- 1School of Tea and Coffee, Pu’er University, Pu’er, China
- 2Yunnan International Union Laboratory for Quality Monitoring and Evaluation of Agricultural Products in China and Malaysia, Pu’er University, Pu’er, China
- 3Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- 4School of Biology and Chemistry, Pu’er University, Pu’er, China
- 5Center for Yunnan Plateau Biological Resources Protection and Utilization & Yunnan International Joint Laboratory of Fungal Sustainable Utilization in South and Southeast Asia, College of Biology and Food Engineering, Qujing Normal University, Qujing, China
- 6Spice and Beverage Research Institute, Chinese Academy of Tropical Agriculture Sciences, Pu’er, China
- 7Department of Plant Breeding and Genetics, Ghazi University, Dera Ghazi Khan, Pakistan
- 8Key Laboratory of Crop Physiology and Ecology, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Ministry of Agriculture and Rural Affairs of China, Beijing, China
In nature, plants are always destined to interact with fungi. Thus, plant–fungal interactions are one of the unavoidable fields of study, particularly for agronomists. Fungi interact with plants in different lifestyles, pathogenic and symbiotic. Pathogenic relationships have adverse effects, causing devastating diseases in plants, while symbiotic interactions provide numerous benefits, promoting the growth and development of plants. The intricate relationship between fungi and plants has been the subject of extensive research, especially in the tropics, where there is a higher plant diversity and a strong positive correlation with fungi diversity. This extensive research has provided us with a wealth of knowledge about these interactions. In this study, we conducted a bibliometric analysis and systematic review, analyzing 733 research articles. A considerable growth was revealed in this field, particularly over the previous decade. Many studies during this period are concentrated in China, with a plethora of emerging researchers. More attention has been paid to genetic/molecular-based work over the last decade. In addition, researchers are promoting the use of plant–fungal interactions for sustainable agriculture, highlighting their crucial importance in mitigating crop stresses under both biotic (pests) and abiotic stresses, such as heavy metal pollutants, nutritional depletion, temperature rises, changes in water regimes, and elevated carbon dioxide concentrations. Considering future studies, further research is needed to elucidate the relationships between plants and fungi, particularly through multi-omics approaches. Network mapping and the influence of indigenous fungi on plant–fungal interactions are other, less-studied, important areas to focus on.
1 Introduction
Plants cannot escape exposure to microbes, from their root systems to their aboveground parts, and are always in contact with a tremendous number of microbes, including fungi, bacteria, oomycetes, protists, protozoa, and viruses (Pandey et al., 2023). A growing body of studies focuses on plant–fungal interactions, primarily examining the importance of these interactions in an agricultural environment, which ultimately affects the country’s economy (Balestrini, 2021; Dai et al., 2023). It has been estimated that the world’s fungal diversity is approximately 2.5 million species; however, only 165,000 species have been identified to date (Baldrian et al., 2022; Niskanen et al., 2023). This highlights the need for a substantial number of studies, as identifying new species is crucial to uncover further advances in the relationship between plants and fungi. According to the theoretical framework of the plant diversity hypothesis, the greater the plant diversity, the greater the microbial diversity, particularly the soil-dwelling organisms (Shen et al., 2021). It is noteworthy that studies on fungi are predominantly conducted in the tropical region; one possible reason could be the higher fungal diversity (along with the higher plant diversity). Moreover, prior studies have indicated that many cryptic species are also present in associated temperate plant species. Therefore, it is crucial to investigate the fungi present in temperate and tropical environments from the standpoint of plant–fungal interactions (Dai et al., 2023).
Fungi live with different lifestyles alongside plants, including saprophytic, pathogenic, or symbiotic. Generally, it is challenging to determine the lifestyle they may have had at the time due to the complex nature of plant–fungal interactions (Priyashantha et al., 2023). The interaction between plants and fungi is either beneficial for both, or positive for one species and negative or neutral for the other. The negative association is described in terms of pathogenicity—the disease-causing ability of fungi to their hosts. It is one of the most threatening scenarios for agriculture, responsible for up to 20% crop losses, posing significant challenges to food security in today’s world (Davies et al., 2021). However, the percentage of crop losses can also vary according to crop variety and environmental conditions, thus increasing the likelihood of severe disease incidents. Like rice blast disease, caused by Magnaporthe oryzae, it can generally result in harvest losses of up to 35% (Godfray et al., 2016).
In the case of extreme conditions, such as disease epidemics, production losses can exceed 60% (Różewicz et al., 2021). Puccinia spp. that attack wheat (Zea mays) can result in up to 70% crop losses, mainly due to stem rust disease (Godfray et al., 2016). In contrast, the beneficial effects of plant–fungal associations are primarily described as the promotion of plant growth and increased survivability under unfamiliar conditions (Dai et al., 2023).
Coming to the point of this study, we have employed bibliometric analysis on plant–fungal interactions related to the field of agriculture over the last 30 years. With a systematic review approach, we have comprehensively discussed the plant-symbiotic fungi interactions, emphasizing their way of creating beneficial effects for the plants. Of this, the beneficial effects of fungi in mitigating unfavorable conditions for crops, including the reduction of pests, pollutants, changes in soil nutrients, temperature rise, changes in soil water content, and carbon dioxide concentration (CO2) in the air, are emphasized. Then, the positive synergistic effect of different fungi on plants is discussed. Finally, challenges and required advancements in the field are briefly presented. Note that, when discussing symbiotic associations, we gave priority to the group of fungi (endophytes or mycorrhizae), which has been extensively discussed in the available literature.
2 Methods
A bibliometric analysis and systematic review were conducted to identify, evaluate, and synthesize studies relevant to plant–fungal interactions from a sustainable agriculture perspective. All the literature data were collected from the Scopus database1 from January 1, 1995, to December 31, 2024. The original research papers were only considered, excluding review papers, conference papers, book chapters, and other gray literature to avoid duplicate records. The terms searched in the Scopus search engine were— “plant–fungal association,” OR “phytopathogenic fungi,” OR “mycorrhizal fungi,” OR “plant-symbiotic fungi” AND “Sustainable agriculture.” Selected terms were filtered in the article title, abstract, and keywords. Initial search results included 1,048 papers, and in order to get the most relevant papers, further articles were filtered based on the fields such as “agriculture and biological sciences,” “environmental science,” “biochemistry,” “genetics and molecular biology,” and “immunology and microbiology” (Supplementary Figure 1). The journal published (and final stage) articles in any language were selected. The obtained papers were further checked for duplicate recordings and irrelevant records, and 315 articles were eliminated. Ultimately, 733 articles were analyzed. VOSviewer (version 1.6.20) software tool was used for bibliometric analysis and to facilitate the construction and visualization of search results. Here, co-authorship analysis, keyword analysis, and bibliographic coupling of documents were conducted.
3 Results
3.1 Publication progress, country, and language-based study projection
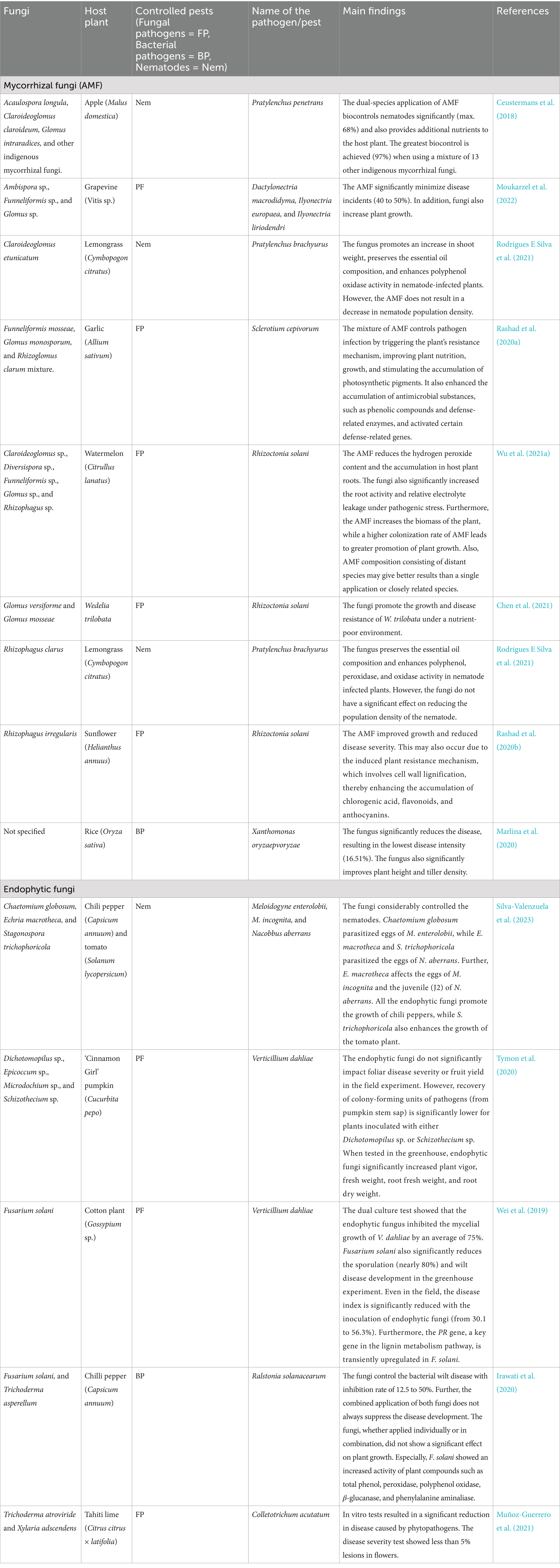
The initial data screening yielded 1,048 publications, comprising 69.9% research papers, 27.0% review articles, 1.0% editorials, 0.8% conference papers, and the remainder (1.3%), which included various types of publications such as conference papers, notes, letters, and errata. As previously mentioned, we have analyzed 733 research articles. In the first phase (1995–2004), only 26 papers were published, and during the second phase (2005–2014), 97 articles were documented in Scopus. Over the last decade, 610 articles have been published, indicating an increasing interest among researchers in this field. In detail, the most significant number of studies was recorded in 2024. Since 2010, there has been an increasing trend in the number of studies, with over 10 papers published per year. Additionally, since 2015, over 25 studies have been published annually showing the trends in this field (Figure 1c). When considering the language of published articles, approximately 96% of articles are published in English, followed by Chinese and Spanish (Figure 1b). Furthermore, the data obtained from the Scopus database indicates that publications originate from 95 countries, demonstrating a global distribution of studies. The majority of studies (68%) have been conducted in temperate or polar countries, while 32% of the work is oriented towards tropical agriculture. Among those, 9.3% of studies are coming from China, making it the leading country in research in this field. India, the United States, Italy, and Iran are among the leading countries in terms of publishing a high number of papers (Figure 1c). Moreover, our systematic review approach reveals that the lion’s share of studies focused on plant-symbiotic fungi interactions (approximately 89%), while fewer studies were conducted on plant-phytopathogenic fungi interactions (approximately 11%). However, there are no considerable trends across the continents in the studies on symbiotic versus pathogenic fungi, where the highest percentage of works are done on plant-symbiotic fungi interactions, with a more or less similar percentage of studies conducted on plant-pathogenic fungi interactions. For instance, out of 89% of studies carried out on plant-symbiotic fungi interactions, North America accounts for about 15%, while out of 11% of plant-phytopathogenic fungi interactions, it accounts for about 14%. In addition, regional viz, particularly a smaller number of studies conducted (less than 10) in Sub-Saharan Africa and Southeast Asia.
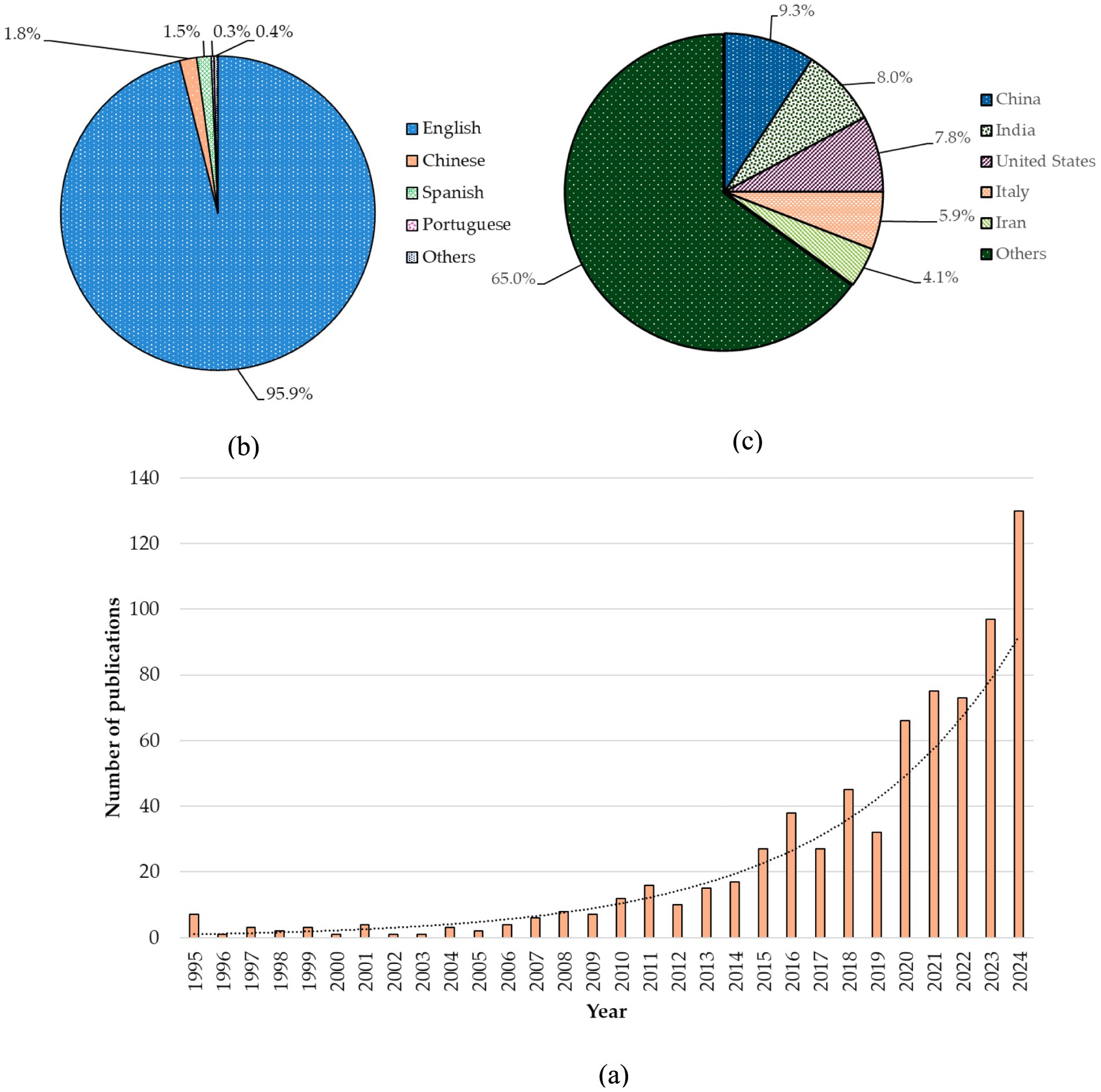
Figure 1. (a) Trends of studies during the last 30 years. (b) The chart shows the percentage of articles published based on language, with the following breakdown: English (95.9%), Chinese (1.8%), Spanish (1.5%), Portuguese (0.3%), and other languages (0.4%), equally shared among Polish, Persian, German, and French. (c) Country-wise studies percentage. Most studies were conducted in China, followed by India, the United States, Italy, and Iran, which collectively accounted for 35% of the total studies. The rest of the research was carried out by 95 other countries.
3.2 Co-authorship analysis
Co-authorship analysis reveals that 3,642 scientists are actively working in this field. In Jung Lee, Muhammad Hamayun, Ying Ma, Luciano Avio, Andress Wiemken, and Helena Freitas are among the top leading scientists with the highest number of citations. Many other researchers are having collaborative works with a considerable number of citations (Figure 2).
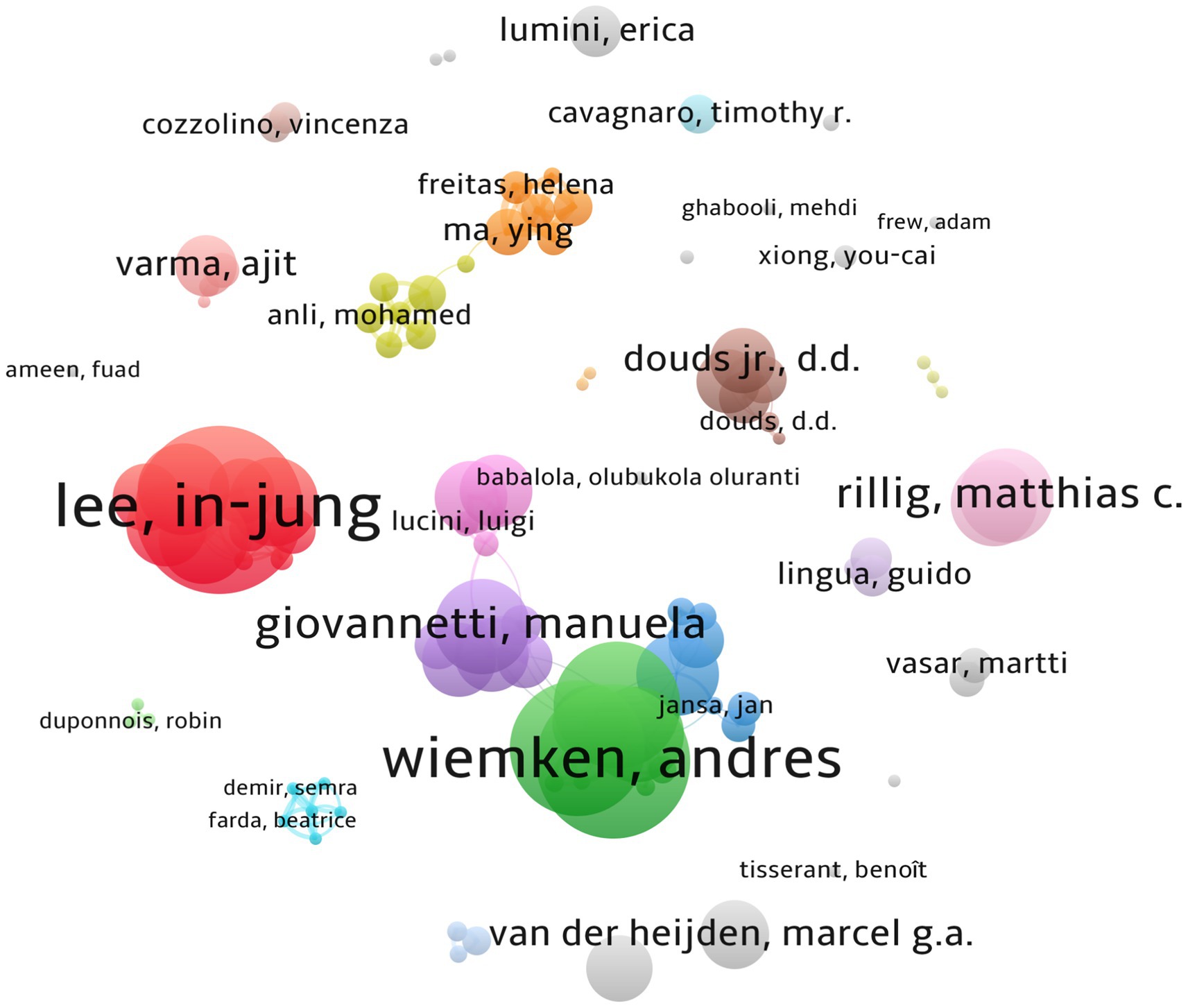
Figure 2. Co-authorship occurrence. A total of 104 items have met the threshold (the minimum number of authors per document is set to three), with 32 clusters, 191 links, and a total link strength (TLS) of 470. The size of the circles indicates the number of papers published by the author. The thickness of the lines represents the closeness of the collaboration between the authors.
