- 1College of Biological and Food Engineering, Chongqing Three Gorges University, Chongqing, China
- 2The Chongqing Engineering Laboratory for Green Cultivation and Deep Processing of the Three Gorges Reservoir Area’s Medicinal Herbs, Chongqing, China
- 3Chongqing Wanzhou Productivity Promotion Center, Chongqing, China
Plumbago auriculata is an important ornamental horticultural plant with high ornamental value. Plumbago auriculata blight was first detected in 2023 in Wanzhou District, Chongqing City, China. This disease seriously reduces the ornamental value of P. auriculata. The disease was characterized by the yellowing and drying up of the apex in the early stage and the drying up and death of the entire aboveground part in the later stage. To identify the pathogenic fungus of P. auriculata blight in Wanzhou district of Chongqing and to screen effective biological pesticides for controlling the disease, the pathogen was isolated and cultured using the tissue separation method. The pathogens were identified by morphology combined with multigene analysis. Cross-pathogenicity experiments were conducted on two other horticultural plants using the pathogen. Biological fungicides were screened by an indoor toxicity test. Combined with the potted plant prevention effect experiment, the control efficacy of the biological fungicide was evaluated. The results showed that isolates L9 and L11 colonies have white cotton flocculent aerial mycelium. The macroconidia are falcate, prominently cell papillate, and hooked. Numerous chlamydia spores were observed through PDA. L9 and L11 were identified by phylogenetic analysis (internal transcribed spacers, RNA polymerase II second largest subunit, translation elongation factor 1 alpha, and calmodulin) and clustered together with Fusarium ipomoeae in the same single clade. This is the first report that F. ipomoeae causes blight on P. auriculata in China. Fusarium ipomoeae was pathogenic to Prunus serrulata and Heptapleurum arboricola. The results of the indoor toxicity test showed that the inhibitory effect of 0.4% osthole SL on F. ipomoeae was significant, with an EC50 value of 1.089 μg/mL. 0.4% osthole SL has a good prevention and control effect on P. auriculata blight, with a control efficacy of 88%. Osthole can be used for the prevention and control of P. auriculata blight. The results provided the foundation for the recognition and green control of P. auriculata blight caused by F. ipomoeae.
1 Introduction
Plumbago auriculata (P. auriculata) is an erect perennial herb in the family Plumbaginaceae and the genus Plumbago. Plumbago auriculata is native to South Africa and has been widely introduced as an ornamental plant in various countries (Shen et al., 2021). It is a commonly used plant in urban landscaping (Vyapari et al., 2007; Ning et al., 2011). With the increase of planting area, the occurrence of P. auriculata diseases has become common. However, there are few reports on P. auriculata disease. Only leaf yellowing in P. auriculata caused by Candidatus Phytoplasma asteris has been reported (Panda et al., 2019).
Blight is a devastating disease of plants. Fusarium spp. (Pandey et al., 2024; Mohamed et al., 2021), Verticillium dahlia (Hassan et al., 2023), Paramyrothecium spp. (Kumar et al., 2025), Plectosphaerella spp. (Yang et al., 2023), Lasiodiplodia spp. (Kwon et al., 2017), Botryosphaeria spp. (Rui et al., 2025) can all cause blight. In 2023, a large area of P. auriculata was found in Wanzhou District, Chongqing, China, with chlorosis of leaf stem and browning of branch vascular bundle, which was similar to the typical symptoms of blight. However, the pathogen that causes the blight of P. auriculata is unclear.
Fusarium spp. is a prevalent pathogenic fungus that infects many hosts and is the primary causal agent of wilt, root rot, and leaf spot. Examples include acacia seedling wilt disease (Soleha et al., 2022), foliar blight on Begonia semperflorens (Lin et al., 2023), chrysanthemum wilt (Balamurugan et al., 2024), root rot in Fatsia japonica (Xu et al., 2024), root rot of Hydrangea (Neupane et al., 2023), leaf spot of Hosta ventricosa (Wang et al., 2021). Often called “plant cancer,” Fusarium wilt disrupts the vascular system (Yao et al., 2025). There have been no reports of Fusarium spp. causing disease on P. auriculata.
In this study, the pathogenic fungi of P. auriculata blight found in the Wanzhou district of Chongqing were isolated and purified. A multi-gene combined phylogenetic tree was constructed using internal transcribed spacers (ITS), RNA polymerase II second largest subunit (RPB2), translation elongation factor 1 alpha (EF–1α), and calmodulin (CAMD), and the pathogenic fungi were identified by combining morphology. Cross-pathogenicity experiments were conducted on two other horticultural plants using the pathogen. Through indoor toxicity tests, suitable biopesticides were selected. Combined with the potted plant prevention effect experiment, the control efficacy of the biological fungicide was evaluated. This study aimed to identify the pathogenic fungus causing P. auriculata blight in Wanzhou district of Chongqing, select effective biological fungicides for better prevention and control effects, and provide a reference for the identification and targeted control of P. auriculata blight.
2 Materials and methods
2.1 Sample collection and fungal isolation
In 2023, a total of 20 leaves and stems of blight on P. auriculata with typical symptoms were collected in Wanzhou District (108°26.4′N,30°45′E), Chongqing City, China. Wash with running water, dry naturally. The disease samples of 5 mm × 5 mm at the junction of disease and healthy were cut with a sterile scalpel, surface sterilized in 75% ethanol for 1 min and then in 3% NaClO for 4 min, rinsed three times in sterilized distilled water. Air-dried on sterilized filter paper, the samples were then placed on antibiotic (streptomycin sulphate, 50 μg/mL) amended potato dextrose agar (PDA) plates and incubated for 5 days at 25°C (Lyu et al., 2024). According to the characteristics of the colony and morphological characteristics, several representative strains were purified and cultured at 25°C on PDA. Inoculate the isolates onto a PDA tube ramp and store at 4°C (Wang et al., 2022).
2.2 Pathogenicity tests
In vitro inoculation was carried out using the mycelium plugs inoculation method and the conidia suspension inoculation method to inoculate detached leaves and stems, respectively (Tang et al., 2022; Ao et al., 2024). In vivo inoculation was carried out using the conidia suspension inoculation method. Inoculate healthy P. auriculata potted plants (Tang et al., 2022).
Prior to in vitro inoculation, rinse the healthy leaves and stems with 75% alcohol for 1 min, wash them with 0.4% sodium hypochlorite solution for 30 s, and then rinse them three times with sterile water (each time for 1 min). Dry naturally. On a healthy leaf, prick the leaf symmetrically with a sterilized needle (the main leaf vein is symmetrical on both sides). The mycelium plugs were dispensed from the representative strain L9 with a sterile punch (5 mm in diameter) and placed in P. auriculata healthy leaves flanking the main veins. Another healthy leaf was taken and inoculated with sterile potato dextrose agar medium as a control. After culturing the representative strain L9 on PDA for 7 days, a 1 × 106 mL conidia suspension was prepared. Spray conidia suspension (1 × 106 conidia/mL) onto P. auriculata healthy stems while using the same procedure with sterile distilled water as a control. All inoculated leaves and stems were placed in 25°C Petri dishes covered with moist sterile filter paper (80% relative humidity).
The potted plants used for in vivo inoculation were healthy P. auriculata that were 3 months old. Before in vivo inoculation, disinfect the surface of the P. auriculata plants with 75% alcohol, rinse them with sterile water, and allow them to dry naturally. Inoculate P. auriculata plants with a conidia suspension by applying an average of 5 mL of conidia suspension (1 × 106 conidia/mL) to each plant until the plant surfaces are completely moist. Healthy plants sprayed with sterile distilled water served as negative controls.
The inoculated materials were placed in a temperature incubator and cultivated at 25°C, with a photoperiod of 12 h and a relative humidity (RH) of 80%. Both the in vitro inoculation experiment and the in vivo inoculation experiment comprised five repetitions. To observe whether the disease symptoms of plants in the temperature incubator were consistent with the field symptoms regularly. After typical symptoms appeared in stems and leaves, the pathogenic fungus was reisolated from the infected leaves and stems. If the isolated strain was consistent with the inoculated strain, it was identified as the pathogen.
2.3 Morphological characterization
Two representative isolates (L9 and L11) obtained were cultured on potato dextrose agar (PDA) at 25°C in the dark for 5 days to observe the morphological characteristics of colonies by optical microscope. Preliminary identification of species was based on morphological characteristics of the pathogenic strains on PDA, including colony color, texture, pigment production, and the morphology of conidiophores and chlamydospores. For each representative fungal isolate, 50 conidia and chlamydospores were randomly selected for measurement.
