- 1STI Unit, Department of Clinical Sciences, Institute of Tropical Medicine, Antwerp, Belgium
- 2Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Parkville, VIC, Australia
- 3Department of Infection Control and Prevention, Faculty of Nursing, Toho University, Tokyo, Japan
- 4Division of Infectious Diseases and HIV Medicine, University of Cape Town, Cape Town, South Africa
There is a striking variation in national doxycycline post exposure prophylaxis (doxyPEP) guidelines for sexually transmitted infections (STIs). Whilst some countries advocate doxyPEP for all men who have sex with men (MSM) and transgender women (TGW) with certain risks, others restrict the use to research settings. In this viewpoint, we argue that part of the explanation for this divergence can be attributed to different underlying conceptual frameworks. For individuals and organizations dominated by biomedical individualist frameworks, the primary goal of STI services is reducing the incidence of STIs. We have good evidence that doxyPEP does this and therefore, particularly in the setting of increasing STI incidence, this framework regards it as logical to roll out doxyPEP as fast as possible. By way of contrast, if organizations and their members operate within an ecosocial framework then their primary goal is the optimization of the sexual and overall health of individuals and populations and not just reducing STI rates. This framework sees the prevalence of STIs as being driven by the connectivity of local sexual networks. Recent increases in STI prevalence are seen as being due to increased network connectivity. The intensive use of antimicrobials such as doxycycline to reduce this prevalence is seen as introducing a selection pressure for the emergence of resistance to tetracyclines and other antimicrobials in N. gonorrhoeae and other species. This plus the other risks of doxyPEP, leads those animated by this framework to tend toward the precautionary principle and restrict the use of doxyPEP to research settings. The differences in these two frameworks thus leads different individuals and organizations with access to the same evidence-base to very different conclusions as to the net risk-benefit of doxyPEP.
Background
Harrison et al. (1979), published the results of what remains the largest ever randomized controlled trial (RCT) to assess if tetracyclines taken post sex could reduce the incidence of gonorrhea. They found that minocycline post exposure prophylaxis (PEP) reduced the incidence of gonorrhea by 54% but advised against the use of minocycline PEP, due to the fact that it would select for tetracycline resistance. More specifically, they found that minocycline was 100% effective at preventing gonococcal infections with low tetracycline MICs but 0% effective against high MIC isolates. They concluded that “minocycline prophylaxis would probably have limited effectiveness as a public-health measure because of the tendency to select resistant gonococci (Harrison et al., 1979).”
More recent RCTs using doxycycline PEP (doxyPEP) to reduce the incidence of sexually transmitted infections (STIs) have had similar findings but reached the opposite conclusion. Three large RCTs in men who have sex with men (MSM) and transgender women (TGW) have found that doxyPEP reduces the incidence of chlamydia and syphilis by approximately 80% and the incidence of gonorrhea by up to 50% (Molina et al., 2018, 2024; Luetkemeyer et al., 2023, 2025; Szondy et al., 2024). These studies found that doxyPEP was associated with the emergence of tetracycline resistance in N. gonorrhoeae, commensal Neisseria species, Staphylococcus aureus and Group A Streptococcus (Luetkemeyer et al., 2023; Molina et al., 2024; Soge et al., 2025). There was also evidence compatible with the selection of methicillin resistant S. aureus) at an ecological level (Vanbaelen et al., 2024c). It should however, be noted that most concerningly was the evidence from the DOXYVAC study that the receipt of doxyPEP was associated with decreased susceptibility to cefixime in gonococci (Bercot et al., n.d.).
These findings would not have surprised the pioneers of antibiotic therapy such as Alexander Fleming who cautioned that the excessive use of antibiotics would select for antimicrobial resistance (AMR) (Rosenblatt-Farrell, 2009). In the subsequent century, a wealth of evidence has emerged to confirm this association and the utility of antimicrobial stewardship – reserving the use of antimicrobial therapy to instances where it is clearly necessary (Bell et al., 2014; Lawes et al., 2017; Spellberg, 2029). Systematic reviews of the short- and long-term use of tetracycline have clearly shown that, as with other classes of antimicrobials, tetracycline use selects for resistance to tetracyclines and other antimicrobials (Bell et al., 2014; Lawes et al., 2017; Truong et al., 2022; Vanbaelen et al., 2024a). That doxyPEP selects for AMR should thus come as no surprise. What is more surprising is that the authors of certain national doxyPEP guidelines have downplayed this concern. In Belgium, national guidance restricts the use of doxyPEP to research settings (De Scheerder et al., 2024). In contrast, the Centers for Disease Control and Prevention (CDC) guidelines in the United States advocate for the use of doxyPEP for all MSM and TGW who have had a bacterial STI in the past year as well to use a “shared decision-making approach” to discuss doxyPEP in other MSM and TGW (Bachmann et al., 2024). DoxyPEP guidelines in countries such as Australia, France, Germany, the Netherlands and the United Kingdom fall between these extremes (Saunders et al., 2021; Mårdh and Plachouras, 2023; Sherrard et al., 2024; Werner et al., 2024). The CDC guidelines note some of the associations between doxyPEP and AMR noted above, but entirely exclude others such as the data from Harrison et al. (1979) study. They conclude their guidance by noting that: “current data suggest overall benefit of the use of doxyPEP, but potential risks related to the development of resistance and changes in the microbiome will need to be monitored as these guidelines are implemented” (Bachmann et al., 2024).
How do we explain this divergence in interpretations of the risks and benefits of doxyPEP in national guidelines? In this perspective piece, we build on previous work to argue that this divergence of opinion about the net risk-benefit of doxyPEP stems in part from a difference in conceptual frameworks (Aral, 1999, 2001; Kenyon, 2020). More specifically it emerges from a difference in the conceptual framework we use to understand the determinants of STI spread and the relationship between antimicrobial consumption, STI prevalence, microbiomes, AMR and health. We also review the recent increases in STI incidence from a historical perspective to provide better context for this debate.
The crucial need for explicit conceptual frameworks- where we stand depends on where we sit
Conceptual frameworks are crucial to structure our ideas and bring facts together in a way that they are able to form a coherent whole (Krieger, 1994; Susser and Susser, 1996; Kenyon et al., 2022). An optimal theory of the determinants of STI prevalence should thus provide an accurate portrayal of all the important determinants in a way that illustrates the interrelationships, the relative importance of the various determinants and facilitates proportionate and effective responses (Susser and Susser, 1996; Aral, 2001; Kenyon et al., 2022).
Biomedical individualism and the argument to roll-out doxyPEP
In previous work, we and others have discerned two dominant conceptual frameworks in the STI field (Aral, 2001; Kenyon, 2020; Kenyon et al., 2022). The biomedical individualistic conceptual framework has dominated the field for much of the past century (Hogben et al., 2020; Kenyon, 2020). This framework focuses on the individual patient and views STIs as obligate pathogens that can and should be eradicated by intensive seek-and-destroy activities (its primary goal) (Kenyon, 2020; Vanbaelen et al., 2025b). Elevated STI prevalences are seen as being primarily due to sexual behaviors and practices, as well as inadequate STI screening and treatment (Hogben et al., 2020; Kenyon, 2020; Kenyon et al., 2022). This framework assumes that STI prevalences can and should be brought to zero (Hogben et al., 2020; Kenyon et al., 2022). In settings of increasing STI prevalence doxyPEP is appealing to individuals operating within this framework, as doxyPEP has been proven to reduce the incidence of bacterial STIs (Bachmann et al., 2024; Figure 1).