3.3 Keyword analysis
There are 5,255 keywords appeared, and according to the network co-occurrence analysis, sustainable agriculture (TLS: 2,520), mycorrhiza (TLS: 3,021) arbuscular mycorrhizal fungi (TLS: 2,065), microbiology (TLS: 3,685), alternative agriculture (TLS: 1,714), plant root (TLS: 2,405), physiology (TLS: 2,083), plant growth (TLS: 2,146), symbiosis (TLS: 1,496), soil microbiology (TLS: 2,114), metabolism (TLS: 1,983), and growth, development and aging (TLS: 1,932) are the top most keywords with occurrence of 198, 151, 148, 141, 102, 92, 86,81,81, 80,80, and 69, respectively, (Figure 3).
Figure 3. Keyword network visualization for the prominent 460 keywords. The minimum number of occurrences of keywords is set as six. The keywords are organized into six clusters, with 31,275 links, and TLS has 58,916. Note that the circle’s size corresponds to the number of keyword occurrences.
When the last 30 years are divided into three phases (1995–2004, 2005–2014, and 2015–2024), the dynamics of the keywords become observable. However, when considering the top five keywords, “arbuscular mycorrhiza” (also known as mycorrhiza) is the most common. Between 1995 and 2004, it was reported that 252 keywords, including sustainable agriculture, arbuscular mycorrhiza (fungi), Zea mays, mycorrhizae, and Triticum aestivum, were the most prevalent (higher TLS) keywords, with occurrences of 12, 6, 5, 4, and 4, respectively (Supplementary Figure 2A). Between 2005 and 2014, 1,292 keywords were identified, with arbuscular mycorrhiza, microbiology, mycorrhiza, alternative agriculture, and plant root being the leading keywords, occurring with frequencies of 37, 24, 24, 27, and 17, respectively (Supplementary Figure 2B). In the previous decade (2015–2024), the leading keywords were sustainable agriculture, arbuscular mycorrhiza, mycorrhiza, microbiology, and plant root, with occurrences of 168, 142, 128, 120, and 76, respectively (Supplementary Figure 2C). Moreover, there is a current trend of addressing the climatic scenario in today’s agricultural system. Here, the keyword “salinity/salt stress” (with occurrence 17) is the leading, followed by drought stress (occurrence:14). Another highlighted fact is the pollution, and heavy metal pollution is the most considered with Zinc (Zn) pollutants (occurrence: 14).
3.4 Bibliographic coupling of documents
The bibliographic coupling shows the usefulness of measuring thematic similarities and relations of publications. The documents by Wright and Upadhyaya (1998) and Waller et al. (2005) are strongly coupled (Figure 4). The cohorts of their studies concentrated on arbuscular mycorrhizal and endophytic fungi, respectively. Other highly bibliographic couplings, as shown by Oehl et al. (2004), discussed the influence of terms, including conventional and organic farming, on the diversity of arbuscular mycorrhizal fungi. Nevertheless, considering the bibliometric analysis outputs, Pellegrino (2014), Bedini (2013), Elliott (2021), Kohil (2016), and Bainard (2012) represent the top five based on TLS of 501, 437, 418, 402, and 399, respectively.
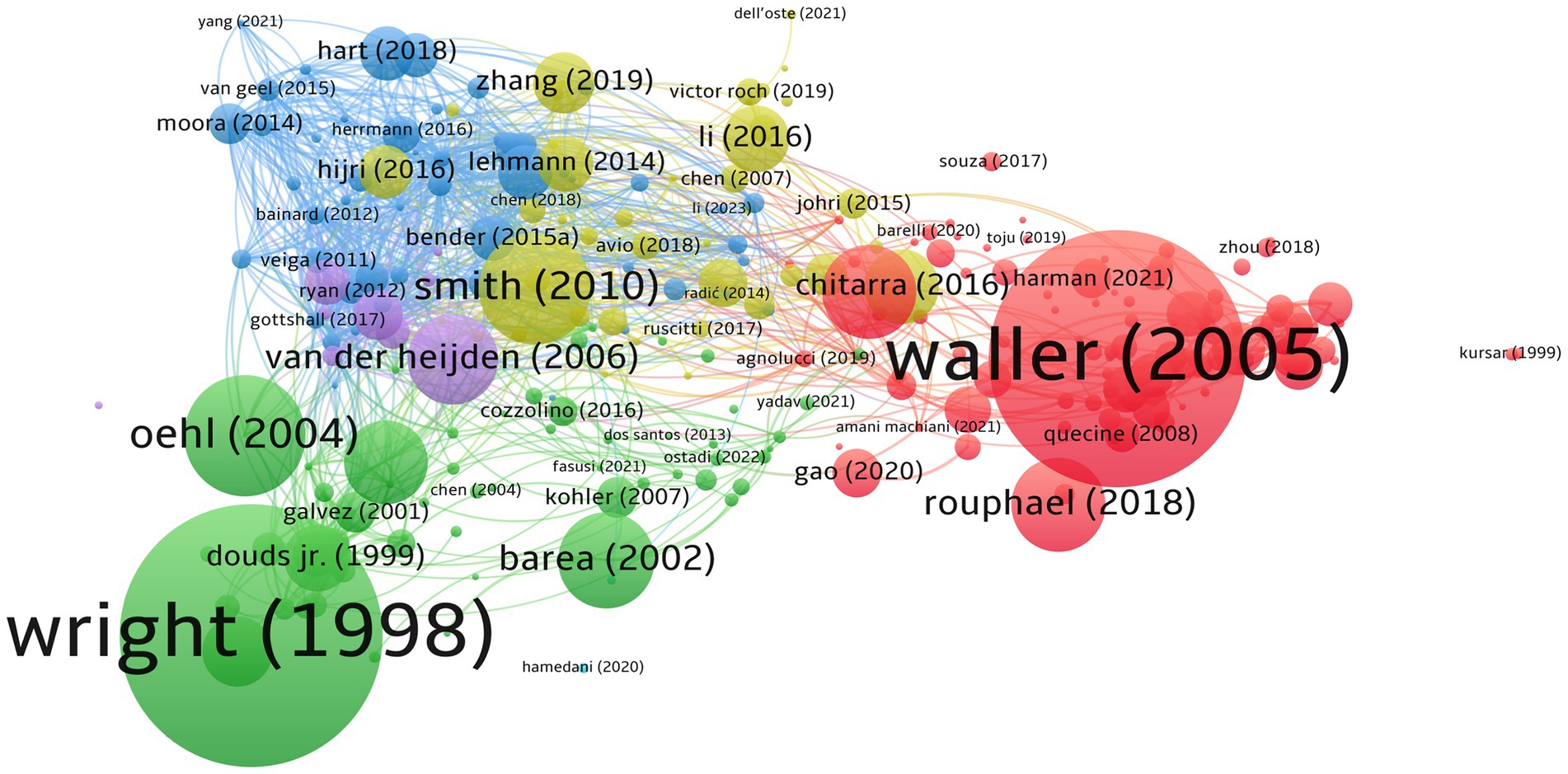
Figure 4. Bibliographic coupling of documents. Out of 733 documents, 279 met the threshold limit of 20 citations, with six distinct clusters, 8,964 links, and 16,196 TLS. This figure represents that the higher the weight citation, the larger the circle. The lines show the relationship links between the articles. Note that, first author of each document only shows in here.
4 Discussion
As previously highlighted, the interaction between plants and fungi is one of the most prominent factors in a healthy or sustainable agricultural system. Phytopathogenic fungi are nightmares to the agriculture sector, while symbiotic fungi are a pleasant dream (Figure 5). Through data analysis, we have also found that approximately 5.4% of studies directly focused on plant–fungal interactions with climate-resilient or low-input agricultural systems, while the majority of other studies indirectly highlighted this. Although highly developed techniques exist to eliminate phytopathogenic fungal diseases, the current world is still unable to eradicate them. Therefore, it is still vital to discuss those fungal interactions with the plants. On the other hand, plant-fungal interactions in the perception of some of the world’s major crops (e.g., cassava, cotton, potato, sorghum, soybean, sugarcane) need to be more thoroughly studied.
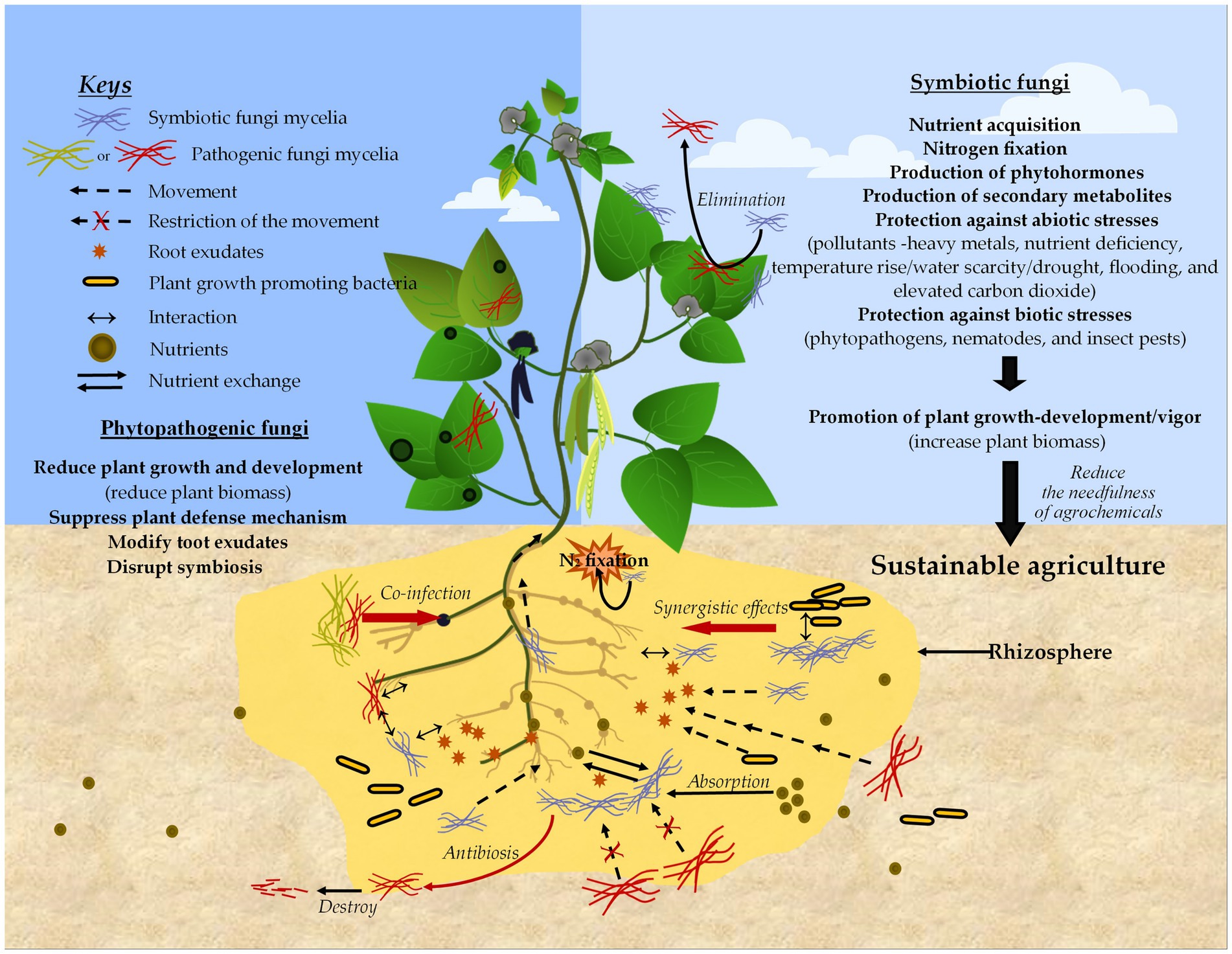
Figure 5. Simplified schematic diagram of plant–fungal interactions illustrating the positive and negative impacts on plants, as well as their contributions to sustainable agriculture.
Studying the plant–fungal interactions are also today more focused on molecular biology. The molecular trends in plant–fungal interactions encompass the intricate signaling pathways and gene expression patterns that govern both beneficial and detrimental relationships between plants and fungi. Although this is not our primary focus in this paper, it is essential to briefly highlight this aspect. In the current trend, the involvement of pathogenic fungi in regulating pathogenicity through surface signal recognition proteins, Mitogen-Activated Protein Kinase (MAPK) signaling pathways, transcription factors, and pathogenic factors during plant infection is being explored by researchers (Li et al., 2022). Pathogenesis-related (PR) proteins, encoded by PR genes, accumulate in response to pathogen attack and play a direct role in plant disease resistance. The development of a hypersensitive response (HR) is another protective reaction provided by plants, which can induce the systemic acquired resistance (SAR) mechanism (Balint-Kurti, 2019), and is still receiving considerable attention among researchers (Ali et al., 2018). In addition, the plant’s resistance mechanism is also induced by the symbiotic fungi in a manner that is beneficial in fighting against pathogens (Constantin et al., 2019). A plethora of recent studies also focused on further understanding of plant innate immunity immune responses in plant-fungal interaction, namely: pattern-triggered immunity (PTI) and effector-triggered immunity (Naveed et al., 2020; Percio and Botella, 2025).
It should also be noted that the interaction between plants and fungi can differ in natural and agricultural ecosystems. In natural ecosystems, such as forests, fungal diversity and richness can be significantly higher than those found in agricultural lands (Öpik et al., 2006). Obviously, this is due to the introduction of various agrochemicals (and other farming practices), leading to shape up of the fungal population, thus influencing their association with plants. In natural ecosystems, symbiotic associations are often more prominent, and there is generally a dynamic balance between symbiotic and pathogenic fungi. In contrast, in an agricultural system, changes in farming operations can significantly alter the fungal population, ultimately affecting plant-fungal interactions (Zeilinger et al., 2016; Priyashantha et al., 2025).
4.1 Phytopathogenic fungi
Based on the mode of nutrition, pathogenic fungi have been classified into three main categories namely, biotrophs (obtain nutrients and energy from living cells), necrotrophs (derive energy from dead or dying cells), and hemibiotrophic (derive nutrients initially from living cells and later transferred into necrotrophic lifestyle and take up nutrients from killing the cells) (Rajarammohan, 2021). All these pathogenic groups may develop diseases following contact with plants; however, this is not always the case. To cause a disease, it requires the presence of specific conditions, as explained in the famous classical disease triangle, which posits that a disease is caused by the combination of three main factors: a susceptible host, a virulent pathogen, and favorable environmental conditions. Elimination of one of those three components will not cause disease to the plants (Gaumann, 1950; Stevens, 1960). Nevertheless, this has been further explained genetically and refined when describing the host-pathogen interaction, as the genotypes of the pathogen and the host, along with environmental factors, combine to determine the outcome of this association (Casadevall and Pirofski, 1999, 2000). Plant survival and pathogen detection also influenced the pathogen-host–environment. The spatial and temporal distribution of pathogens concerning the host depends on the biology of the pathogens, including aspects of pathogen dispersal and overwintering capacity (Benítez et al., 2013). According to the bibliometric analysis, most studies have focused on single-host-single-disease interactions, with less attention given to co-infections. However, by understanding this, today, co-infection is becoming an interesting point for the researchers. Among these, works conducted by Lerch-Olson and Robertson (2020), Fang et al. (2021), and Zhang et al. (2022) can be cited. Host-pathogen, pathogen–pathogen, and host-multiple-pathogen complexes are the three main interactions that cause damage in co-infected plants. Host–pathogen interaction is the most extensively studied, while the other two interactions are relatively obscure. These interactions can yield various outcomes, including antagonism, synergism, coexistence, mutualism, or cooperation (Abdullah et al., 2017). The development of disease due to co-infection remains unresolved, and the crucial factors that determine the outcome of co-infections also remain unclear (Barrett et al., 2021). Nevertheless, in general, co-infection with fungi can have a substantial adverse effect on the host plant, making it more susceptible to diseases. Moreover, the co-occurrence of several fungal species could lead to disease outbreaks (Zhou et al., 2023).
4.2 Symbiotic fungi
Mycorrhizae and endophytes are two main groups of beneficial fungi that have been mostly studied. Endophytic fungi are facultative plant symbionts or biotrophs, and unlike mycorrhizal fungi, their development is not synchronized with that of their respective hosts (Domka et al., 2019). Endophytic fungi primarily colonize the intercellular or intracellular spaces of plants. In addition to the presence of endophytic fungi in aerial parts such as fruits, leaves, peduncles, seeds, and stems, they are primarily associated with the underground parts of the plants (Asad et al., 2023). Among several other mycorrhizal types, arbuscular mycorrhizal fungi (AMF) are the prominently studied group, emphasizing their tremendous benefits towards agriculture. Unlike plant-pathogenic fungi, which cause host destabilization, beneficial fungi provide stability to the host. The interaction of endophytic fungi can alter the metabolic activities of the plants (Usman et al., 2024), supply more nutrients, and protect them from harmful pests, thus plants show improved growth and development, and strengthening the plant against various biotic factors such as high temperature, drought, salinity, acidity, and waterlogged (Zeilinger et al., 2016; Akhtar et al., 2022). Under stressed conditions, the interaction of these symbiotic fungi is crucial, as they produce various stress-responsive molecules. Among these, symbiotic fungi stimulate the production of plant hormones, including gibberellins (GB), cytokinins (CIS), salicylic acid (SA), and indole-3-acetic acid (IAA) (Singh et al., 2023). Furthermore, symbiotic fungi can also produce such hormones and improve support to the plants in mitigating stress conditions (Ikram et al., 2018). Additionally, symbiotic fungi usually enhance the plant synthesis of defense enzymes, antioxidant activity, and expression of SA- and Jasmonic acid (JA)-responsive genes. Furthermore, they minimize reactive oxygen species (ROS) and reactive nitrogen species (RNS), and enhance callose deposition, which is beneficial against pathogenic fungi (Morelli et al., 2020). However, researchers have also found that endophytic fungi can exhibit a distinct pathway of making host plants resistant to phytopathogens, such as endophyte-mediated resistance (EMR). Here, according to the findings of Constantin et al. (2019), the EMR is independent of JA/ET and SA-dependent signaling pathways and distinct from the classical induced systemic disease resistance. Their study implies that antibiosis, competition for niches or nutrients, or a plant response independent from SA, ET, and JA are involved in EMR. Nevertheless, Matušinsky et al. (2022) suggested that the cooperative effect of the induced systemic disease resistance mechanism and EPR exists. They have highlighted the key genes involved in creating disease resistance in studied organisms—B2H2, Chitinase, LOX, PR1, and PR1.1. Recently, a literature review conducted by de Lamo and Takken (2020) provided a comprehensive explanation of EMR and also reported a molecular-level understanding of EMR.
