- 1Department of Clinical Nutrition, Shenzhen Nanshan People’s Hospital, Shenzhen, China
- 2Department of Geriatric Medicine, Shenzhen Nanshan People’s Hospital, Shenzhen, China
Non-alcoholic fatty liver disease (NAFLD) is now the most prevalent chronic liver disease worldwide, ranging from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH) and hepatocellular carcinoma. It poses a significant public health challenge. Growing evidence indicates that the gut microbiota plays a key role in the development and progression of NAFLD. Advances in sequencing technologies, microbiome and metabolomics have helped identify characteristic microbial patterns and microbial-derived metabolites associated with NAFLD. The gut-liver axis has emerged as a central pathway linking intestinal microbes to liver function. Microbiota-derived metabolites, such as short-chain fatty acids, bile acids (BAs), and trimethylamine N-oxide (TMAO), have dual roles in hepatic lipid accumulation, inflammation, and insulin resistance, providing new insight into NAFLD pathogenesis. This review summarizes the mechanisms by which disruptions in the gut-liver axis contribute to NAFLD progression. It also outlines the therapeutic effects and mechanisms of current probiotics, with particular emphasis on next-generation probiotics like Akkermansia muciniphila and the potential benefits of its inactivated forms. Furthermore, we explore the role of prebiotics, plant-derived compounds, and synthetic agents in modulating gut microbiota and liver health. The review highlights key associations between specific bacterial species, microbial metabolites, and NAFLD, offering a theoretical basis for microbiota-targeted precision interventions and new therapeutic directions.
Introduction
NAFLD
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of liver condition characterized by macrovesicular accumulation of triglyceride in hepatocytes without the evidence for ongoing or recent consumption of significant amounts of alcohol (Chalasani et al., 2018). The redefinition of NAFLD as metabolic associated fatty liver disease (MAFLD) reflects its strong association with metabolic disorders, including obesity, dyslipidemia, insulin resistance, hypothyroidism, and obstructive sleep apnea (Eslam et al., 2020). NAFLD is recognized as the most prevalent liver disorder, with a global prevalence of 25% (Younossi et al., 2016). Several systematic reviews have demonstrated that NAFLD affects over 33% of the Asian population as of 2017 with an increasing incidence rate of 29.7 per 1,000 person-years. The progression of NAFLD follows a well-defined spectrum from benign simple steatosis, non-alcoholic fatty liver (NAFL), steatohepatitis and to more advanced disease called non-alcoholic steatohepatitis (NASH) with advanced fibrosis and cirrhosis, ultimately lead to hepatocellular carcinoma, liver failure, and death. The presence of NASH can increase risk to develop fibrosis, cirrhosis or hepatocellular carcinoma. American Association for the Study of Liver Diseases defines NAFL as the presence of greater than 5% hepatic steatosis without evidence of hepatocellular injury while NASH is defined as the presence of >5% hepatic steatosis with inflammation and hepatocyte injury (Chalasani et al., 2018). So far, the pathogenetic mechanisms of NAFLD are complex and not yet fully elucidated.
Noticeably, only a few guidelines recommended pharmacological treatment are available. For example, PPAR-γ ligand drug Pioglitazone, which is primary focus on blood glucose control for diabetes, helps control liver damage (Chalasani et al., 2018; Sheka et al., 2020). Several new medications are currently under research targeting on NAFLD, particularly for its more severe form, NASH. Rezdiffra (resmetirom), a newly FDA-approved treatment for NASH, functions as a thyroid hormone receptor beta (THR-β) agonist, which is thought to aid in reducing liver fat and improving fibrosis in NASH patients (Harrison et al., 2024). Nutritional supplements such as vitamin E at a dose of 800 IU daily and omega-3 fatty acid at a dose of 1 g daily have demonstrated positive effects in reducing inflammation, decreasing fat accumulation and improving liver histology (Sanyal et al., 2010; Nogueira et al., 2016). Apart from medications, the treatment of NAFLD is highly rely on lifestyle intervention at the early stage, with the main objective being weight loss and healthy weight maintenance. Several studies has been demonstrated that a 5% weight reduction is required to decrease hepatic steatosis, while weight loss of 7%–10% can help to improve liver inflammation and fibrosis, even complete resolution of their NASH (Vilar-Gomez et al., 2015). Current pharmacological management of NAFLD faces limited therapeutic options and inability to comprehensively address its multifactorial pathogenesis. Notably, accumulating evidence has demonstrated the therapeutic efficacy of gut microbiota modulation in obesity and type 2 diabetes mellitus (T2DM), providing novel insights for NAFLD intervention (Ng et al., 2022; Zhang et al., 2025).
Currently, it is believed that NAFLD tends to be a multifactorial disease. It involves genetic, metabolic, and environmental factors, including epigenetic modifications, dietary intake, hormones secreted by adipose tissue (leptin, adiponectin), crosstalk or organization between different organs, etc (Trépo and Valenti, 2020). Genome-wide association studies (GWAS) have identified several genetic variants, such as PNPLA3, TM6SF2, MBOAT7 are strongly associated with the severity of NAFLD (Longo et al., 2021). Epigenetic modifications, such as DNA methylation and miRNA regulation, lead to abnormal expression of key metabolic genes, while dysregulation of miRNAs can impact lipid metabolism and inflammatory responses (Juanola et al., 2021). For example, hypomethylation of fibrogenic genes such as TGF-β1, Collagen 1A1 and platelet-derived growth factor, has been observed in advanced stages of NAFLD, accompanied by their transcriptional upregulation to exacerbate lipid accumulation and fibrosis. Moreover, the gut microbiota can recruit DNA methyltransferase 3 (DNMT3) to induce epigenetic modifications of Toll-like receptor 4 (TLR4) in intestinal epithelial cells, thereby impacting hepatic fibrosis and steatosis (Tang et al., 2024). In addition, microbial metabolites TMAO upregulate members of the miR-17/92 cluster in liver, which in turn enhances the expression of target genes associated with inflammation, thus promoting NAFLD progression.
Environmental factors, including high-calorie diets, sedentary lifestyles, smoking, and air pollution, exacerbate NAFLD progression by inducing oxidative stress and inflammation. These factors interact with each other, collectively driving the evolution of NAFLD from simple steatosis NASH and even liver fibrosis (Alferink et al., 2019; Geier et al., 2021; Juanola et al., 2021). Among these risk factors, a growing body of evidence indicates that gut–liver axis is implicated in the onset and progression of NAFLD (Knudsen et al., 2019; Michels et al., 2022). Given the microbiota’s plasticity, targeting gut microbiota composition presents a synergistic effects between microbial interventions and conventional therapies to develop promising therapeutic approach for NAFLD.
Gut microbiome
In recent years, groundbreaking research has illuminated the profound connection between gut microbiota and metabolic diseases, revealing a complex interplay that extends far beyond traditional understanding. Comprehensive investigations have established that the gut microbiome is integral to metabolic regulation, affecting various conditions including obesity, T2DM, and NAFLD (Dugas et al., 2018).
The process of human gut microbiomes colonization starts prenatally and develop across the intestine in early life, therefor to shape physiological and immunological functions throughout an individual’s life (Rodríguez et al., 2015). Approximately 150 to 400 species reside in each person’s gut with mostly species belong to the Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria phyla (Lloyd-Price et al., 2016; Davenport et al., 2017). The composition of gut microbiota is continuously adaptive and dynamic with unhealthy dietary habits, sedentary lifestyle, antibiotics usage, and toxic chemicals exposure can promote sustained modifications of the gut ecosystem homeostasis, leading to dysbiosis. Accumulating evidence demonstrates significantly reduced overall bacterial diversity and richness in NAFLD patients compared to healthy controls. Critically, diminished α-diversity and restructured β-diversity are consistently observed in NAFLD cohorts, with both metrics correlating with disease severity progression. (Shen et al., 2017; Tsai et al., 2020; Zeng et al., 2024). Dysbiosis has been linked to NAFLD through various mechanisms. It is believed that the microbiota profile can influence intestinal permeability, allowing bacteria or their derived factors to reach the liver and contribute to liver injury, steatohepatitis, and fibrosis progression (de Vos et al., 2022; Michels et al., 2022; Hsu and Schnabl, 2023).
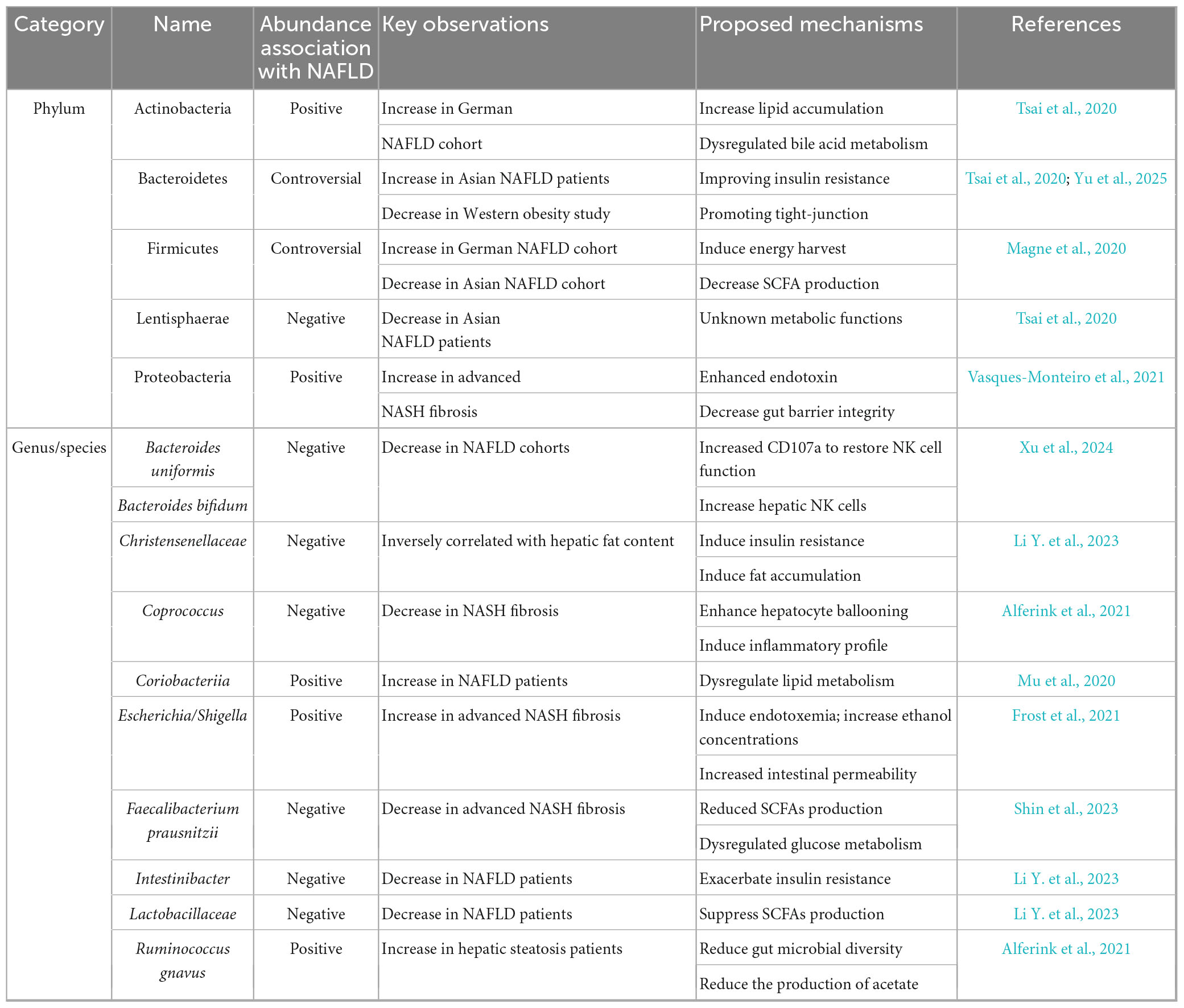
A large number of studies has been focusing on the composition of the gut microbiota in fecal samples among NAFLD patients and related metabolic disease condition. Among animals and human studies, the abundance of certain strains such as Lactobacillaceae, Christensenellaceae, and Intestinibacter have been found to be negatively correlated with NAFLD, while others like Coriobacteriia, Actinomycetales, and Oxalobacteraceae show positive associations with the disease (Li Y. et al., 2023). Moreover, the abundance of Bacteroides, especially B. uniformis and B. bifidum were reported markedly decreased in the NAFLD group (Demir et al., 2020; Xu et al., 2024). At phylum level, one German cross-sectional prospective study found higher abundances of Gram-positive Actinobacteria and Firmicutes comparing with healthy controls. However, the shift of gut microbiota was not consistent among studies. A higher level of the Bacteroidetes and lower levels of Firmicutes and Lentisphaerae were detected among NAFLD patients than in controls in Asian population (Tsai et al., 2020). While the Firmicutes/Bacteroidetes ratio had been reported as a biomarker for obesity, it does not significantly correlate with fibrosis or steatosis in groups other than the obese group (Magne et al., 2020; Jasirwan et al., 2021).