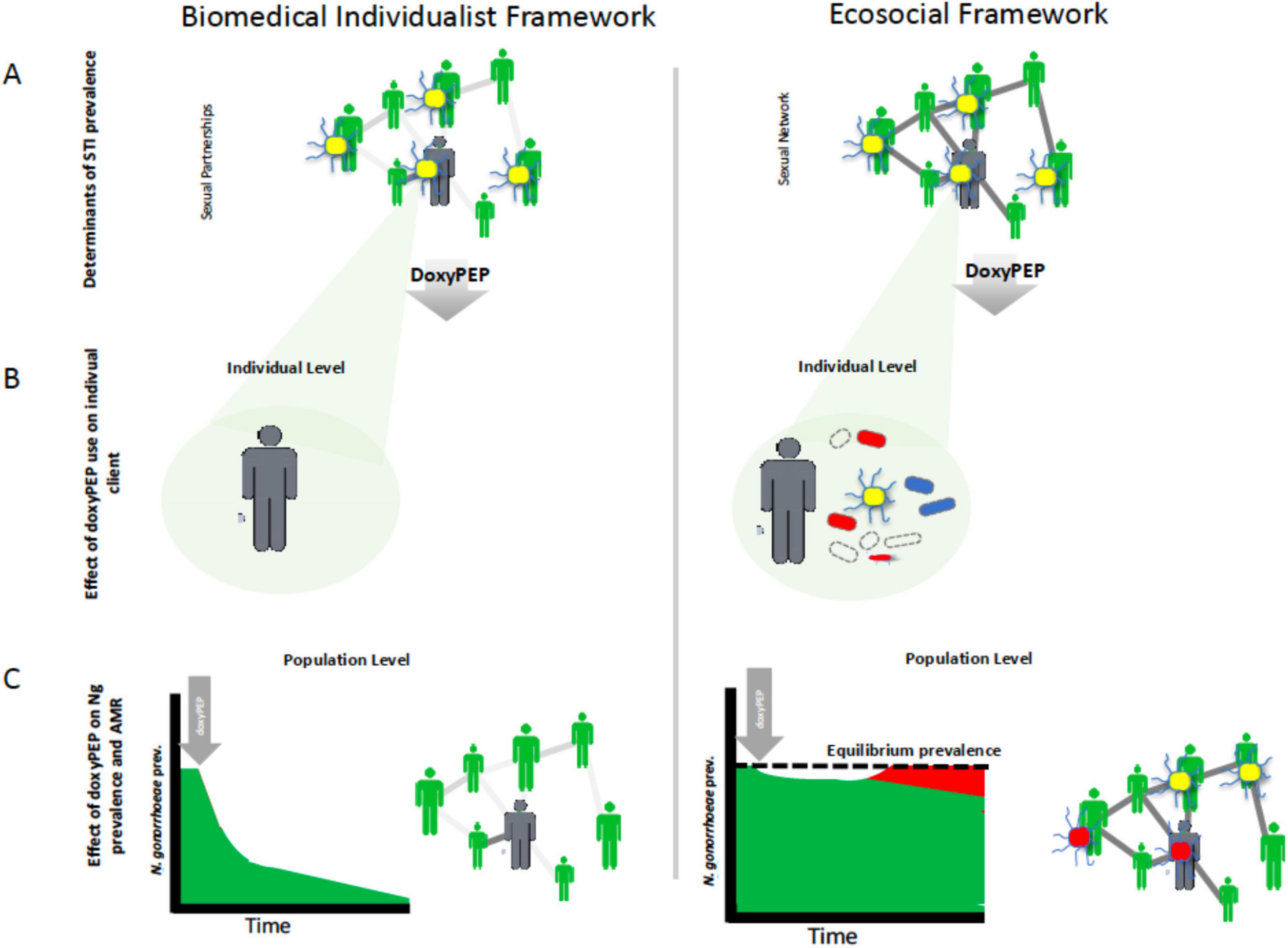
Figure 1. Schematic illustration of how different conceptual frameworks could lead researchers to divergent views on doxycycline postexposure prophylaxis (doxyPEP). The biomedical individualist framework views sexually transmitted infections (STIs) as obligate pathogens that can and should be eradicated by intensive seek-and-destroy activities (A). It focuses on the individual and sees doxyPEP as an effective way to eliminate incident gonococcal infections (yellow diplococci) in individuals (B). By rolling out doxyPEP to enough at-risk individuals it aims to eradicate N. gonorrhoeae and other STIs from a population (C). By contrast, the ecosocial framework sees the high prevalence of STIs such as N. gonorrhoeae in certain populations as being a function of their denser sexual networks. Whilst doxyPEP may be reduce N. gonorrhoeae prevalence in the short term (C), this lowers the prevalence of N. gonorrhoeae to below its equilibrium prevalence which in turn creates a selection pressure for the emergence of tetracycline resistance which will allow the gonococci to retain their equilibrium prevalence (C). The doxyPEP will also select for tetracycline resistance in other bacteria [red oval bacteria in (B)] as well as resistance to other classes of antimicrobials [blue oval bacteria in (B)].
The authors of the CDC doxyPEP guidelines argue that “increasing rates of bacterial STIs” and “the reported high efficacy” of doxyPEP were the main arguments for the decision to roll out doxyPEP (Bachmann et al., 2024). The authors note some concerns about AMR but conclude “systematic reviews of potential harms appear low in the short-term and unknown but potentially concerning in the long-term” (Bachmann et al., 2024). Despite the harms being “potentially concerning” in the long-term this does not stop the authors from actively promoting the roll-out of doxyPEP to a large population of MSM most at risk for gonococcal AMR (Bachmann et al., 2024). Individuals with the highest rates of partner turnover typically are at the highest risk for the emergence of AMR in N. gonorrhoeae and other STIs if heavily exposed to antimicrobials (Lewis, 2013; Kenyon and Schwartz, 2018). This has obvious risks for AMR. Certain proponents of doxyPEP have however, concluded that because doxyPEP would likely reduce exposure to ceftriaxone and azithromycin in this group, it would reduce selection pressure for AMR (Traeger et al., 2023).
The ecosocial perspective and the argument to restrict doxyPEP to research settings
In contrast, the ecosocial framework is an explicitly multilevel framework that views monogamous and non-monogamous norms as equally ethical (Kenyon et al., 2022). STI prevalence is however, seen as largely a function of the connectivity of the local sexual network, and thus populations with high rates of partner turnover or concurrent partnering will have higher equilibrium prevalences of STIs (Kenyon and Delva, 2018; Kenyon et al., 2022). AMR in STIs is seen as typically emerging when populations with high STI prevalence are heavily exposed to antimicrobials – such as via intensive STI screening or doxyPEP (Lewis, 2013; Kenyon and Schwartz, 2018; Figure 1). The primary goal of this eco-social framework is optimizing the health of individuals and populations, which includes stewardship of their microbiomes and resistomes (Kenyon, 2020). A crucial aspect of this is using antibiotics in a way that optimizes cure rates while minimizing unnecessary/inappropriate use (stewardship) (Kenyon, 2020). One component of this approach is that “even in a patient with an obvious bacterial infection, one should only treat when therapy will alter the patient’s clinical course” and where the benefits clearly outweigh the harms (Spellberg, 2029). According to this approach N. gonorrhoeae, C. trachomatis, and M. genitalium infections in MSM that are usually asymptomatic and self-resolving are best classified as occasional pathogens that should be treated only when symptomatic (Kenyon et al., 2023; Vanbaelen et al., 2025b). As a result, physicians practicing within the ecosocial framework have questioned the utility of screening these three infections in MSM (Kenyon et al., 2023). This led them to first stop screening for M. genitalium in a PrEP cohort. This resulted in a 2- and 48-fold decline in macrolide and fluoroquinolone consumption, respectively (Kenyon et al., 2021, 2023). These encouraging results led them to then conduct an RCT to evaluate the effect of stopping screening for N. gonorrhoeae/C. trachomatis in MSM (Vanbaelen et al., 2024e). This RCT found that screening had little or no benefit but resulted in a large increase in antimicrobial consumption. These findings led to the cessation of screening in MSM on PrEP in Belgium (Van Praet et al., 2024). Because asymptomatic N. gonorrhoeae/C. trachomatis infections constitute the majority of the infections that doxyPEP prevents, and the ecosocial framework argues that these infections do not require therapy, the ecosocial views doxyPEP in a less favorable light that the biomedical individualistic framework (Vanbaelen et al., 2024f).
Whilst a range of arguments have been made to restrict doxyPEP to research settings (Figure 2), the most important argument is that doxyPEP will aggravate AMR (Kong et al., 2023). This could occur at both individual and population levels.
