- 1College of Animal Science and Technology, Tarim University, Alar, China
- 2Key Laboratory of Tarim Animal Husbandry Science and Technology, Xinjiang Production and Construction Corps, Alar, China
- 3College of Veterinary Medicine, Huazhong Agricultural University, Wuhan, China
Bovine viral diarrheal virus (BVDV), identified as a serious global pathogen, presents a significant and formidable challenge for the global cattle sector. This presents not only serious economic consequences but also serious immunopathological consequences causing lasting impacts. In the Chinese province of Xinjiang, where the ruminants are high and the topographical features unique and contribute towards complex epidemiology, multiple genotypes of the BVDV are continuously interacting and adapting. This includes the persistent presence of the earlier predominant BVDV1, the rising trend towards the antigenically complex BVDV2, and the recent presence of the HoBi-like (BVDV3) strain, contributing toward complexity. The purpose of this review is to present a detailed analysis relative to the molecular epidemiology, genomic diversity levels, and complex pathology associated with the persistence and spreading of the infection by the BVDV. In light of the country’s inability to execute the efficacious eradication of the infection, stringent biosafety measures need to be adopted, the genomic investigation has to be increased, and the establishment of the multi-valent vaccine for the induction of the local strain of the infection should be encouraged. These steps need to be taken towards the rising danger of the presence of the BVDV, not only towards the province but also towards the larger territories beyond.
1 Introduction
Bovine viral diarrhea (BVD) is considered a widespread infection in cattle accompanied by diarrhea, high fever, necrosis and mucosal erosion of the gastrointestinal tract, leukopenia, and reduced platelets (Peddireddi et al., 2018). BVD is a viral disease caused by bovine viral diarrhea virus (BVDV), which is the enveloped/positively stranded RNA virus and belonging to the genus Pestivirus and the Flaviviridae family. BVDV has three genotypes: BVDV1 (Pestivirus A), BVDV2 (Pestivirus B), and BVDV3 (Pestivirus H); the latter one is also termed a HoBi-like genotype. Cytopathic and non-cytopathic are its two biotypes, which are classified depending upon their impact on cultured cells; the former induces apoptosis (Pedrera et al., 2009; Ran et al., 2019; Wang et al., 2021). BVDV might be sub-clinical, mild, severe, and/or fatal, severely affects dairy industry and infects yaks, buffaloes, goats, pigs, sheep, and deer, resulting in huge economic losses for the cattle industry around the globe, particularly in China (Fulton et al., 2002; Ran et al., 2019; Wang et al., 2021). BVDV causes an average annual production loss of €42.14 to €67.19 per animal (Pinior et al., 2019). In addition, instead of the major symptom of diarrhea, BVDV-infected animals are often susceptible to respiratory infections, reproductive issues, growth retardation, stillbirth, and even mucosal diseases, leading to immunosuppression and disruption of an innate immune response (Tautz et al., 2015). Based on the phylogenetic analysis and genetic characterization, the epidemiological and molecular features of BVDV were investigated, which revealed that BVDV1 and BVDV1b are the most prevalent subtypes of BVDV in China (Wang et al., 2020). Further, in Beijing, the highest prevalence was reported because 93.4% of animals were positive for BVDV antibodies (Weng et al., 2015). The positive rate of BVDV in dairy animals in Fujian, Shaanxi, and Shandong is 90, 88.9, and 83.3%, respectively (Ran et al., 2019). Likewise, in Xinjiang, a 53.68% positive rate of BVDV antibodies is reported (Yang et al., 2023). Xinjiang Uygur Autonomous Region is located in north-western China and has an established animal-husbandry industry consisting of around 4 million cattle in its main districts, including Kuitun, Shawan, Yili, Manas, Aksu, Hami, and Shihezi. As it borders with Kazakhstan, Central Asian countries, and Russia, a higher risk of cross-border BVDV transmission is predicted. Thus, this review aims to assess the genetic characterization, prevalence, epidemiological patterns, pathogenic mechanisms, immunosuppression, and preventive measures for BVDV in Xinjiang compared with other Chinese cities/provinces.
2 Xinjiang might be at a higher risk of BVDV infection
Xinjiang has a larger (57 million hectares) natural grassland area that is naturally cultivatable with fertile land, supporting a diverse group of livestock rearing. Due to its geographical location, Xinjiang is considered among the main pastoral areas of China and is ranked second in contributing cattle production proportion, resulting in rapid progress in developing animal husbandry practices. Thus, most farmers prefer intensive and large-scale animal production. However, due to the continuously expanding breeding scale in Xinjiang, different epidemics have emerged, and BVDV is seriously affecting cattle production at a large scale, posing a continuous risk of BVDV infection for healthy animals (Yang et al., 2023). Additionally, as Xinjiang borders Kazakhstan, Central Asian countries, and Russia and is an immediate neighboring region of China, it is anticipated a higher risk of cross-border BVDV transmission. Although no direct evidence has revealed that Xinjiang’s BVDV prevalence is attributed to its close borders with Asian countries, due to its geographical location and continuous developments in the rapid breeding practices of cattle, it might contribute to the spread of infection. Moreover, the alternative reason why Xinjiang is at higher risk of BVDV infection rate is more convincing. Zhong et al. (2011) reported that BVDV1c has been found circulating in Xinjiang, with higher similarities (94.4%) with the Australian strains. Most of the Holstein cattle in Xinjiang were often imported from Australia, and as BVDV1c is reported to be a predominant subtype of BVDV in Australia, the healthy herd of cattle is also at higher risk, which may lead to increased levels of persistent infection. Therefore, finding a strategy that helps reduce the BVDV risk rate in Xinjiang is an urgent requirement.
3 Genetic characterization and prevalence of BVDV in Xinjiang and other Chinese provinces
It has been established that cattle are the major source of BVDV infection. Back in 1980, it was the first time isolated from cattle in China, termed Changchun184 and named BVDV1b. Subsequently, ZM-95 (BVDV2) was also isolated in 1995 from pigs, and XJ-04 (BVDV2) and JZ05-1 (BVDV2) were isolated from cattle in China. Until 2013, a complete genomic sequence was also available for pigs for the strains SD0806, ZM-95, and SH-28, indicating 70% similarities (Tao et al., 2013). The structural proteins of BVDV viral particles are Nucleocapsid protein C and glycoproteins, i.e., E1, E2, and Erns. These proteins combine with their lipid bilayer, and genomic RNA contains an envelope (Chi et al., 2022). The arrangements of the whole BVDV structure are shown in Figure 1. BVDV genome consists of a single open-reading-frame (ORF) lined with 3′ and 5′ untranslated regions and encodes a polyprotein, which becomes a mature viral protein through undergoing co-translational and post-translational processes. Through phylogenetic analysis (partial sequence) of 5′ untranslated regions of ORF, the N-terminal-autoprotease or envelope-glycoprotein region of BVDV gnome is divided into genetic species, BVDV1 and BVDV2 (Deng et al., 2015).
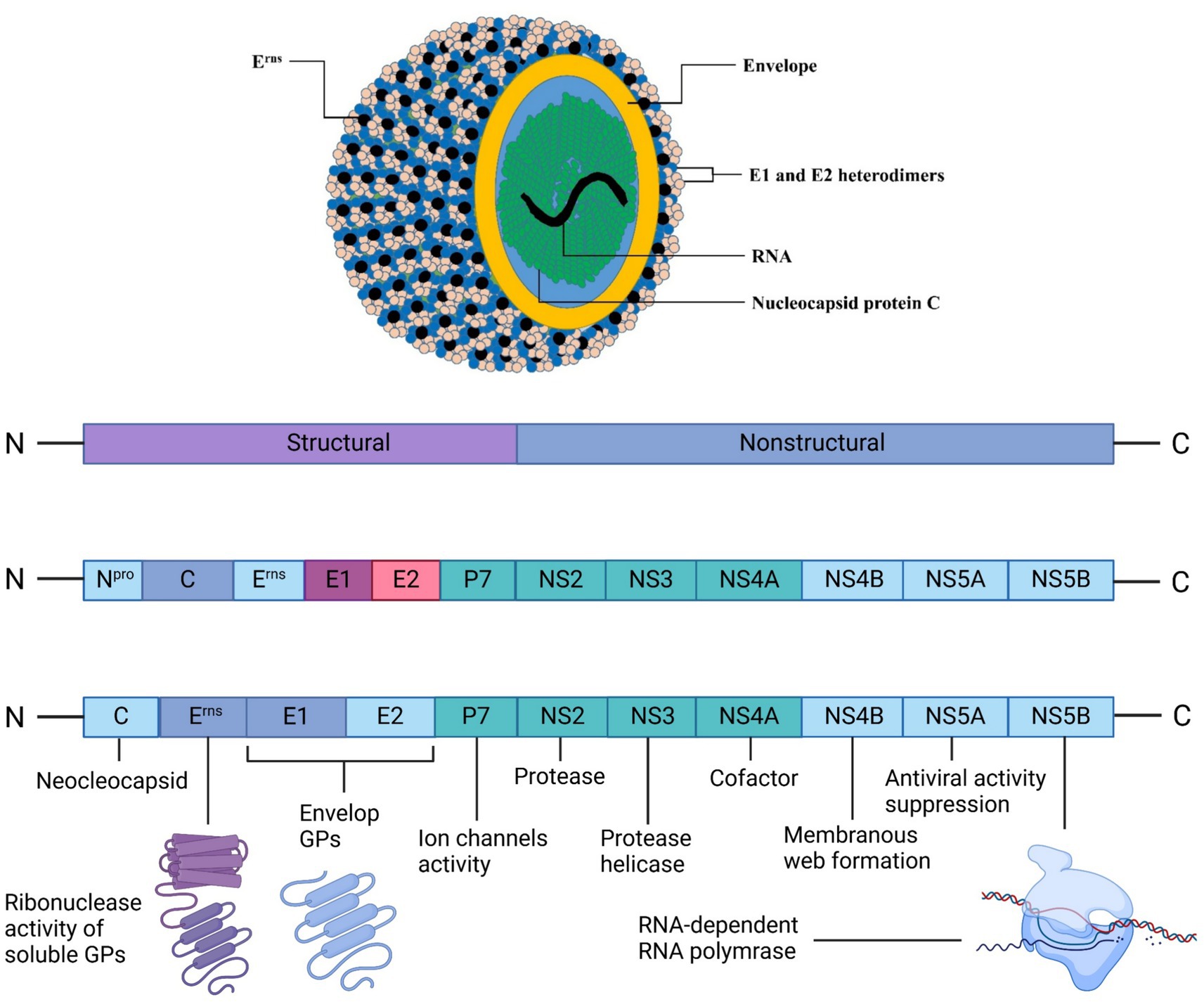
Figure 1. BVDV’s structural morphology and genomic translation into structural and nonstructural proteins for viral assembly. The outermost layer is the lipid envelope, studded with envelope (E) glycoproteins, including the secreted Erns proteins visible as black dots. Beneath the envelope lie the E1 and E2 heterodimers, crucial for viral entry. The yellow layer represents the nucleocapsid protein C, which encapsulates the viral RNA genome (shown in green). Created in BioRender. F. Kulyar, M. (2025) https://BioRender.com/p95yur0.Kulya.