Fewer studies have focused on the changes in gut microbiome profiles during the progression from NAFLD to NASH as well as the advanced degree of fibrosis as determined by liver histology. A decreased in Firmicutes and Faecalibacterium prausnitzii, along with an increase of Proteobacteria and E. coli abundance has been observed in patients with advanced NASH fibrosis (Loomba et al., 2017). Additionally, a recent study also indicated hepatic steatosis was associated with lower microbial alpha diversity and the presence of Coprococcus and Ruminococcus gnavus (Alferink et al., 2021). The association between gut microbiota and NAFLD is summarized in Table 1.
However, it still remains uncertain whether the altered gut dysbiosis observed in NAFLD is merely a consequence results of the disease or if it actually contributes to the disease process itself. A longitudinal study conducted in Germany demonstrated that long-term instability of the gut microbiome over a 5-year interval with dominance of Enterobacteriaceae and Escherichia/Shigella correlated with development of NAFLD and T2DM, suggesting that participants who later developed fatty liver disease or diabetes already showed significant microbiota changes at the outset, even before the diseases were clinically apparent (Frost et al., 2021).
Gut microbiota-derived metabolites critically regulate metabolic homeostasis. For instance, short chain fatty acids (SCFAs), which are primarily produced through the fermentation of dietary fibers by gut microbiota, play a crucial role in maintaining intestinal barrier integrity for gut-liver axis homeostasis. Once production in the gut, SCFAs enter the portal circulation, providing a direct communication channel between the intestine and liver. This allow SCFA to exert metabolic effect including lipid homeostasis and energy expenditure, as well as to activate AMPK pathways, regulating gluconeogenesis and improve insulin sensitivity (Hernández et al., 2019). Besides SCFAs, bile acids (BAs) which are primary produced in liver, play as signaling molecules to activate nuclear receptors, which can exacerbate lipid deposition and fibrosis. Along with imbalances in the gut microbiome that affect bile acid composition, contribute to the progression from simple fatty liver to a more advanced disease state (Perino et al., 2021). Furthermore, the gut microbiota’s role in NAFLD is also seen in its interaction with inflammation factors. Gut-derived pro-inflammatory metabolites can activate inflammatory cytokine signal pathways in the liver, leading to severe inflammation, fibrosis, and liver damage in NAFLD. This interaction between the gut microbiota and the host’s immune system is also a key factor in the pathogenesis of NAFLD (Hammerich and Tacke, 2023).
A growing body of research has revealed that supplementation with probiotics, such as Bifidobacterium, Lactobacillus and Akkermansia, are potential to restore the balance of gut microbiota and to improve the gut microecological environment (Mohamad Nor et al., 2021; Carpi et al., 2022). Additionally, probiotics can enhance gut barrier function, thereby alleviating liver inflammation and damage. For example, Akkermansia muciniphila modulate the expression of tight junction proteins and influence γδT cells and macrophages, which are two key hepatic innate immune cell populations (Han et al., 2023). There is clear strain-specificity for probiotic to become an important component in the treatment of NAFLD, and even emerge as one of the next-generation mainstream therapeutic approaches.
In this review, we provide a synopsis of the connection between the gut microbiota and NAFLD and highlight some recent proposed mechanisms in how gut microbes play a significant role in the development and progression of NAFLD, influencing the disease through its composition, metabolic activities, and potential future therapeutic directions. Understanding these relationships is crucial for developing potential therapeutic strategies focusing on microbiome modulation through probiotics, prebiotics, and personalized nutritional interventions, to prevent and treat NAFLD.
Mechanism of intestinal microbiota affecting NASH and NAFLD
Gut-liver axis
The gut-liver axis refers to the complex biochemical communication between the gut and liver cells, which plays a crucial role in maintaining health and in the progression of diseases. The portal vein delivers approximately 70% of the liver’s blood supply from the gut, which makes the liver the first line of defense against gut-derived substances. After digesting, nutrients and metabolites from the gut were transported to the liver to exert their physiological effects. As the largest mucosal surface of the human body, human gut is continuously exposed to dietary antigens and microbes (Hsu and Schnabl, 2023). This bidirectional relationship is mediated by several factors, including the gut microbiota composition, bile aids circulation, dietary components and circulated metabolites, cytokines etc. The liver influences the structure and function of the gut microbiota through bile secretion, while gut microbes and their metabolites affect hepatic metabolism and immune responses via the portal vein (Pabst et al., 2023). This dynamic between the gut and liver is central to the pathogenesis of NAFLD.
A healthy balanced microbiome is essential in terms of normal digestion and metabolic processes. However, in NAFLD, dysbiosis is commonly observed and can lead to the overgrowth of pathogenic bacteria and a decreased in beneficial microbiota, thus alter the production of metabolites that influence liver function. For example, dysbiosis result in the increased production of endotoxin lipopolysaccharide (LPS) (Di Ciaula et al., 2022). LPS later translocate from the gut to the liver through the portal circulation and activate Kupper cells, a specialized macrophages to regulate innate immune response, leading to inflammation and insulin resistance (Dixon et al., 2013; Wang G. et al., 2021). This phenomenon triggers a systemic inflammatory response, which is directed in the liver. Studies have shown that high-fat diets and excessive fructose intake can impair gut barrier function, increasing the risk of LPS entering blood stream, thus initiating pro-inflammatory cytokine release by activating Toll-like receptor 4 (TLR4) which in turn contribute to liver damage (Cho et al., 2021).
In the context of NAFLD, pro-inflammatory cytokines, including tumor Necrosis Factor (TNF), IFNγ, and IL-1β, and chemokines play key roles in regulating gut barrier function and immune responses. TNF-α promotes hepatocyte apoptosis, and activation of hepatic stellate cells (HSCs), which are involved in fibrosis development (Di Ciaula et al., 2022). Interleukin-1 beta (IL-1β) is another pro-inflammatory cytokine that contribute liver damage by inducing NF-kB pathway. IL-6 activates the JAK-STAT pathway, which leads to hepatocyte damage and fibrosis progression (Lokau et al., 2019). Given the interaction of cytokines within the gut-liver axis, they represent therapeutic targets. Modulating the levels of specific cytokines or their receptors could offer new strategies for treating NAFLD.
The gut-liver axis impacts metabolic function especially insulin resistance which is a hallmark in NAFLD individuals (Tilg et al., 2022). The gut microbiota influence insulin sensitivity by affecting bile acid metabolism, SCFA production, and by modulating the release of gut hormones like glucagon-like peptide 1 (GLP1) (Newsome et al., 2021). Dysbiosis also been shown to increase the production of secondary bile acid, that can disrupt liver function and promote lipid accumulation within hepatocytes (Di Ciaula et al., 2022). The gut-liver axis is involved in every stage of NAFLD from the onset of steatosis to the development of NASH, fibrosis and cirrhosis (Boulangé et al., 2016). Understand the mechanisms underlying gut-liver communication opens to new therapeutic avenues for treating NAFLD by targeting gut microbiota, gut barrier function, and inflammatory signaling pathways.
Short chain fatty acid (SCFA)
Short chain fatty acids, mainly acetate, propionate and butyrate, are end-products of indigestible carbohydrates fermented by gut microbiome. They significantly influence the microbiota-host interaction within the gut-liver axis (Mann et al., 2024). While butyrate and propionate are extensively extracted and metabolized by the liver, a lesser percentage of acetate is taken up by the liver thus reaches the systemic circulation in significantly higher amounts to serve as a significant redistribute carbon source in humans (Bose et al., 2019; Moffett et al., 2020). Multiple factors can influence the production of SCFAs include dietary fiber intake, host health status, medication use, genetic factors, and lifestyle through modifying the microbiome composition, especially to optimize niches for butyrogenic bacteria (Makki et al., 2018; Haak et al., 2019; Maltz et al., 2019). SCFAs help maintain beneficial bacterial populations to support microbial diversity and regulate mucosal barrier integrity, mucosal inflammation, which in turn affects various metabolic functions and nutrient absorption (Mann et al., 2024).
Butyrate once produced, undergoes further metabolism to form glutamate, glutamine, and acetoacetate. Notably, acetoacetate serves as primary energy source for colonocytes supporting their proliferation and differentiation. Butyrate also directly induced mucin expression in polarized goblet cell lines to form a protective mucus layer, acting as a physical barrier shielding epithelial cells from harmful microorganisms and preventing direct contact with the gut contents including ethanol and pro-inflammatory molecules (Martin-Gallausiaux et al., 2021). In addition, SCFAs enhancing epithelial cells tight junctions, which are protein complexes that seals the gaps between cells, by upregulating the expression of protein such as Occludin, Claudins, and Zonula (Zheng et al., 2017).
Microbial SCFAs production is essential to maintain a colonic anaerobic environment (Pral et al., 2021). For instance, butyrate assists in regulating the anaerobic milieu within the colon by activating beta-oxidation in the mitochondria, accounting for over 70% of the oxygen in isolated colonocytes. It achieves this by activating the nuclear receptor peroxisome proliferator-activated receptor gamma (PPARγ) in colonic cells. This activation limits the diffusion of oxygen from colonocytes to the luminal surface, thereby preserving the anaerobic conditions essential for obligate anaerobic organisms (Litvak et al., 2018).
Abundance of literature has extensively documented the supplementation of SCFAs, particularly butyrate, in the treatment of NAFLD and has shown promising results from nutrient metabolism perspectives. Supplementing butyrate for 6 weeks in NAFLD mice model has been shown to alleviate dysbiosis by promoting the abundance of promising bacteria, including Akkermansia, Roseburia, Coprococcus, Corprobacillus, Delftia, Corynebacterium, Sutterella, Bacteroides, Clostridium, and Coriobacteriaceae populations, while concomitantly reducing the abundance of Bilophila and Rikenellaceae. Butyrate also has the ability to prevents liver inflammation and injury, as indicated by decreased pro-inflammatory cytokine genes and activation of Kupffer cells in the liver. Furthermore, the gene expression of peroxisome proliferator activated-receptor (PPAR-γ) was upregulated after supplementation, which promotes fatty acid uptake and increases insulin sensitivity (Ye et al., 2018). SCFAs also known to have downstream effects on the endocrine function influencing the secretion of satiety signals hormones such as GLP-1 and PPY, further affecting insulin resistance and lipid metabolism disorder which accompany with NAFLD most of the time (Canfora et al., 2019). Chronic oral butyrate administration prevented diet-induced obesity, hyperinsulinemia, hypertriglyceridemia and hepatic steatosis via expressing neuropeptide Y in the hypothalamus to attribute a reduction in food intake (Li et al., 2018).
Short chain fatty acids has been demonstrated to modulate the balance between fatty acid synthesis, fatty acid oxidation, and lipolysis in the body. SCFAs can modulate various signaling pathways and gene expression, including activation of AMPK, an essential energy-sensing enzyme in the liver. The activation of AMPK has been shown to inhibit the activity of acetyl-CoA carboxylase and reduce the production of malonyl-CoA, which is a precursor to fatty acid synthesis (Canfora et al., 2019). From animal study, nanoparticle-delivered acetate supplementation decreased lipid accumulation, increased mitochondrial efficiency, and inhibited lipolysis. Additionally, acetate supplementation induced “browning” in white adipose tissue which led to a reduction in body adiposity (Sahuri-Arisoylu et al., 2016). In line with these findings, colonic infusions of SCFAs mixture were found to increase fasting fat oxidation and energy expenditure in humans (Canfora et al., 2017). Besides, SCFAs also act as ligands for G-protein coupled receptors (GPCRs), including GPR41 and GPR43 which are expressed in multiple tissues. This interaction has been shown to induce changes in hepatic gene expression that promote fatty acid oxidation and reduce lipogenesis (Neves et al., 2015).
Bile acids (BAs)
Bile acids are steroid molecules synthesized from cholesterol in the liver forming a critical connection with the gut through multiple mechanisms. Their primary functions extend beyond simple fat digestion and absorption to include metabolic regulation and antimicrobial activity through nuclear receptor signaling (such as FXR and TGR5) (Parséus et al., 2017). In the liver, unconjugated BAs can be processed by conjugating with either taurine or glycine under the action of Bile acyl-CoA synthetase (BACS) and bile acid-CoA:amino acid N-acyltransferase (BAAT) to form conjugated BAs, such as taurocholic acid (TCA) and glycocholic acid (GCA) (Liu et al., 2018). The crosstalk between gut and BAs centers on enterohepatic circulation, where all primary BAs produced by the liver undergo microbial transformation in the gut through processes of deconjugation via bile salt hydrolase (BSH), 7-α-dihydroxylation, oxidation or epimerization, resulting in secondary BAs such as deoxycholic acid (DCA), lithocholic acid (LCA) and ursodeoxycholic acid (UDCA) (Portincasa et al., 2020). Approximately 95% of the BAs in bile acid pool are reabsorbed at the terminal ileum and subsequently recycled back to the liver, establishing a continuous cycle. The amount of BAs that is lost via feces is then replaced by daily BA synthesis. This interaction has profound effects on gut barrier integrity, microbiota composition, and nutrients metabolic function, thus connecting to the progression of NAFLD (Chiang and Ferrell, 2019).
