- Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Taiyuan, China
Acute-on-chronic liver failure (ACLF) is a clinical syndrome that manifests as acute deterioration of liver function due to a series of etiologies and triggers in patients with pre-existing chronic liver diseases. Systemic inflammatory response is the major feature of ACLF. Gut microbiota dysbiosis impairs the intestinal barrier, facilitating the translocation of microorganisms and their metabolites into the liver and thereby exacerbating liver inflammation and disease progression. Recent studies have revealed that bacterial outer membrane vesicles (OMVs) derived from gut microbiota act as key mediators in microbiota-host cell communication. This article elucidates the possible roles of OMVs in ACLF inflammation and their underlying mechanisms.
1 Introduction
Acute-on-chronic liver failure (ACLF) is a clinical syndrome that manifests as an acute decompensation of liver function in patients with pre-existing chronic liver disease, and is characterized by a high case-fatality rate and multiple organ failure (Clària et al., 2023; Br and Sarin, 2023). In ACLF patients, the rapid decline in liver function leads to impaired detoxification and waste disposal, which results in toxin overload and physiological dysfunction, with a series of associated clinical signs and symptoms. Cirrhosis is a common underlying condition of ACLF (Artru and McPhail, 2024) as it will progress to ACLF following an acute injury from sources such as bacterial/viral infection, alcoholism, drugs, or variceal bleeding (Wang et al., 2022; Br and Sarin, 2023; Clària et al., 2023). However, despite its diverse etiologies, ACLF has been typically characterized by a common systemic inflammatory response and immunosuppression (Br and Sarin, 2023).
The pathogenesis of ACLF remains unclear. The systemic inflammation hypothesis has gained widespread recognition, positing that ACLF is an acute flare of pre-existing systemic inflammation in patients with decompensated cirrhosis that can cause immune-mediated tissue damage (Clària et al., 2016; Arroyo et al., 2021; Samuel, 2021). Accordingly, inflammation is a pivotal factor throughout the course of ACLF. The inflammation extends beyond the liver in ACLF, with the gastrointestinal (GI) tract contributing significantly to its pathogenesis (Trebicka et al., 2021a; Kim et al., 2021; Hsu and Schnabl, 2023). The extant literature suggests that gut microbiota dysbiosis is prevalent in patients suffering from cirrhosis and liver failure when compared with healthy populations. The interaction between gut-derived bacteria and their products with the liver is initiated through the gut-liver axis and the intestinal mucosal barrier, which results in the induction of liver inflammation and fibrosis (Trebicka et al., 2021a; Hsu and Schnabl, 2023). In addition, systemic inflammation in ACLF patients has been found to be linked to intestinal flora dysbiosis and altered metabolic pathways (Kim et al., 2021; Moreau et al., 2020).
Recent research has demonstrated an increased interest in the role of outer membrane vesicles (OMVs) released by gut bacteria in disease processes (Chen et al., 2023b; Liang et al., 2022; Liu et al., 2021b; Xie et al., 2023b). OMVs play key roles in mediating gut bacteria-host interactions and can be involved in inflammatory responses and immune regulation (Liang et al., 2022; Han et al., 2024). Gut microbes and their byproducts activate liver inflammation and drive the progression of liver diseases (Hsu and Schnabl, 2023). Gut microbiota-derived OMVs exacerbate liver inflammation and fibrosis (Zahmatkesh et al., 2022; Natsui et al., 2023; Dorner et al., 2024). Therefore, these OMVs may trigger and worsen the progression from cirrhosis and other chronic liver diseases to ACLF, which may inform future intervention strategies.
2 Inflammation in ACLF and its etiologies
ACLF has a variety of etiologies and triggers including bacteria, viruses, alcohol, and toxins, which elicit systemic inflammation that is pivotal to disease progression (Figure 1) (Br and Sarin, 2023). These factors induce hepatocyte necrosis, releaseing of inflammatory mediators and cytokines that amplify liver inflammation, creating a positive feedback loop enhancing liver injury. Additionally, overactivated hepatic immune cells secrete excess inflammatory mediators and cytokines, disrupting immunomodulation and exacerbating liver damage (Albillos et al., 2022; Martin-Mateos et al., 2019). These effects commonly feature inflammatory processes. Studies in mouse models of liver disease (nonalcoholic steatohepatitis (MASH), liver injury, ACLF, etc.) confirmed that inflammation itself can lead to hepatic fibrosis and liver injury and accelerate disease progression (Jiang et al., 2018; Zhang et al., 2023; Cheng et al., 2024; Lv et al., 2024). In the blood and liver tissues of individuals with cirrhosis and ACLF, pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF-α) (Clària et al., 2016; Tilg et al., 1992), as well as important inflammatory pathway proteins, such as nuclear factor- κB (NF-κB) (Zhang et al., 2022b), and thrombospondin-1 (THBS1) (Hassan et al., 2024), and NOD-like receptor protein 3 (NLRP3) inflammasome (Zhang et al., 2024b) are elevated. In essence, all the etiologies and triggers of ACLF have a shared feature in that they promote inflammation.
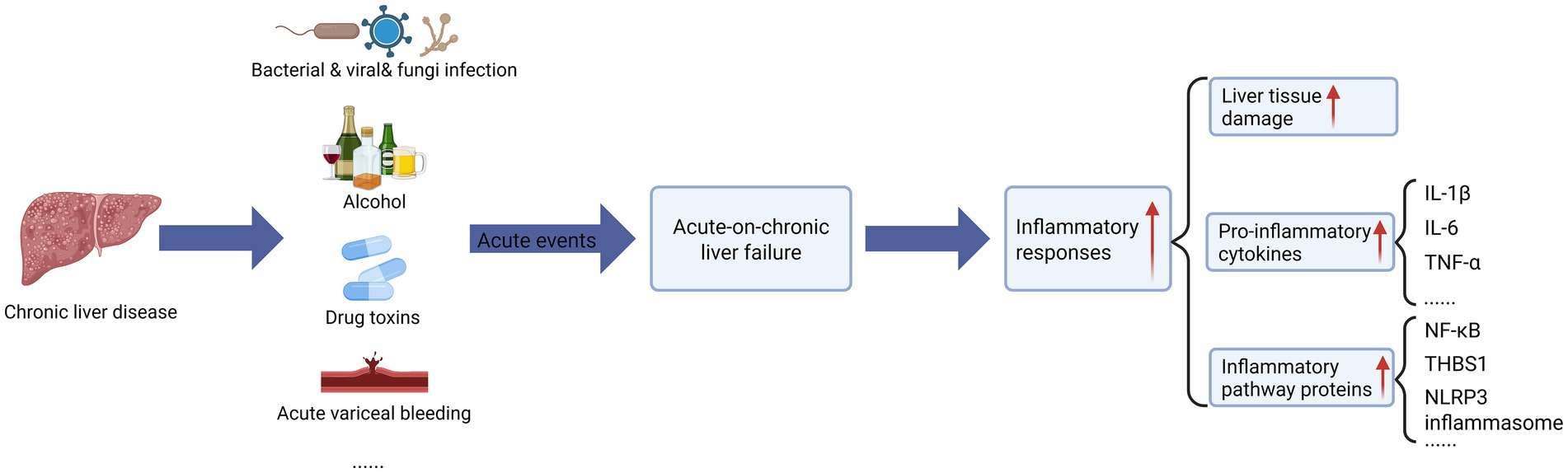
Figure 1. The effect of acute event strikes on acute-on-chronic liver failure (ACLF). On the basis of chronic liver disease, acute liver failure triggered by acute injury events (including bacterial/viral/fungi infection, alcohol abuse, drug/toxicity poisoning, acute vascular rupture and bleeding, etc.) leads to uncontrolled systemic inflammatory response, which is manifested by (1) hepatic tissue injury (hepatocellular necrosis/apoptosis), (2) pro-inflammatory cytokine storm (significant elevation of IL-1β, IL-6, and TNF-α, etc.), (3) activation of inflammatory pathway proteins (NF-κB signaling, upregulation of THBS1 expression, and elevation of NLRP3 inflammasome), which synergistically form a vicious cycle of “inflammation-injury.” The figure was created with BioRender.com (www.biorender.com).
2.1 Pathogen infection
Infection, caused by either bacterial, viral, and fungi pathogens, is the most common etiology and trigger of chronic liver disease in ACLF (Mezzano et al., 2022; Xu et al., 2024; Yang et al., 2025). A multicenter ACLF cohort study (Fernández et al., 2018) revealed that 37% of patients had bacterial infection upon admission, and 46% of others developed infection within 4 weeks, indicating prevalent bacterial infections in ACLF patients, along with an even higher mortality rate among patients with bacterial infection-induced ACLF (Fernández et al., 2018). It has been demonstrated that patients suffering from cirrhosis are susceptible to bacterial infections and are more likely to progress to ACLF than the general population (Wong et al., 2021; Arroyo et al., 2021; Piano et al., 2024). Helicobacter pylori, a common gastric pathogen, is linked to liver disease (Li et al., 2023; Wang et al., 2021a; Mohammadi et al., 2023; Wang et al., 2023), with studies showing its infection correlates with inflammatory markers in cirrhotic patients as H. pylori infection has been observed to stimulate the release of pro-inflammatory cytokines. H. pylori eradication improves inflammatory marker and vascular mediator levels and lowers the incidence of cirrhosis complications (Abdel-Razik et al., 2020; Li et al., 2023). A single-center prospective cohort study by Abdel-Razik et al. also showed that H. pylori infection promotes the development of decompensated cirrhosis such as hepatic decompensation, ascites, deterioration of coagulation indices, and the development of esophagogastric varices, (Abdel-Razik et al., 2020) and induces ACLF progression. Escherichia coli and Klebsiella pneumoniae are also frequently observed in ACLF patients with bacterial infections (Wong et al., 2021; Zhang et al., 2022a). E. coli promotes the progression of cirrhosis and the development of complications, including hepatic encephalopathy by activating immune cells and promoting the release of inflammatory factors (Natsui et al., 2023; Wu et al., 2021b). Viral infections, particularly hepatitis B virus (HBV) and hepatitis E virus, are key triggers of ACLF (Zhu et al., 2022; Wang et al., 2022; Wu et al., 2021a), leading to rapid liver function decline via viral replication, immune dysregulation, immune cell exhaustion, and cytokine storms (Li et al., 2022a; Wang et al., 2022; Zhu et al., 2022). Table 1 lists the major pathogens that trigger ACLF and the substances or mechanisms by which they may act.
2.2 Alcohol
Alcohol overdose is a significant risk factor for ACLF. Long-term or addicted alcohol use is linked to a higher incidence of ACLF (Singal and Shah, 2019). Long-term alcohol use alters gut microbiota composition, disrupts the integrity and permeability of the intestinal mucosal barrier, and facilitates the translocation of pathogen-associated molecular pattern molecules (PAMPs, including bacteria). When the PAMPs enter the liver and the circulation, they act on liver macrophages through lipopolysaccharide (LPS) to induce inflammation, resulting in a series of pathological changes in the liver (Szabo, 2015; Parlesak et al., 2000). Additionally, ethanol metabolism generates reactive oxygen species, damaging mitochondria and inducing apoptosis (Zhou et al., 2001; Lu et al., 2008), thus contributing to liver failure.
2.3 Drug toxins
Drug-induced liver injury is also linked to ACLF progression, beyond chronic liver disease (Devarbhavi et al., 2019). Some drugs can elicit immune system hyperreactivity and oxidative stress, damaging liver cell structure and functions (Qian et al., 2024; Ye et al., 2018). The metabolism of some drugs produces toxic products. For example, acetaminophen (APAP) produces excess N-acetyl-p-benzoquinone imine (NAPQI), which depletes glutathione (GSH), leading to mitochondrial dysfunction, increased oxidative stress, and direct toxicity to hepatocytes, resulting in hepatocellular necrosis (Ye et al., 2018; Li et al., 2022b). In addition, the combination of environmental factors and individual differences can contribute to drug-induced damage, potentially causing ACLF (Yu et al., 2017).
2.4 Acute variceal hemorrhage
Acute variceal hemorrhage is a precipitating factor in liver failure (Wang et al., 2022). Acute variceal hemorrhage leads to liver ischemia, which compromises the integrity of the intestinal barrier. This increases the risk of bacterial infections due to increased bacterial translocation. This can induce a pro-inflammatory or exacerbated inflammatory response (Sarin et al., 2019; Triantos et al., 2022).
Inflammation is a pivotal factor in the clinical course and outcome of ACLF. Chronic liver disease is marked by persistent inflammation and fibrosis progression (Hammerich and Tacke, 2023). In a prospective study (Trebicka et al., 2021b), 65% of ACLF patients enrolled had evident triggers of systemic inflammation. Notably, patients with two or more precipitating factors had higher levels of inflammatory markers (including white blood cell count, neutrophil count, and monocyte count, and C-reactive protein) than in patients with no clinically obvious precipitating factors or a single precipitating factor (Trebicka et al., 2021b). Bacterial infections, alcohol overdose, and viral activity can lead to the release of PAMPs, which triggers inflammation, impairs organ function, and thus induces ACLF. Engelmann et al. also reviewed the core mechanisms of acute decompensation in cirrhosis and the pathophysiology associated with its progression to ACLF, stating that after the occurrence of various triggering factors leading to acute decompensation, a cascade of pathogenetic processes will follow (Engelmann et al., 2021). In short, excessive release of inflammatory mediators can disrupt the “pro-inflammatory and anti-inflammatory” balance and exacerbates liver damage, potentially leading to decompensation (Engelmann et al., 2021). These studies reveal that inflammation both sustains disease and triggers ACLF.
