- 1Department of Dermatology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Guangxi Key Laboratory of AIDS Prevention and Treatment, Guangxi Medical University, Nanning, China
- 3Guangxi Key Laboratory of Mycosis Prevention and Treatment, Nanning, China
- 4Guangxi Scientific and Technological Innovation Cooperation Base of Mycosis Prevention and Control, Nanning, China
- 5Department of Laboratory, Maternity and Child Health Care of Guangxi Zhuang Autonomous Region, Nanning, China
In recent years, some species of Talaromyces have emerged as human pathogens but rarely reported from Southern China, the endemic region of Talaromyces marneffei. To investigate the diversity of Talaromyces species in Southern China, we collected 59 clinical Talaromyces isolates, which were identified as 11 different species through molecular analysis. Notably, several species exhibited phenotypic characteristics similar to T. marneffei, such as red pigment production at 25°C and monoverticillate or biverticillate conidiophores with globose to subglobose conidia, potentially leading to misidentification. Antifungal susceptibility testing revealed distinct drug susceptibility profiles between T. marneffei and other species. Furthermore, seven species demonstrated growth at 37°C and induced inflammatory lung damage in mice, suggesting their pathogenic potential. These emerging Talaromyces pathogens were primarily isolated from respiratory tract samples of immunocompromised patients. Our findings highlight the rich diversity of Talaromyces species clinically in Southern China, emphasizing the critical importance of considering their potentially pathogenic.
1 Introduction
The genus Talaromyces (Trichocomaceae, Eurotiales) is widely distributed in nature and closely associated with human activities. Based on polyphasic taxonomy, it is classified into eight sections: Bacillispori, Helici, Islandici, Purpurei, Subinflati, Tenues, Talaromyces, and Trachyspermi (Yilmaz et al., 2014; Sun et al., 2020). With the advancement of molecular diagnostic techniques, the number of Talaromyces species has rapidly increased, exceeding 180 described species to date.
While Talaromyces marneffei remains a significant cause of high-mortality talaromycosis (Cao et al., 2019; Narayanasamy et al., 2021), emerging pathogenic species within the genus have been increasingly reported. For example, T. indigoticus has been implicated in human skin and nail infections in Panama (Weisenborn et al., 2010), and T. kabodanensis, T. subaurantiacus, T. alveolaris, and T. rapidus have been associated with respiratory tract infections (Guevara-Suarez et al., 2017). Moreover, T. piceum has been linked to fungemia and rib osteomyelitis in patients with X-linked chronic granulomatous disease (Horré et al., 2001; Santos et al., 2006). Notably, phenotypic similarities, such as monoverticillate or biverticillate conidiophores and red pigment production, can lead to the misidentification of other Talaromyces species as T. marneffei. A study from Indonesia demonstrated that nine clinical isolates previously identified as T. marneffei based on phenotype were subsequently confirmed as T. atroroseus through molecular analysis (Surja et al., 2020). Therefore, molecular methods are essential for accurately identifying clinical Talaromyces strains.
In recent years, some clinical Talaromyces species isolated from patients were reported in non-T. marneffei-endemic areas (Li et al., 2019). In this study, we have observed the emergence of Talaromyces species other than T. marneffei in clinical samples from Southern China, a region endemic to T. marneffei. Some of them could grow at 37°C—the temperature of the human body, suggesting a potential pathogenic ability. To investigate the potential pathogenicity of these species, we collected clinical Talaromyces isolates and identified them using molecular analysis. Comparative studies on morphology, drug susceptibility, and clinical features were conducted between T. marneffei and other Talaromyces species. Additionally, based on the principle of Koch’s postulate that strains could be re-isolated from the experimentally infected host (Singh et al., 2016), we assessed the pathogenicity of these Talaromyces species through animal models to inform potential treatment strategies.
2 Materials and methods
2.1 Isolates
Fifty-nine Talaromyces strains were isolated from different patients at The First Affiliated Hospital of Guangxi Medical University between September 2020 and March 2022. Briefly, clinical samples were aseptically collected and subsequently isolated on Sabouraud dextrose agar (SDA) medium at 27°C for 14 days. Pure fungal isolates were obtained through the plate streaking methods in preparation for downstream experimental analyses. All isolates were stored in the Guangxi Key Laboratory of Mycosis Research and Prevention.
2.2 Morphological analysis
Macroscopic characteristics were evaluated on SDA, czapek yeast autolysate agar (CYA) and malt extract agar (MEA). Isolates were cultured at 25°C and 37°C for a 14-day incubation period. Colony texture, color, soluble pigment production, and microscopic features, including conidiophore branching and conidial morphology, were recorded.
2.3 Phylogenetic analysis
Strains were cultured on SDA medium for 7 to 14 days prior to DNA extraction. DNA was extracted using the MightyPrep reagent (Takara, Beijing, China), and PCR amplification was performed with MightyAmp DNA Polymerase Ver.3 (Takara). The ITS, BenA, and CaM genes were amplified following protocols described in previous studies (Houbraken and Samson, 2011; Yilmaz et al., 2014). Phylogenetic analyses were conducted using the Maximum Likelihood (ML) method in MEGA v.7.0 based on combined ITS, BenA, and CaM gene sequences. ML analyses were supported by 1,000 bootstrap replicates, and bootstrap values ≥70 were considered significant.
