- 1International Joint Research Laboratory for Global Change Ecology, School of Life Sciences, Henan University, Kaifeng, Henan, China
- 2Xiaoqinling Ecological Restoration Field Observation and Research Station of the Yellow River Basin, Henan University, Kaifeng, China
Introduction: Water Use Efficiency (WUE) is one of the critical indicators to characterize plant adaptation to arid environments, however, the effects of pathogens infection and Rhizobium symbiosis on WUE are not considered in contexts of water stress.
Methods: A study was conducted in a greenhouse pot to examine the effects of changed soil water conditions on instantaneous Water Use Efficiency (WUEi) and long-term Water Use Efficiency (WUEL) under inoculation Rhizobium, inoculation Fusarium sp., and co-inoculation Rhizobium and Fusarium sp.
Results: The results showed that inoculation Fusarium sp. and co-inoculation Rhizobium and Fusarium sp. reduced WUEi by increasing net photosynthetic rate without drought. Inoculation Fusarium sp. and co-inoculation Rhizobium and Fusarium sp. reduced WUEi by decreasing plant height with drought. Inoculation Rhizobium and Fusarium sp. significantly reduced WUEL by lowering intercellular CO2 concentration without drought. Inoculation Rhizobium reduced WUEL by increasing root nodule number with drought. In contrast, drought had no effect on either WUEi or WUEL without inoculation.
Discussion: The results suggest that Fusarium sp. infection is detrimental to instantaneous Water Use Efficiency while inoculation Rhizobium is unfavorable to long-term Water Use Efficiency, regardless of drought effects. Our findings provide a new insight for developing effective water use strategies after pathogen infection or Rhizobium symbiosis under increased precipitation scenarios.
1 Introduction
Global change has intensified hydrological cycles, increasing the intensity and frequency of drought occurrences (Yang et al., 2020). Drought can inhibit plant growth and development, and even lead to plant mortality by lowing stomatal conductance and reducing photosynthetic rate (McDowell et al., 2013; Furlan et al., 2016). Therefore, it is of great significance to explore effective methods to improve plant water stress tolerance and drought resistance strategies (Mabrouk et al., 2022; Hidalgo-Hidalgo et al., 2022; Felipe et al., 2023).
Improving water use efficiency (WUE) is one of the crucial strategies for plant adaptation to arid environments (Domec et al., 2017). Plants employ various strategies to resist drought, including drought avoidance, drought tolerance, and drought resistance (Jewaria et al., 2021; Gupta et al., 2020). Under drought stress conditions, plants reduce evaporation loss by adjusting the size of their stomata, thereby slowing down the rate of water loss (Sun et al., 2020). They also enhance root growth to increase water uptake, improving tissue water status, and utilizing water resources more efficiently (Nguyen et al., 2022). Furthermore, plants adapt to arid environments by altering physiological characteristics, including chlorophyll content and leaf area (Lauri et al., 2016; Guzzo et al., 2021). Therefore, the mechanisms of plant to resist drought depend on plant morphology and physiological traits.
Pathogens can also alter WUE by affecting plant hydraulic properties and regulating stomatal behavior (Melotto et al., 2006; McElrone et al., 2008). Previous study indicates that pathogens significantly influence photosynthetic parameters in various ways (Gortari et al., 2018; Homet et al., 2019; Li et al., 2022; Murria et al., 2022). For example, the net photosynthesis rate in most plants decreases significantly after infection with pathogens, and this is observed in poplars infected with rust disease (Gortari et al., 2018) and pearl millet [Pennisetum glaucum (L.) R. Br.] infected with Sclerospora graminicola (Murria et al., 2022). In contrast, studies have reported an increase in the net photosynthesis rate of Quercus suber seedlings infected with Phytophthora cinnamomi (Homet et al., 2019) and oat leaves infected with Puccinia graminis f. sp. avenae (Li et al., 2022). Plants can also symbiosis with beneficial microorganisms, such as Rhizobium and Arbusculia, to improve plant drought resistance (Rolli et al., 2015). Rhizobium is a common plant growth promoting bacteria in pulses and other crops. Rhizobium fixes nitrogen in the nodules through symbiosis, supporting the metabolism of plant. Studies have shown that the selection of suitable and effective Rhizobium can improve the symbiotic combination of plants and Rhizobium under drought stress, improving the productivity of legumes (Kibido et al., 2020). Therefore, damage caused by water scarcity can be reduced by inoculating drought-tolerant Rhizobium strains (Barbosa et al., 2018). These findings suggest that complex physiological and biochemical regulatory mechanism works during the interaction of plants and microbes under drought conditions. However, the roles of pathogens infection and symbiosis with beneficial microorganisms in influencing WUE are often overlooked.
As one of the world’s most important oil and cash crops, peanut has significant industrial value and development potential. Elucidating the dynamics of peanut-microbe interactions and their regulating effects on water use efficiency are crucial for maintaining peanut yield. Here, we conducted a greenhouse pot experiment by inoculating peanuts with Rhizobium and Fusarium sp. during pod-setting stages (75 days after inoculation) to investigate how Rhizobium and pathogen infections affect peanut water use efficiency (WUE) both at leaf and whole-plant level under water stress conditions. Specifically, we hypothesize that: (1) water use efficiency at the leaf-level and/or whole-plant level will be enhanced under drought conditions, and inoculation Rhizobium may further promotes the positive effect of drought on water use efficiency by aiding peanuts to fix nitrogen; (2) infection by Fusarium sp. may reduce water use efficiency at the leaf-level and/or whole-plant level by infecting peanuts with diseases, and drought may exacerbate the negative effects of Fusarium sp inoculation on water use efficiency.
2 Materials and methods
2.1 Experimental site
This experiment was carried out in the experimental greenhouse of sustainable agricultural ecology innovation site (114° 18′ 17′′E, 34° 49′ 15′′ N) in Jinming Campus of Henan University. The area is located in the hinterland of North China Plain, which belongs to temperate monsoon climate with four distinct seasons. The average annual temperature is 14.52°C, the average annual precipitation is 627.5 mm, and the precipitation is mostly concentrated in July and August. The soil type used in this experiment is sandy soil, which is suitable for peanut growth.
2.2 Experimental materials
The peanut used in the experiment was the “Kainong 98” variety, which was jointly cultivated by Kaifeng Academy of Agricultural and Forestry Sciences and Henan University. The tested Fusarium sp. was provided by China Agricultural Microbial Strain Storage Management Center, and the strain number was ACCC 36194. Brady Rhizobium hongdouense (ACCC 14082) was provided by China Agricultural Microbiological Culture Collection and Management Center. The sandy soil of 0–30 cm layer in Kaifeng local farmland was used as the culture matrix, and the large soil blocks and plant residues were removed by 2 mm sieve. The gamma ray was used to sterilize soil in Piaohe Longxiang Radiation Technology Co., Ltd. The active microbial strain was not detected in the sterilized soil by the company’s quality inspection. The bottom of the PVC pipe with an inner diameter of 25 cm and a depth of 1 m is sealed as a flowerpot. After the two wire ropes are knotted and closed into a ring, they cross from the bottom and are fixed along the side wall. Two handles are formed on both sides of the flowerpot to facilitate the lifting and weighing of the small gantry crane.