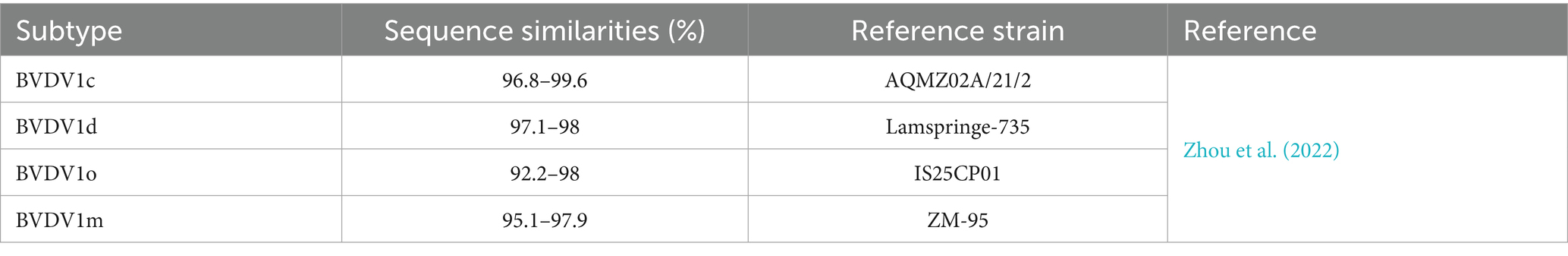
In a recent study, 53 sequences (5´-UTR nucleotide) were determined using the phylogenetic analysis, and all of them belonged to BVDV1 five subtypes, including BVDV1b, BVDV1c, BVDV1d, BVDV1o, and BVDV1m, with the bootstrap support values of more than 75% for the clade assignments. This strong statistical support offers conclusive proof of the circulation of various subtypes of BVDV1 and elucidates the widespread genomic diversity of the BVDV strains in China typically in cattle in eastern China (Shah et al., 2022; Xiao et al., 2025). The sequence similarities of these subtypes are given in Table 1 (Zhou et al., 2022). Another study revealed 21 BVDV1 subtypes (BVDV1a to BVDV1u), four BVDV2 subtypes (BVDV2a to BVDV2d), and two BVDV3 (HoBi-like) subtypes, which are based on their Brazilian and Thai origins Field (Zhang et al., 2022). However, the most prevalent are BVDV1a, BVDV1b, BVDV1c, BVDV1d, BVDV1u, BVDV1m, BVDV1q, BVDV1p, and BVDV1o, which are continuously circulating in dairy cattle in the whole china (Deng et al., 2020). Further, once the host is infected with BVDV, the noncytopathogenic BVDV has the ability to persistently maintain the infection level before establishing an adaptive immune response by the host (Weng et al., 2015). Additionally, cytopathic-BVDV-strains, the derivatives of non-cytopathic-BVDV-strains, are found due to genetic mutations or cytopathic mutation in persistently infected animals, indicating the higher genetic diversity of BVDV (Wu et al., 2023).
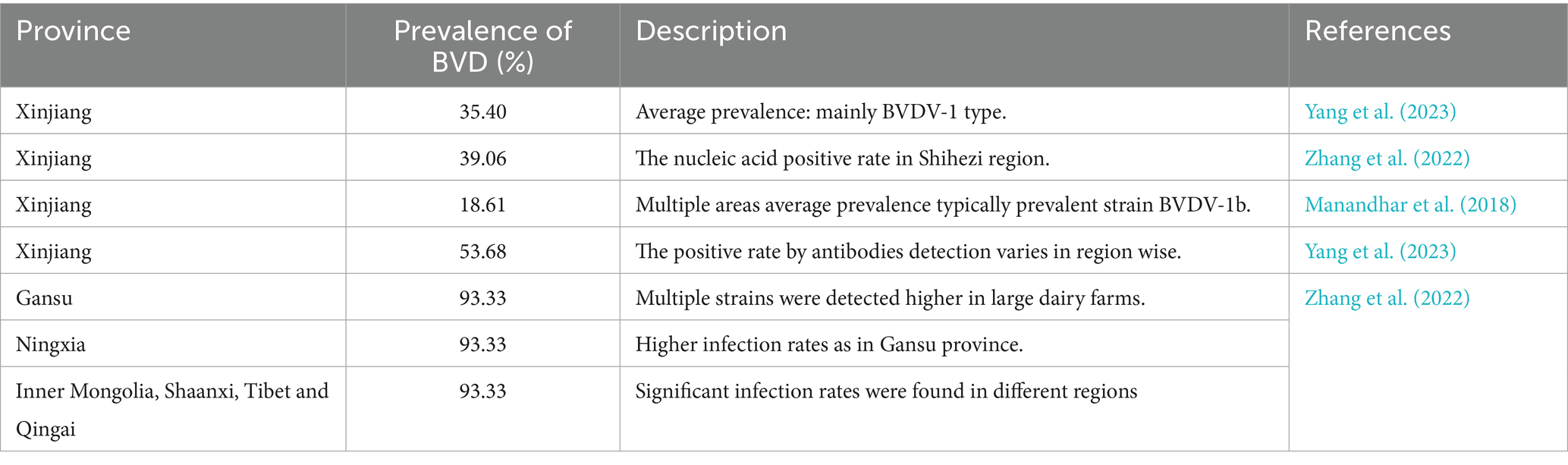
The extent of BVDV infection could be monitored by conducting studies to determine its seroprevalence. Only then, the researchers might implement a control strategy for BVDV. For example, the seroprevalence rate of BVDV in Fujian province for beef cattle is 29.8%, while 92.5% is for dairy cows. Yaks were 53.65 and 72.14% in Tibet and Qinghai provinces, respectively (Yang et al., 2007; Gao et al., 2013). Another study reported BVDV seroprevalence of 14.18, 45.38, 63.27, and 89.49% in water buffaloes, yaks, beef cattle, and dairy cows, respectively (Deng et al., 2015). Alarmingly, even clinically healthy yaks, buffaloes, and cows are 58.09% positive for BVDV antibody, and the BVDV antigen report revealed that around 46.7% of cattle farms were reported positive in China. Overall, a 2.2% rate of persistent infection indicated a considerably higher infection rate in China than other Asian countries (Yang et al., 2023). Several studies have been performed to assess the BVDV infection rate in Xinjiang. For example, an average of 35.40% BVDV prevalence was found in the northern areas of Xinjiang (Yang et al., 2023). Another study conducted a nucleic acid investigation, identified subtype BVDV1b, and found a 39.06% BVDV-positive rate in the Shihezi area of Xinjiang (Li et al., 2009). Yang et al. (2023) conducted an epidemiological investigation in the Kashgar, Aksu, Manas, and Shihezi areas of Xinjiang from 2010 to 2012 and found an 18.61% prevalence rate for BVDV1b. Based on serological investigation, Wang et al. isolated 17 BVDV1 and 4 BVDV2 strains at cattle farms in Xinjiang (Wang et al., 2015). Between 2016 and 2018, Yanhua et al. (2020) found BVDV1q as widespread and prevalent strain at cattle farms of Xinjiang. These reports revealed that BVDV has been spreading in the Xinjiang areas for several previous years, particularly at the cattle farms. In addition, the diverse virus type/subtype poses serious concern on cattle production and causing significant economic losses in animal husbandry in Xinjiang. Thus, by understanding the genetic characterization and prevalence of BVDV could control its dissemination in Xinjiang. Moreover, a comparison among controlled and general BVDV vaccines is made in Table 2.
4 The phylogenetic analysis of BVCV serotypes
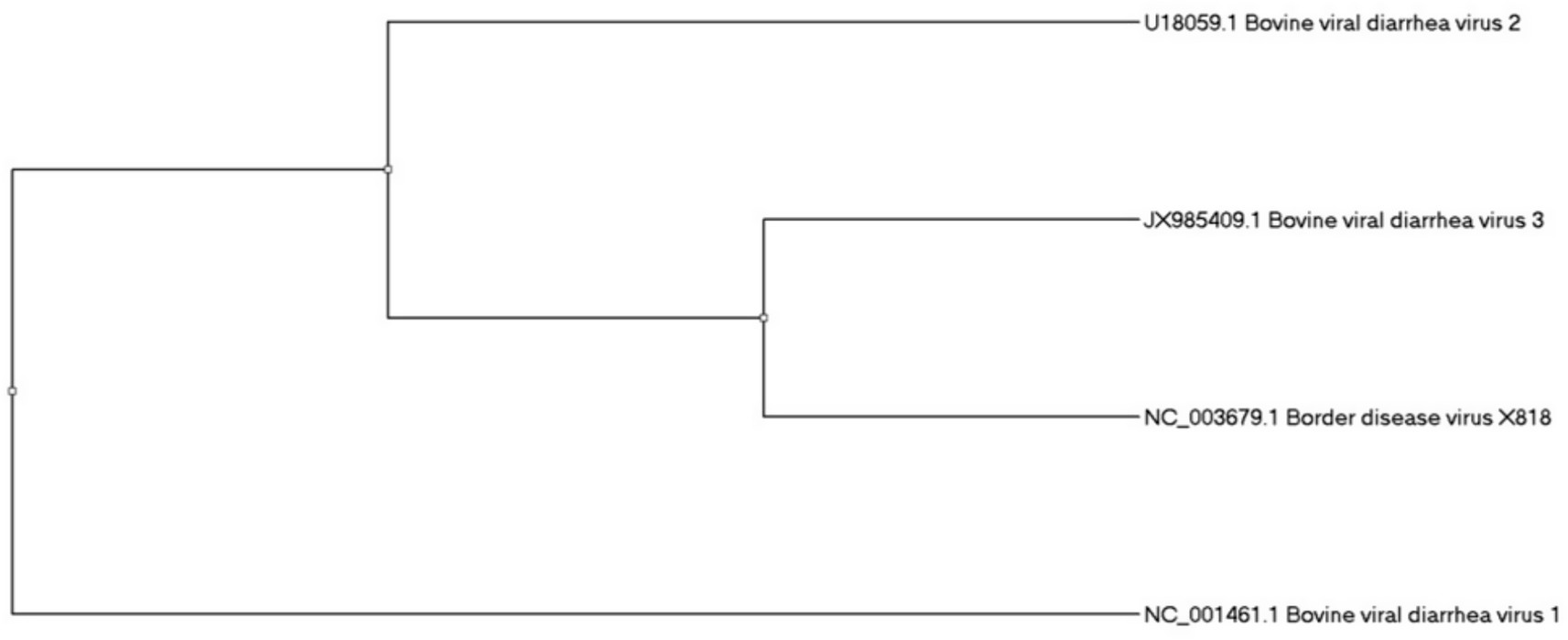
For the understanding of molecular divergence between three BVDV genotypes, we have made a phylogenetic tree (Figure 2). It was made on the base of Npro gene region through Maximum Likelihood method by using reference strains, BVDV1, NADL (NC_001461); BVDV2, 890 (U18059); BVDV3, D32/00_HoBi (JX985409) vs. Border disease virus BDV (X818)1 as an outgroup. The tree’s proper rooting was enabled by the use of BDV as an outgroup and highlighted the genetic difference among BVDV genotypes (Figure 2).
