- 1School of Breeding and Multiplication (Sanya Institute of Breeding and Multiplication), College of Tropical Agriculture and Forestry, Hainan University, Sanya, China
- 2Department of Biological Sciences, Faculty of Science and Technology, Virtual University, Lahore, Pakistan
- 3Department of Plant Pathology, College of Agriculture, University of Sargodha, Sargodha, Pakistan
- 4College of Agriculture, Guangxi University, Nanning, China
- 5Department of Plant Protection, Faculty of Agricultural Sciences and Technologies, Sivas University of Science and Technology, Sivas, Türkiye
- 6Department of Biology, College of Science, King Khalid University, Abha, Saudi Arabia
Arbuscular mycorrhizal fungi (AMF) are the basis symbionts in terrestrial ecosystems, profoundly influencing plant development, nutrient acquisition, and resilience to biotic and abiotic stresses. This review synthesizes current systematic understandings of AMF-mediated augmentation of plant growth and disease resistance, with a particular emphasis on their role in sustainable crop production. AMF improves host plant performance through enhanced phosphorus, nitrogen, and water uptake via extensive extraradical hyphal networks. Moreover, AMF colonization modulates phytohormonal signaling pathways, including salicylic acid, jasmonic acid, abscisic acid, and nitric oxide, priming SR and upregulating defense-related gene expression. Increased biosynthesis of secondary metabolites, reinforcement of cell walls, and activation of antioxidant enzyme systems often accompany these responses. AMF also engage in synergistic interactions with rhizosphere microbiota such as Trichoderma, Pseudomonas, and Bacillus, enhancing their collective biocontrol efficacy against a broad spectrum of soil-borne pathogens, including fungi, bacteria, and nematodes. Through modulation of root exudates, glomalin-mediated soil aggregation, and microbiome restructuring, AMF contributes to the establishment of disease-suppressive soils. Genomic and transcriptomic studies have elucidated key components of the common symbiosis-signaling pathway, supporting AMF-host specificity and functional outcomes. AMF is a promising biotechnological tool for integrated pest, disease, and nutrient management. Advancing their application in field settings requires targeted research on strain-host-environment interactions, formulation technologies, and long-term ecosystem impacts, aligning AMF-based strategies with the goals of resilient and sustainable agriculture.
1 Introduction
Fungi have developed numerous strategies for plant colonization, ranging from beneficial to fatal for the host. Fungi are perhaps the most complex group of economically and ecologically significant threats in terms of plant pathogens. Fungal infections can cause a wide range of symptoms. Today, ~19,000 fungi are globally recognized as causing crop plant diseases. Fungi can be dormant under unfavorable environmental conditions, even when found on live or dead plant tissues. Certain fungi can develop in host plant tissue and be dispersed through the soil, water, wind, and insects to other crop-growing areas (Jain et al., 2019). However, fungi can be mutualistic or pathogenic; a mutualistic relationship with a host involves growth promotion and development, and mycorrhizae form a reciprocal relationship with host root systems. However, pathogenic fungi cause diseases like anthracnose, rusts, smuts, leaf spot, blight, wilt, gall, scab, root rot, damping-off, mildew, canker, and dieback. These fungal diseases contribute to significant yield loss, commercial crop loss, and decreased crop quality (Iqbal et al., 2018). Rapidly recognizing fungal disease symptoms is an efficient strategy for controlling and preventing the spread of fungal diseases. The timely detection and identification of fungal symptoms are crucial for effective management of plant diseases. The process of crop disease management involves assessing the adverse effects of pathogens on crop yield (Baiyee et al., 2019). People rely heavily on consistent and stable farm production, but fungal diseases can pose significant threats to food safety. To ensure the overall health of plants and crops globally, it is necessary to control plant diseases. To date, numerous methods have been developed to protect plants from diseases. Rather than implementing new and improved agricultural procedures, most farmers have primarily focused on pesticides for the past few centuries (Chen et al., 2023). However, numerous advances in cultivation science have occurred over the last century. Due to the extensive use of fungi, bacteria, nematodes, and other pathogens, as well as the use of chemical pesticides in agriculture, ecosystems are becoming increasingly resistant to pesticides (Mubeen et al., 2023). Additionally, a growing number of their natural enemies have been eliminated, leading to an increase in pests and diseases (Sanchez-Bayo, 2021). Chemical pesticides contaminate the soil, water, and air simultaneously, harming the environment and the organisms in the food chain, including insects, and impairing human health (Zhou et al., 2025b). Pesticide and fertilizer-related food safety issues, as well as the ongoing development in people's living standards, have drawn considerable attention (Razak and Gange, 2023). Thus, one of the primary areas of interest for environmental scientists and plant pathologists is the pursuit of eco-friendly technologies to manage plant diseases and insect pests (Begum et al., 2019). One method that has drawn considerable interest is biological control (Van Driesche et al., 2010) due to its outstanding efficiency, low consumption, environmental safety, and diverse applications. Consequently, soil scientists, plant pathologists, and ecologists have extensively investigated it (Prospero et al., 2021). Due to increasing health concerns, an innovative disease management method, biological management, has been implemented, and many microorganisms help keep plant diseases in check (Aria et al., 2025). The use of this method has been revived for the first time in many years due to its minimal environmental and health risks to humans. Soil-borne fungi, known as AMF, can significantly increase plant resilience to various abiotic stressors and nutrient uptake (Sun et al., 2018; Mehmood et al., 2022). Arbuscular mycorrhizal fungi (AMF) is classified into Glomerales, Archaeosporales, Paraglomerales, and Diversisporales. Within these 4 orders, 25 genera are located in the subphylum Glomeromycotina of the phylum Mucoromycota, which encompasses the majority of AMF species (spp.) (Redecker et al., 2013; Goss et al., 2017). It is obligate biotrophs that consume photosynthetic plant products and lipids to complete their life cycle. AMF-induced growth enhances the absorption of water and mineral nutrients from the surrounding soil while safeguarding plants against fungal infections (Ahammed et al., 2023). Therefore, AMFs are vital endosymbionts that influence plant productivity and ecosystem functioning. It is essential to improve crops sustainably (Gianinazzi et al., 2010; Chaudhary et al., 2025). AMF releases hyphal chemicals into the soil to control the hyphosphere that different microorganisms have invaded. The rhizosphere and bulk soil have distinct microbial compositions compared to the hyphosphere (Wang et al., 2025). The shift in the microbiome has an impact on nutrient cycling in the hyphosphere. The organic nutrition cycle is influenced by variations in microbial function, making the hyphosphere a unique and vital functional zone in ecosystems. AMF forms a symbiotic relationship with nearly two-thirds of terrestrial plants, providing them with essential nutrients and supporting their growth. Particular microorganisms are attracted to their hyphosphere by AMF hyphae, the small area of soil that is impacted by hyphal exudates (Wang et al., 2024). It molds this alleged second DNA of AMF, notably assisting in the turnover and mobilization of nutrients. Beneficial interactions between microbes and plants are a natural phenomenon, and there is ample evidence of the potential advantages these interactions offer for plant development and health. Typically, in controlled laboratory settings, some of the mechanisms underlying these advantages have been elucidated (Gruden et al., 2020). AMF establishes intimate mutualistic associations with the roots of most vegetable crops and more than 70% of terrestrial plant spp. (Pozo De La Hoz et al., 2021). AMF induces MIR against various foliar and root diseases and pests, and AMF can also boost resistance or tolerance in plants to biotic stressors (Abarca et al., 2024). It is acknowledged that plants regulate the degree of fungal colonization in response to their requirements and the surrounding environment (Pozo et al., 2015). Consequently, understanding how AMF symbiosis is regulated and the advantages it offers under certain circumstances requires an understanding of its context dependency. Systemic resistance (SR) induced by AMF has been shown in interactions with several pathogens and might be reflected in the systemic autoregulation of mycorrhizal colonization. It has been hypothesized that plants utilize the autoregulation mechanism as a preventative measure against further mycorrhizal colonization, while simultaneously defending against pathogens (Fiorilli et al., 2024). The rhizosphere microbiome is primarily shaped by host resistance, while the microbiomes of the roots have been found to be significantly influenced by pathogenic fungal infections. Fungal networks in the roots are significantly impacted by plant disease and host resistance, as well as a few spp. predominate in the communities from the healthy plants.
2 Role of AMF in enhancing plant growth and stress resistance
The symbiotic relationship between AMF and plants was documented 400 million years ago (Table 1) (Mythili et al., 2025). These connections are formed by a series of biological processes, resulting in numerous advantageous impacts on natural ecosystems and agricultural biotas (Van Der Heijden et al., 2015). The symbiotic relationship of AMF exemplifies a mutualistic interaction that can influence plant growth and development. The mycelial network of fungi spreads beneath plant roots, facilitating the absorption of nutrients that are normally unavailable (Ahmed et al., 2025). The fungal mycelium infuses the roots of numerous plant spp., forming a common mycorrhizal network (CMN) (Figueiredo et al., 2021), and it is considered a central component of the terrestrial ecosystem, intensely affecting several plant communities, principally invasive spp., and permitting the AMF-mediated transfer of nitrogen (N) and phosphorous (P) to plants (Begum et al., 2019). AMF developed synergistic interactions with plants by colonizing their root systems, contributing to enhanced water uptake and nutrient absorption, as well as increased resistance against biotic and abiotic stresses (Boyno et al., 2023). It can improve soil structure and stimulate plant growth in standard and complex conditions. AMF enhances the tolerance of plants in saline soils by enriching soil structure and supporting various plant mechanisms, including the uptake of water and nutrients, antioxidant defense systems, photosynthesis, and the production of secondary metabolites (SMs) (Boorboori and Lackoova, 2024). It is considered a natural biofertilizer that supplies the host with water, nutrients, and pathogen defense in return for photosynthetic byproducts. Therefore, AMF are important biotic components of soil, and their absence or shortage can result in reduced ecosystem competence. Sustainable agriculture can be achieved by reinstating the natural abundance of AMF, as it is a practical alternative to traditional chemical fertilization. The primary method to achieve this goal involves the direct reintroduction of AMF propagules into the specified soil. AMF has no specific host or niche preferences, signifying their potential role in agriculture across a variety of environmental settings (Berruti et al., 2015). AMF inoculation has the potential to maintain and stabilize soil organic carbon (SOC) by promoting the growth of fungal communities. In N-scarce soils, AMF also simultaneously reduce microbial extracellular enzyme activity. AMF contributes to the enrichment of a persistent carbon sink in drylands through its selective influence on SOC components as a rhizospheric carbon engineer (Li et al., 2025). AMF inoculation significantly increased the abundance and diversity of the rhizosphere fungal community, with a more complex co-occurrence network. The abundance and diversity of the rhizosphere bacterial community were reduced significantly (Chang et al., 2021). AMF symbiosis significantly increased the allocation of photosynthetic carbon to the roots and rhizosphere soils of maize plants. AMF inoculation promoted the levels of macro-aggregates in the soil and microbial biomass carbon in low SOC conditions and increased the formation of soil aggregates, as well as the chemical composition of SOC (Li et al., 2024a). The influence of AMF on SOC sequestration is significant, as it alters the quantity and quality of carbon incorporated and the processes regulating its transformation and storage (Liu and Chen, 2024). Glomalin-related soil protein (GRSP) is mainly produced through the decomposition of AMF mycelium and is a varied assortment of plentiful extracellular proteins along with other components (Ling et al., 2025). Elevated concentrations of GRSP in soils signify increased soil aggregate stability and improved long-term SOC and N sequestration. Meanwhile, extended AMF inoculation reduces soil N stocks and inhibits microbial hydrolase synthesis for carbon substrates (Li et al., 2025). GRSP provides a significant source of many macro and micro-elements, such as C, H, O, S, K, P, Ca, Si, Fe, Cu, and Mg, which are vital for plant development and aid in the immobilization of heavy metal pollutants in soils and sediments (Ji et al., 2025). Glomalin protects soil from dehydration by improving its water retention ability. It consists of 30–40% carbon and related chemicals (Sharma et al., 2017). AMF, as natural root symbionts, provide essential inorganic nutrients to host plants, hence improving growth and yield under both stressed and unstressed situations (Begum et al., 2019). The inoculation of AMF influences growth functions, including stomatal conductance, leaf water potential, relative water content (RWC), PSII efficiency, and CO2 assimilation. AMF enhances nutrient absorption, greatly increasing plant resilience to drought, salinity, and heavy metal stress through optimizing water usage efficiency and the modulation of physiological metabolic processes. Additionally, AMF stimulate the plant immune system, augmenting resistance to soil-borne diseases and nematodes, and improving crop safety and quality (Nie et al., 2024). Furthermore, the inoculation of AMF augments water and dry matter absorption, improving plant resilience to stressors such as salinity and desiccation. Employing AMF for plant development across diverse biological environments can significantly enhance organic cultivation, aiming to optimize yield and foster growth.
3 AMF as biocontrol agents against soil-borne plant pathogens
AMFs inhabit the soil and infect plant roots, substantially influencing soil-borne diseases (Cruz and Ishii, 2012; Li et al., 2021b). AMF has been extensively utilized as a biological control strategy against several phytopathogenic fungi (Lin et al., 2021). The biocontrol efficacy of AMF has been documented across various plant spp. and against numerous diseases, predominantly soil-borne fungal pathogens responsible for root rot or wilting. Successful biocontrol has also been documented against aerial infections, including Alternaria solani in tomatoes (Harrier and Watson, 2004). AMF has been reported to reduce both necrotrophic and biotrophic diseases directly or indirectly (Schouteden et al., 2015). AMF establish a symbiotic association with plant roots, thereby playing a crucial role in managing soil-borne diseases (Cruz and Ishii, 2012) and are extensively utilized as biocontrol agents against plant pathogenic fungi (Lin et al., 2021). Mycorrhizal cotton plants have shown superior resistance to infection by the pathogen Thielaviopsis basicola compared to those with sterile roots. Later studies demonstrated that the generation of chlamydospores by T. basicola was inversely correlated with the degree of mycorrhizal infection (Thakur et al., 2024). The interaction between AMF and Rhizobium, alongside two pathogenic fungi, Pythium ultimum and Phytophthora megasper, showed that mycorrhizal fungi reduced the occurrence of plant death caused by P. megasper (Chou and Schmitthenner, 1974; Ghorui et al., 2024). Compared to the control group without AMF inoculation, the illness index and incidence of Ralstonia solanacearum were reduced by 9.7% and 49.8%, respectively, when infected with G. rhizogenes and G. mossie (Steinkellner et al., 2012). G. asciculatum, G. etunicatum, G. macrocarpum, G. Margarita, G. heterogama, and G. calospora in AMF can mitigate diseases induced by pathogenic fungi from the genera Pythium, Phytophthora, Fusarium, Rhizoctonia, Macrophomina, Pyrenochaeta, Thielaviopsis, Phoma, Cylindrocarpum, Ophiobolus, and Sclerotium in barley, peanut, soybean, banana, cotton, kidney bean, onion, tobacco, citrus, peach, poplar, strawberries, red clover, and ginseng (Weng et al., 2022). G. intraradices inhibited the proliferation of the pathogenic fungus F. oxysporum, suggesting that the chemical equilibrium of mycorrhizae suppresses the growth and reproduction of pathogenic fungi (Singh, 2020). Infected peas with Aphanomyces euteiches demonstrate that establishing a complete AMF symbiosis is crucial for plant defense against pathogens (Slezack et al., 2000; Wang et al., 2022). Phytophthora is a typical pathogenic fungus widely employed in the treatment of plant diseases associated with AMF (Krzyzaniak et al., 2021). The application of P and AMF pre-treatment in tomatoes infected with G. intraradices and the pathogen F. oxysporum resulted in diminished disease severity. Factors such as the specific plant disease, the interaction between AMF and host plants, the amount and timing of AMF inoculation, and environmental variables (Weng et al., 2022) all affect the effectiveness of AMF in managing plant diseases. Phytophthora served as a model pathogenic fungus to elucidate the mechanism of AMF-mediated disease control (Krzyzaniak et al., 2021). The efficacy of G. intraradices against F. oxysporum can be enhanced by the use of P, thereby reducing disease severity in tomatoes (Steinkellner et al., 2012). The disease control mechanism of AMF is affected by various aspects, including the pathogenic organism, the symbiotic interaction between AMF and the host, the timing and concentration of AMF inoculation, and environmental conditions.