3 Microbiota in ACLF
In cirrhosis and ACLF patients, the imbalanced intestinal microbiota is characterized by reduced probiotics and increased opportunistic pathogens (e.g., Enterococcus and Bacteroides). The latter can produce toxins, trigger inflammation, and aggravate liver damage (Kim et al., 2021; Solé et al., 2021). Cirrhosis and ACLF patients often exhibit compromised intestinal barrier, allowing endotoxins and bacteria to translocate into the liver, causing infections and systemic inflammation, and worsening liver damage and decompensation (Trebicka et al., 2021c; Kim et al., 2021). The role of gut flora in cirrhosis and ACLF has been widely recognized, and therefore modulating gut flora has also been considered as a potential therapeutic tool (Sharma et al., 2022; Patel et al., 2022). And studies have shown modulating gut microbiota through microbiota transplantation or the use of antibiotics can help to treat or alleviate symptoms and inflammation in cirrhosis or liver failure patients (Sharma et al., 2022, Patel et al., 2022).
3.1 Altered microbiome in ACLF
A study performed over 10 years ago found that increased intestinal bacterial translocation was linked to the development of ACLF, attributing it to the gut-liver axis dysfunction (Verbeke et al., 2011). Increasing studies have characterized gut microbiota alterations in cirrhosis and ACLF (Chen et al., 2011; Bajaj et al., 2014; Qin et al., 2014; Bajaj et al., 2019; Tranah et al., 2021; Bajaj et al., 2012; Chen et al., 2015; Mehtani et al., 2021; Wang et al., 2021b; Yao et al., 2021). The gut microbiome in cirrhosis demonstrates reduced diversity with pathogenic dominance and loss of beneficial taxa. This leads to compromised integrity of the intestinal barrier and increased intestinal permeability. These alterations facilitate the translocation of bacteria and their metabolites (Sharma et al., 2024; Chen et al., 2011; Bajaj et al., 2014; Qin et al., 2014; Bajaj et al., 2019; Tranah et al., 2021), thereby fostering the development and progression of ACLF. Further exploration of the gut microbiome in ACLF revealed reduced abundance of protective anti-inflammatory bacteria (e.g., Bacteroides, Ruminococcae, and Trichalium) and increased abundance of harmful bacteria (e.g., Pasteurella and streptococci) (Chen et al., 2015; Bajaj et al., 2014), which correlated with disease severity and mortality (Chen et al., 2015; Yao et al., 2021; Solé et al., 2021).
Microbiome alteration is an active participant and influencing factor, rather than a passive factor, in disease progression (Sharma et al., 2024; Bajaj et al., 2014; Solé et al., 2021). Relevant studies in cirrhotic mice, mice with liver injury, and other animal models of liver disease have clearly revealed the significant role of microbiota in multiple key pathological processes including intestinal barrier integrity, gut permeability, systemic inflammation, neuroinflammation, and blood–brain barrier integrity (Kang et al., 2024; Liu et al., 2020; Fouts et al., 2012), which jointly contribute to host homeostasis and health. A decline in barrier-maintaining, immunomodulatory, and anti-inflammatory bacteria may enhance the host’s vulnerability to pro-inflammatory damage triggered by harmful gut bacteria, pathogens, or environmental toxins. Such a microecological imbalance worsens the original pathology and can even initiate or advance new disease processes (Chen et al., 2011; Bajaj et al., 2014; Chen et al., 2015).
Microbiome changes are present early in the development of chronic liver disease (Schnabl and Brenner, 2014), which indicates that the intestinal microbiota has been damaged in the prodromal state of ACLF, reinforcing microbial dysbiosis as a functional contributor to ACLF. Recent literature has indicated the close association between intestinal dysbiosis and liver diseases (Bajaj et al., 2019; Stols-Gonçalves et al., 2023; Trebicka et al., 2021a; Hsu and Schnabl, 2023; Solé et al., 2021; Sharma et al., 2022). Studies on the subject of gut microbiota in patients diagnosed with cirrhosis or viral hepatitis revealed signs of microflora dysbiosis in these patients, and the alterations in the composition of their gut microbiota were associated with progression of ACLF (Wang et al., 2021b; Solé et al., 2021). Thus, microbiota dysbiosis is an early marker of ACLF, and microbiome monitoring and intervention in the early stages of ACLF can be valuable for the prevention, timely detection, and effective management of ACLF.
In addition to stool-based gut microbiome changes, small intestinal bacterial overgrowth (SIBO) has also been recognized as another common form of gut dysbiosis in patients diagnosed with cirrhosis and other liver diseases (Pande et al., 2009; Alexiou et al., 2024; Gudan et al., 2022). SIBO impairs nutrient absorption, further taxing compromised liver function (Gudan et al., 2023; Adike and DiBaise, 2018). Furthermore, SIBO fosters gut inflammation and compromises barrier integrity, thus facilitating harmful bacteria and their metabolites entry into the circulation, which exacerbate liver damage and trigger immune responses and systemic inflammation (Lauritano et al., 2010; Pande et al., 2009; Alexiou et al., 2024), finally exacerbating ACLF via the vicious circle of the gut-liver axis. In fact, the cirrhosis-SIBO connection underscores the pivotal function of gut microbiota in advancing liver disease.
Microbiota transplantation studies affirmed the crucial function of gut microbiota in ACLF (Jiang et al., 2024; Zhang et al., 2024c; Bajaj et al., 2018; Sharma et al., 2022). Gut microbiota transplantation can rebalance microbial communities in cirrhotic patients, boosting the diversity of gut bacteria by increasing the relative abundance of potentially beneficial flora (e.g., Bifidobacterium) while reducing the release of pro-inflammatory cytokines (e.g., IL-1β, IL-6). Also, it can help to alleviate inflammation, liver injury, and complications such as ACLF and hepatic encephalopathy in cirrhotic patients (Bajaj et al., 2018, Sharma et al., 2022). In a study on gut microbiota transplantation in alcohol-related ACLF patients, transplantation significantly improved short- to medium-term survival and reduced inflammatory markers (e.g., IL-1β, IL-6) compared to controls (Sharma et al., 2022). And the treatment group also showed more pronounced improvement in hepatic encephalopathy and ascites, as well as a lower incidence of adverse events, such as gastric hemorrhage (Sharma et al., 2022). In addition, an animal-level research has shown that gut microbiota transplantation significantly lowered aspartate transaminase and alanine transaminase levels, mitigated hepatocyte necrosis, enhanced intestinal barrier function, reduced inflammation, and enhanced disease regression (Zhang et al., 2024c; Wang et al., 2017). Zhao et al. (2021) found that administering cirrhotic mice with intragastric bacterial suspension from cirrhosis patients exacerbated intestinal barrier damage and liver injury, accelerating cirrhosis progression (compared to bacterial suspension from healthy individuals). Therefore, the gut microbiome in certain cirrhotic patients may harbor factors that can drive ACLF progression.
3.2 ACLF inflammation and the microbiota-gut-liver/-brain axis
As the gut microbiome significantly impacts human health and disease (Fan and Pedersen, 2021), the gut-liver axis has gained prominence in liver disease research (Albillos et al., 2020; Tilg et al., 2022; Hsu and Schnabl, 2023; Schnabl and Brenner, 2014). The gut-liver axis denotes the intricate network linking the intestine and the liver via anatomic structures, circulation, neural control, and metabolism pathways for bidirectional interactions of the gut and its microbiota with the liver (Hsu and Schnabl, 2023; Albillos et al., 2020). This network plays a crucial role in maintaining homeostasis, particularly in regulating metabolism and immune responses (Hsu and Schnabl, 2023, Albillos et al., 2020). The liver is the first organ affected by gut bacteria and their products after the entry of these pathogens into the intestinal barrier and can be influenced by the gut microbiome in multiple ways (Hsu and Schnabl, 2023; Trebicka et al., 2021a). The significance of microbiota in liver disease, in relation to the translocation of gut bacteria and their byproducts, has increasingly been recognized; however, the mechanism behind gut diversity changes driving disease progression remains unclear.
Most gut bacteria subtly interact with host cells under a healthy gut status. They are confined to the gut lumen and separated from epithelial cells by the mucosal barrier, thus preventing bacterial invasion (Hiippala et al., 2018; Turner, 2009). As a result, their communication with host cells (e.g., epithelial and immune cells) relies on the release of a variety of secretory factors including OMVs. These secretory factors, acting as messengers, convey bacterial information across the mucosal barrier and interact with host cells to influence their physiology (Luo et al., 2021; Dorner et al., 2024; Liang et al., 2022), potentially significantly contributing to ACLF pathology.
Bacteria and their products (e.g., LPS, bacterial DNA, and peptidoglycan) can trigger the immune response (Gasaly et al., 2021), inflammation, and hepatocyte apoptosis, ultimately accelerating liver failure (Won et al., 2021). In the setting of ACLF, the translocation of gut bacteria and their metabolites (e.g., the translocation of LPS into the liver and systemic circulation) modulates the immune response and inflammation in the liver (Hasa et al., 2022; Suriguga et al., 2022; Chen et al., 2021). As a key component of Gram-negative bacteria outer membrane, LPS is also an effective trigger of immune responses. Its blood level is markedly elevated in cirrhotic patients (Simbrunner et al., 2023; Lin et al., 1995). And LPS can be recognized by pattern recognition receptors such as Toll-like receptors (TLRs), which are located on the cell surface of the liver, activate TGF-β and NF-κB signaling pathways, induce strong inflammatory responses and fibrosis, and regulate immune function (Fan et al., 2021; Engelmann et al., 2020). Increased expression of hepatic TLR4 and circulating TLR4 ligands in cirrhotic patients heightens cellular sensitivity to endotoxins generated by translocated Gram-negative bacteria, which is associated with disease development and prognosis (Engelmann et al., 2020). The TLR4 pathway is pivotal in ACLF development, with the recognized LPS triggering intracellular signaling and inflammatory cytokine release (Engelmann et al., 2020). A recent prospective study performed macrogenomic sequencing of fecal samples from patients with cirrhosis, and analysis showed that microbiomic changes significantly impact ACLF incidence (Solé et al., 2021), reinforcing the importance of gut-liver axis in the progression and prognosis of cirrhosis. Furthermore, studies have shown that 40–50% of ACLF patients have unexplained severe systemic inflammation, and it is assumed that gut bacterial translocation and resultant “pathogens” and their metabolites may play pivotal roles (Moreau et al., 2013). All of the above reveal the importance of gut bacteria and their bacterial products in the progression and prognosis of liver disease through the enterohepatic axis.
Studies reveal a bidirectional gut-liver-brain axis communication (Mayer et al., 2015; Liu et al., 2021a), with healthy homeostasis of the axis preventing harmful intestinal contents from reaching the brain via two barriers: the homeostasis of intestinal permeability and the normal liver function (Liu et al., 2021a). Gut microbes can exert direct or indirect effects on neurons to control the production and/or release of neurotransmitters by sending signals to the brain through neurons, endocrine, and immune mediators (Cani and Knauf, 2016). Mounting evidence suggests that changes in the structure of the gut microbiota and the effects of its metabolites, along with local or systemic inflammation, impaired liver metabolism, and leaky gut, can promote the occurrence of liver failure and hepatic encephalopathy (Liu et al., 2021a; Trebicka et al., 2021c; Sharma et al., 2024). Cirrhosis involves systemic inflammation and endotoxin spread, triggering neuroinflammation and causing neurological complications (e.g., cognitive impairment) (Butterworth, 2019). Interestingly, it has been found that H. pylori-derived OMVs can cross the GI, blood–brain, and blood-CSF barriers, reaching the brain to induce peripheral and central inflammation, potentially triggering Alzheimer’s disease (Xie et al., 2023a). As H. pylori is a common bacterium in the GI tract, it is assumed that H. pylori OMVs may also promote the occurrence of hepatic encephalopathy.
It is generally believed that, in the context of ACLF, bacteria-secreted factors may penetrate the intestinal barrier, thus initiating an immune response cascade that likely drives disease progression. Notably, Heetanshi et al. (Jain et al., 2024) isolated LPS-positive extracellular vesicles from portal vein plasma and liver tissues, confirming the presence of OMVs in liver. Accordingly, we propose that OMVs play an equally pivotal role in ACLF. As the main secretions and communication pathways of bacteria, OMVs have the ability to cross biological barriers such as the intestinal barrier and the blood–brain barrier to deliver “cargo,” although their specific mechanisms in ACLF remains understudied. We have created a schematic diagram to illustrate this assumption (Figure 2).