5 BVDV-3 (HoBi-like) vaccine development and constraints in its process
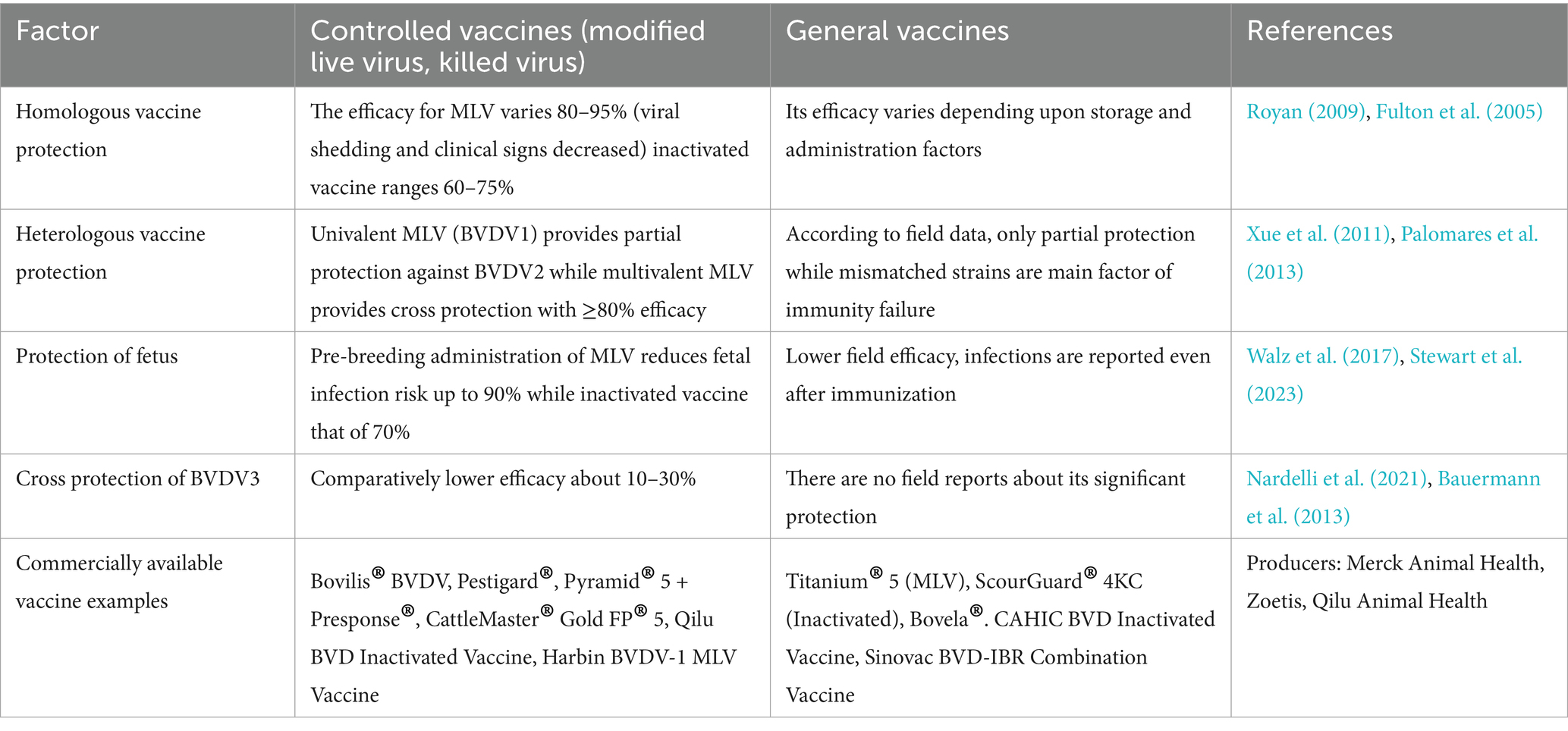
The vaccine development against BVDV-3 (HoBi-like pestivirus) starts from the isolation of the virus from infected animals and whole-genome sequencing, which indicated clear genetic distinction from BVDV-1 and BVDV-2, e.g., a polyprotein of about 3,899 amino acids (Yang et al., 2023). Among the targets of the antigens is glycoprotein E2, which plays a role in viral entry and neutralizing antibody induction; recombinant E2 proteins, with truncations to optimize secretion and immunogenicity, have been successful in case of subunit vaccine (Lo et al., 2023). Immunogenicity experiments shows that recombinant E2 could persuade balanced IgG1/IgG2a responses, a sign of strong T cell-dependent immunity, the experimental animal models validating antibody induction and partial protection from fetal infection (Decaro, 2020; Lo et al., 2023). Naturally acquired BVDV-1 and BVDV-2 immunity does not, however, protect against challenge with BVDV-3, highlighting the extent of limited cross-protection and the necessity for BVDV-3–specific or multivalent vaccines for the Xinjiang region, for instance, where more than one genotype is co-circulating. The novel immunizing strategies are being followed for BVDV-3 implication. But antigenic variation, the failure of current vaccines to provide cross-protection, and difficulties in animal model studies hinder progress. Improved diagnostic tests and site-directed vaccine designs must be developed in order to control successfully this newly emerged pestivirus (Al-Kubati et al., 2021).
6 Assessing the epidemiological patterns of BVDV between Xinjiang and other cattle-producing provinces in China
BVDV is closely related to the loss of productivity in cattle. Assessing which epidemiological factor contribute to this huge loss facilitates the implementation of strategies to reduce the BVDV burden on animals. BVDV initial prevalence, infection risk, intensity of circulation, duration of circulation, testing/culling rate, vaccination, biosecurity, new animals’ introduction to farm, and close contact with the neighboring herds could be measured to investigate the pattern of infection (Pinior et al., 2019). Pinior et al. (2019) calculated €42.14 as an average production loss per animal for BVDV infection. It was demonstrated that higher initial prevalence, infection risk, intensity of circulation, and duration of circulation could significantly increase the average production loss per animal to €67.19, indicating that introducing a new batch of cattle into the healthy cattle herd is the most vital factor that facilitates BVDV transmission. Several analyses revealed that purchasing pregnant cows considerably increases the BVDV infection rate in the healthy cattle herd (Bitsch et al., 2000; Santman-Berends et al., 2017; Pinior et al., 2019). Further, interaction/contact with neighboring animals or cattle herds or contact with the housing of other ruminants is a critical factor that boosts BVDV viral transmission from the infected to the healthy herd and, therefore, leads to production losses. If farmers permitted their cattle to be in contact with other cattle of any other herd or introduced new animals into their farms could substantially contribute to 18% average production losses per animal. Alarmingly, the average production losses per animal are twice as high in dairy herds compared with beef herds annually (Graham et al., 2016; Kaiser et al., 2016; Pinior et al., 2019). Further, average production losses per animal could be 28–29% lower using the biosecurity measures and 8–12% lower by following proper vaccination schedules. Moreover, if the cattle herd is vaccinated with the BVDV vaccine, the fetal infection proportion could be 85% reduced, and the abortion rate could be 45% lower. The reasons farm owners face huge economic losses are either because the farmers fail to follow the vaccine schedule properly or because they administer unprotected vaccines whose efficiency has not been verified. Some studies described that testing/culling of the animals is not a major factor that could change average production losses per animal because it could not be considered an economic strategy (Newcomer et al., 2015; Evans et al., 2019; Pinior et al., 2019). Yaks are of significant importance in China’s cattle industry. The BVDV prevalence, a main epidemiological factor, is found higher (67.5%) in yaks in Xinjiang compared with its prevalence in Qinghai and Sichuan that is 35.5 and 31.16%, respectively, indicating a significant impact of BVDV infection on yaks in Xinjiang (Diao et al., 2020). Another study collected animal serum and found a positive rate of 37.5% in Xinjiang, which is still higher than in Tibet (10.77%), Ningxia (30%), and Shaanxi (4.57%) (Chang et al., 2021). Zhang et al. (2022) reported that in Xinjiang, BVDV infection was significantly spreading (99.33%) in the cattle farms of non-BVDV-vaccinated animals. As the chance of BVDV infection spreading rate increases by importing dairy cattle from Australia to Xinjiang (Zhong et al., 2011), repeated circulation of dairy animals at domestic levels further boosts cross-species BVDV infection (Weng et al., 2015). Lang et al. (2014) identified nine BVDV sub-genotypes that were already circulating in dairy animals in China. These reports exhibit that assessing epidemiological patterns of BVDV infection in Xinjiang could help manage the BVDV infection rate. Moreover, some studies indicating BVDV’s prevalence via different methods are summarized (Table 3).
7 An insight into pathogenic mechanisms of BVDV
BVDV pathogenic mechanism is highly dynamic, including initial entry, attachment, replication, assembly/release, and spreading of the infection (Qi et al., 2022). The whole process is very complex, and the mechanism is still unknown. However, a few studies explain its mechanism (Rajput et al., 2017; Ma et al., 2022). BVDV starts its life cycle after attachment with the host cell, following endocytosis through binding with the host cell receptor. BVDV proteins, i.e., Erns, E1, and E2, help in binding and endocytosis. BVDV RNA constructs its replication complex and structural proteins, which help in BVDV viral assembly and, finally, its release from the host cell (Ma et al., 2022). The possible mechanism is shown in Figure 3.
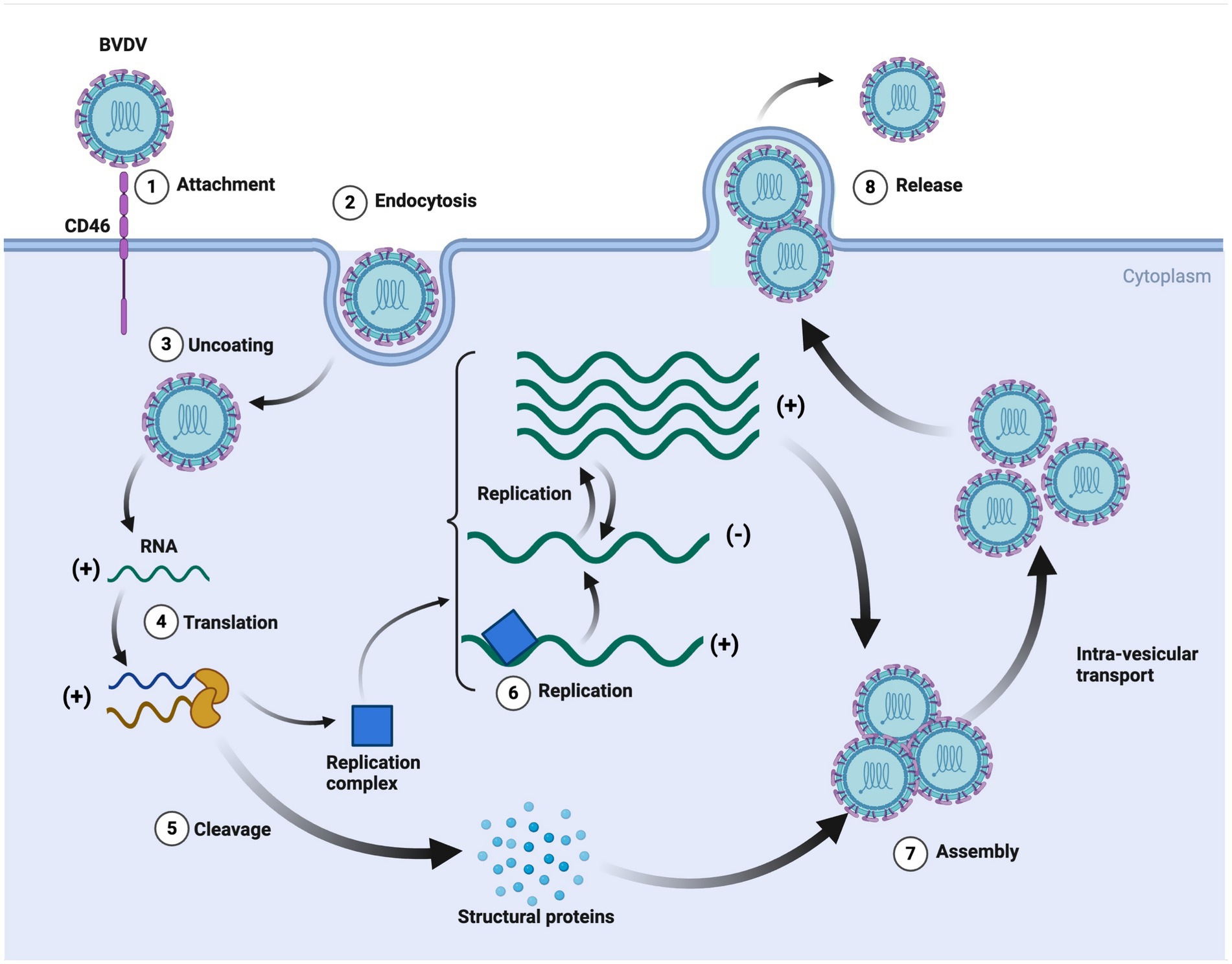
Figure 3. The replication cycle of bovine viral diarrhea virus (BVDV). This detailed schematic illustrates the key steps in BVDV infection and replication. The process also highlights the formation of the replication complex and the production of structural proteins, emphasizing the intricate cellular mechanisms exploited by BVDV for its propagation. It further provides insights into potential targets for antiviral strategies and vaccine development against BVDV. Created in BioRender. F. Kulyar, M. (2025) https://BioRender.com/6penelu.
