- 1Department of Laboratory Medicine, West China Hospital, Sichuan University, Chengdu, China
- 2Clinical Laboratory Medicine Research Center, West China Hospital, Sichuan University, Chengdu, China
- 3Sichuan Clinical Research Center for Laboratory Medicine, Chengdu, China
Background: The rising global burden of invasive fungal infections and the growing issue of antifungal resistance present critical public health threats. By using multicenter surveillance data from Sichuan Province, we conducted the largest five-year study on fungemia to date. Our objective was to gain insights into regional differences in the distribution and resistance patterns of fungal pathogens.
Methods: We performed a retrospective analysis of fungal bloodstream infections (BSIs) from 31 hospitals (2019–2023). Integrated clinical and laboratory data were analyzed using WHONET 5.6 to assess resistance patterns, and Microsoft Excel (with PivotTable functionality) was used to analyze epidemiological trends.
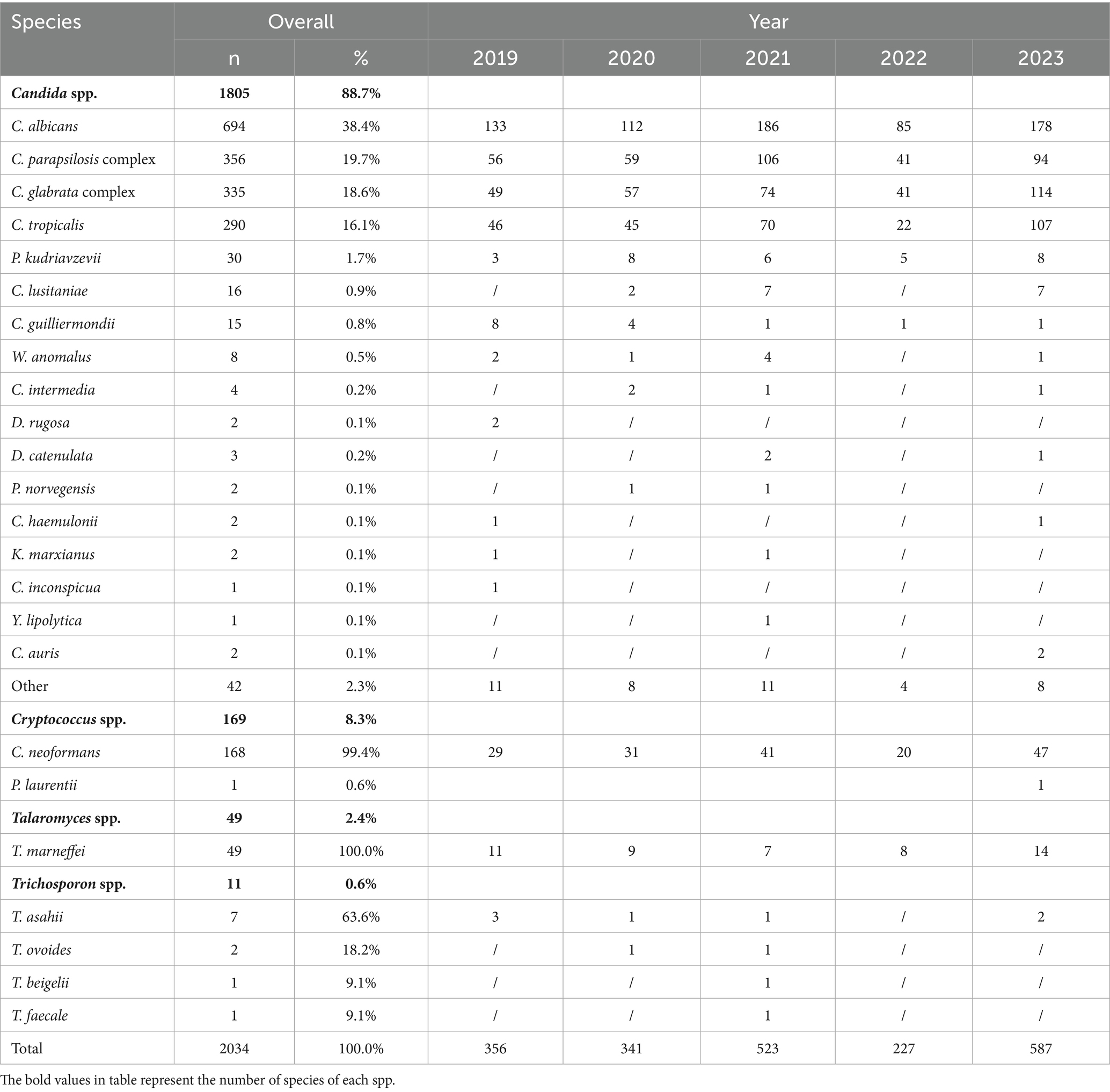
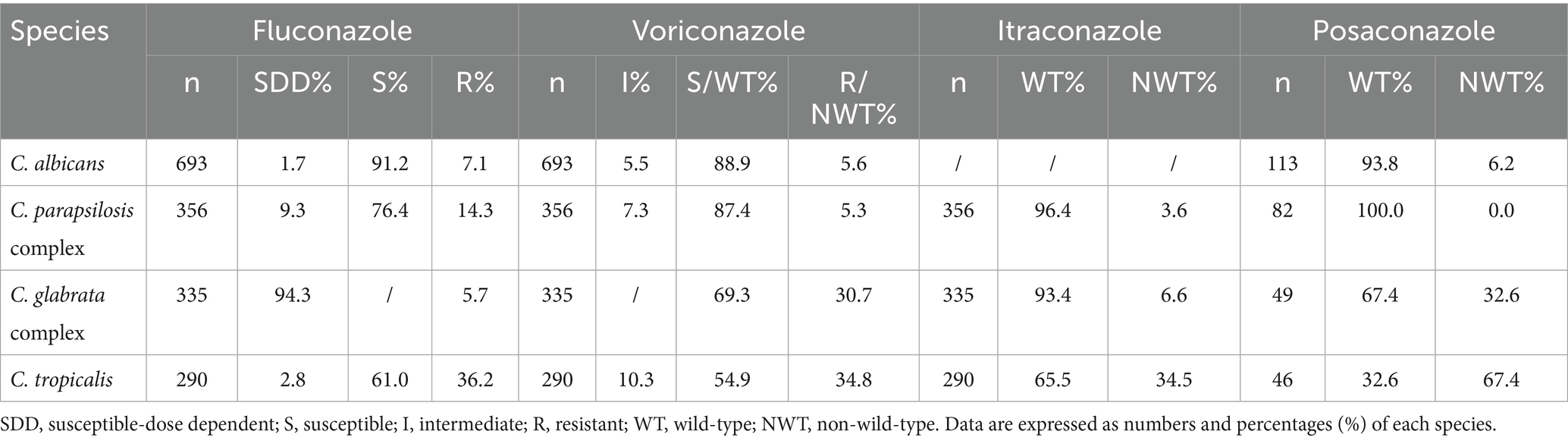
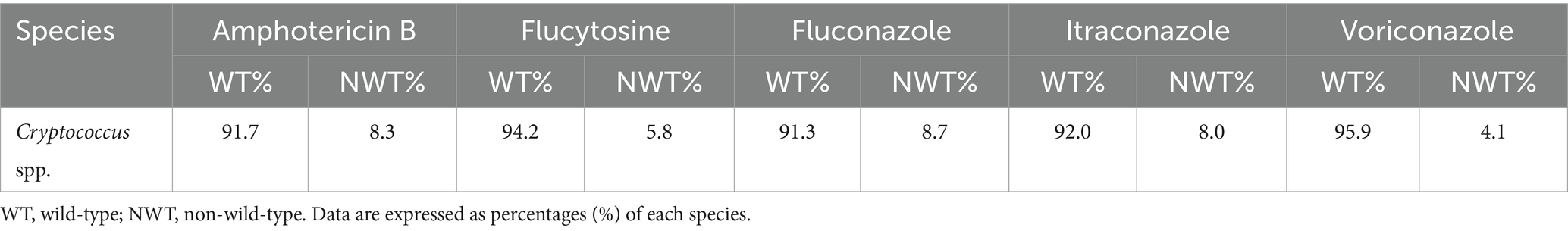
Results: Annual fungal isolations increased steadily over the study period. Candida species accounted for 88.7% (1,805/2,034) of the bloodstream isolates, with C. albicans being the most common (38.4%, 694/1,805). The majority of patients were men (58.6%, 1,191/2,034) and aged 46 years or older (80.0%, 1,627/2,034). Intensive care unit (ICU) cases accounted for 36.8% (748/2,034) of the total. C. albicans showed the highest fluconazole susceptibility (91.2%, 633/694). Both C. albicans and the C. parapsilosis complex maintained >80% voriconazole susceptibility, followed by the voriconazole wild-type C. glabrata complex (69.3%). C. tropicalis exhibited high resistance to fluconazole (36.2%, 21/58) and voriconazole (34.8%, 20/58). Cryptococcus spp. displayed non-wild-type rates to amphotericin B (8.7%), flucytosine (5.8%), fluconazole (8.7%), voriconazole (8.0%), and itraconazole (4.1%). Different hospital types isolated varying fungal species. While C. albicans was the predominant species in 83.9% (26/31) of the hospitals, pediatric specialty centers exhibited distinct microbiological profiles, showing the highest isolation rates of the C. parapsilosis complex (χ2 = 18.34, p = 0.002).
Conclusion: Our research conducted across several centers, revealed significant geographic variations in the spread of fungal diseases and antifungal resistance. It is important to understand local epidemiology to guide antifungal therapy and enhance stewardship programs.
1 Background
Bloodstream infections (BSIs) are life-threatening systemic invasions predominantly caused by bacterial or fungal pathogens (Dolatabadi et al., 2024; Najafzadeh et al., 2024). Notably, fungemia in critically ill patients carries serious prognostic implications, with 30-day attributable mortality rates remaining between 35 and 50% despite advancements in diagnostic modalities and targeted therapies (Oren and Paul, 2014; Ruhnke et al., 2018; Arias et al., 2017). Yeasts of the Candida genus are the predominant etiological agents of invasive fungal diseases (IFDs) in hospitalized populations, accounting for 42.7% of all IFDs (Ruiz-Ruigómez et al., 2018). As the first national surveillance system for IFDs, the China Hospital Invasive Fungal Surveillance Network (CHIF-NET) offers valuable epidemiological evidence. However, our analysis of fungemia data from 2019 to 2023 showed that there are regional differences in the distribution of fungal pathogens and antifungal resistance (Enoch et al., 2017). Understanding the epidemiological patterns and in vitro antifungal susceptibility characteristics of the causes of fungal BSIs in this region is crucial for clinical diagnosis and treatment. These evidence-based findings directly inform clinical decision-making algorithms for selecting empirical antifungal therapies and help optimize provincial-level antimicrobial stewardship programs.
