- 1Medicine Department, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
- 2Medicine Department, Al Faisal University, Riyadh, Saudi Arabia
- 3Research-Jeddah Department, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
- 4Organ Transplant Center of Excellence, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- 5Pathology and Laboratory Medicine Department, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
- 6King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
- 7Medicine Department, King Abdulaziz Medical City, Riyadh, Saudi Arabia
- 8Medicine Department, International Medical Center, Jeddah, Saudi Arabia
Introduction: Solid organ transplant (SOT) recipients, bone marrow transplant (BMT) recipients, and patients with hematological malignancies experience increased morbidity due to infections caused by multidrug-resistant, Carbapenem-resistant Enterobacterales (CRE). The current study aimed to further describe the epidemiology and outcomes associated with CRE infection in high-risk SOT and BMT recipients and in patients with hematological malignancies in Saudi Arabia.
Methods: Patients aged 16 years and older admitted to a participating hospital between October 2018 and August 2024 who received an SOT or a BMT or were diagnosed with a hematological malignancy and had a confirmed CRE infection were included in this retrospective cohort study. A total of 155 eligible patients were included in the study population. The primary outcome of interest was all-cause mortality within 90 days of the date of the first CRE culture.
Results: Among the 155 patients, 118 (76.1%) had received a solid organ transplant, while 37 (23.9%) had either a bone marrow transplant or a hematological malignancy. The BMT recipients and patients with hematological malignancies were 2.84 times more likely to die within 90 days of their first positive culture [adjusted odds ratio (AOR) = 2.84, 95% confidence interval (CI) = 1.01–8.01, p = 0.049]. Compared to the patients with CRE infections carrying the blaNDM gene, after controlling for other predictors, patients with infections harboring the blaOXA-48 gene were 3.97 times more likely to die within 90 days of the first culture (AOR = 3.97, 95%CI = 1.04–15.15, p = 0.044).
Conclusion: The BMT recipients and patients with CRE infections harboring the blaOXA-48 gene were at greater risk for 90-day all-cause mortality in Saudi Arabia, confirming previous findings of high mortality rates associated with CRE infections in immunocompromised populations.
Introduction
Bloodstream infections and other frequently encountered infections in clinical practice, such as pneumonia and complicated urinary tract infections (UTIs), caused by Carbapenem-resistant Enterobacterales (CRE) are associated with increased morbidity and mortality (Wright et al., 2021). More recently, global dissemination of CRE has occurred at an alarmingly rapid pace (Logan and Weinstein, 2017). Internationally, the focus has shifted toward early detection and infection control (Logan and Weinstein, 2017; Patel and Bonomo, 2013). CRE have become endemic in some regions of Europe, Asia, South America, and Africa, with high-risk patients, such as solid organ transplant (SOT) recipients, bone marrow transplant (BMT) recipients, and patients with hematological malignancies, being particularly vulnerable to infection due to frequent therapy with antibiotics and prolonged hospital stays (Pouch and Satlin, 2017; Nordmann et al., 2011).
Solid organ transplant recipients, BMT recipients, and patients with hematological malignancies have experienced increasing morbidity due to infections caused by multidrug-resistant CRE (Kerneis and Lucet, 2019; Patel et al., 2008). Approximately 1.8–2.0% of BMT recipients experienced incident bloodstream infections caused by CRE, and the 30-day mortality rates ranged from 52 to 63% following bacteremia in patients with hematological malignancies (Pouch and Satlin, 2017; Yang et al., 2020). Approximately one-quarter (range of 16–24%) of all patients with CRE bacteremia are patients with hematological malignancies (Pouch and Satlin, 2017).
The incidence of CRE infection among SOT recipients ranges from 3.0 to 23%, and overall mortality rates associated with CRE infection range from 24 to 70% (Freire et al., 2019; Aguado et al., 2018; Gutiérrez-Gutiérrez et al., 2017; Tzouvelekis et al., 2012). Although a recent matched cohort study of patients with CRE found that SOT patients with CRE did not have worse outcomes than non-transplant patients with CRE, 90-day mortality remained substantial among the SOT patients. The study concluded that reducing hospital stay to prevent CRE infection should be a goal for transplant programs (Boutzoukas et al., 2025). Overall, data on patients with CRE infections and immunosuppression remain limited.
A previous study conducted in Saudi Arabia on hospitalized patients showed that OXA-48 and NDM carbapenemases are the most common enzymes found in CRE infections caused by Klebsiella pneumoniae (Alraddadi et al., 2022). Both this multicenter prospective study and a more recent retrospective cohort study conducted in the region provided specific details regarding CRE colonization and infection in high-risk patients (Alraddadi et al., 2022; Alraddadi et al., 2024). The current study aimed to further describe the epidemiology and outcomes associated with CRE infection in high-risk SOT patients, BMT recipients, and patients with hematological malignancies in Saudi Arabia.
Materials and methods
Study design and patient population
Patient data were collected from King Faisal Specialist Hospital & Research Center (KFSH&RC) in Riyadh (n = 73), KFSH&RC in Jeddah (n = 38), and King Abdullah International Medical Research Center (KAIMRC) in Riyadh (n = 44) between October 2018 and August 2024 for this retrospective cohort study. These tertiary care medical centers were included because they are leading centers in Saudi Arabia in terms of both quality of care and the number of SOT and BMT cases. Adult patients aged 16 years and older admitted to the hospital who received a solid organ transplant or a bone marrow transplant or were diagnosed with a hematological malignancy and had a confirmed CRE infection were included, resulting in a total study population of 155 patients. The primary outcome of interest was all-cause mortality within 90 days of the date of the first CRE culture.