4 AMF in managing bacterial and nematode-induced plant diseases
AMF plays a crucial role in regulating bacterial and nematode diseases through diverse molecular mechanisms and signal transduction pathways (Schouteden et al., 2015). SR is induced in host plants by the colonization of AMF through the activation of defense-related genes (DRGs), including those involved in salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) signaling pathways that are fundamental in the resistance against biotrophic and necrotrophic pathogens (Stratton et al., 2022). AMF also regulates the expression of pathogenesis-related proteins and stimulates the activity of antioxidant enzymes, thereby limiting oxidative stress during pathogen attack. AMF also alter root exudation patterns, indirectly inhibiting nematodes and soil-borne pathogens by restructuring the rhizosphere microbiome (Schouteden et al., 2015; Afridi et al., 2024). AMF-mediated suppression of Meloidogyne incognita and R. solanacearum is coupled to the upregulation of defense-related genes (DRGs) such as PR-1, LOX, and PAL and enhanced production of secondary metabolites (SMs), including phenolics and flavonoids (Vos et al., 2013; Zhu et al., 2018). Additionally, pathogen entry is restricted due to AMF-stimulated cell wall modifications and the deposition of callose and lignin (Underwood, 2012). Inoculation of tomato plants with G. intraradices induced the expression of PR-1 and PR-5 genes, thereby enhancing resistance to R. solanacearum (Gao et al., 2004). G. mosseae also enhanced soybean resistance against Heterodera glycines by upregulating JA- or ET-regulated defense genes and antioxidant enzyme activity (Guo et al., 2015). In cucumber, AMF colonization led to the upregulation of lignin biosynthesis and SM genes, which limited M. incognita penetration and gall formation (Schouteden et al., 2015). R. solanacearum induced bacterial wilt in tomatoes (Yuliar et al., 2015), and it can be controlled with mycorrhizal application. Inoculation of mulberry with G. fasciculatum or G. mosseae in combination with 60–90 kg of P per hectare per year reduced the incidence of bacterial blight caused by P. syringae (Imad Khrieba, 2019). AMF application in grape fields has harmed the population of P. fluorescens in the rhizosphere and reduced the likelihood of disease recurrence. G. mosseae suppressed P. syringae and safeguarded soybean and apple seedlings, which can be protected by root treatment with AMF against Actinomycetes. M. incognita and M. javanica can cause total crop failures in tobacco, tomato, sunflower, and pepper, respectively, while AMF symbionts enhance plant tolerance to nematodes (Schouteden et al., 2015). However, it can only inhibit the damage caused by nematodes (Weng et al., 2022). AMF reduces infection and reproduction of root-knot nematodes in crops like tomatoes, bananas, and coffee (Schouteden et al., 2015). G. mosseae and Rhizophagus irregularis reduce infection in bananas by Radopholus similis (Mandou et al., 2023) and controlled M. exigua in coffee plants (Alban et al., 2013). Soybean cyst nematodes parasitised by AMF and the degree of disease-causing ability in soybeans, oats, cucumbers, cotton, kidney beans, tomatoes, citrus, peach, and alfalfa is decreased (Rodrigues et al., 2021). The inoculation of G. mosy controlled the M. incognita population in tobacco and developed disease resistance against nematodes (Liu et al., 2012). Mycorrhizated plants showed fewer galls on the roots of tomato plants than non-mycorrhizated plants, and the infection rate was significantly reduced. AMF colonization can modify host root exudates (Ma et al., 2022) and enhance the antagonistic rhizosphere environment toward pathogens such as Pseudomonas syringae and Agrobacterium tumefaciens, in addition to affecting the levels of phenolic acids in cotton root exudates, hence reducing the incidence of cotton Fusarium wilt (Zhang et al., 2012). Furthermore, AMF enhance callose deposition, cell wall fortification, and detoxification of reactive oxygen species, which are essential at the early stages of pathogen invasion (Nath et al., 2016). Subsequent to AMF colonization, it can limit nematode motility and alleviate nematode infestation in tomatoes by affecting the release of root exudates (Yizhu et al., 2020). AMF colonization can improve resistance by modifying host root exudates. The colonization by AMF affects changes in plant root exudates, and these variations in exudates simultaneously influence the growth and development of AMF, and it interacts with others rather than existing independently (Zhang et al., 2024). The total molecular responses attest to the potential of AMF as an efficient biocontrol agent in agricultural practices.
5 Symbiotic interactions of AMF with microbiota in plant disease management
The synergistic effect of AMF and Trichoderma harzianum is more considerate in the management of severity and incidence of diseases than the use of T. harzianum and AMF alone, and studies showed combined application in the field of Solanum lycopersicum enhanced aboveground biomass by 11.6–69.7% (Weng et al., 2022). Inoculation of F. oxysporum on tomatoes resulted in a disease incidence rate of 70%. After applying Acaulospora laevis and G. mosseae, the decrease was 20%. However, a 10% reduction was found with the inoculation of T. virid and AMF (Tanwar et al., 2013). AMF and Trichoderma can together prevent the occurrence of disease. However, their different combinations have different control effects on plant diseases. If AMF was individually inoculated against Cucumis melo Fusarium wilt, it only reduced disease incidence from 25% to 60% (Martinez-Medina et al., 2010). Furthermore, the same combination of Trichoderma and AMF also has varying effects on the different spp. types. The disease control effect of T. harzianum and G. clarum for HEL246 (a variety of Halianthus tuberosus) was the best. At the same time, AMF alone was the best control for variety JA37 (Sennoi et al., 2013). Synergistic effect of Pseudomonas and AMF refining plant disease resistance rather than application individually. The individual application of G. albida, G. sinosum, or P. fluorescens against the disease induced by Phaseolus vulgaris can only reduce the disease by 50.5 to 52.8%, while the combined application shows a reduction from 68.9 to 69.2%. The combined application increases the P and N contents of plants compared to single inoculation (Neeraj and Singh, 2011). It was discovered that a combination inoculation of G. sinuosum and P. fluorescens was more successful against the diseases caused by F. oxysporum in tomatoes (Srivastava et al., 2010) and papayas (Hernández-Montiel et al., 2013). However, the synergistic effects of P. fluorescens and AMF on plants were not all positive. When applied together, P. fluorescens + G. messeae had a more significant growth-enhancing impact than when applied alone in the absence of pathogenic microorganisms (Behn, 2016). In addition, the combined applications of AMF and P. aeruginosa manage plant diseases, as do the applications of P. fluorescens and AMF. Elaeis guineensis base rot severity was reduced from 15% to 17% when inoculated with AMF alone (G. clarum and G. intraradices). In contrast, if combined with P. aeruginosa, the reduction of severity was found to be 57–80% (Parvin et al., 2020). The synergistic biocontrol effect of Bacillus and AMF on diseases of plant roots is the best control method. The mixed use of Bacillus subtilis and G. mosseae can decrease the disease severity of tomato fusarium root rot from 85% to 93.4%. In addition, it is involved in plant nourishment (N, potassium (K), P, magnesium, calcium, zinc, and iron), total soluble protein, total soluble sugar, total free amino acid content, and leaf pigment (Cai et al., 2021). Single B. vallismortis and G. versiforme can decrease the verticillium wilt index for cotton from 35.7% to 37.7%, respectively. Still, a combined application can reduce the disease by up to 63.3% (Zhang et al., 2012). Additionally, it can be 73.6 to 82.1% effective when applied against F. oxysporum (Cai et al., 2021) and 34.1 to 52.1% effective when applied singly (Rashad et al., 2020). Glomus can enhance the ability of B. subtilis to suppress strawberry Fusarium wilt (Tahmatsidou et al., 2006). Strawberries with a combination inoculation had a 61.7–90.9% increase in fresh weight as compared to a single application (Table 2).
6 Non-symbiotic interactions of AMF with microbiota in plant disease management
AMF participates in non-symbiotic interactions with soil microbiota, which significantly affect plant disease management. While not characterized by direct symbiotic nutrient exchange, these interactions influence microbial community composition and activity, thereby improving plant resilience to pathogens (Purohit et al., 2024). AMF exudates, including strigolactones and glycoproteins, promote the growth of beneficial rhizobacteria and fungi, thereby enhancing a suppressive soil environment (Ghorui et al., 2024). AMF-induced alterations in the rhizosphere microbiome increase the prevalence of Pseudomonas and Bacillus spp., which produce antibiotics and siderophores that inhibit soil-borne diseases (Lahlali et al., 2022). Additionally, AMF hyphae create an environment conducive to microbial colonization, enhancing niche competition and reducing pathogen viability (Yuan et al., 2021). Non-symbiotic interactions enhance plant SR by activating DRGs and phytohormone signaling pathways (Mhlongo et al., 2018). Furthermore, AMF-induced changes in soil aggregation and organic matter breakdown impact microbial habitat dynamics, thereby indirectly reducing the proliferation of pathogens (Frey, 2019). Field studies have demonstrated that AMF-associated microbiota minimize the occurrence of Fusarium wilt and Phytophthora root rot, highlighting their potential as biocontrol agents (Kashyap et al., 2024). Utilizing non-symbiotic AMF-microbiota interactions offers a sustainable strategy for integrated disease management, reducing reliance on chemical fungicides and enhancing soil health.
7 Expanding research on AMF in plant disease control mechanisms
The primary mechanisms associated with the research on utilizing AMF in the control of plant diseases include enhancing the micro-environment of the rhizosphere, modifying the morphological structure of plant roots, improving plant nutrition, sustaining the synthesis of SMs, directly competing with pathogenic microorganisms for invasion sites and nutrients, and inducing the formation of plant defense systems and disease resistance (Figure 1) (Tatsumi et al., 2020; Chen et al., 2021).
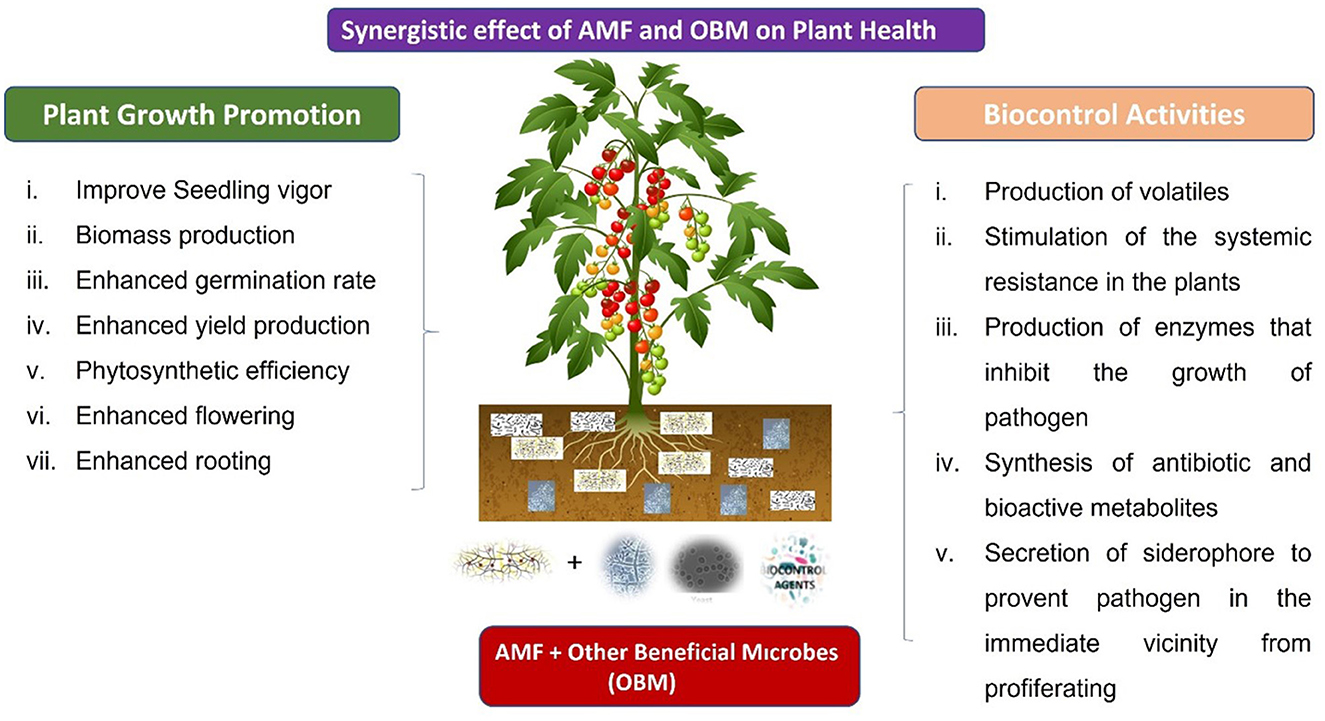
Figure 1. The network highlighted key terms in the article title, abstract, or keywords related to the AMF and their role in biotic stress management. In the network, the same color shows a cluster of interconnected phrases; however, the circle size indicates the number of publications.