2.4 Molecular identification and phylogenetic analysis
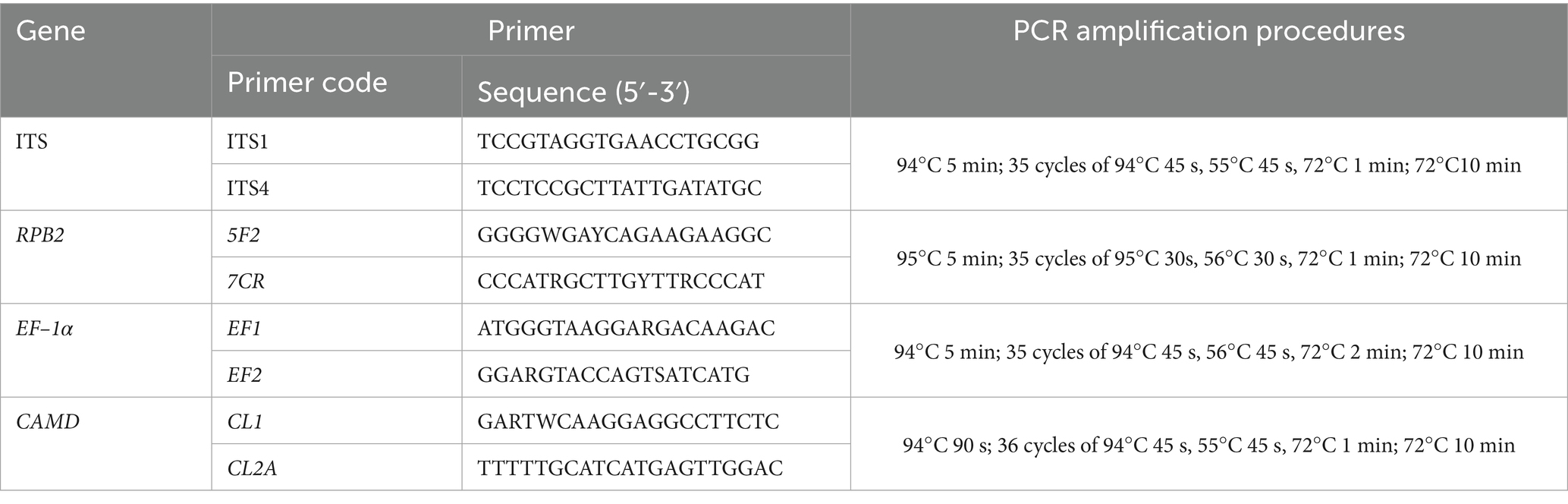
Representative fungal strains were cultured, and mycelium was collected. Plant genomic DNA extraction kit (Cwbio, Jiangsu, China) was used to extract DNA representing strains L9 and L11. Primers ITS1/ITS4 (White et al., 1990), 5F2/7CR (Reeb et al., 2004), EF1/EF2 (O'Donnell et al., 1998), CL1/CL2A (O’Donnell et al., 2000) were used to amplify four loci of L9 and L11, including the internal transcribed spacers (ITS), RNA polymerase II second largest subunit (RPB2), translation elongation factor 1 alpha (EF–1α), and calmodulin (CAMD).
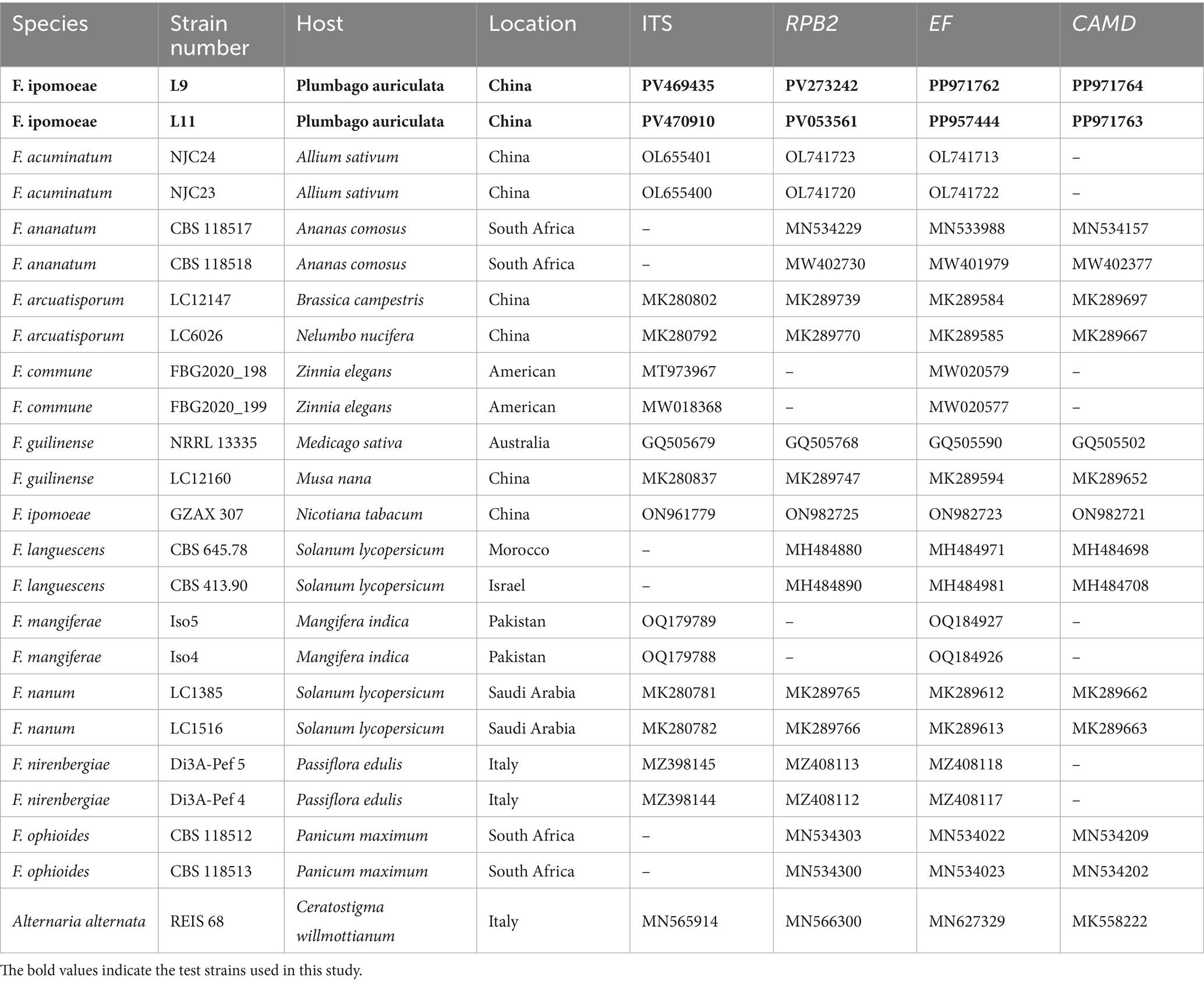
The PCR amplification reaction system was 20 μL, including Taq Master Mix (K1071, Sangon Biotech (Shanghai) Co. Ltd) 10 μL, 10 μmolL−1 upstream and downstream primers 0.3 μL each, DNA template 2 μL, and ddH2O added to 20 μL. The amplification conditions used for the ITS1/ITS4, 5F2/7CR, EF1/EF2, CL1/CL2A, and primer pairs are illustrated in Table 1. After amplification, part of the reactants was detected by 1% agarose gel electrophoresis. The remaining reactants were sent to Sangon Biotech (Shanghai) Co., Ltd. (Chengdu) for sequencing. The obtained sequences were compared and analyzed using GenBank1 to download high-consistency sequences (Table 2). These sequences were combined in PhyloSuite to form the ITS-RPB2-EF–1α-CAMD sequence, and a phylogenetic tree was constructed using the Maximum Likelihood method in MEGA11.0 software, with the repetition value set to 1,000 times. The obtained DNA sequences were uploaded to the National Center for Biotechnology Information to acquire accession numbers.
2.5 Cross-pathogenicity test
Healthy detached leaves were inoculated by mycelium plug inoculation to determine whether the representative strain L9 was also pathogenic to other horticultural plants (Ao et al., 2024). The selected two horticultural plants are Prunus serrulata Lindl. (which belongs to the family Rosaceae and the genus Prunus L.) and Heptapleurum arboricola Hayata (which belongs to the family Araliaceae and the genus Schefflera).
The mycelium plugs inoculation method was carried out as described in section 2.2. The experiment comprised five repetitions. The inoculated leaves were placed in a temperature-controlled incubator and cultivated at 25°C, with a 12-h photoperiod and 80% relative humidity (RH). When re-isolating the pathogen, it matched the pathogen from leaves with lesion symptoms. If the re-isolate was consistent with isolate L9, it confirmed that L9 was pathogenic to P. serrulata and H. arboricola.
2.6 Fungicide assays
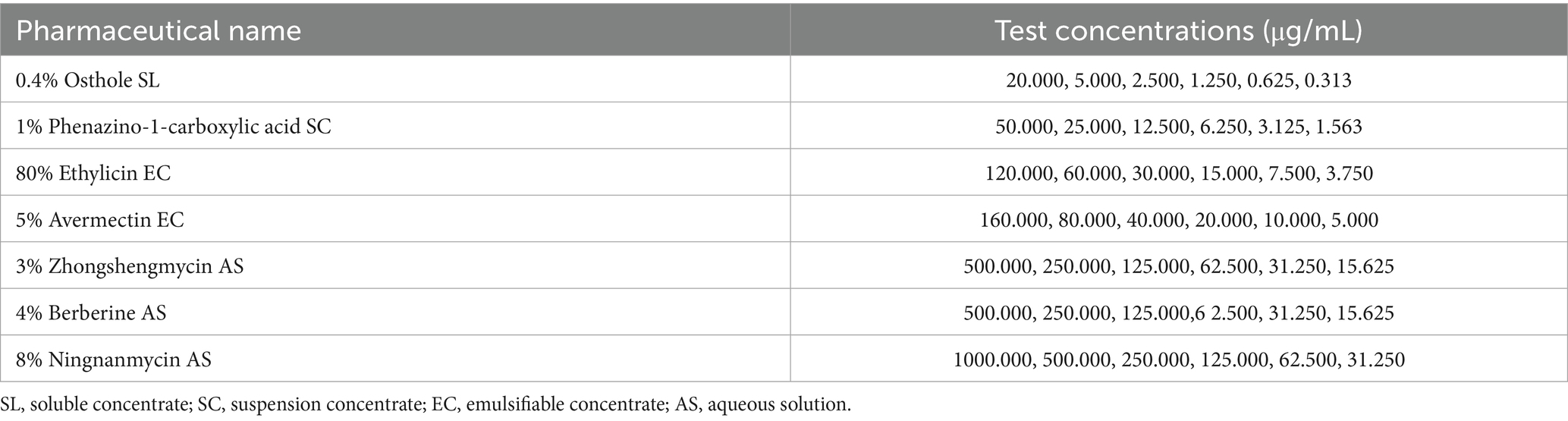
The inhibitory effect of seven types of biological pesticides on pathogenic fungi was measured using the mycelium growth rate method. Each biological pesticide was made into pharmaceutical solutions with different concentration gradients using sterile water (Table 3). The different pharmaceutical solutions concentrations were added to the sterilized PDA medium according to a certain volume ratio. The mixture was thoroughly mixed, and medicine plates were prepared with different test concentrations. Each treatment was repeated five times for each concentration. A sterile punch (5 mm in diameter) was used to extract the mycelium plugs of a representative strain L9 at the edge of the colony. The mycelium plugs were inoculated in the center of the medicine-prepared plates. PDA medium with an equal amount of sterile water served as a control. The plates were incubated at 25°C in darkness within a constant temperature incubator for 5 days. The colony diameter was measured using the cross-shaped intersecting method.