Microbiological procedures
After obtaining the culture, the Vitek 2 system (bioMérieux, Marcy L’étoile, France) was used to perform bacterial identification and susceptibility testing as phenotypic methods for detecting CRE, following the methodology from the Clinical and Laboratory Standards Institute (M-100, 33rd Edition). The Cepheid Xpert Carba-R assay (Cepheid, Sunnyvale, CA, USA) is an automated in vitro diagnostic test for the qualitative detection of the presence of blaKPC, blaNDM, blaVIM, blaOXA-48, and blaIMP gene sequences. It differentiates these carbapenem-resistance–associated sequences in Gram-negative bacteria and provides a non-detected result for other sequences (Bianco et al., 2022). The Xpert Carba-R assay was used to test confirmed isolates from the culture, following recommended procedures to detect and differentiate blaKPC, blaNDM, blaVIM, blaOXA-48, and blaIMP genes.
Data collection and statistical analysis
Information about demographic and clinical characteristics and patient outcomes was abstracted from medical records at the three hospitals. Data were collected for the following variables: date of birth, sex, comorbid conditions, Charlson Comorbidity Index, APACHE II score, Pitt bacteremia score, 30-day history of intensive care unit (ICU) stay and procedures, prior CRE colonization and infection status within 90 days, isolated organism, susceptibility to antibiotics, type of CRE infection, and antibiotic treatment course. These variables were considered potential predictors of 90-day all-cause mortality. In addition to mortality, data were collected on relapse within 30 days, microbiological eradication (defined as documentation of subsequent cultures showing infection clearance, confirmed by negative results), CRE-attributable mortality, acute kidney injury, and clinical cure. CRE-attributable mortality was defined as in-hospital death clearly linked to a CRE infection, with no other obvious cause identified.
Continuous predictor variables were assessed for normality by examining the ratio of mean to median values, evaluating skewness and kurtosis, applying the Kolmogorov–Smirnov test, and by observing the data graphically. Mean and standard deviation (SD) were reported for normally distributed variables, while median and interquartile range (IQR) were reported for non-normally distributed variables. Frequencies were generated for categorical variables. Associations between predictor variables and 90-day all-cause mortality were assessed using Student’s t-test for normally distributed data, the Wilcoxon two-sample test for non-parametrically distributed continuous variables, and the chi-squared and Fisher’s exact test for categorical data. All predictor variables found to be significantly associated with mortality were included in a multivariable logistic model, along with age, to predict 90-day all-cause mortality. A separate model was also created to examine these associations in the patients with bacteremia (n = 72). The analyses were performed using SAS Studio, with the significance level set at α = 0.05. The study was approved by the Institutional Review Board of the KFSH&RC in Jeddah.
Results
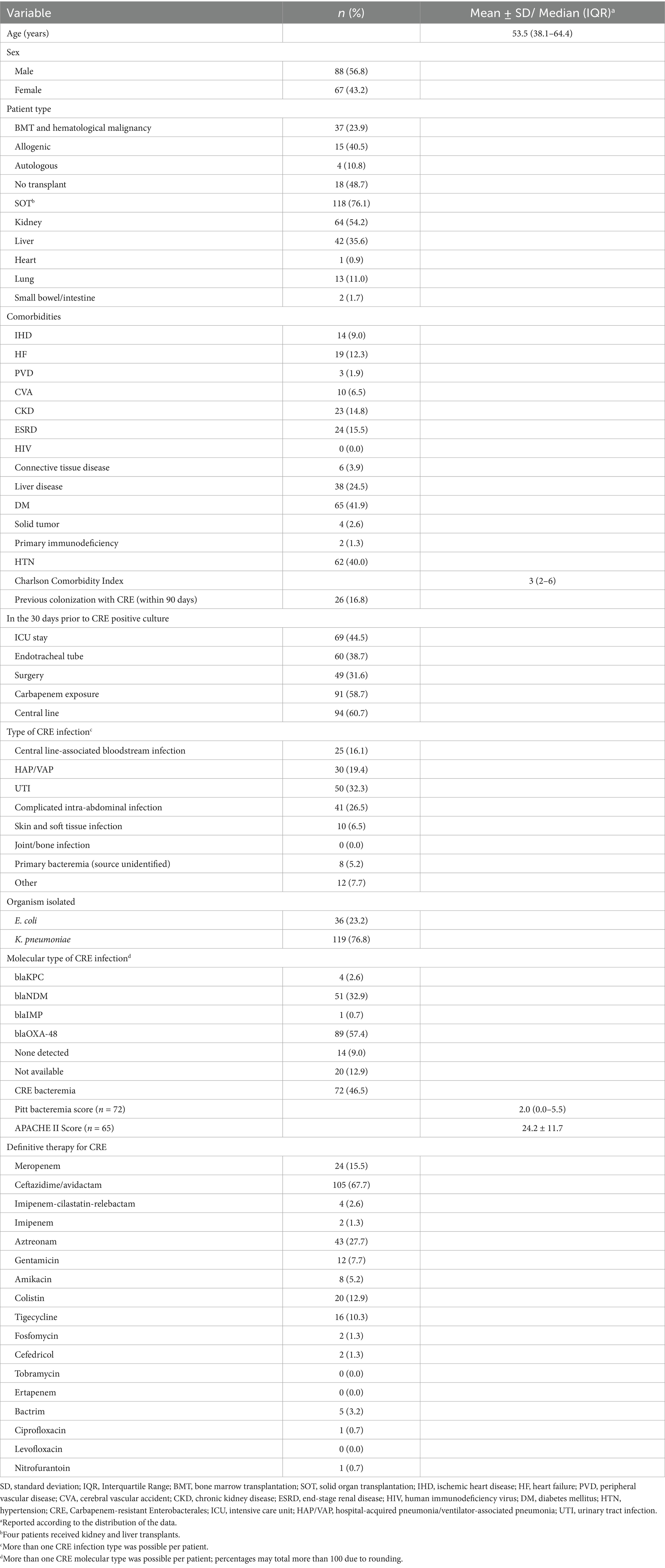
The study population for this retrospective study comprised 155 patients with CRE infections, of whom 118 (76.1%) had received a solid organ transplant (SOT) and 37 (23.9%) had a bone marrow transplant (BMT) or had a hematological malignancy (Table 1). There were 64 (54.2%) patients who received kidney transplants, 42 (35.6%) patients who received liver transplants, one (0.9%) patient who received a heart transplant, 13 (11.0%) patients who received a lung transplant, and two (1.7%) patients who received a small bowel/intestine transplant. In addition, four patients received both kidney and liver transplants. The median [interquartile range (IQR)] age was 53.5 (38.1–64.4) years, and 56.8% of the population was male. The most common comorbidities among the patients were diabetes mellitus (41.9%) and hypertension (40.0%) (Table 1).