2.4 Antifungal susceptibility testing
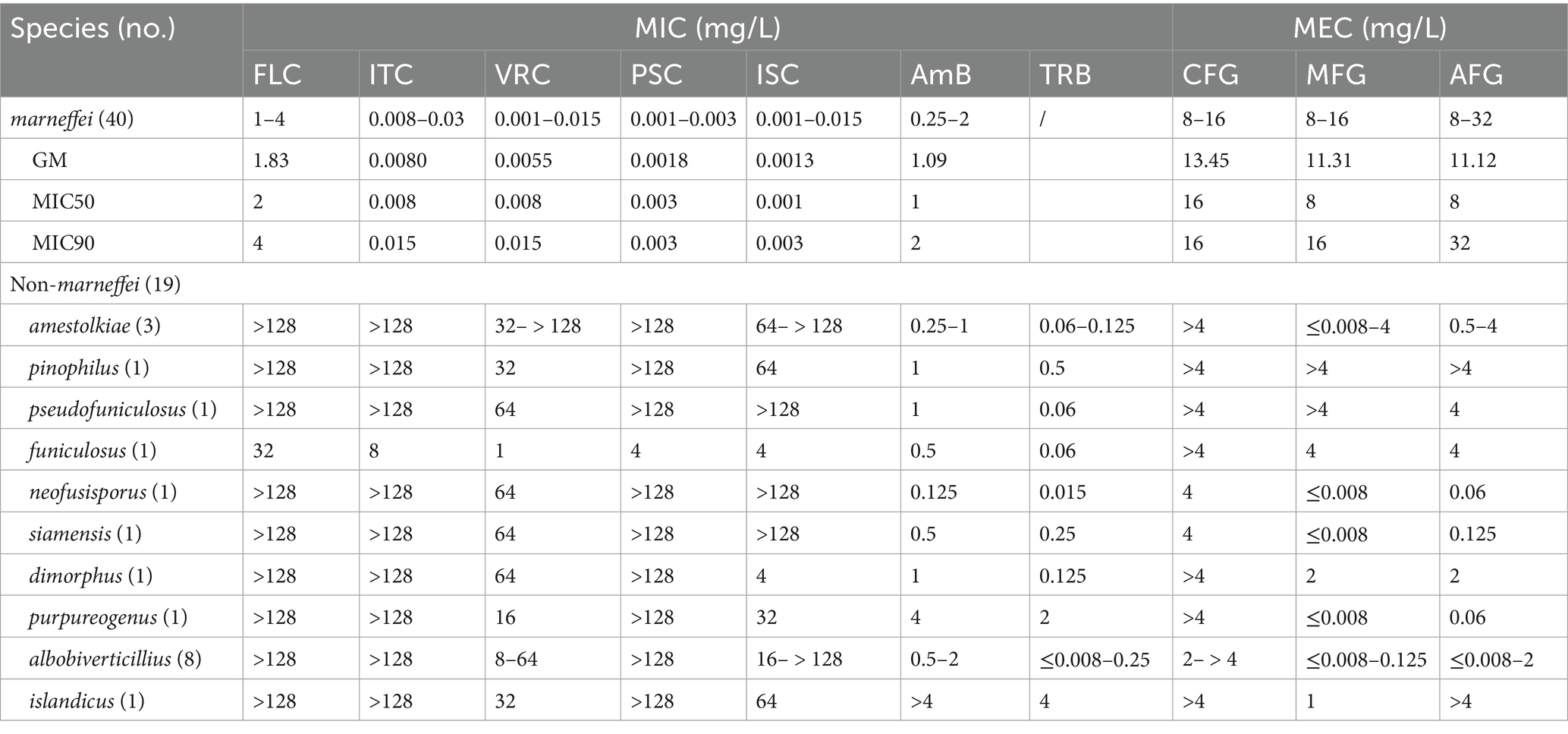
Antifungal susceptibility testing of the isolates was performed using the broth microdilution method as outlined in the Clinical and Laboratory Standards Institute (CLSI) M38-A3 standard for filamentous fungi (Wayne, 2017) and referred to previous studies (Guevara-Suarez et al., 2016; Guo et al., 2021). Briefly, strains were grown on potato dextrose agar for 7 to 10 days at 27°C until good conidiation. The conidia for each strain were resuspended in sterile 0.85% saline to a concentration of 5 × 106 CFU/mL. The susceptibility profiles of itraconazole (ITC), voriconazole (VRC), posaconazole (PSC), isavuconazole (ISC), fluconazole (FLC), caspofungin (CFG), micafungin (MFG), anidulafungin (AFG), amphotericin B (AmB), and terbinafine (TRB) were determined in vitro. FLC, ITC, VRC, PSC, AmB, and TRB were purchased from Sigma-Aldrich. In addition, ISC was purchased from Targetmol. CFG, MFG, and AFG were purchased from MedChemExpress. A volume of 100 μL of twofold of antifungal agents, as well as the 1:50 diluted inoculum suspension, was added to the microdilution wells using 96-well microtiter plates. The last two columns were growth control wells (containing RPMI 1640 culture medium without antifungal agents) and negative control wells (RPMI 1640 only). The drug concentration ranges (from low to high) were: 0.008–4 mg/L for TRB and AmB, 0.25–128 mg/L for FLC, 0.001–0.5 mg/L for triazoles and 0.25–128 mg/L for echinocandins of T. marneffei, 0.008–4 mg/L for echinocandins and 0.25–128 mg/L for triazoles of other Talaromyces species. The minimal inhibitory concentration (MIC) was defined as the lowest drug concentration inhibiting 100% of visible growth for triazoles and AmB, 80% for TRB, and a 50% reduction for FLC compared to the drug-free control. The minimal effective concentration (MEC) for echinocandins was determined as the lowest concentration, producing short, stubby, abnormally branched hyphae. Both MIC and MEC were assessed at 48 h. Quality control was maintained using C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 strains.
2.5 Animal models
Species capable of growth at 37°C were evaluated for pathogenic potential using an animal model. Eight-week-old BALB/c mice were immunosuppressed with cyclophosphamide and triamcinolone acetonide as previously described (Zheng et al., 2019). Immunosuppressed mice received an intraperitoneal injection of 100 μL containing 107 conidia. Four weeks post-inoculation, mice were euthanized, and lung tissue was subjected to fungal culture at 27°C and hematoxylin–eosin staining for histopathological examination.