2 Materials and methods
2.1 Strains and identification of isolates
This retrospective cohort study followed the CHIF-NET protocols to collect fungal BSI data from 31 tertiary care hospitals across Sichuan Province (January 2019–December 2023). Strains of the same type and from the same patient were excluded, and the data of strains with antifungal sensitivity results were included. When duplicate strains had antifungal sensitivity results, the one isolated first was retained. Demographic and microbiological data were collected included gender stratification, age distribution, clinical department classification, fungal speciation, and antifungal susceptibility profiles. All isolates were identified to the species level using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS, Bruker Corporation, Germany), VITEK MS (Bio-Merieux, France).
2.2 Antifungal agents and standards
Antifungal susceptibility testing was conducted using validated commercial microdilution systems: Sensititre YeastOne (Thermo Fisher Scientific, United States) and ATB FUNGUS 3 (bioMérieux, France). Antifungal susceptibility was determined following the Clinical and Laboratory Standards Institute (CLSI) guidelines. Susceptibility testing for C. albicans, the C. parapsilosis complex, C. tropicalis, and the C. glabrata complex (except voriconazole) against fluconazole, voriconazole, anidulafungin, caspofungin, and micafungin was performed using the CLSI M27M44S standards (Clinical and Laboratory Standards Institute, 2022a). Susceptibility of the C. glabrata complex to voriconazole, as well as susceptibility of C. albicans, the C. parapsilosis complex, C. tropicalis, and the C. glabrata complex to amphotericin B, itraconazole, and posaconazole, was analyzed using the epidemiological cutoff value (ECV) outlined in CLSI M57S (Clinical and Laboratory Standards Institute, 2022b). Similarly, susceptibility of Cryptococcus spp. to amphotericin B, flucytosine, fluconazole, voriconazole, and itraconazole was evaluated using the ECV outlined in CLSI M57S. C. albicans ATCC 90028 and C. parapsilosis ATCC 22019 were used as antifungal sensitivity quality controls.
2.3 Software tools and analytical methods
The data were processed using WHONET 5.6 and Microsoft Excel with PivotTable functionality. In addition, we used SPSS 25.0 software to analyze the data, and the count data were expressed as species (n). The χ2 test was performed, and a p-value of < 0.05 was considered statistically significant.
3 Results
3.1 Patient information and departmental distribution
The geographic distribution of the 31 hospitals from 1 January 2019 to 31 December 2023 is shown in Figure 1. Of the 2,034 patients, 58.6% (1,191/2,034) were male individuals and 41.4% (843/2,034) were female individuals. The incidence rates by age group were as follows: 2.7% (55/2,034) for patients aged 0–6 years; 1.3% (26/2,034) for patients aged 7–12 years; 1.1% (22/2,034) for patients aged 13–17 years; 14.9% (304/2,034) for patients aged 18–45 years; 42.0% (854/2,034) for patients aged 46–69 years; and 38.0% (773/2,034) for patients over 69 years. The majority of the patients were admitted to the ICU (36.8%, 748/2,034), followed by the Department of Internal Medicine (27.7%, 564/2,034), Surgical Departments (15.3%, 312/2,034), Emergency Medicine (8.7%, 177/2,034), Outpatient Clinics (4.0%, 81/2,034), Pediatrics (3.9%, 80/2,034), and other departments (e.g., Gynecology, Reproductive Medicine; 3.5%, 72/2,034).