BVDV virus has a complex interaction with the hosts for entering their body and attachment/entry into their cells, which is performed through different affinities and extremely active processes. Before the cellular entry into host cells, the BVDV virus must establish close contact with the host’s cells after traveling to the suitable site. For this purpose, the BVDV virus performs diffusive motion and confined or directed motions (Riedel et al., 2020). It has been found that the BVDV virus requires a receptor (bovine CD46) to enter into host cells. CD46 is ubiquitously expressed, a cofactor, and involved in autophagy modulation and T cell regulation. Some scientists encoded BVDV with mCherry-E2-fusion-protein (BVDV E2) and investigated the attachment/entry mechanism. BVDV E2 binds with CD46 through 30 C-terminal amino acids of complement control protein module-1 (Krey et al., 2006; Riedel et al., 2020). BVDV entry is immediately supported with viral replication and its transmission. Strong et al. (2015) performed strand-specific RT-PCR and demonstrated that the BVDV virus was actively replicating in the blood samples of animals even 85 days after infection, which is crucial and indicates how BVDV persistently infects cattle herds. BVDV virus also has the ability to escape from the host’s immune system, which increases BVDV’s persistent infection rate and inhibits the host’s innate immune response. It has been found that BVDV inhibited the IFN-β production and RLR-signalling pathway by interacting NS4B with 2CARD of the host’s MDA5 domain, resulting in increased BVDV1a proliferation. RLR is the RNA sensor in the cytosol, and RIG-I, MDA5, and LGP2 are its three major members. RLR contains a central helicase domain and CTD, which together play roles in the detection of immunostimulatory RNAs (Rehwinkel and Gack, 2020; Chi et al., 2022). BVDV-mediated inhibition of the RLR signaling pathway is shown in Figure 4.
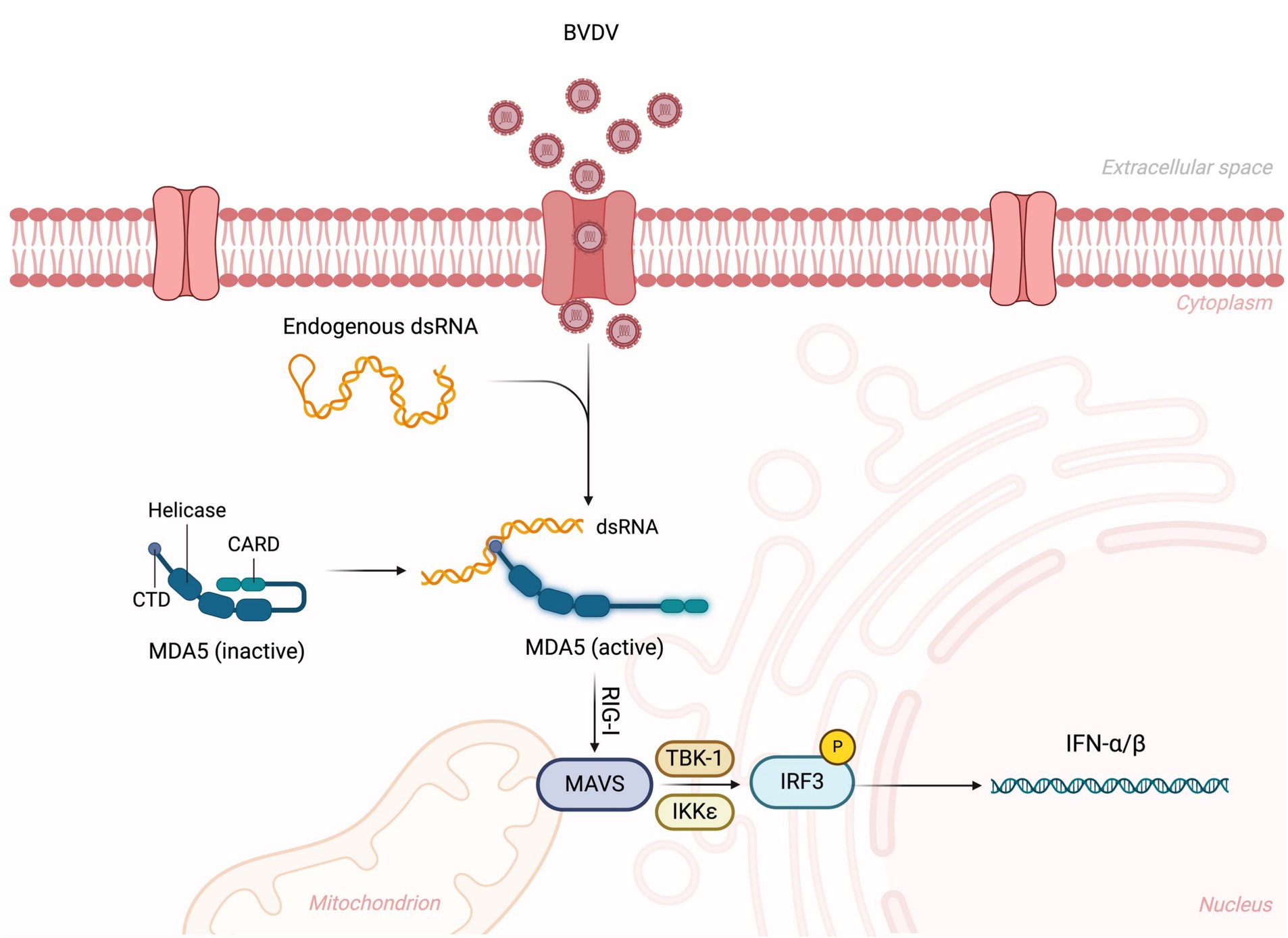
Figure 4. BVDV-mediated inhibition of RLR signaling pathway. The virus is recognized by the RLR sensor MDA5 (melanoma differentiation-associated protein 5), leading to its activation. This activation triggers the downstream signaling cascade, where MDA5 interacts with the mitochondrial antiviral-signaling protein (MAVS), leading to the recruitment of TBK1 and IKKε kinases. These kinases phosphorylate IRF3 (interferon regulatory factor 3), promoting its translocation to the nucleus and the subsequent production of type I interferons (IFN-α/β). Created in BioRender. F. Kulyar, M. (2025) https://BioRender.com/glqkilx.
Based on clinical manifestations, in a study of 177 positive samples of BVDV, 30 and 70% of isolates from cattle were cytopathic and non-cytopathic, respectively. It was also crucial that almost 72% of isolates were obtained from cattle below 1 year of age, and 34% of isolates were recovered from the calves (4–6 months). The clinical signs were 18% for diarrhea, 19% for abortion, and 27% for respiratory syndrome, indicating that BVDV virulence might be affected by several factors (Ahn et al., 2005). BVDV virus caused 33 to 88% and 4 to 8% morbidity and mortality, respectively, and BVDV infection could be divided into four categories, i.e., mild acute, severe acute, chronic, and mucosal disease (Ridpath et al., 2006). BVDV viral antigen was found under the tonsil’s lymphoid tissue, indicating that the tonsil epithelial crypt could be the possible entry site of the non-cytopathic BVDV virus (Liebler-Tenorio et al., 2002). Another study determined that BVDVq1 could increase the size of mesenteric-lymph nodes and cause intestinal and renal hemorrhages and intestinal inflammation in calves in Xinjiang (Yanhua et al., 2020). It has also been found that inoculation of BVDV2 induced acute BVDV infection in calves with symptoms of diarrhea, depression, and fever and also caused recumbency in calves because it significantly decreased lymphocytes and thrombocytes. Other BVDV isolates could also cause severe hemorrhagic diathesis (Liebler-Tenorio et al., 2002). Additionally, Seong et al. (2013) investigated the effects of Korean non-cytopathic BVDV1 and BVDV2 in calves and compared the pathogenetic differences between these isolates that are given in Table 4. Any animal in the cattle herd is susceptible to BVDV infection through horizontal transmission. After exposure, the BVDV incubation period is 6–12 days, but every BVDV strain has some variation in the incubation period, which depends upon the dose of the transmitted virus. Post BVDV infection, the infected animal starts shedding the virus between 3 and 15 days, with a maximum of 3 weeks (Evans et al., 2019). Previous studies indicate that the genotypes of BVDV have different antagonizing abilities with host’s immune system. For example, BVDV1a could potentially overwhelm IFN-β production via IRF-3 as compared to BVDV2a. While, evasion mechanism of BVDV3 remains less prominent. Since there exists sequence difference in immune-modulating proteins like Npro and NS4B among genotypes, molecular docking and in silico modeling of host-pathogen interactions (e.g., Npro–IRF3, NS4B–MDA5) can provide predictive information on genotype-specific immune evasion and rational vaccine development against BVDV3 (Pang et al., 2023; Wang et al., 2024).