2.6 Clinical information
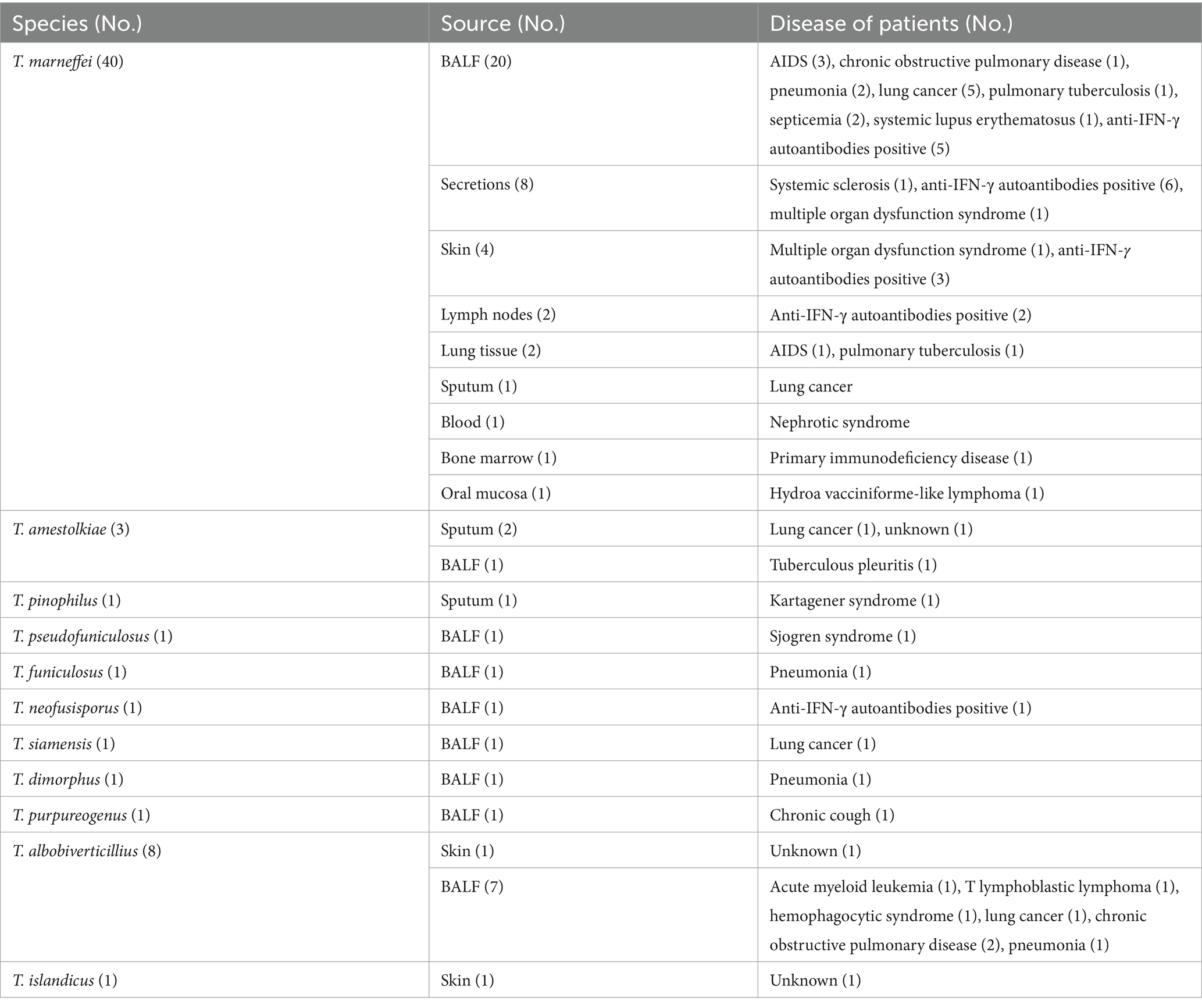
Clinical information was also collected from all 59 patients, including underlying diseases and fungal culture sites.
3 Results
3.1 Molecular and morphological analysis
Molecular analysis revealed that the 59 isolates belonged to three Talaromyces sections: Talaromyces section Talaromyces (n = 50), section Trachyspermi (n = 8), and section Islandici (n = 1).
Of the 59 isolates, 40 were identified as T. marneffei based on ITS sequence analysis using BLAST in NCBI GenBank and checking in the ISHAM-ITS database (Irinyi et al., 2015). The remaining 19 isolates required phylogenetic analysis incorporating ITS, BenA, and CaM sequences for species-level identification. Phylogenetic analysis (Figure 1) revealed these 19 isolates to represent 10 species: T. amestolkiae (n = 3), T. pinophilus (n = 1), T. funiculosus (n = 1), T. pseudofuniculosus (n = 1), T. neofusisporus (n = 1), T. siamensis (n = 1), T. dimorphus (n = 1), T. purpureogenus (n = 1), T. albobiverticillius (n = 8), and T. islandicus (n = 1).