7.1 Structural modifications induced by AMF symbiosis for enhanced plant resistance
In host plants, AMF can lead to the growth, thickening, and branching of the root system, effectively slowing down the virus infecting the roots (Basyal and Emery, 2021; Figure 2A). Symbiotic roots with G. etunicatum and G. mosseae of Gossypium hirsutum increased root xylem structure due to Verticillium dahliae effect, and deformed vessels produced gelatinous substances, shrunken and altered cells, palsied tissue, significant thickening of a cell wall, and deepened color of cells (Weng et al., 2022). The number of vacuoles in cells decreased, the inner folds of mitochondria disappeared, and the root system underwent multiple structural changes, all of which positively improved host resistance against Verticillium dahliae. Mycelial network and callose formed by AMF infection induced papillary structure in the root epi and endodermis with the arrangement of non-esterified pectin (Figure 2B), which is an obstacle for the penetration of pathogen in root cell and tissues and AMF alter the anatomy of tomato roots and changed infection kinetics of Phytophthora (Pozo et al., 2002). Plants and AMF make fully functional symbiotic interactions by establishing surface contact that initiates nutrient exchange and signal transduction. The symbiotic interface is defined as a molecular exchange between plants and AMF cytoplasm via cell walls and plasma membranes (Balestrini and Bonfante, 2014). AMF stimulated the production of hydroxyproline-rich glycoproteins (HRGPs) in mycorrhizal plants (Balestrini and Bonfante, 2014). HRGPs are sugar-containing linear proteins embedded in the plant cell wall. When pathogens attack, these proteins reinforce the cell wall, reducing the breakdown caused by pathogen-secreted enzymes such as proteases, hemicellulases, and cellulases. Additionally, HRGPs function like lectins, acting as adhesive molecules that trap and immobilize invading pathogens, thereby preventing their further penetration into plant cells. AMF also modifies the root system architecture, improving plant resistance against infections (Figure 2C). Some spp. of Glomus, extra-root hyphae, the cell wall of spores, and the germ tube inner wall contain β-1, 3-glucan, while β-1, 3-glucan is not present in the cell wall of Gigaspora or Scutellospora spp. (Ma et al., 2021a). It serves as a structural component and provides a defensive barrier against pathogens. Employing AMF as a biocontrol agent can modify the root's anatomical structure and enhance naturally occurring defensive compounds, thereby boosting the innate resistance in plants to diseases and pests (Silvestri et al., 2020).
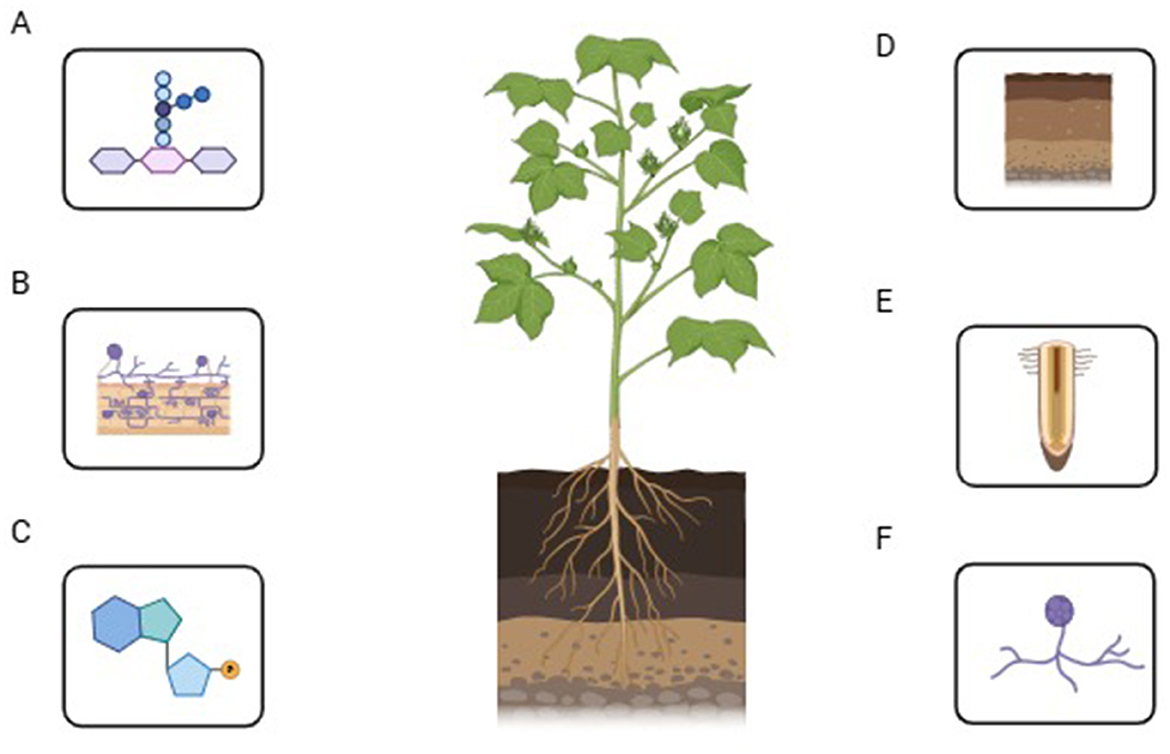
Figure 2. Functional role of AMF within the host roots. (A) Role of AMF in regulating the morphology of plant roots; (B) Mycelial network and callose formed by AMF infection; (C) How the AMF helps the cell wall of the roots of mycorrhizated plants to produce HRGPs and alters the root system; (D–F) The symbiotic association of AMF with the plant roots and their impact on root exudate secretion.
7.2 Enhancement of water and nutrient uptake by AMF for improved plant resistance
AMF enhances the absorption of water and vital mineral elements by plants. Research indicates that AMF establishes a vast mycelial network in the soil, with their extra-radical hyphae interlacing, so considerably enhancing the root system. This network improves the uptake of water, nitrates, phosphates, and other essential nutrients, benefiting numerous plants concurrently. Furthermore, AMF enables the movement of water and nutrients across plants, establishing an alternative and highly efficient mechanism for resource acquisition (De La Rosa-Mera et al., 2011). The 14C labeling technology was used in 1993, and it was found that a very minute quantity of 14C was released around the citrus mycorrhizal roots (Eissenstat, 1993). It was because of the competition between pathogens and AMF for photosynthetic products secreted by host plant roots. AMF utilizes photosynthetic product materials first, which diminishes the pathogen's acquisition chance, thereby reducing its ability to grow and reproduce (Kuila and Ghosh, 2022). By enhancing nutrient and water uptake, mycorrhizae mitigate root damage caused by pathogens, thereby reducing harm and improving plant resilience (Ma et al., 2021a). Tomato inoculation under F. oxysporum stress increases chlorophyll, soluble sugars, branching, and leaf development while improving nutrient absorption (P, N, K, Mn, Zn, Ca), thereby enhancing disease and pest resistance (Liang et al., 2021).
7.3 Mechanism of AMF-induced production of SMs
The presence of SM compounds in AM-colonized plants enhanced the expression of pathogenesis-related genes and increased the production of volatile compounds, including aldehydes, ethers, and alcohols, across different plant parts (Quaglia et al., 2012). Multiple enzymes facilitate the production of these metabolites. Lipoxygenases serve as critical signaling molecules that trigger defense responses in crop plants (Singh et al., 2022). The mechanisms underlying the alteration of the number of SMs remain unknown. Colonization by AMF leads to increased concentrations of phenolic, terpene, and nitrogenous compounds in shoot and root plant tissues (Kumar et al., 2021). The results of the study indicate that symbiotic colonization by AMF improves the nutritional value of the plants due to increased P and N absorption from the soil. The infection of host plants with AMF generally promotes the uptake of P, thereby enhancing the nutritional value and the levels of SMs as well as phytochemicals in the plants (Selwal et al., 2023; Wu et al., 2024). The alteration in SMs production may be an outcome of the introduction of changes by AMF in phytohormone pathway-associated plant pathways (Amani Machiani et al., 2022). The pathways involve those participating in gibberellin acid (GA), abscisic acid (ABA), brassinosteroids (BR), auxin (IAA), SA, JA, cytokinin (CK), and ET. Besides that, the symbiosis enhances the plant's defense mechanism (Schmitz and Harrison, 2014). Cucumber plants inoculated with Gigaspora terrestris contained high levels of IAA, zeatin, and GA. The high IAA levels enhance Rhizoctonia solani resistance by activating the defense mechanisms in plants against pathogen attack (Metwally and Al-Amri, 2020). AMF enhanced GA gene expression in Medicago truncatula (Ortu et al., 2012), JA and GA enhanced the concentration of terpenoid components through the induction of glandular trichomes development and improved expression of sesquiterpenoid biosynthetic genes (Singh and Sharma, 2015). The signaling molecules involved in AMF and host-plant symbiosis have the potential to modulate the content of SMs in plants. A symbiosis between Trifolium repens and G. mosseae enhances the content of signaling molecules such as salicylic acid, nitric oxide, and hydrogen peroxide, which in turn elevates the activity of enzymes related to phenolic biosynthesis (Zhang et al., 2013). Mycorrhizal plants have higher phytohormone levels (ABA, IAA, CK, GA, and ET) in leaves and stems compared to non-mycorrhizal ones. Induction of those participating in the phytohormone pathway, i.e., AMF, directly influences plant growth and indirectly affects resistance. Under stress, these hormones can alter the expression of genes and regulate gene synthesis, thereby enhancing the adaptability of plants (Weng et al., 2022).
7.4 AMF-induced production of SMs for plant disease resistance
A key mechanism by which AMF enhances plant disease resistance is the regulation of SMs production. This occurs as the mycorrhizal symbionts influence the physiological metabolism of plants, altering both the quantity and diversity of these defensive compounds (French, 2017). SMs are advantageous for plants because they help them combat harmful conditions caused by infection. A class of resistant substances known as phytoprotectins is initiated in response to pathogenic infection. The rate and amount of accumulation of these compounds are connected to the ability of plants to resist diseases (Monther Mohumad, 2012). The accumulation of phytophanins serves as a barrier around infected cells to prevent the spread of auxiliary pathogens (Jaiti et al., 2008). G. mosseae enhances phytotoxin production in response to infection, boosting plant resistance. Additionally, inoculation of G. intraradices on cucumber roots promotes callose deposition, which helps protect against the toxic effects of Colletotrichum orbiculare (Bais et al., 2006). AMF infection significantly increases the vinblastine in Catharanthus roseus leaves and protects them against biotic stresses (Martinez-Medina et al., 2010). The compounds belong to the phenolic family, e.g., phenolic carboxylic acids and flavonoids act as signaling molecules in the defense system (Monther Mohumad, 2012). Flavonoids were found to attract AMF toward plants and expand the symbiotic relationship between AMF and plants (Pei et al., 2020). In the roots of Gossypium hirsutum, upon infection of AMF, the production of phenolic substances escalates, and resistance toward Verticillium dahliae rises (Lioussanne et al., 2008). G. mosseae was found to stimulate the higher production of ascorbic acid and polyphenol content in strawberries, while also reducing the severity and disease incidence of C. gloeosporioides and F. oxysporum (Chandanie et al., 2009). The use of the root-splitting technique in tomato plants, SR against Ralstonia solanacearum, can be induced through G. versiforme (Zhu and Yao, 2004). In both uninfected and infected roots, the production of phenolic compounds is significantly increased (Weng et al., 2022). Therefore, plant resistance is based on the enhanced production of phenolic compounds. Conversely, AMF inoculated, and AMF uninoculated Phoenix dactylifera do not show a rise in the production of phenolic compounds upon infection with F. oxysporum, accumulation of derivative of hydroxycinnamic acid by mycorrhizated plants shows the ability to halt chlorosis (Jaiti et al., 2008).
7.5 AMF influence on root exudates, rhizosphere microbiome, and soil properties
Plants and AMF form a symbiotic association that influences the permeability of root cell membranes, the composition and volume of root exudates, the physical and chemical properties of the rhizosphere, the structural makeup of microbial communities, and the overall microbial density within the rhizosphere (Figures 2D–F). Extra-root hyphae of mycorrhizae can pierce from the minute pores present between soil particles and mycorrhizal secretions, e.g., organic acids, Glomus-associated protein (GRSP), and polyamines involved in the soil particles adhesion, stimulate the soil aggregation, mend soil pH, aeration, water permeability, and stability, promote redox potential (Eh), and enhance the growth of plants to resist pathogenic attack (Tatsumi et al., 2020). Reproduction, growth, and development of soil-borne fungi, bacteria, and nematodes are directly affected by the secretion of root exudates, which stimulate the growth of AMF and plant symbiotic relationships (Ghorui et al., 2024). This symbiotic relationship also affected the microbial community in terms of spatial distribution, nature, quantity, structure, and variation. Nematode invasion in roots is controlled through the secretion of root exudates, which paralyze nematodes with AMF infection in tomatoes (Lone et al., 2024), deter the Phytophthora nicotianae zoospores and limit their access to roots (Lioussanne et al., 2008). AMF can form symbiotic relationships with beneficial soil microbes, creating a synergistic effect. This partnership enhances the presence of advantageous microorganisms in the rhizosphere, particularly those that suppress soil-borne pathogens. Trichoderma, Gliocladium, Streptomyces, various antagonistic fungi, Actinomycetes, phosphate-solubilising bacteria, N-fixing bacteria, and plant growth-promoting rhizobacteria (PGPR) (Miransari et al., 2014). These beneficial microorganisms enhance plant disease resistance by decreasing pathogen populations and minimizing the risk of harmful bacterial infections. Furthermore, PGPR can enhance the symbiotic relationship between AMF and plants.
7.6 AMF and soil-borne pathogen competition in the rhizosphere
The ecological habitat and intrusion site shared by soil-borne pathogens and the biotrophic symbiotic microbes (e.g., AMF) in the soil rhizosphere are frequently the same. As a result, under the natural environment conditions, pathogens and AMF must interact in their primary biocontrol function to decrease the initial infection and re-infection of root epidermal pathogens in a spatially competitive manner. G. moshe infection was used to reduce the incidence of Phytophthora nicotianae disease, and it cannot infiltrate arbuscular cells in nearby uninfected root systems. In mycorrhizated plants, mycorrhizated roots and nearby non-mycorrhizated roots had a comparatively low population of H. glycines (Weng et al., 2022). Competition was seen in inoculated pathogenic bacteria, and AMF was aimed at Aquilaria agallocha infection sites (Tabin et al., 2009). The plants of A. agallocha mycorrhizated with G. fasciculatum significantly constrain the damping-off symptoms and morbidity index of the root tissue developed by Pythium aphanidermatum (Zhou et al., 2020). AMF plays a vital role as a parasite of nematodes, and its hyphae, vesicles, and arbuscular incursion are found in nematode galls such as G. polygamyces, which is a parasite of H. glycines and induces infection in their eggs (Keshari et al., 2024). Chlamydospores produced by AMF can colonize the cysts of soybean cyst nematodes, and it is a visible indication that AMF is a parasite of nematodes (Vos et al., 2013; Keshari et al., 2024).