2.3 Experimental design
The top of the experimental greenhouse is covered with a glass pane with good light transmittance, and a rain-blocking mesh cloth is set around to prevent rainwater from entering. The bottom of the greenhouse is a pool with a depth of 1 m. The edge of the pool is 2 m wide from the edge. The width of the pool is 2.4 m and the length is 13.4 m. The PVC flowerpot is neatly placed in the pool. A row of wooden boards is set up between each row of flowerpots to facilitate walking and shading the side wall of the flowerpot.This experiment included water supply (WW: 70% field capacity, natural water conditions; SD: 35% field capacity, water limitation group) and inoculation of microorganisms [no inoculation of microorganisms (recorded as C), inoculation of Rhizobium (recorded as R), inoculation of Fusarium sp. (recorded as X), and simultaneous inoculation of Rhizobium and Fusarium sp. (recorded as XR)], a total of 8 treatment combinations. Each treatment had 10 replicates, and totally of 80 plots. The field water capacity was measured by the determination method of soil specific gravity, 70% field capacity was watered every 3 days and 35% field capacity watered every 5 days to maintain a stable soil water content. The inoculation of rhizobia was completed during the seedling period, and the inoculation of Fusarium sp. was carried out during transplanting. After transplanting, the pots were divided into 10 blocks, and 8 treatments in each block were randomly placed. The harvest was carried out in the pod stage (75 days after inoculation with Fusarium sp.).
2.4 Preparation of Rhizobium and Fusarium sp.
The activated Rhizobium strains were inoculated into liquid medium [sucrose 10 g, K2HPO4 0.5 g, MgSO4⋅7H2O 0.2 g, CaSO4 0.2 g, NaCl 0.1 g, yeast powder 1 g, NaMoO4(1%) l mL, Iron citrate (1%) 1 mL, Boric acid (1%) 1 mL, MnSO4 (1%) 1 mL, H2O 1 L, pH (6.8–7.0)] at 28–30°C for 2.5 days to the logarithmic phase, 480 mL of the bacterial solution was centrifuged at 6,000 r/min for 10 min to collect the bacteria, and then re-suspended with 220 mL of 0.85% NaCl solution to obtain the bacterial suspension. The bacterial suspension was measured by plate colony counting method. The viable bacterial concentration was 7.2 × 109 CFU/mL. Inoculation of Fusarium sp. into liquid medium (peel potato 200 g, cut into small water 1,000 ml boil 30 min block, filter to remove the potato block, the filtrate fill to 1,000 ml, add glucose 20 g, agar 15 g, dissolved after packing, 15 pounds sterilization 30 min) after 2.5 days of shaking culture at 25∼28°C to the logarithmic phase, the bacterial liquid was centrifuged at 8,000 r/min for 10 min to collect the bacteria. After the supernatant was removed, it was re-suspended with 500 mL water to obtain the bacterial suspension. The dry weight method was used to determine the concentration of Fusarium sp., and the measured concentration was 30.7 mg/mL.
2.5 Seedlings, transplanting, and harvests
Peanut seeds and plastic non-porous seedling box (upper diameter 10 cm, lower diameter 8 cm, height 9 cm) were soaked in 70% alcohol for 5 min and 1 h, respectively, and washed three times with sterile water. Distilled water was added to the sterilized soil, and the soil was wet with sterile gloves. Starting from June 5, the seedlings were boxed and inoculated with Rhizobium. After adding 510 g of soil in the seedling basin, two peanut seeds were placed, and then 2 mL of Rhizobium solution was added around the peanut seeds. Finally, 100 g of soil was evenly covered to the upper layer. The inoculation of Fusarium sp. was the same as above, but 6 mL Rhizobium solution was added around the peanut seeds. The treatments without inoculation of Rhizobium and Fusarium sp. were replaced with 2 mL0.85% NaCl solution and 6 mL distilled water, respectively. Before seedling emergence, the seedling box should be covered to avoid excessive temperature or direct sunlight leading to excessive evaporation of soil moisture. During the seedling period, the seedling box should be watered with a spray pot every morning and evening to keep the soil moist.The water content in the soil was measured in advance after the soil was sterilized, and then the sterilized soil and distilled water were added to the stirrer in proportion to stir evenly, so that the soil reached 35% field capacity (35% FC, recorded as SD) and 70% field capacity humidity (70% FC, recorded as WW). The soil was divided into PVC tubes, and the total weight of the tube and soil was recorded. Three peanut seeds with similar weight and good plumpness were selected and planted in the soil of PVC pipe. After the peanut germinated, the other two seedlings were removed to ensure that there was one peanut seedling in each PVC pipe. The weighing method was used to control the water of the pot every 3 days and the watering amount was recorded every time.
Five blocks were randomly selected to harvest plants at the flower needle and podding stage of peanuts, respectively. The peanuts in the PVC pipe were poured out together during harvest, and then the peanut roots were carefully removed from the soil to maintain the integrity of the roots as much as possible.
2.6 Determination of growth traits and WUEL
At the harvest time of the two periods, three individuals of each treatment were randomly selected, and each individual was separated from the junction of the above-ground and below-ground parts of the main stem, washed with tap water, and loaded into a marked envelope. The fresh weight of the leaves was immediately measured after collection to minimize the impact of water evaporation and record the number of nodules in the root (RNN). After measuring the fresh weight, the stems, leaves, pods, and roots of the peanut were dried at 65°C for 48 h before weighing the biomass of each part. Then the root-shoot biomass ratio of each plant was calculated. The concentration or presence of Fusarium sp. in peanut roots is assessed using the agar dilution plate method, which quantifies the number of colony-forming units per gram of dry root weight. Three early morning water potential and noon water potential of peanuts were measured. Pre-dawn water potential measurements were measured between 4:00 and 5:00, and midday water potential measurements were measured between 12:00 and 13:00. Measurements were performed using a pressure chamber instrument. Cut branches or leaves of plants were sealed into the pressure chamber of the device, and pressure was gradually increased until water begins to flow from the cut surface. The pressure at this point represents the water potential of the plant sample. Soil moisture was calculated by the following formula: Soil moisture (%) = [(Wet Weight - Dry Weight)/Dry Weight] × 100%.
2.7 Gas exchange parameters and WUEi
Water use efficiency (WUE) is an important parameter reflecting the water use characteristics of plants, which represents the assimilation amount produced by consuming unit water. Long-term water use efficiency (WUEL) was the ratio of plant biomass to total transpiration water during the experiment (Liu, 2012). Between 9:00–12:00 on sunny days, LI-6400 (Beijing Ligaotai Technology Co., Ltd.) was used to measure the net photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), and intercellular CO2 concentration (Ci) of mature and intact plant leaves for each treatment, with three replicates for each measurement. Instantaneous water use efficiency (WUEi) was calculated as the ratio of the net photosynthetic rate (Pn) to the transpiration rate (Tr).
2.8 Data analysis
One-way variance analysis was used to test the effects of inoculation on peanut WUEi, WUEL, plant height, nodule number (RNN), net photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Gs) and intercellular carbon dioxide concentration (Ci) with drought and without drought in 2022, and inoculation was viewed as fixed factors. The Bonferroni post-hoc test was applied to examine significant differences among the treatments. One-way analysis of variance was also used to examine drought effect in the unvaccinated plots. Linear regression analysis was used to analyze the relationships of RNN, plant height, Pn, Tr, Gs, and Ci with WUEi and WUEL. All statistical analyses were conducted using R software (R Core Team, 2023, version 4.2.3). Structural equation models (SEMs) were used to assess the effects of inoculation on WUEi and WUEL under both drought and non-drought conditions by examining changes in Pn, Gs, and Ci under non-drought conditions, and plant height, RNN, and Ci under drought conditions in 2022. Based on the potential relationship between peanut functional traits, gas exchange parameters, and WUEi and WUEL, a prior model was established. The model fit was assessed using the chi-square test and the minimum Akaike Information Criterion (AIC). SEM analysis was performed using AMOS 24.0.