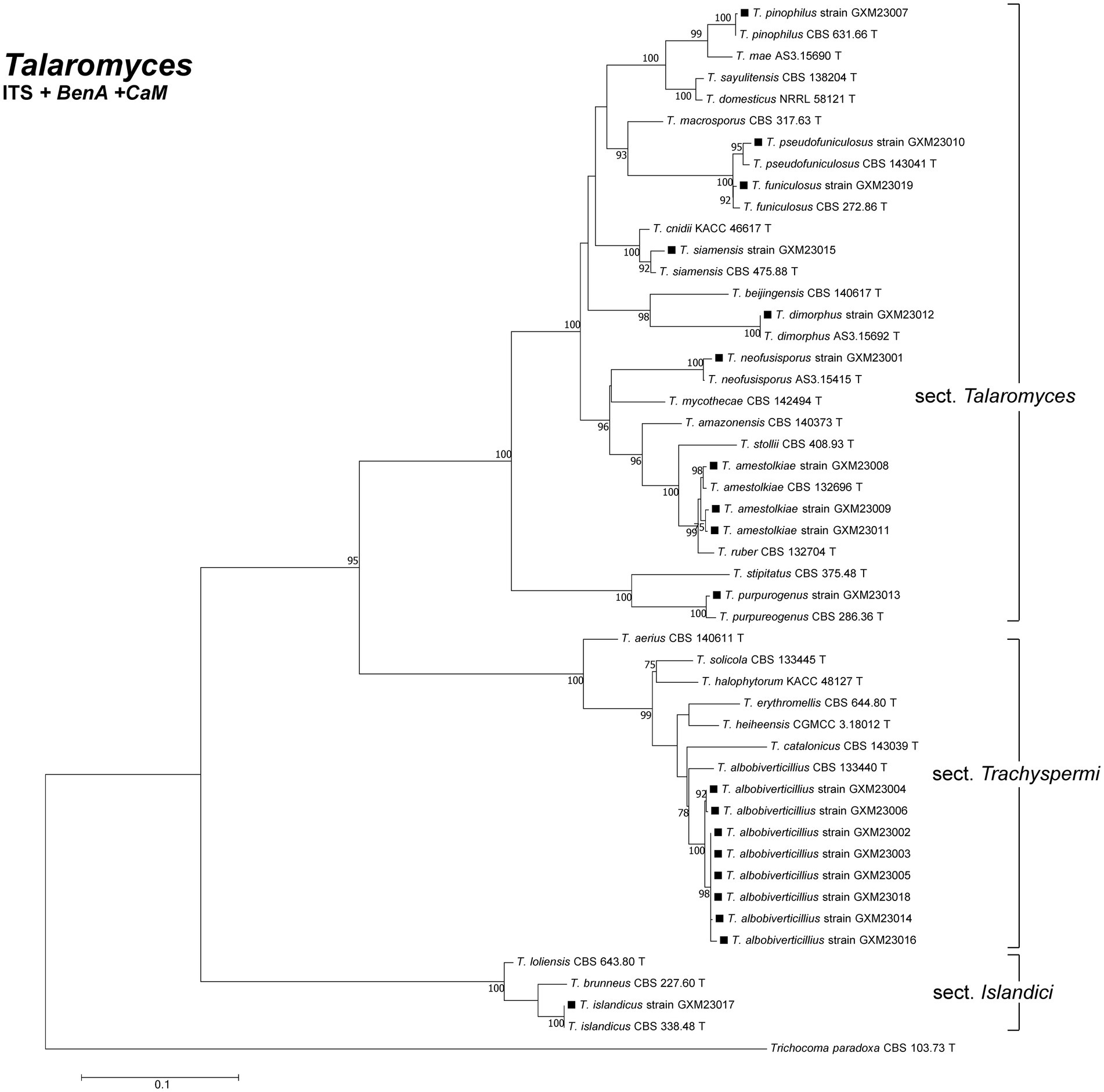
Figure 1. Phylogenetic analysis of clinical Talaromyces species in this study. Bootstrap percentages over 70% derived from 1,000 replicates are indicated at the nodes. Strains belonging to this study are indicated in black squares. Trichocoma paradoxa (CBS 103633.73 T) was chosen as the outgroup of Talaromyces. T: ex-type.
All clinical Talaromyces species in this study exhibited yellow-green to grey-green filamentous colonies at 25°C (Supplementary Figure 1). Notably, in addition to T. marneffei, five species—T. amestolkiae, T. pinophilus, T. neofusisporus, T. purpureogenus, and T. albobiverticillius—produced red pigment on SDA medium at room temperature (Figure 2). Interestingly, the microscopic morphology of these red-pigmented species shared similarities with T. marneffei, characterized by monoverticillate or biverticillate conidiophores and globose to subglobose conidia (Supplementary Table 1 and Supplementary Figure 3).
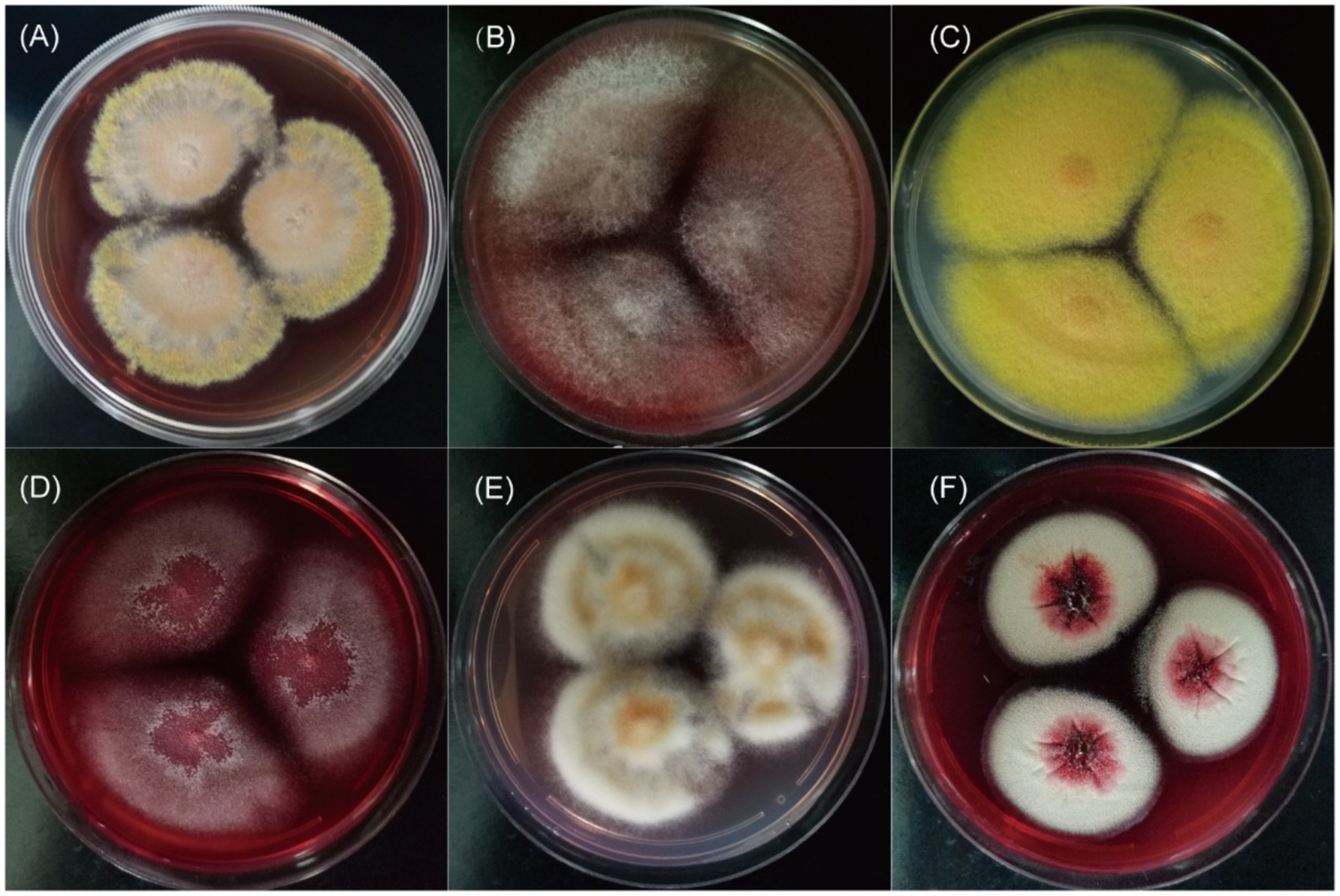
Figure 2. The red pigment of clinical Talaromyces species on SDA medium at 25°C for 14 days. (A–F) Were T. marneffei, T. amestolkiae, T. pinophilus, T. neofusisporus, T. purpureogenus, and T. albobiverticillius, respectively.