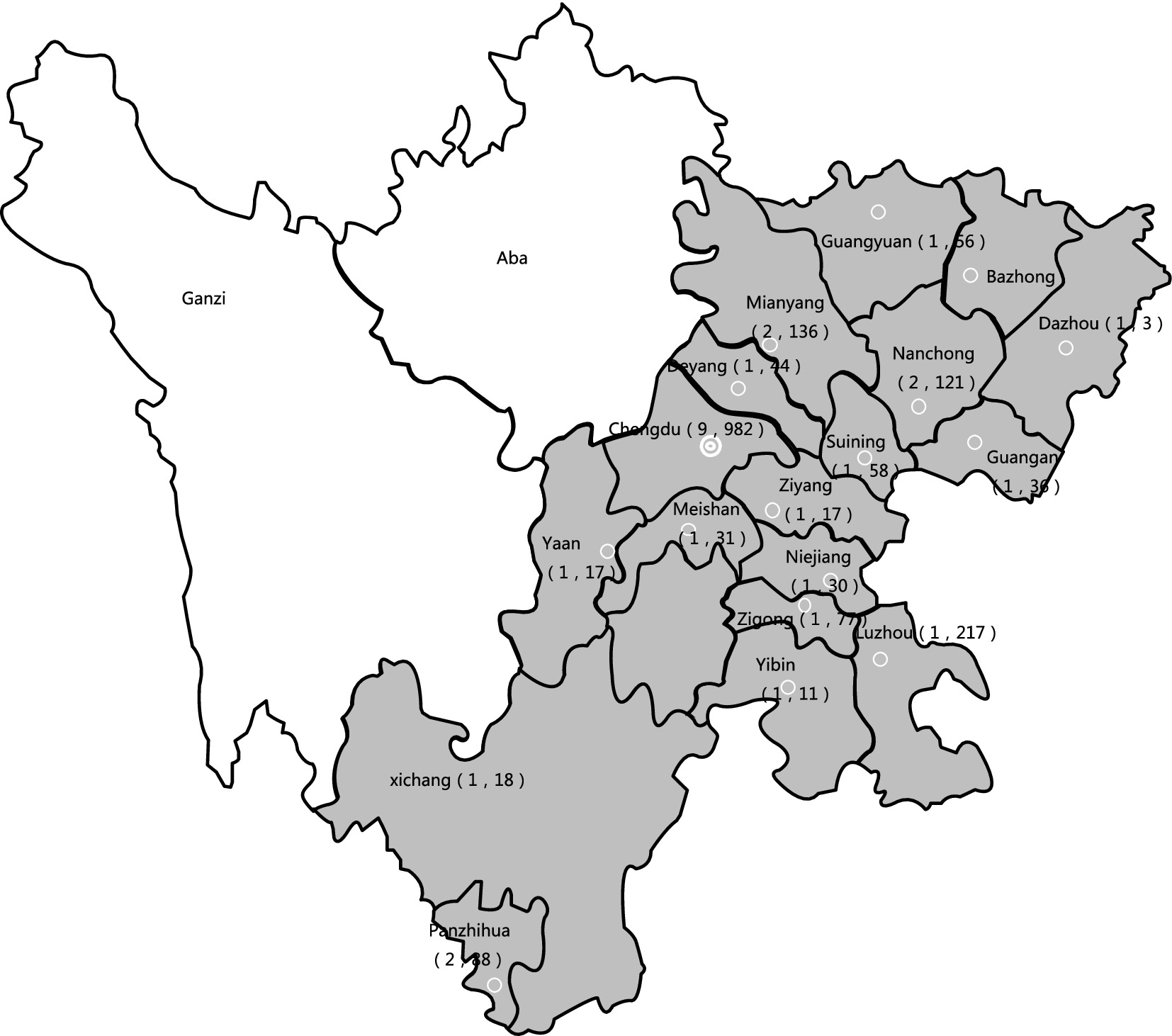
Figure 1. The study’s geographic coverage (18 cities, dark gray). The number of hospitals that participated in the study is indicated by the first number in parentheses under the city name, and the number of isolates gathered is indicated by the second number.
3.2 Strain isolation
Longitudinal analysis revealed a significant upward trend in fungal isolations from 2019 to 2023, with an anomalous decline in 2022 potentially associated with COVID-19 containment measures. Among the 2,034 fungemia cases, Candida species were the predominant etiological agents (88.7%, n = 1,805). C. albicans comprised 38.4% (694/1,805), followed by the C. parapsilosis complex, C. glabrata complex, and C. tropicalis. Cryptococcus spp. ranked fifth among all isolates, accounting for 8.3% (169/2,034). Detailed species distribution and annual isolate counts are provided in Table 1.
3.3 Antifungal susceptibility testing in vitro
The number of tests included in the analysis did not always match the number of strains isolated due to variations in the drug sensitivity kits used at each hospital, which led to the exclusion of certain drugs from the analysis. C. albicans showed the highest susceptibility to fluconazole (91.2%), followed by the C. parapsilosis complex. C. albicans and the C. parapsilosis complex exhibited over 80% susceptibility to voriconazole, followed by the voriconazole wild-type C. glabrata complex (69.3%). A high percentage of wild-type isolates to itraconazole was observed in the C. parapsilosis and C. glabrata complexes, as well as in C. albicans and the C. parapsilosis complex for posaconazole. The resistance rates of C. tropicalis to fluconazole and voriconazole were 36.2 and 34.8%, as shown in Table 2. C. albicans, the C. parapsilosis complex, the C. glabrata complex, and C. tropicalis showed high susceptibility to amphotericin B, caspofungin, micafungin, and anidulafungin, as shown in Table 3. A total of 169 Cryptococcus spp. strains were isolated from 2,034 patients with fungal BSIs. These strains exhibited varying rates of not-wild-type susceptibility to amphotericin B (8.7%), flucytosine (5.8%), fluconazole (8.7%), voriconazole (8.0%), and itraconazole (4.1%), as shown in Table 4.
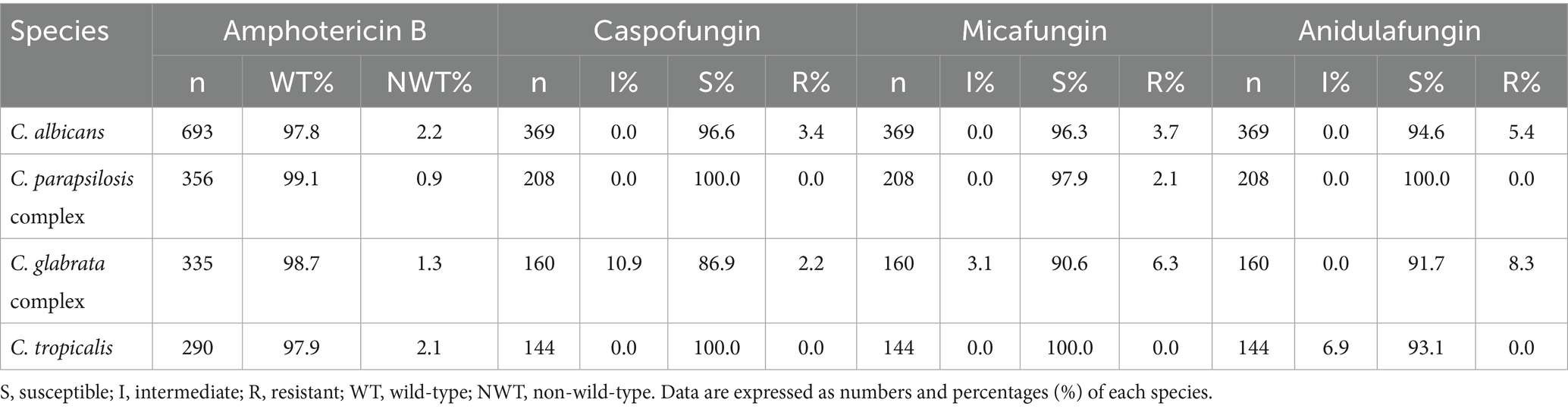
Table 3. In vitro susceptibility of the selected pathogenic fungi to amphotericin B and echinocandins.