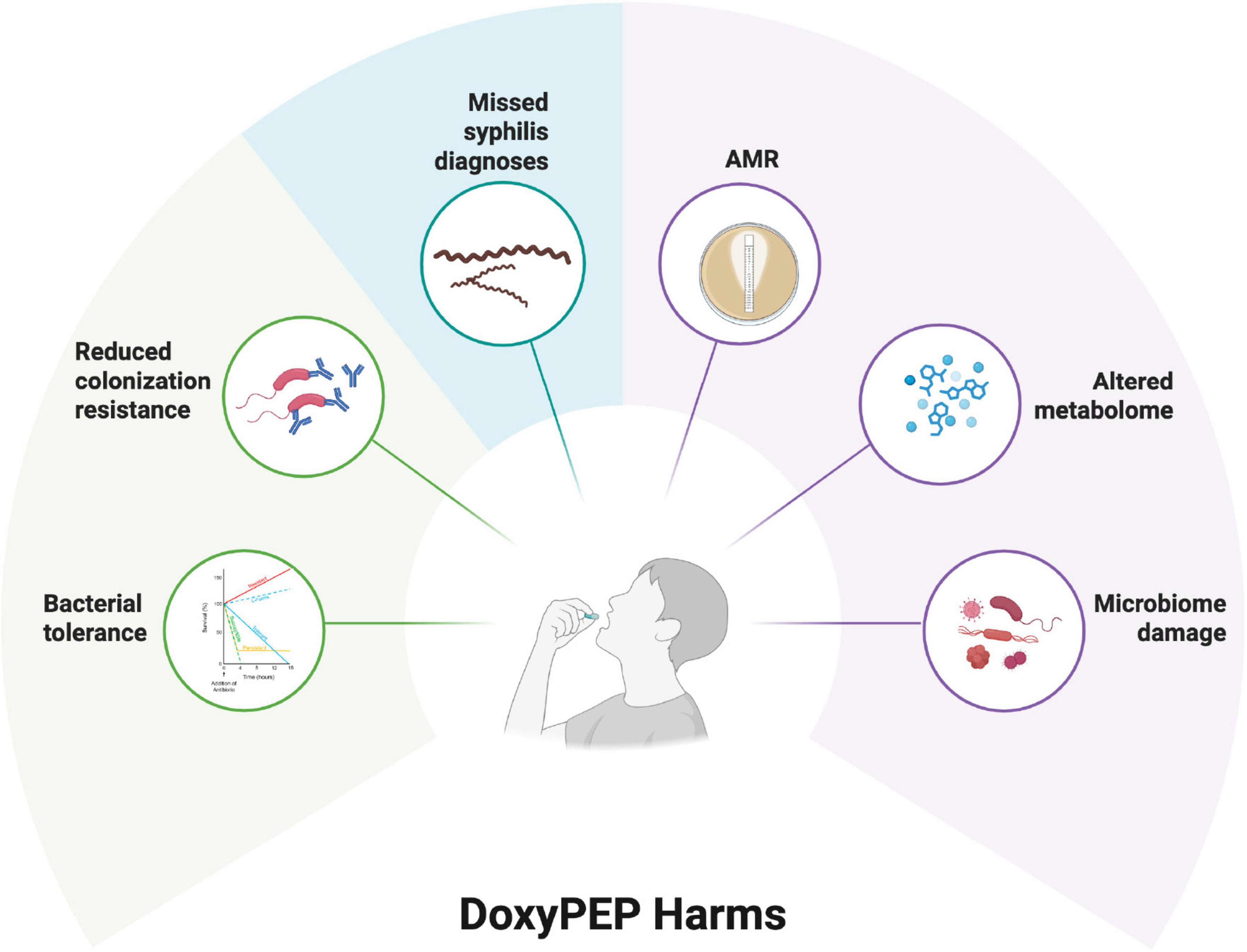
Figure 2. Putative harms of doxycycline postexposure prophylaxis (PEP) as summarized in six domains [see review by Kong et al. (2023)].
Individual level
As noted above, the largest tetracycline PEP RCT thus far, found that minocycline PEP selected for tetracycline resistance in N. gonorrhoeae (Harrison et al., 1979). The more recent DOXYVAC RCT found that doxyPEP had a similar effect on gonococcal tetracycline resistance (Molina et al., 2024). The DoxyPEP RCT did not find this effect but the number of gonococcal isolates assessed was very small (Luetkemeyer et al., 2022, 2025). This study did, however, find that doxyPEP was associated with a higher prevalence of tetracycline resistance in commensal Neisseria spp. and S. aureus (Luetkemeyer et al., 2022, 2025; Vanbaelen et al., 2024a). This increase in S. aureus was however, not statistically significant (Vanbaelen et al., 2024a; Luetkemeyer et al., 2025). The DoxyPEP RCT was the only study to assess the impact of doxyPEP on the resistome using fecal metagenomic sequencing (Chu et al., 2024; Chu et al., 2025). This analysis revealed that doxyPEP had little or no effect on fecal microbiome but was associated with an increase in the abundance of genes conferring tetracyline resistance (Chu et al., 2024).
In a large number of species, including N. gonorrhoeae, tetracycline resistance is strongly associated with resistance to other antimicrobials (Gestels et al., 2023; Kenyon, 2024). By selecting for tetracycline resistance, doxyPEP could therefore inadvertently select for AMR to these other antimicrobial classes (Gestels et al., 2023). Tetracyclines have, for example, previously been shown to select for macrolide resistance in Streptococcus pyogenes (Nielsen et al., 2004). More recently, the DOXYVAC study found that doxyPEP was associated with an increase in gonococcal cefixime MICs (Bercot et al., n.d.; Vanbaelen et al., 2025a). This effect was mediated by selecting for strains with a mosaic penA allelle that has been linked to ceftriaxone resistance (Bercot et al., n.d.).
Population level
The authors of the DOXYVAC study concluded that doxyPEP did not select for extended spectrum β-lactamse (ESBL) producing Escherichia coli or methicillin resistant S. aureus (MRSA) (Molina et al., 2024). They based this conclusion on the fact that there was no increase in the prevalence of these bacteria in the doxyPEP arm compared to the standard care arm (Molina et al., 2024). This analysis is however, limited by only considering individual level selection of AMR (biomedical individualist-based hypothesis testing). A number of analyses have established the importance of population-level selection of AMR (Lipsitch and Samore, 2002; Bell et al., 2014). As an example, differences in country level consumption of penicillin have been shown to explain approximately 80% of the variation in the prevalence of pneumococcal penicillin resistance in Europe (Goossens et al., 2005). Because the participants in both arms of the DOXYVAC study were interacting with one another, it is possible that doxyPEP in one arm could select for AMR in both arms (Vanbaelen et al., 2024c). A reanalysis of the DOXYVAC results using this ecological-hypothesis-testing, found that there was a significant increase in MRSA carriage in the doxyPEP arm (2%–12%) and a delayed and less pronounced increase in MRSA carriage in the standard care arm (2%–10%) (Vanbaelen et al., 2024c). These findings are compatible with the population-selection hypothesis (Vanbaelen et al., 2024c).
The complicated interactions between network connectivity, STI prevalence, antimicrobial consumption and AMR
The evidence that AMR in STIs frequently emerges in sub-populations with dense sexual networks and excessive antimicrobial consumption, provides the rationale for the ecosocial framework to prioritize antimicrobial stewardship in these key populations (Lewis, 2013). It also necessitates an understanding of the links between network connectivity, STI prevalence, and AMR.
Different types of evidence have established that sexual network connectivity is a fundamental determinant of the equilibrium prevalence of STIs and intense STI screening may have little effect on this prevalence (Kenyon and Delva, 2018; Kenyon, 2020). This is illustrated in Figure 3, which represents the N. gonorrhoeae, C. trachomatis, and M. genitalium prevalences of a typical PrEP cohort (Vuylsteke et al., 2019). The cohort report a mean of 5–10 partners per 3 months prevalence, which translates into a dense sexual network which in turn determines the high equilibrium prevalence of the three STIs of ∼ 10% (Vuylsteke et al., 2019). The introduction of three monthly screening for all three STIs at month 0 was perhaps associated with an initial reduction in STI prevalence but this effect was short lived (Vuylsteke et al., 2019). The cessation of M. genitalium screening at month 9 lead to a 50-fold decrease in fluoroquinolone consumption but not an increase in M. genitalium prevalence (Vuylsteke et al., 2019; Kenyon et al., 2021, 2023). A recent RCT has likewise concluded that three monthly screening of MSM taking PrEP had no effect on the incidence of N. gonorrhoeae (compared to non-screening) and a possible small effect on chlamydia incidence (Vanbaelen et al., 2024e). Both studies found that the vast majority of N. gonorrhoeae, C. trachomatis, and M. genitalium infections were asymptomatic and self-resolving (Vuylsteke et al., 2019; Vanbaelen et al., 2024e,2024d). Screening was associated with a large increase in antimicrobial consumption and various lines of evidence suggested that this was associated with the emergence of antimicrobial resistance (Kenyon, 2018; Kenyon et al., 2020; Van Dijck et al., 2020; Vanbaelen et al., 2024e).