4.3 Beneficial aspects of plant-symbiotic fungi towards sustainable agriculture
When it comes to agriculture, plant–fungal interactions are highly influenced by farming practices. The effect may be both direct and indirect, causing either the death of fungi or improved colonization (Begum et al., 2019). Nevertheless, in general speaking, farming practices decrease the natural fungi populations, also dropping the species diversity. However, when compared with different farming systems and conventional and organic practices, the growth of microbial biomass and its diversity is influenced by conventional practices (Manoharan et al., 2017). The farming practices led to changes in the soil’s nutritional regime; moreover, modern farmlands are mostly polluted by various hazardous compounds. In addition to the farming practices, climatic changes contribute to significant changes in plant–fungal associations, due to alterations in temperature, light, water availability, and CO2 concentration (Singh et al., 2023). One of the most intriguing outcomes of the alterations of the factors mentioned above is the lifestyle shift of the fungi. More precisely, these factors may lead to a shift from a pathogenic to a mutualistic lifestyle and vice versa (Priyashantha et al., 2023). Theoretically, the emergence of new pathogenic strains is associated with increased virulence in overcoming host-plant resistance (Singh et al., 2023). Nevertheless, plants may also be exposed to novel effectors despite having extensive defense mechanisms in place, due to the rapid evolution of pathogens, which could also be attributed to changes in environmental factors (Möller and Stukenbrock, 2017). In the case of symbiotic fungi, their importance in agriculture is highly valuable. Moreover, the use of native symbiotic fungi inoculum in agriculture may be more beneficial than the application of exotic species; however, this has not been the primary focus of many studies (Klironomos, 2003). Nevertheless, our aim here is to discuss the significance of plant–symbiotic fungal interactions in addressing both biotic and abiotic stresses.
4.3.1 Pests
Along with diverse bioactive metabolites produced by symbiotic fungi, this creates the possibility of controlling a wide range of pests (Wang et al., 2023), including plant-parasitic nematodes and herbivorous insects (Akram et al., 2023). When mycorrhizal and endophytic fungi are used as biocontrol agents, they yield inconsistent results, including both negative and positive outcomes among studies. This could be due to the condition of the tested environment and differences in the techniques and methodologies followed; however, it is crystal clear that fungi can control phytopathogens and improve plant health (Table 1). On the other hand, most of the works conducted by researchers are limited to the control environment/laboratory scale, and the need for field evaluation needs to be highlighted (Suebrasri et al., 2020; Azuddin et al., 2021; Mota et al., 2021). In a study, Yanan et al. (2015) recognized the enhanced resistance of strawberry plants when inoculated with Glomus mosseae against Fusarium wilt caused by Funneliformis mosseae. The authors found that an increase in cell wall lignin and HRGP (hydroxyproline-rich glycoprotein) proteins, along with changes in the activities of catalase, peroxidase, and superoxide dismutase enzymes, leads to such resistance. Wang et al. (2022a) emphasized the importance of Rhizophagus irregularis against F. oxysporum, as they trigger the plant defense mechanism, induce expression of the JA synthesis genes, such as allene oxide cyclase gene (AOC) and lipoxygenase D gene (LOXD), and increase activities of polyphenol oxidase (PPO) and phenylalanine ammonia lyase (PAL). Khruengsai et al. (2021) showed that Daldinia eschscholtzii MFLUCC 19–0493 isolated from Barleria prionitis inhibits the anthracnose disease fungi Colletotrichum acutatum, through the production of various other volatile compounds—3,5-dimethyl-4-heptanone, benzaldehyde dimethyl acetal, elemicin, ethyl sorbate, methyl geranate, and trans-sabinene hydrate. In contrast to previous works, Odunayo Joseph and Victor Olumide (2022) aimed to identify the additional benefits provided by the fungi beyond pest resistance. Odunayo Joseph and Victor Olumide (2022) showed the importance of Glomus clarum in controlling the tomato early blight disease caused by Alternaria solani. Their results showed that once inoculated with G. clarum, the plants exhibited enhanced resistance to the pathogenic fungus. Moreover, with the enhanced nutrient supply, the crop showed increased production of flowers, fruit weight per plant, leaf condition, and stem growth.
In their in vitro assay, Suebrasri et al. (2020) showed that several endophytes, Diaporthe phaseolorum BUP3/1 and Macrophomina phaseolina BUP2/3, isolated from Jerusalem artichoke (Helianthus tuberosus) and D. eschscholtzii 2NTYL11 and Trichoderma erinaceum ST-KKU2, isolated from Stemona root (Stemona tuberosa) and ginger (Zingiber officinale), control the stem rot fungi (mycelial growth) Sclerotium rolfsii impressively at 76.00, 41.20, 66.67, and 63.63%, respectively. Furthermore, the pot experiment showed that T. erinaceum ST-KKU2 and D. eschscholtzii 2NTYL11 control the disease at a higher level (58.14%) than the others. Furthermore, they have observed predatory mechanisms, and the production of polyketide groups (6-n-pentyl-2H-pyran-2-one/6PAP and 2,3-dihydro-5-hydroxy-2-methyl-4H-1-benzopyran-4-one/DHMB) is a primary factor in controlling the pathogens. Many other studies have also shown the effects of various endophytic fungi in significantly controlling major devastating phytopathogens in crops, e.g., Induratia coffeana inhibits Colletotrichum lindemuthianum at 99.64 ± 0.57 (Mota et al., 2021); T. harzianum inhibits Colletotrichum scovellei at 85.80 ± 5.47 (Azuddin et al., 2021); and Annulohypoxylon sp. inhibits Penicillium digitatum at 72.96 ± 0.58 (Du et al., 2022). The symbiotic fungi also produce various antibacterial compounds, such as acetic acid, acetol, and hexanoic acid, among others (Rashid, 2021). Similarly, a number of compounds produced by symbiotic fungi also control the plant parasitic nematodes, for example, 3-methoxyepicoccone, 4,5,6-trihydroxy-7-methylphthalide, chaetoglobosin A, chaetoglobosin B, and flavoring isolated from Chaetomium globosum YSC5 found to be working against Meloidogyne javanica (Khan et al., 2019). The application of endophytic fungi also exhibits good nematocidal effects due to these compounds. In a study by Yao et al. (2023), it was found that Acremonium sclerotigenum effectively controls M. incognita, with a mortality rate of up to 95.5% in the juvenile (J2 stage) and significant inhibition of egg hatching of up to 43%. Schouteden et al. (2015) emphasized the importance of AMF in controlling nematodes. Adding value to this, Bell et al. (2023) highlighted the effectiveness of various AMFs in controlling potato cyst nematode Globodera pallida. In turn, the importance of symbiotic fungi against plant viruses is still less of a concern. However, available studies have shown their significance (Fakhro et al., 2010; Jaber and Salem, 2014; Kiarie et al., 2020).
It is also important to note that the majority of studies have focused on the application of endophytic fungi to control insect pests, rather than mycorrhizal fungi, despite showing their undeniable importance (Vannette and Hunter, 2009; Jiang et al., 2021). Control of insect pests using fungi has a long history, dating back to the 1980s. Beauveria bassiana is the earliest identified and most recognized entomopathogenic endophyte in controlling a wide range of pests (Islam et al., 2023). Today, in addition to B. bassiana, several other entomopathogenic fungi, including Metarhizium, Isaria, and Lecanicillium, have been used to control insect pests. However, such utilization is still in its early stages of development and requires considerable further study, particularly in identifying new infectious fungal species and strains (Bamisile et al., 2023). Suebrasri et al. (2020) examined the effect of B. bassiana and Purpureocillium lilacinum against the cotton aphid, Aphis gossypii. They have recognized that fungi have an impact on the host’s reproduction. They have found the significant control of aphids by B. bassiana, while P. lilacinum shows considerable but not significant control. Agbessenou et al. (2020) studied the controlling ability of Lepidopteran Tuta absoluta using tomato (Solanum lycopersicum) and nightshade (Solanum scabrum) Trichoderma asperellum M2RT4, B. bassiana ICIPE 706, and Hypocrea lixii F3ST1. They have found the effectiveness of those fungi in reducing the number of eggs laid, mines developed, pupae formed, and adults emerged. In contrast to the above two studies, Pappas et al. (2018) recognized the indirect control of pests by endophytic fungi in their study on Fusarium solani strain K against two-spotted spider mites (Tetranychus urticae) in tomato. They have studied the expression of genes GGPS1, JIP-21, LOXD, PPO-D, PPO-F, PR-1A, PR-P6, and WIPI-II in the pest after fungi colonization, which is important for host defense mechanisms against pests. However, according to their findings, the fungi significantly upregulated the expression of WIPI-II and PPO-D. At the same time, no effects were observed on other mite-defense-related genes, such as GGPS1, JIP-21, LOXD, PPO-F, PR-1A, and PR-P6. The indirect effect was also recognized through the alteration of the plant’s volatile compound emissions, such as decanal, 5-heptene-2-one-6-methyl, and geranyl acetone.
4.3.2 Pollutants
Heavy metal pollutants such as arsenic (As), cadmium (Cd), cobalt (Co), copper (Cu), lead (Pb), magnesium (Mg), manganous (Mn), nickel (Ni), strontium (Sr), and zinc (Zn) are known to be a major threat to agriculture today. The excessive release of heavy metals into the environment leads to biological toxicity and direct environmental pollution (Borymski et al., 2018; Asiminicesei et al., 2024). Furthermore, those metals that are injurious to plants also alter metabolite activities and reduce total productivity (Jańczak-Pieniążek et al., 2022). Moreover, it has been understood that heavy metals impede seed germination, shorten roots and shoots, and lower respiration and photosynthesis rates (Majhi and Sikdar, 2023). A major problem occurs when crops absorb heavy metals, and human consumption of these contaminated crops leads to life-threatening diseases (Boluspayeva et al., 2022). In early studies, microbes and plants have been used separately to eliminate those heavy metal pollutants from the soil prominently; nevertheless, understanding the importance of plant–fungal interactions, combined application of both plants and fungi became a catchier topic nowadays (Flemming et al., 1990; Joshi-Tope and Francis, 1995; Boorboori and Zhang, 2022). The plant-based approach to removing heavy metals, known as phytoremediation, involves the application of selected species that are resistant to heavy metals and accumulate high levels of metal (loid)s in their plant bodies, primarily in the roots and shoots. The plants are selected based on the two assignments: bioconcentration factor (BCF) and translocation factor (TF). The shoot-to-root ratio of heavy metal and the root-to-soil ratio of heavy metal (Sharma et al., 2023).
In general, vascular plants typically have a marginally higher tolerance to heavy metals (Fasani et al., 2022). Plants can alter the number of polysaccharides in their cell walls to withstand the stress caused by heavy metals. The main proteins in cell walls that are involved in cell wall dynamics and plant responses to stressors are called peroxidases. As plant roots grow, they release secretions into the soil, including low-molecular-weight organic acids, which break down soil minerals and release metals (Boorboori and Zhang, 2022). Mycoremediation, the removal of heavy metal stress by fungi, could be a more effective approach because fungi can move across and penetrate toxic or unfavorable zones to reach substrates and nutrients (Antón-Herrero et al., 2023). The underlying mechanism for mitigating the heavy metal threats is not yet well understood (Fan et al., 2022). Nevertheless, according to the findings, the homeostatic systems of fungi regulate the import, export, storage, and transport of heavy metals. It is also known that oxalate crystals produced by mycorrhiza fungi immobilise and detoxify heavy metals. The filamentous hyphae of these fungi enter the deeper soil aggregates and chelate or adsorb heavy metals (Mishra et al., 2017). In more detail, for example, ATP-binding cassette (ABC) transporters regulate several heavy metals, including Cd (Víglaš and Olejníková, 2021); CtrA2, CtrC, and CtrB transporters coordinately work on Cu (Raffa et al., 2019); and SMF1 and SMF2 function on Mn (Mori et al., 2018). Cytoplasmic compounds in fungi transform hazardous metals into less or non-toxic forms, which can then be segregated within the vacuole. Metallothioneins (MTs), a major class of intracellular peptide chelators of metal ions, play a crucial role in cellular tolerance to and detoxification of heavy metals (Gajewska et al., 2022). Moreover, it has been recognized that fungi’s genetic mechanisms of adaptation, or modifying their expression patterns, result in different profiles of gene expression (Traxler et al., 2022). The MT genes of fungi can be induced even by a single heavy metal, triggering the detoxification mechanism (Gajewska et al., 2022).
Lorenzo-Gutiérrez et al. (2019) demonstrated the role of mt1 in conferring tolerance to F. oxysporum against Cd, Cu, and Zn. Gautam et al. (2023) recently showed the metal-binding and heavy metal tolerance mechanisms of metallothionein OsMT-I-Id. Furthermore, their evidence shows the ability of fungi to induce OsMT-I-Id in rice against As, Cd, and Cu. It has also been identified that other genes, particularly ChrA, ChrB, and ChrR, function in response to a specific heavy metal. Those genes facilitate the fungi to tolerate Cr pollutants (Chi et al., 2021; He et al., 2021). In a recent study, Chi et al. (2021) analyzed the genome of P1 in Penicillium janthinellum and annotated 23 genes related to heavy metals.
Substantial investigation has been conducted to examine the significance of fungi in tolerating/degrading heavy metals. Ahmad et al. (2006) found that Aspergillus niger (also Penicillium sp.) tolerates heavy metals like Ni, Cr, and Cd. They have further recognized that fungi tolerance is not just under single-metal conditions but also under multi-metal conditions. Acosta-Rodríguez et al. (2018) later found the tolerability of A. niger against 2,000 ppm of Cr, Zn, Pb, and Hg, 1,200 and 1,000 ppm of Cu, As (III) and (VI), 600 ppm of Co, and 400 ppm of Cd.
Further, their study evident that, for instance, the ability of the fungus to eliminate Zn and Cr (VI) by 100%, followed by Hg (83.2%), Co (71.4%), As (V) (69%), As (III) (66%), Pb (59%), Cd (57%), and Cu (37%). In addition, Acosta-Rodríguez et al. (2018) gave an in-depth understanding of the mycoremediation potential of fungi. They highlighted that A. niger is not always superior in removing heavy metals, as it may remove specific metals (and certain concentrations) that could also depend on temperature and pH of the substrate. In a separate study, Mohamadhasani and Rahimi (2022) found that Pleurotus species grew more under low concentrations of the tested heavy metals (Co and Cu), indicating potential for heavy metal removal. It is noteworthy that when a fungus-like AMF establishes a direct relationship with plants, it increases the immobilization, conversion, detoxification, and extraction of heavy metals (Boorboori and Zhang, 2022). It is also recorded that a wide range of AMF can withstand highly polluted soils, thus, offering future possibilities for finding novel AMF species with greater mycoremediation ability (Suárez et al., 2023).
In a study, Iram et al. (2019) used several Aspergillus spp. (A. niger, A. terreus, and A. flavus) and Penicillium (P. chrysogenum) to remediate the Cr, Cu, Pb, and Cd associated with wheat and sunflower (Helianthus annuus). Plant–fungal interaction significantly helps remove the tested contaminants, enhanced by Aspergillus sp. (Cr < Cu < Pb < Cd). Furthermore, the researchers found that the interaction of P. chrysogenum leads to the storage of a higher amount of Pb in the plant shoot compared to other metals. Fan et al. (2022) found that A. niger (TL-F2) and A. flavus (TL-F3) facilitated the accumulation/subcellular distribution of Cd by annual ryegrass. Further, the researchers observed that ryegrass roots (17.8–37.1 μg pot−1) were significantly more capable of absorbing Cd than the shoots (1.66–5.45 μg pot−1). Overall, it was observed that the accumulation of Cd in different subcellular fractions increased with Cd concentration from 1.96 to 10.2 mg kg−1. Nevertheless, compared with non-fungus ryegrass, cell wall and soluble Cd fractions in fungus-inoculated roots increased by 13.5–44% and decreased by 21.5–26.4%. Additionally, the authors recognized that fungal interaction significantly increases the plant’s biomass. For example, the biomass increases of fungus-inoculated roots range from 14 to 43%. Regarding the increasing Cd solution, in non-fungus-inoculated plants, the dry biomass is decreased by 10–40%. Chen et al. (2007) demonstrated the role of F. mosseae, in removing the effect of Phosphorus (P) and As associated with alfalfa. They inoculated the fungus and observed the increase in dry weight of both shoots and roots of the plants. Furthermore, Chen et al. (2007) observed that F. mosseae significantly decreased As concentrations in both plant shoots and roots, which also correlated with the fungus’s colonization. While fungus significantly increases the P supply to the alfalfa, proving that hyphal uptake of As and Chaturvedi et al. (2021) studied the Pea (Pisum sativum) F. mosseae symbiosis for phytoremediation of soil contaminated with Cd, Pb, and As. It has been found that F. mosseae led to an increase in plant growth, concentration of photosynthetic pigments, carbohydrates, nitrogen (N), and defense antioxidants, as well as a decrease in proline, all of which were statistically significant. Ultimately, those changes also help to protect the plant against hazardous pollutants. Furthermore, this association resulted in the removal of those pollutants from the soil to a considerable extent.
In contrast, a study conducted by Gu et al. (2017) yielded mixed results regarding the interactions between mycorrhizae and plants. Their study showed that showy stonecrop (Hylotelephium spectabile) and Purple Heart (Tradescantia pallida) survive under heavy metal-polluted soil, along with a mycorrhizal association, while exhibiting increased biomass in both roots and shoots. For example, when compared to the non-inoculated condition, stonecrop showed the most significant growth response to mycorrhizal inoculation, with increases in shoot and root biomass of 196 and 263%, respectively. The researchers measured the concentrations of Cd, Cu, Pb, and Zn in the plant’s tissues and recognized that metal concentrations in stonecrop shoots were unaffected by mycorrhiza. In contrast, Pb, Cu, Cd, and Zn concentrations in roots rose considerably by 108, 112, 34, and 19%, in comparison to the non-inoculated control. Furthermore, the study results indicated that the AMF inoculation did not affect the purple Heart plant’s shoot or root metal uptake, except for a 36 and 136% increase in Zn and Cd, respectively, in the roots. The outcomes also showed that AMF had a minimal effect on the uptake of Zn and Cu by the shoots of both plants.