Studies have underscore the importance of the gut microbiome in influencing bile acid signaling and found that primary and secondary BAs were increased in both fecal and serum of patients with NAFLD when compared to healthy controls (Mouzaki et al., 2016). Furthermore, as NAFLD progresses to NASH, ratios of primary to secondary BAs and of conjugated to unconjugated BAs were also increase (Puri et al., 2018). Among conjugated BAs, the GCA to TCA ratio exhibited progressive alterations as it was significantly elevated in NAFL and further increased in NASH but declined sharply upon transition to fibrosis, suggesting altering BSH activity during NAFLD (Chen et al., 2022). Interestingly, plasma taurine levels are decreased in liver disease. Taurine supplementation has shown to reduce pro-inflammatory interleukin expression as well as to maintain the homeostasis of fat metabolism by repressing sterol regulatory element binding proteins-1c (SREBP-1c), thereby alleviating hepatic damage in experimental animal models (Song et al., 2023; Zhu F.-L. et al., 2024). Concurrently, the composition of the gut microbiome was altered in patients with NAFLD, with an 7.2-fold increase increase in bacteria that metabolize taurine and glycine, primarily Escherichia and Bilophila (Jiao et al., 2018; Puri et al., 2018; Zeng et al., 2024). These bacteria deconjugate TCA via BSH to facilitate overproduction of secondary bile acid DCA, a potent farnesoid X receptor (FXR) antagonist, directly suppressing FXR-driven pathways that regulate hepatic lipid and glucose metabolism.
Bile acids has been recently recognized as an endocrine signaling molecules to activate several nuclear receptors located in the gastrointestinal tract including FXR and G-protein-coupled bile acid receptor -1 (TGR5) to modulate epithelial cell proliferation, intestinal barrier integrity and nutrient metabolism (Adorini and Trauner, 2023). The bidirectional relationship between insulin resistance and NAFLD has been well studied as insulin resistance play a crucial role in the initial pathogenesis of NAFLD and in the progression to NASH (Khan et al., 2019). By activating FXR, increased insulin sensitivity and reduced serum markers of liver inflammation and fibrosis were observed in patients with T2DM and NASH (Mudaliar et al., 2013). Moreover, treatment of fexaramine, a selective FXR agonist, has been shown to alter the gut microbiota, increasing the abundance anaerobic Acetatfactor and Bacteroides. These two bacteria that have high 7α- and 7-β-HSDH enzymatic activities, enabling them to convert CDCA to LCA. The metabolic benefits occur because LCA are potent activators of TGR5 receptors in the colon and stimulate secretion of GLP-1, which is an incretin that stimulate insulin secretion from pancreatic beta-cells in response to postprandial glucose thereby suggesting improved insulin sensitivity in obese diabetic mice (Kaur et al., 2015; Pathak et al., 2018).
Given the frequent association of NAFLD with obesity, bariatric surgery, as an effective therapy for obesity, has been suggested to substantially change the concentrations of circulating BAs (Chiang, 2015). Chaudhari et al. (2021) demonstrate that LCA, a microbial metabolites secondary bile acid, is increased in murine portal veins following bariatric surgery, which eventually lead to increased GLP-1 secretion. Conversely, LCA levels in the cecum of post-surgery mice exhibited a decrease in stool samples along with the increasing in the abundance of the phyla Bacteroidetes and Proteobacteria (Chaudhari et al., 2021), and a reduction in the abundance of Clostridia in the gut (Damms-Machado et al., 2015; McGavigan et al., 2017). The reduction in levels of the LCA in the colon, in surprising contrast to the increase observed in the portal vein suggesting increased reabsorption of BAs. Consistent with increased levels of FGF19 and fasting plasma levels of 6α-hydroxylated BAs, which are TGR5 agonists, in post-surgery human patients, together support that bariatric surgery alters bile acid profiles to improve glucose metabolism (Wahlström et al., 2024).
An emerging area of interest is the interaction between SCFAs and BAs in the gut-liver axis. SCFAs may affect bile acid synthesis and metabolism, influencing liver function and the metabolic outcomes of NAFLD (Zhao et al., 2017; Visekruna and Luu, 2021). The interplay between these metabolites may offer synergistic protective effect against liver conditions by promoting liver metabolism and maintaining bile acid homeostasis.
Trimethylamine N-oxide (TMAO)
While SCFAs and BAs dominate gut-liver communication, emerging evidence highlights the role of TMAO in exacerbating NALFD progression through multiple aspects.
Trimethylamine N-oxide, a metabolite produced by gut bacteria from dietary components like choline and L-carnitine, has emerged as a significant player in the development and progression of NAFLD. Gut bacteria including Firmicutes and Proteobacteria metabolize these nutrients commonly found in animal-based foods via TMA lyase to produce trimethylamine (TMA), which is then converted to TMAO by flavin monooxygenase in liver. TMA and TMAO levels have been associated with elevated Firmicute/Bacteroidetes ratio since Bacteroidetes has limited ability to produce TMA. Certain intestinal archaea such as Methanomassiliicoccales has the ability to reduce circulating levels of TMAO to methane (Fadhlaoui et al., 2020). The change of gut microbiota composition indicating TMAO synthesis might be attributed to selected bacterial species (Shang et al., 2016). Elevated peripheral blood TMAO levels are positively correlated with major adverse cardiovascular and renal events. Recently, its relation to metabolic condition such as adipose tissue inflammation, T2DM and NAFLD has been identified (León-Mimila et al., 2021; Andrikopoulos et al., 2023; Wang M. et al., 2023).
The production of TMA/TMAO depends on multiple factors beyond gut microbiota, including host genetics and dietary consumption (Meyer, 2020). Since choline serves as a precursor of gut-microbiota-generated TMA, choline rich food play a important source of variability in serum TMAO levels. Western diets which typically contains a large amount of choline rich foods and are known not only to increase blood but also urine TMAO levels (Romano et al., 2015). In contrast, vegetarian and omnivorous diets were associated with lesser ability to produce TMA, along with the gut microbiota composition alteration especially in Gammaproteobacteria and Erysipelotrichi, reinforcing dietary modulation would have the possible potential to reduce the risk associated with high TMAO levels by limiting liver fat deposition during choline depletion (Lecomte et al., 2015; Tomova et al., 2019).
Elevated TMAO levels can disrupt normal liver function through multiple mechanisms: it interferes with bile acid metabolism and cholesterol homeostasis, impairs the liver’s ability to oxidize fatty acids, and promotes inflammatory responses within liver tissue. In vivo and animal studies experiment reveal that TMAO suppresses bile acid production via inhibiting CYP7A1 activity, the key rate-limiting enzyme that oxidize cholesterol to bile acid thus reduce total bile acid pool size (Koeth et al., 2013). In turn, as mentioned earlier, bile acid has the ability to shaping the gut microbiota composition thus influencing intestinal environment that may affect bacterial TMA production. By administering exogenous TMAO in vitro and animal models, lipid deposition in HepG2 fatty liver cells was promoted suggesting the relationship between TMAO levels and NAFLD initiation. Elevated TMAO level in mice model further damaged intestinal barrier as evidenced by decreased expression levels of tight junction proteins zona occludens-1 (ZO-1) and Occludin, leading to bacterial infiltration from the gut into liver and lipid accumulation. Besides, higher levels of downstream inflammatory factors, such as TNF-α, IL-1β, and IL-6, and elevated serum lipopolysaccharides (LPS) levels indicating TMAO exacerbates intestinal barrier damage in NAFLD rats, plays a negative regulatory role in disease progression (Nian et al., 2024).
Epigenetic refers to changes in gene expression that do not involve alterations to the underlying DNA sequence. These change can be influenced by environmental factors, lifestyles, and diseases, and provides a new perspective on the pathogenesis of NAFLD (Wu Y.-L. et al., 2023). In NAFLD, abnormal DNA methylation patterns may affect genes involved in lipid metabolism(Chen et al., 2020), inflammation (Lai et al., 2020), and insulin resistance (Baumeier et al., 2017a). For example, mice fed a high fat diet for 6 weeks exhibited reduced methylation of four CpG dinucleotides sites and elevated dipeptidyl peptidase 4 (DPP4) expression. The resulting increase in hepatic DPP4 worsen NAFLD progression by disrupting insulin signaling through autocrine and paracrine mechanisms while decreasing GLP-1 levels (Baumeier et al., 2017b). During the methylation processes, choline serves as a precursor to S-adenosylmethionine (SAM) the primary methyl donor. However, when TMA-producing gut microbiota compete with the host for choline, its bioavailability is significantly reduced to maintain proper methylation processes. This competition ultimately lead to host genome hypomethylation and NAFLD initiation and progression (Romano et al., 2017). In addition, acetate can also influence histone acetylation through Acetyl-CoA Synthetase Short Chain Family Member 2 (ACSS2), a nucleo-cytosolic enzyme involved in fat deposition and disrupted cellular signaling in the context of NAFLD, leading to hepatic steatosis or inflammation (El-Kurjieh et al., 2025).
This insight suggests the possibility of using individualized dietary and nutrient interventions to modulate both gut microbiota composition and specific metabolic pathway, including TMAO and acetate synthesis in order to offer promising therapeutic approaches for treating NAFLD and its complications.
The role of probiotic supplementation
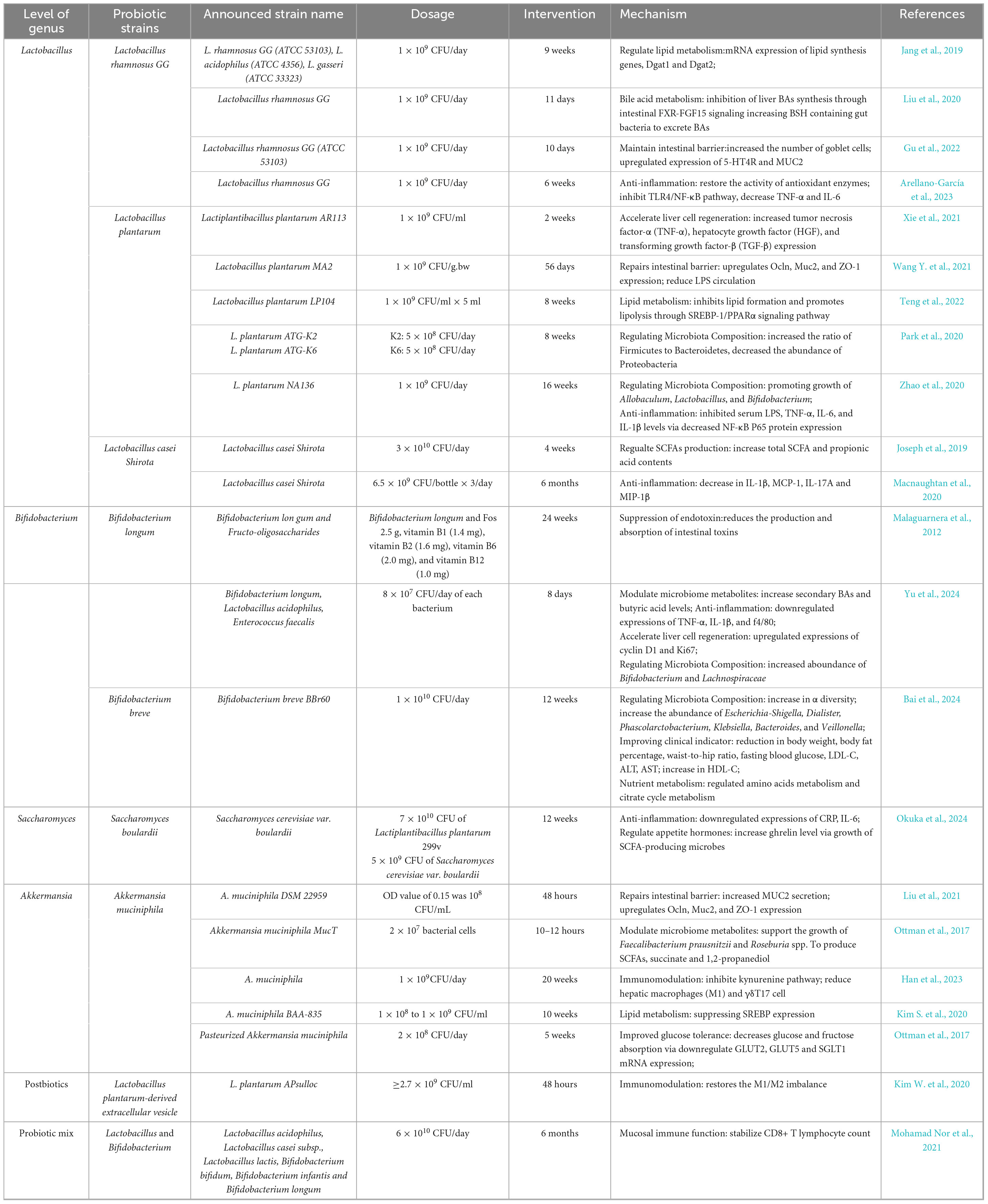
Due to the close relationship between the liver and gastrointestinal tract, it is not surprising that restoring a healthy gut microbiome composition and abundance through targeted interventions such as probiotics, prebiotics, phytochemicals and dietary changes, has the potential to combat the overgrowth of harmful bacteria, bolster the intestinal mucosal barrier, and eventually reduce fat accumulation, and mitigate liver damage. Probiotics defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit,” have been shown to play a significant role in the pathogenesis and progression of NAFLD, as mentioned in this review. Accumulating studies have demonstrated the feasibility of certain probiotic supplementation including Lactobacillus, Bifidobacterium, Polycoccus and Streptococcus against NAFLD and NASH in human and in mouse models (Yoo and Kim, 2016).