8 Mechanisms of host defense activation by AMF
8.1 Role of AMF-induced signaling substances and phytohormones in plant defense
Signal molecules called phytohormones have the potential to be crucial for the functional regulation of the growth, development, and environmental adaptability of plants. Developing the symbiotic relationship between plants and AMF initiates the synthesis of hormones through plants, or AMF can directly produce hormones (Schmitz and Harrison, 2014). AMF initiates the process of synthesis of different signaling substances, for example, JA, nitric oxide (NO), ET, SA, ABA, hydrogen peroxide (H2O2), sugar signal, and Ca2+ signal, once a symbiotic relationship of plant and AMF is established (Schmitz and Harrison, 2014). The signaling substances are functional in developing a symbiotic relationship between plants and AMF, which triggers the plant's defense system (Metwally and Al-Amri, 2020). ET and JA were found to resist saprophytic infections, which have been reported to be triggered by ET and JA, and SA has an inhibiting impact. It was studied for biotrophic pathogens. ET and JA play essential roles in systemic acquired resistance (SAR) in plants, as opposed to systemic induced resistance (ISR) after the establishment of pathogenic infection (Hause et al., 2007). NO was recognized as a signaling substance and initiator of plant defense system-related gene expression (Calcagno et al., 2012). AMF symbiosis has a strong affinity with the NO accumulation in plants, and alfalfa showed NO content in roots and leaves is 1.9 and 3.3 times higher, respectively, than in control treatment when inoculated with G. margarita; it suggested that NO accumulation initiated by AMF symbiosis linked with induced SR (He et al., 2010). F. oxysporum-infected roots of tomato seedlings were inoculated with G. macrocarpum and G. polyphylla. After 20 days, disease severity indexing reduced by 75% and 78%, respectively. AMF-stimulated ISR in plants is primarily due to the signaling substance SA (Dugassa et al., 1996). SA application and the inoculation of G. moses reduced the degree of wilting and disease severity index of F. oxysporum-infected tomato plants. Cantaloupe is a phytohormone deceased upon infection with F. oxysporum, while inoculation of G. rhizogenes on infected plants increases the production of phytohormone cantaloupe, stimulates the SA and JA signaling pathways, and enhances resistance in plants (Steinkellner et al., 2012). However, G. intraradiculae inoculated Nicotiana attenuata showed no appreciable changes in endogenous SA and JA contents while slightly reducing ET content (Kapoor, 2008).
8.2 AMF-regulated expression of DRGs in plants
The symbiotic association between plants and AMF enhances pathogen resistance by upregulating DRGs (Kashyap et al., 2024). AMF can also modulate the expression of specific resistance genes in plants, enhancing defense responses against particular diseases (Badrbani et al., 2024). In wheat leaves, the expression of genes was remodeled explicitly after G. mosseae activated the MIR response against Zymoseptoria tritici, and the rate of foliar protection is 78%. Symbiotic relationship of mycorrhizae with plants before pathogenic infection upregulated the PR1 and Pox genes involved in the process of DRGs. After the establishment of infection, the transcriptome profiling revealed that 5 genes (GST, PAL, PR5, CAD, and CalS) were upregulated along with PR1 and Pox in a biotrophic stage of Z. tritici in leaves (Allario et al., 2025). In soybean plants infected with Heterodera glycines, inoculation with AMF led to upregulation of the DRGs (Chib1 and PAL5). This increased expression was confirmed at the transcriptional level using quantitative reverse transcription PCR (qRT-PCR) and Northern blotting techniques. The activation of these genes contributed to induced resistance against nematodes (Li et al., 2005). The Proteomic profiling depicted that upregulation of the DRGs related to transcription factors (such as WRKY), proteases and kinases receptors, auxins production, and encoding proteins related to disease resistance in response to F. virguliforme induced infection in mycorrhizated soybean plants. However, primed expression was found for DRGs encoding pleiotropic drug resistance and thaumatin-like protein. PODs and modification of cell wall-related DRGs were downregulated in transcriptome analysis of mycorrhizated and non-mycorrhizated soybean plants (Marquez et al., 2019). G. mosseae first colonized susceptible maize cultivars (Gaoyou-115 and Yuenong-9) to establish mycorrhizal symbiosis. After successful colonization, the plants were inoculated with Rhizoctonia solani to induce infection. The study found that mycorrhizal colonization upregulated the expression of DRGs (e.g., PAL, AOS, and PR2a), enhancing resistance against the pathogen. Additionally, BX9, a gene involved in the biosynthesis of benzoxazinoids (including DIMBOA and related compounds), showed increased expression in the leaves of both cultivars, suggesting a role in systemic defense priming (Ma et al., 2021b). Both nonmycorrhizal genotypes of Lycopersicon esculentum (mutant rmc and wild type 76R) infected with R. solani exhibited similar DRGs expression. However, after inoculation with AMF, the mutant rmc showed increased intracellular mRNA levels of GluBAS and Chi9 and higher extracellular PR-1 expression (Gallou et al., 2011).
8.3 AMF-induced defensive enzyme activation in plants
AMF initiate defensive enzymes in plants after the development of symbiotic relationships such as PODs and polyphenol oxidase (PPO) (phenolic substance metabolizer), chalcone synthase (CHS) (flavonoid synthesizer), chalcone isomerase (CHI) (metabolizer of lignin, phytoalexin, and isoflavone/flavonoid biosynthesis), phenylalanine ammonia-lyase (PAL) (metabolizer of proteins related to disease resistance (PR proteins) and phenypropanes) (Isayenkov et al., 2005). PAL is a physiological marker of plant resistance to pathogens. PAL activity was enhanced significantly in leaves and stems when infected F. oxysporum inoculated tomato seedlings were injected with AMF, which resisted disease and infection development (De Román et al., 2010). It was observed that Superoxide dismutase (SOD), 1,1-diphenyl-2-picrylhydrazyl (DPPH), and free radical scavenging activity enhanced in G. mosy-infected strawberry plants. The antioxidant production significantly increased in plants, which improved the resistance against F. oxysporum (Steinkellner et al., 2012). The PR protein, chitinase, glucanase, and other allergic reaction substances are upregulated in mycorrhizated plants with G. mosei. Inoculation with G. clarum, G. monosporum, and G. deserticola significantly enhanced the polyphenol oxidase activity in date palms and resisted chlorosis. G. rhizogenes stimulates the synthesis of sp7 (a defense protein); in the nucleus, it interacts with ERF19 (a protein transcription factor relevant to the disease process). The phrase sp7 helps in symptom alleviation induced by Magnaporthe oryzae (Kloppholz et al., 2011). AMF can enhance disease resistance in plants, either systemically or locally. The mechanisms by which AMF improves plant resistance may involve a single process or the combined effects of multiple pathways (Tabin et al., 2009). The effectiveness of AMF in suppressing diseases depends on the interactions between viruses, host plants, and AMF, which are influenced by abiotic factors such as soil properties (temperature, moisture, pH, and nutrient levels), timing of inoculation, and inoculum dosage. Additionally, agricultural practices, including farming techniques, pest control strategies, and fertilizer application, play a crucial role in determining the success of AMF-mediated biocontrol in farming ecosystems (Figure 3) (Chandanie et al., 2009).
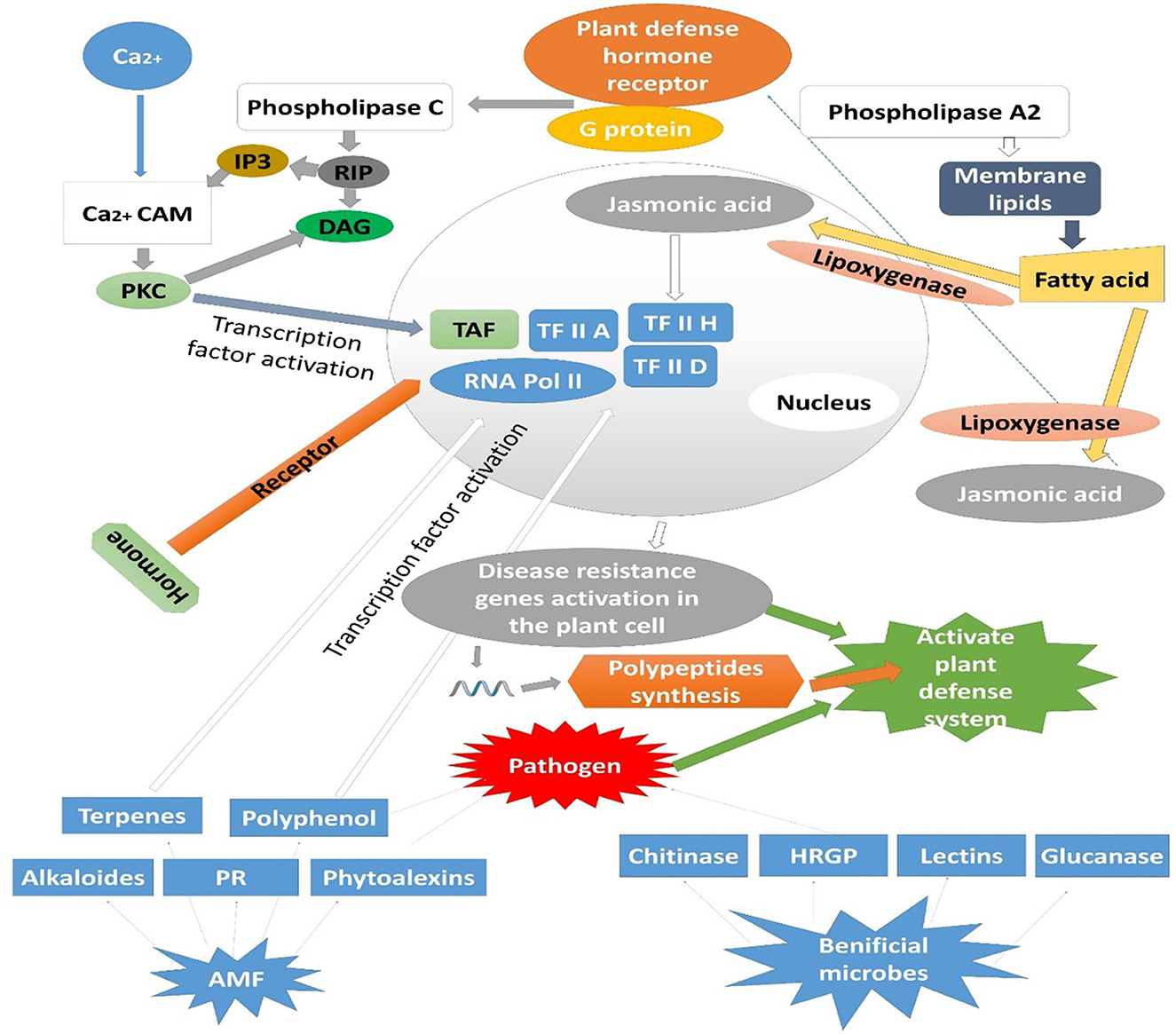
Figure 3. Activation of plant defensive enzymes by pathogen recognition and modulated through colonization of AMF and other beneficial microbes.
8.4 Transcriptome and proteome profiling of AMF-responsive genes in host plants
Transcriptomic and proteomic profiling are crucial for elucidating the molecular mechanisms underlying plant defense responses influenced by AMF (Aslam et al., 2024). Proteomic analysis facilitates the systematic identification and quantification of proteins expressed in plant roots in response to AMF colonization, offering insights into the functional dynamics of the plant-microbe interaction at the protein level (Yu et al., 2023). RNA of 64-day-old AMF RNA sequencing (RNA-seq) inoculated watermelon plants exhibited 2,259 differentially expressed genes (DEGs) related to signal transduction and metabolic pathways and involved in photosynthesis, nutrient transporters, biosynthesis of chlorophyll, and hormone biosynthesis. Proteomic profiling suggested that AMF is involved in the auxin signaling pathway by triggering auxin response factors, auxin-mediated proteins, auxin transporter-like proteins, and auxin-responsive proteins (Ma et al., 2024). Roots of mycorrhizated wheat plants with F. mosseae under water scarcity were analyzed for DEGs, 114,428 DEGs were found those involved in N compound, lipid, and carbohydrate metabolic pathways, cellulose synthase, and chitinase activity, and membrane transports and help plants to tolerate water deficit environment (Moradi Tarnabi et al., 2020). RNA-seq exhibited that the mycorrhizated root of snapdragon plants inhibits the osmotic stress induced by cold stress by enhancing the production of proline, soluble sugars, and proteins. Further, AMF attenuated the damage initiated by reactive oxygen spp. through the boost of GSH and AsA contents, PODs, catalase (CAT), SOD, monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), and ascorbate peroxidase (APX) activities. Furthermore, proteomic profiling identified AMF involved in regulating genes encoding the photosystem I and II related proteins, phytohormones synthesis, transcription factors related to stress, and active oxygen metabolism (Li et al., 2024b). Transcriptome profiling of mycorrhizated soybean plants with F. mosseae and infected with F. oxysporum showed DEGs (24,285), and genes PAL, CCR, CHI, and CYP73A were found upregulated upon infection with pathogenic fungi and triggered the defense response of soybean. In addition, mycorrhizated soybean roots upregulate of isoflavone metabolic pathway and lead to the synthesis of defense compounds by the production of glycine and daidzein along with substantial changes in the ample amount of terpene and phenolic metabolites, phenolic, and amino acids (Lu et al., 2020).