4.3.3 Nutrient
Endophytes solubilize micro- and macronutrients available in the soil, supporting the mobilization and uptake of these nutrients by plants. The activity of endophytic fungi is well recognized in reducing the incidence of Fe deficiency in plants. Around the roots of non-graminaceous plants, certain endophytes synthesize and excrete phytosiderophores that solubilize Fe (III) by binding to mugineic acids (MA). This conjugation results in the formation of Fe (III)-MA, eliminating the need for plants to reduce Fe (III) to Fe (II) in order to absorb rhizospheric metals (Watts et al., 2023). Those fungi are also important in N fixation, as they fix more N within the plant, which is a more favorable environment for N fixation due to the low partial oxygen pressure (Rana et al., 2020). In legume plants, endophytic fungi also promote nodulation and N fixation even under low N availability (Xie et al., 2019). According to the literature, rather than the endophyte associations, the benefits of mycorrhizae in organic N and P accumulation for plants have been well studied. When AMF interacts with plants, it increases the availability of nutrients, particularly P, and with EcM—organic N, P, and other nutrients even under hazardous environmental conditions, promoting greater plant growth (Procter et al., 2014). More precisely, mycorrhizal fungi have been found to enhance crop productivity, increasing plant height, leaf area, biomass, and seed yield, even under lower soil nutrient content (Beslemes et al., 2023). Therefore, numerous studies have been conducted to date on mycorrhizal fungi; thus, here, we primarily discuss the alterations in crop-mycorrhizal interactions, particularly those involving AMF.
In a study, Zhu et al. (2016) demonstrated the response of AMF community composition to fertilization in the rhizosphere soil of maize crops. The findings suggested various fertilization regimes had a considerable impact on AMF diversity in the maize rhizosphere. Furthermore, the addition of organic manure is the most significant factor positively influencing the composition of AMF. Notably, the input of N and P fertilizers is also recognized as the next driving factor for the AMF composition. Furthermore, testing with 2-ethylnaphthalene and 2,6,10-trimethyltetradecane showed an adverse effect on AMF—Glomus relative abundance; however, 3-methylbiphenyl showed a positive correlation with Rhizophagus. This implies that changes in fungal composition may also impact their interaction with host plants. In a separate study, Beslemes et al. (2023) investigated the contribution of AMF inoculation on the growth and productivity of two-rowed barley crops in conventional and organic cropping systems. The researchers also obtained similar results to those of Zhu et al. (2016), as the organic cropping system supports the significant influence of AMF colonization on host plants, leading to increased crop productivity. According to the findings, nutrient availability is sometimes not affected by community composition, particularly in the case of root-associated fungi. However, the effect on plant growth is also supported by the fungal associations, which supply higher nutrients to the plants than those regularly taken (Maciá-Vicente et al., 2022). It is noteworthy to highlight the results of the study conducted by Wang et al. (2022b), as their findings provide additional support for the points discussed above. Here, the researchers have tested different AMF inoculations on cherry tomato crops under varied nutritional conditions, attempting to understand their effect on AMF and host-plant interactions. In their pot experiment, Wang et al. (2022b), used three different nutritional treatments: high nutrition level (1:1, volume ratio of peat soil to sand), medium nutrition level (1:2, volume ratio of peat soil to sand), and low nutrition level (1:3, volume ratio of peat soil to sand). The four fungal inoculation treatments included F. mosseae, Glomus versiforme, Rhizophagus intraradices, and an equal mixture of all three fungi. They have recognized highly significant differences between the various AMF inoculation treatments in the root mycorrhizal infection of cherry tomatoes. Furthermore, Wang et al. (2022b), found that the amount of nutrients in the soil, the type of fungi inoculation, and their interactions all had a significant impact on the accumulation of N and P in S. lycopersicum. Overall, at medium and low nutrient levels, the infection rates of G. versiforme and the mixture were significantly greater than those of F. mosseae and Rp. intraradices, and at high nutrient levels, the infection rates of G. versiforme exceeded those of F. mosseae. Additionally, it is reported that the crop exhibits different growth performances under four fungal treatments and three nutrient levels. Wang et al. (2022a) also observed that the treatments had significant effects on flower number, fruit number, fruit biomass, and reproductive allocation of the crop. By accounting for all these results, the researchers concluded that the nutritional level directly alters the plant-AMF interaction.
4.3.4 Temperature
Temperature plays a crucial role in the metabolite activities of plants, and increased temperature may have a deleterious effect on their physiological functions and growth. Climate change and the temperature rise are among the pressing issues in the agricultural sector, as evidenced by the failure of crop farming and the inability to achieve the expected harvest (Habib-Ur-Rahman et al., 2022; Janni et al., 2023). Moreover, the temperature rise alters the plant–fungal interaction both positive and negative ways, depending on the lifestyle of the plant and the fungus. When plants are associated with pathogens, the disease caused by the fungi may be more prevalent due to an increase in the virulence of the pathogenic fungi, this has been greatly presented by Waheed et al. (2023) in their review. Note that there is an optimum temperature range in each of the plant–fungal interactions at which the disease develops, for instance, 23.8°C is the optimum temperature for the White Rot disease in grape berries caused by Coniella diplodiella (Ji et al., 2021), while 30°C is the optimum temperature for root rot fungi F. solani, and 15°C is the optimum temperature for F. tricinctum to cause the disease in cotyledons, soybean (Yan and Nelson Jr, 2020).
In an earlier study, Park (1990) attempted to understand the infection of Puccinia striiformis in wheat seedlings, as well as the effects of temperature changes under both laboratory and field conditions. Based on the laboratory study, the researchers expected that no infection would occur at or above 20.8 + 0.2°C, where infection declined from 100% at 15.4°C to 0.8% at 20.5°C. However, under field conditions, the infection rate is higher between 19 and 30°C, showing difficulties in predicting which temperature range can exactly influence significant changes in plant–fungal interaction. The molecular-based answer for such changes is still lacking. Onaga et al. (2017) observed that M. oryzae plant fungal biomass is significantly higher at 35°C than at 28°C and also recognized the elevated level of putative fungal effector genes in plants exposed to 35°C, compared to lower temperatures. Considering all those, Onaga et al. (2017) highlighted that raising the temperature may facilitate M. oryzae infection by weakening plant resistance and hastening the pathogen’s colonization of plant tissues.
The adaptation of pathogens to global warming has been reported; however, the patterns and mechanisms of such adaptations in many plant pathogens remain to be understood. To fill this gap, Wu et al. (2022) tested the genotypes of Phytophthora infestans under five temperature regimes. They have noted that phenotypic plasticity contributes ~10 times more than heritability measured by genetic variance. Additionally, the expression of genetic variation and the relationship between local temperature and pathogen aggressiveness have been found to be altered by the experimental temperatures. The variance in aggressiveness is exacerbated by raising the experimental temperature. Pathogens from warmer climates caused less disease than those from cooler climates at low experimental temperatures, whereas the opposite was true at higher experimental temperatures. On the other hand, fungal symbionts provide more benefits to their host plants by protecting them from temperature fluctuations. Mathur et al. (2021) investigated the impact of high-temperature stress on plant physiological traits and mycorrhizal symbiosis in maize. It has been recorded that the vegetative and reproductive growth of maize, from germination to grain filling, is impacted by higher temperatures (35°C and above). The results of their experiment show that AMF interaction helps improve the plant’s photosynthesis rate by increasing N and Mg content, along with enhanced carbohydrate and sugar accumulation. The researchers further reported that AMF prevents damage to the photosynthetic apparatus (PSI and PSII) of the host plant due to the higher temperatures. Furthermore, Mathur et al. (2021) reported that enhanced photosynthesis, accompanied by improved soil quality and crop growth, leads to the mitigation of multiple malformations in the physiological characteristics of the host plant.
4.3.5 Water availability
Both fungal communities and plants respond directly to the soil water regime, thereby affecting their interactions (Erlandson et al., 2016; Xiao et al., 2022; Chen et al., 2023). On one hand, plants generally increase their susceptibility to phytopathogens due to drought stress. On the other hand, they also increase their resistance against those phytopathogens. The morphological characteristics of the plant root are one of the factors that determine its relationship with fungi. It has been recognized that plants with thin root systems exhibit lower dependence on AMFs for water uptake, due to their ability to acquire water efficiently (Lozano et al., 2021). Most EcMFs typically form rhizomorphs and hydrophobic mycelia, which help plants mitigate the impact of drought (Castaño et al., 2023).
Boczoń et al. (2021) described in detail how the plant–fungal interactions change under water scarcity (drought) due to climate change. According to them, drought reduced the overall prominence of mycorrhizal associations with the plants, leading to reduced further water uptake and alterations in metabolic pathways due to ATP and nutritional deficiencies. Next, impairment of plant tissues and chlorosis of the leaves occur, leading to a switch in the fungus’s endophytic lifestyle to pathogenic, causing disease in the hosts. In addition, Boczoń et al. (2021) noted that these changes weaken the plant’s defensive system, even against pests, and make it more susceptible to invertebrate attacks.
Joachin et al. (2023) studied how water availability influences the effective specialization of a fungal pathogen in its interactions with plants. Under low, average, and high-water treatments, they established paired congeners of three native and three non-native species of coastal prairie plants, with or without the pathogenic soil fungus Fusarium incarnatum-equiseti species complex 6b. The Fusarium treatment demonstrated greater adverse and species-specific effects on plant biomass at high water availability than at low water availability for all examined plant species. Furthermore, the results of Joachin et al. (2023) supported the discussions by Benítez et al. (2013). They have emphasized that the effective specialization of pathogens promotes the coexistence of plant species, for instance, via negative plant–soil feedback or the Janzen-Connell hypothesis (Comita et al., 2014). Under water scarcity, endophytic and mycorrhizal fungi show various alterations to host plants, depending on the species and the combination of other environmental factors (Dastogeer, 2018; Ahlawat et al., 2022; Frew, 2023). For instance, drought conditions may lead to the production of various plant biochemical compounds, also with the help of fungi, which mitigates water stress conditions (Boczoń et al., 2021). Hubbard et al. (2014) demonstrated that endophytic Ascomycetous fungi enhance the heat and drought tolerance of wheat (Triticum turgidum) in terms of grain yield and second-generation seed viability. Later, in a more comprehensive study, Miranda et al. (2023) found that the endophytic fungus Zopfiella erostrate substantially colonizes wheat and tomato roots, contributing to enhanced plant nutrient mineralization and water uptake under water deficiency. Their study showed the significant effect of the fungus on plant growth; however, the researchers did not observe any considerable changes in fungal root colonization under water-deficient conditions compared with the well-watered plants. Nevertheless, the fungus exhibited higher photosynthetic efficiency, decreased enzymatic activity (catalase and ascorbate peroxidase), altered glutathione reductase activity, lower hydrogen peroxide concentrations, and a significant drop in lipid peroxide accumulation. The studies indicated that such a change or activation of antioxidant compounds and enzymatic activities worked against oxidative damage generated by water-deficient conditions (Piri et al., 2019; Kaur and Saxena, 2023).
In an experiment with F. mosseae on tomato, Bitterlich et al. (2018) emphasized the importance of AMF in alleviating the drought impact on the plants. They recognized the considerable amount of available water content reserved by F. mosseae. To a great extent, fungal restoration of plant hydraulic status and increased plant transpiration were also observed with the decline in soil water content. In addition to water scarcity, soil waterlogging conditions also influence plant–fungal interactions. According to the study by Calvo-Polanco et al. (2014), the fungus R. irregularis benefits tomato plants under flooded conditions. They have gained a molecular-level understanding, as evidenced by the increase in root hydraulic conductivity due to the fungus, which is related to the upregulation of aquaporin gene expression, specifically GintAQP1 and SlPIP1;7. Further studies have demonstrated that mycorrhizal fungi stimulate the production of 1-aminocyclopropane-1-carboxylic acid (ACC) and ethylene (Et) in plants, thereby enhancing their resistance to flooded conditions (Cruz et al., 2000).
4.3.6 CO2 concentration
The rise of atmospheric CO2 has been observed over recent decades, which is linked to climatic changes. Elevated CO2 (eCO2) is beneficial for crops, as it increases the efficiency of photosynthesis and growth; however, the overall impact is not entirely understood. Furthermore, the interaction between plants and fungi is also affected by changes in CO2 (Smith and Luna, 2023).
Chakraborty and Datta (2003) evaluated the aggressiveness of Colletotrichum gloeosporioides in shrubby stylo (Stylosanthes scabra) that causes anthracnose disease. Considering their 22-year field experiment period, Chakraborty and Datta (2003) found a fourfold difference in disease severity levels between isolates with the lowest and highest disease severity levels. Prior to 1987, both weakly (severity < 0.6) and moderately aggressive (severity 0.6–0.8) isolates were found; however, between 1991 and 1999, the distribution became more uniform, with aggressive isolates (severity 0.8–1.2) and highly aggressive isolates (severity > 1.2) towards the host plant. They have recognized that these changes mainly happen due to the eCO2. Further, they have correlated this scenario with the controlled environment study. The experiment demonstrates that increasing CO2 levels (from 400 ppm to 700 ppm) enhances the pathogen’s aggressiveness. In their experiment, Karnosky et al. (2002) observed that eCO2 (560 ppm, 200 ppm)/O3 (1.5x ambient) occurrence and severity of leaf rust caused by Melampsora medusae on trembling aspen (Populus tremuloides). They have found that seasonal exposures to O3 and CO2, as well as O3 alone, both resulted in significantly increased rust occurrence and severity, three to five times. Leaf surface topography, microroughness, and physicochemical characteristics (e.g., chemical composition and epicuticular wax structure) collectively determine leaf surface properties, such as wettability, and this is recognized as a key consideration for changes in host-fungus interactions. The wettability of the leaf surface significantly increases the incidence of M. medusae by positively influencing the fungal attachment and further infection process. In the contest, some studies reported enhanced disease resistance under eCO2. For instance, Hibberd et al. (1996) and Mikkelsen et al. (2015) found that eCO2 (700 ppm) shows resistance against the Erysiphe graminis and Blumeria graminis, respectively, associated with barley. Williams et al. (2018) recognized the increased resistance of Arabidopsis thaliana against necrotrophic Plectosphaerella cucumerina infection under the eCO2 (1,200 ppm). Furthermore, the study by Williams et al. (2018) added value to Karnosky et al. (2002), as it considered in-depth biochemical mechanisms and molecular bases to explain disease resistance. Of this, Williams et al. (2018) noted a 69.3 and 69.4% increase in SA and JA accumulation under eCO2, respectively. They have further identified that eCO2 alters basal and SA-induced PR1 gene expression, which is responsible for stress resistance. In line with this, Mhamdi and Noctor (2016) also recognized increased disease resistance in A. thaliana (along with other plants, such as Phaseolus vulgaris, or beans) against B. cinerea under 3,000 ppm of CO2. They have also highlighted that eCO2 triggers SA accumulation and is partially linked to metabolic effects involving redox signaling. This is supported by the fact that complete priming of the SA pathway and the corresponding resistance to high CO2 are prevented by genetic modification of redox components, including glutathione levels and NADPH-generating enzymes. Furthermore, they have emphasized that this is also true for other crop plants studied, such as beans. In addition, working with Cochliobolus miyabeanus that infects rice, Dorneles et al. (2020) recognized (under 700 ppm of CO2) higher activity of the enzyme’s ascorbate peroxidase, catalase, chitinase, peroxidase, polyphenoloxidase, superoxide dismutase, enhanced phenolic compounds, and lignin concentration, indicating defense responses.
The interaction between plants and endophytic fungi may vary in response to eCO2. According to their experiment, Christian et al. (2021) found that a community of endophytic fungi declined with eCO2, which is opposite to the findings of Alberton et al. (2010). Liu et al. (2023) investigated the diversity and colonization of eCO2 and AMF. They have recognized that with eCO2, the diversity of AMF increases, while decreasing root colonization with host plants, such as maize and wheat.
4.4 Synergistic effects create more benefits for plant–fungal interactions
The combined application, or co-inoculation, offers a nuanced understanding of plant–fungal interactions, although it can sometimes be extremely challenging to predict the outcomes (Niu et al., 2021). However, several studies have assessed the co-inoculation of different groups of non-pathogenic fungi and found that it has more beneficial effects on plants and crops. In a foremost study, Khaekhum et al. (2021a) tested the growth and yields of sunchoke with co-inoculation of endophytic fungi Exserohilum rostratum NMS1.5 and an AMF Glomus etunicatum UDCN52867 g.5. They found that such application gives significant growth improvement (some parameters), and tuber yield of sunchoke, compared to the application of chemical fertilizers. Furthermore, their results demonstrate superior outcomes compared to those previously reported by Khaekhum et al. (2021b) and Nacoon et al. (2021), indicating a synergistic effect of endophytic fungi and AMF. Co-inoculation is also beneficial to plants, as it helps mitigate the aforementioned biotic and abiotic stresses. In an early study, Leeman et al. (1996) demonstrated the suppression of Fusarium wilt disease in radish by co-inoculation of Pseudomonas spp. and root-colonizing fungi, including Acremonium rutilum, F. oxysporum, and Verticillium lecani. They have found that F. oxysporum and V. lecanii induced systemic disease resistance, hence suppressing the disease. Nevertheless, when co-inoculation of A. rutilum, F. oxysporum, or V. lecanii with Pseudomonas spp. strains WCS358, WCS374, or WCS417, or their pseudobactin-minus mutants, showed a significant suppression of the disease compared to the control treatment.
Seneviratne et al. (2015) evaluated the single and combined application of a gram-negative bacterium and a fungus (Aspergillus sp.) on Z. mays, protecting the plants from growth inhibition. Plants treated with combined microbes increased their uptake of Ni by 13%, Mn by 52%, and Cr by 83%, compared with non-inoculated plants. Further combined application shows a higher heavy metal accumulation factor in plants compared to the single application. For example, the Mn plant accumulation factor is 0.26 ± (0.02) for control, 0.14 ± 0.03 for fungi, 0.18ab ± 0.07 for bacteria, and 0.23b ± 0.05 for combined application. However, the transfer factor (TF) was reported to be lowest in the combined treatment (for Mn, control-0.20 ± 0.05, fungi-0.13a ± 0.03, bacteria-0.11a ± 0.02, combined-0.10a ± 0.04). They further concluded that the combined application resulted in a reduction of heavy metal accumulation in plant shoots, thereby rendering the grains harmless for consumption. While Zou et al. (2024) recorded pronounced heavy metal tolerance (Cd) in Robinia pseudoacacia under an excess N condition due to the co-inoculation of Mesorhizobium huakuii and F. mosseae, compared to the individual application, their study also reported that, although heavy metal accumulation is greater in roots after microbial inoculation, accumulation in shoots was reduced. Furthermore, it demonstrates that such applications enable plants to resist or repair damage caused by Cd effectively. Ważny et al. (2018) demonstrated the importance of the combined application of AMF, Rhizoglomus intraradices, and endophytic fungi, Mucor sp. or T. asperellum, over single inoculation in mitigating the effects of heavy metals (Zn, Cd, Pb) on Lactuca serriola. Other than that, they have concluded that the combined application of these fungi could also support the plants in withstanding drought conditions.