As a widely used probiotic in food industry, Lactobacillus acidophilus has been drawn much attention L. acidophilus NCFM has the potential to improve insulin sensitivity and inflammatory response (Adams et al., 2005). In a separate study, L. acidophilus SNZ 86 was found to ameliorate helps NAFLD in rats induced by western diet by activating AMPK/SIRT-1 signaling pathway, which increase fatty acid oxidation, reduces lipogenesis in the liver, and improve insulin sensitivity (Pant et al., 2023). L. acidophilus KLDS1.0901 administration followed by high-fat diet effectively restored the increased concentrations of cytokines including IL-6, IL-1β, and TNF-α, as well as lowered lipid content in levels of total cholesterol, triglyceride (TG), and low-density lipoprotein cholesterol (Wang Y. et al., 2023). A different species of L. plantarum NA136 also has elucidated the therapeutic potential in mitigating NAFLD via targeting the AMPK/Nrf2 pathway and suppressing the SREBP-1c/FAS signaling pathway in order to inhibit adipogenesis from preadipocytes (Zhao et al., 2020).
Oxidative stress refers to an imbalance between the production of reactive oxygen species and the body’s ability to neutralize them with antioxidants, implicate a key factor in early pathogenesis of NAFLD (Parthasarathy et al., 2020). In vivo, L. plantarum ATG-K2 and L. plantarum ATG-K6 enhanced antioxidant activity by regulating the expression of antioxidant enzymes like superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) via the Nrf2/Keap1 signaling pathway, a key system for regulating the expression of antioxidant and cell-protective genes (Park et al., 2020). Upon activation of Nrf2, its ability to enter the cell nucleus and bind to the antioxidant response element (ARE), thereby promoting the expression of antioxidant enzymes, thereby reducing oxidative stress-induced damage to the liver (Park et al., 2021).
During the progression from NAFLD to NASH, the dysregulation of different innate and adaptive immune cells plays an integral role, of which natural killer (NK) cells are an example. Supplementation of Bifidobacterium uniformis and Bifidobacterium bifidum for 8 weeks significantly upregulate the expression of the NKG2D receptor located on the surface of NK cells and to promote the secretion of cytotoxic enzymes by NK cells, such as granzyme B and perforin to induce apoptosis of target cells directly. NKG2D is an important activating receptor of NK cells, and its ligand recognition can enhance the cytotoxicity and the killing ability of NK cells (Xu et al., 2024). In an animal model, intervention with Bifidobacterium longum has been linked to increased serotonin (5-HT) levels in both serum and feces, attributable to elevated expressions of cyclin D1 and Ki67, pivotal regulators of the transition from the G1 phase to the S phase of the cell cycle. Elevated expression promotes liver cellular proliferation and reduce the formation of scar tissue. Furthermore, an increase in secondary BAs and butyric acid levels after intervention were observed which is in line with the diminishes the expression of proinflammatory factors TNF-a, IL-1b, f4/80 (Yu et al., 2024). A summary of current probiotic strains associated with treatment of NAFLD is shown in Table 2.
Emerging therapeutic candidate: Akkermansia muciniphila
Since its discovery two decades ago, Akkermansia muciniphila, a Gram-negative anaerobic bacterium inhabiting the mucus layer of the gastrointestinal tract has rapidly attracted widespread attention from both the academic and industrial communities due to its potential probiotic beneficial effects in various metabolic diseases, and it is expected to become a star strain of the next-generation therapeutic probiotic. The genus Akkermansia begins to colonize in the early stage of human life, and its relative abundance gradually increases during infancy and early childhood (Collado et al., 2007). During the growth process, the intake of high-fiber foods, polyphenol-rich foods and caloric restriction diet pattern can indeed affect the abundance of A. muciniphila (von Schwartzenberg et al., 2021). Unlike many gut bacteria, A. muciniphila trives in mucin degradation and can produce enzymes that degrade oligosaccharide chains, such as glycosidases, sulfatases, and sialidases, to adapt to the living environment rich in mucin and endogenous glycoproteins in the mucus layer (Liu et al., 2021). At the meantime, the thickness of the mucus layer is related to the abundance of A. muciniphila in the gastrointestinal tract.
A substantial body of research has been dedicated to elucidating the mechanisms through which A. muciniphila exert its systematic influences in the liver of NAFLD. The emergence of A. muciniphila has been demonstrated to modulate gut microbiota, strengthening the intestinal barrier, and regulate immune response that related to NAFLD and its progression to NASH.
Akkermansia muciniphila repairs intestinal barrier function by stimulating mucin production, enhancing tight junction integrity, and modulating immune responses. It promotes the activity of goblet cells, leading to increased secretion of mucins (MUC2), which reinforce the protective mucus layer and prevent harmful microbes and toxins from reaching the gut epithelium (Kim et al., 2021; Liu et al., 2021). Additionally, A. muciniphila upregulates the expression of tight junction proteins Ocln, Muc2, and ZO-1, strengthening epithelial cell connections and reducing intestinal permeability.
Furthermore, during the process of mucin degradation, A. muciniphila releases oligosaccharides and simple sugars that can serve as substrates for other gut bacteria, including SCFA producers Faecalibacterium prausnitzii and Roseburia spp. for further fermentation resulting in the production of SCFAs, succinate and 1,2-propanediol (Ottman et al., 2017; Pichler et al., 2020). In exchange, A. muciniphila receives a vitamin B12 analog as an essential cofactor in DNA synthesis from other bacteria indicating a bidirectional cross-feeding between different microbial species (Ioannou et al., 2024). These mucin metabolites contribute to host’s metabolic health, with acetate and propionate playing key role in modulation of gut hormones like peptide YY and GLP-1, as well as systemic hormones like insulin and glucagon, influencing appetite control and gastric emptying (Hernández et al., 2019; Bridgeman et al., 2020). Besides stimulating SCFAs production, emerging researches have shown that the gut microbiota supports intestinal barrier protection by modulating BAs and tryptophan derivatives (Michaudel and Sokol, 2020; Wu W. et al., 2023). Administration of A. muciniphila reversed the level of tryptophan metabolites such as 3-hydroxykynurenine and 5-hydroxykynurenine, as well as upregulated the gene expression of enzymes in kynurenine pathway which is a primary route of tryptophan degradation in the liver (Han et al., 2023). The kynurenine pathway have been shown to interact with aryl hydrocarbon receptors (AhR), which regulate the function of T cells and macrophages as well as differentiation of Th17cells that contribute to liver inflammation and fibrosis in NASH (Teunis et al., 2022).
In both animal and human trials, high-fat diet and metabolic disease condition are significantly related to the relative gut abundance reduction of A. muciniphila (Rao et al., 2021; Yoon et al., 2021; Zhang et al., 2021; Li T. et al., 2023). A. muciniphila supplementation was found to positively impact lipid metabolism and prevent NAFLD in mice through regulation of genes involved in lipogenesis and the expression of pro-inflammatory cytokines in liver tissue (Han et al., 2023). An additional study revealed that A. muciniphila specifically regulates the transcription of sterol regulatory element-binding protein (SREBP), a family of transcription factors that regulate lipid homoeostasis, to protect liver fat accumulation (Kim S. et al., 2020). A. muciniphila has also been shown to modulate the immune system, reducing hepatic proinflammatory macrophages (M1) and γδT and γδT17 cells in high-fat diet-induced NASH mice. The shift from M1 macrophages to M2 macrophages in the liver were partly regulated by A. muciniphila administration to promoting its anti-inflammatory effect (Han et al., 2023). Futhermore, breast milk-isolated A. muciniphila were shown to prevent NASH from progressing to hepatocellular carcinoma through modulation of immune system, specifically CXCR6+ natural killer T (NKT) cells, thereby enhancing its ability to counteract inflammation-driven tumorigenesis (Li T. et al., 2023).
Interestingly, even pasteurization of A. muciniphila also promisingly exert similar positive effects in mice (Depommier et al., 2020; Wu et al., 2022; Wang Y. et al., 2024). The benefits mostly link to the outer cell membrane protein Amuc-1100 of A. muciniphila. Amuc-1100 has been observed to interact with immune cells, particularly through Toll-like receptors (TLRs) present on intestinal and liver immune cells, leading to a reduction in systemic inflammation. This has been shown to improve fatty acid oxidation and insulin sensitivity, both of which are critical for managing NAFLD (Cani et al., 2022). Notably, comparing to live form, pasteurized form is more effective in upregulating tight junction proteins expression and in inducing higher levels of SCFAs in the ileum (Grajeda-Iglesias et al., 2021). Meanwhile, both alive and pasteurized A. muciniphila promote the growth of beneficial bacteria while reducing the abundance of harmful microbes. This shift in microbial composition contributes to a healthier gut ecosystem, reducing the production of metabolites that could exacerbate inflammation and liver dysfunction (Ashrafian et al., 2021).
Pasteurized A. muciniphila offers significant advantages in the development of novel probiotic products with high commercial value (Plovier et al., 2017). As the bacteria are inactivated, pasteurized A. muciniphila cannot reproduce, eliminating the risk of infection and making them safer for individuals with weakened immune systems (Turck et al., 2021). Additionally, pasteurized A. muciniphila is also more stable during storage and transportation, reducing the need for strict environmental controls and extending its shelf life (Abbasi et al., 2024). Despite these advantages, unlike live bacteria, it lacks the ability to colonize the gut, meaning its effects may be short-lived and require higher doses or more frequent administration to achieve results comparable to live bacteria (Wang B. et al., 2023; Xie et al., 2023; Liu et al., 2024). It highlights the need to carefully consider the patient’s needs when choosing between live and pasteurized supplementation.
Therapeutic potential of other compounds for NAFLD
In addition to the above-mentioned probiotics, prebiotics and certain phytochemicals have also played a positive role in therapeutic potential of NAFLD. Prebiotics are selectively fermented, non-digestible dietary compounds such as inulin, oligosaccharides, have been shown to positively influence the gut microbiota, which is closely linked to NAFLD pathogenesis. The supplementation of oligofructose for 36 weeks has shown statistically significant reduction in hepatic steatosis and NASH score, as well as to improve gut microbiome diversity and to modulate gut microbiota profile (Bomhof et al., 2019; Carpi et al., 2022). Prebiotics exert their beneficial effects by enhancing the growth of beneficial gut bacteria to favor SCFAs production, improving gut barrier function, reducing endotoxemia and modulating inflammatory responses.
Recent research has revealed that certain plant-derived compounds can have a significant impact on NAFLD by modulating the gut microbiota. Nuciferine, a bioactive compound found in lotus leaves, has been shown to enhance gut barrier integrity by upregulating tight junction protein expression and suppressing inflammation via TLR4/MyD88/NF-κB pathway (Zhu X. et al. 2024). Similarly, theabrownin, derived from Pu-erh tea, acts as an intestinal farnesoid X receptor (FXR) antagonist to mitigate NAFLD by inhibiting the intestinal FXR-ceramide axis. The reduction of ceramide levels in the intestine and liver further downregulates ceramide synthase expression, thereby decreasing hepatic lipid accumulation and improving hepatic steatosis in animal models (Wang J. et al., 2024). While these two plant-derived compounds show potential in improving NAFLD by beneficially modulating the gut microbiota. However, excessive consumption of longan fruit, which is rich in free sugars, has been found to disrupt gut homeostasis. It reduces the Bacteroidetes/Firmicutes ratio, increases potentially pathogenic bacteria, decreases beneficial bacteria, and reduces SCFA production, thereby promoting NAFLD development (Wu X. et al., 2023). This indicates that the impact of plant-derived compounds on NAFLD is dual-edged, with effects varying depending on the type and dose of the compound.