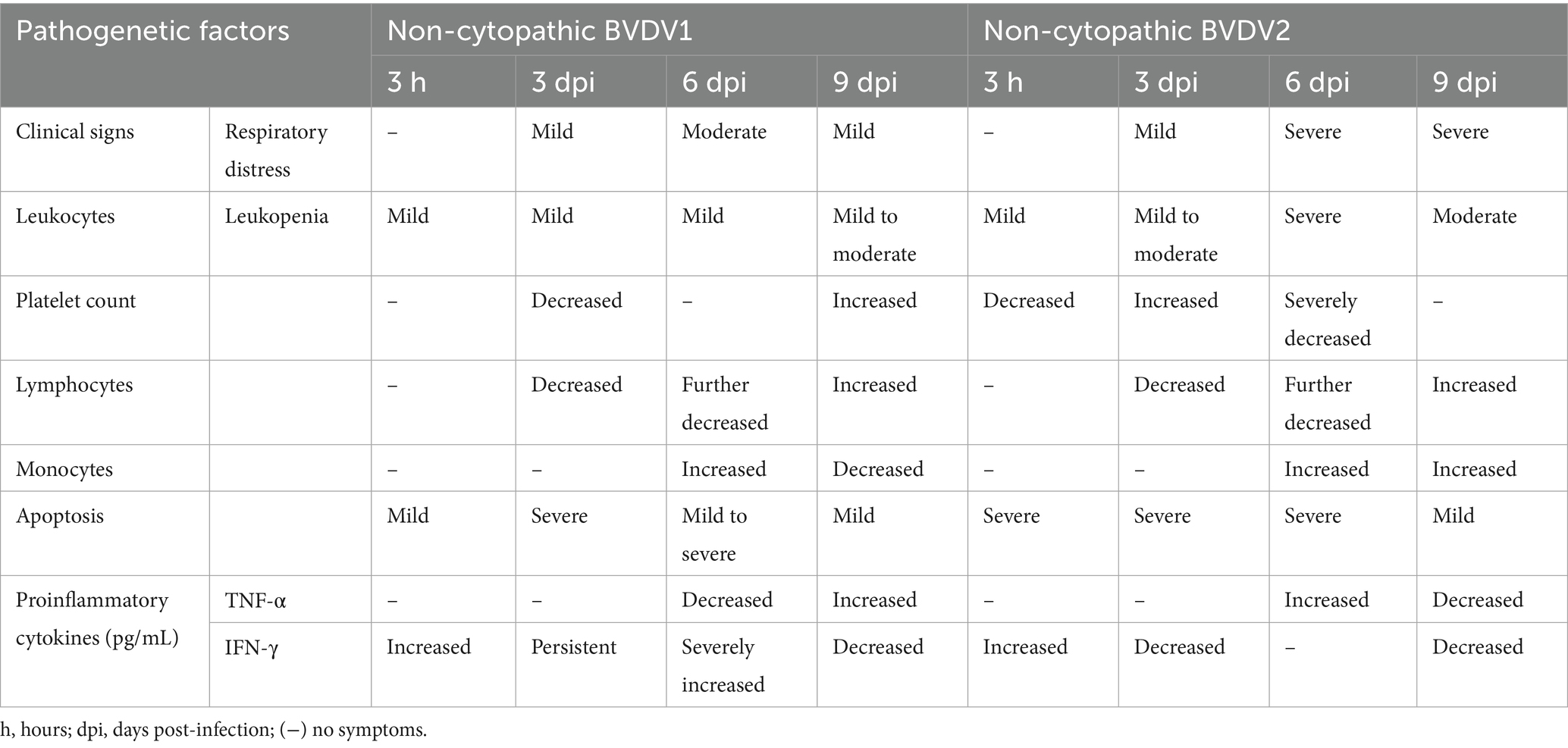
Table 4. Pathogenetic differences between non-cytopathic BVDV1 and BVDV2 in calves (Seong et al., 2013).
8 BVDV-induced immunosuppression and autophagy significantly contribute to secondary infections in the cattle of Xinjiang
It has been reported that BVDV has a higher proportion of inducing mixed infections with other pathogens in the host. BVDV infection suppresses the host immune response, resulting in immunosuppression and an infected animal promotes secondary infections through already present pathogenic organisms in the host. Recently, BVDV-induced secondary infections have become a common issue in Xinjiang. With these mixed infections, Pasteurella multocida and Mycoplasma routinely cause a higher mortality rate in calves and cattle, weakening host immune response in the animals of Xinjiang (Zhang et al., 2022). It has been found that the BVDV virus suppresses the response of IFN α/β of peripheral blood mononuclear cells (monocytes, natural killer, B, and T cells) and decreases T cells (CD4+ and CD8+) and lymphocytes levels in blood circulation, contributing to systemic immunosuppression in the persistently-infected animals (Weng et al., 2015; Liu et al., 2023). Compared with the control group, BVDV infection significantly decreases CD3+ (55.8%), CD4+ (53%), and CD8+ (56.7%) cells in the BVDV-infected group (Liu et al., 2023). BVDV evasion capability maintains persistent infection levels by disrupting adaptive immune response (Weng et al., 2015). In persistently infected animals, BVDV is triggered in early fetal development, and the BVD virus prepares to successfully evade the host’s innate immune response by inhibiting IFN α/β expression. Although some studies explain the BVDV evasion mechanism, its exact details still need to be clarified. For example, IRF-7 and IRF-3 are the main transcriptional factors that regulate type-I-IFN. At first, Erns, a BVDV structural protein, inhibits type-I-IFN through degrading dsRNA (virus). Secondly, Npro, a non-structural BVDV protein, ceases IFN α/β expression through targeting IRF-3. Usually, Npro triggers IRF-3 proteasomal degradation and polyubiquitination and induces its long-term downregulation. BVDV decreases IRF-7 and IRF-3 expression levels in persistently infected cattle, indicating how BVDV disrupts and evades the host’s innate immune response and significantly contributes to host immunosuppression (Figure 5) (Platanias, 2005; Schmid et al., 2010; Weng et al., 2015).
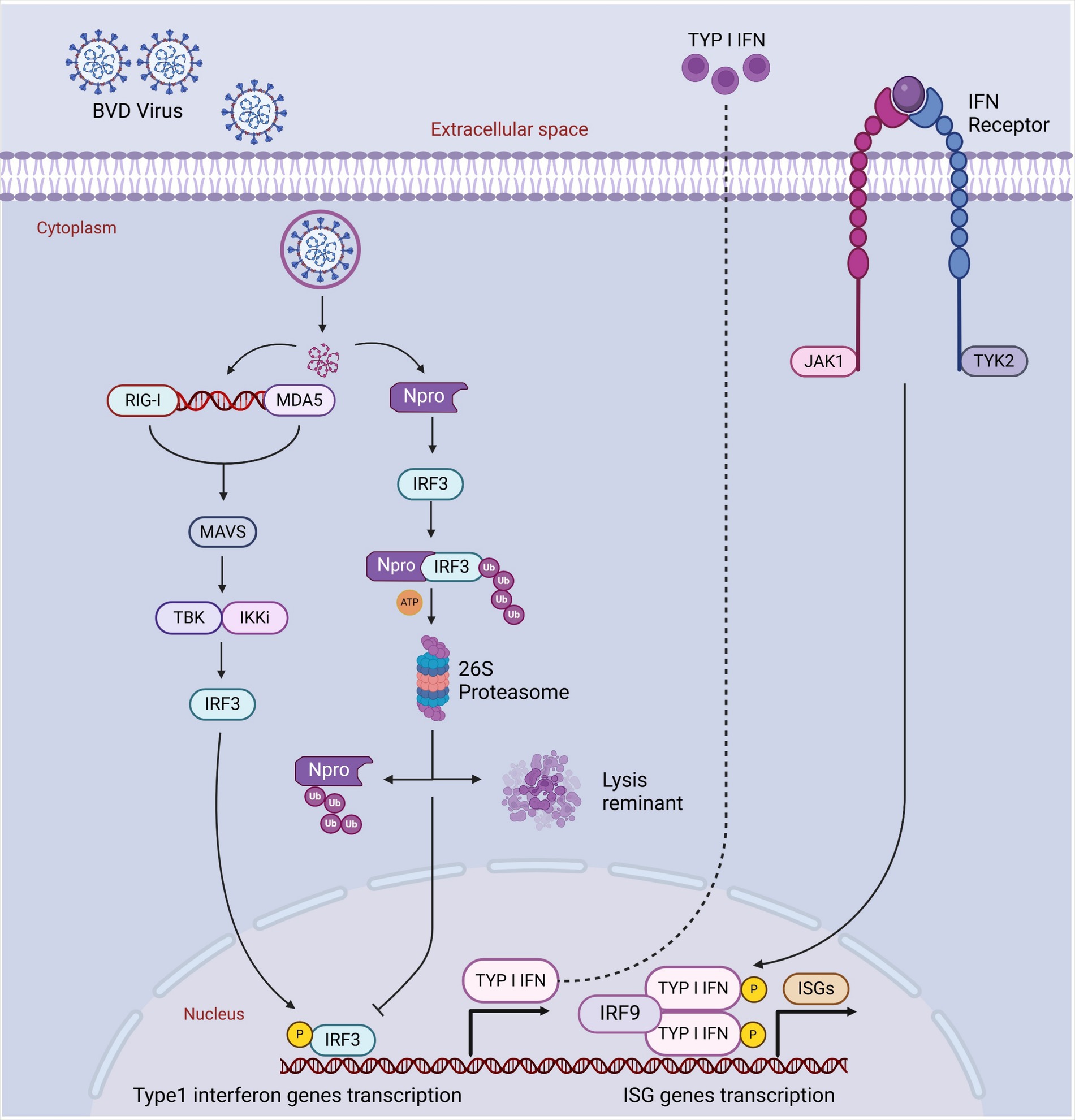
Figure 5. The blockage of host’s IFN activated immune response by Npro and IRF3 degradation. Cellular PRRs recognize PAMPs of viral infection. Such recognition thus triggers a sequence of cellular pathways that eventually lead to the translocation of phosphorylated IRF3 into the nucleus and commence the transcription of type I interferon genes through binding to IFN-α/β promoters. Npro might bind with IRF3 prior to its activation induced by phosphorylation, thereby targeting IRF-3 for ubiquitination and subsequent proteasomal degradation, resulting in a block in the type I interferon response. Created in BioRender. F. Kulyar, M. (2025) https://BioRender.com/j4sbip1.
Another study revealed that Erns of BVDV significantly inhibits lymphocyte protein synthesis but avoids damage in their cell membranes, indicating that BVDV could trigger apoptosis, leading to autophagy (Fu et al., 2014). Fu et al. also described that BVDV infection increased autolysosome, the autophagosome, and ATG14 and Beclin1 expression, suggesting BVDV significantly induced autophagy (Fu et al., 2014). Although autophagy is an important mechanism to minimize the virulence effects of viruses in the hosts, BVDV induces autophagy in host cells to promote its replication process and to suppress the host’s innate immune response (Yang et al., 2022). Further, BVDV also causes severe clinical diseases in wild animals along with cattle and other domestic animals. It may include malformations, mucosal disease, abortions, thrombocytopenia, and reproductive disorders. In acute BVDV infection, a severe inflammatory response has been initiated in the gastrointestinal tract (Li et al., 2019). Thus, immunosuppression, along with non-specified clinical symptoms in the BVDV-infected animals, is also endangering other healthy animals in Xinjiang (Diao et al., 2020; Su et al., 2022). Therefore, corresponding preventive plans are necessary to control BVDV infection dissemination in Xinjiang.
9 Preventive strategies in controlling the spread of BVDV across China
Preventive and control strategies for BVDV are critical in the cattle industry. Although vaccination is a significant measure in controlling BVDV infection, culling the persistently infected cattle could also be adopted. Compared to the European countries, China has not launched any BVDV eradication program (Yang et al., 2023). Currently, the BVDV vaccine developed from conventional cell lines belongs to BVDV1 and BVDV2 strains. Both inactivated and modified-live vaccines (MLV) are available for use against BVDV infection around the globe (Evans et al., 2019). However, the vaccine against BVDV3 (HoBi-like virus) is not commercially available yet, and this virus is also affecting buffaloes, goats, and sheep as well (Kalaiyarasu et al., 2023). BVDV vaccine is helpful in the prevention of developing persistently infected animals, protecting fetuses from infection, and preventing transient infections. Vaccination also boosts the growth rate and regulates the immune responses of the calves exposed to the persistently infected animals, indicating that BVDV-associated production/reproduction losses could be managed using a proper vaccination schedule (Grooms et al., 2014; Evans et al., 2019).
Controlled and coordinated eradication campaigns have been conducted successfully in the European countries. The campaigns aim primarily at the identification and elimination of persistently infected (PI) animals, which are the primary reservoir of BVDV maintenance and transmission in cattle herds (Lindberg et al., 2006). The Scandinavian countries like Norway, started a nationwide voluntary BVDV control program 1992, than to compulsory nation eradication scheme 2005, through serological screening and culling of PI animals as well as the movement restriction (Valle et al., 2005), as a result Norway declared as BVDV free country 2018 (Toplak et al., 2021). Sweden also achieved BVDV free status through similar strategies with a difference, they followed bulk milk antibody testing for identification (Ståhl et al., 2002).