Gasemi et al. (2023) studied the beneficial interaction of the combined application of plant growth-promoting rhizobacteria (Azotobacter, Bacillus lentus, Pseudomonas genus, and Pseudomonas putida) and AMF (G. etunicatum, Rp. intraradices, and G. mossea) with Dracocephalum kotschyi. The findings suggested that co-inoculation of those fungi creates more positive effects on the host plant than independent applications, such as an increase in plant height, leaf number, yield, essential oil content, chlorophyll content, carotenoid concentration, relative water content, total soluble sugars, and proline content. The most important consideration of their study is that those co-inoculated microbes deliver the aforesaid benefits to the host plants under water deficit stress conditions. In a similar study, Barzegari Barogh et al. (2023) co-inoculated Azotobacter chroococcum and Pseudomonas putida with AMF (F. mosseae, and Rp. intraradices) on potato plants. In addition to the beneficial effects reported by Gasemi et al. (2023), Barzegari Barogh et al. (2023) highlighted the importance of improving nutrient content. For example, compared with the control, the interaction of P. putida at 100 mL and Rp. intraradices increase the potato minituber number (116%), minituber weight (181%), shoot dry weight (248%), root dry weight (120%), chlorophyll content (57%), carotenoid content (10%), ascorbic acid (8%), proline (18%), total soluble solids (TSS, 49%), TSS to titration acidity (46%), phosphorus (72%), potassium (27%), zinc (24%), and Fe (17%). Zai et al. (2021) tested two fungi species, AMF- F. mosseae, and a phosphate-solubilizing fungus, Apophysomyces spartima, with Beach plum (Prunus maritima) host to understand the helpfulness of interaction in nutrient uptake and photosynthesis under a salt stress environment. The findings are also significant for nutrient uptake (P and N), and higher values for photosynthesis. Similarly, the study by Lee et al. (2015) demonstrates the alleviation of salt stress and improved maize growth through the combined application of G. etunicatum and Methylobacterium oryzae CBMB20.
4.5 Challenges and required advancement
4.5.1 Multiomics approaches
The study of multiomic techniques, including the integration of genome, metagenomics, metabolomics, volatilomics, and spectranomics data, in plant–fungal interactions appears to be scarce. However, such an application will provide an in-depth understanding of the biology and physiology of both the plant and the fungus when they are associated (Shakeel et al., 2022). These multiomic strategies can unravel the functional roles of different fungal species within agricultural ecosystems, improve our understanding of how co-infecting fungi may synergistically modulate the plant’s defense mechanisms, often leading to enhanced pathogenicity and altered nutrient acquisition strategies. Analyzing the microbial community dynamics beneath the plant surface through this methodology sheds light on how co-infection scenarios can lead to unique fungal interactions that ultimately impact plant productivity and health, including the identification of microbial modulation of key plants’ genetic potentials and metabolic pathways (Hori et al., 2021; Doni et al., 2022; Luo et al., 2022). Also, multiomics can facilitate the discovery of novel bioactive compounds produced during interaction, further expanding the toolkit for sustainable agriculture (Kumar et al., 2023). For instance, genomics studies can identify genes in microbes that are responsible for nutrient acquisition or phytohormone production, which in turn enhance plant growth (Wójcik et al., 2023). Transcriptomics investigations reveal how plants respond to microbes at the gene expression level, highlighting signaling pathways activated during symbiosis or pathogen defense (Van Dillewijn, 2008; Mukherjee, 2022). There are several limitations to using multiomic approaches. Such as in gene expression, replication of environmental conditions, complexity of microbial communities, and difficulties in obtaining sufficient materials (e.g., Rhizosphere fungi), its wide use is restricted (Chiquito-Contreras et al., 2024). Overall, in multitopic approaches, several limitations exist, including gaps in taxonomic or chemical compound databases, which can make comparison and verification challenging. Instead, higher costs for sample analysis, advanced technologies with specific infrastructure facilities, and a lack of expertise are the main considerations, particularly in developing countries. Those factors further restricted the application of multiomic techniques (Crandall et al., 2020).
4.5.2 Network mapping
Network mappings of plant–fungal interactions are crucial for understanding how microbes influence plant metabolism, stress responses, and immune systems (He et al., 2021). Of this, genome-wide association studies (GWAS) have been a recent focus; for example, Bergelson et al. (2019) identified a few significant Quantitative Trait Loci (QTLs) associated with Arabidopsis thaliana root microbial species richness and community structure. These QTLs are implicated in plant immunity, cell wall integrity, and the development of roots and root hairs. A study by He et al. (2021) developed a behavioral ecology model to define the strengths of mutualism, antagonism, aggression, and altruism between each pair of root microbes of A. thaliana. It showed the importance of such studies in selecting microbes that benefit sustainable plant growth. De Vries et al. (2018) indicated that the inherent mechanisms of microbial relationships in response to environmental disturbances can be uncovered by examining the properties of co-occurrence networks. Agler et al. (2016) noted that recognizing network hubs and their significance in microbial community structure has important implications for understanding interactions between microbes and can aid in the development of targeted biocontrol strategies in the future.
4.5.3 Influence of indigenous fungi
One of the overlooked aspects of plant–fungal interactions is the impact of indigenous fungi on plant growth and protection. Studying native fungi is limited, which could be due to their lower infection potential, and they may not be able to get sufficient inoculum that non-native fungi offer (Deguchi et al., 2021; Wu et al., 2021b). However, several studies have revealed that native fungi also have significant potential to enhance crop productivity in various dimensions, making their interaction with plants a crucial consideration (Koziol and Bever, 2023). One such example is the communication of plants through fungal mycelial networks. This is widely discussed in relation to mycorrhizal fungi; the fungal mycelia allow signals to be sent between plants. Recent research has demonstrated that these mycorrhizal networks serve as an information highway, facilitating the exchange of defense signals, allelochemicals, and nutritional resources (Luo et al., 2023). Nevertheless, this aspect has not been extensively discussed in the agricultural context, although it has been explored in other ecosystems (Gorzelak et al., 2015). On the one hand, this could be due to the shift in paradigm towards intensive, input-driven practices that prioritize high-yield crop production. Synthetic fertilizers and pesticides became the standard tools for increasing productivity, as they provided immediate and visible benefits to plant growth. However, these activities may alter soil properties and accumulate pollutants, which could negatively impact indigenous or native fungi, thereby hindering their potential benefits (Semenov et al., 2022; Steiner et al., 2024). On the other hand, the intricacy of biological interactions is not attracting researchers, as it is a challenging task (Aleklett et al., 2021).
4.5.4 Commercial fungal formulations
It has long been recognized that the importance of fungal biocontrol agents is higher among academic communities (Wallis and Sisterson, 2024). In contrast, the commercial application is still not widely adopted worldwide due to its limitations, including a short shelf life, reduced efficacy, commercialization issues, and legislative procedures (Bamisile et al., 2021; Palmieri et al., 2022). Production quality control is one of the crucial steps in fungi formulations. The quality control process initiates with evaluating the inoculants, microbial strains, carriers, and the final product, as well as ensuring proper labeling and commercialization. To obtain legal acceptance, it is essential to properly identify fungi species or strains prior to mass cultivation. Furthermore, the cultures must be sterile, with high viability and germination capacity. Regulation regimes in the European Union and India specifically concern minimum viable spores and acceptable variation in microorganism concentrations (Ghorui et al., 2025).
When discussing commercial formulations, it has been recognized that the importance of developed fungal strains extends beyond indigenous fungi. In the current complex agricultural system, indigenous fungi may not be the most competitive option; however, it is also essential to identify more effective species for future formulations (Gohel et al., 2022; Díaz- Urbano et al., 2023; Erdoğan and Sağlan, 2023). Keep in mind that one of the foremost things to discuss in the fungal formulations is the viability of fungi. It is typically expected to be effective for at least 6 months and preferably for about 2 years. The formulation could be liquid-based or solid-based; however, both forms have their advantages and disadvantages. The liquid media could be easily controlled and supplemented with nutrients, although it required complex fermentation equipment. The solid-based formulations are relatively simple; in contrast, it is a challenge to supply the necessary nutrients to the fungi, and maintaining the viability of the fungal inoculum under low water content in the formulations can be complex (Teixidó et al., 2022). The researchers also made significant efforts to enhance the packaging and shelf life of fungal formulations. For example, Jeong et al. (2022) utilized modified atmosphere packaging (MAP) to prolong the shelf life of Metarhizium anisopliae conidia. They have found that MAP with 30% CO2 + 70% N2 retains 80.5% conidial viability after 28 days. However, the challenge here is the storage condition, as achieving higher shelf life with higher conidial viability requires cold storage facilities. Jeong et al. (2022) reported that MAP-treated conidia have a longer half-life when stored at 4°C compared to 25°C. In an independent study, Silva et al. (2024) attempted to extend the shelf life of a rice flour-granular formulation based on Trichoderma conidial biopesticide. Similar to the result of Jeong et al. (2022), Silva et al. (2024) evidenced that cold storage of the formulation is necessary for a longer shelf life (up to 24 months), which may not always be convenient for sellers or farmers in practice, particularly in developing countries.
Scientific evidence also indicated that the demerits of such applications—could be highly specialized against the targeted disease or the pathogen, and the controlling ability is highly dependent on the various biotic (e.g., host plant, metabolites and enzymes synthesized by host plant, and natural microflora of the host plant and their interaction) and abiotic (e.g., microelements, nutrients, pH, temperature, ultraviolet (UV) radiation, and water activity) conditions (Bonaterra et al., 2012). Therefore, the prediction of the effectiveness when applied to the field could be reduced, inconsistent, and dependent (Boro et al., 2022). For example, the study by Wei et al. (2019) can provide insight; the researchers tested the endophytic fungus Fusarium solani against Verticillium dahliae in Cotton plants. They have found that the biocontrol potential of the fungi is higher in efficacy in the greenhouse experiment. In contrast, when tested in the field, the efficacy decreased (Table 1). Nevertheless, numerous studies have shown that fungi biocontrol agents can be effective against phytopathogens; however, these findings are limited to greenhouse experiments (Herrera et al., 2020; Catambacan and Cumagun, 2021; Safari Motlagh et al., 2022).
5 Prospects
Along with the advancement of technology, a significant understanding of plant-fungal interactions has been gained today. The mechanisms behind phytopathogenic fungal infection, host immunity, and disease susceptibility have been extensively studied at the molecular level. However, due to the complex nature of those associations, considerable studies still need to be conducted. For instance, studies on plant–fungal interactions under co-infection and co-inoculation (synergistic) of beneficial fungi are limited and have not revealed the proper association.
Comparing plant–fungal interactions between natural and agricultural systems is important for future research. Such comparisons can help identify general mechanisms of the interaction that cope with environmental stresses, especially with the aid of modern omics technologies. Additionally, there is a potential for obtaining fungal strains that are beneficial for crop health. Understanding and harnessing the interactions between plants and fungi can significantly improve agricultural practices. Nevertheless, understanding the conditions that need to be overcome and carefully selecting the type of symbiotic fungi group is vital to achieving the best results.
Furthermore, future studies should prioritize identifying new symbiotic relationships, unraveling the mechanisms of plant interaction, and exploring the various effects of these relationships on plants. Additionally, the use of indigenous fungi to enhance agricultural productivity and crop protection through their interaction with host plants could be a promising area for future research. In the context of disease control, a critical but often overlooked consideration is the prevention of virus infections in plants. However, a few studies have highlighted the potential of plant–fungal interactions in controlling these viruses, underscoring the importance of this issue and the need for further research.
6 Conclusion
The pathogenic association between a fungus and a plant is one of the most recognized phenomena in nature. The use of the Scopus database only to extract data could hinder the precise development of the field, as many publications are not included in this database. Nevertheless, the platform is also vital for extracting the most acknowledged studies in the field, as well as for gaining an overall picture of the field. According to the bibliometric analysis, it is evident that studies on the interaction between plants and fungi have increased steadily over the last three decades. Notably, China has emerged as a leading contributor to the field of study, accompanied by a dynamic cohort of early-career and emerging authors/researchers, a pattern that mirrors the global shift toward “green” agricultural paradigms. Yet, despite this momentum, the evidence base remains uneven across climatic zones and production systems, underscoring the need to expand inquiry across tropical and temperate agriculture contexts and to prioritize research on the world’s top-ranked major crops and high-value crops to maximize impact. On the one hand, plant–pathogen interactions continue to impose significant yield and quality losses due to the phytopathogens, reinforcing the need for improved surveillance, resistance breeding, and integrated disease management. On the other hand, mutualistic partnerships—particularly with mycorrhizal fungi and endophytes—offer compelling avenues to enhance plant growth and development, increase efficiency of water and nutrient acquisition, modulate host immunity, and confer tolerance to abiotic stressors such as drought, salinity, and heat, thus playing a significant role in climate-resilience. Strategically fostering these beneficial symbioses can reduce reliance on synthetic fertilizers and pesticides, with attendant co-benefits for soil health, biodiversity, and farm profitability. Realizing this potential, however, requires extended field-based experimental designs, standardized metadata and reporting, and integrative approaches that connect molecular mechanisms. Although it is essential to conduct advanced research (e.g., multi-omics studies), this has not been practiced adequately, particularly in developing countries, due to limited technological and financial resources; thus, the need for such involvement is pressing. Collectively, our mapping and synthesis highlight both the opportunities and constraints of plant–fungal interactions, advancing a research agenda that can translate fundamental insights into scalable, environmentally responsible strategies to safeguard productivity while reducing external inputs.
Data availability statement
The data used for bibliometric analysis can be obtained by writing to the corresponding author.
Author contributions
SA: Project administration, Writing – review & editing, Supervision, Visualization, Validation, Conceptualization. MC: Writing – review & editing, Formal Analysis. AP: Software, Methodology, Conceptualization, Writing – review & editing, Writing – original draft. PG: Writing – review & editing, Formal Analysis. JL: Writing – review & editing, Formal Analysis. ZS: Writing – review & editing, Formal Analysis. ST: Validation, Writing – review & editing, Supervision. CN: Writing – review & editing, Formal Analysis. MQ: Investigation, Writing – review & editing. MA: Writing – review & editing, Investigation. XK: Writing – review & editing, Investigation. YX: Writing – review & editing, Data curation. ZL: Writing – review & editing, Investigation. SK: Writing – review & editing, Conceptualization, Supervision, Validation, Visualization. JZ: Funding acquisition, Data curation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the International Union Laboratory of China and Malaysia for Quality Monitoring and Evaluation of Agricultural Products in Yunnan (Grant number: 202403AP140027); the Research on Coffee Quality Control and Utilization of Residues Based on Nano multifunctional Materials and Endophytic Fungi (Grant number: FWCY-ZNT2024030); Young Talents Special Fund of Yunnan Provincial “Promoting Yunnan Talents,” (Grant number: XDYC-QNRC-2022-0108); Basic Research in Local Undergraduate Universities, Fund of Yunnan Provincial Science and Technology Department Nos. 202101BA070001-025; 202301BA070001-126, and “Jianqiang Zhang funded the APC”.