Tauroursodeoxycholic Acid (TUDCA), a synthetic bile acid derived from the conjugation of UDCA with taurine, has been widely recognized for its therapeutic potential in cholestatic liver diseases (Torres et al., 2019). Beyond its conventional uses, TUDCA intervention is positively associated with the abundance of beneficial bacteria such as Allobaculum and Bifidobacterium in NAFLD animal models. It also influences bile acid metabolism by upregulating key enzymes cholesterol 7α-hydroxylase (CYP7A1) and sodium taurocholate cotransporting polypeptide (NTCP). This results in increased hepatic bile acid levels, which facilitate cholesterol conversion to BAs and promote their enterohepatic circulation, thereby alleviating hepatic lipid accumulation (Wang H. et al., 2024). Medium to high dose of TUDCA (50 and 100 mg/kg/day) have shown to enhance hepatocyte proliferation by upregulating GATA3 activity, and to alleviates liver fibrosis by attenuating hepatic stellate cell activation in animal study (Bai et al., 2025). These findings underscore the significant therapeutic potential of synthetic BAs compounds in NAFLD treatment.
Bridging the gaps: current limitations
Despite the promising findings, there are significant limitations in current research that need to be addressed. First, there is significant heterogeneity among human studies in terms of design protocols, diagnostic criteria, patient population characteristics, and methods of flora analysis, making cross-study comparisons difficult. Second, although microbial-derived metabolites (e.g., SCFAs, BAs, TMAO) have been demonstrated to be associated with NAFLD pathogenesis, their clinical translational application has not been clarified and causality remains unestablished. Human intervention trials with isotope-labeled metabolites or fecal microbiota transplantation (FMT) are needed to validate mechanisms. In addition, current microbiota studies primarily rely on 16S rRNA sequencing, which lacks resolution at the species level and functional insights. While metagenomics resolves these limitations, its high cost and computational demands hinder widespread clinical application. More importantly, comprehensive databases integrating microbiome and multi-omics data (e.g., metabolomics, lipidomics) as well as disease phenotypes have not yet been developed. Future studies should integrate multi-omics approaches including metagenomics or metatranscriptomics to link specific flora to NAFLD progression. This review has not addressed translational applications in clinical practice or causal relationships supported by existing databases. Given the existing methodological limitations, screening of diagnostic markers through computational science and integrated multi-omics approaches represents a critical direction of exploration in the future.
Conclusion
Non-alcoholic fatty liver disease is a multifactorial metabolic disorder with significant public health implications, potentially progressing to NASH, fibrosis, and even hepatocellular carcinoma. The progression of NAFLD is driven by a complex interplay between genetic, metabolic, and environmental factors drives its progression, with the gut-liver axis emerging as a critical pathway in its pathogenesis. Accumulating evidence highlights the pivotal role of the gut microbiota in modulating hepatic lipid deposition, inflammation, and insulin resistance through microbiota-derived metabolites, including SCFAs, BAs, and TMAO offering new insights into the progression of the disease. Emerging therapeutic strategies targeting the gut microbiota offer promising avenues for NAFLD management. Probiotics, such as Lactobacillus, Bifidobacterium, and Akkermansia muciniphila, have shown significant benefits in restoring microbial balance, enhancing gut barrier function, modulating nutrient metabolism, and reducing inflammation. The integration of probiotics, prebiotics, plant-derived compound, and TUDCA leverages the complementary mechanisms to address the multifactorial nature of NAFLD and to position them as next-generation probiotics for NAFLD treatment. Future research could focus on optimizing the combinations and dosages of these therapies, validating their efficacy in large-scale clinical trials, and exploring personalized treatment regimens based on individual gut microbiota profiles.
In summary, the targeting of the gut microbiota and its metabolites presents a promising strategy for the prevention and treatment of NAFLD. However, challenges persist. The heterogeneity of NAFLD and the complex interactions between the gut microbiota and host metabolism underscore the necessity for personalized therapeutic approaches. Additionally, the long-term efficacy and safety of probiotic interventions, particularly in diverse patient populations, require rigorous evaluation. Consequently, there is an imperative for further research to develop precision-based therapeutic approaches, and exploring the potential synergistic effects of combining probiotics, prebiotics, plant-derived compounds and BAs with existing pharmacological treatments in managing this multifaceted disease.
Author contributions
SS: Writing – original draft, Writing – review and editing. YL: Writing – review and editing. NW: Writing – review and editing. ZH: Writing – review and editing, Funding acquisition, Resources. GD: Funding acquisition, Resources, Writing – review and editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Shenzhen Science and Technology Innovation Committee (grant number JCYJ20240813114522030) and Sanming Project of Medicine (grant number SZSM202103011).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbasi, A., Bazzaz, S., Da Cruz, A. G., Khorshidian, N., Saadat, Y. R., Sabahi, S., et al. (2024). A critical review on Akkermansia muciniphila: Functional mechanisms, technological challenges, and safety issues. Probiotics Antimicrob. Proteins 16, 1376–1398. doi: 10.1007/s12602-023-10118-x
Adams, L. A., Lymp, J. F., St Sauver, J., Sanderson, S. O., Lindor, K. D., Feldstein, A., et al. (2005). The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 129, 113–121. doi: 10.1053/j.gastro.2005.04.014
Adorini, L., and Trauner, M. (2023). FXR agonists in NASH treatment. J. Hepatol. 79, 1317–1331. doi: 10.1016/j.jhep.2023.07.034
Alferink, L. J. M., Radjabzadeh, D., Erler, N. S., Vojinovic, D., Medina-Gomez, C., Uitterlinden, A. G., et al. (2021). Microbiomics, metabolomics, predicted metagenomics, and hepatic steatosis in a population-based study of 1,355 adults. Hepatology 73, 968–982. doi: 10.1002/hep.31417
Alferink, L. J., Kiefte-de Jong, J. C., Erler, N. S., Veldt, B. J., Schoufour, J. D., de Knegt, R. J., et al. (2019). Association of dietary macronutrient composition and non-alcoholic fatty liver disease in an ageing population: The rotterdam study. Gut 68, 1088–1098. doi: 10.1136/gutjnl-2017-315940
Andrikopoulos, P., Aron-Wisnewsky, J., Chakaroun, R., Myridakis, A., Forslund, S. K., Nielsen, T., et al. (2023). Evidence of a causal and modifiable relationship between kidney function and circulating trimethylamine N-oxide. Nat. Commun. 14:5843. doi: 10.1038/s41467-023-39824-4
Arellano-García, L., Trepiana, J., Martínez, J. A., Portillo, M. P., and Milton-Laskibar, I. (2023). Beneficial effects of viable and heat-inactivated Lactobacillus rhamnosus GG administration on oxidative stress and inflammation in diet-induced NAFLD in rats. Antioxidants (Basel) 12:717. doi: 10.3390/antiox12030717
Ashrafian, F., Keshavarz Azizi, Raftar, S., Shahryari, A., Behrouzi, A., Yaghoubfar, R., et al. (2021). Comparative effects of alive and pasteurized Akkermansia muciniphila on normal diet-fed mice. Sci. Rep. 11:17898. doi: 10.1038/s41598-021-95738-5
Bai, C., Song, X., Yan, J., Xu, J., Zhou, Y., Sun, Z., et al. (2025). Tauroursodeoxycholic acid induces liver regeneration and alleviates fibrosis through GATA3 activation. Biomedicines 13:910. doi: 10.3390/biomedicines13040910
Bai, Z., Wu, Y., Gao, D., Dong, Y., Pan, Y., and Gu, S. (2024). Gut microbiome and metabolome alterations in overweight or obese adult population after weight-loss bifidobacterium breve BBr60 intervention: A randomized controlled trial. Int. J. Mol. Sci. 25:10871. doi: 10.3390/ijms252010871
Baumeier, C., Saussenthaler, S., Kammel, A., Jähnert, M., Schlüter, L., Hesse, D., et al. (2017a). Hepatic DPP4 DNA methylation associates with fatty liver. Diabetes 66, 25–35. doi: 10.2337/db15-1716
Baumeier, C., Schlüter, L., Saussenthaler, S., Laeger, T., Rödiger, M., Alaze, S. A., et al. (2017b). Elevated hepatic DPP4 activity promotes insulin resistance and non-alcoholic fatty liver disease. Mol. Metab. 6, 1254–1263. doi: 10.1016/j.molmet.2017.07.016
Bomhof, M. R., Parnell, J. A., Ramay, H. R., Crotty, P., Rioux, K. P., Probert, C. S., et al. (2019). Histological improvement of non-alcoholic steatohepatitis with a prebiotic: A pilot clinical trial. Eur. J. Nutr. 58, 1735–1745. doi: 10.1007/s00394-018-1721-2
Bose, S., Ramesh, V., and Locasale, J. W. (2019). Acetate metabolism in physiology. cancer, and beyond. Trends Cell. Biol. 29, 695–703. doi: 10.1016/j.tcb.2019.05.005
Boulangé, C. L., Neves, A. L., Chilloux, J., Nicholson, J. K., and Dumas, M.-E. (2016). Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 8:42. doi: 10.1186/s13073-016-0303-2
Bridgeman, S. C., Northrop, W., Melton, P. E., Ellison, G. C., Newsholme, P., and Mamotte, C. D. S. (2020). Butyrate generated by gut microbiota and its therapeutic role in metabolic syndrome. Pharmacol. Res. 160:105174. doi: 10.1016/j.phrs.2020.105174
Canfora, E. E., Meex, R. C. R., Venema, K., and Blaak, E. E. (2019). Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 15, 261–273. doi: 10.1038/s41574-019-0156-z
Canfora, E. E., van der Beek, C. M., Jocken, J. W. E., Goossens, G. H., Holst, J. J., Olde Damink, S. W. M., et al. (2017). Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: A randomized crossover trial. Sci. Rep. 7:2360. doi: 10.1038/s41598-017-02546-x
Cani, P. D., Depommier, C., Derrien, M., Everard, A., and de Vos, W. M. (2022). Author correction: Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 19:682. doi: 10.1038/s41575-022-00650-6
Carpi, R. Z., Barbalho, S. M., Sloan, K. P., Laurindo, L. F., Gonzaga, H. F., Grippa, P. C., et al. (2022). The effects of probiotics, prebiotics and synbiotics in non-alcoholic fat liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): A systematic review. Int. J. Mol. Sci. 23:8805. doi: 10.3390/ijms23158805
Chalasani, N., Younossi, Z., Lavine, J. E., Charlton, M., Cusi, K., Rinella, M., et al. (2018). The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357. doi: 10.1002/hep.29367
Chaudhari, S. N., Luo, J. N., Harris, D. A., Aliakbarian, H., Yao, L., Paik, D., et al. (2021). A microbial metabolite remodels the gut-liver axis following bariatric surgery. Cell Host Microbe 29, 408–424.e7. doi: 10.1016/j.chom.2020.12.004
Chen, H.-C., Chen, Y.-Z., Wang, C.-H., and Lin, F.-J. (2020). The nonalcoholic fatty liver disease-like phenotype and lowered serum VLDL are associated with decreased expression and DNA hypermethylation of hepatic ApoB in male offspring of ApoE deficient mothers fed a with Western diet. J. Nutr. Biochem. 77:108319. doi: 10.1016/j.jnutbio.2019.108319