3 Results
3.1 Rhizobium and Fusarium sp. inoculation
Nodules were only present in peanuts inoculated with Rhizobium (R) and co-inoculated with Fusarium sp. and Rhizobium (XR) under both 35 and 70% field capacity (Figure 1a). The average number of nodules for R- and XR-inoculated peanuts was 54.04 and 35.50, respectively, under 70% field capacity. The average number of nodules for R- and XR-inoculated peanuts was 31.47 and 30.30, respectively, under 35% field capacity (Figure 1a). Fusarium sp. was detected in peanuts inoculated with Fusarium sp. and co-inoculated with Fusarium sp. and Rhizobium (XR) in both 35 and 70% field capacity. The concentration of Fusarium sp. was 1976.34 and 1391.04 CFU/g in peanuts when inoculation with X and XR, respectively, under 70% field capacity. The concentrations were 2795.13 and 1764.79 CFU/g in peanuts when inoculation with X and XR, respectively, under 35% field capacity (Figure 1b).
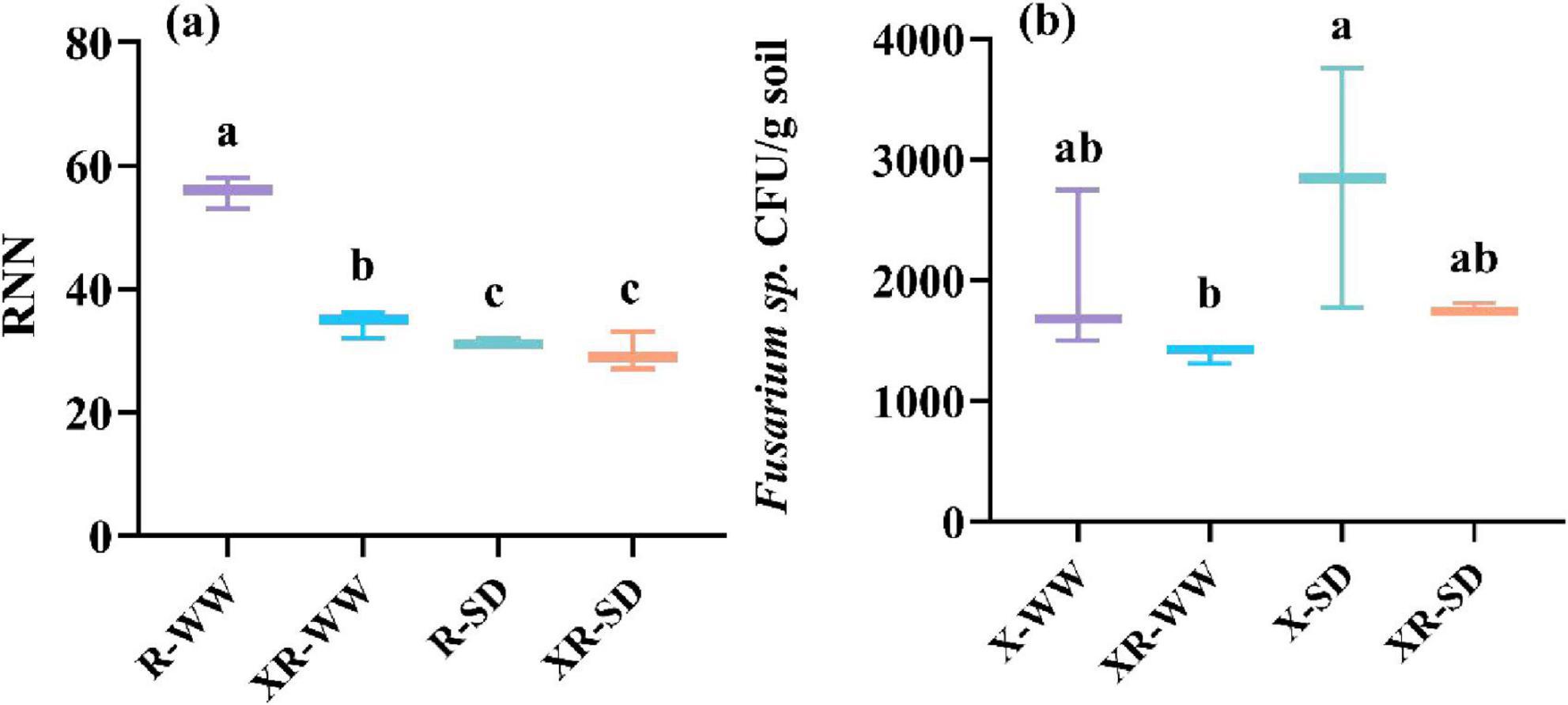
Figure 1. The number of nodules (a) and the concentration of Fusarium sp. CFU/g soil (b) after inoculated with Rhizobium (R) or Fusarium sp. (X) and simultaneous inoculated with the two microorganisms (XR) during the pod stage under SD and WW conditions. RNN: the number of Rhizobium nodules in peanut roots; Fusarium sp. CFU/g soil: the number of Fusarium sp. colony-forming units per gram of soil; WW: 70% field capacity, natural water conditions; SD: 35% field capacity, water limitation group. Different letters indicate significant differences at p < 0.05.
3.2 Leaf water potential and soil water content
Pre-dawn water potential, midday water potential, and soil moisture were −0.50 MPa, −0.56 MPa, and 12.62%, respectively, under 70% field capacity. Drought conditions (35% field capacity) increased the pre-dawn water potential by 138.47% (P < 0.001) and decreased the field water content by 34.98% (P < 0.001; Figures 2a–c). Inoculation X and XR reduced the field water content by 47.33% and 39.94% (P < 0.001; Figure 2c), respectively. Inoculation R decreased the field water content by 22.95% (P < 0.001; Figure 2c), inoculation X and XR increased the pre-dawn water potential by 76.61% (P < 0.01) and 143.46% (P < 0.001; Figure 2a), respectively, under 35% field capacity. Neither drought nor inoculation affected the midday water potential (Supplementary Table S1).
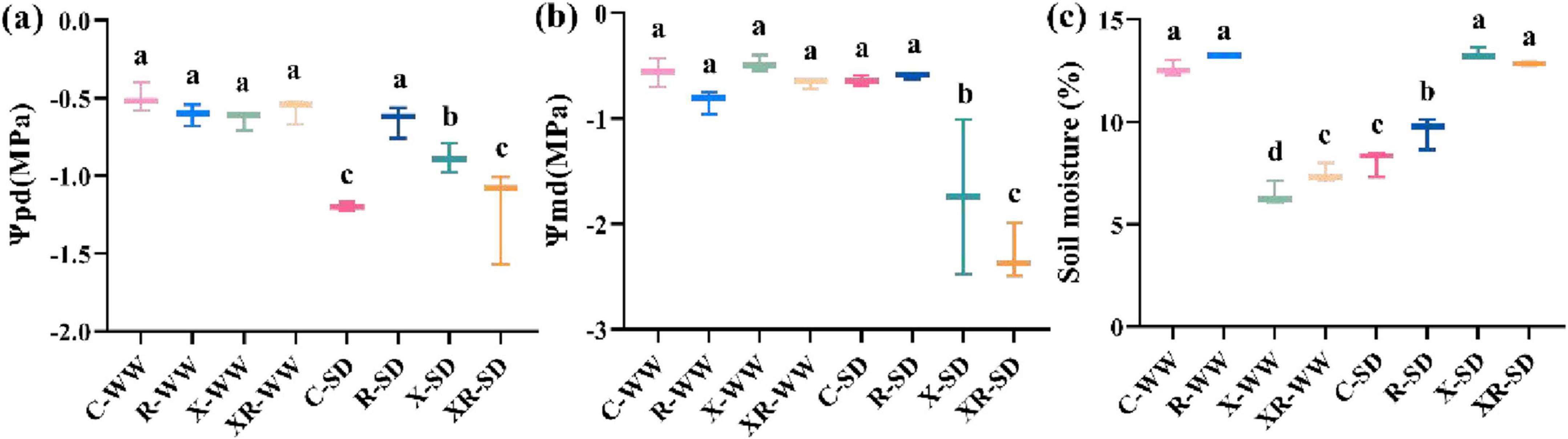
Figure 2. Ψpd (a), Ψmd (b), and soil moisture (c) under uninoculated (C), inoculated with Rhizobium (R) or Fusarium sp. (X) and simultaneous inoculated with the two microorganisms (XR) at the pod stage under SD and WW conditions. Ψpd: predawn water potential; Ψmd: midday water potential; WW: 70% field capacity, natural water conditions; SD: 35% field capacity, water limitation group. Different letters indicate significant differences at p < 0.05.