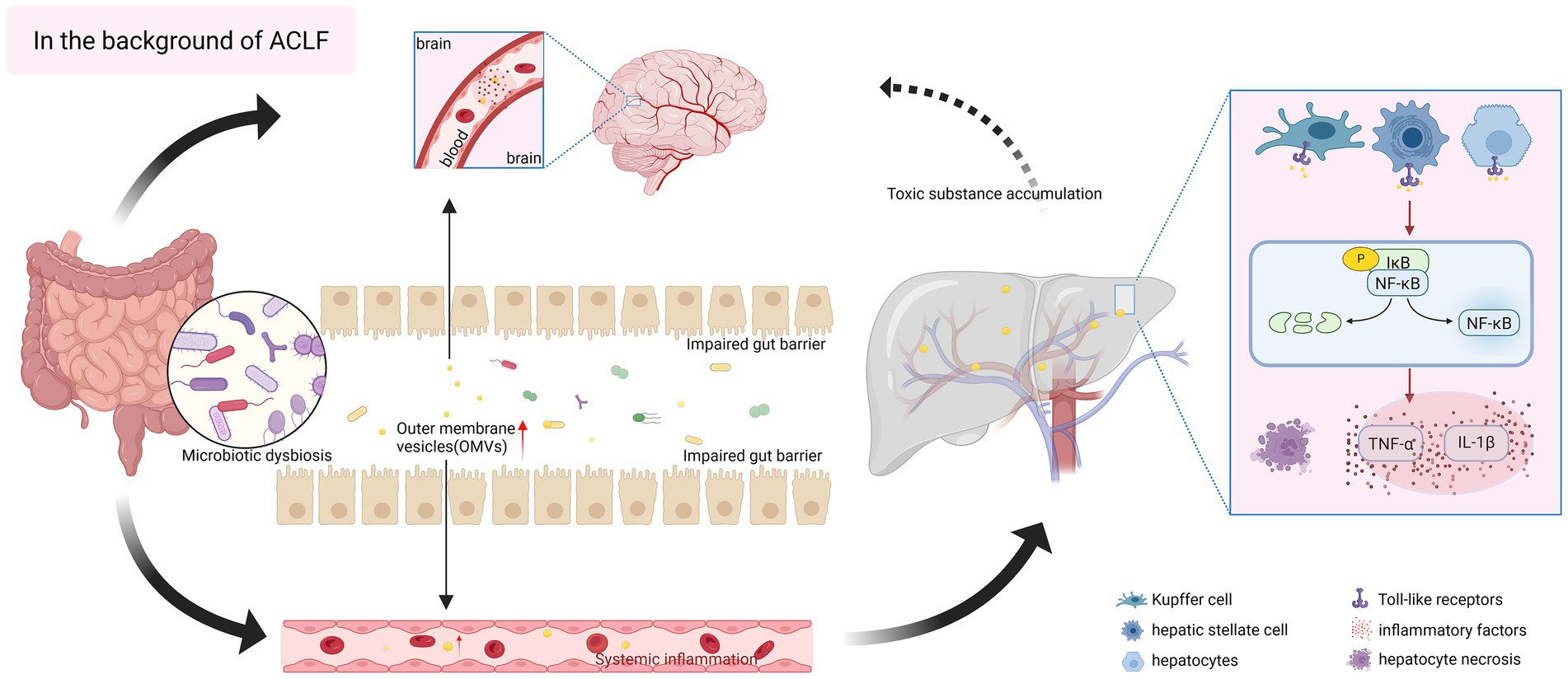
Figure 2. Hypothesized pro-inflammatory role of bacterial outer membrane vesicles (OMVs) in acute-on-chronic liver failure (ACLF). In the context of ACLF, the composition of gut microorganisms is disordered, and intestinal bacteria release a large number of OMVs, which are rich in pathogen-associated molecular patterns (PAMPs), and at the same time, there is damage to the gut barrier, and intestinal permeability is enhanced, which is more conducive for OMVs to cross the gastrointestinal barrier, and to reach liver tissues through the gut-liver axis, and they can act on a wide variety of cells in the liver, and they can act on hepatocytes directly, inducing OMVs can act on various cells in the liver, directly affecting hepatocytes, inducing oxidative stress and apoptosis, and exacerbating liver injury; they can also act on Kupffer cell and hepatic stellate cells, and bind to Toll-like receptors on the surface of the cells, activating inflammatory signaling pathways, such as NF-κB, and releasing a large number of pro-inflammatory cytokines (IL-1β, TNF-α), exacerbating hepatic fibrosis, and triggering systemic inflammatory responses. The inflammation within the liver mediated by OMVs can further spread to induce multi-organ failure. In addition, OMVs can also cross the blood–brain barrier, invade the brain and activate neuroinflammation, while the severely impaired liver detoxification function promotes the accumulation of ammonia and other toxic substances, affecting the nervous system, a series of chain reactions, which lay the groundwork for the occurrence of hepatic encephalopathy. The figure was created with BioRender.com (www.biorender.com).
4 Bacterial OMVs
OMVs were first reported in the 1960s, with researchers observing spherical vesicles from E. coli under electron microscopy, initially believed to be apoptotic fragments (Bishop and Work, 1965). These vesicles were later termed outer membrane vesicles (OMVs). Bacteria extracellular vesicles (bEVs) are a general term for nanoscale vesicles with a lipid bilayer membrane structure secreted by bacteria, and encompasses a variety of types of vesicles, such as cytoplasmic membrane vesicles (CMVs), OMVs, and so on (Ho et al., 2024). Among them, OMVs are extracellular vesicles derived from Gram-negative bacteria. OMVs, 20–300 nm in size (Avila-Calderón et al., 2021), are vesicle-like structures that are shed from the bacterial cells surface and released into the extracellular area bacteria during the growth of Gram-negative, and are primarily composed of parental proteins, lipids, and nucleic acids (Avila-Calderón et al., 2021; Toyofuku et al., 2019; Toyofuku et al., 2023). For the purpose of this review, we focus on OMVs, the extracellular membrane vesicles released by Gram-negative bacteria. Of course, we also recognize the potential relevance of extracellular vesicles (CMVs) released by Gram-positive bacteria in disease [see review for details (Xu et al., 2023, Sangiorgio et al., 2024)].
OMVs are produced by Gram-negative bacteria through a typical model of outer membrane vesiculation. There are multiple mechanisms by which Gram-negative bacteria shed OMVs, including a decrease in the number of lipoproteins attached to the peptidoglycan layer leading to outer membrane bulging, peptidoglycan residues with autolysin leading to outer membrane protrusion, and the effect of the charge carried by the LPS (Avila-Calderón et al., 2021). The secretion of OMVs is impacted by multiple factors, including bacterial growth stage, surrounding environmental conditions, cell physiological state, antibiotic exposure, and microbial interactions (Toyofuku et al., 2019). Most OMVs carry specific “cargo” molecules, conferring various functions such as virulence factor transmission, enhancing bacterial survival and pathogenicity, signal transduction, promoting interbacterial communication, and mediating bacteria-host information exchange (Jan, 2017; Sartorio et al., 2021). Compared with bacteria themselves, OMVs are more likely to cross tight junctions and biological barriers. As the “long-range weapons” for bacteria, they attack host tissues, facilitate bacterial colonization, impair host cell function, and compromise host defenses, thus playing key roles in the pathogenic processes (Toyofuku et al., 2023). OMVs can spread to distant tissues and are found in blood (Tulkens et al., 2020; Luo et al., 2021), gastric mucosa (Luo et al., 2021), liver (Jain et al., 2024), brain (Xie et al., 2023a), and feces (Park et al., 2018).
4.1 Roles of OMVs in immunomodulation
Gut microbiota-derived OMVs modulate the host’s immunity in ACLF. The OMVs elicit local and systemic pro-inflammatory immune responses by engaging innate and adaptive immune cells including macrophages, dendritic cells, and neutrophils (Tiku and Tan, 2021; Spari et al., 2024). OMVs are abundant in PAMPs, including DNA, RNA, lipoproteins, LPS, and toxins, which can bind to pattern recognition receptors located on the surface of the host’s immune cells, leading to complex mechanisms of immune activation (Liang et al., 2022; Liu et al., 2021b; Xie et al., 2023b) and triggering a cascade of biological responses. For instance, Yang et al. treated mouse bone marrow-derived macrophages (BMDMs) with the OMVs secreted by Salmonella typhimurium, in which promoted flagellin act as a stimulus for the activation of NLRC4 inflammatory vesicles, mediating caspase-1 activation and IL-1β secretion (Yang et al., 2020). Additionally, an investigation into the functions of OMVs from another flagellated bacterial pathogen, Pseudomonas aeruginosa, revealed the similar outcomes. Robust caspase-1 activation and IL-1β secretion were also observed (Yang et al., 2020). Davis et al. found that the OMVs derived from pathogenic E. coli contain a bacterial toxin known as cytotoxic necrotizing factor 1, which negatively affects the phagocytic and chemotactic capabilities of neutrophils (Davis et al., 2006). Salmonella typhimurium OMVs have been observed to induce an increase in the expression of CD86 and MHC class II molecules in dendritic cells, and to promote the production of pro-inflammatory mediators TNF-α and IL-12 (Alaniz et al., 2007). Furthermore, the OMVs have been shown to possess antigenic properties that mediate the immune response of protective B cells and T cells (Alaniz et al., 2007). It has also been demonstrated that intraperitoneal injection of OMVs from LPS- and outer membrane protein-rich E. coli or fecal bacterial extracellular vesicles containing Gram-negative and Gram-positive bacterial EVs induced sepsis-like systemic inflammation in mice (Park et al., 2010; Park et al., 2018). Subsequent research has further elucidated that E. coli OMVs can induce the release of macrophage inflammatory mediators in both in vitro and in vivo experimental models, and there are components other than LPS in E. coli OMVs mediating the pro-inflammatory effects (Svennerholm et al., 2017).
The OMVs are replete with LPS (Kaparakis-Liaskos and Ferrero, 2015), and they possess the capacity to deliver LPS to a variety of cell types including macrophages, neutrophils, and natural killer cells. Through interaction with the Toll-like receptor 4 (TLR4)/myeloid differentiation protein 2 (MD2)/CD14 receptor complex on the cell surface, LPS activates the NF-κB signaling pathway (Ciesielska et al., 2021). This activation triggers the secretion of pro-inflammatory cytokines (e.g., TNF-α and IL-1β) and enhances the production of free radicals, including nitric oxide and superoxide, ultimately exacerbating hepatic inflammation (Ciesielska et al., 2021). Furthermore, OMVs, as a vector for the intracellular transport of LPS, can activate caspases and the NLRP3 inflammasomes, further potentiating the inflammatory response (Vanaja et al., 2016). Several studies have confirmed the pro-inflammatory signaling pathway of OMVs described above (Zhang et al., 2024a; Engevik et al., 2021; Chen et al., 2023a; Gong et al., 2022). LPS-containing fusobacterium nucleatum OMVs have been shown to induce intestinal inflammation through the activation of TLR4 and subsequent downstream targets NF-κB and extracellular signal-regulated kinase (ERK) (Engevik et al., 2021). In a rat model of periodontitiss, the addition of the OMVs resulted in an upregulation of dendritic cell, T cell, and macrophage markers (Zhang et al., 2024a). Furthermore, it was observed that the secretion of inflammatory factors was promoted by the triggering of NF-κB signaling pathway, the activation of NLRP3 inflammasomes, and the exertion of pathogenicity (Zhang et al., 2024a). The diabetic rat model demonstrated that OMVs can be transported into the renal tubular mesenchyme through the disrupted intestinal vascular barrier (Chen et al., 2023a). In this model, abundant LPS has been shown to target renal tubular epithelial cells, inducing tubular mesenchymal inflammation and renal injury through the caspases-11 pathway (Chen et al., 2023a). In vitro experimentation with colorectal cancer cells treated with adherent invasive E. coli OMVs resulted in the up-regulation of TLR2/TLR4 expression, the down-regulation of cell junction-associated proteins, and the induction of inflammatory factor release (Nadalian et al., 2024). Notably, OMVs emitted by E. coli have the capacity to elicit mitochondrial dysfunction, mitochondria-mediated apoptosis, and inflammatory activation (Deo et al., 2020), which may be implicated in the pathogenesis of ACLF given that mitochondrial impairment is also a hallmark of ACLF (Moreau et al., 2020).
The heightened abundance of pro-inflammatory Gram-negative bacteria and the enhanced production of LPS in ACLF (Cesaro et al., 2011; Clària et al., 2016; Wu et al., 2022; Pan et al., 2012) suggest that the gut microbiome in ACLF may be a significant source of LPS-rich OMVs. Elevated levels of LPS have been detected in the blood of ACLF patients. However, the methodologies employed in these studies did not differentiate between cell-free, monomeric LPS and OMVs-contained LPS. The LPS detected in the blood of ACLF patients may be OMV-associated.
OMVs engage with host cells through the binding of ligands to pattern recognition receptors (PRRs; including TLRs) located on the cell surface or to intracellular receptors such as nucleotide-binding oligomerization domain (NOD) proteins (Thay et al., 2014; Marion et al., 2019). In the pathological trajectory of ACLF, the predominant PRR families involved in the recognition of PAMPs and damage-associated molecular patterns (DAMPs) are TLRs and NOD-like receptors (NLRs) (Zhang et al., 2021; Engelmann et al., 2020). Alterations in TLR2 and TLR4 within the TLR family are associated with the pathological processes of ACLF. As a key component of the TLR family, TLR2 plays a central role in detecting bacterial cell wall components, lipoproteins, and other PAMPs from both Gram-positive and Gram-negative bacteria. Research has evidenced that TLR2 expression was upregulated in the immune cells of patients with hepatitis B virus-related ACLF (HBV-ACLF) (Xu et al., 2017), facilitating the secretion of inflammatory cytokines. Moreover, the enhanced expression of TLR2 was positively associated with the degree of liver injury and the severity of the disease in ACLF patients (Xu et al., 2017). The activation of TLR4 facilitates the recruitment of inflammatory cells and regulates the expression of inflammatory signaling pathways (Seki et al., 2007). Evidence indicates that TLR4 signaling is a crucial determinant in the pathogenesis of ACLF (Engelmann et al., 2020). TLR4 mediates LPS-induced tissue damage and potentiates cellular sensitivity to LPS derived from Gram-negative bacteria (Engelmann et al., 2020). In rodent models of ACLF, treatment with TAK-242, a TLR4 antagonist, has been demonstrated to mitigate LPS-induced hepatocyte apoptosis, decrease cytokine production in hepatocytes and monocytes, lower circulating IL-1β levels, and ameliorate systemic inflammation (Engelmann et al., 2020; Engelmann et al., 2022). NLR proteins, including the NLRP3 inflammasome complex which recognizes a spectrum of MAMPs and DAMPs, have been implicated in the pathogenesis of ACLF (Li and Jiang, 2021; Zhang et al., 2024b; Taru et al., 2024). In a prospective controlled study, Li et al. found elevated levels of NLRP3 in both serum and peripheral blood mononuclear cells of patients with ACLF, and increased expression of the related signaling pathway molecules procaspase-1 and pro-1β, which were positively correlated with disease severity (Li and Jiang, 2021).