While 16.8% of the patients had documented CRE colonization within the last 90 days, almost one-third of the patients (32.3%) had a UTI (Table 1). The median (IQR) Charlson Comorbidity Index was 3 (2–6). Among the 72 (46.5%) patients with CRE bacteremia and a Pitt bacteremia score, the median score was 2.0 (0.0–5.5). More than three-quarters of the isolated organisms (76.8%) were K. pneumoniae, and the blaOXA-48 (57.4%) gene was the most common molecular type, followed by blaNDM (32.9%).
Following the hospital protocol for treatment, definitive therapy for CRE infection included ceftazidime/avibactam for 67.7% of the patients, while 27.7% received aztreonam. The majority of the patients with the blaOXA-48 gene received ceftazidime/avibactam, while those with the blaNDM gene were treated with a combination of ceftazidime/avibactam and aztreonam (Table 1). Among the 61 patients with available sensitivity data for ceftazidime/avibactam and the blaOXA-48 molecular type, 17 (27.9%) showed resistance to this treatment regimen, while 44 patients (72.1%) had susceptible isolates. However, when analyzing the subgroup of 43 patients with only the blaOXA-48 gene, only two patients (4.7%) demonstrated resistance to ceftazidime/avibactam. The median (IQR) MIC value for ceftazidime/avibactam for patients with NDM and patients with OXA-48 was 16 (1–16).
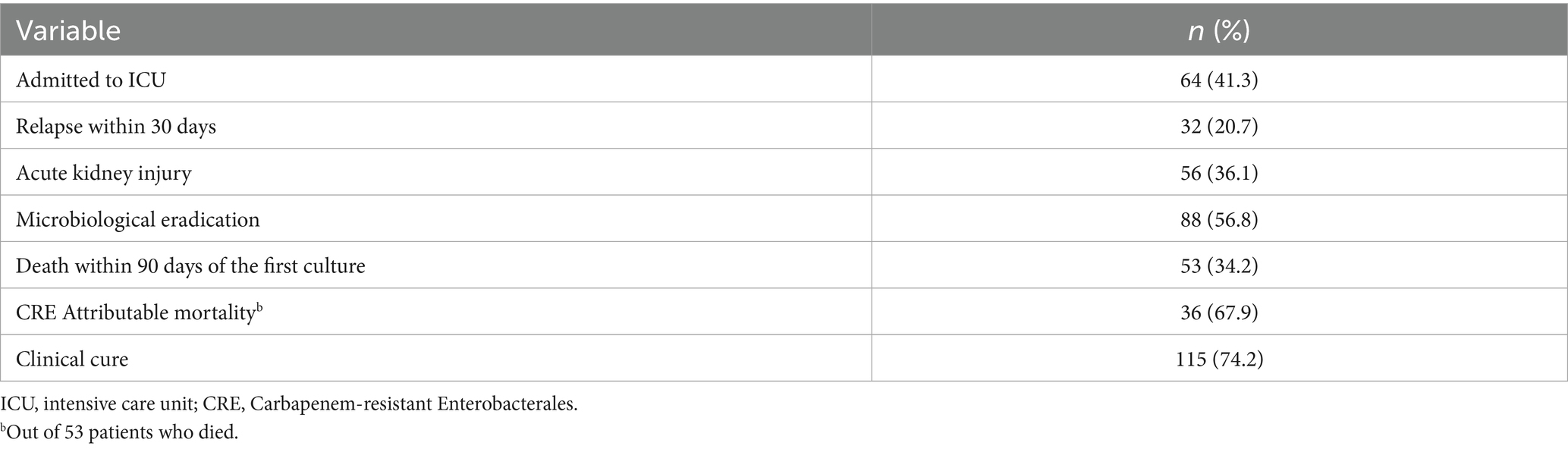
There were 64 patients (41.3%) admitted to the intensive care unit (ICU) during their hospital stay, and over one-third of all patients (36.1%) experienced acute kidney injury (Table 2). Just over one-third (34.2%) of patients died within 90 days of their first CRE culture, with 67.9% of these deaths being attributable to CRE (Table 2). Attributable mortality was defined as death directly linked to CRE infection, including cases with no other clear cause of death or cases where the patient had persistent infection without clinical improvement until death.