9 Common symbiosis signaling pathway
The common symbiosis-signaling pathway (CSSP) is a conserved signaling cascade that is required for the development of AMF symbiosis and the activation of nutrient exchange between plants and AMF (Maclean et al., 2017). It is activated upon the perception of fungal-derived lipochitooligosaccharides (e.g., Myc factors) and by plant LysM receptor-like kinases, including LYR3 from Medicago truncatula (Fliegmann et al., 2016). This interaction induces a downstream signaling cascade involving DMI1, DMI2, DMI3, and CCaMK (Mitra et al., 2004). DMI1 is an ion channel localized in the nucleus that facilitates calcium spiking, a key second messenger in AMF symbiosis (Jiang and Ding, 2023). Patch-clamp electrophysiological experiments revealed that DMI1 played an essential role in the generation of rhythmic calcium oscillations in root epidermal cells after AM fungus recognition (Tian et al., 2020). DMI2, a leucine-rich repeat receptor-like kinase, functions downstream of Myc factor perception and, together with the symbiosis receptor kinase SYMRK, assembles into a complex to activate the CSSP (Zhou et al., 2025a). Phosphoproteomic analysis indicates that DMI2 is quickly auto-phosphorylated in response to fungal signals, thereby activating a phosphorelay cascade that propagates the symbiotic signal (Ivanov and Harrison, 2024). Calcium-activated protein kinase CCaMK, which DMI3 encodes, decodes the calcium-spiking rhythm via calmodulin binding and transcription factor phosphorylation (Dhanker et al., 2020). Structural studies through cryo-electron microscopy (cryo-EM) have also unraveled how calcium-calmodulin binding relieves the auto-inhibitory domain of CCaMK, thereby facilitating downstream transcriptional reprogramming (Yuan et al., 2022). CCaMK also interacts with DELLA proteins, thereby coordinating gibberellin signaling to regulate AM fungal colonization (Yuan et al., 2022). New single-cell RNA-seq data reveal that DMI genes are cell-type-expressed explicitly in the root cortex, where arbuscule formation occurs, during their spatial regulation during symbiosis (Somoza et al., 2024). Epigenetic studies reveal that histone deacetylases regulate DMI expression to maintain proper signaling intensity under different phosphate conditions (Li et al., 2024c). CSSP land plant conservation prioritizes its evolutionary importance as comparative genomics dictates the existence of orthologs in non-legumes, postulating a potential role in promoting AMF symbiosis for sustainable agriculture (Vernie et al., 2025).
10 Genomic and pangenomic studies of AMF
The genomic and pangenomic research on AMF has significantly advanced our understanding of their evolutionary biology, symbiosis, and functional diversity (Liu and Chen, 2024). The genomic sequencing of the fungus Rhizophagus irregularis (genome accession no.: DAOM-197198) provided the first complete genome of an AMF (Masclaux et al., 2019). This analysis exhibited a diminished suite of plant cell wall-degrading enzymes with an expanded suite of symbiotic signaling genes, including those in the common symbiosis pathway (Tisserant et al., 2013). Pangenomic analyses of Rhizophagus and Gigaspora spp. have likewise revealed significant genomic plasticity, in which strain-specific genes are associated with host adaptation and nutrient exchange (Oliveira et al., 2024). The research uncovered evidence of horizontal gene transfer from bacterial origins, which accounts for the metabolic versatility of AMF (Li et al., 2018). Pangenomic approaches unveiled the core and accessory genomes, with a focus on the contribution of transposable elements to genomic development (He et al., 2024). The findings emphasize the need for more extensive sampling approaches to achieve the genomic diversity of AMF and inform subsequent research on their ecological and agricultural significance.
11 Conclusion
This comprehensive review underscores the pivotal role of AMF in sustainable crop disease management and yield enhancement. AMF establishes intricate symbiotic associations with plant roots that greatly boost nutrient uptake, water absorption, and protection from biotic and abiotic stresses. Besides their conventional role as a facilitator of nutrient mobilization, AMF induces SR through hormonal signaling, upregulation of DRGs, and SMs biosynthesis. Their symbiotic and non-symbiotic interactions with beneficial rhizosphere microbiota also enhance their biocontrol activity against a range of phytopathogens. AMF-mediated root exudate alteration, porosity of soil, and structure of microbial community create a suppressive soil status that is unfavorable for pathogens. Molecular studies, including proteomics and transcriptomics, have explained the potential of AMF in modulating host plant immunity at biochemical and genetic levels. Most generally, AMF offers a promising, sustainable alternative to chemical inputs to modern agriculture that is consistent with global sustainability and food security goals.
12 Future aspects
Future research must endeavor to optimize AMF inoculants for diverse agroecosystems through the identification of host-specific strains and environmental tolerance. Metagenomics and transcriptomics can resolve tripartite interactions among AMF, plants, and pathogens. Field trials with varying climatic and soil conditions will validate efficacy, while precision agriculture tools can integrate AMF for pinpoint delivery. Investigation of synergistic effects from interaction with other biocontrol agents (e.g., PGPR, Trichoderma) through combinatorial testing will enhance disease control measures. Long-term studies on soil fertility and carbon sequestration through glomalin production are a must. Lastly, the scaling up of AMF production processes will enable commercial viability for sustainable agriculture.
Author contributions
MU: Methodology, Data curation, Writing – original draft, Writing – review & editing. NA: Validation, Writing – review & editing. MM: Methodology, Investigation, Data curation, Software, Conceptualization, Writing – original draft, Writing – review & editing. YL: Project administration, Writing – review & editing. AA: Visualization, Writing – review & editing. MA: Formal analysis, Writing – review & editing. PL: Resources, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the China NSFC Research Fund for International Young Scientists (grant number: 32250410291) and a Special Project for the Academician Team Innovation Centre of Hainan Province (grant number: YSPTZX202206).
Acknowledgments
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number RGP2/342/45.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abarca, C., Fernandez Bidondo, L., Bompadre, J., and Velázquez, M. S. (2024). Arbuscular mycorrhizal fungi in tomato tolerance to pathogens and nematodes: a comprehensive review. Sci. Hortic. 329:112969. doi: 10.1016/j.scienta.2024.112969
Afridi, M. S., Kumar, A., Javed, M. A., Dubey, A., De Medeiros, F. H. V., and Santoyo, G. (2024). Harnessing root exudates for plant microbiome engineering and stress resistance in plants. Microbiol. Res. 279:127564. doi: 10.1016/j.micres.2023.127564
Ahammed, G. J., Shamsy, R., Liu, A., and Chen, S. (2023). Arbuscular mycorrhizal fungi-induced tolerance to chromium stress in plants. Environ. Pollut. 327:121597. doi: 10.1016/j.envpol.2023.121597
Ahmed, N., Li, J., Li, Y., Deng, L., Deng, L., Chachar, M., et al. (2025). Symbiotic synergy: how Arbuscular Mycorrhizal Fungi enhance nutrient uptake, stress tolerance, and soil health through molecular mechanisms and hormonal regulation. IMA Fungus 16:e144989. doi: 10.3897/imafungus.16.144989
Alaux, P.-L. (2020). Does the Arbuscular Mycorrhizal Fungus Rhizophagus irregularis Mitigate Late Blight in Potato plants? Belgium: Presses Universitaires de Louvain.
Alban, R., Guerrero, R., and Toro, M. (2013). Interactions between a root knot nematode (Meloidogyne exigua) and arbuscular mycorrhizae in coffee plant development (Coffea arabica). Am. J. Plant Sci. 4, 19–23. doi: 10.4236/ajps.2013.47A2003
Allario, T., Krzyzaniak, Y., Magnin-Robert, M., Tisserant, B., Fontaine, J., Courty, P.-E., et al. (2025). Defense responses related to mycorrhizal-induced resistance in wheat against Zymoseptoria tritici. Biol. Control 203:105729. doi: 10.1016/j.biocontrol.2025.105729
Amani Machiani, M., Javanmard, A., Habibi Machiani, R., and Sadeghpour, A. (2022). Arbuscular mycorrhizal fungi and changes in primary and secondary metabolites. Plants 11:2183. doi: 10.3390/plants11172183
Andrade, S. A., Silveira, A. P., and Mazzafera, P. (2010). Arbuscular mycorrhiza alters metal uptake and the physiological response of Coffea arabica seedlings to increasing Zn and Cu concentrations in soil. Sci. Total Environ. 408, 5381–5391. doi: 10.1016/j.scitotenv.2010.07.064
Aria, F. R., Karimi, F., Fakoor, M. Y., Faizi, G. R., and Sun, X. (2025). An overview of biological control of plant disease in Afghanistan. Biol. Control 204:105753. doi: 10.1016/j.biocontrol.2025.105753
Aslam, N., Li, Q., Bashir, S., Yuan, L., Qiao, L., and Li, W. (2024). Integrated review of transcriptomic and proteomic studies to understand molecular mechanisms of rice's response to environmental stresses. Biology 13:659. doi: 10.3390/biology13090659
Aylward, J., Roets, F., Dreyer, L. L., and Wingfield, M. J. (2023). Unseen fungal biodiversity and complex inter-organismal interactions in Protea flower heads. Fungal Biol. Rev. 45:100317. doi: 10.1016/j.fbr.2023.100317
Badrbani, A. H., Amini, J., Sharifi, R., and Karimi, K. (2024). Arbuscular mycorrhizal fungi, induce resistance in tomato plant against Fusarium wilt through transferring underground warning signal. Physiol. Mol. Plant Pathol. 133:102380. doi: 10.1016/j.pmpp.2024.102380
Bais, H. P., Weir, T. L., Perry, L. G., Gilroy, S., and Vivanco, J. M. (2006). The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266. doi: 10.1146/annurev.arplant.57.032905.105159
Baiyee, B., Ito, S.-I., and Sunpapao, A. (2019). Trichoderma asperellum T1 mediated antifungal activity and induced defense response against leaf spot fungi in lettuce (Lactuca sativa L.). Physiol. Mol. Plant Pathol. 106, 96–101. doi: 10.1016/j.pmpp.2018.12.009
Balestrini, R., and Bonfante, P. (2014). Cell wall remodeling in mycorrhizal symbiosis: a way towards biotrophism. Front. Plant Sci. 5:237. doi: 10.3389/fpls.2014.00237
Basyal, B., and Emery, S. M. (2021). An arbuscular mycorrhizal fungus alters switchgrass growth, root architecture, and cell wall chemistry across a soil moisture gradient. Mycorrhiza 31, 251–258. doi: 10.1007/s00572-020-00992-6
Begum, N., Qin, C., Ahanger, M. A., Raza, S., Khan, M. I., Ashraf, M., et al. (2019). Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front. Plant Sci. 10:1068. doi: 10.3389/fpls.2019.01068
Behn, O. (2016). Influence of Pseudomonas fluorescens and arbuscular mycorrhiza on the growth, yield, quality and resistance of wheat infected with Gaeumannomyces graminis. J. Plant Dis. Protect. 115, 4–8. doi: 10.1007/BF03356232
Berruti, A., Lumini, E., Balestrini, R., and Bianciotto, V. (2015). Arbuscular mycorrhizal fungi as natural biofertilizers: let's benefit from past successes. Front. Microbiol. 6:1559. doi: 10.3389/fmicb.2015.01559
Boorboori, M. R., and Lackoova, L. (2024). Arbuscular mycorrhizal fungi and salinity stress mitigation in plants. Front. Plant Sci. 15:1504970. doi: 10.3389/fpls.2024.1504970
Boyno, G., Demir, S., and Danesh, Y. R. (2022). Effects of some biological agents on the growth and biochemical parameters of tomato plants infected with Alternaria solani (Ellis and Martin) Sorauer. Eur. J. Plant Pathol. 162, 19–29. doi: 10.1007/s10658-021-02398-2
Boyno, G., Rezaee Danesh, Y., Demir, S., Teniz, N., Mulet, J. M., and Porcel, R. (2023). The complex interplay between arbuscular mycorrhizal fungi and strigolactone: mechanisms, sinergies, applications and future directions. Int. J. Mol. Sci. 24:16774. doi: 10.3390/ijms242316774
Cai, X., Zhao, H., Liang, C., Li, M., and Liu, R. (2021). Effects and mechanisms of symbiotic microbial combination agents to control tomato Fusarium crown and root rot disease. Front. Microbiol. 12:629793. doi: 10.3389/fmicb.2021.629793
Calcagno, C., Novero, M., Genre, A., Bonfante, P., and Lanfranco, L. (2012). The exudate from an arbuscular mycorrhizal fungus induces nitric oxide accumulation in Medicago truncatula roots. Mycorrhiza 22, 259–269. doi: 10.1007/s00572-011-0400-4
Chandanie, W. A., Kubota, M., and Hyakumachi, M. (2009). Interactions between the arbuscular mycorrhizal fungus Glomus mosseae and plant growth-promoting fungi and their significance for enhancing plant growth and suppressing damping-off of cucumber (Cucumis sativus L.). Appl. Soil Ecol. 41, 336–341. doi: 10.1016/j.apsoil.2008.12.006
Chang, J., Sun, Y., Tian, L., Ji, L., Luo, S., Nasir, F., et al. (2021). The structure of rhizosphere fungal communities of wild and domesticated rice: changes in diversity and co-occurrence patterns. Front. Microbiol. 12:610823. doi: 10.3389/fmicb.2021.610823
Chaudhary, A., Poudyal, S., and Kaundal, A. (2025). Role of arbuscular mycorrhizal fungi in maintaining sustainable agroecosystems. Appl. Microbiol. 5:6. doi: 10.3390/applmicrobiol5010006
Chen, D., Saeed, M., Ali, M. N. H. A., Raheel, M., Ashraf, W., Hassan, Z., et al. (2023). Plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi combined application reveals enhanced soil fertility and rice Production. Agronomy 13:550. doi: 10.3390/agronomy13020550
Chen, Q., Wu, W. W., Qi, S. S., Cheng, H., Li, Q., Ran, Q., et al. (2021). Arbuscular mycorrhizal fungi improve the growth and disease resistance of the invasive plant Wedelia trilobata. J. Appl. Microbiol. 130, 582–591. doi: 10.1111/jam.14415
Chou, L., and Schmitthenner, A. (1974). Effect of Rhizobium japonicum and Endogone mosseae on soybean root rot caused by Phythium ultimum and Phytophthora megasperma var. sojae. Plant Dis. Report.