Acknowledgments
Jianqiang Zhang thanks for the financial support from the Science and Technology Plan Project of Yunnan Provincial Science and Technology Department; the Basic Research in Local Undergraduate Universities Fund of Yunnan Provincial Science and Technology Department (202101BA070001-025; 202301BA070001-126) and Yunnan China–Laos International Joint Research and Development Center for Energy and Mineral Analysis and Technology Innovation (202303AP140019). Samantha C. Karunarathna and Saowaluck Tibpromma thank the National Natural Science Foundation of China (NSFC 32260004), the Yunnan Revitalization Talents Support Plan (“High-End Foreign Experts” Program), and the Key Laboratory of Yunnan Provincial Department of Education of the Deep-Time Evolution on Biodiversity from the Origin of the Pearl River, for their support. Suhail Asad thanks Start-up funding for the high-level talent research, Pu’er University, China (2023142500).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1612428/full#supplementary-material
Footnotes
References
Abdullah, A. S., Moffat, C. S., Lopez-Ruiz, F. J., Gibberd, M. R., Hamblin, J., and Zerihun, A. (2017). Host–multi-pathogen warfare: pathogen interactions in co-infected plants. Front. Plant Sci. 8:806. doi: 10.3389/fpls.2017.01806
Acosta-Rodríguez, I., Cárdenas-González, J. F., Rodríguez Pérez, A. S., Oviedo, J. T., and Martínez-Juárez, V. M. (2018). Bioremoval of different heavy metals by the resistant fungal strain Aspergillus niger. Bioinorg. Chem. Appl. 2018, 1–7. doi: 10.1155/2018/3457196
Agbessenou, A., Akutse, K. S., Yusuf, A. A., Ekesi, S., Subramanian, S., and Khamis, F. M. (2020). Endophytic fungi protect tomato and nightshade plants against Tuta absoluta (Lepidoptera: Gelechiidae) through a hidden friendship and cryptic battle. Sci. Rep. 10:22195. doi: 10.1038/s41598-020-78898-8
Agler, M. T., Ruhe, J., Kroll, S., Morhenn, C., Kim, S.-T., Weigel, D., et al. (2016). Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 14:e1002352. doi: 10.1371/journal.pbio.1002352
Ahlawat, O. P., Yadav, D., Kashyap, P. L., Khippal, A., and Singh, G. (2022). Wheat endophytes and their potential role in managing abiotic stress under changing climate. J. Appl. Microbiol. 132, 2501–2520. doi: 10.1111/jam.15375
Ahmad, I., Ansari, M. I., and Aqil, F. (2006). Biosorption of Ni, Cr and cd by metal tolerant Aspergillus niger and Penicillium sp. using single and multi-metal solution. Indian J. Exp. Biol. 44, 73–76
Akhtar, N., Wani, A. K., Dhanjal, D. S., and Mukherjee, S. (2022). Insights into the beneficial roles of dark septate endophytes in plants under challenging environment: resilience to biotic and abiotic stresses. World J. Microbiol. Biotechnol. 38:79. doi: 10.1007/s11274-022-03264-x
Akram, S., Ahmed, A., He, P., He, P., Liu, Y., Wu, Y., et al. (2023). Uniting the role of endophytic fungi against plant pathogens and their interaction. J. Fungi 9, 72, doi: 10.3390/jof9010072
Alberton, O., Kuyper, T. W., and Summerbell, R. C. (2010). Dark septate root endophytic fungi increase growth of scots pine seedlings under elevated CO2 through enhanced nitrogen use efficiency. Plant Soil 328, 459–470. doi: 10.1007/s11104-009-0125-8
Aleklett, K., Ohlsson, P., Bengtsson, M., and Hammer, E. C. (2021). Fungal foraging behaviour and hyphal space exploration in micro-structured soil chips. ISME J. 15, 1782–1793. doi: 10.1038/s41396-020-00886-7
Ali, S., Mir, Z. A., Bhat, J. A., Tyagi, A., Chandrashekar, N., Yadav, P., et al. (2018). Isolation and characterization of systemic acquired resistance marker gene PR1 and its promoter from Brassica juncea. 3 Biotech 8:10. doi: 10.1007/s13205-017-1027-8
Antón-Herrero, R., Chicca, I., García-Delgado, C., Crognale, S., Lelli, D., Gargarello, R. M., et al. (2023). Main factors determining the scale-up effectiveness of mycoremediation for the decontamination of aliphatic hydrocarbons in soil. J. Fungi 9:1205. doi: 10.3390/jof9121205
Asad, S., Priyashantha, A. K. H., Tibpromma, S., Luo, Y., Zhang, J., Fan, Z., et al. (2023). Coffee-associated endophytes: plant growth promotion and crop protection. Biology 12:911. doi: 10.3390/biology12070911
Asiminicesei, D.-M., Fertu, D. I., and Gavrilescu, M. (2024). Impact of heavy metal pollution in the environment on the metabolic profile of medicinal plants and their therapeutic potential. Plants 13:913. doi: 10.3390/plants13060913
Azuddin, N. F., Mohd, M. H., Rosely, N. F. N., Mansor, A., and Zakaria, L. (2021). Molecular phylogeny of endophytic fungi from rattan (Calamus castaneus Griff.) spines and their antagonistic activities against plant pathogenic fungi. J. Fungi 7:301. doi: 10.3390/jof7040301
Baldrian, P., Větrovský, T., Lepinay, C., and Kohout, P. (2022). High-throughput sequencing view on the magnitude of global fungal diversity. Fungal Divers. 114, 539–547. doi: 10.1007/s13225-021-00472-y
Balestrini, R. (2021). Grand challenges in fungi-plant interactions. Front. Fungal Biol. 2:750003. doi: 10.3389/ffunb.2021.750003
Balint-Kurti, P. (2019). The plant hypersensitive response: concepts, control and consequences. Mol. Plant Pathol. 20, 1163–1178. doi: 10.1111/mpp.12821
Bamisile, B. S., Afolabi, O. G., Siddiqui, J. A., and Xu, Y. (2023). Endophytic insect pathogenic fungi-host plant-herbivore mutualism: elucidating the mechanisms involved in the tripartite interactions. World J. Microbiol. Biotechnol. 39:326. doi: 10.1007/s11274-023-03780-4
Bamisile, B. S., Siddiqui, J. A., Akutse, K. S., Ramos Aguila, L. C., and Xu, Y. (2021). General limitations to endophytic entomopathogenic fungi use as plant growth promoters, pests and pathogens biocontrol agents. Plants 10:2119. doi: 10.3390/plants10102119
Barrett, L. G., Zala, M., Mikaberidze, A., Alassimone, J., Ahmad, M., McDonald, B. A., et al. (2021). Mixed infections alter transmission potential in a fungal plant pathogen. Environ. Microbiol. 23, 2315–2330. doi: 10.1111/1462-2920.15417
Barzegari Barogh, R., Hassanpanah, D., Esmaeilpour, B., Godehkahriz, S. J., and Kalateh Jari, S. (2023). Co-inoculation of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria improve growth, biochemical attributes, and nutritional status of potato (Solanum tuberosum L.) minitubers. J. Soil Sci. Plant Nutr. 23, 3447–3460. doi: 10.1007/s42729-023-01262-y
Begum, N., Qin, C., Ahanger, M. A., Raza, S., Khan, M. I., Ashraf, M., et al. (2019). Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front. Plant Sci. 10:1068. doi: 10.3389/fpls.2019.01068
Bell, C. A., Magkourilou, E., Barker, H., Barker, A., Urwin, P. E., and Field, K. J. (2023). Arbuscular mycorrhizal fungal-induced tolerance is determined by fungal identity and pathogen density. Plants People Planet 5, 241–253. doi: 10.1002/ppp3.10338
Benítez, M.-S., Hersh, M. H., Vilgalys, R., and Clark, J. S. (2013). Pathogen regulation of plant diversity via effective specialization. Trends Ecol. Evol. 28, 705–711. doi: 10.1016/j.tree.2013.09.005
Bergelson, J., Mittelstrass, J., and Horton, M. W. (2019). Characterizing both bacteria and fungi improves understanding of the Arabidopsis root microbiome. Sci. Rep. 9:24. doi: 10.1038/s41598-018-37208-z
Beslemes, D., Tigka, E., Roussis, I., Kakabouki, I., Mavroeidis, A., and Vlachostergios, D. (2023). Effect of arbuscular mycorrhizal fungi on nitrogen and phosphorus uptake efficiency and crop productivity of two-rowed barley under different crop production systems. Plants 12:1908. doi: 10.3390/plants12091908
Bitterlich, M., Sandmann, M., and Graefe, J. (2018). Arbuscular mycorrhiza alleviates restrictions to substrate water flow and delays transpiration limitation to stronger drought in tomato. Front. Plant Sci. 9:154. doi: 10.3389/fpls.2018.00154
Boczoń, A., Hilszczańska, D., Wrzosek, M., Szczepkowski, A., and Sierota, Z. (2021). Drought in the forest breaks plant–fungi interactions. Eur. J. Forest Res. 140, 1301–1321. doi: 10.1007/s10342-021-01409-5
Boluspayeva, L., Jakubus, M., Spychalski, W., Abzhalelov, A., and Bitmanov, Y. (2022). Health risk of heavy metals related to consumption of vegetables in areas of industrial impact in the republic of Kazakhstan—case study for Oskemen. Int. J. Environ. Res. Public Health 20:275. doi: 10.3390/ijerph20010275
Bonaterra, A., Badosa, E., Cabrefiga, J., Francés, J., and Montesinos, E. (2012). Prospects and limitations of microbial pesticides for control of bacterial and fungal pomefruit tree diseases. Trees (Berl. West) 26, 215–226. doi: 10.1007/s00468-011-0626-y
Boorboori, M. R., and Zhang, H.-Y. (2022). Arbuscular mycorrhizal fungi are an influential factor in improving the phytoremediation of arsenic, cadmium, lead, and chromium. J. Fungi 8:176. doi: 10.3390/jof8020176
Boro, M., Sannyasi, S., Chettri, D., and Verma, A. K. (2022). Microorganisms in biological control strategies to manage microbial plant pathogens: a review. Arch. Microbiol. 204:666. doi: 10.1007/s00203-022-03279-w
Borymski, S., Cycoń, M., Beckmann, M., Mur, L. A. J., and Piotrowska-Seget, Z. (2018). Plant species and heavy metals affect biodiversity of microbial communities associated with metal-tolerant plants in metalliferous soils. Front. Microbiol. 9:1425. doi: 10.3389/fmicb.2018.01425
Calvo-Polanco, M., Molina, S., Zamarreño, A. M., García-Mina, J. M., and Aroca, R. (2014). The symbiosis with the arbuscular mycorrhizal fungus Rhizophagus irregularis drives root water transport in flooded tomato plants. Plant Cell Physiol. 55, 1017–1029. doi: 10.1093/pcp/pcu035
Casadevall, A., and Pirofski, L. A. (1999). Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect. Immun. 67, 3703–3713. doi: 10.1128/IAI.67.8.3703-3713.1999
Casadevall, A., and Pirofski, L.-A. (2000). Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect. Immun. 68, 6511–6518. doi: 10.1128/iai.68.12.6511-6518.2000
Castaño, C., Suarez-Vidal, E., Zas, R., Bonet, J. A., Oliva, J., and Sampedro, L. (2023). Ectomycorrhizal fungi with hydrophobic mycelia and rhizomorphs dominate in young pine trees surviving experimental drought stress. Soil Biol. Biochem. 178:108932. doi: 10.1016/j.soilbio.2022.108932
Catambacan, D. G., and Cumagun, C. J. R. (2021). Weed-associated fungal endophytes as biocontrol agents of Fusarium oxysporum f. sp. cubense TR4 in Cavendish banana. J. Fungi 7:224. doi: 10.3390/jof7030224
Ceustermans, A., Van Hemelrijck, W., Van Campenhout, J., and Bylemans, D. (2018). Effect of arbuscular mycorrhizal fungi on Pratylenchus penetrans infestation in apple seedlings under greenhouse conditions. Pathogens 7:76. doi: 10.3390/pathogens7040076
Chakraborty, S., and Datta, S. (2003). How will plant pathogens adapt to host plant resistance at elevated CO2 under a changing climate? New Phytol. 159, 733–742. doi: 10.1046/j.1469-8137.2003.00842.x
Chaturvedi, R., Favas, P. J. C., Pratas, J., Varun, M., and Paul, M. S. (2021). Harnessing Pisum sativum–Glomus mosseae symbiosis for phytoremediation of soil contaminated with lead, cadmium, and arsenic. Int. J. Phytoremediation 23, 279–290. doi: 10.1080/15226514.2020.1812507
Chen, X., Wang, Y., Wang, Y., Zhang, Y., Shen, Y., He, X., et al. (2023). A natural moisture gradient affects soil fungal communities on the south shore of Hulun Lake, Inner Mongolia, China. J. Fungi 9:549. doi: 10.3390/jof9050549
Chen, Q., Wu, W.-W., Qi, S.-S., Cheng, H., Li, Q., Ran, Q., et al. (2021). Arbuscular mycorrhizal fungi improve the growth and disease resistance of the invasive plant Wedelia trilobata. J. Appl. Microbiol. 130, 582–591. doi: 10.1111/jam.14415
Chen, B., Xiao, X., Zhu, Y.-G., Smith, F. A., Miao Xie, Z., and Smith, S. E. (2007). The arbuscular mycorrhizal fungus Glomus mosseae gives contradictory effects on phosphorus and arsenic acquisition by Medicago sativa Linn. Sci. Total Environ. 379, 226–234. doi: 10.1016/j.scitotenv.2006.07.038
Chi, B.-B., Lu, Y.-N., Yin, P.-C., Liu, H.-Y., Chen, H.-Y., and Shan, Y. (2021). Sequencing and comparative genomic analysis of a highly metal-tolerant Penicillium janthinellum P1 provide insights into its metal tolerance. Front. Microbiol. 12:663217. doi: 10.3389/fmicb.2021.663217
Chiquito-Contreras, C. J., Meza-Menchaca, T., Guzmán-López, O., Vásquez, E. C., and Ricaño-Rodríguez, J. (2024). Molecular insights into plant-microbe interactions: a comprehensive review of key mechanisms. Front. Biosci. (Elite Ed.) 16:9. doi: 10.31083/j.fbe1601009
Christian, N., Espino Basurto, B., Toussaint, A., Xu, X., Ainsworth, E. A., Busby, P. E., et al. (2021). Elevated carbon dioxide reduces a common soybean leaf endophyte. Glob. Chang. Biol. 27, 4154–4168. doi: 10.1111/gcb.15716
Comita, L. S., Queenborough, S. A., Murphy, S. J., Eck, J. L., Xu, K., Krishnadas, M., et al. (2014). Testing predictions of the Janzen–Connell hypothesis: a meta-analysis of experimental evidence for distance- and density-dependent seed and seedling survival. J. Ecol. 102, 845–856. doi: 10.1111/1365-2745.12232
Constantin, M. E., de Lamo, F. J., Vlieger, B. V., Rep, M., and Takken, F. L. W. (2019). Endophyte-mediated resistance in tomato to Fusarium oxysporum is independent of ET, JA, and SA. Front. Plant Sci. 10:979. doi: 10.3389/fpls.2019.00979
Crandall, S. G., Gold, K. M., Jiménez-Gasco, M. D. M., Filgueiras, C. C., and Willett, D. S. (2020). A multi-omics approach to solving problems in plant disease ecology. PLoS One 15:e0237975. doi: 10.1371/journal.pone.0237975
Cruz, A. F., Ishii, T., and Kadoya, K. (2000). Effects of arbuscular mycorrhizal fungi on tree growth, leaf water potential, and levels of 1-aminocyclopropane-1-carboxylic acid and ethylene in the roots of papaya under water-stress conditions. Mycorrhiza 10, 121–123. doi: 10.1007/s005720000067
Dai, D.-Q., Suwannarach, N., Bamunuarachchige, T. C., and Karunarathna, S. C. (2023). Editorial: plant-fungal interactions. Front. Microbiol. 14:394. doi: 10.3389/fmicb.2023.1236394
Dastogeer, K. M. G. (2018). Influence of fungal endophytes on plant physiology is more pronounced under stress than well-watered conditions: a meta-analysis. Planta 248, 1403–1416. doi: 10.1007/s00425-018-2982-y
Davies, C. R., Wohlgemuth, F., Young, T., Violet, J., Dickinson, M., Sanders, J.-W., et al. (2021). Evolving challenges and strategies for fungal control in the food supply chain. Fungal Biol. Rev. 36, 15–26. doi: 10.1016/j.fbr.2021.01.003
de Lamo, F. J., and Takken, F. L. W. (2020). Biocontrol by Fusarium oxysporum using endophyte-mediated resistance. Front. Plant Sci. 11:37. doi: 10.3389/fpls.2020.00037
de Vries, F. T., Griffiths, R. I., Bailey, M., Craig, H., Girlanda, M., Gweon, H. S., et al. (2018). Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 9:3033. doi: 10.1038/s41467-018-05516-7
Deguchi, S., Yagi, T., and Ohtomo, R. (2021). Low indigenous AM fungal activity would be a necessary but not sufficient condition for effective utilization of exogenous AM fungal inoculum to forage corn. Soil Sci. Plant Nutr. 67, 50–56. doi: 10.1080/00380768.2020.1838234
Díaz- Urbano, M., Goicoechea, N., Velasco, P., and Poveda, J. (2023). Development of agricultural bio-inoculants based on mycorrhizal fungi and endophytic filamentous fungi: co-inoculants for improve plant-physiological responses in sustainable agriculture. Biol. Control 182:105223. doi: 10.1016/j.biocontrol.2023.105223
Domka, A. M., Rozpaądek, P., and Turnau, K. (2019). Are fungal endophytes merely mycorrhizal copycats? The role of fungal endophytes in the adaptation of plants to metal toxicity. Front. Microbiol. 10:371. doi: 10.3389/fmicb.2019.00371
Doni, F., Miranti, M., Mispan, M. S., Mohamed, Z., and Uphoff, N. (2022). Multi-omics approaches for deciphering the microbial modulation of plants’ genetic potentials: what’s known and what’s next? Rhizosphere 24:100613. doi: 10.1016/j.rhisph.2022.100613
Dorneles, K. R., Refatti, J. P., Pazdiora, P. C., de Avila, L. A., Deuner, S., and Dallagnol, L. J. (2020). Biochemical defenses of rice against Bipolaris oryzae increase with high atmospheric concentration of CO2. Physiol. Mol. Plant Pathol. 110:101484. doi: 10.1016/j.pmpp.2020.101484
Du, T.-Y., Karunarathna, S. C., Zhang, X., Dai, D.-Q., Mapook, A., Suwannarach, N., et al. (2022). Endophytic fungi associated with Aquilaria sinensis (agarwood) from China show antagonism against bacterial and fungal pathogens. J. Fungi 8:1197. doi: 10.3390/jof8111197
Erdoğan, O., and Sağlan, Z. (2023). Antifungal activity of local isolates of Beauveria bassiana (Balsamo) Vuillemin against Verticillium dahliae Kleb. Causing wilt disease of cotton. Egypt. J. Biol. Pest Control 33:684. doi: 10.1186/s41938-023-00684-1
Erlandson, S. R., Savage, J. A., Cavender-Bares, J. M., and Peay, K. G. (2016). Soil moisture and chemistry influence diversity of ectomycorrhizal fungal communities associating with willow along an hydrologic gradient. FEMS Microbiol. Ecol. 92:fiv148. doi: 10.1093/femsec/fiv148
Fakhro, A., Andrade-Linares, D. R., von Bargen, S., Bandte, M., Büttner, C., Grosch, R., et al. (2010). Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens. Mycorrhiza 20, 191–200. doi: 10.1007/s00572-009-0279-5
Fan, T., Liu, R., Pan, D., Liu, Y., Ye, W., Lu, H., et al. (2022). Accumulation and subcellular distribution of cadmium in rygegrass induced by Aspergillus niger TL-F2 and Aspergillus flavus TL-F3. Int. J. Phytoremediation 24, 263–270. doi: 10.1080/15226514.2021.1932734
Fang, X., Zhang, C., Wang, Z., Duan, T., Yu, B., Jia, X., et al. (2021). Co-infection by soil-borne fungal pathogens alters disease responses among diverse alfalfa varieties. Front. Microbiol. 12:385. doi: 10.3389/fmicb.2021.664385
Fasani, E., Li, M., Varotto, C., Furini, A., and DalCorso, G. (2022). Metal detoxification in land plants: from bryophytes to vascular plants. State of the art and opportunities. Plants 11:237. doi: 10.3390/plants11030237
Flemming, C. A., Ferris, F. G., Beveridge, T. J., and Bailey, G. W. (1990). Remobilization of toxic heavy metals adsorbed to bacterial wall-clay composites. Appl. Environ. Microbiol. 56, 3191–3203. doi: 10.1128/aem.56.10.3191-3203.1990
Frew, A. (2023). Water availability alters the community structure of arbuscular mycorrhizal fungi and determines plant mycorrhizal benefit. Plants People Planet 5, 683–689. doi: 10.1002/ppp3.10372
Gajewska, J., Floryszak-Wieczorek, J., Sobieszczuk-Nowicka, E., Mattoo, A., and Arasimowicz-Jelonek, M. (2022). Fungal and oomycete pathogens and heavy metals: an inglorious couple in the environment. IMA Fungus 13:6. doi: 10.1186/s43008-022-00092-4
Gasemi, S., Mahdavikia, H., Rezaei-Chiyaneh, E., Banaei-Asl, F., Dolatabadian, A., and Sadeghpour, A. (2023). Co-inoculation of mycorrhizal fungi and plant growth-promoting rhizobacteria improve growth, biochemical and physiological attributes in Dracocephalum kotschyi Boiss. Under water deficit stress. PeerJ 11:e16474. doi: 10.7717/peerj.16474
Gautam, N., Tiwari, M., Kidwai, M., Dutta, P., and Chakrabarty, D. (2023). Functional characterization of rice metallothionein OsMT-I-id: insights into metal binding and heavy metal tolerance mechanisms. J. Hazard. Mater. 458:131815. doi: 10.1016/j.jhazmat.2023.131815
Ghorui, M., Chowdhury, S., and Burla, S. (2025). Recent advances in the commercial formulation of arbuscular mycorrhizal inoculants. Front. Ind. Microbiol. 3:472. doi: 10.3389/finmi.2025.1553472
Godfray, H. C. J., Mason-D’Croz, D., and Robinson, S. (2016). Food system consequences of a fungal disease epidemic in a major crop. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 371:20150467. doi: 10.1098/rstb.2015.0467
Gohel, N. M., Raghunandan, B. L., Patel, N. B., Parmar, H. V., and Raval, D. B. (2022). “Role of fungal biocontrol agents for sustainable agriculture” in Fungal Biology. ed. N. M. Gohel (Singapore: Springer Nature Singapore), 577–606.