The association between patient type and mortality was significant in this patient population, where 34.0% of the patients who died were BMT recipients compared to 18.6% of survivors who were BMT recipients (p = 0.03) (Table 3). Among the patients who died, 35.9% had hospital-acquired pneumonia/ventilator-associated pneumonia (HAP/VAP), compared to 10.8% of the patients who survived with this infection (p < 0.001). The association between the type of carbapenemase found in the CRE isolates from the patients’ infections and mortality was also significant. Among the deaths, 75.5% (n = 53) occurred in the patients with isolates harboring blaNDM, blaOXA-48, or both blaNDM and blaOXA-48. While 62.3% of the patients who died developed CRE bacteremia, 38.2% of survivors were bacteremic (p = 0.003) (Table 3).
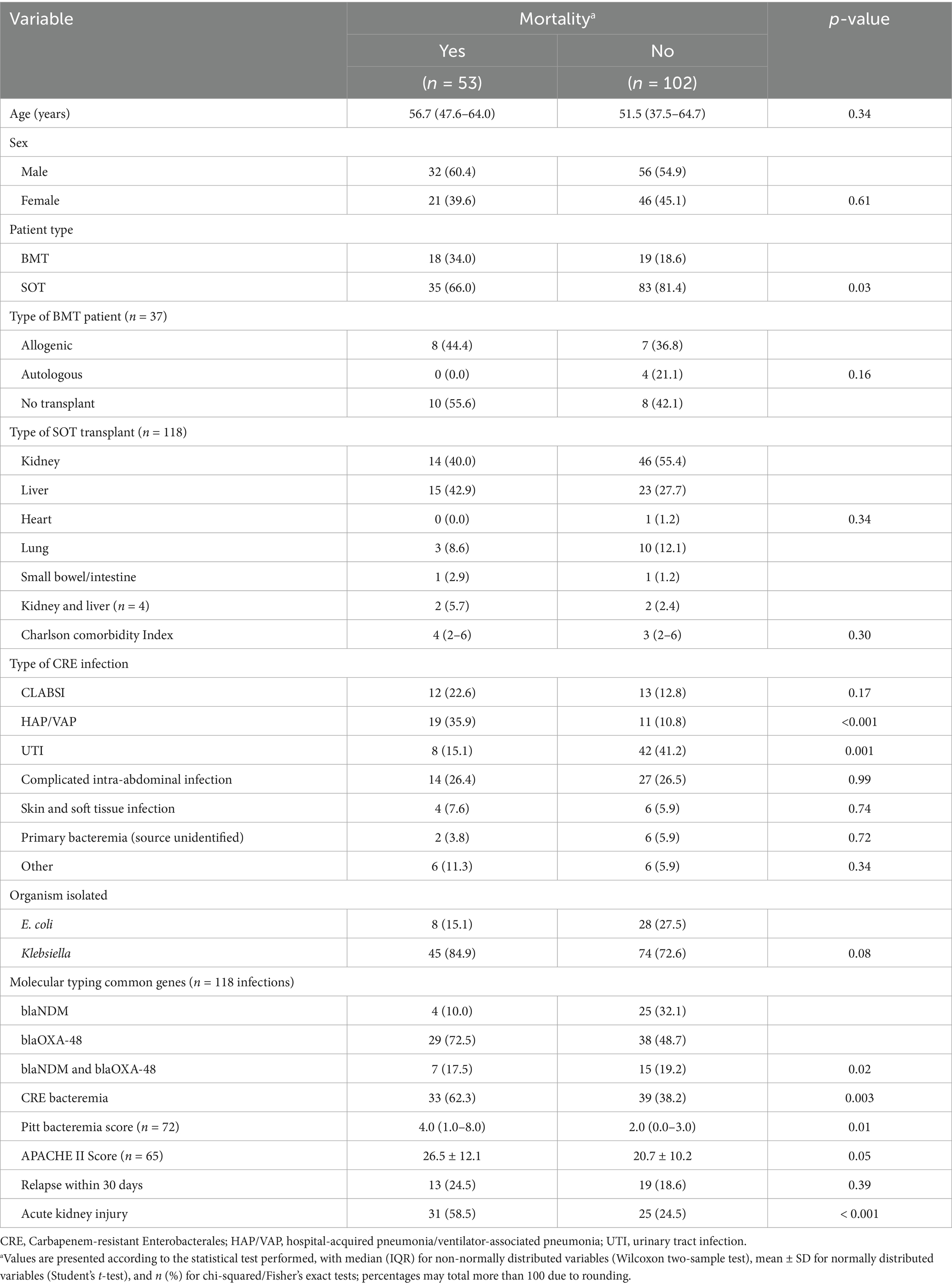
Table 3. Univariate analyses of the associations between demographic/clinical variables and mortality.