Cruz, A. F., and Ishii, T. (2012). Arbuscular mycorrhizal fungal spores host bacteria that affect nutrient biodynamics and biocontrol of soil-borne plant pathogens. Biol. Open 1, 52–57. doi: 10.1242/bio.2011014
De La Rosa-Mera, C. J., Ferrera-Cerrato, R., Alarcón, A., De Jesús Sánchez-Colín, M., and Muñoz-Muñiz, O. D. (2011). Arbuscular mycorrhizal fungi and potassium bicarbonate enhance the foliar content of the vinblastine alkaloid in Catharanthus roseus. Plant Soil 349, 367–376. doi: 10.1007/s11104-011-0883-y
De Román, M., Fernández, I., Wyatt, T., Sahrawy, M., Heil, M., and Pozo, M. J. (2010). Elicitation of foliar resistance mechanisms transiently impairs root association with arbuscular mycorrhizal fungi. J. Ecol. 99, 36–45. doi: 10.1111/j.1365-2745.2010.01752.x
Dhanker, R., Chaudhary, S., Kumari, A., Kumar, R., and Goyal, S. (2020). Symbiotic signaling: insights from arbuscular mycorrhizal symbiosis. Plant Microbe Symbiosis 75–103. doi: 10.1007/978-3-030-36248-5_5
Diagne, N., Ndour, M., Djighaly, P. I., Ngom, D., Ngom, M. C. N., Ndong, G., et al. (2020). Effect of plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) on salt stress tolerance of Casuarina obesa (Miq.). Fron. Sust.Syst. 4:601004. doi: 10.3389/fsufs.2020.601004
Dugassa, G. D., Von Alten, H., and Schönbeck, F. (1996). Effects of arbuscular mycorrhiza (AM) on health of Linum usitatissimum L. infected by fungal pathogens. Plant Soil 185, 173–182. doi: 10.1007/BF02257522
Eissenstat, D. (1993). Carbon economy of sour orange in relation to mycorrhizal colonization and phosphorus status. Ann. Bot. 71, 1–10. doi: 10.1006/anbo.1993.1001
Emmanuel, O. C., and Babalola, O. O. (2020). Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria. Microbiol Res 239:126569. doi: 10.1016/j.micres.2020.126569
Fayaz, F., and Zahedi, M. (2021). Beneficial effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) nutritional status and tolerance indices under soil salinity stress. J. Plant Nutr. 45, 185–201. doi: 10.1080/01904167.2021.1952228
Figueiredo, A. F., Boy, J., and Guggenberger, G. (2021). Common mycorrhizae network: a review of the theories and mechanisms behind underground interactions. Front. Fungal Biol. 2:735299. doi: 10.3389/ffunb.2021.735299
Fiorilli, V., Martinez-Medina, A., Pozo, M. J., and Lanfranco, L. (2024). Plant immunity modulation in arbuscular mycorrhizal symbiosis and its impact on pathogens and pests. Annu. Rev. Phytopathol. 62, 127–156. doi: 10.1146/annurev-phyto-121423-042014
Fliegmann, J., Jauneau, A., Pichereaux, C., Rosenberg, C., Gasciolli, V., Timmers, A. C., et al. (2016). LYR3, a high-affinity LCO-binding protein of Medicago truncatula, interacts with LYK3, a key symbiotic receptor. FEBS Lett. 590, 1477–1487. doi: 10.1002/1873-3468.12191
Frac, M., Jedryczka, M., and Hannula, E. S. (2023). Soil fungal biodiversity for plant and soil health, volume II. Front. Media SA. 14:1170312. doi: 10.3389/fmicb.2023.1170312
French, K. E. (2017). Engineering mycorrhizal symbioses to alter plant metabolism and improve crop health. Front. Microbiol. 8:1403. doi: 10.3389/fmicb.2017.01403
Frey, S. D. (2019). Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu. Rev. Ecol. Evol. Syst. 50, 237–259. doi: 10.1146/annurev-ecolsys-110617-062331
Fuentes-Quiroz, A., Herrera, H., Soto, J., Campos-Vargas, R., Ortiz, J., and Arriagada, C. (2022). Rhizosphere fungi regulate the expression of metal tolerance genes in Solanum lycopersicum L. (Solanaceae) growing in a metal(loid)-contaminated soil. Rhizosphere 24:100599. doi: 10.1016/j.rhisph.2022.100599
Gallou, A., Lucero Mosquera, H. P., Cranenbrouck, S., Suárez, J. P., and Declerck, S. (2011). Mycorrhiza induced resistance in potato plantlets challenged by Phytophthora infestans. Physiol. Mol. Plant Pathol. 76, 20–26. doi: 10.1016/j.pmpp.2011.06.005
Gao, L.-L., Knogge, W., Delp, G., Smith, F. A., and Smith, S. E. (2004). Expression patterns of defense-related genes in different types of arbuscular mycorrhizal development in wild-type and mycorrhiza-defective mutant tomato. Mol. Plan Microbe Interact. 17, 1103–1113. doi: 10.1094/MPMI.2004.17.10.1103
Garg, N., and Cheema, A. (2021). Relative roles of Arbuscular Mycorrhizae in establishing a correlation between soil properties, carbohydrate utilization and yield in Cicer arietinum L. under as stress. Ecotoxicol. Environ. Saf. 207:111196. doi: 10.1016/j.ecoenv.2020.111196
Ghorui, M., Chowdhury, S., Balu, P., Das, K., and Sunar, K. (2024). “Boosting plant immunity: the functional role and mechanism of arbuscular mycorrhizal fungi in resistance,” in Plant Microbiome and Biological Control (Cham: Springer), 195–219. doi: 10.1007/978-3-031-75845-4_9
Gianinazzi, S., Gollotte, A., Binet, M. N., Van Tuinen, D., Redecker, D., and Wipf, D. (2010). Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20, 519–530. doi: 10.1007/s00572-010-0333-3
Goss, M. J., Carvalho, M., and Brito, I. (2017). “Diversity in arbuscular mycorrhizal fungi * with Clarisse Brígido,” in Functional Diversity of Mycorrhiza and Sustainable Agriculture, eds. M. J. Goss, M. Carvalho, and I. Brito (Cambridge, MA: Academic Press), 59–79. doi: 10.1016/B978-0-12-804244-1.00004-6
Gruden, K., Lidoy, J., Petek, M., Podpecan, V., Flors, V., Papadopoulou, K. K., et al. (2020). Menage a trois: unraveling the mechanisms regulating plant-microbe-arthropod interactions. Trends Plant Sci. 25, 1215–1226. doi: 10.1016/j.tplants.2020.07.008
Guo, X., Chronis, D., De La Torre, C. M., Smeda, J., Wang, X., and Mitchum, M. G. (2015). Enhanced resistance to soybean cyst nematode Heterodera glycines in transgenic soybean by silencing putative CLE receptors. Plant Biotechnol. J. 13, 801–810. doi: 10.1111/pbi.12313
Harrier, L. A., and Watson, C. A. (2004). The potential role of arbuscular mycorrhizal (AM) fungi in the bioprotection of plants against soil-borne pathogens in organic and/or other sustainable farming systems. Pest Manag. Sci. 60, 149–157. doi: 10.1002/ps.820
Hause, B., Mrosk, C., Isayenkov, S., and Strack, D. (2007). Jasmonates in arbuscular mycorrhizal interactions. Phytochemistry 68, 101–110. doi: 10.1016/j.phytochem.2006.09.025
He, X., Qi, Z., Liu, Z., Chang, X., Zhang, X., Li, J., et al. (2024). Pangenome analysis reveals transposon-driven genome evolution in cotton. BMC Biol. 22:92. doi: 10.1186/s12915-024-01893-2
He, Z., Li, H., and Tang, H. (2010). Effect of arbuscular mycorrhizal fungi on endogenous in cucumber after Rhizoctonia solani inoculation. Chin. Agric. Sci. Bull 26, 187–190. doi: 10.11924/j.issn.1000-6850.2010-0610
Hernández-Montiel, L. G., Rueda-Puente, E. O., Cordoba-Matson, M. V., Holguín-Peña, J. R., and Zulueta-Rodríguez, R. (2013). Mutualistic interaction of rhizobacteria with arbuscular mycorrhizal fungi and its antagonistic effect on Fusarium oxysporum in Carica papaya seedlings. Crop Prot. 47, 61–66. doi: 10.1016/j.cropro.2013.01.008
Igiehon, N. O., Babalola, O. O., Cheseto, X., and Torto, B. (2021). Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiol Res 242:126640. doi: 10.1016/j.micres.2020.126640
Imad Khrieba, M. (2019). Mycorrhizae's role in plant nutrition and protection from pathogens. Curr. Investig. Agric. Curr. Res. 8, 1037–1045. doi: 10.32474/CIACR.2019.08.000277
Iqbal, Z., Khan, M. A., Sharif, M., Shah, J. H., Ur Rehman, M. H., and Javed, K. (2018). An automated detection and classification of citrus plant diseases using image processing techniques: a review. Comput. Electr. Agric. 153, 12–32. doi: 10.1016/j.compag.2018.07.032
Isayenkov, S., Mrosk, C., Stenzel, I., Strack, D., and Hause, B. (2005). Suppression of allene oxide cyclase in hairy roots of Medicago truncatula reduces jasmonate levels and the degree of mycorrhization with Glomus intraradices. Plant Physiol. 139, 1401–1410. doi: 10.1104/pp.105.069054
Ivanov, S., Austin, J. 2nd, Berg, R. H., and Harrison, M. J. (2019). Extensive membrane systems at the host-arbuscular mycorrhizal fungus interface. Nat Plants 5, 194–203. doi: 10.1038/s41477-019-0364-5
Ivanov, S., and Harrison, M. J. (2024). Receptor-associated kinases control the lipid provisioning program in plant-fungal symbiosis. Science 383, 443–448. doi: 10.1126/science.ade1124
Jain, A., Sarsaiya, S., Wu, Q., Lu, Y., and Shi, J. (2019). A review of plant leaf fungal diseases and its environment speciation. Bioengineered 10, 409–424. doi: 10.1080/21655979.2019.1649520
Jaiti, F., Kassami, M., Meddich, A., and El Hadrami, I. (2008). Effect of arbuscular mycorrhization on the accumulation of hydroxycinnamic acid derivatives in date palm seedlings challenged with Fusarium oxysporum f. sp. albedinis. J. Phytopathol. 156, 641–646. doi: 10.1111/j.1439-0434.2008.01411.x
Jaizme-Vega, M. C., Tenoury, P., Pinochet, J., and Jaumot, M. (1997). Interactions between the root-knot nematode Meloidogyne incognita and Glomus mosseae in banana. Plant Soil 196, 27–35. doi: 10.1023/A:1004236310644
Ji, Q., Cheng, G., Zhang, X., Wang, W., Guo, X., and Wang, H. (2025). Tree species diversity and tree growth affected element compositions in glomalin-related soil protein–soil pH interaction. Sustainability 17:801. doi: 10.3390/su17020801
Jiang, Y., and Ding, P. (2023). Calcium signaling in plant immunity: a spatiotemporally controlled symphony. Trends Plant Sci. 28, 74–89. doi: 10.1016/j.tplants.2022.11.001
Kapoor, R. (2008). Induced resistance in mycorrhizal tomato is correlated to concentration of jasmonic acid. Online J. Biol. Sci. 8, 49–56. doi: 10.3844/ojbsci.2008.49.56
Kashyap, P., Sharma, I., Kashyap, S., and Agarwala, N. (2024). “Arbuscular mycorrhizal fungi (AMF)-mediated control of foliar fungal diseases,” in Arbuscular Mycorrhizal Fungi and Higher Plants (Singapore: Springer), 193–223. doi: 10.1007/978-981-99-8220-2_9
Keshari, N., Kranti, K. V. V. S.K., Gunda, N. K., and Ansari, R. A. (2024). “Arbuscular mycorrhizal fungi: a potential agent for phytonematodes management in diverse agro-climatic zones,” in Mycorrhizal Symbiosis and Agroecosystem Restoration (Springer), 147–169. doi: 10.1007/978-981-99-5030-0_7
Kloppholz, S., Kuhn, H., and Requena, N. (2011). A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy. Curr. Biol. 21, 1204–1209. doi: 10.1016/j.cub.2011.06.044
Krzyzaniak, Y., Magnin-Robert, M., Randoux, B., Fontaine, J., and Lounès-Hadj Sahraoui, A. (2021). Combined use of beneficial bacteria and arbuscular mycorrhizal fungi for the biocontrol of plant cryptogamic diseases: evidence, methodology, and limits. Symbiot. Soil Microorg. Biol. Applic. 60, 429–468. doi: 10.1007/978-3-030-51916-2_24
Kuila, D., and Ghosh, S. (2022). Aspects, problems and utilization of Arbuscular Mycorrhizal (AM) application as bio-fertilizer in sustainable agriculture. Curr. Res. Microb. Sci. 3:100107. doi: 10.1016/j.crmicr.2022.100107
Kumar, S., Arora, N., and Upadhyay, H. (2021). “Arbuscular mycorrhizal fungi: source of secondary metabolite production in medicinal plants,” in New and Future Developments in Microbial Biotechnology and Bioengineering, eds. J. Singh and P. Gehlot (Amsterdam: Elsevier), 155–164. doi: 10.1016/B978-0-12-821005-5.00011-9
Kumari, S. M. P., and Prabina, B. J. (2019). Protection of tomato, Lycopersicon esculentum from wilt pathogen, Fusarium oxysporum f. sp. lycopersici by arbuscular mycorrhizal fungi, Glomus sp. Int. J. Curr. Microbiol. Appl. Sci. 8, 1368–1378. doi: 10.20546/ijcmas.2019.804.159
Kumari, S. M. P., and Srimeena, N. (2019). Arbuscular mycorrhizal fungi (AMF) induced defense factors against the damping-off disease pathogen, Pythium aphanidermatum in chilli (Capsicum annum). Int. J. Curr. Microbiol. Appl. Sci. 8, 2243–2248. doi: 10.20546/ijcmas.2019.806.267
Lahlali, R., Ezrari, S., Radouane, N., Kenfaoui, J., Esmaeel, Q., El Hamss, H., et al. (2022). Biological control of plant pathogens: a global perspective. Microorganisms 10:596. doi: 10.3390/microorganisms10030596
Li, H., Liu, R., Shu, H., and Li, Y. (2005). Chib1 and PAL5 directly involved in the defense responses induced by the arbuscular mycorrhizal fungus Glomus fasciculatus against nematode. Mycosystema 24, 385–393.