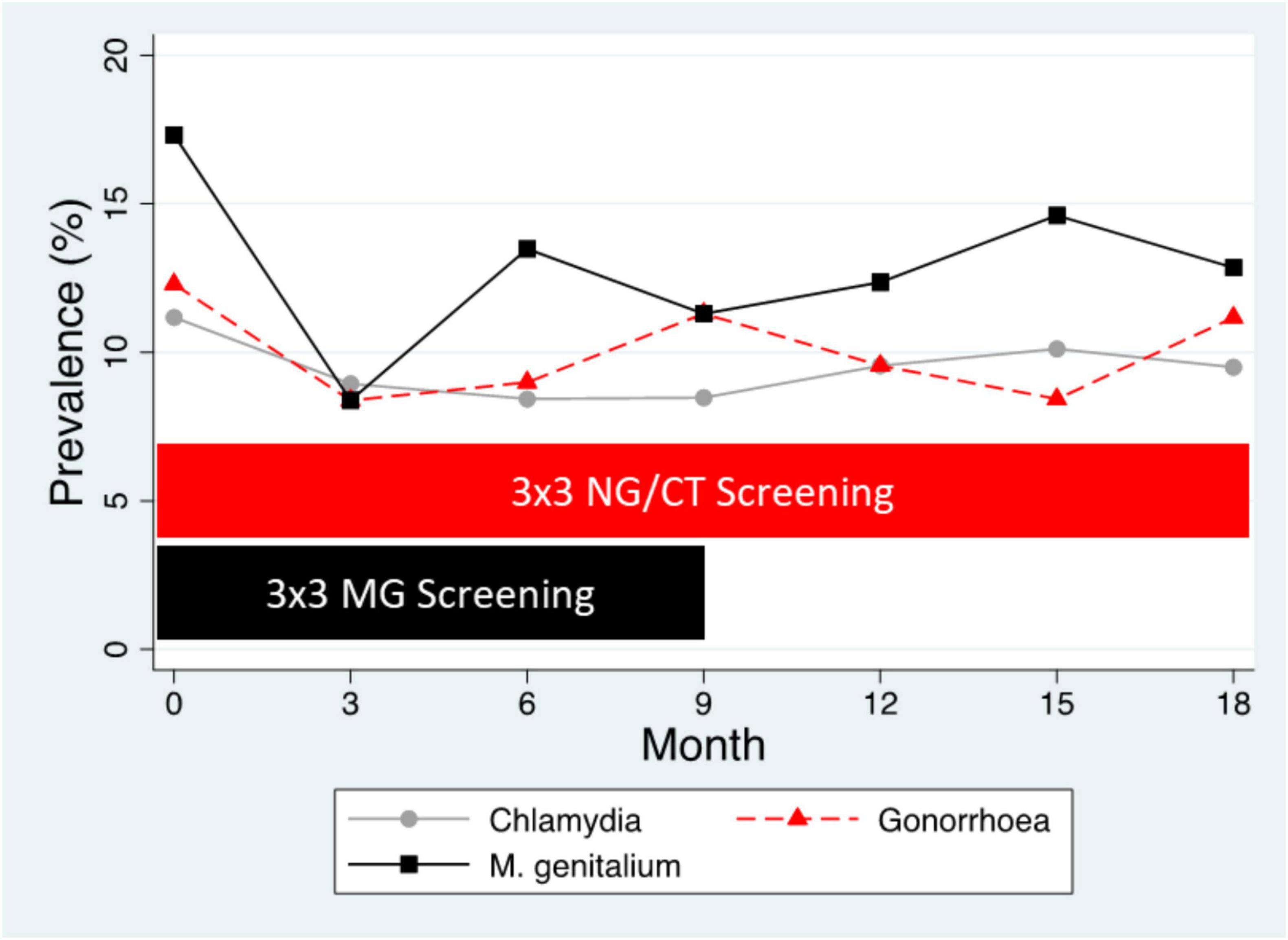
Figure 3. Prevalence of N. gonorrhoeae (NG), C. trachomatis (CT), and M. genitalium (MG) in the BePrEPared Study. This was an observational study of HIV PrEP in men who have sex with men who were tested for these three STIs at three-sites every 3 months. All infections were treated except those for M. genitalium after 9 months [3 × 3 screening– three site (anorectal, urethral, oropharynx), three monthly screening] (Reyniers et al., 2018).
Increases in network connectivity also offer a parsimonious explanation for the recent increases in multiple bacterial STIs noted in many countries (Kenyon and Delva, 2018; Kenyon et al., 2022). This is particularly evident if we consider data from the past 50 years. Using Denmark, the United States and the United Kingdom as examples, the incidence of bacterial STIs dropped precipitously in the 1980’s in response to the AIDS epidemic, which shattered sexual networks via behavior change and removing central nodes from the networks (Chesson et al., 2003; Figures 4, 5). The introduction of antiretroviral therapy for the treatment and prevention (PrEP) of HIV as well as other factors have been associated with an increase in sexual network connectivity (Chesson and Gift, 2008; Figures 4, 5). This has been followed by an increase in the incidence of syphilis, gonorrhea and other bacterial STIs (Mitjà et al., 2023). In the case of Denmark despite the dramatic increase in incidence in N. gonorrhoeae between 2020 and 2023 (46% in 2022 alone), the incidence remains below the pre-AIDS “equilibrium prevalence.” Seen from the ecosocial framework, this patterning would suggest the importance of not relying on the extremely low STI rates of the post-AIDS period (early 1990’s – Figure 4) as the baseline used for defining increases in STI incidence, which in turn justify doxyPEP rollout (Bachmann et al., 2024). This low STI incidence in the post-AIDS period (late 1980’s, early 1990’s) is depicted in Figure 4 where the incidence of syphilis in MSM in the United States fell to close to zero.
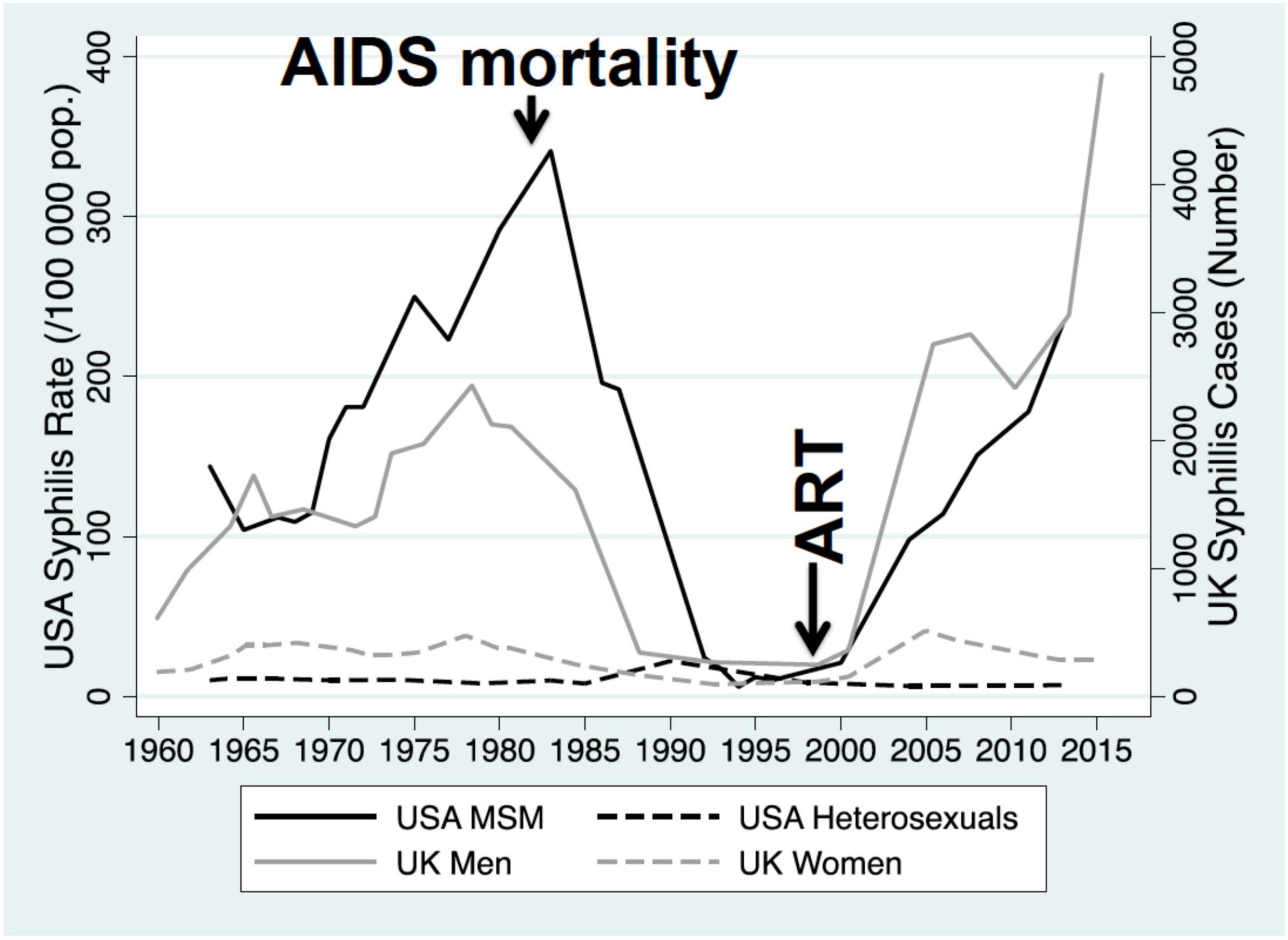
Figure 4. Incidence of primary and secondary syphilis among men who have sex with men (MSM) and heterosexuals in the United States, 1963–2013, and incidence of primary/secondary syphilis in men and women in the United Kingdom, 1960–2015. The AIDS epidemic and introduction of antiretroviral therapy (ART) are indicated with vertical arrows [modified from Kenyon (2020)].