The percent inhibition of mycelial growth (PIMG) was calculated using the following formula (where F is the diameter of the fungal plug, C is the radial growth diameter of the fungus in the control, and T is the radial growth diameter of the fungus in the treatment group) (Ao et al., 2024).
Microsoft Excel 2019 was used to calculate a toxicity regression equation (where x is the logarithm of the fungicide concentration (μg/mL) and y is the inhibition rate) and correlation coefficient for each fungicide. SPSS 26.0 was used to calculate the half maximal effective concentration (EC50) value of each fungicide. We used ANOVA with post-hoc Duncan’s test in SPSS 26.0 to compare inhibition rates across treatments at different concentrations among the same fungicides. And mark the statistical differences with lowercase letters.
2.7 Potted plant prevention effect test
Based on the indoor toxicity test results, the fungicide 0.4% osthole SL, which showed an obvious antibacterial effect, was selected for the pot control effect test. The treatment was carried out with three concentration gradients of 0.4% osthole SL EC50, EC70, and EC90 (1.089 μg/mL, 2.071 μg/mL, and 5.244 μg/mL, respectively). The treatment without any pharmaceutical application, but inoculated with the isolate L9, served as the negative control. Each treatment was set up with five replicates.
After culturing the representative strain L9 on PDA for 7 days, 1 × 106 mL of conidia suspension was prepared in advance. Sterilize the soil in the potted plants in advance. Select healthy P. auriculata plant seedlings of the same size and age of 3 months and transfer them into seedling pots. After they stand upright and grow normally, disinfect the surface of P. auriculata plants with 75% ethanol and rinse with sterile water. Inoculate an average of 5 mL of the conidia suspension per plant until the surface of the plants is completely moist. The potted plants were inoculated and sprayed with 0.4% osthole SL early in the disease for prevention and control. Fungicides were applied once every 7 days for a total of 4 times. Spray each plant with a micro sprayer to ensure even application of the medicine. The potted plants were placed in a greenhouse and cultivated at 25°C, with a photoperiod of 12 h and a relative humidity (RH) of 80%. The disease severity was investigated 1 day before the application of the pesticide and on the 7th day after the application ended, and the disease severity (DS) and control efficacy (CE) were calculated, respectively (Wang et al., 2024).
The DS was scored for each plant according to the percentage of leaves and stems with yellowing or necrosis where 0 = No evidence of lesions, 1 = 33% of leaf and stem area infected, 2 = 34–66% of leaf and stem area infected, 3 = 67–100% of leaf and stem area infected and 4 = dead plant (Díaz-Gutiérrez et al., 2021). The disease severity index (DSI) was calculated for each treatment using the formula of Promwee et al. (2017).
The control efficacy (CE) was calculated for each treatment using the formula of Wang et al. (2024) (where D is the disease severity index in the blank control area, and d is the disease severity index of the treatment area).
Data were collated using Microsoft Excel 2019 software, and the Duncan’s test in SPSS 26.0 statistical software was used for the significance analysis of differences.
3 Results
3.1 Symptom characteristics
According to the investigation, the incidence of wilt disease of P. auriculata was 50–60% in the field. The disease started in April; the worst onset was in September to October. The initial symptoms of the disease are the yellowing and wilting of the top stems and young leaves. The leaves lost water, curled, and the junction between disease and health appeared dark brown (Figure 1a). The symptoms gradually spread downwards. The leaves became dry, curly, drooping, and dying. The later symptoms were manifested as the withering and death of the entire plant (Figure 1b).

Figure 1. (a,b) Symptoms of disease in Plumbago auriculata in the field. (a) Early field diseases occur at the tip of the stem; (b) In the later stages of the disease, the aboveground parts died. (c) Colony morphology. (d,e) Negative controls, healthy detached leaves and stems. (f–h) Symptoms on healthy detached stems inoculated with F. ipomoeae (at 4, 8, and 12 days post-inoculation, respectively). (i–k) Symptoms on healthy detached leaves inoculated with F. ipomoeae (at 4, 8, and 12 days post-inoculation, respectively). (l) Negative controls, healthy potted plants of P. auriculata. (m–o) Symptoms on healthy potted plants inoculated with F. ipomoeae (at 5, 10, and 15 days post-inoculation, respectively). (p,q) Macroconidia. (r) Microconidia. (s,t) Chlamydospore. (u) Macroconidia form on the sporophore. (d–o) Scale bars = 50 mm.
3.2 Morphological characteristics of the pathogen
Fungal colonies have white cotton flocculent aerial mycelium after 7 days of incubation under dark conditions on PDA. The back of the colony is pale pink. The edges of the colony are uneven (Figure 1c). The macroconidia are falcate, prominently cell papillate or hooked, and have 4–6 septa, measuring 40–68 × 4–7 μm (Figures 1p,q). Microconidia are oval and have either one or no septa. Microconidia 8–10 × 3–5 μm (Figure 1r). A lot of chlamydia spores are observed on PDA. Chlamydospores were produced in chains or pairs, globose, and thick-walled (Figures 1s,t). The conidiophores in the sporodochia vary in length and are branched verticillately. The hyphae are thin-walled and hyaline (Figure 1u). These characteristics suggest the pathogenic fungus was Fusarium spp.
3.3 Pathogenicity test
Four to five days after inoculation with the representative strain L9, the detached stems showed chlorosis and yellowing (Figure 1f). Brown spots were observed on the detached leaves (Figure 1i). Wilting and yellowing occurred in leaves and at the tips of the stems of P. auriculata potted plants (Figure 1m). After 8–10 days, the outer layer of the stems turned light brown (Figure 1g). The browning area of the detached leaves expanded, and some gradually died (Figure 1j). The leaves of potted plants have become dry and curly (Figure 1n). After 12–15 days, the outer layer of the detached stems turned dark brown (Figure 1h). The inoculated site of the detached leaves completely turned brown and withered (Figure 1k). The symptoms of withering in potted plants gradually spread downward, and the drooping leaves completely withered and died. White mycelial layers appeared on the surface of diseased plant tissues (Figure 1o). The boundary between diseased and healthy plant tissues appeared dark brown. The symptoms of the indoor pathogenicity test were consistent with those of the field diseases. In contrast, all the control groups remained healthy on the 15th day (Figures 1d,e,l).
The inoculated and diseased P. auriculata leaves and stems were subjected to pathogen re-isolation and identification. The re-isolated pathogens were consistent with the inoculated pathogens. Thus, we identified L9 as the pathogen causing blight in P. auriculata.
3.4 Molecular identification and phylogenetic analyses
The gene regions of ITS, RPB2, EF–1α and CAMD were PCR amplified and sequenced. The obtained sequences of ITS, RPB2, EF–1α and CAMD were deposited in GenBank. The combined dataset of ITS, RPB2, EF–1α and CAMD genes was used for phylogenetic analysis by MEGA11.0. Alternaria alternata strain REIS 68 was used as an outgroup. The ML tree demonstrated that L9 and L11 isolates were placed in the F. ipomoeae (GZAX 307) group, supported by a 100% bootstrap value (Figure 2). Representative isolates L9 and L11 were confirmed as F. ipomoeae based on morphological characteristics and molecular identification.