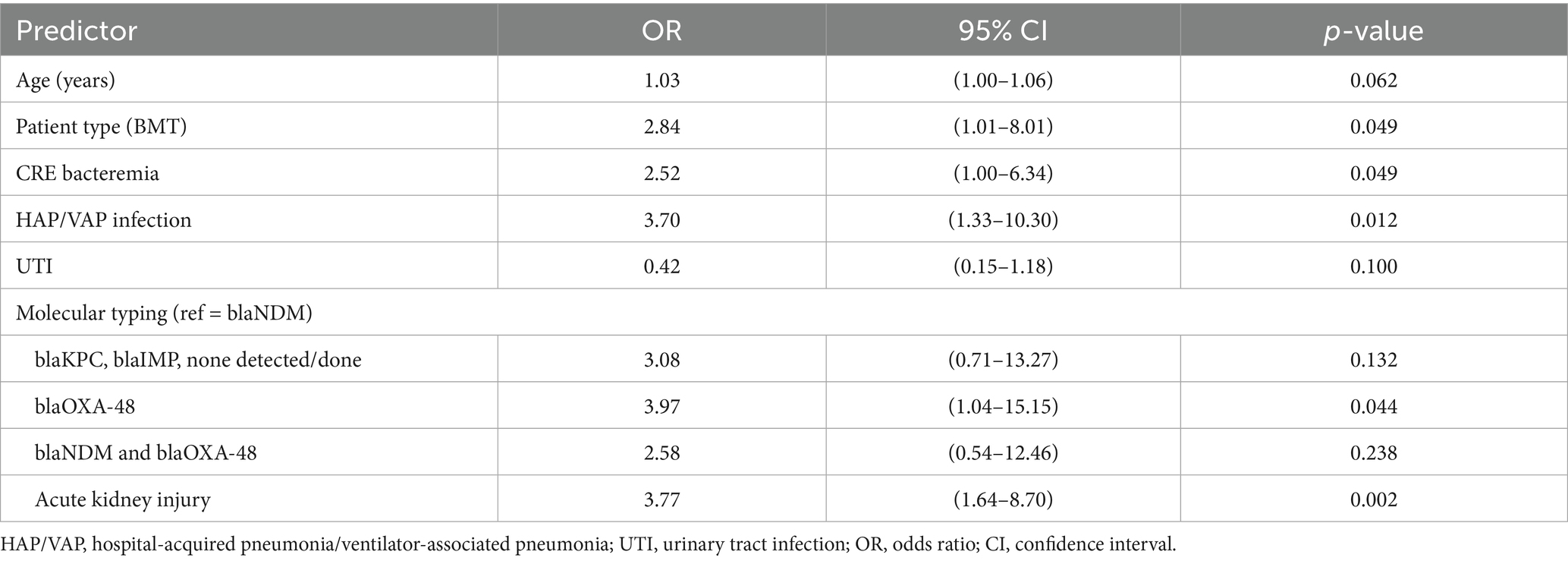
Predicting mortality using a multivariable logistic regression model indicated that the BMT recipients and patients with hematological malignancies were 2.84 times more likely to die within 90 days of their first positive culture compared to the SOT recipients [adjusted odds ratio (AOR) = 2.84, 95% confidence interval (CI) = 1.01–8.01, p = 0.049] (Table 4). The participants who developed HAP/VAP were also significantly more likely to experience 90-day all-cause mortality (AOR = 3.70, 95% CI = 1.33–10.30, p = 0.012). Compared to the patients with the blaNDM gene, the patients with an infection harboring the blaOXA-48 gene were 3.97 times more likely to die within 90 days of the first culture (AOR = 3.97, 95%CI = 1.04–15.15, p = 0.044). Having CRE bacteremia and acute kidney injury were also associated with an increased likelihood of experiencing 90-day all-cause mortality (Table 4). A multivariable model analyzing the patients with CRE bacteremia (n = 72) revealed that the BMT recipients (n = 29 of the 72, 40.3%) had an increased likelihood of experiencing 90-day mortality compared to the SOT recipients (AOR = 3.71, 95% CI = 1.05–13.14, p = 0.042). In addition, for every one-unit increase in the Pitt bacteremia score, the patients were 1.32 times more likely to die within 90 days of their first positive culture (p = 0.005) (Table 5).
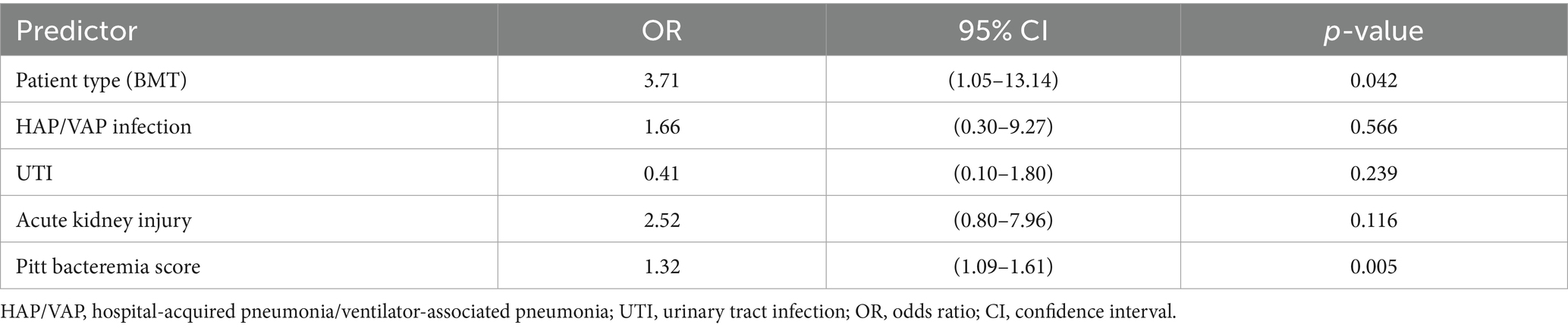
Table 5. Multivariable analysis predicting mortality by predictor variables in the bacteremic patients (n = 72).
Discussion
This study reveals that mortality is alarmingly high among immunosuppressed SOT patients and BMT recipients, with more than one-third of the population dying within 90 days of their first positive culture and just over two-thirds of these deaths being attributable to CRE infection. Furthermore, BMT recipients, patients with hematological malignancies, and patients infected with CRE carrying the blaOXA-48 gene are at increased risk of 90-day mortality. Given the high prevalence of blaOXA-48 CRE infections in the region, this is particularly concerning due to the heightened risk these high-risk patients face of developing infections, compounded by recurrent antibiotic use and prolonged hospital stays. Despite the availability of effective antibiotics for CRE, it is alarming that mortality in this patient population remains high. This is likely due to delays in initiating the appropriate antibiotic therapy or the development of resistance. These factors highlight the urgent need for rapid diagnostic tools with quick turnaround times to detect CRE and the current unmet needs regarding the limited treatment options for CRE.