Li, M., Hou, S., Wang, J., Hu, J., and Lin, X. (2021a). Arbuscular mycorrhizal fungus suppresses tomato (Solanum lycopersicum Mill.) Ralstonia wilt via establishing a soil–plant integrated defense system. J. Soils Sediments 21, 3607–3619. doi: 10.1007/s11368-021-03016-8
Li, M., Zhao, J., Tang, N., Sun, H., and Huang, J. (2018). Horizontal gene transfer from bacteria and plants to the arbuscular mycorrhizal fungus rhizophagus irregularis. Front. Plant Sci. 9:701. doi: 10.3389/fpls.2018.00701
Li, M.-Y., Wang, W., Yin, H.-H., Chen, Y., Ashraf, M., Tao, H.-Y., et al. (2025). The functional role of arbuscular mycorrhizal fungi in enhancing soil organic carbon stocks and stability in dryland. Soil Till. Res. 248:106443. doi: 10.1016/j.still.2024.106443
Li, S., Yang, W., Hu, J., Guo, M., Li, Y., Wang, Y., et al. (2024a). Effects of arbuscular mycorrhizal fungi on organic carbon allocation, sequestration, and decomposition in black soils. Can. J. Soil Sci. 104, 456–468. doi: 10.1139/cjss-2024-0048
Li, W., Wu, H., Hua, J., Zhu, C., and Guo, S. (2024b). Arbuscular mycorrhizal fungi enhanced resistance to low-temperature weak-light stress in snapdragon (Antirrhinum majus L.) through physiological and transcriptomic responses. Front. Plant Sci. 15:1330032. doi: 10.3389/fpls.2024.1330032
Li, W., Zhang, X., Zhang, Q., Li, Q., Li, Y., Lv, Y., et al. (2024c). PICKLE and HISTONE DEACETYLASE6 coordinately regulate genes and transposable elements in Arabidopsis. Plant Physiol. 196, 1080–1094. doi: 10.1093/plphys/kiae369
Li, Y., Duan, T., Nan, Z., and Li, Y. (2021b). Arbuscular mycorrhizal fungus alleviates alfalfa leaf spots caused by Phoma medicaginis revealed by RNA-seq analysis. J. Appl. Microbiol. 130, 547–560. doi: 10.1111/jam.14387
Liang, M., Shi, L., Burslem, D., Johnson, D., Fang, M., Zhang, X., et al. (2021). Soil fungal networks moderate density-dependent survival and growth of seedlings. New Phytol. 230, 2061–2071. doi: 10.1111/nph.17237
Lin, P., Zhang, M., Wang, M., Li, Y., Liu, J., and Chen, Y. (2021). Inoculation with arbuscular mycorrhizal fungus modulates defense-related genes expression in banana seedlings susceptible to wilt disease. Plant Signal. Behav. 16:1884782. doi: 10.1080/15592324.2021.1884782
Ling, Q., Wu, H., Xie, L., Zhao, Y., Huang, Q., Zhang, Q., et al. (2025). Advances, challenges, and perspectives in glomalin-related soil protein research. Microorganisms 13:740. doi: 10.3390/microorganisms13040740
Lioussanne, L., Jolicoeur, M., and St-Arnaud, M. (2008). Mycorrhizal colonization with Glomus intraradices and development stage of transformed tomato roots significantly modify the chemotactic response of zoospores of the pathogen Phytophthora nicotianae. Soil Biol. Biochem. 40, 2217–2224. doi: 10.1016/j.soilbio.2008.04.013
Liu, R., and Chen, Y. (2024). “Mycorrhizal symbiosis: evolution, opportunities, challenges, and prospects,” in Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Inoculum Production and Application (Singapore: Springer), 1–35. doi: 10.1007/978-981-97-0296-1_1
Liu, R., Dai, M., Wu, X., Li, M., and Liu, X. (2012). Suppression of the root-knot nematode [Meloidogyne incognita (Kofoid and White) Chitwood] on tomato by dual inoculation with arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria. Mycorrhiza 22, 289–296. doi: 10.1007/s00572-011-0397-8
Lone, R., Mushtaq, G., Hassan, N., Malla, N. A., Rohella, G. K., and Khan, S. (2024). “Role of phenolics in establishing mycorrhizal association in plants for management of biotic stress,” in Plant Phenolics in Biotic Stress Management (Singapore: Springer), 35–74. doi: 10.1007/978-981-99-3334-1_2
Lu, C. C., Guo, N., Yang, C., Sun, H. B., and Cai, B. Y. (2020). Transcriptome and metabolite profiling reveals the effects of Funneliformis mosseae on the roots of continuously cropped soybeans. BMC Plant Biol. 20:479. doi: 10.1186/s12870-020-02647-2
Ma, J., Wang, W., Yang, J., Qin, S., Yang, Y., Sun, C., et al. (2022). Mycorrhizal symbiosis promotes the nutrient content accumulation and affects the root exudates in maize. BMC Plant Biol. 22:64. doi: 10.1186/s12870-021-03370-2
Ma, J., Zhao, Q., Zaman, S., Anwar, A., and Li, S. (2024). The transcriptomic analysis revealed the molecular mechanism of Arbuscular Mycorrhizal Fungi (AMF) inoculation in watermelon. Sci. Hortic. 332:113184. doi: 10.1016/j.scienta.2024.113184
Ma, X., Li, X., and Ludewig, U. (2021a). Arbuscular mycorrhizal colonization outcompetes root hairs in maize under low phosphorus availability. Ann. Bot. 127, 155–166. doi: 10.1093/aob/mcaa159
Ma, Y., Zhang, H., Wang, D., Guo, X., Yang, T., Xiang, X., et al. (2021b). Differential responses of arbuscular mycorrhizal fungal communities to long-term fertilization in the wheat rhizosphere and root endosphere. Appl. Environ. Microbiol. 87:e0034921. doi: 10.1128/AEM.00349-21
Maclean, A. M., Bravo, A., and Harrison, M. J. (2017). Plant signaling and metabolic pathways enabling arbuscular mycorrhizal symbiosis. Plant Cell 29, 2319–2335. doi: 10.1105/tpc.17.00555
Mandou, M. S., Souleymanou, A., and Chotangui, A. H. (2023). Arbuscular mycorrhizal fungi inoculation and intercropping combine to control nematodes in bananas. Int. J. Plant Soil Sci. 35, 136–146. doi: 10.9734/ijpss/2023/v35i143029
Marquez, N., Giachero, M. L., Gallou, A., Debat, H. J., Declerck, S., and Ducasse, D. A. (2019). Transcriptome analysis of mycorrhizal and nonmycorrhizal soybean plantlets upon infection with Fusarium virguliforme, one causal agent of sudden death syndrome. Plant Pathol. 68, 470–480. doi: 10.1111/ppa.12964
Martinez-Medina, A., Pascual, J. A., Perez-Alfocea, F., Albacete, A., and Roldan, A. (2010). Trichoderma harzianum and Glomus intraradices modify the hormone disruption induced by Fusarium oxysporum infection in melon plants. Phytopathology 100, 682–688. doi: 10.1094/PHYTO-100-7-0682
Masclaux, F. G., Wyss, T., Pagni, M., Rosikiewicz, P., and Sanders, I. R. (2019). Investigating unexplained genetic variation and its expression in the arbuscular mycorrhizal fungus Rhizophagus irregularis: a comparison of whole genome and RAD sequencing data. PLoS ONE 14:e0226497. doi: 10.1371/journal.pone.0226497
Mehmood, H., Arif Ali, M., Hussain, S., Shehzad Baig, K., Farooq, U., Ajmal, M., et al. (2022). Synchronization of arbuscular mycorrhizae fungi inoculation with different zinc application methods for improvement in BASMATI rice growth and yield in alkaline calcareous soil. J. King Saud Univ. Sci. 34:102053. doi: 10.1016/j.jksus.2022.102053
Metwally, R. A., and Al-Amri, S. M. (2020). Individual and interactive role of Trichoderma viride and arbuscular mycorrhizal fungi on growth and pigment content of onion plants. Lett. Appl. Microbiol. 70, 79–86. doi: 10.1111/lam.13246
Mhlongo, M. I., Piater, L. A., Madala, N. E., Labuschagne, N., and Dubery, I. A. (2018). The chemistry of plant-microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front. Plant Sci. 9:112. doi: 10.3389/fpls.2018.00112
Miransari, M., Abrishamchi, A., Khoshbakht, K., and Niknam, V. (2014). Plant hormones as signals in arbuscular mycorrhizal symbiosis. Crit. Rev. Biotechnol. 34, 123–133. doi: 10.3109/07388551.2012.731684
Mitra, R. M., Gleason, C. A., Edwards, A., Hadfield, J., Downie, J. A., Oldroyd, G. E., et al. (2004). A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: gene identification by transcript-based cloning. Proc. Natl. Acad. Sci. U. S. A. 101, 4701–4705. doi: 10.1073/pnas.0400595101
Monther Mohumad, T. (2012). Ultrastructural changes of tomatoes (Lycopersicon esculentum) root colonized by Glomus mosseae and Ralstonia solanacearum. Afr. J. Biotechnol. 11, 6681–6686. doi: 10.5897/AJB11.2960
Moradi Tarnabi, Z., Iranbakhsh, A., Mehregan, I., and Ahmadvand, R. (2020). Impact of arbuscular mycorrhizal fungi (AMF) on gene expression of some cell wall and membrane elements of wheat (Triticum aestivum L.) under water deficit using transcriptome analysis. Physiol. Mol. Biol. Plants 26, 143–162. doi: 10.1007/s12298-019-00727-8
Mubeen, I., Fawzi Bani Mfarrej, M., Razaq, Z., Iqbal, S., Naqvi, S. A. H., Hakim, F., et al. (2023). Nanopesticides in comparison with agrochemicals: outlook and future prospects for sustainable agriculture. Plant Physiol. Biochem. 198:107670. doi: 10.1016/j.plaphy.2023.107670
Mythili, M., Ramalakshmi, A., and Gopal, N. O. (2025). “Arbuscular mycorrhizal fungi for sustainable agriculture ecosystem,” in Encyclopedia of Green Materials, eds. C. Baskar, S. Ramakrishna, and A. D. L. Rosa (Singapore: Springer Nature Singapore), 96–104. doi: 10.1007/978-981-97-4618-7_250
Nath, M., Bhatt, D., Prasad, R., Gill, S. S., Anjum, N. A., and Tuteja, N. (2016). Reactive oxygen species generation-scavenging and signaling during plant-arbuscular mycorrhizal and Piriformospora indica interaction under stress condition. Front. Plant Sci. 7:1574. doi: 10.3389/fpls.2016.01574
Neeraj and Singh, K.. (2011). Organic amendments to soil inoculated arbuscular mycorrhizal fungi and Pseudomonas fluorescens treatments reduce the development of root-rot disease and enhance the yield of Phaseolus vulgaris L. Eur. J. Soil Biol. 47, 288–295. doi: 10.1016/j.ejsobi.2011.07.002
Nie, W., He, Q., Guo, H., Zhang, W., Ma, L., Li, J., et al. (2024). Arbuscular mycorrhizal fungi: boosting crop resilience to environmental stresses. Microorganisms 12:2448. doi: 10.3390/microorganisms12122448
Oliveira, J., Yildirir, G., and Corradi, N. (2024). From chaos comes order: genetics and genome biology of arbuscular mycorrhizal fungi. Annu. Rev. Microbiol. 78, 147–168. doi: 10.1146/annurev-micro-041522-105143
Ortu, G., Balestrini, R., Pereira, P. A., Becker, J. D., Kuster, H., and Bonfante, P. (2012). Plant genes related to gibberellin biosynthesis and signaling are differentially regulated during the early stages of AM fungal interactions. Mol. Plant 5, 951–954. doi: 10.1093/mp/sss027
Parvin, W., Govender, N., Othman, R., Jaafar, H., Rahman, M., and Wong, M. Y. (2020). Phenazine from Pseudomonas aeruginosa UPMP3 induced the host resistance in oil palm (Elaeis guineensis Jacq.)-Ganoderma boninense pathosystem. Sci. Rep. 10:15621. doi: 10.1038/s41598-020-72156-7
Pei, Y., Siemann, E., Tian, B., and Ding, J. (2020). Root flavonoids are related to enhanced AMF colonization of an invasive tree. AoB Plants 12:plaa002. doi: 10.1093/aobpla/plaa002
Pozo De La Hoz, J., Rivero, J., Azcon-Aguilar, C., Urrestarazu, M., and Pozo, M. J. (2021). Mycorrhiza-induced resistance against foliar pathogens is uncoupled of nutritional effects under different light intensities. J. Fungi 7:402. doi: 10.3390/jof7060402
Pozo, M. J., Cordier, C., Dumas-Gaudot, E., Gianinazzi, S., Barea, J. M., and Azcon-Aguilar, C. (2002). Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J. Exp. Bot. 53, 525–534. doi: 10.1093/jexbot/53.368.525
Pozo, M. J., Lopez-Raez, J. A., Azcon-Aguilar, C., and Garcia-Garrido, J. M. (2015). Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 205, 1431–1436. doi: 10.1111/nph.13252
Prospero, S., Botella, L., Santini, A., and Robin, C. (2021). Biological control of emerging forest diseases: how can we move from dreams to reality? For. Ecol. Manage. 496:119377. doi: 10.1016/j.foreco.2021.119377
Purohit, H. J., Pandit, P., Pal, R., Warke, R., and Warke, G. M. (2024). Soil microbiome: an intrinsic driver for climate smart agriculture. J. Agric. Food Res. 18:101433. doi: 10.1016/j.jafr.2024.101433
Quaglia, M., Fabrizi, M., Zazzerini, A., and Zadra, C. (2012). Role of pathogen-induced volatiles in the Nicotiana tabacum-Golovinomyces cichoracearum interaction. Plant Physiol. Biochem. 52, 9–20. doi: 10.1016/j.plaphy.2011.11.006
Rashad, Y. M., Abbas, M. A., Soliman, H. M., Abdel-Fattah, G., and Abdel-Fattah, G. (2020). Synergy between endophytic Bacillus amyloliquefaciens GGA and arbuscular mycorrhizal fungi induces plant defense responses against white rot of garlic and improves host plant growth. Phytopathol. Mediterr. 59, 169–186. doi: 10.36253/phyto-11019
Razak, N. A., and Gange, A. C. (2023). Multitrophic interactions between arbuscular mycorrhizal fungi, foliar endophytic fungi and aphids. Microb. Ecol. 85, 146–156. doi: 10.1007/s00248-021-01937-y
Redecker, D., Schussler, A., Stockinger, H., Sturmer, S. L., Morton, J. B., and Walker, C. (2013). An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 23, 515–531. doi: 10.1007/s00572-013-0486-y
Rodrigues, E. S. M. T., Calandrelli, A., Miamoto, A., Rinaldi, L. K., Pereira Moreno, B., Da Silva, C., et al. (2021). Pre-inoculation with arbuscular mycorrhizal fungi affects essential oil quality and the reproduction of root lesion nematode in Cymbopogon citratus. Mycorrhiza 31, 613–623. doi: 10.1007/s00572-021-01045-2
Sałata, A., and Buczkowska, H. B. (2020). Inoculation with arbuscular mycorrhizal fungi (AMF) and plant irrigation with yield – forming factors in organic sweet pepper (Capsicum annuum L.) cultivation. Acta Sci. Polonor. Hortor. Cultus 19, 125–138. doi: 10.24326/asphc.2020.6.11