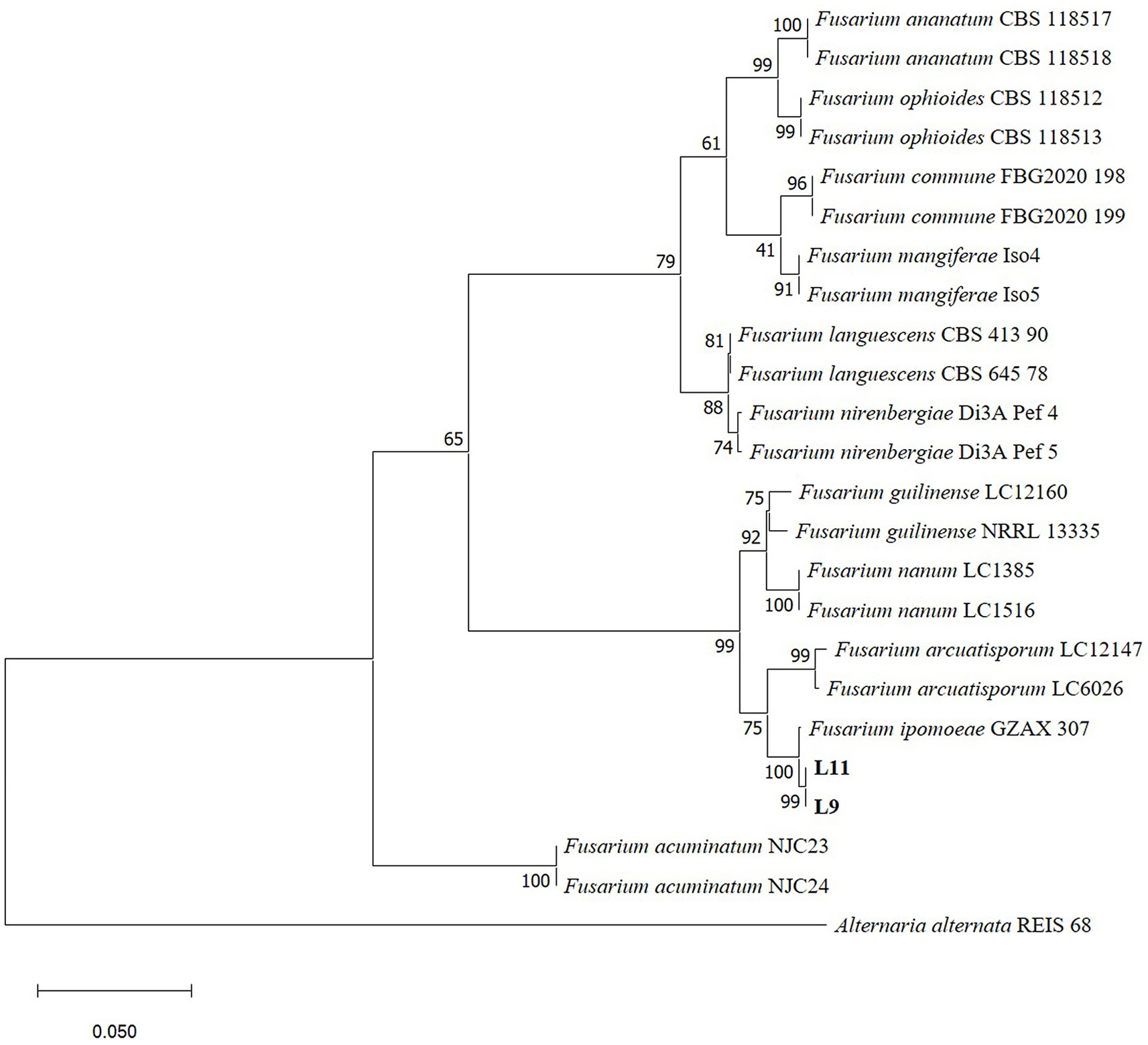
Figure 2. Phylogenetic tree calculated from the alignment of concatenated sequences of the internal transcribed spacer (ITS), RNA polymerase II second largest subunit (RPB2), translation elongation factor 1 alpha (EF–1α) and calmodulin (CAMD) genes using Maximum likelihood method. L9 and L11 are the isolates described in this study. Bootstrap values > 50% (1,000 replications) are given at the nodes. Bar = 0.05 substitution per nucleotide position. Alternaria alternata strain REIS 68 was used as an outgroup.
3.5 Cross-pathogenicity test
After in vitro inoculation of the isolated plant leaves of P. serrulata and H. arboricola, the inoculated parts of the plant leaves showed symptoms of disease to varying degrees. Strain L9 had a relatively strong pathogenicity to P. serrulata. Four days after inoculation, 3–4 mm brown small lesions appeared at the pinhole. After 12 days of inoculation, the lesion size reached 17–25 mm (Figures 3a–d). Conversely, the strain L9 had weaker pathogenicity against H. arboricola. Twelve days after inoculation, the size of the lesion was 9–11 mm (Figures 3e–h). Twelve days later, none of the control group showed any symptoms of the disease. Re-isolate the pathogen from the diseased leaves, and the resulting pathogen was consistent with that of L9. The isolate L9 was pathogenic to both P. serrulata and H. arboricola. Fusarium ipomoeae isolated from P. auriculata blight can infect other horticultural plants.
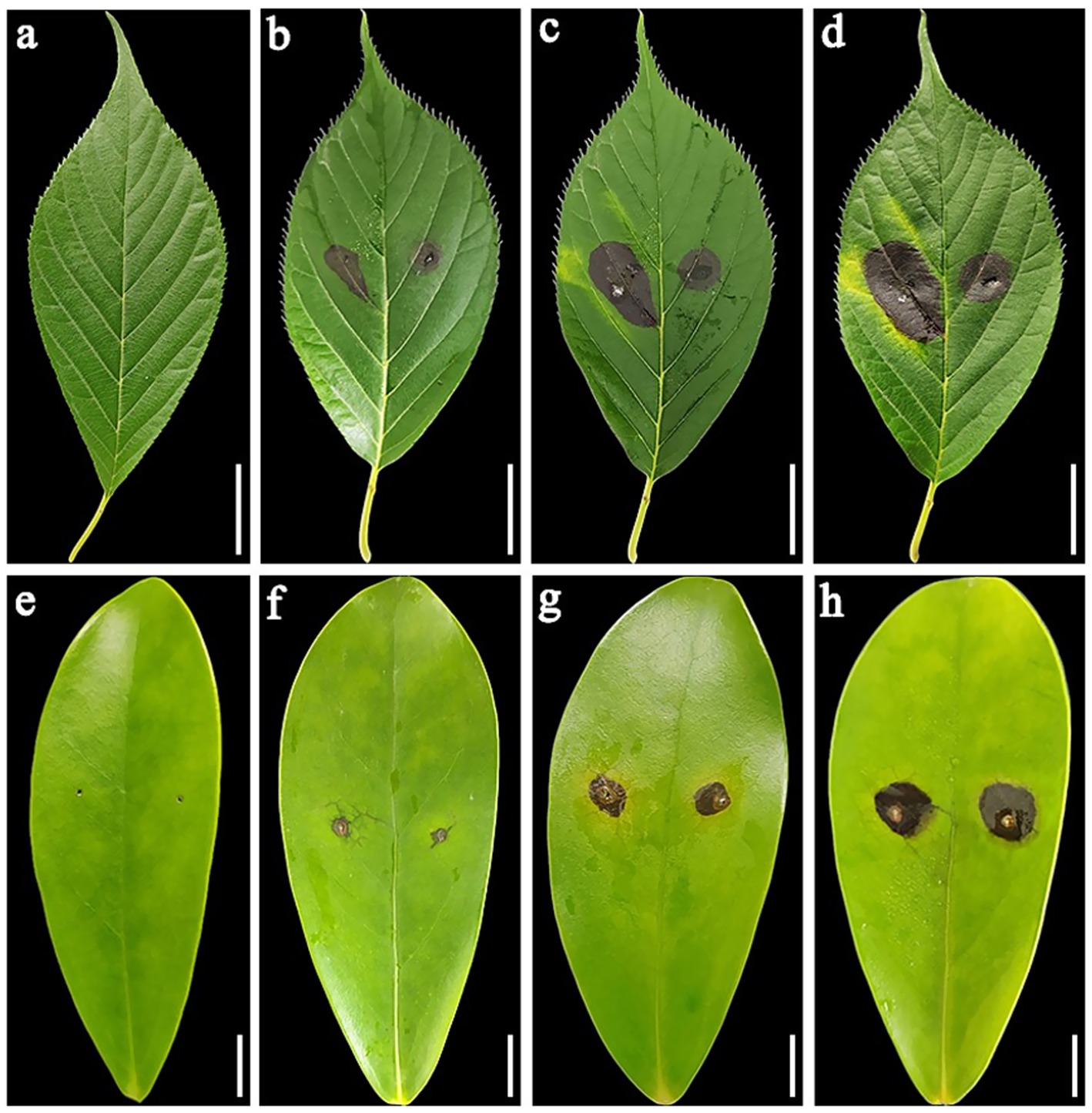
Figure 3. (a) Negative controls, healthy detached leaves of Prunus serrulate. (b–d) Symptoms on Prunus serrulate healthy detached leaves inoculated with Fusarium ipomoeae (at 4, 8, and 12 days post-inoculation, respectively). (e) Negative controls, healthy detached leaves of Heptapleurum arboricola. (f–h) Symptoms on Heptapleurum arboricola healthy detached leaves inoculated with F. ipomoeae (at 4, 8, and 12 days post-inoculation, respectively). (a–d) Scale bars = 20 mm. (e–h) Scale bars = 10 mm.
3.6 Fungicide assays
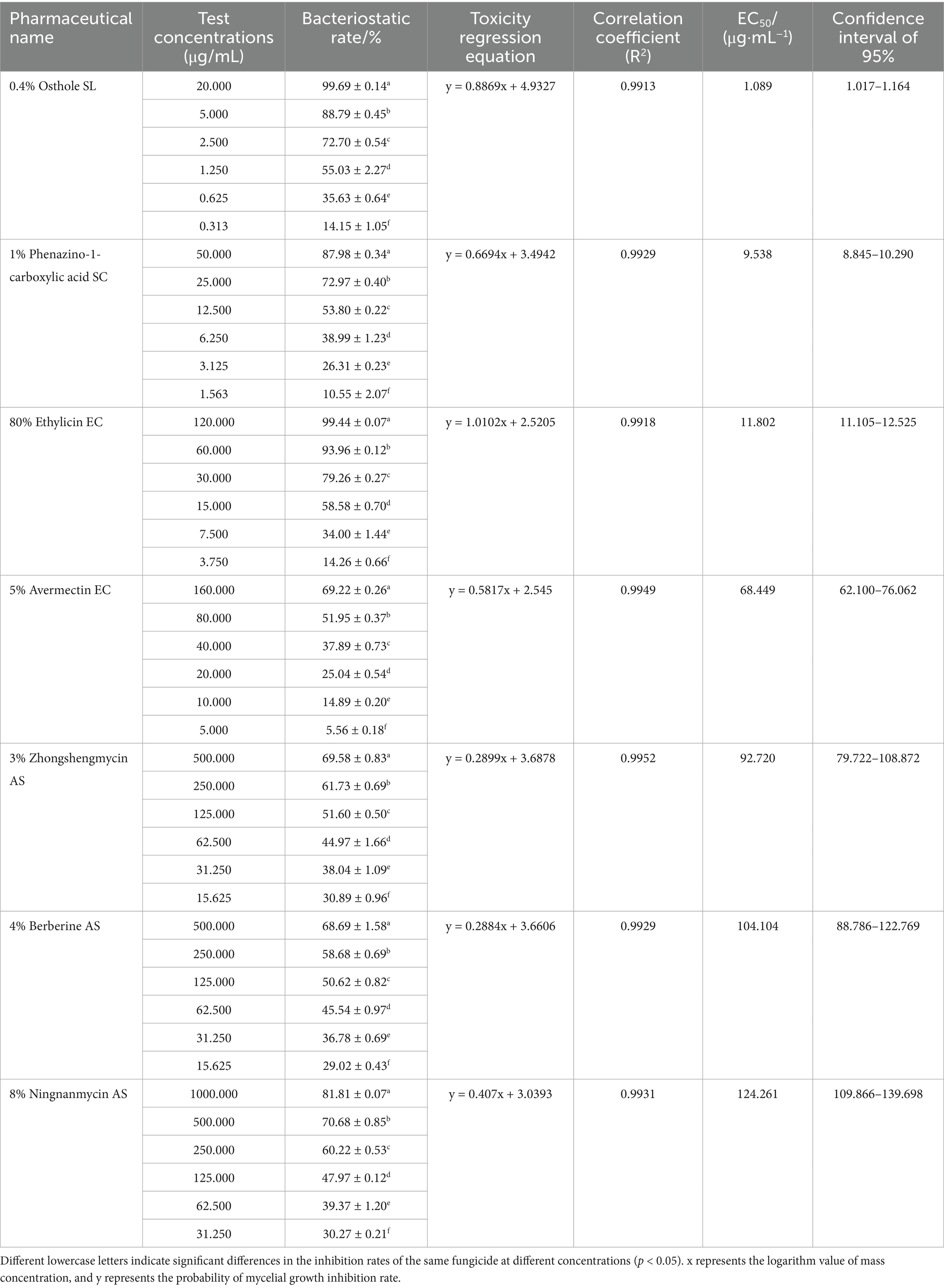
The biological pesticides treated with different fungicide types and concentrations could inhibit the mycelial growth of F. ipomoeae to different degrees (Figure 4). For each biopesticide used in the fungicide assays, the original fungicide concentration, formulation type, toxicity regression equation, correlation coefficient, EC50 value, and 95% confidence intervals are shown in Table 4.