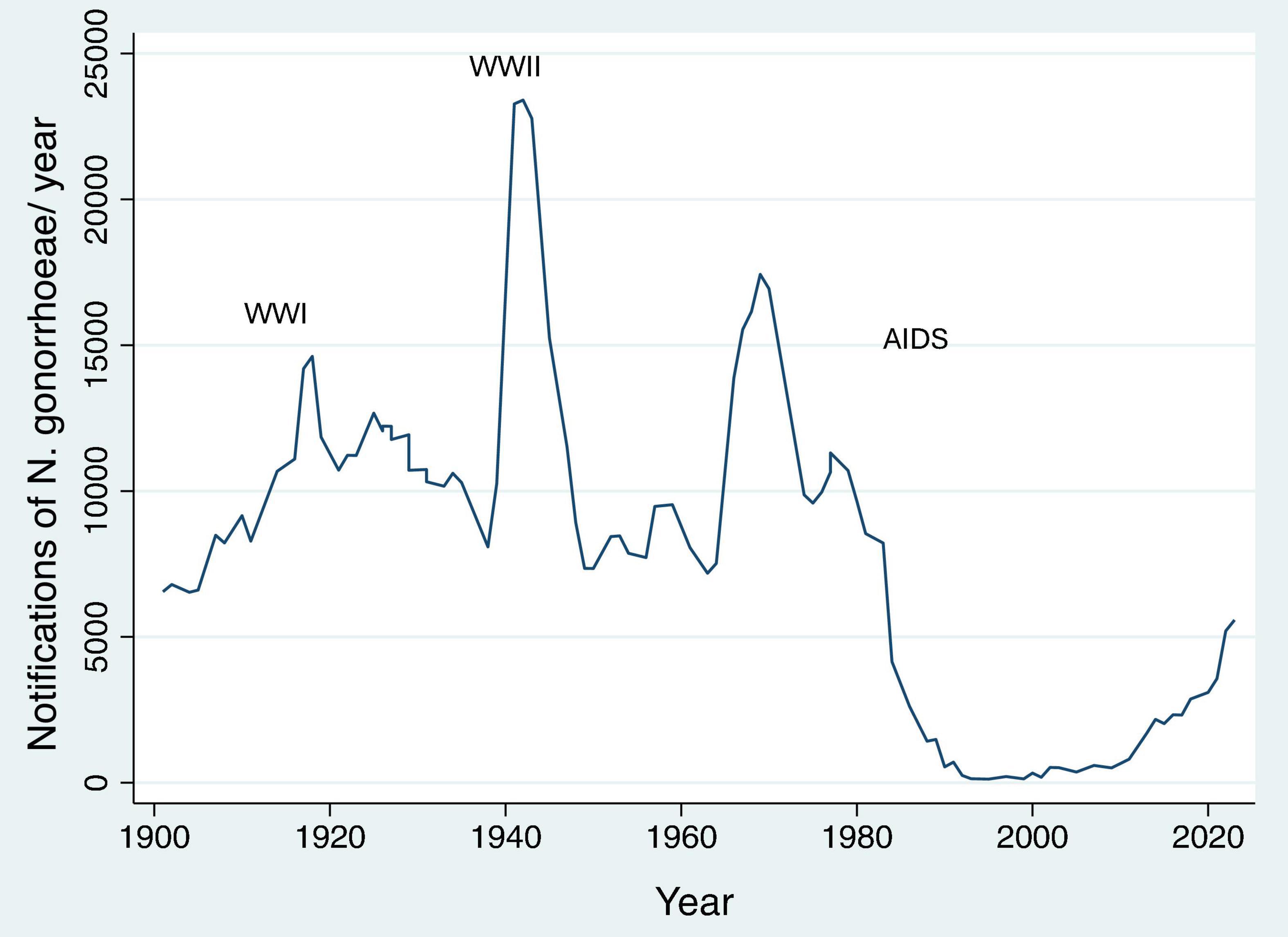
Figure 5. Number of N. gonorrhoeae notifications per year in Denmark between 1901 and 2023 [WWI/II – World War I/II; data from Statens Serum Institute (2023)].
The ecosocial perspective highlights an important concern. Even if doxyPEP lowers the prevalence of N. gonorrhoeae and other bacterial pathogens in PrEP cohorts this is not necessarily a positive outcome, as it is from a biomedical individualist perspective. This is because if doxyPEP lowers the prevalence of N. gonorrhoeae below its equilibrium prevalence, then this will create a selection pressure for N. gonorrhoeae to acquire resistance conferring mutations that will enable to return to its equilibrium prevalence (Figure 1). The larger the decline in equilibrium prevalence the larger this risk will be. The same principles apply to other pathogens such as S. aureus whose prevalence has been reduced in certain doxyPEP RCTs (Luetkemeyer et al., 2023). We acknowledge that this concern is based on theoretical reasoning and the results of mathematical modeling and therefore requires empirical testing (Tsoumanis et al., 2023).
These considerations reveal a fundamental difference between the two perspectives. The biomedical individualist perspective aims for a maximum doxyPEP induced decline in STI prevalence and as such recommends targeting doxyPEP to those with the highest STI incidence such as MSM with the highest rates of partner turnover (Bachmann et al., 2024). The ecosocial perspective cautions that gonococcal AMR has typically emerged in key populations with high rates of partner turnover exposed to high antimicrobial consumption (Lewis, 2013). Targeting these same populations with intensive doxycycline, will once again place selection pressure for AMR emergence in these populations. This effect is congruent with that seen from other interventions involving the intensive use of antimicrobials through screening and mass treatment of STIs (Vanbaelen et al., 2024b). Particularly in MSM, these interventions result in at best a temporary decline in STI incidence at the expense of a long-term increase in AMR (Vanbaelen et al., 2024b). Similarly, previous attempts to use tetracyclines to prevent travelers’ diarrhea were halted by the emergence of AMR (Diptyanusa et al., 2018). A systematic review of the long-term use of tetracyclines for acne found a lack of high-quality evidence as to the risk of selection of AMR (Bhate et al., 2021). This review and other studies did however, find that long-term use of tetracyclines was associated with an increased risk of upper respiratory tract infections and pharyngitis. This effect could be explained by tetracycline-induced reduced colonization resistance (Kong et al., 2023). A more recent systematic review found that tetracycline use for various indications was associated with the selection of AMR (Truong et al., 2022).
The ecosocial perspective is therefore cautious about the use of antimicrobials to reduce STI prevalence in dense sexual networks. Rather it advocates for the use of vaccines, barrier contraception, condom use, and non-antibiotic compounds such as probiotics, chewing gums and bacteriophages as safer ways to reduce STI prevalence (Kenyon, 2020; Laumen et al., 2022; Adamczyk-Popławska et al., 2024). Noting the fact that most N. gonorrhoeae and C. trachomatis infections are asymptomatic and self-resolving in MSM, it is more inclined to a disease control approach that only tests and treats these STIs when they are symptomatic (van Bergen et al., 2021; Kenyon et al., 2023; Williams et al., 2023). In this sense it complies with the principles of optimal use of antimicrobial’s outlined by Spellberg (2029), such as limiting the use of antimicrobials to bacterial infections where treatment will alter the patient’s clinical course.
Using doxyPEP to reduce syphilis incidence
Reducing the incidence of syphilis is the strongest argument for the use of doxyPEP for both perspectives. The Australian doxyPEP guidelines explicitly state that “doxy-PEP should be considered primarily for the prevention of syphilis in GBMSM” (Cornelisse et al., 2024). DoxyPEP is highly efficacious in this regard, involves little risk of inducing AMR in T. pallidum and the consequences of a missed syphilis infection can be severe (Kong et al., 2023; Cornelisse et al., 2024). The ecosocial perspective does however, note that doxyPEP used for syphilis prevention could still select for AMR in off target species. A further consideration is that doxyPEP may obscure the diagnosis of syphilis (Kong et al., 2023; Raccagni et al., 2024; Chircop et al., 2025). Treatment for syphilis with doxycycline requires 14–28 days therapy. Doxycycline taken intermittently may be sufficient to prevent the clinical signs of syphilis and prevent the normal serological response (Kong et al., 2023). This may result in missed or delayed diagnoses (Kong et al., 2023; Chircop et al., 2025).