Gorzelak, M. A., Asay, A. K., Pickles, B. J., and Simard, S. W. (2015). Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AoB Plants 7:lv050. doi: 10.1093/aobpla/plv050
Gu, H.-H., Zhou, Z., Gao, Y.-Q., Yuan, X.-T., Ai, Y.-J., Zhang, J.-Y., et al. (2017). The influences of arbuscular mycorrhizal fungus on phytostabilization of lead/zinc tailings using four plant species. Int. J. Phytoremediation 19, 739–745. doi: 10.1080/15226514.2017.1284751
Habib-ur-Rahman, M., Ahmad, A., Raza, A., Hasnain, M. U., Alharby, H. F., Alzahrani, Y. M., et al. (2022). Impact of climate change on agricultural production; issues, challenges, and opportunities in Asia. Front. Plant Sci. 13:548. doi: 10.3389/fpls.2022.925548
He, X., Zhang, Q., Li, B., Jin, Y., Jiang, L., and Wu, R. (2021). Network mapping of root–microbe interactions in Arabidopsis thaliana. NPJ Biofilms Microbiomes 7:72. doi: 10.1038/s41522-021-00241-4
Herrera, W., Valbuena, O., and Pavone-Maniscalco, D. (2020). Formulation of Trichoderma asperellum TV190 for biological control of Rhizoctonia solani on corn seedlings. Egypt. J. Biol. Pest Control 30:246. doi: 10.1186/s41938-020-00246-9
Hibberd, J. M., Whitbread, R., and Farrar, J. F. (1996). Effect of elevated concentrations of CO2 on infection of barley by Erysiphe graminis. Physiol. Mol. Plant Pathol. 48, 37–53. doi: 10.1006/pmpp.1996.0004
Hori, Y., Fujita, H., Hiruma, K., Narisawa, K., and Toju, H. (2021). Synergistic and offset effects of fungal species combinations on plant performance. Front. Microbiol. 12:713180. doi: 10.3389/fmicb.2021.713180
Hubbard, M., Germida, J. J., and Vujanovic, V. (2014). Fungal endophytes enhance wheat heat and drought tolerance in terms of grain yield and second-generation seed viability. J. Appl. Microbiol. 116, 109–122. doi: 10.1111/jam.12311
Ikram, M., Ali, N., Jan, G., Jan, F. G., Rahman, I. U., Iqbal, A., et al. (2018). IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS One 13:e0208150. doi: 10.1371/journal.pone.0208150
Iram, S., Basri, R., Ahmad, K. S., and Jaffri, S. B. (2019). Mycological assisted phytoremediation enhancement of bioenergy crops Zea mays and Helianthus annuus in heavy metal contaminated lithospheric zone. Soil Sediment Contam. 28, 411–430. doi: 10.1080/15320383.2019.1597011
Irawati, A. F. C., Mutaqin, K. H., Suhartono, M. T., and Widodo, W. (2020). The effect of application endophytic fungus Trichoderma spp. and Fusarium spp. to control bacterial wilt in chilli pepper. Walailak J. Sci. Tech. 17, 559–569. doi: 10.48048/wjst.2020.3432
Islam, S. M. N., Chowdhury, M. Z. H., Mim, M. F., Momtaz, M. B., and Islam, T. (2023). Biocontrol potential of native isolates of Beauveria bassiana against cotton leafworm Spodoptera litura (Fabricius). Sci. Rep. 13:8331. doi: 10.1038/s41598-023-35415-x
Jaber, L. R., and Salem, N. M. (2014). Endophytic colonisation of squash by the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) for managing zucchini yellow mosaic virusin cucurbits. Biocontrol Sci. Tech. 24, 1096–1109. doi: 10.1080/09583157.2014.923379
Jańczak-Pieniążek, M., Cichoński, J., Michalik, P., and Chrzanowski, G. (2022). Effect of heavy metal stress on phenolic compounds accumulation in winter wheat plants. Molecules 28:241. doi: 10.3390/molecules28010241
Janni, M., Maestri, E., Gullì, M., Marmiroli, M., and Marmiroli, N. (2023). Plant responses to climate change, how global warming may impact on food security: a critical review. Front. Plant Sci. 14:1297569. doi: 10.3389/fpls.2023.1297569
Jeong, S.-G., Kim, H. M., Kim, J., Kim, J. S., and Park, H. W. (2022). Effect of storage conditions on the shelf-life extension of fungus-colonized substrates based on Metarhizium anisopliae using modified atmosphere packaging. Sci. Rep. 12:423. doi: 10.1038/s41598-021-04232-5
Ji, T., Languasco, L., Li, M., and Rossi, V. (2021). Effects of temperature and wetness duration on infection by Coniella diplodiella, the fungus causing white rot of grape berries. Plants 10:1696. doi: 10.3390/plants10081696
Jiang, D., Tan, M., Wu, S., Zheng, L., Wang, Q., Wang, G., et al. (2021). Defense responses of arbuscular mycorrhizal fungus-colonized poplar seedlings against gypsy moth larvae: a multiomics study. Hortic. Res. 8:245. doi: 10.1038/s41438-021-00671-3
Joachin, J., Kritzell, C., Lagueux, E., Luecke, N. C., and Crawford, K. M. (2023). Climate change and plant-microbe interactions: water-availability influences the effective specialization of a fungal pathogen. Fungal Ecol. 66:101286. doi: 10.1016/j.funeco.2023.101286
Joshi-Tope, G., and Francis, A. J. (1995). Mechanisms of biodegradation of metal-citrate complexes by Pseudomonas fluorescens. J. Bacteriol. 177, 1989–1993. doi: 10.1128/jb.177.8.1989-1993.1995
Karnosky, D. F., Percy, K. E., Xiang, B., Callan, B., Noormets, A., Mankovska, B., et al. (2002). Interacting elevated CO2 and tropospheric O3 predisposes aspen (Populus tremuloides Michx.) to infection by rust (Melampsora medusae f. sp. tremuloidae). Glob. Chang. Biol. 8, 329–338. doi: 10.1046/j.1354-1013.2002.00479.x
Kaur, R., and Saxena, S. (2023). Penicillium citrinum, a drought-tolerant endophytic fungus isolated from wheat (Triticum aestivum L.) leaves with plant growth-promoting abilities. Curr. Microbiol. 80:184. doi: 10.1007/s00284-023-03283-3
Khaekhum, S., Ekprasert, J., Suebrasri, T., Mongkolthanaruk, W., Riddech, N., Jogloy, S., et al. (2021a). The first member of Exserohilum rostratum beneficial for promoting growth and yield of sunchoke (Helianthus tuberosus L.). Rhizosphere 19:100379. doi: 10.1016/j.rhisph.2021.100379
Khaekhum, S., Ekprasert, J., Suebrasri, T., Seemakram, W., Mongkolthanaruk, W., Riddech, N., et al. (2021b). Co-inoculation of an endophytic and arbuscular mycorrhizal fungus improve growth and yield of Helianthus tuberosus L. under field condition. J. Fungi 7:976. doi: 10.3390/jof7110976
Khan, B., Yan, W., Wei, S., Wang, Z., Zhao, S., Cao, L., et al. (2019). Nematicidal metabolites from endophytic fungus Chaetomium globosum YSC5. FEMS Microbiol. Lett. 366:169. doi: 10.1093/femsle/fnz169
Khruengsai, S., Pripdeevech, P., Tanapichatsakul, C., Srisuwannapa, C., D’Souza, P. E., and Panuwet, P. (2021). Antifungal properties of volatile organic compounds produced by Daldinia eschscholtzii MFLUCC 19-0493 isolated from Barleria prionitis leaves against Colletotrichum acutatum and its post-harvest infections on strawberry fruits. PeerJ 9:e11242. doi: 10.7717/peerj.11242
Kiarie, S., Nyasani, J. O., Gohole, L. S., Maniania, N. K., and Subramanian, S. (2020). Impact of fungal endophyte colonization of maize (Zea mays L.) on induced resistance to Thrips- and aphid-transmitted viruses. Plants 9:416. doi: 10.3390/plants9040416
Klironomos, J. N. (2003). Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84, 2292–2301. doi: 10.1890/02-0413
Koziol, L., and Bever, J. D. (2023). Crop productivity boosters: native mycorrhizal fungi from an old-growth grassland benefits tomato (Solanum lycopersicum) and pepper (Capsicum annuum) varieties in organically farmed soils. Microorganisms 11:2012. doi: 10.3390/microorganisms11082012
Kumar, U., Raj, S., Sreenikethanam, A., Maddheshiya, R., Kumari, S., Han, S., et al. (2023). Multi-omics approaches in plant–microbe interactions hold enormous promise for sustainable agriculture. Agronomy 13:1804. doi: 10.3390/agronomy13071804
Lee, Y., Krishnamoorthy, R., Selvakumar, G., Kim, K., and Sa, T. (2015). Alleviation of salt stress in maize plant by co-inoculation of arbuscular mycorrhizal fungi and Methylobacterium oryzae CBMB20. J. Korean Soc. Appl. Biol. Chem. 58, 533–540. doi: 10.1007/s13765-015-0072-4
Leeman, M., Den Ouden, F. M., Van Pelt, J. A., Cornelissen, C., Matamala-Garros, A., Bakker, P. A. H. M., et al. (1996). Suppression of Fusarium wilt of radish by co-inoculation of fluorescent Pseudomonas spp. and root-colonizing fungi. Eur. J. Plant Pathol. 102, 21–31. doi: 10.1007/bf01877112
Lerch-Olson, E. R., and Robertson, A. E. (2020). Effect of co-inoculations with Pythium and Fusarium species on seedling disease development of soybean. Can. J. Plant Pathol. 42, 408–418. doi: 10.1080/07060661.2019.1668858
Li, L., Zhu, X.-M., Zhang, Y.-R., Cai, Y.-Y., Wang, J.-Y., Liu, M.-Y., et al. (2022). Research on the molecular interaction mechanism between plants and pathogenic fungi. Int. J. Mol. Sci. 23:4658. doi: 10.3390/ijms23094658
Liu, Z., Yu, Z., Song, B., Li, Y., Fang, J., Guo, Y., et al. (2023). Elevated CO2 and temperature increase arbuscular mycorrhizal fungal diversity, but decrease root colonization, in maize and wheat. Sci. Total Environ. 873:162321. doi: 10.1016/j.scitotenv.2023.162321
Lorenzo-Gutiérrez, D., Gómez-Gil, L., Guarro, J., Roncero, M. I. G., Fernández-Bravo, A., Capilla, J., et al. (2019). Role of the Fusarium oxysporum metallothionein Mt1 in resistance to metal toxicity and virulence. Metallomics 11, 1230–1240. doi: 10.1039/c9mt00081j
Lozano, Y. M., Aguilar-Trigueros, C. A., Roy, J., and Rillig, M. C. (2021). Drought induces shifts in soil fungal communities that can be linked to root traits across 24 plant species. New Phytol. 232, 1917–1929. doi: 10.1111/nph.17707
Luo, X., Liu, Y., Li, S., and He, X. (2023). Interplant carbon and nitrogen transfers mediated by common arbuscular mycorrhizal networks: beneficial pathways for system functionality. Front. Plant Sci. 14:1169310. doi: 10.3389/fpls.2023.1169310
Luo, F., Yu, Z., Zhou, Q., and Huang, A. (2022). Multi-omics-based discovery of plant signaling molecules. Meta 12:76. doi: 10.3390/metabo12010076
Maciá-Vicente, J. G., Bai, B., Qi, R., Ploch, S., Breider, F., and Thines, M. (2022). Nutrient availability does not affect community assembly in root-associated fungi but determines fungal effects on plant growth. mSystems 7:e0030422. doi: 10.1128/msystems.00304-22
Majhi, S., and Sikdar, M. (2023). How heavy metal stress affects the growth and development of pulse crops: insights into germination and physiological processes. 3 Biotech 13:585. doi: 10.1007/s13205-023-03585-0
Manoharan, L., Rosenstock, N. P., Williams, A., and Hedlund, K. (2017). Agricultural management practices influence AMF diversity and community composition with cascading effects on plant productivity. Appl. Soil Ecol. 115, 53–59. doi: 10.1016/j.apsoil.2017.03.012
Marlina, R., Fikrinda, F., and Suzana, S. (2020). Effect of mycorrhizal fungi in controlling bacterial leaf disease in lowland rice caused by Xanthomonas oryzae pv oryzae bacteria. Ann. RSCB 2020, 1–9.
Mathur, S., Agnihotri, R., Sharma, M. P., Reddy, V. R., and Jajoo, A. (2021). Effect of high-temperature stress on plant physiological traits and mycorrhizal symbiosis in maize plants. J. Fungi 7:867. doi: 10.3390/jof7100867
Matušinsky, P., Sedláková, B., and Bleša, D. (2022). Compatible interaction of Brachypodium distachyon and endophytic fungus Microdochium bolleyi. PLoS One 17:e0265357. doi: 10.1371/journal.pone.0265357
Mhamdi, A., and Noctor, G. (2016). High CO2 primes plant biotic stress defences through redox-linked pathways. Plant Physiol. 172:1129. doi: 10.1104/pp.16.01129
Mikkelsen, B. L., Olsen, C. E., and Lyngkjær, M. F. (2015). Accumulation of secondary metabolites in healthy and diseased barley, grown under future climate levels of CO2, ozone and temperature. Phytochemistry 118, 162–173. doi: 10.1016/j.phytochem.2015.07.007
Miranda, V., Silva-Castro, G. A., Ruiz-Lozano, J. M., Fracchia, S., and García-Romera, I. (2023). Fungal endophytes enhance wheat and tomato drought tolerance in terms of plant growth and biochemical parameters. J. Fungi 9:384. doi: 10.3390/jof9030384
Mishra, J., Singh, R., and Arora, N. K. (2017). Alleviation of heavy metal stress in plants and remediation of soil by rhizosphere microorganisms. Front. Microbiol. 8:706. doi: 10.3389/fmicb.2017.01706
Mohamadhasani, F., and Rahimi, M. (2022). Growth response and mycoremediation of heavy metals by fungus Pleurotus sp. Sci. Rep. 12:19947. doi: 10.1038/s41598-022-24349-5
Möller, M., and Stukenbrock, E. H. (2017). Erratum: evolution and genome architecture in fungal plant pathogens. Nat. Rev. Microbiol. 15:771. doi: 10.1038/nrmicro.2017.143
Morelli, M., Bahar, O., Papadopoulou, K. K., Hopkins, D. L., and Obradović, A. (2020). Editorial: role of endophytes in plant health and defense against pathogens. Front. Plant Sci. 11:1312. doi: 10.3389/fpls.2020.01312
Mori, T., Nagai, Y., Kawagishi, H., and Hirai, H. (2018). Functional characterization of the manganese transporter Smf2 homologue gene, PsMnt, of Phanerochaete sordida YK-624 via homologous overexpression. FEMS Microbiol. Lett. 365:50. doi: 10.1093/femsle/fny050
Mota, S. F., Pádua, P. F., Ferreira, A. N., de Barros Wanderley Gomes, L., Dias, M. A., Souza, E. A., et al. (2021). Biological control of common bean diseases using endophytic Induratia spp. Biol. Control 159:104629. doi: 10.1016/j.biocontrol.2021.104629
Moukarzel, R., Ridgway, H. J., Liu, J., Guerin-Laguette, A., and Jones, E. E. (2022). AMF community diversity promotes grapevine growth parameters under high black foot disease pressure. J. Fungi 8:250. doi: 10.3390/jof8030250
Mukherjee, A. (2022). What do we know from the transcriptomic studies investigating the interactions between plants and plant growth-promoting bacteria? Front. Plant Sci. 13:308. doi: 10.3389/fpls.2022.997308
Muñoz-Guerrero, J., Guerra-Sierra, B. E., and Alvarez, J. C. (2021). Fungal endophytes of Tahiti lime (Citrus citrus × latifolia) and their potential for control of Colletotrichum acutatum J. H. Simmonds causing anthracnose. Front. Bioeng. Biotechnol. 9:650351. doi: 10.3389/fbioe.2021.650351
Nacoon, S., Ekprasert, J., Riddech, N., Mongkolthanaruk, W., Jogloy, S., Vorasoot, N., et al. (2021). Growth enhancement of sunchoke by arbuscular mycorrhizal fungi under drought condition. Rhizosphere 17:100308. doi: 10.1016/j.rhisph.2021.100308
Naveed, Z. A., Wei, X., Chen, J., Mubeen, H., and Ali, G. S. (2020). The PTI to ETI continuum in Phytophthora-plant interactions. Front. Plant Sci. 11:593905. doi: 10.3389/fpls.2020.593905
Niskanen, T., Lücking, R., Dahlberg, A., Gaya, E., Suz, L. M., Mikryukov, V., et al. (2023). Pushing the frontiers of biodiversity research: unveiling the global diversity, distribution, and conservation of fungi. Annu. Rev. Environ. Resour. 48, 149–176. doi: 10.1146/annurev-environ-112621-090937
Niu, X.-Y., Wang, S.-K., Zhou, J., Di, D.-L., Sun, P., and Huang, D.-Z. (2021). Inoculation with indigenous rhizosphere microbes enhances aboveground accumulation of lead in Salix integra Thunb. By improving transport coefficients. Front. Microbiol. 12:686812. doi: 10.3389/fmicb.2021.686812
Odunayo Joseph, O., and Victor Olumide, O. (2022). The effect of AMF (Glomus clarum) on tomato resistance to early leaf blight (Alternaria solani) on tomato. Global J. Agric. Sci. 21, 87–92. doi: 10.4314/gjass.v21i1.11
Oehl, F., Sieverding, E., Mäder, P., Dubois, D., Ineichen, K., Boller, T., et al. (2004). Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia 138, 574–583. doi: 10.1007/s00442-003-1458-2
Onaga, G., Wydra, K. D., Koopmann, B., Séré, Y., and von Tiedemann, A. (2017). Elevated temperature increases in planta expression levels of virulence related genes in Magnaporthe oryzae and compromises resistance in Oryza sativa cv. Nipponbare. Funct. Plant Biol. 44, 358–371. doi: 10.1071/FP16151
Öpik, M., Moora, M., Liira, J., and Zobel, M. (2006). Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe: arbuscular mycorrhizal fungal communities around the globe. J. Ecol. 94, 778–790. doi: 10.1111/j.1365-2745.2006.01136.x
Palmieri, D., Ianiri, G., Del Grosso, C., Barone, G., De Curtis, F., Castoria, R., et al. (2022). Advances and perspectives in the use of biocontrol agents against fungal plant diseases. Horticulturae 8:577. doi: 10.3390/horticulturae8070577
Pandey, P., Tripathi, A., Dwivedi, S., Lal, K., and Jhang, T. (2023). Deciphering the mechanisms, hormonal signaling, and potential applications of endophytic microbes to mediate stress tolerance in medicinal plants. Front. Plant Sci. 14:1250020. doi: 10.3389/fpls.2023.1250020
Pappas, M. L., Liapoura, M., Papantoniou, D., Avramidou, M., Kavroulakis, N., Weinhold, A., et al. (2018). The beneficial endophytic fungus Fusarium solani strain K alters tomato responses against spider mites to the benefit of the plant. Front. Plant Sci. 9:1603. doi: 10.3389/fpls.2018.01603
Park, R. F. (1990). The role of temperature and rainfall in the epidemiology of Puccinia striiformis f.sp. tritici in the summer rainfall area of eastern Australia. Plant Pathol. 39, 416–423. doi: 10.1111/j.1365-3059.1990.tb02517.x
Percio, F., and Botella, M. A. (2025). Hidden in plain sight: fungal effector evades plant immune responses by relocating to the vacuole. Plant Cell Environ. 48, 6490–6492. doi: 10.1111/pce.15628
Piri, R., Moradi, A., Balouchi, H., and Salehi, A. (2019). Improvement of cumin (Cuminum cyminum) seed performance under drought stress by seed coating and biopriming. Sci. Hortic. (Amsterdam) 257:108667. doi: 10.1016/j.scienta.2019.108667
Priyashantha, A. K. H., Dai, D.-Q., Bhat, D. J., Stephenson, S. L., Promputtha, I., Kaushik, P., et al. (2023). Plant-fungi interactions: where it goes? Biology 12:809. doi: 10.3390/biology12060809
Priyashantha, A. K. H., Tibpromma, S., Manike, G. D. N., Dissanayake, D. M., Dai, D. Q., Yang, J. Y., et al. (2025). Emerging fungal pathogens warfare, fungi-like organisms, and the world’s major crops: lessons from the past and solutions for the future. Curr. Microbiol. 82:440. doi: 10.1007/s00284-025-04406-8
Procter, A. C., Ellis, J. C., Fay, P. A., Polley, H. W., and Jackson, R. B. (2014). Fungal community responses to past and future atmospheric CO2 differ by soil type. Appl. Environ. Microbiol. 80, 7364–7377. doi: 10.1128/aem.02083-14
Raffa, N., Osherov, N., and Keller, N. P. (2019). Copper utilization, regulation, and acquisition by Aspergillus fumigatus. Int. J. Mol. Sci. 20:1980. doi: 10.3390/ijms20081980
Rajarammohan, S. (2021). Redefining plant-necrotroph interactions: the thin line between hemibiotrophs and necrotrophs. Front. Microbiol. 12:673518. doi: 10.3389/fmicb.2021.673518
Rana, K. L., Kour, D., Kaur, T., Devi, R., Yadav, A. N., Yadav, N., et al. (2020). Endophytic microbes: biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Van Leeuwenhoek 113, 1075–1107. doi: 10.1007/s10482-020-01429-y
Rashad, Y. M., Abbas, M. A., Soliman, H. M., Abdel-Fattah, G., and Abdel-Fattah, G. (2020a). Synergy between endophytic Bacillus amyloliquefaciens GGA and arbuscular mycorrhizal fungi induces plant defense responses against white rot of garlic and improves host plant growth. Phytopathol. Mediterr. 59, 169–186. doi: 10.36253/phyto-11019
Rashad, Y., Aseel, D., Hammad, S., and Elkelish, A. (2020b). Rhizophagus irregularis and Rhizoctonia solani differentially elicit systemic transcriptional expression of polyphenol biosynthetic pathways genes in sunflower. Biomolecules 10:379. doi: 10.3390/biom10030379
Rashid, T. S. (2021). Bioactive metabolites from tomato endophytic fungi with antibacterial activity against tomato bacterial spot disease. Rhizosphere 17:100292. doi: 10.1016/j.rhisph.2020.100292
Rodrigues E Silva, M. T., Calandrelli, A., Miamoto, A., Rinaldi, L. K., Pereira Moreno, B., da Silva, C., et al. (2021). Pre-inoculation with arbuscular mycorrhizal fungi affects essential oil quality and the reproduction of root lesion nematode in Cymbopogon citratus. Mycorrhiza 31, 613–623. doi: 10.1007/s00572-021-01045-2
Różewicz, M., Wyzińska, M., and Grabiński, J. (2021). The most important fungal diseases of cereals—problems and possible solutions. Agronomy 11:714. doi: 10.3390/agronomy11040714
Safari Motlagh, M. R., Jahangiri, B., Kulus, D., Tymoszuk, A., and Kaviani, B. (2022). Endophytic fungi as potential biocontrol agents against Rhizoctonia solani J.G. Kühn, the causal agent of rice sheath blight disease. Biology 11:1282. doi: 10.3390/biology11091282
Schouteden, N., De Waele, D., Panis, B., and Vos, C. M. (2015). Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: a review of the mechanisms involved. Front. Microbiol. 6:280. doi: 10.3389/fmicb.2015.01280
Semenov, M. V., Krasnov, G. S., Semenov, V. M., and van Bruggen, A. (2022). Mineral and organic fertilizers distinctly affect fungal communities in the crop rhizosphere. J. Fungi 8:251. doi: 10.3390/jof8030251
Seneviratne, M., Seneviratne, G., Madawala, H. M. S. P., Iqbal, M. C. M., Rajakaruna, N., Bandara, T., et al. (2015). A preliminary study of the role of bacterial–fungal co-inoculation on heavy metal phytotoxicity in serpentine soil. Aust. J. Bot. 63:261. doi: 10.1071/bt14270
Shakeel, Q., Bajwa, R. T., Li, G., Long, Y., Wu, M., Zhang, J., et al. (2022). “Multiomics approaches and plant–fungal interactions” in Phytomycology and Molecular Biology of Plant–Pathogen Interactions. ed. Q. Shakeel (Boca Raton: CRC Press), 213–237.