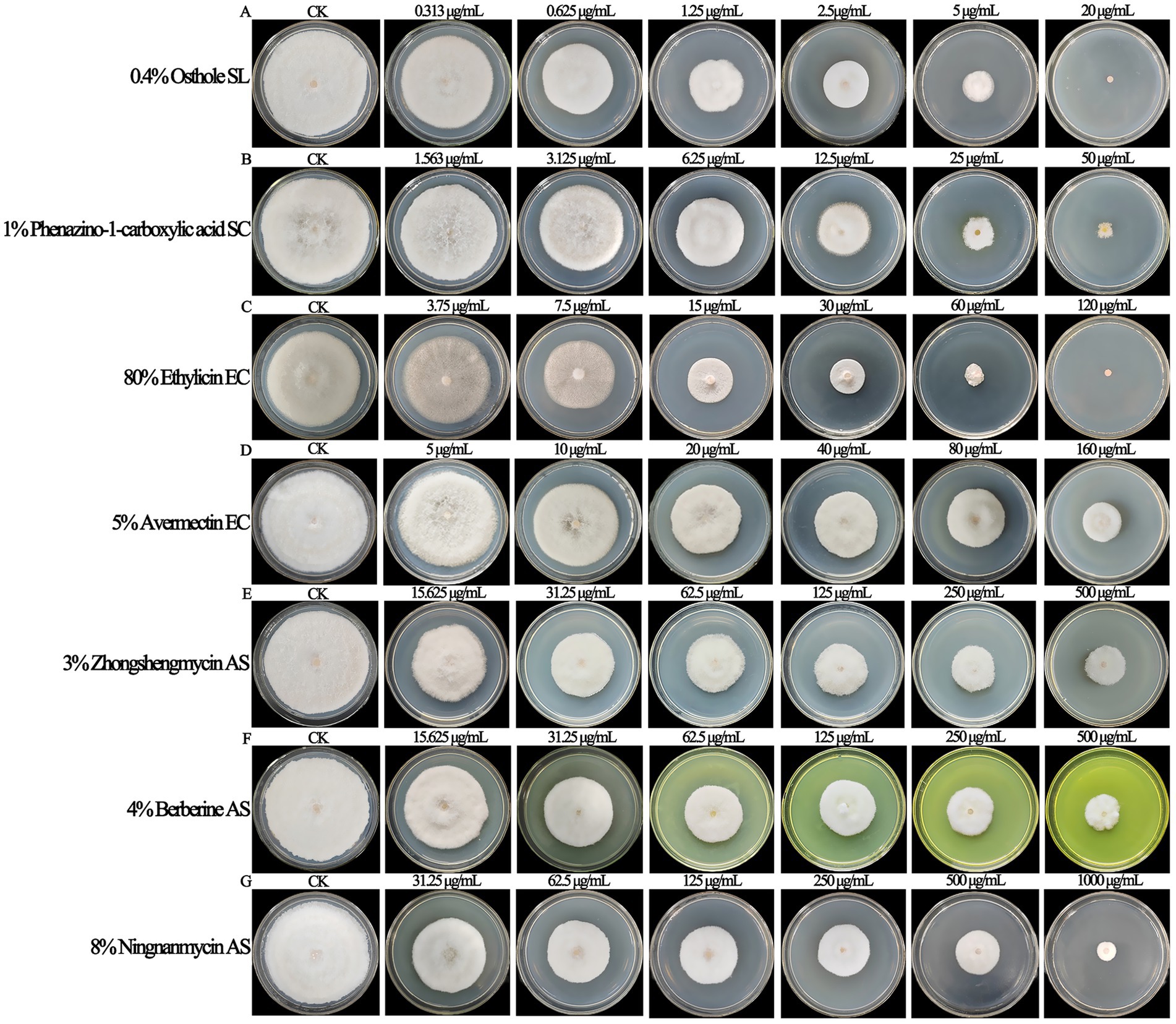
Figure 4. The biological pesticides treated with different fungicide types and concentrations could inhibit the mycelial growth of Fusarium ipomoeae to different degrees. (A) 0.4% Osthole SL; (B) 1% Phenazino-1-carboxylic acid SC; (C) 80% Ethylicin EC; (D) 5% Avermectin EC; (E) 3% Zhongshengmycin AS; (F) 4% Berberine AS; (G) 8% Ningnanmycin AS.
Among the seven biological pesticides, the inhibitory effect of 0.4% osthole SL on F. ipomoeae is the best, with an EC50 value of 1.089 μg/mL. 1% phenazino-1-carboxylic acid SC and 80% ethylicin EC show good inhibitory effect on mycelia growth, with EC50 values of 9.538 μg/mL and 11.802 μg/mL, respectively. Followed by 5% avermectin EC and 3% zhongshengmycin AS, with EC50 values of 68.449 μg/mL and 92.720 μg/mL, respectively. The inhibitory effect of 4% berberine AS and 8% ningnanmycin AS is poor, with EC50 values of 104.104 μg/mL and 124.261 μg/mL, respectively.
3.7 Potted plant prevention effect tests
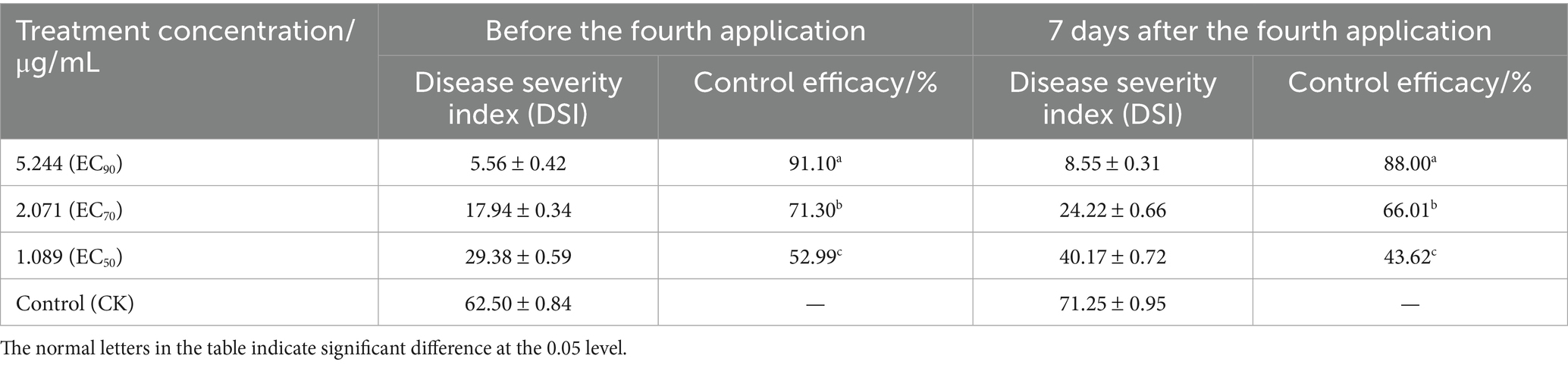
The control effects of different concentrations of 0.4% osthole SL on the blight of P. auriculata potted plants caused by F. ipomoeae were evaluated (Table 5; Figure 5). The results showed that 0.4% osthole SL has a good control efficacy on P. auriculata blight caused by F. ipomoeae. However, three different concentrations of osthole treated had significantly different control efficacy on P. auriculata blight. Before the fourth application, 0.4% osthole SL had the highest control efficacy at a concentration of 5.244 μg/mL (EC90), reaching 91.10%, significantly higher than 2.071 μg/mL (EC70) and 1.089 μg/mL (EC50). After the 4th application for 7 days, the control efficacy remained good at a concentration of 5.244 μg/mL of 0.4% osthole SL, reaching 88.00%. In contrast, the control effects of 0.4% osthole SL concentrations of 2.071 μg/mL (EC70) and 1.089 μg/mL (EC50) were weaker, with 66.01 and 43.62%, respectively. 0.4% osthole SL can be used as a biological pesticide for the field control of P. auriculata blight caused by F. ipomoeae, and the recommended application concentration is 5.244 μg/mL.