Chen, T., Zhou, K., Sun, T., Sang, C., Jia, W., and Xie, G. (2022). Altered bile acid glycine: Taurine ratio in the progression of chronic liver disease. J. Gastroenterol. Hepatol. 37, 208–215. doi: 10.1111/jgh.15709
Chiang, J. Y. L. (2015). Sphingosine-1-phosphate receptor 2: A novel bile acid receptor and regulator of hepatic lipid metabolism? Hepatology 61, 1118–1120. doi: 10.1002/hep.27616
Chiang, J. Y. L., and Ferrell, J. M. (2019). Bile acids as metabolic regulators and nutrient sensors. Annu. Rev. Nutr. 39, 175–200. doi: 10.1146/annurev-nutr-082018-124344
Cho, Y.-E., Kim, D.-K., Seo, W., Gao, B., Yoo, S.-H., and Song, B.-J. (2021). Fructose promotes leaky gut, endotoxemia, and liver fibrosis through ethanol-inducible cytochrome P450-2E1-mediated oxidative and nitrative stress. Hepatology 73, 2180–2195. doi: 10.1002/hep.30652
Collado, M. C., Derrien, M., Isolauri, E., de Vos, W. M., and Salminen, S. (2007). Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol. 73, 7767–7770. doi: 10.1128/AEM.01477-07
Damms-Machado, A., Mitra, S., Schollenberger, A. E., Kramer, K. M., Meile, T., Königsrainer, A., et al. (2015). Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed. Res. Int. 2015:806248. doi: 10.1155/2015/806248
Davenport, E. R., Sanders, J. G., Song, S. J., Amato, K. R., Clark, A. G., and Knight, R. (2017). The human microbiome in evolution. BMC Biol. 15:127. doi: 10.1186/s12915-017-0454-7
de Vos, W. M., Tilg, H., Van Hul, M., and Cani, P. D. (2022). Gut microbiome and health: Mechanistic insights. Gut 71, 1020–1032. doi: 10.1136/gutjnl-2021-326789
Demir, M., Lang, S., Martin, A., Farowski, F., Wisplinghoff, H., Vehreschild, M. J. G. T., et al. (2020). Phenotyping non-alcoholic fatty liver disease by the gut microbiota: Ready for prime time? J. Gastroenterol. Hepatol. 35, 1969–1977. doi: 10.1111/jgh.15071
Depommier, C., Van Hul, M., Everard, A., Delzenne, N. M., De Vos, W. M., and Cani, P. D. (2020). Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes 11, 1231–1245. doi: 10.1080/19490976.2020.1737307
Di Ciaula, A., Bonfrate, L., and Portincasa, P. (2022). The role of microbiota in nonalcoholic fatty liver disease. Eur. J. Clin. Invest. 52:e13768. doi: 10.1111/eci.13768
Dixon, L. J., Barnes, M., Tang, H., Pritchard, M. T., and Nagy, L. E. (2013). Kupffer cells in the liver. Compr. Physiol. 3, 785–797. doi: 10.1002/cphy.c120026
Dugas, L. R., Lie, L., Plange-Rhule, J., Bedu-Addo, K., Bovet, P., Lambert, E. V., et al. (2018). Gut microbiota, short chain fatty acids, and obesity across the epidemiologic transition: The METS-Microbiome study protocol. BMC Public Health 18:978. doi: 10.1186/s12889-018-5879-6
El-Kurjieh, A., Al-Arab, R., Hachem, Q. A., Ibrahim, J.-N., and Kobeissy, P. H. (2025). ACSS2 and metabolic diseases: From lipid metabolism to therapeutic target. Lipids Health Dis. 24, 1–17. doi: 10.1186/s12944-025-02491-z
Eslam, M., Newsome, P. N., Sarin, S. K., Anstee, Q. M., Targher, G., Romero-Gomez, M., et al. (2020). A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73, 202–209. doi: 10.1016/j.jhep.2020.03.039
Fadhlaoui, K., Arnal, M.-E., Martineau, M., Camponova, P., Ollivier, B., O’Toole, P. W., et al. (2020). Archaea, specific genetic traits, and development of improved bacterial live biotherapeutic products: Another face of next-generation probiotics. Appl. Microbiol. Biotechnol. 104, 4705–4716. doi: 10.1007/s00253-020-10599-8
Frost, F., Kacprowski, T., Rühlemann, M., Pietzner, M., Bang, C., Franke, A., et al. (2021). Long-term instability of the intestinal microbiome is associated with metabolic liver disease, low microbiota diversity, diabetes mellitus and impaired exocrine pancreatic function. Gut 70, 522–530. doi: 10.1136/gutjnl-2020-322753
Geier, A., Tiniakos, D., Denk, H., and Trauner, M. (2021). From the origin of NASH to the future of metabolic fatty liver disease. Gut 70, 1570–1579. doi: 10.1136/gutjnl-2020-323202
Grajeda-Iglesias, C., Durand, S., Daillère, R., Iribarren, K., Lemaitre, F., Derosa, L., et al. (2021). Oral administration of Akkermansia muciniphila elevates systemic antiaging and anticancer metabolites. Aging (Albany NY) 13, 6375–6405. doi: 10.18632/aging.202739
Gu, Y., Qin, X., Zhou, G., Wang, C., Mu, C., Liu, X., et al. (2022). Lactobacillus rhamnosus GG supernatant promotes intestinal mucin production through regulating 5-HT4R and gut microbiota. Food Funct. 13, 12144–12155. doi: 10.1039/d2fo01900k
Haak, B. W., Lankelma, J. M., Hugenholtz, F., Belzer, C., de Vos, W. M., and Wiersinga, W. J. (2019). Long-term impact of oral vancomycin, ciprofloxacin and metronidazole on the gut microbiota in healthy humans. J. Antimicrob. Chemother. 74, 782–786. doi: 10.1093/jac/dky471
Hammerich, L., and Tacke, F. (2023). Hepatic inflammatory responses in liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 20, 633–646. doi: 10.1038/s41575-023-00807-x
Han, Y., Ling, Q., Wu, L., Wang, X., Wang, Z., Chen, J., et al. (2023). Akkermansia muciniphila inhibits nonalcoholic steatohepatitis by orchestrating TLR2-activated γδT17 cell and macrophage polarization. Gut Microbes 15:2221485. doi: 10.1080/19490976.2023.2221485
Harrison, S. A., Bedossa, P., Guy, C. D., Schattenberg, J. M., Loomba, R., Taub, R., et al. (2024). A Phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N. Engl. J. Med. 390, 497–509. doi: 10.1056/NEJMoa2309000
Hernández, M. A. G., Canfora, E. E., Jocken, J. W. E., and Blaak, E. E. (2019). The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 11:1943. doi: 10.3390/nu11081943
Hsu, C. L., and Schnabl, B. (2023). The gut-liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 21, 719–733. doi: 10.1038/s41579-023-00904-3
Ioannou, A., Berkhout, M. D., Geerlings, S. Y., and Belzer, C. (2024). Akkermansia muciniphila: Biology, microbial ecology, host interactions and therapeutic potential. Nat. Rev. Microbiol. 23, 162–177. doi: 10.1038/s41579-024-01106-1
Jang, H. R., Park, H.-J., Kang, D., Chung, H., Nam, M. H., Lee, Y., et al. (2019). A protective mechanism of probiotic Lactobacillus against hepatic steatosis via reducing host intestinal fatty acid absorption. Exp. Mol. Med. 51:95. doi: 10.1038/s12276-019-0293-4
Jasirwan, C. O. M., Muradi, A., Hasan, I., Simadibrata, M., and Rinaldi, I. (2021). Correlation of gut Firmicutes/Bacteroidetes ratio with fibrosis and steatosis stratified by body mass index in patients with non-alcoholic fatty liver disease. Biosci. Microbiota Food Health 40, 50–58. doi: 10.12938/bmfh.2020-046
Jiao, N., Baker, S. S., Chapa-Rodriguez, A., Liu, W., Nugent, C. A., Tsompana, M., et al. (2018). Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 67, 1881–1891. doi: 10.1136/gutjnl-2017-314307
Joseph, N., Vasodavan, K., Saipudin, N. A., Yusof, B. N. M., Kumar, S., and Nordin, S. A. (2019). Gut microbiota and short-chain fatty acids (SCFAs) profiles of normal and overweight school children in Selangor after probiotics administration. J. Funct. Foods 57, 103–111. doi: 10.1016/j.jff.2019.03.042
Juanola, O., Martínez-López, S., Francés, R., and Gómez-Hurtado, I. (2021). Non-alcoholic fatty liver disease: Metabolic, genetic, epigenetic and environmental risk factors. Int. J. Environ. Res. Public Health 18:5227. doi: 10.3390/ijerph18105227
Kaur, A., Patankar, J. V., de Haan, W., Ruddle, P., Wijesekara, N., Groen, A. K., et al. (2015). Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes 64, 1168–1179. doi: 10.2337/db14-0716
Khan, R. S., Bril, F., Cusi, K., and Newsome, P. N. (2019). Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology 70, 711–724. doi: 10.1002/hep.30429
Kim, S., Lee, Y., Kim, Y., Seo, Y., Lee, H., Ha, J., et al. (2020). Akkermansia muciniphila prevents fatty liver disease, decreases serum triglycerides, and maintains gut homeostasis. Appl. Environ. Microbiol. 86:e03004-19. doi: 10.1128/AEM.03004-19
Kim, S., Shin, Y.-C., Kim, T.-Y., Kim, Y., Lee, Y.-S., Lee, S.-H., et al. (2021). Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 13, 1–20. doi: 10.1080/19490976.2021.1892441
Kim, W., Lee, E., Bae, I., Myoung, K., Kim, S., Park, P., et al. (2020). Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J. Extracell. Vesicles 9:1793514. doi: 10.1080/20013078.2020.1793514
Knudsen, C., Neyrinck, A. M., Lanthier, N., and Delzenne, N. M. (2019). Microbiota and nonalcoholic fatty liver disease: Promising prospects for clinical interventions? Curr. Opin. Clin. Nutr. Metab. Care 22, 393–400. doi: 10.1097/MCO.0000000000000584
Koeth, R. A., Wang, Z., Levison, B. S., Buffa, J. A., Org, E., Sheehy, B. T., et al. (2013). Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585. doi: 10.1038/nm.3145
Lai, Z., Chen, J., Ding, C., Wong, K., Chen, X., Pu, L., et al. (2020). Association of hepatic global DNA methylation and serum one-carbon metabolites with histological severity in patients with NAFLD. Obesity 28, 197–205. doi: 10.1002/oby.22667
Lecomte, V., Kaakoush, N. O., Maloney, C. A., Raipuria, M., Huinao, K. D., Mitchell, H. M., et al. (2015). Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One 10:e0126931. doi: 10.1371/journal.pone.0126931
León-Mimila, P., Villamil-Ramírez, H., Li, X. S., Shih, D. M., Hui, S. T., Ocampo-Medina, E., et al. (2021). Trimethylamine N-oxide levels are associated with NASH in obese subjects with type 2 diabetes. Diabetes Metab. 47:101183. doi: 10.1016/j.diabet.2020.07.010
Li, T., Lin, X., Shen, B., Zhang, W., Liu, Y., Liu, H., et al. (2023). Corrigendum: Akkermansia muciniphila suppressing nonalcoholic steatohepatitis associated tumorigenesis through CXCR6+ natural killer T cells. Front. Immunol. 14:1297103. doi: 10.3389/fimmu.2023.1297103
Li, Y., Liang, X., Lyu, Y., Wang, K., Han, L., Wang, Y., et al. (2023). Association between the gut microbiota and nonalcoholic fatty liver disease: A two-sample Mendelian randomization study. Digestive Liver Dis. 55, 1464–1471. doi: 10.1016/j.dld.2023.07.014
Li, Z., Yi, C.-X., Katiraei, S., Kooijman, S., Zhou, E., Chung, C. K., et al. (2018). Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 67, 1269–1279. doi: 10.1136/gutjnl-2017-314050
Litvak, Y., Byndloss, M. X., and Bäumler, A. J. (2018). Colonocyte metabolism shapes the gut microbiota. Science 362:eaat9076. doi: 10.1126/science.aat9076
Liu, H., Huang, R., Shen, B., Huang, C., Zhou, Q., Xu, J., et al. (2024). Live Akkermansia muciniphila boosts dendritic cell retinoic acid synthesis to modulate IL-22 activity and mitigate colitis in mice. Microbiome 12:275. doi: 10.1186/s40168-024-01995-7
Liu, X., Zhao, F., Liu, H., Xie, Y., Zhao, D., and Li, C. (2021). Transcriptomics and metabolomics reveal the adaption of Akkermansia muciniphila to high mucin by regulating energy homeostasis. Sci. Rep. 11:9073. doi: 10.1038/s41598-021-88397-z
Liu, Y., Chen, K., Li, F., Gu, Z., Liu, Q., He, L., et al. (2020). Probiotic Lactobacillus rhamnosus GG prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice. Hepatology 71, 2050–2066. doi: 10.1002/hep.30975
Liu, Z., Zhang, Z., Huang, M., Sun, X., Liu, B., Guo, Q., et al. (2018). Taurocholic acid is an active promoting factor, not just a biomarker of progression of liver cirrhosis: Evidence from a human metabolomic study and in vitro experiments. BMC Gastroenterol. 18:112. doi: 10.1186/s12876-018-0842-7