3.4 Changes in resistance to fluconazole and voriconazole
Among the four common Candida species, C. albicans exhibited the lowest resistance rates to fluconazole and voriconazole, with both rates declining annually. In contrast, fluconazole resistance in the C. parapsilosis complex initially increased but subsequently declined, while voriconazole resistance decreased consistently each year. C. tropicalis displayed the highest resistance rates to both antifungal agents in 2021, exceeding 50% (fluconazole: 55.5%; voriconazole: 50.8%; p < 0.01), but these rates declined over the following 2 years (p < 0.05), as shown in Figure 2.
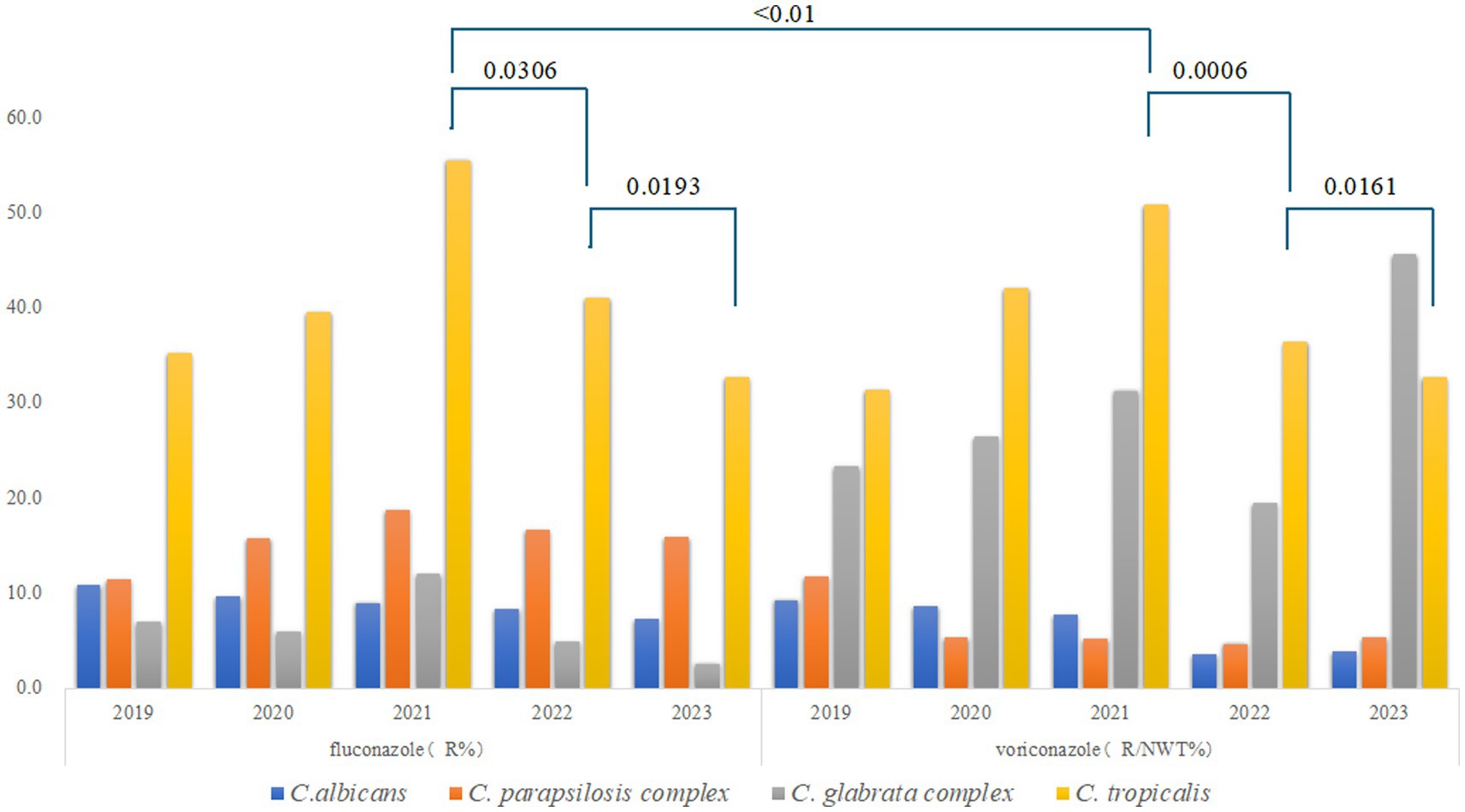
Figure 2. Trends in the resistance of the common Candida species to fluconazole and voriconazole. R for Resistant; NWT for Non-wild-type. Different colors represent distinct Candida species: blue for C. albicans, orange for the C. parapsilosis complex, gray for the C. glabrata complex, and yellow for C. tropicalis.
3.5 Differences in the distribution of fungi across hospitals
C. albicans, the C. parapsilosis complex, the C. glabrata complex, and C. tropicalis were the predominant species causing fungal BSIs across the 31 hospitals. There was some variation in the ranking of the fungal species isolated across hospitals, although C. albicans predominated in most institutions. However, in certain hospitals, such as Panzhihua Central Hospital, Zigong First People’s Hospital, and Xichang People’s Hospital, the C. glabrata complex accounted for the highest number of isolates. The highest number of C. tropicalis isolates was reported at the Affiliated Hospital of Sichuan North Medical College, while the C. parapsilosis complex was the most frequently isolated fungal species at the Second West China Hospital of Sichuan University. Figure 3 shows the differences in the distribution of fungal isolates responsible for bloodstream infection across hospitals.