Community perspectives
For both perspectives it is crucial to take the opinions of the affected populations into account. A number of studies have confirmed that a high proportion of MSM are interested in using doxyPEP (Chow and Fairley, 2019; Evers et al., 2020; Hornuss et al., 2023). There is however, considerable concern about side effects (Evers et al., 2020). One study, for example, found that around 80% of the participants initially reported being willing to use doxyPEP, and 50% reported being concerned about side effects (Vanbaelen et al., 2025c). This study then provided participants with information about the risks of AMR. After receipt of this information, willingness to use doxyPEP decreased to 60% and concerns of side effects increased to 70%. These results suggest that the way individuals and communities view doxyPEP is influenced by how the intervention is framed and the net risk-benefit attributed to its use by experts.
Conclusion
The available evidence shows that doxyPEP clearly reduces the incidence of bacterial STIs in MSM and TGW. Its impressive reduction in syphilis incidence could translate into large declines in the prevalence of symptomatic syphilis in not only this population but the general population as well (Szondy et al., 2024). It also clearly selects for AMR and some of the other deleterious outcomes listed in Figure 2. It is hard to combine these positive and negative outcomes into a single measure to evaluate the net cost-benefit of doxyPEP. One could try and calculate disability adjusted life years for these outcomes. This would however, to a great extent, involve assuming what these outcomes will be. This is because it is far from clear how quickly various STIs will rebound following rollout of doxyPEP and what the extent of doxyPEP-induced-adverse-effects such as AMR will be (Reichert and Grad, 2024). It has been the thesis of this manuscript that, in the absence of this information, individuals and organizations have fallen back onto their core ideologies. For those from a STI control (biomedical individualist) background where combating STIs is paramount, doxyPEP’s proven efficacy at reducing STIs makes it attractive for rapid roll out. For those from backgrounds such as infectious diseases and microbiology where ecological concepts and antimicrobial stewardship are foundational principles (Spellberg, 2029), the concerns of AMR have led to a more cautious approach. There are, of course, also a large number of other factors which could explain variations in attitudes to doxyPEP and therefore many exceptions to this claim are possible. It is also important to acknowledge that most individuals fall somewhere on the spectrum between the biomedical individualist and ecosocial approaches (Kenyon, 2020). Dividing individuals into more biomedical individualist and ecosocial frameworks does however, provide one way to help scientists understand how their colleagues may have very different opinions as to the rollout of doxyPEP. Ultimately, it is merely a hypothesis that requires empirical testing.
Whatever the reasons for the divergence in the rollout of doxyPEP, a benefit of this divergence is that it has set up a large natural experiment with some countries rolling out doxyPEP and others not. This should enable us to compare the individual- and population-level effects of doxyPEP in populations where it has and has not been rolled out. It would be useful to put more thought into collecting standardized samples and data from sentinel sites from these areas in a way that this comparison could be carried out in a meaningful way.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TV: Writing – review and editing. FK: Writing – review and editing. IK: Writing – review and editing. SM-B: Writing – review and editing. CK: Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adamczyk-Popławska, M., Golec, P., Piekarowicz, A., and Kwiatek, A. (2024). The potential for bacteriophages and prophage elements in fighting and preventing the gonorrhea. Crit. Rev. Microbiol. 50, 769–784. doi: 10.1080/1040841X.2023.2274849
Aral, S. O. (1999). Sexual network patterns as determinants of STD rates: Paradigm shift in the behavioral epidemiology of STDs made visible. Sex Transm. Dis. 26, 262–264. doi: 10.1097/00007435-199905000-00004
Aral, S. O. (2001). Sexually transmitted diseases: Magnitude, determinants and consequences. Int. J. STD AIDS 12, 211–215. doi: 10.1258/0956462011922814
Bachmann, L. H., Barbee, L. A., Chan, P., Reno, H., Workowski, K. A., Hoover, K., et al. (2024). CDC clinical guidelines on the use of doxycycline postexposure prophylaxis for bacterial sexually transmitted infection prevention. United States, 2024. MMWR. Recommendations Rep. 73, 1–8. doi: 10.15585/mmwr.rr7302a1
Bell, B. G., Schellevis, F., Stobberingh, E., Goossens, H., and Pringle, M. (2014). A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 14:13. doi: 10.1186/1471-2334-14-13
Bercot, B., Assoumou, L., Caméléna, F., and Molina, J. (n.d.). Antimicrobial Resistance in Neisseria Gonorrhoeae Infections Among MSM using Doxycycline Post-Exposure Prophylaxis. A Substudy of the ANRS DOXYVAC Trial. 37th IUSTI. Zagreb.
Bhate, K., Lin, L.-Y., Barbieri, J. S., Leyrat, C., Hopkins, S., Stabler, R., et al. (2021). Is there an association between long-term antibiotics for acne and subsequent infection sequelae and antimicrobial resistance? A systematic review. BJGP Open 5:BJGO.2020.0181. doi: 10.3399/BJGPO.2020.0181
Chesson, H. W., and Gift, T. L. (2008). Decreases in AIDS mortality and increases in primary and secondary syphilis in men who have sex with men in the United States. J. Acquired Immune Defic. Syndr. 47, 263–264. doi: 10.1097/QAI.0b013e31815e4062
Chesson, H. W., Dee, T. S., and Aral, S. O. (2003). AIDS mortality may have contributed to the decline in syphilis rates in the United States in the 1990s. Sex Transm. Dis. 30, 419–424. doi: 10.1097/00007435-200305000-00008
Chircop, O., Jaggers, C., Spiteri, M., Schembri, A., and Padovese, V. (2025). DOXY do, or DOXY Don’t? Syphilis and doxycycline post-exposure prophylaxis: A case report. Int. J. STD AIDS 36, 324–326. doi: 10.1177/09564624241308026
Chow, E. P. F., and Fairley, C. K. (2019). Use of doxycycline prophylaxis among gay and bisexual men in Melbourne. Lancet HIV 6, e568–e569. doi: 10.1016/S2352-3018(19)30186-9
Chu, V. T., Glascock, A., Donnell, D., Grabow, C., Brown, C. E., Ward, R., et al. (2025). Impact of doxycycline post-exposure prophylaxis for sexually transmitted infections on the gut microbiome and antimicrobial resistome. Nat. Med. 31, 207–217. doi: 10.1038/s41591-024-03274-2
Chu, V., Luetkemeyer, A., and Langelier, C. (2024). “Impact of doxycycline as STI PEP on the gut microbiome and antimicrobial resistance gene expression,” in Proceedings of the Conference on Retroviruses and Opportunistic Infections, (Denver).