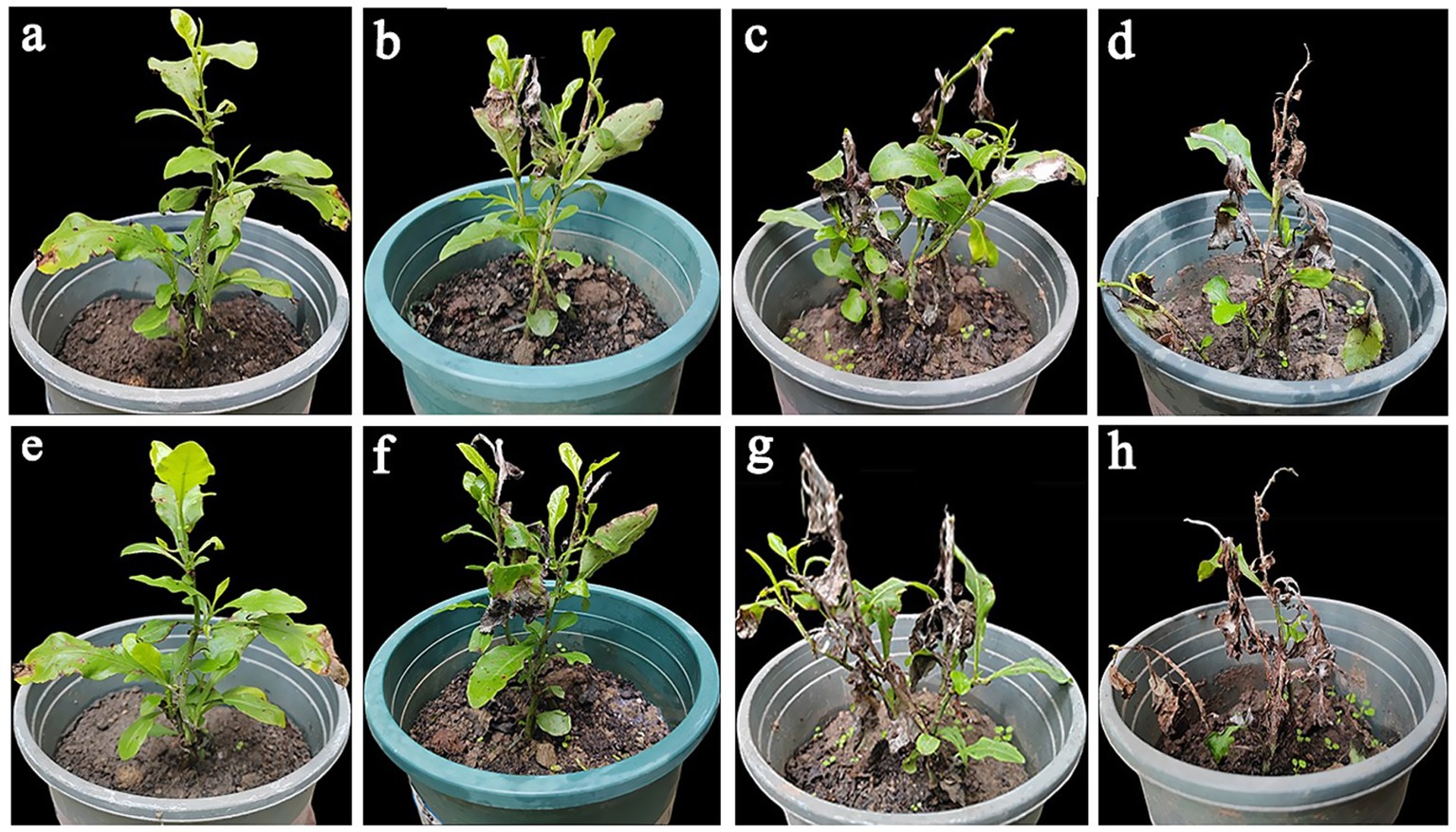
Figure 5. The potted control effect of osthole on Plumbago auriculata blight. (a–c) The control effect of potted plants before the fourth application of osthole (EC90, EC70, and EC50, respectively). (d) The control group before the fourth application of the osthole. (e–g) The control effect of potted plants 7 days after the fourth application of osthole (EC90, EC70, and EC50, respectively). (h) The control group was 7 days after the fourth application of the osthole.
4 Discussion and conclusion
This study used morphological observation, a pathogenicity test, and molecular identification to identify the pathogen of P. auriculata blight disease. It was determined that the pathogen causing P. auriculata blight was F. ipomoeae. This is the first report of F. ipomoeae causing wilt on P. auriculata in China.
Fusarium ipomoeae is a member of the Fusarium incarnatum-equiseti species complex (FIESC). The holotype of F. ipomoeae was isolated from Ipomoea aquatica by L. Cai in 2016, named after the host genus, Ipomoea (Wang et al., 2019). Fusarium ipomoeae has a wide range of hosts and causes many plant disease symptoms after infection, causing leaf spot diseases on Bletilla striata (Zhou et al., 2020), peanut (Xu et al., 2021), tobacco (Wang et al., 2022) and sweet cherries (Zhou et al., 2022), and can also cause Fusarium wilt on soybean (Choi et al., 2022), maize leaf blight (Xu et al., 2022) and head blight of wheat (Al-Hashimi et al., 2025). In addition, F. ipomoeae can also cause rot in different parts of the plant. Rhizome rot of tobacco (Liu et al., 2022), crown rot of wheat (Ma et al., 2024), root rot of Medicago sativa (Suo et al., 2024), leaf rot on Hosta plantaginea (Zhou L. et al., 2023), fruit rot disease on Cucumis melo (Zhang et al., 2024), strawberry fruit rot (Li et al., 2023), fruit rot of Cucurbita maxima (Kitabayashi et al., 2023), pod rot of Vigna mungo (Verma et al., 2023).
At present, there are many types of fungicides for the control of plant diseases caused by Fusarium spp., but there are few studies on the screening of fungicides for F. ipomoeae. Suo et al. (2024) found that F. ipomoeae was most suitable for growth under the conditions of temperature 30°C, pH 6–10, and low nitrogen, and could withstand 150 g/L NaCl stress, showing strong environmental adaptability. Chemical fungicide fludioxonil had an obvious inhibitory effect on the colony growth of F. ipomoeae. Median effect concentration is 0.142 μg/mL. Compared with traditional chemical pesticides, biopesticides are highly selective, safe for humans and animals, safe for the natural ecological environment, and pollution-free. However, there are no reports on the screening of F. ipomoeae biopesticides. In this study, we evaluated the inhibitory effects of seven biopesticides on the growth of F. ipomoeae, a pathogen of P. auriculata blight. Through the study, the inhibitory effect of 0.4% osthole SL on F. ipomoeae was screened to be the most significant, with an EC50 value of 1.119 μg/mL. Osthole can reduce the activity of chitinase and β-1, 3-glucanase in Fusarium, to destroy the integrity and homeostasis of the Fusarium cell wall, thus inhibiting the normal growth of mycelia (Hu et al., 2023). Osthole has been proven to have a broad spectrum of antibacterial activity (Zhang et al., 2016). It is reported that osthole has a good inhibitory effect on growth on Alternaria alternata (Gu et al., 2024), Erysiphe paeoniae (Wu et al., 2023), Cladobotryum asterophorum (Yuan et al., 2023), Nothophoma quercina (Sun et al., 2024), Puccinia helianthi (Zhang et al., 2023), Athelia rolfsii (Wang et al., 2023). Some studies have reported that osthole has a good preventive and control effect on other plant diseases. Osthole has a good prevention and control effect on apple spotted leaf fall disease (Zhai et al., 2024), crown rot of wheat (Zhou F. et al., 2023), and gray mold of Paris polyphylla (Tang et al., 2021).
Although biopesticides are more environmentally friendly than chemical pesticides, they are not completely immune to the problem of drug resistance. Fungal drug resistance is an ongoing issue in both agriculture and medicine. Mechanisms of fungal drug resistance involve target alterations and non-target alterations. In Fusarium, the target alterations include generation of point mutations and overexpression of target genes, and the non-target alterations mainly involve drug efflux and biofilm formation (Zhao et al., 2021). Hengwei et al. (2018) observed that the Y137H mutation results in the resistance of F. graminearum to tebuconazole. The absence of CYP51C can increase the sensitivity of F. graminearum to tebuconazole and other drugs (Liu et al., 2011). In Fusarium, efflux pump research mainly focuses on ABC transporters, which use the energy generated by ATP hydrolysis to transport substances into or out of cell membranes (Locher, 2016). The ABC family in Fusarium regulates the resistance to azole drugs. Specifically, FcABC1 in Fusarium culmorum (Hellin et al., 2018), FgABC3, FgABC4 and FgABC-C9 in Fusarium graminearum are all related to the resistance of azole drugs (Abou Ammar et al., 2013; Qi et al., 2018).
Although this study screened out highly efficient and low-toxicity biological fungicides, repeated use of 0.4% osthole SL may select for resistant F. ipomoeae strains, compromising long-term disease management. Díaz-Gutiérrez et al. (2021) verified the fungicidal or fungistatic action of Trichoderma asperellum by sub-culturing the mycelia of the phytopathogenic fungi. In subsequent studies, it is necessary to conduct sub-culturing of pathogenic fungi. Monitor shifts in EC50 values across generations and analyze expression of resistance-associated genes. Future work remains essential to clarify the mechanisms involved in F. ipomoeae drug resistance and to develop more effective drugs.