Lloyd-Price, J., Abu-Ali, G., and Huttenhower, C. (2016). The healthy human microbiome. Genome Med. 8:51. doi: 10.1186/s13073-016-0307-y
Lokau, J., Schoeder, V., Haybaeck, J., and Garbers, C. (2019). Jak-stat signaling induced by interleukin-6 family cytokines in hepatocellular carcinoma. Cancers (Basel) 11:1704. doi: 10.3390/cancers11111704
Longo, M., Meroni, M., Paolini, E., Erconi, V., Carli, F., Fortunato, F., et al. (2021). TM6SF2/PNPLA3/MBOAT7 loss-of-function genetic variants impact on NAFLD development and progression both in patients and in in vitro models. Cell. Mol. Gastroenterol. Hepatol. 13, 759–788. doi: 10.1016/j.jcmgh.2021.11.007
Loomba, R., Seguritan, V., Li, W., Long, T., Klitgord, N., Bhatt, A., et al. (2017). Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell. Metab. 25, 1054–1062.e5. doi: 10.1016/j.cmet.2017.04.001
Macnaughtan, J., Figorilli, F., García-López, E., Lu, H., Jones, H., Sawhney, R., et al. (2020). A double-blind, randomized placebo-controlled trial of probiotic Lactobacillus casei Shirota in stable cirrhotic patients. Nutrients 12:1651. doi: 10.3390/nu12061651
Magne, F., Gotteland, M., Gauthier, L., Zazueta, A., Pesoa, S., Navarrete, P., et al. (2020). The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 12:1474. doi: 10.3390/nu12051474
Makki, K., Deehan, E. C., Walter, J., and Bäckhed, F. (2018). The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23, 705–715. doi: 10.1016/j.chom.2018.05.012
Malaguarnera, M., Vacante, M., Antic, T., Giordano, M., Chisari, G., Acquaviva, R., et al. (2012). Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig. Dis. Sci. 57, 545–553. doi: 10.1007/s10620-011-1887-4
Maltz, R. M., Keirsey, J., Kim, S. C., Mackos, A. R., Gharaibeh, R. Z., Moore, C. C., et al. (2019). Social stress affects colonic inflammation, the gut microbiome, and short-chain fatty acid levels and receptors. J. Pediatr. Gastroenterol. Nutr. 68, 533–540. doi: 10.1097/MPG.0000000000002226
Mann, E. R., Lam, Y. K., and Uhlig, H. H. (2024). Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 24, 577–595. doi: 10.1038/s41577-024-01014-8
Martin-Gallausiaux, C., Marinelli, L., Blottière, H. M., Larraufie, P., and Lapaque, N. (2021). SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 80, 37–49. doi: 10.1017/S0029665120006916
McGavigan, A. K., Garibay, D., Henseler, Z. M., Chen, J., Bettaieb, A., Haj, F. G., et al. (2017). TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut 66, 226–234. doi: 10.1136/gutjnl-2015-309871
Meyer, K. A. (2020). Population studies of TMAO and its precursors may help elucidate mechanisms. Am. J. Clin. Nutr. 111, 1115–1116. doi: 10.1093/ajcn/nqaa068
Michaudel, C., and Sokol, H. (2020). The gut microbiota at the service of immunometabolism. Cell Metab. 32, 514–523. doi: 10.1016/j.cmet.2020.09.004
Michels, N., Zouiouich, S., Vanderbauwhede, B., Vanacker, J., Indave Ruiz, B. I., and Huybrechts, I. (2022). Human microbiome and metabolic health: An overview of systematic reviews. Obes. Rev. 23:e13409. doi: 10.1111/obr.13409
Moffett, J. R., Puthillathu, N., Vengilote, R., Jaworski, D. M., and Namboodiri, A. M. (2020). Acetate revisited: A key biomolecule at the nexus of metabolism, epigenetics and oncogenesis—part 1: Acetyl-CoA, acetogenesis and Acyl-CoA short-chain synthetases. Front. Physiol. 11:580167. doi: 10.3389/fphys.2020.580167
Mohamad Nor, M., Ayob, N., Mokhtar, N., Raja Ali, R., Tan, G., Wong, Z., et al. (2021). The effect of probiotics (MCP® BCMC® Strains) on hepatic steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease. Nutrients 13:3192. doi: 10.3390/nu13093192
Mouzaki, M., Wang, A. Y., Bandsma, R., Comelli, E. M., Arendt, B. M., Zhang, L., et al. (2016). Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS One 11:e0151829. doi: 10.1371/journal.pone.0151829
Mu, H., Zhou, Q., Yang, R., Zeng, J., Li, X., Zhang, R., et al. (2020). Naringin attenuates high fat diet induced non-alcoholic fatty liver disease and gut bacterial dysbiosis in mice. Front. Microbiol. 11:585066. doi: 10.3389/fmicb.2020.585066
Mudaliar, S., Henry, R. R., Sanyal, A. J., Morrow, L., Marschall, H.-U., Kipnes, M., et al. (2013). Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145, 574–582.e1. doi: 10.1053/j.gastro.2013.05.042
Neves, A. L., Chilloux, J., Sarafian, M. H., Rahim, M. B. A., Boulangé, C. L., and Dumas, M.-E. (2015). The microbiome and its pharmacological targets: Therapeutic avenues in cardiometabolic diseases. Curr. Opin. Pharmacol. 25, 36–44. doi: 10.1016/j.coph.2015.09.013
Newsome, P. N., Buchholtz, K., Cusi, K., Linder, M., Okanoue, T., Ratziu, V., et al. (2021). A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 384, 1113–1124. doi: 10.1056/NEJMoa2028395
Ng, S. C., Xu, Z., Mak, J. W. Y., Yang, K., Liu, Q., Zuo, T., et al. (2022). Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: A 24-week, double-blind, randomised controlled trial. Gut 71, 716–723. doi: 10.1136/gutjnl-2020-323617
Nian, F., Chen, Y., Xia, Q., Zhu, C., Wu, L., and Lu, X. (2024). Gut microbiota metabolite trimethylamine N-oxide promoted NAFLD progression by exacerbating intestinal barrier disruption and intrahepatic cellular imbalance. Int. Immunopharmacol. 142:113173. doi: 10.1016/j.intimp.2024.113173
Nogueira, M. A., Oliveira, C. P., Ferreira Alves, V. A., Stefano, J. T., Rodrigues, L. S. D. R., Torrinhas, R. S., et al. (2016). Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 35, 578–586. doi: 10.1016/j.clnu.2015.05.001
Okuka, N., Milinkovic, N., Velickovic, K., Polovina, S., Sumarac-Dumanovic, M., Minic, R., et al. (2024). Beneficial effects of a new probiotic formulation on adipocytokines, appetite-regulating hormones, and metabolic parameters in obese women. Food Funct. 15, 7658–7668. doi: 10.1039/d4fo01269k
Ottman, N., Davids, M., Suarez-Diez, M., Boeren, S., Schaap, P. J., and Martins Dos, et al. (2017). Genome-scale model and omics analysis of metabolic capacities of Akkermansia muciniphila reveal a preferential mucin-degrading lifestyle. Appl. Environ. Microbiol. 83:e01014-17. doi: 10.1128/AEM.01014-17
Pabst, O., Hornef, M. W., Schaap, F. G., Cerovic, V., Clavel, T., and Bruns, T. (2023). Gut-liver axis: Barriers and functional circuits. Nat. Rev. Gastroenterol. Hepatol. 20, 447–461. doi: 10.1038/s41575-023-00771-6
Pant, R., Sharma, N., Kabeer, S. W., Sharma, S., and Tikoo, K. (2023). Selenium-enriched probiotic alleviates western diet-induced non-alcoholic fatty liver disease in rats via modulation of autophagy through AMPK/SIRT-1 pathway. Biol. Trace Elem. Res. 201, 1344–1357. doi: 10.1007/s12011-022-03247-x
Park, E.-J., Lee, Y.-S., Kim, S. M., Park, G.-S., Lee, Y. H., Jeong, D. Y., et al. (2020). Beneficial effects of Lactobacillus plantarum strains on non-alcoholic fatty liver disease in high fat/high fructose diet-fed rats. Nutrients 12:542. doi: 10.3390/nu12020542
Park, M., Park, E.-J., Kim, S.-H., and Lee, H.-J. (2021). Lactobacillus plantarum ATG-K2 and ATG-K6 ameliorates high-fat with high-fructose induced intestinal inflammation. Int. J. Mol. Sci. 22:4444. doi: 10.3390/ijms22094444
Parséus, A., Sommer, N., Sommer, F., Caesar, R., Molinaro, A., Ståhlman, M., et al. (2017). Microbiota-induced obesity requires farnesoid X receptor. Gut 66, 429–437. doi: 10.1136/gutjnl-2015-310283
Parthasarathy, G., Revelo, X., and Malhi, H. (2020). Pathogenesis of nonalcoholic steatohepatitis: An overview. Hepatol. Commun. 4, 478–492. doi: 10.1002/hep4.1479
Pathak, P., Xie, C., Nichols, R. G., Ferrell, J. M., Boehme, S., Krausz, K. W., et al. (2018). Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68, 1574–1588. doi: 10.1002/hep.29857
Perino, A., Demagny, H., Velazquez-Villegas, L., and Schoonjans, K. (2021). Molecular physiology of bile acid signaling in health, disease, and aging. Physiol. Rev. 101, 683–731. doi: 10.1152/physrev.00049.2019
Pichler, M. J., Yamada, C., Shuoker, B., Alvarez-Silva, C., Gotoh, A., Leth, M. L., et al. (2020). Butyrate producing colonic Clostridiales metabolise human milk oligosaccharides and cross feed on mucin via conserved pathways. Nat. Commun 11:3285. doi: 10.1038/s41467-020-17075-x
Plovier, H., Everard, A., Druart, C., Depommier, C., Van Hul, M., Geurts, L., et al. (2017). A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113. doi: 10.1038/nm.4236
Portincasa, P., Di Ciaula, A., Garruti, G., Vacca, M., De Angelis, M., and Wang, D. Q.-H. (2020). Bile acids and GPBAR-1: Dynamic interaction involving genes, environment and gut microbiome. Nutrients 12:3709. doi: 10.3390/nu12123709
Pral, L. P., Fachi, J. L., Corrêa, R. O., Colonna, M., and Vinolo, M. A. R. (2021). Hypoxia and HIF-1 as key regulators of gut microbiota and host interactions. Trends Immunol. 42, 604–621. doi: 10.1016/j.it.2021.05.004
Puri, P., Daita, K., Joyce, A., Mirshahi, F., Santhekadur, P. K., Cazanave, S., et al. (2018). The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 67, 534–548. doi: 10.1002/hep.29359
Rao, Y., Kuang, Z., Li, C., Guo, S., Xu, Y., Zhao, D., et al. (2021). Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes 13, 1–19. doi: 10.1080/19490976.2021.1927633
Rodríguez, J. M., Murphy, K., Stanton, C., Ross, R. P., Kober, O. I., Juge, N., et al. (2015). The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 26:26050. doi: 10.3402/mehd.v26.26050
Romano, K. A., Martinez-Del Campo, A., Kasahara, K., Chittim, C. L., Vivas, E. I., Amador-Noguez, D., et al. (2017). Metabolic, epigenetic, and transgenerational effects of gut bacterial choline consumption. Cell Host Microbe 22, 279–290.e7. doi: 10.1016/j.chom.2017.07.021
Romano, K. A., Vivas, E. I., Amador-Noguez, D., and Rey, F. E. (2015). Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 6:e02481. doi: 10.1128/mBio.02481-14
Sahuri-Arisoylu, M., Brody, L. P., Parkinson, J. R., Parkes, H., Navaratnam, N., Miller, A. D., et al. (2016). Reprogramming of hepatic fat accumulation and “browning” of adipose tissue by the short-chain fatty acid acetate. Int. J. Obes. 40, 955–963. doi: 10.1038/ijo.2016.23
Sanyal, A. J., Chalasani, N., Kowdley, K. V., McCullough, A., Diehl, A. M., Bass, N. M., et al. (2010). Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362, 1675–1685. doi: 10.1056/NEJMoa0907929
Shang, Y., Wang, X., Shang, X., Zhang, H., Liu, Z., Yin, T., et al. (2016). Repetitive transcranial magnetic stimulation effectively facilitates spatial cognition and synaptic plasticity associated with increasing the levels of BDNF and synaptic proteins in Wistar rats. Neurobiol. Learn. Mem. 134, 369–378. doi: 10.1016/j.nlm.2016.08.016
Sheka, A. C., Adeyi, O., Thompson, J., Hameed, B., Crawford, P. A., and Ikramuddin, S. (2020). Nonalcoholic Steatohepatitis: A review. JAMA 323, 1175–1183. doi: 10.1001/jama.2020.2298
Shen, F., Zheng, R.-D., Sun, X.-Q., Ding, W.-J., Wang, X.-Y., and Fan, J.-G. (2017). Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 16, 375–381. doi: 10.1016/S1499-3872(17)60019-5
Shin, J.-H., Lee, Y., Song, E.-J., Lee, D., Jang, S.-Y., Byeon, H. R., et al. (2023). Faecalibacterium prausnitzii prevents hepatic damage in a mouse model of NASH induced by a high-fructose high-fat diet. Front. Microbiol. 14:1123547. doi: 10.3389/fmicb.2023.1123547