Among the species producing abundant red pigment at room temperature, T. albobiverticillius (n = 8) exhibited limited growth at 37°C. In contrast, T. amestolkiae, T. pinophilus, T. siamensis, T. islandicus, T. neofusisporus, T. purpureogenus, T. funiculosus, and T. marneffei exhibited growth at 37°C. However, T. marneffei was the only species demonstrating thermal dimorphism, adopting a yeast-form growth at 37°C (Supplementary Figure 2).
3.2 Antifungal susceptibility testing
Antifungal susceptibility testing was performed on all isolates. The results demonstrated distinct drug susceptibility profiles between T. marneffei and the other species (Table 1).
Azoles, except for FLC, demonstrated superior activity against T. marneffei strains compared to echinocandins and AmB. ITC, VRC, PCS, and ISC exhibited MIC values <0.03 mg/L, while AmB MICs ranged from 0.25 to 2 mg/L. Echinocandins, including CFG, MFG, and AFG, had MEC values ≥8 mg/L.
In contrast to T. marneffei, echinocandins displayed superior antifungal activity against other Talaromyces species. MEC values of MFG and AFG were ≤0.008–2 mg/L for most strains, while FLC, ITC, and PSC MIC values exceeded 128 mg/L, except for a single T. funiculosus isolate.
However, susceptibility to echinocandins varied among strains. T. amestolkiae, T. pinophilus, T. funiculosus, and T. pseudofuniculosus (n = 1 for each) exhibited high MEC of ≥4 mg/L for all echinocandins. TRB exhibited good antifungal activity against these strains (MIC range ≤0.008–4 mg/L), while AmB showed intermediate activity (MIC range 0.125– > 4 mg/L).
3.3 Animal models
Murine models of invasive infection were established to assess the pathogenicity of species capable of growth at 37°C. Survival rates, lung fungal cultures, and histopathological examination of mice were used to evaluate disease progression.
Except for T. pinophilus, all other groups, including T. amestolkiae, T. siamensis, T. islandicus, T. neofusisporus, T. purpureogenus, and T. funiculosus, exhibited mouse mortality within 4 weeks after infection. All infected mice died for the groups of T. amestolkiae and T. funiculosus, and most for T. islandicus (5/ 6), T. neofusisporus (5/6), and T. siamensis (4/6) infection groups. Histopathological examination of lung tissue from these seven groups showed perivascular edema with lymphocyte infiltration, and infiltration of eosinophilic flocs and macrophages in the alveolar space revealed inflammatory damage (Figure 3). Lung fungal cultures were positive for all mice infected with T. islandicus and T. purpureogenus, as well as most the mice of T. pinophilus (5/6), T. siamensis (4/6) T. funiculosus (4/6) infection groups (Supplementary Table 2).
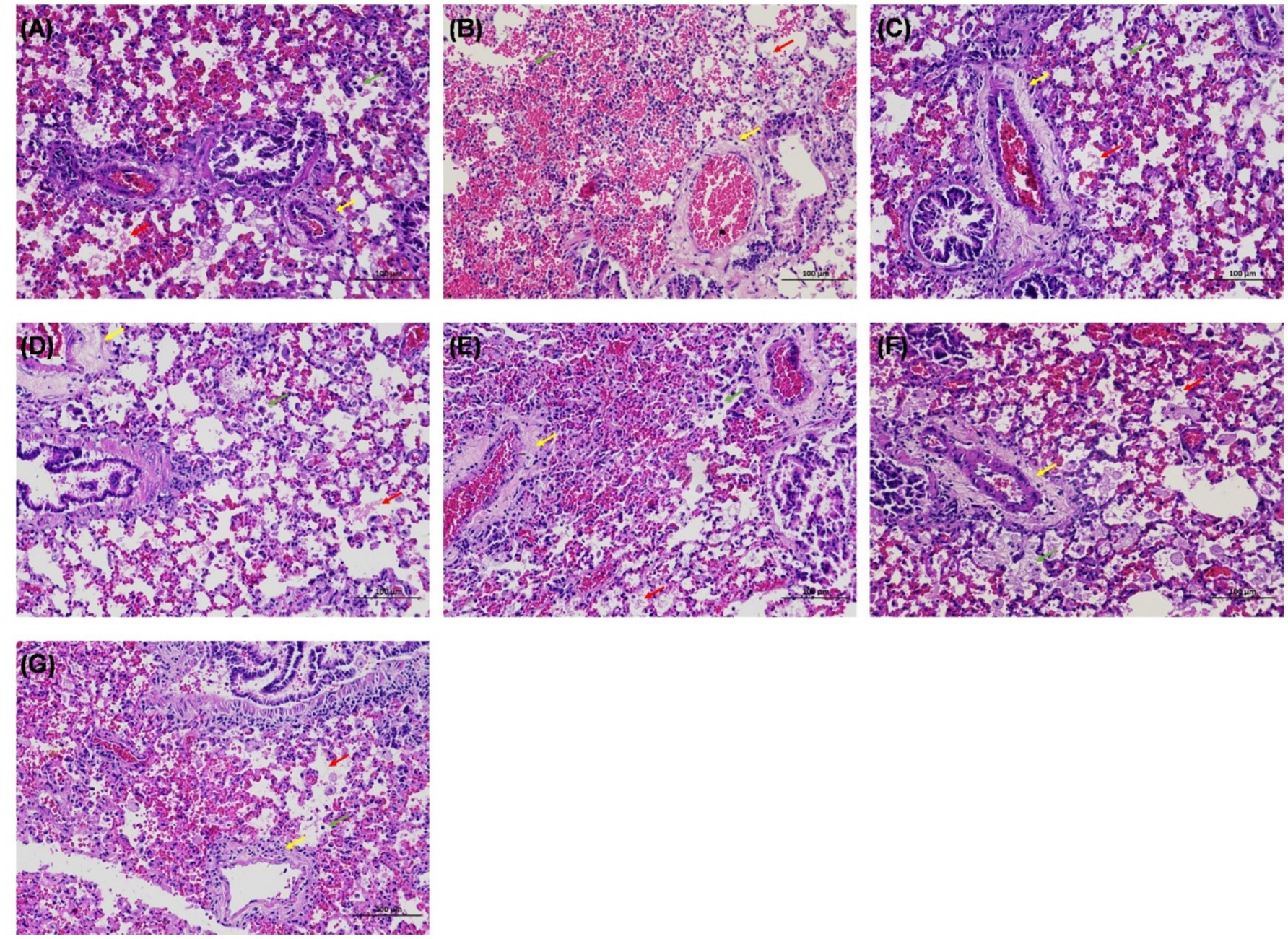
Figure 3. Hematoxylin–eosin (HE) staining of the lung in mice infected with Talaromyces. (A–G) Were infected by T. amestolkiae, T. pinophilus, T. neofusisporus, T. funiculosus, T. purpureogenus, T. siamensis, and T. islandicus, respectively. HE staining showed perivascular edema with lymphocyte infiltration (yellow arrows), and infiltration of eosinophilic flocs (red arrows) and macrophages (green arrows) in the alveolar space.
3.4 Clinical information
The distribution of clinical Talaromyces strains across sample types is summarized in Table 2. Among the 11 Talaromyces species identified, T. marneffei exhibited the broadest range of sample origins, including bronchoalveolar lavage fluid (BALF), sputum, lung tissue, and extrapulmonary specimens such as secretions, skin, lymph nodes, blood, bone marrow, and oral mucosa. In contrast, most (89.5%, 17/19) of Talaromyces isolates were recovered from respiratory tract samples (BALF or sputum), with only T. albobiverticillius and T. islandicus isolated from the skin. Ten strains, primarily T. albobiverticillius (n = 8), but also including T. pseudofuniculosus and T. dimorphus, were categorized as environmental contaminants due to their inability to grow at human body temperature (37°C).