As BVDV has the potential to spread across borders, bovine viral diarrhea (BVD) is enlisted as a notifiable infection in the Office International des Es (OIE) list. Every country has certain principles which are strictly followed to restrict those animal trading that has an unknown bovine viral diarrhea status (Marschik et al., 2018). Preventing the development of new persistently infected animals and reducing the prevalence of these animals in any cattle herd are the fundamental principles in controlling and eradicating BVD. It includes (a) testing (identifying) and culling (removing) of the persistently-infected animals, (b) improving biosecurity (reducing virus transmission in the population), and (c) vaccination (protecting the fetus from BVDV infection), which reduces the development of persistently-infected animals. Further, strictly controlling the semen quality and embryo transferring protocols could also control BVD at the farm level by following the closed-herd policy. Upon failing the closed-herd-policy, direct or indirect BVDV transmission routes are controlled. For this, purchasing persistently-infected animals and introducing dams containing persistently-infected fetuses are immediately stopped. Further, putting a double fence on the boundary wall, keeping recently purchased animals in quarantine, and cleaning the shared equipment/vehicles between the herds were strictly followed (Evans et al., 2019; Benavides et al., 2021; Schweizer et al., 2021). The dissemination of the BVDV virus shed from BVDV-infected animals is shown in Figure 6.
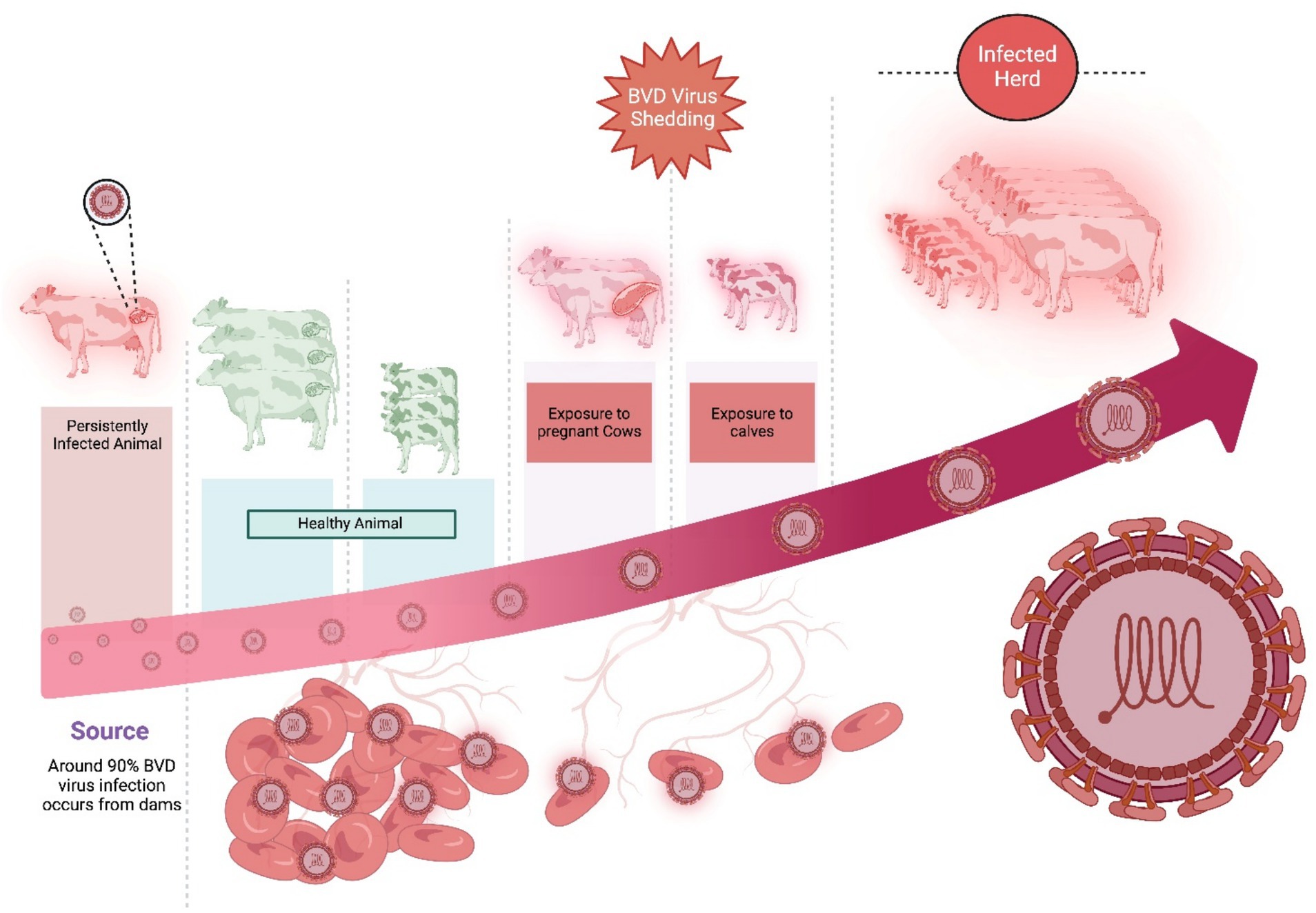
Figure 6. The dissemination of BVDV from persistently infected animals to other healthy animals and calves. Created in BioRender. F. Kulyar, M. (2025) https://BioRender.com/m5lufia.
The application of control strategies for BVDV in Xinjiang and rural China in general is hindered by a number of factors. Cattle production in Xinjiang is largely extensive or semi-nomadic in nature, and fencing and separation of the animals is not feasible. Furthermore, poor farmer education on viral spread, lack of accessibility of veterinary diagnostics, and uncontrolled inter-farm cattle movement significantly decrease the efficacy of such control measures. Focused education campaigns and government-funded infrastructural development are desperately necessary to enable the institution of these protocols in rural and pastoral communities. Moreover, inadequate veterinary extension services and limited knowledge regarding PI animal recognition prevents early detection and culling. Such systemic limitations lead to the failure of compliance with vaccination schedules, especially in remote counties like Hami and Manas. In the absence of increasing farmer education, access to diagnostic infrastructure, and affordability of vaccine delivery, regional control programs will remain inadequate despite apparent epidemiologic justification.
Unfortunately, non-systematic vaccine approaches have also been extensively applied and whether these approaches substantially reduce the infection rate is unknown. Limited data regarding such vaccination approaches indicates several issues in the recently available vaccines. For example, it is unknown whether fetal protection is 100% or not because the BVDV virus crosses the placenta, resulting in reproductive and fetal health issues. To protect the fetus from BVDV infection, either cell-mediated immunity or antibody neutralization is useful, but it is also not clear (Evans et al., 2019). Thus, further investigations are still required on how dam or fetus health could be protected from BVDV infection.
10 Conclusion
BVD is enlisted as a notifiable infection in the Office International des Epizootics (OIE) list because BVDV has the potential to spread across borders. BVDV infection has appeared as a serious threat to the cattle industry, particularly in Xinjiang, China. It infects cows, buffaloes, pigs, goats, yaks, and sheep and is contributing to the huge economic losses in this industry. BVD virus has dynamic genetic characteristics, and rapidly discovering new biotypes are due to cytopathic mutation in persistently infected animals, indicating the interconversion of non-cytopathic to cytopathic. This nature of BVDV increases the infection rate in Xinjiang compared with its prevalence in Beijing, Fujian, Shaanxi, and Shandong. The pattern of BVDV infection depends upon the infection risk, intensity/duration of circulation, testing/culling rate, arrival of new animals, and close contact with the neighboring herds. Thus, identifying and removing the persistently infected animals and improving biosecurity, i.e., reducing virus transmission in the population, could minimize the BVDV infection rate. Although vaccination is a useful tool to prevent the BVDV infection rate, applying non-systematic vaccine approaches should strictly be avoided. Further, the BVDV virus’s diffusive, confined, and directed motions facilitate its replication and transmission. Its active replication in the blood samples of BVDV-infected animals, even 85 days after infection, indicates virulence of BVDV persistently infects cattle herds, which increases morbidity and mortality rates. Additionally, immunosuppression and autophagy, along with non-specified clinical symptoms in the BVDV-infected animals, also endanger other healthy animals in Xinjiang.
Attention must be paid to the preventive measures of BVDV infection. Preventing the development of new persistently-infected animals, controlling the semen quality, maintaining the embryo transferring protocols, following closed-herd-policy, prohibiting the introduction of dams containing persistently infected fetuses, putting double-fence on the boundary wall, keeping recently purchased animals in quarantine, and cleaning the shared equipment/vehicles between the herds should be strictly followed. Xinjiang should be particularly focused because its major districts Kuitun, Shawan, Yili, Manas, Aksu, Hami, and Shihezi contains 4 million cattle, it borders with the Kazakhstan, Central Asian countries, and Russia, and most of the Holstein cattle in Xinjiang were often imported from Australia where BVDV1c is reported a predominant subtype. It is predicted that the above factors could increase the BVDV infection rate in the healthy herd of cattle in Xinjiang. Thus, the risk of BVDV infection could be minimized by decreasing the prevalence rate, understanding its epidemiology and pathogenesis, and using approved and verified BVDV vaccines in cattle.
Author contributions
JG: Conceptualization, Data curation, Writing – original draft. LK: Data curation, Methodology, Software, Writing – review & editing. JY: Methodology, Resources, Writing – review & editing. MZ: Data curation, Formal analysis, Software, Writing – review & editing. MK: Formal analysis, Software, Validation, Writing – review & editing. CH: Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Huyang Yingcai Project (Doctoral Talent Research Start-up Fund of Tarim University, No. TDZKBS202418), Xinjiang Corps Guiding Science and Technology Plan Project, No. 2024ZD084.
Acknowledgments
The authors are grateful to Aluna Schrift for their support regarding scholarly services.
Conflict of interest
JG, JY, and MZ were employed by Xinjiang Production and Construction Corps.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BVD, Bovine viral diarrhea; BVDV, Bovine viral diarrhea Virus; BVDV1, Bovine viral diarrhea Virus 1; BVDV2, Bovine viral diarrhea Virus 2; BVDV3, Bovine viral diarrhea Virus 3; OIE, Office International des Es; dsRNAs, Double-stranded RNAs; ORF, open-reading-frame; CD46, Cluster of differentiation 46; OIE, Office International des Epizootics.
Footnotes
References
Ahn, B. C., Walz, P., Kennedy, G. A., and Kapil, S. (2005). Biotype, genotype, and clinical presentation associated with bovine viral diarrhea virus (BVDV) isolates from cattle. J. Appl. Res. Vet. Med. 3:319–325.