4.2 OMVs and intestinal epithelium
OMVs released from intestinal lumen can cross the intestinal mucosal barrier and adhere to epithelial cells (Liang et al., 2022; Liu et al., 2021b). Such an effect may be exacerbated in ACLF patients, as these patients frequently exhibit a compromised intestinal barrier function. The interactions of OMVs with epithelial cells can precipitate the secretion of cytokines, which regulates cellular proliferation and impacts the expression of tight junction proteins (Fiocca et al., 1999). Critically, studies have demonstrated that OMVs are capable of traversing the epithelial layer and accessing the lamina propria through either paracellular or transcellular routes, where they subsequently engage with resident immune cells (Krsek et al., 2023; Kaparakis-Liaskos and Ferrero, 2015). OMVs derived from diverse bacterial species, including Campylobacter jejuni, H. pylori, and Fusobacterium nucleatum, among others, are capable of down-regulating the expression of key tight junction proteins (Elmi et al., 2016; Turkina et al., 2015; Nie et al., 2022; Liu et al., 2021b). This downregulation directly contributes to attenuation of the epithelial barrier function and potentially facilitates the translocation of bacteria and their products from the intestinal lumen to the deeper intestinal layers (Elmi et al., 2016; Turkina et al., 2015; Nie et al., 2022; Liu et al., 2021b). Tulkens et al. (2020) discovered that individuals exhibiting compromised intestinal barrier functionality exhibited a markedly elevated concentration of bacterial extracellular vesicles within their plasma as compared to healthy controls. Furthermore, there was a robust positive correlation between the abundance of these vesicles and the increased plasma level of zonulin-1, a pivotal biomarker indicative of intestinal barrier dysfunction (Tulkens et al., 2020). In an in vitro model of simulated colitis, the concentration of bacterial extracellular vesicles detected on the basal side increased with the decrease in zonulin-1 level, which further confirmed the capability of OMVs to translocate via the paracellular route (Tulkens et al., 2020). OMVs in ACLF have the potential to downregulate the expression of critical tight junction proteins, thereby compromising the integrity of the epithelial barrier; as a result, bacterial byproducts can be translocated into the liver and other organs more easily, thereby amplifying the systemic inflammatory response.
4.3 OMVs and liver
OMVs can affect the functionality of diverse cell types, and their translocation beyond the intestinal lumen will precipitate enduring and extensive impacts. The diffuse spread of OMVs throughout the body enables gut-resident bacteria to exert indirect, long-distance effects (Juodeikis and Carding, 2022; Hendrix and De Wever, 2022; Tulkens et al., 2020). An investigation into OMVs biodistribution indicated that, following oral administration of labeled OMVs to mice, the in vivo distribution of OMVs was predominantly localized to the GI tract and liver, suggesting that OMVs are capable of reaching and accumulating in the liver via GI translocation in the host (Jones et al., 2020). Significantly, this experiment was conducted in healthy mice, implying that OMVs can traverse the intestinal barrier even without the presence of intestinal injury (Jones et al., 2020). Furthermore, Dorner et al. (2024) found that in both patients and murine models of primary sclerosing cholangitis (PSC) complicated by inflammatory bowel disease (IBD), OMVs secreted by intestinal commensal bacteria crossed the intestinal mucosal barrier and accumulated within the liver. By activating the TLR4 and NLRP3-GSDMD signaling pathways, these OMVs lead to the activation of hepatocytes and stellate cells, promoting liver inflammation and fibrosis.
Evidence suggests that individuals exhibiting compromised GI barriers exhibit elevated levels of circulating bacterial extracellular vesicles relative to healthy individuals (Tulkens et al., 2020). Although this finding has not been corroborated in patients with ACLF, enhanced intestinal permeability and reduced intestinal tight junction protein expression are frequently noted in individuals with ACLF (Assimakopoulos et al., 2012; Kim et al., 2021; Hasa et al., 2022). It is plausible to hypothesize that the concentration of circulating bacterial extracellular vesicles, including OMVs, may be increased in this patient population. These OMVs, which potentially harbor inflammatory genes, could contribute to exacerbation of the inflammatory response observed in ACLF.
Within the intestinal milieu, OMVs demonstrate a superior capacity for traversing biological barriers compared to intact bacterial cells, thereby facilitating their access to liver tissues with greater ease. Throughout their transit, OMVs have the potential to encapsulate and transport toxins, antigens, and bioactive compounds present on the bacterial surface. Upon reaching the liver, these vesicles can elicit a cascade of immune responses and induce pathological alterations. Initially, several studies have demonstrated that liver macrophages are capable of internalizing microbe-originated EVs (Luo et al., 2021). Notably, EVs isolated from the feces of mice subjected to a high-fat diet are enriched with bacterial DNA. This bacterial DNA has the potential to induce hepatocellular inflammation and to activate hepatic stellate cell fibrosis through the cGAS/STING pathway, indicating the hepatotropic impact of microbial DNA contained within intestinal bacteria-derived EVs (Luo et al., 2022; Luo et al., 2021). Intraperitoneal administration of Porphyromonas gingivalis-derived OMVs to mice results in the accumulation of OMVs within the murine liver. Within this context, gingival proteases contained within the OMVs may modulate inflammation in macrophages and the immune response, consequently compromising hepatocyte metabolic function (Seyama et al., 2020). As previously discussed, an association exists between H. pylori infection and the pathogenesis of ACLF. Among the virulence factors of H. pylori, OMVs are particularly significant (Zahmatkesh et al., 2022). Bolori et al. (2023) and Shegefti et al. (2023) co-cultured H. pylori OMVs with LX-2 cells to assess the expression of hepatic fibrosis markers (E-cadherin, vimentin, β-catenin, α-SMA, and Snail) and autophagy inhibition markers (PI3K, AKT, and mTOR). Their findings revealed that H. pylori OMVs promoted liver fibrosis, inhibited autophagy of hepatic stellate cells, and mediated liver injury.
Natsui et al. (2023) isolated hepatocytes from wild-type mice and cultured them on Petri dishes. In comparison to cultures treated with LPS, the expression of Clec4e, TLR2, TLR3, and TLR4 was markedly elevated in hepatocyte cultures following the addition of E. coli OMVs. Furthermore, in vitro experiments showed that OMVs elicited inflammatory responses mediated by macrophages, neutrophils, and hepatic stellate cells in a dose-dependent manner, which can result in hepatocyte injury. In the in vivo experiments conducted in cirrhotic mice, OMVs triggered the activation of hepatic macrophages, leading to increased liver inflammation; in addition, OMVs activated hepatic stellate cells, causing the progression of fibrosis (Natsui et al., 2023). The OMVs were also detected in the ascitic fluid of cirrhotic patients (Natsui et al., 2023). All these findings suggested that OMVs were involved in the pathogenetic mechanisms of cirrhosis. Therefore, within the context of cirrhosis, the presence of OMVs may serve to augment hepatic injury and hasten the progression towards ACLF.
4.4 Prospects for the clinical anti-inflammatory therapy of OMVs
In addition to being pro-inflammatory and pathogenic, OMVs are promising therapeutic targets because they lack the ability to replicate, unlike intact bacteria. However, they have a similar structural composition, a unique nanoscale structure, and good biocompatibility, safety, immunogenicity, and targeting capabilities for disease treatment. OMVs can mediate intercellular information transfer for disease treatment due to their good biocompatibility, safety, immunogenicity, and targeting ability.
A substantial body of research has demonstrated that probiotic OMVs carry certain specific components that can ameliorate inflammation. It has been demonstrated that mucinophilic Ackermannia EVs have the capacity to attenuate liver injury and hepatic fibrosis in mice with liver injury (Keshavarz Azizi Raftar et al., 2021; Raftar et al., 2022). Furthermore, these EVs have been shown to modulate inflammation by restoring the composition of the intestinal flora and the barrier function (Keshavarz Azizi Raftar et al., 2021). In addition, polysaccharide A (PSA) from the OMVs of B. fragilis has been demonstrated to promote the synthesis of the anti-inflammatory factor IL-10 by binding to the TLR2 receptor in dendritic cells (Shagaleeva et al., 2024). This binding has been shown to alleviate DSS-induced colitis and to modulate the intestinal flora (Shagaleeva et al., 2024). In addition, OMVs derived from Escherichia coli Nissle 1917 have been found to express a PD1 agonist, a property that has been demonstrated to reduce the release of inflammatory factors by suppressing immune overactivation and, consequently, to ameliorate colitis (Hu et al., 2025).
In certain conditions, pathogenic OMVs have the potential to be utilized as a therapeutic agent, thereby transforming “enemies” into “friends.” Pan et al. employed the adhesive chemotaxis of Stenotrophomonas maltophilia OMVs to colon tissues and ice chips to construct hybrid liposomes for targeted drug delivery to restore intestinal homeostasis by alleviating inflammation and modulating the dysregulated intestinal epithelial barrier, redox balance, and intestinal microbiota for the treatment of IBD (Pan et al., 2025). In addition, there is considerable potential for OMVs in the domain of vaccine development (Sartorio et al., 2021), given their ability to stimulate the production of high levels of antibodies. A number of OMVs have already been successfully implemented in clinical settings, including the group B meningococcal OMV vaccine and the Neisseria meningitidis OMV vaccine (Petousis-Harris et al., 2017; Gan et al., 2021). Research is underway to develop vaccines for Klebsiella pneumoniae OMVs, Acinetobacter baumannii OMVs, and Bordetella pertussis OMVs (Huang et al., 2019; Assoni et al., 2021; Pschunder et al., 2024).
Many challenges remain in the clinical application of OMVs. However, continued exploration of the pathophysiological mechanisms of OMVs in the body and their translation into effective treatments is expected to lead to the integration of key inflammatory modulators or pathogens associated with ACLF disease into OMVs. This will facilitate the development of novel vaccines that target ACLF disease, attenuate inflammation, and prevent disease progression in future clinical practice.
5 Conclusion
ACLF is a clinical syndrome characterized by an intense systemic inflammatory response and is associated with a complex etiology and pathogenesis. Previous studies have demonstrated that the gut microbiota dysbiosis and impaired barrier function in ACLF drives the translocation of bacteria and their by-products through the gut-liver axis. This process subsequently activates the systemic immune system and induces excessive inflammatory responses and hepatocellular injury.
In this review, we emphasize that OMVs released by gut bacteria act as a “long-range weapon” of bacteria, delivering active ingredients and exacerbating systemic inflammation through activation of TLR signaling pathway, NLRP3 inflammasome, and mitochondrial dysfunction, echoing the pro-inflammatory effects of bacterial metabolites in previous studies. This finding aligns with the pro-inflammatory effects of bacterial metabolites previously observed in studies.
The study focuses on the contribution of OMVs in ACLF inflammation in the context of the disease background of ACLF. It reveals the mechanisms by which OMVs contribute to liver injury, including the activation of macrophages, neutrophils, and hepatic stellate cells. These exacerbate the storm of inflammatory factors and promote hepatic fibrosis and metabolic dysfunction. It is imperative to elucidate the potential pivotal functions of OMVs in the progression of inflammation in ACLF. This review systematically constructs a theoretical framework for the relationship between OMVs and inflammation in ACLF, providing a novel perspective to interpret the precipitous decline in liver function and uncontrolled inflammation in ACLF patients.
Furthermore, OMVs have been demonstrated to enhance disease progression and treatment outcomes through mechanisms such as drug loading, vaccine development, and OMVs modulation. Additionally, studies have explored the use of OMVs in the suppression of hepatic and intestinal inflammation. In light of the transport and biological activity of OMVs, future studies must further analyze the role of OMVs in ACLF. This analysis will provide a theoretical basis for the subsequent development of therapeutic strategies targeting OMVs to improve the severity and progression of ACLF.
Author contributions
XQ: Writing – original draft, Writing – review & editing, Investigation, Visualization. SW: Writing – review & editing, Investigation. ZY: Writing – review & editing, Investigation. NZ: Writing – review & editing, Supervision. JY: Writing – review & editing, Conceptualization, Funding acquisition, Project administration, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Research and Innovation Team Project for Scientific Breakthroughs at Shanxi Bethune Hospital (2024ZHANCHI06); Science and Technology Cooperation and Exchange Special Project of Shanxi Province (202304041101048); Science and Technology Achievements Transformation Guidance Special Project of Shanxi Province (202404021301057); Chinese Group for the Study of Severe Liver Diseases, CGSLD (iGandanF-1082023-CGSLD); and Beijing iGandan Foundation of Funding Support (iGandanF-1082023-GSH018).