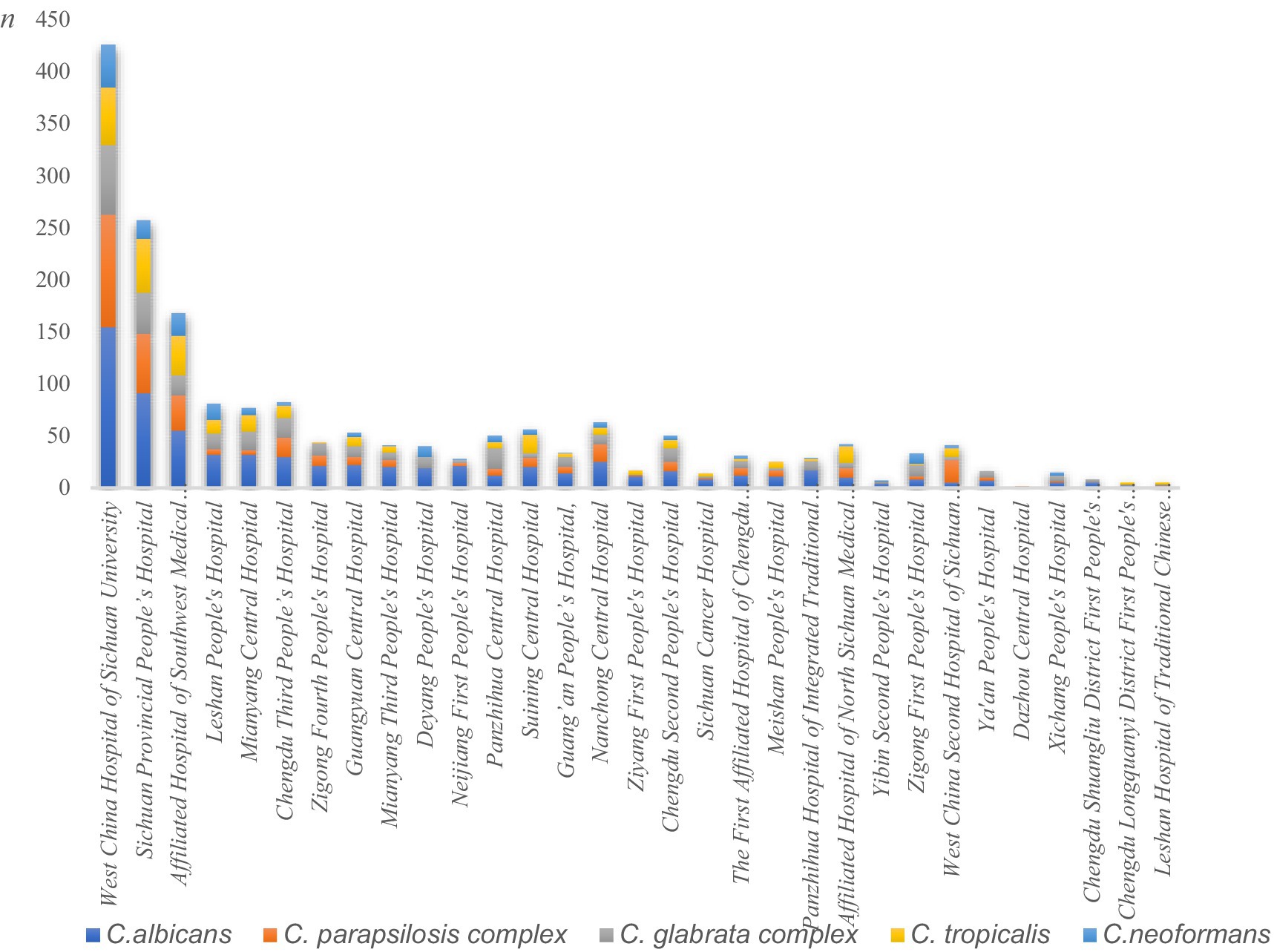
Figure 3. Differences in the distribution of fungal isolates across the 31 hospitals. The Y-axis represents isolate counts, explicitly labeled as “n” (number of isolates). Different colors represent distinct Candida species: blue for C. albicans, orange for the C. parapsilosis complex, gray for the C. glabrata complex, yellow for C. tropicalis, and light blue for C. neoformans.
4 Discussion
The number of patients with invasive mycoses is rising due to increasing risk factors such as immunocompromised states, neoplastic diseases, leukemia, and invasive operations (Pappas et al., 2018; Puig-Asensio et al., 2014). The annual number of fungal BSIs reported from 31 hospitals in the Sichuan provincial center of the CHIF-NET increased steadily from 2019 to 2023, with the exception of 2022 when a decline occurred likely due to COVID-19-related disruptions. This is the largest recent study of fungemia in Sichuan. Few other studies in China have conducted province-wide surveillance over a 5-year period, particularly in comparing different types of hospitals. In our study, men accounted for 58.6% (1,191/2,034) of the patients, with 80.0% (1,627/2,034) being middle-aged or elderly patients (>46 years old). Previous studies have shown that fungal BSIs are highly prevalent in middle-aged and elderly populations. In addition, 36.8% (748/2,034) of the cases were from ICUs, followed by the Department of Internal Medicine and Surgical Departments. IFDs in ICUs represent a critical clinical challenge, with a global incidence increasing by 6.8% annually. This rise is largely due to the severity of illness in ICU patients and risk factors such as invasive procedures, prolonged bed rest, and extended antifungal use, all of which contribute to an increased likelihood of infection (Vincent et al., 2009).
Candida species dominated the fungal distribution (88.7%, 1,805/2,034). The most common species was C. albicans, followed by the C. parapsilosis complex, the C. glabrata complex, and C. tropicalis. This distribution differs from reports in other countries and shows regional variation compared to national data from the CHIF-NET (Xiao et al., 2018a). The pathogenicity of fungal BSIs shifts from C. albicans to non-albicans Candida species, and there is also variation in the virulence of different Candida species (Arendrup, 2013). In this study, non-albicans Candida species outnumbered C. albicans as causes of BSIs. This shift in pathogen distribution may reflect the widespread use of antifungal agents, which can alter the epidemiology of fungal BSIs (Pfaller et al., 2019). Two strains of C. auris were detected in 2023, representing only 0.1% (2/2,034) of the cases. However, C. auris is an emerging fungus that can cause serious illnesses and is easily spread among patients in healthcare facilities. The emergence and rapid increase of C. auris should be considered a threat. C. auris was first discovered in China in 2018 (Wang et al., 2018; Chen et al., 2018). As of December 2023, C. auris has been reported in 10 provinces across China. A study reported 312 cases of C. auris infections across 18 hospitals. The researchers found significant differences in prevalence between years, with the lowest number of cases occurring in 2020–2021. Notably, the number of infections increased dramatically to 182 in 2023, marking a 450% rise compared to 2022 (33 cases) (Bing et al., 2024). C. auris causes serious infections including bacteremia, wound infections, and catheter-associated infections (Tsay et al., 2017), and it can also colonize the urinary tract, respiratory system, digestive tract, and central nervous system (Eyre et al., 2018). The fungus persists in hospital environments and colonizes patients’ skin, posing significant infection control risks. Primarily affecting immunocompromised individuals through nosocomial transmission, C. auris carries a high mortality rate (up to 60% in BSI cases) (Boutin and Luong, 2024). Its propensity for causing hospital outbreaks jeopardizes the safety of patients and healthcare workers. On the other hand, C. auris is naturally prone to carry antifungal resistance-related genes, and more than 90% of clinical isolates of C. auris are resistant to azole antifungal agents (Chowdhary et al., 2023). Resistance to other antifungal agents (e.g., amphotericin B or echinocandins) is also frequently observed, complicating treatment strategies for C. auris infections and significantly increasing healthcare costs, compared to other fungal infections.