5 Conclusion
This study identified the pathogenic fungi, F. ipomoeae, responsible for causing P. auriculata blight in Wanzhou District, Chongqing. This is the first report that F. ipomoeae causes blight on P. auriculata in China. Fusarium ipomoeae isolated from P. auriculata blight can infect other horticultural plants. And screen out biological fungicides that have inhibitory effects on F. ipomoeae. The efficacy of 0.4% osthole SL was tested under controlled greenhouse conditions. The results of this study provide a reference for the identification of P. auriculata blight and precise green control.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
ML: Writing – review & editing. TG: Writing – review & editing, Writing – original draft. HY: Writing – review & editing. YY: Writing – review & editing. ZX: Writing – review & editing. YL: Writing – review & editing. SZ: Writing – review & editing. FL: Writing – review & editing. SJ: Writing – review & editing. FY: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was financially supported by the Science and Technology Research Program of Chongqing Municipal Education Commission (KJZD-K202201207).
Acknowledgments
We would like to thank all the growers who allowed us to collect soil samples on their farms.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Abou Ammar, G., Tryono, R., Döll, K., Karlovsky, P., Deising, H. B., and Wirsel, S. G. (2013). Identification of ABC transporter genes of Fusarium graminearum with roles in azole tolerance and/or virulence. PLoS One 8:e79042. doi: 10.1371/journal.pone.0079042
Al-Hashimi, A., Daniel, A. I., Aina, O., Du Plessis, M., Keyster, M., and Klein, A. (2025). Survey and identification of Fusarium head blight pathogens of wheat in the Western cape region of South Africa. Pathogens. 14:80. doi: 10.3390/pathogens14010080
Ao, X., Shi, T., Yang, W., Ouyang, H., Fan, R., Siddiqui, J. A., et al. (2024). Biological characterization and in vitro fungicide screening of a new causal agent of walnut leaf spot in Guizhou province, China. Front. Microbiol. 15:487. doi: 10.3389/fmicb.2024.1439487
Balamurugan, A., Ashajyothi, M., Shanu, K., Charishma, K., Varun, H., Gunjeet, K., et al. (2024). Chrysanthemum wilt caused by Fusarium incarnatum: etiology unveiled through polyphasic taxonomic methods. Physiol. Mol. Plant Pathol. 129:102214. doi: 10.1016/j.pmpp.2023.102214
Choi, H. W., Ryu, H., Lee, Y., Jang, Y. W., Yi, H., Hong, S. K., et al. (2022). First report of Fusarium ipomoeae causing fusarium wilt on Glycine max in South Korea. Plant Dis. 107:575. doi: 10.1094/pdis-07-21-1499-pdn
Díaz-Gutiérrez, C., Arroyave, C., Llugany, M., Poschenrieder, C., Martos, S., and Peláez, C. (2021). Trichoderma asperellum as a preventive and curative agent to control Fusarium wilt in Stevia rebaudiana. Biol. Control 155:104537. doi: 10.1016/j.biocontrol.2021.104537
Gu, Q., Huang, C., Wu, Z., Zhang, Y., Huang, Y., and Shen, J. (2024). Identification,Biological characteristics and botanical pesticide screening of the Pathogenof black spot on Paeonia lactiflora. J Chin Med Mater. 2024, 2428–2433. doi: 10.13863/j.issn1001-4454.2024.10.004
Hassan, O., Ryu, H., and Choi, H. W. (2023). First report of Verticillium wilt caused by Verticillium dahliae on chilli in South Korea. Plant Dis. 108:219. doi: 10.1094/pdis-07-23-1437-pdn
Hellin, P., King, R., Urban, M., Hammond-Kosack, K. E., and Legrève, A. (2018). The adaptation of Fusarium culmorum to DMI fungicides is mediated by major transcriptome modifications in response to azole fungicide, including the overexpression of a PDR transporter (FcABC1). Front. Microbiol. 9:1385. doi: 10.3389/fmicb.2018.01385
Hengwei, Q., Juan, D., Mengyu, C., Xiaomei, S., Wenxing, L., Jinguang, H., et al. (2018). The Y137H mutation in the cytochrome P450 FgCYP51B protein confers reduced sensitivity to tebuconazole in Fusarium graminearum. Pest Manag. Sci. 74, 1472–1477. doi: 10.1002/ps.4837
Hu, K., Li, R., Mo, F., Ding, Y., Zhou, A., Guo, X., et al. (2023). Natural product osthole can significantly disrupt cell wall integrity and dynamic balance of Fusarium oxysporum. Pestic. Biochem. Physiol. 196:105623. doi: 10.1016/j.pestbp.2023.105623
Kitabayashi, S., Kawaguchi, A., Yoshida, M., Kami, D., Sugiyama, K., and Kawakami, A. (2023). First report of Fusarium ipomoeae and F. citri causing postharvest fruit rot of winter squash (Cucurbita maxima). J. Gen. Plant Pathol. 89, 61–66. doi: 10.1007/s10327-022-01103-3
Kumar, S., Milton, B., Mufeeda, K. T., and Singh, R. (2025). Paramyrothecium travancorense: a novel fungal pathogen causing leaf spots and blights on Coffea travancorensis in Kerala, India. Physiol. Mol. Plant Pathol. 137:102609. doi: 10.1016/j.pmpp.2025.102609
Kwon, J.-H., Okhee, C., Byeongsam, K., Yeyeong, L., Jiyeong, P., Dong-Wan, K., et al. (2017). Identification of Lasiodiplodia pseudotheobromae causing mango dieback in Korea. Can. J. Plant Pathol. 39, 241–245. doi: 10.1080/07060661.2017.1329231
Li, Z., Yu, X., Zhang, W., Han, R., Zhang, J., Ma, Y., et al. (2023). Identification, characterization, and pathogenicity of fungi associated with strawberry fruit rot in Shandong Province, China. Plant Dis. 107, 3773–3782. doi: 10.1094/pdis-04-23-0696-re
Lin, S., Liu, Z., Guo, J., Zhu, L., Xu, W., Wu, W., et al. (2023). Identification of Fusarium sacchari causing foliar blight on Begonia semperflorens in China. Plant Dis. 107:3279. doi: 10.1094/pdis-01-23-0124-pdn
Liu, X., Li, Y., Cai, L., Zhang, C., Yin, J., and Wang, H. (2022). Isolation and identification of pathogenic fungi from the rhizomes of tobacco. Chin. Tob. Sci. 43, 45–52. doi: 10.13496/j.issn.1007-5119.2022.06.007
Liu, X., Yu, F., Schnabel, G., Wu, J., Wang, Z., and Ma, Z. (2011). Paralogous cyp51 genes in Fusarium graminearum mediate differential sensitivity to sterol demethylation inhibitors. Fungal Genet. Biol. 48, 113–123. doi: 10.1016/j.fgb.2010.10.004
Locher, K. P. (2016). Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat. Struct. Mol. Biol. 23, 487–493. doi: 10.1038/nsmb.3216
Lyu, Y., Dong, X., Sun, H., Li, R., Li, S., Hu, L., et al. (2024). First report of Alternaria leaf spot caused by Alternaria alternata on Ulmus parvifolia in Jiangsu, China. Plant Dis. 108:807. doi: 10.1094/pdis-10-23-2038-pdn
Ma, G., Wang, H., Qi, K., Ma, L., Zhang, B., Zhang, Y., et al. (2024). Isolation, characterization, and pathogenicity of Fusarium species causing crown rot of wheat. Front. Microbiol. 15:1405115. doi: 10.3389/fmicb.2024.1405115
Mohamed, R. A., Al-Bedak, O. A., and Hassan, S. H. A. (2021). First record in upper Egypt of vascular wilt on pomegranate caused by Fusarium oxysporum, its molecular identification and artificial pathogenicity. J. Plant Dis. Prot. 128, 311–316. doi: 10.1007/s41348-020-00385-z
Neupane, S., Alexander, L., and Baysal-Gurel, F. (2023). Evaluation of Hydrangea cultivars for tolerance against root rot caused by Fusarium oxysporum. Plant Dis. 107, 3967–3974. doi: 10.1094/pdis-11-22-2712-re
Ning, H., Zhang, J., Shao, F., Wu, X., and Fan, Y. (2011). Investigation on landscape application of blue flower plants in Hangzhou. J. Northwest For. Univ. 26, 173–176.