Acknowledgments
We would like to thank MedE (http://www.meditorexpert.com) for English language editing. We would like to thank Biorender for providing the professionally designed figure elements to the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Razik, A., Mousa, N., Elhelaly, R., Elzehery, R., Hasan, A. S., Abdelsalam, M., et al. (2020). Helicobacter pylori as an initiating factor of complications in patients with cirrhosis: a single-center observational study. Front. Med. (Lausanne) 7:96. doi: 10.3389/fmed.2020.00096
Adike, A., and Dibaise, J. K. (2018). Small intestinal bacterial overgrowth: nutritional implications, diagnosis, and management. Gastroenterol. Clin. N. Am. 47, 193–208. doi: 10.1016/j.gtc.2017.09.008
Al-Aalim, A. M., Al-Iedani, A. A., and Hamad, M. A. (2021). Study of the effects of Escherichia coli lipopolysaccharide on innate immunity: the expression profile of Tlr4 and Cd14 genes in rat liver. Open Vet. J. 11, 771–779. doi: 10.5455/OVJ.2021.v11.i4.30
Alaniz, R. C., Deatherage, B. L., Lara, J. C., and Cookson, B. T. (2007). Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 179, 7692–7701. doi: 10.4049/jimmunol.179.11.7692
Albillos, A., De Gottardi, A., and Rescigno, M. (2020). The gut-liver axis in liver disease: pathophysiological basis for therapy. J. Hepatol. 72, 558–577. doi: 10.1016/j.jhep.2019.10.003
Albillos, A., Martin-Mateos, R., Van Der Merwe, S., Wiest, R., Jalan, R., and Álvarez-Mon, M. (2022). Cirrhosis-associated immune dysfunction. Nat. Rev. Gastroenterol. Hepatol. 19, 112–134. doi: 10.1038/s41575-021-00520-7
Alexiou, O., Despotis, G., Kalambokis, G., Tsiakas, I., Christaki, M., Tsiouris, S., et al. (2024). Impact of small intestinal bacterial overgrowth on systemic inflammation, circulatory and renal function, and liver fibrosis in patients with cirrhosis and ascites. Ann. Gastroenterol. 37, 348–355. doi: 10.20524/aog.2024.0881
Arroyo, V., Angeli, P., Moreau, R., Jalan, R., Clària, J., Trebicka, J., et al. (2021). The systemic inflammation hypothesis: towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J. Hepatol. 74, 670–685. doi: 10.1016/j.jhep.2020.11.048
Artru, F., and Mcphail, M. J. (2024). Immunopathogenesis of acute on chronic liver failure. Am. J. Transplant. 24, 724–732. doi: 10.1016/j.ajt.2024.02.001
Assimakopoulos, S. F., Tsamandas, A. C., Tsiaoussis, G. I., Karatza, E., Triantos, C., Vagianos, C. E., et al. (2012). Altered intestinal tight junctions' expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur. J. Clin. Investig. 42, 439–446. doi: 10.1111/j.1365-2362.2011.02609.x
Assoni, L., Girardello, R., Converso, T. R., and Darrieux, M. (2021). Current stage in the development of Klebsiella pneumoniae vaccines. Infect. Dis. Ther. 10, 2157–2175. doi: 10.1007/s40121-021-00533-4
Avila-Calderón, E. D., Ruiz-Palma, M. D. S., Aguilera-Arreola, M. G., Velázquez-Guadarrama, N., Ruiz, E. A., Gomez-Lunar, Z., et al. (2021). Outer membrane vesicles of gram-negative Bacteria: an outlook on biogenesis. Front. Microbiol. 12:557902. doi: 10.3389/fmicb.2021.557902
Bajaj, J. S., Heuman, D. M., Hylemon, P. B., Sanyal, A. J., White, M. B., Monteith, P., et al. (2014). Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol. 60, 940–947. doi: 10.1016/j.jhep.2013.12.019
Bajaj, J. S., Kakiyama, G., Savidge, T., Takei, H., Kassam, Z. A., Fagan, A., et al. (2018). Antibiotic-associated disruption of microbiota composition and function in cirrhosis is restored by fecal transplant. Hepatology 68, 1549–1558. doi: 10.1002/hep.30037
Bajaj, J. S., O'leary, J. G., Reddy, K. R., Wong, F., Olson, J. C., Subramanian, R. M., et al. (2012). Second infections independently increase mortality in hospitalized patients with cirrhosis: the north American consortium for the study of end-stage liver disease (Nacseld) experience. Hepatology 56, 2328–2335. doi: 10.1002/hep.25947
Bajaj, J. S., Vargas, H. E., Reddy, K. R., Lai, J. C., O'leary, J. G., Tandon, P., et al. (2019). Association between intestinal microbiota collected at hospital admission and outcomes of patients with cirrhosis. Clin. Gastroenterol. Hepatol. 17, 756–765.e3. doi: 10.1016/j.cgh.2018.07.022
Bishop, D. G., and Work, E. (1965). An extracellular glycolipid produced by Escherichia coli grown under lysine-limiting conditions. Biochem. J. 96, 567–576. doi: 10.1042/bj0960567
Bolori, S., Shegefti, S., Baghaei, K., Yadegar, A., Moon, K. M., Foster, L. J., et al. (2023). The effects of Helicobacter pylori-derived outer membrane vesicles on hepatic stellate cell activation and liver fibrosis in vitro. Biomed. Res. Int. 2023:4848643. doi: 10.1155/2023/4848643
Br, V. K., and Sarin, S. K. (2023). Acute-on-chronic liver failure: terminology, mechanisms and management. Clin. Mol. Hepatol. 29, 670–689. doi: 10.3350/cmh.2022.0103
Buti, M., Ruiz-Cobo, J. C., Esteban, R., and Riveiro-Barciela, M. (2025). Hepatitis E as a trigger for acute-on-chronic liver failure. Clin. Mol. Hepatol. 31, S196–s204. doi: 10.3350/cmh.2024.0758
Butterworth, R. F. (2019). Hepatic encephalopathy in cirrhosis: pathology and pathophysiology. Drugs 79, 17–21. doi: 10.1007/s40265-018-1017-0
Cani, P. D., and Knauf, C. (2016). How gut microbes talk to organs: the role of endocrine and nervous routes. Mol Metab 5, 743–752. doi: 10.1016/j.molmet.2016.05.011
Cesaro, C., Tiso, A., Del Prete, A., Cariello, R., Tuccillo, C., Cotticelli, G., et al. (2011). Gut microbiota and probiotics in chronic liver diseases. Dig. Liver Dis. 43, 431–438. doi: 10.1016/j.dld.2010.10.015
Chang, Y., Jeong, S. W., and Jang, J. Y. (2021). Hepatitis B virus reactivation associated with therapeutic interventions. Front. Med. (Lausanne) 8:770124. doi: 10.3389/fmed.2021.770124
Chen, Y., Guo, J., Qian, G., Fang, D., Shi, D., Guo, L., et al. (2015). Gut dysbiosis in acute-on-chronic liver failure and its predictive value for mortality. J. Gastroenterol. Hepatol. 30, 1429–1437. doi: 10.1111/jgh.12932
Chen, S., Lei, Q., Zou, X., and Ma, D. (2023b). The role and mechanisms of gram-negative bacterial outer membrane vesicles in inflammatory diseases. Front. Immunol. 14:1157813. doi: 10.3389/fimmu.2023.1157813
Chen, S. N., Tan, Y., Xiao, X. C., Li, Q., Wu, Q., Peng, Y. Y., et al. (2021). Deletion of Tlr4 attenuates lipopolysaccharide-induced acute liver injury by inhibiting inflammation and apoptosis. Acta Pharmacol. Sin. 42, 1610–1619. doi: 10.1038/s41401-020-00597-x
Chen, Y., Yang, F., Lu, H., Wang, B., Chen, Y., Lei, D., et al. (2011). Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 54, 562–572. doi: 10.1002/hep.24423
Chen, P. P., Zhang, J. X., Li, X. Q., Li, L., Wu, Q. Y., Liu, L., et al. (2023a). Outer membrane vesicles derived from gut microbiota mediate tubulointerstitial inflammation: a potential new mechanism for diabetic kidney disease. Theranostics 13, 3988–4003. doi: 10.7150/thno.84650
Cheng, K., Liu, K., Liu, S., Zhao, Y., and Wang, Q. (2024). Igf2bp3 regulates macrophage-induced inflammation and liver damage in acute-on-chronic liver failure via the Rorα-Nf-κB signaling axis. Int. Immunopharmacol. 142:113030. doi: 10.1016/j.intimp.2024.113030
Ciesielska, A., Matyjek, M., and Kwiatkowska, K. (2021). Tlr4 and Cd14 trafficking and its influence on Lps-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 78, 1233–1261. doi: 10.1007/s00018-020-03656-y
Clària, J., Arroyo, V., and Moreau, R. (2023). Roles of systemic inflammatory and metabolic responses in the pathophysiology of acute-on-chronic liver failure. JHEP Rep. 5:100807. doi: 10.1016/j.jhepr.2023.100807
Clària, J., Stauber, R. E., Coenraad, M. J., Moreau, R., Jalan, R., Pavesi, M., et al. (2016). Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology 64, 1249–1264. doi: 10.1002/hep.28740
Davis, J. M., Carvalho, H. M., Rasmussen, S. B., and O'brien, A. D. (2006). Cytotoxic necrotizing factor type 1 delivered by outer membrane vesicles of uropathogenic Escherichia coli attenuates polymorphonuclear leukocyte antimicrobial activity and chemotaxis. Infect. Immun. 74, 4401–4408. doi: 10.1128/IAI.00637-06
De Kimpe, S. J., Kengatharan, M., Thiemermann, C., and Vane, J. R. (1995). The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. USA 92, 10359–10363. doi: 10.1073/pnas.92.22.10359
Deo, P., Chow, S. H., Han, M. L., Speir, M., Huang, C., Schittenhelm, R. B., et al. (2020). Mitochondrial dysfunction caused by outer membrane vesicles from gram-negative bacteria activates intrinsic apoptosis and inflammation. Nat. Microbiol. 5, 1418–1427. doi: 10.1038/s41564-020-0773-2
Devarbhavi, H., Choudhury, A. K., Sharma, M. K., Maiwall, R., Al Mahtab, M., Rahman, S., et al. (2019). Drug-induced acute-on-chronic liver failure in Asian patients. Am. J. Gastroenterol. 114, 929–937. doi: 10.14309/ajg.0000000000000201
Dorner, H., Stolzer, I., Mattner, J., Kaminski, S., Leistl, S., Edrich, L. M., et al. (2024). Gut Pathobiont-derived outer membrane vesicles drive liver inflammation and fibrosis in primary Sclerosing cholangitis-associated inflammatory bowel disease. Gastroenterology 167, 1183–1197.e16. doi: 10.1053/j.gastro.2024.06.032
Elmi, A., Nasher, F., Jagatia, H., Gundogdu, O., Bajaj-Elliott, M., Wren, B., et al. (2016). Campylobacter jejuni outer membrane vesicle-associated proteolytic activity promotes bacterial invasion by mediating cleavage of intestinal epithelial cell E-cadherin and occludin. Cell. Microbiol. 18, 561–572. doi: 10.1111/cmi.12534
Engelmann, C., Clària, J., Szabo, G., Bosch, J., and Bernardi, M. (2021). Pathophysiology of decompensated cirrhosis: portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J. Hepatol. 75, S49–s66. doi: 10.1016/j.jhep.2021.01.002
Engelmann, C., Habtesion, A., Hassan, M., Kerbert, A. J., Hammerich, L., Novelli, S., et al. (2022). Combination of G-Csf and a Tlr4 inhibitor reduce inflammation and promote regeneration in a mouse model of Aclf. J. Hepatol. 77, 1325–1338. doi: 10.1016/j.jhep.2022.07.006
Engelmann, C., Sheikh, M., Sharma, S., Kondo, T., Loeffler-Wirth, H., Zheng, Y. B., et al. (2020). Toll-like receptor 4 is a therapeutic target for prevention and treatment of liver failure. J. Hepatol. 73, 102–112. doi: 10.1016/j.jhep.2020.01.011
Engevik, M. A., Danhof, H. A., Ruan, W., Engevik, A. C., Chang-Graham, A. L., Engevik, K. A., et al. (2021). Fusobacterium nucleatum secretes outer membrane vesicles and promotes intestinal inflammation. mBio 12:e02706-20. doi: 10.1128/mBio.02706-20
Fan, Y., Li, Y., Chu, Y., Liu, J., Cui, L., and Zhang, D. (2021). Toll-like receptors recognize intestinal microbes in liver cirrhosis. Front. Immunol. 12:608498. doi: 10.3389/fimmu.2021.608498
Fan, Y., and Pedersen, O. (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71. doi: 10.1038/s41579-020-0433-9
Fernández, J., Acevedo, J., Wiest, R., Gustot, T., Amoros, A., Deulofeu, C., et al. (2018). Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut 67, 1870–1880. doi: 10.1136/gutjnl-2017-314240
Fiocca, R., Necchi, V., Sommi, P., Ricci, V., Telford, J., Cover, T. L., et al. (1999). Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 188, 220–226. doi: 10.1002/(SICI)1096-9896(199906)188:2<>3.0.CO;2-C
Fouts, D. E., Torralba, M., Nelson, K. E., Brenner, D. A., and Schnabl, B. (2012). Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J. Hepatol. 56, 1283–1292. doi: 10.1016/j.jhep.2012.01.019