Due to sample size considerations, the SOT patients and the BMT recipients were combined in the analyses. Overall, 29.7% of the SOT patients died compared to 48.6% of the BMT recipients, which was not unexpected considering the severity of illness among many BMT recipients. While more than three-quarters of all patients had a K. pneumoniae infection, 37.8% of these patients died, compared to 22.2% of the patients with an E. coli infection. Running the multivariable model with only the SOT patients revealed similar findings, except that the association with 90-day mortality was no longer significant for the patients with the blaOXA-48 isolate or for the bacteremic patients. When the lung, heart, and small bowel SOT patients (n = 16) were removed from the SOT model, the results were similar to the original model, except that the association between blaOXA-48 isolates and mortality was no longer significant.
A matched case–control study compared patients with carbapenem-resistant K. pneumoniae to patients with carbapenem-susceptible K. pneumoniae infections as controls (Patel et al., 2008). Carbapenem-resistant K. pneumoniae infection was found to be associated with recent organ or stem-cell transplantation, and the cases were more likely than the controls to die during hospitalization and from the infection (Patel et al., 2008). SOT patients have increased susceptibility to infections related to long-term immunosuppression. Although there are specific guidelines, it is still challenging to determine how this condition can be managed in recipients with CRE infections (Pérez-Nadales et al., 2022).
Carbapenem resistance has been shown to be a threat to allograft and patient survival, with limited antibiotics available for treatment (Patel et al., 2010). Among SOT recipients in CRE-endemic areas, 3.0–10% develop infections correlated with the transplanted organ (Satlin et al., 2014). Active surveillance is needed to monitor and prevent infection in immunocompromised patients, especially considering that empirical therapy is not active against CRE and its identification can take 2–4 days (Satlin et al., 2014; Blaschke et al., 2012). In addition, several studies have revealed high in-hospital mortality rates for CRE bacteremia among greatly immunocompromised BMT recipients and patients with hematological malignancies, with the majority of deaths being attributable to the infection (Satlin et al., 2014; Satlin et al., 2013; Muchtar et al., 2012; Snitkin et al., 2012). Analyses from a study indicated that considering genomic and epidemiological data simultaneously may assist in controlling CRE hospital-acquired infections (Snitkin et al., 2012). More information is needed regarding factors that may be specific to immunocompromised patients to facilitate CRE prevention in hospitals (Satlin et al., 2014).
The results from our study revealed that a number of factors were independently associated with 90-day all-cause mortality, including the type of patient (BMT compared to SOT), bacteremia, hospital-acquired pneumonia/ventilator-associated pneumonia (HAP/VAP), CRE infection carrying blaOXA-48, and acute kidney injury. A multivariable model for the bacteremic patients (n = 72) indicated that being a BMT patient (reference SOT) and a higher Pitt bacteremia score were significant predictors of mortality.
A recent study with some similar findings reported that 30-day mortality of CRE infection was independently associated with higher Simplified Acute Physiology Score II, sepsis at the time of diagnosis, pneumonia, monotherapy, and inappropriate empiric antibiotic therapy (So-Ngern et al., 2023). Additional studies have shown that factors associated with 14-, 28-, and 30-day mortality, infection-related mortality, and overall hospital mortality in patients with CRE infections include chronic renal failure, high APACHE II score, presentation with septic shock and severe sepsis, neutropenia, dialysis, age over 60 years, ultimately fatal disease, and rapidly fatal underlying diseases (Li et al., 2019; Tumbarello et al., 2015; de Maio Carrilho et al., 2016; Daikos et al., 2014). In our study, we were not able to determine why patients with infections caused by CRE-producing OXA-48 were more likely to die compared to those infected with other carbapenemase-producing strains. It is not clear whether this is due to the development of further antimicrobial resistance or the associations with other types of resistance genes. The most plausible explanation is the presence of unmeasured confounders not captured in our analysis, most likely related to the timing of antibiotic initiation and the appropriateness of the empirical regimen for this specific carbapenemase enzyme.
The current study benefited from the inclusion of a decent sample size of immunocompromised patients with molecular testing for CRE infection, confirming previous findings in the region regarding molecular identification for CRE infection. These studies also showed that a positive CRE screening test increased the likelihood of 30-day mortality in high-risk patients (Alraddadi et al., 2024). To date, this is the largest reported cohort of post-transplant patients with CRE in the region. In addition, our study confirmed that factors such as pneumonia and bacteremia (n = 72) are independently associated with mortality in patients with CRE, in line with findings from previous studies. Furthermore, we demonstrated that the BMT recipients, compared to the SOT patients, and patients with molecular identification of blaOXA-48 in their CRE infections were at greater risk for 90-day all-cause mortality in Saudi Arabia.
Although we were able to collect data on several potential predictor variables associated with CRE infection and mortality, the retrospective nature of our study and reliance on medical records may have limited our ability to measure or account for certain relevant factors in the analyses. It is possible that relevant clinical differences exist between BMT recipients and patients with hematological malignancies, although these patients were combined in the analysis due to sample size constraints. In addition, sequencing was not performed in this study, which may have predicted outcomes from CRE infection more accurately. The absence of sequencing may have limited our ability to detect virulence factors or resistance mechanisms that could have contributed to the observed increase in mortality. In conclusion, our study confirmed previous findings regarding the high mortality rate associated with CRE infection in immunocompromised populations. Future research should focus on developing effective strategies to care for immunocompromised patients with prolonged hospital stays to reduce the risk of acquiring CRE infections.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board KFSH&RC Jeddah. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study was a retrospective chart review.