Song, Q., Guo, J. X., Ma, Y. X., Ou, T., Zhang, J., Li, H. Z., et al. (2023). Taurine alleviated hepatic steatosis in oleic acid-treated-HepG2 cells and rats fed a high-fat diet. Heliyon 9:e16401. doi: 10.1016/j.heliyon.2023.e16401
Tang, R., Liu, R., Zha, H., Cheng, Y., Ling, Z., and Li, L. (2024). Gut microbiota induced epigenetic modifications in the non-alcoholic fatty liver disease pathogenesis. Eng. Life Sci. 24:2300016. doi: 10.1002/elsc.202300016
Teng, Y., Wang, Y., Guan, W., Wang, C., Yu, H., Li, X., et al. (2022). Effect of Lactobacillus plantarum LP104 on hyperlipidemia in high-fat diet induced C57BL/6N mice via alteration of intestinal microbiota. J. Funct. Foods 95:105176. doi: 10.1016/j.jff.2022.105176
Teunis, C., Nieuwdorp, M., and Hanssen, N. (2022). Interactions between tryptophan metabolism, the gut microbiome and the immune system as potential drivers of non-alcoholic fatty liver disease (NAFLD) and metabolic diseases. Metabolites 12: 514. doi: 10.3390/metabo12060514
Tilg, H., Adolph, T. E., and Trauner, M. (2022). Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 34, 1700–1718. doi: 10.1016/j.cmet.2022.09.017
Tomova, A., Bukovsky, I., Rembert, E., Yonas, W., Alwarith, J., Barnard, N. D., et al. (2019). The effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 6:47. doi: 10.3389/fnut.2019.00047
Torres, S., Baulies, A., Insausti-Urkia, N., Alarcón-Vila, C., Fucho, R., Solsona-Vilarrasa, E., et al. (2019). Endoplasmic reticulum stress-induced upregulation of STARD1 promotes acetaminophen-induced acute liver failure. Gastroenterology 157, 552–568. doi: 10.1053/j.gastro.2019.04.023
Trépo, E., and Valenti, L. (2020). Update on NAFLD genetics: From new variants to the clinic. J. Hepatol. 72, 1196–1209. doi: 10.1016/j.jhep.2020.02.020
Tsai, M.-C., Liu, Y.-Y., Lin, C.-C., Wang, C.-C., Wu, Y.-J., Yong, C.-C., et al. (2020). Gut microbiota dysbiosis in patients with biopsy-proven nonalcoholic fatty liver disease: A cross-sectional study in Taiwan. Nutrients 12:820. doi: 10.3390/nu12030820
Turck, D., Bohn, T., Castenmiller, J., De Henauw, S., Hirsch-Ernst, K. I., Maciuk, A., et al. (2021). Safety of pasteurised Akkermansia muciniphila as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 19:e06780. doi: 10.2903/j.efsa.2021.6780
Vasques-Monteiro, I. M. L., Silva-Veiga, F. M., Miranda, C. S., de Andrade Gonçalves, ÉC. B., Daleprane, J. B., and Souza-Mello, V. (2021). A rise in Proteobacteria is an indicator of gut-liver axis-mediated nonalcoholic fatty liver disease in high-fructose-fed adult mice. Nutr. Res. 91, 26–35. doi: 10.1016/j.nutres.2021.04.008
Vilar-Gomez, E., Martinez-Perez, Y., Calzadilla-Bertot, L., Torres-Gonzalez, A., Gra-Oramas, B., Gonzalez-Fabian, L., et al. (2015). Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, 367–378.e5. doi: 10.1053/j.gastro.2015.04.005
Visekruna, A., and Luu, M. (2021). The role of short-chain fatty acids and bile acids in intestinal and liver function, inflammation, and carcinogenesis. Front. Cell. Dev. Biol. 9:703218. doi: 10.3389/fcell.2021.703218
von Schwartzenberg, R. J., Bisanz, J. E., Lyalina, S., Spanogiannopoulos, P., Ang, Q. Y., Cai, J., et al. (2021). Caloric restriction disrupts the microbiota and colonization resistance. Nature 595, 272–277. doi: 10.1038/s41586-021-03663-4
Wahlström, A., Aydin, Ö, Olsson, L. M., Sjöland, W., Henricsson, M., Lundqvist, A., et al. (2024). Alterations in bile acid kinetics after bariatric surgery in patients with obesity with or without type 2 diabetes. EBioMedicine 106:105265. doi: 10.1016/j.ebiom.2024.105265
Wang, B., Chen, X., Chen, Z., Xiao, H., Dong, J., Li, Y., et al. (2023). Stable colonization of Akkermansia muciniphila educates host intestinal microecology and immunity to battle against inflammatory intestinal diseases. Exp. Mol. Med. 55, 55–68. doi: 10.1038/s12276-022-00911-z
Wang, G., Jin, S., Huang, W., Li, Y., Wang, J., Ling, X., et al. (2021). LPS-induced macrophage HMGB1-loaded extracellular vesicles trigger hepatocyte pyroptosis by activating the NLRP3 inflammasome. Cell Death Discov. 7, 1–11. doi: 10.1038/s41420-021-00729-0
Wang, H., Guo, Y., Han, W., Liang, M., Xiao, X., Jiang, X., et al. (2024). Tauroursodeoxycholic acid improves nonalcoholic fatty liver disease by regulating gut microbiota and bile acid metabolism. J. Agric. Food Chem. 72, 20194–20210. doi: 10.1021/acs.jafc.4c04630
Wang, J., Zheng, D., Ge, K., Huang, F., Li, Y., Zheng, X., et al. (2024). Theabrownin alleviates nonalcoholic fatty liver disease by inhibiting the intestinal farnesoid X receptor–ceramide axis. Food Front. 5, 1559–1570. doi: 10.1002/fft2.394
Wang, M., Li, X. S., Wang, Z., de Oliveira Otto, M. C., Lemaitre, R. N., Fretts, A., et al. (2023). Trimethylamine N-oxide is associated with long-term mortality risk: The multi-ethnic study of atherosclerosis. Eur. Heart J. 44, 1608–1618. doi: 10.1093/eurheartj/ehad089
Wang, Y., Huang, Z., Gui, Z., Yang, B., You, F., Yang, G., et al. (2024). Supplementation with Akkermansia muciniphila improved intestinal barrier and immunity in zebrafish (Danio rerio). Fish. Shellfish. Immunol. 154:109935. doi: 10.1016/j.fsi.2024.109935
Wang, Y., Wang, Z., Wan, Y., Jin, F., Shi, X., Xing, Z., et al. (2023). Assessing the in vivo ameliorative effects of Lactobacillus acidophilus KLDS1.0901 for induced non-alcoholic fatty liver disease treatment. Front. Nutr. 10:1147423. doi: 10.3389/fnut.2023.1147423
Wang, Y., Zhang, Y., Yang, J., Li, H., Wang, J., and Geng, W. (2021). Lactobacillus plantarum MA2 ameliorates methionine and choline-deficient diet induced non-alcoholic fatty liver disease in rats by improving the intestinal microecology and mucosal barrier. Foods 10:3126. doi: 10.3390/foods10123126
Wu, W., Kaicen, W., Bian, X., Yang, L., Ding, S., Li, Y., et al. (2023). Akkermansia muciniphila alleviates high-fat-diet-related metabolic-associated fatty liver disease by modulating gut microbiota and bile acids. Microb. Biotechnol. 16, 1924–1939. doi: 10.1111/1751-7915.14293
Wu, X., Huang, H., Li, M., Wang, Y., Wu, X., Wang, Q., et al. (2023). Excessive consumption of the sugar rich longan fruit promoted the development of nonalcoholic fatty liver disease via mediating gut dysbiosis. Food Front. 4, 491–510. doi: 10.1002/fft2.185
Wu, Y.-L., Lin, Z.-J., Li, C.-C., Lin, X., Shan, S.-K., Guo, B., et al. (2023). Epigenetic regulation in metabolic diseases: Mechanisms and advances in clinical study. Signal. Transduct. Target. Ther. 8:98. doi: 10.1038/s41392-023-01333-7
Wu, Z., Xiao, Y., Zhou, F., Chen, J., Chen, X., Hou, A., et al. (2022). Pasteurized akkermansia muciniphila reduces fat accumulation via nhr-49-mediated nuclear hormone signaling pathway in Caenorhabditis elegans. Molecules 27:6159. doi: 10.3390/molecules27196159
Xie, C., Zhang, Z., Yang, M., Cao, C., Zhou, Y., Zhu, Z., et al. (2021). Lactiplantibacillus plantarum AR113 exhibit accelerated liver regeneration by regulating gut microbiota and plasma glycerophospholipid. Front. Microbiol. 12:800470. doi: 10.3389/fmicb.2021.800470
Xie, S., Li, J., Lyu, F., Xiong, Q., Gu, P., Chen, Y., et al. (2023). Novel tripeptide RKH derived from Akkermansia muciniphila protects against lethal sepsis. Gut 73, 78–91. doi: 10.1136/gutjnl-2023-329996
Xu, J., Xia, Q., Wu, T., Shao, Y., Wang, Y., Jin, N., et al. (2024). Prophylactic treatment with Bacteroides uniformis and Bifidobacterium bifidum counteracts hepatic NK cell immune tolerance in nonalcoholic steatohepatitis induced by high fat diet. Gut Microbes 16:2302065. doi: 10.1080/19490976.2024.2302065
Ye, J., Lv, L., Wu, W., Li, Y., Shi, D., Fang, D., et al. (2018). Butyrate protects mice against methionine-choline-deficient diet-induced non-alcoholic Steatohepatitis by improving gut barrier function, attenuating inflammation and reducing endotoxin levels. Front. Microbiol. 9:1967. doi: 10.3389/fmicb.2018.01967
Yoo, J. Y., and Kim, S. S. (2016). Probiotics and prebiotics: Present status and future perspectives on metabolic disorders. Nutrients 8:173. doi: 10.3390/nu8030173
Yoon, H. S., Cho, C. H., Yun, M. S., Jang, S. J., You, H. J., Kim, J.-H., et al. (2021). Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 6, 563–573. doi: 10.1038/s41564-021-00880-5
Younossi, Z. M., Koenig, A. B., Abdelatif, D., Fazel, Y., Henry, L., and Wymer, M. (2016). Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84. doi: 10.1002/hep.28431
Yu, J., Wu, Y., Zhu, Z., and Lu, H. (2025). The impact of dietary patterns on gut microbiota for the primary and secondary prevention of cardiovascular disease: A systematic review. Nutr. J. 24:17. doi: 10.1186/s12937-024-01060-x
Yu, J., Zhu, P., Shi, L., Gao, N., Li, Y., Shu, C., et al. (2024). Bifidobacterium longum promotes postoperative liver function recovery in patients with hepatocellular carcinoma. Cell Host Microbe 32, 131–144.e6. doi: 10.1016/j.chom.2023.11.011
Zeng, F., Su, X., Liang, X., Liao, M., Zhong, H., Xu, J., et al. (2024). Gut microbiome features and metabolites in non-alcoholic fatty liver disease among community-dwelling middle-aged and older adults. BMC Med. 22:104. doi: 10.1186/s12916-024-03317-y
Zhang, J., Ni, Y., Qian, L., Fang, Q., Zheng, T., Zhang, M., et al. (2021). Decreased abundance of Akkermansia muciniphila leads to the impairment of insulin secretion and glucose homeostasis in lean type 2 diabetes. Adv. Sci. 8:e2100536. doi: 10.1002/advs.202100536
Zhang, Y., Liu, R., Chen, Y., Cao, Z., Liu, C., Bao, R., et al. (2025). Akkermansia muciniphila supplementation in patients with overweight/obese type 2 diabetes: Efficacy depends on its baseline levels in the gut. Cell Metab. 37, 592–605.e6. doi: 10.1016/j.cmet.2024.12.010
Zhao, Y., Liu, J., Hao, W., Zhu, H., Liang, N., He, Z., et al. (2017). Structure-specific effects of short-chain fatty acids on plasma cholesterol concentration in male syrian hamsters. J. Agric. Food Chem. 65, 10984–10992. doi: 10.1021/acs.jafc.7b04666
Zhao, Z., Chen, L., Zhao, Y., Wang, C., Duan, C., Yang, G., et al. (2020). Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation. Appl. Microbiol. Biotechnol. 104, 5273–5282. doi: 10.1007/s00253-020-10633-9
Zheng, L., Kelly, C. J., Battista, K. D., Schaefer, R., Lanis, J. M., Alexeev, E. E., et al. (2017). Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of Claudin-2. J. Immunol. 199, 2976–2984. doi: 10.4049/jimmunol.1700105
Zhu, F.-L., Huang, T., Lv, Z.-L., Liang, G., Yao, Z., Lan, L.-C., et al. (2024). Taurine regulates the expression of interleukin -17/10 and intestinal flora and protects the liver and intestinal mucosa in a nonalcoholic fatty liver disease rat model. Diabetes Metab. Syndr. Obes. 17, 675–689. doi: 10.2147/DMSO.S440978
Keywords: NAFLD (non-alcoholic fatty liver disease), metabolic associated fatty liver disease (MAFLD), prebiotics and probiotics, gut-liver axis, bile acid
Citation: Shen S, Liu Y, Wang N, Huang Z and Deng G (2025) The role of microbiota in nonalcoholic fatty liver disease: mechanism of action and treatment strategy. Front. Microbiol. 16:1621583. doi: 10.3389/fmicb.2025.1621583
Received: 01 May 2025; Accepted: 03 July 2025;
Published: 30 July 2025.
Edited by:
Yan Liu, Southwest University, ChinaCopyright © 2025 Shen, Liu, Wang, Huang and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guifang Deng, bWlzeWZseUAxNjMuY29t
 Siwen Shen1
Siwen Shen1 Guifang Deng
Guifang Deng