O’Donnell, K., Nirenberg, H. I., Aoki, T., and Cigelnik, E. (2000). A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additional phylogenetically distinct species. Mycoscience 41, 61–78. doi: 10.1007/BF02464387
O'Donnell, K., Kistler, H. C., Cigelnik, E., and Ploetz, R. C. (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 95, 2044–2049. doi: 10.1073/pnas.95.5.2044
Panda, P., Rihne, T., Reddy, M. G., and Rao, G. P. (2019). First report of the association of a ‘Candidatus Phytoplasma asteris’-related strain with Plumbago auriculata leaf yellowing in India. New Dis. Rep. 40:15. doi: 10.5197/j.2044-0588.2019.040.015
Pandey, A. K., Hubballi, M., Sharma, H. K., Ramesh, R., Roy, S., Dinesh, K., et al. (2024). Molecular delineation and genetic diversity of Fusarium species complex causing tea dieback in India and their sensitivity to fungicides. Crop Prot. 181:106707. doi: 10.1016/j.cropro.2024.106707
Promwee, A., Yenjit, P., Issarakraisila, M., Intana, W., and Chamswarng, C. (2017). Efficacy of indigenous Trichoderma harzianum in controlling Phytophthora leaf fall (Phytophthora palmivora) in Thai rubber trees. J. Plant Dis. Prot. 124, 41–50. doi: 10.1007/s41348-016-0051-y
Qi, P. F., Zhang, Y. Z., Liu, C. H., Zhu, J., Chen, Q., Guo, Z. R., et al. (2018). Fusarium graminearum ATP-binding cassette transporter gene FgABCC9 is required for its transportation of salicylic acid, fungicide resistance, mycelial growth and pathogenicity towards wheat. Int. J. Mol. Sci. 19:351. doi: 10.3390/ijms19082351
Reeb, V., Lutzoni, F., and Roux, C. (2004). Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol. Phylogenet. Evol. 32, 1036–1060. doi: 10.1016/j.ympev.2004.04.012
Rui, L., Zhang, Q.-Q., Kong, W.-L., Ni, H., and Wu, X.-Q. (2025). First report of Botryosphaeria dothidea causing leaf blight on Yulania denudata in China. Crop Prot. 193:107180. doi: 10.1016/j.cropro.2025.107180
Shen, P., Gao, S., Hu, J., Li, Y., Lei, T., and Shi, L. (2021). In vitro flowering of the distylous plant Plumbago auriculata lam. S. Afr. J. Bot. 137, 492–498. doi: 10.1016/j.sajb.2020.11.018
Soleha, S., Muslim, A., Suwandi, S., Kadir, S., and Pratama, R. (2022). The identification and pathogenicity of Fusarium oxysporum causing acacia seedling wilt disease. J. For. Res. 33, 711–719. doi: 10.1007/s11676-021-01355-3
Sun, Y., Jiao, J., Li, G., Guo, T., Ding, J., Guo, M., et al. (2024). Identification, biological characteristics and sensitivity to different fungicides of the pathogen causing stem canker of Pinus bungeana. J Northwest Forest Univ. 39, 18–25+66. doi: 10.3969/j.issn.1001-7461.2024.03.03
Suo, X., Niu, Q., Guo, C., Gai, Y., Chen, L., and Yin, S. (2024). Isolation of a novel alfalfa Fusarium root rot pathogen FIESC and characterization of strain biology. Acta Agrestia Sin. 32, 1327–1338. doi: 10.11733/j.issn.1007-0435.2024.05.003
Tang, T., Wang, F., Duan, Y., Guo, X., Guo, J., and You, J. (2021). Control effect of thirteen biological fungicides on the graymold of Paris polyphylla. Agrochemical. 60, 297–300. doi: 10.16820/j.cnki.1006-0413.2021.04.017
Tang, X., Yangjing, G., Zhuoma, G., Guo, X., Cao, P., Yi, B., et al. (2022). Biological characterization and in vitro fungicide screenings of a new causal agent of wheat Fusarium head blight in Tibet, China. Front. Microbiol. 13:734. doi: 10.3389/fmicb.2022.941734
Verma, R., Kushwaha, K. P. S., Chakrawarti, N., Kumar, S., Kaur, M., Gupta, P. K., et al. (2023). First report of Fusarium incarnatum-equiseti species complex as the causal agent of pod rot of black gram (Vigna mungo) in India. Plant Dis. 107:2855. doi: 10.1094/pdis-02-23-0363-pdn
Vyapari, S., Scheiber, S. M., and Thralls, E. L. (2007). Pre-transplant root ball condition influences growth of Plumbago during establishment. HortTechnology. 17, 486–490. doi: 10.21273/HORTTECH.17.4.486
Wang, M. M., Chen, Q., Diao, Y. Z., Duan, W. J., and Cai, L. (2019). Fusarium incarnatum-equiseti complex from China. Persoonia 43, 70–89. doi: 10.3767/persoonia.2019.43.03
Wang, Q., Huang, J., Li, S., Zhang, Y., Li, Z., Liu, Y., et al. (2024). Identification of the pathogen of sorghum anthracnose in Chongqing and fungicides screening. Plant Prot. 50, 323–330+367. doi: 10.16688/j.zwbh.2023458
Wang, C. X., Hulei, Z., Shenhai, W., and Shengfeng, M. (2021). Leaf spot of Hosta ventricosa caused by Fusarium oxysporum in China. PeerJ. 9:e12581. doi: 10.7717/PEERJ.12581
Wang, H., Li, Y., Li, W., Cai, L., Meng, J., Xia, G., et al. (2022). Morphological and molecular identification of Fusarium ipomoeae as the causative agent of leaf spot disease in tobacco from China. Microorganisms. 10:1890. doi: 10.3390/microorganisms10101890
Wang, F., Tang, T., He, Y., Guo, X., Duan, Y., and You, J. (2023). Identification, biological characterisation, and fungicides screening of pathogen causing southern blight of Coptis chinensis in Lichuan city. Act Phytopathol Sin. 53, 789–795. doi: 10.13926/j.cnki.apps.000846
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. eds. M. A. Innis, D. H. Gelfand, J. J. Sninsky and T. J. White, PCR Protocols. 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wu, Z., Zhang, Y., Chang, H., Yang, K., Xu, Z., Wang, L., et al. (2023). Pathogen identification and field efficacy test of powdery mildew on Paeonia lactiflora in Hexi area of Gansu Province. Agrochemiaals. 62, 758–762+776. doi: 10.16820/j.nyzz.2023.1045
Xu, X., Dai, T., and Wu, C. (2024). First report of Fusarium vanettenii causing fusarium root rot in Fatsia japonica in China. Forests 15:805. doi: 10.3390/f15050805
Xu, X., Zhang, L., Yang, X., Shen, G., Wang, S., Teng, H., et al. (2022). Fusarium species associated with maize leaf blight in Heilongjiang Province, China. J Fungi. 8:170. doi: 10.3390/jof8111170
Xu, M., Zhang, X., Yu, J., Guo, Z., Li, Y., Wu, J., et al. (2021). First report of Fusarium ipomoeae causing peanut leaf spot in China. Plant Dis. 105:3754 doi: 10.1094/pdis-01-21-0226-pdn
Yang, L., Gao, W., Zhang, C., Huo, J., and Wang, Y. (2023). Identification of strawberry wilt caused by Plectosphaerella cucumerina in China. Plant Dis. 107:3290. doi: 10.1094/pdis-03-23-0544-pdn
Yao, L., Ji, C., Wang, Z., Liu, Y., Li, W., Wang, M., et al. (2025). Enhancing Astragalus mongholicus performance through endophytic fungi improvement for Fusarium wilt resistance. Physiol. Mol. Plant Pathol. 136:102532. doi: 10.1016/j.pmpp.2024.102532
Yuan, X., Peng, K., Zhao, Z., Wang, Y., Tian, F., and Li, Y. (2023). Identification of the pathogen causing cobweb disease on black termite mushroom Hymenopellis raphanipes in Guizhou Province, with analysis of its biologicalcharacteristics and screening of fungicides for its control. J. Plant Prot. 50, 780–790. doi: 10.13802/j.cnki.zwbhxb.2023.2021145
Zhai, Y., Zheng, G., Li, J., Xu, B., Zhang, Z., Hui, N., et al. (2024). The control effects of 12 kinds of biogenic agents on apple spot leaf drop disease(Alternaria alternata). Acta Agric. Boreali-Occid. Sin. 33, 1559–1566. doi: 10.7606/j.issn.1004-1389.2024.08.017
Zhang, A., Lu, Y., Huang, J., and Jing, L. (2023). Control effects of 9 botanical fungicides on Puccinia helianthi. Chin. J. Oil Crop Sci. 45, 623–628. doi: 10.19802/j.issn.1007-9084.2022090
Zhang, S. L., Wang, J. J., Shi, C., Shi, Z. R., and Luan, F. (2024). First report of fruit rot disease on melon (Cucumis melo) caused by Fusarium ipomoeae in China. Plant Dis. 108:2923. doi: 10.1094/pdis-02-24-0386-pdn
Zhang, M. Z., Zhang, R. R., Wang, J. Q., Yu, X., Zhang, Y. L., Wang, Q. Q., et al. (2016). Microwave-assisted synthesis and antifungal activity of novel fused Osthole derivatives. Eur. J. Med. Chem. 124, 10–16. doi: 10.1016/j.ejmech.2016.08.012
Zhao, B., He, D., and Wang, L. (2021). Advances in Fusarium drug resistance research. J. Glob. Antimicrob. Resist. 24, 215–219. doi: 10.1016/j.jgar.2020.12.016
Zhou, L., He, W., Long, Y., Li, Y., Ma, W., Yin, F., et al. (2023). First report of Fusarium ipomoeae causing leaf rot on Hosta plantaginea in China. Plant Dis. 108:227. doi: 10.1094/pdis-09-23-1749-pdn
Zhou, F., Luo, A., Han, A., Li, G., Xu, L., Zhang, F., et al. (2023). Study on the antibacterial activity of eight botanical fungicides against Fusarium pseudograminearum and control effects on Fursarium crown rot of wheat. J. Triticeae Crops. 43, 1629–1635. doi: 10.7606/jissn.1009-10412023.12.15
Zhou, L. Y., Yang, S. F., Wang, S. M., Lv, J. W., Wan, W. Q., Li, Y. H., et al. (2020). Identification of Fusarium ipomoeae as the causative agent of leaf spot disease in Bletilla striata in China. Plant Dis. 105:1214. doi: 10.1094/pdis-09-20-1974-pdn
Keywords: Plumbago auriculata, Fusarium ipomoeae, blight, pathogen, multigene phylogeny, biological fungicides screening, control efficacy
Citation: Liu M, Guo T, Yan H, Yuan Y, Xiao Z, Liu Y, Zhang S, Lyu F, Jing S and Yin F (2025) Pathogen identification and biological fungicides screening for Plumbago auriculata blight in China. Front. Microbiol. 16:1609944. doi: 10.3389/fmicb.2025.1609944
Edited by:
Md. Motaher Hossain, Bangabandhu Sheikh Mujibur Rahman Agricultural University, BangladeshCopyright © 2025 Liu, Guo, Yan, Yuan, Xiao, Liu, Zhang, Lyu, Jing and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuqiang Yin, MjAyMDAwMDJAc2FueGlhdS5lZHUuY24=
†These authors have contributed equally to this work
 Ming Liu1,2†
Ming Liu1,2† Shaotian Zhang
Shaotian Zhang Fuqiang Yin
Fuqiang Yin