Cornelisse, V. J., Riley, B., and Medland, N. A. (2024). Australian consensus statement on doxycycline post-exposure prophylaxis (doxy-PEP) for the prevention of syphilis, chlamydia and gonorrhoea among gay, bisexual and other men who have sex with men. Med. J. Australia 220, 381–386. doi: 10.5694/mja2.52258
De Scheerder, M., Libois, A., Van Praet, J., and Kenyon, C. (2024). Doxy Post-Exposure Prophylaxis for STI Not Endorsed by BREACH. Belgium Research on AIDS and HIV Consortium. Available at: https://breach-hiv.be/wp-content/uploads/2024/03/DoxyPEP-Breach-statement-AL.pdf
Diptyanusa, A., Ngamprasertchai, T., and Piyaphanee, W. (2018). A review of antibiotic prophylaxis for traveler’s diarrhea: Past to present. Trop. Dis. Travel Med. Vaccines 4:14. doi: 10.1186/s40794-018-0074-4
Evers, Y. J., van Liere, G. A. F. S., Dukers-Muijrers, N. H. T. M., and Hoebe, C. J. P. A. (2020). Use of doxycycline and other antibiotics to prevent STIs among men who have sex with men visiting sexual health clinics in the Netherlands. Sex Transm. Infect. 96, 550–551. doi: 10.1136/sextrans-2019-054325
Gestels, Z., Manoharan-Basil, S. S., and Kenyon, C. (2023). Doxycycline post exposure prophylaxis could select for cross-resistance to other antimicrobials in various pathogens: An in silico analysis. Int. J. STD AIDS 34, 962–968. doi: 10.1177/09564624231190108
Goossens, H., Ferech, M., Vander Stichele, R., Elseviers, M., and Group, E. P. (2005). Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 365, 579–587. doi: 10.1016/S0140-6736(05)17907-0
Harrison, W. O., Hooper, R. R., Wiesner, P. J., Campbell, A. F., Karney, W. W., Reynolds, G. H., et al. (1979). A trial of minocycline given after exposure to prevent gonorrhea. N. Engl. J. Med. 300, 1074–1078. doi: 10.1056/NEJM197905103001903
Hogben, M., Leichliter, J., and Aral, S. O. (2020). “An overview of social and behavioral determinants of STI,” in Sexually Transmitted Infections, eds A. Cristado and M. Giuliani (Cham: Springer International Publishing), 25–45. doi: 10.1007/978-3-030-02200-6_3
Hornuss, D., Mathé, P., Usadel, S., Zimmermann, S., Müller, M., and Rieg, S. (2023). Already current practice? A snapshot survey on doxycycline use for prevention of sexually transmitted infections in parts of the German MSM community. Infection 51, 1831–1834. doi: 10.1007/s15010-023-02086-9
Kenyon, C. (2018). Risks of antimicrobial resistance in N. gonorrhoeae associated with intensive screening programs in PrEP programs. Clin. Infect. Dis. 67, 154–155. doi: 10.1093/cid/ciy048
Kenyon, C. (2020). Does intense STI screening cause or prevent antimicrobial resistance in STIs? It depends on one’s underlying epistemology. A viewpoint. Sex Transm. Dis. 47, 506–510. doi: 10.1097/OLQ.0000000000001199
Kenyon, C. (2024). Doxycycline post-exposure prophylaxis could theoretically select for resistance to various antimicrobials in 19 pathobionts: An in silico analysis. Int. J. Infect. Dis. 142:106974. doi: 10.1016/j.ijid.2024.02.017
Kenyon, C. R., and Delva, W. (2018). It’s the network, stupid: A population’s sexual network connectivity determines its STI prevalence. F1000Res 7:1880. doi: 10.12688/f1000research.17148.2
Kenyon, C. R., and Schwartz, I. S. (2018). Effects of sexual network connectivity and antimicrobial drug use on antimicrobial resistance in Neisseria gonorrhoeae. Emerg. Infect. Dis. 24, 1195–1203. doi: 10.3201/eid2407.172104
Kenyon, C., Baetselier, I., and Wouters, K. (2020). Screening for STIs in PrEP cohorts results in high levels of antimicrobial consumption. Int. J. STD AIDS 31, 1215–1218. doi: 10.1177/0956462420957519
Kenyon, C., De Baetselier, I., Vanbaelen, T., Buyze, J., and Florence, E. (2021). The population-level effect of screening for M. genitalium on antimicrobial resistance: A quasi-experimental study. Sex Transm. Dis. 48, 629–634. doi: 10.1097/OLQ.0000000000001404
Kenyon, C., Herrmann, B., Hughes, G., and de Vries, H. J. C. (2023). Management of asymptomatic sexually transmitted infections in Europe: Towards a differentiated, evidence-based approach. Lancet Regional Health Eur. 34:100743. doi: 10.1016/j.lanepe.2023.100743
Kenyon, C., Vanbaelen, T., and Van Dijck, C. (2022). Recent insights suggest the need for the STI field to embrace a more eco-social conceptual framework: A viewpoint. Int. J. STD AIDS 33, 404–415. doi: 10.1177/09564624211064133
Kong, F. Y. S., Kenyon, C., and Unemo, M. (2023). Important considerations regarding the widespread use of doxycycline chemoprophylaxis against sexually transmitted infections. J. Antimicrob. Chemother. 78, 1561–1568. doi: 10.1093/jac/dkad129
Krieger, N. (1994). Epidemiology and the web of causation: Has anyone seen the spider? Soc. Sci. Med. 39, 887–903. doi: 10.1016/0277-9536(94)90202-x
Laumen, J. G. E., Abdellati, S., Manoharan-Basil, S. S., Van Dijck, C., Van den Bossche, D., De Baetselier, I., et al. (2022). Screening of anorectal and oropharyngeal samples fails to detect bacteriophages infecting Neisseria gonorrhoeae. Antibiotics 11:268. doi: 10.3390/antibiotics11020268
Lawes, T., Lopez-Lozano, J. M., Nebot, C. A., Macartney, G., Subbarao-Sharma, R., Wares, K. D., et al. (2017). Effect of a national 4C antibiotic stewardship intervention on the clinical and molecular epidemiology of Clostridium difficile infections in a region of Scotland: A non-linear time-series analysis. Lancet Infect. Dis. 17, 194–206. doi: 10.1016/S1473-3099(16)30397-8
Lewis, D. A. (2013). The role of core groups in the emergence and dissemination of antimicrobial-resistant N gonorrhoeae. Sex Transm. Infect. 89, iv47–iv51. doi: 10.1136/sextrans-2013-051020
Lipsitch, M., and Samore, M. H. (2002). Antimicrobial use and antimicrobial resistance: A population perspective. Emerg. Infect. Dis. 8, 347–354. doi: 10.3201/eid0804.010312
Luetkemeyer, A. F., Donnell, D., Cohen, S. E., Dombrowski, J. C., Grabow, C., Haser, G., et al. (2025). Doxycycline to prevent bacterial sexually transmitted infections in the USA: Final results from the DoxyPEP multicentre, open-label, randomised controlled trial and open-label extension. Lancet Infect. Dis. doi: 10.1016/S1473-3099(25)00085-4 Online ahead of print.
Luetkemeyer, A. F., Donnell, D., Dombrowski, J. C., Cohen, S., Grabow, C., Brown, C. E., et al. (2023). Postexposure doxycycline to prevent bacterial sexually transmitted infections. New Engl. J. Med. 388, 1296–1306. doi: 10.1056/NEJMoa2211934
Luetkemeyer, A., Dombrowski, J., Cohen, S., Donnell, D., Grabow, C., Brown, C., et al. (2022). Doxycycline Post-Exposure Prophylaxis for STI Prevention Among MSM and Transgender Women on HIV PrEP or Living with HIV: High Efficacy to Reduce Incident STI’s in a Randomized Trial., in JAIDS. 25:226.
Mårdh, O., and Plachouras, D. (2023). Using doxycycline for prophylaxis of bacterial sexually transmitted infections: Considerations for the European Union and European Economic Area. Euro Surveill. 28:2300621. doi: 10.2807/1560-7917.ES.2023.28.46.2300621
Mitjà, O., Padovese, V., Folch, C., Rossoni, I., Marks, M., Rodríguez i Arias, M. A., et al. (2023). Epidemiology and determinants of reemerging bacterial sexually transmitted infections (STIs) and emerging STIs in Europe. Lancet Regional Health Eur. 34:100742. doi: 10.1016/j.lanepe.2023.100742
Molina, J.-M., Bercot, B., Assoumou, L., Rubenstein, E., Algarte-Genin, M., Pialoux, G., et al. (2024). Doxycycline prophylaxis and meningococcal group B vaccine to prevent bacterial sexually transmitted infections in France (ANRS 174 DOXYVAC): A multicentre, open-label, randomised trial with a 2× 2 factorial design. Lancet Infect. Dis. 24, 1093–1104. doi: 10.1016/S1473-3099(24)00236-6
Molina, J.-M., Charreau, I., Chidiac, C., Pialoux, G., Cua, E., Delaugerre, C., et al. (2018). Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: An open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect. Dis. 18, 308–317. doi: 10.1016/S1473-3099(17)30725-9
Nielsen, H. U. K., Hammerum, A. M., Ekelund, K., Bang, D., Pallesen, L. V., and Frimodt-Møller, N. (2004). Tetracycline and macrolide co-resistance in Streptococcus pyogenes: Co-selection as a reason for increase in macrolide-resistant S. pyogenes? Microb. Drug Resist. 10, 231–238. doi: 10.1089/mdr.2004.10.231
Raccagni, A. R., Bruzzesi, E., Castagna, A., and Nozza, S. (2024). Doxycycline postexposure prophylaxis may delay seroconversion in incident syphilis. Sex Transm. Infect. 100, 397–397. doi: 10.1136/sextrans-2024-056240
Reichert, E., and Grad, Y. H. (2024). Effects of doxycycline post-exposure prophylaxis for prevention of sexually transmitted infections on gonorrhoea prevalence and antimicrobial resistance among men who have sex with men in the USA: A modelling study. Lancet Microbe 5:100926. doi: 10.1016/S2666-5247(24)00168-X
Reyniers, T., Nostlinger, C., Laga, M., De Baetselier, I., Crucitti, T., Wouters, K., et al. (2018). Choosing between daily and event-driven pre-exposure prophylaxis: Results of a belgian PrEP demonstration project. J. Acquir. Immune Defic. Syndr. 79, 186–194. doi: 10.1097/QAI.0000000000001791
Rosenblatt-Farrell, N. (2009). The landscape of antibiotic resistance. Environ. Health Perspect. 117, A244–A250. doi: 10.1289/ehp.117-a244
Saunders, J., Kohli, M., and Medland, N. (2021). Position Statement on Doxycycline as Prophylaxis for Sexually Transmitted Infections 2021 Update. bashh.orgJM Saunders, M Kohli, NA Medland, H Fifer, D taken as PreBASHH: Lichfield, UK, 2021∙bashh.org. Available online at: https://www.bashh.org/_userfiles/pages/files/sigs/position_statement_refresh_2021_v10_1.pdf (Accessed May 20, 2025).