Of the 49 patients whose isolates grew at 37°C, 95.9% (47/49) had underlying diseases. The distribution of underlying diseases differed between patients infected with T. marneffei and those infected with other Talaromyces species. Among the 40 patients infected with T. marneffei, 40% were positive for anti-IFN-γ autoantibodies. In contrast, respiratory diseases were the primary underlying condition in patients infected with other Talaromyces species.
4 Discussion
Emerging human pathogenic fungi that were previously thought to be environmental could be driven by warming (Casadevall, 2023). Southern China’s warm and humid climate fosters a diverse fungal environment, making it a hotspot for fungal infections, including talaromycosis caused by T. marneffei (Hu et al., 2013; Cao et al., 2019). Although other Talaromyces species are commonly reported in the natural environment, several clinical cases have shown their potential pathogenicity. While T. marneffei is prevalent, clinical cases involving other Talaromyces species are underreported in this region. Our study identified 11 Talaromyces species from three distinct sections among clinical samples: T. amestolkiae, T. pinophilus, T. funiculosus, T. pseudofuniculosus, T. neofusisporus, T. siamensis, T. dimorphus, T. purpureogenus, T. albobiverticillius, T. islandicus, and T. marneffei. Although T. marneffei was the most common, other species constituted 32.2% of the isolates, demonstrating the region’s rich Talaromyces diversity in a clinical setting.
Red pigment production at room temperature is a common characteristic among Talaromyces species, including T. purpureogenus, T. albobiverticillius, T. minioluteus, and T. atroroseus (Yilmaz et al., 2012; Frisvad et al., 2013). Relying solely on gross morphology and red pigment production at 25°C for species identification can lead to the misidentification of these species as T. marneffei. In this study, we observed red pigment production in several clinical Talaromyces species other than T. marneffei, specifically T. amestolkiae, T. pinophilus, T. neofusisporus, T. purpureogenus, and T. albobiverticillius. Consequently, heightened attention should be given to these species, particularly in the context of T. marneffei research. To differentiate these species from T. marneffei, cultivating fungal isolates at both 25°C and 37°C is crucial, as T. marneffei exhibits yeast-form growth at 37°C.
Understanding antifungal drug susceptibility is crucial for guiding the clinical management of fungal infections. Previous studies have demonstrated significant differences in drug susceptibility profiles between T. marneffei and other Talaromyces species (Liu et al., 2013; Guevara-Suarez et al., 2016; Lau et al., 2017; Guo et al., 2021). Consistent with these findings, our study revealed high triazole susceptibility but strong echinocandin resistance in T. marneffei strains. Conversely, most other Talaromyces species exhibited high susceptibility to echinocandins, moderate susceptibility to AmB and TRB, and lower susceptibility to azoles. Notably, the antifungal susceptibility for echinocandins of some strains in our study was low, probably due to the potential variability in antifungal susceptibility among different Talaromyces species and geographic regions. Therefore, antifungal susceptibility testing of clinical Talaromyces strains is necessary for appropriate antifungal treatment to avoid toxic side effects caused by unnecessary drug exposure. However, due to the limitations of the sample of this study, it is expected that more in-depth research will be conducted in the future to verify this view. Consequently, tailoring antifungal treatment based on drug susceptibility testing is recommended. In the absence of timely susceptibility data, TRB, AmB, MFG and AFG among the echinocandins may be considered as initial empiric therapy.