Gan, Y., Li, C., Peng, X., Wu, S., Li, Y., Tan, J. P. K., et al. (2021). Fight bacteria with bacteria: bacterial membrane vesicles as vaccines and delivery nanocarriers against bacterial infections. Nanomedicine 35:102398. doi: 10.1016/j.nano.2021.102398
Gasaly, N., De Vos, P., and Hermoso, M. A. (2021). Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front. Immunol. 12:658354. doi: 10.3389/fimmu.2021.658354
Gong, T., Chen, Q., Mao, H., Zhang, Y., Ren, H., Xu, M., et al. (2022). Outer membrane vesicles of Porphyromonas gingivalis trigger Nlrp3 inflammasome and induce neuroinflammation, tau phosphorylation, and memory dysfunction in mice. Front. Cell. Infect. Microbiol. 12:925435. doi: 10.3389/fcimb.2022.925435
Gudan, A., Jamioł-Milc, D., Hawryłkowicz, V., Skonieczna-Żydecka, K., and Stachowska, E. (2022). The prevalence of small intestinal bacterial overgrowth in patients with non-alcoholic liver diseases: Nafld, Nash, fibrosis, cirrhosis-a systematic review, Meta-analysis and Meta-regression. Nutrients 14:5261. doi: 10.3390/nu14245261
Gudan, A., Kozłowska-Petriczko, K., Wunsch, E., Bodnarczuk, T., and Stachowska, E. (2023). Small intestinal bacterial overgrowth and non-alcoholic fatty liver disease: what do we know in 2023? Nutrients 15:1323. doi: 10.3390/nu15061323
Hammerich, L., and Tacke, F. (2023). Hepatic inflammatory responses in liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 20, 633–646. doi: 10.1038/s41575-023-00807-x
Han, H. S., Hwang, S., Choi, S. Y., Hitayezu, E., Humphrey, M. A., Enkhbayar, A., et al. (2024). Roseburia intestinalis-derived extracellular vesicles ameliorate colitis by modulating intestinal barrier, microbiome, and inflammatory responses. J Extracell Vesicles 13:e12487. doi: 10.1002/jev2.12487
Hartmann, P., and Schnabl, B. (2023). Fungal infections and the fungal microbiome in hepatobiliary disorders. J. Hepatol. 78, 836–851. doi: 10.1016/j.jhep.2022.12.006
Hasa, E., Hartmann, P., and Schnabl, B. (2022). Liver cirrhosis and immune dysfunction. Int. Immunol. 34, 455–466. doi: 10.1093/intimm/dxac030
Hassan, H. M., Liang, X., Xin, J., Lu, Y., Cai, Q., Shi, D., et al. (2024). Thrombospondin 1 enhances systemic inflammation and disease severity in acute-on-chronic liver failure. BMC Med. 22:95. doi: 10.1186/s12916-024-03318-x
Hendrix, A., and De Wever, O. (2022). Systemically circulating bacterial extracellular vesicles: origin, fate, and function. Trends Microbiol. 30, 213–216. doi: 10.1016/j.tim.2021.12.012
Hiippala, K., Jouhten, H., Ronkainen, A., Hartikainen, A., Kainulainen, V., Jalanka, J., et al. (2018). The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 10:988. doi: 10.3390/nu10080988
Ho, M. Y., Liu, S., and Xing, B. (2024). Bacteria extracellular vesicle as nanopharmaceuticals for versatile biomedical potential. Nano Converg 11:28. doi: 10.1186/s40580-024-00434-5
Hsu, C. L., and Schnabl, B. (2023). The gut-liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 21, 719–733. doi: 10.1038/s41579-023-00904-3
Hu, M., Li, T., Xu, M., Dong, A., Zhang, C., Wang, L., et al. (2025). Engineering Escherichia coli Nissle 1917 carrying Pd1 agonists resolves intestinal inflammation via local immune modulation. ACS Appl. Mater. Interfaces 17:4339. doi: 10.1021/acsami.5c04339
Huang, W., Zhang, Q., Li, W., Chen, Y., Shu, C., Li, Q., et al. (2019). Anti-outer membrane vesicle antibodies increase antibiotic sensitivity of Pan-drug-resistant Acinetobacter baumannii. Front. Microbiol. 10:1379. doi: 10.3389/fmicb.2019.01379
Jain, H., Kumar, A., Almousa, S., Mishra, S., Langsten, K. L., Kim, S., et al. (2024). Characterisation of Lps+ bacterial extracellular vesicles along the gut-hepatic portal vein-liver axis. J Extracell Vesicles 13:e12474. doi: 10.1002/jev2.12474
Jan, A. T. (2017). Outer membrane vesicles (Omvs) of gram-negative Bacteria: a perspective update. Front. Microbiol. 8:1053. doi: 10.3389/fmicb.2017.01053
Jiang, M. P., Xu, C., Guo, Y. W., Luo, Q. J., Li, L., Liu, H. L., et al. (2018). Β-Arrestin 2 attenuates lipopolysaccharide-induced liver injury via inhibition of Tlr4/Nf-κB signaling pathway-mediated inflammation in mice. World J. Gastroenterol. 24, 216–225. doi: 10.3748/wjg.v24.i2.216
Jiang, H., Yu, Y., Hu, X., Du, B., Shao, Y., Wang, F., et al. (2024). The fecal microbiota of patients with primary biliary cholangitis (Pbc) causes Pbc-like liver lesions in mice and exacerbates liver damage in a mouse model of Pbc. Gut Microbes 16:2383353. doi: 10.1080/19490976.2024.2383353
Jones, E. J., Booth, C., Fonseca, S., Parker, A., Cross, K., Miquel-Clopés, A., et al. (2020). The uptake, trafficking, and biodistribution of Bacteroides thetaiotaomicron generated outer membrane vesicles. Front. Microbiol. 11:57. doi: 10.3389/fmicb.2020.00057
Juodeikis, R., and Carding, S. R. (2022). Outer membrane vesicles: biogenesis, functions, and issues. Microbiol. Mol. Biol. Rev. 86:e0003222. doi: 10.1128/mmbr.00032-22
Kanda, T., Sasaki, R., Masuzaki, R., Takahashi, H., Mizutani, T., Matsumoto, N., et al. (2020). Co-occurrence of hepatitis a infection and chronic liver disease. Int. J. Mol. Sci. 21:6384. doi: 10.3390/ijms21176384
Kang, E. J., Cha, M. G., Kwon, G. H., Han, S. H., Yoon, S. J., Lee, S. K., et al. (2024). Akkermansia muciniphila improve cognitive dysfunction by regulating Bdnf and serotonin pathway in gut-liver-brain axis. Microbiome 12:181. doi: 10.1186/s40168-024-01924-8
Kaparakis-Liaskos, M., and Ferrero, R. L. (2015). Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 15, 375–387. doi: 10.1038/nri3837
Keshavarz Azizi Raftar, S., Ashrafian, F., Yadegar, A., Lari, A., Moradi, H. R., Shahriary, A., et al. (2021). The protective effects of live and pasteurized Akkermansia muciniphila and its extracellular vesicles against HFD/Ccl4-induced liver injury. Microbiol. Spectr. 9:e0048421. doi: 10.1128/Spectrum.00484-21
Kim, S.-E., Park, J. W., Kim, H. S., Jang, M.-K., Suk, K. T., and Kim, D. J. (2021). The role of gut Dysbiosis in acute-on-chronic liver failure. Int. J. Mol. Sci. 22:1680. doi: 10.3390/ijms222111680
Krsek, D., Yara, D. A., Hrbáčková, H., Daniel, O., Mančíková, A., Schüller, S., et al. (2023). Translocation of outer membrane vesicles from enterohemorrhagic Escherichia coli O157 across the intestinal epithelial barrier. Front. Microbiol. 14:1198945. doi: 10.3389/fmicb.2023.1198945
Kumar, M., Sharma, B. C., and Sarin, S. K. (2008). Hepatitis E virus as an etiology of acute exacerbation of previously unrecognized asymptomatic patients with hepatitis B virus-related chronic liver disease. J. Gastroenterol. Hepatol. 23, 883–887. doi: 10.1111/j.1440-1746.2007.05243.x
Lauritano, E. C., Valenza, V., Sparano, L., Scarpellini, E., Gabrielli, M., Cazzato, A., et al. (2010). Small intestinal bacterial overgrowth and intestinal permeability. Scand. J. Gastroenterol. 45, 1131–1132. doi: 10.3109/00365521.2010.485325
Li, Q., Chen, F., and Wang, F. (2022b). The immunological mechanisms and therapeutic potential in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Cell Biosci. 12:187. doi: 10.1186/s13578-022-00921-4
Li, Z., and Jiang, J. (2021). The Nlrp3 inflammasome mediates liver failure by activating procaspase-1 and pro-Il-1 β and regulating downstream Cd40-Cd40L signaling. J. Int. Med. Res. 49:3000605211036845. doi: 10.1177/03000605211036845
Li, J., Liang, X., Jiang, J., Yang, L., Xin, J., Shi, D., et al. (2022a). Pbmc transcriptomics identifies immune-metabolism disorder during the development of Hbv-Aclf. Gut 71, 163–175. doi: 10.1136/gutjnl-2020-323395
Li, L., Xu, X., Cheng, P., Yu, Z., Li, M., Yu, Z., et al. (2025). Klebsiella pneumoniae derived outer membrane vesicles mediated bacterial virulence, antibiotic resistance, host immune responses and clinical applications. Virulence 16:2449722. doi: 10.1080/21505594.2025.2449722
Li, J., Yu, H., Wang, Y., Wang, B., Zhang, R., Chen, S., et al. (2023). A meta-analysis of the association between Helicobacter pylori infection and risk of hepatic encephalopathy. J. Public Health (Oxf.) 45, 321–329. doi: 10.1093/pubmed/fdac078
Li, B., Zhao, Y., Liu, C., Chen, Z., and Zhou, D. (2014). Molecular pathogenesis of Klebsiella pneumoniae. Future Microbiol. 9, 1071–1081. doi: 10.2217/fmb.14.48
Liang, X., Dai, N., Sheng, K., Lu, H., Wang, J., Chen, L., et al. (2022). Gut bacterial extracellular vesicles: important players in regulating intestinal microenvironment. Gut Microbes 14:2134689. doi: 10.1080/19490976.2022.2134689
Lin, R. S., Lee, F. Y., Lee, S. D., Tsai, Y. T., Lin, H. C., Lu, R. H., et al. (1995). Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J. Hepatol. 22, 165–172. doi: 10.1016/0168-8278(95)80424-2
Liu, R., Kang, J. D., Sartor, R. B., Sikaroodi, M., Fagan, A., Gavis, E. A., et al. (2020). Neuroinflammation in murine cirrhosis is dependent on the gut microbiome and is attenuated by fecal transplant. Hepatology 71, 611–626. doi: 10.1002/hep.30827
Liu, L., Liang, L., Yang, C., Zhou, Y., and Chen, Y. (2021b). Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting Ripk1-mediated cell death pathway. Gut Microbes 13, 1–20. doi: 10.1080/19490976.2021.1902718
Liu, J., Xu, Y., and Jiang, B. (2021a). Novel insights into pathogenesis and therapeutic strategies of hepatic encephalopathy, from the gut microbiota perspective. Front. Cell. Infect. Microbiol. 11:586427. doi: 10.3389/fcimb.2021.586427
Lu, Y., Zhuge, J., Wang, X., Bai, J., and Cederbaum, A. I. (2008). Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology 47, 1483–1494. doi: 10.1002/hep.22222
Luo, Z., Ji, Y., Gao, H., Gomes Dos Reis, F. C., Bandyopadhyay, G., Jin, Z., et al. (2021). Crig (+) macrophages prevent gut microbial Dna-containing extracellular vesicle-induced tissue inflammation and insulin resistance. Gastroenterology 160, 863–874. doi: 10.1053/j.gastro.2020.10.042
Luo, Z., Ji, Y., Zhang, D., Gao, H., Jin, Z., Yang, M., et al. (2022). Microbial Dna enrichment promotes liver steatosis and fibrosis in the course of non-alcoholic steatohepatitis. Acta Physiol (Oxf.) 235:e13827. doi: 10.1111/apha.13827
Lv, T., Fan, X., He, C., Zhu, S., Xiong, X., Yan, W., et al. (2024). Slc7A11-Ros/αkg-Ampk axis regulates liver inflammation through mitophagy and impairs liver fibrosis and Nash progression. Redox Biol. 72:103159. doi: 10.1016/j.redox.2024.103159
Marion, C. R., Lee, J., Sharma, L., Park, K. S., Lee, C., Liu, W., et al. (2019). Toll-like receptors 2 and 4 modulate pulmonary inflammation and host factors mediated by outer membrane vesicles derived from Acinetobacter baumannii. Infect. Immun. 87:e00243-19. doi: 10.1128/IAI.00243-19
Martin-Mateos, R., Alvarez-Mon, M., and Albillos, A. (2019). Dysfunctional immune response in acute-on-chronic liver failure: it takes two to tango. Front. Immunol. 10:973. doi: 10.3389/fimmu.2019.00973
Mayer, E. A., Tillisch, K., and Gupta, A. (2015). Gut/brain axis and the microbiota. J. Clin. Invest. 125, 926–938. doi: 10.1172/JCI76304
Mehtani, R., Roy, A., and Singh, V. (2021). Gut microbial metagenomics in Aclf: the causality-association conundrum. Gastroenterology 160:2205. doi: 10.1053/j.gastro.2020.12.055
Mezzano, G., Juanola, A., Cardenas, A., Mezey, E., Hamilton, J. P., Pose, E., et al. (2022). Global burden of disease: acute-on-chronic liver failure, a systematic review and meta-analysis. Gut 71, 148–155. doi: 10.1136/gutjnl-2020-322161