3.3 Effects of inoculation and drought on peanut WUE
WUEi and WUEL were 1.659 μmol CO2 mmol–1 H2O and 0.588 g⋅kg–1, respectively, under natural water conditions (70% field capacity). Drought had no effect on either WUEi or WUEL (Supplementary Table S1 and Supplementary Figure S1). Under natural water conditions, inoculation X and XR decreased WUEi by 14.56% (P = 0.066) and 26.19% (P < 0.01, Figure 3a), respectively, while inoculation R did not affect WUEi; inoculation R and X significantly reduced WUEL by 32.56% (P < 0.001) and 22.77% (P < 0.01), respectively, whereas inoculation XR did not affect WUEL (Figure 3b). Under drought conditions, inoculation X and XR significantly reduced WUEi by 27.31% (P < 0.01) and 18.26% (P < 0.05, Figure 3a), respectively; Inoculation R reduced WUEL by 27.82% (P < 0.001), whereas inoculation XR did not affect WUEL (Figure 3b).
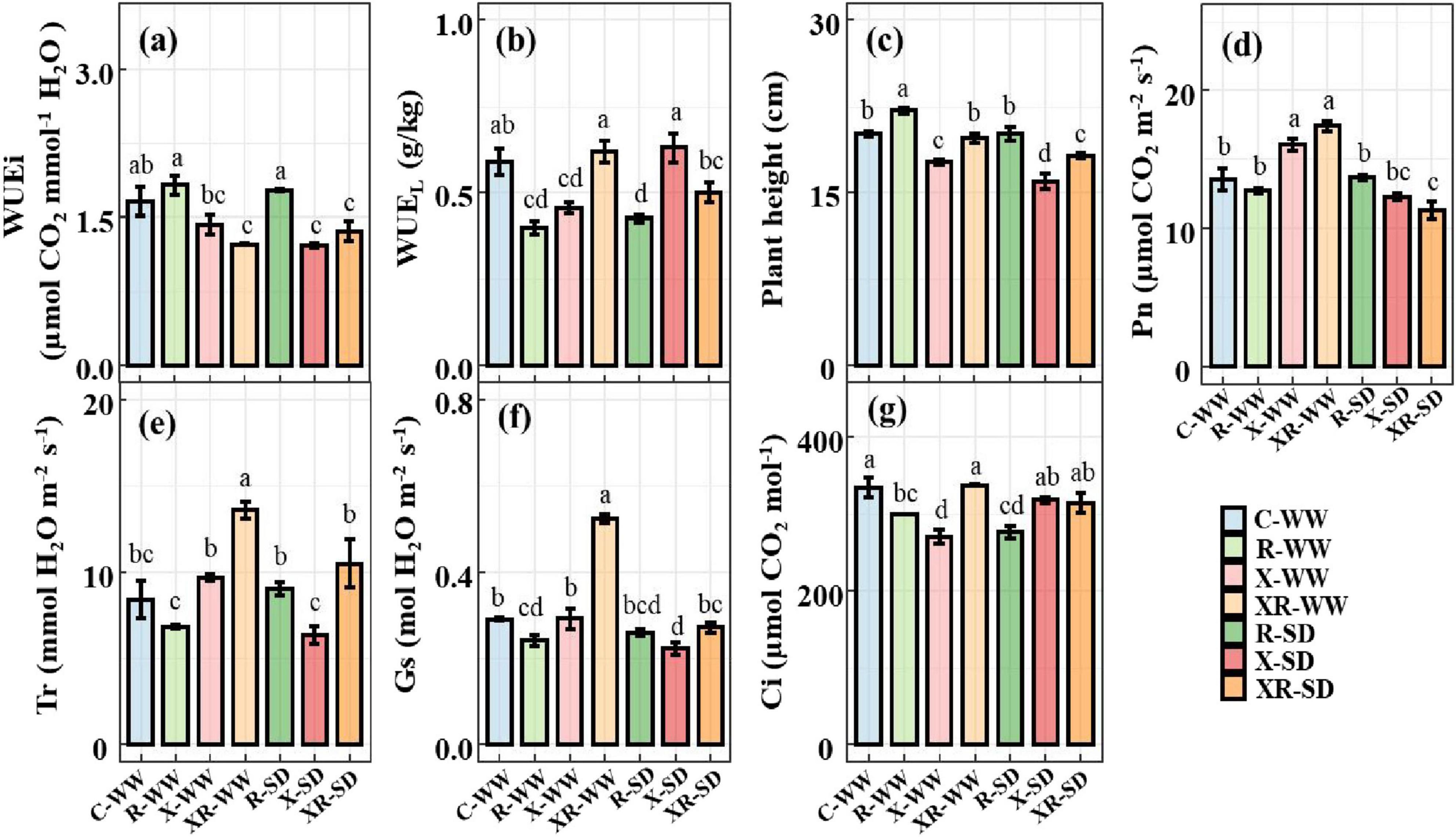
Figure 3. WUEi (a), WUEL (b), plant height (c), Pn (d), Tr (e), Gs (f), and Ci (g) of peanut without inoculation (C), inoculation Rhizobium (R) or Fusarium sp. (X), and simultaneous inoculation the two microorganisms (XR) at the pod stage under WW and SD, the values were mean ± standard error (n = 3). WW: 70% field capacity, natural water conditions; SD: 35% field capacity, water restriction group. Different letters indicate significant differences at p < 0.05.
3.4 Effects of inoculation and drought on peanut morphological and physiological traits
Drought decreased plant height (P < 0.05) and Pn (P < 0.01), but did not affect root nodule number, Tr, Gs, or Ci (Supplementary Table S2). Under natural water conditions, inoculation R increased plant height (P < 0.01), and decreased Gs and Ci (P < 0.05, Figures 3c,f,g); inoculation X increased Pn (P < 0.01, Figure 3d), and decreased plant height (P < 0.001, Figure 3c) and Ci (P < 0.001, Figure 3g); inoculation XR increased Pn, Tr, and Gs (P < 0.001, Figures 3d–f). Under drought conditions, inoculation R significantly decreased Ci (P < 0.001, Figure 3g); inoculation X significantly reduced plant height (P < 0.001) and Gs (P < 0.01, Figures 3c,f); inoculation XR significantly reduced plant height and Pn (P < 0.01 Figures 3c,d).
