00388810
Wu, G., Gao, J., Li, H., Ren, F., Liang, D., and Li, X. (2023). Shifts in plant and soil C, N, and P concentrations and C: N: P stoichiometry associated with environmental factors in alpine marshy wetlands in West China. CATENA 221:106801. doi: 10.1016/j.catena.2022.106801
Xue, X., Ren, C., Zhao, C., Wang, W., Luo, X., Zhang, Y., et al. (2025). Litter removal and nitrogen deposition alter soil microbial community composition and diversity in a typical rubber (Hevea brasiliensis) plantation of Hainan, China. Appl. Soil Ecol. 208:105969. doi: 10.1016/j.apsoil.2025.105969
Yang, A., Song, B., Zhang, W., Zhang, T., Li, X., Wang, H., et al. (2024). Chronic enhanced nitrogen deposition and elevated precipitation jointly benefit soil microbial community in a temperate forest. Soil Biol. Biochem. 193:109397. doi: 10.1016/j.soilbio.2024.109397
Yang, S., Sun, J., Liu, C., Li, S., Wang, C., Wei, G., et al. (2024). Stellera chamaejasme expansion promotes the restoration of soil microbial diversity and ecosystem multifunctionality in degraded grasslands. CATENA 241:108020. doi: 10.1016/j.catena.2024.108020
Yu, F., Li, P., Li, S., and He, W. (2010). Kobresia tibetica tussocks facilitate plant species inside them and increase diversity and reproduction. Basic Appl. Ecol. 11, 743–751. doi: 10.1016/j.baae.2010.09.005
Zhang, Y., Cao, C., Cui, Z., Qian, W., Liang, C., and Wang, C. (2019). Soil bacterial community restoration along a chronosequence of sand-fixing plantations on moving sand dunes in the Horqin sandy land in Northeast China. J. Arid Environ. 165, 81–87. doi: 10.1016/j.jaridenv.2019.04.003
Zhang, S., Jian, Y., Yan, B., Jin, J., Wu, J., Liang, C., et al. (2022). Land consolidation with seedling cultivation could decrease soil microbial PLFA diversity. Phyton 91:1076. doi: 10.32604/phyton.2022.021076
Zhang, H., Zhou, M., Dong, L., Liu, H., and Wang, W. (2023). Soil bacterial community mediates temporal stability of plant community productivity in degraded grasslands. Appl. Soil Ecol. 182:104725. doi: 10.1016/j.apsoil.2022.104725
Zhao, R., Hui, R., Liu, L., Xie, M., and An, L. (2018). Effects of snowfall depth on soil physical–chemical properties and soil microbial biomass in moss–dominated crusts in the Gurbantunggut Desert, northern China. CATENA 169, 175–182. doi: 10.1016/j.catena.2018.05.042
Zhao, J., Li, X., Li, R., Tian, L., and Zhang, T. (2016a). Effect of grazing exclusion on ecosystem respiration among three different alpine grasslands on the central Tibetan plateau. Ecol. Eng. 94, 599–607. doi: 10.1016/j.ecoleng.2016.06.112
Zhao, J., Luo, T., Li, R., Li, X., and Tian, L. (2016b). Grazing effect on growing season ecosystem respiration and its temperature sensitivity in alpine grasslands along a large altitudinal gradient on the central Tibetan plateau. Agric. For. Meteorol. 218, 114–121. doi: 10.1016/j.agrformet.2015.12.005
Zhao, F., Ren, C., Shelton, S., Wang, Z., Pang, G., Chen, J., et al. (2017). Grazing intensity influence soil microbial communities and their implications for soil respiration. Agric. Ecosyst. Environ. 249, 50–56. doi: 10.1016/j.agee.2017.08.007
Zheng, D., Zhang, Q., and Wu, S. (2000). Mountain geoecology and sustainable development of the Tibetan plateau. New York: Springer Science & Business Media.
Zheng, L., Zhao, Q., Lin, G., Hong, X., and Zeng, D. (2023). Nitrogen addition impacts on soil phosphorus transformations depending upon its influences on soil organic carbon and microbial biomass in temperate larch forests across northern China. CATENA 230:107252. doi: 10.1016/j.catena.2023.107252
Zhou, X., Wang, X., Ma, Y., Wang, Y., Ma, Y., and Xie, L. (2023). Fertilization can accelerate the pace of soil microbial community response to rest-grazing duration in the three-river source region of China. Ecol. Evol. 13:e10734. doi: 10.1002/ece3.10734
Zong, N., Shi, P., Niu, B., Jiang, J., Song, M., Zhang, X., et al. (2014). Effects of nitrogen and phosphorous fertilization on community structure and productivity of degraded alpine meadows in northern Tibet, China. Ying Yong Sheng Tai Xue Bao 25, 3458–3468. doi: 10.13287/j.1001-9332.20140930.005
Keywords: fertilization, rest-grazing, soil microbial community, Carex tibetikobresia meadow, nutrient limitation
Citation: Zhou X, Wang X, Ma Y, Wang Y, Ma Y and Xie L (2025) Fertilization decreases the effect of ammonium nitrogen on microorganisms in Chinese Carex tibetikobresia meadows during rest-grazing. Front. Microbiol. 16:1608011. doi: 10.3389/fmicb.2025.1608011
Edited by:
Kamran Malik, Lanzhou University, ChinaReviewed by:
Muhammad Abaid-Ullah, COMSATS Institute of Information Technology, PakistanRistina Siti Sundari, Universitas Perjuangan Tasikmalaya, Indonesia
Copyright © 2025 Zhou, Wang, Ma, Wang, Ma and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoli Wang, d3hsLnl1QDE2My5jb20=
†ORCID: Xuanbo Zhou, https://orcid.org/0009-0004-0599-6828
 Xuanbo Zhou
Xuanbo Zhou Xiaoli Wang
Xiaoli Wang Yushou Ma
Yushou Ma Lele Xie
Lele Xie