The isolation rates of different fungi varied across hospitals. For example, the C. parapsilosis complex was the most frequently isolated fungal pathogen at the Second Hospital of West China, Sichuan University, accounting for 53.6% (22/41) of cases. This may be because the hospital is a specialized children’s hospital (Yılmaz-Ciftdoğan et al., 2021). The C. parapsilosis complex comprises C. parapsilosis, C. metapsilosis, and C. orthopsilosis. Although these species were not individually listed in the Results section of this study, it should be noted that they exhibit differences in antimicrobial susceptibility breakpoints and phenotypic profiles. Similar considerations apply to the C. glabrata complex. Unlike in adults, the C. parapsilosis complex may be the predominant pathogen causing fungal BSIs in children. Among Candida species, the C. parapsilosis complex ranks second only to C. albicans in biofilm-forming capability among clinically relevant species (Mancera et al., 2021). Biofilms formed by this complex are structurally less complex and thinner than those of C. albicans, facilitating their attachment to medical devices (Larkin et al., 2018). In children, the gut is not fully developed, allowing the C. parapsilosis complex to enter the bloodstream through damaged mucosal barriers. This leads to infections in children. It can also be transmitted to immunocompromised children by healthcare workers through contact.
An important limitation of our study is the variability in antifungal susceptibility testing methods among the participating hospitals, which may affect the comparability of resistance rates for certain agents. Nevertheless, the data presented in this study can still truly reflect the prevalence of fungal BSIs in Sichuan Province. In this study, the best in vitro susceptibility of Candida to the antifungal agents analyzed was observed with echinocandins, followed by amphotericin B. Among the azoles, fluconazole (except C. albicans) and voriconazole showed sensitivities of no more than 90%. This may be due to the extensive clinical use of fluconazole, which has driven natural selection in Candida itself, leading to mutations in certain genes that make it less sensitive to fluconazole (Andes et al., 2006). The C. parapsilosis complex showed resistance rates of 14.3 and 5.3% to fluconazole and voriconazole, respectively, both of which were higher than those reported in 20 consecutive years of surveillance data from the SENTRY Antimicrobial Surveillance Program (Pfaller et al., 2019). C. tropicalis exhibited resistance rates of 36.2 and 34.8% to fluconazole and voriconazole, respectively, with rates exceeding 50% in 2021. These values are notably higher than those reported by the CHIF-NET (fluconazole 21.0% vs. voriconazole 21.4%) (Xiao et al., 2018a). As with the threat posed by the emergence and rapid spread of C. auris, the increasing azole resistance rate of C. tropicalis also warrants serious attention. The rate of drug resistance in C. tropicalis has been increasing annually, as shown in some studies (Fan et al., 2017). The increase in isolation rates of azole-resistant strains was not associated with the amount of azole antifungal used. However, several mechanisms of resistance to azoles in C. tropicalis have been identified, including mutations and overexpression of the ERG11 gene (Morio et al., 2017), mutations and overexpression of the UPC2 gene (Choi et al., 2016), and overexpression of the MDR1 and CDR1 genes (Fan et al., 2019). The observed increase in fluconazole resistance poses a direct threat to the effectiveness of first-line therapy for C. tropicalis infections. In regions where resistance rates exceed 10%, Infectious Diseases Society of America (IDSA) guidelines recommend avoiding fluconazole for the empirical treatment of BSIs. Our data suggest that local stewardship programs should prioritize the use of echinocandins or voriconazole in high-risk patients (Pappas et al., 2016). In this study, resistance rates to fluconazole and voriconazole were higher than those observed for itraconazole. Further investigation is needed to determine the underlying reasons for this difference. The IDSA guidelines recommend echinocandins as the best treatment for IFDs because resistance to azole antifungal agents has increased. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) has not established a breakpoint for caspofungin because susceptibility results for caspofungin vary significantly across laboratories. The EUCAST also does not recommend using caspofungin MIC values for clinical evaluation. Some laboratories found that certain C. glabrata complex isolates were susceptible to anidulafungin but not to caspofungin when tested using methods such as the E-test and Sensititre YeastOne for sensitization. This discrepancy may be related to the variability of the in vitro assay for caspofungin (Arendrup and Pfaller, 2012; Eschenauer et al., 2014). Therefore, the EUCAST recommends using anidulafungin and micafungin as markers for caspofungin susceptibility. Until 2018, when the first case of echinocandin-resistant C. glabrata infection was reported in China. The main mechanism of its resistance to echinocandins is a mutation in FKS1/FKS2, and the FKS2 E655K mutation (Fks2 HS1) is a recently identified echinocandin resistance site (Xiao et al., 2018b). Therefore, when testing for caspofungin, the result can be directly reported as “Sensitive.” When an “Intermediate” or “Resistant” result is obtained, it is necessary to confirm the result using the following methods: 1. Additional testing for micafungin or anidulafungin; 2. DNA sequencing analysis, including screening for FKS1 point mutations in all Candida species and FKS2 point mutations specifically in C. glabrata; and 3. sending the isolate to a reference laboratory for further confirmation. In addition, Candida spp. should report resistance to all echinocandins (including caspofungin) if they are resistant to anidulafungin or micafungin or carry an FKS point mutation.
The preferred treatment for Cryptococcus infections is amphotericin B (Iyer et al., 2021). In our study, 8.3% (14/169) of Cryptococcus strains were classified as non-wild-type for amphotericin B, which is inconsistent with the results of a global drug sensitivity study of 3,590 novel Cryptococcus strains conducted by Espinel-Ingroff et al. (2012). A total of five Cryptococcus strains exhibited non-wild-type susceptibility to amphotericin B (four strains with an MIC = 1 μg/mL; one strain with an MIC = 2 μg/mL). This reduced susceptibility may reflect prolonged amphotericin B exposure in patients with disseminated cryptococcosis, where antifungal pressure selects for resistant subpopulations (Córdoba et al., 2016). In addition, since most laboratories use Sensititre YeastOne, which is not the gold standard (broth microdilution method), this may result in the observation of higher levels of not-wild-type strains.
Our findings reveal a critical geospatial divergence in both fungal pathogen distribution and resistance profiles. Given the high prevalence of azole-resistant C. tropicalis and the emergence of C. auris, empirical treatment with echinocandins should be considered for high-risk patients, particularly in settings where resistance rates exceed established thresholds. Accurate knowledge of the regional epidemiology of pathogenic fungi and suspected fungal BSIs is essential for the early selection of appropriate empirical antifungal treatment or intervention, even before definitive pathogenic evidence is obtained.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
JD: Supervision, Methodology, Data curation, Formal analysis, Writing – original draft. YL: Writing – original draft, Methodology, Investigation. LS: Methodology, Writing – original draft. DZ: Writing – original draft, Investigation, Methodology. YX: Project administration, Writing – review & editing, Resources. YM: Funding acquisition, Writing – review & editing, Validation. MK: Supervision, Writing – review & editing, Visualization, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Ministry of Science and Technology of the People’s Republic of China, grant nos. 2020AAA0109405.