3.5 Factors affecting WUEL and WUEi
Linear correlation showed that WUEi was negatively correlated with Pn (R2 = 0.717, p < 0.001), Tr (R2 = 0.739, p < 0.001), and Gs (R2 = 0.553, p < 0.01), and WUEL was positively correlated with Tr (R2 = 0.466, p < 0.01), Gs (R2 = 0.379, p < 0.05), and Ci (R2 = 0.519, p < 0.01) without drought. WUEi was positively correlated with plant height (R2 = 0.624, p < 0.01), and WUEL was positively correlated with Ci (R2 = 0.347, p < 0.05) and negatively correlated with root nodule number (R2 = 0.588, p < 0.01) and plant height (R2 = 0.174, p = 0.098) under drought (Figure 4).
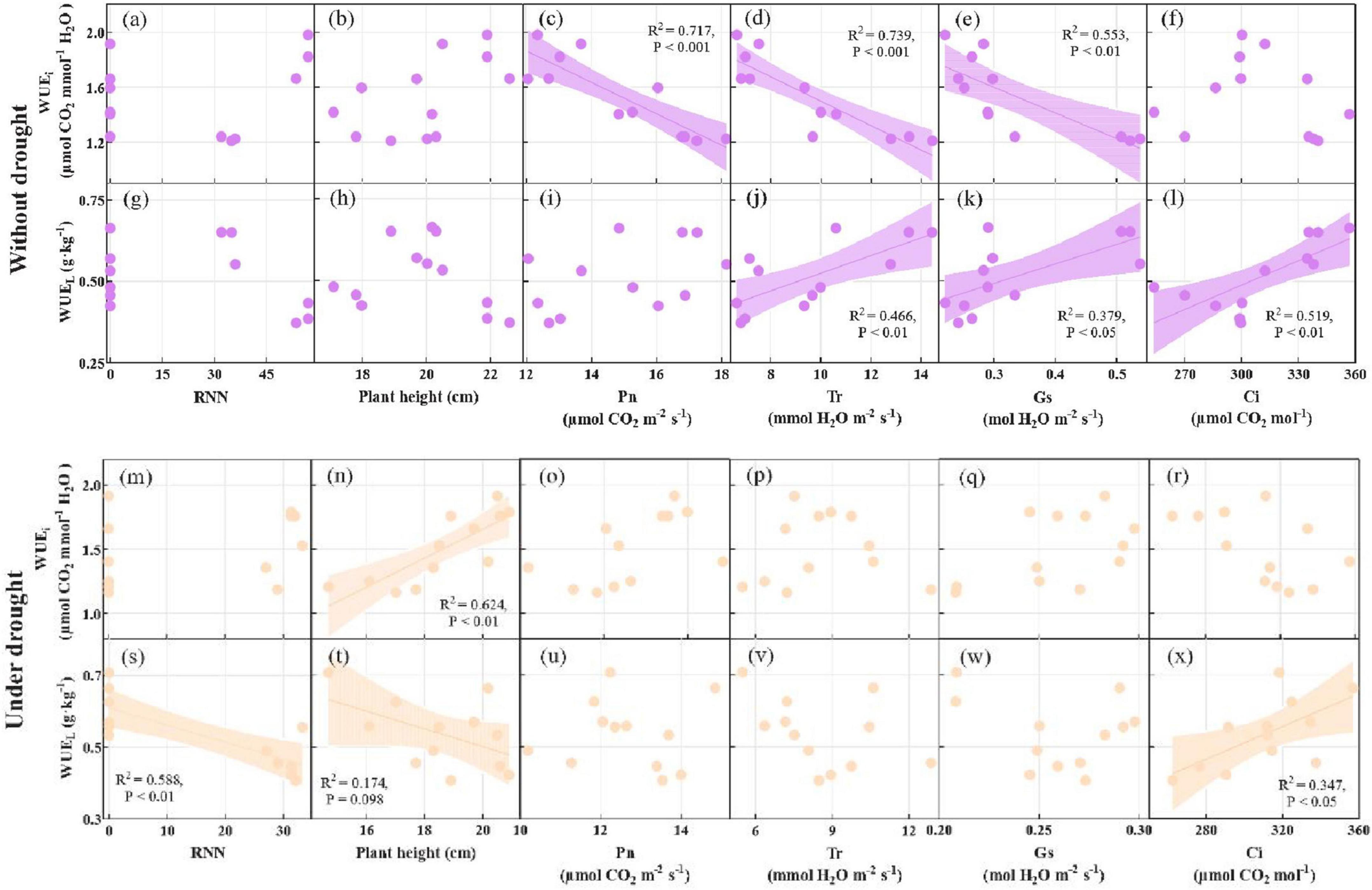
Figure 4. The linear correlation analysis of RNN, plant height, Pn, Tr, Gs, and Ci with WUEi and WUEL without drought (a–l) and under drought (m–x) at the pod stage. WUEL, long term water use efficiency; WUEi, instant water use efficiency; RNN, root nodule number; Pn, net photosynthetic rate; Tr, transpiration rate; Gs, stomatal conductivity; Ci, intercellular CO2 concentration.
Under ambient water conditions, the SEM model showed that inoculation X and XR reduced WUEi by increasing Pn, while inoculation R and X decreased WUEL by lowering Ci (χ2 = 11.370, P = 0.251). Under drought, SEM model showed that inoculation X and XR reduced WUEi by reducing plant height, and inoculation R reduced WUEL by increasing the number of nodules (χ2 = 8.523, P = 0.130, Figure 5).