Sanchez-Bayo, F. (2021). Indirect effect of pesticides on insects and other arthropods. Toxics 9:177. doi: 10.3390/toxics9080177
Schmitz, A. M., and Harrison, M. J. (2014). Signaling events during initiation of arbuscular mycorrhizal symbiosis. J. Integr. Plant Biol. 56, 250–261. doi: 10.1111/jipb.12155
Schouteden, N., De Waele, D., Panis, B., and Vos, C. M. (2015). Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: a review of the mechanisms involved. Front. Microbiol. 6:1280. doi: 10.3389/fmicb.2015.01280
Selwal, N., Rahayu, F., Herwati, A., Latifah, E., Supriyono, S.uhara, C., Kade Suastika, I. B., et al. (2023). Enhancing secondary metabolite production in plants: exploring traditional and modern strategies. J. Agric. Food Res. 14:100702. doi: 10.1016/j.jafr.2023.100702
Sennoi, R., Singkham, N., Jogloy, S., Boonlue, S., Saksirirat, W., Kesmala, T., et al. (2013). Biological control of southern stem rot caused by Sclerotium rolfsii using Trichoderma harzianum and arbuscular mycorrhizal fungi on Jerusalem artichoke (Helianthus tuberosus L.). Crop Prot. 54, 148–153. doi: 10.1016/j.cropro.2013.08.011
Sharma, M., Saini, I., Kaushik, P., Aldawsari, M. M., Balawi, T. A., and Alam, P. (2021). Mycorrhizal fungi and Pseudomonas fluorescens application reduces root-knot nematode (Meloidogyne javanica) infestation in eggplant. Saudi J. Biol. Sci. 28, 3685–3691. doi: 10.1016/j.sjbs.2021.05.054
Sharma, S., Prasad, R., Varma, A., and Sharma, A. (2017). Glycoprotein associated with Funneliformis coronatum, Gigaspora margarita and Acaulospora scrobiculata suppress the plant pathogens in vitro. Asian J. Plant Pathol. 11, 199–202. doi: 10.3923/ajppaj.2017.199.202
Silvestri, A., Perez-Tienda, J., and Lopez-Raez, J. A. (2020). Arbuscular mycorrhizal fungal gene expression analysis by real-time PCR. Methods Mol. Biol. 2146, 157–170. doi: 10.1007/978-1-0716-0603-2_12
Singh, B., and Sharma, R. A. (2015). Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 5, 129–151. doi: 10.1007/s13205-014-0220-2
Singh, H. K. (2020). Current Research and Innovations in Plant Pathology. AkiNik, India. doi: 10.22271/ed.book.794
Singh, P., Arif, Y., Miszczuk, E., Bajguz, A., and Hayat, S. (2022). Specific roles of lipoxygenases in development and responses to stress in plants. Plants 11:979. doi: 10.3390/plants11070979
Slezack, S., Dumas-Gaudot, E., Paynot, M., and Gianinazzi, S. (2000). Is a fully established arbuscular mycorrhizal symbiosis required for a bioprotection of Pisum sativum roots against Aphanomyces euteiches? Mol. Plant Microbe Interact. 13, 238–241. doi: 10.1094/MPMI.2000.13.2.238
Somoza, S. C., Bonfante, P., and Giovannetti, M. (2024). Breaking barriers: improving time and space resolution of arbuscular mycorrhizal symbiosis with single-cell sequencing approaches. Biol. Direct 19:67. doi: 10.1186/s13062-024-00501-1
Spagnoletti, F. N., Cornero, M., Chiocchio, V., Lavado, R. S., and Roberts, I. N. (2020). Arbuscular mycorrhiza protects soybean plants against Macrophomina phaseolina even under nitrogen fertilization. Eur. J. Plant Pathol. 156, 839–849. doi: 10.1007/s10658-020-01934-w
Srivastava, R., Khalid, A., Singh, U. S., and Sharma, A. K. (2010). Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt. Biol. Control 53, 24–31. doi: 10.1016/j.biocontrol.2009.11.012
Steinkellner, S., Hage-Ahmed, K., Garcia-Garrido, J. M., Illana, A., Ocampo, J. A., and Vierheilig, H. (2012). A comparison of wild-type, old and modern tomato cultivars in the interaction with the arbuscular mycorrhizal fungus Glomus mosseae and the tomato pathogen Fusarium oxysporum f. sp. lycopersici. Mycorrhiza 22, 189–194. doi: 10.1007/s00572-011-0393-z
Stratton, C. A., Ray, S., Bradley, B. A., Kaye, J. P., Ali, J. G., and Murrell, E. G. (2022). Nutrition vs association: plant defenses are altered by arbuscular mycorrhizal fungi association not by nutritional provisioning alone. BMC Plant Biol. 22:400. doi: 10.1186/s12870-022-03795-3
Sun, Z., Song, J., Xin, X., Xie, X., and Zhao, B. (2018). Arbuscular mycorrhizal fungal 14-3-3 proteins are involved in arbuscule formation and responses to abiotic stresses during AM symbiosis. Front. Microbiol. 9:91. doi: 10.3389/fmicb.2018.00091
Tabin, T., Arunachalam, A., Shrivastava, K., and Arunachalam, K. (2009). Effect of arbuscular mycorrhizal fungi on damping-off disease in Aquilaria agallocha Roxb. seedlings. Trop. Ecol. 50:243.
Tahmatsidou, V., O'sullivan, J., Cassells, A. C., Voyiatzis, D., and Paroussi, G. (2006). Comparison of AMF and PGPR inoculants for the suppression of Verticillium wilt of strawberry (Fragaria×ananassa cv. Selva). Appl. Soil Ecol. 32, 316–324. doi: 10.1016/j.apsoil.2005.07.008
Tanwar, A., Aggarwal, A., and Panwar, V. (2013). Arbuscular mycorrhizal fungi and Trichoderma viride mediated Fusariumwilt control in tomato. Biocontrol Sci. Technol. 23, 485–498. doi: 10.1080/09583157.2013.772561
Tatsumi, C., Hyodo, F., Taniguchi, T., Shi, W., Koba, K., Fukushima, K., et al. (2020). Arbuscular mycorrhizal community in roots and nitrogen uptake patterns of understory trees beneath ectomycorrhizal and non-ectomycorrhizal overstory trees. Front. Plant Sci. 11:583585. doi: 10.3389/fpls.2020.583585
Thakur, M., Sharma, D., Thakur, A., Bhardwaj, S., Angurana, R., Katoch, V., et al. (2024). “Success story of arbuscular mycorrhizal fungi as a bio protectant against major plant pathogens,” in Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Nutrient and Crop Management (Singapore: Springer), 321–336. doi: 10.1007/978-981-97-0300-5_14
Tian, W., Wang, C., Gao, Q., Li, L., and Luan, S. (2020). Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat Plants 6, 750–759. doi: 10.1038/s41477-020-0667-6
Tisserant, E., Malbreil, M., Kuo, A., Kohler, A., Symeonidi, A., Balestrini, R., et al. (2013). Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc. Nat. Acad. Sci. U. S. A. 110, 20117–20122. doi: 10.1073/pnas.1313452110
Underwood, W. (2012). The plant cell wall: a dynamic barrier against pathogen invasion. Front. Plant Sci. 3:85. doi: 10.3389/fpls.2012.00085
Van Der Heijden, M. G. A., Martin, F. M., Selosse, M. A., and Sanders, I. R. (2015). Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205, 1406–1423. doi: 10.1111/nph.13288
Van Driesche, R., Carruthers, R., Center, T., Hoddle, M., Hough-Goldstein, J., Morin, L., et al. (2010). Classical biological control for the protection of natural ecosystems. Biol. Control 54, S2–S33. doi: 10.1016/j.biocontrol.2010.03.003
Vandegrift, R., Newman, D. S., Dentinger, B. T. M., Batallas-Molina, R., Duenas, N., Flores, J., et al. (2023). Richer than gold: the fungal biodiversity of reserva los cedros, a threatened Andean cloud forest. Bot. Stud. 64:17. doi: 10.1186/s40529-023-00390-z
Vernie, T., Rich, M., Pellen, T., Teyssier, E., Garrigues, V., Chauderon, L., et al. (2025). Conservation of symbiotic signaling since the most recent common ancestor of land plants. Proc. Natl. Acad. Sci. U. S. A. 122:e2408539121. doi: 10.1073/pnas.2408539121
Vos, C., Schouteden, N., Van Tuinen, D., Chatagnier, O., Elsen, A., De Waele, D., et al. (2013). Mycorrhiza-induced resistance against the root–knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biolistry 60, 45–54. doi: 10.1016/j.soilbio.2013.01.013
Wahid, F., Fahad, S., Danish, S., Adnan, M., Yue, Z., Saud, S., et al. (2020). Sustainable Management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture 10:334. doi: 10.3390/agriculture10080334
Wang, J., Zhai, L., Ma, J., Zhang, J., Wang, G. G., Liu, X., et al. (2020). Comparative physiological mechanisms of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects on leaves and roots of Zelkova serrata. Mycorrhiza 30, 341–355. doi: 10.1007/s00572-020-00954-y
Wang, L., George, T. S., and Feng, G. (2024). Concepts and consequences of the hyphosphere core microbiome for arbuscular mycorrhizal fungal fitness and function. New Phytol. 242, 1529–1533. doi: 10.1111/nph.19396
Wang, L., Zhang, L., George, T. S., and Feng, G. (2025). Hyphosphere core taxa link plant-arbuscular mycorrhizal fungi combinations to soil organic phosphorus mineralization. Soil Biol. Biochem. 201:109647. doi: 10.1016/j.soilbio.2024.109647
Wang, Y., Li, Y., and Duan, T. (2022). Arbuscular mycorrhizal fungus changes alfalfa response to pathogen infection activated by pea aphid infestation. Front. Microbiol. 13:1074592. doi: 10.3389/fmicb.2022.1074592
Weng, W., Yan, J., Zhou, M., Yao, X., Gao, A., Ma, C., et al. (2022). Roles of arbuscular mycorrhizal fungi as a biocontrol agent in the control of plant diseases. Microorganisms 10:1266. doi: 10.3390/microorganisms10071266
Wu, Y., Chen, C., and Wang, G. (2024). Inoculation with arbuscular mycorrhizal fungi improves plant biomass and nitrogen and phosphorus nutrients: a meta-analysis. BMC Plant Biol. 24:960. doi: 10.1186/s12870-024-05638-9
Yizhu, L., Imtiaz, M., Ditta, A., Rizwan, M. S., Ashraf, M., Mehmood, S., et al. (2020). Response of growth, antioxidant enzymes and root exudates production towards as stress in Pteris vittata and in Astragalus sinicus colonized by arbuscular mycorrhizal fungi. Environ. Sci. Pollut. Res. Int. 27, 2340–2352. doi: 10.1007/s11356-019-06785-5
Yu, H., Ji, C., Zheng, Z., Yu, M., Liu, Y., Xiao, S., et al. (2023). Comparative proteomic analysis identifies proteins associated with arbuscular mycorrhizal symbiosis in Poncirus trifoliata. Front. Plant Sci. 14:1294086. doi: 10.3389/fpls.2023.1294086
Yuan, M. M., Kakouridis, A., Starr, E., Nguyen, N., Shi, S., Pett-Ridge, J., et al. (2021). Fungal-bacterial cooccurrence patterns differ between arbuscular mycorrhizal fungi and nonmycorrhizal fungi across soil niches. mBio 12:e03509-03520. doi: 10.1128/mBio.03509-20
Yuan, P., Luo, F., Gleason, C., and Poovaiah, B. W. (2022). Calcium/calmodulin-mediated microbial symbiotic interactions in plants. Front. Plant Sci. 13:984909. doi: 10.3389/fpls.2022.984909
Yuan, S., Li, M., Fang, Z., Liu, Y., Shi, W., Pan, B., et al. (2016). Biological control of tobacco bacterial wilt using Trichoderma harzianum amended bioorganic fertilizer and the arbuscular mycorrhizal fungi Glomus mosseae. Biol. Control 92, 164–171. doi: 10.1016/j.biocontrol.2015.10.013
Yuliar, Nion, Y. A., and Toyota, K. (2015). Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ. 30, 1–11. doi: 10.1264/jsme2.ME14144
Zhang, G., Raza, W., Wang, X., Ran, W., and Shen, Q. (2012). Systemic modification of cotton root exudates induced by arbuscular mycorrhizal fungi and Bacillus vallismortis HJ-5 and their effects on Verticillium wilt disease. Appl. Soil Ecol. 61, 85–91. doi: 10.1016/j.apsoil.2012.02.003
Zhang, R. Q., Zhu, H. H., Zhao, H. Q., and Yao, Q. (2013). Arbuscular mycorrhizal fungal inoculation increases phenolic synthesis in clover roots via hydrogen peroxide, salicylic acid and nitric oxide signaling pathways. J. Plant Physiol. 170, 74–79. doi: 10.1016/j.jplph.2012.08.022
Zhang, S., Li, S., Meng, L., Liu, X., Zhang, Y., Zhao, S., et al. (2024). Root exudation under maize/soybean intercropping system mediates the arbuscular mycorrhizal fungi diversity and improves the plant growth. Front. Plant Sci. 15:1375194. doi: 10.3389/fpls.2024.1375194
Zhou, J., Chai, X., Zhang, L., George, T. S., Wang, F., and Feng, G. (2020). Different arbuscular mycorrhizal fungi cocolonizing on a single plant root system recruit distinct microbiomes. mSystems 5, e00929–e00920. doi: 10.1128/mSystems.00929-20
Zhou, J., Lin, S., Luo, X., Sun, L., Chen, J., Cheng, B., et al. (2025a). SYMRK significantly affected AMF symbiosis and plant growth in maize. Plant Sci. 353:112427. doi: 10.1016/j.plantsci.2025.112427
Zhou, W., Li, M., and Achal, V. (2025b). A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg. Contam. 11:100410. doi: 10.1016/j.emcon.2024.100410
Zhu, H., and Yao, Q. (2004). Localized and systemic increase of phenols in tomato roots induced by Glomus versiforme inhibits Ralstonia solanacearum. J. Phytopathol. 152, 537–542. doi: 10.1111/j.1439-0434.2004.00892.x
Keywords: arbuscular mycorrhizae, plant disease management, induced systemic resistance, sustainable crop production, biotic and abiotic stress mitigation
Citation: Umer M, Anwar N, Mubeen M, Li Y, Ali A, Alshaharni MO and Liu P (2025) Roles of arbuscular mycorrhizal fungi in plant growth and disease management for sustainable agriculture. Front. Microbiol. 16:1616273. doi: 10.3389/fmicb.2025.1616273
Received: 22 April 2025; Accepted: 30 June 2025;
Published: 23 July 2025.
Edited by:
Renu S., Indian Council of Agricultural Research (ICAR), IndiaReviewed by:
Chandra Mohan Singh, Banda University of Agriculture and Technology, IndiaJayalakshmi K., Directorate of Onion and Garlic Research (ICAR), India
Copyright © 2025 Umer, Anwar, Mubeen, Li, Ali, Alshaharni and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Li, Z3h1bGl5dW5AZ3h1LmVkdS5jbg==; Pingwu Liu, aG51bHB3QGhhaW5hbnUuZWR1LmNu
†These authors have contributed equally to this work
 Muhammad Umer1†
Muhammad Umer1† Naureen Anwar
Naureen Anwar Mustansar Mubeen
Mustansar Mubeen Amjad Ali
Amjad Ali Pingwu Liu
Pingwu Liu