Al-Kubati, A. A., Hussen, J., Kandeel, M., Al-Mubarak, A. I., and Hemida, M. G. (2021). Recent advances on the bovine viral diarrhea virus molecular pathogenesis, immune response, and vaccines development. Front. Vet. Sci. 8:665128. doi: 10.3389/fvets.2021.665128
Bauermann, F. V., Ridpath, J. F., Weiblen, R., and Flores, E. F. (2013). HoBi-like viruses: an emerging group of pestiviruses. J. Vet. Diagn. Invest. 25, 6–15. doi: 10.1177/1040638712473103
Benavides, B., Casal, J., Diéguez, J., Yus, E., Moya, S. J., and Allepuz, A. (2021). Quantitative risk assessment of introduction of BVDV and BoHV-1 through indirect contacts based on implemented biosecurity measures in dairy farms of Spain. Prev. Vet. Med. 188:105263. doi: 10.1016/j.prevetmed.2021.105263
Bitsch, V., Hansen, K.-E., and Rønsholt, L. (2000). Experiences from the Danish programme for eradication of bovine virus diarrhoea (BVD) 1994–1998 with special reference to legislation and causes of infection. Vet. Microbiol. 77, 137–143. doi: 10.1016/S0378-1135(00)00270-4
Chang, L., Qi, Y., Liu, D., Du, Q., Zhao, X., and Tong, D. (2021). Molecular detection and genotyping of bovine viral diarrhea virus in Western China. BMC Vet. Res. 17:66. doi: 10.1186/s12917-021-02747-7
Chi, S., Chen, S., Jia, W., He, Y., Ren, L., and Wang, X. (2022). Non-structural proteins of bovine viral diarrhea virus. Virus Genes 58, 491–500. doi: 10.1007/s11262-022-01914-8
Decaro, N. (2020). HoBi-like pestivirus and reproductive disorders. Front. Vet. Sci. 7:622447. doi: 10.3389/fvets.2020.622447
Deng, M., Chen, N., Guidarini, C., Xu, Z., Zhang, J., Cai, L., et al. (2020). Prevalence and genetic diversity of bovine viral diarrhea virus in dairy herds of China. Vet. Microbiol. 242:108565. doi: 10.1016/j.vetmic.2019.108565
Deng, M., Ji, S., Fei, W., Raza, S., He, C., Chen, Y., et al. (2015). Prevalence study and genetic typing of bovine viral diarrhea virus (BVDV) in four bovine species in China. PLoS One 10:e0121718. doi: 10.1371/journal.pone.0121718
Diao, N.-C., Gong, Q.-L., Li, J.-M., Zhao, D., Li, D., Zhao, B., et al. (2020). Prevalence of bovine viral diarrhea virus (BVDV) in yaks between 1987 and 2019 in mainland China: a systematic review and meta-analysis. Microb. Pathog. 144:104185. doi: 10.1016/j.micpath.2020.104185
Evans, C. A., Pinior, B., Larska, M., Graham, D., Schweizer, M., Guidarini, C., et al. (2019). Global knowledge gaps in the prevention and control of bovine viral diarrhoea (BVD) virus. Transbound. Emerg. Dis. 66, 640–652. doi: 10.1111/tbed.13068
Fu, Q., Shi, H., Shi, M., Meng, L., Bao, H., Zhang, G., et al. (2014). Roles of bovine viral diarrhea virus envelope glycoproteins in inducing autophagy in MDBK cells. Microb. Pathog. 76, 61–66. doi: 10.1016/j.micpath.2014.09.011
Fulton, R. W., Ridpath, J. F., Ore, S., Confer, A. W., Saliki, J., Burge, L. J., et al. (2005). Bovine viral diarrhoea virus (BVDV) subgenotypes in diagnostic laboratory accessions: distribution of BVDV1a, 1b, and 2a subgenotypes. Vet. Microbiol. 111, 35–40. doi: 10.1016/j.vetmic.2005.10.002
Fulton, R. W., Ridpath, J. F., Saliki, J. T., Briggs, R. E., Confer, A. W., Burge, L. J., et al. (2002). Bovine viral diarrhea virus (BVDV) 1b: predominant BVDV subtype in calves with respiratory disease. Can. J. Vet. Res. 66, 181–190
Gao, J., Liu, M., Meng, X., Han, Z., Zhang, D., Hou, B., et al. (2013). Seroprevalence of bovine viral diarrhea infection in yaks (Bos grunniens) on the Qinghai-Tibetan plateau of China. Trop. Anim. Health Prod. 45, 791–793. doi: 10.1007/s11250-012-0290-2
Graham, D., Clegg, T., Thulke, H.-H., O’sullivan, P., McGrath, G., and More, S. (2016). Quantifying the risk of spread of bovine viral diarrhoea virus (BVDV) between contiguous herds in Ireland. Prev. Vet. Med. 126, 30–38. doi: 10.1016/j.prevetmed.2016.01.017
Grooms, D. L., Brock, K. V., Bolin, S. R., Grotelueschen, D. M., and Cortese, V. S. (2014). Effect of constant exposure to cattle persistently infected with bovine viral diarrhea virus on morbidity and mortality rates and performance of feedlot cattle. J. Am. Vet. Med. Assoc. 244, 212–224. doi: 10.2460/javma.244.2.212
Kaiser, V., Nebel, L., Schüpbach-Regula, G., Zanoni, R. G., and Schweizer, M. (2016). Influence of border disease virus (BDV) on serological surveillance within the bovine virus diarrhea (BVD) eradication program in Switzerland. BMC Vet. Res. 13, 1–13. doi: 10.1186/s12917-016-0932-0
Kalaiyarasu, S., Mishra, N., Subramaniam, S., Moorthy, D., Sudhakar, S. B., Singh, V. P., et al. (2023). Whole-genome-sequence-based evolutionary analyses of HoBi-like pestiviruses reveal insights into their origin and evolutionary history. Viruses 15:733. doi: 10.3390/v15030733
Krey, T., Himmelreich, A., Heimann, M., Menge, C., Thiel, H. -Jr, Maurer, K., et al. (2006). Function of bovine CD46 as a cellular receptor for bovine viral diarrhea virus is determined by complement control protein 1. J. Virol. 80, 3912–3922. doi: 10.1128/JVI.80.8.3912-3922.2006
Lang, Y., Gao, S., Du, J., Shao, J., Cong, G., Lin, T., et al. (2014). Polymorphic genetic characterization of E2 gene of bovine viral diarrhea virus in China. Vet. Microbiol. 174, 554–559. doi: 10.1016/j.vetmic.2014.10.018
Li, N., Han, M., Huang, X., Bo, X., Wang, X., Zhao, Y., et al. (2009). Epidemiology investigation of bovine viral diarrhea virus in Shihezi, Xinjiang. J. Shihezi Univ. (Nat. Sci.). 27, 706–711.
Li, S., Hu, X., Tian, R., Guo, Y., Chen, J., Li, Z., et al. (2019). RNA-Seq-based transcriptomic profiling of primary interstitial cells of Cajal in response to bovine viral diarrhea virus infection. Vet. Res. Commun. 43, 143–153. doi: 10.1007/s11259-019-09754-y
Liebler-Tenorio, E. M., Ridpath, J. E., and Neill, J. D. (2002). Distribution of viral antigen and development of lesions after experimental infection with highly virulent bovine viral diarrhea virus type 2 in calves. Am. J. Vet. Res. 63, 1575–1584. doi: 10.2460/ajvr.2002.63.1575
Lindberg, A., Brownlie, J., Gunn, G., Houe, H., Moennig, V., Saatkamp, H., et al. (2006). The control of bovine viral diarrhoea virus in Europe: today and in the future. Rev. Sci. Tech. 25, 961–980.
Liu, Z., Zhang, Y., Zhao, D., Chen, Y., Meng, Q., Zhang, X., et al. (2023). Application of flow cytometry in the diagnosis of bovine epidemic disease. Viruses 15:1378. doi: 10.3390/v15061378
Lo, Y. T., Ryan, M. D., Luke, G. A., Chang, W. C., and Wu, H. C. (2023). Immunogenicity of a secreted, C-terminally truncated, form of bovine viral diarrhea virus E2 glycoprotein as a potential candidate in subunit vaccine development. Sci. Rep. 13:296. doi: 10.1038/s41598-022-26766-y
Ma, Y., Wang, L., Jiang, X., Yao, X., Huang, X., Zhou, K., et al. (2022). Integrative transcriptomics and proteomics analysis provide a deep insight into bovine viral diarrhea virus-host interactions during BVDV infection. Front. Immunol. 13:862828. doi: 10.3389/fimmu.2022.862828
Marschik, T., Obritzhauser, W., Wagner, P., Richter, V., Mayerhofer, M., Egger-Danner, C., et al. (2018). A cost-benefit analysis and the potential trade effects of the bovine viral diarrhoea eradication programme in Styria, Austria. Vet. J. 231, 19–29. doi: 10.1016/j.tvjl.2017.11.010
Manandhar, S., Yadav, G. P., and Singh, D. K. (2018). OIE bulletin news feed, and undefined 2018. n.d. ‘Epidemiological Survey of Bovine Viral Diarrhea in Dairy Cattle in Nepal’. Oiebulletin.Com. Available at: https://oiebulletin.com/wp-content/uploads/2018/08/OIE_NF-002-MANANDHAR.pdf (Accessed 2 July 2025).
Nardelli, S., Decaro, N., Belfanti, I., Lucente, M. S., Giammarioli, M., Mion, M., et al. (2021). Do modified live virus vaccines against bovine viral diarrhea induce fetal cross-protection against HoBi-like Pestivirus? Vet. Microbiol. 260:109178. doi: 10.1016/j.vetmic.2021.109178
Newcomer, B. W., Walz, P. H., Givens, M. D., and Wilson, A. E. (2015). Efficacy of bovine viral diarrhea virus vaccination to prevent reproductive disease: a meta-analysis. Theriogenology 83, 360–365.e1. doi: 10.1016/j.theriogenology.2014.09.028
Palomares, R. A., Marley, S. M., Givens, M. D., Gallardo, R. A., and Brock, K. V. (2013). Bovine viral diarrhea virus fetal persistent infection after immunization with a contaminated modified-live virus vaccine. Theriogenology 79, 1184–1195. doi: 10.1016/j.theriogenology.2013.02.017
Pang, F., Long, Q., and Wei, M. (2023). Immune evasion strategies of bovine viral diarrhea virus. Front. Cell. Infect. Microbiol. 13:1282526. doi: 10.3389/fcimb.2023.1282526
Peddireddi, L., Foster, K. A., Poulsen, E. G., An, B., Hoang, Q. H., O'Connell, C., et al. (2018). Molecular detection and characterization of transient bovine viral diarrhea virus (BVDV) infections in cattle commingled with ten BVDV persistently infected cattle. J. Vet. Diagn. Invest. 30, 413–422. doi: 10.1177/1040638717753962
Pedrera, M., Sánchez-Cordón, P. J., Romero-Trevejo, J. L., Risalde, M. A., Greiser-Wilke, I., Núñez, A., et al. (2009). Morphological changes and virus distribution in the ileum of colostrum-deprived calves inoculated with non-cytopathic bovine viral diarrhoea virus genotype-1. J. Comp. Pathol. 141, 52–62. doi: 10.1016/j.jcpa.2009.03.004
Pinior, B., Garcia, S., Minviel, J. J., and Raboisson, D. (2019). Epidemiological factors and mitigation measures influencing production losses in cattle due to bovine viral diarrhoea virus infection: a meta-analysis. Transbound. Emerg. Dis. 66, 2426–2439. doi: 10.1111/tbed.13300
Platanias, L. C. (2005). Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386. doi: 10.1038/nri1604
Qi, S., Wo, L., Sun, C., Zhang, J., Pang, Q., and Yin, X. (2022). Host cell receptors implicated in the cellular tropism of BVDV. Viruses 14:2302. doi: 10.3390/v14102302
Rajput, M. K. S., Abdelsalam, K., Darweesh, M. F., Braun, L. J., Kerkvliet, J., Hoppe, A. D., et al. (2017). Both cytopathic and non-cytopathic bovine viral diarrhea virus (BVDV) induced autophagy at a similar rate. Vet. Immunol. Immunopathol. 193–194, 1–9. doi: 10.1016/j.vetimm.2017.09.006
Ran, X., Chen, X., Ma, L., Wen, X., Zhai, J., Wang, M., et al. (2019). A systematic review and meta-analysis of the epidemiology of bovine viral diarrhea virus (BVDV) infection in dairy cattle in China. Acta Trop. 190, 296–303. doi: 10.1016/j.actatropica.2018.08.031
Rehwinkel, J., and Gack, M. U. (2020). RIG-I-like receptors: their regulation and roles in RNA sensing. Nat. Rev. Immunol. 20, 537–551. doi: 10.1038/s41577-020-0288-3
Ridpath, J. F., Neill, J. D., Vilcek, S., Dubovi, E. J., and Carman, S. (2006). Multiple outbreaks of severe acute BVDV in North America occurring between 1993 and 1995 linked to the same BVDV2 strain. Vet. Microbiol. 114, 196–204. doi: 10.1016/j.vetmic.2005.11.059
Riedel, C., Chen, H.-W., Reichart, U., Lamp, B., Laketa, V., and Rümenapf, T. (2020). Real time analysis of bovine viral diarrhea virus (BVDV) infection and its dependence on bovine CD46. Viruses 12:116. doi: 10.3390/v12010116
Royan, G. (2009). Comparison of the BVDV, BHV-1, and BRSV anamnestic response to modified-live or inactivated vaccines in calves previously vaccinated with a modified-live virus vaccine. Bov. Pract. 43, 44–50.