Mohammadi, M., Attar, A., Mohammadbeigi, M., Peymani, A., Bolori, S., and Fardsanei, F. (2023). The possible role of Helicobacter pylori in liver diseases. Arch. Microbiol. 205:281. doi: 10.1007/s00203-023-03602-z
Moreau, R., Clària, J., Aguilar, F., Fenaille, F., Lozano, J. J., Junot, C., et al. (2020). Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J. Hepatol. 72, 688–701. doi: 10.1016/j.jhep.2019.11.009
Moreau, R., Jalan, R., Gines, P., Pavesi, M., Angeli, P., Cordoba, J., et al. (2013). Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 144:1437.e1-9, 1426–1437. doi: 10.1053/j.gastro.2013.02.042
Nadalian, B., Nadalian, B., Zali, M. R., and Yadegar, A. (2024). Outer membrane vesicles derived from adherent-invasive Escherichia coli induce inflammatory response and alter the gene expression of junction-associated proteins in human intestinal epithelial cells. Can. J. Infect. Dis. Med. Microbiol. 2024:2701675. doi: 10.1155/2024/2701675
Natsui, K., Tsuchiya, A., Imamiya, R., Osada-Oka, M., Ishii, Y., Koseki, Y., et al. (2023). Escherichia coli-derived outer-membrane vesicles induce immune activation and progression of cirrhosis in mice and humans. Liver Int. 43, 1126–1140. doi: 10.1111/liv.15539
Nie, Y., Xie, X. Q., Zhou, L., Guan, Q., Ren, Y., Mao, Y., et al. (2022). Desulfovibrio fairfieldensis-derived outer membrane vesicles damage epithelial barrier and induce inflammation and pyroptosis in macrophages. Cells 12:89. doi: 10.3390/cells12010089
Okushin, K., Tsutsumi, T., Ikeuchi, K., Kado, A., Enooku, K., Fujinaga, H., et al. (2018). Helicobacter pylori infection and liver diseases: epidemiology and insights into pathogenesis. World J. Gastroenterol. 24, 3617–3625. doi: 10.3748/wjg.v24.i32.3617
Pan, C., Gu, Y., Zhang, W., Zheng, Y., Peng, L., Deng, H., et al. (2012). Dynamic changes of lipopolysaccharide levels in different phases of acute on chronic hepatitis B liver failure. PLoS One 7:e49460. doi: 10.1371/journal.pone.0049460
Pan, X., Xian, P., Li, Y., Zhao, X., Zhang, J., Song, Y., et al. (2025). Chemotaxis-driven hybrid liposomes recover intestinal homeostasis for targeted colitis therapy. J. Control. Release 380, 829–845. doi: 10.1016/j.jconrel.2025.02.036
Panda, S. K., and Varma, S. P. (2013). Hepatitis e: molecular virology and pathogenesis. J. Clin. Exp. Hepatol. 3, 114–124. doi: 10.1016/j.jceh.2013.05.001
Pande, C., Kumar, A., and Sarin, S. K. (2009). Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment. Pharmacol. Ther. 29, 1273–1281. doi: 10.1111/j.1365-2036.2009.03994.x
Park, K. S., Choi, K. H., Kim, Y. S., Hong, B. S., Kim, O. Y., Kim, J. H., et al. (2010). Outer membrane vesicles derived from Escherichia coli induce systemic inflammatory response syndrome. PLoS One 5:e11334. doi: 10.1371/journal.pone.0011334
Park, K. S., Lee, J., Lee, C., Park, H. T., Kim, J. W., Kim, O. Y., et al. (2018). Sepsis-like systemic inflammation induced by Nano-sized extracellular vesicles from feces. Front. Microbiol. 9:1735. doi: 10.3389/fmicb.2018.01735
Parlesak, A., Schäfer, C., Schütz, T., Bode, J. C., and Bode, C. (2000). Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J. Hepatol. 32, 742–747. doi: 10.1016/S0168-8278(00)80242-1
Patel, V. C., Lee, S., Mcphail, M. J. W., Da Silva, K., Guilly, S., Zamalloa, A., et al. (2022). Rifaximin-α reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: Rifsys randomised controlled trial. J. Hepatol. 76, 332–342. doi: 10.1016/j.jhep.2021.09.010
Petousis-Harris, H., Paynter, J., Morgan, J., Saxton, P., Mcardle, B., Goodyear-Smith, F., et al. (2017). Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 390, 1603–1610. doi: 10.1016/S0140-6736(17)31449-6
Piano, S., Bunchorntavakul, C., Marciano, S., and Rajender Reddy, K. (2024). Infections in cirrhosis. Lancet Gastroenterol. Hepatol. 9, 745–757. doi: 10.1016/S2468-1253(24)00078-5
Pschunder, B., Locati, L., López, O., Martin Aispuro, P., Zurita, E., Stuible, M., et al. (2024). Outer membrane vesicles derived from Bordetella pertussis are potent adjuvant that drive Th1-biased response. Front. Immunol. 15:1387534. doi: 10.3389/fimmu.2024.1387534
Qian, Y., Zhao, J., Wu, H., and Kong, X. (2024). Innate immune regulation in inflammation resolution and liver regeneration in drug-induced liver injury. Arch. Toxicol. 99, 115–126. doi: 10.1007/s00204-024-03886-0
Qin, N., Yang, F., Li, A., Prifti, E., Chen, Y., Shao, L., et al. (2014). Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64. doi: 10.1038/nature13568
Raftar, S. K. A., Ashrafian, F., Abdollahiyan, S., Yadegar, A., Moradi, H. R., Masoumi, M., et al. (2022). The anti-inflammatory effects of Akkermansia muciniphila and its derivates in Hfd/Ccl4-induced murine model of liver injury. Sci. Rep. 12:2453. doi: 10.1038/s41598-022-06414-1
Samuel, D. (2021). Systemic inflammation and liver cirrhosis complications: driving or secondary event? How to square the circle? J. Hepatol. 74, 508–510. doi: 10.1016/j.jhep.2021.01.001
Sangiorgio, G., Nicitra, E., Bivona, D., Bonomo, C., Bonacci, P., Santagati, M., et al. (2024). Interactions of gram-positive bacterial membrane vesicles and hosts: updates and future directions. Int. J. Mol. Sci. 25:2904. doi: 10.3390/ijms25052904
Sarin, S. K., Choudhury, A., Sharma, M. K., Maiwall, R., Al Mahtab, M., Rahman, S., et al. (2019). Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (Apasl): an update. Hepatol. Int. 13, 353–390. doi: 10.1007/s12072-019-09946-3
Sartorio, M. G., Pardue, E. J., Feldman, M. F., and Haurat, M. F. (2021). Bacterial outer membrane vesicles: from discovery to applications. Ann. Rev. Microbiol. 75, 609–630. doi: 10.1146/annurev-micro-052821-031444
Schnabl, B., and Brenner, D. A. (2014). Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146, 1513–1524. doi: 10.1053/j.gastro.2014.01.020
Seki, E., De Minicis, S., Osterreicher, C. H., Kluwe, J., Osawa, Y., Brenner, D. A., et al. (2007). Tlr4 enhances Tgf-beta signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332. doi: 10.1038/nm1663
Seyama, M., Yoshida, K., Yoshida, K., Fujiwara, N., Ono, K., Eguchi, T., et al. (2020). Outer membrane vesicles of Porphyromonas gingivalis attenuate insulin sensitivity by delivering gingipains to the liver. Biochim. Biophys. Acta Mol. basis Dis. 1866:165731. doi: 10.1016/j.bbadis.2020.165731
Shagaleeva, O. Y., Kashatnikova, D. A., Kardonsky, D. A., Efimov, B. A., Ivanov, V. A., Smirnova, S. V., et al. (2024). Bacteroides vesicles promote functional alterations in the gut microbiota composition. Microbiol Spectr 12:e0063624. doi: 10.1128/spectrum.00636-24
Sharma, S. P., Gupta, H., Kwon, G. H., Lee, S. Y., Song, S. H., Kim, J. S., et al. (2024). Gut microbiome and metabolome signatures in liver cirrhosis-related complications. Clin. Mol. Hepatol. 30, 845–862. doi: 10.3350/cmh.2024.0349
Sharma, A., Roy, A., Premkumar, M., Verma, N., Duseja, A., Taneja, S., et al. (2022). Fecal microbiota transplantation in alcohol-associated acute-on-chronic liver failure: an open-label clinical trial. Hepatol. Int. 16, 433–446. doi: 10.1007/s12072-022-10312-z
Shegefti, S., Bolori, S., Nabavi-Rad, A., Dabiri, H., Yadegar, A., and Baghaei, K. (2023). Helicobacter pylori-derived outer membrane vesicles suppress liver autophagy: a novel mechanism for H. pylori-mediated hepatic disorder. Microb. Pathog. 183:106319. doi: 10.1016/j.micpath.2023.106319
Shen, B., Gu, T., Shen, Z., Zhou, C., Guo, Y., Wang, J., et al. (2023). Escherichia coli promotes endothelial to mesenchymal transformation of liver sinusoidal endothelial cells and exacerbates nonalcoholic fatty liver disease via its Flagellin. Cell. Mol. Gastroenterol. Hepatol. 16, 857–879. doi: 10.1016/j.jcmgh.2023.08.001
Shin, E. C., and Jeong, S. H. (2018). Natural history, clinical manifestations, and pathogenesis of hepatitis a. Cold Spring Harb. Perspect. Med. 8:31708. doi: 10.1101/cshperspect.a031708
Simbrunner, B., Caparrós, E., Neuwirth, T., Schwabl, P., Königshofer, P., Bauer, D., et al. (2023). Bacterial translocation occurs early in cirrhosis and triggers a selective inflammatory response. Hepatol. Int. 17, 1045–1056. doi: 10.1007/s12072-023-10496-y
Singal, A. K., and Shah, V. H. (2019). Current trials and novel therapeutic targets for alcoholic hepatitis. J. Hepatol. 70, 305–313. doi: 10.1016/j.jhep.2018.10.026
Solé, C., Guilly, S., Da Silva, K., Llopis, M., Le-Chatelier, E., Huelin, P., et al. (2021). Alterations in gut microbiome in cirrhosis as assessed by quantitative metagenomics: relationship with acute-on-chronic liver failure and prognosis. Gastroenterology 160, 206–218.e13. doi: 10.1053/j.gastro.2020.08.054
Spari, D., Schmid, A., Sanchez-Taltavull, D., Murugan, S., Keller, K., Ennaciri, N., et al. (2024). Released bacterial Atp shapes local and systemic inflammation during abdominal sepsis. eLife 13:96678. doi: 10.7554/eLife.96678
Stols-Gonçalves, D., Mak, A. L., Madsen, M. S., Van Der Vossen, E. W. J., Bruinstroop, E., Henneman, P., et al. (2023). Faecal microbiota transplantation affects liver Dna methylation in non-alcoholic fatty liver disease: a multi-omics approach. Gut Microbes 15:2223330. doi: 10.1080/19490976.2023.2223330
Surewaard, B. G. J., Thanabalasuriar, A., Zeng, Z., Tkaczyk, C., Cohen, T. S., Bardoel, B. W., et al. (2018). Α-Toxin induces platelet aggregation and liver injury during Staphylococcus aureus Sepsis. Cell Host Microbe 24, 271–284.e3. doi: 10.1016/j.chom.2018.06.017
Suriguga, S., Li, M., Luangmonkong, T., Boersema, M., De Jong, K. P., Oosterhuis, D., et al. (2022). Distinct responses between healthy and cirrhotic human livers upon lipopolysaccharide challenge: possible implications for acute-on-chronic liver failure. Am. J. Physiol. Gastrointest. Liver Physiol. 323, G114–G125. doi: 10.1152/ajpgi.00243.2021
Svennerholm, K., Park, K. S., Wikström, J., Lässer, C., Crescitelli, R., Shelke, G. V., et al. (2017). Escherichia coli outer membrane vesicles can contribute to sepsis induced cardiac dysfunction. Sci. Rep. 7:17434. doi: 10.1038/s41598-017-16363-9
Szabo, G. (2015). Gut-liver axis in alcoholic liver disease. Gastroenterology 148, 30–36. doi: 10.1053/j.gastro.2014.10.042
Taru, V., Szabo, G., Mehal, W., and Reiberger, T. (2024). Inflammasomes in chronic liver disease: hepatic injury, fibrosis progression and systemic inflammation. J. Hepatol. 81, 895–910. doi: 10.1016/j.jhep.2024.06.016
Thay, B., Damm, A., Kufer, T. A., Wai, S. N., and Oscarsson, J. (2014). Aggregatibacter actinomycetemcomitans outer membrane vesicles are internalized in human host cells and trigger Nod1- and Nod2-dependent Nf-κB activation. Infect. Immun. 82, 4034–4046. doi: 10.1128/IAI.01980-14
Tiku, V., and Tan, M. W. (2021). Host immunity and cellular responses to bacterial outer membrane vesicles. Trends Immunol. 42, 1024–1036. doi: 10.1016/j.it.2021.09.006
Tilg, H., Adolph, T. E., and Trauner, M. (2022). Gut-liver axis: pathophysiological concepts and clinical implications. Cell Metab. 34, 1700–1718. doi: 10.1016/j.cmet.2022.09.017
Tilg, H., Wilmer, A., Vogel, W., Herold, M., Nölchen, B., Judmaier, G., et al. (1992). Serum levels of cytokines in chronic liver diseases. Gastroenterology 103, 264–274. doi: 10.1016/0016-5085(92)91122-K
Toyofuku, M., Nomura, N., and Eberl, L. (2019). Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 17, 13–24. doi: 10.1038/s41579-018-0112-2