Author contributions
BA: Writing – review & editing, Supervision, Writing – original draft, Methodology, Conceptualization. EH: Writing – original draft, Data curation, Formal analysis, Writing – review & editing. RA: Writing – review & editing, Methodology, Data curation. SahA: Data curation, Writing – review & editing, Methodology. AhA: Writing – review & editing, Methodology, Data curation. SalA: Writing – review & editing, Methodology, Data curation. AbA: Methodology, Writing – review & editing, Data curation. LH: Writing – review & editing, Methodology, Data curation. MA: Data curation, Methodology, Writing – review & editing. IB: Methodology, Writing – review & editing, Data curation. RT: Methodology, Writing – review & editing, Data curation. EA: Methodology, Writing – review & editing, Data curation. MB: Writing – review & editing, Methodology, Data curation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aguado, J. M., Silva, J. T., Fernández-Ruiz, M., Cordero, E., Fortún, J., Gudiol, C., et al. (2018). Management of multidrug resistant gram-negative bacilli infections in solid organ transplant recipients: SET/GESITRA-SEIMC/REIPI recommendations. Transplant. Rev. (Orlando) 32, 36–57. doi: 10.1016/j.trre.2017.07.001
Alraddadi, B. M., Heaphy, E. L. G., Aljishi, Y., Ahmed, W., Eljaaly, K., Al-Turkistani, H. H., et al. (2022). Molecular epidemiology and outcome of carbapenem-resistant Enterobacterales in Saudi Arabia. BMC Infect. Dis. 22:542. doi: 10.1186/s12879-022-07507-y
Alraddadi, B. M., Heaphy, E. L. G., Alzahrani, M. S., Alqadi, M., Qashqari, M. S., Alhuthali, M. S., et al. (2024). Association between Carbapenem-resistant Enterobacterales (CRE) colonization status at time of hospital admission and the subsequent development of CRE infection and mortality in high-risk patients. Infect. Drug Resist. 17, 4655–4664. doi: 10.2147/IDR.S479487
Bianco, G., Boattini, M., Comini, S., Leone, A., Bondi, A., Zaccaria, T., et al. (2022). Implementation of chromatic super CAZ/AVI(®) medium for active surveillance of ceftazidime-avibactam resistance: preventing the loop from becoming a spiral. Eur. J. Clin. Microbiol. Infect. Dis. 41, 1165–1171. doi: 10.1007/s10096-022-04480-x
Blaschke, A. J., Heyrend, C., Byington, C. L., Fisher, M. A., Barker, E., Garrone, N. F., et al. (2012). Rapid identification of pathogens from positive blood cultures by multiplex polymerase chain reaction using the FilmArray system. Diagn. Microbiol. Infect. Dis. 74, 349–355. doi: 10.1016/j.diagmicrobio.2012.08.013
Boutzoukas, A. E., Dai, W., Cober, E., Abbo, L. M., Komarow, L., Chen, L., et al. (2025). Carbapenem-resistant Enterobacterales in solid organ transplant recipients. Am. J. Transplant. 25, 848–859. doi: 10.1016/j.ajt.2024.10.020
Daikos, G. L., Tsaousi, S., Tzouvelekis, L. S., Anyfantis, I., Psichogiou, M., Argyropoulou, A., et al. (2014). Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob. Agents Chemother. 58, 2322–2328. doi: 10.1128/AAC.02166-13
de Maio Carrilho, C. M., de Oliveira, L. M., Gaudereto, J., Perozin, J. S., Urbano, M. R., Camargo, C. H., et al. (2016). A prospective study of treatment of carbapenem-resistant Enterobacteriaceae infections and risk factors associated with outcome. BMC Infect. Dis. 16:629. doi: 10.1186/s12879-016-1979-z
Freire, M. P., de Oliveira Garcia, D., Cury, A. P., Francisco, G. R., Dos Santos, N. F., Spadão, F., et al. (2019). The role of therapy with aminoglycoside in the outcomes of kidney transplant recipients infected with polymyxin-and carbapenem-resistant Enterobacteriaceae. Eur. J. Clin. Microbiol. Infect. Dis. 38, 755–765. doi: 10.1007/s10096-019-03468-4
Gutiérrez-Gutiérrez, B., Salamanca, E., de Cueto, M., Hsueh, P. R., Viale, P., Paño-Pardo, J. R., et al. (2017). Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect. Dis. 17, 726–734. doi: 10.1016/S1473-3099(17)30228-1
Kerneis, S., and Lucet, J. C. (2019). Controlling the diffusion of multidrug-resistant organisms in intensive care units. Semin. Respir. Crit. Care Med. 40, 558–568. doi: 10.1055/s-0039-1696980
Li, C., Li, Y., Zhao, Z., Liu, Q., and Li, B. (2019). Treatment options and clinical outcomes for carbapenem-resistant Enterobacteriaceae bloodstream infection in a Chinese university hospital. J. Infect. Public Health 12, 26–31. doi: 10.1016/j.jiph.2018.08.002
Logan, L. K., and Weinstein, R. A. (2017). The epidemiology of Carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J. Infect. Dis. 215, S28–S36. doi: 10.1093/infdis/jiw282
Muchtar, E., Paul, M., Horowitz, A., Shpilberg, O., and Raanani, P. (2012). Persistent carbapenem-resistant Klebsiella pneumoniae bacteremia in a patient with acute lymphoblastic leukemia. Isr. Med. Assoc. J. 14, 195–197.