Santman-Berends, I., Mars, M., Van Duijn, L., Van den Broek, K., and Van Schaik, G. (2017). A quantitative risk-analysis for introduction of bovine viral diarrhoea virus in the Netherlands through cattle imports. Prev. Vet. Med. 146, 103–113. doi: 10.1016/j.prevetmed.2017.08.003
Schmid, S., Mordstein, M., Kochs, G., García-Sastre, A., and Tenoever, B. R. (2010). Transcription factor redundancy ensures induction of the antiviral state. J. Biol. Chem. 285, 42013–42022. doi: 10.1074/jbc.M110.165936
Schweizer, M., Stalder, H., Haslebacher, A., Grisiger, M., Schwermer, H., and Di Labio, E. (2021). Eradication of bovine viral diarrhoea (BVD) in cattle in Switzerland: lessons taught by the complex biology of the virus. Front. Vet Sci. 8:702730. doi: 10.3389/fvets.2021.702730
Seong, G., Oem, J.-K., and Choi, K.-S. (2013). Pathogenetic differences after experimental infection of calves with Korean non-cytopathic BVDV-1 and BVDV-2 isolates. Vet. Immunol. Immunopathol. 156, 147–152. doi: 10.1016/j.vetimm.2013.09.010
Shah, P. T., Nawal Bahoussi, A., Ahmad, A., Sikandar, M., and Xing, L. (2022). Bovine viral diarrhea virus in China: a comparative genomic and phylogenetic analysis with complete genome sequences. Front. Vet. Sci. 9:992678. doi: 10.3389/fvets.2022.992678
Ståhl, K., Rivera, H., Vågsholm, I., and Moreno-López, J. (2002). Bulk milk testing for antibody seroprevalences to BVDV and BHV-1 in a rural region of Peru. Prev. Vet. Med. 56, 193–202. doi: 10.1016/S0167-5877(02)00161-7
Stewart, J. L., Currin, J., Clark, S. G., Redifer, T., Givens, M. D., and Mercadante, V. R. G. (2023). Assessing pregnancy outcomes in cow-calf operations after administration of modified-live or killed virus vaccinations at the initiation of synchronization for fixed-time AI. Theriogenology 200, 43–48. doi: 10.1016/j.theriogenology.2023.01.027
Strong, R., La Rocca, S. A., Paton, D., Bensaude, E., Sandvik, T., Davis, L., et al. (2015). Viral dose and immunosuppression modulate the progression of acute BVDV-1 infection in calves: evidence of Long term persistence after intra-nasal infection. PLoS One 10:e0124689. doi: 10.1371/journal.pone.0124689
Su, N., Wang, Q., Liu, H. Y., Li, L. M., Tian, T., Yin, J. Y., et al. (2022). Prevalence of bovine viral diarrhea virus in cattle between 2010 and 2021: a global systematic review and meta-analysis. Front. Vet. Sci. 9:1086180. doi: 10.3389/fvets.2022.1086180
Tao, J., Wang, Y., Wang, J., Wang, J.-y., and Zhu, G.-q. (2013). Identification and genetic characterization of new bovine viral diarrhea virus genotype 2 strains in pigs isolated in China. Virus Genes 46, 81–87. doi: 10.1007/s11262-012-0837-3
Tautz, N., Tews, B. A., and Meyers, G. (2015). The molecular biology of pestiviruses. Adv. Virus Res. 93, 47–160. doi: 10.1016/bs.aivir.2015.03.002
Toplak, I., Hostnik, P., Černe, D., Mrkun, J., and Starič, J. (2021). The principles of the voluntary programme for the control and elimination of bovine viral diarrhoea virus (BVDV) from infected herds in Slovenia. Front. Vet. Sci. 8:676473. doi: 10.3389/fvets.2021.676473
Valle, P. S., Skjerve, E., Martin, S. W., Larssen, R. B., Østerås, O., and Nyberg, O. (2005). Ten years of bovine virus diarrhoea virus (BVDV) control in Norway: a cost-benefit analysis. Prev. Vet. Med. 72, 189–207. doi: 10.1016/j.prevetmed.2005.07.017
Walz, P. H., Givens, M. D., Rodning, S. P., Riddell, K. P., Brodersen, B. W., Scruggs, D., et al. (2017). Evaluation of reproductive protection against bovine viral diarrhea virus and bovine herpesvirus-1 afforded by annual revaccination with modified-live viral or combination modified-live/killed viral vaccines after primary vaccination with modified-live viral vaccine. Vaccine 35, 1046–1054. doi: 10.1016/j.vaccine.2017.01.006
Wang, L., Liang, X., Ji, X., Shi, Q., and Rang, D. (2015). Bovine viral diarrhea virus isolation identification and molecular epidemiology investigation in some areas in Xinjiang. Xinjiang Agric. Sci. 52, 334–338.
Wang, Z., Liu, M., Zhao, H., Wang, P., Ma, W., Zhang, Y., et al. (2021). Induction of robust and specific humoral and cellular immune responses by bovine viral diarrhea virus virus-like particles (BVDV-VLPs) engineered with baculovirus expression vector system. Vaccine 9:350. doi: 10.3390/vaccines9040350
Wang, S., Wei, R., Ma, X., Guo, J., Aizaz, M., Li, F., et al. (2024). The host protein CALCOCO2 interacts with bovine viral diarrhoea virus Npro, inhibiting type I interferon production and thereby promoting viral replication. Virulence 15:2289764. doi: 10.1080/21505594.2023.2289764
Wang, L., Wu, X., Wang, C., Song, C., Bao, J., and Du, J. (2020). Origin and transmission of bovine viral diarrhea virus type 1 in China revealed by phylodynamic analysis. Res. Vet. Sci. 128, 162–169. doi: 10.1016/j.rvsc.2019.11.015
Weng, X. G., Song, Q. J., Wu, Q., Liu, M. C., Wang, M. L., and Wang, J. F. (2015). Genetic characterization of bovine viral diarrhea virus strains in Beijing, China and innate immune responses of peripheral blood mononuclear cells in persistently infected dairy cattle. J. Vet. Sci. 16, 491–500. doi: 10.4142/jvs.2015.16.4.491
Wu, Y., Zhang, G., Jiang, H., Xin, T., Jia, L., Zhang, Y., et al. (2023). Molecular characteristics of bovine viral diarrhea virus strains isolated from persistently infected cattle. Vet. Sci. 10:413. doi: 10.3390/vetsci10070413
Xiao, Y., Liu, Y., Chi, T., Jiang, W., He, T., Xu, L., et al. (2025). Prevalence and genetic characterization of bovine viral diarrhea virus in dairy cattle in northern China. BMC Vet. Res. 21:250. doi: 10.1186/s12917-025-04491-8
Xue, W., Mattick, D., and Smith, L. (2011). Protection from persistent infection with a bovine viral diarrhea virus (BVDV) type 1b strain by a modified-live vaccine containing BVDV types 1a and 2, infectious bovine rhinotracheitis virus, parainfluenza 3 virus and bovine respiratory syncytial virus. Vaccine 29, 4657–4662. doi: 10.1016/j.vaccine.2011.04.129
Yang, N., Hu, N., Zhang, J., Yi, J., Wang, Z., Wang, Y., et al. (2022). Bta-miR-2904 inhibits bovine viral diarrhea virus replication by targeting viral-infection-induced autophagy via ATG13. Arch. Virol. 168:11. doi: 10.1007/s00705-022-05630-4
Yang, N., Xu, M., Ma, Z., Li, H., Song, S., Gu, X., et al. (2023). Detection of emerging HoBi-like Pestivirus (BVD-3) during an epidemiological investigation of bovine viral diarrhea virus in Xinjiang: a first-of-its-kind report. Front. Microbiol. 14:1222292. doi: 10.3389/fmicb.2023.1222292
Yang, D.-S., Yin, H., Jin, Y.-H., Guan, Y.-F., Liu, M.-R., and Ye, Y.-J. (2007). Serological investigation of bovine viral diarrhea in Fujian Province in 2006. Prog. Vet. Med. 9:012.
Yanhua, H., Xusheng, M., Huang, X., Sheng, J., Zhong, F., Xinxia, Z., et al. (2020). Detection of BVDV 1q in China: genetic characterization and experimental infection for the investigation of it. Kafkas Univ. Vet. Fak. Derg. 26, 377–384. doi: 10.9775/kvfd.2019.23273
Zhang, K., Zhang, J., Qiu, Z., Zhang, K., Liang, F., Zhou, Q., et al. (2022). Prevalence characteristic of BVDV in some large scale dairy farms in Western China. Front. Vet. Sci. 9:961337. doi: 10.3389/fvets.2022.961337
Zhong, F., Li, N., Huang, X., Guo, Y., Chen, H., Wang, X., et al. (2011). Genetic typing and epidemiologic observation of bovine viral diarrhea virus in Western China. Virus Genes 42, 204–207. doi: 10.1007/s11262-010-0558-4
Keywords: bovine viral diarrhea, bovine viral diarrhea virus, prevalence, epidemiology, pathogenesis, Xinjiang
Citation: Gao J, Kuang L, Yin J, Zhang M, Kulyar MF and Hu C (2025) Molecular epidemiology and immunopathogenesis of bovine viral diarrhea virus: a growing threat to regional cattle industry of Xinjiang, China. Front. Microbiol. 16:1617998. doi: 10.3389/fmicb.2025.1617998
Edited by:
Shengwei Ji, Obihiro University of Agriculture and Veterinary Medicine, JapanReviewed by:
Aruna Pal, West Bengal University of Animal and Fishery Sciences, IndiaOmer Baris Ince, Necmettin Erbakan University, Türkiye
Copyright © 2025 Gao, Kuang, Yin, Zhang, Kulyar and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jindong Gao, SmluZG9uZy1HYW9AdGFydS5lZHUuY24=; Changmin Hu, aGNtQG1haWwuaHphdS5lZHUuY24=
 Jindong Gao1,2*
Jindong Gao1,2* Md. F. Kulyar
Md. F. Kulyar Changmin Hu
Changmin Hu