Toyofuku, M., Schild, S., Kaparakis-Liaskos, M., and Eberl, L. (2023). Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 21, 415–430. doi: 10.1038/s41579-023-00875-5
Tranah, T. H., Edwards, L. A., Schnabl, B., and Shawcross, D. L. (2021). Targeting the gut-liver-immune axis to treat cirrhosis. Gut 70, 982–994. doi: 10.1136/gutjnl-2020-320786
Trebicka, J., Bork, P., Krag, A., and Arumugam, M. (2021a). Utilizing the gut microbiome in decompensated cirrhosis and acute-on-chronic liver failure. Nat. Rev. Gastroenterol. Hepatol. 18, 167–180. doi: 10.1038/s41575-020-00376-3
Trebicka, J., Fernandez, J., Papp, M., Caraceni, P., Laleman, W., Gambino, C., et al. (2021b). Predict identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J. Hepatol. 74, 1097–1108. doi: 10.1016/j.jhep.2020.11.019
Trebicka, J., Macnaughtan, J., Schnabl, B., Shawcross, D. L., and Bajaj, J. S. (2021c). The microbiota in cirrhosis and its role in hepatic decompensation. J. Hepatol. 75, S67–s81. doi: 10.1016/j.jhep.2020.11.013
Triantos, C., Kalafateli, M., Assimakopoulos, S. F., Karaivazoglou, K., Mantaka, A., Aggeletopoulou, I., et al. (2022). Endotoxin translocation and gut barrier dysfunction are related to variceal bleeding in patients with liver cirrhosis. Front Med (Lausanne) 9:836306. doi: 10.3389/fmed.2022.836306
Tulkens, J., Vergauwen, G., Van Deun, J., Geeurickx, E., Dhondt, B., Lippens, L., et al. (2020). Increased levels of systemic Lps-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut 69, 191–193. doi: 10.1136/gutjnl-2018-317726
Turkina, M. V., Olofsson, A., Magnusson, K. E., Arnqvist, A., and Vikström, E. (2015). Helicobacter pylori vesicles carrying CagA localize in the vicinity of cell-cell contacts and induce histone H1 binding to Atp in epithelial cells. FEMS Microbiol. Lett. 362:76. doi: 10.1093/femsle/fnv076
Turner, J. R. (2009). Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809. doi: 10.1038/nri2653
Vanaja, S. K., Russo, A. J., Behl, B., Banerjee, I., Yankova, M., Deshmukh, S. D., et al. (2016). Bacterial outer membrane vesicles mediate cytosolic localization of Lps and Caspase-11 activation. Cell 165, 1106–1119. doi: 10.1016/j.cell.2016.04.015
Verbeke, L., Nevens, F., and Laleman, W. (2011). Bench-to-beside review: acute-on-chronic liver failure - linking the gut, liver and systemic circulation. Crit. Care 15:233. doi: 10.1186/cc10424
Verma, N., Garg, P., Bhardwaj, A., Sarohi, V., and Singh, S. (2023). Candida leucine aminopeptidase: a novel mycoprotein linked to invasive candidiasis and mortality in acutely decompensated cirrhosis. J. Hepatol. 79, e68–e71. doi: 10.1016/j.jhep.2023.03.037
Waluga, M., Kukla, M., Żorniak, M., Bacik, A., and Kotulski, R. (2015). From the stomach to other organs: helicobacter pylori and the liver. World J. Hepatol. 7, 2136–2146. doi: 10.4254/wjh.v7.i18.2136
Wang, J., Dong, F., Su, H., Zhu, L., Shao, S., Wu, J., et al. (2021a). H. pylori is related to Nafld but only in female: a Cross-sectional study. Int. J. Med. Sci. 18, 2303–2311. doi: 10.7150/ijms.50748
Wang, C., Li, W., Shao, L., Zhou, A., Zhao, M., Li, P., et al. (2023). Both extracellular vesicles from Helicobacter pylori-infected cells and Helicobacter pylori outer membrane vesicles are involved in gastric/extragastric diseases. Eur. J. Med. Res. 28:484. doi: 10.1186/s40001-023-01458-z
Wang, T., Tan, W., Wang, X., Zheng, X., Huang, Y., Li, B., et al. (2022). Role of precipitants in transition of acute decompensation to acute-on-chronic liver failure in patients with Hbv-related cirrhosis. JHEP Rep 4:100529. doi: 10.1016/j.jhepr.2022.100529
Wang, W. W., Zhang, Y., Huang, X. B., You, N., Zheng, L., and Li, J. (2017). Fecal microbiota transplantation prevents hepatic encephalopathy in rats with carbon tetrachloride-induced acute hepatic dysfunction. World J. Gastroenterol. 23, 6983–6994. doi: 10.3748/wjg.v23.i38.6983
Wang, K., Zhang, Z., Mo, Z. S., Yang, X. H., Lin, B. L., Peng, L., et al. (2021b). Gut microbiota as prognosis markers for patients with Hbv-related acute-on-chronic liver failure. Gut Microbes 13, 1–15. doi: 10.1080/19490976.2021.1921925
Wang, G., Zhao, G., Chao, X., Xie, L., and Wang, H. (2020). The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health 17:6278. doi: 10.3390/ijerph17176278
Won, S. M., Park, E., Jeong, J. J., Ganesan, R., Gupta, H., Gebru, Y. A., et al. (2021). The gut microbiota-derived immune response in chronic liver disease. Int. J. Mol. Sci. 22:8309. doi: 10.3390/ijms22158309
Wong, F., Piano, S., Singh, V., Bartoletti, M., Maiwall, R., Alessandria, C., et al. (2021). Clinical features and evolution of bacterial infection-related acute-on-chronic liver failure. J. Hepatol. 74, 330–339. doi: 10.1016/j.jhep.2020.07.046
Wu, Y., He, Y., Wang, F., Yao, N., Zhao, Y., and Tian, Z. (2022). Lipopolysaccharide inhibits autophagy and promotes inflammatory responses via p38 Mapk-induced proteasomal degradation of Atg13 in hepatic stellate cells. Mediat. Inflamm. 2022:9603989. doi: 10.1155/2022/9603989
Wu, J., Ling, B., Guo, N., Zhai, G., Li, M., and Guo, Y. (2021a). Immunological manifestations of hepatitis E-associated acute and chronic liver failure and its regulatory mechanisms. Front Med (Lausanne) 8:725993. doi: 10.3389/fmed.2021.725993
Wu, W., Sun, S., Wang, Y., Zhao, R., Ren, H., Li, Z., et al. (2021b). Circulating neutrophil dysfunction in Hbv-related acute-on-chronic liver failure. Front. Immunol. 12:620365. doi: 10.3389/fimmu.2021.620365
Xie, J., Cools, L., Van Imschoot, G., Wonterghem, E., Pauwels, M. J., Vlaeminck, I., et al. (2023a). Helicobacter pylori-derived outer membrane vesicles contribute to Alzheimer's disease pathogenesis via C3-C3aR signalling. J. Extracell. Vesicles 12:e12306. doi: 10.1002/jev2.12306
Xie, J., Haesebrouck, F., Van Hoecke, L., and Vandenbroucke, R. E. (2023b). Bacterial extracellular vesicles: an emerging avenue to tackle diseases. Trends Microbiol. 31, 1206–1224. doi: 10.1016/j.tim.2023.05.010
Xu, C., Lu, Y., Zheng, X., Feng, X., Yang, X., Timm, J., et al. (2017). Tlr2 expression in peripheral Cd4+ T cells promotes Th17 response and is associated with disease aggravation of hepatitis B virus-related acute-on-chronic liver failure. Front. Immunol. 8:1609. doi: 10.3389/fimmu.2017.01609
Xu, Y., Xie, C., Liu, Y., Qin, X., and Liu, J. (2023). An update on our understanding of gram-positive bacterial membrane vesicles: discovery, functions, and applications. Front. Cell. Infect. Microbiol. 13:1273813. doi: 10.3389/fcimb.2023.1273813
Xu, Z., Zhang, X., Chen, J., Shi, Y., and Ji, S. (2024). Bacterial infections in acute-on-chronic liver failure: epidemiology, diagnosis, pathogenesis, and management. J. Clin. Transl. Hepatol. 12, 667–676. doi: 10.14218/JCTH.2024.00137
Yang, J., Hwang, I., Lee, E., Shin, S. J., Lee, E. J., Rhee, J. H., et al. (2020). Bacterial outer membrane vesicle-mediated cytosolic delivery of Flagellin triggers host Nlrc4 canonical Inflammasome signaling. Front. Immunol. 11:581165. doi: 10.3389/fimmu.2020.581165
Yang, X., Li, J., Yang, Y., Zhang, L., Dan, X., Cai, D., et al. (2025). Early prediction of invasive fungal infection risk in acute-on-chronic liver failure: a prediction model based on admission indicators. BMC Microbiol. 25:131. doi: 10.1186/s12866-025-03819-6
Yao, X., Yu, H., Fan, G., Xiang, H., Long, L., Xu, H., et al. (2021). Impact of the gut microbiome on the progression of hepatitis B virus related acute-on-chronic liver failure. Front. Cell. Infect. Microbiol. 11:573923. doi: 10.3389/fcimb.2021.573923
Ye, H., Nelson, L. J., Gómez Del Moral, M., Martínez-Naves, E., and Cubero, F. J. (2018). Dissecting the molecular pathophysiology of drug-induced liver injury. World J. Gastroenterol. 24, 1373–1385. doi: 10.3748/wjg.v24.i13.1373
Yu, Y. C., Mao, Y. M., Chen, C. W., Chen, J. J., Chen, J., Cong, W. M., et al. (2017). Csh guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol. Int. 11, 221–241. doi: 10.1007/s12072-017-9793-2
Zahmatkesh, M. E., Jahanbakhsh, M., Hoseini, N., Shegefti, S., Peymani, A., Dabin, H., et al. (2022). Effects of exosomes derived from Helicobacter pylori outer membrane vesicle-infected hepatocytes on hepatic stellate cell activation and liver fibrosis induction. Front. Cell. Infect. Microbiol. 12:857570. doi: 10.3389/fcimb.2022.857570
Zhang, X., Hu, Y., Wang, W., Ji, R., Li, Z., Yu, W., et al. (2024b). Irgm/Irgm1 increases autophagy to inhibit activation of Nlrp3 inflammasome in inflammatory injury induced acute liver failure. Cell Death. Discov. 10:272. doi: 10.1038/s41420-024-02052-w
Zhang, Y., Li, P., Chen, B., and Zheng, R. (2024c). Therapeutic effects of fecal microbial transplantation on alcoholic liver injury in rat models. Clin. Res. Hepatol. Gastroenterol. 48:102478. doi: 10.1016/j.clinre.2024.102478
Zhang, Q., Shi, B., and Wu, L. (2022a). Characteristics and risk factors of infections in patients with Hbv-related acute-on-chronic liver failure: a retrospective study. PeerJ 10:e13519. doi: 10.7717/peerj.13519
Zhang, Y., Wu, W., Wang, Y., Tong, L., Hong, M., Xia, Q., et al. (2021). Gene profiling of toll-like receptor signalling pathways in neutrophils of patients with acute-on-chronic liver failure. J. Transl. Med. 19:465. doi: 10.1186/s12967-021-03135-3
Zhang, Z., Yuan, Y., Hu, L., Tang, J., Meng, Z., Dai, L., et al. (2023). Angptl8 accelerates liver fibrosis mediated by Hfd-induced inflammatory activity via Lilrb2/Erk signaling pathways. J. Adv. Res. 47, 41–56. doi: 10.1016/j.jare.2022.08.006
Zhang, L., Zhang, D., Liu, C., Tang, B., Cui, Y., Guo, D., et al. (2024a). Outer membrane vesicles derived from Fusobacterium nucleatum trigger periodontitis through host overimmunity. Adv. Sci. (Weinh.). 11:e2400882. doi: 10.1002/advs.202400882
Zhang, X., Zhang, Y., Zhou, P., Ai, J., Liu, X., Zhang, Q., et al. (2022b). Down-regulated cylindromatosis enhances Nf-κB activation and aggravates inflammation in Hbv-Aclf patients. Emerg. Microbes Infect. 11, 1586–1601. doi: 10.1080/22221751.2022.2077128
Zhao, Y., Guo, Y., Huang, N., Chen, X., Zhang, A., Liu, L., et al. (2021). Gut microbiota of patients with cirrhosis promotes cirrhosis development by injuring intestinal mucosal barrier in mice. Chin. J. Exp. Surg. 38, 1567–1570. doi: 10.3760/cma.j.cn421213-20201210-00905
Zhou, Z., Sun, X., and Kang, Y. J. (2001). Ethanol-induced apoptosis in mouse liver: Fas- and cytochrome c-mediated caspase-3 activation pathway. Am. J. Pathol. 159, 329–338. doi: 10.1016/S0002-9440(10)61699-9
Keywords: acute-on-chronic liver failure, bacteria, outer membrane vesicles, inflammation, cirrhosis, gut-liver axis
Citation: Qin X, Wang S, Yan Z, Zhao N and Yao J (2025) The role of bacterial outer membrane vesicles in inflammatory response of acute-on-chronic liver failure. Front. Microbiol. 16:1608137. doi: 10.3389/fmicb.2025.1608137
Edited by:
Michal Letek, University of León, SpainReviewed by:
Saugata Majumder, Albany Medical College, United StatesSinem Oktem Okullu, Acibadem Mehmet Ali Aydinlar University, Türkiye
Shreya Banerjee, Boston University, United States
Copyright © 2025 Qin, Wang, Yan, Zhao and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Yao, eWFvamlhMjAwNkAxNjMuY29t
 Xiaojing Qin
Xiaojing Qin Shuang Wang
Shuang Wang Zhanyao Yan
Zhanyao Yan Ninghui Zhao
Ninghui Zhao Jia Yao
Jia Yao