Clinically, unlike the diverse sources of T. marneffei isolates, other Talaromyces species are predominantly recovered from respiratory specimens. In this study, a substantial proportion (89.5%, 17/19) of Talaromyces strains originated from BALF or sputum. This finding aligns with the growing body of evidence implicating Talaromyces species in respiratory tract infections. For instance, T. amestolkiae has been isolated from BALF in both an AIDS patient and an immunocompetent female with pneumonia (Yilmaz et al., 2012). Additionally, T. pinophilus, T. funiculosus, T. purpureogenus, T. albobiverticillius, and T. islandicus have been reported in human respiratory tract infections (Yilmaz et al., 2012; Guevara-Suarez et al., 2016; Li et al., 2019; Guo et al., 2021). Generally, the capacity to thrive at human body temperature is a prerequisite for fungal infections in humans. Our study found that patients infected with Talaromyces species capable of growth at 37°C predominantly exhibited underlying conditions associated with immunosuppression, such as lung cancer, Kartagener syndrome, and anti-IFN-γ autoantibodies. These findings align with previous research demonstrating a correlation between immunocompromised states and infections caused by these fungi (Atalay et al., 2016; Villanueva-Lozano et al., 2017; Guo et al., 2021). These results suggest a predilection for respiratory tract infections and a reliance on a host with a compromised immune system for successful colonization. And they indirectly indicate that there are similarities in the pathogenesis of clinical Talaromyces species in different regions. However, whether the same species in different regions show this trend needs to be explored in the future. To explore the potential pathogenicity and validate Koch’s postulates experimentally, we established a murine infection model using immunosuppressed hosts, and clinical Talaromyces strains re-isolated from diseased mice were successfully. The observed lung inflammation in mice infected with these Talaromyces species further supports this hypothesis. Notably, in several species, the mortality in mice did not agree with the lung fungal cultures due to the low sensitivity of fungal cultures and the low toxicity of some species.
Our study has certain limitations that should be acknowledged. Due to institutional constraints regarding specialized instrumentation and heightened biosafety considerations, we use intraperitoneal injection to study pulmonary infection in murine models. Notably, in T. marneffei infection models, intraperitoneal injection has been widely adopted and validated (Zheng et al., 2019; He et al., 2022). These investigations demonstrated pulmonary involvement with fungal culture or histopathological evidence despite extra-respiratory inoculation by intraperitoneal injection. The respiratory tract infection models of these emerging Talaromyces species challenge as a future research direction. And secondly, it is the small sample size derived from a single center. To enhance the generalizability of our findings and potentially identify additional Talaromyces pathogen species, future studies should involve larger sample sizes collected from multiple centers.
In conclusion, Southern China exhibits a diverse clinical spectrum of Talaromyces species, some of which can be easily misidentified as T. marneffei. Certain Talaromyces species can grow at 37°C and have demonstrated pathogenicity in animal models. Since T. marneffei and other Talaromyces species require different drug treatments, misdiagnosis can result in inappropriate therapy and treatment failure. Consequently, when Talaromyces strains are isolated from respiratory tract specimens of immunocompromised individuals, clinicians should consider their potential pathogenicity and initiate appropriate treatment.
Data availability statement
The sequences used for phylogenetic analysis in this study have been deposited at NCBI GenBank. The accession numbers were as follows: OQ550077-OQ550095 and PQ37528-PQ37567 for ITS region, PP869295-PP869313 and PQ756998-PQ757037 for BenA, PQ095599-PQ095617 and PQ661880-PQ661919 for CaM.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. The animal study was approved by the First Affiliated Hospital of Guangxi Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. KP: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. DZ: Data curation, Resources, Writing – review & editing. SW: Methodology, Visualization, Writing – review & editing. WW: Data curation, Resources, Writing – review & editing. CC: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Key Research and Development Program of China (2022YFC2504800); and the Natural Science Foundation of Guangxi Province of China under Grant (AD23026325 AB24010134).
Acknowledgments
The authors would like to thank Dr. Liyan Xi, Professor of the Department of Dermatology, Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University, for her reading of the manuscript and for providing valuable suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1610481/full#supplementary-material
References
Atalay, A., Koc, A. N., Akyol, G., Cakir, N., Kaynar, L., and Ulu-Kilic, A. (2016). Pulmonary infection caused by Talaromyces purpurogenus in a patient with multiple myeloma. Infez. Med. 24, 153–157.