Sherrard, J., Gokengin, D., Winter, A., Marks, M., Unemo, M., Jensen, J. S., et al. (2024). IUSTI Europe position statement on use of DoxyPEP: June 2024. Int. J. STD AIDS 35, 1087–1089. doi: 10.1177/09564624241273801
Soge, O. O., Thibault, C. S., Cannon, C. A., McLaughlin, S. E., Menza, T. W., Dombrowski, J. C., et al. (2025). Potential impact of doxycycline post-exposure prophylaxis on tetracycline resistance in Neisseria gonorrhoeae and colonization with tetracycline-resistant Staphylococcus aureus and Group A Streptococcus. Clin. Infect. Dis. doi: 10.1093/cid/ciaf089 Online ahead of print.
Spellberg, B. (2029). “Principles of anti infective therapy,” in Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th Edn, eds J. E. Bennett, R. Dolin, and M. J. Blaser (Amsterdam: Elsevier).
Statens Serum Institute (2023). Available online at: https://en.ssi.dk/surveillance-and-preparedness/surveillance-in-denmark/annual-reports-on-disease-incidence/g/gonorrhoea. (Accessed March 2, 2025).
Susser, M., and Susser, E. (1996). Choosing a future for epidemiology: II. From black box to Chinese boxes and eco-epidemiology. Am. J. Public Health 86, 674–677. doi: 10.2105/ajph.86.5.674
Szondy, I., Meznerics, F. A., Lőrincz, K., Kemény, L. V., Walter, A., Mohammed, A. A., et al. (2024). Doxycycline prophylaxis for the prevention of sexually transmitted infections: A systematic review and meta-analysis of randomized controlled trials. Int. J. Infect. Dis. 147:107186. doi: 10.1016/j.ijid.2024.107186
Traeger, M. W., Mayer, K. H., Krakower, D. S., Gitin, S., Jenness, S. M., and Marcus, J. L. (2023). Potential impact of doxycycline post-exposure prophylaxis prescribing strategies on incidence of bacterial sexually transmitted infections. Clin. Infect. Dis. doi: 10.1093/cid/ciad488 Online ahead of print.
Truong, R., Tang, V., Grennan, T., and Tan, D. H. S. (2022). A systematic review of the impacts of oral tetracycline class antibiotics on antimicrobial resistance in normal human flora. JAC Antimicrob. Resist. 4:dlac009. doi: 10.1093/jacamr/dlac009
Tsoumanis, A., Van Dijck, C., Hens, N., and Kenyon, C. (2023). Rethinking screening intensity in terms of reducing prevalence or increasing selection pressure for the emergence of resistant gonorrhea: A modeling study of men who have sex with men in Belgium. Open Forum Infect. Dis. 10:ofad165. doi: 10.1093/ofid/ofad165
van Bergen, J. E. A. M., Hoenderboom, B. M., David, S., Deug, F., Heijne, J. C. M., van Aar, F., et al. (2021). Where to go to in chlamydia control? From infection control towards infectious disease control. Sex Transm. Infect. 97, 501–506. doi: 10.1136/sextrans-2021-054992
Van Dijck, C., Laumen, J., Zlotorzynska, M., Manoharan-Basil, S. S., and Kenyon, C. (2020). Association between STI screening intensity in men who have sex with men and gonococcal susceptibility in 21 States in the USA: An ecological study. Sex Transm. Infect. 96, 537–540. doi: 10.1136/sextrans-2019-054313
Van Praet, J. T., Henrard, S., Kenyon, C., Libois, A., Meuwissen, A., Sauvage, A.-S., et al. (2024). Belgian 2024 guidance on the use of pre-exposure prophylaxis. Acta Clin. Belg. 79, 121–129. doi: 10.1080/17843286.2024.2356337
Vanbaelen, T., Manoharan-Basil, S. S., and Kenyon, C. (2024a). 45 years of tetracycline post exposure prophylaxis for STIs and the risk of tetracycline resistance: A systematic review and meta-analysis. BMC Infect. Dis. 24:376. doi: 10.1186/s12879-024-09275-3
Vanbaelen, T., Manoharan-Basil, S. S., and Kenyon, C. (2024b). Effect of mass treatment on the long-term prevalence of gonorrhoea, chlamydia and syphilis-a systematic review. Int. J. STD AIDS 35, 550–564. doi: 10.1177/09564624241239994
Vanbaelen, T., Manoharan-Basil, S. S., and Kenyon, C. (2024c). Studies of post-exposure prophylaxis with doxycycline should consider population-level selection for antimicrobial resistance. Lancet Infect. Dis. 24, e606–e607. doi: 10.1016/S1473-3099(24)00502-4
Vanbaelen, T., Manoharan-Basil, S. S., and Kenyon, C. (2024d). Treatment of asymptomatic chlamydia and gonorrhoea drives antibiotic consumption in PrEP cohorts. Sex Transm. Infect. 101, 207–208. doi: 10.1136/sextrans-2024-056381
Vanbaelen, T., Manoharan-Basil, S. S., and Kenyon, C. (2025a). Doxy-PEP could select for ceftriaxone resistance in Neisseria gonorrhoeae. Lancet Infect. Dis. 25:e316. doi: 10.1016/S1473-3099(25)00234-8
Vanbaelen, T., Manoharan-Basil, S. S., and Kenyon, C. (2025b). Stop classifying Neisseria gonorrhoeae as an obligate pathogen in men who have sex with men: A viewpoint. Int. J. STD AIDS 36, 337–340. doi: 10.1177/09564624241306600
Vanbaelen, T., Rotsaert, A., De Baetselier, I., Platteau, T., Hensen, B., Reyniers, T., et al. (2025c). Doxycycline post-exposure prophylaxis among men who have sex with men and transgender women in Belgium: Awareness, use and antimicrobial resistance concerns in a cross-sectional online survey. Sex Transm. Infect. 101, 34–40. doi: 10.1136/sextrans-2024-056261
Vanbaelen, T., Tsoumanis, A., and Kenyon, C. (2024f). Total antimicrobial consumption in doxyPEP cohorts depends on the intensity of screening for bacterial sexually transmitted infections. Clin. Infect. Dis. 78, 803–805. doi: 10.1093/cid/ciad553
Vanbaelen, T., Tsoumanis, A., Florence, E., Van Dijck, C., Huis in’t Veld, D., Sauvage, A. S., et al. (2024e). Effect of screening for Neisseria gonorrhoeae and Chlamydia trachomatis on incidence of these infections in men who have sex with men and transgender women taking HIV pre-exposure prophylaxis (the Gonoscreen study): Results from a randomised, multicentre, controlled trial. Lancet HIV 11, e233–e244. doi: 10.1016/S2352-3018(23)00299-0
Vuylsteke, B., Reyniers, T., De Baetselier, I., Nöstlinger, C., Crucitti, T., Buyze, J., et al. (2019). Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: Results of a prospective cohort measuring adherence, sexual behaviour and STI incidence. J. Int. AIDS Soc. 22:e25407. doi: 10.1002/jia2.25407
Werner, R. N., Schmidt, A. J., Potthoff, A., Spornraft-Ragaller, P., and Brockmeyer, N. H. (2024). Position statement of the German STI society on the prophylactic use of doxycycline to prevent STIs (Doxy-PEP, Doxy-PrEP). J. Deutschen Dermatol. Gesellschaft 22, 466–478. doi: 10.1111/ddg.15282
Keywords: doxyPEP, doxycycline, STI - science, chlamydia, gonorrhea
Citation: Vanbaelen T, Kong F, Kanesaka I, Manoharan-Basil SS and Kenyon C (2025) Where we stand on doxyPEP depends on where we sit: a viewpoint. Front. Microbiol. 16:1616111. doi: 10.3389/fmicb.2025.1616111
Received: 22 April 2025; Accepted: 04 June 2025;
Published: 20 June 2025;
Corrected: 15 July 2025.
Edited by:
Takashi Azuma, Osaka Medical College, JapanReviewed by:
Shawnalyn Sunagawa, University of Nebraska Medical Center, United StatesChase Cannon, University of Washington, United States
Copyright © 2025 Vanbaelen, Kong, Kanesaka, Manoharan-Basil and Kenyon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chris Kenyon, Y2tlbnlvbkBpdGcuYmU=
 Thibaut Vanbaelen1
Thibaut Vanbaelen1 Fabian Kong
Fabian Kong Izumo Kanesaka
Izumo Kanesaka Sheeba Santhini Manoharan-Basil
Sheeba Santhini Manoharan-Basil Chris Kenyon
Chris Kenyon