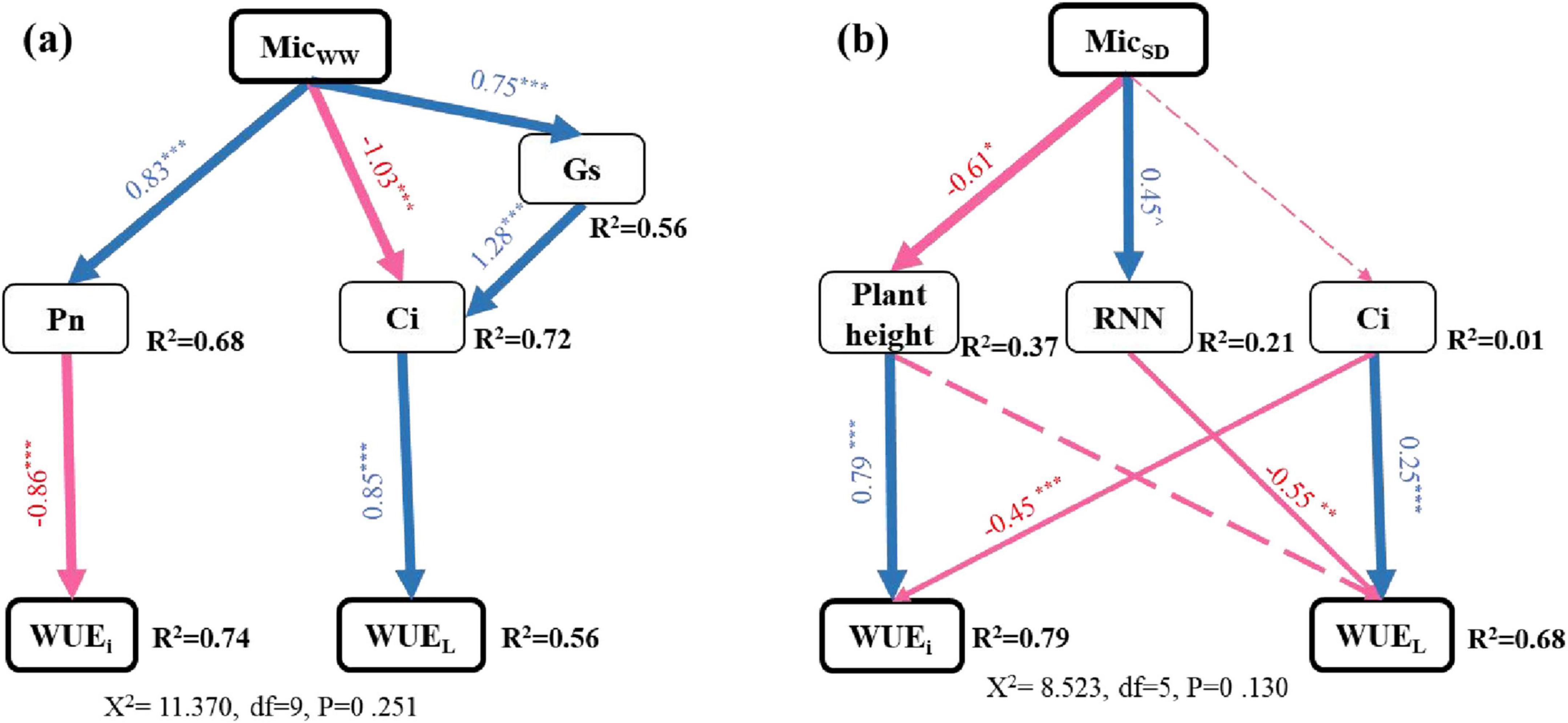
Figure 5. Structural equation models described the direct and indirect effects of inoculated with different microorganisms without drought (a) and under drought (b) on WUEi and WUEL of peanut at the pod stage. The arrow represents the direct influence determined by the path coefficient (i.e., the normalized factor load). R2 values represent the proportion of variance explained for each variable. The solid line arrows represent important relationships. The dotted arrow indicates an insignificant relationship. The blue arrow indicates a positive correlation, and the red arrow indicates a negative correlation. ***, **, and * represent significant level of 0.001, 0.01, and 0.05, respectively.
4 Discussion
4.1 Effect of inoculation on WUEL and WUEi under natural water condition
Inoculation Fusarium sp. and co-inoculation Rhizobium and Fusarium sp. decreased WUEi, and inoculation Rhizobium. and Fusarium sp. decreased WUEL. Pathogen infection reduced tomato development and diminished its water use efficiency (Buhtz et al., 2017). Pathogen infection (such as Fusarium sp.) may affect WUE mainly through two interrelated pathways. First, plants may regulate stomatal opening and closing (Huang et al., 2017), which directly affects gas exchange parameters (such as changes in stomatal conductance observed in this study), thereby affecting the calculation basis of WUEi. The enhancement of stomatal conductance may be an adaptation to the changes induced by inoculation, increasing carbon dioxide absorption. Therefore, it is essential to consider how external environmental factors (such as moisture, light, temperature, etc.) interact with the changes in inoculation and stomatal conductance, which may greatly influence the direction of the effect of inoculation on intercellular carbon dioxide concentration under different water conditions. In addition, plants might allocate more photosynthetic products (carbon resources) to defense responses, such as the synthesis of antimicrobial secondary metabolites (Kumari et al., 2024), and thereby redistribution resources away from growth and development. The transfer of this resource from growth and development (including potential water absorption and utilization efficiency optimization structure) is a key mechanism leading to long-term decline in WUEL and WUEi (as shown in the biomass change trend observed in this study). Although inoculation with Rhizobium does not increase WUE under natural water conditions, it is worth noting that the selection of suitable rhizobia strains may indeed improve water use (Del-Canto et al., 2023), especially under mild water stress (Bahador et al., 2023). However, nodule symbiosis itself requires resource input (such as maintaining nodules) and may change root structure and leaf physiology. The presence of rhizobia generally benefits plants, but their activity may inadvertently attract or increase plant susceptibility to certain pathogens and pests (Fahde et al., 2023; Liu et al., 2024), and thus affect the growth and biomass production. Following inoculation rhizobia, plants often undergo physiological adjustments to accommodate the new symbiotic relationship. These adjustments can include changes in root architecture and leaf area (Fahde et al., 2023). Although physiological changes are geared toward optimizing nitrogen fixation, they may simultaneously influence the overall accumulation of plant biomass. The benefits of promoting nitrogen fixation may not fully offset the cost of symbiosis or the potential consumption in resisting pathogens (such as co-inoculation), leading to neutral WUE.
4.2 Effects of inoculation on WUEL and WUEi under drought condition
WUEi is decreased after inoculating with Fusarium sp. and co-inoculating with Rhizobium and Fusarium sp., and WUEL is reduced after inoculation Rhizobium under drought conditions, which highlights the superimposed effect of drought and biotic stress. Drought itself forces plants to prioritize the basic metabolic functions required for survival at the expense of growth and efficiency (such as reduced WUE) (He et al., 2023). When superimposing pathogen infection, the situation is more severe. The damage of Fusarium to roots under drought (such as root structure damage and water transport disorder) directly limits the water absorption capacity, which is closely related to the observed decrease of WUEi. In order to cope with double stress of drought and Fusarium infection, plants need to mobilize more resources for osmotic regulation and synthesis of defensive secondary metabolites (such as flavonoids, phenols) (Khare et al., 2020; Ahmed et al., 2021; Khasin et al., 2021), causing the redistribution of carbon resources (from growth to defense and maintenance) and the decline in WUEL (Condon, 2020).
Efficient water use allows plants to allocate more resources to support stem growth and expansion, particularly in environments where resources are scarce (Mundim and Pringle, 2018; Szczepaniec and Finke, 2019). However, both plant height and WUE decrease because energy and resources are reallocated to resist water stress in this study. In this study, inoculation of rhizobia under drought results in a decrease in WUEL, which is related to the cost of symbiotic nitrogen fixation in the absence of resources. In the period of reproductive growth, plants preferentially allocate resources to reproductive organs and nodules (Dolezal et al., 2021), and the effect is exacerbated by the limited resources under drought stress, resulting in a decrease in WUEL after inoculation of rhizobia. The combined effects from prolonged drought and pathogen infection can induce significant physiological and metabolic challenges, further reduce photosynthetic efficiency and WUE. The destruction of root structure and function by drought reduces the ability of plants to absorb water and nutrients, which is also contributes to the decline in WUEi (Farooq et al., 2019). Under severe drought, resources (especially water and carbon) are extremely limited (Zhang et al., 2021). Maintaining symbiotic nitrogen fixation and activating defense responses (rhizobia may also induce defense) consume a large amount of resources that can be used to maintain higher WUEL, resulting in a significant decrease in growth and WUEL. Consequently, a decline in WUEi may mirror the decline in the plant’s photosynthetic capacity and water management efficacy. Moreover, the sustained reduction in leaf gas exchange parameters observed at podding stages underscores the irreversible harm on peanut leaves by severe drought (Zhang et al., 2021).
It should be noted that the experiment is carried out in a semi-open greenhouse environment (four sides ventilated, top glass covered). The measured data shows that the light intensity inside the greenhouse was about 80% of the natural field conditions, and the day and night temperature is significantly different from the field environment (temperature difference ≤ 1.5°C). Therefore, the semi-open greenhouse environment may amplify or weaken the observed drought effects by affecting plant transpiration rate, photosynthetic efficiency, and the intensity of stress response signals (Wang and Wang, 2023). For example, higher greenhouse temperature may accelerate soil water evaporation and plant transpiration, exacerbating the degree of water stress felt by plants (Zhang et al., 2022); specific light conditions may affect the ability of photosynthetic carbon assimilation, which in turn affects the total amount of resources that plants can use for defense and osmotic regulation (Heath et al., 2020). Therefore, it is necessary to consider the possible effects of specific light and temperature conditions in the greenhouse, and future studies consider the effects of greenhouses on light and temperature will be very valuable for accurately predicting the impact of drought.