Acknowledgments
The co-principal investigators in the 31 hospitals participating in the China Hospital Invasive Fungal Surveillance Network (CHIF-NET) Study Group (Sichuan, 2019 to 2023) are as follows: (1) Lin Yin, Sichuan Provincial People’s Hospital, Chengdu. (2) Haiyan Jiang, Guang’an People’s Hospital, Guang’an. (3) Linghan Kuang, West China Second Hospital of Sichuan University, Chengdu. (4) Xi Peng, Chengdu Third People’s Hospital, Chengdu. (5) Xianggui Yang, The First Affiliated Hospital of Chengdu Medical College, Chengdu. (6) Sujiao Ni, Sichuan Cancer Hospital, Chengdu. (7) Bangqin Zhang, Affiliated Hospital of Southwest Medical University, Luzhou. (8) Jinfang Feng, Guangyuan Central Hospital, Guangyuan. (9) Yanling Wang, Leshan People’s Hospital, Leshan. (10) Yushan Ma, Mianyang Third People’s Hospital, Mianyang. (11) Zongyao Chen, Deyang People’s Hospital, Deyang. (12) Hanyu Zhong, Neijiang First People’s Hospital, Neijiang. (13) Xianli Wu, Panzhihua Central Hospital, Panzhihua. (14) Kun Li, Suining Central Hospital, Suining. (15) Ling Wang, Xichang Municipal People’s Hospital Xichang. (16) Wei Gao, Ya’an Municipal People’s Hospital Ya’an. (17) Xueqiang Yang, Yibin Second People’s Hospital Yibin. (18) Jun Zhu, Ziyang First People’s Hospital Ziyang. (19) Yu Chen, Zigong First People’s Hospital Zigong. (20) Xian Zhang, Chengdu Second People’s Hospital Chengdu. (21) Changjun Sun, Dazhou Central Hospital Dazhou. (22) Jun Luo, Mianyang Central Hospital Mianyang. (23) Yumei Li, Zigong Fourth People’s Hospital Zigong. (24) Yan Li, Meishan People’s Hospital Meishan. (25) Bin Zhang, Nanchong Central Hospital, Nanchong. (26) Ning Xie, Affiliated Hospital of North Sichuan Medical College, Nanchong. (27) Jun Wang, Panzhihua Hospital of Integrated Traditional Chinese and Western Medicine, Panzhihua. (28) Peiling Guo, Chengdu Shuangliu District First People’s Hospital, Chengdu. (29) Yanchun Huang, Chengdu Longquanyi District First People’s Hospital, Chengdu. (30) Rongchun Shu, Leshan Hospital of Traditional Chinese Medicine, Leshan. (31) Ya Liu and Ling Shu, West China Hospital of Sichuan University, Chengdu.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andes, D., Forrest, A., Lepak, A., Nett, J., Marchillo, K., and Lincoln, L. (2006). Impact of antimicrobial dosing regimen on evolution of drug resistance in vivo: fluconazole and Candida albicans. Antimicrob. Agents Chemother. 50, 2374–2383. doi: 10.1128/AAC.01053-05
Arendrup, M. C. (2013). Candida and candidaemia. Susceptibility and epidemiology. Dan. Med. J. 60:B4698.
Arendrup, M. C., and Pfaller, M. A. (2012). Caspofungin etest susceptibility testing of Candida species: risk of misclassification of susceptible isolates of C. Glabrata and C. krusei when adopting the revised CLSI caspofungin breakpoints. Antimicrob. Agents Chemother. 56, 3965–3968. doi: 10.1128/AAC.00355-12
Arias, S., Denis, O., Montesinos, I., Cherifi, S., Miendje Deyi, V. Y., and Zech, F. (2017). Epidemiology and mortality of candidemia both related and unrelated to the central venous catheter: a retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 36, 501–507. doi: 10.1007/s10096-016-2825-3
Bing, J., Du, H., Guo, P., Hu, T., Xiao, M., Lu, S., et al. (2024). Candida auris-associated hospitalizations and outbreaks, China, 2018-2023. Emerg. Microbes Infect. 13:2302843. doi: 10.1080/22221751.2024.2302843
Boutin, C. A., and Luong, M. L. (2024). Update on therapeutic approaches for invasive fungal infections in adults. Ther. Adv. Infect. Dis. 11:20499361231224980. doi: 10.1177/20499361231224980
Chen, Y., Zhao, J., Han, L., Qi, L., Fan, W., Liu, J., et al. (2018). Emergency of fungemia cases caused by fluconazole-resistant Candida auris in Beijing, China. J. Infect. 77, 561–571. doi: 10.1016/j.jinf.2018.09.002
Choi, M. J., Won, E. J., Shin, J. H., Kim, S. H., Lee, W. G., Kim, M. N., et al. (2016). Resistance mechanisms and clinical features of fluconazole-nonsusceptible Candida tropicalis isolates compared with fluconazole-less-susceptible isolates. Antimicrob. Agents Chemother. 60, 3653–3661. doi: 10.1128/AAC.02652-15
Chowdhary, A., Jain, K., and Chauhan, N. (2023). Candida auris genetics and emergence. Ann. Rev. Microbiol. 77, 583–602. doi: 10.1146/annurev-micro-032521-015858
Clinical and Laboratory Standards Institute. (2022a) Performance standards for antifungal susceptibility testing of yeasts M27M44S. Available online at: https://clsi.org/shop/standards/m27m44s/
Clinical and Laboratory Standards Institute. (2022b) Epidemiological cutoff values for antifungal susceptibility testing M57S. Available online at: https://clsi.org/shop/standards/m57s/
Córdoba, S., Isla, M. G., Szusz, W., Vivot, W., Altamirano, R., and Davel, G. (2016). Susceptibility profile and epidemiological cut-off values of Cryptococcus neoformans species complex from Argentina. Mycoses 59, 351–356. doi: 10.1111/myc.12479
Dolatabadi, S., Najafzadeh, M. J., Raeisabadi, A., Zarrinfar, H., Jalali, M., Spruijtenburg, B., et al. (2024). Epidemiology of Candidemia in Mashhad, Northeast Iran: a prospective multicenter study (2019-2021). J Fungi 10:481. doi: 10.3390/jof10070481
Enoch, D. A., Yang, H., Aliyu, S. H., and Micallef, C. (2017). The changing epidemiology of invasive fungal infections. Methods Mol. Biol. 1508, 17–65. doi: 10.1007/978-1-4939-6515-1_2
Eschenauer, G. A., Nguyen, M. H., Shoham, S., Vazquez, J. A., Morris, A. J., Pasculle, W. A., et al. (2014). Real-world experience with echinocandin MICs against Candida species in a multicenter study of hospitals that routinely perform susceptibility testing of bloodstream isolates. Antimicrob. Agents Chemother. 58, 1897–1906. doi: 10.1128/AAC.02163-13
Espinel-Ingroff, A., Aller, A. I., Canton, E., Castañón-Olivares, L. R., Chowdhary, A., Cordoba, S., et al. (2012). Cryptococcus neoformans-Cryptococcus gattii species complex: an international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob. Agents Chemother. 56, 5898–5906. doi: 10.1128/AAC.01115-12