Nordmann, P., Naas, T., and Poirel, L. (2011). Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17, 1791–1798. doi: 10.3201/eid1710.110655
Patel, G., and Bonomo, R. A. (2013). "stormy waters ahead": global emergence of carbapenemases. Front. Microbiol. 4:48. doi: 10.3389/fmicb.2013.00048
Patel, G., Huprikar, S., Factor, S. H., Jenkins, S. G., and Calfee, D. P. (2008). Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect. Control Hosp. Epidemiol. 29, 1099–1106. doi: 10.1086/592412
Patel, G., Perez, F., and Bonomo, R. A. (2010). Carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii: assessing their impact on organ transplantation. Curr. Opin. Organ Transplant. 15, 676–682. doi: 10.1097/MOT.0b013e3283404373
Pérez-Nadales, E., Fernández-Ruiz, M., Gutiérrez-Gutiérrez, B., Pascual, Á., Rodríguez-Baño, J., Martínez-Martínez, L., et al. (2022). Extended-spectrum β-lactamase-producing and carbapenem-resistant Enterobacterales bloodstream infection after solid organ transplantation: recent trends in epidemiology and therapeutic approaches. Transpl. Infect. Dis. 24:e13881. doi: 10.1111/tid.13881
Pouch, S. M., and Satlin, M. J. (2017). Carbapenem-resistant Enterobacteriaceae in special populations: solid organ transplant recipients, stem cell transplant recipients, and patients with hematologic malignancies. Virulence 8, 391–402. doi: 10.1080/21505594.2016.1213472
Satlin, M. J., Calfee, D. P., Chen, L., Fauntleroy, K. A., Wilson, S. J., Jenkins, S. G., et al. (2013). Emergence of carbapenem-resistant Enterobacteriaceae as causes of bloodstream infections in patients with hematologic malignancies. Leuk. Lymphoma 54, 799–806. doi: 10.3109/10428194.2012.723210
Satlin, M. J., Jenkins, S. G., and Walsh, T. J. (2014). The global challenge of carbapenem-resistant Enterobacteriaceae in transplant recipients and patients with hematologic malignancies. Clin. Infect. Dis. 58, 1274–1283. doi: 10.1093/cid/ciu052
Snitkin, E. S., Zelazny, A. M., Thomas, P. J., Stock, F., Henderson, D. K., Palmore, T. N., et al. (2012). Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci. Transl. Med. 4:148ra16. doi: 10.1126/scitranslmed.3004129
So-Ngern, A., Osaithai, N., Meesing, A., and Chumpangern, W. (2023). Mortality rate and factors associated with mortality of carbapenem-resistant Enterobacteriaceae infection. Drug Target Insights 17, 120–125. doi: 10.33393/dti.2023.2622
Tumbarello, M., Trecarichi, E. M., De Rosa, F. G., Giannella, M., Giacobbe, D. R., Bassetti, M., et al. (2015). Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J. Antimicrob. Chemother. 70, 2133–2143. doi: 10.1093/jac/dkv086
Tzouvelekis, L. S., Markogiannakis, A., Psichogiou, M., Tassios, P. T., and Daikos, G. L. (2012). Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin. Microbiol. Rev. 25, 682–707. doi: 10.1128/CMR.05035-11
Wright, H., Harris, P. N. A., Chatfield, M. D., Lye, D., Henderson, A., Harris-Brown, T., et al. (2021). Investigator-driven randomised controlled trial of Cefiderocol versus standard therapy for healthcare-associated and hospital-acquired gram-negative bloodstream infection: study protocol (the GAME CHANGER trial): study protocol for an open-label, randomised controlled trial. Trials 22:889. doi: 10.1186/s13063-021-05870-w
Yang, T. T., Luo, X. P., Yang, Q., Chen, H. C., Luo, Y., Zhao, Y. M., et al. (2020). Different screening frequencies of carbapenem-resistant Enterobacteriaceae in patients undergoing hematopoietic stem cell transplantation: which one is better? Antimicrob. Resist. Infect. Control 9:49. doi: 10.1186/s13756-020-0706-0
Keywords: CRE genes, epidemiology, bone marrow transplant (BMT), solid organ transplant (SOT), Enterobacterales
Citation: Alraddadi BM, Heaphy ELG, Almaghrabi R, Althawadi S, Alshahrani AM, Almosallam S, Alraddadi A, Hefni L, Almannaei MS, Bahabri I, Taha R, Alamoudi E and Bosaeed M (2025) Epidemiology and outcomes of Carbapenem-resistant Enterobacterales infection in high-risk patients in Saudi Arabia. Front. Microbiol. 16:1619611. doi: 10.3389/fmicb.2025.1619611
Edited by:
Arryn Craney, Petrified Bugs LLC, United StatesReviewed by:
Reem AlOmar, Imam Abdulrahman Bin Faisal University, Saudi ArabiaJoana Moreira Da Silva, Instituto Superior de Ciências da Saúde Egas Moniz, Portugal
Copyright © 2025 Alraddadi, Heaphy, Almaghrabi, Althawadi, Alshahrani, Almosallam, Alraddadi, Hefni, Almannaei, Bahabri, Taha, Alamoudi and Bosaeed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emily L. G. Heaphy, ZW1pbHkubGcuaGVhcGh5QGdtYWlsLmNvbQ==
†ORCID: Emily L. G. Heaphy, http://orcid.org/0009-0000-3692-1168
 Basem M. Alraddadi
Basem M. Alraddadi Emily L. G. Heaphy
Emily L. G. Heaphy Reem Almaghrabi4
Reem Almaghrabi4 Ahmad M. Alshahrani
Ahmad M. Alshahrani Ibrahim Bahabri
Ibrahim Bahabri Ebtihal Alamoudi
Ebtihal Alamoudi Mohammed Bosaeed
Mohammed Bosaeed