Cao, C., Xi, L., and Chaturvedi, V. (2019). Talaromycosis (Penicilliosis) due to Talaromyces (Penicillium) marneffei: insights into the clinical trends of a major fungal disease 60 years after the discovery of the pathogen. Mycopathologia 184, 709–720. doi: 10.1007/s11046-019-00410-2
Casadevall, A. (2023). Global warming could drive the emergence of new fungal pathogens. Nat. Microbiol. 8, 2217–2219. doi: 10.1038/s41564-023-01512-w
Frisvad, J. C., Yilmaz, N., Thrane, U., Rasmussen, K. B., Houbraken, J., and Samson, R. A. (2013). Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS One 8:e84102. doi: 10.1371/journal.pone.0084102
Guevara-Suarez, M., Sutton, D. A., Cano-Lira, J. F., García, D., Martin-Vicente, A., Wiederhold, N., et al. (2016). Identification and antifungal susceptibility of Penicillium-like Fungi from clinical samples in the United States. J. Clin. Microbiol. 54, 2155–2161. doi: 10.1128/jcm.00960-16
Guevara-Suarez, M., Sutton, D. A., Gené, J., García, D., Wiederhold, N., Guarro, J., et al. (2017). Four new species of Talaromyces from clinical sources. Mycoses 60, 651–662. doi: 10.1111/myc.12640
Guo, L. N., Yu, S. Y., Wang, Y., Liu, Y. L., Yuan, Y., Duan, S. M., et al. (2021). Species distribution and antifungal susceptibilities of clinical isolates of Penicillium and Talaromyces species in China. Int. J. Antimicrob. Agents 58:106349. doi: 10.1016/j.ijantimicag.2021.106349
He, J., Li, J. S., Xu, H. Y., Kuang, Y. Q., Li, J., Li, H. B., et al. (2022). A reliable murine model of disseminated infection induced by Talaromyces Marneffei. Mycopathologia 187, 53–64. doi: 10.1007/s11046-021-00596-4
Horré, R., Gilges, S., Breig, P., Kupfer, B., de Hoog, G. S., Hoekstra, E., et al. (2001). Case report. Fungaemia due to Penicillium piceum, a member of the Penicillium marneffei complex. Mycoses 44, 502–504. doi: 10.1046/j.1439-0507.2001.00710.x
Houbraken, J., and Samson, R. A. (2011). Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud. Mycol. 70, 1–51. doi: 10.3114/sim.2011.70.01
Hu, Y., Zhang, J., Li, X., Yang, Y., Zhang, Y., Ma, J., et al. (2013). Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia 175, 57–67. doi: 10.1007/s11046-012-9577-0
Irinyi, L., Serena, C., Garcia-Hermoso, D., Arabatzis, M., Desnos-Ollivier, M., Vu, D., et al. (2015). International Society of Human and Animal Mycology (ISHAM)-ITS reference DNA barcoding database--the quality controlled standard tool for routine identification of human and animal pathogenic fungi. Med. Mycol. 53, 313–337. doi: 10.1093/mmy/myv008
Lau, S. K., Lo, G. C., Lam, C. S., Chow, W. N., Ngan, A. H., Wu, A. K., et al. (2017). In vitro activity of posaconazole against Talaromyces marneffei by broth microdilution and Etest methods and comparison to itraconazole, voriconazole, and anidulafungin. Antimicrob. Agents Chemother. 61:e01480-16. doi: 10.1128/aac.01480-16
Li, L., Chen, K., Dhungana, N., Jang, Y., Chaturvedi, V., and Desmond, E. (2019). Characterization of clinical isolates of Talaromyces marneffei and related species, California, USA. Emerg. Infect. Dis. 25, 1765–1768. doi: 10.3201/eid2509.190380
Liu, D., Liang, L., and Chen, J. (2013). In vitro antifungal drug susceptibilities of Penicillium marneffei from China. J. Infect. Chemother. 19, 776–778. doi: 10.1007/s10156-012-0511-7
Narayanasamy, S., Dat, V. Q., Thanh, N. T., Ly, V. T., Chan, J. F., Yuen, K. Y., et al. (2021). A global call for talaromycosis to be recognised as a neglected tropical disease. Lancet Glob. Health 9, e1618–e1622. doi: 10.1016/s2214-109x(21)00350-8
Santos, P. E., Piontelli, E., Shea, Y. R., Galluzzo, M. L., Holland, S. M., Zelazko, M. E., et al. (2006). Penicillium piceum infection: diagnosis and successful treatment in chronic granulomatous disease. Med. Mycol. 44, 749–753. doi: 10.1080/13693780600967089
Singh, V. P., Proctor, S. D., and Willing, B. P. (2016). Koch's postulates, microbial dysbiosis and inflammatory bowel disease. Clin. Microbiol. Infect. 22, 594–599. doi: 10.1016/j.cmi.2016.04.018
Sun, B. D., Chen, A. J., Houbraken, J., Frisvad, J. C., Wu, W. P., Wei, H. L., et al. (2020). New section and species in Talaromyces. MycoKeys 68, 75–113. doi: 10.3897/mycokeys.68.52092
Surja, S. S., Adawiyah, R., Houbraken, J., Rozaliyani, A., Sjam, R., Yunihastuti, E., et al. (2020). Talaromyces atroroseus in HIV and non-HIV patient: a first report from Indonesia. Med. Mycol. 58, 560–563. doi: 10.1093/mmy/myz090
Villanueva-Lozano, H., Treviño-Rangel, R. J., Renpenning-Carrasco, E. W., and González, G. M. (2017). Successful treatment of Talaromyces amestolkiae pulmonary infection with voriconazole in an acute lymphoblastic leukemia patient. J. Infect. Chemother. 23, 400–402. doi: 10.1016/j.jiac.2016.12.017
Wayne, P. A. (2017). CLSI. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. 3rd ed. CLSI standard M38.
Weisenborn, J. L., Kirschner, R., Cáceres, O., and Piepenbring, M. (2010). Talaromyces indigoticus Takada & Udagawa, the first record for Panama and the American continent. Mycopathologia 170, 203–208. doi: 10.1007/s11046-010-9305-6
Yilmaz, N., Houbraken, J., Hoekstra, E. S., Frisvad, J. C., Visagie, C. M., and Samson, R. A. (2012). Delimitation and characterisation of Talaromyces purpurogenus and related species. Persoonia 29, 39–54. doi: 10.3767/003158512x659500
Yilmaz, N., Visagie, C. M., Houbraken, J., Frisvad, J. C., and Samson, R. A. (2014). Polyphasic taxonomy of the genus Talaromyces. Stud. Mycol. 78, 175–341. doi: 10.1016/j.simyco.2014.08.001
Keywords: Talaromyces , Southern China, diversity, drug susceptibility, pathogenicity
Citation: Liao L, Pan K, Zheng D, Wang S, Wu W and Cao C (2025) Exploring the diversity and pathogenicity of Talaromyces species isolated from clinical in Southern China. Front. Microbiol. 16:1610481. doi: 10.3389/fmicb.2025.1610481
Edited by:
Joshua J. Obar, Dartmouth College, United StatesReviewed by:
Valeria Carvalho Santos-Ebinuma, São Paulo State University, BrazilFang Wang, University of Macau, China
Sirida Youngchim, Chiang Mai University, Thailand
Copyright © 2025 Liao, Pan, Zheng, Wang, Wu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cunwei Cao, Y2FvY3Vud2VpQHllYWgubmV0
 Liuwei Liao
Liuwei Liao Kaisu Pan
Kaisu Pan Dongyan Zheng1,3,4
Dongyan Zheng1,3,4 Shuangjie Wang
Shuangjie Wang Cunwei Cao
Cunwei Cao