Eyre, D. W., Sheppard, A. E., Madder, H., Moir, I., Moroney, R., Quan, T. P., et al. (2018). A Candida auris outbreak and its control in an intensive care setting. N. Engl. J. Med. 379, 1322–1331. doi: 10.1056/NEJMoa1714373
Fan, X., Xiao, M., Liao, K., Kudinha, T., Wang, H., Zhang, L., et al. (2017). Notable increasing trend in azole non-susceptible Candida tropicalis causing invasive candidiasis in China (august 2009 to July 2014): molecular epidemiology and clinical azole consumption. Front. Microbiol. 8:464. doi: 10.3389/fmicb.2017.00464
Fan, X., Xiao, M., Zhang, D., Huang, J. J., Wang, H., Hou, X., et al. (2019). Molecular mechanisms of azole resistance in Candida tropicalis isolates causing invasive candidiasis in China. Clin. Microbiol. Infect. 25, 885–891. doi: 10.1016/j.cmi.2018.11.007
Iyer, K. R., Revie, N. M., Fu, C., Robbins, N., and Cowen, L. E. (2021). Treatment strategies for cryptococcal infection: challenges, advances and future outlook. Nat. Rev. Microbiol. 19, 454–466. doi: 10.1038/s41579-021-00511-0
Larkin, E. L., Dharmaiah, S., and Ghannoum, M. A. (2018). Biofilms and beyond: expanding echinocandin utility. J. Antimicrob. Chemother. 73, i73–i81. doi: 10.1093/jac/dkx451
Mancera, E., Nocedal, I., Hammel, S., Gulati, M., Mitchell, K. F., Andes, D. R., et al. (2021). Evolution of the complex transcription network controlling biofilm formation in Candida species. eLife 10:e64682. doi: 10.7554/eLife.64682
Morio, F., Jensen, R. H., Le Pape, P., and Arendrup, M. C. (2017). Molecular basis of antifungal drug resistance in yeasts. Int. J. Antimicrob. Agents 50, 599–606. doi: 10.1016/j.ijantimicag.2017.05.012
Najafzadeh, M. J., Shaban, T., Zarrinfar, H., Sedaghat, A., Hosseinikargar, N., Berenji, F., et al. (2024). COVID-19 associated candidemia: from a shift in fungal epidemiology to a rise in azole drug resistance. Med. Mycol. 62:myae031. doi: 10.1093/mmy/myae031
Oren, I., and Paul, M. (2014). Up to date epidemiology, diagnosis and management of invasive fungal infections. Clin. Microbiol. Infect. 20, 1–4. doi: 10.1111/1469-0691.12642
Pappas, P. G., Kauffman, C. A., Andes, D. R., Clancy, C. J., Marr, K. A., Ostrosky-Zeichner, L., et al. (2016). Clinical practice guideline for the Management of Candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 62, e1–e50. doi: 10.1093/cid/civ933
Pappas, P. G., Lionakis, M. S., Arendrup, M. C., Ostrosky-Zeichner, L., and Kullberg, B. J. (2018). Invasive candidiasis. Nat. Rev. Dis. Primers 4:18026. doi: 10.1038/nrdp.2018.26
Pfaller, M. A., Diekema, D. J., Turnidge, J. D., Castanheira, M., and Jones, R. N. (2019). Twenty years of the SENTRY antifungal surveillance program: results for Candida species from 1997-2016. Open Forum Infect. Dis. 6, S79–s94. doi: 10.1093/ofid/ofy358
Puig-Asensio, M., Padilla, B., Garnacho-Montero, J., Zaragoza, O., Aguado, J. M., Zaragoza, R., et al. (2014). Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clin. Microbiol. Infect. 20, O245–O254. doi: 10.1111/1469-0691.12380
Ruhnke, M., Behre, G., Buchheidt, D., Christopeit, M., Hamprecht, A., Heinz, W., et al. (2018). Diagnosis of invasive fungal diseases in haematology and oncology: 2018 update of the recommendations of the infectious diseases working party of the German society for hematology and medical oncology (AGIHO). Mycoses 61, 796–813. doi: 10.1111/myc.12838
Ruiz-Ruigómez, M., Dueñas, C., Hernandez, C., Vinuesa, D., Coronado-Álvarez, N. M., Portillo-Tuñón, V., et al. (2018). Clinical predictors of candidemia in medical non-neutropenic, non-ICU patients. The CaMed score. Int. J. Clin. Pract. 72:e13275. doi: 10.1111/ijcp.13275
Tsay, S., Welsh, R. M., Adams, E. H., Chow, N. A., Gade, L., Berkow, E. L., et al. (2017). Notes from the field: ongoing transmission of Candida auris in health care facilities - United States, June 2016-may 2017. MMWR Morb. Mortal Wkly. Rep. 66, 514–515. doi: 10.15585/mmwr.mm6619a7
Vincent, J. L., Rello, J., Marshall, J., Silva, E., Anzueto, A., Martin, C. D., et al. (2009). International study of the prevalence and outcomes of infection in intensive care units. JAMA 302, 2323–2329. doi: 10.1001/jama.2009.1754
Wang, X., Bing, J., Zheng, Q., Zhang, F., Liu, J., Yue, H., et al. (2018). The first isolate of Candida auris in China: clinical and biological aspects. Emerg. Microbes Infect. 7:93. doi: 10.1038/s41426-018-0095-0
Xiao, M., Fan, X., Hou, X., Chen, S. C., Wang, H., Kong, F., et al. (2018b). Clinical characteristics of the first cases of invasive candidiasis in China due to pan-echinocandin-resistant Candida tropicalis and Candida glabrata isolates with delineation of their resistance mechanisms. Infect. Drug Resist. 11, 155–161. doi: 10.2147/IDR.S152785
Xiao, M., Sun, Z. Y., Kang, M., Guo, D. W., Liao, K., Chen, S. C., et al. (2018a). Five-year national surveillance of invasive candidiasis: species distribution and azole susceptibility from the China hospital invasive fungal surveillance net (CHIF-NET) study. J. Clin. Microbiol. 56:e00577–18. doi: 10.1128/JCM.00577-18
Keywords: fungal bloodstream infections, antifungal susceptibility, distribution characteristics, epidemiology, public health
Citation: Deng J, Liu Y, Shu L, Zhou D, Xie Y, Ma Y and Kang M (2025) The prevalence, patterns, and antifungal drug resistance of bloodstream infection-causing fungi in Sichuan Province, China (2019–2023): a retrospective observational study using national monitoring data. Front. Microbiol. 16:1616013. doi: 10.3389/fmicb.2025.1616013
Edited by:
John Osei Sekyere, University of Pretoria, South AfricaReviewed by:
Hossein Zarrinfar, Mashhad University of Medical Sciences, IranHazrat Bilal, Jiangxi Cancer Hospital, China
Laxmi Shanker Rai, Gandhi Institute of Technology and Management, Bengaluru, India
Copyright © 2025 Deng, Liu, Shu, Zhou, Xie, Ma and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Ma, bWF5aW5nNzJAaG90bWFpbC5jb20=Mei Kanga2FuZ21laUBzaW5hLmNvbQ==
 Jin Deng
Jin Deng Ya Liu
Ya Liu Ling Shu1,2,3
Ling Shu1,2,3 Yi Xie
Yi Xie Mei Kang
Mei Kang