Sharma, J. K., Kumar, N., Singh, N. P., and Santal, A. R. (2023). Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: an approach for a sustainable environment. Front. Plant Sci. 14:876. doi: 10.3389/fpls.2023.1076876
Shen, C., Wang, J., He, J.-Z., Yu, F.-H., and Ge, Y. (2021). Plant diversity enhances soil fungal diversity and microbial resistance to plant invasion. Appl. Environ. Microbiol. 87:21. doi: 10.1128/AEM.00251-21
Silva, L. G., Camargo, R. C., Mascarin, G. M., Favaro, C. P., Nunes, P. S. O., Farinas, C. S., et al. (2024). Innovative sustainable bioreactor-in-a-granule formulation of Trichoderma asperelloides. Appl. Microbiol. Biotechnol. 108:458. doi: 10.1007/s00253-024-13261-9
Silva-Valenzuela, M., Rojas-Martínez, R. I., Manzanilla-López, R. H., Macías-Rubalcava, M. L., Aranda-Ocampo, S., and Zavaleta-Mejía, E. (2023). Antagonistic potential of endophytic fungi against Meloidogyne enterolobii, M. incognita and Nacobbus aberrans sensu lato. Biol. Control 186:105343. doi: 10.1016/j.biocontrol.2023.105343
Singh, B. K., Delgado-Baquerizo, M., Egidi, E., Guirado, E., Leach, J. E., Liu, H., et al. (2023). Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 21, 640–656. doi: 10.1038/s41579-023-00900-7
Smith, F., and Luna, E. (2023). Elevated atmospheric carbon dioxide and plant immunity to fungal pathogens: do the risks outweigh the benefits? Biochem. J. 480, 1791–1804. doi: 10.1042/bcj20230152
Steiner, M., Falquet, L., Fragnière, A.-L., Brown, A., and Bacher, S. (2024). Effects of pesticides on soil bacterial, fungal and protist communities, soil functions and grape quality in vineyards. Ecol. Sol. Evid. 5:327. doi: 10.1002/2688-8319.12327
Stevens, R. B. (1960). ‘‘Cultural practices in disease control,’’ in Plant Pathology, An Advanced Treatise, vol. 3. ed. J. G. Horsfall and A. E. Dimond (New York and London: Academic Press), 357–429.
Suárez, J. P., Herrera, P., Kalinhoff, C., Vivanco-Galván, O., and Thangaswamy, S. (2023). Generalist arbuscular mycorrhizal fungi dominated heavy metal polluted soils at two artisanal and small-scale gold mining sites in southeastern Ecuador. BMC Microbiol. 23:42. doi: 10.1186/s12866-022-02748-y
Suebrasri, T., Somteds, A., Harada, H., Kanokmedhakul, S., Jogloy, S., Ekprasert, J., et al. (2020). Novel endophytic fungi with fungicidal metabolites suppress sclerotium disease. Rhizosphere 16:100250. doi: 10.1016/j.rhisph.2020.100250
Teixidó, N., Usall, J., and Torres, R. (2022). Insight into a successful development of biocontrol agents: production, formulation, packaging, and shelf life as key aspects. Horticulturae 8:305. doi: 10.3390/horticulturae8040305
Traxler, L., Shrestha, J., Richter, M., Krause, K., Schäfer, T., and Kothe, E. (2022). Metal adaptation and transport in hyphae of the wood-rot fungus Schizophyllum commune. J. Hazard. Mater. 425:127978. doi: 10.1016/j.jhazmat.2021.127978
Tymon, L. S., Morgan, P., Gundersen, B., and Inglis, D. A. (2020). Potential of endophytic fungi collected from Cucurbita pepo roots grown under three different agricultural mulches as antagonistic endophytes to Verticillium dahliae in western Washington. Microbiol. Res. 240:126535. doi: 10.1016/j.micres.2020.126535
Usman, M., Shah, I. H., Sabir, I. A., Malik, M. S., Rehman, A., Murtaza, G., et al. (2024). Synergistic partnerships of endophytic fungi for bioactive compound production and biotic stress management in medicinal plants. Plant Stress 11:100425. doi: 10.1016/j.stress.2024.100425
Van Dillewijn, P. (2008). What gets turned on in the rhizosphere?: what gets turned on in the rhizosphere? Microb. Biotechnol. 1, 341–342. doi: 10.1111/j.1751-7915.2008.00054.x
Vannette, R. L., and Hunter, M. D. (2009). Mycorrhizal fungi as mediators of defence against insect pests in agricultural systems. Agric. For. Entomol. 11, 351–358. doi: 10.1111/j.1461-9563.2009.00445.x
Víglaš, J., and Olejníková, P. (2021). An update on ABC transporters of filamentous fungi - from physiological substrates to xenobiotics. Microbiol. Res. 246:126684. doi: 10.1016/j.micres.2020.126684
Waheed, A., Haxim, Y., Islam, W., Ahmad, M., Muhammad, M., Alqahtani, F. M., et al. (2023). Climate change reshaping plant-fungal interaction. Environ. Res. 238:117282. doi: 10.1016/j.envres.2023.117282
Waller, F., Achatz, B., Baltruschat, H., Fodor, J., Becker, K., Fischer, M., et al. (2005). The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. U. S. A. 102, 13386–13391. doi: 10.1073/pnas.0504423102
Wallis, C. M., and Sisterson, M. S. (2024). Opportunities for optimizing fungal biological control agents for long-term and effective management of insect pests of orchards and vineyards: a review. Front. Fungal Biol. 5:1443343. doi: 10.3389/ffunb.2024.1443343
Wang, L., Chen, X., Du, Y., Zhang, D., and Tang, Z. (2022b). Nutrients regulate the effects of arbuscular mycorrhizal fungi on the growth and reproduction of cherry tomato. Front. Microbiol. 13:3010. doi: 10.3389/fmicb.2022.843010
Wang, H., Hao, Z., Zhang, X., Xie, W., and Chen, B. (2022a). Arbuscular mycorrhizal fungi induced plant resistance against Fusarium wilt in jasmonate biosynthesis defective mutant and wild type of tomato. J. Fungi 8:422. doi: 10.3390/jof8050422
Wang, Z., Wang, L., Pan, Y., Zheng, X., Liang, X., Sheng, L., et al. (2023). Research advances on endophytic fungi and their bioactive metabolites. Bioprocess Biosyst. Eng. 46, 165–170. doi: 10.1007/s00449-022-02840-7
Watts, D., Palombo, E. A., Jaimes Castillo, A., and Zaferanloo, B. (2023). Endophytes in agriculture: potential to improve yields and tolerances of agricultural crops. Microorganisms 11:1276. doi: 10.3390/microorganisms11051276
Ważny, R., Rozpądek, P., Jędrzejczyk, R. J., Śliwa, M., Stojakowska, A., Anielska, T., et al. (2018). Does co-inoculation of Lactuca serriola with endophytic and arbuscular mycorrhizal fungi improve plant growth in a polluted environment? Mycorrhiza 28, 235–246. doi: 10.1007/s00572-018-0819-y
Wei, F., Zhang, Y., Shi, Y., Feng, H., Zhao, L., Feng, Z., et al. (2019). Evaluation of the biocontrol potential of Endophytic fungus Fusarium solani CEF559 against Verticillium dahliae in cotton plant. Biomed. Res. Int. 2019:3187943. doi: 10.1155/2019/3187943
Williams, A., Pétriacq, P., Schwarzenbacher, R. E., Beerling, D. J., and Ton, J. (2018). Mechanisms of glacial-to-future atmospheric CO2 effects on plant immunity. New Phytol. 218, 752–761. doi: 10.1111/nph.15018
Wójcik, M., Koper, P., Żebracki, K., Marczak, M., and Mazur, A. (2023). Genomic and metabolic characterization of plant growth-promoting rhizobacteria isolated from nodules of clovers grown in non-farmed soil. Int. J. Mol. Sci. 24:16679. doi: 10.3390/ijms242316679
Wright, S. F., and Upadhyaya, A. (1998). A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 198, 97–107. doi: 10.1023/A:1004347701584
Wu, N., Li, Z., and Tang, M. (2021b). Impact of salt and exogenous AM inoculation on indigenous microbial community structure in the rhizosphere of dioecious plant, Populus cathayana. Sci. Rep. 11:674. doi: 10.1038/s41598-021-97674-w
Wu, E.-J., Wang, Y.-P., Yang, L.-N., Zhao, M.-Z., and Zhan, J. (2022). Elevating air temperature may enhance future epidemic risk of the plant pathogen Phytophthora infestans. J. Fungi 8:808. doi: 10.3390/jof8080808
Wu, M., Yan, Y., Wang, Y., Mao, Q., Fu, Y., Peng, X., et al. (2021a). Arbuscular mycorrhizal fungi for vegetable (VT) enhance resistance to Rhizoctonia solani in watermelon by alleviating oxidative stress. Biol. Control 152:104433. doi: 10.1016/j.biocontrol.2020.104433
Xiao, X., Chen, J., Liao, X., Yan, Q., Liang, G., Liu, J., et al. (2022). Different arbuscular mycorrhizal fungi established by two inoculation methods improve growth and drought resistance of Cinnamomum migao seedlings differently. Biology 11:220. doi: 10.3390/biology11020220
Xie, X.-G., Zhang, F.-M., Yang, T., Chen, Y., Li, X.-G., and Dai, C.-C. (2019). Endophytic fungus drives nodulation and N2 fixation attributable to specific root exudates. MBio 10:19. doi: 10.1128/mbio.00728-19
Yan, H., and Nelson, B. Jr. (2020). Effect of temperature on Fusarium solani and F. tricinctum growth and disease development in soybean. Can. J. Plant Pathol. 42, 527–537. doi: 10.1080/07060661.2020.1745893
Yanan, W., Xusheng, Z., Baozhong, Y., Wenchao, Z., and Jintang, G. (2015). Biochemical defenses induced by mycorrhizae fungi Glomus mosseae in controlling strawberry Fusarium wilt. Open Biomed. Eng. J. 9, 301–304. doi: 10.2174/1874120701509010301
Yao, Y., Huo, J., Ben, H., Gao, W., Hao, Y., Wang, W., et al. (2023). Biocontrol efficacy of endophytic fungus, Acremonium sclerotigenum, against Meloidogyne incognita under in vitro and in vivo conditions. Biologia 78, 3305–3313. doi: 10.1007/s11756-023-01505-4
Zai, X.-M., Fan, J.-J., Hao, Z.-P., Liu, X.-M., and Zhang, W.-X. (2021). Effect of co-inoculation with arbuscular mycorrhizal fungi and phosphate solubilizing fungi on nutrient uptake and photosynthesis of beach palm under salt stress environment. Sci. Rep. 11:5761. doi: 10.1038/s41598-021-84284-9
Zeilinger, S., Gupta, V. K., Dahms, T. E. S., Silva, R. N., Singh, H. B., Upadhyay, R. S., et al. (2016). Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 40, 182–207. doi: 10.1093/femsre/fuv045
Zhang, Y., Chen, C., Mai, Z., Lin, J., Nie, L., Maharachchikumbura, S. S. N., et al. (2022). Co-infection of Fusarium aglaonematis sp. nov. and Fusarium elaeidis causing stem rot in Aglaonema modestum in China. Front. Microbiol. 13:790. doi: 10.3389/fmicb.2022.930790
Zhou, J., Kolb, S., and Zhou, X. (2023). Editorial: pathogen co-infections and plant diseases. Front. Microbiol. 14:476. doi: 10.3389/fmicb.2023.1189476
Zhu, C., Ling, N., Guo, J., Wang, M., Guo, S., and Shen, Q. (2016). Impacts of fertilization regimes on arbuscular mycorrhizal fungal (AMF) community composition were correlated with organic matter composition in maize rhizosphere soil. Front. Microbiol. 7:840. doi: 10.3389/fmicb.2016.01840
Keywords: co-infection, endophytic fungi, gene expression, mycorrhiza, sustainable agriculture, synergistic effect
Citation: Asad S, Chen M, Priyashantha AKH, Gu P, Liu J, Shan Z, Tibpromma S, Niu C, Qadir M, Akhtar M, Kan X, Xu Y, Liu Z, Karunarathna SC and Zhang J (2025) Mapping of plant–fungal interactions on agriculture perception: a bibliometric analysis and systematic review. Front. Microbiol. 16:1612428. doi: 10.3389/fmicb.2025.1612428
Edited by:
Mubasher Hussain, Guangdong Pest Control Technology Group, ChinaReviewed by:
Gamal Ammar, City of Scientific Research and Technological Applications, EgyptZichao Wang, Henan University of Technology, China
Atif Khurshid Wani, Lovely Professional University, India
Huu-Thanh Nguyen, An Giang University, Vietnam
Copyright © 2025 Asad, Chen, Priyashantha, Gu, Liu, Shan, Tibpromma, Niu, Qadir, Akhtar, Kan, Xu, Liu, Karunarathna and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samantha C. Karunarathna, c2FtYW50aGFrYXJ1bmFyYXRobmFAZ21haWwuY29t; Jianqiang Zhang, ZHJqcXpoYW5nQGZveG1haWwuY29t
†These authors have contributed equally to this work
 Suhail Asad
Suhail Asad Mei Chen1†
Mei Chen1† Alviti Kankanamalage Hasith Priyashantha
Alviti Kankanamalage Hasith Priyashantha Jie Liu
Jie Liu Zhiguo Shan
Zhiguo Shan Saowaluck Tibpromma
Saowaluck Tibpromma Masood Qadir
Masood Qadir Muhammad Akhtar
Muhammad Akhtar Samantha C. Karunarathna
Samantha C. Karunarathna