5 Conclusion
This study explored the impact of rhizobia and Fusarium sp. inoculation, both individually and in combination, on leaf-level efficiency (WUEL) and instantaneous efficiency (WUEi) under different water conditions during the pod-filling stages. However, drought did not affect WUEi and WUEL without inoculation. In contrast, simultaneous inoculation Rhizobium and Fusarium sp. decreased WUEi when inoculation of either Rhizobium or Fusarium sp alone. significantly reduced WUEL. Inoculation Fusarium sp. and co-inoculation Rhizobium and Fusarium sp. led to a decrease in WUEi, and inoculation Rhizobium decreased WUEL under drought. The findings suggest that Rhizobium inoculation has crucial effects on long-term water use efficiency and Fusarium sp. inoculation largely regulates short-term water use efficiency. These insights are crucial for elucidating the dynamics of plant-microbe interactions and their regulating effects on water use efficiency under climate change scenarios.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
GR: Writing – original draft, Writing – review and editing. YZ: Investigation, Writing – original draft. YS: Methodology, Writing – review and editing. LZ: Investigation, Writing – review and editing. JZ: Investigation, Writing – review and editing. YL: Project administration, Writing – review and editing. GL: Supervision, Writing – review and editing. RX: Writing – review and editing. ZY: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was sponsored by the Natural Science Foundation of Henan (252300421030 and 222300420001), National Natural Science Foundation of China (32271645 and 32201333), National Science Foundation for Post-Doctoral of China (2022M711044), Natural Science Foundation of Henan Province (222300420108), and Key Scientific Research Project Plan of Colleges and Universities in Henan Province (23A180013).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1612341/full#supplementary-material
References
Ahmed, U., Rao, M. J., Qi, C., Xie, Q., Noushahi, H. A., Yaseen, M., et al. (2021). Expression profiling of flavonoid biosynthesis genes and secondary metabolites accumulation in populus under drought stress. Molecules 26:5546. doi: 10.3390/molecules26185546
Bahador, M., Tadayon, M. R., Soureshjani, H. K., and Ghaffari, H. (2023). Radiation and water use efficiencies of mycorrhizal inoculated hemp under water-deficit stress. J. Soil Sci. Plant Nutrit. 23, 2202–2214. doi: 10.1007/s42729-023-01173-y
Barbosa, D. D., Brito, S. L., Fernandes, P. D., Fernandes-Júnior, P. I., and Lima, L. M. (2018). Can Bradyrhizobium strains inoculation reduce water deficit effects on peanuts? World J. Microbiol. Biotechnol. 34:87. doi: 10.1007/s11274-018-2474-z
Buhtz, A., Hohe, A., Schwarz, D., and Grosch, R. (2017). Effects of Verticillium dahliae on tomato root morphology considering plant growth response and defence. Plant Pathol. 66, 667–676. doi: 10.1111/ppa.12595
Condon, A. G. (2020). Drying times: Plant traits to improve crop water use efficiency and yield. J. Exp. Bot. 71, 2239–2252. doi: 10.1093/jxb/eraa002
Del-Canto, A., Sanz-Saez, Á, Sillero-Martínez, A., Mintegi, E., and Lacuesta, M. (2023). Selected indigenous drought tolerant rhizobium strains as promising biostimulants for common bean in Northern Spain. Front. Plant Sci. 14:1046397. doi: 10.3389/fpls.2023.1046397
Dolezal, J., Jandova, V., Macek, M., and Liancourt, P. (2021). Contrasting biomass allocation responses across ontogeny and stress gradients reveal plant adaptations to drought and cold. Funct. Ecol. 35, 32–42. doi: 10.1111/1365-2435.13687
Domec, J. C., Smith, D. D., and McCulloh, K. A. (2017). A synthesis of the effects of atmospheric carbon dioxide enrichment on plant hydraulics: Implications for whole-plant water use efficiency and resistance to drought. Plant Cell Environ. 40, 921–937. doi: 10.1111/pce.12843
Fahde, S., Boughribil, S., Sijilmassi, B., and Amri, A. (2023). Rhizobia: A promising source of plant growth-promoting molecules and their non-legume interactions: Examining applications and mechanisms. Agriculture-Basel 13:1279. doi: 10.3390/agriculture13071279
Farooq, M., Hussain, M., Ul-Allah, S., and Siddique, K. H. (2019). Physiological and agronomic approaches for improving water-use efficiency in crop plants. Agricult. Water Manag. 219, 95–108. doi: 10.1016/j.agwat.2019.04.010
Felipe, A. J. B., Alejo, L. A., Balderama, O. F., and Rosete, E. A. (2023). Climate change intensifies the drought vulnerability of river basins: A case of the Magat River Basin. J. Water Climate Change 14, 1012–1038. doi: 10.2166/wcc.2023.005
Furlan, A., Bianucci, E., Del Carmen Tordable, M., Kleinert, A., Valentine, A., and Castro, S. (2016). Dynamic responses of photosynthesis and the antioxidant system during a drought and rehydration cycle in peanut plants. Funct. Plant Biol. 43, 337–345. doi: 10.1071/FP15206
Gortari, F., Guiamet, J. J., and Graciano, C. (2018). Plant-pathogen interactions: Leaf physiology alterations in poplars infected with rust (Melampsora medusae). Tree Physiol. 38, 925–935. doi: 10.1093/treephys/tpx174
Gupta, A., Rico-Medina, A., and Caño-Delgado, A. I. (2020). The physiology of plant responses to drought. Science 368, 266–269. doi: 10.1126/science.aaz7614
Guzzo, M. C., Costamagna, C., Salloum, M. S., Rotundo, J. L., Monteoliva, M. I., and Luna, C. M. (2021). Morpho-physiological traits associated with drought responses in soybean. Crop Sci. 61, 672–688. doi: 10.1002/csc2.20314
He, J. Q., Hu, W., Zhu, H. H., Li, Y., Zou, J., and Khattak, W. A. (2023). Changes in cotton water use efficiency and its mechanism during drought and subsequent rehydration. Environ. Exp. Botany 209:105285. doi: 10.1016/j.envexpbot.2023.105285
Heath, K. D., Podowski, J. C., Heniff, S., Klinger, C. R., Burke, P. V., Weese, D. J., et al. (2020). Light availability and rhizobium variation interactively mediate the outcomes of legume–rhizobium symbiosis. Am. J. Botany 107, 229–238. doi: 10.1002/ajb2.1347
Hidalgo-Hidalgo, J.-D., Collados-Lara, A.-J., Pulisurname>Pulido-Velazquez, D., Rueda, F. J., and Pardo-Igúzquiza, E. (2022). Analysis of the potential impact of climate change on climatic droughts, snow dynamics, and the correlation between them. Water 14:1081. doi: 10.3390/w14071081
Homet, P., González, M., Matías, L., Godoy, O., Pérez-Ramos, I. M., García, L. V., et al. (2019). Exploring interactive effects of climate change and exotic pathogens on Quercus suber performance: Damage caused by Phytophthora cinnamomi varies across contrasting scenarios of soil moisture. Agricult. Forest Meteorol. 276-277:107605. doi: 10.1016/j.agrformet.2019.06.004
Huang, H. P., Jing, X. H., Wu, L. Y., and Huang, J. (2017). Chitin elicitor receptor kinase 1 (CERK1) is required for the non-host defense response of Arabidopsis to Fusarium oxysporum f. Sp cubense. Eur. J. Plant Pathol. 147, 571–578. doi: 10.1007/s10658-016-1026-3
Jewaria, P. K., Hänninen, H., Li, X., Bhalerao, R. P., and Zhang, R. (2021). A hundred years after: Endodormancy and the chilling requirement in subtropical trees. New Phytol. 231, 565–570. doi: 10.1111/nph.17382
Khare, S., Singh, N. B., Singh, A., Hussain, I., Niharika, K., Yadav, V., et al. (2020). Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 63, 203–216. doi: 10.1007/s12374-020-09245-7
Khasin, M., Bernhardson, L. F., O’Neill, P. M., Palmer, N. A., Scully, E. D., Sattler, S. E., et al. (2021). Pathogen and drought stress affect cell wall and phytohormone signaling to shape host responses in a sorghum COMT bmr12 mutant. BMC Plant Biol. 21:391. doi: 10.1186/s12870-021-03149-5
Kibido, T., Kunert, K., Makgopa, M., Greve, M., and Vorster, J. (2020). Improvement of rhizobium-soybean symbiosis and nitrogen fixation under drought. Food Energy Secur. 9:e177. doi: 10.1002/fes3.177
Kumari, S., Nazir, F., Maheshwari, C., Kaur, H., Gupta, R., Siddique, K. H. M., et al. (2024). Plant hormones and secondary metabolites under environmental stresses: Enlightening defense molecules. Plant Physiol. Biochem. 206:108238. doi: 10.1016/j.plaphy.2023.108238
Lauri, P. -É, Barigah, T. S., Lopez, G., Martinez, S., Losciale, P., and Zibordi, M. (2016). Genetic variability and phenotypic plasticity of apple morphological responses to soil water restriction in relation with leaf functions and stem xylem conductivity. Trees 30, 1893–1908. doi: 10.1007/s00468-016-1408-3
Li, Y., Liu, J., Lv, P., Mi, J., and Zhao, B. (2022). Silicon improves the photosynthetic performance of oat leaves infected with Puccinia graminis f. sp. avenae. Front. Plant Sci. 13:1037136. doi: 10.3389/fpls.2022.1037136
Liu, B. H. (2012). Evaluation of water use efficiency and the physiological mechanism and regulation of efficient water use in young apple genetic resources. China: Northwest A&F Universit.
Liu, X., Chu, H., Godoy, O., Fan, K., Gao, G. F., Yang, T., et al. (2024). Positive associations fuel soil biodiversity and ecological networks worldwide. Proc. Natl. Acad. Sci. U. S. A. 121:e2308769121. doi: 10.1073/pnas.2308769121
Mabrouk, E. H., Moursy, F. I., and Morsy, M. M. A. (2022). Assessment of climate characteristics and long-term trends of rainfall and drought in the Congo River Basin. J. Water Climate Change 13, 3906–3933. doi: 10.2166/wcc.2022.241
McDowell, N. G., Ryan, M. G., Zeppel, M. J. B., and Tissue, D. T. (2013). Feature: Improving our knowledge of drought-induced forest mortality through experiments, observations, and modeling. New Phytol. 200, 289–293. doi: 10.1111/nph.12502
McElrone, A. J., Jackson, S., and Habdas, P. (2008). Hydraulic disruption and passive migration by a bacterial pathogen in oak tree xylem. J. Exp. Bot. 59, 2649–2657. doi: 10.1093/jxb/ern124
Melotto, M., Underwood, W., Koczan, J., Nomura, K., and He, S. Y. (2006). Plant stomata function in innate immunity against bacterial invasion. Cell 126, 969–980. doi: 10.1016/j.cell.2006.06.054
Mundim, F. M., and Pringle, E. G. (2018). Whole-Plant metabolic allocation under water stress. Front. Plant Sci. 9:852. doi: 10.3389/fpls.2018.00852
Murria, S., Gupta, N., Kaur, N., Bhardwaj, R., and Sharma, A. B. (2022). Impact of Sclerospora graminicola infection on physiological mechanisms and hormonal regulation of floral reversion in pearl millet Pennisetum glaucum (L.) R. Br. Physiol. Mol. Plant Pathol. 118:101806. doi: 10.1016/j.pmpp.2022.101806
Nguyen, L. V., Bertero, D., Hoang, D. T., and Long, N. V. (2022). Variation in quinoa roots growth responses to drought stresses. J. Agron. Crop Sci. 208, 830–840. doi: 10.1111/jac.12528
R Core Team. (2023). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/.
Rolli, E., Marasco, R., Vigani, G., Ettoumi, B., Mapelli, F., Deangelis, M. L., et al. (2015). Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ. Microbiol. 17, 316–331. doi: 10.1111/1462-2920.12439
Sun, Y., Wang, C., Chen, H. Y. H., and Ruan, H. (2020). Response of plants to water stress: A meta-analysis. Front. Plant Sci. 11:978. doi: 10.3389/fpls.2020.00978
Szczepaniec, A., and Finke, D. (2019). Plant-Vector-Pathogen interactions in the context of drought stress. Front. Ecol. Evol. 7:262. doi: 10.3389/fevo.2019.00262
Wang, Z., and Wang, C. (2023). Interactive effects of elevated temperature and drought on plant carbon metabolism: A meta-analysis. Glob. Chang Biol. 29, 2824–2835. doi: 10.1111/gcb.16639
Yang, Z., Wei, Y., Fu, G., Song, H., Li, G., and Xiao, R. (2020). Asymmetric effect of increased and decreased precipitation in different periods on soil and heterotrophic respiration in a semiarid grassland. Agric. For. Meteorol. 291:108039. doi: 10.1016/j.agrformet.2020.108039
Zhang, J. X., Wang, Q. Q., Xia, G. M., Wu, Q., and Chi, D. (2021). Continuous regulated deficit irrigation enhances peanut water use efficiency and drought resistance. Agricult. Water Manag. 255:106997. doi: 10.1016/j.agwat.2021.106997
Keywords: peanuts, drought, greenhouse pot, pathogen, symbiosis, water use efficiency
Citation: Regassa GB, Zhang Y, Shen Y, Zhang L, Zhang J, Liu Y, Li G, Xiao R and Yang Z (2025) Effects of pathogen infection and Rhizobium inoculation on instantaneous and long-term water use efficiency of peanut with and without drought. Front. Microbiol. 16:1612341. doi: 10.3389/fmicb.2025.1612341
Received: 15 April 2025; Accepted: 31 May 2025;
Published: 24 June 2025.
Edited by:
Xu Liu, Nanjing Agricultural University, ChinaReviewed by:
Congcong Shen, Chinese Academy of Sciences (CAS), ChinaZhanbiao Li, Guangxi Academy of Agricultural Sciences, China
Copyright © 2025 Regassa, Zhang, Shen, Zhang, Zhang, Liu, Li, Xiao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Xiao, eGlhb3IxMTMwQDE2My5jb20=; Zhongling Yang, eWFuZ196aGwwNkAxMjYuY29t
†These authors have contributed equally to this work
 Girmaye Benti Regassa1†
Girmaye Benti Regassa1† Yifan Shen
Yifan Shen Yinzhan Liu
Yinzhan Liu Rui Xiao
Rui Xiao Zhongling Yang
Zhongling Yang